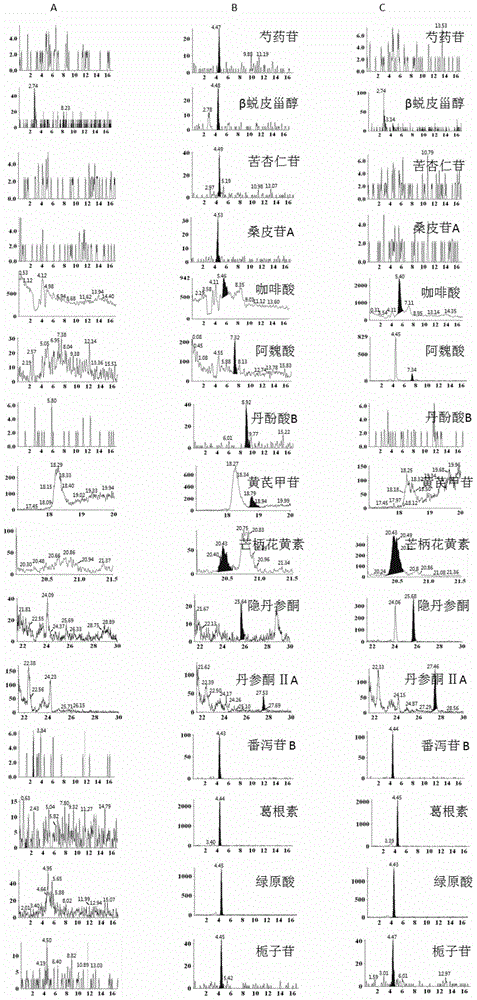
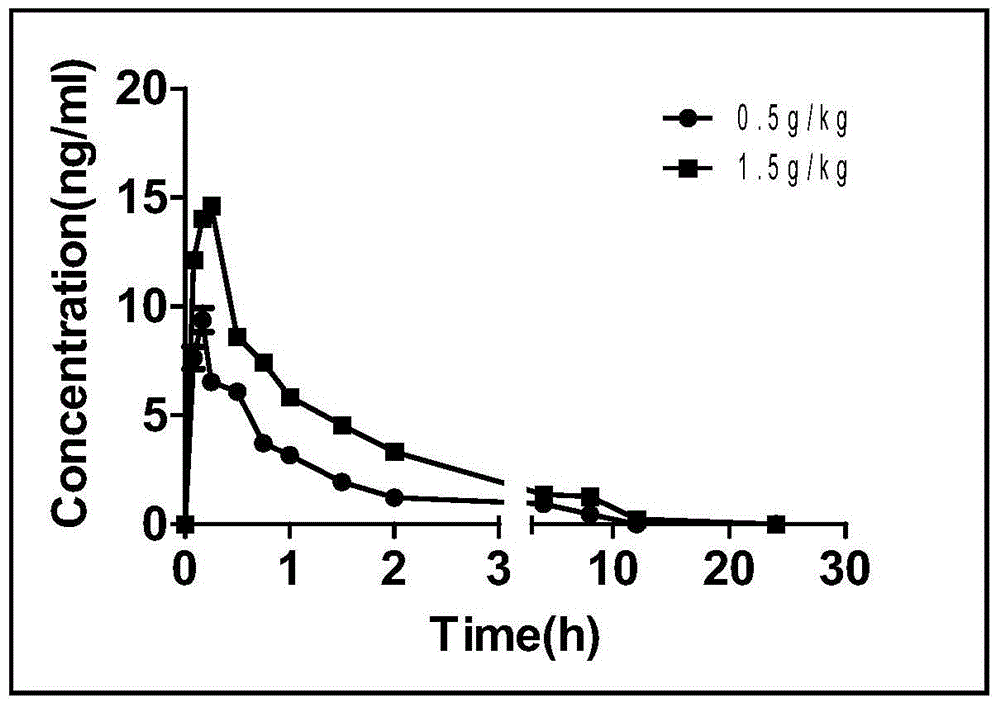
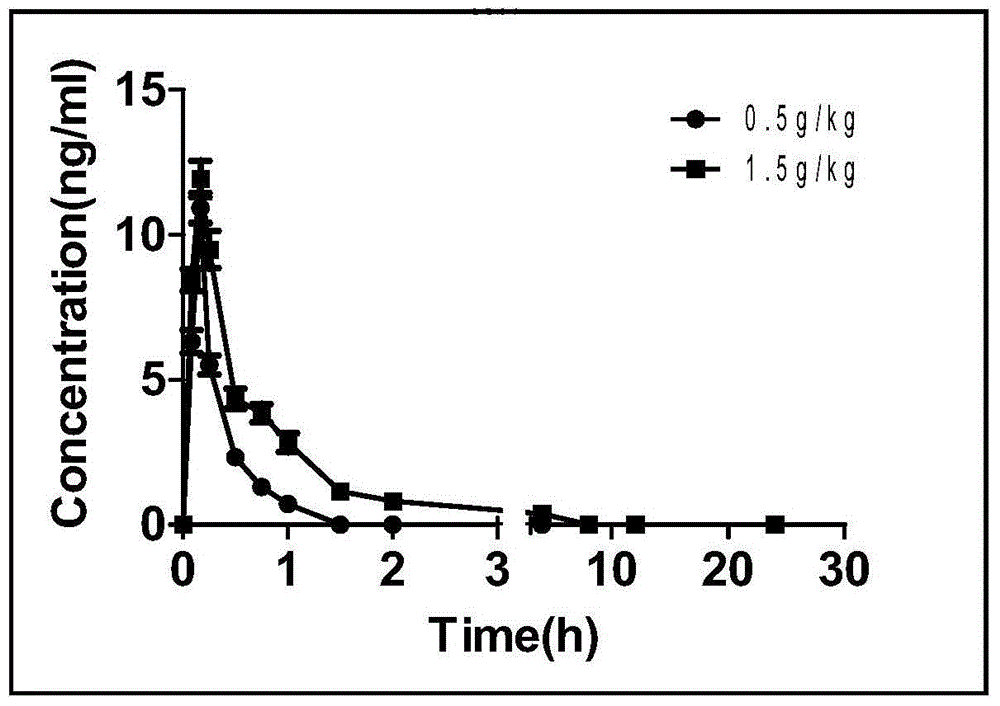
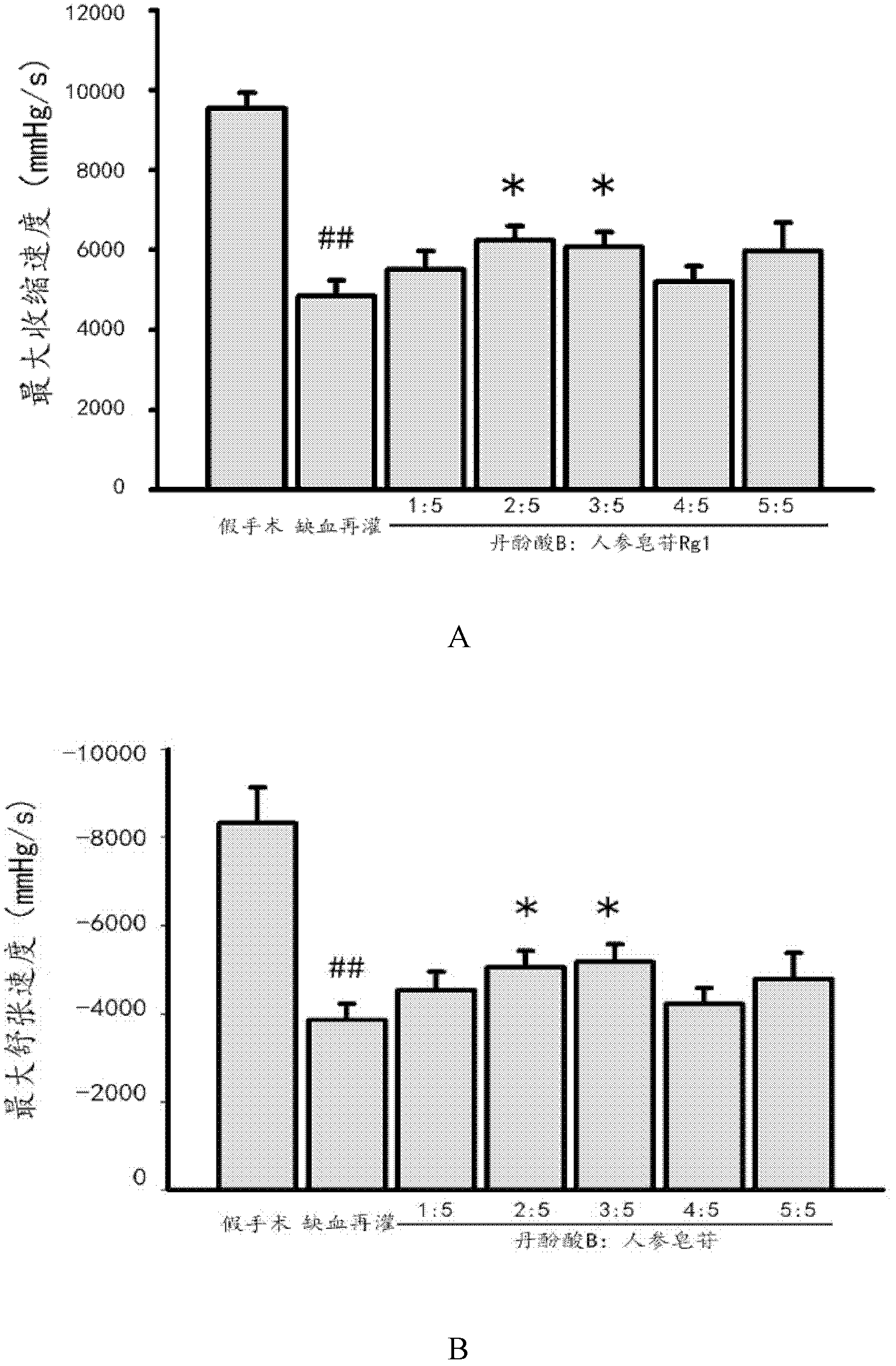
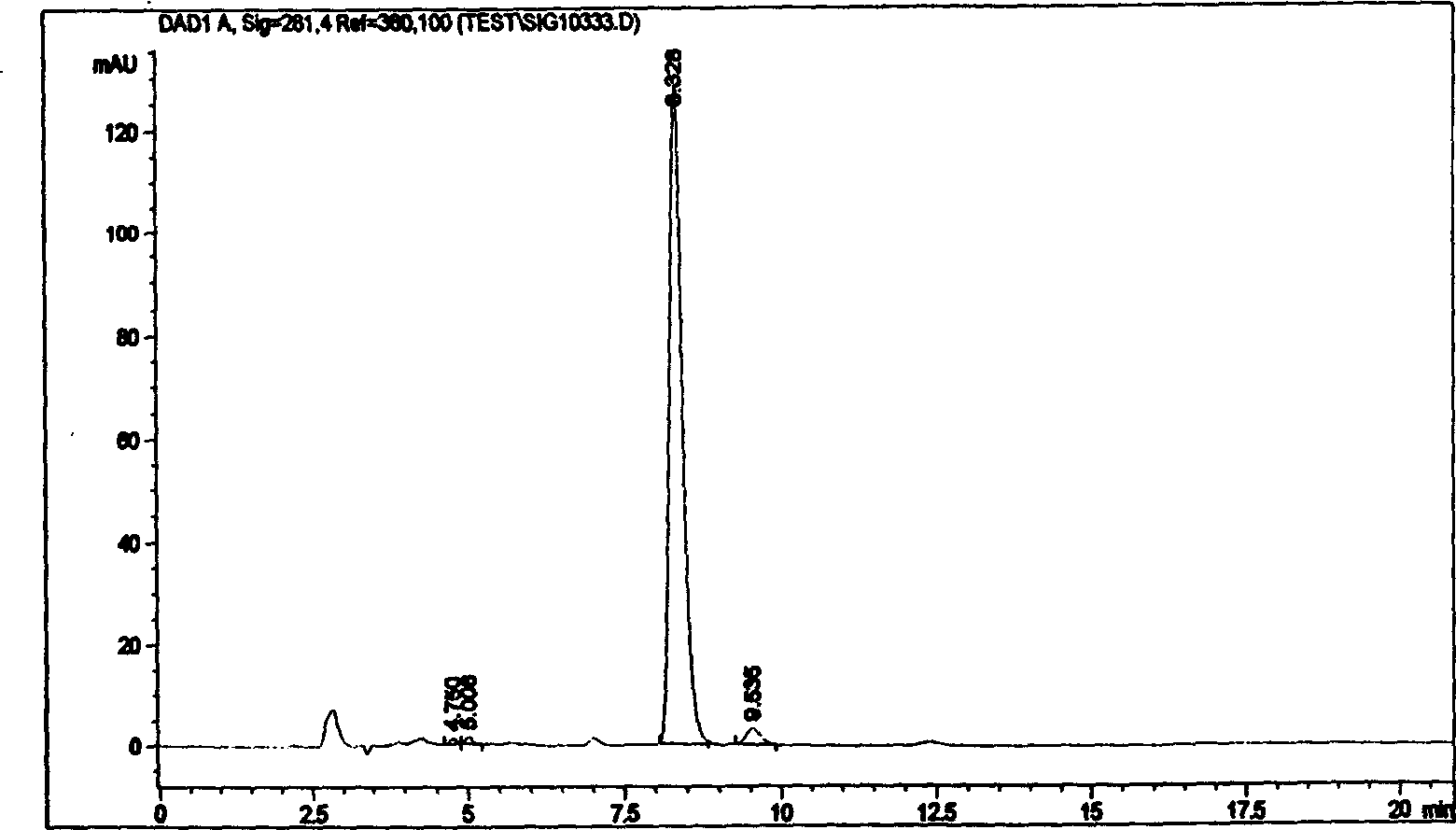
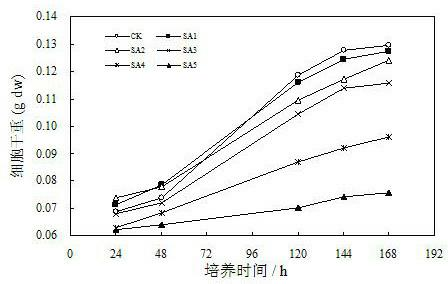
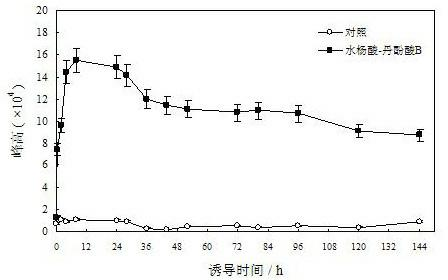
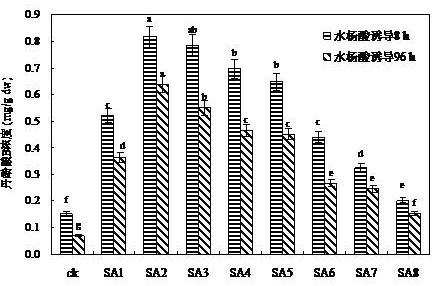
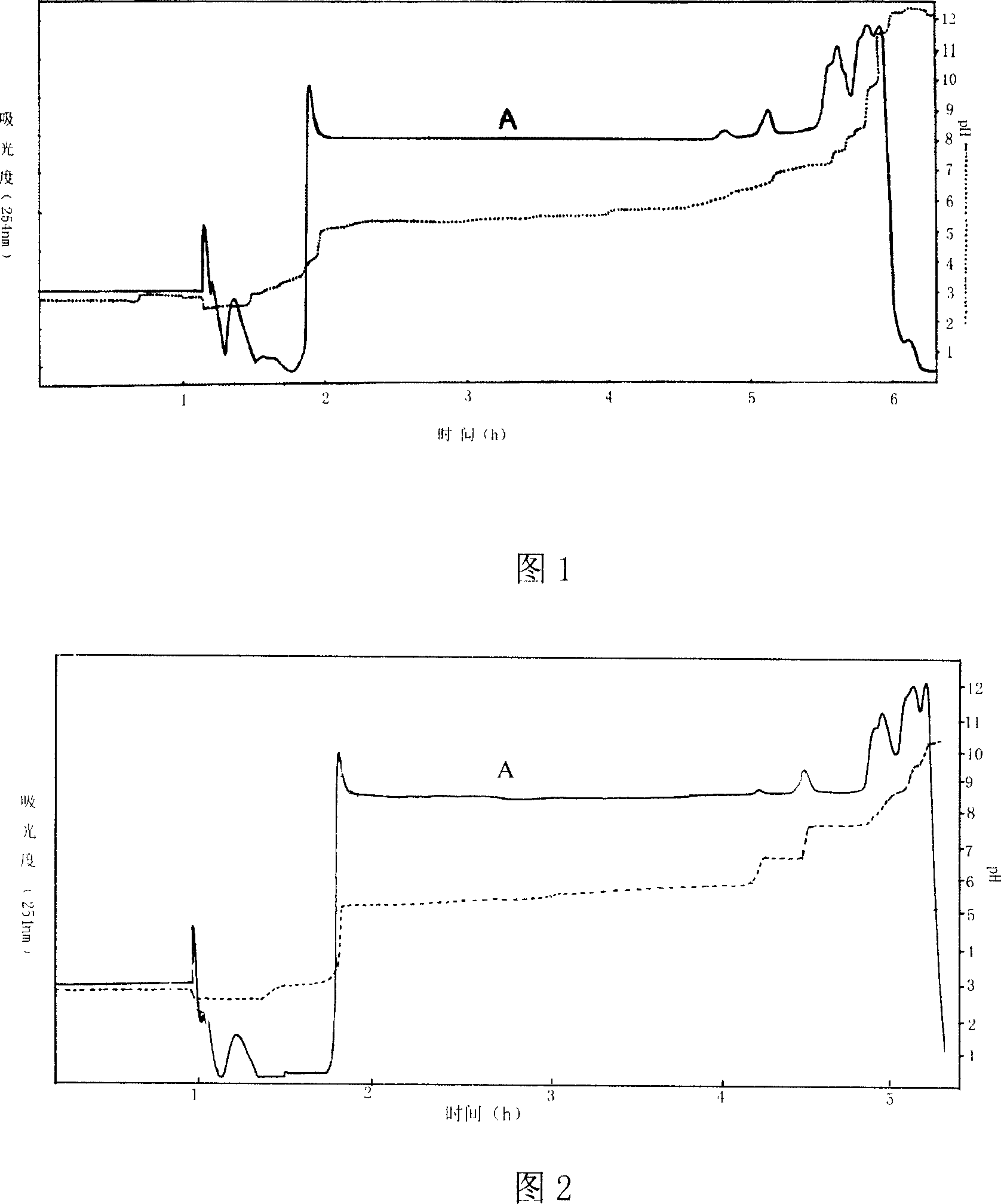
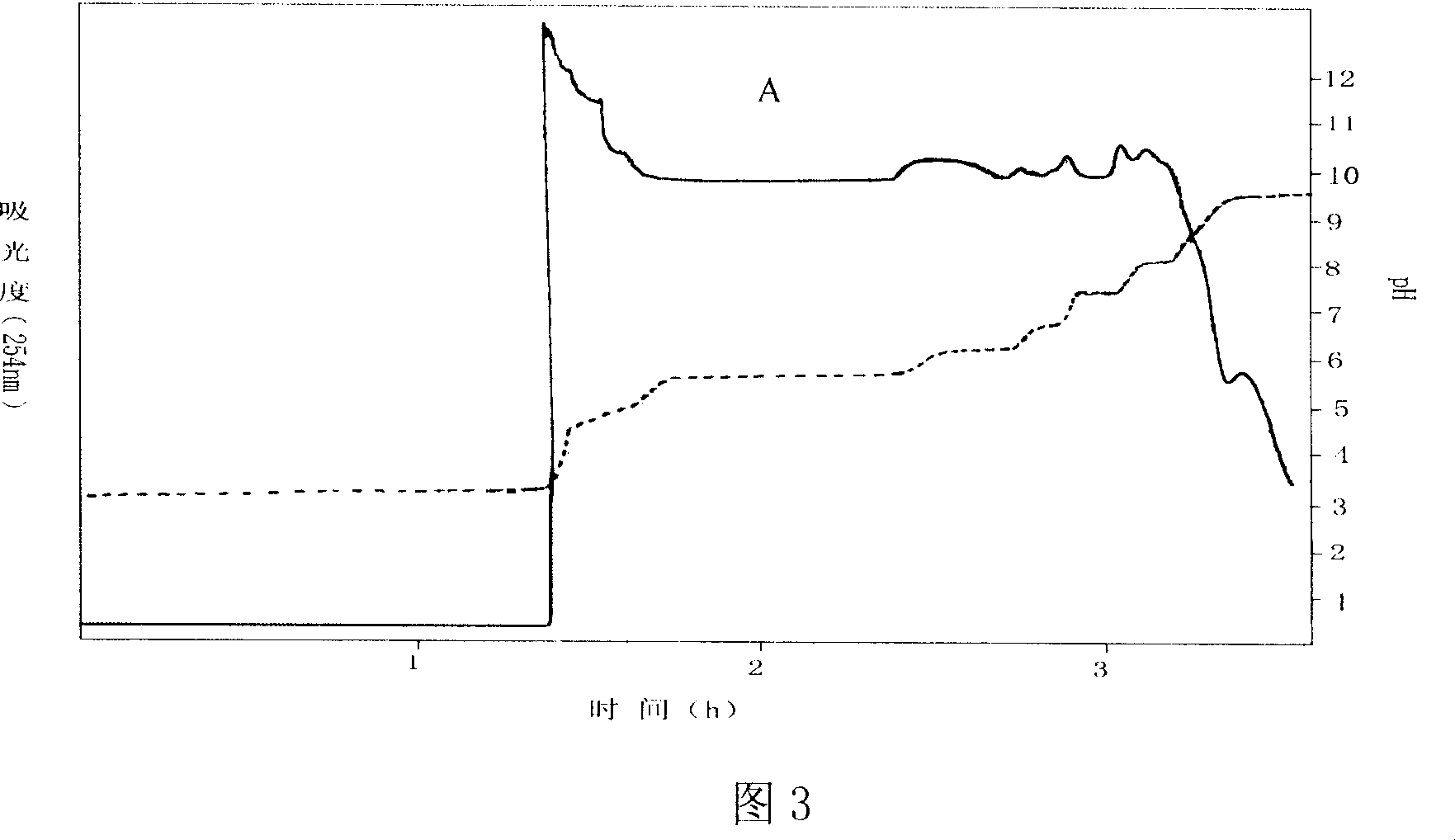
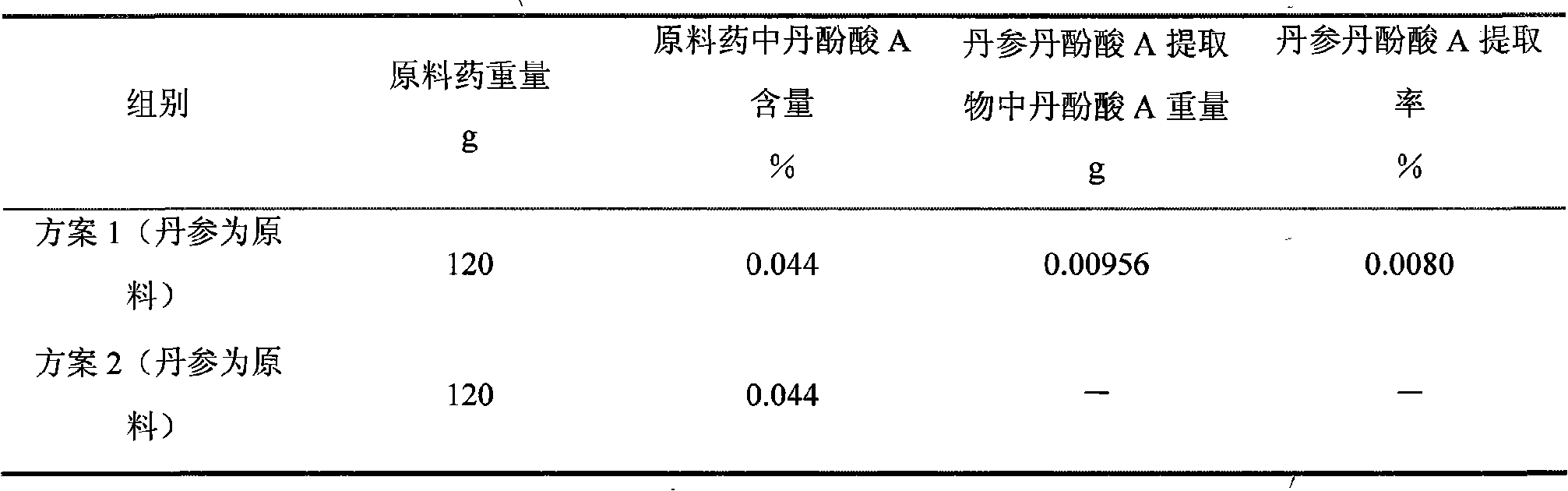
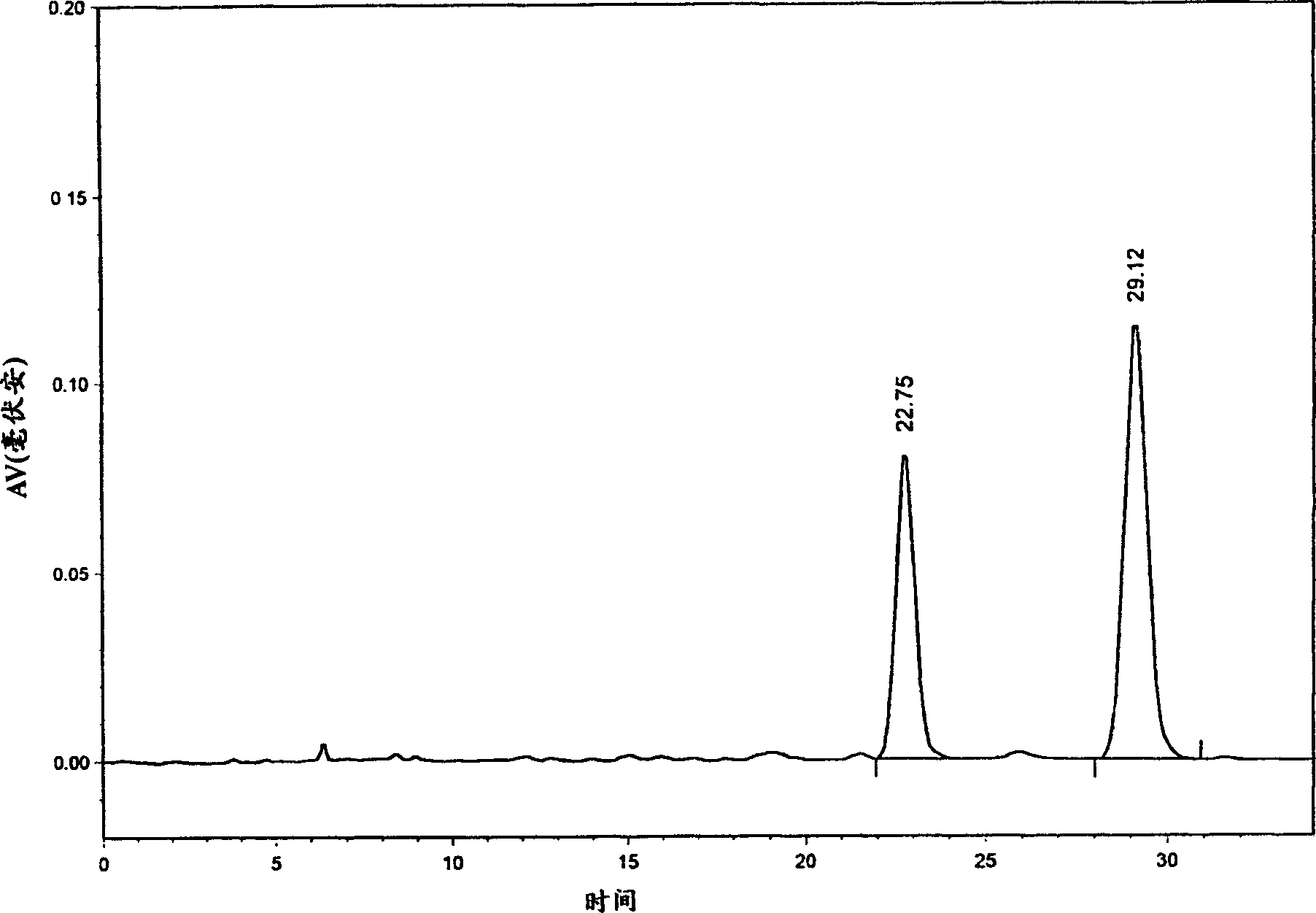
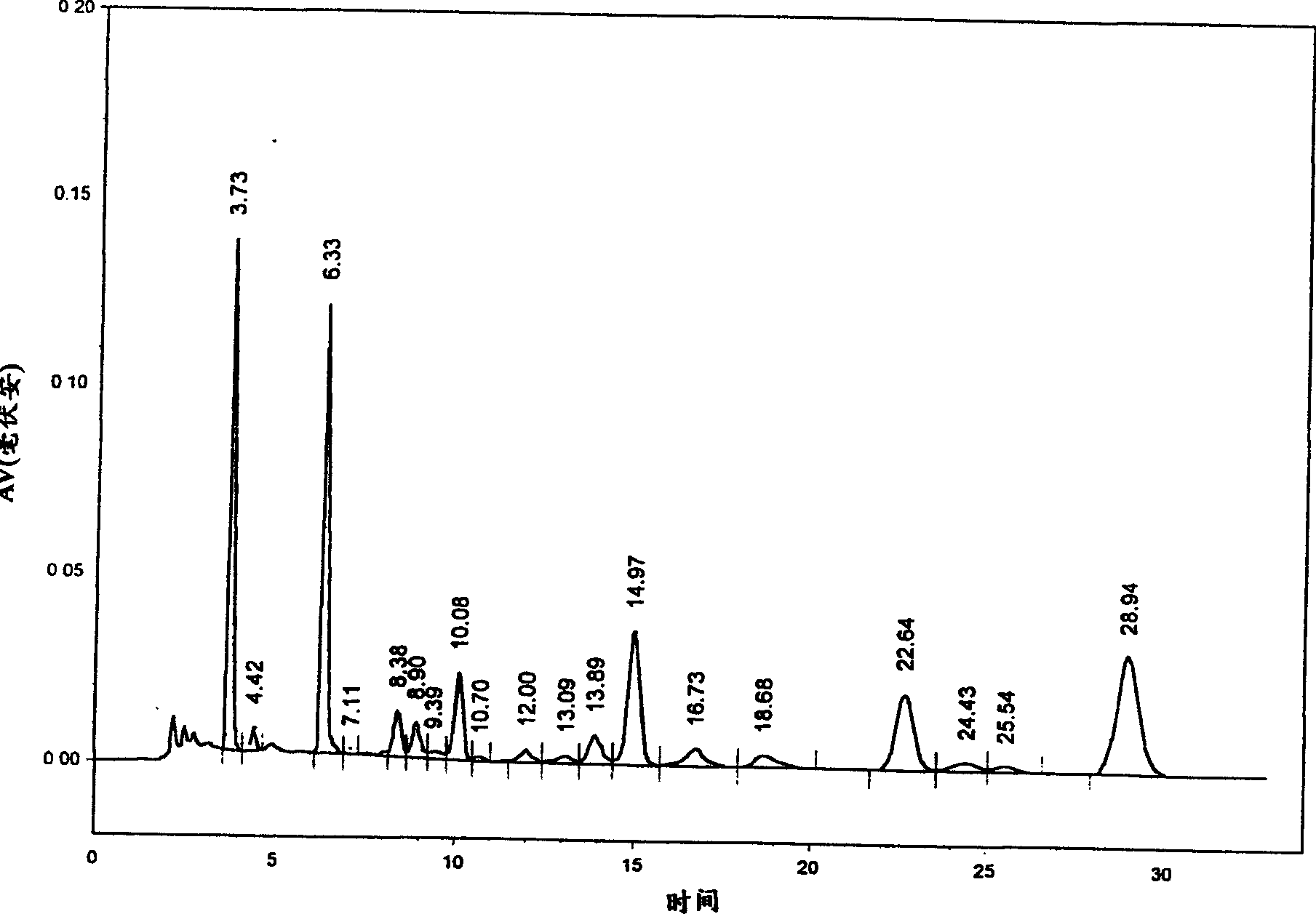
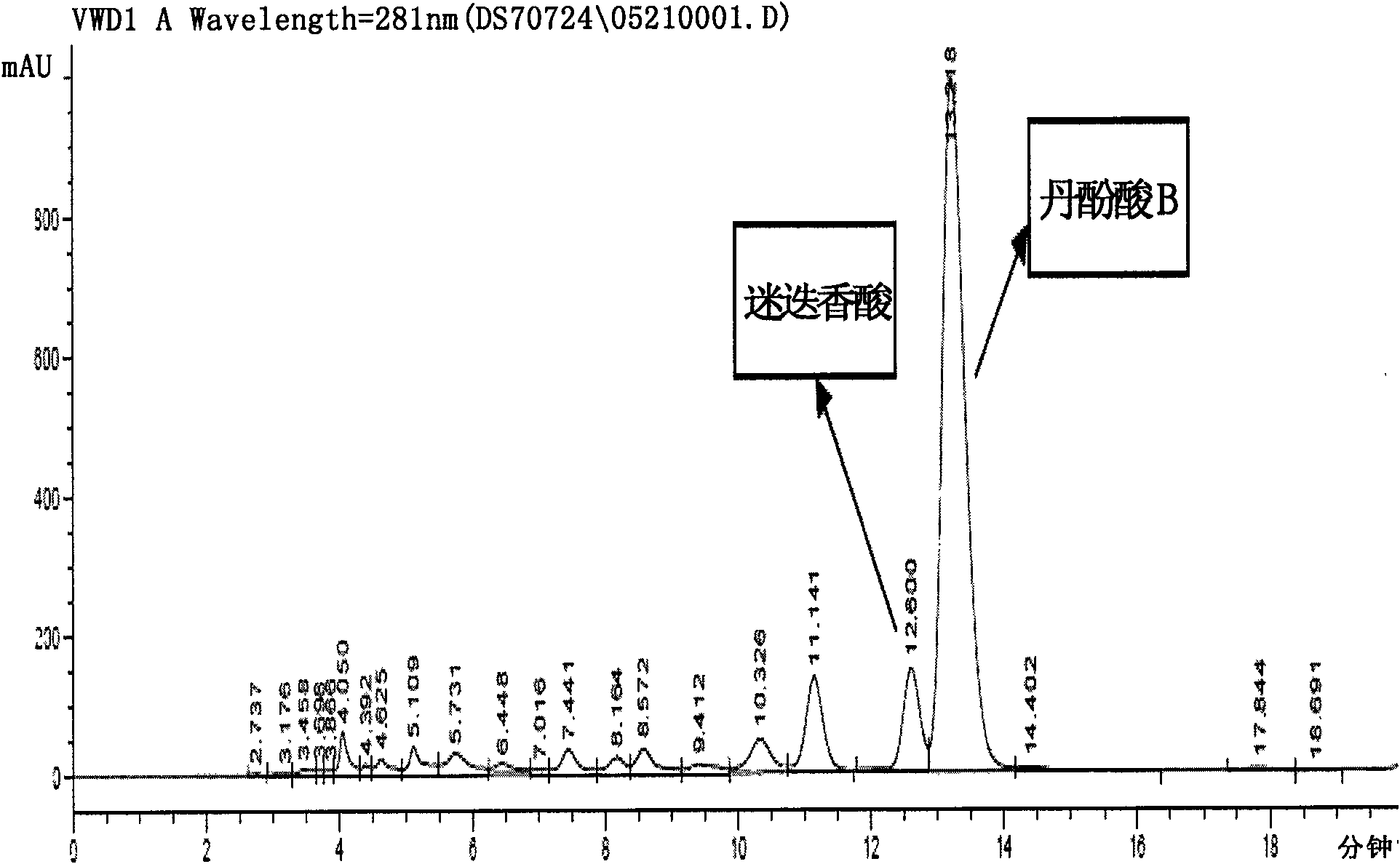
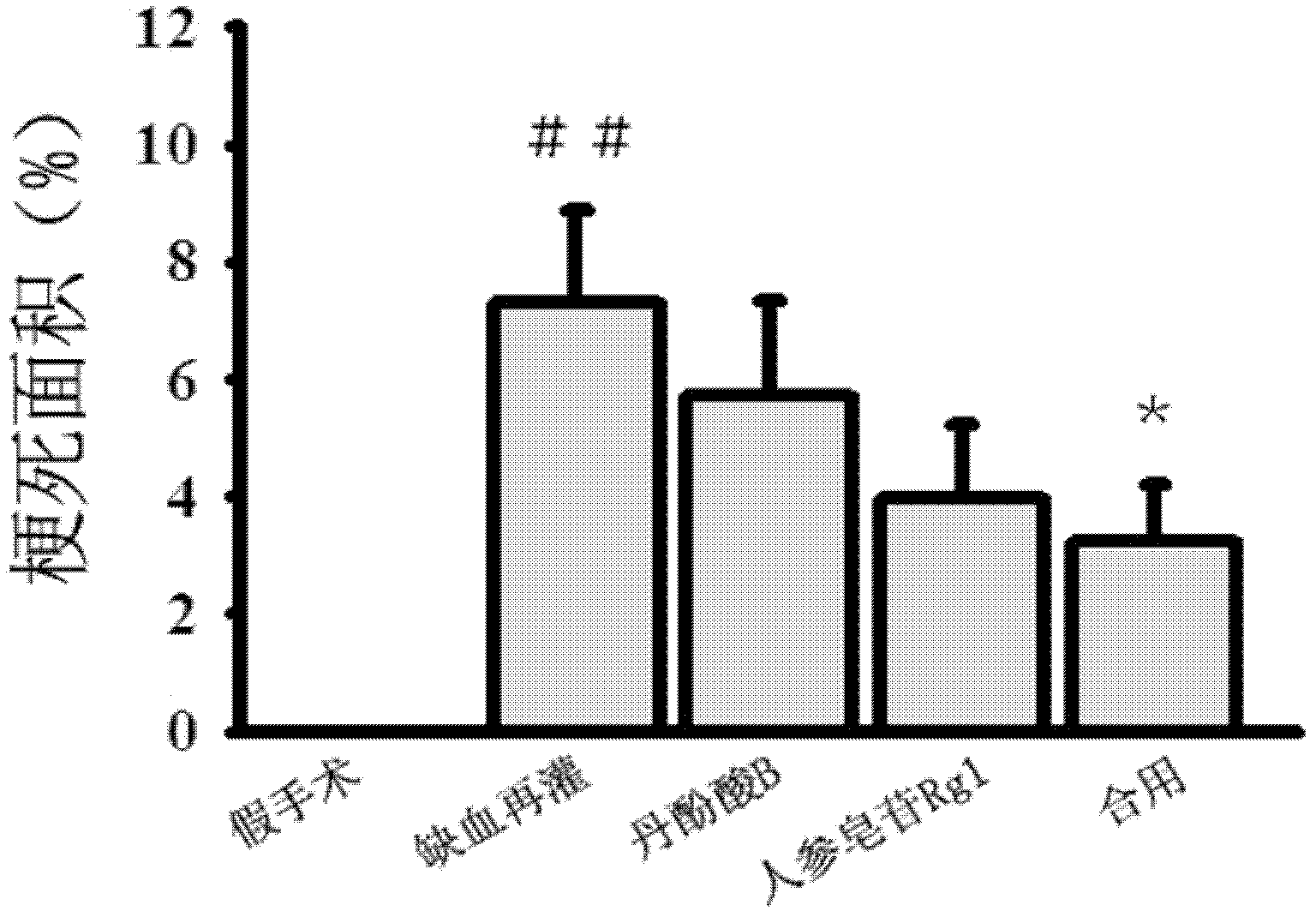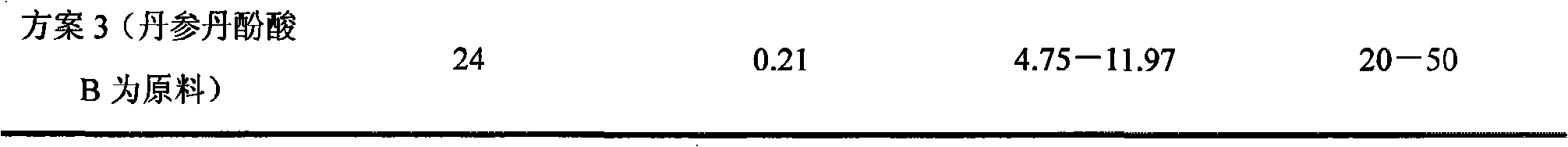Patents
Literature
527 results about "Salvianolic acid B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
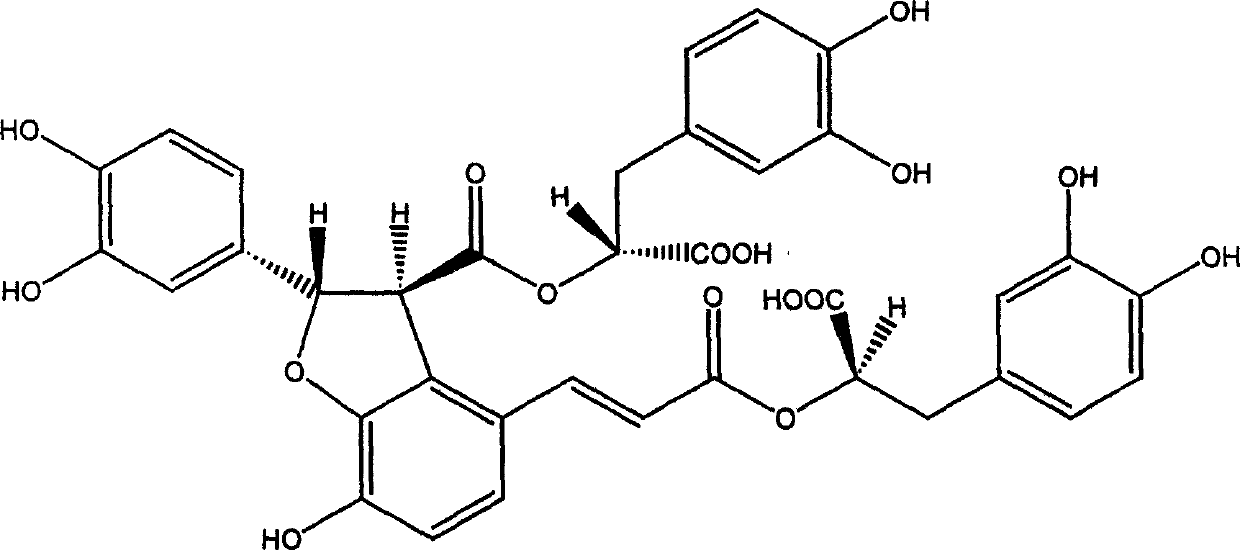
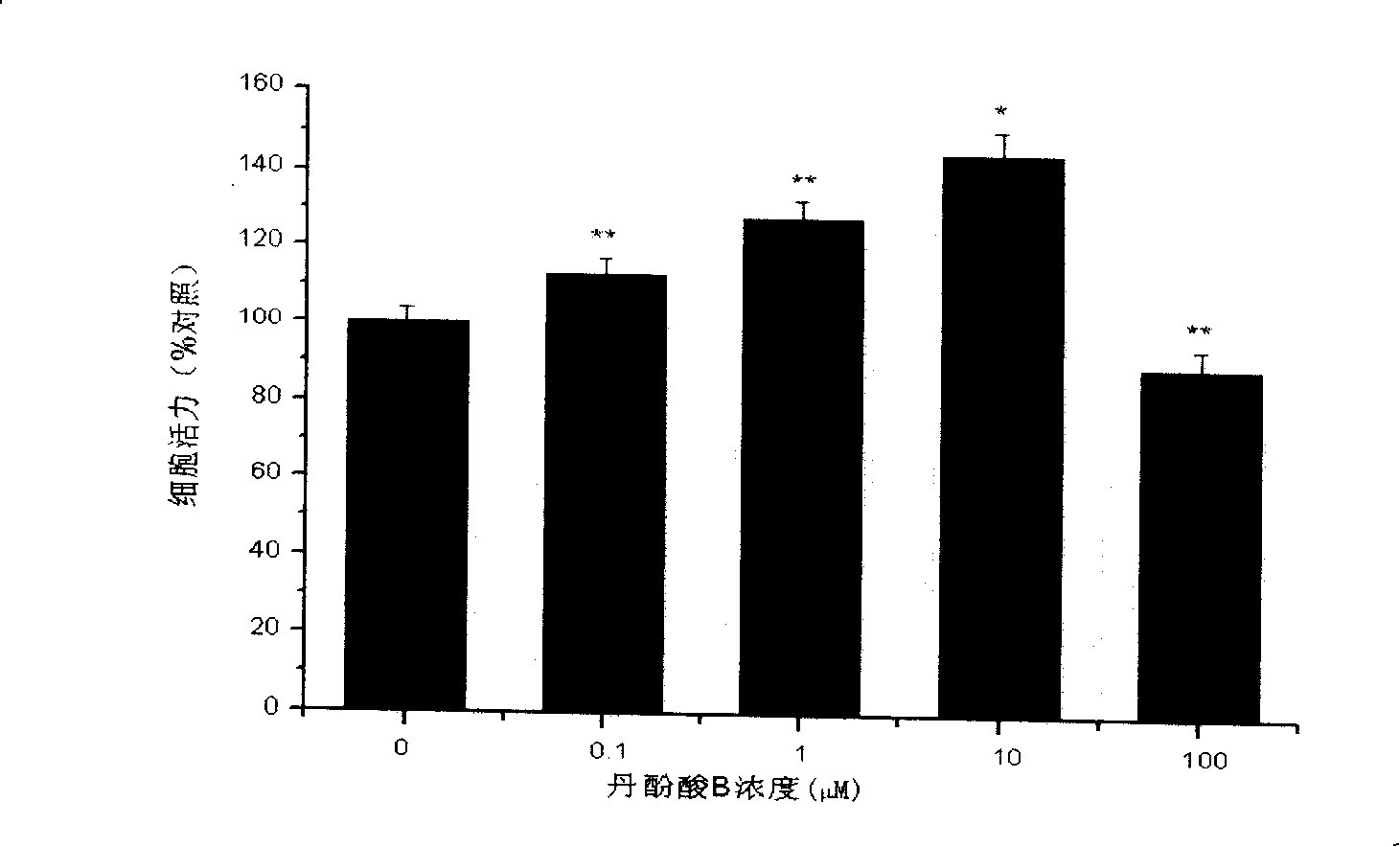
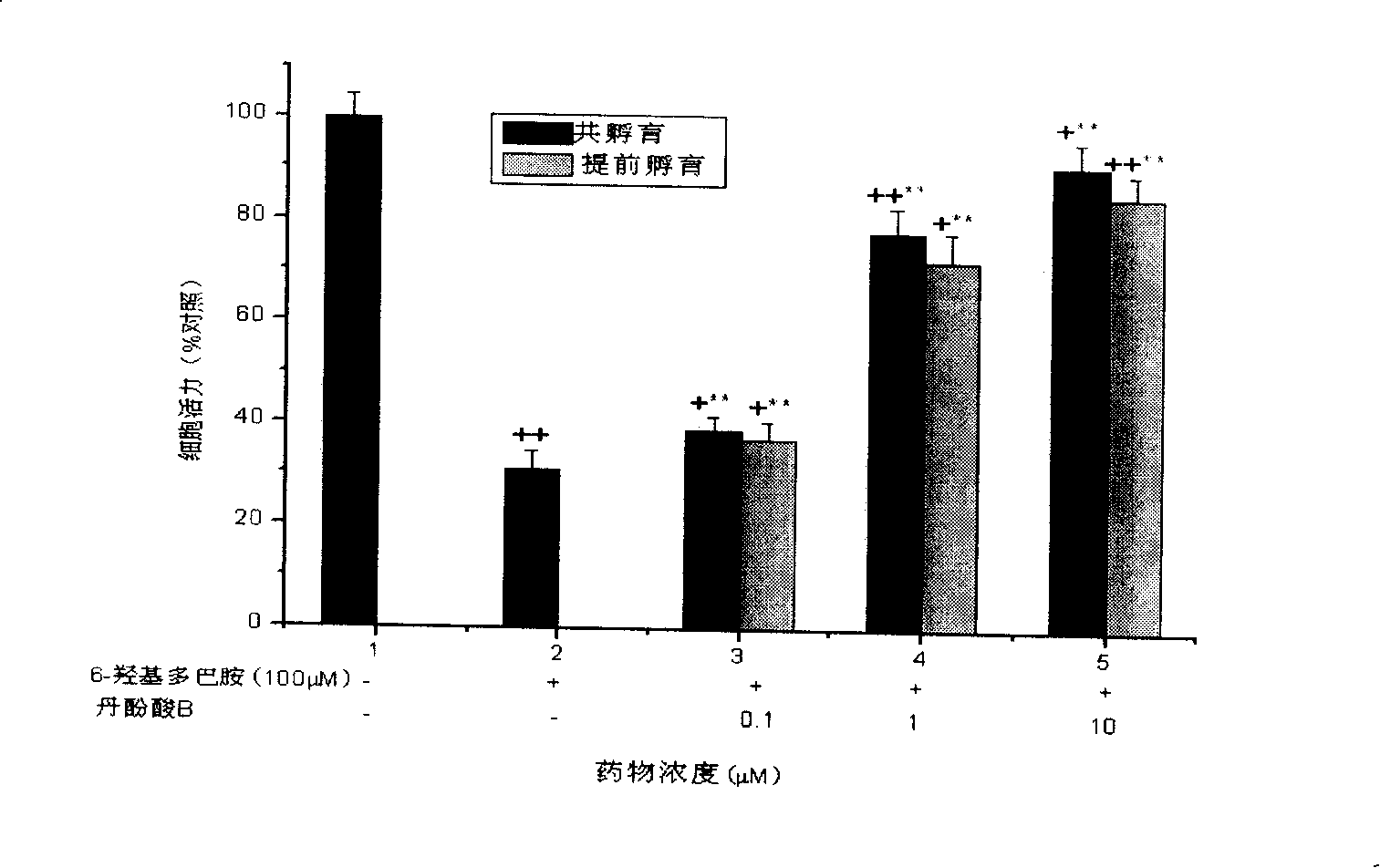
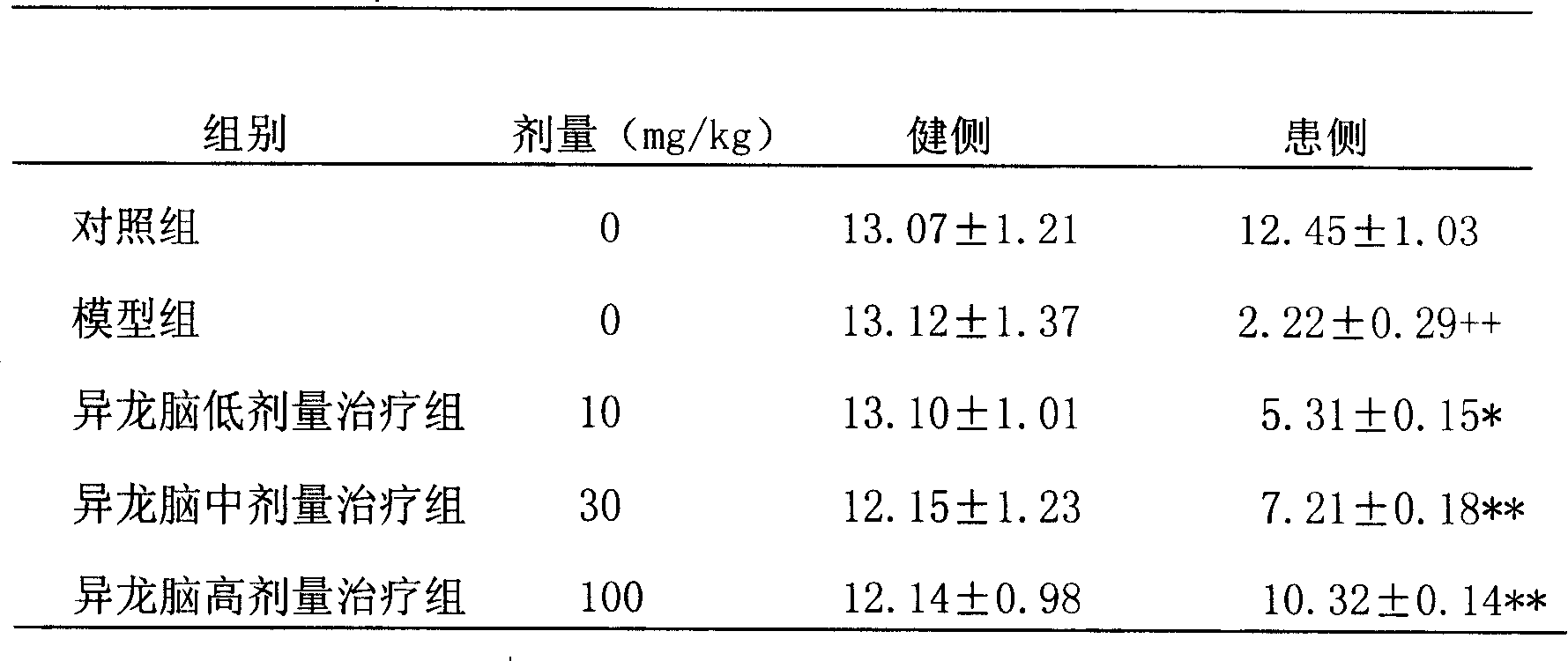
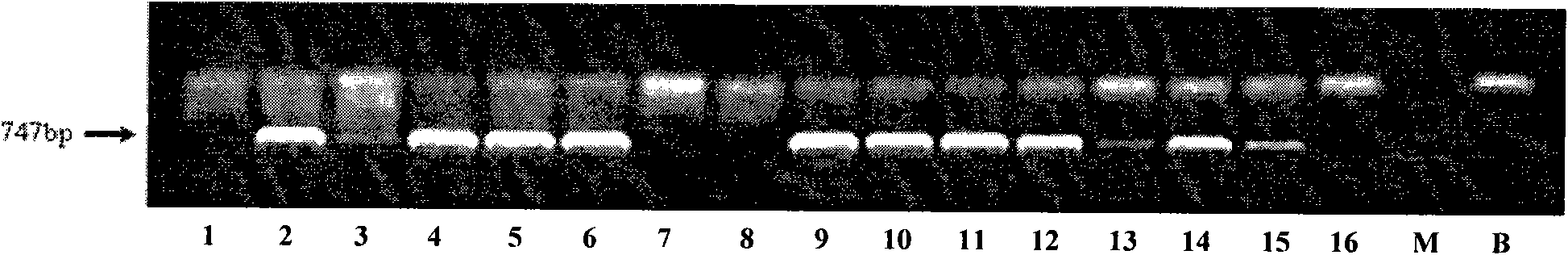
Salvianolic acid B is an active ingredient of Salvia miltiorrhiza, which has been widely applied in China for the management of various microcirculation-related disorders, such as cardiovascular disease, cerebrovascular disease, and diabetic vascular complication. For research use only.
Method for preparing salvianolic acid A by catalytically converting salvianolic acid B
InactiveCN102212004AShorten the timeHigh yieldOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid KSalvianolic acid B
The invention discloses a method for preparing salvianolic acid A by catalytically converting salvianolic acid B. The method is characterized in that the converted raw material is a salvia miltiorrhiza aqueous extract (salvianolic acid B=>50%) primarily purified through combined chromatography; the concentration of the raw material salvianolic acid B is 0.5-2%; urea is taken as the catalyst; the mole ratio of urea to the salvianolic acid B is 0.3-0.7; the conversion reaction temperature is 100-125 DEG C; and the reaction time is 3-6 hours. The method has the following beneficial effects: urea is taken as the catalyst, thus greatly shortening the time for which the salvianolic acid B is in easily destroyed state and remarkably increasing the yield of the salvianolic acid A; the primarily purified salvia miltiorrhiza extract is taken as the converted raw material, thus not only removing the metal ions which are not beneficial to conversion but also removing most colloid-like impurities and frontal impurities which are not beneficial to following separation of the salvianolic acid; and the directional conversion rate of the salvianolic acid B to the salvianolic acid A prepared by the method is not less than 10% and even reaches 60%.
Owner:SUZHOU LEINA PHARMA RES DEV +1
Process for preparing danshen salviandic acid
InactiveCN1425659AAvoid destructionHigh purityOrganic chemistryChromatographic separationSalvianolic acid B
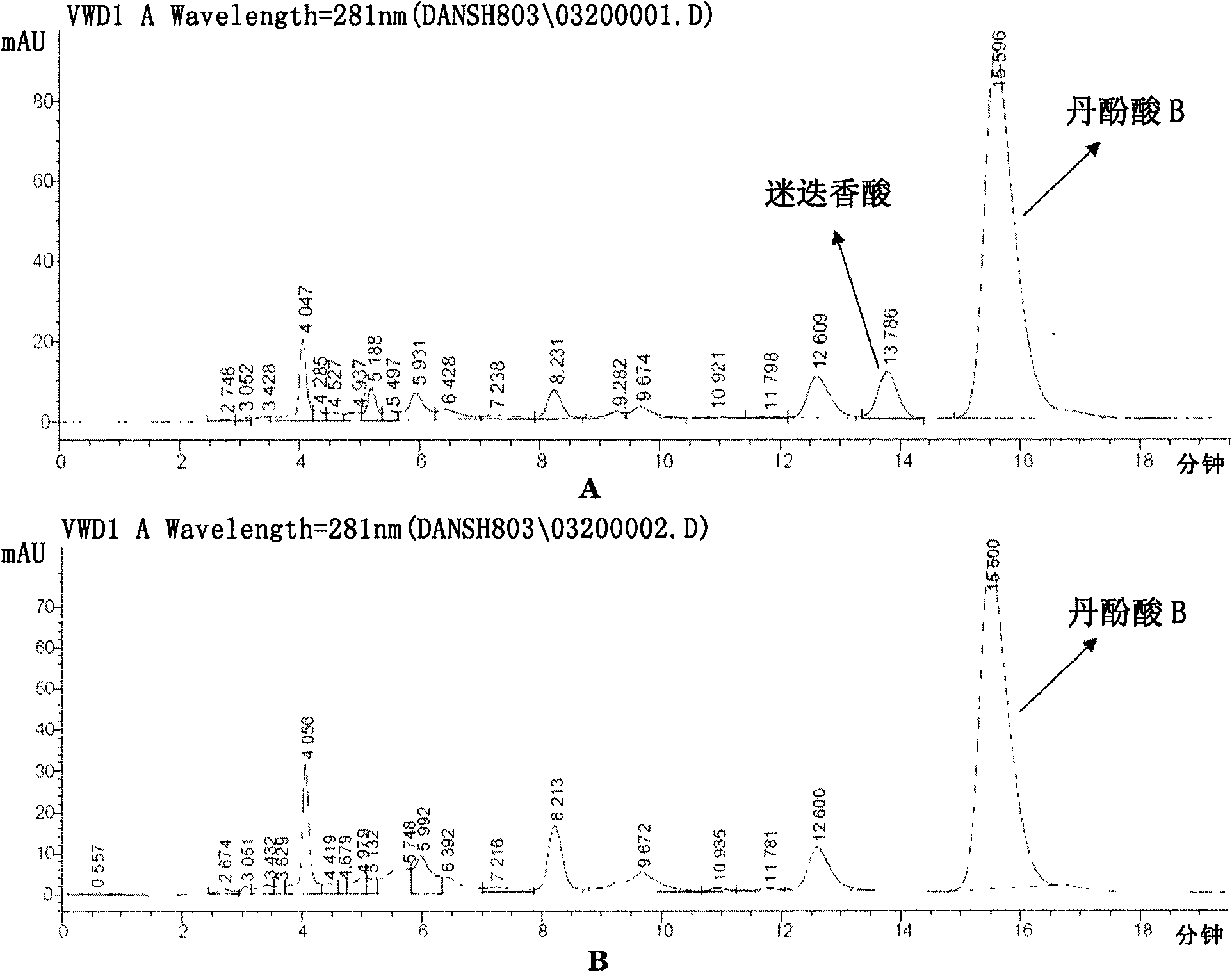
The present invention process of danshen salvianolic acid B includes the technological steps of: water extraction; acidifying extractive liquid; column chromatographic separation and purification of salvianolic acid B; and drying. The said process has high extraction rate and high purity, and the extracted product has salvianolic acid B content as high as 90% and stale quality. The present invention is suitable for large scale production.
Owner:江苏新本草医药研究院有限公司
Total tanshinone and total phenolic acid extract in red-rooted salvia root and its production
The invention is concerned with a kind of extract of total ketone of salviae miltiorrhizae and total phenolic acid and its produce method form radix Salviae Miltiorrhizae. The extract of total ketone of salviae miltiorrhizae has cryptotanshinone, tanshinone I, tanshinone IIA, methyl Tanshinon, dihydrotanshinon I and ramification. The extract of total phenolic acid has salvianolic acid A, salvianolic acid B, protocatechuic aldehyde and ramification. The extract can be got form one or arbitrary compound of extraction with solvent method, macroporous resin method, column chromatography and liquid-liquid counter-current chromatography. The summation of the content to each total ketone of salviae miltiorrhizae is 20 to 100 percnte (w / w) of the extract of total ketone of salviae miltiorrhizae, the contene of cryptotanshinone, tanshinone I and tanshinone IIA is 5 to 100 percent (w / w) of whole content of total ketone of salviae miltiorrhizae. The summation of the content to each total phenolic acid is 5 to 100 percent (w / w) of the extract of the radix salviae miltiorrhizae total phenolic acid. The content of salvianolic acid B is the 5 to 100 percent (w / w) of the whole salvianolic acid.
Owner:石任兵 +1
Method for extracting salvianolic acid B and hydroxysafflor Yellow A and preparation method of Danhong for injection
ActiveCN101168539AAvoid thermal decompositionFully extractedOrganic chemistryPlant ingredientsSalvianolic acid BSalvianolic acid
The invention discloses an extraction method of salvianolic acid B and hydroxy safflor yellow A as well as a preparation method of danhong for injection, and relates to the extraction method of the salvianolic acid and the hydroxy safflor yellow as well as the preparation method of the danhong preparation. The invention solves the problems that the extraction of the salvianolic acid B and the hydroxy safflor yellow A is not thorough, and the transportation and the storage of the prepared danhong injection are inconvenient. The salvianolic acid B of the invention is extracted according to the following steps: backflow, acidification and adsorption; thus the salvianolic acid B is obtained. The hydroxy safflor yellow A of the invention is extracted according to the following steps: boil out, acidification and adsorption; thus the hydroxy safflor yellow A is obtained. The danhong for injection is extracted according to the following steps: compounding ratio and standing, filtrating and lyophylization; thus the danhong for injection of the invention is obtained. The extraction rate of the salvianolic acid B and the hydroxy safflor yellow A of the invention is high, and the transportation and the storage of the prepared danhong injection are convenient.
Owner:哈药集团中药有限公司
Method for transforming danshinolic acid B into tanshinol
ActiveCN102701956AIncrease contentOrganic compound preparationCarboxylic compound preparationSalvianolic acid BPhases of clinical research
The invention relates to tanshinol, in particular to transformation of danshinolic acid B in a red-rooted salvia root-extracted extract into tanshinol. At an extract sterilizing stage, danshinolic acid B in a red-rooted salvia root extract containing mixed components of danshinolic acid B and tanshinol is completely transformed into tanshinol at one time under a high temperature condition. The tanshinol content of a preparation prepared from the red-rooted salvia root extract which is transformed at a high temperature is kept stable before and after sterilization. Due to the adoption of a method disclosed by the invention, an extract of tanshinol is taken as a target component, a purifying process in an extracting process is reduced, and the content of target tanshinol is increased greatly. The equipment investment is small, and the method is suitable for industrial production.
Owner:GUIZHOU JINGFENG INJECTION
Ultrafine wall-breaking grinding method for salviae miltiorrhizae
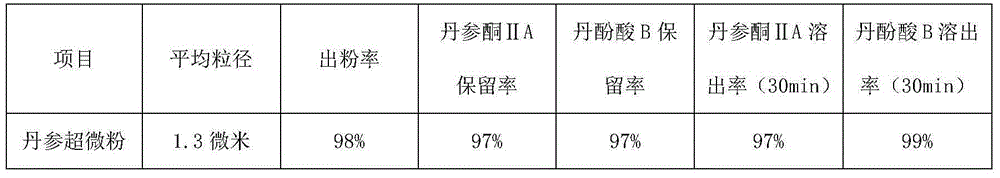
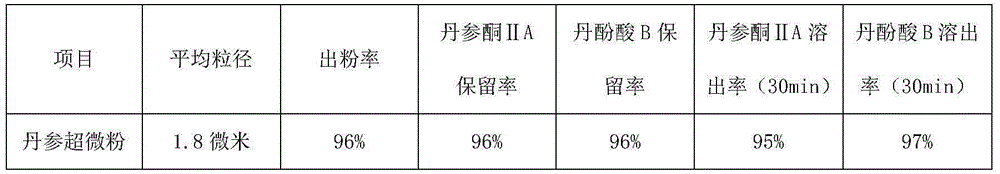
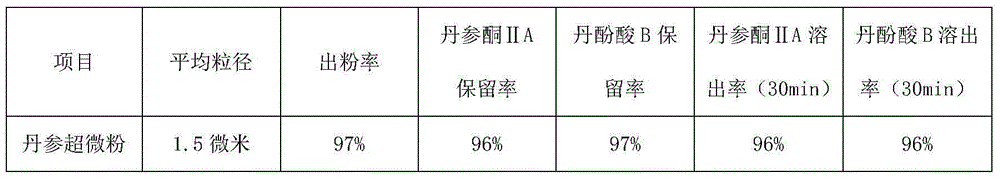
ActiveCN105030896AShort processing timeIncrease powder yieldPowder deliveryPharmaceutical non-active ingredientsSalvianolic acid BMetallurgy
The invention provides an ultrafine wall-breaking grinding method for salviae miltiorrhizae, and belongs to the field of traditional Chinese medicines. The ultrafine wall-breaking grinding method for salviae miltiorrhizae provided by the invention comprises the following steps: (1) conventionally crushing salviae miltiorrhizae, and filtering through an 80-100mesh sieve to obtain salviae miltiorrhizae powder; (2) mixing sorbitol with phenylalanine evenly, wherein the mass ratio of the sorbitol to the phenylalanine preferably is 1 to 1, and obtaining sorbitol-phenylalanine mixed powder; and (3) mixing the salviae miltiorrhizae powder with the sorbitol-phenylalanine mixed powder evenly, wherein the mass ratio of the salviae miltiorrhizae powder to the sorbitol-phenylalanine mixed powder preferably is (200-100) to 1, crushing the powder until the mean grain size is smaller than 2 microns by an ultrafine grinder, so as to obtain ultrafine salviae miltiorrhizae wall-broken powder. Compared with a conventional ultrafine wall-breaking grinding method, the ultrafine wall-breaking grinding method for salviae miltiorrhizae provided by the invention has the advantages of short processing time, high powder extraction rate, high retention rate and dissolution rate of tanshinone IIA and salvianolic acid B, and small mean grain size of powder.
Owner:深圳市盛元堂生物科技有限公司
Method for separating and purifying salvianolic acid from red sage root liquid extract by one step
InactiveCN101186572ACarboxylic compound separation/purificationPlant ingredientsProcess dynamicsSalvianolic acid B
The invention relates to a method for furthering separating and purifying salvianolic acid from Danshen extract fluid, which comprises that prepares, breaks and extracts Danshen via water solution, acidifies extract to adjust pH and adds salt to process post-treatment, processes dynamic continuous adsorption and elution on the treated extract at chromatography column stuffed with resin adsorbent, elutes via water, collects and concentrates eluent, elutes via gradient ethanol solution, segmented collects and concentrates eluent, and dries the concentrates solution to obtain product. The invention can simply, effectively and quickly separate and purify various salvianolic acids, wherein test on different salvianolic acid products shows that the highest yields of tanshinol, alkannic acid, rosmarinic acid, salvianolic acid A and salvianolic acid B are 35. 55%, 70.65%, 99.27%, 82.78%, and 89.34%, and relative highest purities are 95.32%, 65.05%, 29.40%, 33.93%, and 82.35%, which are near or higher than the result of purification on the goal of single component.
Owner:TIANJIN UNIV
Method for simultaneously detecting main components of Naoxintong capsule in plasma
ActiveCN104614456AInhibit aggregationImprove neurological deficitsComponent separationAstragalosideSalvianolic acid B
The invention provides a method for simultaneously detecting main components of paeoniflorin, beta ecdysterone, laetrile, mulberroside A, caffeic acid, ferulic acid, salvianolic acid B, astragaloside, formononetin, cryptotanshinone and tanshinone IIA of a Naoxintong capsule in a plasma sample by a liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). In a liquid chromatogram, a mobile phase consists of acetonitrile and a formic acid aqueous solution of which the volume fraction is 0.1%, and gradient elution is used. A mass spectrum uses a quick positive and negative ions switching and analyzing mode and an MRM (Multiple Reaction Monitoring) scanning manner. After the Naoxintong capsule is taken, the situations of the changes of the blood-medicine concentration of several kinds of main components in the plasma of a rat are detected at the same time. The methodological survey results indicate that the established method conforms to determination requirements on biological samples in a body; the method is good in sensitivity, high in specificity, stable, reliable, and suitable for detecting substances with lower contents.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
Medicinal composition and application thereof
ActiveCN102908355AOrganic active ingredientsCardiovascular disorderVascular diseaseSalvianolic acid B
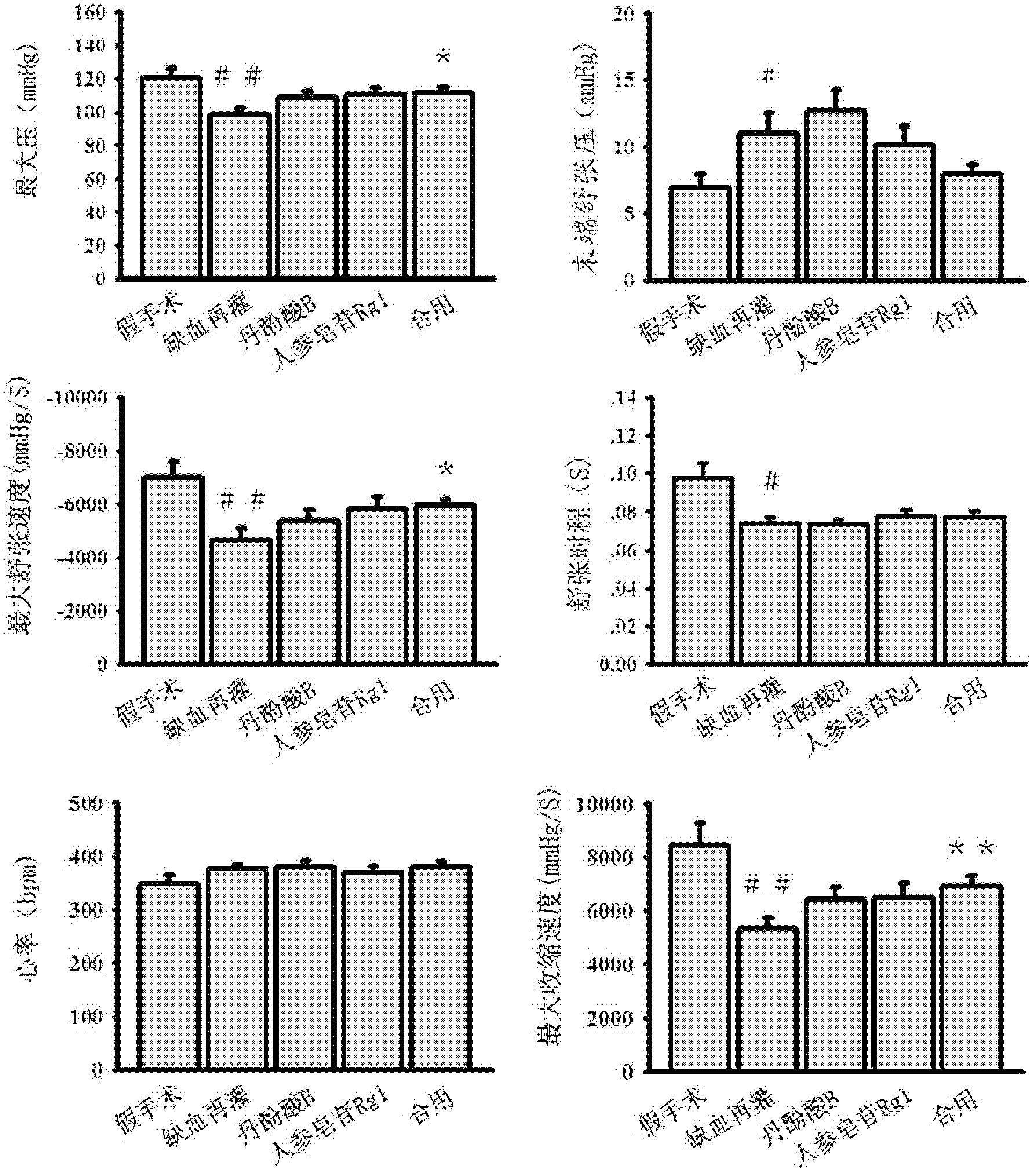
The invention discloses a medicinal composition, which is mainly prepared from compound salvianolic acid B and ginsenoside Rg1 according to a certain weight part ratio. The medicinal composition has a protection effect on cardiac muscle in myocardial ischemia and reperfusion and can be used for treating cardiovascular diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Technical method for extracting salvianolic acid B from radix salivae miltiorrhizae
The invention is a technical method to extract salvianolic acid B from tanshen, organically combining several methods to extract salvianolic acid B, to develop a new technique proper to separate and purify salvianolic acid B. It firstly extracts by water, then flocculates by chitosan, successively makes alcohol precipitation, then extracts by petroleum ether and acetic ester, respectively, and finally adopts silica gel column chromatography to obtain higher purity salvianolic acid B. It is applied to industrialized production.
Owner:EAST CHINA UNIV OF SCI & TECH
Quality detection method for compound prepn of red sage and notoginseng
InactiveCN1772041ASimple methodGood precisionComponent separationBlood disorderHplc dadSalvianolic acid B
The present invention relates to Chinese medicine quality detecting technology, and is especially the quality detection method for compound preparation of red sage and notoginseng. HPLC-DAD process is adopted to measure the contents of protocatechuic aldehyde, salvianolic acid B, cryptotanshinone, neotanshinone IIA, arasaponin R1, ginsenoside Rg1 and ginsenoside Rb1 in compound red sage preparation simultaneously. The process is simple, precise, repeatable and reliable, and may be used in the quality control of compound red sage preparation.
Owner:CHINA PHARM UNIV
Pharmaceutical composition for treating senile dementia
ActiveCN1879697AOrganic active ingredientsNervous disorderSalvianolic acid BHigh volume manufacturing
Disclosed is a medicinal composition for treating senile dementia, wherein 1g of the dried active sites comprises tanshinone IIA 1.79-9.01mg, salvianolic acid B 35.87-225.23mg, danshensu 4.93-45.05mg, protocatechuic aldehyde 0.10-4.50mg, and notoginseng saponin R 13.14-22.50mg.
Owner:GUANGZHOU BAIYUSN HUTCHISON WHAMPOA CHINESE MEDICINE
Method for improving yield of salvianolic acid B in savia miltiorrhiza suspension culture cells by inducing
InactiveCN102428871ASimple processShort induction timePlant tissue cultureHorticulture methodsBiotechnologySalvianolic acid B
The invention discloses a method for improving yield of salvianolic acid B in savia miltiorrhiza suspension culture cells by inducing, specifically comprising the following steps: 1) cultivating the aseptic seedlings; 2) inducing the callus; 3) sub-cultivating the callus; 4) cultivating the callus by suspending; 5) inducing to produce the salvianolic acid B; and 6) harvesting the culture cells. The method solves the problem that the salvianolic acid B is not produced after the inducing factors, such as yeast extract, galacturonic acid, mycelia extract and the like are added. The content of the salvianolic acid B in the savia miltiorrhiza suspension culture cells is increased by about 5 times by using the method. The technology condition provided by the invention is suitable for industrial production of the salvianolic acid B on a large scale by a cell culture method after being expanded.
Owner:NORTHWEST A & F UNIV
Separating preparation process of salvianolic acid B
ActiveCN1995027ANo lossShort separation timeOrganic chemistryPlant ingredientsAlcoholSalvianolic acid B
The invention discloses a separating method of salviol acid in the salvia miltiorrhizae extract, which is characterized by the following: grinding medicine materical crude slice of radix salvia miltiorrhizae; extracting through alcohol; sucking; decompressing filtrate; condensing; adding water in the condensate; adjusting pH value through alkaline to 7. 5; extracting through ether; adjusting pH value through water to 3; extracting through ether; recycling solvent; obtaining red powder-shaped rough extract of radix salvia miltiorrhizae; applying counter-current chromatographic fractionation method of pH value band to make salviol acid B.
Owner:山东蓝城分析测试有限公司
Method for preparing red sage root salviandic acid A
InactiveCN101311160AOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid BSalvianolic acid A
The invention discloses a preparation method for salvianolic acid A in salvia miltiorrhiza and is characterized in that the method takes salvianolic acid B in the salvia miltiorrhiza as the raw material and converts the salvianolic acid B into the salvianolic acid A in the salvia miltiorrhiza after adjusting pH value to 3.5-6.0 and chemical reaction, thus greatly increasing the extraction yield of the salvianolic acid A; the pharmacological tests prove that a preparation prepared by the salvianolic acid A in the salvia miltiorrhiza has excellent pharmacological action.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Compound red sage root freezing-dried powder injection containing salvianolic acid B and its preparation method
InactiveCN101085000AIncrease workIncrease cardiac outputPowder deliveryHydroxy compound active ingredientsSalvianolic acid BFreeze-drying
The invention provides a compound red sage root freeze dried powder injection containing salvianolic acid B and its preparing process, wherein the injection comprises root of red rooted saliva, notoginseng and borneol, and is prepared through extracting root of red rooted saliva and notoginseng to obtain red sage root extract, pseudo-ginseng extract, dissolving baras camphor with right amount of ethanol, coating with beta-cyclodextrin, charging injection for water into red sage root extract, notoginseng or red sage root and notoginseng extracts for dissolving, boiling, cooling down, filtering, mixing filtrate with baras camphor beta-CD inclusion solution, charging mannitol, adding right amount of water for injection, adjusting pH, and freeze-drying. In the obtained freeze dried injection, calculated by salvianolic acid B (C36H30O16), the content of red sage root 6.25-100.00mg / g freeze-dried powder solid.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Extraction, purification and preparation method of high-purity salvianolic acid B
The invention discloses an extraction, purification and preparation method of high-purity salvianolic acid B. The method comprises the following steps: (1) smashing a salvia miltiorrhiza medical material into fine powder, and screening with a sieve of 20-60 meshes; (2) adding an ethanol solution, soaking at the temperature below 5 DEG C, and carrying out ultrasonic extraction, wherein the extraction process temperature is controlled below 25 DEG C; (3) centrifuging extract, filtering, recovering ethanol at reduced pressure until the extract has no alcohol odor, concentrating the extract and filtering; (4) clarifying the extract, enriching through macroporous resin, and purifying through chromatographic column chromatography so as to obtain salvianolic acid B fraction; and (5) carrying out vacuum drying or freeze drying so as to obtain extractive. In the new process disclosed by the invention, cost is low, only ethanol is used as an organic solvent, and more than 90% of salvianolic acid B can be obtained through separation and purification, thus the method disclosed by the invention is suitable for industrial production.
Owner:GUANGZHOU HANFANG PHARMA
Injection formulation containing raw material herb red sage root and its quality control method
ActiveCN1788748AEnsure safetyGuaranteed validityOrganic active ingredientsCardiovascular disorderSalvianolic acid BMedicine
The present invention is one kind of injection containing medicine material red sage, and each preparation unit contains salvianolic acid A 0.5-7.5 mg and salvianolic acid B 0.5-7.5 mg. The present invention also provides the quality control method for the injection, and the contents of salvianolic acid A and salvianolic acid B are controlled to ensure the safety and effectiveness of the injection. The detection method is advanced, accurate, simple and suitable for conventional analysis. The present invention is significant in controlling the quality of different kinds of injection containing red sage component.
Owner:YAAN THREE NINE PHARMA
Method for preparing high-purity danshinolic acid B
InactiveCN101638401AHigh purityEfficient removalOrganic chemistryMicroorganism based processesSalvianolic acid BAqueous solution
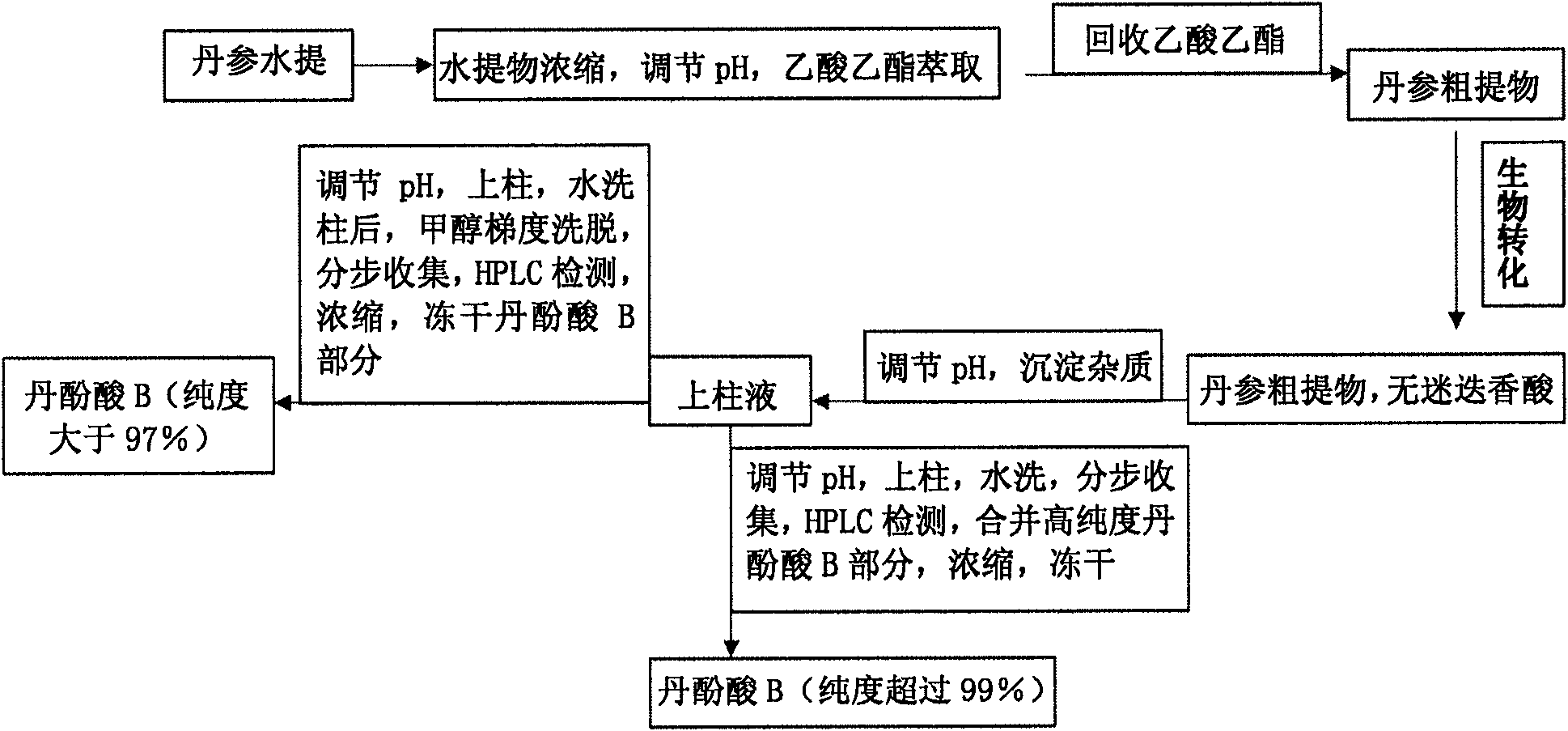
The invention discloses a method for preparing high-purity danshinolic acid B, which comprises the following steps: (1) mixing a red sage root aqueous extract with Fusarium graminearum resting cells,and performing biotransformation at a temperature of between 20 and 35 DEG C for 24 to 90 hours to obtain a red sage root aqueous extract without rosmarinic acid; and (2) adsorbing the red sage root aqueous extract without the rosmarinic acid by anti-phase resin, and eluting the red sage root aqueous extract without the rosmarinic acid to obtain the high-purity danshinolic acid B. The purity of the danshinolic acid B prepared by the method is more than or equal to 99 percent, the recovery percent is over60 percent, the process is simple, and the cost is low.
Owner:SHANGHAI INST OF PHARMA IND
Establishment method of traditional Chinese medicine composite fingerprint
The invention provides an establishment method of a traditional Chinese medicine composite fingerprint, and particularly relates to an establishment method of a Weilikang granule fingerprint. Pure water is added to Weilikang granules, so as to dissolve, and paeoniflorin, naringin, rutin, salvianolic acid B, rosmarinic acid, emodin-8-O-beta-D glucopyranoside and glycyrrhizic acid are used as reference substances, so as to establish the fingerprint by referring to high-performance liquid chromatography. The method is more comprehensive and objective than the current quality standard, and the current quality standard is increased and improved, and more information is provided for the internal quality control of the Weilikang granules.
Owner:SICHUAN LVYE BAO GUANG PHARMA IND
Preparation method of high-content salvianolic acid B
ActiveCN104130226ACreativeReduce manufacturing costOrganic active ingredientsOrganic chemistryInorganic saltsOrganic solvent
The invention discloses a preparation method of a high-content salvianolic acid B. The method comprises the following two steps: separating total salvianolic acids and preparing the high-content salvianolic acid B. According to the method, water phase is selectively removed via a method of organic solvent extraction without affecting on water-soluble components, and the rest of water phase is acidized and then extracted, so that the water-soluble components with high polarity such as inorganic salt, tannin, sugar and protein and can be selectively removed; finally, the high-content total salvianolic acid is obtained. Based on the obtained total salvianolic acid, salvianolic acid B product having content being more than 95% is finally obtained with the final yield about 3% by carrying out the steps of regulating pH of the total salvianolic acid solution within 6.0 to 8.0, separating the total salvianolic acid solution via macroporous resin and the like. The method is simple to develop, effective and low in cost, and has an industrial value.
Owner:王萍
Method for preparing salvianolic acid B and rosmarinic acid by adopting high-speed counter-current chromatography separation and purification process
ActiveCN104031013ASimple and fast operationImprove efficiencyCarboxylic acid esters separation/purificationChromatographic separationSalvianolic acid B
The invention discloses a method for preparing salvianolic acid B and rosmarinic acid by adopting a high-speed counter-current chromatography separation and purification process. The method comprises the following steps: grinding salvia miltiorrhiza, performing ethanol extraction, enriching through macroporous adsorption resin to obtain a crude extract, and performing high-speed counter-current chromatography separation on the crude extract by a high-speed counter-current solvent system consisting of solvents A, B, C and D to prepare salvianolic acid B and rosmarinic acid respectively, wherein the volume ratio of the solvent A to the solvent B to the solvent C to the solvent D is (1-5) to (5-10) to (1-5) to (1-10); the solvent A is methanol, ethanol, n-hexane or iso-hexane; the solvent B is ethyl acetate, propyl acetate or n-butyl acetate; the solvent C is methanol, ethanol or acetone; the solvent D is water; an upper phase is a stationary phase; a lower phase is a mobile phase. According to the method, the salvianolic acid B and the rosmarinic acid are prepared by adopting the high-speed counter-current chromatography process, inreversible adsorption is not existent, and the sample loss is avoided; the method has the characteristics of having a good separation effect and being low in solvent consumption, pollution-free, efficient and quick.
Owner:ANHUI YONSENT PHARMA
Method for preparing salvianolic acid B magnesate
The invention provides a manufacturing method of red phenolic acid B magnesium salts. Crushes the radix salviae miltiorrhizae, immerses and extracts with alcohol containing water or acetone, condenses, eliminates the sediment. Then uses macropore to absorb resin chromatography, washes them with water, then wash with alcohol. The coarse product is obtained after condensed, then carries on chromatography with polyamide, and washes with water or alcohol. There gets the 90% purity product through condensation, cooling, drying.
Owner:上海天甲生物医药有限公司 +1
Use of salvianolic acid B and its salt in treating parkinson's disease
InactiveCN101239057AWide variety of sourcesRaw materials are easy to getOrganic active ingredientsNervous disorderSalvianolic acid BParkinsonism
The invention relates to a new usage of Salvianolic acid B and the salt used in curing parkinsonism, the usages of the medicine combined material of the Salvianolic acid B as well as the salt, and the medicine combined material or each unit being jointly used with other current medicines curing parkinsonism used for curing parkinsonism.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
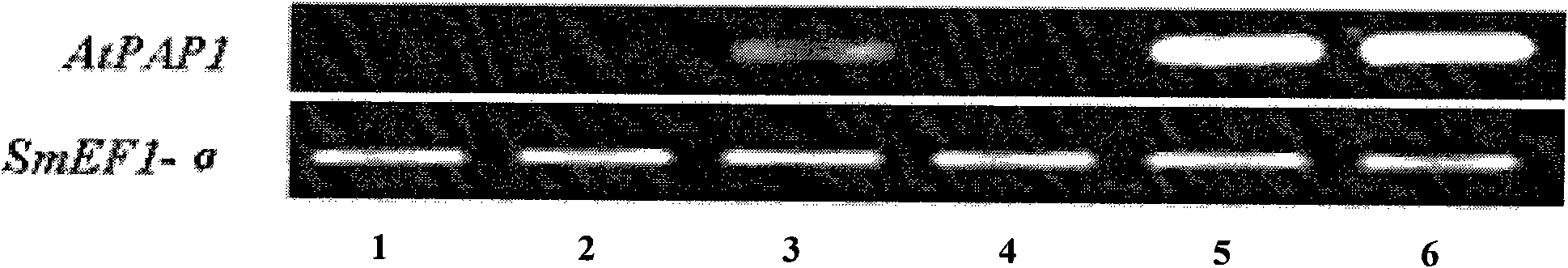
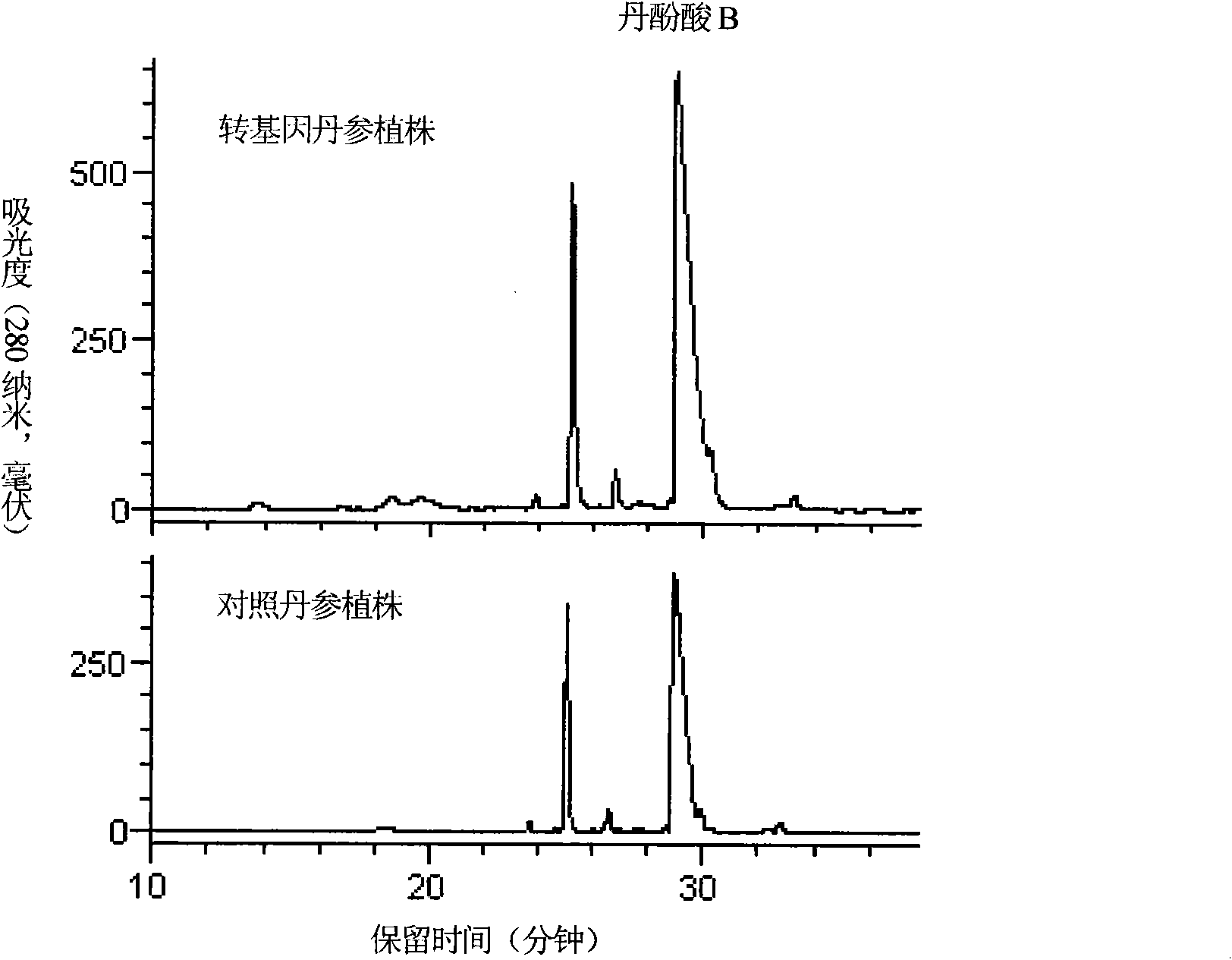
Transgenic method for improving salvianolic acid B content in root of red-rooted salvia
InactiveCN102061297AIncreased salvianolic acid B contentComponent separationMicrobiological testing/measurementSalvianolic acid BBiology
The invention discloses a transgenic method for improving salvianolic acid B content in root of red-rooted salvia, which comprises the following steps of: obtaining cDNA overall length of arabidopsis anthocyanidin generation transcription factor 1 gene through gene cloning; constructing a plant expression vector containing the genes; transforming agrobactrium tumefaciens EHA105 by using the vector so as to obtain an agrobactrium tumefaciens strain containing the genes; transforming the root of red-rooted salvia by using the agrobactrium tumefaciens strain, and culturing to obtain a transgenicplant of the root of red-rooted salvia detected through a polymerase chain reaction; detecting the expression of the arabidopsis anthocyanidin generation transcription factor 1 gene in the transgenicroot of red-rooted salvia through a semi-quantitative reverse transcription-polymerase chain reaction; and performing high performance liquid chromatography on the salvianolic acid B content in the transgenic root of red-rooted salvia subjected to the gene expression, and screening to obtain a transgenic plant of the root of red-rooted salvia with the improved salvianolic acid B content. Due to the obtained transgenic plant of the root of red-rooted salvia, the salvianolic acid B content is 73.27mg / g in dry roots when the transgenic plant of the root of red-rooted salvia grows for 165 days, wherein the salvianolic acid B content is two times of the salvianolic acid B content in non-transformed common dry roots of the root of red-rooted salvia.
Owner:SHAANXI NORMAL UNIV
Method for pre processing sample in use for measuring concentration of salvianolic acid B, in biologic body fluid
InactiveCN1959409AMeet the requirements of the analysisHigh sensitivityComponent separationOther chemical processesPretreatment methodSalvianolic acid B
A method for pre-treating analysis sample of red phenolic acid B concentration in body fluid on living beings includes utilizing water- methanol to spray-wash then utilizing aqueous ammonia-methauol to elute Waters Oasis HLB small column obtained by activating sample containing red phenolic acid with water and methanil, enriching eluted liquid, using high efficiency of chromatography to carry out pretreatment on living beings sample containing red phenolic acid B after enriched matter is redissloved by flowing phase of 1% trifluoroacetic acid: methanol: acetonitrile.
Owner:SHANGHAI UNIV OF T C M
Traditional Chinese medicine active part composition for curing cardio and cerebral vascular disease and its preparing method
InactiveCN1425430AGood treatment effectMyocardial infarct size improvedUnknown materialsCardiovascular disorderClinical efficacySalvianolic acid B
The composition of Chinese medicine effective parts for treating cardiac and cerebral vascular diseases consists of general salvianolic acid and general astragalus saponin in certain ratio. The compound medicine has raised pharmacological effect and clinical curative effect and reduced impurity content. It may be prepared into various preparation forms. It is used in treating coronary heart disease, angina pectoris, myocardial ischemia, heart failure, arrhythmia, cerebral infarction and cerebral ischemia; and is safe, effective stable, controllable and convenient.
Owner:山东希尔康泰药业有限公司
Preparation method and quality control method of traditional Chinese medicine Danmo capsule
ActiveCN102210781AAnthropod material medical ingredientsComponent separationMedicinal herbsSalvianolic acid B
The invention relates to a preparation method and a quality control method of a traditional Chinese medicine Danmo capsule for treating chronic kidney disease. The prescription of the traditional Chinese medicine compound preparation is composed of 9 traditional Chinese medicines, namely yerbadetajo, glossy privet fruit, salvia root, honeysuckle, motherwort, rehmannia root, madder, wolfberry bark and scorpion. The preparation method of the Danmo capsule comprises the steps of: pretreatment by water extraction and alcohol precipitation, forming of capsule content particles by one-step granulation, encapsulation, sub-packaging and external packaging. In the quality control method, thin layer identification of the yerbadetajo, the glossy privet fruit, the salvia root and the honeysuckle is established, and the content of salvianolic acid B is determined by high performance liquid chromatography. Compared with the original preparation process and quality standard, the traditional Chinese medicine Danmo capsule prepared in the invention is the I capsule, so that the compliance of patients in administration is improved, the moisture resistance of the capsule is improved, the content of the effective component salvianolic acid B is also greatly improved, and the quality standard of the preparation is improved.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Qianliexin capsule quality evaluation method based on multi-index active ingredient measurement
ActiveCN106353430AQuality reflectionQuality assuranceComponent separationHplc fingerprintChlorogenic acid
The invention discloses a Qianliexin capsule quality evaluation method based on multi-index active ingredient measurement. The Qianliexin capsule quality evaluation method is characterized by comprising the following steps: on the basis of an efficient liquid chromatography-electrospray time of flight mass spectrum technology, measuring eight active ingredients in the Qianliexin capsule, and constructing a Qianliexin capsule HPLC (High Performance Liquid Chromatography) fingerprint chromatogram, wherein the eight active ingredients are respectively gallic acid, chlorogenic acid, caffeic acid, vaccarin, isoquercitrin, salvianolic acid B, salvianolic acid A and cryptotanshinone. The efficient liquid chromatography-electrospray time of flight mass spectrum technology is simultaneously adopted to measure the eight active ingredients in the Qianliexin capsule and construct the Qianliexin capsule HPLC fingerprint chromatogram, and meanwhile, the Qianliexin capsule quality evaluation method is used for evaluating the quality of a Qianliexin capsule medicine, and the eight active ingredients and the chromatogram realize mutual corroboration so as to more comprehensively reflect the quality of the Qianliexin capsule medicine to be favorable for researching and guaranteeing the quality of Qianliexin capsule medicine raw materials and preparations.
Owner:SHANDONG ANALYSIS & TEST CENT
Carrier capable of simultaneously improving rosmarinic acid and salvianolic acid B content of red sage root and use thereof
InactiveCN103834685AIncrease contentFermentationVector-based foreign material introductionBiotechnologySalvianolic acid B
The invention discloses a carrier capable of simultaneously improving rosmarinic acid and salvianolic acid B content of red sage root and a use thereof. The carrier contains an over-expressed AtPAP1 transcription factor and can realize RNA interference of a SmCOMT gene. Dried root of three-month-old of the transgenic red sage root plant obtained by the carrier has rosmarinic acid content of 9.59+ / -0.36mg / g which is 1.15 times that of dried root of the same old of the common red sage root plant, and salvianolic acid B content of 55.87+ / -0.59mg / g which is 1.79 times that of the dried root of the same old of the common red sage root plant.
Owner:SHAANXI NORMAL UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com