Red sage root salvianolic acid A injection formulation for treating cardiovascular diseases and preparation process thereof
A technology of salvianolic acid and injection preparations of salvia miltiorrhiza, applied in the field of traditional Chinese medicine pharmacy, can solve the problems of inability to have quantitative relationship, difficult preparation process, wide selection of excipients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
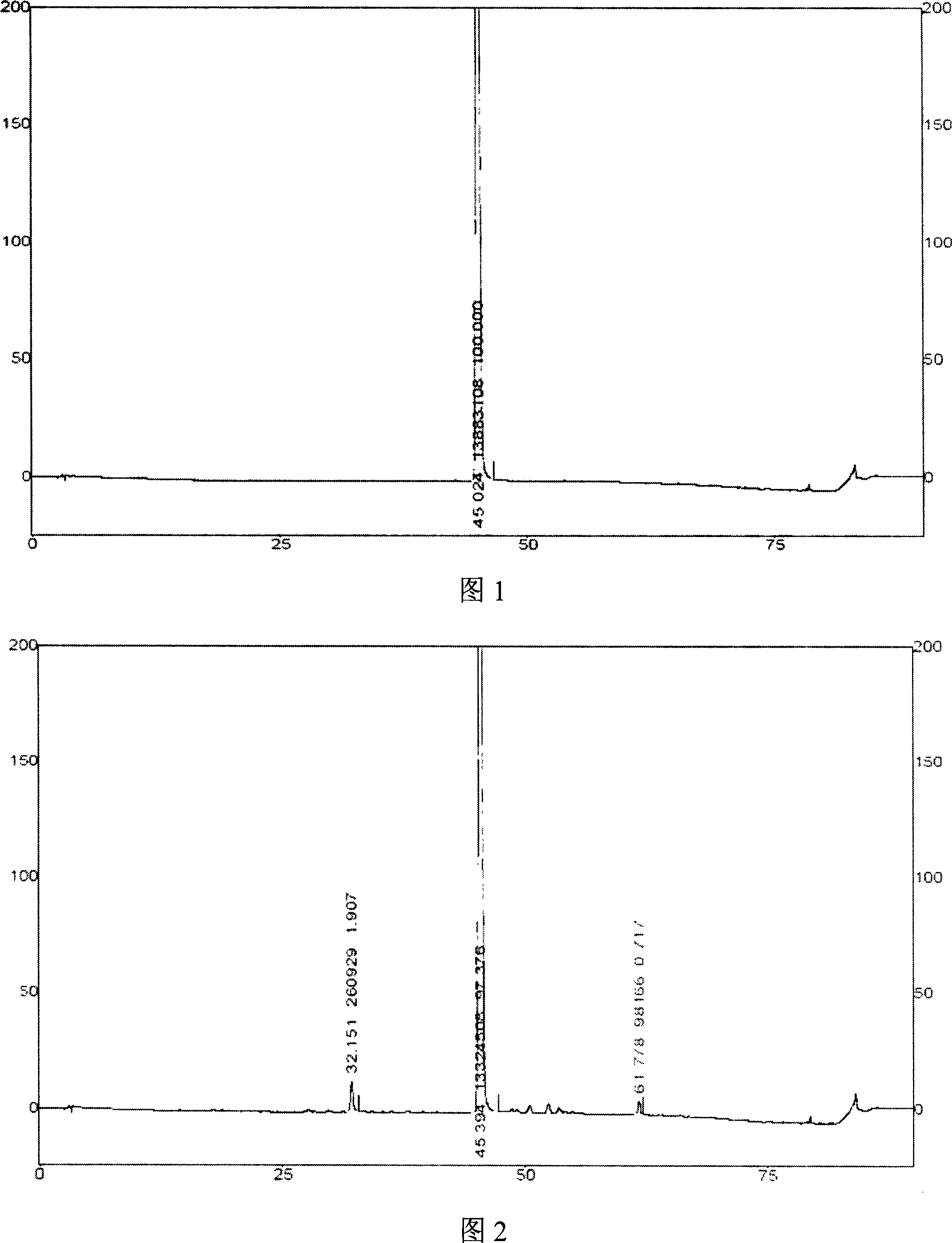
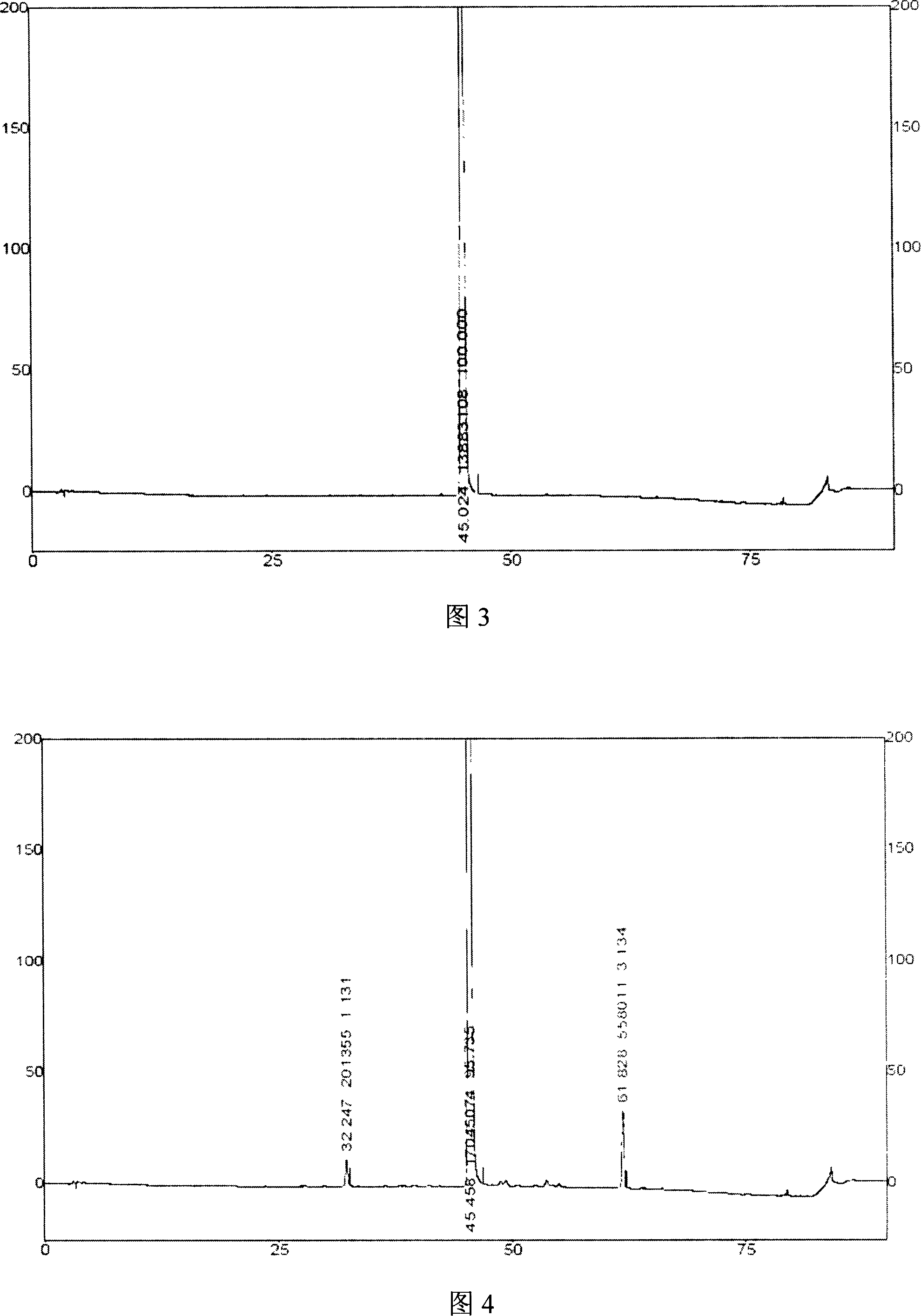
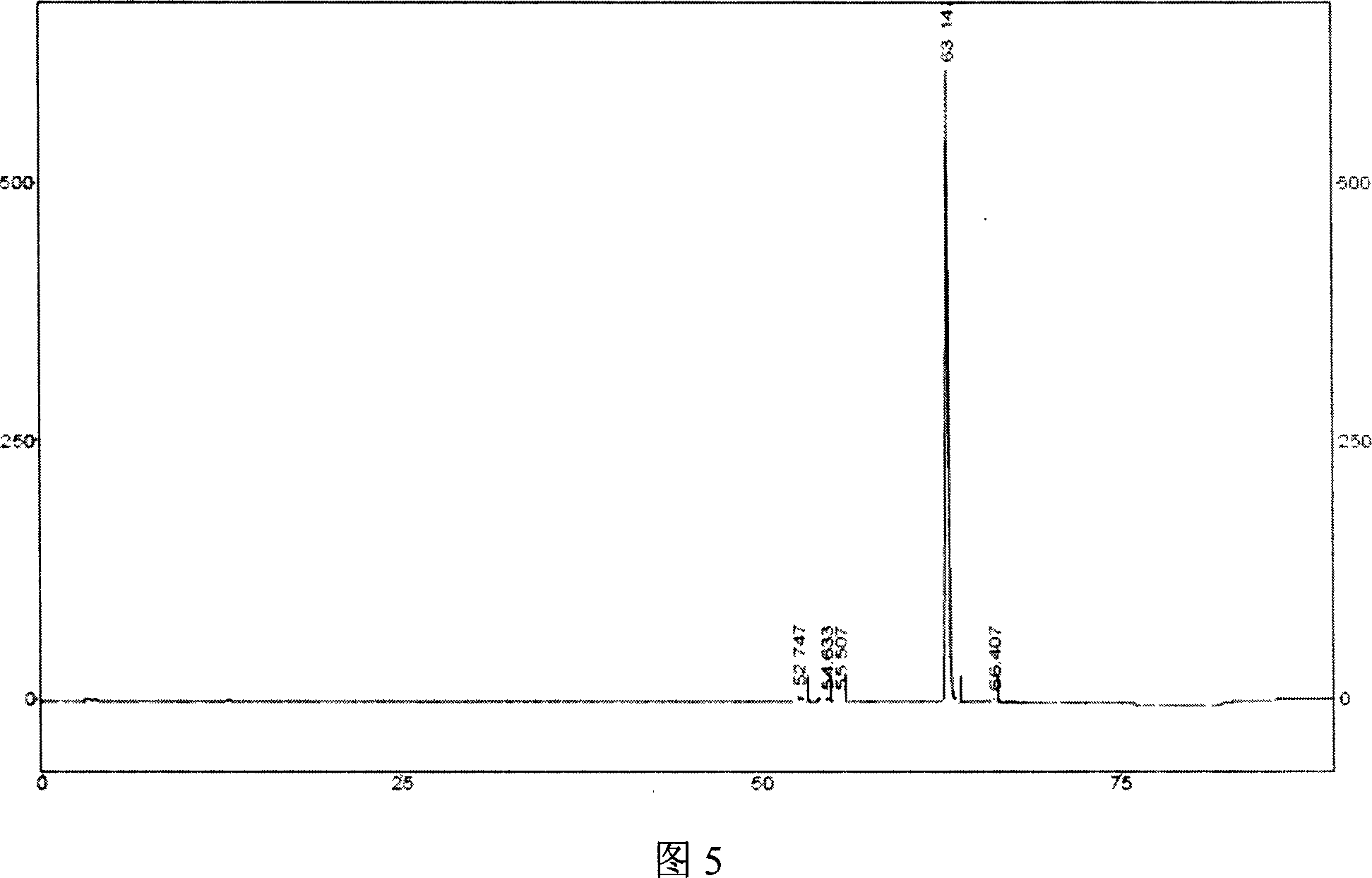
Image
Examples
Embodiment 1
[0127] Extraction and purification of salvianolic acid A:
[0128] Extract the salvia miltiorrhiza with water to obtain the water extract, adjust the pH value to 3.5, 110°C temperature, 0.05MPa pressure, and heat for 1 hour; the solution is filtered, and the filtrate is separated by HPD-100 macroporous resin column chromatography, firstly water, 10% dilute ethanol Elution, remove impurities, and then elute with 30% ethanol, collect the part containing salvianolic acid A, concentrate the eluate until it has no alcohol smell; the concentrated solution is separated by polyamide chromatography column, firstly use water, 20% ethanol solution Elution, the eluent was discarded, and then eluted with 95% ethanol solution, the eluent was collected, and the ethanol was concentrated to the utmost; the concentrated solution was adjusted to a pH value of 2, extracted with ethyl acetate, and the organic solvent phase was separated to obtain the drug-containing solution, concentrated, dried o...
Embodiment 2
[0130] Extraction and purification of salvianolic acid A:
[0131] Salvia miltiorrhiza was extracted with ethanol solution to obtain alcohol extract, the ethanol was concentrated until exhausted, the pH value was adjusted to 6.0, the temperature was 130°C, the pressure was 0.17MPa, and heating was carried out for 6 hours; the solution was filtered, and the filtrate was separated by HPD-100A macroporous resin column chromatography. Elute with water and 30% dilute ethanol to remove impurities, then elute with 70% ethanol to collect the part containing salvianolic acid A, and concentrate the eluate until it has no alcohol smell; use Sephadex LH-20 for the concentrate , first eluted with water and 20% ethanol solution, the eluent was discarded, and then eluted with 95% ethanol solution; the eluent was collected, and the ethanol was concentrated to the utmost; Extract and separate the organic solvent phase to obtain a drug-containing solution, concentrate, dry or freeze-dry to obta...
Embodiment 3
[0133] Extraction and purification of salvianolic acid A:
[0134] Extract the salvia miltiorrhiza with water to obtain the water extract, adjust the pH value to 5.0, 120°C temperature, 0.10MPa pressure, and heat for 4 hours; the solution is filtered, and the filtrate is separated by HPD-300 macroporous resin column chromatography, firstly water, 20% dilute ethanol Elution, remove impurities, and then elute with 50% ethanol, collect the part containing salvianolic acid A, concentrate the eluent until it has no alcohol smell; Elute, discard the eluent, and then elute with 75% ethanol solution, concentrate the ethanol to the utmost; adjust the pH value of the concentrated solution to 3, extract with the organic solvent butyl acetate, separate the organic solvent phase, and obtain the drug-containing solution, concentrate , dried or freeze-dried to obtain salvianolic acid A;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com