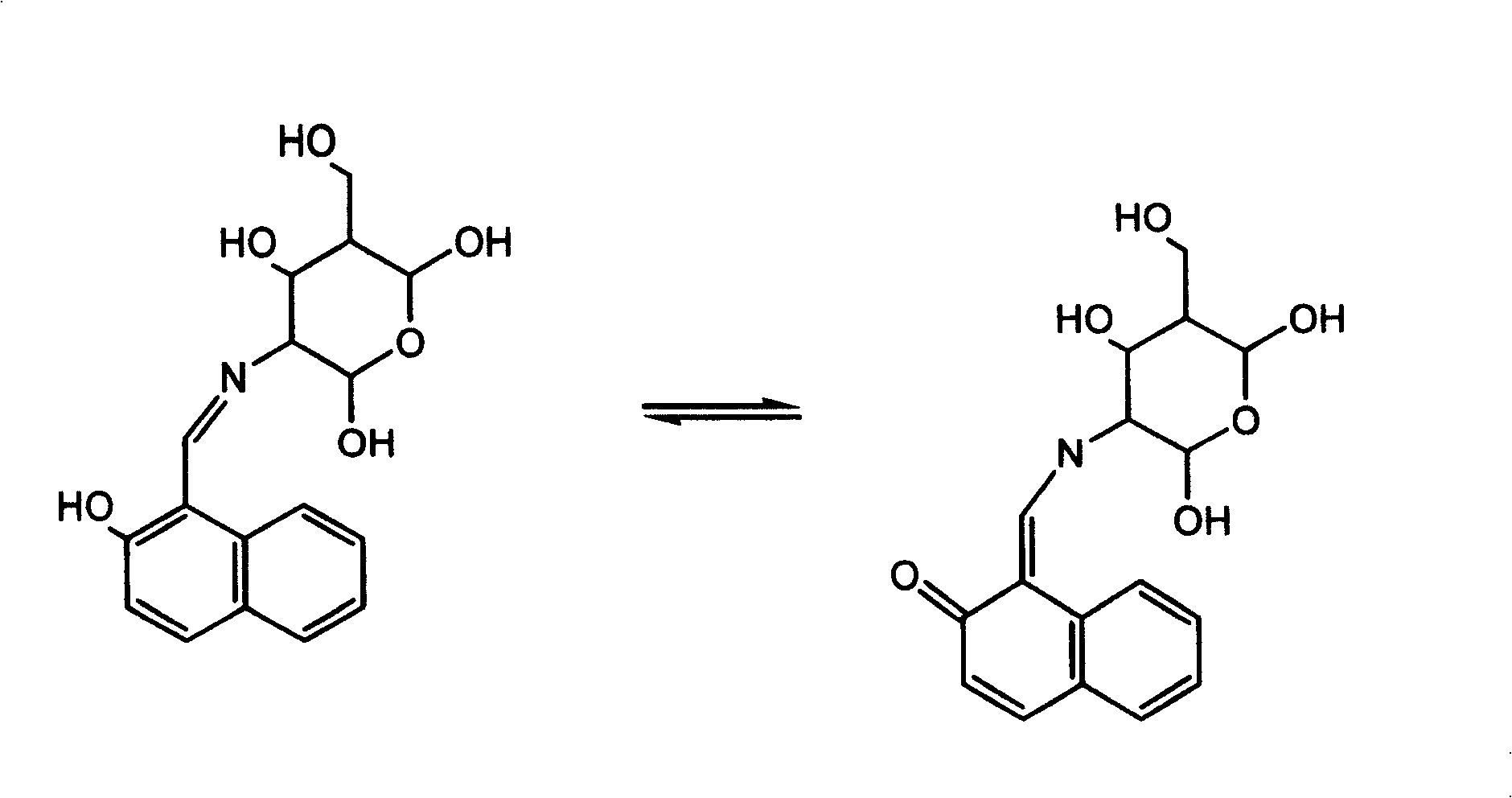
Aluminium ion investigating method using glycosyl naphthol
An aluminum ion and determination method technology, applied in the field of ion detection, can solve the problems affecting the measurement accuracy, no selectivity and interference of the color reaction, and achieve the effect of high selectivity, high fluorescence intensity and high sensitivity test
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (synthesis of fluorescent compound)
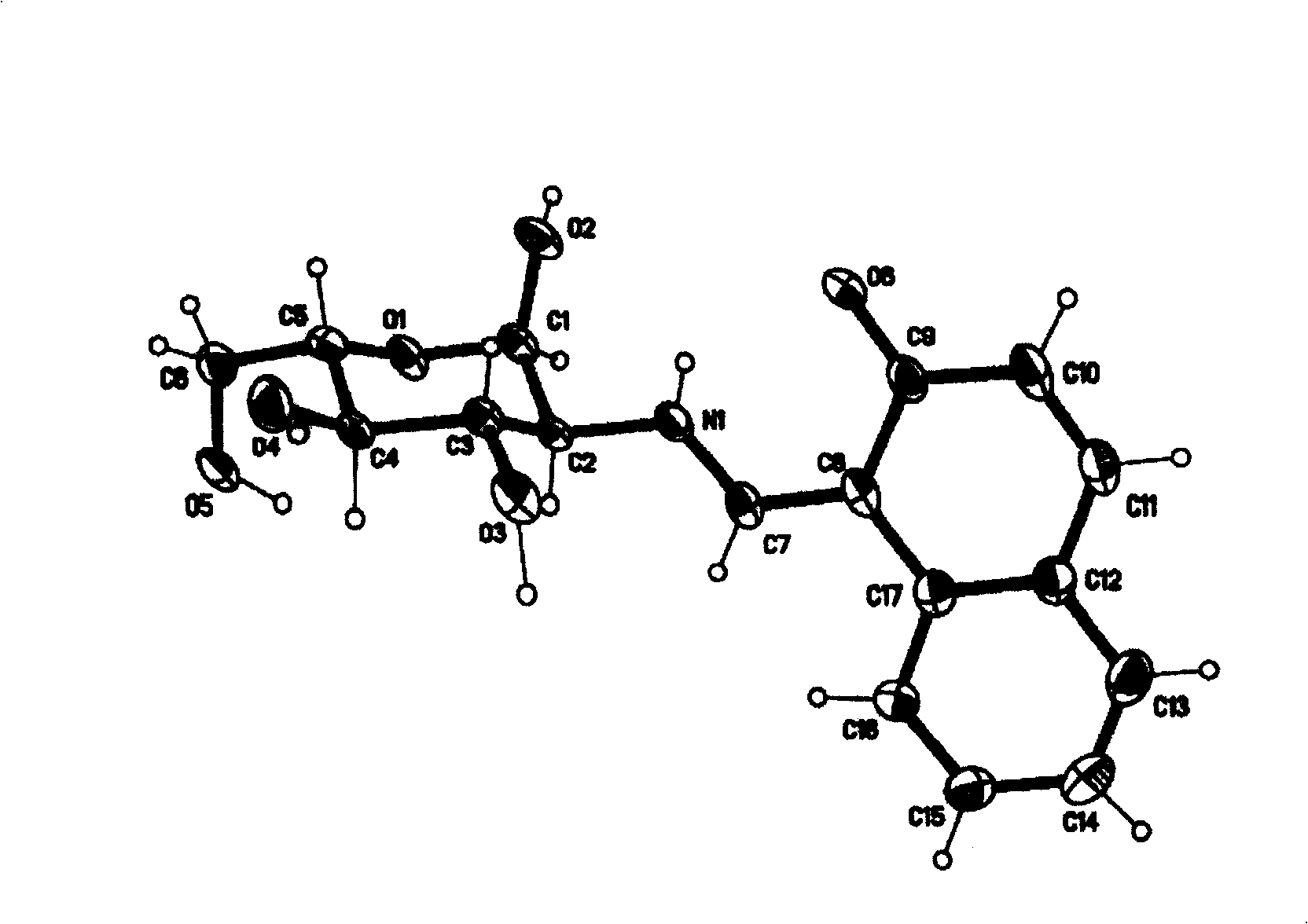
[0024] Dissolve 1.722g (10mmol) of 2-hydroxyl-1-naphthalene aldehyde in 100ml of anhydrous methanol, add 2.152g (10mmol) of D-glucosamine hydrochloride and 2ml of triethylamine, heat to reflux for 60 minutes, and cool to room temperature , filtered, and the light yellow precipitate was collected, washed with water, methanol, and anhydrous ether several times. Vacuum dry. Yield: 60%. Elemental Analysis: Calculated Value C 17 h 19 NO 6 C 61.26, H 5.75, N 4.20% Tested: C 61.36H 5.82N 4.17%. After single crystal cultivation, the single crystal structure was measured (see attached figure 2 ).
Embodiment 2
[0025] Embodiment 2 (absorption spectrum after reacting with aluminum ion)
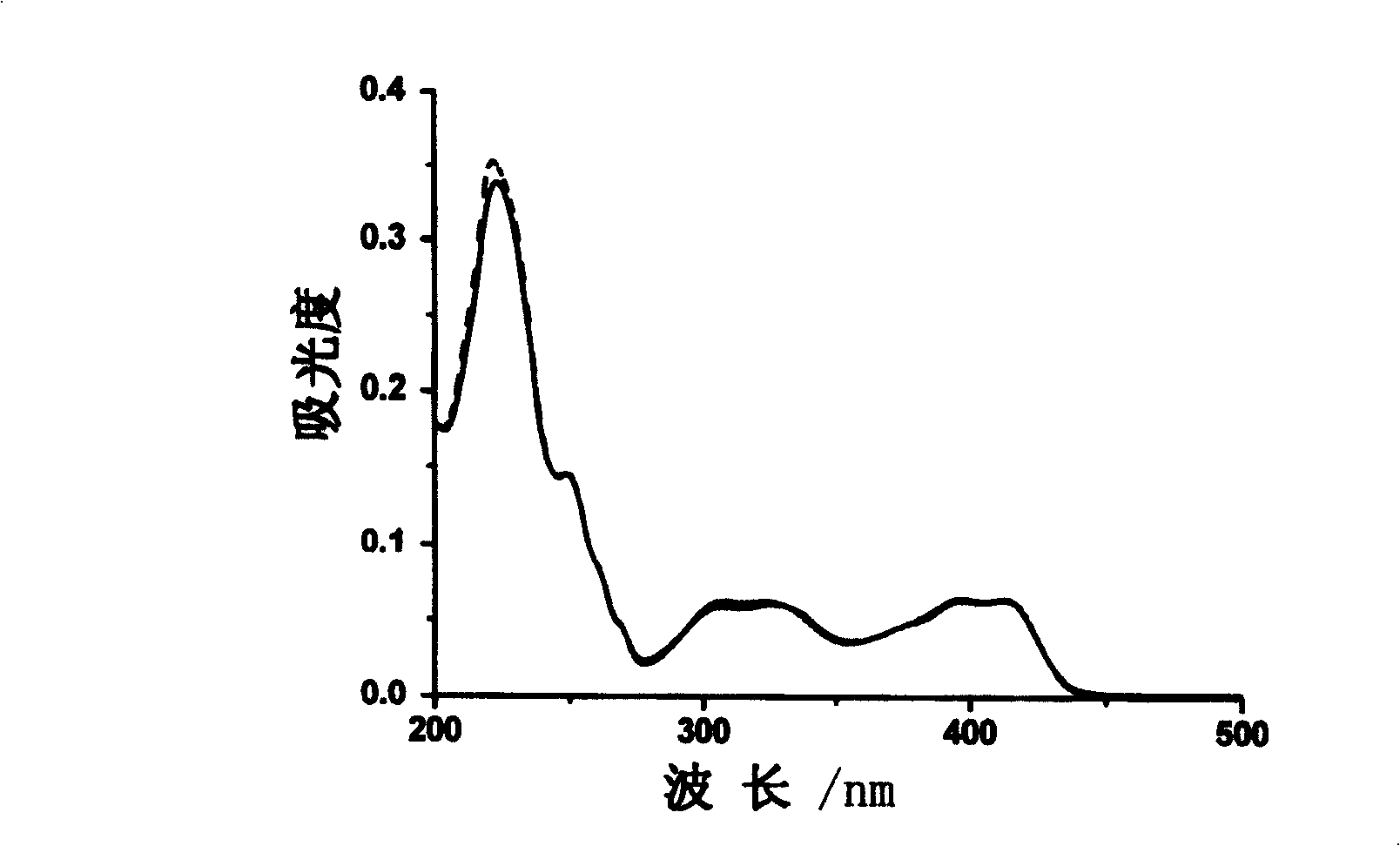
[0026] Get the compound of Example 1, configure it into an aqueous solution, and test the UV-visible spectrum after 20 times of aluminum ions. The concentration of the compound of Example 1 is 1×10 -5 mol·1 -1 . See image 3 .
Embodiment 3
[0027] Embodiment 3 (fluorescence spectrum and concentration intensity working curve)
[0028] Take by weighing the compound 0.0017 gram of embodiment 1, be dissolved in water, be mixed with 1 * 10 with 500ML volumetric flask -5 M standard stock solution (L). Weigh 0.0150 g of aluminum nitrate nonahydrate, dissolve in water, and prepare 4.0×10-3 Standard stock solution of M (F). Measure 2 milliliters of stock solution L, add the stock solution F of calculated amount, be mixed with standard test solution, scan and record fluorescence spectrum (see attached Figure 4 ), excited at 370nm, and tested its fluorescence intensity at 431nm (see attached Figure 5 ). The binding constant of the compound of Example 1 to aluminum ions was calculated by nonlinear least square method as log K=5.67±0.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com