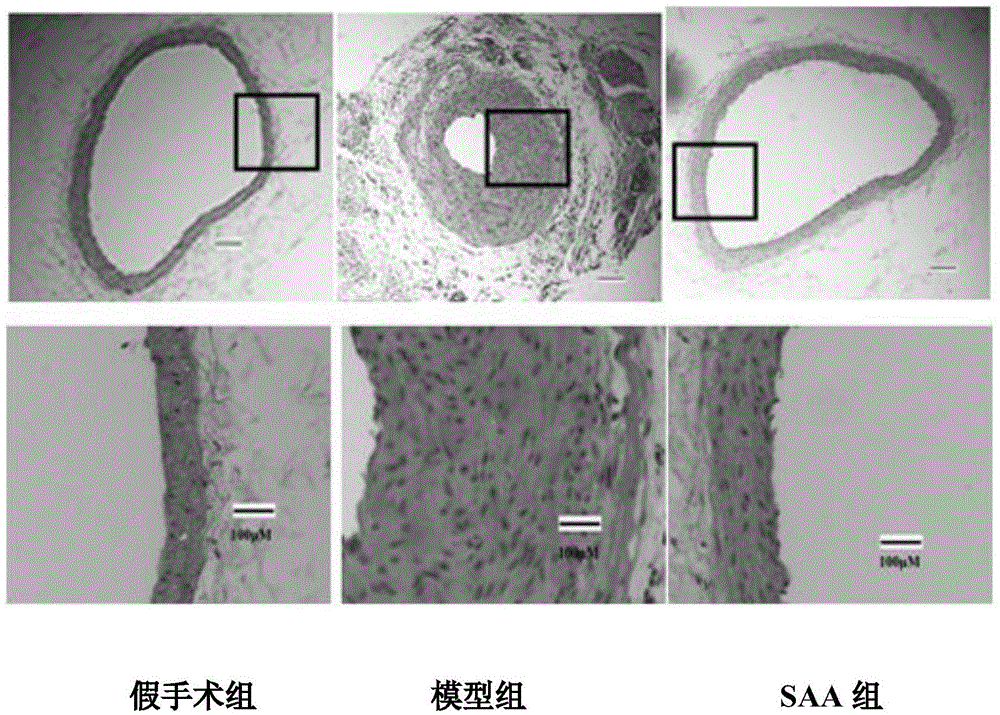
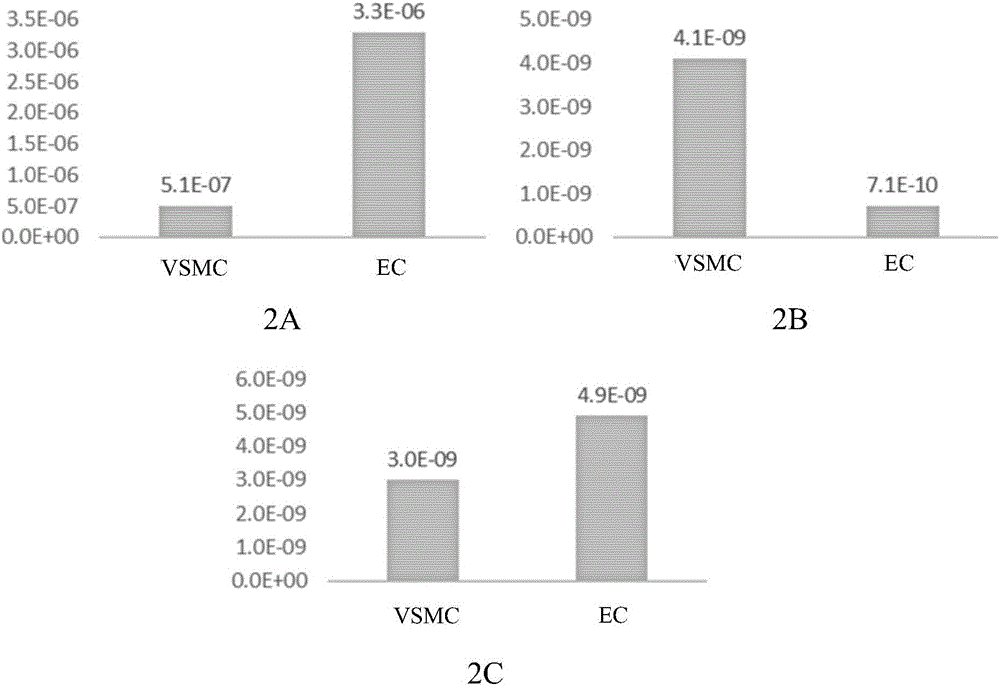
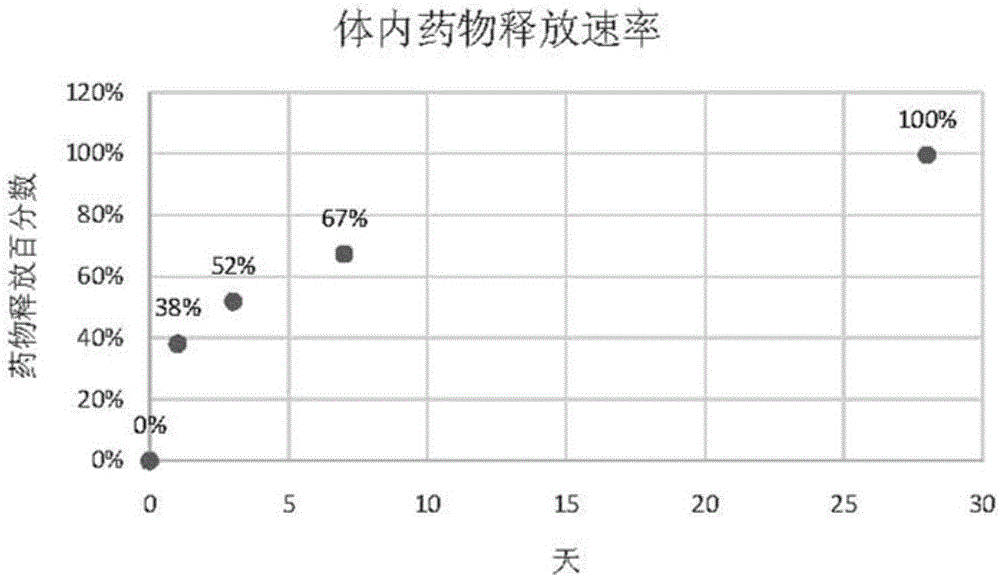
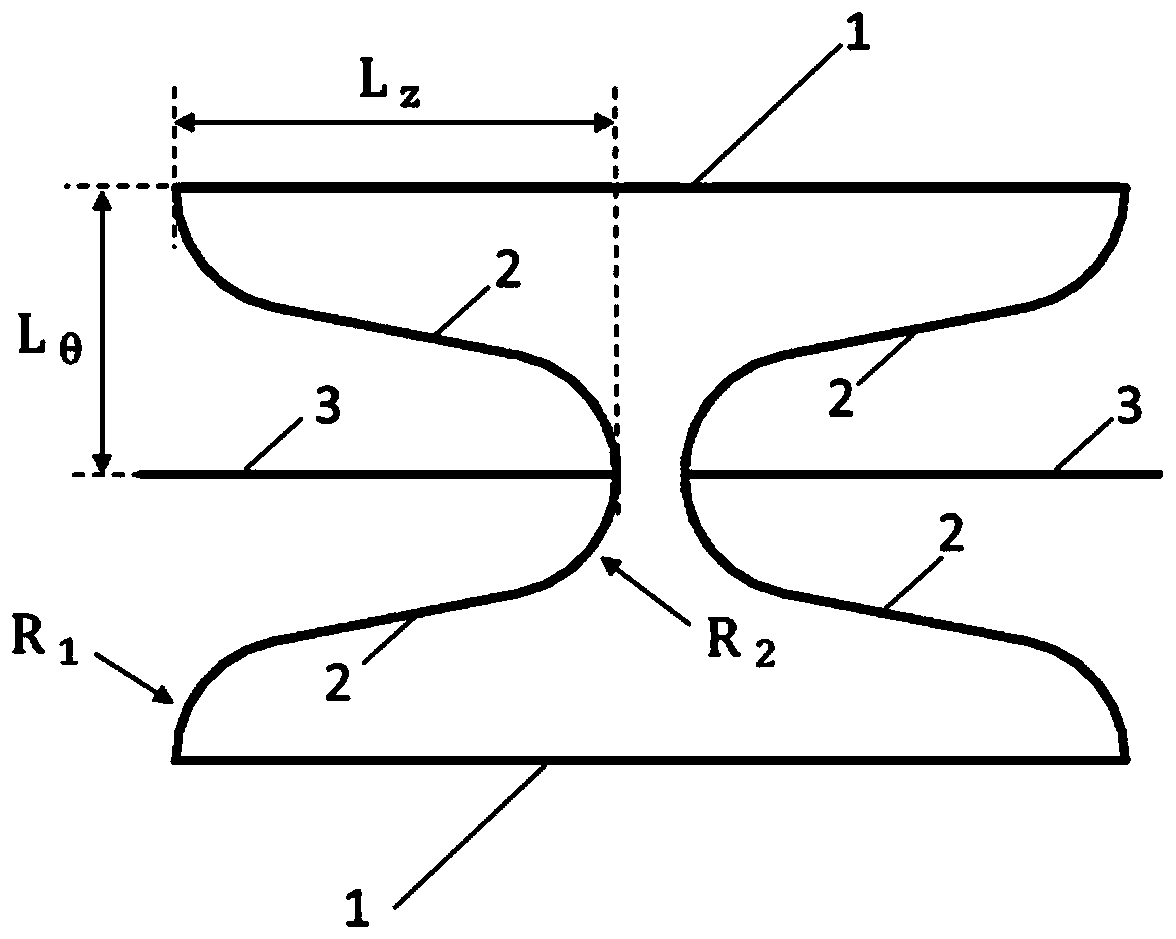
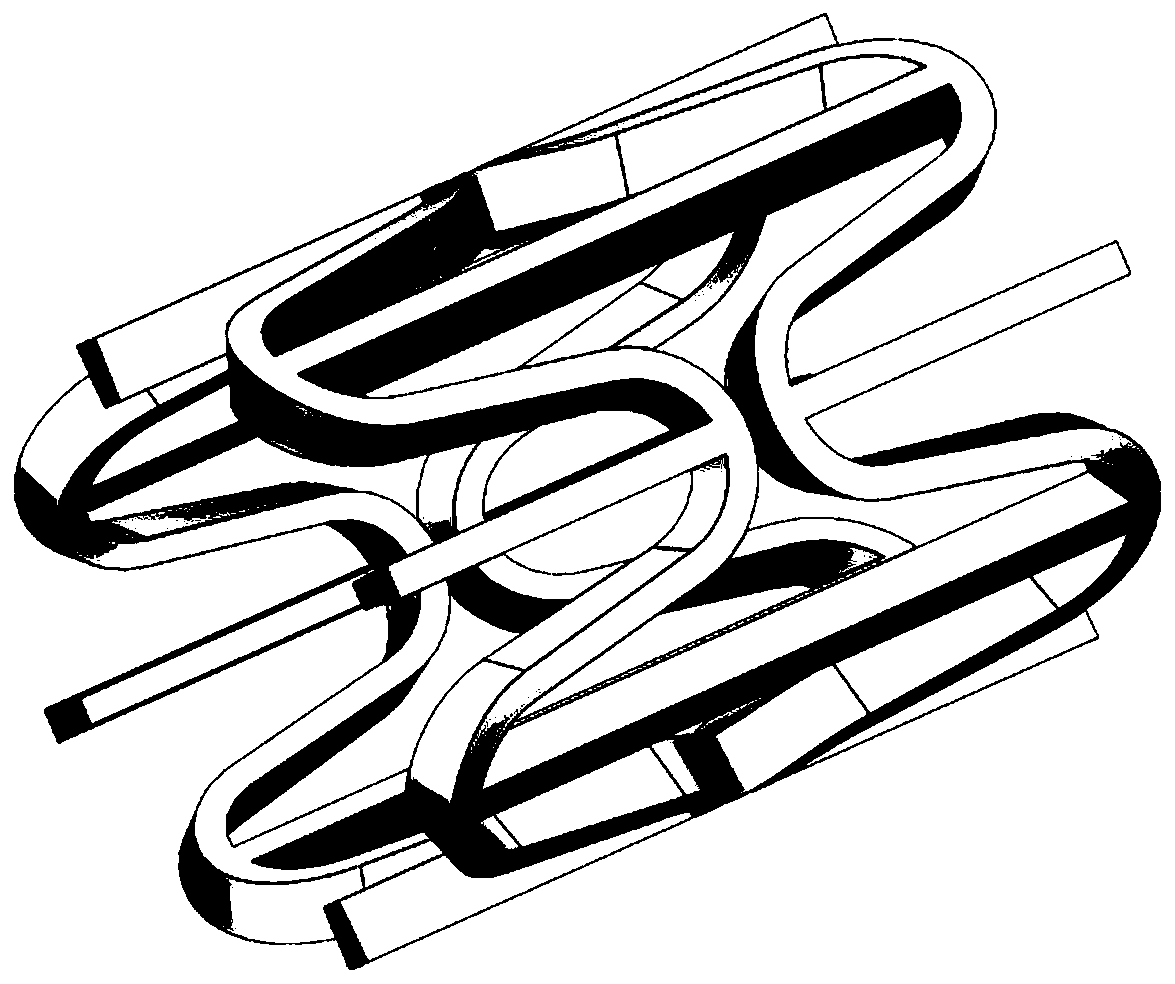
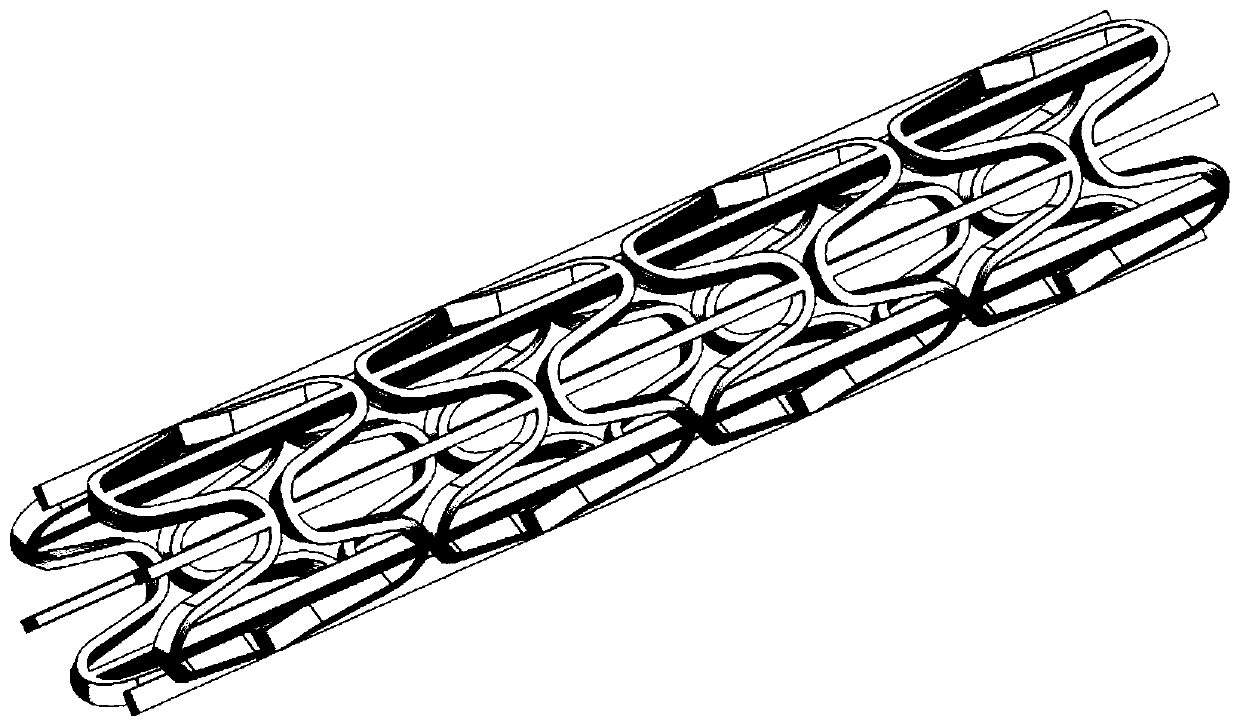
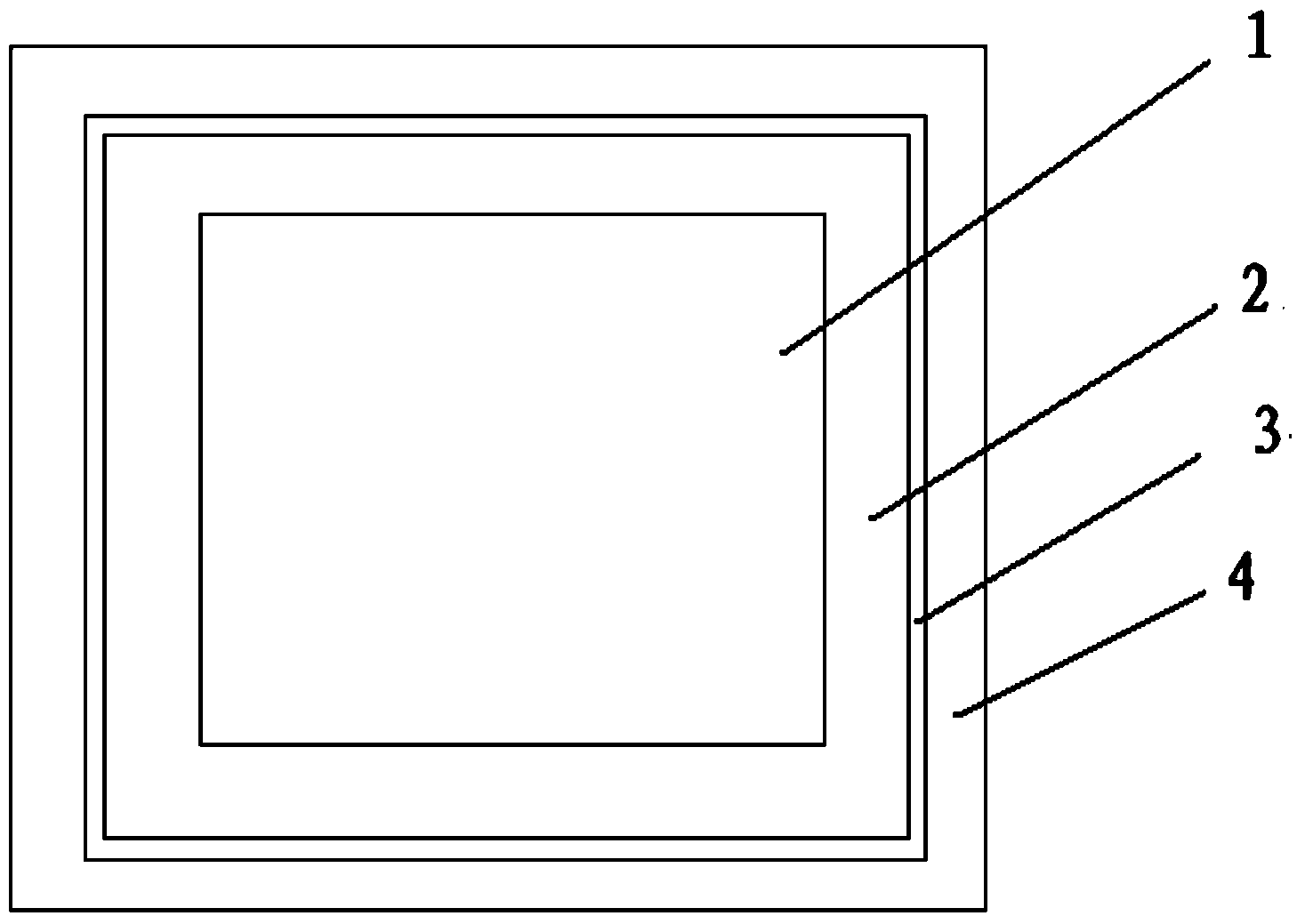
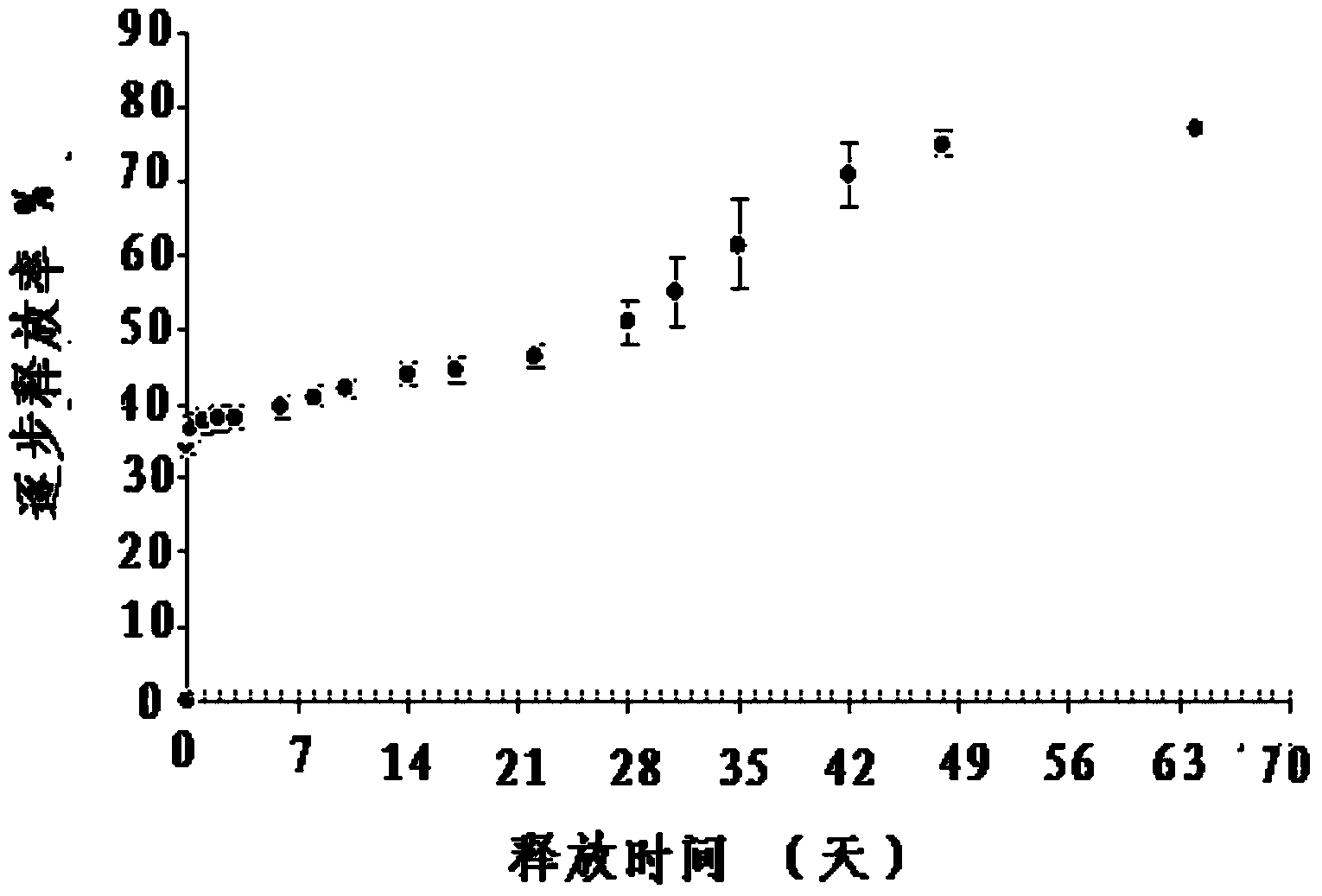
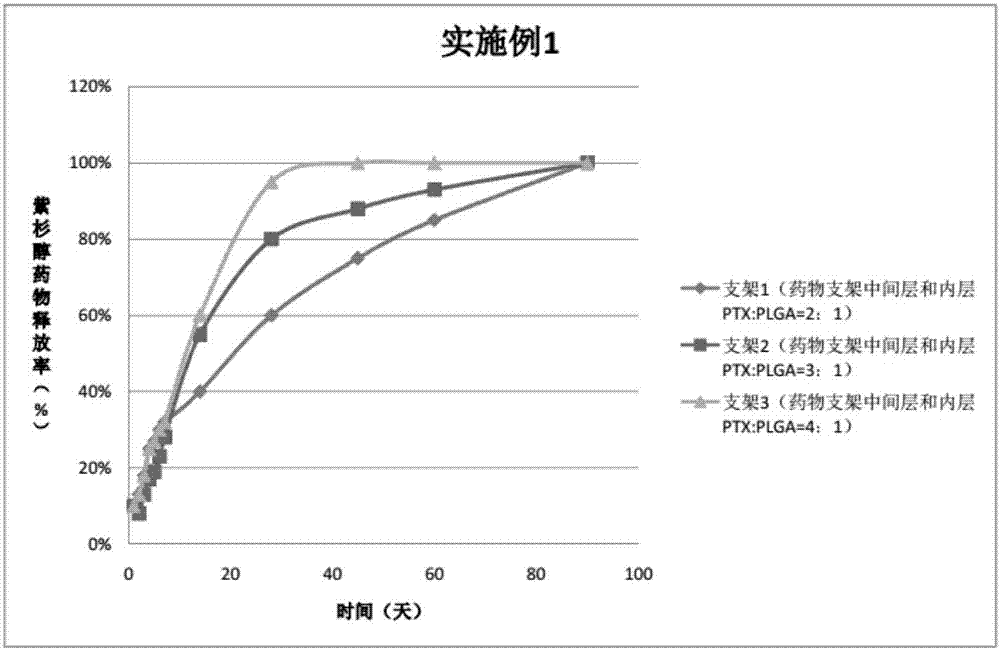
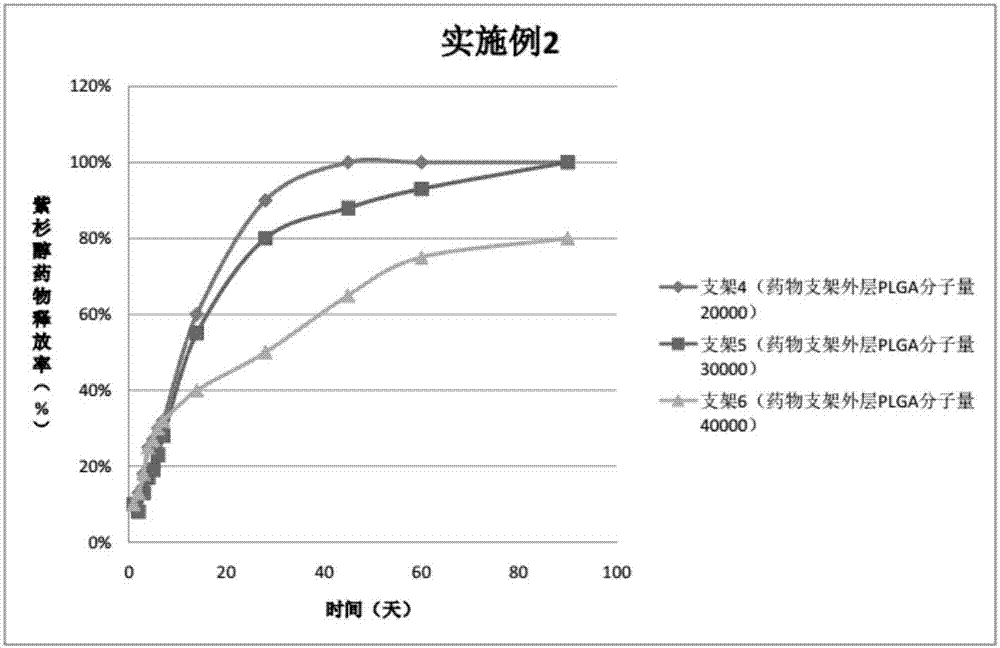
Patents
Literature
57 results about "In stent restenosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
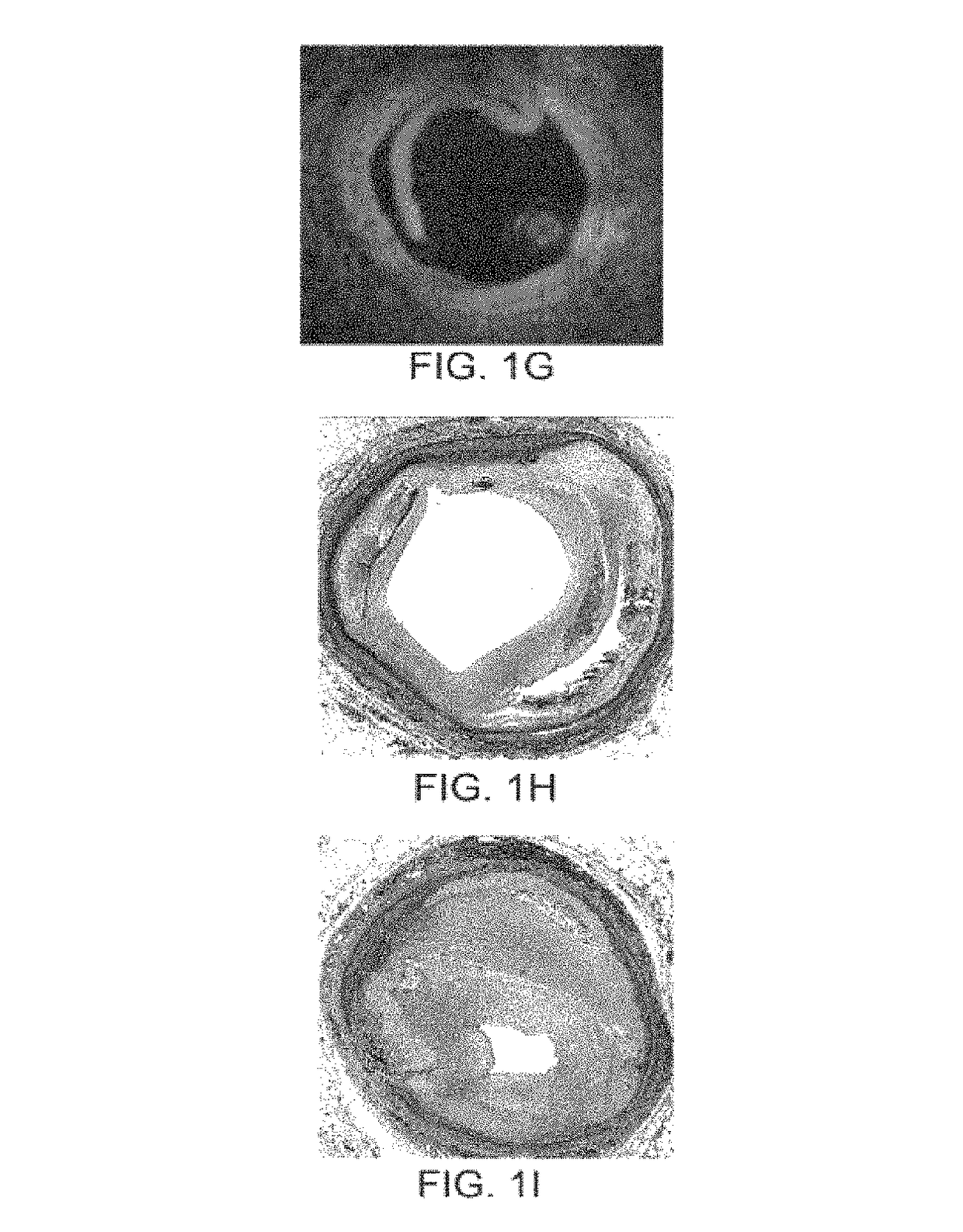
In-stent restenosis (ISR) Angioplasty, a type of percutaneous coronary intervention (PCI), is a procedure used to open up blocked arteries. During the procedure, a small metal scaffold, called a cardiac stent, is almost always placed in the artery where it was reopened.
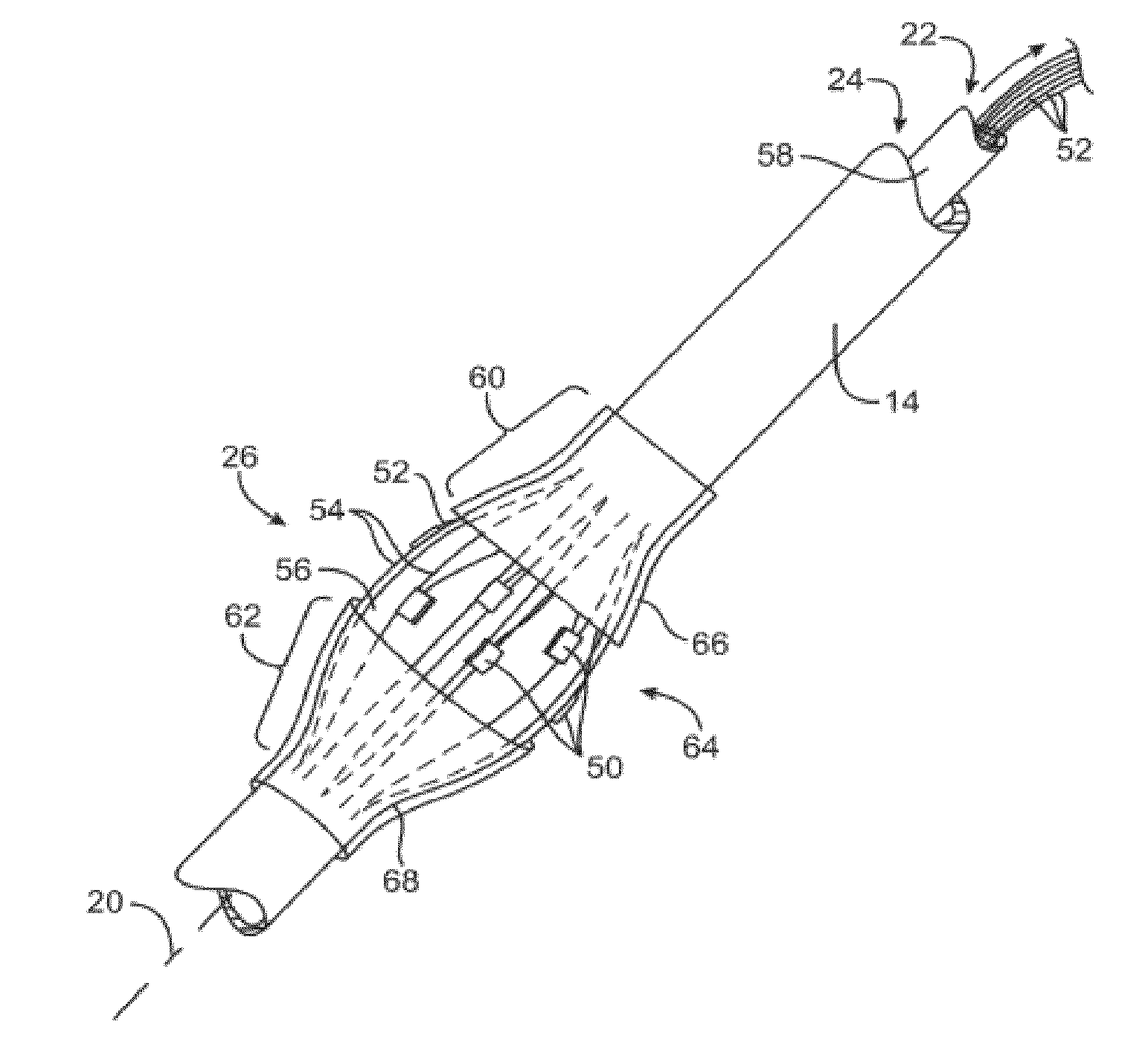
Apparatus and Method for Treatment of In-Stent Restenosis
ActiveUS20130282084A1Big vessel lumenIncrease blood flowSurgical instrument detailsLight therapyInsertion stentCatheter
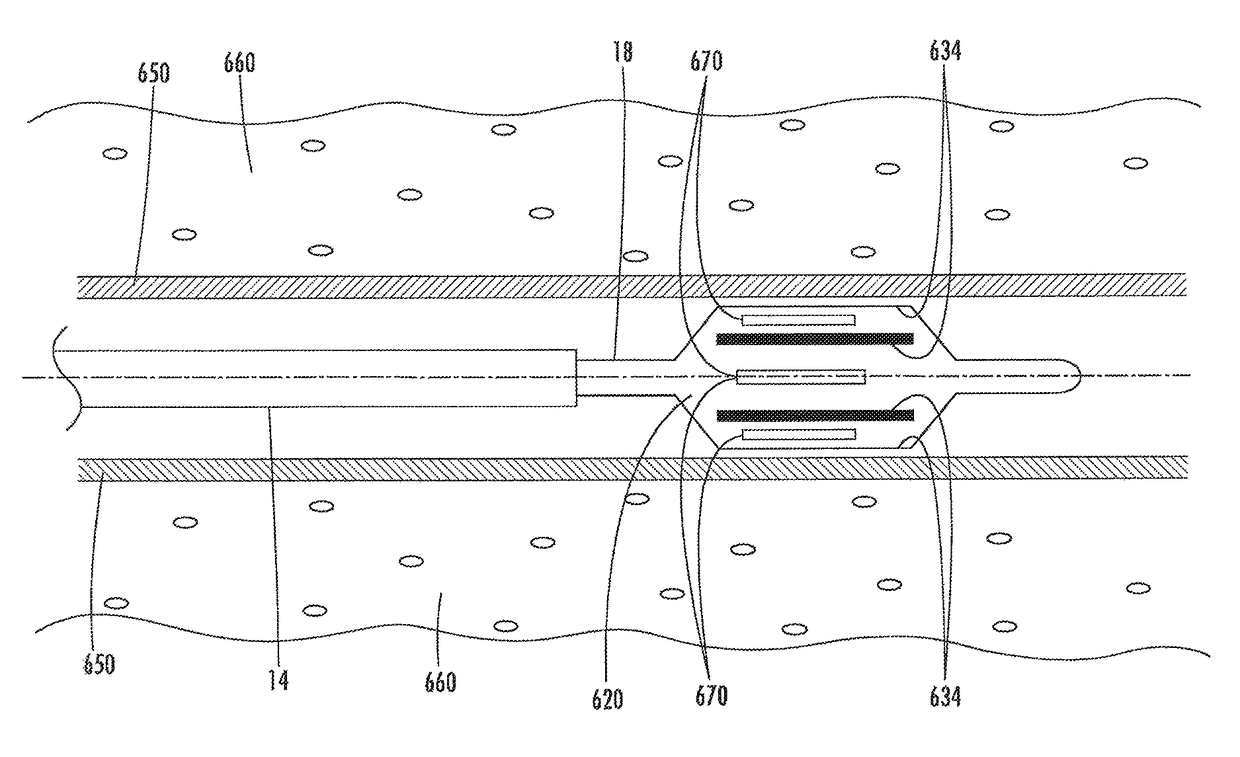
A catheter and catheter system can use energy tailored for remodeling and / or removal of target material proximate to a body lumen, often of stenotic material or tissue in the luminal wall of a blood vessel of a patient. An elongate flexible catheter body with a radially expandable structure may have a plurality of electrodes or other electrosurgical energy delivery surfaces to radially engage the luminal wall when the structure expands. Feedback using one or parameters of voltage, current, power, temperature, impedance magnitude, impedance phase angle, and frequency may be used to selectively control the delivery of energy.
Owner:BOSTON SCI SCIMED INC
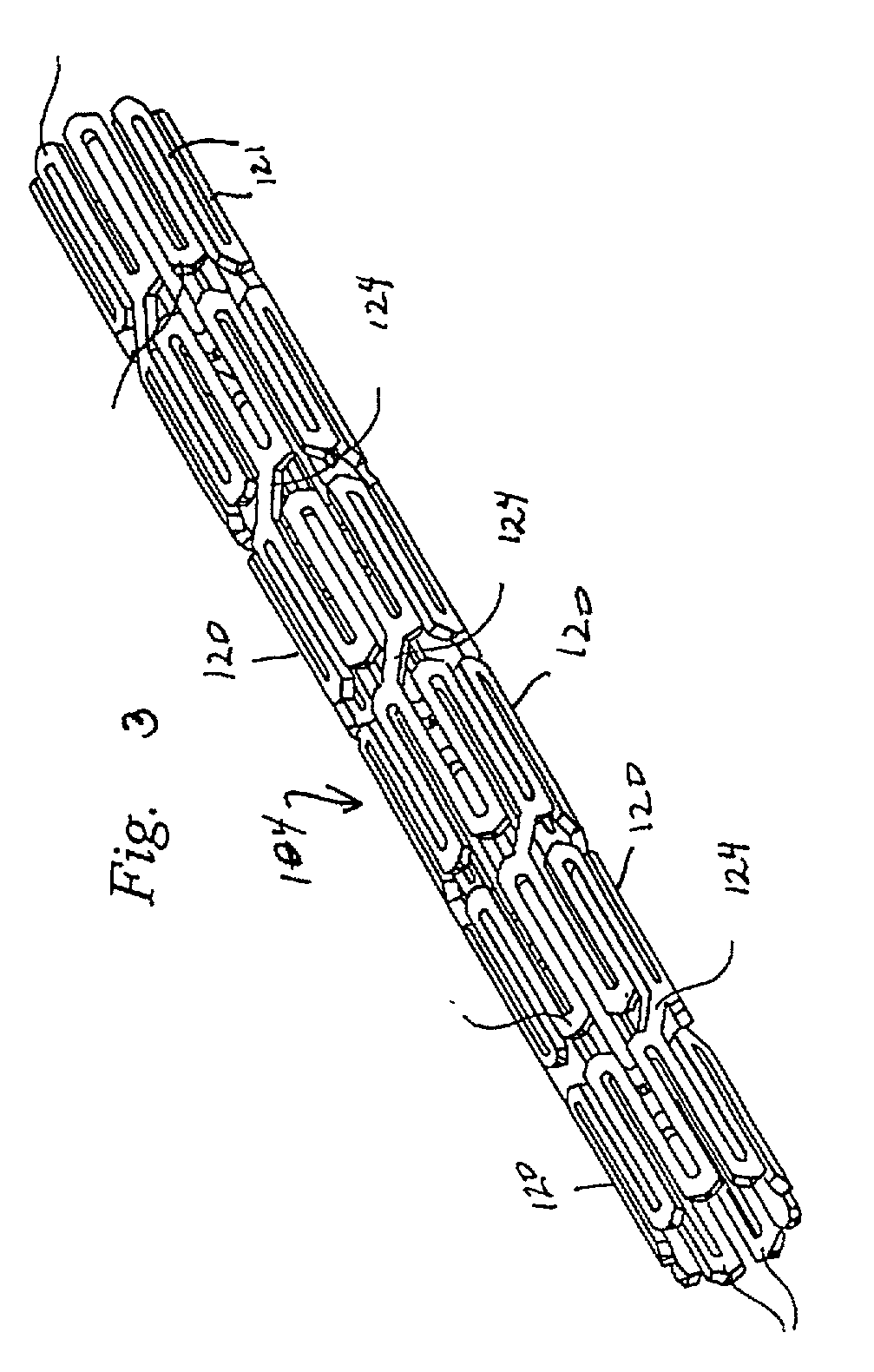
Stent for in-stent restenosis
A second stent having a wall thickness of 0.002 inches or less or a second stent having a tubular surface with openings therethrough where the ratio of the area of the surface to the area of the openings is at least 3:10 may be implanted in a first stent which has been previously implanted in a bodily vessel.
Owner:BOSTON SCI SCIMED INC
Micromantled drug-eluting stent
InactiveUS20060195142A1Avoid complicationsEliminate direct impactSuture equipmentsStentsElectrospinningMedicine
Pharmacologically active, easy-to-deploy, biomechanically compatible, inflatable endovascular, drug-eluting stent are formed of a primary expandable polymeric or metallic construct, intimately mantled with a biomechanically compatible, polymeric microporous, microfibrous, compliant, stretchable fabric formed by direct electrospinning onto the outside surface of the primary construct using at least one polymer solution containing at least one active compound, selected from those expected to control key biological events leading to in-stent restenosis.
Owner:POLY MED
Apparatus and method for treatment of in-stent restenosis
ActiveUS9713730B2Avoid injuryGood curative effectSurgical instruments for heatingLight therapyProximateBlood vessel
A catheter and catheter system can use energy tailored for remodeling and / or removal of target material proximate to a body lumen, often of stenotic material or tissue in the luminal wall of a blood vessel of a patient. An elongate flexible catheter body with a radially expandable structure may have a plurality of electrodes or other electrosurgical energy delivery surfaces to radially engage the luminal wall when the structure expands. Feedback using one or parameters of voltage, current, power, temperature, impedance magnitude, impedance phase angle, and frequency may be used to selectively control the delivery of energy.
Owner:BOSTON SCI SCIMED INC
Micromantled drug-eluting stent
InactiveUS7416559B2Avoid complicationsEliminate direct impactSuture equipmentsStentsElectrospinningMedicine
Owner:POLY MED
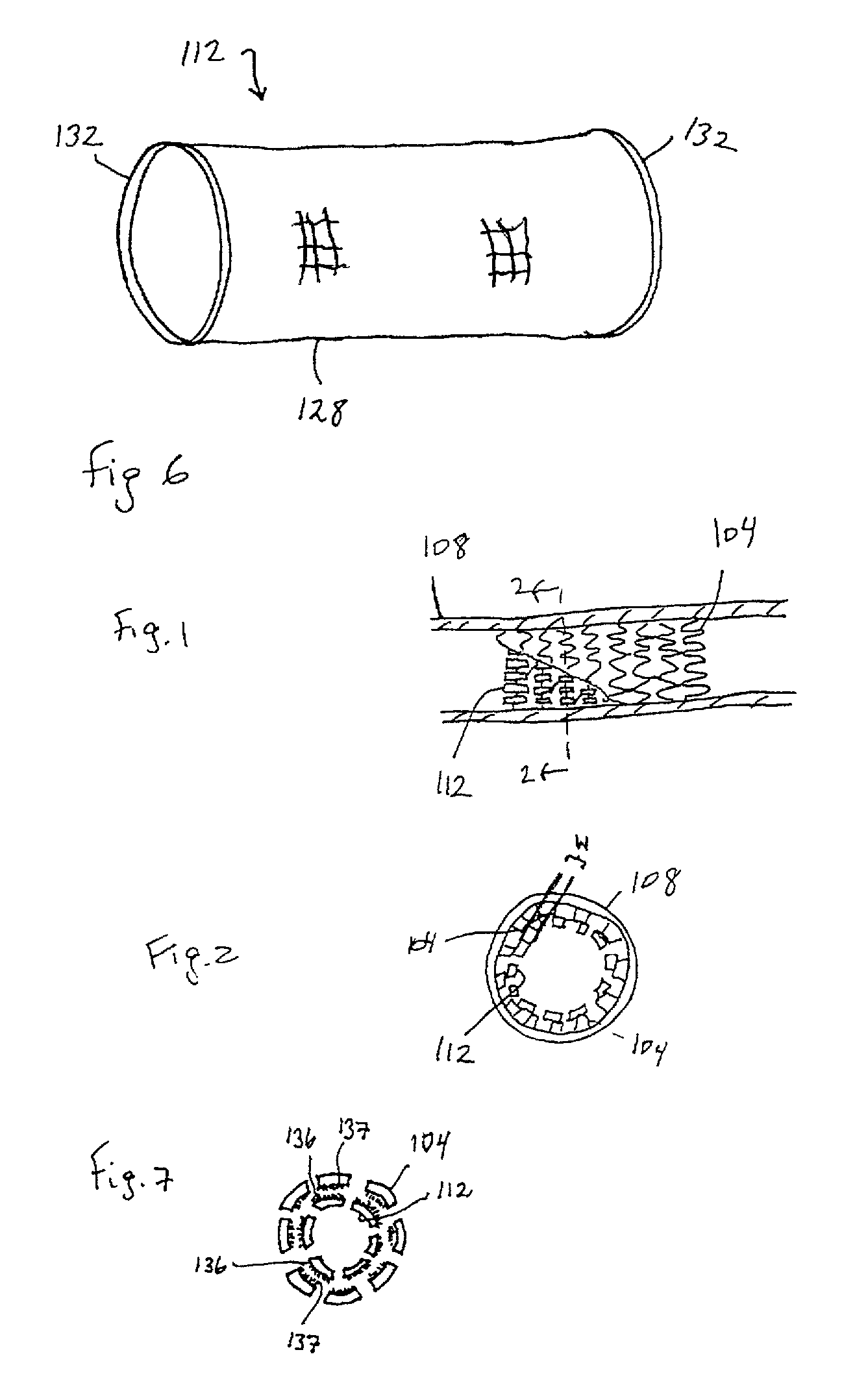
Method for manufacturing biodegradable shape-memory polymer intravascular stent additive with negative poisson ratio
InactiveCN106236338APrecise positioningInhibit migrationStentsAdditive manufacturing apparatusManufacturing technologyIntravascular stent
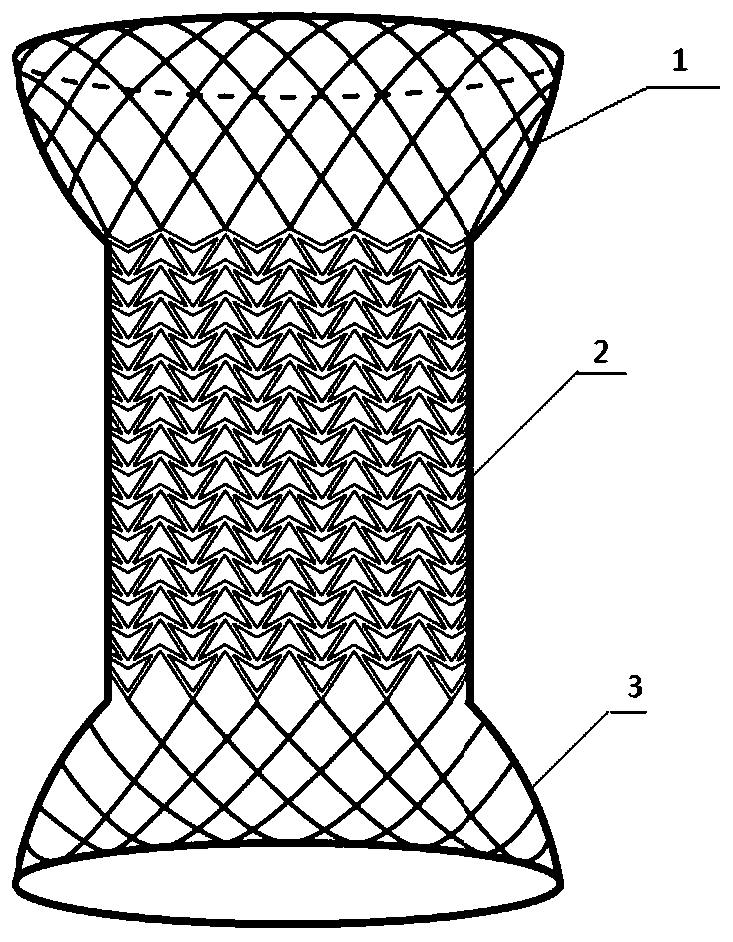
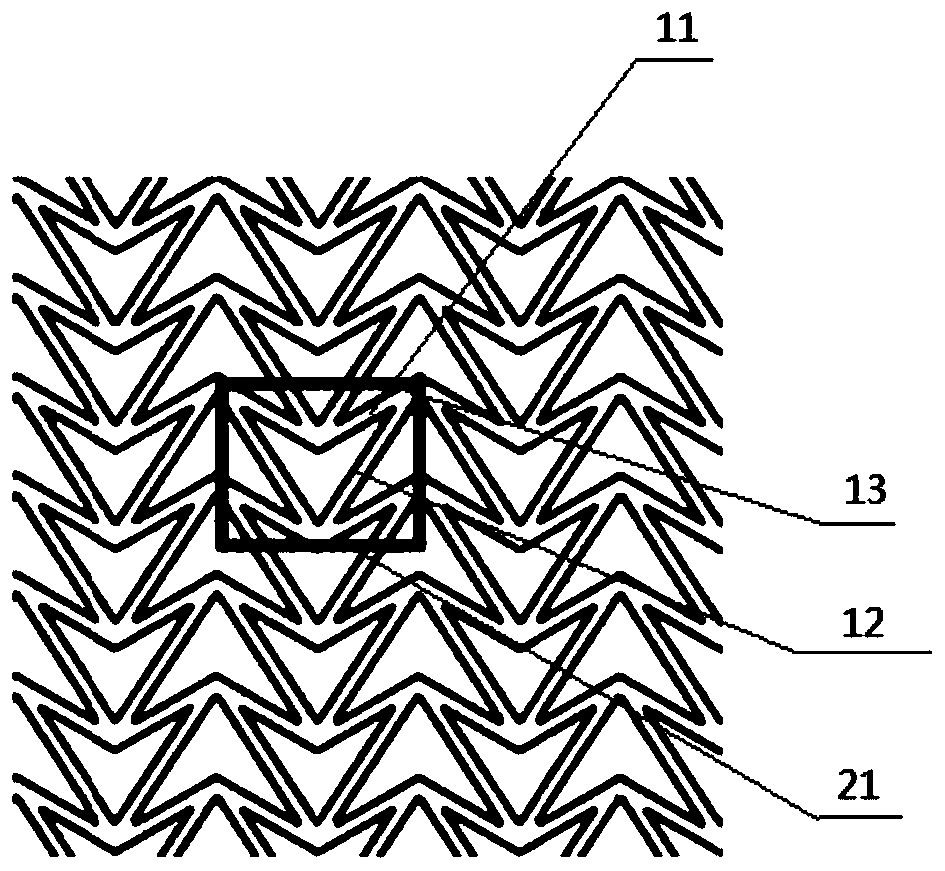
The invention relates to a method for manufacturing a biodegradable shape-memory polymer intravascular stent additive with a negative poisson ratio. The method comprises the following steps: firstly, designing an intravascular stent original configuration with negative poisson ratio in radial and axial directions based on a negative poisson ratio structural unit; then, manufacturing additive of the biodegradable shape-memory polymer intravascular stent additive with a negative poisson ratio by adopting a micro-droplet jetting additive technology; and finally, testing the performance of the intravascular stent. According to the method, a complex geometric intravascular stent from micro-droplet jetting additive, so that the shape and performance controlling capacity of the intravascular stent can be effectively improved; minimal invasive implantation of the intravascular stent can be realized by utilizing the negative poisson ratio; in the stent expanding process, the vascular wall is uniformly stressed, so that injury can be effectively avoided, and stent migration is avoided due to no axial contraction; and the shape-memory spontaneous gradual expansion and biodegradability of the biodegradable shape-memory polymer intravascular stent can be realized under dynamic excitation, and the problems of in-stent restenosis, taking-out difficulty after implantation and the like can be effectively avoided.
Owner:JILIN UNIV
VEGF variant that lacks VEGFR-1 binding activity and its use in promotion of re-endothelization and prevention of in-stent restenosis
InactiveUS20090291891A1Organic active ingredientsPeptide/protein ingredientsPercent Diameter StenosisCancer research
A VEGF145 polypeptide devoid of a VEGFR-1 binding activity and methods of making and using same in preventing and / or treating restenosis are provided.
Owner:TECHNION RES & DEV FOUND LTD
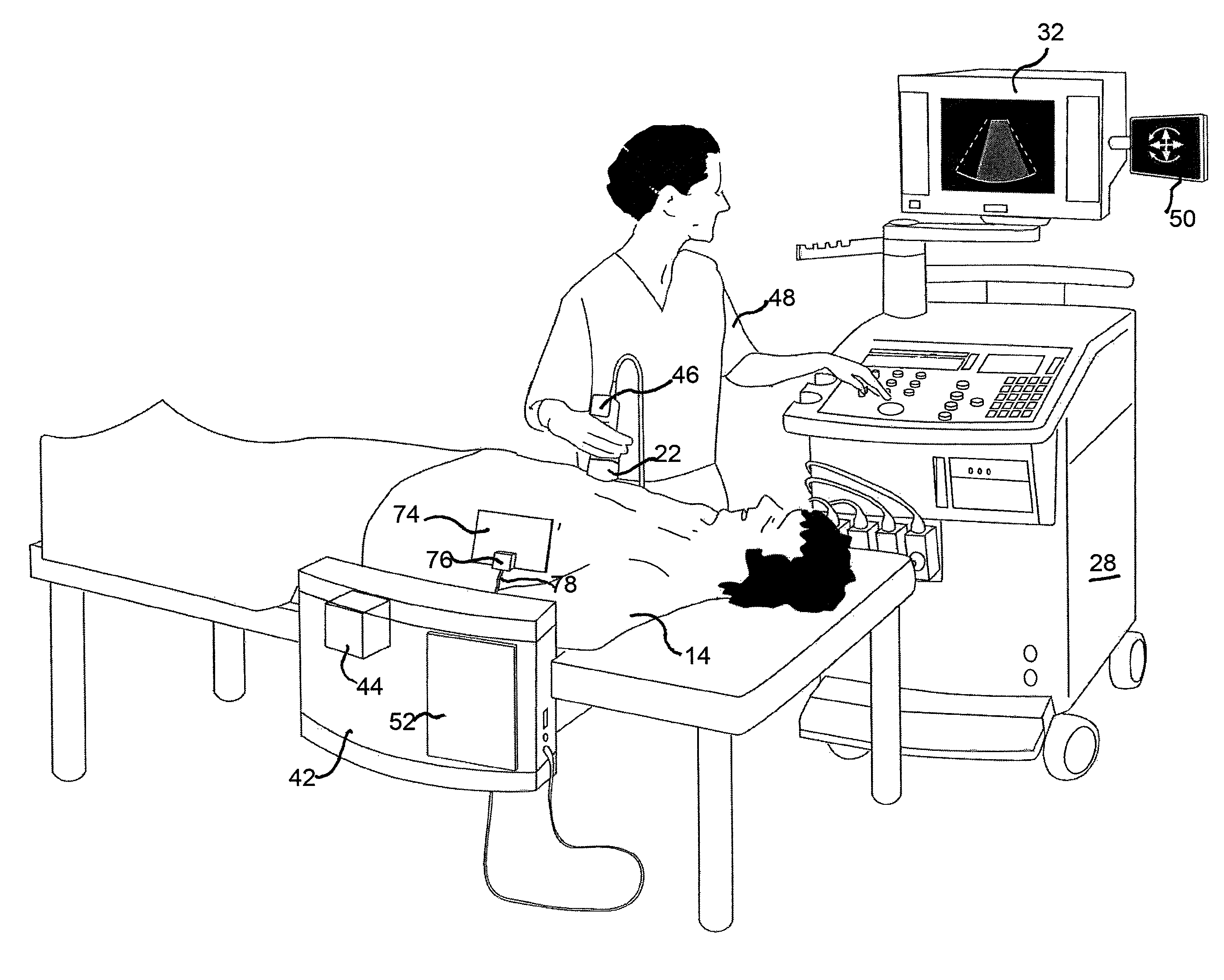
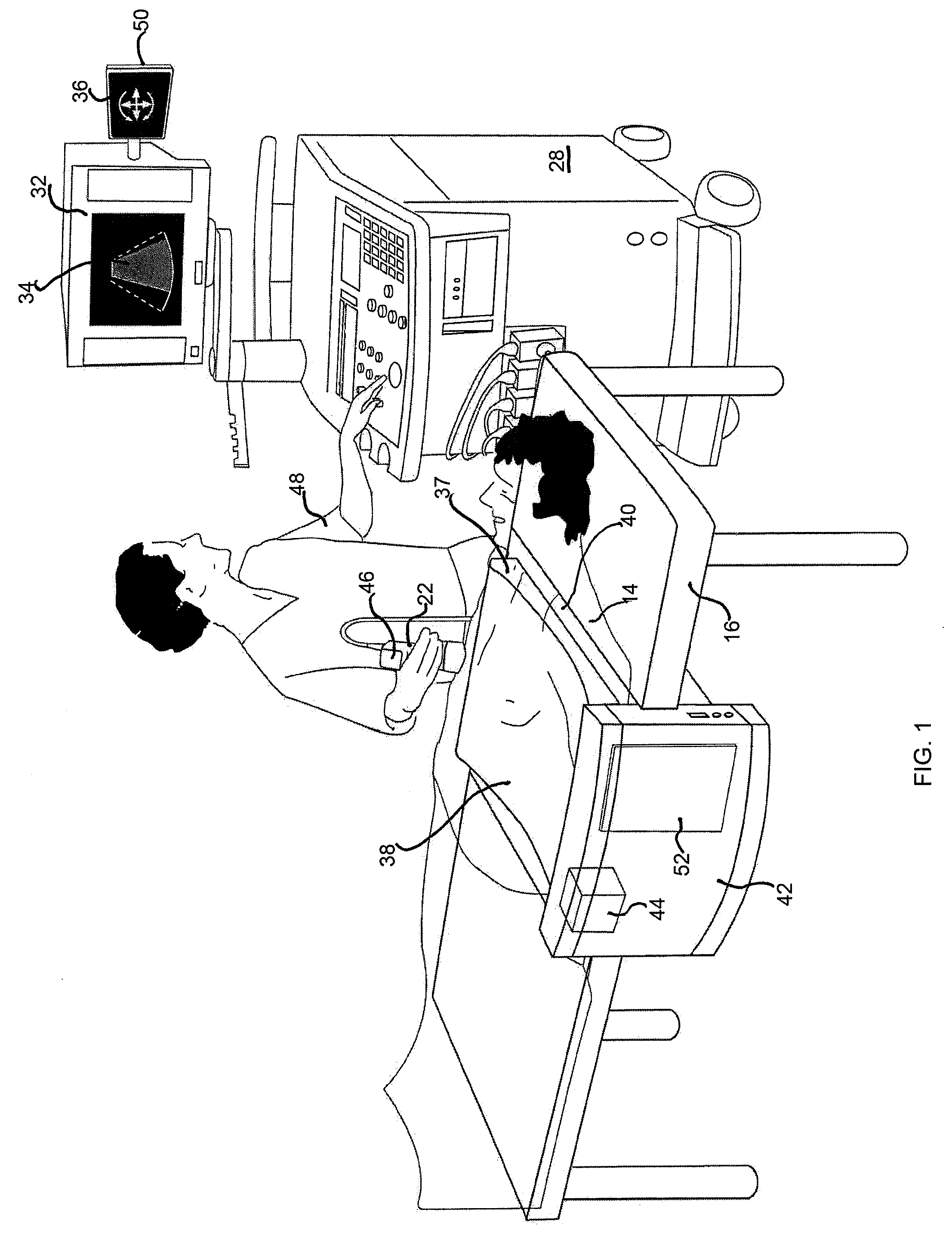
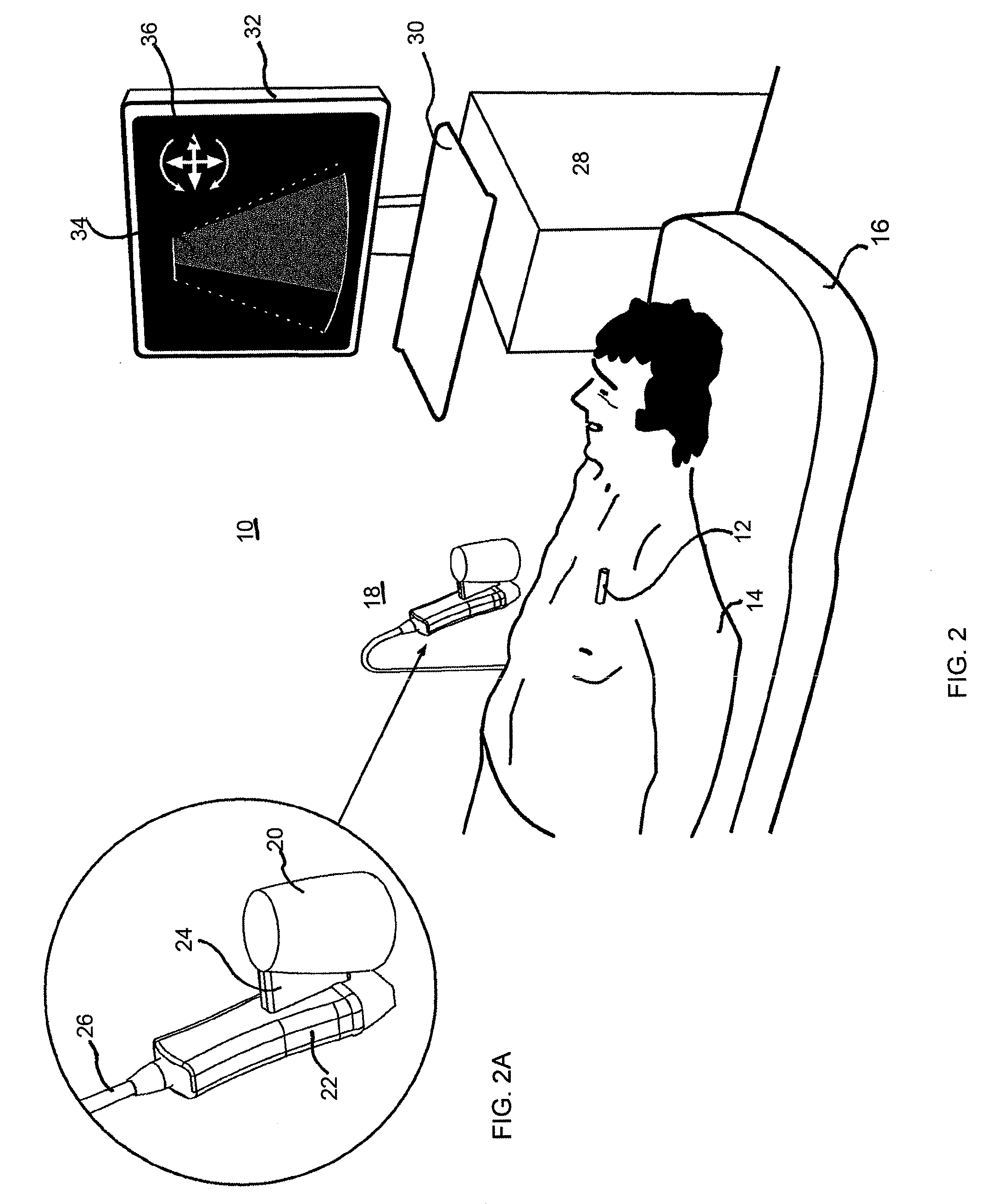
Location tracking of a metallic object in a living body using a radar detector and guiding an ultrasound probe to direct ultrasound waves at the location
ActiveUS8352015B2Facilitates direct vascular flow measurementPrecise determinationDiagnostic probe attachmentBlood flow measurement devicesDiagnostic Radiology ModalityEcg gating
A method and apparatus are provided for determining and tracking location of a metallic object in a living body, and then directing a second modality such as ultrasound waves to the determined location. The metal detector may be a radar detector adapted to operate on a living body. The adaption may include disposing a transfer material having electromagnetic properties similar to the body between the radar detector and the living body, ECG gating the radar detector, and / or employing an optimal estimator with a model of expected stent movement in a living body. Applications include determination of extent of in-stent restenosis, performing therapeutic thrombolysis, or determining operational features of a metallic implant.
Owner:ZOLL MEDICAL ISRAEL LTD
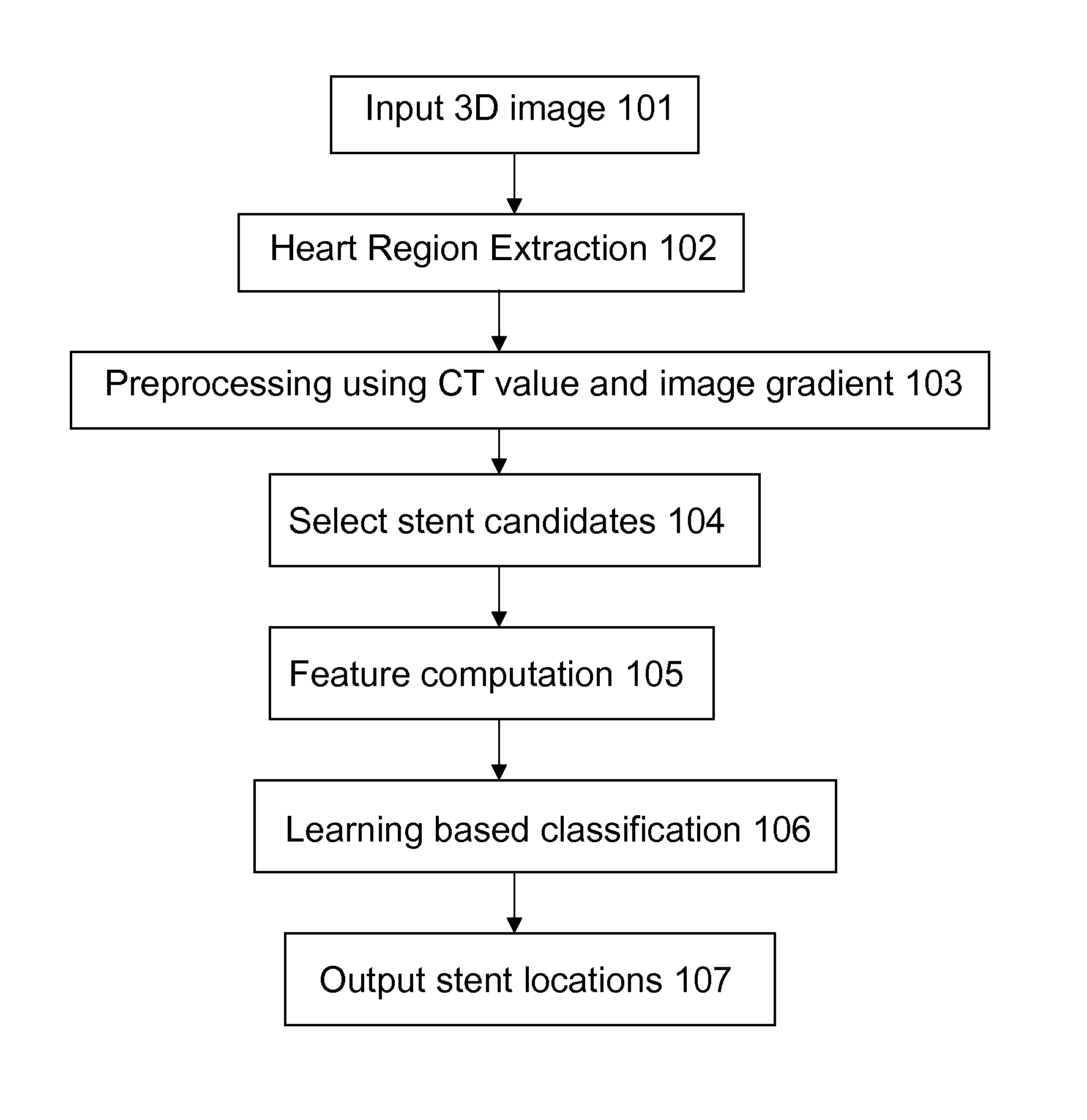
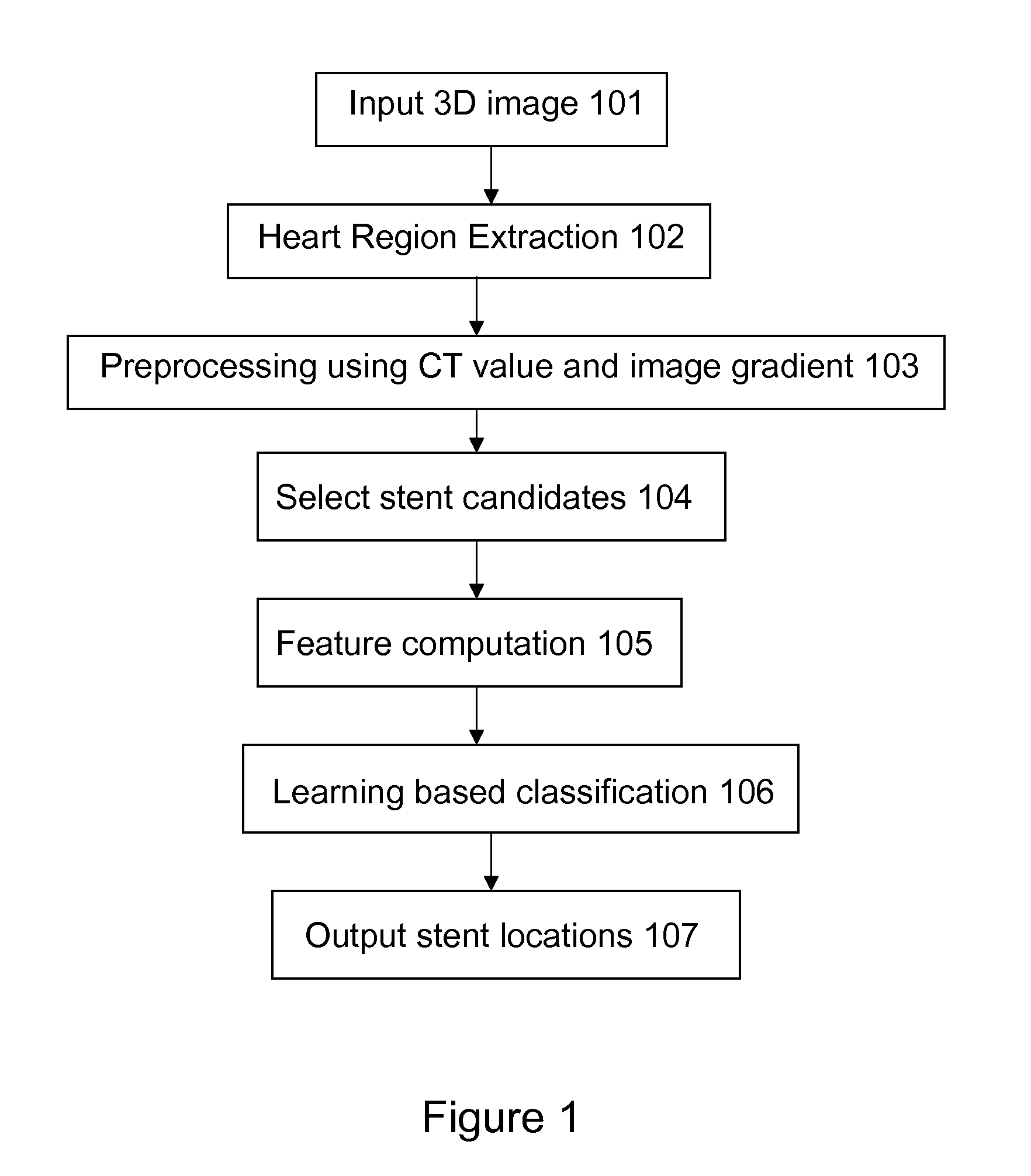
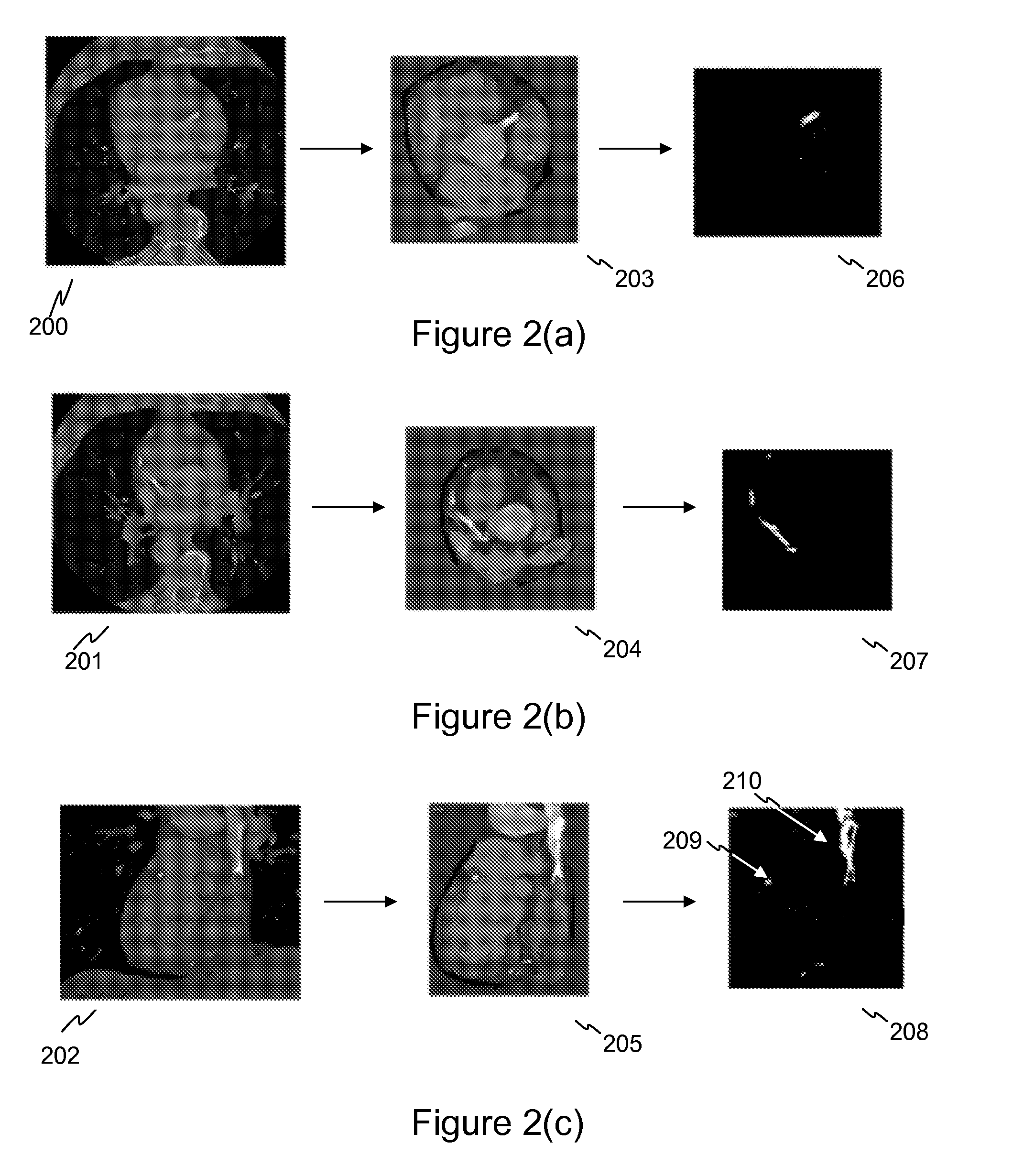
Stent localization in 3D cardiac images
Systems and methods are described for automatically identifying coronary stents within a 3D cardiac image. Based on feature analysis on a cardiac image, coronary stents can be detected by filtering the cardiac images for stent candidates, applying a score based on factors related to coronary stents and applying a threshold. Once coronary stents are identified, in-stent restenosis and stent fractures can be further detected.
Owner:FUJIFILM CORP
Method for preparing personalized bionic drug eluting coronary stent by using 3D printing technology
InactiveCN105877881AAvoid influenceImprove the quality of lifeStentsAdditive manufacturing apparatusThrombusCoronary angiogram
The invention discloses a method for preparing a personalized bionic drug eluting coronary stent by using a 3D printing technology and a product. The method for preparing the personalized bionic drug eluting coronary stent comprises the step that according to image data of coronary angiogram, adopting a QCA technique for measuring the diameter of a diseased coronary artery and reconstructing in a three-dimensional manner. According to indexes such as lesion vascular diameter, lesion length and lesion vascular pattern, a personalized coronary stent can be made for each patient in a customized manner and a stent most suitable for the lesion state of a patient can be prepared. The personalized bionic drug eluting coronary stent can be made of degradable poly L-lactic acid (PLLA) or other materials, after 3D printing molding, the surface of the stent can be coated with a polymer with anti-proliferation medicines by using a conventional process, and in-stent restenosis can be reduced (the polymer is prepared by mixing a medicine with PDLLA in a ratio of the medicine to PDLLA being 1:1). The most suitable personalized bionic drug eluting coronary stent can be customized for the patient according to diseased coronary arteries of different states, requirements of different lesions can be met, customization of coronary stents can be achieved, and complications such as vascular injury, thrombus and coronary arterial dissection caused by mismatching of the diameter of the stent and that of the blood vessel can be reduced.
Owner:周玉杰 +3
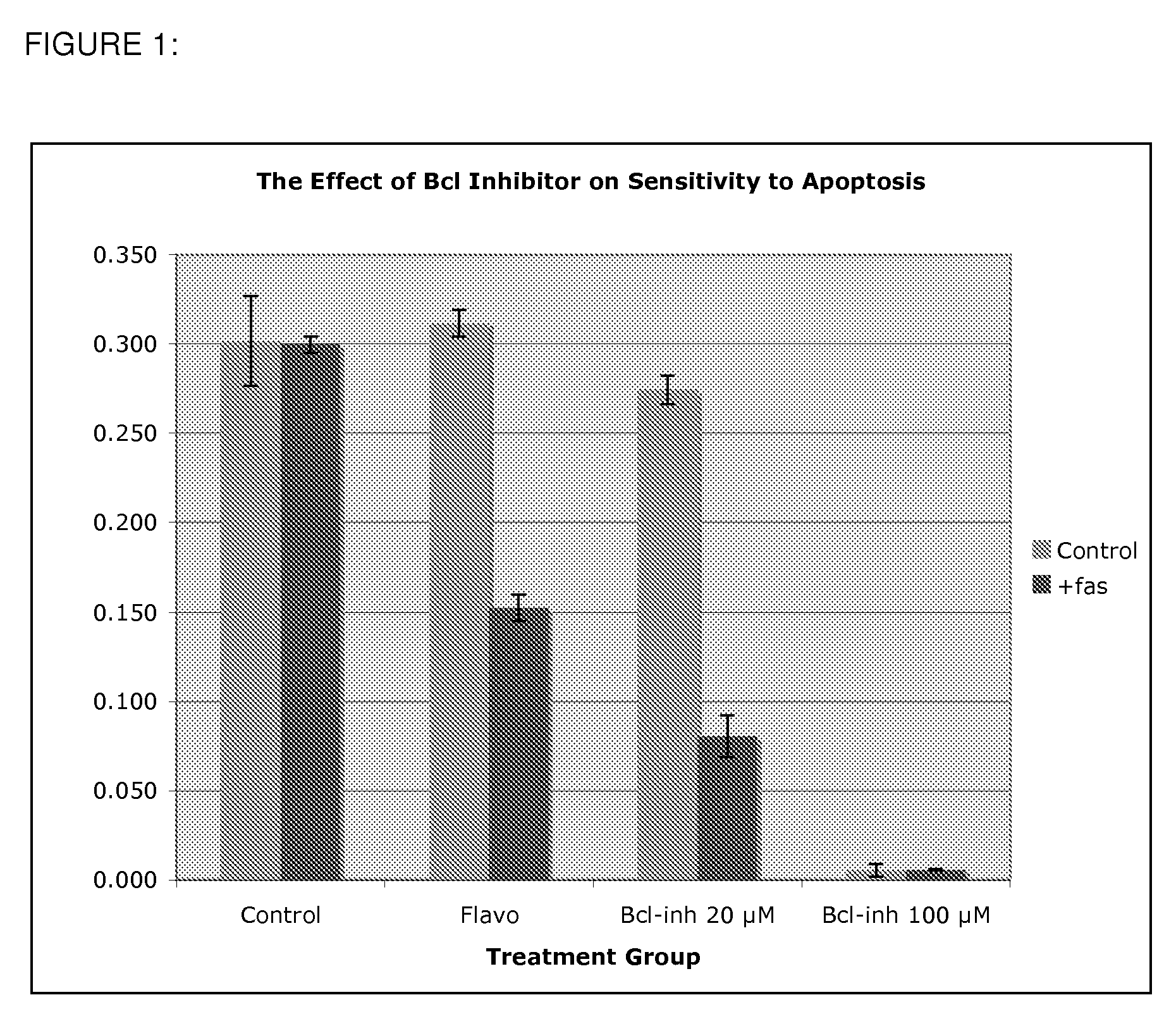
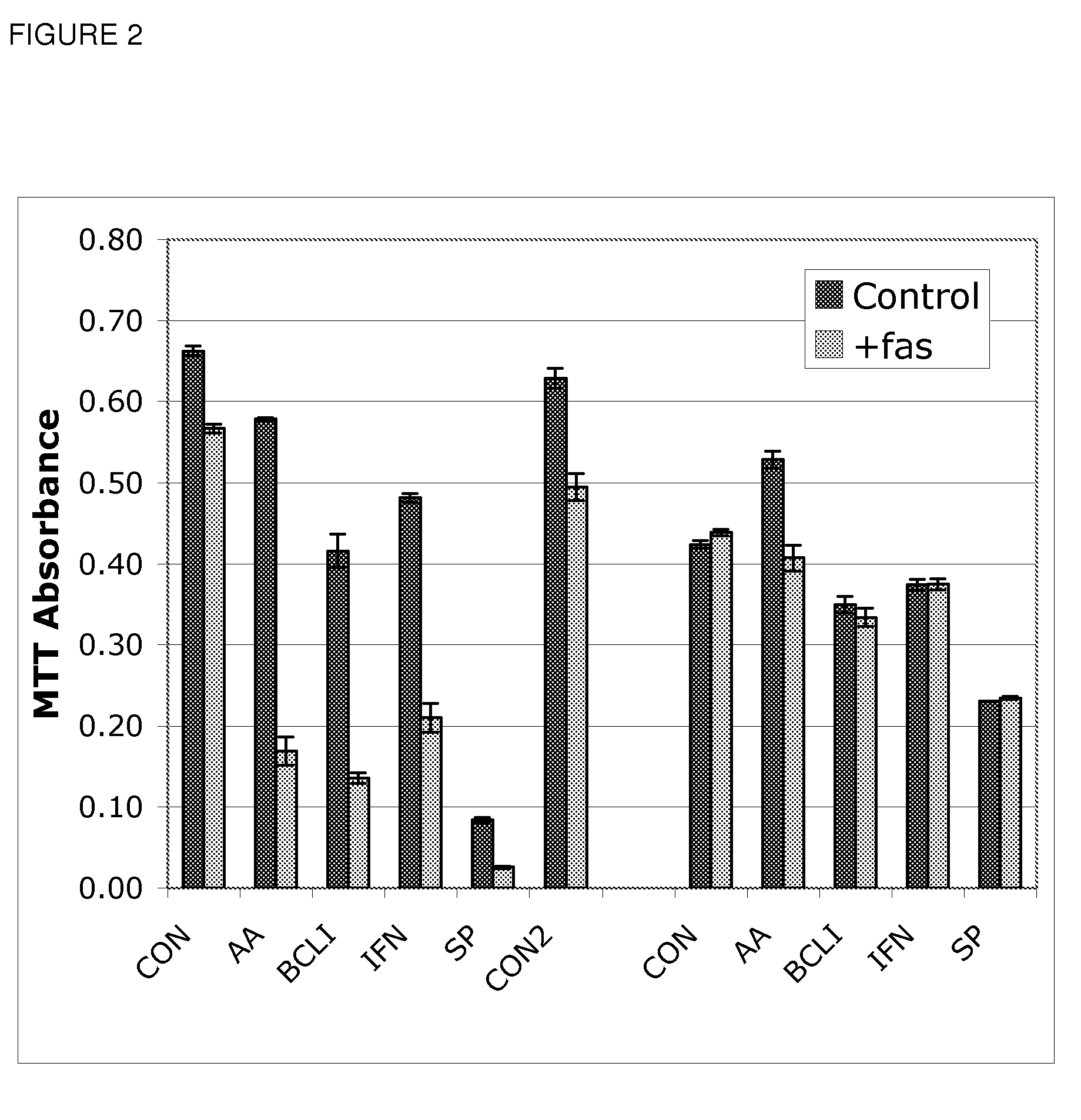
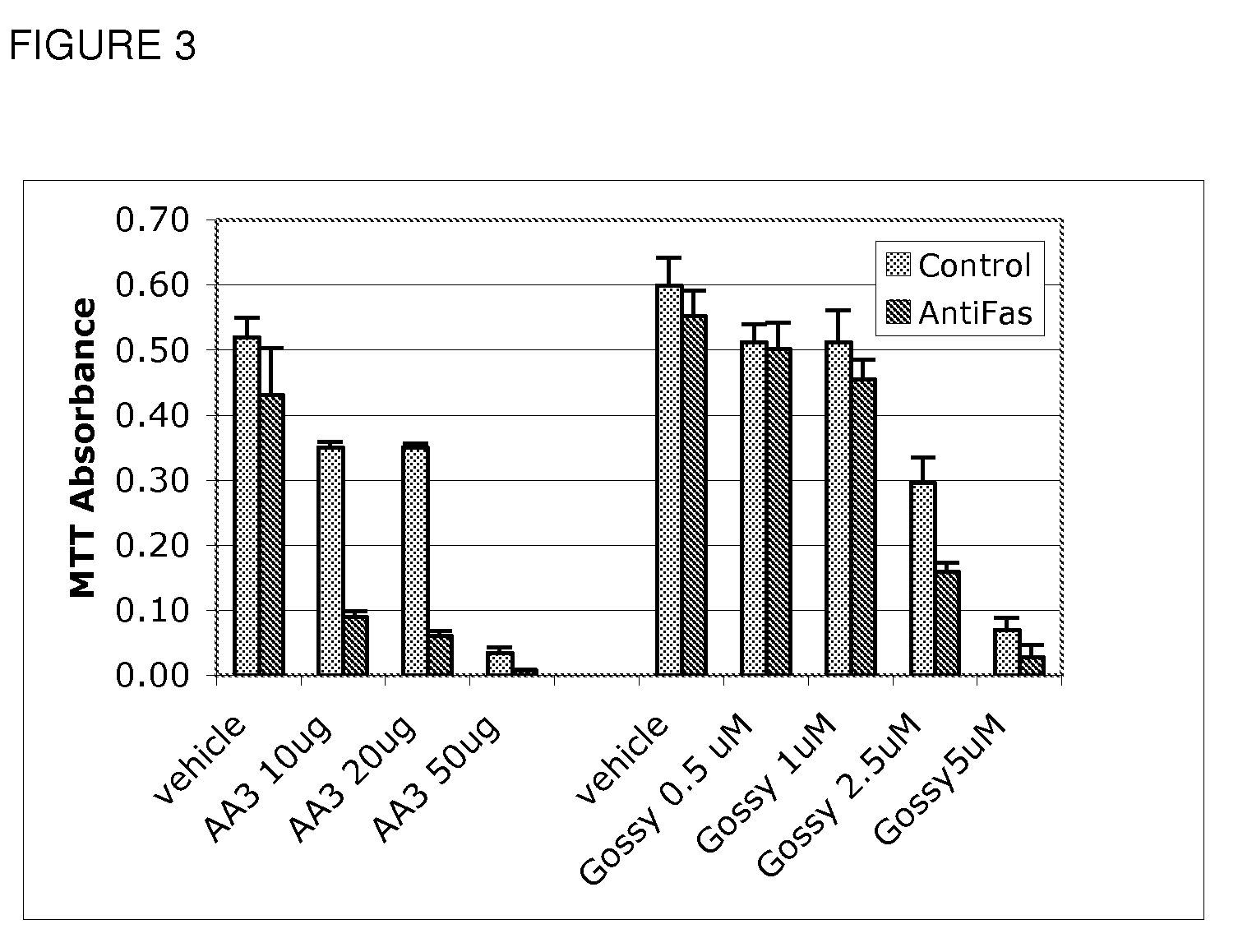
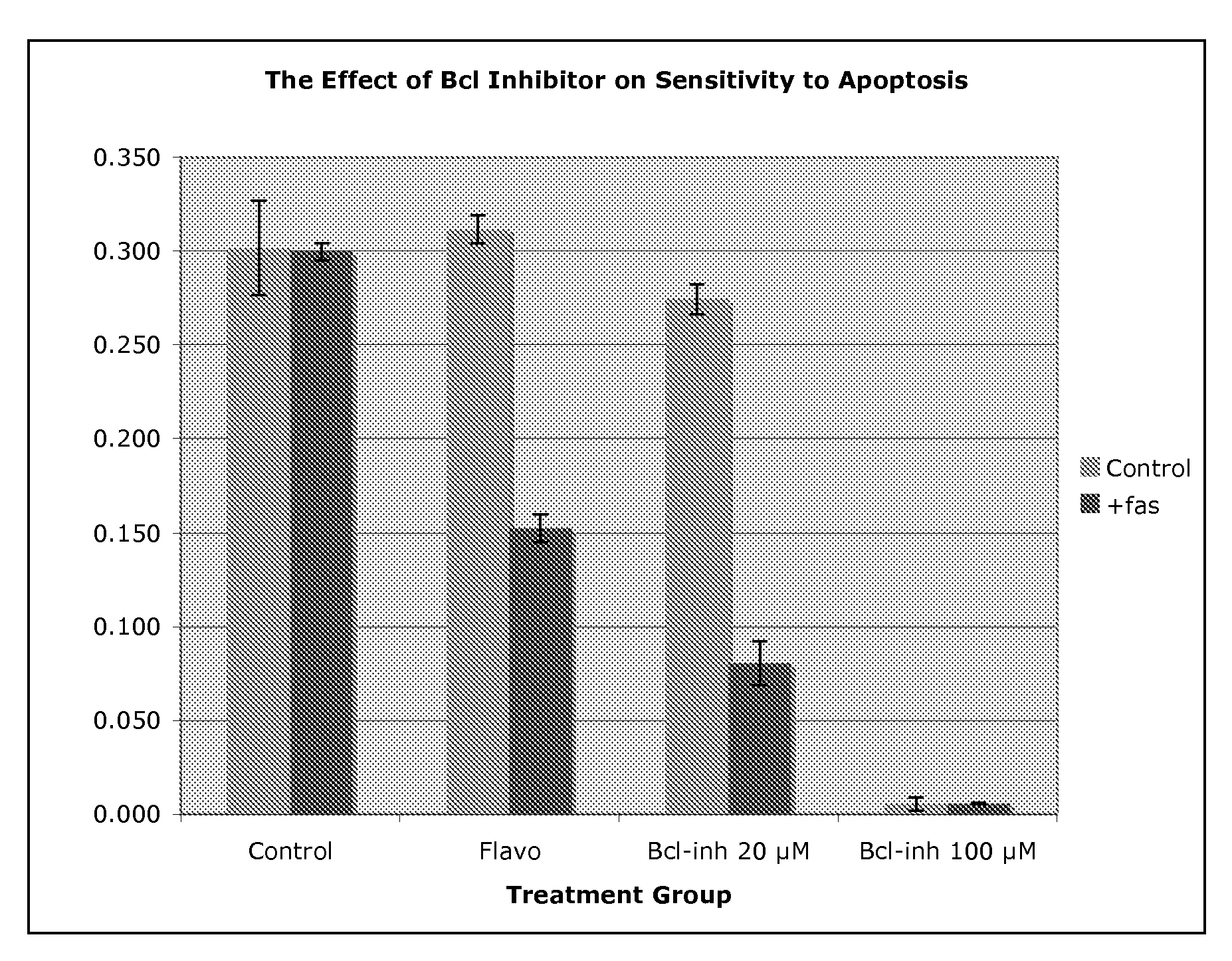
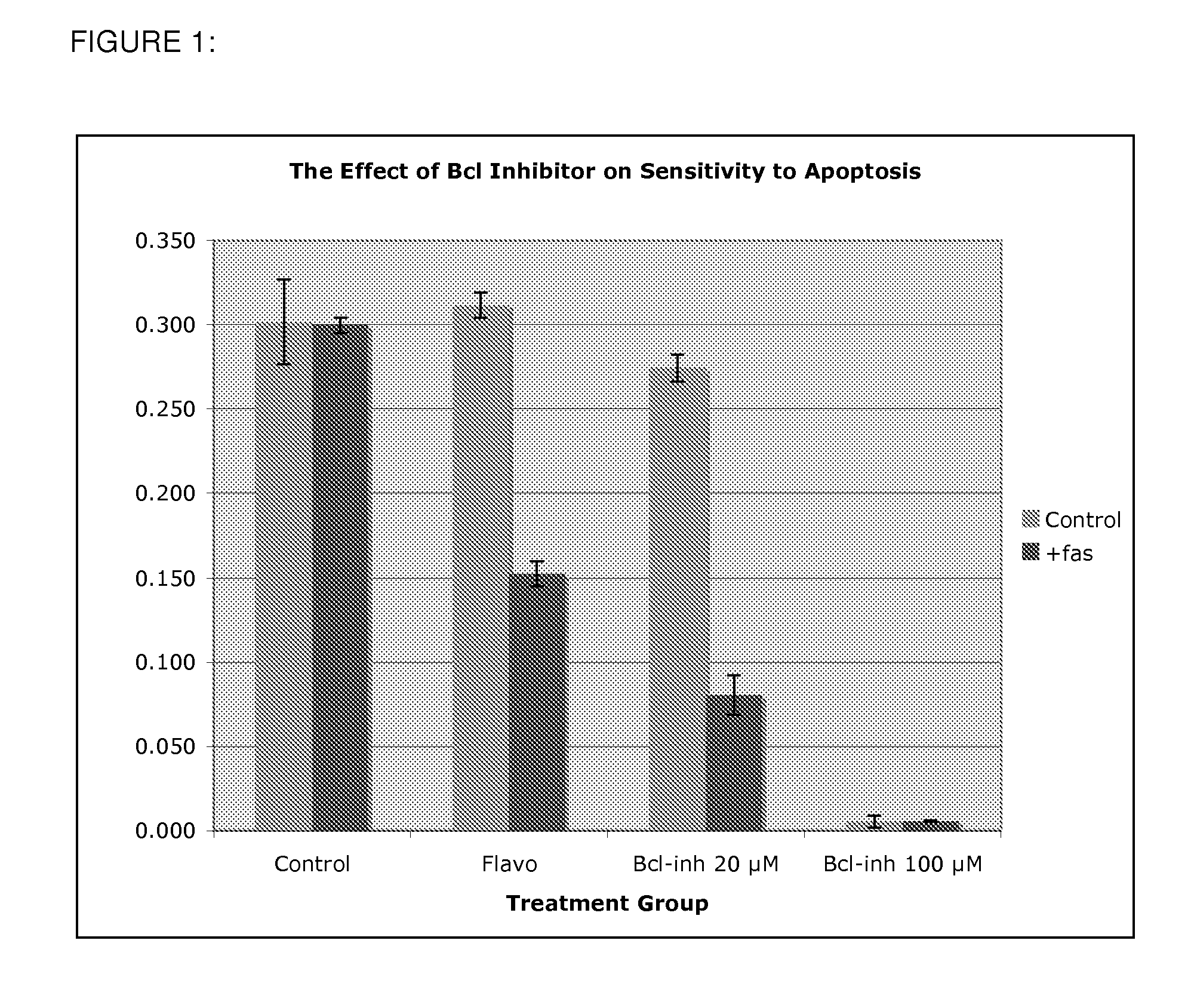
Use of Bcl inhibitors for the prevention of fibroproliferative reclosure of dilated blood vessels and other iatrogenic fibroproliferative disorders
Owner:MCCAFFREY TIMOTHY A +2
Apparatus and method for treatment of in-stent restenosis
InactiveCN104125815AGreat vessel lumenIncrease blood flowSurgical instrument detailsBlood vesselsInsertion stentCatheter
A catheter and catheter system can use energy tailored for remodeling and / or removal of target material proximate to a body lumen, often of stenotic material or tissue in the luminal wall of a blood vessel of a patient. An elongate flexible catheter body with a radially expandable structure may have a plurality of electrodes or other electrosurgical energy delivery surfaces to radially engage the luminal wall when the structure expands. Feedback using one or parameters of voltage, current, power, temperature, impedance magnitude, impedance phase angle, and frequency may be used to selectively control the delivery of energy.
Owner:VESSIX VASCULAR
Application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and/or in-stent restenosis
InactiveCN105434417AReduce the degree of intimal hyperplasiaPrevent proliferationOrganic active ingredientsCardiovascular disorderBalloon injuryPercent Diameter Stenosis
The invention discloses application of salvianolic acid A in preparation of medicine for resisting tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis. Experimental research finds that salvianolic acid A can restrain proliferation of umbilical cord artery blood vessel smooth muscle cells of a person and reduce the tunica intima thickening rate and the blood vessel lumen restenosis rate of a tunica intima thickening animal model after balloon injury, has the remarkable effects of resisting tunica intima thickening and post-angioplasty restenosis and / or in-stent restenosis, can be used for preparing the medicine for restraining tunica intima thickening, post-angioplasty restenosis and / or in-stent restenosis and is particularly used for preparing medicine for preventing post-percutaneous coronary angioplasty restenosis and in-stent restenosis or used for preparing a drug eluting stent. In this way, efficient, safe and economical prevention and treatment medicine and an efficient, safe and economical solution are provided for prevention and treatment of post-angioplasty, particularly post-percutaneous coronary angioplasty restenosis and in-stent restenosis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Intracranial drug eluting stent system and preparation method thereof
ActiveCN105833358AReduce late thrombosisReduce restenosis catch-up problemSurgeryCoatingsDiseaseIntracranial Artery
The invention relates to an intracranial drug eluting stent system and a preparation method thereof, in particular to an intracranial drug eluting stent used for treating the intracranial atherosclerotic stenosis disease .The intracranial drug eluting stent is composed of a metal stent body and a coating structure covering the surface of the metal stent body, the coating structure comprises one or more stent substrate coatings and drug coatings, and the drug coatings contain a biodegradable drug carrier and a drug inhibiting excessive proliferation of VSMCs .According to the intracranial drug eluting stent, the diseased artery is expanded with the stent to improve intracranial artery blood perfusion, the drug carried by the stent can prevent excessive proliferation of endangium, and thus the probability of in-stent restenosis is lowered; meanwhile, stent healing in the artery can be rapidly achieved, and thus long-term safety and effectiveness are ensured for patients.
Owner:赛诺神畅医疗科技有限公司
Degradable vascular stent structure with negative Poisson ratio
ActiveCN109893295AAvoid long-term retentionAvoid damageStentsBlood vesselsInjury causeMedical equipment
The invention discloses a degradable vascular stent structure with a negative Poisson ratio to make up defects of design of degradable vascular stents in the prior art and belongs to the field of medical equipment. The vascular stent structure is formed by arranging concave hexagonal base structure units in the annular and axial directions of a stent and is made of a degradable material. The vascular stent structure is designed on the specificity of the degradable material and has a negative Poisson ratio effect. A degradable vascular stent with the structure can be matched with the negative Poisson ratio effect of tunica intima tissue after being implanted into blood vessels, so that injury caused by the stent to vascular tissue and the probability of in-stent restenosis are reduced, thestent can be completely degraded in vivo finally, and degradation products of the stent are absorbed and metabolized by the human body.
Owner:BEIHANG UNIV
Nano multi-coating medicine stent and preparation method thereof
InactiveCN103656763AReduce the incidence of restenosisReduce medical burdenStentsSurgeryVascular endotheliumCeramic coating
The invention discloses a nano multi-coating medicine stent and a preparation method thereof. A stainless steel metal stent is covered by a nano ceramic coating, wherein an REDV (Arg-Glu-Asp-Val) mono-molecule layer is covered outside the nano ceramic coating; a PLGA (Polylactic-co-Glycolic Acid)-rapamycin coating is covered outside the REDV mono-molecule layer. The preparation method of the nano multi-coating medicine stent comprises the following steps: 1) manufacturing the stainless steel metal stent; 2) preparing a TiO2 coating; 3) forming a polydopamine film on the TiO2 coating; 4) creating the REDV mono-molecule layer on the surface of the polydopamine film; 5) preparing a PLGA-RAPA (Rapamycin) solution; 6) forming a medicine coating. The nano multi-coating medicine stent and the preparation method thereof have the advantages of further reducing occurrence rate of in-stent restenosis after a stent implanting operation, suppressing proliferation of smooth muscle cells, accelerating vascular endothelium process, reducing hemorrhage risk caused due to long-term antithrombotic therapy, reducing medical cost of operators and being significant in economic and social benefits.
Owner:中国医科大学附属第四医院
Peripheral drug eluting stent and preparation and application thereof
ActiveCN107496998AThe release rate is easy to controlPrevent restenosisSurgeryPretreated surfacesBare-metal stentTubular stenosis
The invention provides a peripheral drug eluting stent. A drug coating of the peripheral drug eluting stent is divided into an outer sustained-release layer, an intermediate drug sustained-release mixed layer and an inner drug sustained-release mixed layer. The outer sustained-release layer is PLGA with molecular weight being 30000, and PLA:PGA=70:30. The intermediate drug sustained-release mixed layer is a mixture of PTX and PLGA, wherein the molecular weight of PLGA is 20000, PLA:PGA=50:50, and the mass ratio of PTX to PLGA is 3:1. The inner drug sustained-release mixed layer is a mixture of PTX and PLGA, wherein the molecular weight of PLGA is 20000, PLA:PGA=80:20, and the mass ratio of PTX to PLGA is 3:1. The invention further relates to preparation and application of the peripheral drug eluting stent. The peripheral drug eluting stent can effectively treat peripheral artery stenosis, and the problem of in-stent restenosis after a traditional peripheral bare metal stent is implanted into the peripheral vessel is avoided.
Owner:北京永益润成科技有限公司
Bidirectional double-drug eluting stent and preparation method thereof
InactiveCN108114326APrevent restenosisInhibition of Proliferation RepairSurgeryCoatingsSmooth muscleBlood vessel spasm
The invention relates to a bidirectional double-drug eluting stent and a preparation method thereof. The bidirectional double-drug eluting stent is used for carrying out percutaneous coronary intervention operations so as to supply expansion support to heart and blood vessels of a patient. An A drug layer containing a drug capable of inhibiting the proliferation of smooth muscles is applied to theouter side surface, namely the surface in contact with an inner surface of a blood vessel after mounting, of the stent; and a B drug layer containing a drug capable of accelerating the reendothelialization of the blood vessel and stabilizing or reversing plaques is applied to the side surface, namely the surface deviated from the inner surface of the blood vessel after mounting, of the stent. According to the bidirectional double-drug eluting stent, the cell proliferation of the smooth muscles can be inhibited, so that the in-stent restenosis and the rapid endothelialization are inhibited, the plaques can be stabilized and reversed, the new atherosclerosis is prevented, and the occurring risk of later-period thrombus is reduced.
Owner:SOUTHWEST JIAOTONG UNIV
Medical application of berberine
ActiveCN106822117APrevention and treatment of myocardial infarctionReduce mean pressureOrganic active ingredientsRespiratory disorderArterial smooth muscle cellsBerberine
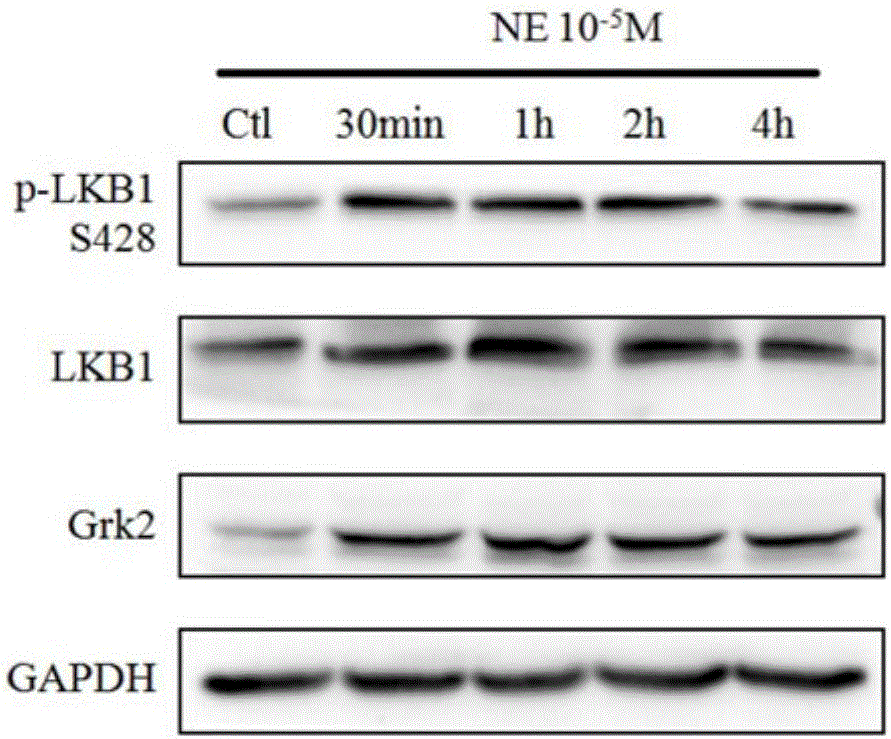
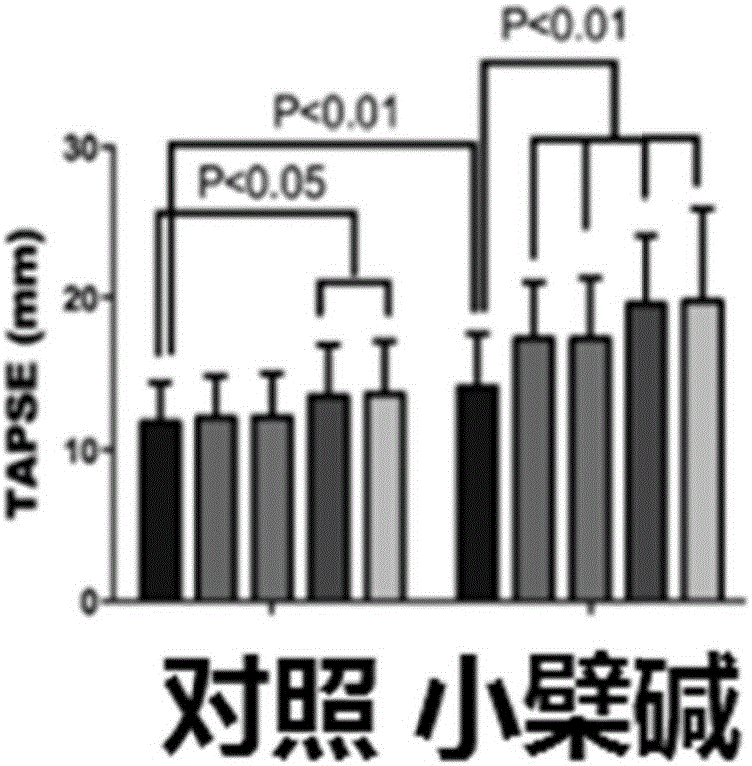
The invention discloses medical application of berberine. The berberine has prominent functions of resisting noradrenaline and stimulating pulmonary arterial smooth muscle cells and is capable of effectively improving the function of a right ventricular, and meanwhile, the berberine is capable of remarkably reducing mean pulmonary arterial pressure; the functions of preventing in-stent restenosis and stent thrombosis are obtained; the berberine has a unique function of preventing and treating myocardial infarction; the berberine is capable of effectively preventing and treating ischemic cardiomyopathy and cardiac failure caused thereby.
Owner:陈绍良
Traditional Chinese medicine preparation for treating stenocardia, myocardial infarction and preventing in-stent restenosis
InactiveCN101244173AInhibit aggregationThe effect of promoting blood circulation and removing stasis is obviousAnthropod material medical ingredientsCardiovascular disorderSide effectAdditive ingredient
The invention discloses a Chinese herbal medicine preparation for curing angina pectoris, myocardial infarction and preventing in-stent restenosis, which is developed by a cardiovascular disease curing expert and President Shang Xiaoming through the understanding of the pathogenesis of chest stuffiness based on traditional Chinese medical science and the combination of modern pharmacological knowledge. The Chinese herbal medicine preparation comprises the following active pharmaceutical ingredients: danshen root, astragalus, red peony root, angelica sinensis, rhizomaligusticichuanxiong, peach kernel, safflower, hirudo, earth worm and rhizoma pinelliae. The drug is compatible with the dialectic theory of Chinese traditional medicine, which has multiple effects of activating blood circulation to dissipate blood stasis, improving blood circulation, nourishing pneuma and benefiting marrow, soothing nerves and promoting intelligence, can expand peripheral vascular, and improve microcirculation, and can prolong the aggregation time of fibrinogen remarkably and inhibit myocardial fibrosis effectively. Thereby, the Chinese herbal medicine preparation has the advantages of low price, good efficacy, no poison and side effect, and broad market prospects.
Owner:尚小明
Anti-skid, self-dilating and degradable esophagus stent with negative Poisson's ratio structure and preparation of stent
The invention provides an anti-skid, self-dilating and degradable esophagus stent with a negative Poisson's ratio structure. The anti-skid, self-dilating and degradable esophagus stent with the negative Poisson's ratio structure is characterized by comprising a main stent body made of a totally or partially degradable material, wherein the main stent body comprises an upper end part, a middle partand a lower end part which are connected in sequence, an upper port and a lower port are formed in the top end of the upper end part and the bottom end of the lower end part respectively, and inner diameter of the upper port and inner diameter of the lower port are both larger than inner diameter of the middle part. Minimally invasive implanting of the esophagus stent is realized by the negativePoisson's ratio effect, and the esophagus wall is uniformly stressed in the stent dilating process, so that damage is effectively prevented, axial shrinkage is avoided and the stent is prevented fromdisplacing; meanwhile, by means of the negative Poisson's ratio structure, discomfort of a patient in an eating process can be relieved by the radial process of the stent while displacement is avoidedin the eating and swallowing process of the patient. The esophagus stent dilates automatically and gradually and is biodegradable under external motivation, and the problems of in-stent restenosis, difficulty in taking-out after implantation and the like are effectively solved.
Owner:DONGHUA UNIV
Medicament for preventing in-stent restenosis after coronary stent implantation and preparation method
ActiveCN102872455AReduce the incidence of restenosisReduce long-term thrombosis riskHydroxy compound active ingredientsPeptide/protein ingredientsSide effectClinical efficacy
The invention discloses a medicament for preventing in-stent restenosis after coronary stent implantation and a preparation method. The medicament is prepared into 1000 parts from, by weight, 800-1200 parts of tanshinone, 200-300 parts of hirudin, 20-30 parts of camphol and borneol and 6-9 parts of musk ketone. The medicament has the advantages of effective treatment, no toxic and side effects and capabilities of decreasing occurrence rate of in-stent restenosis after coronary stent implantation, obviously improving clinical symptoms of patients and reducing long-term risks of thrombosis of the patients, and is obvious in clinical effect. The medicament provides a new choice for preventing ISR (in-stent restenosis) after coronary stent implantation.
Owner:贵州中医药大学
Encephalic drug eluting stent
InactiveCN103948458AInhibit the inflammatory responsePrevent restenosisStentsSurgeryDiseaseAntiplatelet drug
The invention discloses an encephalic drug eluting stent in the field of medicines, and particularly relates to the drug eluting stent used for curing the encephalic atherosclerotic stenosis disease. Diseased blood vessels are expanded by the stent to improve the condition of stenosis, so as to improve the blood flow state, meanwhile, drug carried by the stent can be used for preventing a tunica elastica interna from hyperplasia, and the probability of in-stent restenosis can be lowered. The encephalic drug eluting stent comprises a conveying system, a stent body and a stent drug carrying layer, and is characterized in that one or more drug carrying layers are attached to the surface of the encephalic drug eluting stent; each drug carrying layer is composed of a polymer and an active ingredient, the active ingredient is an antiplatelet drug cilostazol, and each drug carrying layer comprises the following components by weight percent: 5 to 50 percent of the polymer and the balance of active ingredients; the active ingredients in all medicine carrying layers are the same or different. The encephalic drug eluting stent provided by the invention has the advantages that the functions of protecting blood vessels, restraining inflammation reactions, expanding blood vessels and the like can be achieved, and the purpose of preventing encephalic vascular in-stent restenosis can be achieved.
Owner:ACHIEVA MEDICAL SHANGHAI
Beta-elemene controlled-release eluting stent for preventing and treating coronary artery in-stent restenosis
InactiveCN106237492AWith thrombosisInhibits thrombosisStentsMedical devicesAdditive ingredientRelease time
The invention belongs to the technical field of biomedicine and medical materials, and relates to a beta-elemene controlled-release eluting stent for preventing and treating coronary artery in-stent restenosis. The medicine stent takes a stent as a medicine carrying tool, the effective medicine ingredient beta-elemene achieves controlled releasing through a coating, and the in-stent restenosis is prevented and treated by inhibiting smooth muscle cell proliferation and inducing cell apoptosis; meanwhile, in-stent late thrombosis is prevented by protecting endothelial cells against damage and inhibiting the thrombosis effect. According to the medicine controlled-release eluting stent, in-vitro controlled-release test results show that more than half of the medicine is still not released after six months, the releasing time is significantly prolonged compared with a homologous pure polymer, and the sustained action of the medicine is guaranteed; animal experiments prove that the beta-elemene controlled-release eluting stent can effectively prevent and control the coronary artery in-stent restenosis and thrombosis and has the safety and feasibility.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
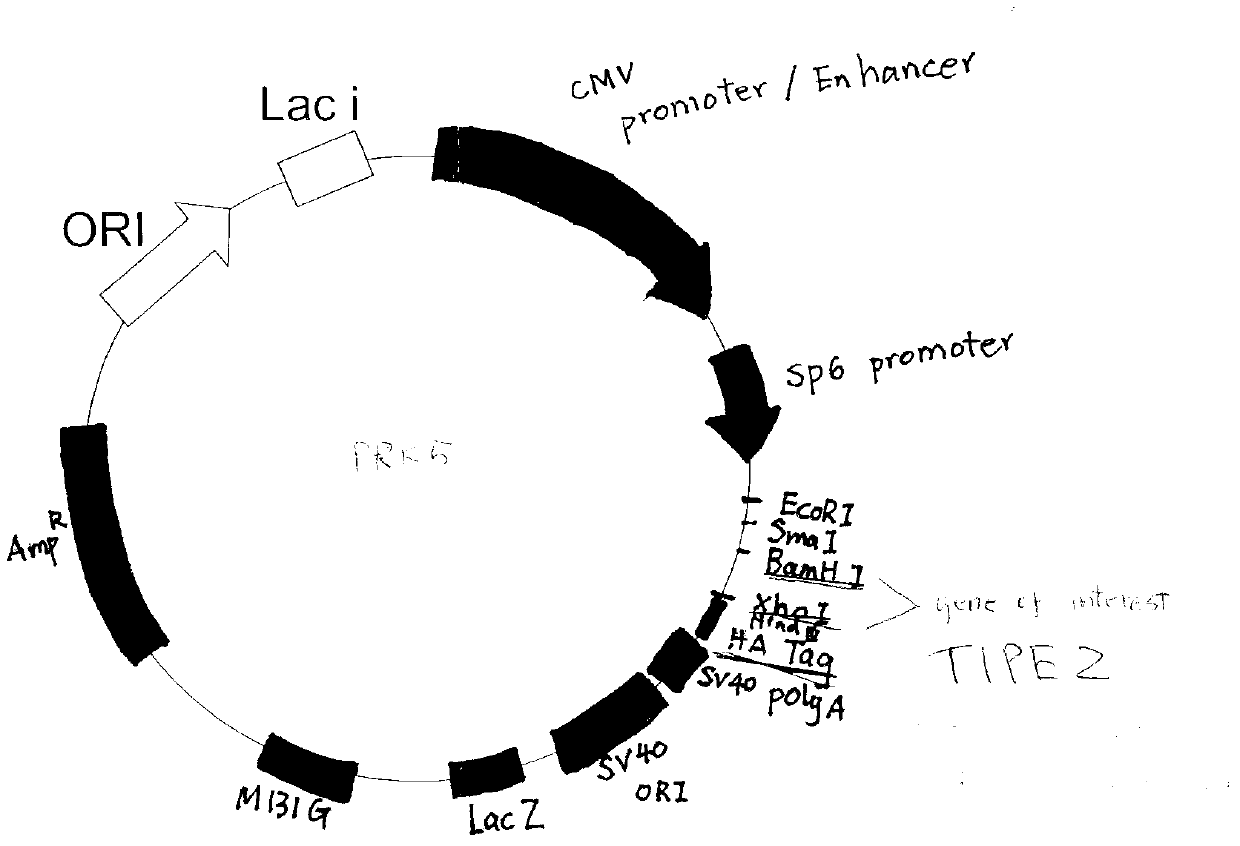
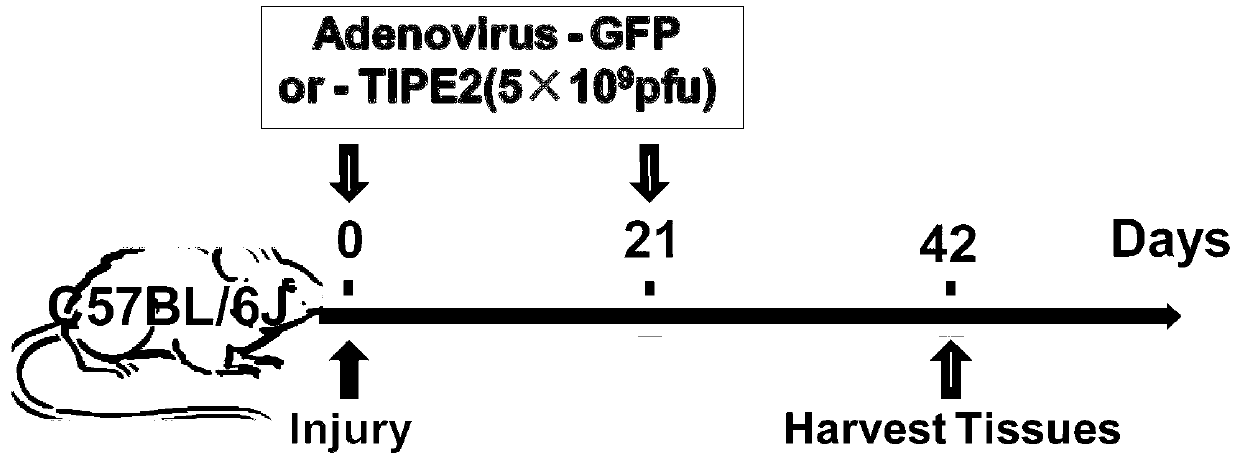
Application of immune regulation markers TIPE2 (Tumor Necrosis Factor-a Induced Protein 8 like 2) in preparation of medicine used for treating vascular proliferation diseases
ActiveCN103341159AInhibit hyperproliferationEasy to put inSenses disorderPeptide/protein ingredientsDiseaseVascular proliferation
The invention discloses an application of immune regulation markers TIPE2 (Tumor Necrosis Factor-a Induced Protein 8 like 2) in preparation of a medicine used for treating vascular proliferation diseases, and also discloses a recombinant adenovirus for expressing the TIPE2. The recombinant adenovirus for the TIPE2 is adopted to realize the in-vivo continuous high expression of TIPE2 protein, which shows the function on inhibiting excess proliferation of a vascular smooth muscle cell, inhibits the hyperplasia of neointima caused by the vascular injury in order to reduce vascular stenting postoperative complication and prognosis and other postoperative complications and prognoses, creates a new path with a wide prospect on effectively preventing and treating the vascular in-stent restenosis of acute coronary syndrome and intervening other vascular proliferation diseases on the cytological basis of vascular smooth muscle proliferation in clinic, and also provides the reference for developing a novel medicine.
Owner:SHANDONG UNIV
Use of Bcl inhibitors For The Prevention Of Fibroproliferative Reclosure Of Dilated Blood Vessels And Other Iatrogenic Fibroproliferative Disorders
The present invention relates to new use of inhibitors of bcl-2 / bcl-XI family of anti-apoptotic compounds for the treatment of post-angioplasty restenosis and in-stent restenosis. Small molecules of Bcl inhibitors are incorporated into a stent, typically by a fine polymeric coating over the metal, which would be placed during angioplasty of occluded blood vessels, to act on fibroproliferative in-growth. Hollow organs such as urethras, Fallopian tubes and vascular access grafts could be treated in a similar manner to prevent their closure due to fibrosis. Other iatrogenic fibrosis such as adhesions after surgery could also be blocked by this therapy.
Owner:MCCAFFREY TIMOTHY A +2
Stent for In-Stent Restenosis
A second stent having a wall thickness of 0.002 inches or less or a second stent having a tubular surface with openings therethrough where the ratio of the area of the surface to the area of the openings is at least 3:7 may be implanted in a first stent which has been previously implanted in a bodily vessel.
Owner:BOSTON SCI SCIMED INC
PLGA-S1P nanometer material and preparation method of PLGA-S1P nanometer coating stent
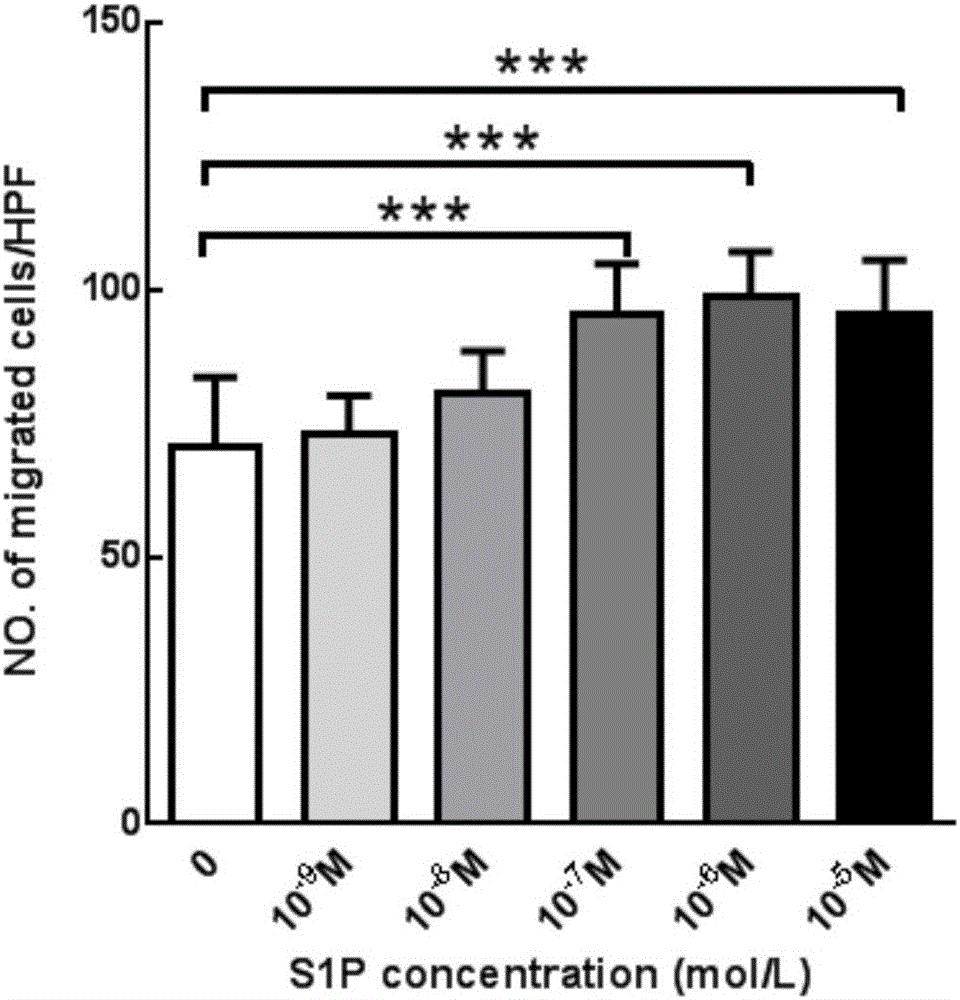
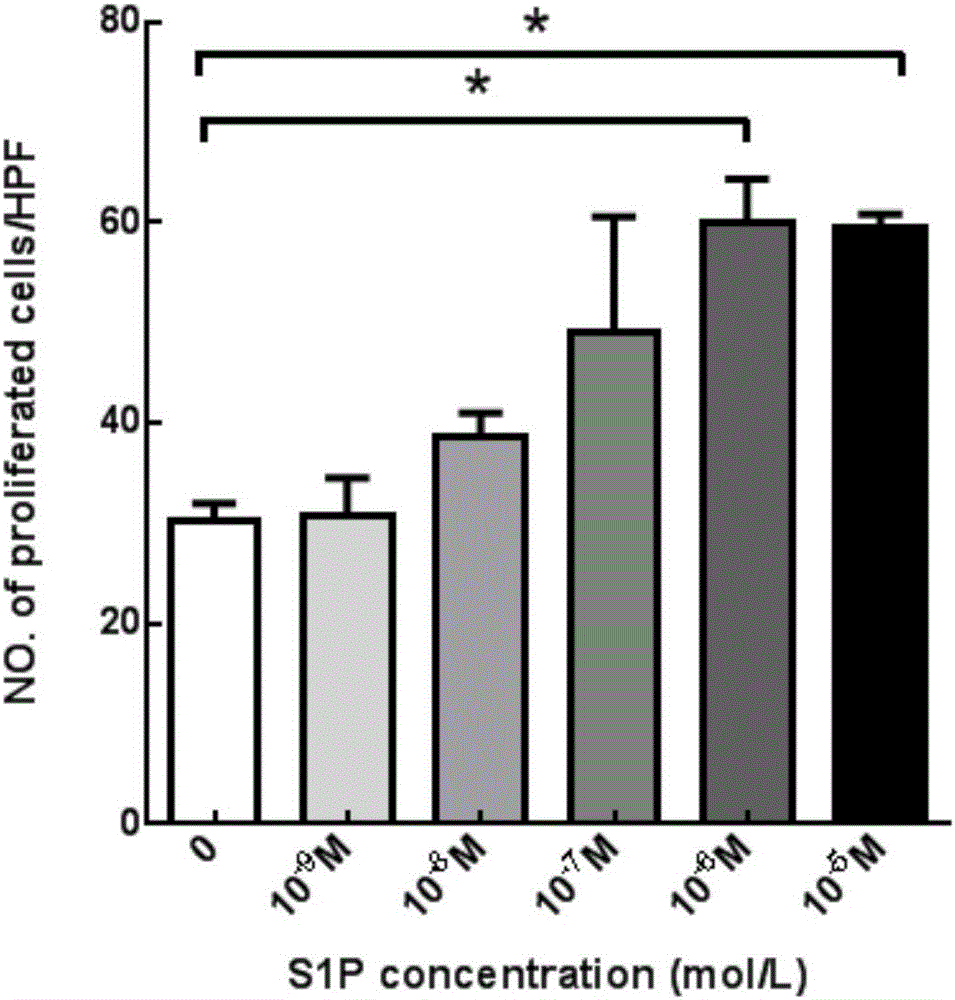
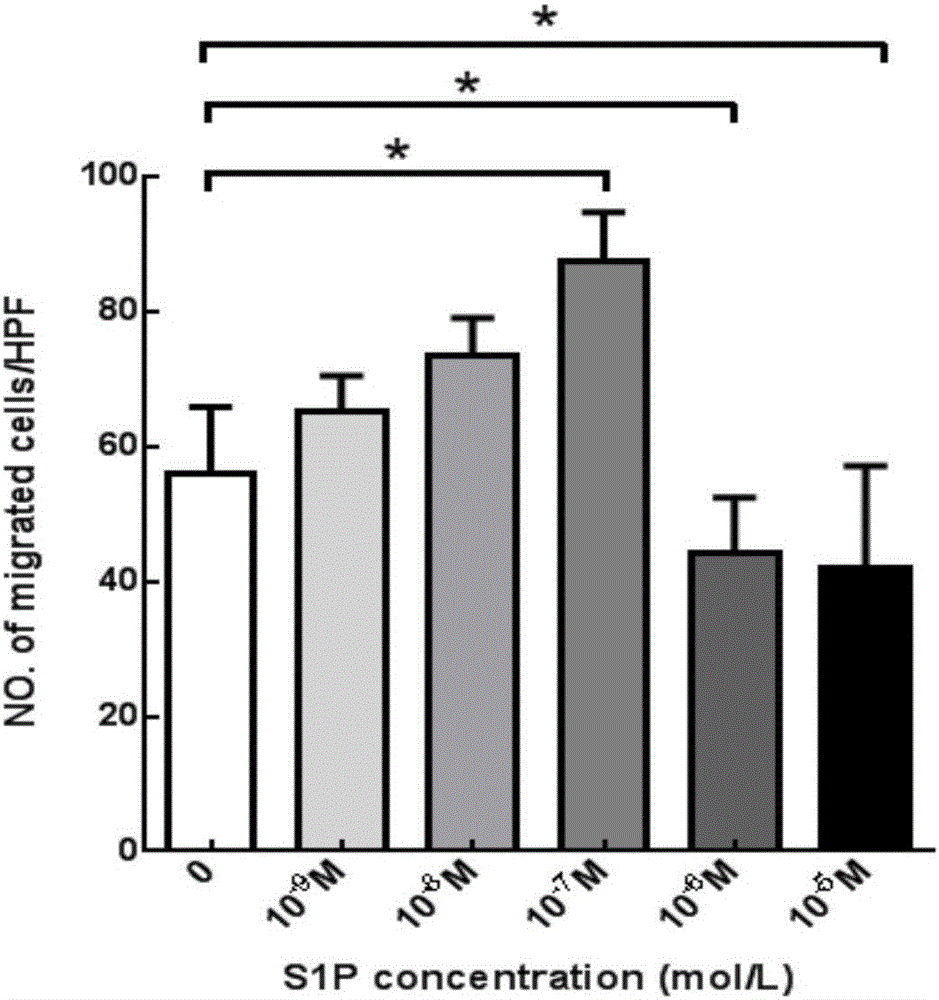
The invention aims at providing a PLGA-S1P nanometer material and a preparation method of a PLGA-S1P nanometer coating stent. The preparation method is characterized by comprising the following steps that S1P is subjected to PLGA coating by a solvent emulsifying method by using an ultrasonic dispersion method, and the ratio of the S1P to PLGA is 1:(10 to 200) (w / w); then, an organic solvent is removed by a method of diffusing an emulsifying agent to water; and finally, freeze-dried powder is prepared by using a freeze-drying concentration method. The PLGA-S1P nanometer particle freeze-dried powder is added into the solvent; after the ultrasonic dissolution, the solution is used as a medicine carrying coating solution; a stainless steel stent is cleanly cleaned; the medicine carrying coating solution is repeatedly sprayed and coated on the surface of the stent by a high-voltage electric spinning method / an ultrasonic spray coating technology; the coating thickness is controlled to be 5 to 20 <mu>m; a de-ionized water / organic solvent volatilization method is used for cleaning; and the PLGA-S1P nanometer coating stent is obtained after the sterilization. The PLGA-S1P nanometer particles are used as the surface coating material of a metal bare stent; and the goal of inhibiting the in-stent restenosis can be achieved. The invention provides a novel method for the interventional therapy for preventing arteriostenosis / occlusion diseases.
Owner:THE FIRST HOSPITAL OF CHINA MEDICIAL UNIV
Medicine for preventing restenosis after intracoronary stent implantation and preparation method thereof
InactiveCN103055122AImprove symptoms such as chest tightness and chest painImprove the quality of lifeCardiovascular disorderPlant ingredientsSalvia miltiorrhizaTrichosanthes kirilowii
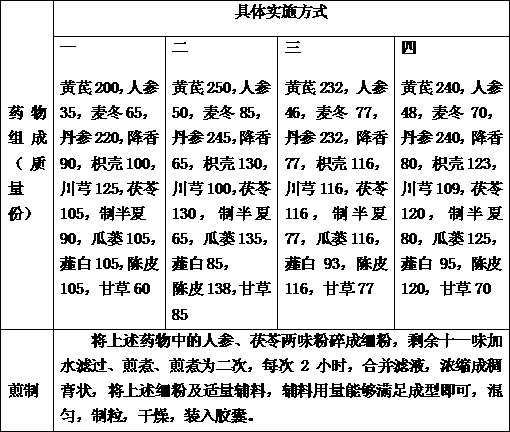
The invention discloses a medicine for preventing restenosis after the intracoronary stent implantation, which is characterized by comprising the components in parts by mass as follows: 200-250 parts of astragalus mongholicus, 35-50 parts of ginseng, 65-85 parts of radix ophiopogonis, 220-245 parts of salvia miltiorrhiza, 65-90 parts of lignum dalbergiae odoriferae, 100-130 parts of fructus aurantii, 100-125 parts of ligusticum wallichii, 105-130 parts of poria cocos, 65-90 parts of processed rhizoma pinelliae, 105-135 parts of trichosanthes kirilowii maxim, 85-105 parts of allium macrostemon, 105-138 parts of pericarpium citri reticulatae and 60-85 parts of liquorice. Compared with the prior art, the medicine can prevent a restenosis phenomenon after the intracoronary stent implantation, effectively relieve angina, and prevent and treat in-stent restenosis.
Owner:张新元
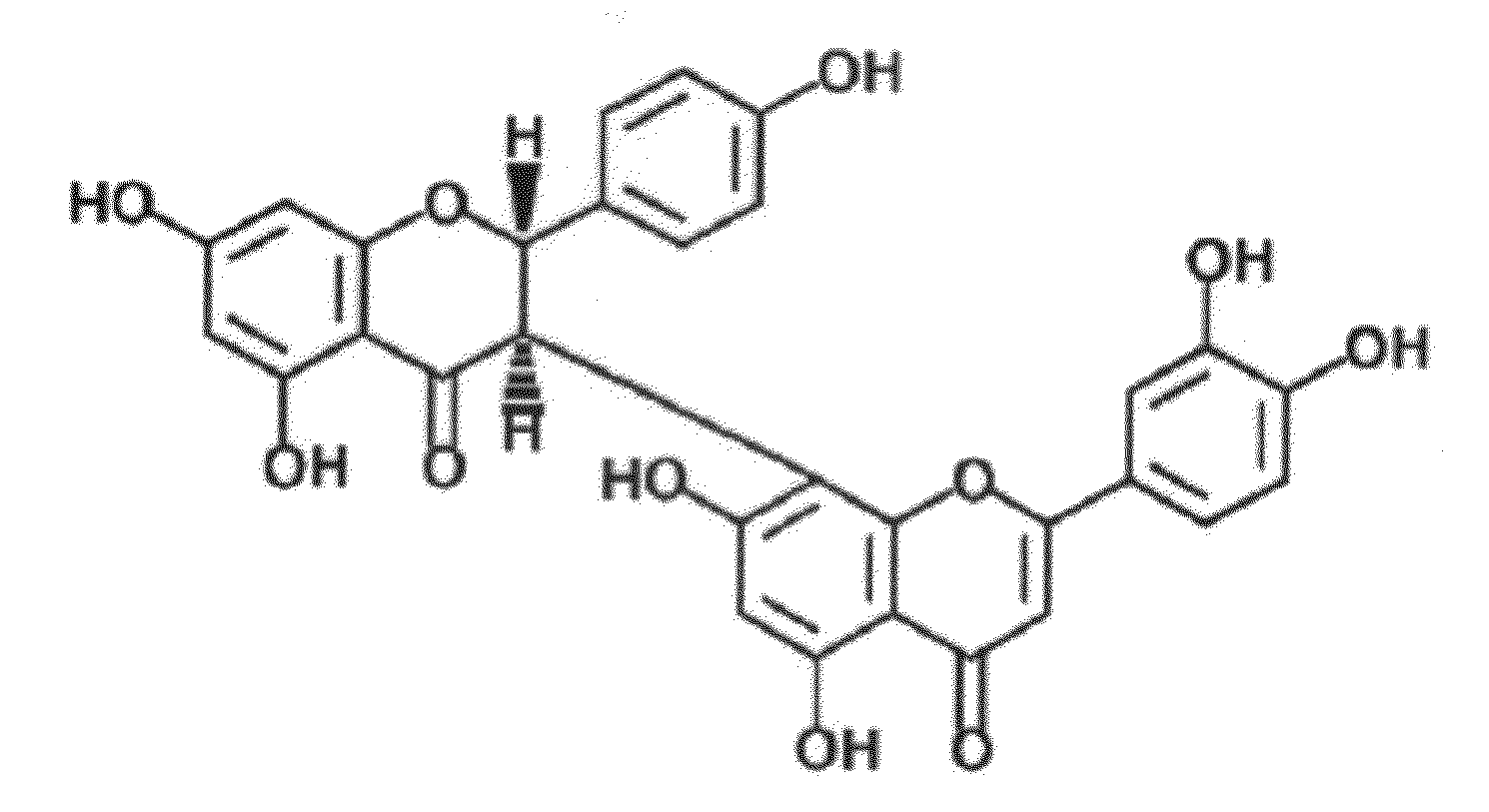
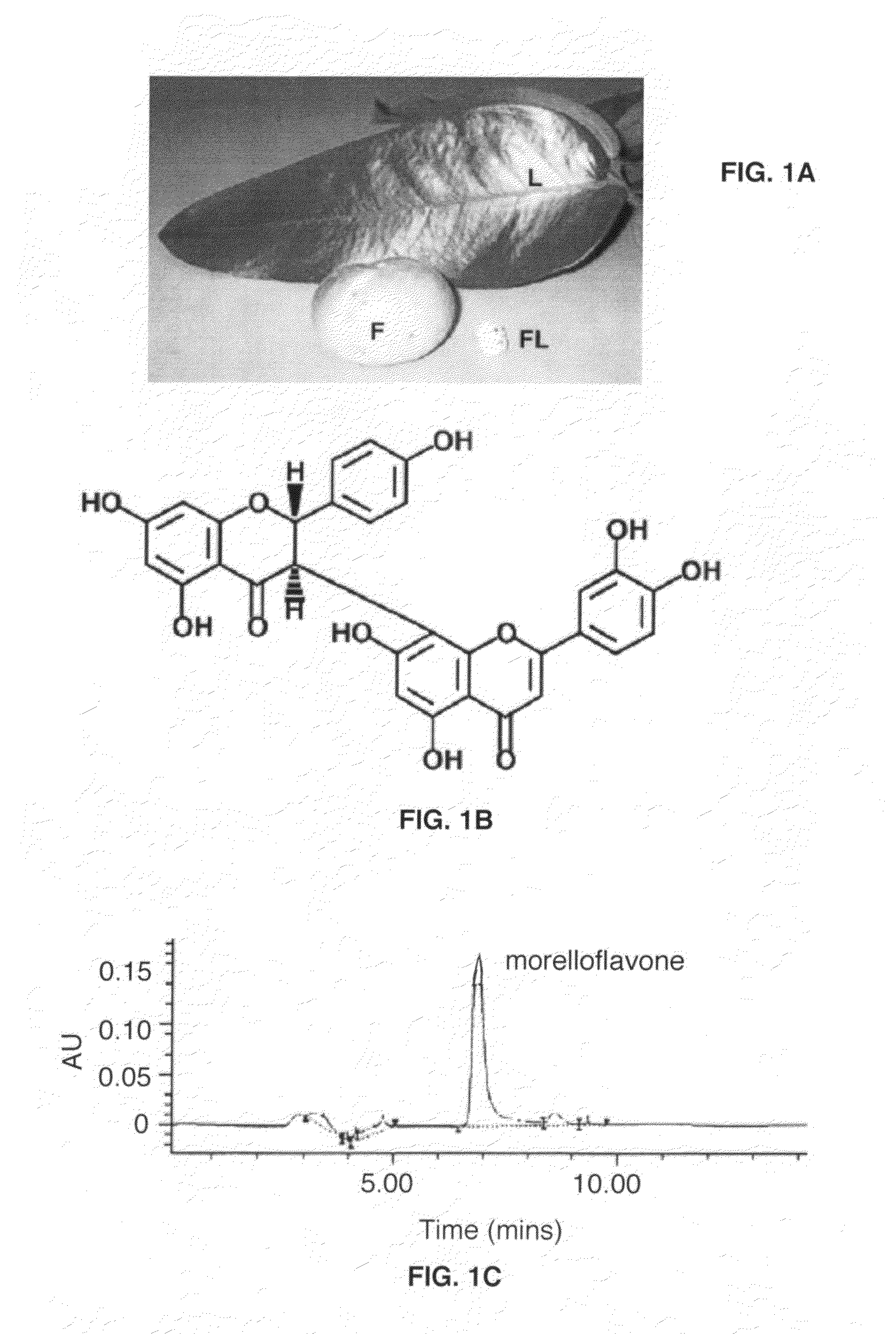
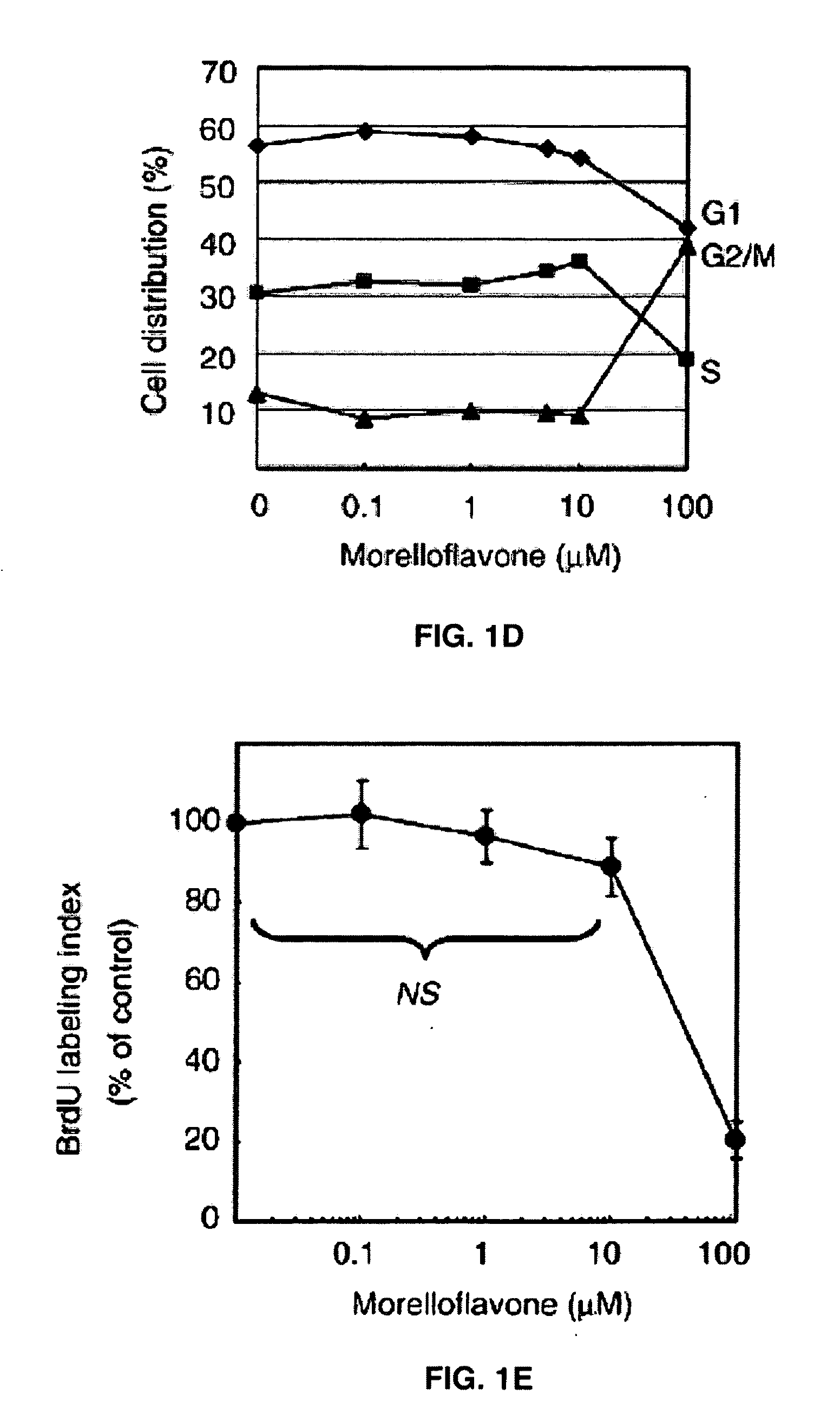
Uses of morelloflavone
Provided herein are methods of treating postangiplasty, in-stent restenosis or atherosclerosis in an individual in need of such treatment, comprising the step of administering to said individual an effective dose of morelloflavone with or with a effective dose of one or more of an HMG-CoA reductase inhibitor or a hypolipidemic agent or lipid-lowering agent or other lipid agent or lipid modulating agent or anti-atherosclerotic agent. Also provided are pharmaceutical compositions comprising a morelloflavone or pharmaceutical combinations comprising a morelloflavone and one of an HMG-CoA reductase inhibitor or a hypolipidemic agent or lipid-lowering agent or other lipid agent or lipid modulating agent or anti-atherosclerotic agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com