Tebuconazole nano controlled release preparation and preparation method thereof
A technology of tebuconazole and sustained-release agent, applied in the field of tebuconazole nano sustained-release agent and preparation thereof, can solve the problems of uneven application, poor biodegradability, environmental pollution and the like, and achieves good sustained-release effect, strong The effect of adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Mesoporous SiO 2 Preparation of microspheres.
[0033] Dissolve CTAB in a mixed solution of ethanol and water, add ammonia and TEOS to the above-mentioned mixed solution containing CTAB under stirring conditions, react at 35°C for 24 hours, centrifuge, and wash with ethanol to obtain SiO 2 White powder. The molar ratio of ethyl orthosilicate: cetyltrimethylammonium bromide: 25wt% ammonia: water: ethanol is 1:0.0922:2.96:621:115.
[0034] The resulting SiO 2 SiO is obtained by transferring the powder to water 2 Dispersion liquid, placed for 150h, centrifuged, washed with a mixed solution of hydrochloric acid and ethanol several times. The concentration of hydrochloric acid is 36wt%, and the volume ratio of hydrochloric acid to ethanol is 1:500.
[0035] SiO 2 The white powder is dried and calcined at 500℃ for 5h to obtain ordered mesoporous SiO with hollow structure 2 Microspheres.
[0036] figure 1 Mesoporous SiO 2 The TEM image of the microspheres shows that the pr...
Embodiment 2
[0037] Example 2: Preparation of Pentanolol Nano Sustained Release Agent.
[0038] A saturated toluene solution of pentotholol is added to the silica microspheres, shaken in a constant temperature shaker at 25° C. for 24 hours, and centrifuged to obtain a pentotholol nano sustained release agent.
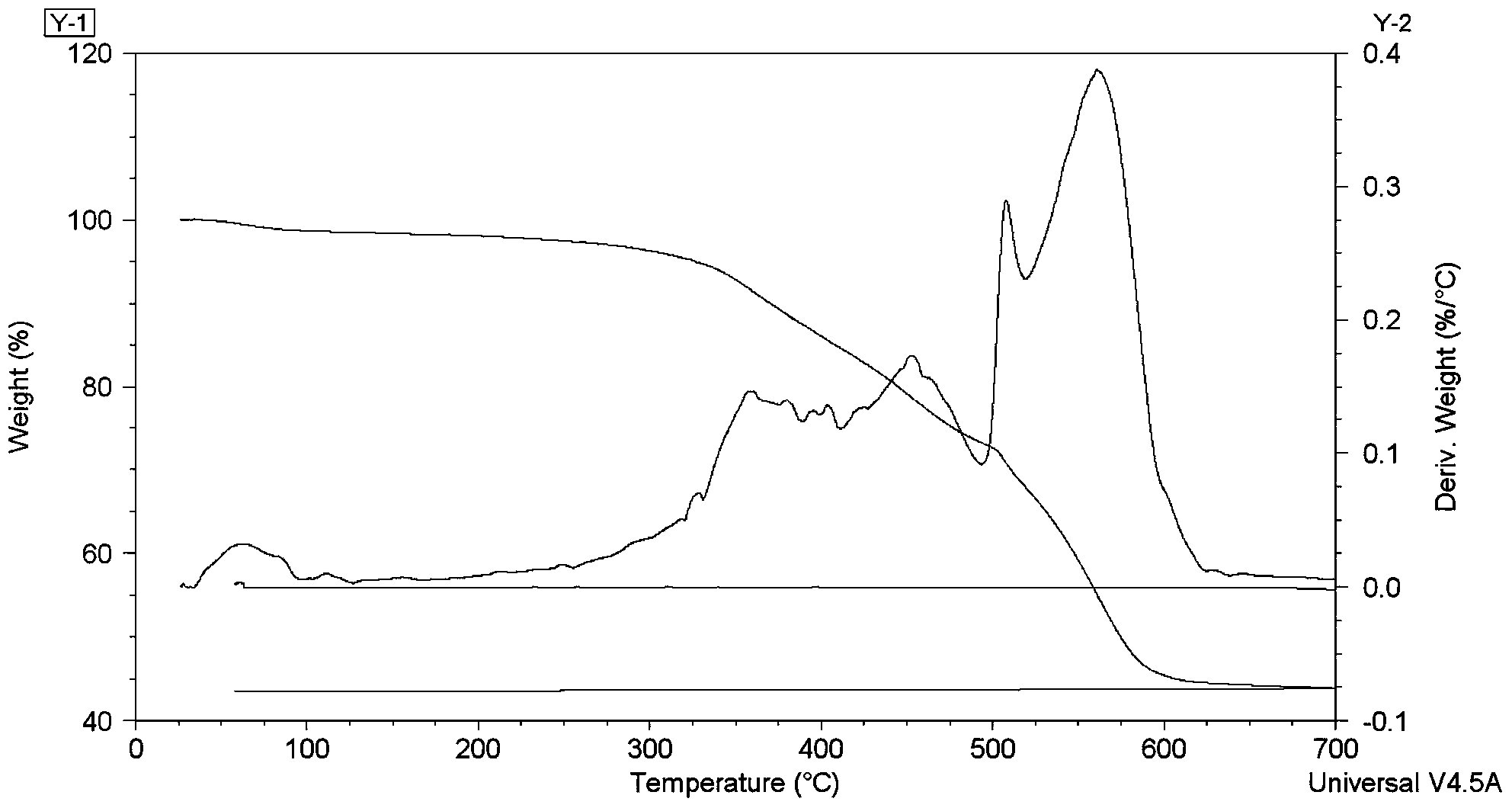
[0039] figure 2 Is pentanol and hollow mesoporous SiO 2 The thermogravimetric analysis results of the nano drug-loading system showed that the sample weight loss was not obvious before 300℃. When the temperature was higher than 300℃, the pentanolol began to decompose, and the weight loss was obvious. The mass was constant at 600~700℃, indicating that the pentanolol had completely decomposed. . It can be concluded from the figure that the drug loading amount of pentanolol in the nano drug loading system is about 46wt%.
Embodiment 3
[0040] Example 3: Determination of the sustained-release performance of the pentanolol nano sustained-release agent.
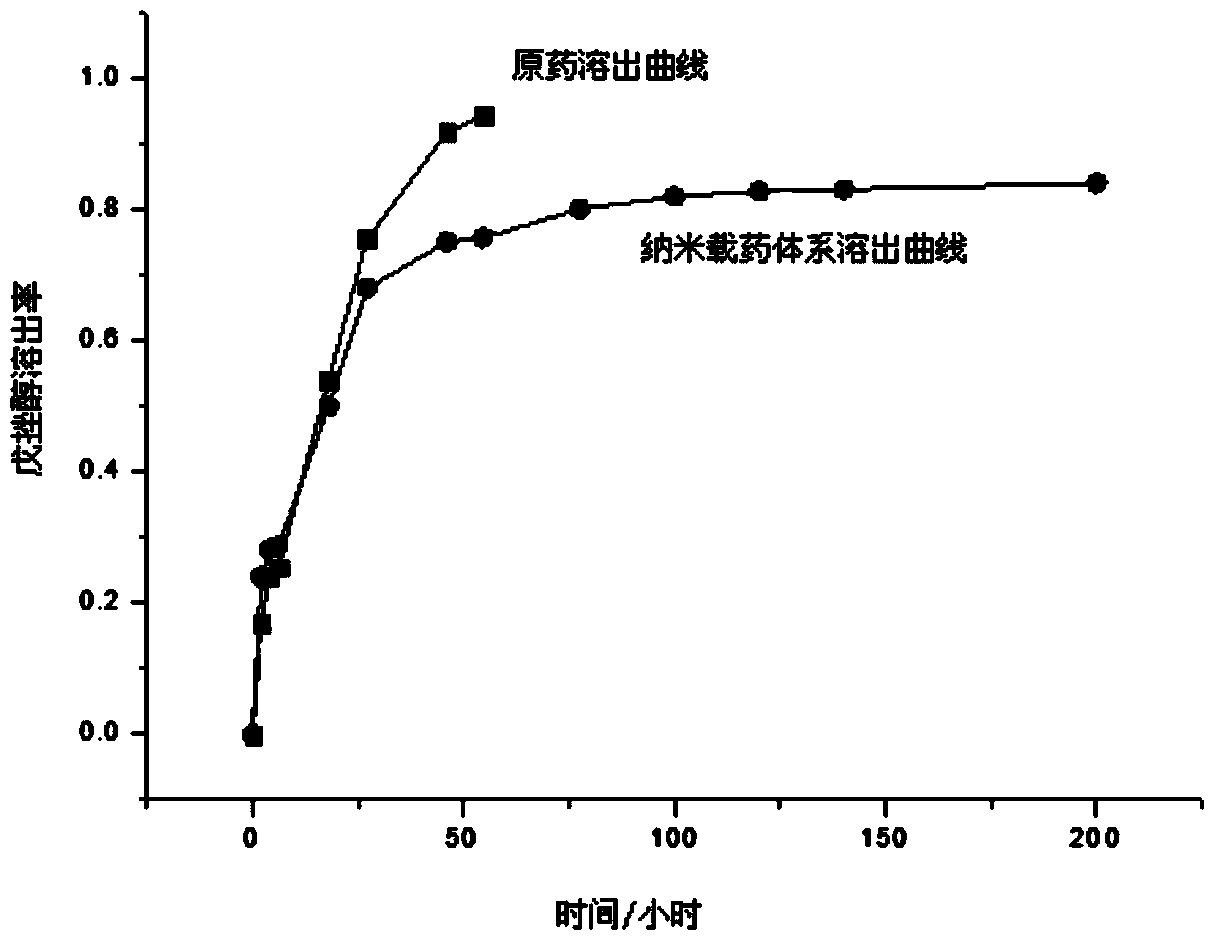
[0041] Precisely weigh 40mg pentoxolol and 1.3g pendoxolol nano drug-loaded particles, place them in a cylindrical dialysis bag, clamp the two ends with dialysis clips, and suspend them in 800mL ethanol solution containing 50(v / v)% by volume In the Erlenmeyer flask, perform magnetic stirring at room temperature, take out a small amount of liquid regularly, and supplement it with the same amount of liquid to keep the total volume of the liquid unchanged. The content of pentoxolol in the solution was measured by ultraviolet-visible spectrophotometer, and the curve of pentoxolol over time was drawn, and compared with the dissolution curve of pentoxolol original drug to characterize the sustained release effect.
[0042] image 3 It is the release kinetic curve of pentozolol technical drug and nano drug delivery system in 50% (v / v) ethanol / water solution. It can be s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com