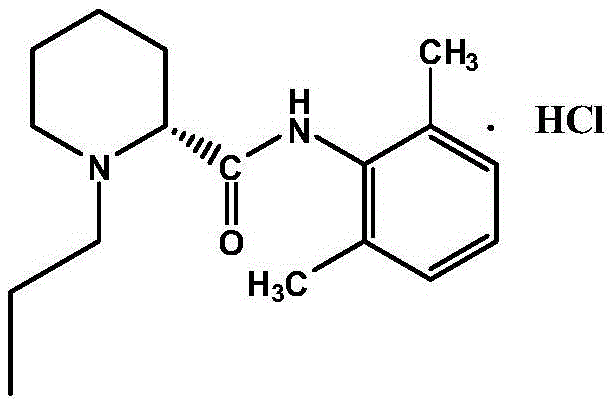
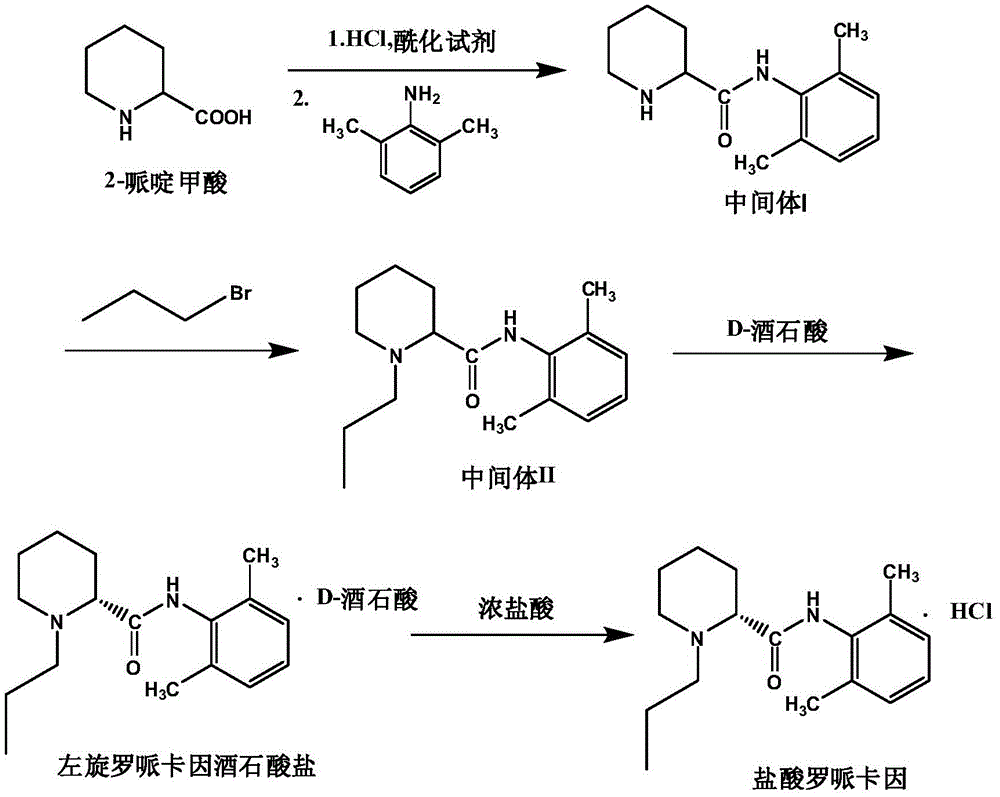
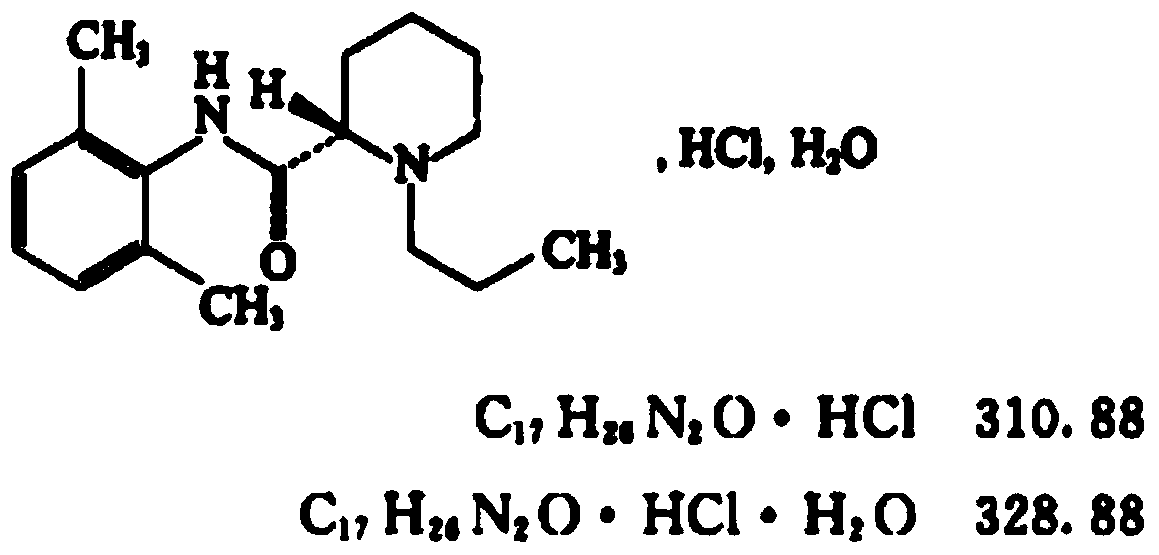
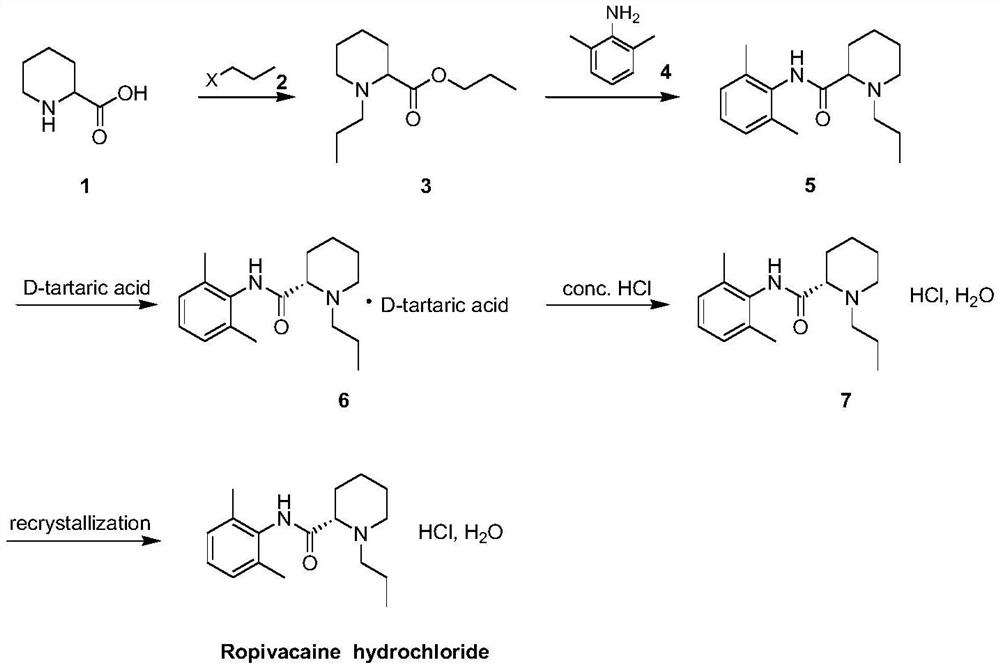
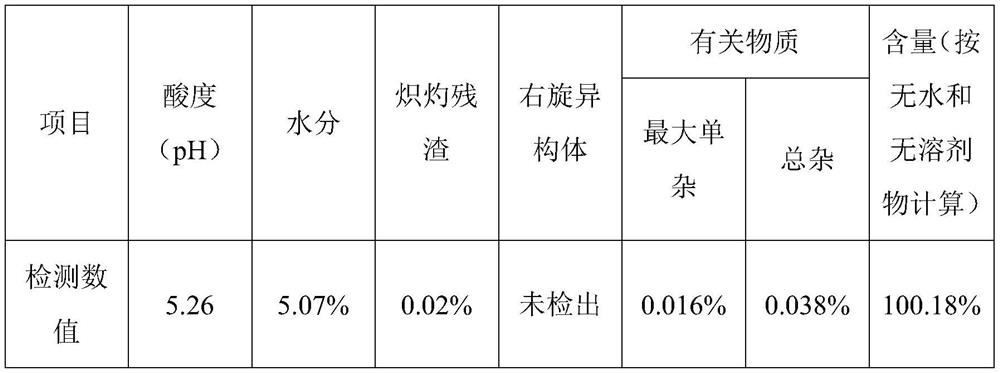
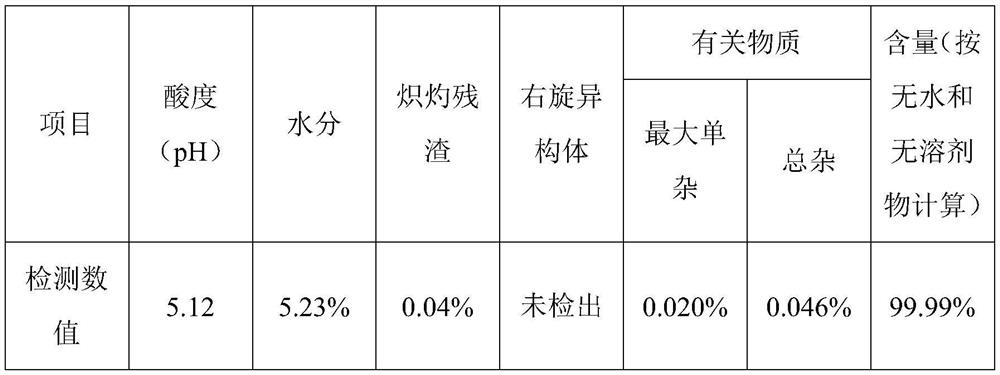
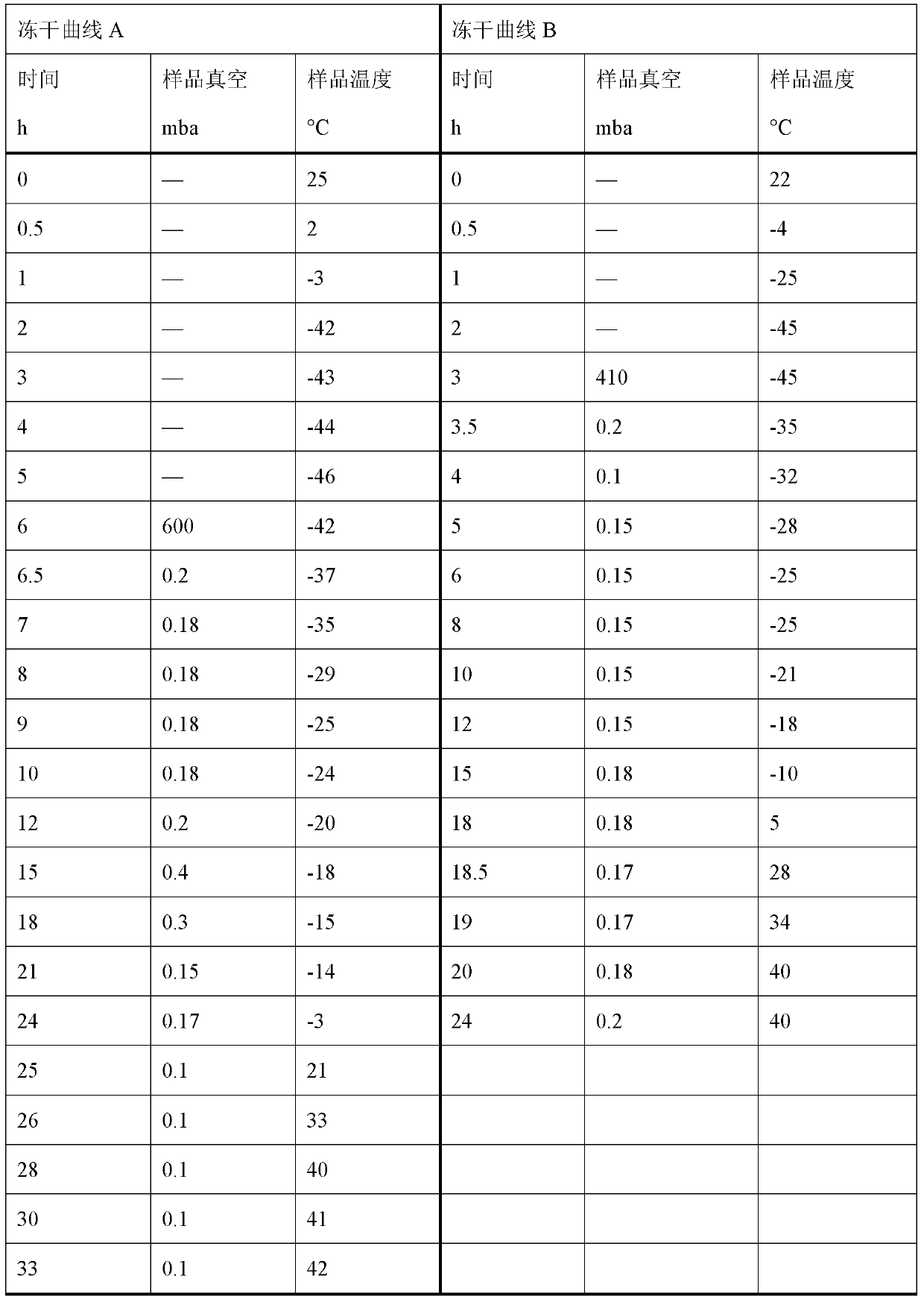
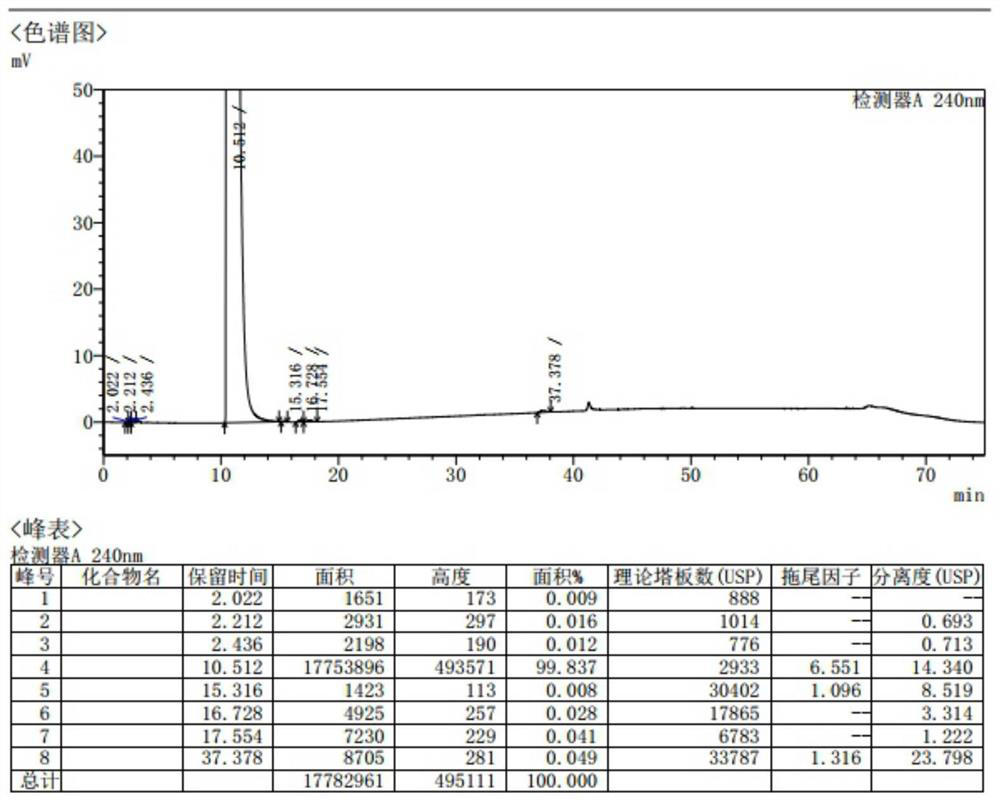
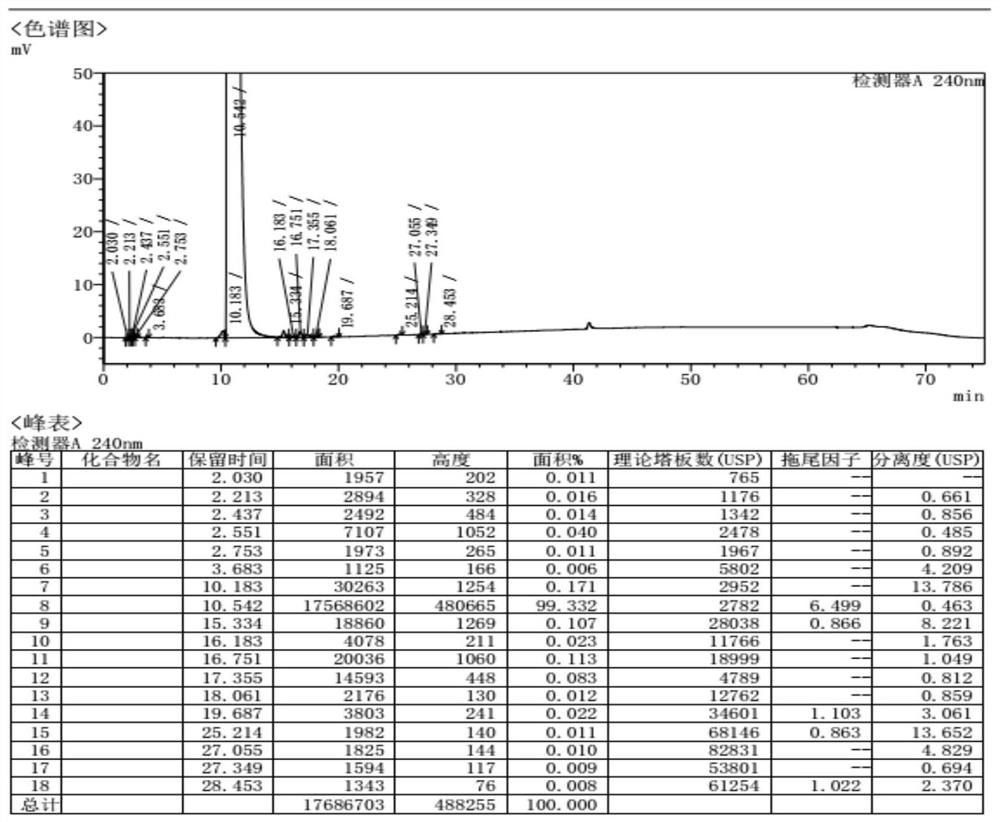
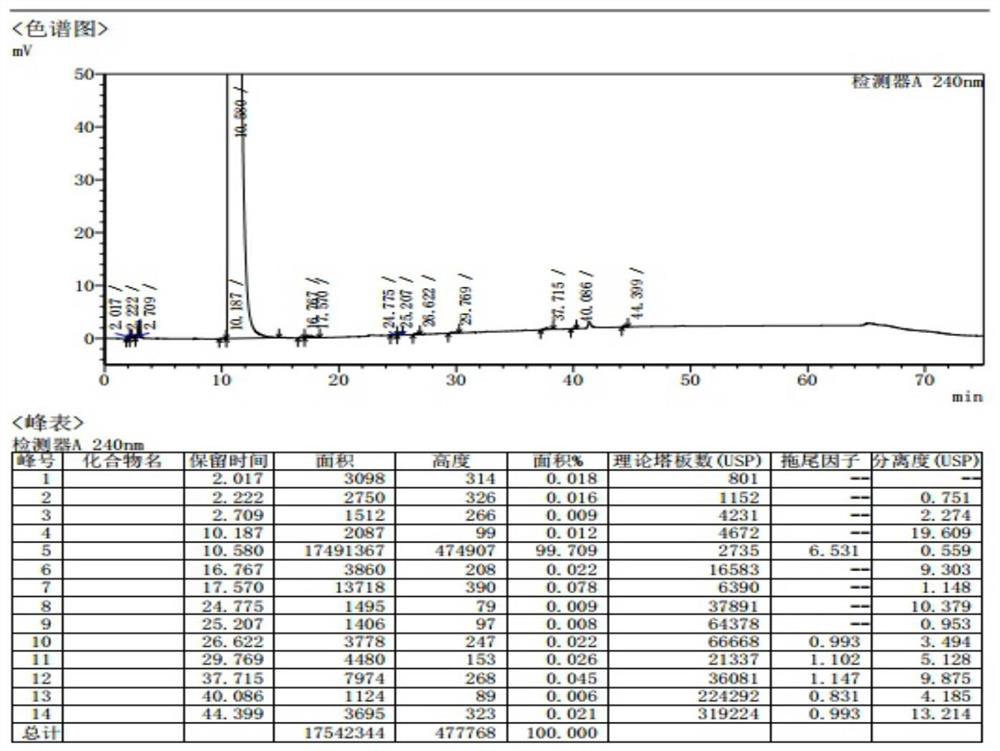
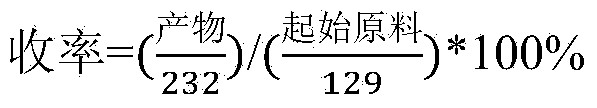Patents
Literature
38 results about "Ropivacaine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
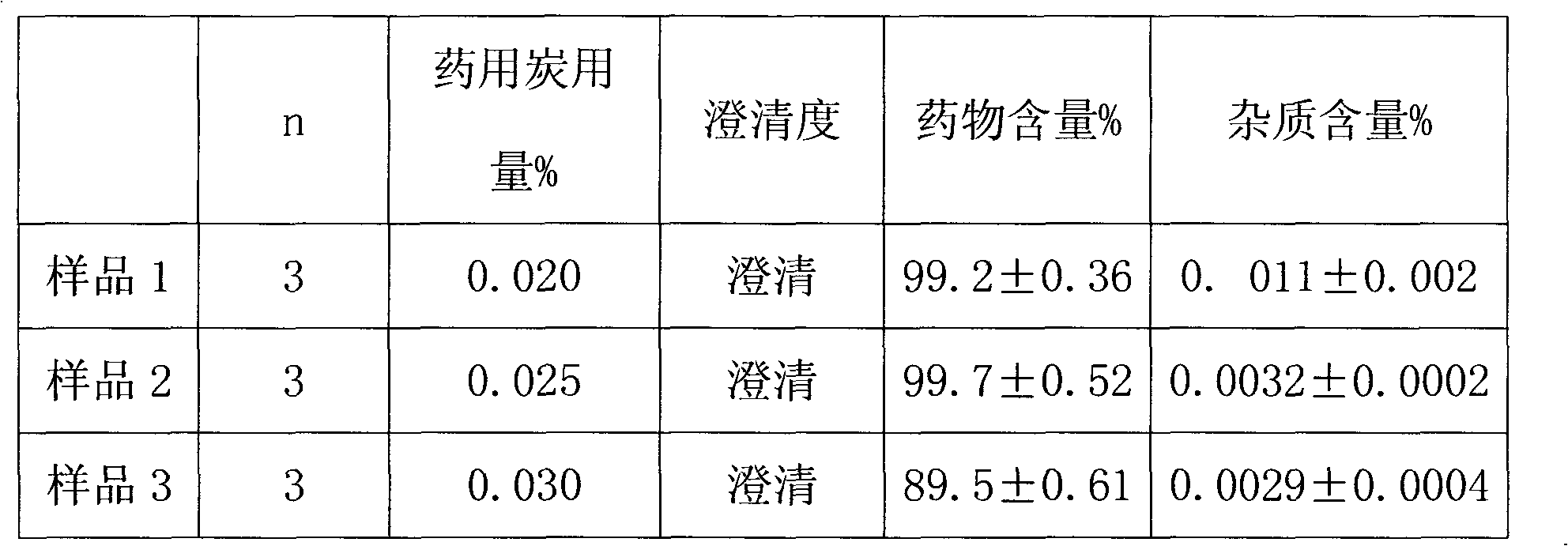
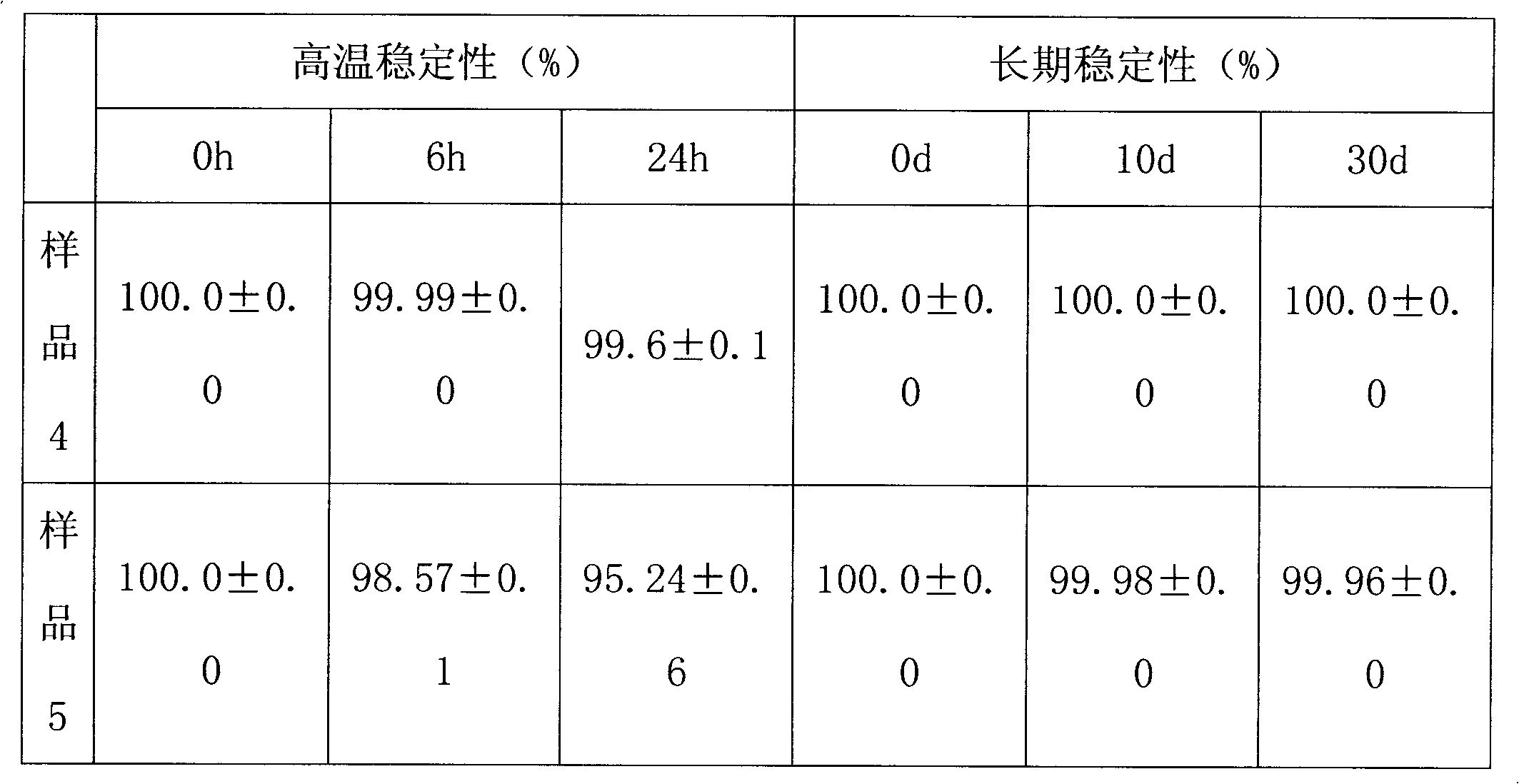
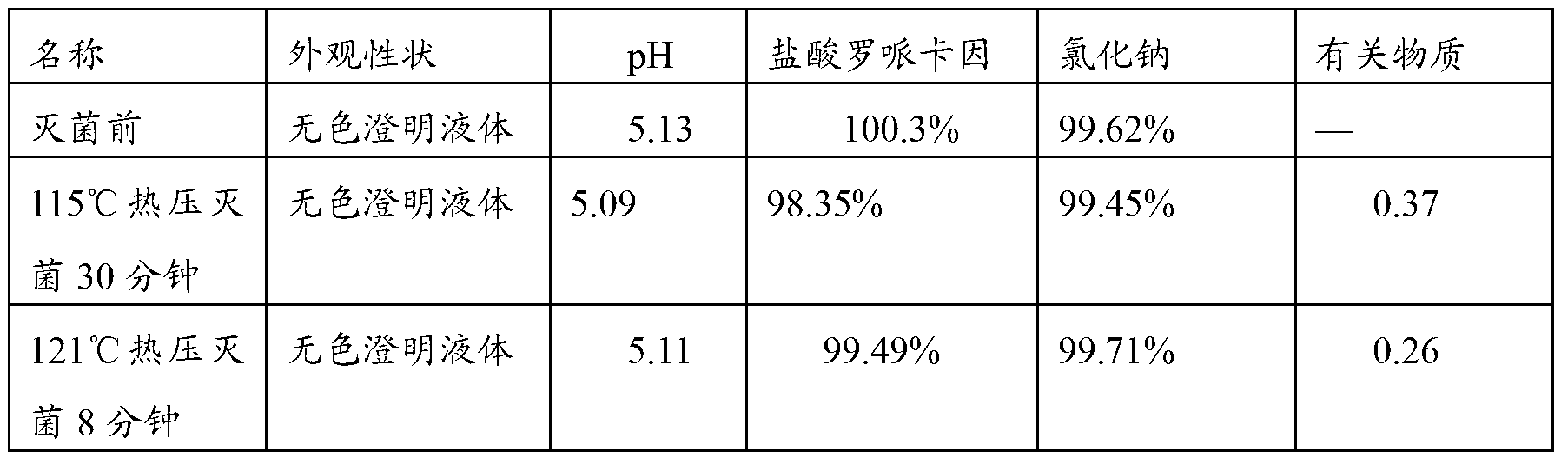
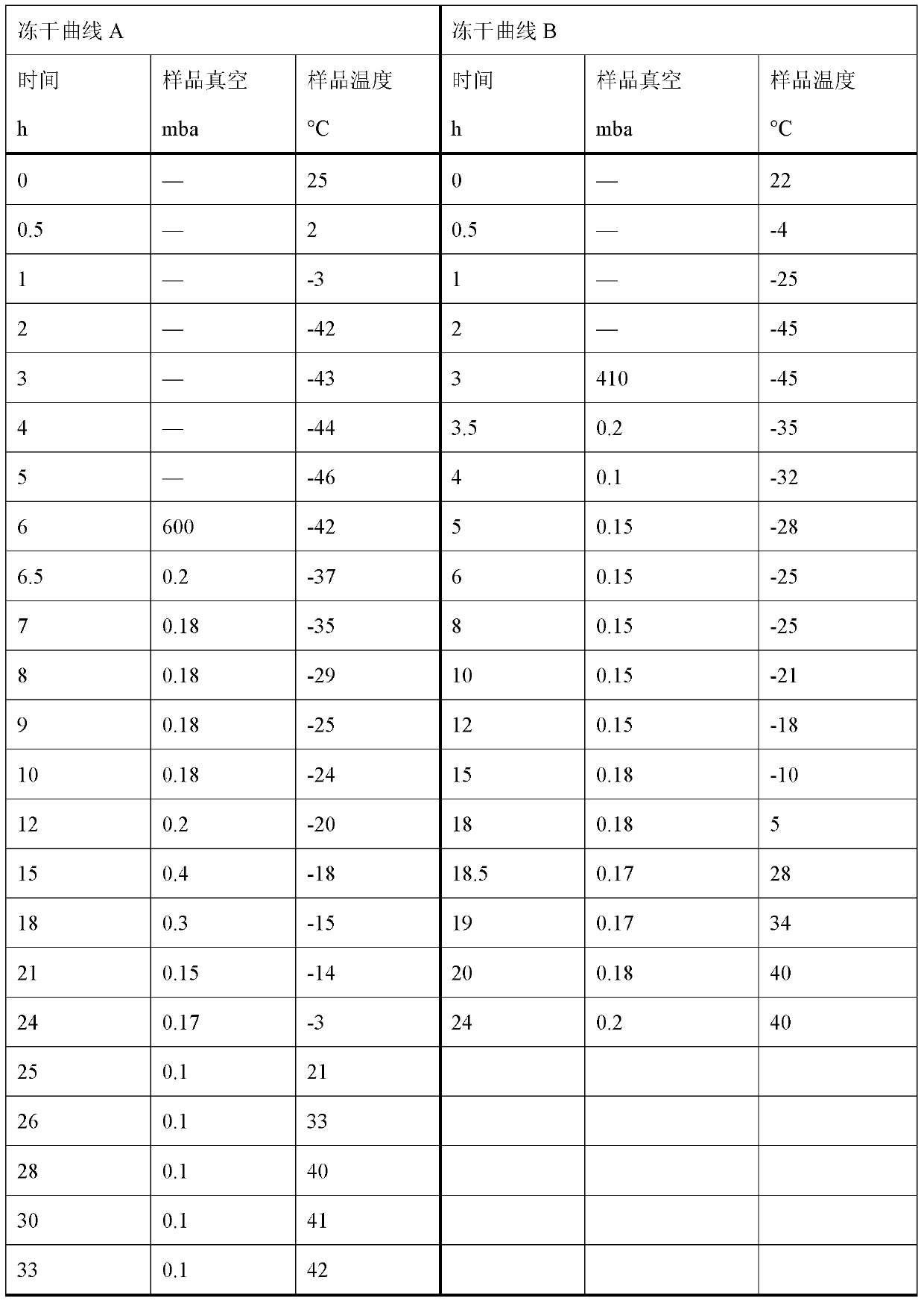
Ropivacaine has been used for up to 24 hours in clinical trials {01} {02}. A cumulative dose of 770 mg in 24 hours for postoperative pain management is well tolerated by most adults {01} {02}. Parenteral Dosage Forms ROPIVACAINE HYDROCHLORIDE INJECTION
High-safety ropivacaine hydrochloride injection and preparation method thereof
ActiveCN102552126AImprove adsorption capacityGuaranteed physical stabilityPharmaceutical delivery mechanismAnaestheticsDrug contentInjection solution
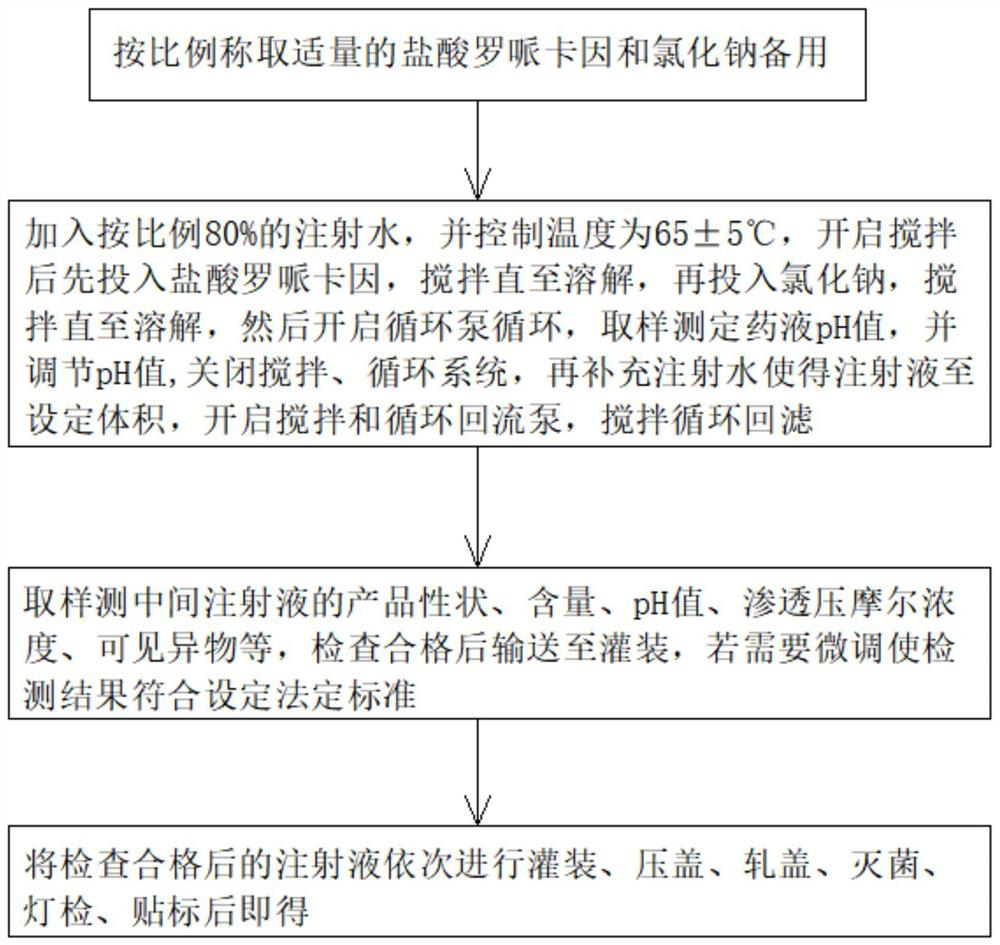
The invention relates to a high-safety ropivacaine hydrochloride injection and a preparation method of the high-safety ropivacaine hydrochloride injection. The formula of the high-safety ropivacaine hydrochloride injection comprises 20-200g of ropivacaine hydrochloride, 70-100g of sodium chloride, appropriate amount of sodium hydroxide or hydrochloric acid and 10000ml of water for injection. The formula is prepared into 1000 injections, and the pH value of the injection is 4.0-6.0. The injection has good stability, high drug content and safe and reliable effect.
Owner:GUANGDONG JIABO PHARM CO LTD
Ropivacaine freeze-dried powder and injection preparation in use for injection and preparation method
InactiveCN1626081AAvoid decompositionReduce moisture contentPowder deliveryAnaestheticsFreeze-dryingDextran
A freeze-dried injection of ropivacaine is prepared from the ropivacaine methanesulfonate (or hydrochloride), diluent chosen from mannitol, lactose, sodium chloride, dextran, glucose, glycine, hydrolytic gelatin and povidone, isotonic regulator and pH regulator through dissolving them in the water for injection, stirring, cooling, adding the water for injection, adding activated carbon, adsorption, filtering for removing carbon, fitlering by millipore filter and freeze drying.
Owner:BEIJING BOERDA BIO TECH DEV
Ropivacaine hydrochloride in use for injection and preparation technique
InactiveCN1660094ASimple compositionSmall toxicityPowder deliveryAnaestheticsFreeze-dryingCurative effect
A freeze-dried powder injection of ropivacaine hydrochloride is prepared from ropivacaine hydrochloride and the pharmacologically suporting materials including material, lactose, glucose and dextran. Its preparing process features use of low-temp aseptic vacuum spray drying for shortening time.
Owner:黄健鹏 +1
Method for preparing hydrochloric acid ropivacaine
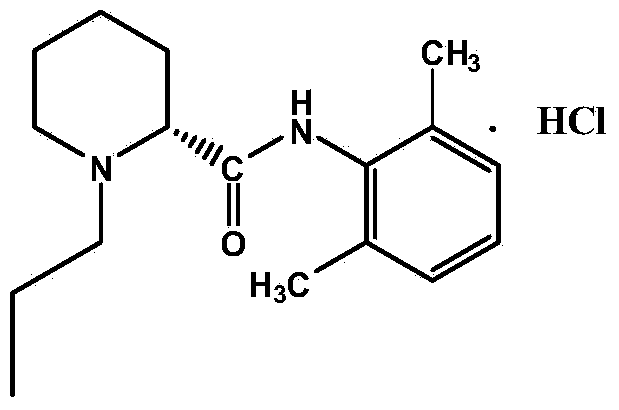
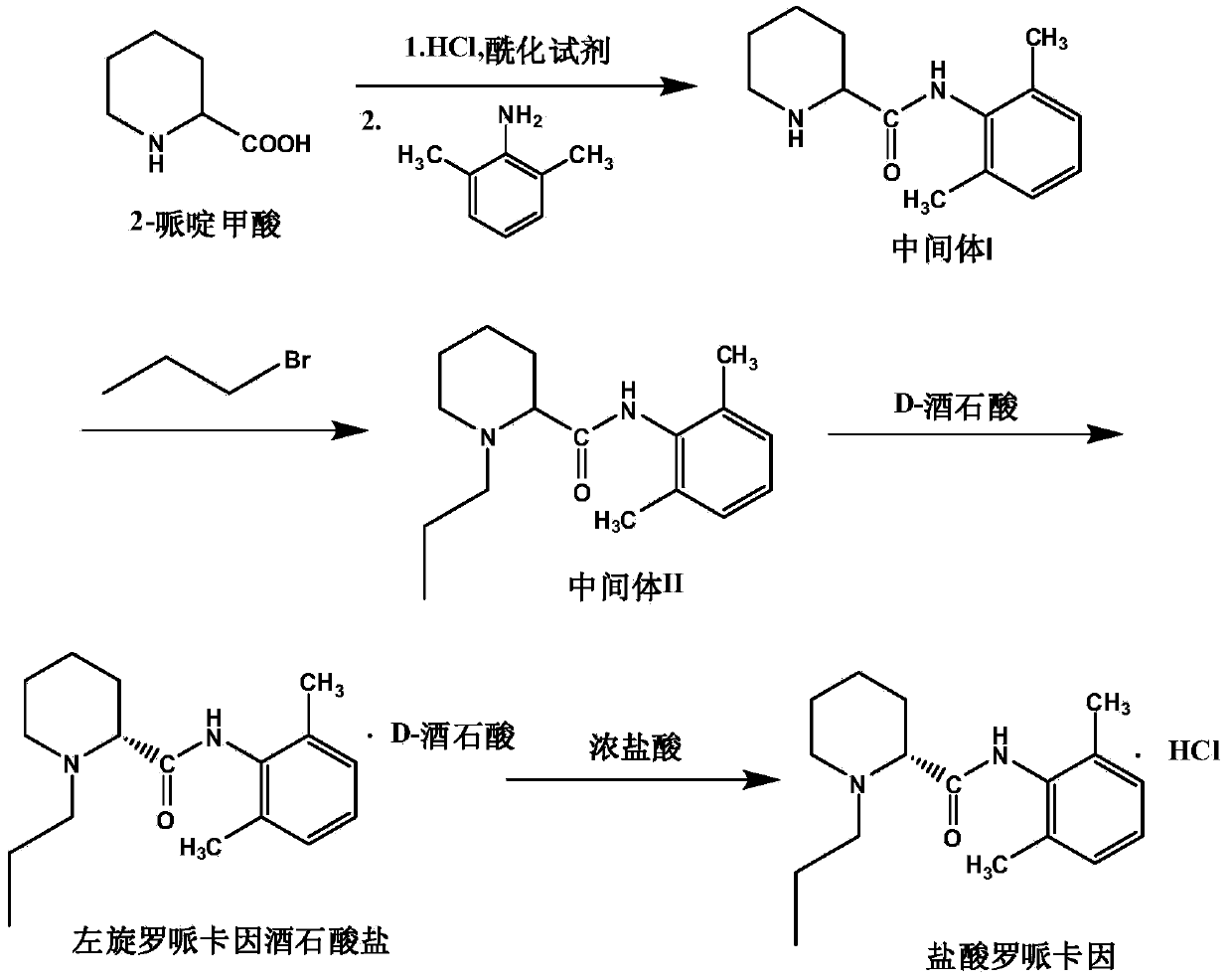
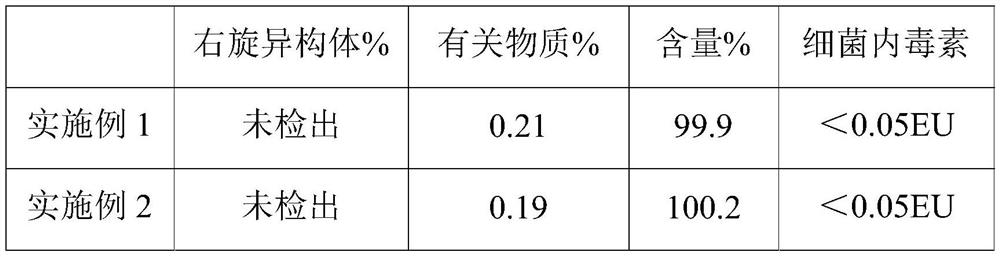
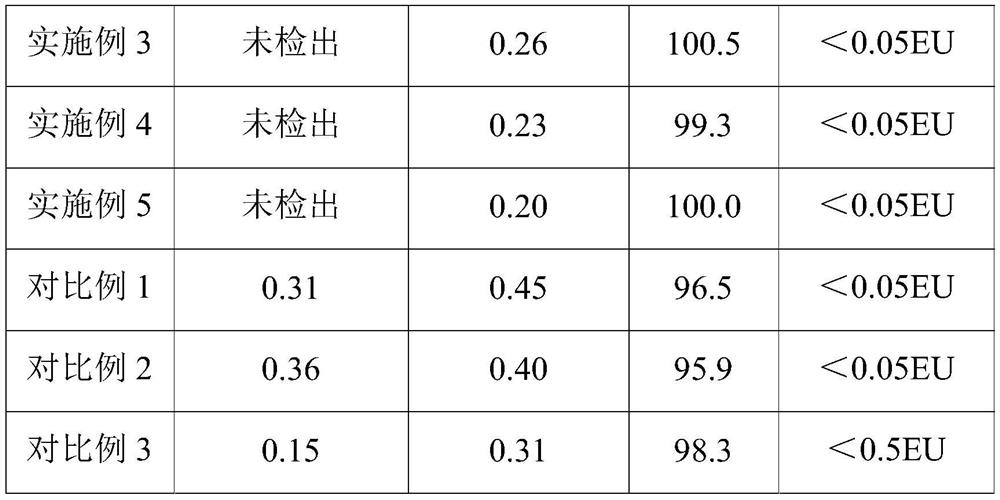
The invention provides a method for preparing hydrochloric acid ropivacaine. Part of parameters and conditions in the prior art are improved, and optimization is performed through the following steps that intermediate (I) separation pH and separation extracting solvent are selected; a catalyst and the usage quantity of the catalyst in a resolution agent are selected; refining solvent is selected. In this way, the yield and purity of the prepared hydrochloric acid ropivacaine are high, the purity reaches up to over 99% under the optimal condition, the percentage of dextrorotary isomer is reduced below 0.5%, standard requirements are completely met, and the hydrochloric acid ropivacaine is suitable for industrial production.
Owner:SHANDONG JINHE DRUG RES DEV
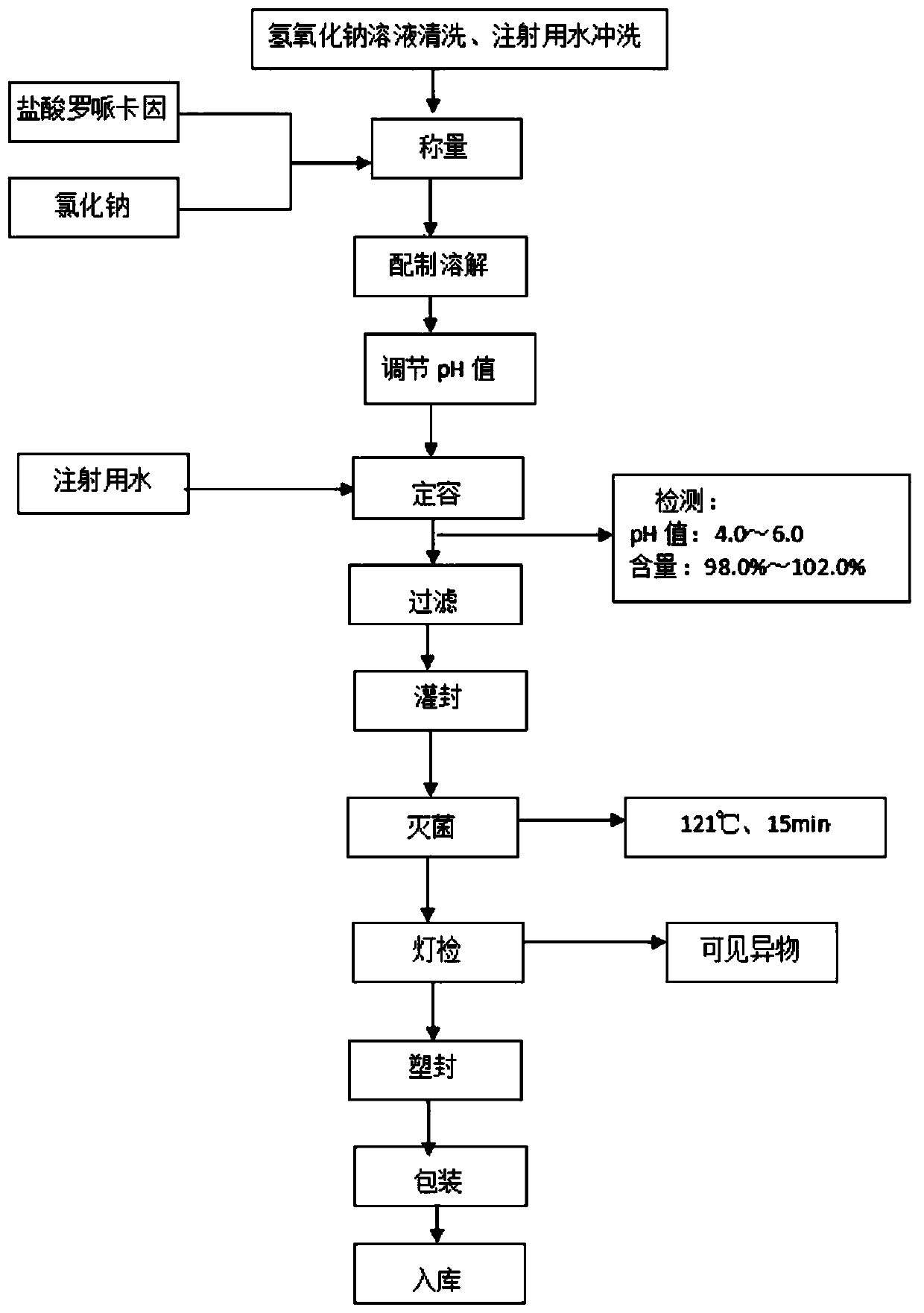
Ropivacaine hydrochloride sodium chloride injection and preparation method thereof
ActiveCN102670489AImprove sterilization effectImprove product qualityPharmaceutical delivery mechanismAnaestheticsMulti injectionFreeze-drying
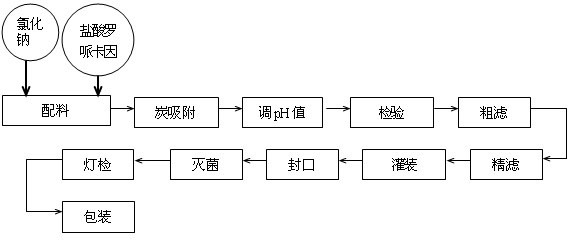
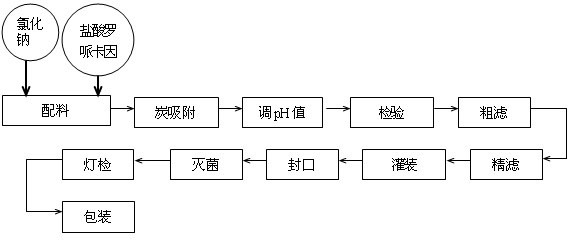
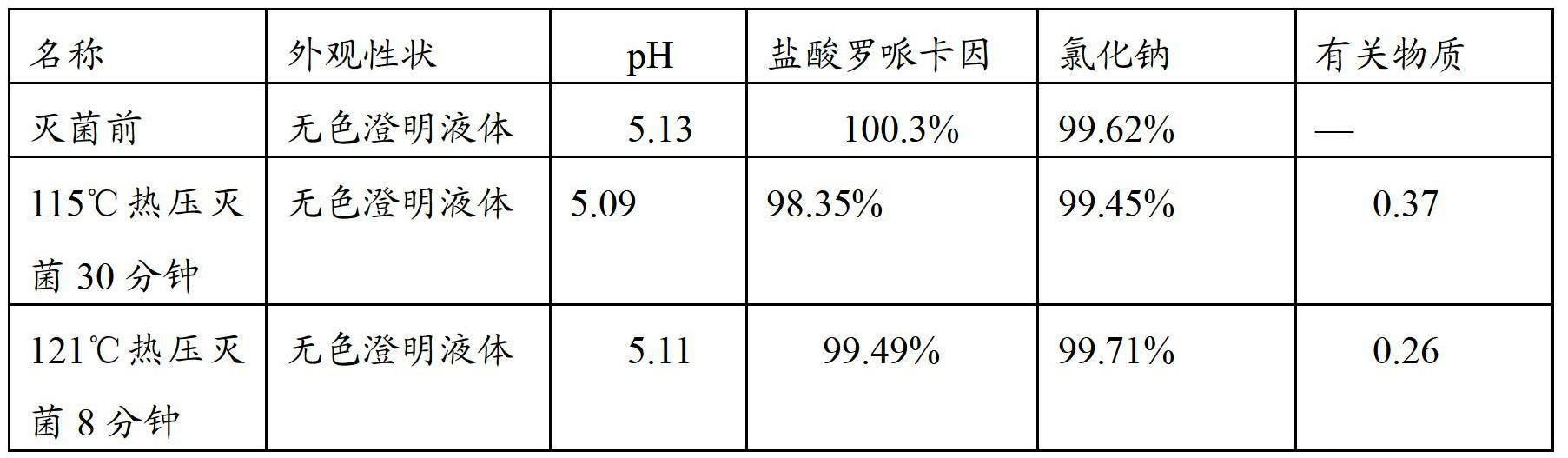
The invention provides a ropivacaine hydrochloride sodium chloride injection and a preparation method thereof. The method comprises the process steps of: burdening, carbon adsorption, rough filtering, fine filtering, filling, sterilizing, lamp detecting, packing and the like. The prepared ropivacaine hydrochloride sodium chloride injection has a definite medicinal effect and high safety; the testresult of each index in a stability test is accordant with specifications, and the defects of the requirement of repeated injection, inconvenience in using, easiness in increasing secondary pollutionby freeze-dried powder and the like existing in a ropivacaine water injection are overcome; the ropivacaine hydrochloride sodium chloride injection is particularly suitable for a patient using an analgesia pump; during use in a large volume, an instant stable concentration is kept for a medicinal liquid, so that a remarkable postoperative analgesic effect is achieved, and the requirement of an opioid medicament is remarkably lowered; and moreover, the effect can be enhanced rapidly by administrating in a self-pressing way.
Owner:HUAREN PHARMA (RIZHAO) CO LTD
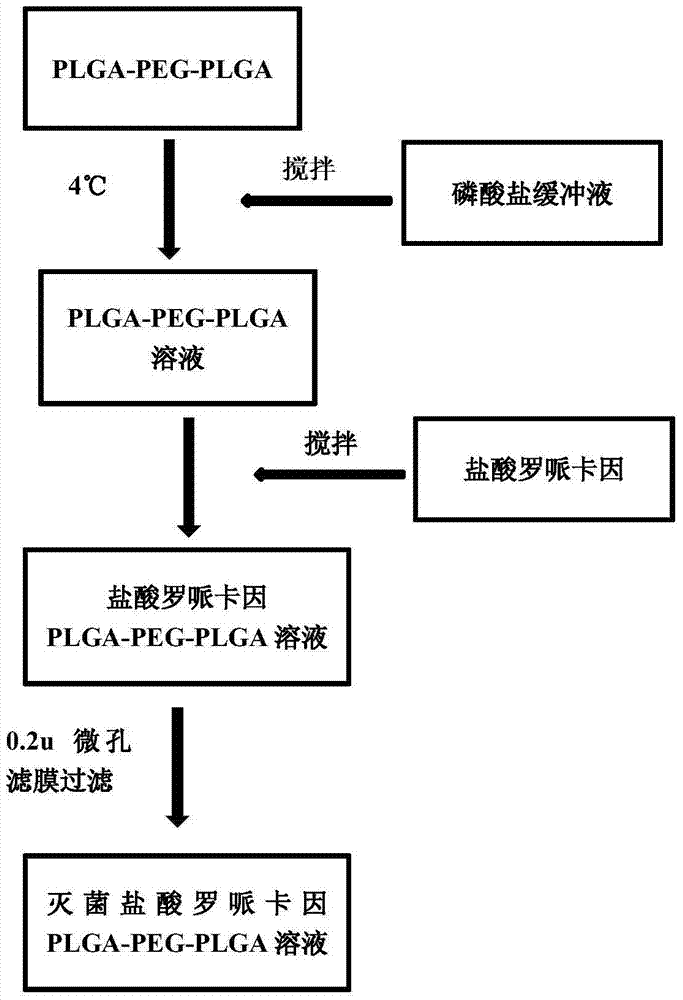
Temperature-sensitive gel containing ropivacaine and used for long-acting injection and preparation method of temperature-sensitive gel
ActiveCN104606129AEasy to administerSimple preparation processAntipyreticAerosol deliveryLactidePh regulation
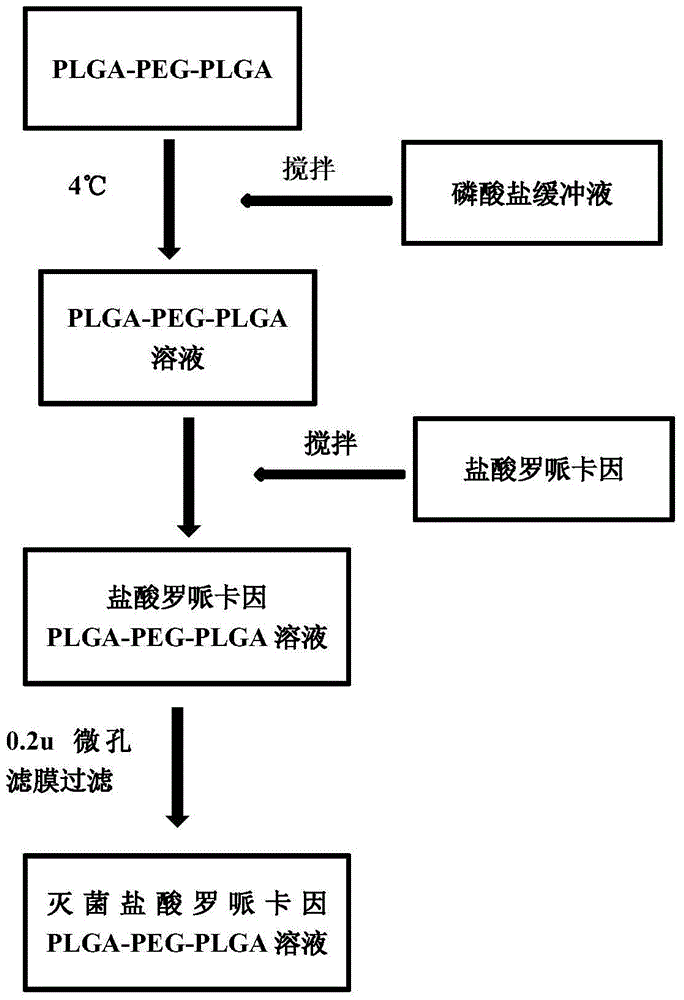
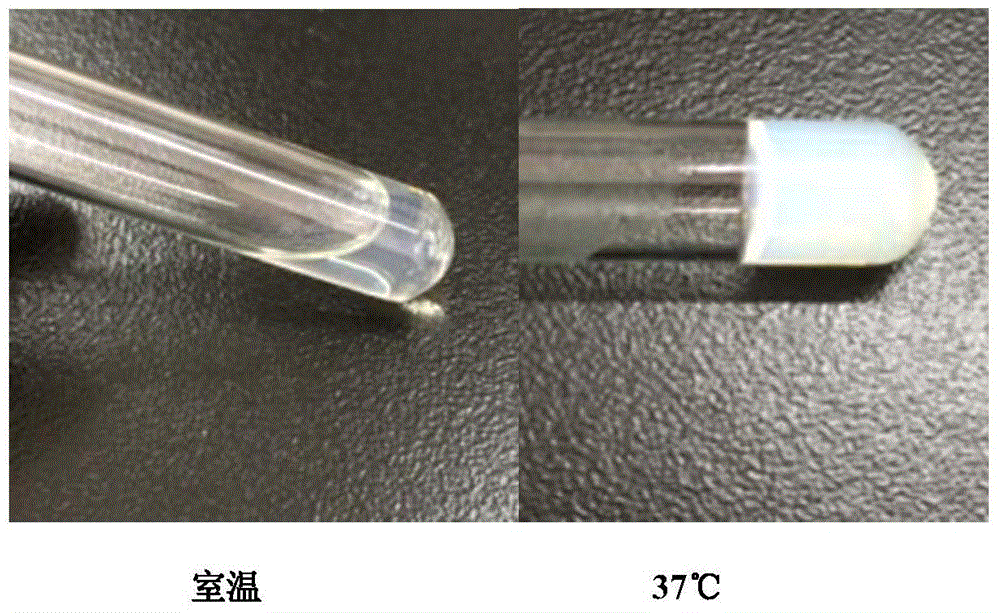
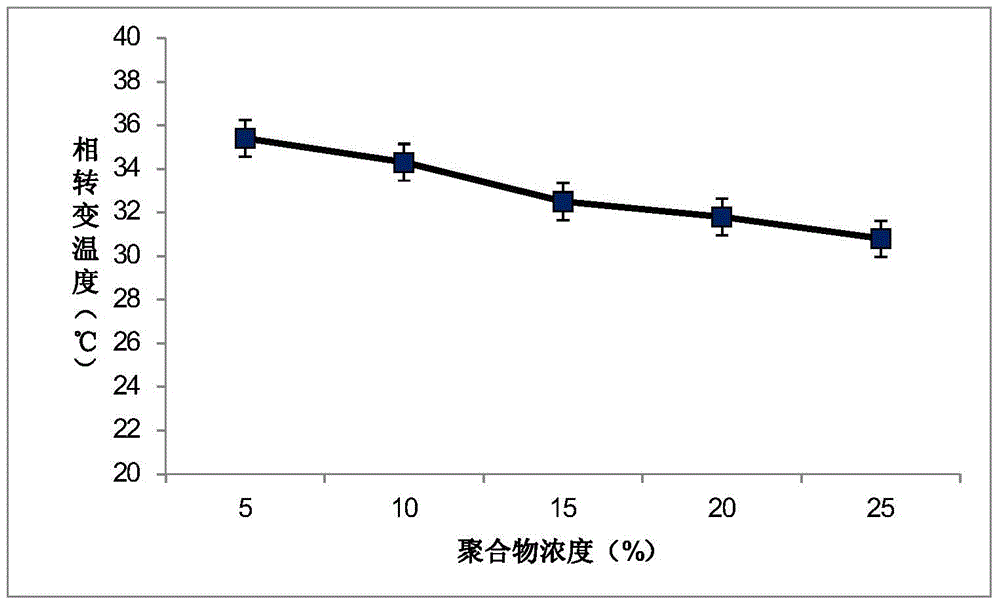
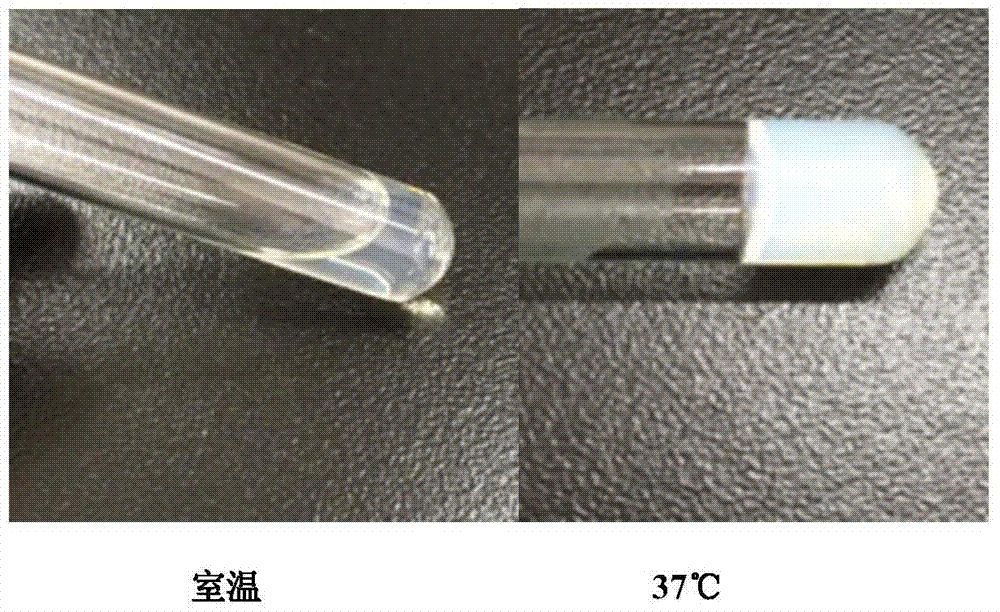
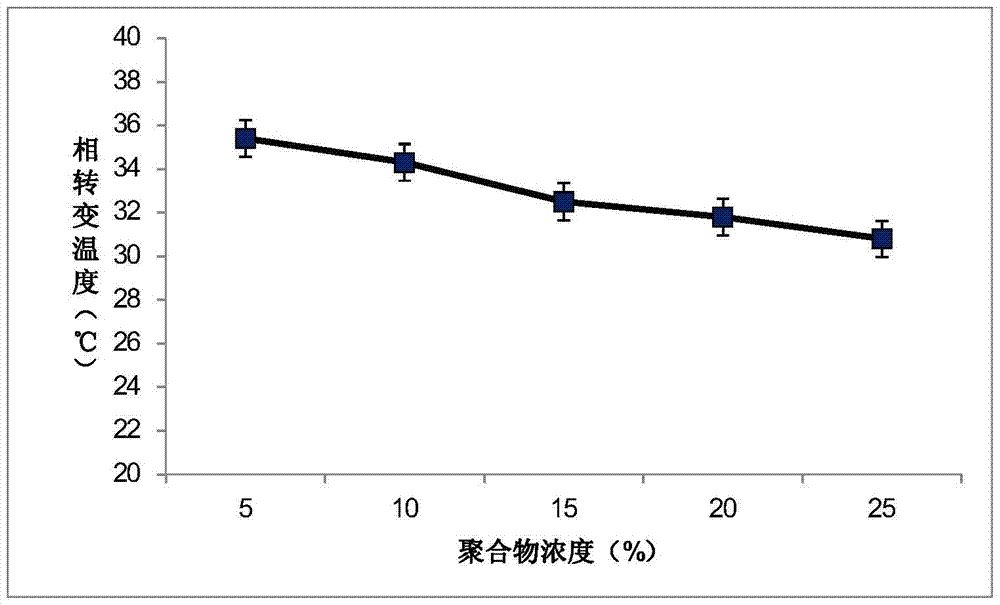
The invention discloses temperature-sensitive gel containing ropivacaine and used for long-acting injection and a preparation method of the temperature-sensitive gel. The gel comprises a main drug ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate, a PLGA (poly (lactic-co-glycolic acid)) -PEG (polyethylene glycol)-PLGA copolymer as well as water, normal saline or pH regulation solution for injection, wherein the mole ratio of lactide to glycolide is (2-3): 1, and the PEG molecular weight of the PLGA-PEG-PLGA copolymer is 1,000. The preparation method comprises the following steps: the copolymer is sufficiently swelled in a solvent firstly, then the main drug is added, and sterilization is performed through a filter membrane. The temperature-sensitive gel facilitates drug delivery and can gel rapidly at human body temperature so as to play a slow release role, smaller than or equal to 35%-45% of the drug is released in vitro after 12 h, larger than or equal to 65%-75% of the drug is released after 48 h, larger than or equal to 80% of the drug is released after 72 h, and the design requirement for postoperative continuous analgesia for 48 h through single injection of a local anesthesia drug is met. Pharmacodynamics study shows that a temperature-sensitive gel group containing ropivacaine can prolong the efficacy maintaining time remarkably and continuously play 48 h analgesic effect.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Hyperbaric injection solution of ropivacaine hydrochloride and process for preparation thereof
ActiveUS11224593B2Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsBiochemistryBaricity
Disclosed herein is hyperbaric solution for injection of Ropivacaine Hydrochloride which comprises Ropivacaine Hydrochloride; a base / acid to adjust the pH and a baricity adjuster to modify Baricity of the injection solution.
Owner:NEON LAB
Preparation method of ropivacaine hydrochloride
InactiveCN107325041AHigh purityHigh recovery rateOrganic chemistryMedicinal chemistryRopivacaine hydrochloride
The invention discloses a preparation method of ropivacaine hydrochloride. The preparation method comprises steps as follows: (1) preparation of an intermediate (I), (2) preparation of an intermediate (II), (3) preparation of a crude product and (4) refining. The preparation method has the advantages as follows: the provided preparation method adopts simple steps and is lower in cost and suitable for industrial production, and prepared ropivacaine hydrochloride has high purity and high recovery rate.
Owner:广州市桐晖药业有限公司
Local externally-applied antalgic compound preparation composition for skin and preparation method thereof
InactiveCN102846610AImprove complianceRelieve painAntipyreticAnalgesicsSide effectPharmaceutic Adjuvant
The invention relates to local externally-applied antalgic compound preparation composition for skin and a preparation method of the compound preparation composition. The compound preparation composition comprises the following components: (a) lidocaine hydrochloride; (b) ropivacaine hydrochloride monohydrate and conventional pharmaceutic adjuvant. The invention further relates to the preparation method of the compound preparation composition, which comprises the following steps of: mixing the lidocaine hydrochloride and the ropivacaine hydrochloride monohydrate through a conventional technological way and the conventional pharmaceutic adjuvant, and carrying out split charging to obtain the compound preparation composition. According to the compound preparation composition and the preparation method, the effect is significant, the efficacy time is long, the side effect is reduced, and the compliance of the patients is improved, so that the pain of the patients is alleviated. The compound preparation method composition provided by the invention can be applied to the pains caused by various factors, and has a good application prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Preparation and purification method of ropivacaine hydrochloride intermediate
ActiveCN109503465AEasy to operateHigh yieldOrganic chemistry methodsPurification methodsDimethylaniline N-oxide
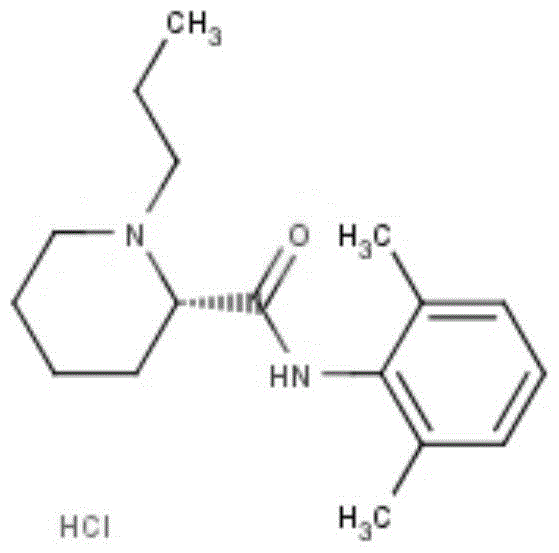
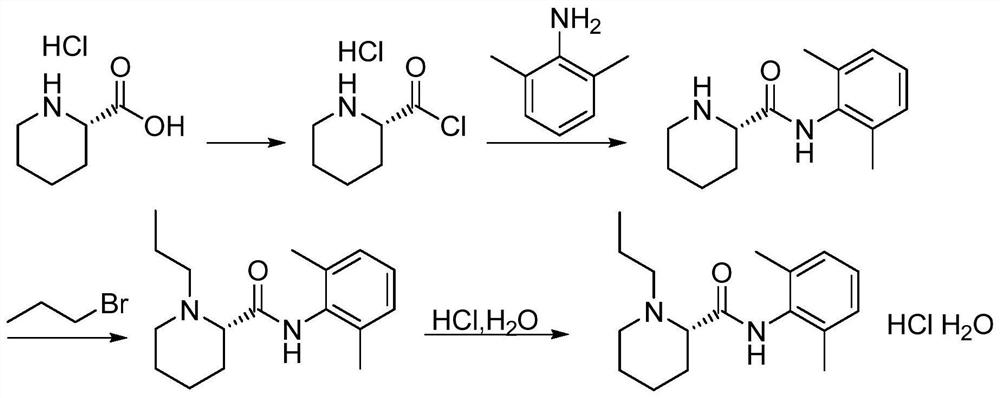
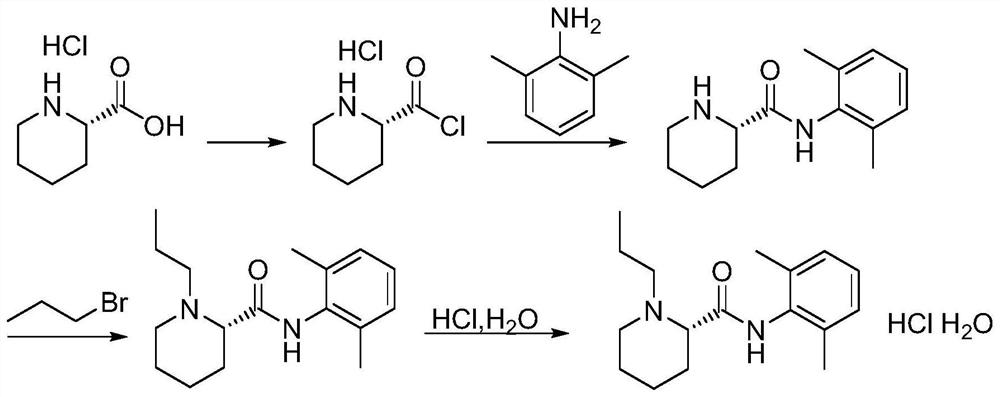
The invention relates to a preparation and purification method of a ropivacaine hydrochloride intermediate. According to the method, single chiral intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide is prepared from single chiral raw material L-pipecolic acid hydrochloride and 2,6-dimethylaniline, and a resolution step can be omitted; a nonpolar solvent is further adopted to refine the intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide, and related impurities are reduced. The preparation method is simple in aftertreatment, product purity is high, purity reaches 99.5% or higher after beating refining, content of single impurities is lower than 0.1%, the total yield is higher than 80%, and the method is suitable for large-scale industrial production.
Owner:HEBEI YIPIN PHARMA +1
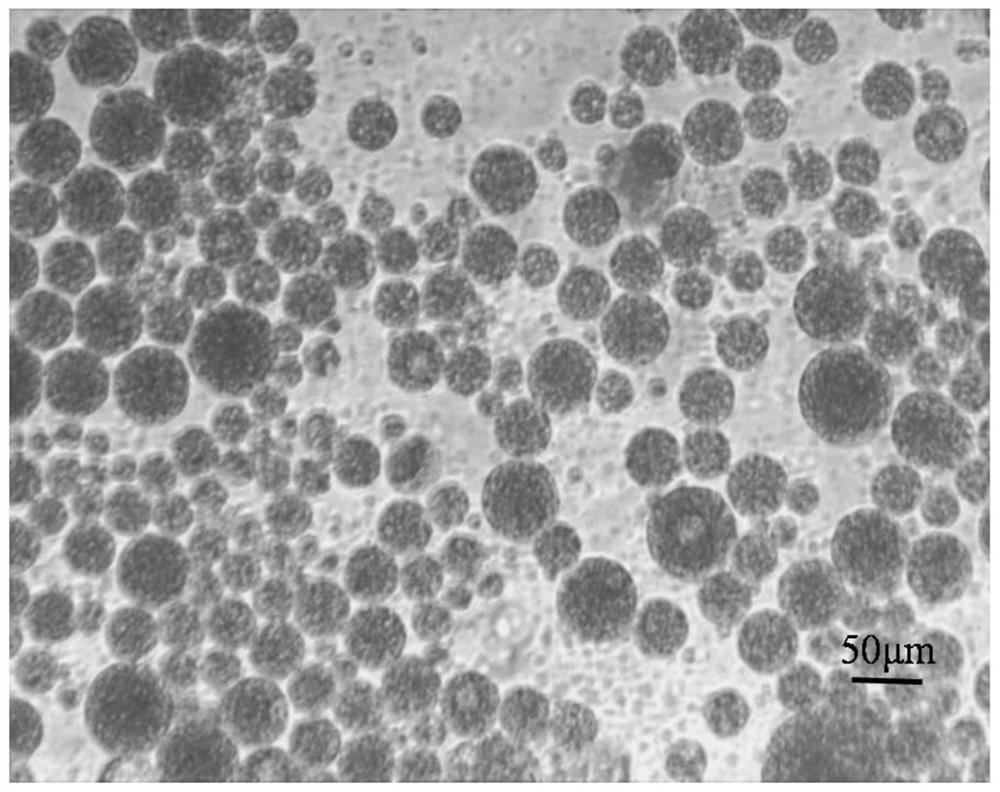
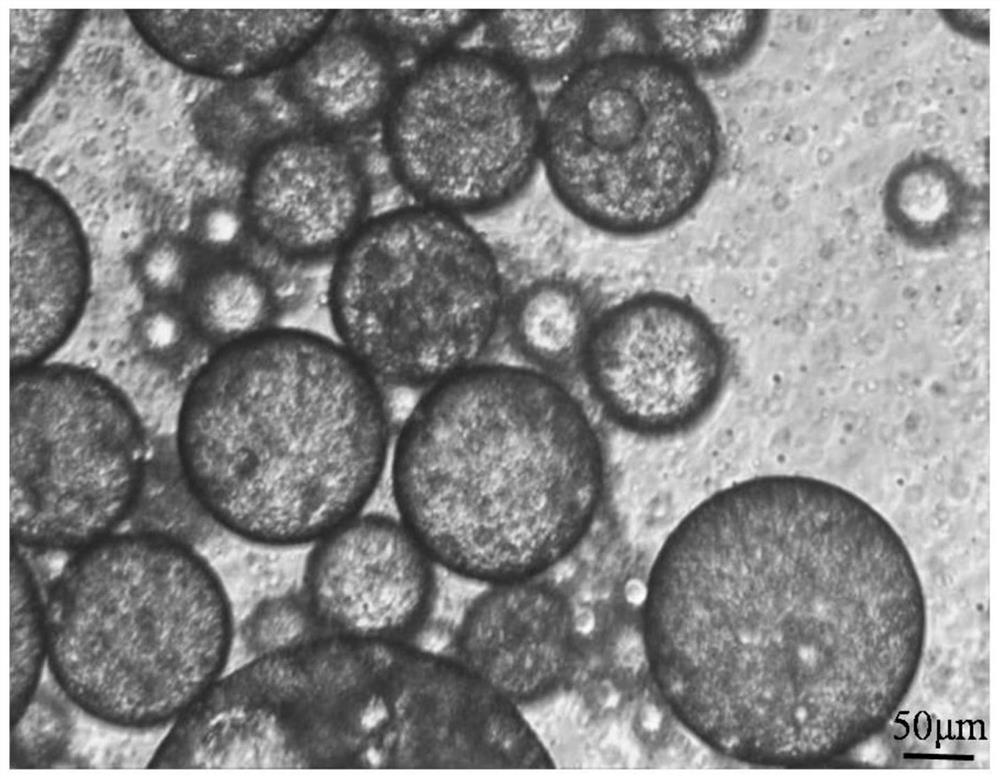
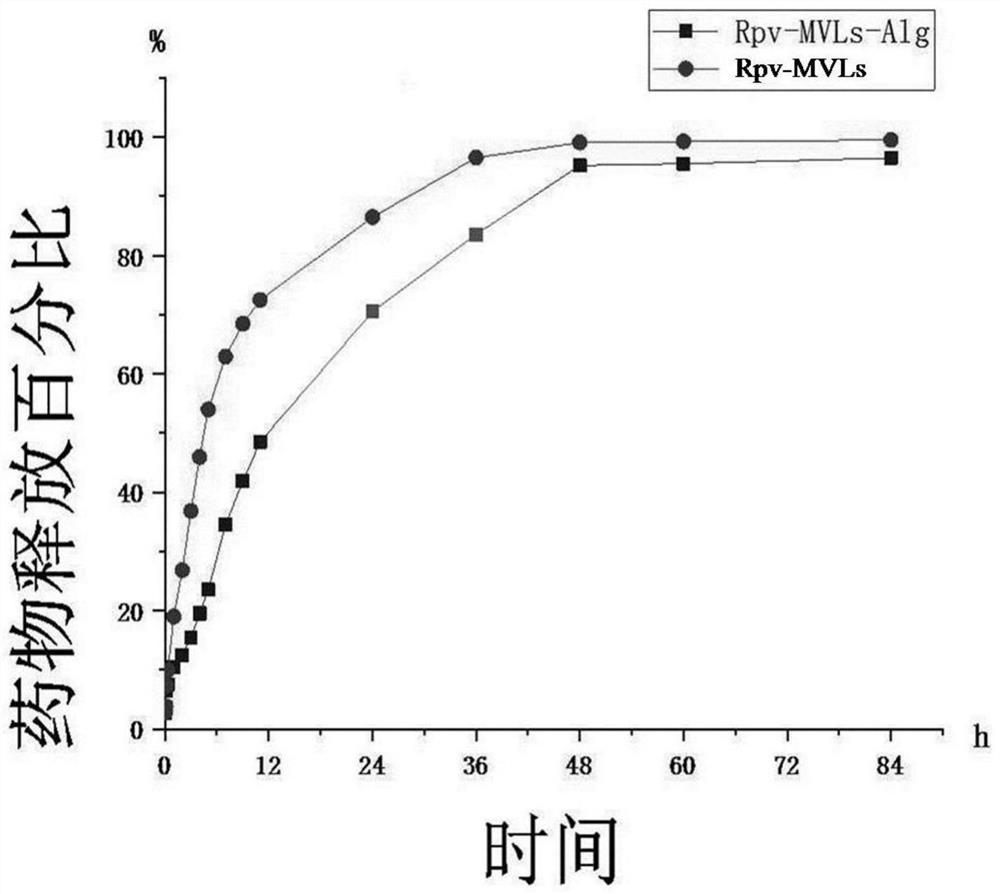
Sodium alginate modified ropivacaine hydrochloride multivesicular liposome microsphere as well as preparation method and application thereof
ActiveCN113171354AGuarantee structureEvenly dispersedAnaestheticsPharmaceutical non-active ingredientsMicrosphereOrganosolv
The invention discloses a sodium alginate modified ropivacaine hydrochloride multivesicular liposome microsphere as well as a preparation method and an application thereof. The preparation method comprises the following steps: firstly, uniformly mixing and dispersing a nano calcium carbonate gel initiator into liquid paraffin, adding multivesicular liposome under the condition of uniform stirring, then adding a sodium alginate solution, and uniformly mixing; and then adjusting the pH value to 3.0-5.8, and rapidly initiating gelation reaction by free calcium ions to form the uniform gel-coated multi-vesicular liposome microspheres with a net-shaped cross-linked structure. The multi-vesicular liposome microsphere has the characteristics of clear structure, uniform particle size, stable state, long slow release time and the like. Compared with an existing preparation method, the preparation method has the advantages that the encapsulation effect of the final multi-vesicular liposome is guaranteed, the surface of the multi-vesicular liposome is uniformly covered with the sodium alginate, the problem that the sodium alginate is connected into a sheet is solved, the stability of the multi-vesicular liposome is enhanced, and therefore the slow release effect is improved. Meanwhile, the use amount of chloroform in the organic solvent can be reduced.
Owner:SOUTH CHINA UNIV OF TECH
Hyperbaric injection solution of ropivacaine hydrochloride and process for preparation thereof
ActiveUS20190054075A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsBaricityInjection solution
Disclosed herein is hyperbaric solution for injection of Ropivacaine Hydrochloride which comprises Ropivacaine Hydrochloride; a base / acid to adjust the pH and a baricity adjuster to modify Baricity of the injection solution.
Owner:NEON LAB
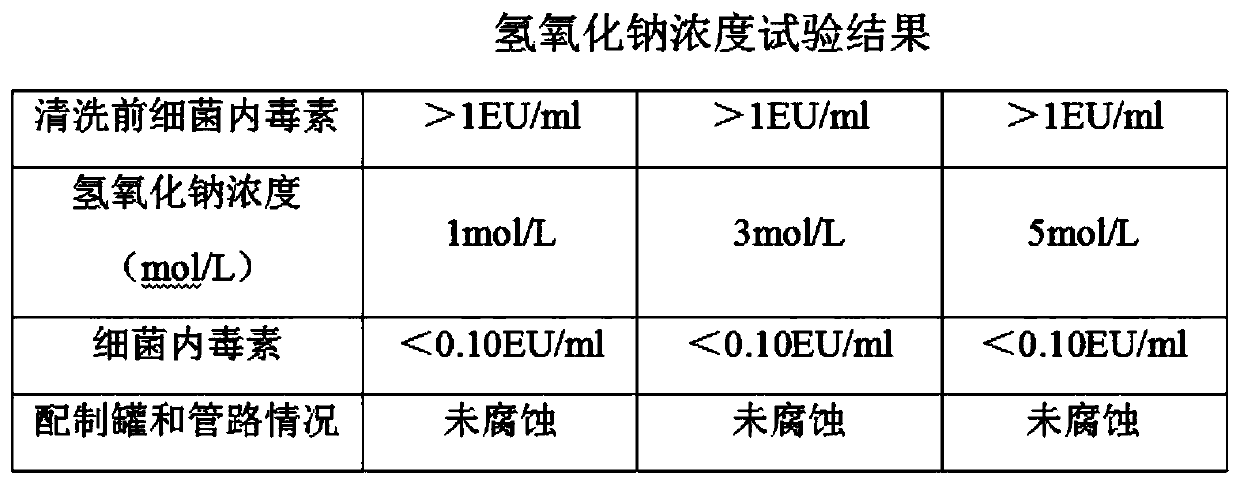
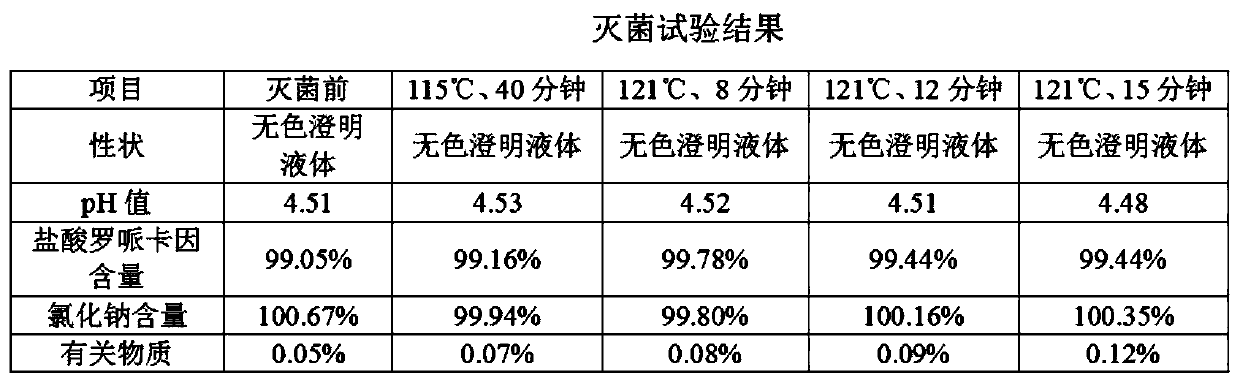
Ropivacaine hydrochloride and sodium chloride injection and preparation method
InactiveCN111067864AAvoid influenceReduce drug safetyInorganic non-active ingredientsPharmaceutical product form changeActivated carbonUse medication
The invention relates to the field of medicines, in particular to a ropivacaine hydrochloride and sodium chloride injection and a preparation method. The ropivacaine hydrochloride and sodium chlorideinjection is prepared from ropivacaine hydrochloride, sodium chloride and water for injection. The preparation process is mature, the product quality is stable and the use safety is high. Influences of activated carbon on element impurities of an injector, on insoluble microparticles of the injector and the content of a main drug of the injector are avoided, besides, the medication safety of a patient is greatly reduced, and various risks caused by an activated carbon process are avoided.
Owner:济南康桥医药科技有限公司
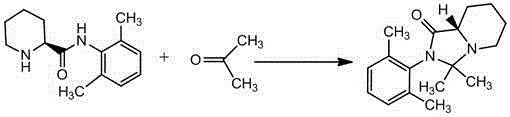
Preparation method of ropivacaine hydrochloride impurity F
ActiveCN105646482AHigh puritySolving quality control challengesOrganic chemistryChemical synthesisKetone
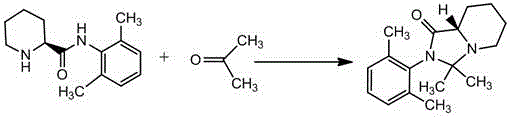
The invention belongs to the field of chemical synthesis technology, and concretely relates to a preparation method of a ropivacaine hydrochloride impurity F (8aS)-2-(2,6-dimethylphenyl)-3,3-dimethyl-imidazo[1,5-a]pyridine-1(5H)-ketone. (S)-N-(2',6'-dimethylphenl)-2-piperidine carboxamide and acetone are used as raw materials, a condensation reaction is carried out with an acidic condition in order to remove one water molecule, a solution is distilled, treatment is adjusted with alkaline, the impurity F crude product is obtained, then refining is carried out, and the high-purity ropivacaine hydrochloride impurity F is obtained. The method has the advantages of short synthetic route, simple operation, and high product purity; and a qualified impurity reference substance is provided for quality control of ropivacaine hydrochloride.
Owner:山东诚汇双达药业有限公司
Thermosensitive gel for ropivacaine long-acting injection and preparation method thereof
ActiveCN104606129BEasy to administerSimple preparation processAntipyreticAerosol deliveryLactideSingle injection
The invention discloses a temperature-sensitive gel for ropivacaine long-acting injection and a preparation method thereof. The gel comprises main ingredients ropivacaine, ropivacaine hydrochloride or ropivacaine mesylate; Glycolide molar ratio 2-3:1, PLGA-PEG-PLGA copolymer with PEG molecular weight of 1000, PEG accounts for 18-30% of the total mass of the copolymer; water for injection, normal saline or pH adjustment solution. The preparation method is to fully swell the copolymer in a solvent, add the main drug, and sterilize through a filter membrane. The temperature-sensitive gel is convenient for administration, gels rapidly at body temperature and exerts a slow-release effect. The drug release in vitro is ≤35-45%, 48 hours ≥65%-75%, and 72 hours ≥80%, which is in line with local anesthetics. After a single injection of continuous analgesia 48h design requirements. Pharmacodynamic studies have shown that the ropivacaine temperature-sensitive gel group can significantly prolong the maintenance time of the drug effect, and can continuously exert an analgesic effect for 48 hours.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Ropivacaine hydrochloride injection and preparation method thereof
InactiveCN105982848APharmaceutical delivery mechanismHeterocyclic compound active ingredientsBalance waterFood and drug administration
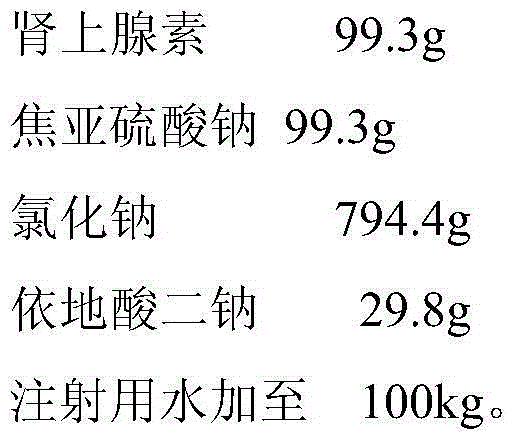
The invention provides a ropivacaine hydrochloride injection and a preparation method thereof. A formula of the ropivacaine hydrochloride injection contains 75 g of ropivacaine hydrochloride, 60 g of sodium chloride and the balance water for injection in 10000 ml injection. The quality of the ropivacaine hydrochloride injection meets the stipulations of 'Notification' issued by State Food and Drug Administration in 2008.
Owner:上海禾丰制药有限公司
Ropivacaine hydrochloride injection and preparation method thereof
The invention provides a ropivacaine hydrochloride injection and a preparation method thereof, and the method comprises the following steps: dissolving sodium chloride and ropivacaine hydrochloride in water for injection, and adding a pH regulator; under the protection of A-level laminar flow, sequentially carrying out blow molding by adopting a blowing-filling-sealing three-in-one mode to form an empty polypropylene ampoule, filling and sealing to obtain the ropivacaine hydrochloride injection; in the step of forming the empty polypropylene ampoule through blow molding, the heating, melting and extruding temperature of polypropylene particles is 190-230 DEG C, the supporting air flow is 1600-2700 L / min, and the forming time is 3-4 s. By limiting the extrusion temperature for preparing the polypropylene ampoule bottle, the supporting air flow and the forming time, all parameters and steps are matched with one another, the prepared polypropylene ampoule bottle is low in overall oxygen permeation rate, and the ropivacaine hydrochloride injection is good in stability.
Owner:SHIJIAZHUANG NO 4 PHARMA
A kind of ropivacaine hydrochloride injection and preparation method thereof
ActiveCN104208020BImprove stabilityImproved light and heat resistancePharmaceutical delivery mechanismPharmaceutical non-active ingredientsBiological propertyTrehalose
The invention relates to a ropivacaine hydrochloride injection and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The injection contains ropivacaine hydrochloride and a stabilizer, the mass ratio of the two is 1:1-1.6; the stabilizer is one of trehalose and trehalose sodium sulfate or a mixture of the two in any proportion . The ropivacaine hydrochloride injection of the present invention, due to the structural characteristics of trehalose and its unique biological characteristics, can inhibit the enantiomeric transformation of ropivacaine hydrochloride in aqueous solution, and enhance the strength of ropivacaine hydrochloride. The stability of the injection solves the problems of poor stability, light and heat resistance, and easy freezing of the ropivacaine hydrochloride injection, and obtains satisfactory technical results.
Owner:HEBEI YIPIN PHARMA
Ropivacaine hydrochloride impurity and preparation method thereof
ActiveCN111925317AGood anesthesiaEasy to operateOrganic chemistryChemical compoundPhysical chemistry
The invention provides a ropivacaine hydrochloride impurity and a preparation method thereof. The structural formula of the ropivacaine hydrochloride impurity is shown as a formula I, the ropivacainehydrochloride impurity disclosed by the invention is a new compound discovered by an inventor in a research process; the ropivacaine hydrochloride impurity has important significance in quality control in the ropivacaine hydrochloride synthesis process, and the inventor finds that the ropivacaine hydrochloride impurity with the structural formula shown in the formula I has excellent anesthetic performance through research and can be used for a new anesthetic drug. The method is simple to operate, the preparation method has the advantages of simple operation, mild conditions, realization of thereaction generation of the ropivacaine hydrochloride impurity only by mixing the reaction raw materials, no temperature control, low cost, no side reaction, high purity and high yield of the ropivacaine hydrochloride impurity prepared through the reaction, and facilitation of the reduction of the quality control analysis cost of ropivacaine hydrochloride.
Owner:GUANGDONG JIABO PHARM CO LTD
Ropivacaine hydrochloride sodium chloride injection and preparation method thereof
ActiveCN102670489BImprove sterilization effectImprove product qualityPharmaceutical delivery mechanismAnaestheticsAnalgesia postoperativeOpioidergic
The invention provides a ropivacaine hydrochloride sodium chloride injection and a preparation method thereof. The method comprises the process steps of: burdening, carbon adsorption, rough filtering, fine filtering, filling, sterilizing, lamp detecting, packing and the like. The prepared ropivacaine hydrochloride sodium chloride injection has a definite medicinal effect and high safety; the test result of each index in a stability test is accordant with specifications, and the defects of the requirement of repeated injection, inconvenience in using, easiness in increasing secondary pollution by freeze-dried powder and the like existing in a ropivacaine water injection are overcome; the ropivacaine hydrochloride sodium chloride injection is particularly suitable for a patient using an analgesia pump; during use in a large volume, an instant stable concentration is kept for a medicinal liquid, so that a remarkable postoperative analgesic effect is achieved, and the requirement of an opioid medicament is remarkably lowered; and moreover, the effect can be enhanced rapidly by administrating in a self-pressing way.
Owner:HUAREN PHARMA (RIZHAO) CO LTD
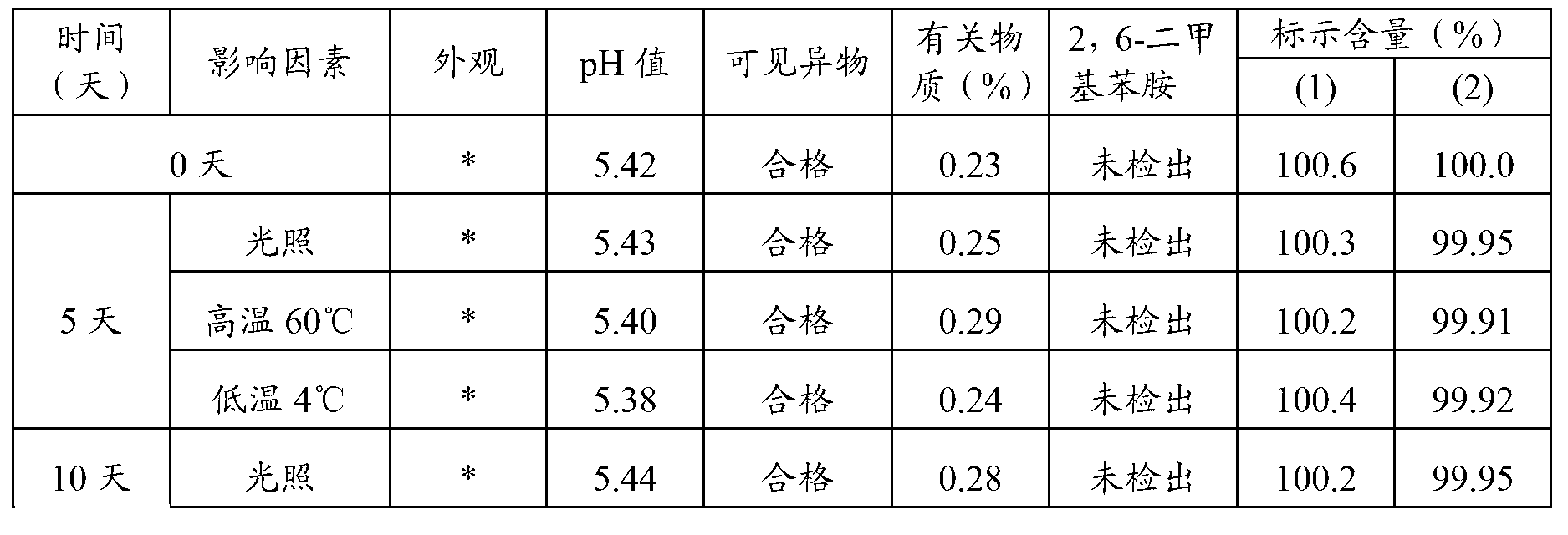
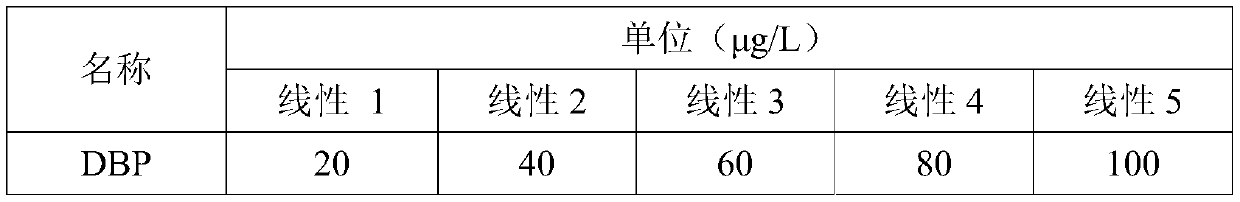
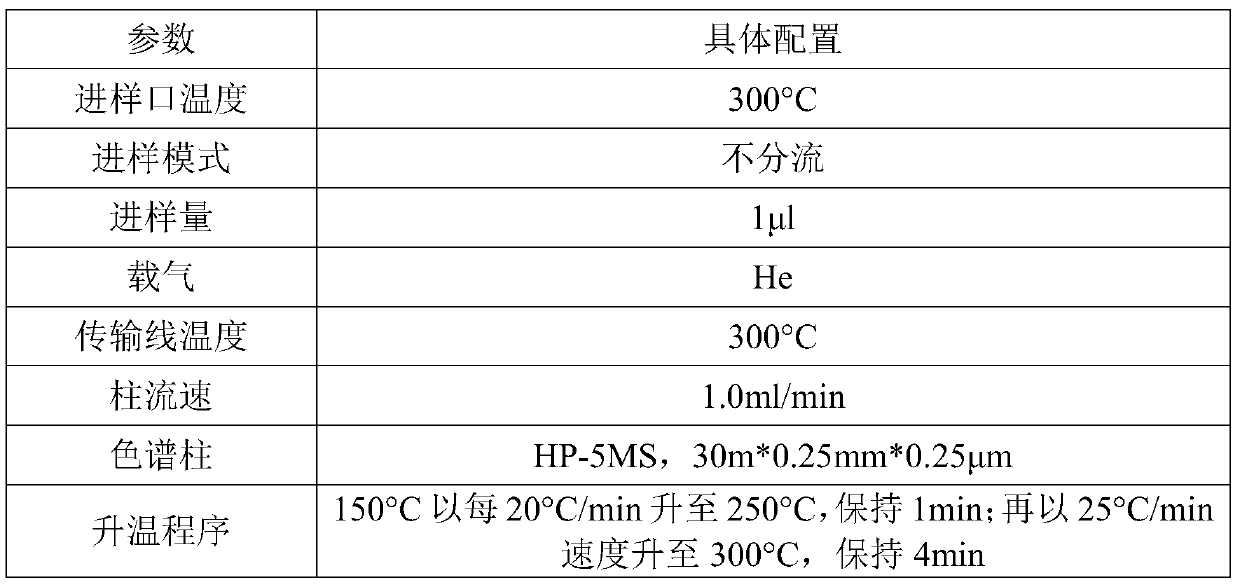
Anesthetic analysis method
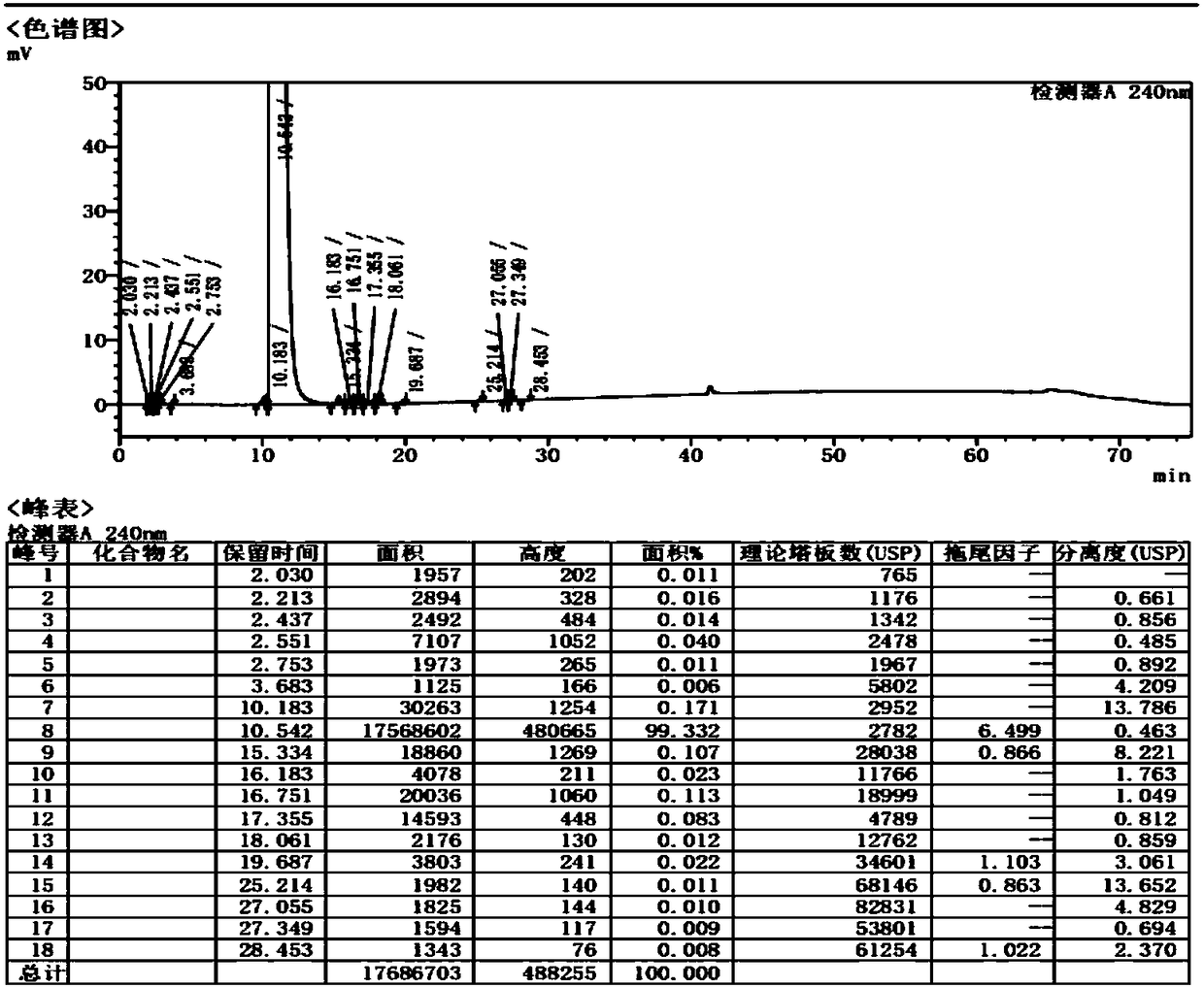
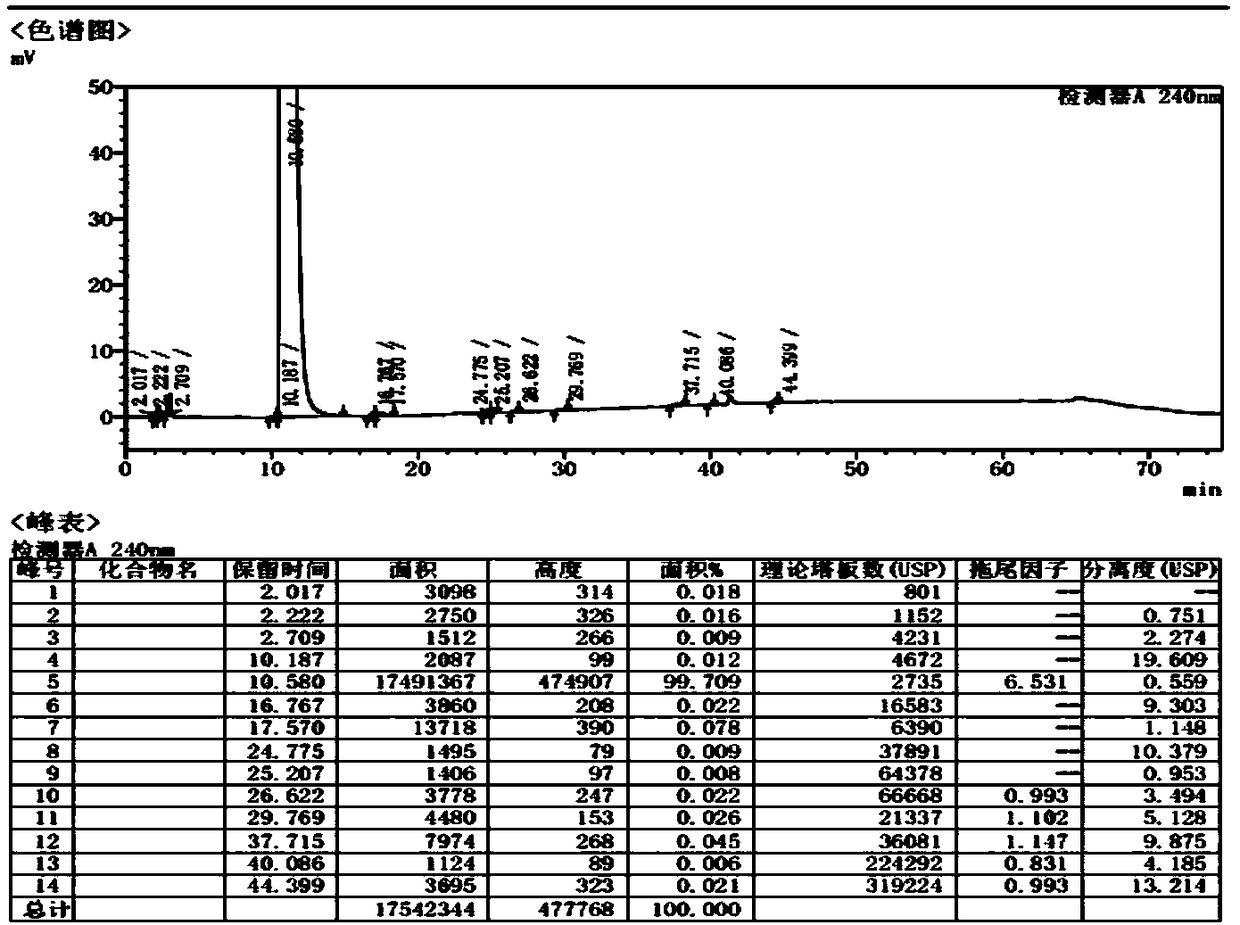
PendingCN111122746AAvoid SNR DegradationImprove accuracyComponent separationRelative standard deviationEngineering
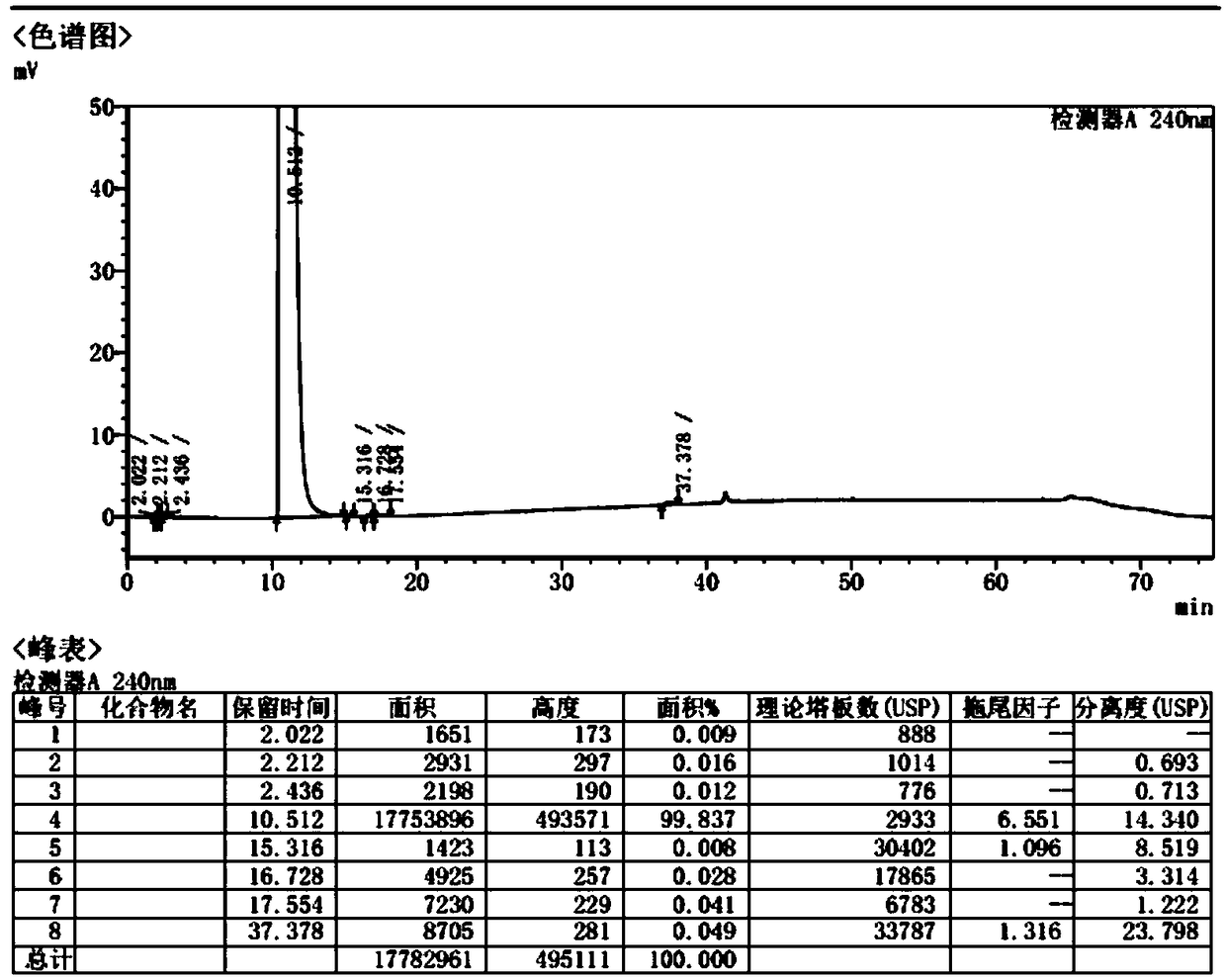
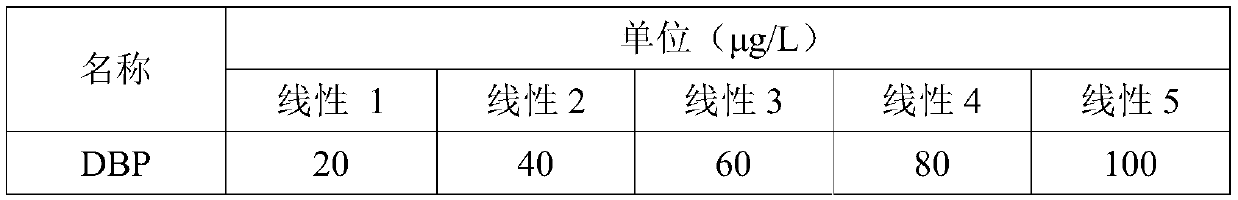
The invention provides an anesthetic analysis method. The anesthetic analysis method comprises the following steps of: (1) solution preparation; (2) sample testing; and (3) performance verification; wherein the solution preparation comprises five steps of: preparation of a linear solution, preparation of a sample mother solution, preparation of a sample solution, preparation of a quantitation limit solution and preparation of an accuracy solution. The anesthetic analysis method has good accuracy, can still separate different substances at a higher heating rate, reaches a good separation effect, and avoids the situations that a signal-to-noise ratio is reduced, the relative standard deviation is increased and the like. The method can be used as a test method for determining the DBP in a ropivacaine hydrochloride process liquid in the biological medicine business division.
Owner:上海微谱检测科技集团股份有限公司
A kind of ropivacaine hydrochloride impurity and preparation method thereof
ActiveCN111925317BGood anesthesiaEasy to operateOrganic chemistryChemical compoundStructural formula
The invention provides a ropivacaine hydrochloride impurity and a preparation method thereof. The structural formula of the ropivacaine hydrochloride impurity is shown in formula I. The ropivacaine hydrochloride impurity of the present invention is a kind of impurity discovered by the inventor during the research process. A new compound, the impurity of ropivacaine hydrochloride of the present invention is of great significance for quality control in the synthesis process of ropivacaine hydrochloride, and the inventors have found through research that the impurity of ropivacaine hydrochloride with structural formula such as formula I has excellent The anesthetic performance can be used for a new kind of anesthetic. The method of the present invention is simple in operation and mild in conditions. It only needs to mix the reaction raw materials to generate ropivacaine hydrochloride impurities. It does not need to control temperature and other conditions, and the preparation method has low cost and no side reactions. The impurity of pivacaine has high purity and high yield, which is beneficial to reduce the cost of quality control analysis of ropivacaine hydrochloride.
Owner:GUANGDONG JIABO PHARM CO LTD
A kind of preparation method of ropivacaine hydrochloride
The invention provides a method for preparing hydrochloric acid ropivacaine. Part of parameters and conditions in the prior art are improved, and optimization is performed through the following steps that intermediate (I) separation pH and separation extracting solvent are selected; a catalyst and the usage quantity of the catalyst in a resolution agent are selected; refining solvent is selected. In this way, the yield and purity of the prepared hydrochloric acid ropivacaine are high, the purity reaches up to over 99% under the optimal condition, the percentage of dextrorotary isomer is reduced below 0.5%, standard requirements are completely met, and the hydrochloric acid ropivacaine is suitable for industrial production.
Owner:SHANDONG JINHE DRUG RES DEV
Freeze-dried ropivacaine hydrochloride composition for injection
ActiveCN105560195BDuration of low actionHas a peripheral vasoconstrictor effectPowder deliveryAntipyreticActivated carbonPenicillin
The invention relates to a freeze-dried ropivacaine hydrochloride composition for injection. The freeze-dried ropivacaine hydrochloride composition comprises ropivacaine hydrochloride and lactose, for example, 75 parts by weight of ropivacaine hydrochloride and 20-60 parts by weight of lactose. The composition is basically prepared by a method comprising the following steps: weighing a formulated amount of ropivacaine hydrochloride and lactose, adding an appropriate amount of injection water, and stirring for dissolution; adding active carbon into a medicinal solution obtained in the previous step, stirring, and filtering to remove carbon; refilling the injection water to a full formulated amount, stirring uniformly, measuring the pH value of the solution and optionally measuring the content of active ingredients, and adjusting the pH value to 4.0-6.0 by using a pH value regulator if necessary; carrying out sterilization and filtration on the medicinal solution, and filling the solution into a penicillin bottle; and carrying out freeze-drying to remove water, and carrying out tamponment. The freeze-dried ropivacaine hydrochloride composition for injection, provided by the invention, has excellent pharmaceutical properties as described in the specification.
Owner:CHENGDU TIANTAISHAN PHARMA
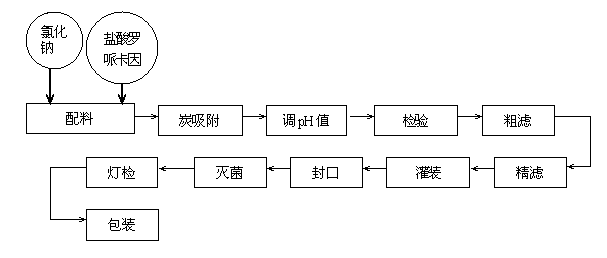
Ropivacaine hydrochloride sodium chloride injection and preparation process thereof
PendingCN114796106AClear efficacyImprove securitySynchronising machinesAntipyreticSodium Chloride InjectionDrug efficiency
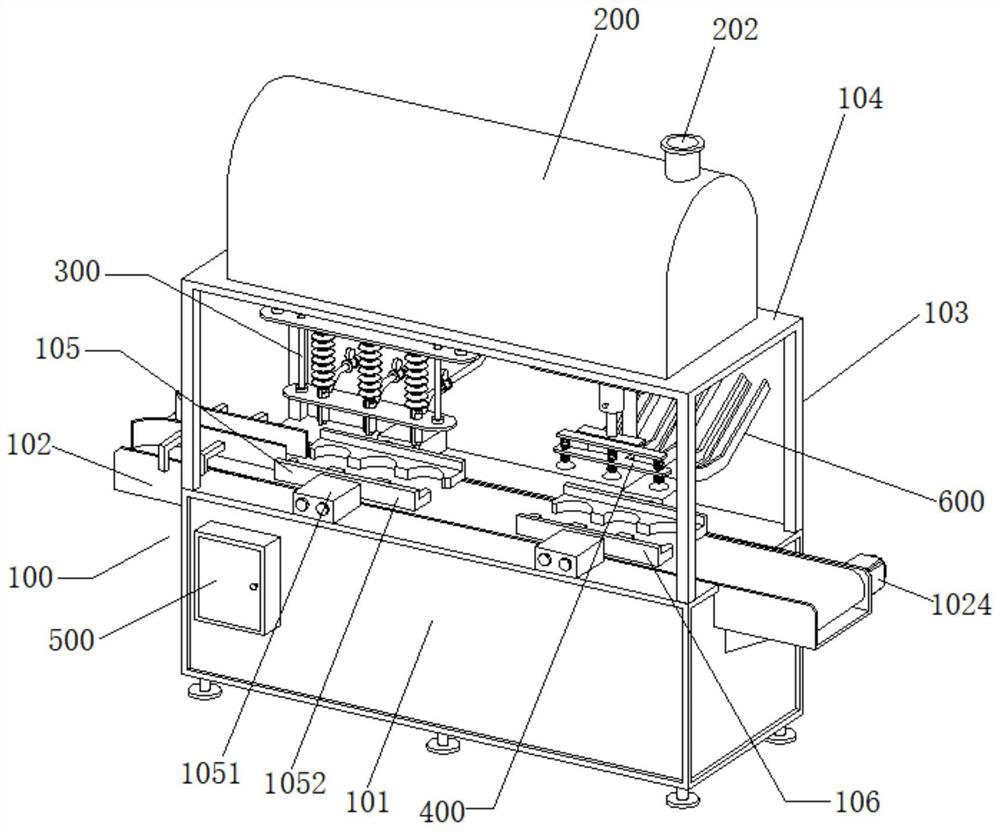
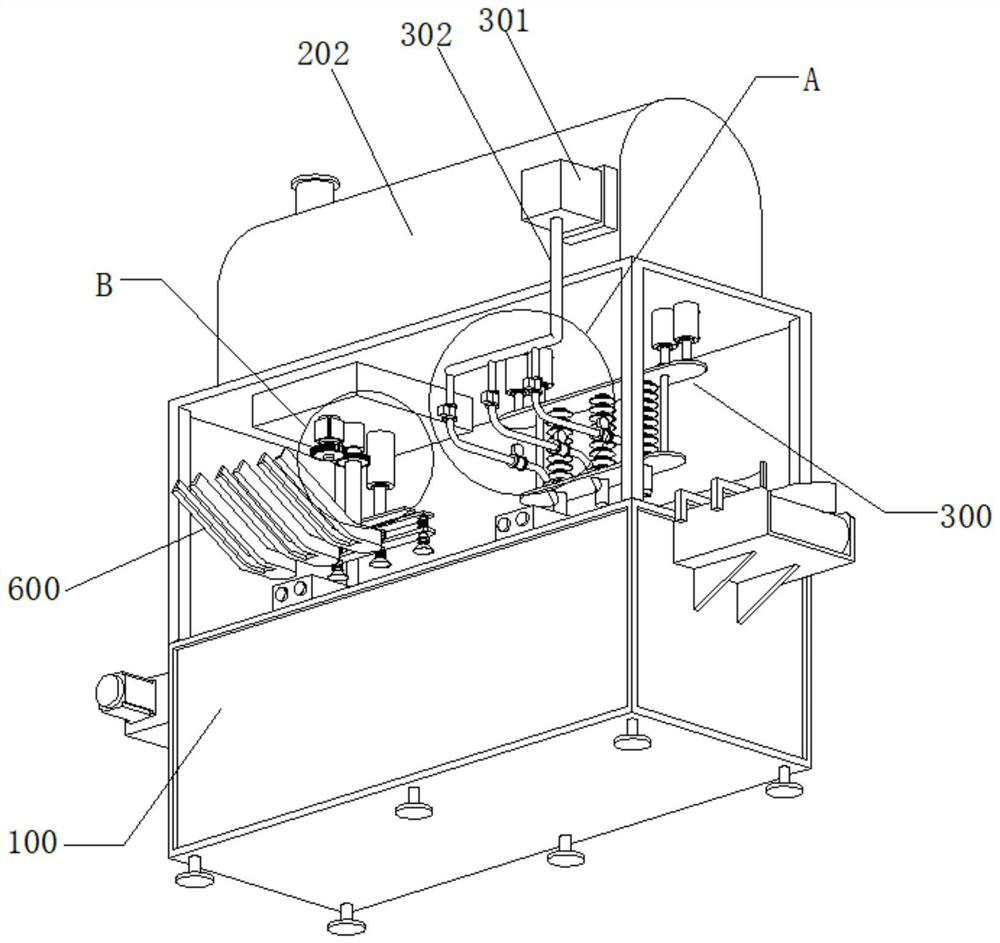
The invention belongs to the technical field of injection production, and particularly relates to a ropivacaine hydrochloride sodium chloride injection and a preparation process. The ropivacaine hydrochloride injection comprises 0.40 g of ropivacaine hydrochloride and 1.72 g of sodium chloride in every 200 ml of the ropivacaine hydrochloride injection, and injection water is added to 200 ml of the ropivacaine hydrochloride injection. In addition, the preparation device comprises a machine body assembly, an injection storage box, a sterile filling mechanism, a cover pressing mechanism and a control box, the sterile filling mechanism comprises a conveying pump, the conveying pump is connected with a liquid conveying main pipe, and the lower end of the liquid conveying main pipe is connected with a plurality of liquid conveying branch pipes at intervals; the ropivacaine hydrochloride sodium chloride injection provided by the invention is clear in drug effect and high in safety, determination results of various indexes in a stability test all meet regulations, and the preparation device ensures the product quality of the prepared injection, so that the drug effect of the injection is clear, and the safety is high.
Owner:江苏长江药业有限公司
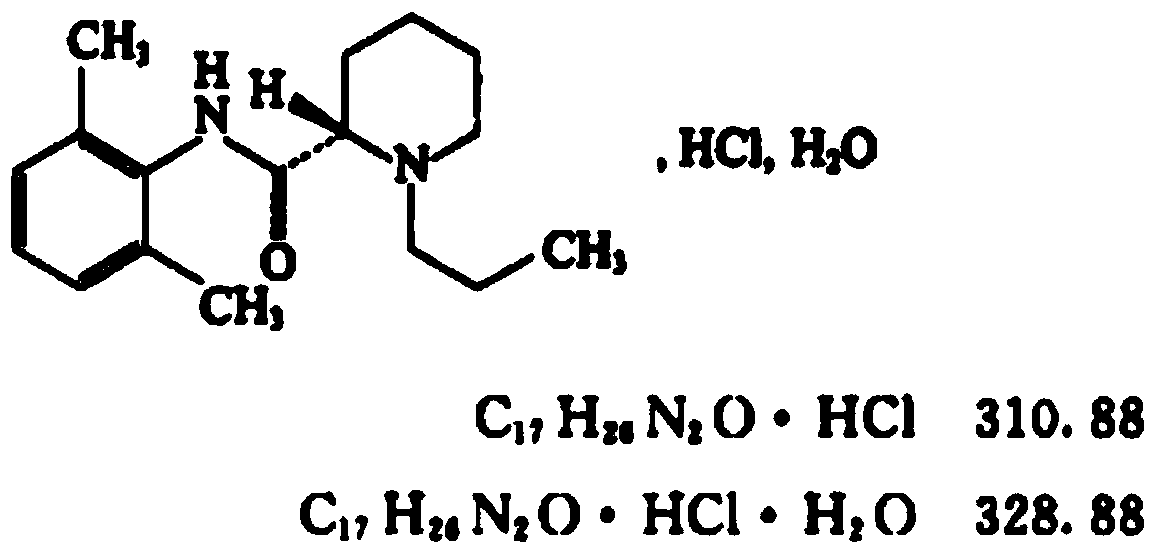
Industrial preparation method of ropivacaine hydrochloride monohydrate
ActiveCN113105386AShort reaction pathExtensive sources of raw materialsOptically-active compound separationOrganic racemisationOrganosolvTransesterification reaction
The invention discloses an industrial synthesis method of ropivacaine hydrochloride monohydrate. The method comprises the following steps: dissolving racemized 2-piperidinecarboxylic acid serving as a starting material in an organic solvent, and carrying out alkylation reaction, transesterification, chiral resolution and salification refining to obtain a target product. The raw materials are cheap and easy to obtain, a one-pot method is adopted, organic reagents are easy to recycle and reuse, the reaction safety coefficient is high, the technological operation is simple, few three wastes are generated, and the obtained ropivacaine hydrochloride monohydrate is high in yield and high in purity.
Owner:HEBEI YIPIN PHARMA
A kind of preparation method of ropivacaine hydrochloride impurity f
ActiveCN105646482BHigh puritySolving quality control challengesOrganic chemistryChemical synthesisQuality control
The invention belongs to the field of chemical synthesis technology, and concretely relates to a preparation method of a ropivacaine hydrochloride impurity F (8aS)-2-(2,6-dimethylphenyl)-3,3-dimethyl-imidazo[1,5-a]pyridine-1(5H)-ketone. (S)-N-(2',6'-dimethylphenl)-2-piperidine carboxamide and acetone are used as raw materials, a condensation reaction is carried out with an acidic condition in order to remove one water molecule, a solution is distilled, treatment is adjusted with alkaline, the impurity F crude product is obtained, then refining is carried out, and the high-purity ropivacaine hydrochloride impurity F is obtained. The method has the advantages of short synthetic route, simple operation, and high product purity; and a qualified impurity reference substance is provided for quality control of ropivacaine hydrochloride.
Owner:山东诚汇双达药业有限公司
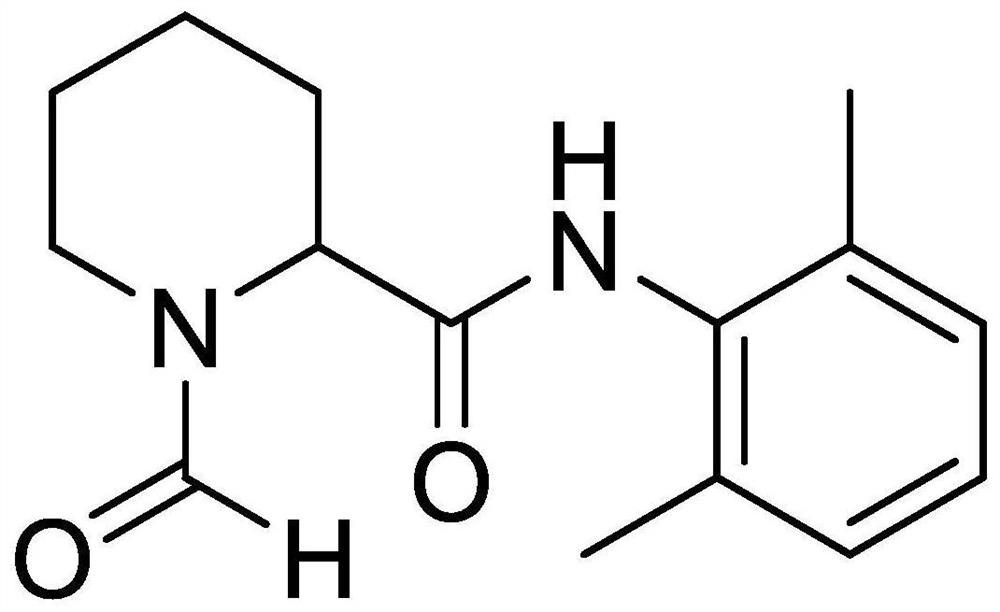
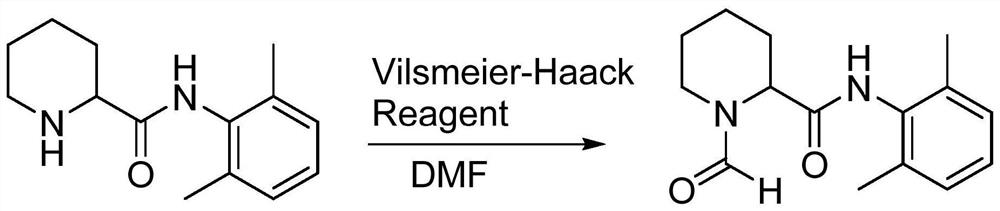
Preparation method of ropivacaine hydrochloride impurities
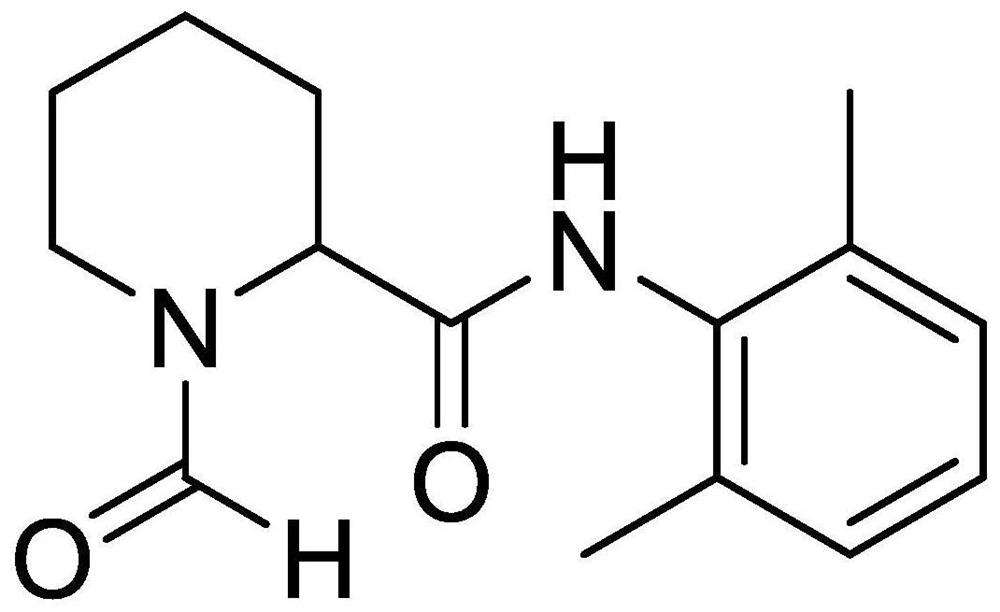
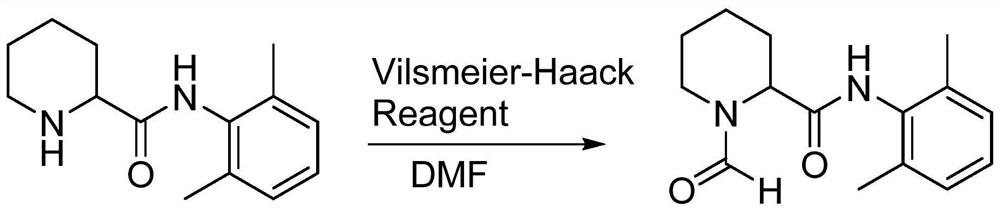
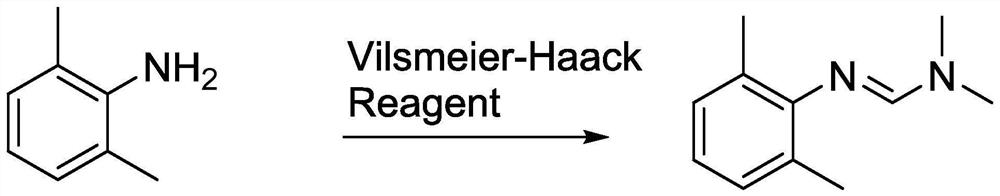
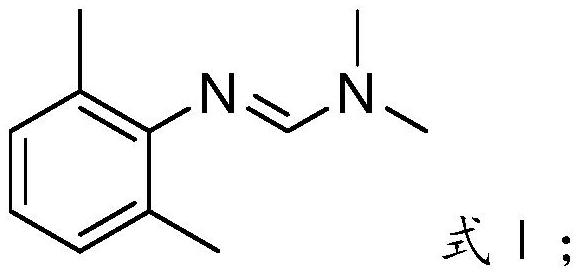
The invention provides a preparation method of a ropivacaine hydrochloride impurity, and the preparation method of the ropivacaine hydrochloride impurity comprises the following steps: mixing 2, 6-dimethylaniline and a Vilsmeier-Haack reagent, reacting and purifying to obtain the ropivacaine hydrochloride impurity. The preparation method of the ropivacaine hydrochloride impurity is low in raw material cost and mild in reaction condition, the ropivacaine hydrochloride impurity can be generated through reaction only by mixing the reaction raw materials, conditions such as temperature control arenot needed, the preparation method is low in cost, no side reaction occurs, and the ropivacaine hydrochloride impurity prepared through the reaction is high in purity and yield.
Owner:GUANGDONG JIABO PHARM CO LTD
Freeze-dried ropivacaine hydrochloride composition for injection and its quality control method
ActiveCN105816432BDuration of low actionHas a peripheral vasoconstrictor effectPowder deliveryComponent separationActivated carbonPenicillin
Owner:CHENGDU TIANTAISHAN PHARMA
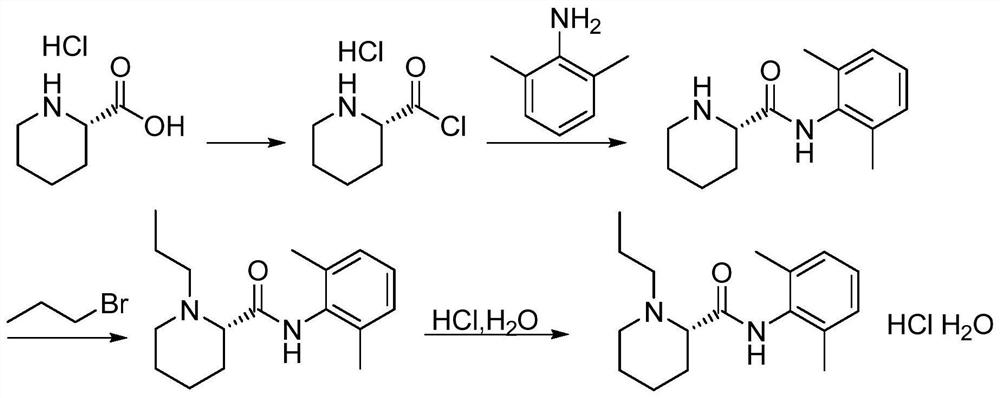
A kind of preparation and purification method of ropivacaine hydrochloride intermediate
The invention relates to a method for preparing and purifying a ropivacaine hydrochloride intermediate. The method uses a single chiral raw material L-piperidine formic acid hydrochloride and 2,6-dimethylaniline to prepare a single chiral intermediate Body (-)-(2S)-N-(2,6-dimethylphenyl) piperidine-2-carboxamide can reduce resolution steps; the present invention further adopts non-polar solvent to intermediate (-) -(2S)-N-(2,6-dimethylphenyl) piperidine-2-carboxamide is refined to reduce related impurities. The preparation method of the present invention has simple and convenient post-processing, high product purity, and After refining, the purity reaches over 99.5%, the single impurity is less than 0.1%, and the total yield is greater than 80%, which is suitable for large-scale industrial production.
Owner:HEBEI YIPIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com