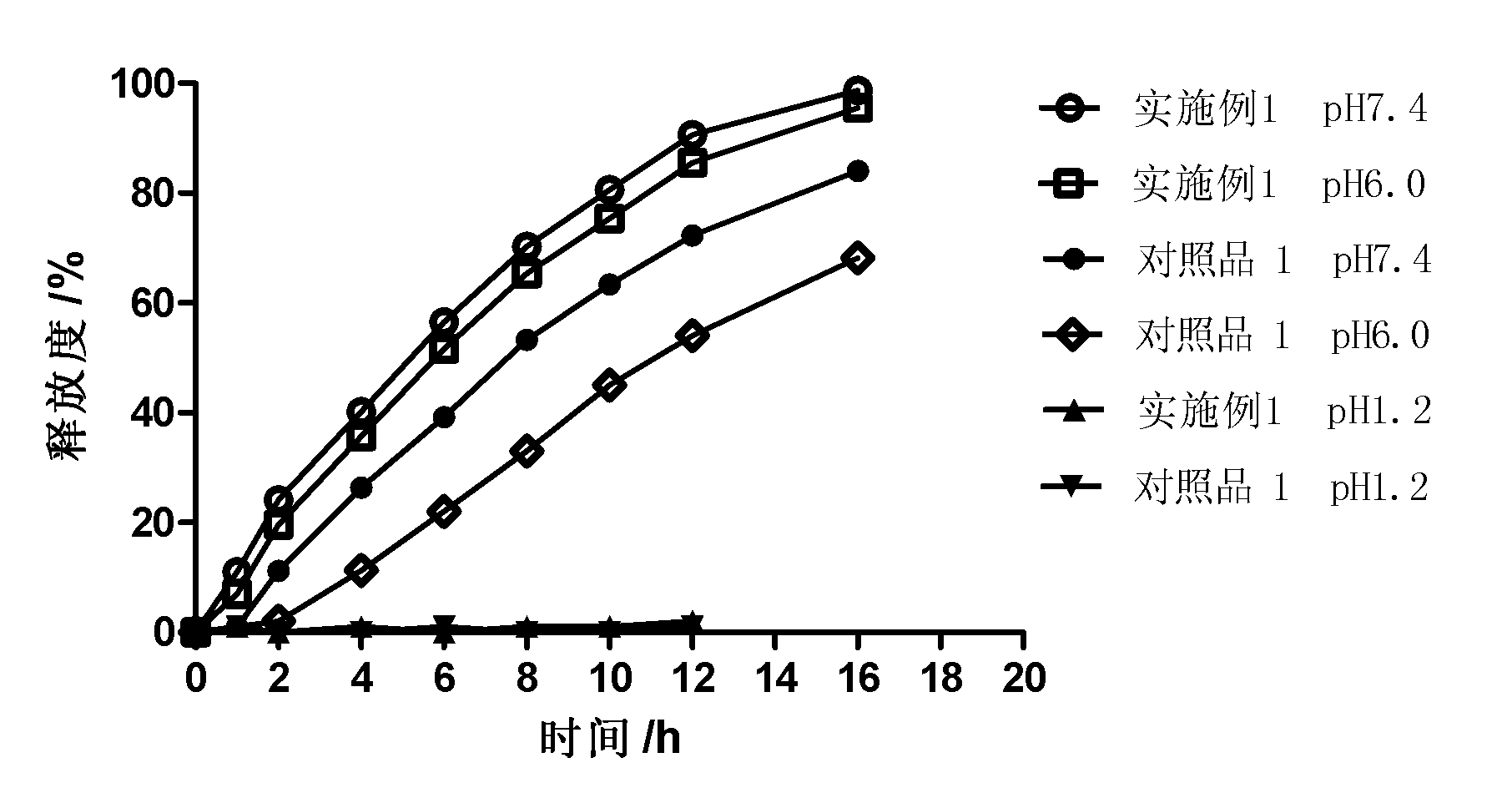
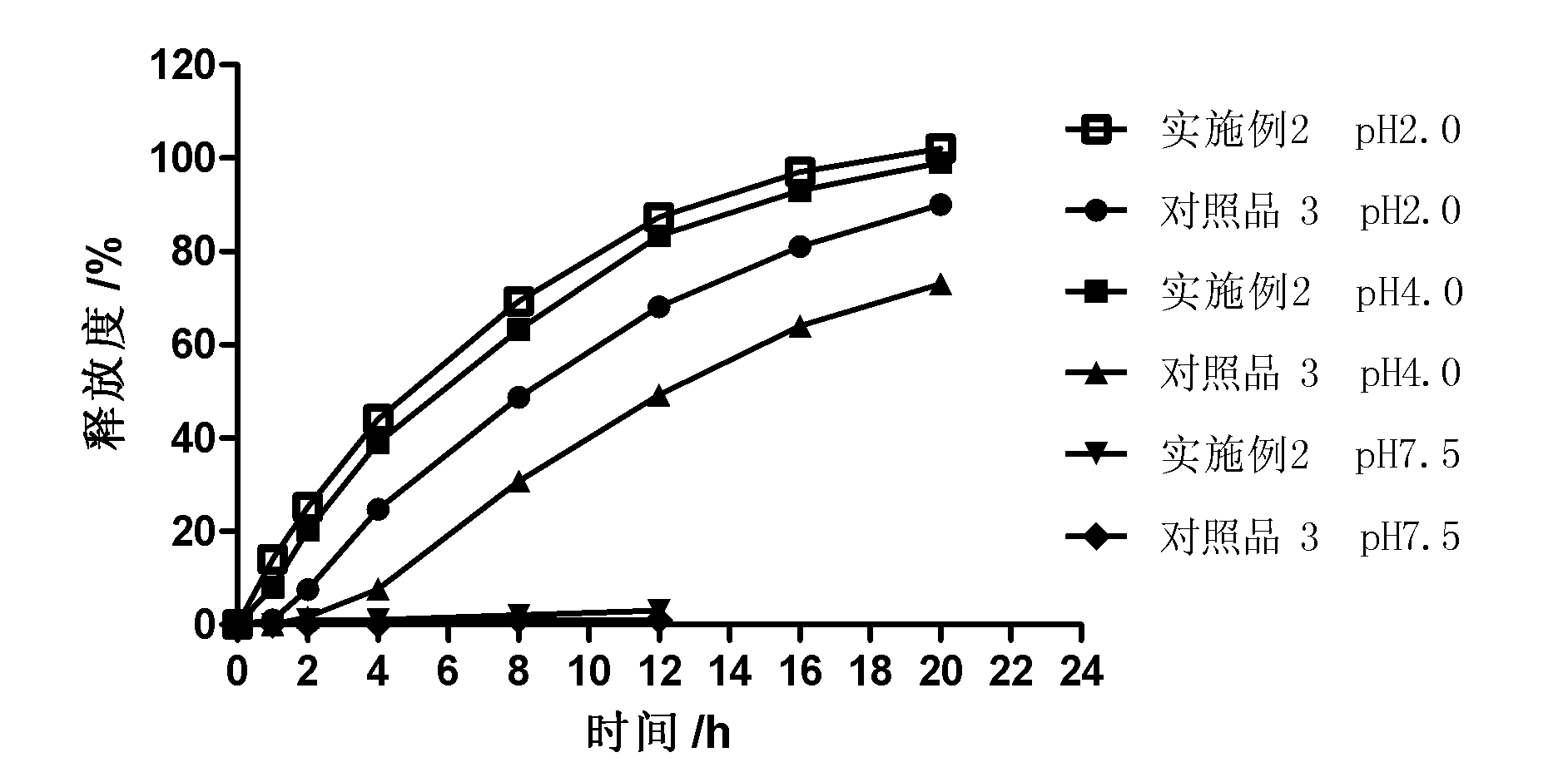
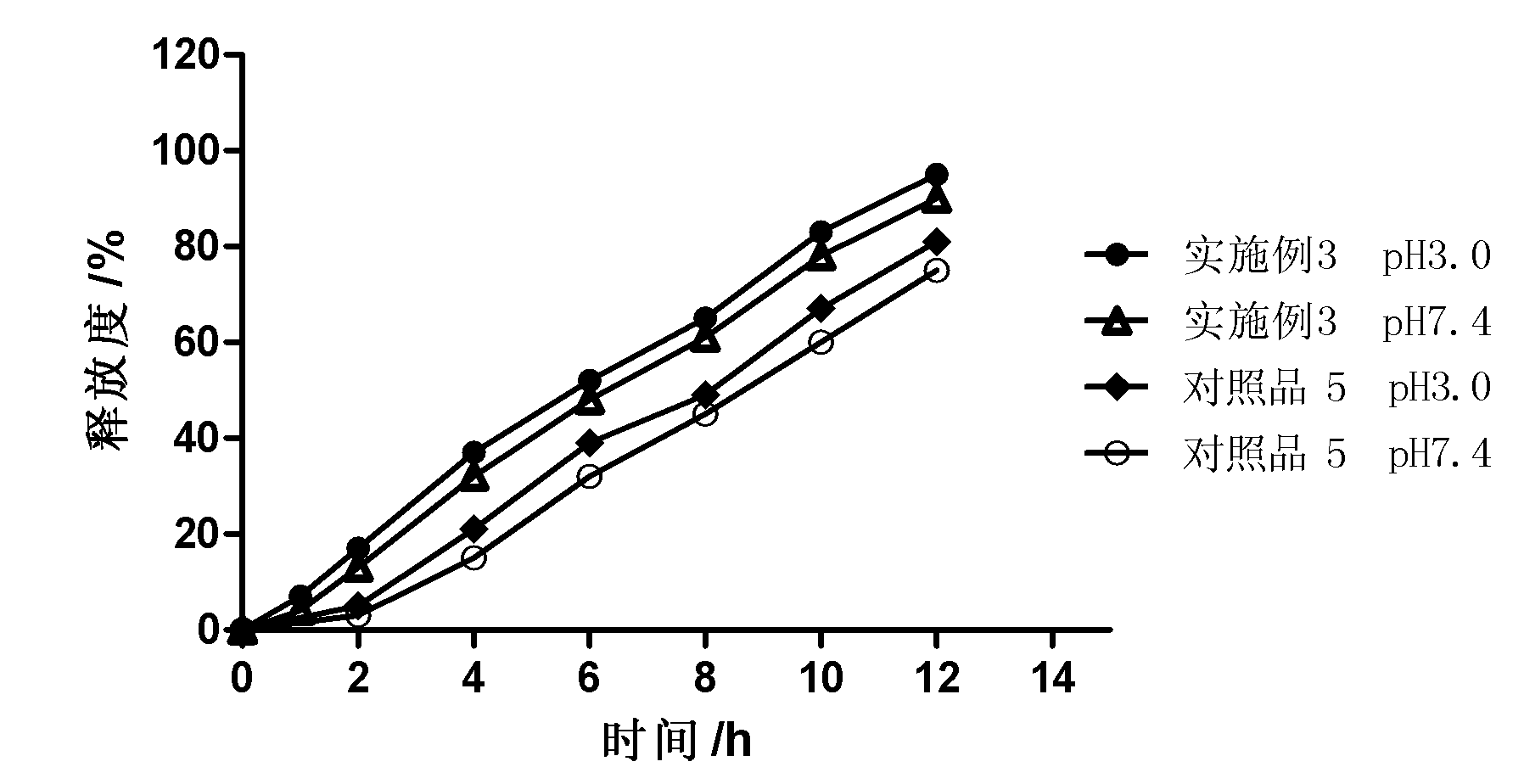
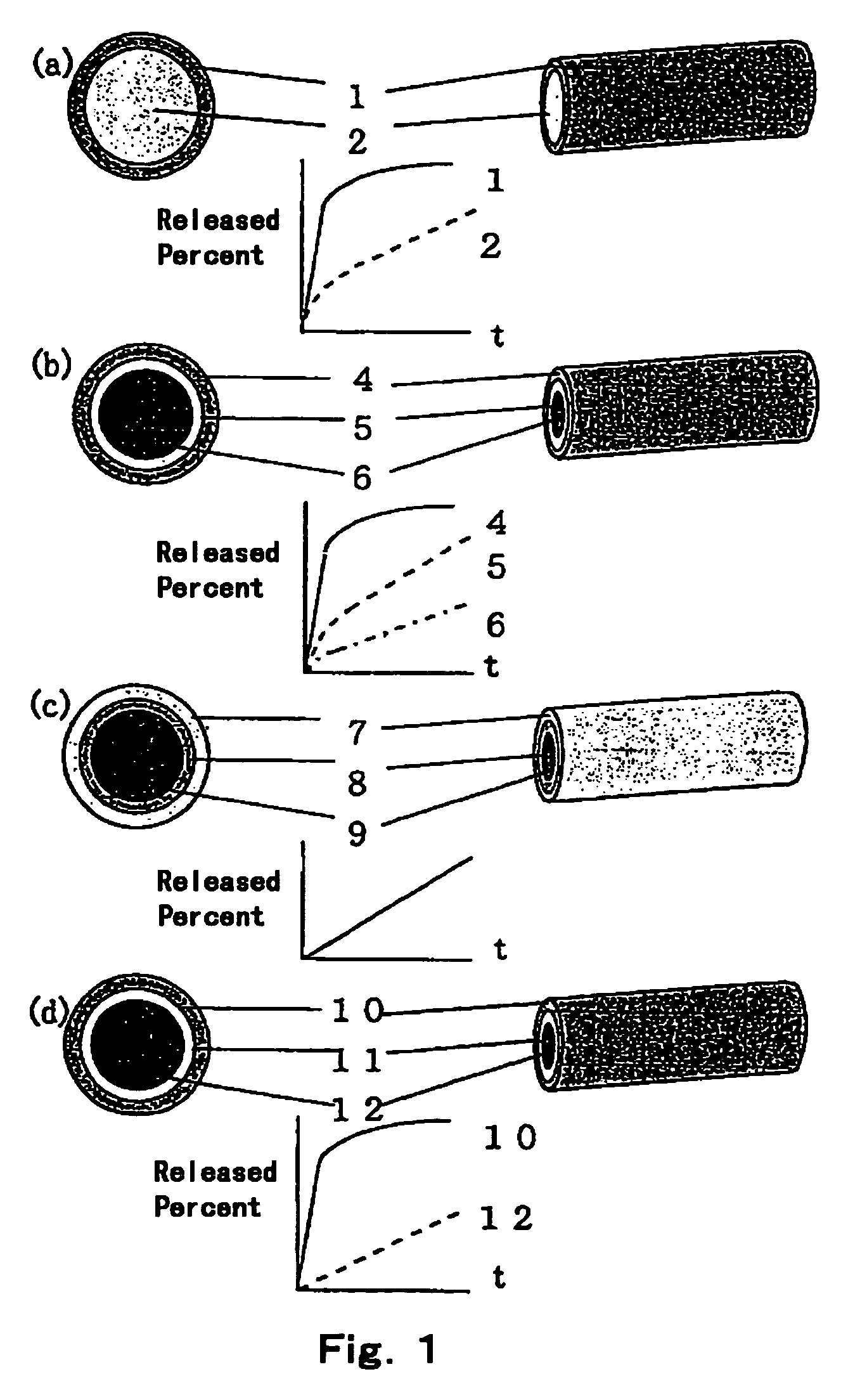
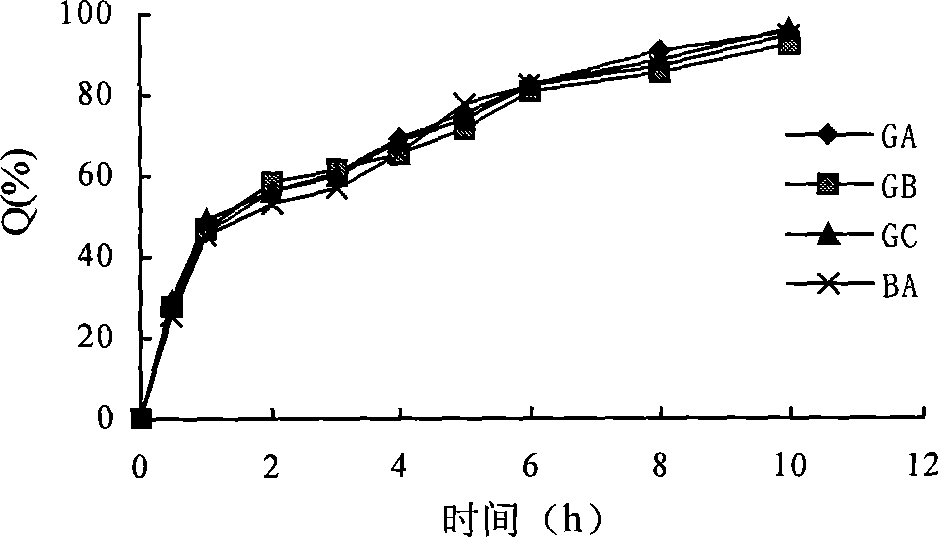
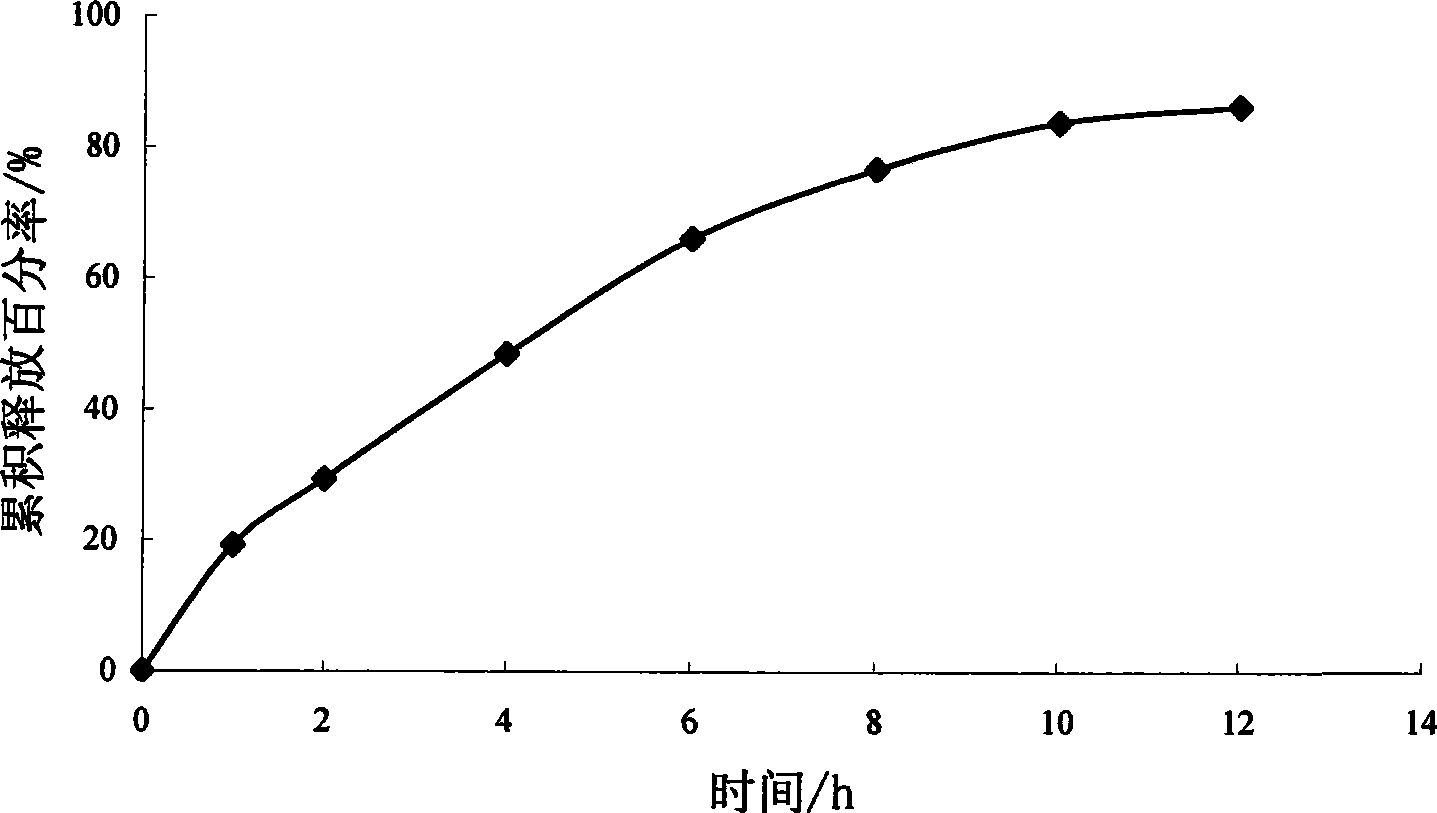
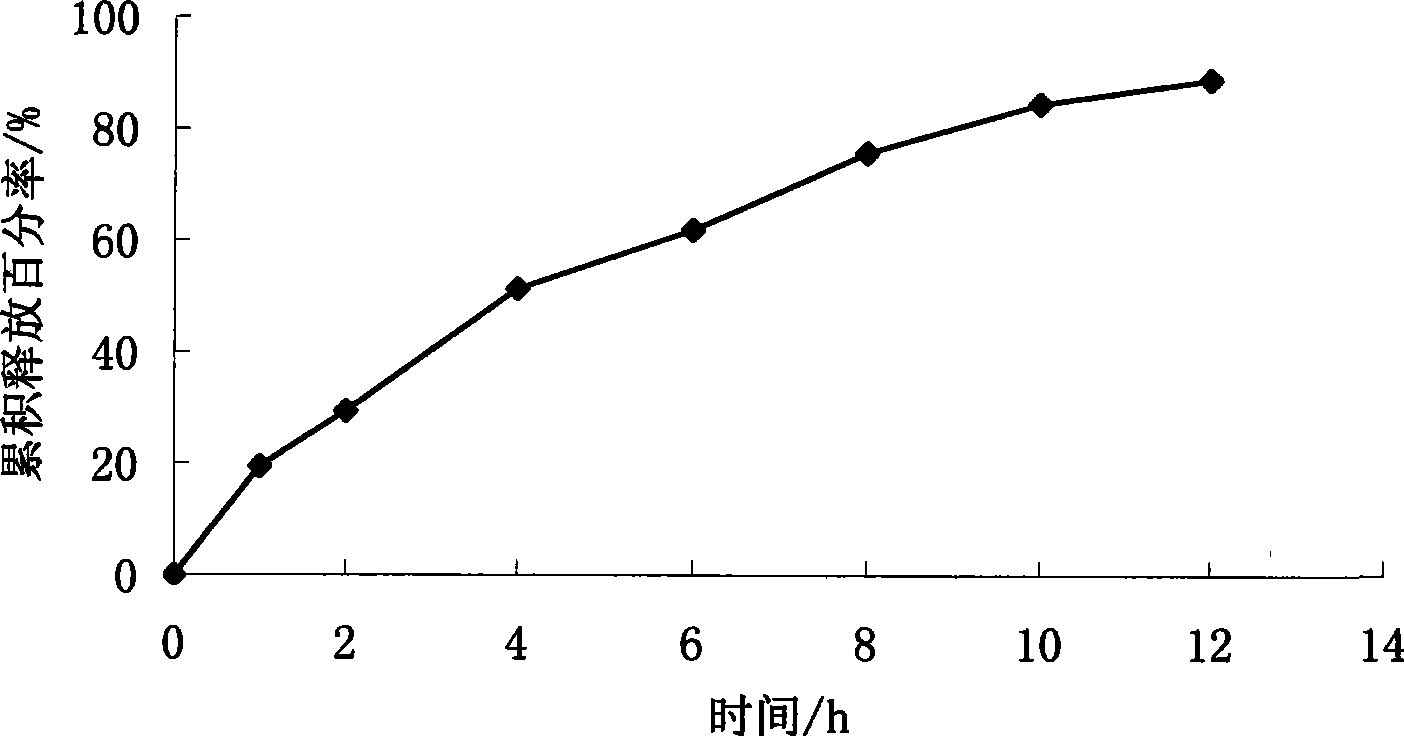
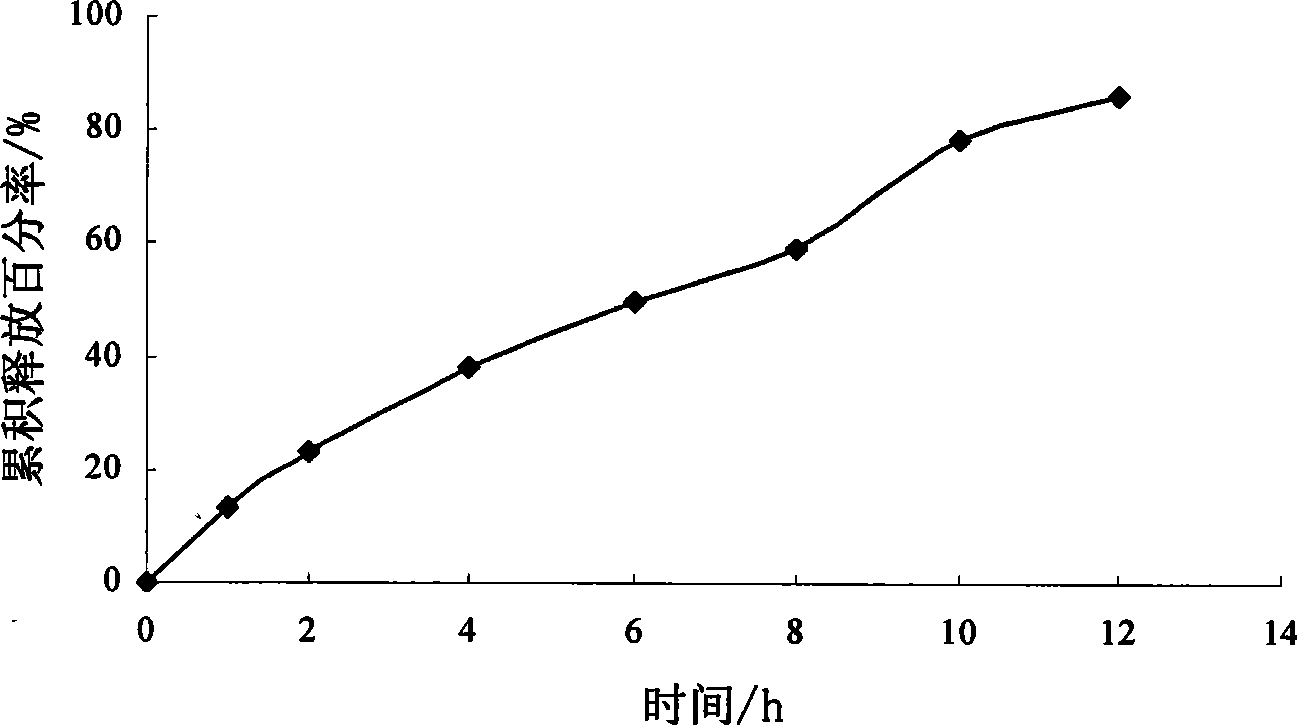
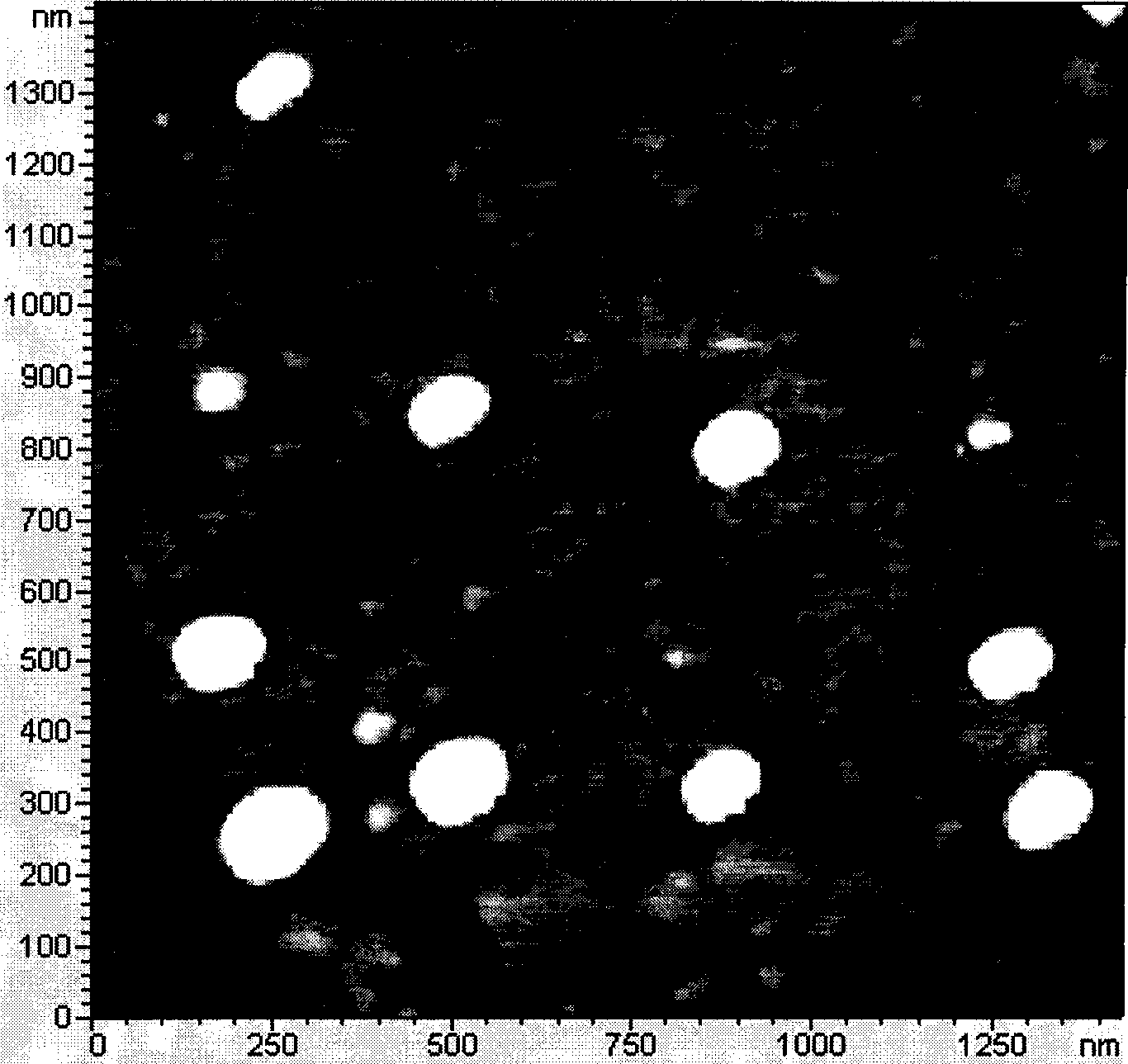
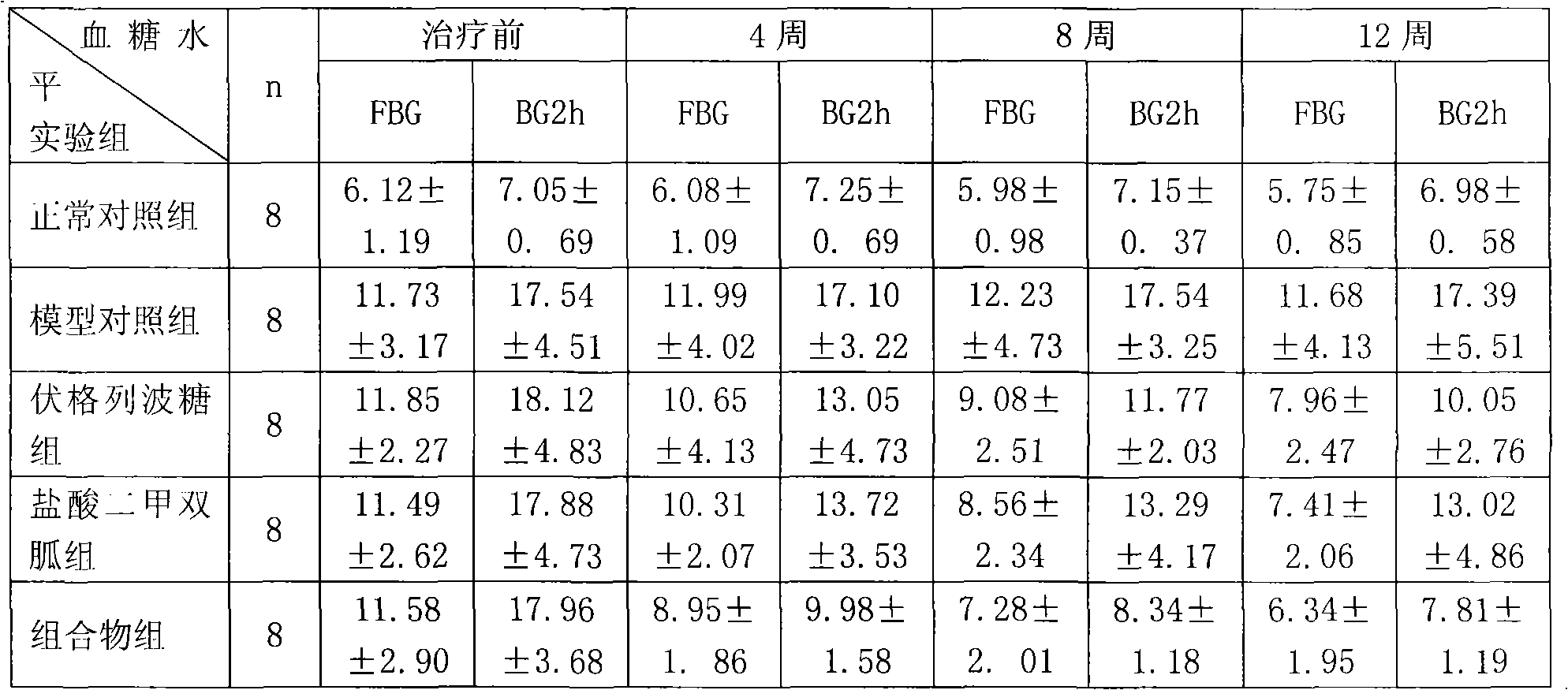
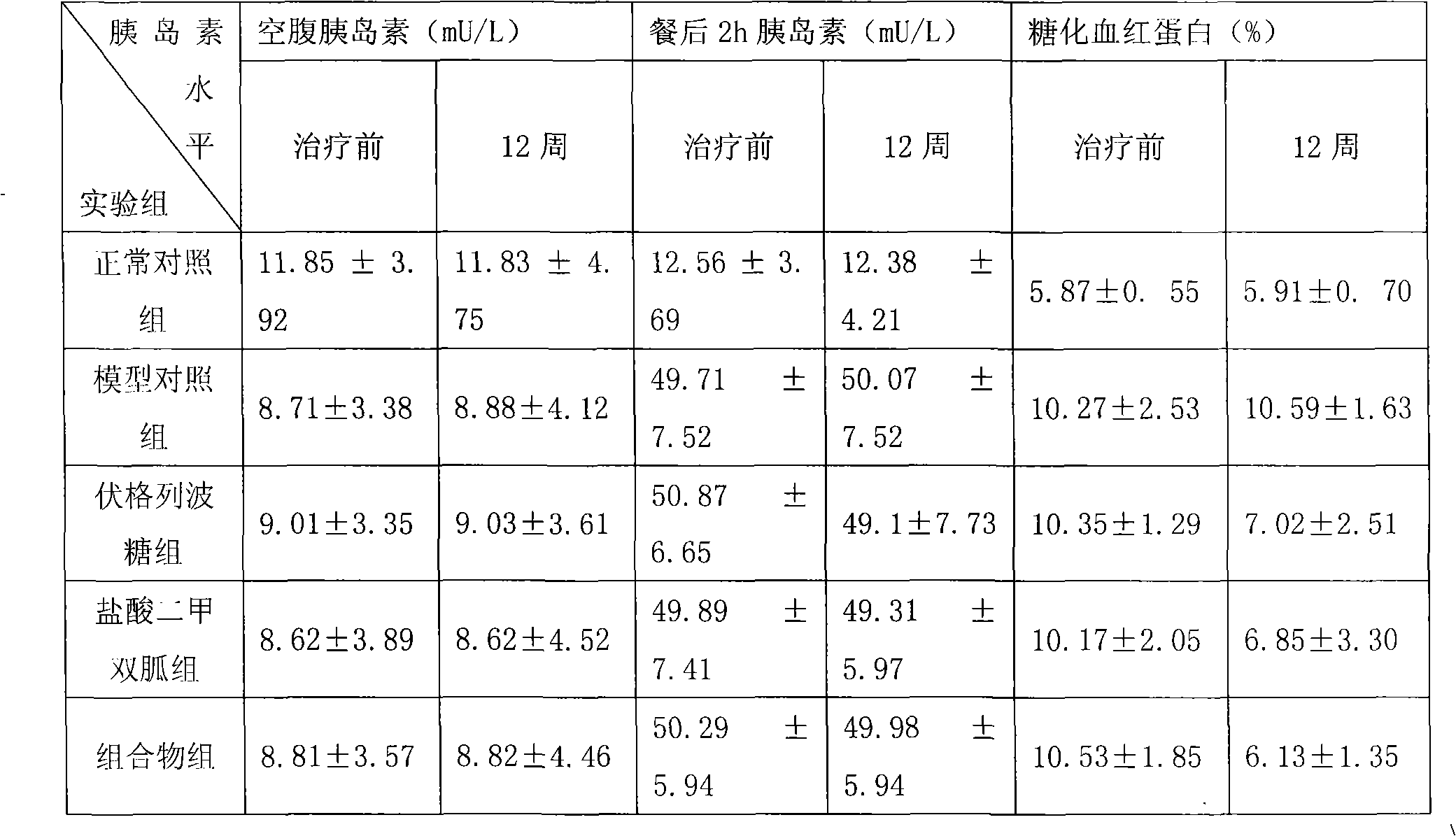
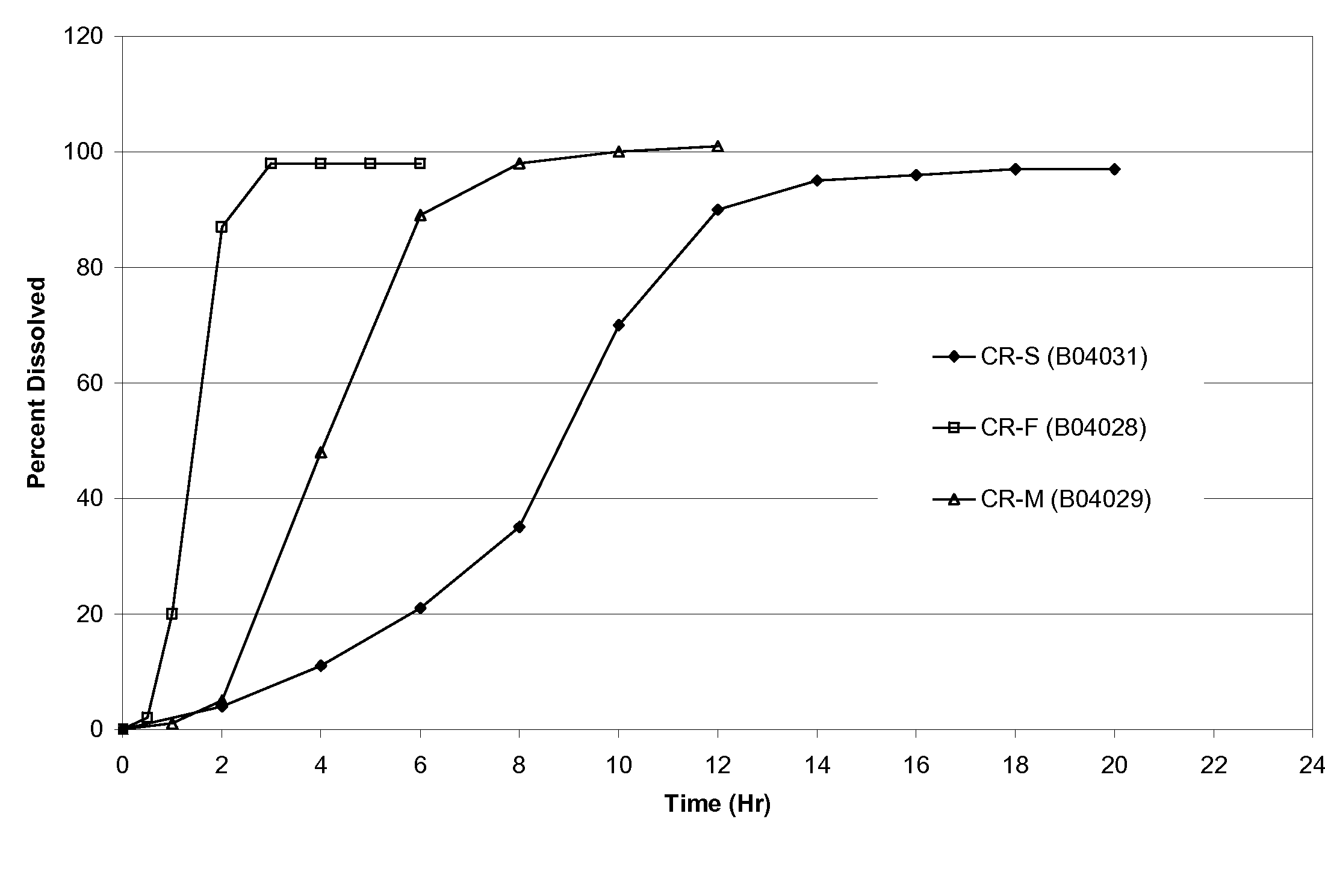
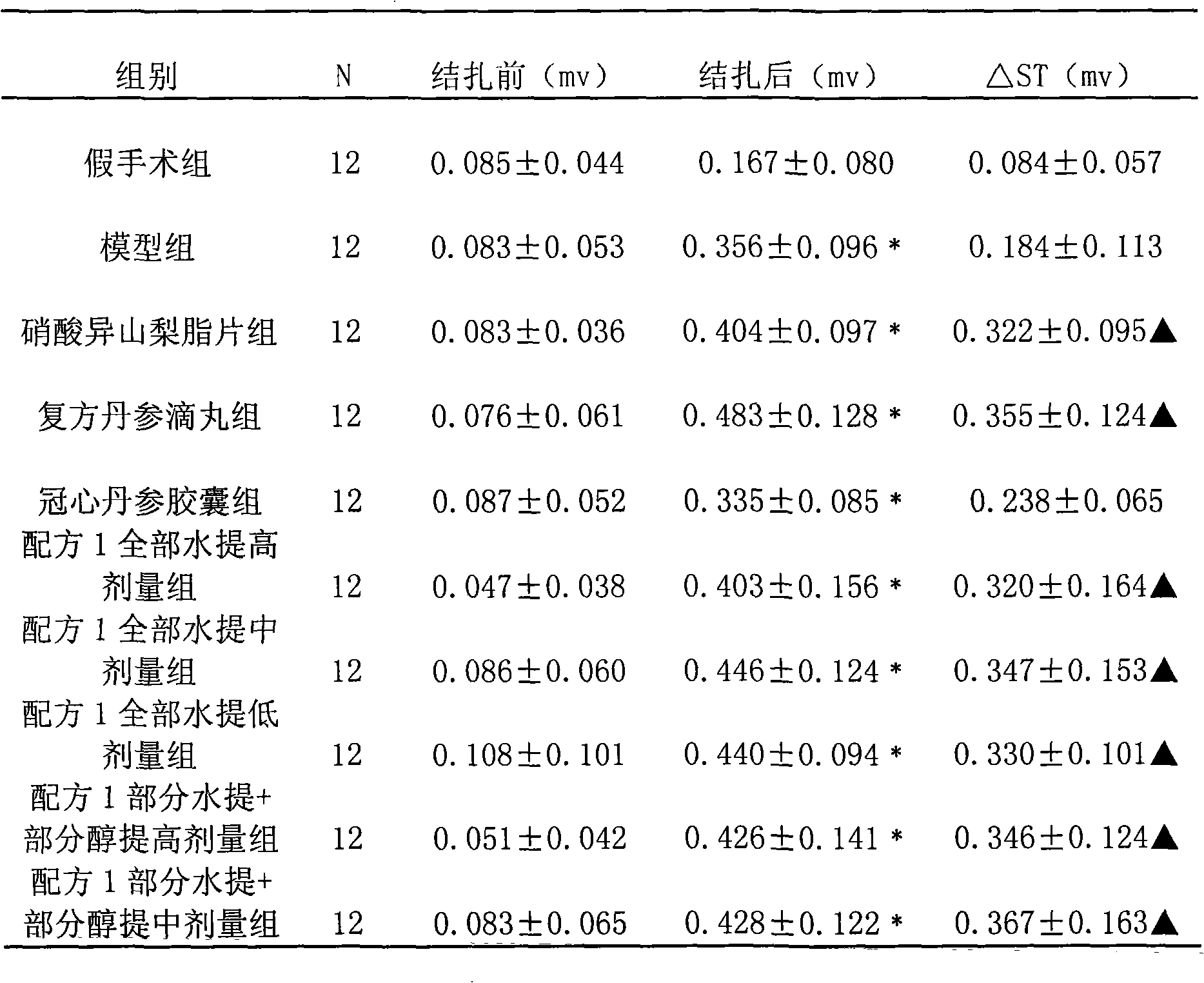
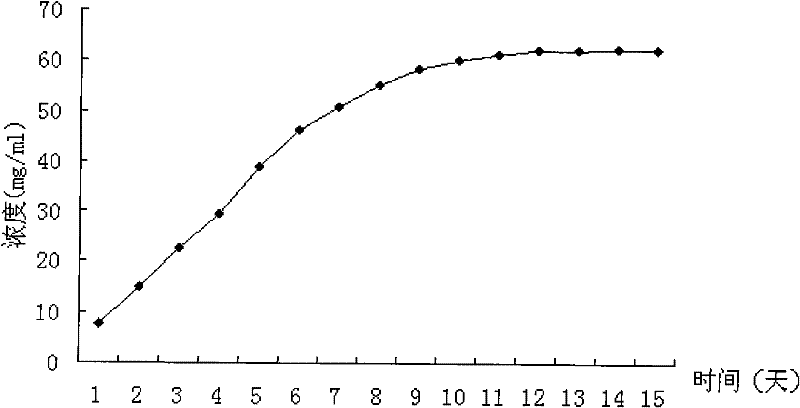
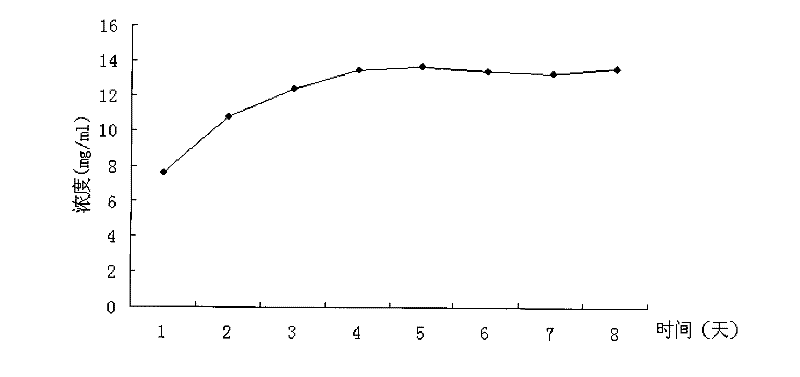
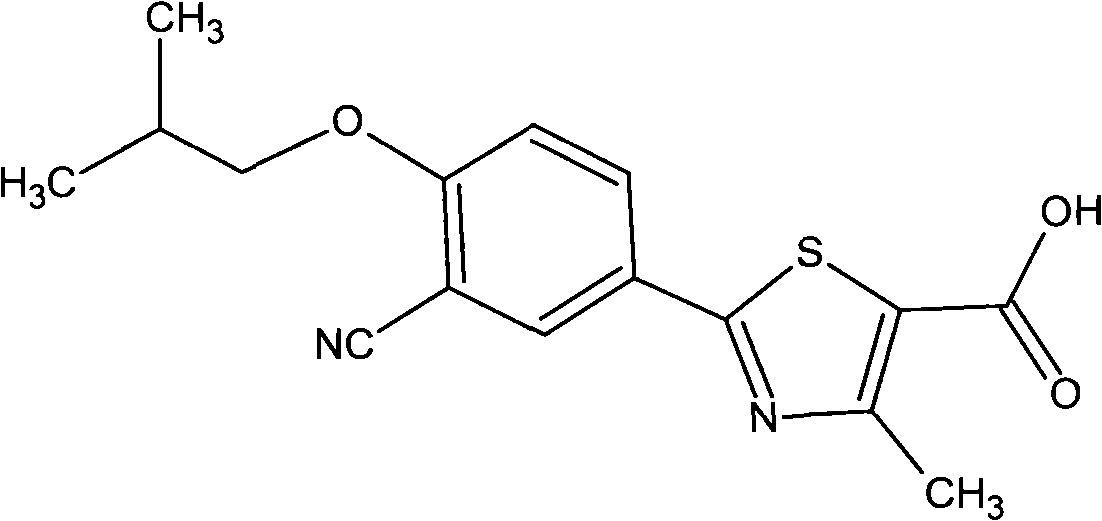
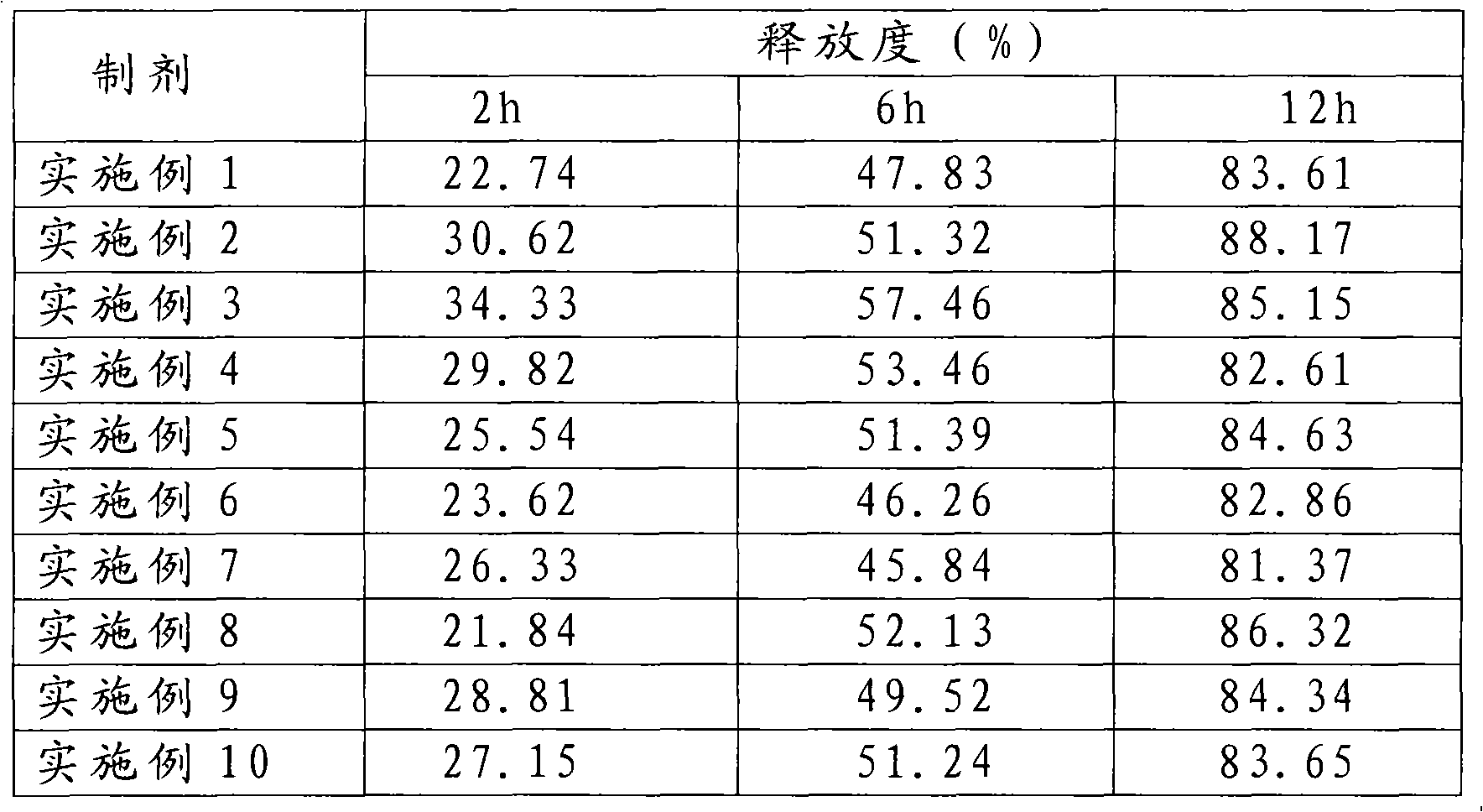
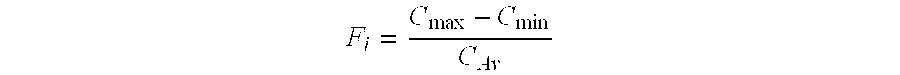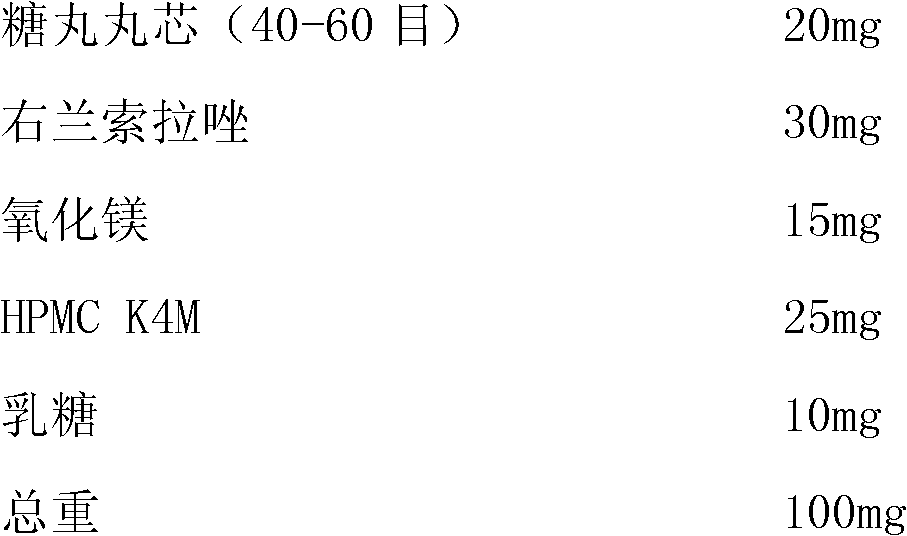Patents
Literature
345 results about "Controlled-Release Preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
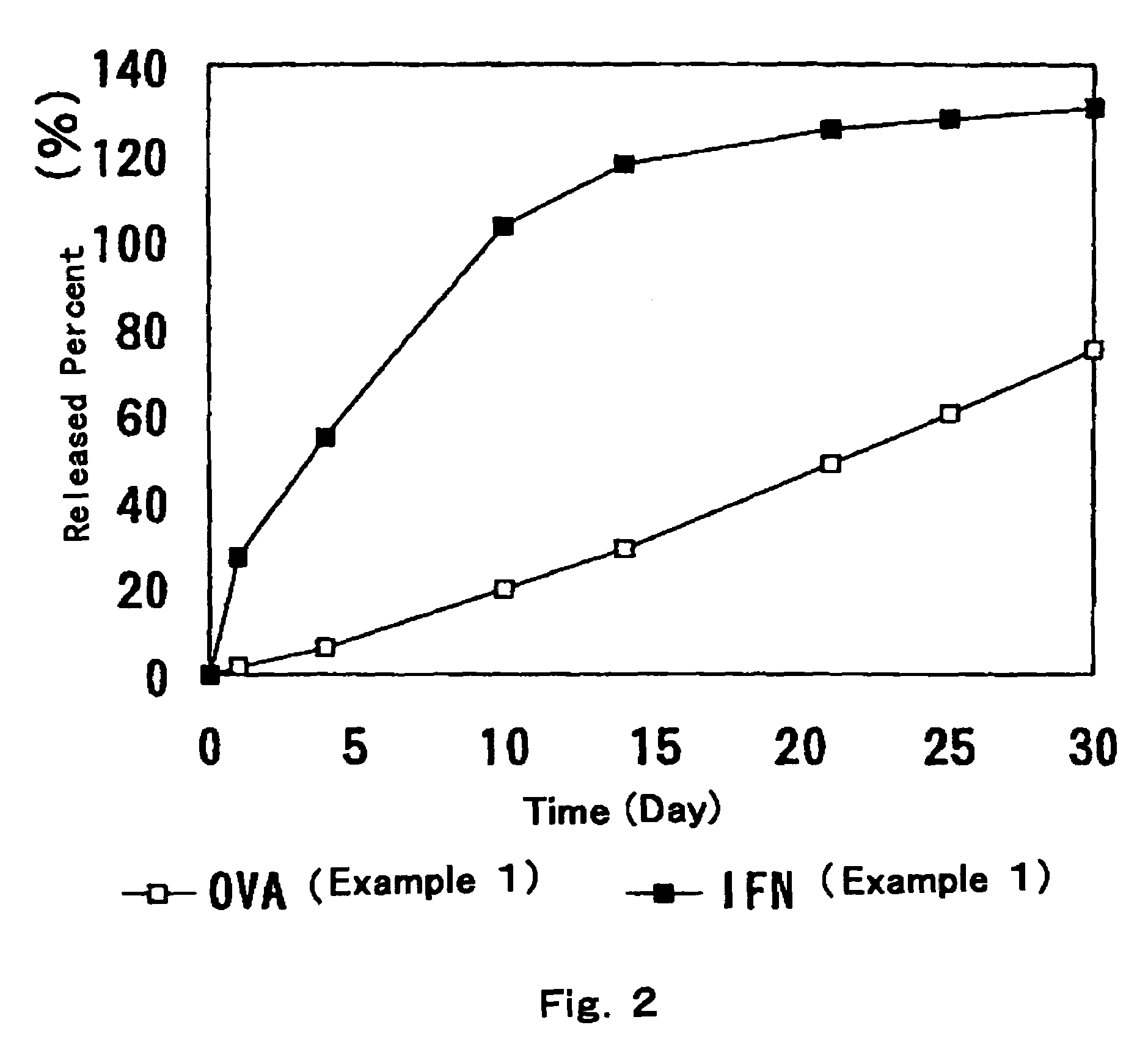
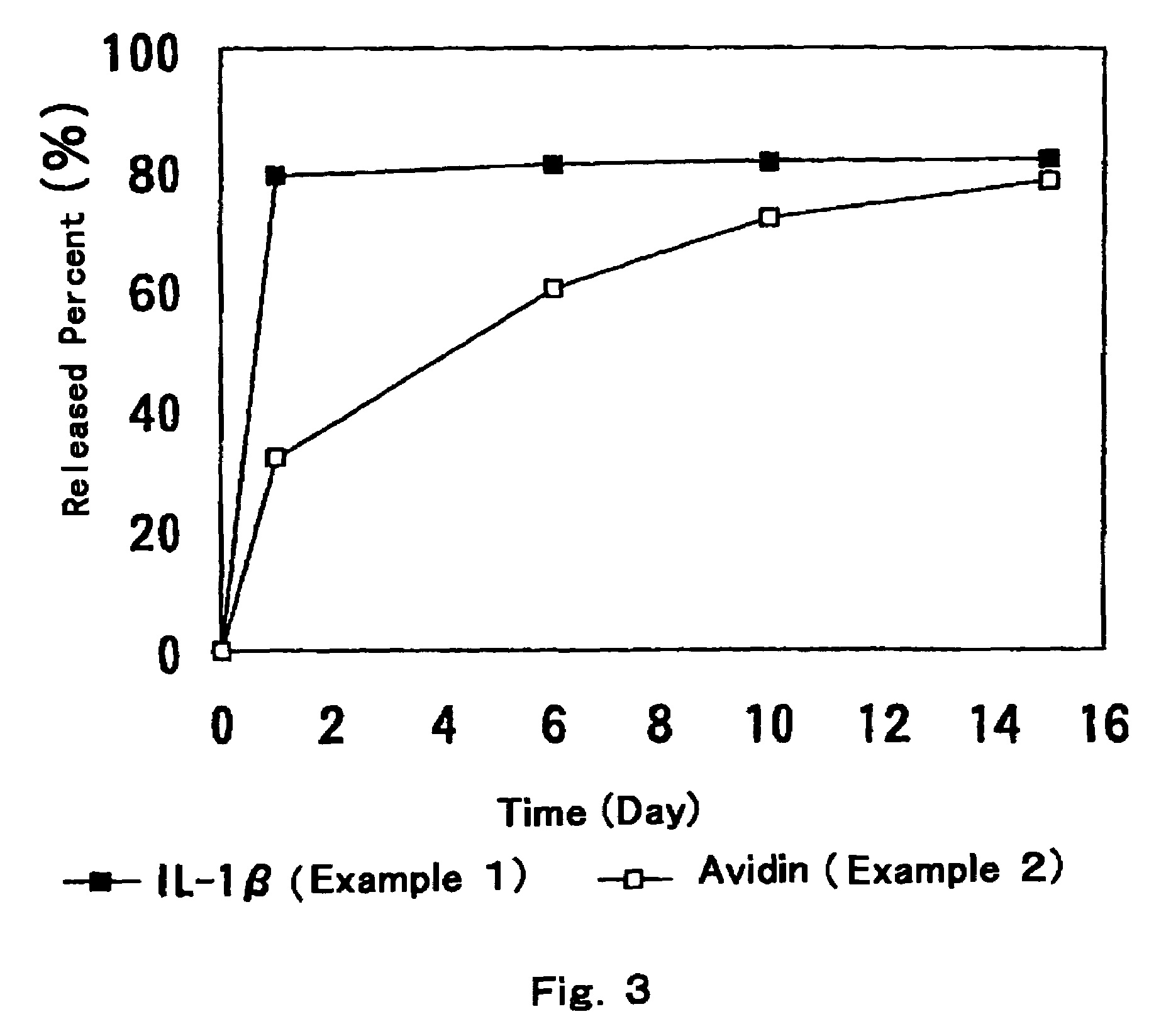
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Controlled release preparation
Controlled release preparations and capsules are provided. Also provided are emulsions and suspensions, including compositions and methods of manufacturing controlled release capsules, where the fill contains a suspension and / or an emulsion.
Owner:PATHEON SOFTGELS INC
Method for producing a controlled-release preparation
InactiveUS6974591B2Improve mechanical stabilityBiocideInorganic non-active ingredientsWater insolubleMechanical stability
The invention concerns a method for producing a controlled-release pharmaceutical preparation with a particle-containing coating, the coating being derived from an aqueous dispersion of a film-forming water insoluble polymer and a water soluble pore-forming agent. By suspending, instead of dissolving the pore-forming agent, the resulting coating will contain particles of the pore-formers with a predetermined size that creates, when disintegrated or dissolved in the body fluid, canals or a network of pores through the polymer film. Due to this network, the film will get a good mechanical stability and are left intact after the release of the drug.
Owner:WATSON LAB INC
Controlled release preparations having multi-layer structure
Preparations whereby two or more drugs can be released separately at appropriate speeds depending on the disease or the release behaviors of one or more drugs can be precisely controlled, which consist of an outer layer wherein a water-soluble drug is dispersed in a carrier made of a biologically non-degradable and hydrophobic polymer material, and one or more inner layers wherein a water-soluble drug, differing in kind or concentration from the one contained in the outer layer, is dispersed in a carrier made of a biologically non-degradable and hydrophobic polymer material, and in which the outer and inner layers are concentrically located in diametral direction of rod-like preparations and both or one of the ends in the axial direction are opened so as to directly come into contact with the environment.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Sirolimos sustained and controlled release preparation and preparation method thereof
InactiveCN101361703AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityCyclodextrin
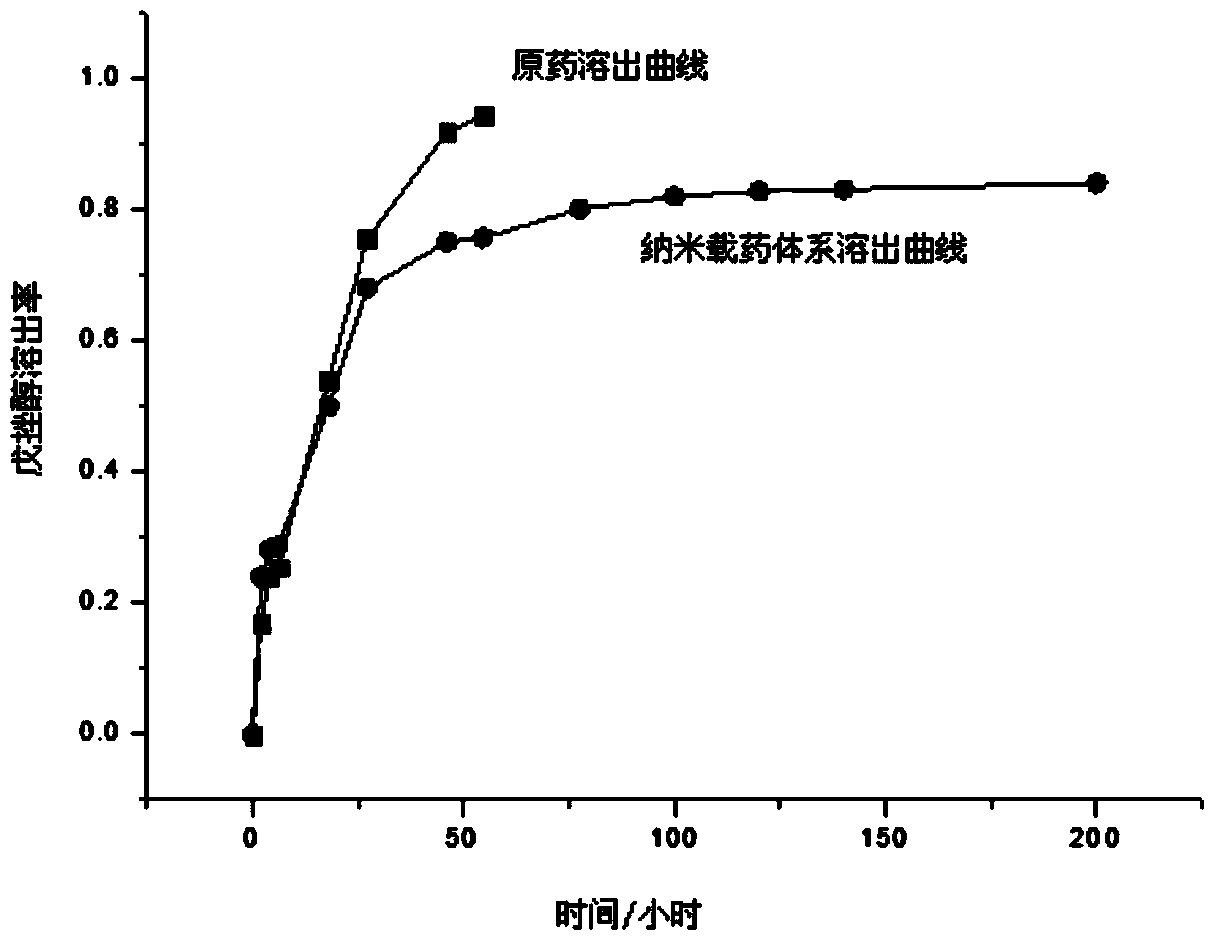
The invention provides a Sirolimus slow controlled release preparation and a preparation method thereof. By adopting the solubilizing methods including a solid dispersion technology or cyclodextrin inclusion technology or adding one or a plurality of surface active agents after micronizing drugs and the like, the solubility is dramatically improved and further the bioavailability is improved, therefore, a matrix type slow controlled release preparation is made by adding one or a plurality of framework materials and other accessories, or a diaphragm-controlled or osmotic pump type slow controlled release preparation is made by adopting slow controlled release materials for coating. The Sirolimus slow controlled release preparation has better solubility and dissolution rate, high bioavailability as well as slow controlled release effect, thus being capable of maintaining steady blood concentration, bringing down the concentration of peak, reducing the occurrence of adverse reaction and improving the safety of clinical medicines, in addition, the raw materials can be acquired easily, the preparation process is simple and feasible with high yield rate and low cost, and the large-scale industrial production can be realized and the economic benefit is marked.
Owner:宋洪涛
Oil-soluble pesticide nano capsules and preparation method thereof
InactiveCN101461358AMild conditionsEase of industrial productionBiocideAnimal repellantsCypermethrinAbamectin
Disclosed is an oil soluble pesticide nm capsule and preparation method, having weight ration of: pesticide: 0.5-4%; high molecular compound: 8-25%; mixed-surfactant: 8-20%; cosurfactant 3-10%; crosslinking agent 0.5-1.0%; organic solvent 3-10%; water: added to 100%, wherein the high molecular compound is selected from sodium lignosulfonates, chitosan, gum arabic or glutin, one or two is / are selected for agglutination reaction, forming capsule skin of the nm capsule; the capsule core is pesticide bulk drug, selected from diflubenzuron, emamectin benzoate, abamectin, imidacloprid, ivermectin or highly active cypermethrin. The mixed-surfactant adopts nonionic surfactant and anionic surfactant for compound. The pesticide nm controlled release preparation prepared by the invention is easy in storage, high in stability, large in drug-loading rate and high in dispersion.
Owner:NANJING NORMAL UNIVERSITY
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
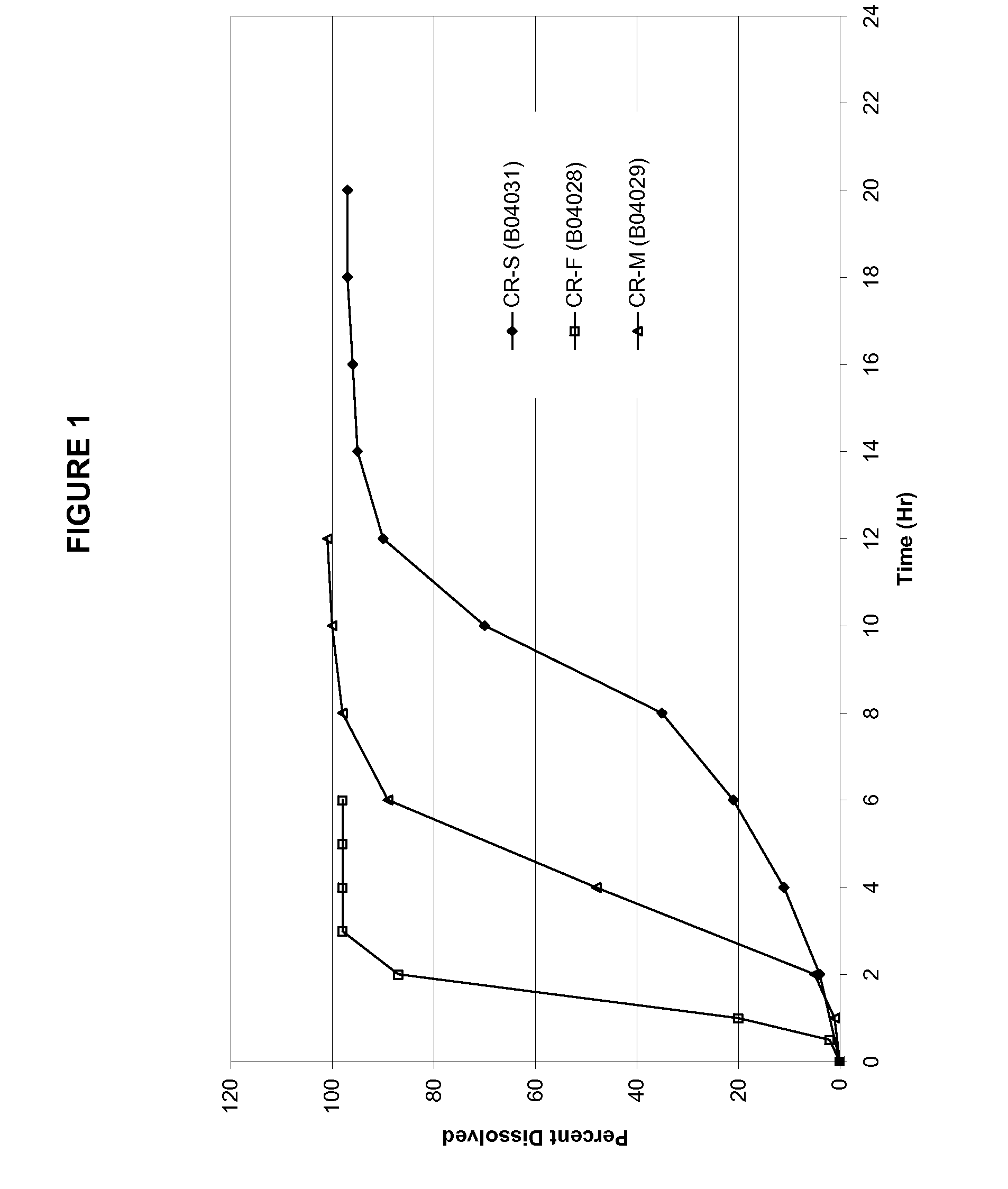
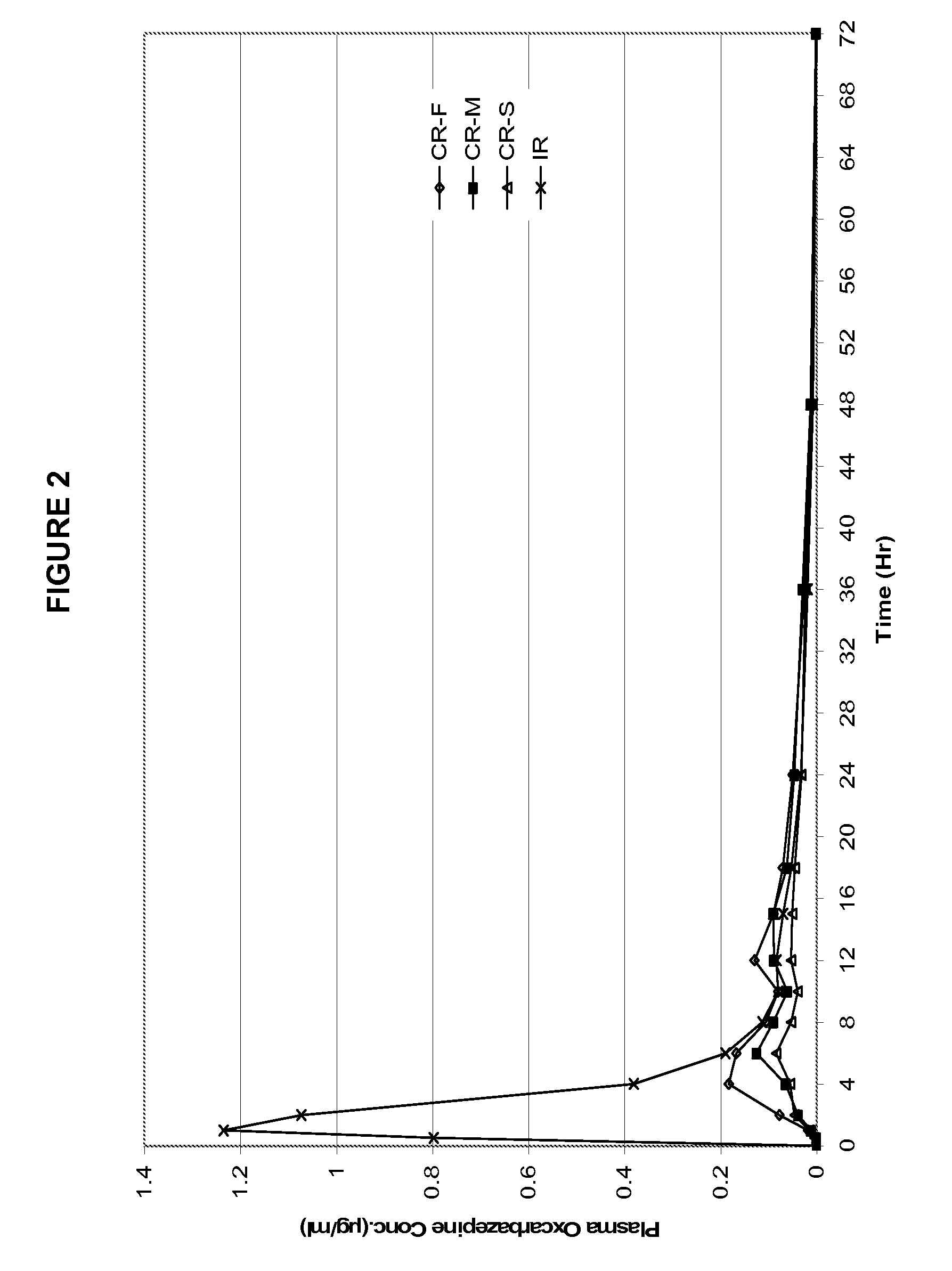
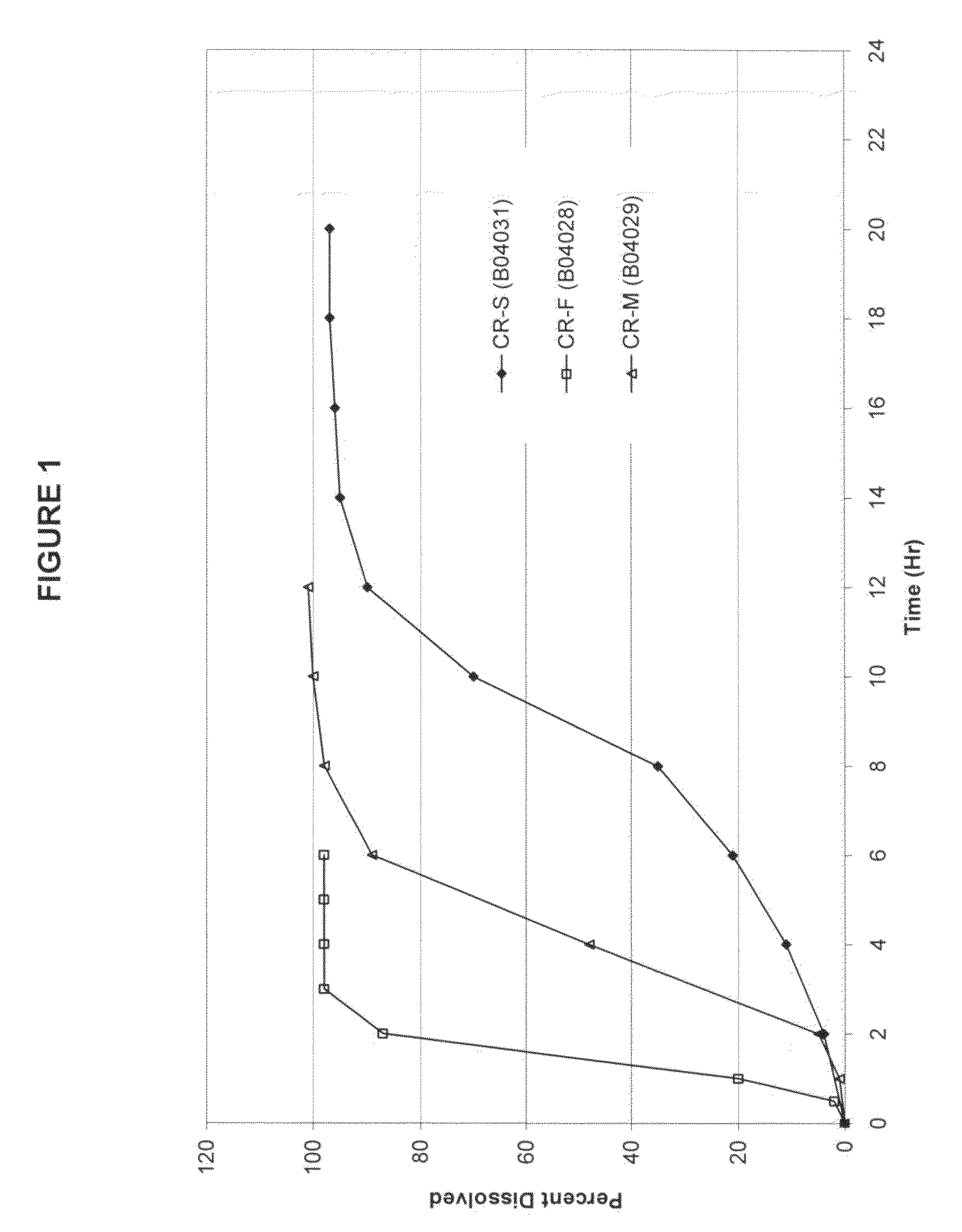
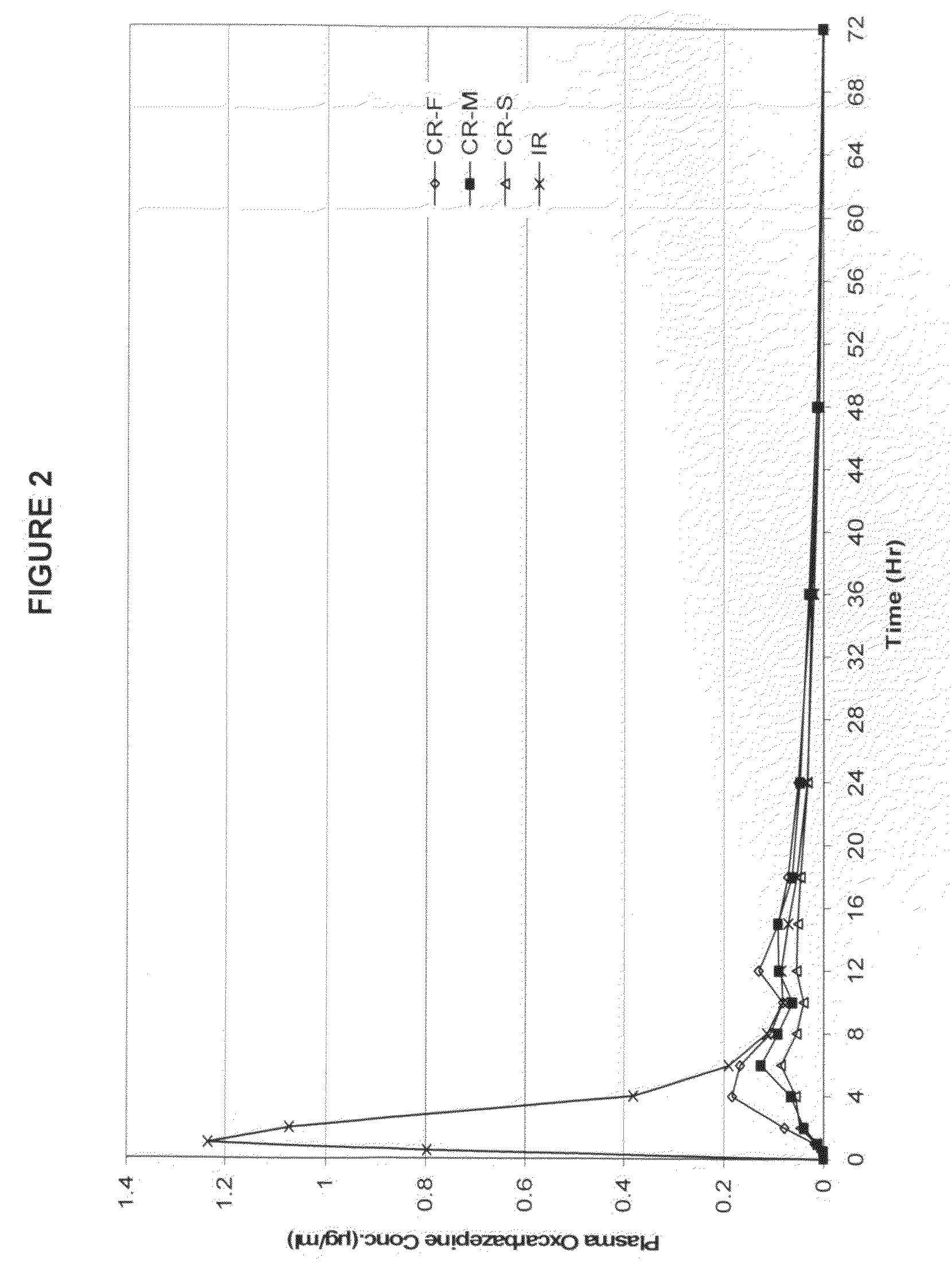
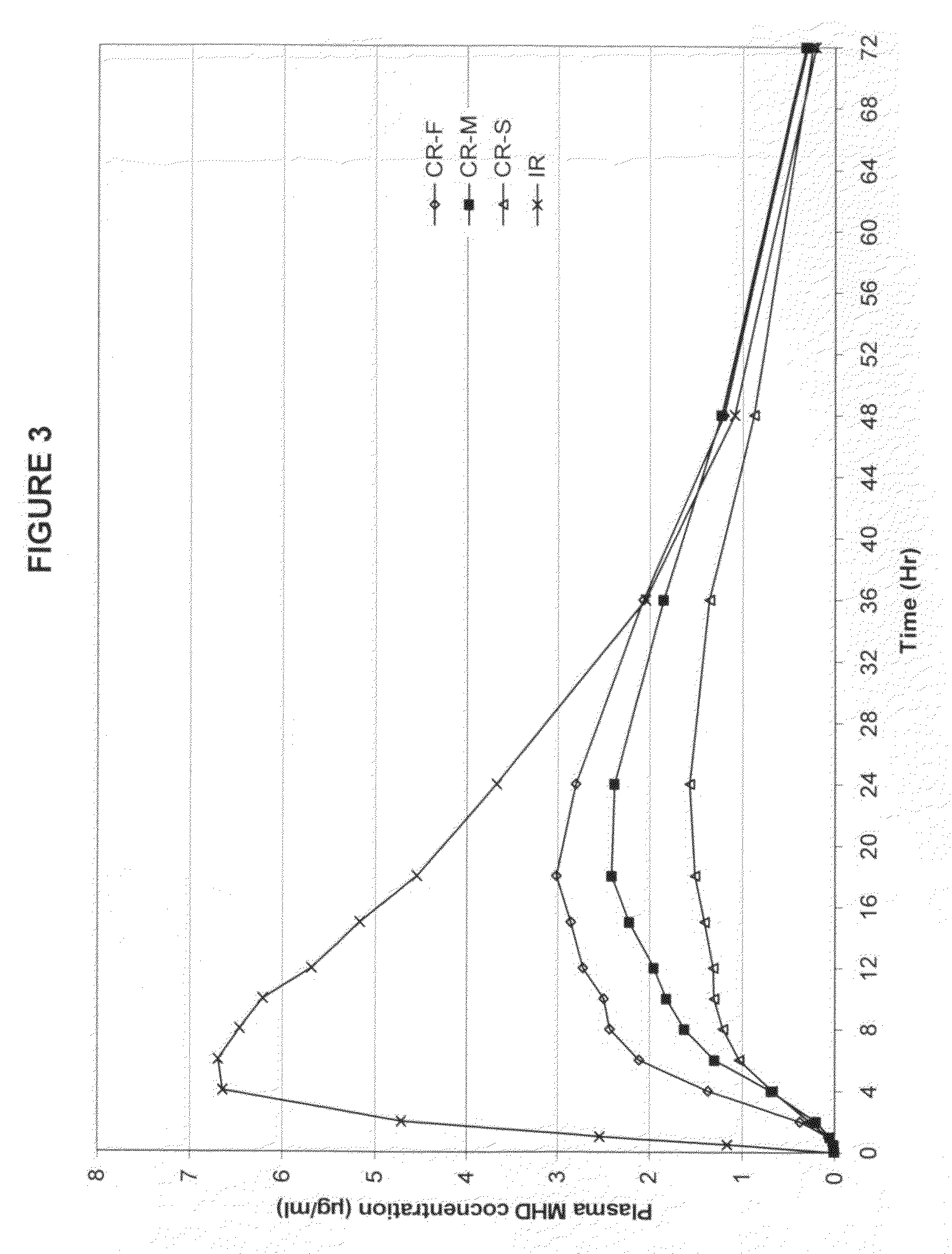
Modified-release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20070254033A1Reduce volatilityBetter therapeutic profilePowder deliveryNervous disorderSolubilityOxcarbazepine
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility-and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
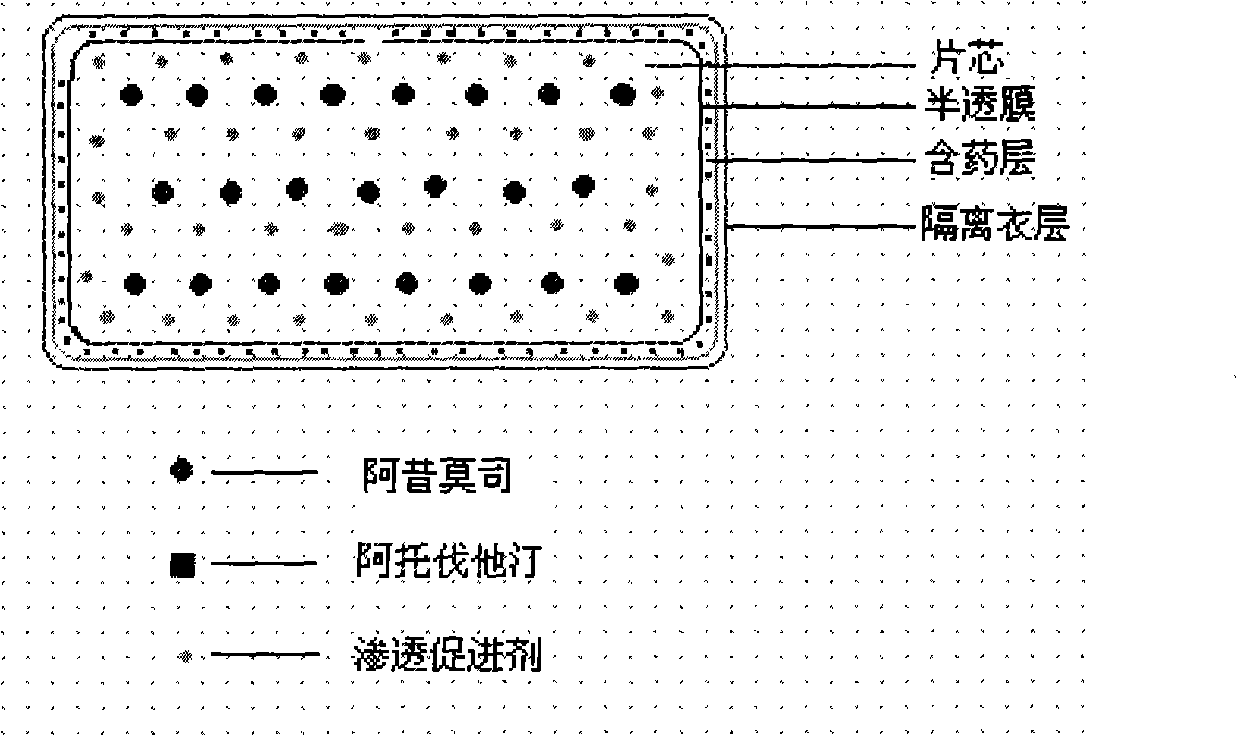
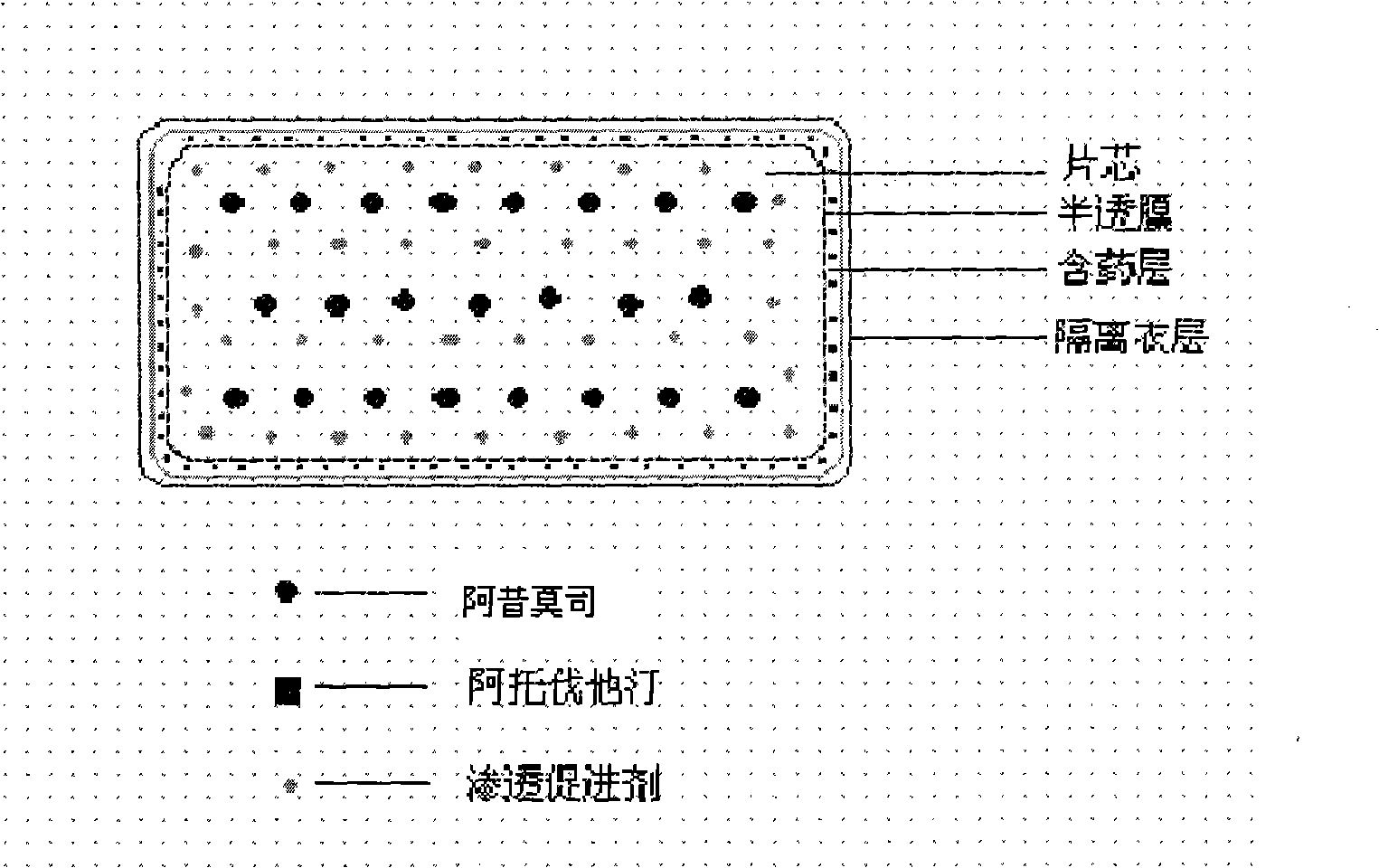
Osmotic pump controlled release preparation composition and preparation thereof
ActiveCN101352426AFully functionalSmall toxicityMetabolism disorderPharmaceutical delivery mechanismSide effectBULK ACTIVE INGREDIENT
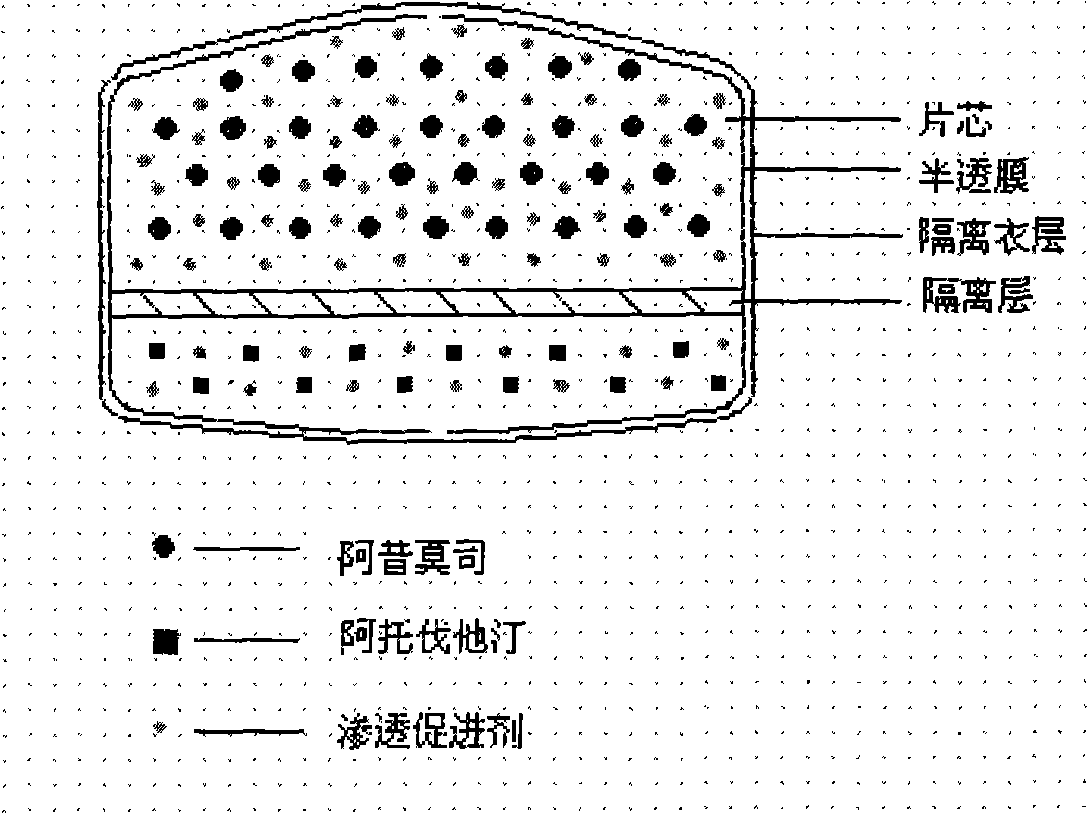
The invention relates to an osmotic pump controlled release pharmaceutical composition which contains two medicine active ingredients and is used for treating hyperlipoidemia and a preparation method thereof, the pharmaceutical composition has a nicotinic acids medicine such as acipimox and the other one estatina medicine such as atorvastatin; wherein, the nicotinic acids medicine such as acipimox is a controlled release part, and the estatina medicine such as atorvastatin is a quick release part; or both the acipimox and the atorvastatin are the controlled release part. The compound osmotic pump preparation of the invention has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
A explosive core composition of controlled release administer drug and controlled release preparation as well as its preparing method
ActiveCN101161242AImprove securityImprove effectivenessInorganic non-active ingredientsOsmotic deliverySolubilityMedicine
A medicine core composition used for low solubility active medicine controlling release administration is provided in the present invention, wherein, at least comprising medicine-containing layer and boosting layer, the medicine-containing layer comprises low solubility active medicine and hydrophilic polymer carrier, the boosting layer at least comprises penetration enhancing polymer, insoluble polymer and osmotic pressure promoter; a osmotic pump containing the medicine core composition is also provided in the present invention, wherein also comprising semi-transparent material film coating outer of the medicine core; the medicine core composition of the present invention can controlled speed release medicine, leading the preparation containing the medicine core to achieve aim of administration one time every day, that is about in 24 hours to release active medicine.
Owner:OCEAN STAR INT
Method for producing a controlled-release preparation
InactiveUS20010038853A1Effective mechanical strengthEffective release rateBiocideInorganic non-active ingredientsWater insolubleMechanical stability
Owner:WATSON LAB INC
Controlled release preparations comprising tramadol and topiramate
This invention relates to an oral pharmaceutical preparation, suitable for dosing every 24 hours, comprising a substrate, which substrate comprises a pharmaceutically effective amount of tramadol or a salt thereof and a pharmaceutically effective amount of topiramate and wherein said substrate may be coated with a controlled release coating; said preparation having a specific dissolution rate in vitro.
Owner:CILAG
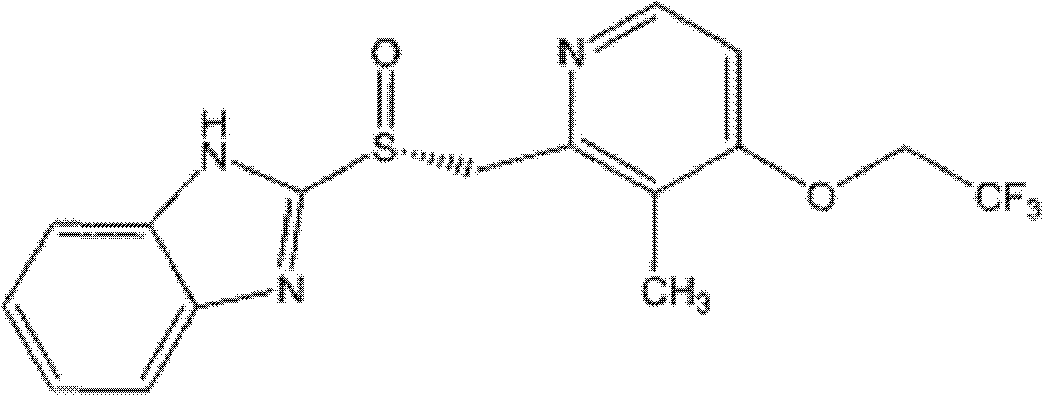
Dexlansoprazole enteric-coated slow controlled-release pellet tablets
InactiveCN103565770AEasy product industrializationTrouble swallowingAntibacterial agentsOrganic active ingredientsDexlansoprazoleBULK ACTIVE INGREDIENT
The invention discloses dexlansoprazole enteric-coated slow controlled-release pellet tablets belonging to the field of slow controlled-release preparations, and in particular relates to a preparation method of acid-sensitive proton pump inhibitor (PPI) slow controlled-release pellet tablets. The pellet tablets are prepared by tabletting and coating two types of pellets with different release speeds as well as a filler, a disintegrating agent and a lubricant, and release active ingredients at different release speeds, wherein one type of the pellets are enteric-coated quick-release pellets, and the other type of the pellets are enteric-coated slow-release pellets.
Owner:FUKANGREN BIO PHARMA
Traditional Chinese medicine preparation for treating cardiovascular and cerebrovascular diseases
InactiveCN101524421ALow costImprove efficacyCardiovascular disorderPlant ingredientsDiseaseSalvia miltiorrhiza
The invention relates to a traditional Chinese medicine preparation for treating cardiovascular and cerebrovascular diseases, mainly containing one or more of Salvia miltiorrhiza, folium ginkgo, panax notoginseng, kudzuvine root Pueraria lobata, rhizoma ligustici wallichii, hawthorn and lotus leaf. The preparation method comprises the steps of selecting one or more of the Salvia miltiorrhiza, the folium ginkgo, the panax notoginseng, the kudzuvine root Pueraria lobata, the rhizoma ligustici wallichii and the hawthorn and lotus leaf, decocting, extracting active ingredients and concentrating the extracting solution to a certain concentration to obtain the Chinese traditional medicine preparation. In addition, the concentrated solution can be further made into capsules, tablets, granules, oral liquid, pills, pulvis, injection and sustained and controlled release preparations. Toxicologic and pharmacologic agent experiments show that the invention has no toxic side effect, is safe to use, has the functions of promoting blood circulation, remove blood stasis, reducing fat, freeing channels and eliminating phlegm and is suitable for phlegm stasis internal-block type cardiovascular and cerebrovascular diseases.
Owner:JIANGXI HERBFINE HI TECH
Composition of paeoniflorin and peony lactone glycoside with function of increasing leukocyte
InactiveCN1706397AImprove indexClassification normalOrganic active ingredientsPavoniaWhite blood cell
The present invention relates to medicine composition extracted from Paeonia lactifora Pall and containing the active components paeoniflorin and albiflorin in the total content of 50-95 % and weight ratio of 0.1-10. The composition is suitable for being prepared into various medicine preparations, including delayed releasing preparation, controlled releasing preparation, body cavity administrated preparation, spray, transdermal preparation, oral preparation, injection and transfused fluid preparation alone or together with other medicine components. The composition is suitable for preparing medicine for treating leucopenia, throbocytopenia and achreocythemia of different causes.
Owner:SHENYANG PHARMA UNIVERSITY
Biodegradability hydrogel controlled-release preparation and its preparation method and application
InactiveCN101283966ASimple manufacturing methodControl release speedPharmaceutical delivery mechanismLactidePLA-PEG-PLA
The invention relates to a biodegradable hydrogel controlled release preparation and a preparation and an application thereof, belonging to the technology field of medicines. The preparation method comprises the steps of: synthesizing poly(lactic acid)-polyethylene glycol-poly(lactic acid) (PLA-PEG-PLA) triblock copolymer by inducing L-lactide and D-lactide to respectively conduct ring-expansion polymerization by using zinc powder, zinc lactate or stannous octoate as catalyst and polyethylene glycol (PEG) 2,000-20,000; respectively dissolving poly-L-lactic acid-polyethylene glycol-poly-L-lactic acid and poly-D-lactic acid-polyethylene glycol-poly-D-lactic acid in water to obtain solutions with concentration of 0.05-0.5g / mL, swelling, mixing, and allowing gelatinization under constant temperature to obtain PLA-PEG-PLA triblock copolymer hydrogel for injection by complexing reaction. The hydrogel has good biological compatibility and biodegradability, can be used for embedding water-soluble drugs, and is an ideal drug controlled release carrier.
Owner:FUDAN UNIV
Modified release preparations containing oxcarbazepine and derivatives thereof
ActiveUS20090004263A1Improve bioavailabilityMinimize fluctuationBiocidePowder deliverySolubilityDrug release
Controlled-release preparations of oxcarbazepine and derivatives thereof for once-a-day administration are disclosed. The inventive compositions comprise solubility- and / or release enhancing agents to provide tailored drug release profiles, preferably sigmoidal release profiles. Methods of treatment comprising the inventive compositions are also disclosed.
Owner:SUPERNUS PHARM INC
Sustained control release preparation of nano porous active carbon-carrying agricultural antibiotic and preparation method thereof
The invention discloses a sustained control release preparation of a nano porous active carbon-carrying agricultural antibiotic and a preparation method thereof. The sustained control release preparation consists of the following components in percentage by weight: 15 to 40 percent of nano porous carbon, 50 to 80 percent of agricultural antibiotic, 0.25 to 1.0 percent of emulsifying agent, 0.5 to1.0 percent of thickening agent, 0.25 to 1.0 percent of dispersing agent, 0.25 to 1.0 percent of quickly penetrating agent and the balance of filler. The preparation method comprises the following steps of: after the agricultural antibiotic is dissolved, absorbing the agricultural antibiotic by using the nano porous active carbon; recrystallizing; adding the components according to the ratio; mixing completely and uniformly; and grinding and crushing the mixture to obtain the sustained control release preparation. The sustained control release preparation can effectively avoid illumination and degradation of microorganisms and can greatly increase the utilization ratio of the agricultural antibiotic.
Owner:INST OF ENVIRONMENT & SUSTAINABLE DEV IN AGRI CHINESE ACADEMY OF AGRI SCI
Oral slow/controlled-release preparation containing febuxostat and preparation method thereof
ActiveCN101773498AImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderBlood concentrationBurst effect
The invention provides an oral slow / controlled-release preparation containing febuxostat and a preparation method thereof, which prepare the febuxostat into the long-acting oral slow / controlled-release preparation and can solve the problem that the incidence of an adverse reaction is increased because of the quicker dissolving-out and the burst effect of a common preparation existing in the prior art. The invention has the technical scheme that the oral slow / controlled-release preparation containing the febuxostat comprises the following components by weight percent: 5 to 60 percent of febuxostat, 10 to 50 percent of slow / controlled-release material, 20 to 80 percent of filling auxiliary material, 0.3 to 20 percent of adhesive and 0.1 to 7 percent of lubricant or glidant. Compared with a common quick-release preparation, the slow / controlled-release preparation can keep the effective and stable blood concentration for a longer time, avoids the burst effect of the quick-release preparation, lowers the incidence of the adverse reaction and enhances the application safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Felodipine controlled-release preparation
InactiveCN1640400AAvoid damageStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveSemipermeable membrane
The present invention discloses a felodipine control-released preparation. It comprises core body and film. Said core body comprises feldodipine with effective therapeutic dose and physiologically-acceptable osmotic pressure regulating agent, osmotic accelerator and adhesive, and its film is made up by using semipermeable membrane material and pore-forming material.
Owner:HEBEI MEDICAL UNIVERSITY +1
Preparation method of ivermectin sustained-release microspheres
ActiveCN102302457AMicrosphere surface roundingImprove uniformityOrganic active ingredientsPharmaceutical non-active ingredientsMass ratioMicrosphere
The invention relates to ivermectin sustained-release microspheres used as an animal medicine for animal injection, and a preparation method thereof. The invention belongs to an interdisciplinary field of biomedical polymer materials and controlled release preparations. The invention discloses ivermectin sustained-release microspheres, which are characterized in that: the ivermectin sustained-release microspheres have particle sizes of 0.1 to 10mum, a medicine loading capacity of 20% to 35%, and an entrapment rate of above 80%. The preparation method of the ivermectin sustained-release microspheres comprises steps that: a carrier material and the medicine ivermectin with a mass ratio of 1:1-10 are dissolved in an organic solvent; the solution is processed through supersonic wave or mechanical stirring, such that the solution is emulsified into an oil phase, wherein a volume ratio of oil phase to water phase is 1:5-10; under a temperature of -10 DEG C to 30 DEG C, the oil phase is injected into a water phase disperse medium solution step-by-step; constant-temperature magnetic stirring is carried out with a rotation speed of 400 to 10000rpm, the mixture is sufficiently emulsified, such that an S / O / W type emulsion is obtained; the emulsion is stirred under room temperature for 3 to 4 hours, such that the organic solvent is sufficiently volatilized; the obtained product is centrifuged, washed, collected, and is vacuum-dried with a pressure of 0.01 to 0.04MPa under room temperature for 10 to 12 hours or is lyophilized under a temperature of -0 DEG C to -20 DEG C. With the processes, ivermectin sustained-release microspheres with particle sizes of 0.1 to 10mum are obtained.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
Preparation method for controlled release preparation, especial for zero-order release controlled release preparation
ActiveCN101987083AHigh moisture absorptionPharmaceutical non-active ingredientsGranular deliveryControl releaseMedicine
The invention discloses a preparation method for a controlled release preparation which has improved drug releasing property and mechanical property and increased production repeatability and is externally coated with a controlled release coat film comprising drug released pores filled with air, especial for a zero-order release controlled release preparation. The preparation method comprises thefollowing steps of firstly preparing core material; secondly, coating a controlled release coat film for the core material with solution of polymer containing sublimable matters and / or matter particles capable of being degraded into harmless gases or a dispersion liquid; thirdly, subjecting the polymer coat film to healing processing until the coating core material has a stable dissolution characteristic endpoint; and fourthly, volatilizing and / or degrading the sublimable matters and / or the matters capable of being degraded into harmless gases, wherein the fourth step is finished after the third step.
Owner:OVERSEAS PHARMA +1
Pregabalin controlled release preparation, and preparation method thereof
InactiveCN103585098AExtended stayProlong the action timeOrganic active ingredientsNervous disorderSodium bicarbonateCellulose
The invention relates to a pregabalin controlled release preparation, and a preparation method thereof. The pregabalin controlled release preparation comprises following ingredients, by weight, 30 to 75% of pregabalin, 5 to 40% of a filling material, 5 to 30% of a sustained-release material, 1 to 10% of an adhesive, and the balance an auxiliary material. The auxiliary material is one or more selected from magnesium stearate, talc, superfine silica powder, cetanol, octadecanol, liquid paraffin, stearic acid, sodium bicarbonate, magnesium carbonate and calcium carbonate; the sustained-release material is one or a mixture of more selected from ethyl cellulose, carbomer, polycarbophil, alginate, hydroxypropyl methyl cellulose, polyacrylic resin, and carnauba wax. The pregabalin controlled release preparation is capable of prolonging residence time of medicine in stomach, releasing long-lasting release and absorption of medicine, and further increasing bioavailability of medicine.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
Tebuconazole nano controlled release preparation and preparation method thereof
ActiveCN103636598AGood sustained release effectImprove adsorption capacityBiocideFungicidesMicrosphereMedicine
The invention discloses a tebuconazole nano controlled release preparation which takes tebuconazole as an active ingredient of a pesticide, and takes a hollow mesoporous nano-silica microsphere as a carrier. The invention further discloses a preparation method of the nano controlled release preparation. According to the tebuconazole nano controlled release preparation disclosed by the invention, the hollow mesoporous SiO2 nano-particles have stronger adsorption ability to the tebuconazole, and has adsorption drug loading capacity of 46%; the prepared tebuconazole nano controlled release preparation can control release time to over 100 hours in a continuously-stirred dissolution medium, and therefore, the novel tebuconazole nano controlled release preparation is indicated to have a very good controlled release effect.
Owner:JIANGSU ESSENCE AGROCHEM
Budesonide intestines sustained release dextromethorphan pellets and method of manufacturing the same
InactiveCN101108171ARegulated release rateGood film formingOrganic active ingredientsAntipyreticSustained release pelletsMedicine
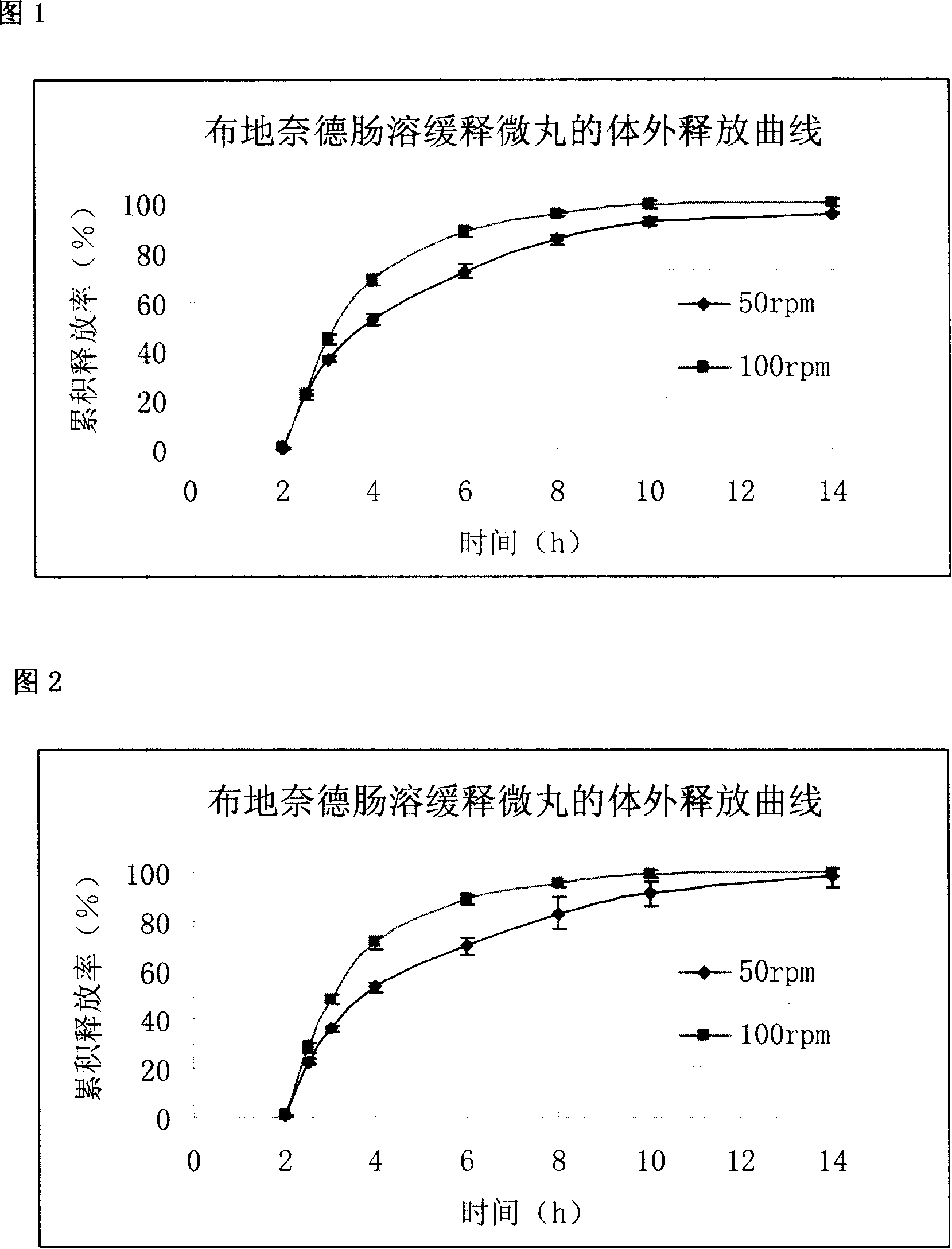
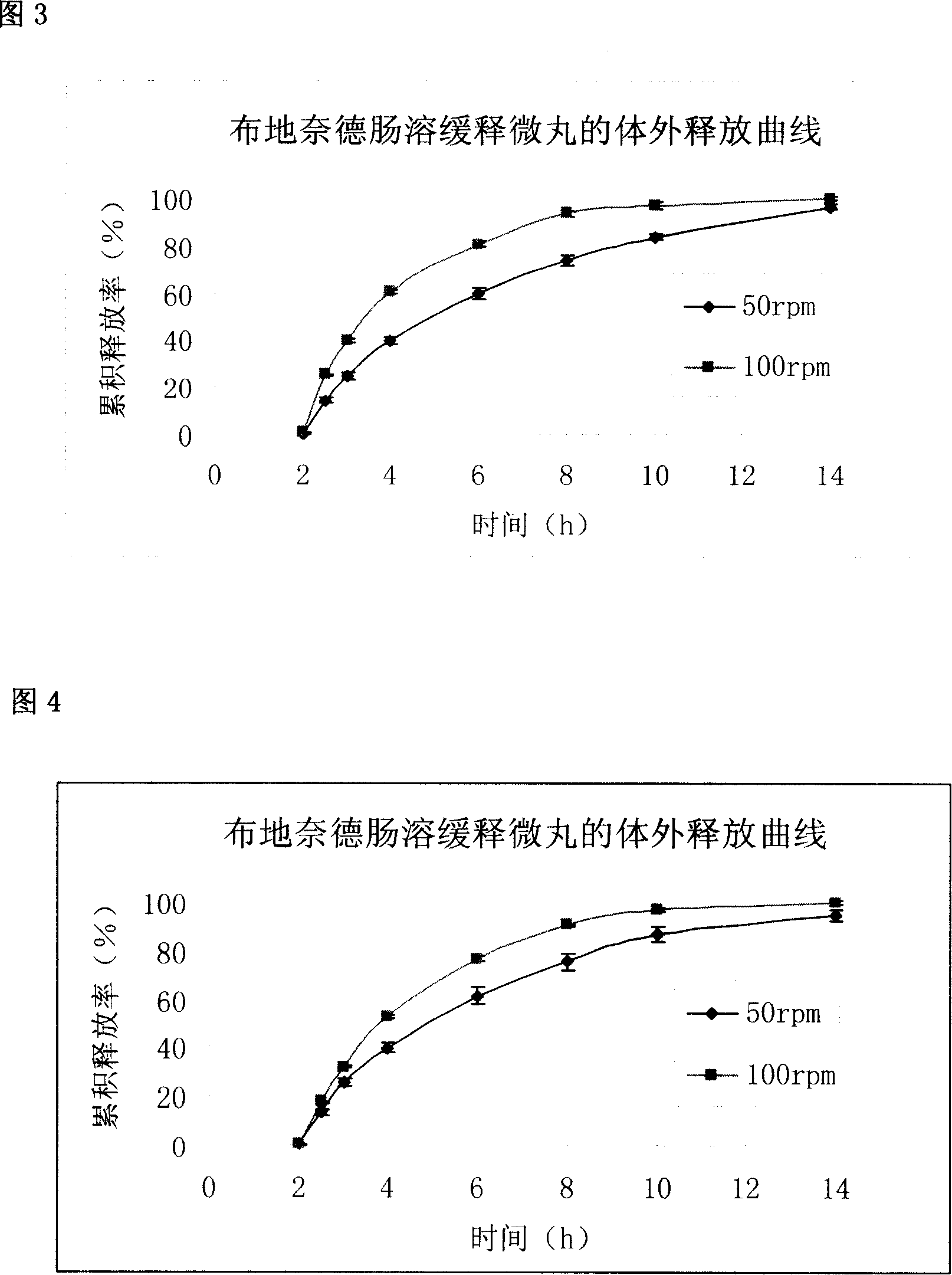
The invention belongs to the technical field of medicinal preparation and relates to an enteric preparation and sustained or controlled release preparation, in particular to an enteric controlled release micro-granule with budesonide and its preparation method. The invention, which comprises a quick release celphere, a controlled release layer and an enteric layer, takes budesonide as active ingredient of the drug to coordinate with medicinal carrier supplementary materials. After the release level testing, it is proved that the enteric controlled release micro-granule, which adopts different enteric materials, controlled release material and different coating conditions, does not release in stomach but began to release slowly after entering the small intestine with even release level. Therefore, the enteric controlled release micro-granule in the invention can effectively prevent the release in stomach, ensure the slow release in small intestine and the release of drug in the affected section of small incestine, ileocecus and colon.
Owner:FUDAN UNIV
A controlled-release preparation for eliminating Mikania micrantha and its preparation method
InactiveCN102283196AReduce dosageRemarkable killing effectBiocideAnimal repellantsMikania micranthaControl release
The invention discloses a controlled release preparation for removing mikania micrantha and a preparation method thereof. The controlled release preparation consists of a controlled release microsphere and a function medium with suspension capability and attaching and film-forming capability. The controlled release microsphere comprises the following components calculated according to mass percentage: 20-50 percent of weedicide, 20-40 percent of release rate mediator and 30-50 percent of high polymer carrier materials; and the function medium with the suspension capability and attaching and film-forming capability is a water solution consisting of a cross-linking agent and a functional polymer. The preparation method of the controlled release preparation comprises the steps of respectively preparing the controlled release microsphere and the function medium and then dispersing the controlled release microsphere in the function medium to obtain the controlled release preparation. The dosage of the controlled release preparation for treating can be reduced to be 0.01 / m<2>; the controlled release preparation has favorable safety for the environment and operators; and the effect of the controlled release preparation for removing the mikania micrantha is thorough compared with that of a traditional method.
Owner:SOUTH CHINA AGRI UNIV
Epimedium extract and preparation method, preparation and use thereof
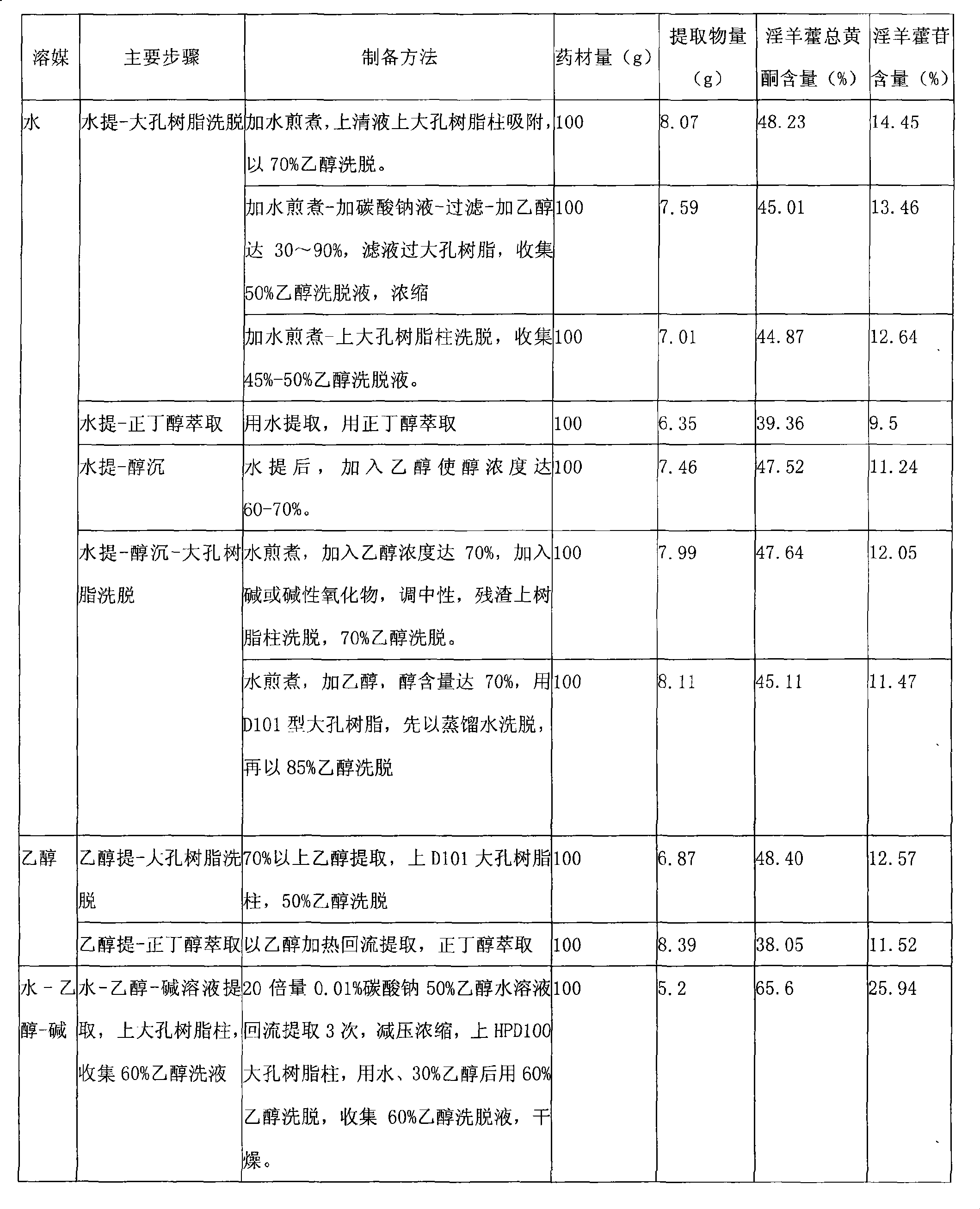
The invention provides a herba epimedii extract, in particular to a high-content herba epimedii total flavonoids extract, and the preparation method thereof mainly comprises the following steps: (1) alkali-ethanol-water is taken as a menstruum for refluxing extraction; (2) decompression and concentration are carried out; (3) pH value is adjusted; (4) macroporous resin column elution is carried out; and (5) an eluant is dried and the content of the obtained total flavonoids is over 65 percent and the content of icariin is over 20 percent. The extract can be prepared into various oral preparations according to the requirements in pharmacy, which can be hard capsules, soft capsules, conventional tablets, orally disintegrating tablets, buccal tablets, dispersible tablets, sustained-release preparations, controlled release preparations, granules, water-paste pills, dripping pills, honeyed pills, and the like. The extract and effective medicines containing the extract can be applied to preparing brain tissue protective agents, especially to preparing medicines that prevent and treat cerebral infarction, cerebral infarction sequela and other ischemic cerebrovascular diseases.
Owner:南京宇道科技开发有限公司
Compound pioglitazone hydrochloride/metformin hydrochloride bilayer osmotic pump controlled release preparation and preparation method thereof
InactiveCN102008472AImprove controllabilityStable storageOrganic active ingredientsMetabolism disorderCoated tabletsControl release
The invention provides a compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The compound preparation structurally comprises the following parts from inside to outside in sequence: a tablet core comprising a drug layer and a digestive layer, an insulation coating layer, a controlled release coating film with drug release holes, a rapid pioglitazone hydrochloride release layer and an unnecessary attractive coating. The invention also provides a preparation method of the compound preparation of pioglitazone hydrochloride and metformin hydrochloride bilayer osmotic pump controlled release tablets. The preparation method comprises the following steps of: (1) preparing the drug layer; (2) preparing the digestive layer; (3) tabletting the tablet core; (4) coating insulation coating layer; (5) coating the controlled release coating; (6) punching the coated tablet: (7) coating the rapid pioglitazone hydrochloride release layer; and (8) coating the attractive coating.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Chinese medicine granule containing dalbergia heartwood oil and preparation method thereof
ActiveCN101439076AReduce dosageReduce fearGranular deliveryCardiovascular disorderControlled-Release PreparationsDalbergia
The invention provides a traditional Chinese medicine granular preparation with dalbergia wood oil and a preparation method thereof. Traditional Chinese medicine spheroidized particles are prepared by using a traditional Chinese medicine extract and a pharmaceutically acceptable carrier. The shapes of the traditional Chinese medicine spheroidized particles are spherical or similar to spheres, and the density of the traditional Chinese medicine spheroidized particles ranges from 0.6 g / ml to 1.3 g / ml. A single dose of the spheroidized particles is small, the medicine taking is convenient, and the granular preparation can be used as a semifinished product of capsules and can also be used for developing sustained and controlled release preparations of traditional Chinese medicine.
Owner:TIANJIN TASLY PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com