Injection formulation containing raw material herb red sage root and its quality control method
A quality control method and injection technology, applied in the field of traditional Chinese medicine, can solve problems such as insufficient stability, and achieve the effects of ensuring safety, advanced detection methods, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 salvianolic acid A and salvianolic acid B reference substance
[0040]Take 5 kg of Salvia miltiorrhiza, crush it, add four times the amount of water to decoct for 1 hour, let it cool, filter, decoct twice in the same way, combine the filtrate, concentrate to about 5 liters, add ethanol until the alcohol content is 75%, left overnight, filtered, took the filtrate, evaporated ethanol, extracted 3 times with chloroform, and discarded the chloroform solution. The aqueous layer was adjusted to pH 3 with hydrochloric acid, extracted three times with ethyl acetate, the ethyl acetate solution was combined, and the solvent was evaporated to obtain a yellow crystalline powder, which was the crude product.
[0041] The crude product was separated by silica gel column chromatography, the mobile phase was chloroform-ethyl acetate-formic acid (volume ratio 3:2:0.4), collected in sections, monitored by thin-layer chromatography, and the sections of the ...
Embodiment 2
[0058] The assay method of embodiment 2 salvianolic acid A and salvianolic acid B
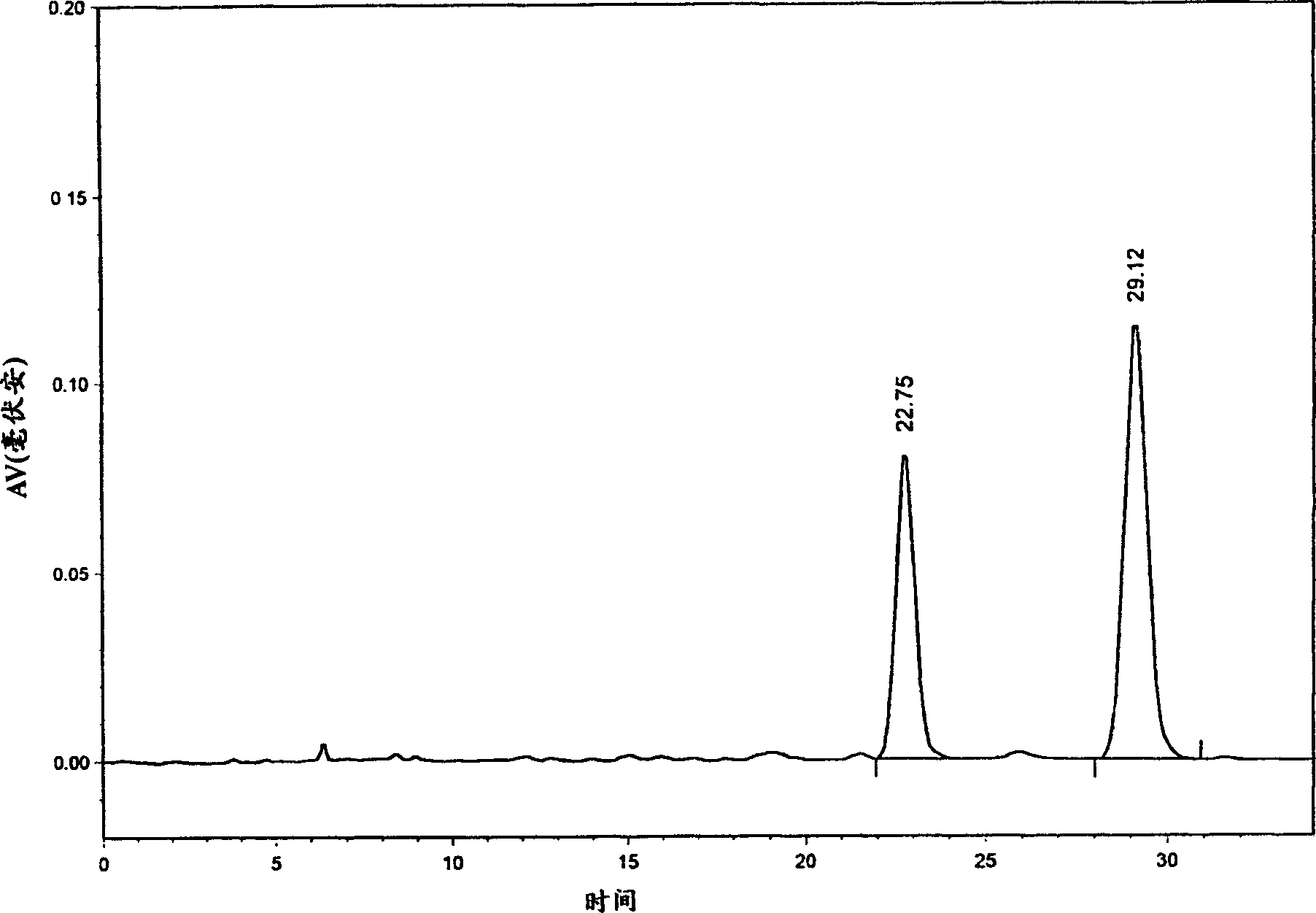
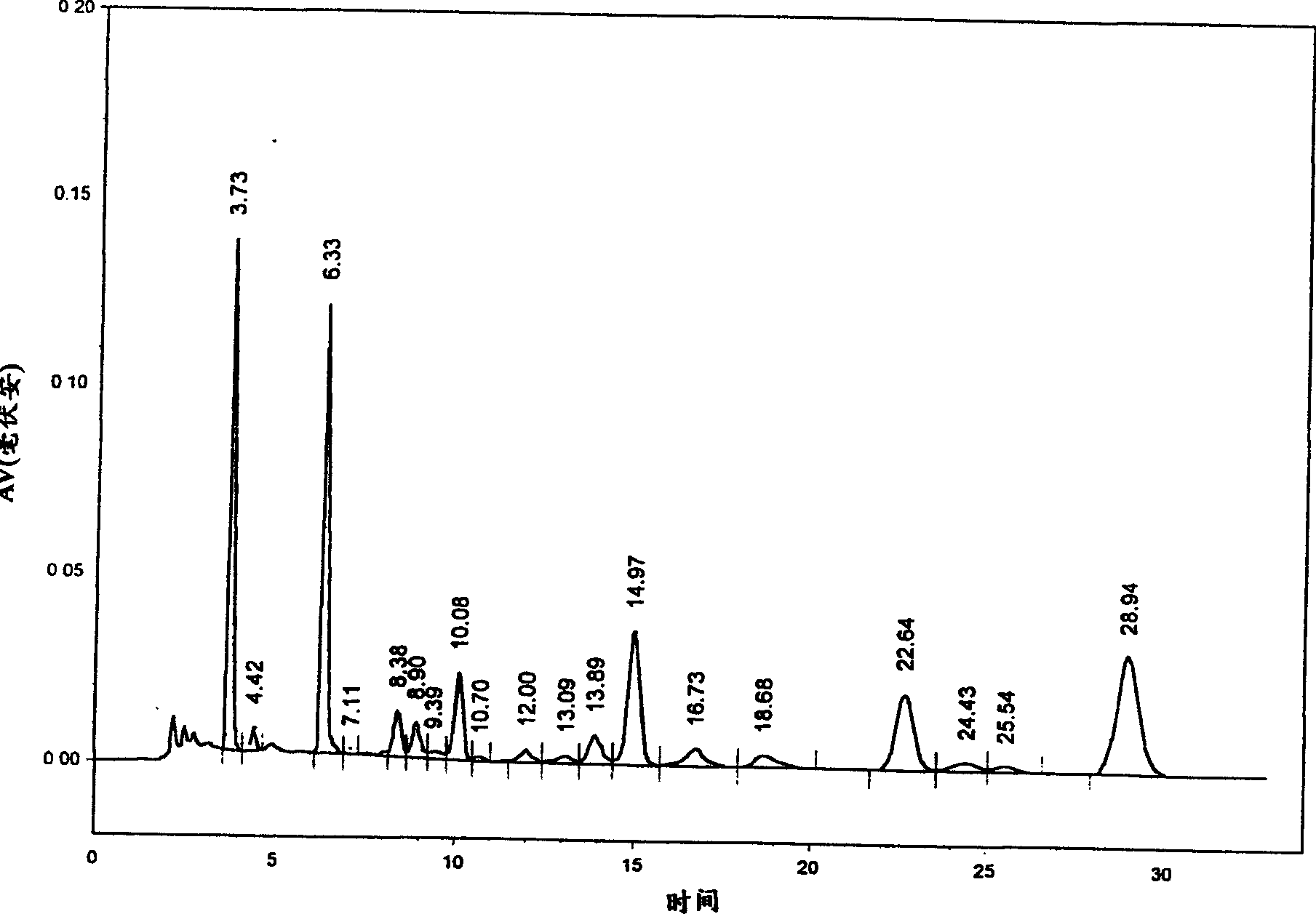
[0059] Chromatographic conditions: use Inertsil C8-3 column 5um (250×4.6mm); acetonitrile-water-formic acid (volume ratio 24:76:0.8) as mobile phase; column temperature 25°C; flow rate: 1.0ml / min; detection wavelength: 280nm.
[0060] Preparation of reference solution: Accurately weigh about 5 mg of salvianolic acid A reference substance and salvianolic acid B reference substance respectively, put them in the same 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark.
[0061] Preparation of the test solution: Precisely measure 2ml of Xiangdan injection or Danshen injection, put it in a 25ml measuring bottle, add mobile phase to dilute to the mark, shake well, filter, and take the filtrate for later use.
[0062] Determination method: Accurately draw 10 μl each of the reference substance solution and the test solution, inject it into the liquid chromatograph, calculate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com