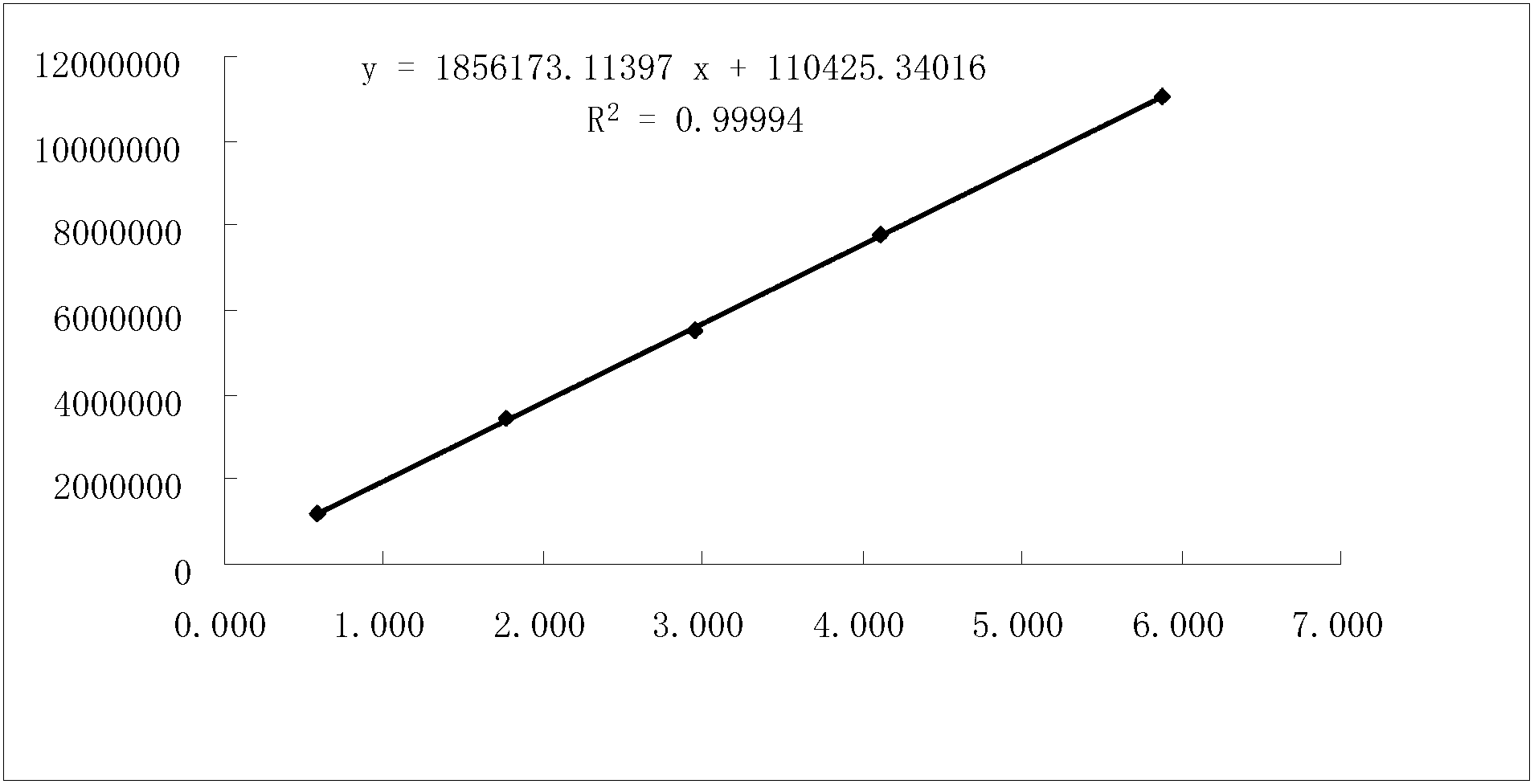Patents
Literature
90 results about "Famotidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Famotidine is known as an H2 blocker. It works by reducing the amount of acid in your stomach. It is used to prevent and treat heartburn and other symptoms caused by too much acid in the stomach (acid indigestion). Check the ingredients on the label even if you have used the product before. The manufacturer may have changed the ingredients.
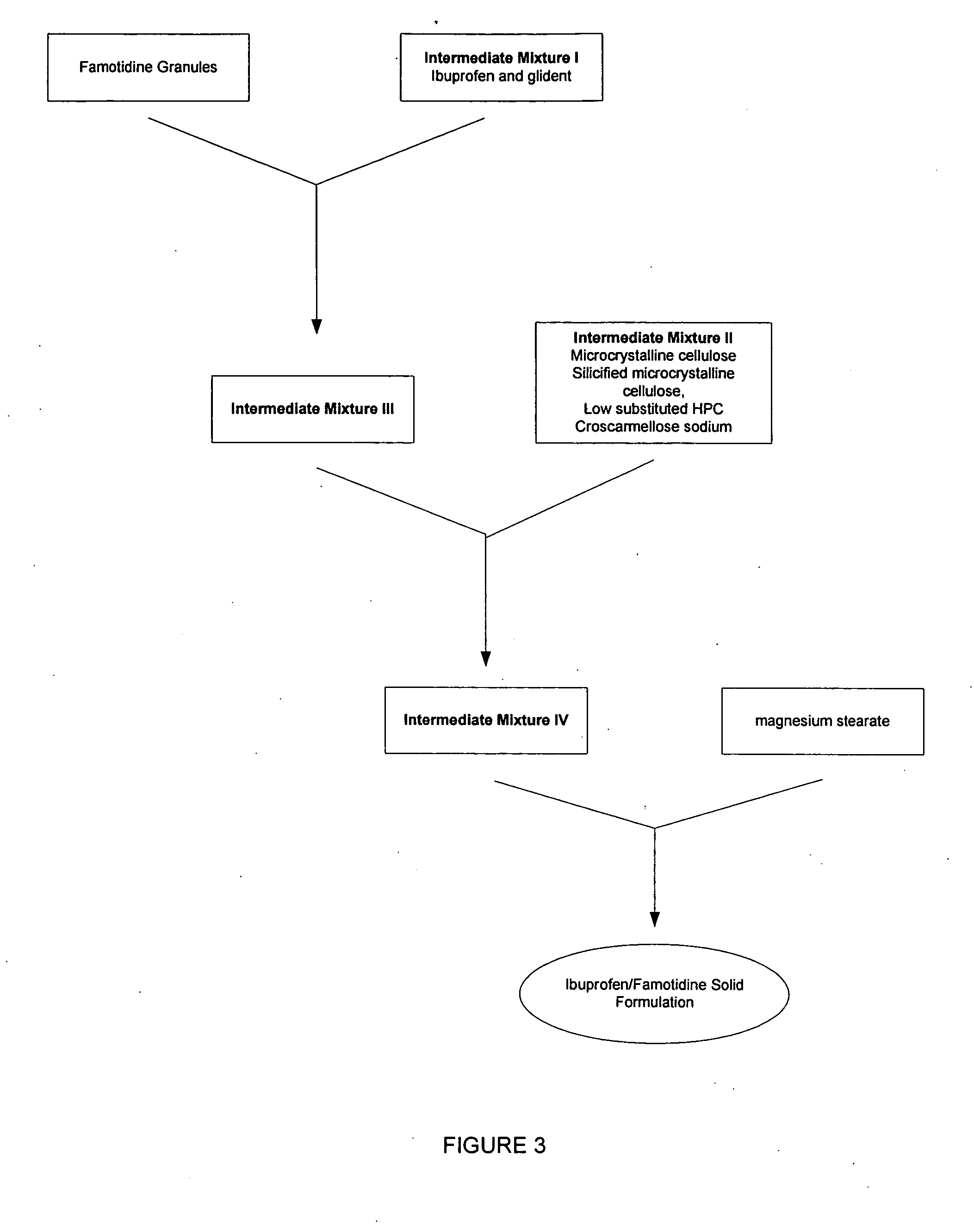
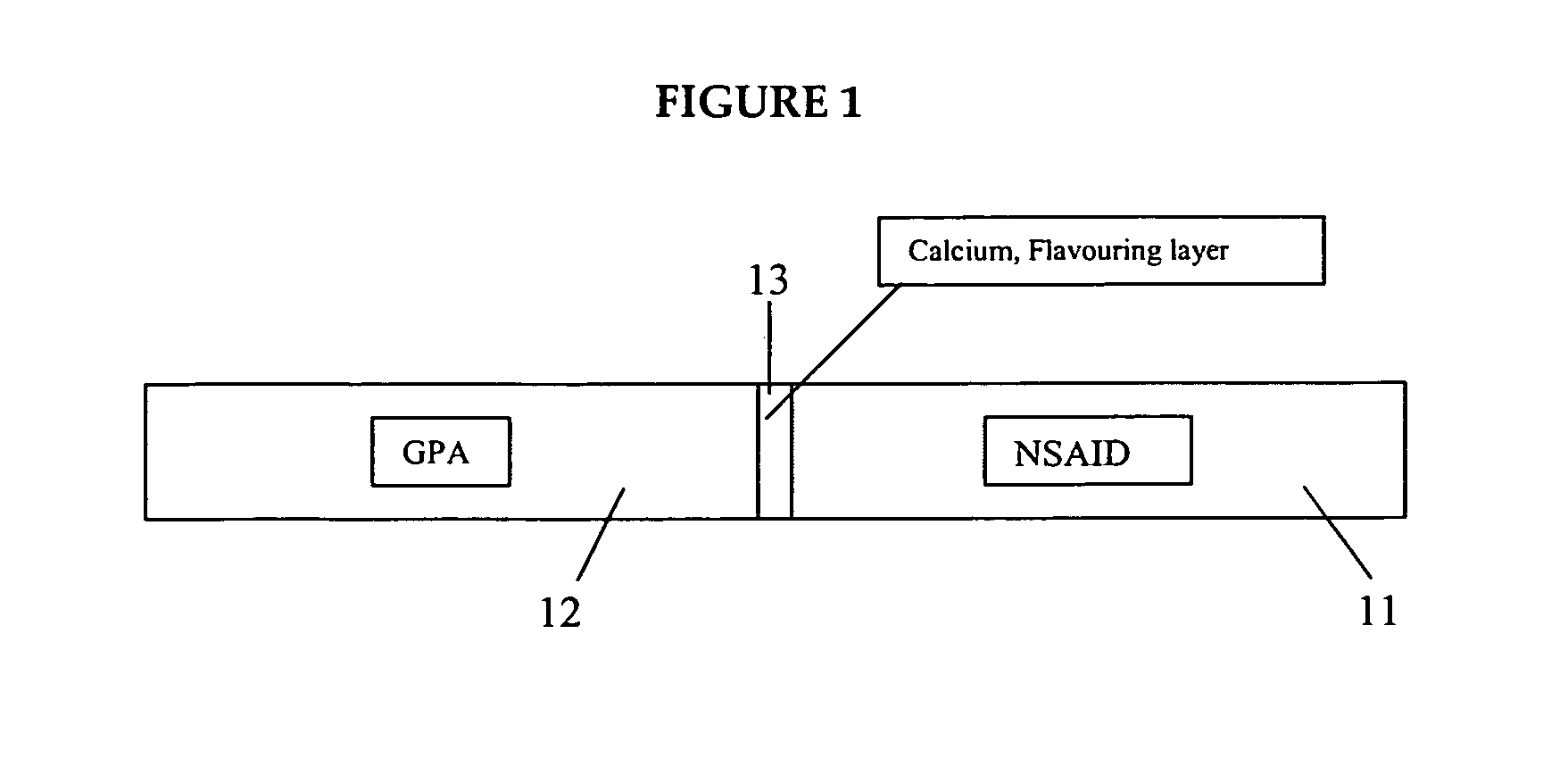
Rapid disintegrating tablets (RDTs) for pharmaceutical use and method for preparing the same
InactiveUS20050053655A1Disintegrates quicklyBiocideSalicyclic acid active ingredientsAdditive ingredientActive agent
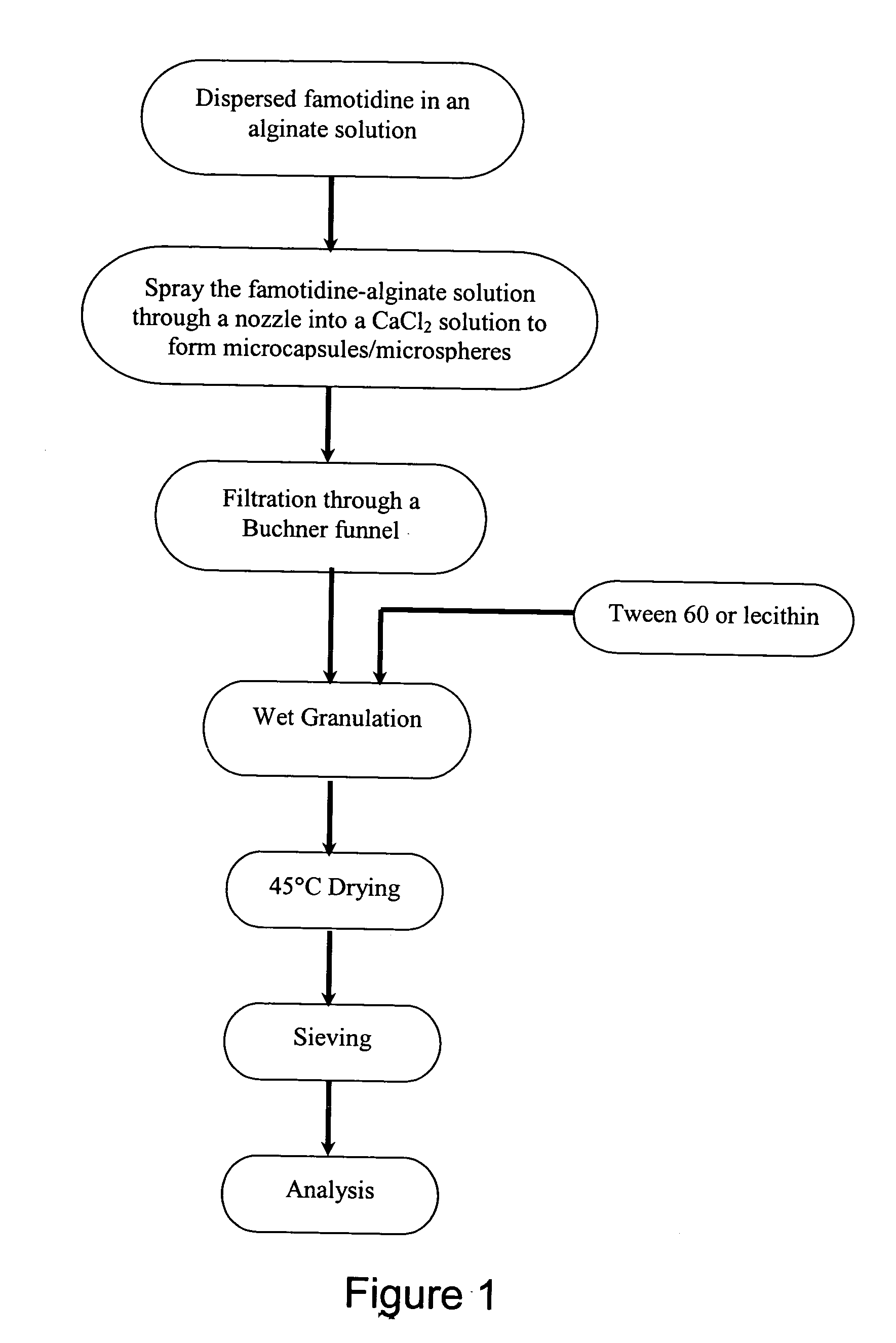
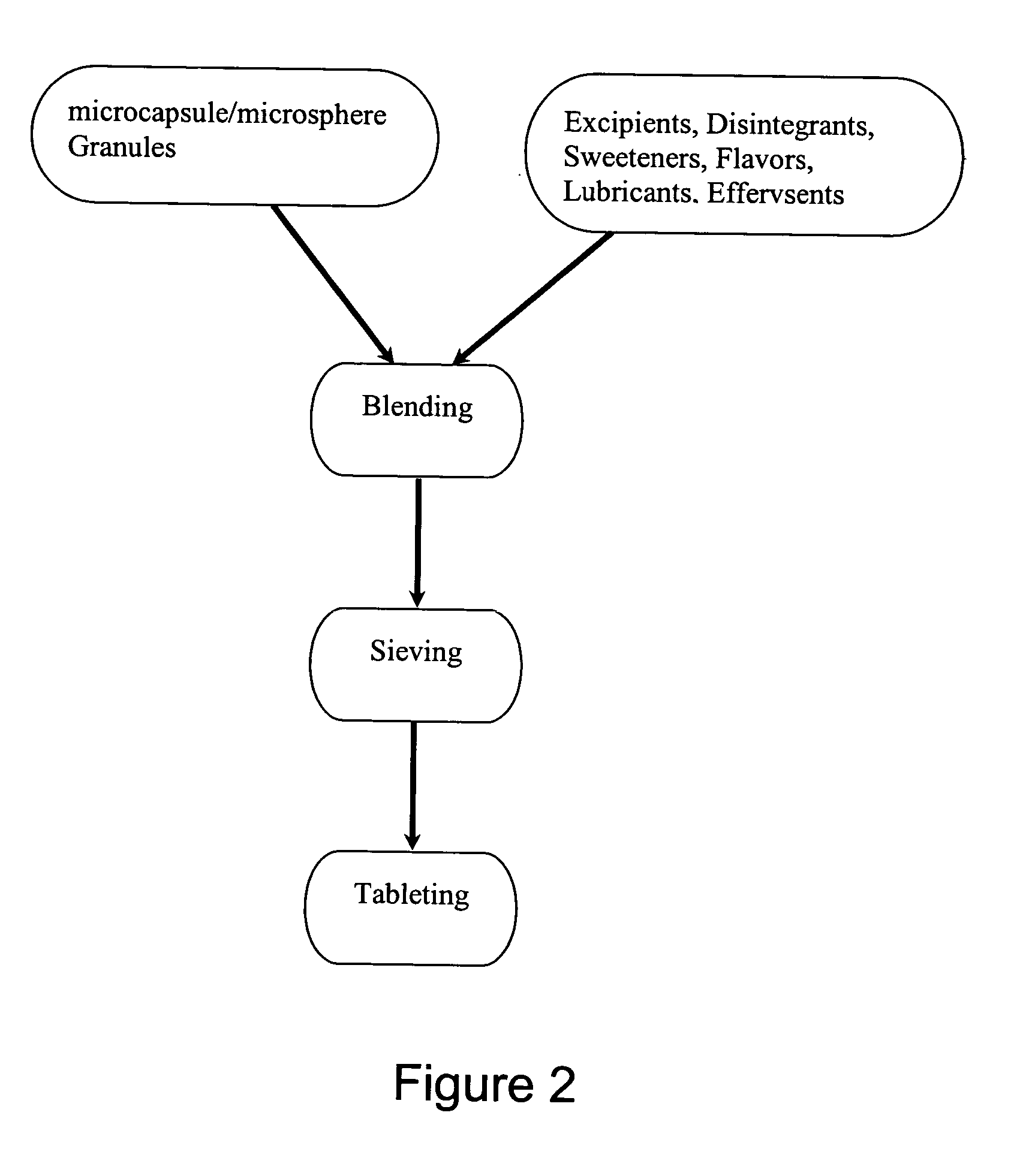
The present invention provides a fast-disintegrating tablet (RDT) and the method of preparing the RDT. The RDT contains a plurality of microcapsules which contains an active pharmaceutical ingredient surrounded by a polymeric matrix formed by a hydrogel. The microcapsules are separated from each other by a surfactant, particularly lecithin. The RDT is particularly suitable for use as a drug delivery system for antiacid or antiulcer drugs, such as famotidine. The RDT is further characterized by their its fast disintegration time of about 3 second to 3 minutes.
Owner:MEDICAL & PHARMA IND TECH & DEV CENT
Methods and Medicaments for Administration of Ibuprofen
ActiveUS20080063706A1Reduce the possibilityReducing patient-to-patient variabilityBiocideAntipyreticFamotidineIbuprofen
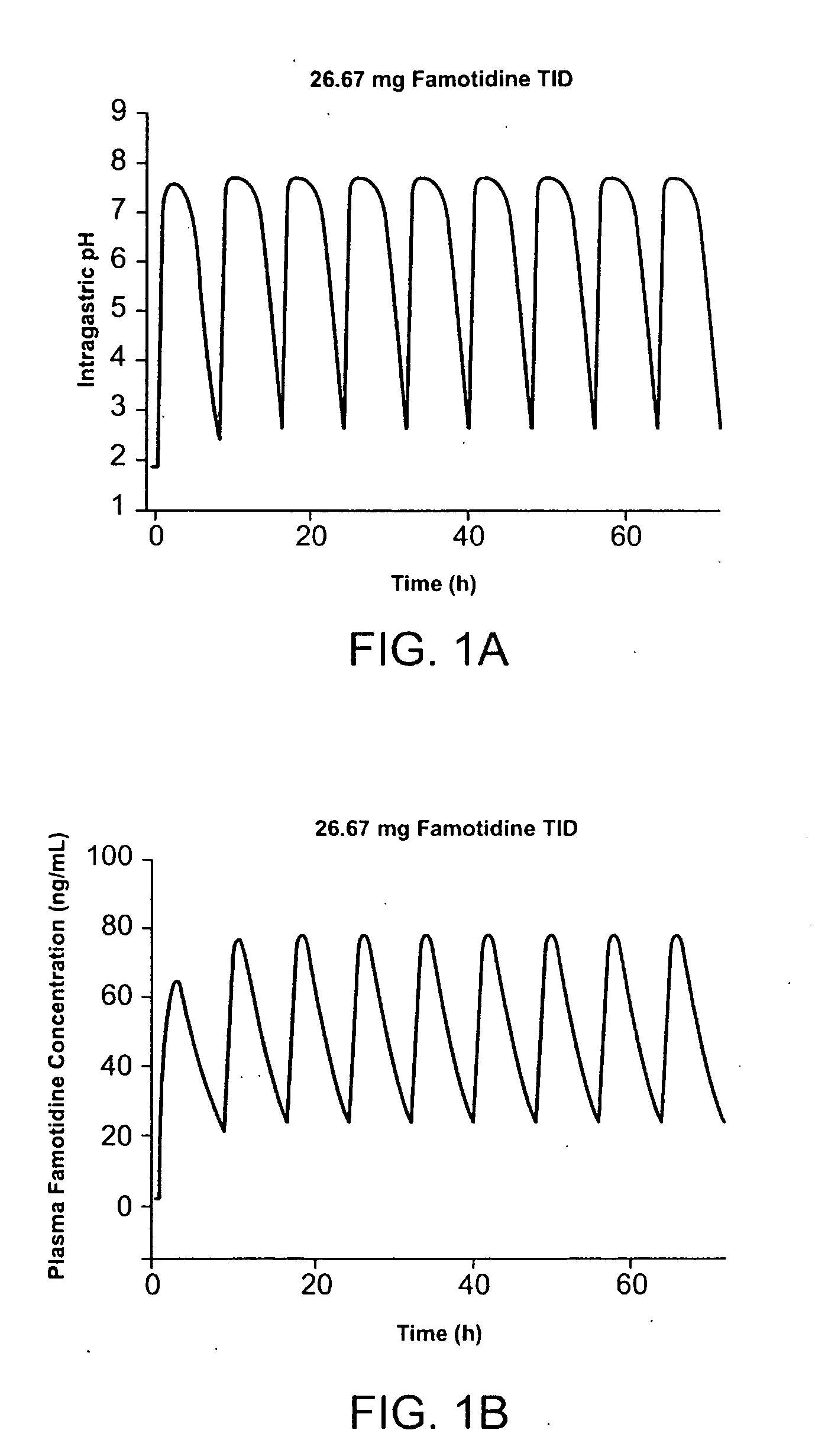
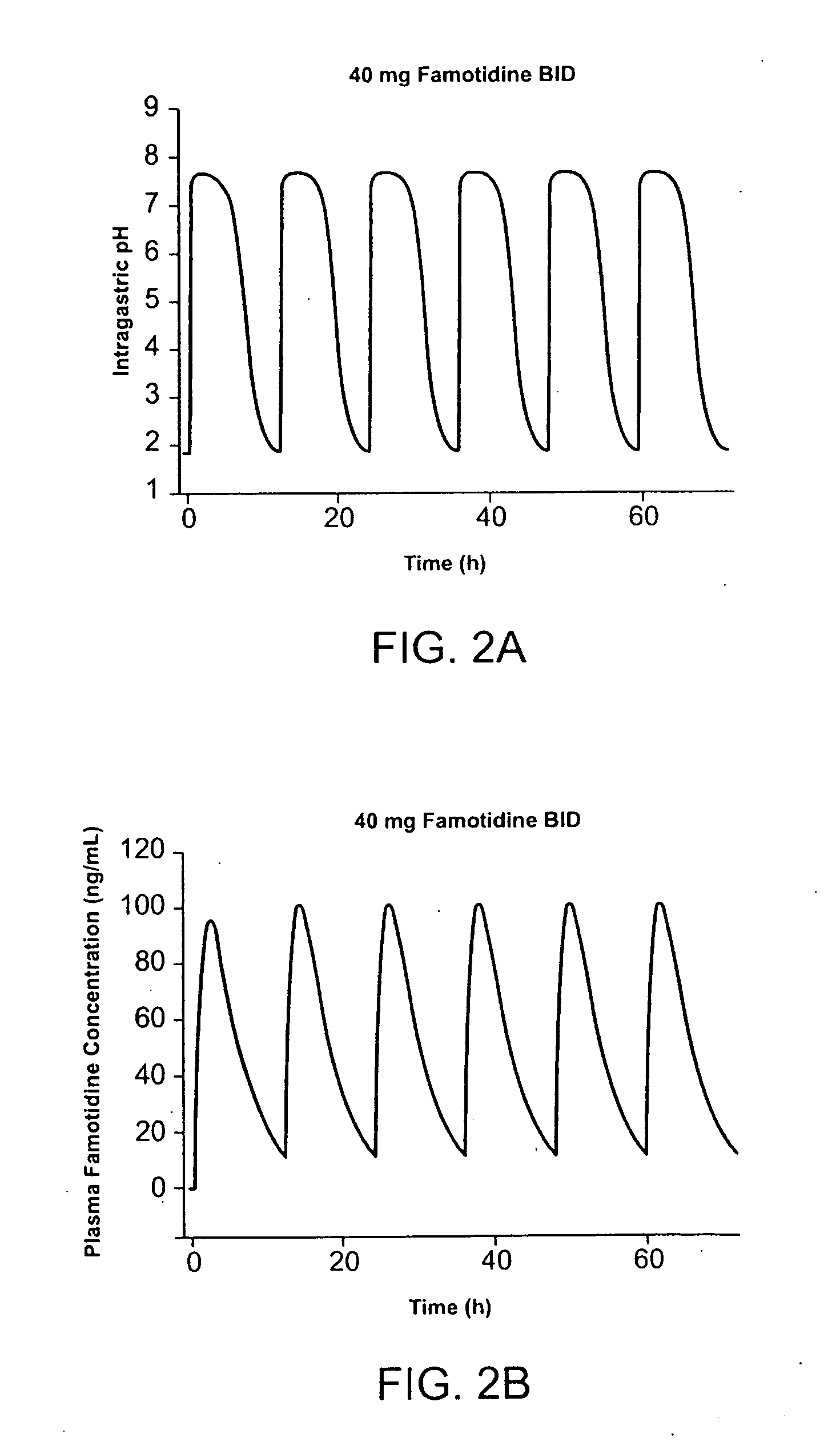
A method for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine is administered three times per day.
Owner:HORIZON MEDICINES LLC
Stable Compositions of Famotidine and Ibuprofen
Stable pharmaceutical compositions of famotidine and ibuprofen in a single unit dosage form are disclosed herein. The compositions comprise a famotidine core having a reduced or minimal surface area surrounded by a layer of ibuprofen. In some embodiments, the ibuprofen is in direct physical contact with the famotidine.
Owner:HORIZON MEDICINES LLC
Medicaments containing famotidine and ibuprofen and administration of same
InactiveUS20070043097A1Reduce gastrointestinal side effectsImprove protectionBiocideAntipyreticFamotidineIbuprofen
A method for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine, in admixture, is administered three times per day.
Owner:HORIZON MEDICINES LLC
Unit dose form for administration of ibuprofen
An oral dosage form for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine, in separate compartments, in amounts suitable for three times per day administration.
Owner:HORIZON MEDICINES LLC
Medicated gumstick for treatment in anti-inflammatory conditions and prophylaxis against NSAID gastropathy
InactiveUS20070003490A1Promote absorptionMinimize contactAntipyreticAnalgesicsDiseaseMedicated chewing-gum
A stick of gum is provided containing therapeutic benefits of non-steroid anti-inflammatory drugs for inflammation in conditions such as arthritis, and also alleviates subsequent side effects of NSAID administration, as well as antacid effects from compounds such as an H2 antagonist (ranitidine, cimetidine, famotidine) and / or a proton pump inhibitor (such as lansoprazole, pantoprazole, omeprazole, esomeprazole or rabeprazole) and / or an acid pump antagonist selected from the group of soraprazan, AZD0865, YH1885 and CS-526.
Owner:MEDICAL FUTURES
Methods and Medicaments for Administration of Ibuprofen
A method for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine is administered three times per day.
Owner:HORIZON MEDICINES LLC
Compound medicine contg. famotidine cyclodextrin clathrate, and its prepn. method
InactiveCN1868473AMask bitternessImprove medication complianceOrganic active ingredientsDigestive systemMedicineFamotidine
A compound medicine taken orally is prepared from famotidine, cyclodextrin and antiacid through including the famotidine particles by cyclodextrin, and mixing the inclusion compound with antiacid.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Method for preparing compound famotidine chewing tablet
ActiveCN1768744AImprove stabilityClinical application safetyOrganic active ingredientsDigestive systemTherapeutic effectFamotidine
The invention relates to a process for preparing compound Famotidine chewable tablets, which comprises separating Famotidin from calcium carbonate and magnesium hydroxide, thus no reactions with be produced between the effective compositions, thus the product stability can be improved.
Owner:四川泰华堂制药有限公司
Medicine composition for preparing canker and preparing method thereof
InactiveCN1552409ARaise the ratioLow costDigestive systemMolluscs material medical ingredientsAlcoholCuttlefish
A Chinese medicine in the form of powder for treating gastric ulcer and duodenal ulcer is prepared through decocting astragalus root, white peony root and liquorice root, filtering, concentrating, depositing in alcohol, distilling to remove alcohol, concentrating, mixing with cuttlefish bone powder and famotidine, drying and pulverizing. Its advantages are high cure rate and low cost.
Owner:黄伟
Famotidine composition for injection and preparation method thereof
InactiveCN101972248AUniform and stable contentUniform and accurate contentOrganic active ingredientsDigestive systemFreeze-dryingMedicine
The invention relates to a famotidine composition for injection. The famotidine composition comprises famotidine and L-aspartic acid; and the preparation method of the composition comprises the following steps of: 1) preparing: placing the famotidine and the L-aspartic acid in a preparation tank in a weight proportion of 1 to 0.4, filling injection water, stirring to completely dissolve the materials and uniformly mixing; 2) performing aseptic filtration, sub-packaging and partially stoppering; and 3) freeze-drying under vacuum to prepare the composition. The composition has a simple formula, an advanced process, good appearance molding, uniform and stable quality, uniform and accurate content, no moisture and high stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Methods and medicaments for administration of ibuprofen
A method for administration of ibuprofen to a subject in need of ibuprofen treatment is provided, in which an oral dosage form comprising a therapeutically effective amount of ibuprofen and a therapeutically effective amount of famotidine is administered three times per day.
Owner:HORIZON MEDICINES LLC
Famotidine composition for injection and preparation method thereof
InactiveCN101972248BUniform and stable contentUniform and accurate contentOrganic active ingredientsDigestive systemFreeze-dryingFiltration
The invention relates to a famotidine composition for injection. The famotidine composition comprises famotidine and L-aspartic acid; and the preparation method of the composition comprises the following steps of: 1) preparing: placing the famotidine and the L-aspartic acid in a preparation tank in a weight proportion of 1 to 0.4, filling injection water, stirring to completely dissolve the materials and uniformly mixing; 2) performing aseptic filtration, sub-packaging and partially stoppering; and 3) freeze-drying under vacuum to prepare the composition. The composition has a simple formula,an advanced process, good appearance molding, uniform and stable quality, uniform and accurate content, no moisture and high stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Famotidine high density type gastric retention osmotic pump controlled release preparation and preparation method thereof
InactiveCN101380313ARelease completelyPromote absorptionOrganic active ingredientsDigestive systemAbsorption capacityRelease time
The invention belongs to the technical field of pharmaceutical preparation and discloses a high-density gastric stasis osmotic pump controlled release famotidine preparation and a preparation method thereof. The osmotic pump controlled release preparation is made from a tablet core containing famotidine and a semi-permeable coating membrane with drug release pores which is coated outside the tablet core; the tablet core is made from 400mg of the famotidine, 100-150mg of medicinal ferrous powder, 30-90mg of a suspension, 20-80mg of an osmotic pressure active material and 0.5-2mg of a lubricant; the semi-permeable coating membrane is made from 10-20g of a semi-permeable polymer material and 2-5g of a water-soluble pore making agent, and accounts for 5-9% of the weight of the tablet core; and the drug release pores are drilled at one side or two sides of a coating tablet by laser and by mechanical means. The medicinal ferrous powder is added to the tablet core to cause the drug to be more completely released from the drug release pores; meanwhile, the medicinal ferrous powder increases the density of the preparation; therefore, the preparation is stagnated in the folds of the lower part of the stomach, which greatly prolongs the release time of the pharmaceutical preparation in the stomach and improves the absorption capacity of the pharmaceutical preparation.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation of composite motidine dispersant pills
InactiveCN1456160AMeet medication needsDisintegrates quicklyOrganic active ingredientsDigestive systemCross-linkAdhesive
A dispersing tablet of famotidine features that on the basis of its chewing tablet, the special additives are used for it, such as excellent disintegrant (cross-linked povidone, sodium laurylsulfate, or microcrystal cellulose) and high-performance adhesive (povidone K30) are used in it. Its advantages are high disintegrating speed, high curative effect and high safety.
Owner:金日制药(中国)有限公司
Lafutidine injecta and its prepn
InactiveCN1421204AImprove stabilityImprove bioavailabilityOrganic active ingredientsDigestive systemVeinTreatment effect
The present invention is Lafutidine injection for intravenous injection and intramuscular injection. The recipe of the Lafutidine injection includes Lafutidine, organic solvent, surfactant, antioxidant, osmotic pressure regulator and water for injection. Compared with Lafutidine tablet, the Lafutidine injection has fast effect, short peak reaching time in the blood high medicine concentration andhigh treating effect; and compared with Famotidine or Ranitidine injection, the Lafutidine injection has less dosage and higher curative effect in treating gastric ulcer.
Owner:黄振华
Method for preparing famotidine sodium chloride injection by inclusion method and product prepared by using the same
ActiveCN102225050BImprove solubilityImprove stabilityOrganic active ingredientsDigestive systemAcetic acidSodium Chloride Injection
The invention relates to a method for preparing a famotidine sodium chloride injection by an inclusion method and a product prepared by using the same. The method is characterized in that sulfobutyl ether-beta-cyclodextrin is used as an including agent of famotidine, and famotidine is firstly dissolved in glacial acetic acid and then added into a sulfobutyl ether-beta-cyclodextrin saturated solution to allow sulfobutyl ether-beta-cyclodextrin to include famotidine, and the sulfobutyl ether-beta-cyclodextrin including famotidine is dissolved in water; the weight part ratio of famotidine to sulfobutyl ether-beta-cyclodextrin is (1:5)-(1:10); the quantity of glacial acetic acid is 5-7 times of that of famotidine based on weight part; and excessive sterilization at 121 DEG C for 15 minutes isadopted. The method provided by the invention has the following advantages: related substances are controlled within 2%, which is far lower than 5% of domestic like-products, and the sterilization ischanged from a residual probability method to an excessive sterilization method, which ensures 100% sterilization, greatly improves product quality and enhances the safety of medicine in clinical applications.
Owner:HUIYINBI GRP JIANGXI EAST ASIA PHARMA CO LTD
Stable compositions of famotidine and ibuprofen
Owner:HORIZON MEDICINES LLC
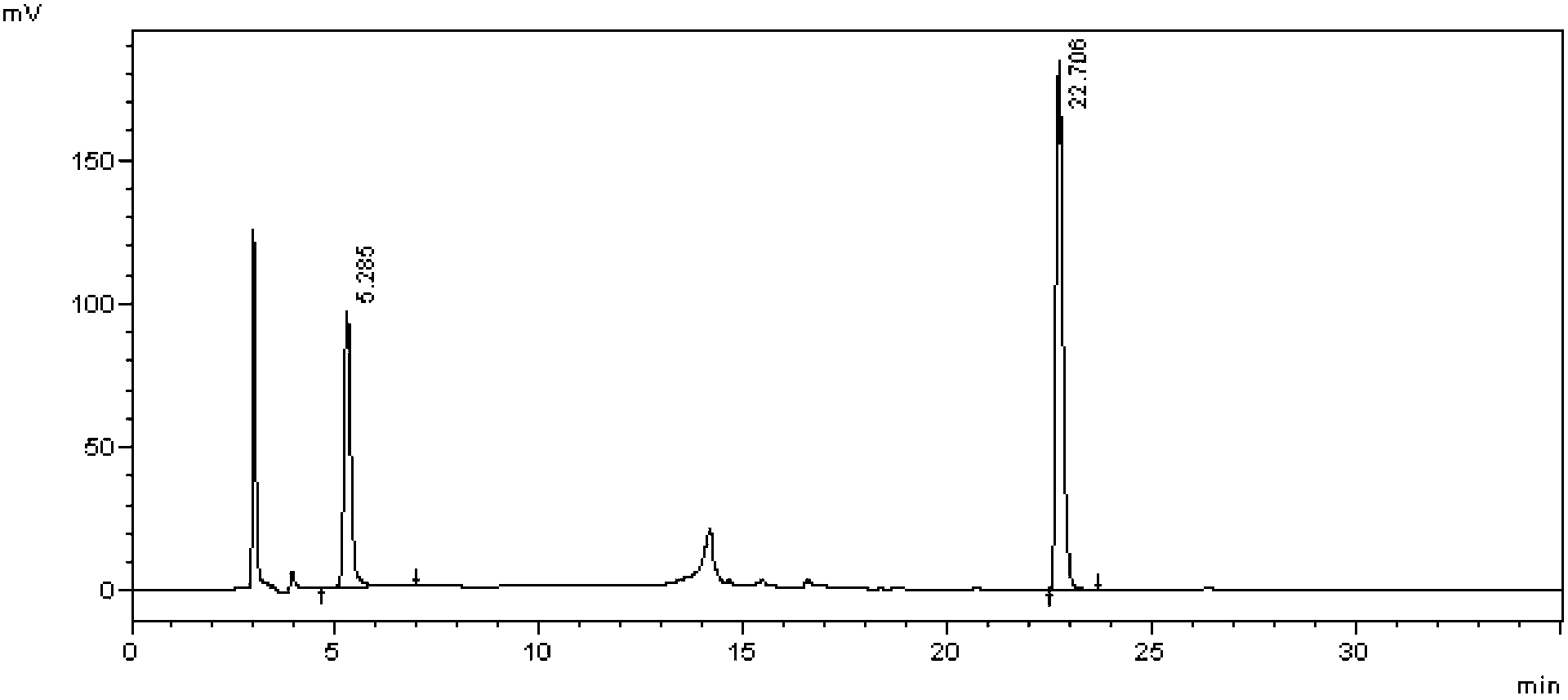
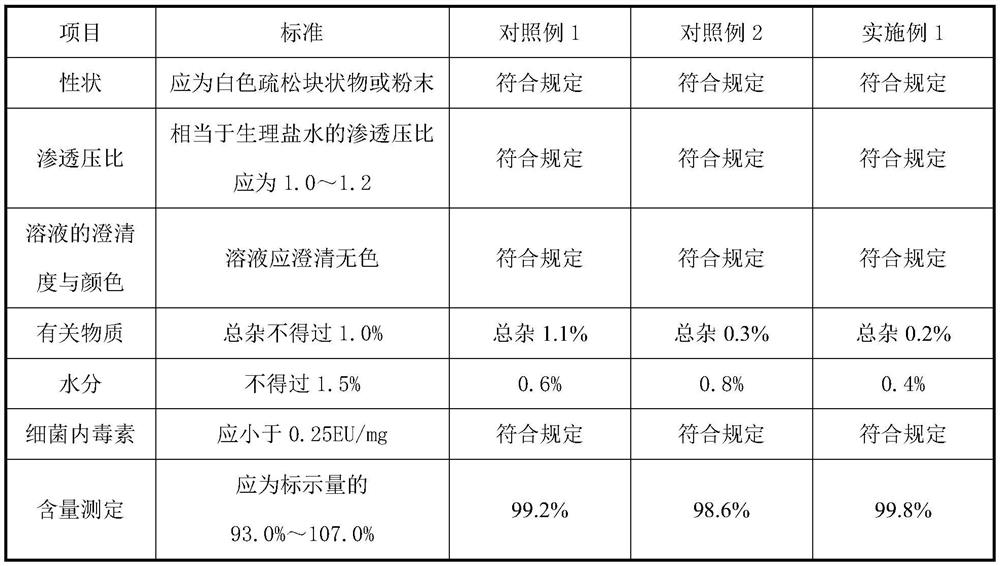
Method for measuring buprofen/famotidine compound preparation content
The invention discloses a method for measuring a buprofen / famotidine compound preparation content. The method comprises the following steps of: dissolving a compound preparation sample by methanol as a solvent; measuring by using a high performance liquid chromatography, wherein the measurement wavelength is 265nm; and with an octadecyl silane bonded silica gel filler as a stationary phase, performing gradient elution according to the following mobile phase conditions: the mobile phase A is a 0.02mol / L sodium acetate solution, pH is regulated to be 4.0 by using phosphoric acid, and the mobile phase B is acetonitrile. The method provided by the invention is time-saving and labor-saving, high in precision, accurate in content determination result and excellent in repeatability, and can be used for conventional analysis and quality control on the buprofen / famotidine compound preparation sample.
Owner:NANJING CORE TECH CO LTD
Preparation method of famotidine preparation for injection
PendingCN112006999ALow content of related substancesFast dissolutionPowder deliveryOrganic active ingredientsActivated carbonMannitol
The invention provides a preparation method of a famotidine preparation for injection, and relates to the technical field of medicaments. The method comprises the following steps: s1, weighing a prescription amount of aspartic acid, adding a proper amount of water for injection, keeping the liquid preparation temperature within the range of 20-30 DEG C, performing stirring to dissolve the asparticacid, sequentially adding prescription amounts of famotidine, mannitol and poloxamer-188, and adding the water for injection to full dose; s2, adding activated carbon, and performing stirring at normal temperature, wherein the dosage of the activated carbon is 0.1% (g / mL) of the total amount of the liquid medicine; and s3, after sterilization, filtration and split charging, adopting a rubber plughalf-pressure plug of which the drying weight loss is controlled to be 0.15% or below after cleaning and sterilization, and performing freeze-drying according to set freeze-drying parameters. According to the invention, moisture can be kept stable in the storage period, the main drug dissolving speed can be increased, and the liquid preparation time is obviously shortened.
Owner:江苏大同盟制药有限公司
Famotidine composition for injection
InactiveCN103301122AGood treatment effectAvoid instabilityOrganic active ingredientsPowder deliveryOral medicationHalf-life
The invention provides a famotidine composition for injection, and relates to the technical field of medicine manufacturing. The main medicine of the composition comprises famotidine and melatonin, wherein the melatonin comprises a quick release part and a cyclodextrin-included slow release part. According to the famotidine composition for injection provided by the invention, the therapeutic effect of famotidine is improved, instability caused by oral administration of MT (Melatonin) is avoided and MT is quick to distribute and eliminate and the like, and the first pass effect of MT is reduced. The design of dosage combining quick release and slow release is in accordance with secretion characteristic of MT, so that the problem of half-life period of MT is solved and the bioavailability of melatonin is improved. Melatonin combined with famotidine can be used for effectively inhibiting the secretion of gastric acid, so that the composition has a good synergistic effect for treating benign active gastric ulcer.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Famotidine inclusion compound and preparation method
InactiveCN103656649AHydrophobicWith anti-oxidationOrganic active ingredientsDigestive systemWater bathsAbsorption capacity
The invention provides a famotidine inclusion compound and a preparation method. The inclusion compound comprises famotidine and glyceryl behenate. The preparation method comprises the following steps: the glyceryl behenate is heated to 80 DEG C through water bath or steam, stirring and fusion are performed on the glyceryl behenate at the temperature of 80 DEG C; the famotidine, which is equal to the glyceryl behenate in weight, is added into the fused glyceryl behenate, stirring and mixing are performed for 25-35 minutes at the temperature of 80 DEG C or homogenizing is performed through a homogenizer; the mixture prepared through the step 2 is fed into a spray dryer for drying before cooling, so that the famotidine inclusion compound is obtained. The famotidine inclusion compound has the benefits as follows: because the glyceryl behenate has the characteristics of hydrophobicity, anti-oxidation, hydrolysis prevention and the like, and is used for famotidine inclusion, the water absorption capacity of the famotidine self is reduced, and the famotidine is separated from other substances in the compound, the stability of the drugs is enhanced, the drug therapeutic effect is maintained, the bitter taste of the famotidine is covered up, and the bad taste during taking is reduced.
Owner:吉林修正药业新药开发有限公司
Composition and method for direct visualization of the human appendix
InactiveUS20060251577A1Rapid and direct and unambiguous visualizationImprove bioavailabilityIn-vivo radioactive preparationsX-ray constrast preparationsMeglumine diatrizoateSodium Diatrizoate
A positive contrast agent composition containing meglumine diatrizoate, sodium diatrizoate, simethicone, famotidine and aspartame in predetermined amounts that is orally administered to a patient for clinical evaluations of appendicitis wherein a positive contrast effect is achieved. Methods of use include orally administering individual doses of the composition approximately 50 minutes prior to appendix visualization using computerized axial tomography.
Owner:VINCON RESEARC ENTERPRISES
Famotidine calcium magnesium chewable tablet
The invention relates to a famotidine calcium magnesium chewable tablet. The famotidine calcium magnesium chewable tablet contains famotidine, calcium carbonate and magnesium hydroxide. In particular, the invention belongs to the technical field of medicine, and relates to a medicinal composition for treating stomach disease, in particular to a compound medicinal composition of famotidine, calcium carbonate and magnesium hydroxide, in particular to a famotidine calcium magnesium chewable tablet which has superior medicinal property and contains famotidine, calcium carbonate and magnesium hydroxide. The famotidine calcium magnesium chewable tablet has superior pharmaceutical property and superior chemical stability. The famotidine calcium magnesium chewable tablet can be clinically used for treating or preventing acid regurgitation, gastric acid, heartburn (stomach burning), stomachache, stomach distension, belching and other stomach discomfort. A preparation method of the famotidine calcium magnesium chewable tablet is further related.
Owner:YANTAI RONGCHANG PHARMA
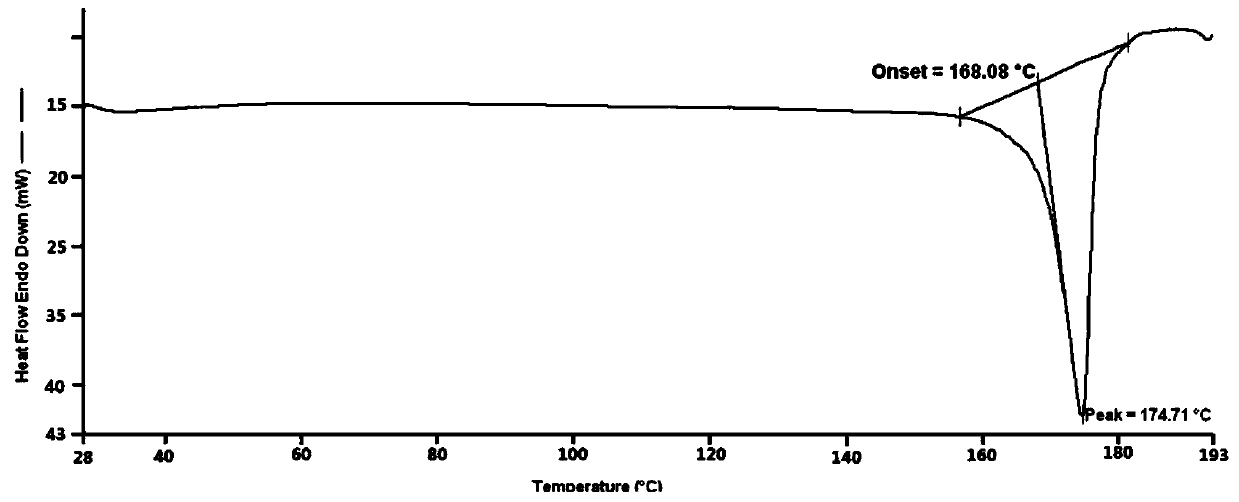
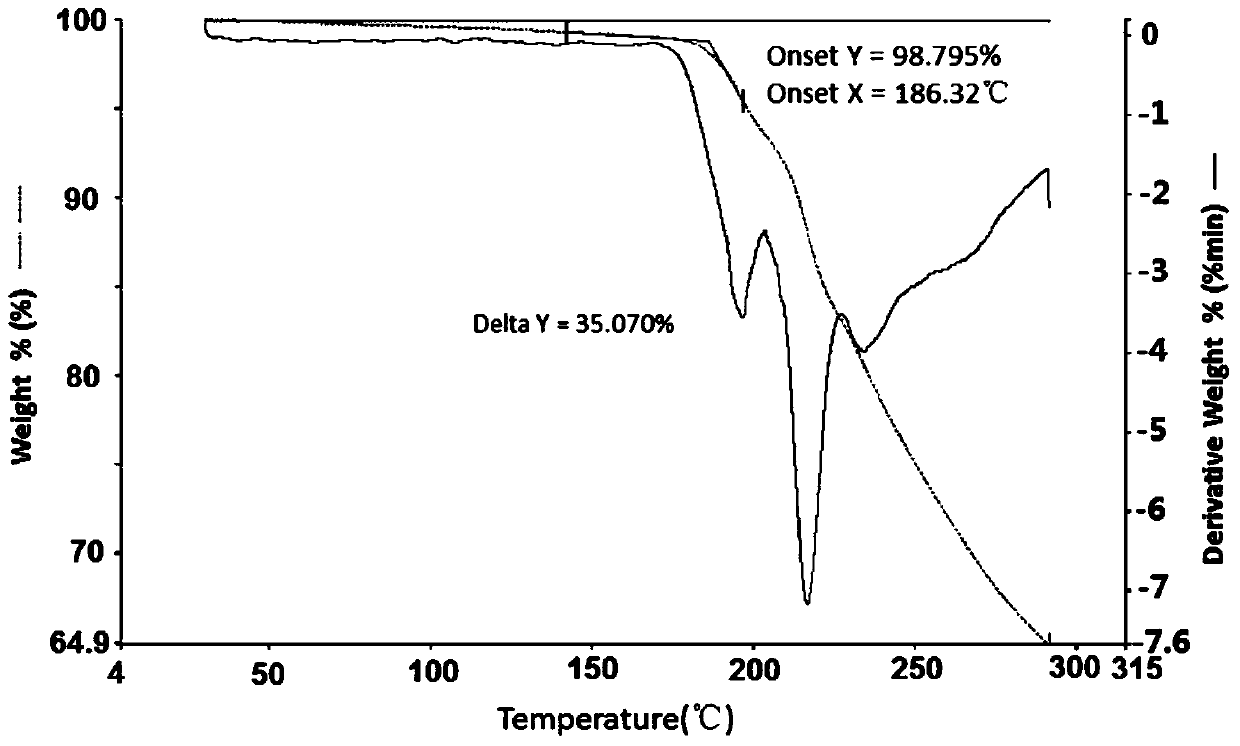
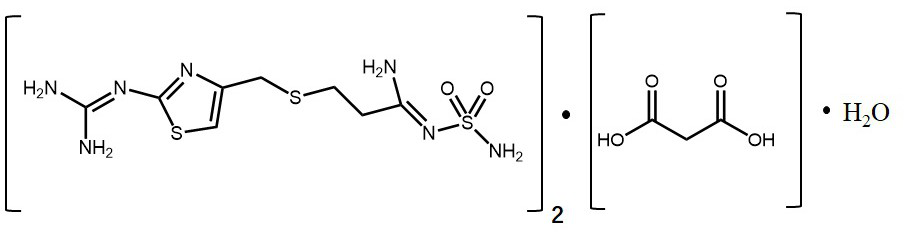
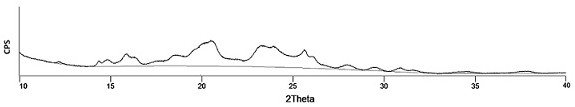
Famotidine-malic acid eutectic crystal and preparation method thereof
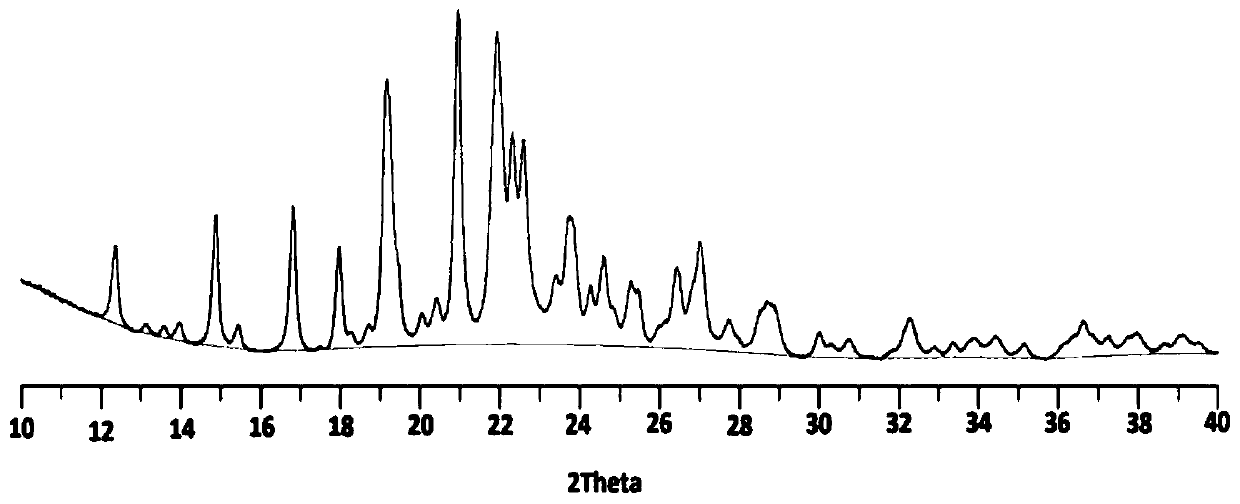
ActiveCN111592503AStability has no effectImprove solubilityOrganic active ingredientsOrganic chemistry methodsHigh humidityPhysical chemistry
The invention discloses a famotidine-malic acid eutectic crystal and a preparation method thereof. The molar ratio of famotidine to malic acid in the eutectic crystal is 2:1. The invention also discloses the preparation method of the eutectic crystal. The famotidine-malic acid eutectic crystal provided by the invention has a characteristic melting point, an infrared spectrum, an X-ray powder diffraction pattern and the like. The eutectic crystal improves the solubility of famotidine in water, does not change the stability of famotidine under the conditions of artificial gastric juice, high temperature and high humidity, and has the potential of improving the bioavailability of famotidine. The preparation method of the famotidine-malic acid eutectic crystal is simple in step, easy to operate and good in reproducibility, and has great commercial application value.
Owner:青岛市食品药品检验研究院
Medicine for curing pain syndrome of late cancer and its preparation
InactiveCN1391952ACurbing rapid growthRelieve painHydroxy compound active ingredientsUnknown materialsCancer cellPain syndrome
The medicine for treating pain syndrome of later stage cancer is one Chinese and Western combined medicine, which is prepared with astragalus root, privet fruit, sealwort and other Chinese medicinal materials as well as Indomethacin, Dexamethasone, Spironolactone, Famotidine and Diazepam. It has the functions of resisting cancer, diminishing inflammation, relieving pain, promoting diuresis and raising immunity; and in can inhibit the growth of cancer cell, relieve pain of the later stage cancer patient, raise survical quality and prolong life.
Owner:潘远志
Refining technique of famotidine raw material
The present invention belongs to the field of medicine producing technology, and is especially process of refining famotidine material. The technological scheme of the present invention is that the famotidine material refining process includes the following steps: weighing famotidine material in certain ratio, adding water solution of alcohol, heating reflux to form light orange solution, hot filtering, cooling to room temperature, cooling in cold storage room of refrigerator, suction filtering, washing and vacuum drying to obtain white powder. The present invention has the advantages of simple technological process, high safety, no toxicity and capacity of eliminating impurity from famotidine material effectively.
Owner:HAINAN PULIN PHARMA +1
Eutectic of famotidine and adipic acid and preparation method thereof
ActiveCN113234038AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistry methodsAdipic acidFamotidine
The invention discloses a famotidine and adipic acid eutectic crystal and a preparation method thereof. The eutectic crystal is a hydrate containing one molecule of water, and the molar ratio of famotidine to adipic acid is 2: 1. The invention also discloses a preparation method of the eutectic crystal. The famotidine-adipic acid eutectic crystal provided by the invention has a characteristic melting point, an X-ray powder diffraction pattern and the like. According to the eutectic crystal, the solubility of famotidine in water is improved, the stability of famotidine under high-temperature and high-humidity conditions is improved, and the eutectic crystal has the potential of improving the bioavailability of famotidine. The preparation method of the famotidine-adipic acid eutectic crystal is simple in step, easy to operate and good in reproducibility, and has great commercial application value.
Owner:青岛市食品药品检验研究院
Prpearation method of H2 receptor antagonist composite salt and its application
The present invention relates to a preparation method of H2 receptor antagonist compound salt and its application. The optimized H2 receptor antagonist is famotidine, and the optimized metal ions forming compound salt are bismuth and ammonium. Said compound salt is applicable for curing gastrointestinal disorder, specially for curing gastroduodenal diseases. It also has the stronger protecting action for rat acute injury of gastric mucosa due to ethyl alcohol, and has the action of raising gastric juice acidity and reducing gastric acid secretion for gastric secretion due to pyloric ligation.
Owner:漆又毛
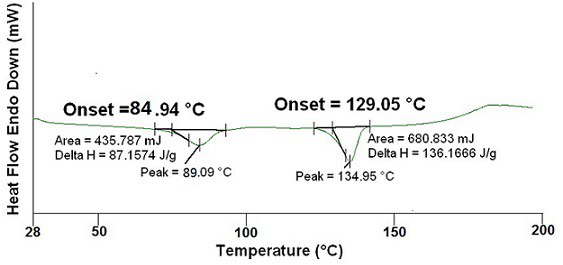
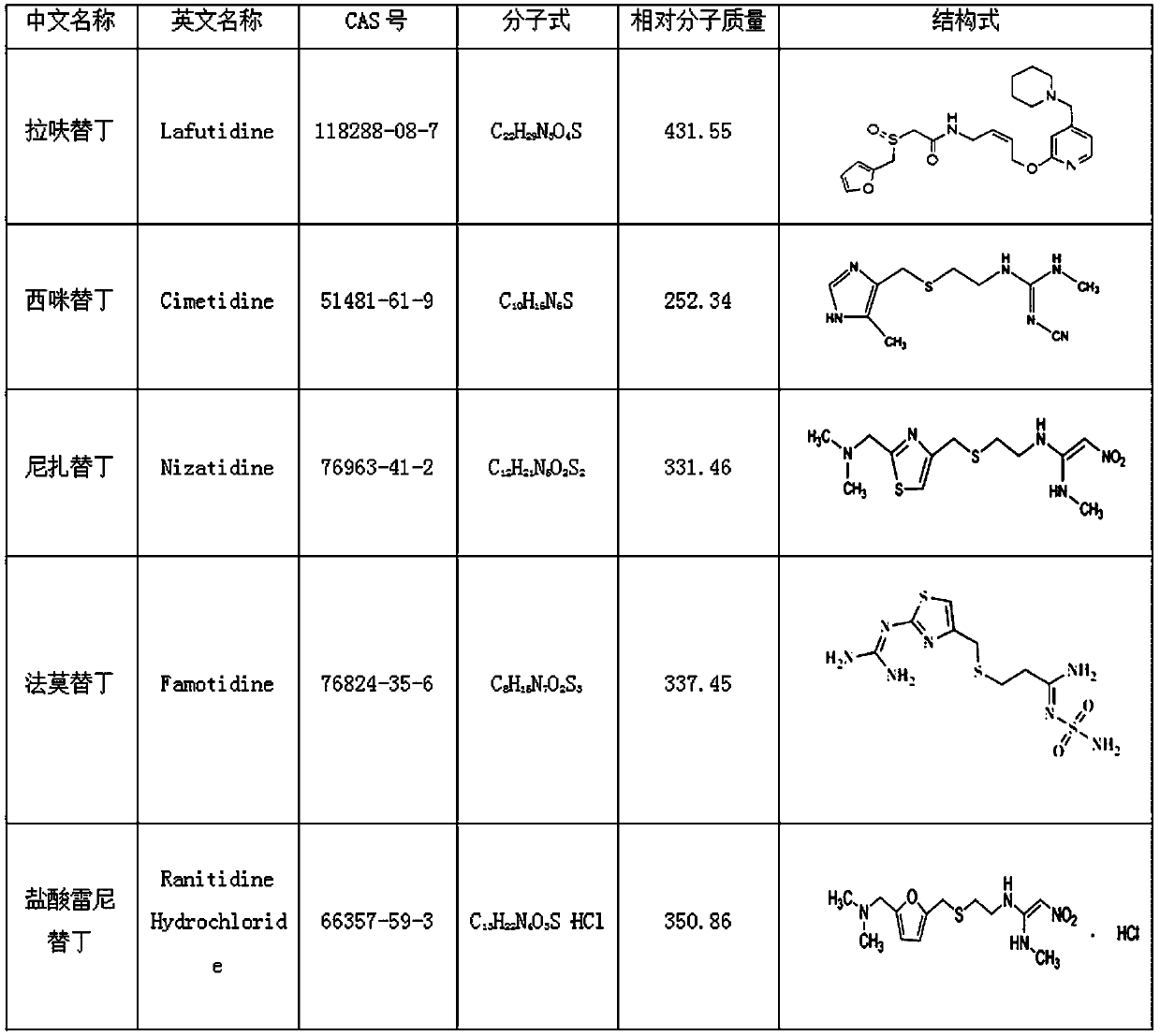
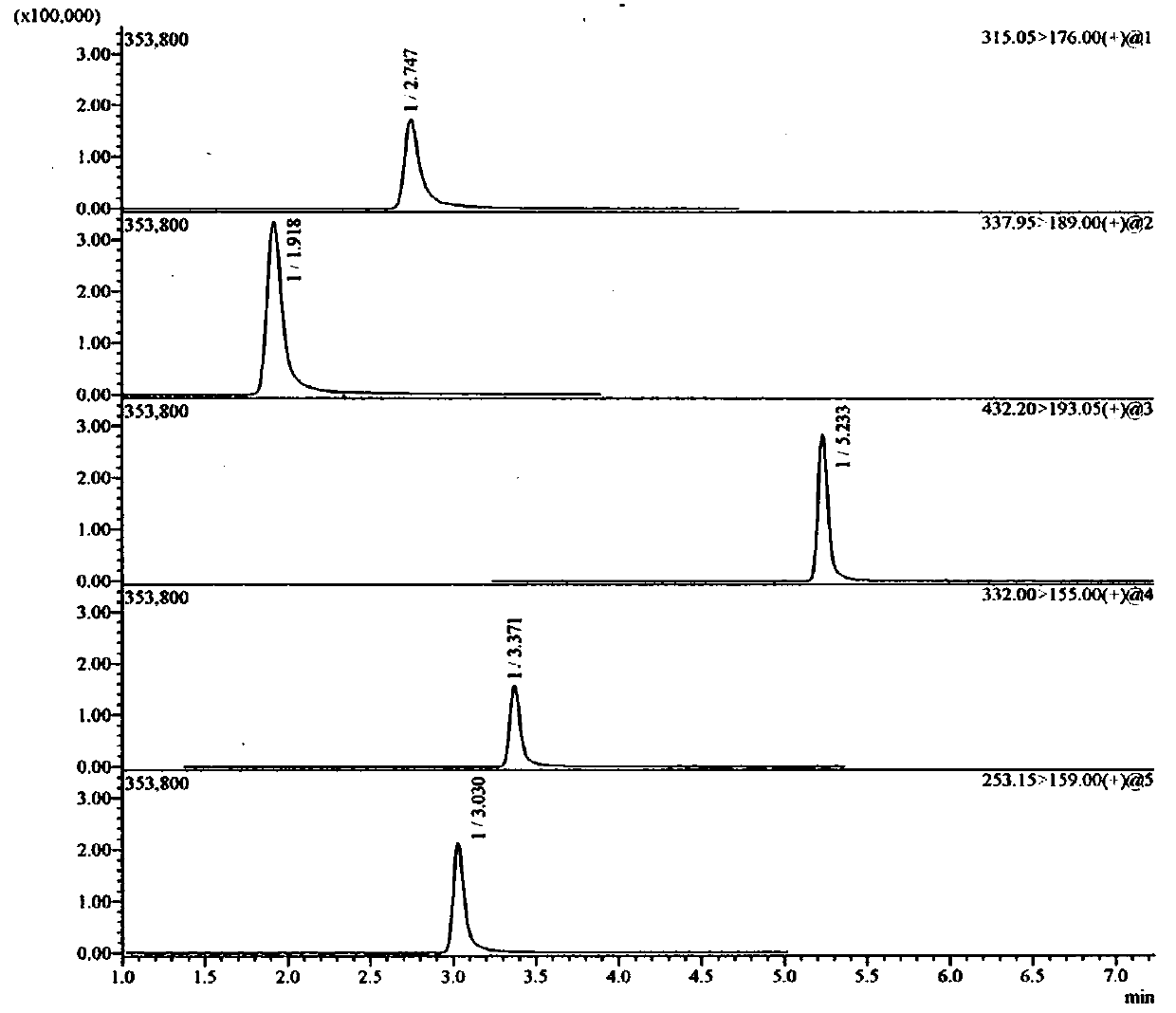
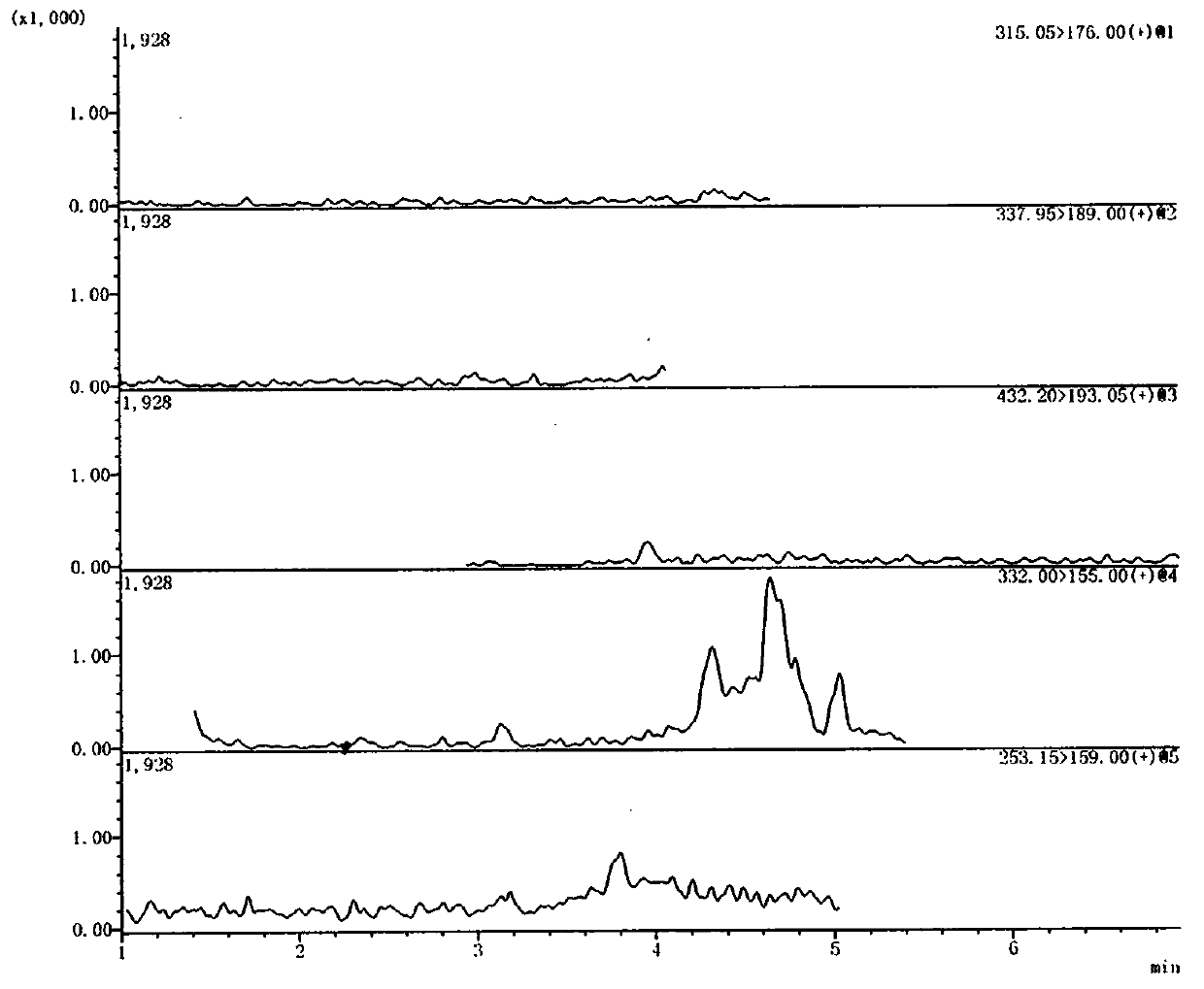
Method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine
The invention discloses a method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine. The method comprises the following steps: (1) selecting chromatographic conditions; (2) selecting mass spectrometric conditions; (3) preparing a standard solution; (4), preparing samples; (5), detecting and calculating. Tests prove that the method is rapid and high in specificity; the method is suitable for detecting the illegally added ranitidine hydrochloride, famotidine, lafutidine, nizatidine, cimetidine and other chemical medicines in Chinese patent medicines and health-care foods which have the effects of invigorating the stomach, improving the gastrointestinal function (having an auxiliary protection effect on gastric mucosal injury) and having an auxiliary protection effect on gastric mucosal injury or in other foods illegally claimed to have said functions.
Owner:太原市食品药品检验所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com