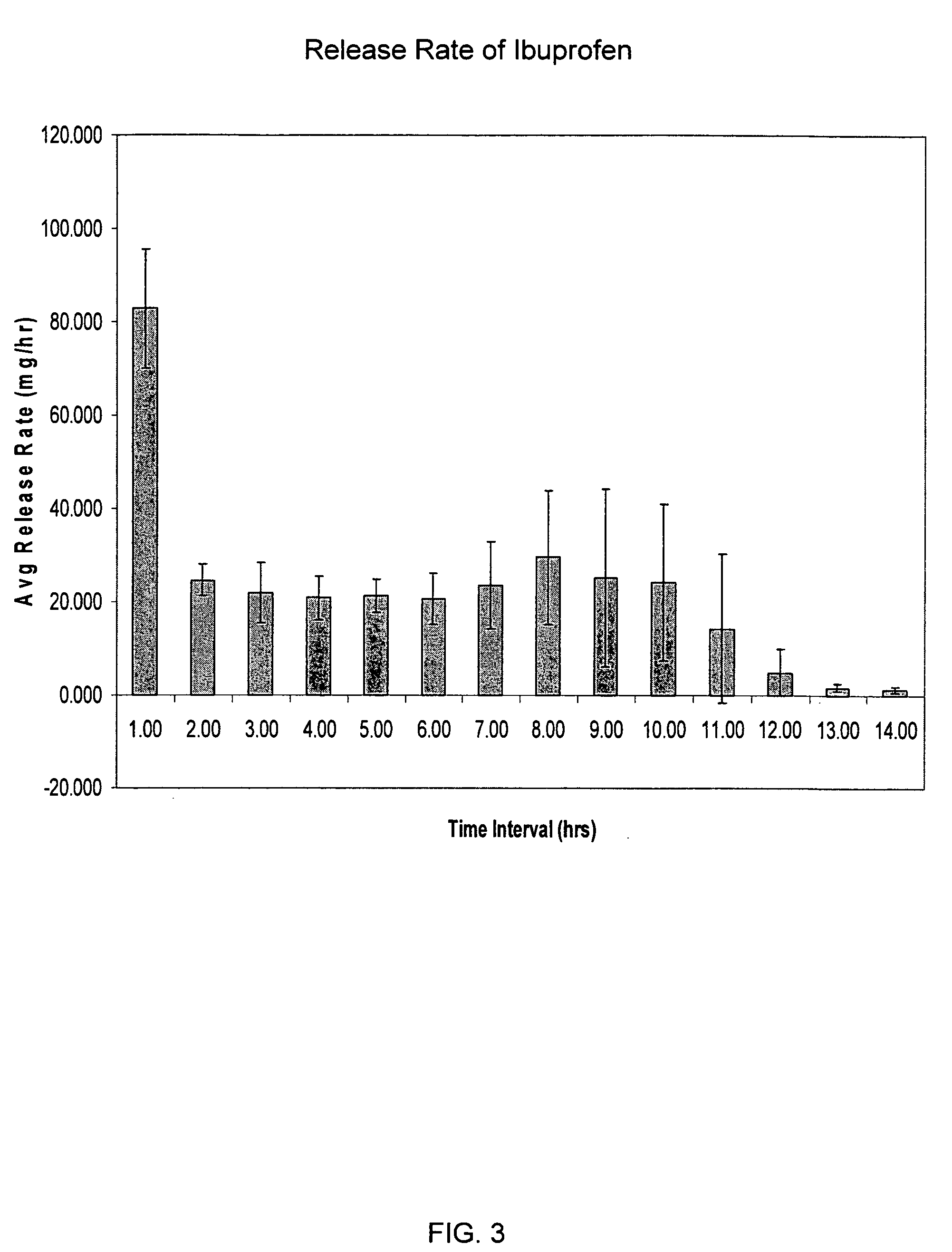
Patents
Literature
586 results about "Drug coating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Electromagnetic photonic catheter for reducing restenosis
InactiveUS6962584B1Reduce spreadImprove scalabilitySurgical instrument detailsCatheterPhotonicsPercent Diameter Stenosis
The method of vascular treatment for restenosis or vulnerable plaque after an invasive procedure, such as for example angioplasty, stenting with or without drug coating, or drug delivery, comprises: inserting a catheter or hollow guide wire to the treatment location; delivering light through the catheter in the wavelength range of about 700–2500 nm; and moving the light to treat the affected region.
Owner:STONE GREGG W +5
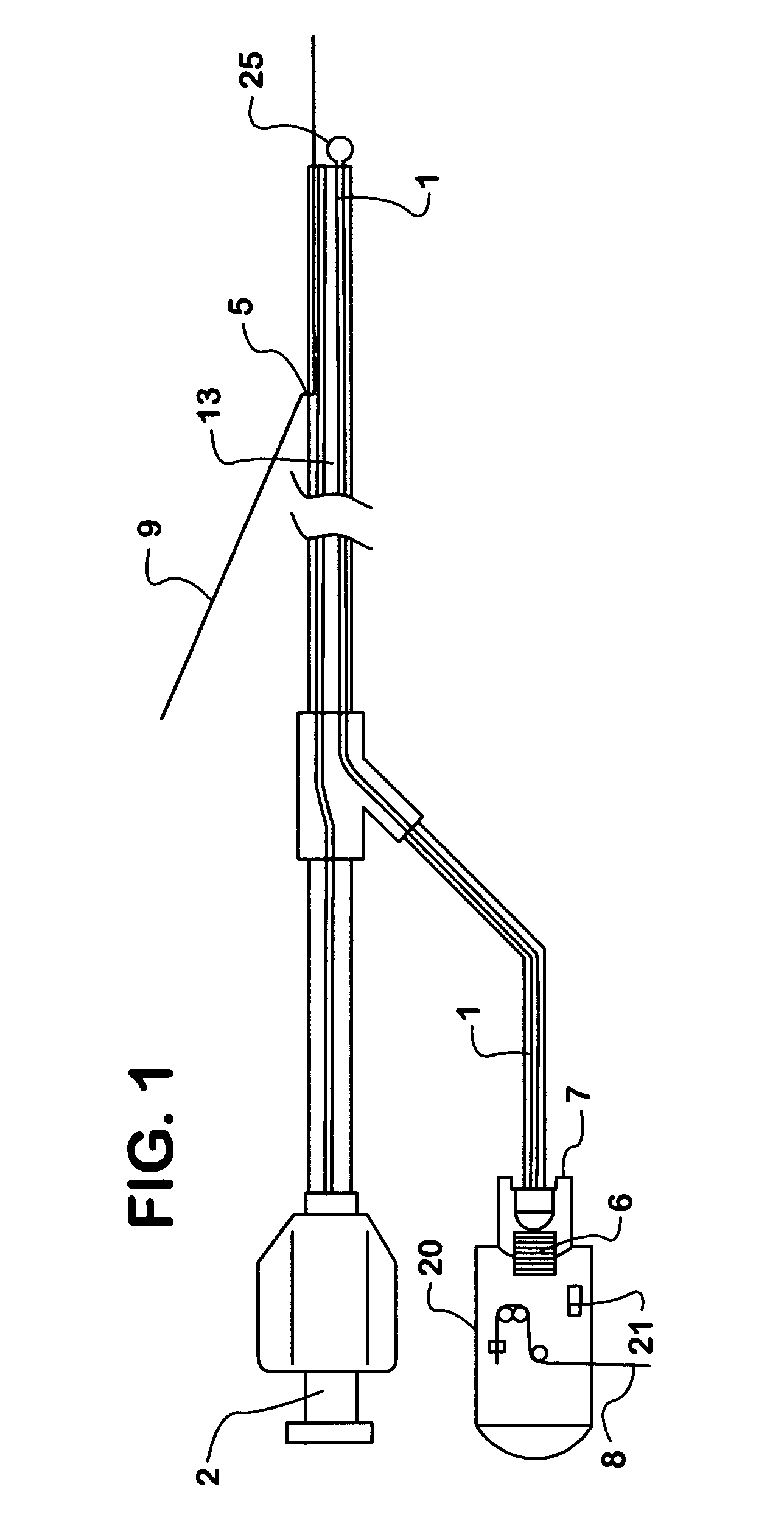
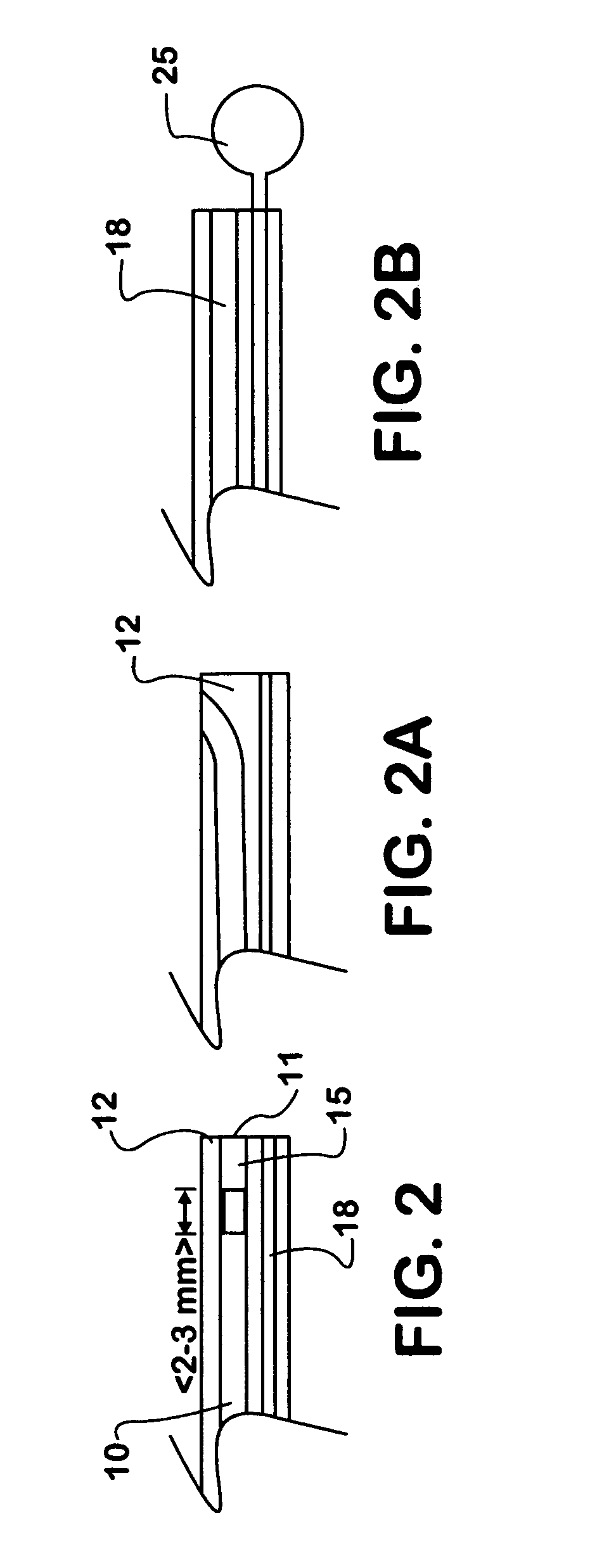
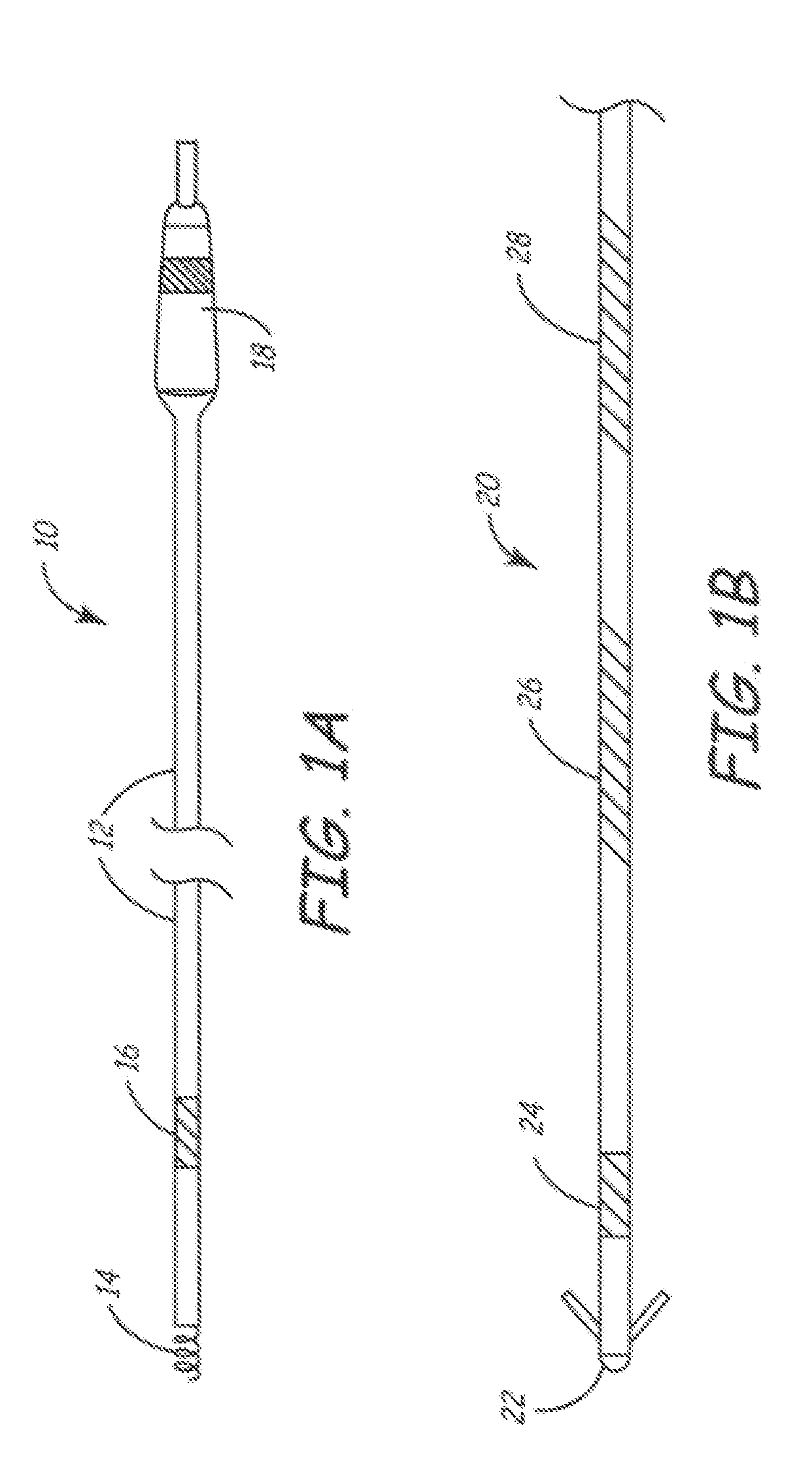
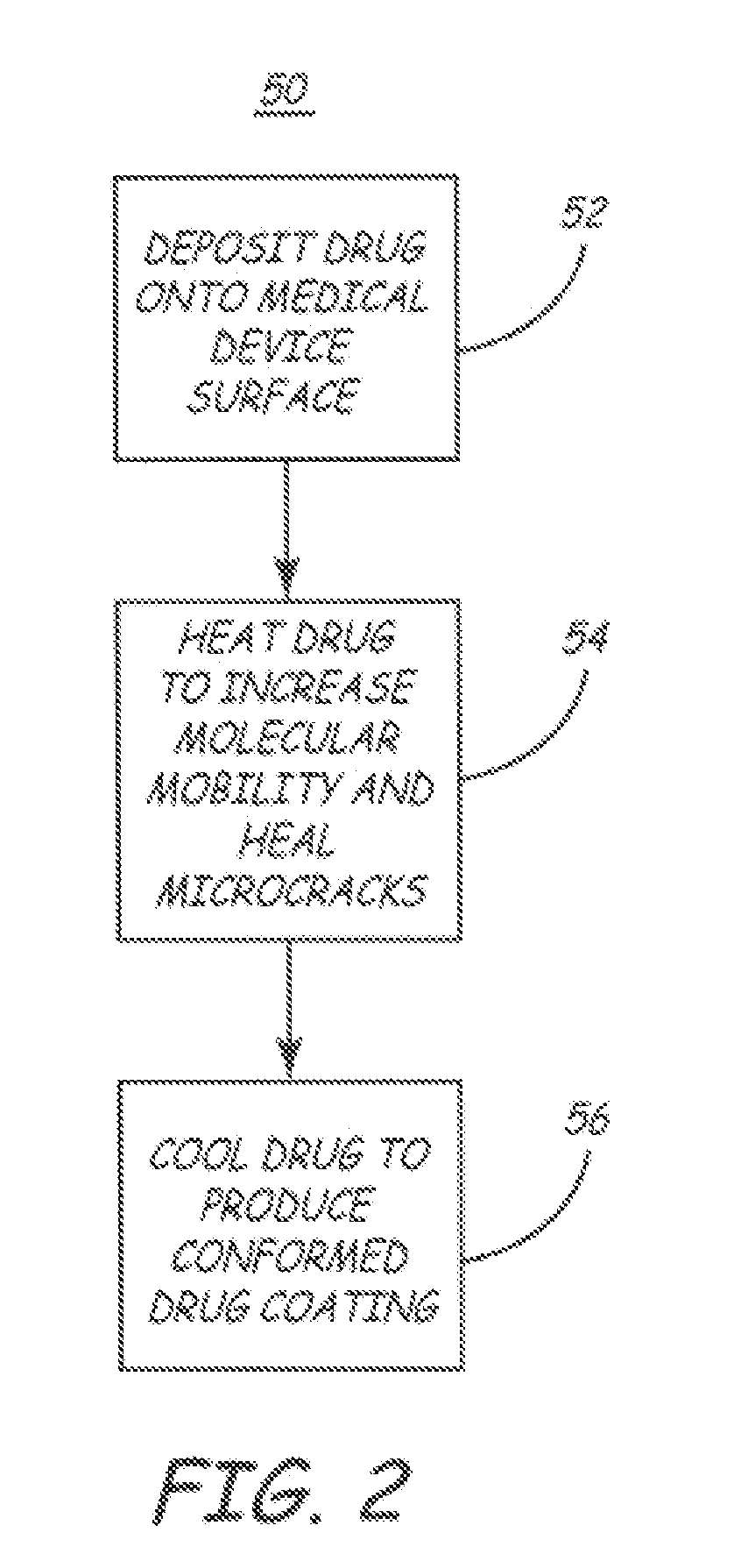
Method for applying a drug coating to a medical device
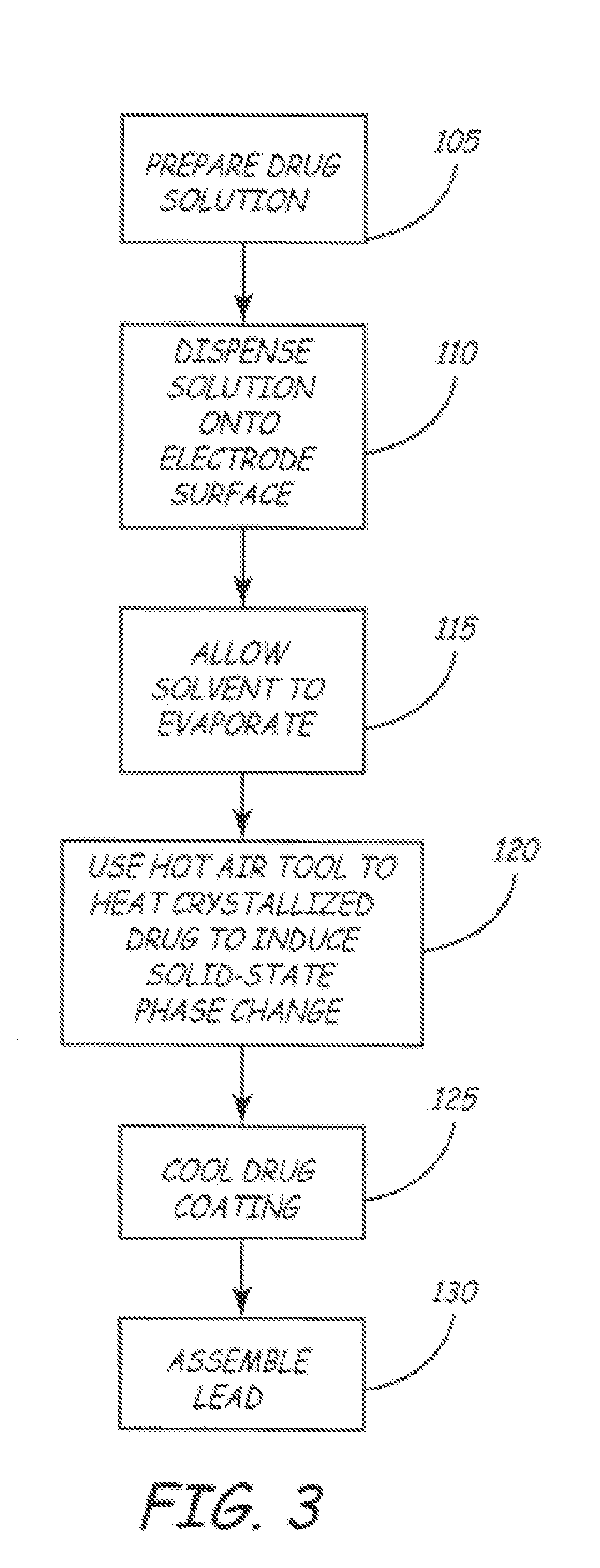
A method for coating a medical device with a drug is provided. Energy, preferably thermal energy, is applied to a crystalline deposit of a drug on the surface of a medical device to increase the molecular mobility and form a conformable drug coating with a low density of micro-cracks and other mechanical defects that can degrade the coating toughness and effective adhesion to the device surface. In a preferred embodiment, solution evaporation methods are used to deposit a crystalline coating of an anti-inflammatory steroid on a medical electrode. Heat applied at a controlled temperature, for a predetermined amount of time, induces a solid-state phase change of the drug coating providing a smooth, uniform, well-attached, conformable coating to form a layer that will elute from the electrode over time when implanted in a patient's body.
Owner:MEDTRONIC INC
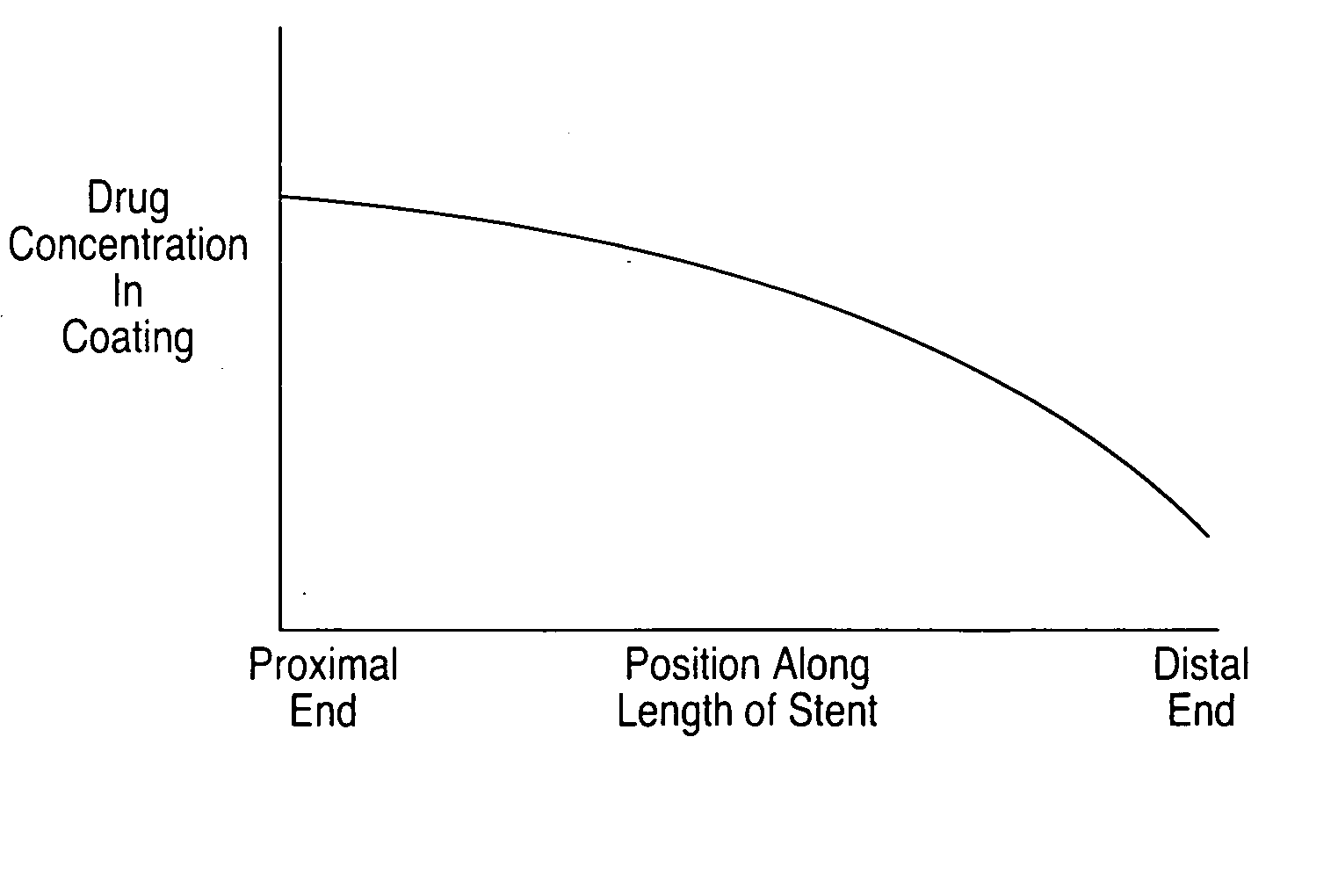
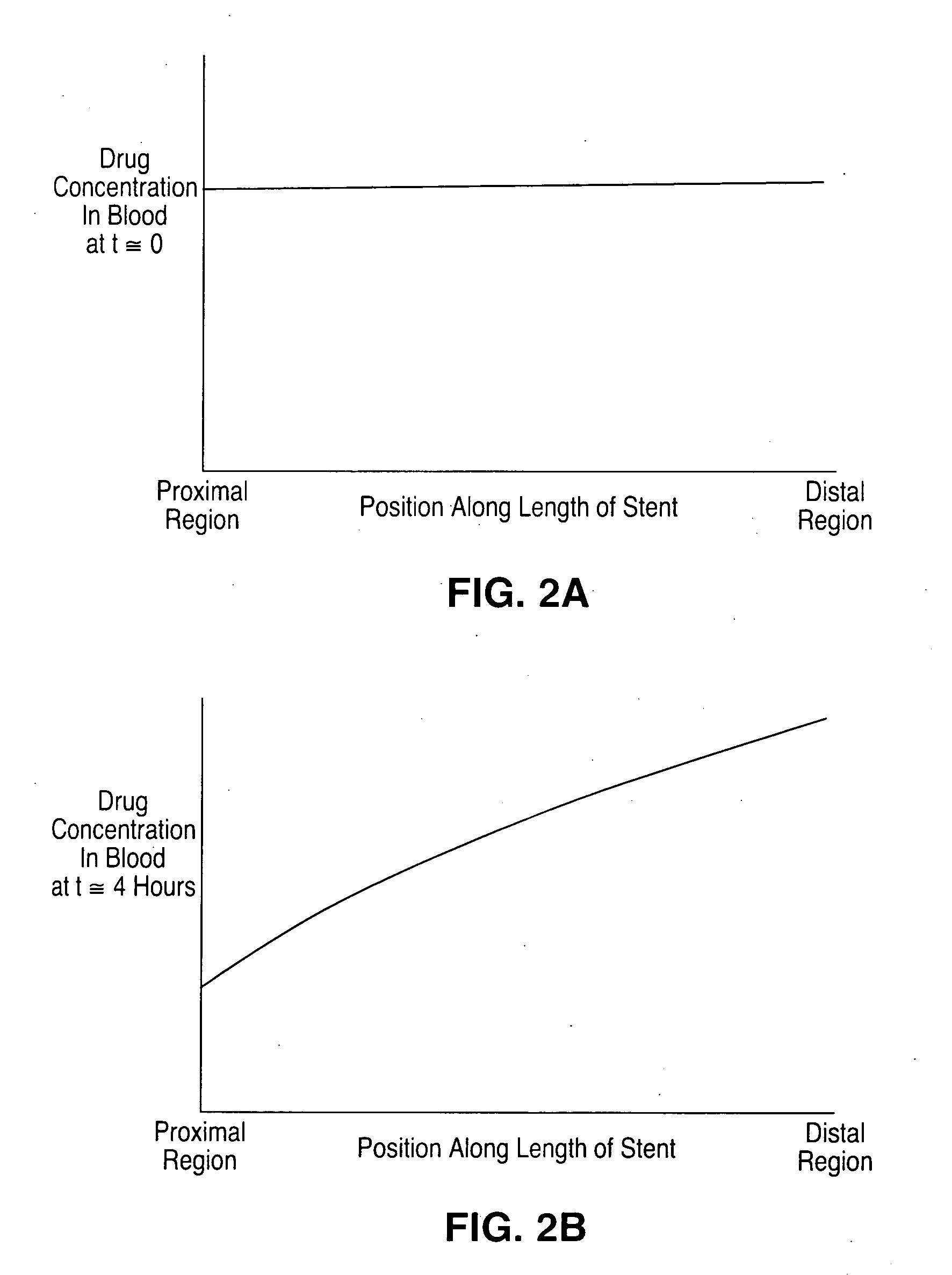
Optimized dosing for drug coated stents
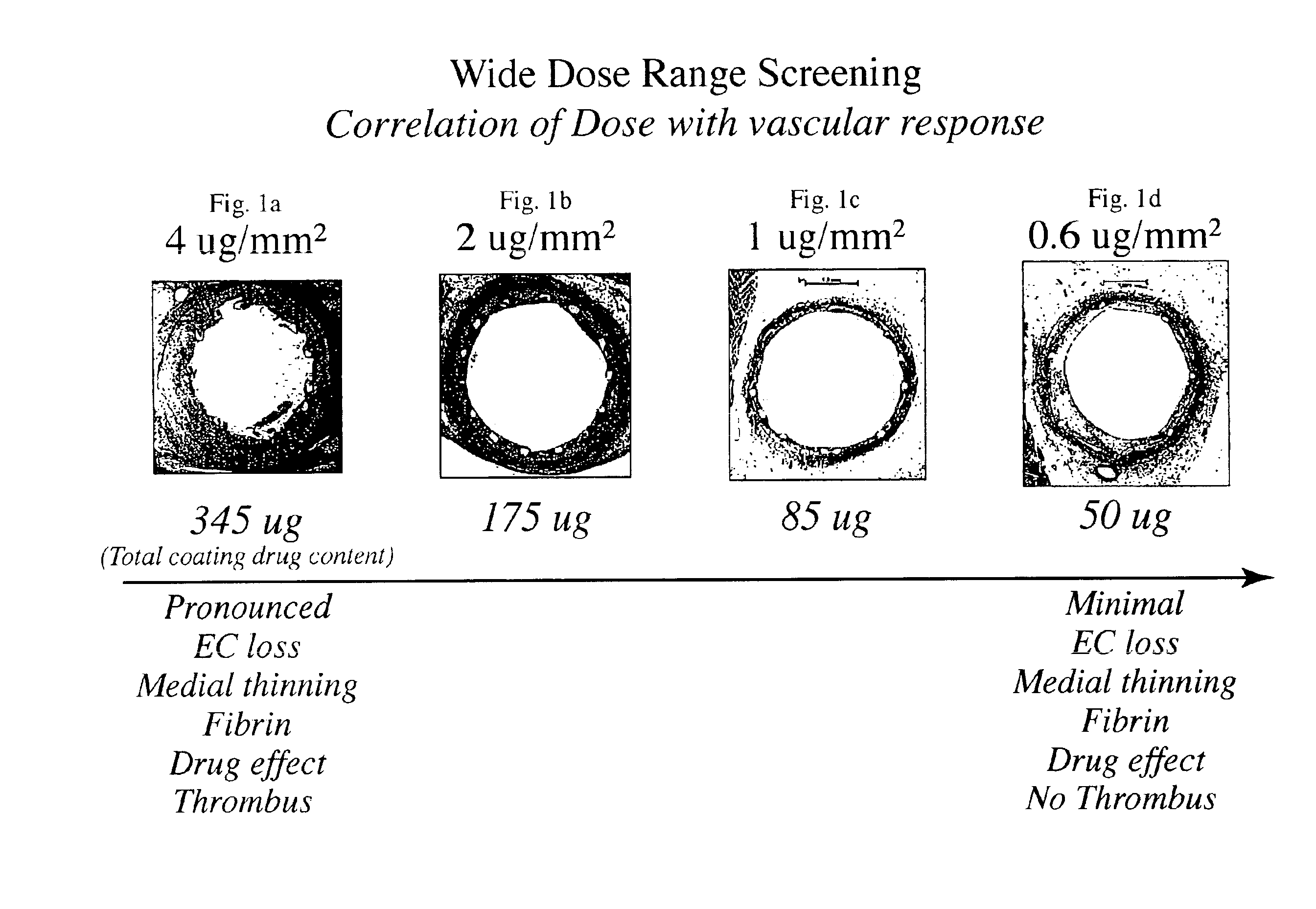
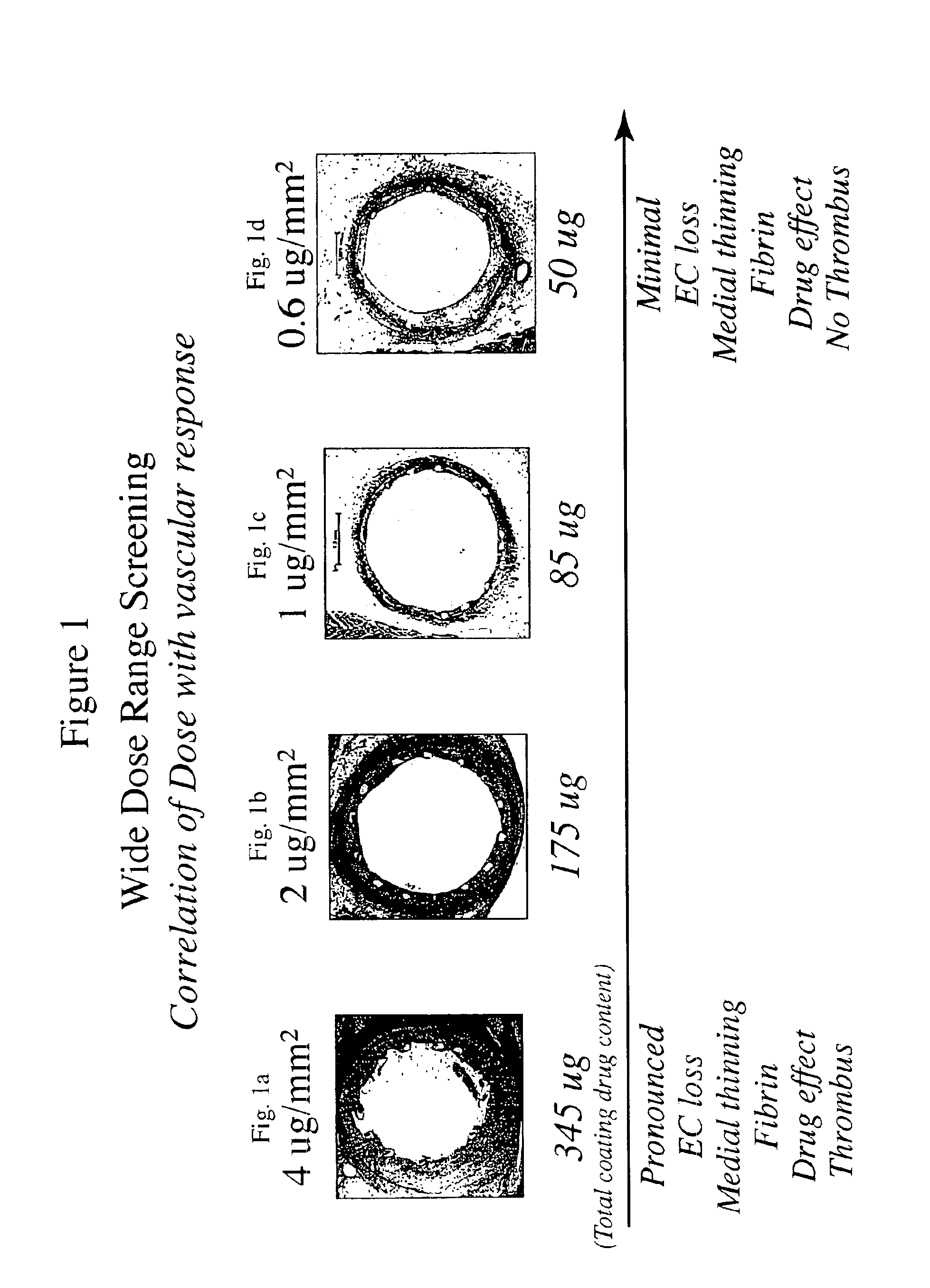
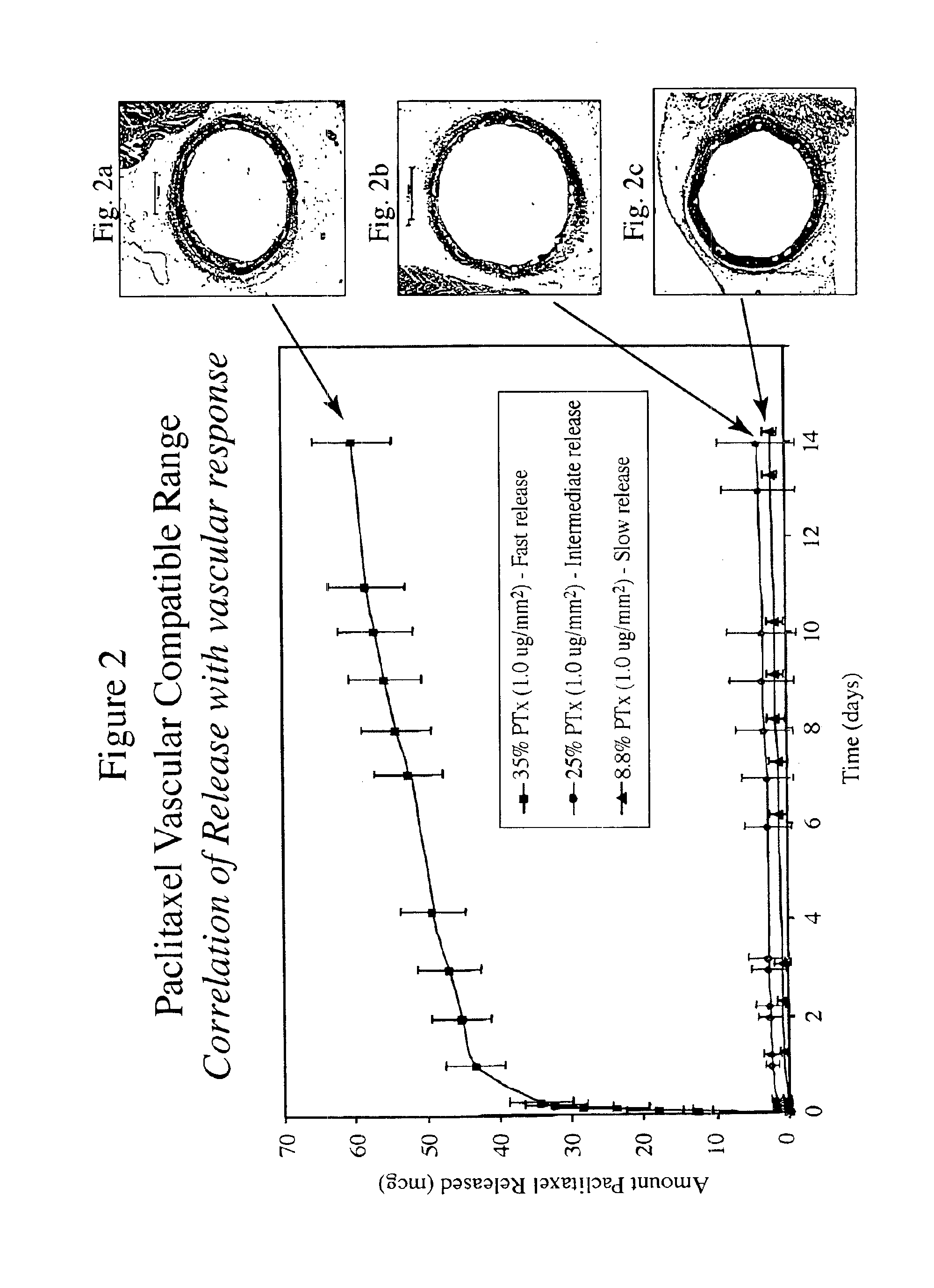
The inventors have found that both the drug dose and drug release profiles are significant factors for the safety and efficacy of drug coated stents. The inventors have identified optimum dosing and release kinetics for drug coated stents. In particular, the inventors have determined dosing and release kinetics that permit the delivery of the lowest effective drug dosage, thus enhancing patient safety and minimizing any side effects from the drug.
Owner:BOSTON SCI SCIMED INC
Plug for use in left atrial appendage
A plug or insert occludes the left atrial appendage (LAA), thus preventing blood from entering. The plug is formed in one piece without separately movable parts, and may be monolithic. A drug coating can be provided, with or without a plug.
Owner:NMT MEDICAL
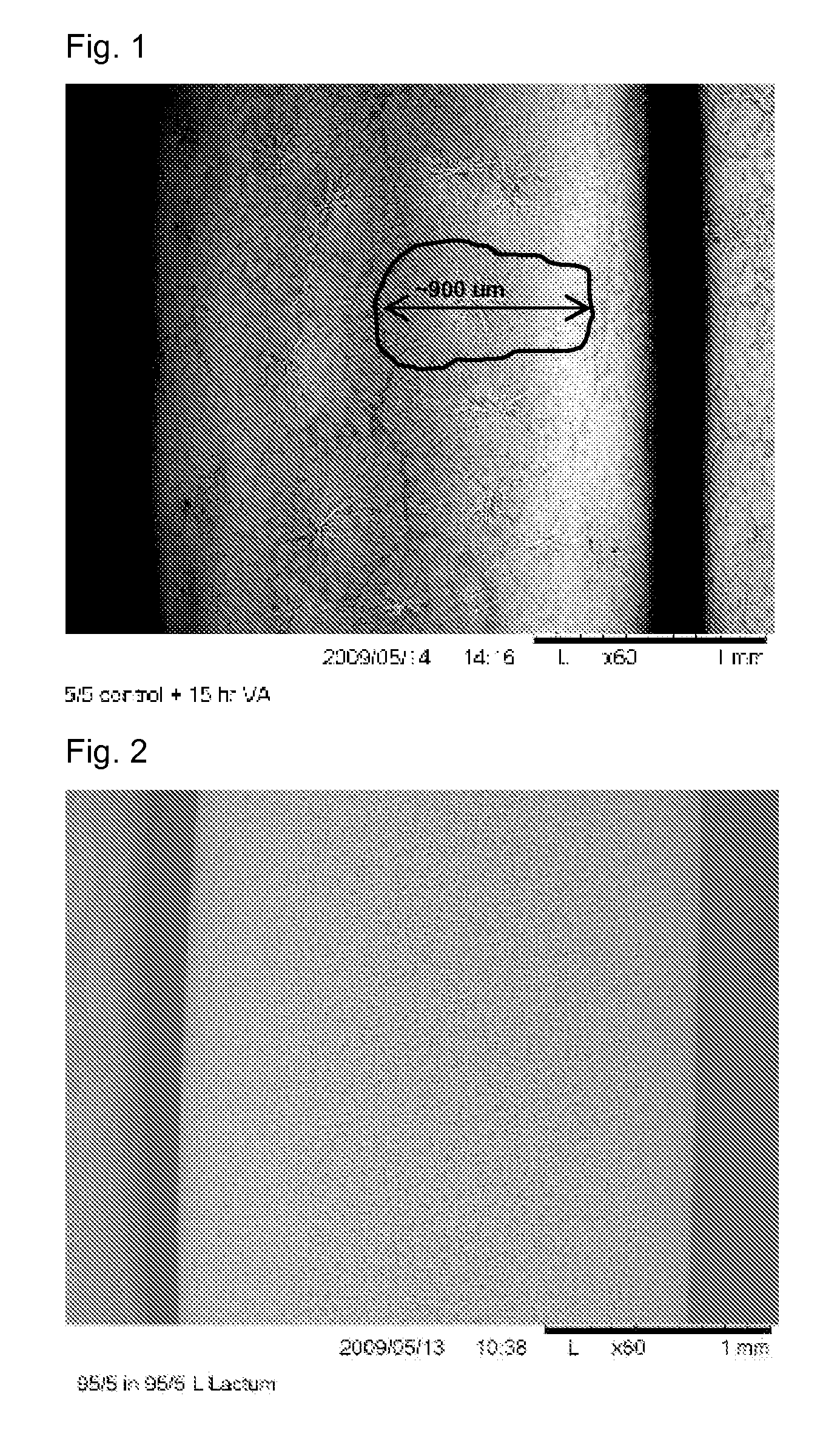
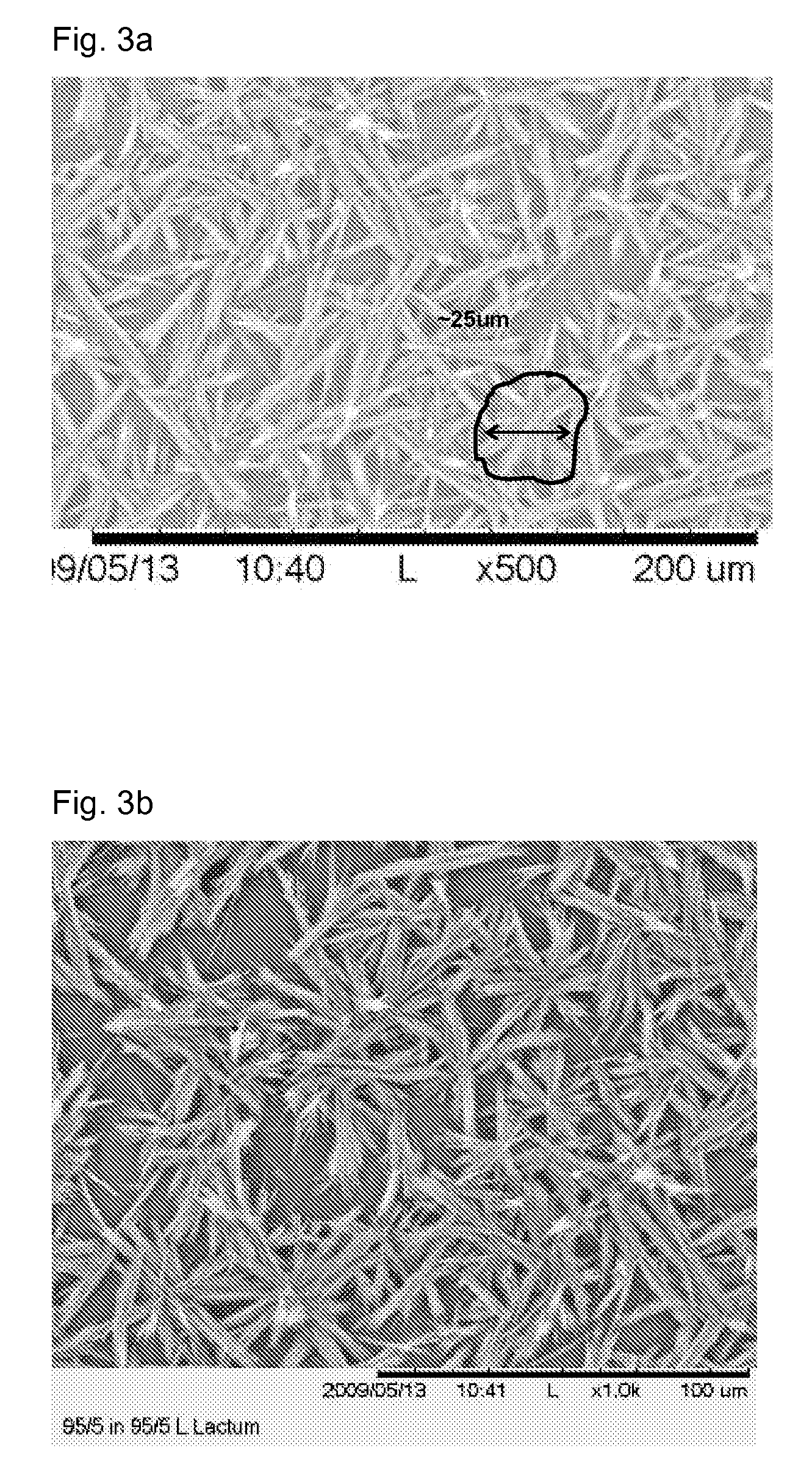
Nucleation of Drug Delivery Balloons to Provide Improved Crystal Size and Density
Drug delivery balloons have densely packed crystals of small particle size of the drug thereon. An amorphous drug coating is applied to a balloon surface and annealed to provide the crystals. The balloon surface is nucleated to induce formation of drug crystals in the annealing step to provide the crystals in high density with small size.
Owner:BOSTON SCI SCIMED INC
Use of Drug Polymorphs to Achieve Controlled Drug Delivery From a Coated Medical Device
When making a medical device having a drug coating thereon, the drug having a plurality of characteristic morphological forms, the manufacturing process is controlled to produce a predetermined ratio of said morphological forms on the device. The process has application to drug coated balloons.
Owner:BOSTON SCI SCIMED INC
Flat process of drug coating for stents
InactiveUS20070077347A1Electropolishing step can be eliminatedExquisite designStentsSurgeryInsertion stentDrug-Coated Stents
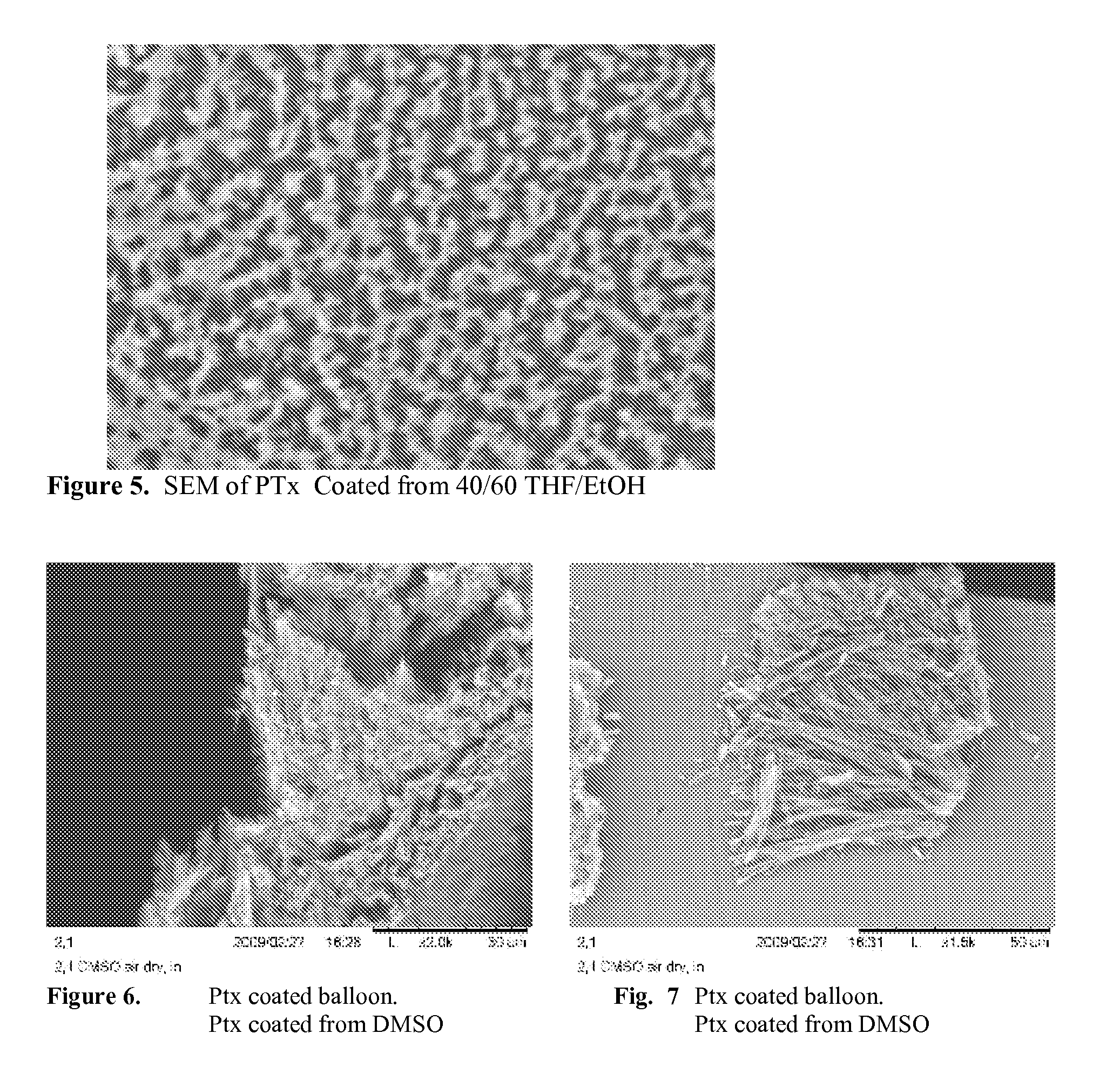
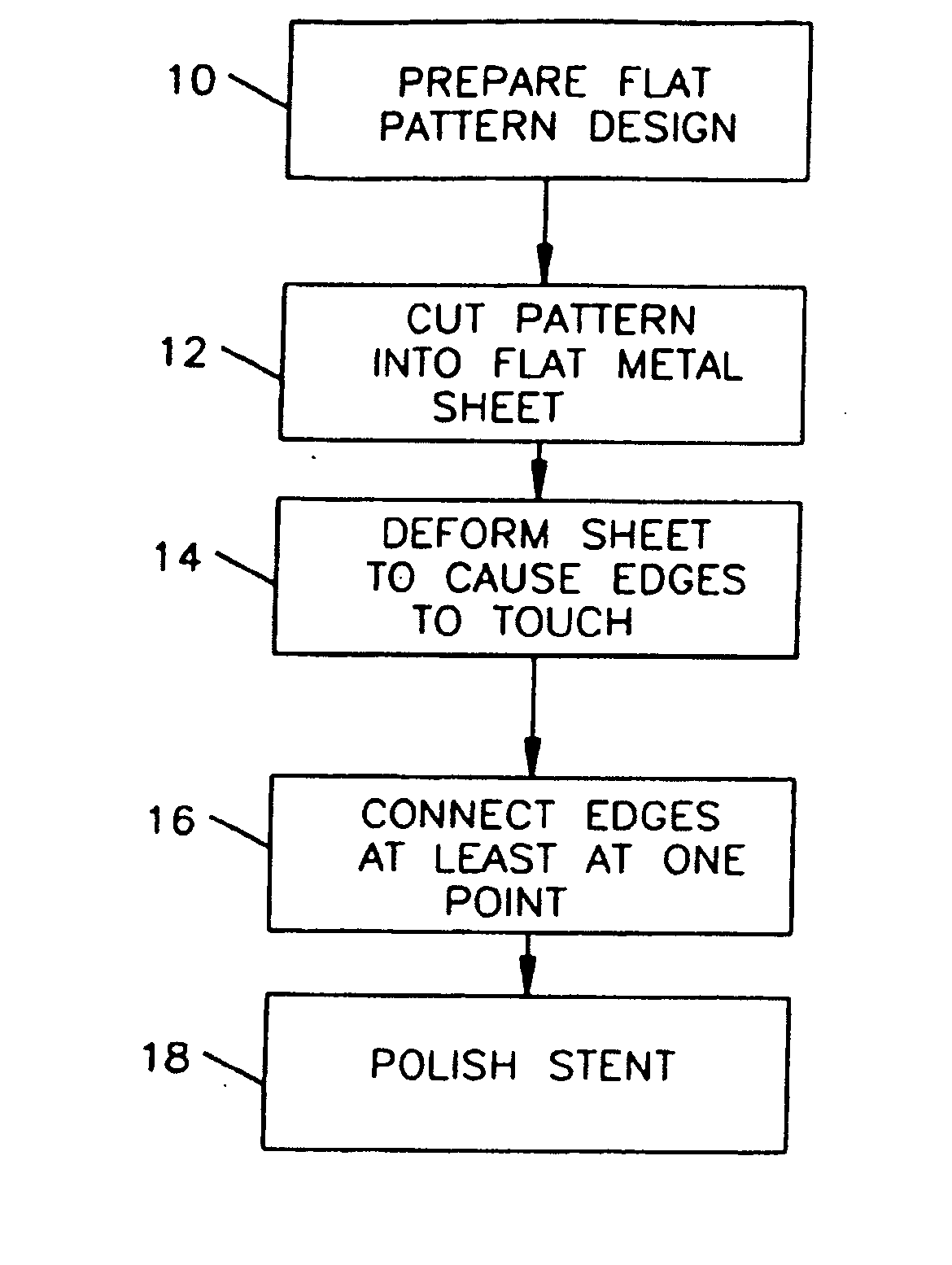
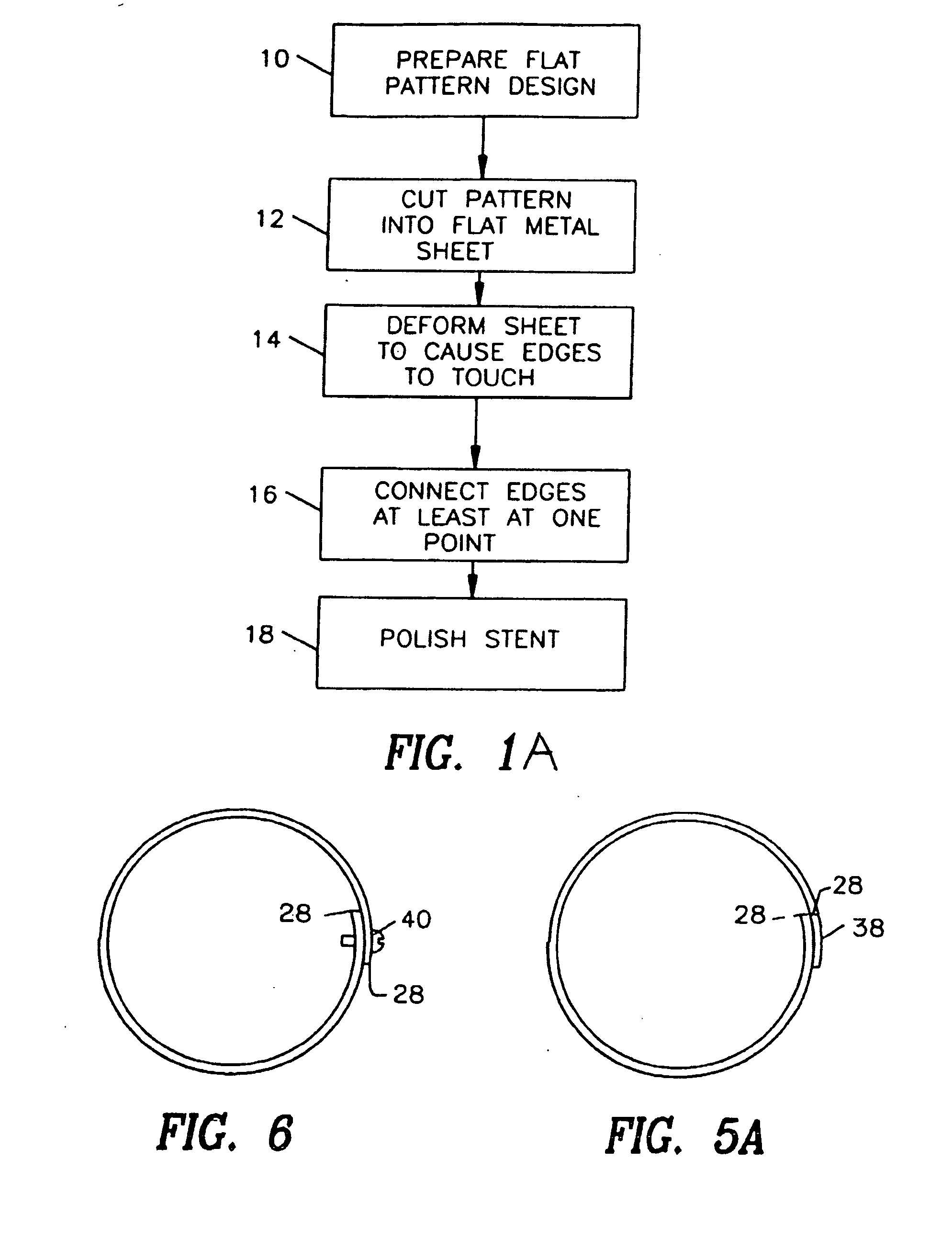
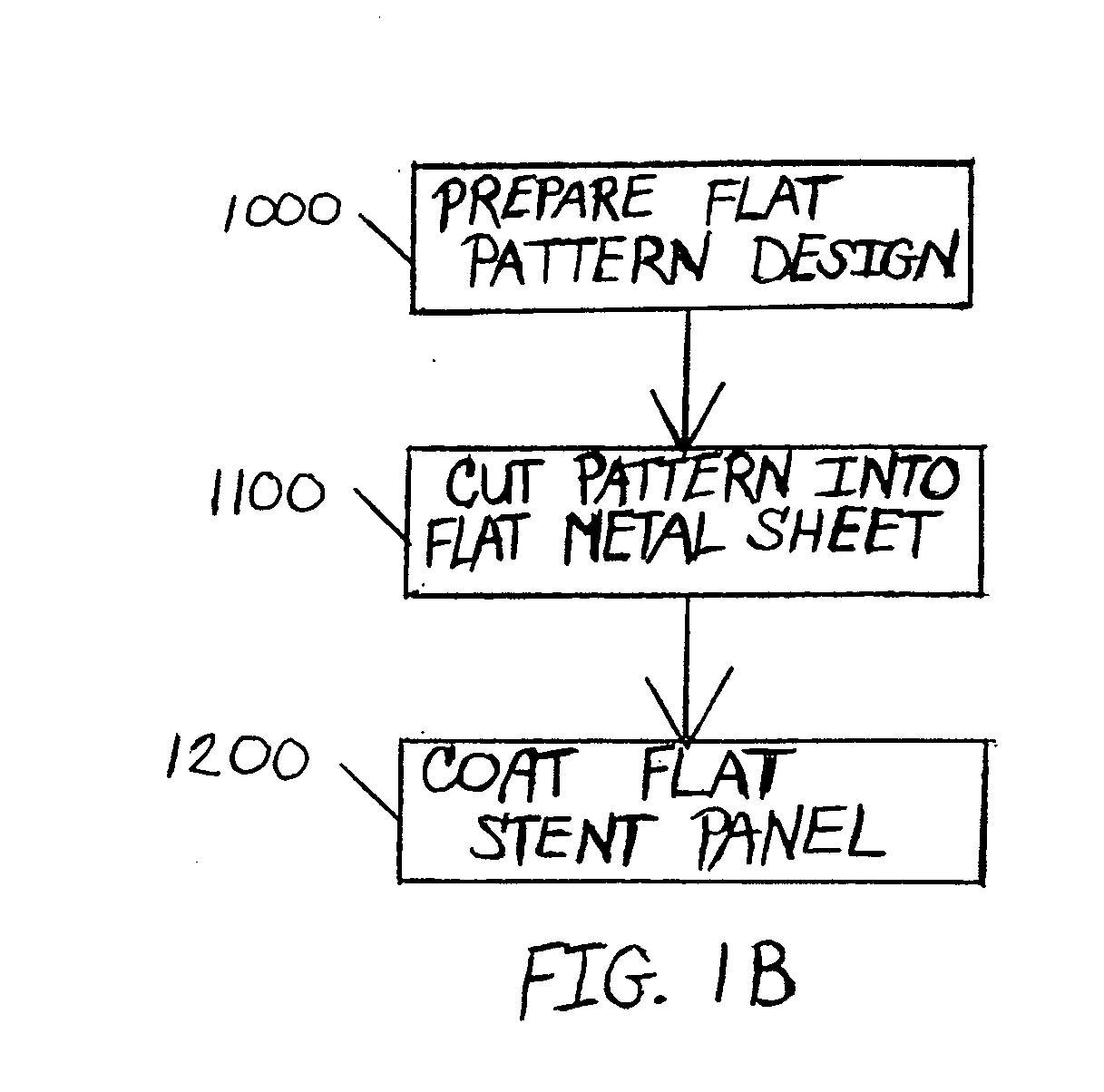
A drug-coated stent and a method for fabricating the stent are disclosed. The stent has an originally flat pattern and connection points where the sides of the flat pattern are joined. The method includes the steps of a) cutting a stent pattern into a flat piece of metal thereby to produce a metal pattern, b) spraying the flat metal stent pattern with a polymer and a drug, c) deforming the metal pattern so as to cause two opposing sides to meet, and d) joining the two opposing sides at least at one point. Substantially no portion of the stent projects into the lumen of the stent when the stent is expanded against the internal wall of a blood vessel.
Owner:MEDINOL LTD
Hydrophobic therapueutic agent and solid emulsifier coating for drug coated balloon
InactiveUS20110144578A1Improved coating transfer efficiencyPromote absorptionSurgeryGlovesSolubilityMedicine
The disclosed subject matter is directed to a coated medical device such as a balloon or stent and methods of manufacturing the device, where the device has a working length disposed between a distal end and a proximal end thereof; and a coating applied to at least a length of the body. The coating includes a hydrophobic therapeutic agent having a water solubility less than about 15.0 μg / ml and an emulsifier that is a solid at ambient temperature.
Owner:ABBOTT CARDIOVASCULAR
Medical devices, drug coatings and methods for maintaining the drug coatings thereon
InactiveUS7056550B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyMedical device
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC +1
Local drug delivery devices and methods for maintaining the drug coatings thereon
InactiveUS7261735B2Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsMedical deviceDrug coating
Local drug delivery medical devices are utilized to deliver therapeutic dosages of drugs, agents or compounds directly to the site where needed. The local drug delivery medical devices utilize various materials and coating methodologies to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
Hinged sheath assembly and method of use
A sheath assembly is provided for protecting a stent mounted on a catheter. An inner tubular member is positioned over the stent without longitudinal movement of the inner tubular member along the stent surface thereby eliminating the possibility of scraping or scratching a drug coating or polymer coating on the stent surface. An outer tubular member slides over the inner tubular member to firmly compress it onto the stent for further protection. In use, the outer tubular member is removed from over the inner tubular member so that the inner tubular member can open similar to a clamshell opening radially outwardly away from the stent without longitudinal movement along the stent surface.
Owner:ABBOTT LAB INC
Anti-adhesion agents for drug coatings
InactiveUS20070134288A1Lower and prevents adhesionLower and prevents and tackStentsSurgeryAnti-Adhesion AgentMedical device
Coated medical devices and methods for coating such devices are disclosed. The invention is directed to the use of an anti-adhesion agent in a coating for a medical device. More particularly, the invention is directed to a medical device comprising an anti-adhesion agent that prevents the self-adhesion of different portions of a coating disposed on the surface of the medical device. Additionally, this invention is directed to methods for coating such a medical device.
Owner:BOSTON SCI SCIMED INC
Medical devices, drug coatings and methods of maintaining the drug coatings thereon
InactiveUS20060222756A1Reduce drug toxicityGood curative effectSuture equipmentsOrganic active ingredientsBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH LLC
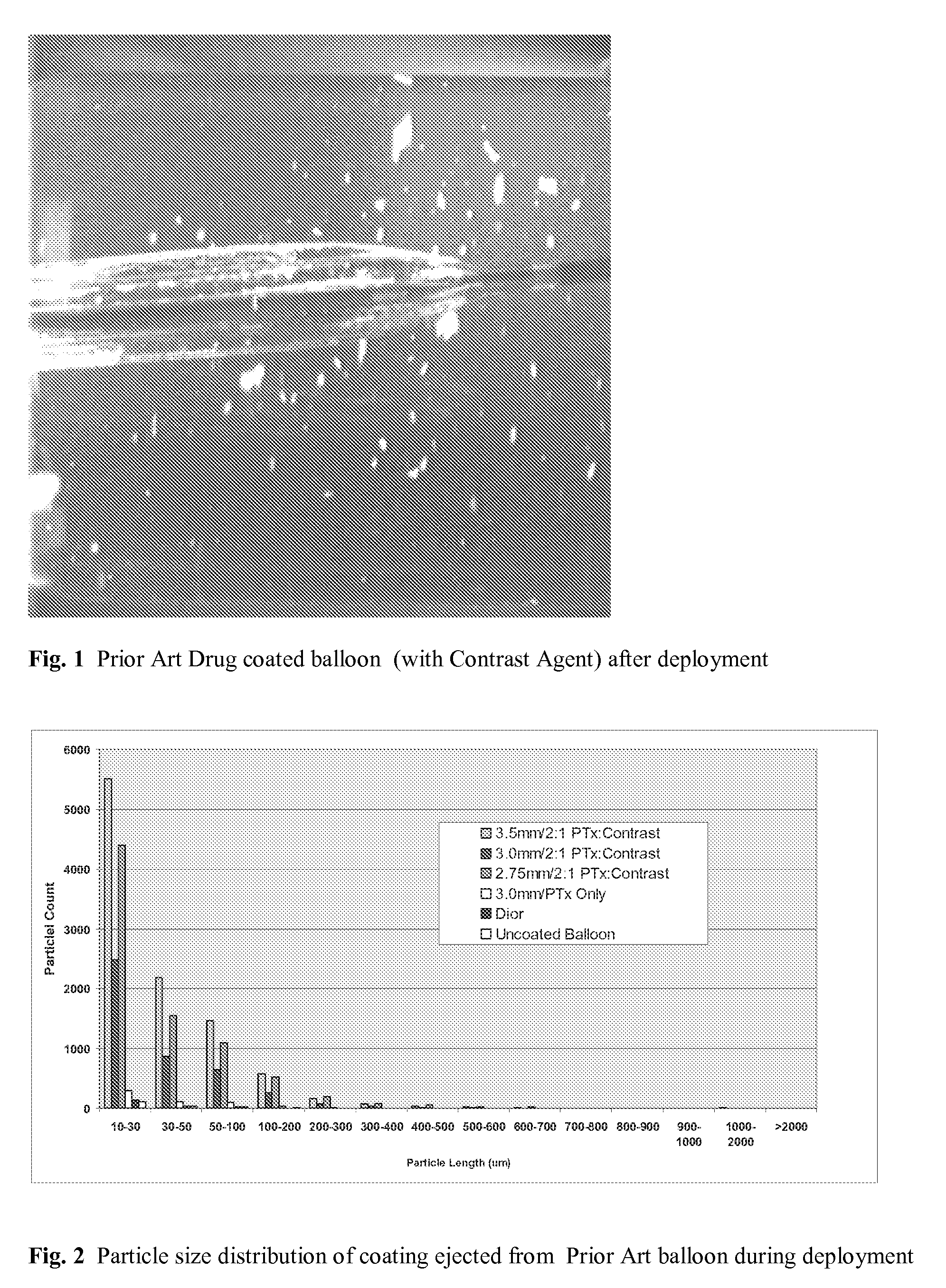
Contrast coated stent and method of fabrication
The invention provides a system for treating a vascular condition. The system includes a catheter and a stent disposed on the catheter. The system further includes a stent with at least an outer portion of the stent framework coated with a drug polymer solution and a contrast medium substantially covering at least an outer surface of the drug coating disposed on the outer surface of the stent framework. A method of manufacturing the contrast coated stent and a method of using the contrast coated stent is included.
Owner:MEDTRONIC VASCULAR INC
Drug coating providing high drug loading and methods for providing same
InactiveUS20050112195A1High viscosityEasy to produceBiocideNervous disorderDrug coatingInsoluble drug
The present invention is directed to aqueous drug coatings that include at least one insoluble drug, wherein the drug accounts for about 85 wt % to about 97 wt % of the drug coatings. A drug coating according to the present invention may include only one insoluble drug, two or more insoluble drugs, or one or more insoluble drugs in combination with one or more soluble drugs. The present invention also includes drug coating formulations suitable for providing drug coatings according to the present invention and dosage forms that include a drug coating according to the present invention.
Owner:ALZA CORP
Taste masked pharmaceutical compositions
InactiveUS20060182796A1Fast absorptionQuick releaseDispersion deliveryPill deliveryOral medicationBULK ACTIVE INGREDIENT
A pharmaceutical composition for oral administration containing a pharmaceutically active ingredient coated with an amount of a polymer combination of an enteric polymer and an ammonio methacrylate copolymer to effectively mask the taste of the medicament. In a preferred embodiment, the ratio of the enteric polymer to the ammonio methacrylate copolymer is about 40:60 to about 90:10, preferably about 60:40, by weight of polymer. The pharmaceutical coating composition is soluble in the acidic environment of the stomach, which generally has a pH value of about 1.0 to 3.0, but relatively insoluble at higher pH values of the mouth. The coatings provide for rapid release and absorption of the drug after it passes through the mouth, and is particularly desirable in the case of liquid dosage forms.
Owner:ABRIKA PHARMA
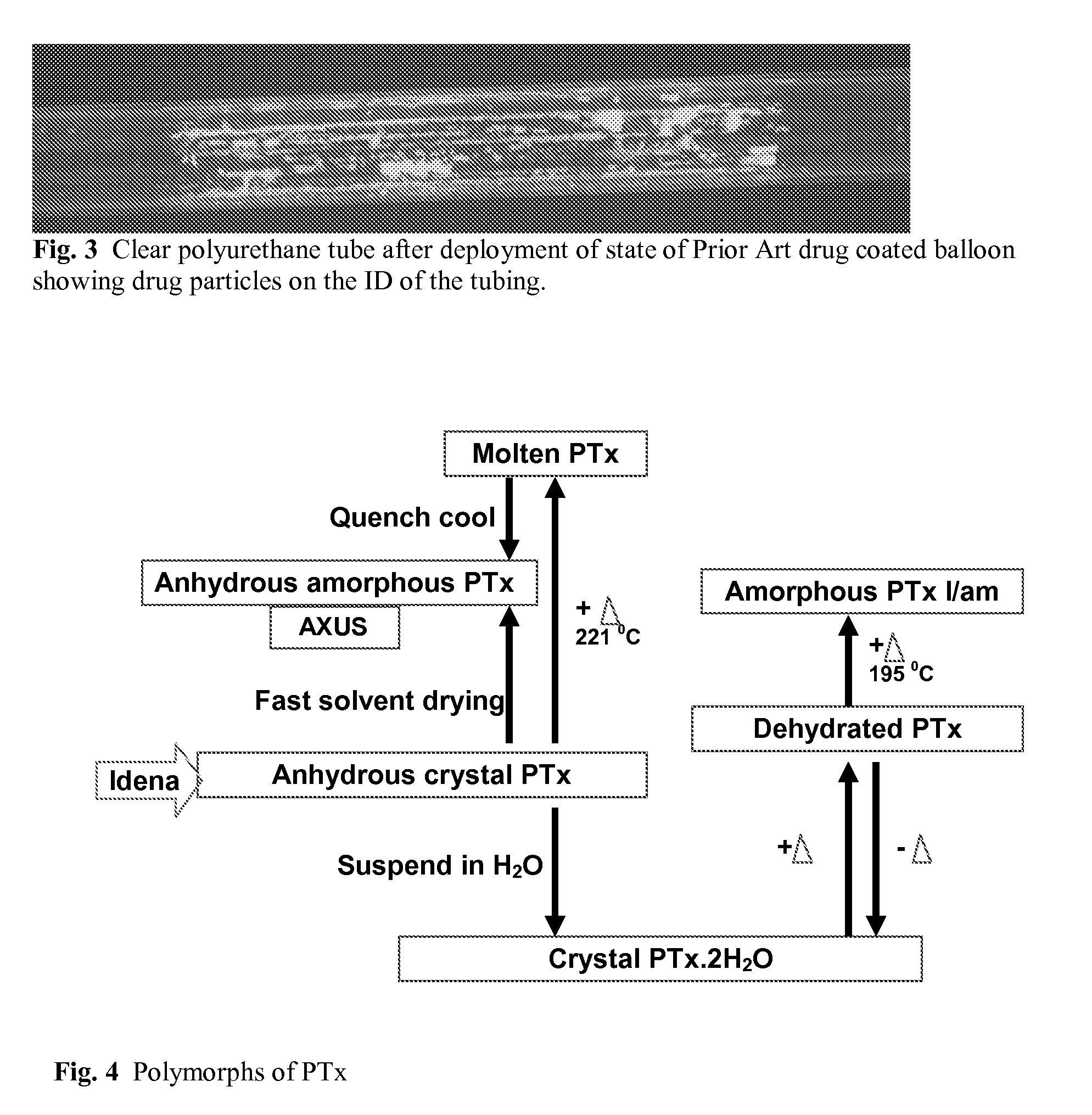
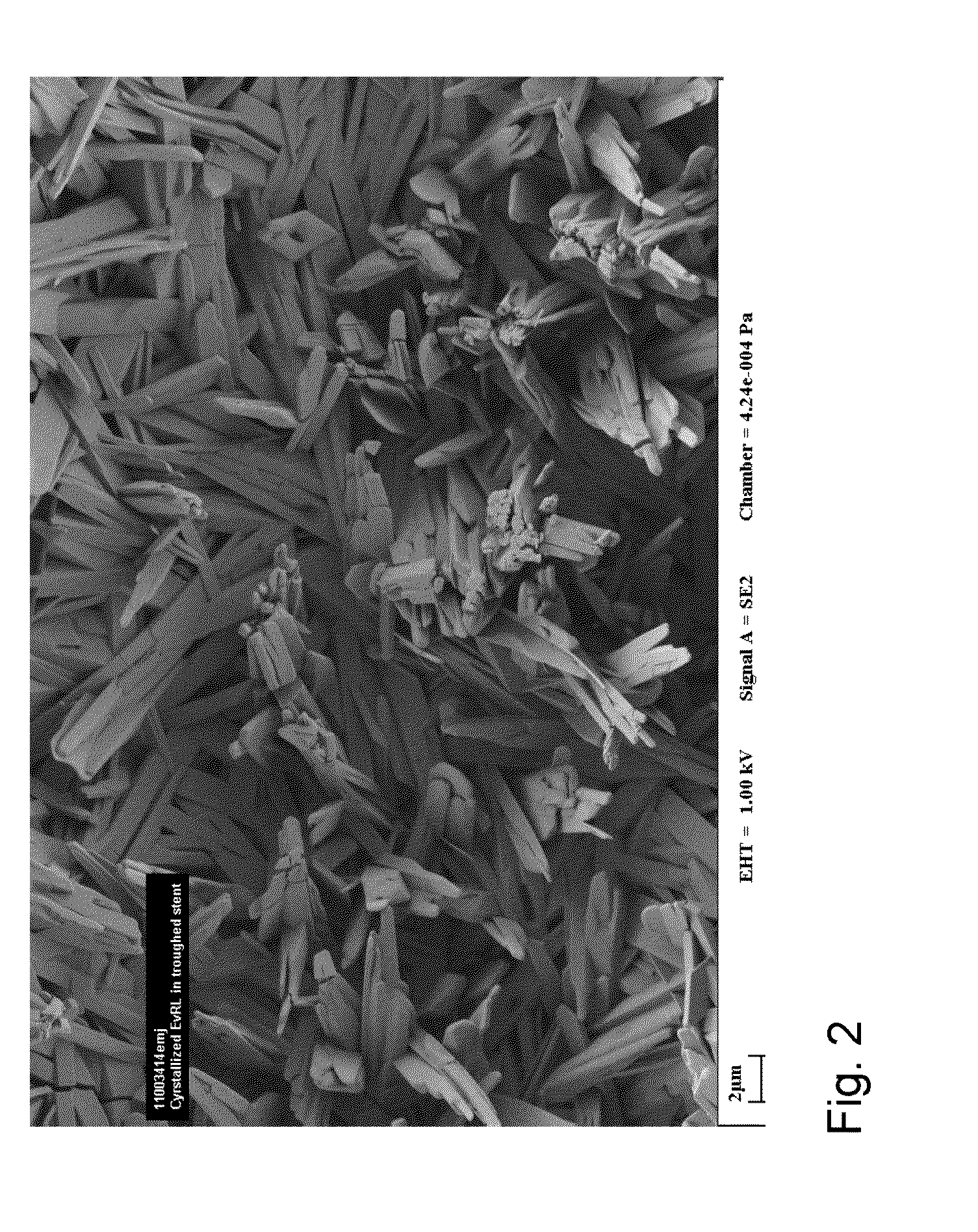
Medical Device with Crystalline Drug Coating
A medical device having a polymer-free outer surface layer comprising a crystalline drug selected from the group consisting of everolimus, tacrolimus, sirolimus, zotarolimus, biolimus, and rapamycin. The device may be produced by a method comprising the steps of providing a medical device; applying a solution of the drug to said portion of the outer surface to form a coating of amorphous drug; and vapor annealing the drug with a solvent vapor to form crystalline drug; wherein a seed layer of a crystalline form of said drug having a maximum particle size of about 10 μm or less is applied to at least said portion of the outer surface of the device before or after applying the drug solution, but before vapor annealing the amorphous coating.
Owner:BOSTON SCI SCIMED INC
Iontophoresis device and method of producing the same
InactiveUS20070066930A1Reduce material lossSimple processElectrotherapyLamination ancillary operationsBiological bodyElectrical polarity
An iontophoresis device and method of producing the same may reduce material loss during the course of production of a conventional iontophoresis device, and may allow for easy automation of production processes and increases in production scale. The iontophoresis device may be used for administering drug ions of a first polarity generated by dissociation of a drug to a living body, and may comprise: a first conductive layer formed on a surface of a first substrate; a drug layer made of a drug coating containing the drug, the drug layer being laminated on the first conductive layer; and a first ion exchange layer made of an ion exchange coating containing an ion exchange resin having an exchange group introduced thereto, the ion exchange group having a counter ion to the first polarity ions, the first ion exchange layer being laminated on the drug layer.
Owner:TITI ELLEBEAU INC
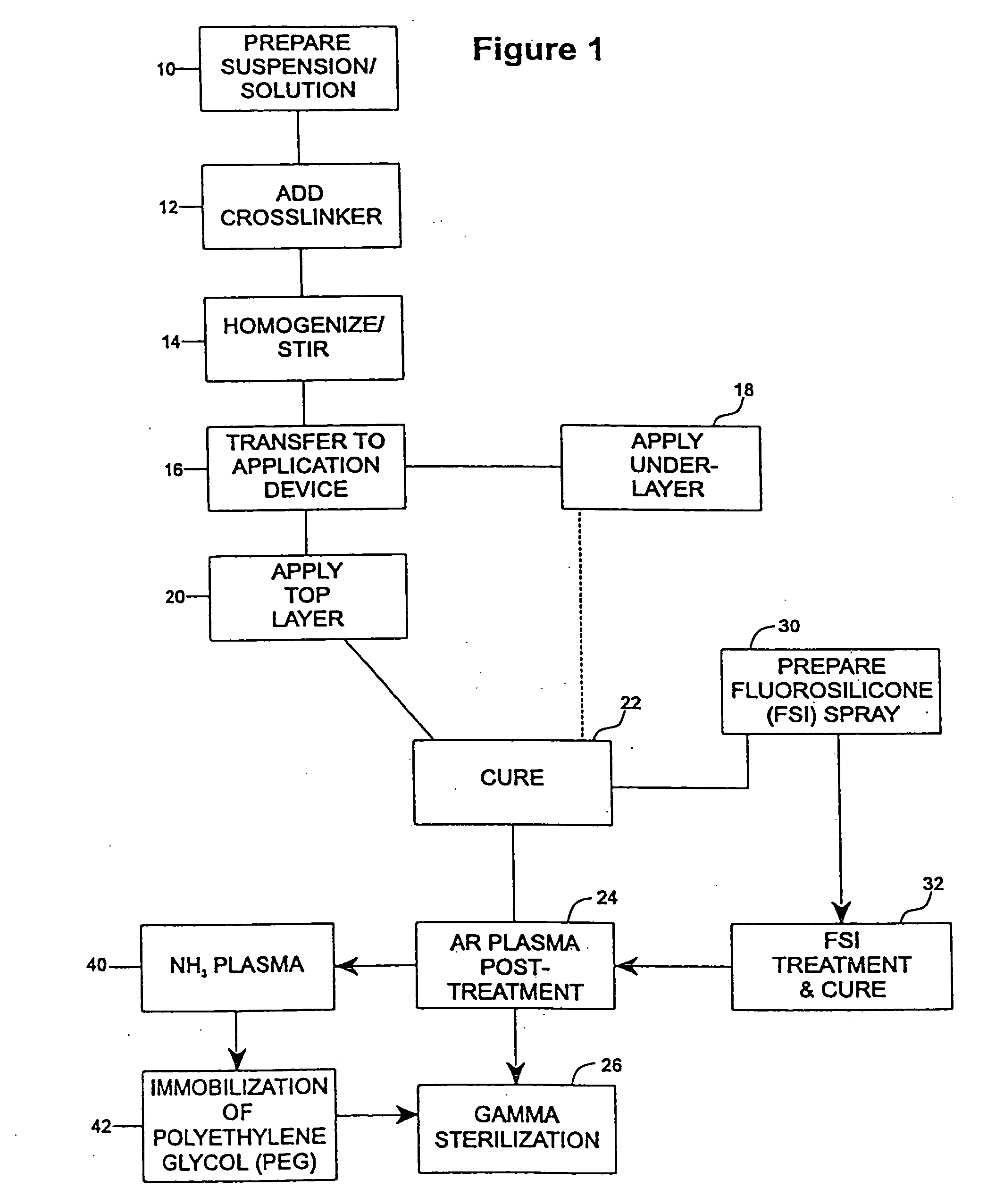
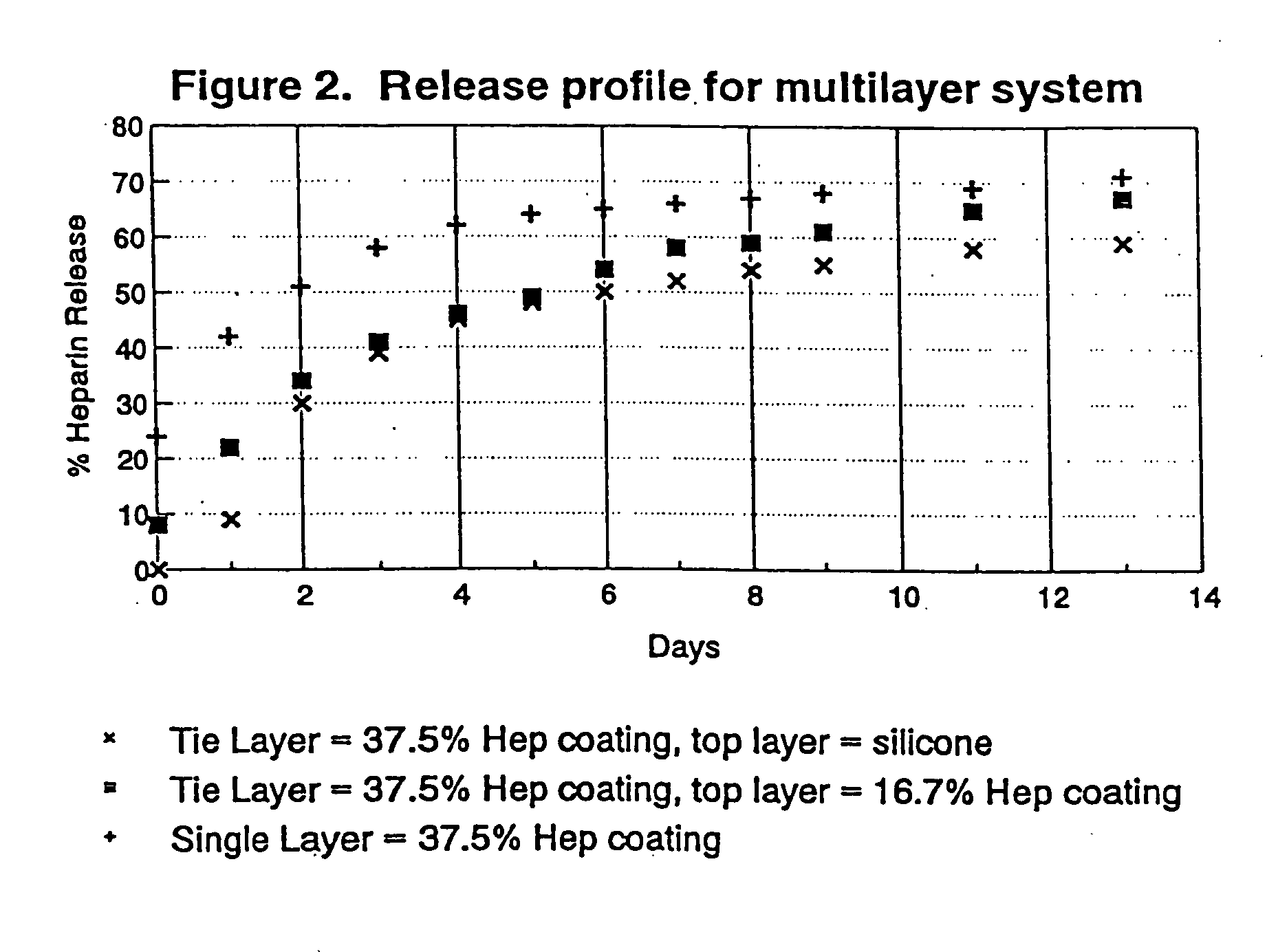
Drug coating with topcoat
InactiveUS20050187611A1Long release timeReduced burst effectStentsSurgeryThrombogenicityDrug coating
A coating and method for a coating an implantable device or prostheses are disclosed. The coating includes an undercoat of polymeric material containing an amount of biologically active material, particularly heparin, dispersed herein. The coating further includes a topcoat which covers less than the entire surface of the undercoat and wherein the topcoat comprises a polymeric material substantially free of pores and porosigens. The polymeric material of the topcoat can be a biostable, biocompatible material which provides long term non-thrombogenicity to the device portion during and after release of the biologically active material.
Owner:BOSTON SCI SCIMED INC
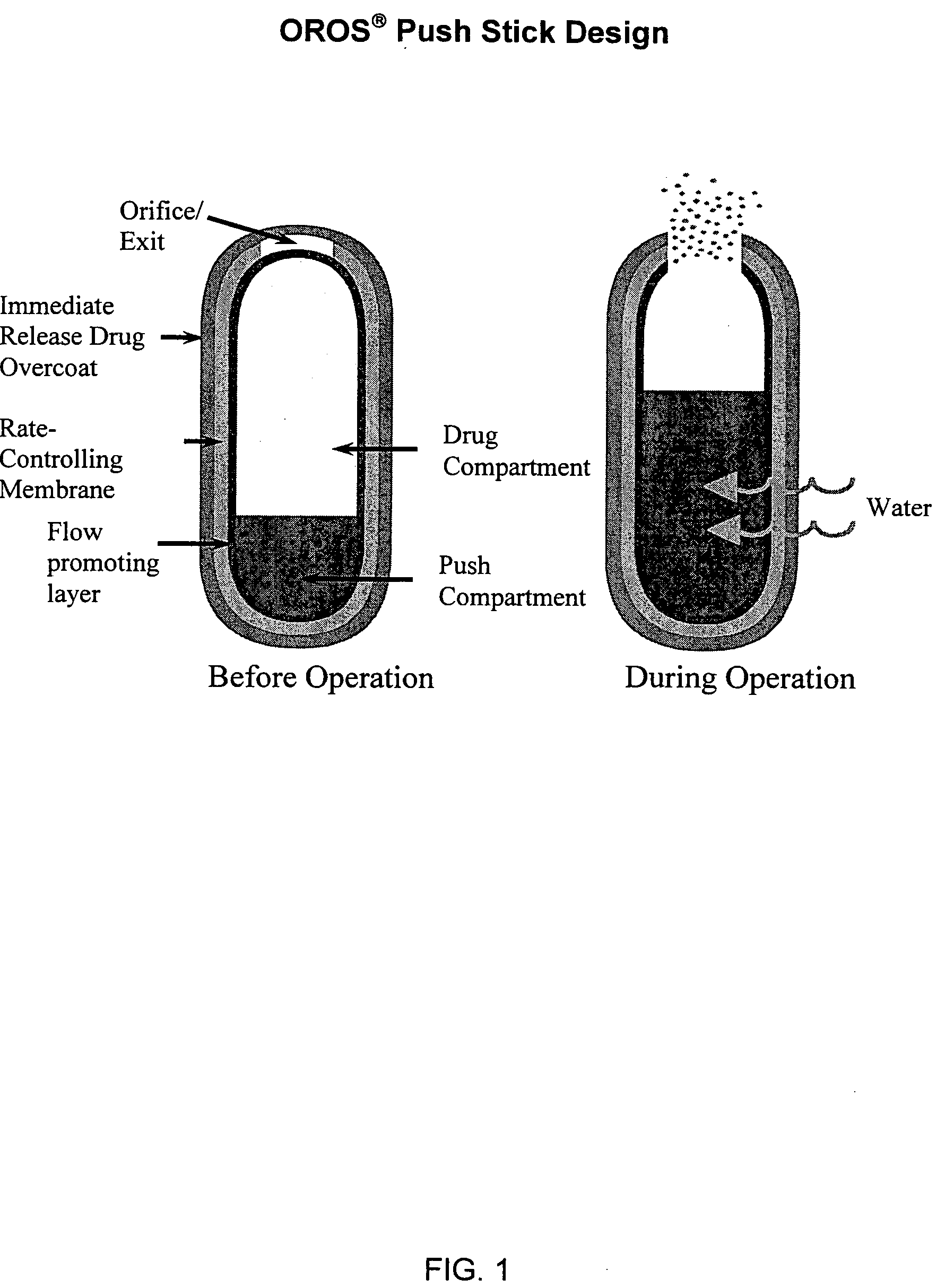
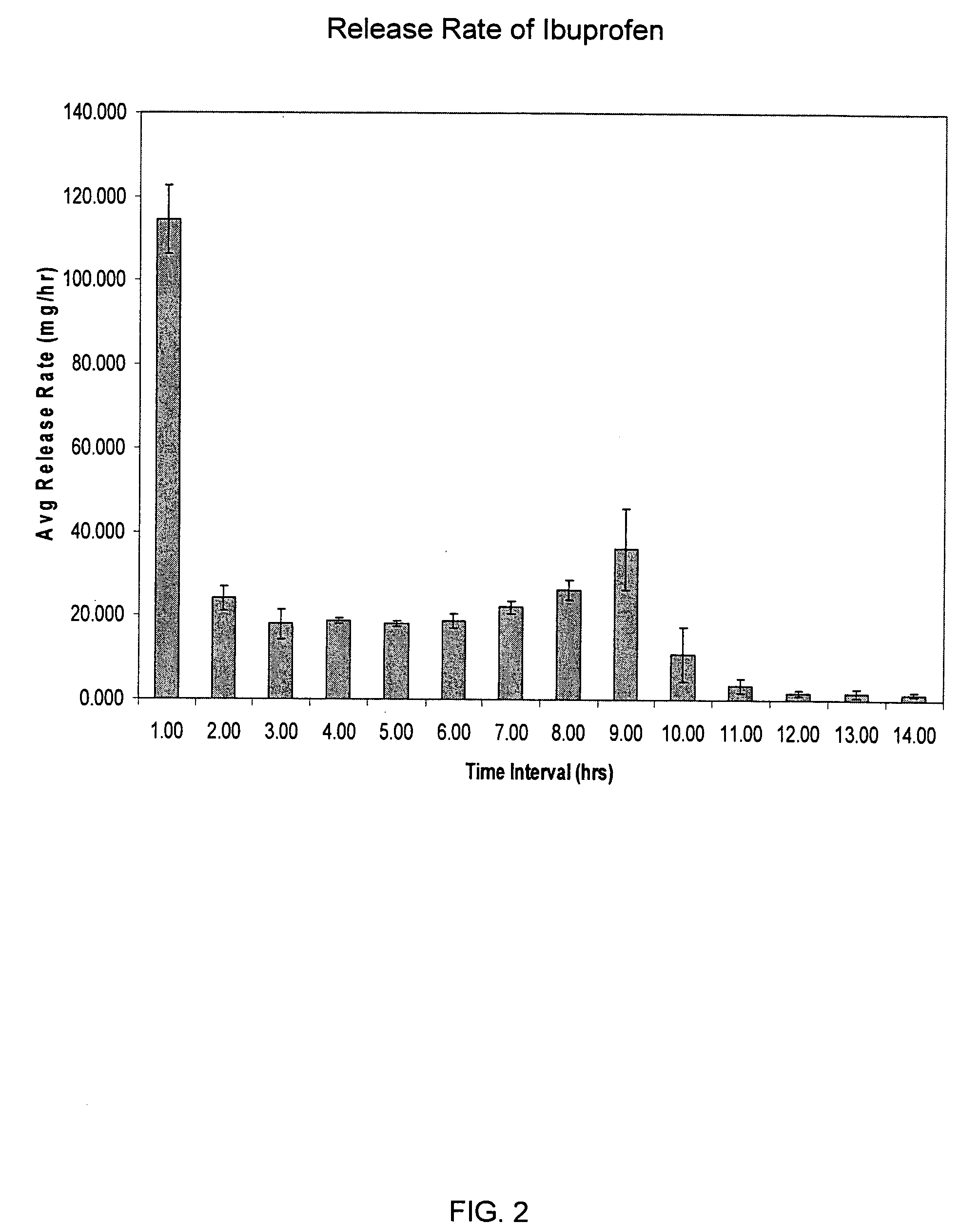
Oros push-stick for controlled delivery of active agents
InactiveUS20050089570A1Faster initial rate of releaseFast releaseOrganic active ingredientsPill deliveryDiseaseSustained release drug
A sustained release dosage form is provided comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides burst release of the pharmaceutically active agent without the use of an immediate release drug coating. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents at a controlled rate. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:ALZA CORP
Biodegradable vascular filter
InactiveUS20070112372A1Preventing recurrent pulmonary embolismEfficient trappingStentsSurgeryVascular filterInsertion stent
Novel enhanced products and processes for trapping emboli utilize self-expanding skeletons and biodegradable polymer systems, for example stent-like Nitinol® elements and PLGA, to address longstanding issues related to thrombus capture without deleterious impacts on the vasculature or other negative artifacts of the procedure by at least partial post-use dissolution in situ. Drug coating and elution technologies are included as would be known to those skilled in the art.
Owner:COVIDIEN GROUP +1
Dual sheath assembly and method of use
A sheath assembly is provided for protecting a stent mounted on a catheter. An inner tubular member is positioned over the stent without longitudinal movement of the inner tubular member along the stent surface thereby eliminating the possibility of scraping or scratching a drug coating or polymer coating on the stent surface or balloon surface. An outer tubular member slides over the inner tubular member to firmly compress it onto the stent for further protection. In use, the outer tubular member is removed from over the inner tubular member so that the two half-cylindrical portions of the inner tubular member can fall away from the stent without longitudinal movement along the stent surface.
Owner:ABBOTT LAB INC
Medical Devices, Drug Coatings and Methods for Maintaining the Drug Coatings Thereon
InactiveUS20080051885A1Reduce drug toxicityGood curative effectStentsSurgeryBiological bodyCompound (substance)
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:CORDIS CORP
Medicated coatings for implantable medical devices including polyacrylates
A polymer for a medical device, particularly for a drug eluting stent, is described. The polymer can be derived from n-butyl methacrylate and can have a degree of an elongation at failure from about 20% to about 500%
Owner:ABBOTT CARDIOVASCULAR
Method for preparing medicine sustained-releasing bracket
ActiveCN101185779ANo oxidative decompositionThickness is easy to controlStentsSurgeryDrug release rateSustained release drug
A preparation method of a drug sustained-release stent includes a preparation stent, a drug sustained-release stent constituted by drug coating on the stent, and the drug coating of the stent is composed of the following steps sequentially: (1) the preparation of a sustained-release drug coating layer; (2) the coating of the sustained-release drug coating layer; (3) drying. The dosages of the drug components on the sustained-release drug stent of the invention are in line with the designed dosages, and the drug can be released according to the stipulated dosages in the stipulated time of the design requirements completely in vitro tests. The invention has good biocompatibility, low cost of spraying process, high yield, controllable thickness of the coating layer, and slow and stable drug release rate.
Owner:上海赢生医疗科技有限公司
Osmotic device containing amantadine and an osmotic salt
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
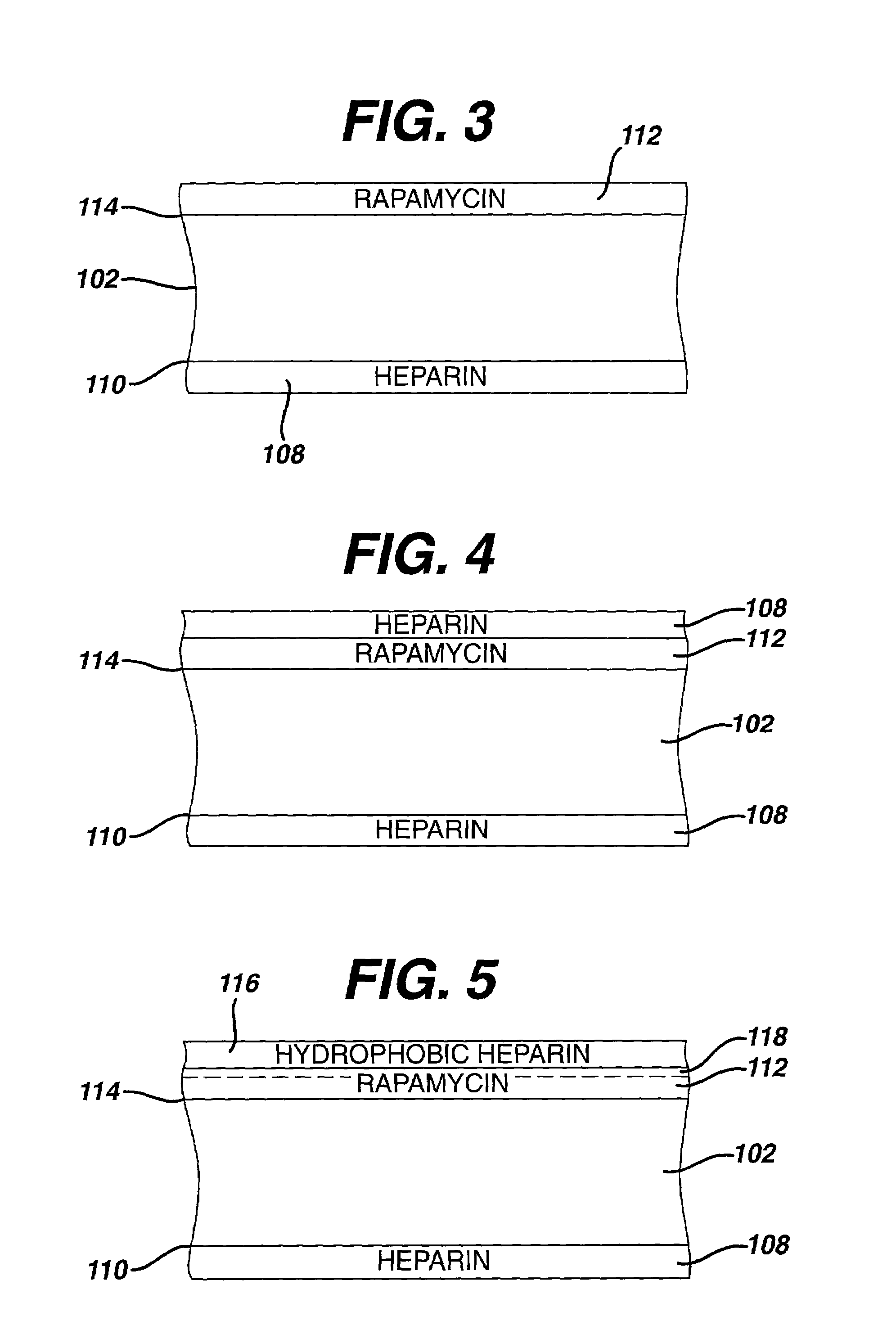
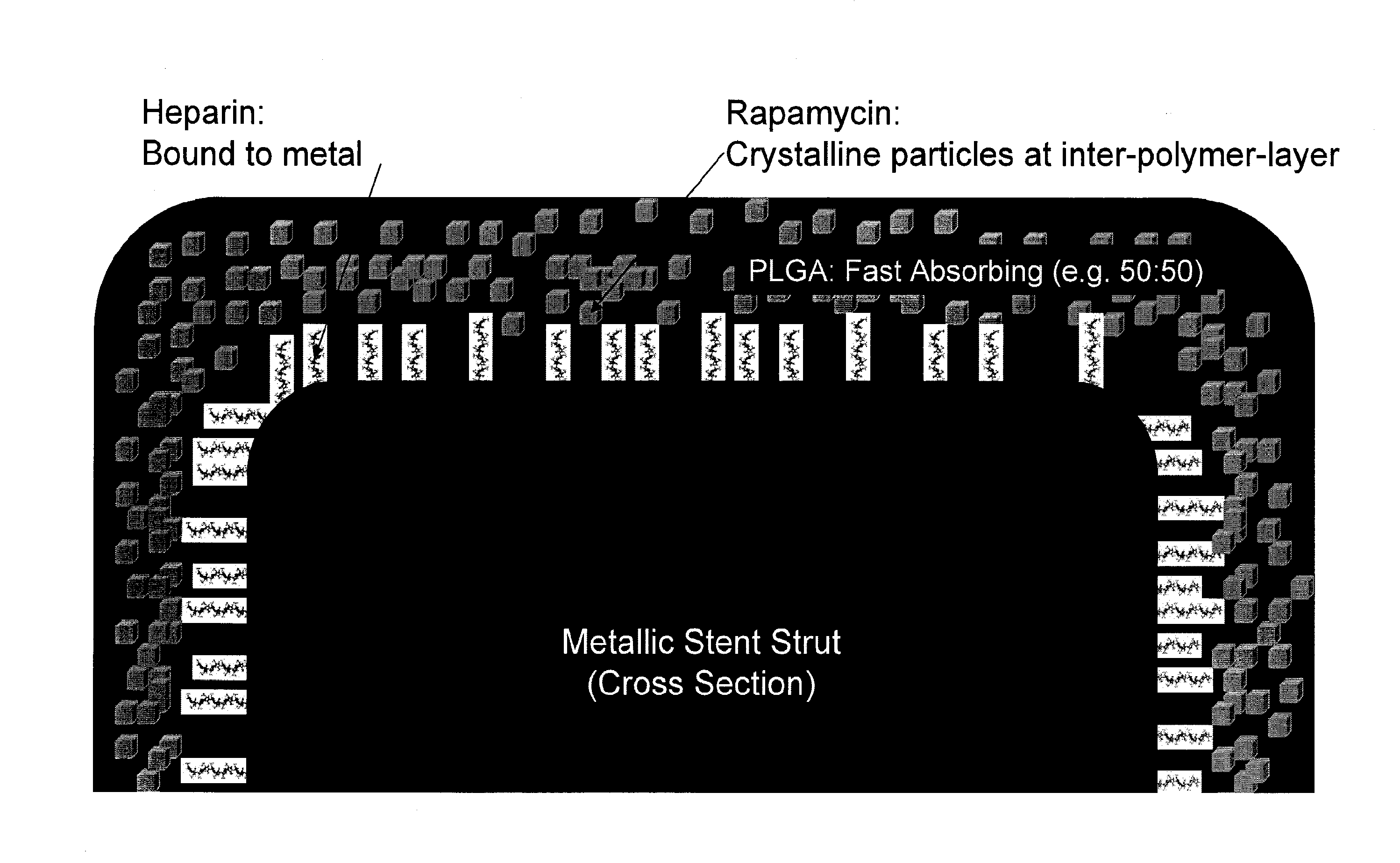
Drug Coated Stents
Provided herein is a coated coronary stent, comprising: a stent framework; heparin molecules attached to the stent framework; and a rapamycin-polymer coating wherein at least part of rapamycin is in crystalline form. In one embodiment, the rapamycin-polymer coating comprises one or more resorbable polymers.
Owner:MICELL TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com