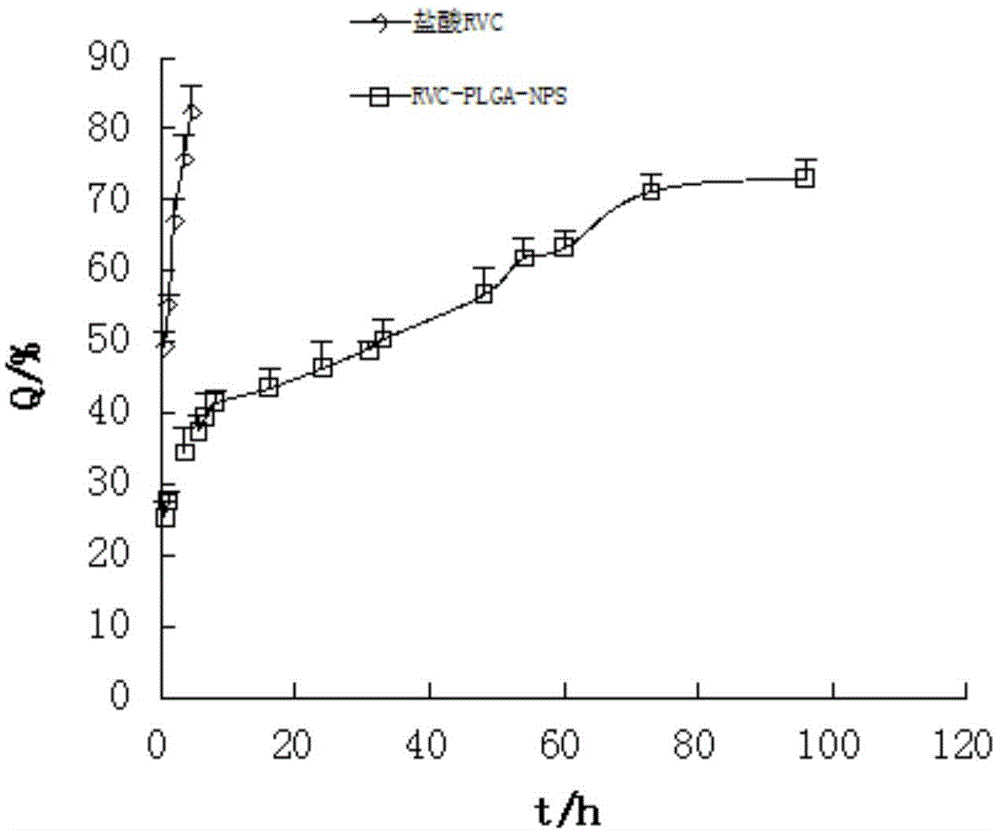
Ropivacaine nano particle, preparation method thereof and optimizing experimental method of effect of the ropivacaine nano particle
A technology of ropivacaine and nanoparticles, applied in the field of ropivacaine nanoparticles and its preparation, can solve the problems of catheter infection, short half-life, discount, etc., and achieve smooth and smooth appearance, long sustained release time, and sustained release effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of ropivacaine nanoparticles is as follows:
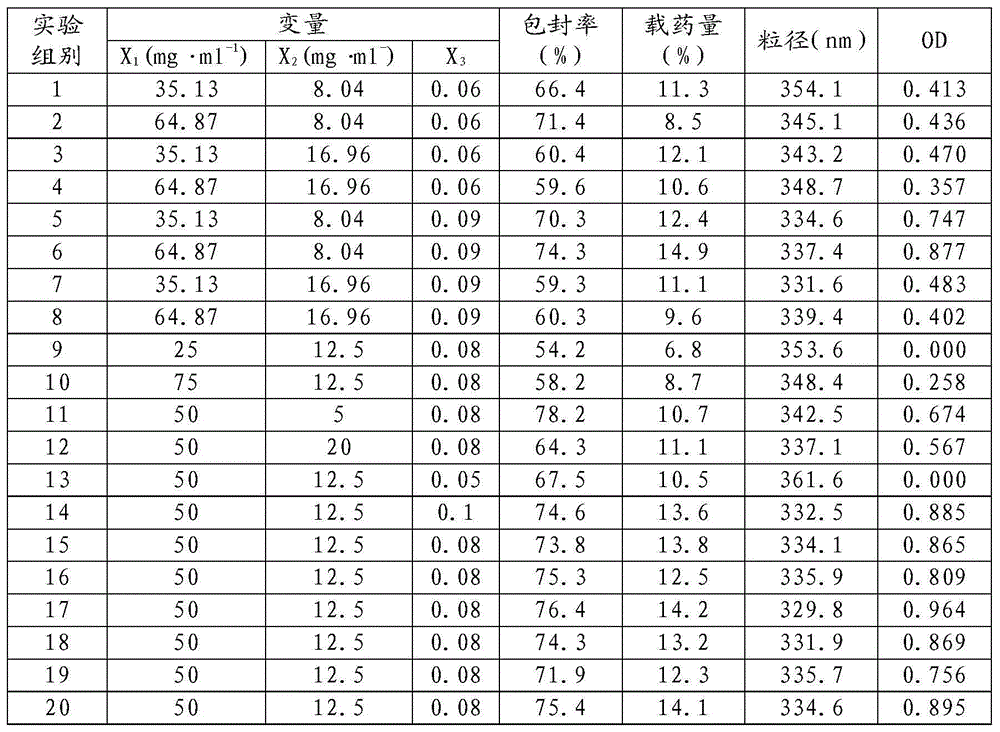
[0030] (A) dissolving ropivacaine free base and poly(lactic-co-polyglycolic acid) block copolymer in dichloromethane as organic phase, polyvinyl alcohol solution as water phase, and slowly adding organic phase into water phase Ultrasonic treatment at 0°C yielded a white emulsion, in which the mass concentration of PLGA in the organic phase was 35 mg / ml, the mass concentration of RVC was 5 mg / ml, and the oil-water volume ratio of the organic phase to the aqueous phase was 0.05.
[0031] (B) Evaporating the white emulsion at 30°C to remove the organic phase to obtain a light blue opalescent suspension;
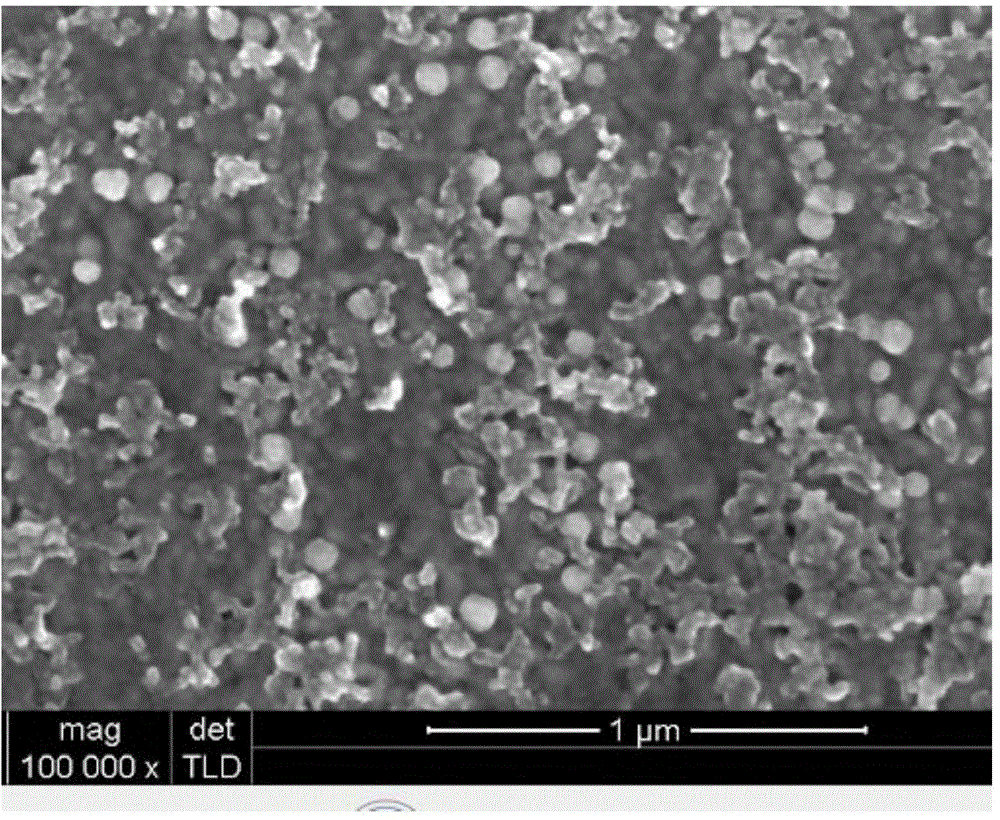
[0032] (C) The light blue opalescent suspension was centrifuged to obtain a precipitate, and the precipitate was washed, ultrasonically dispersed, and vacuum freeze-dried to obtain ropivacaine nanoparticles RVC-PLGA-NPS.
Embodiment 2
[0034] The preparation method of ropivacaine nanoparticles is as follows:
[0035](A) dissolving ropivacaine hydrochloride in water to make a saturated aqueous solution, adding ammonia water to obtain a precipitate, washing and drying the precipitate to obtain ropivacaine free base;
[0036] (B) dissolving ropivacaine free base and polylactic acid polyglycolic acid block copolymer in dichloromethane as the organic phase, the mass percent concentration is 1% polyvinyl alcohol solution as the water phase, and the organic phase is slowly Add dropwise into the water phase and ultrasonically treat at 5°C to obtain a white emulsion, in which the mass concentration of PLGA in the organic phase is 75 mg / ml, the mass concentration of RVC is 17 mg / ml, and the oil-water volume ratio of the organic phase to the water phase is 0.1.
[0037] (C) Evaporate the white emulsion at 40°C with a rotary evaporator to remove the organic phase to obtain a light blue opalescent suspension, and the ro...
Embodiment 3
[0040] The preparation method of ropivacaine nanoparticles is as follows:
[0041] (A) Dissolve ropivacaine hydrochloride in water to make a saturated aqueous solution, add ammonia water with a mass concentration of 0.9g / ml to completely precipitate to obtain a precipitate, wash the precipitate to neutrality, and then store it at 42°C Dry to constant weight to get ropivacaine free base;
[0042] (B) dissolving ropivacaine free base and polylactic acid polyglycolic acid block copolymer in dichloromethane as organic phase, mass percent concentration is 1.5% polyvinyl alcohol solution as water phase, and organic phase slowly Add dropwise into the water phase and ultrasonically treat at 2°C to obtain a white emulsion, in which the mass concentration of PLGA in the organic phase is 65 mg / ml, the mass concentration of RVC is 15 mg / ml, and the oil-water volume ratio of the organic phase to the water phase is 0.08.
[0043] (C) Evaporate the white emulsion at 37° C. with a rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com