Patents
Literature
111 results about "Ceftriaxone Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
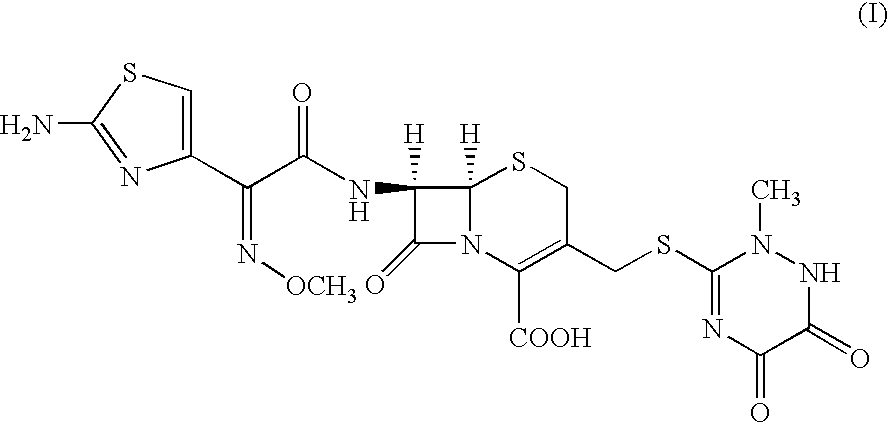
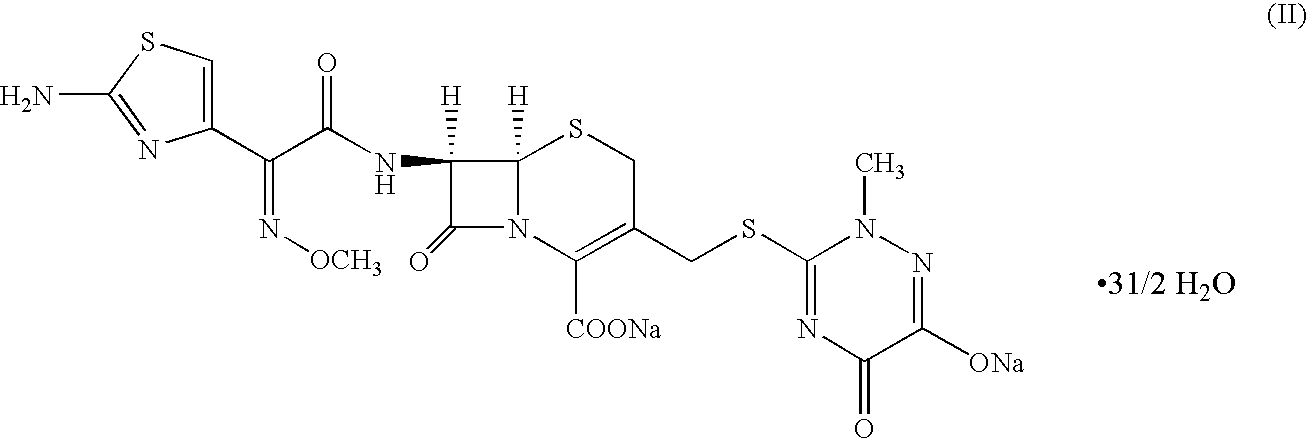
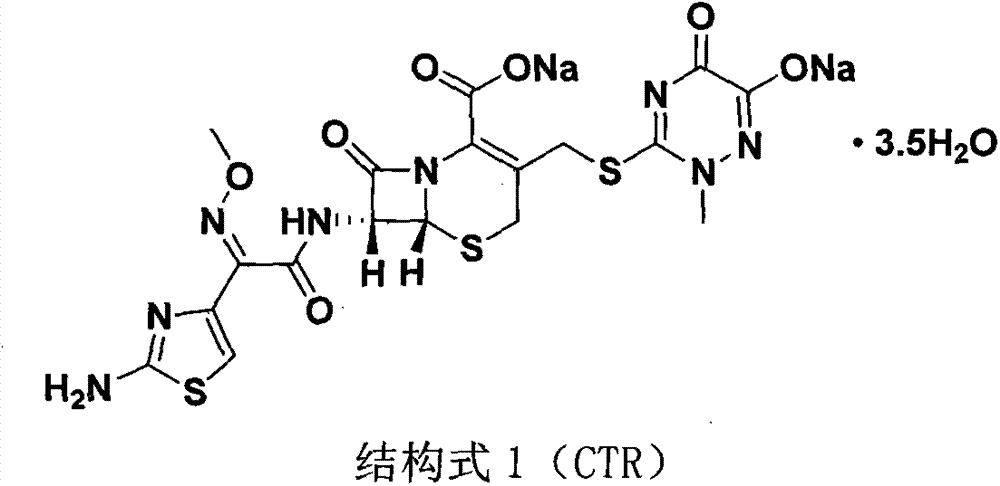
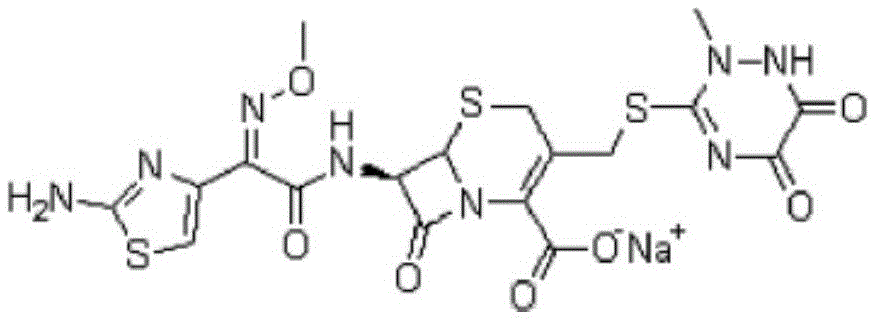
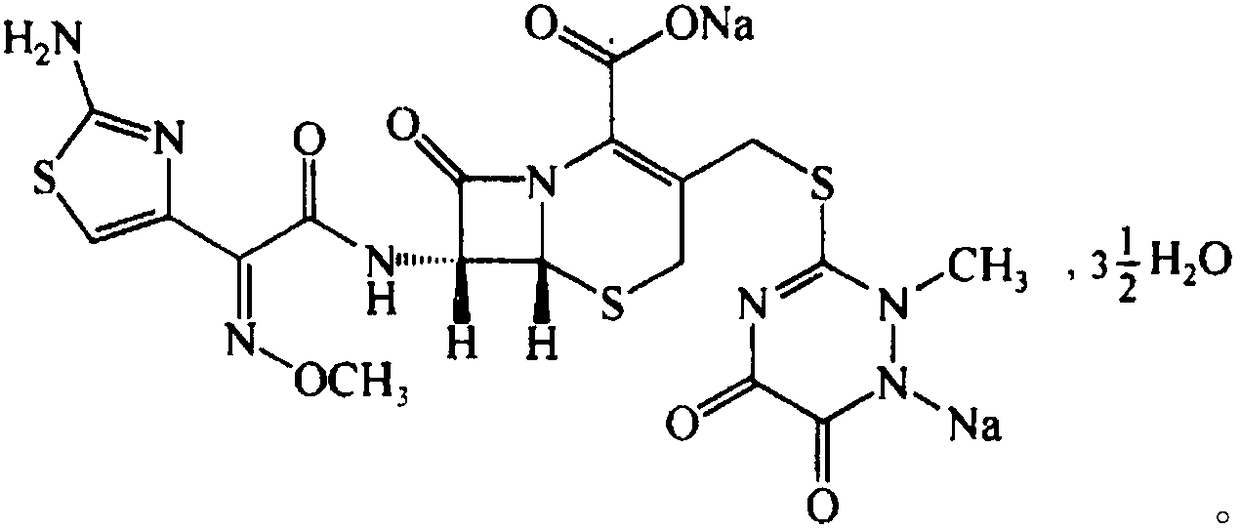
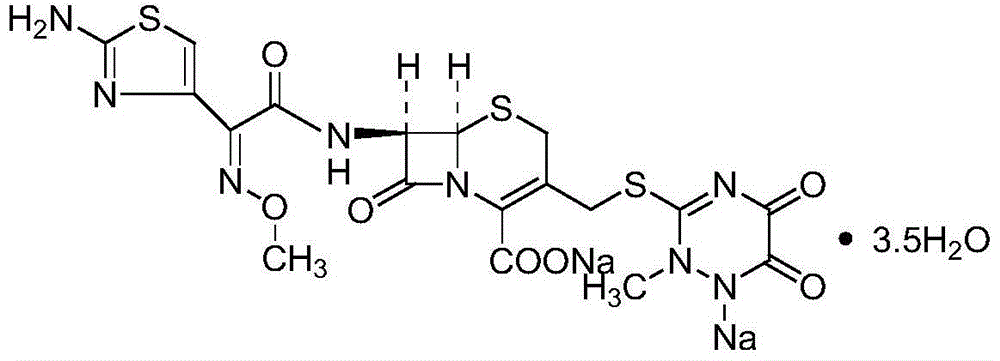
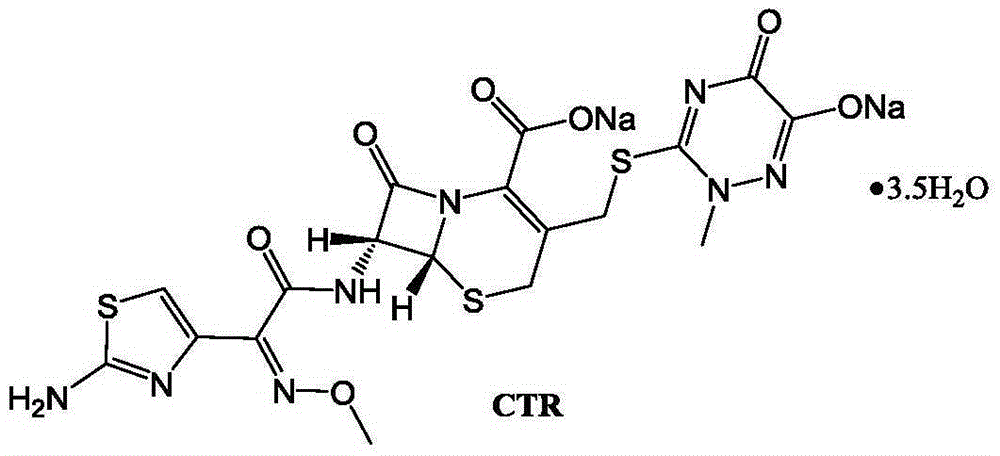
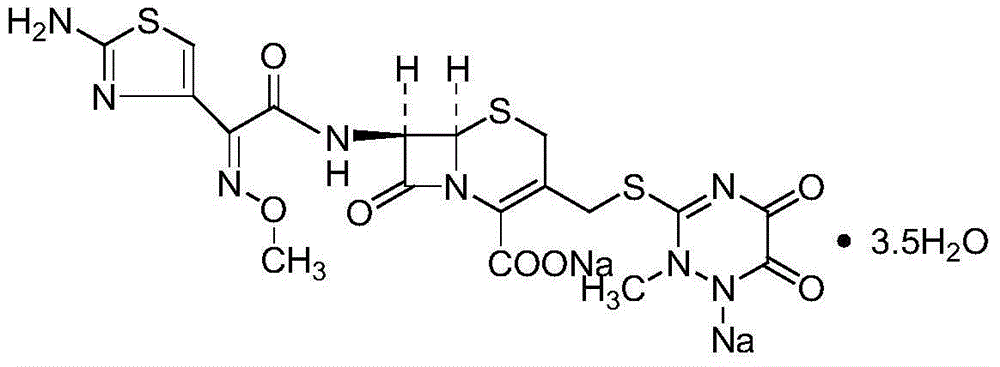
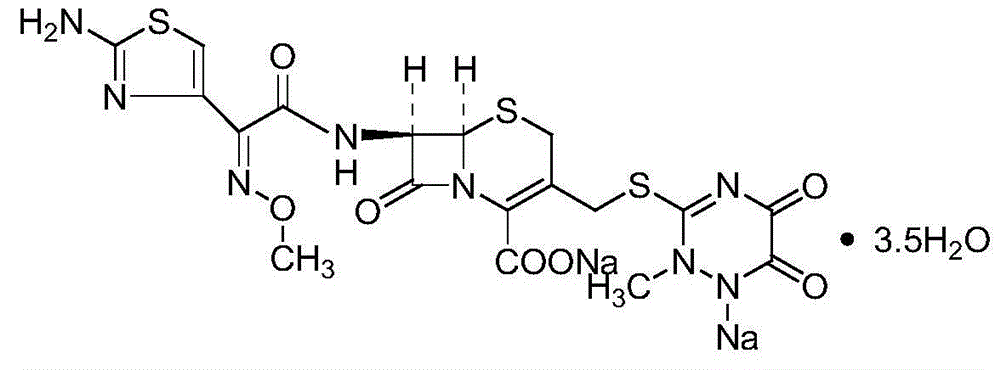
The sodium salt form of ceftriaxone, a beta-lactam, third-generation cephalosporin antibiotic with bactericidal activity. Ceftriaxone binds to and inactivates penicillin-binding proteins (PBP) located on the inner membrane of the bacterial cell wall. PBPs participate in the terminal stages of assembling the bacterial cell wall, and in reshaping the cell wall during cell division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. Compared to the second and first generation cephalosporins, ceftriaxone is more active against gram-negative bacteria and less active against gram-positive bacteria. Ceftriaxone also crosses the blood-brain barrier and reaches therapeutic concentrations in the central nervous system (CNS).
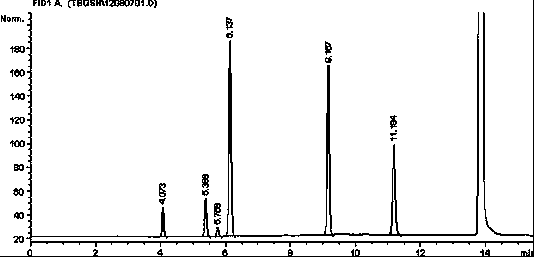
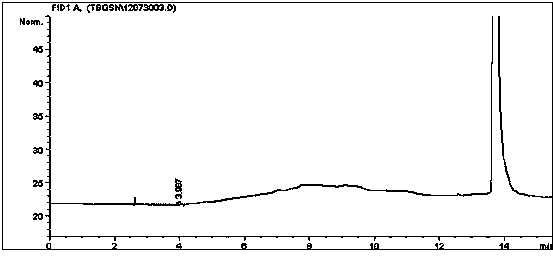
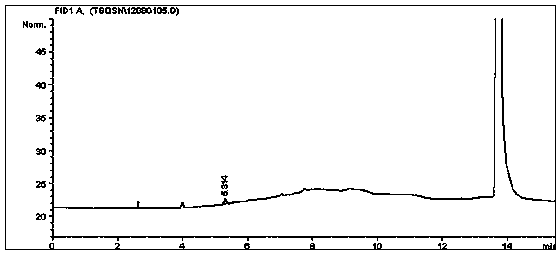
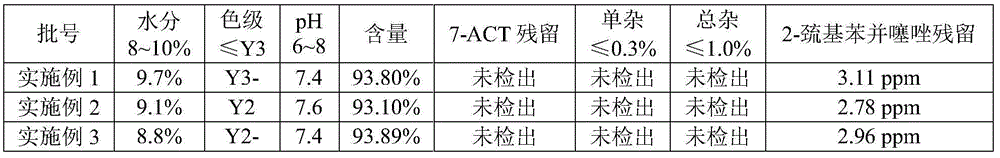
Method for simultaneously detecting multiple residual solvents in ceftriaxone sodium
ActiveCN103487541AHigh sensitivityHigh precisionComponent separationCeftriaxonumGas liquid chromatographic
The invention relates to a method for simultaneously detecting multiple residual solvents in ceftriaxone sodium, which adopts a gas chromatographic analysis process to simultaneously detect six organic solvents in medicines, including methanol, ethanol, acetonitrile, acetone, ethyl acetate and triethylamine. The gas chromatographic process is adopted to simultaneously detect the contents of the six organic solvents, and has the advantages of high sensitivity, favorable repetitiveness and high precision; by using a standard addition process, the method does not need any additional standard substance as an internal standard, only needs a pure substance for predicting components, can counteract the matrix effect, and is simple to operate; by using a headspace sampling process, the method prevents the direct solution sample from interfering with detection and from polluting the chromatographic column; and a programmed heating process is adopted to effectively separate low-boiling-point organic solvents from high-boiling-point organic solvents.
Owner:SHANGHAI NEW ASIA PHARMA
Method for manufacture of ceftriaxone sodium
Owner:LUPIN LTD
Method for preparing ceftriaxone sodium
ActiveCN103539803AIncrease surface tensionImprove solubilityOrganic chemistryBoron trifluorideAcetonitrile
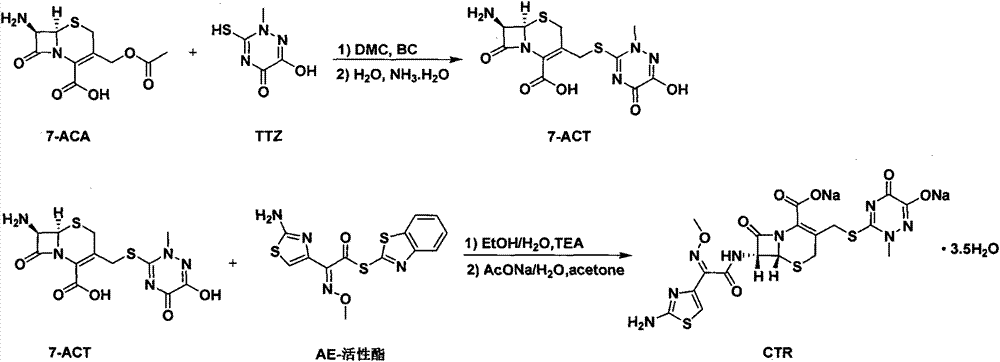
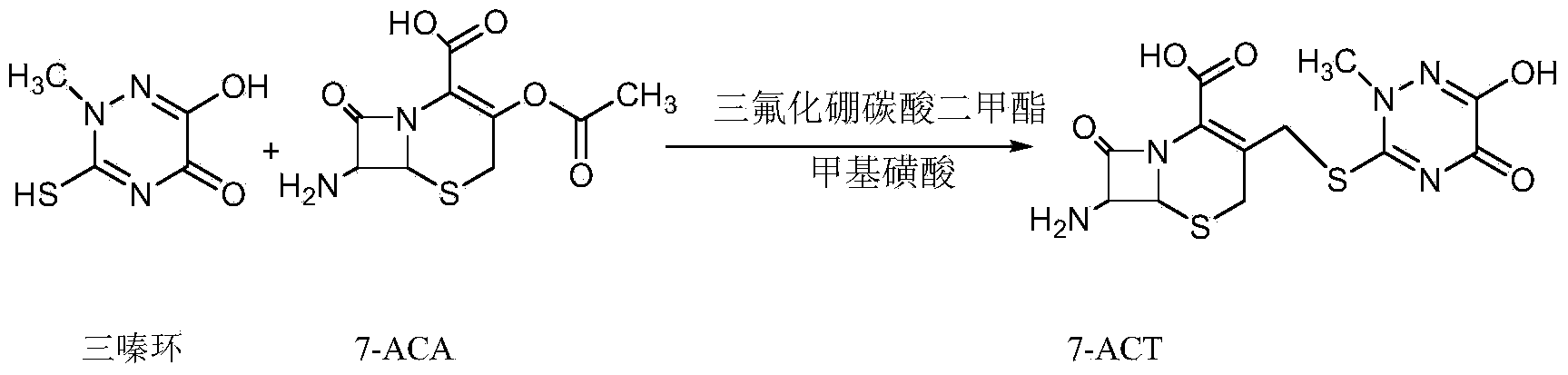
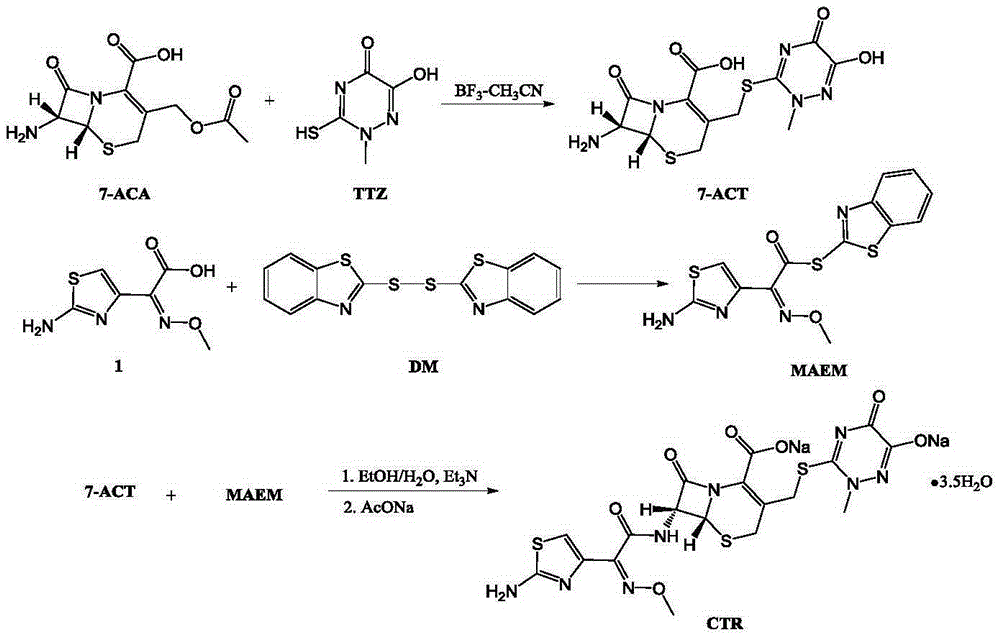
The invention relates to a novel green low-cost method for synthesizing ceftriaxone sodium. Firstly, in the 7-ACT synthesis process, the dimethyl carbonate is used as the reaction solvent, and the boron trifluoride-dimethyl carbonate is used as the catalyst, thereby substituting the use of the high-cost high-toxicity acetonitrile; and secondly, in the ceftriaxone sodium synthesis process, the ethanol-water reaction system is adopted to obtain the high-yield high-quality ceftriaxone sodium.
Owner:珠海保税区丽珠合成制药有限公司
Pithecellobium clypearia extracts and application of extract in preparation of medicines for treating methicillin-resistant staphylococcus aureus
ActiveCN103385912AHas a sensitizing effectRealize comprehensive utilizationAntibacterial agentsPlant ingredientsEthyl acetatePharmaceutical Substances
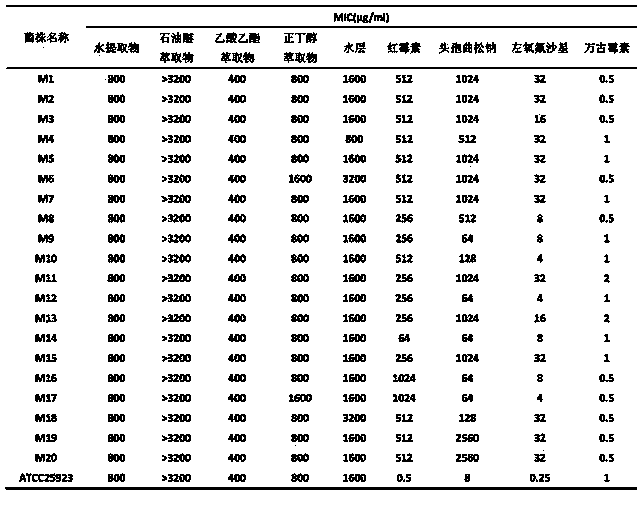
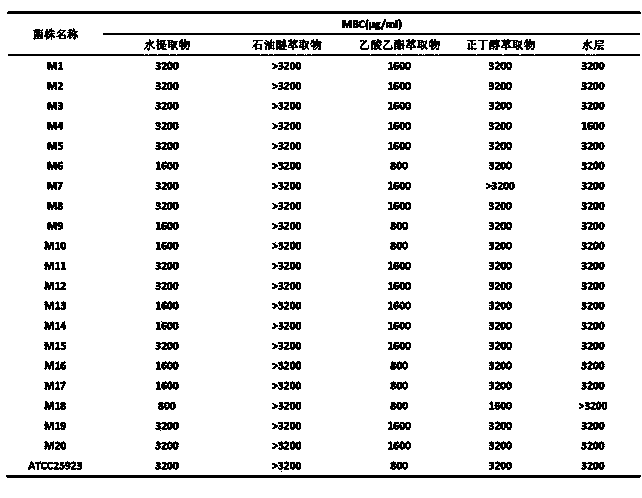
The invention discloses water, ethanol and ethanol aqueous extracts of traditional Chinese medicine pithecellobium clypearia and application of corresponding petroleum ether, ethyl acetate, normal butanol and a water extractants in preparation of medicines for treating methicillin-resistant staphylococcus aureus (MRSA) and antibiotics anti-MRSA sensitization medicines. Meanwhile, the invention further discloses a preparation method of the extracts or extractants. Experimental results show that water, 10% ethanol, 30% ethanol, 60% ethanol, 95% ethanol extracts of pithecellobium clypearia and corresponding ethyl acetate, normal butanol and water extractants have stronger anti-MRSA effect, wherein the activity of the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia is the strongest, and the ethyl acetate extracting part of the 60% ethanol extract of pithecellobium clypearia has sensitization effect on erythrocin, ceftriaxone sodium, levofloxacin for treating MRSA.
Owner:HUACHENG PHARMA FACTORY GAUNGZHOU
Process for preparing ceftriaxone sodium
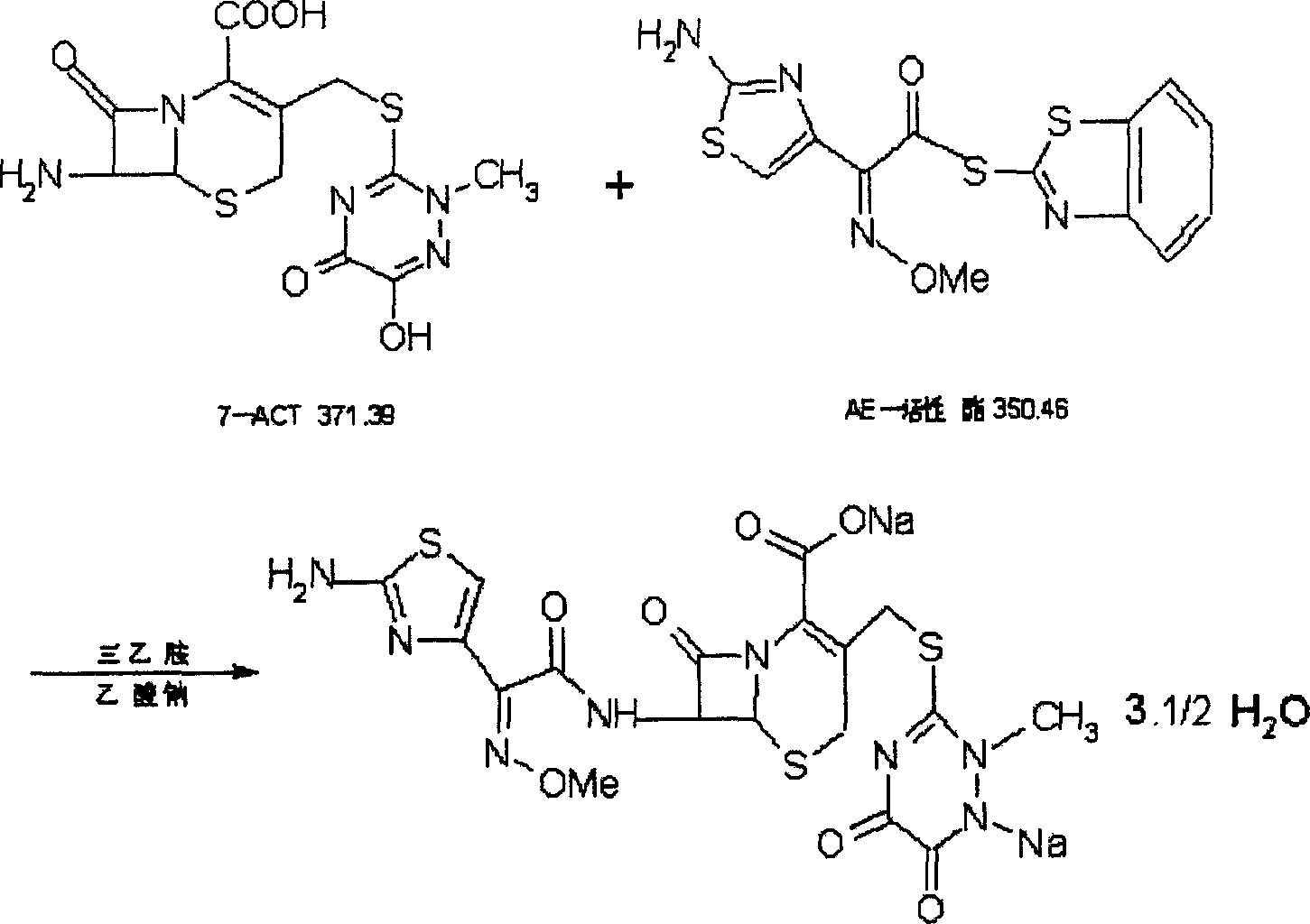
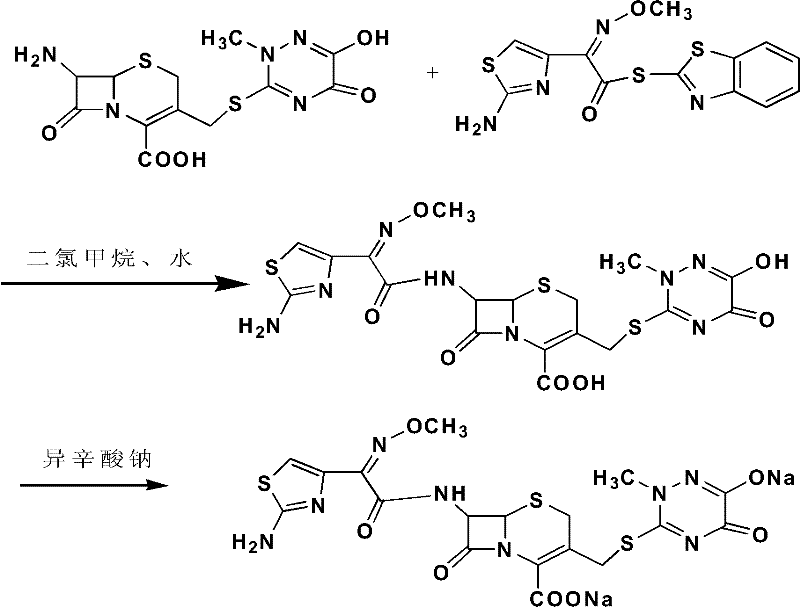
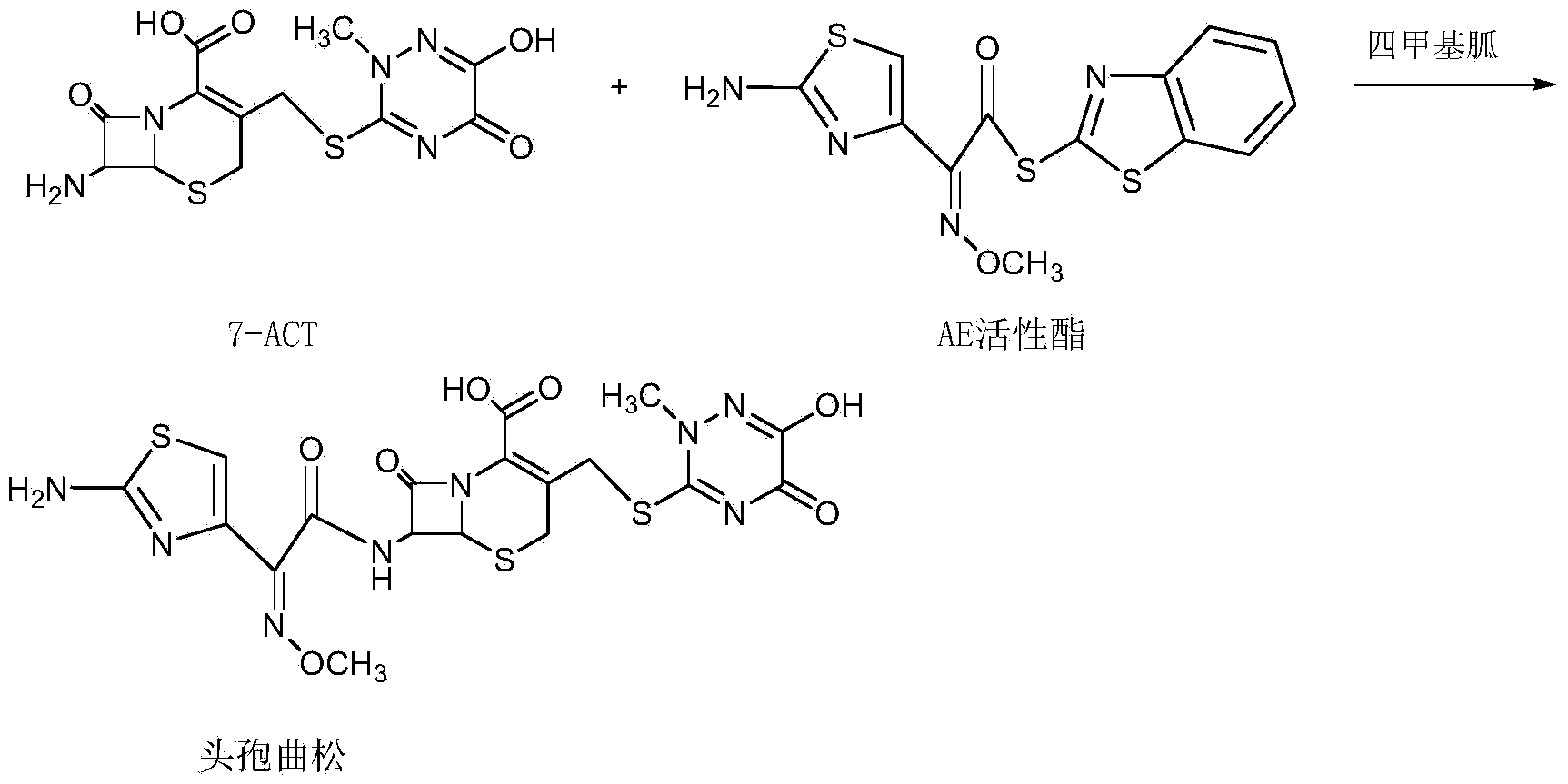
Disclosed is a process for preparing ceftriaxone sodium belonging to compound preparation technical field. Protected by nitrogen, 7-ACT3 reacts with AE-active ester under the action of amine intermediate reactant in a solvent. Then a sodium salt forming agent is added in, and the cefotaxime sodium is obtained after reaction and seedout. The solvent is a mixed solvent composed of alkane halocarbon, ethyl acetate, or acetone and alcohol solvent and water. The solvent dosage is small, and the product yield is high.
Owner:REYOUNG PHARMA
Refining process for crude product of ceftriaxone sodium
The invention relates to an art for refining a ceftriaxone sodium crude product, which includes the following steps: the ceftriaxone sodium crude product is put into injection water to dissolve till clarification; the insoluble organic solvent of the ceftriaxone sodium is added after the solution is sterilized and filtered; then an aseptic ceftriaxone sodium crystal seed is added, and a crystal nourishing treatment is carried out after a crystal is precipitated; the insoluble organic solvent of the ceftriaxone sodium is added continuously; and finally the ceftriaxone sodium finished product is obtained after normal crystallization, washing and drying. The art for refining the ceftriaxone sodium crude product is characterized in that: the insoluble organic solvent of the ceftriaxone sodium is isopropanol. By adopting a single isopropanol solvent, the art for refining the ceftriaxone sodium crude product reduces the use amount of the solvent sharply, which is 57 percent of the former art, and has the advantages of convenient operation, easy control, short production cycle (shortened by 4 hours compared with the former art), low labor strength, low production cost, and stable color which is weaker than the color of the mixed solvent crystal by 0.5 to 1 size. Meanwhile, the art for refining the ceftriaxone sodium crude product can improve the production yield by 2 percent with the yield being 98 percent, and reduces the damage of the solvent to the operators owing to the weak solvent toxicity.
Owner:REYOUNG PHARMA
Preparation method of ceftriaxone sodium
InactiveCN102702233ASimple production processReduce manufacturing costOrganic chemistryOrganic basePotassium
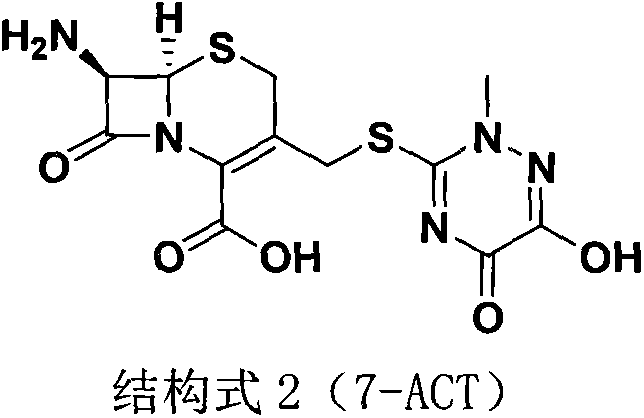
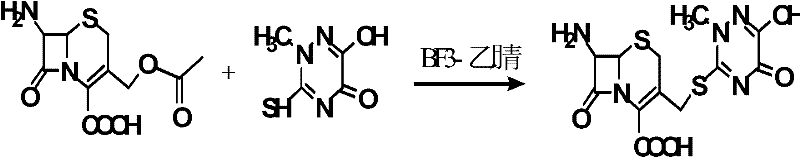
The invention discloses a preparation method of ceftriaxone sodium. The method comprises the following steps: with 7-ACA and triazine ring as raw materials, reacting under the catalysis of boron trifluoride acetonitrile, crystallizing to obtain 7-ACT, recovering potassium fluoborate and acetonitrile from the mother solution, dissolving 7-ACT and AE-active ester in a unitary or binary solvent system, reacting under catalysis of an organic base, adding a salifying agent and a water-soluble organic solvent in the water phase to separate out sodium salt and obtain ceftriaxone sodium, and recovering 2-mercaptobenzothiazole from the organic phase. The preparation method of ceftriaxone sodium provided by the invention has simple production process and low production cost, the yields in two steps are both higher than 90 percent, the solvents, organic matters, inorganic salts and the like in the reaction system can be recycled, and no or little waste water is generated.
Owner:苏州盛达药业有限公司
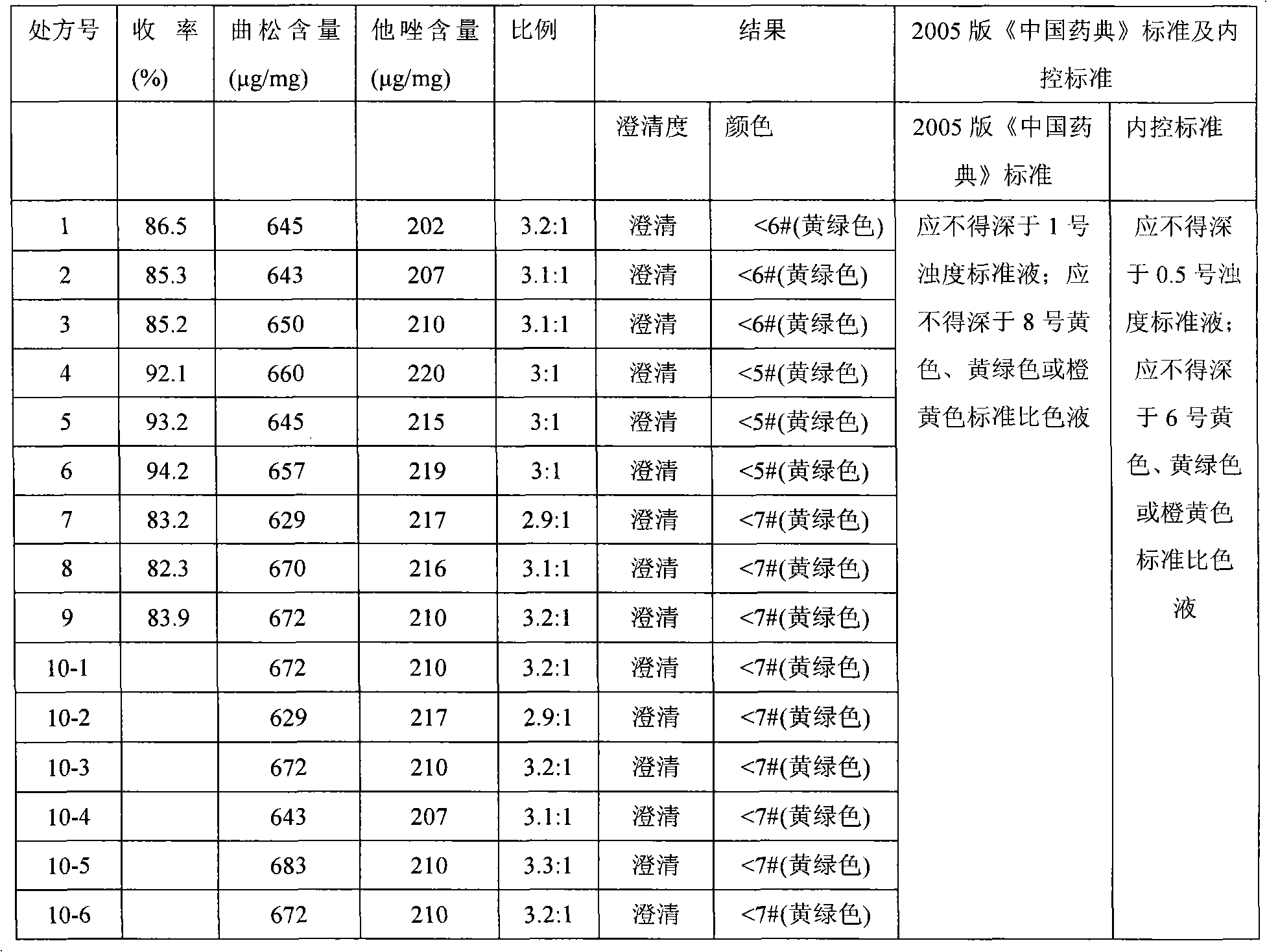
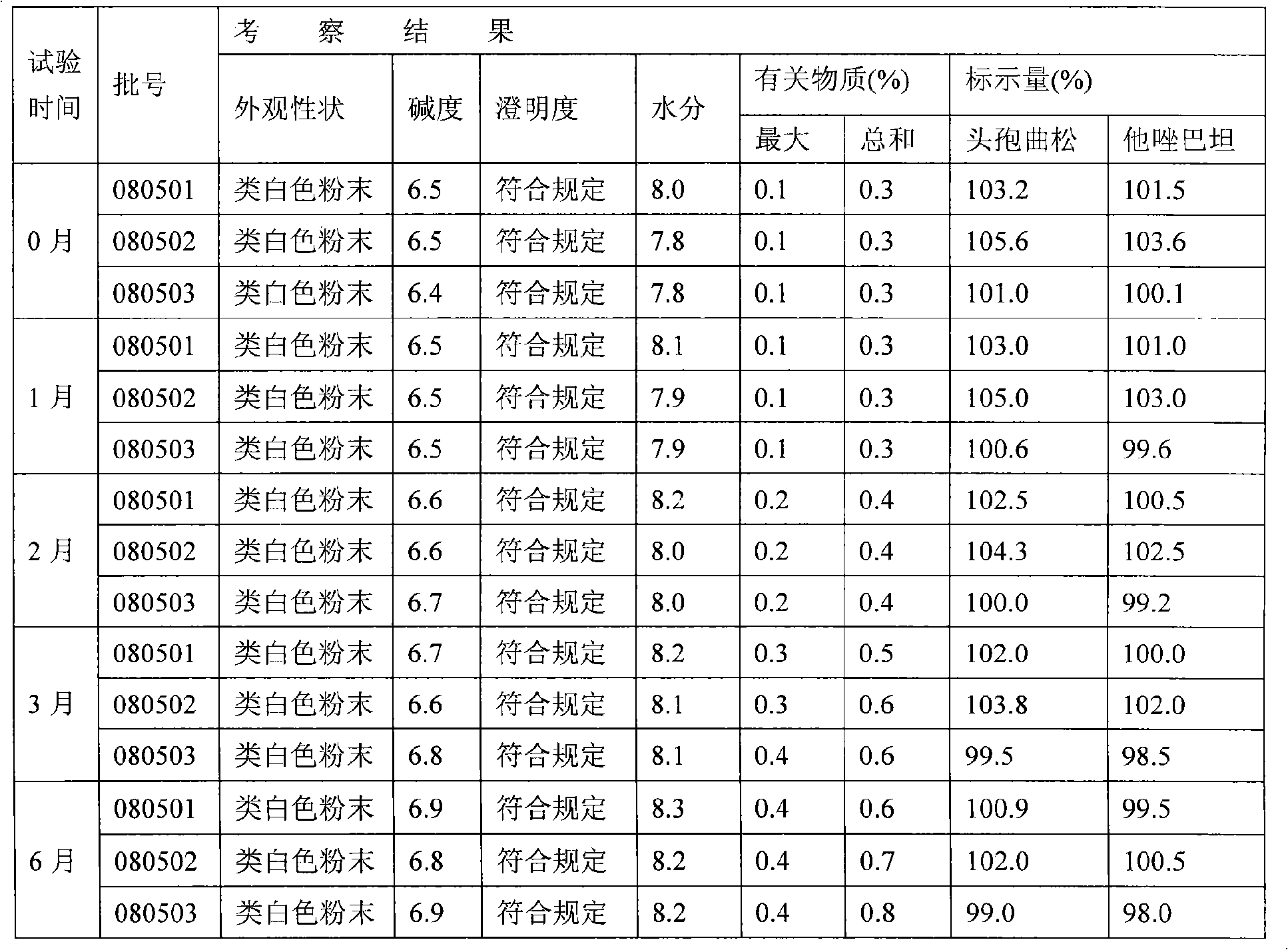
Medicinal composition consisting of ceftriaxone sodium and sulbactam sodium and preparation method thereof
ActiveCN102462684AStable contentEasy to storeAntibacterial agentsPowder deliverySolubilityCurative effect
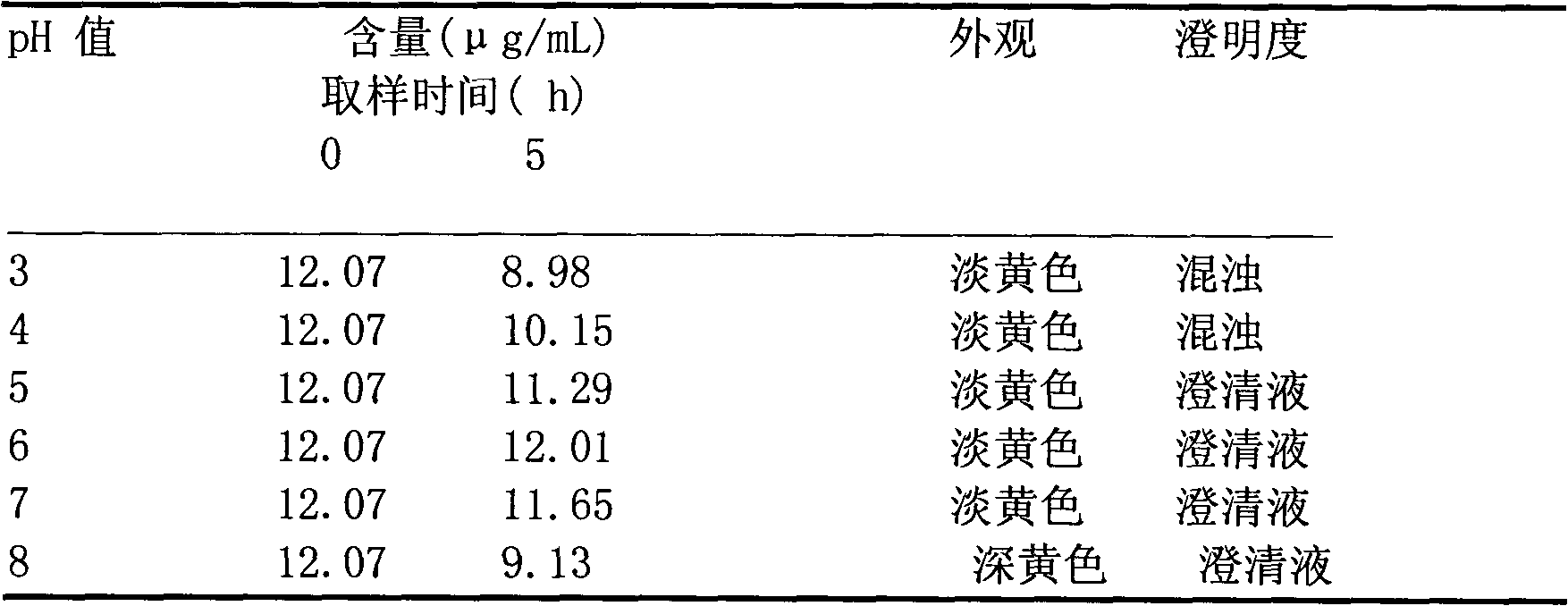
The invention aims to provide a medicinal composition consisting of ceftriaxone sodium and sulbactam sodium, which does not have any sensitization effect, has high stability and is efficient, and a preparation method thereof. The weight ratio of the ceftriaxone sodium to the sulbactam sodium to a stable conditioning agent component in the medicinal composition is (1-100):(0.25-100):(0.0005-9). The medicinal composition provided by the invention is in a good crystal form, and has stable and controllable quality at the temperature between 20 DEG C below zero and 60 DEG C in production and transportation processes and a good curative effect; and an effective period can be up to 36 months. During clinical administration, the medicinal composition is dissolved and diluted through the conventional transfusion, the ceftriaxone sodium and the sulbactam sodium in the medicinal composition have stable content, high solubility and indecomposability, insoluble crystals and sensitized high polymers are not produced, the influences of temperature and illumination are small, high stability is achieved, and the effective period is 36 months, so that a better curative effect is achieved. A preparation method of the medicinal composition provided by the invention is scientific and reasonable, is convenient to operate, and is suitable for large-scale industrial production.
Owner:XIANGBEI WELMAN PHARMA CO LTD
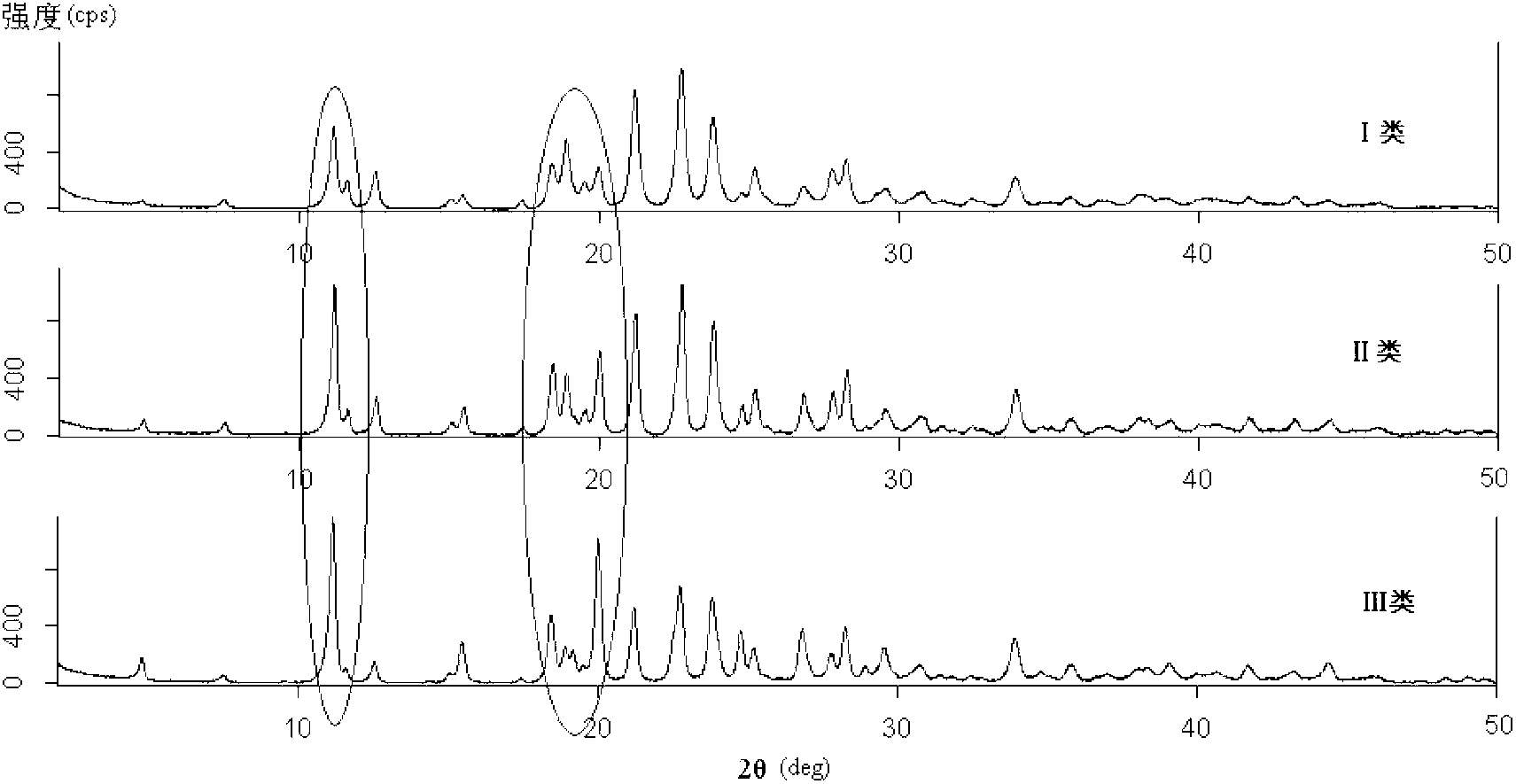
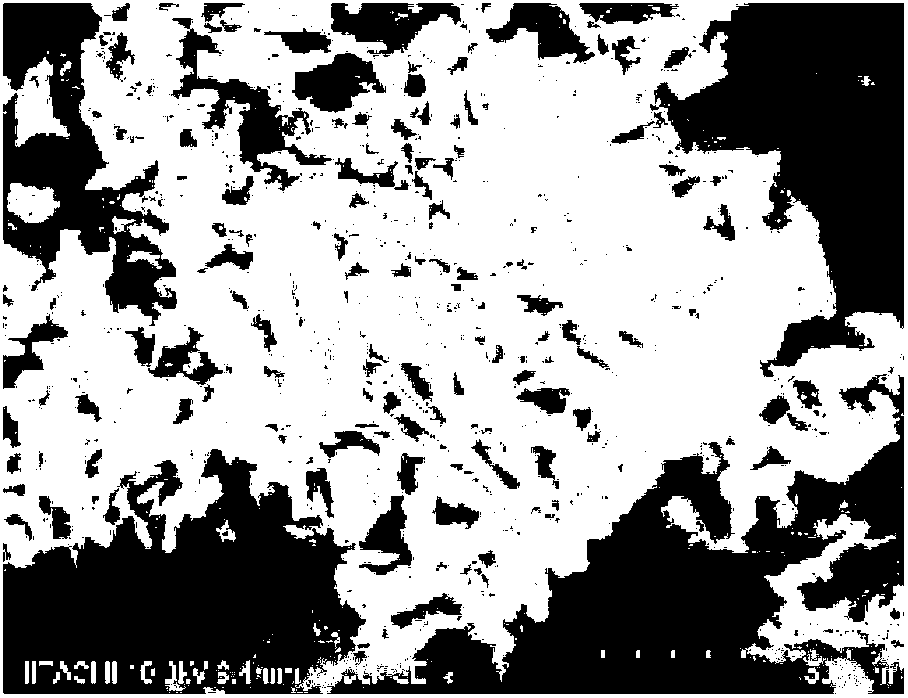
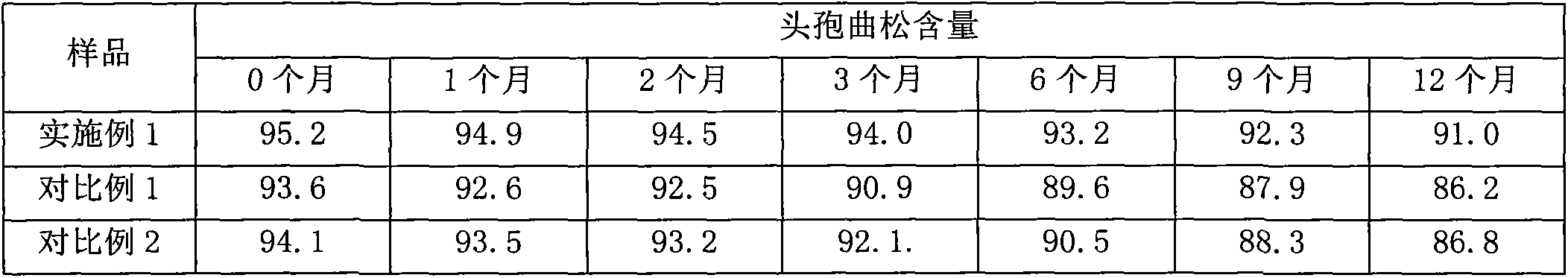
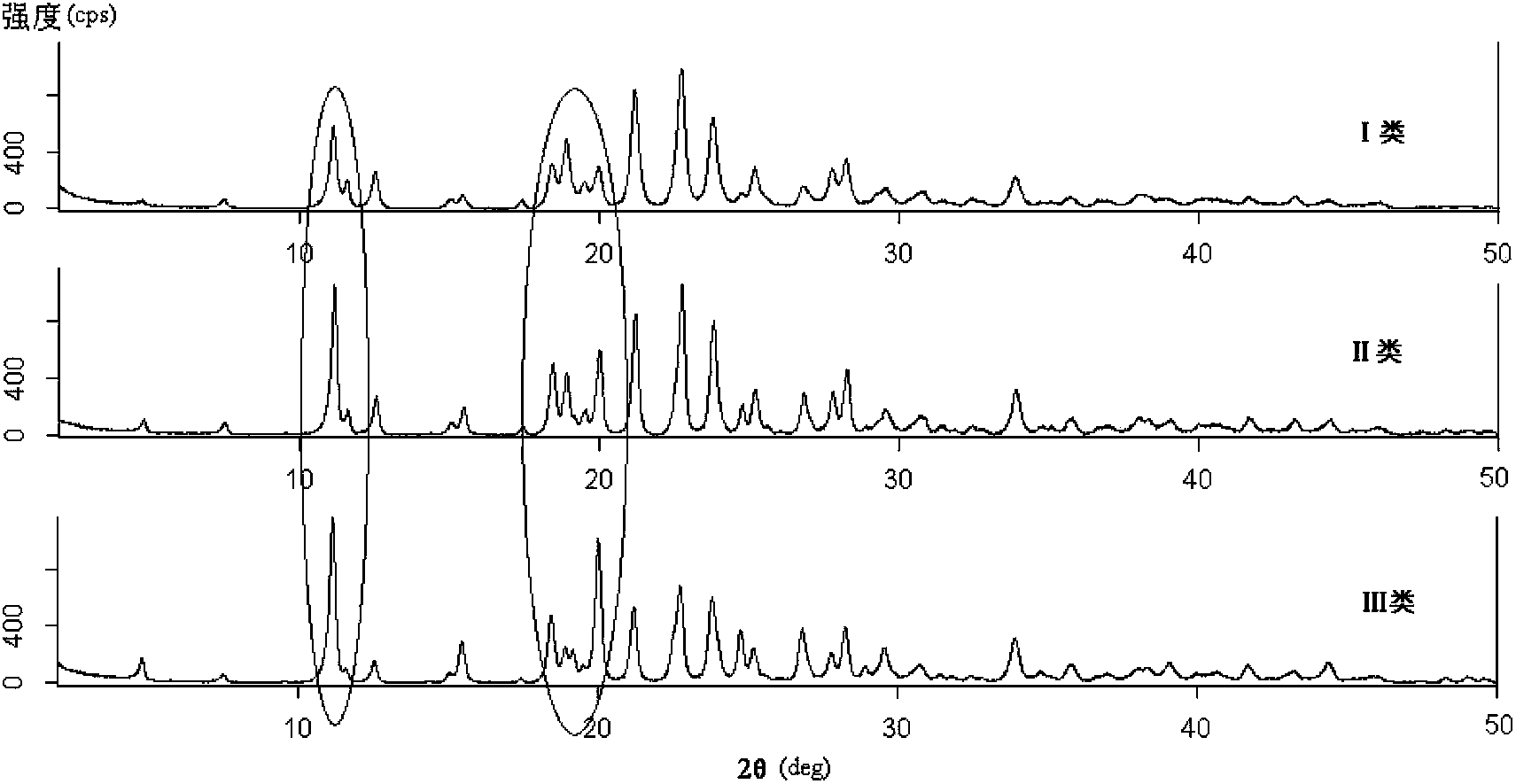
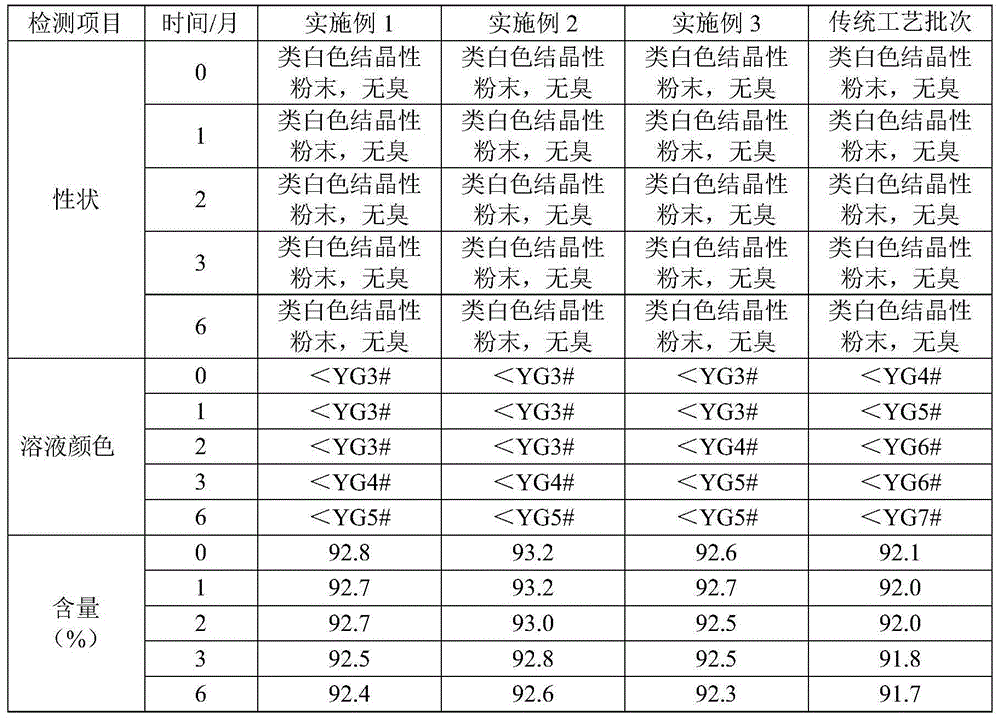
Preparation method of ceftriaxone sodium crystal and evaluation method of ceftriaxone sodium aqueous solution turbidity
ActiveCN102993215AReduce the incidence of allergic reactionsHigh clarityOrganic chemistryMaterial analysis using radiation diffractionCLARITYHypersensitive response
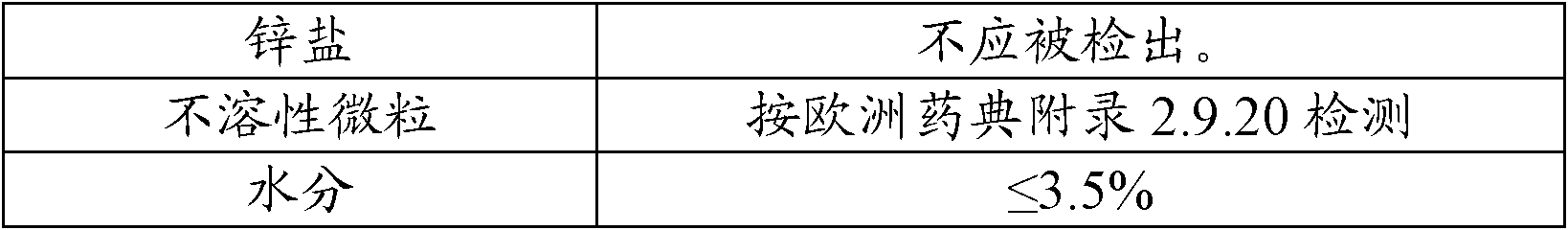
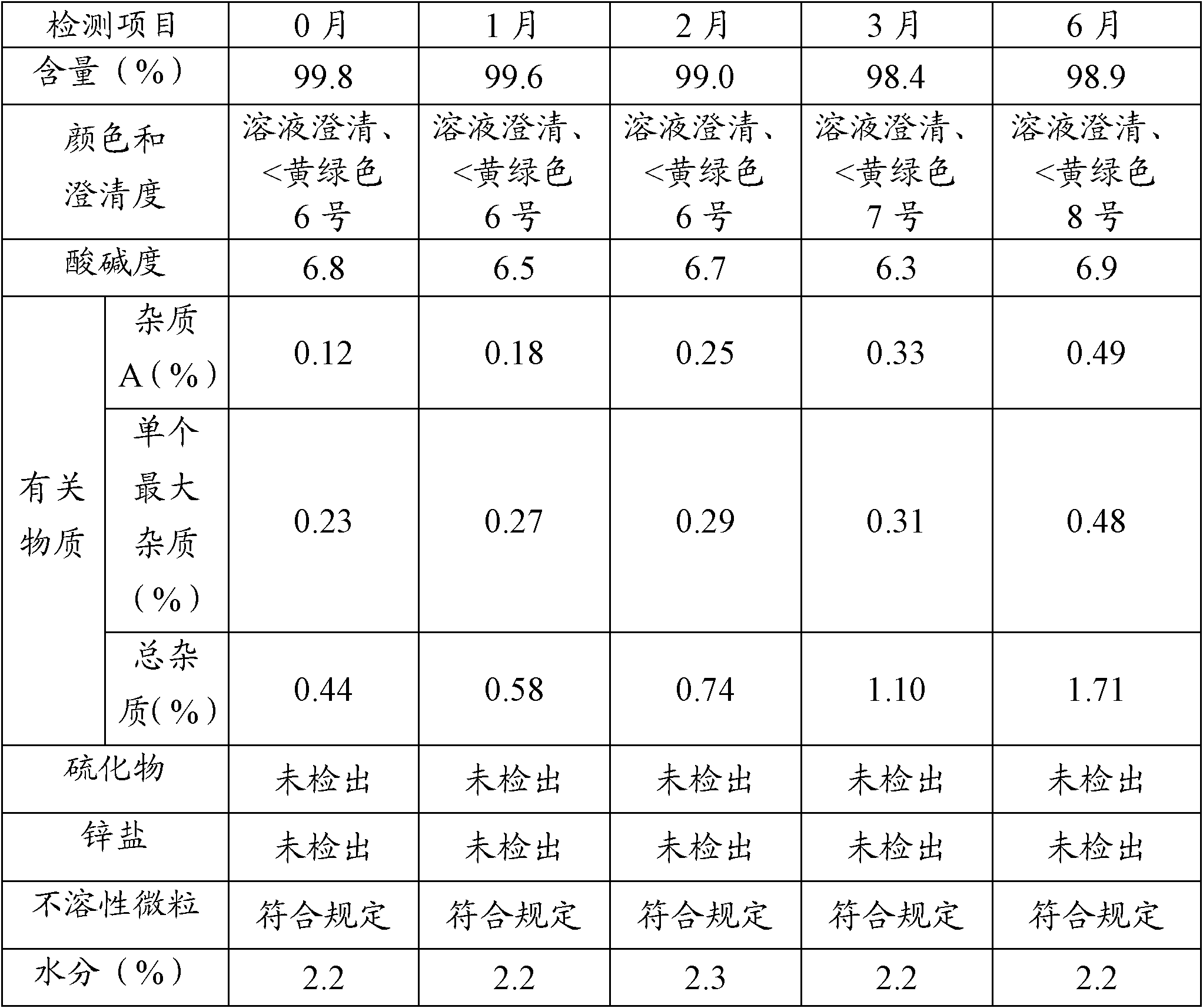
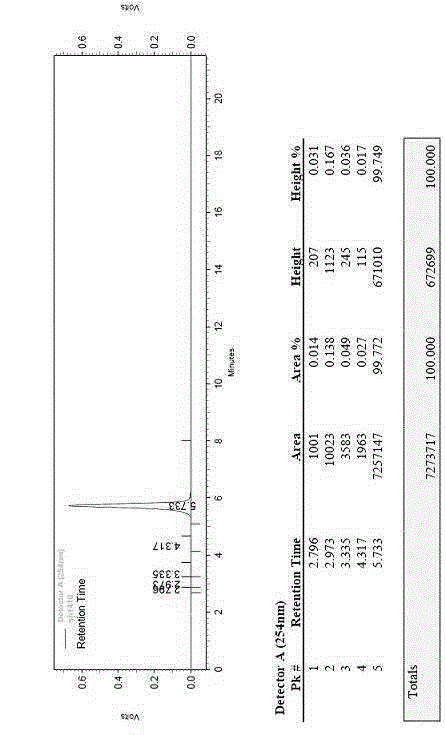
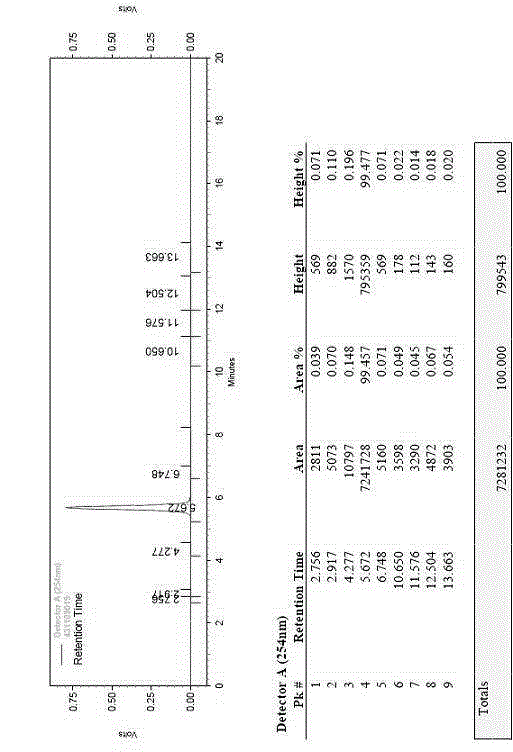
The invention relates to a preparation method of ceftriaxone sodium crystal, wherein the obtained ceftriaxone sodium is the crystal of the same subtype, and the subtype crystal has the best clarity, the lowest anaphylaxis occurrence rate and the highest safety in comparison with all crystals. The preparation method is simple in technology and excellent in reproducibility. The invention further relates to an evaluation method of ceftriaxone sodium aqueous solution turbidity, wherein a powder X-ray diffraction technology and a scanning electron microscope technology are used, in combination with statistical methods, a method for evaluating the ceftriaxone sodium crystal form is established, three subtypes of the ceftriaxone sodium crystal form is defined for the first time, and the relation between the subtypes and the aqueous solution turbidity is established, thus, rapid evaluation on crystal form and aqueous solution turbidity of ceftriaxone sodium samples becomes possible, and the method has great practical value.
Owner:YOUCARE PHARMA GROUP +1
Ceftriaxone sodium powder-injection for injection
ActiveCN104873466ALess impuritiesImprove stabilityAntibacterial agentsPowder deliveryNorth chinaSolvent
The invention discloses a ceftriaxone sodium powder-injection for injection. the powder-injection is prepared by the following steps: (1) weighing a ceftriaxone sodium crude raw material at 20 DEG C, adding water, stirring and dissolving, adding active carbon, decolouring, filtering, and washing with mixed solvents; (2) by a particle process crystal product molecular assembly and shape-state optimization technology of North China Pharmaceutical Hebei Huamin Pharmaceutical Co., Ltd., adding a solventing-out agent acetone according to a stream acceleration table at 15 DEG C at the stirring speed of 300 r / min; (3) carrying out suction filtration, washing a filter cake with acetone, putting the filter cake into a vacuum drying oven and carrying out vacuum drying at 30-40 DEG C; and (4) sub-packaging preparations of different specifications, and controlling environmental temperature and humidity until temperature is 20-24 DEG C and humidity is less than 40% so as to obtain the ceftriaxone sodium for injection. In comparison with a traditional technology, ceftriaxone sodium prepared by the above preparation method has advantages of less impurity, high stability and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Synthetic method of ceftriaxone sodium crude salt
InactiveCN102559829AHigh purityHigh yieldOrganic chemistryFermentationChemical synthesisEnzymatic hydrolysis
The invention belongs to the field of chemical synthesis, and particularly relates to a synthetic method of a ceftriaxone sodium crude salt. The method comprises the following steps of: (1) performing electrophilic substitution on 7-ACA and a triazine ring by taking a BF3-acetonitrile solution as a catalyst to obtain 7-ACT at last; and (2) undergoing an N-acylation reaction on 7-ACT and AE-active ester in an organic phase, adding sodium iso-octoate, and undergoing a salt forming reaction to obtain the ceftriaxone sodium crude salt. The method is characterized in that: the 7-ACT is prepared with an enzymatic hydrolysis method after electrophilic substitution in the step (1); and a flocculating agent is added after the N-acylation reaction is completed in the step (2). The method has the advantages that: the product yield and purity are raised; the 7-ACT is prepared with the enzymatic hydrolysis method in the first step, and the enzymatic hydrolysis method has the characteristics of specificity and high efficiency, so that side reactions are avoided, the yield is increased by over 8 percent, and can be up to 88 percent, and the product purity is raised; and the flocculating agent is added in the second step, so that insoluble matters in a reaction liquid are removed, and a high-purity ceftriaxone sodium crude salt is obtained finally.
Owner:YIYUAN XINQUAN CHEM
Method for synthesizing ceftriaxone sodium
The invention discloses a method for synthesizing ceftriaxone sodium. The method comprises the steps of firstly carrying out condensation on 7-ACA and triazine ring by taking dimethyl carbonate and organic acid as a mixed solvent and taking boron trifluoride dimethyl carbonate as a catalyst, so as to produce 7-ACT, then, enabling 7-ACT and AE (Active Ester) to react by taking tetramethyl guanidine as a cosolvent, so as to produce ceftriaxone, and finally, adding sodium acetate, thereby synthesizing the sodium salt. The method has the advantages that the operating method is simple, the conditions are mild, the raw materials are easily available, the pollution is little, the cost is very low, the purity of the finally obtained ceftriaxone sodium product is over 99.6%, and the molar yield is over 94%.
Owner:哈药集团股份有限公司 +1
Preparation method of ceftriaxone sodium
The invention provides a preparation method of ceftriaxone sodium. The preparation method includes steps of (1) cooling dichloromethane, methyl alcohol and water mixed solvent, adding 7-ACT and AE (active ester) and trimethylamine, controlling the temperature and timing to react; (2) sequentially adding sodium hydrogen sulfite, sodium acetate and sodium hydroxide and stirring; adding extractant, stirring, standing and splitting phase; extracting extractant by water and splitting phase, and combining water phase; (3) adding extractant under water-phase stirring, transferring to and filling in a pressure container, removing air bubbles and vibrating hermetically, and taking out after freezing with temperature controlled; (4) removing organic phase, adding activated carbon after solids are melted, stirring to decolorize, and filtering in a decarbonized and aseptic manner; adding filtrate into solvent to crystalize, filtering, washing, drying and packaging. The preparation method is low in technique cost, moderate and safe in reaction conditions, convenient to operate and facilitates industrial production, and the obtained product conforms to quality requirements and has the advantages of few impurities and good crystal forms.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Pharmaceutical packaging composition for injection and preparation method thereof
InactiveCN102670400AGuaranteed sterilityTotal quality stabilityPharmaceutical containersMedical packagingPolyesterCephazolin sodium
The invention provides a pharmaceutical packaging composition for injection, which comprises the combination of an aluminum-plastic composition cover containing a coating plastic plug, a sterile antibiotic glass bottle and pharmaceutical sterile powder for injection, wherein the coating material of the coating plastic plug is polydimethylsiloxane coating, polyparaxylene coating, polytetrafluoroethylene coating, ethylene-tetrafluoroethylene copolymer coating, polyester coating, polyethylene coating or polypropylene coating; and the pharmaceutical sterile powder for injection is cephalo-type pharmaceutical sterile powder, such as cefuroxime sodium, cefoxitin sodium, cephazolin sodium and ceftriaxone sodium. The pharmaceutical packaging composition for injection is compatible with the pharmaceutical sterile powder for injection, so that the transition incidence rate is lower, and the quality risks of unqualified solution clarity due to compatibility and addition of related substances are reduced. The invention further provides a preparation method for the pharmaceutical packaging composition for injection, so that the obtained pharmaceutical packaging composition for injection can solve the compatibility problem very well and guarantee the stability of pharmaceutical quality.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
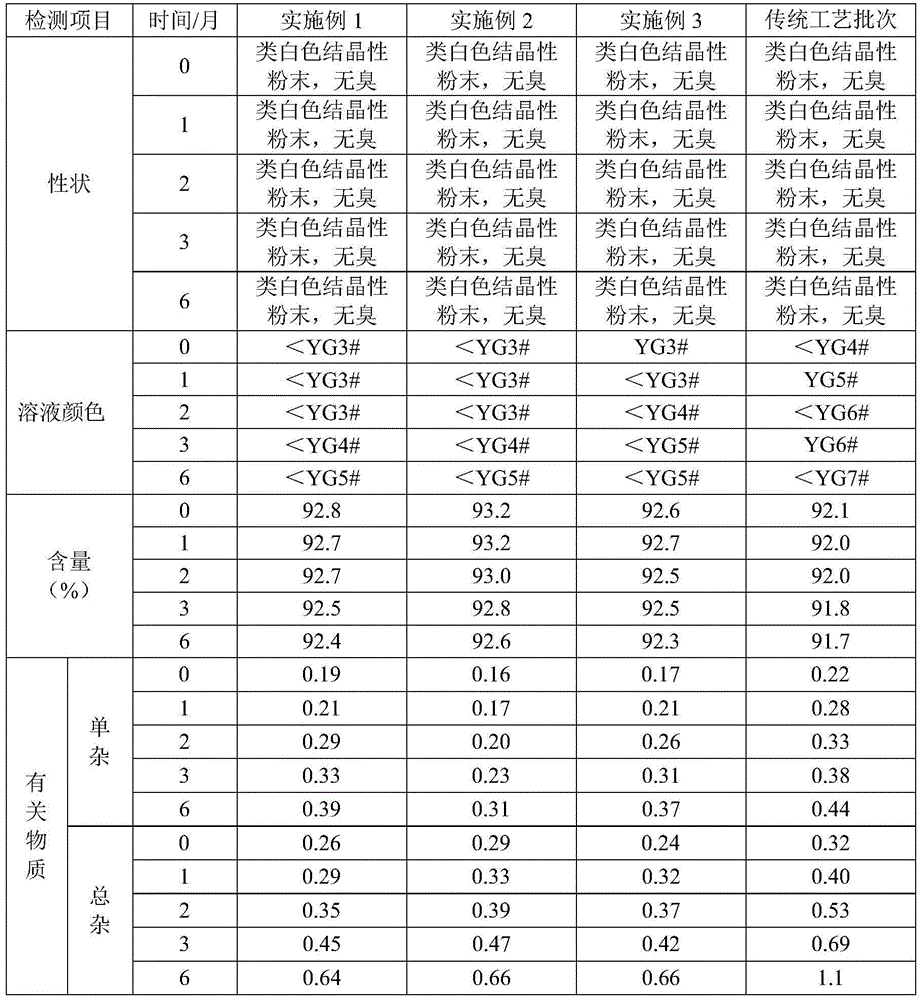
A kind of refining method of ceftriaxone sodium crude product
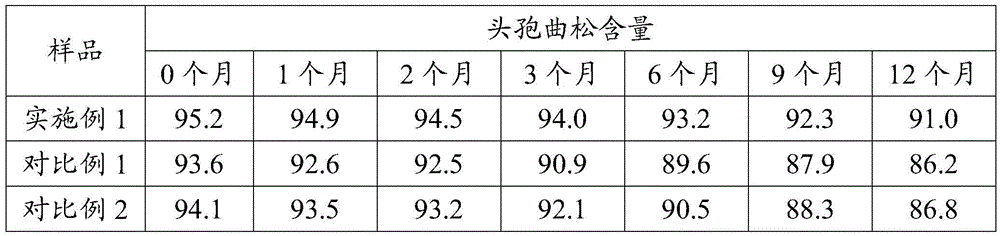
ActiveCN104031067BImprove crystal qualityReduce degradationOrganic chemistryCrystallizationCeftriaxone Sodium
The invention provides a refinement method of a ceftriaxone sodium crude product. Ceftriaxone sodium can be easily subjected to degradation reaction in the crystallization process; and the ceftriaxone sodium is not stable and can be easily subjected to oxidative degradation to generate a degradation product mainly in a pure water system, thereby causing high color grade of the product and directly influencing the quality of the ceftriaxone sodium. The refinement method provided by the invention can lower the degradation of the ceftriaxone sodium in the crystallization process.
Owner:LIVZON PHARM GRP INC +1
Preparation method of ceftriaxone sodium spherical crystals
ActiveCN108440569AImprove liquidityGood compressibilityOrganic chemistry methodsCompressibilitySolvent
The invention belongs to the technical field of pharmacy, and in particular relates to a preparation method of ceftriaxone sodium spherical crystals. The method comprises the following steps: providing ceftriaxone sodium crude drug, a bridging agent and a solventing-out agent; dissolving the ceftriaxone sodium crude drug into a polar solvent to obtain a first ceftriaxone sodium solution; mixing the bridging agent with the first ceftriaxone sodium solution to obtain a second ceftriaxone sodium solution; mixing the solventing-out agent with the second ceftriaxone sodium solution so as to separate out the ceftriaxone sodium spherical crystals. The preparation method provided by the invention is suitable for aseptic continuous operation of the actual industry, and not only simplifies the production process, has high single-pass yield and is easy in realization of industrial production, but also obtains coalesced particles with good fluidity, compressibility and stability.
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
Oral dosage form of ceftriaxone and methods of use
InactiveUS20090123537A1Inhibits enteric degradationAvoid degradationGranular deliveryCoatingsSmall intestineDissolution
The present invention contemplates a novel oral dosage form for the intestinal delivery of ceftriaxone sodium. The oral dosage form inhibits enteric degradation of the therapeutic compound by encapsulation within an inner core region and having an outer shell, preventing its dissolution until reaching the small intestine. Furthermore, the enzymatic degradation of the compound is substantially inhibited until absorption at the intestinal mucosa.
Owner:PHERMACEUTICA
Refinement method of ceftriaxone sodium crude product
ActiveCN104031067AImprove crystal qualityReduce degradationOrganic chemistryCrystallizationChemistry
The invention provides a refinement method of a ceftriaxone sodium crude product. Ceftriaxone sodium can be easily subjected to degradation reaction in the crystallization process; and the ceftriaxone sodium is not stable and can be easily subjected to oxidative degradation to generate a degradation product mainly in a pure water system, thereby causing high color grade of the product and directly influencing the quality of the ceftriaxone sodium. The refinement method provided by the invention can lower the degradation of the ceftriaxone sodium in the crystallization process.
Owner:LIVZON PHARM GRP INC +1
Method for synthesizing coarse salt of ceftriaxone sodium by phase transfer catalysis method
ActiveCN101747346AHighlight substantiveSignificant progressAntibacterial agentsOrganic chemistry7-ACABoron trifluoride
The invention relates to a method for synthesizing coarse salt of ceftriaxone sodium, which comprises the following steps: (1) taking 7-aminoce-phalosporanic acid (7-ACA) and a triazine ring as raw materials, adopting boron trifluoride acetonitrile as a catalyst for carrying out electrophilic substitution and finally, preparing 7-ACT by a zymohydrolysis method at a proper pH value; and (2) putting the 7-ACT and AE-active ester into an organic phase, adding a phase transfer catalyst for carrying out an N-acidylating reaction, salifying and crystallizing to obtain the coarse salt of the ceftriaxone sodium. The invention prepares the 7-ACT by the zymohydrolysis method, avoids the generation of a side reaction, enhances the yield and enhances the product purity. The reaction is carried out in the organic phase by the phase transfer catalyst, a product is transferred to a water phase, the continuous contact with a reactant is avoided, the generation of impurities with large molecular weight is lowered, and the coarse salt of the ceftriaxone sodium with high purity and high yield is finally obtained.
Owner:YIYUAN XINQUAN CHEM
Preparation method of ceftriaxone sodium crystal and evaluation method of ceftriaxone sodium aqueous solution turbidity
ActiveCN102993215BReduce the incidence of allergic reactionsHigh clarityOrganic chemistryMaterial analysis using radiation diffractionCLARITYX-ray
The invention relates to a preparation method of ceftriaxone sodium crystal, wherein the obtained ceftriaxone sodium is the crystal of the same subtype, and the subtype crystal has the best clarity, the lowest anaphylaxis occurrence rate and the highest safety in comparison with all crystals. The preparation method is simple in technology and excellent in reproducibility. The invention further relates to an evaluation method of ceftriaxone sodium aqueous solution turbidity, wherein a powder X-ray diffraction technology and a scanning electron microscope technology are used, in combination with statistical methods, a method for evaluating the ceftriaxone sodium crystal form is established, three subtypes of the ceftriaxone sodium crystal form is defined for the first time, and the relation between the subtypes and the aqueous solution turbidity is established, thus, rapid evaluation on crystal form and aqueous solution turbidity of ceftriaxone sodium samples becomes possible, and the method has great practical value.
Owner:YOUCARE PHARMA GROUP +1
Rapid propagation method of Paris polyphylla plant
InactiveCN103548682ARealize mass productionPlant tissue cultureHorticulture methodsActivated carbonHigh volume manufacturing
The invention discloses a rapid propagation method of a Paris polyphylla plant. The method comprises the steps of inducing calluses, differentiating adventitious buds, and carrying out rooting culture, seedling hardening and transplanting, wherein a culture medium for inducing and rapidly propagating the adventitious buds comprises MS, 1.0-4.0 mg.L<-1> of 6-BA, 0.1-1.0 mg.L<-1> of IAA, 0.1-1.0 mg.L<-1> of KT and 200-500 mg.L<-1> of ceftriaxone sodium, and the rooting culture medium comprises 1 / 2 MS, 0.1-1.0 mg.L<-1> of IBA, 0.1-0.5 mg.L<-1> of NAA and 0.1%-1.0% of activated carbon. According to the rapid propagation method, methods for inducing and rapidly propagating the adventitious buds and rooting, hardening and transplanting rootless seedlings are further presented based on the callus induction of the Paris polyphylla for the first time, a novel way is provided for the culturing of fine varieties of the Paris polyphylla and the mass and rapid propagation of tissue culture seedlings, and the batch production of the Paris polyphylla plant can be realized under the conditions of short time and low cost.
Owner:CHENGDU UNIV
Breeding method for experimental macaque
ActiveCN104542475AImprove sexual functionImprove pregnancy rateAnimal feeding stuffAnimal housingCefotaximeCvd risk
The invention relates to the technical field of animal breeding, in particular to a breeding method for an experimental macaque. Main feed containing vitamin E is fed to the macaque in at least one of a suckling period, a growth period and an adult period; cefotaxime or ceftriaxone sodium or ceftazidime is added into the feed continuously for 5-7 days every 1-2 months in at least one of the growth period and the adult period; a young macaque is independently fed in the suckling period, feeding quality is further improved, the health condition of the macaque can be timely discovered and managed, the survival rate of the young macaque is improved, and the young macaque is normal in growth and development, flexible in limbs and physically strong. Moreover, the probability and the risk of bacterial dysentery infection of the macaque are reduced, and common health of the macaque and workers is ensured. A normalized experimental animal breeding standard is provided, and the breeding method is easy to popularize and use.
Owner:四川横竖生物科技股份有限公司
Children pharmaceutical composition containing ceftriaxone sodium and low-sodium carrier
InactiveCN104887621AIncrease internal pressureImprove solubilityAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionKidney
The invention relates to a children pharmaceutical composition containing ceftriaxone sodium, namely a pharmaceutical combined preparation containing ceftriaxone sodium and a low-sodium carrier infusion solution, and particularly relates to a combined package. The children pharmaceutical composition comprises ceftriaxone sodium for injection and the low-sodium carrier infusion solution. The low-sodium carrier infusion solution contains a glucose and sodium chloride injection (15-200):1, a glucose and sodium chloride potassium chloride injection (15-200):1: (0-1) and the like. Compared with a mixture of ceftriaxone sodium and the low-sodium carrier infusion solution, the children pharmaceutical composition has the advantages that clinical application steps are simplified, clinical risks generated because kidneys of children are not developed to be mature and overmuch sodium in blood cannot be metabolized are reduced, and the clinical application quality and safety performance of children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
Method for refining ceftriaxone sodium crude product
ActiveCN102432629BImprove crystal qualityImprove antioxidant capacityOrganic chemistrySodium metabisulfiteSolvent
The invention relates to a method for refining a ceftriaxone sodium crude product. The method comprises the following steps of: adding the ceftriaxone sodium crude product and sodium metabisulfite into a mixed solvent of water for injection and methanol, and dissolving until the solution is clarified; sterilizing the solution, filtering, and adding a leaching agent; adding ceftriaxone sodium seed crystals, and cultivating crystals after the crystals are precipitated; and continuing to add the leaching agent, precipitating the crystals completely, washing the crystals and drying to obtain a ceftriaxone sodium refined product. In the method, the mixed solvent is used as an initial solvent of the ceftriaxone sodium to improve the stability of the ceftriaxone sodium in a single hydrosolvent system; and in the presence of the sodium metabisulfite, the product is low in total impurities, less in degradation and stable in colors, the color of the ceftriaxone sodium refined product is No.1 lighter than that of crystals in the single hydrosolvent system, and the purity and quality of the product are improved.
Owner:QILU ANTIBIOTICS PHARMA +1
Ceftriaxone sodium compound and preparation method thereof
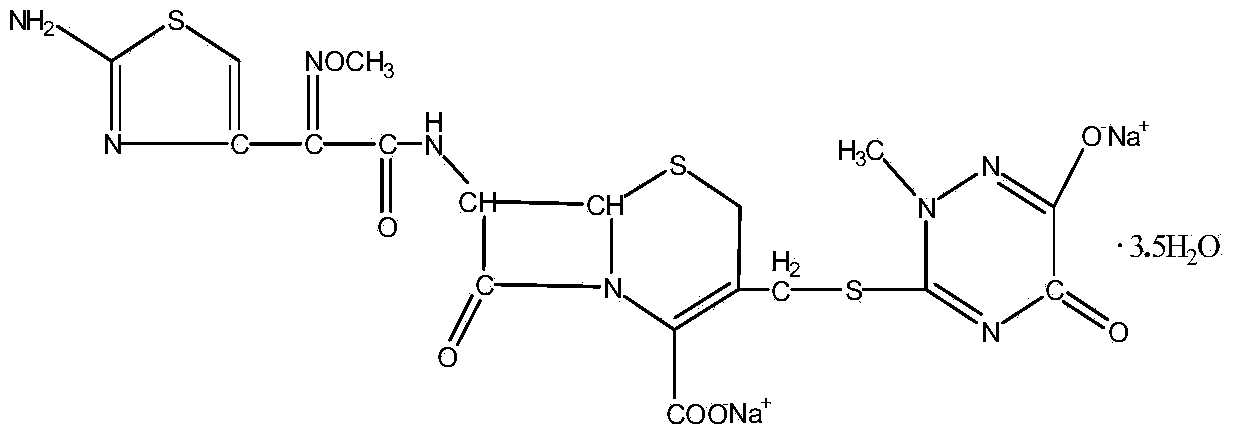
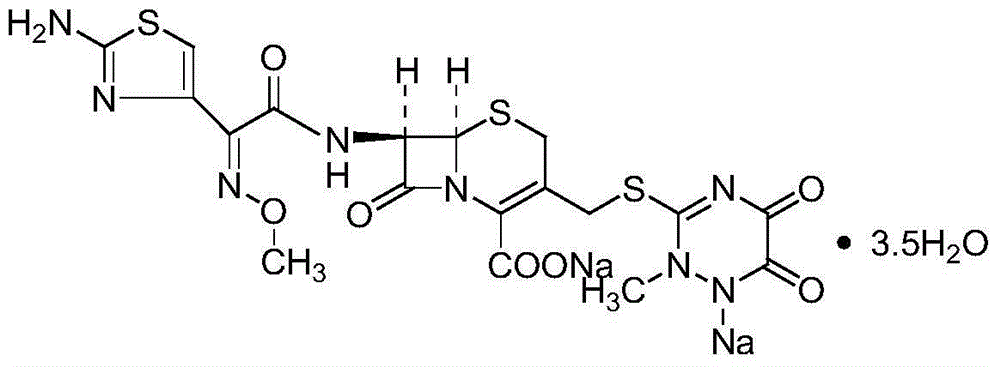
ActiveCN102617605BEasy to operateImprove product qualityAntibacterial agentsOrganic active ingredientsTriazineBULK ACTIVE INGREDIENT
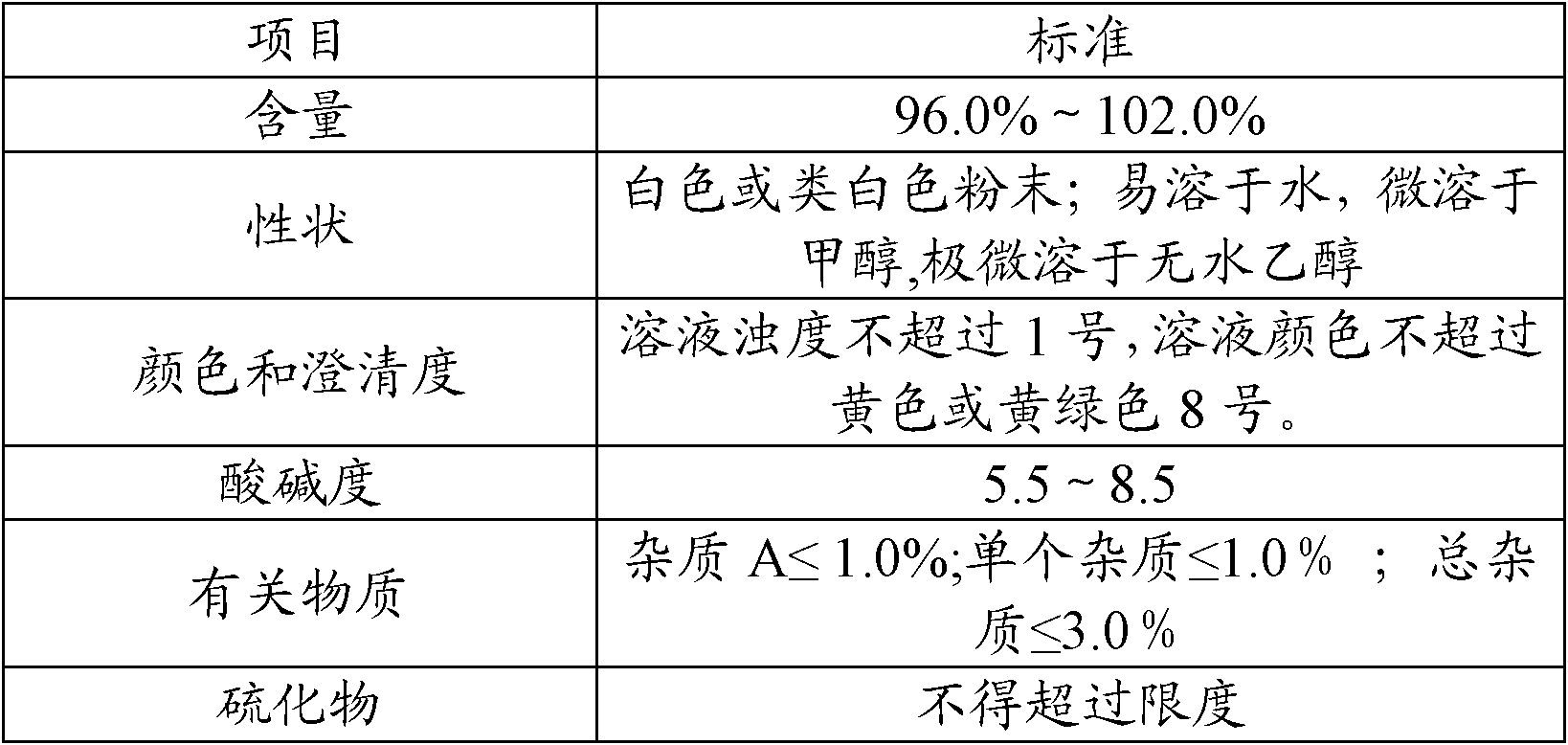
The invention relates to a ceftriaxone sodium compound and a preparation method thereof. The compound comprises active ingredient ceftriaxone sodium, impurities and ceftriaxone polymer, wherein the content of ceftriaxone sodium is not less than 90.0% by calculation on the basis of an anhydride, the total weight percent of the impurities is not larger than 0.1% on the basis of the weight percent of ceftriaxone, the impurities comprise 7-ACT and triazine ring, and the weight percent of the ceftriaxone polymer is not larger than 0.1% on the basis of the weight percent of ceftriaxone. The preparation method of the compound is simple, economical and environment-friendly, the contents of the impurities and the polymer in a product are low, adverse reactions are remarkably reduced, and safety of clinical medication of a ceftriaxone sodium preparation can be guaranteed well.
Owner:石药集团中诺药业(石家庄)有限公司 +1
Original-quality ceftriaxone sodium and pharmaceutical preparation thereof
ActiveCN105418641AHarm reductionToxicAntibacterial agentsOrganic active ingredientsChloroformateAntibiotic Y
The invention discloses original-quality ceftriaxone sodium and a pharmaceutical preparation thereof. The key technology and industrialization of the third generation of cephalosporin antibiotics active ester intermediate wins the second prize of National Scientific and Technological Progress Award, and the third generation of cephalosporin antibiotics intermediate AE active ester is a key factor for affecting the internal quality of the ceftriaxone sodium. A preparation method comprises the steps that 1, boron trifluoride-acetonitrile serves as a catalyst, and on the condition that acetonitrile serves as solvent, a triazine ring is reacted with 7-ACA to generate 7-ACT; 2, triethylamine and aminothiazoly loximate are added into the solvent, a chloroformate activator is dropwise added slowly during cooling mixing, the 7-ACT is added for a one-pot reaction after stirring is conducted, and ceftriaxone is obtained; 3, a salt-forming agent is added, and the ceftriaxone sodium is obtained. According to the preparation method, use of a condensing agent with higher price is avoided, the process route is shortened, operation is easy, the reaction condition is mild, the product yield is high, the purity is good, and industrial production is easy.
Owner:广东金城金素制药有限公司 +1
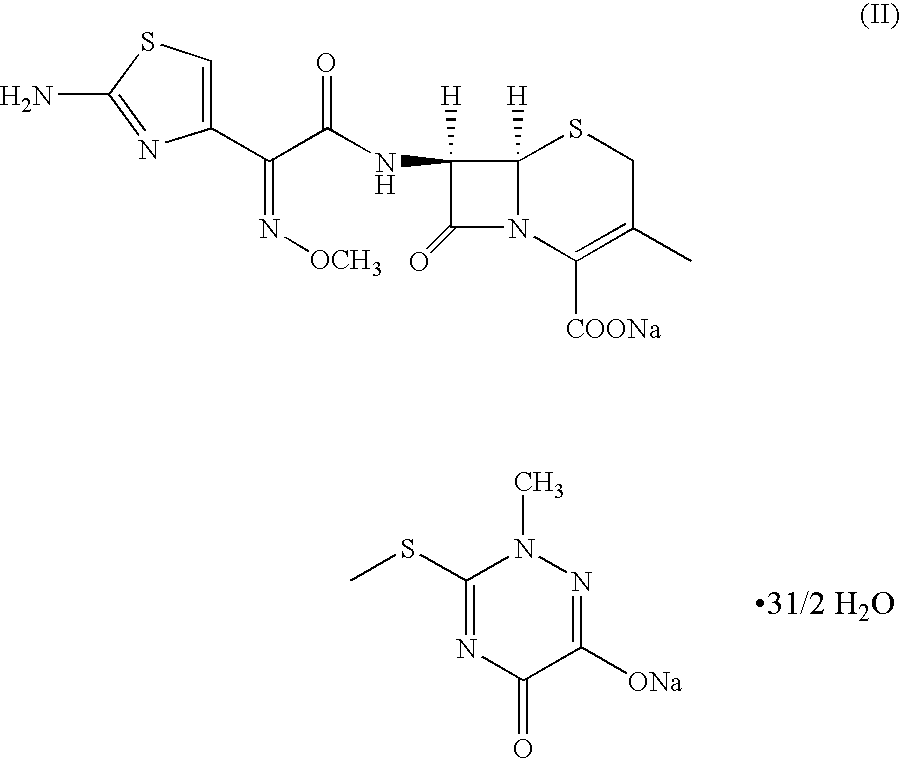
Process for the preparation of a cephalosporin antibiotic
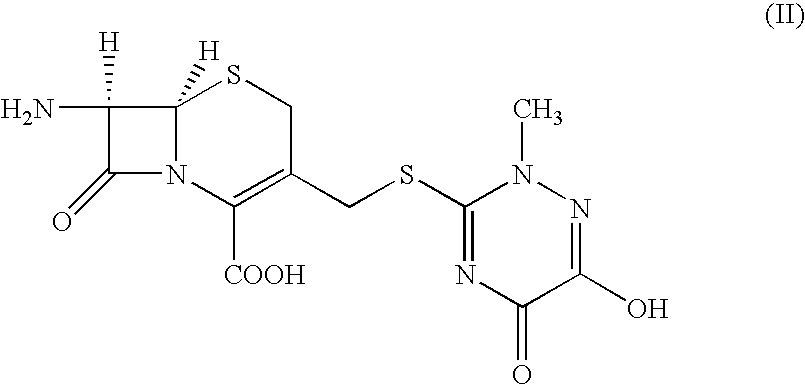
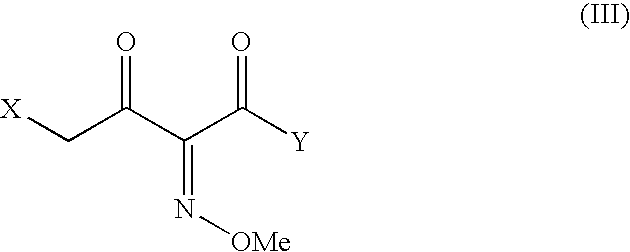
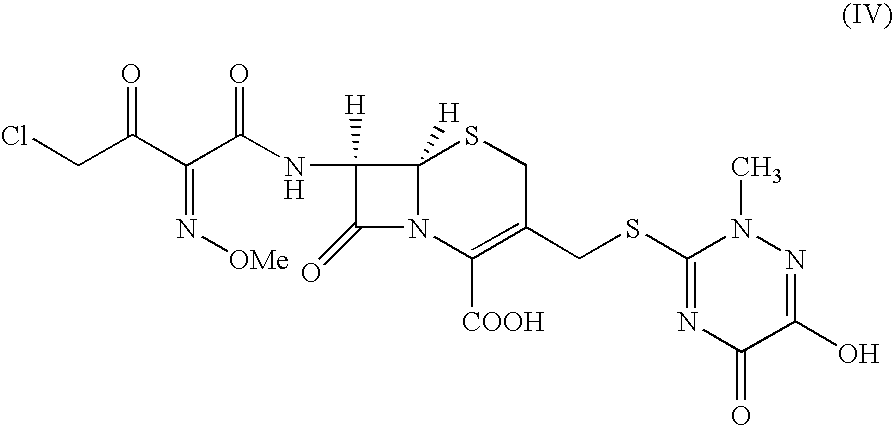
An improved process for the preparation of ceftriaxone sodium comprising the steps of: i) reacting the 3-cephem derivative of formula (II) with halo acid derivative of formula (III) wherein X represents halogen and Y represent halogen in the presence of silylating agent and methylene chloride at −25 to 10° C., to produce (IV), ii) quenching the reaction by pouring the reaction mixture into water or in a aqueous solution of sodium carbonate, iii) preparing sodium salt solution of (IV) by adding sodium carbonate and separating the organic layer, iv) cyclizing the sodium salt of (IV) in the aqueous solution with thiourea at a temperature in the range of 0 to 30° C., v) adjusting the pH to 1.5 to 2.5 to precipitate the ceftriaxone free acid, vi) converting the ceftriaxone free acid to sodium salt using sodium-2-ethyl hexanoate in water and vii) precipitating and isolating the ceftriaxone sodium.
Owner:ORCHID CHEM & PHARM LTD
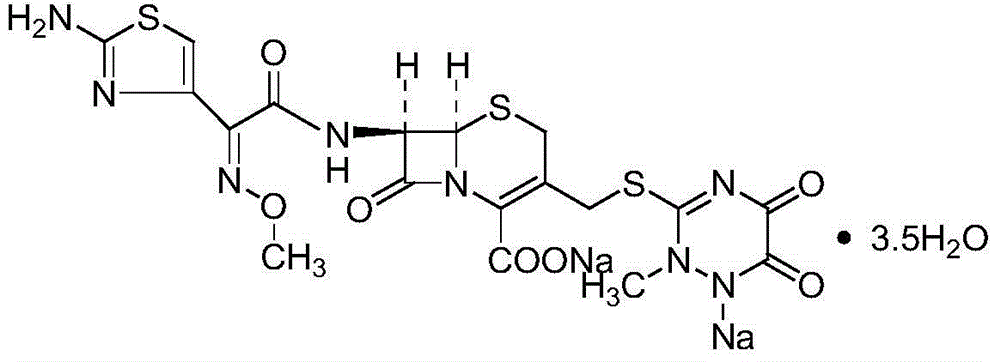
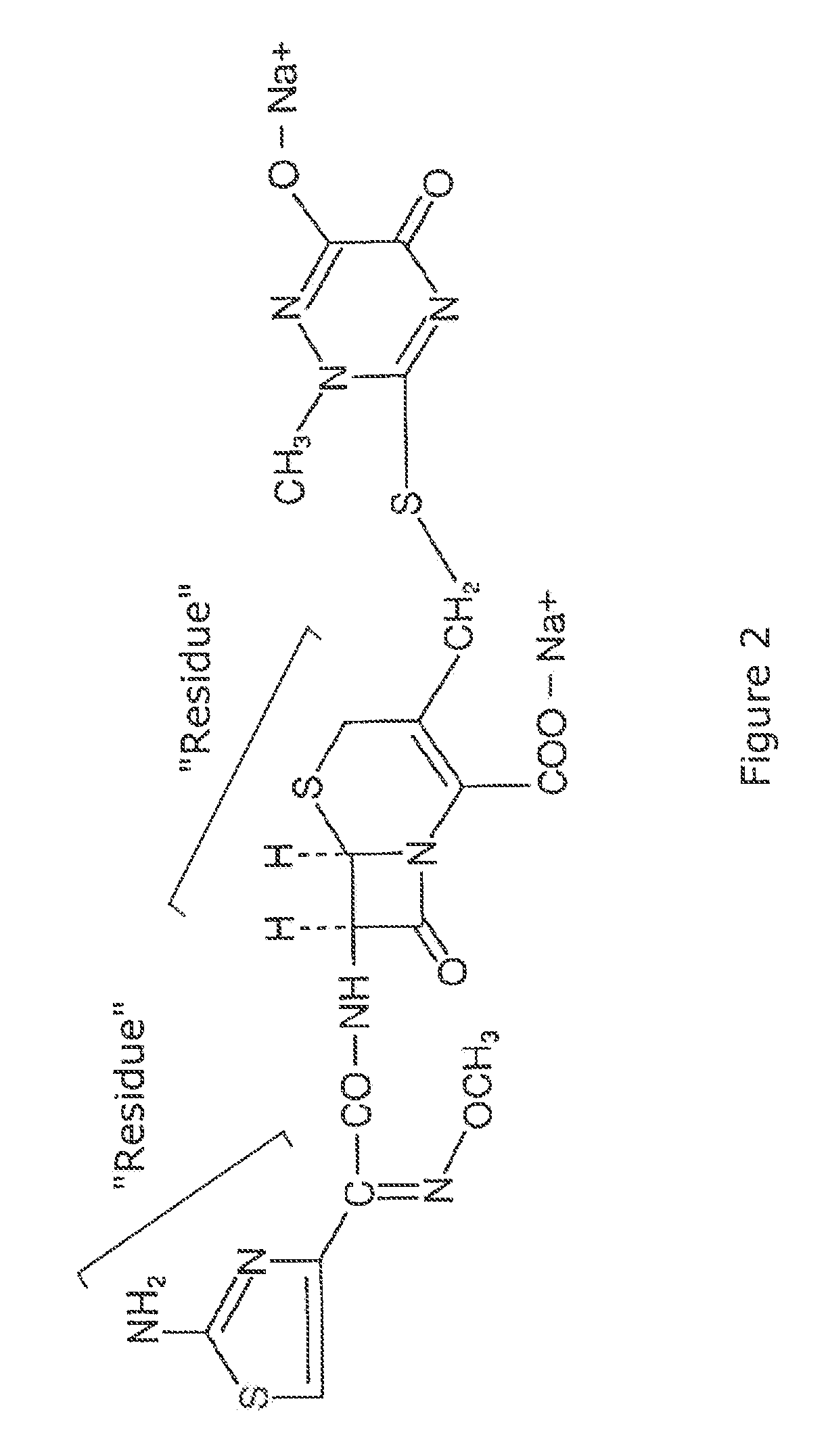
Ceftriaxone sodium compound entity for children and preparation for ceftriaxone sodium compound entity for children
InactiveCN104926834AMaintain stabilityIncrease internal pressureAntibacterial agentsPowder deliveryActivated carbonTemperature control
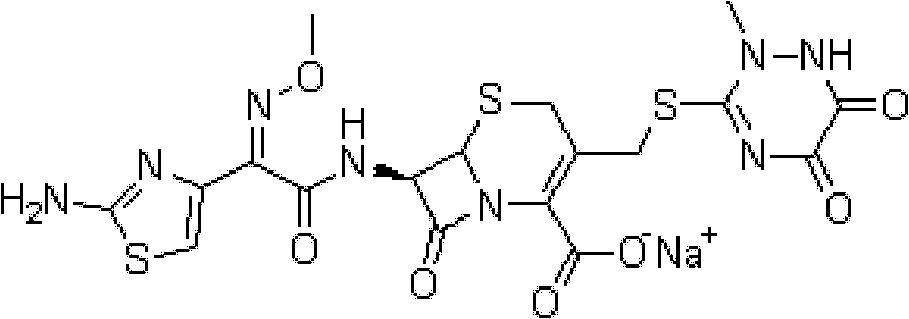
The invention provides a ceftriaxone sodium compound entity for children. A structural formula of the ceftriaxone sodium compound entity is as follows: formula as shown in the specification. The ceftriaxone sodium compound entity is prepared by the following steps: (1) dissolving a ceftriaxone crude product into water, adding activated carbon, stirring and discoloring, and filtering; (2) adding an extraction agent into filtrate to obtain a mixture, transferring and filling the mixture into a pressure-resistant container, and carrying out temperature-controlled freezing on the mixture and taking out the mixture after removing air bubbles; and (3) removing an organic phase of the mixture, dropwise adding acetone at 10-15 DEG C after solids are molten, slowly stirring, growing crystals, filtering, washing, carrying out vacuum drying and packaging preparations of different specifications. Compared with ceftriaxone sodium prepared by a conventional process, the ceftriaxone sodium prepared by the preparation method has the advantages of few impurities, high stability and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA
Production process of compound preparation of ceftriaxone sodium and tazobactam sodium for injection
ActiveCN101537009AHigh purityImprove the safety of useAntibacterial agentsPharmaceutical delivery mechanismFiltrationDissolution
The invention discloses a production process of compound preparation of ceftriaxone sodium and tazobactam sodium for injection, comprising the steps of weighing raw materials of ceftriaxone sodium, tazobactam sodium, sterilized water for injection, mixed liquid of ethyl acetate and isopropanol, and anhydrous ethanol based on the weight ratio of 3-5:1:2:5:9; conducing dissolution and filtration; crystallizing and washing; and lyophilizing to obtain the compound preparation of ceftriaxone sodium and tazobactam sodium for injection. The invention is applicable to the compound preparation of ceftriaxone sodium and tazobactam sodium for injection, which is produced according to the ratio of 3:1-5:1 of ceftriaxone to tazobactam; and the invention adopts evenly mixing of liquid phase, can achieve good mixing uniformity, effectively increase the purity of the preparations simultaneously, and further guarantee the advantage of high safety of clinic use.
Owner:HAIKOU QILI PHARMA
Chinese and western composite medicine for treating neoplastic diseases
InactiveCN102949712AToxic reductionEasy drainage and exclusionOrganic active ingredientsPeptide/protein ingredientsSide effectAdditive ingredient
A Chinese and western composite medicine for treating neoplastic diseases is characterized by comprising Chinese herbal medicinal ingredients and a western medicinal ingredient which are mixed, the Chinese herbal medicinal ingredients include 5 polyzyme tablets and a stomach invigorating and digestion aiding tablet, each polyzyme tablet comprises pepsin, trypsin, pancreatic lipase and amylopsin, and the stomach invigorating and digestion aiding tablet comprises radix pseudostellariae, tangerine peels, Chinese yam, (roasted) malt and hawthorn; the western medicinal ingredient is ceftriaxone sodium injection solution; and the weight of each polyzyme tablet is 0.313g, the total weight of the polyzyme tablets is 1.57g, the weight of the stomach invigorating and digestion aiding tablet is 0.8g, the weight of the ceftriaxone sodium injection solution is 0.75g, and the Chinese herbal medicinal ingredients and the western medicinal ingredient are combined to form the prescription. The Chinese and western composite medicine for treating the neoplastic diseases is particularly used for suppressing and killing pathogenic bacteria capable of causing benign tumor and malignant tumor infection of tissue cells of a human body, the composite medicine is directly dripped into the oral cavity or an affected part of the human body when the human body suffers from the tumor infection, and 3-4 drops of the composite medicine are applied every time. The Chinese and western composite medicine has few side effects, becomes effective within 30 minutes and does not injure the liver, the brain and the kidneys of the human body.
Owner:李红彬
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com