Patents
Literature
131 results about "Huperzine A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
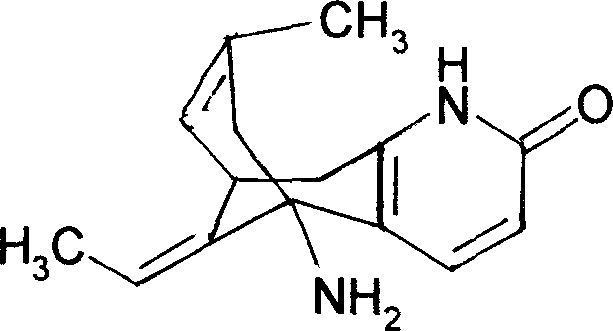
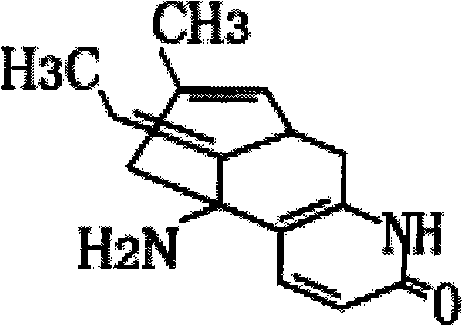
Huperzine A is a naturally occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinesterase. It is commonly available over the counter as a nutrient supplement, and is marketed as a cognitive enhancer for improving memory and concentration.
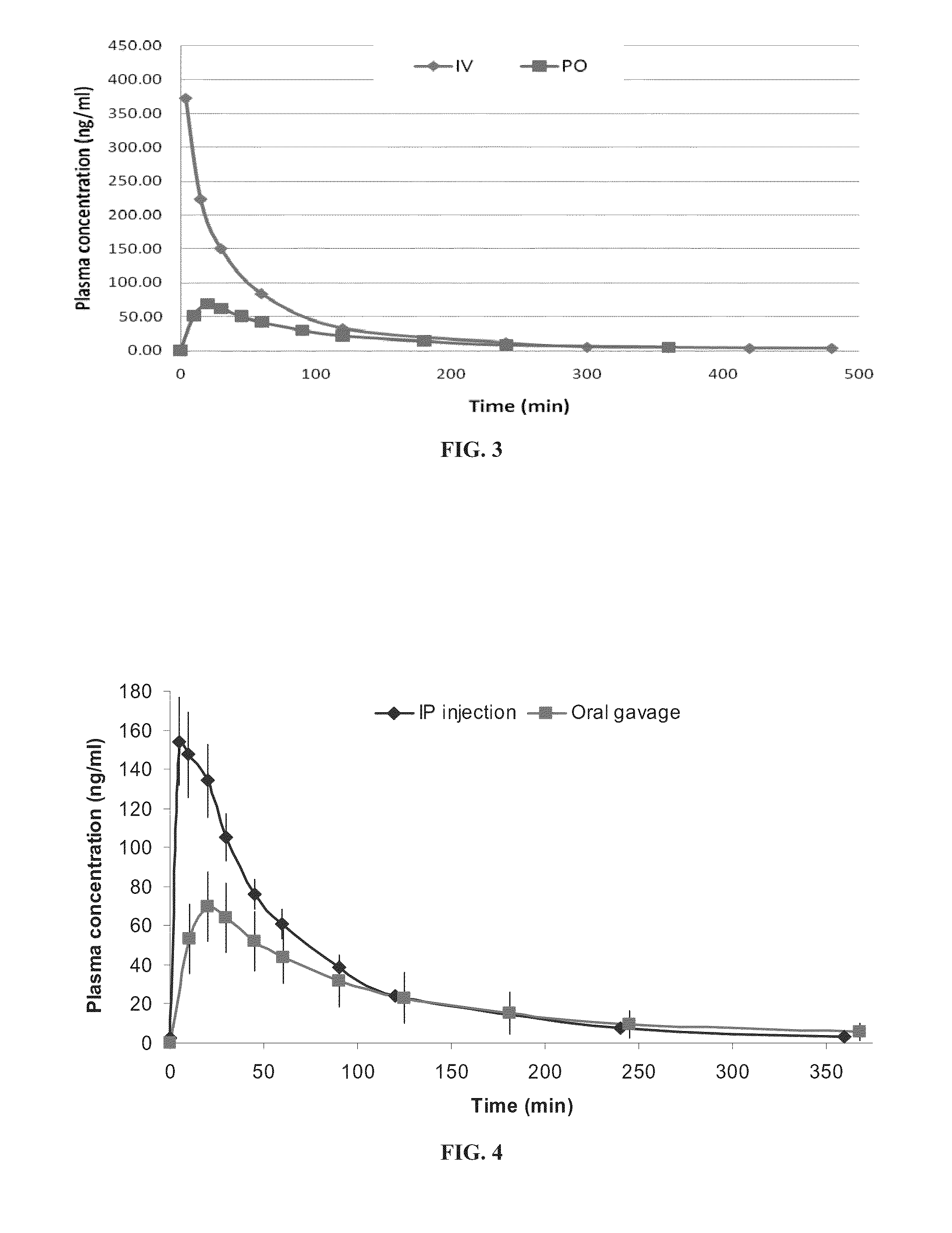
Transdermal rate-controlled delivery of Huperzine A for treatment of alzheimer's disease
This invention relates to a novel transdermal drug delivery system whereby Huperzine A ("Hup A"), a naturally occurred Acetylcholine esterase inhibitor traditionally used to alleviate memory problem, is formulated for transdermal administration suitable for the treatment of Alzheimer's Disease ("AD") to increase the efficacy and convenience for outpatient care of AD patients. A controlled-release skin patch designed for once-a-week application of Hup A is provided for easy AD medication according to the invention.
Owner:SAGITTARIUS LIFE SCI
Composition and method for enhancing neuromuscular facilitation and cognitive functions
InactiveUS20070248696A1Function increaseIncrease in the neurotransmitter acetylcholineBiocideCarbohydrate active ingredientsGriffonia simplicifoliaDietary supplement
A composition and a method for improving neuromuscular facilitation, also known as “muscle memory,” and enhancing cognitive functions, such as memory and mental focus. The dietary supplement, and the method for the administration thereof, increases acetylcholine levels, which improves neuromuscular facilitation. In various embodiments, factors other than increasing acetylcholine levels within the body further contribute to the overall effect of the dietary supplement composition. In a preferred embodiment, the invention is a composition, and a method for the administration thereof, comprising effective amounts of choline, dimethylaminoethanol, cytidine 5′-diphosphocholine, turmeric extract, decaffeinated green tea extract, vitamin B1, vitamin B5, vitamin B6, vitamin B12, folic acid, dimethylglycine, huperzine A, Griffonia simplicifolia extract, and 1-phenylalanine, magnesium stearate, and silicon dioxide.
Owner:MIND SPORTS NUTRITION
Composition to retard the onset of symptoms of alzheimer's disease
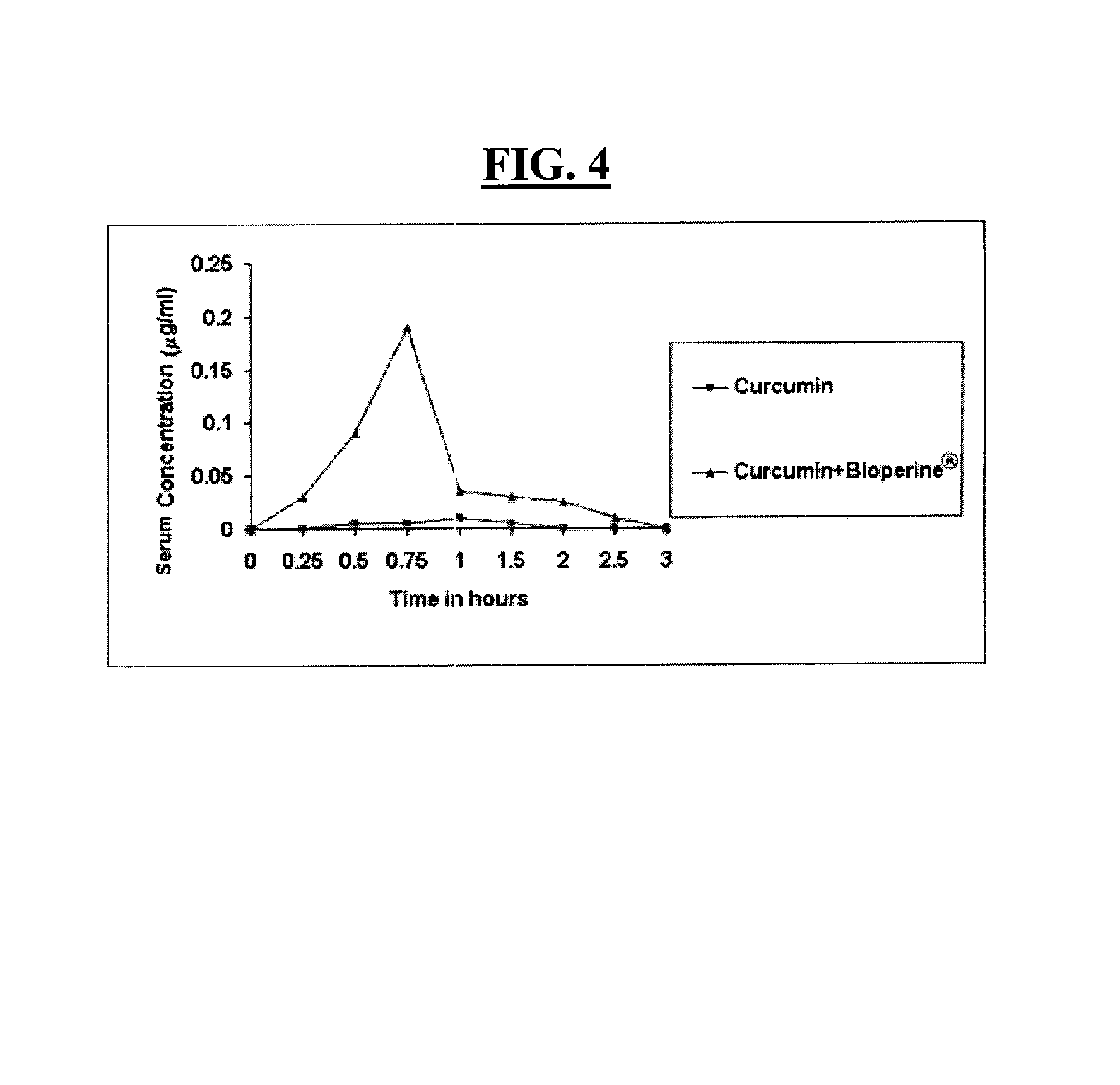
A composition and a method for using the composition to delay the onset of the symptoms of Alzheimer's disease in humans, comprising curcumin, piperine, oleic acid, oleanolic acid, ursolic acid, galantamine, and huperzine A, among other compounds. Curcumin is an antioxidant, while galantamine and huperzine A inhibit the activity of acetylcholinesterase in the brain. Piperine and oleic acid increase the bioavailability and gastrointestinal absorption of curcumin, galantamine, huperzine A, and other nutrients.
Owner:CONSEAL INT
Slow-release small pill prparation containing huperzine A and method for making same
InactiveCN101081217AStable blood concentrationRelease stabilityOrganic active ingredientsNervous disorderSmall intestineTreatment level
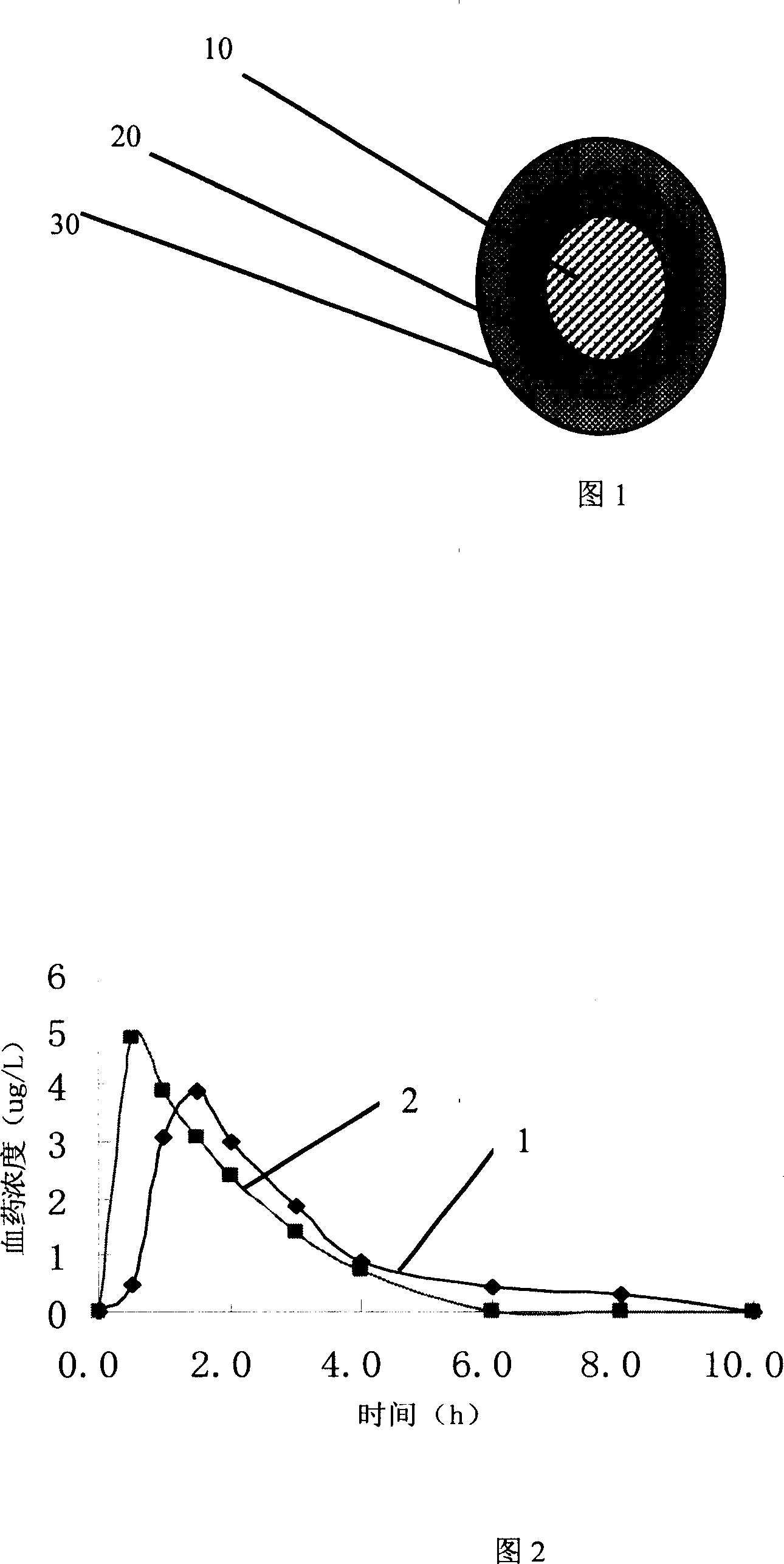
The slow released Huperzine A pillet comprising one neutral core, one active layer containing Huperzine A and coating the core, and one slow releasing layer coating the active layer. It is prepared through a solvent dispersing process to coat the Huperzine A medicine onto the neutral core and subsequent coating the slow releasing layer. The present invention has raised medicine content homogeneity, ensures the stable, slow and complete release of the medicine, makes Huperzine A be absorbed in stomach, small intestine and colon to maintain the treating concentration of Huperzine A in blood and raises the compliance of the patient greatly.
Owner:上海特敏生物医药科技有限公司
Preparing method for sustained releasing huperzine preparation
InactiveCN1456151ASafe Drug TherapySafe and long-acting drug therapyNervous disorderOil/fats/waxes non-active ingredientsChemical synthesisDrug release
A slowly releasing huperzine for treating sanile dementia by oral application features that its active components are huperzine A and its humolog, or their salts or ester derivatives. Its advantages are slow release (more than 12 hr), and high curative effect and safety.
Owner:解健博
Method for extracting and separating Huperzine from huperzine serrate
ActiveCN101134743AHigh yieldAvoid extraction emulsificationOrganic chemistryChemical recyclingChromatographic separationReflux
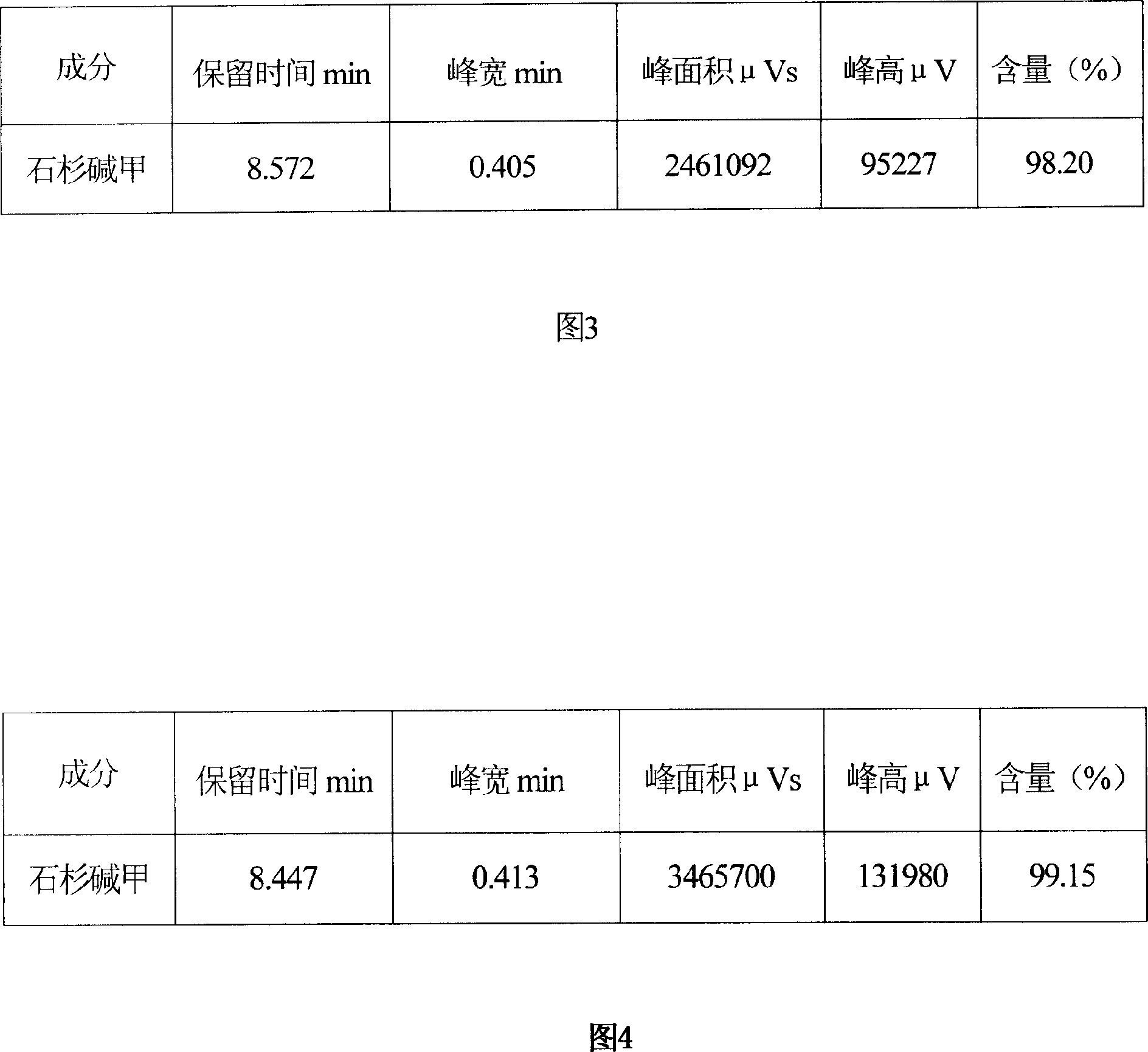
The process of extracting and separating huperzine A from huperzine serrate includes the steps of crushing huperzine serrate, reflux extracting in organic solvent, regulating acidity, filtering, chromatographic separation of the filtrate with cationic exchange resin, eluting, concentrating to obtain extractum, eluting on silica gel column with mixed eluant, collecting the eluted liquid, concentrating, letting stand to separate out huperzine A, and re-crystallizing to obtain refined huperzine A product. The present invention has high yield, low production cost and huperzine A product purity as high as 98 %.
Owner:SHAANXI JIAHE PHYTOCHEM
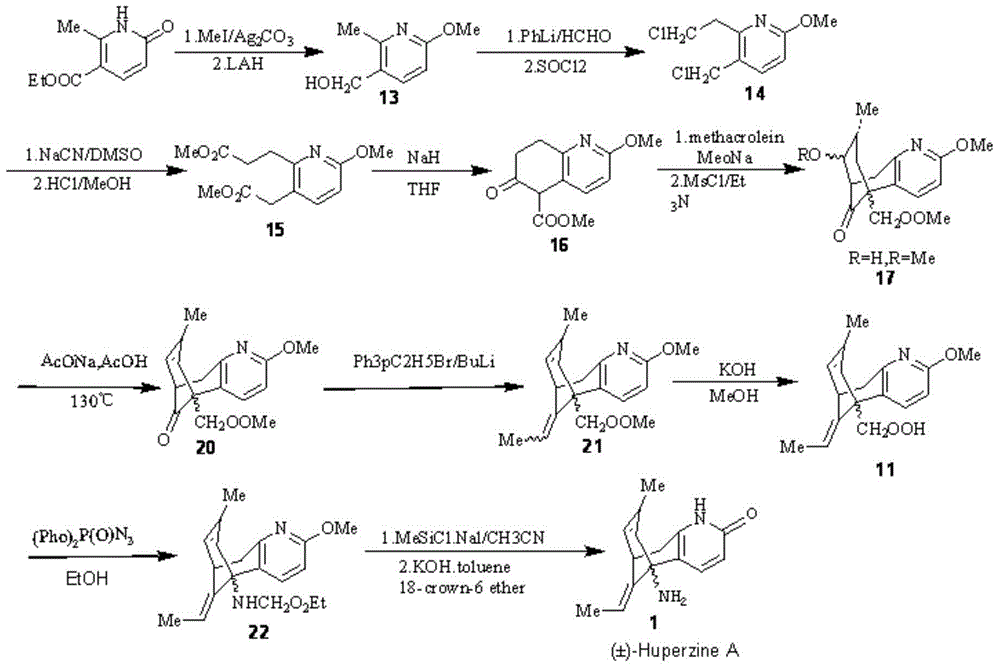
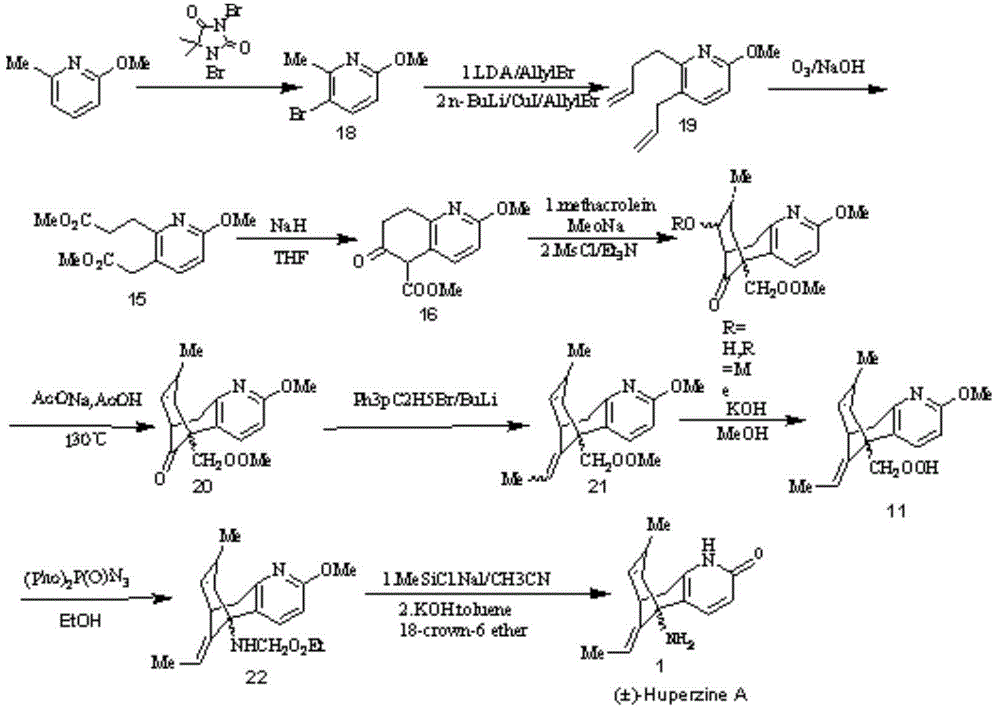
Asymmetric synthesis for chiral huperzine A
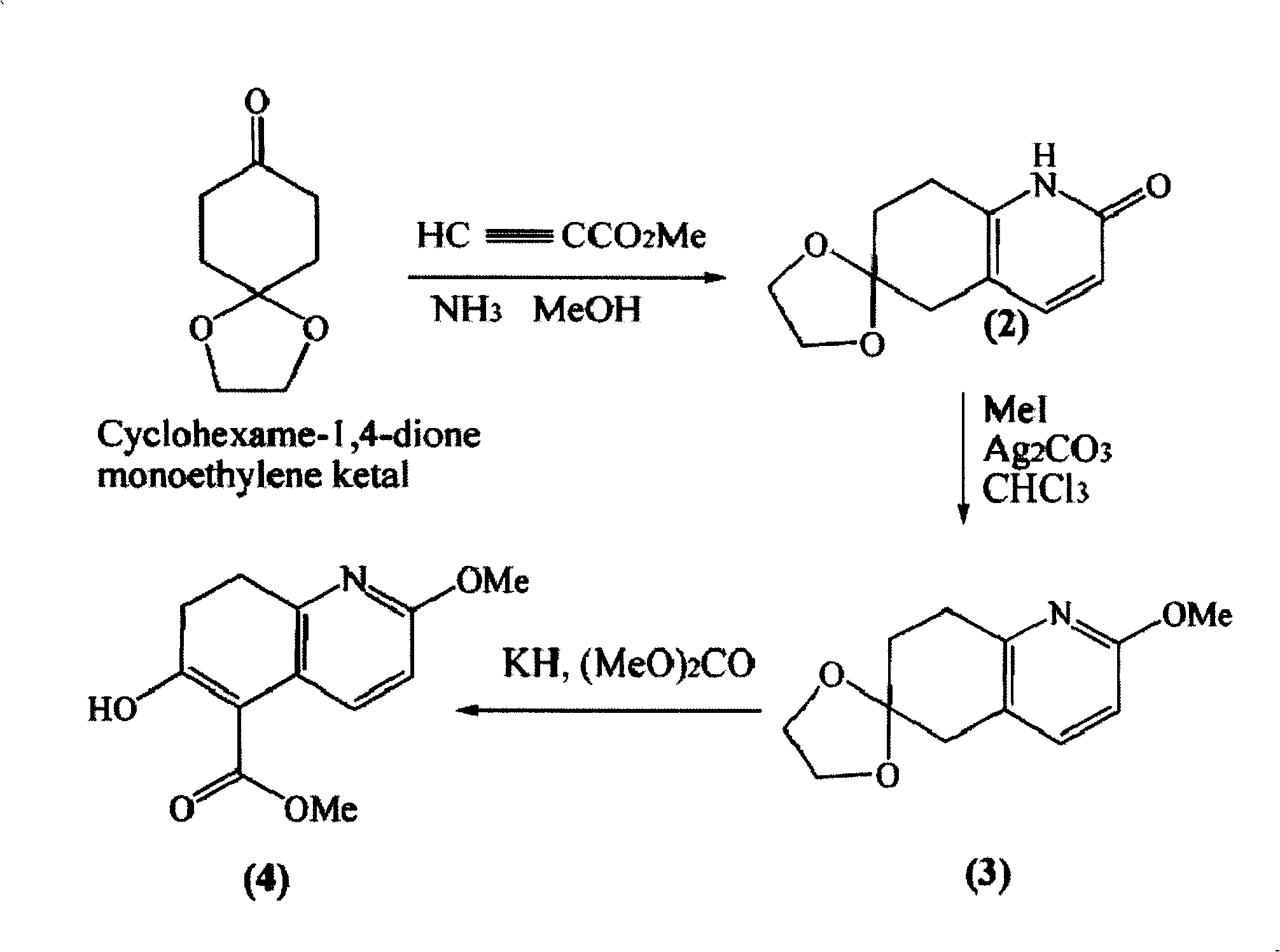
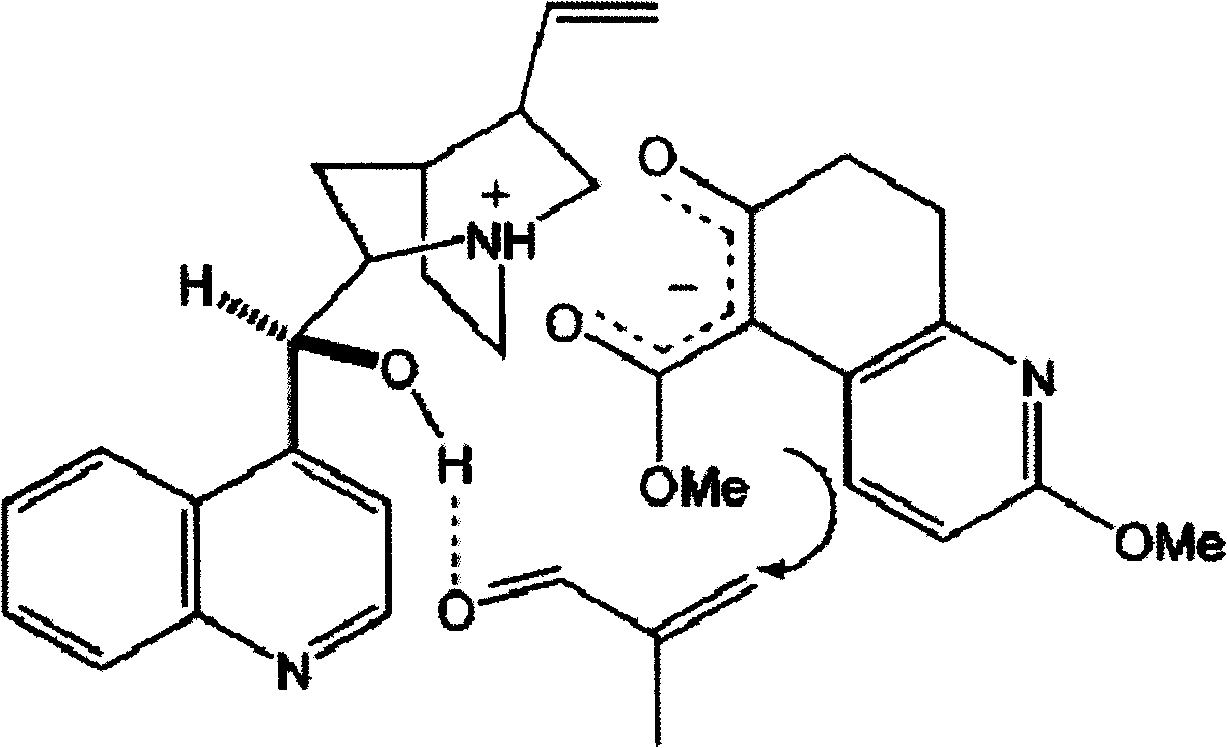
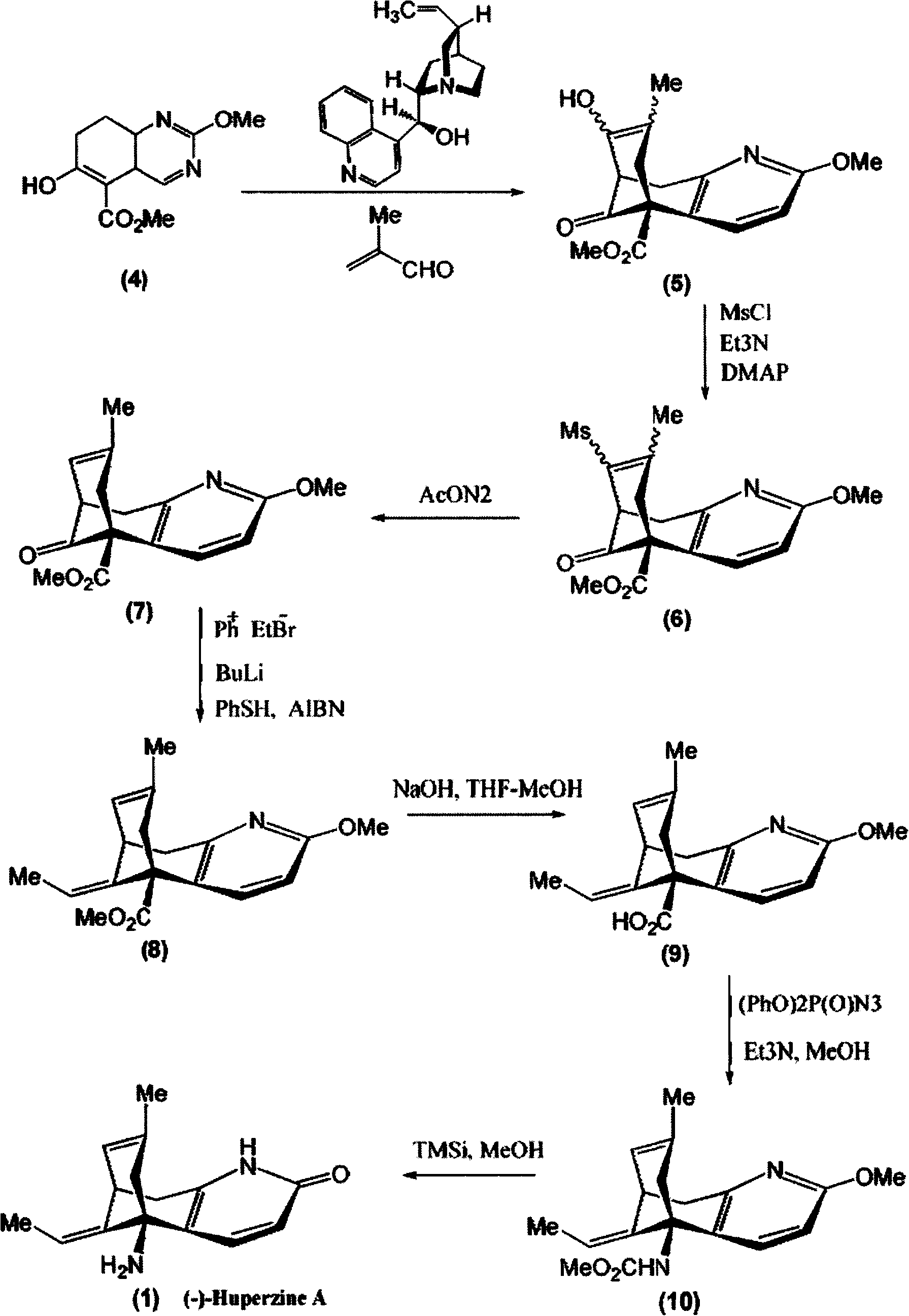
Disclosed is an asymmetric total-synthesis method for chiral huperzine a. The method takes 1,4-dihydro-spiro (4,5) -8 -decanone as starting material to get benzoate through hydroxymethylation and the treatment of benzoyl chloride and K2CO3; the benzoate is reacted with hydrogen sulfate O-methyl iso urea to get quinazoline; after the ketal is eliminated, Mander reagent is used for methyl esterification reaction so as to obtain beta-keto ester. Chiral ammonia, such as cinchona alkaloid, is used to promote the tandem asymmetric Michael addition / aldol condensation reaction of beta-keto esters and methyl acrolein. The compound carboxylate of diastereoisomer is reacted with MsCl, triethylamine and DMAP to get transformed. Through TMSI and MeOH processing, the protective group is removed so as to obtain optically pure-chiral huperzine a.
Owner:京山瑞生制药有限公司 +1
Enteric soluble coating slow releasing tablet containing huperzine A and preparing method
InactiveCN1682719AReduce adverse effectsGood curative effectOrganic active ingredientsNervous disorderRelease timeReversible anticholinesterase
The present invention discloses a kind of slow releasing enteric soluble coated tablet containing hyperzine A and its preparation process. Enteric soluble coating technology is adopted to overcome the bad effect of lower pH in stomach on the diffusion of medicine in skeleton tablet to ensure the stable, slow and complete release of medicine. The slow releasing tablet contains hyperzine A as reversible anticholinesterase and has one layer of enteric soluble polymer film coating outside the skeleton slow releasing layer. The active medicine component may be also various pharmaceutically acceptable acid salts of hyperzine A. Each tablet contains hyperzine A I n10-500 microgram and has sustained release time of 20 hr.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of huperzine A
InactiveCN101602727AHigh purityConducive to mass production operationsNervous disorderOrganic chemistryAbsorption columnChloroform
The invention relates to a preparation method of huperzine A, which is characterized by simple operation and little pollution and comprises the following processing steps: taking huperzine serrate dry coarse powder, adding 2-30 times amount of acid solution for ultrasonic extraction, with power of 100-2000W and extraction time of 10-30min, filtering extracts by anion exchange resins, extracting liquid medicines by chloroform, combining chloroform layers, recovering the chloroform at reduced pressure and concentrating the chloroform, adding the chloroform to a macroporous resin absorption column, using water for elution and impurity removal, using 50-90% of ethanol for elution, collecting eluent, recovering the ethanol at reduced pressure, adding acetone-chloroform for crystallization, washing and drying the crystal, thus obtaining the huperzine A. By adopting the invention to prepare the huperzine A, the product is high in purity and industrialization enlargement is easy to realize.
Owner:SUZHOU PAITENG BIOLOGICAL MEDICAL TECH
Process of extracting lycopdine A from plant
InactiveCN1861580AImprove solubilityImprove the extraction effectOrganic chemistryActivated carbonChloroform
A process for extracting huperzine A from clubmoss herb, cryptomeria, or masson fir includes such steps as breaking, immersing in acid solution, concentrating, decoloring by activated carbon, regulating pH value, extracting by chloroform, vacuum recovering, volatilizing, eluting out, vacuum recovering, crystallizing and drying.
Owner:HENAN TALOPH PHARMACEUTICAL STOCK CO LTD
Method for purifying huperzine A
The invention relates to a method for purifying huperzine A, which is easy for industrialized production. The method comprises the following production steps of: crushing of raw materials, acid water heating and extraction, membrane filtration, impurity removal and condensation, aminated chloroform extraction and condensation, medium-pressure alumina column chromatography, chloroform methanol recrystallization, and drying of a finished product. The method for producing the huperzine A has low energy consumption and short period.
Owner:NANJING ZELANG AGRI DEV
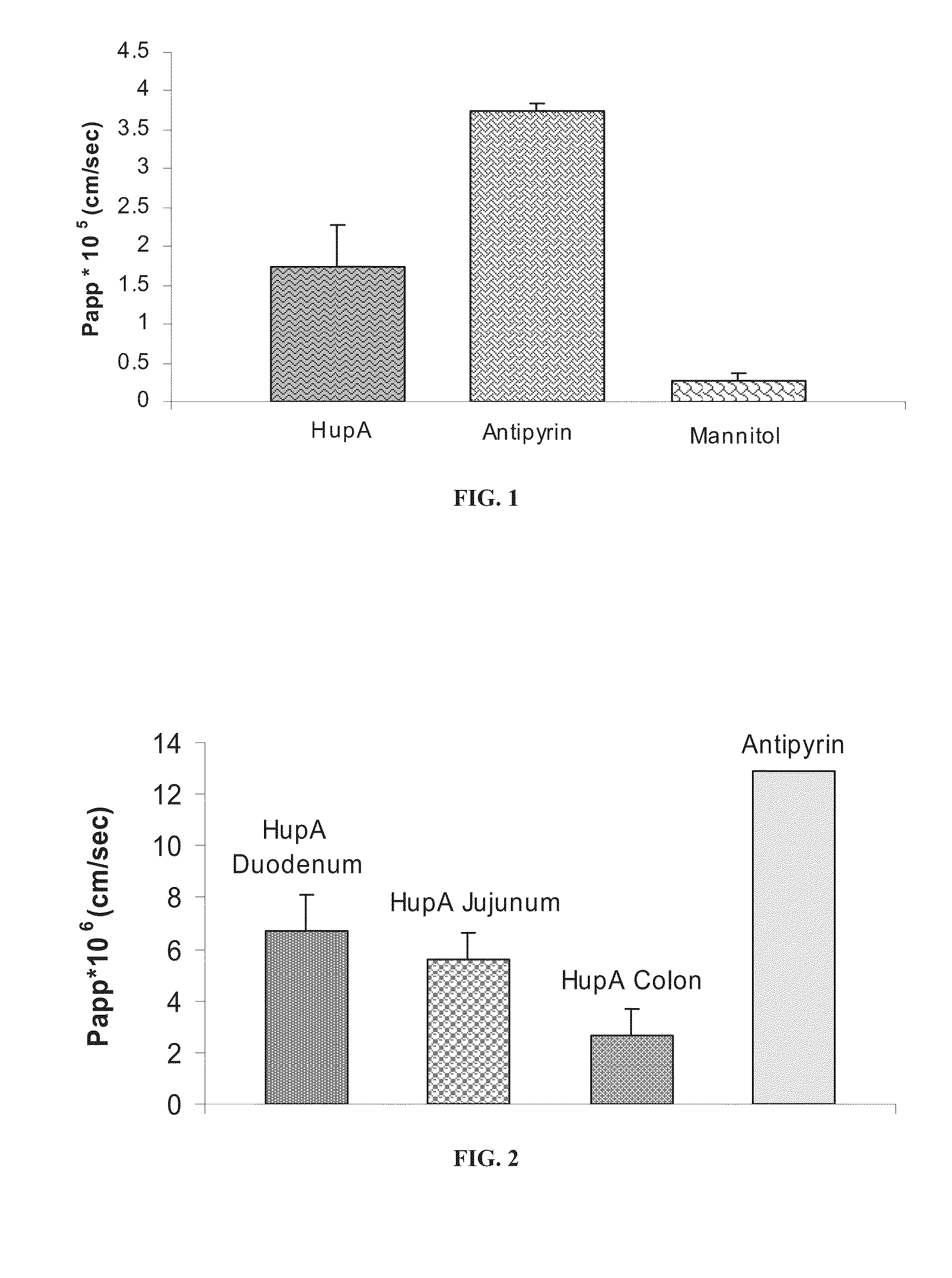
Transdermal administration of huperzine
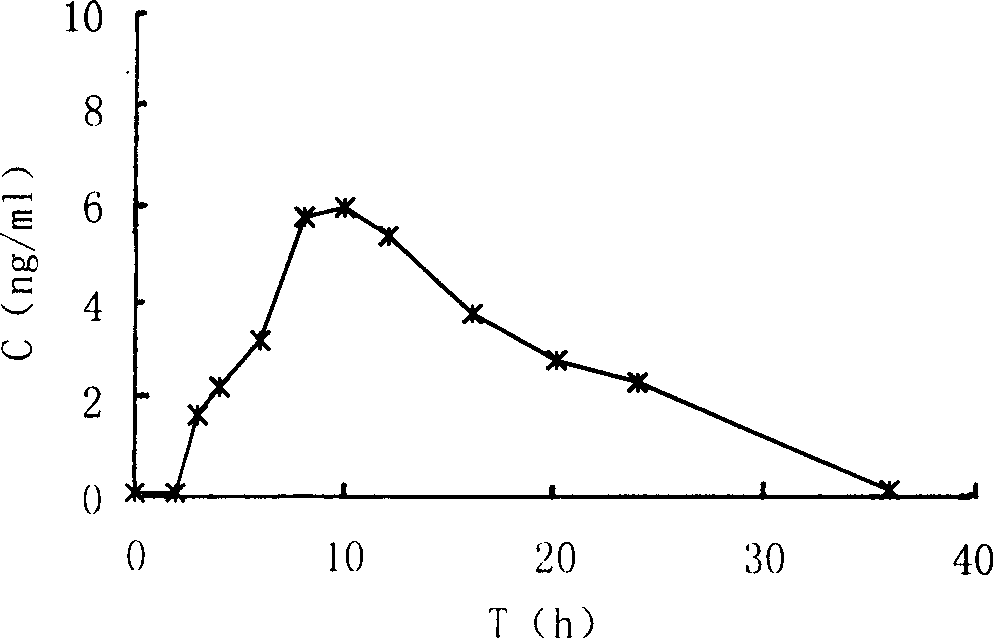
The present invention provides a composition of transdermally administered huperzine for improving memory and cognitive function. In one aspect, huperzine is delivered in a sufficient amount to achieve and maintain a blood plasma Huperzine level of about 0.1 ng / mL to about to 30 ng / mL. Huperzine may be delivered by itself, or in combination with other positive health promoting substances, such as vitamins, minerals, other cholinesterase inhibitors, etc. Various formulations for the transdermal delivery of huperzine are disclosed, and may include selected penetration enhancers.
Owner:美国爱科赛尔制药有限公司
Shishan alkaloid drip pill and its preparation method
InactiveCN1493287ADisintegration and dissolution fastHigh dissolution rateOrganic active ingredientsNervous disorderAlkaloidDrugs stability
A dripping pill of huperzine A and its superfine pulverizing process for preparing it are disclosed. Its advantages are high dissolving and disintegrating speed, high stripping percentage, quickly taking its effect, stability and low cost.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
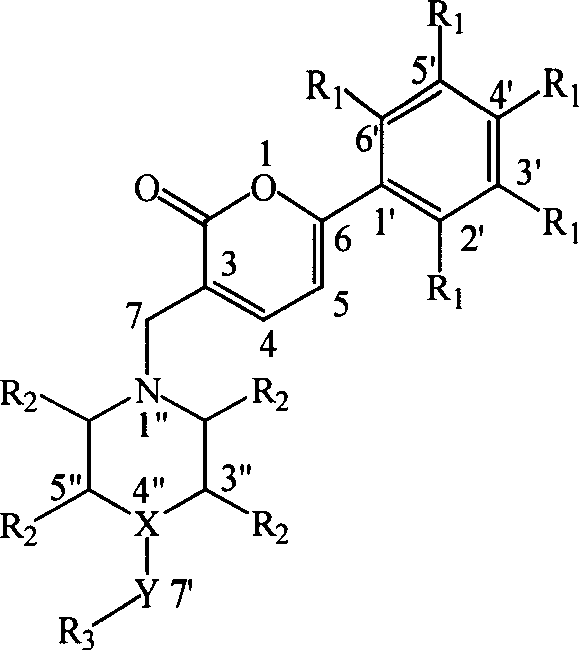
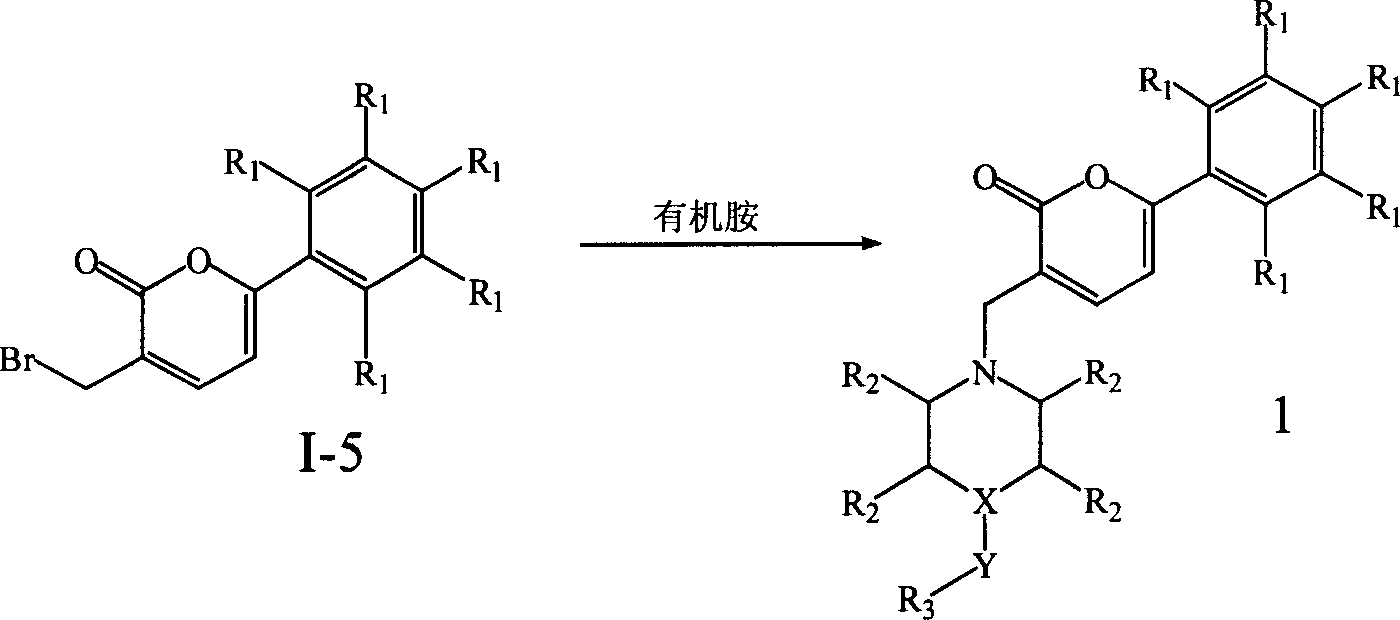
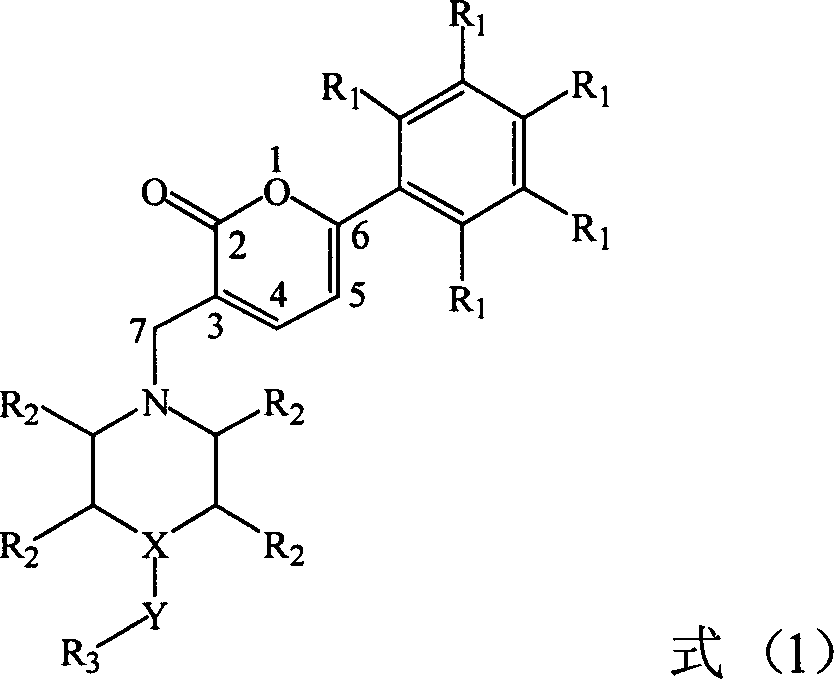
Preparation and application of a category of 6 - aryl - 3 - cycroamido methyl pyrone derviation
This invention relates to compounds with 6-aryl-3-cycloaminomethyl pyrone structure, their preparation method and their application. Pharmacological activity experiments show that the compounds have a high activity in selectively inhibiting AChE while no obvious activity in inhibiting BuChE. The activity of the compounds in inhibiting AChE is higher than huperzine A. Mouse in vivo tests show that the compounds do not have acute toxicity or abnormal reaction phenomenon, and the compounds can be used as a kind of new drugs for treating Alzheimer's disease.
Owner:赵昱
Sustained releasing huperzine preparation
InactiveCN1208054CContinuous and stable releaseRelease stabilityNervous disorderOil/fats/waxes non-active ingredientsChemical synthesisDrug release
A slowly releasing huperzine for treating sanile dementia by oral application features that its active components are huperzine A and its humolog, or their salts or ester derivatives. Its advantages are slow release (more than 12 hr), and high curative effect and safety.
Owner:解健博
Reversible acetylcholinesterase inhibitor huperzine-A synthesis method
InactiveCN105399672AHigh ee valueLow costOrganic chemistryCholinesterase inhibitionSynthesis methods
The present invention discloses a reversible acetylcholinesterase inhibitor huperzine-A synthesis method, wherein the route is defined in the specification. The method of the present invention has advantages of easily-available raw materials, simple operation, high yield, low cost, high purity of the final product, easy quality control and the like, and is suitable for industrial production.
Owner:SHANGHAI HONGJING BIOTECH CO LTD
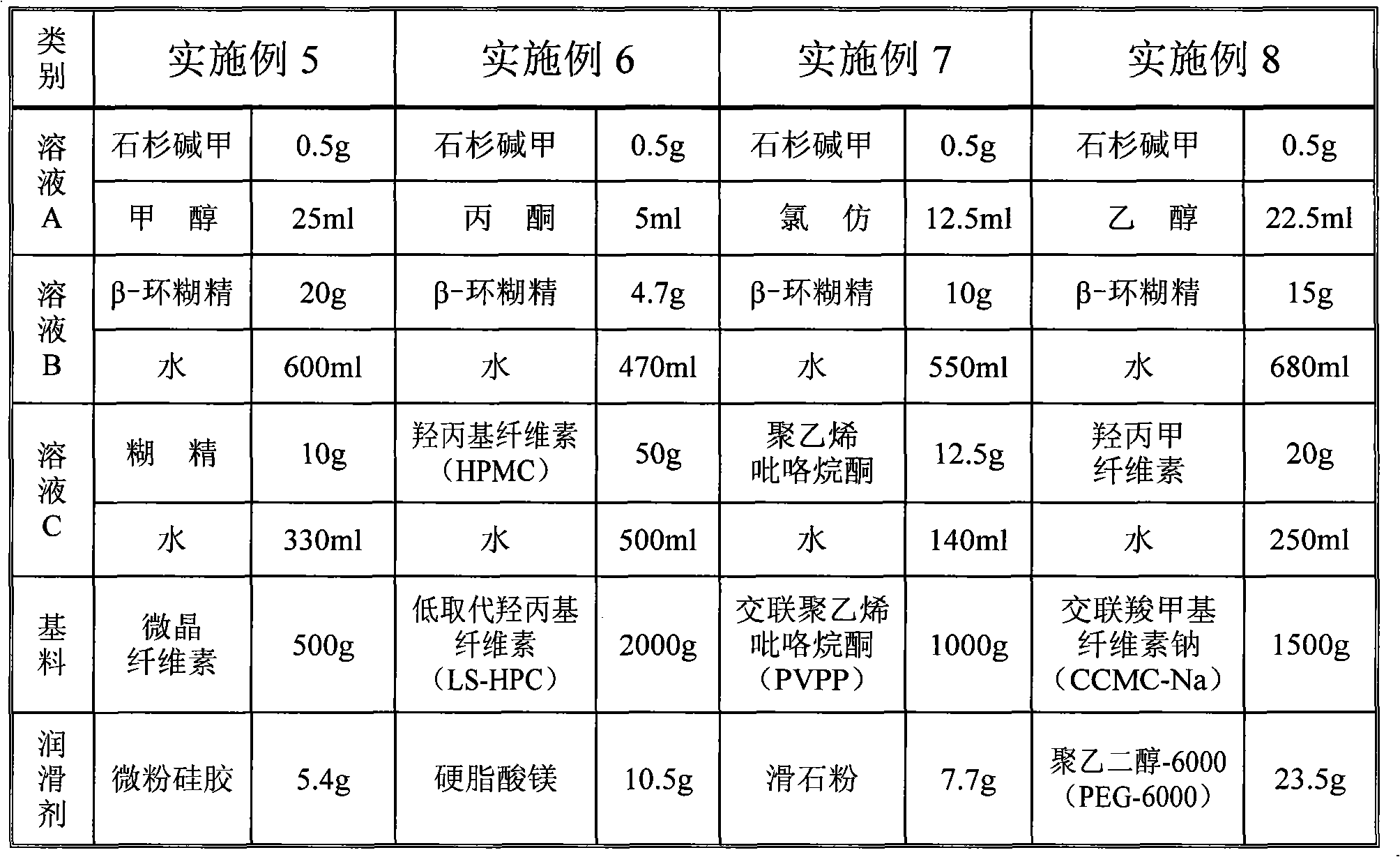
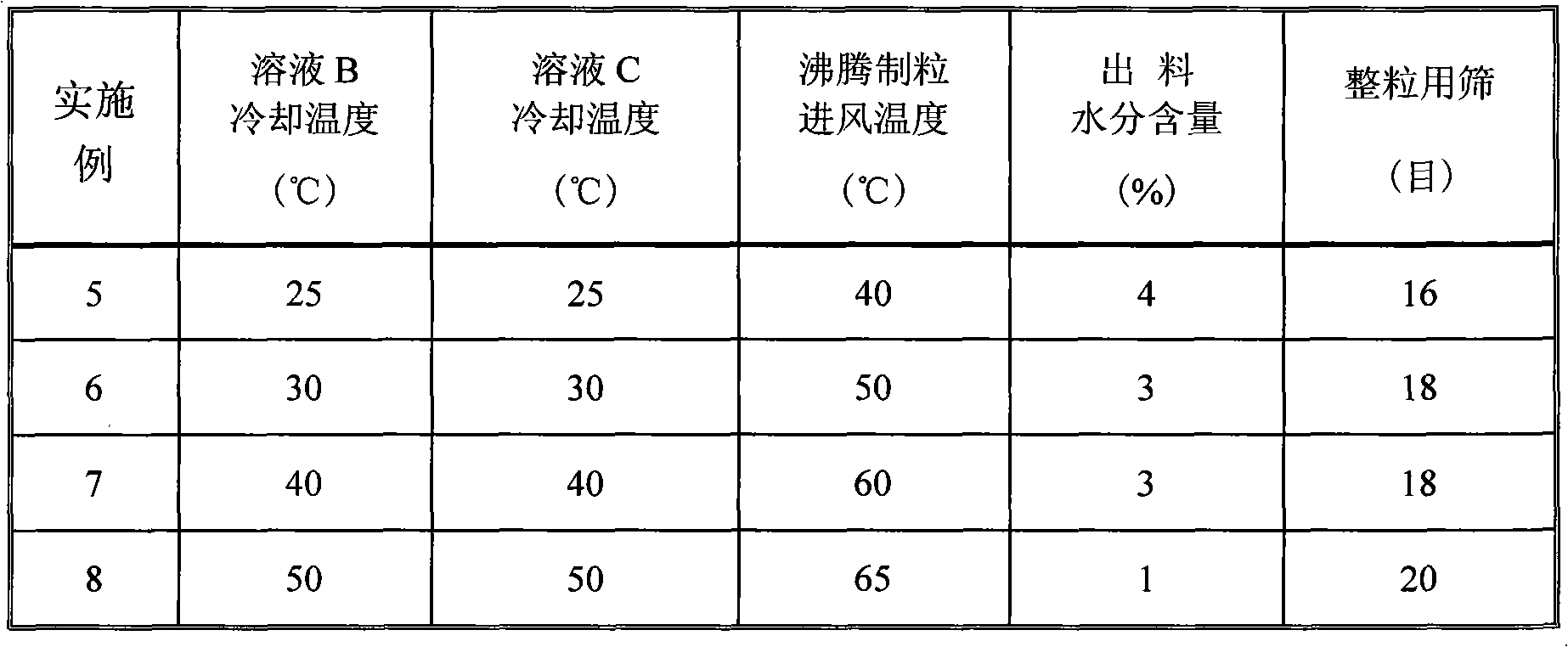
Beta-cyclodextrin inclusion compound of huperzine A, and preparation method and preparation thereof
InactiveCN101991859AReduce lossGood content uniformityOrganic active ingredientsNervous disorderOrganic solventWater insoluble
The invention discloses a beta-cyclodextrin inclusion compound of huperzine A, and a preparation method and preparations thereof. The beta-cyclodextrin inclusion compound of the huperzine A consists of 2.5 to 9.5 weight percent of huperzine A and 97.5 to 90.5 weight percent of beta-cyclodextrin. The preparation method comprises the following steps of: a) dissolving the huperzine A in an pharmaceutically acceptable organic solvent to prepare solution A, and heating and dissolving the beta-cyclodextrin in water to prepare solution B; and b) slowly adding the solution A into the solution B with stirring, and continuing to perform stirring until the mixed solution is uniform to prepare the beta-cyclodextrin inclusion compound of the huperzine A. The beta-cyclodextrin inclusion compound of the huperzine A further can be mixed with water-soluble carriers with viscosity, such as starch and the like, and then is pelletized, tabletted or filled into capsules with substrates. In the method, an extremely small amount of water-insoluble huperzine A is fully, effectively and uniformly dispersed, so the dissolution rate of the huperzine A is increased and the stability of the huperzine A is improved. The provided beta-cyclodextrin inclusion compound of the huperzine A can be used for preparing solid preparations and liquid preparations, operating steps are reduced and the production cost is remarkably reduced.
Owner:上海复旦复华药业有限公司 +1
Pharmaceutical composition for nose administered in-situ gel spray of Huperzine A, preparation process and use thereof
InactiveCN1961879AIncrease brain selectivityEnhance memoryOrganic active ingredientsNervous disorderNasal cavityNose
The invention relates to a compound of original gel atomizer of Huperzine nose, and relative preparation, wherein it is external liquid, which can be atomized into nose as gel state. The invention use Huperzine as active component, assisted with original gel agent, pH adjuster, water, etc. The invention can treat senile dementia, and improve memory level of children. The invention has simple application, while it can be atomized into nose. And its cerebrospinal fluid and jordonet snyder utilization is 2.2-2.7 times of oral agent, and its effect is 2-4 times of oral agent, with low toxic.
Owner:SHANGHAI INST OF PHARMA IND
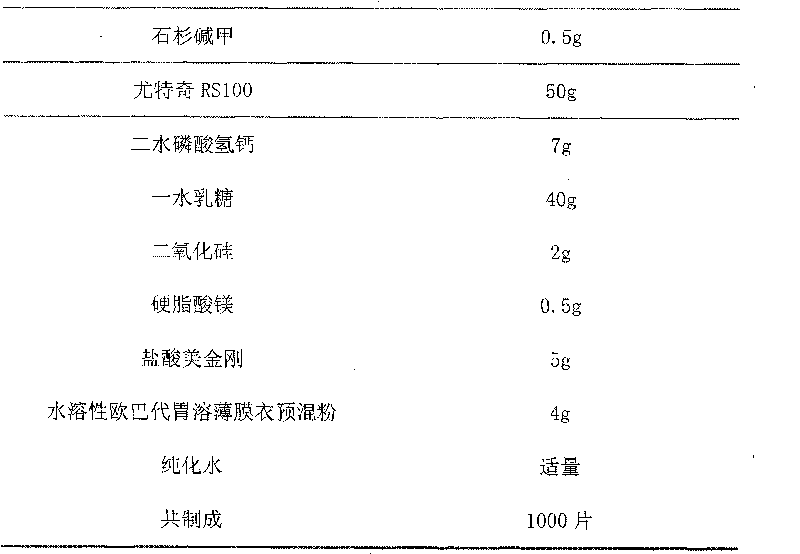
Compound preparation for treating Alzheimer's disease and preparation method thereof
ActiveCN102151268AImprove solubilityAvoid first pass effectOrganic active ingredientsNervous disorderDiseaseNervous system
The invention relates to a compound preparation for treating Alzheimer's disease, which is prepared by combining huperzine A and ligustrazine phosphate according to certain proportion. The compound preparation has reasonable compatibility; all components in the compound preparation have the role of collaboratively protecting the nervous system and can carry out intervention on the pathogenesis of the Alzheimer's disease from multiple targets; and the compound preparation disclosed by the invention has an important clinical application meaning.
Owner:王义明
Injection containing huperzine for sulci venosi and method of preparing the same
The invention provides intravenous injections of Huperzine and process for preparation, which include the dose forms of injections, fluid infusions and freeze dried injections.
Owner:ZHEJIANG WANBANG PHARMA
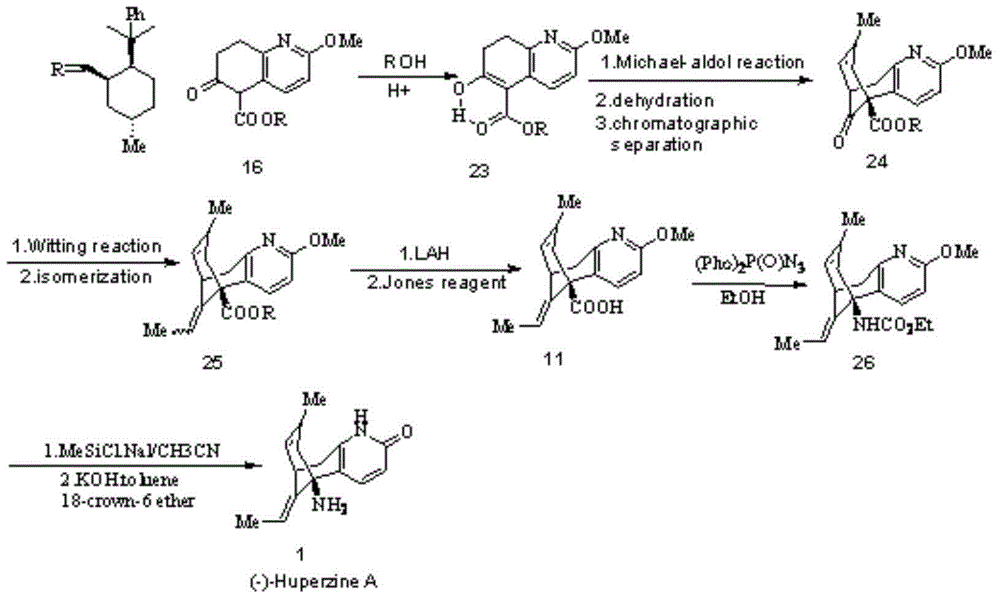
Preparation method of (-)-huperzine A
InactiveCN103224467AHigh purityLow purityOrganic active ingredientsNervous disorderChemical synthesisOrganic solvent
The invention relates to a preparation method of (-)-huperzine A, which comprises the following steps: preparing (+)-huperzine A mixture obtained by chemical synthesis and chiral acid into huperzine A chiral acid salt under optimum conditions, recrystallizing the chiral acid salt with organic solvent, and carrying out alkali ionization to obtain optically pure (-)-huperzine A. The method is convenient to operate and suitable for industrial production; and the chemical purity and optical purity of the (-)-huperzine A obtained by the method are respectively greater than 99.5%, thereby satisfying the requirements for the purity of the active pharmaceutical ingredients in pharmaceutical industry.
Owner:ZHEJIANG WANBANG PHARMA
Method for extracting and separating huperzine A from huperizia serrata
InactiveCN101759641AReduce pollutionIncrease productivityIon-exchange process apparatusOrganic chemistryAcetic acidSilica gel
The invention relates to a method for extracting and separating huperzine A from huperizia serrata. Aiming at the disadvantages of the existing method of lower yield, higher cost and weak market competitive power, the invention establishes the method for extracting and separating the huperzine A from the huperizia serrata, which comprises the following steps: grinding raw materials, extracting by 2% acetic acid, separating by SP825 micro-pore adsorption resin, column chromatographic separating by alumina B, preparative chromatography purifying by nitrile-group bonding silica gel, concentrating and drying, so as to obtain a finished product. The invention has the benefit that as the new extraction process is adopted, by simplifying operation, reducing loss, improving yield and lowering cost, the market competitive power of the product can be enhanced.
Owner:ENSHI QINGJIANG BIO ENG
Method for extracting huperzine A from huperzine serrate
ActiveCN102702101APromote dissolutionHigh recovery rateOrganic chemistryReversed-Phase Liquid ChromatographyDissolution
The invention discloses a method for extracting huperzine A from huperzine serrate. The method comprises the following steps of: preparing materials, extracting, leaching, performing column chromatography, crystallizing and the like, wherein according to the extracting step, a continuous countercurrent extraction method is adopted, so that the dissolution of active ingredients can be accelerated, the recovery rate of the huperzine A can be improved, and the extraction time can be shortened; according to the column chromatography, reversed phase chromatography is performed by cyano bonded silica gel resin, so that the recovery rate and purity can be improved by over 90 percent; and the reversed phase chromatography is performed by the cyano bonded silica gel resin, and the toxicity of an eluent is small.
Owner:CHANGSHA HUIRUI BIO TECH
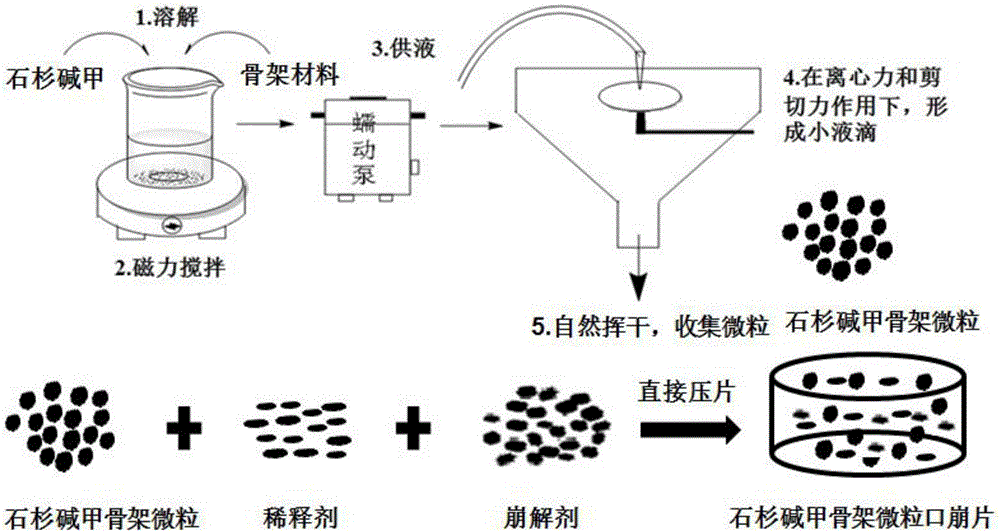
Huperzine-A framework particles, orally disintegrating tablets and preparation methods thereof
ActiveCN106511348AImprove uniformitySolve the problem of uneven contentOrganic active ingredientsNervous disorderOrally disintegrating tabletPolyethylene glycol
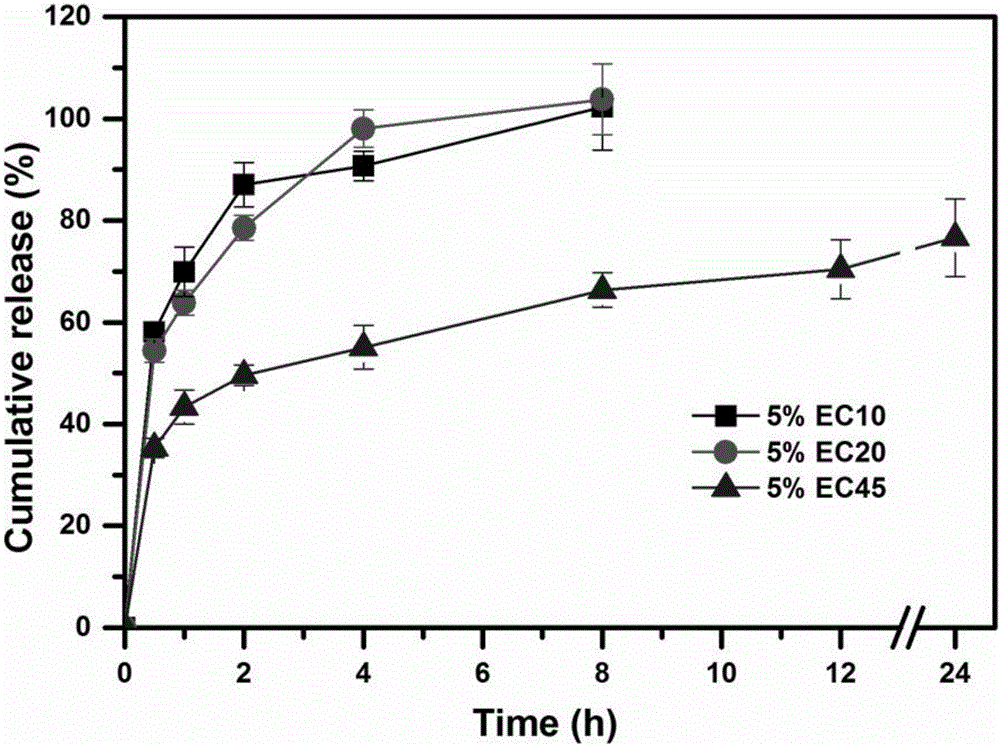
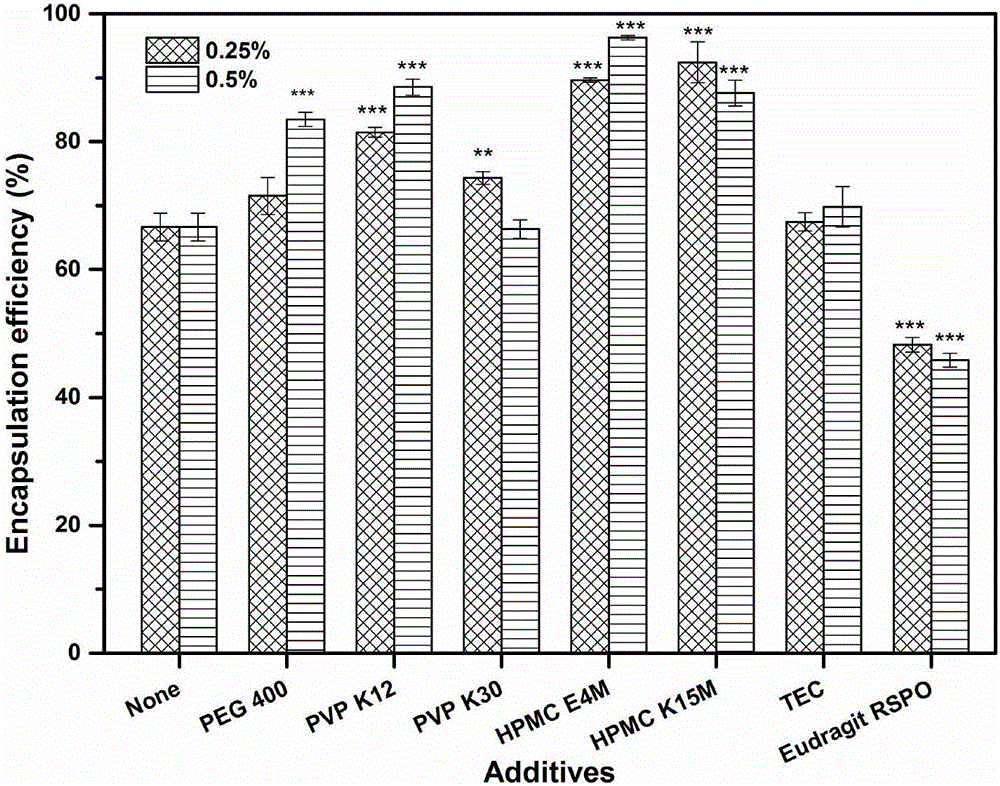
The invention relates to huperzine-A framework particles, orally disintegrating tablets and preparation methods thereof. The huperzine-A framework particles are prepared by an ultrafine particle preparation system (UPPS) and through taking huperzine-A, a framework material and an ethanol aqueous solution as raw materials, wherein the mass ratio of the huperzine-A to the framework material is (0.01 to 2): 100; the framework material is ethyecellulose or a mixture of ethyecellulose and an additive with a mass ratio of (2 to 40): 1; and the additive is selected from at least one of triethyl citrate, polyethylene glycol, hydroxypropyl methyl cellulose and povidone. The huperzine-A framework particles disclosed by the invention are high in medicine entrapment efficiency, high in formability and compact in structure, have obvious rough surfaces, and are high in mixing uniformity thereamong, high in compressibility and good in sustained-release effect. The orally disintegrating tablets disclosed by the invention are high in medicine uniformity, and keep the medicine release behaviour of the huperzine-A framework particles before tabletting.
Owner:SUN YAT SEN UNIV
Huperzine A pellets preparation and its preparation method
The invention relates to a kind of huperzine A parvule preparation and it's preparing method which is made of huperzine A and certain diluents bases. The preparation is applicable to patients who have problem with degluting tablets or capsules; it can treat nonmalignant dysmnesia and can increase the patients' ability of directing memory, thought association, image recall, nonsense figure recognition and portrait recall; it can also improve the dysmnesia caused by brain organic affection or dementia.
Owner:BEIJING QI YUAN YI DE PHARMA RESEARCH CENTER
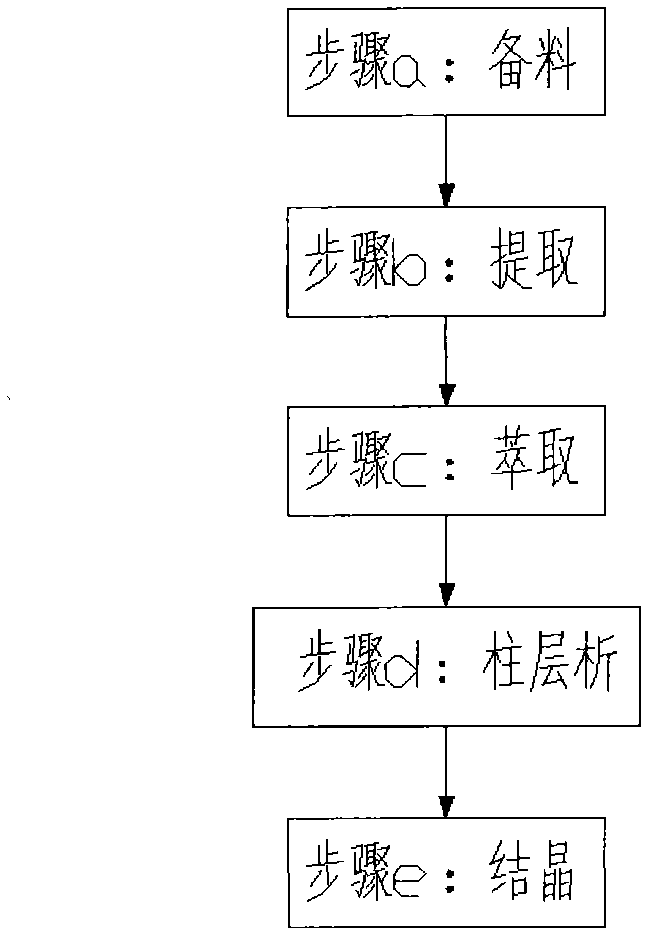
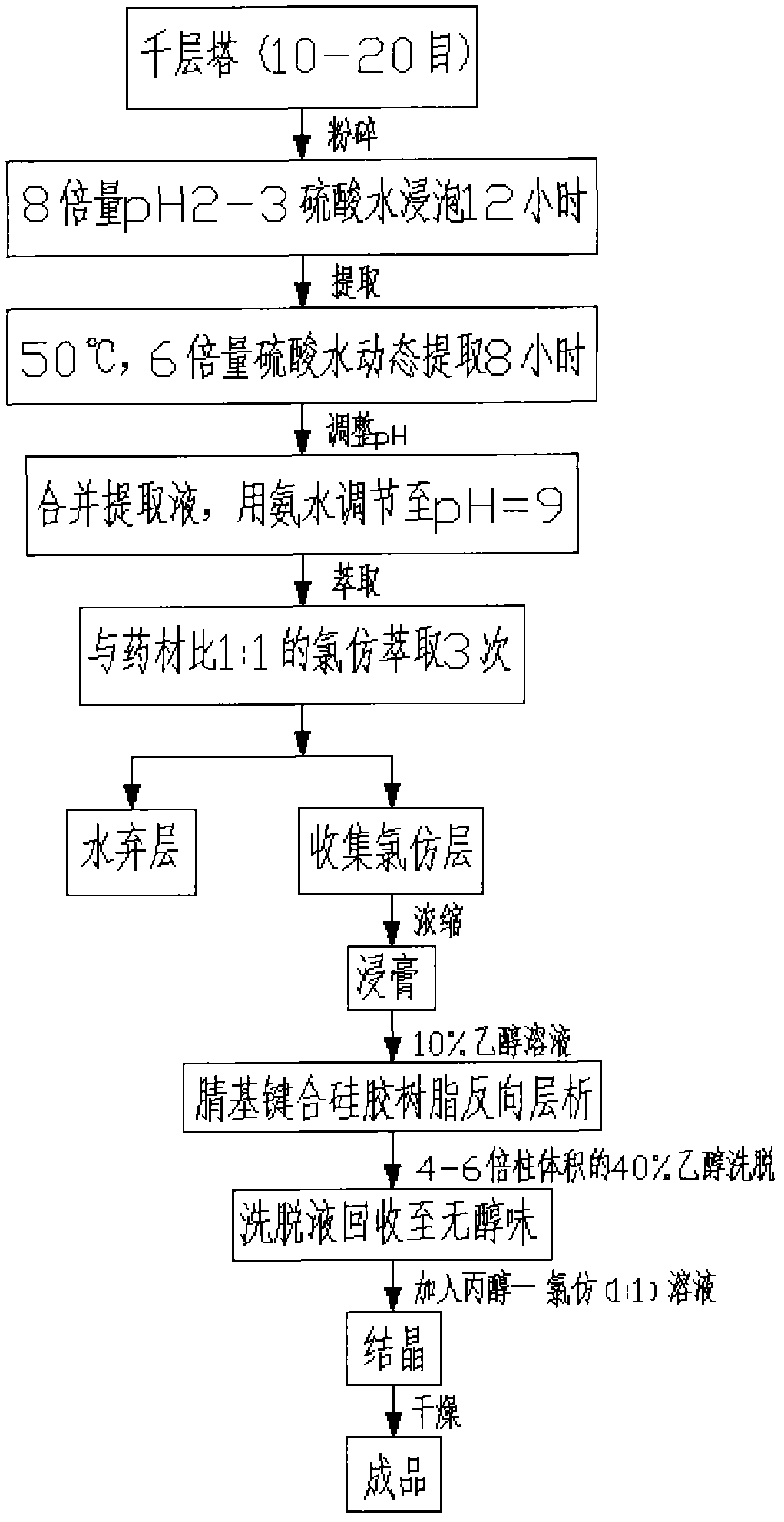
Method of extracting Huperzine A from Chinese herbal medicine multi-layer tower
InactiveCN1207285CDissolution is completeLow yieldOrganic chemistryPresent methodInternational market
A method for extracting huperzine A from Chinese herbal medicine Melaleuca, which comprises the following process steps in sequence: raw material treatment and crushing → impregnation → concentration → extraction (repeated) → column chromatography → crystallization → high performance liquid chromatography selection → concentration and drying → Finished product. This method adopts superfine powder as its raw material, and utilizes the preparative high-performance liquid chromatograph to carry out separation and purification, can make the yield reach 7 to 9 / 100,000, improve the yield by 30%, and the purity is also improved, reaching 98 More than %, the production can reach the scale of industrialization, the product quality is high, and the competitiveness in the international market is strong.
Owner:HENAN TALOPH PHARMACEUTICAL STOCK CO LTD
Dietary supplement compositions
ActiveUS20160193273A1Good for healthImproved well-beingBiocideKetone active ingredientsCholinesterase inhibitionDietary supplement
This document provides dietary supplement compositions. For example, dietary supplement compositions having an acetylcholinesterase inhibitor (e.g., huperzine A), a Bacopa monnieri extract, acetyl-L-carnitine or acetyl CoA, and a curcuminoid (e.g., curcumin) are provided.
Owner:MELALEUCA INC
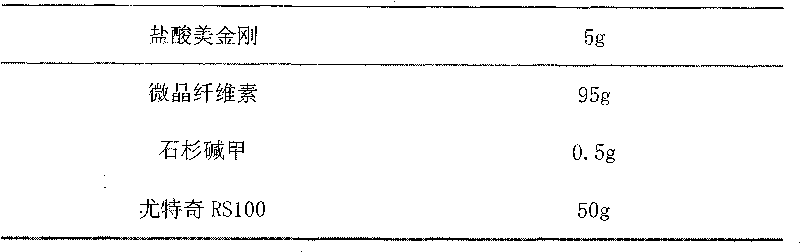
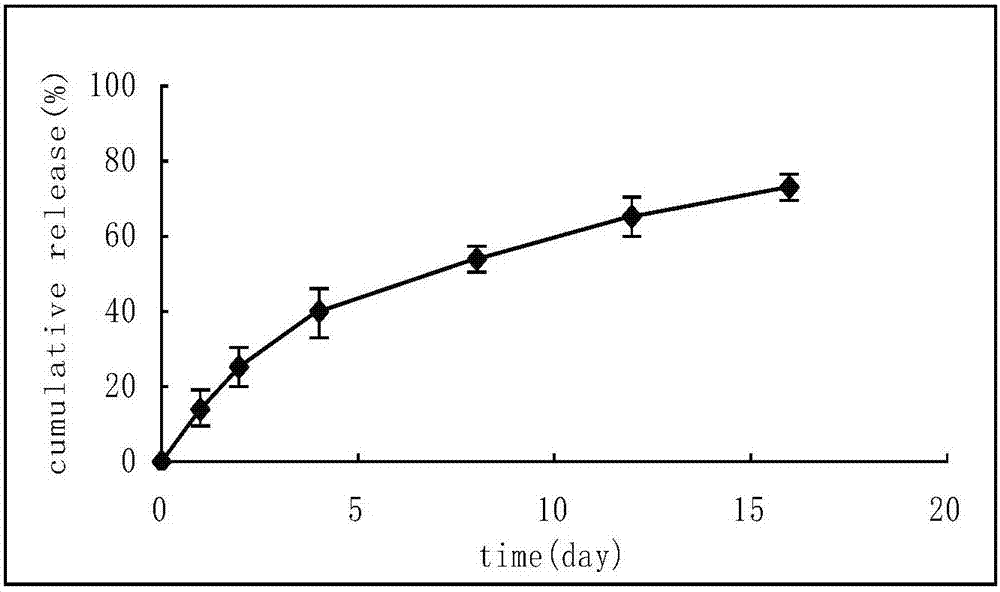
Sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof
InactiveCN101756970ANervous disorderAmine active ingredientsMemantine HydrochlorideImmediate release
The invention relates to sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof. The sustained release preparation includes quick release component and slow release component, wherein the memantine hydrochloride is the quick release component which represents the characteristic of quick release in vitro dissolution test and can be released by more 80% 30min later, while huperzine A represents the characteristic of slow release in vitro dissolution test and is released by 10-30% in the first hour, by 40-60% in 6 hours, by 60-80% in 12 hours and by more than 80% in 18 hours. The sustained release preparation is mild, has long-term effect and can reduce the application time (only once a day) and improve the compliance of the patient. The invention further discloses the vitro release characteristic and the preparation method of the sustained release preparation.
Owner:北京利乐生制药科技有限公司
Application and preparation method of huperzine A gelatin nanoparticle loaded microspheres
ActiveCN107149595ASolve the sudden release problemPowder deliveryOrganic active ingredientsNanoparticleMedicine
The invention discloses application and a preparation method of huperzine A gelatin nanoparticle loaded microspheres. According to drug release amount requirements of huperzine A and drug release characteristics of nanoparticles and microspheres, nanoparticles and microsphere preparations are combined so as to solve the problem of burst release of huperzine A sustained-release preparations, and sustained release is realized.
Owner:YANTAI UNIV
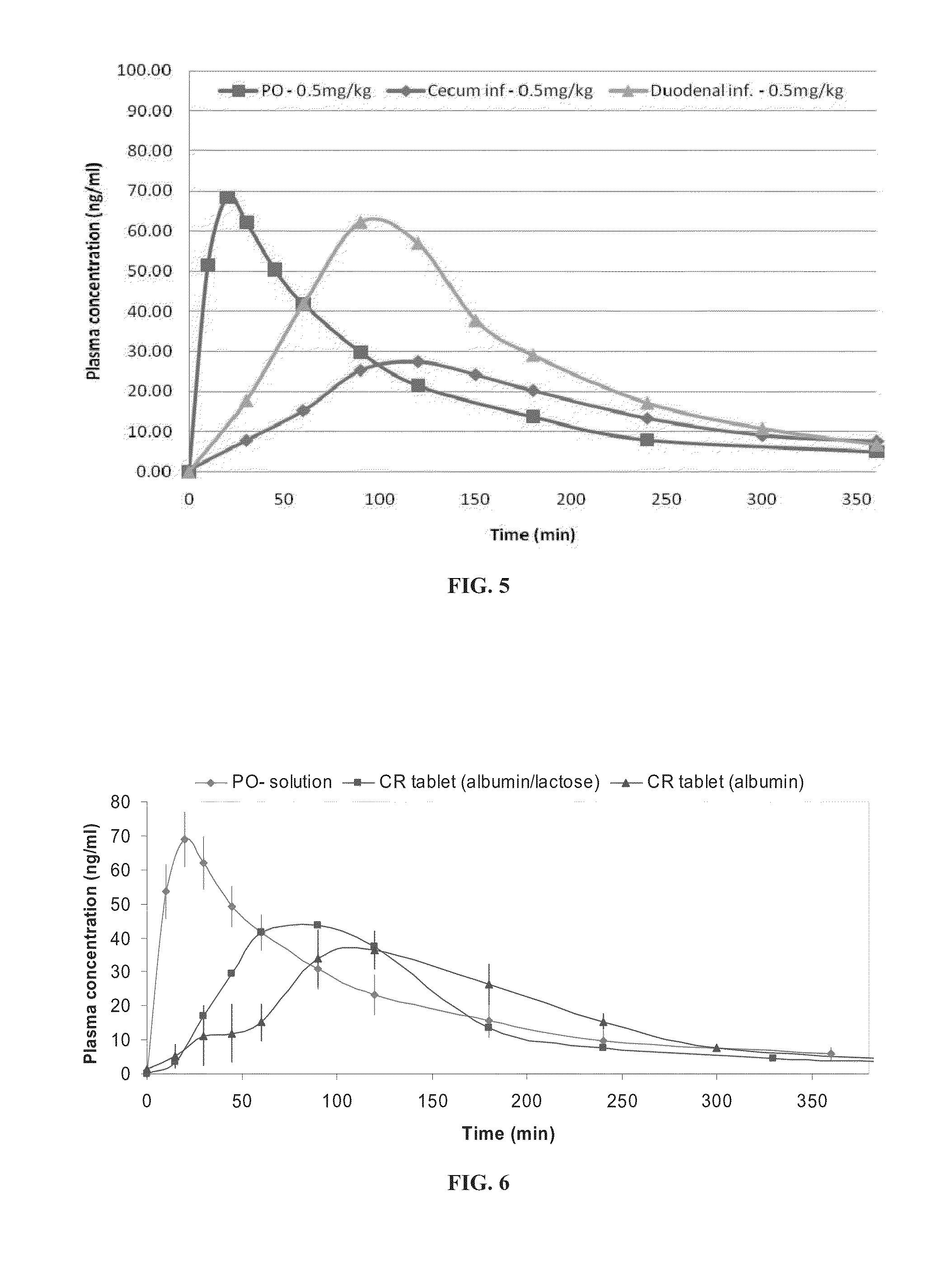
Oral sustained release formulation of huperzine a
Sustained-release formulations comprising huperzine A are disclosed herein. The formulations are for oral administration, and contain a carrier which comprises native albumin. Unit dosage forms of the formulations, and kits comprising such unit dosage forms are also disclosed herein. Methods utilizing the formulations for treating a medical condition treatable by huperzine A are also disclosed herein, as well as processes for preparing the formulations, and uses of huperzine A and albumin in the manufacture of a medicament.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com