Famotidine high density type gastric retention osmotic pump controlled release preparation and preparation method thereof
A technology of osmotic pump controlled release and famotidine, which is applied in the field of famotidine high-density gastric retention osmotic pump controlled release preparation and its preparation, which can solve the problems of low bioavailability and short residence time of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
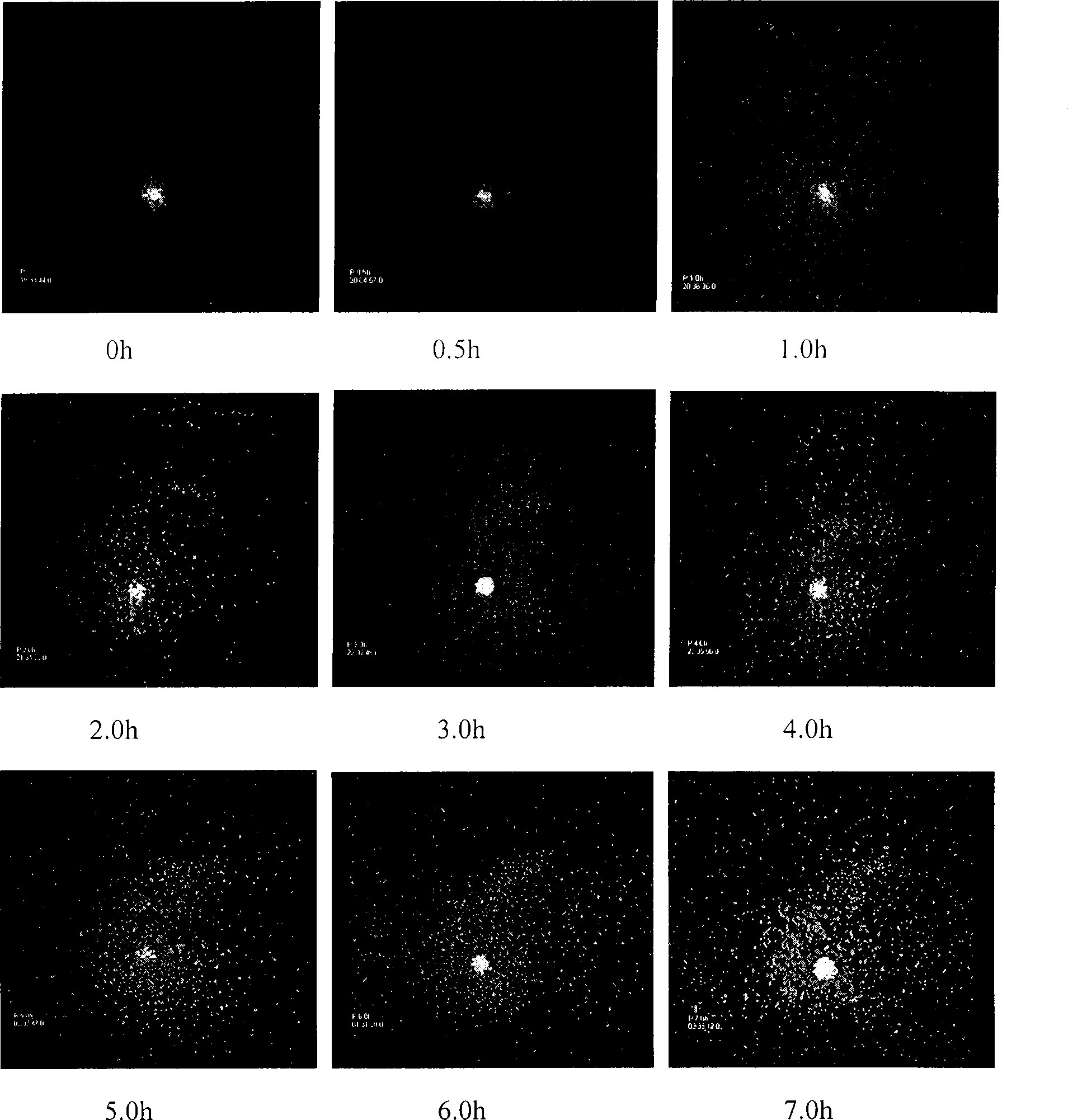
Image
Examples
Embodiment 1
[0025] Tablet prescription:
[0026] Famotidine 40mg
[0027] N12K 30mg
[0028] NaCl 50mg
[0029] Medicinal iron powder 150mg
[0031] Semi-permeable coating film prescription:
[0032] Cellulose acetate 20g
[0033] PEG4000 3g
[0034] Solvent prescription for dissolving film coating material:
[0035] Acetone 1000ml
[0036] water 20ml
[0037] Preparation Process:
[0038] Pass the prescribed amount of medicine, N12K, NaCl, medicinal iron powder and magnesium stearate through an 80-mesh sieve, mix evenly, and directly use No. 7 punched tablets to obtain tablet cores. Dissolve cellulose acetate and PEG4000 in acetone and water respectively and mix evenly, coat the tablet core with a coating pan, with a weight gain of 7%, and cure in an oven at 40°C for 12 hours after coating. Then prepare a 0.8 mm diameter release hole with a mechanical drill on one side of the coated tablet to obtain the controlled-release preparation of the famo...
Embodiment 2
[0040] Tablet prescription:
[0041] Famotidine 40mg
[0042] Sodium Alginate 80mg
[0043] Lactose 20mg
[0044] Medicinal iron powder 137.5mg
[0045] Micronized silica gel 1mg
[0046] Semi-permeable coating film prescription:
[0047] Cellulose acetate 15g
[0048] PEG6000 2g
[0049] Solvent prescription for dissolving film coating material:
[0050] Acetone 500ml
[0051] water 30ml
[0052] Preparation Process:
[0053]Pass the prescribed amount of medicine, sodium alginate, lactose, medicinal iron powder and micronized silica gel through an 80-mesh sieve, mix evenly, and directly use No. 7 punched tablets to obtain tablet cores. Dissolve cellulose acetate and PEG6000 in acetone and water respectively and mix evenly, coat the tablet core with a coating pot, the weight gain is 8%, and cure in an oven at 40°C for 12 hours after coating. Then, a 0.6 mm diameter drug release hole was prepared with a mechanical drill on both sides of the coated tablet to obtain th...
Embodiment 3
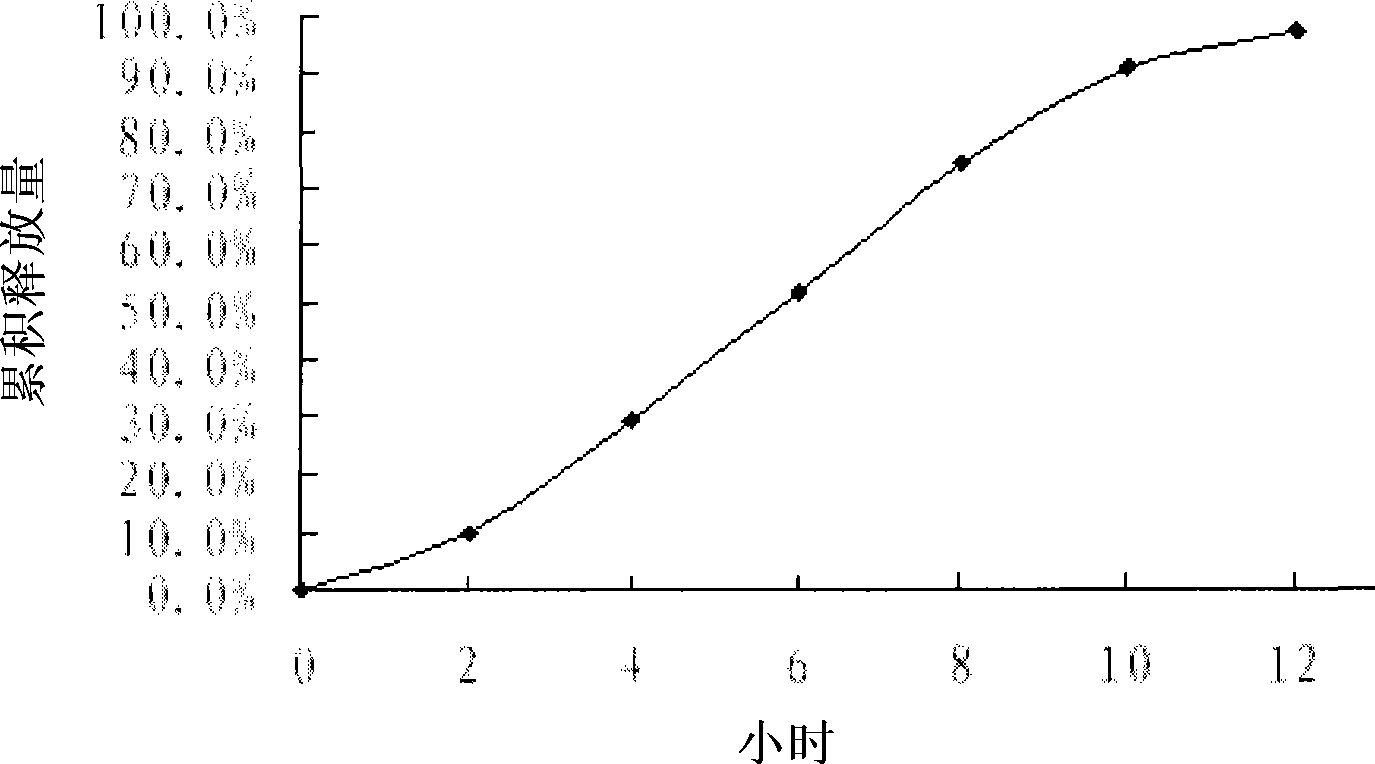
[0055] In vitro cumulative release of famotidine high density gastric retention osmotic pump tablets
[0056] According to "Pharmacopoeia of the People's Republic of China" 2005 edition two release determination method first method, the release medium is 900mL 0.1mol L -1 HCl, speed 100r·min -1 , Medium temperature (37±0.5)℃. 5 mL samples were taken at 2, 4, 6, 8, 10 and 12 hours respectively, and an equal volume of isothermal release medium was added at the same time. Dilute appropriately after passing through a 0.8μm microporous membrane, take the release medium as a blank, measure the absorbance at 266nm, and calculate the cumulative release.
[0057] attached figure 1 Represented is the in vitro cumulative 12-hour release percentage-time curve of the famotidine high-density gastric retention osmotic pump tablet prepared according to Example 1, and the results show that although the present invention makes a single-layer osmotic pump preparation for insoluble drugs, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com