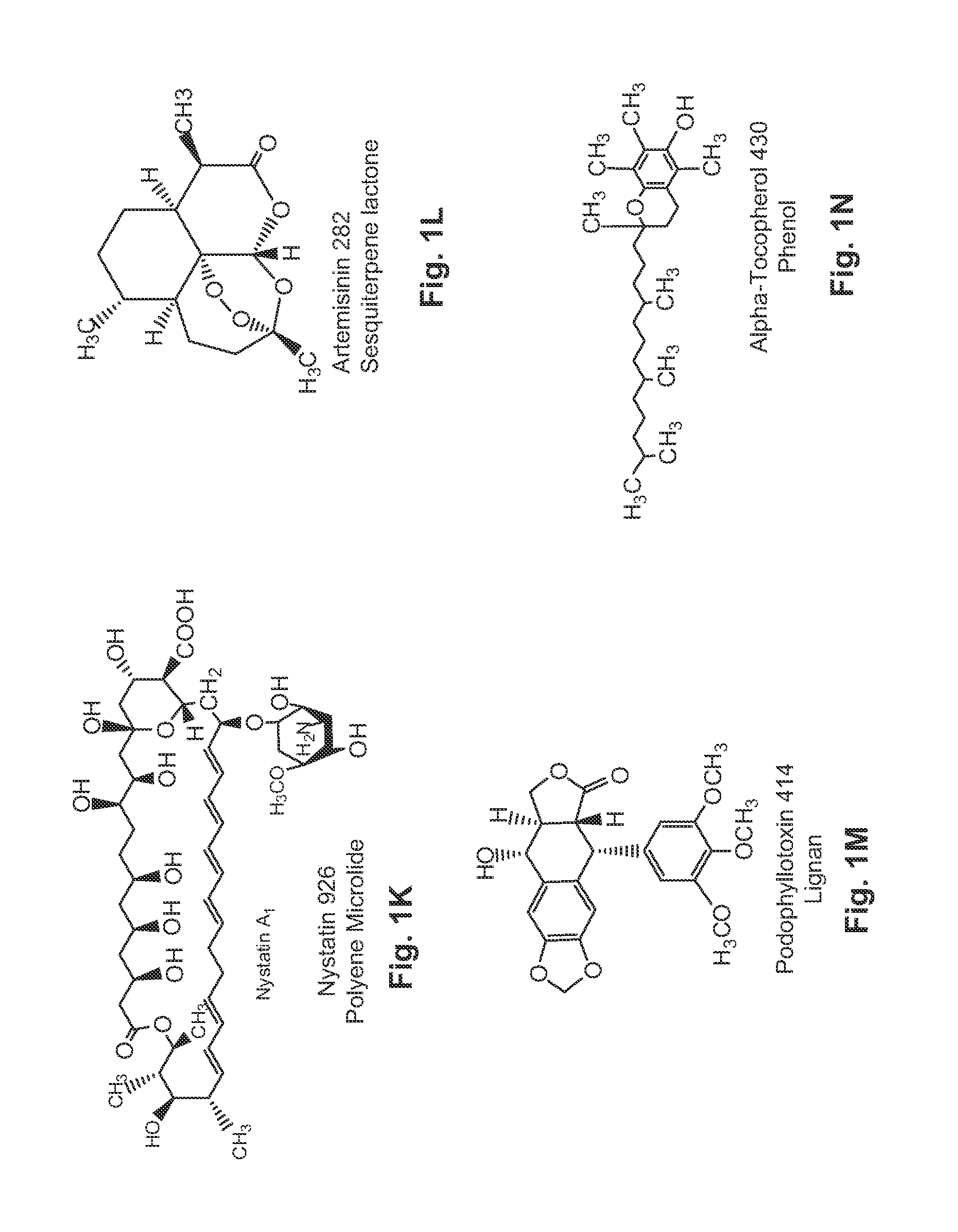
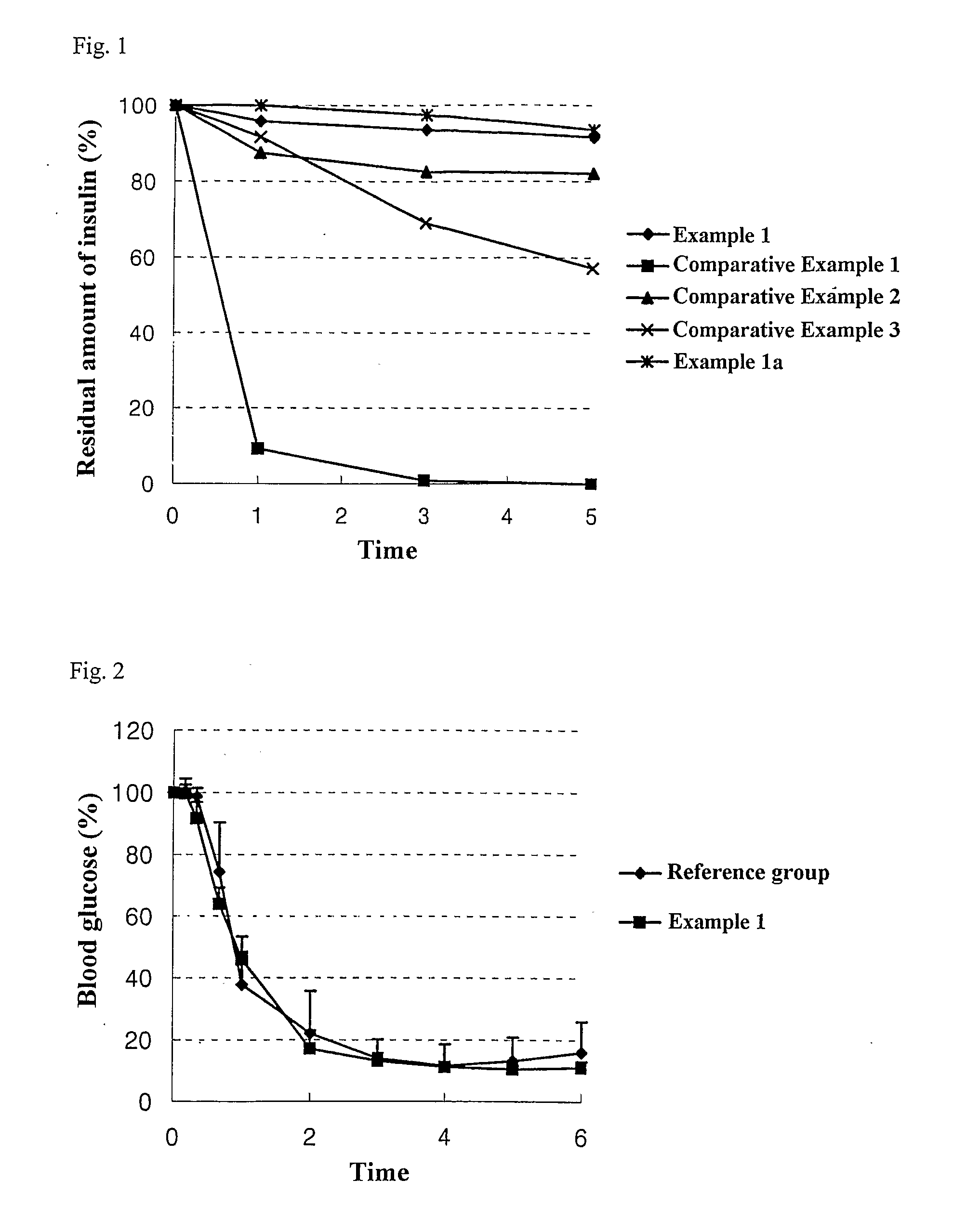
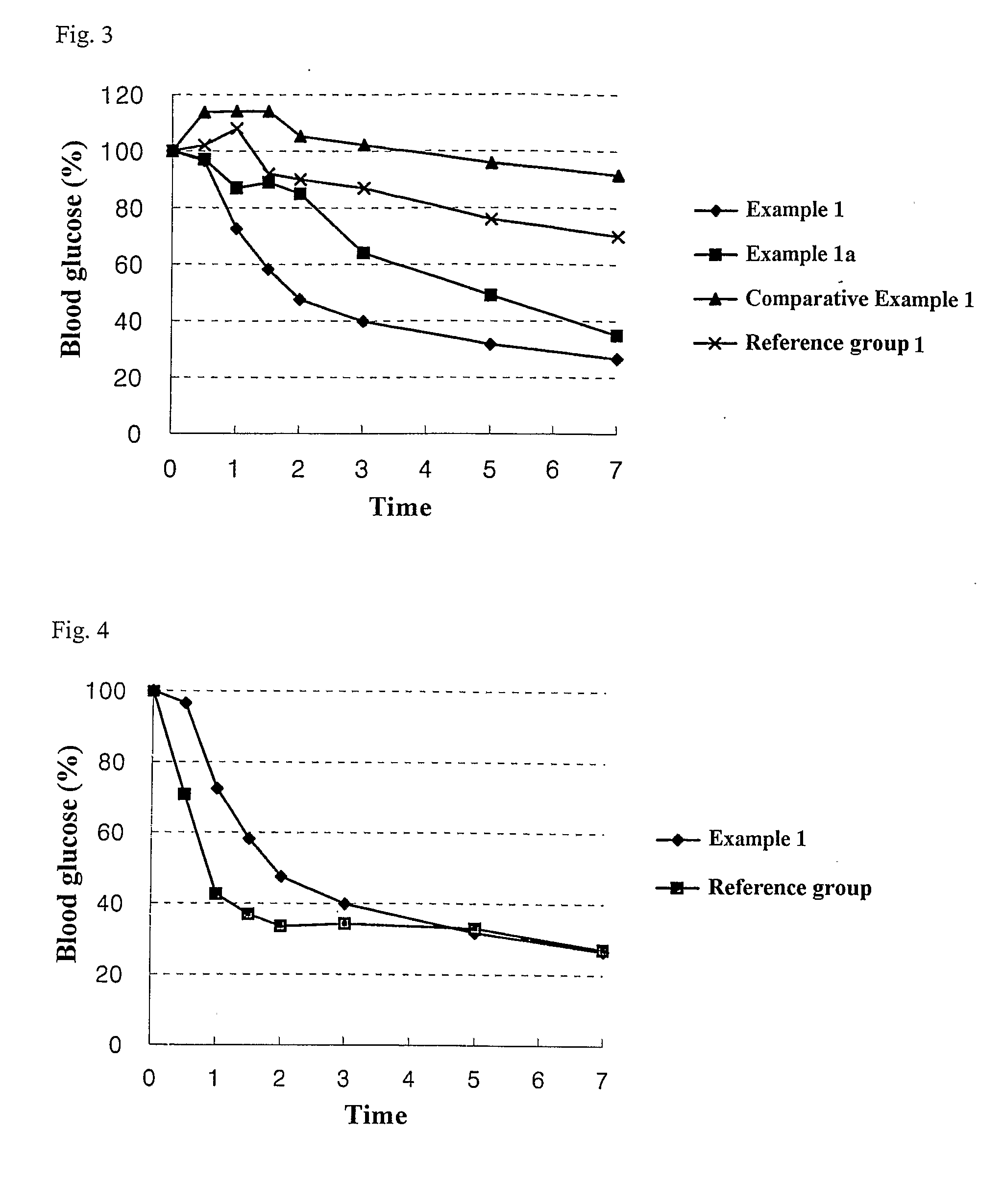
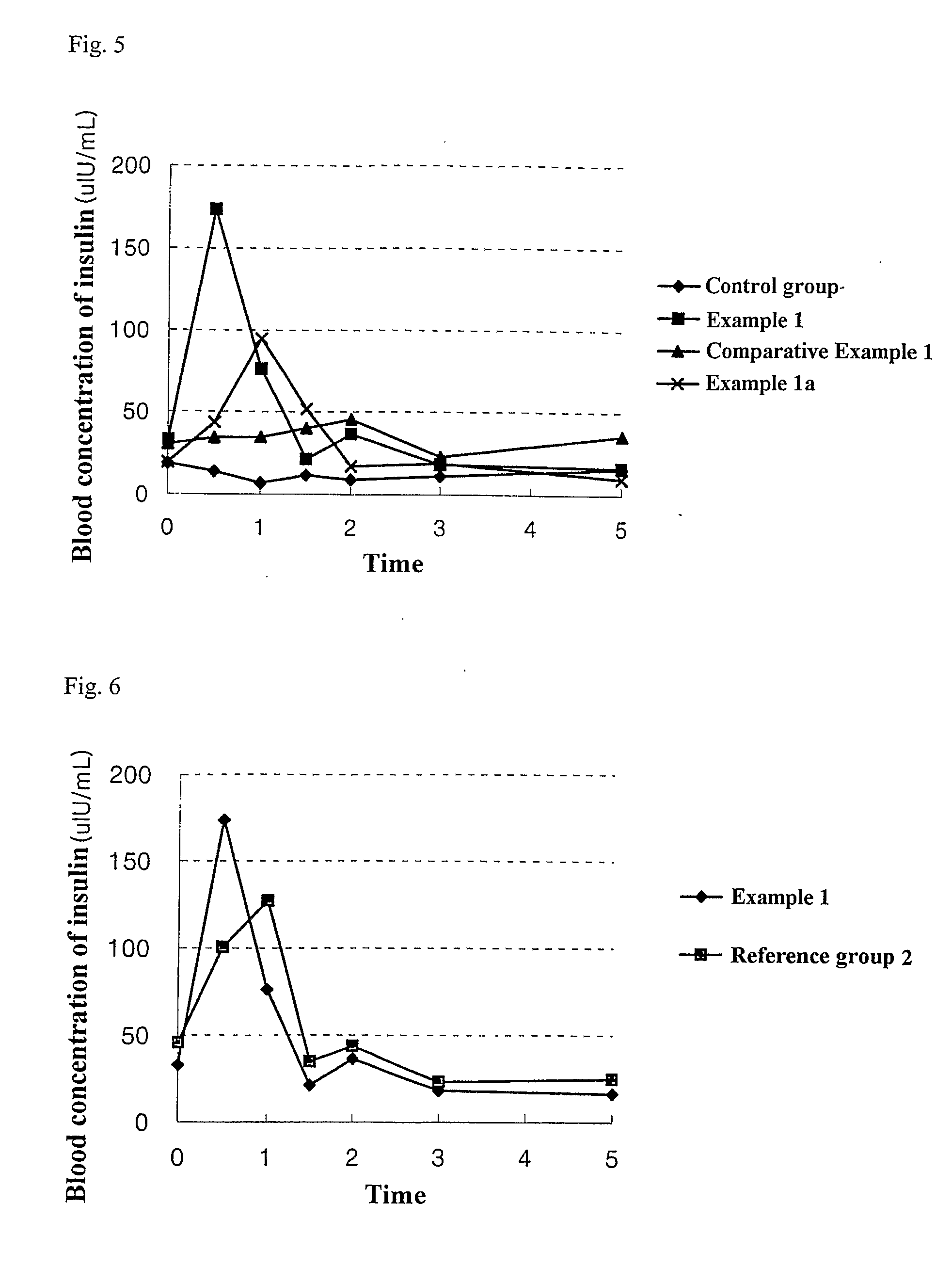
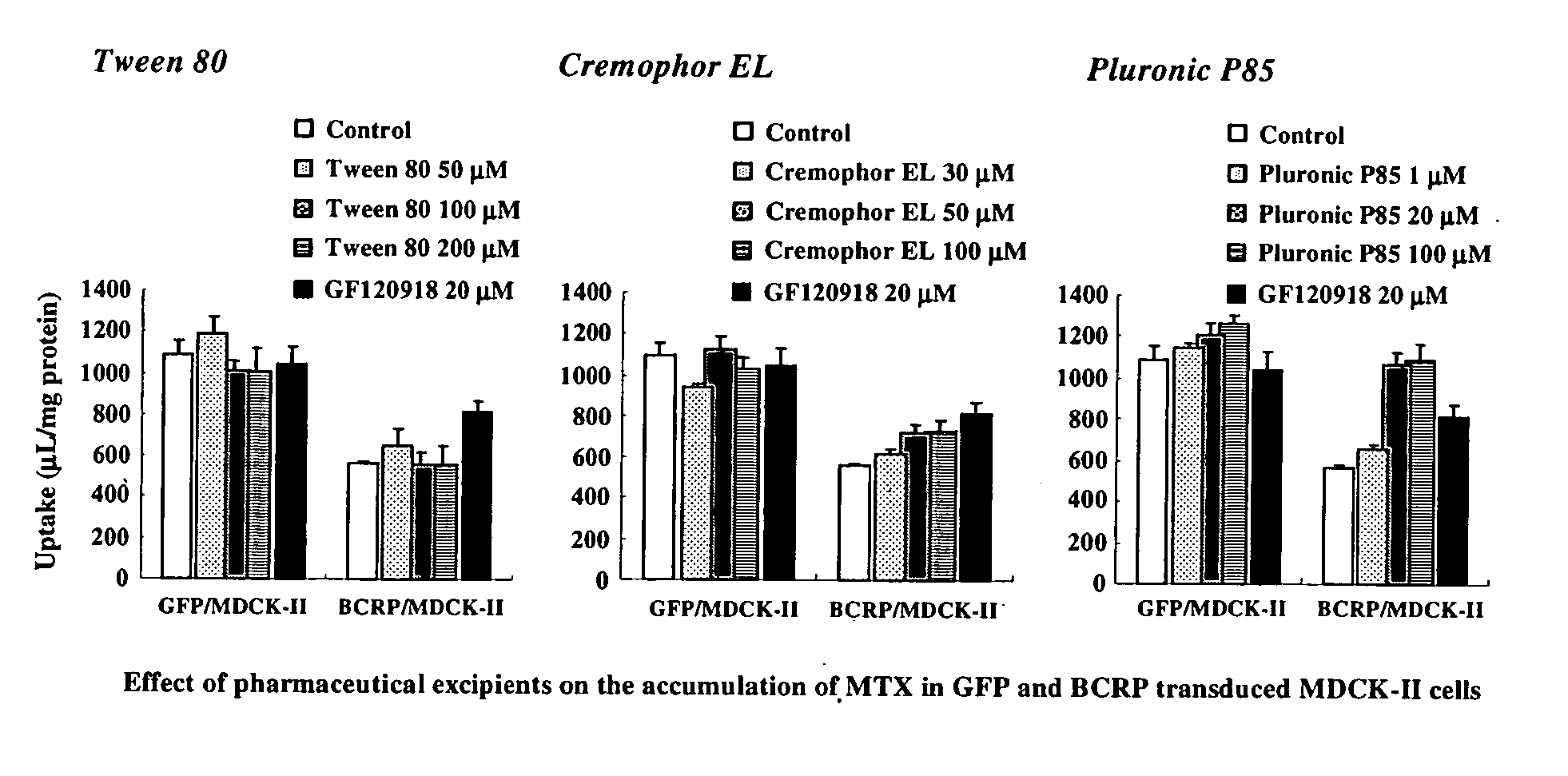
Patents
Literature
123 results about "Gastrointestinal absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dosage forms for administering combinations of drugs
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Dosage forms for administering combinations of drugs
InactiveUS20070207200A1Efficient and rapid deliveryImpairs absorptionOrganic active ingredientsNervous disorderCo administrationGastrointestinal absorption
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
Water Soluble Drug-Solubilizer Powders and Their Uses
InactiveUS20120329738A1Improve gastrointestinal absorptionEfficient processingBiocideCarbohydrate active ingredientsWater soluble drugGastrointestinal absorption
Enhanced methods have been discovered, using either sonication or homogenization followed by increased temperature and pressure, to solubilize compounds using diterpene glycosides and to produce a powder form of the compound-solubilizer complex than can be reconstituted in water. Without the diterpene glycoside, the compounds were insoluble or sparingly soluble in water, including some fat-insoluble vitamins. Water solutions of these compounds were made using a diterpene glycoside solubilizer, for example, rubusoside. The compound-solubilizer complex was then dehydrated to a stable powder that could then be reconstituted with water. A reconstituted drug-solubilizer complex (curcumin-rubusoside) was shown to be effective on reconstitution. In addition, the diterpene glycoside, rubusoside, was shown to be an inhibitor of permeability glycoprotein (P-gp), and will thus increase gastrointestinal absorption of certain drugs administered with rubusoside.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Nanoparticle compositions of water-soluble drugs for oral administration and preparation methods thereof
InactiveUS20070154559A1Increase ratingsIncrease resistanceAntibacterial agentsCosmetic preparationsLipid formationOral medication
The present invention relates to an orally administrable composition containing nanoparticles with the particle size of 500 nm or less, comprising 0.1˜30 weight % of a complex of a water-soluble drug and a counter-ion substance in which the charged water-soluble drug is bonded with the counter-ion substance, 0.5˜80 weight % of a lipid, 0.5˜80 weight % of a polymer, and 1˜80 weight % of an emulsifier, wherein the weight ratio of said lipid and said polymer is in the range of 1:0.05˜3, and a preparation method thereof. The composition of the present invention has high gastrointestinal absorption rate upon oral administration, and has high drug entrapping rate in the nanoparticle, and is also stable against lipases.
Owner:SAMYANG BIOPHARMLS CORP
Anti-aging composition containing resveratrol and method of administration
InactiveUS20090163580A1Improve athletic performanceBiocideNervous disorderActive agentExercise performance
Formulations and methods of treatment and putative prevention for aging (anti-aging composition) and for diseases or conditions of all reactive oxygen species-dependant illnesses, such as Alzheimer's disease, Parkinson's disease, diabetes mellitus, cardiovascular disease, cancer, hepatitis, and disorders associated with estrogen deficiencies including osteoporosis and breast cancer and for improving athletic performance of humans include resveratrol and two (2) or more of the following features or additional active ingredients: (1) slow release formulation of resveratrol; (2) pterostilbene; (3) quercetin; (4) fisetin, and (5) naringenin. Slow release is defined for the purposes of the present invention as releasing 95% of the active agent or agents in eight (8) hours through normal human gastrointestinal absorption.
Owner:NATROL
Formulation and method for enhancement of gastrointestinal absorption of pharmaceutical agents
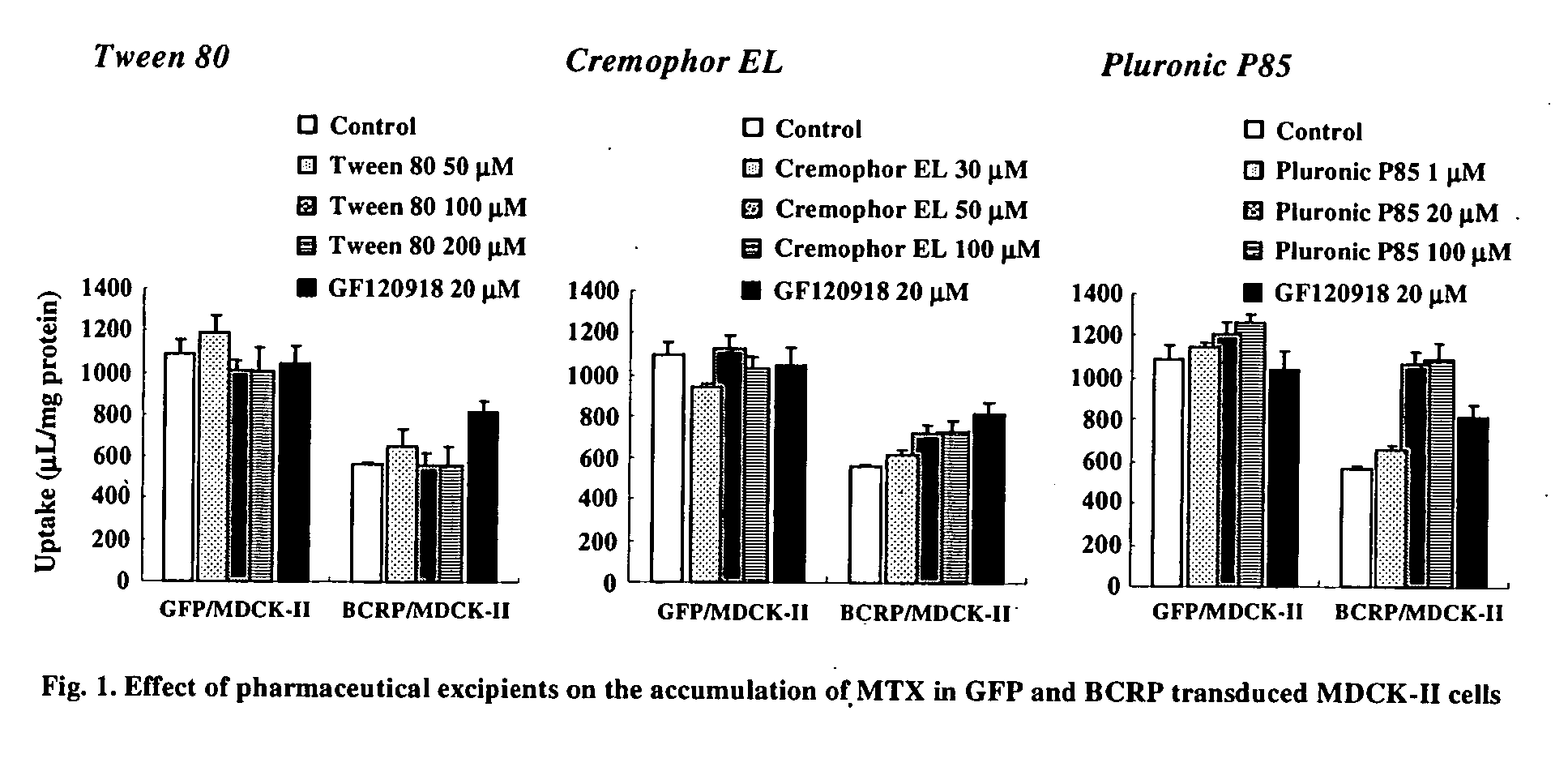
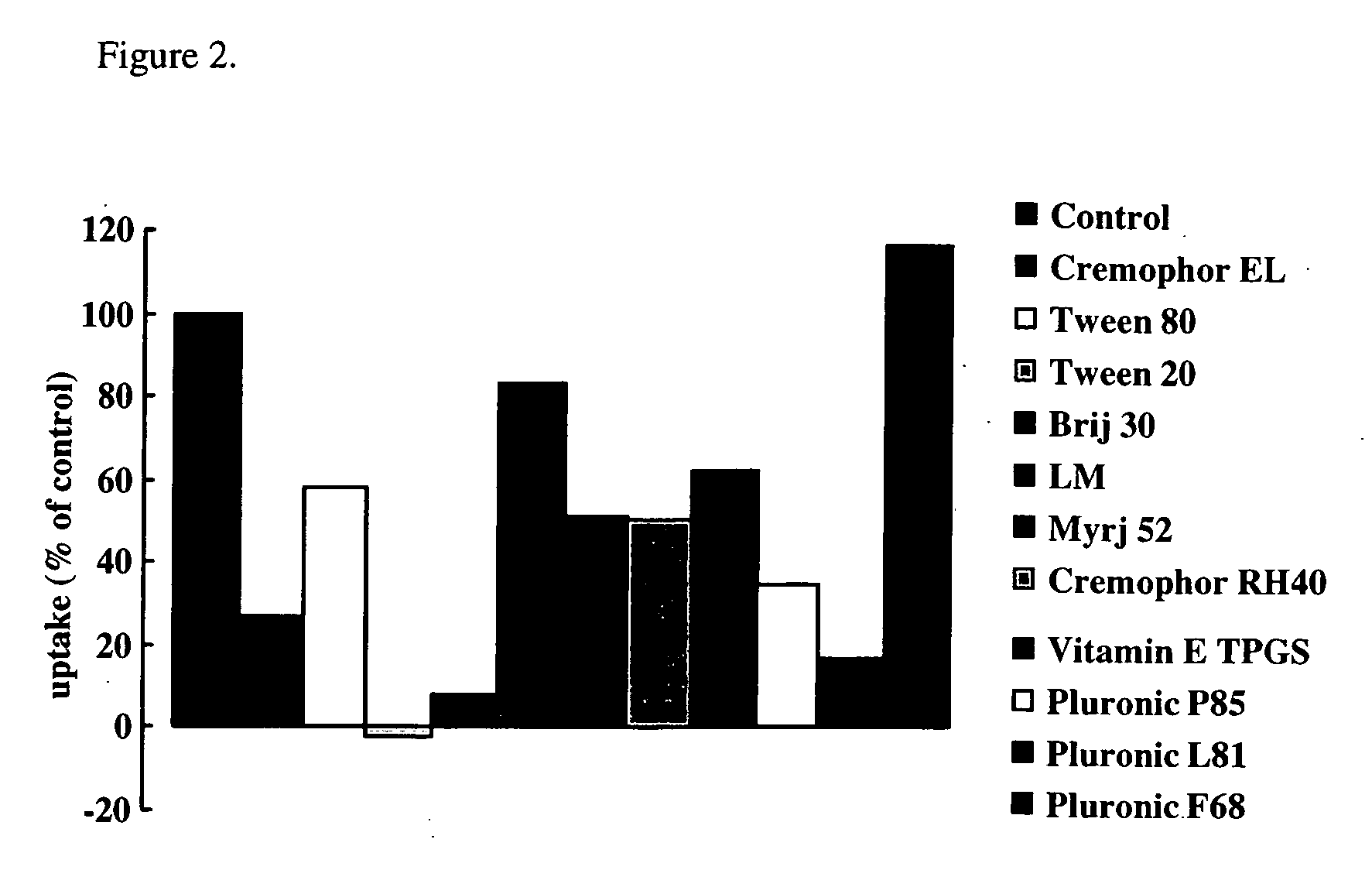
InactiveUS20070053869A1BiocideMetabolism disorderCritical micelle concentrationGastrointestinal absorption
The present invention relates to a method of enhancing absorption of a pharmaceutical agent by administering the agent in combination with an inhibitor of BCRP / ABCG2 wherein the amount of the inhibitor is about the critical micelle concentration of the inhibitor or less than the critical micelle concentration. The invention also relates to a formulation suitable for use to enhance absorption of a pharmaceutical agent. The pharmaceutical agent can be a chemotherapeutic agent. The invention also relates to capsules containing the formulation.
Owner:WARNER-LAMBERT CO
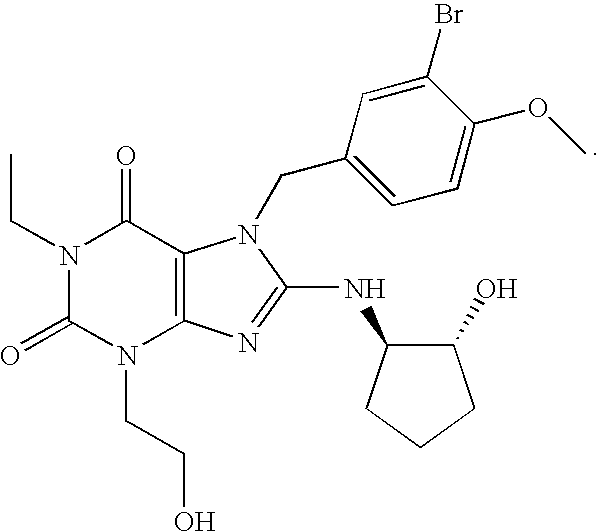
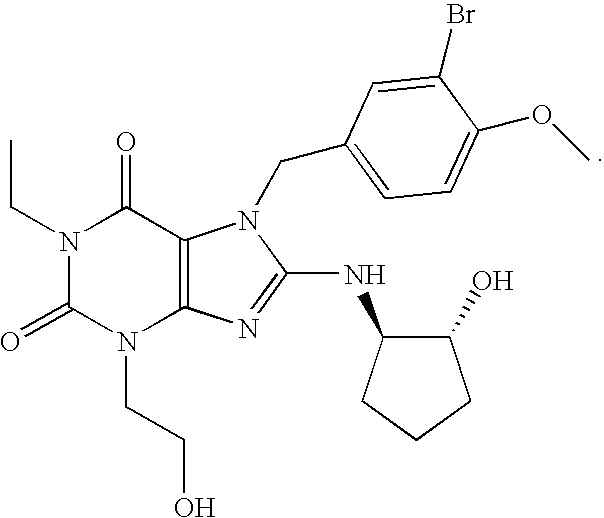
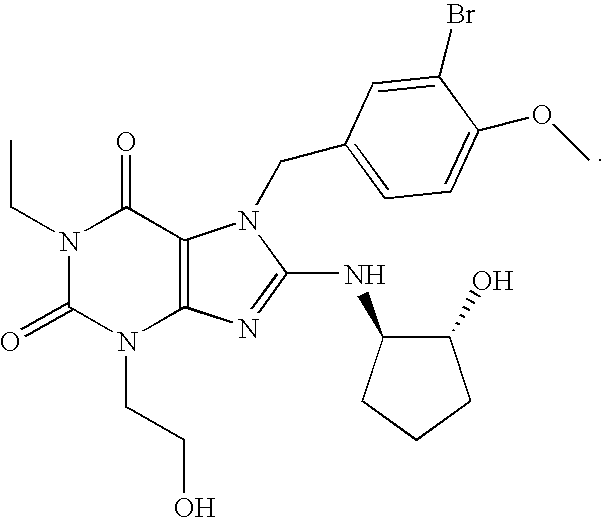
Rapidly absorbing oral formulations of PDE 5 inhibitors
The present invention encompasses oral formulations of a PDE5 inhibitor which provide rapid disintegration after introduction to the oral cavity, followed by buccal and / or sublingual absorption. The orally disintegrating formulations can be in a variety of dosage forms including lingual strip, sublingual strip, oral mist, rapidly disintegrating tablet, lyophilized wafer, granulated particles and gum. The formulations can include an extended release component that allows the PDE5 inhibitor to be swallowed for gastrointestinal absorption. Combination therapies with a second pharmaceutical agent known to cause a PDE5-treatable condition as a side effect, such as erectile dysfunction, are also described. The PDE5 inhibitor of the following chemical structure is particularly favored for these formulations:
Owner:SCHERING CORP
Encapsulated calcium acetate caplet and a method for inhibiting gastrointestinal phosphorous absorption
InactiveUS6875445B2Reduce absorptionReduce phosphorous absorptionBiocidePill deliveryAcetic acidGastrointestinal absorption
A composition for inhibiting gastrointestinal absorption of phosphorous in an individual. The composition includes a quantity of calcium acetate sufficient to bind the phosphorous in the gastrointestinal tract of the individual. The calcium acetate has a bulk density of between 0.50 kg / L and 0.80 kg / L and is dimensioned to form a caplet for fitting within a capsule in a manner that optimizes the volume of the capsule. Also provided is a method for administering the calcium acetate composition of the present invention to an individual to reduce phosphorous absorption by binding with the phosphorous in their gastrointestinal tract.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Composition to retard the onset of symptoms of alzheimer's disease
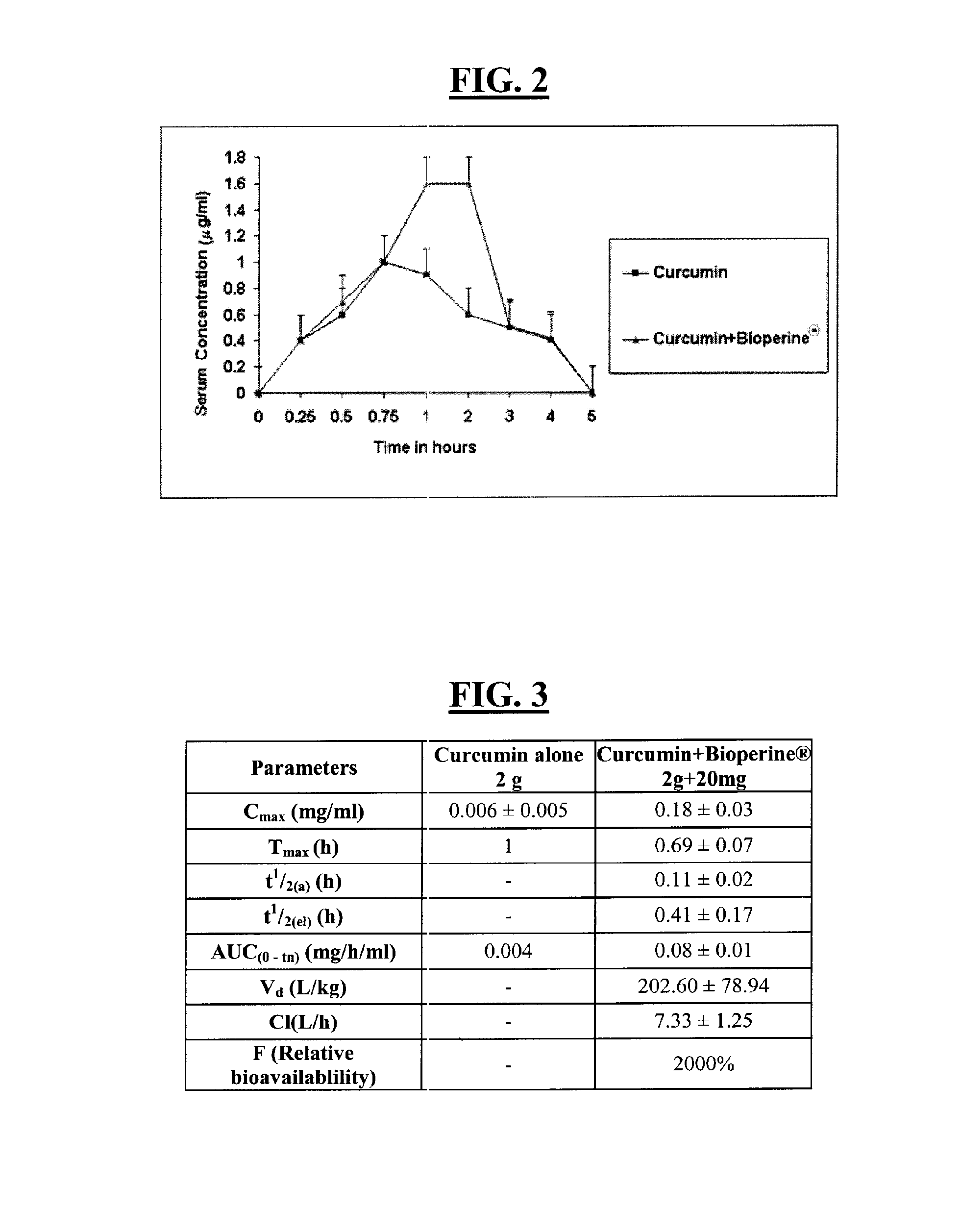
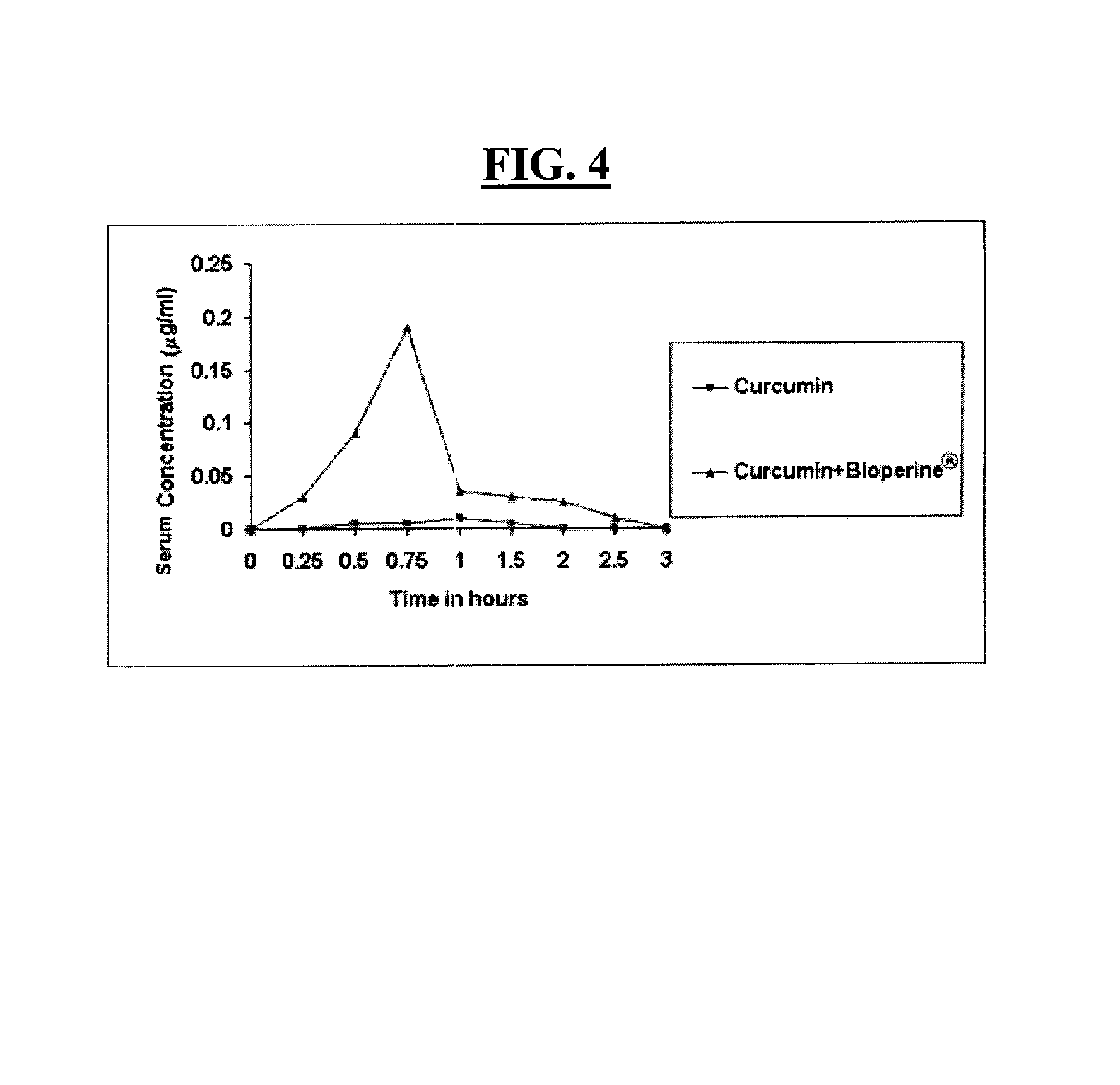
A composition and a method for using the composition to delay the onset of the symptoms of Alzheimer's disease in humans, comprising curcumin, piperine, oleic acid, oleanolic acid, ursolic acid, galantamine, and huperzine A, among other compounds. Curcumin is an antioxidant, while galantamine and huperzine A inhibit the activity of acetylcholinesterase in the brain. Piperine and oleic acid increase the bioavailability and gastrointestinal absorption of curcumin, galantamine, huperzine A, and other nutrients.
Owner:CONSEAL INT
Facilitated transport of bisphosphonates by vitamin C
ActiveUS8247572B2Minimize occurrenceUltrasonic/sonic/infrasonic diagnosticsBiocidePhosphateActive agent
Compounds of the formula: are described wherein: A1 is ascorbic acid, dehydroascorbic acid, ascorbyl-2-phosphate, an analog thereof, or a salt thereof; L is a linking group coupled to A1 at the C5 or C6 position thereof; and B1 is an active agent such as an imaging agent or therapeutic agent (e.g. a bisphosphonate), along with pharmaceutically acceptable salts and prodrugs thereof. The compounds are useful for, among other things, improving cartilage uptake of active agents administered for joint diseases such as osteoarthritis and rheumatoid arthritis, and for improving gastrointestinal absorption of bisphosphonates.
Owner:DUKE UNIV
Nutritional supplements including meal replacements and related methods
InactiveUS20140127351A1Lose weightVitamin food ingredientsAnimal feeding stuffDiseaseDietary supplement
A nutritional or dietary supplement composition useful for formulating meal replacements, particularly beverage-based meal replacements. The composition is absorbed well in patients with compromised gastrointestinal absorption status, such as those having undergone bariatric surgery, other gastrointestinal surgery, those undergoing chemotherapy, and those with other gastrointestinal absorption disorders. Moreover, the compositions are nutritious foods for healthy people. Lastly, the compositions are useful for people with insulin disorders, difficulty maintaining healthy body weight, or inflammation-related disorders. Related methods are also provided.
Owner:OHIO STATE INNOVATION FOUND
Dosage forms for administering combinations of drugs
Owner:POZEN INC
Health care pu'er tea capable of resisting HIV virus and improving human body immunity
InactiveCN101213998AEnhanced inhibitory effectIncrease the number ofPre-extraction tea treatmentAntiviralsAnti virusSide effect
The invention provides healthcare Puer tea with the function of anti-HIV and improving the immunity. The healthcare Puer tea comprises the components with the following mass ratio: 30 to 50 percent of Puer tea, 10 to 20 percent of dendrobium, 10 to 20 percent of ginseng, 5 to 10 percent of tuckahoe, 10 to 20 percent of rhizoma gastrodiae and 8 to 18 percent of lucid ganoderma. The tea is post-fermentation tea, in the process of fermentation, in addition to the nutrient solution of biological enzyme is added, the fungal substance including Lucid Ganoderma and Rhizoma Gastrodiae as well as the medicine with refreshing and invigorating qi is added. Besides of the function of alleviating the damage on the immune cell, promoting the restore of the impaired immune cell, adjusting the absorption environment of intestinal tract and improving the immune system of human body and the gastrointestinal absorption, the invention has the important function of the strong inhibition role on AIDS virus i.e. HIV. Based on the clinical observation, the invention can reduce the occurrence rates of toxic and the side effects caused by the anti-medicine taken by patients, especially the gastrointestinal symptoms and the liver toxicity, promote the medication compliance of patients, significantly increase the number of human CD4 immune cells, do not has the toxic and side effects, thereby promoting the efficacy of anti-virus therapy.
Owner:王乐观
Method of preparing shiitake fungus polyoses oral liquid
InactiveCN101244093APromote absorptionQuality assuranceOrganic active ingredientsFungi medical ingredientsSide effectAlcohol
The invention relates to a preparation method of mushroom polysaccharide oral liquid, which comprises the following steps: slicing mushroom, heating extraction, concentration, alcohol precipitation, high-speed centrifugation, concentration and getting small molecular weight mushroom polysaccharide oral liquid. The oral liquid is characterized in that the obtained mushroom polysaccharide oral liquid removes big molecular structure unsuitable for absorption, therefore, the preparation method has the advantages of preventing gastrointestinal inflation, promoting gastrointestinal absorption, enhancing effect of the product and generating no side effect, facilitating the use, and guaranteeing the quality.
Owner:上海慈瑞医药科技股份有限公司
Salvia miltiorrhiza total phenolic acid gastric stasis preparation
InactiveCN101229225AExtended stayProlong gastrointestinal absorptionOrganic active ingredientsNervous disorderDiseaseRetention time
The invention belongs to the medicine technical field, in particular to a gastric retention preparation containing total salvianolic acid. The invention adopts the effective position of the Chinese medicine of total salvianolic acid as the main component, and the stomach retention preparation is made with acceptable auxiliary materials in pharmacy. The preparation of the invention is gastric floating preparation or stomach mucoadhesive preparation; the invention can significantly prolong the retention time of the total salvianolic acid on the stomach and the upper part of small intestine, enhance stability of medicine, and prolong effective absorption time and improve the utilizing degree of salvianolic acid biology through the double effect of enhancing the medicine stability and prolonging the effective absorption time. The invention can not only improve gastrointestinal absorption, but also have better efficacy in treating gastric ulcer and some other diseases; the invention also has simple production technique and is easy for industrialization; the invention is easy for the patients to take with good compliance.
Owner:FUDAN UNIV
Dosage forms for administering combinations of drugs
InactiveUS20080175897A1Impairs absorptionGood treatment effectBiocideAnimal repellantsCo administrationTreatment pain
The present invention is directed to dosage forms that can be used in therapeutic methods involving the oral co-administration of a combination of at least two drugs, one of which impairs gastrointestinal absorption and one of which does not. The dosage forms are designed so that the drug impairing absorption is not released into the gastrointestinal tract of a patient until after the drugs that do not impair absorption have been released and substantially absorbed. The invention may be used in treatment of migraine using a combination of triptans and NSAIDs or in the treatment of pain using a combination of NSAIDs and opioid analgesics.
Owner:POZEN INC
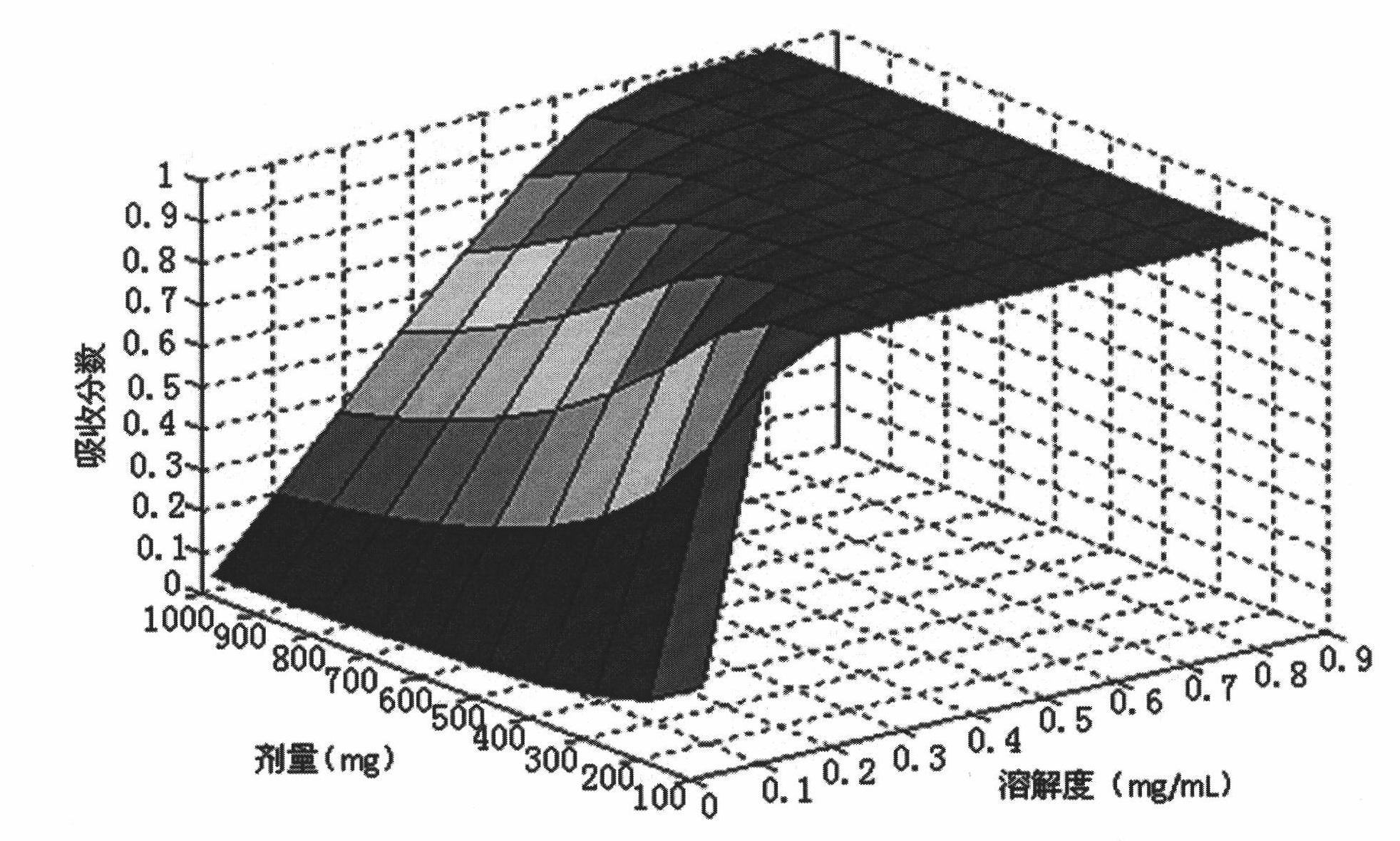
A molding and using method for a bulk drug gastrointestinal absorption prediction BSPK model
InactiveCN102043892ASpeed up screeningPromote absorptionIn-vivo testing preparationsSpecial data processing applicationsSolubilityMedicine
The invention discloses a molding and using method for a bulk drug gastrointestinal absorption prediction BSPK model, which establishes a series of functions based on the bulk drug dissolution, settlement and absorption in multiple gastrointestinal compartments, and which takes into account many factors and variables as follows: drug logP, logD, pKa, solubility, dissolution rate, density, partical diameter, particle forms, particle diameter distribution, settlement rate, settlement particle diameter, settlement time, permeability and human gastrointestinal physiological conditions, etc. The method is applicable to acidic, alkalic, neutral and acidic-alkalic drug absorption prediction in different gastrointestinal parts. The invention, which is used in active compound gastrointestinal absorption prediction, not only raises the early stage drug screening accuracy, but also reduces the workload of preformulation researches. In this way, medicine clinical application process is accelerated with great scientific research value and application prospect.
Owner:SHANGHAI YUNYI HEALTHCARE MANAGEMENT
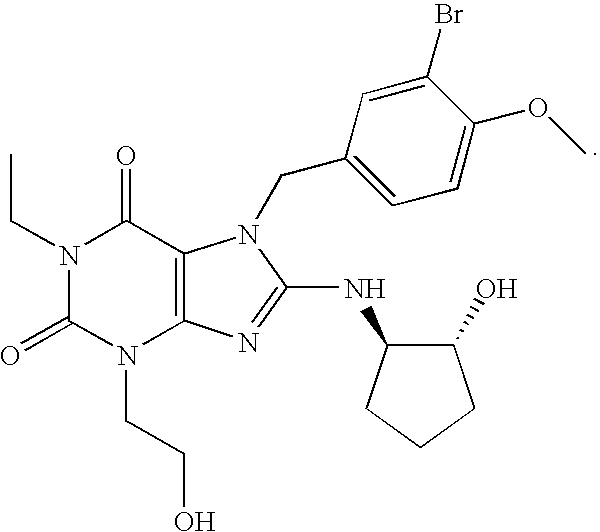
Rapidly absorbing oral formulations of PDE 5 inhibitors
The present invention encompasses oral formulations of a PDE5 inhibitor which provide rapid disintegration after introduction to the oral cavity, followed by buccal and / or sublingual absorption. The orally disintegrating formulations can be in a variety of dosage forms including lingual strip, sublingual strip, oral mist, rapidly disintegrating tablet, lyophilized wafer, granulated particles and gum. The formulations can include an extended release component that allows the PDE5 inhibitor to be swallowed for gastrointestinal absorption. Combination therapies with a second pharmaceutical agent known to cause a PDE5-treatable condition as a side effect, such as erectile dysfunction, are also described. The PDE5 inhibitor of the following chemical structure is particularly favored for these formulations:
Owner:SCHERING CORP
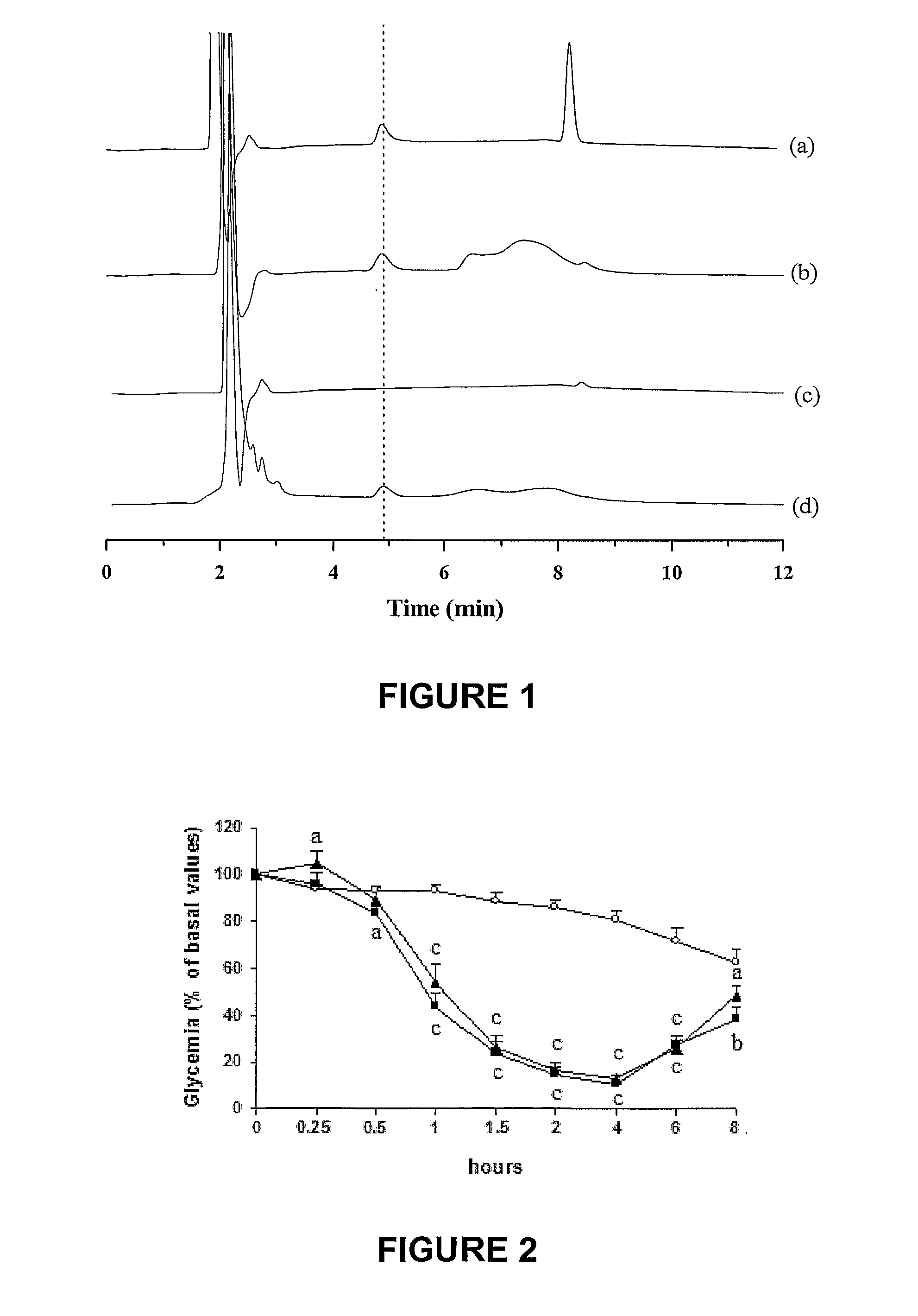
Oral submicron particle delivery system for proteins and process for its production
InactiveUS20100143484A1Powder deliveryPeptide/protein ingredientsGastrointestinal absorptionEnzymatic degradation
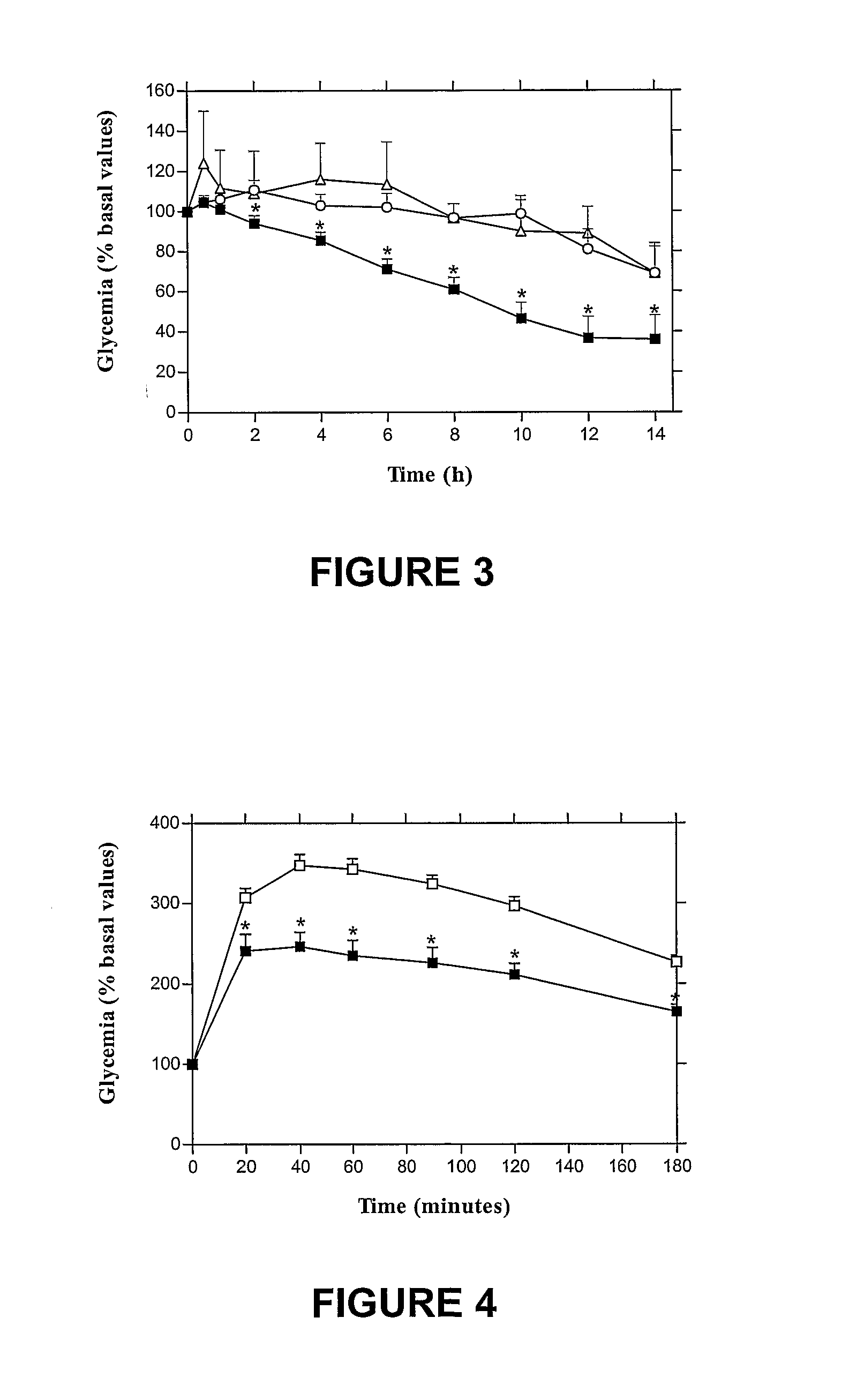
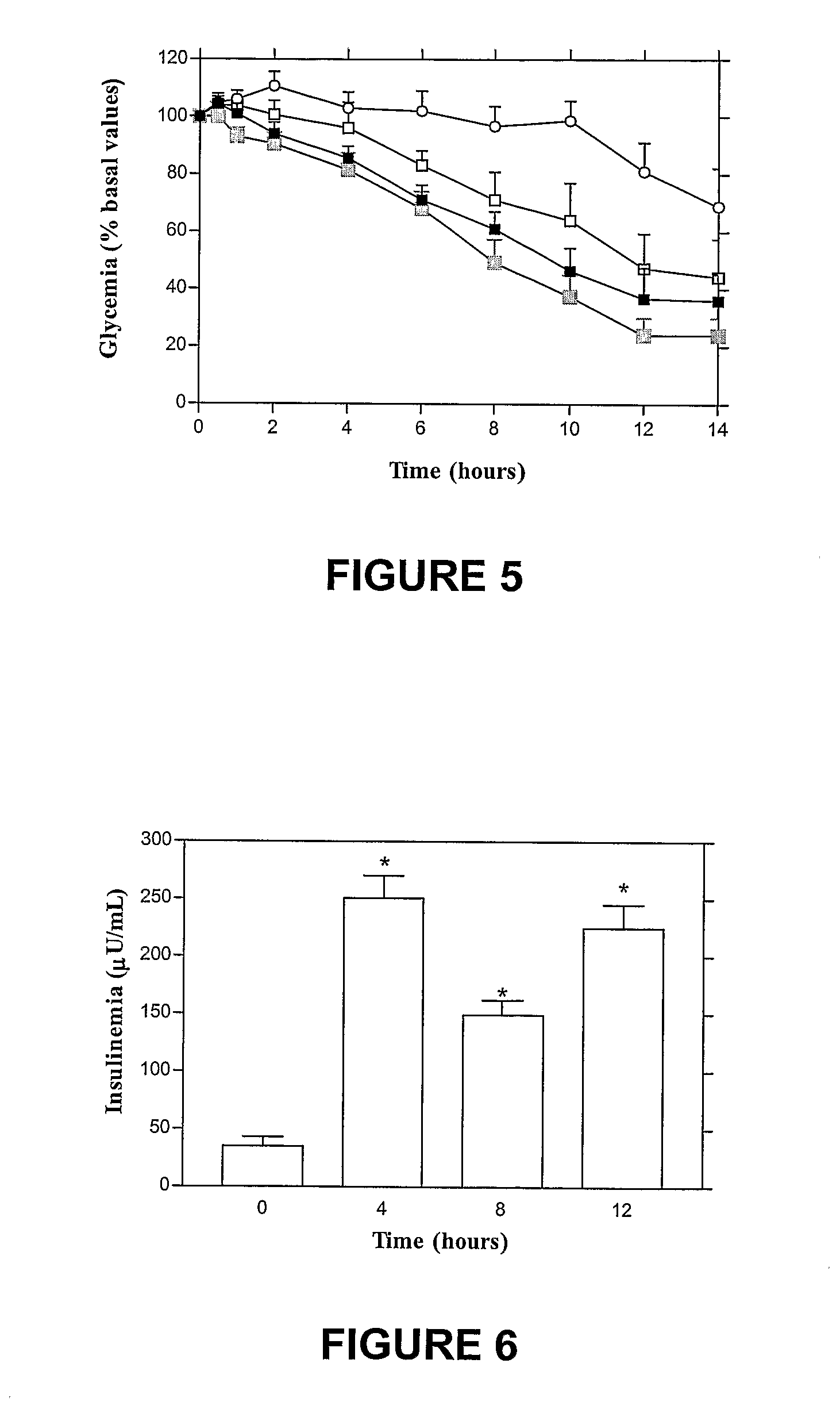
The invention provides a novel submicron system for the oral administration of proteins. An effective oral carrier for proteins should shield its content against the gastrointestinal tract proteases and be capable of facilitating the uptake of the protein drug across the gastrointestinal epithelium. The present invention relates to production of gelled particles which comprises a protein drug susceptible to enzymatic degradation by enzymes and acid conditions in the stomach, a polymeric matrix which undergoes precipitation-swelling process, and two-layer-coating materials which are themselves capable of enhancing absorption of said drug across the intestinal mucosal tissues and of inbihiting degradation of said drug by gastric enzymes. Insulin-loaded particles with appropriate submicron size for gastrointestinal absorption were made of natural occurring polymers by emulsification-based method and proved to be gastric pH and protease protective. Effects on glycemia were observed during 14 h after their oral single administration to rats, achieving 42% of pharmacological activity compared to subcutaneous administration. Postprandial rise in blood glucose was suppressed and insulinemia levels increased by a factor of seven. The relative oral bioavailability of insulin calculated over 8 h by comparison with a subcutaneous injection of free insulin was 34%.
Owner:UNIVE DE COIMBRA
Houttuynia cordata thunb beverage
InactiveCN101530228APromote absorptionPrevent oxidationPre-extraction tea treatmentFood preparationHouttuyniaGastrointestinal absorption
The invention discloses a houttuynia cordata thunb beverage belonging to the food field. The production method thereof is as follows: (1) fresh houttuynia cordata thunb is juiced by being added with 2-3 times of water, and tea leaves are added with 2-3 times of water for soaking or the fresh houttuynia cordata thunb is crushed into 300 to 1000 items and then added with 4-5 times of water; (2) 10 to 20 percent of houttuynia cordata thunb juice, 10 to 20 percent of tea, 1 to 5 percent of citric acid, 5 to 9 percent of sugar and the balance of 50 to 60 percent of water are mixed, evenly stirred, filtered and then packed, thus obtaining the beverage. The beverage has better mouthfeel, can accelerate gastrointestinal absorption and effectively protect lung cells, prevent and cure hyperlipoidemia and cerebral apoplexy, and has the functions of cellular oxidation inhibiting and anti-aging and health care.
Owner:唐晨光
Nanoparticle compositions of water-soluble drugs for oral administration and preparation methods thereof
InactiveUS7674767B2Increase ratingsIncrease resistanceAntibacterial agentsPowder deliveryOral medicationGastrointestinal absorption
The present invention relates to an orally administrable composition containing nanoparticles with the particle size of 500 nm or less, comprising 0.1-30 weight % of a complex of a water-soluble drug and a counter-ion substance in which the charged water-soluble drug is bonded with the counter-ion substance, 0.5-80 weight % of a lipid, 0.5-80 weight % of a polymer, and 1-80 weight % of an emulsifier, wherein the weight ratio of said lipid and said polymer is in the range of 1:0.05-3, and a preparation method thereof. The composition of the present invention has high gastrointestinal absorption rate upon oral administration, and has high drug entrapping rate in the nanoparticle, and is also stable against lipases.
Owner:SAMYANG BIOPHARMLS CORP
Stabilized insulinotropic peptides and methods of use
ActiveUS9296805B2Superior and unexpected benefitEnhanced alpha-helicityPeptide/protein ingredientsMetabolism disorderCross-linkAbnormal glucose homeostasis
The present invention provides stably cross-linked insulionotropic polypeptides having superior and unexpected benefits in the treatment of conditions involving abnormal glucose homeostasis, e.g., type 2 diabetes and conditions relating to type 2 diabetes. Such benefits include, but are not limited to, extended polypeptide half-life, enhanced alpha-helicity, improved thermal stability and protease resistance, increased functional activity and pharmacologic properties, improved bioavailability when administered by any route, and improved bioavailability and gastrointestinal absorption when delivered orally, as compared to the corresponding unmodified polypeptides. The invention also provides compositions for administering the polypeptides of the invention, as well as methods for preparing and evaluating the polypeptides of the invention.
Owner:DANA FARBER CANCER INST INC
Chinese medicine paste for treating infantile diarrhoea due to cold exposure
InactiveCN102370955AAvoid painAvoid side effectsDigestive systemSheet deliverySide effectLiver and kidney
The invention discloses a Chinese medicine paste for treating infantile diarrhoea due to cold exposure, comprising the following ingredients: 2-4 weight portions of evodia rutaecarpa, 2-4 weight portions of fructus psoraleae, 3-4 weight portions of long pepper extract, 5-8 weight portions of clove, 5-8 weight portions of rhizoma zingiberis, 5-8 weight portions of acalypha australis, 3-5 weight portions of bighead atractylodes rhizome, 5-8 weight portions ofmutmeg, 5-8 weight portions of cimicifuga foetida, and 3-5 weight portions of pericarpium granati. According to the invention, the medicinal paste is used for treating infantile diarrhoea, and the physiological characteristic of strong absorption function of infantile umbilical region is mainly utilized; the use of the unique way of pasting on navel prevents the pain of injection and taking medicines of children; simultaneously, the treatment of the Chinese medicine paste has no need of gastrointestinal absorption and liver and kidney metabolism, thus the possible side effect of medicines is avoided. The present invention has the advantages of good therapeutic efficacy, quick cure speed and convenient use.
Owner:潘立芝
Composition and feed additive and preparation methods thereof
InactiveCN107047975AGood antibacterial effectVolatileAccessory food factorsBiotechnologyAquaculture industry
The present invention belongs to the field of compositions and particularly relates to a composition and a feed additive and preparation methods thereof. The provided feed additive comprises cortex cinnamomi essential oil, acetic acid and mannan oligosaccharide, wherein the cortex cinnamomi essential oil has volatility and broad spectrum antibacterial activity, the acetic acid can maintain gastrointestinal acid-base balance, inhibit harmful bacteria and promote probiotics growth, and a combination of the acetic acid and cortex cinnamomi essential oil enhances antibacterial effect of the composition, reduces harm of the cortex cinnamomi essential oil on the gastrointestinal probiotics, and promotes gastrointestinal absorption; and at the same time, the three materials of the cortex cinnamomi essential oil, acetic acid and mannan oligosaccharide are reasonable in compatibility, have synergies, improve effects of the composition in inhibiting growth of intestinal pathogens, enhance body immunity functions and protect the intestinal probiotics. The feed additive is simple in preparation method, low in costs of the components, can effectively replace feed antibiotics applied in aquaculture industry, and reduces production costs.
Owner:GUANGDONG UNIV OF TECH
Glucosamine formulations
InactiveCN101926808AImprove bioavailabilityOrganic active ingredientsDispersion deliveryCyclodextrinGastrointestinal absorption
Glucosamine supplements are widely used for the treatment of osteoarthritis. However, glucosamine has poor and variable gastrointestinal absorption although it is usually taken orally. The current invention provides new formulations and / or methods that could increase the plasma concentration by 1.3 to 4 times after oral administration of glucosamine with an additional absorption enhancer. In the current invention, chitosans and cyclodextrins were identified as suitable absorption enhancers for glucosamine.
Owner:JIWA PHARMA
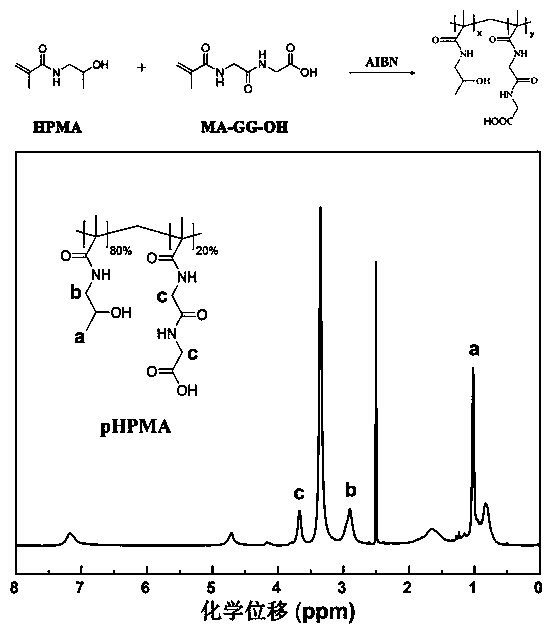
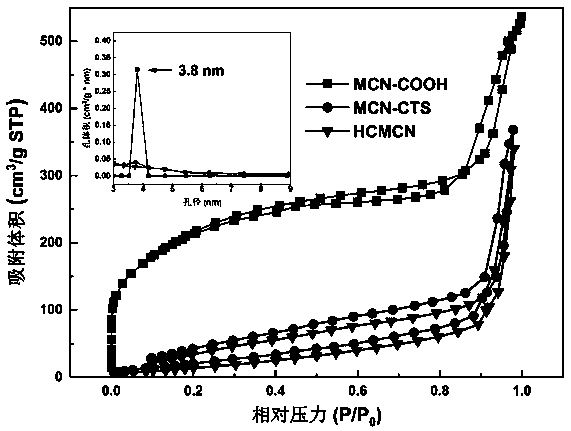
Polymer functionalized modified mesoporous carbon nanoparticles and preparation and application thereof
ActiveCN110201178APowder deliveryInorganic non-active ingredientsGastrointestinal absorptionNanometer size
The invention belongs to the technical field of medicines, relates to polymer functionalized modified mesoporous carbon nanoparticles and preparation and application thereof, particularly relates to polymer functionalized modified mesoporous carbon nanoparticles capable of overcoming multiple gastrointestinal absorption barriers and a drug delivery system prepared from the nanoparticles, and alsorelates to application of the nanoparticles as insoluble drug carriers in promoting oral drug absorption. According to the invention, firstly, chitosan is covalently modified on the surfaces of the mesoporous carbon nanoparticles for improving intestinal uptake of carriers; and a hydrophilic and negatively charged N-(2-hydroxypropyl) methacrylamide copolymer (pHPMA) is adsorbed to the surfaces ofthe nano carriers through electrostatic force, so that the mucus permeability of the carriers is improved. According to an administration system constructed by the invention, drug is highly dispersedin mesoporous pore channels of the carriers in a nanometer size, so that the drug exists in an amorphous state, and oral absorption of the insoluble drug is promoted.
Owner:SHENYANG PHARMA UNIVERSITY
Veterinary-use intestinal targeted antibacterial pellet and preparation method thereof
ActiveCN102600184AReduce first pass effectImprove bioavailabilityAntibacterial agentsTetracycline active ingredientsFormularyChlortetracycline Hydrochloride
The invention discloses a veterinary-use intestinal targeted antibacterial pellet. The veterinary-use intestinal targeted antibacterial pellet comprises a main drug and auxiliary drugs, wherein the main drug is composed of valnemulin hydrochloride and chlortetracycline hydrochloride; the mass of the valnemulin hydrochloride is 1-5% of that of the pellet; the mass of the chlortetracycline hydrochloride is 5-16 times of that of the valnemulin hydrochloride; the pellet sequentially comprises a valnemulin hydrochloride core, an intestinal targeted coating layer, a chlortetracycline hydrochloride drug layer and an outer coating layer from inside to outside. The invention further discloses a preparation method of the veterinary-use intestinal targeted antibacterial pellet. Through the combination of the method and the formula, the valnemulin hydrochloride and the chlortetracycline hydrochloride are adopted for preparing the pellet; the pellet is released in a gradient form in an animal digestion system; the chlortetracycline hydrochloride is firstly released and absorbed by the stomach and the intestine; the valnemulin hydrochloride is rapidly released and absorbed by the intestinal tract with the pH value larger than or equal to 6.8, so that an excellent intestinal tract sterilization effect is achieved, the drug absorbed by the colon can be prevented from suffering from the first-pass effect of the liver and the intestine, the haemoconcentration and bioavailability of the valnemulin hydrochloride can be improved, and an excellent treatment effect for systemic diseases is obtained.
Owner:山东胜利生物工程有限公司
Selenium-rich health-preserving flavored tea and preparation method thereof
InactiveCN107019066AEfficient dryingEffective dissolutionPre-extraction tea treatmentTrace elementMedicine
The invention relates to the technical field of tea processing, in particular to a selenium-rich health-preserving flavored tea and a preparation method thereof. Boat-fruited sterculia, lophatherum gracile and mango peel with heat-clearing, toxin removing, antibacterial and health care effects are added into the selenium-rich health-preserving flavored tea to improve the gastrointestinal environment in human body and enhance the gastrointestinal absorption capacity; then hawthorn and kudzu vine root are added to reduce blood glucose and blood lipid; Chinese wolfberry, red dates and longan are added to invigorate qi and blood; and chrysanthemum and cassia seeds are added to remove liver fire and improve eyesight. The above 10 raw materials cooperate with selenium-rich black tea, and can effectively increase the content of zinc, selenium and other trace elements in the tea soup, all the components coordinate with each other to improve the internal environment in the human body, promote the absorption of effective components, relieve people's mental pressure and other discomfort conditions, and improve human immune functions, thus finally reaching the purpose of improving and treating sub-health. As different processing methods are employed for the different raw materials, the dissolution rates of various components are different, so that the tea soup can have rich taste and layered sense, and enjoy wide popularity.
Owner:BAISE UNIV
Compound-type fitness powder for quickly restoring strength and gaining muscles
ActiveCN107485019APromote muscle gainProtection absorption functionSugar food ingredientsFood ingredient functionsAdditive ingredientWhey protein powder
The invention discloses compound-type fitness powder for quickly restoring strength and gaining muscles and belongs to the technical field of functional food. Every 100g of the compound-type fitness powder contains 40g-50g of concentrated hydrolyzed whey protein and soy protein isolate, wherein the ratio of the soy protein isolate to the concentrated hydrolyzed whey protein is 1:(1-1.5); the protein content of the concentrated hydrolyzed whey protein powder is 80% or higher; the hydrolysis degree of the whey protein is 5%-12%; the protein content of the soy protein isolate is 90% or higher ; and the hydrolysis degree of the soy protein isolate is 5%-12%. The fitness powder provided by the invention is applicable for supplementing various kinds of nutrients to restore the strength after keep-fit exercises and can significantly increase muscle weight and improve athletic ability; through selecting the concentrated hydrolyzed whey protein and the soy protein isolate as ingredients, inner heat and diarrhea caused by lactose intolerance are reduced so as to protect gastrointestinal absorption functions; and with scientifically added taurine, betaine and creatine and intensified vitamin B components, basic nutrient demands after exercises can be fulfilled.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Stabilized insulinotropic peptides and methods of use
ActiveUS20130157942A1Superior and unexpected benefitEnhanced alpha-helicityPeptide/protein ingredientsMetabolism disorderCross-linkAbnormal glucose homeostasis
The present invention provides stably cross-linked insulionotropic polypeptides having superior and un-expected benefits in the treatment of conditions involving abnormal glucose homeostasis, e.g., type 2 diabetes and conditions relating to type 2 diabetes. Such benefits include, but are not limited to, extended polypeptide half-life, enhanced alpha-helicity, improved thermal stability and protease resistance, increased functional activity and pharmacologic properties, improved bioavailability when administered by any route, and improved bioavailability and gastrointestinal absorption when delivered orally, as compared to the corresponding unmodified polypeptides. The invention also provides compositions for administering the polypeptides of the invention, as well as methods for pre-paring and evaluating the polypeptides of the invention.
Owner:DANA FARBER CANCER INST INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com