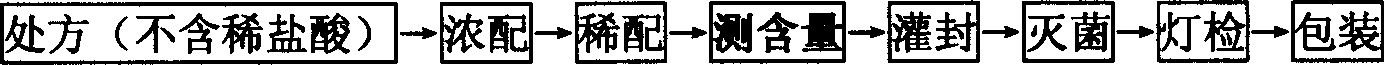Patents
Literature
362 results about "Sodium Chloride Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers for intravenous administration.
Milrinone sodium chloride injection and production thereof
ActiveCN1679566AImprove toleranceSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneSodium Chloride Injection
A milrinone-sodium chloride injection for treating congestive heart failure is proportionally prepared from milrinone, sodium chloride and lactic acid.
Owner:LUNAN BETTER PHARMA
Method for preparing cefozopran hydrochloride, cefozopran hydrochloride powder injection and preparation method thereof
ActiveCN101265267AAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionSodium carbonate anhydrous
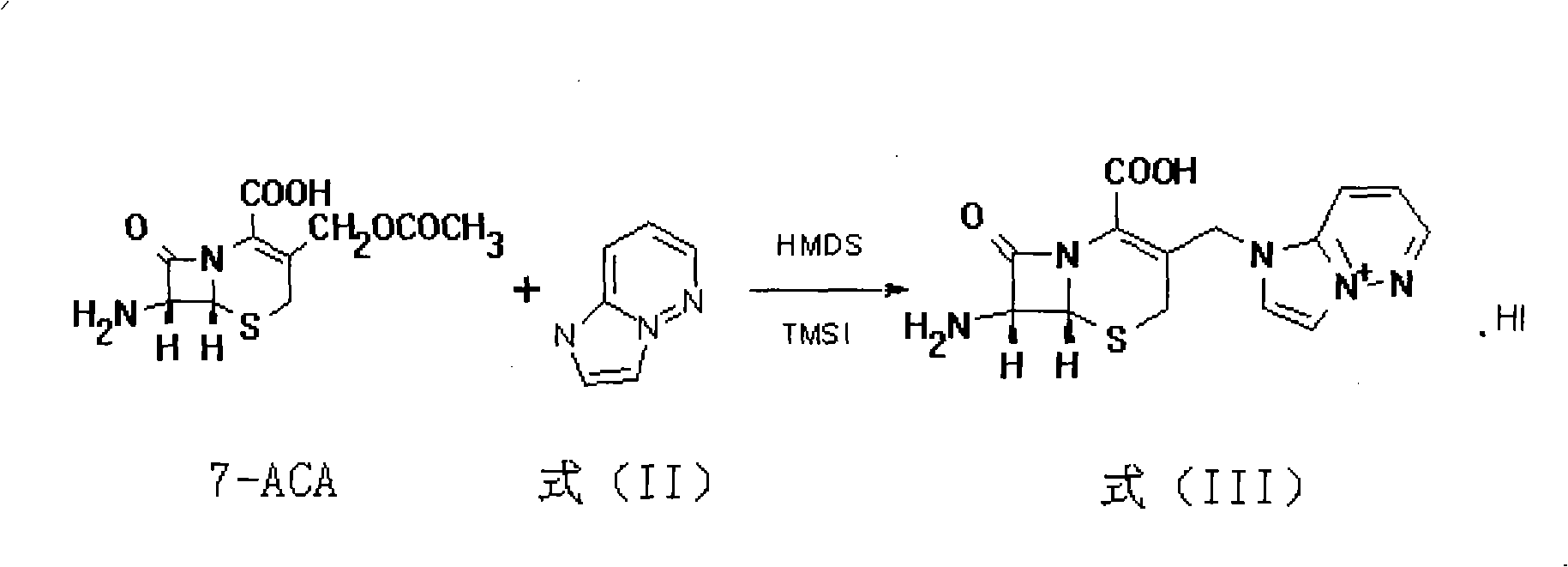
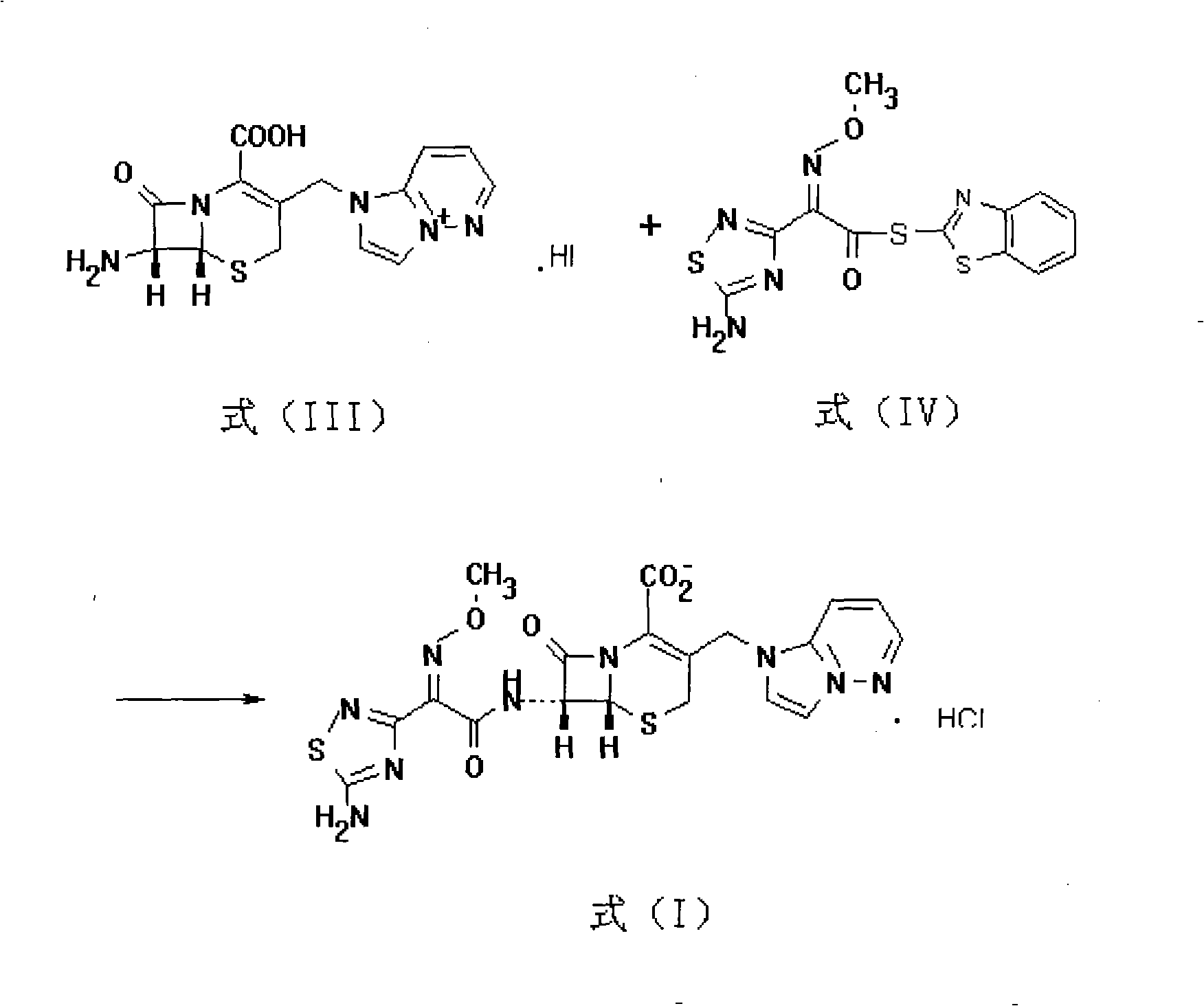
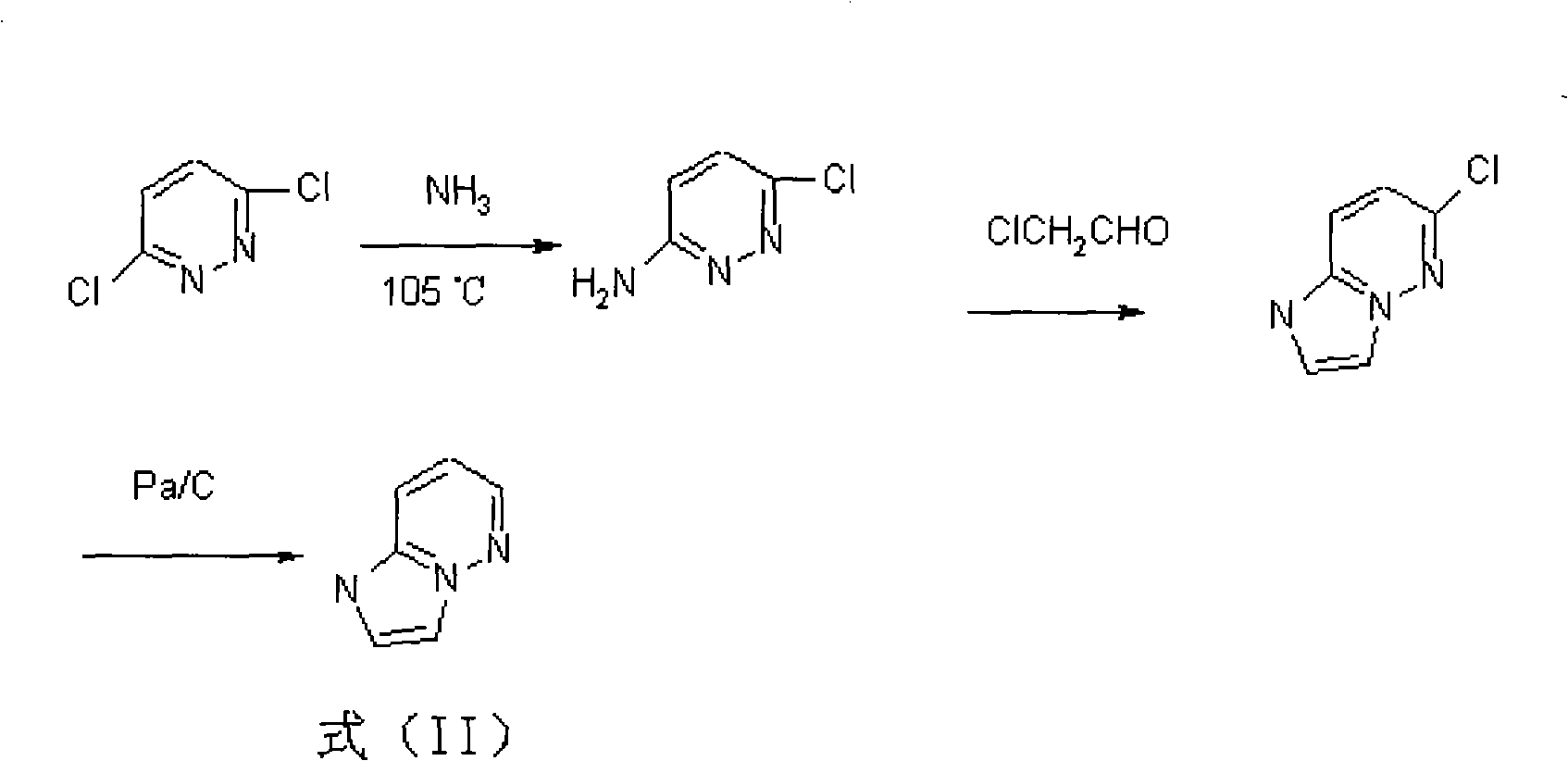
The invention relates to a preparation method of Cefozopran Hydrochloric acid, Cefozopran Hydrochloric acid powder for injection and preparation method thereof. The preparation method includes allowing 7-ACA as raw material and compound in the formula (II) to react in the presence of solvent such as dichloromethane, HMDS and TMSI, and post-treating to obtain compound in the formula (III); condensing with the compound in the formula (IV), post-treating to obtain the Cefozopran, salifying with hydrochloric acid to obtain product, centrifuging, water washing, and drying to obtain Cefozopran Hydrochloric acid crude product; and refining to obtain Cefozopran Hydrochloric acid refined product. The preparation method of the Cefozopran Hydrochloric acid provided by the invention has easily available raw material, high yield, and suitability for industrialized production. The Cefozopran Hydrochloric acid is mixed with anhydrous sodium carbonate and sodium chloride, and sub-packaged to obtain powder for injection having good stability. The obtained powder for injection can be used together with 5% glucose injection, and 0.9% sodium chloride injection.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Kit for processing human marrow, cord blood and peripheral blood cells and cell processing method
The invention relates to a kit for processing human marrow, cord blood and peripheral blood stem cells and a stem cell processing method. The kit radically solves the problems of high manufacture cost, low cell activity, indefinite reaction of markers in a human body, mobilizing agent injection causing patient pain, long obtainment time, complication, clinical unsuitability, and the like in the prior cell separation technology. The invention has the technical scheme that the kit comprises the following three reagents: (1) a diluent comprising 0.9 percent of sodium chloride injection or PBS solution, (2) a precipitator comprising 6 percent of hydroxyethyl starch or 0.2-1 percent of methyl cellulose and (3) a separating solution which has the density of 1.074-1.076 and is prepared by saccharosan and diatrizoate. The kit is easy to store and transport and convenient and rapid to use and can be produced in industrialization.
Owner:BEI ZHENG STEM CELLS BIOLOGICAL TECH CO LTD BEIJING
Ibuprofen injection and preparation method thereof
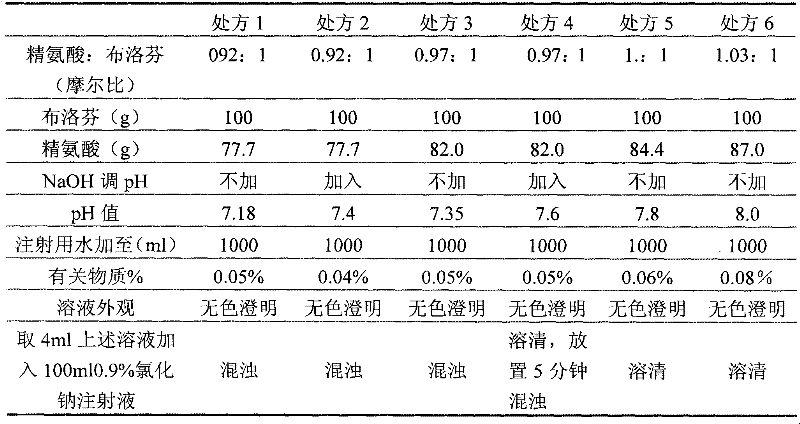
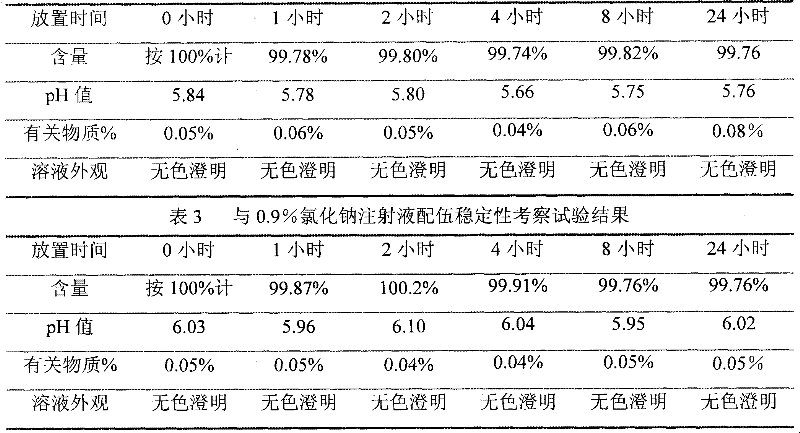
The invention relates to an ibuprofen injection and a preparation method thereof. The ibuprofen injection is characterized in that the concentration of ibuprofen in the injection is 100mg / ml, arginine is used as a cosolvent, and the molar ratio of the arginine to the ibuprofen is greater than or equal to 1:1. The invention also provides a method for preparing the injection. The prepared injection which has good dissolvability is added into a 0.9% sodium chloride injection or a 5% glucose injection, the concentration of the ibuprofen is 4mg / ml or less than 4mg / ml, and the solution is clear and is stabilized for a long time. The ibuprofen injection is suitable for intravenous drip for patients for whom the oral administration is not suitable.
Owner:付正香
Vinpocetine containing high-capacity sodium chloride injection and preparation method thereof
InactiveCN101991530AImprove bioavailabilityQuality improvementOrganic active ingredientsNervous disorderVinpocetineSodium Chloride Injection
The invention provides a vinpocetin containing high-capacity sodium chloride injection and a preparation method thereof, aiming at solving the problems that vinpocetin is mainly applied to middle aged and elderly patients, high-capacity glucose injection contains massive glucose and is not beneficial to treating patients suffering from diabetes at the same time. The invention has the key point that the injection is mainly composed of vinpocetin, osmotic pressure regulator, cosolvent and pH value regulator. The vinpocetin high-capacity sodium chloride injection of the invention has high bioavailability and stable quality, is convenient to use and is applicable to treatment of patients suffering from neurologic disease caused by cerebral circulation restriction, especially the patients suffering from diabetes at the same time.
Owner:SHENYANG ZHIYING PHARMA FACTORY
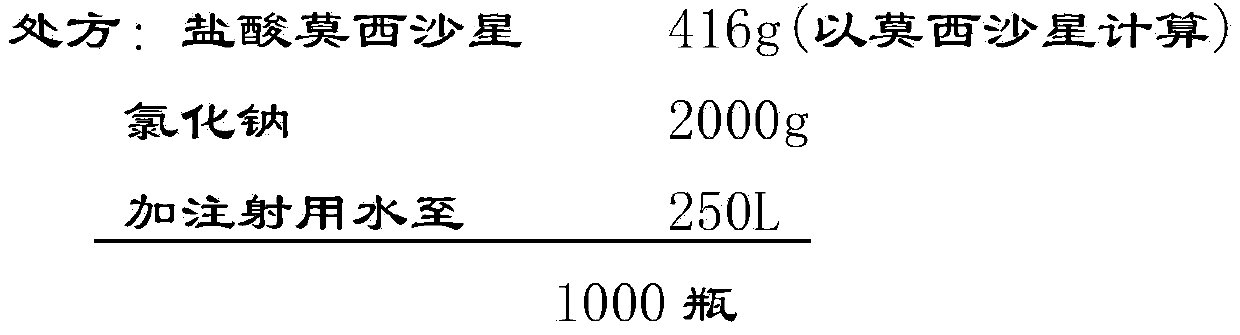
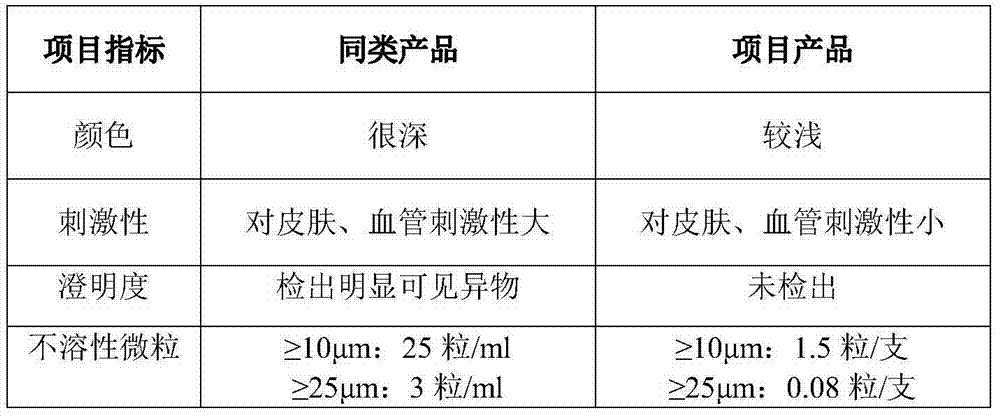
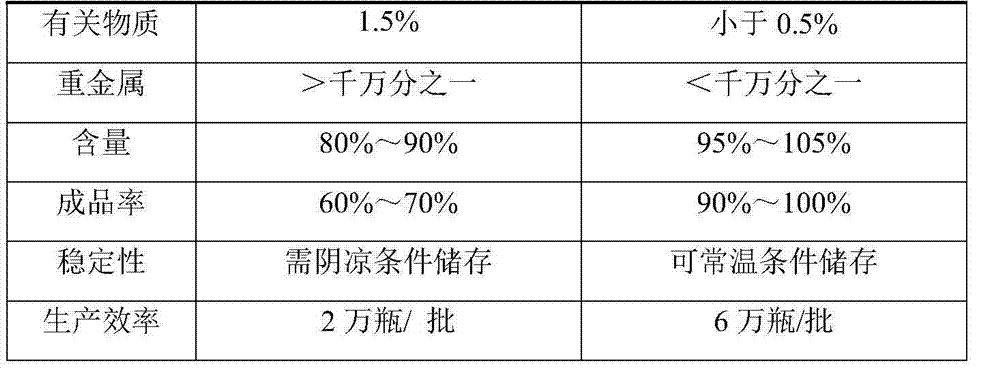
Stable moxifloxacin hydrochloride injection
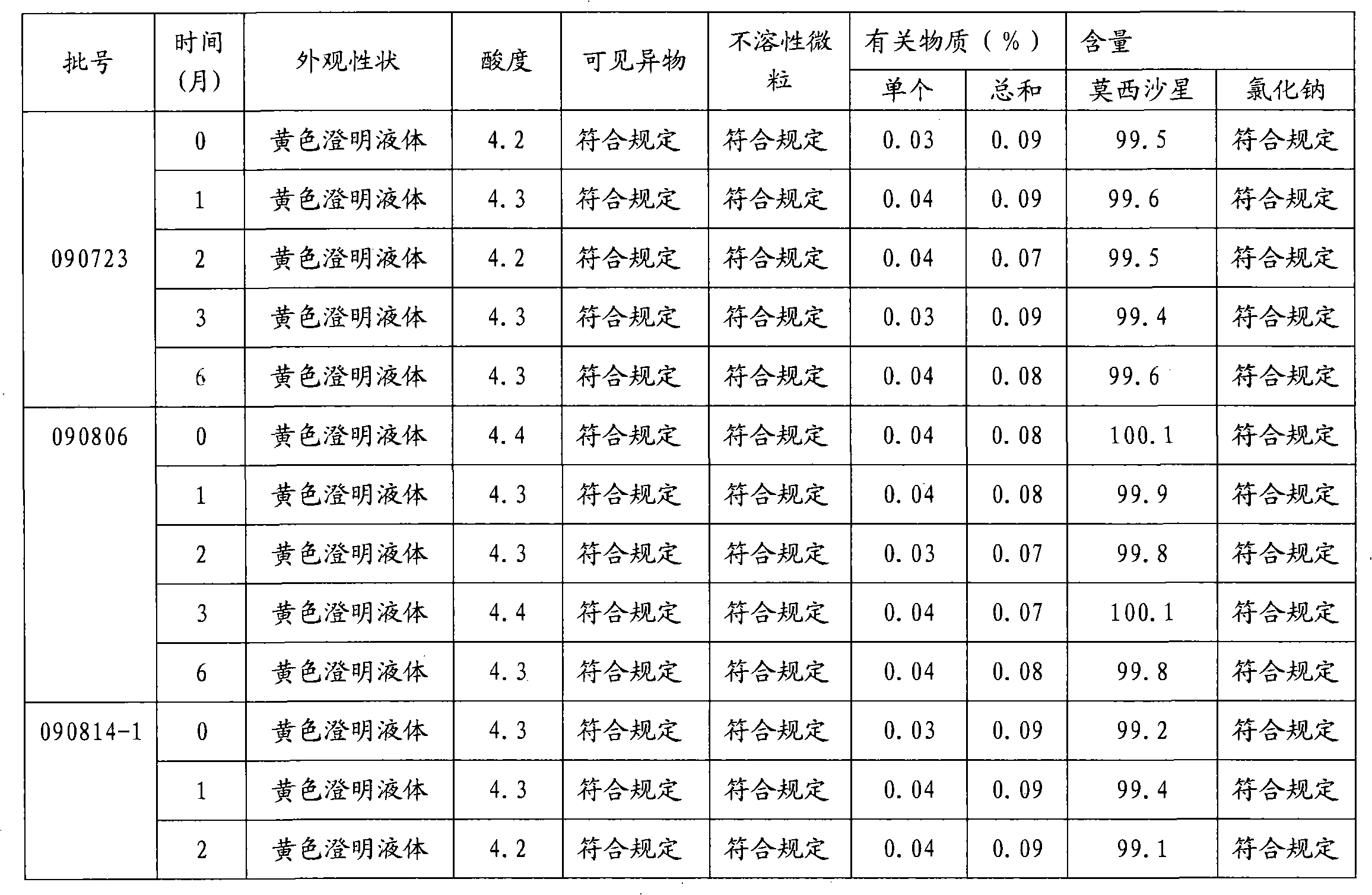
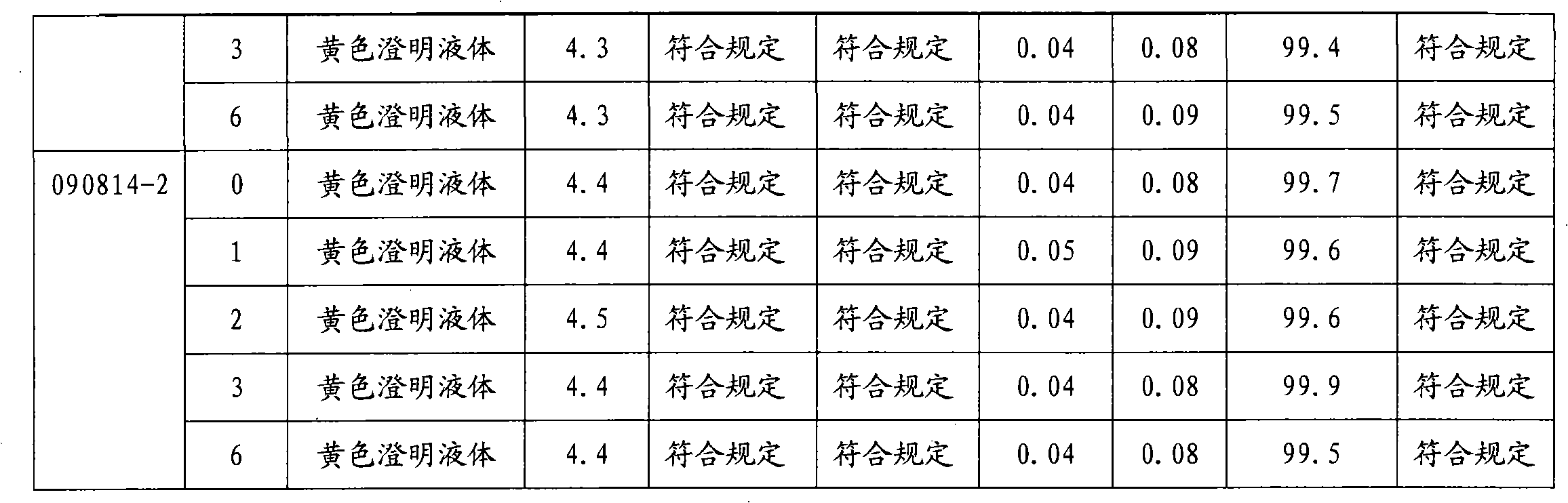
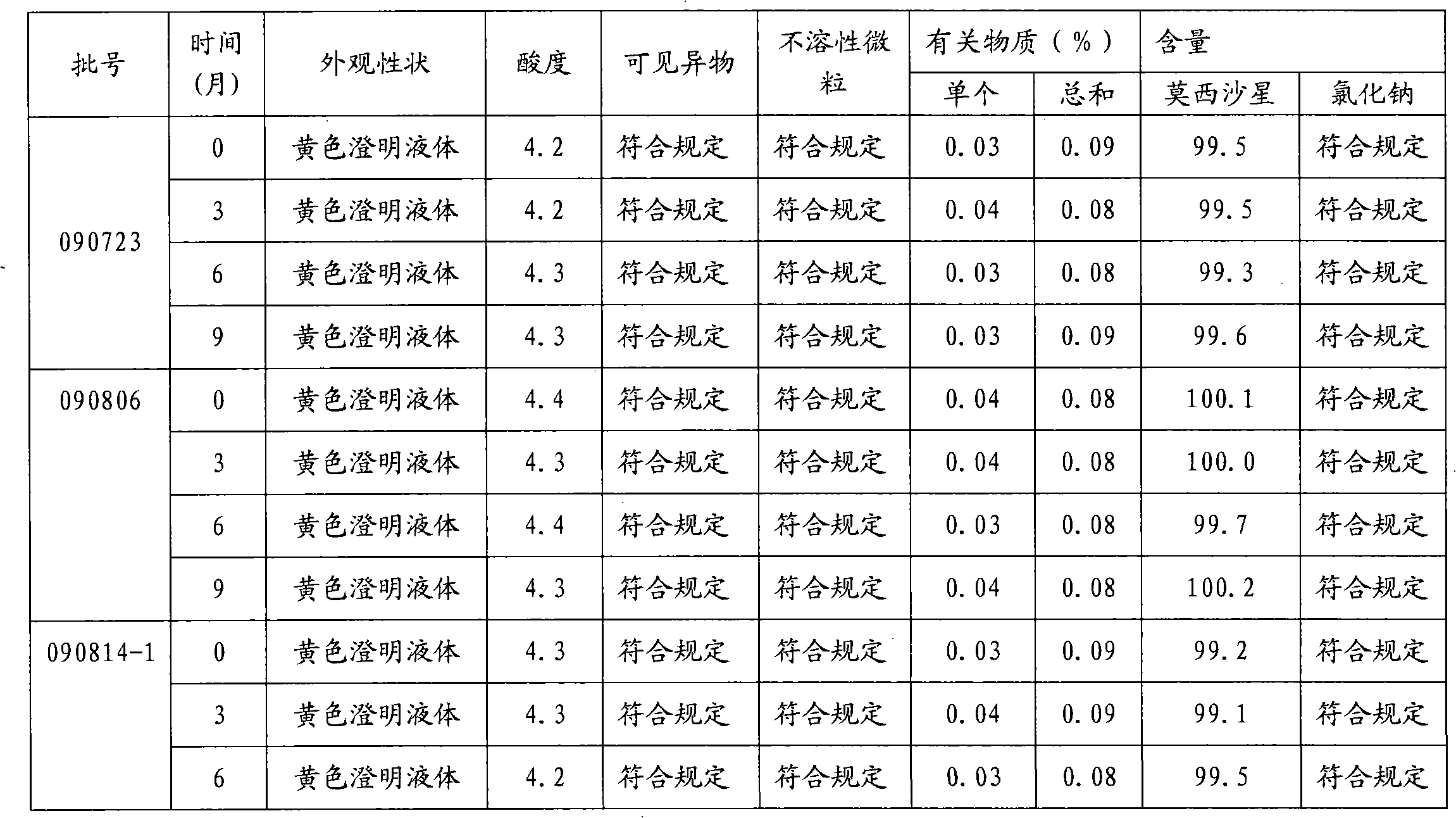
InactiveCN102688183AImprove stabilityReduce formationAntibacterial agentsOrganic active ingredientsFreeze-dryingSodium Chloride Injection
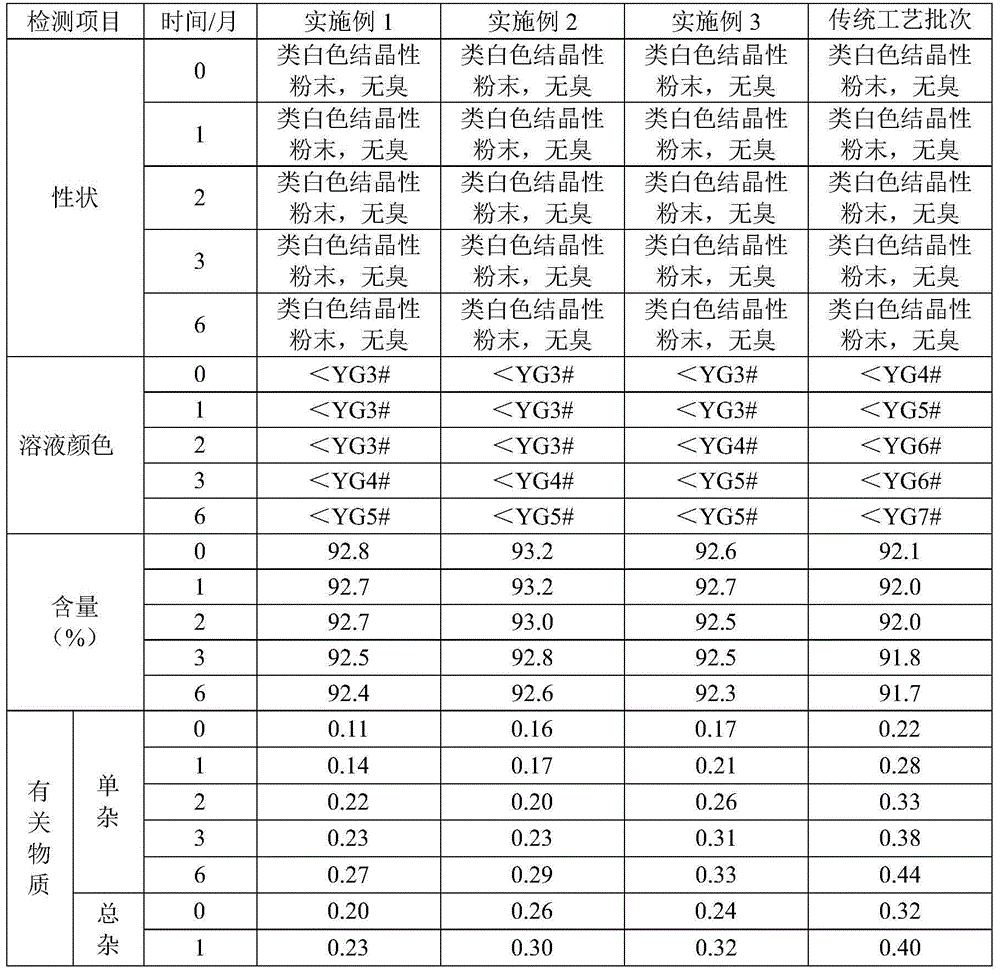
The invention relates to a stable moxifloxacin hydrochloride injection, which concretely contains moxifloxacin hydrochloride and xylitol. The injection can be in the forms of an injection, a transfusion and a freeze-drying powder injection. By the adoption of the stable moxifloxacin hydrochloride injection, stability of moxifloxacin hydrochloride-related substances, solution color, insoluble microparticles and the like during preparation, storage and usage processes of a moxifloxacin hydrochloride glucose or moxifloxacin hydrochloride sodium chloride injection in the present products or technologies is improved. The preparation process has no demanding requirements on equipment and is easy for industrial production.
Owner:CHONGQING PHARMA RES INST
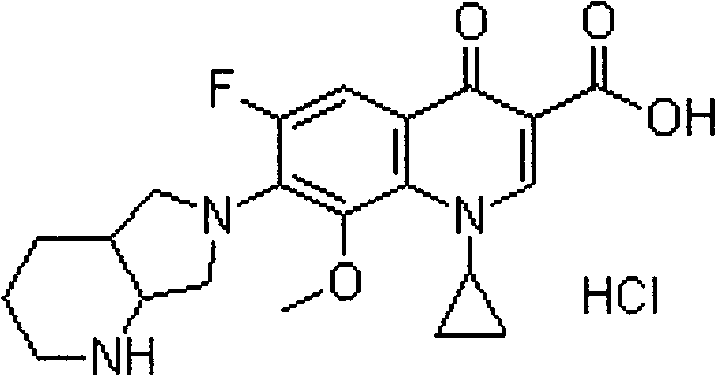
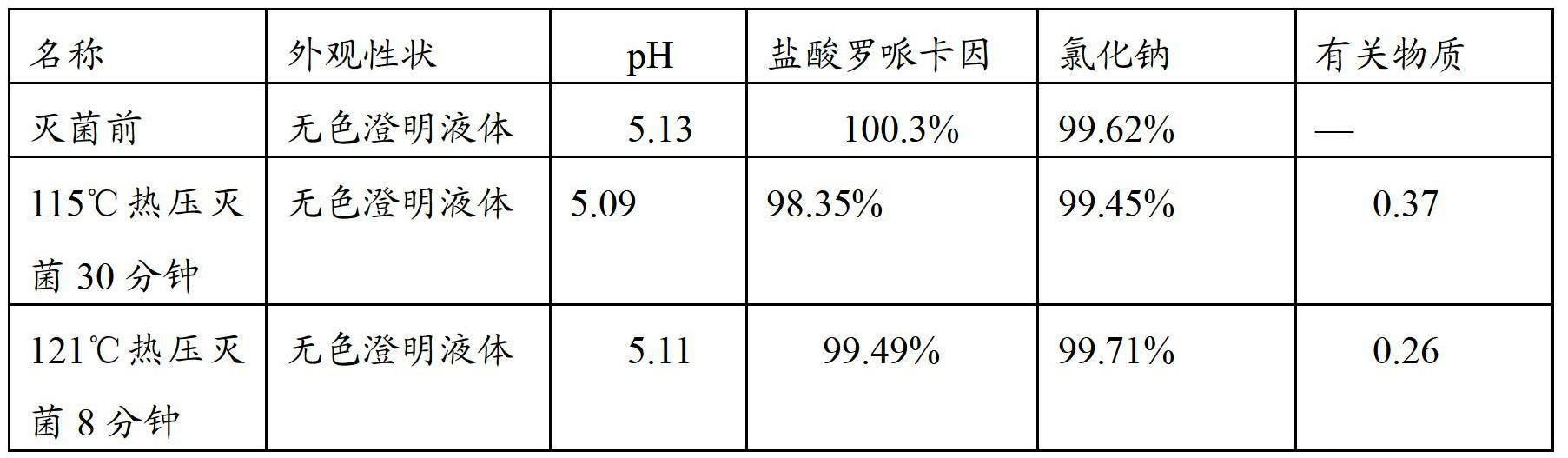
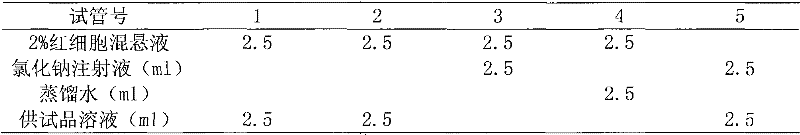
Ropivacaine hydrochloride sodium chloride injection and preparation method thereof
ActiveCN102670489AImprove sterilization effectImprove product qualityPharmaceutical delivery mechanismAnaestheticsMulti injectionFreeze-drying
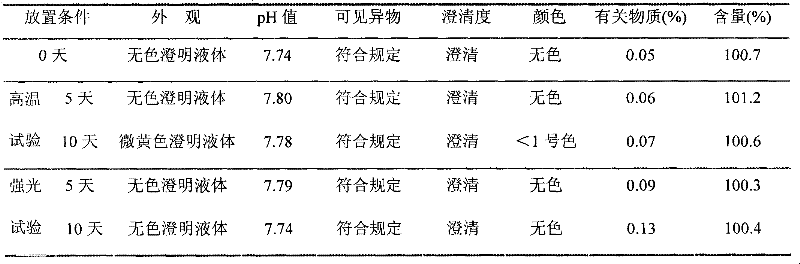
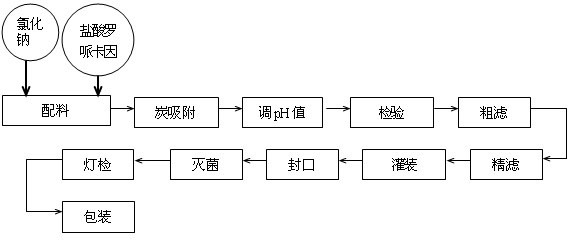
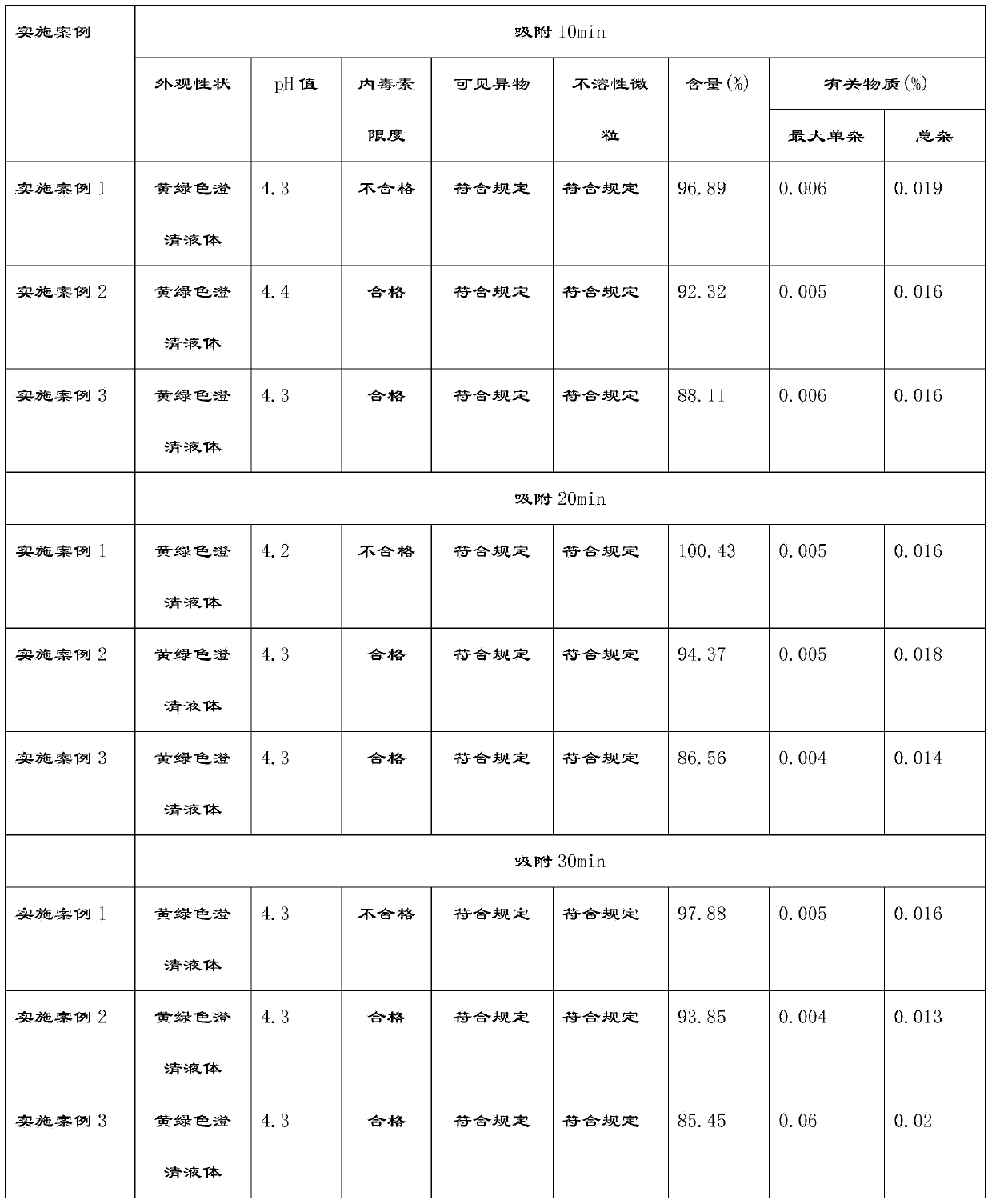
The invention provides a ropivacaine hydrochloride sodium chloride injection and a preparation method thereof. The method comprises the process steps of: burdening, carbon adsorption, rough filtering, fine filtering, filling, sterilizing, lamp detecting, packing and the like. The prepared ropivacaine hydrochloride sodium chloride injection has a definite medicinal effect and high safety; the testresult of each index in a stability test is accordant with specifications, and the defects of the requirement of repeated injection, inconvenience in using, easiness in increasing secondary pollutionby freeze-dried powder and the like existing in a ropivacaine water injection are overcome; the ropivacaine hydrochloride sodium chloride injection is particularly suitable for a patient using an analgesia pump; during use in a large volume, an instant stable concentration is kept for a medicinal liquid, so that a remarkable postoperative analgesic effect is achieved, and the requirement of an opioid medicament is remarkably lowered; and moreover, the effect can be enhanced rapidly by administrating in a self-pressing way.
Owner:HUAREN PHARMA (RIZHAO) CO LTD
Glycerin fructose sodium chloride injection and preparation method thereof
ActiveCN103417568AMedication safetyOrganic active ingredientsPharmaceutical delivery mechanismSodium Chloride InjectionHydroxymethylfurfural
The invention provides glycerin fructose sodium chloride injection and a preparation method thereof and belongs to the technical field of medicine. The invention mainly solves the problem that, in the prior art, absorbance exceeds standard during measurement of 5-hydroxymethyl furfural. The glycerin fructose sodium chloride injection is characterized in that 1,000 ml of the prescription comprises the following raw and auxiliary materials: 99 to 101 g of glycerin, 49 to 51 g of fructose and 8 to 10 g of sodium chloride, and fresh water for injection is added until the volume is 1,000 ml. The preparation method comprises the following steps: sequentially adding the sodium chloride, the glycerin and the fructose according to the prescription quantity into a thick mixing tank with the water for injection which is 30 percent of the total volume of the prescription; opening the liquid outlet of the thick mixing tank and the liquid inlet of a diluting mixing tank after the raw materials are completely dissolved; putting the mixture into the diluting mixing tank; supplementing the mixture with water for injection until the volume is 99 percent and adjusting the pH value to be 3.8 to 4.2; filtering by a terminal filter with a 0.22-micron polyether sulfone filter element and filling to obtain the injection, wherein a stabilizing agent is not added into the product and the absorbance of the 5-hydroxymethyl furfural is less than 0.6.
Owner:SHANXI NUOCHENG PHARMA
Cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion
InactiveCN108552159AAvoid lostReduce workloadInorganic non-active ingredientsPharmaceutical delivery mechanismClinical gradeHydroxyethyl starch
The invention discloses a cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion. The cryopreserving solution comprisesthe following raw materials: dimethyl sulfoxide, a glycerol fructose sodium chloride injection, an invert sugar electrolyte injection, a dextran 40 glucose injection, a hydroxyethyl starch 130 / 0.4 electrolyte injection, a vitamin C injection, a human serum albumin injection and a 0.9 % sodium chloride injection; the cryopreserving solution has the pH value of 6.8-7.0. When a formula for the clinical-grade CAR-T cryopreservation and capable of being directly reinfused through the intravenous infusion is used for cryopreserving CAR-T cells, the thawed cells and the thawed cryopreserving solutiondo not need centrifugation, resuspension, fluid exchange and other processes and can be directly reinfused through the intravenous infusion, so that the loss of cell varieties due to pollution in-vitro repeated proliferation of the CAR-T cells, or changes of cell morphologies and cell functions are effectively avoided, medical equipment can be also significantly simplified and the workload of medical workers can be reduced.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Single nitrate isosorbide sodium chloride injection
InactiveCN1679537AFast and reliableGood curative effectPharmaceutical delivery mechanismHeterocyclic compound active ingredientsCoronary artery diseaseAngina
A liquid sodium chloride injection of isosorbide mononitrate for treating coronary heart disease and angina pectoris is prepared from isosorbide mononitrate and sodium chloride. It can quickly take its high curative effect.
Owner:LUNAN PHARMA GROUP CORPORATION
Compound monoammonium glycyrrhizinate S pharmaceutical composition and method for preparing high-capacity injection
The invention belongs to the technical field of pharmaceutical preparations, in particular to a compound monoammonium glycyrrhizinate S pharmaceutical composition and a method for preparing a high-capacity injection. The composition is a compound monoammonium glycyrrhizinate S sodium chloride injection. The injection is characterized by comprising the following components: 40-160g of monoammonium glycyrrhizinate S, 30-120g of cysteine hydrochloride, 400-1,600g of glycine, 40-160g of anhydrous sodium sulphite, 4-16g of sodium citrate, 900-1,800g of sodium chloride, a proper amount of sodium hydroxide and 100-200L of water for injection. The high-capacity injection is more stable and safer.
Owner:九瑞健康股份有限公司
Process for preparing glucose injection and glucose sodium chloride injection
InactiveCN1799551AOvercoming technical biasImprove productivityOrganic active ingredientsMetabolism disorderSodium Chloride InjectionD-Glucose
Owner:樊武良
Preparation method of high-purity breviscapinun material used by breviscapinun injection
ActiveCN102188440AHigh purityImprove securityPowder deliveryOrganic active ingredientsAlcoholSodium Chloride Injection
The invention provides a preparation method of high-purity breviscapinun material used by a breviscapinun injection. The method comprises the preparation steps of: carrying out dissolving, alcohol precipitation, acid precipitation, ultrafitration and the like on the breviscapinun to obtain the high-purity breviscapinun material. The purity of the high-purity breviscapinun material can achieve more than 98 percent by detection through high performance liquid chromatography, and the high-purity breviscapinun material can be used for preparing large-capacity injection such as breviscapinun sodium chloride injection or breviscapinun glucose Injection.
Owner:吉林四长制药有限公司
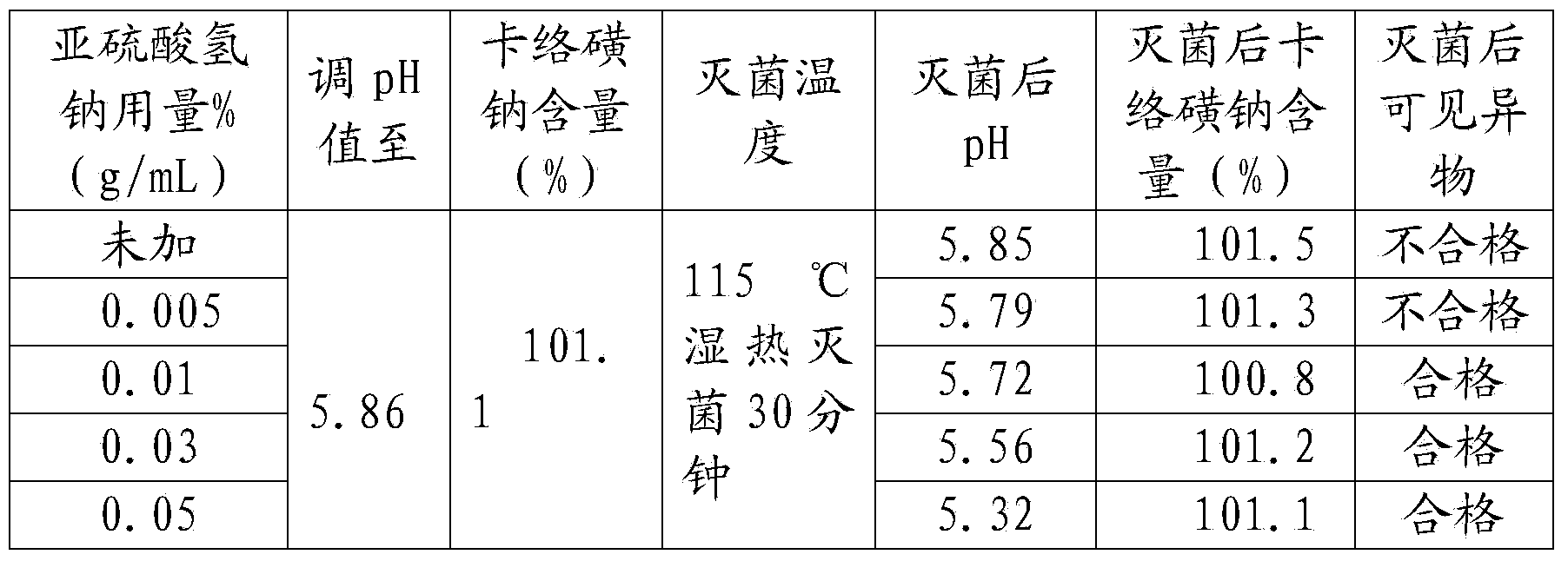
Carbazochrome sodium sulfonate sodium chloride injection and preparation method thereof
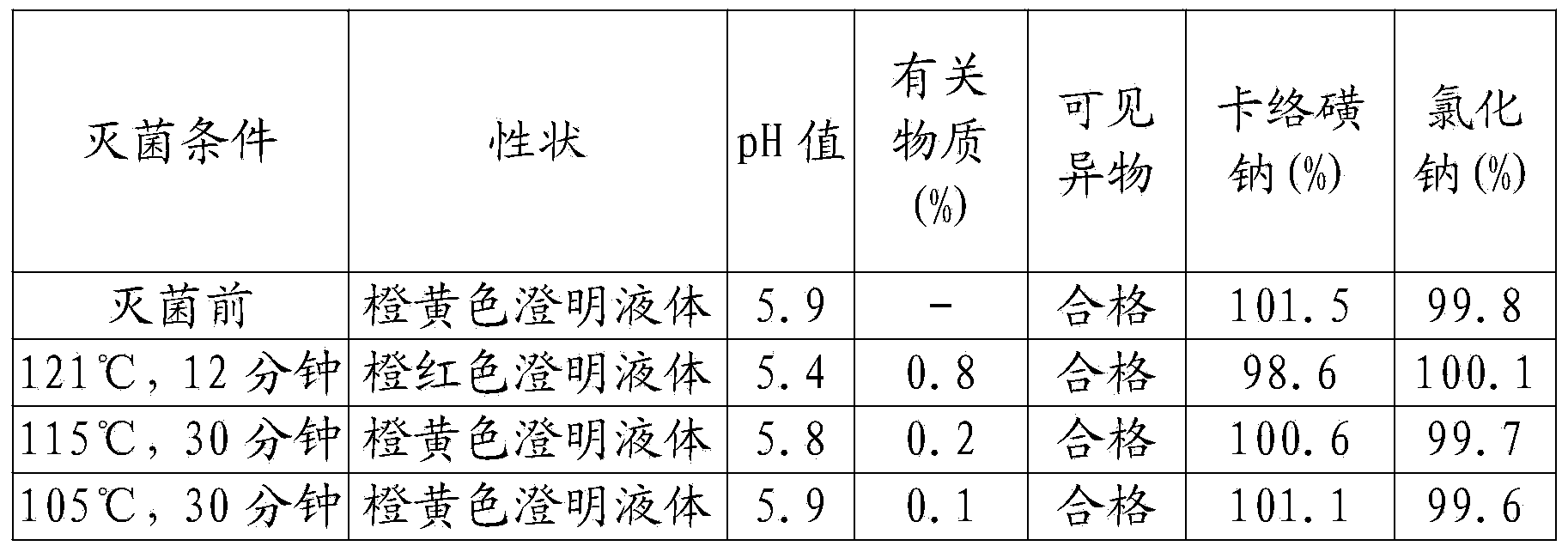
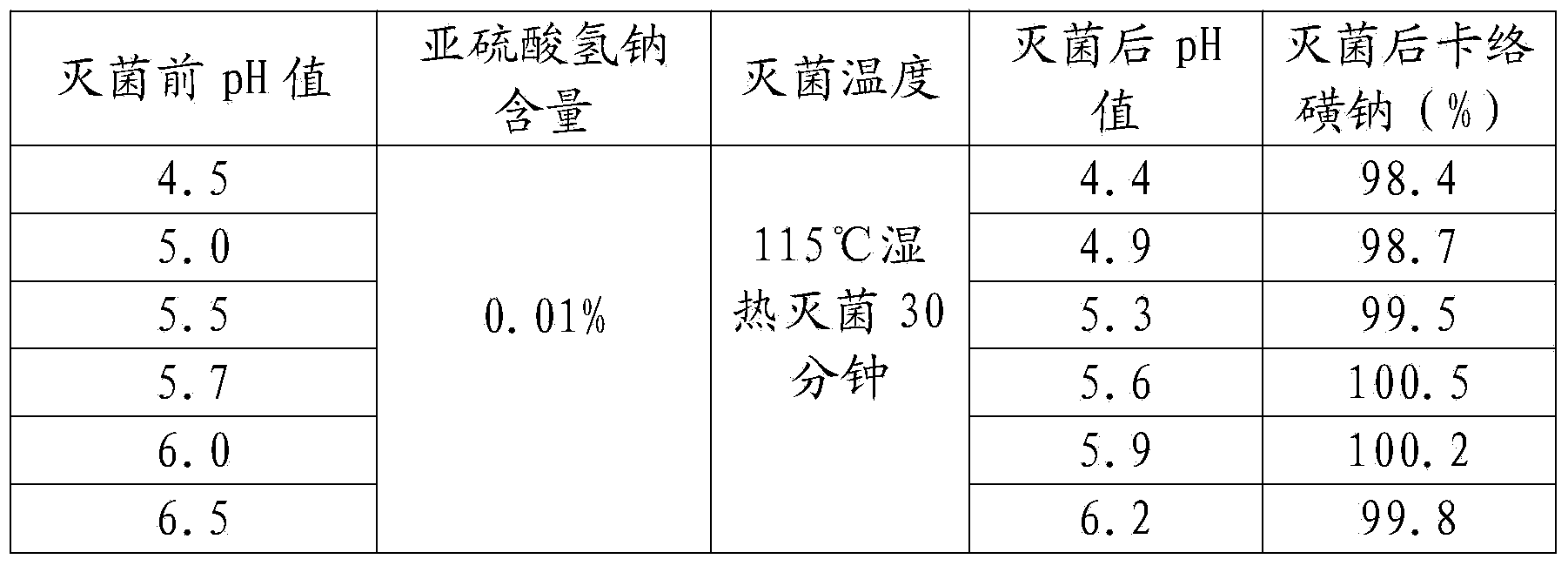
ActiveCN103961310ASolve the sterilization that cannot tolerate F0≥8Solve productivityOrganic active ingredientsPharmaceutical delivery mechanismCarbazochrome Sodium SulfonateSodium Chloride Injection
The invention discloses a carbazochrome sodium sulfonate sodium chloride injection and a preparation method thereof. The product quality and stability are significantly improved by adding a stabilizer A and a stabilizer B, and controlling the pH value and the sterilization condition in the production process. The carbazochrome sodium sulfonate sodium chloride injection is prepared by adopting the formula, and the problems that the carbazochrome sodium sulfonate sodium chloride injection in the prior art cannot tolerate sterilization of which F0 is greater than or equal to 8, and the product is poor in stability in the production and storage processes are solved.
Owner:HUAREN PHARMA (RIZHAO) CO LTD
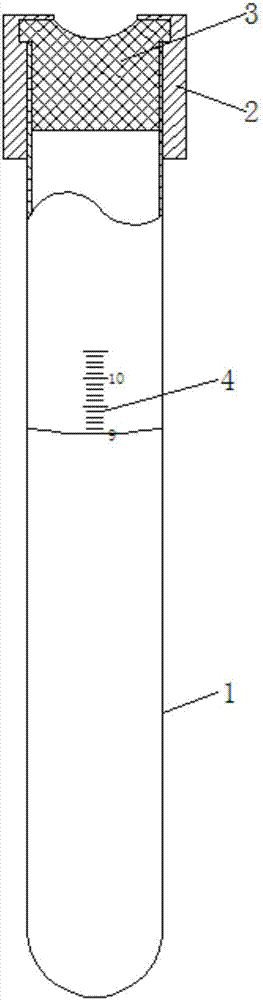
Method for pre-diluting vacuum blood collection tube and for measuring platelet number
ActiveCN106913348AEasy to readThe number is accurate and reliableDiagnostic recording/measuringSensorsBlood Collection TubeVenous blood
The invention discloses a pre-diluted vacuum blood collection tube, which comprises a tube body and a tube plug, wherein a tube cap is arranged at the outer side of the tube plug; the tube body, which is sealed, is under a vacuum state; scale marks, which are used for marking capacity of the tube body, are arranged on the outer wall of the tube body; scale values, indicated by the scale marks, range from 9mL to 10.5mL, and a minimum division value is 0.1mL; and 9mL of sodium chloride injection or compound electrolyte injection is contained in the tube body. The invention also discloses a method for measuring platelet number in a patient with EDTA-dependent pseudo-thrombocytopenia with the application of the pre-diluted vacuum blood collection tube. The pre-diluted vacuum blood collection tube provided by the invention is simple in structure; the pre-diluted vacuum blood collection tube is designed with the precise scale marks; venous blood is diluted by liquid which is free from an anticoagulation, so that autologous antigen in an anticoagulation-induced and activated platelet membrane glycoprotein GPIIb structure, and a purpose of inhibiting platelet aggregation is achieved; and the pre-diluted vacuum blood collection tube, through a mode of vacuum blood collection, simplifies operations and avoids pollution, caused by open blood collection, to operators and environment.
Owner:汪学耀
Stable bromhexine hydrochloride sodium chloride injection composition
ActiveCN104434786AGuaranteed sterilityOrganic active ingredientsPharmaceutical delivery mechanismAcetic acidSodium Chloride Injection
The invention provides a high-capacity bromhexine hydrochloride sodium chloride injection composition. The bromhexine hydrochloride sodium chloride injection provided by the invention is prepared from bromhexine hydrochloride, sodium chloride, tartaric acid, glacial acetic acid and injection water. The bromhexine hydrochloride sodium chloride injection provided by the invention has the advantages of low content of related substances, high stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Ibuprofen medicinal composition
ActiveCN101978945AGood water solubilityImprove stabilityOrganic active ingredientsAntipyreticSodium lactateArginine
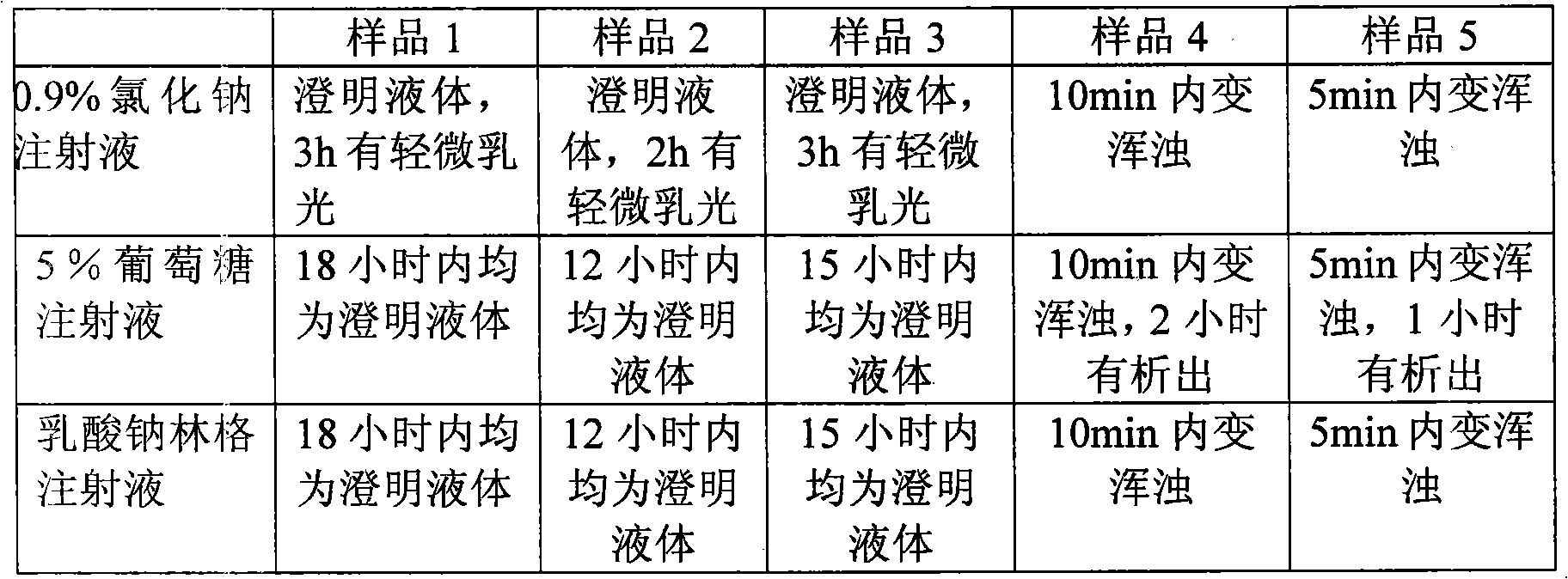
The invention discloses an ibuprofen medicinal composition for injection. By adding tromethamine into solution of ibuprofen and arginine, the stability of the ibuprofen medicinal composition in clinical application is improved. Experiments prove that: after being diluted by the sodium chloride injection or glucose injection or sodium lactate Ringers injection, the composition solution can be stably stored for at least 12 hours.
Owner:茂裕环保科技南通有限公司
Process for preparing cerebroprotein hydrolysate NaCl injection
InactiveCN101204576AEasy to useImprove securityNervous disorderHydrolysed protein ingredientsUltrafiltrationSodium Chloride Injection
The invention discloses a method for preparing sodium chloride injection liquid of brain protein hydrolysate.The preparation includes the processes: weighing the sodium chloride of 90g and adding injection water of 3,000ml dissolve the sodium chloride; adding activated carbon, standing and filtering for removing the carbon; adding the injection water to 8,000ml to prepare sodium chloride solution; weighing stock solution of the brain protein hydrolysate of 1,000ml to ultrafilter by 8,000 dalton ultrafiltration membrane; adding the filtering liquid into the sodium chloride and adding the injection water to 10,000ml, stirring and mixing uniformly and inletting nitrogen into mixed liquid; respectively filtering through filteration membrane of 0.45Mum and 0.22Mum, filling, nitrogen-inletting, encapsulating and sterilizing the mixed liquid. The injection liquid provided by the invention can be directly used in clinic and is unnecessary to be diluted again, thus avoiding secondary pollution, improving the safety of the medicine and convenient for the clinical use; the operation is simplified due to one-time ultrafiltration in the production.
Owner:XINXIANG QILI BIOENG TECH
Adetphos sodium chloride injection and its preparation method
InactiveCN1485043AImprove the level of clinical applicationImprove securityOrganic active ingredientsDigestive systemCITRATE ESTERPhosphate
Owner:刘小清
Novel route for administering hairy holly injection and preparation process thereof
The invention relates to the novel administration methods of traditional medicinal injection for treating coronary atherosclerotic heart disease, thrombolysis in myocardial infarction, center syrup liquid chorioretintis and children's pneumonia, and the process for its preparation. The invention is prepared from pubescent holly root, freeze-dried powder, germ-free powder, 5% glucose injection, 5% sodium chloride injection through extracting with water, decompression concentrating, alcohol depositing, filtrating, adjusting pH, filtrating, charging water for injection into the filter liquor. A novel administration method of intra-vascular injection is provided.
Owner:FUKANGREN BIO PHARMA
Glycerin fructose sodium chloride injection and preparation process thereof
ActiveCN104666340AFix stability issuesFix security issuesNervous disorderHydroxy compound active ingredientsActivated carbonUltrafiltration
The invention discloses a glycerin fructose sodium chloride injection and a preparation process thereof. The glycerin fructose sodium chloride injection is prepared by the following step: preparing 50-150 parts of glycerinum, 5-15 parts of sodium chloride and 10-100 parts of fructose for injection at a low temperature and carrying out ultrafiltration, wherein low temperature refers to the temperature at 30-60 DEG C. According to the glycerin fructose sodium chloride injection, a new technology is used. By adopting a new low-temperature preparation and ultrafiltration technology, no auxiliaries and active carbon are added in the preparation process, so that the problem that fructose is unstable (5-hydroxymethylfurfural is generated) and the problem on safety of residues of active carbon are solved, and the clinical potential safety hazards are eliminated.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Isosorbide mononitrate sodium chloride injection
ActiveCN101708157AEnsure safetyEnsure effectivenessInorganic non-active ingredientsPharmaceutical delivery mechanismSodium Chloride InjectionIsosorbide mononitrate
The invention relates to isosorbide mononitrate sodium chloride injection which belongs to the field of medical preparation. The isosorbide mononitrate sodium chloride injection is prepared from 10-40mg of isosorbide mononitrate, 450-1800mg of sodium chloride, an amount of pH regulator which regulates the pH to 4.0-7.0 and water for injection, wherein the volume of the water for injection is fixed to 100ml. The isosorbide mononitrate sodium chloride injection has high stability and simple preparation process.
Owner:LUNAN PHARMA GROUP CORPORATION
DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid
PendingCN112772637ASo as not to damageReduce adverse effectsDead animal preservationSodium Chloride InjectionMesenchymal stem cell
The invention discloses a DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid, which is characterized by being prepared from the following raw materials in parts by volume: 50 to 60 parts of compound electrolyte injection, 20 to 40 parts of dextran 40 glucose injection, 1 to 10 parts of sodium chloride injection, 1 to 10 parts of glucose injection, 30 to 50 parts of human serum albumin, and 1 to 10 parts of a mesenchymal stem cell serum-free medium. The cryopreservation liquid does not contain DMOS or serum, so that the risk of clinical use is reduced, the influence of the uncertainty of serum components and the instability of serum culture on the normal induced differentiation function of the mesenchymal stem cells is avoided, and the cryopreservation liquid enables the human umbilical cord mesenchymal stem cells to keep a good cryopreservation effect, and the human umbilical cord mesenchymal stem cells have high survival rate after cryopreservation and resuscitation. In addition, the cells cryopreserved by the cryopreservation liquid can be directly diluted and then applied clinically, components of the cryopreservation liquid do not need to be removed through centrifugation, and the cryopreservation liquid can be used as an auxiliary material and directly applied to clinical administration, so that the cryopreservation liquid is more convenient to use.
Owner:朱灏
Method for detecting related substances in azithromycin for injection
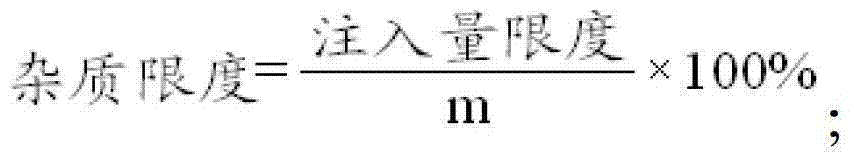
The invention relates to the field of a drug, and in particular relates to a method for detecting related substances in azithromycin for injection. The method comprises the steps of: diluting an azithromycin B reference substance into a solution by sodium chloride injection, orderly carrying out an abnormal toxicity control method test on the solution according to the ascending order of the injection amount, and setting the injection amount in a previous test of the test to be injection amount extreme of the azithromycin B until a mice death result appears in a retest of the test of the azithromycin B injection amount; measuring the injection extremes of azithromycin Gx and erythrocin A iminoether according to the same method; carrying out quantitative analysis on the content C of three substances and the azithromycin in the drug to be tested; calculating according to each maximal dosage and formula to obtain the impurity extremes W1, W2 and W3 of three related substances; if the content of one related substance is not more than W1, not more than W2 and not more than W3, judging that the content of the related substance of the drug achieves the safety level, or else, judging that the content does not achieve the safety level. The target of quantitative evaluation is achieved by the method.
Owner:峨眉山通惠制药有限公司
Children pharmaceutical composition containing ceftriaxone sodium and low-sodium carrier
InactiveCN104887621AIncrease internal pressureImprove solubilityAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionKidney
The invention relates to a children pharmaceutical composition containing ceftriaxone sodium, namely a pharmaceutical combined preparation containing ceftriaxone sodium and a low-sodium carrier infusion solution, and particularly relates to a combined package. The children pharmaceutical composition comprises ceftriaxone sodium for injection and the low-sodium carrier infusion solution. The low-sodium carrier infusion solution contains a glucose and sodium chloride injection (15-200):1, a glucose and sodium chloride potassium chloride injection (15-200):1: (0-1) and the like. Compared with a mixture of ceftriaxone sodium and the low-sodium carrier infusion solution, the children pharmaceutical composition has the advantages that clinical application steps are simplified, clinical risks generated because kidneys of children are not developed to be mature and overmuch sodium in blood cannot be metabolized are reduced, and the clinical application quality and safety performance of children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Intravenous administration preparation extracted from marsdenia tenacissima stem
ActiveCN100434088CPharmaceutical delivery mechanismAntineoplastic agentsVeinSodium Chloride Injection
The present invention relates to a venous administration preparation extracted from Chinese medicinal material marsdenia tenacissima. Its preparation process includes the following steps: firstly, extracting marsdenia tenacissima to obtain its extract, then adding medicinal material into said extract to make them into intravenous drip administration dosage forms, including injection preparation, glucose injection preparation and sodium chloride injection preparation.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Glycerin fructose sodium chloride injection and preparation process thereof
InactiveCN102908360AFix stability issuesFix security issuesNervous disorderHydroxy compound active ingredientsActivated carbonFructose
The invention discloses a glycerin fructose sodium chloride injection and a preparation process thereof. According to the invention, the preparation method of the glycerin fructose sodium chloride injection comprises the following steps: preparing 50-150 parts of glycerin, 5-15 parts of sodium chloride and 10-100 parts of injection fructose by weight at a low temperature; and ultra-filtering a prepared product, wherein the low temperature is 30-60 DEG C. As the glycerin fructose sodium chloride injection disclosed by the invention is prepared by using a new technology and using a novel low temperature preparation and ultrafiltration technology, and any auxiliary material and active carbon are not needed in the preparation process, the instability problem of the fructose (5-hydroxymethyl furfural is generated) and the safety problem of active carbon residue are solved, and clinical potential safety hazards are eliminated.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
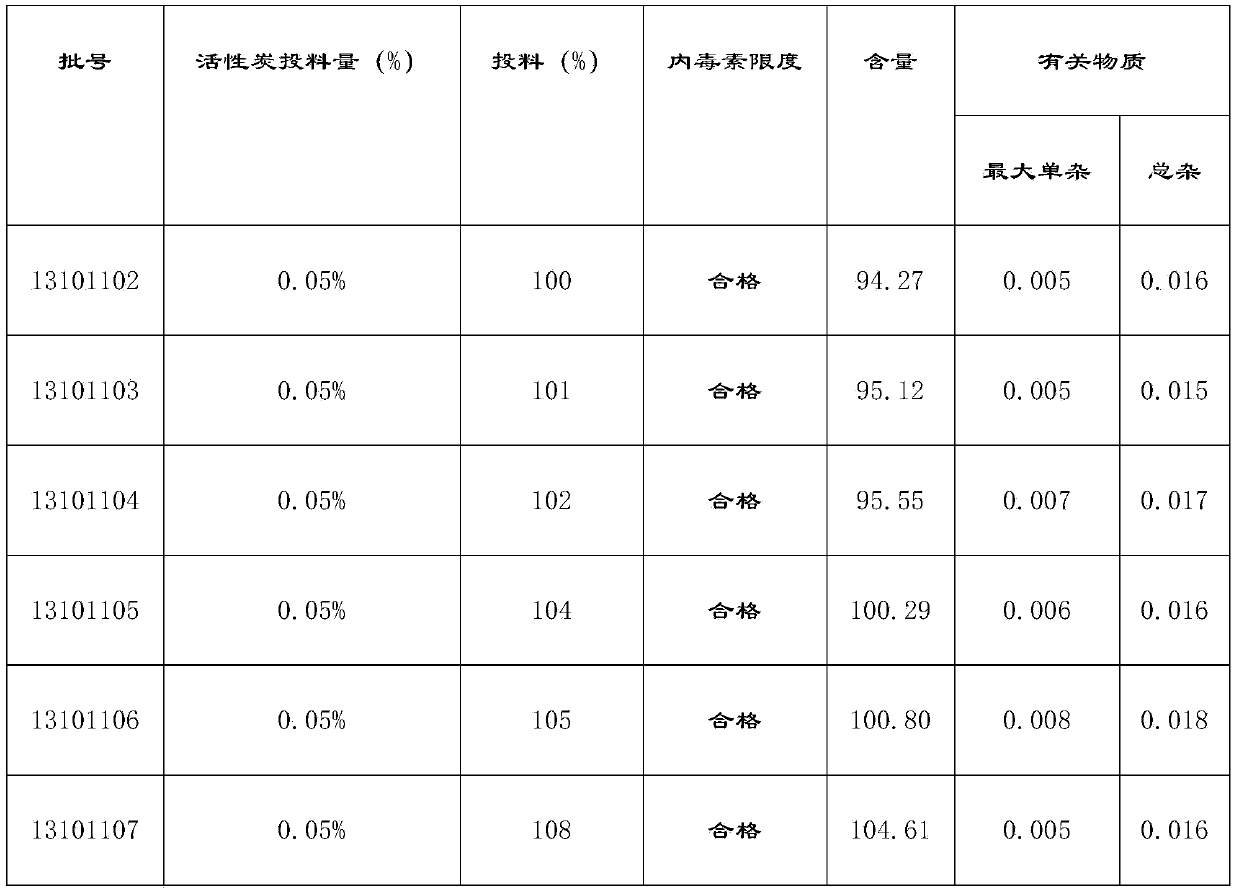
Moxifloxacin hydrochloride sodium chloride injection and preparation method thereof
ActiveCN103989630AImprove adsorption capacityAntibacterial agentsOrganic active ingredientsActivated carbonSodium Chloride Injection
The invention relates to moxifloxacin hydrochloride sodium chloride injection and a preparation method thereof, and aims at overcoming the shortage of the prior art, solving the problem that the detection result shows that moxifloxacin content is inconsistent in final moxifloxacin hydrochloride sodium chloride injection in the background technology, as well as solving the problem of complexity of the existing process. The preparation method, provided by the invention, is a novel preparation method capable of avoiding product pollution and ensuring the safety and stability of the product quality. The moxifloxacin hydrochloride sodium chloride injection is characterized by containing 0.05 to 0.3% (w / v) of moxifloxacin based on the amount of moxifloxacin, 0.8 to 0.85% (w / v) of sodium chloride, and water of injection, wherein the concentrated preparation volume is 70 to 80%, 0.025 to 0.1% (w / v) of activated carbon is added and agitated for 10 to 30 minutes, and hydrochloric acid or sodium hydroxide is used as a pH modifier. The preparation method is simple and feasible in process and can be used for producing medicines for treating or preventing human or animal bacterial infection.
Owner:WUHAN WUYAO SCI & TECH
Method for preparing levofloxacin hydrochloride sodium chloride injection
InactiveCN103479522ASubstance reductionHigh clarityAntibacterial agentsOrganic active ingredientsWater bathsSodium Chloride Injection
The invention provides a method for preparing levofloxacin hydrochloride sodium chloride injection. By researching a liquid medicine preparation method, medicinal charcoal pretreatment and a terminal sterilization process, product quality is ensured by the aid of technologies of a concentrated solution and diluted solution method, charcoal slurry preparation from medicinal charcoal, water bath sterilization and the like, production is facilitated, obtained finished products have higher stability, so that the storage life of the finished products can be prolonged, and clinical use effects are better.
Owner:HAINAN HOTMED TIANYA PHARMA
Moxifloxacinhydrochloride sodium chloride injection, preparation method thereof and use thereof
InactiveCN101884613AWill not crystallizePrecipitation does not occurAntibacterial agentsOrganic active ingredientsPhosphateSodium Chloride Injection
The invention relates to moxifloxacinhydrochloride sodium chloride injection. The injection comprises the following components in percentage by weight / volume: 0.03 to 1 percent of moxifloxacinhydrochloride, 0.01 to 3 percent of weak acid, 0.01 to 3 percent of salt of weak acid, 0.01 to 3 percent of phosphoric acid, 0.01 to 3 percent of amino acid or phosphate and 0.65 to 0.95 percent of sodium chloride.
Owner:HC SYNTHETIC PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com