Application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and/or in-stent restenosis
An angioplasty and vascular intima technology is applied in the application field of salvianolic acid A in the preparation of anti-intimal thickening, anti-angioplasty and/or in-stent restenosis drugs, and can solve the problem of unseen dandruff. Phenolic acid A application reports and other issues, to achieve significant vascular intimal thickening and lumen restenosis, reduce restenosis rate, anti-intimal thickening and lumen restenosis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
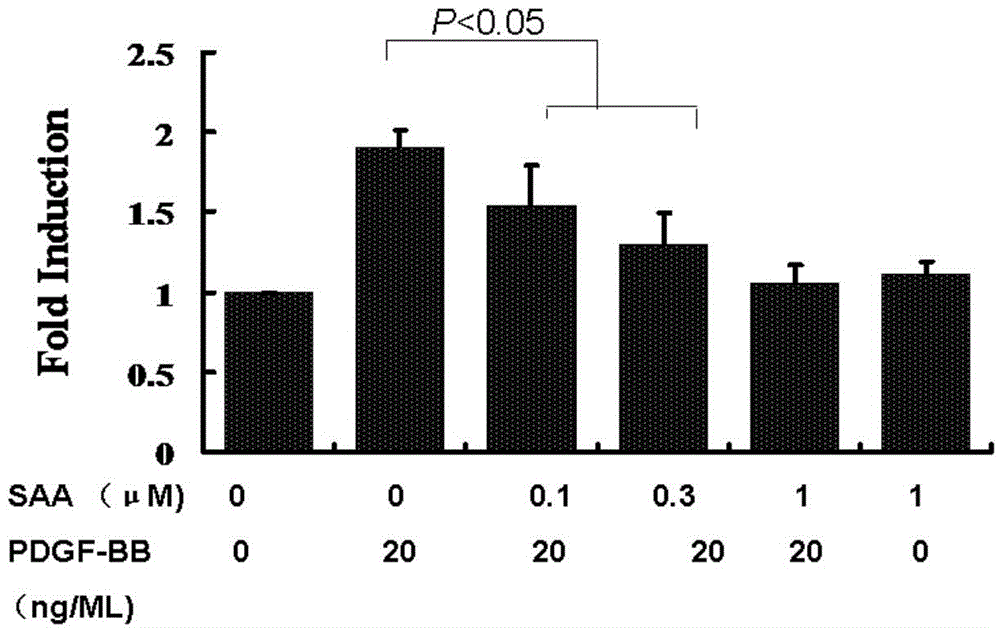
[0037] Example 1. SAA inhibits the proliferation of human umbilical artery smooth muscle cells induced by PDGF-BB
[0038] Experimental Materials:
[0039] Human umbilical artery smooth muscle cells (hUASMCs) were purchased from ScienCell (Carlsbad, Calif). Smooth muscle cell culture medium: ScienCell (Carlsbad, Calif), fetal bovine serum FBS (Gibco). PDGF-BB was purchased from R&D Company. SAA was dissolved in DMSO into a 20mM stock solution, and the final concentration of DMSO was less than 0.1%, which had no effect on the growth of smooth muscle cells. The stock solution was aliquoted and stored in a -20°C refrigerator in the dark.
[0040] human umbilical artery smooth muscle cells 4 The density of each well was planted in a 96-well plate until 50% confluence, the serum was withdrawn and incubated for 48 hours, then according to the experimental design, 0, 0.1, 0.3 and 1 μM SAA were added for pre-incubation for 24 hours, and then 20ng / ml PDGF-BB were respectively conti...
Embodiment 2
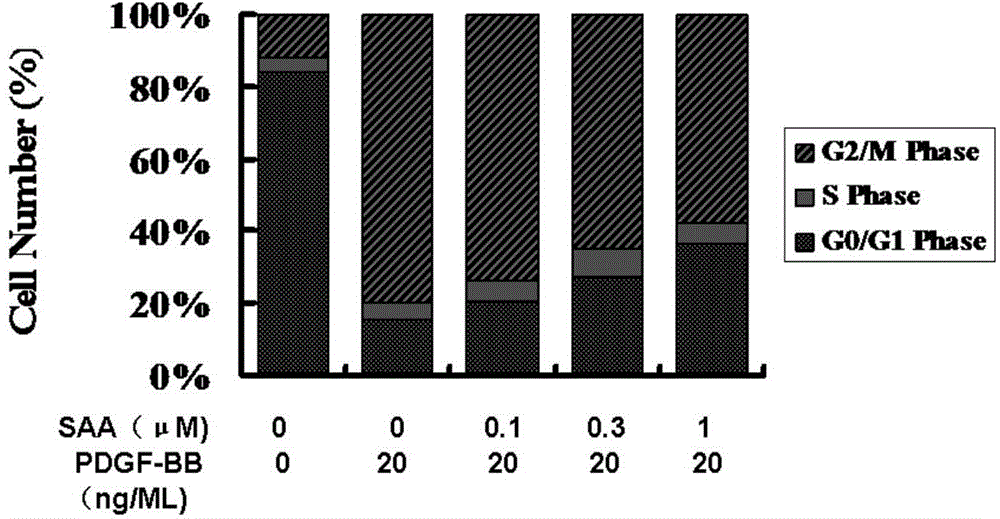
[0044] Example 2. SAA inhibits the cell cycle of human umbilical artery smooth muscle cells
[0045] Cell culture method is the same as embodiment 1. smooth muscle cells at 5×10 6 The density was planted in a 100mm petri dish until it was 50% confluent, and cultured in serum-free DMEM medium for 48h. Give different concentrations of SAA for pre-incubation for 24 hours, then give 20ng / ml PDGF-BB stimulation, and continue to incubate for 48 hours; collect the cell supernatant, digest the cells with 0.25% trypsin, collect the cell suspension, centrifuge at 1000r / min×5min, discard supernatant. Fix with 70% cold ethanol pre-cooled at 4°C for 18h, then adjust the cell concentration to 10 6 / ml, stained with propidium iodide (PI) and incubated at 37°C for 30 min, followed by flow cytometry analysis, and the percentage of cells in each phase of the cell cycle was analyzed by using ModFitLT3.0 software; the cell proliferation index (PI) was calculated, PI = (S+G2 / M) / (G0 / G1+S+G2 / M)....
Embodiment 3
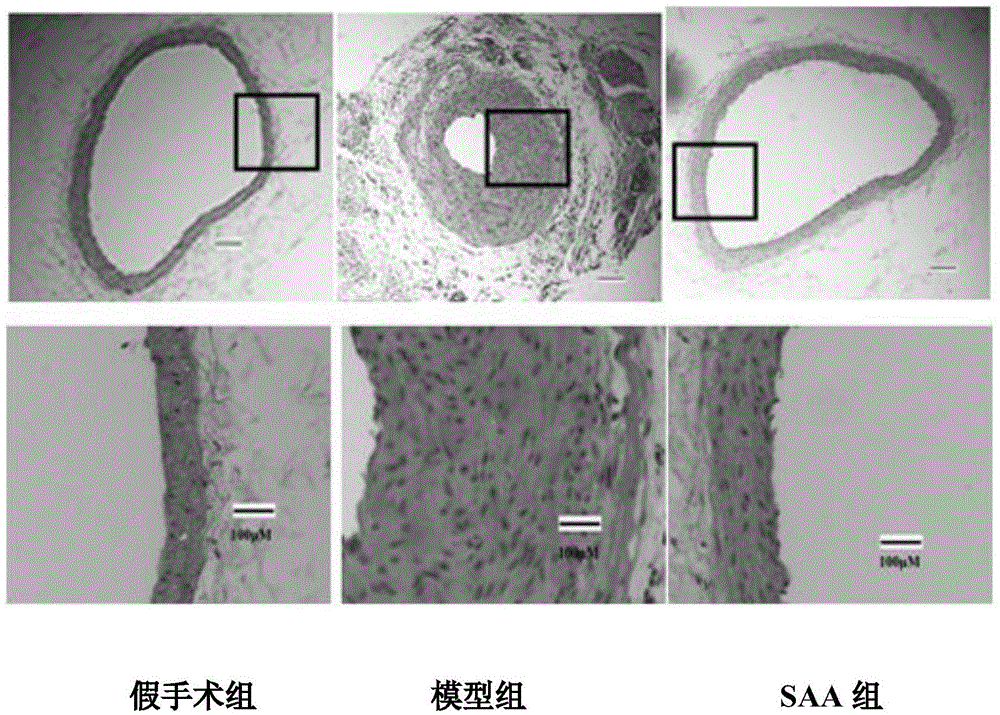
[0050] Example 3. Experimental study of SAA against restenosis after balloon-injured common carotid artery in rats
[0051] The restenosis model of rat common carotid artery balloon injury is an internationally recognized classic animal model of restenosis after PTA and in-stent restenosis.
[0052] Experimental materials: salvianolic acid A (purity ≥ 98%), made into 0.01mg / mL with triple distilled water; male SD rats with a body weight of 300-350g (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) .
[0053] Experimental method: the rats were randomly divided into 3 groups, namely the sham operation group, the model group and the salvianolic acid A group; after intraperitoneal anesthesia with 30% chloral hydrate 0.1mL / 100g. The skin and subcutaneous tissue were incised in the front of the neck, the common carotid artery and the internal carotid artery were clamped with arterial clips, a wedge-shaped incision was made in the external carotid arter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com