Patents
Literature
4146 results about "Control release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of 'controlled-release'. controlled-release in the Pharmaceutical Industry. A controlled-release drug or preparation is released into the body in specified amounts over a specified period of time. The controlled-release system is designed to release the drug's active ingredient gradually over the day.
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
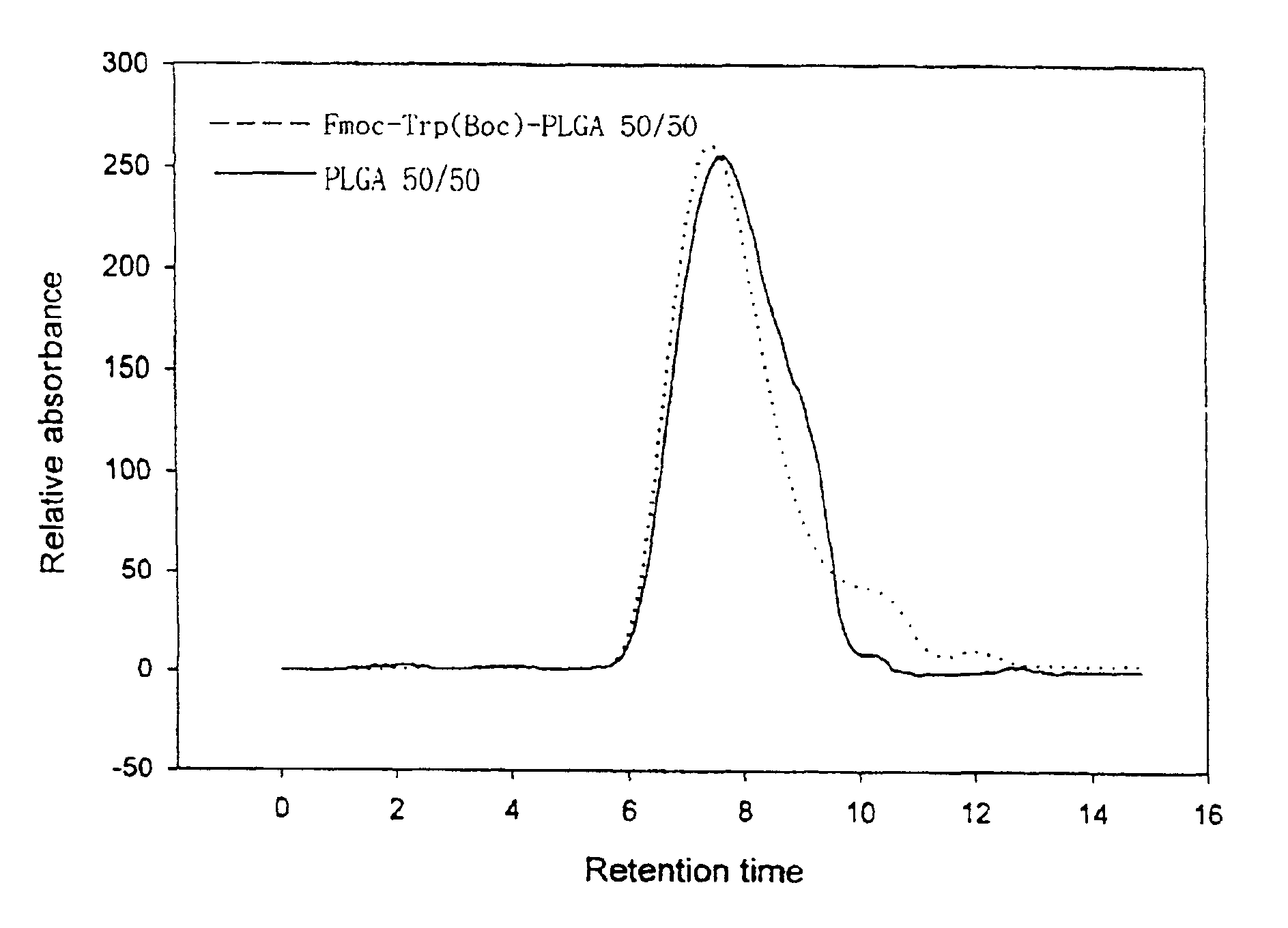
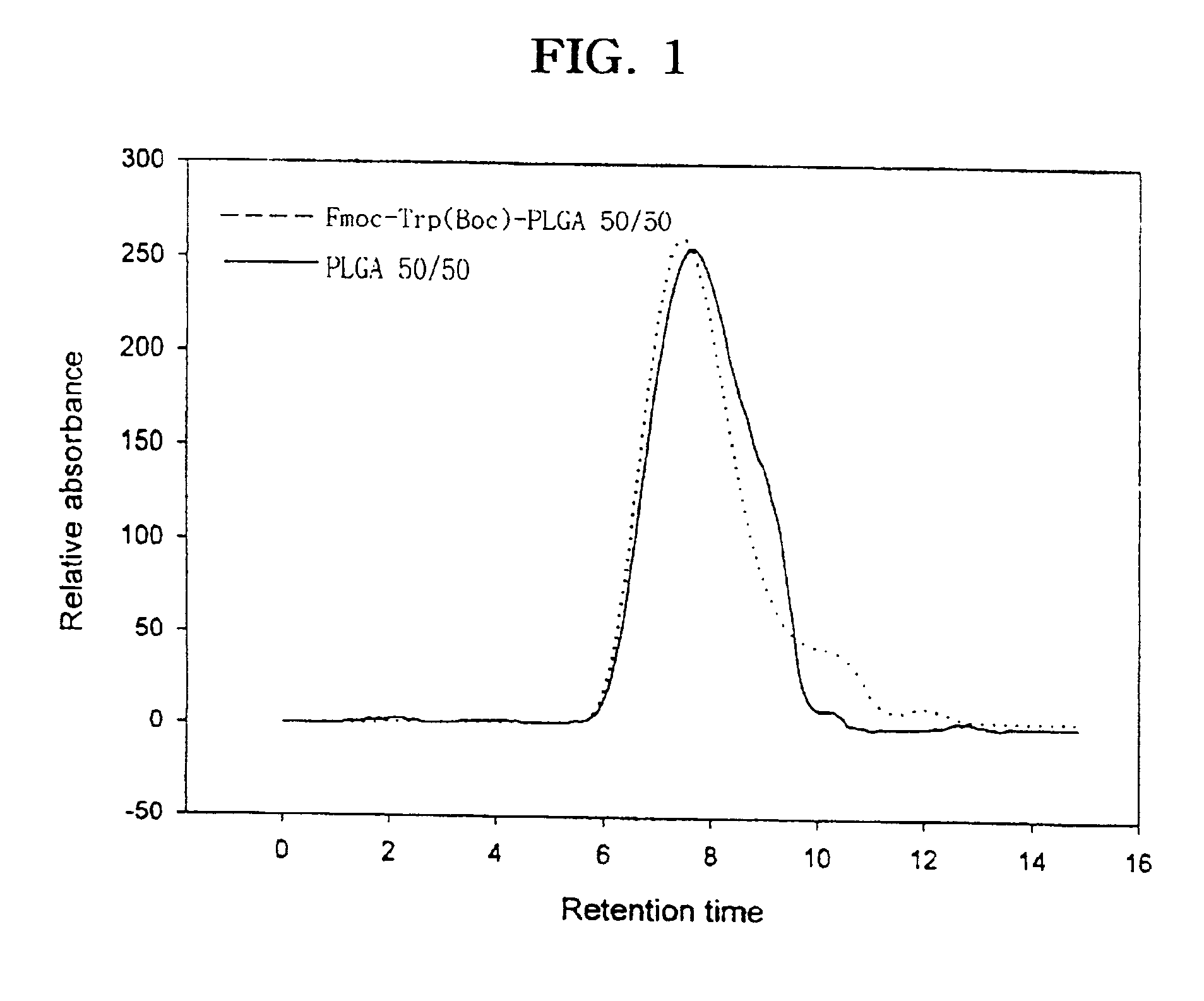
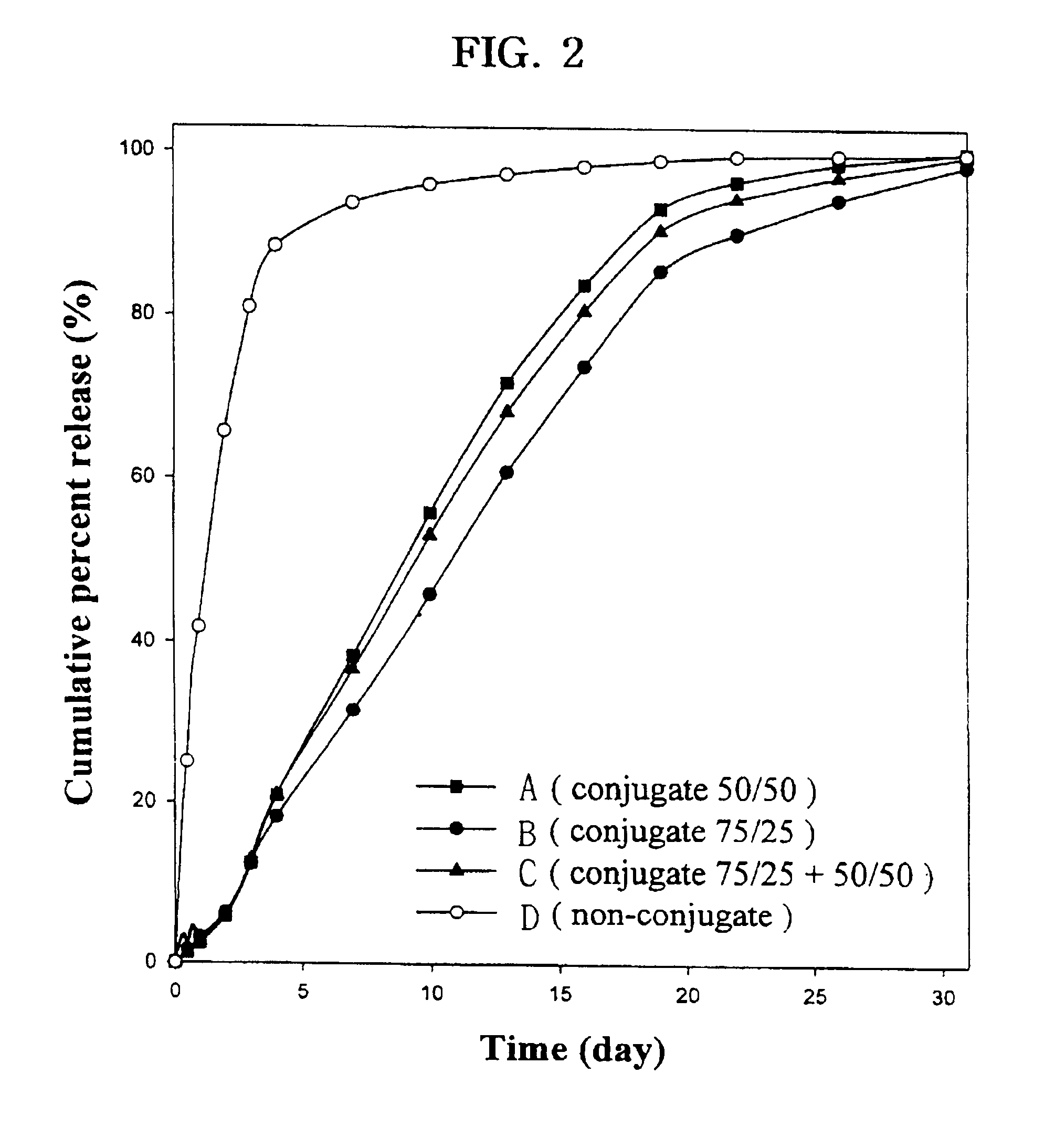
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
High molecular wegiht polymers, devices and method for making and using same
Anhydride polymers that release active or activatable agent(s) have pre-selected properties such as molecular weight, flexibility, hardness, adhesiveness, and other valuable properties. The polymers are suitable for use in compositions, formulations, coatings, devices, and the like that benefit from the controlled release of an agent(s) over a period of time. The polymers are prepared by a process involving various alternative and sequential steps that allow the design a priori of products with specific characteristics. The polymers are suitable as delivery systems, either by themselves, as compositions, formulations or devices.
Owner:RUTGERS THE STATE UNIV
Resorbable matrices for delivery of bioactive compounds
This invention relates generally to the production and use of inorganic-conditioning agent complexes for the controlled release of compounds including medicinals. Advantageously, the inorganic used is calcium sulfate and the conditioning agent is calcium stearate.
Owner:ROYER BIOMEDICAL INC
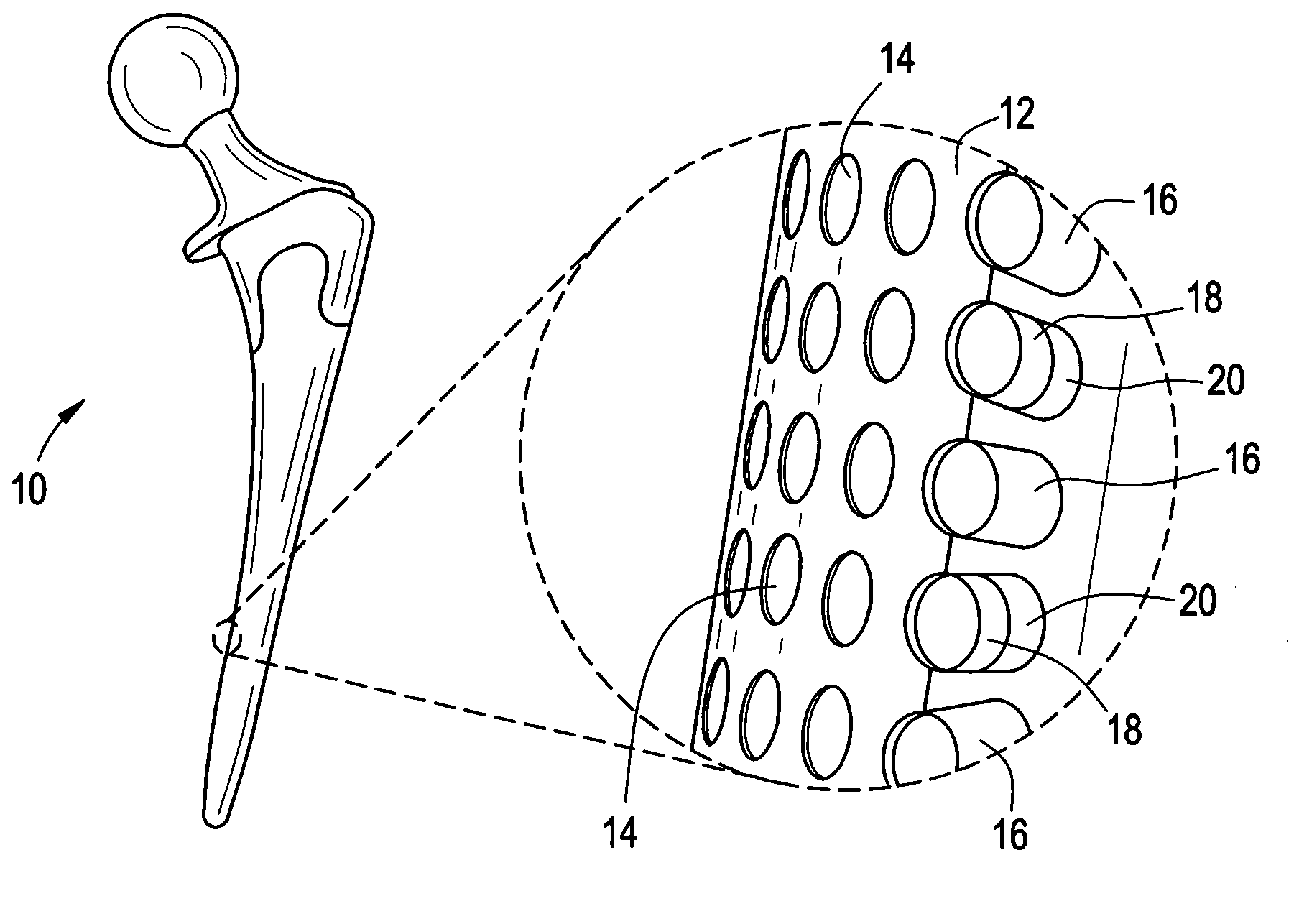
Orthopedic and dental implant devices providing controlled drug delivery
Implantable prosthetic devices are provided for controlled drug delivery, for orthopedic and dental applications. The device may include a prosthetic device body having at least one outer surface area; two or more discrete reservoirs located in spaced apart positions across at least a portion of the outer surface area, the reservoirs formed with an opening at the surface of the device body and extending into the device body; and a release system disposed in the reservoirs which comprises at least one therapeutic or prophylactic agent, wherein following implantation into a patient the therapeutic or prophylactic agent is released in a controlled manner from the reservoirs. The prosthetic device body preferably is a joint prosthesis or part thereof, such as a hip prosthesis, a knee prosthesis, a vertebral or spinal disc prosthesis, or part thereof. Optional reservoir caps may further control release kinetics.
Owner:MICROCHIPS INC
Controlled regional oral delivery
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
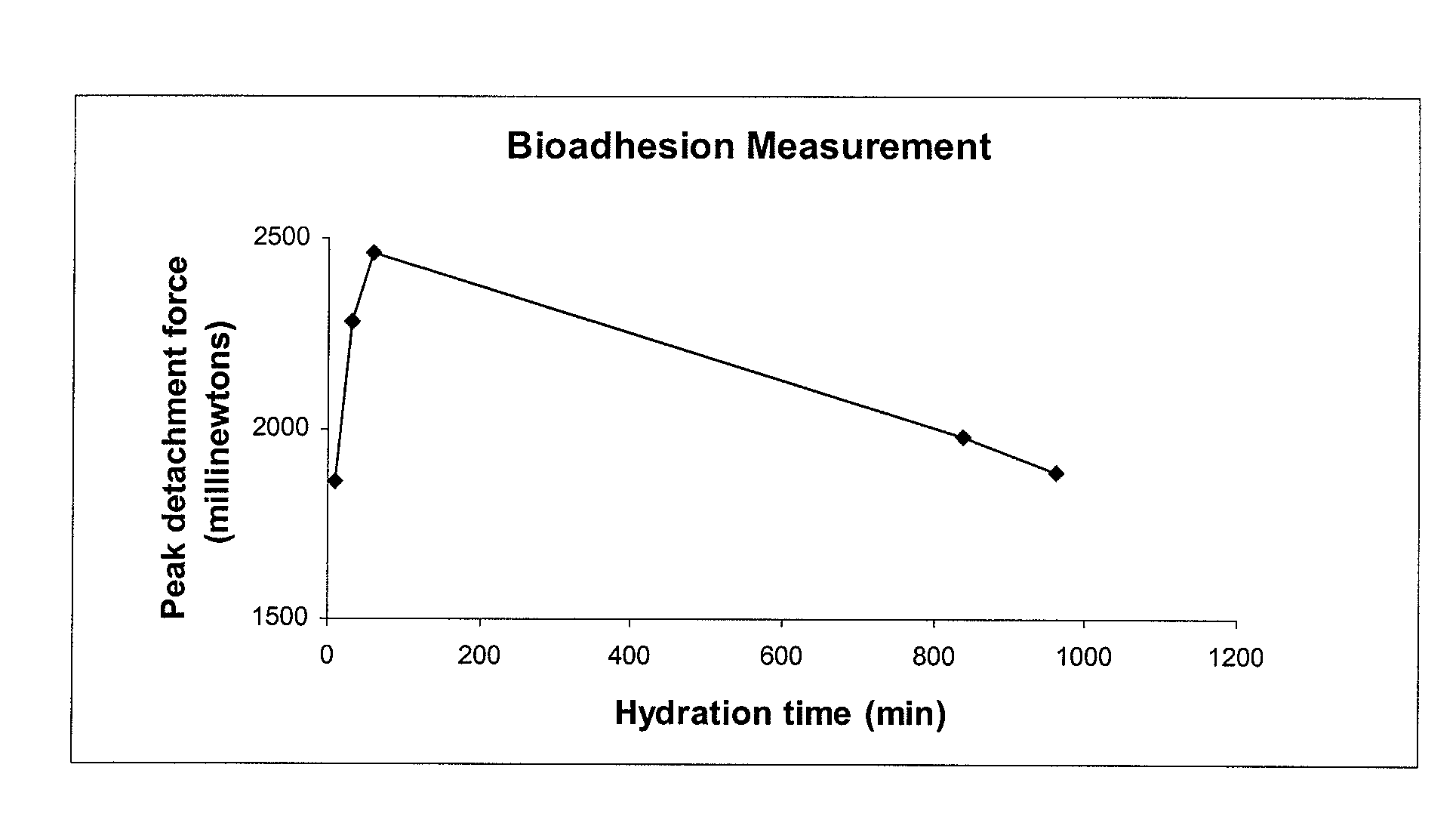
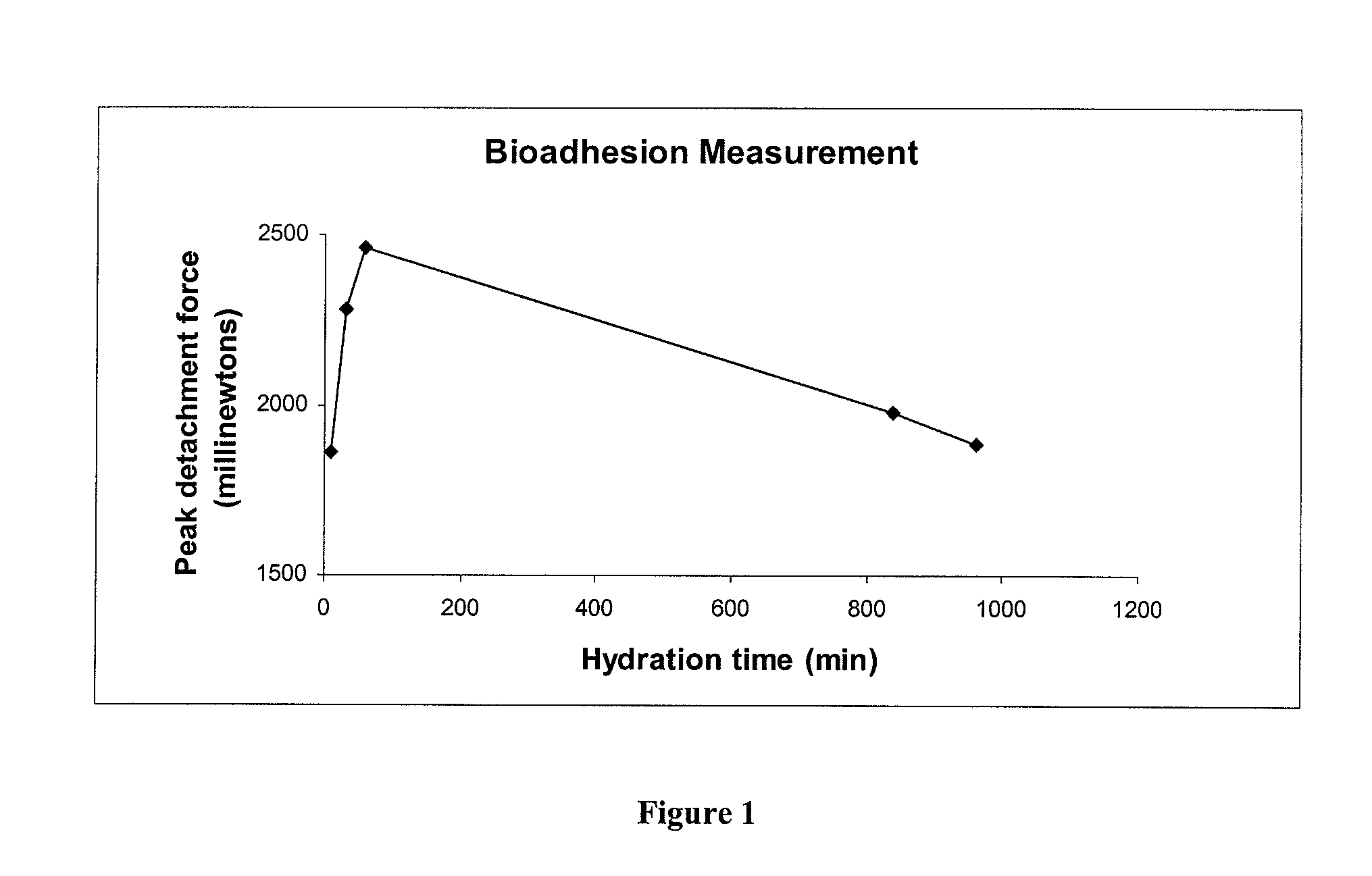
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
High load formulations and methods for providing prolonged local anesthesia
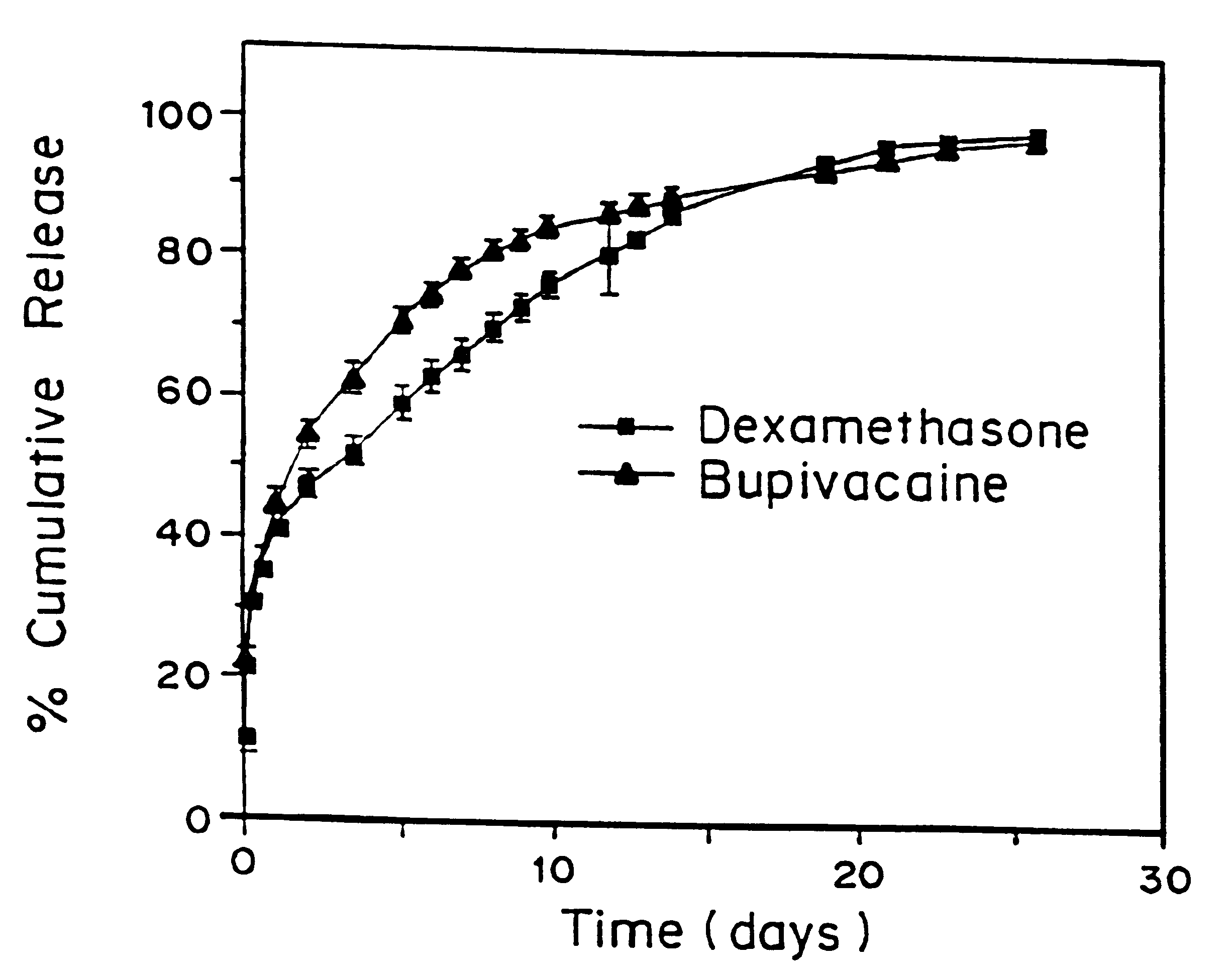
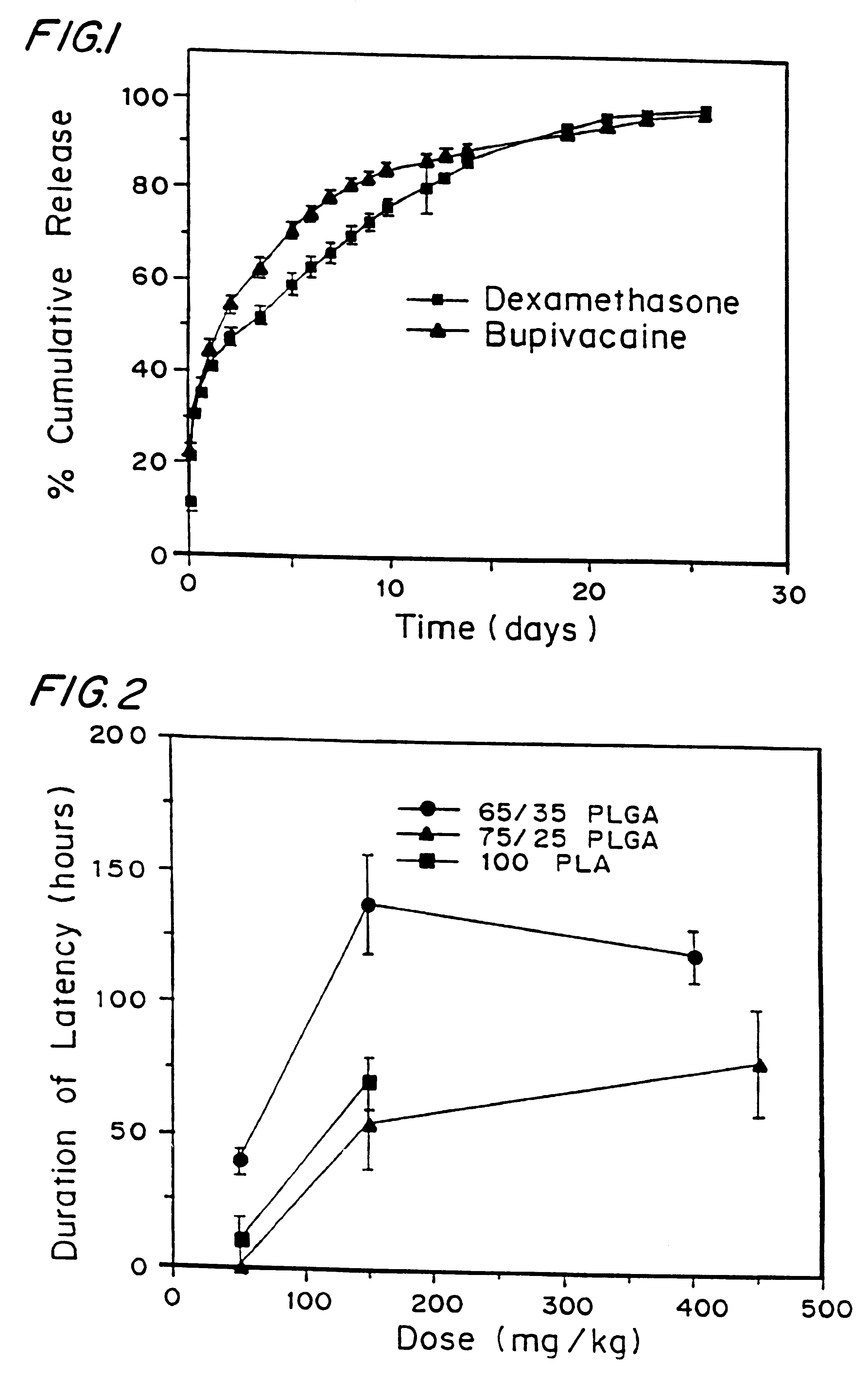
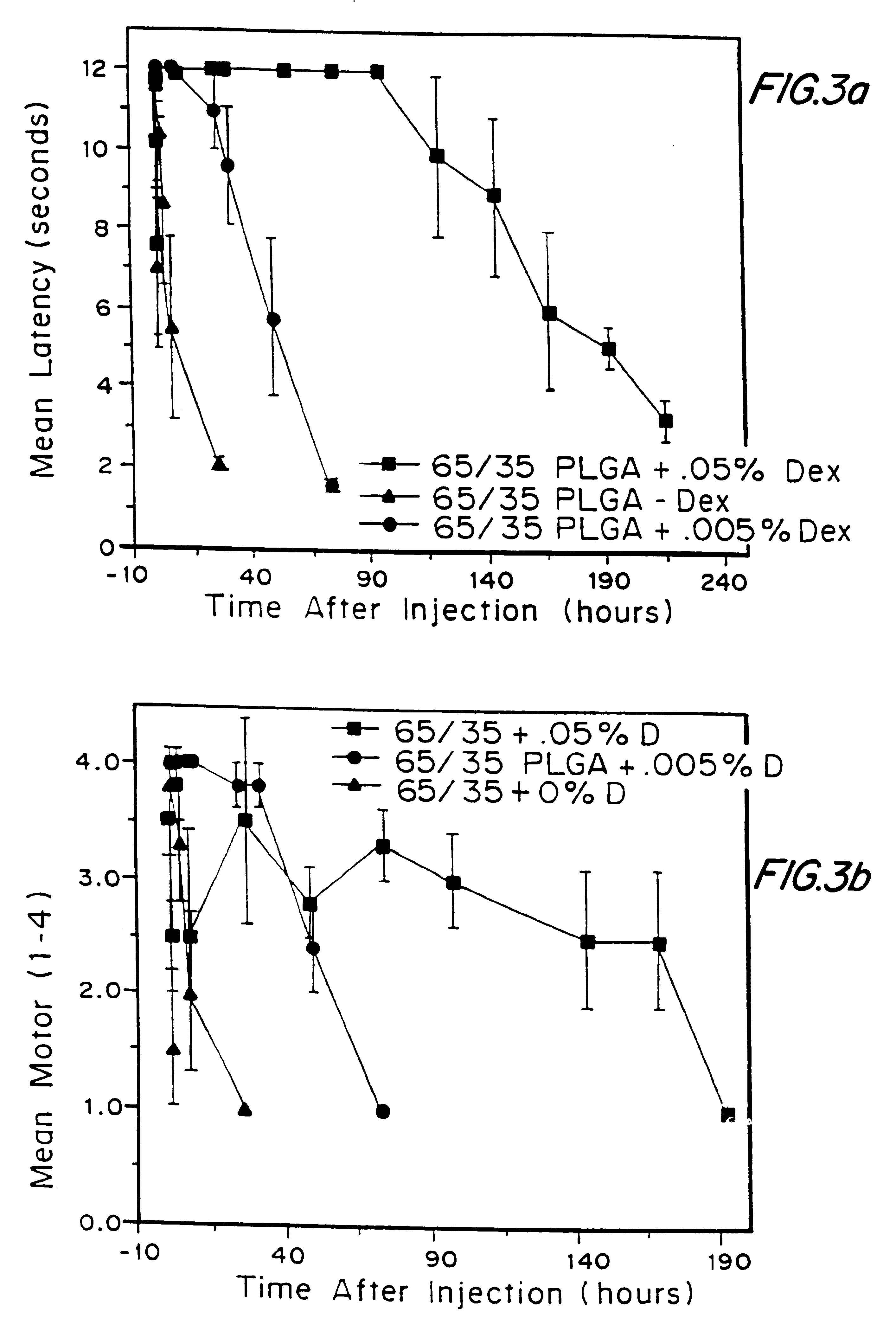
A formulation for inducing sustained local anesthesia in a patient comprising a substrate comprising a high load of local anesthetic by weight and an effective amount of a biocompatible, controlled release material to obtain a. reversible nerve blockade or anesthesia effect when implanted or injected in a patient, and a non-toxic glucocorticosteroid agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the glucocorticosteroid agent.
Owner:CHILDRENS MEDICAL CENT CORP
Compositions having a combination of immediate release and controlled release characteristics
InactiveUS6908626B2Rapid in vivo dissolutionSlower in vivo dissolutionPowder deliveryAerosol deliveryControlled releaseNanoparticle
Disclosed are compositions exhibiting a combination of immediate release and controlled release characteristics. The compositions comprise at least one poorly soluble active ingredient having a nanoparticulate particle size, at least one surface stabilizer adsorbed onto the surface of the nanoparticulate active agent particles, and at least one active ingredient having a microparticulate particle size.
Owner:BAUDAX BIO INC +1
Barrier layer with underlying medical device and one or more reinforcing support structures
A barrier layer device is formed of an underlying biocompatible structure having a barrier layer coating that can exhibit anti-inflammatory properties, non-inflammatory properties, and / or adhesion-limiting properties, as well as generate a modulated healing effect on injured tissue. As implemented herein, the barrier layer is a non-polymeric cross-linked gel derived at least in part from a fatty acid compound, and may include a therapeutic agent. The underlying structure can be in the form of a surgical mesh. The barrier device is further provided with anchoring reinforcements to aid with the fastening of the barrier device for implantation purposes and reinforcing truss sections or portions that prohibit or substantially reduce the occurrence of excessive stretching and tearing. The barrier device is implantable in a patient for short term or long term applications, and can include controlled release of the therapeutic agent.
Owner:ATRIUM MEDICAL
Protein matrix materials, devices and methods of making and using thereof
InactiveUS7662409B2Enhances strength and durabilityFacilitated releaseBiocidePowder deliveryActive agentProtein materials
The present invention relates to protein matrix materials and devices and the methods of making and using protein matrix materials and devices. More specifically the present invention relates to protein matrix materials and devices that may be utilized for various medical applications including, but not limited to, drug delivery devices for the controlled release of pharmacologically active agents, encapsulated or coated stent devices, vessels, tubular grafts, vascular grafts, wound healing devices including protein matrix suture material and meshes, skin / bone / tissue grafts, biocompatible electricity conducting matrices, clear protein matrices, protein matrix adhesion prevention barriers, cell scaffolding and other biocompatible protein matrix devices. Furthermore, the present invention relates to protein matrix materials and devices made by forming a film comprising one or more biodegradable protein materials, one or more biocompatible solvents and optionally one or more pharmacologically active agents. The film is then partially dried, rolled or otherwise shaped, and then compressed to form the desired protein matrix device.
Owner:PETVIVO HLDG INC
Controlled release pharmaceutical compositions for prolonged effect
InactiveUS20100239667A1Simple wayFew stepsOrganic active ingredientsAntipyreticEfferalganControl release
Layered pharmaceutical composition suitable for oral use in the treatment of diseases where absorption takes place over a large part of the gastrointestinal tract. The composition comprising A) a solid inner layer comprising i) an active substance, and ii) one or more disintegrants / exploding agents, one of more effervescent agents or a mixture thereof. the solid inner layer being sandwiched between two outer layers B1) and B2), each outer layer comprising iii) a substantially water soluble and / or crystalline polymer or a mixture of substantially water soluble and / or crystalline polymers, the polymer being a polyglycol in the form of one of a) a homopolymer having a MW of at least about 100,000 daltons, and b) a copolymer having a MW of at least about 2,000 daltons, or a mixture thereof, and iv) an active substance, which is the same as in said solid inner layer A), and layer A being different from layer B, the layered composition being coated with a coating C) that has at least one opening exposing at least one surface of said outer layer, the coating being substantially insoluble in and impermeable to fluids and comprising a polymer, and the composition having a cylindrical form optionally with one or more tapered ends, wherein the ratio between the surface area of one end surface of the cylinder and the length of the cylinder is in a range of from 0.02 to 45 mm.
Owner:EGALET LTD
Coated medical device
A stent that is adapted for introduction into a body passageway and which releases at least one biological agent after being inserted in the body passageway. The release of the at least one biological agent can be a controlled release via molecular diffusion through a non-porous polymer layer.
Owner:MIRUS LLC
Controlled release preparation
InactiveCN101987081AImprove stabilityRelease impact mitigationInorganic non-active ingredientsSuppositories deliveryParticulatesChemical reaction
The invention discloses a controlled release preparation with improved performance. The controlled release preparation comprises a core containing medicament and a controlled release film covering the outside of the core and being almost insoluble in water as well as stomach and intestines digestive juice. The controlled release film comprises particulate matters of a water soluble medicinal additive, the water-soluble medicinal additive is covered by a polymer film which can be soluble in the stomach and / or intestines digestive juice but almost insoluble in water, the polymer and the medicinal additive can not produce chemical reaction or can produce chemical reaction but do not produce water-insoluble non-gaseous products and the pharmaceutically unacceptable products, and the amount of the polymer is no more 700% of that of the medicinal additive. The invention also discloses a preparation method of the controlled release preparation. The controlled release preparation has the advantages of improved medicament release reproducibility, reduced medicament release lag time, accelerated medicament release and improved bioavailability, can realize located controlled release, delayed controlled release and interval type or pulse type controlled release of the medicament in the gastrointestinal tract, and the like.
Owner:钟术光
Conversion of liquid filled gelatin capsules into controlled release systems by multiple coatings
InactiveUS6929803B2Pretreated surfacesMacromolecular non-active ingredientsControl releaseActive agent
A dosage form comprising a gelatin capsule formed with a composite wall and containing a liquid, active agent formulation where the wall comprises a barrier layer formed over the external surface of the gelatin capsule, an expandable layer formed over the barrier layer and a semipermeable layer formed over the expandable layer is described. The dosage forms and methods provide for the conversion of standard gelatin, liquid formulation capsules into controlled, release dosage forms that permit the controlled release of the active agent into the environment of use over time.
Owner:ENCINAL PHARMA INVESTMENTS
Controlled release bait material and preparation method thereof
InactiveCN103004717AQuick and long-lasting releaseFast and long-lasting effectOther angling devicesWater insolubleMicrosphere
The invention relates to a controlled release bait material and a preparation method thereof. The controlled release bait material refers to small pills, micro-pills, microcapsules, microspheres, particles or sticks prepared from raw materials including a basic material, a feed attractant, a bonding agent, a hydrophilic high-polymer material, a water-insoluble high-polymer material, a quick-release material, a filler, a colorant, a flavoring agent and the like through a preparation means; and the controlled release bait material can be coated for improving the controlled release effect. The invention further discloses a preparation method of the bait material. The controlled release material has rapid and lasting attracting force on fishes, the fish attracting time can be adjusted according to the habit of a fisherman and the habits of fishes, the food ration and frequency of fishes are increased, the fishing success rate can be increased, the food intake of fishes in cultivation of fishes can be increased, the corrosion ratios of remnant feeds and feeds in water bodies are reduced, the utilization ratios of feeds is increased, and the water body environmental pollution is lowered. The preparation method provided by the invention is suitable for industrial production.
Owner:李群益 +2
Dual controlled release dosage form
A dosage form that provides a controlled release of at least two different active agents is provided. Particular embodiments include a dosage form that provides therapeutically effective levels of a first active agent and a second active agent in a mammal for an extended period of time following oral administration. An osmotic device containing a bi-layered core is provided. The osmotic device provides a dual controlled release of both drugs from the core. The layers of the core are in stacked, substantially concentric or substantially eccentric arrangement.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Disintegrant assisted controlled release technology
InactiveUS20060024361A1Controlled release rateEasy to controlOrganic active ingredientsBiocideControlled releaseWater insoluble
A disintegrant assisted controlled release device is disclosed. The device is a combination of a swelling disintegrant or super-disintegrant and water insoluble polymer or water soluble polymer, or both, and one or more water soluble or water insoluble active pharmaceutical ingredient(s). The said device is stabilized by a humectant or trehalose.
Owner:INTELLIPHARMACEUTICS
Controlled delivery system for fabric care products
InactiveUS7119060B2Improve impact performanceIncrease depositionCationic surface-active compoundsOrganic detergent compounding agentsControl releaseMedicine
The present invention relates to a controlled delivery system that can be incorporated in liquid, as well as, dry granular, or powder, fabric care products, such as fabric softeners, laundry detergents, rinse added products, and other fabric care products, to enhance fragrance performance. The controlled delivery system of the present invention is a solid, substantially spherical particle comprising hydrophobic cationic charge enhancing agents in conjunction with cationic fabric softening agents that assist in adhering the particles onto fabric. The particles can also include a fragrance. The particle can have an average particle diameter of from about 1 micron to about 500 microns. The controlled delivery system of the present invention can be utilized to deliver a broad range of fragrance ingredients onto fabric and prolong fragrance release from the dry laundered fabric over an extended period of time, or yield a high impact fragrance “burst” upon ironing the fabric. The invention also pertains to fabric care products comprising the controlled release system of the present invention.
Owner:SALVONA
Gastric retention controlled drug delivery system
ActiveUS20040180088A1Maintain it physical integrityMaintain physical integrityOrganic active ingredientsNervous disorderDrug deliveryDrug
The present invention provides a gastric retention controlled drug delivery system comprising: (a) a controlled release core comprising a drug, a highly swellable polymer and a gas generating agent, said core being capable of swelling and achieving floatation rapidly while maintaining its physical integrity in gastrointestinal fluids for prolonged periods, and (b) a rapidly releasing coat composition comprising the same drug as in the core and pharmaceutically acceptable excipients, wherein the coating composition surrounds the core such that the system provides a biphasic release of the drug in gastrointestinal fluids.
Owner:SUN PHARMA INDS
Pharmaceutical composition having reduced abuse potential
ActiveUS20090232887A1Efficiently employedReduce decreaseBiocideOrganic active ingredientsControl releaseAdditive ingredient
A pharmaceutical paste composition comprising an active ingredient such as an addictive substance, a controlled release agent, and a pharmaceutically suitable aqueous or non-aqueous carrier. The composition may comprise one or more of a clay, or an oily, waxy, or fatty substance. The composition may be filled into a capsule or other dispensing device. The composition may reduce dose dumping of an active ingredient. Methods of making and using the composition are also described.
Owner:INTELLIPHARMACEUTICS
Metering and packaging of controlled release medication
InactiveUS7404968B2Powder deliveryPeptide/protein ingredientsControlled releaseBiomedical engineering
Controlled quantities of powdered medication are formed in controlled release packages using electrostating metering. Also provided are combination medication therapy delivery packages comprising two or more active pharmaceuticals segregated from one another in a single delivery package.
Owner:MICRODOSE THERAPEUTX INC
Heavy polymer coated slow-release fertilizer with sulfide as bottom coat
InactiveCN1569774AStrong impact resistanceImprove wear resistanceFertiliser formsUrea compound fertilisersControl releaseCoated urea
The invention relates to an enveloped controlled release fertilizer and method for preparation which consists of, preheating the urea particles to a predetermined temperature, spraying the molten liquid state sulfur to the urea particles, forming a layer of smooth and compact sulfur-coated urea, charging hot-curing resin component on the sulfur-coated urea for even distribution onto the urea particle surface and fast formation.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Controlled and directed local delivery of anti-inflammatory compositions
InactiveUS20060046961A1Relieve painLimiting bone lossOrganic active ingredientsPeptide/protein ingredientsSkeletal injuryControl release
The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by controlled and directed delivery of one or more biological response modifiers to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by implantable or infusion pumps, implantable controlled release devices, or by sustained release compositions comprising biological response modifiers.
Owner:SDGI HLDG
Smoke-free substitute cigarette product
ActiveUS20100126505A1Straightforwardly be carriedReduce weightTobacco treatmentTobacco devicesFlammable gasCigarillo
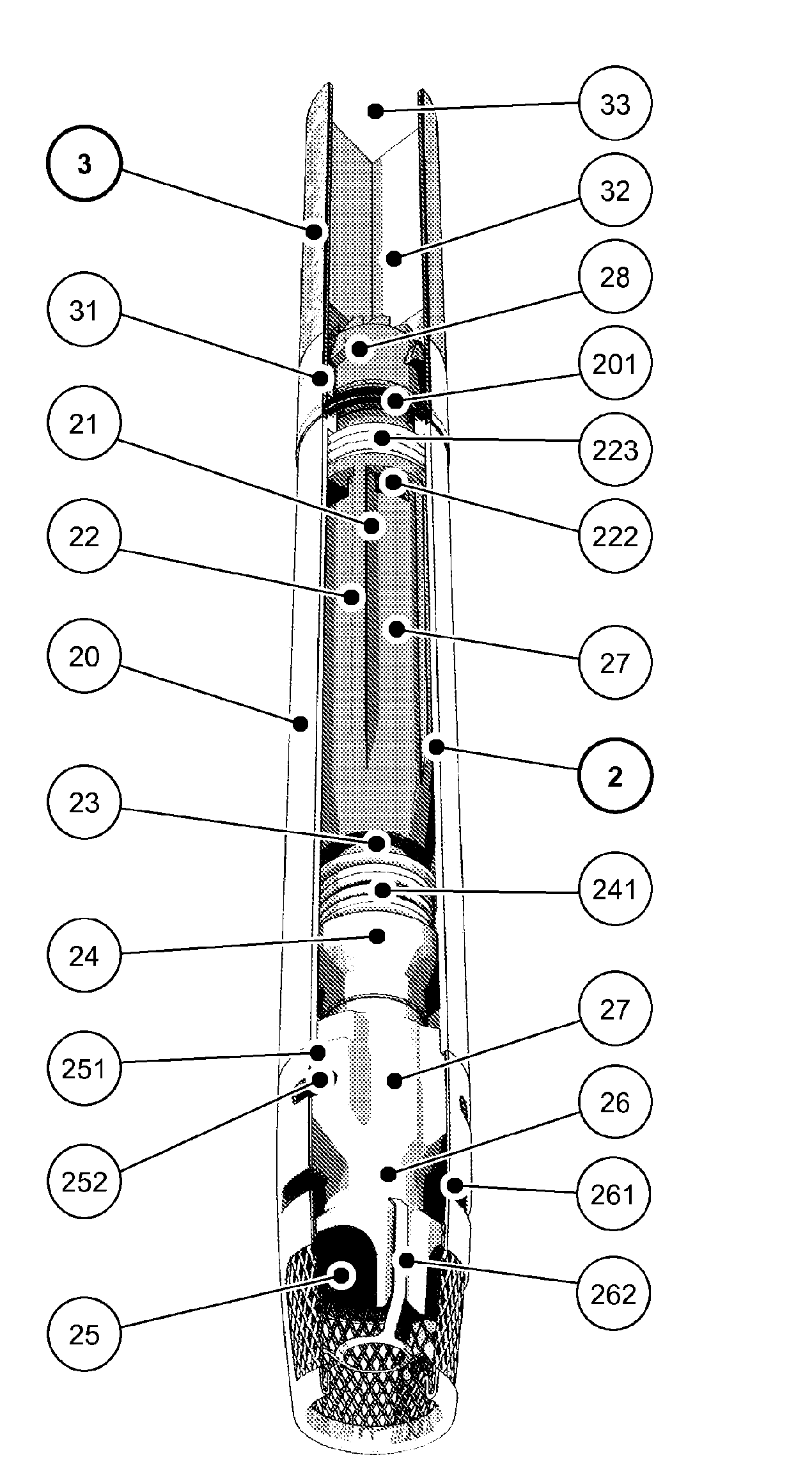
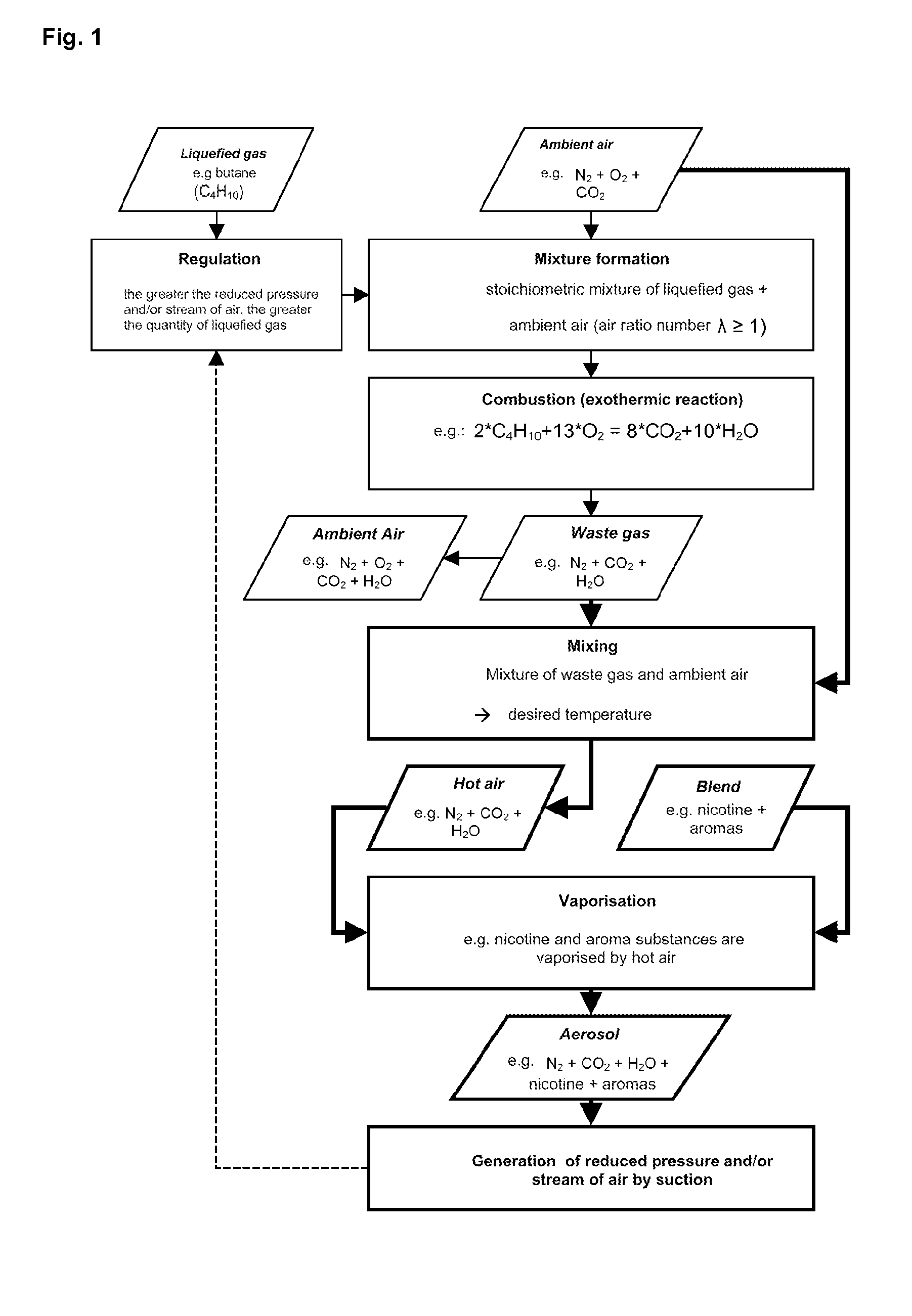
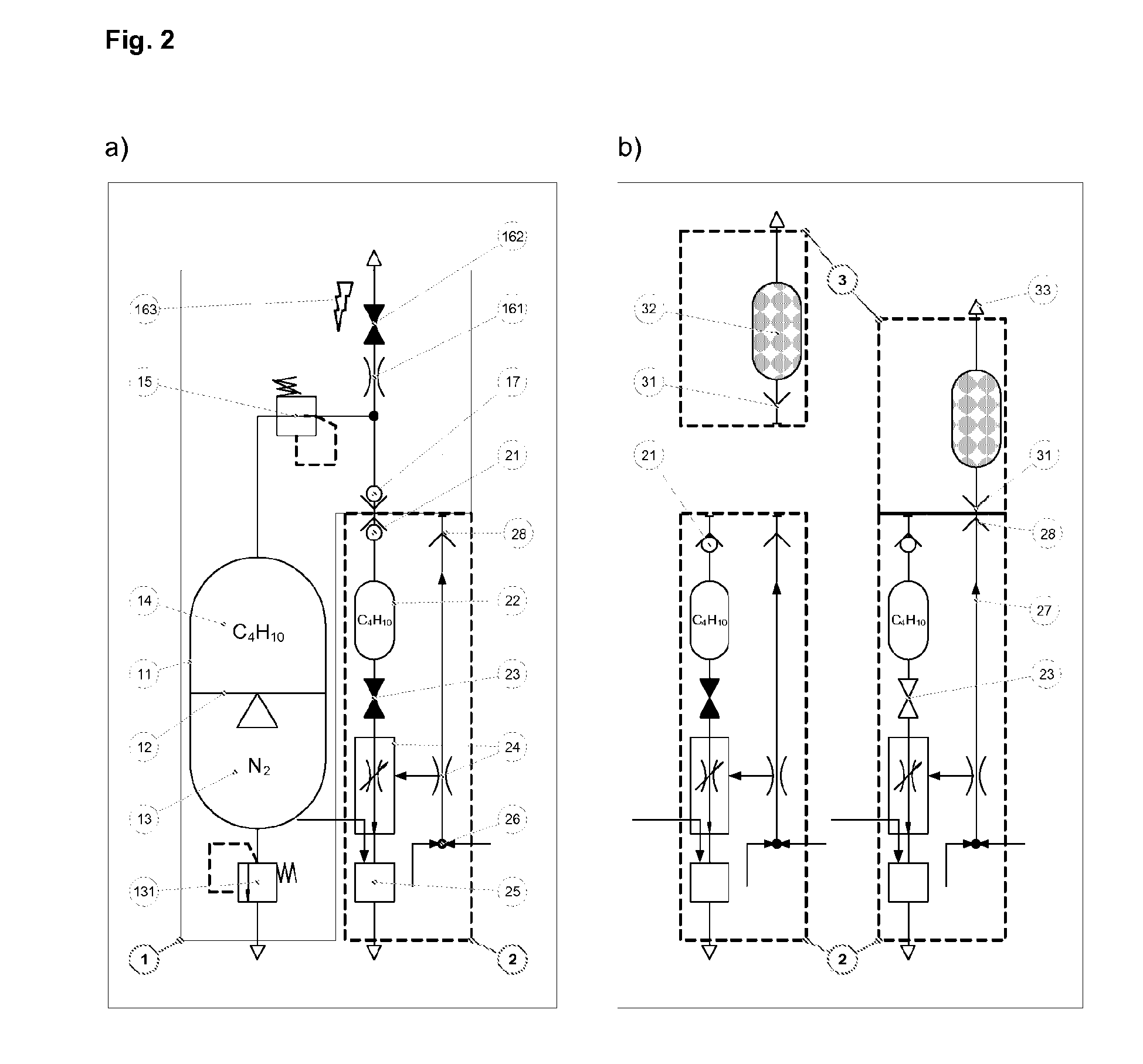
A method for volatilising active and / or aroma substances for the purpose of releasing an inhalable aerosol, wherein combustion gases of a flammable gas, which is preferably combusted with an excess of air, are passed partially or entirely, optionally mixed with ambient air, through an active and / or aroma substance depot and wherein a desired temperature is selectable by the proportion of combustion gases and optionally by the mixing ratio of said combustion gases with ambient air and the device implementing the method in the shape and dimensions of a cigarette or cigar for releasing an inhalable aerosol, comprising a mouthpiece (3) containing an active and / or aroma substance depot (32), a heating member (2) with a housing sleeve with one or more air inlets and one or more hot air outlets at the mouthpiece end, a filling valve (21) for filling a gas tank (22) with a flammable gas, preferably propane or butane gas, a regulating valve (24) for the controlled release of the gas from the gas tank (22) to a burner (25) and a mass transfer exchanger (26) for heating the air by the hot combustion gases produced by means of the burner (24), wherein the mouthpiece (3) is detachably connected to the heating member (2) and control of the regulating valve (24) is effected by means of the reduced pressure and / or stream of air generated by a user's suction on the mouthpiece (3). Fuelling station for such a device.
Owner:PHILIP MORRIS PROD SA
Method of microencapsulation
InactiveUS6932984B1Easily adaptedEasy to adaptCosmetic preparationsOrganic active ingredientsEmulsionMicroencapsulations
A method for microencapsulation of substances is provided. The substance(s) is / are dissolved or dispersed in an organic solvent of the kind that is partially miscible in water media. This organic solution is then mixed with an aqueous solution, which is saturated with an organic solvent and an emulsifier to form an emulsion. The emulsion is then poured into water under continuous agitation for the extraction of residual solvent. The formation of the solid capsules takes place during this extraction process. The capsules are undergone to further purification, whereby the microcapsules can be separated from the water and dried. By conditions of incubation of microcapsules in water-containing formulations the wall-softening process takes place. The unique system for controlled releasing the ingredients from microcapsules is based on the above-mentioned process.
Owner:TAGRA BIOTECH
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
A kind of stable controlled-release granular medicinal fertilizer
ActiveCN102276356AAvoid stabilityAchieve controlled releaseFertilizer mixturesControl releaseCore shell
The invention relates to a stable controlled release particle pesticide-containing fertilizer. The pesticide-containing fertilizer has a core-shell structure, is granular and comprises a core which is composed of a fertilizer and a microcapsule pesticide or pesticide pulvis adsorbed by a porous material, and a shell which is used as a controlled release layer. The invention enables the pesticide and the fertilizer to stably exist and to be slowly released, frequency of pesticide application and fertilizer application in growth stages of crops to be reduced and field labor to be saved.
Owner:GAUNGXI TIANYUAN BIOCHEM
Elastomeric article with antimicrobial coating
ActiveUS20070104766A1Low water solubilitySustainable antimicrobial activityBiocideStentsWater basedSolubility
A first aspect of the present invention is directed to a surface treatment for elastomeric articles such as medical gloves, comprising a water-based coating formulation and antimicrobial agent(s) embraced therein in an essentially powder-free composition coating. A second aspect of the present invention is directed to a water-based coating formulation for elastomeric articles such as gloves comprising at least one non-volatile water-soluble antimicrobial agent in a controlled-release matrix comprising a blend of a hydrophilic polymer and a hydrophobic component. The controlled-release matrix / blend requirements include: compatibility with the antimicrobial agent, formation of a reservoir of antimicrobial agent, coating film flexibility and lower water-solubility than the antimicrobial agent. A third aspect of the present invention is directed to a method for treating medical gloves comprising dipping, spraying, tumbling or other means for applying to the glove surface a composition to provide medical gloves with sustainable antimicrobial activity.
Owner:ALLEGIANCE CORP
Devices for intrabody delivery of molecules and systems and methods utilizing same
InactiveUS20040032187A1Piezoelectric/electrostriction/magnetostriction machinesMicromachined deliveryControl releaseTransducer
A device for controlled release of molecules is provided. The device including: (a) a device body having at least one reservoir therein for containing the molecules, the at least one reservoir being formed with a barrier impermeable to the molecules thereby preventing release thereof from the at least one reservoir; and (b) at least one acoustic transducer being attached to, or forming a part of, the device body, the at least one acoustic transducer being for converting an acoustic signal received thereby into an electrical signal, the electrical signal leading to barrier permeabilization and therefore release of the molecules from the at least one reservoir.
Owner:REMON MEDICAL TECH
Pharmaceutical compositions of rifaximin
ActiveUS20090028940A1Extended stayImprove complianceAntibacterial agentsBiocideImmediate releasePharmaceutical medicine
A pharmaceutical composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s) and release controlling agent(s). Pharmaceutical composition of rifaximin comprising: at least two entities wherein one entity is an immediate release or fast release and the other is controlled release. The pharmaceutical composition in the form of multilayer tablet comprising, at least one layer comprising, therapeutically effective amount of rifaximin or pharmaceutically acceptable salt or enantiomer or polymorph thereof, pharmaceutically acceptable excipient(s); said layer providing controlled release rifaximin; and at least one layer which provides increased residence time of the dosage form in the gastrointestinal tract. The pharmaceutical formulation comprising rifaximin having an in vitro dissolution profile, wherein about 70% of rifaximin is released in about 24 hours. The composition comprising therapeutically effective amount of rifaximin or pharmaceutically acceptable salt(s) or enantiomer(s) or polymorph(s) thereof, one or more release controlling agent(s) and pharmaceutically acceptable excipient(s) causing pathogenic eradication.
Owner:LUPIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com