Production process of compound preparation of ceftriaxone sodium and tazobactam sodium for injection
A technology of ceftriaxone sodium and tazobactam sodium, which is applied in the field of compound preparations of ceftriaxone sodium and tazobactam sodium for injection, which can solve the problem of poor mixing uniformity, different specific volumes, and effects on the content of sterile products and the color level of the solution And other problems, to achieve high safety, good mixing uniformity, and improve the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
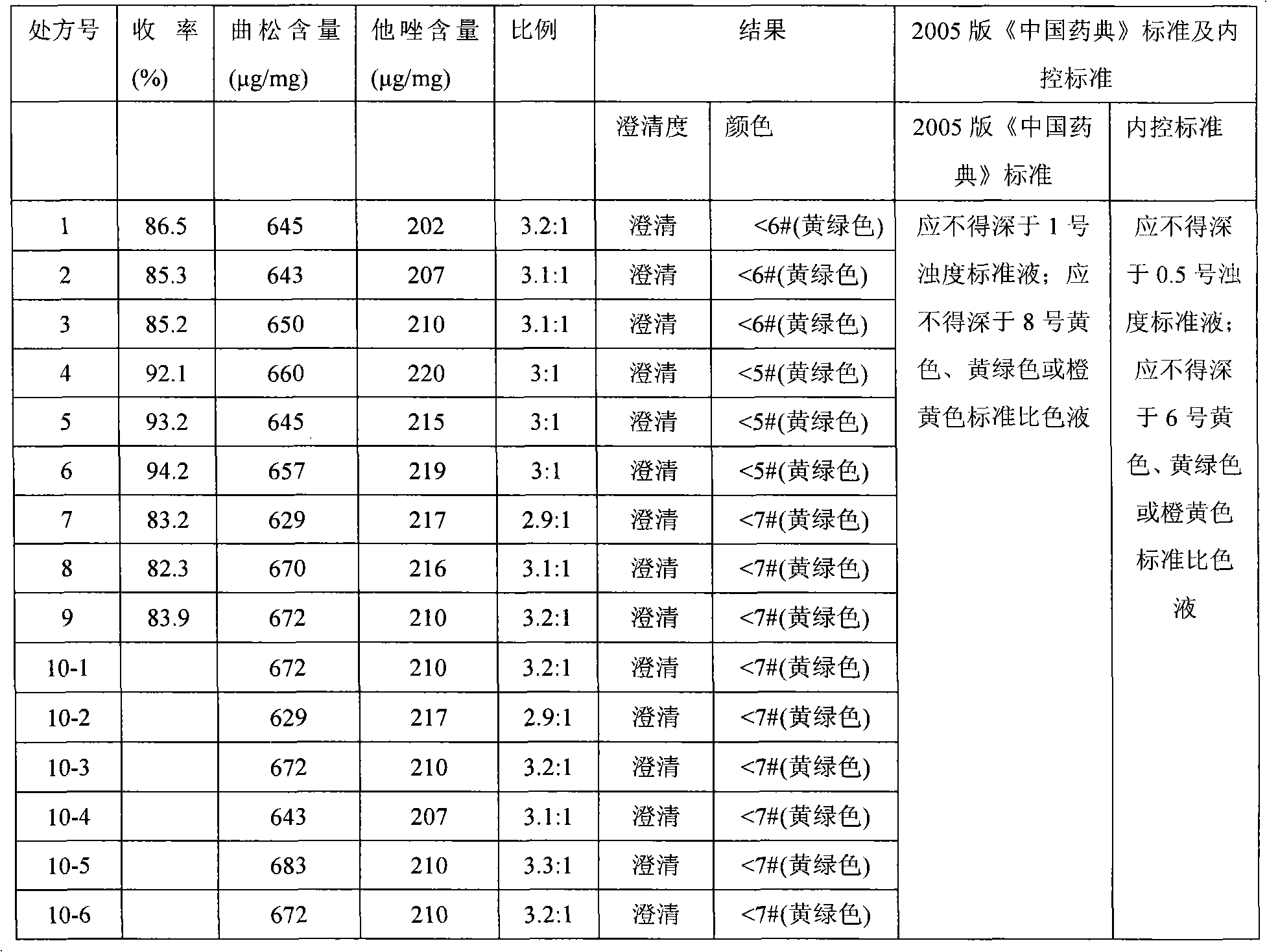
[0022] A kind of production technology of ceftriaxone sodium tazobactam sodium compound preparation for injection, this production technology comprises steps as follows:
[0023] 1. Choice of prescription
[0024] Prescription 1, and ratio: specification 1.0g
[0025] Material name
prescription
Proportion
894.0g (equivalent to 750g ceftriaxone)
3
294.0g (equivalent to 250g tazobactam)
1
Sterile water for injection
500g
2
Ethyl acetate and isopropanol mixture (1:2)
1250
5
Absolute ethanol
2250
9
co-production
1000 bottles
[0026] Prescription 2, and ratio: specification 1.0g
[0027] Material name
prescription
Proportion
894.0g (equivalent to 750g ceftriaxone)
3
Tazobactam Sodium
294.0g (equivalent to 250g tazobactam)
1
Sterile water fo...
Embodiment 2
[0066] A kind of production technology of ceftriaxone sodium tazobactam sodium compound preparation for injection, this production technology comprises steps as follows:
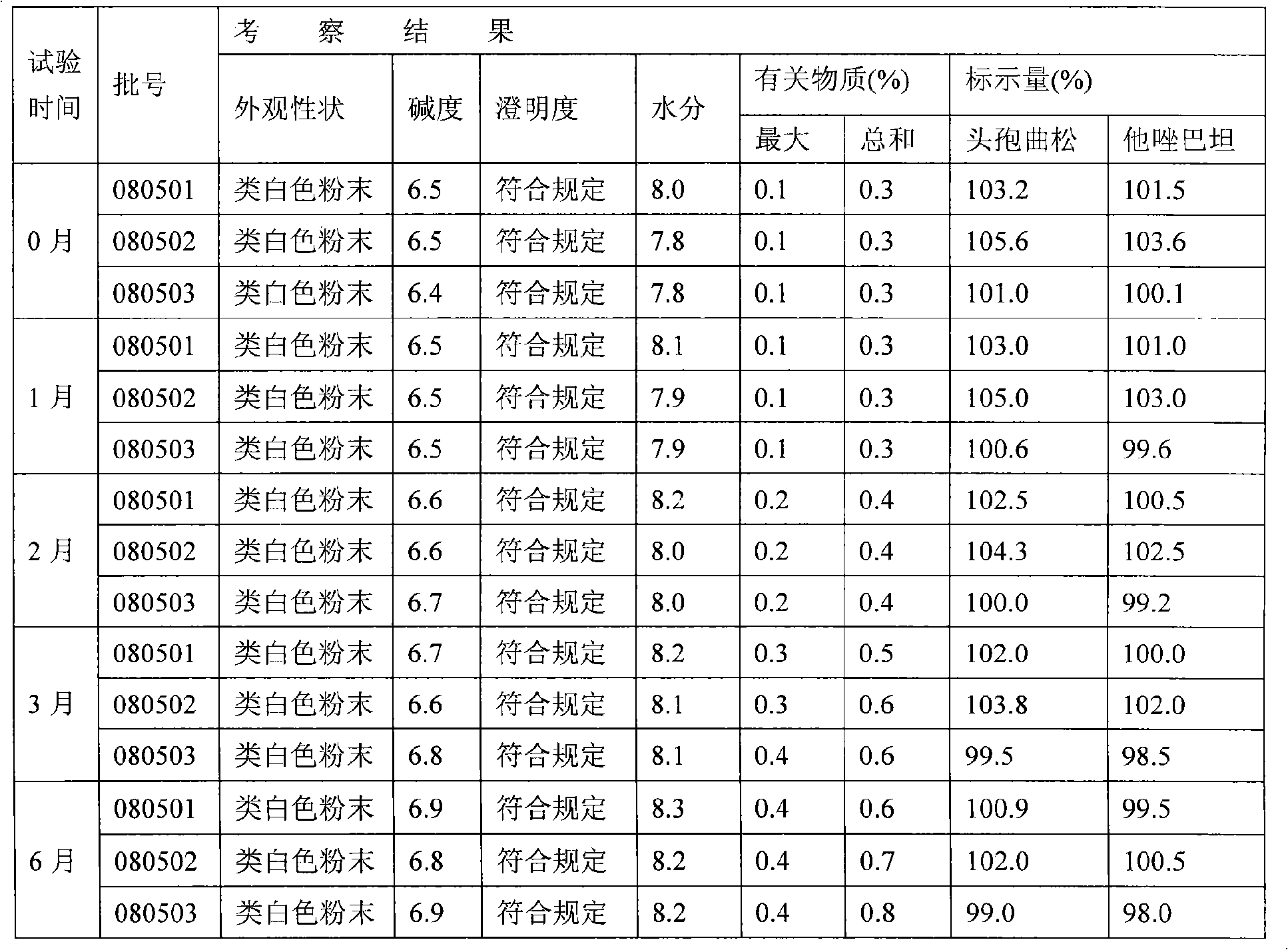
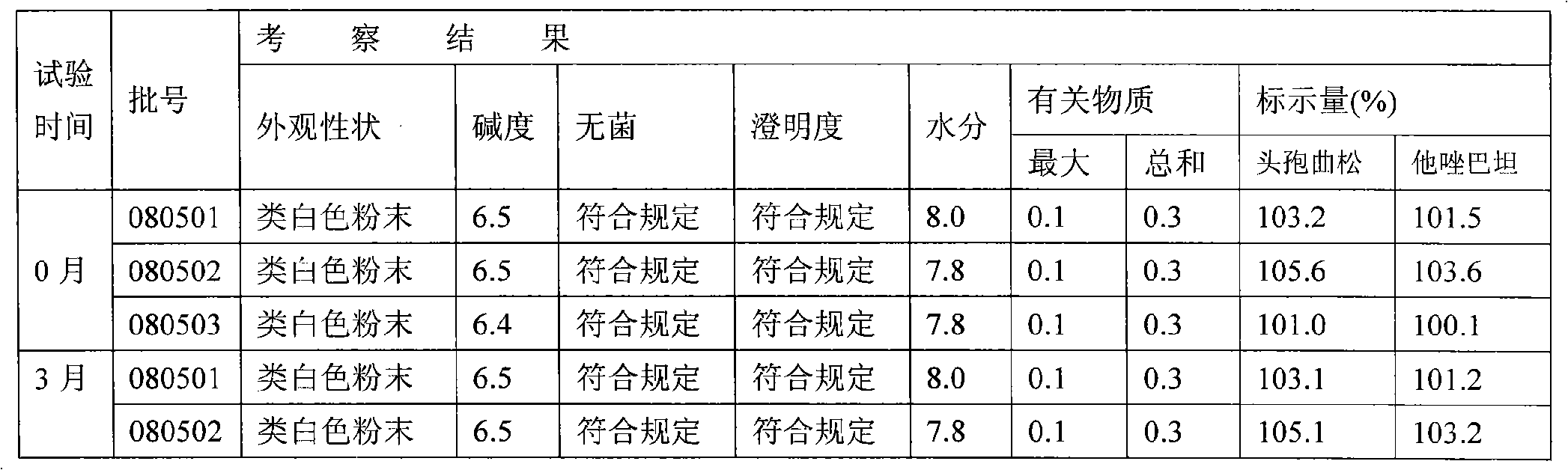
[0067](1), raw material: press ceftriaxone sodium: tazobactam sodium: sterile water for injection: ethyl acetate and isopropanol mixed solution: the weight ratio of dehydrated alcohol is 5: 1: 2: 5: 9, Weigh the above raw materials respectively;
[0068] Wherein, the mixed solution of ethyl acetate and isopropanol is that ethyl acetate and isopropanol are mixed in a volume ratio of 1:2;
[0069] (2) Dissolving and filtering: add sterile water for injection into the dissolving tank, 8°C to 12°C, then add ceftriaxone sodium and tazobactam sodium and stir to dissolve, add activated carbon, and the amount added is 0.2% of the liquid weight in the tank , and filtered after stirring for 30 minutes: pass the mixed solution through a 0.45 micron titanium rod filter, 0.22 micron and 0.01 micron filter elements for u...
Embodiment 3
[0077] A kind of production technology of ceftriaxone sodium tazobactam sodium compound preparation for injection, this production technology comprises steps as follows:
[0078] (1), raw material: press ceftriaxone sodium: tazobactam sodium: sterile water for injection: ethyl acetate and isopropanol mixed solution: the weight ratio of dehydrated alcohol is 5: 1: 2: 5: 9, Weigh the above raw materials respectively;
[0079] Wherein, the mixed solution of ethyl acetate and isopropanol is that ethyl acetate and isopropanol are mixed in a volume ratio of 1:4;
[0080] (2) Dissolving and filtering: add sterile water for injection into the dissolving tank, 8°C to 12°C, then add ceftriaxone sodium and tazobactam sodium and stir to dissolve, add activated carbon, and the amount added is 0.2% of the liquid weight in the tank , and filtered after stirring for 30 minutes: pass the mixed solution through a 0.45 micron titanium rod filter, 0.22 micron and 0.01 micron filter elements for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com