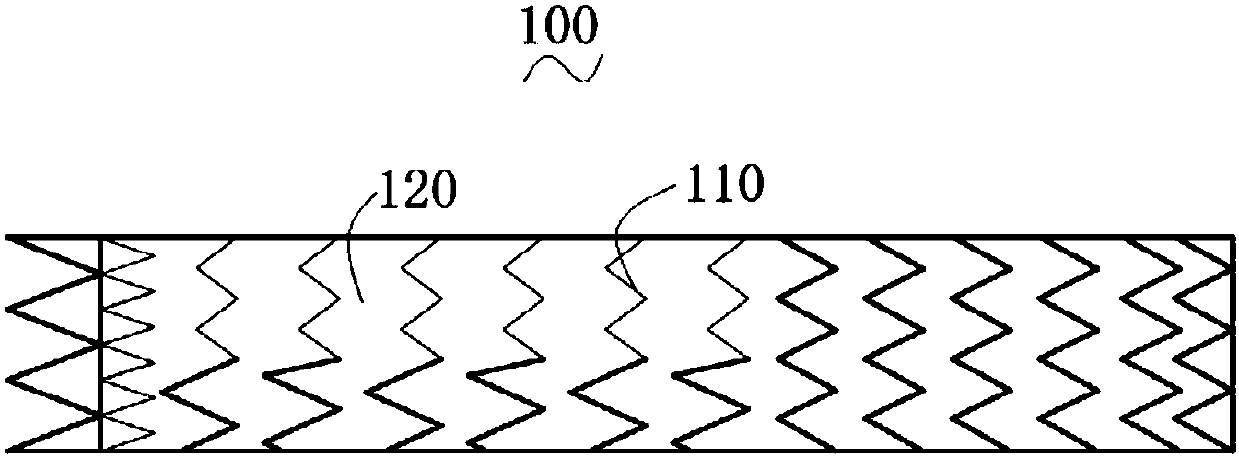
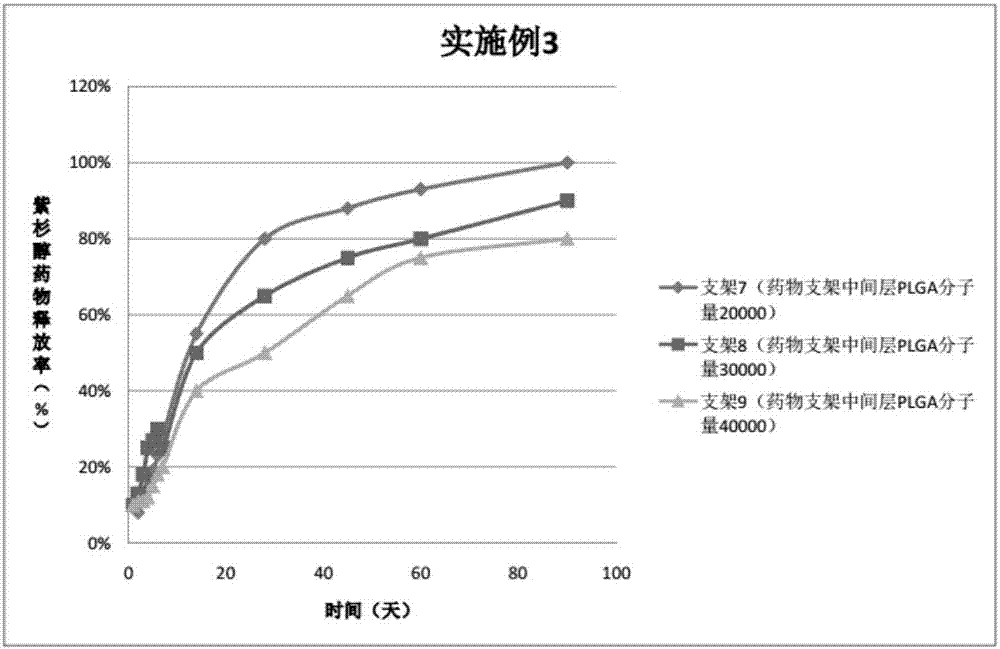
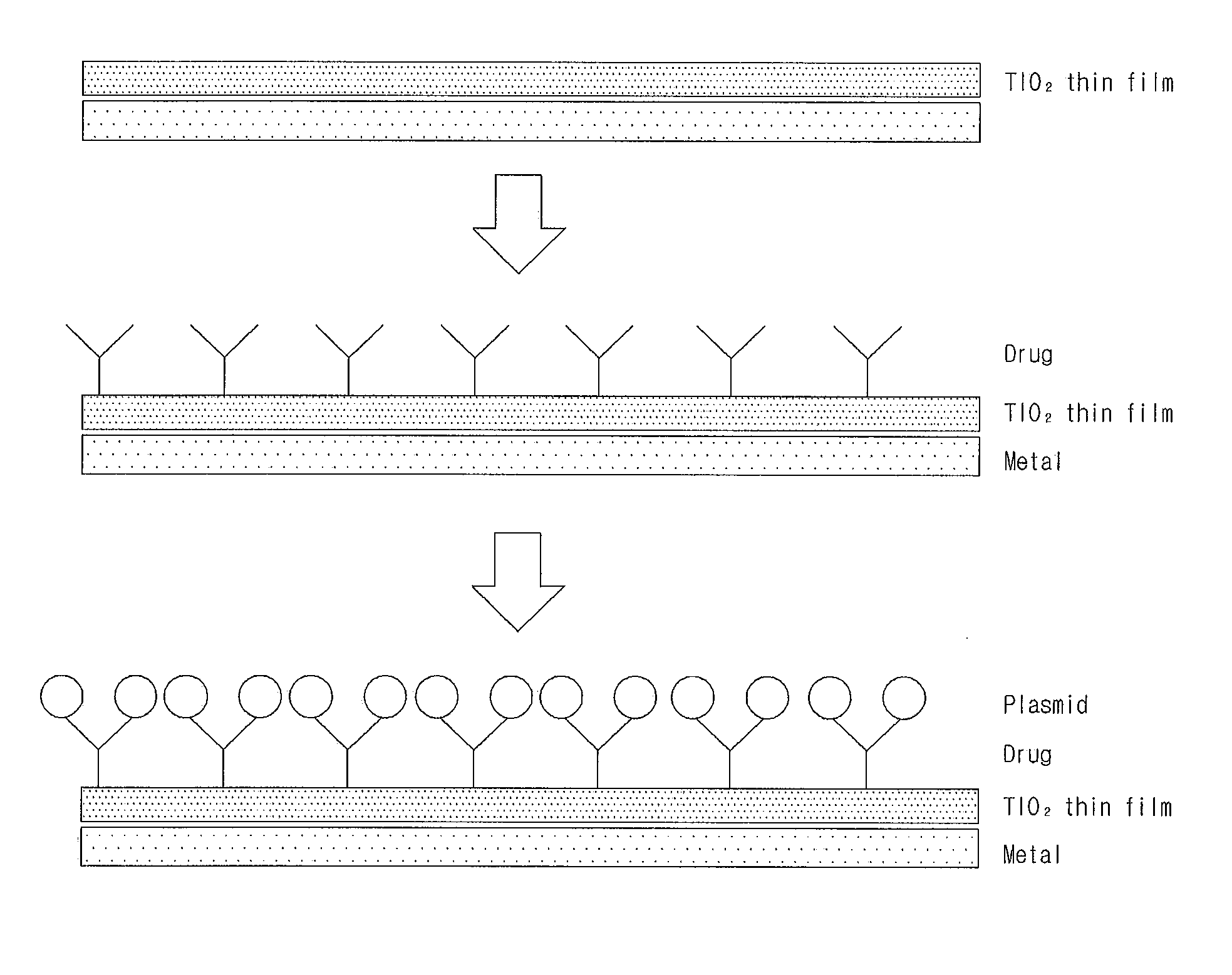
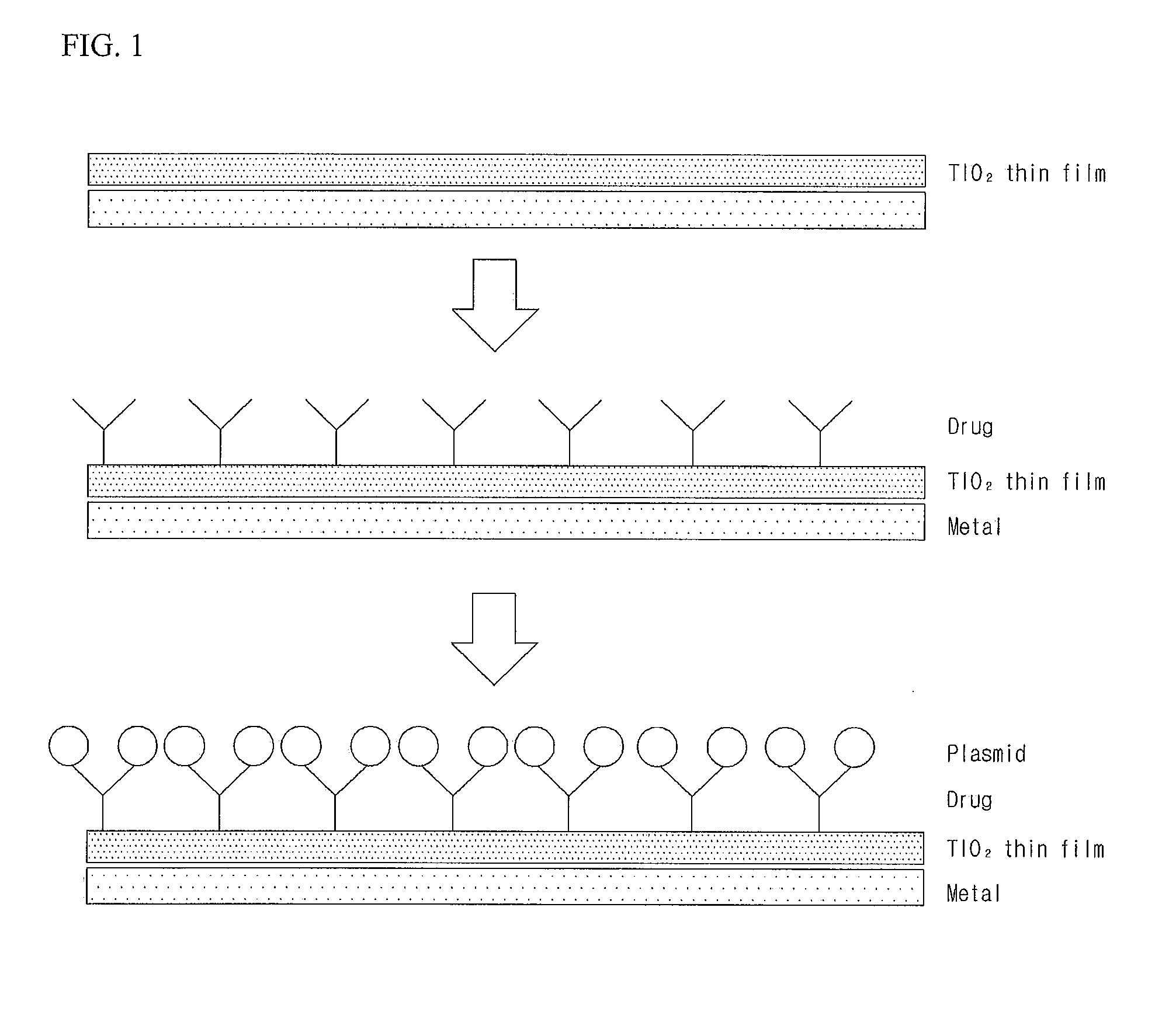
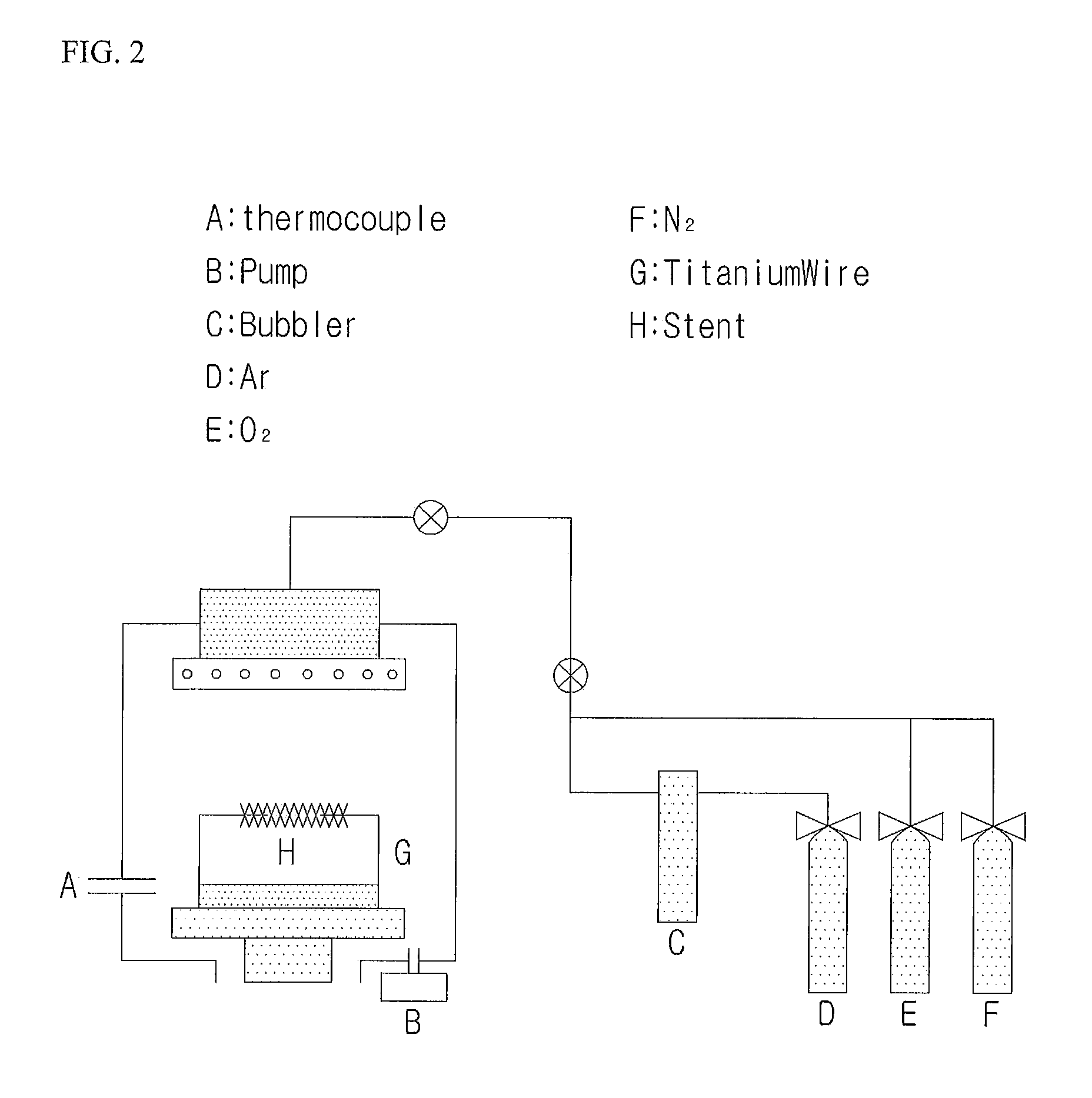
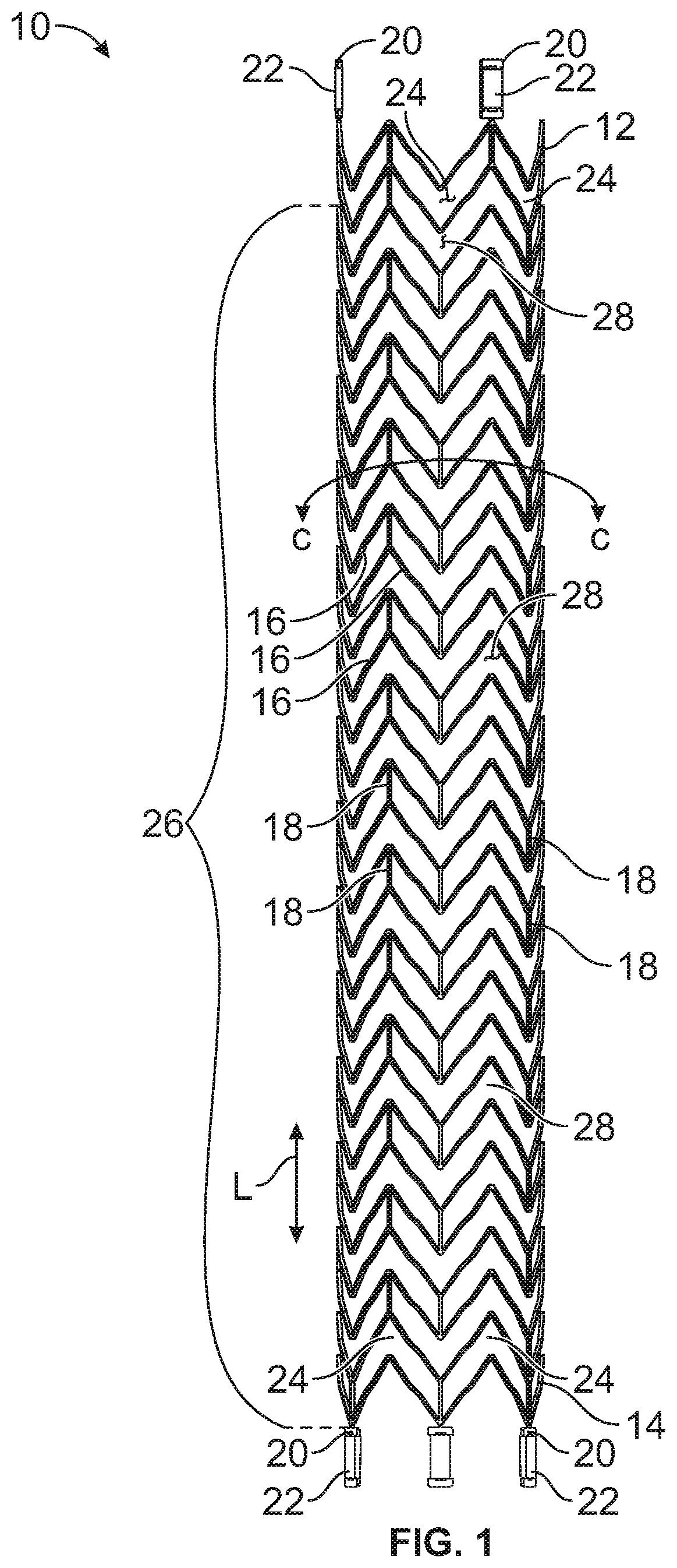
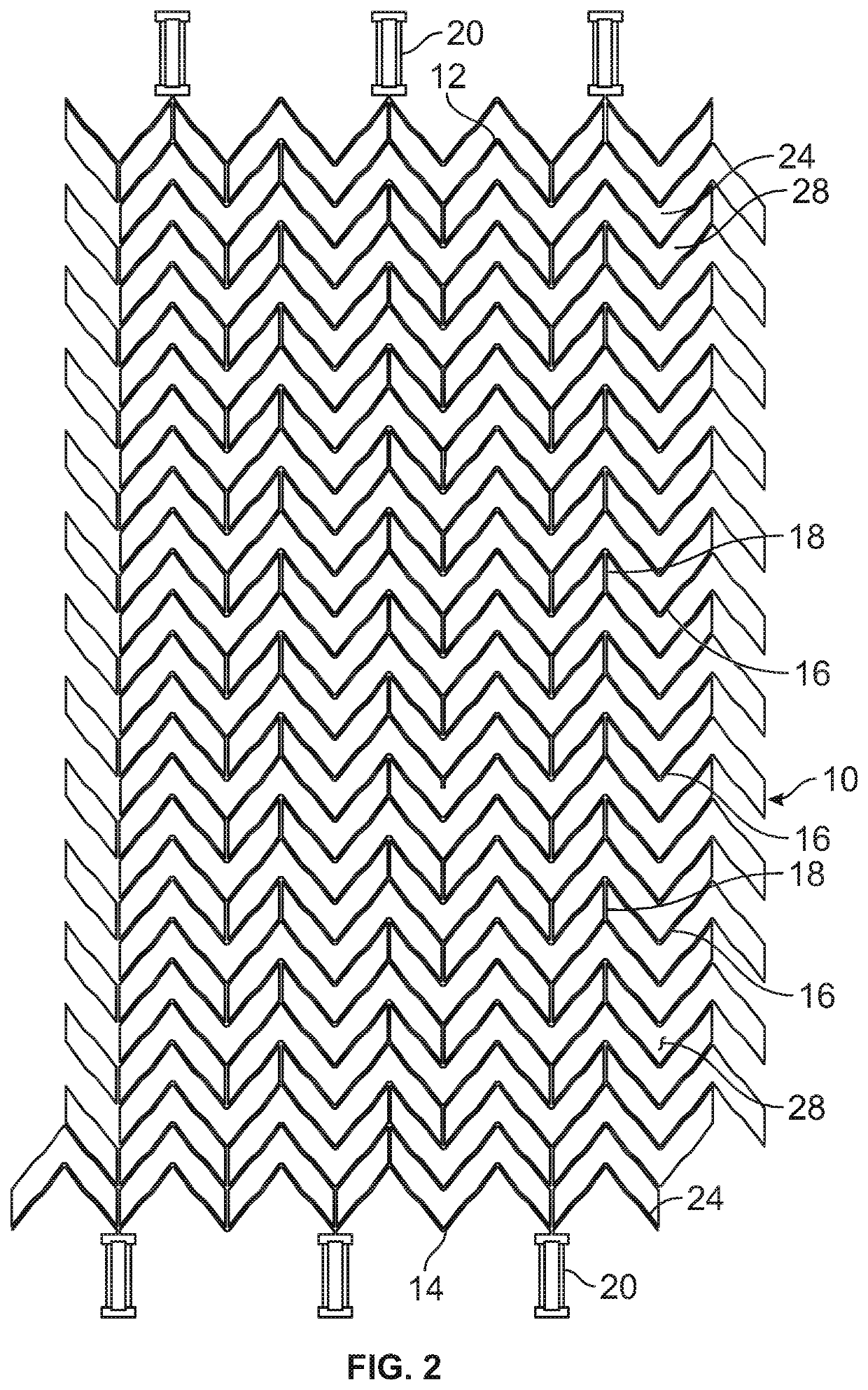
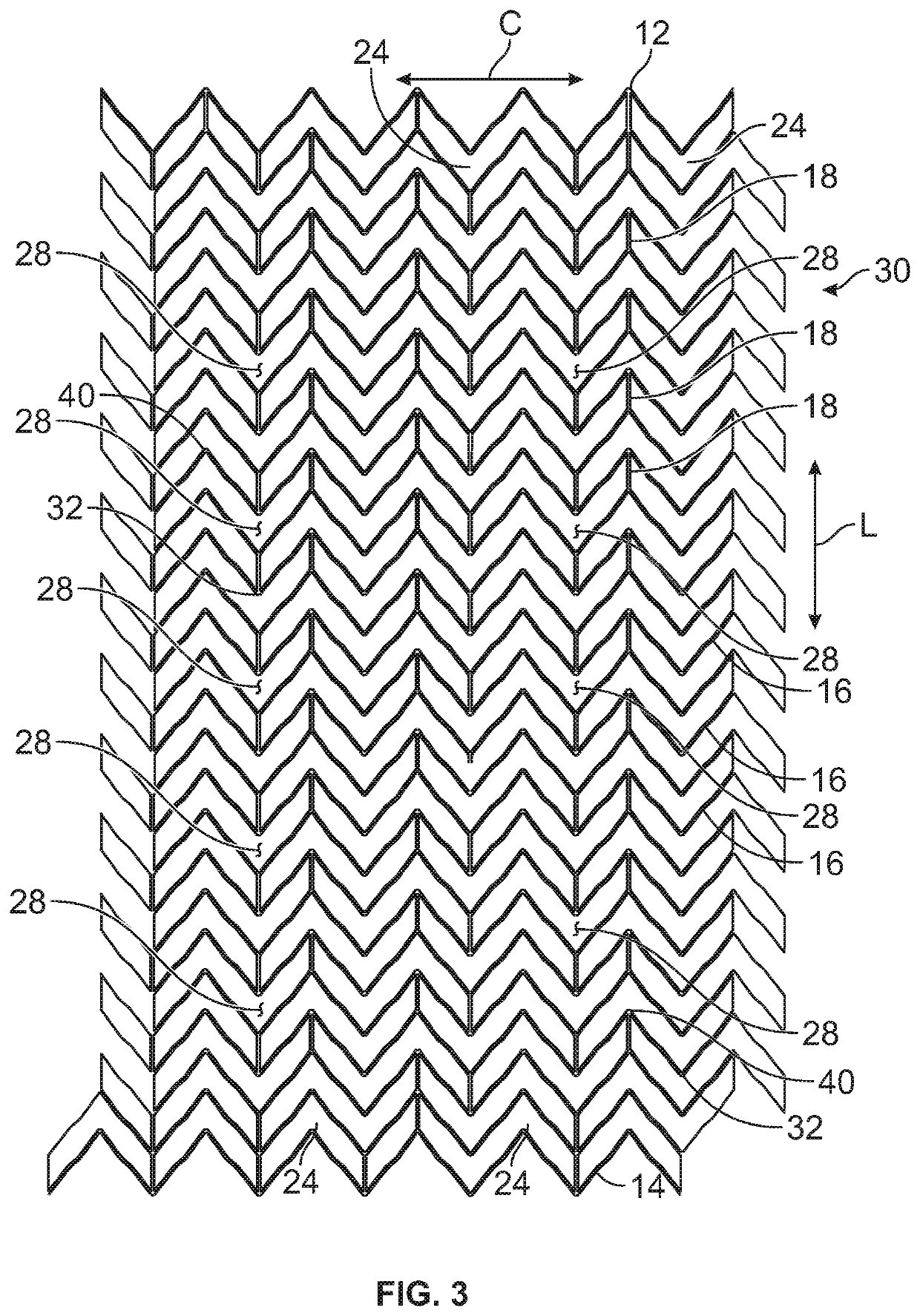
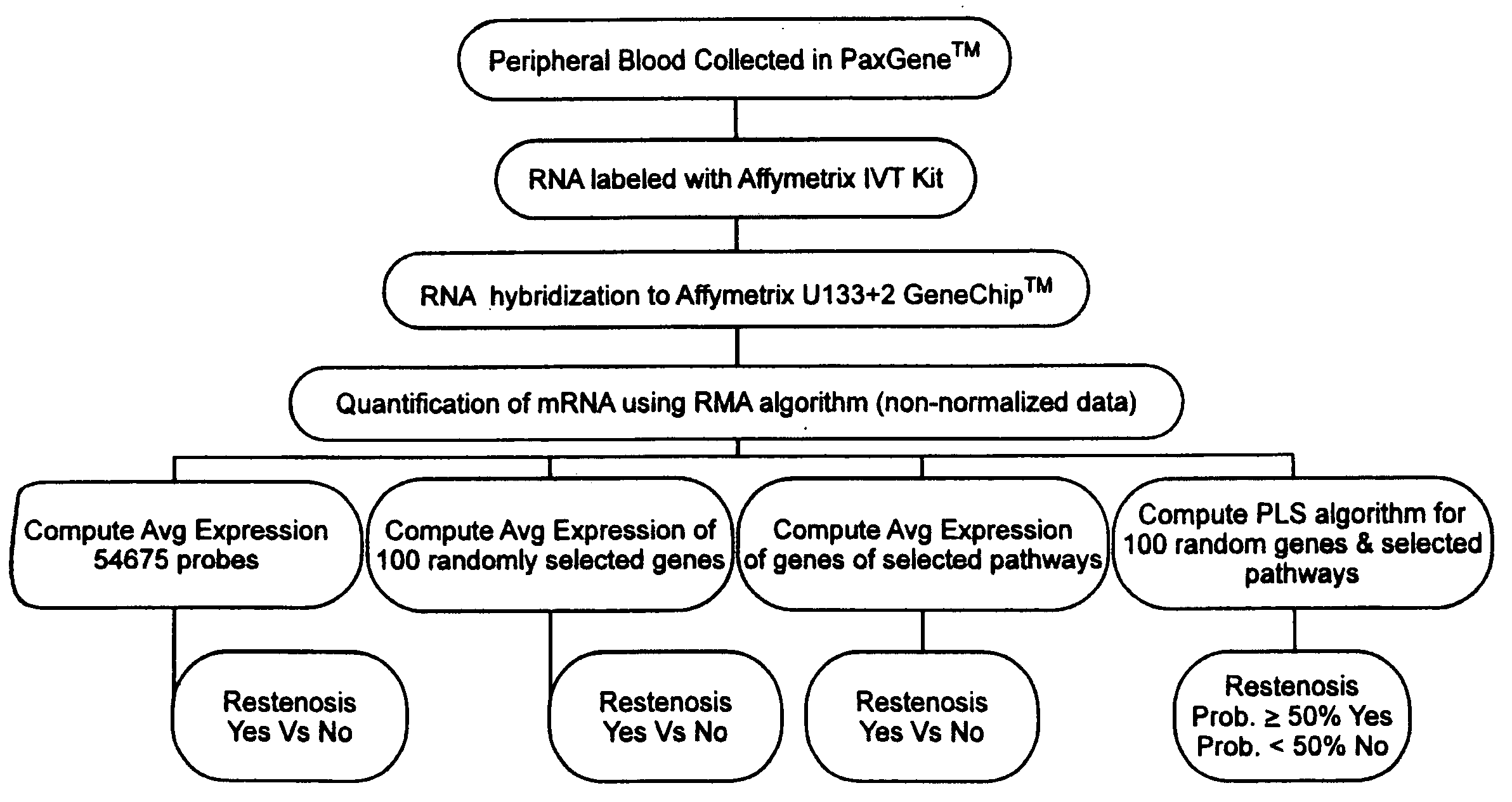
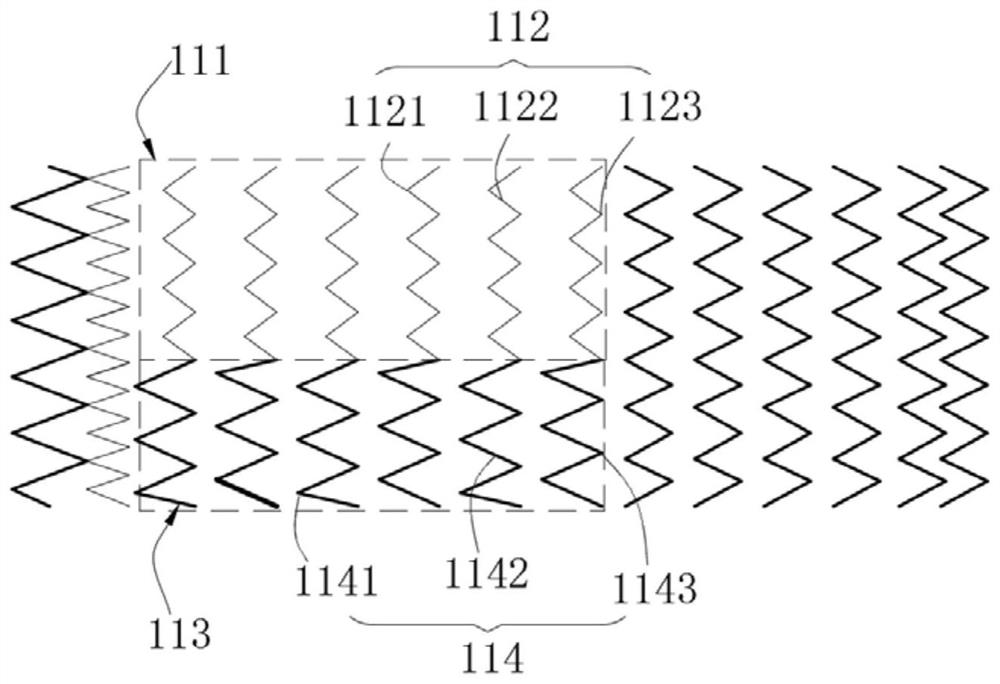
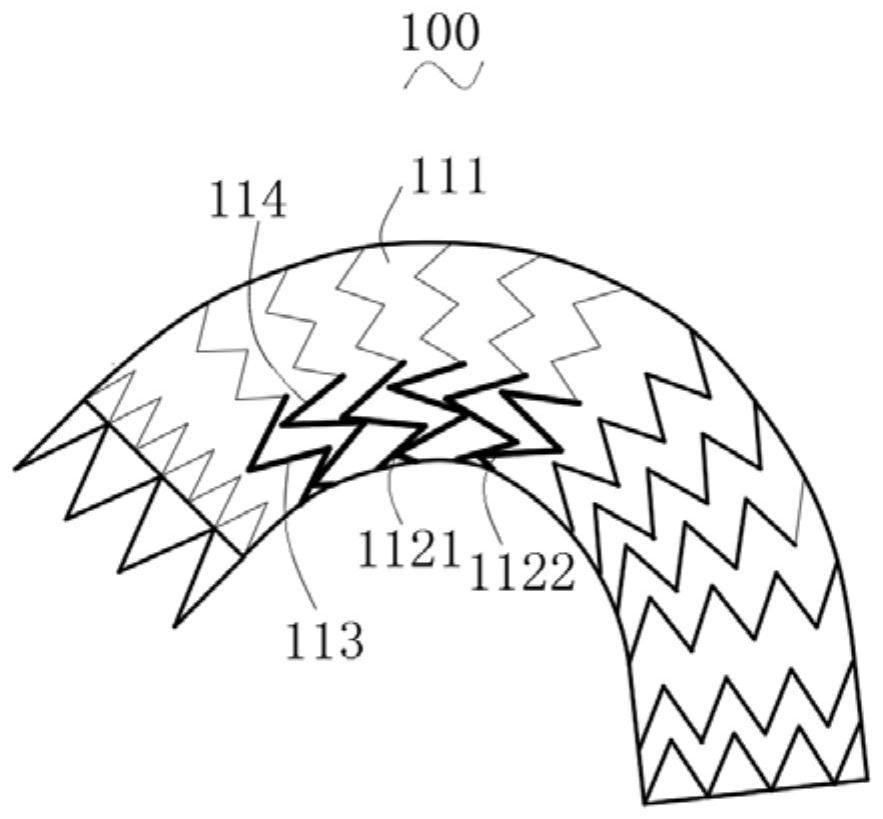
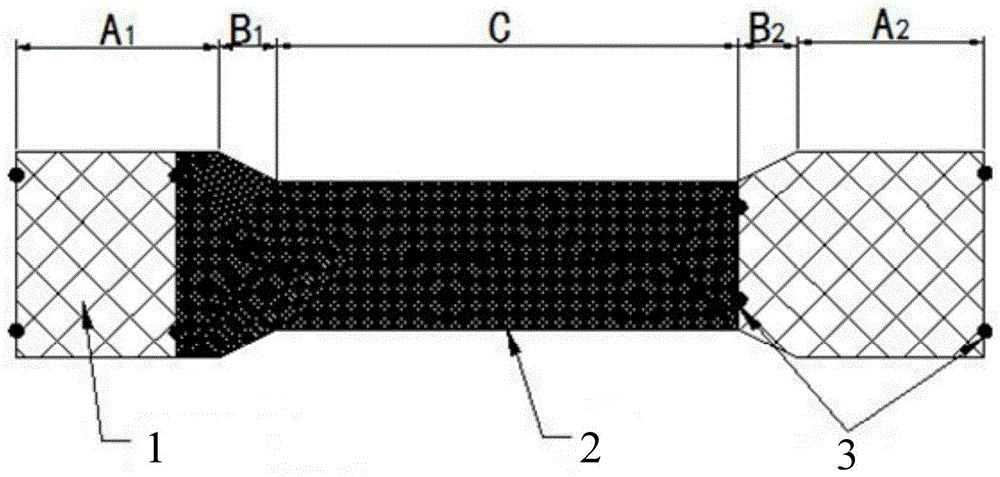
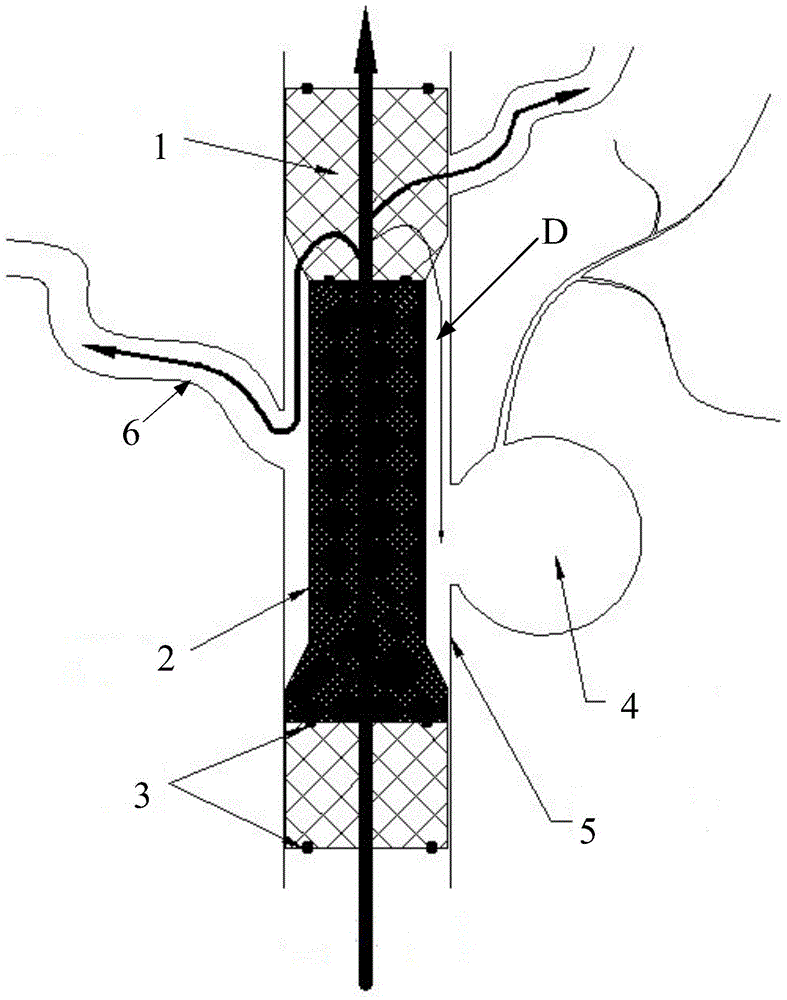
Patents
Literature
42 results about "Bare-metal stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
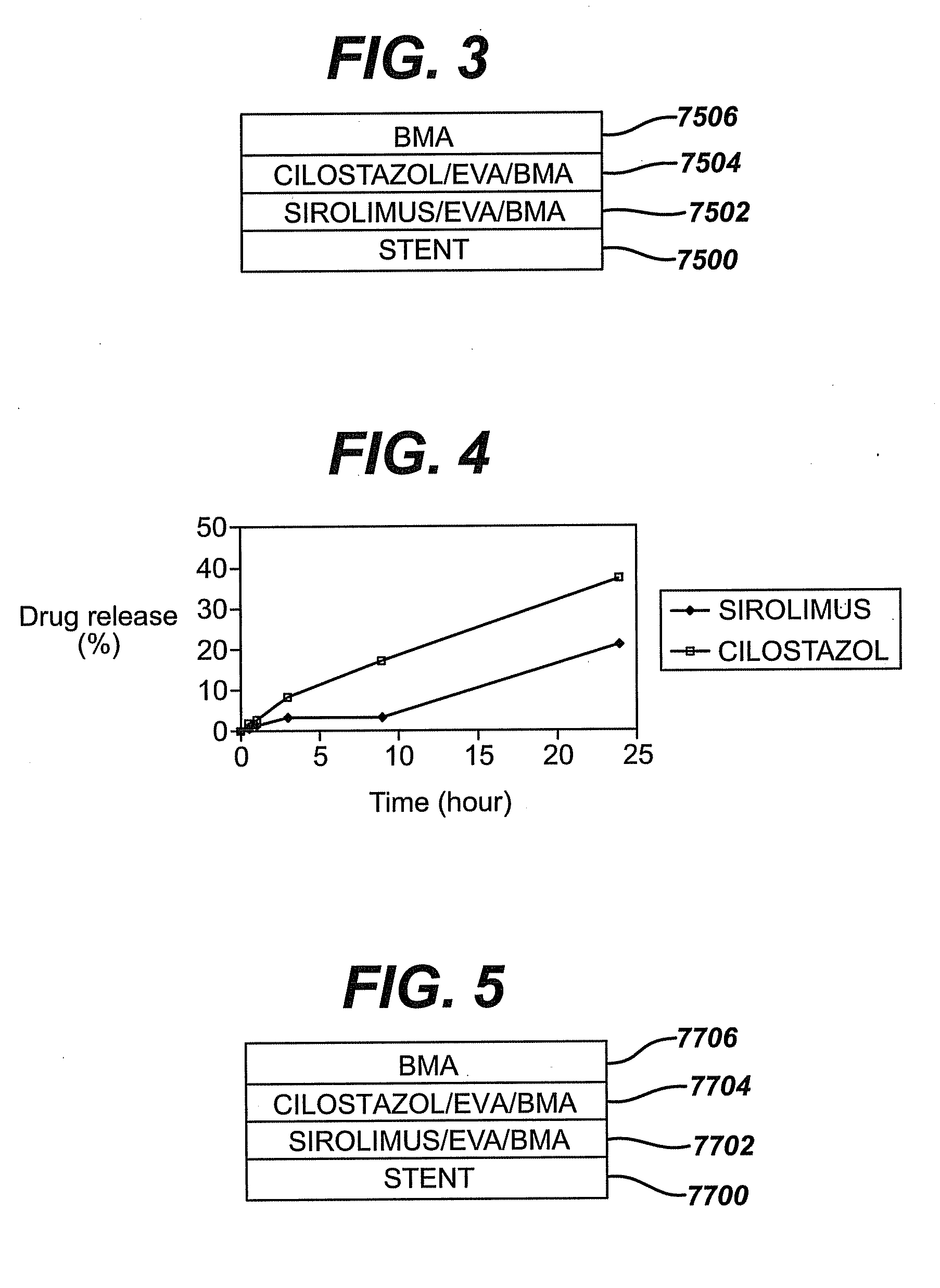
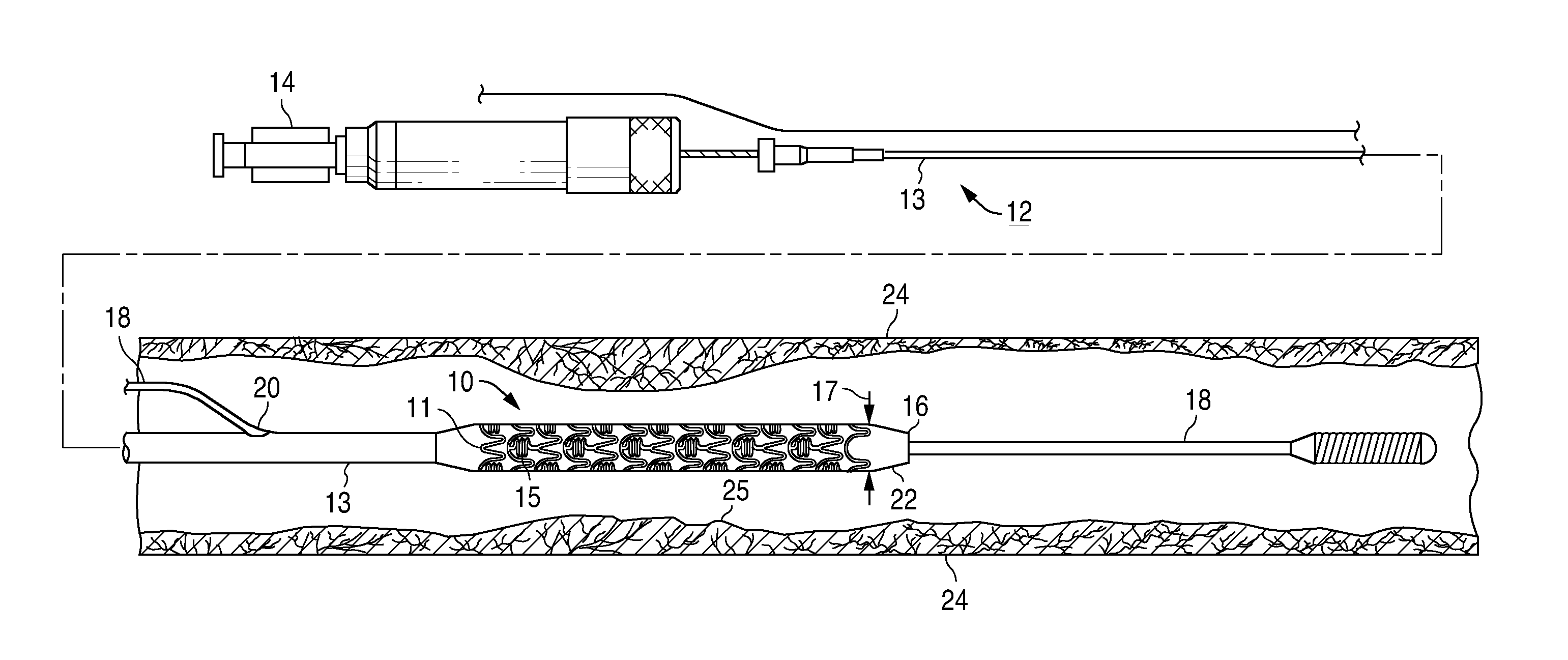
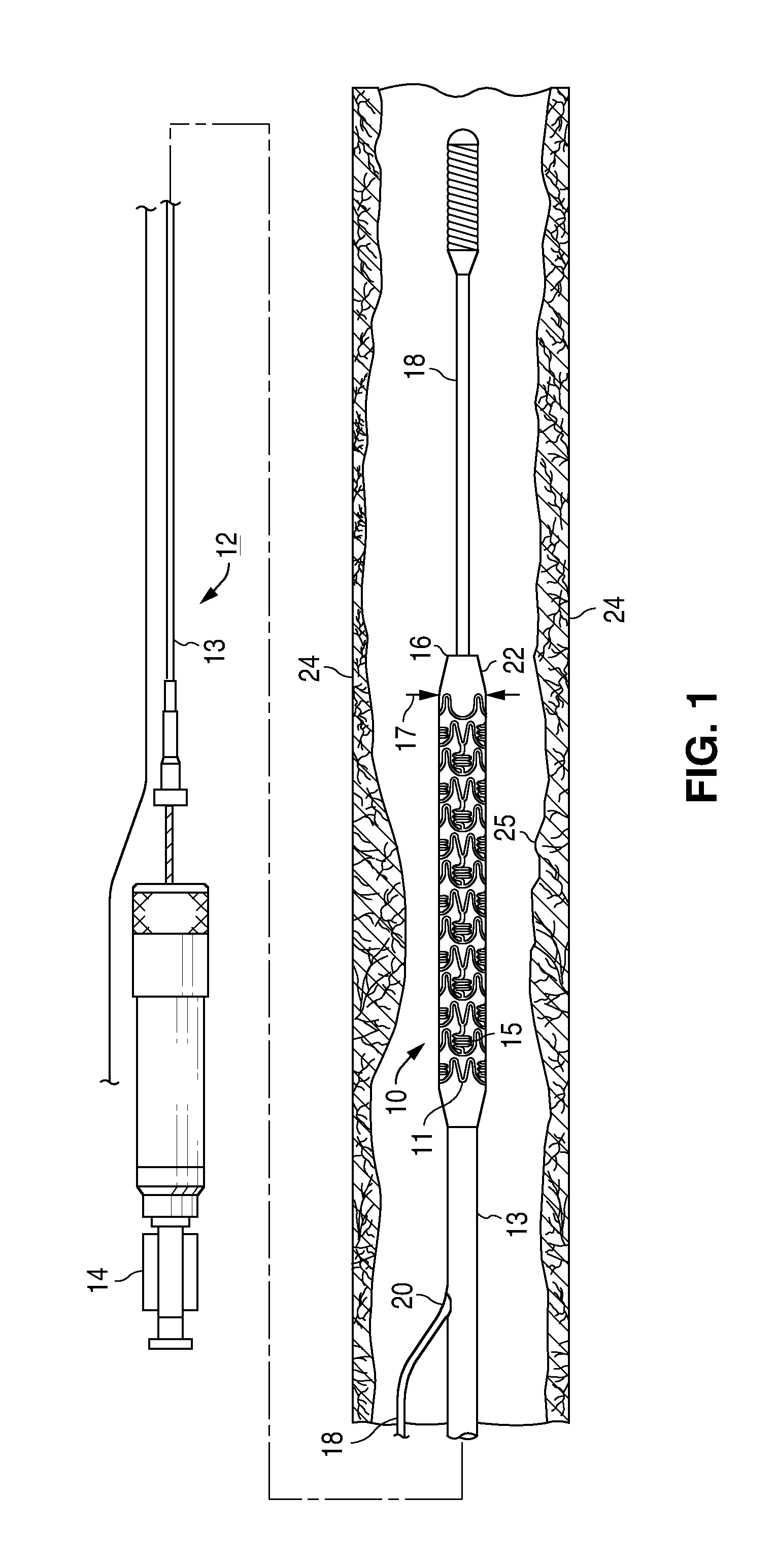
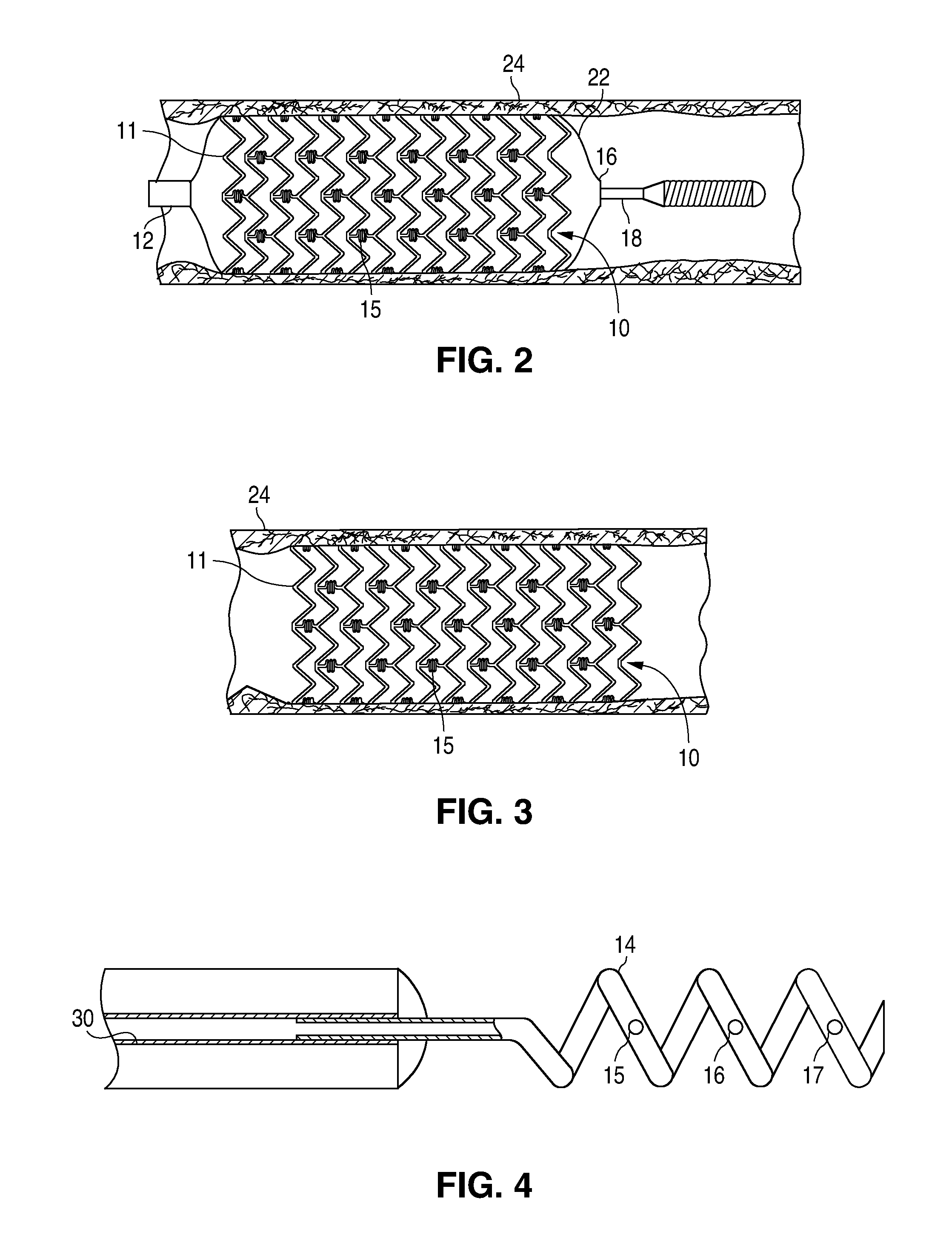
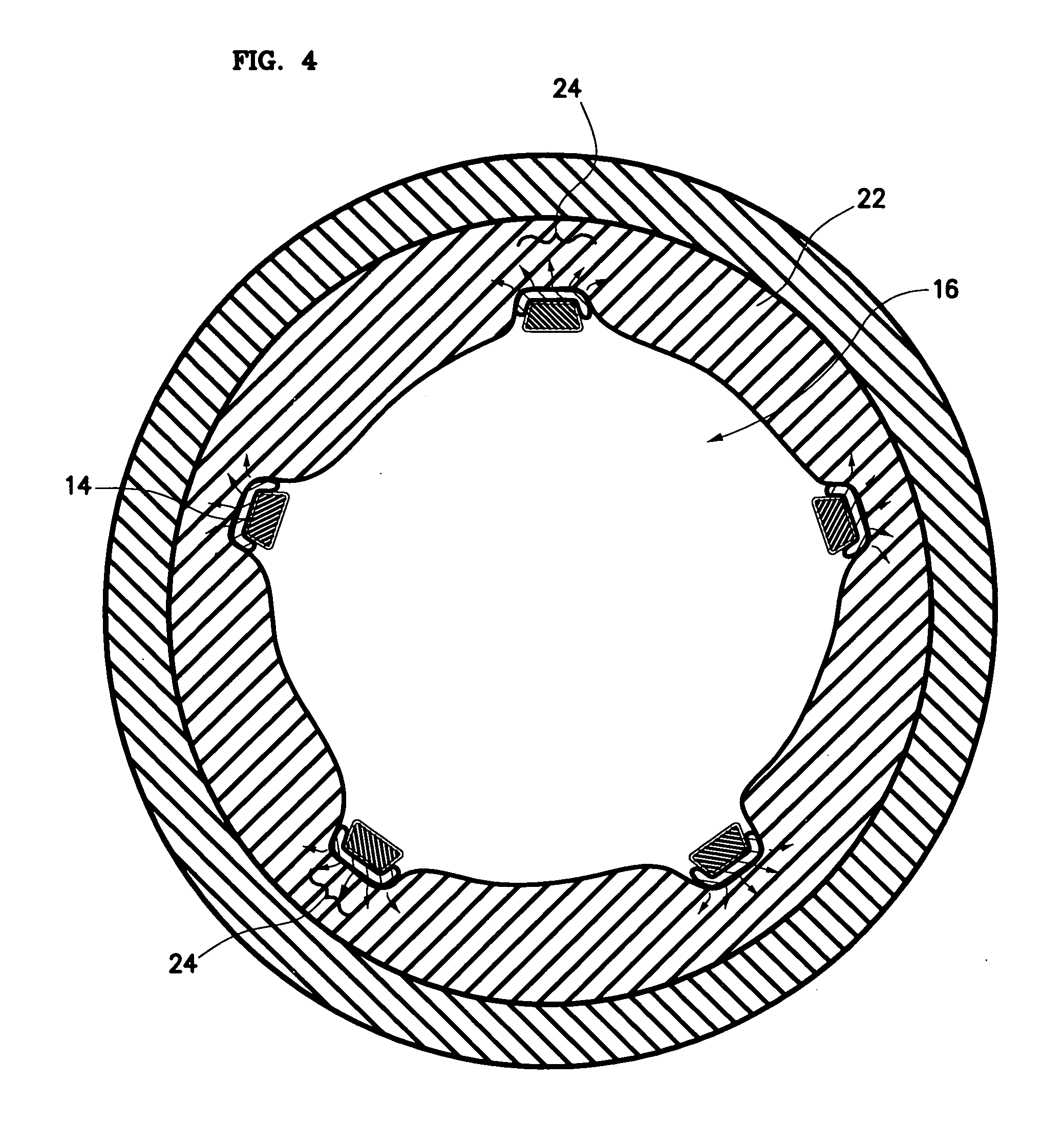
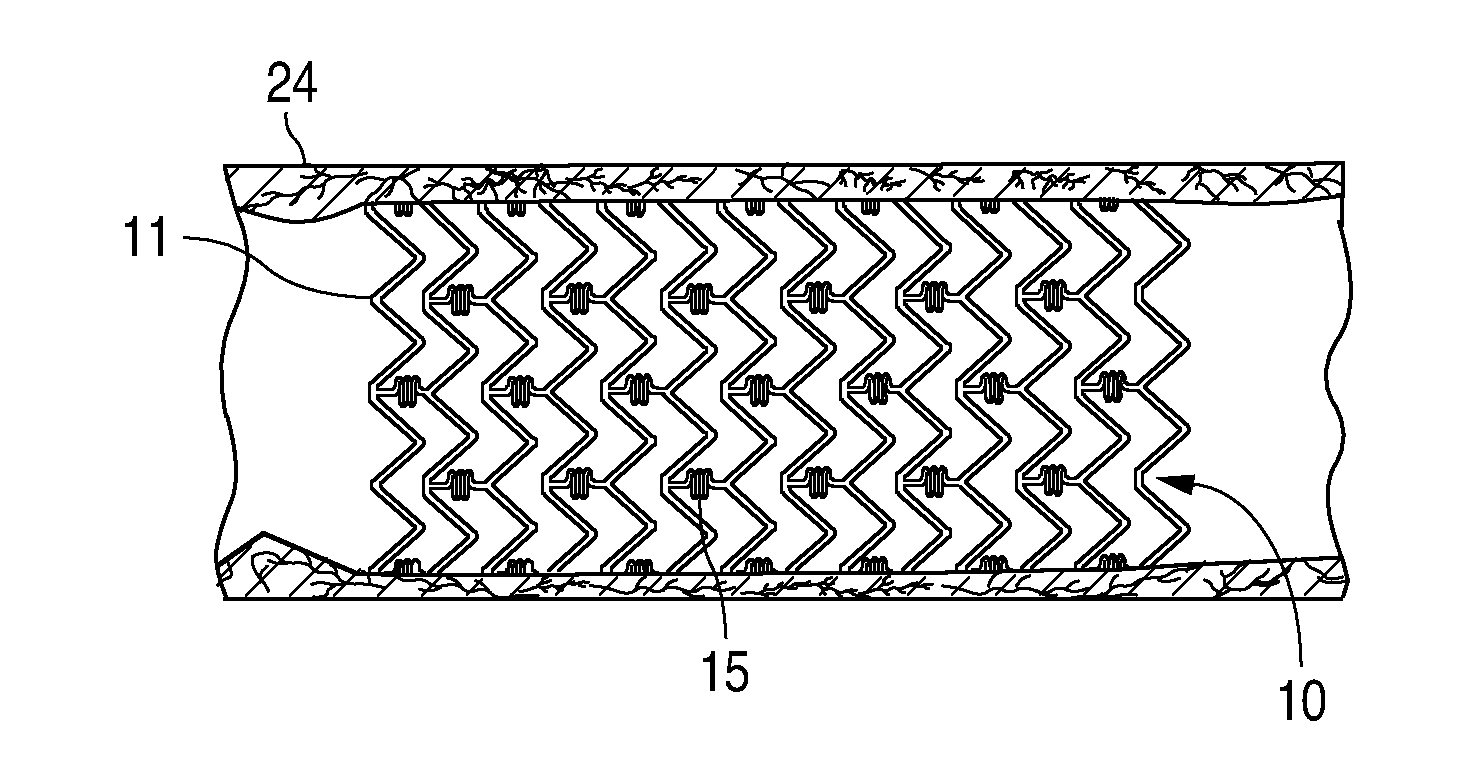
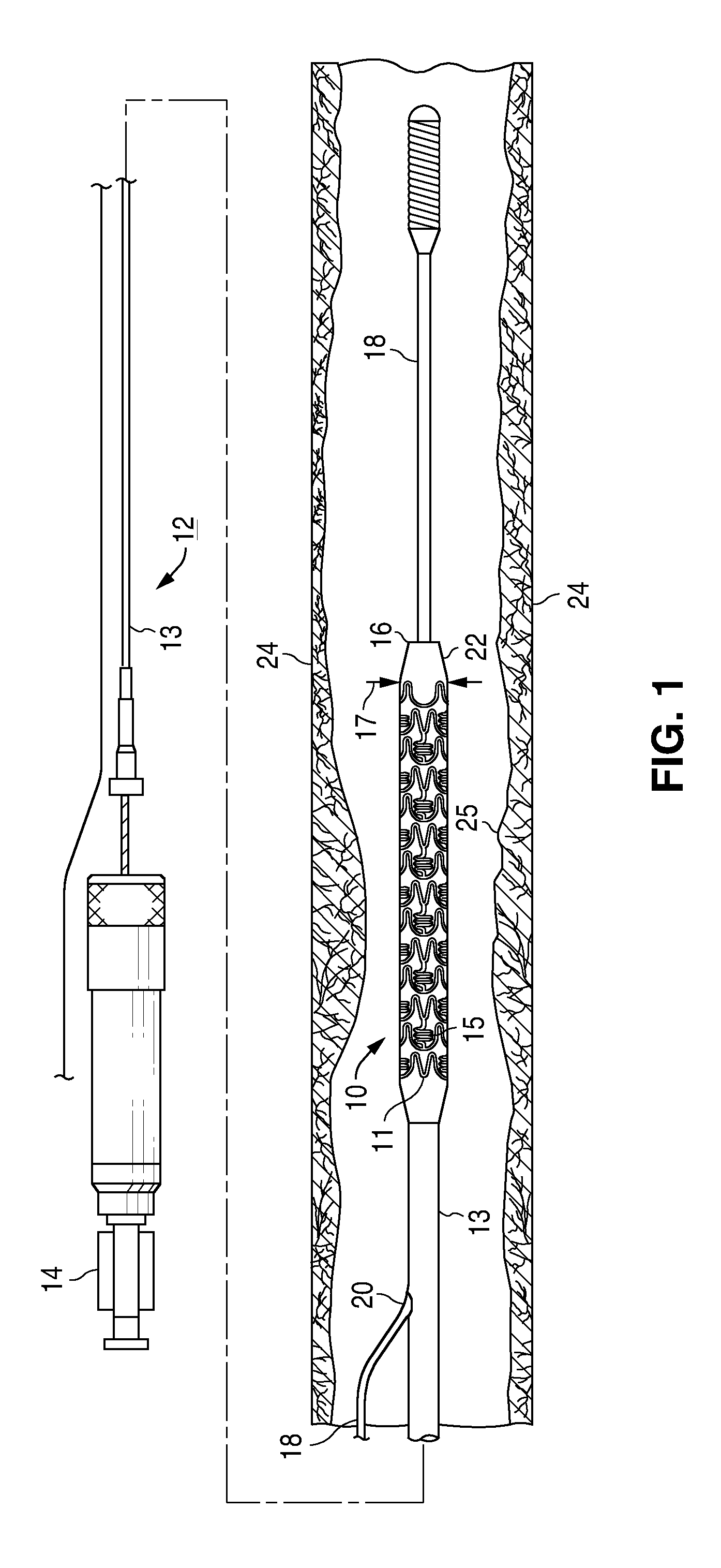
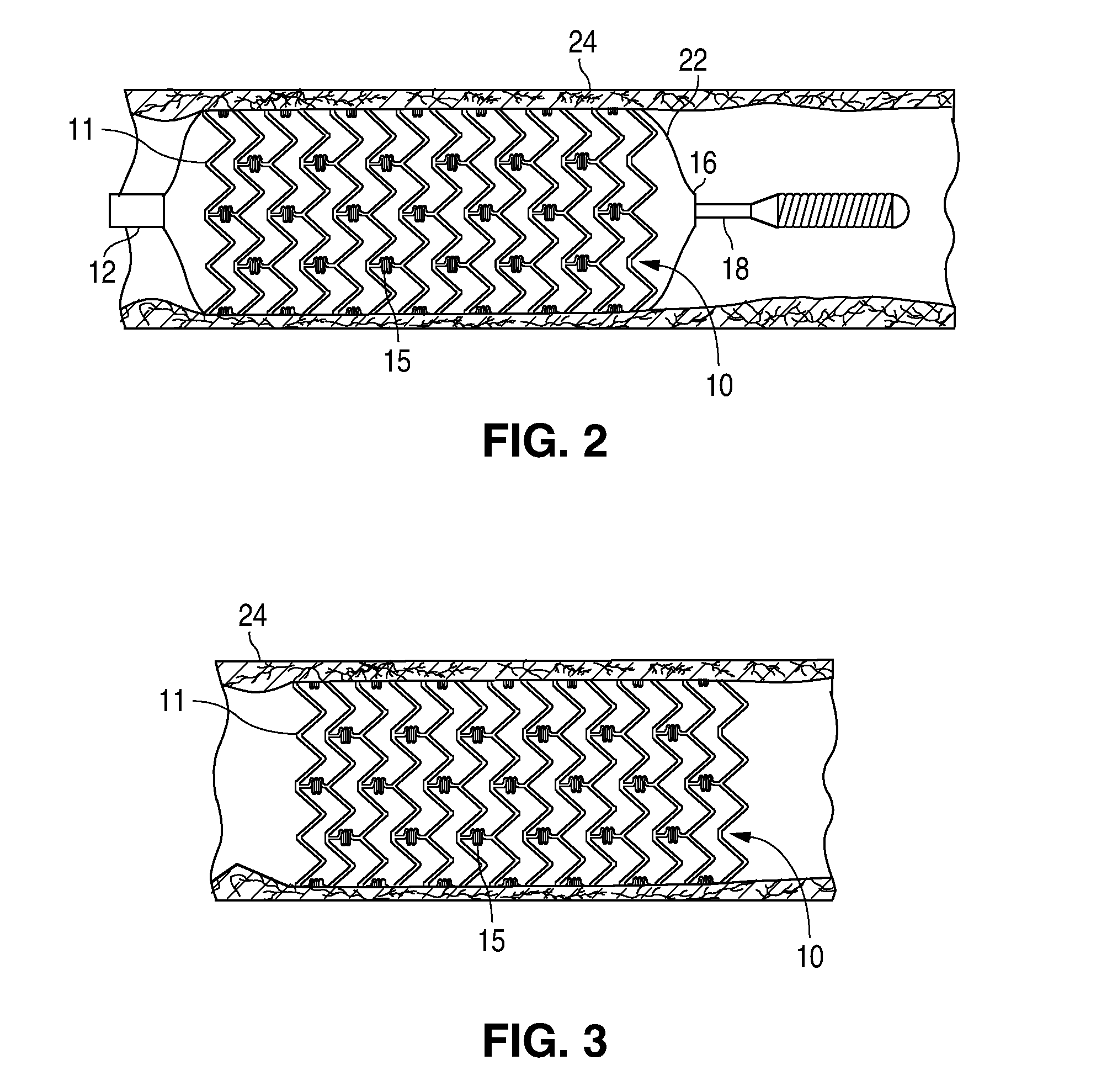
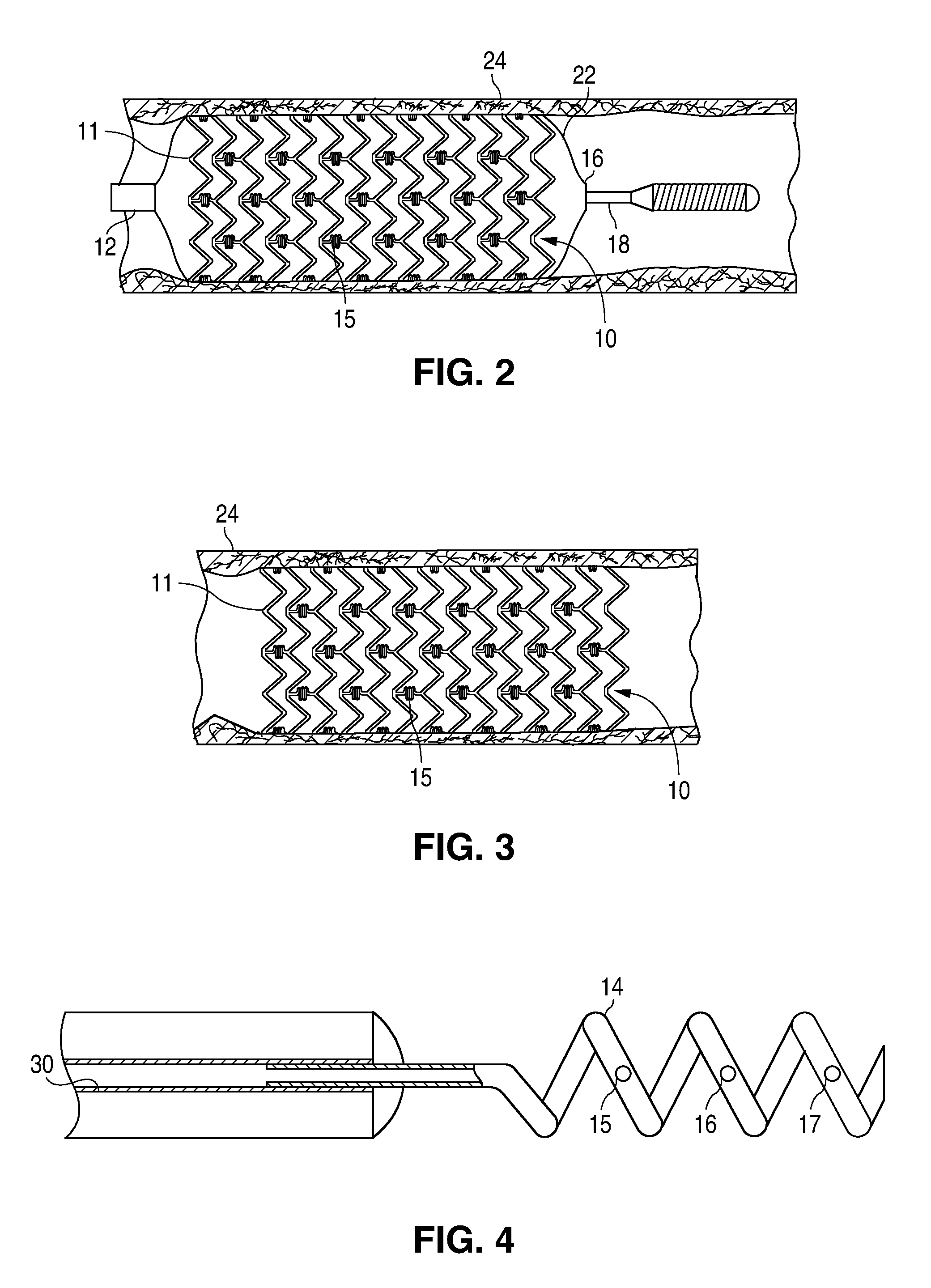
Bare-metal stent is a stent without a coating or covering (as used in covered stents drug-eluting stents). It is a mesh-like tube of thin wire. The first stents licensed for use in cardiac arteries were bare metal – often 316L stainless steel. More recent ('2nd generation') stents use cobalt chromium alloy. The first stents used in gastrointestinal conditions of the esophagus, gastroduodenum, biliary ducts, and colon were plastic; bare metal stents were first brought into the clinic in the 1990s.
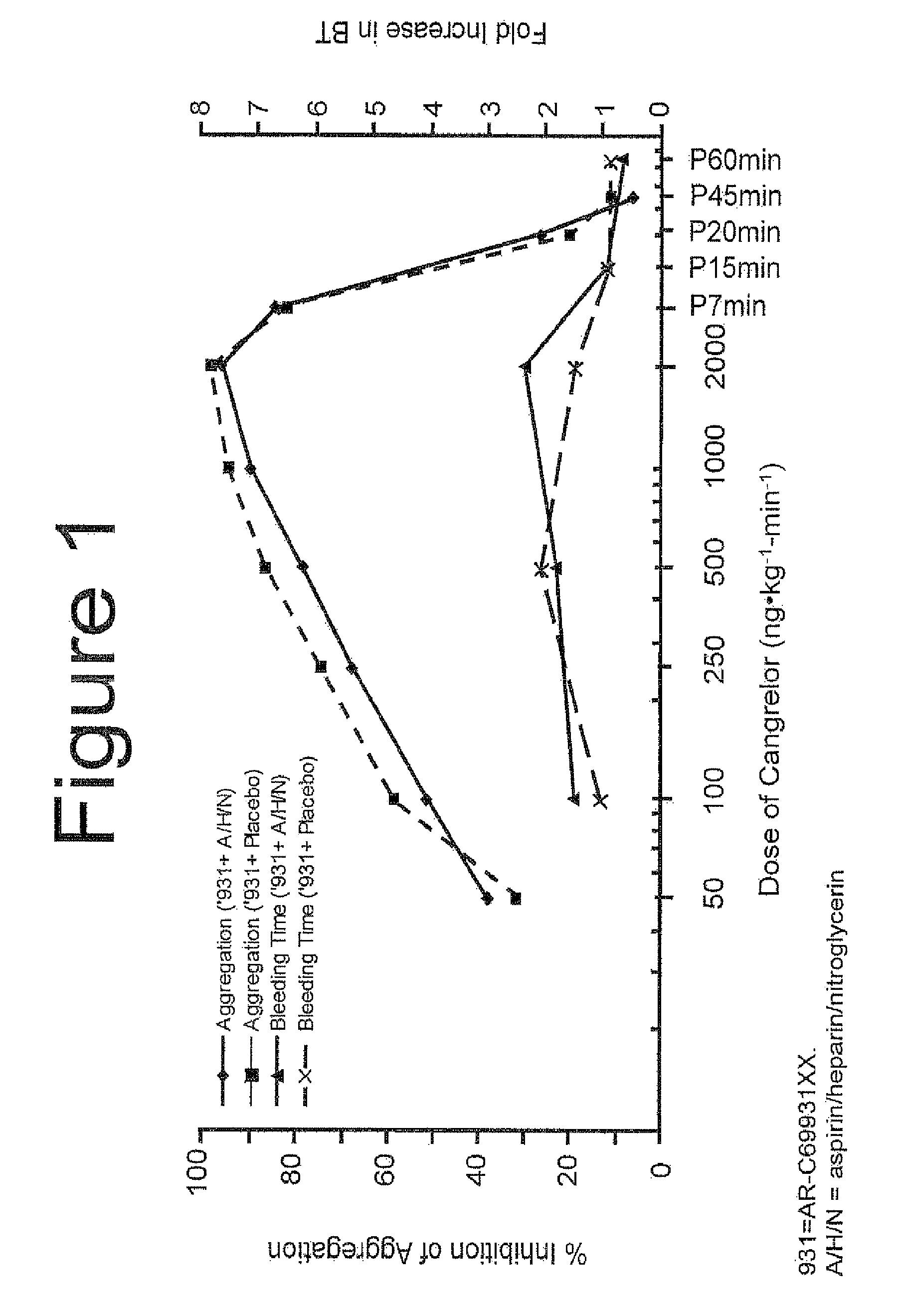
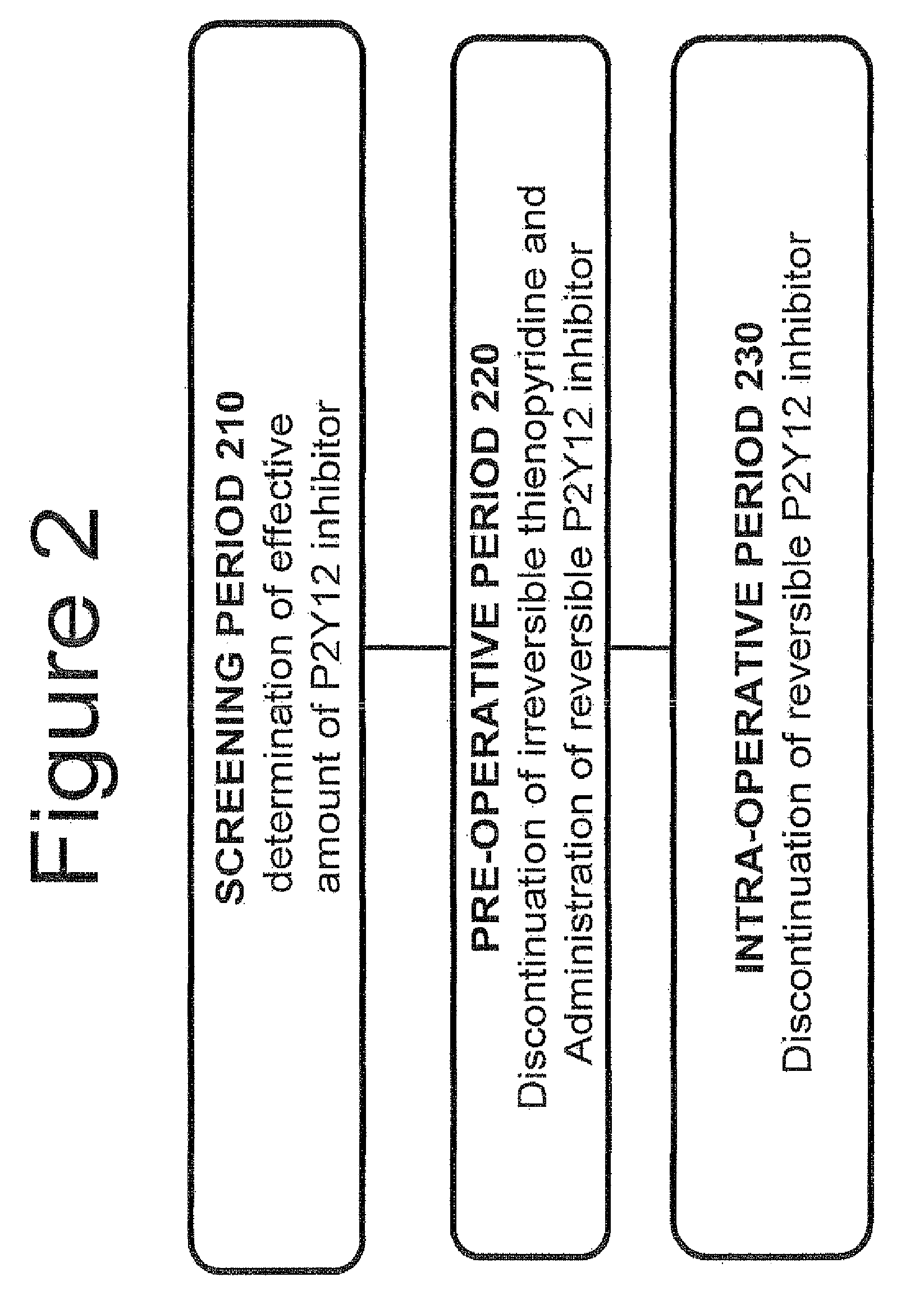
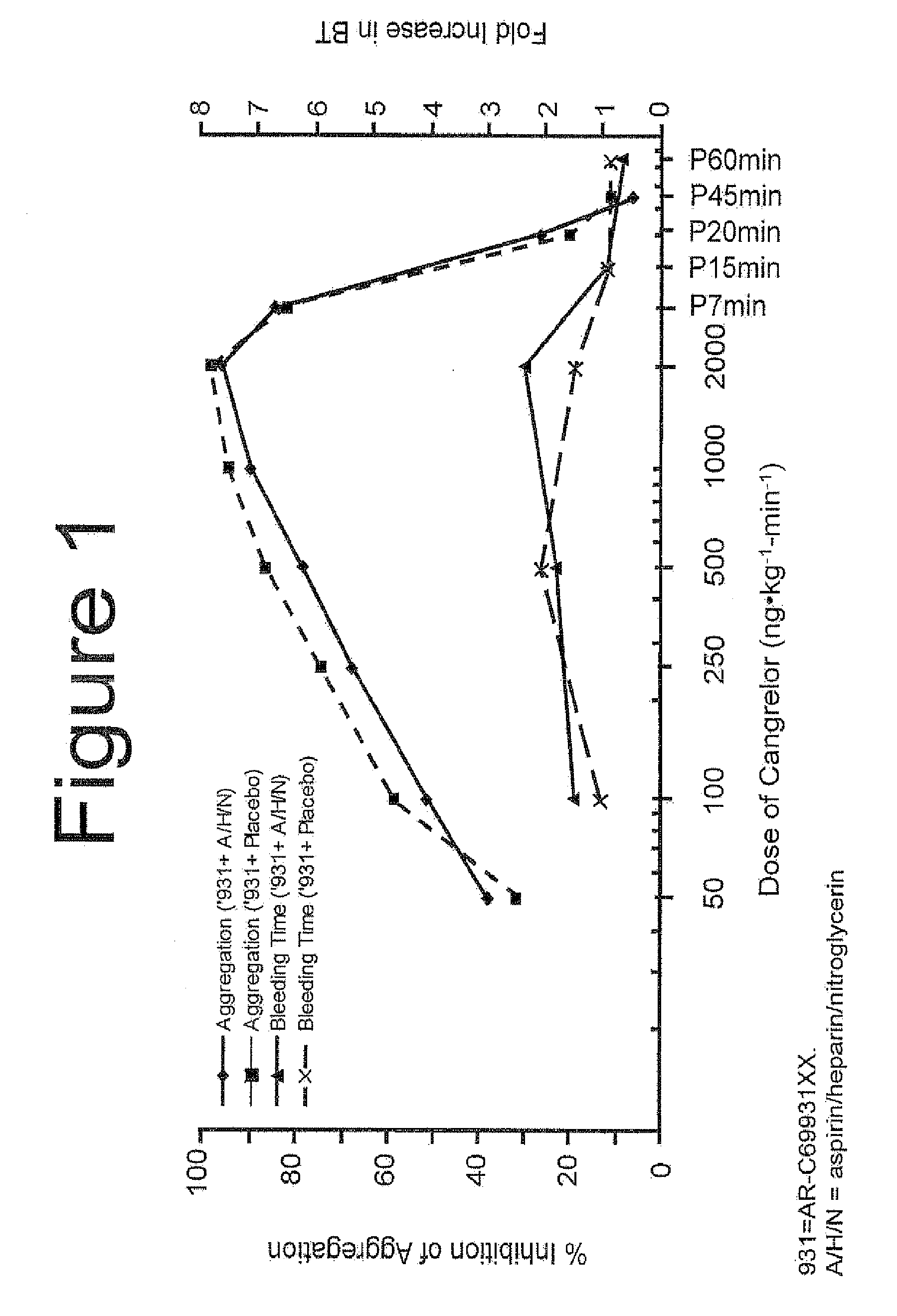
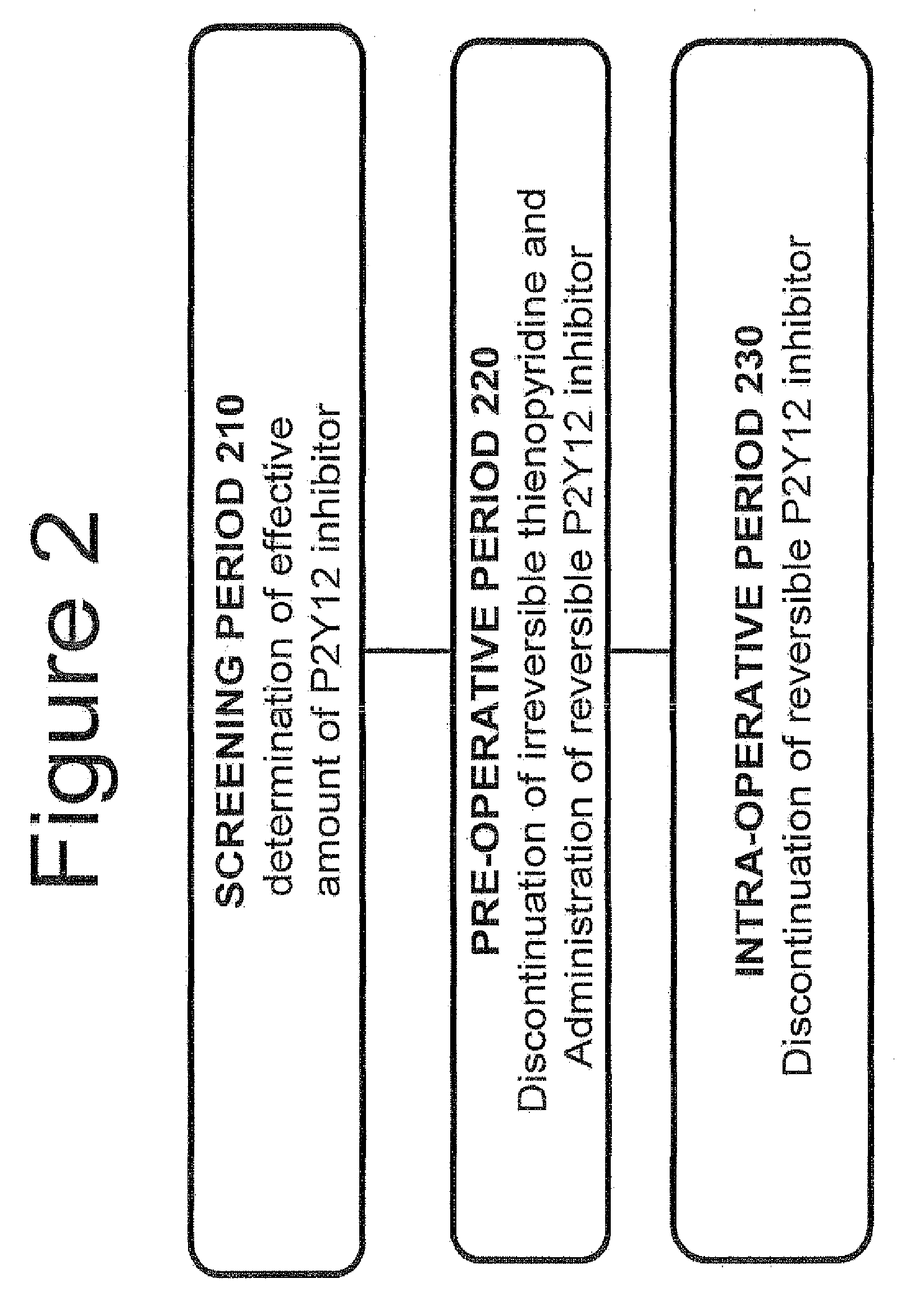
Maintenance of platelet inhibition during antiplatelet therapy
A method of treating or preventing a disease or condition in a subject that was previously treated with at least one thienopyridine is described. The method includes administering to the subject an effective amount of at least one reversible, short-acting P2Yi2 inhibitor. The described method can be used for subjects diagnosed with symptoms such as stable or unstable angina, vascular ischemic events, atherosclerosis, acute coronary syndrome, as well as STEMi or N-STEMI. The described method can also be used for patients having previously received a stent, such as a bare metal stent or a drug-eluting stent, and the treatment or prevention of stent thrombosis. The method can be used prior to, during, or after an invasive procedure such as coronary artery bypass grafting, percutaneous coronary intervention, or other general surgical procedure.
Owner:CHIESI FARM SPA
Bare metal stent with drug eluting reservoirs
Implantable medical devices may be utilized to locally delivery one or more drugs or therapeutic agents to treat a wide variety of conditions, including the treatment of the biological organism's reaction to the introduction of the implantable medical device. These therapeutic agents may be released under controlled and directional conditions from a stent so that the one or more therapeutic agents reach the correct target area, for example, the surrounding tissue.
Owner:CORDIS CORP
Treatment Of Diabetic Patients With Stent And Locally Administered Adjunctive Therapy
Embodiments of the present invention include methods of treating, preventing, and / or ameliorating a vascular disease and / or disorder in a diabetic or pre-diabetic patient. The methods include implanting a stent in a vascular region in a diabetic or pre-diabetic patient, and prior to and / or during the implantation procedure, delivering a lubricant formulation to the vascular region. The stent may be a bare metal stent, or a drug eluting stent, such as a metal stent with a coating including a drug, such as everolimus or sirolimus.
Owner:ABBOTT CARDIOVASCULAR
Maintenance of platelet inhibition during antiplatelet therapy
A method of treating or preventing a disease or condition in a subject that was previously treated with at least one thienopyhdine is described. The method includes administering to the subject an effective amount of at least one reversible, short-acting P2Yi2 inhibitor. The described method can be used for subjects diagnosed with symptoms such as stable or unstable angina, vascular ischemic events, atherosclerosis, acute coronary syndrome, as well as STEMi or N-STEMI. The described method can also be used for patients having previously received a stent, such as a bare metal stent or a drug-eluting stent, and the treatment or prevention of stent thrombosis. The method can be used prior to, during, or after an invasive procedure such as coronary artery bypass grafting, percutaneous coronary intervention, or other genera! surgical procedure.
Owner:CHIESI FARM SPA
Drug eluting stent coating with extended duration of drug release
A stent having a drug eluting formulation has three components: 1) Anti-neointimal hyperplasia or anti-restenosis agent 2) Main polymer 3) Additive polymer The anti-neointimal hyperplasia or anti-restenosis agent includes, but not limited to, Paclitaxel, Taxol, Rapamycin, Tacrolimus, Actinomycin D, Methotrexate, Doxorubicin, cyclophosphamide, and 5-fluorouracil, 6-mercapatopurine, 6-thioguanine, cytoxan, cyclosporine, cytarabinoside, cis-platin, chlorambucil, busulfan, and any other drug that can inhibit cell proliferation, and combinations thereof. The main polymer includes, but not limited to, polystyrene, parylene and polyurethane. The additive polymer includes, but not limited to, polyethylene glycol capped with diisocyanate moiety (NCO-PEG). TABLERatio between three components without solvent%ComponentformulationAgent1-10%Main polymer80-98% Additive1-19%polymer9.0 g of parylene, 0.6 g of tacrolimus, 0.4 g of NCO-PEG and 0.01 g of triethylene amine were dissolved in 90 g of tetrahydrofuran. The resulting mixture was heated at 40° C. for 30 minutes and cooled to room temperature. To the solution was added 0.1 g of pH 8.0 aqueous solution and mixed thoroughly. The resulting solution is applied to bare metal stents for coating.
Owner:HAHN SOONKAP
Treatment Of Diabetic Patients With A Stent And An Adjunctive Drug Formulation
Embodiments of the present invention include methods of treating, preventing, or ameliorating a vascular disease and / or disorder in a diabetic or pre-diabetic patient. The methods include implanting a stent in a vascular region in a diabetic patient, and during the implantation procedure, delivering a drug formulation from a source other than the stent to the vascular region. The stent may be a bare metal stent, or a drug eluting stent, such as a metal stent having a coating including a drug. The drug may be everolimus, sirolimus, or a combination thereof. The drug formulation may include dexamethasone, paclitaxel, or a combination thereof.
Owner:ABBOTT CARDIOVASCULAR
Gene-releasing type stent and preparation method thereof
InactiveCN102441192AEffective treatmentControl release speedStentsSurgeryBare-metal stentGene Solution
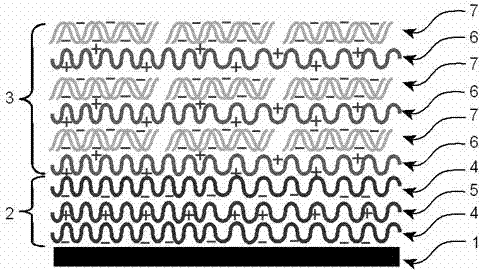
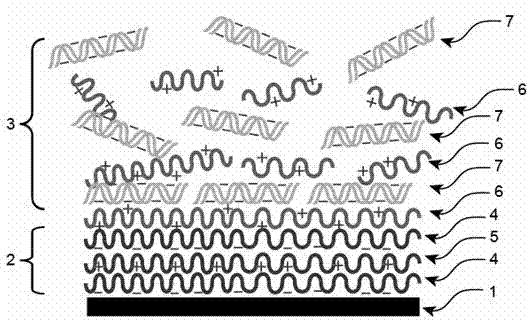
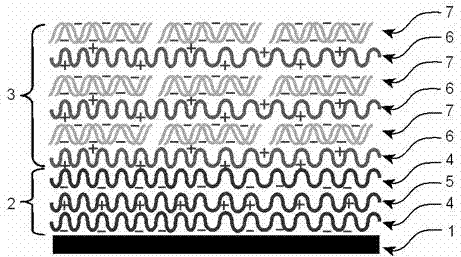
The invention relates to a gene-releasing type stent and a preparation method thereof. The stent comprises a bare metal stent, a substrate layer and a gene-loading coating, wherein the substrate layer is formed by alternating two layers or more substrate polyanion carrier layers and polycation carrier layers; the gene-loading coating is formed by alternating two layers or more biodegradable polycation carrier layers and gene layers; and all layers are mutually combined by electrostatic attraction. The preparation method for the gene-releasing type stent is characterized in that the metal stent is repeatedly and alternatively dipped into a polycation carrier solution and a gene solution with a solution dipping method, and therefore, the carrier layers and the gene layers can be alternatively coated on the surface of the stent. The stent disclosed by the invention is used for loading all genes (comprising DNA (Deoxyribo Nucleic Acid) and RNA (Ribose Nucleic Acid)) with negative charges to slowly release or quickly release gene drug. After the gene-releasing type stent is implanted in a human body, the gene-releasing type stent not only can mechanically expand a narrow channel but also can release gene therapy drug to the lesion tube wall tissue direction. The gene-releasing type stent is used for treating benign or malignant stenosis diseases of the physiological channel of the human body.
Owner:SHANGHAI JIAO TONG UNIV
Intravascular stent with PLGA blend medicament eluting surface coating
The invention provides an intravascular stent coated with a PLGA polymer blend drug-eluting coating on the surface. The stent comprises a bare-metal stent, and on the surface of the bare-metal stent, the PLGA polymer blend drug-eluting coating is applied; the polymer blend of the PLGA polymer blend drug-eluting coating is obtained through the physical blend of L-typed or DL-typed PLGA with a molecular weight of 10,000 to 20,000 and PEG or PEO with a molecular weight of 4,000 to 800,000 and the component proportion of PEG or PEO to PLGA is 1 to 40 percent. The intravascular stent coated with the PLGA polymer blend drug-eluting coating on the surface prepared by the invention can realize the gradual and slow release of drugs and guarantee the good combination performance of the drug coating and the metal stent.
Owner:HARBIN ENG UNIV
Internal lumen inner bracket with bilateral medicine-releasing performance and preparation method thereof
InactiveCN101810889ALong-term topical treatmentAvoid stickingSurgeryCoatingsBare-metal stentMedical equipment
The invention discloses an internal lumen inner bracket with bilateral medicine-releasing performance and a preparation method thereof, relating to the technical field of medical equipment. The bracket consists of a naked metal bracket and an enveloped film, wherein the enveloped film comprises an outer layer medicine-releasing layer, an insulating layer and an inner layer medicine-releasing layer; the outer layer medicine-releasing layer is positioned at the outermost side of the bracket; and the bracket is inwards sequentially provided with the insulating layer and the inner layer medicine-releasing layer. The preparation method of the bracket adopts a solution dipping method or a molten press film packing method, wherein the dissolvent method comprises the following steps of dissolvingmedicine and high polymer material in the dissolvent, and sequentially dipping the naked metal bracket in a coating film in solution with different components; and the molten press film packing method comprises the following steps of mixing the high polymer material with the medicine under high temperature, hot pressing the mixture into a film, and packing the film on the naked metal bracket. After being implanted into human body, the bracket can enlarge a narrow pipeline, outward releases anti-tumor drug and inward releases antibiotic drug, realizes long-term local anti-tumor treatment, and avoids infection and salts sedimentation.
Owner:SHANGHAI JIAO TONG UNIV
Vertebral artery stent and manufacturing method thereof
InactiveCN107049571AImprove control release abilityLittle side effectsStentsSurgeryBare-metal stentSide effect
The invention provides a vertebral artery stent and a manufacturing method thereof. The vertebral artery stent comprises multiple unit ring structures, multiple connection structures and a medicament coating. The multiple unit ring structures are arrayed in an axial direction. The multiple connection structures are connected to the multiple unit ring structures to form a net-post-shaped structure. The medicament coating is only coated on the outer surface of the net-post-shaped structure. The medicament coating contains anti-stenosis medicament components. The vertebral artery stent and the manufacturing method thereof have the following beneficial effects: by coating the medicament coating on the outer surface of the net-post-shaped structure, anti-stenosis medicament can be released by the medicament coating as compared with a conventional bare metal support used for vertebral artery so that restenosis is effectively prevented; in addition, the medicament coating is only coated on the outer surface of the net-post-shaped structure; compared with an intravascular stent with inner and outer sides having coatings or the overall coating, the control releasing capability of medicament is enhanced by a single-layer coating; adverse effect of medicament is reduced; and treatment efficiency of medicament is improved.
Owner:MICROPORT NEUROTECH SHANGHAI
Method for fixing antibody on the surface of medical instrument
ActiveUS20100112189A1Improve smoothnessHigh antibody activitySurgeryPharmaceutical containersBare-metal stentMicro arc oxidation
A method for fixing antibody on the surface of medical instrument, mainly includes: 1) pre-treating the instrument surface; 2) preparing holes: preparing multicrystal phase structure which has same size holes in the surface of the instrument by chemical corrosion, electrochemical corrosion, anodic oxidation, micro-arc oxidation, micro-arc nitridation; 3) post-treating the instrument surface; 4) fixing the antibody: immerging the bare metal scaffold which has holes in surface into a buffer solution containing antibody, adjusting the pH value of the antibody buffer solution, fixing the antibody on the surface of the instrument by the attraction between positive and negative charge and hole effect; and 5) confirming the effectiveness of the fixed antibody by artificial simulation hemodynamics and detecting method of antibody activity in scaffold surface. The method can promote the firm degree of the antibody which is fixed on the surface of the instrument, and keep high activity of the antibody on the surface of medical instrument.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Covered stent
ActiveCN109966018AHigh elongationReduce distortionStentsBlood vesselsBare-metal stentInsertion stent
The invention relates to a covered stent. The covered stent comprises a bare metal stent and a membrane covering the bare metal stent. The covered stent further comprises a first area and a second area connected with the first area along the peripheral direction, wherein the first area and the second area extend along the axial direction of the covered stent; and the ductility of the membrane in the first area is less than that in the second area. According to the covered stent, after a window is formed in the membrane of the first area, the membrane at the window has relatively small deformation under the long-term effect of a branch stent since the ductility of the membrane in the first area is relatively small, so that the risk of endoleak of the covered stent at the window in a long term can be reduced; and the membrane of the second area has relatively large ductility and relatively good flexibility, so that the covered stent can easily fit the bent shape of a blood vessel.
Owner:LIFETECH SCIENTIFIC (SHENZHEN) CO LTD
Blood vessel support for curing coronary heart disease
ActiveCN101249287AFunction increaseAvoid influenceStentsSurgeryBare-metal stentCoronary artery disease
The invention discloses a vascular stent that can treat the coronary heart disease. The stent comprises a bare metal stent, a coating that contacts one side of a vascular wall of the bare metal stent and a coating that contacts a side of a vascular cavity of the bare metal stent; the coating that contacts the side of the vascular wall of the bare metal stent is a mixture of a recombinant expression vector that can express human vascular endothelial growth factors or human fibroblasts and degradable polymers; the coating that contacts the side of the vascular cavity of the bare metal stent is a mixture of genes that promote the vascular endothelialization and degradable polymers. The vascular stent that can treat the coronary heart disease of the sent optimizes the functions of the stent more through an unsymmetrical coating manufacture techniques, meanwhile, a series of problems caused by smooth muscle cell proliferation, vascular endothelialization delay and polymer permanent residue, thus improving the treatment effect of the coronary heart disease stent in the maximal degree.
Owner:JW MEDICAL SYSTEMS LTD
Combined medical instrument formed by drug coated balloon and stent
InactiveCN102940543AAvoid elastic recoilAvoid long-term useStentsMedical devicesBare-metal stentInsertion stent
The invention discloses a combined medical instrument formed by a drug coated balloon and a stent. The combined medical instrument comprises the balloon and the stent, a drug-containing coating is coated outside the balloon, the stent is pressed on the outer wall of the balloon in a held manner, a conduit communicated with the balloon is arranged at one end of the balloon, and the far end of the conduit is connected with a conduit base. By the structure, the novel medical instrument is formed by the drug coated balloon (DCB) carrying drugs and the bare metal stent (BMS), so that the therapeutic effect of low restenosis, similar to that of a drug eluting stent (DES), is achieved through a series of novel design of the balloon and the BMS; meanwhile, late stent thrombosis possibly appearing after the DES is used, the risks brought about by taking anticoagulants for a long period of time, and the financial burden caused by taking the anticoagulants can be avoided; and the novel instrument can be used in a way similar to that of the existing DES, so that the same convenience is achieved.
Owner:苏州爱瑞德医疗科技有限公司
Cerebral aneurysm intracavity blood vessel rebuilding device
ActiveCN104758086AAvoid expansionReduce oppressive effectBlood vesselsBare-metal stentTectorial membrane
The invention discloses a cerebral aneurysm intracavity blood vessel rebuilding device which comprises a bare metal stent and a tectorial membrane. The bare metal stent comprises a near supporting end, a far supporting end and a middle part, the near supporting end and the far supporting end are located at the two ends of the bare metal stent and used for supporting a aneurysm-carried cerebral artery blood vessel wall, the length of the middle part is slightly larger than the width of a cerebral aneurysm neck, and the middle part is arranged between the two supporting ends. The radial size of the middle part is smaller than that of the two supporting ends, and a blood supply cavity is formed between the middle part and the aneurysm-carried cerebral artery blood vessel wall. The tectorial membrane covers the near supporting end and part of the middle part so as to isolate the cerebral aneurysm from receiving the forward blood flowing, large in vector, of an aneurysm-carried cerebral artery and allow the far supporting end without the tectorial membrane to provide backward blood flow, small in vector, from the far end of the aneurysm-carried cerebral artery. The cerebral aneurysm intracavity blood vessel rebuilding device solves the problems that according to very large and huge cerebral aneurysm stent-assisted coil embolization, the aneurysmal obliteration rate is low and the recurrence rate is high, side branch and perforating branch blood supply is ensured, the cerebral aneurysm rupture is effectively prevented, and the space occupying effect of the cerebral aneurysm is reduced.
Owner:APT MEDICAL HUNAN INC +1
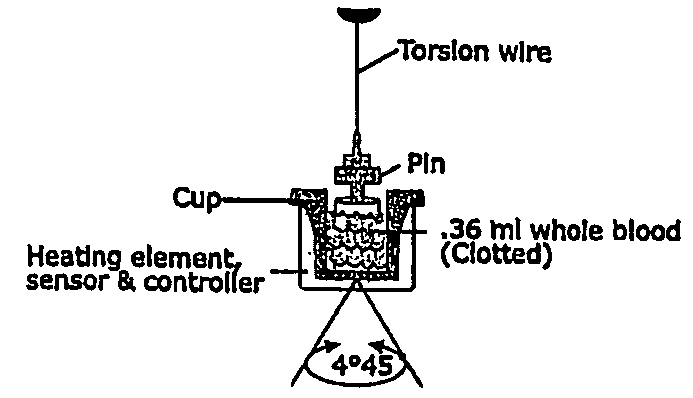
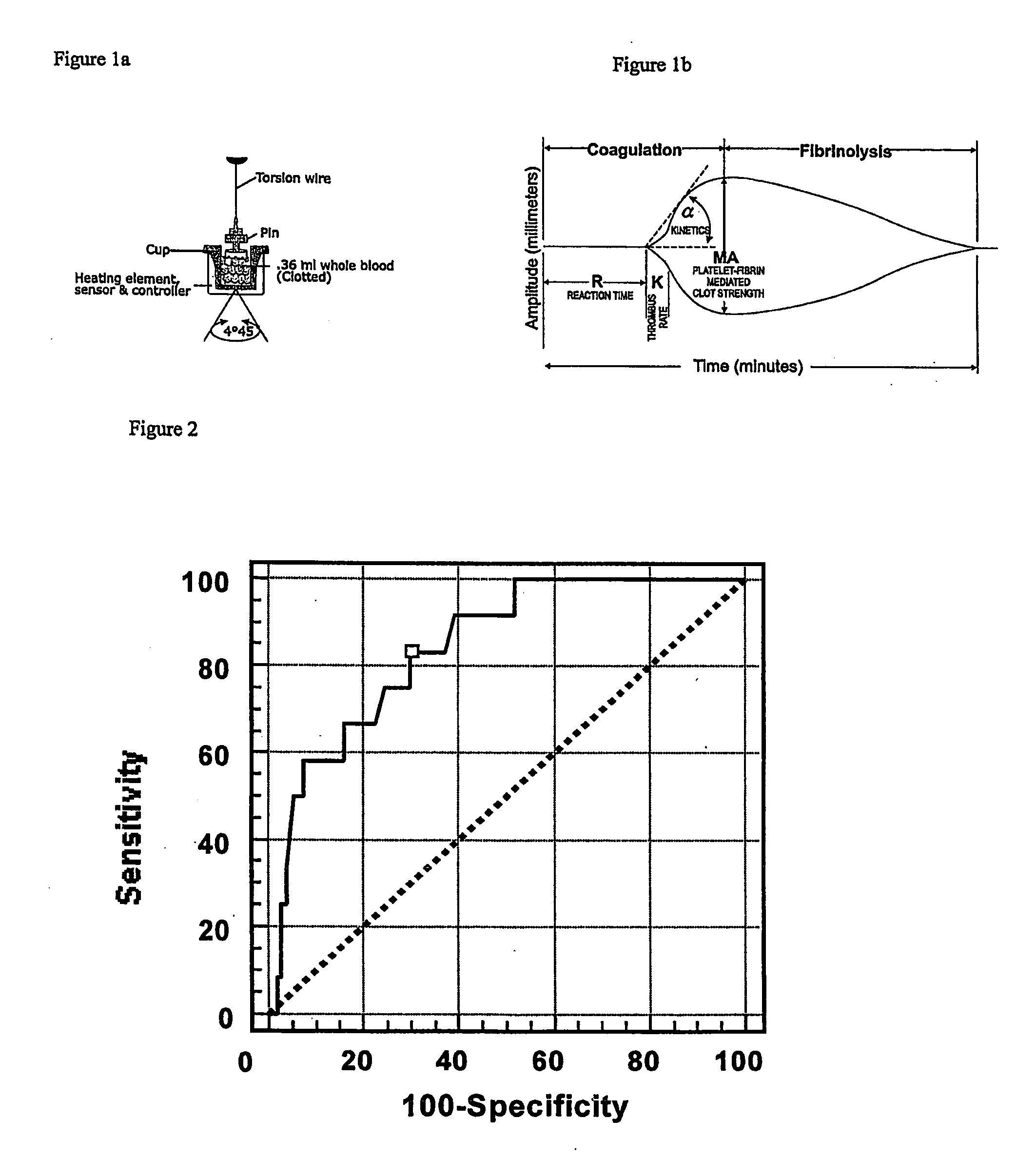
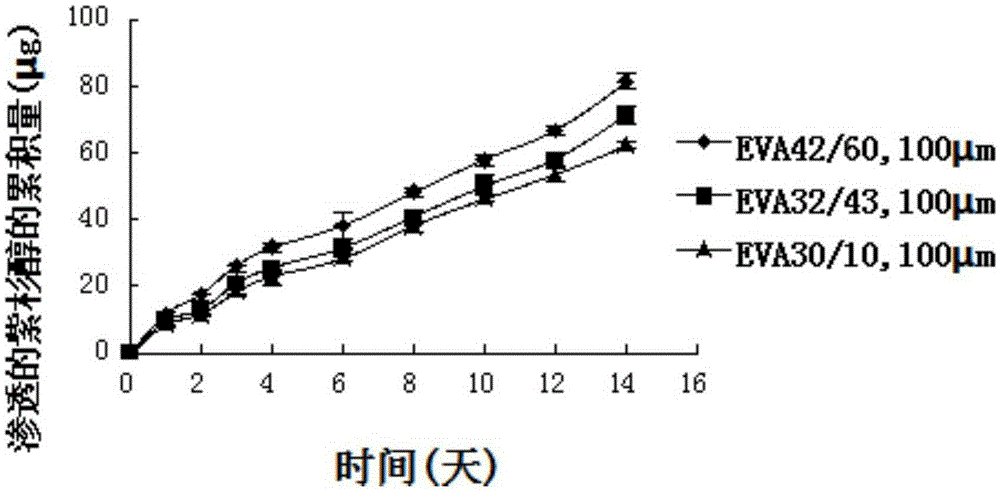
Detection of restenosis risk in patients receiving a stent by measuring the characteristics of blood clotting including the measurement of maximum thrombin-induced clot strength
ActiveUS20090198120A1Avoid complicationsReadily availableDisease diagnosisSensorsBare-metal stentPercent Diameter Stenosis
A method of selecting a stent for implantation in the circulatory system of a human being includes the steps of determining a threshold level of platelet hyper-coaguability (PHC). One marker of PHC is platelet-fibrin mediated clot strength (MA) of blood. MA above a threshold demonstrates a risk of restenosis that is relatively high. In practicing the method, a sample of blood is obtained from a patient who requires implantation of a stent, the blood sample is tested for its platelet-fibrin mediated clot strength, the clot strength of the blood sample is compared with the threshold level; if the blood sample has a clot strength below the threshold level, selecting a bare metal stent, and if the blood sample has a clot strength at or above the threshold level, selecting a drug-eluting stent. The method includes, in a preferred embodiment, use of a viscoelastic monitor to perform the testing step. Other markers of PHC may also be employed such as measurement of thrombin generation and / or platelet reactivity.
Owner:GURBEL PAUL A
Paclitaxel-loaded ethylene-vinyl acetate esophageal stent and preparation method thereof
InactiveCN105232182AGood mechanical supportImprove mechanical propertiesSurgeryTubular organ implantsBare-metal stentMechanical property
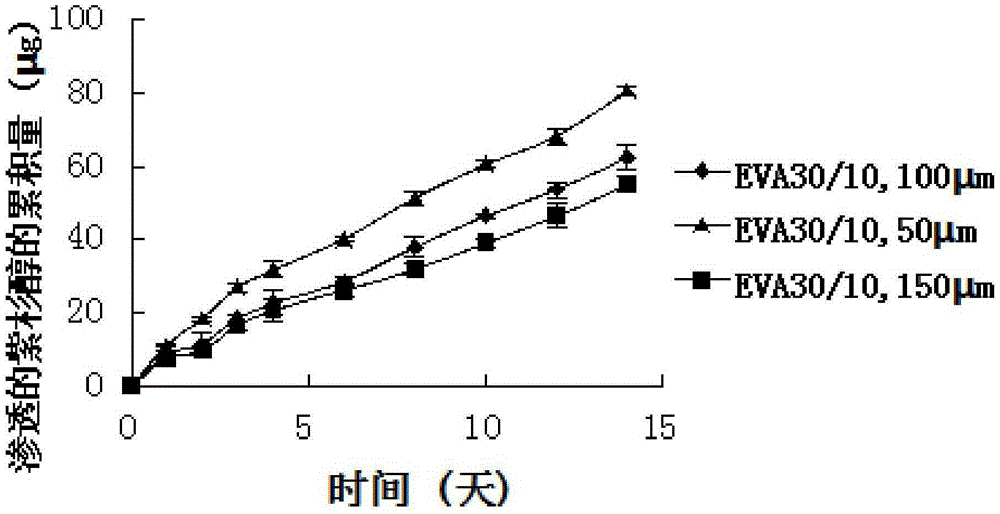
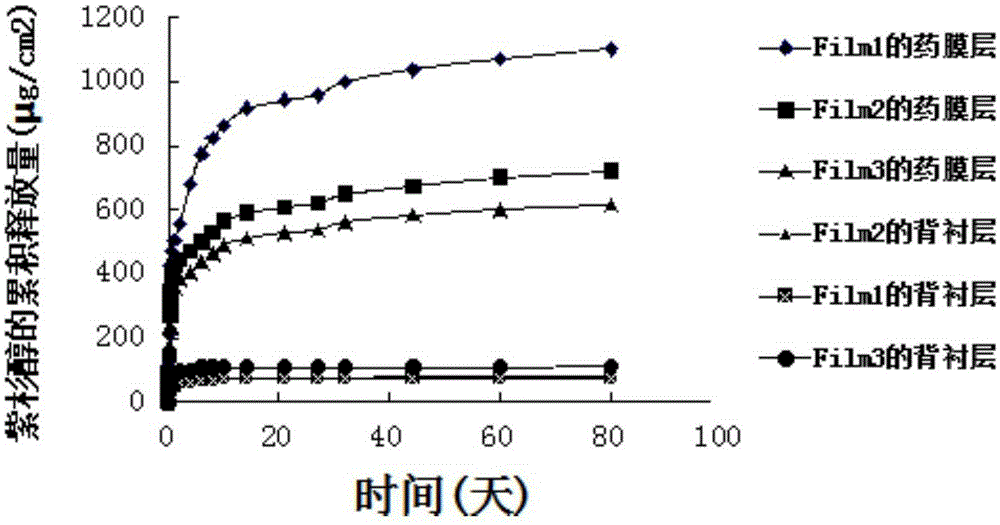
The invention pertains to the field of medical instruments and discloses a paclitaxel-loaded ethylene-vinyl acetate esophageal stent and a preparation method thereof. The stent is composed of a bare metal stent and a covering film covering the bare metal stent. The covering film comprises an ethylene-vinyl acetate esophageal backing layer and a paclitaxel medicine film layer. The inside of the ethylene-vinyl acetate esophageal backing layer is closely adhered to the bare metal stent. The outside of the ethylene-vinyl acetate esophageal backing layer is adhered to the paclitaxel medicine film layer. The stent is 50% in the theoretical drug-loading amount ranges from 48.26% -50.81% in the measured drug-loading amount. Within 80d, paclitaxel ranges from 615.274-1101.368 [mu]m / cm2 in the accumulative releasing amount and ranges from 57.93-108359 [mu]m / cm2 and also ranges from 57.93-108359 [mu]m / cm2 in the infiltration amount from the backing layer. The paclitaxel-loaded ethylene-vinyl acetate esophageal stent and the preparation method thereof have following beneficial effects: the preparation method is easily and conveniently applied; the good mechanical property is obtained; the slow release function can be fulfilled for a long time; and the stent has good functions of mechanical support and prevention against growth inside of tumors.
Owner:SHANGHAI JIAO TONG UNIV
Peripheral drug eluting stent and preparation and application thereof
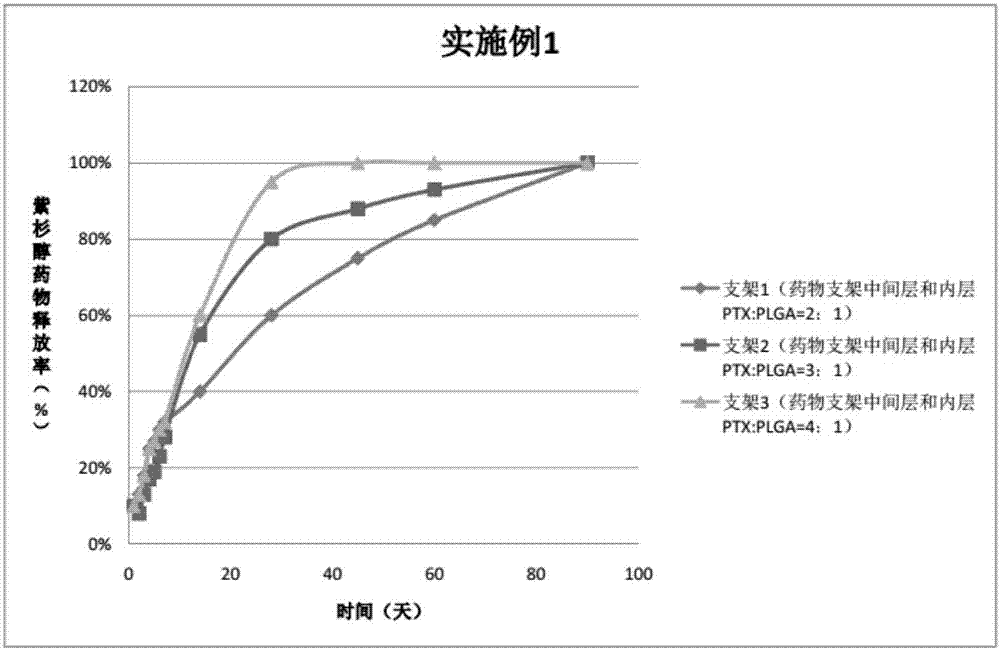
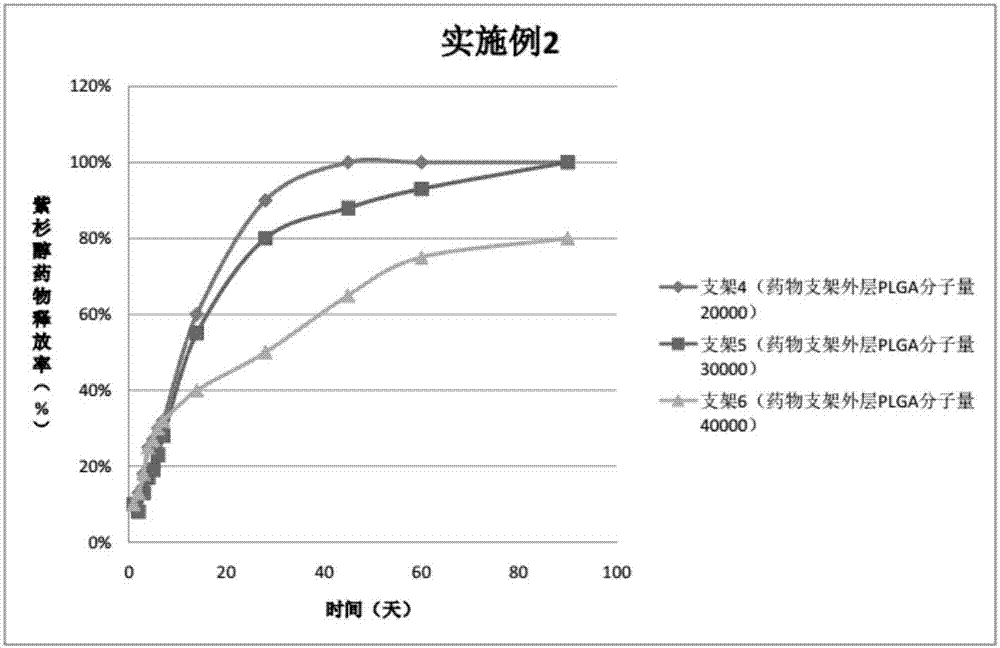
ActiveCN107496998AThe release rate is easy to controlPrevent restenosisSurgeryPretreated surfacesBare-metal stentTubular stenosis
The invention provides a peripheral drug eluting stent. A drug coating of the peripheral drug eluting stent is divided into an outer sustained-release layer, an intermediate drug sustained-release mixed layer and an inner drug sustained-release mixed layer. The outer sustained-release layer is PLGA with molecular weight being 30000, and PLA:PGA=70:30. The intermediate drug sustained-release mixed layer is a mixture of PTX and PLGA, wherein the molecular weight of PLGA is 20000, PLA:PGA=50:50, and the mass ratio of PTX to PLGA is 3:1. The inner drug sustained-release mixed layer is a mixture of PTX and PLGA, wherein the molecular weight of PLGA is 20000, PLA:PGA=80:20, and the mass ratio of PTX to PLGA is 3:1. The invention further relates to preparation and application of the peripheral drug eluting stent. The peripheral drug eluting stent can effectively treat peripheral artery stenosis, and the problem of in-stent restenosis after a traditional peripheral bare metal stent is implanted into the peripheral vessel is avoided.
Owner:北京永益润成科技有限公司
Gene Delivery Stent Using Titanium Oxide Thin Film Coating, and Method for Fabricating Same
ActiveUS20140018908A1Reducing late thrombosisReducing metal allergyStentsSurgeryBare-metal stentGene delivery
The present invention relates to a gene delivery stent using titanium oxide thin film coating and a method for fabricating the gene delivery stent. The gene delivery stent according to the present invention may be loaded with a drug having anti-inflammatory and anti-thrombotic effects and simultaneously deliver a gene capable of inhibiting proliferation of vascular smooth muscle cells. Accordingly, late thrombosis and metal allergy may be reduced, and vascular restenosis in the stent region may be prevented, thereby making it possible to increase treatment effects of the bare metal stent.
Owner:CHONNAM NAT UNIV HOSPITAL
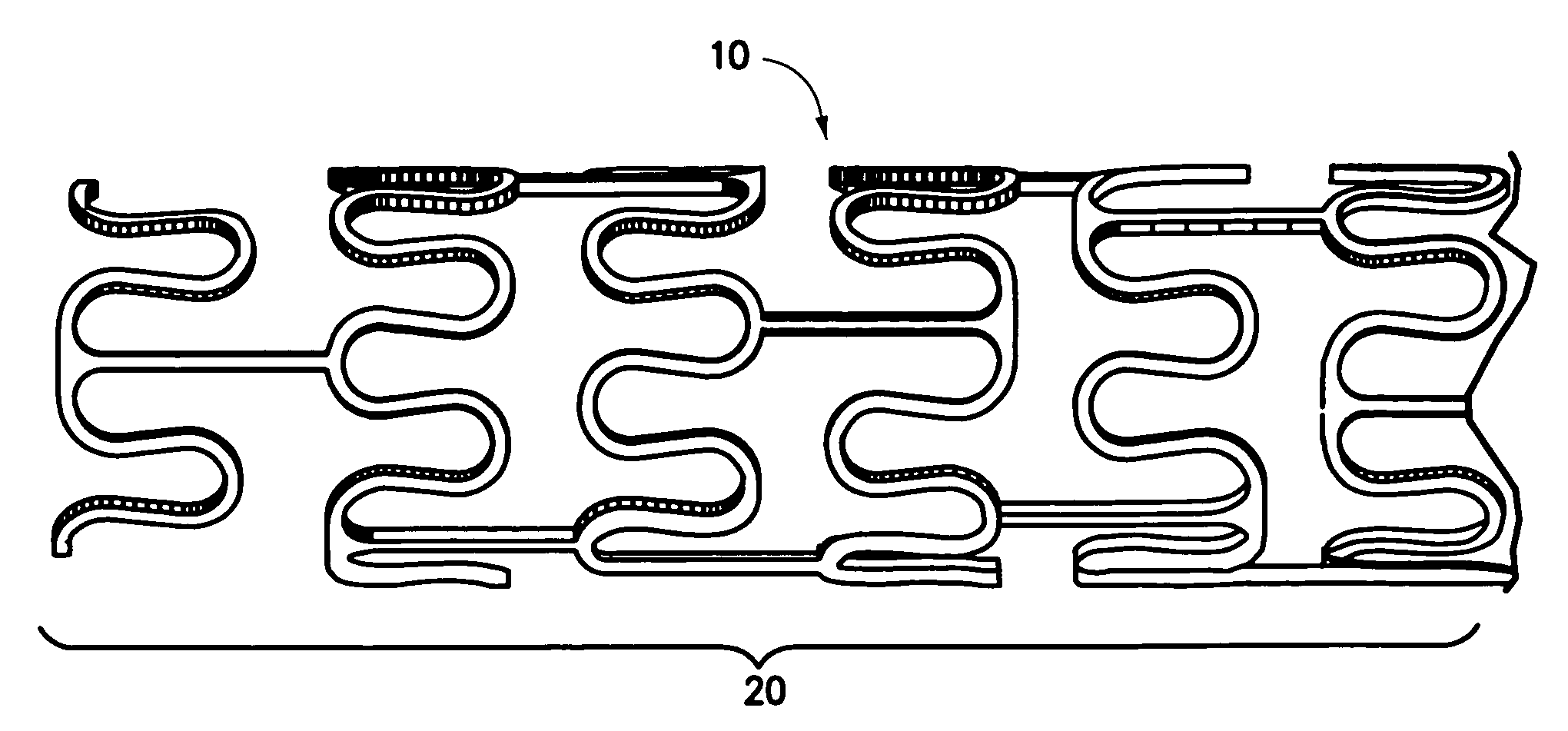
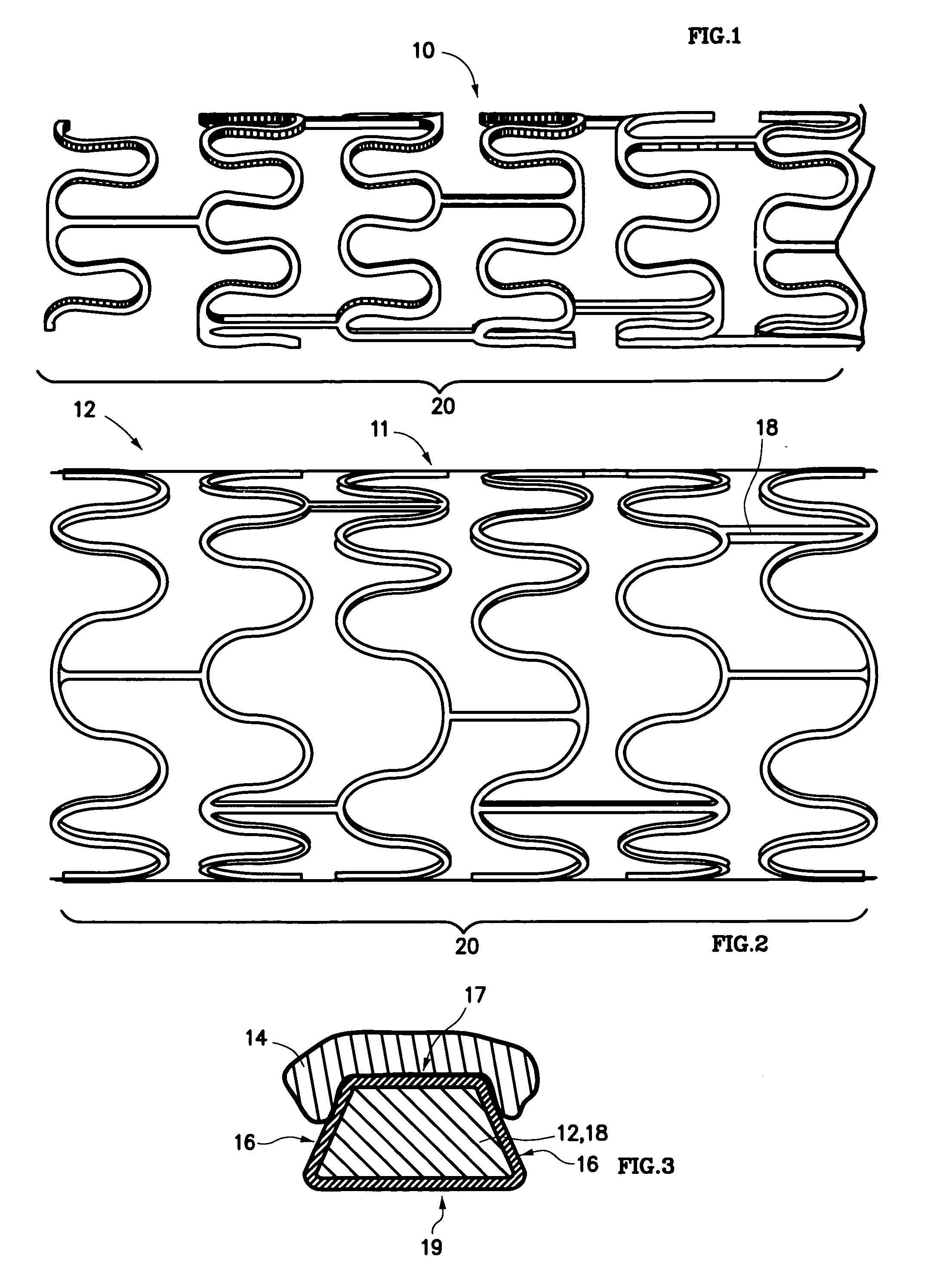
Stent and method of making same
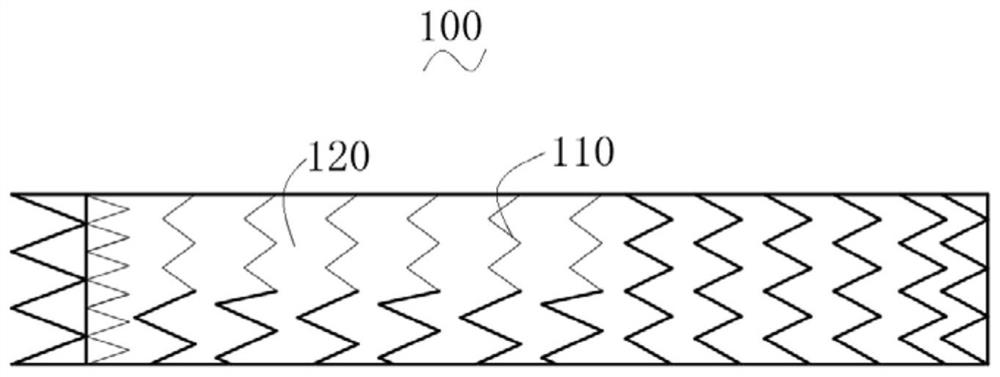
A stent for vascular interventions having a hybrid open cell geometry. Variants of the stent include bare metal stents and drug-eluting stents. Embodiments of the stent include end projections for radiopaque markers or a discontinuous partial radiopaque coating on low-stress or low-strain regions of the peripheral stent. The stents of the invention are characterized by having thin walls, nested rows of struts, high expansion ratio, high and uniform radial force over entire diametric size and length of device, crush resistance up to and including about 90% of its fully expanded diameter, high fatigue resistance and high corrosion resistance.
Owner:VACTRONIX SCI LLC
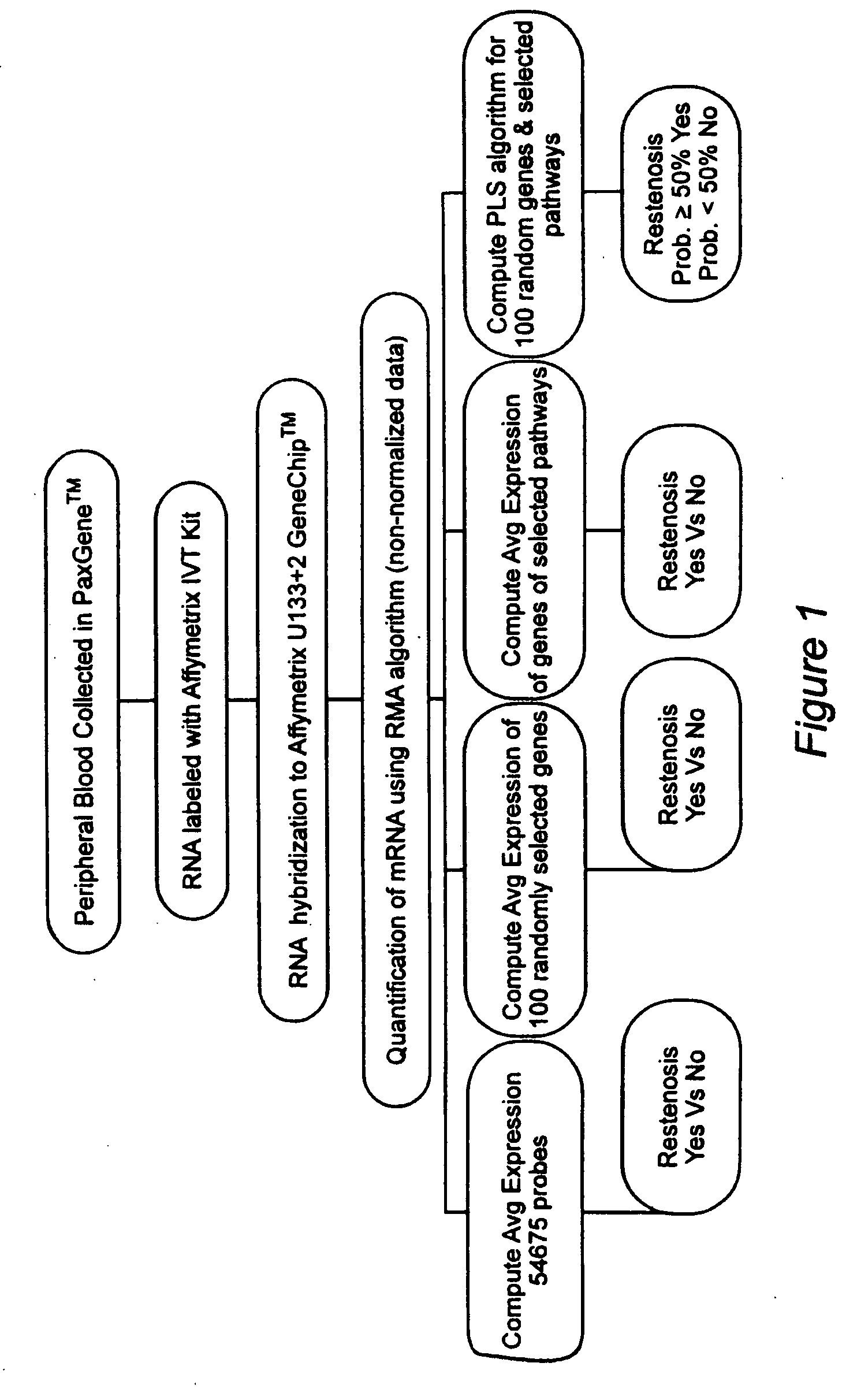
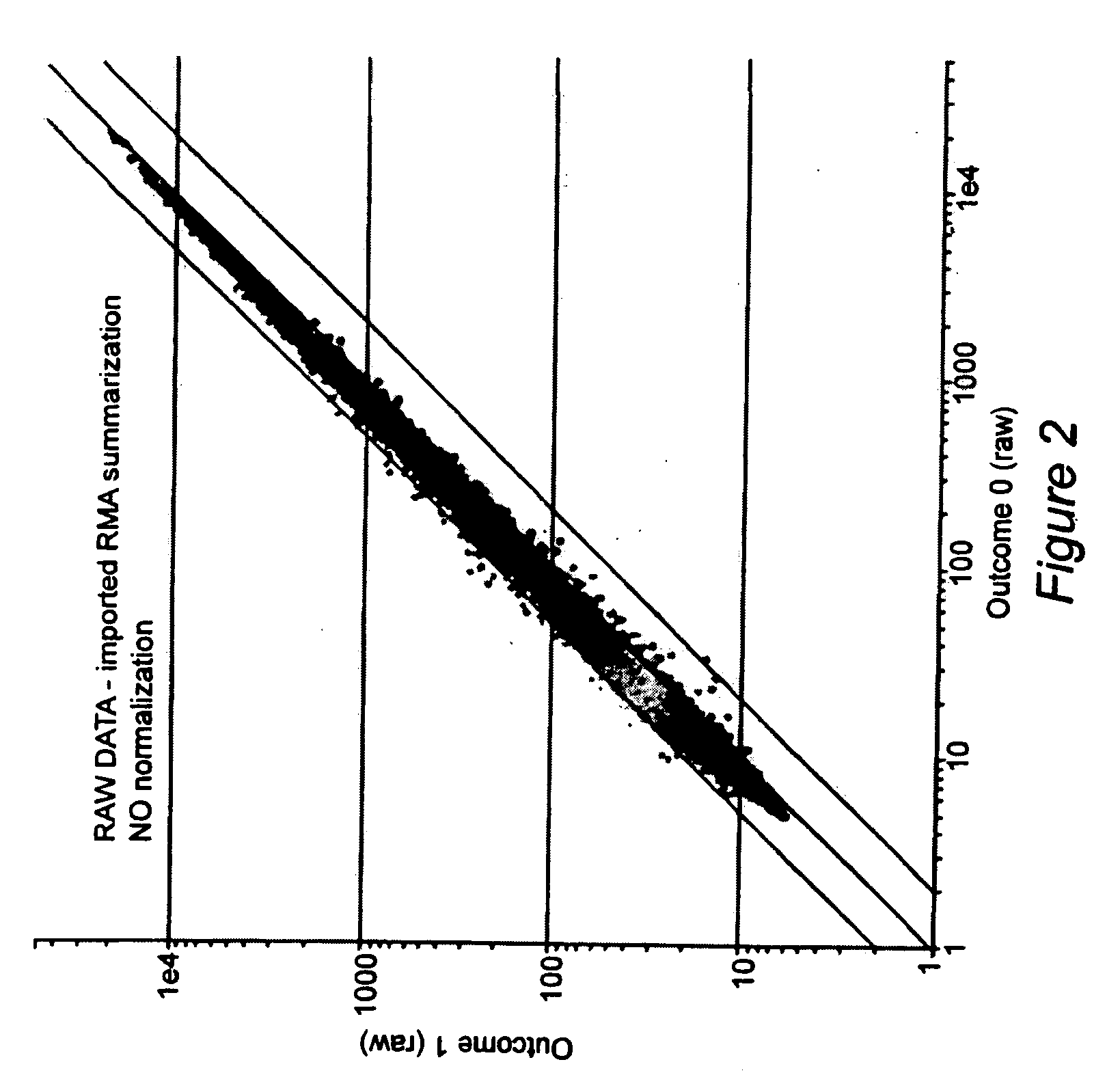
Prediction of bare metal stent restenosis
InactiveUS20090142756A1Increased gene activation/up-regulationCosts of majorBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentBare-metal stent
Methods for predicting whether or not a patient is likely to experience restenosis after the placement of a bare metal stent are provided. The methods involve detecting and analyzing gene expression patterns of the cellular components of whole blood, where activation of selected genes has been found to be indicative of a high probability of restenosis. The method thus allows the identification, prior to placement of a stent, of patients who are i) likely to experience restenosis, and thus should receive a stent that includes anti-restenosis agents; or ii) unlikely to experience restenosis, and thus should receive a stent without anti-stenosis agents.
Owner:CAPGEN SCI
Stent and method of making same
A stent for vascular interventions having a hybrid open cell geometry. Variants of the stent include bare metal stents and drug-eluting stents. Embodiments of the stent include end projections for radiopaque markers or a discontinuous partial radiopaque coating on low-stress or low-strain regions of the peripheral stent. The stents of the invention are characterized by having thin walls, nested rows of struts, high expansion ratio, high and uniform radial force over entire diametric size and length of device, crush resistance up to and including about 90% of its fully expanded diameter, high fatigue resistance and high corrosion resistance.
Owner:VACTRONIX SCI LLC
Drug-coated intravascular stent capable of preventing in-stent restenosis and preparation method of drug-coated intravascular stent
ActiveCN113244462AEasy to capturePromote differentiationStentsSurgeryBare-metal stentAntiendomysial antibodies
The invention discloses a composite drug intravascular stent which comprises a bare metal stent (prepared from a nickel-titanium memory alloy material), recombinant human collagen and folic acid are loaded on a stent body, HMGB1 fragment peptide and a VEGFR-2 antibody are crosslinked on the surface of the bare metal stent. Specifically, a dynamic liquid electrospinning technology is utilized to load a composite spinning solution of recombinant human collagen, folic acid, HMGBI fragment peptide and the VEGFR-2 antibody onto a nickel-titanium memory alloy bare metal stent to form an intravascular stent loaded with the recombinant human collagen and folic acid and cross-linked with the HMGBI fragment peptide and the VEGFR-2 antibody, so that EPCs capture, homing, proliferation and differentiation are promoted, and rapid endothelialization of the blood vessel is realized. The stent not only can be used for coronary stents, but also can be used for cerebrovascular stents, renal artery stents, main artery stents and the like.
Owner:TAIYUAN UNIV OF TECH +1
Blood vessel support for curing coronary heart disease
ActiveCN101249287BFunction increaseAvoid bad consequencesStentsSurgeryBare-metal stentCoronary artery disease
The invention discloses a vascular stent that can treat the coronary heart disease. The stent comprises a bare metal stent, a coating that contacts one side of a vascular wall of the bare metal stent and a coating that contacts a side of a vascular cavity of the bare metal stent; the coating that contacts the side of the vascular wall of the bare metal stent is a mixture of a recombinant expression vector that can express human vascular endothelial growth factors or human fibroblasts and degradable polymers; the coating that contacts the side of the vascular cavity of the bare metal stent is a mixture of genes that promote the vascular endothelialization and degradable polymers. The vascular stent that can treat the coronary heart disease of the sent optimizes the functions of the stent more through an unsymmetrical coating manufacture techniques, meanwhile, a series of problems caused by smooth muscle cell proliferation, vascular endothelialization delay and polymer permanent residue, thus improving the treatment effect of the coronary heart disease stent in the maximal degree.
Owner:JW MEDICAL SYSTEMS LTD
Bare metal stent with drug eluting reservoirs having improved drug retention
ActiveUS20120109284A1Reduced elutionMaintain vessel patencyStentsMetal rolling stand detailsBare-metal stentInsertion stent
Implantable medical devices may be utilized to locally delivery one or more drugs or therapeutic agents to treat a wide variety of conditions, including the treatment of the biological organism's reaction to the introduction of the implantable medical device. These therapeutic agents may be released under controlled and directional conditions from a stent having reservoirs so that the one or more therapeutic agents reach the correct target area, for example, the surrounding tissue. Features may be incorporated into the walls and bases of these reservoirs to improve securement of the drug construct.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Treatment of diabetic patients with a stent and locally administered adjunctive therapy
Embodiments of the present invention include methods of treating, preventing, and / or ameliorating a vascular disease and / or disorder in a diabetic or pre-diabetic patient. The methods include implanting a stent in a vascular region in a diabetic or pre-diabetic patient, and prior to and / or during the implantation procedure, delivering a lubricant formulation to the vascular region. The stent may be a bare metal stent, or a drug eluting stent, such as a metal stent with a coating including a drug, such as everolimus or sirolimus.
Owner:ABBOTT CARDIOVASCULAR
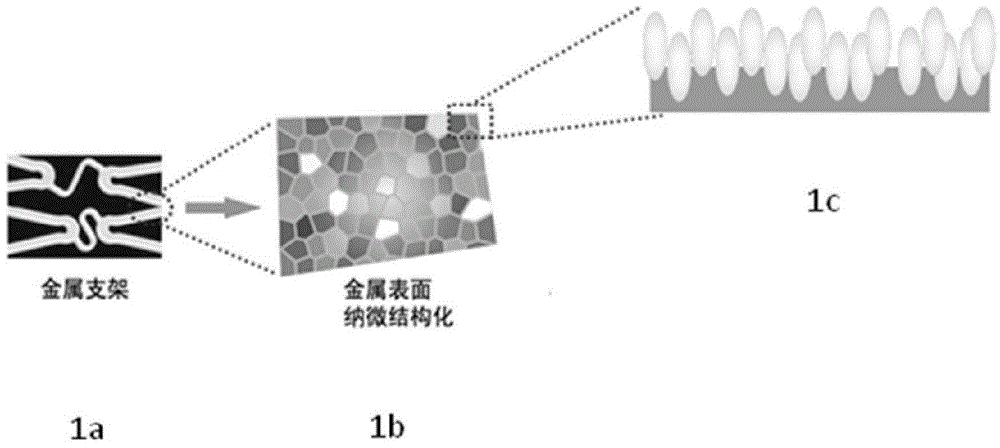
Metal support with micro-nano structure on surface
The invention relates to a metal support with a micro-nano structure on the surface and belongs to the technical field of intravascular intervention in the department of cardiology. According to the metal support with the micro-nano structure on the surface, the support structure is the same as that of an existing bare metal support. The metal support with the micro-nano structure on the surface is characterized in that the micro-nano structure is arranged on the surface of the metal support. The structure and the material of the metal support are the same as those of a metal support of a drug eluting support (DES) which is not coated with a coating in the prior art. According to the difference, the micro-nano structure needs to be machined on the surface of the metal support in the prior art. The metal support has the advantages that due to the construction of the micro-nano structure on the surface of the support, growth of endothelial cells is facilitated, and the micro-nano structure is consistent with the coronary artery hemodynamics; the bionic structure is arranged on the surface of the support and is closer to the growth environment in a human body, the endothelial cells can be attached to the bionic structure easily, re-endothelialization is accelerated, and restenosis caused by hyperplasia of vascular smooth muscle cells and thrombus formed in an anaphase coronary stent are avoided.
Owner:JILIN UNIV
Stent Graft
ActiveCN109966020BImprove the ability to resist expansion in the circumferential directionReduced Risk of Functional FailureStentsBlood vesselsBare-metal stentCovered stent
The invention relates to a covered stent, comprising a bare metal stent and a covering film covering the bare metal stent, the covering film includes an inner membrane and an adventitia, and the bare metal stent is arranged on the inner membrane and the outer membrane. Between the adventitia, the intima and at least one surface of the adventitia are provided with binding wires distributed along the circumferential direction of the stent-graft, and the stent-graft includes a first region and a The second region connected to the first region, the first region and the second region both extend along the axial direction of the stent-graft, and the coverage of the binding wire in the first region is zero. In the stent-graft, since the binding wire is arranged in the second region, the ability of the stent-graft to resist expansion in the circumferential direction can be improved, and the risk of the stent-graft function failing due to the expansion of the stent-graft can be reduced.
Owner:LIFETECH SCIENTIFIC (SHENZHEN) CO LTD
Cerebral aneurysm endovascular reconstruction device
ActiveCN104758086BAvoid expansionReduce oppressive effectBlood vesselsBare-metal stentTectorial membrane
The invention discloses a cerebral aneurysm intracavity blood vessel rebuilding device which comprises a bare metal stent and a tectorial membrane. The bare metal stent comprises a near supporting end, a far supporting end and a middle part, the near supporting end and the far supporting end are located at the two ends of the bare metal stent and used for supporting a aneurysm-carried cerebral artery blood vessel wall, the length of the middle part is slightly larger than the width of a cerebral aneurysm neck, and the middle part is arranged between the two supporting ends. The radial size of the middle part is smaller than that of the two supporting ends, and a blood supply cavity is formed between the middle part and the aneurysm-carried cerebral artery blood vessel wall. The tectorial membrane covers the near supporting end and part of the middle part so as to isolate the cerebral aneurysm from receiving the forward blood flowing, large in vector, of an aneurysm-carried cerebral artery and allow the far supporting end without the tectorial membrane to provide backward blood flow, small in vector, from the far end of the aneurysm-carried cerebral artery. The cerebral aneurysm intracavity blood vessel rebuilding device solves the problems that according to very large and huge cerebral aneurysm stent-assisted coil embolization, the aneurysmal obliteration rate is low and the recurrence rate is high, side branch and perforating branch blood supply is ensured, the cerebral aneurysm rupture is effectively prevented, and the space occupying effect of the cerebral aneurysm is reduced.
Owner:APT MEDICAL HUNAN INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com