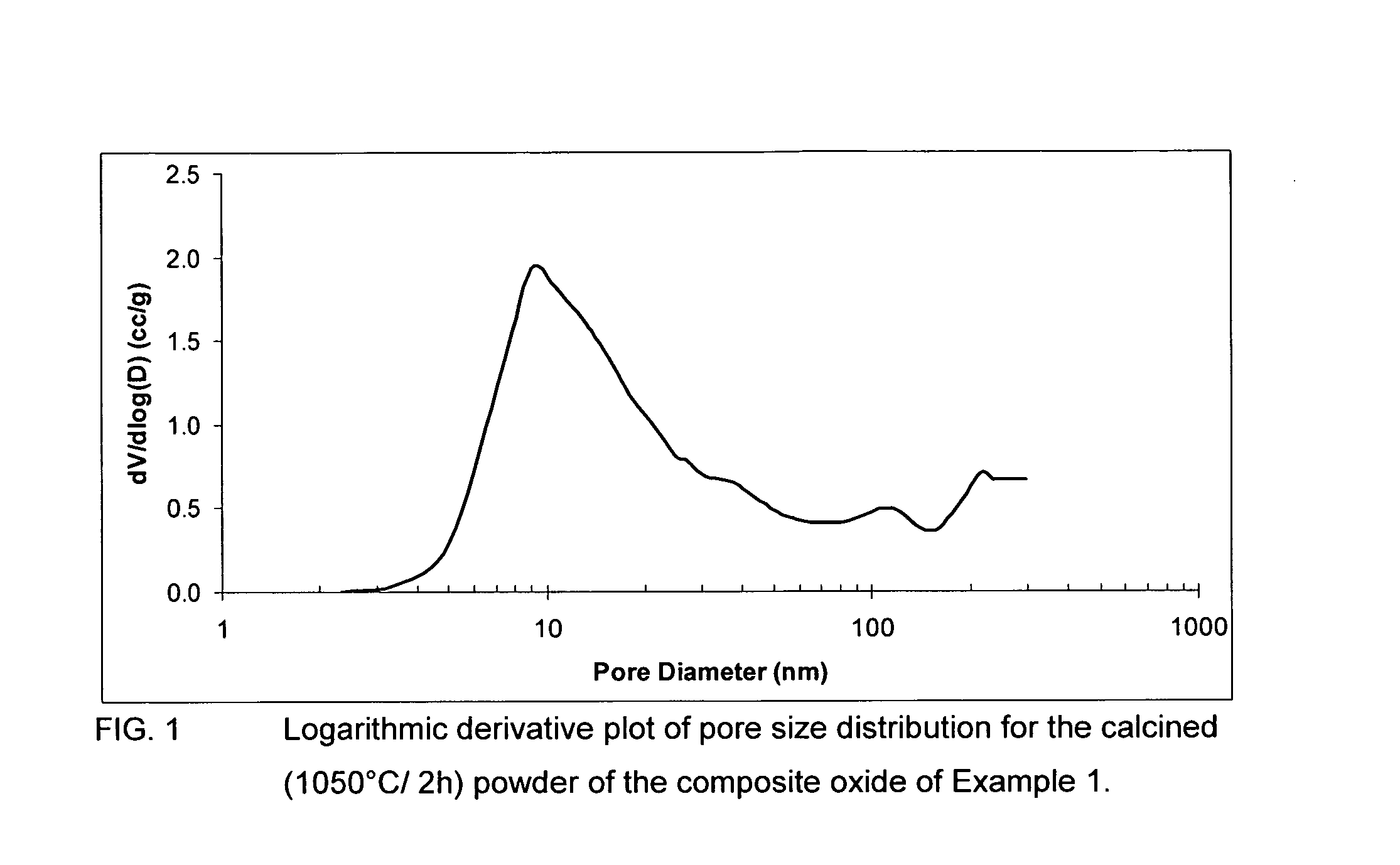
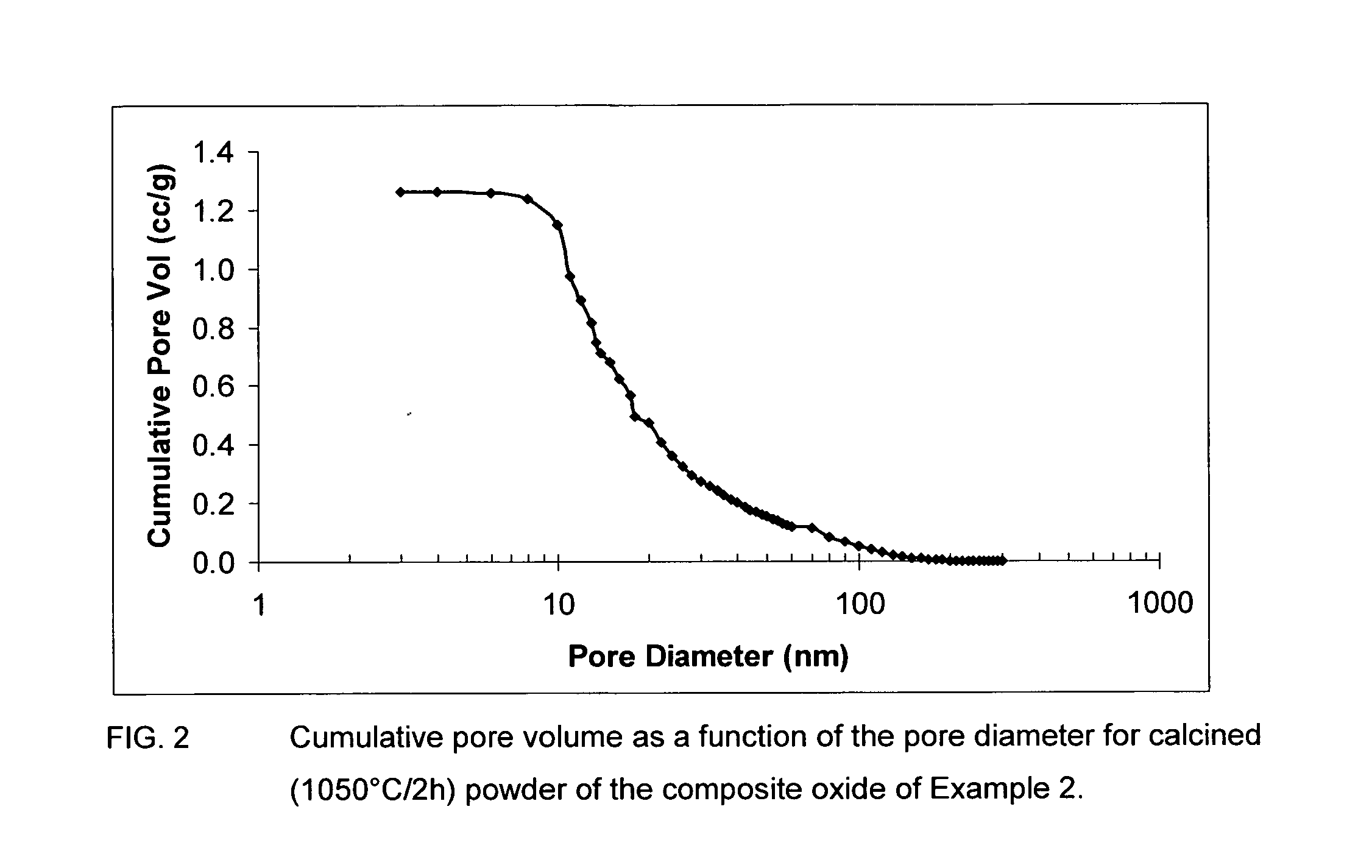
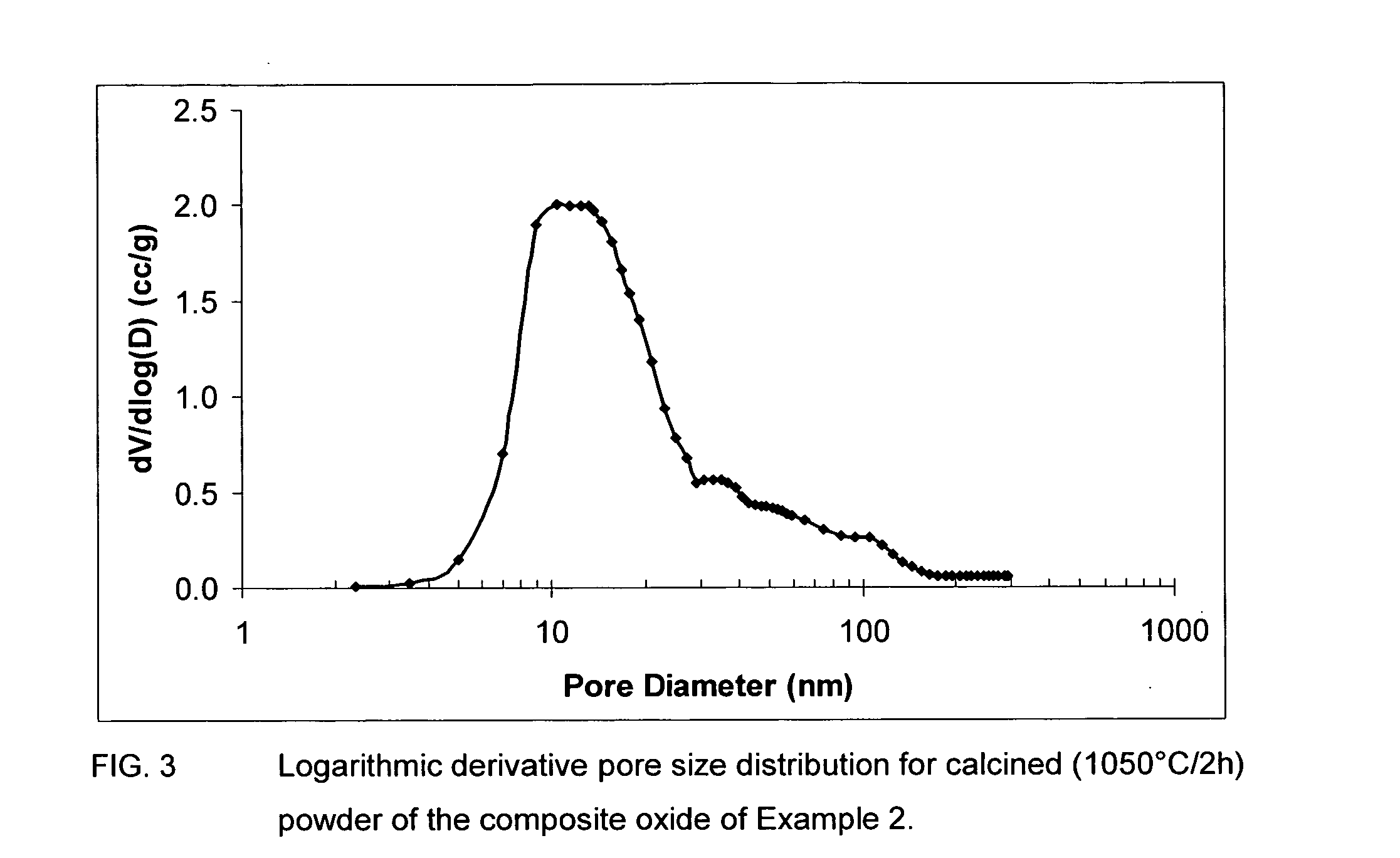
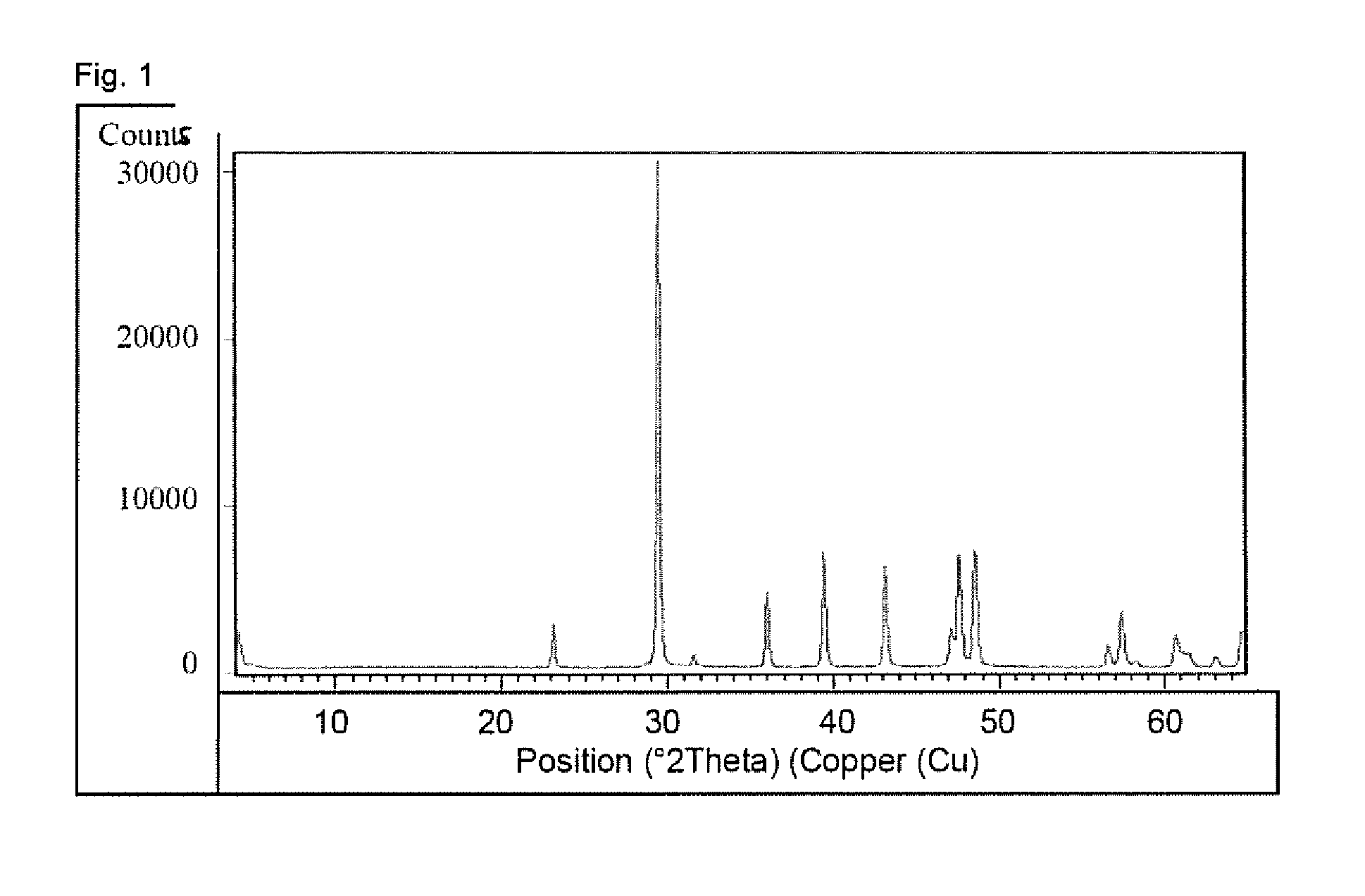
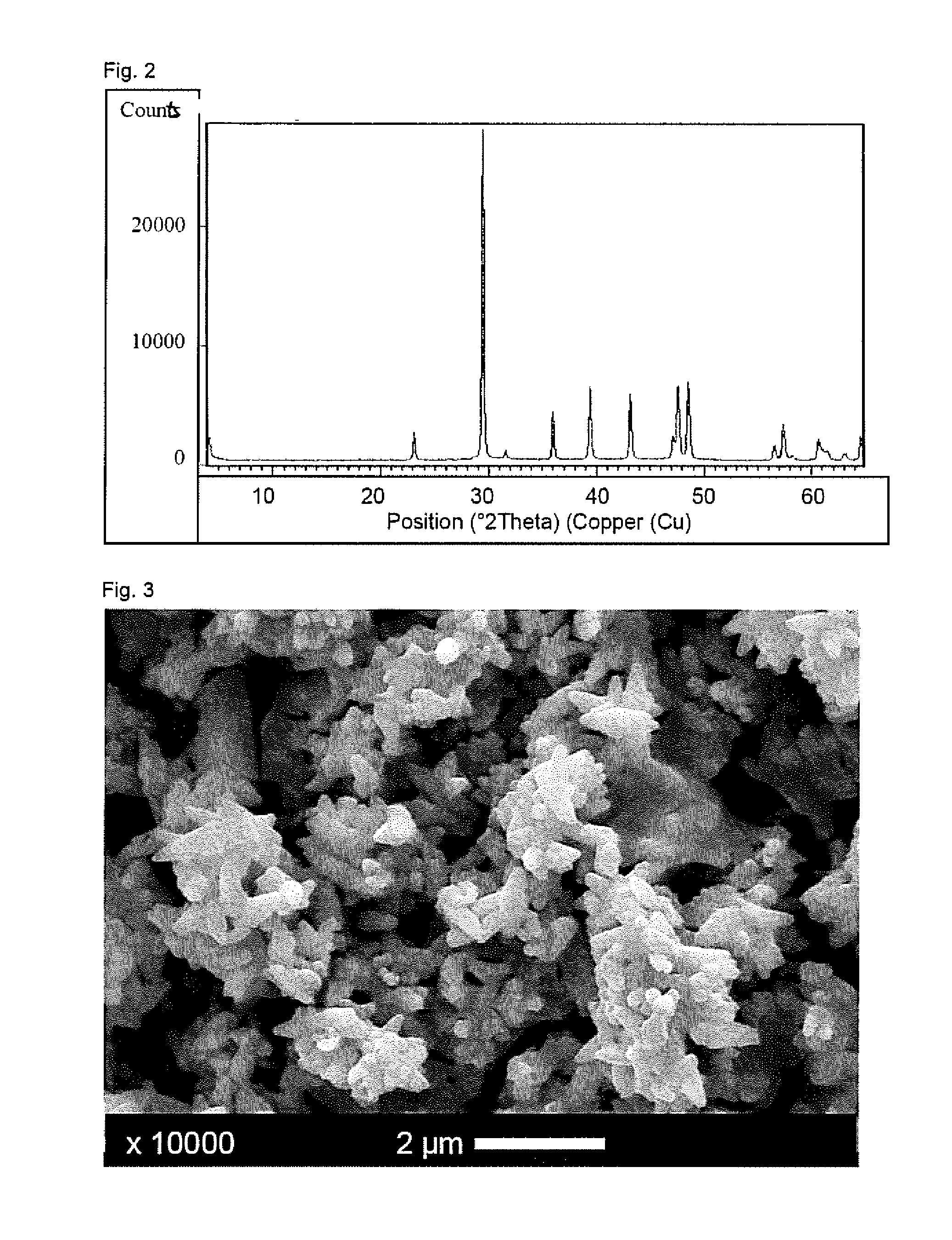
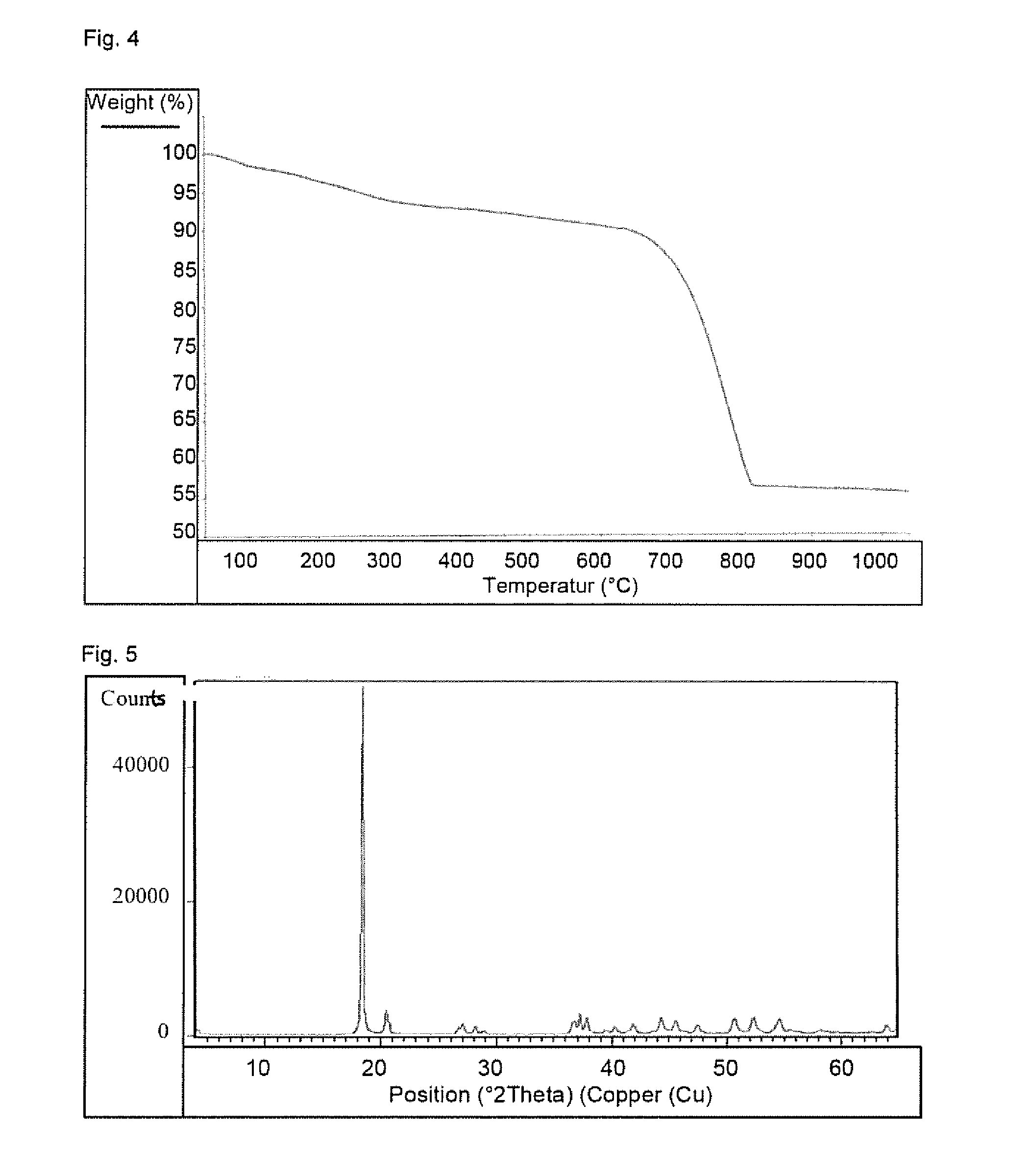
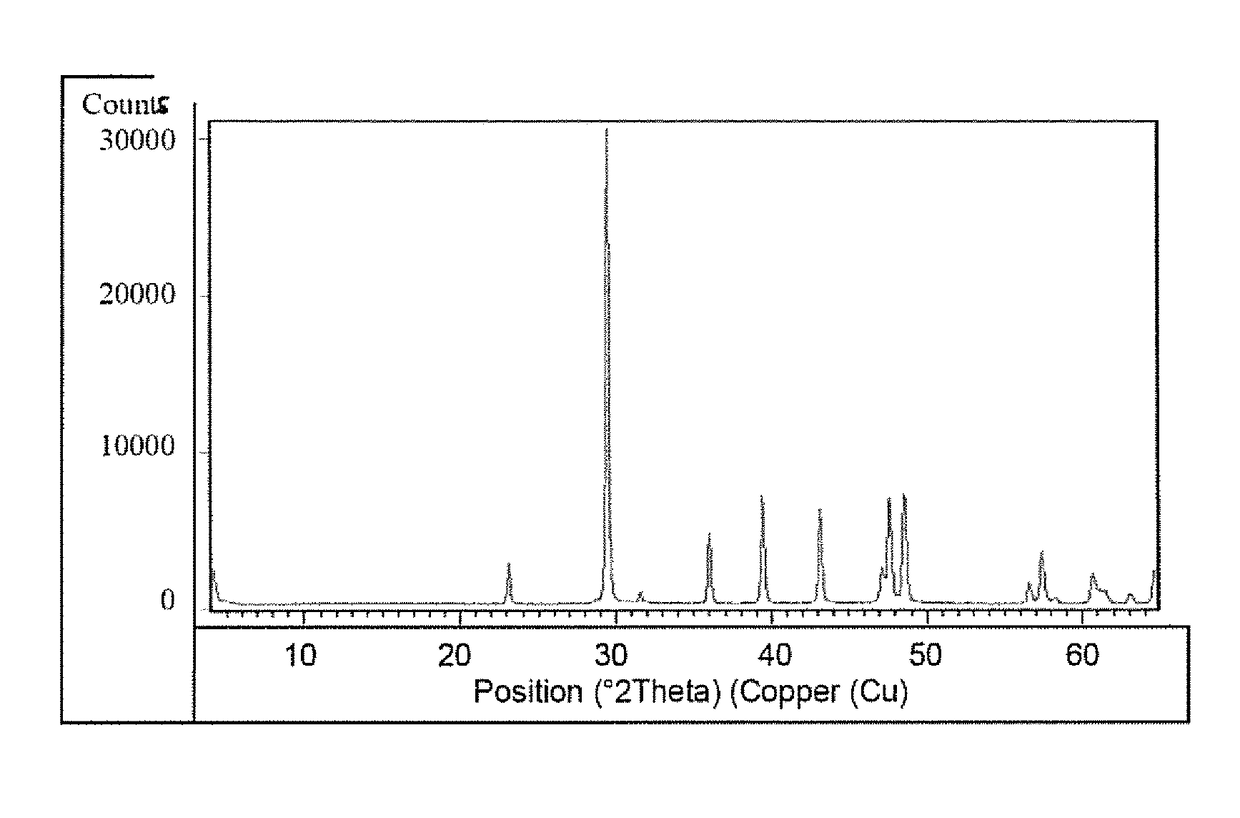
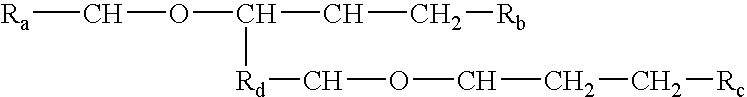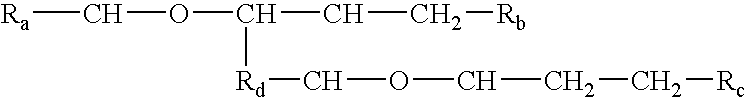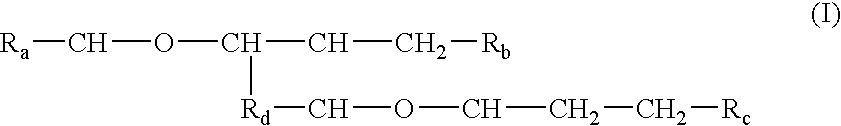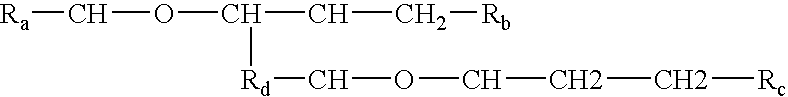Patents
Literature
30 results about "Aluminum Cation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aluminum ion requiring three electrons to return to its elemental state.
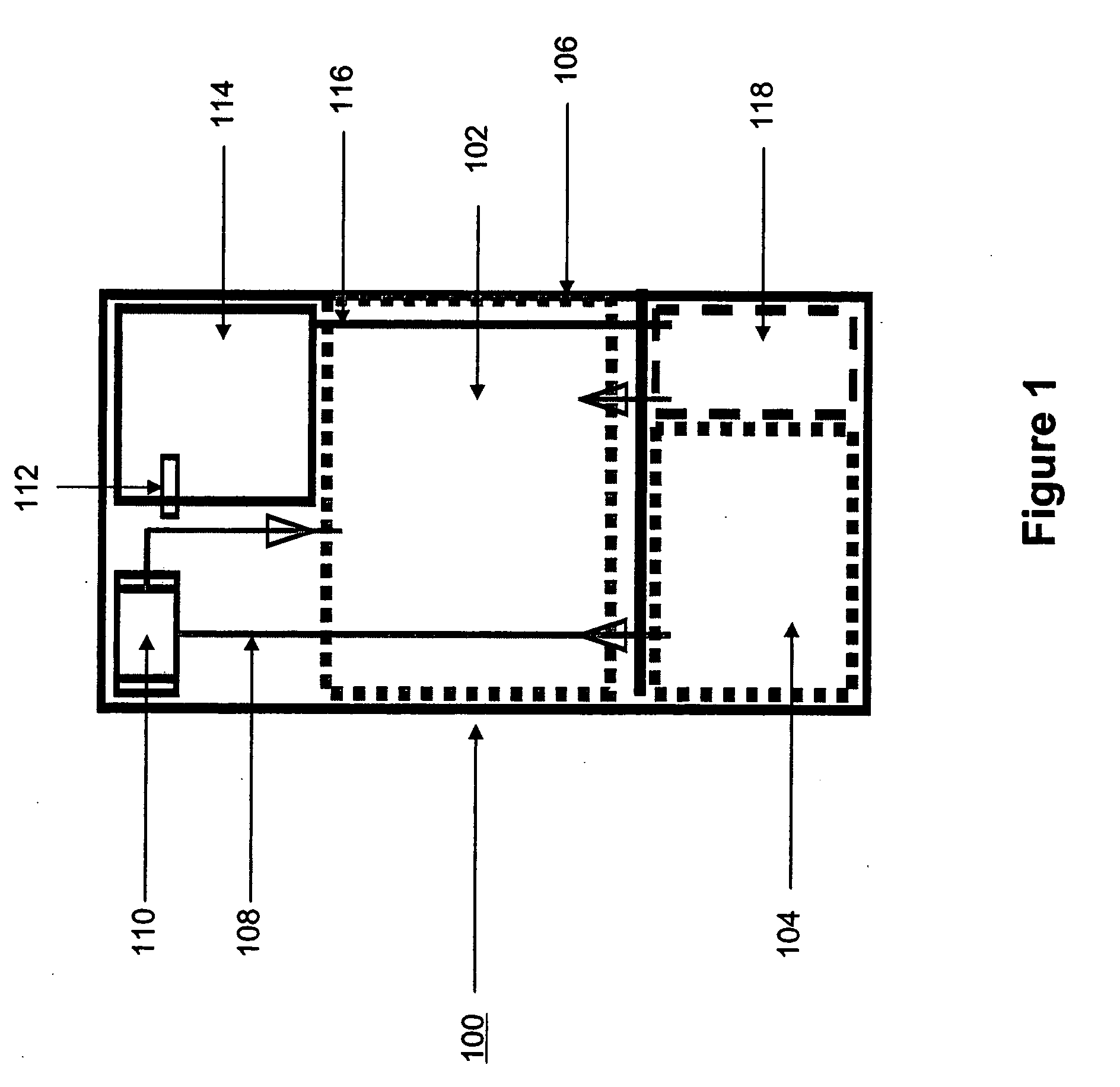
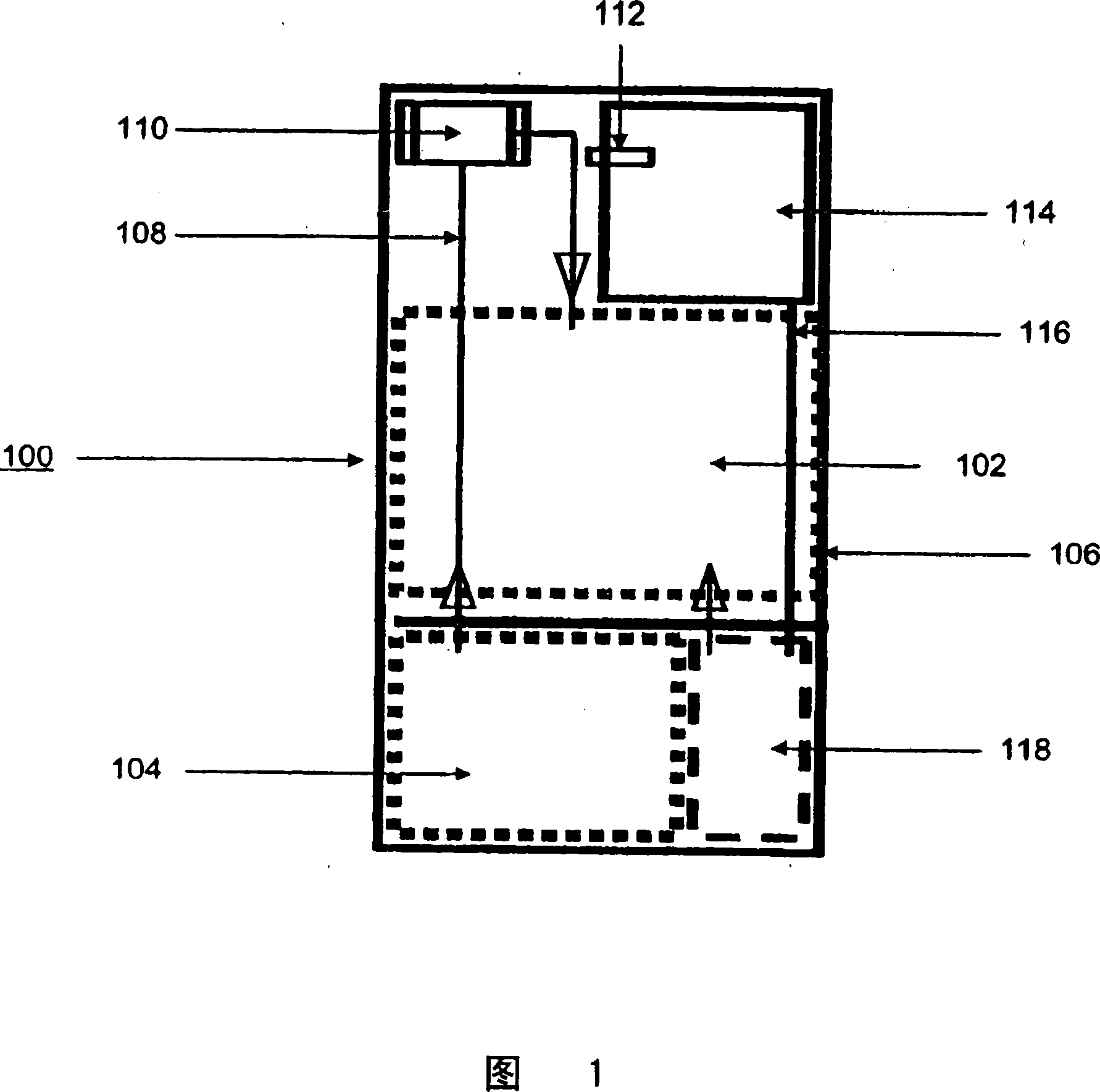
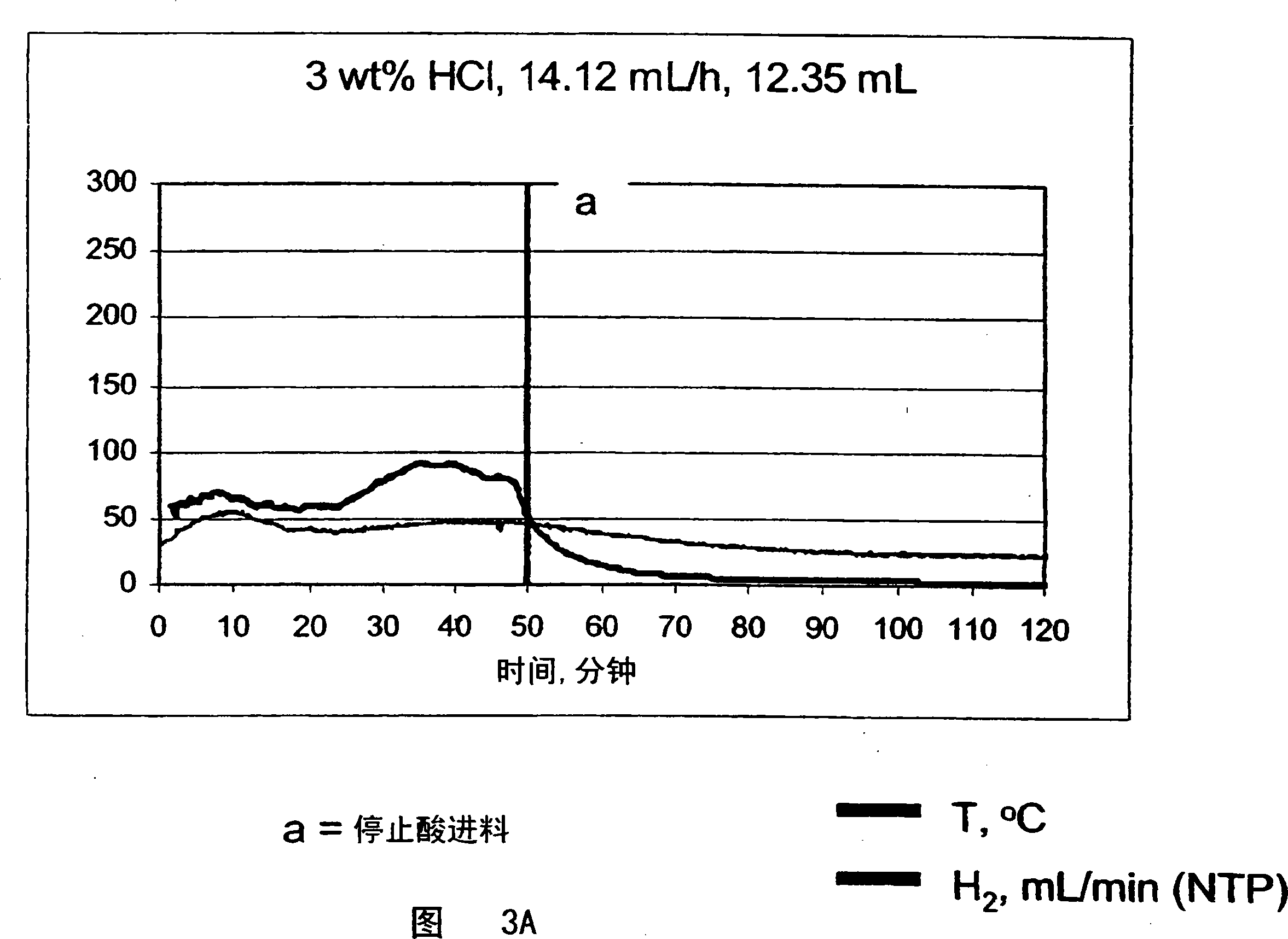
Systems and methods for hydrogen generation from solid hydrides
InactiveUS20050238573A1Regulate rateReactant parameters controlHydrogen productionO-Phosphoric AcidAlkaline earth metal
A system is disclosed for hydrogen generation based on hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
Borohydride fuel compositions and methods
Solid self-stabilized borohydride fuel compositions, fuel cartridges, related methods of preparation and hydrogen generation are provided. The fuel compositions comprise mixtures of at least one borohydride salt with a cation selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation, preferably sodium borohydride, and at least one hydroxide salt with a cation selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation, preferably sodium hydroxide.
Owner:MILLENNIUM CELL
Dechlorinating agent used for removing HCl from gas by dry method and preparation method thereof
InactiveCN101773768AImprove dechlorination activityHigh activityOther chemical processesAluminium silicatesPorosityCross-link
The invention relates to a dechlorinating agent used for removing HCl from a gas by using a dry method and a preparation method thereof. The dechlorinating agent is prepared from Na2CO3, CaCO3, CaO and MaO as active constituents, crosslinked bentonite as a porous auxiliary agent, and methyl cellulose as a foaming agent and an auxiliary extrusion agent through extrusion forming, drying and roasting. The cross-linked bentonite is prepared by exchanging large-size poly aluminum cation with small-size simple cation, so that the crosslinked bentonite has great porosity factor and large specific surface. The specific surface and the pore volume of the crosslinked bentonite are larger than those of non-crosslinked bentonite. By using the crosslinked bentonite as the porous auxiliary agent, the specific surface of the dechlorinating agent is enlarged, and the dechlorinating activity and the chlorosity of the dechlorinating agent are increased. The dechlorinating agent prepared by using the crosslinked bentonite has a pore volume of 0.3-0.4 mL / g, a specific surface of 70-90 m<2> / g and a crushing strength of 60-80 N / cm, not only has lower price than pseudo-boehmite and a molecular sieve, but also has simple preparation process, high dechlorinating activity and great low-temperature penetration chlorosity.
Owner:长春惠工净化工业有限公司
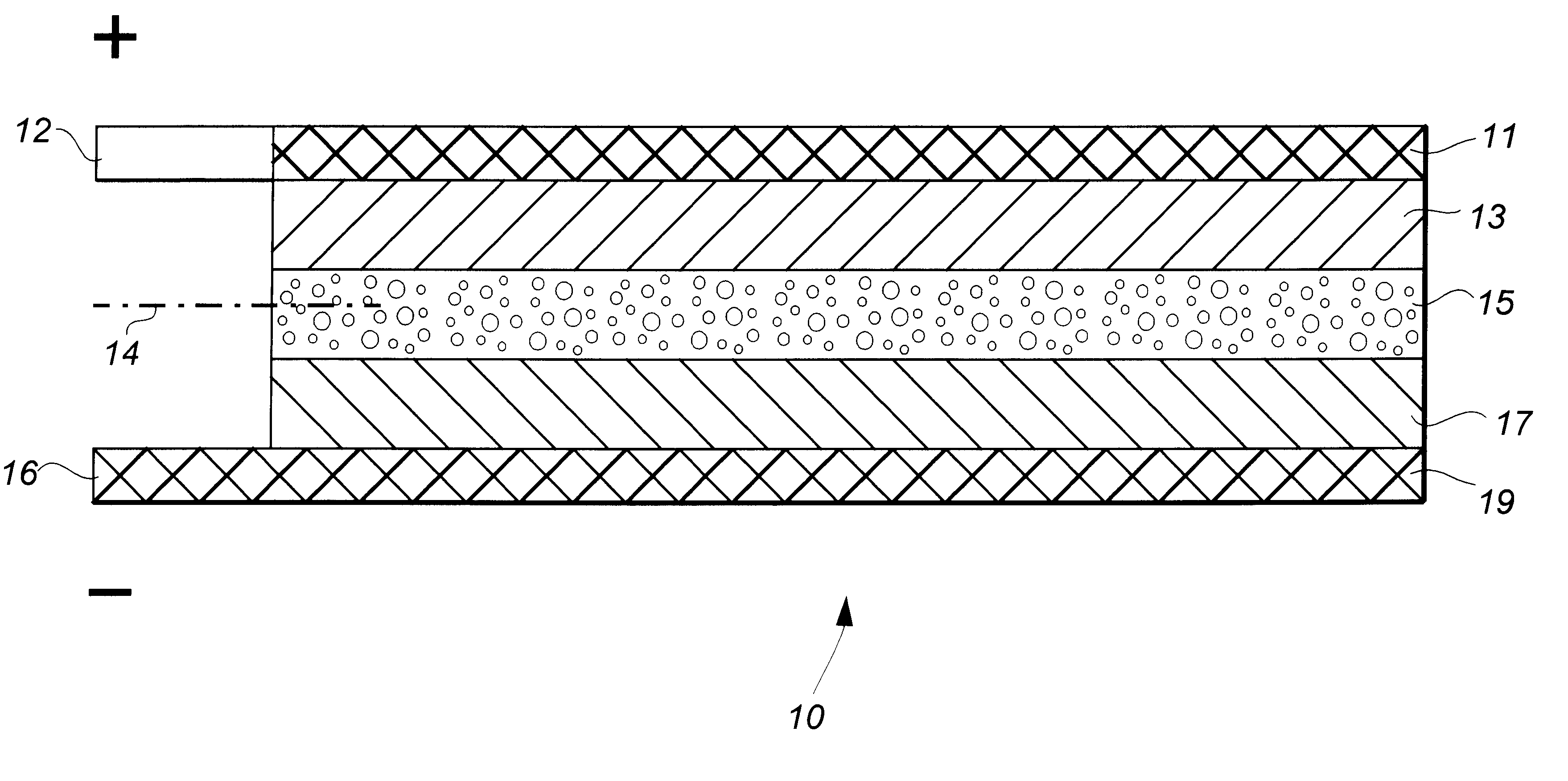
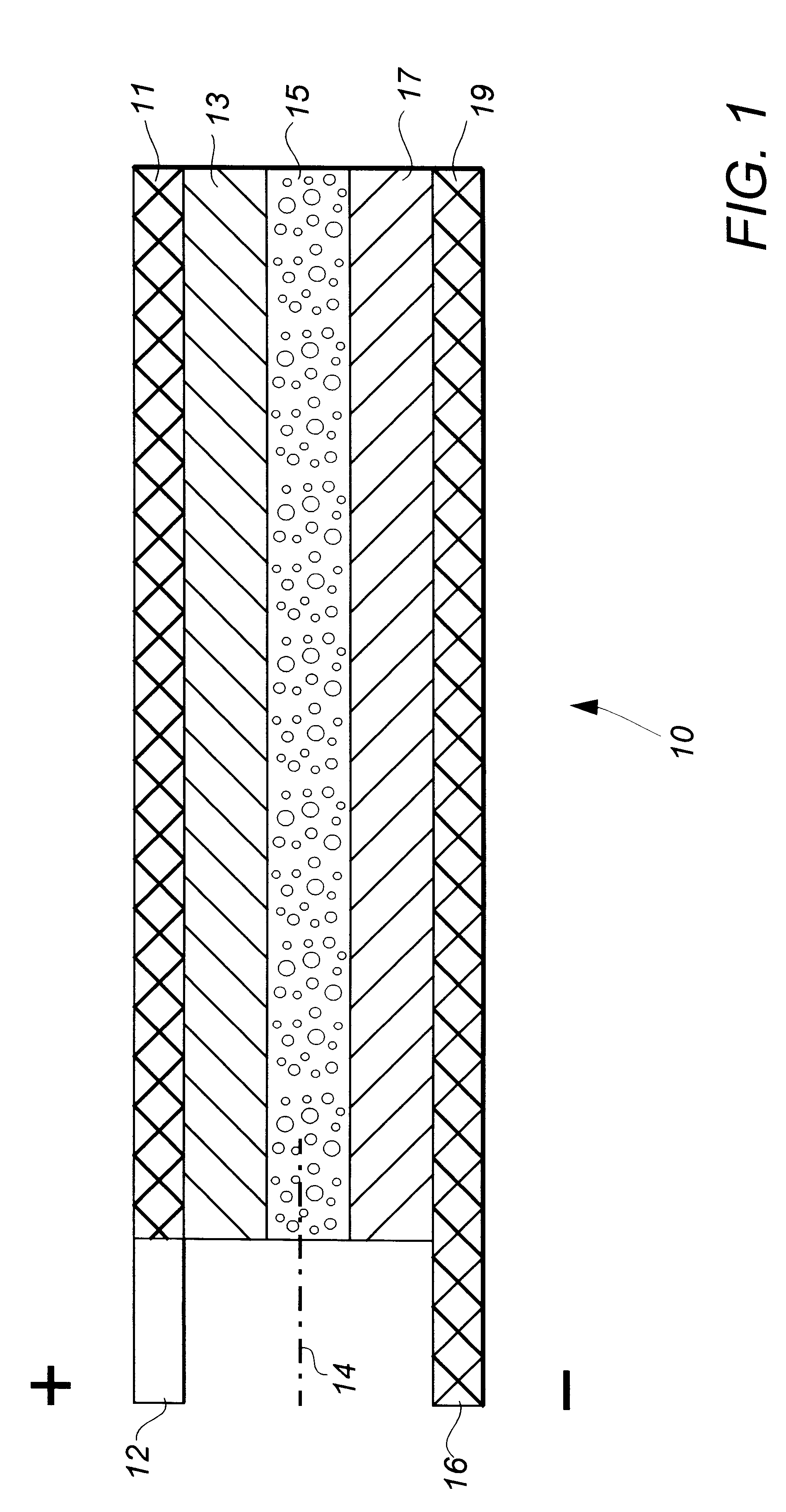
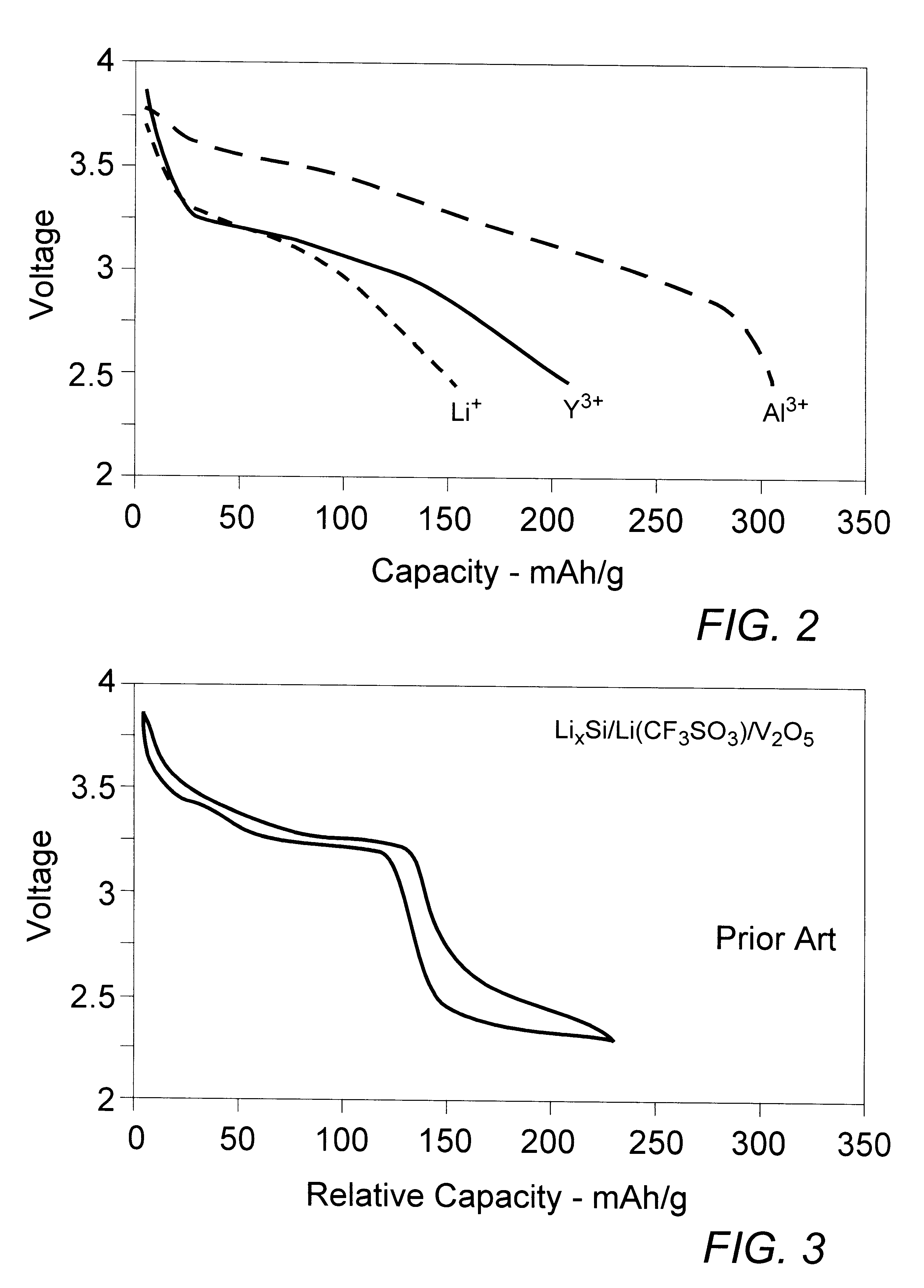
Lithium-aluminum dual-cation rechargeable electrochemical battery cell
InactiveUS6482548B2Lower potentialIncrease working voltageOrganic electrolyte cellsSolid electrolyte cellsLithiumAluminum Cation
A rechargeable battery cell (10) having high operating voltage and significantly increased specific capacity comprises a positive electrode member (13), a negative electrode member (17), and an interposed separator member (15) containing an electrolyte comprising a solution of a polyvalent aluminum cation solute in a non-aqueous solvent. The positive electrode member comprises an active material which reversibly takes up and releases the reactive polyvalent cation species during operation of the cell while the active material of the negative electrode contemporaneously reversibly releases into and takes up from the electrolyte solvent a monovalent cation species. Preferred cation species are those of aluminum, such as Al.sup.3+, and alkali metals, such as Li.sup.+.
Owner:RUTGERS THE STATE UNIV
Bacteria capturing treatment for fibrous webs
A fibrous web containing a composition capable to tract and / or trap negatively charged matter, such as bacteria and other pathogens, is generally disclosed. The bacteriostatic composition can be a multivalently charged metal ion, such as an aluminum cation, ligated to at least one surfactant. The surfactant can be an anionic surfactant, such as an alkyl sulfate. Also, methods of forming a fibrous web capable of trapping negatively charged matter is generally provided.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Host-guest processes and formulations for delivering bio-affecting compounds
InactiveUS20040228887A1Increase concentrationCosmetic preparationsBiocideSolubilityChemical structure
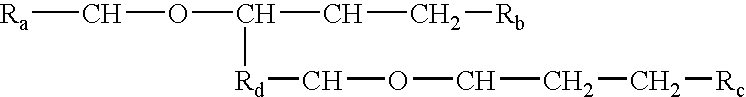
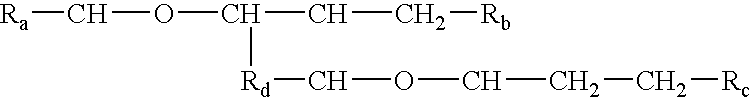
Processes and formulations are provided that are capable of protecting, stabilizing, and / or topically delivering one or more bio-affecting compounds. More particularly, the invention relates to processes of making a composition having a host compound capable of accepting one or more bio-affecting guest compounds, and new compositions formed by the processes. The processes and formulations can be used to protect and stabilize bio-affecting compounds of widely-different solubility characteristics. The processes include formulating a host composition having a host capable of accepting a guest, the processes comprising mixing, in any order: (i) a non-ionic surfactant s elected from the group consisting of compounds having a chemical structure: where "-CH-O-CH-" represents an epoxide group, where Ra and Rb are hydrocarbons that can be the same or different, where at least one of the Ra and Rb hydrocarbons includes an epoxide group within 3 carbons of the hydrocarbon attachment to contribute to the desired hydrolypid balance of 7-9, where Rc is hydrogen or a methyl group, and where Rd is a methylene group (-CH2-), an ethyl group (-CH2-CH2-), or a structurally equivalent link with a bond length range about the same as or shorter than that provided by an ethyl group, and having a hydro-lipid balance in the range of 7-9; (ii) an amphoteric surfactant selected from the group consisting of organic compounds having the chemical formula NH3-R-COOH, where R is a straight, branched, or aromatic hydrocarbon structure having 6-24 carbons; (iii) a solvent for the amphoteric surfactant; (iv) an aromatic selected from the group consisting of compounds having at least one aromatic five or six-member ring; (v) an aluminum cation; (vi) a Lewis acid that is not a Bronsted-Lowry acid; and (vii) a Bronsted-Lowry acid. According to a further aspect of the invention, one or more compounds are selected to be sequentially mixed with the host composition to form a stable molecular environment, which is sometimes referred to herein as a process of molecular stacking.
Owner:CHAMP CHARLES W +1
Inorganic intercalated vermiculite insulation refractory material and preparation method thereof
The invention relates to an inorganic intercalated vermiculite insulation refractory material and a preparation method thereof. In the technical scheme, the preparation method comprises the following steps: calcinating and activating raw vermiculite ore for 2-8 hours at the temperature of 500-650 DEG C, modifying for 2-10 hours in acid solution of 0.1-1.0mol / l, eluting and drying; preparing 2-5 weight percent of suspension with the eluted and dried vermiculite and poly-hydroxy-aluminum cation precursor solution, stirring for 4-8 hours at the temperature of 80-100 DEG C, eluting and drying, thereby obtaining poly-hydroxy-aluminum cation precursor intercalated vermiculite; calcinating the poly-hydroxy-aluminum cation precursor intercalated vermiculite for 4-6 hours at the temperature of 500-650 DEG C, thereby obtaining inorganic intercalated vermiculite; and finally mixing 40-80 weight percent of inorganic intercalated vermiculite and 20-60 weight percent of forsterite, adding 10-20 weight percent of aluminium dihydrogen phosphate, forming through stirring and pressing, and carrying out heat treatment at the temperature of 500-650 DEG C, thereby obtaining the inorganic intercalated vermiculite insulation refractory material. The invention has the advantages of low heat conduction coefficient at high temperature, high strength and wide application scope.
Owner:WUHAN UNIV OF SCI & TECH
Host-guest processes and formulations for delivering bio-affecting compounds
InactiveUS6676951B1Increase concentrationCosmetic preparationsBiocideChemical structureAluminum Cation
The invention relates to processes of making a composition having a host compound capable of accepting one or more bio-affecting guest compounds, and compositions formed by the processes. The processes comprise mixing, in any order: (i) a non-ionic surfactant selected from the group consisting of compounds having chemical structure (I) where "-CH-O-CH-" represents an epoxide group, where Ra and Rb are hydrocarbons that can be the same or different, where at least one of the Ra and Rb hydrocarbons includes an epoxide group within 3 carbons of the hydrocarbon attachment to contribute to the desired hydrolipid balance of 7-9, where Rc is hydrogen or a methyl group, and where Rd is a methylene group, and ethyl group, or a structurally equivalent link with a bond length range about the same as or shorter than that provided by an ethyl group, and having a hydro-lipid balance in the range 7-9; (ii) an amphoteric surfactant selected from the group consisting of organic compounds having the chemical formula NH3-R-COOH, where R is a straight, branched, or aromatic hydrocarbon structure having 6-24 carbons; (iii) a solvent for the amphoteric surfactant; (iv) an aromatic selected from the group consisting of compounds having at (I) least one aromatic five or six-member ring; (v) an aluminum cation; (vi) a Lewis acid that is not a Bronsted-Lowry acid; and (vii) a Bronsted-Lowry acid.
Owner:CHAMP CHARLES WALTON +1
Sulfur tolerant alumina catalyst support
InactiveUS20120122670A1Improve anti-sulfur poisoning performanceExtreme simplicityDispersed particle separationCatalyst activation/preparationCounterionSulfur
The present invention is directed to a method for making a sulfur tolerant alumina, that includes the steps of: forming aluminum hydrate from one or more water soluble aluminum salts, said salts each comprising an aluminum cation or aluminum anion and an oppositely charged counterion, in an aqueous medium, contacting the aluminum hydrate with a silica precursor in the aqueous medium and in the presence of counterions of the one or more aluminum salts, isolating silica precursor-contacted aluminum hydrate particles from the aqueous medium, and calcining the silica precursor-contacted aluminum hydrate particles to form particles of the sulfur tolerant alumina.
Owner:RHODIA OPERATIONS SAS
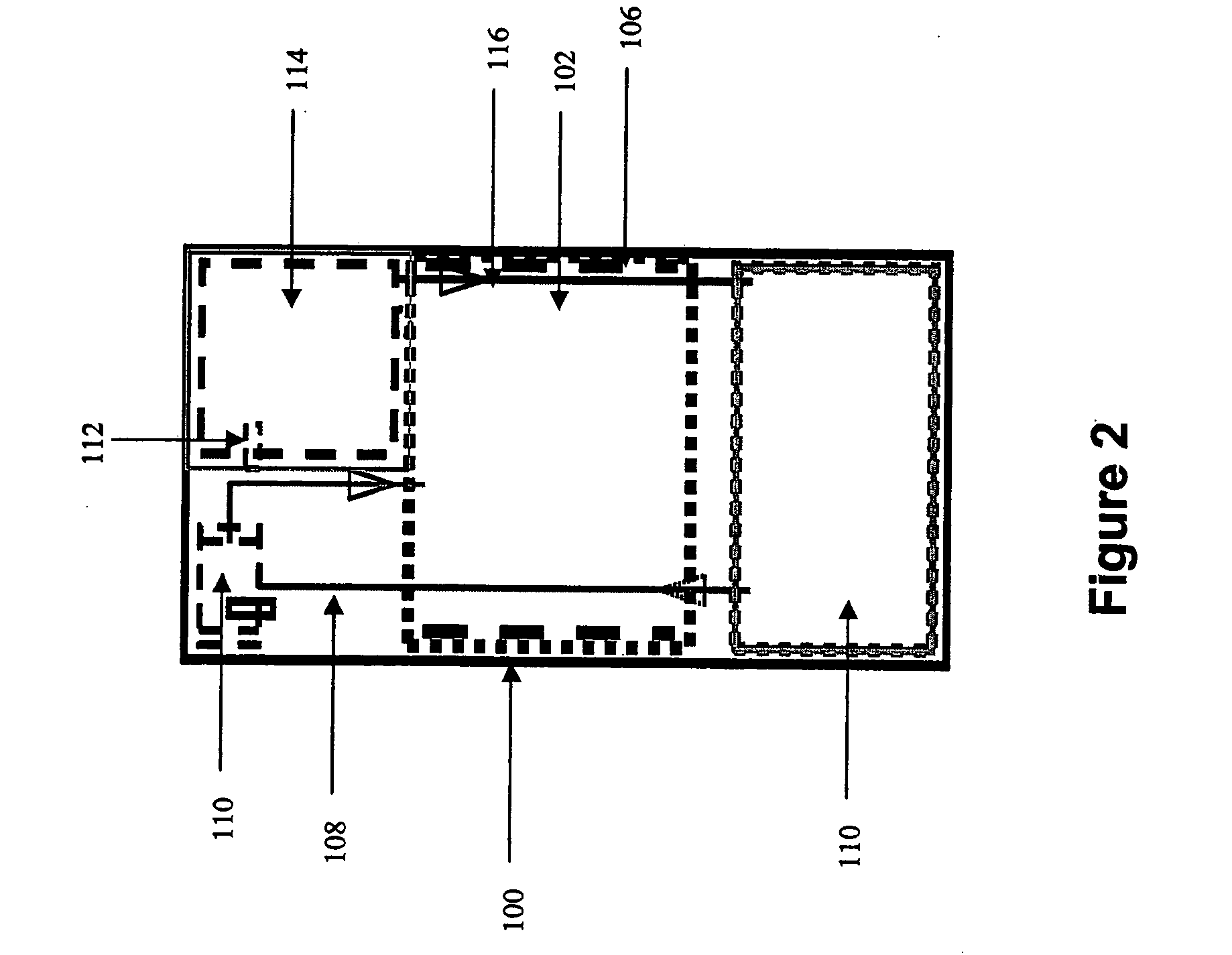
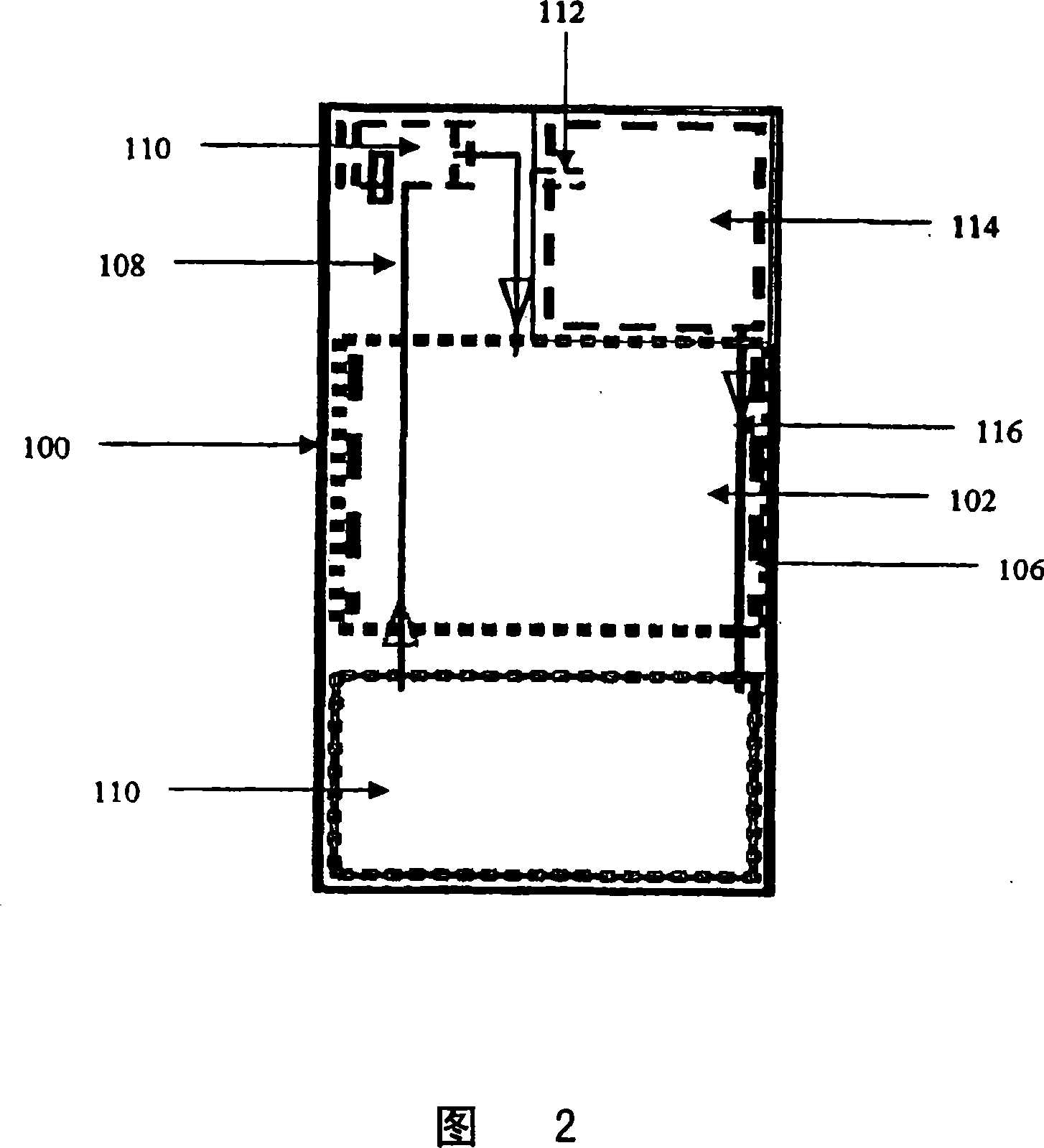
Systems and methods for hydrogen generation from solid hydrides
A system is disclosed for hydrogen generation based on the hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
Antiperspirant Active Compositions and Manufacture Thereof
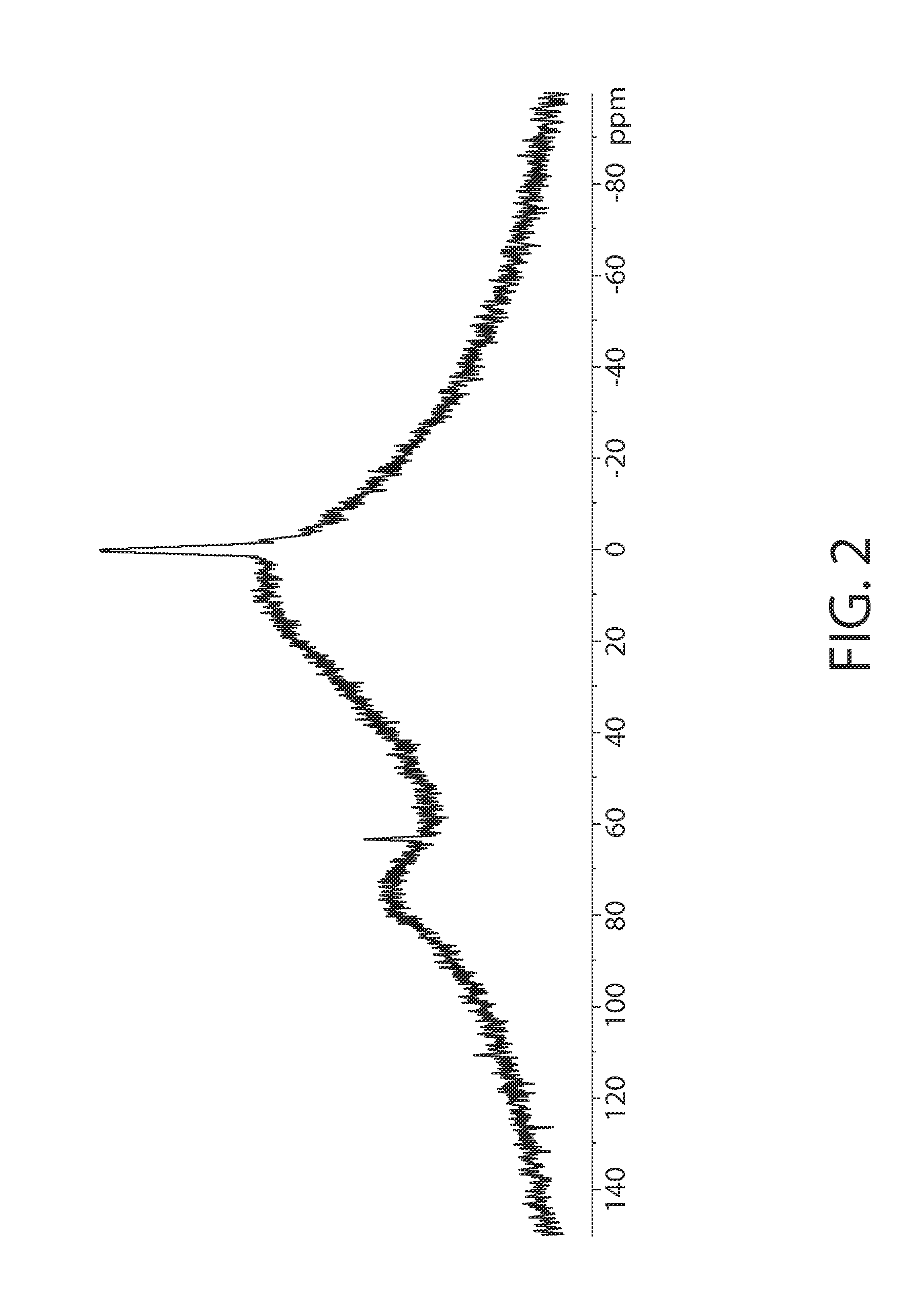
InactiveUS20130209387A1High antiperspirant efficacyHighly stable antiperspirant activeCosmetic preparationsAluminium compoundsAluminum CationChloride
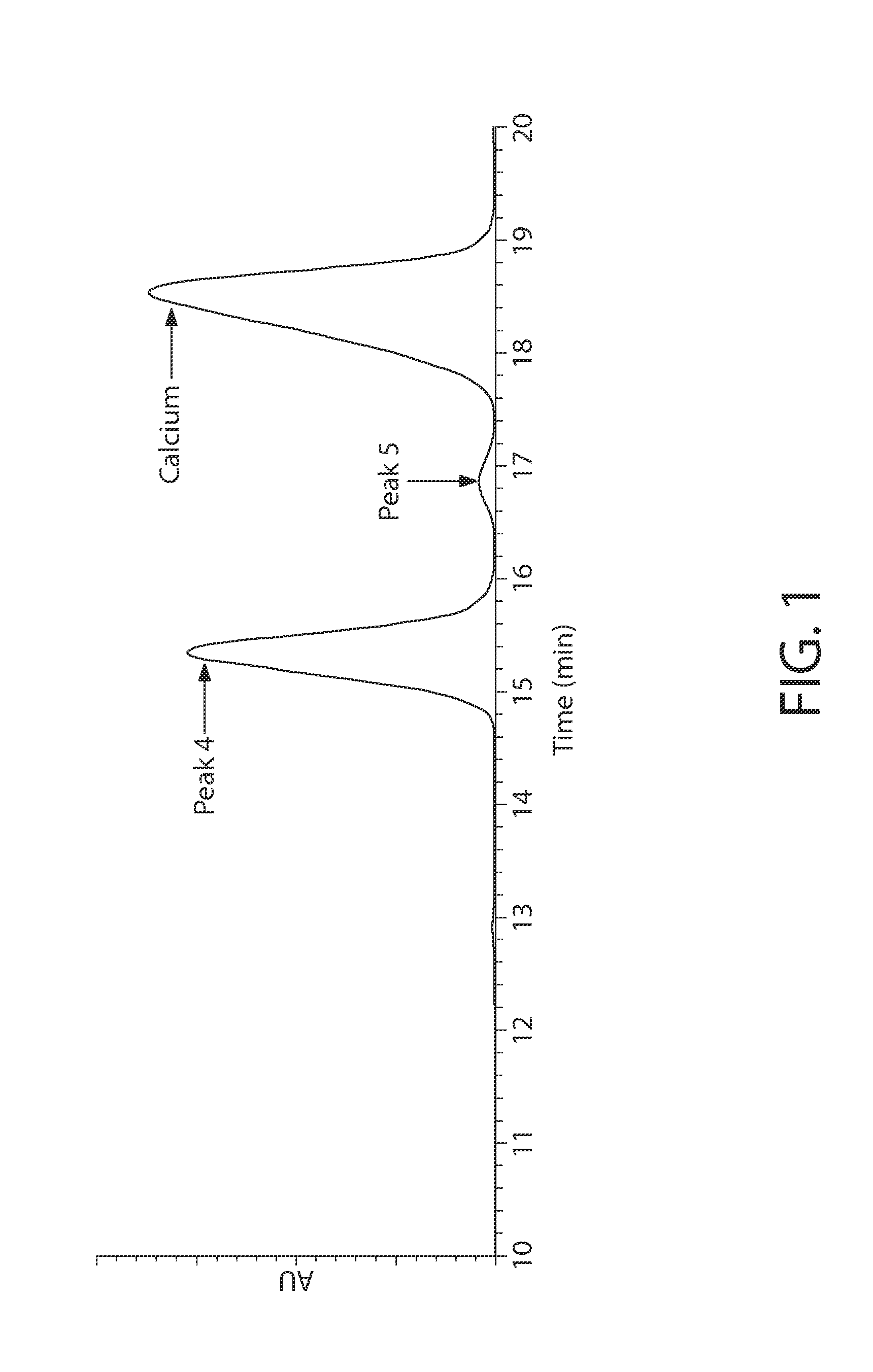
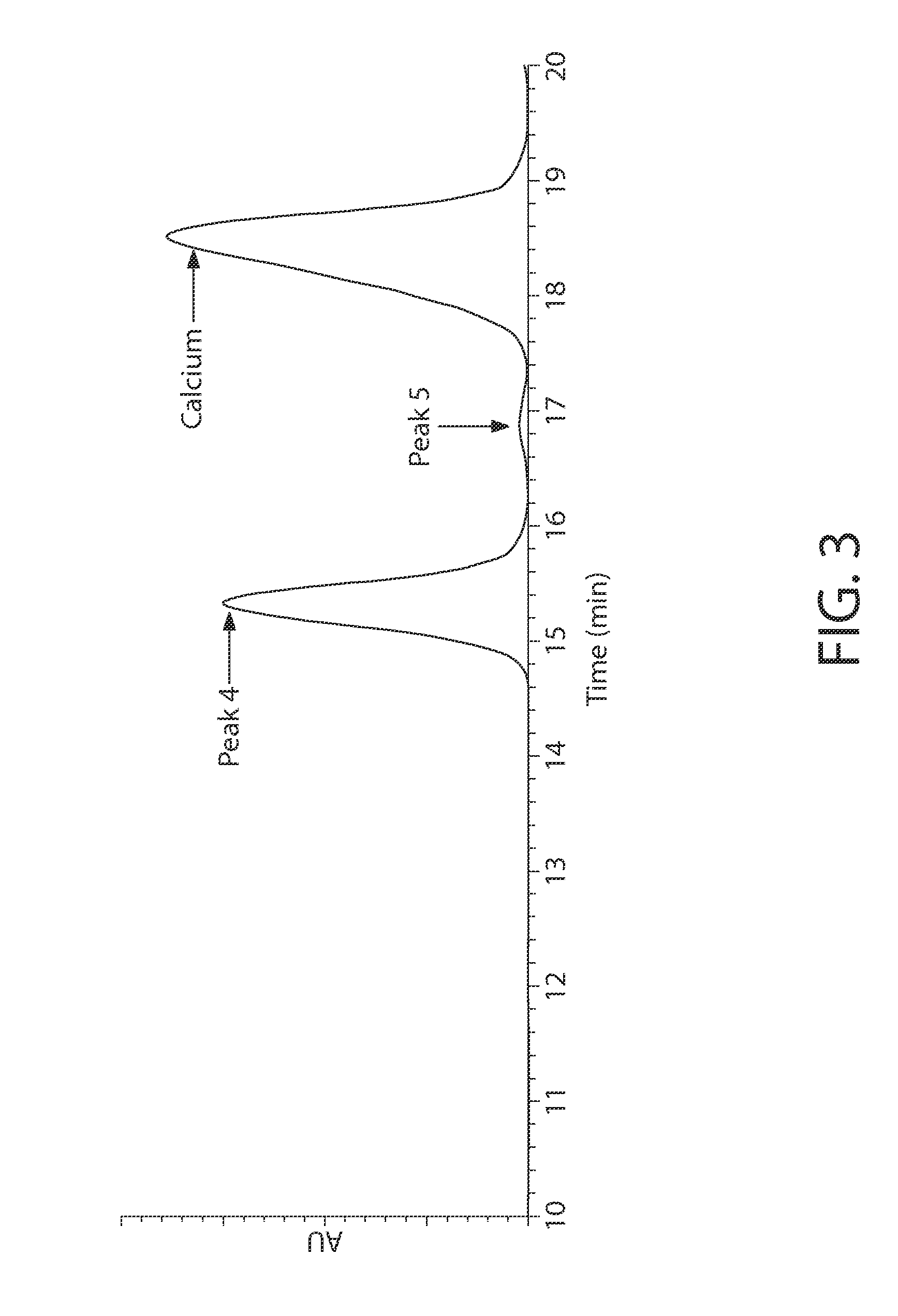
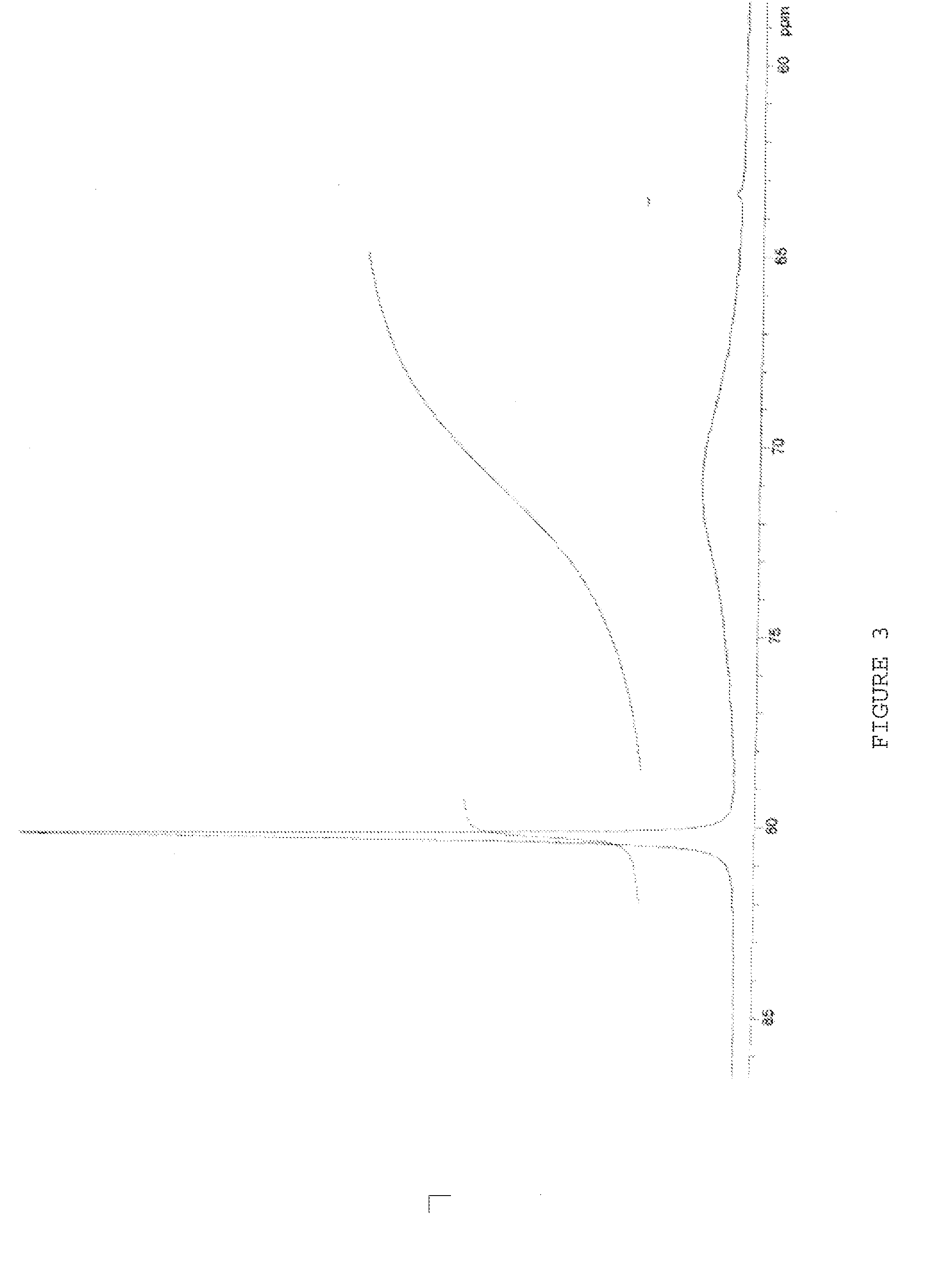
An antiperspirant active composition comprising an aluminum salt, the aluminum salt (i) having an aluminum to chloride molar ratio of 0.3:1 to 3:1; and (ii) exhibiting an 27Al NMR spectrum with a species distribution including at least 90% A130 polyhydroxyoxoaluminum cation as the predominant species detectable by 27Al NMR within the aluminum salt. The composition can optionally include zirconium. Also disclosed are a method of making an antiperspirant active composition and the use of a heating step at elevated temperature to convert Al13 polyhydroxyoxoaluminum cations in the species detectable by 27Al NMR within an aqueous aluminum salt solution into Al30 polyhydroxyoxoaluminum cations in the species detectable by 27Al NMR without increasing a SEC Peak 3 area in the SEC chromatogram of the aluminum salt.
Owner:COLGATE PALMOLIVE CO
Sulfur tolerant alumina catalyst support
InactiveCN103260748AImprove toleranceToleratedDispersed particle separationCatalyst activation/preparationCounterionSulfur
The present invention is directed to a method for making a sulfur tolerant alumina, that includes the steps of: forming aluminum hydrate from one or more water soluble aluminum salts, said salts each comprising an aluminum cation or aluminum anion and an oppositely charged counterion, in an aqueous medium, contacting the aluminum hydrate with a silica precursor in the aqueous" medium and in the presence of counterions of the one or more aluminum salts, isolating silica precursor-contacted aluminum hydrate particles from the aqueous medium, and calcining the silica precursor-contacted aluminum hydrate particles to form particles of the sulfur tolerant alumina.
Owner:RHODIA OPERATIONS SAS
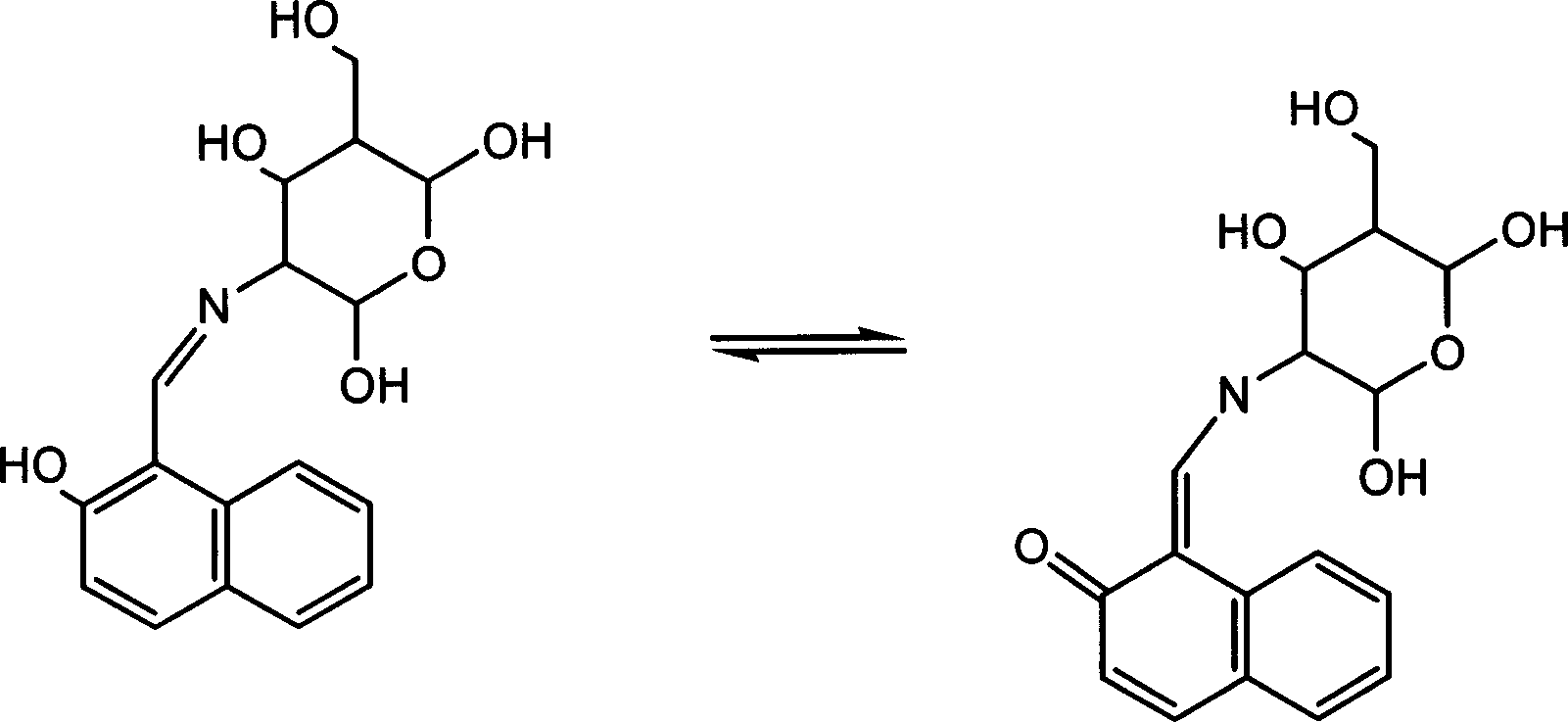
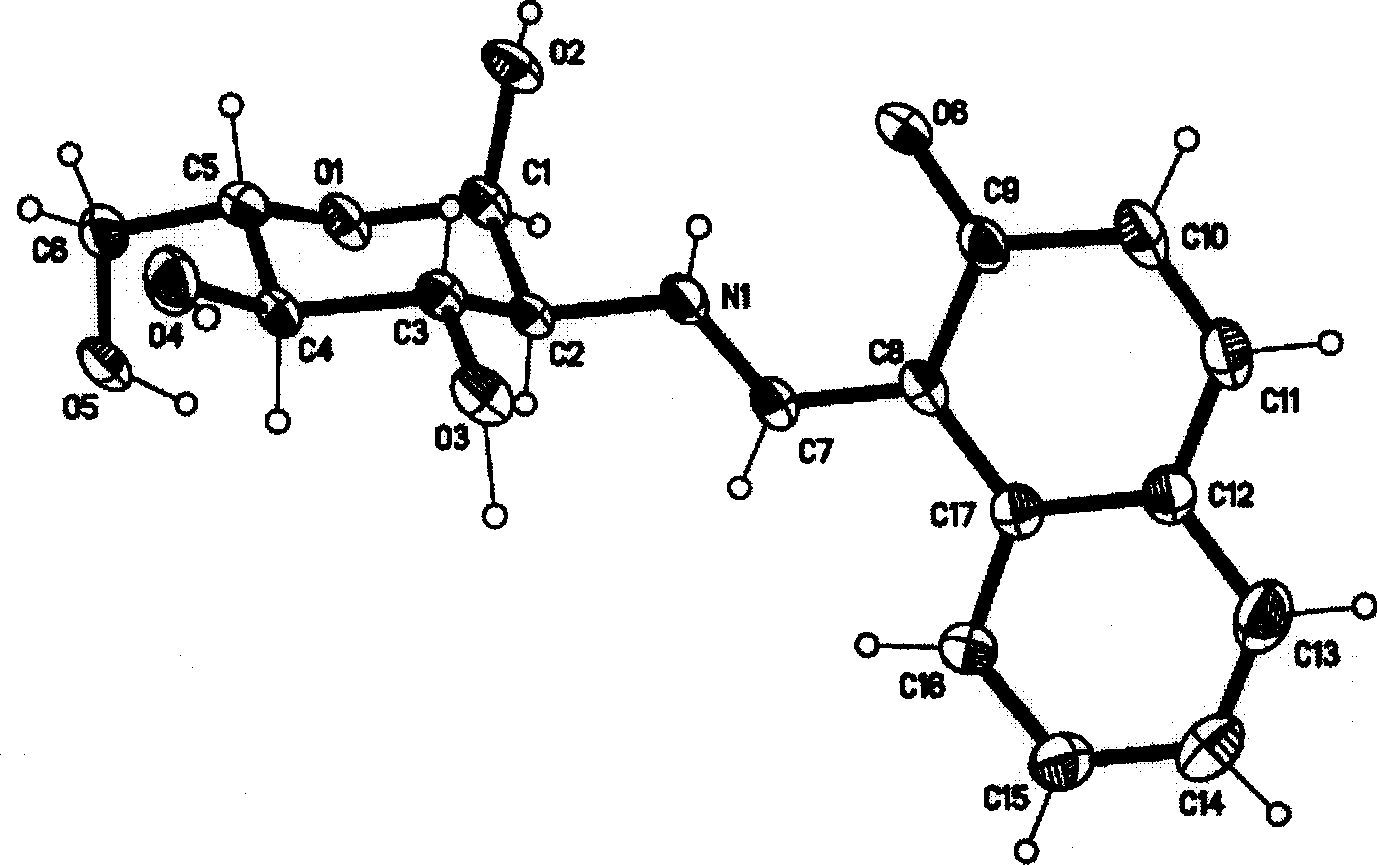
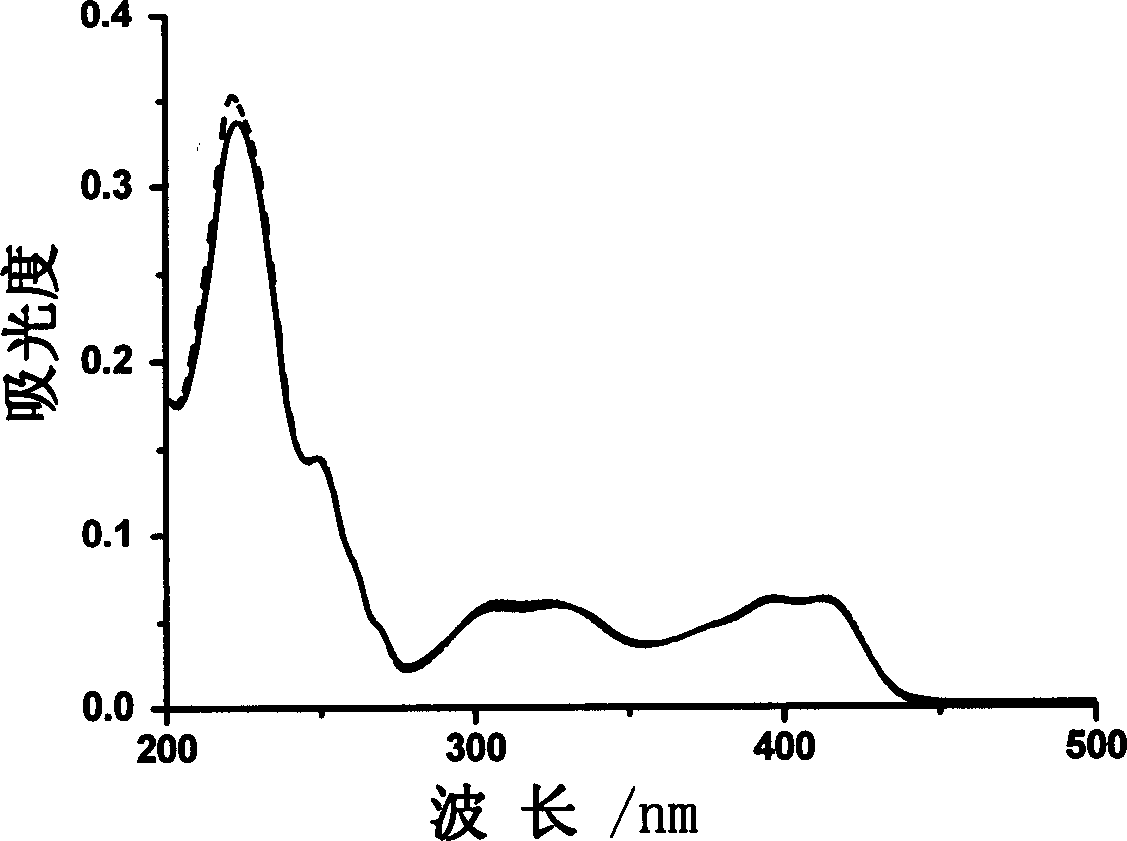
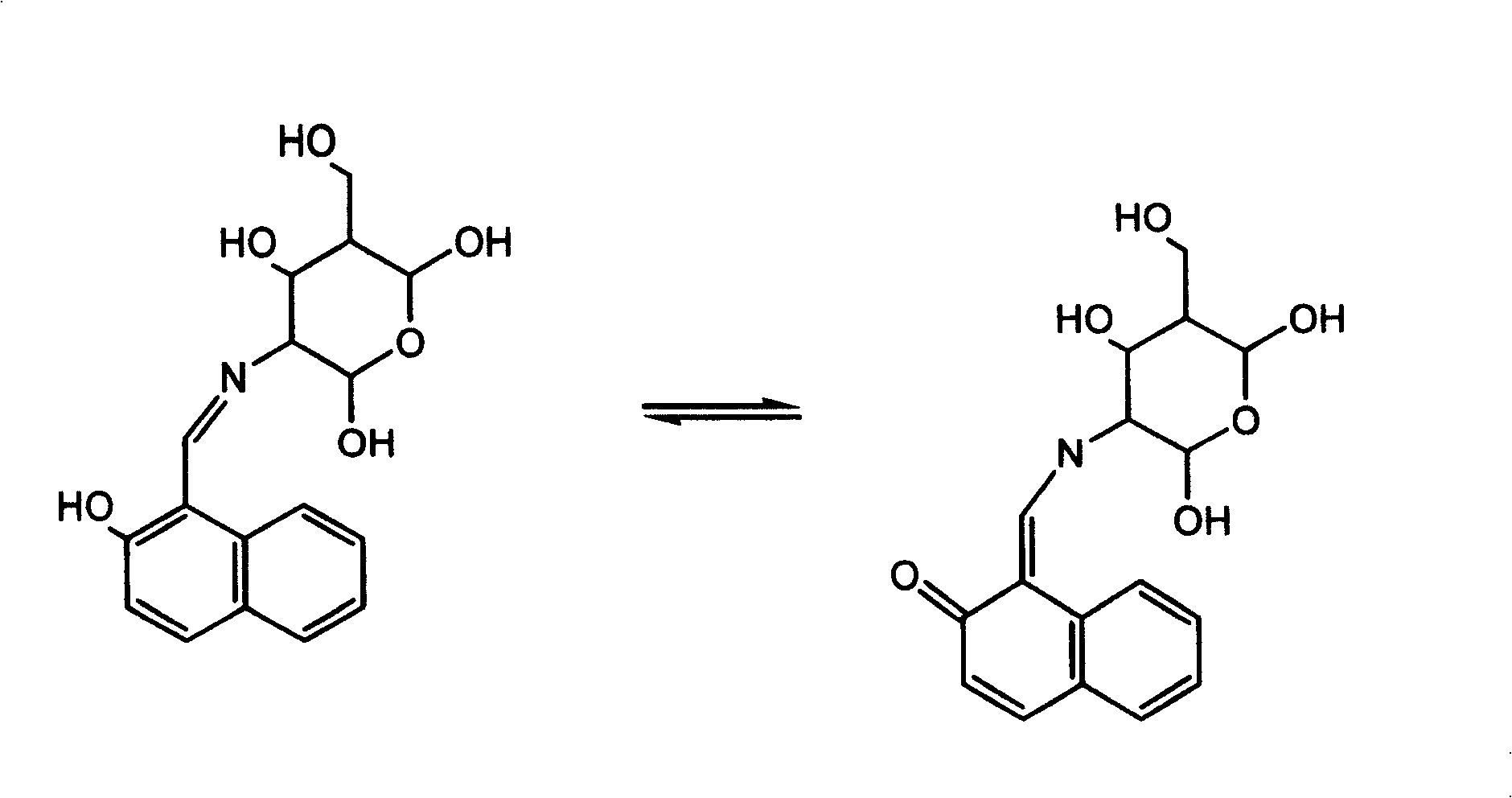
Aluminium ion investigating method using glycosyl naphthol
InactiveCN1869661AHigh selectivityHigh fluorescence intensityMaterial analysis by observing effect on chemical indicatorBiological testingAluminum IonFluorescence
The invention discloses a method for making aluminum ion fluorescent detection based on selective aluminum cation recognizing and binding of 2-desoxy-2- [(E)-[2-hydroxyl-1-2-hydroxyl-1-naphthoicmethylene]amino] -D-glucose compound obtained by condensation of naphthoic aldehyde and D-glucosamine, where the compound can make high-selectivity aluminum ion fluorescent detection, and can obviously detect aluminum ion concentration down to 0.013ppm, without interferences of other ions such as Li(I),Na(I),K(I),Ag(I),Mg(II),Ca(II),Cu(II),Ba(II),Pb(II),Mn(II),Co(II),Ni(II),Cd(II),Zn(II) and Hg(II). And it provides a method for detecting aluminum ions in water sample in high selectivity and high sensitivity.
Owner:NANJING UNIV
Preparation method of high-dispersion light-stable titanium white powder
InactiveCN110373048AHigh fluorescence quantum yieldImprove stabilityPigment treatment with macromolecular organic compoundsPigment treatment with non-polymer organic compoundsQuantum yieldSolubility
The invention discloses a preparation method of high-dispersion light-stable titanium white powder, and belongs to the technical field of chemical engineering. Coumarin prepared by the preparation method of the high-dispersion light-stable titanium white powderis high in fluorescence quantum yield and high in stability, a quaternary ammonium group is introduced throughreaction with 2,3-epoxypropyltrimethylammoniumchloride, the ultraviolet absorption rate of a light stabilizer is increased, and the quaternary amino group can be combined with hydroxyl radicals in water and adsorbed onto the surface of titanium dioxide to form a light-stable protective layer to prevent photooxidation of the titanium dioxide of the titanium white powder; theprepared organic light-stable coumarin on the surfaceof the titanium white powder is poor in water solubility,thus moisture absorption of the titanium white powder can be reduced,the coumarin in the light stabilizer fills between laminates of zinc-aluminum talcumpowder under theelectrostatic repulsion effect of highly charged zinc-aluminum cations in the talcum powder due to the fact that the quaternary amino group is introduced, thusthe inner surface of the titanium white powder is waterproof, so that the water dispersibility of the titanium white powder is improved, an organic dispersant is added to improve the water dispersibility of the titanium white powder in aqueous coating.
Owner:王志胜
Process for curing aminoplast resins
InactiveUS7208540B2Improve toughnessHigh strengthOther chemical processesAnti-corrosive paintsInorganic particleAluminum Cation
Aminoplast resins are cured using inorganic particles as curing agents. The inorganic particles have a laminated structure and include interlamellarly exchangeable cations such as alkali cations, alkaline-earth cations, aluminum cations, iron cations and / or manganese cations. The aminoplast resins may be cured to produce semi-finished products and molding materials.
Owner:AGROLINZ MELAMIN
Isotopically labeled polyfluorinated compound useful as an imaging tracer
A polyfluorinated compound is provided inclusive of at least one 18F atom having a formula: CF3(CF2)nR1 (I) where R1 is —C(O)OR2, —C(O)N(R3)2, C—N(R3)2, —C(NR3)R2, C-QR3, -QR3, —N+(R3)3, X, C1-C30 alkyl, C1-C30 haloalkyl, C1-C30 alkoxyl, or C1-C30 perhaloalkyl; R2 is MZ+, H, C1-C12 alkyl, C1-C12 alkyl, C1-C12 perhalo alkyl, C6-C30 aryl, C6-C30 perhaloaryl, and a substituted form thereof where one or more protons or halogens is replaced with a plasma solubility enhancing moiety of —N+(R3)3, —SO3H, —SO2N(R3)2, or -QR3; R3 is independently in each occurrence MZ+, —SO3H, —SO2N(R3)2, or -QR3; Q is O or S; MZ+ is a cation that forms a net neutral compound with an anionic (CF3(CF2)nC(O)O−)z and is an alkali metal cation, an alkali earth cation, a transition metal cation, ammonium, and aluminum cations; Z is an integer value of between 1 and 3 inclusive; X is a fluorine, chlorine, bromine or iodine atom; halo denotes a replacement of at least one and not all protons with X; perhalo denotes a replacement of all protons with X; and n is an integer value of between 1 and 30 inclusive.
Owner:PERKINELMER HEALTH SCIENCES INC
Tobacco Product Wrapping Material with Controlled Burning Properties
InactiveUS20150114414A1Effective controlEasy to handleNatural cellulose pulp/paperTobacco treatmentSolubilityBack burns
A tobacco product wrapping, material comprising composite particles is described, the composite particles being obtainable by a method in which an aqueous suspension containing calcium carbonate particles is prepared, and a metal salt comprising an aluminum cation is added. The metal salt is able to form a basic metal component in the suspension; and has a solubility of greater than 9.0 mg / L in water, measured at the pH value of the prepared suspension and at a temperature of 20° C. The invention further relates to a method for the production of the tobacco product wrapping material, the use of such tobacco product wrapping material for the production of tobacco products, and the tobacco products produced with the tobacco product wrapping material.
Owner:JULIUS GLATZ GMBH
Tobacco product wrapping material with controlled burning properties
InactiveUS9775377B2Low costUniversally applicableCigar manufactureInorganic compound additionSolubilityBack burns
A tobacco product wrapping, material comprising composite particles is described, the composite particles being obtainable by a method in which an aqueous suspension containing calcium carbonate particles is prepared, and a metal salt comprising an aluminum cation is added. The metal salt is able to form a basic metal component in the suspension; and has a solubility of greater than 9.0 mg / L in water, measured at the pH value of the prepared suspension and at a temperature of 20° C. The invention further relates to a method for the production of the tobacco product wrapping material, the use of such tobacco product wrapping material for the production of tobacco products, and the tobacco products produced with the tobacco product wrapping material.
Owner:JULIUS GLATZ GMBH
Antiperspirant active compositions and manufacture thereof
An antiperspirant active composition comprising an aluminum salt, the aluminum salt (i) having an aluminum to chloride molar ratio of 0.3:1 to 3:1; and (ii) having a species of polyhydroxyoxoaluminum cation detectable it 76 ppm by 27Al NMR that is present in a relative abundance on a 27Al NMR spectrograph that is greater than an other polyhydroxyoxoaluminum cation detectable by 27Al NMR. Also, disclosed are methods of making the antiperspirant active.
Owner:COLGATE PALMOLIVE CO
Aqueous composition for coating grain-oriented steel
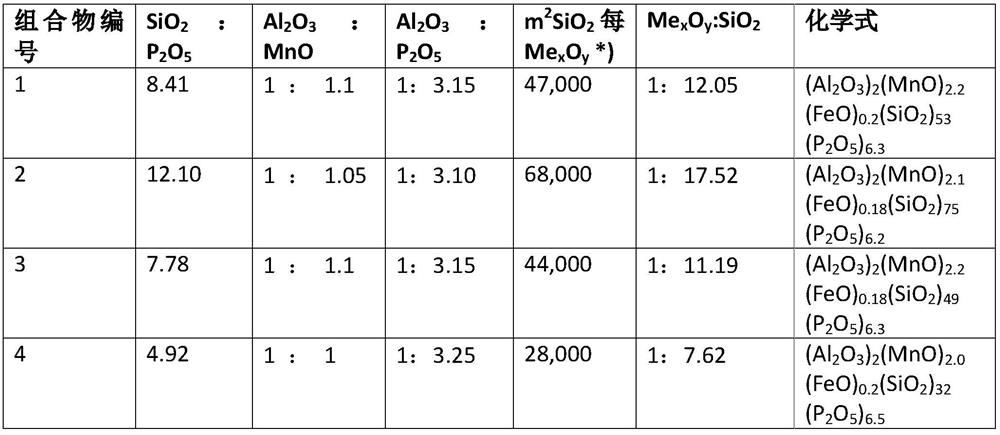
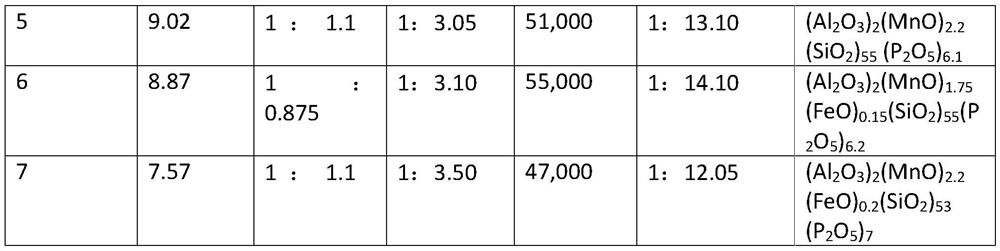
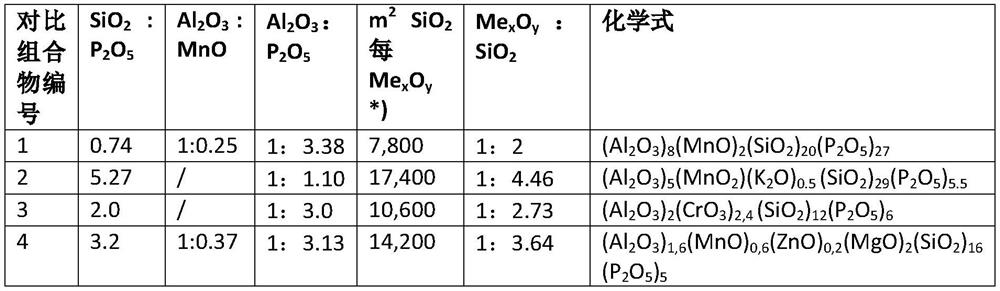
PendingUS20220112605A1Inorganic material magnetismMetallic material coating processesColloidal silicaHydrogen phosphate
The present patent application relates to an aqueous composition for coating grain oriented steel, comprisingaluminium cations,manganese cations,dihydrogen phosphate, hydrogen phosphate and / or phosphate anions,colloidal silica andoptionally iron cations, wherein the aluminium cations, expressed as Al2O3, manganese cations, expressed as MnO, dihydrogen phosphate, hydrogen phosphate and / or phosphate anions, expressed as P2O5, colloidal silica, expressed as SiO2, and optionally iron cations, expressed as FeO, which are present in the composition, give the sum formula of (Al2O3)2(MnO)1,8-2,4(FeO)0-0,2(P2O5)5-7(SiO2)≥30.
Owner:KANSAI HELIOS AUSTRIA GMBH
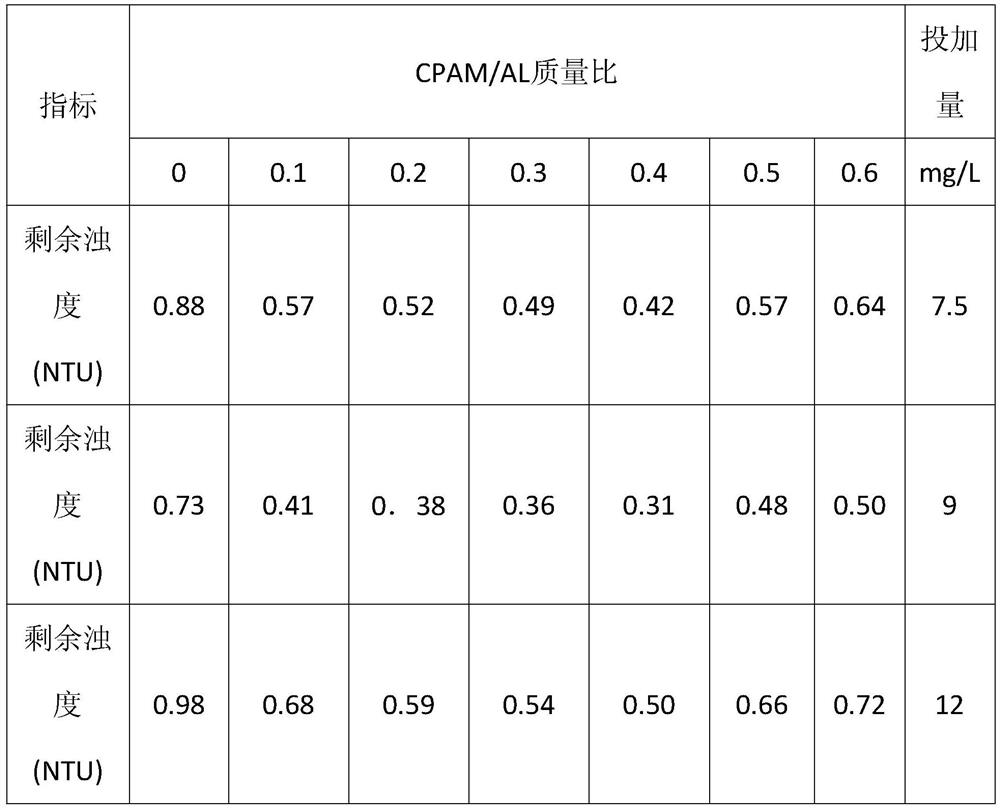
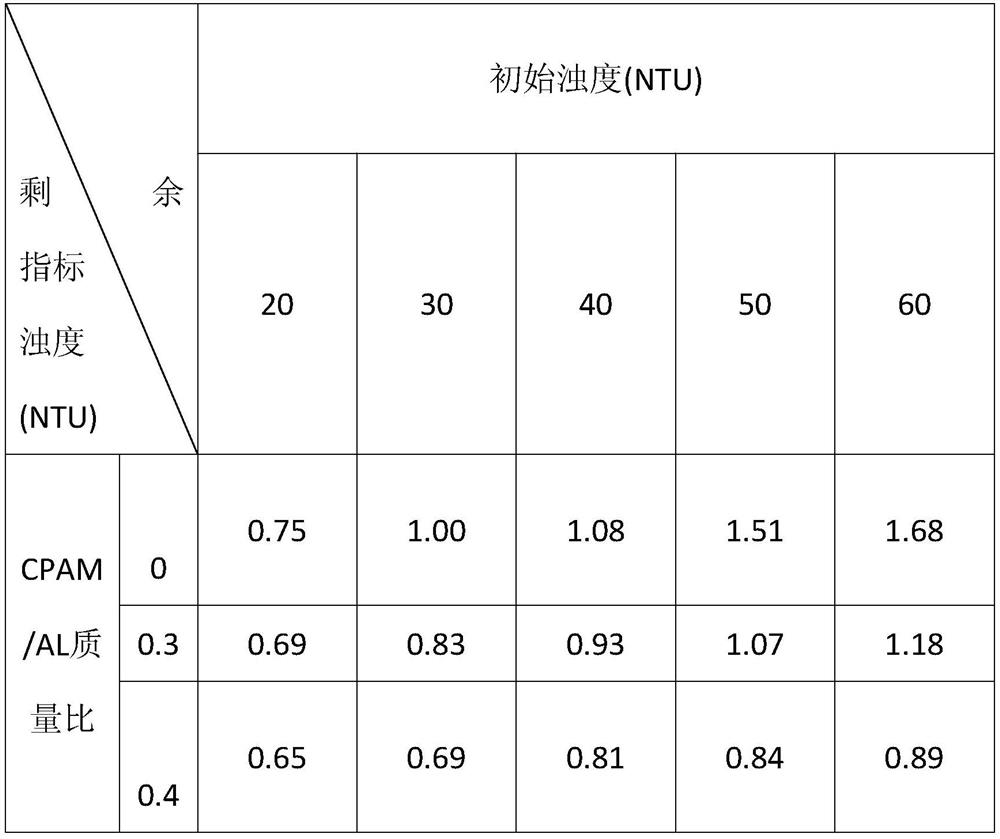
Preparation method and application of polytitanium aluminum chloride-cationic polyacrylamide composite flocculant
ActiveCN107176661BImprove stabilityImprove the coagulation effectWater/sewage treatment by flocculation/precipitationWater/sewage treatment by sorptionAluminium chlorideAluminum Cation
The invention relates to a preparation method and application of a titanium aluminum polychloride-cationic polyacrylamide composite flocculating agent and belongs to the technical field of water treatment. The preparation method comprises the following steps: preparing a titanium aluminum polychloride solution; adding TiCl4 into the titanium aluminum polychloride solution obtained in step (1); sufficiently stirring and dropwise adding a sodium hydroxide solution; adding water into cationic polyacrylamide and sufficiently stirring and dissolving to obtain a colorless and transparent sticky solution; preserving a cationic polyacrylamide solution which is sufficiently dissolved at a temperature of -2 DEG C to 45 DEG C; adding the cationic polyacrylamide solution into the titanium aluminum polychloride solution according to the mass ratio of cationic polyacrylamide / Al of (0.1-0.6):1; and continually stirring to obtain the titanium aluminum polychloride-cationic polyacrylamide composite flocculating agent. The preparation method and application of the titanium aluminum polychloride-cationic polyacrylamide composite flocculating agent have the advantages of simple process and low cost; the obtained flocculating agent has stronger adsorption and electrical neutralization, and adsorption bridging and net capturing capabilities; and the flocculating agent has a good coagulation effect, a wide application range and a small addition amount.
Owner:舒朋士环境科技(常州)股份有限公司 +1
A waterborne ultra-thin steel structure fireproof coating based on aluminum diethylphosphinate/montmorillonite hybrid and its preparation method and application
ActiveCN109627877BIncreased durabilityGood weather resistanceFireproof paintsAluminum CationEngineering
The invention discloses a water-based ultra-thin steel structure fireproof coating based on aluminum diethylphosphinate / montmorillonite hybrid, a preparation method and application thereof. The composition and weight percent of the fireproof coating are: ammonium polyphosphate, aluminum diethylphosphinate / montmorillonite hybrid, melamine, pentaerythritol, polymer emulsion, water, titanium dioxide, silicon dioxide, kaolin, dispersant , defoamer, coalescent and thickener. The present invention adopts the aluminum diethylphosphinate / montmorillonite hybrid as the important component in the ammonium polyphosphate intumescent flame retardant system, utilizes the P-C structure in the aluminum diethylphosphinate structure to have Superior high temperature oxidation resistance, excellent hydrolysis resistance, and high phosphorus content make it highly efficient flame retardant, and the aluminum cation in its structure has the function of inorganic flame retardant, showing good Smoke suppression effect.
Owner:广州市新稀冶金化工有限公司 +1
Isotopically labeled polyfluorinated compound useful as an imaging tracer
A polyfluorinated compound is provided inclusive of at least one 18F atom having a formula:CF3(CF2)nR1 (I)where R1 is —C(O)OR2, —C(O)N(R3)2, C—N(R3)2, —C(NR3)R2, C-QR3, -QR3, —N+(R3)3, X, C1-C30 alkyl, C1-C30 haloalkyl, C1-C30 alkoxyl, or C1-C30 perhaloalkyl; R2 is MZ+, H, C1-C12 alkyl, C1-C12 alkyl, C1-C12 perhalo alkyl, C6-C30 aryl, C6-C30 perhaloaryl, and a substituted form thereof where one or more protons or halogens is replaced with a plasma solubility enhancing moiety of —N+(R3)3, —SO3H, —SO2N(R3)2, or -QR3; R3 is independently in each occurrence MZ+, —SO3H, —SO2N(R3)2, or -QR3; Q is O or S; MZ+ is a cation that forms a net neutral compound with an anionic (CF3(CF2)nC(O)O−)Z and is an alkali metal cation, an alkali earth cation, a transition metal cation, ammonium, and aluminum cations; Z is an integer value of between 1 and 3 inclusive; X is a fluorine, chlorine, bromine or iodine atom; halo denotes a replacement of at least one and not all protons with X; perhalo denotes a replacement of all protons with X; and n is an integer value of between 1 and 30 inclusive.
Owner:PERKINELMER HEALTH SCIENCES INC
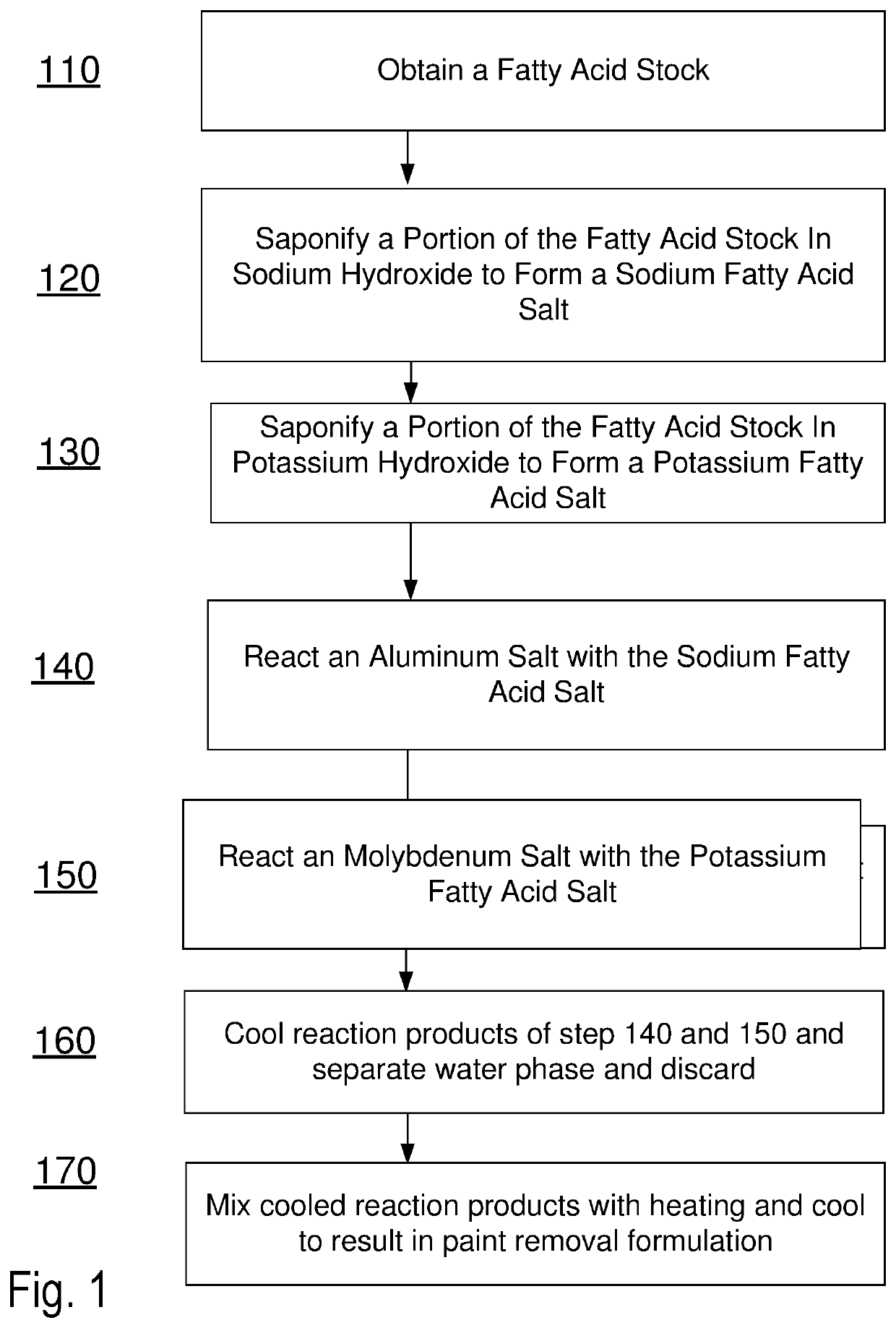
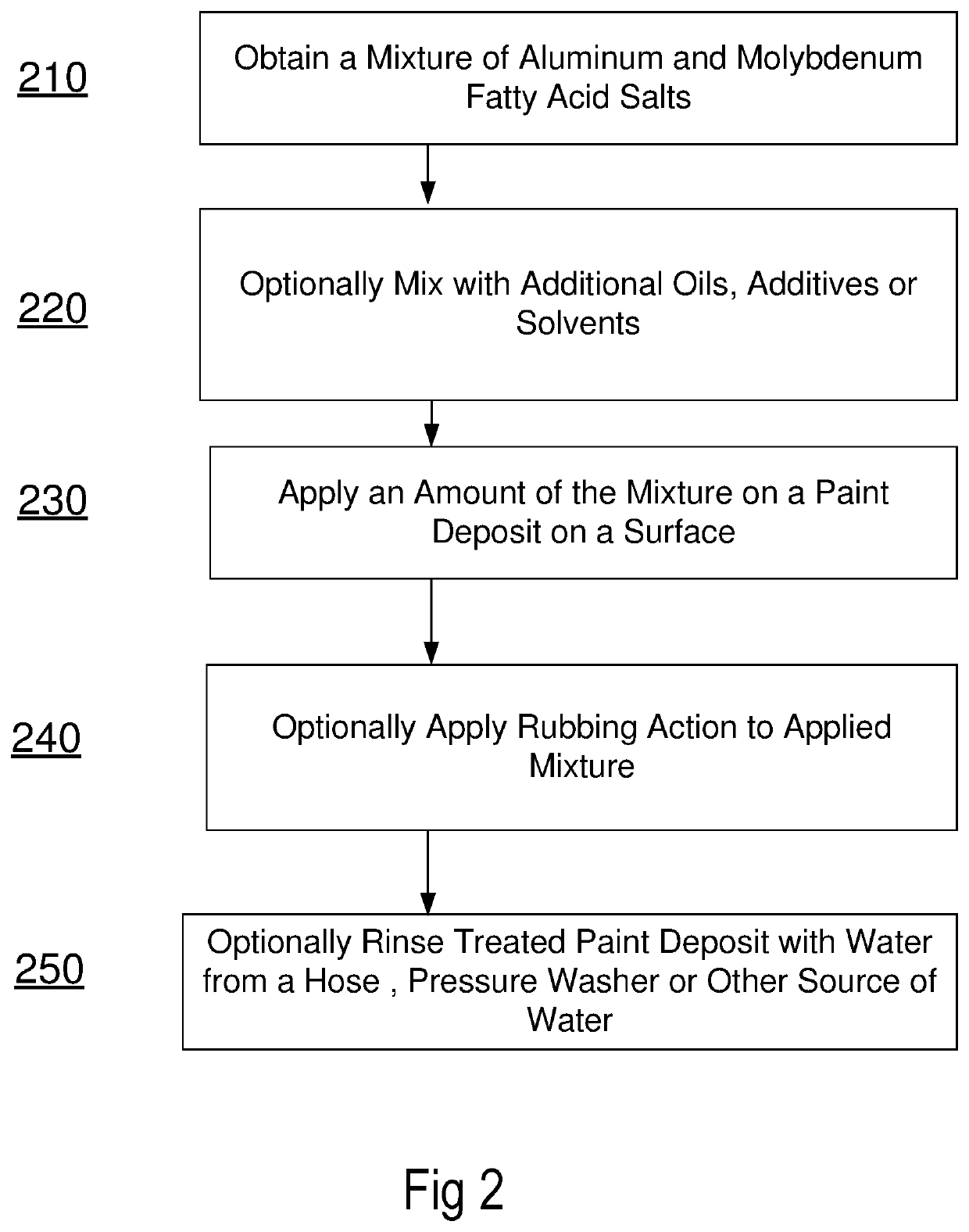
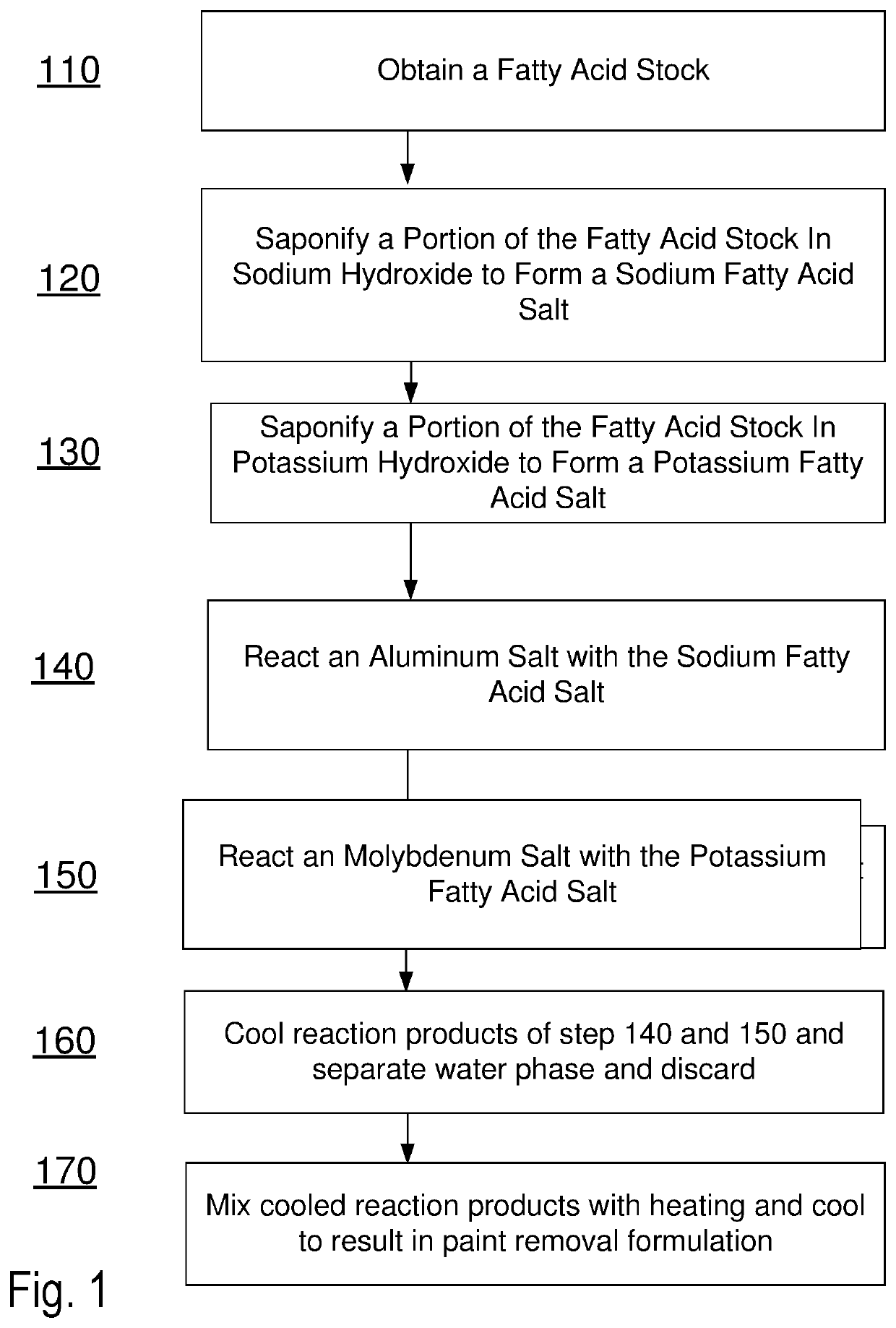
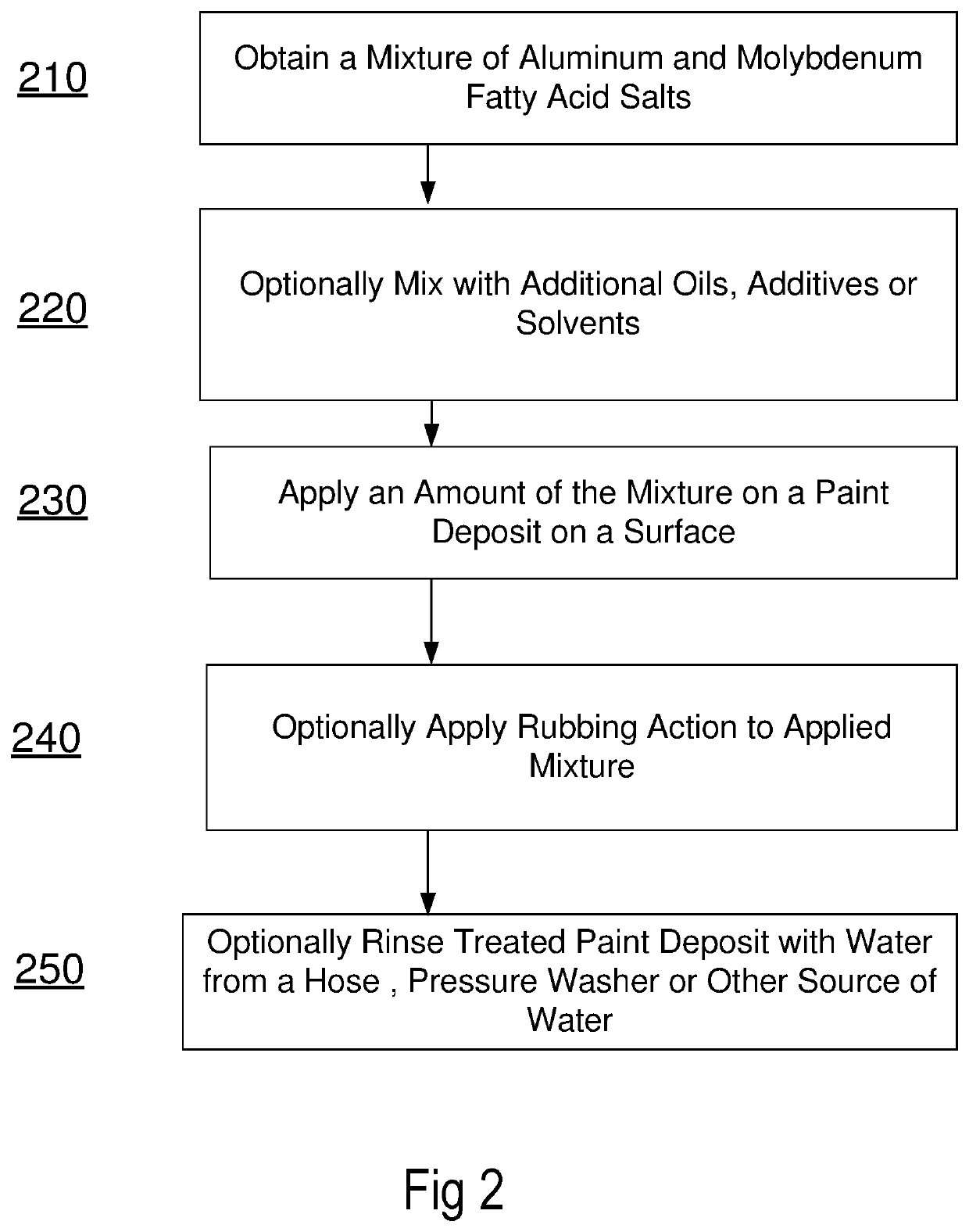
Methods and formulations for paint deposit removal
ActiveUS10822506B2Safely affordableSafely usableChemical paints/ink removersAluminum CationMolybdenum
Owner:PODSTAWA MICHAEL +1
Metal-phosphate binders
A metal-phosphate binder is provided. The binder may include an aqueous phosphoric acid solution, a metal-cation donor including a metal other than aluminum, an aluminum-cation donor, and a non-carbohydrate electron donor.
Owner:CATERPILLAR INC
Aqueous composition for coating grain-oriented steel
ActiveCN113412343AInorganic material magnetismMetallic material coating processesHydrogen phosphateManganese
The present invention relates to an aqueous composition for coating grain-oriented steel, comprising -aluminum cations, -manganese cations,-dihydrogen phosphate, hydrogen phosphate and / or phosphate anions, -colloidal silicon dioxide and-optionally iron cations, wherein the aluminum cations present in the composition, calculated as Al2O3, the manganese cations, calculated as MnO, the dihydrogen phosphate, hydrogen phosphate and / or phosphate anions, calculated as P2O5, colloidal silicon dioxide, calculated as SiO2, and optionally the iron cations, calculated as FeO, result in the molecular formula (Al2O3)2(MnO)1,8-2,4(FeO)0-0,2(P2O5)5-7(SiO2)>=30.
Owner:伦勃朗涂料公司
Aluminium ion investigating method using glycosyl naphthol
InactiveCN100422721CHigh selectivityHigh fluorescence intensityMaterial analysis by observing effect on chemical indicatorBiological testingAluminum IonFluorescence
The invention discloses a method for making aluminum ion fluorescent detection based on selective aluminum cation recognizing and binding of 2-desoxy-2- [(E)-[2-hydroxyl-1-2-hydroxyl-1-naphthoicmethylene]amino] -D-glucose compound obtained by condensation of naphthoic aldehyde and D-glucosamine, where the compound can make high-selectivity aluminum ion fluorescent detection, and can obviously detect aluminum ion concentration down to 0.013ppm, without interferences of other ions such as Li(I),Na(I),K(I),Ag(I),Mg(II),Ca(II),Cu(II),Ba(II),Pb(II),Mn(II),Co(II),Ni(II),Cd(II),Zn(II) and Hg(II). And it provides a method for detecting aluminum ions in water sample in high selectivity and high sensitivity.
Owner:NANJING UNIV
Inorganic intercalated vermiculite insulation refractory material and preparation method thereof
The invention relates to an inorganic intercalated vermiculite insulation refractory material and a preparation method thereof. In the technical scheme, the preparation method comprises the following steps: calcinating and activating raw vermiculite ore for 2-8 hours at the temperature of 500-650 DEG C, modifying for 2-10 hours in acid solution of 0.1-1.0mol / l, eluting and drying; preparing 2-5 weight percent of suspension with the eluted and dried vermiculite and poly-hydroxy-aluminum cation precursor solution, stirring for 4-8 hours at the temperature of 80-100 DEG C, eluting and drying, thereby obtaining poly-hydroxy-aluminum cation precursor intercalated vermiculite; calcinating the poly-hydroxy-aluminum cation precursor intercalated vermiculite for 4-6 hours at the temperature of 500-650 DEG C, thereby obtaining inorganic intercalated vermiculite; and finally mixing 40-80 weight percent of inorganic intercalated vermiculite and 20-60 weight percent of forsterite, adding 10-20 weight percent of aluminium dihydrogen phosphate, forming through stirring and pressing, and carrying out heat treatment at the temperature of 500-650 DEG C, thereby obtaining the inorganic intercalated vermiculite insulation refractory material. The invention has the advantages of low heat conduction coefficient at high temperature, high strength and wide application scope.
Owner:WUHAN UNIV OF SCI & TECH
Methods and formulations for paint deposit removal
ActiveUS20200299528A1Safely affordableSafely usableChemical paints/ink removersAluminum CationMolybdenum
The present disclosure provides for preparing formulations to clear and clean paint deposits from surfaces. In some examples, the paint deposits may be spray paint deposits on concrete, brick and metal surfaces amongst others. In some examples, the formulations may include active salt agents comprising molybdenum and aluminum cations.
Owner:PODSTAWA MICHAEL +1
Antiperspirant active compositions and manufacture thereof
An antiperspirant active composition comprising an aluminum salt, the aluminum salt (i) having an aluminum to chloride molar ratio of 0.3:1 to 3:1; and (ii) having a species of polyhydroxyoxoaluminum cation detectable at 76 ppm by 27Al NMR that is present in a relative abundance on a 27Al NMR spectrograph that is greater than any other polyhydroxyoxoaluminum cation detectable by 27Al NMR. Also, disclosed are methods of making the antiperspirant active.
Owner:COLGATE PALMOLIVE CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com