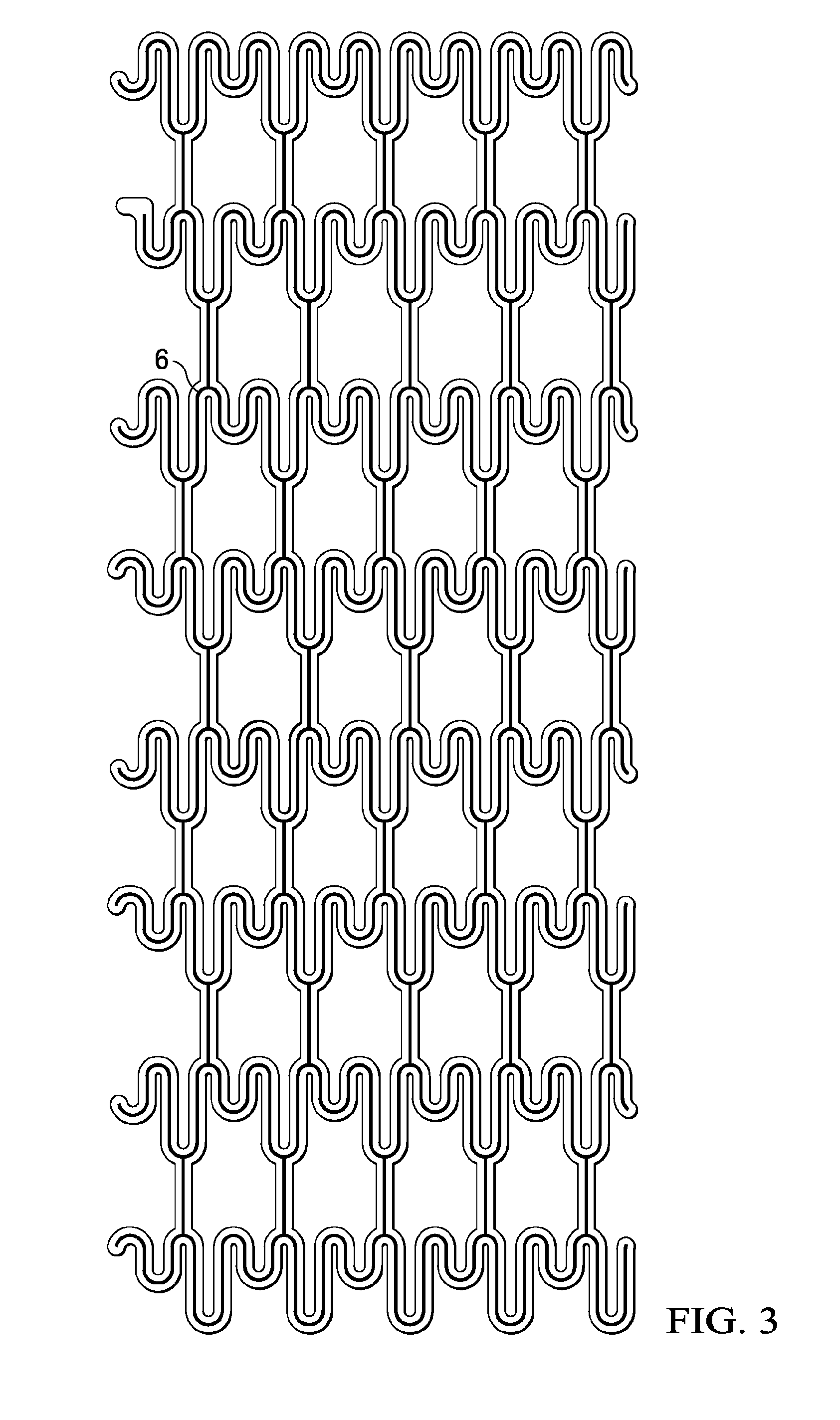Patents
Literature
33 results about "Zero order kinetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
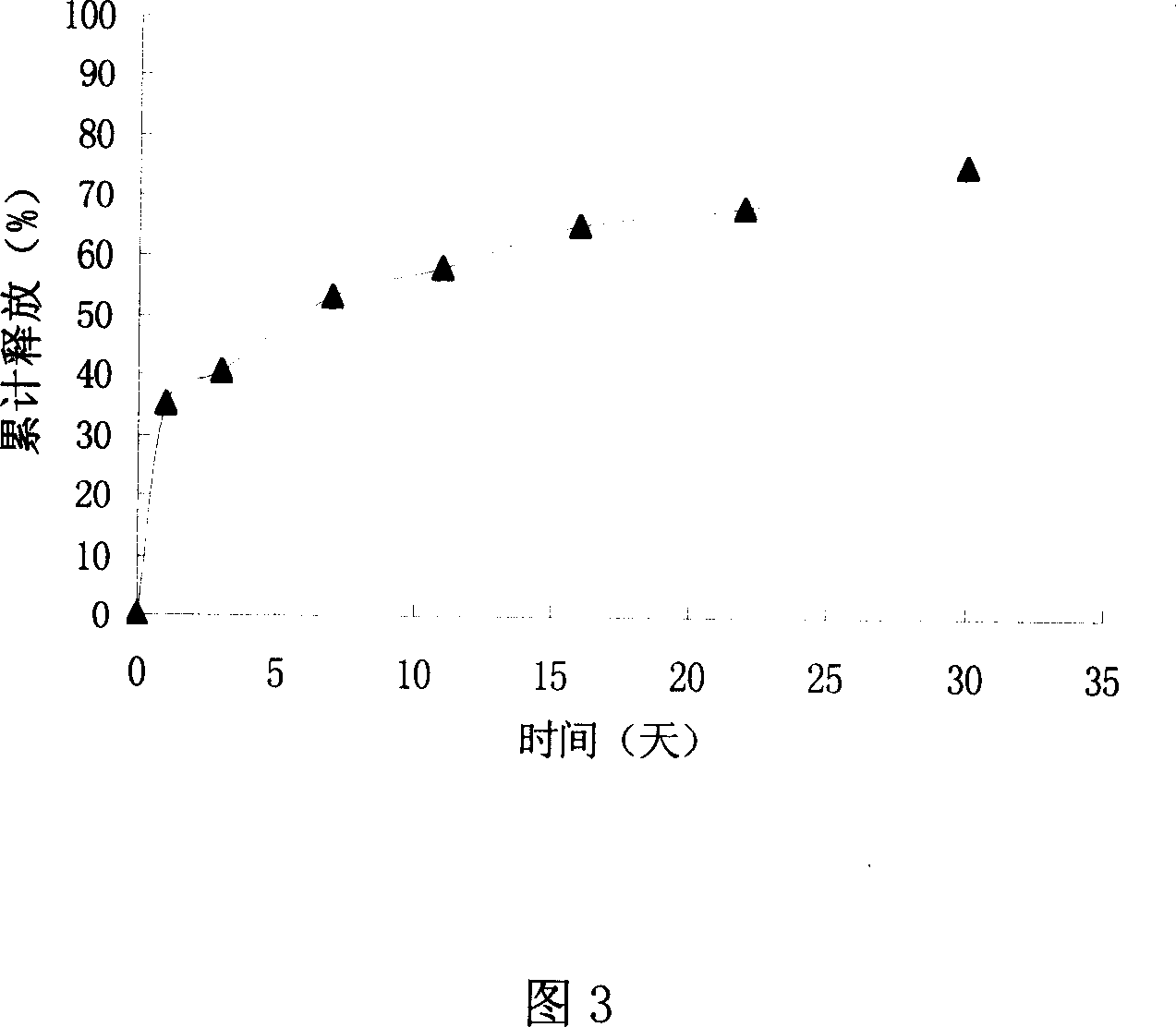
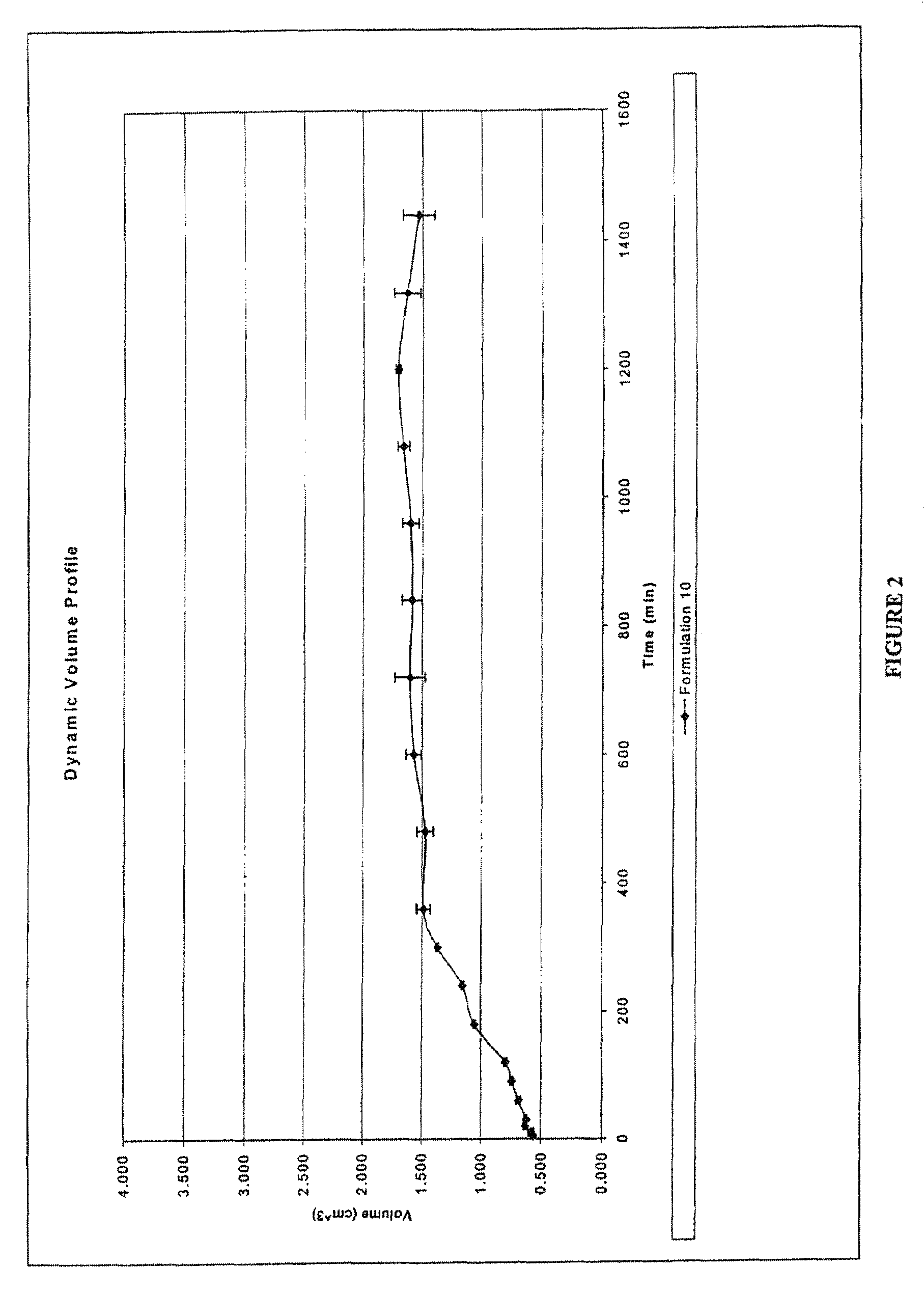
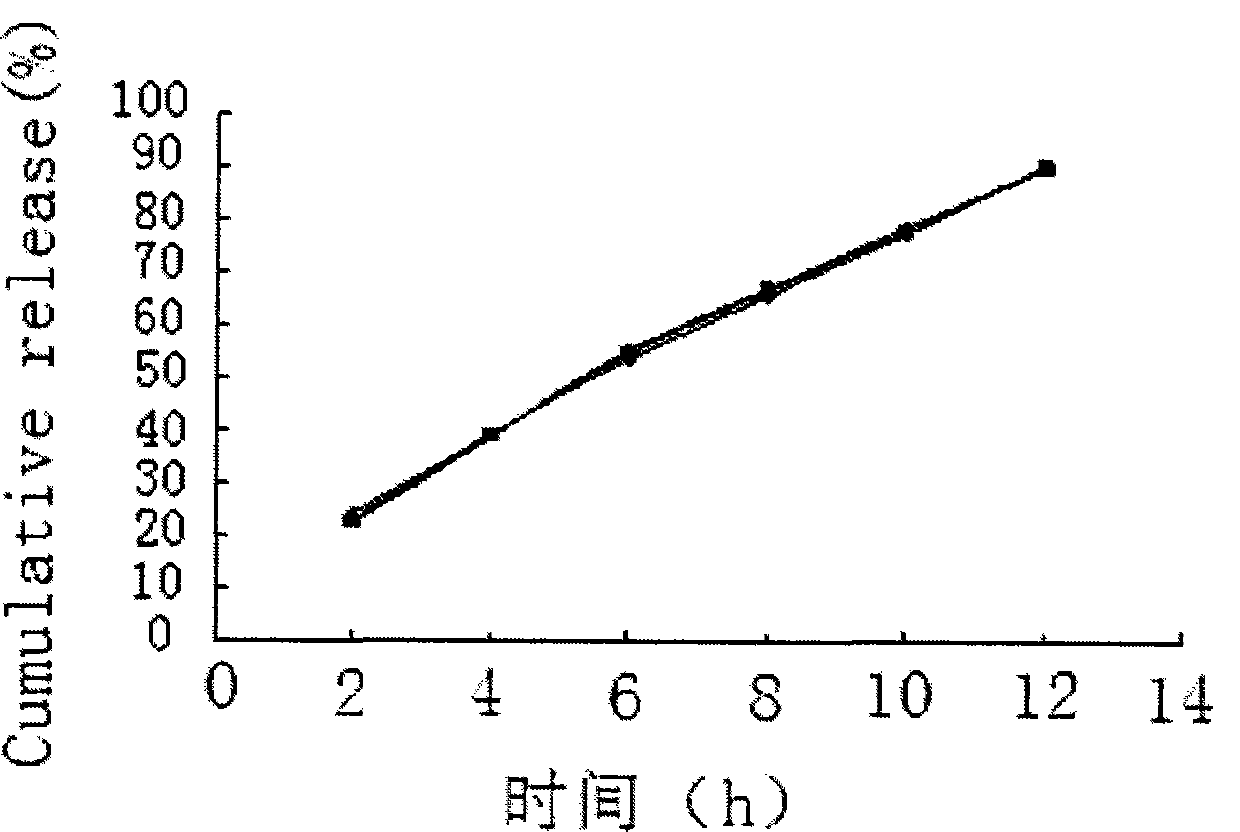
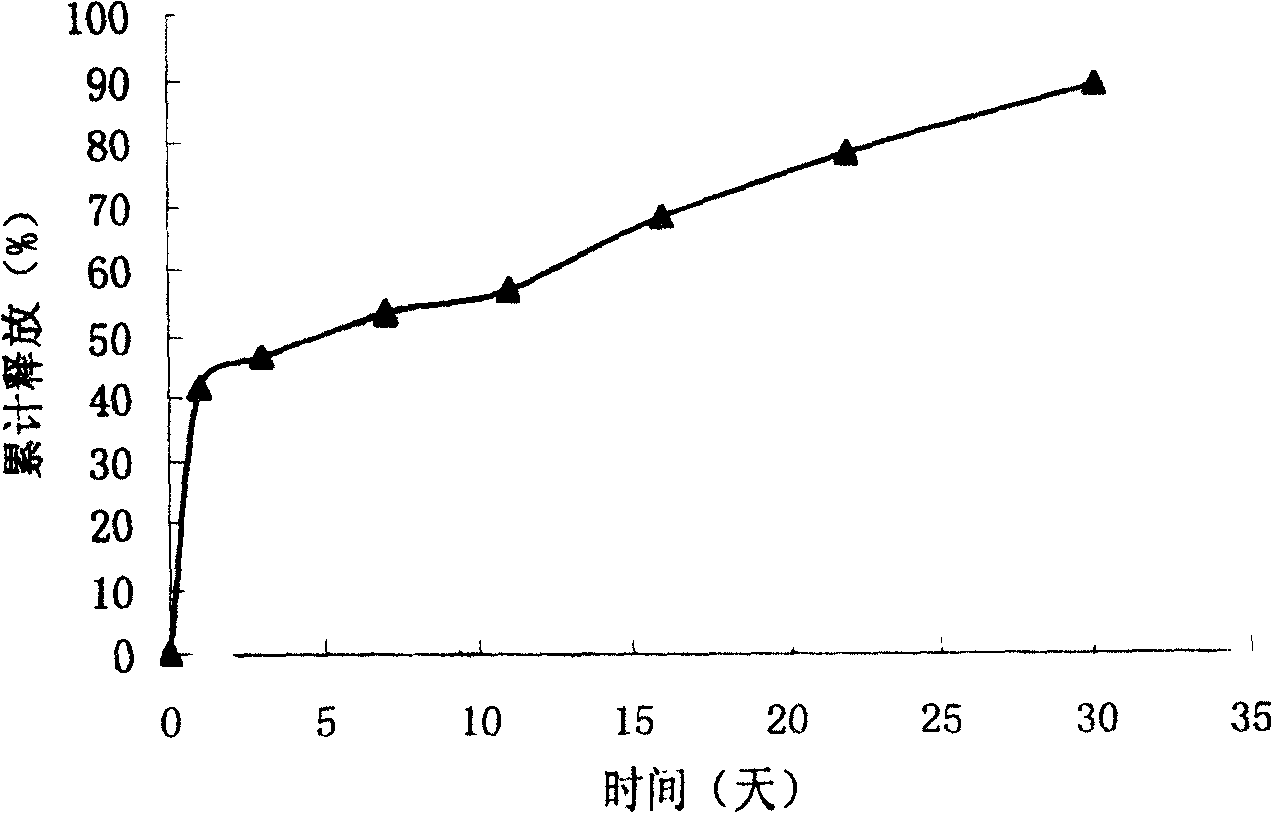
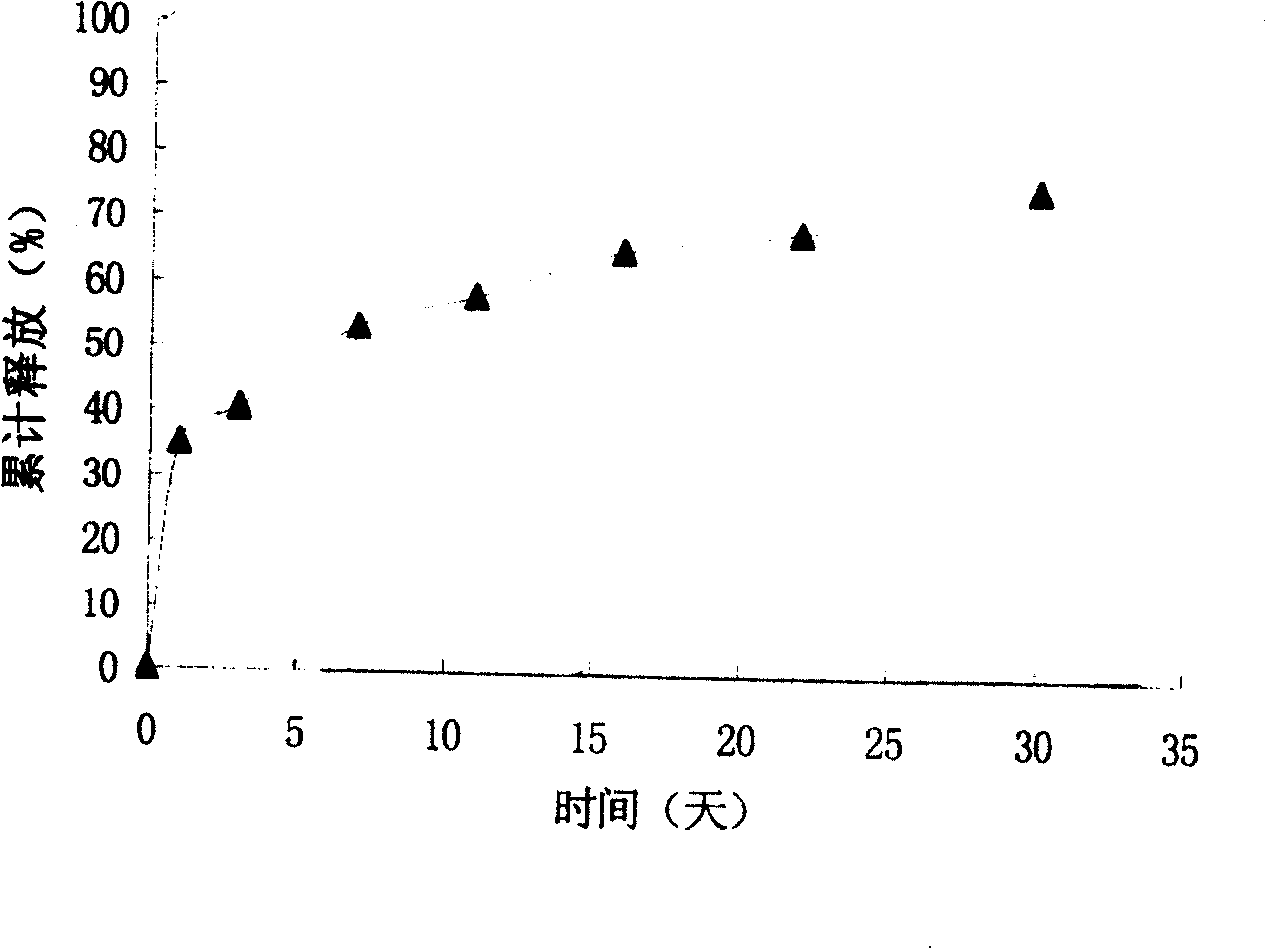
Zero order kinetics is a way of describing how the body uses and breaks down some medicines. While the rate at which the body eliminates most drugs is proportional to the concentration administered, known as first order kinetics, drugs that work by zero order kinetics work at a predictable, constant rate.
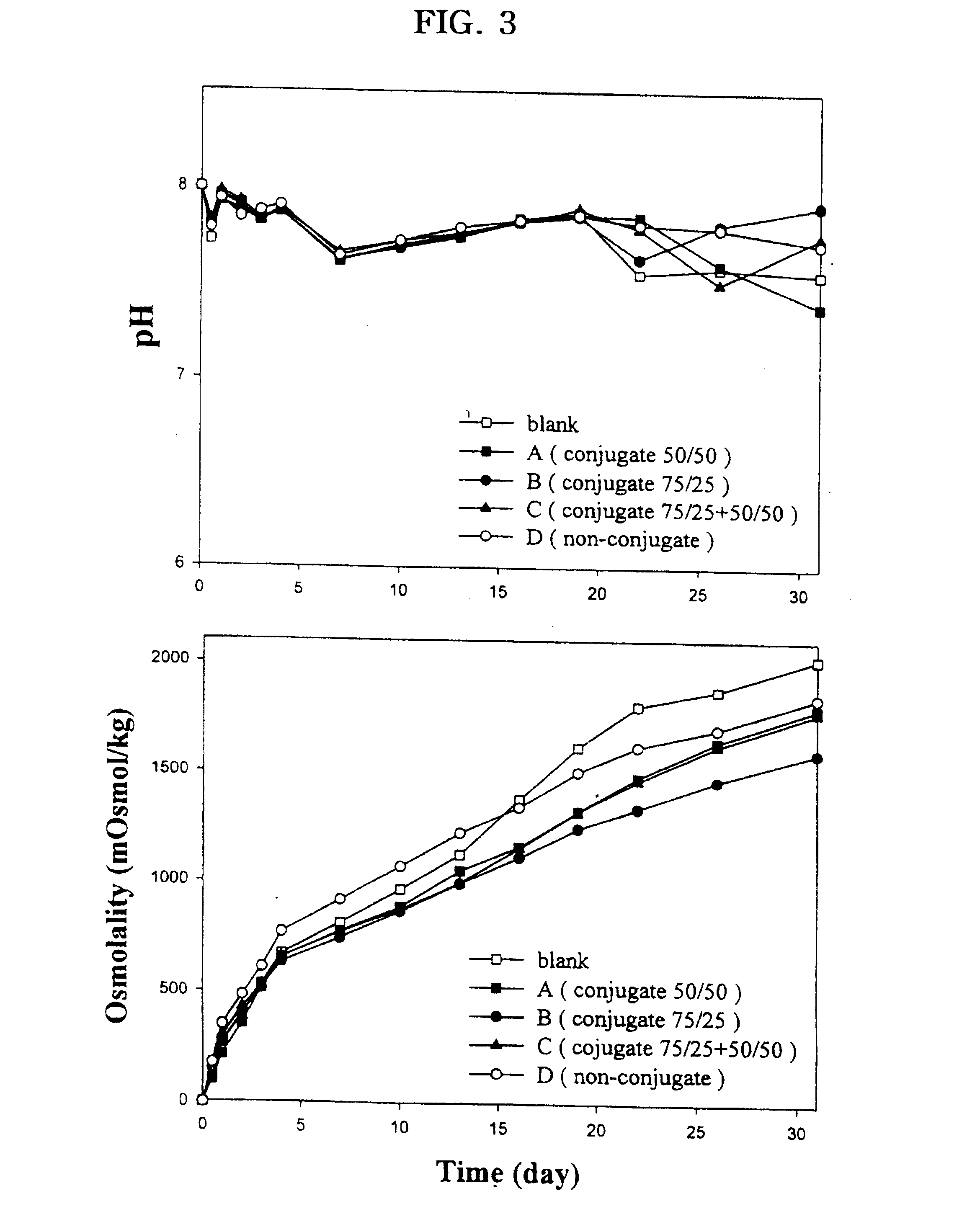
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
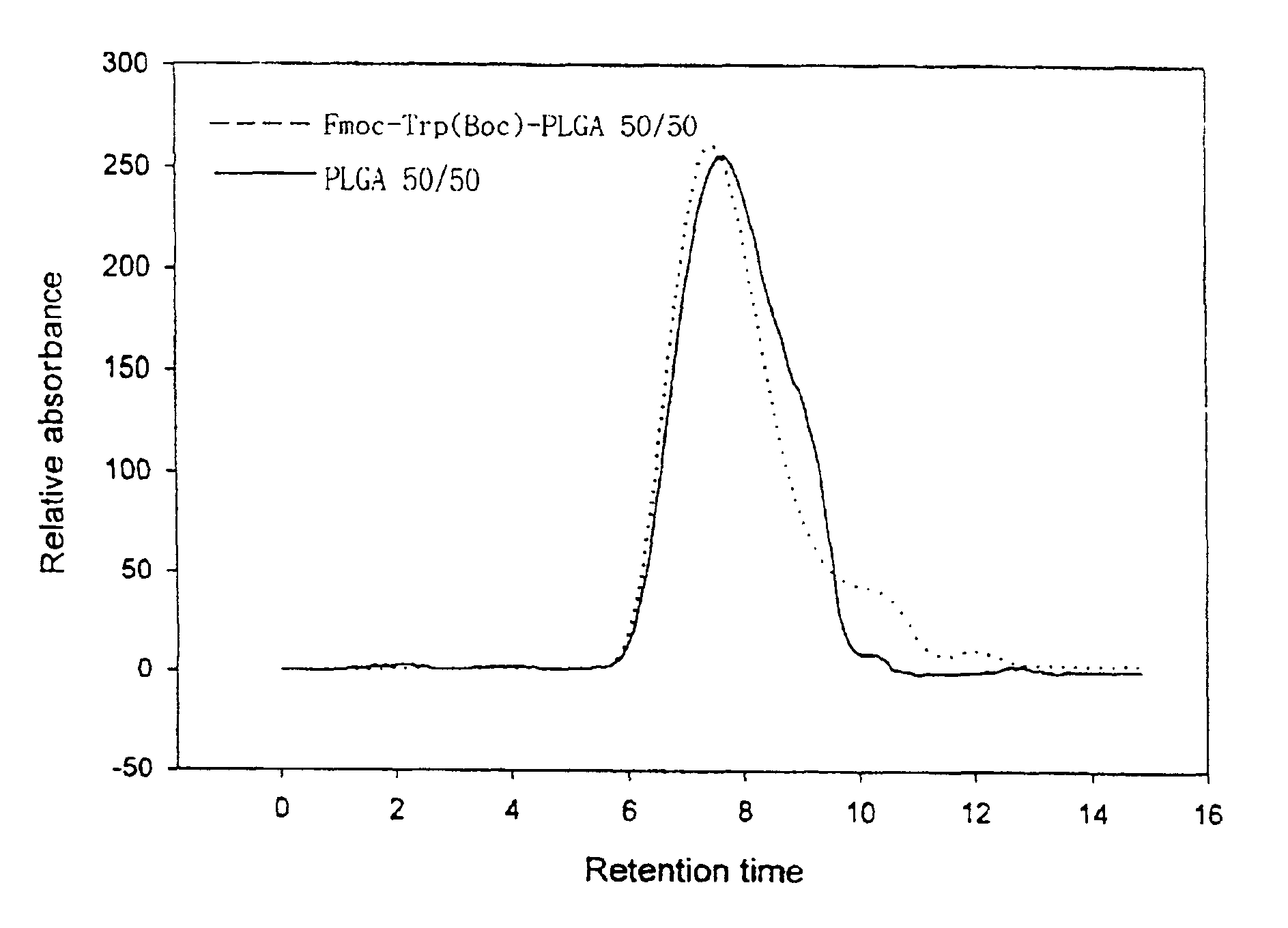
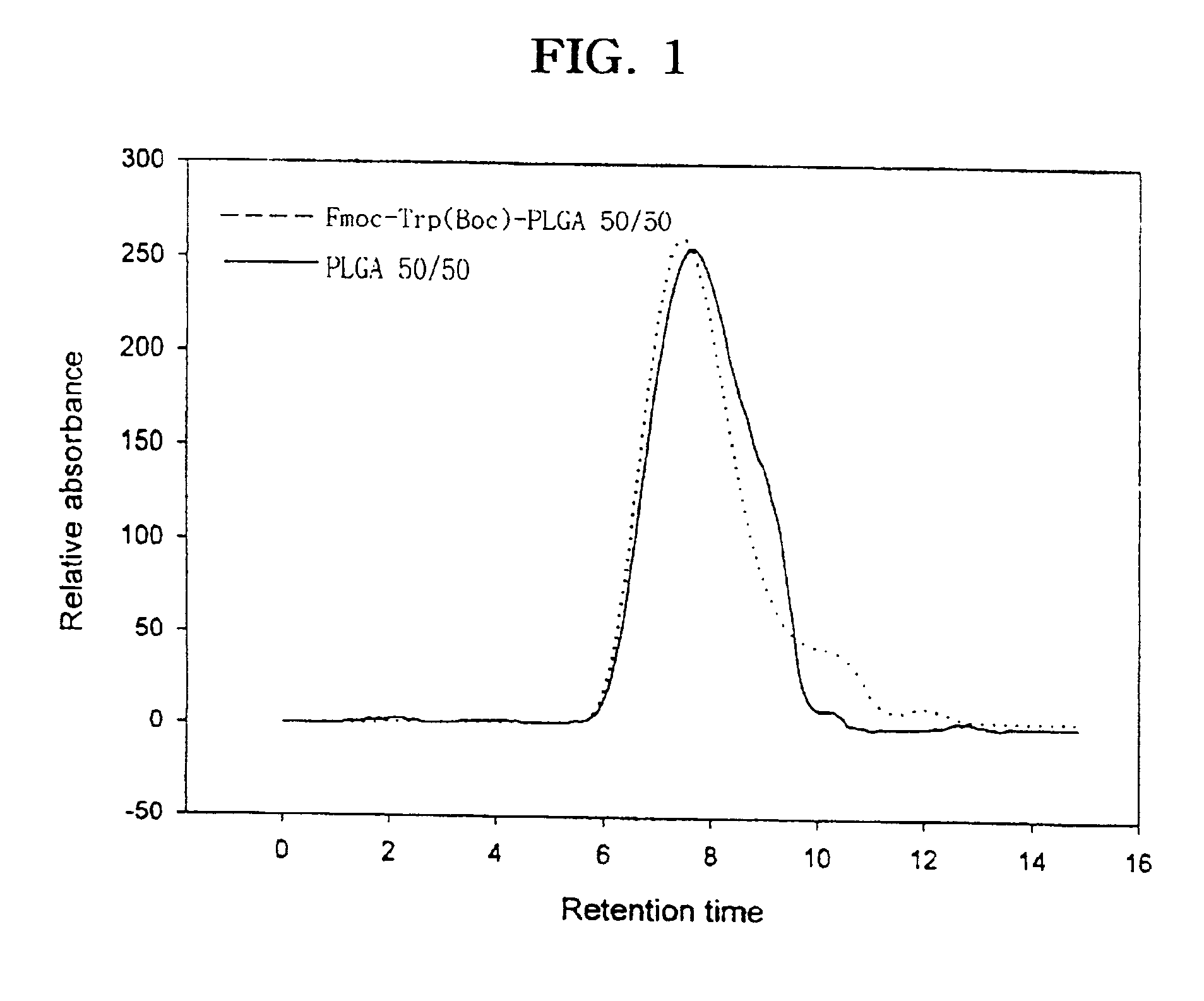
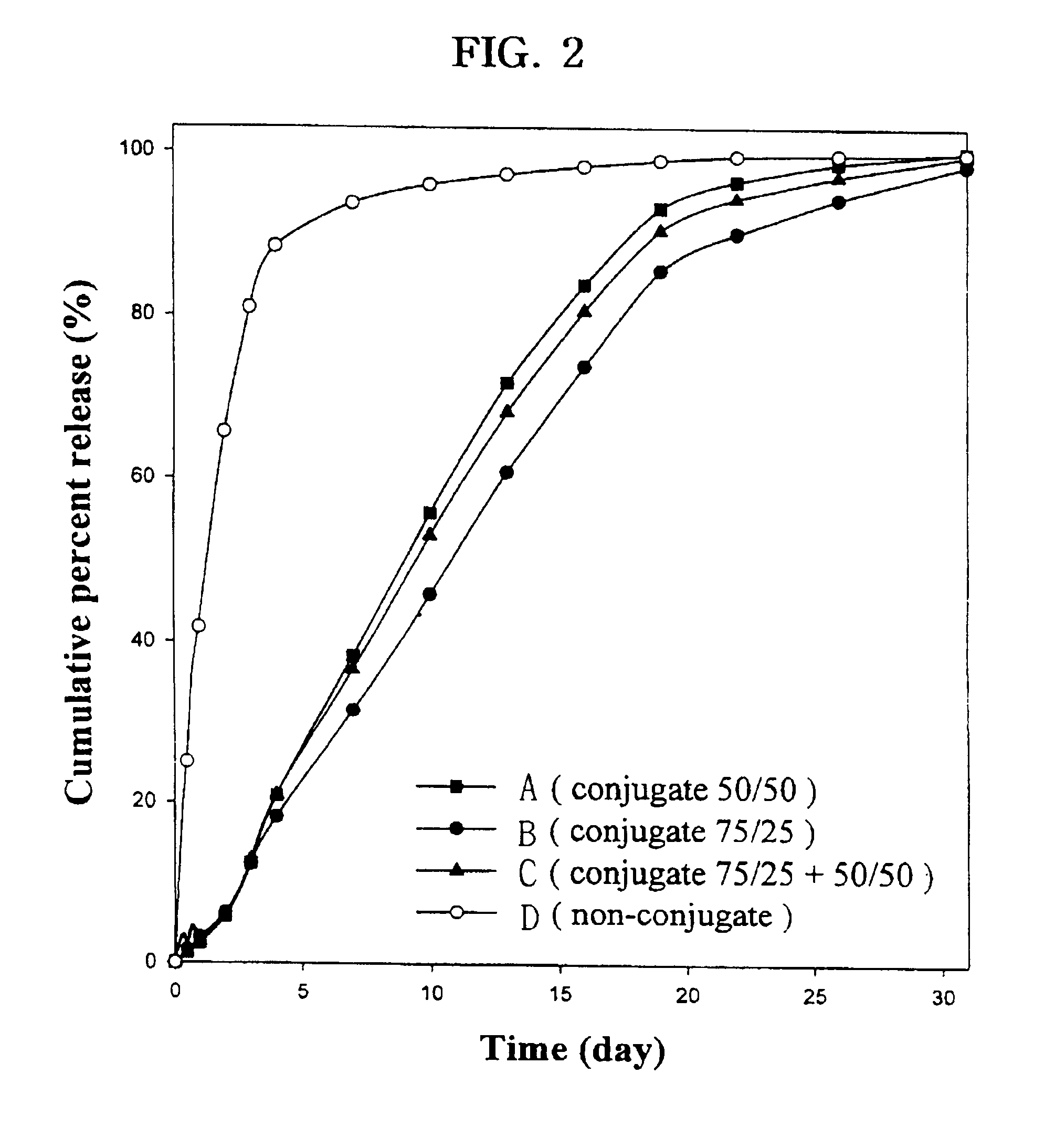
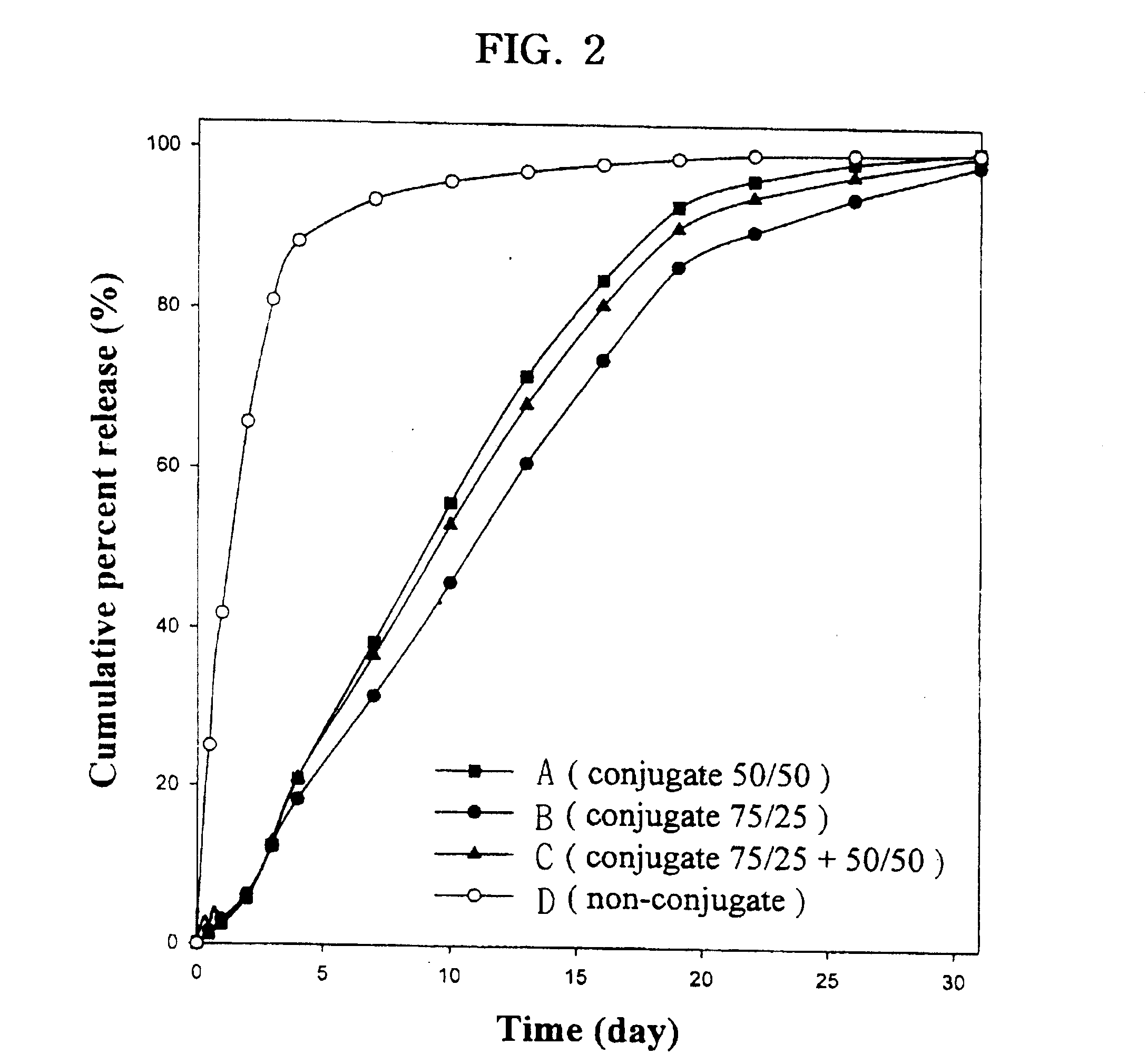
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Subcutaneous implant
InactiveUS6126956AEasy to modifyEasy for flexibilityOrganic active ingredientsNervous disorderControl releaseSubcutaneous implant
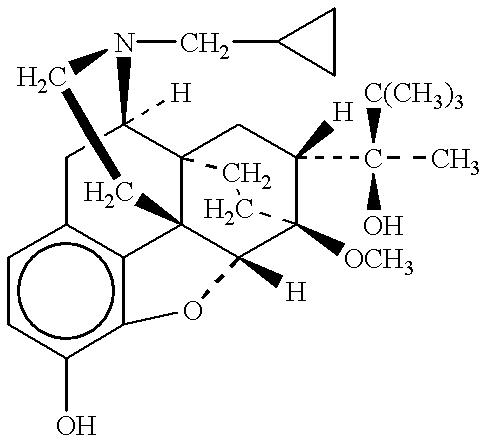
A non-abusable, non-inflammatory, biocompatible, non-biodegradable, subcutaneous, polymeric implant for the prolonged, controlled release of hydromorphone with near zero-order kinetics is described. Methods of alleviating cancer pain and treating opioid drug addiction with the implant are also described.
Owner:AXXIA PHARMA
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
The present invention relates to the molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect. Moreover, the high loading efficiency of hydrophilic drugs can be achieved.
Owner:OH JONG EUN +3
Methods of providing sustained treatment with opioids
InactiveUS6231886B1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationPlasma glucose
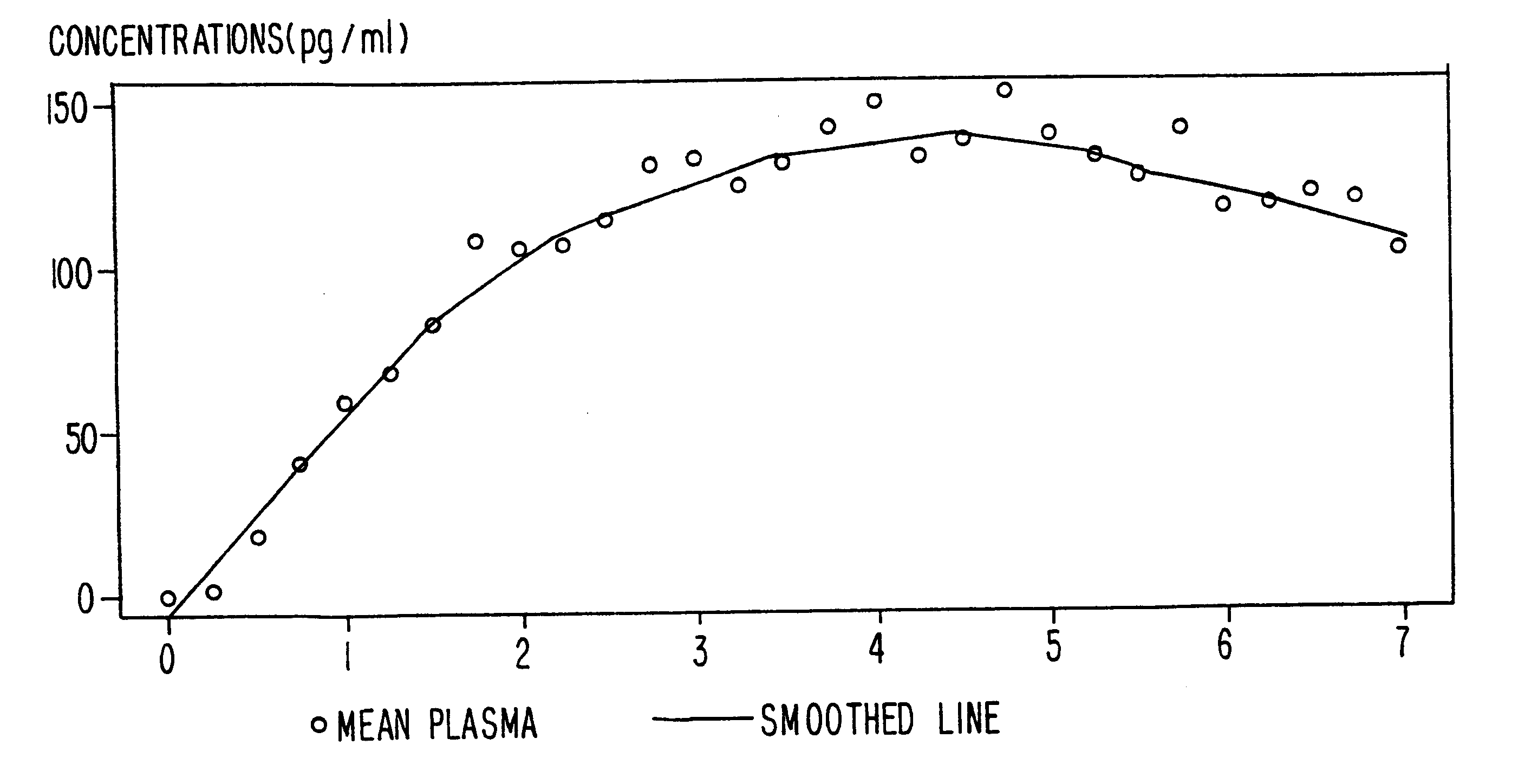
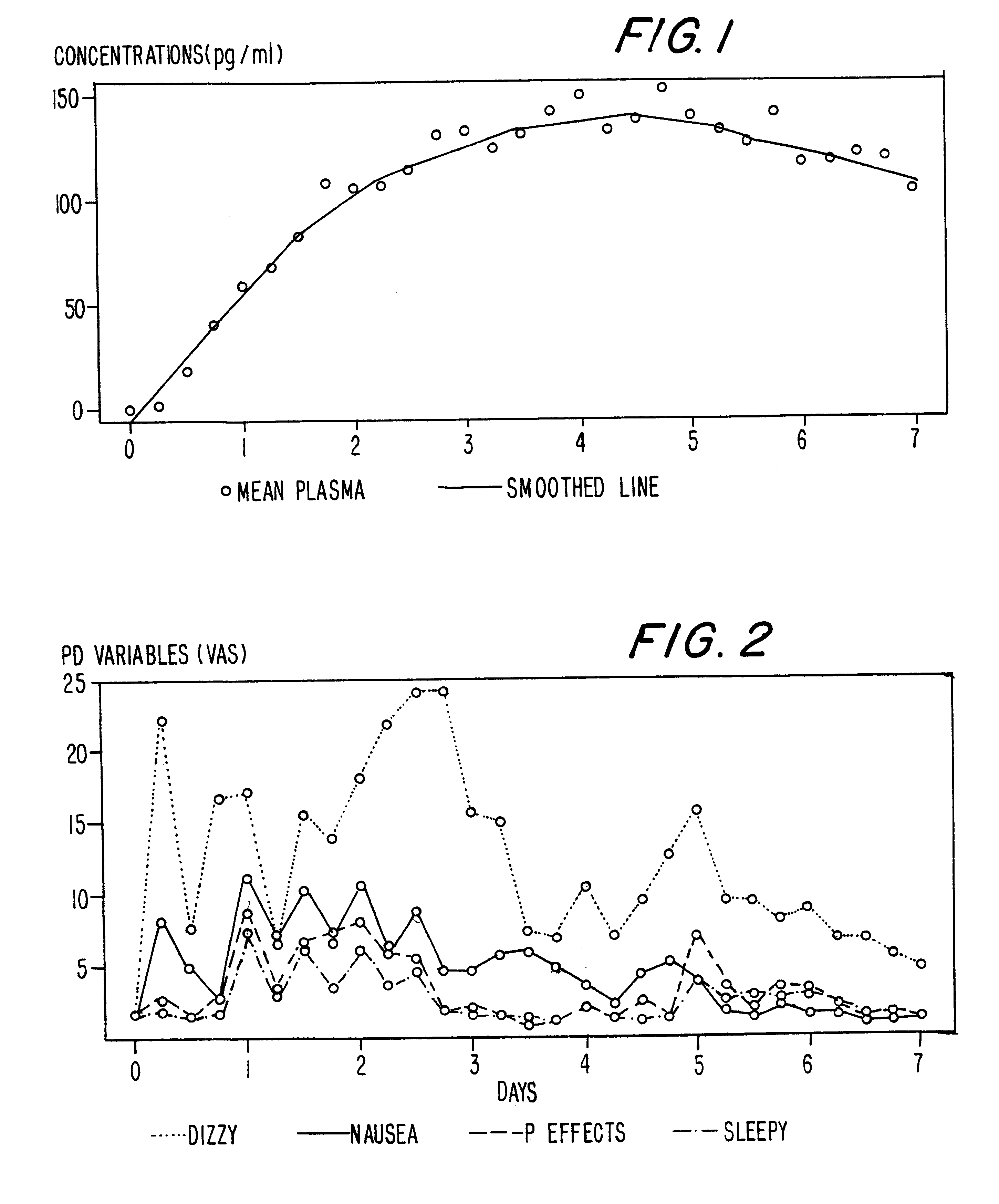
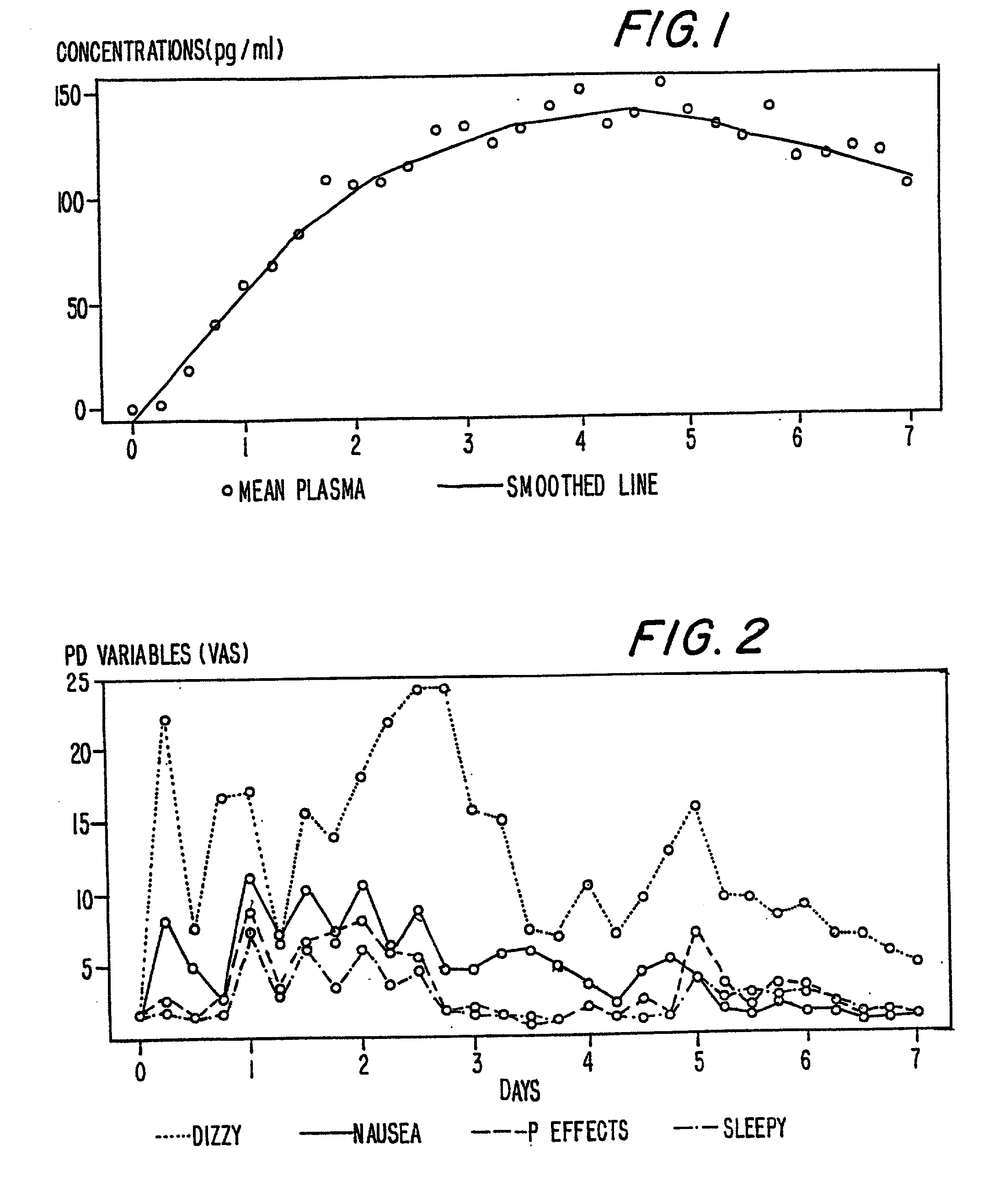
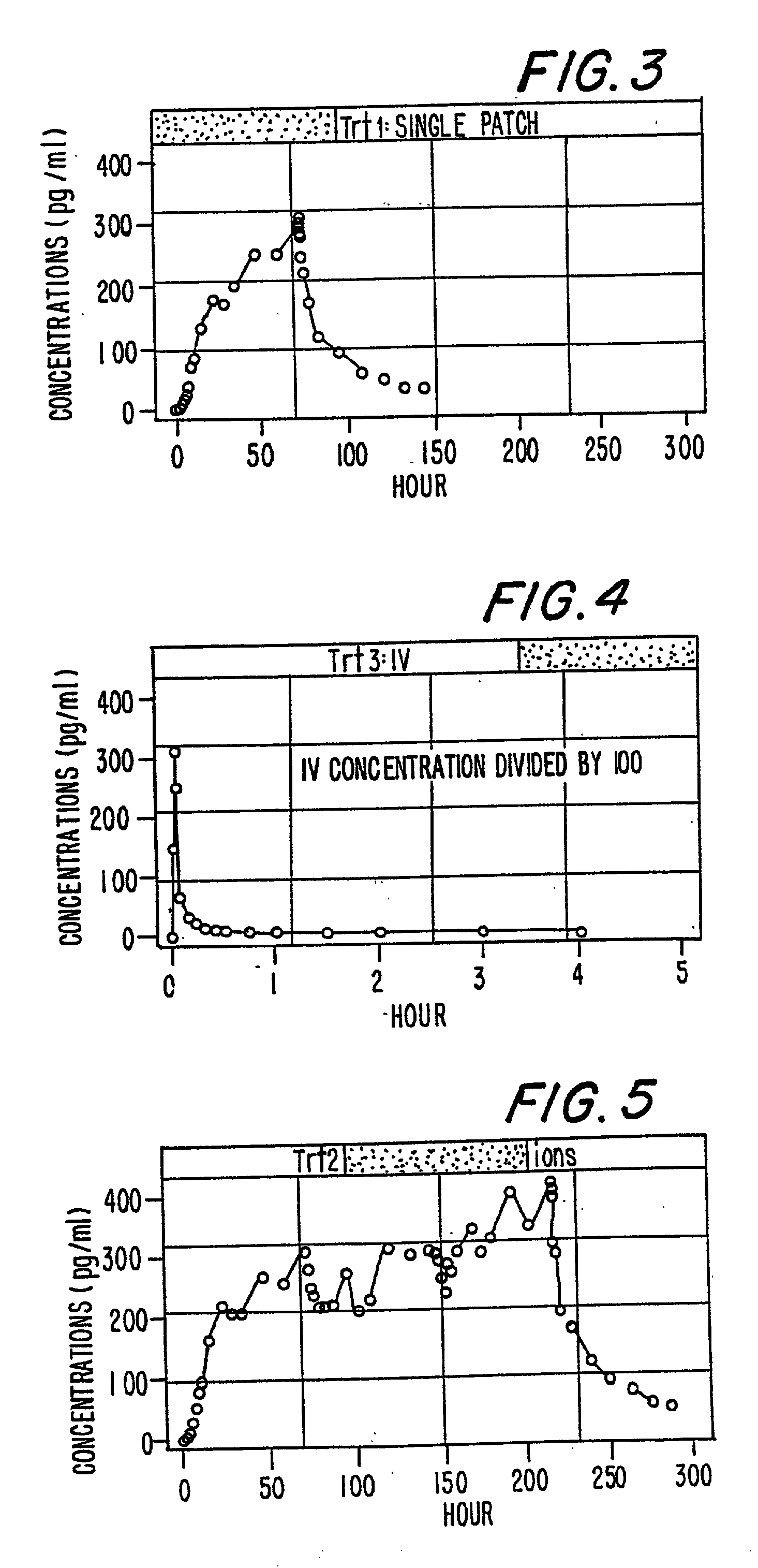
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an addition two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Method of providing sustained analgesia with buprenorphine
InactiveUS20010002259A1Reduced plasma concentrationEfficient managementOrganic active ingredientsNervous disorderPlasma concentrationZero order kinetics
A method of effectively treating pain in humans is achieved by administering buprenorphine in accordance with first order kinetics over an initial three-day dosing interval, such that a maximum plasma concentration from about 20 pg / ml to about 1052 pg / ml is attained, and thereafter maintaining the administration of buprenorphine for at least an additional two-day dosing interval in accordance with substantially zero order kinetics, such that the patients experience analgesia throughout the at least two-day additional dosing interval.
Owner:PURDUE PHARMA LP
Methods for Making Controlled Delivery Devices Having Zero Order Kinetics
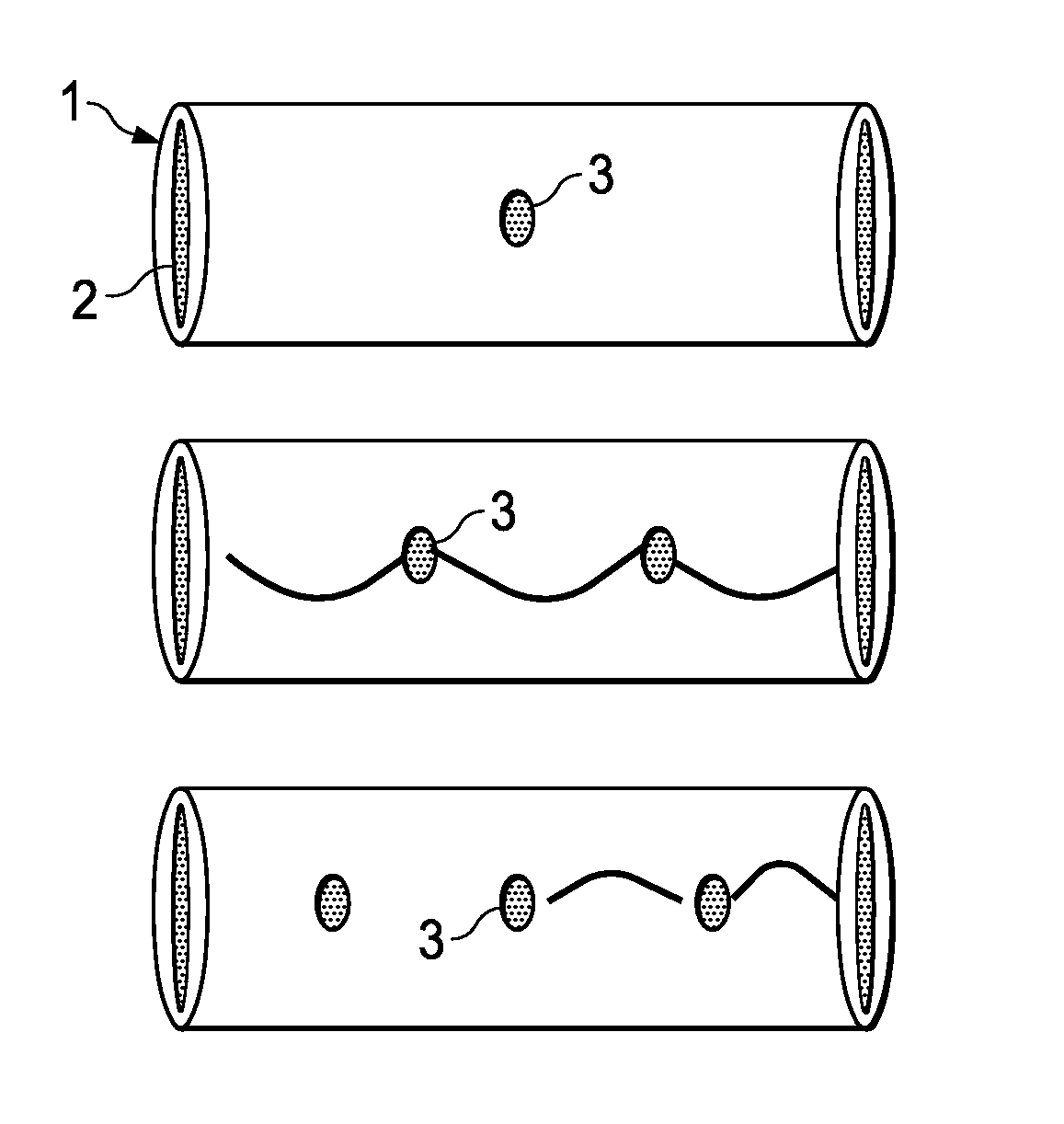
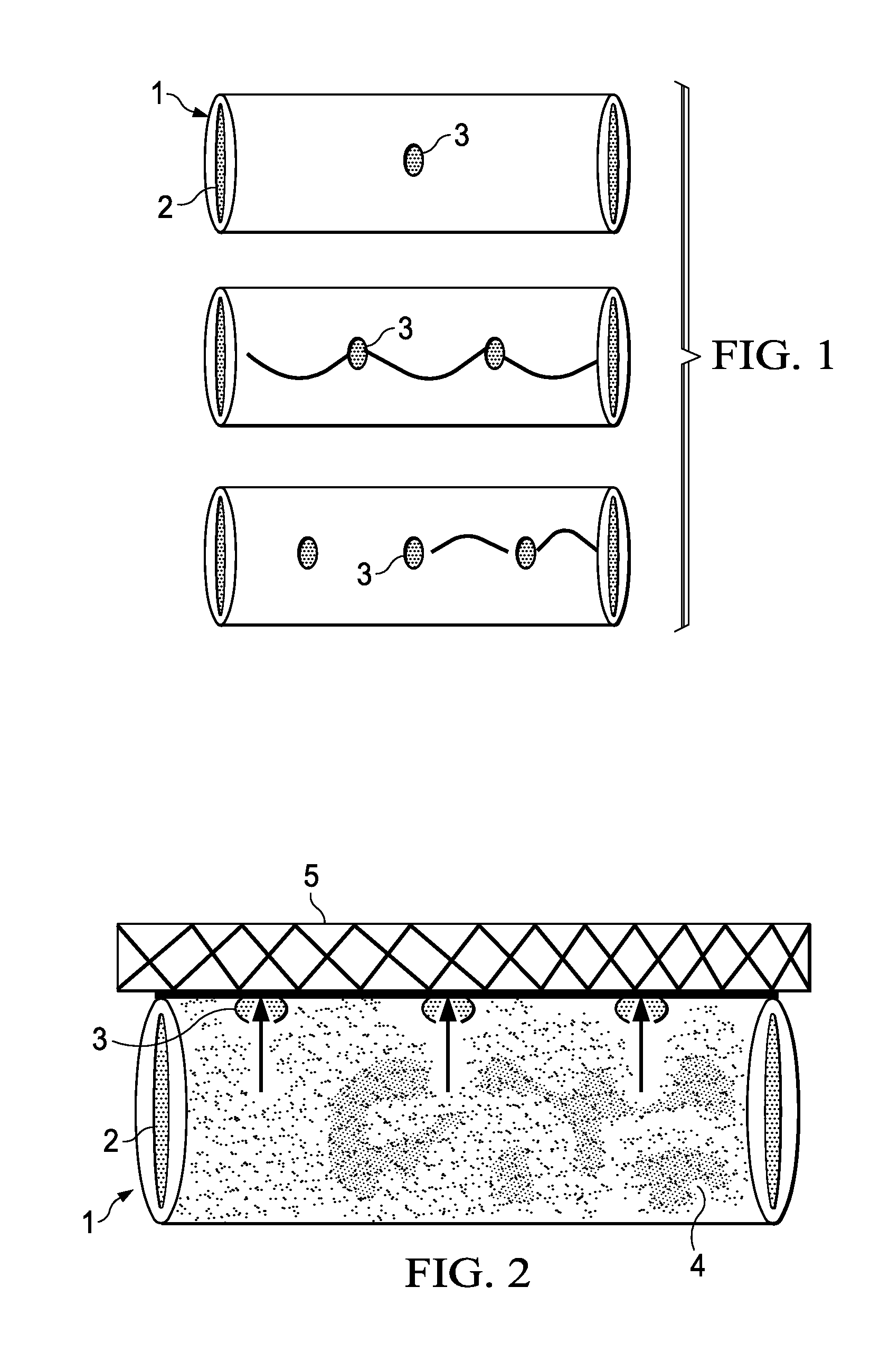
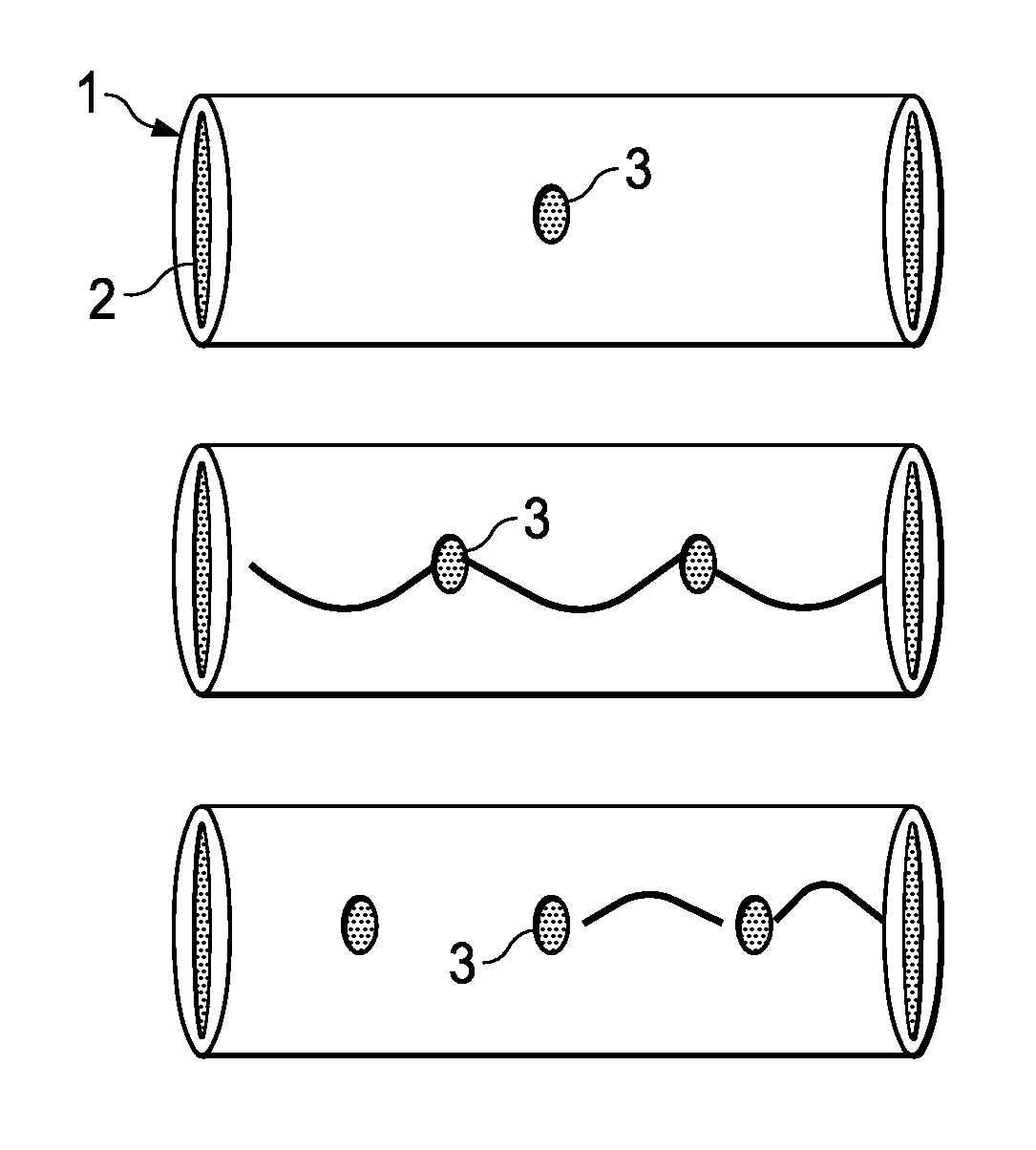
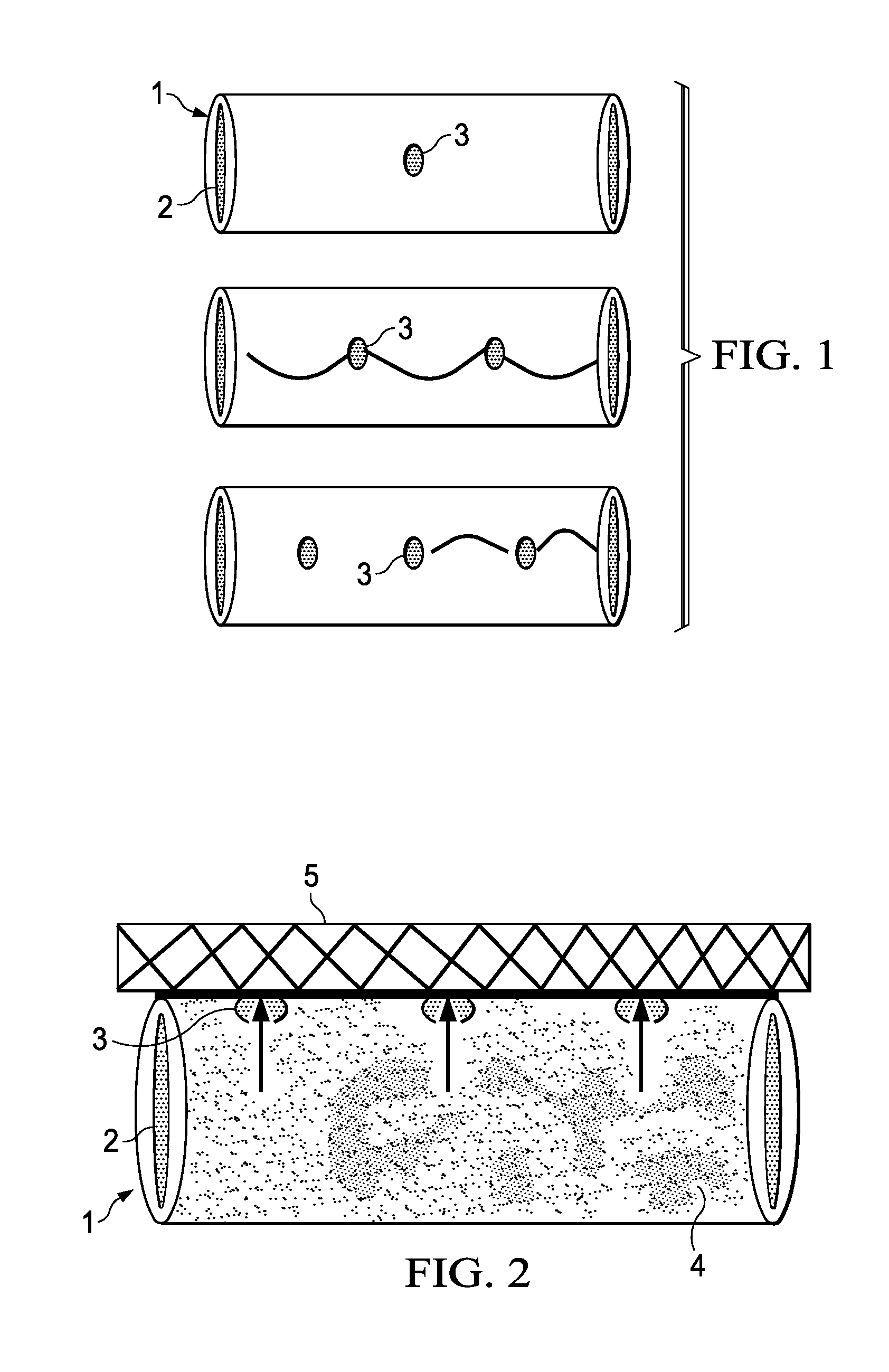
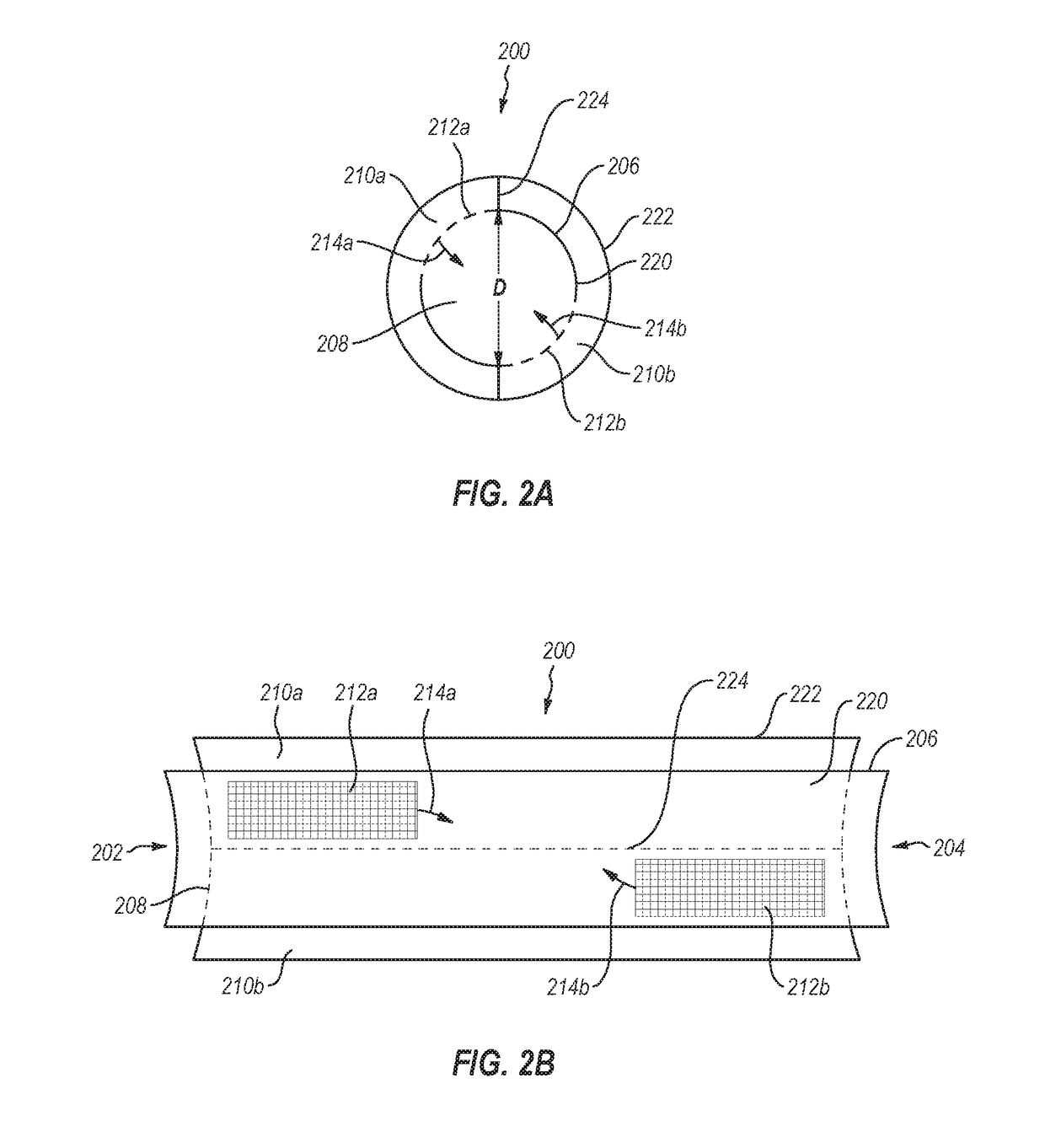
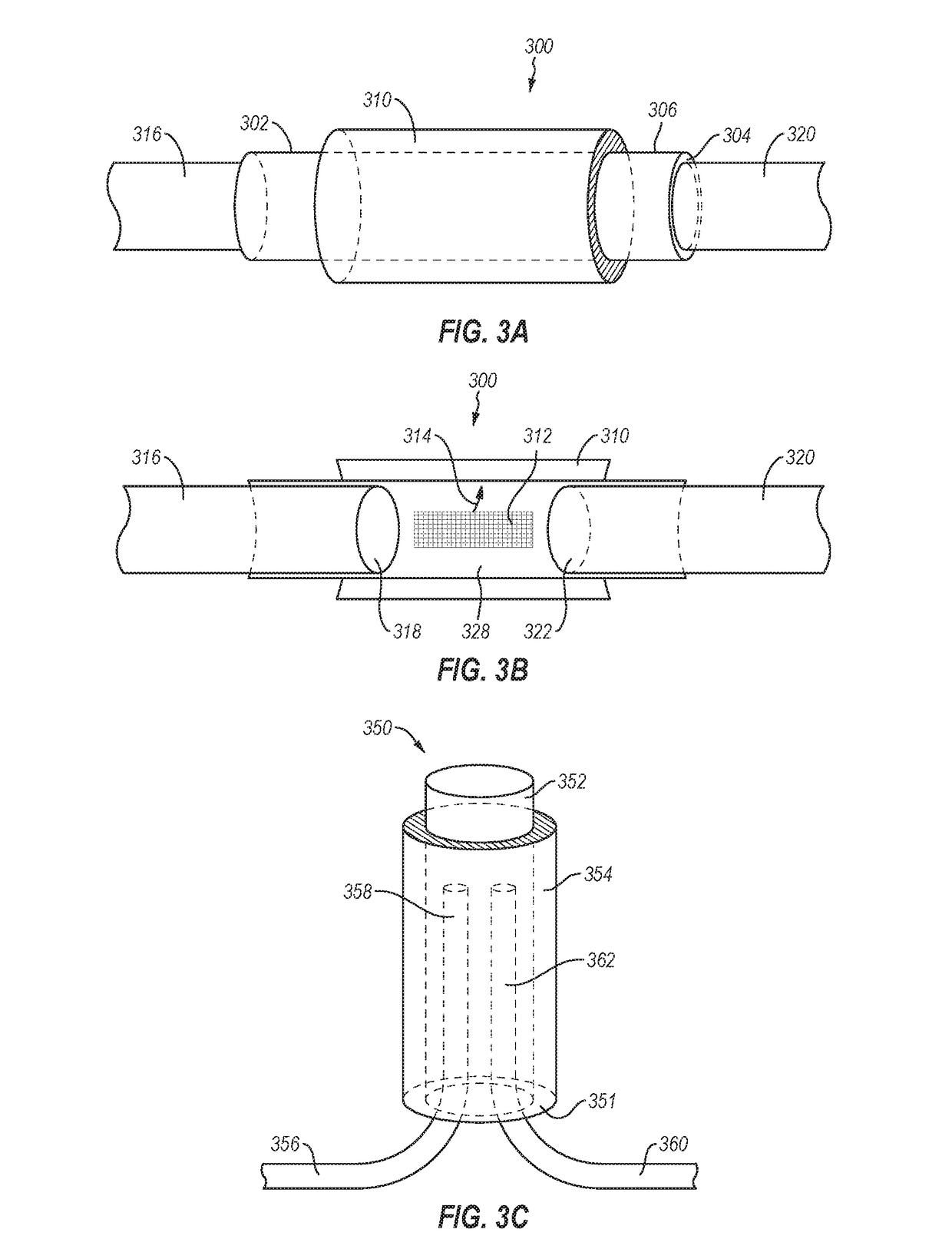
A method of making an injectable or implantable active agent delivery device capable of delivering a diagnostic, therapeutic, and / or prophylactic agent to a desired targeted site having orifice(s) on the surface is disclosed herein providing unidirectional release of the agent at a controlled desirable rate. The agent may include, but is not limited to, drugs, proteins, peptides, biomarkers, bioanalytes, and / or genetic material. The technology of the invention is based on parallel processing to fabricate micro-holes on tubes employing photo-lithography and reactive ion etching techniques and also incorporates a simple molding method to form the micro-holes on flexible polymer tubes, including bio-degradable tubes. The parallel processing method of the instant invention is fast, economical and well suited for mass production. The developed device, due to its composite structure, has the ability to combine several release mechanisms, leading to zero-order release kinetics for most of the time.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
VEGF slowly releasing injection microsphere support and its prepn and use
InactiveCN1973828AImprove survival ratePromote formationPeptide/protein ingredientsGranular deliverySerum protein albuminMicrosphere
The VEGF slowly releasing injection microsphere support includes matrix, VEGF, protectant and ammonium bicarbonate. It features the matrix of polylactic acid-hydroxyl acetate copolymer of polylactic acid / hydroxyl acetate ratio 25:75 to 75:25; the protectant comprising zinc carbonate, serum albumin, mycose and mannitol; the weight ratio between VEGF and polylactic acid-hydroxyl acetate copolymer of 1:100000 to 1:10; and the microsphere size of 50-1000 microns. It is prepared through one W / O / W solvent volatilizing process or one fine medicine powder adding W / O / W solvent volatilizing process. The preparation process is simple, easy in operation and high in repeatability. In the microsphere support, protein medicine VEGF may be in vitro released for over 4 weeks in approached zero order kinetic mode. The present invention may be used in the repair and treatment of different kinds of tissue and blood vessel damage.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
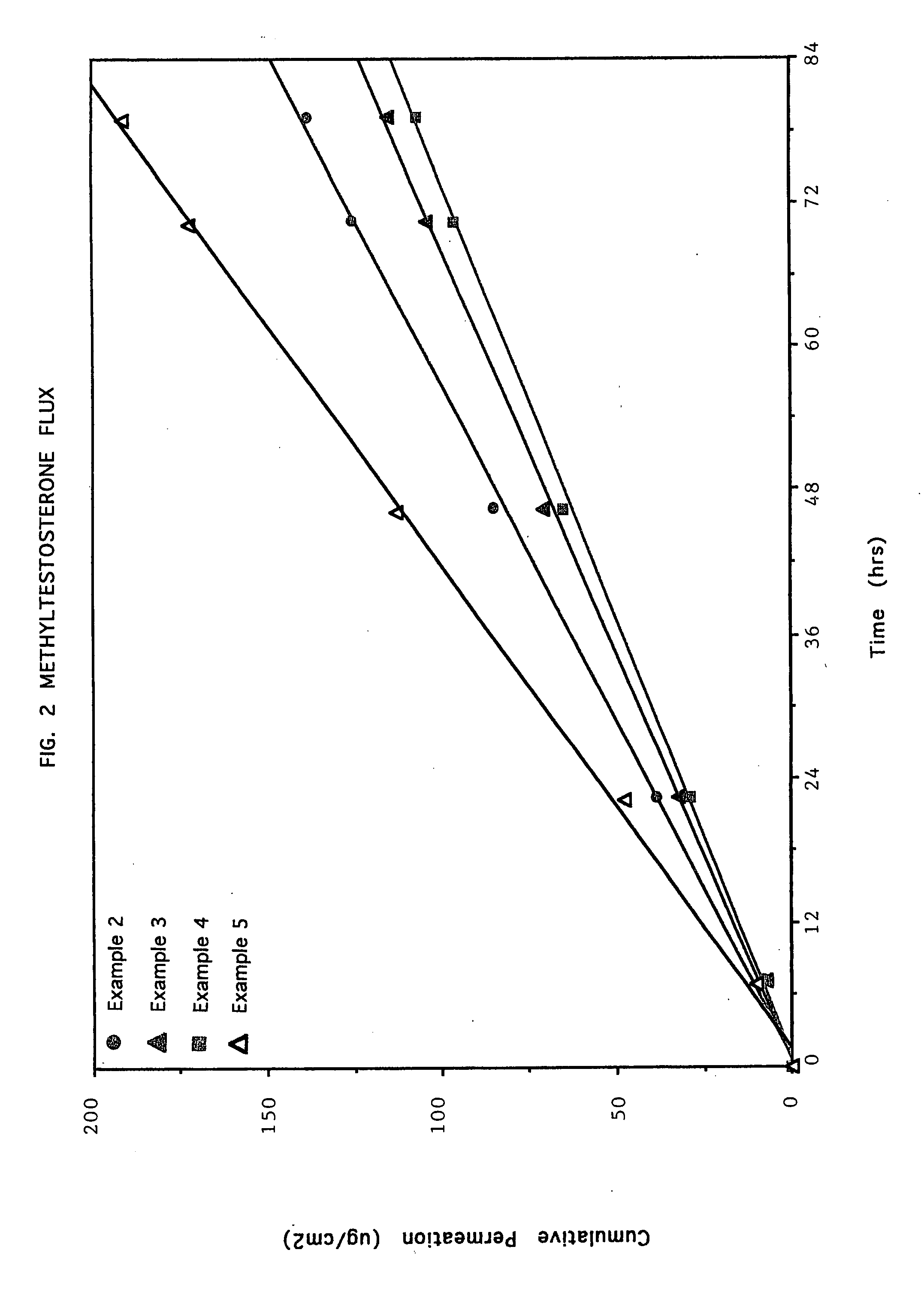
Crystallization inhibition of drugs in transdermal drug delivery systems and methods of use
InactiveUS20080063698A1Suppress and prevent crystallizationGood physical adhesive propertyBiocideOrganic active ingredientsActive agentRosin
The invention relates to compositions and methods for making a transdermal drug delivery system capable of achieving substantially zero-order kinetics for delivery of the active agent over a period of time in excess of 24 hours and at least 72 hours, comprising a pharmaceutically acceptable active agent carrier and a rosin ester which provides a crystal inhibiting and drug stabilizing effect on the active agents incorporated therein.
Owner:NOVEN PHARMA
Methods for making controlled delivery devices having zero order kinetics
A method of making an injectable or implantable active agent delivery device capable of delivering a diagnostic, therapeutic, and / or prophylactic agent to a desired targeted site having orifice(s) on the surface is disclosed herein providing unidirectional release of the agent at a controlled desirable rate. The agent may include, but is not limited to, drugs, proteins, peptides, biomarkers, bioanalytes, and / or genetic material. The technology of the invention is based on parallel processing to fabricate micro-holes on tubes employing photo-lithography and reactive ion etching techniques and also incorporates a simple molding method to form the micro-holes on flexible polymer tubes, including bio-degradable tubes. The parallel processing method of the instant invention is fast, economical and well suited for mass production. The developed device, due to its composite structure, has the ability to combine several release mechanisms, leading to zero-order release kinetics for most of the time.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Nanoparticle composition and methods to make and use the same
ActiveUS20130209566A1Ability to encapsulateNarrow distributionOrganic active ingredientsBiocideDiseaseWhole body
The present invention provides a novel nanoparticle drug delivery system generated from poly(ortho ester) polymers with sustained drug release capability and can be functionalized to allow for systemic delivery to various organ systems throughout the body. One important aspect of this invention is that the nanoparticle drug delivery system generated from poly(ortho ester) polymers encapsulate several types of drugs in poly(ortho ester) nanoparticles, including but not limited to lipophilic, hydrophilic small and large molecules and also hydrophilic and lipophilic dyes by adopting appropriate emulsion techniques. These poly(ortho ester) nanoparticles are biodegradable, biocompatible and controlled release drug delivery system with zero order kinetics, which can be used in various biomedical applications such as eye-related diseases, cancer, arthritis, etc.
Owner:UNIV OF TENNESSEE RES FOUND
Methods and devices for connecting nerves
ActiveUS20140336681A1Increase ratingsDegree of improvementTissue regenerationProsthesisDrug reservoirSemipermeable membrane
A nerve repair conduit configured to be secured on first and second portions of a selected nerve. The nerve repair conduit includes a polymeric body having a proximal end, a distal end, an exterior surface and an interior surface defining an interior lumen. In addition, the nerve conduit includes at least one drug reservoir to hold agent(s) that may, for example, facilitate nerve regeneration. The drugs diffuse from the drug reservoir(s) into the nerve repair conduit through an outlet (e.g., a semipermeable membrane) in proximity to the first and second portions of a selected nerve. The nerve repair conduit may be configured to deliver the agent(s) at a rate having substantially zero-order kinetics and / or at a constant rate over a selected period of time (e.g., at least 1 week).
Owner:UNIV OF UTAH RES FOUND
Medical Devices Having Nanoporous Coatings for Controlled Therapeutic Agent Delivery
According to an aspect of the present invention, implantable or insertable medical devices are provided which contain (a) one or more depressions that contain at least one therapeutic agent, and (b) a nanoporous coating, disposed over the therapeutic-agent-containing depressions, which regulate transport of species between the therapeutic-agent-containing depressions and the exterior of the device. The implantable or insertable devices are configured to preform a role beyond mere drug delivery, for example, providing mechanical and / or electrical functions within the body, among other functions. An advantage of the present invention is that medical devices may be provided, which release therapeutic agents in quantities far exceeding the void volume within the nanoporous coating, while at the same time providing functionality that extends beyond drug delivery. Such release may further approach or achieve a zero order kinetic drug release profile.
Owner:BOSTON SCI SCIMED INC
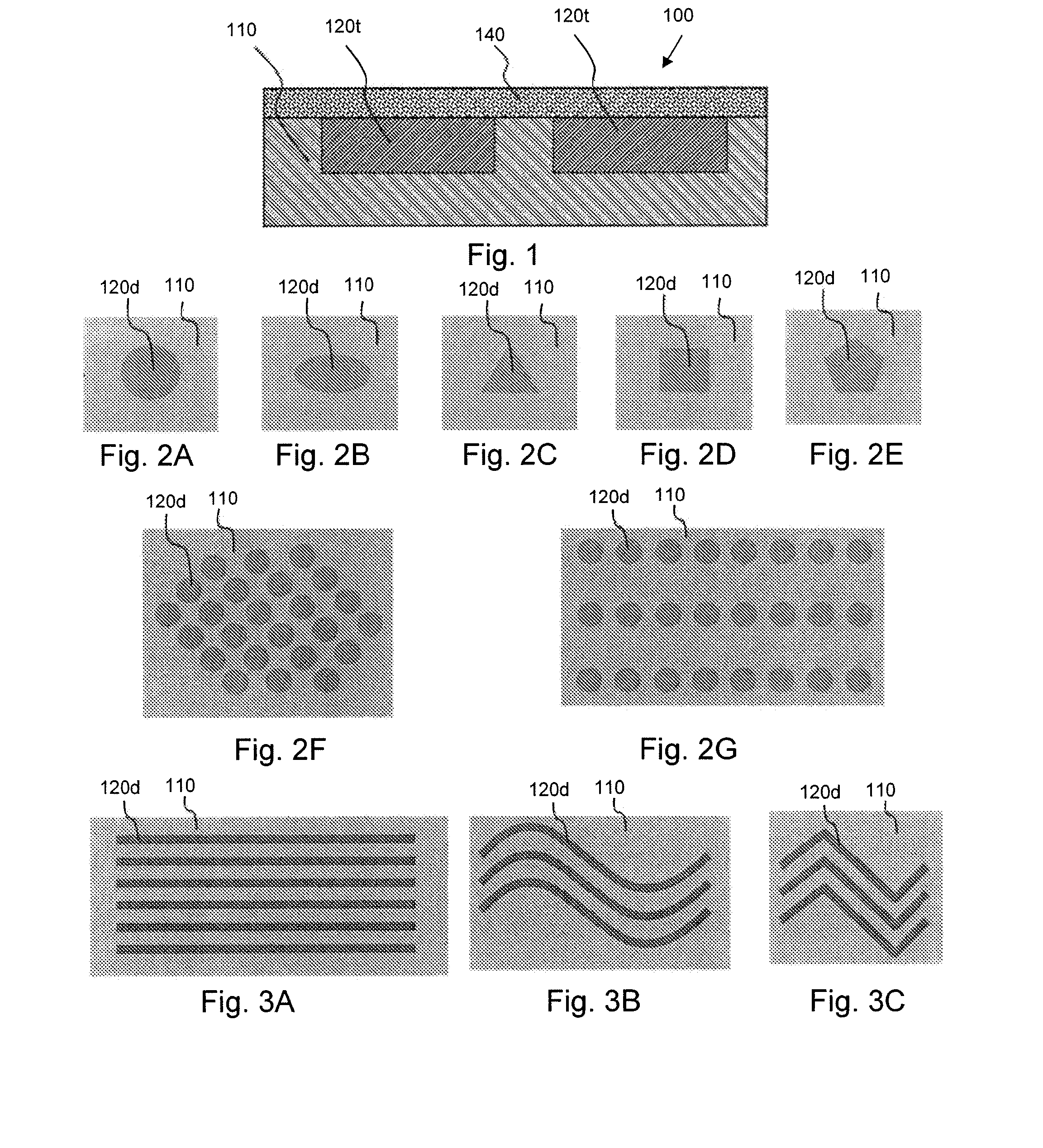
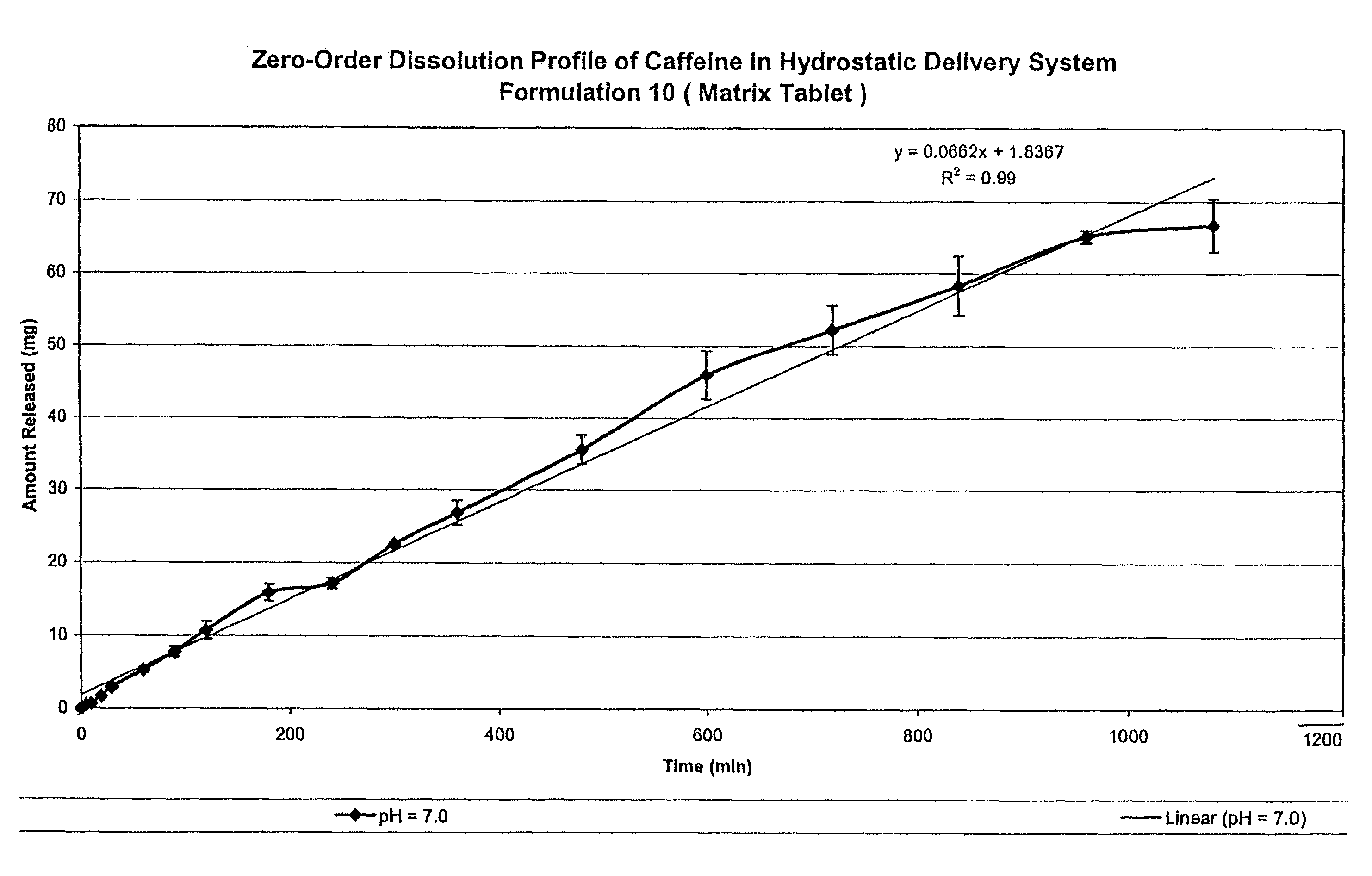
Hydrostatic delivery system for controlled delivery of agent
The present invention provides a hydrostatic delivery system comprising a hydrostatic couple and an agent of interest. The hydrostatic couple comprises, at least one hydrodynamic fluid-imbibing polymer, and at least one hydrostatic pressure modulating agent. This delivery system has the ability to control the release of one or more agent of interest within a fluid environment following zero-order kinetics.
Owner:MACGREGOR ALEXANDER
Method for reducing hexavalent chromium through micromolecular diketone-ultraviolet light
ActiveCN105731587APromote reductionGood removal effectWater/sewage treatment by irradiationWater treatment compoundsDiketoneUltraviolet lights
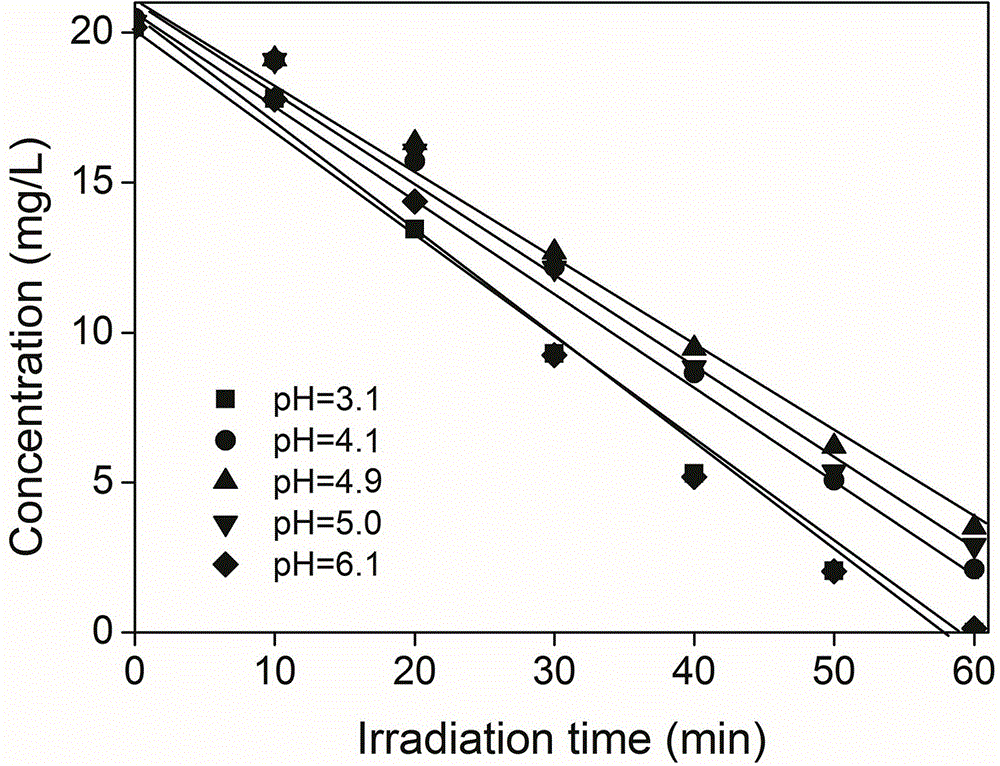
The invention discloses a method for reducing hexavalent chromium through micromolecular diketone-ultraviolet light. According to the method, micromolecular diketone with the final concentration of 0.1-1 mM is put in water containing hexavalent chromium, the pH of the solution is regulated to be 3-6, the solution is placed under an ultraviolet light source for irradiation, and then hexavalent chromium in the water can be reduced into trivalent chromium. According to the UV / micromolecular diketone system established through the method, hexavalent chromium in the water can be quickly reduced into trivalent chromium which is low in toxicity and easier to remove through a physical method; the reaction conforms to the zero order kinetics, and the removal rate of hexavalent chromium is tens of that obtained through a UV / TiO2 method; a homogeneous reaction is performed in the method, operation is simple and convenient, and compared with the UV / TiO2 method and the like, the solution pH applicable range is broader, and the method is little affected by co-existing ions in the solution and can be widely applied to treatment of industrial wastewater containing hexavalent chromium.
Owner:NANJING UNIV
Method of predicting shelf life of sauce ducks
InactiveCN108399467ATimely processingPrediction is fast and accurateForecastingDesign optimisation/simulationEscherichia coliColony number
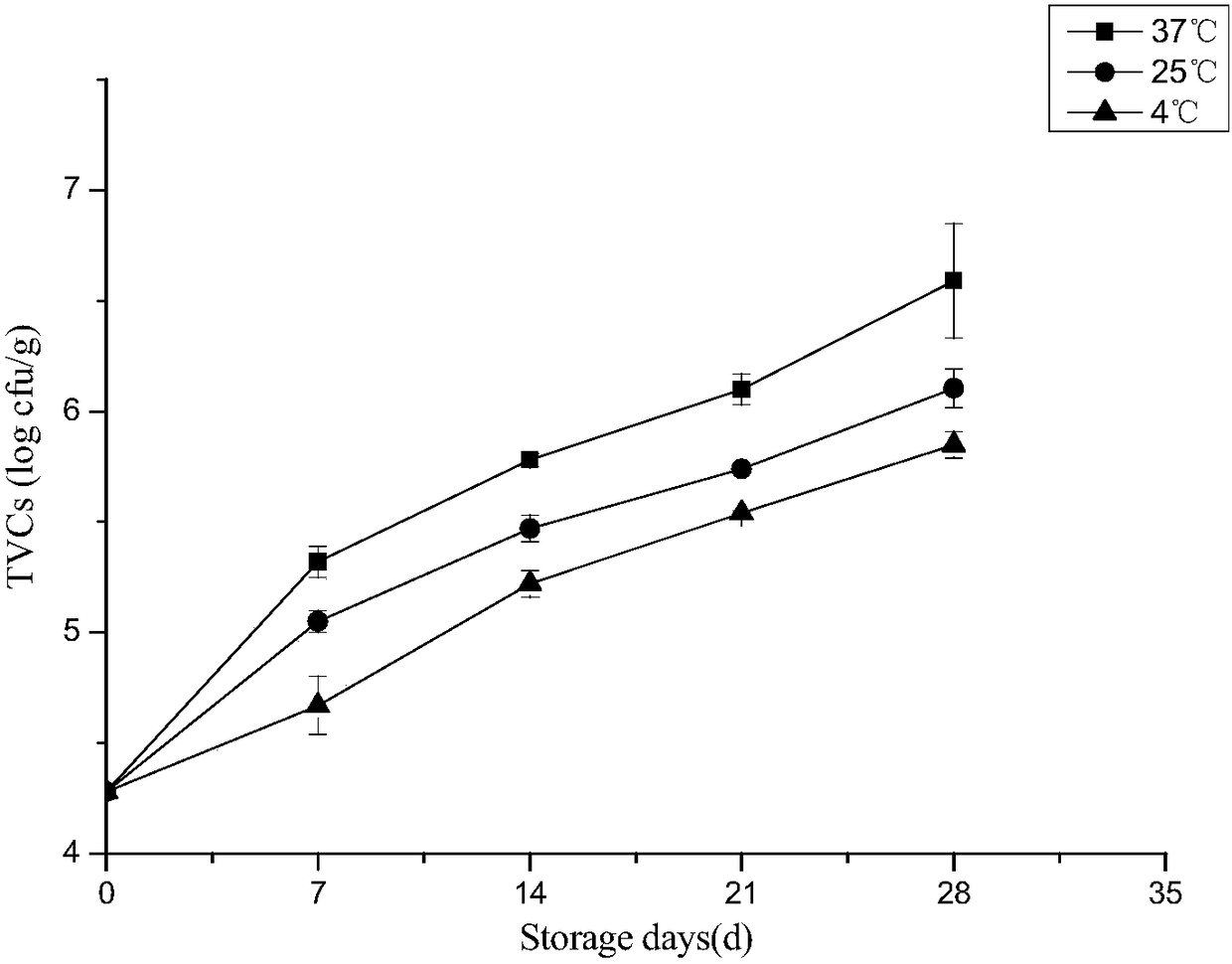
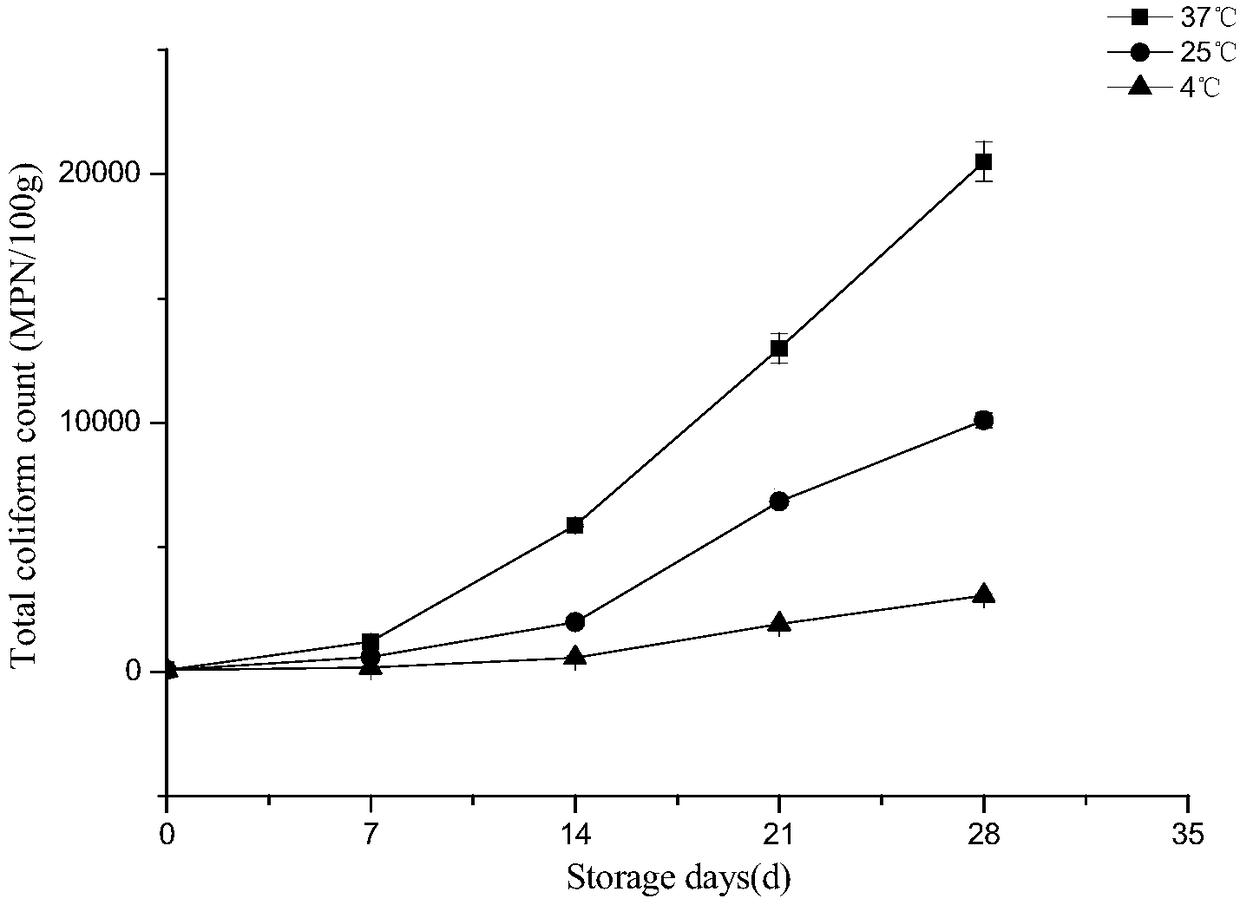
The invention discloses a method of predicting shelf life of sauce ducks. Quality indexes of sauce-duck products stored under different temperatures are studied, a kinetic model of sauce-duck qualitychanges is determined through determining, by measurement, laws of changes of total colony numbers of the sauce ducks, escherichia coli, mould, acid values, peroxide values, thiobarbituric acid reactive substances (TBARS) and sensory quality with time, then quality change key-indexes of the mould and the TBARS in a sauce-duck storage period are screened out according to Pearson correlation analysis, and the change laws accord with a zero-order kinetic-model. An Arrhenius equation is combined to establish a shelf-life prediction model of the sauce ducks according to mould and TBARS values of the sauce-duck quality indexes. According to the method, remaining shelf life of sauce ducks in a temperature range of 0-40 DEG C can be quickly and effectively predicted, guidance can be provided for storage, transportation and sales processes to facilitate correspondingly processing sauce-duck products of different freshness in time, and costs can be saved.
Owner:ZHEJIANG UNIV OF TECH
Hydrostatic delivery system for controlled delivery of agent
The present invention provides a hydrostatic delivery system including a hydrostatic couple and an agent of interest. The hydrostatic couple includes at least one hydrodynamic fluid-imbibing polymer, and at least one hydrostatic pressure modulating agent. This delivery system has the ability to control the release of one or more agents of interest within a fluid environment following zero-order kinetics.
Owner:MACGREGOR ALEXANDER
Salvianolic acid controlled porosity osmotic pump tablets and method of preparing the same
ActiveCN101278920AStable blood concentrationProlong the effective timeOrganic active ingredientsPharmaceutical delivery mechanismPorositySalvianolic acid B
The invention discloses a salvianolic acid micropore osmotic pump controlled release tablet, comprising a tablet core and a coating; the tablet core is composed of salvianolic acid bulk drug, controlled release supplementary material, lubricant and moderate anhydrous ethanol; the weight ratio of the salvianolic acid bulk drug, the controlled release supplementary material and the lubricant is 50:150:1; the coating is composed of cellulose acetate, polyethylene glycol 400 solution and diethyl ester phthalate. In the invention, on the basis of controlling the content of 50-90% of salvianolic acid B, the content of 5-15% of tanshinol, protocatechualdehyde, salvianolic acid C, etc. in raw materials and by the optimal screening of penetrating agent, pore-forming agent, plasticizer and film thickness, an osmotic pump controlled release tablet which releases 85%-95% of a drug for 12 hours and conforms to the release characteristic of zero order kinetics is successfully prepared and used for preparing the drug for treating coronary heart disease and cerebral arteriosclerosis, which is good for the drug to form steady blood concentration in a body and lengthening the effective acting time of the drug, thus having good clinical compliance and treatment quality.
Owner:惠州市九惠药业有限公司
Calciparine/sodium salt nano oral preparation and preparation technique thereof
InactiveCN101249063AImprove stabilityHigh nano encapsulation efficiencyOrganic active ingredientsAntineoplastic agentsHigh concentrationHigh absorption
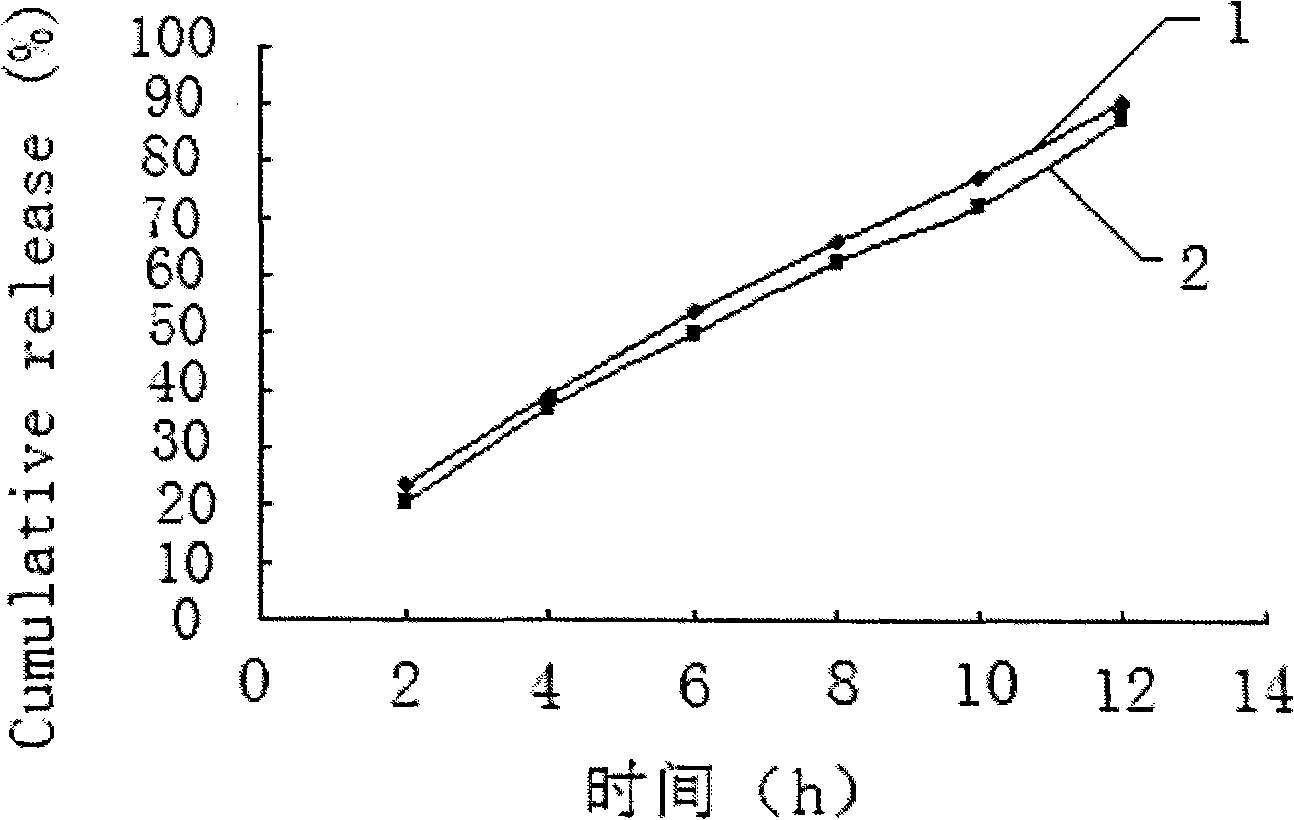
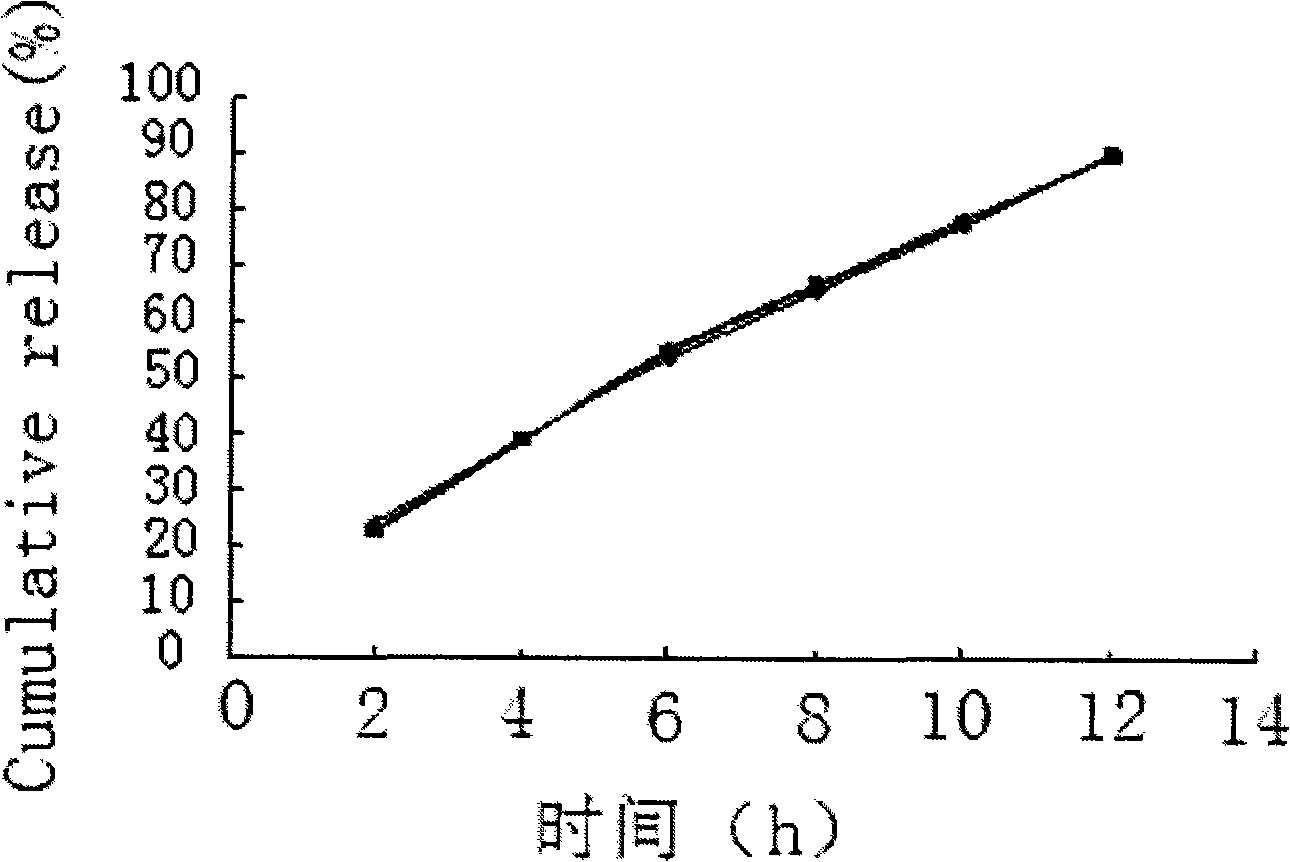
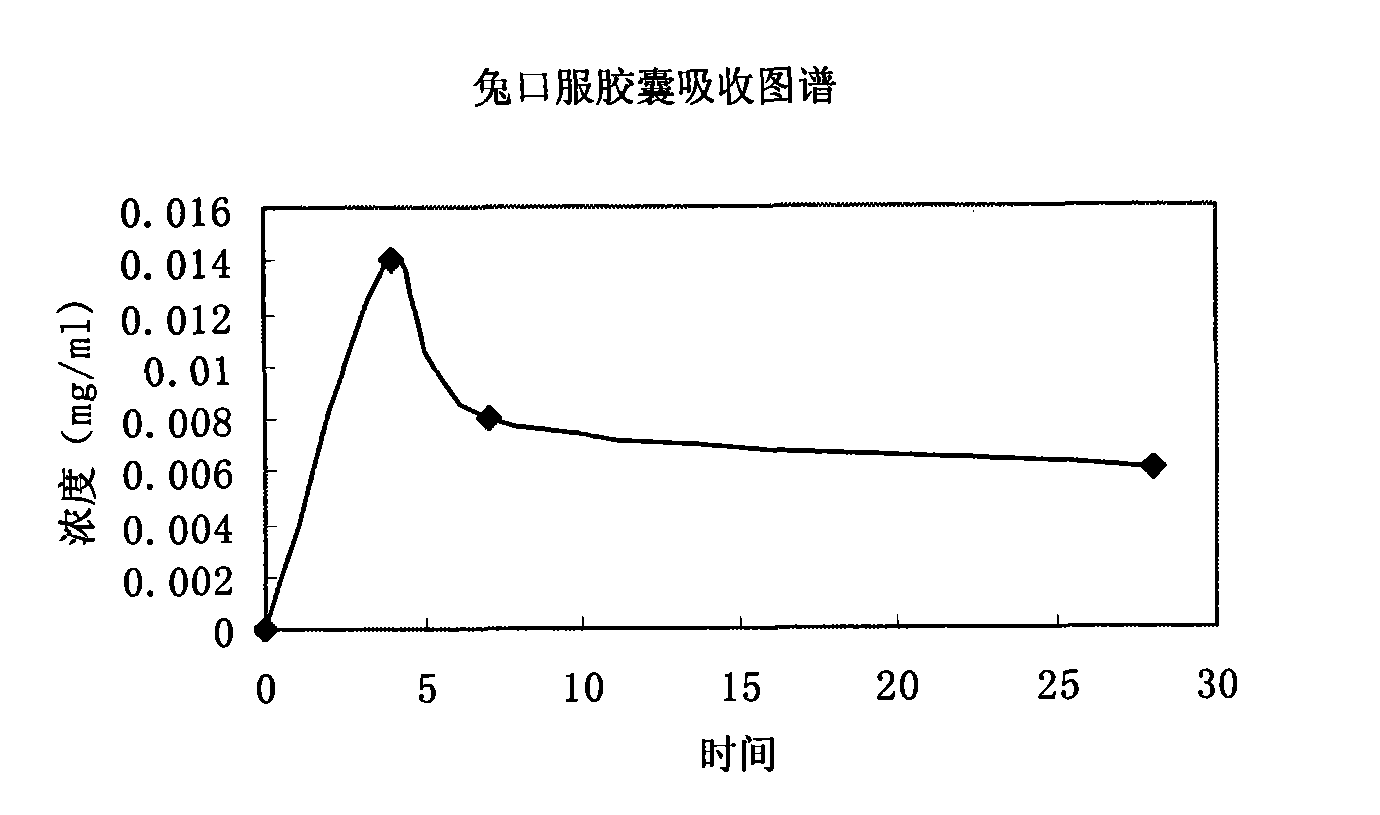
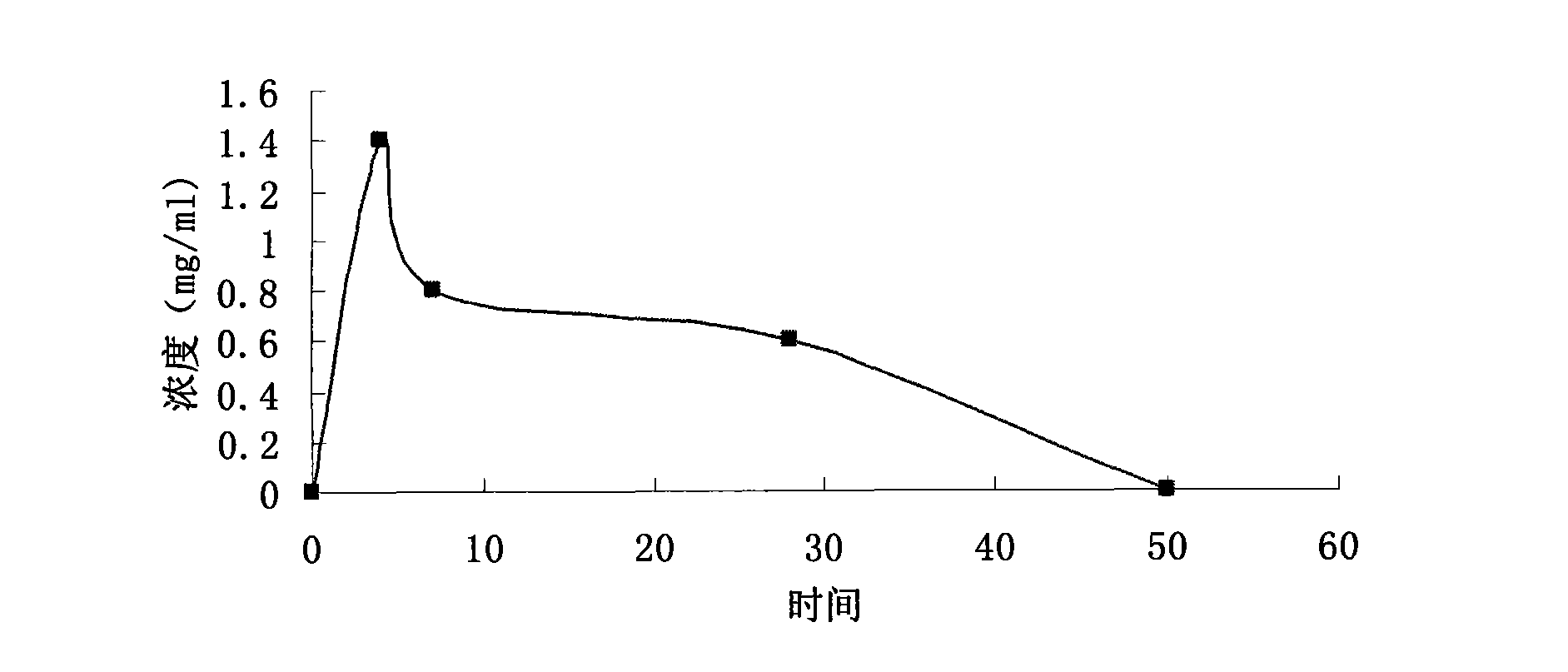
The invention relates to a calcium heparin / sodium salt nano-sized oral preparation and a preparation method thereof, and a calcium heparin / sodium salt nano-sized liposome with high encapsulation rate for preparing the calcium heparin / sodium salt nano-sized oral preparation. The calcium heparin / sodium salt nano-sized liposome is obtained by the following steps: dissolving chitosan in 1-10% diluted solution of acetic acid (the usage amount of chitosan is 0.5-5g per 100ml acetic acid solution) to obtain an acidified chitosan solution, filtering, collecting filtrate, adding dropwise 1-99% aqueous solution of calcium heparin / sodium salt into the filtrate under magnetic stirring until milk white precipitate occurs, centrifugating the resulating suspension, collecting precipitates, and freezes-drying to obtain calcium heparin / sodium salt nano-sized liposome. The calcium heparin / sodium salt nano-sized oral preparation is characterized in that the preparation has good stability, high encapsulation rate (up to 98%), and high in vitro release rate (up to 25% at hour 4); the preparation is absorbed in the intestinal tract with high absorption rate and high concentration; the half life is prolonged upon the increase of oral dosage, showing zero-order kinetics; the tissue concentration is larger than blood concentration; the preparation is released sustainedly; and study in rabbits (20mg oral intake) shows that the blood concentration in vivo is kept high for over 50 hours, thereby proving sustained release.
Owner:褚红女
Nanoparticle composition and methods to make and use the same
InactiveUS9149426B2Ability to encapsulateNarrow distributionOrganic active ingredientsBiocideDiseaseWhole body
The present invention provides a novel nanoparticle drug delivery system generated from poly(ortho ester) polymers with sustained drug release capability and can be functionalized to allow for systemic delivery to various organ systems throughout the body. One important aspect of this invention is that the nanoparticle drug delivery system generated from poly(ortho ester) polymers encapsulate several types of drugs in poly(ortho ester) nanoparticles, including but not limited to lipophilic, hydrophilic small and large molecules and also hydrophilic and lipophilic dyes by adopting appropriate emulsion techniques. These poly(ortho ester) nanoparticles are biodegradable, biocompatible and controlled release drug delivery system with zero order kinetics, which can be used in various biomedical applications such as eye-related diseases, cancer, arthritis, etc.
Owner:UNIV OF TENNESSEE RES FOUND
Methods and devices for connecting nerves
ActiveUS9931121B2Increase ratingsHigh degreeSurgeryTissue regenerationDrug reservoirSemipermeable membrane
A nerve repair conduit configured to be secured on first and second portions of a selected nerve. The nerve repair conduit includes a polymeric body having a proximal end, a distal end, an exterior surface and an interior surface defining an interior lumen. In addition, the nerve conduit includes at least one drug reservoir to hold agent(s) that may, for example, facilitate nerve regeneration. The drugs diffuse from the drug reservoir(s) into the nerve repair conduit through an outlet (e.g., a semipermeable membrane) in proximity to the first and second portions of a selected nerve. The nerve repair conduit may be configured to deliver the agent(s) at a rate having substantially zero-order kinetics and / or at a constant rate over a selected period of time (e.g., at least 1 week).
Owner:UNIV OF UTAH RES FOUND
Hydrostatic delivery system for controlled delivery of agent
The present invention provides a hydrostatic delivery system including a hydrostatic couple and an agent of interest. The hydrostatic couple includes at least one hydrodynamic fluid-imbibing polymer, and at least one hydrostatic pressure modulating agent. This delivery system has the ability to control the release of one or more agents of interest within a fluid environment following zero-order kinetics.
Owner:MACGREGOR ALEXANDER
Method for detecting skin permeability of metronidazole gel
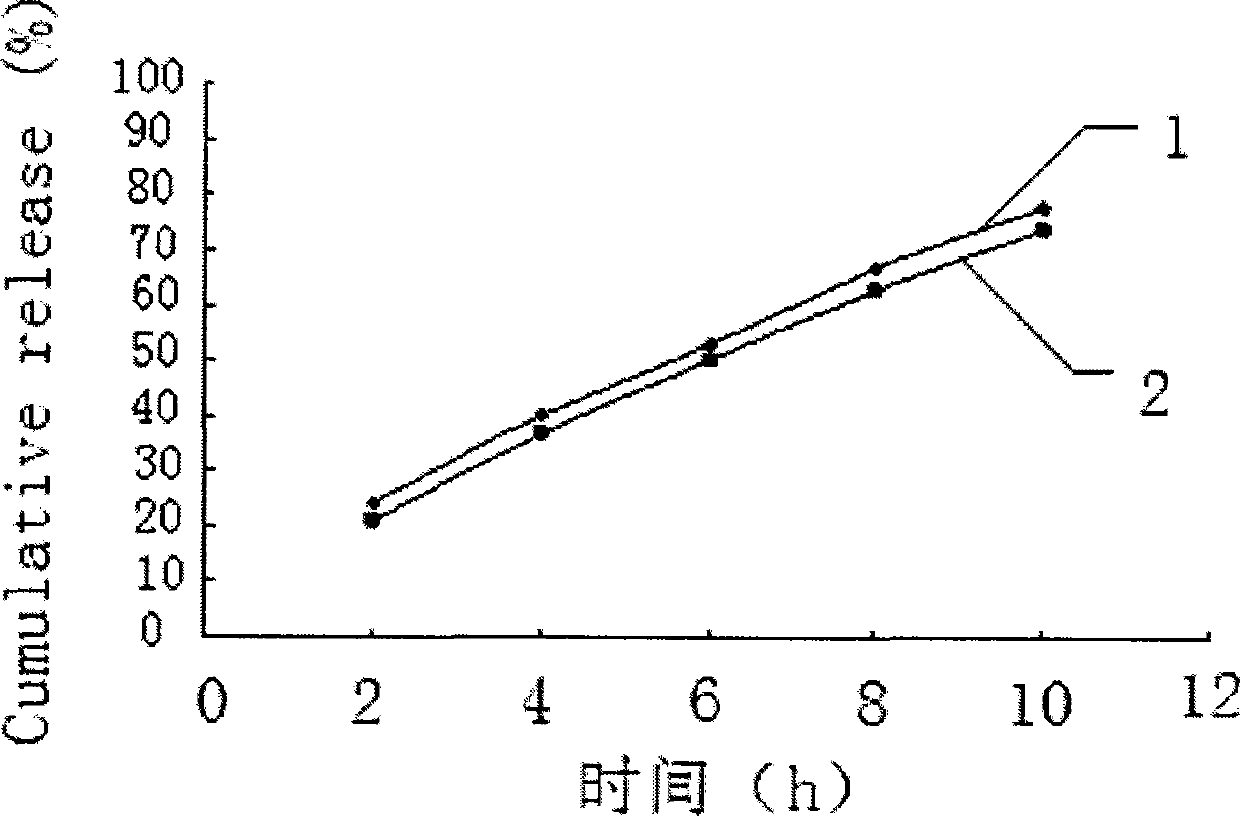
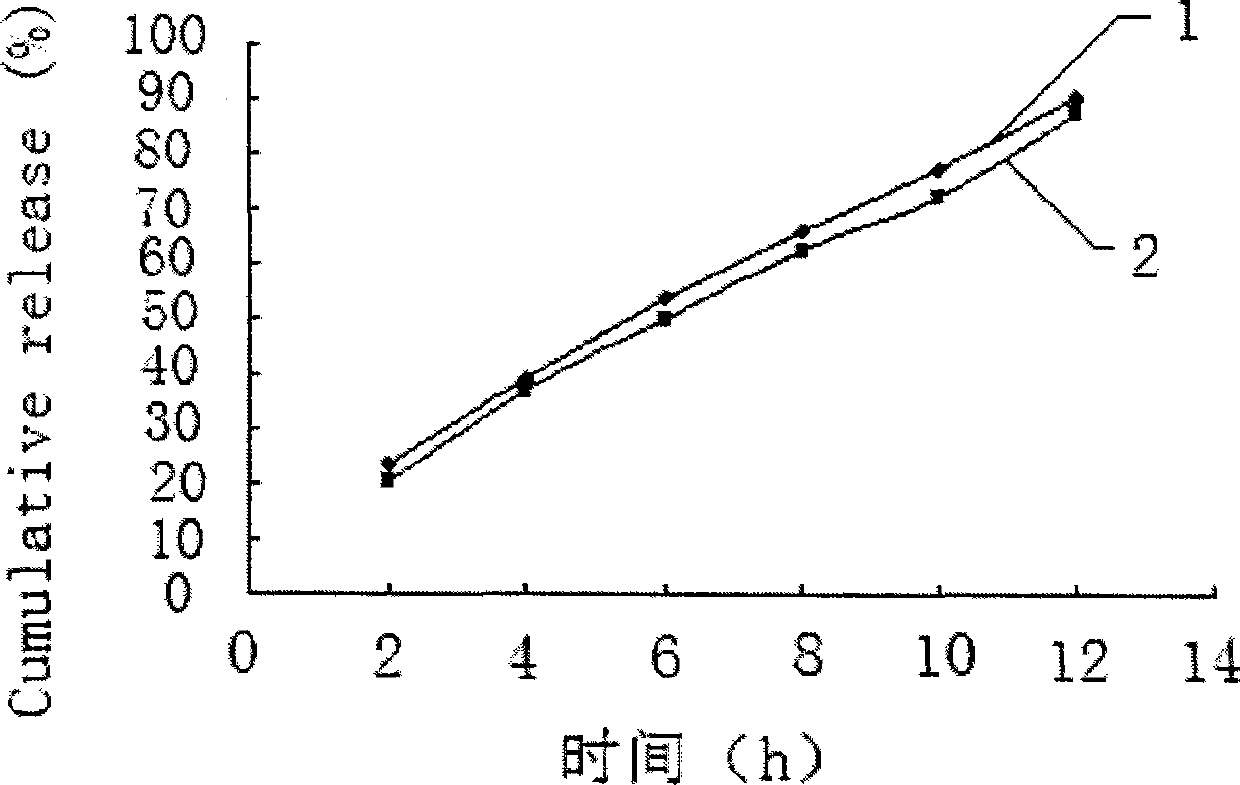
The invention relates to a method for detecting skin permeability of metronidazole gel. The method comprises the following steps: (1) uniformly coating metronidazole gel samples for test on the surface of one side of a test skin, contacting the surface of the other side with a receiving liquid that is a 0.8-1% sodium chloride aqueous solution; and making the samples for test pass through the testskin and enter into the receiving liquid in a constant temperature water bath environment of 31 to 33 degrees centigrade; (2) taking multiple sets of receiving samples from the receiving liquid regularly; (3) respectively injecting each set of the receiving samples obtained by timed sampling into a high performance liquid chromatograph, and measuring the concentration of metronidazole in each setof the receiving samples using an external standard method by regarding metronidazole as a reference substance; and (4) calculating the cumulative permeation of metronidazole, and calculating to determine whether it meets the zero-order kinetic release law. The detection method provided by the invention is convenient, efficient and accurate, can be used for evaluating the transdermal absorption effect of metronidazole gel in industrial production, and then used for evaluating the product quality of metronidazole gel.
Owner:ZHUZHOU QIANJIN PHARMA
Apparatus and method for zero order drug delivery from multilayer amphiphilic co-networks
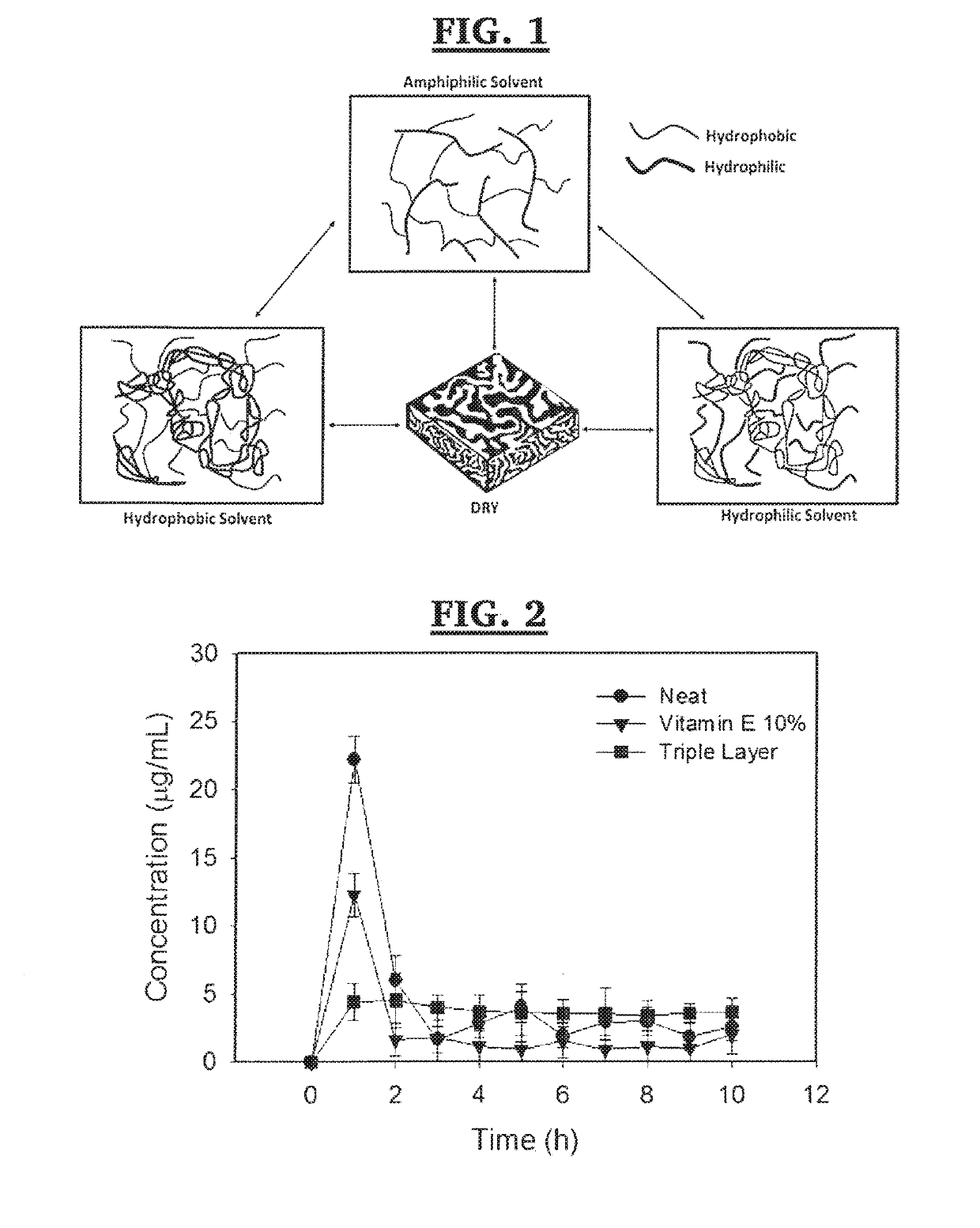
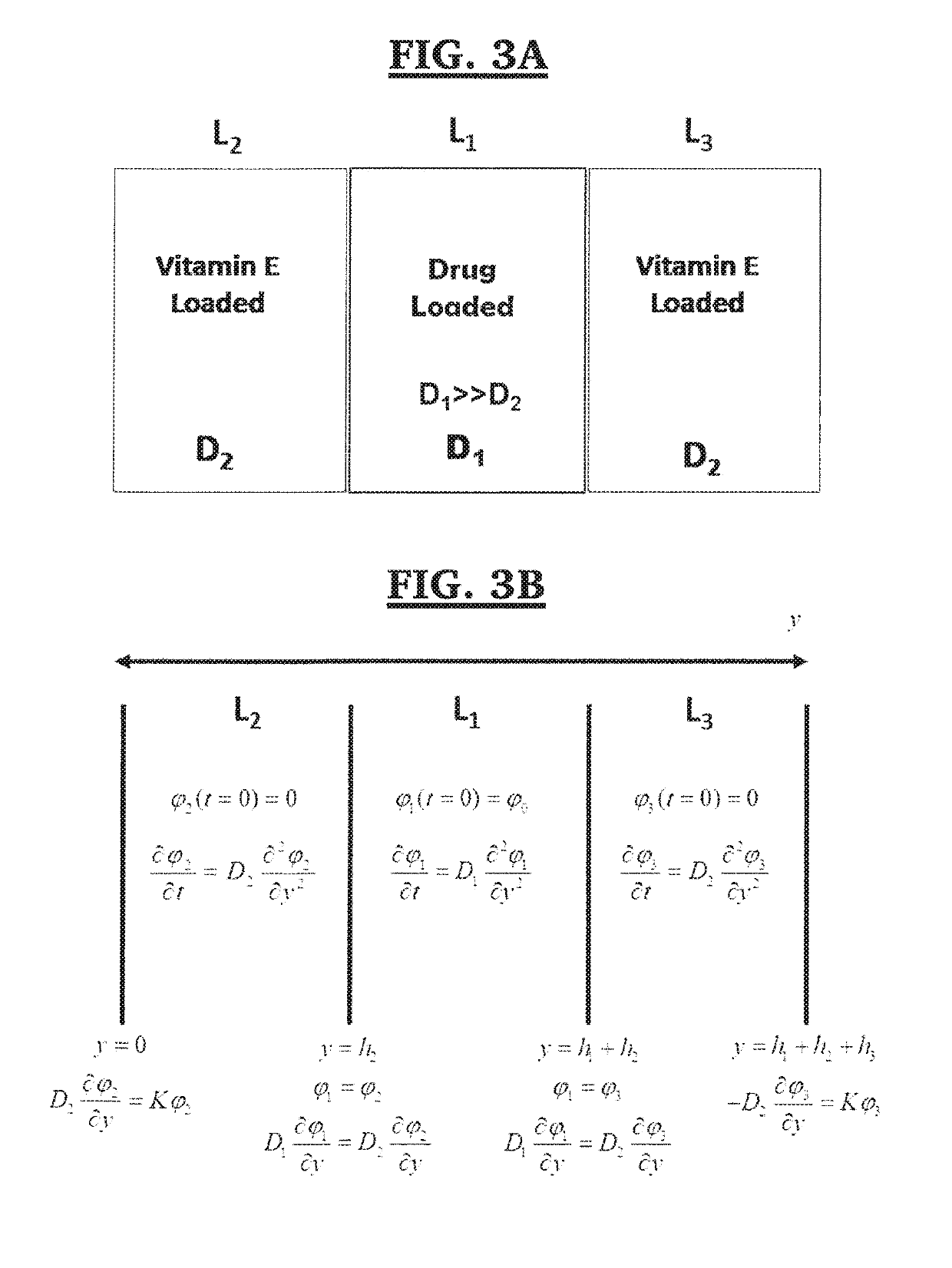
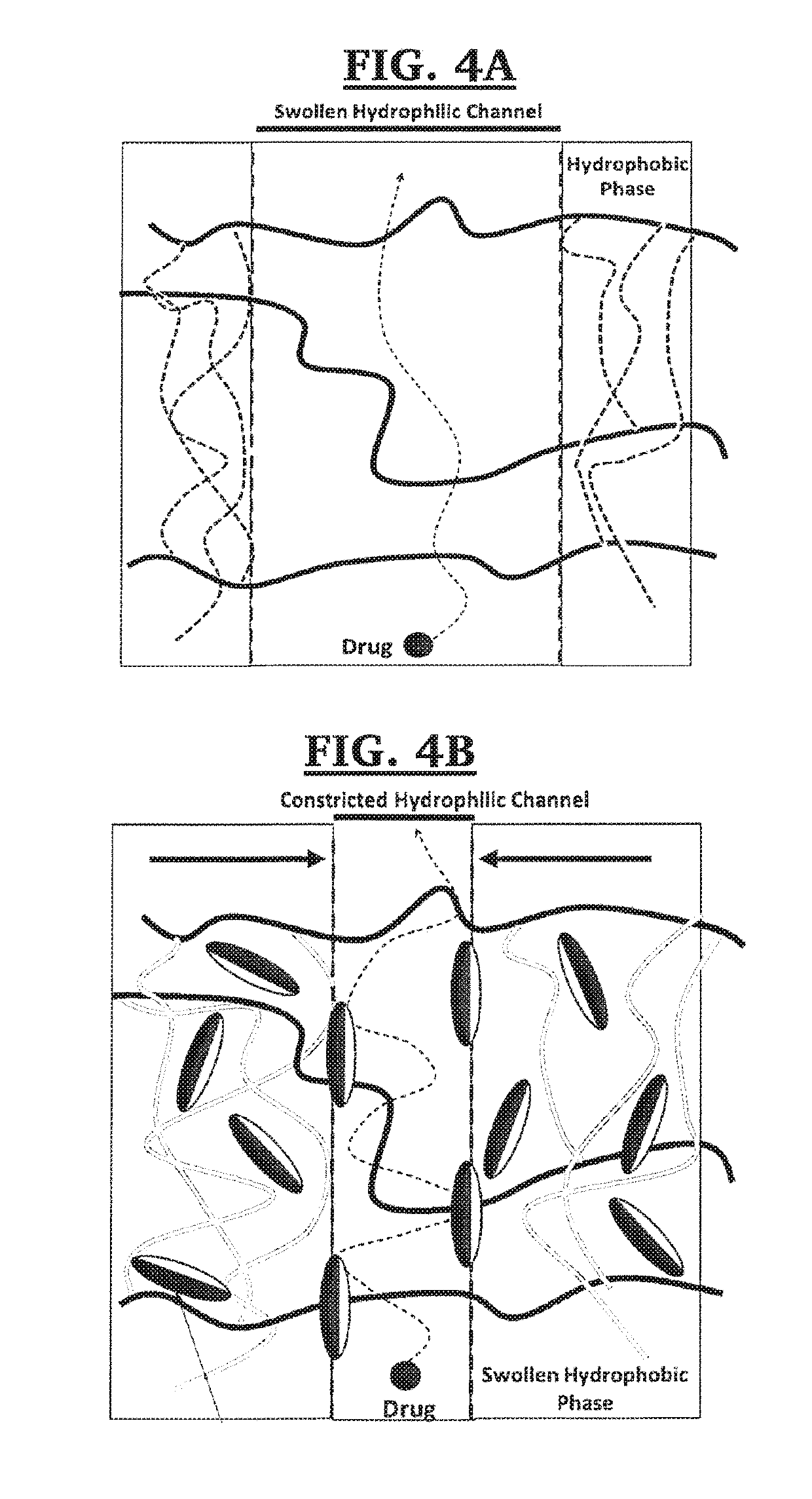
In one or more embodiments the present invention provides a three layer bimodal amphiphilic co-network (β-APCN) based drug delivery device and methods for its making and use. In various embodiments, the system is based on a three-layer scheme. A center layer is composed of a β-APCN matrix containing a high drug loading and exhibiting high drug diffusivity and two outer layers which are also β-APCN-based, contain no-drug and are instead loaded with a diffusional barrier such as vitamin E, which considerably slows drug diffusion through these outer layers. Both modeling and experimental data demonstrates that the combined effect of non-uniform distribution of drug loading and diffusion constants within the three-layer systems of various embodiments of the present invention is capable of maintaining a low local drug concentration at the polymer-fluid interface, thus achieving zero-order kinetics.
Owner:THE UNIVERSITY OF AKRON
Methods and devices for connecting nerves
ActiveUS20190038290A1Increase ratingsDegree of improvementSurgeryTissue regenerationDrug reservoirSemipermeable membrane
A nerve repair conduit configured to be secured on first and second portions of a selected nerve. The nerve repair conduit includes a polymeric body having a proximal end, a distal end, an exterior surface and an interior surface defining an interior lumen. In addition, the nerve conduit includes at least one drug reservoir to hold agent(s) that may facilitate nerve regeneration. The drugs diffuse from the drug reservoir(s) into the nerve repair conduit through an outlet (such as a hole or a semipermeable membrane) in proximity to the first and second portions of a selected nerve. The nerve repair conduit may be configured to deliver the agent(s) at a rate having substantially zero-order kinetics and / or at a constant rate over a selected period of time.
Owner:UNIV OF UTAH RES FOUND
Salvianolic acid controlled porosity osmotic pump tablets and method of preparing the same
ActiveCN101278920BStable blood concentrationProlong the effective timeOrganic active ingredientsPharmaceutical delivery mechanismPorositySalvianolic acid B
The invention discloses a salvianolic acid micropore osmotic pump controlled release tablet, comprising a tablet core and a coating; the tablet core is composed of salvianolic acid bulk drug, controlled release supplementary material, lubricant and moderate anhydrous ethanol; the weight ratio of the salvianolic acid bulk drug, the controlled release supplementary material and the lubricant is 50:150:1; the coating is composed of cellulose acetate, polyethylene glycol 400 solution and diethyl ester phthalate. In the invention, on the basis of controlling the content of 50-90% of salvianolic acid B, the content of 5-15% of tanshinol, protocatechualdehyde, salvianolic acid C, etc. in raw materials and by the optimal screening of penetrating agent, pore-forming agent, plasticizer and film thickness, an osmotic pump controlled release tablet which releases 85%-95% of a drug for 12 hours and conforms to the release characteristic of zero order kinetics is successfully prepared and used for preparing the drug for treating coronary heart disease and cerebral arteriosclerosis, which is good for the drug to form steady blood concentration in a body and lengthening the effective acting time of the drug, thus having good clinical compliance and treatment quality.
Owner:惠州市九惠药业有限公司
VEGF slowly releasing injection microsphere support and its preparation and use
InactiveCN100457187CImprove survival ratePromote formationPeptide/protein ingredientsGranular deliverySerum protein albuminMicrosphere
The VEGF slowly releasing injection microsphere support includes matrix, VEGF, protectant and ammonium bicarbonate. It features the matrix of polylactic acid-hydroxyl acetate copolymer of polylactic acid / hydroxyl acetate ratio 25:75 to 75:25; the protectant comprising zinc carbonate, serum albumin, mycose and mannitol; the weight ratio between VEGF and polylactic acid-hydroxyl acetate copolymer of 1:100000 to 1:10; and the microsphere size of 50-1000 microns. It is prepared through one W / O / W solvent volatilizing process or one fine medicine powder adding W / O / W solvent volatilizing process. The preparation process is simple, easy in operation and high in repeatability. In the microsphere support, protein medicine VEGF may be in vitro released for over 4 weeks in approached zero order kinetic mode. The present invention may be used in the repair and treatment of different kinds of tissue and blood vessel damage.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Sustained Release Formulation Containing Selective Serotonin Reuptake Inhibitor and Method for the Preparation Thereof
A sustained release formulation of a selective serotonin reuptake inhibitor (SSRI), comprising the SSRI as an active ingredient; a matrix comprising a water-soluble and erodible polymer, a hydrogel for shape sustenance and a pharmaceutically acceptable additive, the SSRI being embedded in the matrix, is capable of maintaining a constant drug level in the blood owing to the fact that the release of the SSRI follows zero order kinetics without initial burst release and such a release pattern is little affected by external factors such as gastrointestinal movements.
Owner:AMOREPACIFIC CORP
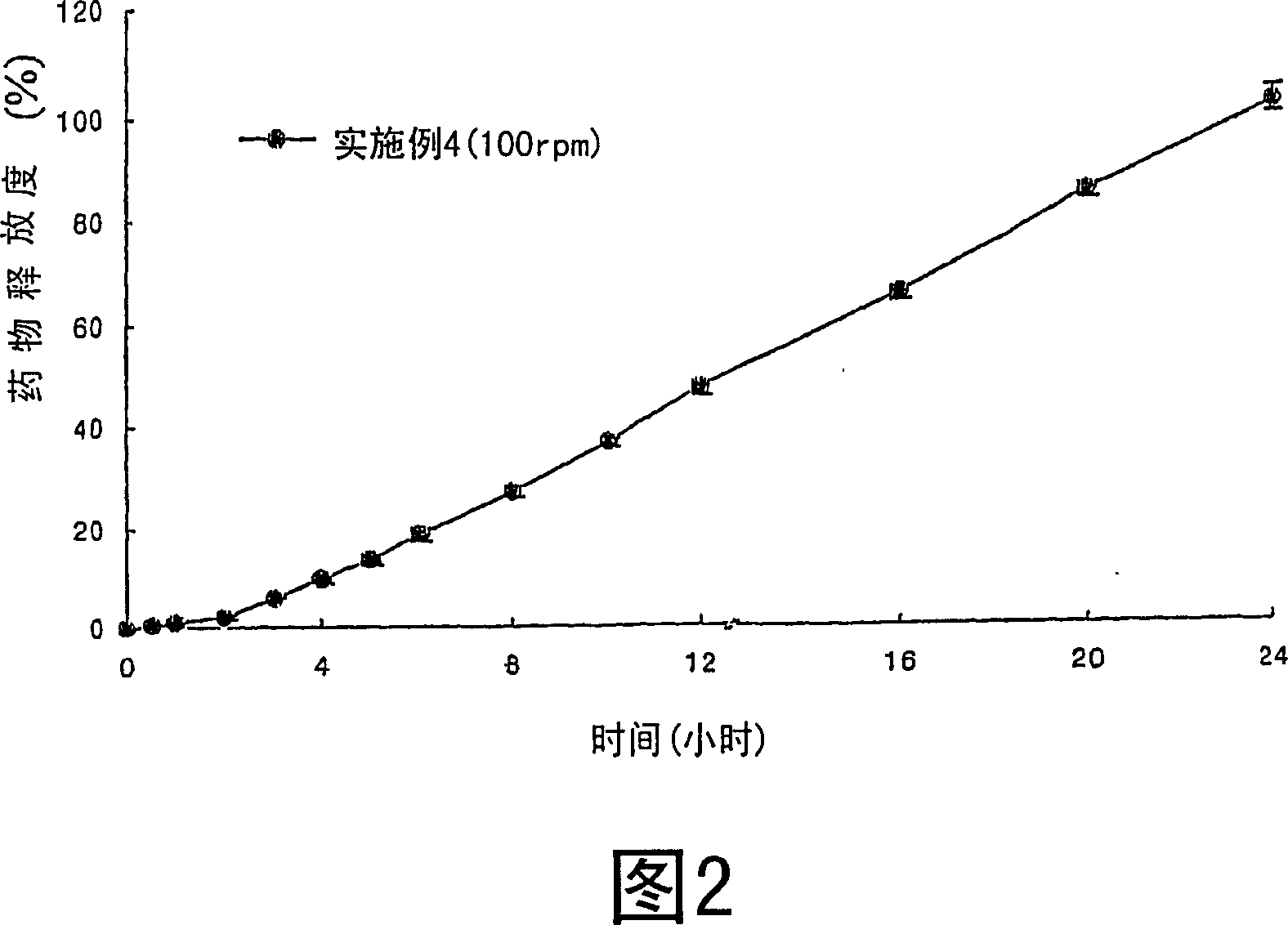
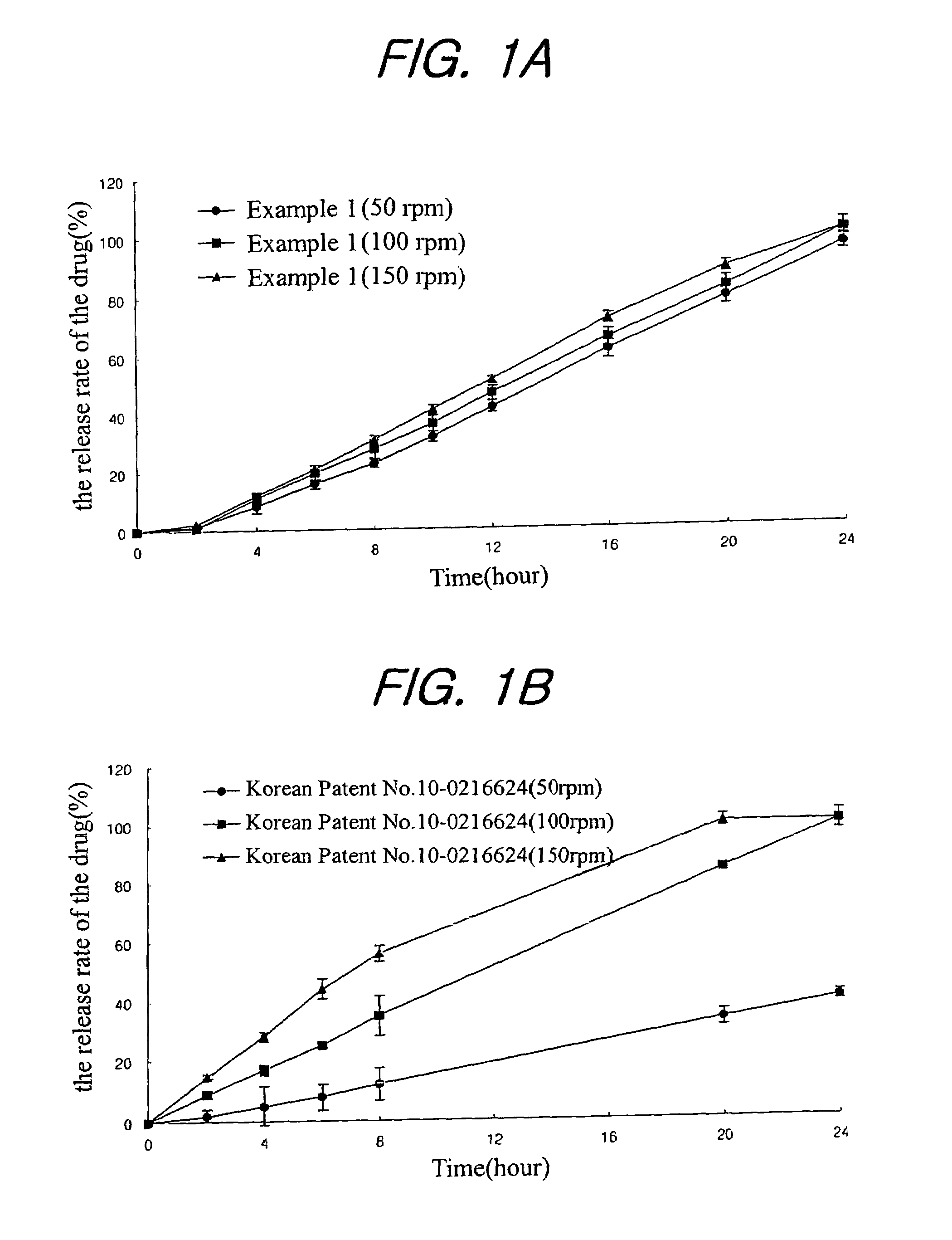
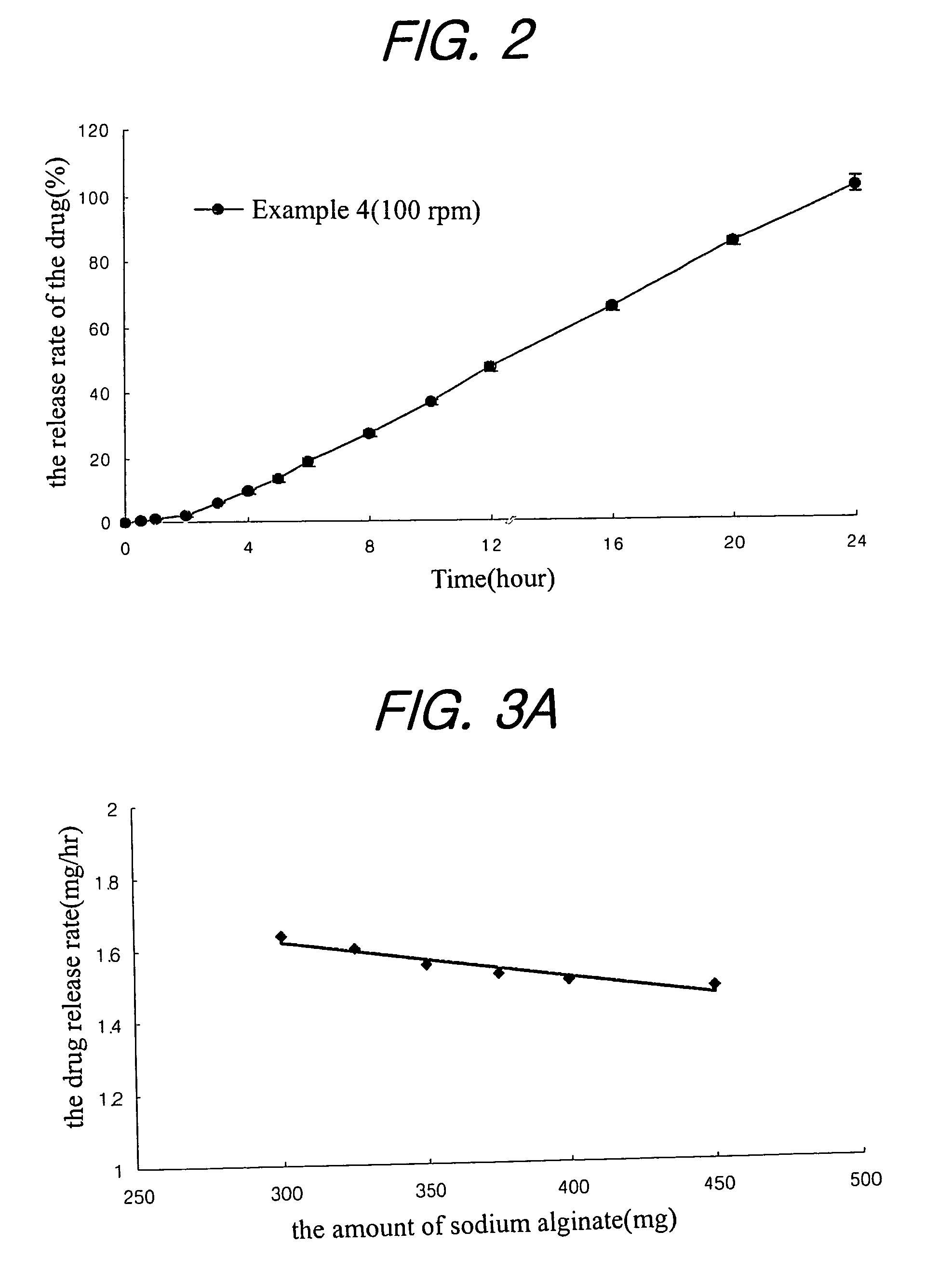
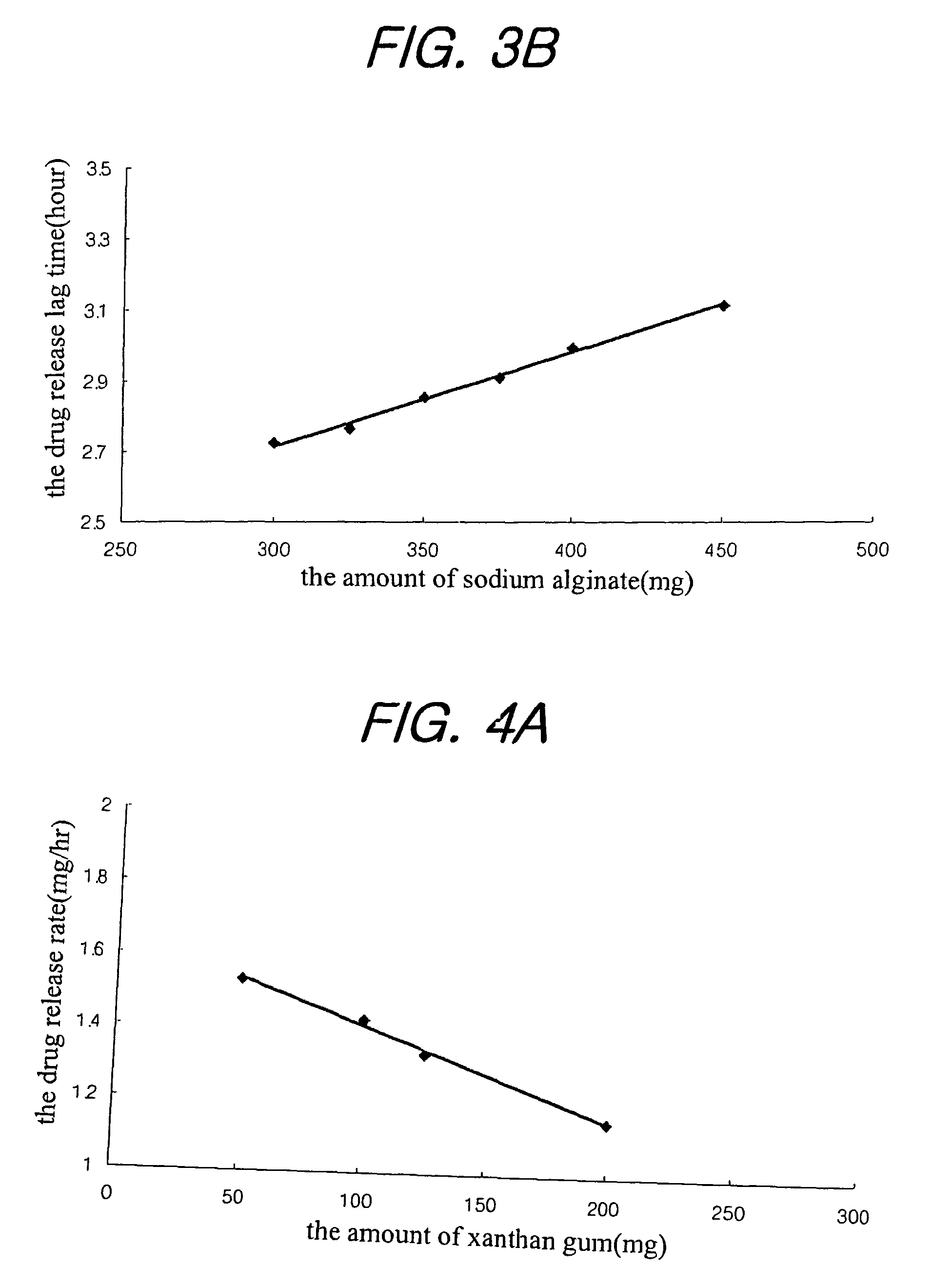
Sustained release composition for oral administration of drugs
A sustained-release composition for oral administration of a drug, comprising the drug, a mixture of sodium alginate and xanthan gum as a carrier for sustained release and a mixture of hydroxypropyl methylcellulose and propylene glycol alginate as a gel hydration accelerator, which is capable of maintaining a constant drug level in blood for 24 hours or more owing to the fact that the drug release rate follows zero order kinetics and does not significantly vary with the degree of gastrointestinal motility due to rapid gel hydration without forming a non-gelated core.
Owner:HANMI PHARMA
Sustained release composition for oral administration of drugs
A sustained-release composition for oral administration of a drug, comprising the drug, a mixture of sodium alginate and xanthan gum as a carrier for sustained release and a mixture of hydroxypropyl methylcellulose and propylene glycol alginate as a gel hydration accelerator, which is capable of maintaining a constant drug level in blood for 24 hours or more. Due to rapid gel hydration without forming a non-gelated core, the drug release rate follows zero order kinetics and does not significantly vary with the degree of gastrointestinal motility.
Owner:HANMI PHARMA
Calciparine/sodium salt nano oral preparation and preparation technique thereof
InactiveCN101249063BImprove stabilityHigh nano encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationHigh absorption
The invention relates to a calcium heparin / sodium salt nano-sized oral preparation and a preparation method thereof, and a calcium heparin / sodium salt nano-sized liposome with high encapsulation rate for preparing the calcium heparin / sodium salt nano-sized oral preparation. The calcium heparin / sodium salt nano-sized liposome is obtained by the following steps: dissolving chitosan in 1-10% dilutedsolution of acetic acid (the usage amount of chitosan is 0.5-5g per 100ml acetic acid solution) to obtain an acidified chitosan solution, filtering, collecting filtrate, adding dropwise 1-99% aqueoussolution of calcium heparin / sodium salt into the filtrate under magnetic stirring until milk white precipitate occurs, centrifugating the resulating suspension, collecting precipitates, and freezes-drying to obtain calcium heparin / sodium salt nano-sized liposome. The calcium heparin / sodium salt nano-sized oral preparation is characterized in that the preparation has good stability, high encapsulation rate (up to 98%), and high in vitro release rate (up to 25% at hour 4); the preparation is absorbed in the intestinal tract with high absorption rate and high concentration; the half life is prolonged upon the increase of oral dosage, showing zero-order kinetics; the tissue concentration is larger than blood concentration; the preparation is released sustainedly; and study in rabbits (20mg oral intake) shows that the blood concentration in vivo is kept high for over 50 hours, thereby proving sustained release.
Owner:褚红女
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com