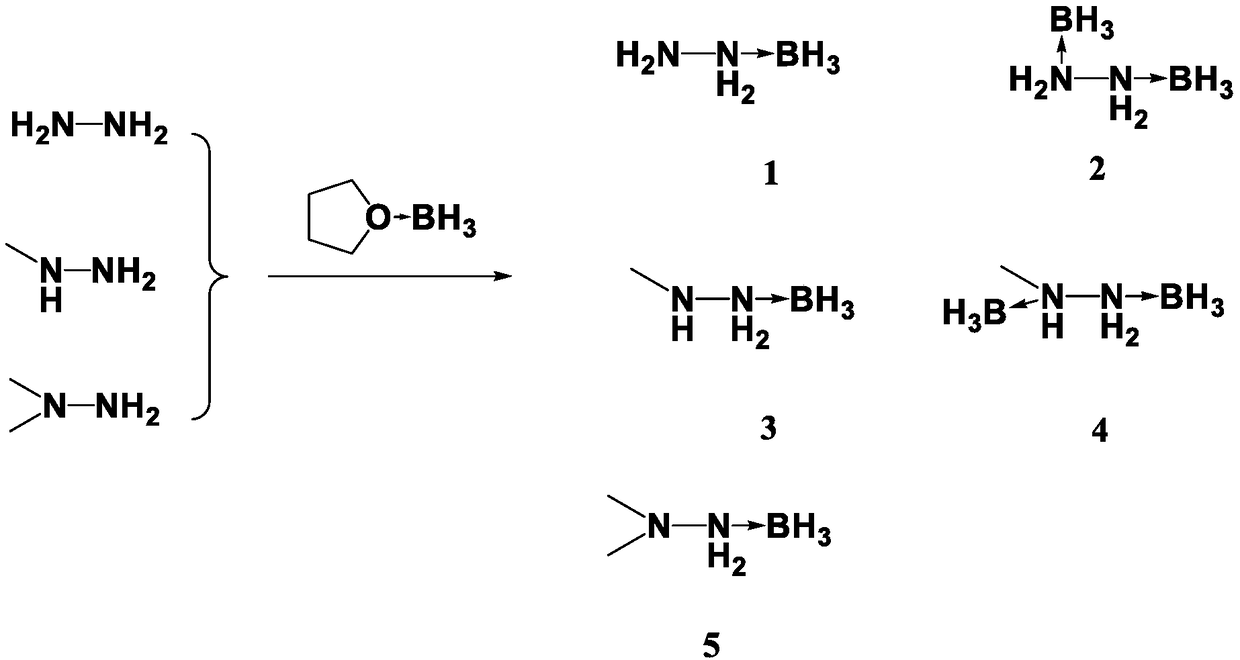
Preparation method for propellant fuel
A technology of propellant and fuel, applied in the field of propellant fuel and its preparation, can solve the problems of harmfulness to the environment and human health, increase in cost, high volatility, etc., and achieve the effects of reducing hazard, high safety and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
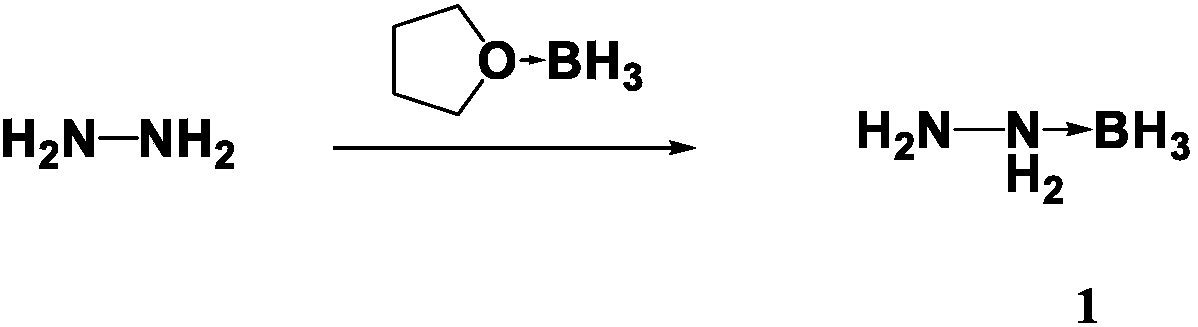
[0018] Dissolve 1.6ml of hydrazine (32g / mol) in 30ml of anhydrous tetrahydrofuran solution, stir and mix evenly, slowly add 75ml of borane tetrahydrofuran solution with a concentration of 1M at room temperature, stir and mix with magnetic force, and react at room temperature after the borane solution is added After 2h, the temperature was slowly raised to 70°C for 8h. After cooling down to room temperature, the tetrahydrofuran solution was spin-dried, and 50 ml of dichloromethane was added. After washing with acetone for several times, the dichloromethane was spin-dried to obtain a colorless transparent liquid 1, and the product yield was 89%. 1 H NMR(600MHz,DMSO-d6):δppm:1.5(s,2H),7.2(s,2H),0.04–0.40(m,3H,BH3).Elemental analysis,calcd(%)forBH7N2(46.07):H :15.38,N:61.06,Found,H:14.99,N:61.43.
Embodiment 2
[0020]
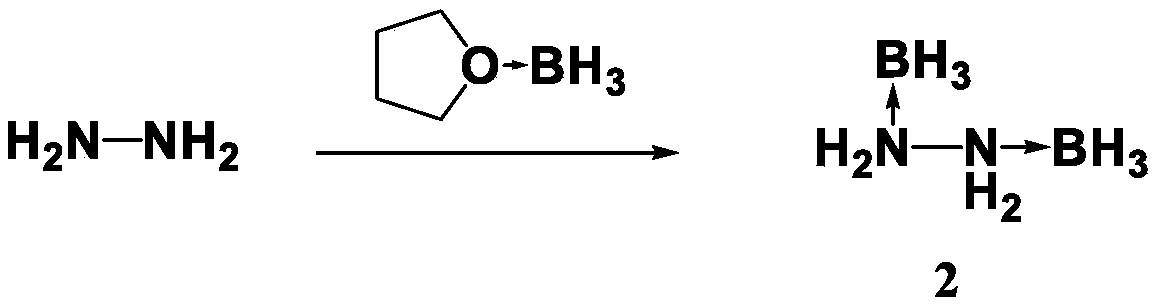
[0021] Dissolve 1.6ml of hydrazine (32g / mol) in 30ml of anhydrous tetrahydrofuran solution, stir and mix evenly, slowly add 110ml of borane tetrahydrofuran solution with a concentration of 1M at room temperature, stir and mix with magnetic force, and react at room temperature after the borane solution is added After 3h, the temperature was slowly raised to 70°C for 4h, and then the temperature was raised to 80°C for 10h. After cooling down to room temperature, spin dry the tetrahydrofuran solution, add 70ml of dichloromethane, wash with acetone for several times, spin dry the dichloromethane to obtain off-white liquid 2, and the product yield is 90.5%. 1 HNMR(600MHz,DMSO-d6):δppm:7.2(s,4H),0.09–0.42(m,6H,BH3).Elemental analysis,calcd(%)for B2H10N2(60.10):H:16.88,N:46.91, Found, H: 17.02, N: 47.22.
Embodiment 3
[0023]
[0024] Dissolve 2.3ml of monomethylhydrazine (46g / mol) in 30ml of anhydrous tetrahydrofuran solution, stir and mix evenly, slowly add 60ml of borane tetrahydrofuran solution with a concentration of 1M at room temperature, stir and mix with magnetic force, after the borane solution is added, After 2 hours of reaction at room temperature, the temperature was slowly raised to 70°C for 6 hours. After cooling down to room temperature, the tetrahydrofuran solution was spin-dried, and 40 ml of dichloromethane was added. After repeated washing with acetone, the dichloromethane was spin-dried to obtain a colorless transparent liquid 3, and the product yield was 87.3%. 1 H NMR(600MHz,DMSO-d6):δppm:1.5(s,H),2.47(m,3H,CH3),7.2(s,2H),0.04–0.40(m,3H,BH3).13C NMR(150MHz ,DMSO-d6):δ:ppm:34.15.Elemental analysis,calcd(%)for CH9BN2(60.09):C:20.05,H:15.14,N:46.76,Found,C:19.70,H:15.39,N:47.13 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com