Patents
Literature
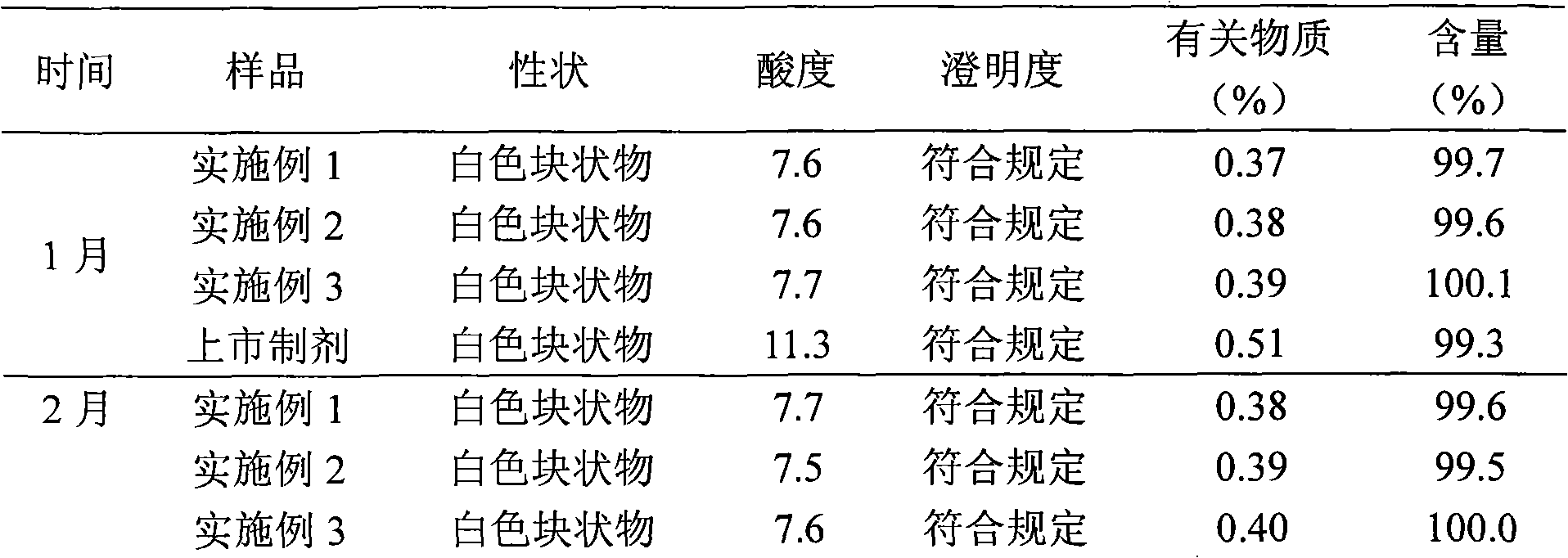
223 results about "Pantoprazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pantoprazole is used to treat certain stomach and esophagus problems (such as acid reflux).
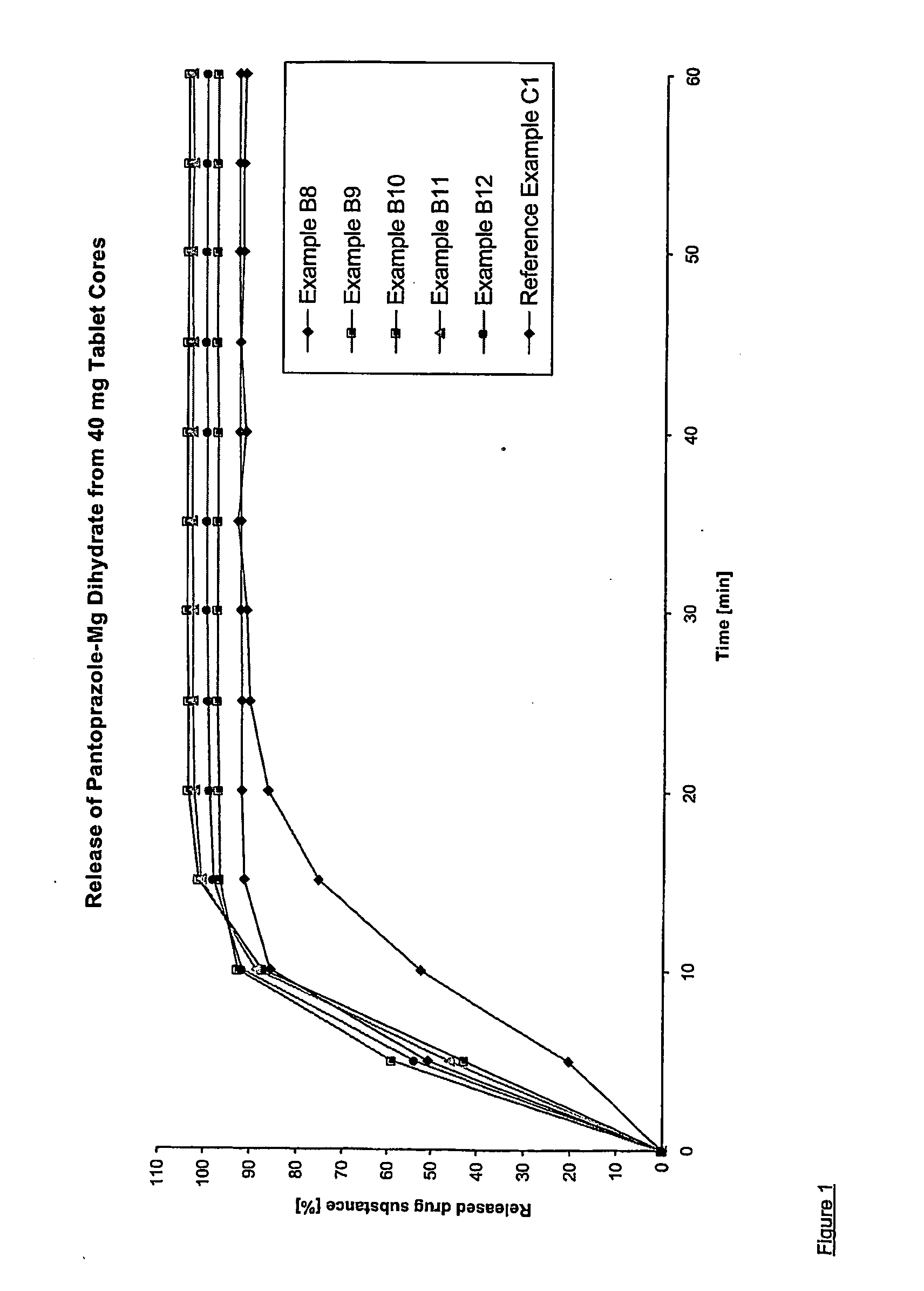
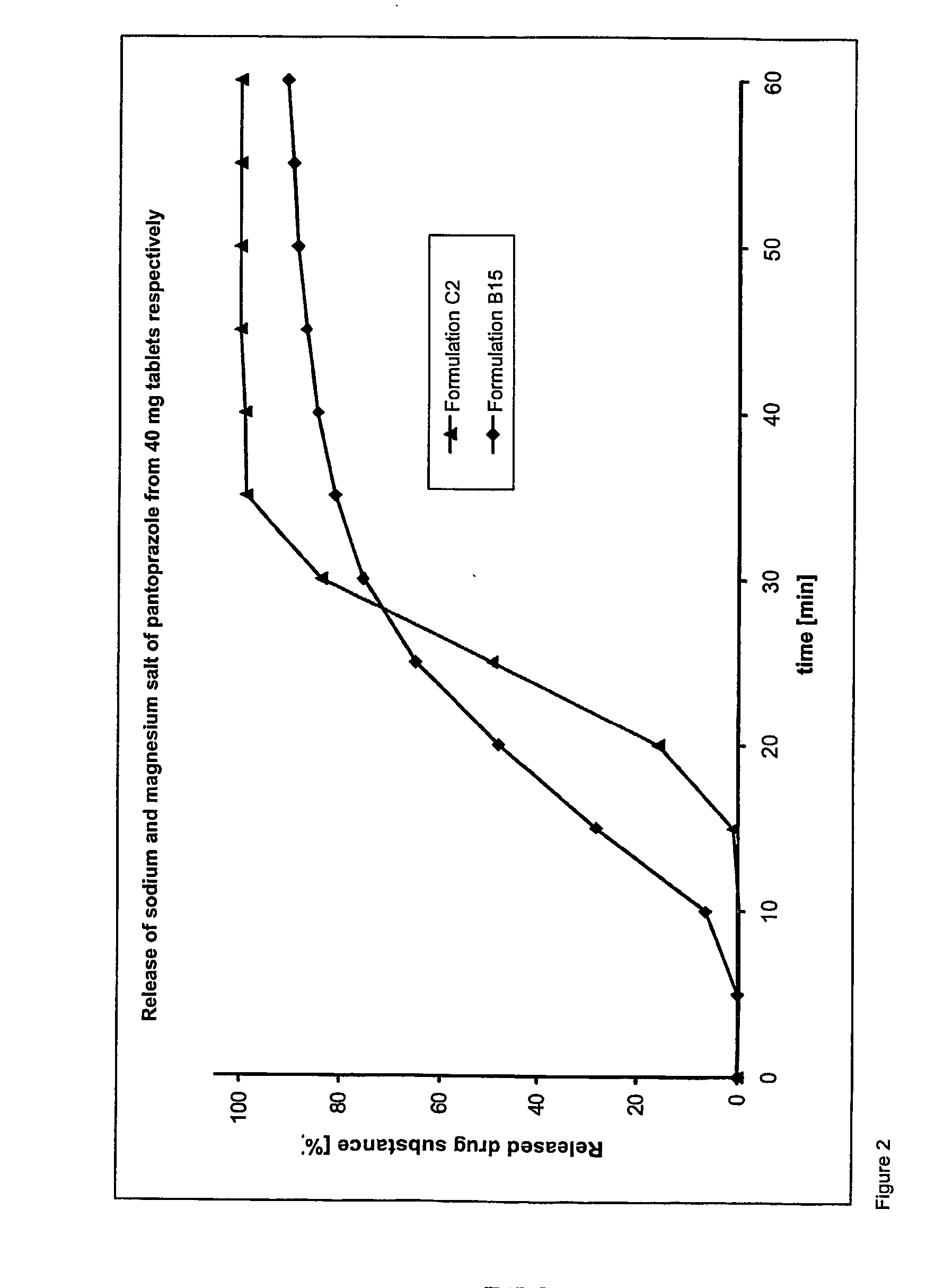
Oral pharmaceutical composition with delayed release of active ingredient for pantoprazole
An oral pharmaceutical composition comprises an acid-labile irreversible proton pump inhibitor in pellet or tablet form, wherein the irreversible proton pump inhibitor is at least partly in slow-release form. On combined administration with an anti-microbially-active ingredient, the composition is distinguished by imparting an enhanced action of rapid onset against disorders caused by Helicobacter.
Owner:TAKEDA GMBH
Salt form of pantoprazole
Owner:TAKEDA GMBH
Pantoprazole multiparticulate formulations
ActiveUS20050129761A1Low variabilityProlong the action timeBiocideDispersion deliveryMedicineEnantiomer
Pantoprazole sodium multiparticulates are described which avoid sticking to nasogastric and gastronomy tubes. The pantoprazole multiparticulates have a spheroid core of pantoprazole or an enantiomer thereof, or a salt thereof, a surfactant, and a distintegrant; a sub coat which is comprised of hydroxypropyl methylcellulose (hypromellose) and water, an enteric coat on the sub-coat, and a final seal coat over the enteric coat, which is composed of hydroxypropyl methylcellulose (hypromellose) and water.
Owner:WYETH LLC
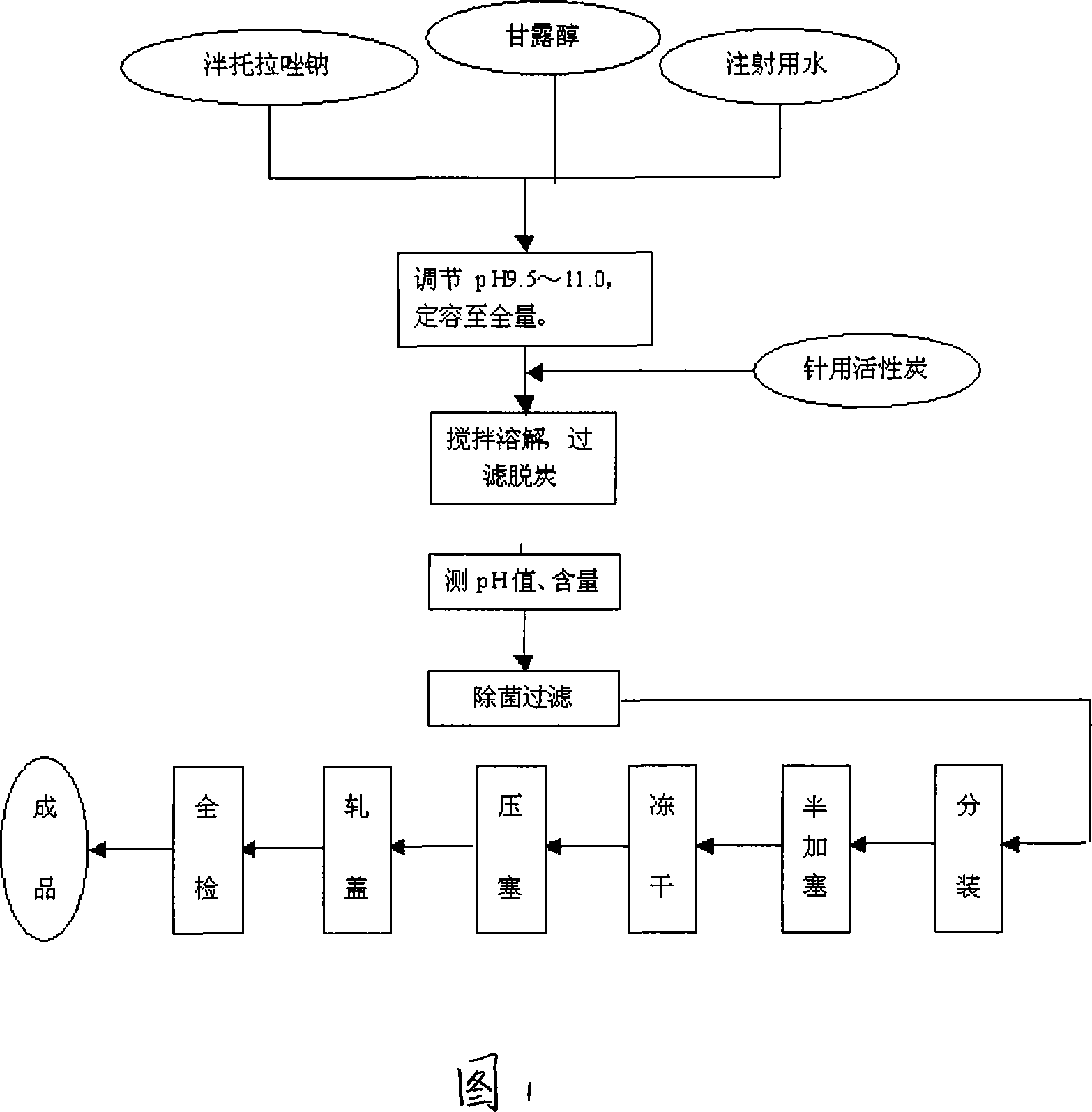
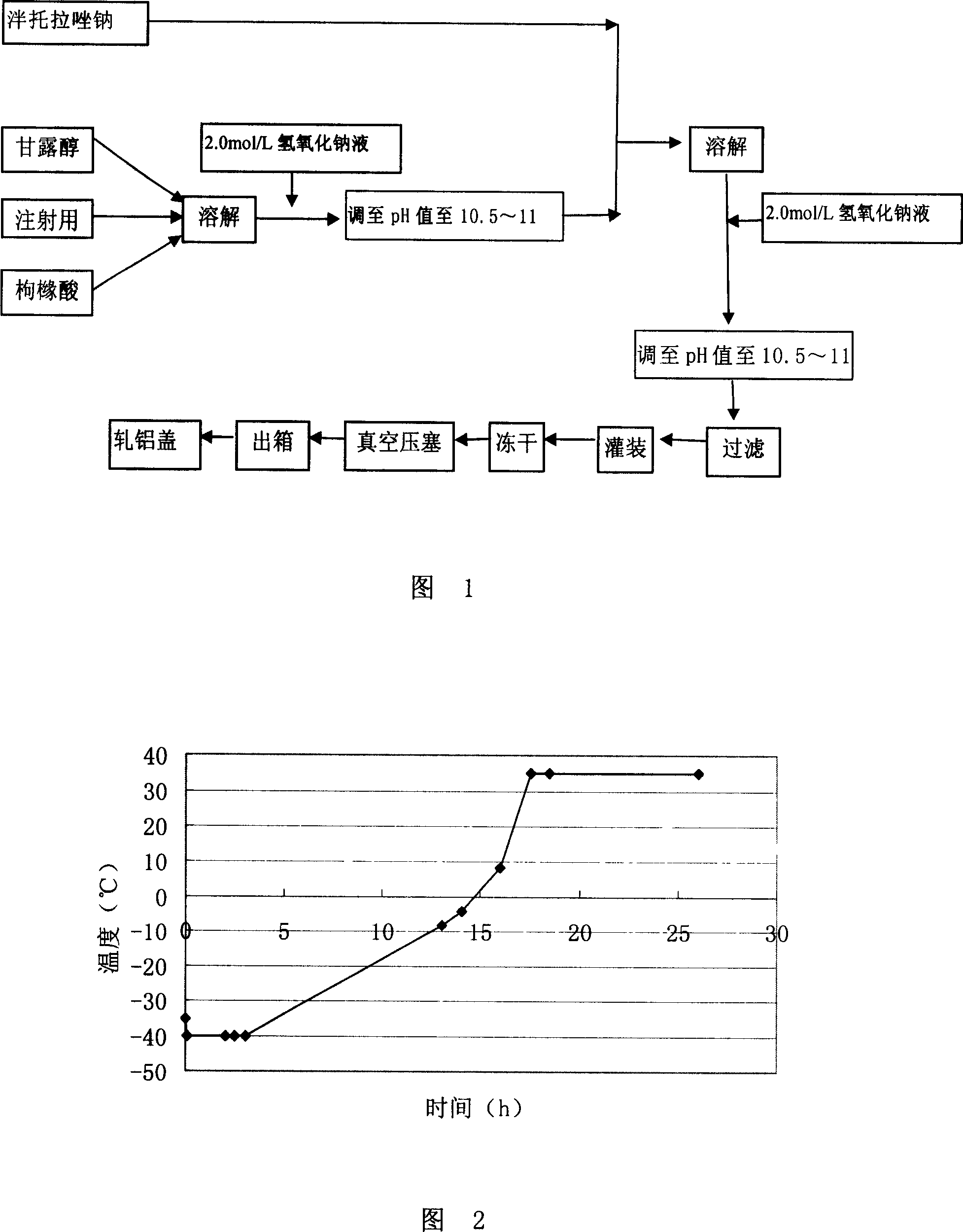
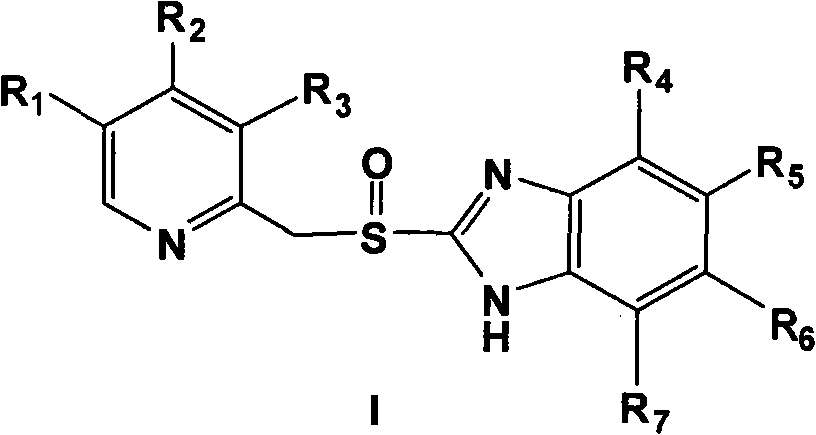
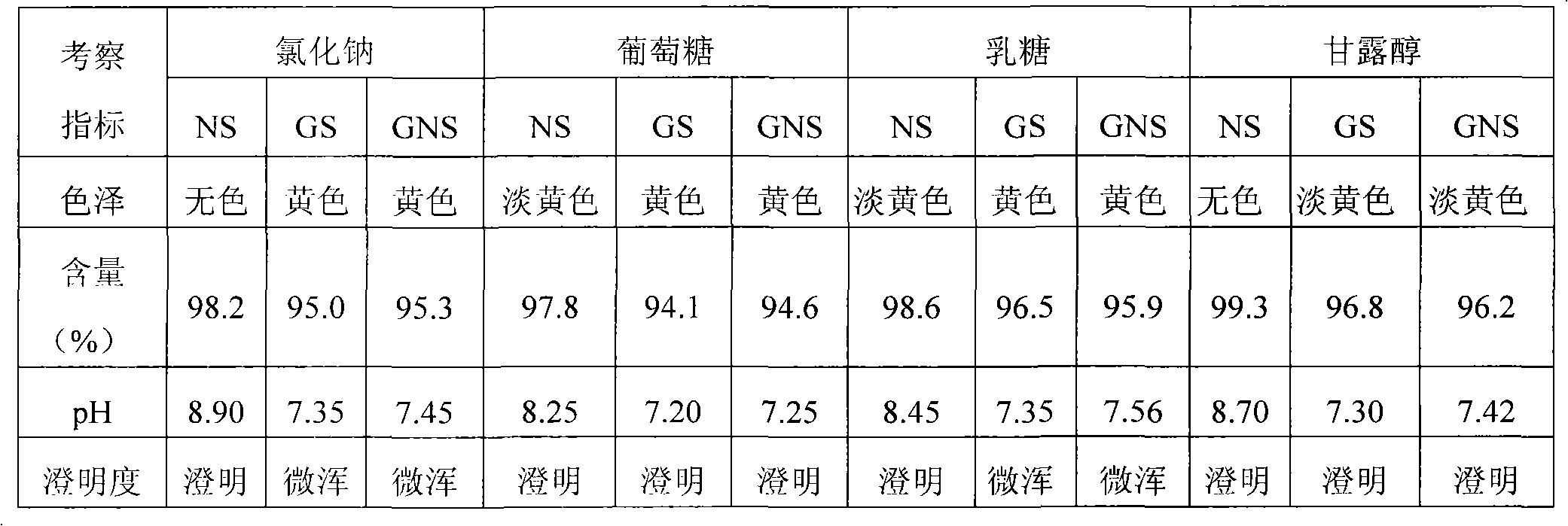
Pantoprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229138ASimple recipeLittle side effectsPowder deliveryOrganic active ingredientsSolubilityMANNITOL/SORBITOL
The invention aims at providing a pantoprazole sodium freeze-dried powder injection and comprises pantoprazole sodium and mannitol with the weight ratio of 1: 2 to 5. The invention is simple in formula and little in side effect; products prepared by the method are plump in appearance, good in complex solubility and excellent in quality with the adoption of an advanced freezing and drying process.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Pantoprazole sodium freeze dried injection and preparation method thereof
InactiveCN101011397AReduce dosageIncrease dosagePowder deliveryOrganic active ingredientsInorganic saltsFreeze-drying
The invention relates to a method for preparing batoracosodium freeze dried, whose pH value is 9.5-11.5. And the invention comprises 1 deal of batoracosodium, 0.5-1 deals of supporting agent, 0-0.06 deals of weak-acid strong-alkali salt, and some inorganic alkali. And the preparation comprises that 1, preparing materials; 2, dissolving the supporting agent and weak-acid strong-alkali salt via injection water, using inorganic salt to adjust the pH value to 9.5-11.5, adding batoracosodium, dissolving and using inorganic salt to adjust the pH value to 9.5-11.5; 3, filtering; 4, freezing and drying to obtain the final product. The invention can be used treat peptic ulcer, ulcer bleed, or the like.
Owner:LIVZON PHARM GRP INC
Freeze-dried powder injection of pantoprazole sodium and its preparation
ActiveCN1679563ALittle side effectsImprove stabilityOrganic active ingredientsPowder deliveryDisodium EdetateFreeze-drying
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +1
Novel method for preparing chiral sulphoxide compound
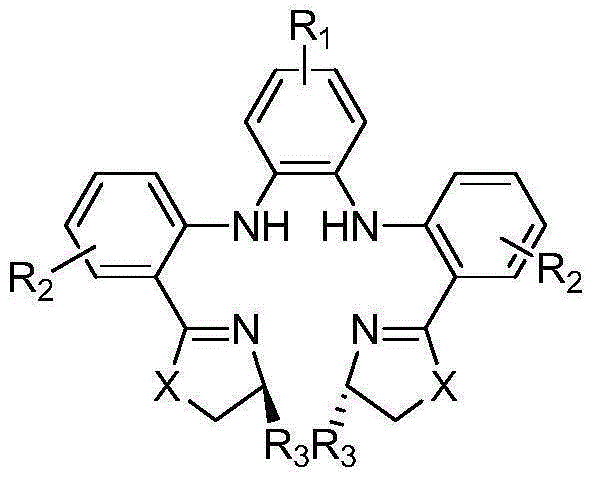
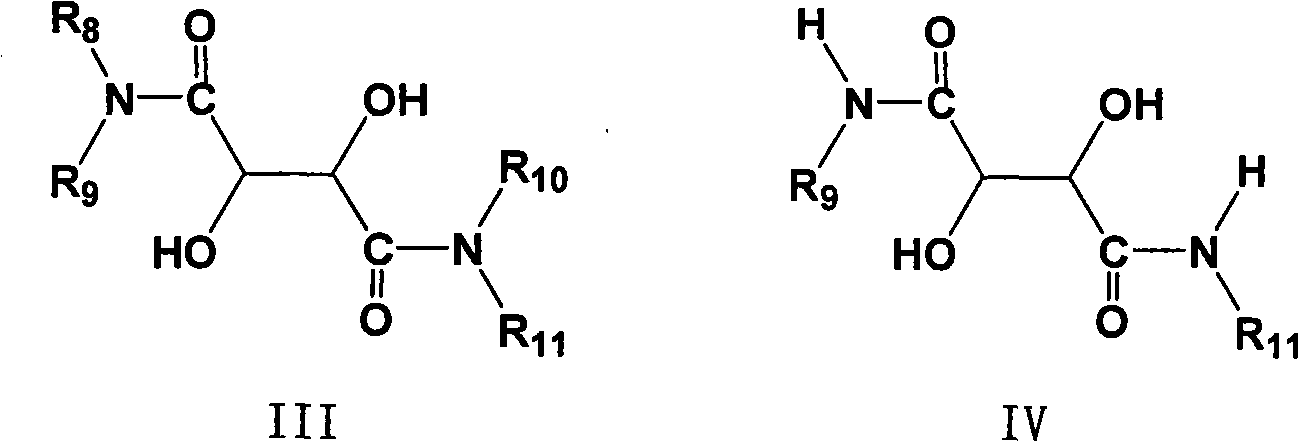
The invention provides a novel method for preparing optically pure substituted [(pyridyl methylene) sulfinyl]-1H-benzimidazole sulphoxide compound by enantioselective synthesis. The method requiring protection is to directly and asymmetrically oxidize prochiral thioether into a corresponding optically pure sulphoxide compound or a sulphoxide compound rich in single enantiomer by a mild and cheap oxidizing agent in the presence of a complex compound catalyst formed by an accessible and stable (+)- or (-)- tartaric acid diamide ligand shown in a general formula and titanium. Therefore, optically pure omeprazole, lansoprazole and pantoprazole can be obtained, wherein R8, R9, R10 and R11 are the same or different, and are selected from hydrogen, alkyl, aralkyl, aryl, organic polymers or a silica loading body.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI +1
Dosage form containing (s)-pantoprazole as active ingredient
Owner:ALTANA PHARMA
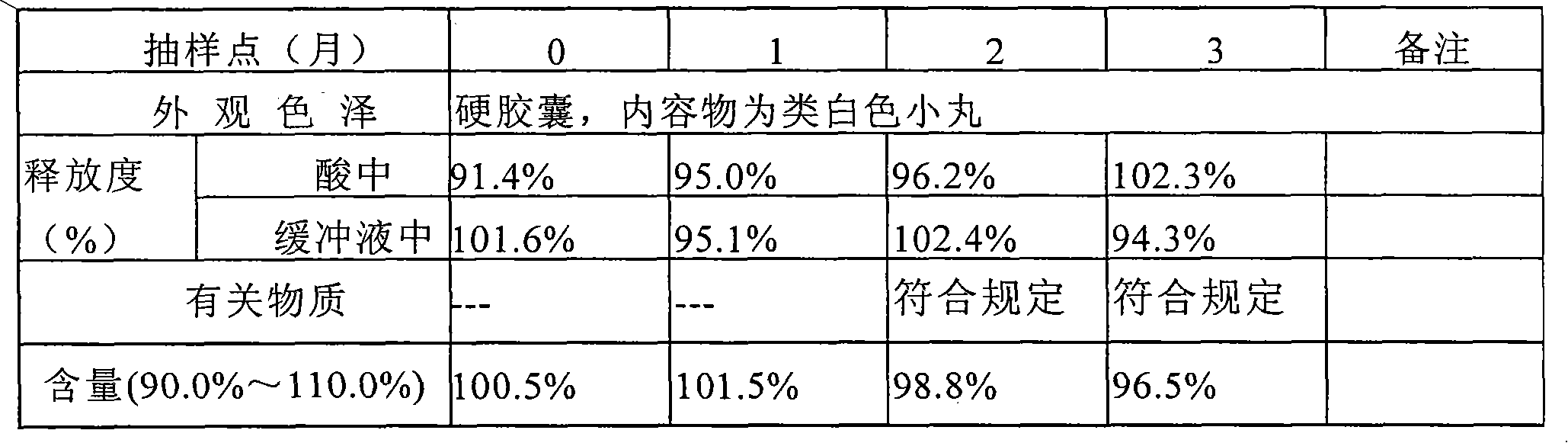
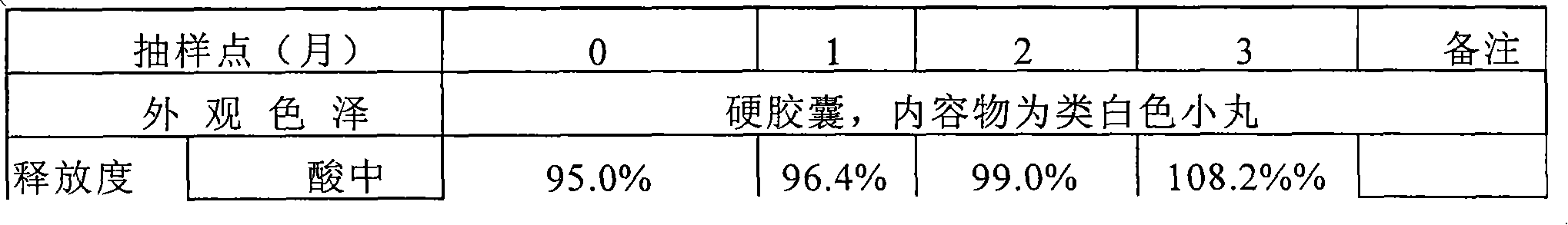
Pantoprazole sodium enteric tablet and preparation method thereof
InactiveCN101461809AOne-sided bright and tidyQuality improvementOrganic active ingredientsDigestive systemPantoprazole SodiumSilicon dioxide
The invention relates to a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is prepared by a pantoprazole sodium plain tablet which is coated with an insulating layer and an enteric coating layer, and the pantoprazole sodium plain tablet contains 0.5 to 5 percent of silicon dioxide and 0.5 to 5 percent of talcum powder. The method solves the tablet pressing problem of extremely easy sticking during the pressing of the pantoprazole sodium plain tablet.
Owner:YAOPHARMA CO LTD
Pantoprazole sodium enteric-coated pellet
ActiveCN101596165AImprove stabilityAbsorb evenlyOrganic active ingredientsDigestive systemBioavailabilityPantoprazole Sodium
The invention discloses a pantoprazole sodium enteric-coated pellet which contains the following components in percentage by weight from inside to outside: 20-60 percent of blank core pellet, 4-38 percent of medicinal layer containing pantoprazole sodium and one or more medicinal excipients, 2-15 percent of isolated layer and 15-26 percent of enteric-coated layer. The invention also discloses a preparation method and an application of the enteric-coated pantoprazole sodium pellet. The pantoprazole sodium enteric-coated capsule has the advantages of better stability, uniform absorption, smaller difference of bioavailability among individuals, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
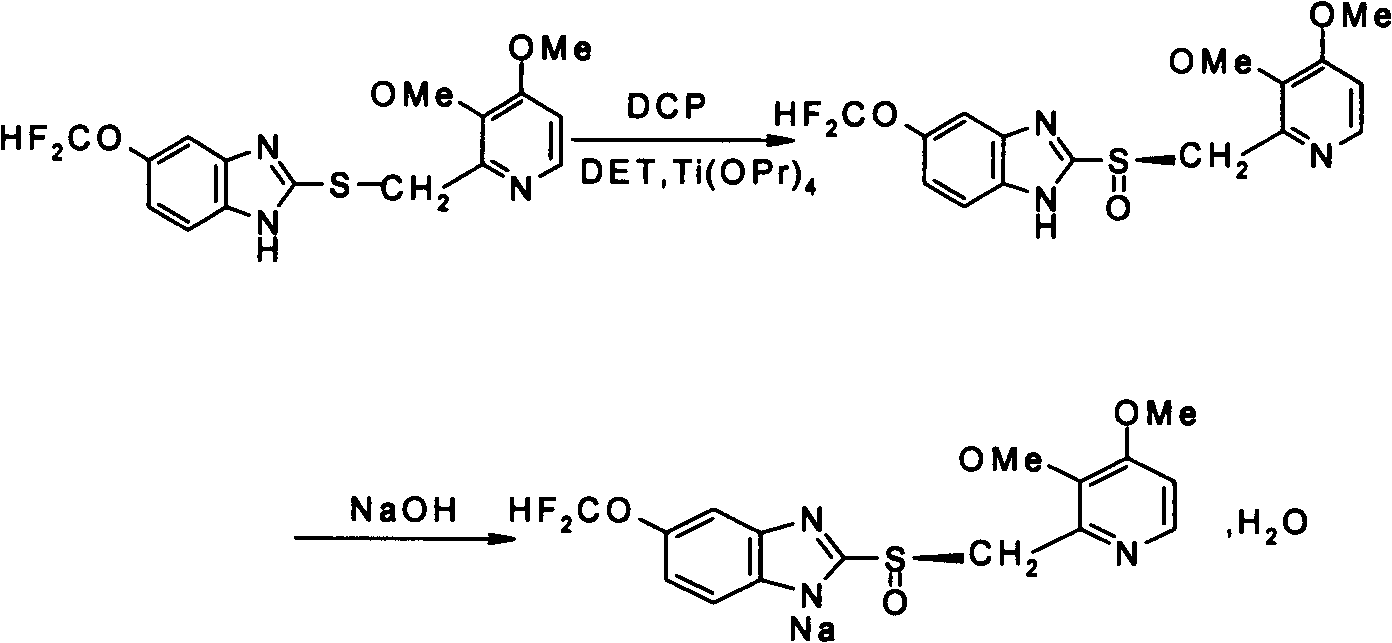
Method for preparing and purifying (L)-pantoprazole sodium
The invention provides a method for preparing (L)-pantoprazole sodium, which comprises the following steps of: oxidizing 5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]thio}-1H-benzimidazole by using 3,5-diisopropylbenzene hydroperoxide under the catalysis of a tetraisopropyl titanate, D-(-)-diethyl tartrate and N,N-diisopropylethylamine system to obtain S-(-)-5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole, namely (L)-pantoprazole, refining the (L)-pantoprazole, and preparing a salt to obtain the (L)-pantoprazole sodium.
Owner:HC SYNTHETIC PHARMA CO LTD
Dosage form containing pantoprazole as active ingredient
InactiveUS20060240100A1Without great technical complexityEnhance the imageBiocideDigestive systemOral medicationMagnesium salt
Owner:NYCOMED GMBH
Pantoprazole sodium freeze-drying medicinal composition for injection and preparation method thereof
ActiveCN101810588AImprove stabilityEliminate side effectsPowder deliveryOrganic active ingredientsFreeze-dryingSulfite salt
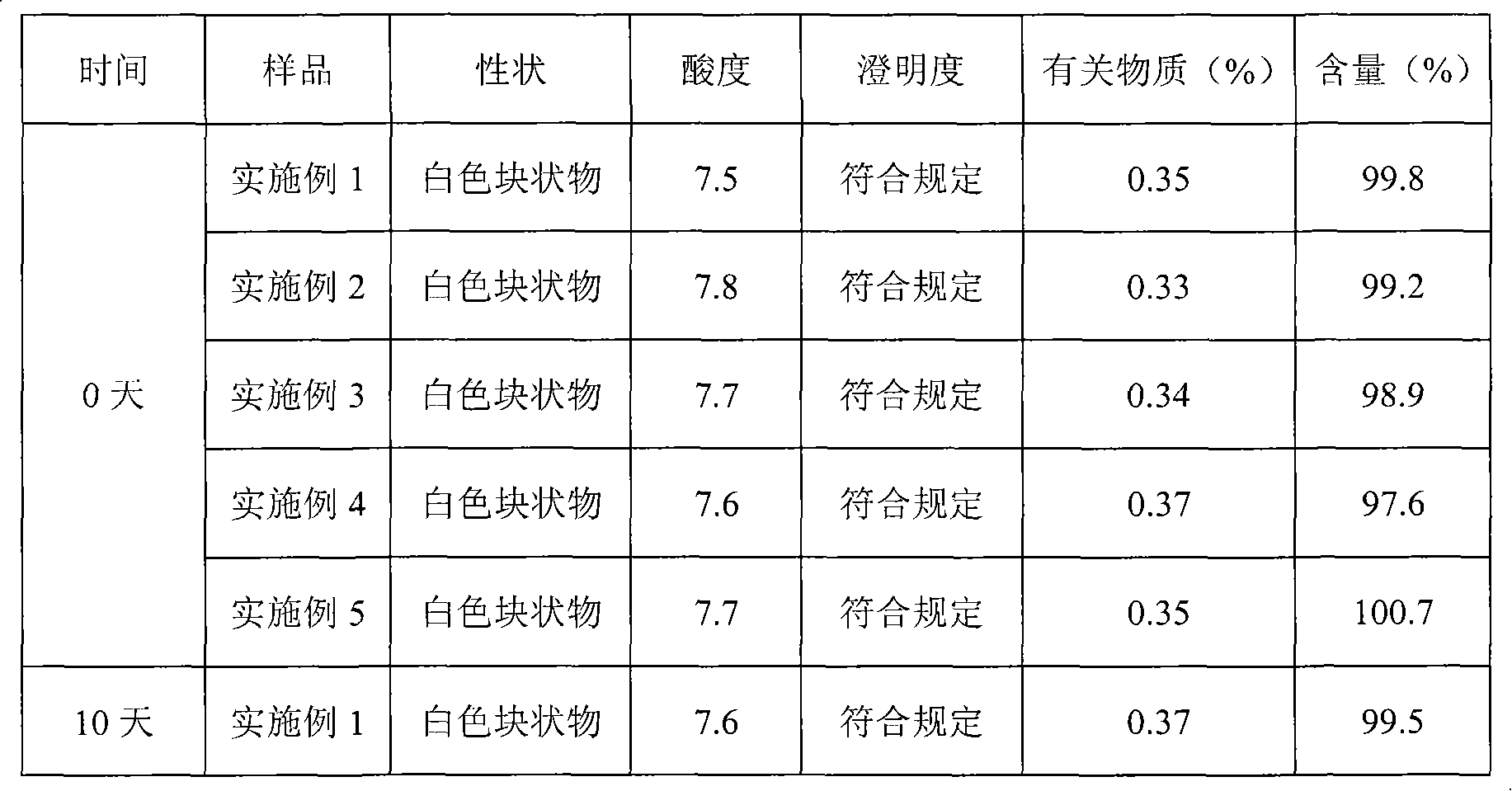
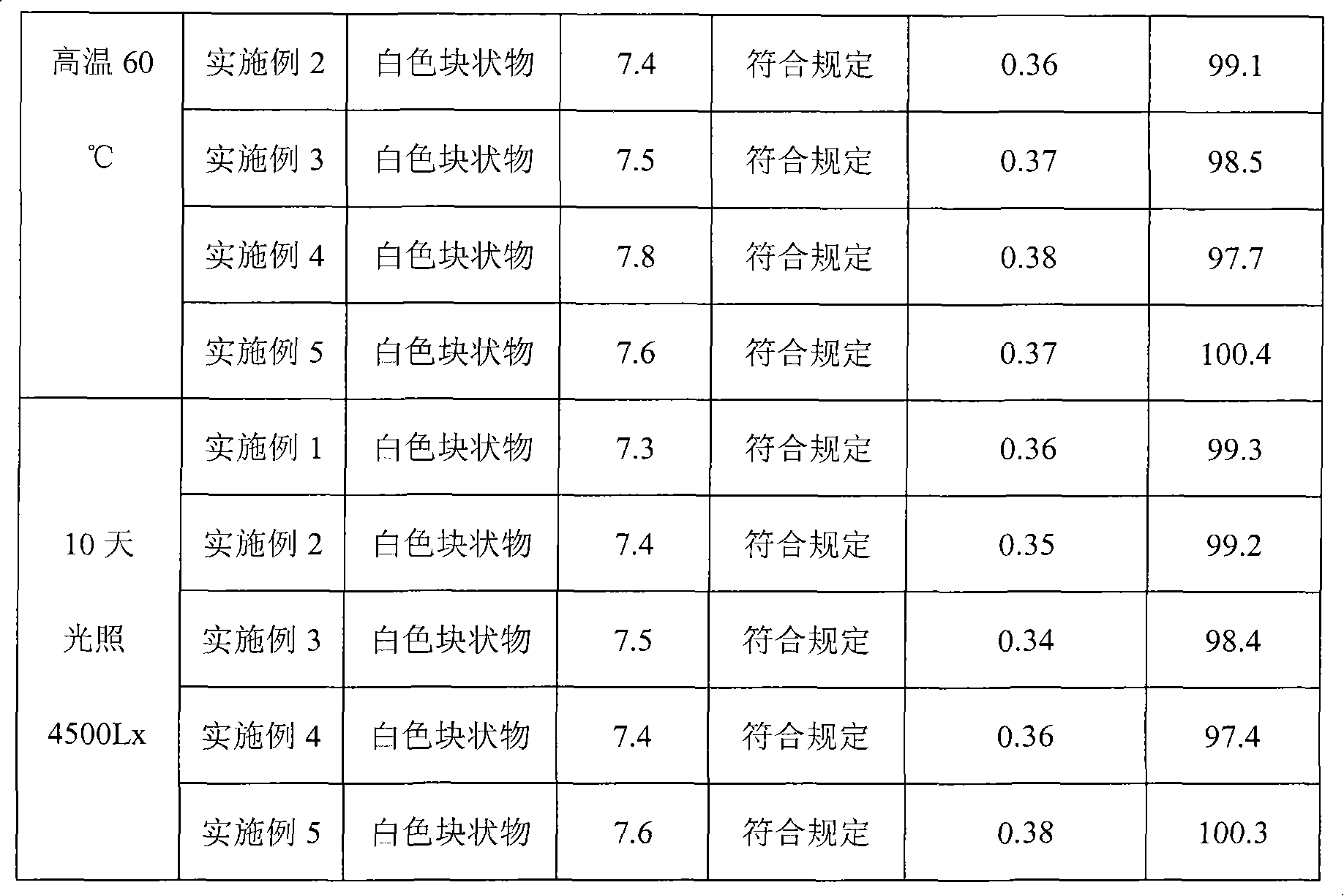
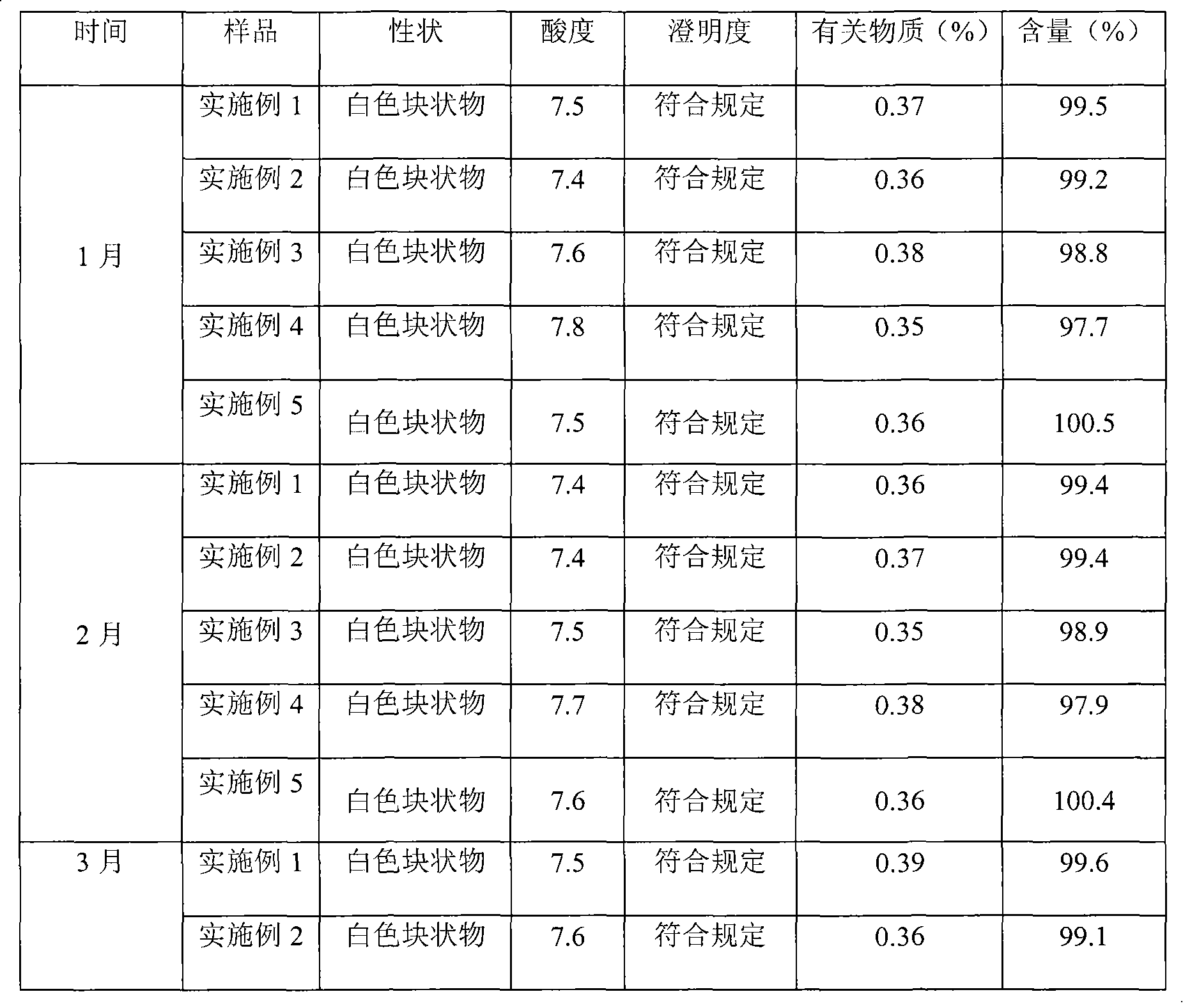
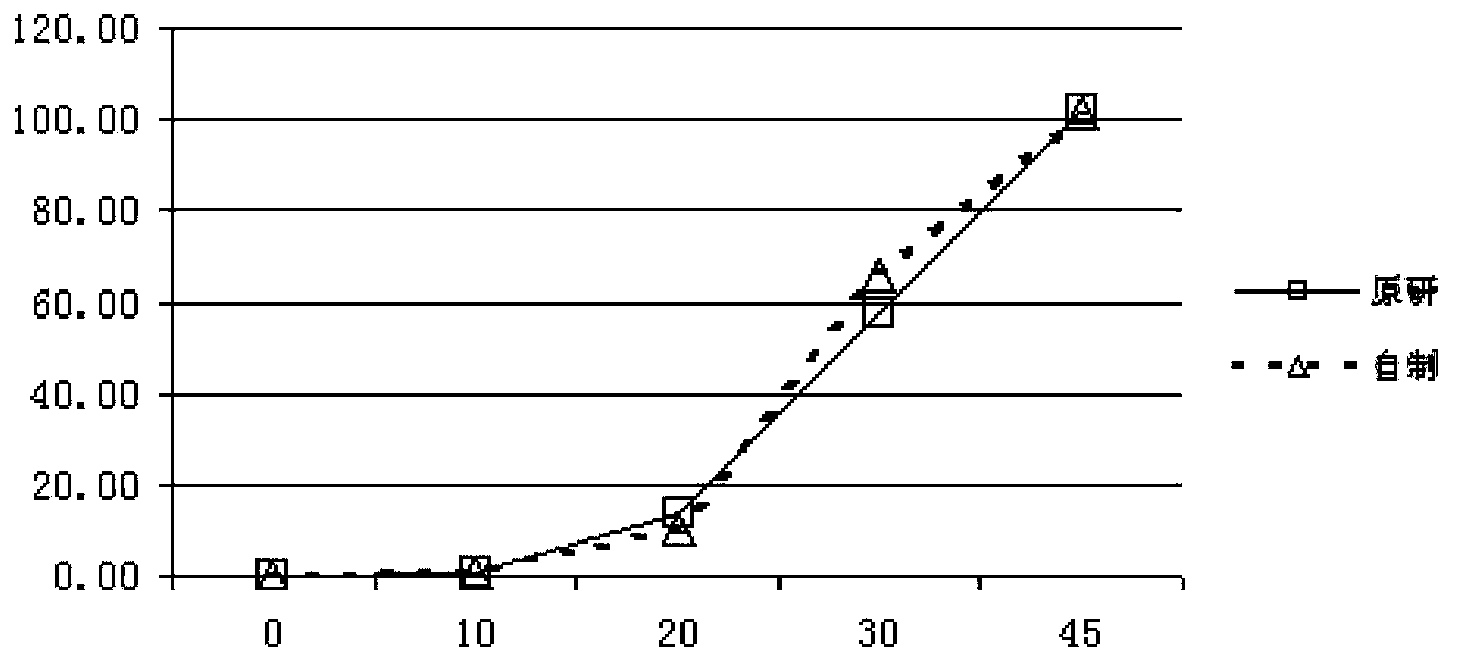
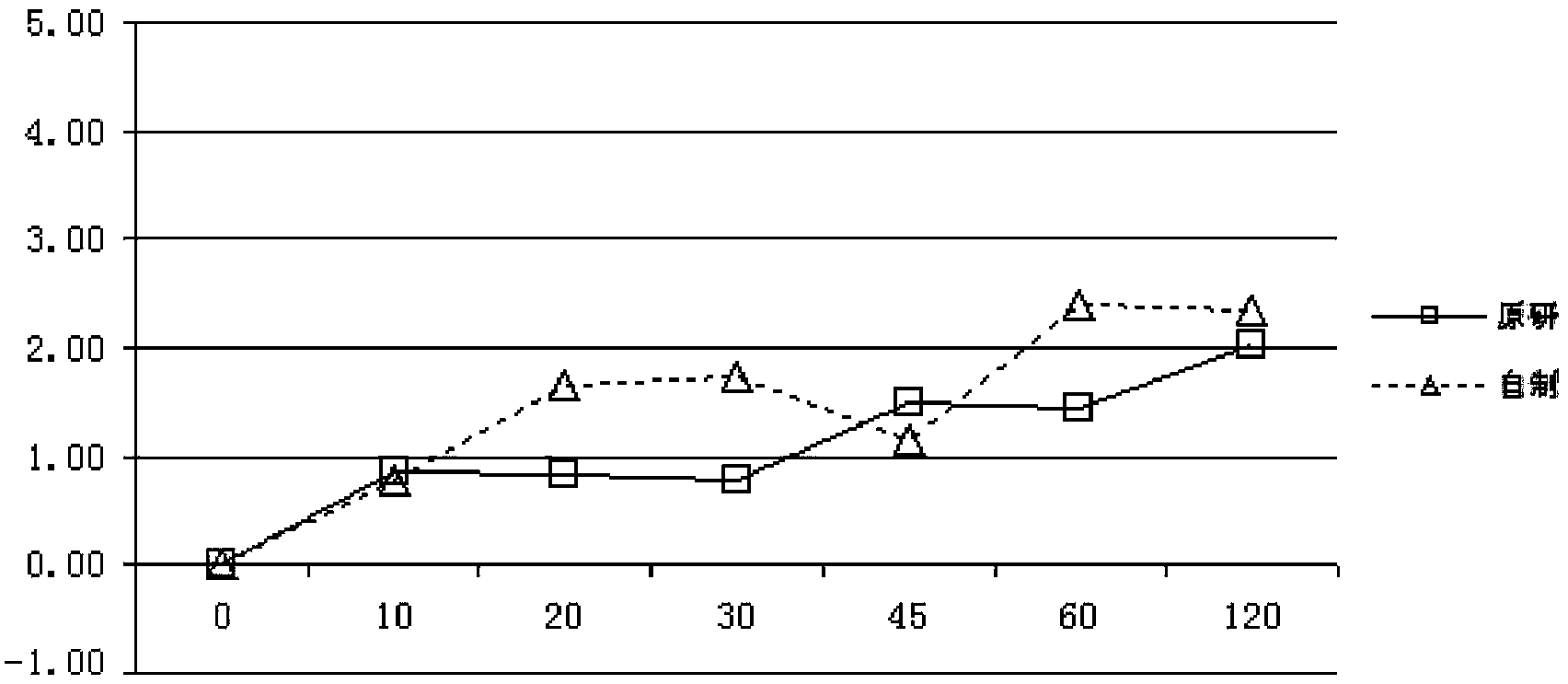
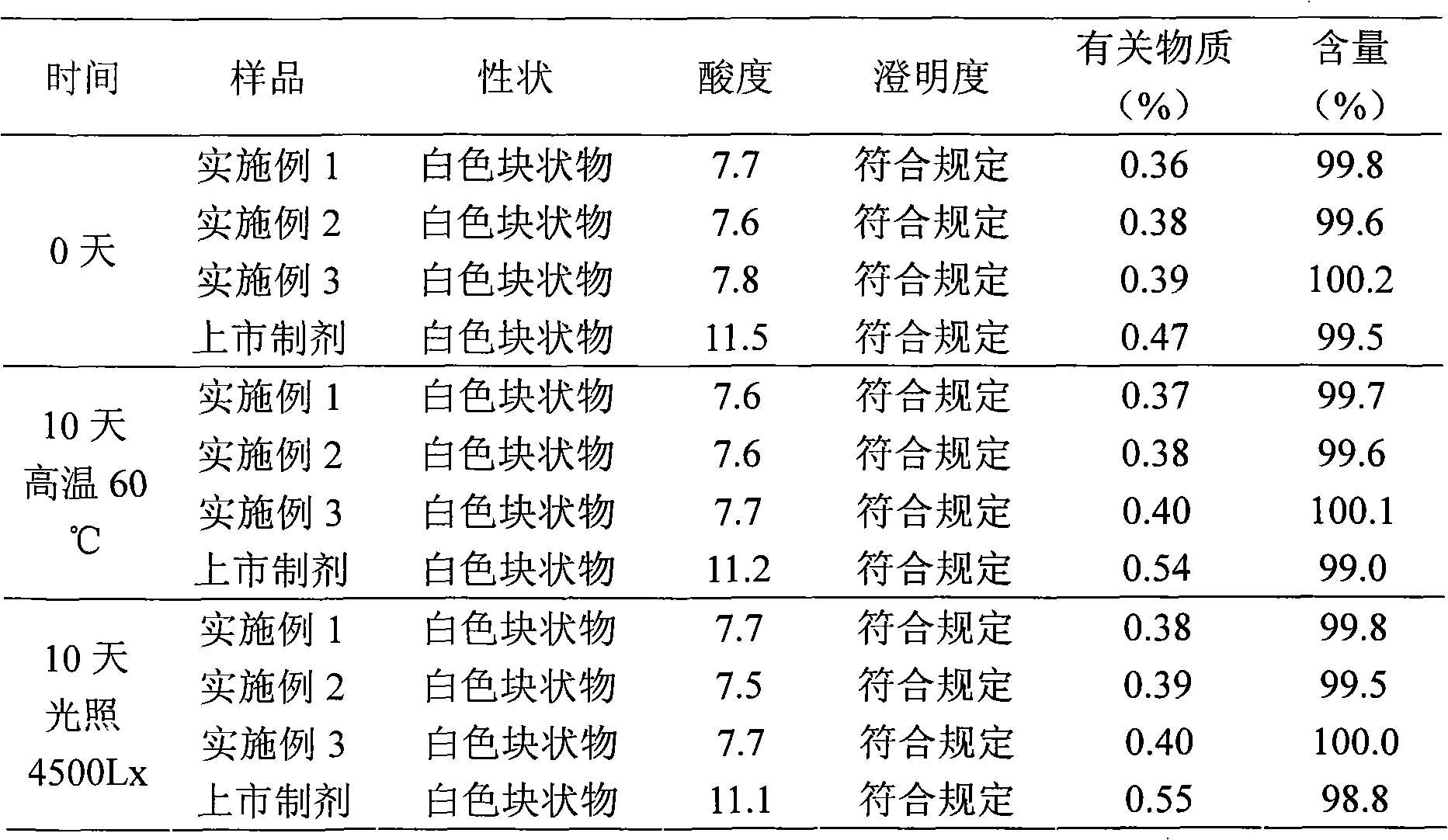
The invention relates to a pantoprazole sodium freeze-drying medicinal composition for injection and a preparation method thereof. The pantoprazole sodium freeze-drying medicinal composition for injection comprises the following components in part by weight: 1 part of pantoprazole sodium, 0.01 to 0.1 part of mannitol, 0.02 to 0.03 part of natrium adetate, 0.07 to 0.10 part of sodium sulfite and 0 to 0.1 part of sodium citrate. In the method, the stability of the solution of the pantoprazole sodium is improved, related matters of the solution of the pantoprazole sodium in the process of preparation, packaging or freeze-drying during preparation are not increased obviously, the content of the related matters is not reduced obviously; the prepared pantoprazole sodium freeze-drying powder injection is good in stability in the process of transportation and storage; solution mixed with the injection during clinical use can be placed for a long time, so that the clinical use is more convenient; and simultaneously, hidden troubles of the medication safety of patients due to the increase of impurities (related matters) and the problem of the curative effect on the patients due to content reduction are reduced greatly.
Owner:福建康成医药有限公司
Polymorphs of pantoprazole sodium salt and process for the preparation thereof
Novel crystalline forms of pantoprazole sodium salt solvate with ketone solvents, a process for the preparation thereof, the use of the forms for the purification of pantoprazol, pharmaceutical compositions therefrom and the use thereof in therapy.
Owner:DIPHARMA SPA
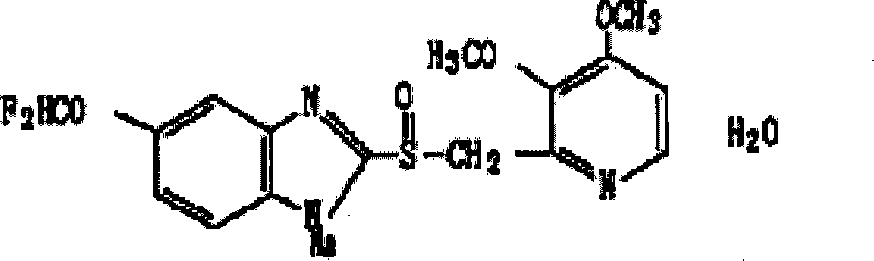
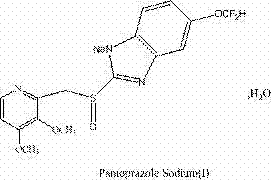
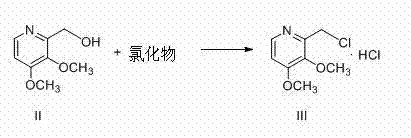
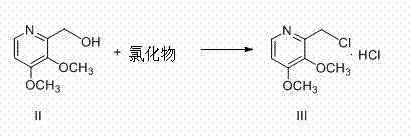
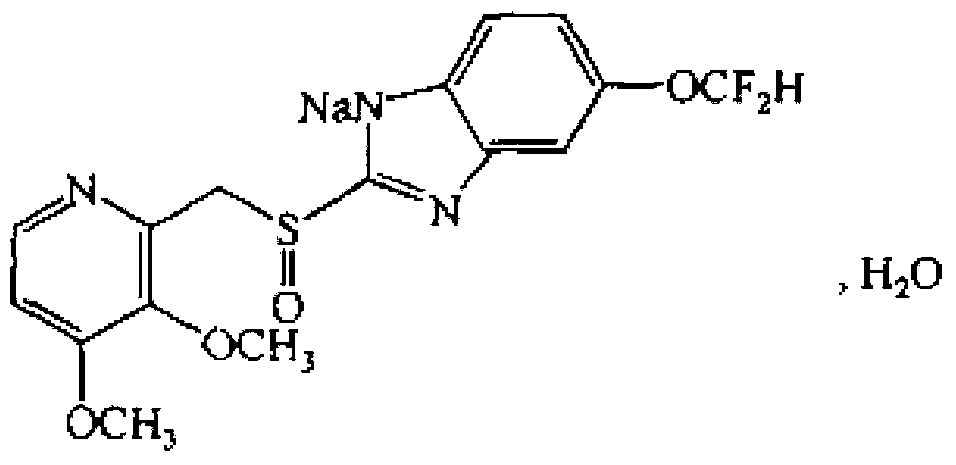
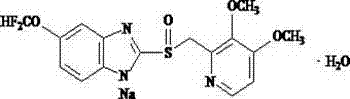
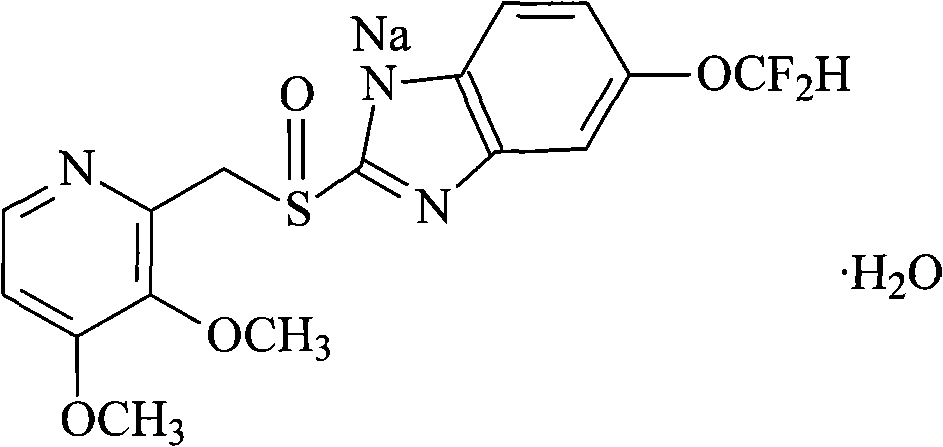
Preparation method of pantoprazole sodium
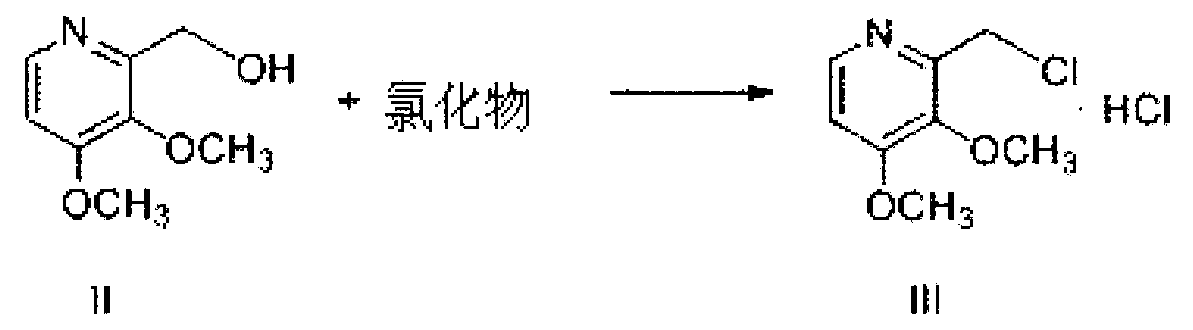
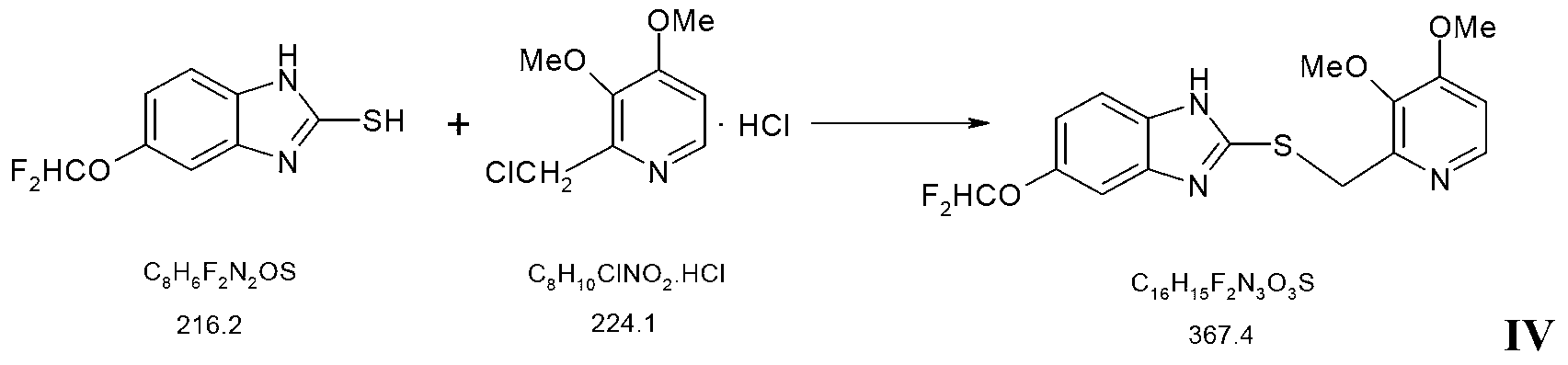
The invention provides a preparation method of pantoprazole sodium. The method comprises the following steps of: 1) generating 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (III) by taking 2-hydroxymethyl-3,4-dimethoxypyridine (II) as the start raw material in the presence of chloride; 2) condensing the obtained compound (III) with 5-difluoromethoxy-2-sulfydryl-1H-benzimidazole in alkaline conditions in the presence of inorganic base to generate 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thioxo-1H-benzimidazole (IV); and 3) salifying the obtained compound (IV) with sodium hydroxide, and oxidizing with an oxidizing agent to generate 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]sulfinyl-1H-benzimidazole sodium, namely pantoprazole sodium (I). According to the invention, the operation is simple and efficient, the reaction conditions are mild, the safety is high, the control is easy, the yield is relatively high, and the method is suitable for industrial production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
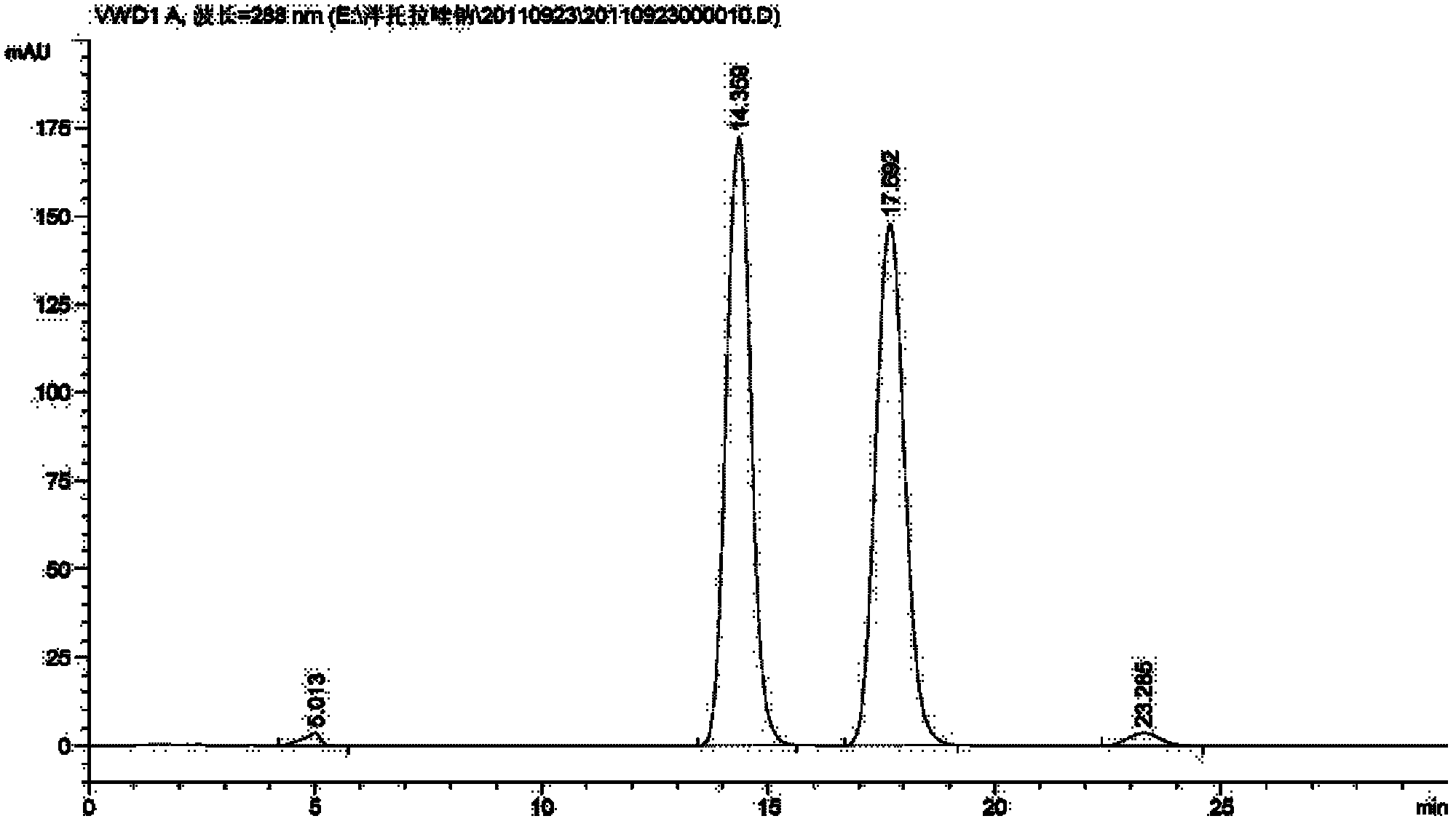
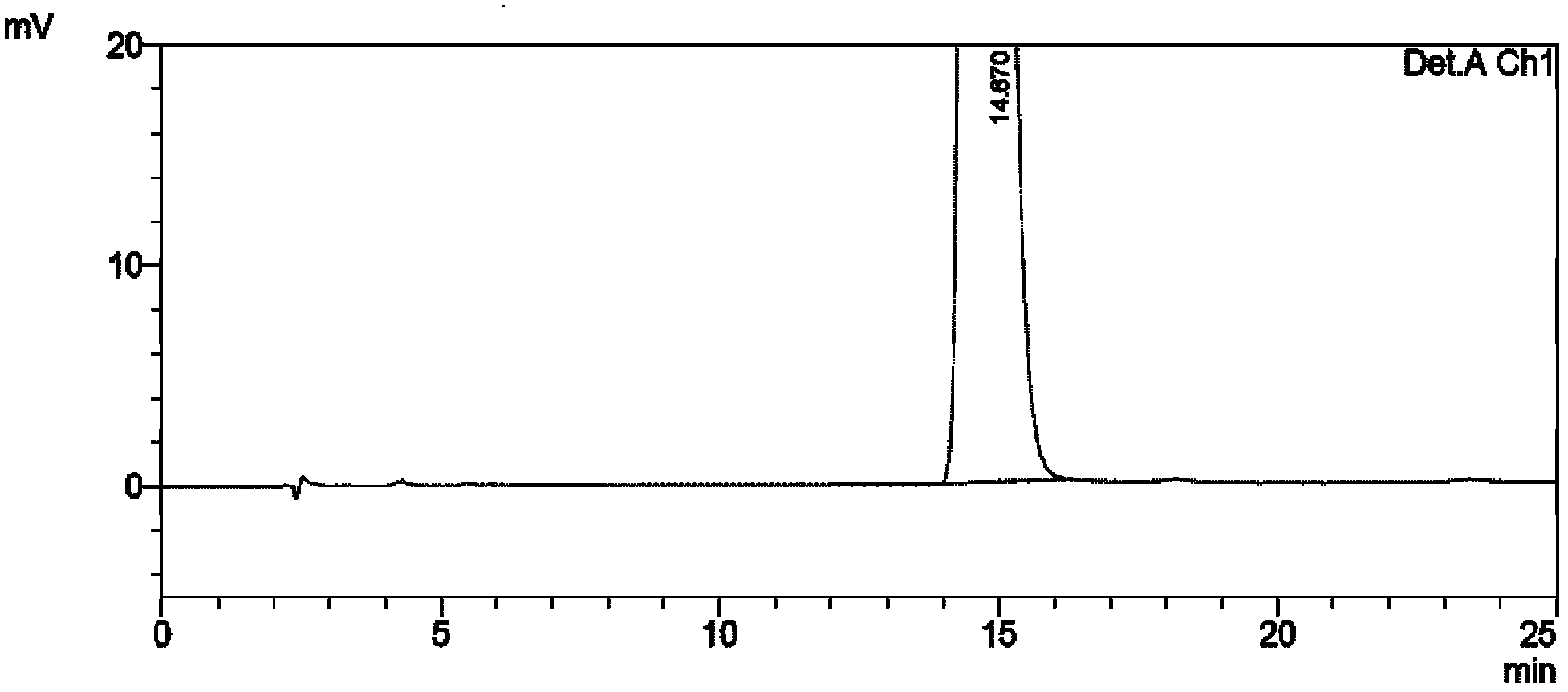
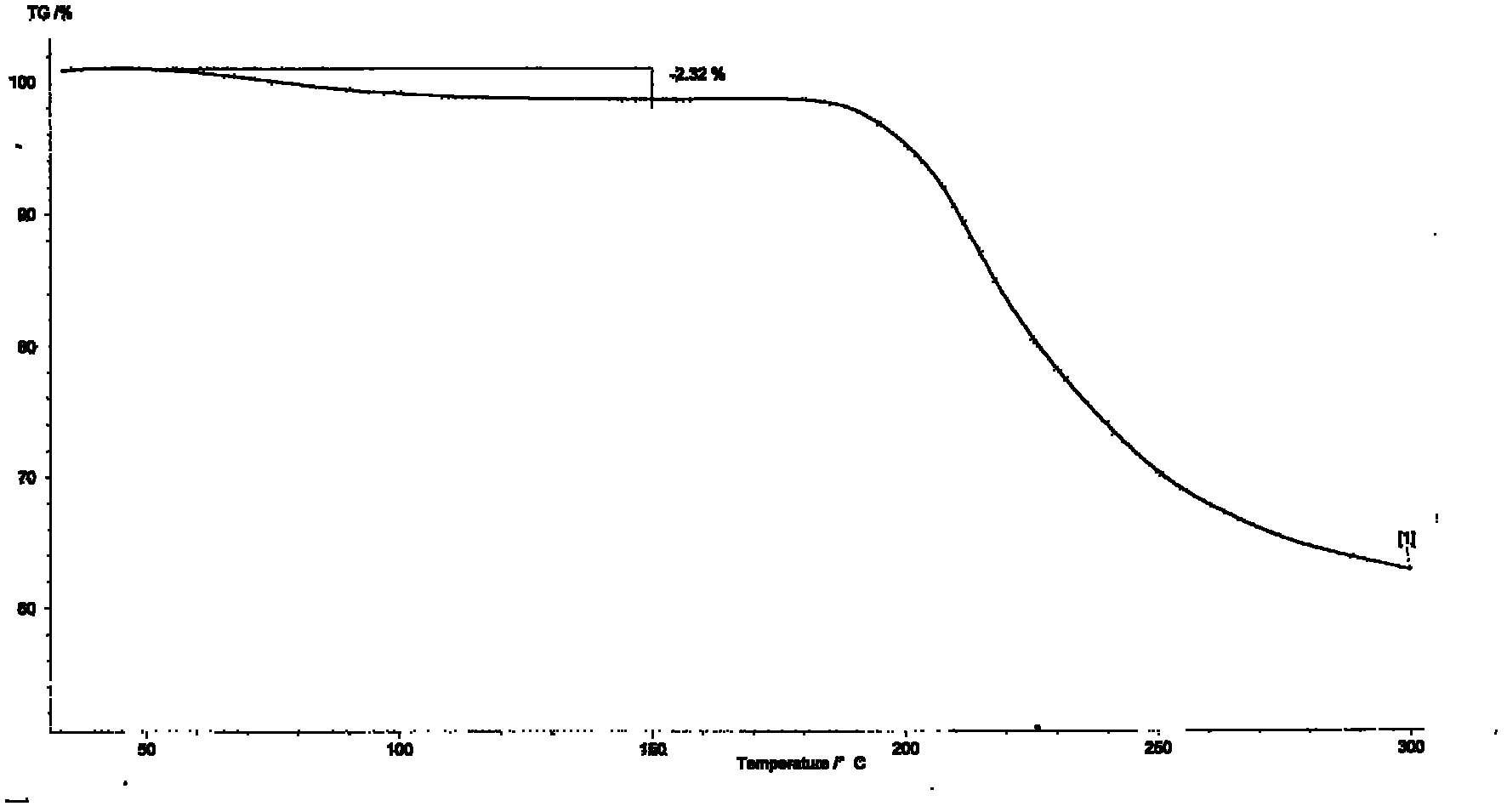
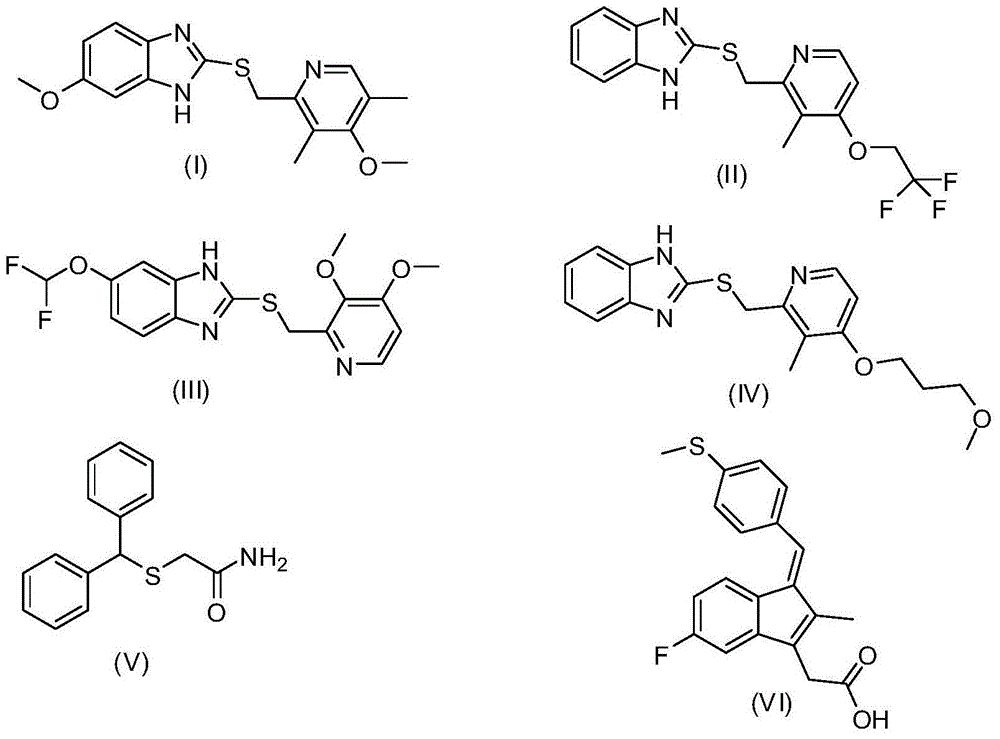
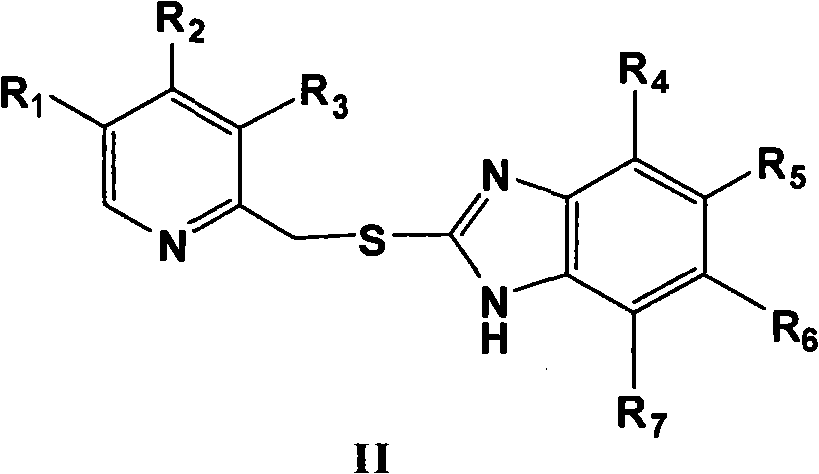
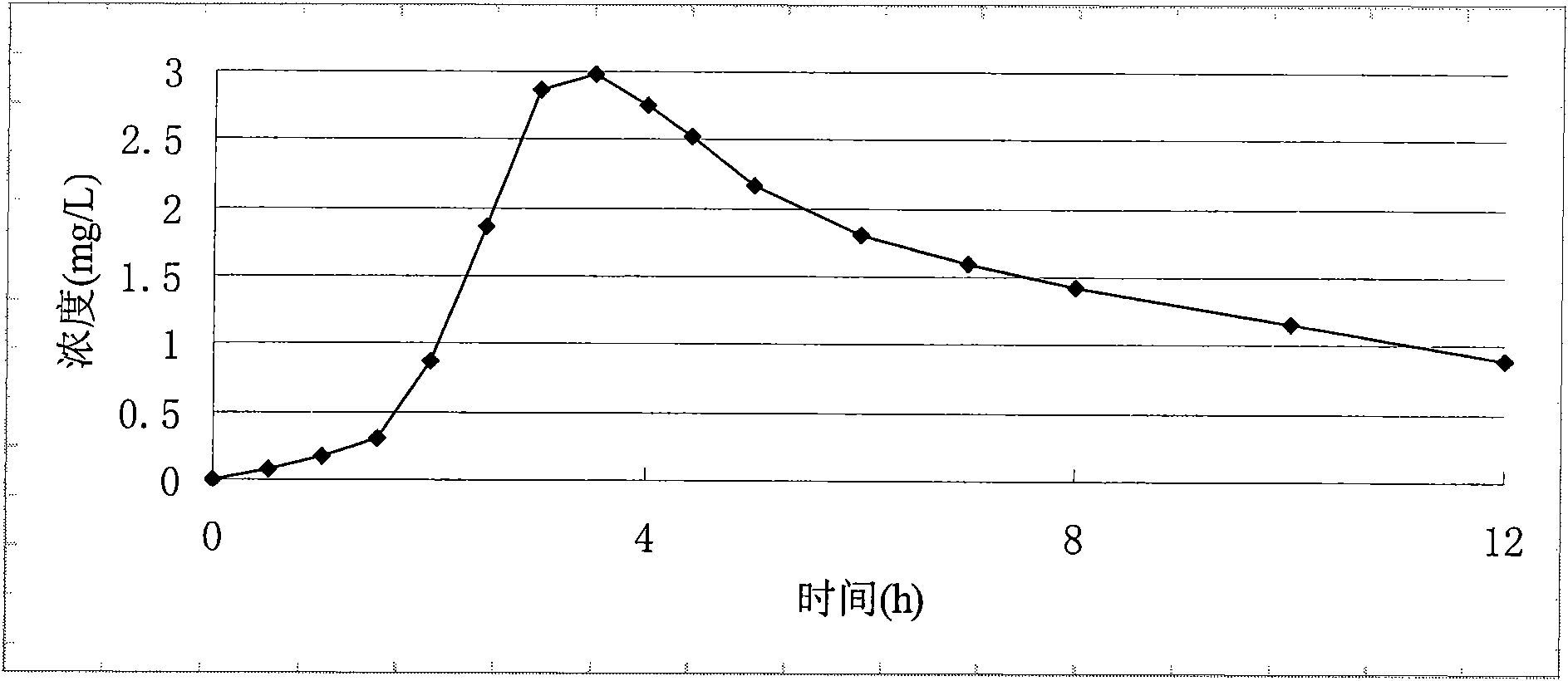
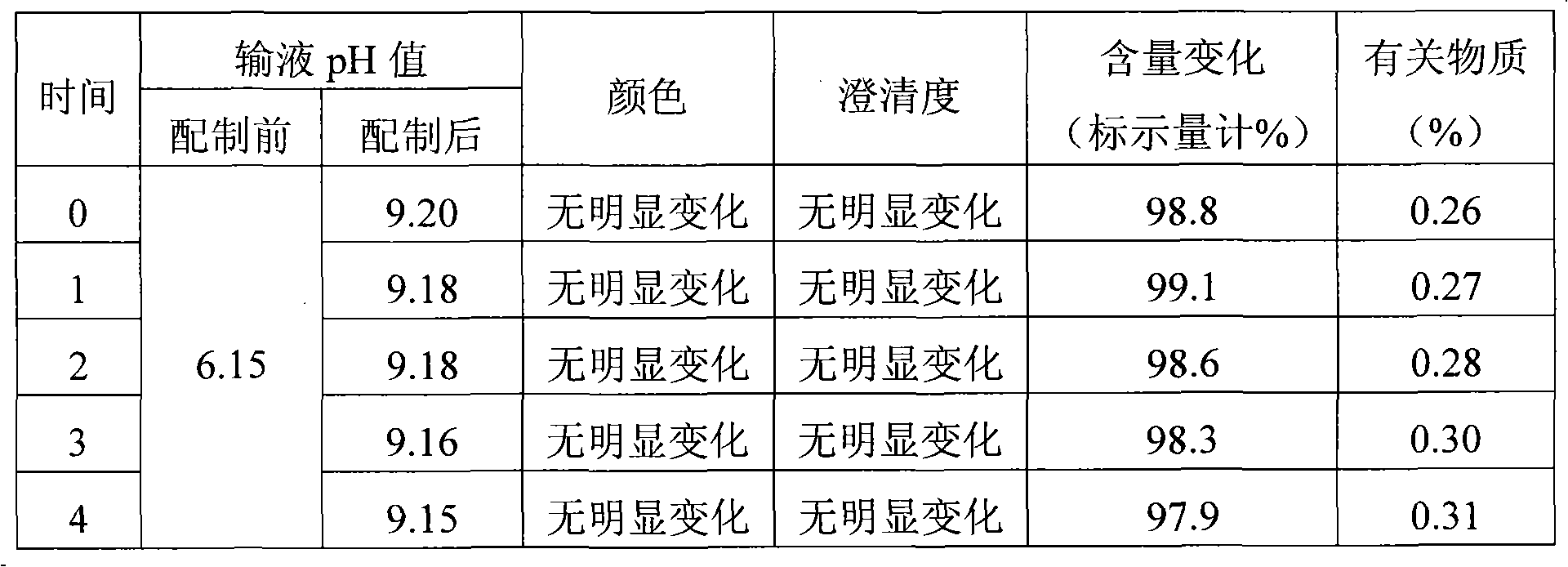
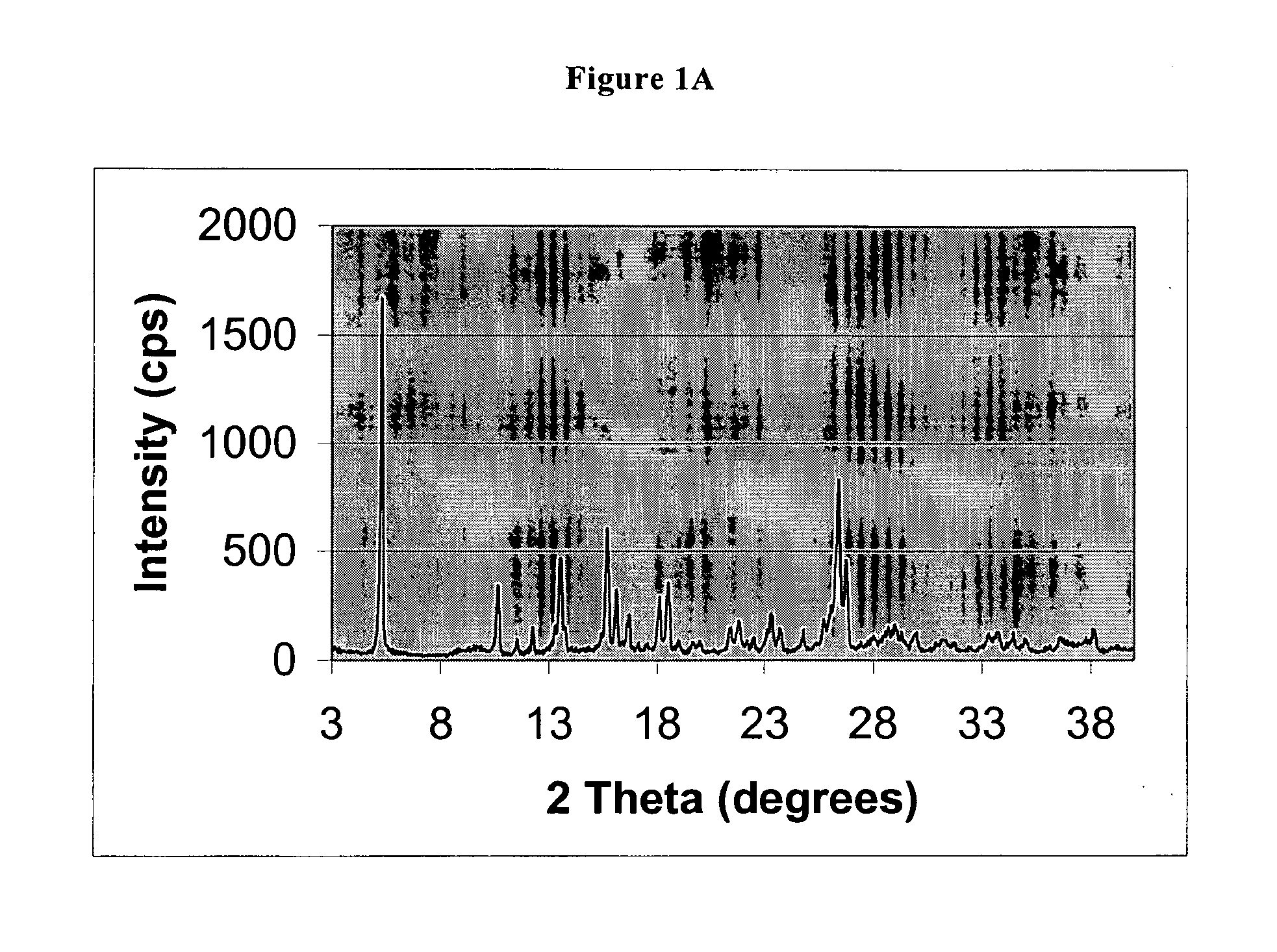
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
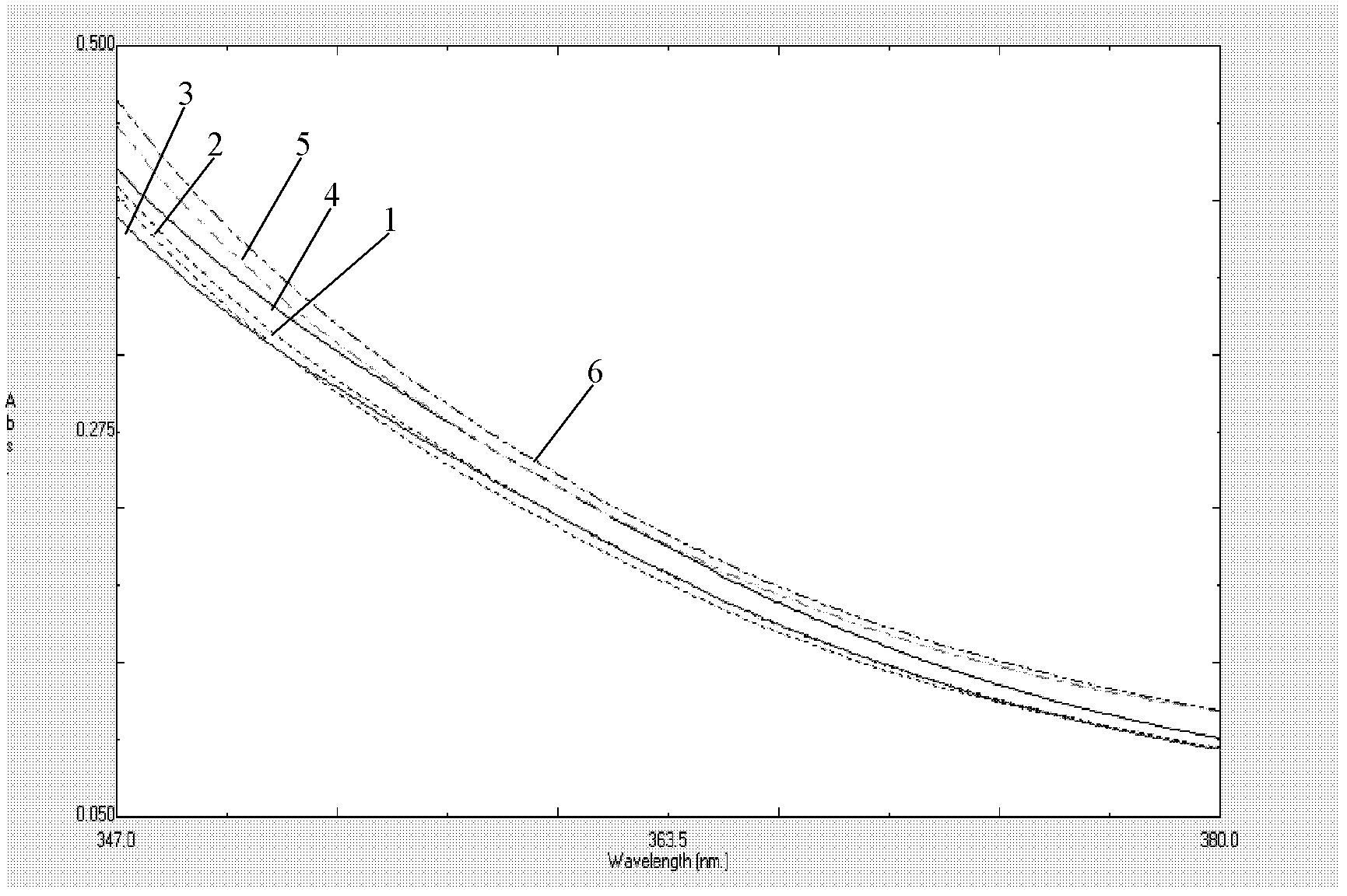
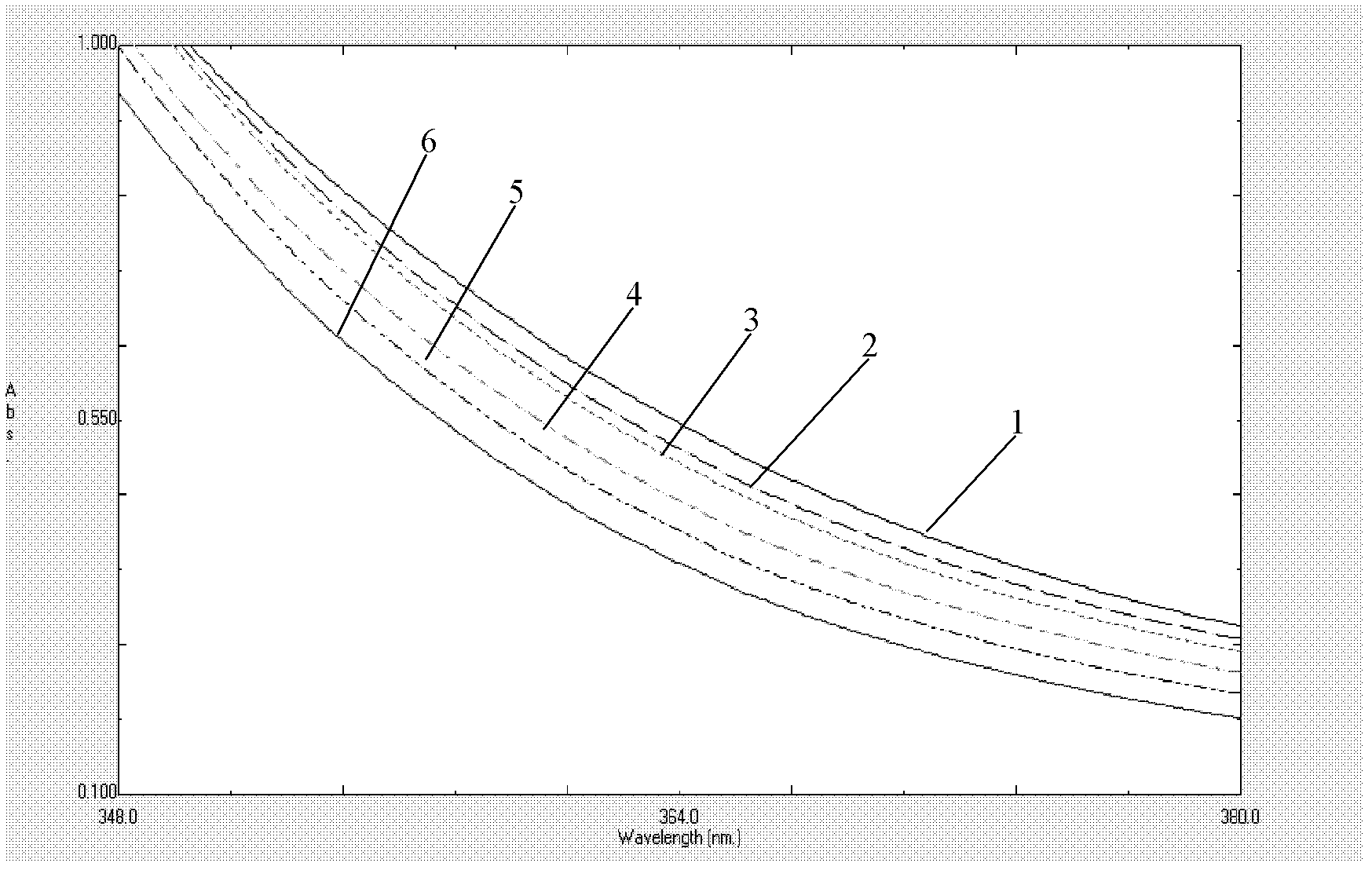
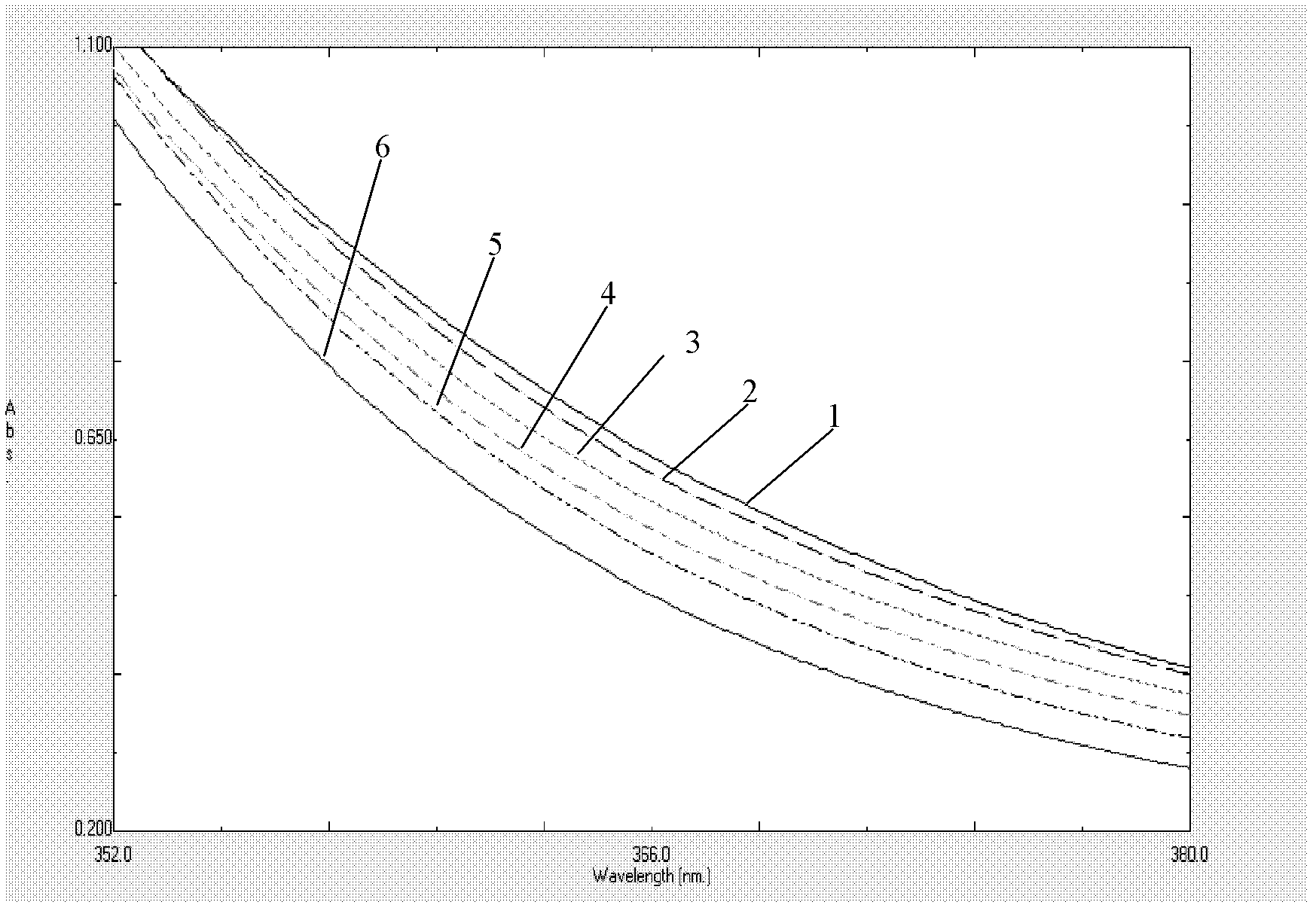
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H- benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)chlorohydroxymethyl] sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:TEVA PHARMA IND LTD
Pantoprazole sodium and preparation method thereof
The invention relates to pantoprazole sodium and a preparation method thereof and in particular relates to a method for preparing the pantoprazole sodium. The preparation method comprises the following steps of: (1) with 2-hydroxymethyl-3,4-dimethoxyl pyridine (II) as a starting material, generating 2-chloromethyl-3,4-dimethoxyl pyridine hydrochloride (III) under the action of chlorides; (2) carrying out condensation on the obtained compound (III) and 5-difluoromethoxyl-2-sulfydryl-1H-benzimidazole in the presence of inorganic base to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfenyl-1H-benzimidazole (IV); (3) oxidizing the obtained compound (IV) by using an oxidant to generate 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole; (4) enabling the 5-difluoromethoxyl-2-[(3,4-dimethoxyl-2-pyridyl) methyl] sulfinyl-1H-benzimidazole to react with sodium hydroxide to generate a salt, namely the pantoprazole sodium (I); and optionally (5) refining the prepared pantoprazole sodium. According to the preparation method, the prepared pantoprazole sodium product has high purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Pantoprazole sodium drug composition and preparation method thereof
ActiveCN102512418AAvoid side effectsReduce dosageOrganic active ingredientsPowder deliverySucrosePharmaceutical Substances
The invention provides a pantoprazole sodium drug composition and a preparation method thereof. The pantoprazole sodium drug composition is characterized by comprising the raw materials in parts by weight as follows: 1 part of pantoprazole sodium, 0.3-0.6 parts of sucrose, 0.005-0.05 parts of metal ion complexing agent and a proper amount of inorganic base. Meanwhile, the invention further provides a preparation method of a pantoprazole sodium freeze-dried powder injection.
Owner:SHANDONG LUYE PHARMA CO LTD
Pantoprazole sodium freeze-dried preparation for injection and preparation method thereof
ActiveCN102670524AImprove stabilityWell formedOrganic active ingredientsPowder deliveryFreeze-dryingD-Glucose
The invention relates to a pantoprazole sodium freeze-dried preparation for injection and a preparation method thereof. The freeze-dried preparation is formed by preparing components containing the following parts by weight into liquor for freeze drying after dissolving into water for injection: 60 to 80 parts of pantoprazole sodium, 100 to 300 parts of glycocoll, 50 to 100 parts of glucose and aright amount of sodium hydroxide. According to the pantoprazole sodium freeze-dried preparation for the injection, which is disclosed by the invention, the problem of stability of a preparation is solved; and moreover, an adverse reaction existing in a traditional product is lowered.
Owner:NANJING CHIA TAI TIANQING PHARMA
Pantoprazole and its sodium salt enteric sustained-release pellet preparation
InactiveCN101428005AEliminate local irritationLarge distribution areaOrganic active ingredientsDigestive systemSustained release pelletsIsolation layer
The invention discloses pantoprazole and the sodium salt enteric sustained-release micropill preparation thereof. The preparation comprises pantoprazole-containing micropills, an isolation layer, a sustained-release layer, another isolation layer and an enteric layer from inside to outside in sequence; the weight of the first isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, the weight of the sustained-release layer is 5% to 100% of that of the pantoprazole-containing micropills, the weight of the second isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, and the weight of the enteric layer is 20% to 200% of that of the pantoprazole-containing micropills. The pantoprazole enteric sustained-release micropills can stably release the drug.
Owner:ZHEJIANG UNIV
Pantoprazole sodium liposomes freeze-dry preparations and method of preparing the same
InactiveCN101249073ASolve the problem of quality stabilitySmall toxicityOrganic active ingredientsDigestive systemFreeze-dryingCholesterol
The invention discloses a freeze-dried preparation of pantoprazole sodium liposomes, wherein pantoprazole sodium is encapsulated in antioxidant-containing liposomes made of soybean lecithin and cholesterol. The freeze-dried preparation is administered intravenously with stable quality, less toxicity and high effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pantoprazole sodium enteric-coated tablet and preparation method thereof
ActiveCN103006613AImprove stabilityReduced stabilityOrganic active ingredientsDigestive systemAdhesivePantoprazole Sodium
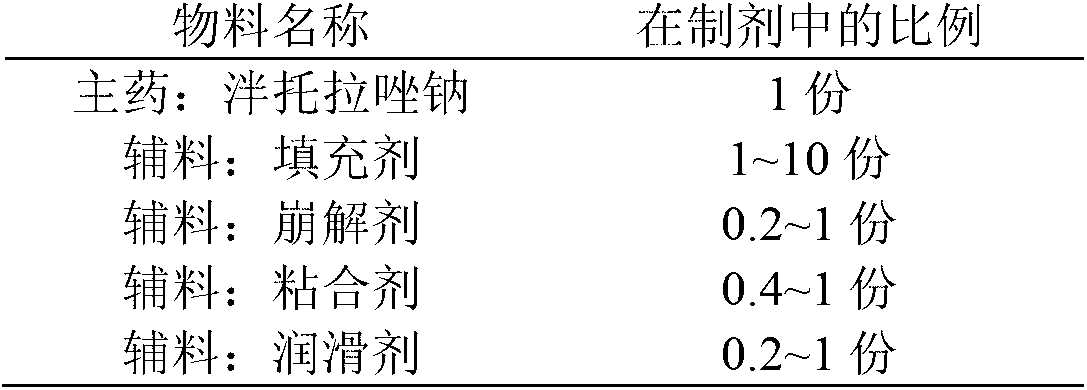
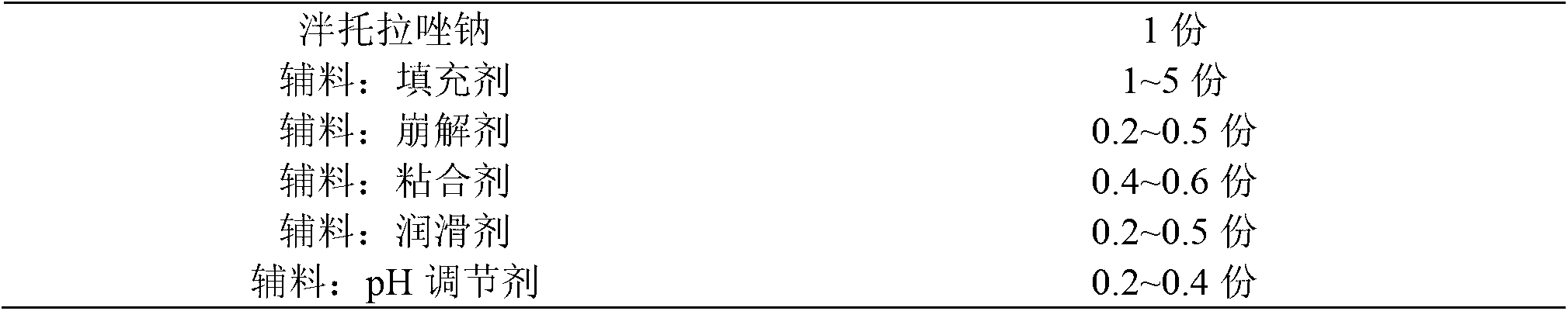
The invention provides a pantoprazole sodium enteric-coated tablet and a preparation method thereof. The pantoprazole sodium enteric-coated tablet comprises a pantoprazole sodium tablet, an isolated layer and an enteric-coated layer, wherein the pantoprazole sodium tablet comprises main drug pantoprazole sodium and auxiliaries; and the auxiliaries include a filler, a disintegrant, a lubricating agent, an adhesive, a pH regulating agent, and the like. The pantoprazole sodium enteric-coated tablet accelerates the disintegration time of the pantoprazole sodium enteric-coated tablet and solves the problem of drug instability during a storage period.
Owner:SHIJIAZHUANG HUAXIN PHARMA
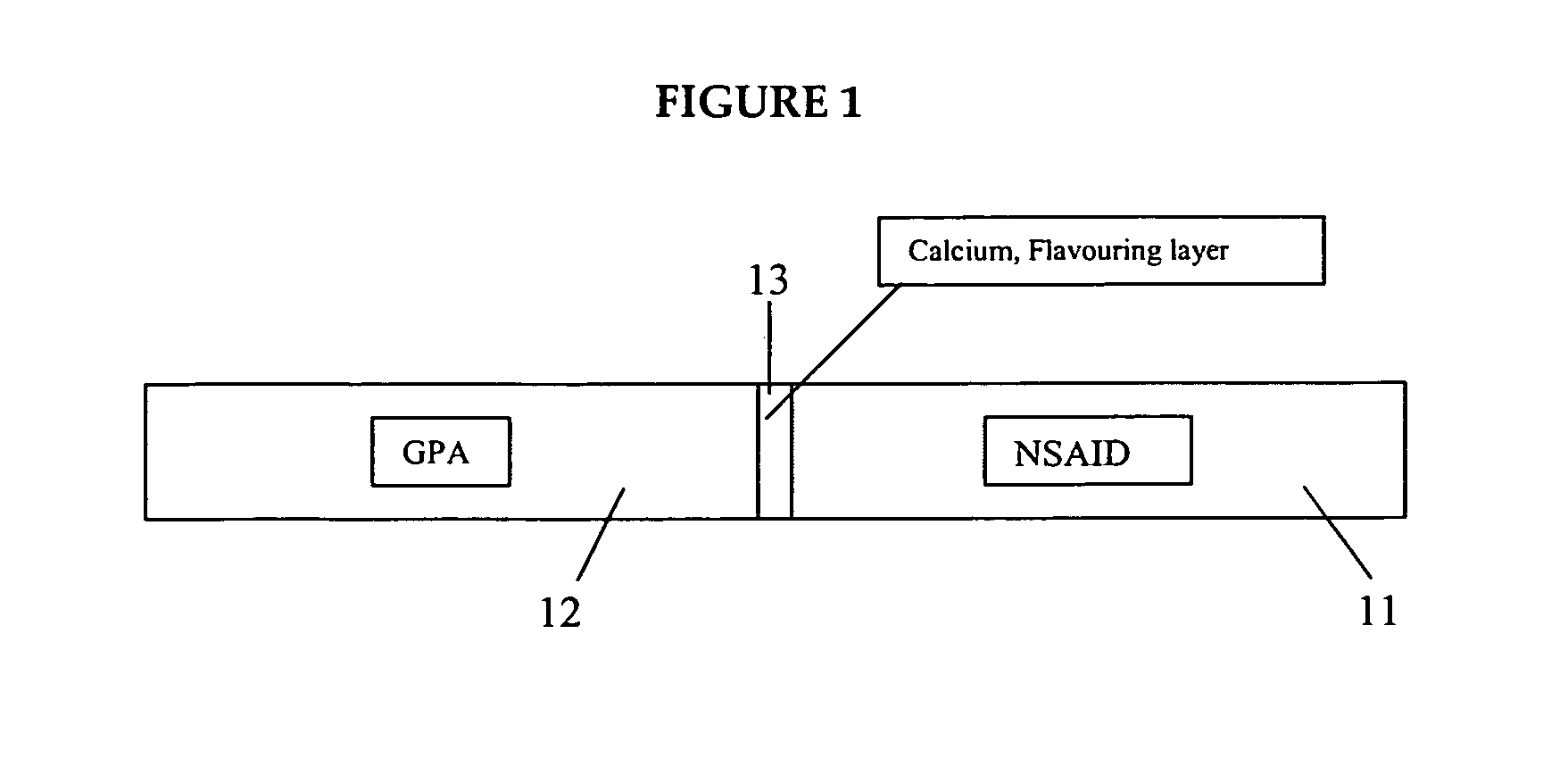
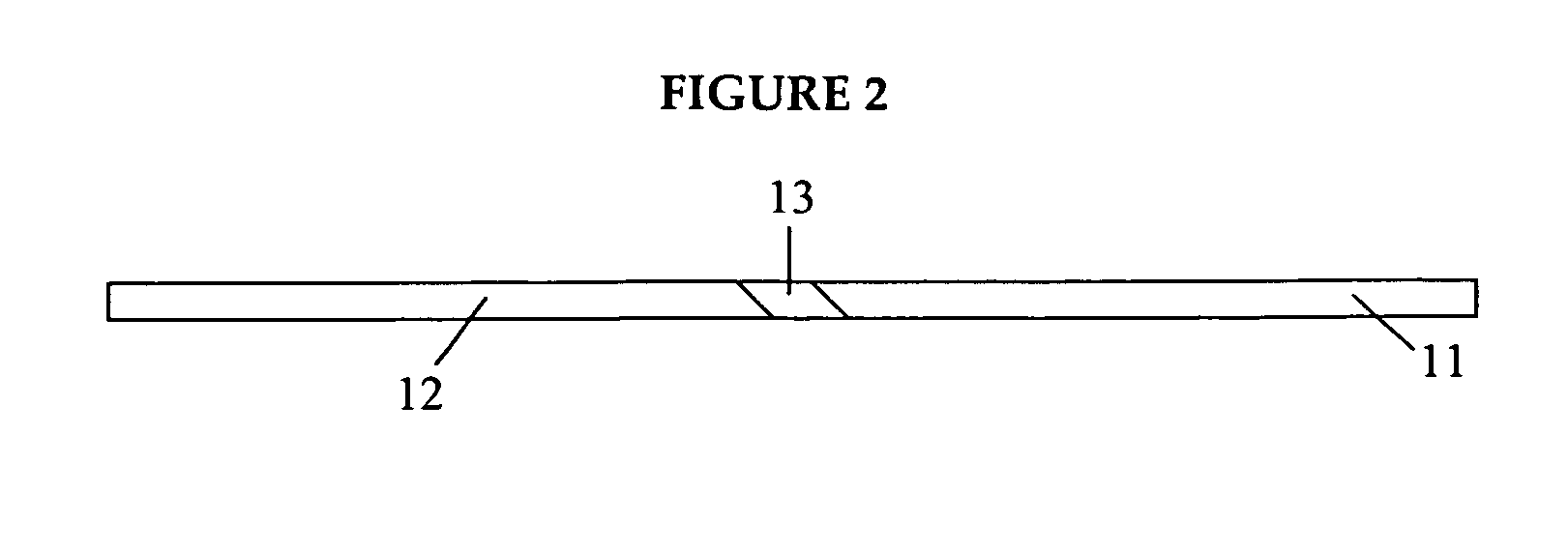
Medicated gumstick for treatment in anti-inflammatory conditions and prophylaxis against NSAID gastropathy
InactiveUS20070003490A1Promote absorptionMinimize contactAntipyreticAnalgesicsDiseaseMedicated chewing-gum
A stick of gum is provided containing therapeutic benefits of non-steroid anti-inflammatory drugs for inflammation in conditions such as arthritis, and also alleviates subsequent side effects of NSAID administration, as well as antacid effects from compounds such as an H2 antagonist (ranitidine, cimetidine, famotidine) and / or a proton pump inhibitor (such as lansoprazole, pantoprazole, omeprazole, esomeprazole or rabeprazole) and / or an acid pump antagonist selected from the group of soraprazan, AZD0865, YH1885 and CS-526.
Owner:MEDICAL FUTURES
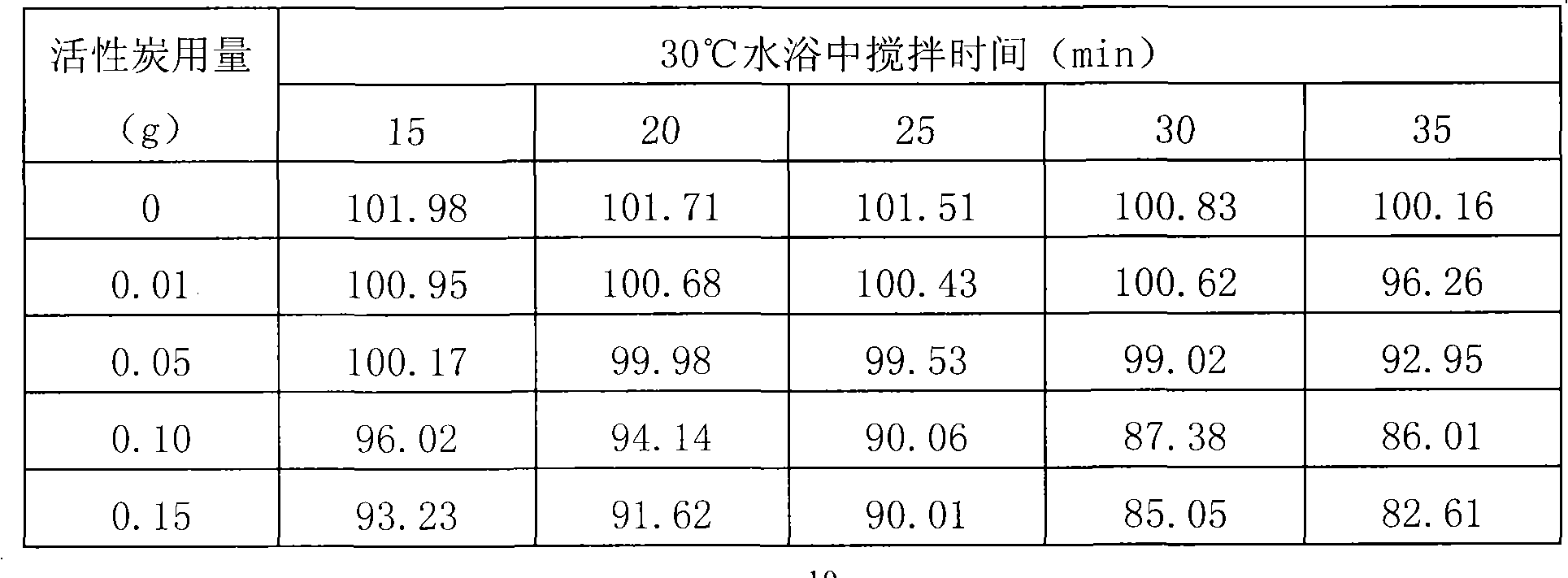
Pantoprazole sodium composition for injection
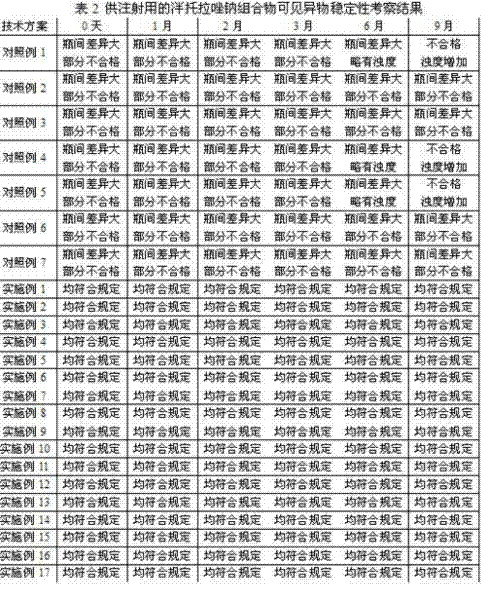
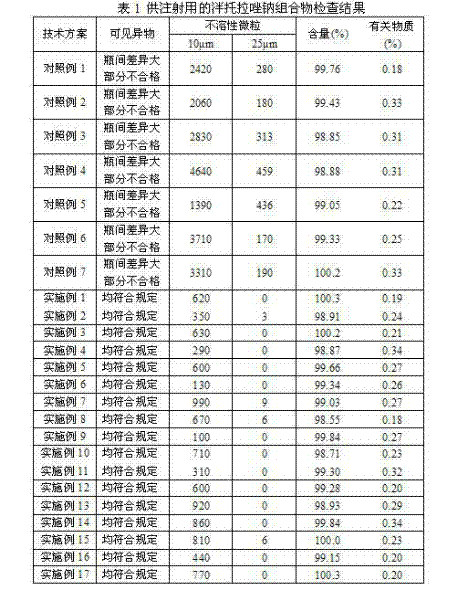
ActiveCN102225063AWon't releaseAvoid reactionOrganic active ingredientsPowder deliveryForeign matterSide effect
The invention provides a pantoprazole sodium composition for injection. The composition contains pantoprazole sodium and disodium ethylene diamine tetraacetate that are in a proportion of 1:0.02-0.1 by weight. And the composition is prepared by the steps of: 1) liquid medicine preparation: placing pantoprazole sodium and disodium ethylene diamine tetraacetate in a preparation pot, adding water for injection and stirring to make the above agents dissolved and mixed well with the water, adjusting the pH value of the mixture to 10.5-12.5; 2) rubber stopper treatment; 3) aseptic filtration and separate packing; 4) vacuum freeze drying, thus obtaining the composition. For pantoprazole sodium medicaments extremely easy to react with exudates from a rubber stopper, the pantoprazole sodium composition for injection in the invention can simultaneously guarantee the visible foreign matters and insoluble particles in products meeting the requirement of an injection. According to the invention, quality level of the composition product is improved, and the hidden trouble that unqualified visible foreign matters and insoluble particles threat patient clinical medication safety can be avoided. Additionally, the composition of the invention has better curative effects and less clinical side effects.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
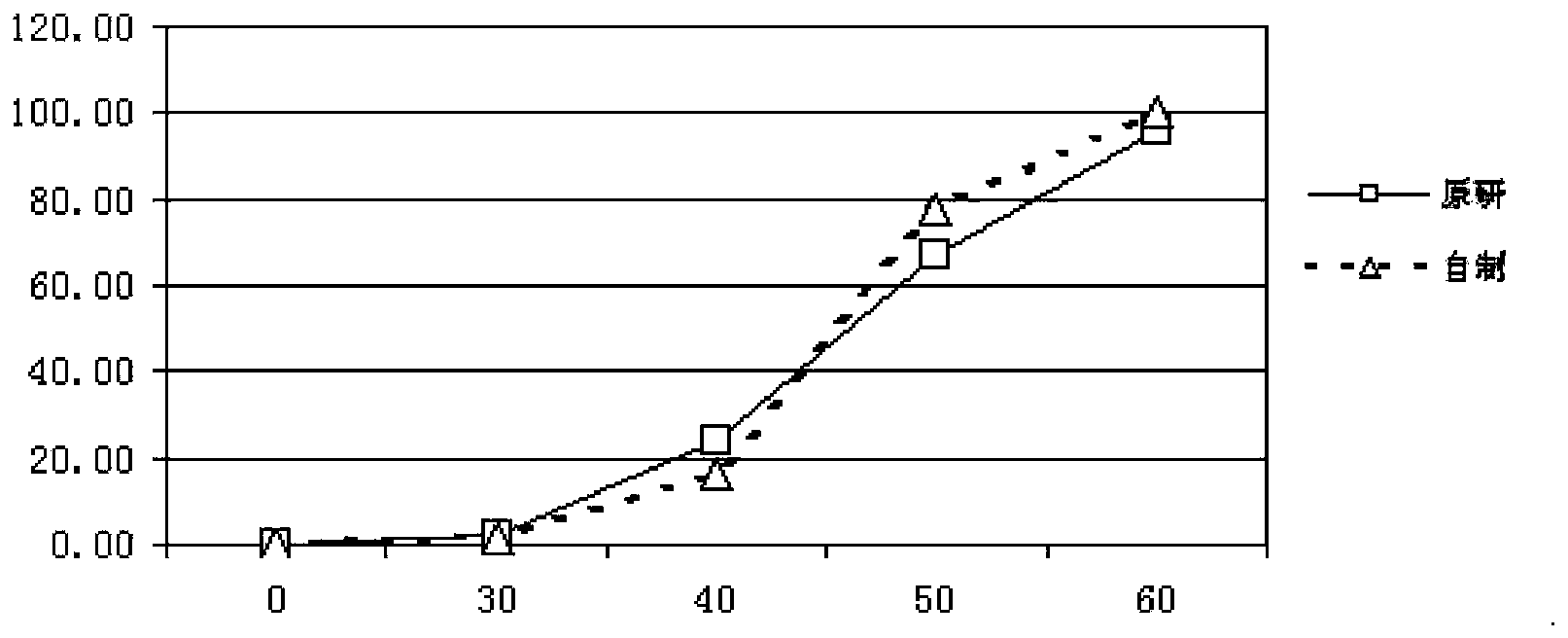
Pantoprazole sodium enteric-coated tablet and preparation method thereof
ActiveCN104382875ACreativeMeet the requirements of consistency evaluationOrganic active ingredientsDigestive systemCoated tabletsOrganic chemistry
The invention provides a pantoprazole sodium enteric-coated tablet. The pantoprazole sodium enteric-coated tablet is prepared by coating a pantoprazole sodium tablet with an isolation layer and an enteric-coated layer, wherein the pantoprazole sodium tablet comprises the following components in percentage by weight: 35-45% of pantoprazole sodium, 30-59% of a filling agent, 0.2-3% of a binder, 8.1-18% of a disintegrant, 5-15% of a stabilizer and 0.2-1.5% of a lubricant. According to the pantoprazole sodium enteric-coated tablet and the preparation method thereof provided by the invention, a dissolution curve of the pantoprazole sodium enteric-coated tablet in each of four dissolution media is consistent with that of an originally developed preparation, thereby being in line with the requirements of consistency evaluation; and furthermore, the stability of the pantoprazole sodium enteric-coated tablet is better than that of the originally developed preparation.
Owner:HANGZHOU CONBA PHARMA
Sub-micro emulsion frozen preparation of pantoprazole sodium prepared by using multiple emulsion method
InactiveCN101548957AImprove stabilityGuarantee product qualityPowder deliveryOrganic active ingredientsEmulsionEngineering
The invention provides a sub-micro emulsion frozen preparation of pantoprazole sodium and a preparation method thereof. The sub-micro emulsion frozen preparation of pantoprazole sodium is prepared mainly with the following components according to portions by weight: 1-10 portions of pantoprazole sodium, 5-50 portions of biodegradable polymer, 1-20 portions of emulsifying agent, 5-40 portions of skeleton supporting agent and 1-20 portions of stabilizing agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
An enteric coated mini-pill of pantoprazole sodium
ActiveCN1883460AHigh feasibilityImprove stabilityOrganic active ingredientsDigestive systemCellulosePantoprazole Sodium
The invention discloses pantoprazole sodium enteric pellets which comprise cores containing pantoprazole sodium and enteric coating containing cellulose substances. The invention also discloses the process for preparing the cores. The invention realize higher preparation stability and facilitated mass production.
Owner:HANGZHOU ZHONGMEI HUADONG PHARMA
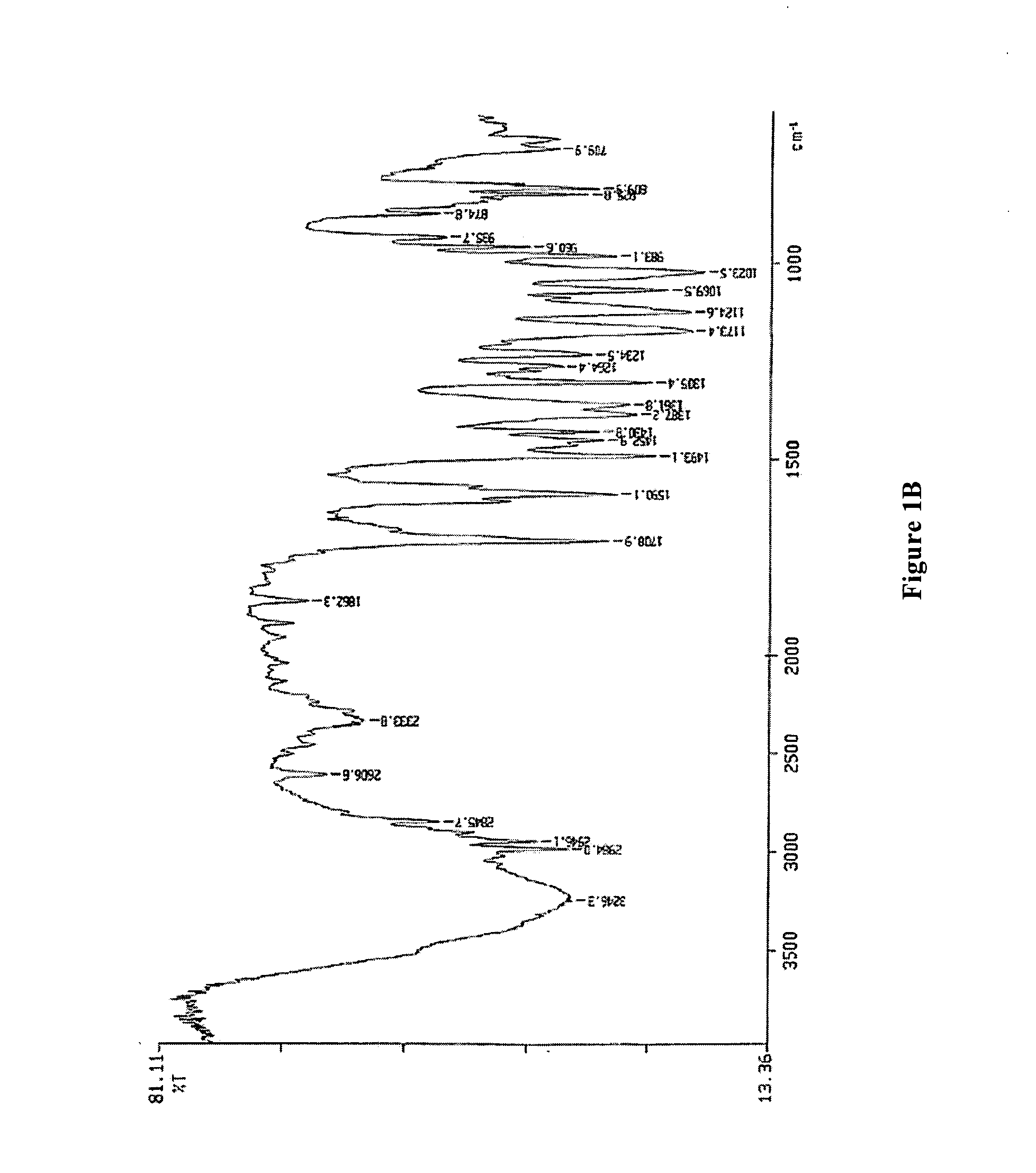
Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole
The present invention provides a process comprising admixing a thioether with about 1.05 to about 1.6 molar equivalents of an active chlorine-containing oxidant, preferably sodium hypochlorite, and about 2.5 to about 5.0 molar equivalents of an alkali metal base; and recovering a sulfoxide that is preferably pantoprazole, lansoprazole, omeprazole, or rabeprazole. The process may further comprise contacting the sulfoxide with a source of sodium ions, preferably sodium hydroxide, to produce the sodium salt of the sulfoxide. The invention also relates to novel chlorinated derivatives of pantoprazole including 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chloromethyl]sulfinyl]-1H-benzimidazole and 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)-chlorohydroxymethyl]sulfinyl]-1H-benzimidazole and processes for making them. The invention also relates to processes of quantifying and identifying a compound other than pantoprazole in a mixture of pantoprazole and at least one other compound.
Owner:TEVA PHARM USA INC
Method for preparing (S)-pantoprazole in high-enantioselectivity way
The invention relates to the field of medicinal chemistry, in particular to a method for preparing (S)-pantoprazole in a high-enantioselectivity way. The method is characterized in that any organic alkali is not added under the condition of chiral reagent existence, and oxidants are used for directly oxidizing 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridyl)-methyl]-sulfenyl]-1H-benzimidazole. The ee value of the (S)-pantoprazole obtained by using the method provided by the invention can reach 100 percent.
Owner:GUANGDONG HUANAN PHARMACEUTICAL GROUP CO LTD
Preparation method of chiral sulfoxide medicament though catalysis of asymmetric oxidation of sulfides compound
ActiveCN104447692AEasy to synthesizeRaw materials are easy to getOrganic chemistryOrganic compound preparationManganeseSulfide compound
The invention provides a preparation method of a chiral sulfoxide medicament though catalysis of asymmetric oxidation of sulfides compounds. A chiral complex formed by quadridentate nitrogen organic ligand and metal manganese compound as a catalyst and hydrogen peroxide as an oxidant are used for asymmetric catalytic oxidation of prochiral thioether compound, so as to obtain the corresponding chiral sulfoxide medicament compounds including S-omeprazole, S-lansoprazole, S-pantoprazole, S-rabeprazole, R-Modafinil and R-sulindac. The reaction has the advantages of cleaness, mild reaction conditions, high conversion rate and antipodal selectivity, and shows industrial prospects.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900401.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900411.PNG)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/a8916e2e-db80-4459-98c4-e5f80d1ab692/A20048002223900421.PNG)




![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00001.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00002.png)
![Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole Process for preparing 2-[(pyridinyl)methyl]sulfinyl-substituted benzimidazoles and novel chlorinated derivatives of pantoprazole](https://images-eureka.patsnap.com/patent_img/f276dec5-fdf8-4bc3-88d5-ce5cab9c91ec/US20050075370A1-20050407-D00003.png)