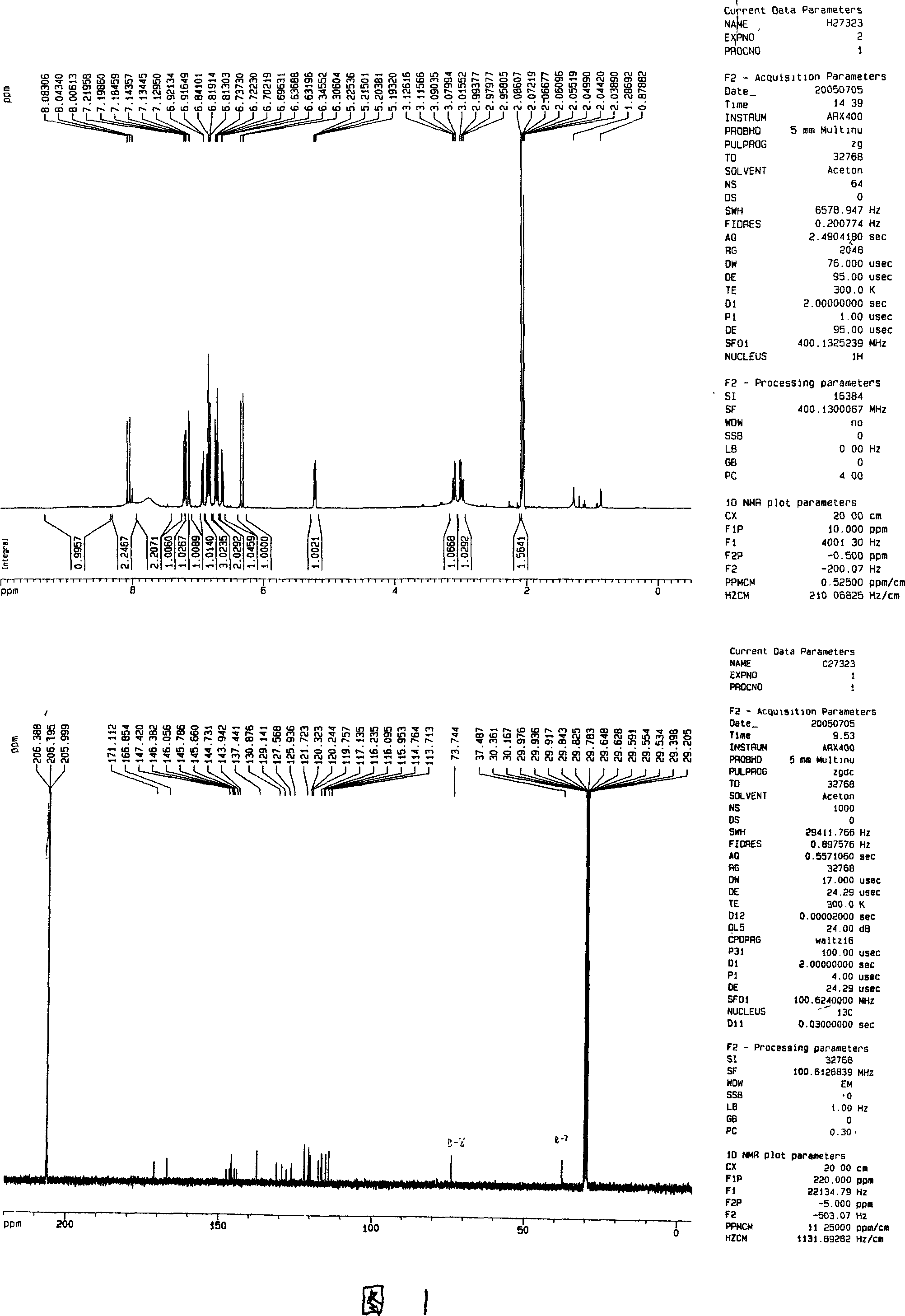
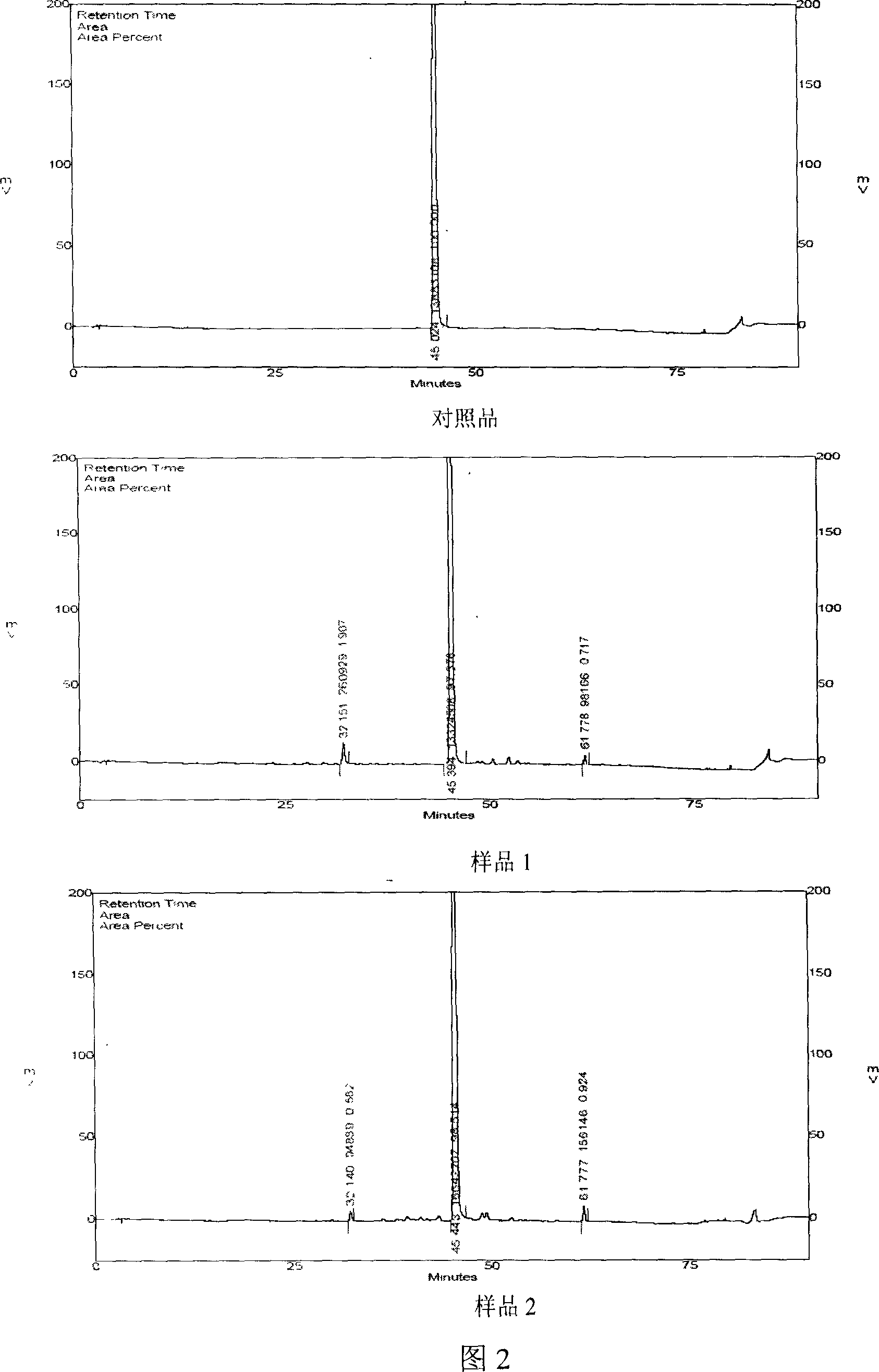
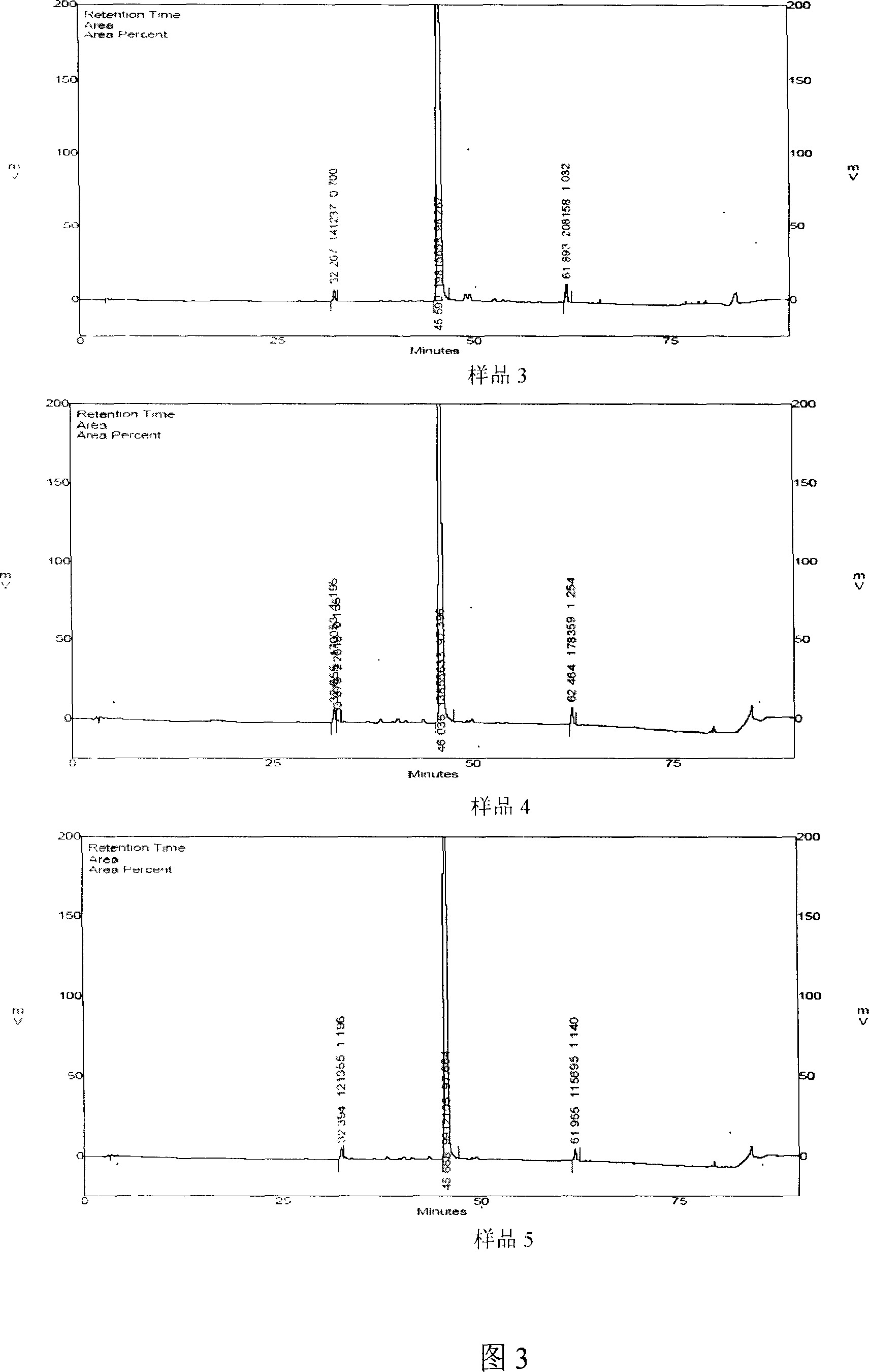
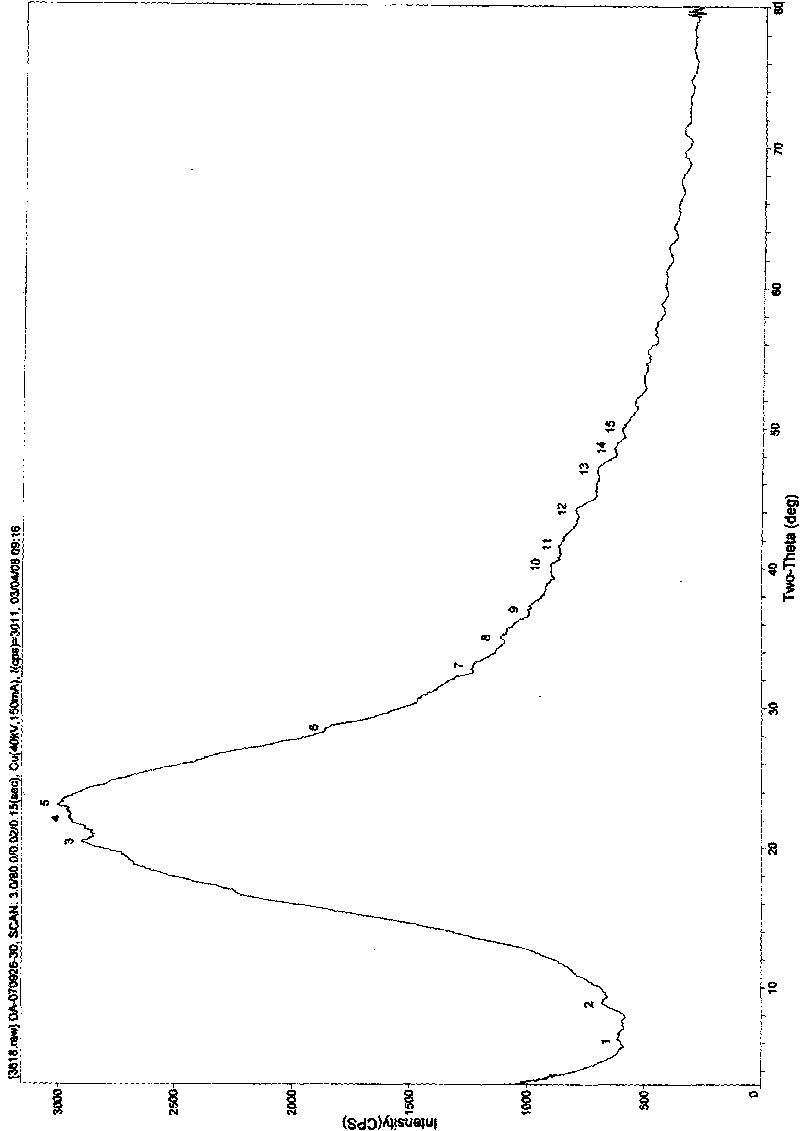
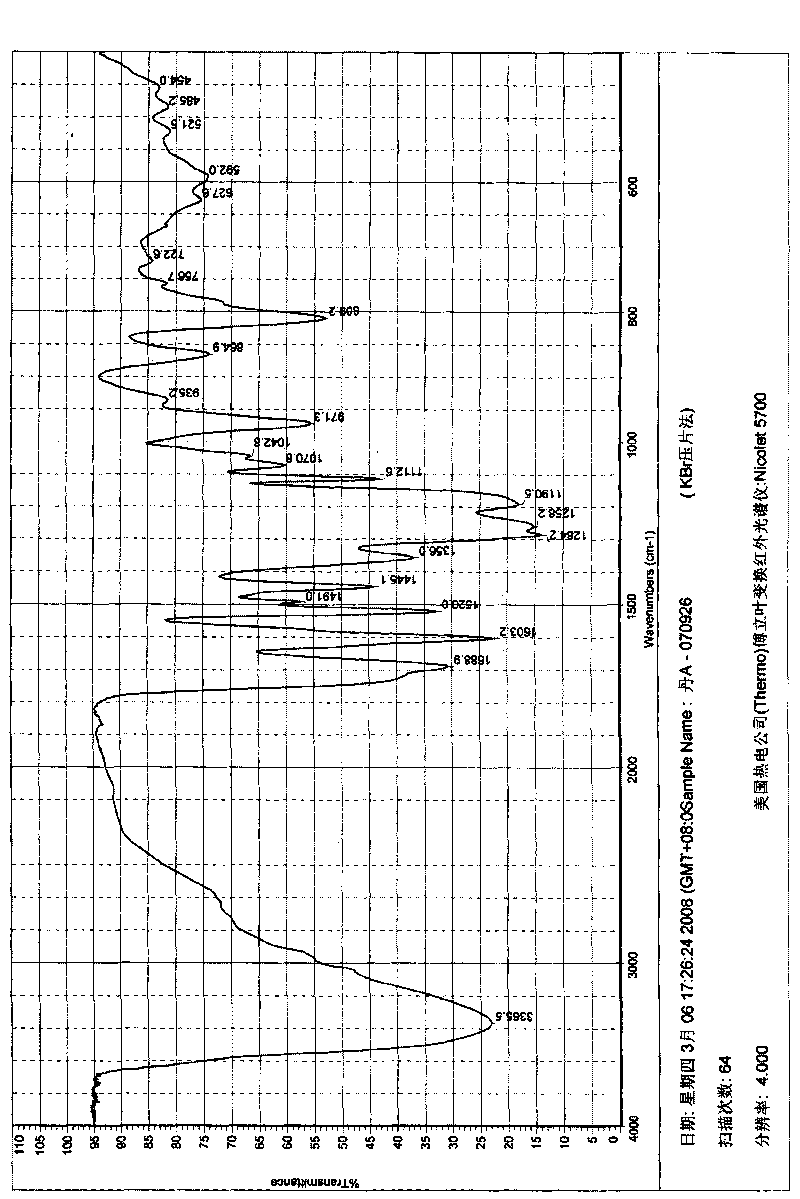
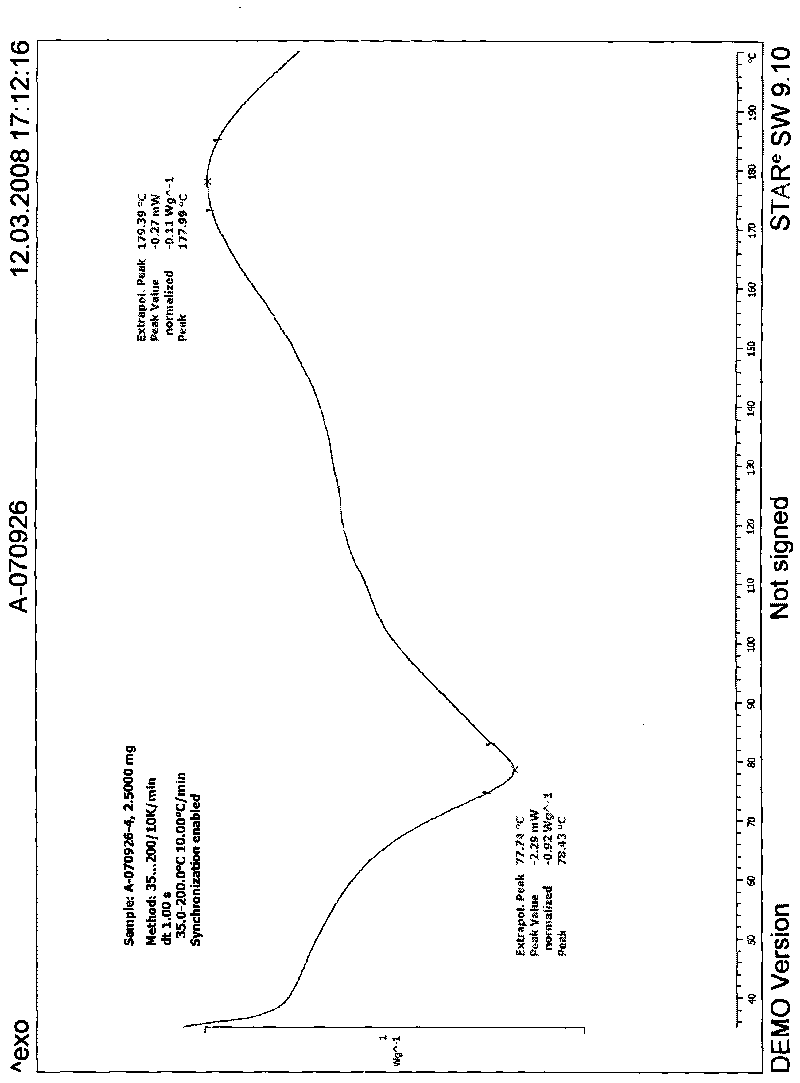
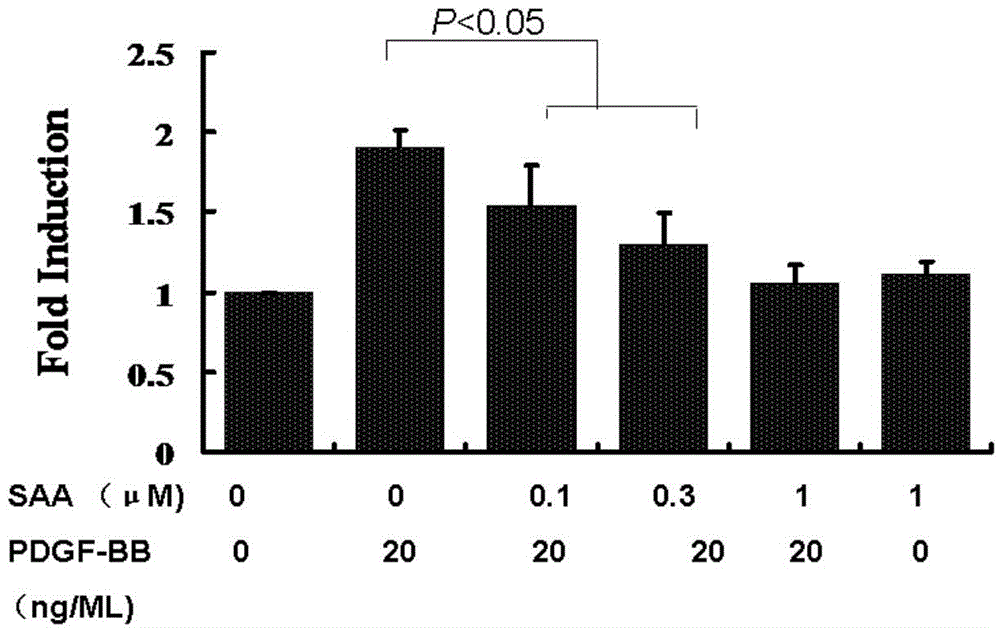
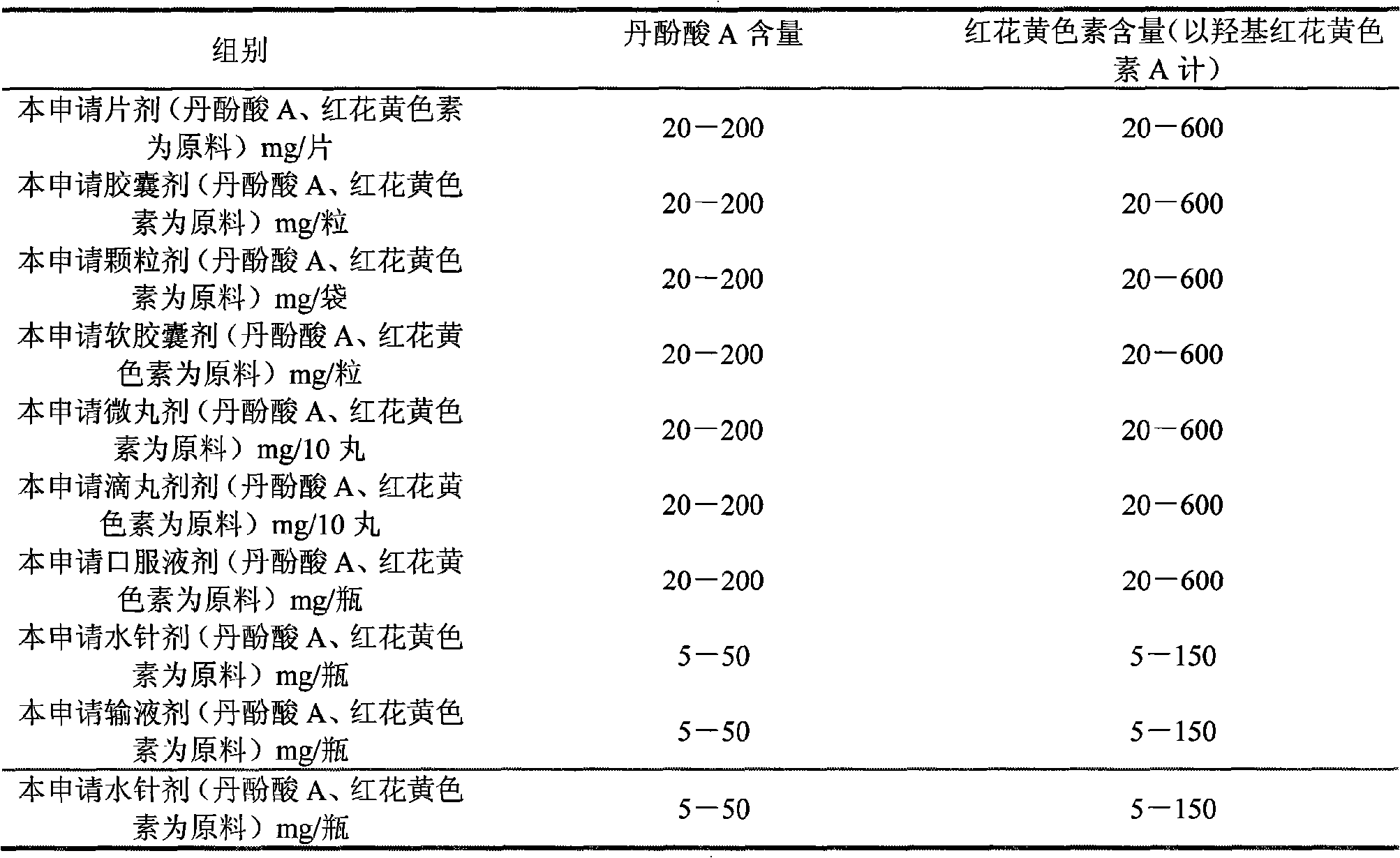
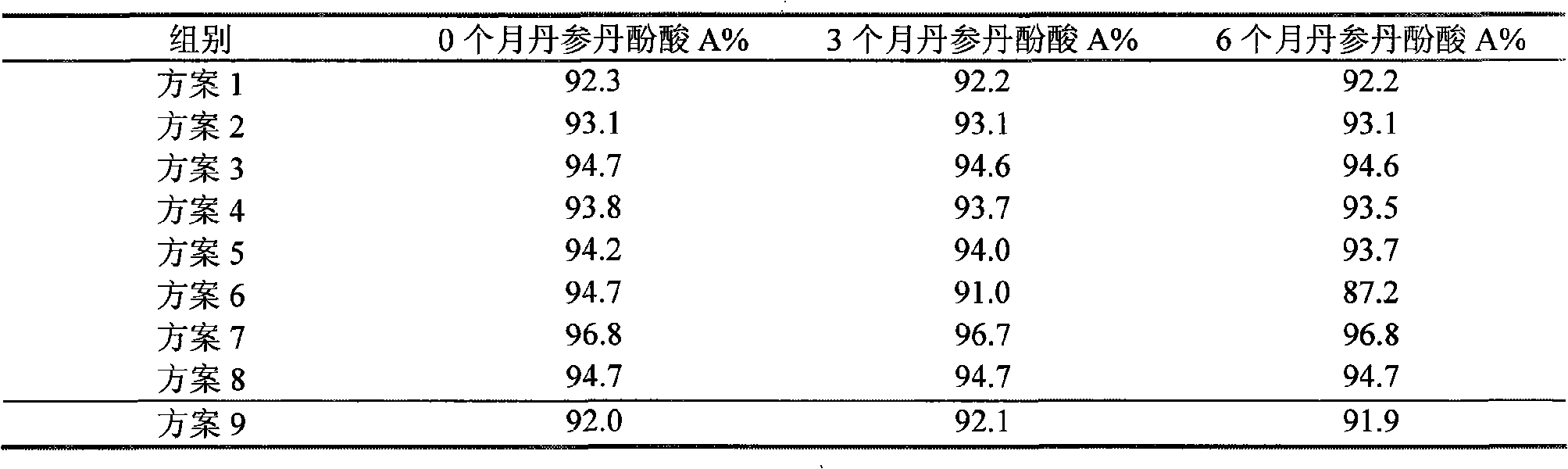
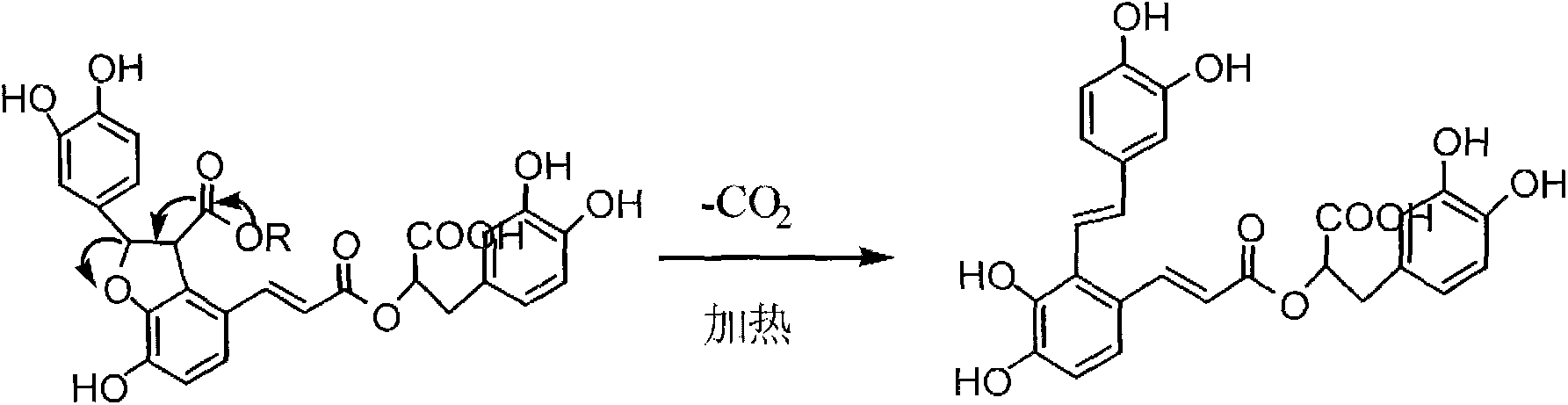
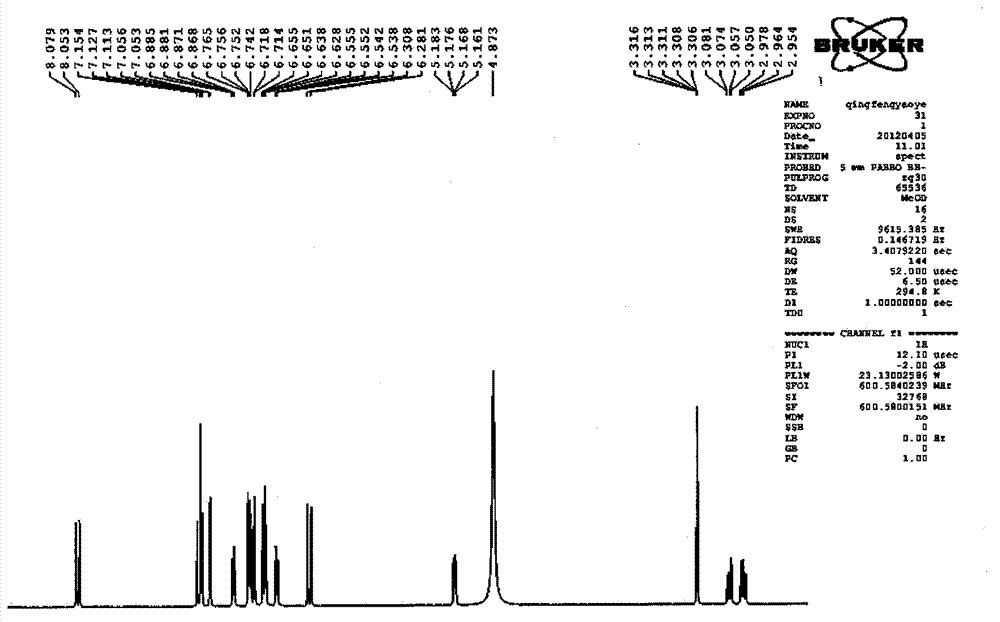
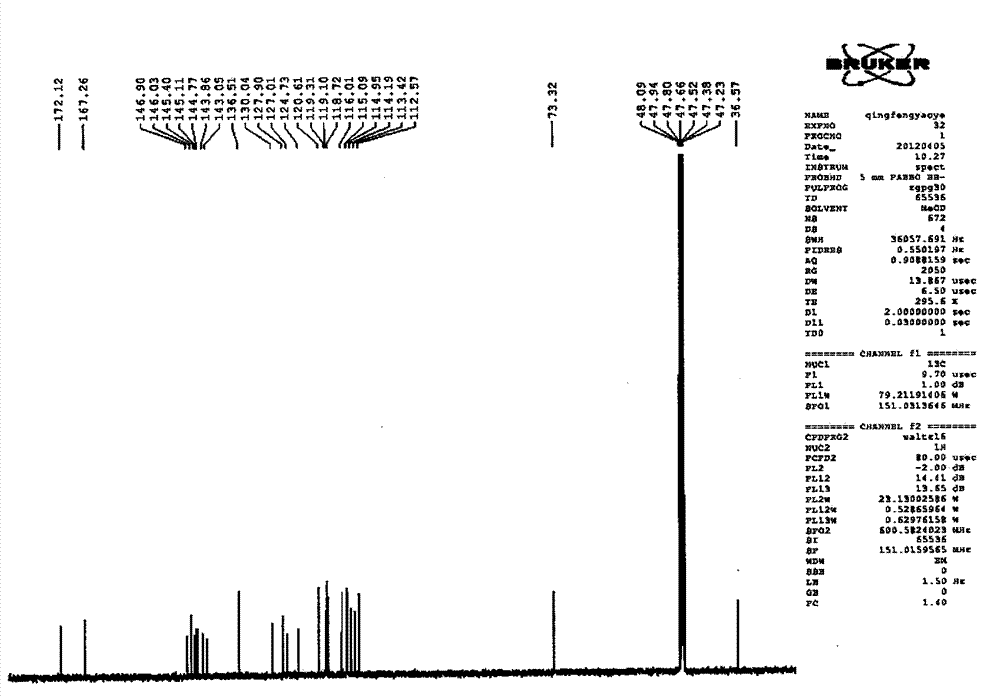
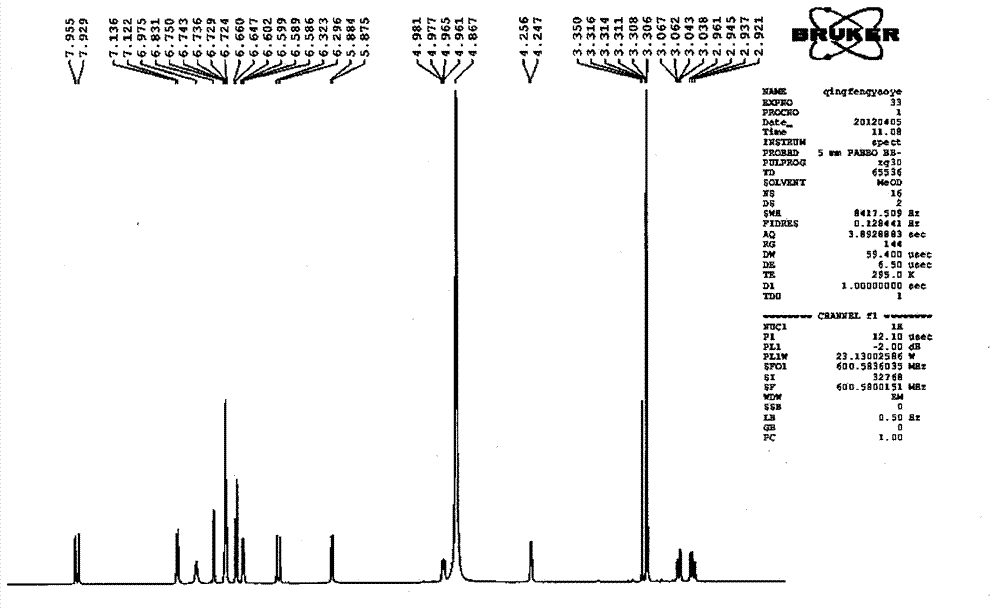
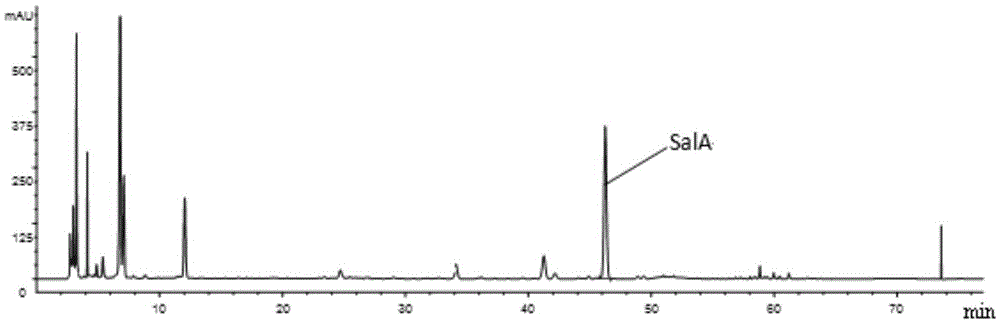
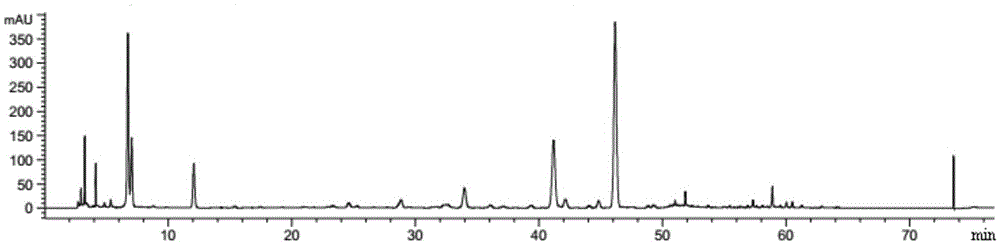
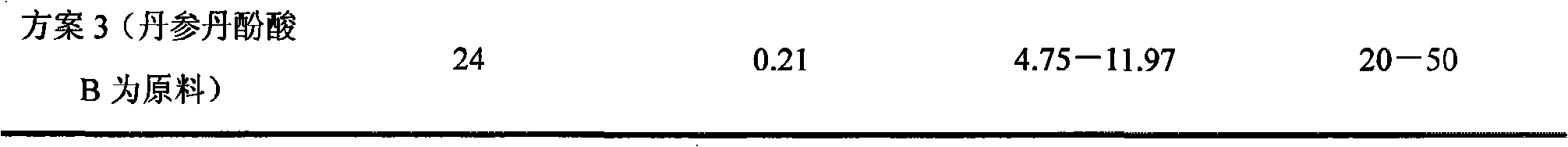Patents
Literature
275 results about "Salvianolic acid A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
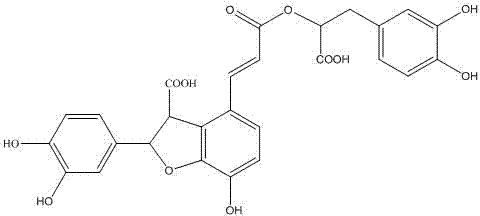
Salvia minium phenolic acid A and process of preparing preparation and use
InactiveCN100999470AImprove conversion rateGood repeatabilityOrganic active ingredientsOrganic chemistryMedicineCurative effect
This invention concerns the method of extracting salvianolic acid A from Chinese crude drug: danshen root, and the quality control methods and drug combinations, and the application of this drug. It can be used in the preparation of the prevention drugs for cardiovascular disease, liver damage, liver fibrosis, pulmonary fibrosis and other.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
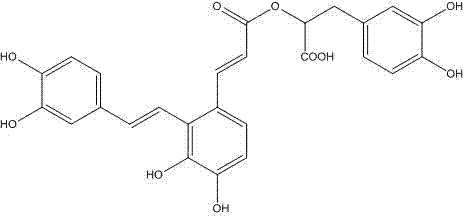
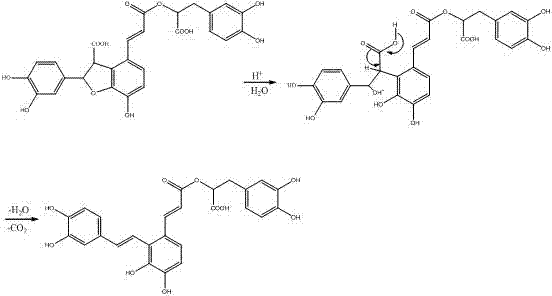
Method for preparing salvianolic acid A by catalytically converting salvianolic acid B
InactiveCN102212004AShorten the timeHigh yieldOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid KSalvianolic acid B
The invention discloses a method for preparing salvianolic acid A by catalytically converting salvianolic acid B. The method is characterized in that the converted raw material is a salvia miltiorrhiza aqueous extract (salvianolic acid B=>50%) primarily purified through combined chromatography; the concentration of the raw material salvianolic acid B is 0.5-2%; urea is taken as the catalyst; the mole ratio of urea to the salvianolic acid B is 0.3-0.7; the conversion reaction temperature is 100-125 DEG C; and the reaction time is 3-6 hours. The method has the following beneficial effects: urea is taken as the catalyst, thus greatly shortening the time for which the salvianolic acid B is in easily destroyed state and remarkably increasing the yield of the salvianolic acid A; the primarily purified salvia miltiorrhiza extract is taken as the converted raw material, thus not only removing the metal ions which are not beneficial to conversion but also removing most colloid-like impurities and frontal impurities which are not beneficial to following separation of the salvianolic acid; and the directional conversion rate of the salvianolic acid B to the salvianolic acid A prepared by the method is not less than 10% and even reaches 60%.
Owner:SUZHOU LEINA PHARMA RES DEV +1
Total tanshinone and total phenolic acid extract in red-rooted salvia root and its production
The invention is concerned with a kind of extract of total ketone of salviae miltiorrhizae and total phenolic acid and its produce method form radix Salviae Miltiorrhizae. The extract of total ketone of salviae miltiorrhizae has cryptotanshinone, tanshinone I, tanshinone IIA, methyl Tanshinon, dihydrotanshinon I and ramification. The extract of total phenolic acid has salvianolic acid A, salvianolic acid B, protocatechuic aldehyde and ramification. The extract can be got form one or arbitrary compound of extraction with solvent method, macroporous resin method, column chromatography and liquid-liquid counter-current chromatography. The summation of the content to each total ketone of salviae miltiorrhizae is 20 to 100 percnte (w / w) of the extract of total ketone of salviae miltiorrhizae, the contene of cryptotanshinone, tanshinone I and tanshinone IIA is 5 to 100 percent (w / w) of whole content of total ketone of salviae miltiorrhizae. The summation of the content to each total phenolic acid is 5 to 100 percent (w / w) of the extract of the radix salviae miltiorrhizae total phenolic acid. The content of salvianolic acid B is the 5 to 100 percent (w / w) of the whole salvianolic acid.
Owner:石任兵 +1
Red sage root salvianolic acid A injection formulation for treating cardiovascular diseases and preparation process thereof
The invention discloses a salvianolic acid A for injection and its preparing process, wherein high efficiency liquid chromatography method is employed to determine salvianolic acid A in the raw material medicaments, the content of salvianolic acid A is between 92% and 100%, two foreign substances are present. The mass ratio of salvianolic acid A and anti-oxidizing agent in the preparation is 2-50:1, the pH of the injection is controlled to 3-7.
Owner:CHIATAI QINGCHUNBAO PHARMA
Salvianolic acid A preparing process
InactiveCN1887849AGood curative effectHigh yieldOrganic chemistryPlant ingredientsOrganic solventAlcohol
The present invention discloses one salvianolic acid A preparing process. The salvianolic acid A preparing process includes extract red sage material with water or alcohol, concentrating the extracted liquid, purifying salvianolic acid A in chromatographic column, extracting with organic solvent in regulated pH value, concentrating and drying to obtain salvianolic acid A. The said process has high salvianolic acid A extracting rate, and high salvianolic acid A product purity, up to 90 %, stable quality, and is suitable for large scale production.
Owner:CHIATAI QINGCHUNBAO PHARMA
Preparation method of salvia miltiorrhiza tanshinoate A
InactiveCN101230003AOrganic active ingredientsCarboxylic acid esters separation/purificationMedicineSalvianolic acid
The invention discloses a method for manufacturing salvianolic acid A of danshen root, which is characterized in that total salvianolic acid in the danshen root is transformed into salvianolic acid A by adopting a certain method. The method increases greatly the extraction yield of salvianolic acid A. The salvianolic acid A is prepared into tablets, capsule, granula, soft capsule, micro pill, dripping pill and oral liquid, which have good pharmacological action indicated by pharmacological experiments.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for separating and purifying salvianolic acid from red sage root liquid extract by one step
InactiveCN101186572ACarboxylic compound separation/purificationPlant ingredientsProcess dynamicsSalvianolic acid B
The invention relates to a method for furthering separating and purifying salvianolic acid from Danshen extract fluid, which comprises that prepares, breaks and extracts Danshen via water solution, acidifies extract to adjust pH and adds salt to process post-treatment, processes dynamic continuous adsorption and elution on the treated extract at chromatography column stuffed with resin adsorbent, elutes via water, collects and concentrates eluent, elutes via gradient ethanol solution, segmented collects and concentrates eluent, and dries the concentrates solution to obtain product. The invention can simply, effectively and quickly separate and purify various salvianolic acids, wherein test on different salvianolic acid products shows that the highest yields of tanshinol, alkannic acid, rosmarinic acid, salvianolic acid A and salvianolic acid B are 35. 55%, 70.65%, 99.27%, 82.78%, and 89.34%, and relative highest purities are 95.32%, 65.05%, 29.40%, 33.93%, and 82.35%, which are near or higher than the result of purification on the goal of single component.
Owner:TIANJIN UNIV
Method for preparing red sage root salviandic acid A
InactiveCN101311160AOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid BSalvianolic acid A
The invention discloses a preparation method for salvianolic acid A in salvia miltiorrhiza and is characterized in that the method takes salvianolic acid B in the salvia miltiorrhiza as the raw material and converts the salvianolic acid B into the salvianolic acid A in the salvia miltiorrhiza after adjusting pH value to 3.5-6.0 and chemical reaction, thus greatly increasing the extraction yield of the salvianolic acid A; the pharmacological tests prove that a preparation prepared by the salvianolic acid A in the salvia miltiorrhiza has excellent pharmacological action.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Control method for preparing salvianolic acid A
InactiveCN101121658AIncrease contentReduce performanceCarboxylic acid esters separation/purificationPlant ingredientsAcetic acidOrganic solvent
The invention discloses a control method for the preparation of salvianolic acid; in the method, the proper quantity of solvent is added into the salvia drinking piece or the smashed salvia to be used as the menstruum; after the extraction and the solid-liquid separation, the extracted liquid reacts at the high temperature and a high pressure; then the medical liquid is added into the chromatography column; after eluted by water, elute with the eluant; collect the high-purity salvianolic acid A solution; compress the eluant until the eluant has no organic solvent under the reduced pressure or the normal pressure; the alkali is used to adjust the pH value of the acquired solution; the solution is extracted with the chloroform or ether organic solvent; after the organic solvent are extracted, the acetic acid ester and similar organics solvents are added into the water for extraction; the medical liquid is compressed and dried with the acetic acid ester and similar organics solvents and then the salvianolic acid A product of 50 to 84 percent is gotten. Through controlling the content of the salvianolic acid A in the final extracted materials, the performance of the final extracted materials are more stable; and the invention is low in the requirements for the chemical medicine, more universal, and more suitable for the industrialization.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Medicine composition containing salvianolic acid A, preparation method and application thereof as well as freeze-dried powder injection and water injection containing composition
InactiveCN101596182AGood water solubilityImprove stabilityPowder deliveryOrganic active ingredientsDiseaseDistillation
The invention relates to a medicine composition containing salvianolic acid A, the application and a preparation method thereof as well as freeze-dried powder injection and water injection containing the composition. The preparation method of the medicine composition comprises the following steps: taking salvianolic acid A to dissolve or disperse into water or ethanol, taking one or a plurality of alkaline sodium salt and alkaline kali salt to dissolve into water, mixing and fully stirring two solutions to be settled completely, and generally freeze-drying or decompression-drying by distillation to obtain a finished product, or directly adding water solution into pharmaceutic adjuvant to make the freeze-dried powder injection or water injection. The salvianolic acid A composition provided by the invention has strong stability, high dissolubility and uneasy oxidation in the water solution; the preparation method has good manufacturability, simple operation and low cost and can be used for preparing medicines for curing ischemic cardiovascular and cerebrovascular diseases; and the freeze-dried powder injection and water injection containing the salvianolic acid A have good stability and high uniformity and are accordant with requirements of medicines.
Owner:YANTAI TARGET DRUG RES
Injection formulation containing raw material herb red sage root and its quality control method
ActiveCN1788748AEnsure safetyGuaranteed validityOrganic active ingredientsCardiovascular disorderSalvianolic acid BMedicine
The present invention is one kind of injection containing medicine material red sage, and each preparation unit contains salvianolic acid A 0.5-7.5 mg and salvianolic acid B 0.5-7.5 mg. The present invention also provides the quality control method for the injection, and the contents of salvianolic acid A and salvianolic acid B are controlled to ensure the safety and effectiveness of the injection. The detection method is advanced, accurate, simple and suitable for conventional analysis. The present invention is significant in controlling the quality of different kinds of injection containing red sage component.
Owner:YAAN THREE NINE PHARMA
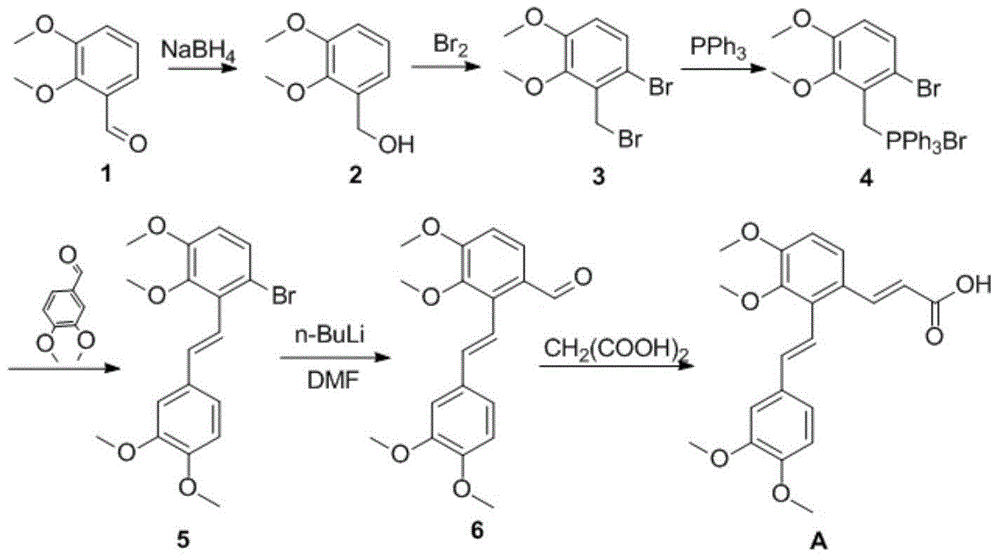
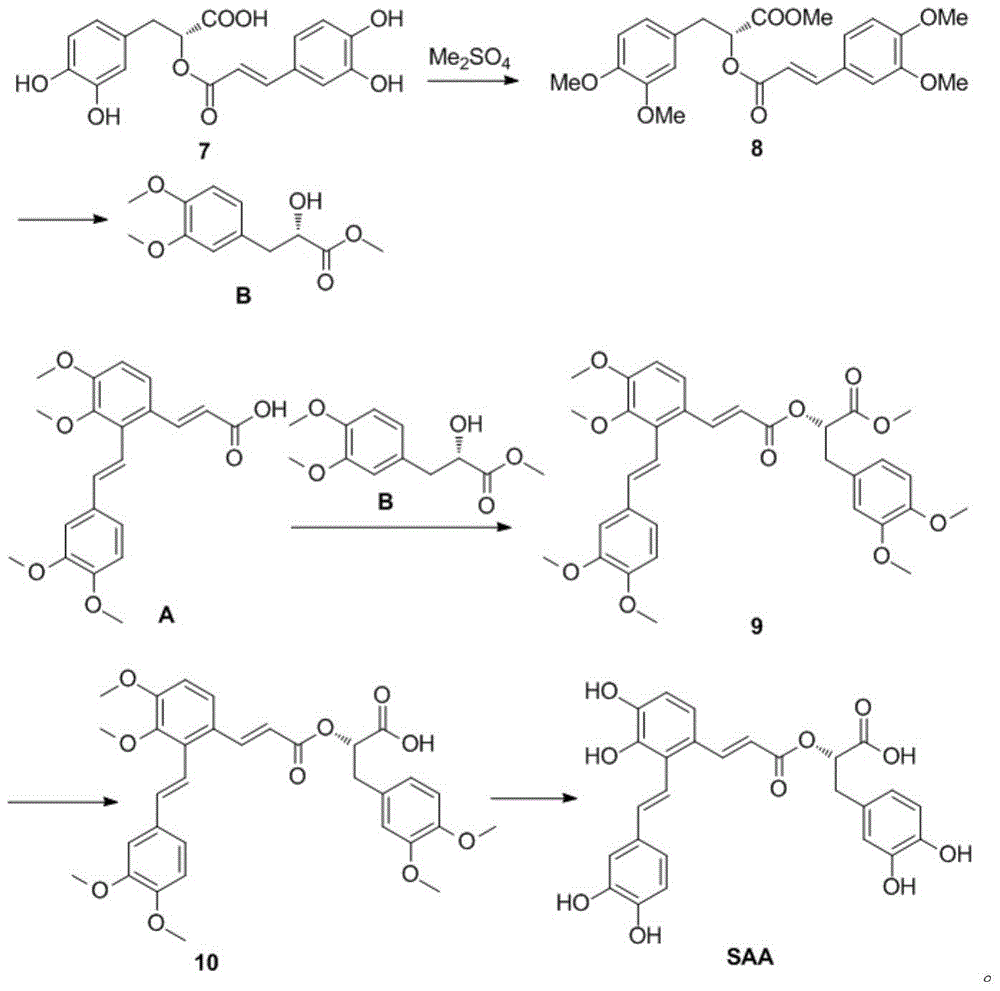
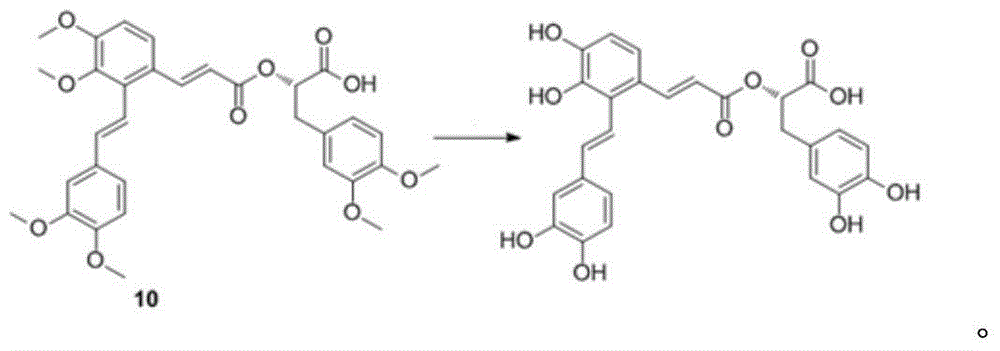
Synthetic method for salvianolic acid A
InactiveCN105085267AHigh purityOrganic compound preparationCarboxylic acid esters preparationPreparative hplcSalvianolic acid A
The invention provides a synthetic method for salvianolic acid A. The synthetic method is characterized by comprising a step of subjecting a salvianolic acid A synthetic precursor (10) to demethylation so as to prepare salvianolic acid A. A synthetic route is described in the specification. The prepared salvianolic acid A has a purity of 90 to 94%; and the purity of the prepared salvianolic acid A can be increased to 97% after preparative HPLC purification.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Rapid separation liquid chromatography detection method for naoxintong capsules
ActiveCN105241980AInhibit bindingQuality assuranceComponent separationChlorogenic acidGallic acid ester
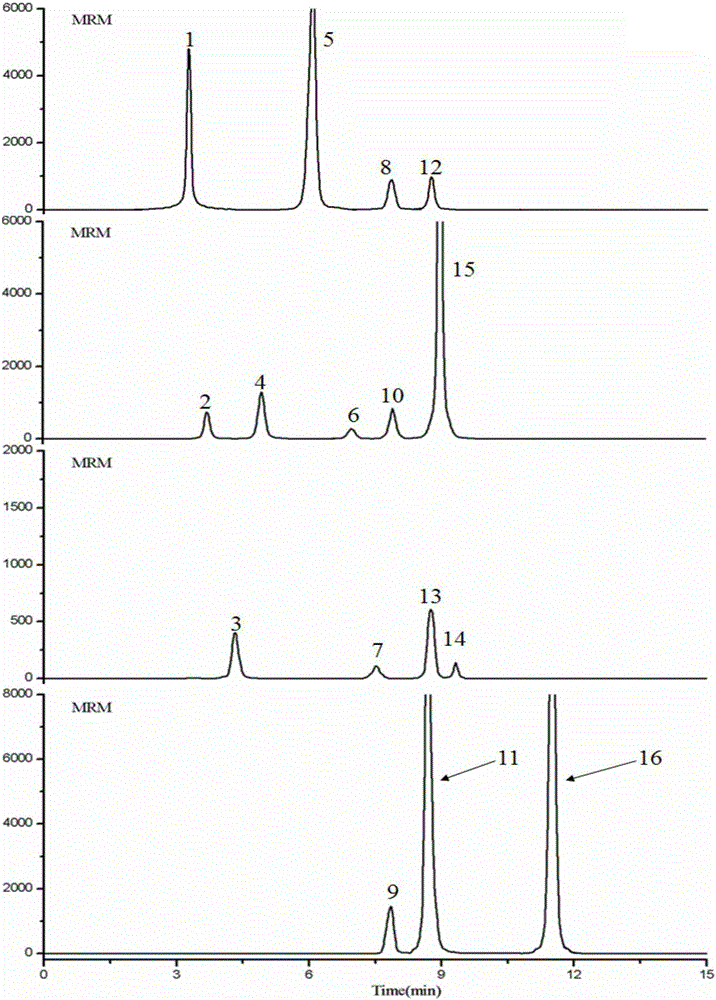
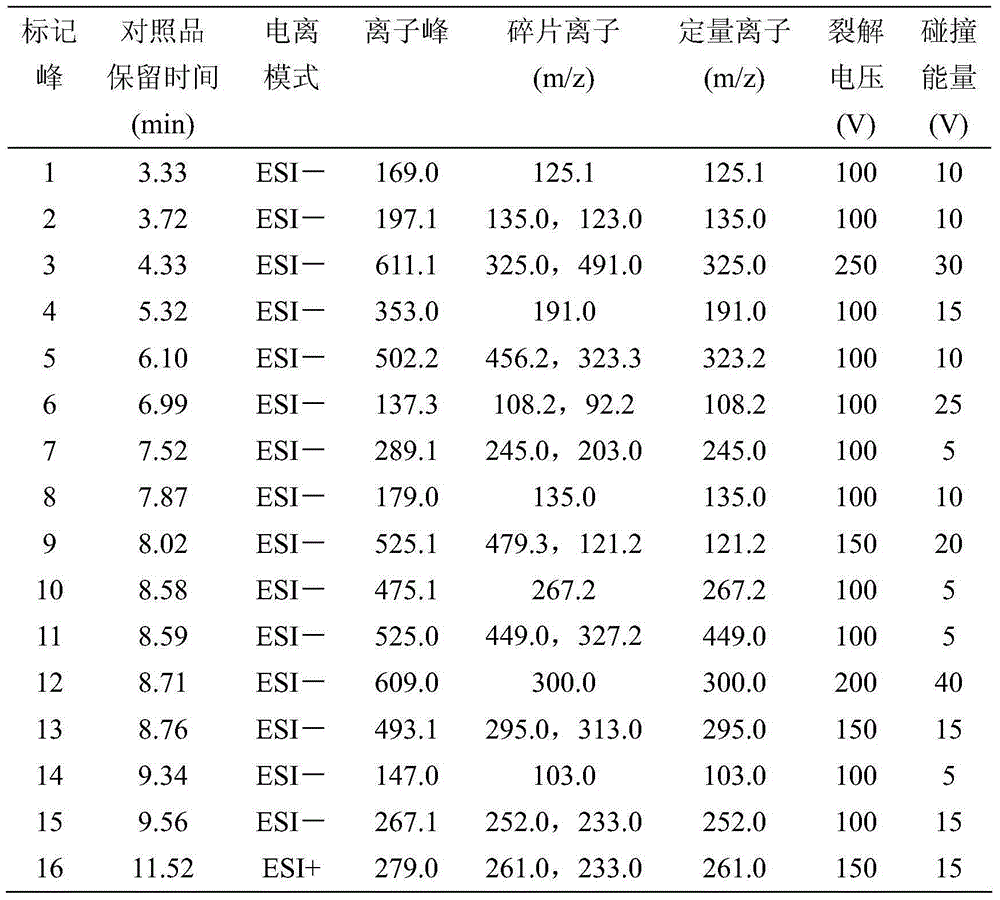
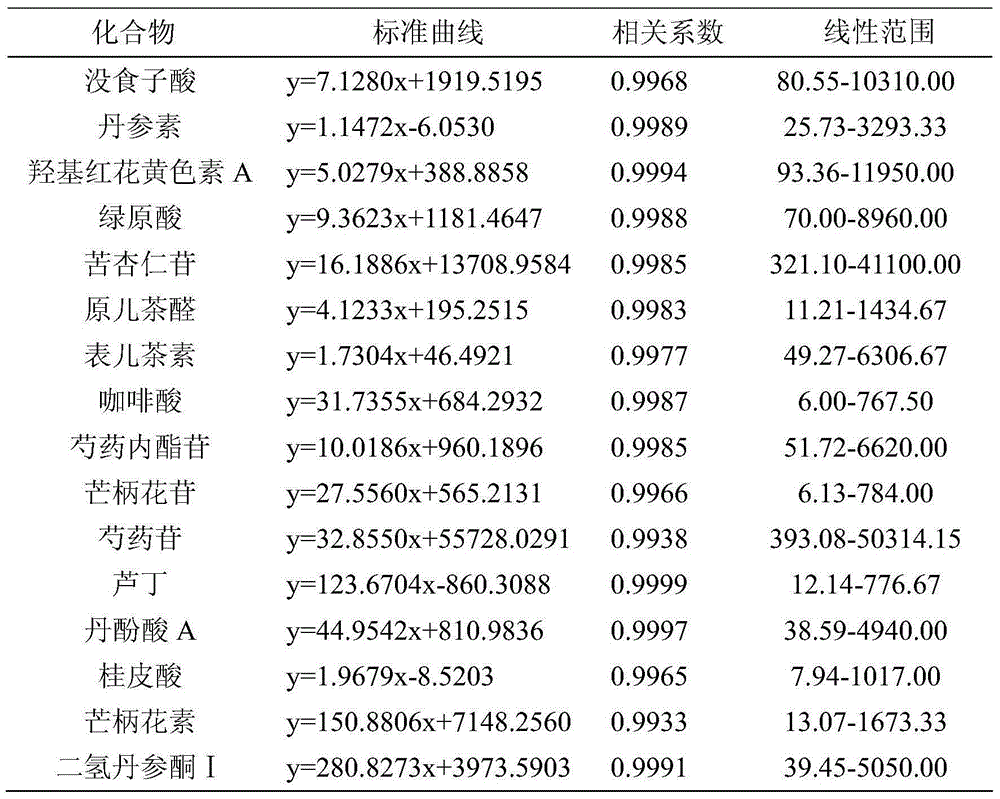
The invention relates to a rapid separation liquid chromatography detection method for naoxintong capsules. The method comprises the chromatographic conditions that octadecyl bonded silica gel columns serve as filling agents, 0.1% formic acid water serves as an A mobile phase, acetonitrile serves as a B mobile phase, the volume ratio of the A mobile phase to the B mobile phase is 10-85:15-90, and linear gradient elution is performed, the flow velocity is set at 0.20 mL.min<-1>, the column temperature is set at 25 DEG C, and the sample introduction volume is 5 microliters. The content of gallic acid, tanshinol, hydroxysafflor yellow A, chlorogenic acid, amygdalin, protocatechualdehyde, epicatechin, caffeic acid, albiflorin std, ononin, paeoniflorin, rutin, salvianolic acid A, cinnamic acid, fermlononetin, dihydrotanshinone I in the naoxintong capsules is detected. The detection method has the advantages of being rapid, stable and accurate.
Owner:SHAANXI BUCHANG PHARMA
Method for extracting and purifying salvianolic acid A
InactiveCN101130498AHigh purityReach the purpose of separating and purifying salvianolic acid ACarboxylic acid esters separation/purificationPlant ingredientsAqueous acetoneSolvent
The invention discloses an extracting and purifying method of salviol acid A, which comprises the following steps: adopting salvia miltiorrhizae medicine materical crude slice or powder as raw material; using solvent to extract; condensing to obtain the condensate of salvia miltiorrhizae; obtaining high-purity product through protein adsorption and solvent extracting method; using gelatin or egg albumin to sediment salviol acid A; dissolving the salviol acid A through acetone solution; analyzing; extracting through organic solvent to obtain the high-purity product with receiving rate over 0. 3% and purity over 90%. The invention has advantages of reasonable design, high extraction rate, good purity, stable property, simple technique and low manufacturing cost without column chromatography, which is fit for industrial manufacturing.
Owner:ZHEJIANG UNIV
Preparation method of high-purity salvianolic acid A
InactiveCN102249920AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationProcess engineeringLithospermum
The invention relates to a preparation method of high-purity salvianolic acid A. The preparation method is characterized in that: by taking lithospermum as a raw material, salvianolic acid A is obtained through the processes of water heating and extraction, acid regulation and transformation, resin absorption, elution and separation as well as purification and concentration. In the preparation method, the lithospermum is used as the raw material for preparing the salvianolic acid A, thereby expanding the sources of medicinal raw materials, saving the original salvia mitiorrhiza material and greatly reducing the use cost of the raw material; and simultaneously, the preparation method of the salvianolic acid A by taking the lithospermum as the raw material has a simple technological process and is suitable for large scale production, and the salvianolic acid A prepared by the method has high yield and purity and low cost.
Owner:上海朗萨医药科技有限公司
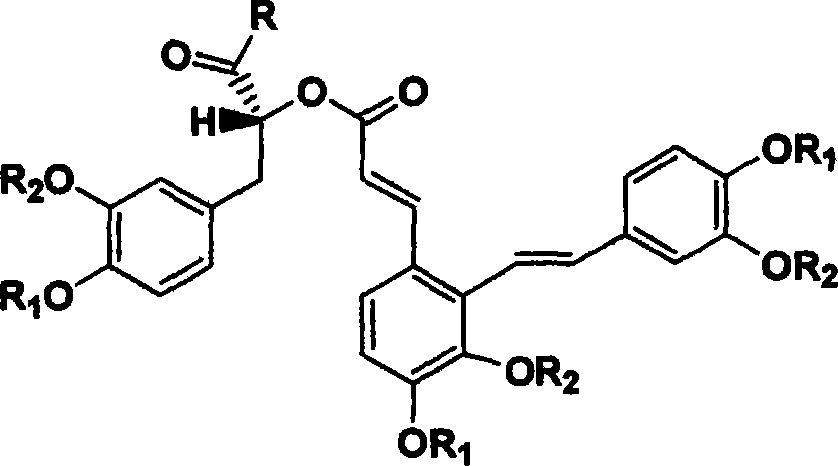
Derivant of salviol acidn A and its application as antioxidant
InactiveCN101134726AInhibitor synthesisAntioxidantOrganic active ingredientsOrganic chemistryAntioxidantOrgan transplant rejection
The present invention provides salvianolic acid A derivative and its application as antioxidant based on the physical and chemical properties and bioactivity of natural salvianolic acid. The salvianolic acid A derivative has the pharmacological effects of resisting oxidation, resisting inflammation, protecting endothelial cell, lowering blood fat, raising HDL, etc. It may be applied in preventing and treating atheroscleorsis, cardiac and cerebral vascular diseases, rheumatoid arthritis, diabetes, tumor and other diseases.
Owner:何克江
Qianliexin capsule quality evaluation method based on multi-index active ingredient measurement
ActiveCN106353430AQuality reflectionQuality assuranceComponent separationHplc fingerprintChlorogenic acid
The invention discloses a Qianliexin capsule quality evaluation method based on multi-index active ingredient measurement. The Qianliexin capsule quality evaluation method is characterized by comprising the following steps: on the basis of an efficient liquid chromatography-electrospray time of flight mass spectrum technology, measuring eight active ingredients in the Qianliexin capsule, and constructing a Qianliexin capsule HPLC (High Performance Liquid Chromatography) fingerprint chromatogram, wherein the eight active ingredients are respectively gallic acid, chlorogenic acid, caffeic acid, vaccarin, isoquercitrin, salvianolic acid B, salvianolic acid A and cryptotanshinone. The efficient liquid chromatography-electrospray time of flight mass spectrum technology is simultaneously adopted to measure the eight active ingredients in the Qianliexin capsule and construct the Qianliexin capsule HPLC fingerprint chromatogram, and meanwhile, the Qianliexin capsule quality evaluation method is used for evaluating the quality of a Qianliexin capsule medicine, and the eight active ingredients and the chromatogram realize mutual corroboration so as to more comprehensively reflect the quality of the Qianliexin capsule medicine to be favorable for researching and guaranteeing the quality of Qianliexin capsule medicine raw materials and preparations.
Owner:SHANDONG ANALYSIS & TEST CENT
Salvianolic acid A magnesium salt, preparation method and use of the salvianolic acid A magnesium salt, and salvianolic acid A magnesium salt-containing freeze-dried powder injection composition
InactiveCN102432467AImprove solubilityLess irritatingOrganic active ingredientsPowder deliveryMagnesium saltFreeze-drying
The invention discloses a salvianolic acid A magnesium salt, specially, relates to the salvianolic acid A magnesium salt, a preparation method and a use of the salvianolic acid A magnesium salt, and a salvianolic acid A magnesium salt-containing freeze-dried powder injection composition, and belongs to the technical field of medicines. The preparation method of the salvianolic acid A magnesium salt comprises the following steps of adding magnesium hydroxide into water, feeding CO2 into the magnesium hydroxide solution until the magnesium hydroxide solution is clarified, removing the CO2 in the magnesium hydroxide solution by ultrasonic waves to obtain magnesium hydrogen carbonate, adding salvianolic acid A into the obtained magnesium hydrogen carbonate so that the salvianolic acid A and the obtained magnesium hydrogen undergo a reaction at a temperature of 20 to 40 DEG C for 20 to 40 minutes to produce a reaction product solution, adding ethyl acetate into the reaction product solution, wherein the volume of the added ethyl acetate is equal to that of the reaction product solution, carrying out vortexing, and then carrying out centrifugal separation and freeze drying to obtain the salvianolic acid A magnesium salt. The salvianolic acid A magnesium salt has strong stability in an aqueous solution, high solubility and small irritation. The preparation method of the salvianolic acid A magnesium salt adopts a good preparation technology and has the simple steps. The salvianolic acid A magnesium salt-containing freeze-dried powder injection composition has good stability and high uniformity, and satisfies medicine rehydration capability requirements. The salvianolic acid A magnesium salt can be utilized for preparation of medicines for treating ischemic stroke.
Owner:吴谢军 +2
Method for preparing salvianolic acid A of salvia miltiorrhiza
InactiveCN102219685AOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid KGradient elution
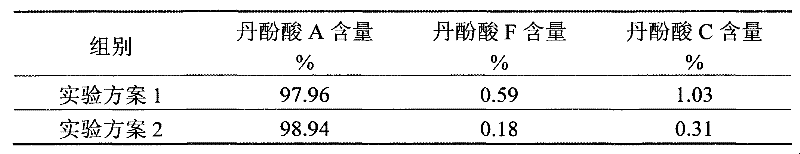
The invention discloses a method for extracting salvianolic acid A, which is characterized in that a new process method is adopted to obtain salvianolic acid A extract, the yield of the salvianolic acid A in the salvianolic acid A extract prepared with the method is more than five thousandth, thus saving a great quantity of resources. The invention further discloses a method for preparing the salvianolic acid A, which comprises the steps of adopting a medium pressure liquid phase preparation method, wherein eluent has two phases to carry out isocratic or gradient elution to obtain high-purity salvianolic acid A with related substances, such as salvianolic acid F and salvianolic acid C, with content lower than 0.5 percent. The quality of the salvianolic acid A obtained by using the method is more uniform and more stable.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Application of polymer salvianolic acid in preparation of drugs used for inhibiting occurrence or development of aortic aneurysm or aortic dissection
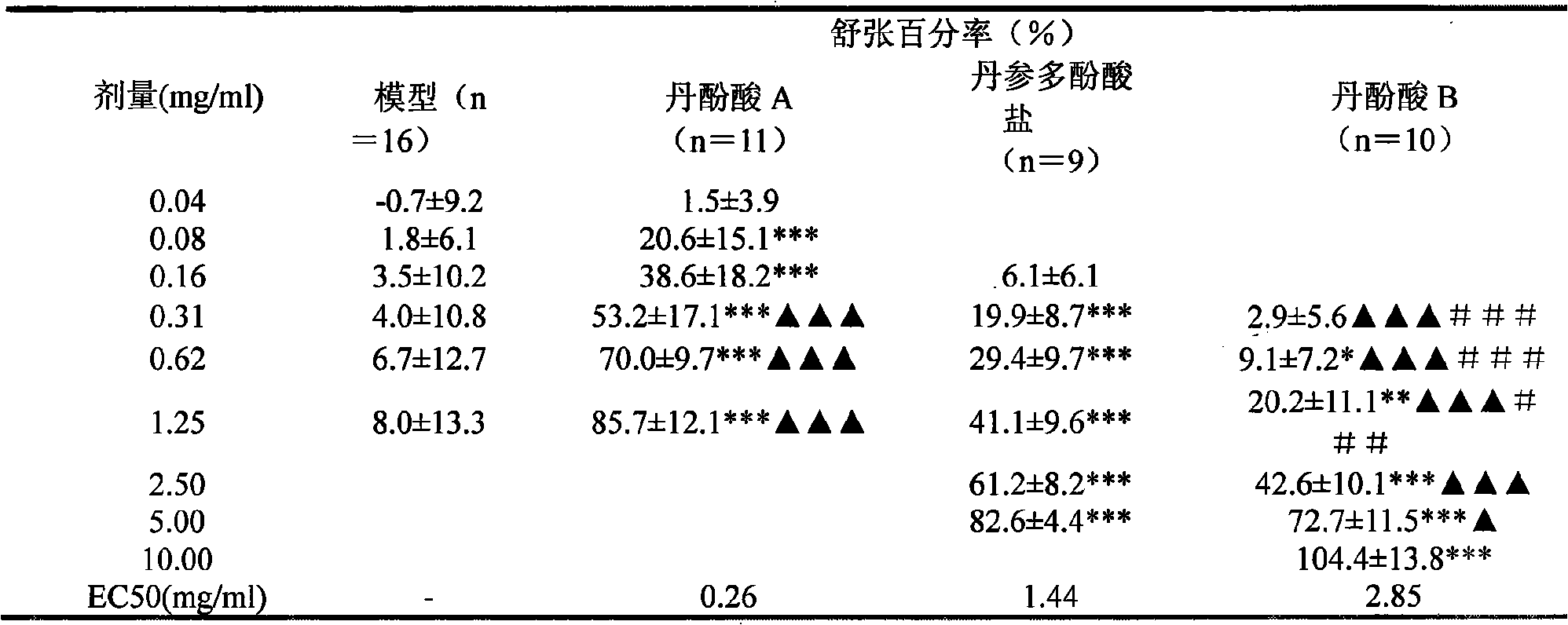
The invention relates to application of polymer salvianolic acid in preparation of drugs used for inhibiting occurrence or development of aortic aneurysm or aortic dissection and a pharmaceutical composition comprising the polymer salvianolic acid used as a pharmaceutical active substance, related desired adjuvant and the like. The pharmaceutical composition can be used for inhibiting occurrence of aortic aneurysm or aortic dissection. The polymer salvianolic acid is dimer salvianolic acid, trimer salvianolic acid or tetramer salvianolic acid, wherein the dimer salvianolic acid is rosmarinic acid, the trimer salvianolic acid is salvianolic acid C, alkannic acid or salvianolic acid A, and the tetramer salvianolic acid is salvianolic acid B.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
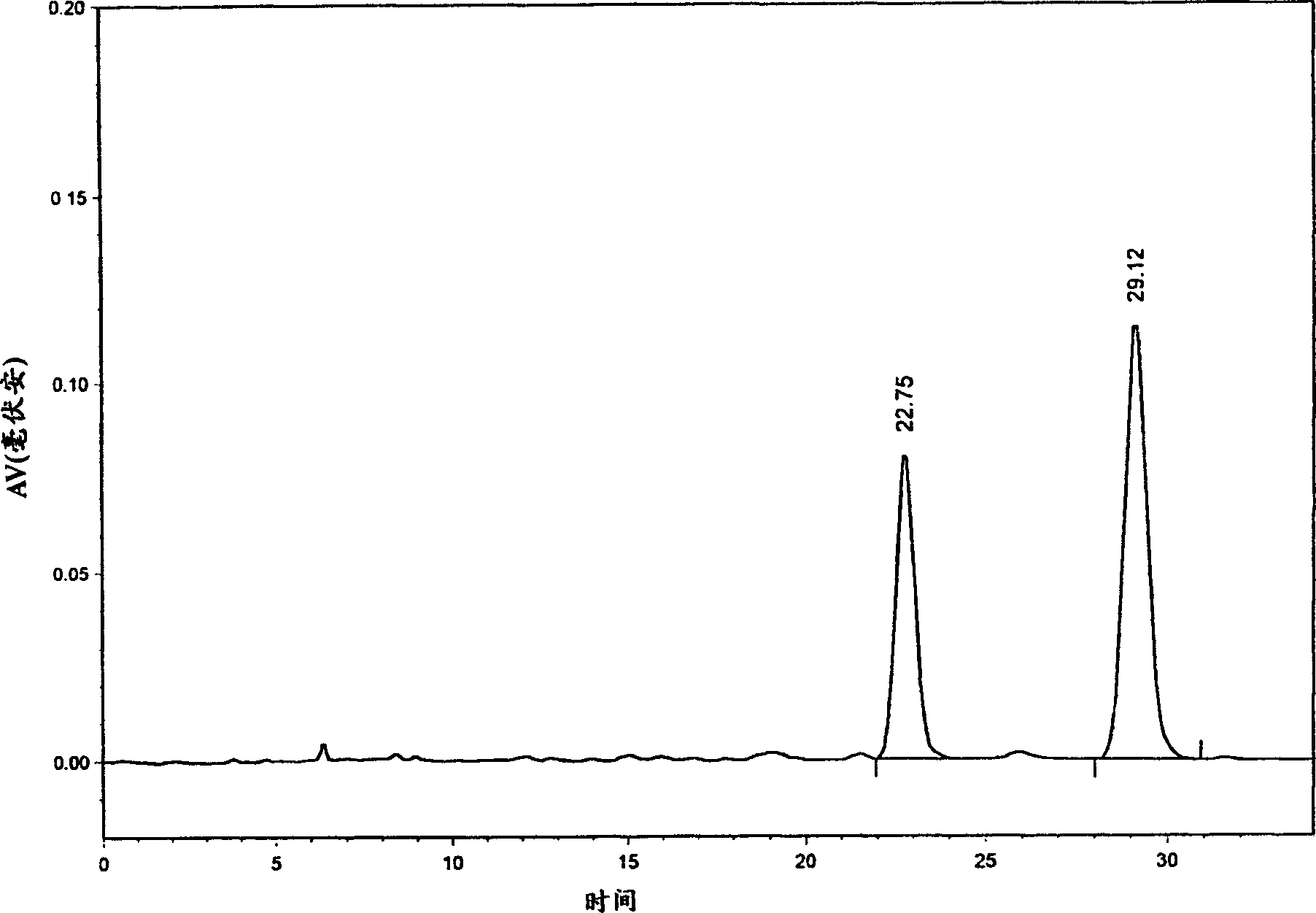
Two crystal form materials of salvianolic acid A, preparation method as well as medicine compound and application thereof
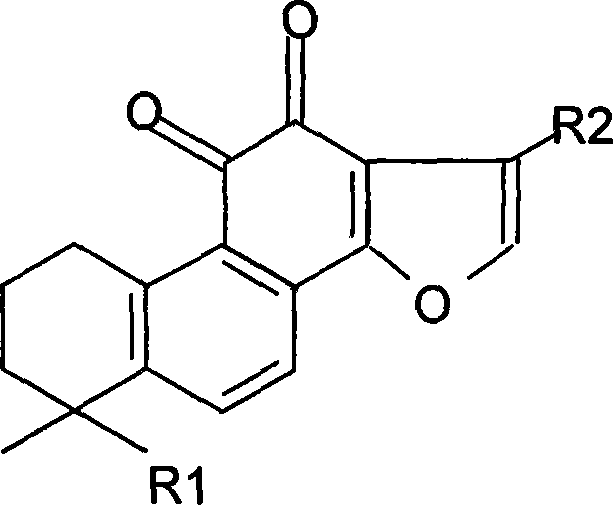
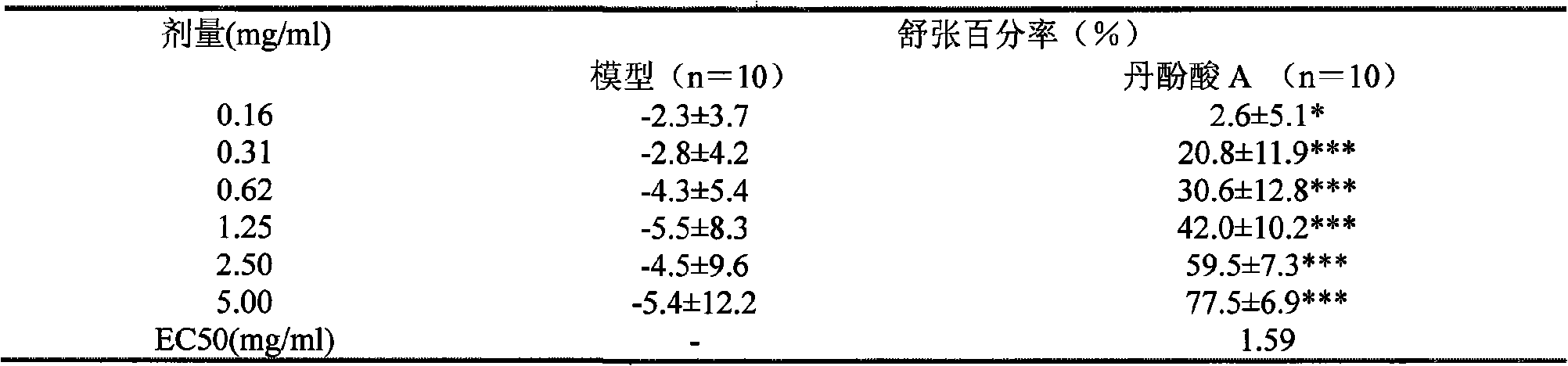
ActiveCN101712618ALower blood sugarPromote absorptionOrganic active ingredientsOrganic compound preparationDiabetes mellitusDisease
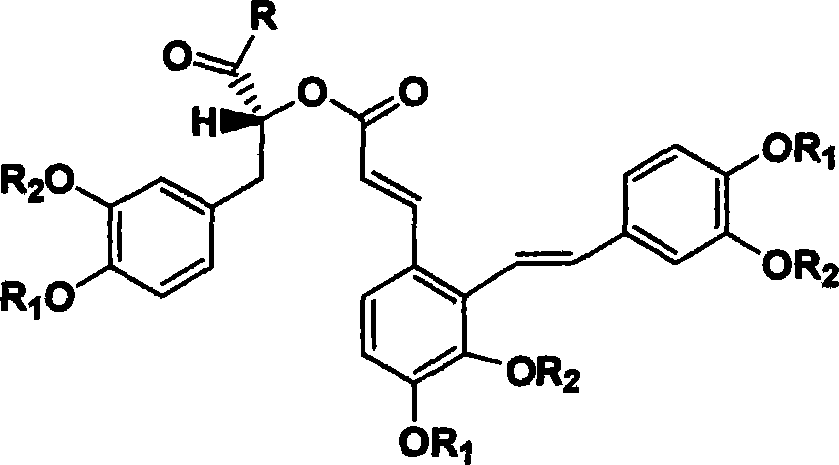
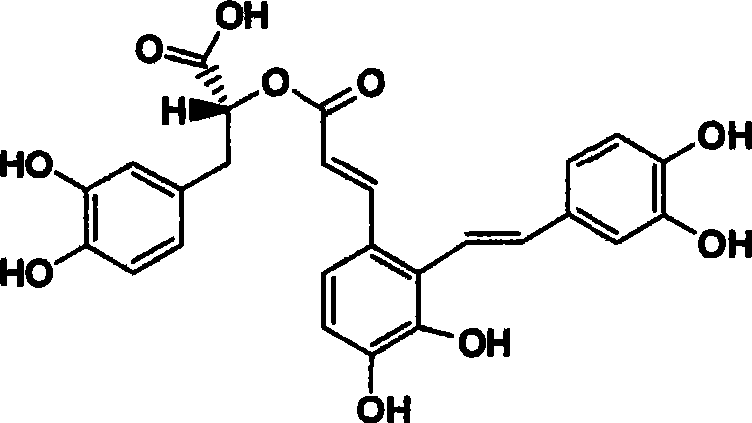
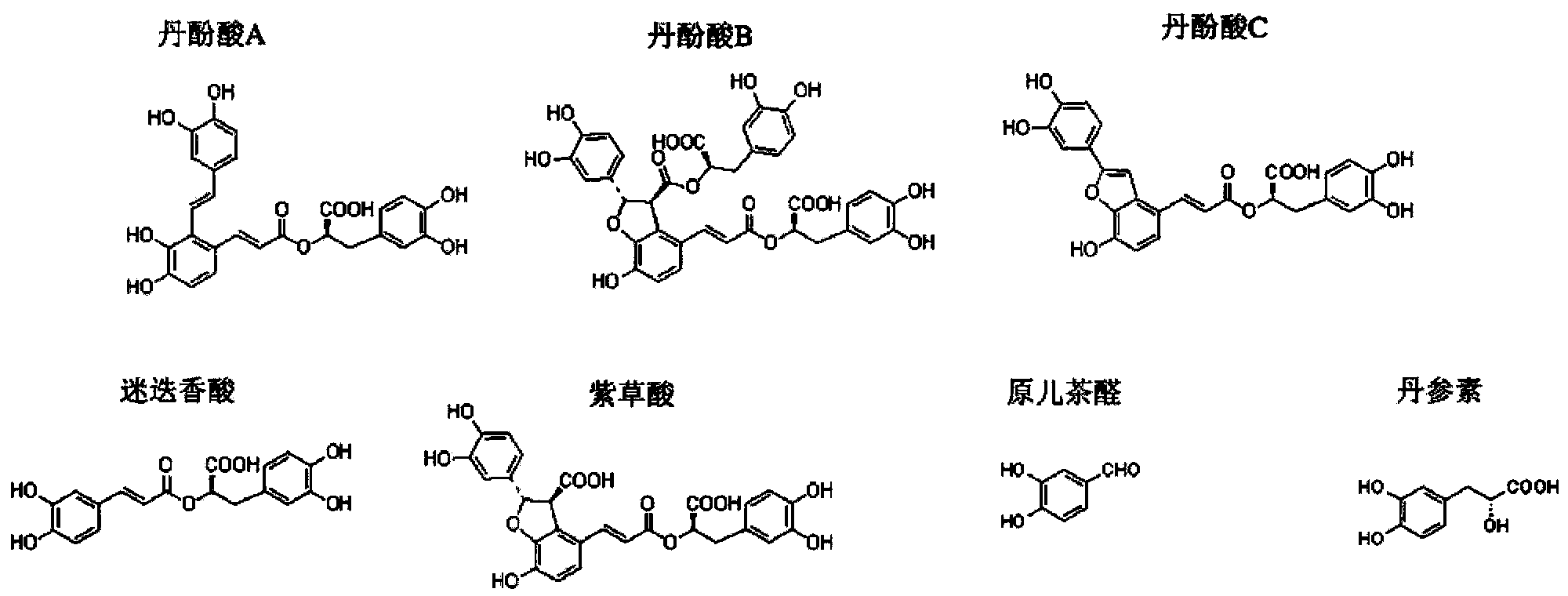
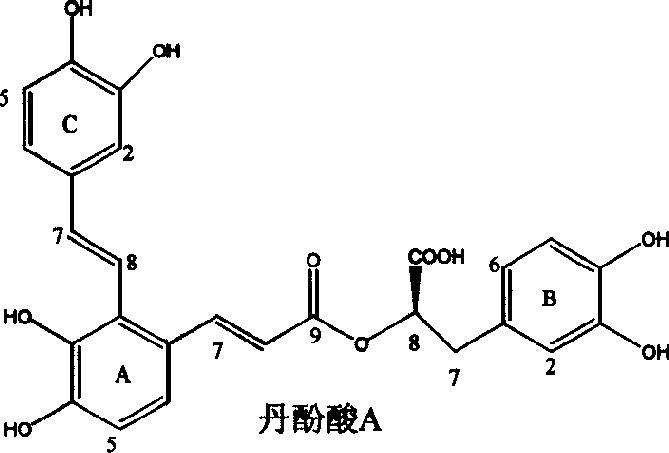
The invention discloses two crystal form solid materials of compound salvianolic acid A, and also discloses a preparation method of the two crystal form solid material samples. The invention relates to a medicine compound which is prepared and developed by adopting crystal form materials as medicine active constituents, and applications thereof to the aspects in preventing and treating diseases of cardio-cerebral-vascular system, immunity system, hyperlipemia, diabetes mellitus and complicating diseases thereof, and the like. A molecular structure of the salvianolic acid A is shown in the formula.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI +1
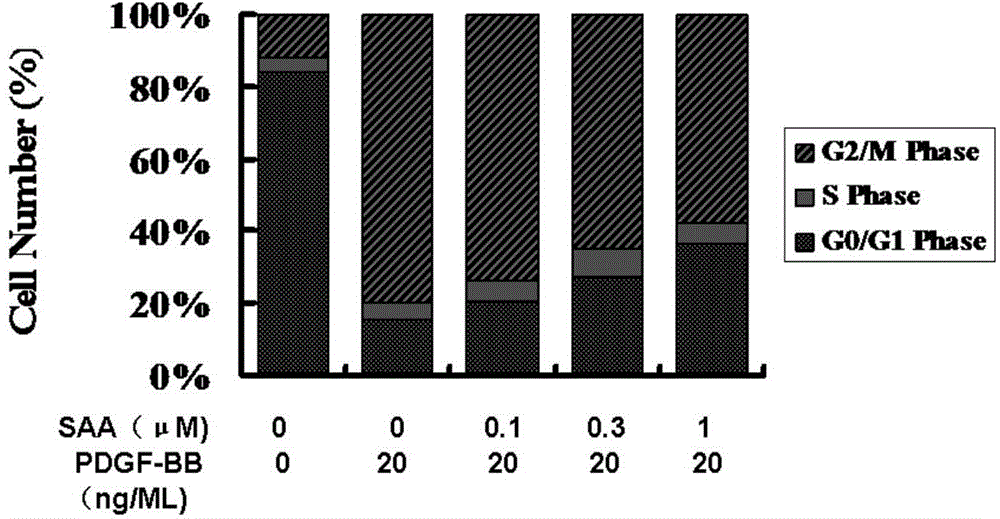
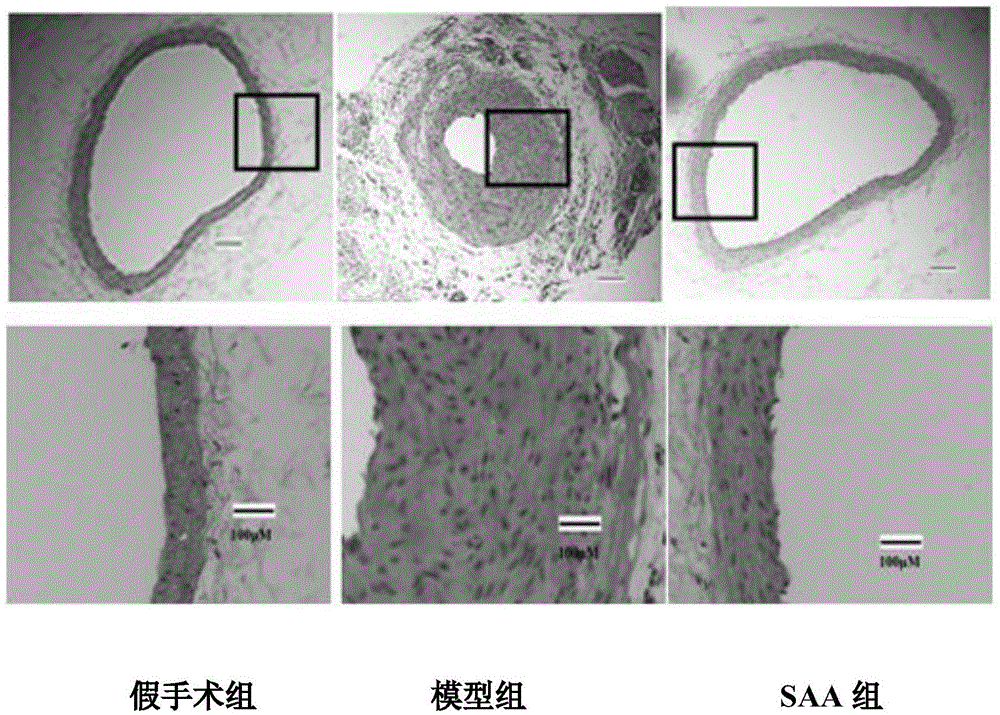
Application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and/or in-stent restenosis
InactiveCN105434417AReduce the degree of intimal hyperplasiaPrevent proliferationOrganic active ingredientsCardiovascular disorderBalloon injuryPercent Diameter Stenosis
The invention discloses application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis. Experimental research finds that salvianolic acid A can restrain proliferation of umbilical cord artery blood vessel smooth muscle cells of a person and reduce the tunica intima thickening rate and the blood vessel lumen restenosis rate of a tunica intima thickening animal model after balloon injury, has the remarkable effects of resisting tunica intima thickening and post-angioplasty restenosis and / or in-stent restenosis, can be used for preparing the medicine for restraining tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis and is particularly used for preparing medicine for preventing post-percutaneous coronary angioplasty restenosis and in-stent restenosis or used for preparing a drug eluting stent. In this way, efficient, safe and economical prevention and treatment medicine and an efficient, safe and economical solution are provided for prevention and treatment of post-angioplasty, particularly post-percutaneous coronary angioplasty restenosis and in-stent restenosis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Solid preparation of salvianolic acid A of red sage root and preparation process thereof
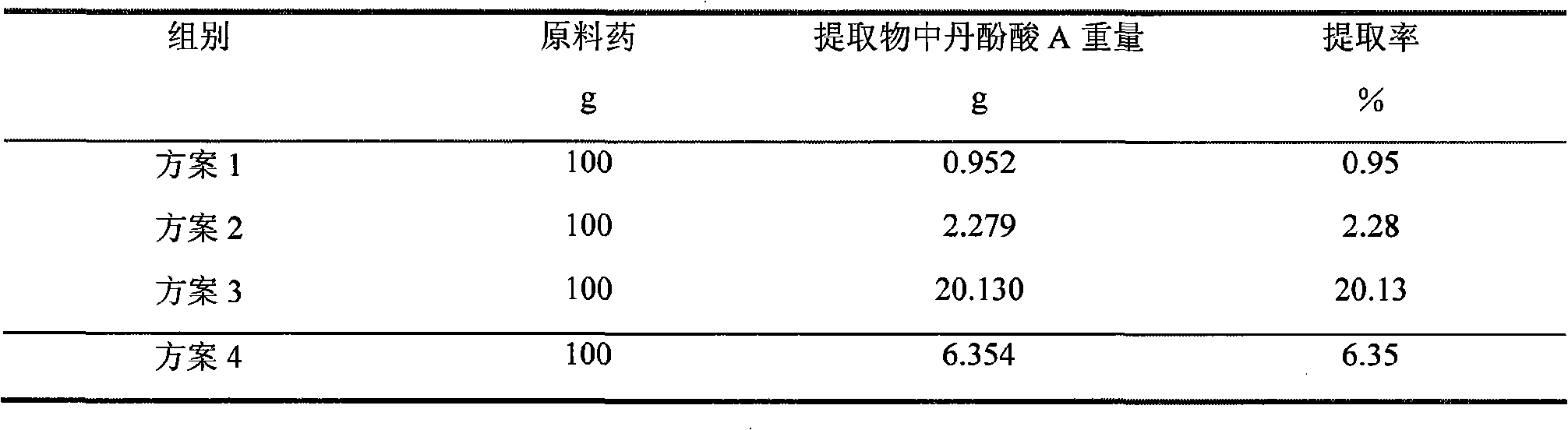
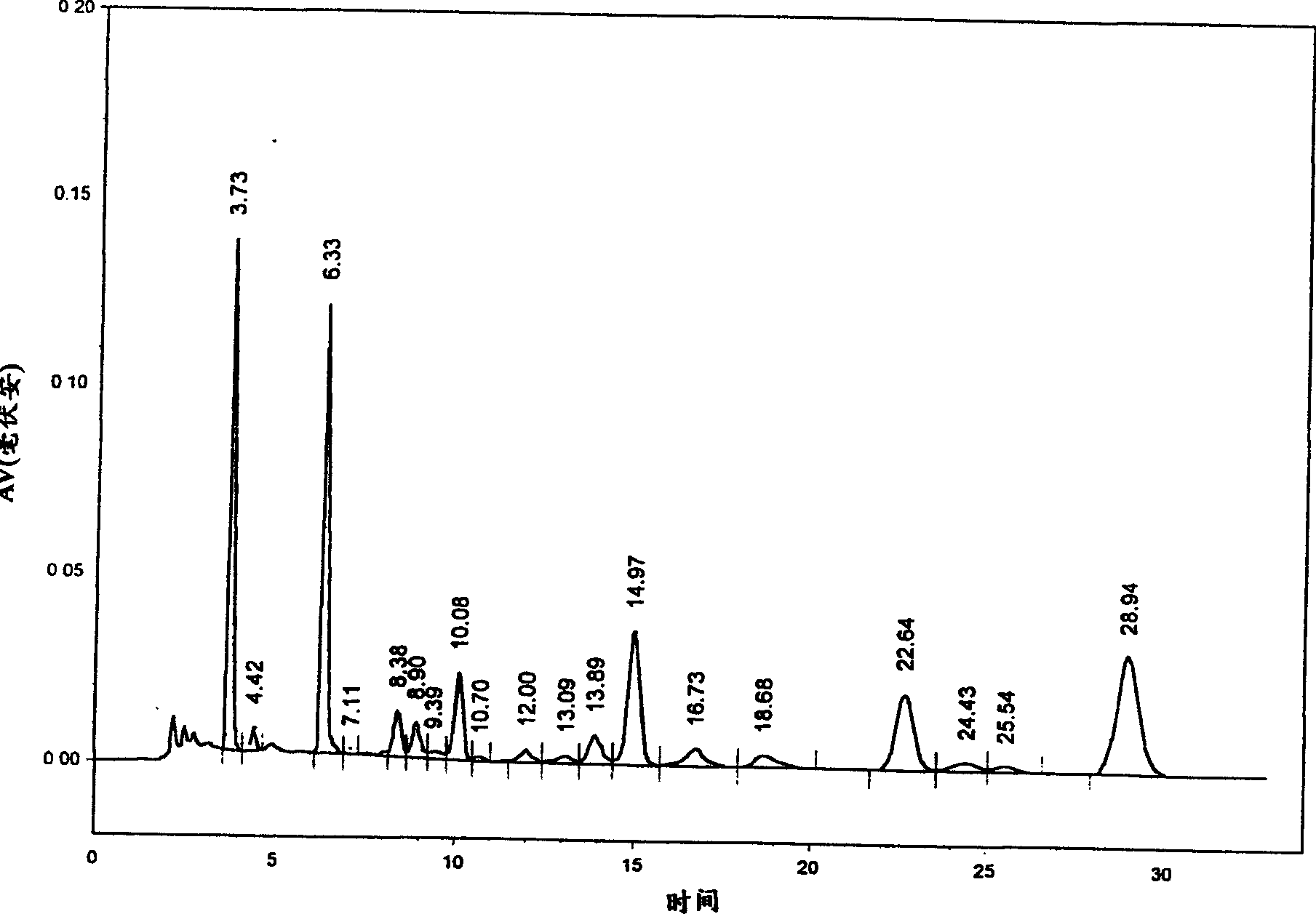
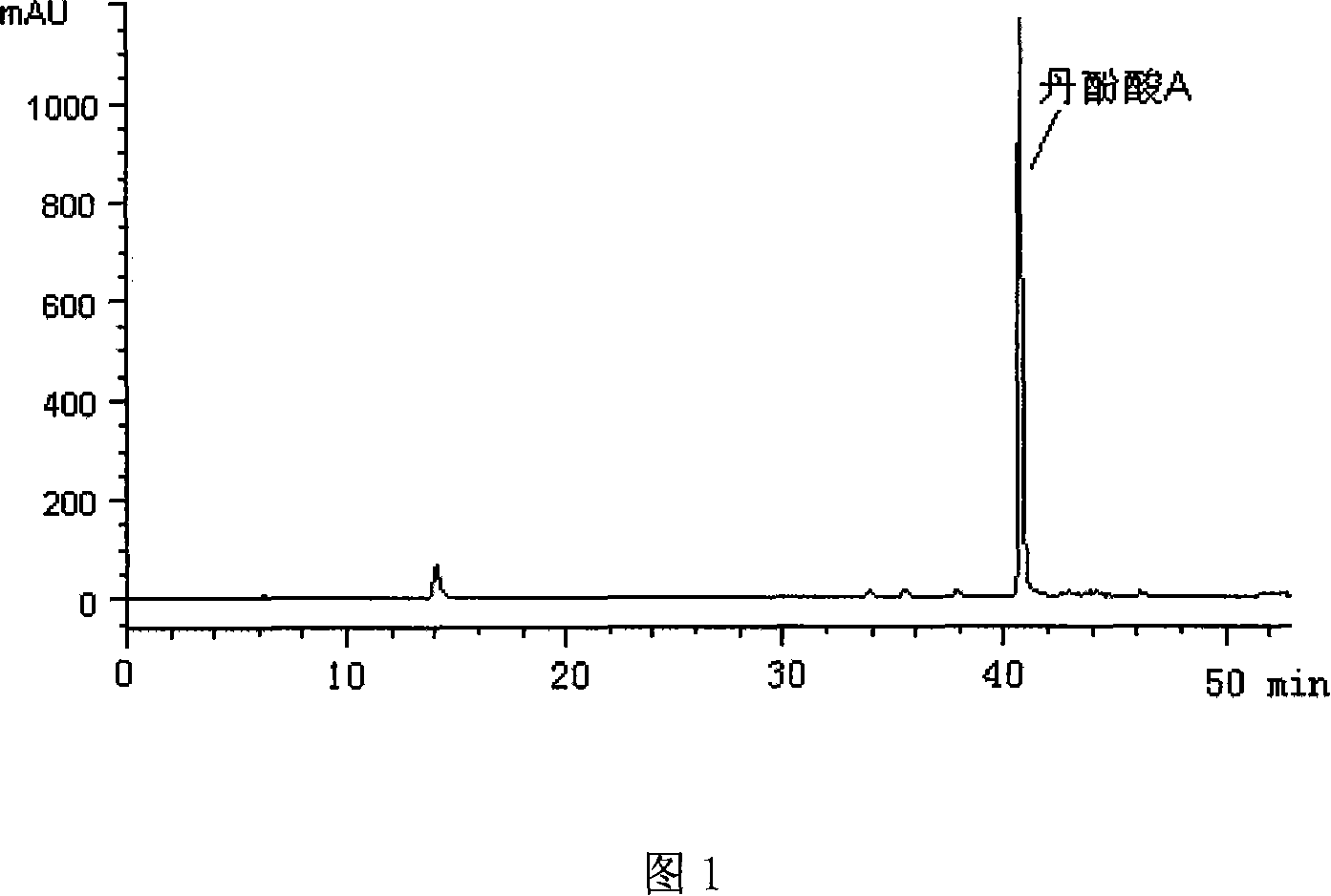
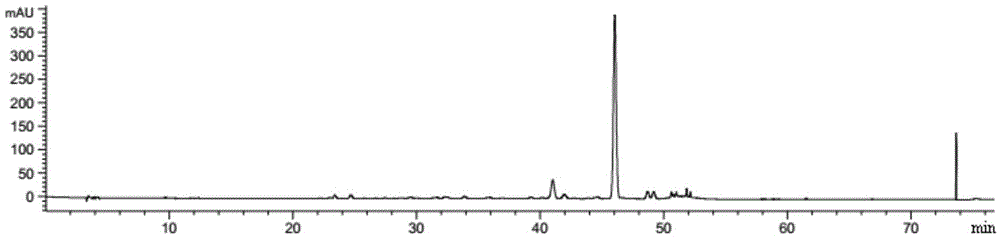
The invention relates to a method for producing Danshen acid a solid agent, wherein it uses high-effect liquid spectrum to test, to obtain three share peaks; uses Danshen acid A as reference, while its held time is 1 and the held times of two foreign materials are 0.71 and 1. 36; using Danshen acid A as contrast, the foreign material with held time as 0.71 is 0.10-1. 96 content; the content of foreign material whose held time is 1. 36 is 0.11-5. 97%, as Danshen acid C; the whole content of foreign materials is not higher than 10%. The inventive agent comprises Danshen acid A and drug findings, without oxidization resistance; therefore, said agent is stable and safe.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Medicament composition
InactiveCN101301300AOrganic active ingredientsSolution deliveryDose–response relationshipTraditional medicine
The invention discloses a medicine composition which is characterized in that: the medicine composition is determined through a dose-response relationship experiment, a harmacodynamic verification experiment, a pharmacokinetic experiment and a toxicological experiment, wherein, the medicine composition comprises the following compositions in portion by weight: 1 to 10 portions of salvianolic acid A from salvia miltiorrhiza, and 1 to 50 portions of blood-quickening and stasis-dispelling effective constituents of traditional Chinese medicines; or preferable 1 to 5 portions of the salvianolic acid A from salvia miltiorrhiza, and 20 to 50 portions of the blood-quickening and stasis-dispelling effective compositions of the traditional Chinese medicines; or preferable 6 to 10 portions of the salvianolic acid A from salvia miltiorrhiza, and 1 to 19 portions of the blood-quickening and stasis-dispelling effective compositions of the traditional Chinese medicines. As shown in a pharmacologic experiment of the composition, the medicine composition has good pharmacologic effect.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
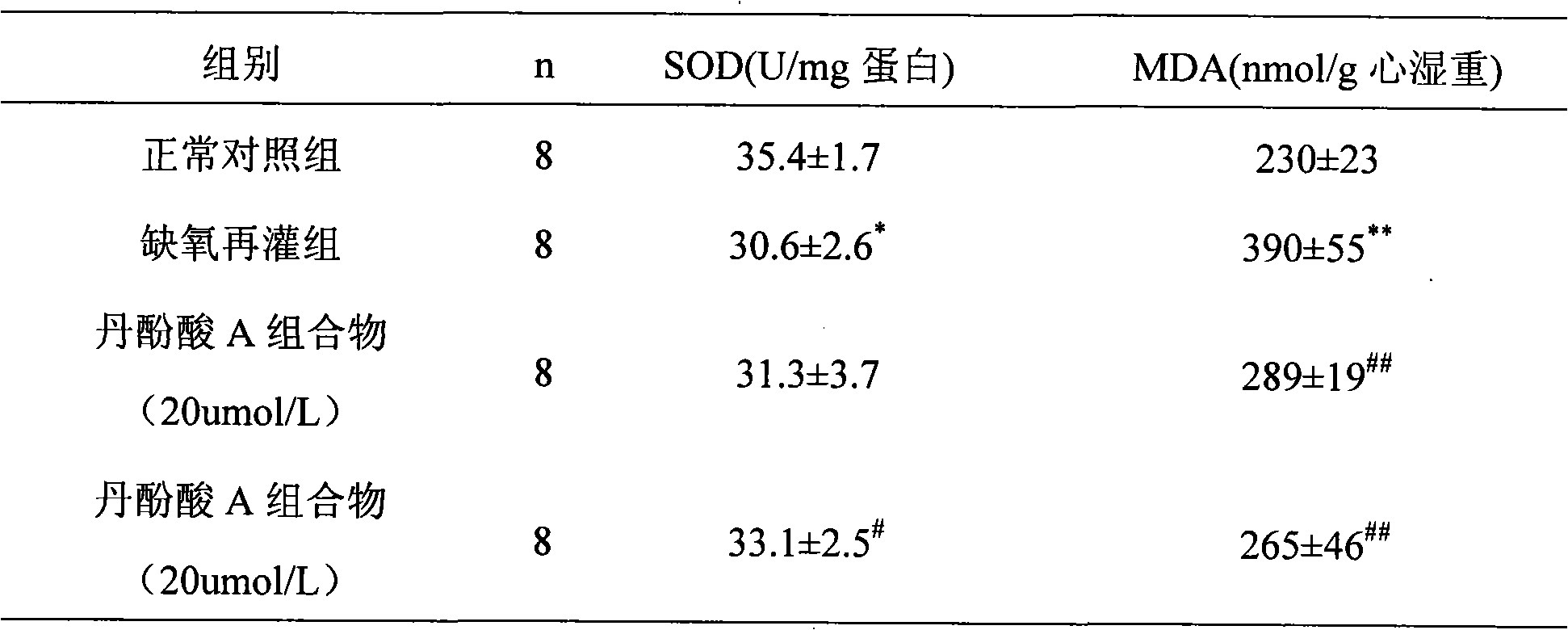
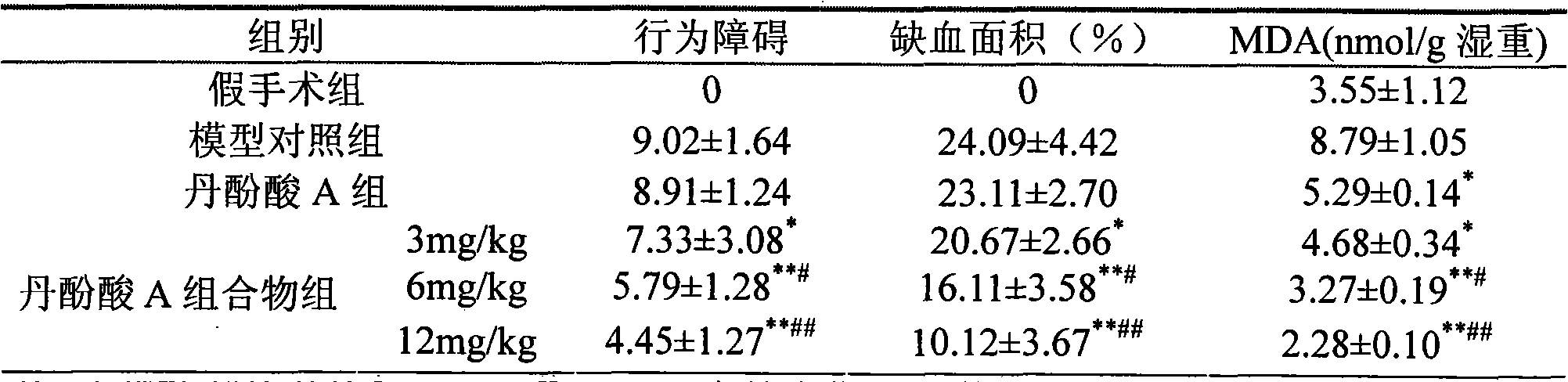
Application of salvianolic acid A composition in preparing medicines for improving neural function symptom after cerebral ischemia
ActiveCN103083297AGood water solubilityNot destroyedOrganic active ingredientsNervous disorderSalvianolic acid BMedicine
The invention relates to application of a salvianolic acid A composition in preparing medicines for improving neural function symptom after cerebral ischemia. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Medicinal composition mainly for curing cardiovascular and cerebrovascular diseases and preparation method thereof
The invention discloses a pharmaceutical composition for treating cardiovascular and cerebrovascular diseases and a preparation method thereof. Through a stability test, a pharmacodynamic screening and verification test and a toxicological test, the composition is determined to comprise the following components of (by weight parts): salvianolic acid A 1-10 and safflower yellow 1-30, preferably salvianolic acid A 1-5 and safflower yellow 20-30, or salvianolic acid A 6-10 and safflower yellow 1-19. The invention also discloses a detection method and an application of the pharmaceutical composition and the preparations thereof. The pharmacological experiment shows that the pharmaceutical composition has good pharmacological effect.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Method for preparing salvianolic acid A
ActiveCN103242161APrevent oxidationLow impurity contentOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid KSalvianolic acid B
The invention discloses a method for preparing salvianolic acid A. The method comprises the following steps of: (1), with a substance containing salvianolic acid B as a material, adding an acid substance with weight 0.1-10 times that of the raw material and concentration of 0.01%(v / v)-100%(v / v); adding an organic solvent with weight 0.01-10 times that of the acid substance and boiling point higher than 100 DEG C; heating for 3-5 hours at the temperature of 110-130 DEG C to obtain salvianolic acid A enrichment liquid, wherein the salvianolic acid B accounts for 2%-100% by weight in the substance containing the salvianolic acid B; and (2), cooling the salvianolic acid A enrichment liquid, adding water which is 2-6 times of the enrichment liquid to dilute and purifying to obtain the salvianolic acid A. The method for preparing salvianolic acid A can be used for preventing the salvianolic acid A from oxidizing in a high-temperature aqueous solution, improving the conversion rate of the salvianolic acid B substance, and achieving the material extracting rate of 20%-60%. Meanwhile, the impurity content and purifying difficulty of the final product salvianolic acid A are lowered, so that the prepared salvianolic acid A has purity higher than 90% and can be used for carrying out industrial production well.
Owner:CHENGDU KEYUAN BIOTECH
Application of salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage
The invention relates to application of a salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
An extracting, separating and purifying method of salvianolic acid A and a preparing method of salvianolic acid salts
InactiveCN105523926AEasy to prepareSimple and time-saving preparation methodOrganic compound preparationCarboxylic acid esters separation/purificationMagnesium saltPolyamide
The invention relates to an extracting, separating and purifying method of salvianolic acid A and a preparing method of salvianolic acid A salts. The extracting, separating and purifying method adopts salvia miltiorrhiza bunge var.alha or danshen root as a raw material and includes steps of solvent extraction, solvent extraction, concentration for drying, separation, purification, MCI column separation, crystallization and recrystallization to obtain the salvianolic acid A. Compared with macroporous resin columns, polyamide column chromatography adopted by the extracting, separating and purifying method can purify phenolic acid components more effectively and has better purification effects. A salvianolic acid A magnesium salt complexing process is adopted, and therefore chromatographic processes using macroporous resin or polyamide column chromatography can be omitted, and the purifying method is simpler, more time-saving and lower in cost. The preparing method of the salvianolic acid A magnesium salt is simple and suitable for large-scale production. A salvianolic acid A ammonium salt is a novel salvianolic acid A salt compound.
Owner:SHANDONG UNIV
Application of salvianolic acid A composition for preparing medicines for preventing and/or treating cerebral thrombosis
The invention relates to application of a salvianolic acid A composition for preparing medicines for preventing and / or treating cerebral thrombosis. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com