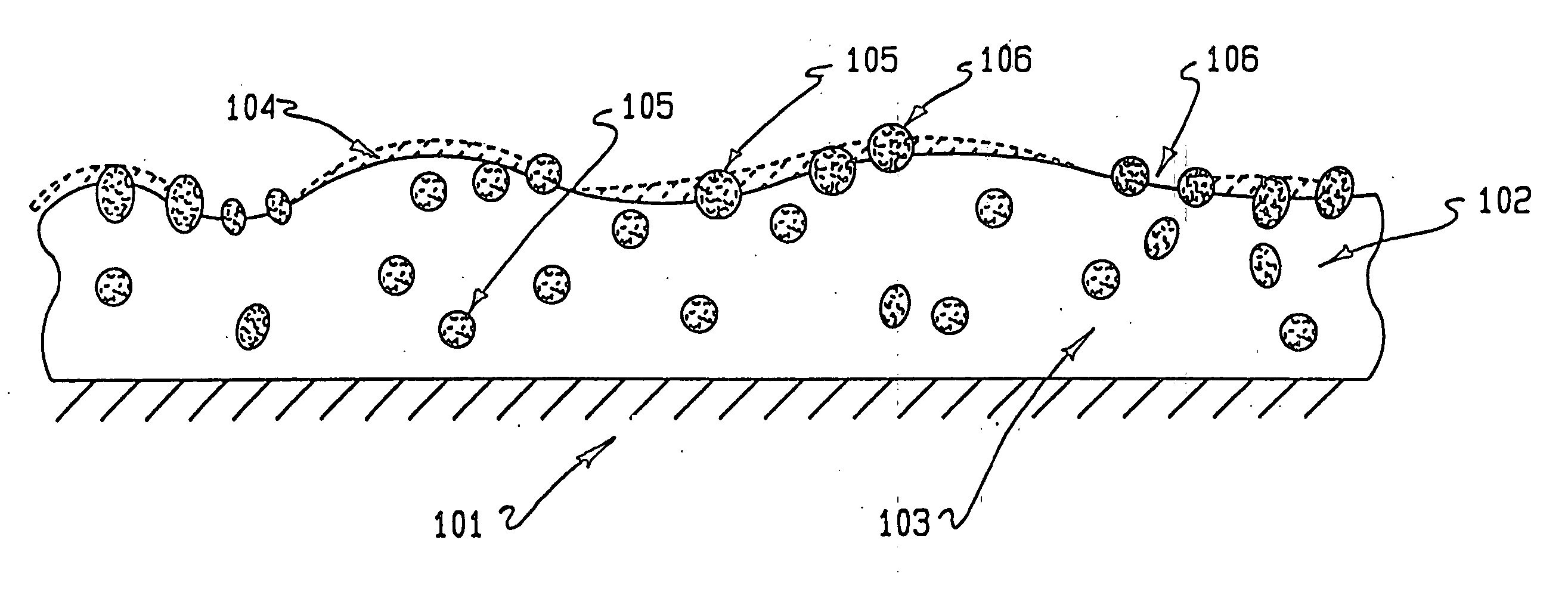
Drug coating with topcoat
a topcoat and drug technology, applied in the field of topcoats for drugs, can solve the problems of increasing the thickness of the coating, reducing the effective drug loading, and increasing the coating thickness, so as to reduce the burst effect, prolong the release time, and reduce the effect of drug elution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fluorosilicone Surface Treatment of Eluting Heparin Coating
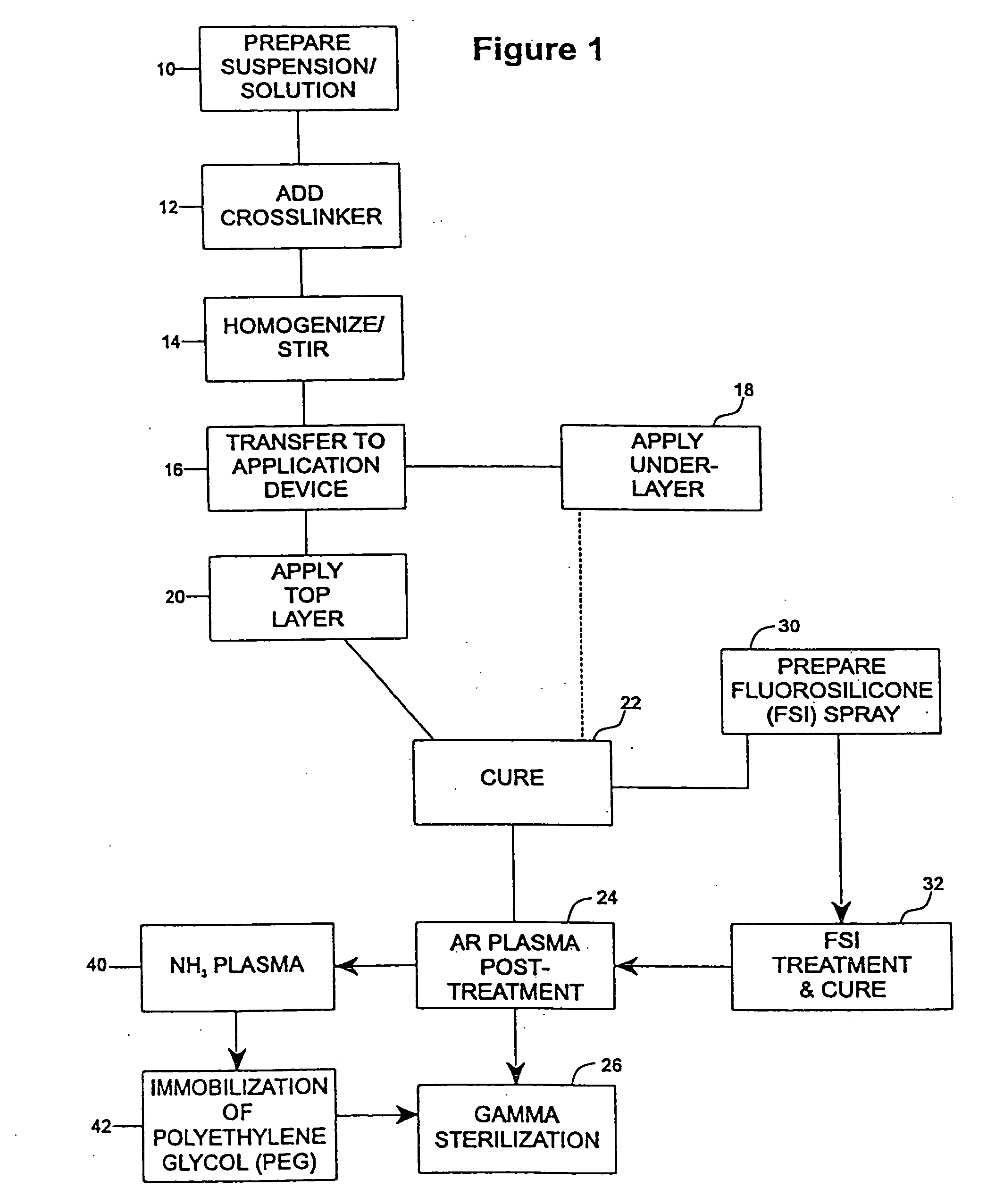
[0078] The undercoat of a stent was coated as multiple applied layers as described above thereafter and cured as described at 22. The heparin content of the undercoat was 37.5% and the coating thickness was about 30-40μ. Fluorosilicone (FSi) spray solution was prepared at 30 from a fluorosilicone suspension (Applied Silicone #40032) by weighing an amount of fluorosilicone suspension and adding tetrahydrofuran (THF) according to the relation equation of VTHF=1.2× the weight of fluorosilicone suspension. The solution was stirred very well and spray-coated on the stent at 32 using the technique of the application of the undercoat process at 18 and the coated stents were cured at 90° C. for 16 hours. The coated stents are argon plasma treated prior to gamma sterilization according to the procedures described above in accordance with steps 22-26.
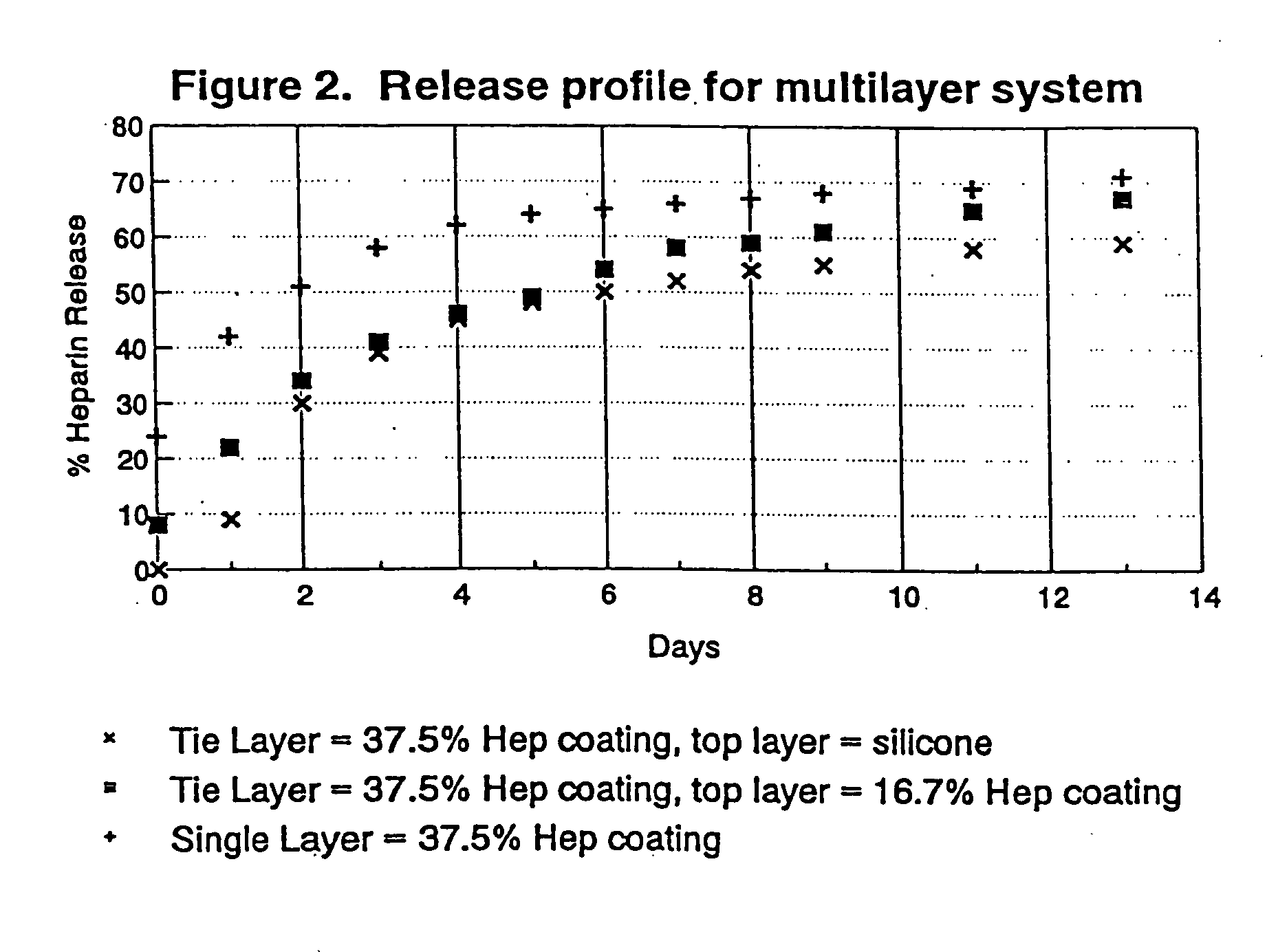
[0079]FIG. 7 is a plot of heparin release kinetics in phosphate buffer system with ...
example 2
Immobilization of Polyethylene Glycol (PEG) on Drug Eluting Undercoat
[0081] An undercoat was coated on a stent and cured at 22 as in Example 1. The stent was then treated by argon gas plasma as at 24 and ammonium gas plasma at 40. The equipment and the process of argon gas plasma treatment was as has been described above. The ammonium plasma treatment was implemented immediately after the argon gas plasma treatment, to aminate the surface of the coating. The ammonium flow rate was in the range of 100-700 cubic centimeter per minute (ccM) in preferably in the range of 500-600 ccM. The power output of radio frequency plasma was in the range of 50-500 watts, preferably in ˜200 watts. The process time was in the range of 30 sec-10 min, preferably ˜5 min.
[0082] Immediately after amination, the stents were immersed into electrophilically activated polyethylene glycol (PEG) solution it 42. PEG is known to be an inhibitor of protein absorption. Examples of electrophilically activated PEG ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com