Preparation method of protein drug long-acting preparation using polyketal as matrix
A polyketal and drug technology, applied in the field of medicine, can solve the problems of incomplete release and low bioavailability of long-acting sustained-release preparations, and achieve low bioavailability, improved stability, improved release and bioavailability degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
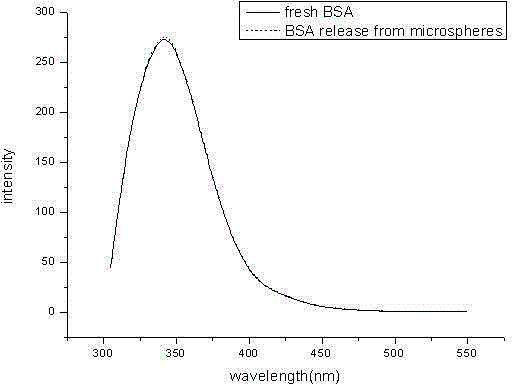
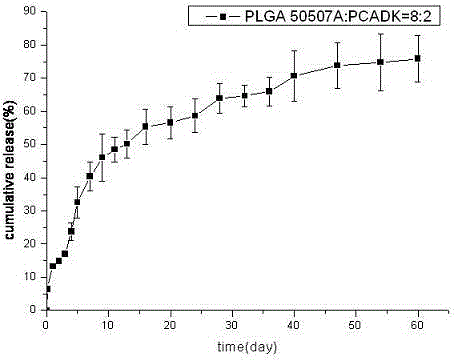
[0029] Weigh 60mg of BSA protein, dissolve it in 150ul 1%PVA, this is the inner water phase; weigh 400mg of PLGA50507A and polyketal PCADK 100mg, dissolve it in 2.25ml of dichloromethane, this is the oil phase; add the inner water phase to the oil phase In the process, homogenize at 10000rpm for 30s to form colostrum, slowly and uniformly add colostrum into 100ml 1%NaCl 1%PVA solution, and homogenize with a homogenizer at 7500rpm for 30s to form double milk. Stir the double emulsion for 3 hours, wait for the organic solvent to evaporate completely, wash and collect the microspheres, freeze-dry and store at 4°C to obtain a long-acting preparation of BSA protein drugs. figure 1 . Its fluorescence spectrum see figure 2 ; In vitro release rate-time curve of microspheres see image 3 , conclusion: the shape of the microspheres is round and uniform, the mechanical properties are strong, and the release is relatively complete.
Embodiment 2
[0031] Weigh 60mg of BSA, dissolve in 150ul 1%PVA, this is the internal water phase; weigh 350mg of PLGA75257E and polyketal PCADK 150mg, dissolve in 2.25ml of dichloromethane, this is the oil phase; add the internal water phase to the oil phase , perform homogenization at 10000rpm for 30s to form colostrum, slowly and uniformly add colostrum into 100ml 1%NaCl 1%PVA solution, and homogenize at 10000rpm for 30s with a homogenizer to form double emulsion. Stir the double emulsion for 3 hours, wait for the organic solvent to evaporate completely, wash and collect the microspheres, freeze-dry and store at 4°C to obtain a long-acting preparation of BSA protein drugs. Figure 4 . Its fluorescence spectrum see Figure 5 ; In vitro release rate-time curve of microspheres see Figure 6 , conclusion: the shape of the microspheres is round and uniform, the mechanical properties are strong, and the release is relatively complete.
Embodiment 3
[0033] 200ul 65mg / ml desalted recombinant human growth hormone (rHGH) solution is the inner water phase; weigh PLGA5050 3A 400mg and polyketal PCADK 100mg and dissolve it in 2ml dichloromethane, this is the oil phase; add the inner water phase to the oil phase In the process, homogenize at 4000rpm for 90s to form colostrum, slowly and uniformly add colostrum into 100ml 4%NaCl 1%PVA solution, and homogenize with a homogenizer at 4000rpm for 120s to form double milk. Stir the double emulsion for 3 hours, wait for the organic solvent to evaporate completely, wash and collect the microspheres, freeze-dry and store at 4°C to obtain a long-acting preparation of recombinant human growth hormone (rHGH). Figure 7 . Its fluorescence spectrum see Figure 8 ; In vitro release rate-time curve of microspheres see Figure 9 , conclusion: the shape of the microspheres is round and uniform, the mechanical properties are strong, and the release is relatively complete.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com