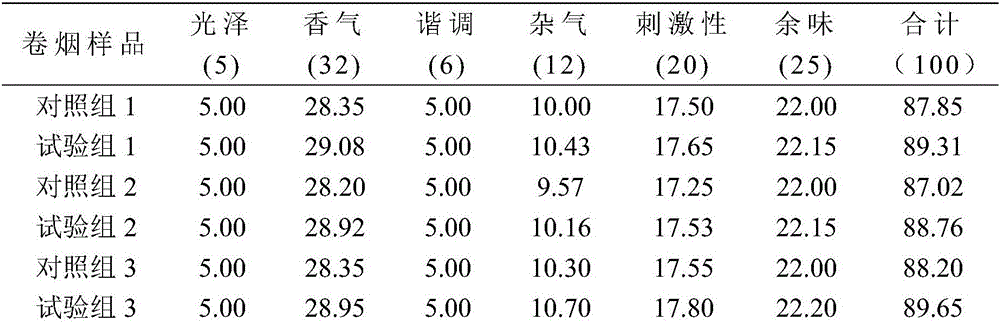
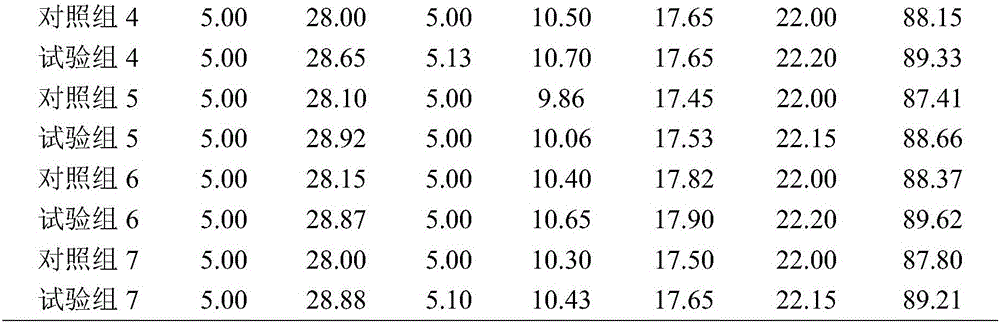
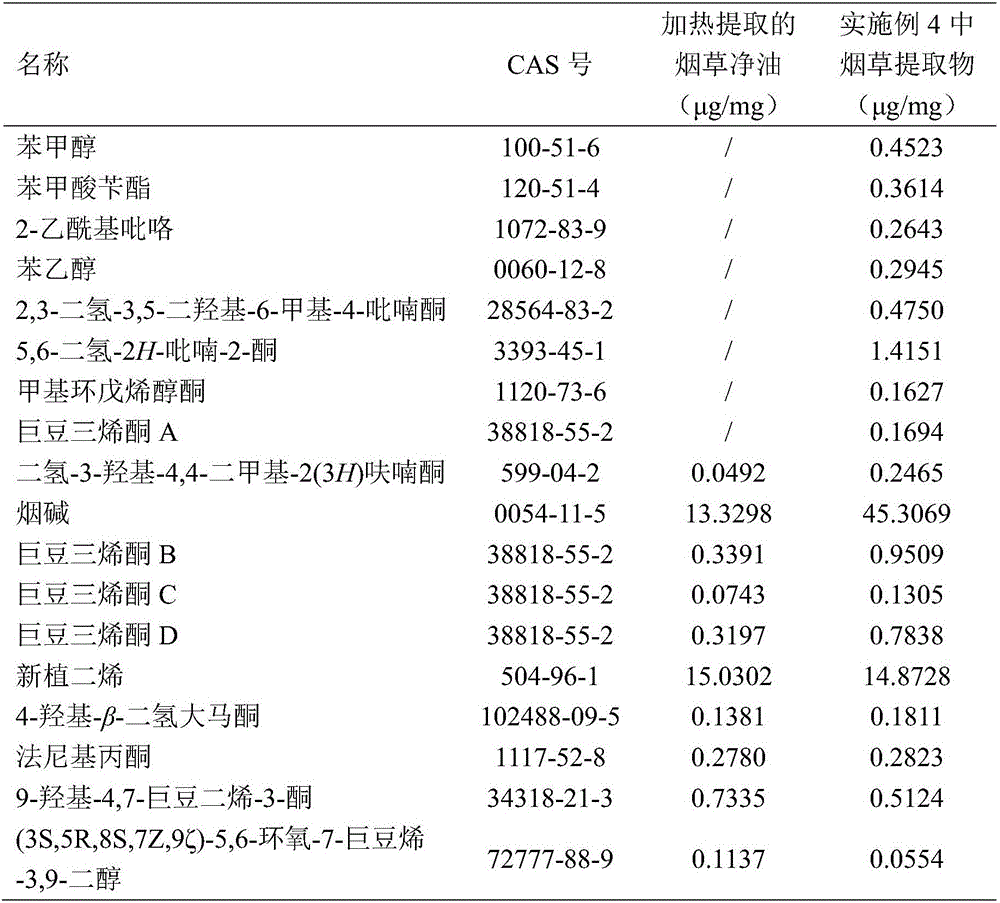
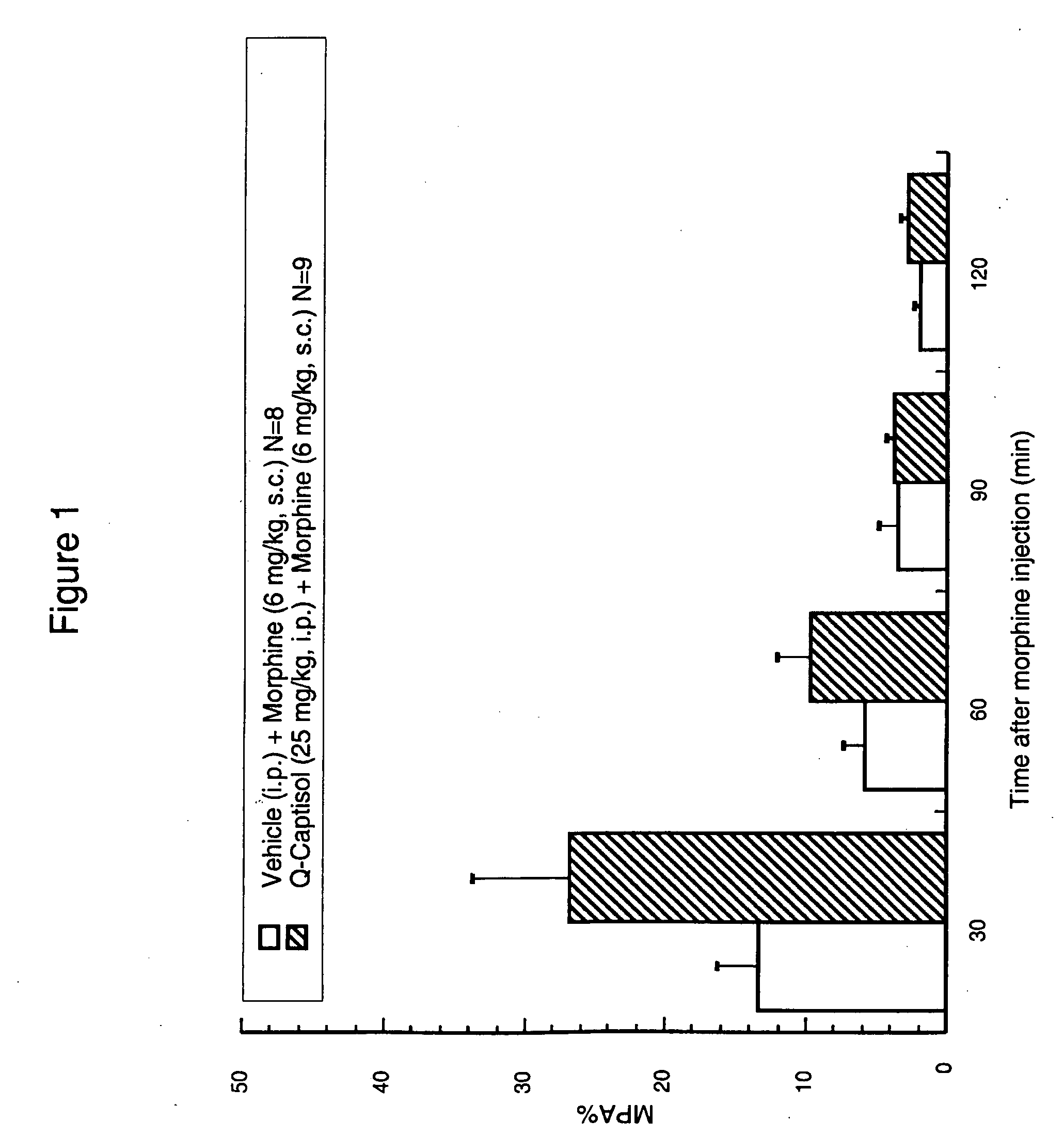
Patents
Literature
312 results about "Pyrone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
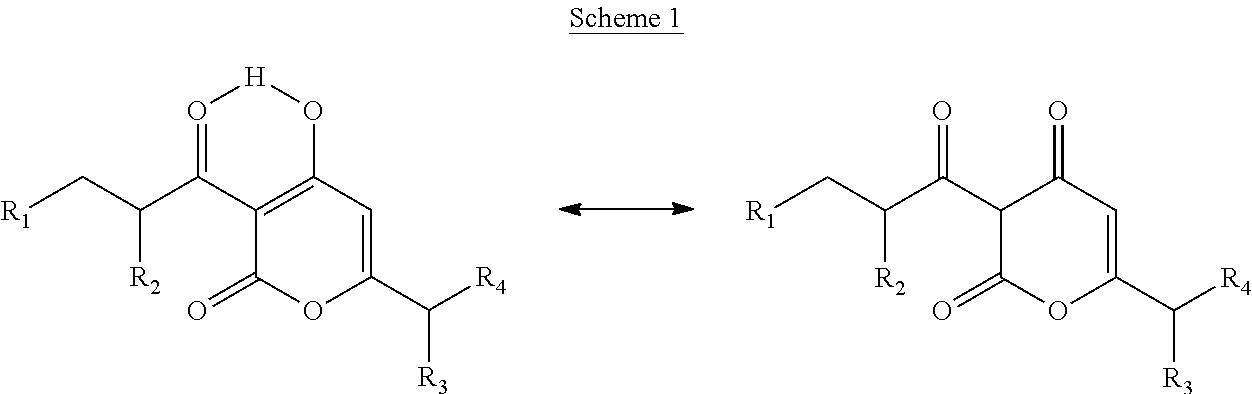
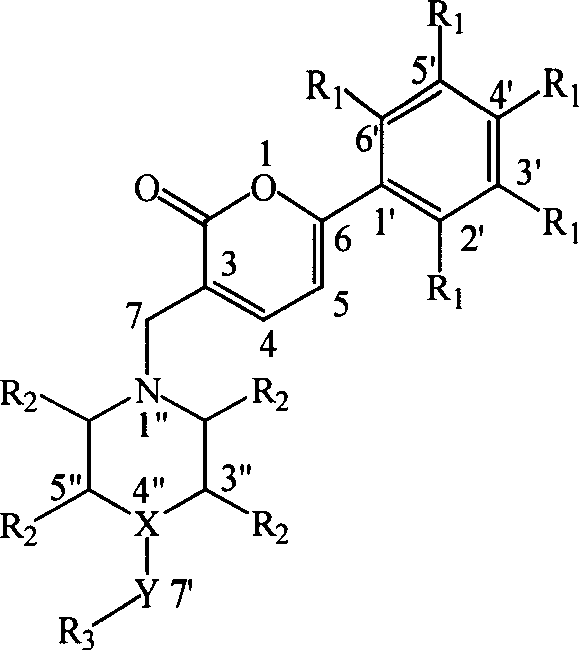
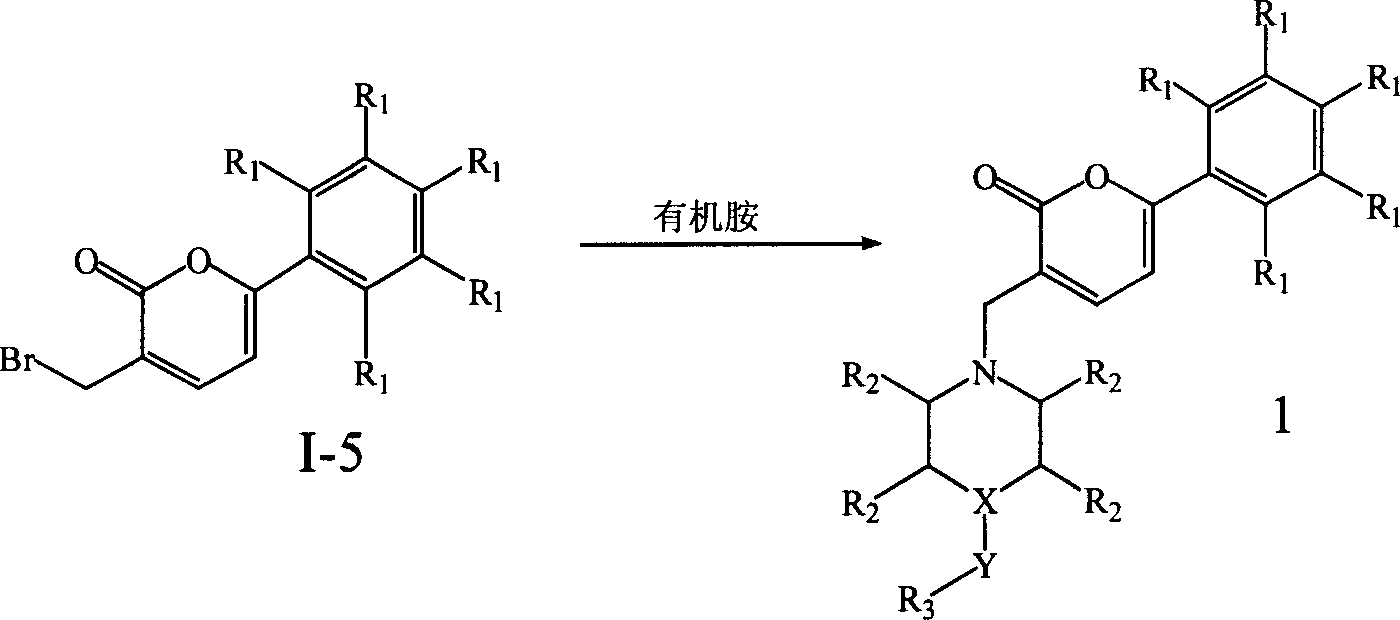
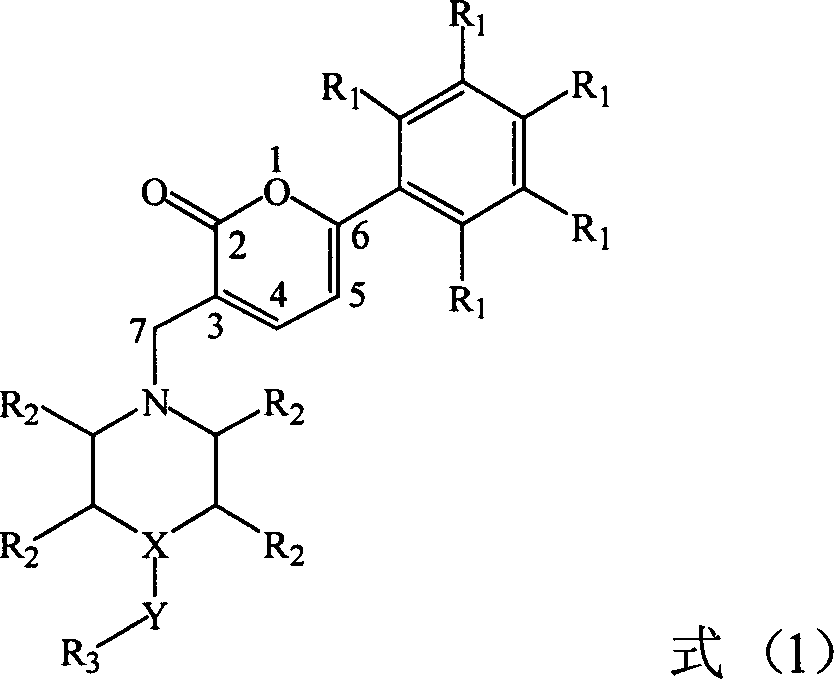
Pyrones or pyranones are a class of heterocyclic chemical compounds. They contain an unsaturated six-membered ring containing one oxygen atom and a ketone functional group. There are two isomers denoted as 2-pyrone and 4-pyrone. The 2-pyrone (or α-pyrone) structure is found in nature as part of the coumarin ring system. 4-Pyrone (or γ-pyrone) is found in some natural chemical compounds such as chromone, maltol and kojic acid.
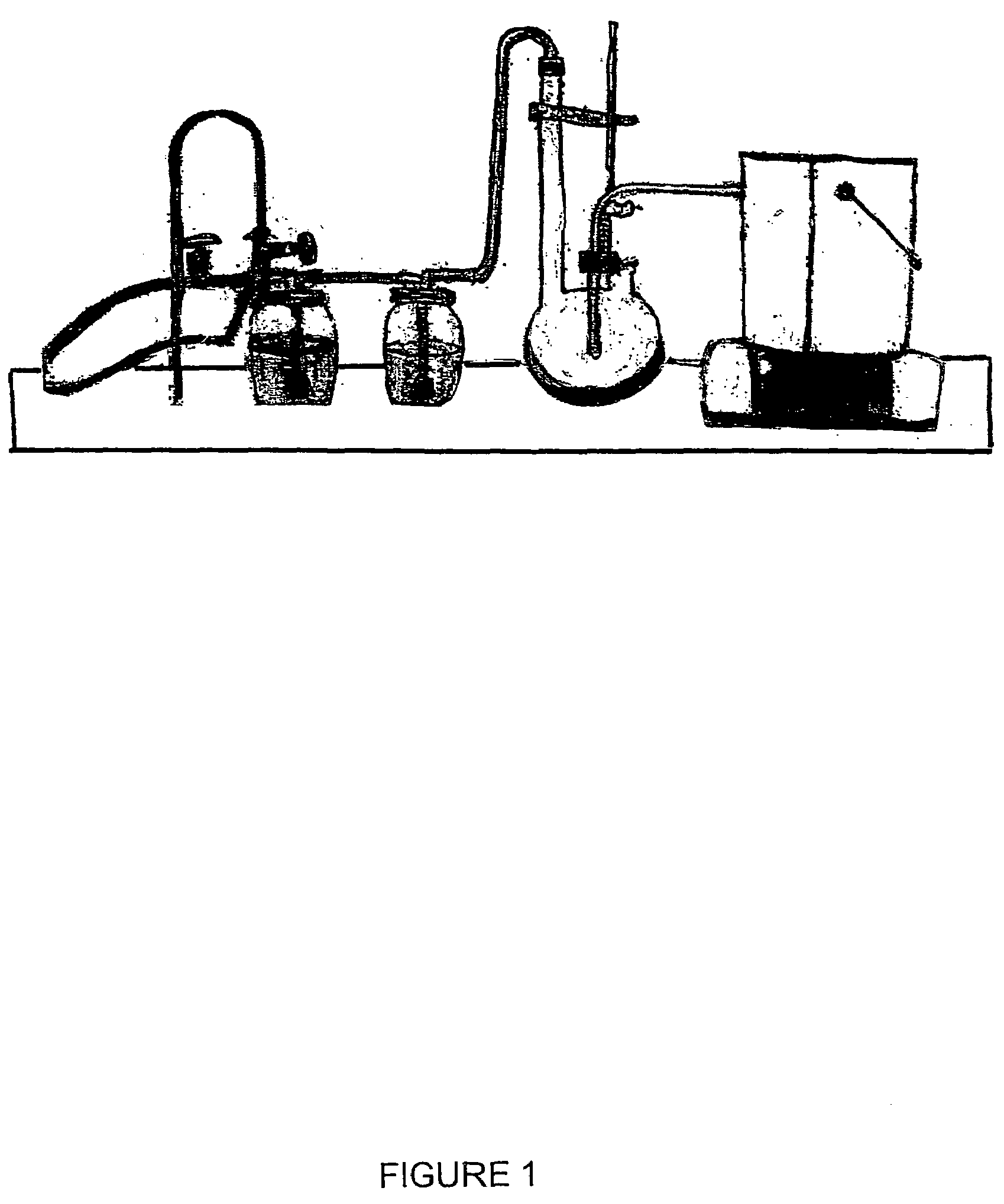
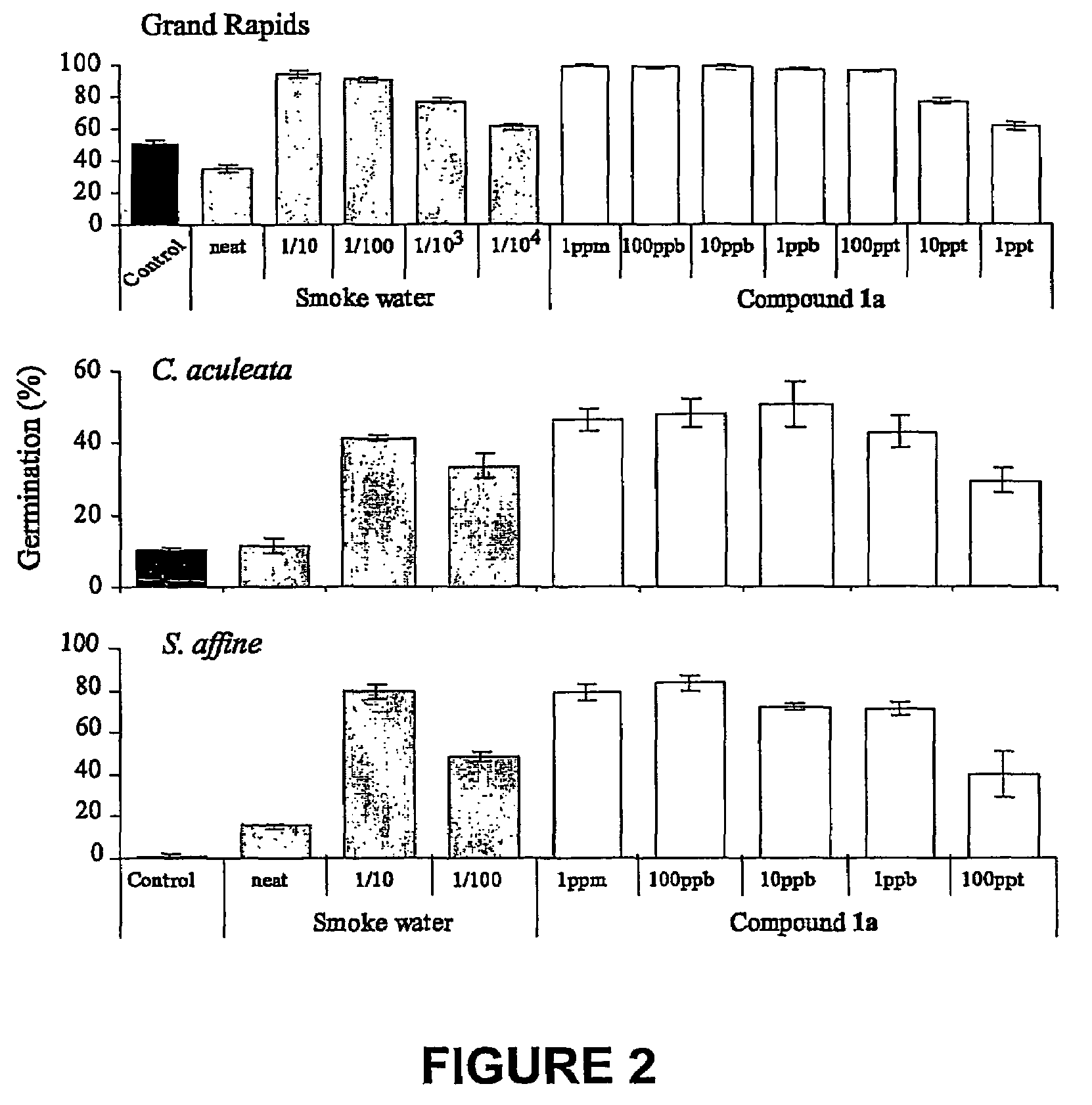
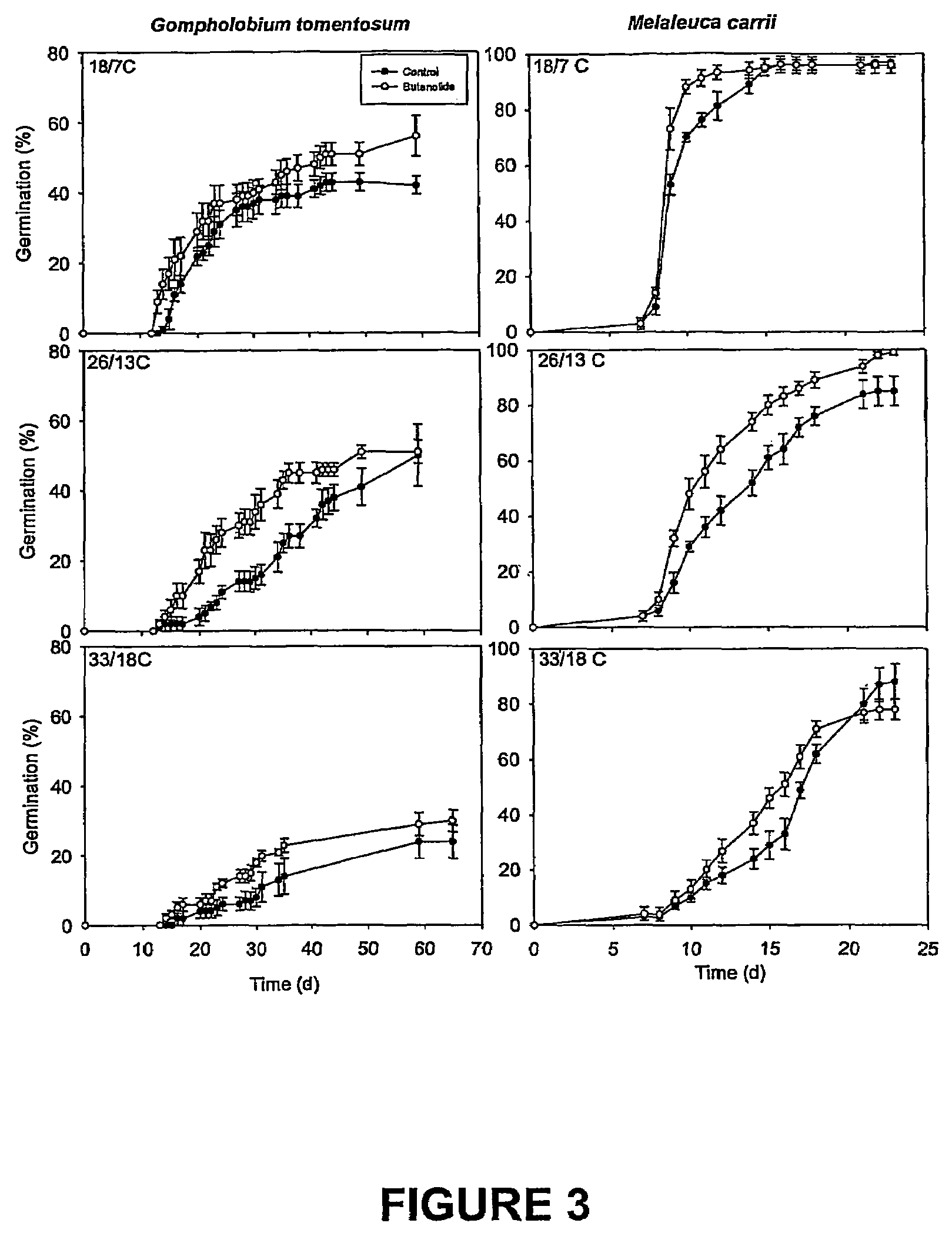
Vinylogous 4H-pyrones and their use in promoting plant growth
Owner:UNIV OF WESTERN AUSTRALIA
Gallium complexes of 3-hydroxy-4-pyrones to treat cancer
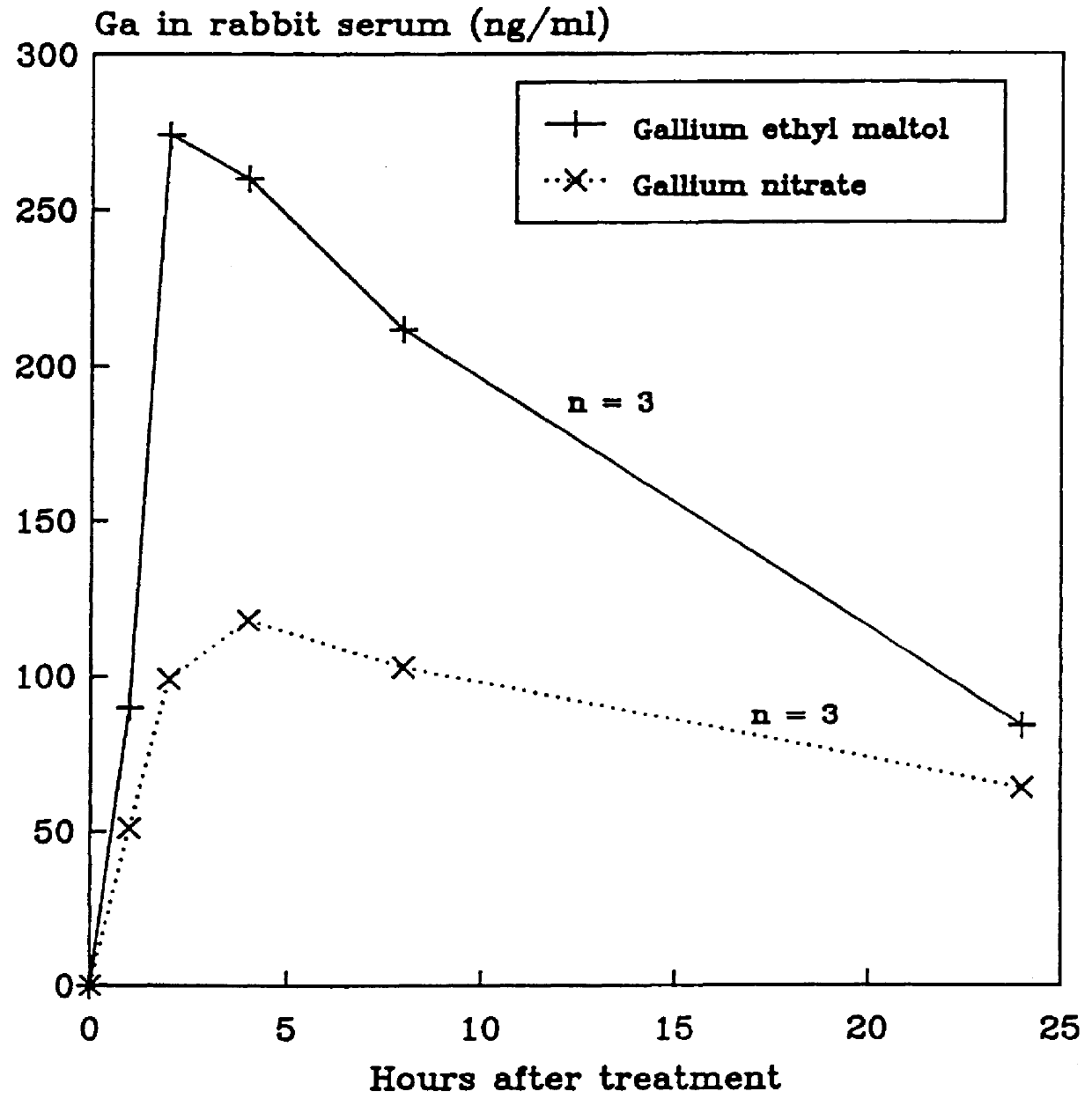
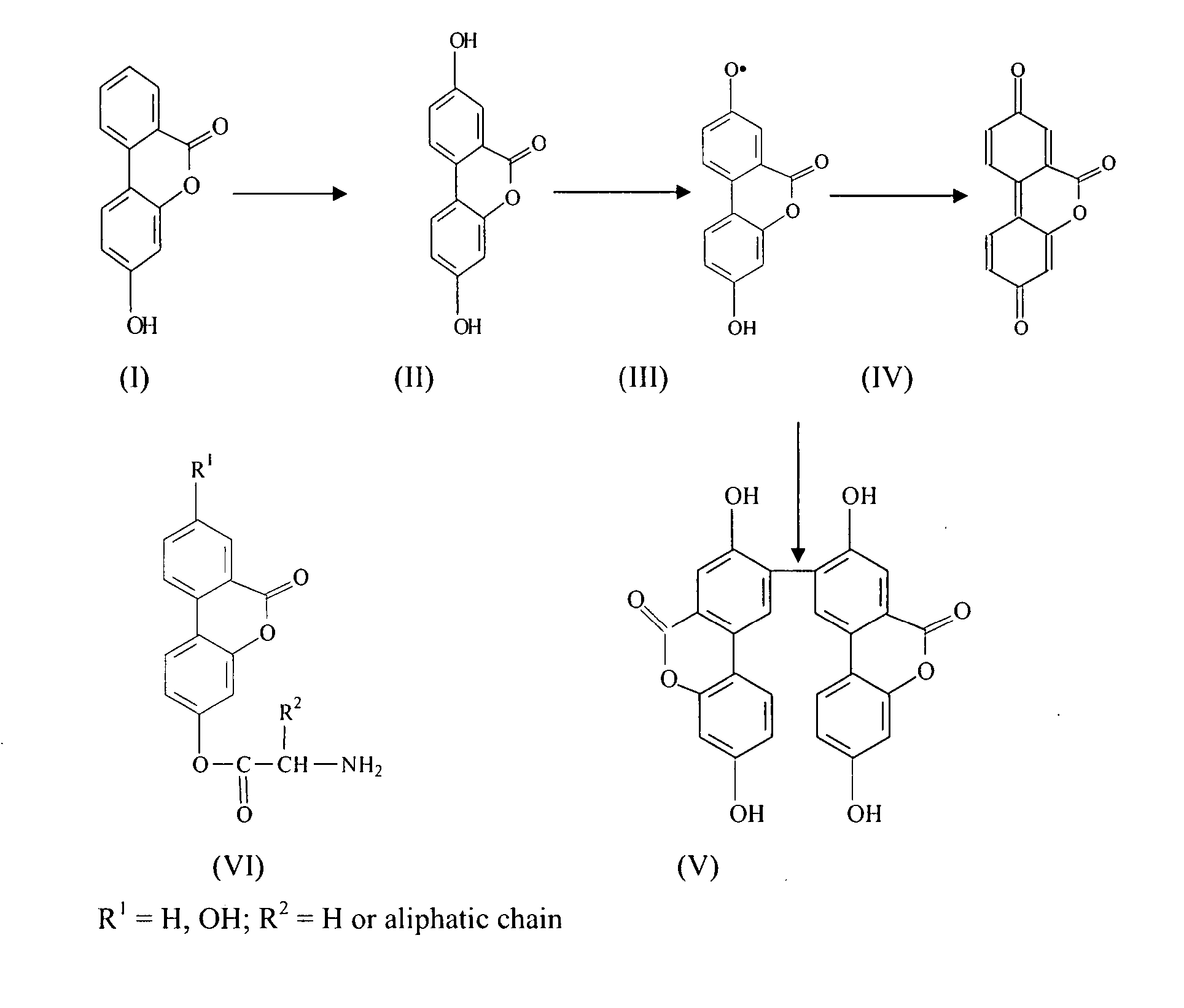
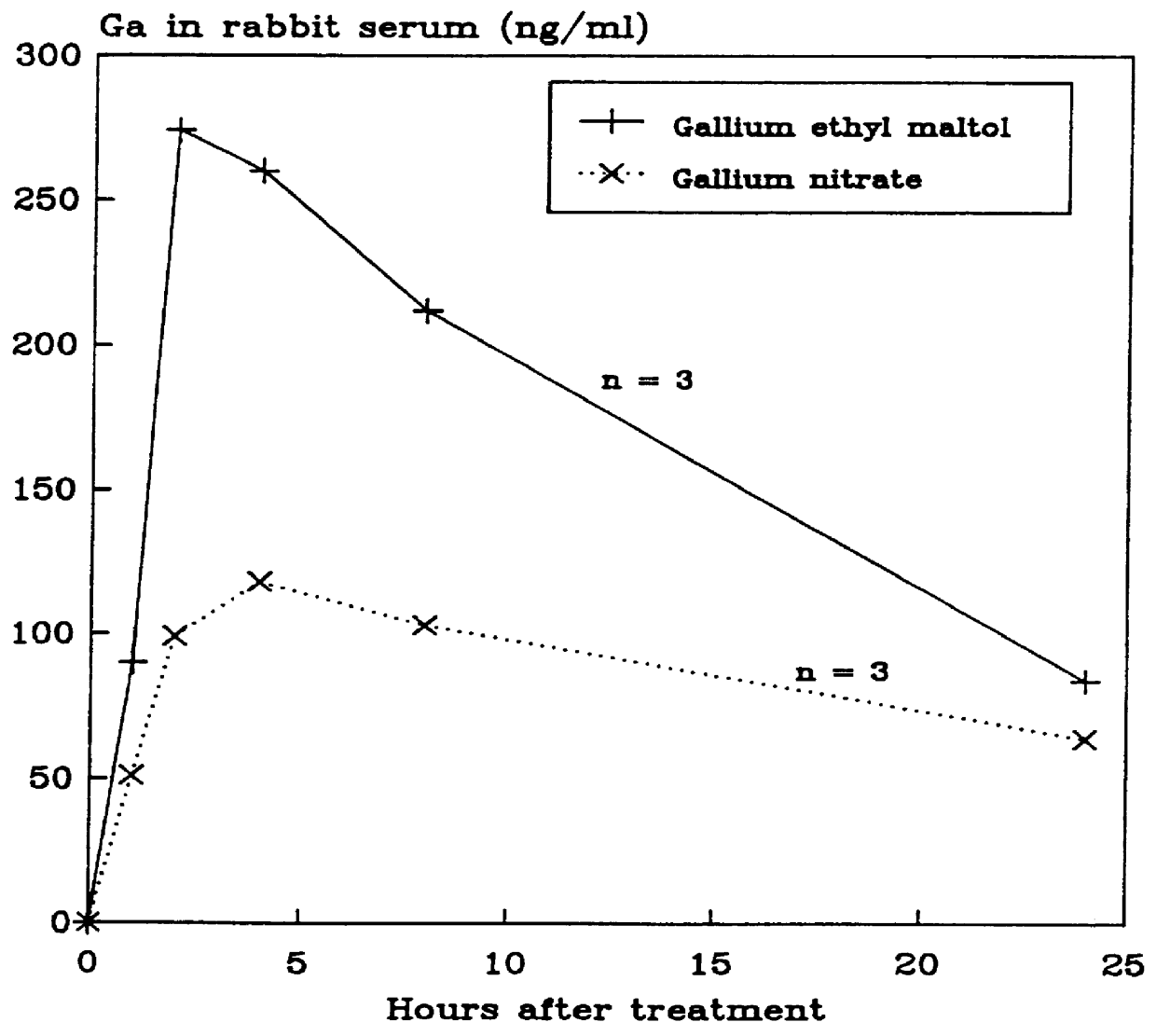
Disclosed are pharmaceutical compositions that comprise gallium complexes of 3-hydroxy-4-pyrones. These compositions provide enhanced gallium bioavailability particularly when orally administered as compared to the gallium bioavailability achieved by use of pharmaceutical compositions containing gallium salts. Compositions included in this invention are useful in providing gallium to humans and other mammals for a wide variety of medical and veterinary applications, including the treatment, prevention, or diagnosis of hypercalcemia, certain cancers, certain disorders of calcium homeostasis, and certain bone diseases including osteoporosis, osteopenia, and Paget's disease.
Owner:BERNSTEIN LAWRENCE RICHARD
Mitochondria-targeted antioxidants
ActiveUS20080031862A1Protect mitochondrionHigh energyCosmetic preparationsBiocideDiseasePersonal care
The present invention relates to a pharmaceutical or veterinary or nutritional or personal care composition comprising coenzyme Q10 (CoQ10), reduced CoQ10, or mixtures thereof and oxygenated dibenzo-α-pyrone or an amino acyl ester thereof. The composition of the present invention is able to support and / or provide therapy to individuals at risk and / or under treatment for dysfunctions of energy metabolism, and specifically, for mitochondrial diseases.
Owner:NATREON INC +1
Solid pharmaceutical compositions for the oral administration of gallium
The subjects of this invention are pharmaceutical compositions that comprise gallium complexes of 3-hydroxy-4-pyrones. The compositions have been developed to provide pharmaceutically acceptable gallium bioavailability together with low toxicity, particularly for oral administration. Compositions included in this invention should be useful in providing gallium to humans and other animals for a wide variety of medical and veterinary applications, including the treatment, prevention, or diagnosis of certain bone diseases, certain cancers, and certain disorders of calcium homeostasis.
Owner:BERNSTEIN LAWRENCE RICHARD
Compositions of stable bioactive metabolites of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids
InactiveUS20050282781A1Affect rate of absorptionOptimal moisture rangeBiocideNervous disorderMetaboliteBenzopyrone
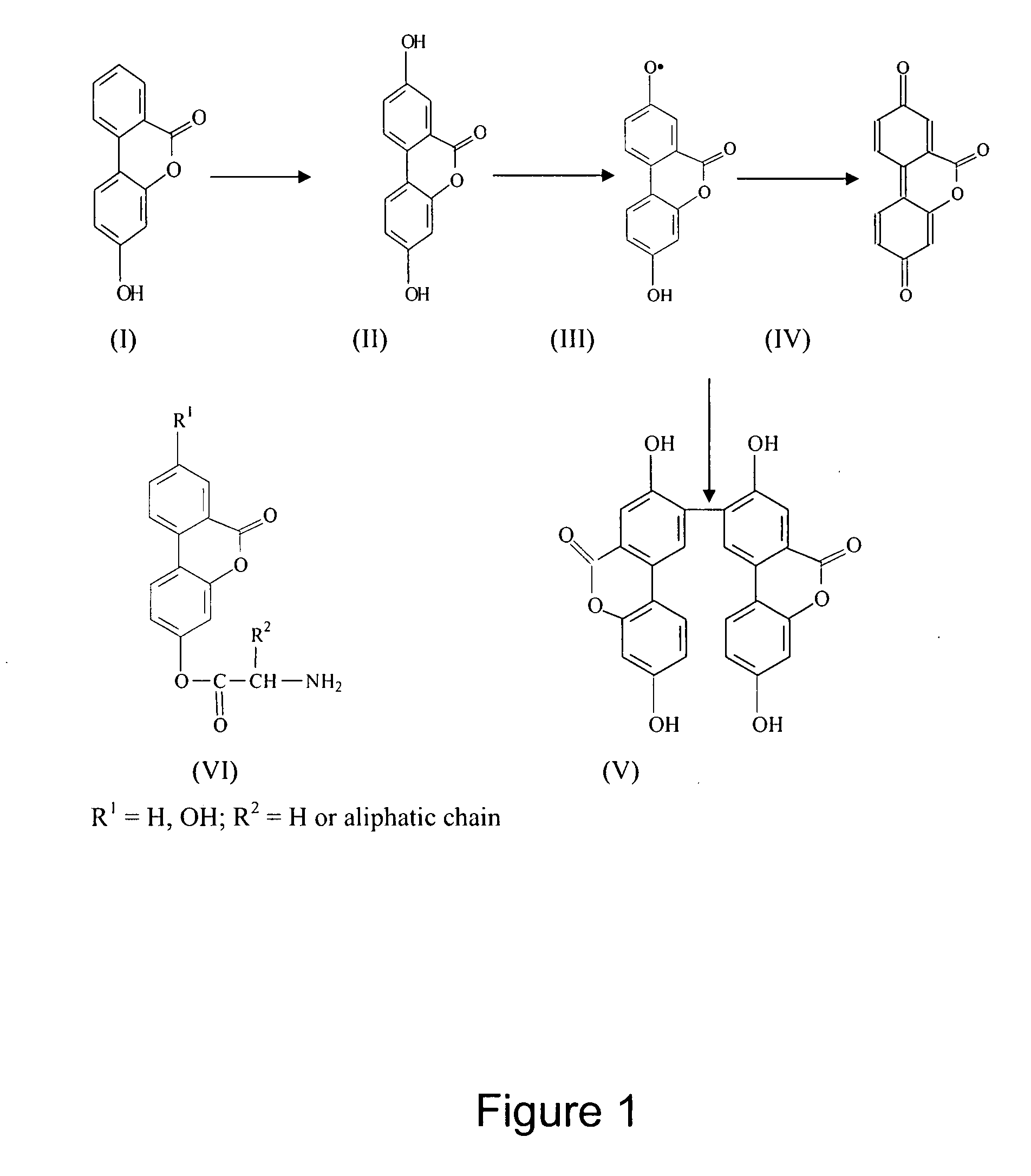
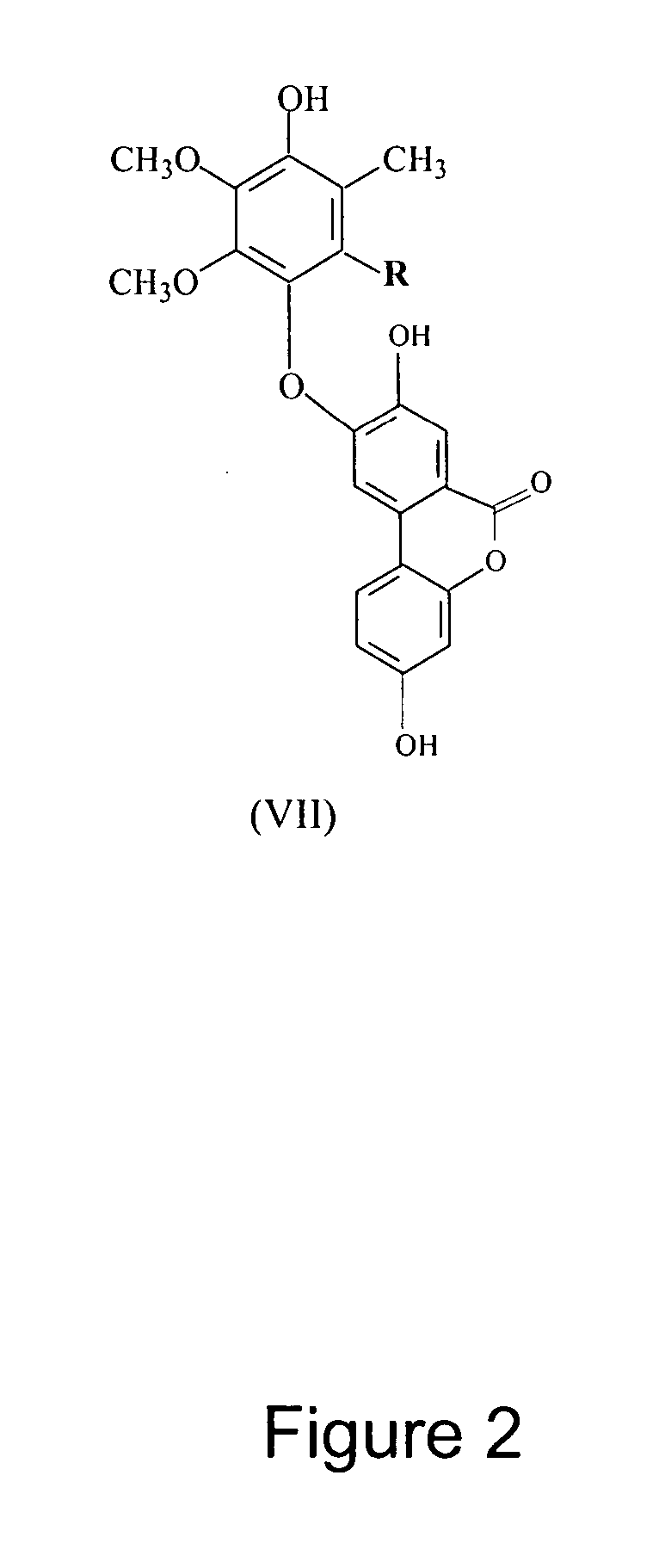
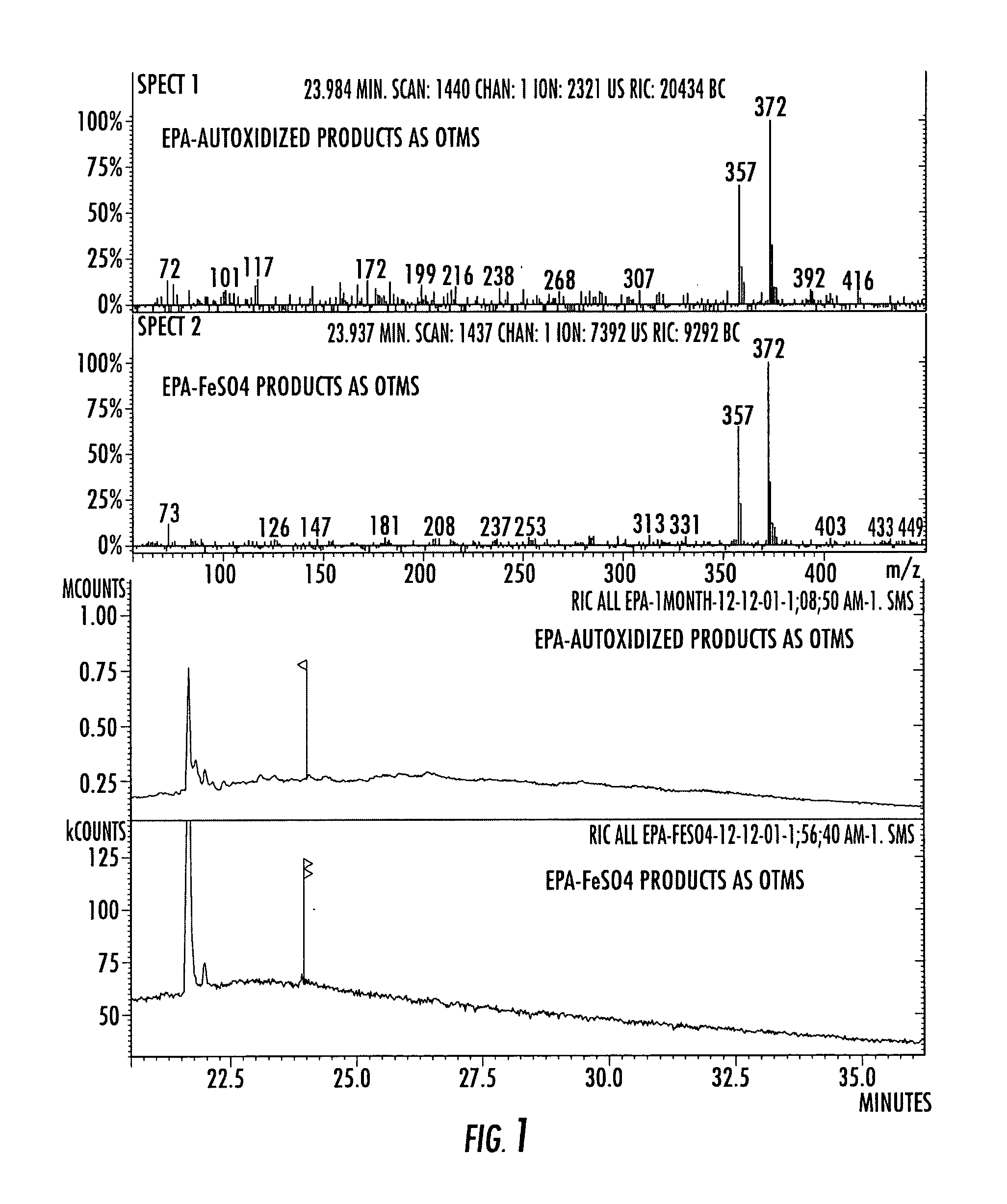
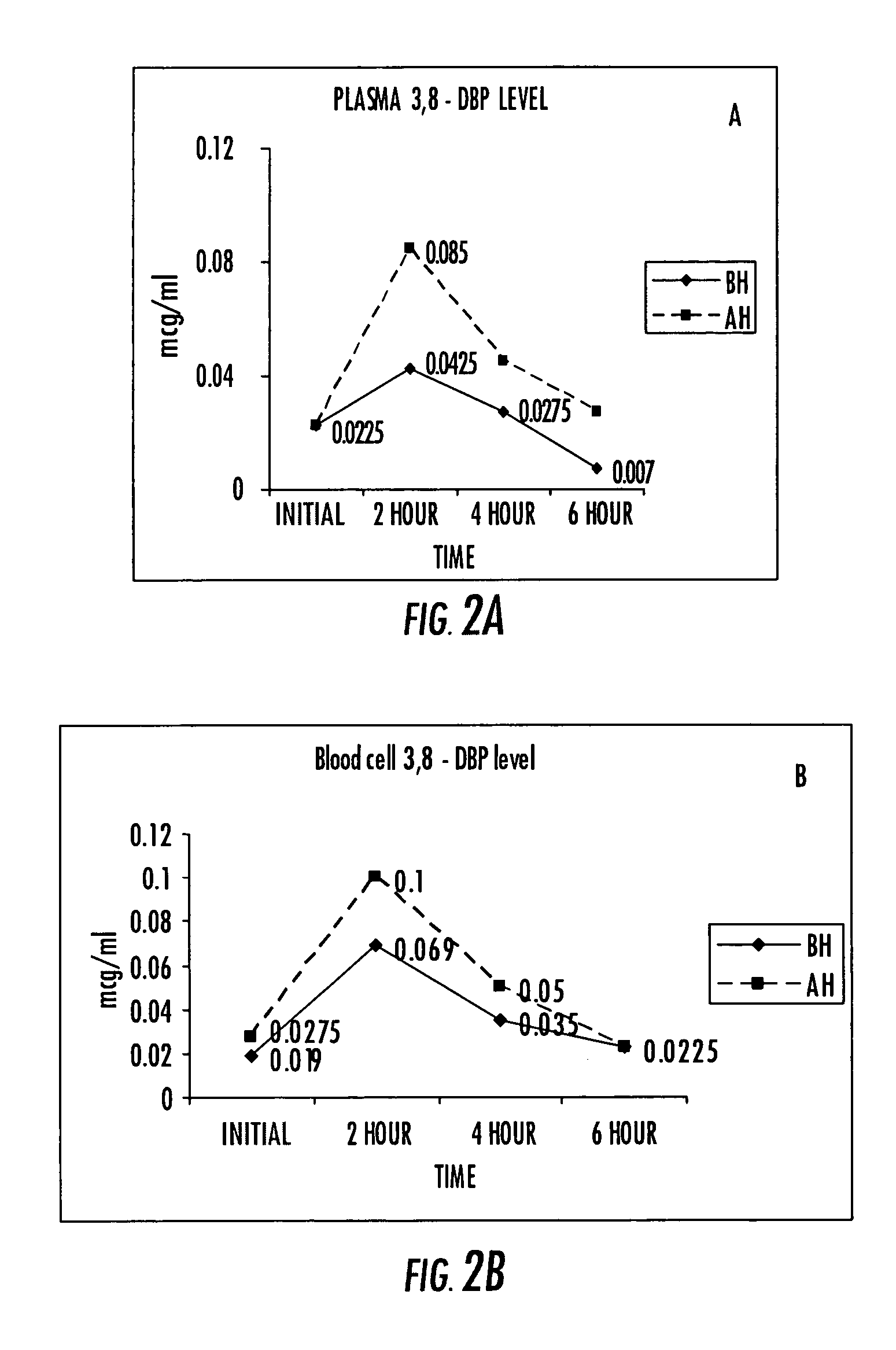
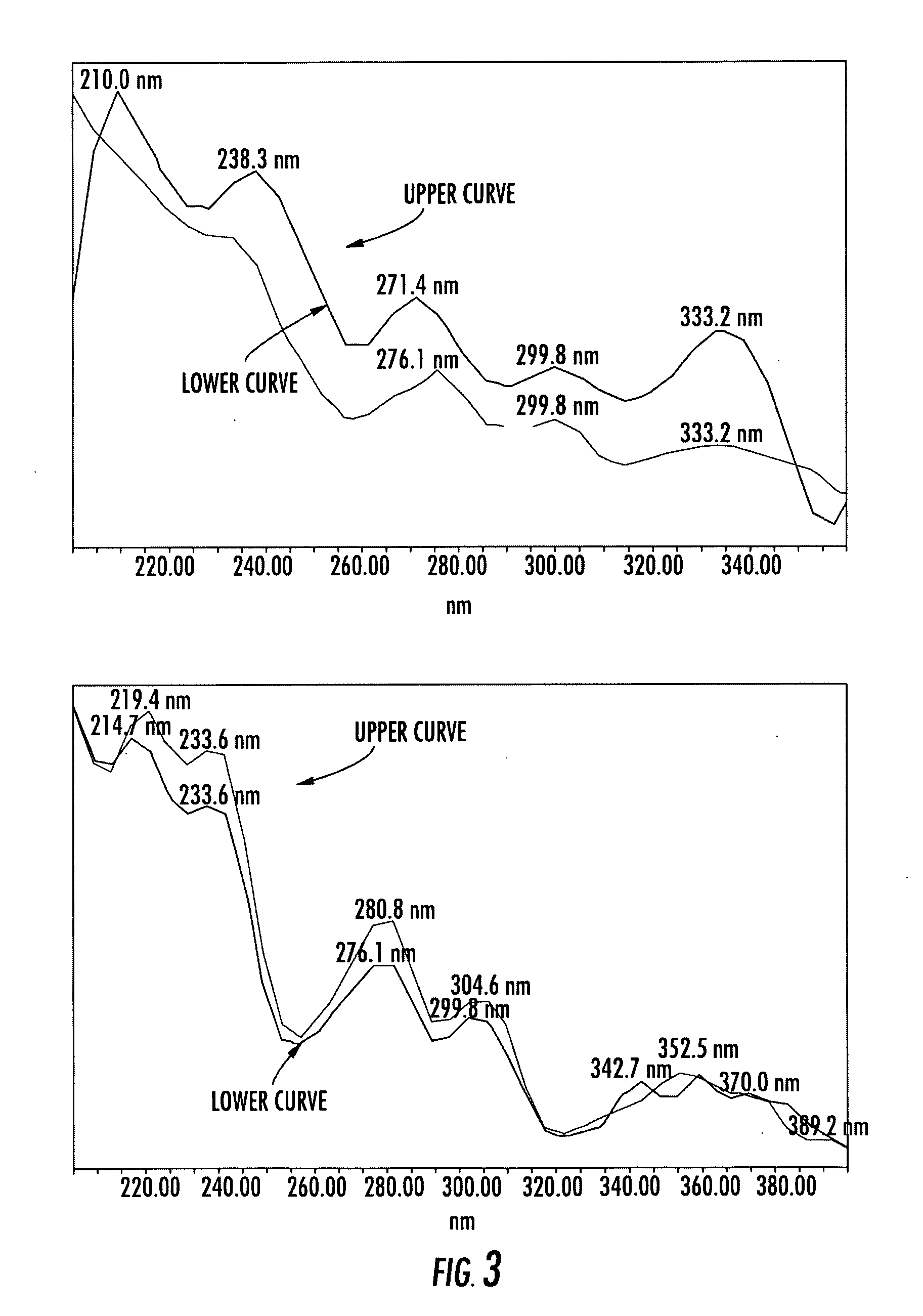
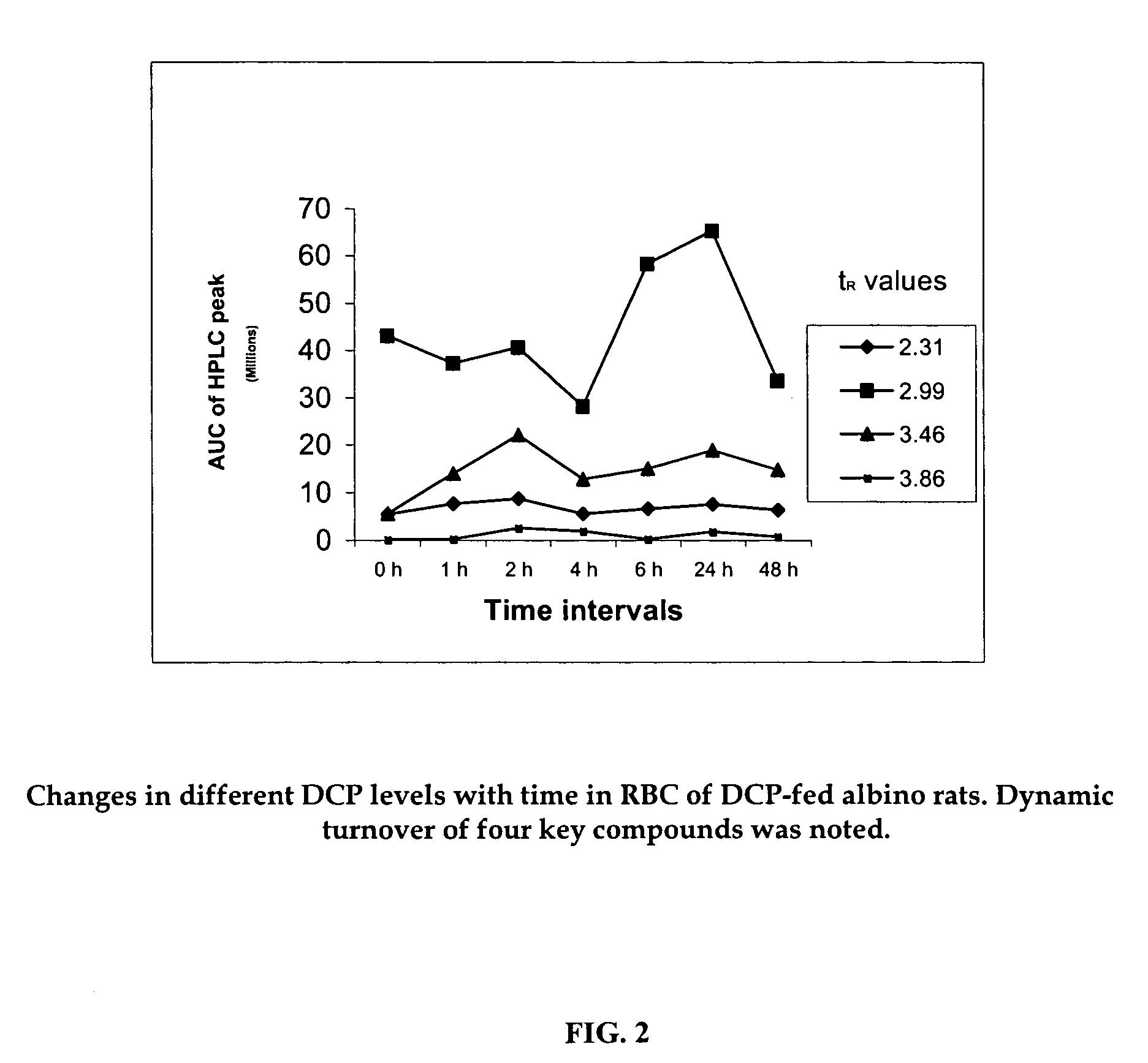
An invention that adduces cogent evidence to establish that oxygenated dibenzo-α-pyrones (DBPs and their conjugates), the major bioactives of shilajit (Ayurvedic vitalizer), have their origin, at least partly, in EPA and DHA. Earlier research has shown that, in mammals, C-20 PUFAs are metabolized by oxygenases and other enzymes to produce short-lived prostaglandins, leukotrienes and thromboxanes that bind to specific G-protein-coupled receptors and signal cellular responses, e.g., inflammation, vasodilation, blood pressure, pain etc. But never before it was suggested / shown that C20:5n-3 (and C22:6 n-3) PUFAs, e.g., EPA (and DHA), are transformed into stable aromatic metabolites, DBPs, which elicit a large array of bioactivities in the producer organisms and also control the synthesis and metabolism of arachidonate-derived prostaglandins. The major beneficial effects attributed to EPA and DHA are now found to be largely contributed by DBPs and their aminoacyl conjugates and the dibenzo-α-pyrone-chromoproteins (DCPs). Because of the highly unstable nature of EPA and DHA, when administered, they are metabolized into a large array of uncontrolled products, several of which are systemically undesirable. By contrast, DBPs, because of their stability, perform the biological response modifier (BRM) functions in a directed and sustained way. Many of the biological effects of DBPs described in this invention, were earlier attributed to EPA and DHA,—the precursors of DBPs.
Owner:NATREON INC
Preparation method of high-nicotine tobacco extract
ActiveCN106509981AHigh extraction rateAvoid destructionTobacco treatmentMaillard reactionDistillation
The invention discloses a preparation method of a high-nicotine tobacco extract. The preparation method of the high-nicotine tobacco extract belongs to the field of tobacco additives, and mainly comprises three steps of raw material pretreatment, subcritical extraction and molecular distillation. A baking process is adopted for pretreating tobacco leaves, so that reducing sugar of tobacco and a nitrogen-containing compound are promoted to generate maillard reaction, substances such as pyrone and furanone are produced, the baking aroma is improved, the reducing sugar content is reduced, the suction offensive odor is reduced, meanwhile, combined-state nicotine in the tobacco leaves is converted into a free state, and the content of the free-state nicotine is improved. A weak polar solvent is adopted for subcritical extraction, so that the extraction rate of tobacco feature aroma substances and the free-state nicotine can be improved; and low-temperature molecular distillation is combined, so that low-boiling-point aroma substances and the nicotine can be effectively enriched, the damage on thermosensitive substances due to high temperature is avoided, and meanwhile, the content of macromolecular substances such as pectin and protein in an extract is reduced. The method is simple in preparation process, simple and convenient to operate, and suitable for industrial production and application.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
High-nicotine tobacco extract and application thereof
ActiveCN106387990ACater to physiological needsIncrease contentTobacco treatmentNicotiana tabacumDamascone
The invention discloses a high-nicotine tobacco extract and application thereof, and belongs to the field of tobacco additives. The extract is rich in aroma components such as megastigmatrienone, damascone, pyrones and furanones in tobacco, can provide tobacco aroma and roasting aroma, further contains certain nicotine, can cater for physiological needs of consumers, and improves satisfaction. Content of macromolecular substances such as proteins, pectin and polysaccharides in the extract is extremely low, solubleness is good, the high-nicotine tobacco extract can be completely dissolved into MCT (medium chain triglyceride), and can be used for perfuming a filter tip. Besides, the high-nicotine tobacco extract is added into cut stems or reconstituted tobaccos, so that stimulus can be reduced, strength is improved, a blending rate, in a tobacco group, of the cut stems or reconstituted tobaccos is increased, and content of cigarette tar is reduced while fragrance is invigorated and impurities are covered.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Tobacco extract, extracting method and application
The invention discloses a tobacco extract, an extracting method and application and belongs to the field of tobacco additives. The tobacco extract is extracted with the method including the following steps that firstly, propylene glycol is added into tobacco raw materials, and then baking is carried out after standing; secondly, smashing is carried out, and a solvent is added into powder materials for extraction; thirdly, extract liquid passes through an ultrafiltrtion membrane, penetrating liquid is obtained and subjected to reduced pressure distillation, and concentrate is obtained; fourthly, the concentrate is subjected to secondary separation with a molecule distillation method, light components are collected, and the tobacco extract is obtained. The extract contains rich megastigmatrienone, Damascone, neophytadiene, phenylcarbinol, phenethyl alcohol, farnesylacetone and pyrone and furanone aroma components, and the tobacco extract is thick in baking aroma and tobacco aroma, good in penetration, small in irritation and miscellaneous gas, high in satisfaction, capable of improving the smoking quality of cigarettes and providing a certain amount of nicotine to meet the physiological needs of consumers when used.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
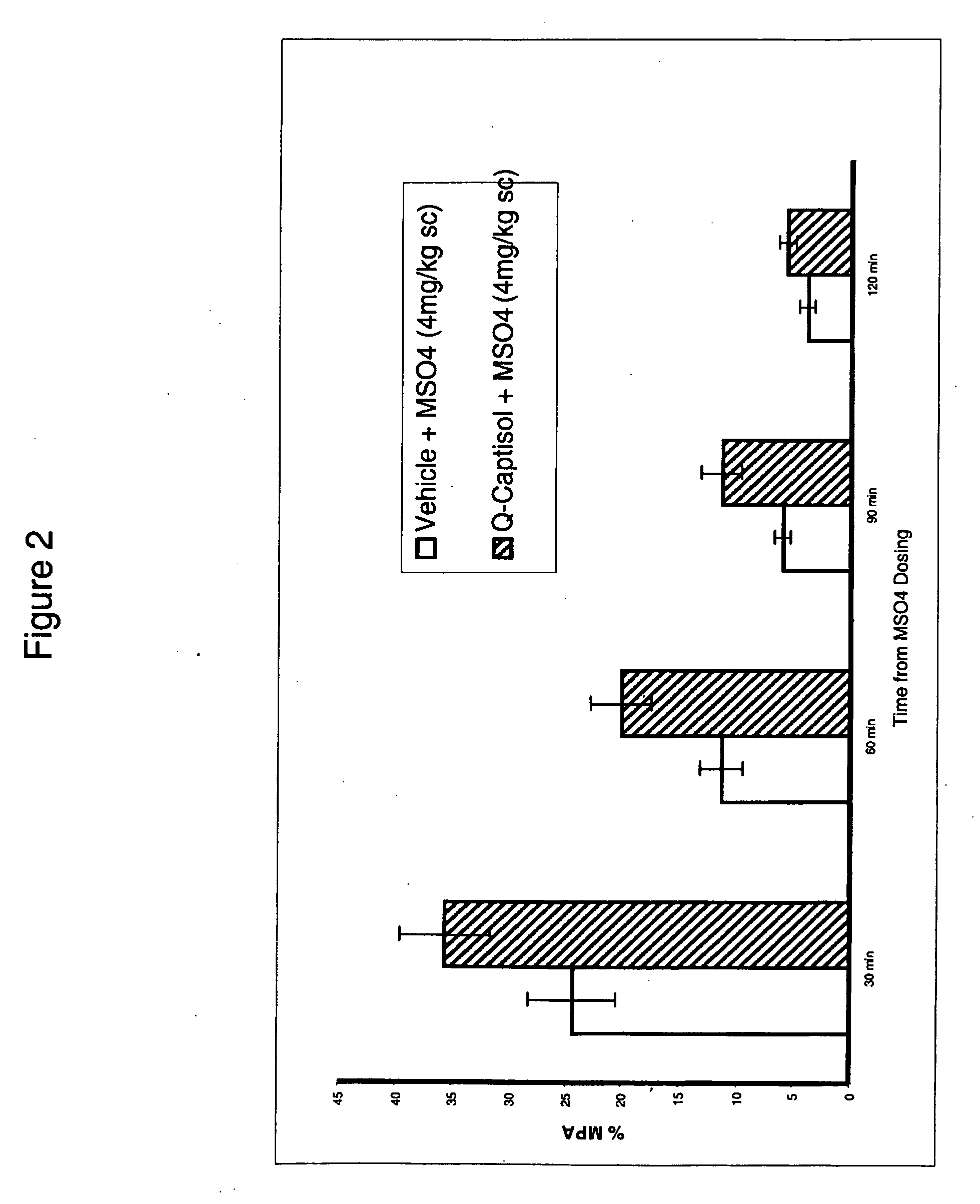
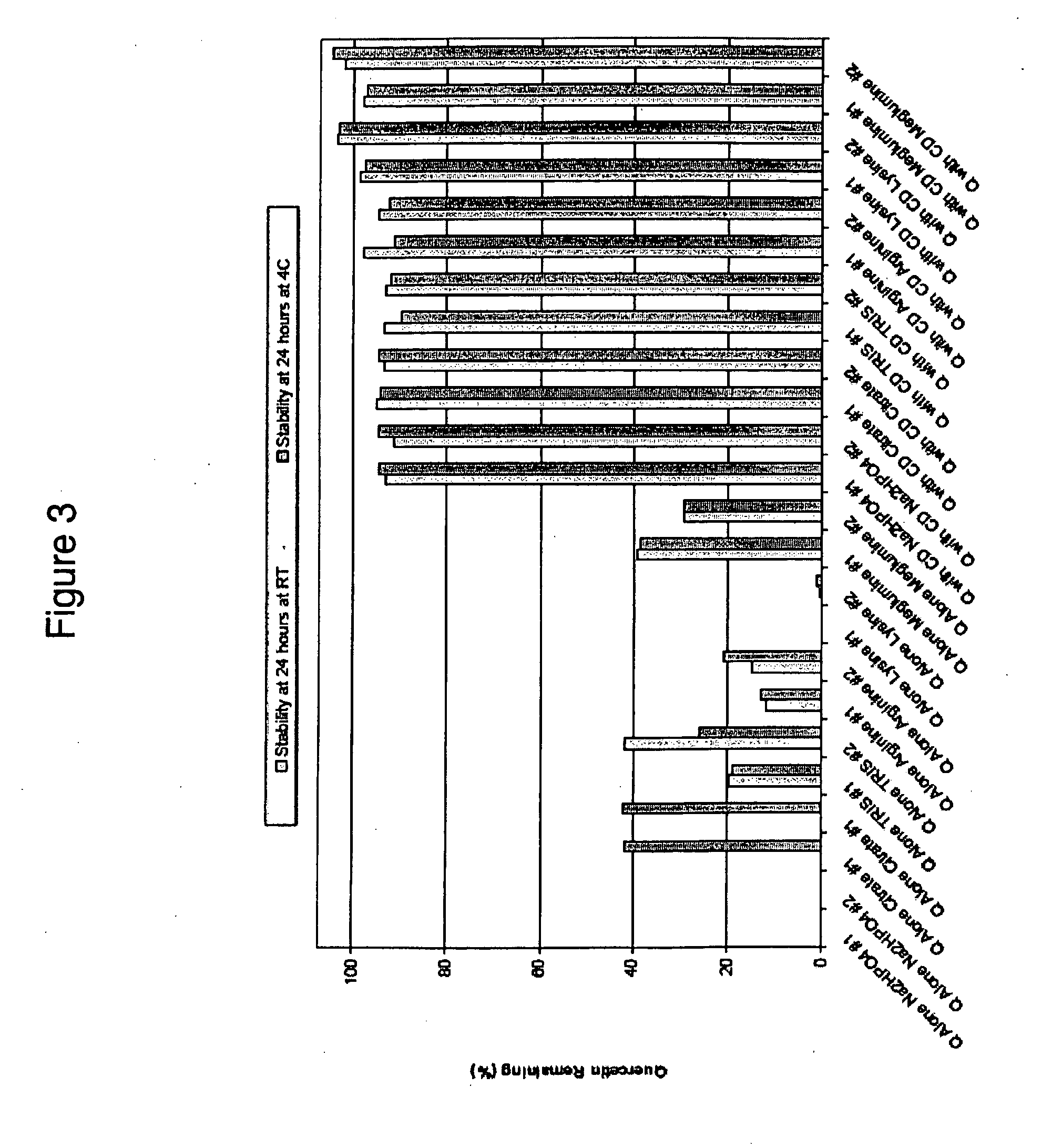
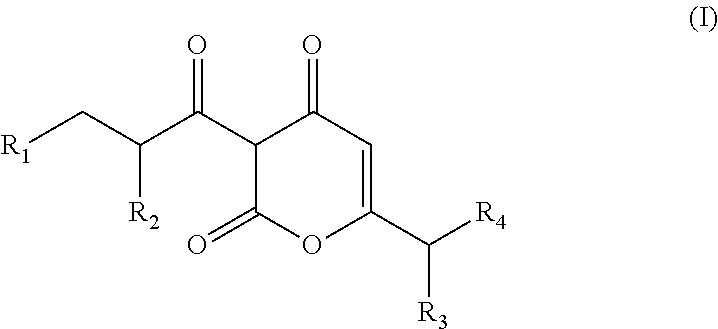
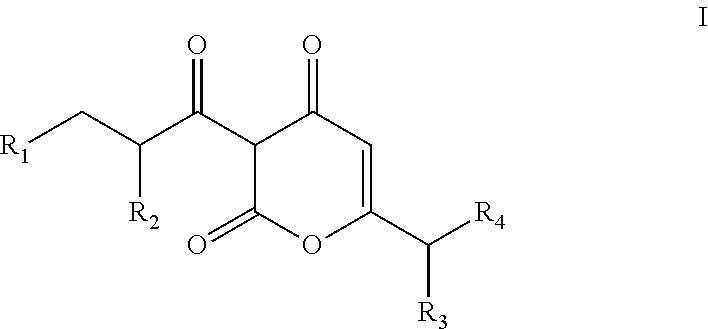
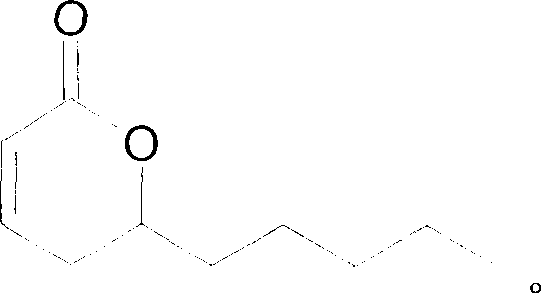
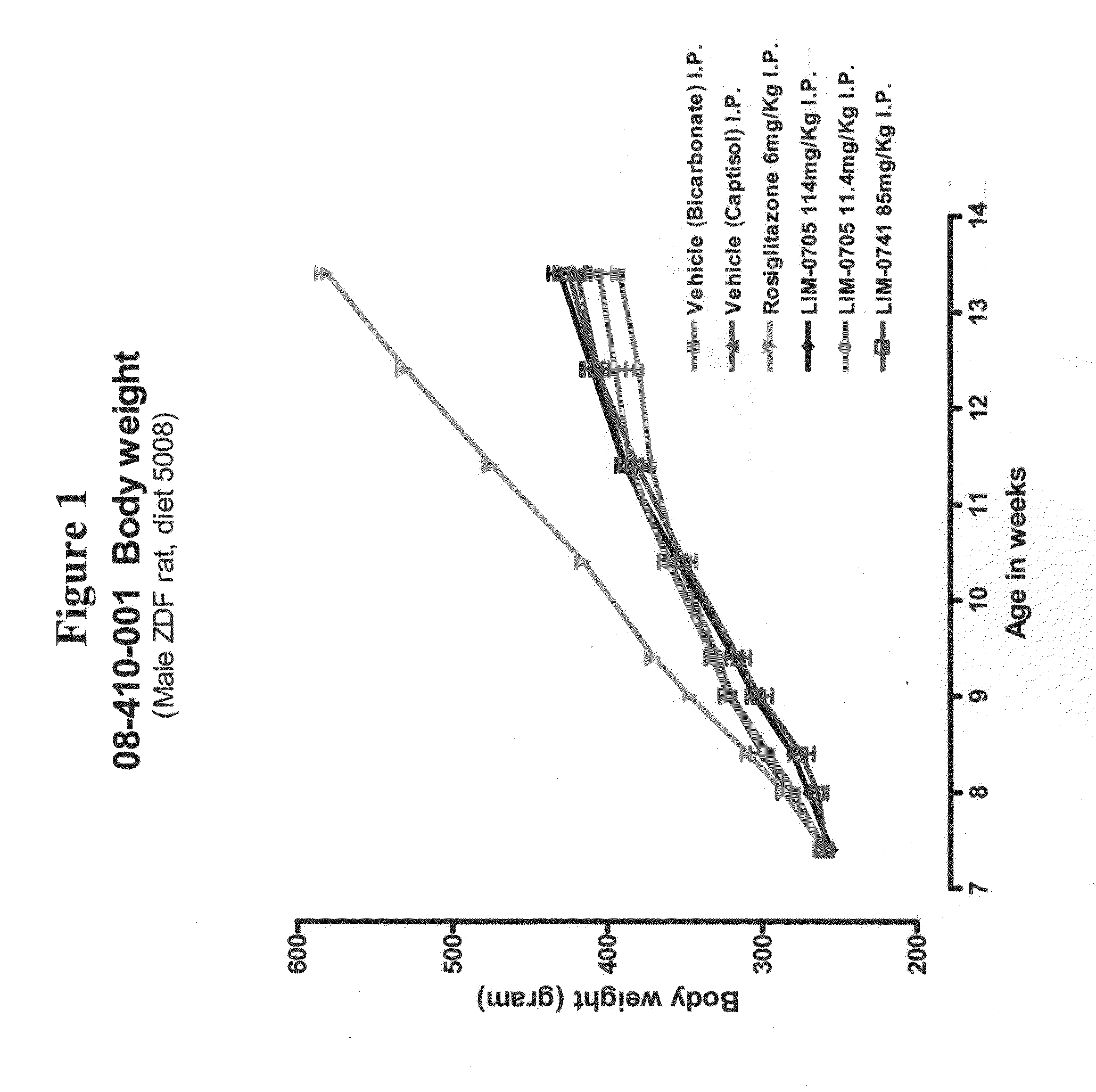
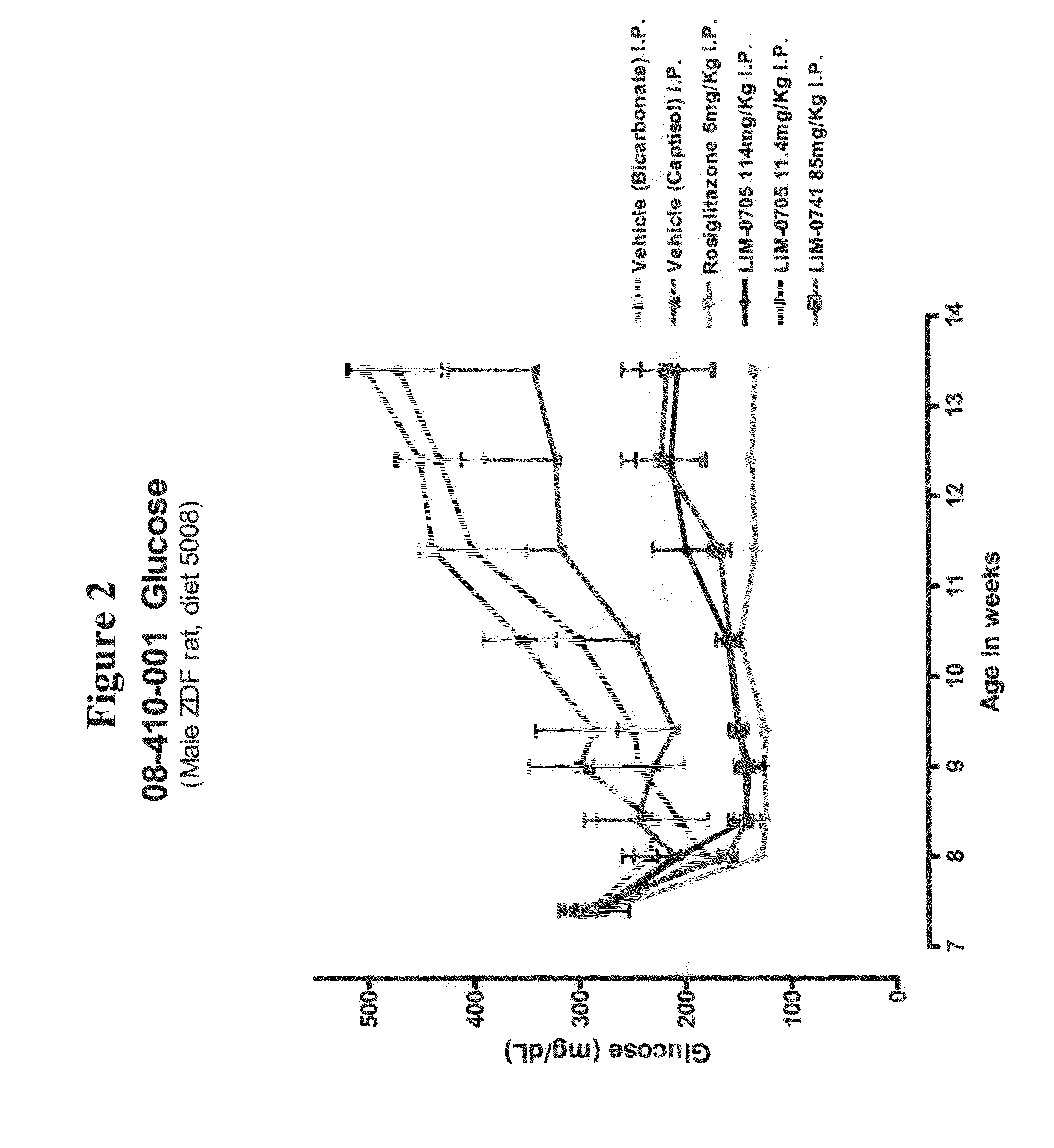
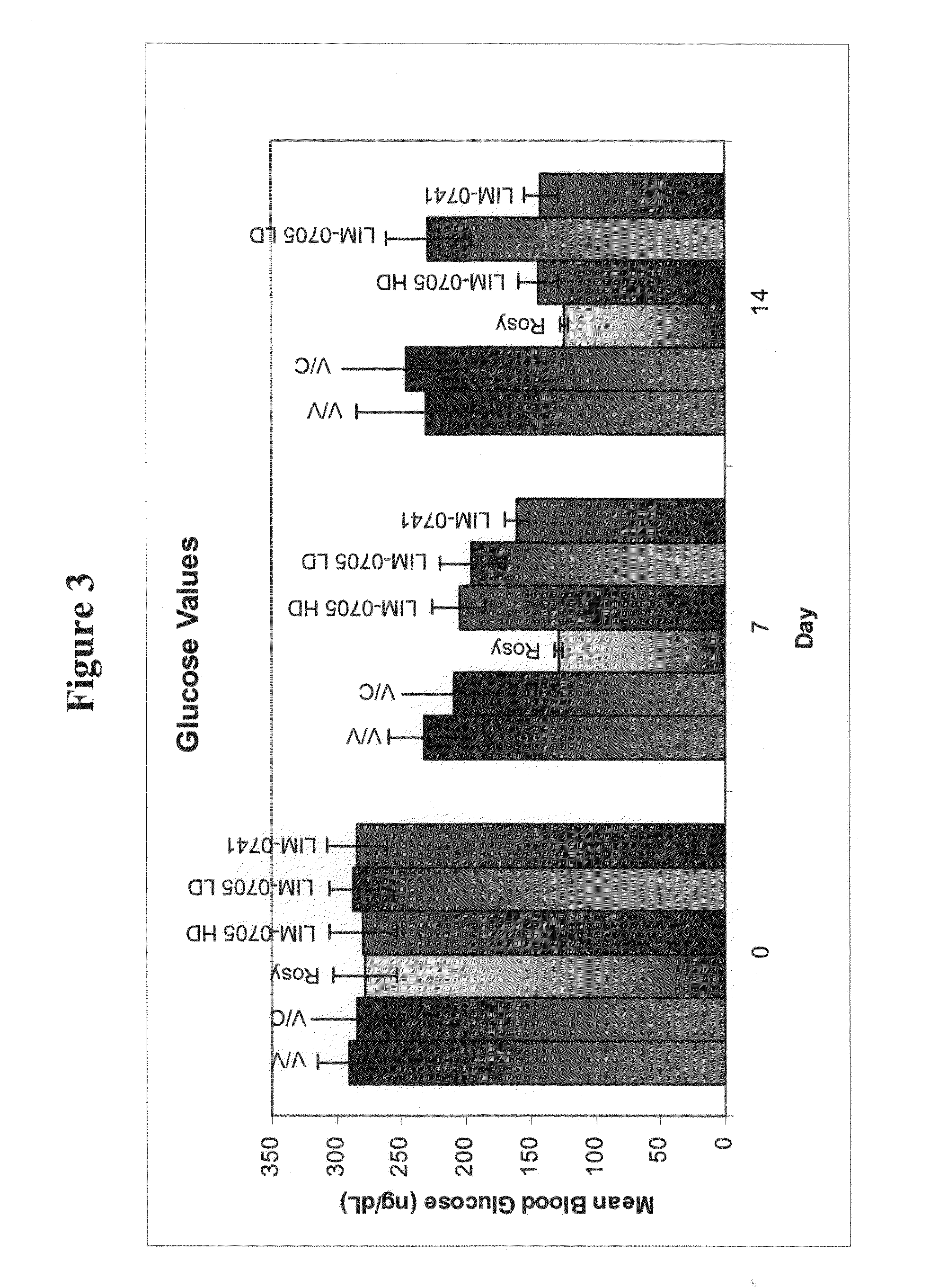
Soluble pyrone analogs methods and compositions
InactiveUS20090082400A1Reduce appetiteWeaken energyBiocideAntipyreticQuercetin derivativesCyclodextrin
Methods and compositions are described that comprise pyrone analogs such as flavonoids and cyclodextrins including quercetin and quercetin derivatives and sulfoalkyl ether cyclodextrins. In some cases the compounds of the invention are administered with a therapeutic agent such as an analgesic. In some cases, treatment with the compositions of the invention can result in the modulation of central nervous system and / or fetal effects of substances. Methods and compositions are described for the modulation of efflux transporter activity to increase the efflux of drugs and other compositions out of a physiological compartment and into an external environment. In particular, the methods and compositions disclosed herein provide for the increase of efflux transporter activity at blood-brain, blood-CSF and placental-maternal barriers to increase the efflux of drugs and other compositions from physiological compartments, including central nervous system and fetal compartments.
Owner:LIMERICK BIOPHARMA INC
Arylpropionyl-alpha-pyrone antibacterial agents
Owner:EBRIGHT RICHARD H
Essence microcapsule wall material for cigarettes and essence microcapsule
InactiveCN103146480AExtended stayFacilitated releaseEssential-oils/perfumesMicroballoon preparationMaillard reactionFuran
The invention provides an essence microcapsule wall material for cigarettes. The material is a whey protein-glucose grafting product which is prepared through the maillard reaction of whey protein and glucose, wherein the mass ratio of the whey protein to glucose is 1:(1-3). The essence microcapsule wall material can generate heterocyclic compounds such as pyrazine, furan, pyrrole, pyrone and the like during combustion pyrolysis, and aroma components are harmony with the aroma of tobacco, so that adverse effects which are caused by adding a conventional wall material into the cigars are avoided. Moreover, an essence microcapsule prepared by the wall material has the advantages of uniform particles, dense capsule wall, high embedding rate, good stability, good releasing property and the like, and is suitable for various volatile cigar essences. In addition, the essence microcapsule prepared by the wall material has a certain releasing function, so that the essence durability can be prolonged, the phenomenon of strong aroma at the beginning and obviously weak aroma during a later period in a smoking process is avoided, and the essence microcapsule has strong practicability.
Owner:HUBEI CHINA TOBACCO IND
E-cigarette nicotine liquid and preparation method thereof
ActiveCN106617265ASimple preparation processSimple and fast operationTobacco treatmentAdditive ingredientLiquid smoke
The invention discloses E-cigarette nicotine liquid and a preparation method thereof and belongs to the technical field of E-cigarette nicotine liquids. The E-cigarette nicotine liquid is made from high-nicotine tobacco extract, tobacco flavor and the like, wherein the tobacco extract has rich tobacco characteristic fragrant ingredients (such as megastigmatrienone, beta-damascone, pyrones, and furanones) and nicotine, can provide tobacco aroma and curing aroma and meet the physiological needs of consumers, and provides better satisfaction. The high-nicotine tobacco extract has very low contents of high-boiling-point ingredients and macromolecular substances (such as proteins, pectin, and polysaccharides), is free of burnt smell and low in sweetness, and can supplement aroma and cover peculiar odors, lower stimulus and improve taste strength. By being used with tobacco flavors, the E-cigarette nicotine liquid has enhanced curing aroma and tobacco aroma, and has strong aroma and good volatility. The preparation process of the E-cigarette nicotine liquid is simple, is simple and easy to perform, and is applicable to large-scale industrial production and application.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Preparation and application of a category of 6 - aryl - 3 - cycroamido methyl pyrone derviation
This invention relates to compounds with 6-aryl-3-cycloaminomethyl pyrone structure, their preparation method and their application. Pharmacological activity experiments show that the compounds have a high activity in selectively inhibiting AChE while no obvious activity in inhibiting BuChE. The activity of the compounds in inhibiting AChE is higher than huperzine A. Mouse in vivo tests show that the compounds do not have acute toxicity or abnormal reaction phenomenon, and the compounds can be used as a kind of new drugs for treating Alzheimer's disease.
Owner:赵昱
Substituted methylene pyrones derivatives and their preparing process and use
The present invention relates to compound with 6-aryl-3-substituted methylene-pyrone structure, derivative with 6-aryl-3-methyl-4-acryloxy tetrahydro pyrone skeleton, and their preparation process and use. Pharmacological activity test shows that the compound possesses acetylcholinesterase inhibiting activity and is expected to be used as medicine for senile dementia. The compound also shows relatively high activity of inhibiting tumor cells of prostate cancer, oral epithelium cancer, lung cancer and cervical cancer and thus is expected to be used as antitumor medicine.
Owner:浙江海正集团有限公司
Phosphorylated pyrone analogs and methods
InactiveUS20110028437A1Reduce appetiteWeaken energyBiocideOrganic active ingredientsSubstance useSide effect
The invention relates to phosphorylated polyphenols, phosphorylated flavonoids, and phosphorylated pyrone analogs. Methods and compositions for the modulation of side effects of substances using such phosphorylated compounds are described. Methods and compositions are described for the modulation of blood-tissue barrier (BTB) transporter activity to increase the efflux of drugs and other compounds out of a physiological compartment and into an external environment. In particular, the methods and compositions disclosed herein provide lowered side effects when phosphorylated pyrone analogs are coadministered with therapeutic agents.
Owner:LIMERICK BIOPHARMA INC
Pyronone antibiotic its preparation method and application
The present invention relates to a pyrone antibiotic, its preparation method and application. Its molecular formula is C10H16O2, and its molecular weight is 168. Said invention also provides its molecular structure diagram. Its preparation method includes the following steps: using solid culture medium to culture green trichoderma LTR-2, obtaining spore powder; soaking spore powder in dichloromethane with 5-10deg.C, making the soaking liquor successively undergo the processes of adsorption with active carbon, washing by using sodium sulfate solution, dehydration by using anhydrous sodium sulfate and reduced pressure concentration to obtain concentrate containing antibiotic pyrone. It can be used as effective component to prepare an agricultural preparation-wettability powder preparation whose composition includes diatomite, concentrate containing pyrone, sodium dodecyl benzene sulfonate and Tween 80.
Owner:BIOTECH CENT OF SHANDONG ACAD OF SCI
Pyrone analogs for therapeutic treatment
InactiveUS20100189653A1Modulate activityGood effectBiocideOrganic active ingredientsLipid transporter activityDisease
Methods are described for the treatment and prevention of metabolic disorders or other diseases by administering a pyrone analog or a derivative thereof. Methods are also described for the treatment and prevention of metabolic disorders and other diseases by administering a pyrone analog, or a derivative thereof, in combination with one or more additional agents such as, for example, lipid lowering agents or glucose lowering agents. Methods are described for the modulation of lipid transporter activity to increase the efflux of lipid from a physiological compartment into an external environment. Methods disclosed herein may be used to assess treatment or prevention of a metabolic disorder following administration of a pyrone analog or a derivative thereof.
Owner:LIMERICK BIOPHARMA INC
Methods of treating cataracts and diabetic retinopathy with tricyclic pyrones
InactiveUS6916824B1Improve solubilityFacilitate slow-releaseBiocidePharmaceutical delivery mechanismDiabetic retinopathyWater soluble
Owner:KANSAS STATE UNIV RES FOUND
Tricyclic pyrones
InactiveUS7935726B1Improve solubilityFacilitate slow-releaseBiocideOrganic chemistryPhenyl groupMedicinal chemistry
Provided are compounds of formula IA or IB:wherein R1 and R8 are independently optionally substituted hydrocarbyl groups; wherein(a) R1 contains a carbonyl group and a phenyl group,(b) R8 contains an optionally substituted adenine group, or(c) R8 contains an alkenyl group with from two to six carbon atoms;R10 is H, —OH, —OR or ═O; R6 is selected from the group consisting of: H, OH, alkyl, alkenyl, alkynyl, an aromatic ring system, amino, sulfhydryl, sulfonyl, NH2 and OCOR; R2 is selected from the group consisting of: H, —OH and lower alkyl; R is H or an optionally substituted hydrocarbyl group, and pharmaceutically acceptable salts or esters of the foregoing, as well as isomers thereof.
Owner:KANSAS STATE UNIV RES FOUND
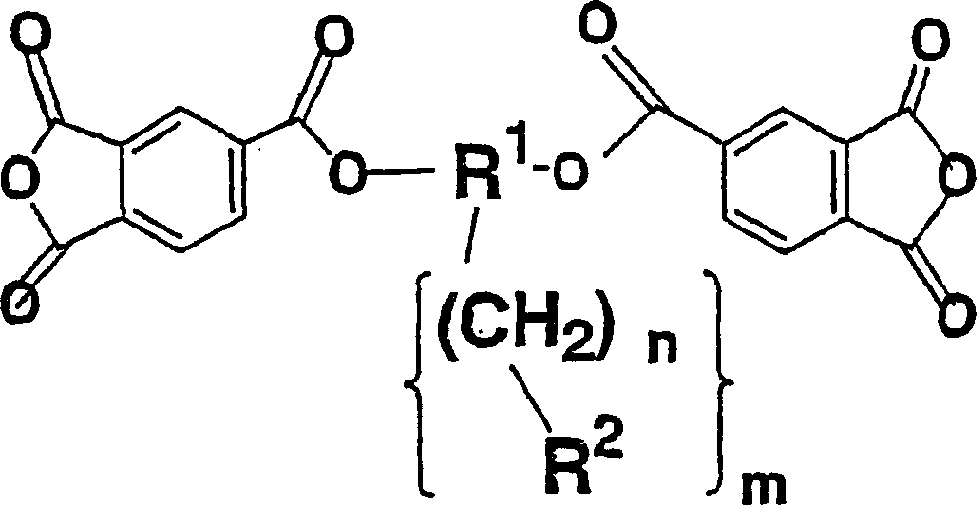
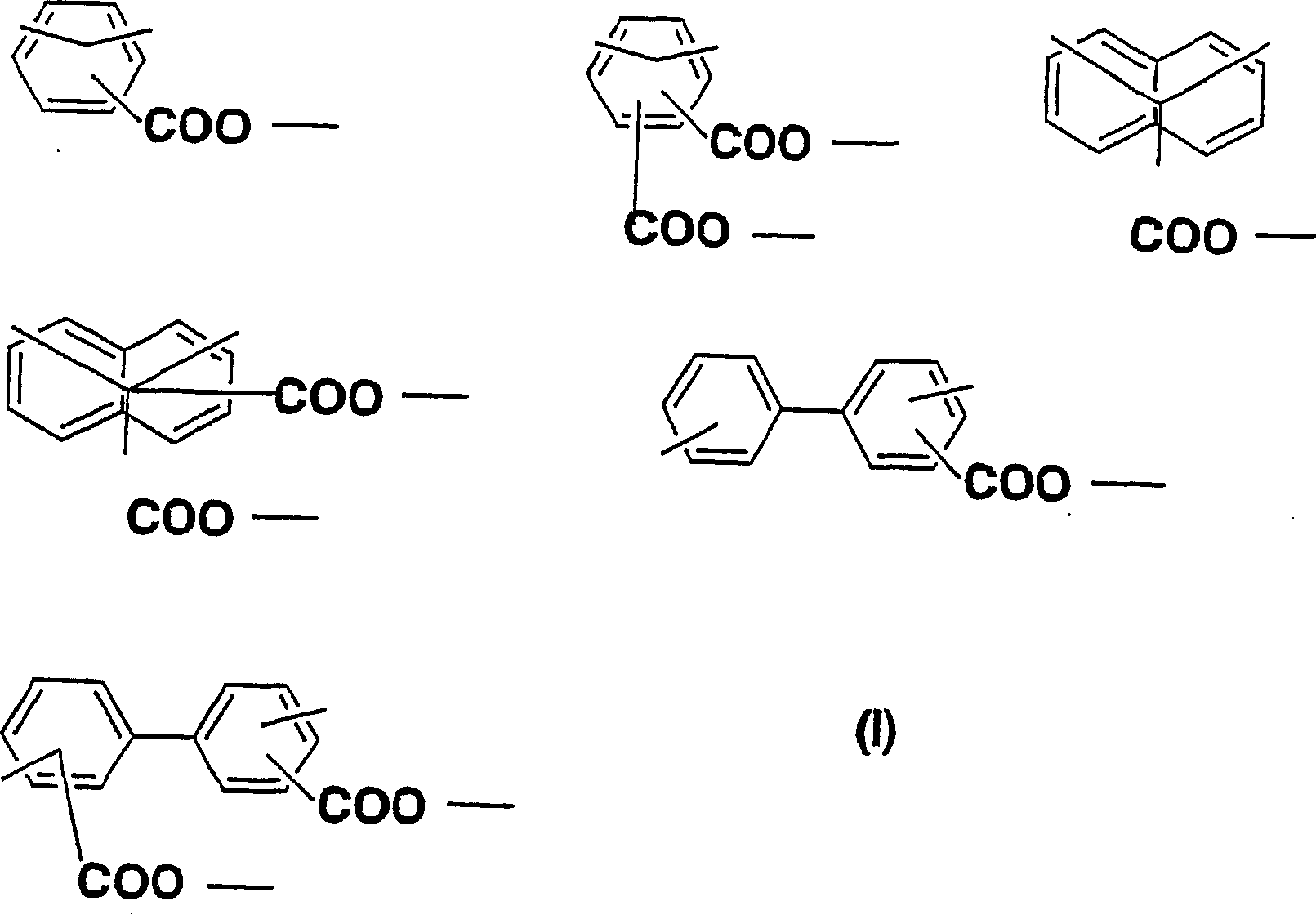
Diamine, acid dianhydride, polyimide composition having reactive group obtained therefrom, and processes for producing these
InactiveCN1501921AOrganic compound preparationAmino-carboxyl compound preparationOrganic groupBis-diamine
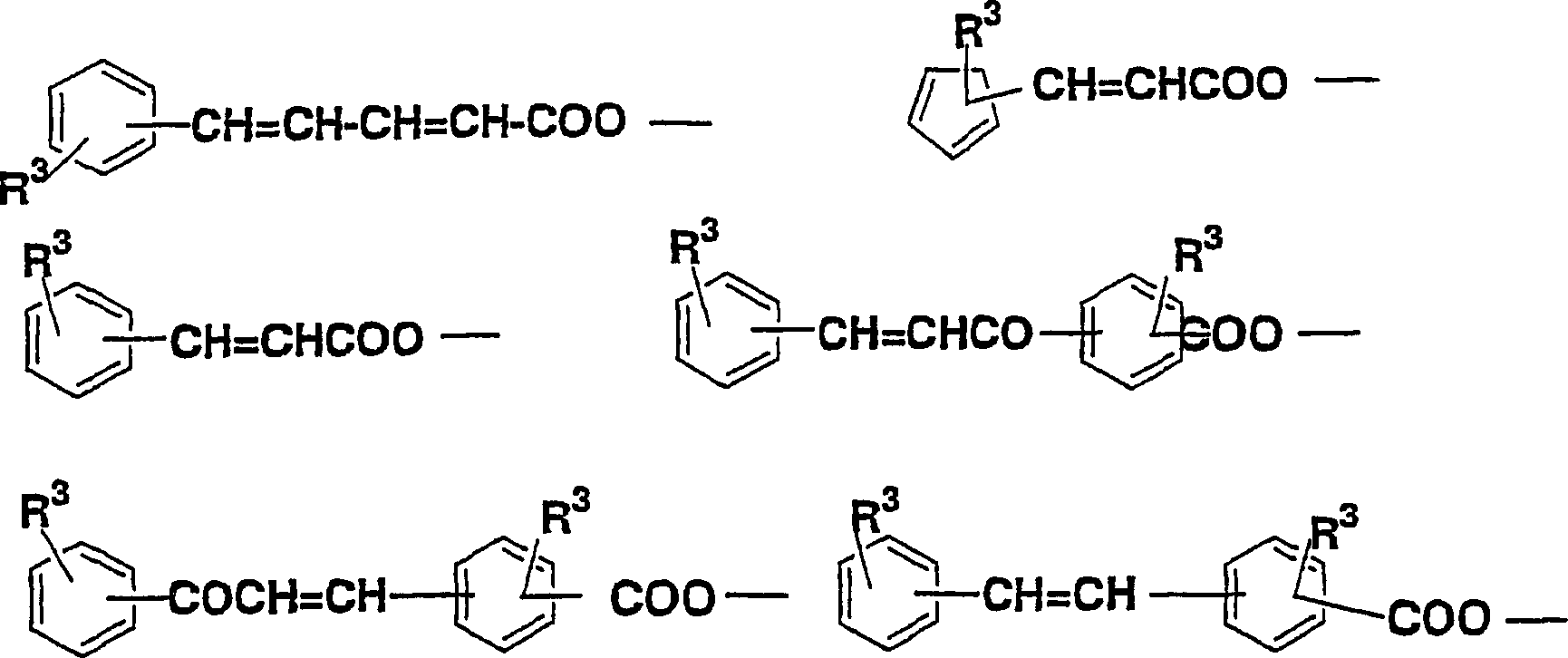
The present invention relates to a novel diamine, a novel acid dianhydride, and a novel composition containing a polyimide produced from such diamine and acid dianhydride. Specifically, the present invention relates to an acid dianhydride that has a photosensitive group bonded through the mediation of an alkylene group or a fluoroalkylene group, a diamine that has a reactive group bonded through the mediation of an alkylene group or a fluoroalkylene group, and a polyimide composition that contains a novel polyimide having at least one of such acid dianhydride residue and diamine residue in a molecule thereof. Particularly, an object of the present invention is to provide a novel polyimide that has both photoreactivity and thermoreactivity specific to the reactive group and whose reactive group is selected from the group consisting of: organic groups derived from cinnamic acid, chalcone, furylacryloyl, benzalacetophenone, stilbene, coumarin, and pyrone; allyl; propargyl; ethinyl; CH2=CH-; CH2=C(CH3)-; and skeletons derived therefrom, and it to provide a novel diamine and a novel acid dianhydride that may be contained in the polyimide composition.
Owner:KANEKA CORP
Mitochondria-targeted antioxidants
The present invention relates to a pharmaceutical or veterinary or nutritional or personal care composition comprising coenzyme Q10 (CoQ10), reduced CoQ10, or mixtures thereof and oxygenated dibenzo-α-pyrone or an amino acyl ester thereof. The composition of the present invention is able to support and / or provide therapy to individuals at risk and / or under treatment for dysfunctions of energy metabolism, and specifically, for mitochondrial diseases.
Owner:NATREON INC +1
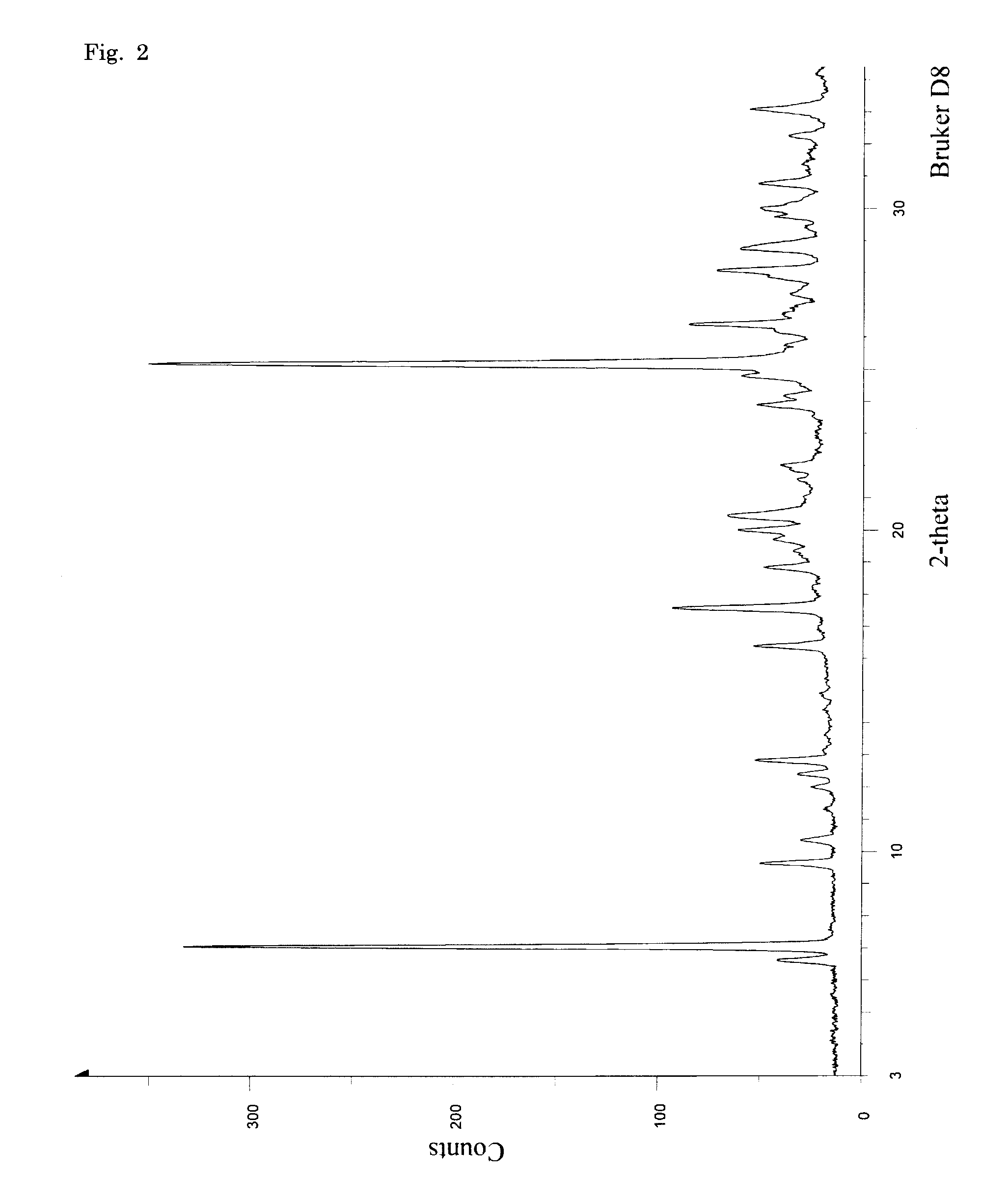
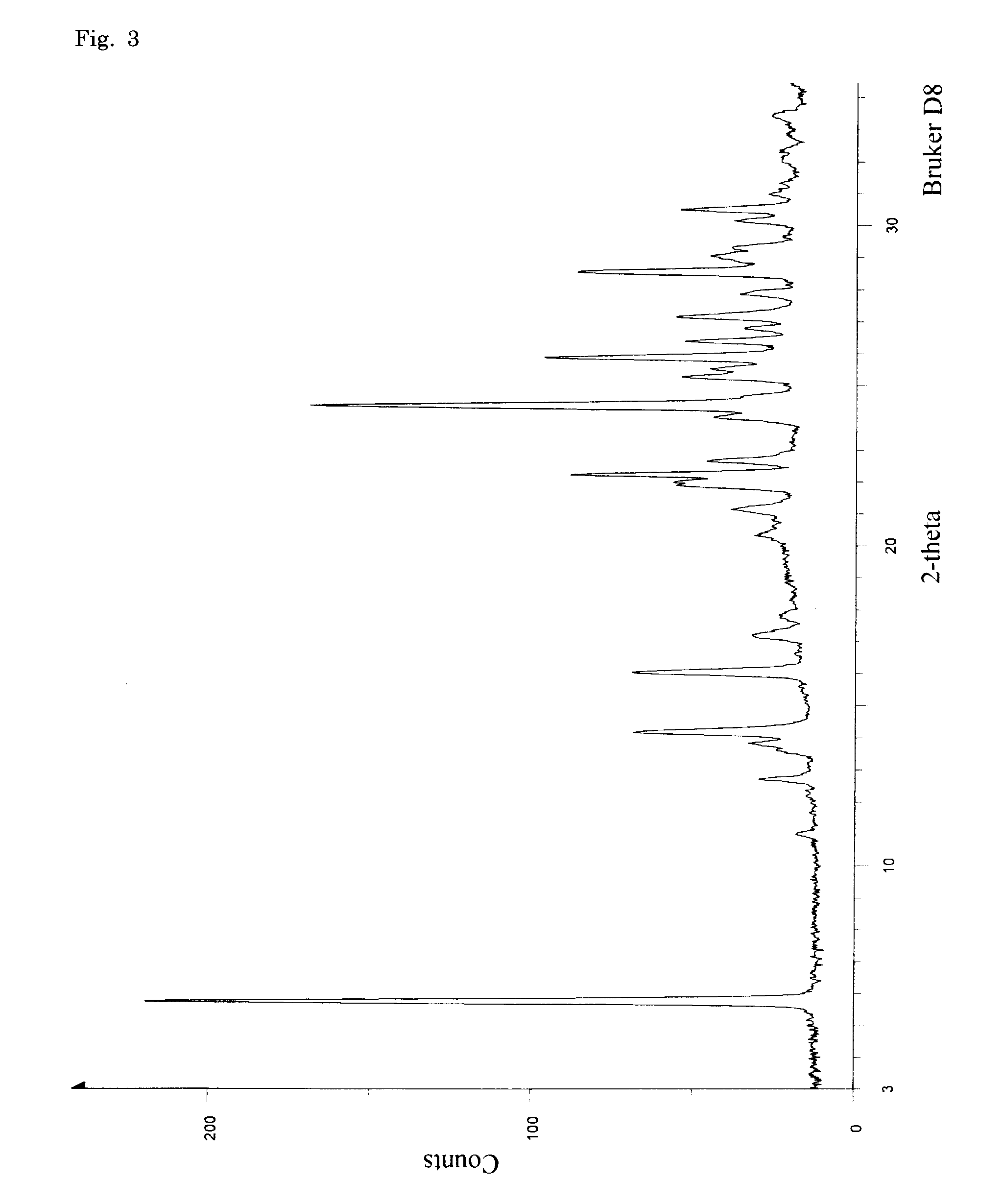
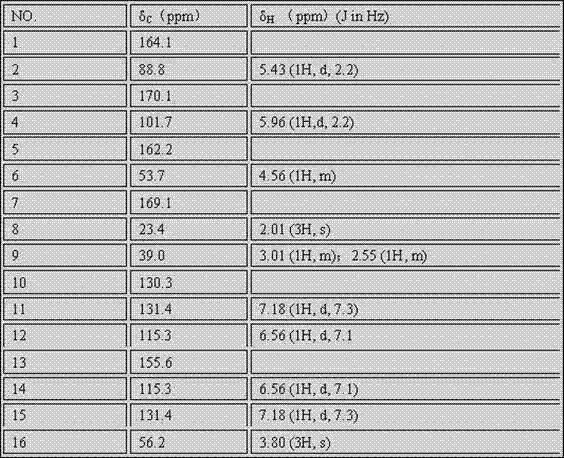
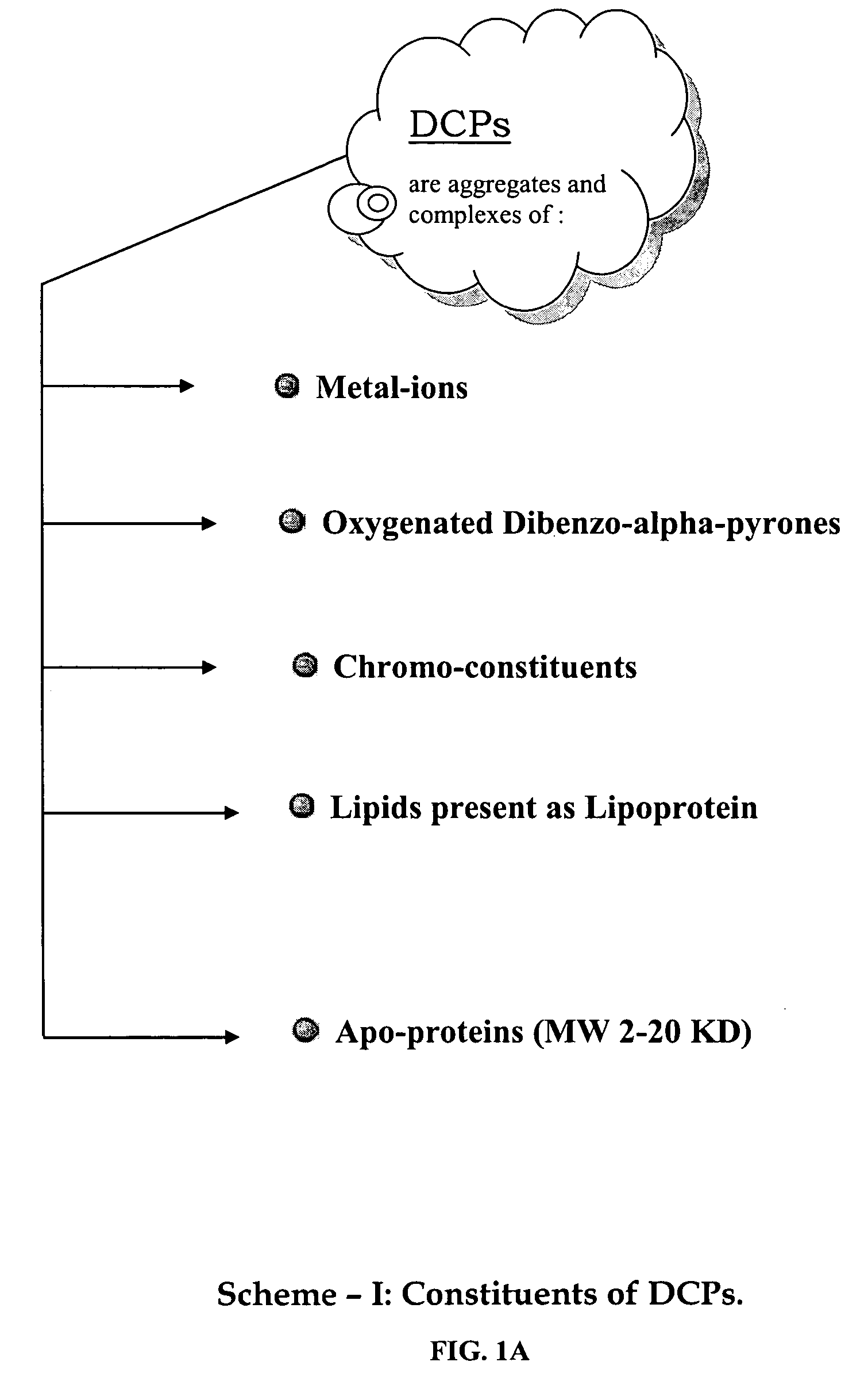
Oxygenated dibenzo-alpha-pyrone chromoproteins
InactiveUS20050245434A1Increasing cognition learningIncrease awarenessCosmetic preparationsHair cosmeticsPersonal careSterol
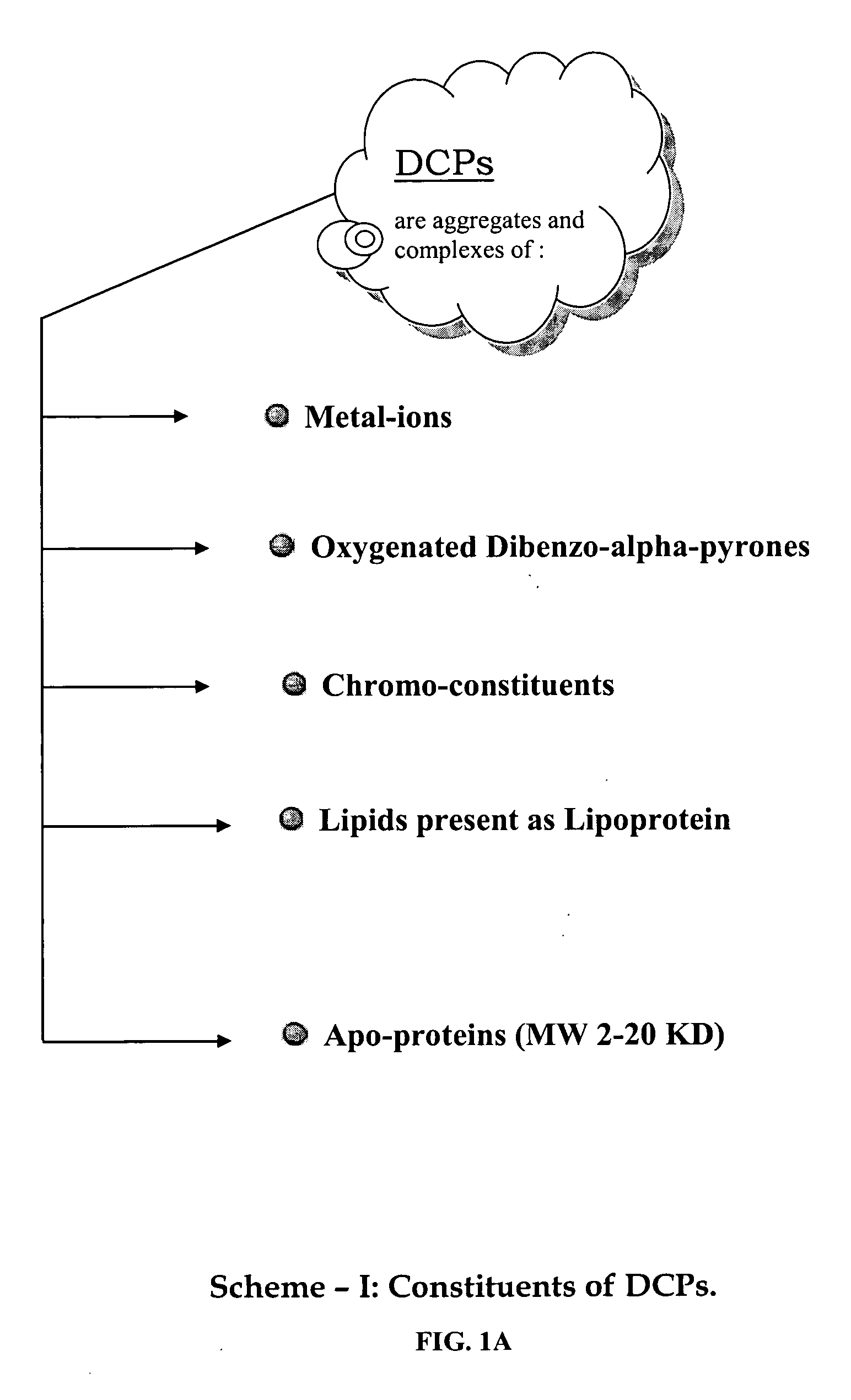
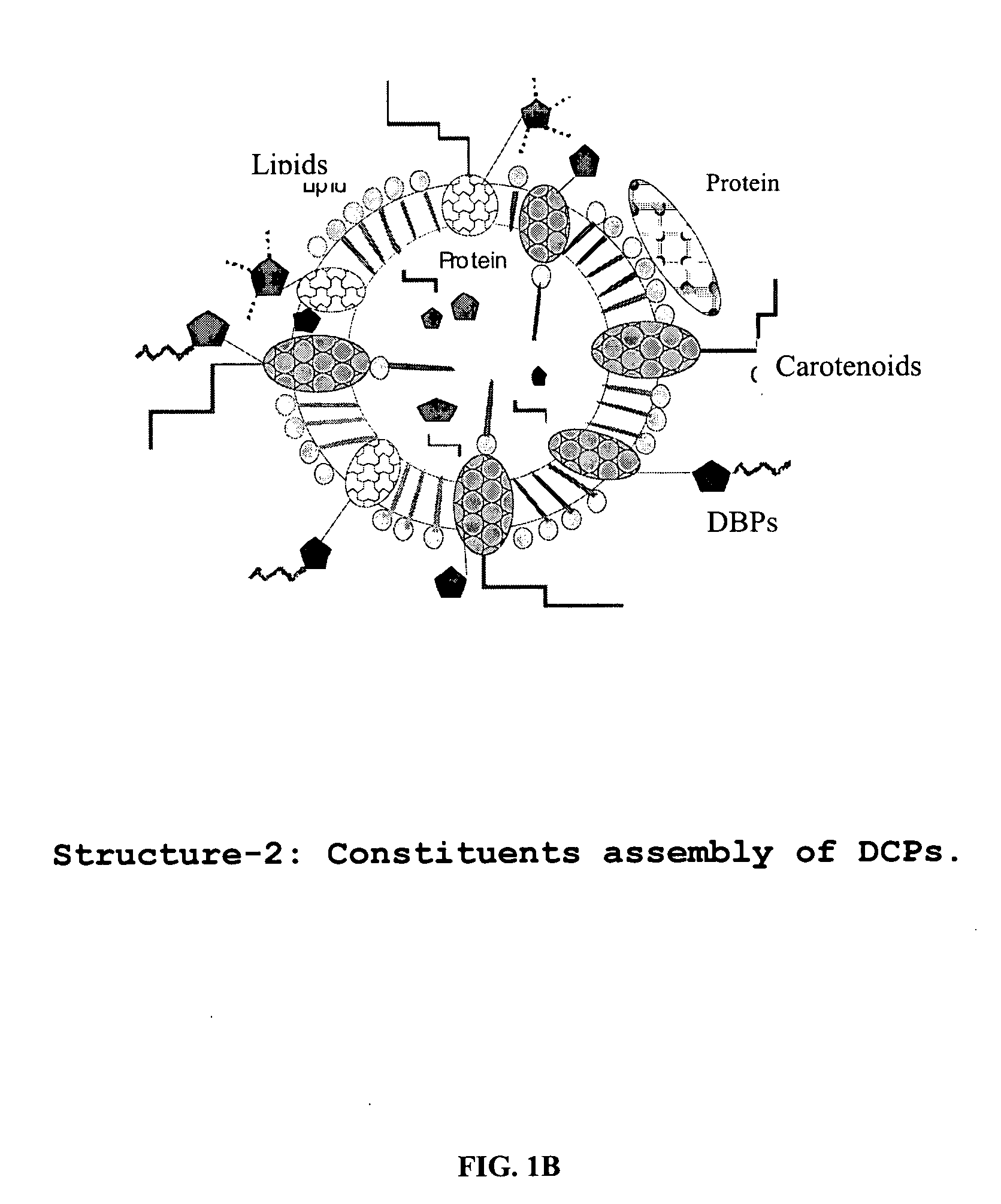
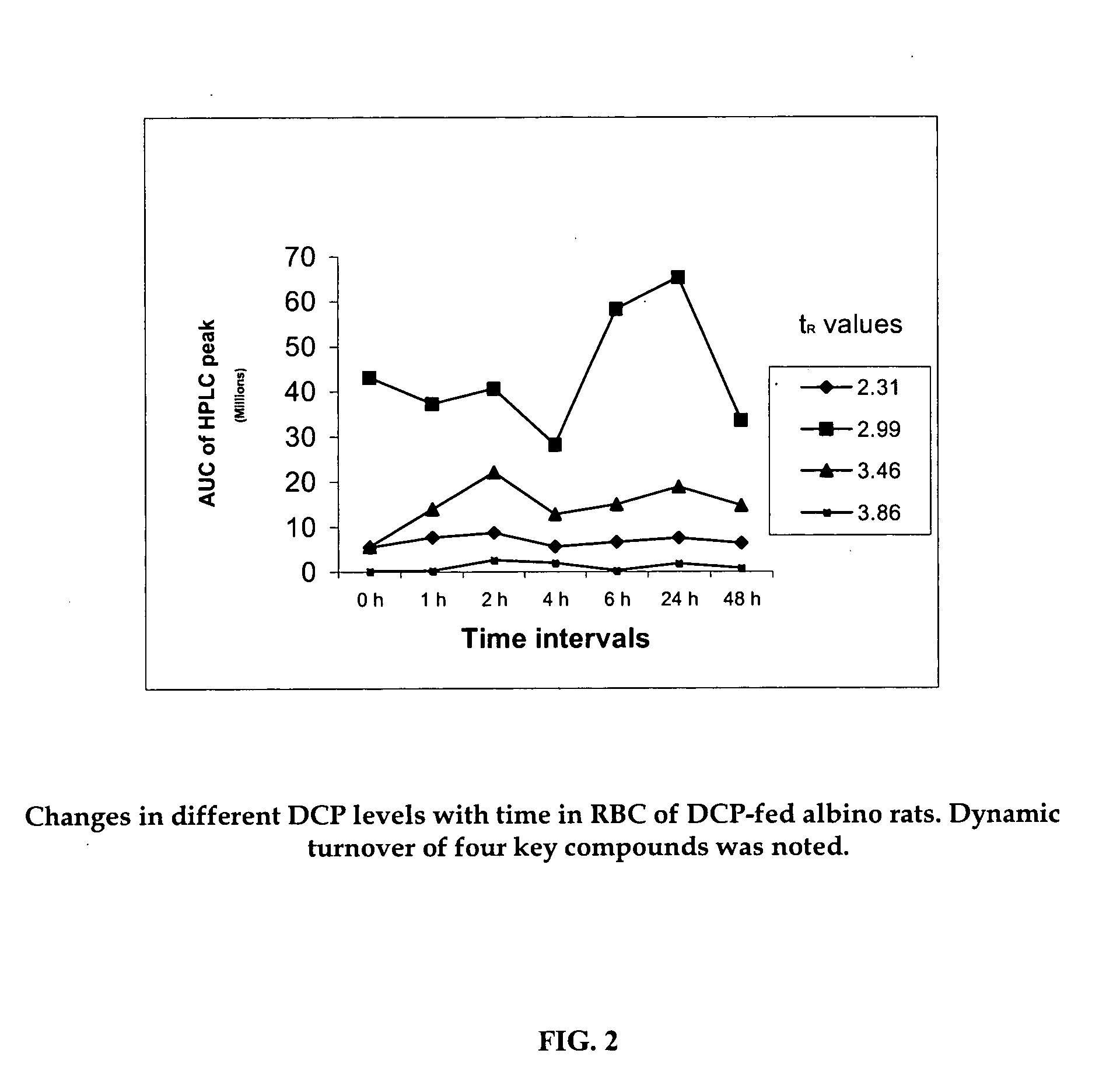
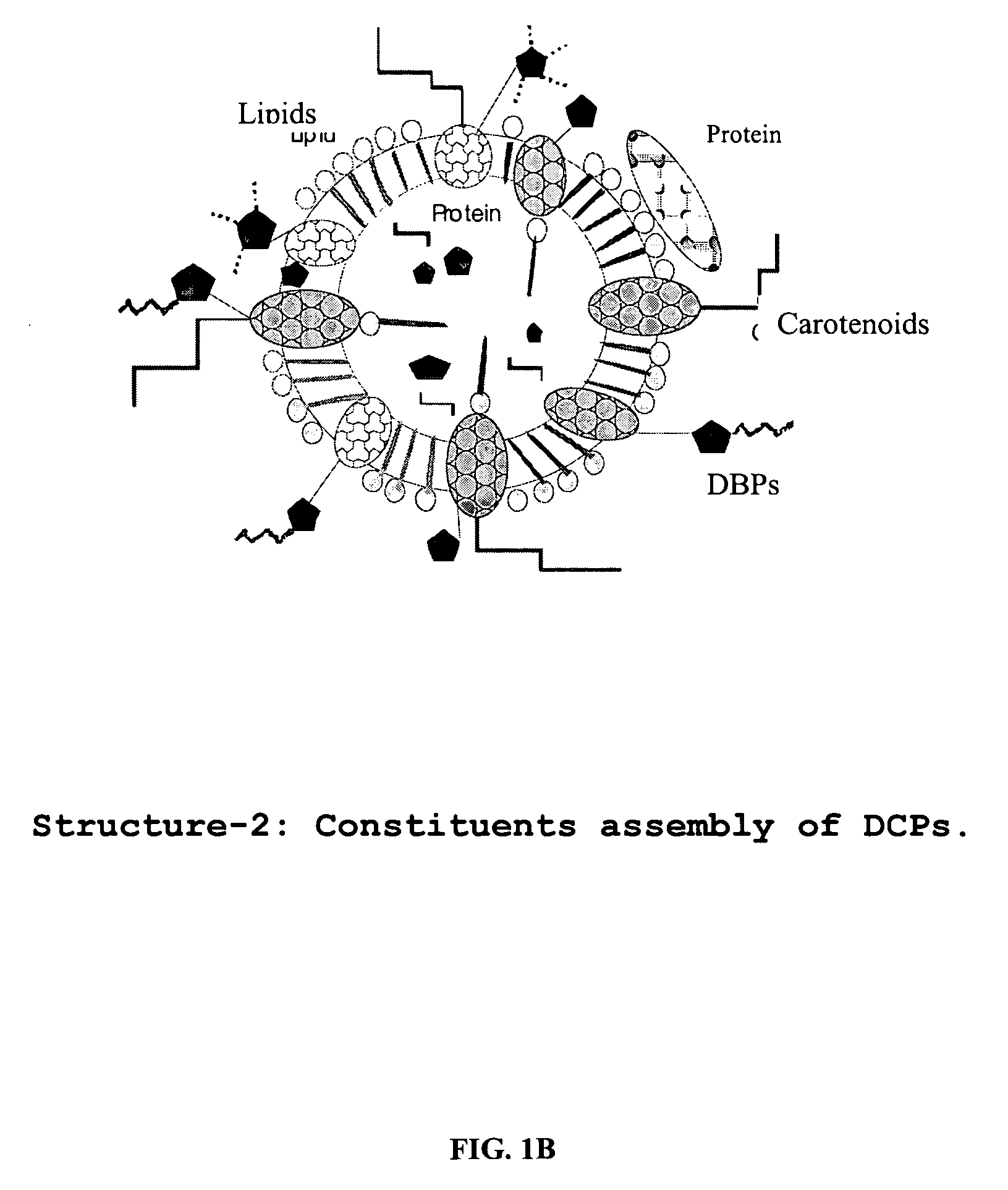
A composition of oxygenated dibenzo-alpha-pyrone chromoproteins (DCP) and their isolation from shilajit, fossils of ammonites, corals and other invertebrates. More particularly, to the description of DCP-composition comprising oxygenated dibenzo-alpha-pyrone or its conjugates, phosphocreatine, proteins, fatty acyl esters of glycerol and other small ligands, e.g., carotenoids, sterols and aromatic acids, as core structural fragments, and their biological functions. Pharmaceutical, nutritional, skin care and personal care formulations are also described. These findings establish DCPs as the major bioactives of shilajit.
Owner:NATREON INC
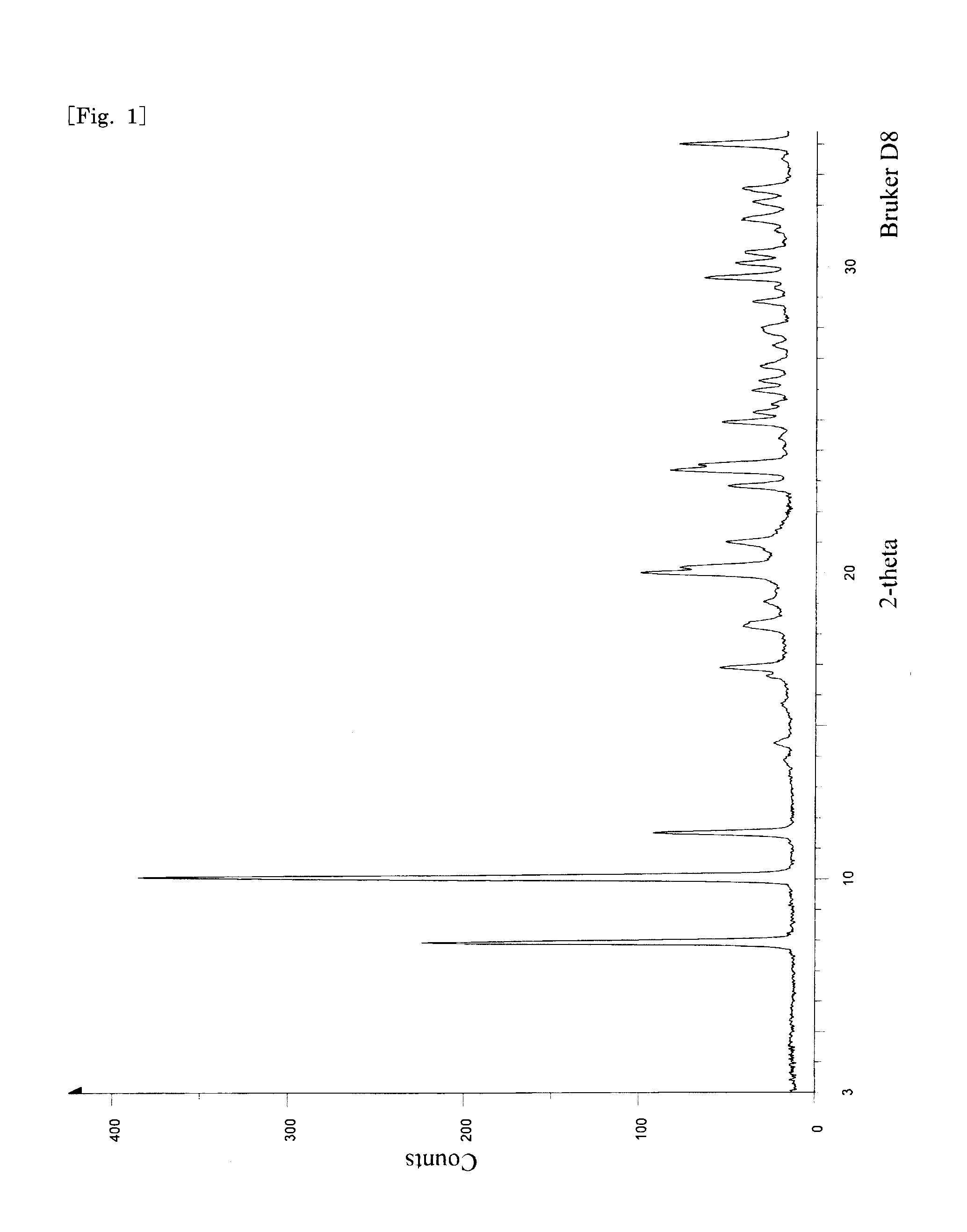
Alpha-pyrone derivative, preparation method and application thereof
ActiveCN106967024AHas anti-Candida albicans effectAntimycoticsOrganic chemistryEthyl acetateSilica gel
The invention discloses an alpha-pyrone derivative, a preparation method and application thereof. The alpha-pyrone derivative is characterized by having a structural formula shown as I. The preparation method includes the steps of: using penicillium dipodomyicola with a preservation number of CCTCC No. M2014087 to conduct fermentation culture so as to obtain a fermentation product containing new alpha-pyrone derivative, then conducting methanol soaking and ethyl acetate extraction on the fermentation product to obtain a crude extract, and carrying out pressure reduction silica gel column chromatography and reversed-phase semi-preparative high performance liquid chromatography separation and purification on the crude extract, thus obtaining the alpha-pyrone derivative. The alpha-pyrone derivative has anti-Candida albicans effect, and can be used as a new pharmaceutical component or lead compound of a Candida albicans inhibitor.
Owner:NINGBO UNIV
Oxygenated dibenzo-alpha-pyrone chromoproteins
InactiveUS20050233942A1Increasing cognition learningIncrease awarenessBiocideNervous disorderPersonal careSterol
A composition of oxygenated dibenzo-alpha-pyrone chromoproteins (DCP) and their isolation from shilajit, fossils of ammonites, corals and other invertebrates. More particularly, to the description of DCP-composition comprising oxygenated dibenzo-alpha-pyrone or its conjugates, phosphocreatine, proteins, fatty acyl esters of glycerol and other small ligands, e.g., carotenoids, sterols and aromatic acids, as core structural fragments, and their biological functions. Pharmaceutical, nutritional, skin care and personal care formulations are also described. These findings establish DCPs as the major bioactives of shilajit.
Owner:NATREON INC
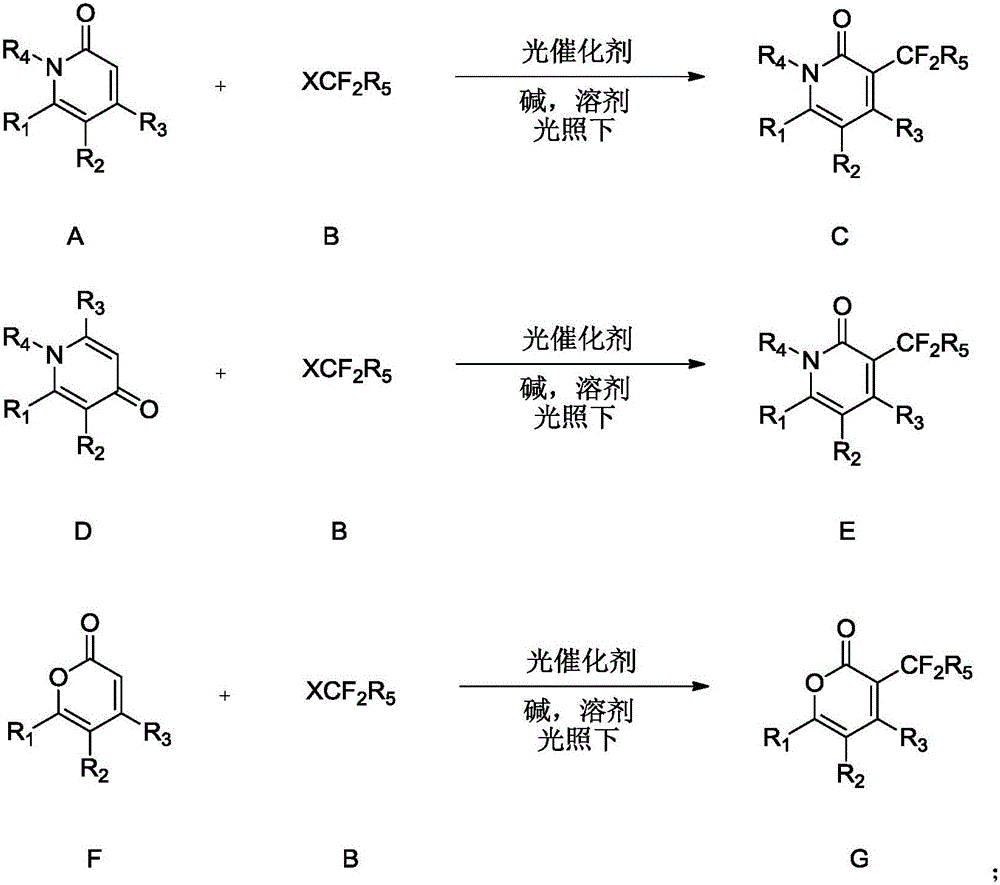
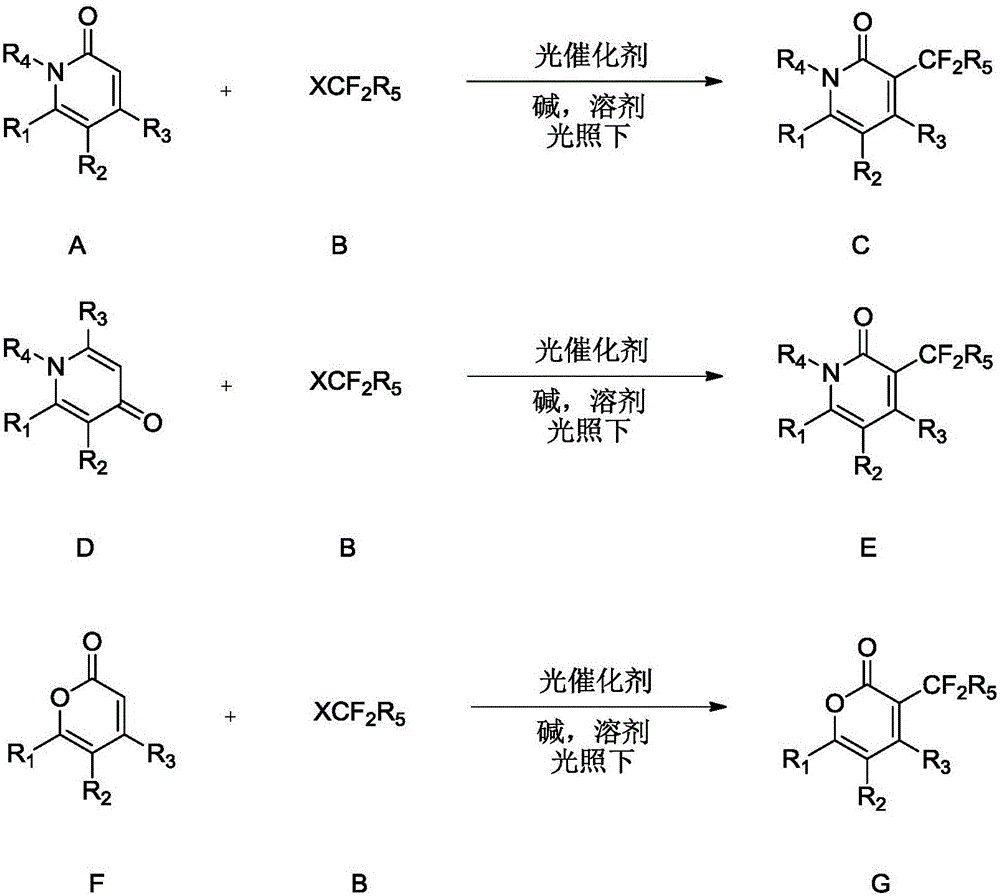
Synthesis method of difluoroalkyl substituted pyridone or pyrone
InactiveCN105669546AShort reaction stepsReact Green ConciseOrganic chemistryIridiumSynthesis methods
The invention discloses a synthesis method of difluoroalkyl substituted pyridone or pyrone under simple conditions.The method specifically comprises the steps that simple pyridine, pyrone and halogenated difluoroalkyl compounds (chloro, bromo and iodo) are used as raw materials, complexes with iridium or ruthenium as cores are used as catalysts, and various kinds of difluoroalkyl substituted pyridone or pyrone are obtained with high efficiency.According to the method, the pyridine, pyrone and halogenated difluoroalkyl compounds (chloro, bromo and iodo) which are easy to obtain are used as the raw materials, and therefore the method has the advantages that the catalyst dosage is small, the substrate application range is wide, operation is easy and convenient, and the reaction efficiency is high.The obtained structure is the important fluorine-containing building blocks at present, and has the wide application prospect in the field of biological medicine.
Owner:ZUNYI MEDICAL UNIVERSITY
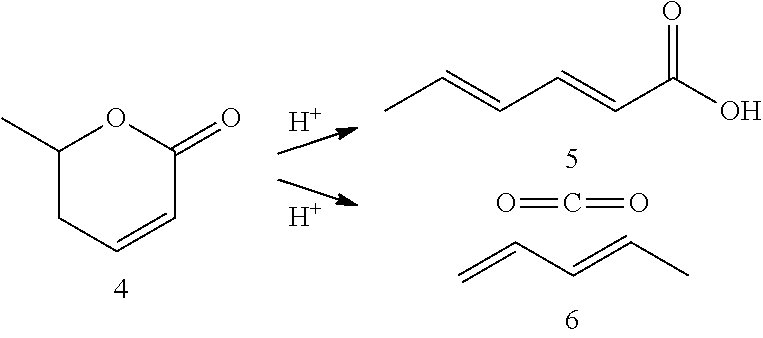
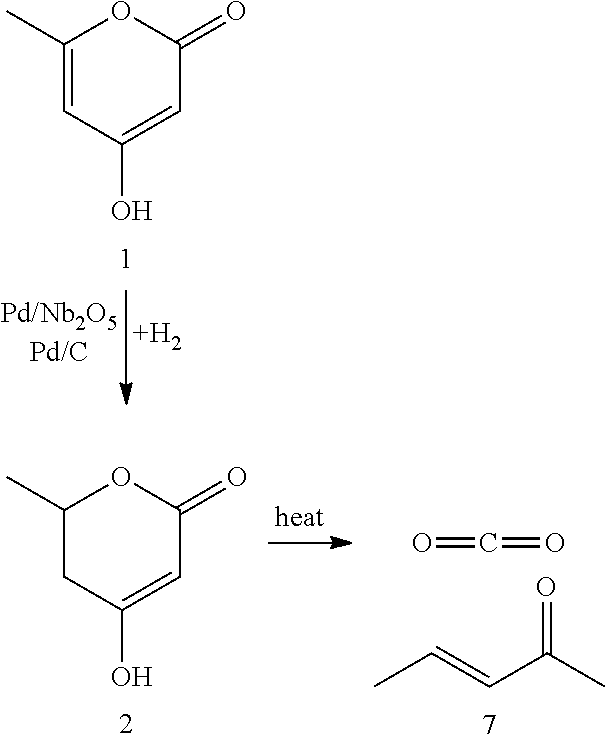
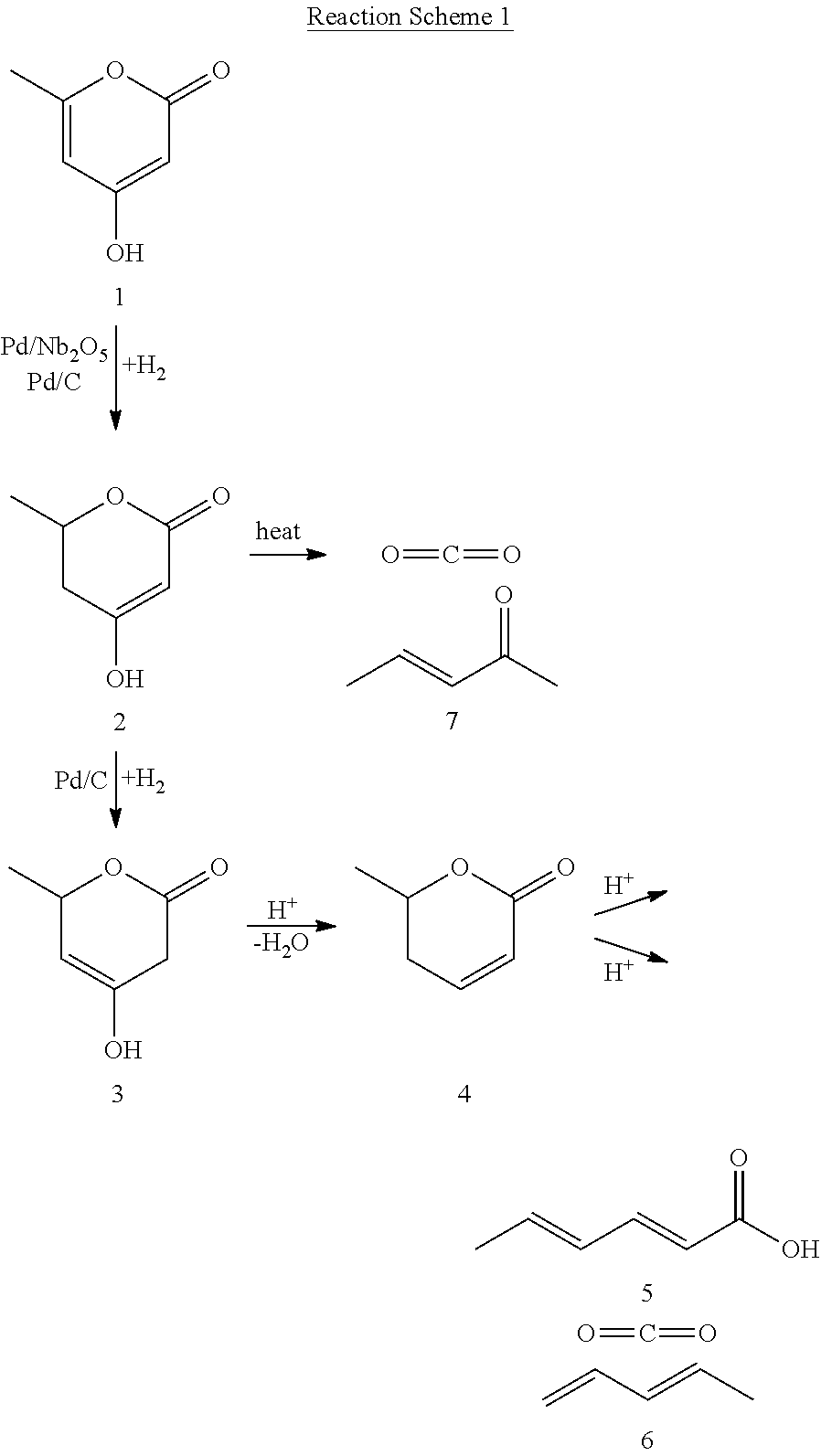
Production of 2,4-hexadienoic acid and 1,3-pentadiene from 6-methyl-5,6-dihydro-2-pyrone
ActiveUS20120116119A1Organic compound preparationPreparation from carboxylic acid esters/lactonesPtru catalystHexadienoic Acid
Described is a method of making sorbic acid, pentadiene, or 3-penten-2-one. The method includes partially hydrogenating 4-hydroxy-6-methyl-2-pyrone (HMP) to yield 5,6-dihydro-4-hydroxy-6-methyl-2H-pyran-2-one (4-DHMMP). Then, if 3-penten-2-one is desired, thermally decomposing the 4-DHMMP to yield 3-penten-2-one. If sorbic acid or pentadiene are desired, the 4-DHMMP is hydrogenated to yield 4-hydroxy-6-methyltetrahydro-2-pyrone (4-HMTHP). The 4-HMTHP is then dehydrated by contacting it with a solid acid catalyst to yield parasorbic acid (PSA). The PSA can then be ring-opened by contacting it with a solid acid catalyst. The reaction conditions of the ring-opening reaction can be controlled to yield sorbic acid and / or pentadiene.
Owner:WISCONSIN ALUMNI RES FOUND
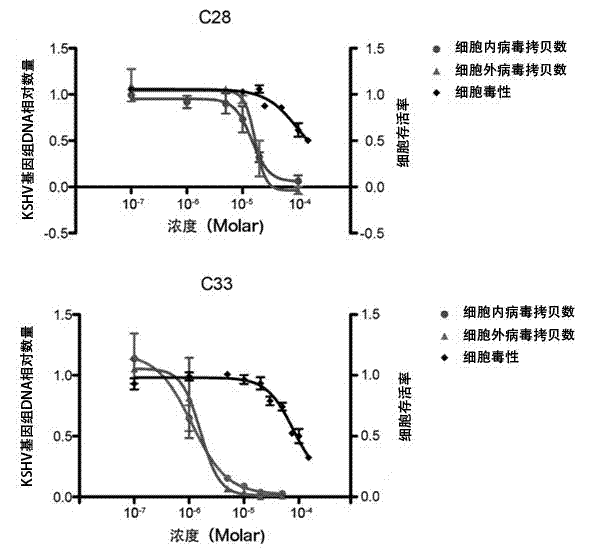
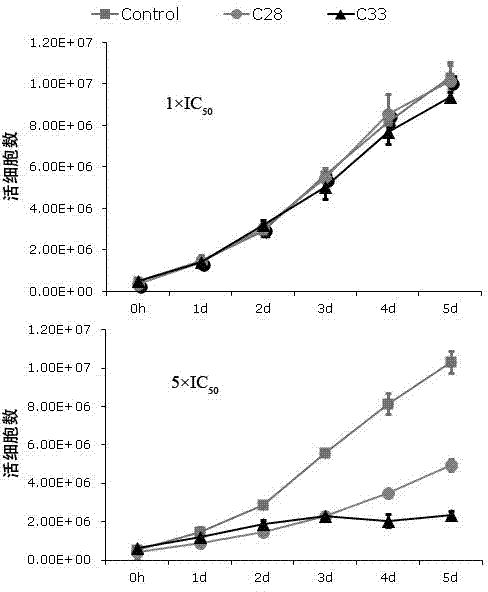
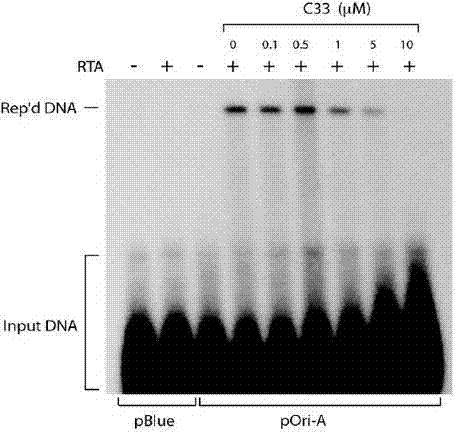
Application of benzo Alpha-pyrone compound as Gamma-type human herpes virus-resistant medicine
ActiveCN103070858AStrong inhibitory activityLow toxicityAntiviralsHeterocyclic compound active ingredientsDiseaseNovobiocin
The invention discloses an application of a benzo Alpha-pyrone compound as a Gamma-type human herpes virus-resistant medicine. According to the invention, the compound resistant to Gamma-type human herpes virus infection is higher in activity as compared with a novobiocin which is a marketed medicine, and is less in toxicity based on a cytoxicity test. The compound can be used for curing or preventing related diseases caused by KSHV infection.
Owner:SUN YAT SEN UNIV
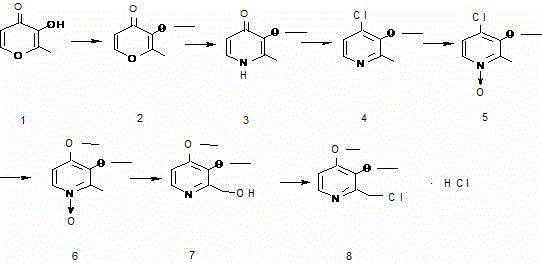
Preparation method of pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride
The invention belongs to the technical field of medicine, and particularly relates to a preparation method of a pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride. The preparation method comprises the following steps: using 3-hydroxyl-2-methyl-4-pyrone as a starting raw material, and then only performing five-step reaction to obtain the pantoprazole intermediate 2-chloromethyl-3,4-dimethoxy pyridine hydrochloride. The preparation method reduces the reaction steps, shortens the reaction cycle, improves the working efficiency, and increases the yield coefficient.
Owner:SHOUGUANG FUKANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com