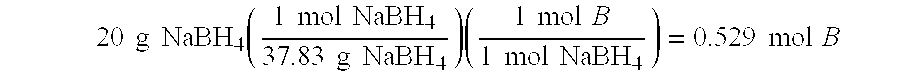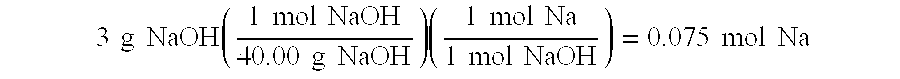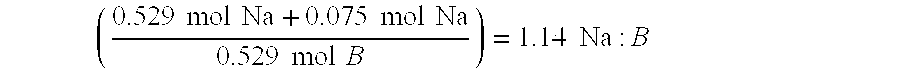Fuel blends for hydrogen generators
a hydrogen generator and fuel blend technology, applied in the field of fuel blends for hydrogen generators, can solve the problems of reduced hydrogen storage capacity, reduced water present and available for maintaining the borate product in solution, and reduced water content for hydrogen generation, so as to facilitate fuels, maximize the solubility of borate, and high gravimetric hydrogen storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generic Procedure for Hydrogen Generation from Fuel Blends
[0029] A generic description of a hydrogen generation test is described, using sodium borohydride, decaborane, sodium hydroxide and water in the fuel blend as a sample system. Fuel blends are made in open air. The water and stabilizing sodium hydroxide are initially mixed, decaborane is added thereto, followed by the proper amount of sodium borohydride to deliver the desired ratio.
[0030] To catalytically discharge the fuel blend, a Parr reactor resting on a hot plate is employed. The reactor incorporates two thermocouples that operated continuously during the run, one measuring the temperature of the fuel solution, and the other measuring the temperature of the head-space near the top of the reactor. The second thermocouple controls a cooling loop circulating through the reactor that is activated when the second thermocouple records a threshold temperature of 95° C. As a practical matter, however, even with the hot plate t...
example 2
7.0 wt-% Hydrogen, +IC / B Ratio=0.8
[0032] The following were mixed according to the procedure of Example 1 to yield 100 g of a fuel blend capable of delivering 7.0 weight-% H2, with a sodium to boron ratio of 0.8: sodium borohydride 27.16 g; sodium hydroxide 3 g; decaborane(14) 3.34 g and water 66.5 g. Each mole of sodium borohydride yields four moles of hydrogen according to the equation (1) above
NaBH4+2H2O→NaBO2+4H2 (1)
And each mole of decaborane(14) yields twenty-two moles of hydrogen according to equation (2)
B10H14+15H2O→5B2O3+22H2 (2)
[0033] The hydrogen storage capacity of this blend is determined by calculating the total number of moles of H2 produced and divided by the initial weight of the blend. 27.16 g NaBH4(1 mol NaBH437.83 g NaBH4)(4 mol H21 mol NaBH4)(2.0158 g H21 mol H2)=5.79 g H2 from NaBH43.34 g B10H14(1 mol B10H14122.21 g B10H14)(22 mol H21 mol B10H14)(2.0158 g H21 mol H2)=1.21 g ...
example 3
6.8 wt-% Hydrogen, +IC / B Ratio=0.25
[0035] The following were mixed according to the procedure of Example 1 to yield 100 g of a fuel blend capable of delivering 6.8 weight-% H2, with a sodium to boron ratio of 0.25: sodium triborohydride 13.74 g; sodium dodecahydrododecaborate 13.74 g and water 76.09 g. Each mole of sodium dodecahydrododecaborate yields twenty-five moles of hydrogen according to equation (3)
Na2B12H12+19H2O→2NaBO2+5B2O3+25H2 (3)
And each mole of sodium triborohydride yields nine moles of hydrogen according to equation (4)
NaB3H8+5H2O→NaBO2+B2O3+9H2 (4)
[0036] The hydrogen storage capacity of this blend is determined by calculating the total number of moles of H2 produced and divided by the initial weight of the blend. 10.17 g Na2B12H12(1 mol Na2B12H12187.79 g Na2B12H12)(25 mol H21 mol Na2B12H12) (2.0158 g H21 mol H2)=2.87 g H2 from Na2B12H1213.74 g NaB3H8(1 mol NaB3H863.48 g NaB3H8)(9 mol H21...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com