Method for detecting polymer impurities of ceftriaxone sodium
A ceftriaxone sodium and detection method technology, which is applied in the detection field of ceftriaxone sodium polymer impurities, can solve the problems of poor specificity, poor peak shape, low number of theoretical plates, etc., and achieve good specificity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Solution configuration
[0038] Need testing solution: get ceftriaxone sodium crude drug, accurately weighed, add water to dissolve and quantitatively dilute and make the solution that contains ceftriaxone 0.5mg in every 1mL about, as need testing solution.
[0039] Reference substance solution: take an appropriate amount of ceftriaxone sodium reference substance, accurately weighed, add water to dissolve and quantitatively dilute to make a solution containing about 5 μg per 1 mL as the reference substance solution.
[0040] 2. Chromatographic conditions
[0041] Chromatographic column: TSKgel G2000SWXL 7.8×300mm, 5μm;
[0042] Mobile phase A: 0.14% triethylamine solution, adjust the pH value to 6.0 with phosphoric acid solution;
[0043] Mobile phase B: acetonitrile;
[0044] Mobile phase A:B=95:5;
[0045] Column temperature: 30°C;
[0046] Detection wavelength: 254nm;
[0047] Flow rate: 0.8mL / min.
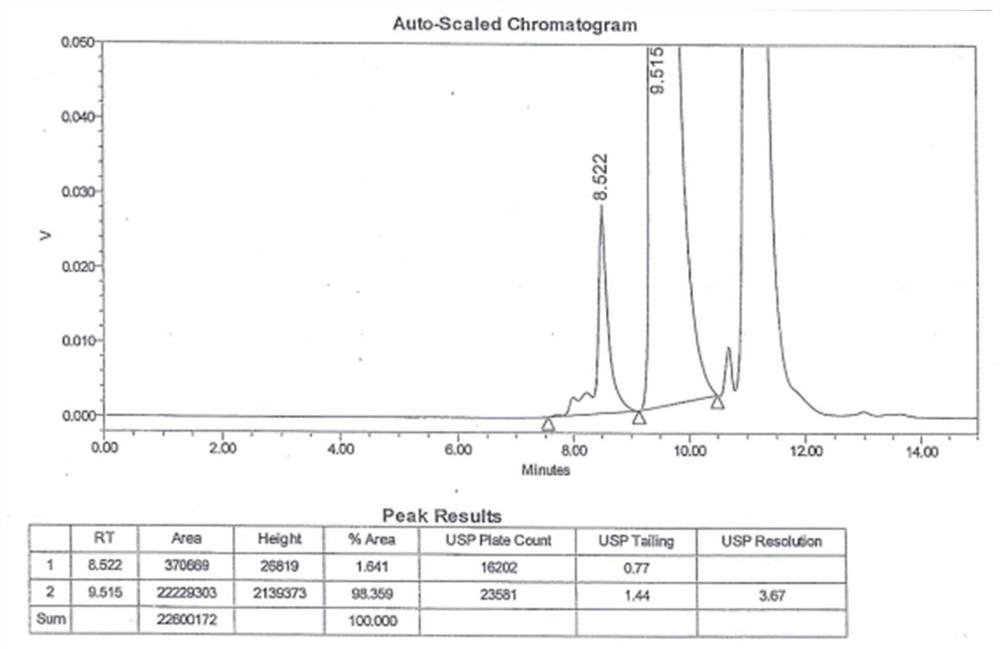
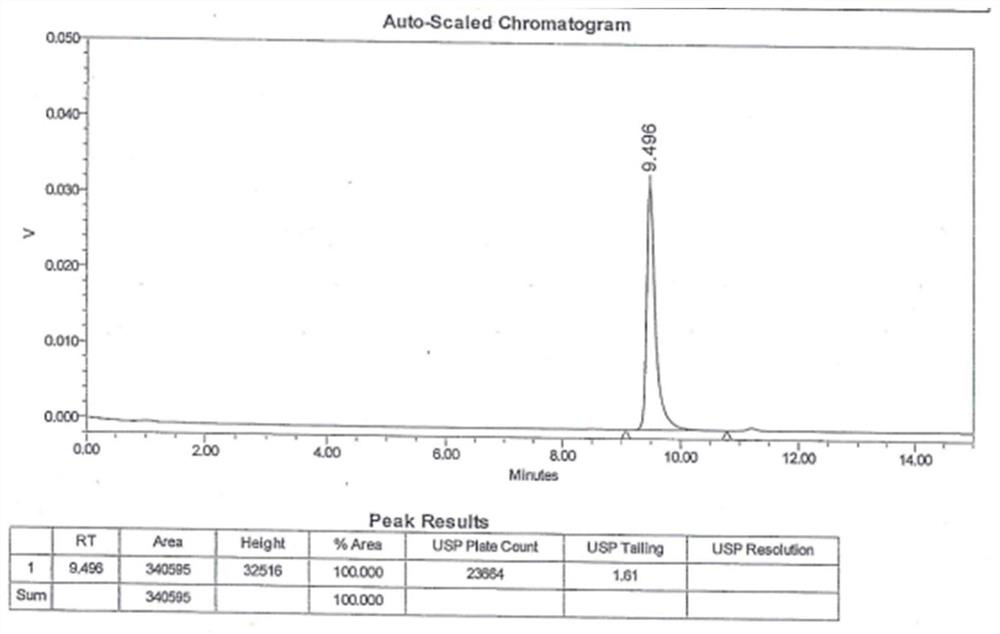
[0048] 3. Sample determination
[0049] Get 20 μl of nee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com