Ropivacaine hydrochloride sodium chloride injection and preparation process thereof
A technology for the preparation of ropivacaine hydrochloride, sodium chloride, and injection, which is applied in liquid treatment, liquid bottling, bottle filling and other directions, can solve problems such as poor stability, and achieves quality assurance, mature preparation technology, and clear efficacy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
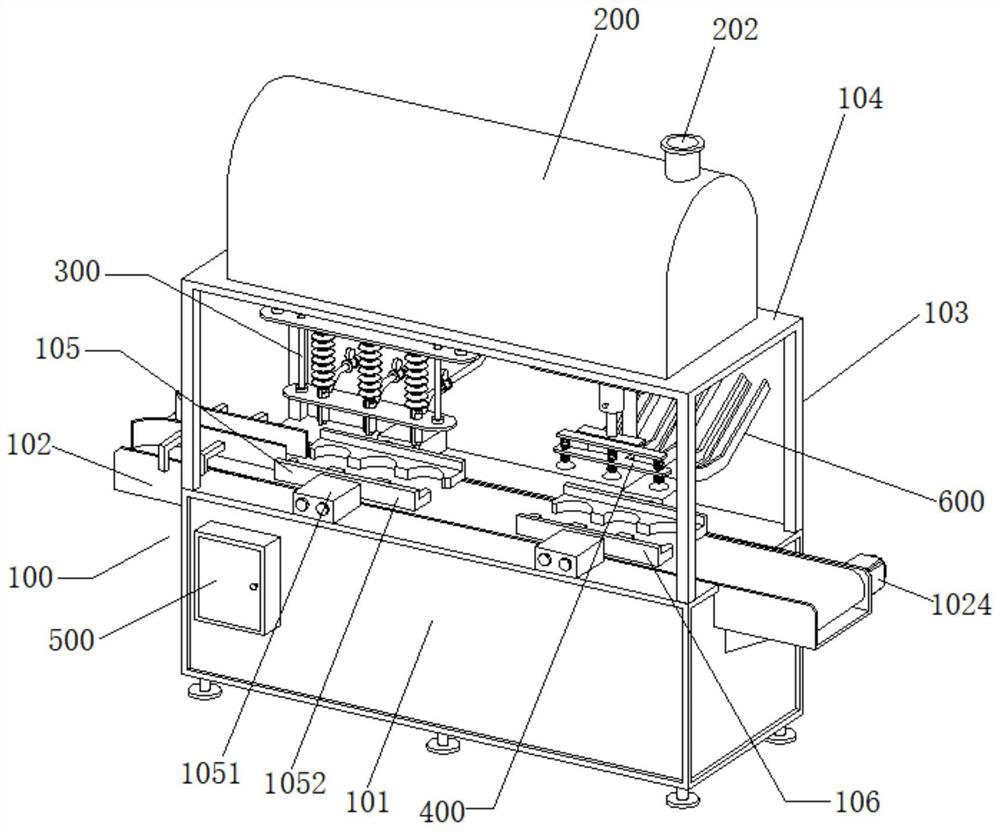
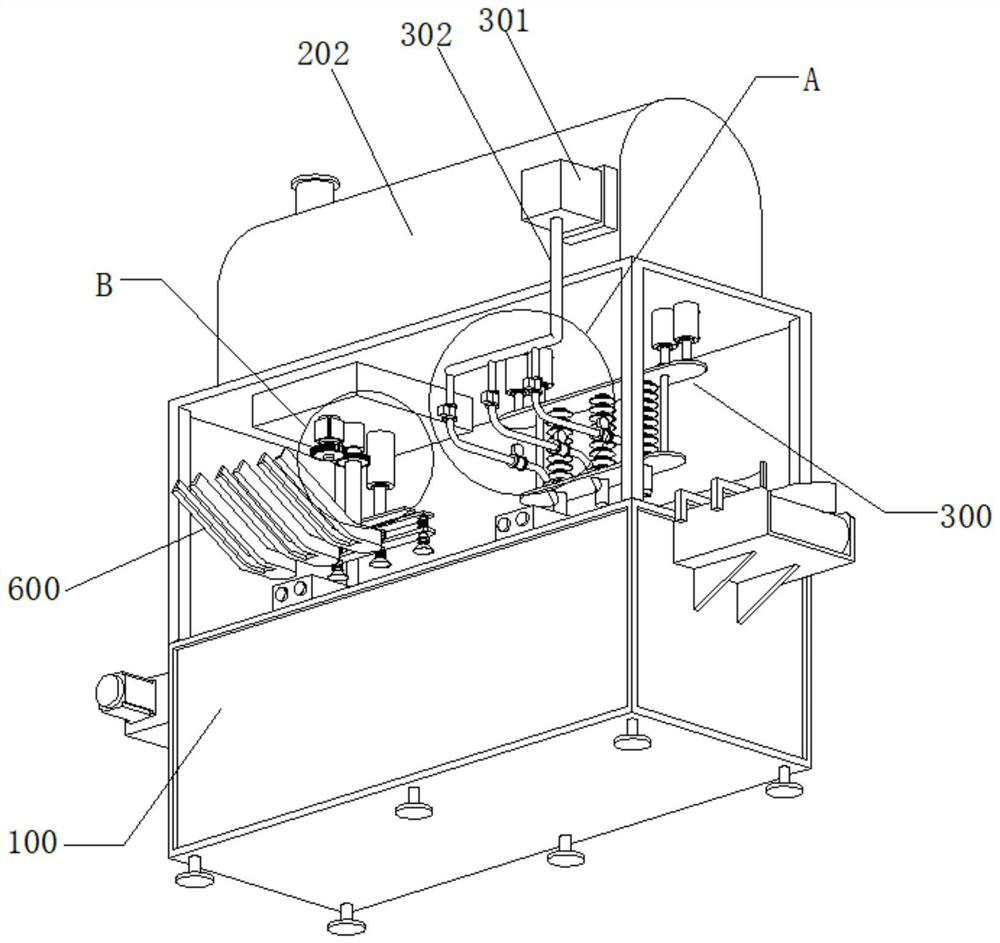
Image
Examples
Embodiment 1
[0051] Embodiment 1 discloses a ropivacaine hydrochloride and sodium chloride injection. The injection includes the following components: 0.40g of ropivacaine hydrochloride, 1.72g of sodium chloride, and water for injection are mixed in every 200ml of the injection. to 200ml. In addition, the injection also includes appropriate amounts of dilute hydrochloric acid and sodium hydroxide, and the pH value of the injection is controlled at 4.0-6.0 by the added dilute hydrochloric acid and sodium hydroxide.
[0052] Wherein, the preparation technology of this Ropivacaine Hydrochloride Sodium Chloride Injection comprises the following steps:
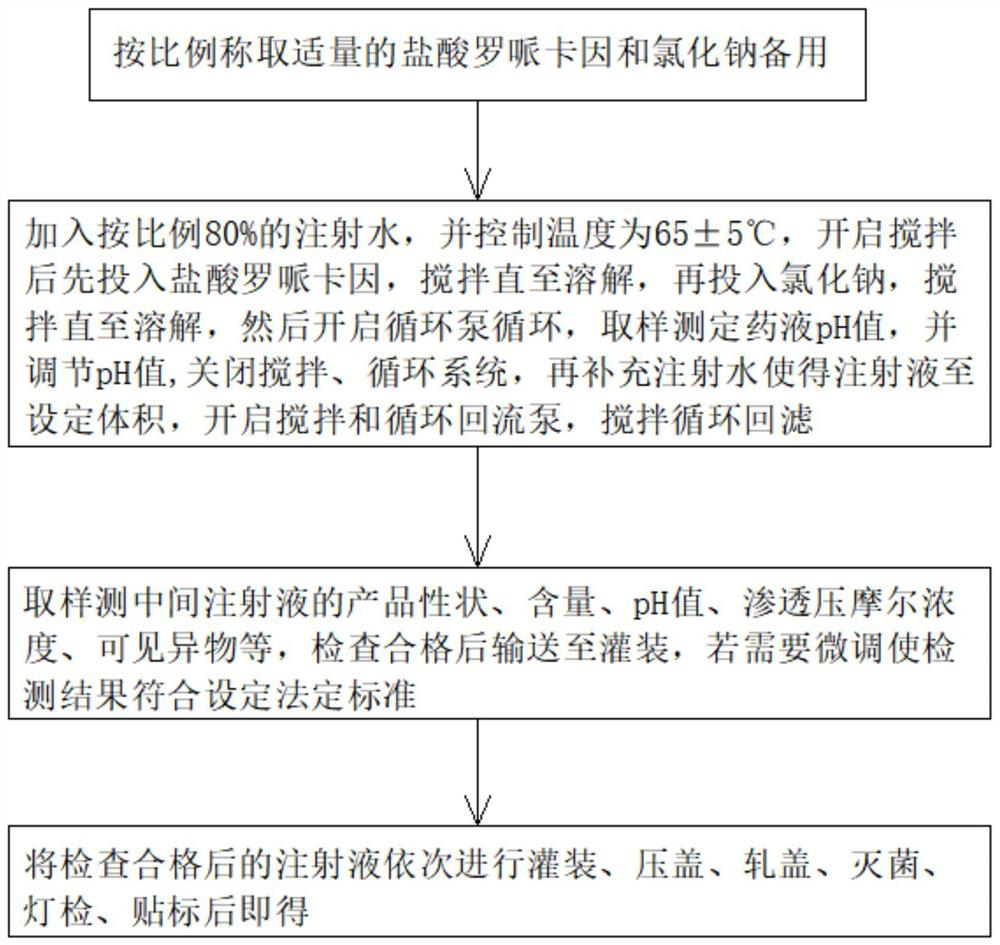
[0053] S1: Weigh an appropriate amount of ropivacaine hydrochloride and sodium chloride according to the above ratio for use.
[0054] S2: Add 80% water for injection to the liquid preparation tank, and control the temperature to be 65 ° C. After starting the stirring, first put in ropivacaine hydrochloride, stir until dissolved, then put in s...
Embodiment 2
[0058] Embodiment 2 discloses a ropivacaine hydrochloride and sodium chloride injection, which includes the following components: 0.40 g of ropivacaine hydrochloride, 1.72 g of sodium chloride, and 0.40 g of ropivacaine hydrochloride, 1.72 g of sodium chloride, and water for injection in every 200 ml of the injection. to 200ml. In addition, the injection also includes appropriate amounts of dilute hydrochloric acid and sodium hydroxide, and the pH value of the injection is controlled at 4.0-6.0 by the added dilute hydrochloric acid and sodium hydroxide.
[0059] Wherein, the preparation technology of this Ropivacaine Hydrochloride Sodium Chloride Injection comprises the following steps:
[0060] S1: Weigh an appropriate amount of ropivacaine hydrochloride and sodium chloride according to the above ratio for use.
[0061] S2: Add 80% water for injection to the liquid preparation tank, and control the temperature to be 60°C. After turning on the stirring, first put in ropivacai...
Embodiment 3
[0065] Embodiment 3 discloses a ropivacaine hydrochloride and sodium chloride injection. The injection includes the following components: 0.40g of ropivacaine hydrochloride, 1.72g of sodium chloride, and 0.40g of ropivacaine hydrochloride, 1.72g of sodium chloride, and water for injection in every 200ml of the injection. to 200ml. In addition, the injection also includes appropriate amounts of dilute hydrochloric acid and sodium hydroxide, and the pH value of the injection is controlled at 4.0-6.0 by the added dilute hydrochloric acid and sodium hydroxide.
[0066] Wherein, the preparation technology of this Ropivacaine Hydrochloride Sodium Chloride Injection comprises the following steps:
[0067] S1: Weigh an appropriate amount of ropivacaine hydrochloride and sodium chloride according to the above ratio for use.
[0068] S2: Add 80% water for injection to the dosing tank, and control the temperature to be 70°C. After turning on the stirring, first add ropivacaine hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com