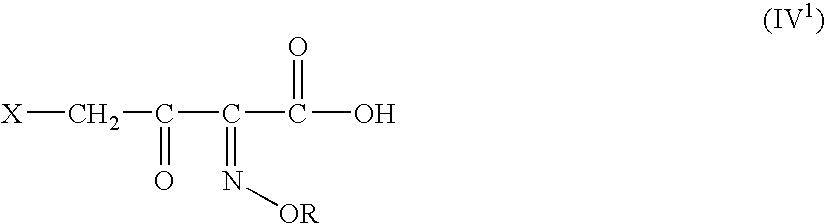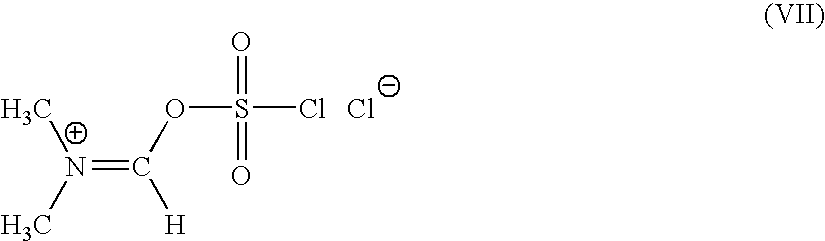Patents
Literature
84 results about "Ceftriaxone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
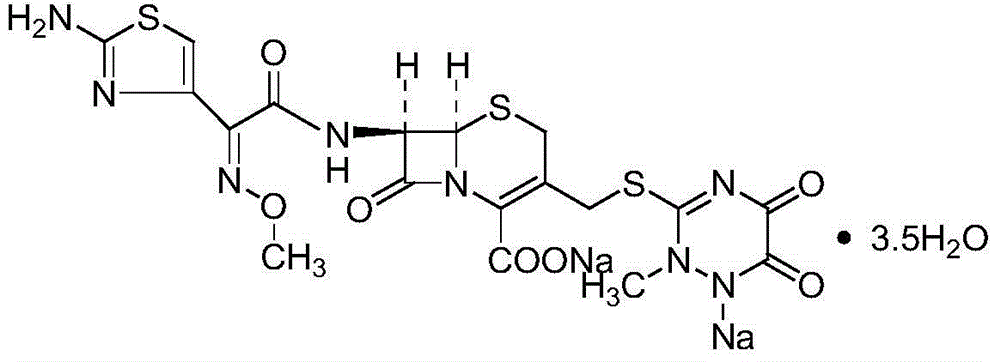
Ceftriaxone is used to treat a wide variety of bacterial infections.
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
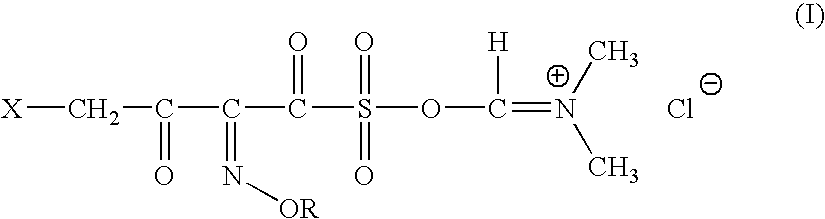
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
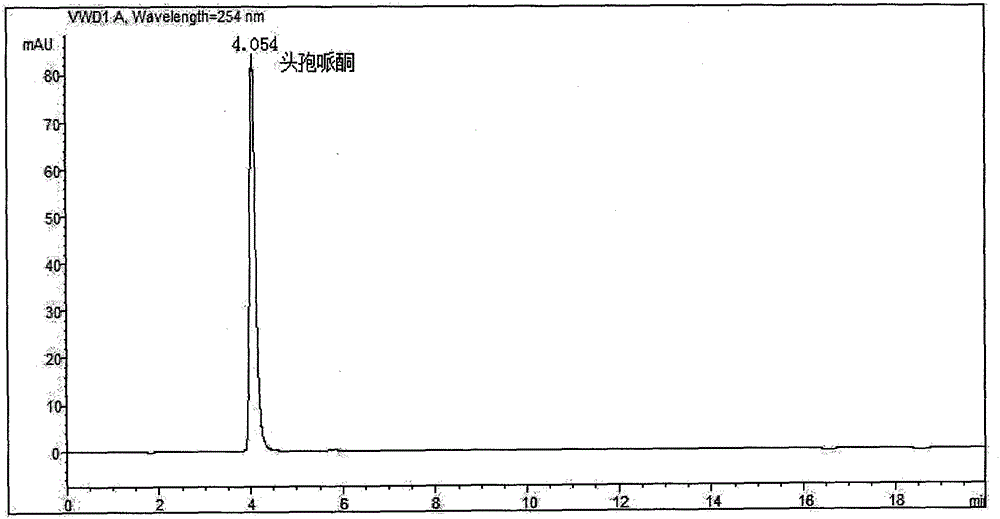
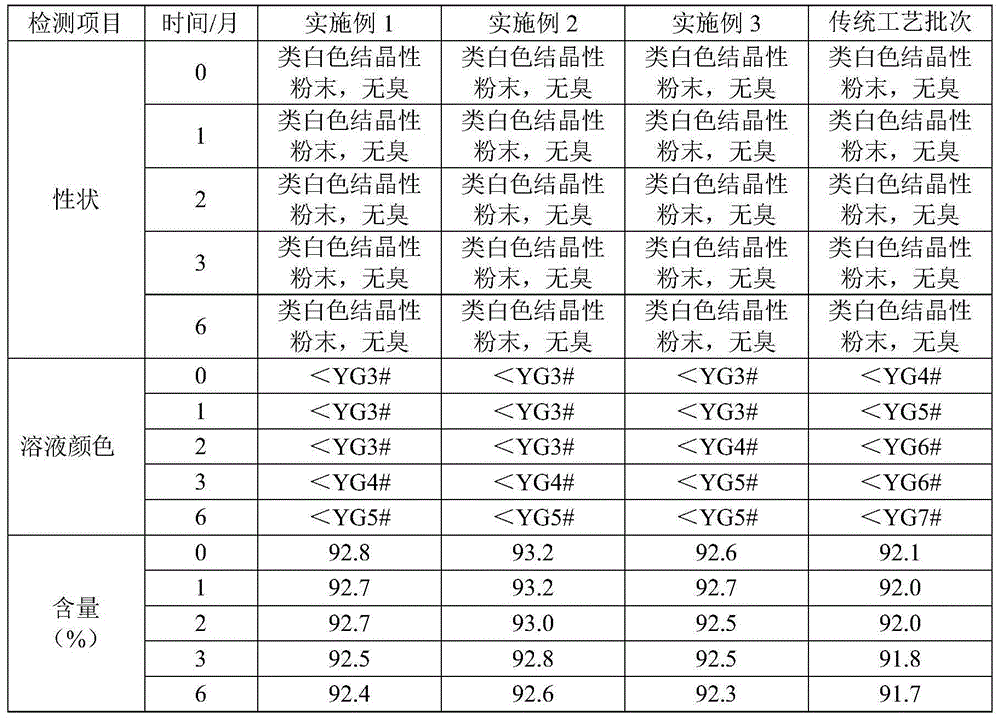
Ceftriaxone sodium powder-injection for injection
ActiveCN104873466ALess impuritiesImprove stabilityAntibacterial agentsPowder deliveryNorth chinaSolvent
The invention discloses a ceftriaxone sodium powder-injection for injection. the powder-injection is prepared by the following steps: (1) weighing a ceftriaxone sodium crude raw material at 20 DEG C, adding water, stirring and dissolving, adding active carbon, decolouring, filtering, and washing with mixed solvents; (2) by a particle process crystal product molecular assembly and shape-state optimization technology of North China Pharmaceutical Hebei Huamin Pharmaceutical Co., Ltd., adding a solventing-out agent acetone according to a stream acceleration table at 15 DEG C at the stirring speed of 300 r / min; (3) carrying out suction filtration, washing a filter cake with acetone, putting the filter cake into a vacuum drying oven and carrying out vacuum drying at 30-40 DEG C; and (4) sub-packaging preparations of different specifications, and controlling environmental temperature and humidity until temperature is 20-24 DEG C and humidity is less than 40% so as to obtain the ceftriaxone sodium for injection. In comparison with a traditional technology, ceftriaxone sodium prepared by the above preparation method has advantages of less impurity, high stability and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Treatment and/or prevention of parkinson's disease dementia with ceftriaxone
Disclosed herein is a method for treatment of a human subject having or suspected of having Parkinson's disease dementia, which includes administering to the human subject ceftriaxone at a daily dosage ranging from about 1.5 mg to about 35 mg per kilogram of a body weight of the human subject.
Owner:BRAINX PHARMA CO LTD +1
Parenteral Combination Therapy For Infective Conditions With Drug Resistant Bacterium
InactiveUS20080188403A1Antibacterial agentsOrganic active ingredientsBacteroidesHospitalized patients
The invention describes a pharmaceutical composition to combat multiple-drug-resistant bacteria in non-ocular infective conditions. Compositions comprising glycopeptides, in particular vancomycin, and cephalosporins, in particular ceftriaxone, are disclosed. Such compositions are found to be useful for parenteral administration for hospitalized patients with serious infections. Specifically, this invention also discloses a pharmaceutical composition further including an excipient such as CVMC agent and is available in dry powder form for reconstitution before injection with a suitable solvent. The pharmaceutical compositions of this invention have been found normally to enhance resistance to precipitation in solutions to be administered parenterally. The invention also gives details of the dosage forms stored in sealed containers to be reconstituted before use. The invention further provides a process to manufacture these compositions and also a method of treating a subject having non-ocular infective conditions due to multi drug resistant bacterium.
Owner:VENUS REMEDIES LTD
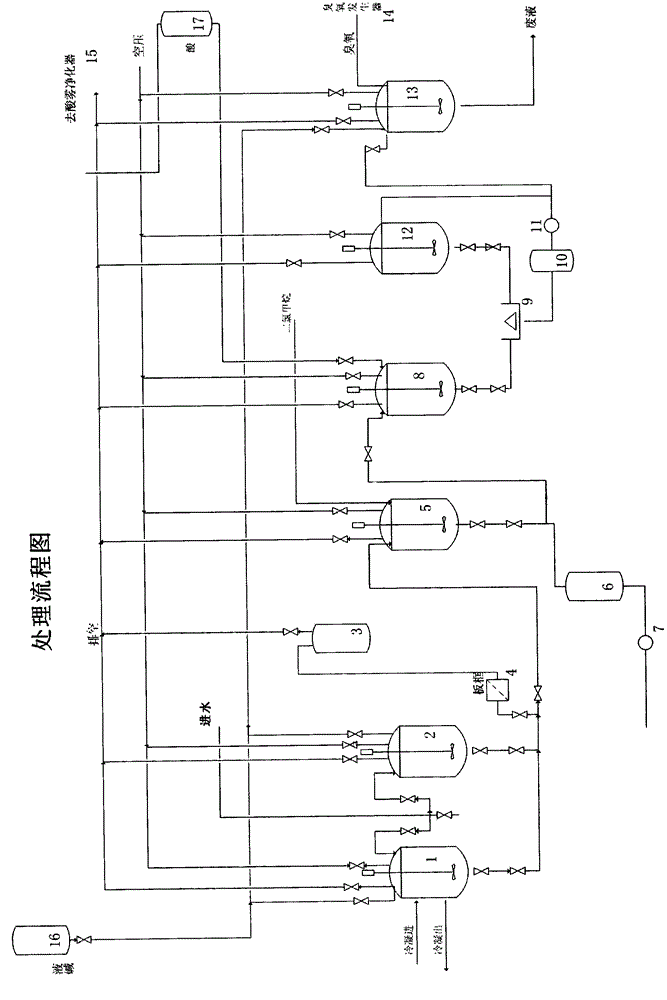
Treatment method of cephalosporin synthesizing pharmaceutical production wastewater
ActiveCN104628199AHigh purityReduce performanceMultistage water/sewage treatmentNature of treatment waterActivated carbonStrong acids
The invention relates to a treatment method of ceftriaxone wastewater. The treatment method comprises the following steps: 1) adding a strong base to ceftriaxone kettle residue to regulate the pH to the range of 13.0-13.5, stirring, standing for layering, and separating the organic phase of the upper layer from the water phase of the lower layer; 2) adding dichloromethane to the organic phase, stirring, extracting the organic matter, and standing to separate out the organic phase; 3) adding strong acid to the water phase to regulate the pH to the range of 1.9 to 2.1, stirring and centrifugally separating out 2-mercaptobenzothiazole; and 4) adding activated carbon to the water phase, and introducing ozone under the irradiation of an ultraviolet lamp for oxidization for 60-90 minutes.
Owner:珠海保税区丽珠合成制药有限公司
Antibiotic carbon steel pickling corrosion inhibitor and application thereof
InactiveCN101994122APrevent over erosionAvoid excessive consumptionSimple Organic CompoundsCleansing Agents
The invention discloses a carbon steel pickling corrosion inhibitor and application thereof. The carbon steel pickling corrosion inhibitor is used for preventing unnecessary corrosion and acid liquid consumption in a pickling process of carbon steel and products thereof. The corrosion inhibitor is one or a combination of antibiotic organic compounds such as ceftriaxone and amoxicillin. The application of the carbon steel pickling corrosion inhibitor comprises the following steps of: adding a 0.010 to 2.0 kg / m<3> cleaning agent into 0.10 to 1.0 kmol / m<3> dilute hydrochloric acid or dilute sulphuric acid serving as cleaning liquid; controlling the temperature to be about 25 DEG C; adding the carbon steel and the products thereof to be cleaned; and immersing for 30 minutes to 4 hours. The product of the invention is used for cleaning the surfaces of the carbon steel and the products thereof, can prevent the excessive corrosion of metals and the excessive consumption of acid liquid in a cleaning process, and has the outstanding advantages of low dosage, high efficiency and good continuous effect.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Treatment device of ceftriaxone synthesis pharmaceutical production waste water
ActiveCN104803503AReduce CODImprove biodegradabilityMultistage water/sewage treatmentNature of treatment waterTreatment systemCeftriaxone
The invention discloses a treatment device of ceftriaxone synthesis pharmaceutical production waste water. The treatment device comprises four sets of reaction tanks, three sets of storage tanks, one set of liquid caustic soda tank, one set of liquid acid tank, a separation and purification device, a recovery device, and a pipeline pump and a valve. According to a treatment method, ceftriaxone crude product kettle residue liquid is delivered into a treatment system via the first set reaction tank, and then is delivered through the second set reaction tank, the third set reaction tank, and the fourth set reaction tank respectively.
Owner:珠海保税区丽珠合成制药有限公司
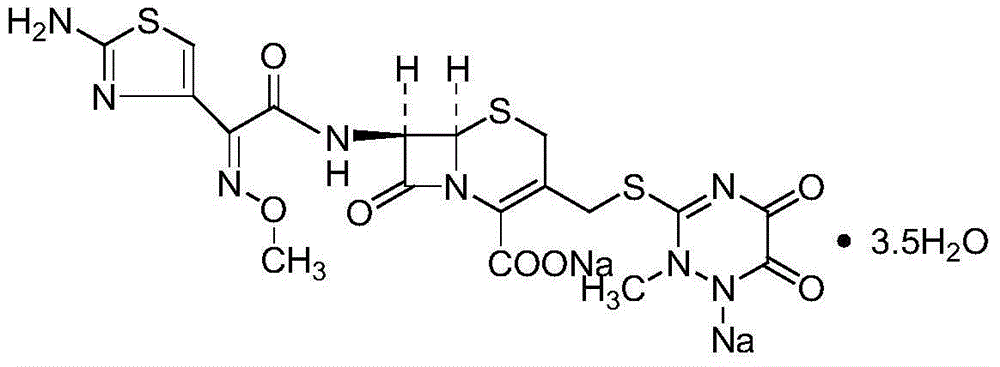
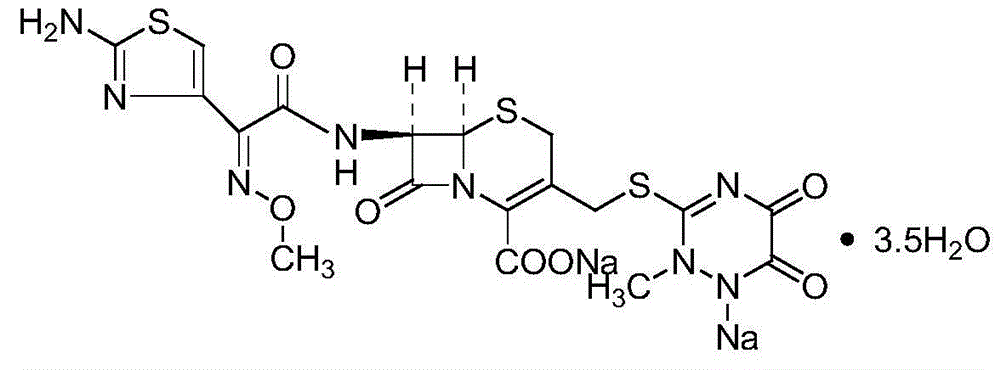
Original-quality ceftriaxone sodium and pharmaceutical preparation thereof
ActiveCN105418641AHarm reductionToxicAntibacterial agentsOrganic active ingredientsChloroformateAntibiotic Y
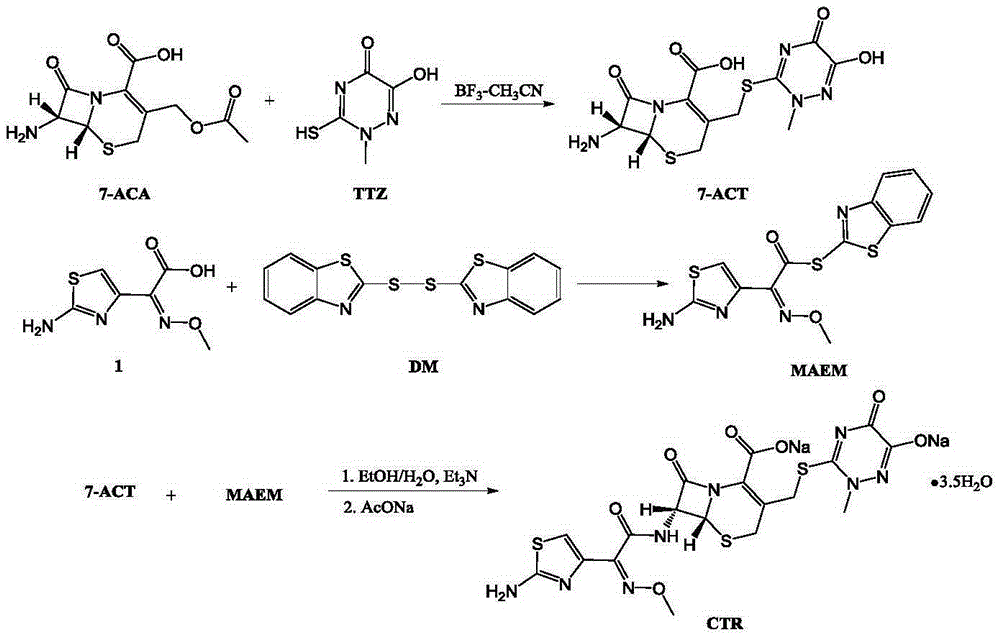
The invention discloses original-quality ceftriaxone sodium and a pharmaceutical preparation thereof. The key technology and industrialization of the third generation of cephalosporin antibiotics active ester intermediate wins the second prize of National Scientific and Technological Progress Award, and the third generation of cephalosporin antibiotics intermediate AE active ester is a key factor for affecting the internal quality of the ceftriaxone sodium. A preparation method comprises the steps that 1, boron trifluoride-acetonitrile serves as a catalyst, and on the condition that acetonitrile serves as solvent, a triazine ring is reacted with 7-ACA to generate 7-ACT; 2, triethylamine and aminothiazoly loximate are added into the solvent, a chloroformate activator is dropwise added slowly during cooling mixing, the 7-ACT is added for a one-pot reaction after stirring is conducted, and ceftriaxone is obtained; 3, a salt-forming agent is added, and the ceftriaxone sodium is obtained. According to the preparation method, use of a condensing agent with higher price is avoided, the process route is shortened, operation is easy, the reaction condition is mild, the product yield is high, the purity is good, and industrial production is easy.
Owner:广东金城金素制药有限公司 +1
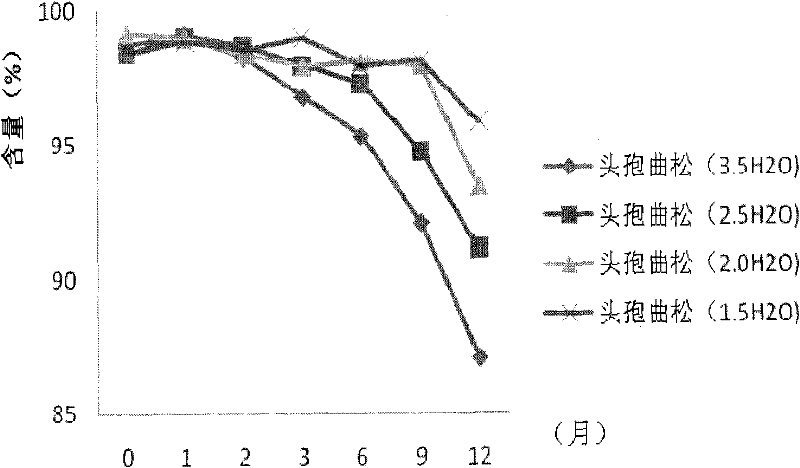
Low crystal-water ceftriaxone crystals and preparation method thereof
The invention relates to the field of chemosynthesis, particularly discloses low crystal-water ceftriaxone crystals containing 1.5-2.5 water molecules. The invention also provides a preparation method of the low crystal-water ceftriaxone crystals. Compared with the ceftriaxone crystals in the prior art, the low crystal-water ceftriaxone crystals have better stability and wide clinical application prospect at the same time of remaining the same dynamics characteristics and antibiosis activities.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Multi-detection method of residual of cephalo-type drugs in milk product
ActiveCN105116063AGuaranteed SensitivityAvoid interferenceComponent separationCefotaximeRelative standard deviation
The invention belongs to the technical field of detection of residual of drugs in an animal source food, and relates to a method for determining the residual quantity of cephalo-type drugs in a milk product through a high performance liquid chromatograph. The method comprises the steps of sample pre-extraction, standard curve drafting and apparatus detection analysis, the detection method is a multi-detection method carried out by using high performance liquid chromatography, and detected drugs comprise cefoperazone, cefotaxime, ceftriaxone and cephalothin. The cefoperazone detection limit of the method is 0.26mg / Kg, the cefotaxime detection limit of the method is 0.01mg / Kg, the ceftriaxone detection limit of the method is 0.10mg / Kg, and the cephalothin detection limit of the method is 0.07mg / Kg; the method has good linear relationship in an addition concentration range of 1-50mg / kg, and the recovery rate is 90-105%; and the intra-batch relative standard deviation is not greater than 15%, and the inter-batch relative standard deviation is not greater than 20%. The method has the advantages of short analysis time, low detection limit, high precision and multi-detection, and is of great significance to accurately monitor the residual quantity of the cephalo-type drugs in milk.
Owner:山东世通检测评价技术服务有限公司
Ceftriaxone sodium compound entity for children and preparation for ceftriaxone sodium compound entity for children
InactiveCN104926834AMaintain stabilityIncrease internal pressureAntibacterial agentsPowder deliveryActivated carbonTemperature control
The invention provides a ceftriaxone sodium compound entity for children. A structural formula of the ceftriaxone sodium compound entity is as follows: formula as shown in the specification. The ceftriaxone sodium compound entity is prepared by the following steps: (1) dissolving a ceftriaxone crude product into water, adding activated carbon, stirring and discoloring, and filtering; (2) adding an extraction agent into filtrate to obtain a mixture, transferring and filling the mixture into a pressure-resistant container, and carrying out temperature-controlled freezing on the mixture and taking out the mixture after removing air bubbles; and (3) removing an organic phase of the mixture, dropwise adding acetone at 10-15 DEG C after solids are molten, slowly stirring, growing crystals, filtering, washing, carrying out vacuum drying and packaging preparations of different specifications. Compared with ceftriaxone sodium prepared by a conventional process, the ceftriaxone sodium prepared by the preparation method has the advantages of few impurities, high stability and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cephalosporin antibiotics
ActiveCN104558187AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureAntibiotic YCephalosporin Antibiotic
The invention discloses a specific monoclonal antibody capable of resisting various cephalosporin antibiotics such as ceftiofur, ceftriaxone and the like. The invention further discloses an enzyme-linked immunosorbent assay method and kit for detecting the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like. According to the invention, the monoclonal antibody is secreted by a hybridoma cell 4D5 of which the preservation number is CCTCC No. C201341. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the various cephalosporin antibiotics such as the ceftiofur, the ceftriaxone and the like at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
Preparation method of liposome of medicine composition of ceftriaxone sodium and sulbactam sodium
ActiveCN102973568APromote crystallizationHigh dosageAntibacterial agentsLiposomal deliveryDrugSulbactam Sodium
The invention relates to a preparation method of liposome of a medicine composition of ceftriaxone sodium and sulbactam sodium. According to the method, the liposome is prepared in a combining form of medicines, and the specification proportion of the ceftriaxone sodium to the sulbactam sodium is equal to (1-4):1. The preparation method comprises the following main steps of: preparing blank liposome from phospholipid and cholesterol, preparing the liposome from the ceftriaxone sodium and the sulbactam sodium which are respectively subjected to passive loading, and performing freeze drying on the prepared liposome to obtain a finished product; or dissolving the ceftriaxone sodium and the sulbactam sodium in phosphate buffer with the pH ranging from 6.0 to 6.5, and adding phospholipid cholesterol and a freeze-dried support agent to the solution in an active loading manner to prepare a freeze-drying preparation of the liposome.
Owner:JIANGSU SKYRUN PHARMA CO LTD
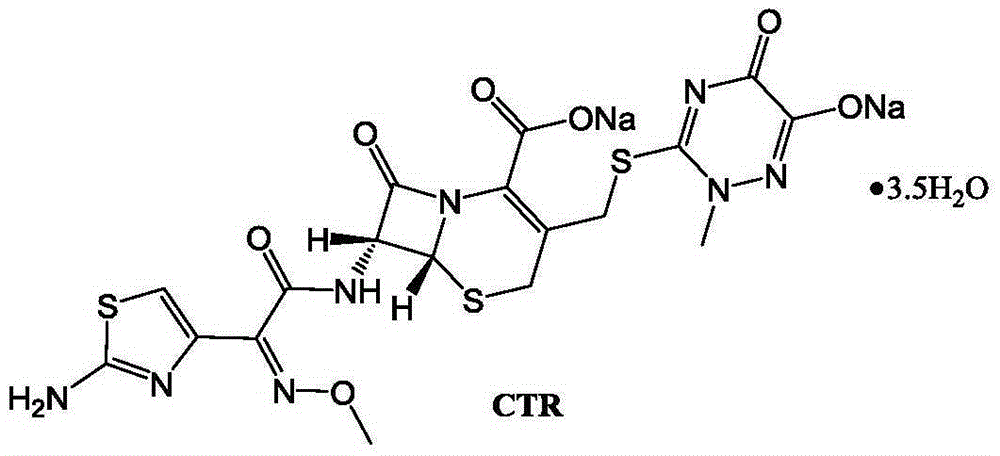
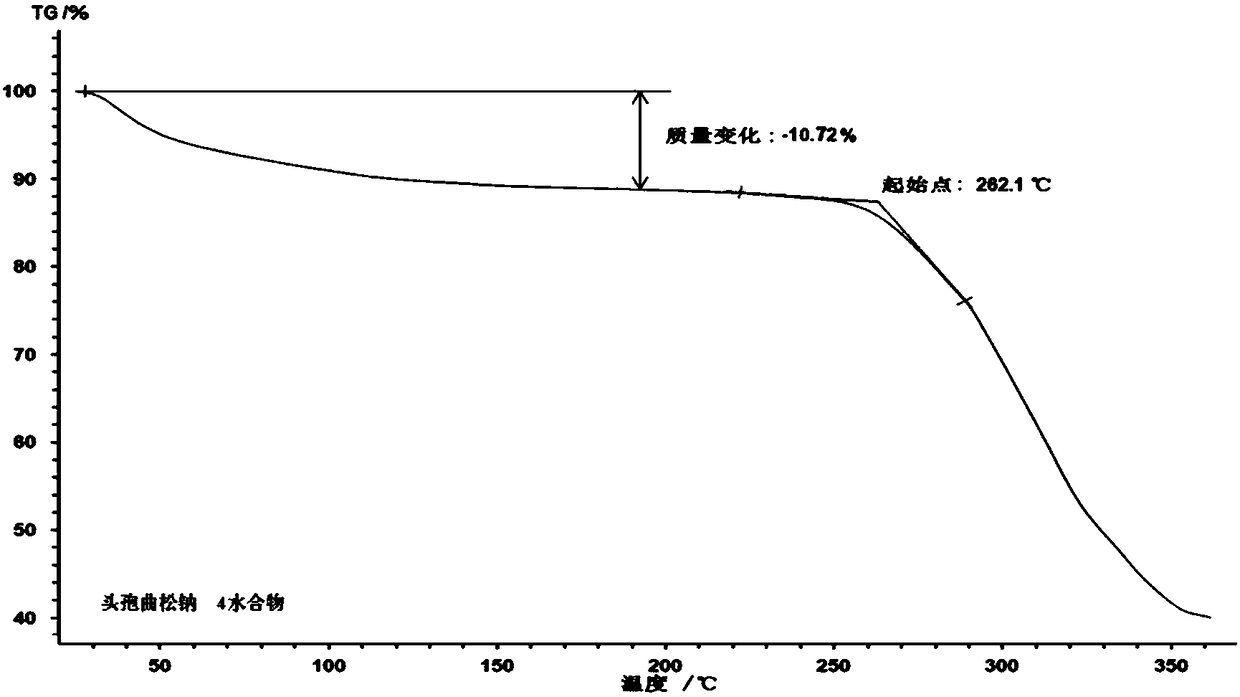
Ceftriaxone sodium tetrahydrate compound
The invention discloses a ceftriaxone sodium tetrahydrate compound and a preparation method thereof. Each mole of ceftriaxone sodium contains four mole of water. According to the compound, sulfochlorides and dimethylformamide are adopted to be reacted to generate an activator, the activator is directly reacted with 7-aminoceftriazine tetramethylguanidine salt to obtain the ceftriaxone sodium tetrahydrate compound. The operation is simple, the obtaining of a reactant is easy, the reaction condition is milder, and the yield is high. The ceftriaxone sodium tetrahydrate compound has the advantagesof low hygroscopicity, low impurity content, good fluidity, good thermodynamic stability and more extensive application prospects.
Owner:陕西顿斯制药有限公司 +2
Chinese and western composite medicine for treating neoplastic diseases
InactiveCN102949712AToxic reductionEasy drainage and exclusionOrganic active ingredientsPeptide/protein ingredientsSide effectAdditive ingredient
A Chinese and western composite medicine for treating neoplastic diseases is characterized by comprising Chinese herbal medicinal ingredients and a western medicinal ingredient which are mixed, the Chinese herbal medicinal ingredients include 5 polyzyme tablets and a stomach invigorating and digestion aiding tablet, each polyzyme tablet comprises pepsin, trypsin, pancreatic lipase and amylopsin, and the stomach invigorating and digestion aiding tablet comprises radix pseudostellariae, tangerine peels, Chinese yam, (roasted) malt and hawthorn; the western medicinal ingredient is ceftriaxone sodium injection solution; and the weight of each polyzyme tablet is 0.313g, the total weight of the polyzyme tablets is 1.57g, the weight of the stomach invigorating and digestion aiding tablet is 0.8g, the weight of the ceftriaxone sodium injection solution is 0.75g, and the Chinese herbal medicinal ingredients and the western medicinal ingredient are combined to form the prescription. The Chinese and western composite medicine for treating the neoplastic diseases is particularly used for suppressing and killing pathogenic bacteria capable of causing benign tumor and malignant tumor infection of tissue cells of a human body, the composite medicine is directly dripped into the oral cavity or an affected part of the human body when the human body suffers from the tumor infection, and 3-4 drops of the composite medicine are applied every time. The Chinese and western composite medicine has few side effects, becomes effective within 30 minutes and does not injure the liver, the brain and the kidneys of the human body.
Owner:李红彬
Microsphere injection of ceftriaxone sodium/tazobactam sodium drug composition
InactiveCN101804060AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryDrugChemistry
The invention discloses microsphere injection of a ceftriaxone sodium / tazobactam sodium drug composition, which comprises ceftriaxone sodium, tazobactam sodium, PLA, sodium glycocholate, PEG600 and trehalose. In the preferential technical scheme, the microsphere injection comprises 3 parts of ceftriaxone sodium, 1 part of tazobactam sodium, 2.5-7 parts of PLA, 3-6 parts of sodium glycocholate, 2-5 parts of PEG600 and 8-15 parts of trehalose. Compared with the prior art, the invention has the advantages of good stability, high encapsulation efficiency, preparation process suitable for industrial production and proper slow-release effect.
Owner:HAINAN MEIDA PHARMA
Method and kit for multiple detection of drug resistance sites of neisseria gonorrhoeae
PendingCN110643722AFast and Sensitive AnalysisImprove accuracyMicrobiological testing/measurementMicroorganism based processesHigh Resolution Melt AnalysisRelated gene
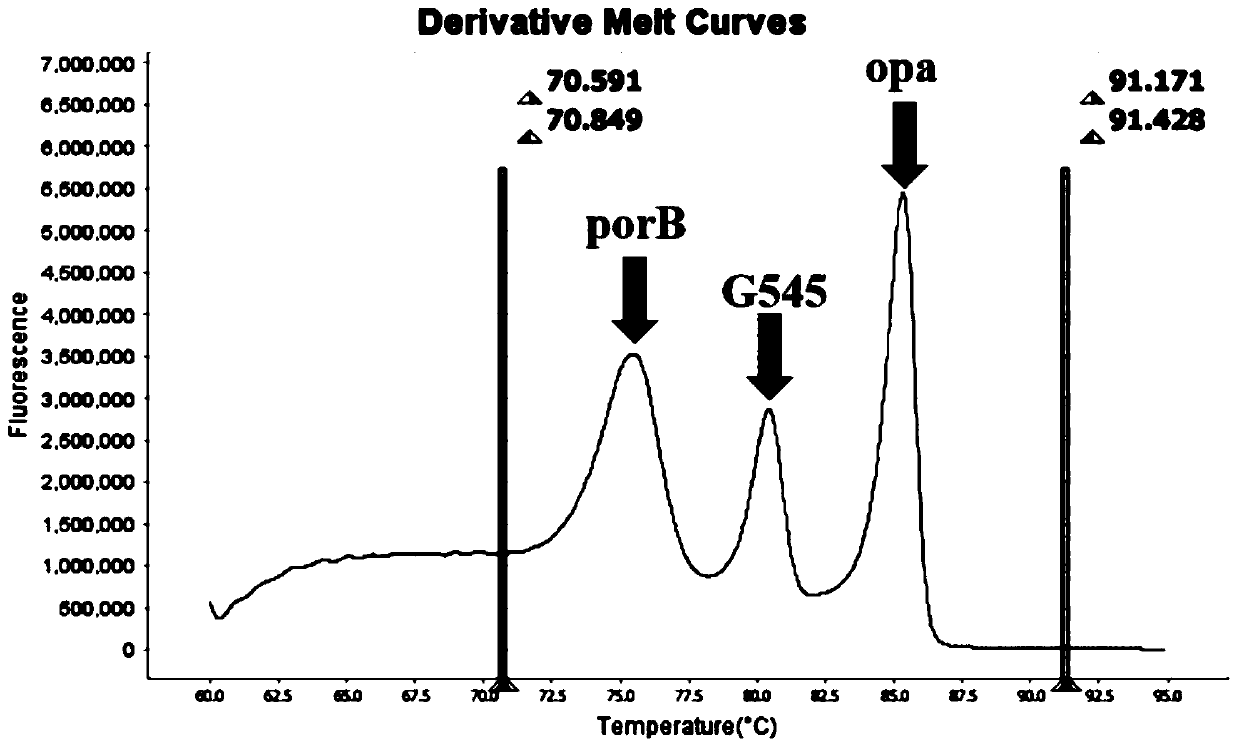
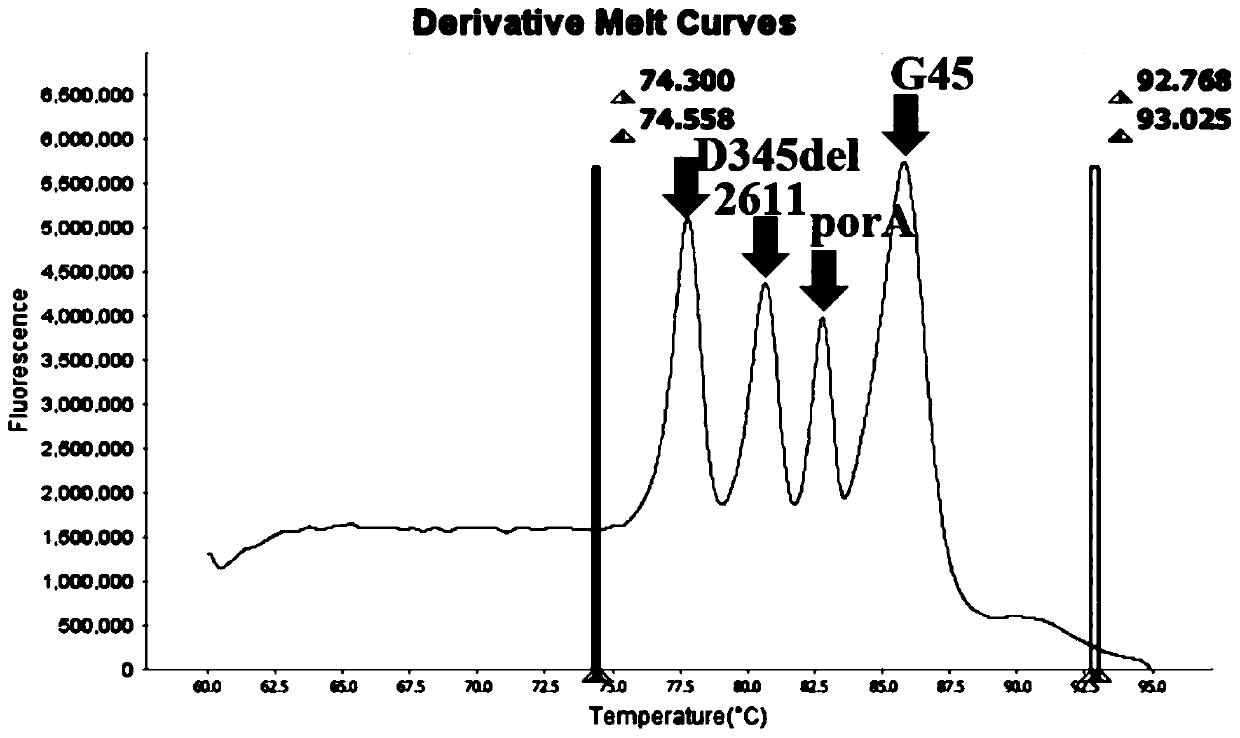
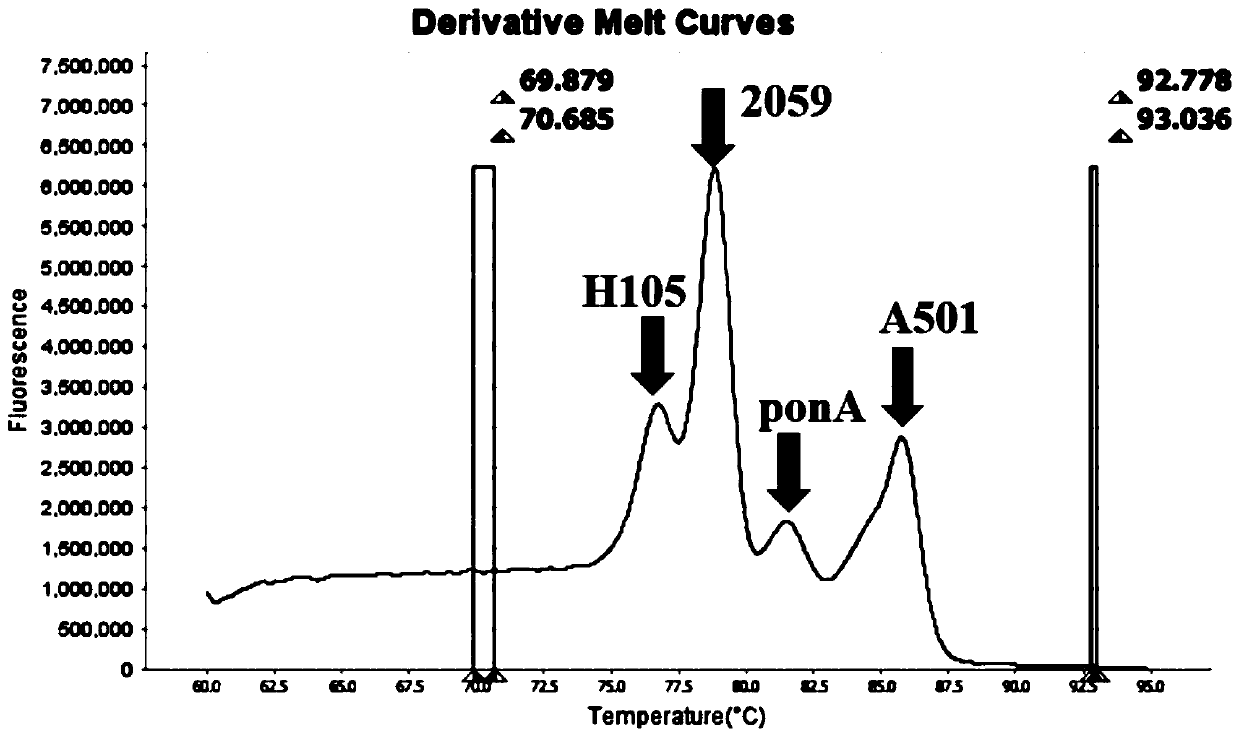
The invention belongs to the technical field of molecular biology detection, and relates to a detection method for drug resistance sites, in particular to a method and a kit for multiple detection ofthe drug resistance sites of neisseria gonorrhoeae. A provided specific primer for the multiple detection of the drug resistance sites of the neisseria gonorrhoeae selects drug-resistance-related genes of two drug combinations (ceftriaxone and azithromycin) as target genes for detection, and includes penA, ponA, porB, mtrR, and 23S rRNA. Based on high resolution melting curve analysis technique, the DNA demulsification process is monitored in real time through high resolution melting of PCR products, the mutation condition of the genes is analyzed according to the characteristic change of a melting curve, and thus a basis is provided for determining the drug-resistant condition of the neisseria gonorrhoeae.
Owner:USTAR BIOTECHNOLOGIES (HANGZHOU) CO LTD
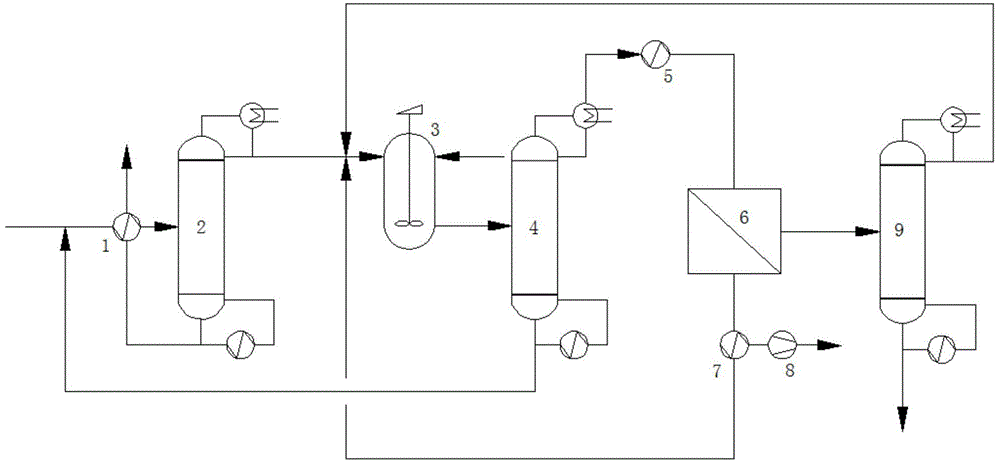
Method and device for recycling and refining acetonitrile in ceftriaxone sodium synthesis
ActiveCN104926690ASimple processImprove securityCarboxylic acid nitrile purification/separationSodium ceftriaxonePhysical chemistry
The invention relates to a method and device for recycling and refining acetonitrile in ceftriaxone sodium synthesis. The method includes the following steps: acetonitrile waste liquid in ceftriaxone sodium synthesis is fed into a first rectifying tower, and parts of water and heavy components are removed in a rectifying mode; rectified acetonitrile liquid is obtained after concentrating is carried out and fed into a neutralization tank for pH value adjustment; the neutralized rectified acetonitrile liquid is fed into a second rectifying tower for further impurity removing, the rectified acetonitrile liquid enters a pervaporation membrane separation unit after impurities are removed in the second rectifying tower, residual liquid in a kettle is returned to the first rectifying tower, and the acetonitrile in the residual liquid is recycled; the crude acetonitrile is obtained after the liquid is separated through the pervaporation membrane separation unit, water and a small amount of acetonitrile in a solution on the feed liquid side permeate a pervaporation membrane in a steam mode to obtain permeating liquid, the permeating liquid is condensed to be returned to the neutralization tank, and the acetonitrile in the permeating liquid is recycled; the crude acetonitrile obtained through the pervaporation membrane separation unit is fed into a third rectifying tower to be refined, the finished acetonitrile is obtained, acetonitrile-water azeotrope obtained after rectifying of the third rectifying tower is returned to the second rectifying tower, and the acetonitrile in the acetonitrile-water azeotrope is recycled.
Owner:JIANGSU NINE HEAVEN HIGH TECH
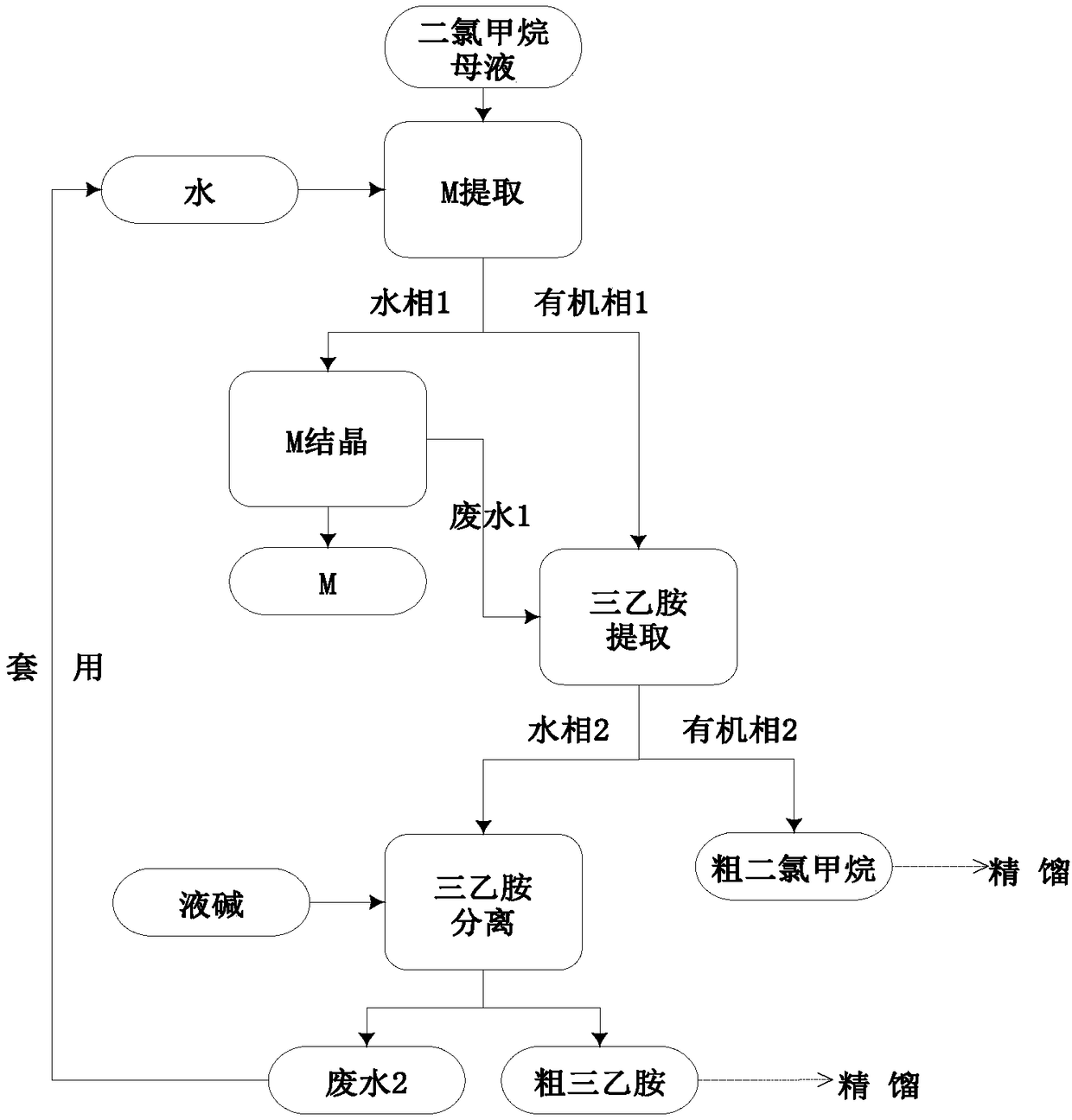
Comprehensive recycling method of 2-mercaptobenzothiazole, triethylamine, and dichloromethane in ceftriaxone sodium dichloromethane mother liquid
ActiveCN109053627AMeet the requirements of production recyclingReduce dosageAmino compound purification/separationHalogenated hydrocarbon preparationThiazoleSodium ceftriaxone
The invention discloses a comprehensive recycling method of 2-mercaptobenzothiazole, triethylamine, and dichloromethane in a ceftriaxone sodium dichloromethane mother liquid. The method provided by the invention comprises: (1) recycling 2-mercaptobenzothiazole, (2) recysling triethylamine, and (3) recycling dichloromethane. The invention relates to a complete set of comprehensive recycling schemeof the ceftriaxone sodium dichloromethane mother liquid. According to the invention, the method provided by the invention has the advantages of simple operation, high yield, low cost, little pollutionand the like; the comprehensive recycling of 2-mercaptobenzothiazole, triethylamine, and dichloromethane can be implemented; the recycling of organic matter is maximized, and organic pollution environment is reduced; waste water generated from a recycling process is applied for reducing the dosage of acid and alkali in an extraction process, and meanwhile, the discharge of waste water is also controlled. The method provided by the invention has a good practical prospect.
Owner:SHANXI WEIQIDA PHARMA IND
Detection method for antioxidant in ceftriaxone sodium for injection
InactiveCN104198618AAuxiliary control of product qualityAvoid product qualityComponent separationSodium ceftriaxoneAnti oxidant
The invention discloses a detection method for an antioxidant in ceftriaxone sodium for injection. According to the method, the antioxidant in ceftriaxone sodium for injection can be detected in an appropriate detection concentration through the establishment of a linear equation and the determination of precision degree, so that the product quality of the ceftriaxone sodium for injection can be further controlled in an auxiliary mode; the method is practical and simple, and a new direction of controlling the product quality of the ceftriaxone sodium for injection is developed.
Owner:SICHUAN PHARMA
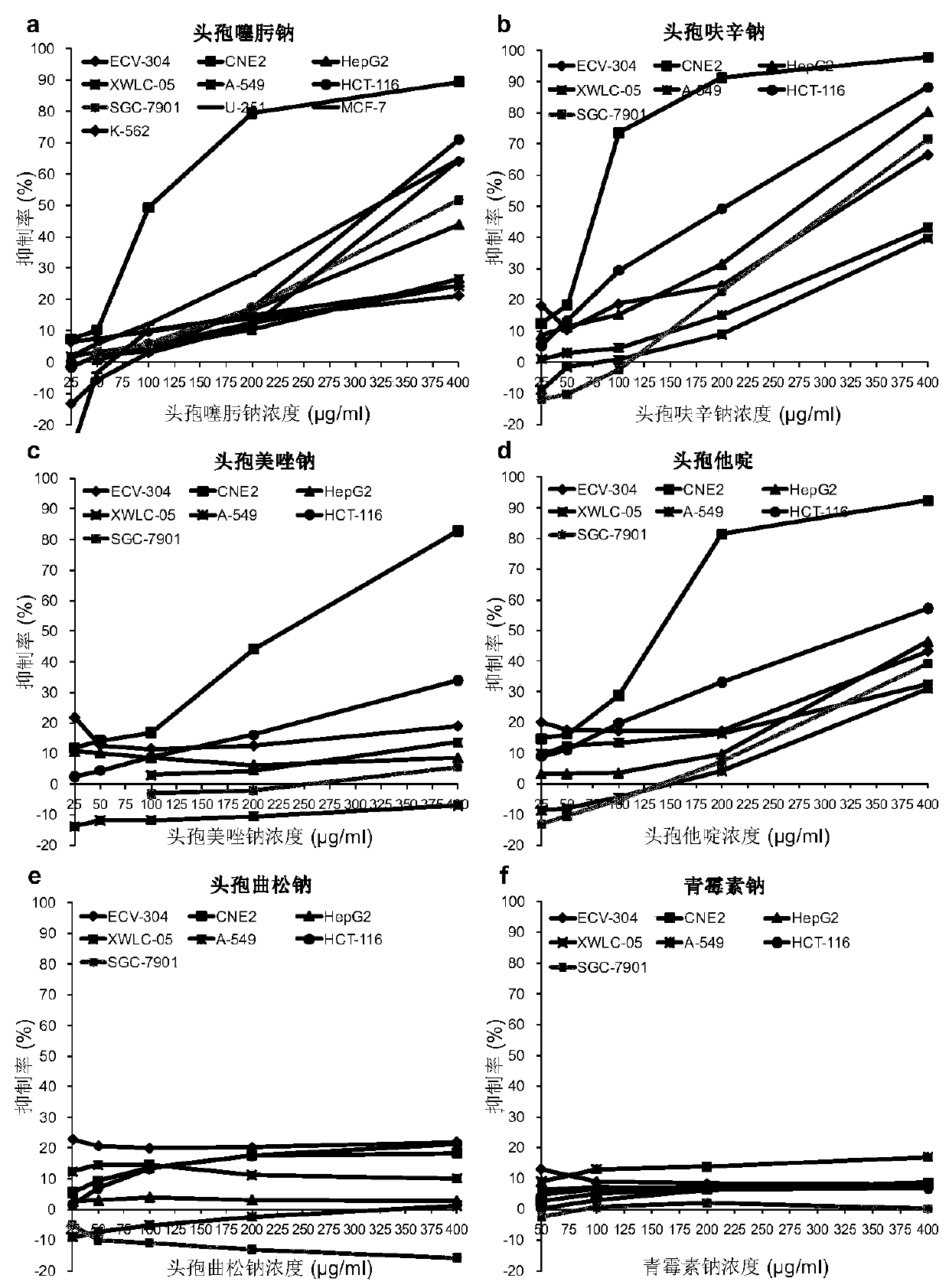
Application of cephalosporin antibiotics as anticancer medicines and anticancer medicines
ActiveCN110327349AHas selective anticancer effectNo anticancer effectOrganic chemistryAntineoplastic agentsChemical structureCancer cell
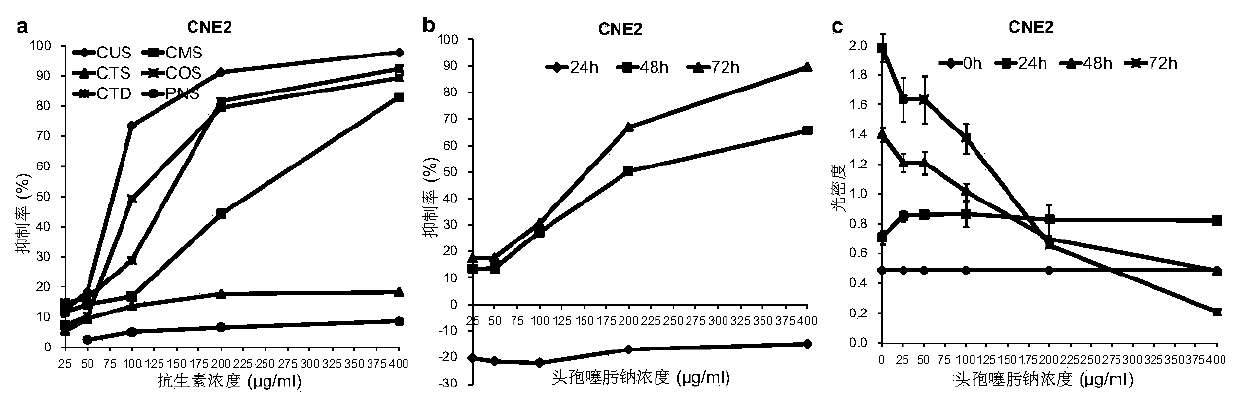
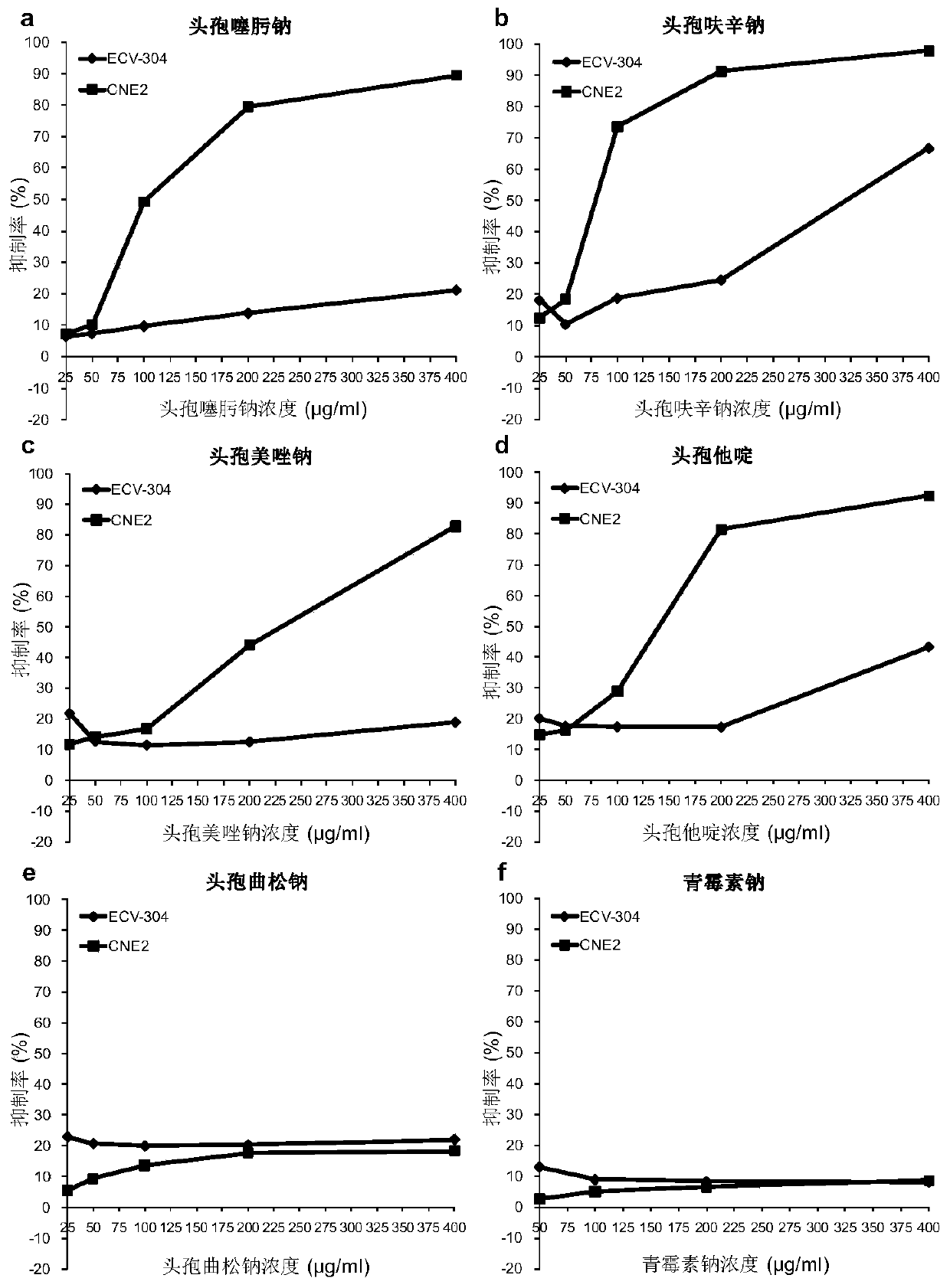
The invention relates to an application of cephalosporin antibiotics as anticancer medicines and the anticancer medicines, and belongs to the technical field of medicine research. The cephalosporin antibiotics have a high specific anticancer effect on nasopharyngeal carcinoma, also have obvious anticancer effects on other various cancers, and have no toxicity to normal cells or have toxicity, farlower than toxicity to cancer cells, to normal cells. The cephalosporin antibiotics remarkably inhibit growth of nasopharyngeal carcinoma in vivo, and do not influence body weight development of animals. The combined effects of the cephalosporin antibiotics with clinical anticancer medicines on different cancers are different. The cephalosporin antibiotics have great differences in anticancer activity, and ceftriaxone does not have anticancer activity. The anti-cancer activity and selectivity of the cephalosporin antibiotics are determined by side chains of chemical structures of the cephalosporin antibiotics, and external structure modification of mother nuclei is a way for finding and synthesizing novel anticancer substances. The result shows that the cephalosporin antibiotics except ceftriaxone are specific anticancer medicines for nasopharyngeal carcinoma, can also be used as therapeutic medicines for other cancers, can be used as a combined anticancer medicine, or can be used as health-care products for adjuvant therapy of cancers.
Owner:KUNMING MEDICAL UNIVERSITY
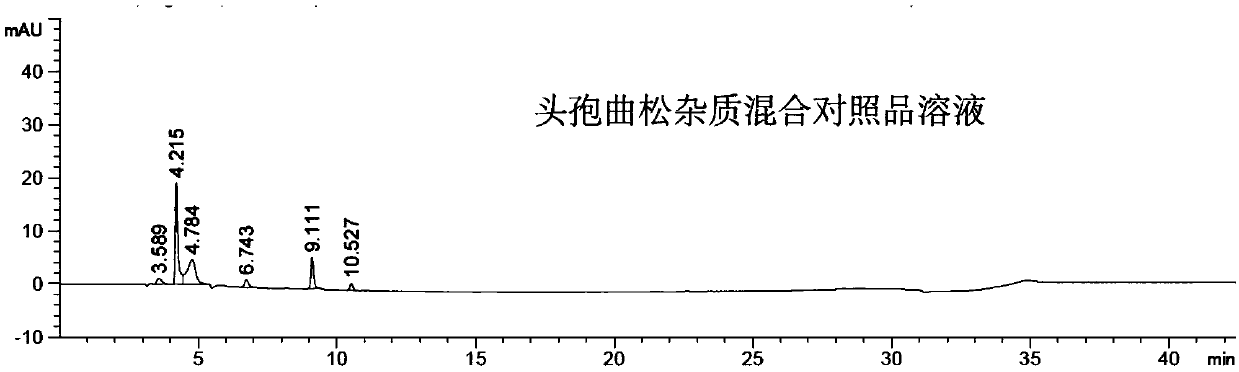
Method for detecting impurity 2-mercaptobenzothiazole in ceftriaxone sodium
ActiveCN111208215AStrong detection specificityGood linear fitComponent separationThiamazolumPhosphate
The invention relates to a method for detecting impurity 2-mercaptobenzothiazole in ceftriaxone sodium by using high performance liquid chromatography. The detection method adopts the following chromatographic conditions: a mobile phase A: a mixed solution of a phosphate buffer solution with the pH value of 6.05-6.45 and methanol; and a mobile phase B: an acetonitrile aqueous solution; wherein themobile phase A and the mobile phase B are used for gradient elution. The method is good in detection specificity, good in linear fitting, low in detection limit, high in sensitivity, good in quantification limit repeatability and good in durability.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Antibacterial medicinal composition
InactiveCN1679585AAntibacterial agentsHeterocyclic compound active ingredientsCurative effectPharmacology
A composite antibacterial medicine with durable and high curative effect is proportionally prepared from ceftriaxone and tazobactam, or their soduim salts.
Owner:HAIKOU QILI PHARMA
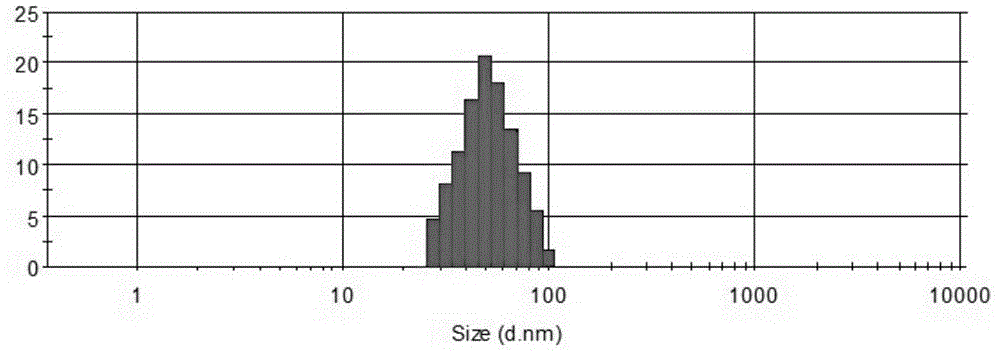
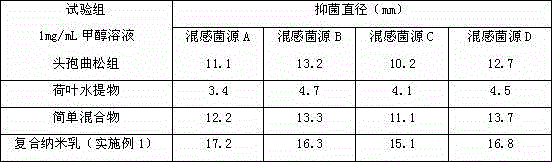
Oil-in-water type compound ceftriaxone nanoemulsion antimicrobial medicine
InactiveCN104367636ANarrow particle size distributionSystem transparencyAntibacterial agentsOrganic active ingredientsDistilled waterDrug product
The invention relates to an oil-in-water type compound ceftriaxone nanoemulsion antimicrobial medicine which is a nanoemulsion preparation taking ceftriaxone and an aqueous extract of lotus leaves as effective components. The medicine is prepared from the following components in parts by weight: 1 to 25 parts of ceftriaxone, 15 to 35 parts of surfactant, 0 to 20 parts of cosurfactant, 1 to 20 parts of aqueous extract of lotus leaves, 1 to 20 parts of oil and 20 to 70 parts of distilled water. The medicine is good in pharmacological action and excellent in antimicrobial effect via combination of the ceftriaxone and the aqueous extract of lotus leaves, and is very simple in preparation method and easy to popularize.
Owner:HENAN SOAR VETERINARY PHARMA
Amino butanetriol salt of cephalosporin compounds and preparing method
InactiveCN101012235AReduce intakeAvoid the risk of hypernatremiaAntibacterial agentsOrganic active ingredientsCefuroximeCefazolin
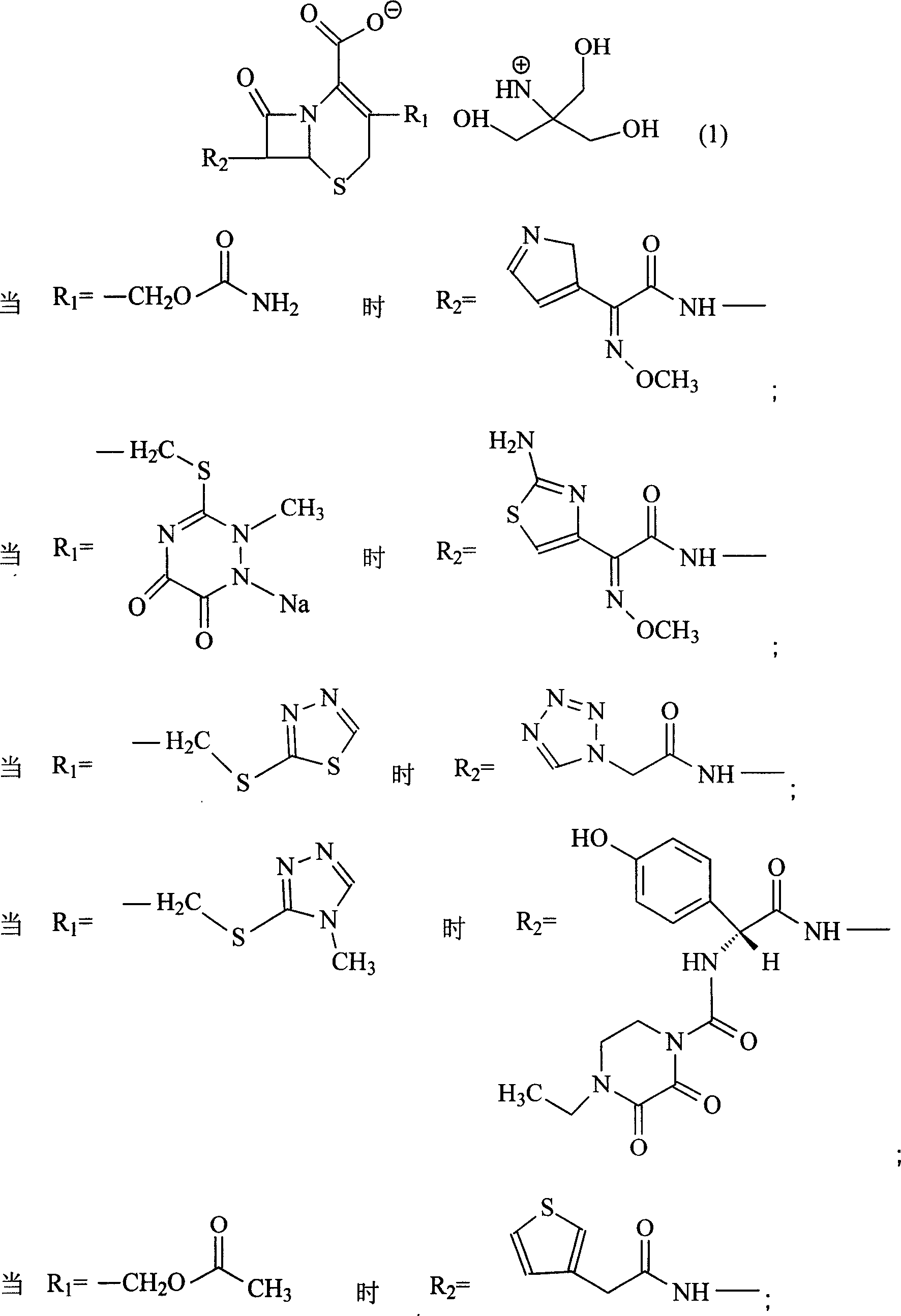
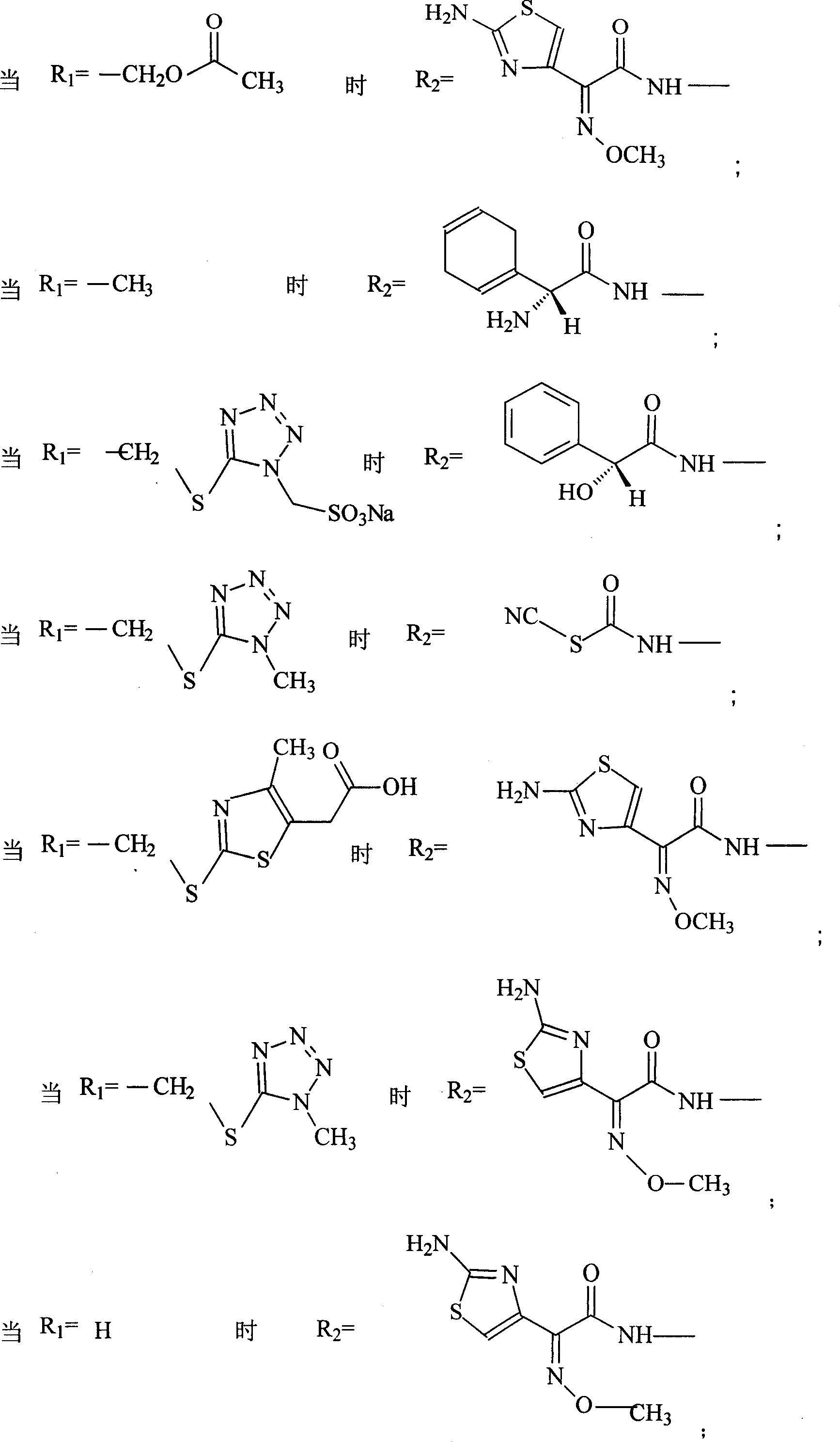
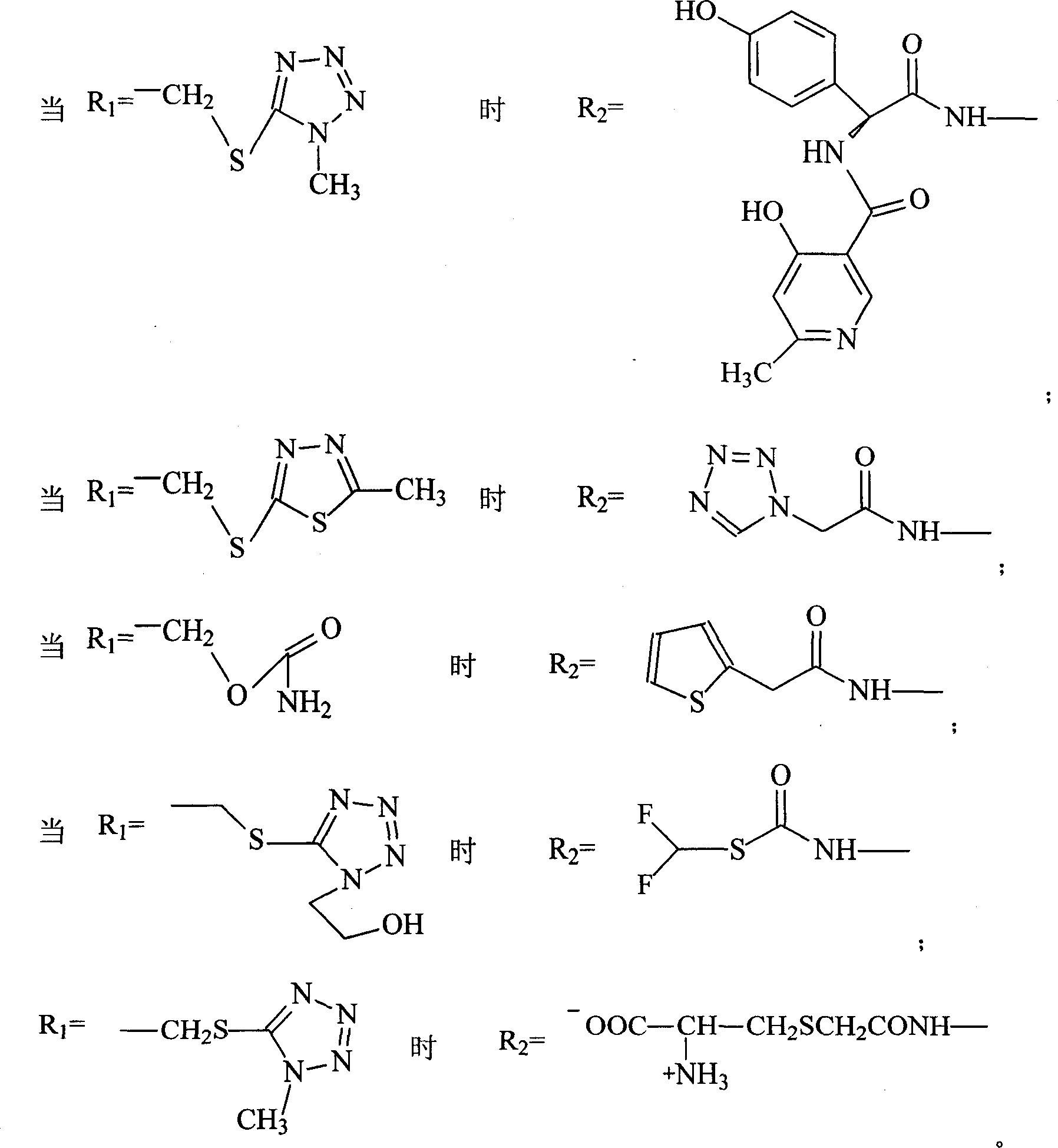
The invention discloses a pehanorm salt or hydrate with chemical formula as picture (1) and drug composition and application to treat bacterial infection, which comprises the following parts: cefuroxime oxtatromethane, cepham qusong tromethane, cepham thiotepa tromethane, cefoperazone tromethane, cephalothin tromethane, cefotaxime tromethane, cefolading tromethane, cefonixin tromethane, cefameizin tromethane, cefadizine tromethane, cefuroxime tromethane, cefazolin tromethane, cefapamine tromethane, cefazoline tromethane, cefaadid tromethane, cefaoxofluoride tromethane, cefaminol tromethane and their hydrate.
Owner:GUANGDONG ZHONGKE DRUG R&D
Antibacterial composite medicine
An antibacterial composite medicine is prepared from ceftriaxone and cefradine in the radio of 1:(0.1-10) and they have cooperation effect. Its medical application is also disclosed.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Method for detecting polymer impurities of ceftriaxone sodium
PendingCN112798705AStrong specificityImprove linearityComponent separationO-Phosphoric AcidSilica gel
The invention belongs to the technical field of drug detection, and particularly relates to a method for detecting polymer impurities of ceftriaxone sodium. According to the method, a ceftriaxone sodium raw material medicine is taken to prepare a test solution, HPLC detection is carried out, and the detection conditions are as follows: a chromatographic column with globular protein hydrophilic modified silica gel as a filler is adopted as a stationary phase; and the mobile phase is a mixed solution composed of a mobile phase A and a mobile phase B in a volume ratio of (93-97): (7-3), the mobile phase A is a 0.1-0.2% triethylamine solution, the pH value is adjusted to 5.5-6.5 by using a phosphoric acid solution, and the mobile phase B is acetonitrile. The method for detecting the polymer impurities in the ceftriaxone sodium is high in specificity, good in operability and good in accuracy and precision.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Ceftriaxone combinatorial drug
InactiveCN103908673AGood treatment effectPyrogenic reaction noAntibacterial agentsOrganic active ingredientsCurative effectBULK ACTIVE INGREDIENT
The invention provides a ceftriaxone combinatorial drug. The ceftriaxone combinatorial drug is characterized in that the ceftriaxone combinatorial drug is prepared from ceftriaxone, 2-hydroxypropyl-beta-cyclodextrin and tiopronin as active ingredients according to a weight part ratio of ceftriaxone, 2-hydroxypropyl-beta-cyclodextrin to tiopronin of 53-98: 5-11: 6-19. The invention also provides a preparation method of the ceftriaxone combinatorial drug. The ceftriaxone combinatorial drug is superior to the existing drug prepared by the prior art in safety, stability and curative effects and can be prepared by an energy-saving and eco-friendly preparation method.
Owner:邓学峰
Preparation method of high-selectivity ceftriaxone sodium magnetic molecularly imprinted polymer
ActiveCN111269366ASuperparamagneticImprove adsorption capacityOther chemical processesAlkali metal oxides/hydroxidesPolymer scienceFunctional monomer
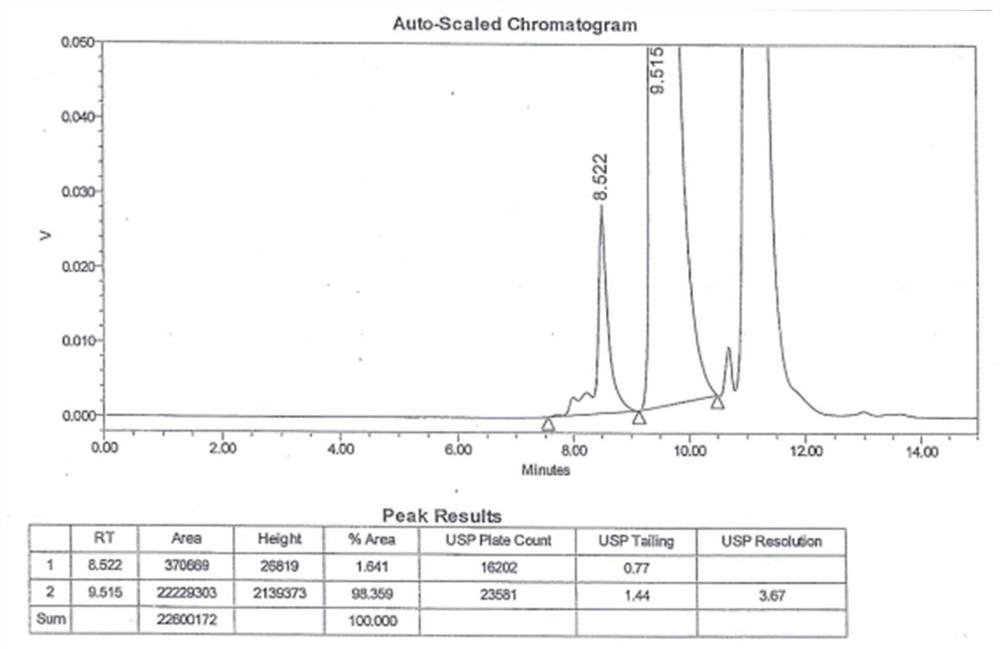
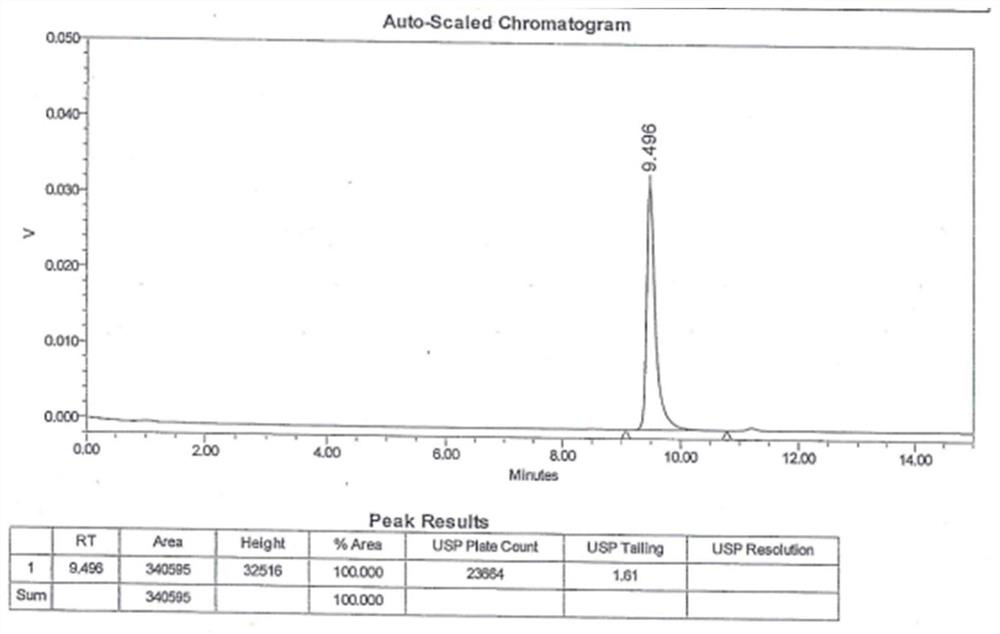
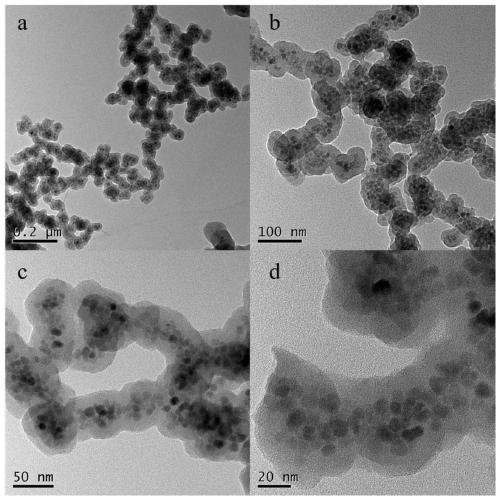
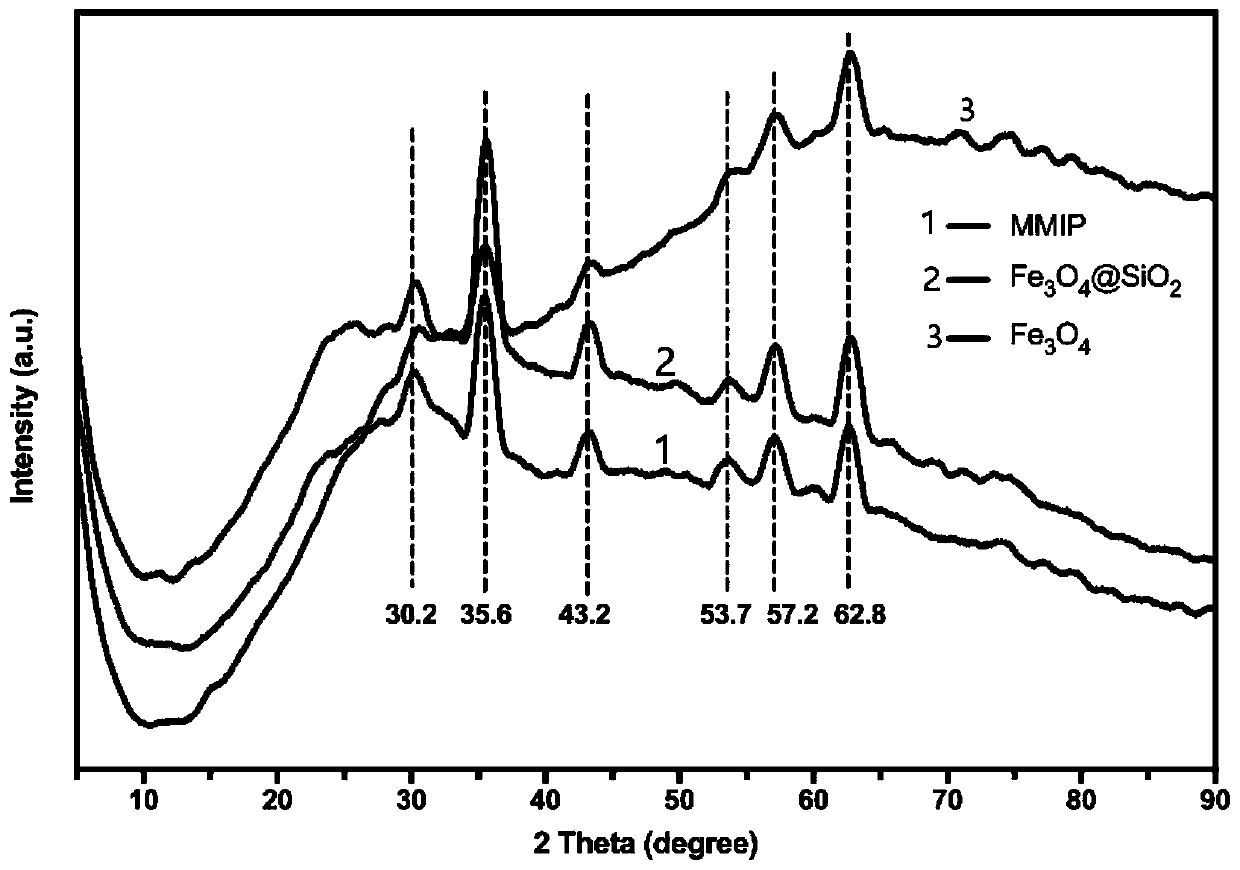
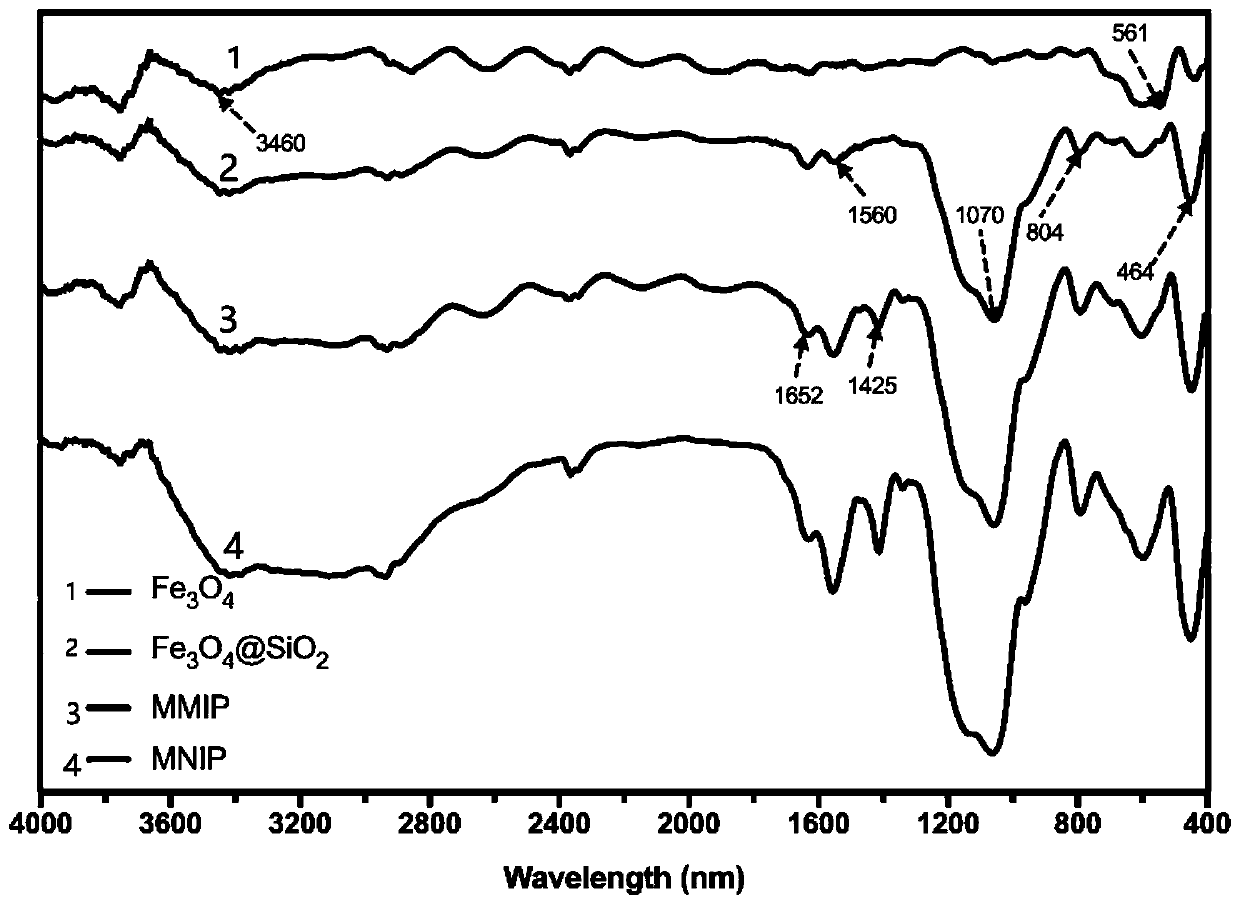
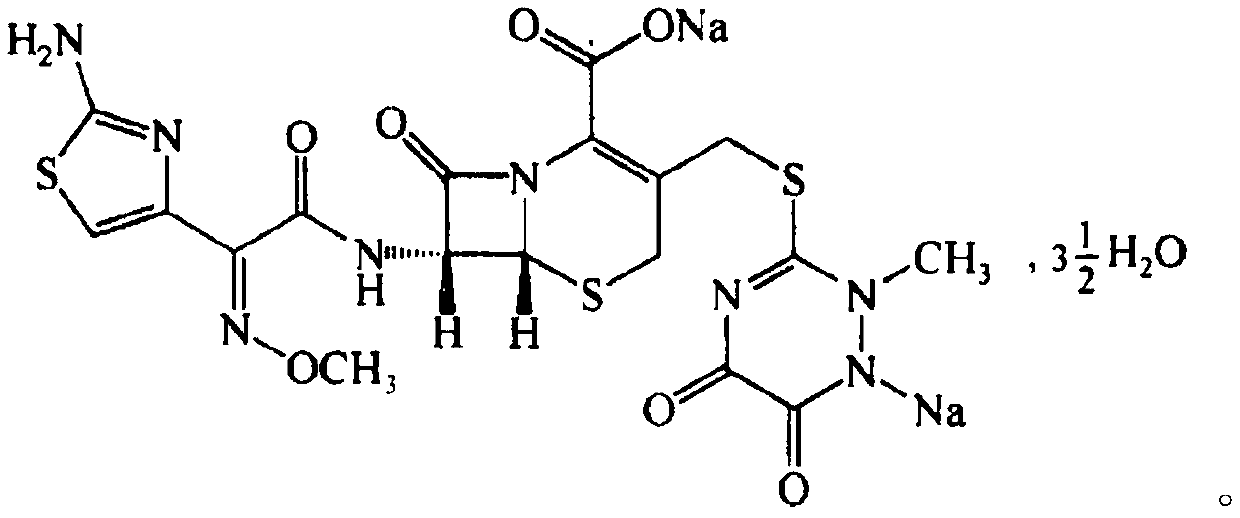
The invention discloses a preparation method of a high-selectivity ceftriaxone sodium magnetic molecularly imprinted polymer. The preparation method comprises the following steps: firstly, synthesizing superparamagnetic Fe3O4 nanoparticles with hydroxylated surfaces in one step by taking triethylene glycol and ferric acetylacetonate as raw materials through a polyol method; then taking the superparamagnetic Fe3O4 nano particles, tetraethoxysilane and ammonia water as raw materials, preparing Fe3O4@SiO2 nano particles by a sol-gel method, and modifying amino groups on the surfaces of the nano particles by utilizing 3-aminopropyltriethoxysilane; then with the ceftriaxone sodium as a template molecule, methacrylic acid as a functional monomer, acetonitrile and methanol as pore-foaming agents,ethylene glycol dimethacrylate as a cross-linking agent and azodiisobutyronitrile as an initiator, preparing the magnetic molecularly imprinted polymer with specific adsorption performance on ceftriaxone sodium. The superparamagnetism and specific adsorption capacity of the ceftriaxone sodium magnetic molecularly imprinted nanoparticles are effectively improved. The preparation method is simple,the cost is low, and the ceftriaxone sodium magnetic molecularly imprinted nanoparticles have wide application prospects.
Owner:HANGZHOU DIANZI UNIV
Preparation method of ceftriaxone sodium spherical crystal
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com