Patents
Literature
511 results about "Drug detection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Procedures to detect the presence of drugs or their side products in blood, urine, breath, or other biological samples; do not confuse with DRUG SCREENING/EVALUATION.
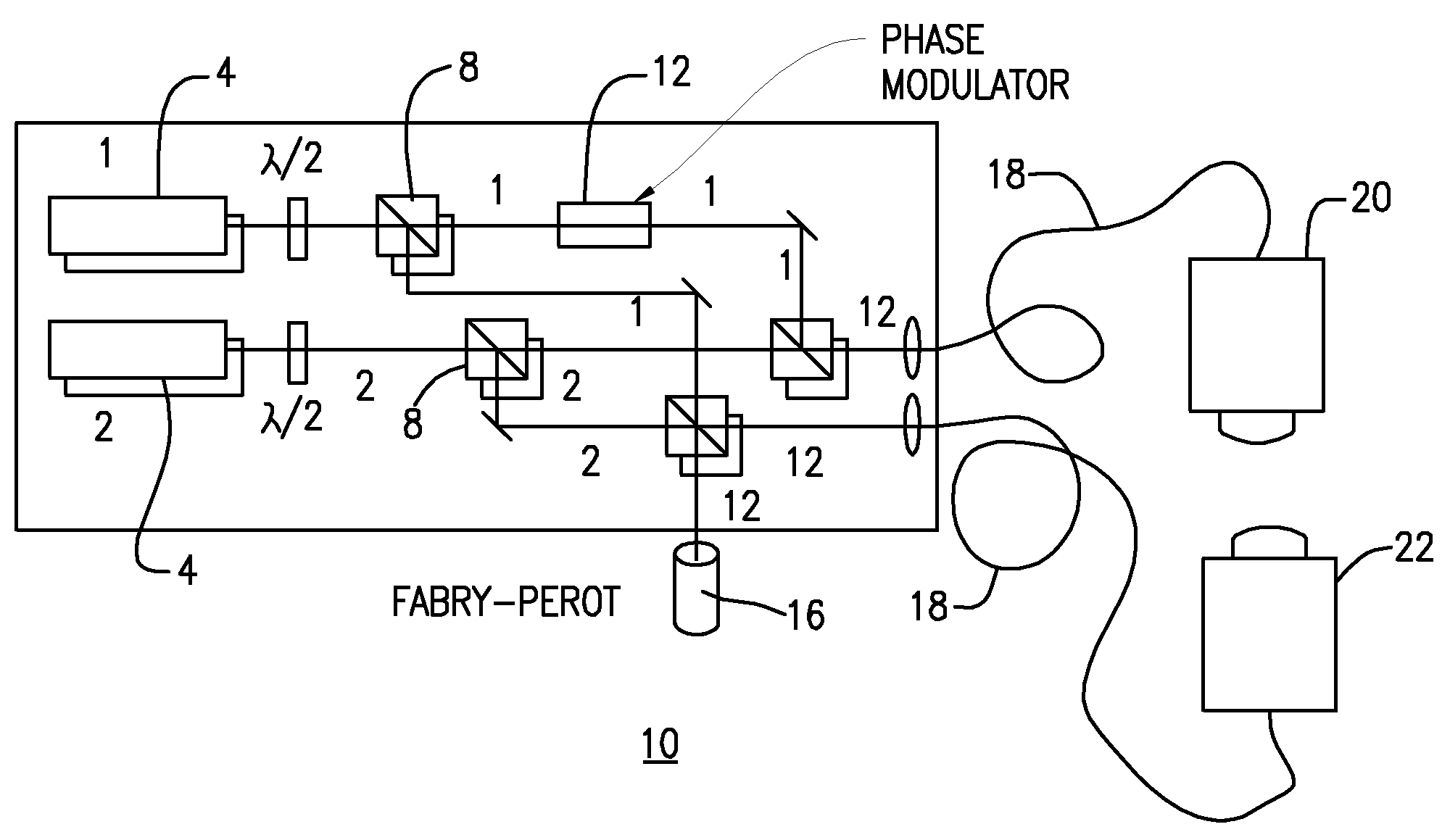
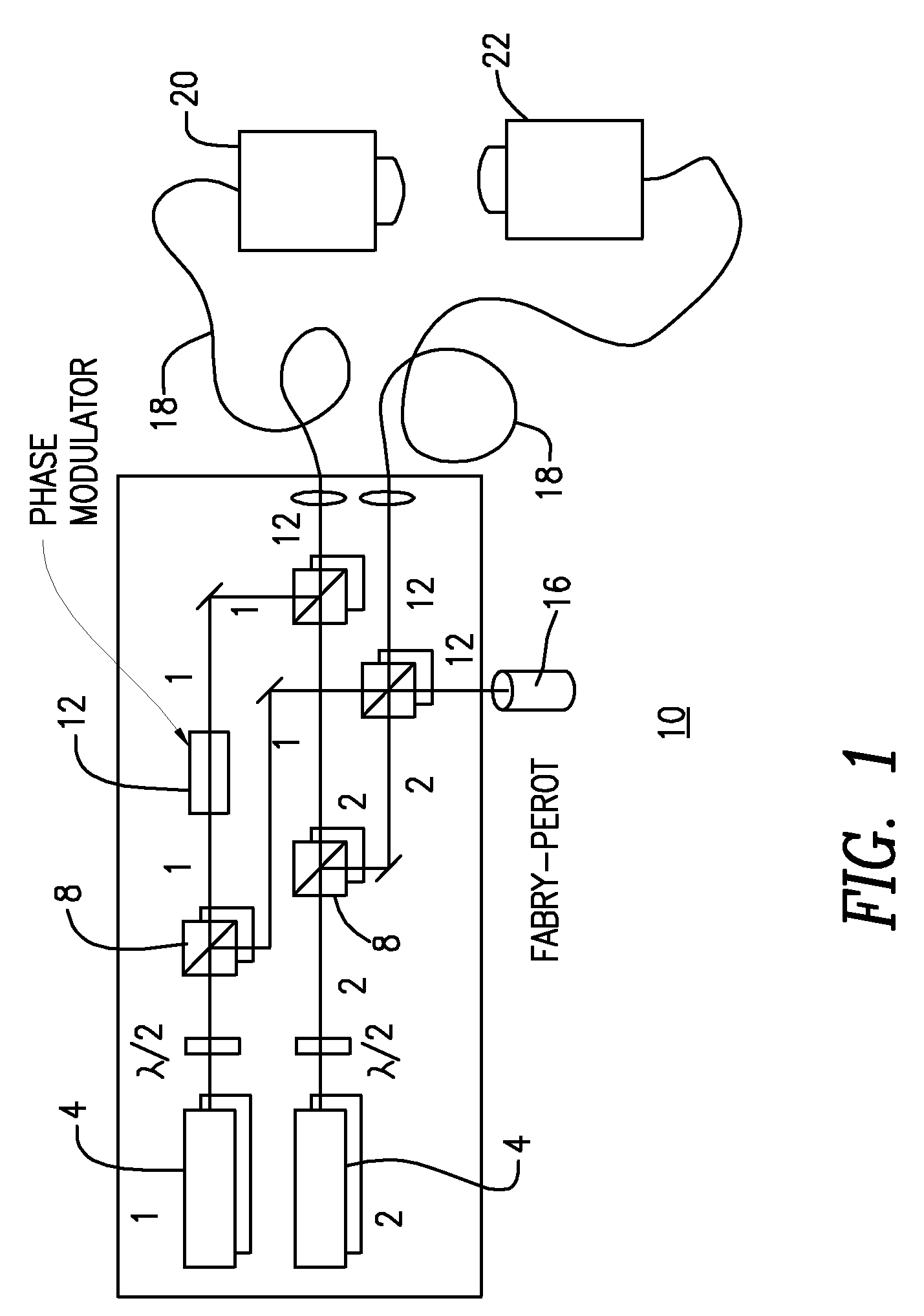
ILS sensors for drug detection within vehicles
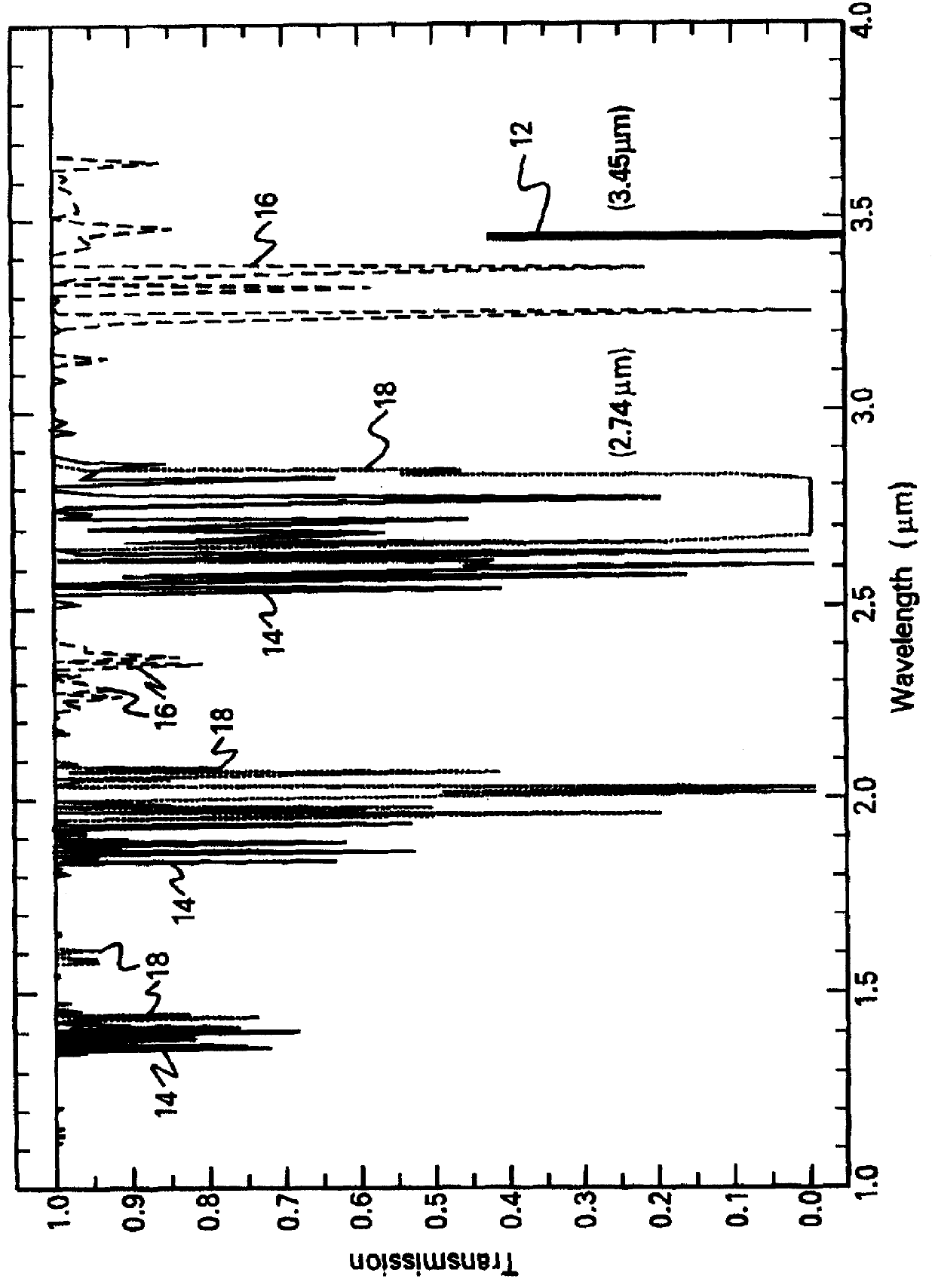
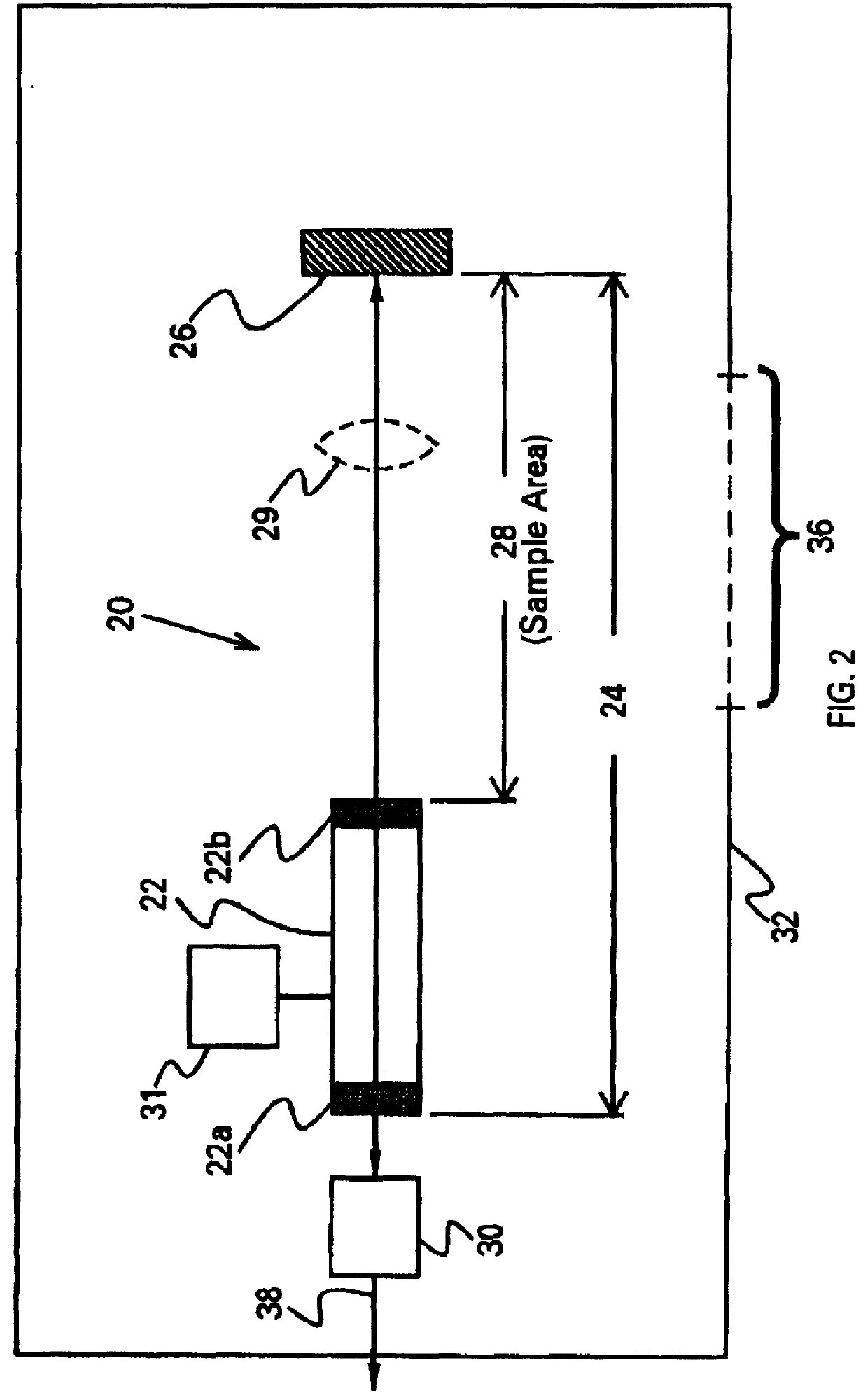
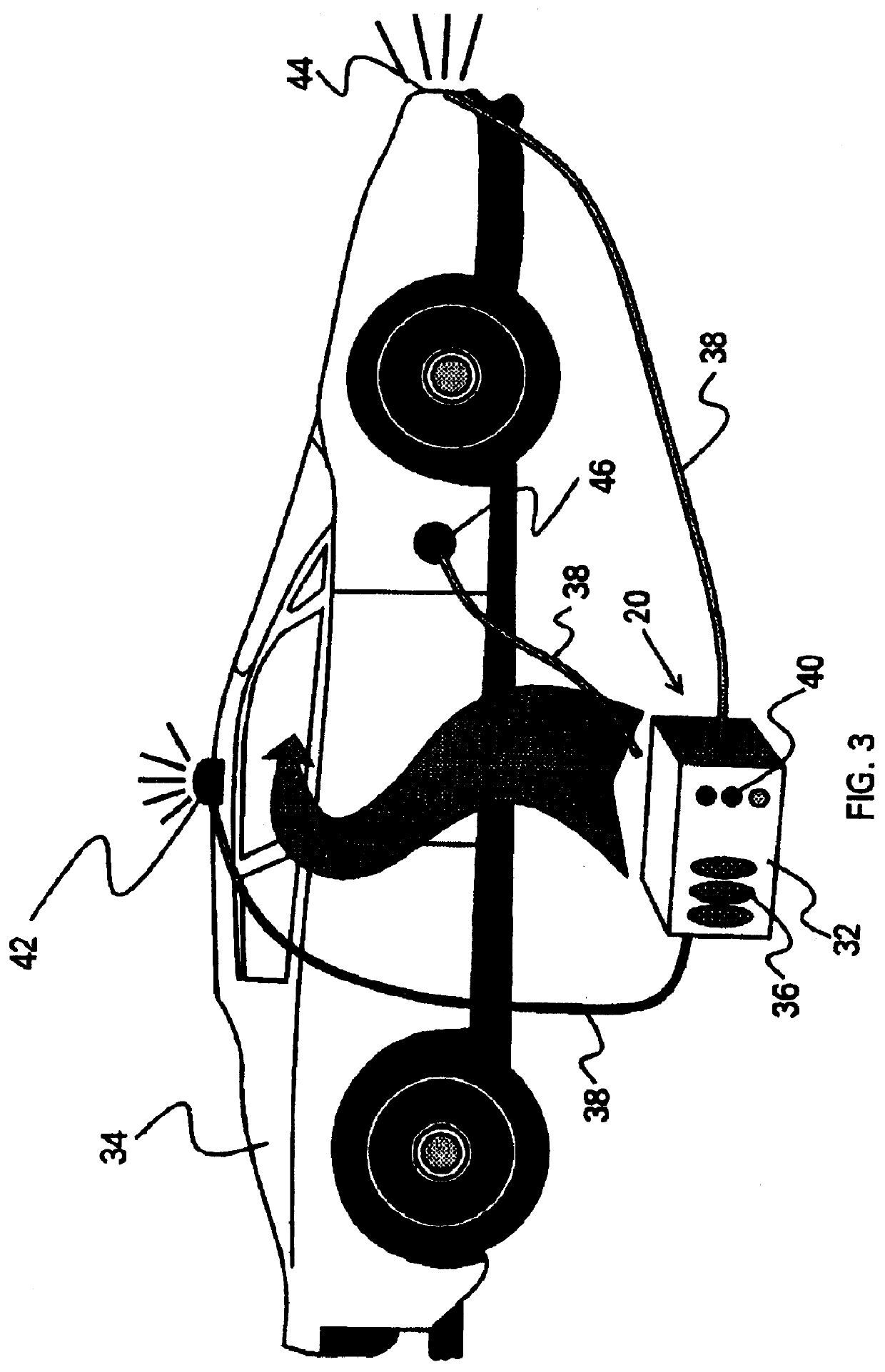
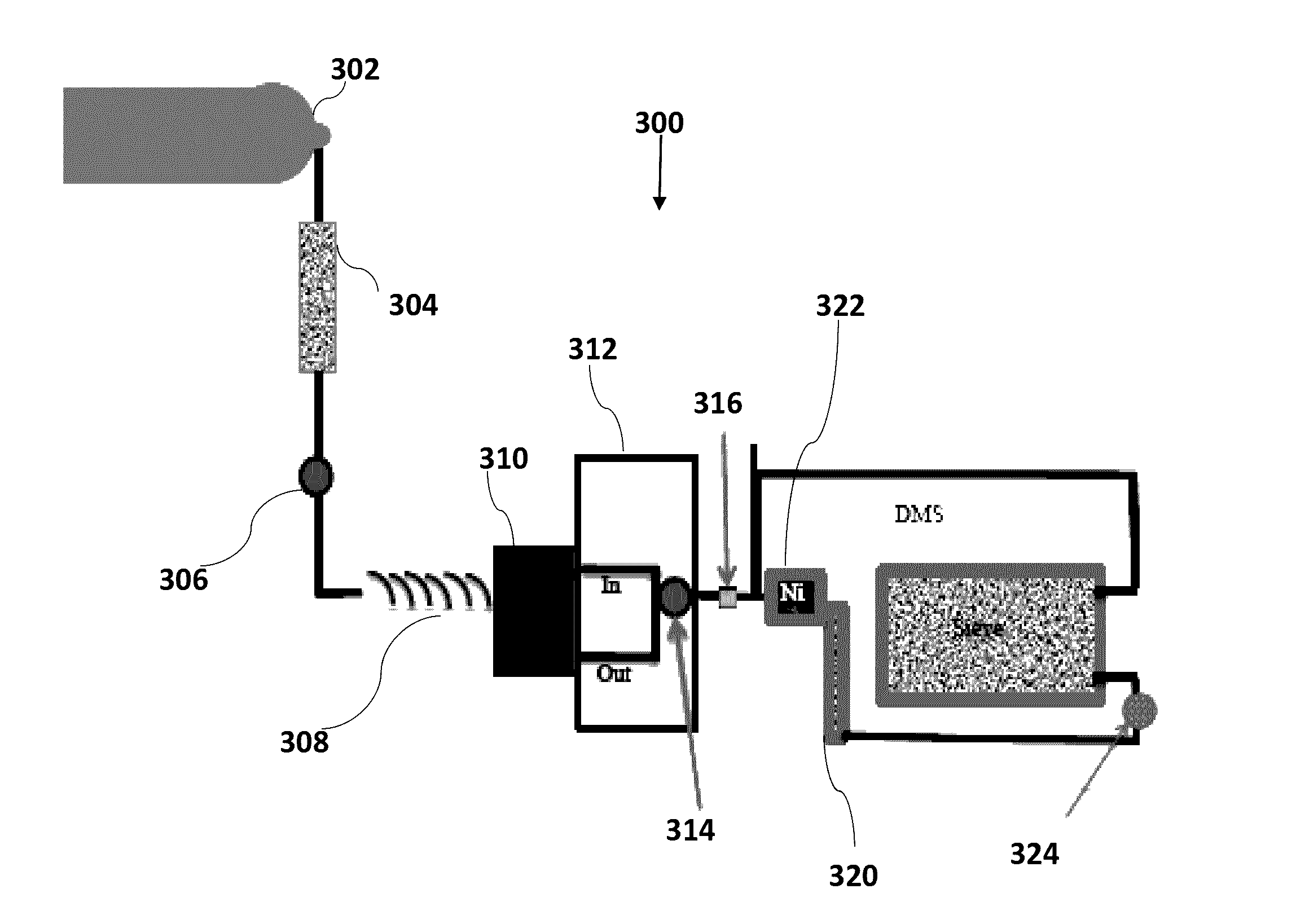
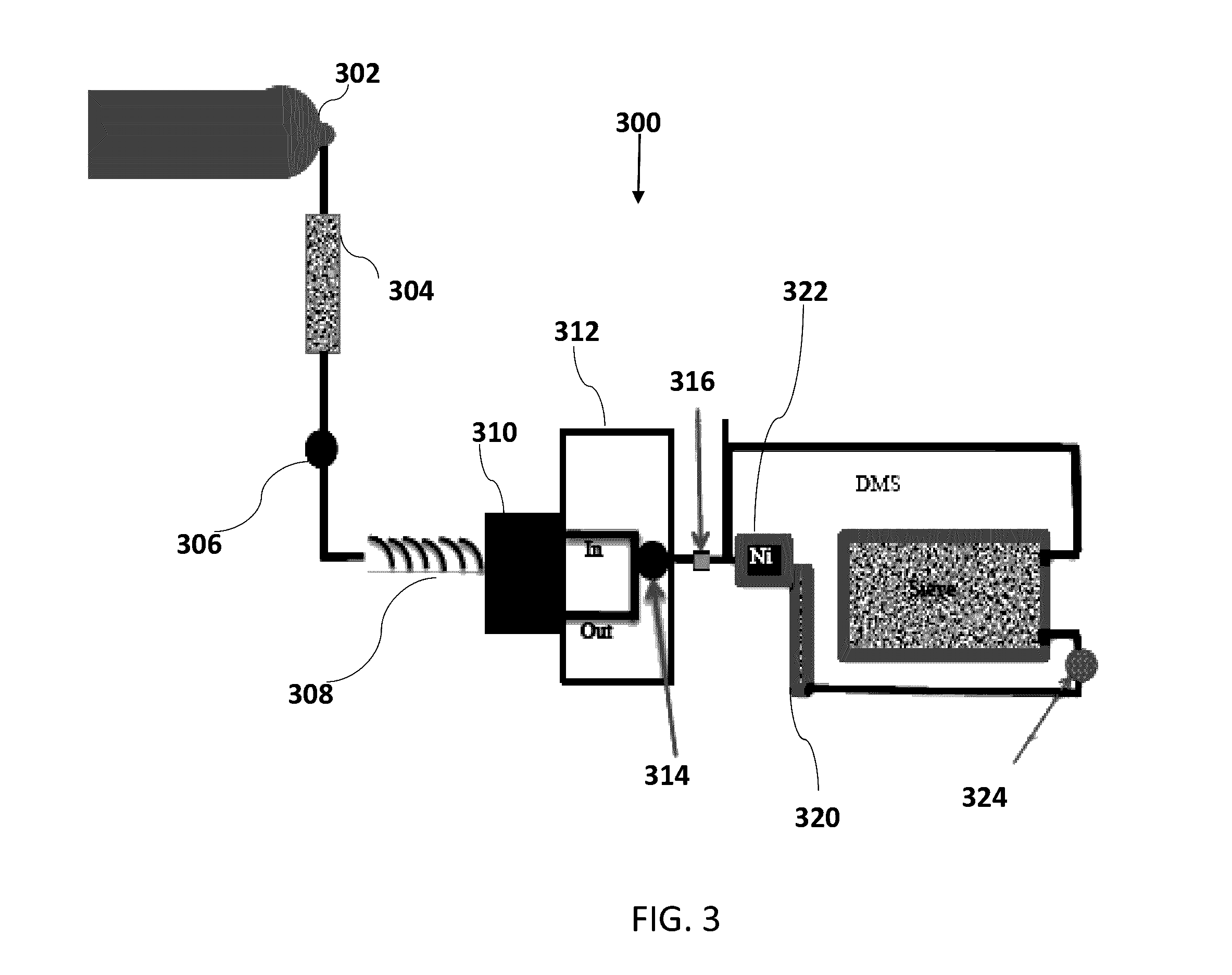
On-board ILS sensors for detecting illegal drugs and based on intracavity laser spectroscopy (ILS) are provided for detecting the presence of drugs and their metabolized by-product vapors in an enclosed space, such as a vehicle. The sensor comprises: (a) a laser comprising a gain medium having two opposed facets within a laser resonator and functioning as an intracavity spectroscopic device having a first end and a second end, the first end operatively associated with a partially reflecting (i.e., partially transmitting) surface; (b) a reflective or dispersive optical element (e.g., a mirror or a diffraction grating) operatively associated with the second end to define a broadband wavelength laser resonator between the optical element and the first end and to thereby define an external cavity region between at least one facet of the gain medium and either the first end or the second end or both ends; (c) the external cavity region being exposed to air in the enclosed space to enable any drugs or their metabolized by-product molecules to enter thereinto; (d) a detector spaced from the first end; (e) appropriate electronics for measuring and analyzing the detector signal; (f) a housing for containing at least the laser, the partially reflecting surface, and the optical element, the housing being configured to prevent escape of stray radiation into the enclosed space and to permit air from the enclosed space to continuously circulate through the external cavity region for analysis; and (g) means for driving the laser (e.g., electrical or optical). A method is provided for measuring concentration of drug vapors and their metabolized by-product vapors in the vehicle or other enclosed space employing the on-board sensor. The method comprises: (1) sensing any drugs and their metabolized by-product vapors in the enclosed space by the on-board sensor; and (2) providing a signal indicative of presence of any drugs or metabolized vapors.
Owner:INNOVATIVE LASERS
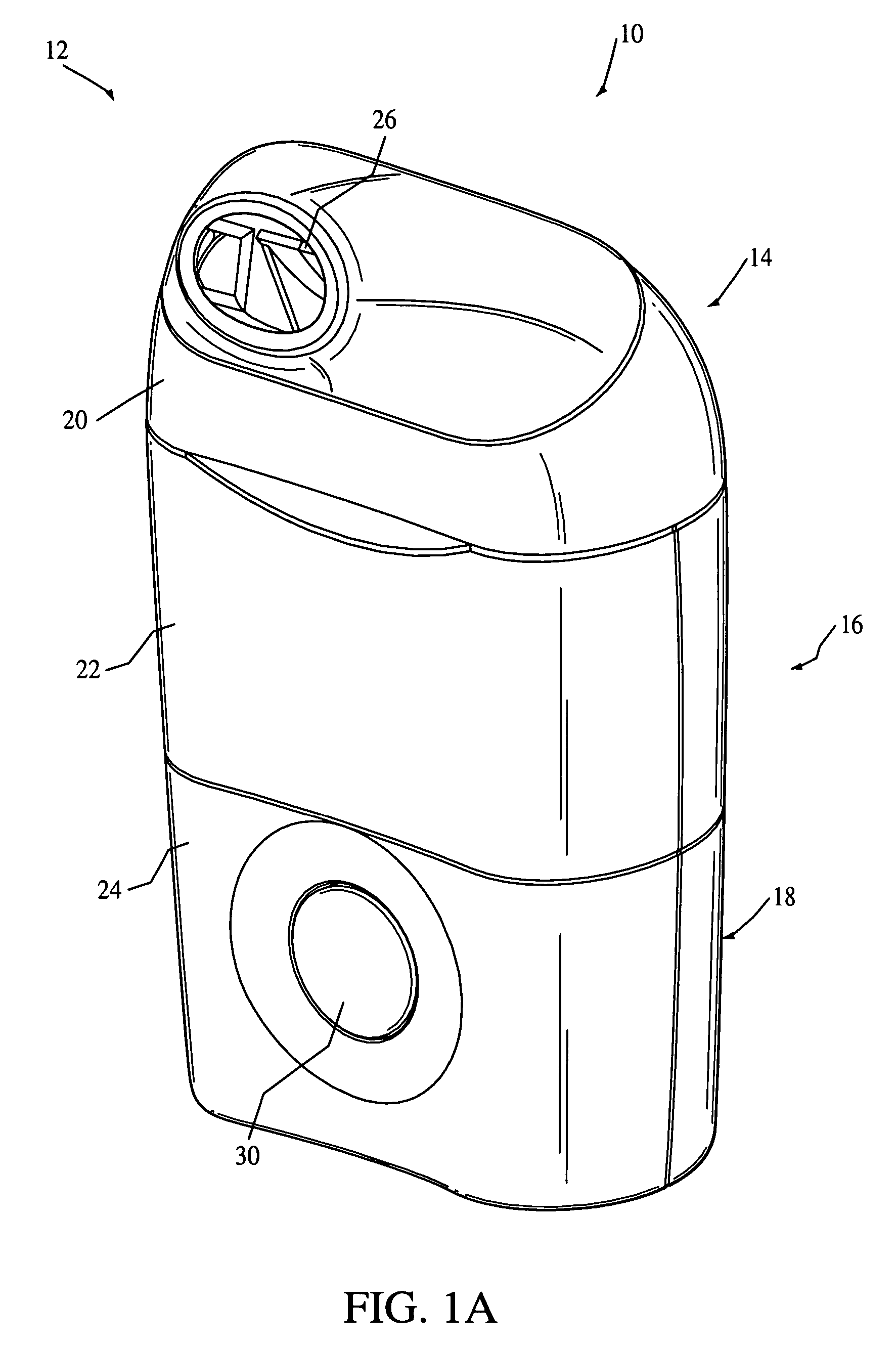
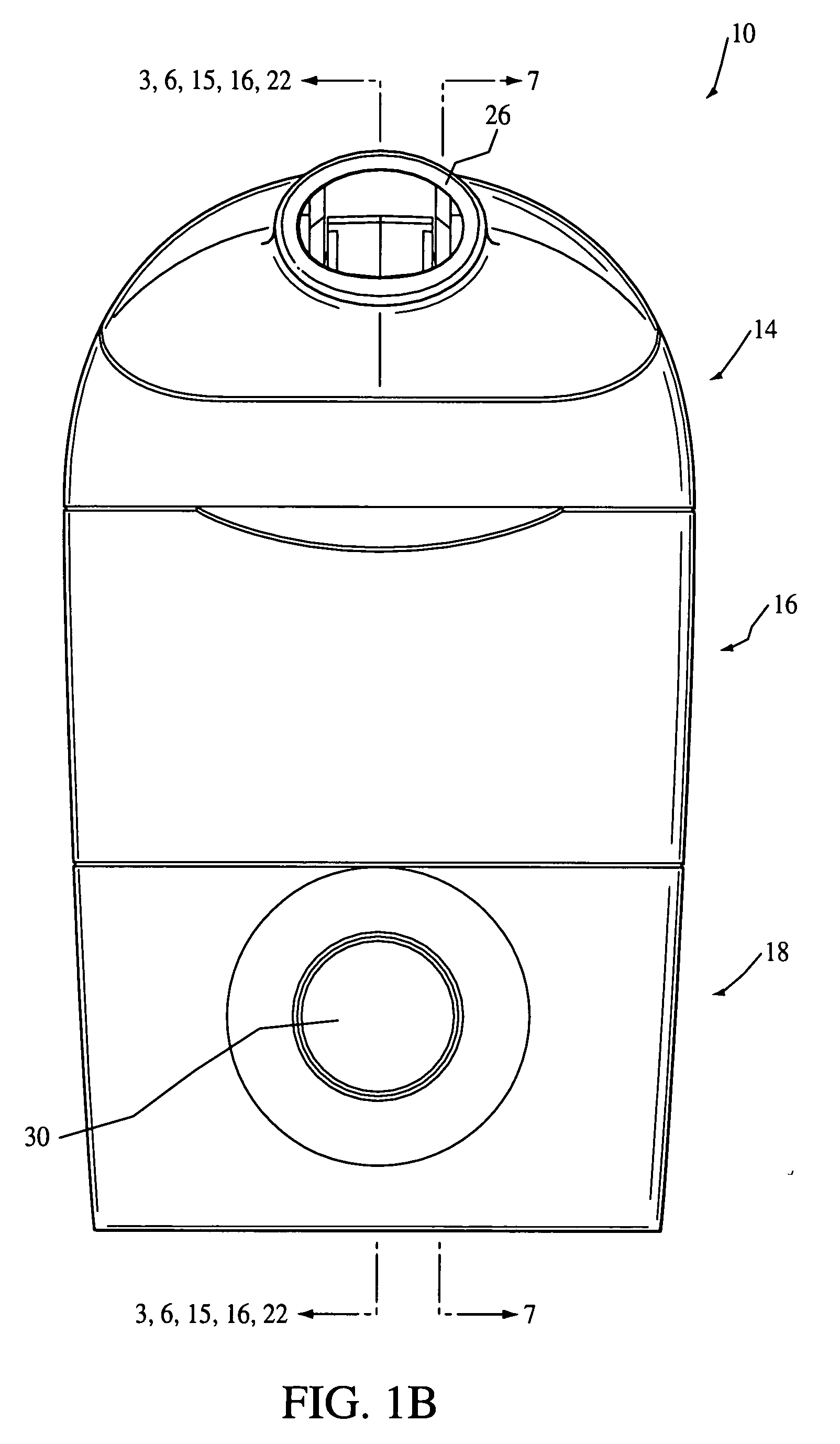
Nebulizing drug delivery device with barrier
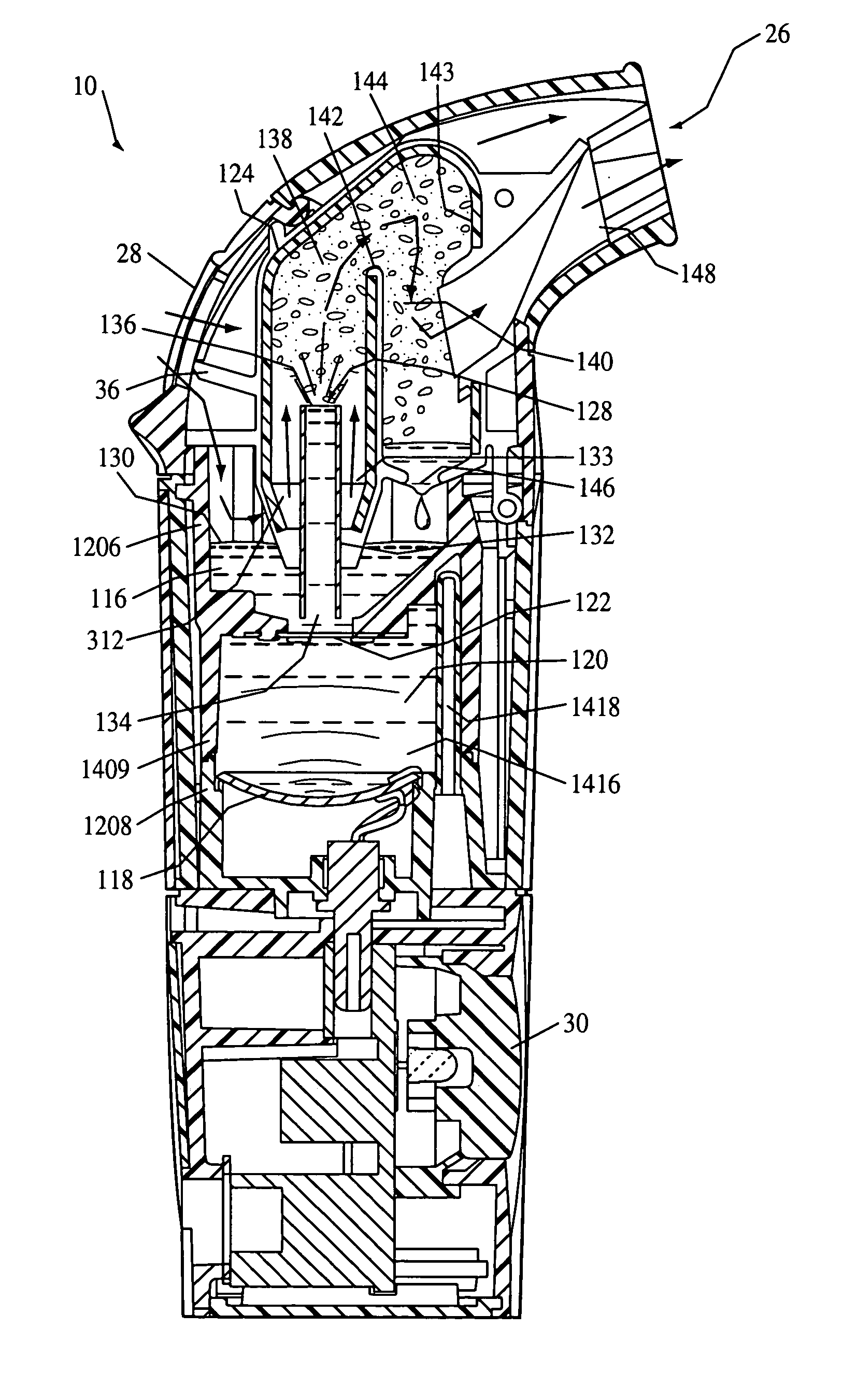
The present invention provides a drug delivery device that uses an aerosol generator to nebulize a drug solution. The drug delivery device includes differently sized guide tubes and separator walls to provide substantially consistent particles that can be varied in size by using different guide tubes. The drug delivery device also includes a barrier that separates a fluid contained in the device from the drug solution at least a portion of the barrier is formed from Polyetheretherketone. The present invention also has a drug detection device that can detect the volume of drug in the device or whether the barrier has been compromised.
Owner:RIC INVESTMENTS LLC
System and Method for Drug Detection in Exhaled Breath
ActiveUS20120302907A1Improve robustnessReliable resultsEngine manufactureBlade accessoriesEnvironmental healthDrug detection
Owner:SENSA BUSB
Immunochromatographic quantitative test reagent based on near-infrared fluorescent marker
ActiveCN102680692AHigh detection sensitivityHigh sensitivityMaterial analysisMicroorganismFood safety
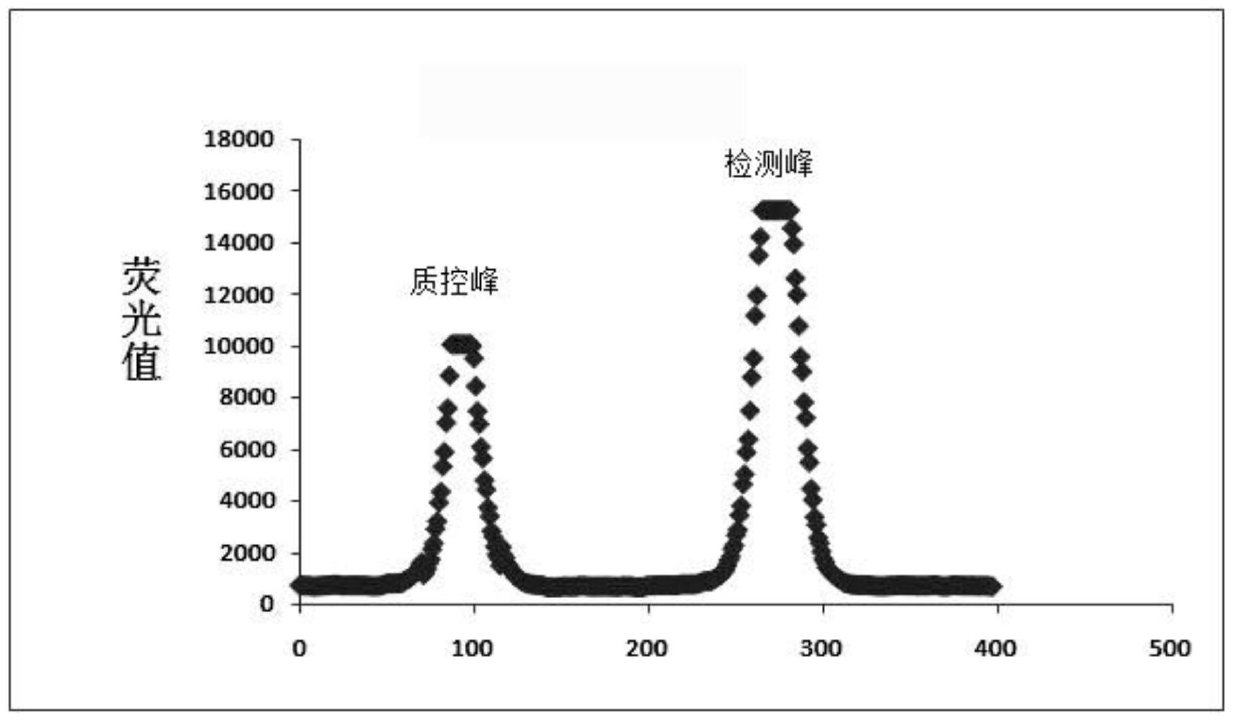
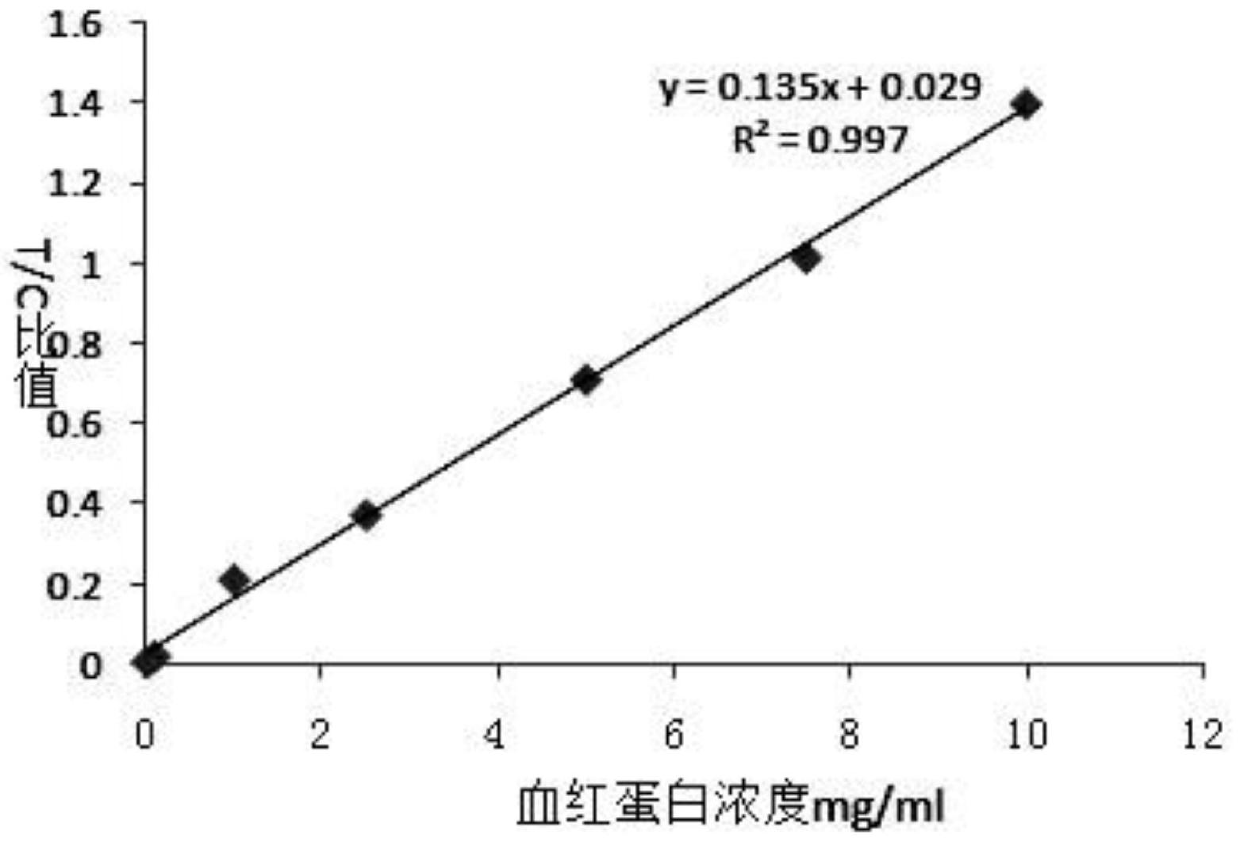
The invention relates to an immunochromatographic quantitative test reagent based on a near-infrared fluorescent marker. A near-infrared fluorescent molecule is adopted as a marker, and the immunochromatographic technology is adopted to prepare a near-infrared fluorescent immunochromatographic test strip. In the test process, a conventional near-infrared test instrument is used for respectively scanning a quality control line and a sample line through near-infrared rays, and after being corrected by utilizing the fluorescent strength of the quality control line, the fluorescent strength of a detection line is substituted in a standard curved line in a fluorescent analysis instrument, so that the concentration of an object to be tested in a test specimen can be analyzed. The reagent can be applied to the detection of microorganism, the food safety detection, the drug detection and the quick detection of dangerous chemicals. The immunochromatographic quantitative test reagent has characteristics of high sensitivity, accuracy in quantization and convenience in operation.
Owner:BEIJING RUNBO FUDE BIOLOGICAL TECH DEV
Portable Device and Method for On-Site Detection and Quantification of Drugs
The present invention discloses a portable, reliable, automated and simple device using Spectral Fluorescence Signature technology (SFS) for fast and accurate drug detection, quantification and data storage. The present also discloses a method for using Spectral Fluorescence Signature technology (SFS) for fast and accurate drug detection, quantification and data storage. Such device and method needing not highly skilled personnel or specific background to run the tests.
Owner:NARTEST
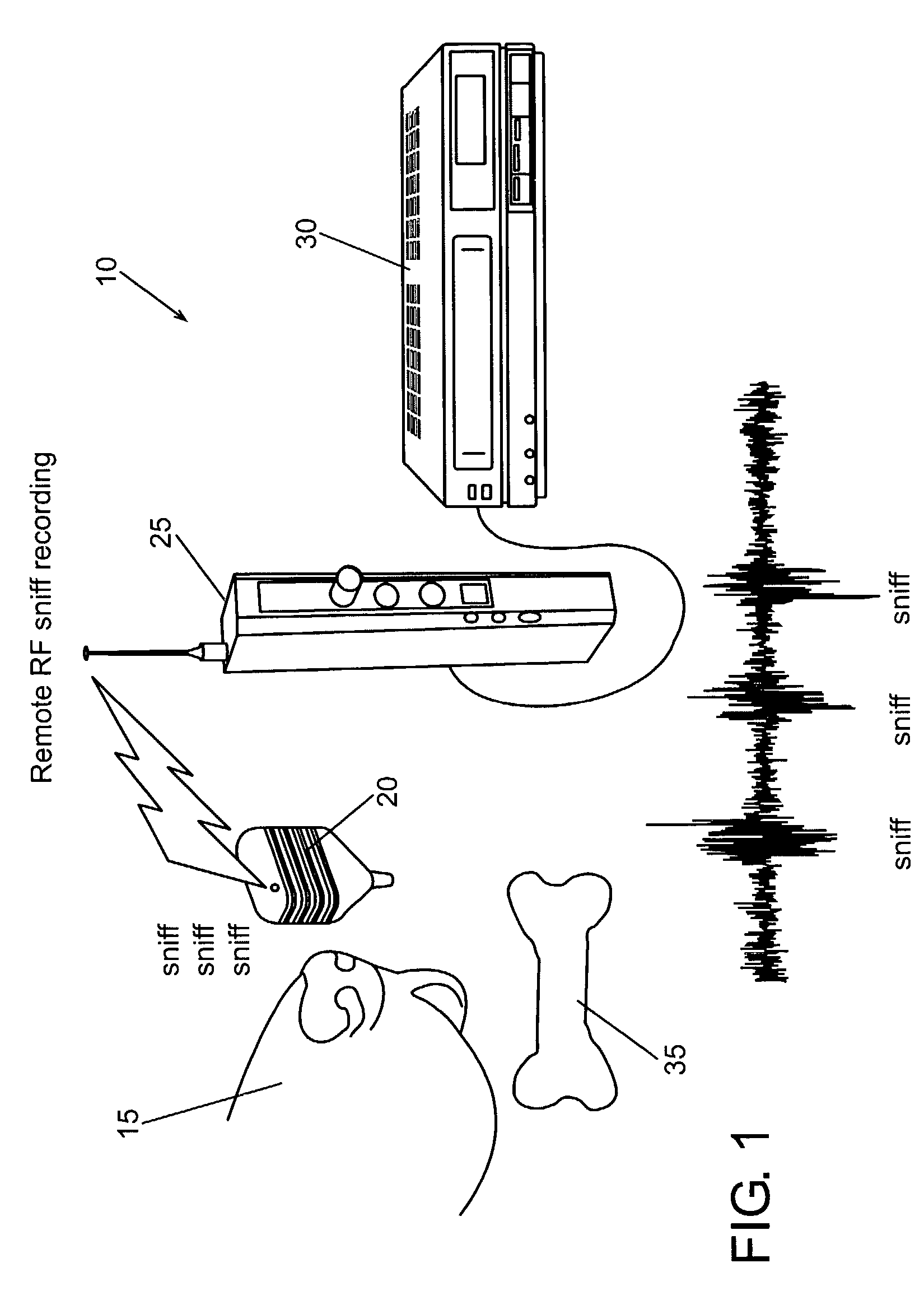
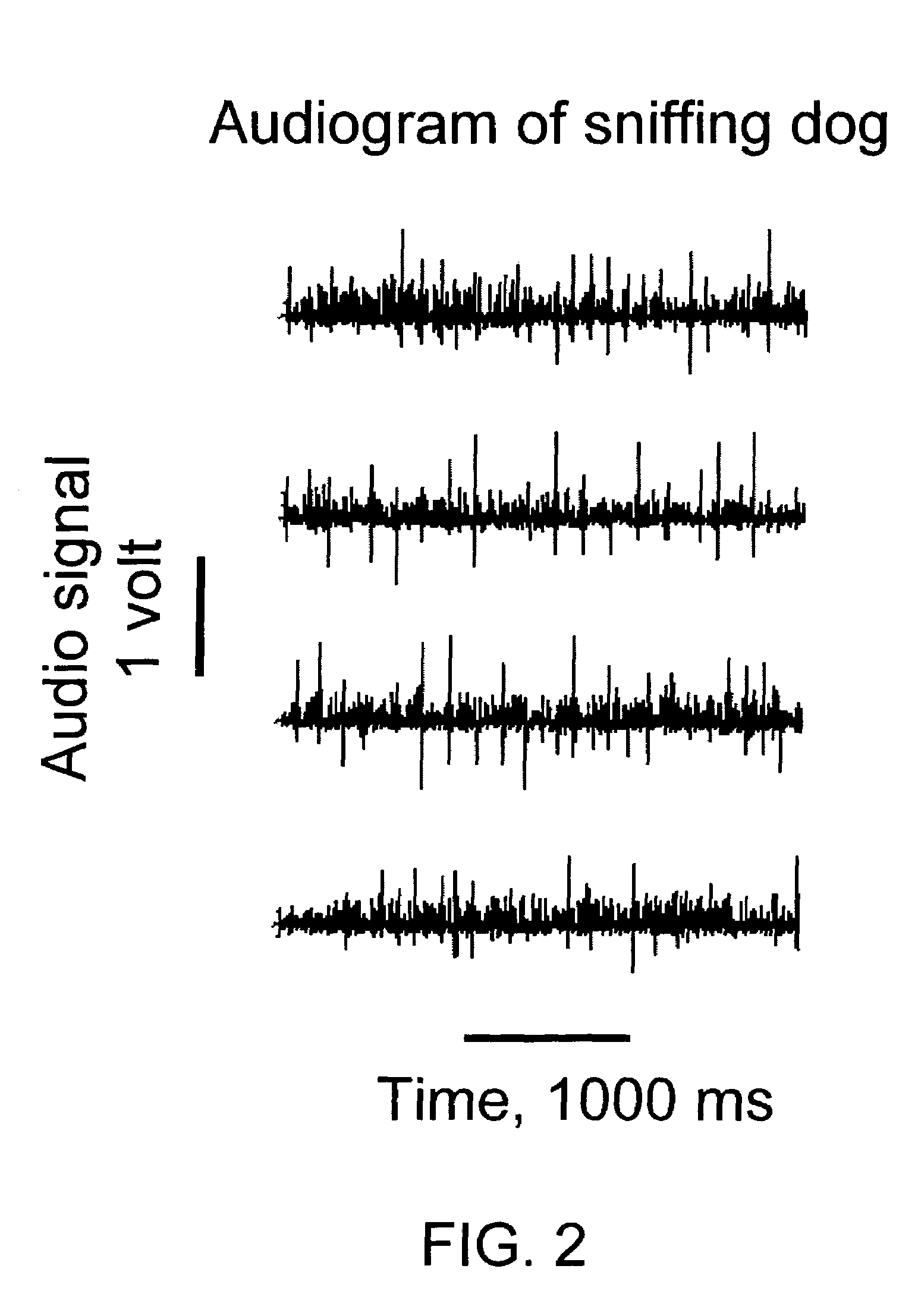
Apparatus and method for the measurement of the aerodynamics of olfaction in animals and man
A system and method for observing, collecting and analyzing olefactory characteristics of a human or animal subject, such as sniffing, breathing and respiratory patterns and sounds. Example applications include the evaluation and training of dogs for explosive and drug detection, clinical diagnostics, scientific research, and identification.
Owner:AUBURN UNIV
Nebulizing drug delivery device with barrier
The present invention provides a drug delivery device that uses an aerosol generator to nebulize a drug solution. The drug delivery device includes differently sized guide tubes and separator walls to provide substantially consistent particles that can be varied in size by using different guide tubes. The drug delivery device also includes a barrier that separates a fluid contained in the device from the drug solution at least a portion of the barrier is formed from Polyetheretherketone. The present invention also has a drug detection device that can detect the volume of drug in the device or whether the barrier has been compromised.
Owner:RIC INVESTMENTS LLC
Preparation and applications of clenbuterol monoclonal antibody
ActiveCN103012593AHigh recovery rateProcessing method saves timeOrganic compound preparationImmunoglobulinsAssayPharmaceutical drug
The present invention provides a clenbuterol monoclonal antibody and applications thereof. The present invention discloses a clenbuterol monoclonal antibody preparation method, wherein clenbuterol hapten is synthesized, the synthesized clenbuterol hapten and carrier protein are coupled to obtain clenbuterol antigen, and the clenbuterol antigen is adopted to immunize animals to obtain a high specificity monoclonal antibody. The present invention further provides a method for application of the clenbuterol monoclonal antibody in a clenbuterol enzyme-linked immunosorbent assay kit to detect clenbuterol. The present invention further provides a method for application of the clenbuterol monoclonal antibody in a clenbuterol colloidal gold test paper card to detect clenbuterol. The prepared clenbuterol monoclonal antibody has characteristics of high specificity and low cost, wherein the clenbuterol drug detection clenbuterol enzyme-linked immunosorbent assay kit prepared by using the clenbuterol monoclonal antibody and the clenbuterol drug detection clenbuterol colloidal gold test paper card prepared by using the clenbuterol monoclonal antibody have characteristics of convenient operation, high specificity, high sensitivity, high accuracy, high precision, fast detection and the like.
Owner:BEIJING KWINBON BIOTECH
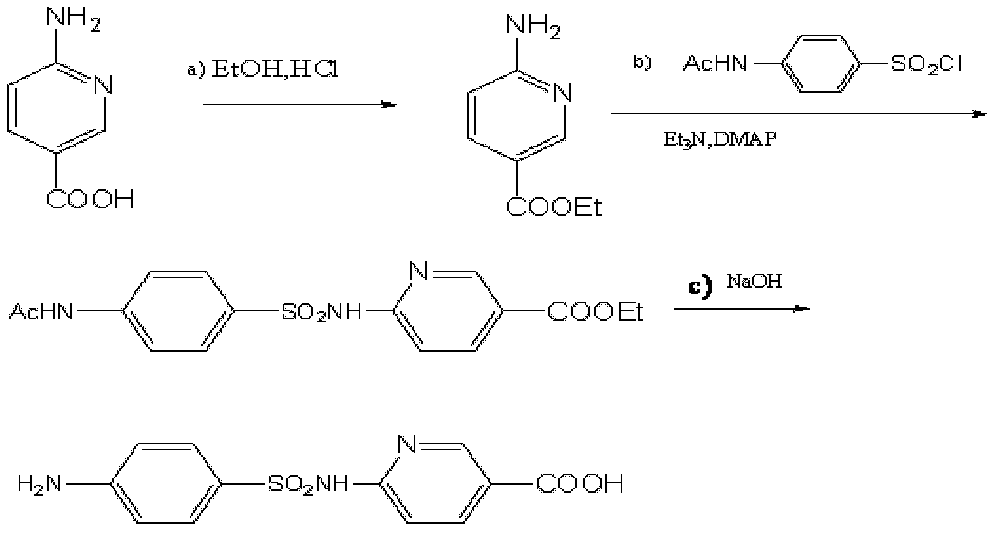
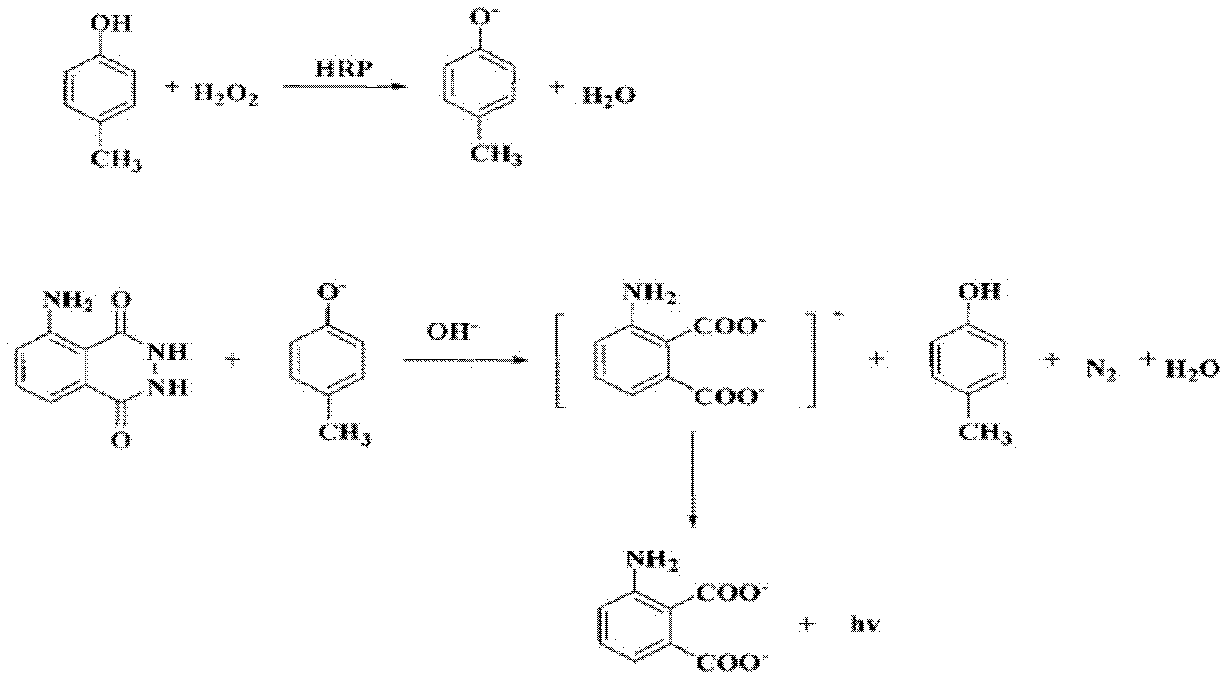
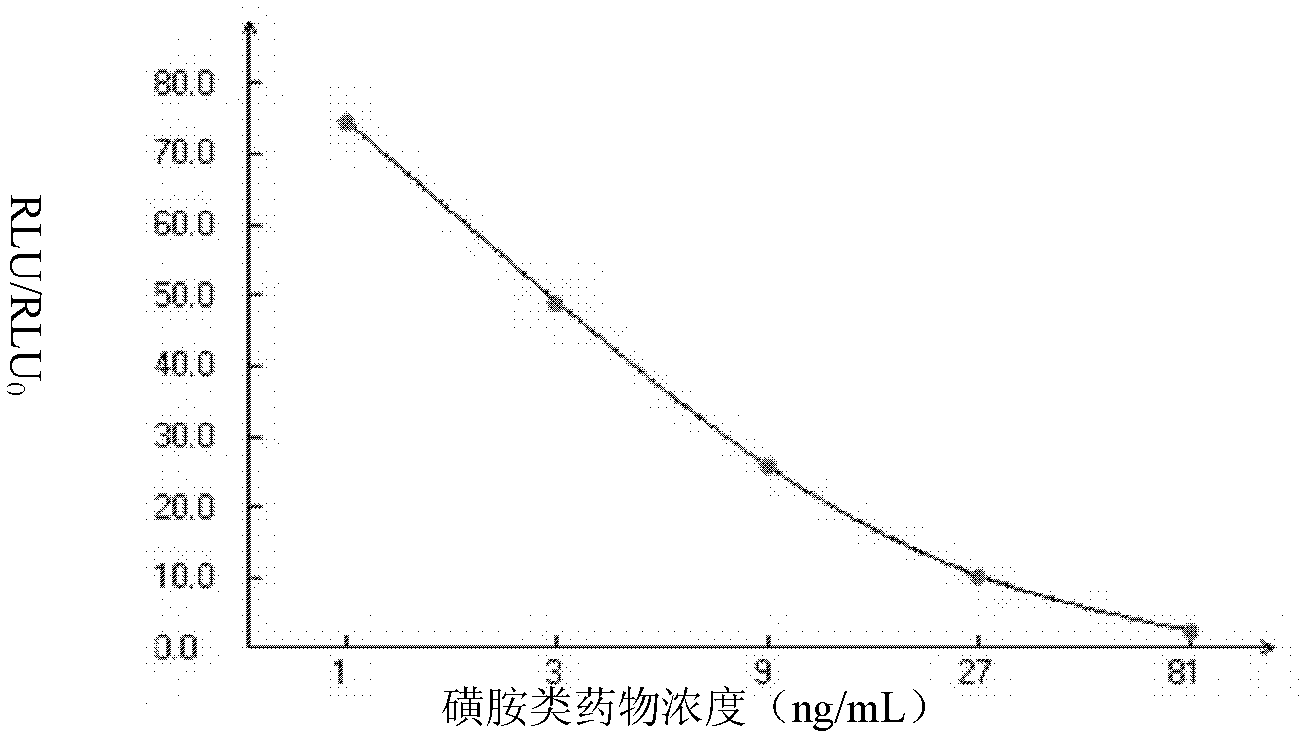
Sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit
The present invention discloses a sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit, which comprises a kit body, an enzyme label plate placed inside the kit body, and reagents placed inside the kit body, and is characterized in that every hole of the enzyme label plate is coated with coating antigen, the coating antigen is a sulfanilamide mother nucleus and carrier protein conjugate, and the reagents comprise sulfanilamide monoclonal antibody, horseradish peroxidase-labeled goat anti-mouse antibody, a series of sulfanilamide standard solutions, a concentrated phosphate buffer, a concentrated washing solution and a chemiluminescence solution. The sulfanilamide drug chemiluminescence enzyme-linked immunodetection kit has characteristics of high sensitivity, simple and rapid detection, high accuracy, and more drug detection types, provides a substantially reduced operation time compared to the conventional colorimetric ELISA method, and can be used for detection of residues of the 17 sulfanilamide drugs in animal tissues (pork, chicken, pork liver and chicken liver), aquatic products (fish and shrimp), eggs, milk and milk powder.
Owner:BEIJING KWINBON BIOTECH
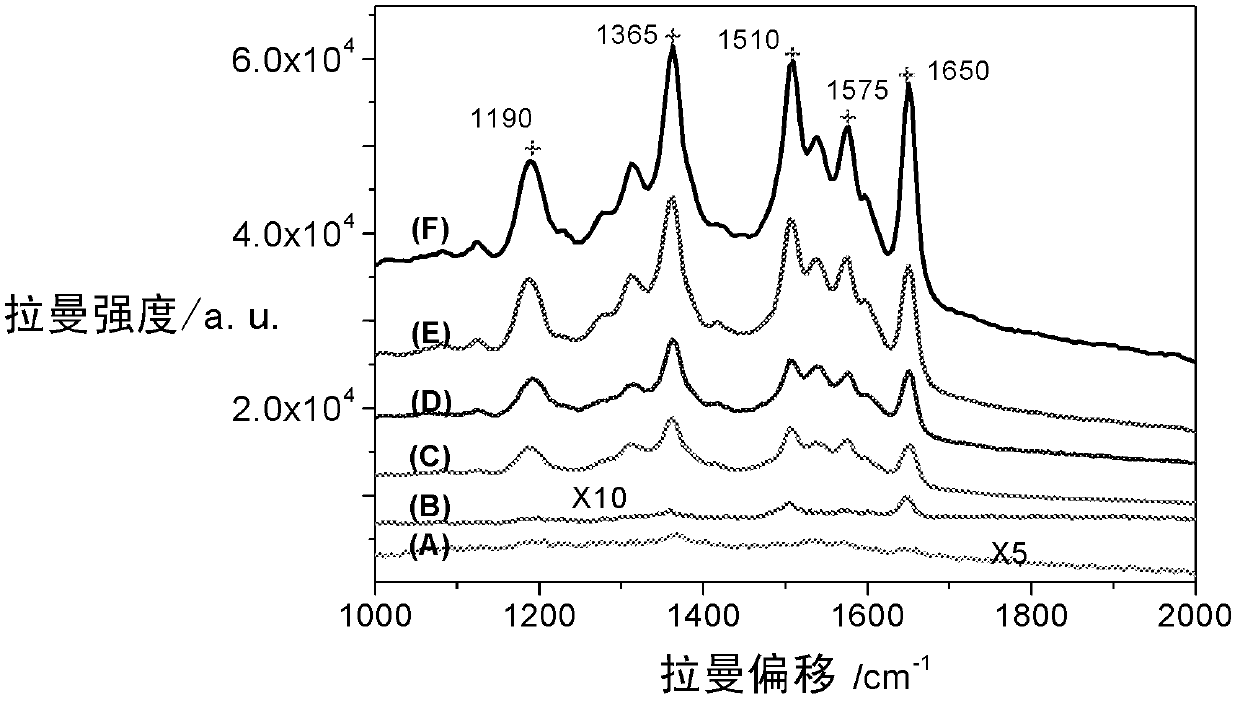
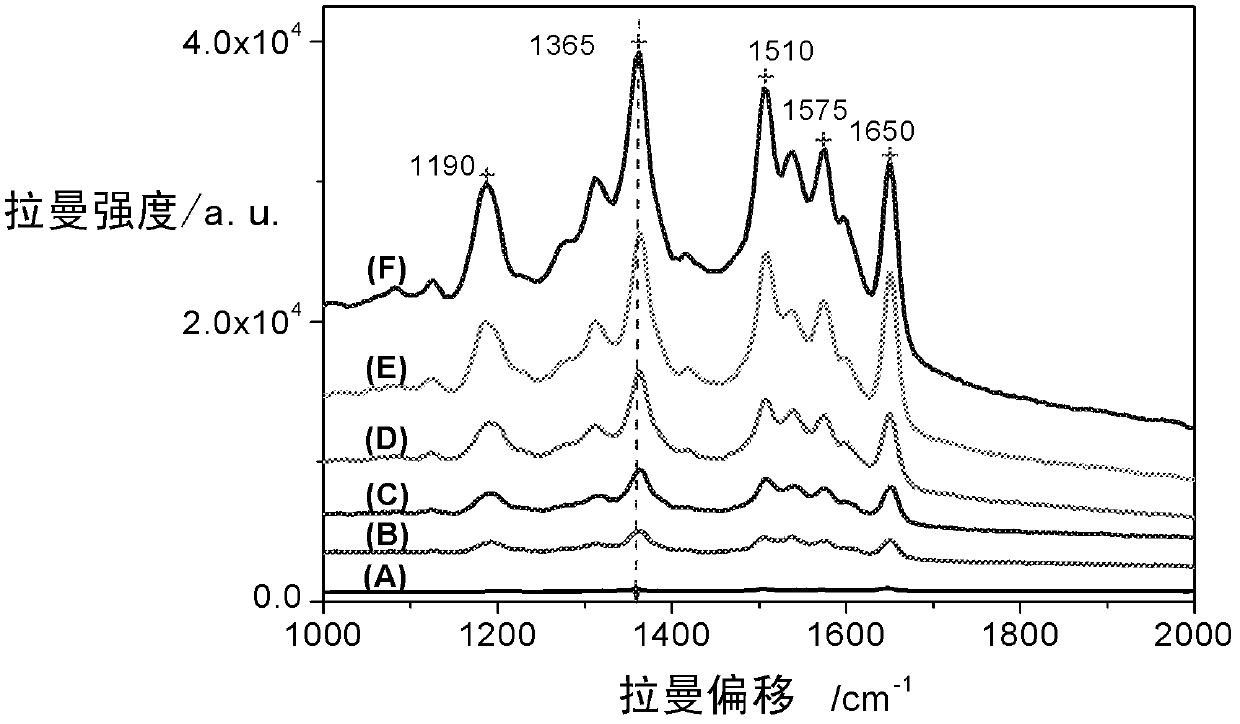
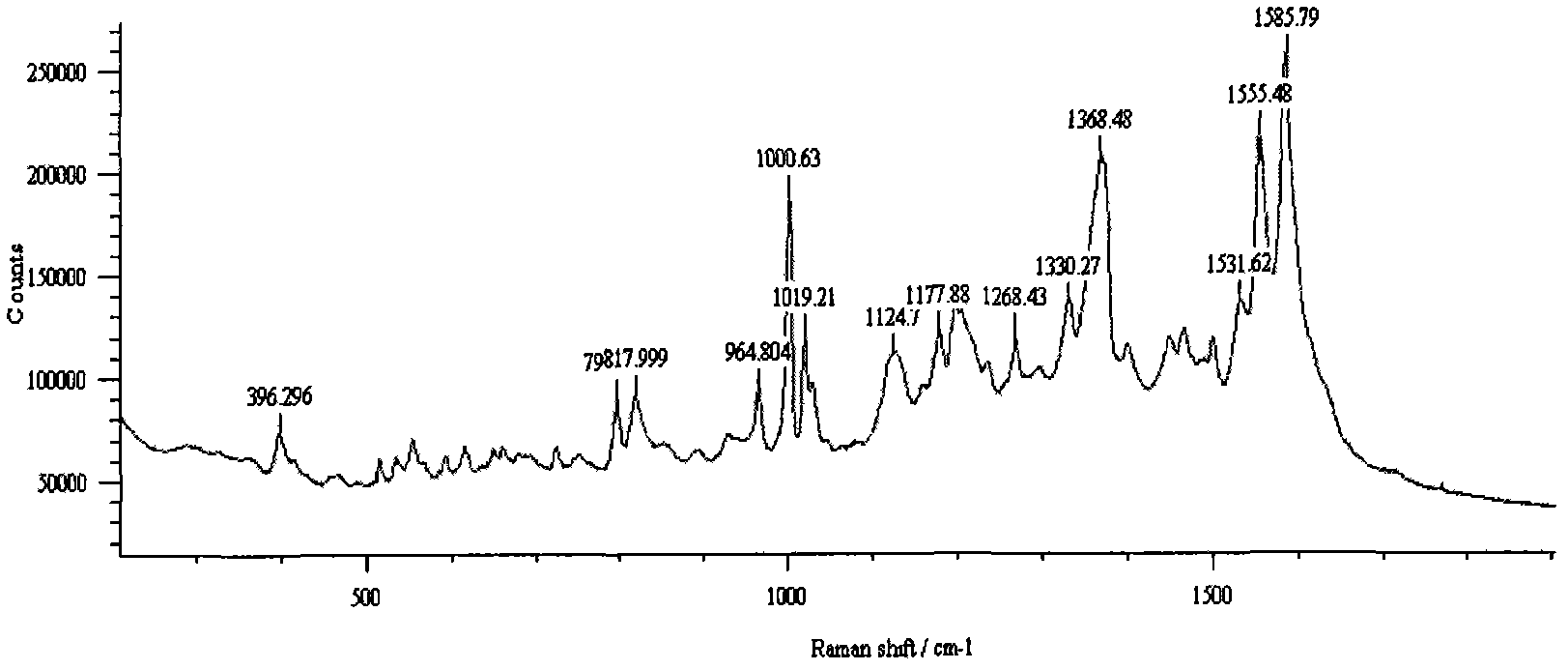
High-sensitivity SERS (surface enhanced Raman scattering) sensor active-substrate for drug detection and preparation method thereof
ActiveCN102886933AImprove stabilityLow costRaman scatteringMetal layered productsDrug detectionLaser raman
The invention belongs to the technical field of laser-Raman spectrum and trace drug detection, and in particular relates to a high-sensitivity SERS (surface enhanced Raman scattering) sensor active-substrate for drug detection and a preparation method thereof. The invention provides a SERS active-substrate, the SERS active-substrate is a precious metal nano cone array structure coated on a chip, and the precious metal material is silver and / or gold. The invention also provides a method for preparing the SERS active-substrate by using an argon ion impact method. The high-sensitivity SERS sensor active-substrate disclosed by the invention has surface enhanced Raman activity and is high in repetition rate, and can be applied to the detection of trace compounds such as drugs and explosives and the like.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI +1
Methods and apparatus for rapid scanning continuous wave terahertz spectroscopy and imaging
Methods and apparatus are provided employing rapid scanning continuous wave terahertz spectroscopy and imaging for the non-destructive evaluation of materials such as animal hides and natural cork, and explosive detection, concealed weapon detection, and drug detection. A system employing an aperiodic detector array and implementing phase modulation at 100 kHz significantly reduces the imaging time and enables interferometric images of a THz point source to be obtained at several frequencies between 0.3 and 0.95 THz.
Owner:NEW JERSEY INSTITUTE OF TECHNOLOGY
Method for preparing polymer-coated nano-cluster core-shell microsphere
InactiveCN101559922AIncrease concentrationIncrease with increasing concentrationNanostructure manufacturePolymer scienceIn situ polymerization
The invention relates to a method for preparing a core-shell microsphere taking nano-cluster as a core and polymer as a shell by an in-situ polymerization method. The method comprises three steps of preparing nano-particles, preparing the nano-cluster and preparing the core-shell microsphere, wherein the organic phase nano-particles can be prepared by a two-phase method, a phase transfer method or a high-temperature thermal decomposition method; the nano-cluster is prepared by a method of taking oil drops as a template; the polymer shell is prepared by adopting the in-situ polymerization method; the size of the microsphere can be regulated by changing the amount of surfactant; and the thickness of the polymer shell can be achieved by adjusting the concentration of polymeric monomer. The method is a novel method combining the nano-luster with the polymer. The polymer-coated nano-cluster core-shell microsphere prepared by the method not only improves the stability and biocompatibility of the nano-cluster, but also introduces new chemical functions so as to integrate the functions of the nano-cluster and the polymer. Therefore, the method provides the novel multifunctional material with good stability for research on biomarkers, drug detection and sensors.
Owner:JILIN UNIV
Drug detection kit based on bicompective immunochromatographic method and preparation technology thereof
InactiveCN107478829AHigh sensitivityLow costMaterial analysisAntibody antigen reactionsAntigen-antibody reactions
The invention provides a detection kit applied to colloidal gold competition law and a preparation technology thereof. According to the preparation technology, an antigen-antibody reaction with high degree of specificity is combined with an immune colloidal gold chromatography technique, the preparation technology is improved on the basis of the prior art, fluorescent microspheres are introduced to compete together with colloidal gold marked antibodies for antigens of materials to be detected in a sample, and the fluorescent materials are not visible to naked eyes, so that the sensitivity of the competition law colloidal god detection kit is improved.
Owner:BOZHOU CITY THE NEW HEALTH TECH CO LTD
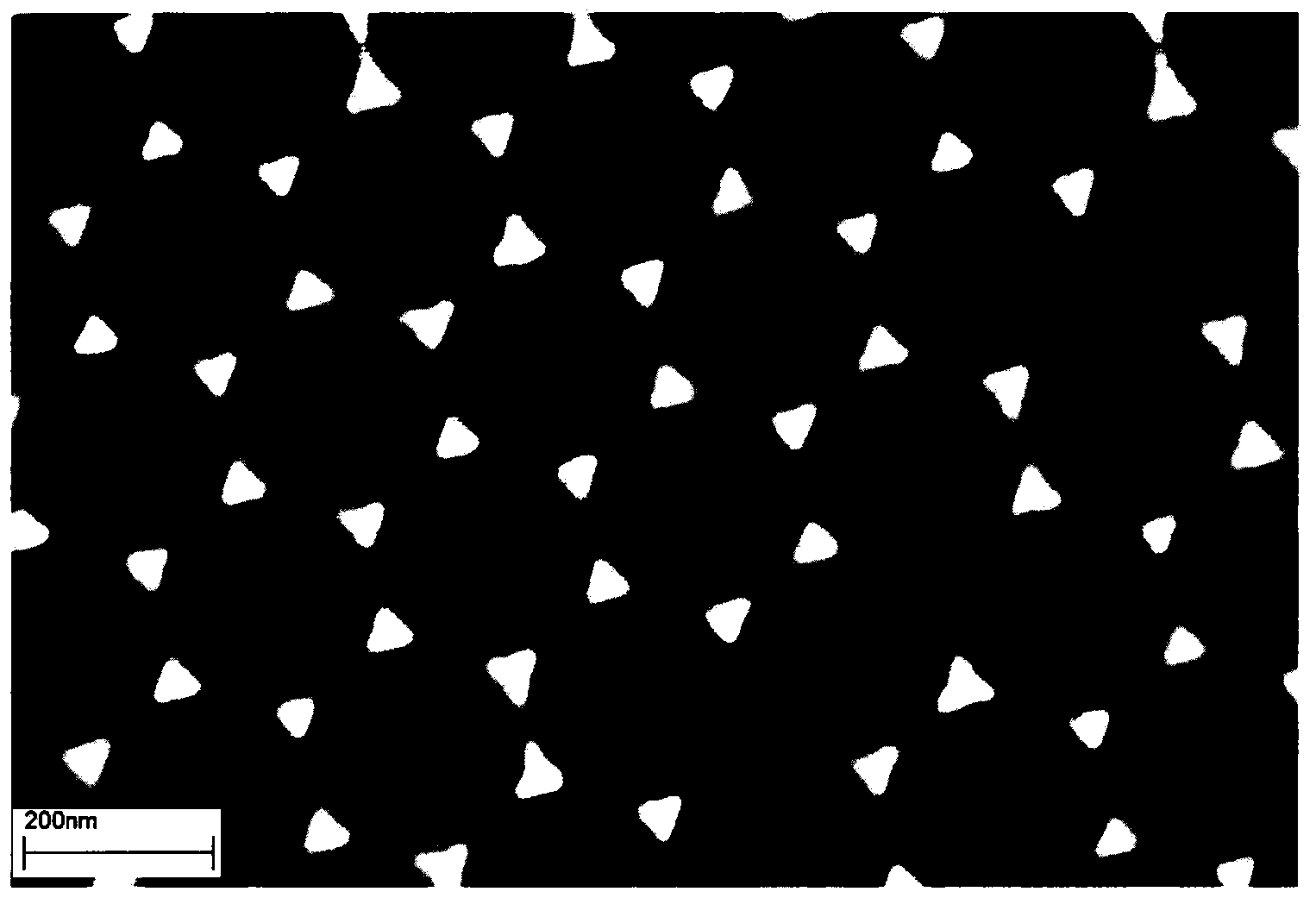
Rapid preparation method of regular triangular pyramid SERS active substrate
Belonging to the technical field of laser Raman spectroscopy and trace drug detection, the invention in particular relates to a high sensitivity SERS (furface enhanced Raman scattering) sensor active substrate for drug detection and a preparation method thereof. The regular triangular pyramid SERS active substrate provided in the invention is a noble metal nano-pyramid array structure coated on a base chip. The noble metal material can be silver, copper and / or gold. The high sensitivity SERS sensor active substrate provided in the invention has surface enhanced Raman activity and high repetition rate, and can be used for detection of trace compounds such as drugs, explosives and the like.
Owner:苏州扬清芯片科技有限公司
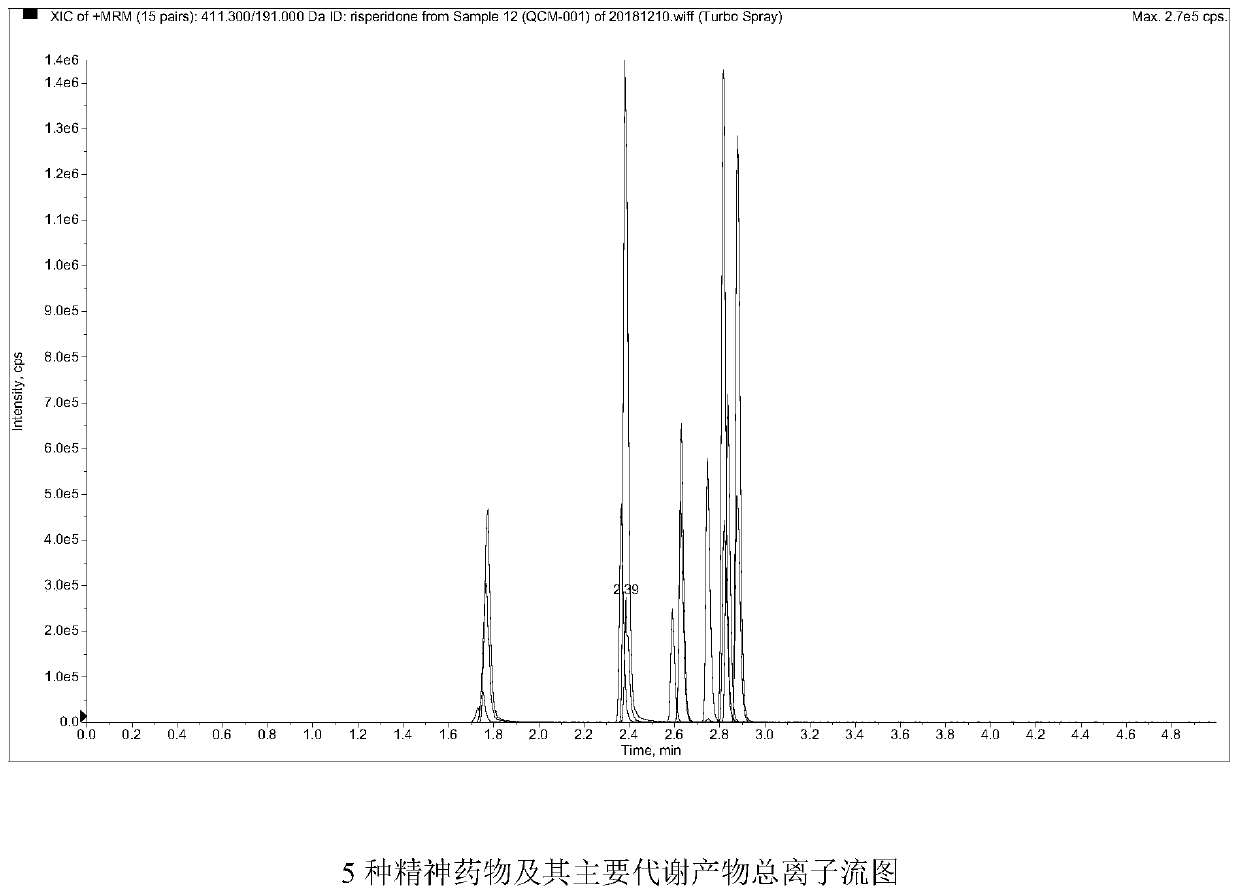
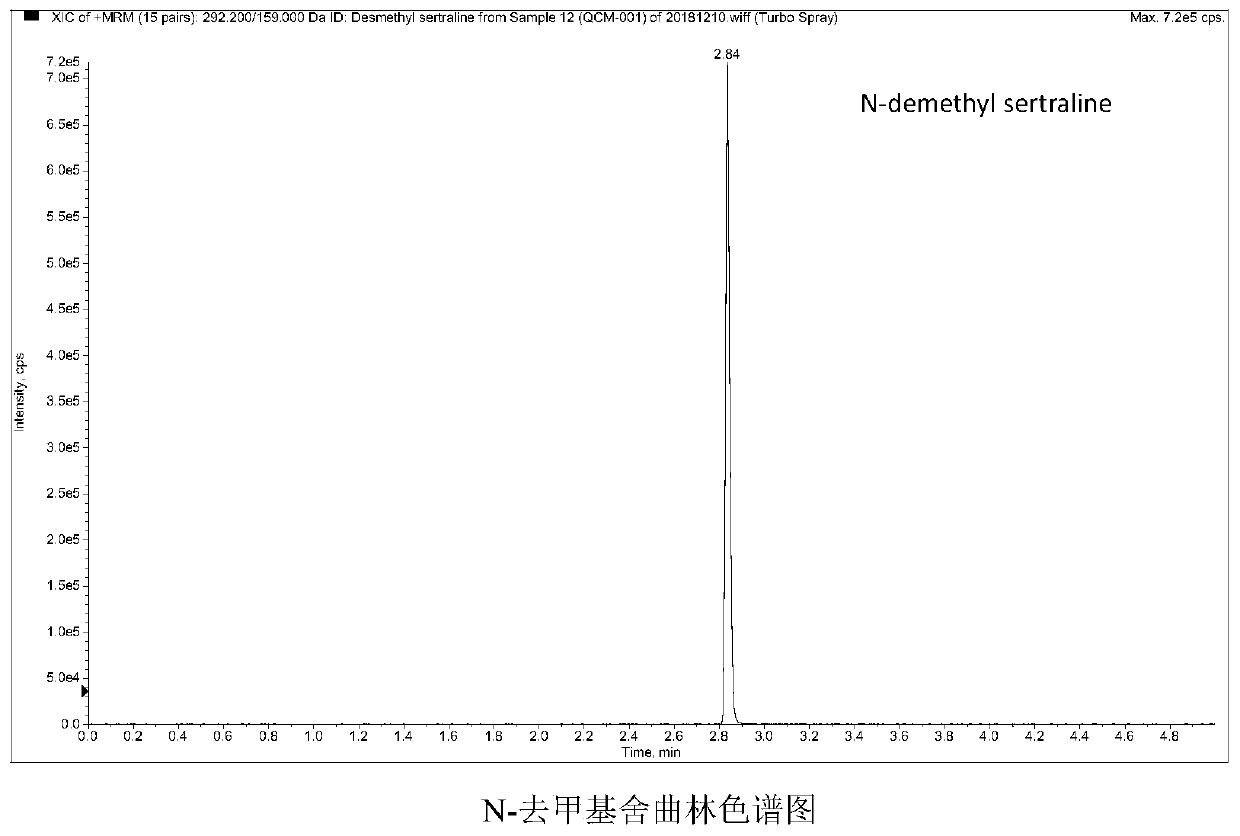
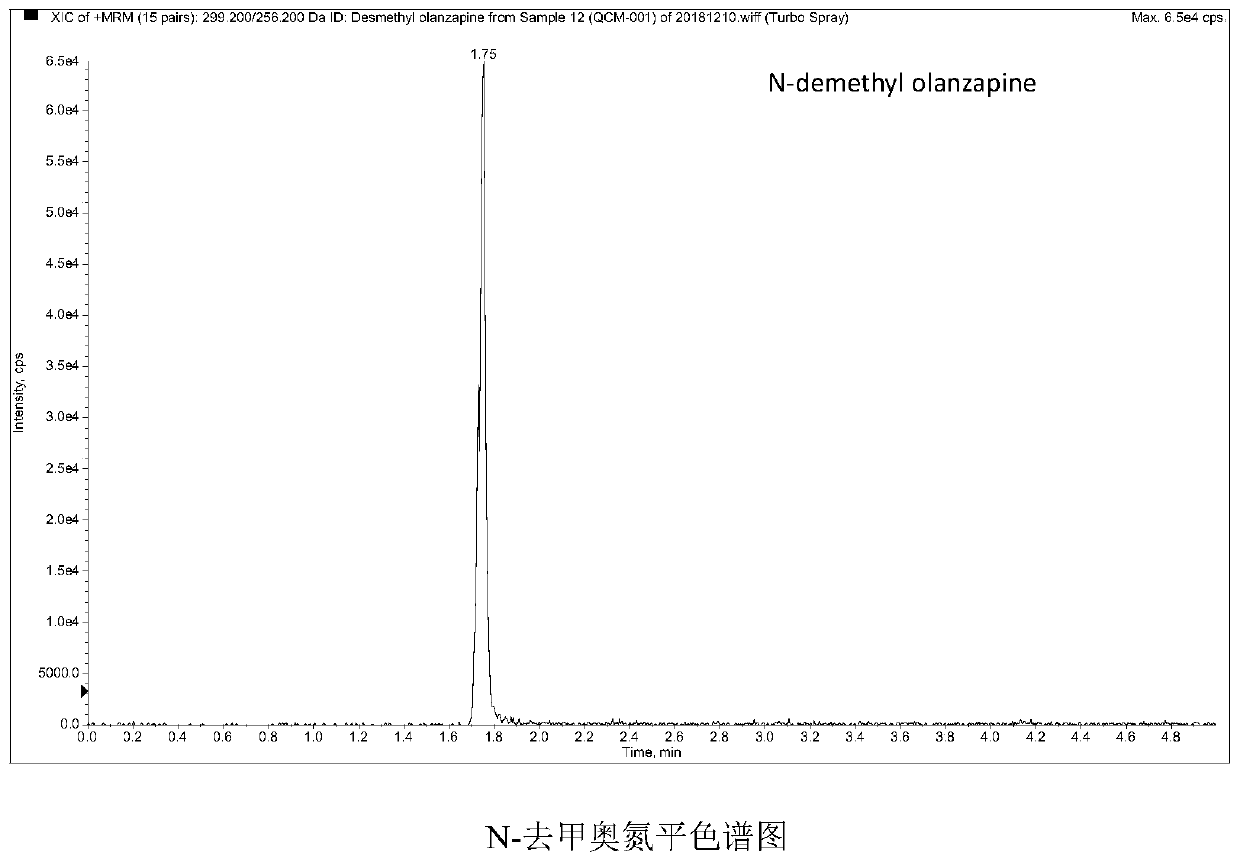
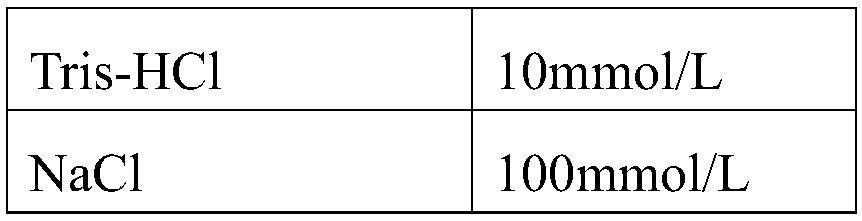
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
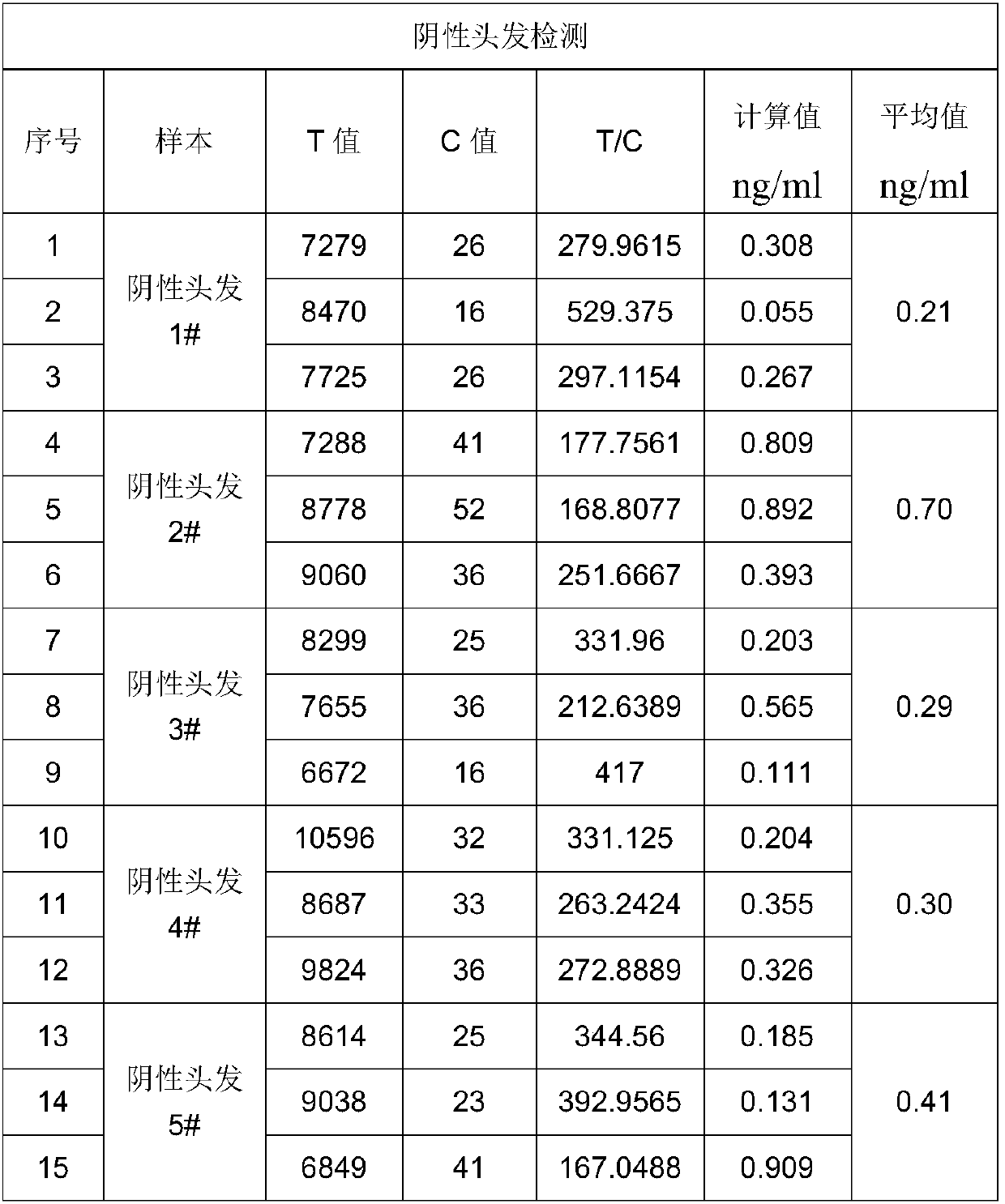
Rapid quantitative detection method for drugs in hair
InactiveCN109613230ARelease fullyAccurate and rapid detection and analysisBiological testingReagent stripAnalyte
The invention relates to a rapid quantitative detection method for drugs in hair. The rapid quantitative detection method comprises the following steps that (1) hair extraction is conducted, specifically, the drugs in the hair are fully released through mechanical grinding and bioenzyme extraction; (2) a sample is prepared from a to-be-detected object treated in the step (1), and the sample is dropwise added onto a reagent strip of a hair drug fluorescence immunochromatographic kit; and (3) the hair drug fluorescence immunochromatographic kit is detected through a dry immunofluorescence analyzer, and the content of an analyzed object in the to-be-detected sample is calculated. According to the rapid quantitative detection method, the advantages that sampling for hair detecting is easy, preservation is easy, the detecting time limit is long (from several weeks to several months), and information is comprehensive are reserved; the defects that conventional hair detection is complex in pretreatment, cumbersome operation, long in result analysis time and the like are overcome; the detecting time of the drugs in the hair is shortened from several hours and several days to fifteen to twenty minutes, the operation steps and technical requirements are greatly simplified, and thus non-professional technical personnel can also operate and use the rapid quantitative detection method.
Owner:SHANGHAI VENTURE BIOTECH CO LTD
Salbutamol drug detection colloidal gold test paper card and detection method
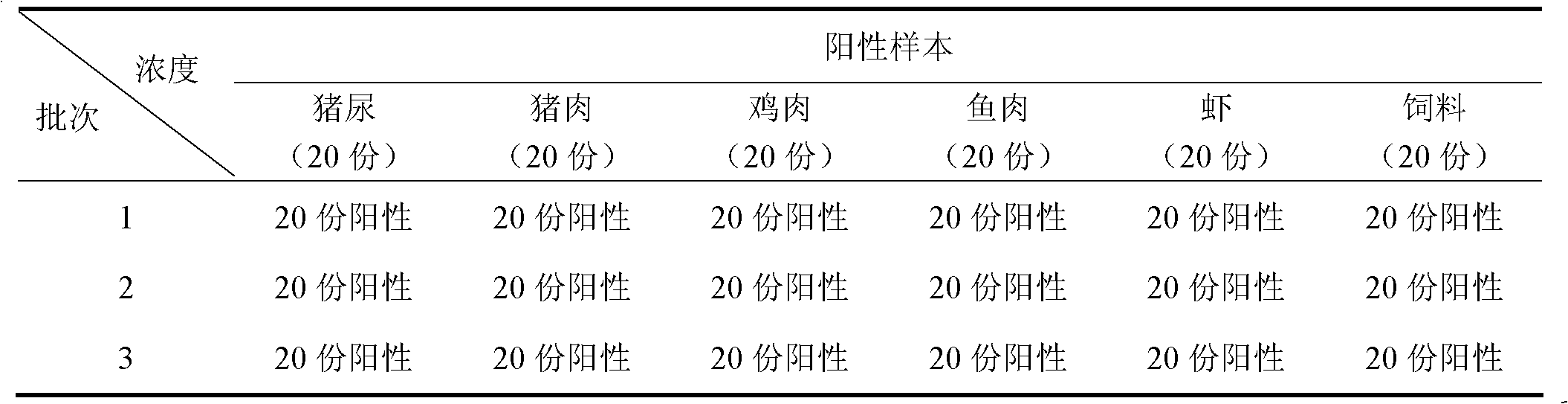
The present invention provides a salbutamol drug detection colloidal gold test paper card, which comprises a sample absorption pad, a conjugate releasing pad, a reaction membrane, a water absorption pad and a backing, wherein the reaction membrane is provided with a detection line formed by coating a salbutamol-carrier protein conjugate and a quality control line formed by coating goat anti-mouse anti-antibody, and the conjugate releasing pad is coated with a salbutamol monoclonal antibody-colloidal gold marker. The present invention further provides a method for adopting the salbutamol colloidal gold test paper card to detect salbutamol drugs. The salbutamol drug detection colloidal gold test paper card can be used for detection of salbutamol drug residues in urine, pork, chicken, fish, shrimp and feed samples, has characteristics of simple operation, high sensitivity, rapid detection and low cost, and is suitable for screening and on-site monitoring of a large number of samples.
Owner:BEIJING KWINBON BIOTECH
Drug detection kit
InactiveCN107167593ASimple preparation processLow costBiological testingAntibody antigen reactionsControl line
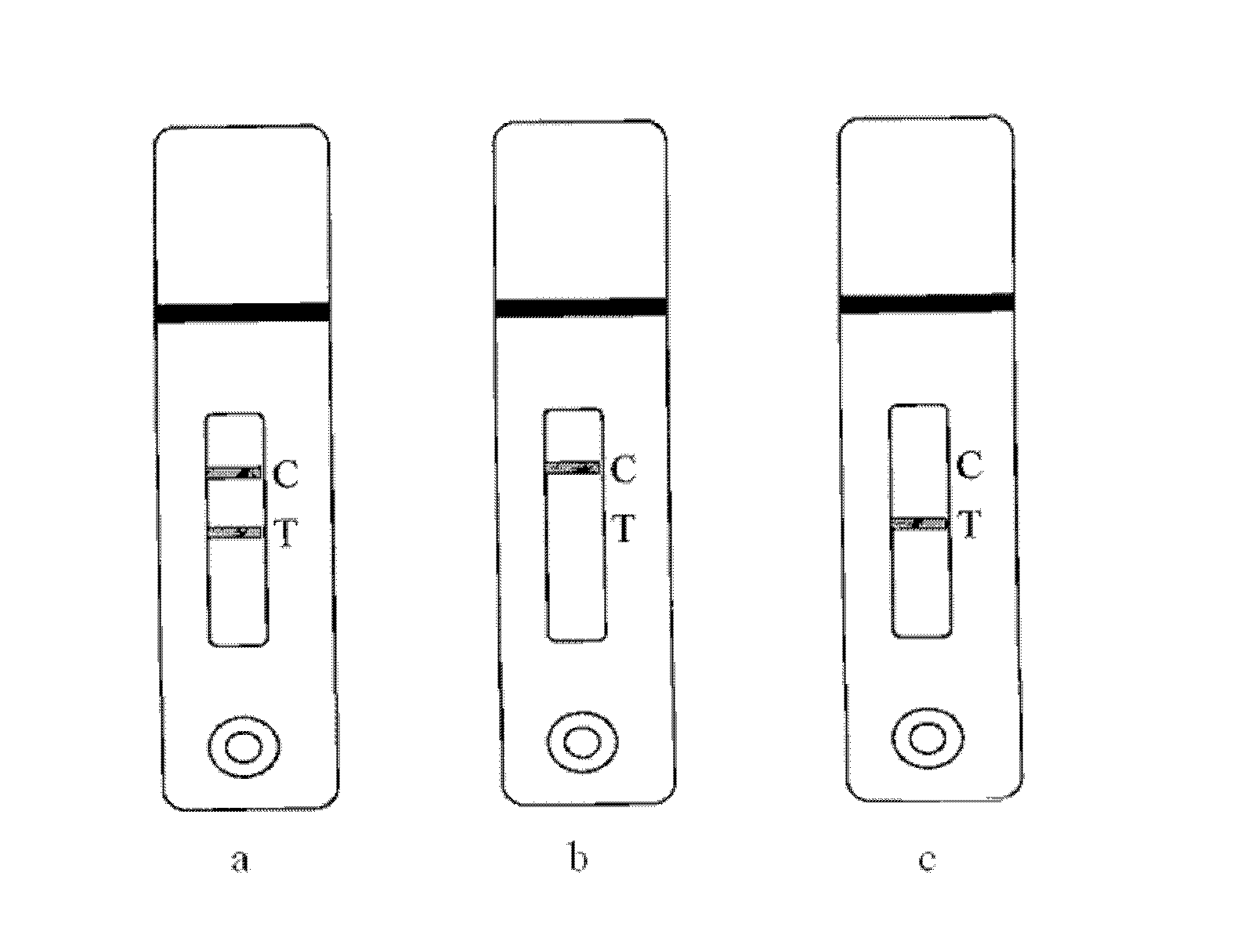
The invention provides a drug detection kit and its preparation process, which combines highly specific antibody antigen reaction with immune colloidal gold chromatography technology, improves on the basis of this prior art, and detects antibody in colloidal gold labeling At the same time, mark the control line antibody, and correspondingly point the second antibody on the membrane at the quality control area (C line) for color reaction with the colloidal gold-labeled control line antibody, so as to ensure that the C line will always develop color, and the color will develop The degree will not change, so it can be judged whether one or more drugs are contained by observing whether the detection area (T line) is colored, and one or more drugs can be semi-quantitatively compared by observing the degree of color development of the T line . The drug detection kit has the characteristics of qualitative and semi-quantitative comparison of drugs, and also has the characteristics of rapidity, sensitivity, easy operation, low cost, and no need for professional detection.
Owner:BOZHOU CITY THE NEW HEALTH TECH CO LTD
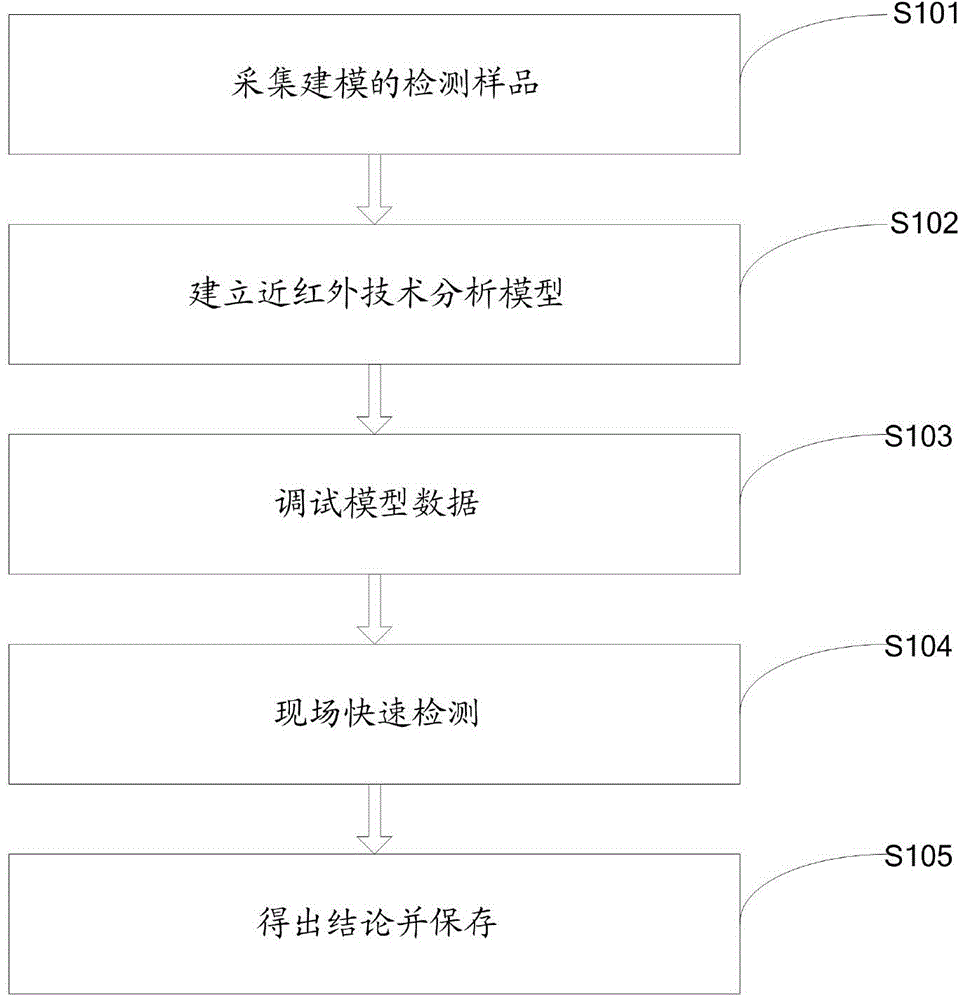
Rapid food and drug detection method based on near infrared technology
InactiveCN104390933AConsumables cost NoneMaintenance cost noMaterial analysis by optical meansMaterial resourcesQuality safety
The invention discloses a rapid food and drug detection method based on a near infrared technology. The method comprises the following steps: collecting a modeling detection sample, establishing a near infrared technology analysis model, debugging the model data, performing rapid field detection, drawing a conclusion and preserving the conclusion. According to the method disclosed by the invention, the rapid field detection is performed by utilizing a near infrared spectrum, and the information is simple and rich; nondestructive sampling is adopted, direct sample introduction is realized, the operation is simple, pretreatment of the sample is not needed, the sample is green and environmental-friendly, and the performance is reliable; only the detection amount of 0.1mg is needed, related consumables and maintenance cost are avoided, the operating cost and environmental protection risk are reduced, lots of manpower, material resources and financial resources are saved, actual problems such as long-term hidden grass-roots criminal activities, wide supervision range, large quantity and high difficulty are effectively solved, and the aim of guaranteeing the quality safety of foods and drugs is achieved.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
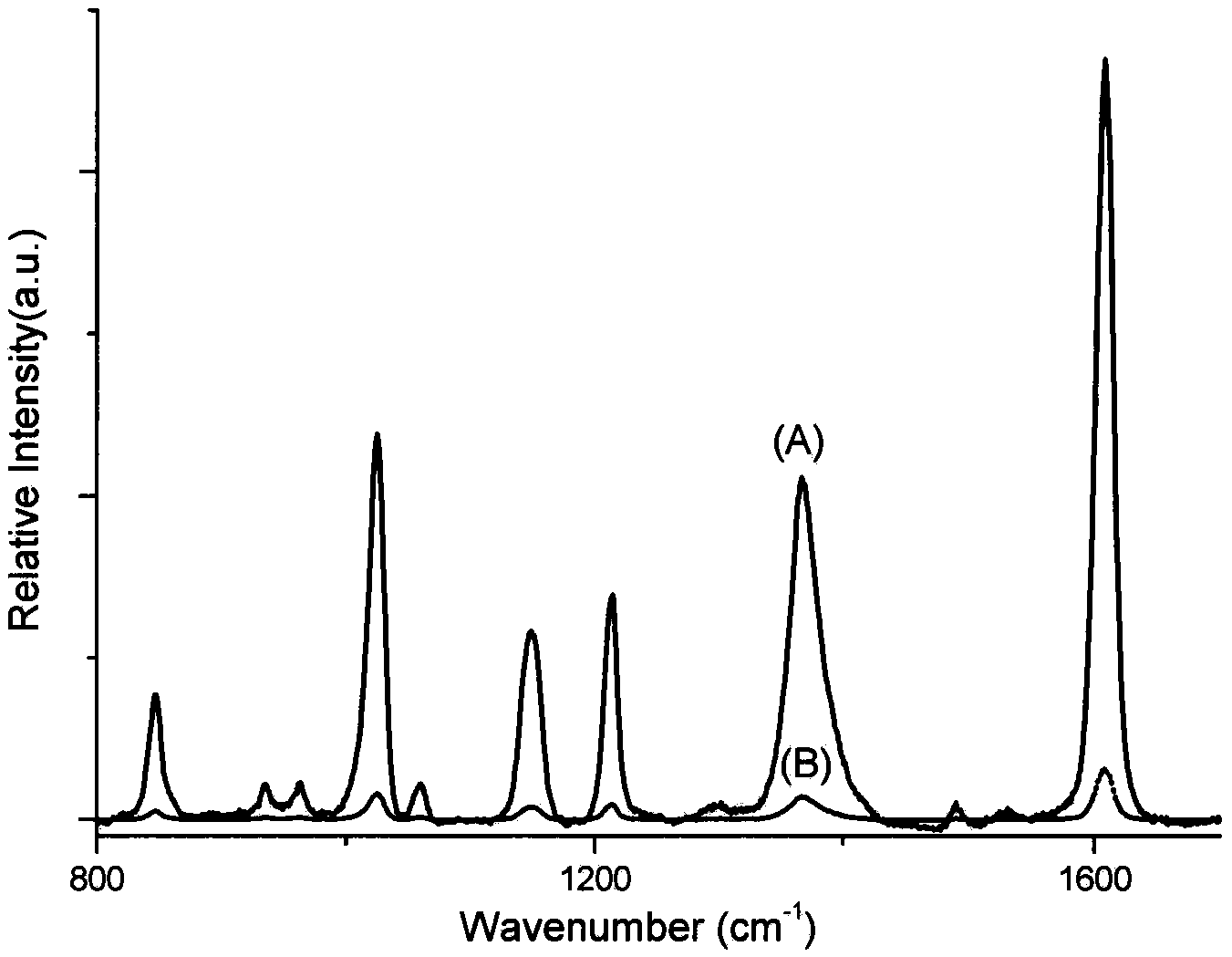
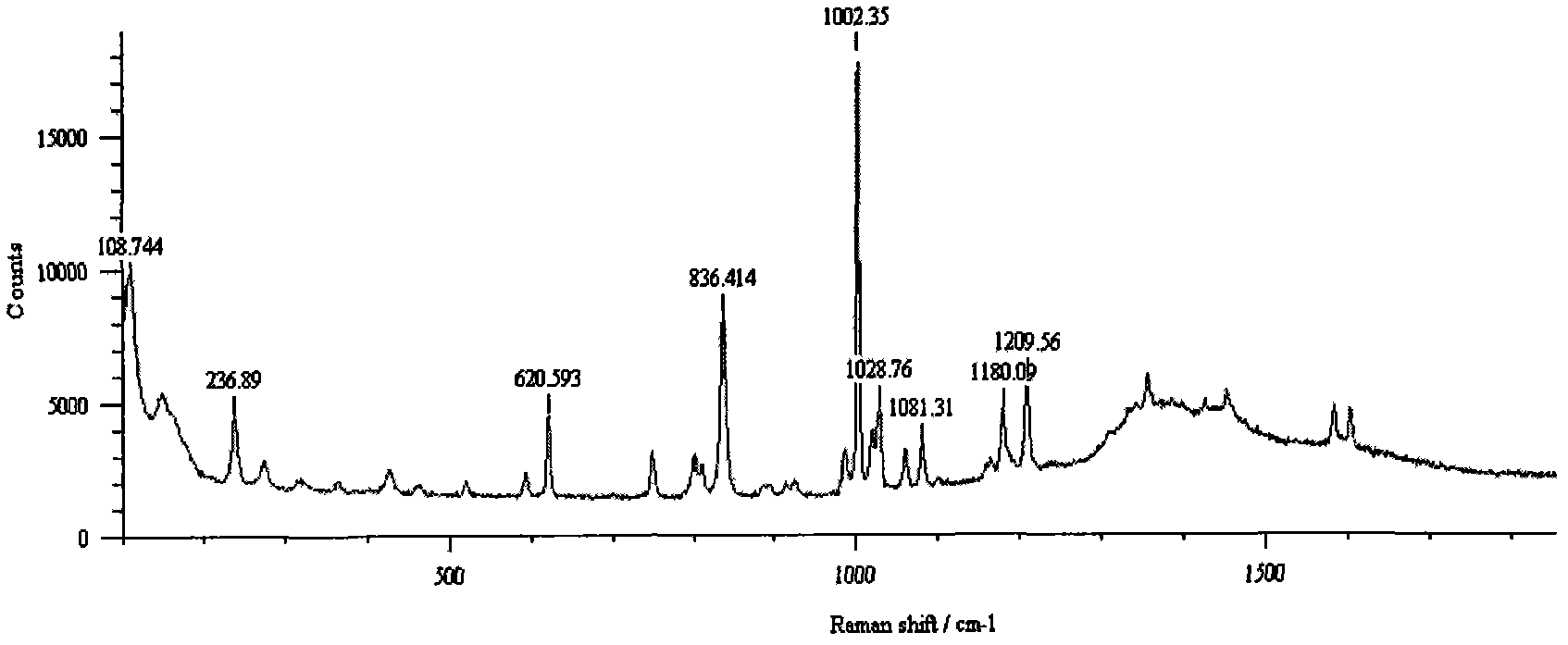
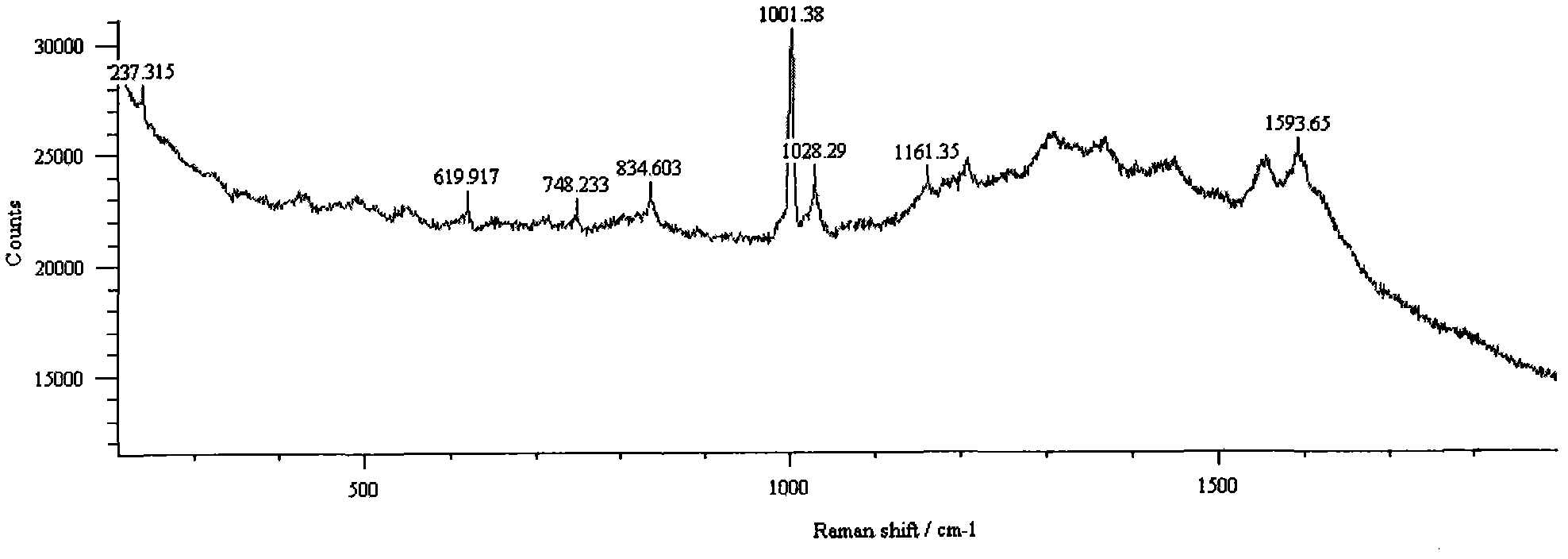
Method for detecting methamphetamine through surface enhanced Raman spectroscopy
InactiveCN102590177AQuick Qualitative AnalysisSimple and fast operationRaman scatteringSurface-enhanced Raman spectroscopyTest sample
The invention belongs to the technical field of drug detection, and particularly relates to a method for detecting methamphetamine through surface enhanced Raman spectroscopy, which includes the following steps: step I, pretreating a to-be-detected sample, so as to form sample solution; step II, preparing gold sol or silver sol; step III, uniformly mixing the test sample solution obtained through the step I and the gold sol or the silver sol obtained through the step II, so as to form the to-be-detected sample that is on standing; step IV, enabling the mixed to-be-detected sample obtained through the step III to be dripped on a slide and naturally evaporated for Raman spectrometry, thereby obtaining the SERS spectrogram of the gold sol and the to-be-detected sample or the SERS spectrogram of the silver sol and the to-be-detected sample; and step V, comparing the SERS spectrogram of the to-be-detected sample with the standard SERS spectrogram of the methamphetamine, so as to determine whether the sample contains the methamphetamine. The method for detecting the methamphetamine is simple to operate and quick to detect, has low sample consumption and detection cost, and can be widely applied to the detection field of the drug.
Owner:CHINA UNIVERSITY OF POLITICAL SCIENCE AND LAW
Narcotic detection paper based on microfluid capillary structure and preparing method thereof
InactiveCN106501520ASpread evenlyNo background fluorescenceMaterial analysisBackground fluorescenceFluorescence immunoassay
The invention discloses narcotic detection paper based on a microfluid capillary structure and a preparing method thereof. The narcotic detection paper is based on the fluorescence immunoassay principle, and the narcotic content in human body saliva can be accurately and quantitatively measured. The s narcotic detection paper is of the microfluid capillary structure, the problems that traditional fluorescence immunoassay detection paper is high in background fluorescence, and saliva samples are difficult to evenly diffuse are solved, and the advantages that the fluorescence immunoassay technology is high in sensitivity, and quantitative analysis can be carried out are fully developed. Shaping of the microfluid capillary structure of the narcotic detection paper is carried out with the reel-to-reel heat photoetching technology; by means of the method, the preparing cost can be greatly reduced, the preparing period is shortened, and large-scale production of the narcotic detection paper is achieved.
Owner:成都市亿泰科技有限公司
Particle-based drug detection methods
Disclosed herein are embodiments of methods for detecting the presence of and amount of drugs in a sample, particularly a particle sample obtained from a subject. In particular disclosed embodiments, the particle samples are skin particle samples, saliva particle samples, and / or mucous samples isolated from a subject and analyzed using thermal desorption methods combined with a selected detection method.
Owner:WASHINGTON STATE UNIVERSITY
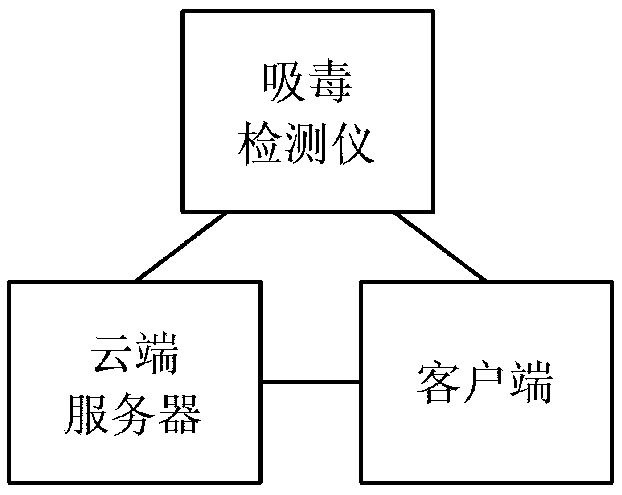
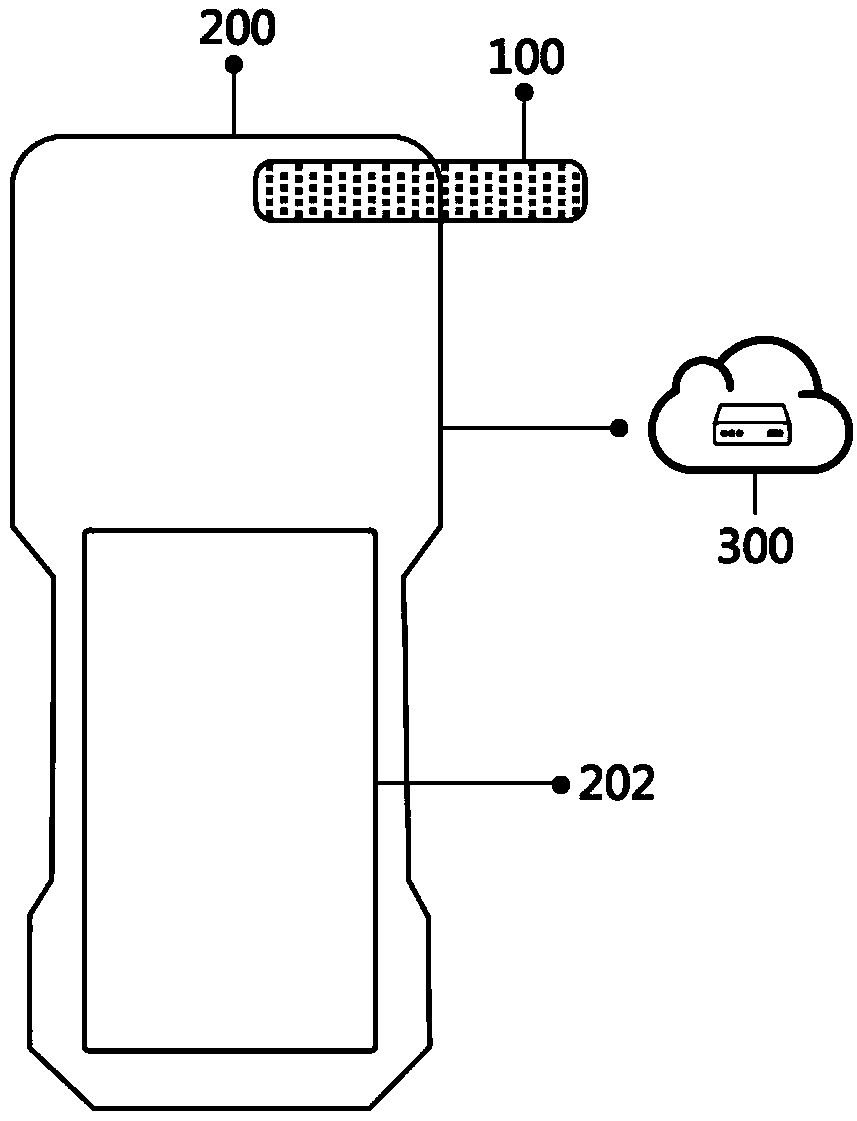
Detection system for rapidly inspecting drug taking
InactiveCN109342407AImprove visibilityFast and Accurate InterpretationMethod using image detector and image signal processingMaterial analysis by observing effect on chemical indicatorLaser scanningDrug detection
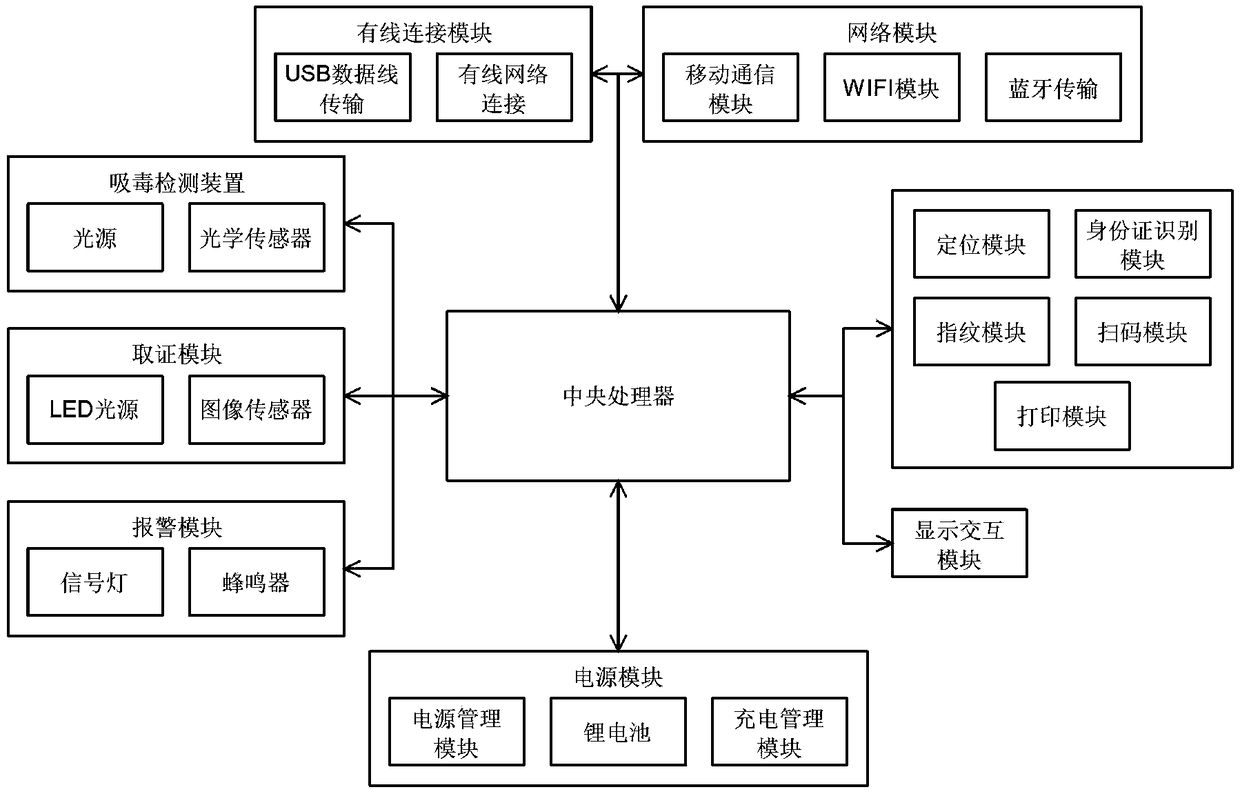
The invention relates to the technical field of the drug detection. The detection system comprises the following contents: S1, an on-site detection system for the drug taking is provided for recordingand uploading in real time identity information of law enforcement personnel and suspects, a detection result and related information in the law enforcement process and performing the intelligent analysis; S2, the detection system is composed of three parts of a drug taking detector, a cloud server and a client; S3, the drug taking detector comprises a central processing unit, a drug taking detection device, an evidence collecting module, a positioning module, a communication module, a printing module and the like; S6, the evidence collecting module comprises an LED light source and an imagesensor; S7, the positioning module is one or more of a Beidou, a GPS, a GLONASS and a Galileo system; S8, a code scanning module is a laser scanner or a red light scanner; and S9, the communication module comprises a network module and a wired connection module. The detection system performs the interpretation on the detection result of a drug detection card quickly and accurately by an optical sensor, can improve the recognition degree for the interpretation of the drug detection card, and can reduce the threshold and the grayscale area of a detection limit.
Owner:合肥五号速子科技有限公司
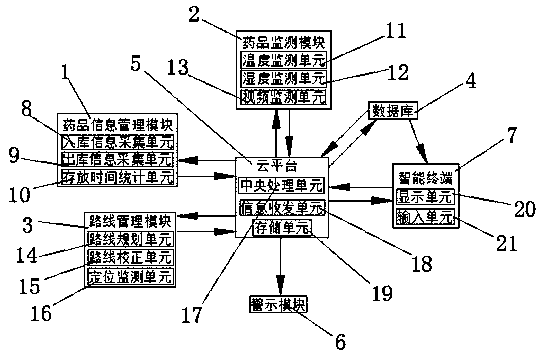
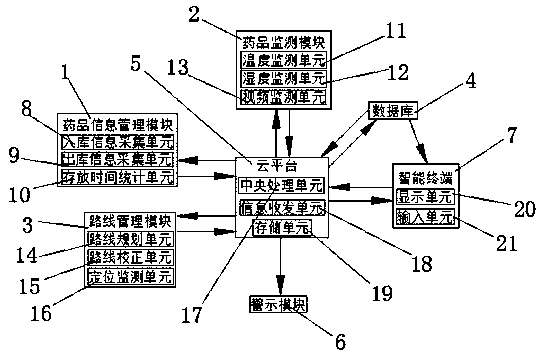
Drug cold chain monitoring and information intelligent management system and use method
The invention discloses a drug cold chain monitoring and information intelligent management system. The system comprises a medicine information management module, a medicine monitoring module, a routemanagement module, a database, a cloud platform, a warning module and an intelligent terminal. wherein the drug information management module comprises a warehouse-in information acquisition unit, awarehouse-out information acquisition unit and a storage time statistics unit, the drug detection module comprises a temperature monitoring unit, a humidity monitoring unit and a video monitoring unit, and the route management module comprises a route planning unit, a route correction unit and a positioning monitoring unit; the system can collect temperature, humidity and video data of cold-chaindrugs in real time, manage warehouse-in and warehouse-out information and storage time of the cold-chain drugs, plan transportation routes of the cold-chain drugs and carry out real-time positioning and monitoring. The invention further provides a using method of the drug cold chain monitoring and information intelligent management system, operation is convenient and fast, and popularization is convenient.
Owner:赖成龙
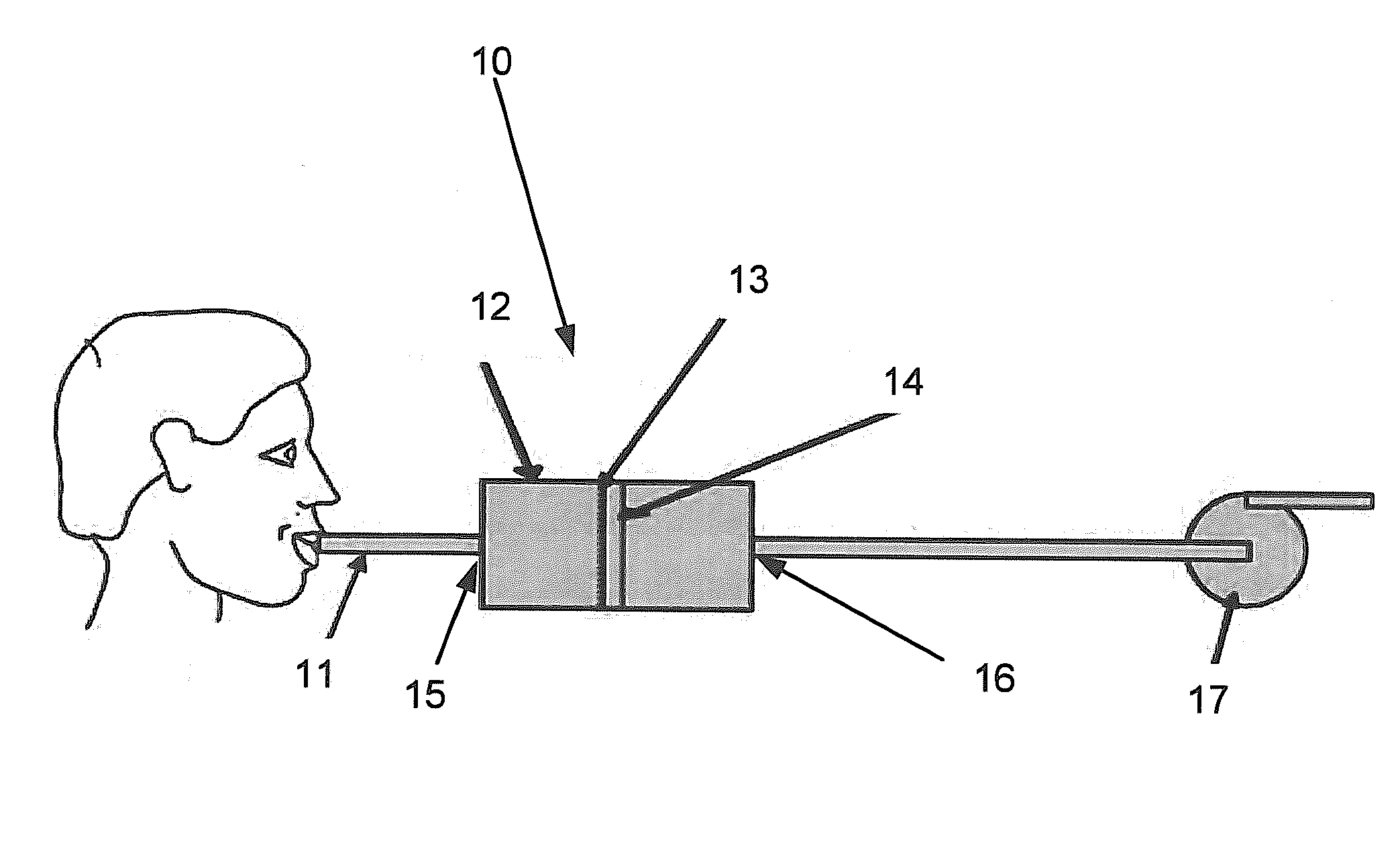
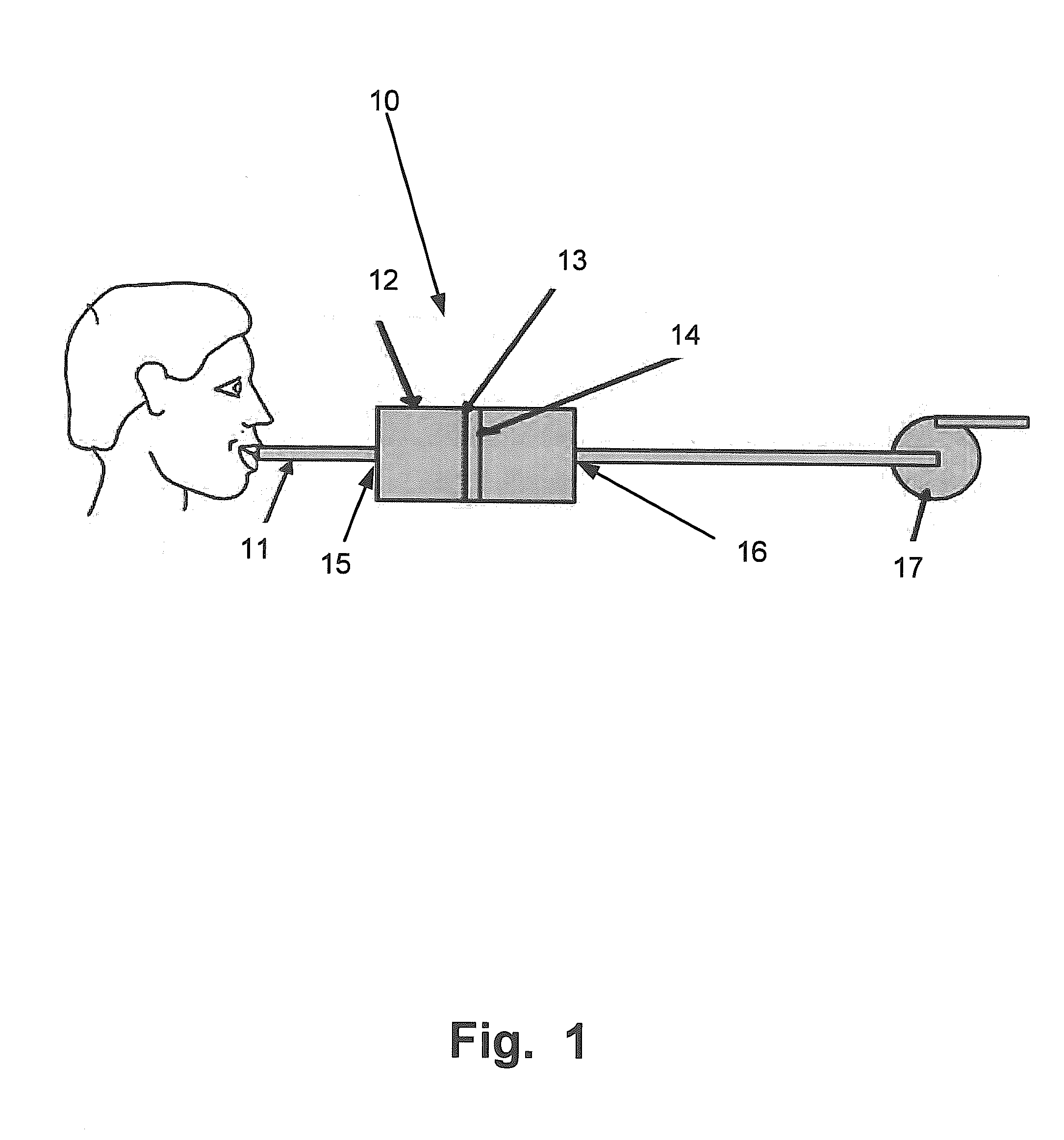
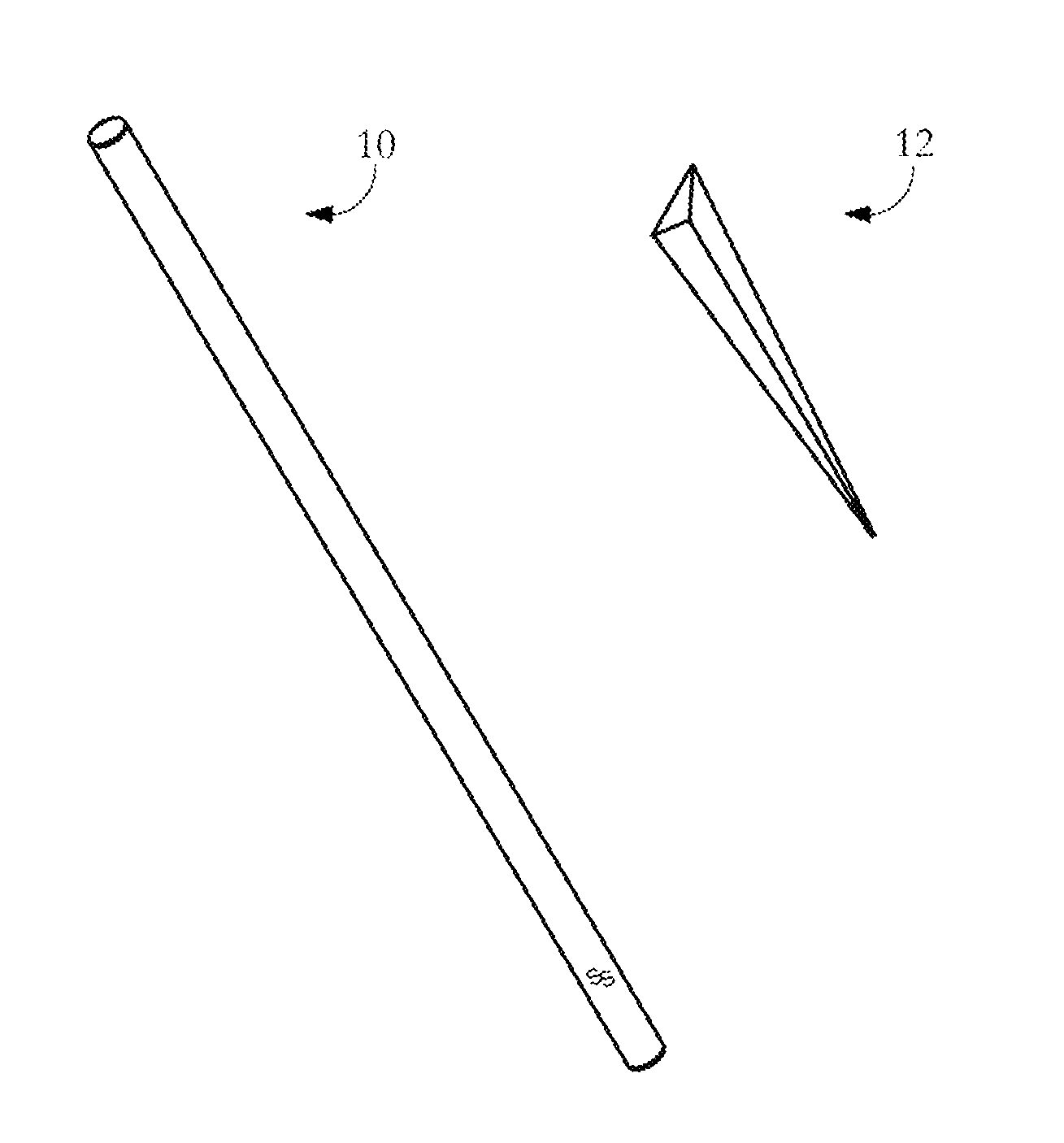
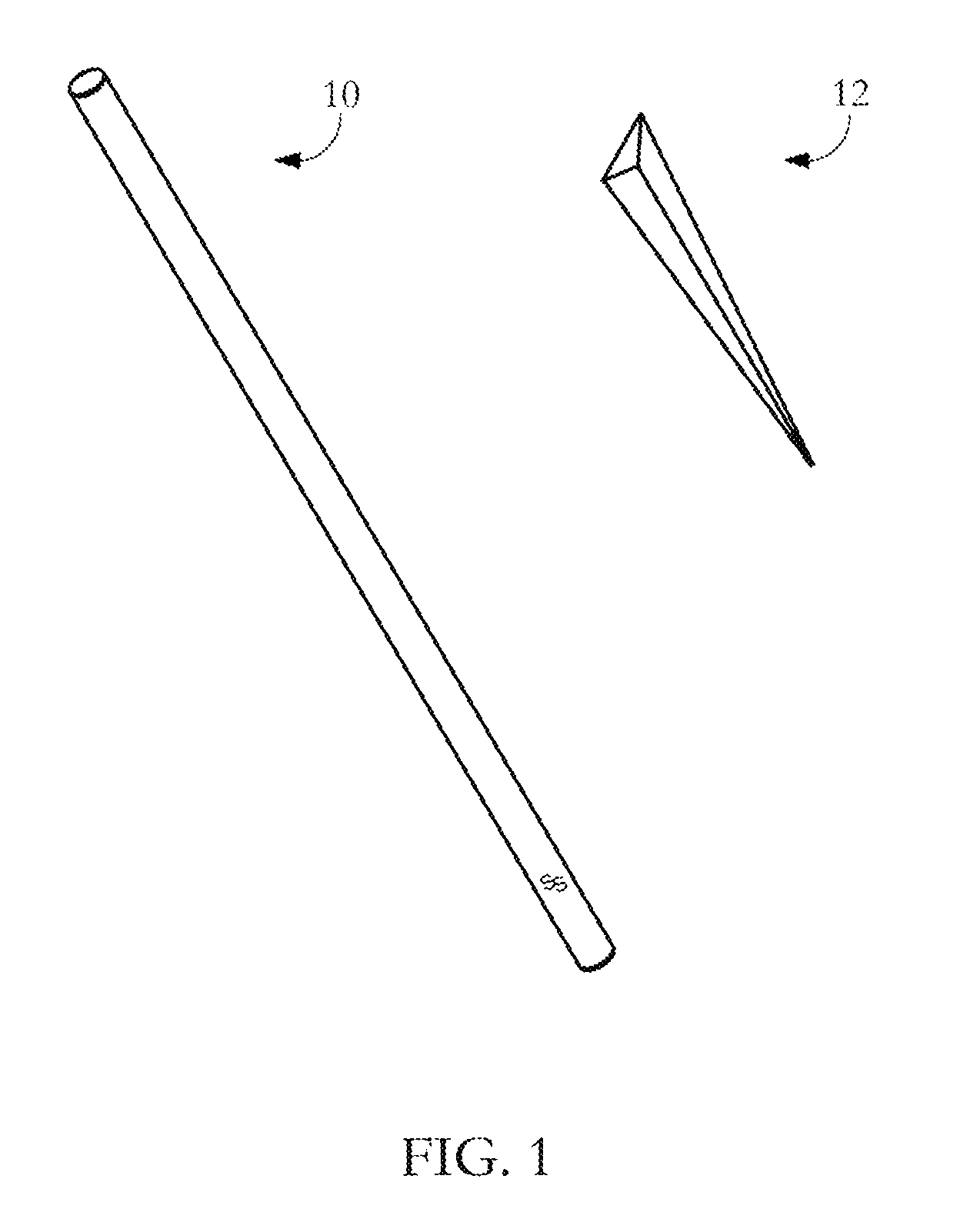
Drug detection straw
InactiveUS20110039346A1Material analysis by observing effect on chemical indicatorDwelling equipmentChange colorDrug detection
The present invention relates to a drug detection device for detecting the presence of a date rape drug in a beverage comprising: a straw; and a chemical reagent, where said chemical reagent coats the surface of the straw and changes color upon contact with a date rape drug. In one exemplary embodiment, the drug detection device may be used to test for Gamma Hydoxybutyrate (GHB) or ROHYPNOL® (Flunitrazepam). A stirring straw may be used as a component of the drug detection device as opposing to a conventional straw. The present invention also includes a method of date rape drug detection comprising the steps of: coating a stirring device with a chemical reagent; inserting the stirring device in a beverage; and observing the stirring device to determine an indication of the presence of a date rape drug.
Owner:BRADLEY MICHAEL +1
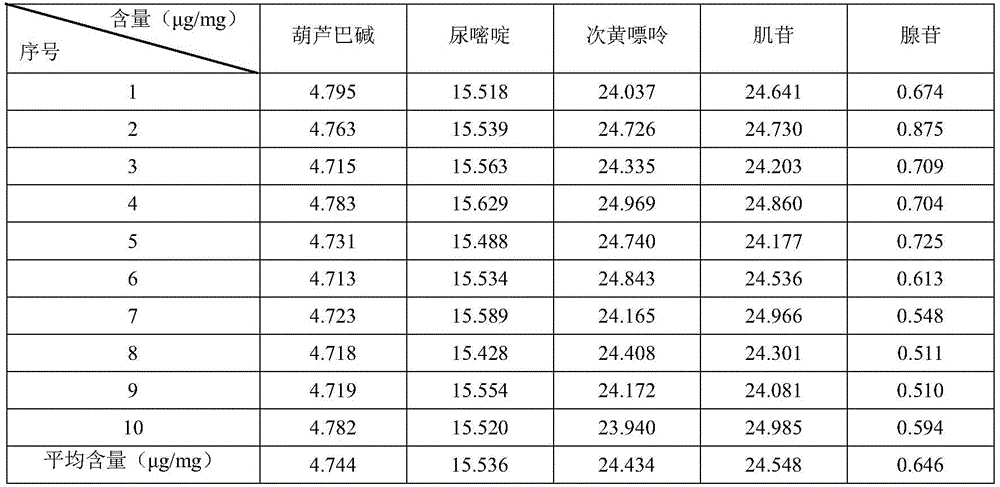
Kangfuxin solution preparation fingerprint quality determination method and standard fingerprint
InactiveCN106370739AAvoid one-sidednessAccurate and objective quality controlComponent separationQuality controlRepeatability
The invention relates to a drug detection method and especially relates to a Kangfuxin solution preparation fingerprint quality determination method and a standard fingerprint. Through high performance liquid chromatography detection, a Kangfuxin solution fingerprint is obtained. The method comprises a, contrast solution preparation, b, sample solution preparation and c, fingerprint making. The method builds a Kangfuxin solution chemical fingerprint with good chromatographic peak resolution, repeatability and stability and can be used as a Kangfuxin solution preparation quality control method. The method provides scientific basis for scientific and effective evaluation and control of qualities of periplaneta americana medical raw materials and a Kangfuxin solution preparation.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit
Rapid drug detection is a regular detection item for public security departments, drug control organizations, sports events, enlistment and entry departments. At present, colloidal gold test paper is a basic choice for rapid drug detection, but the sensitivity of the colloidal gold test paper is low, the possibility of fake positive and fake negative results is high, the repeatability is low and the quantification is impossible. The invention discloses a cloned enzyme-donor immunoassay (CEDIA) ImmunoChip drug detecting kit. The kit comprises an immuno chip on which the combination of an enzyme donor and an enzyme receptor is immobilized, wherein the enzyme donor can be coupled with a drug micromolecule by a chemical bond to form an enzyme-donor-labeled exogenous antigen so as to be used for detecting whether a specific anti-drug antibody which can be competitively combined with the enzyme-donor-labeled exogenous antigen exists in a sample to be detected by a CEDIA ImmunoChip drug detecting method and calibration is carried out by using a novel fluorogenic substrate. The kit has the advantages of sensitive reaction, high accuracy, no cross reaction and high repeatability, and is simple and convenient to operate.
Owner:GUANGZHOU YIHANG BIOTECH
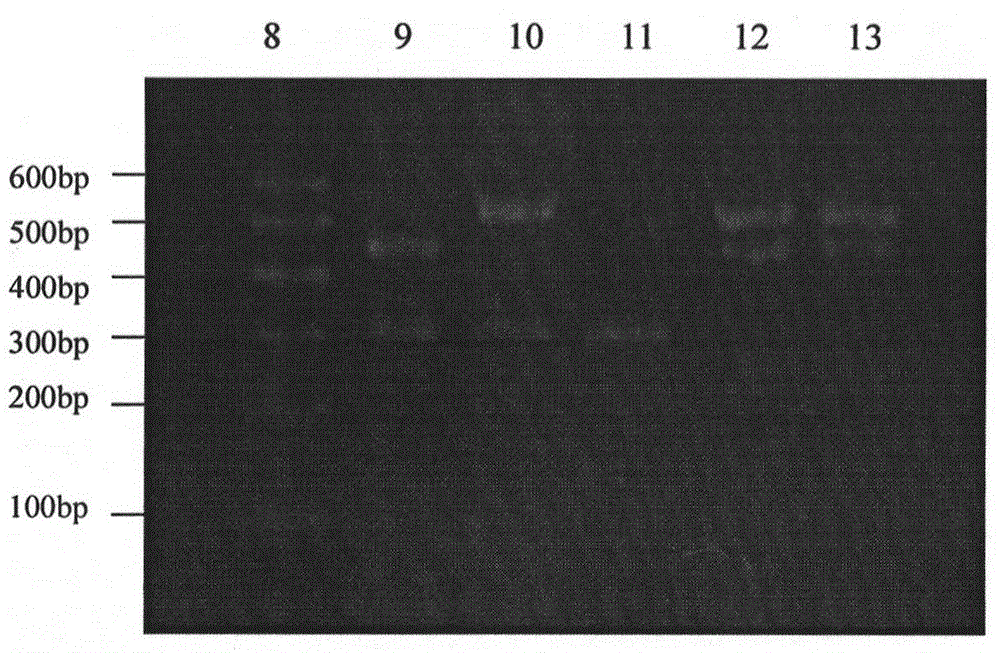
Semi-nested-multiple PCR (Polymerase Chain Reaction) method for discerning authenticity of donkey-hide gelatin
InactiveCN104988231AHigh detection sensitivityGuaranteed Assay SpecificityMicrobiological testing/measurementSulfonateElectrophoresis
The invention relates to the field of food and drug detection technologies, in particular to a semi-nested-multiple PCR (Polymerase Chain Reaction) method for discerning the authenticity of donkey-hide gelatin, and the semi-nested-multiple PCR method is used for discerning whether components of horse, pig and cattle are doped in the donkey-hide gelatin, comprises the steps of adopting a modified SDS (Sodium Dodecyl Sulfonate) method to extract high quality DNA from the donkey-hide gelatin treated by liquid nitrogen grinding, and then designing universal primers P1 and P2 to perform the first round of amplification, wherein an amplification product is taken as a template of the second round of PRC; performing the second round of multiple PCR amplification by using mixed primers including the universal primer P1 and specific primers A, B, C and D of four species (donkey, horse, pig and cattle), detecting an amplification product of the second round by cataphoresis to discern whether the donkey-hide gelatin is adulterated according to the size of an electrophoretic band. By adopting the technical scheme of the invention, the detection sensitivity of the authenticity detection method of the donkey-hide gelatin on the basis of the existing PCR technology is improved in premise of ensuring the detection specificity, meanwhile, the detection time is shortened.
Owner:山东世通检测评价技术服务有限公司
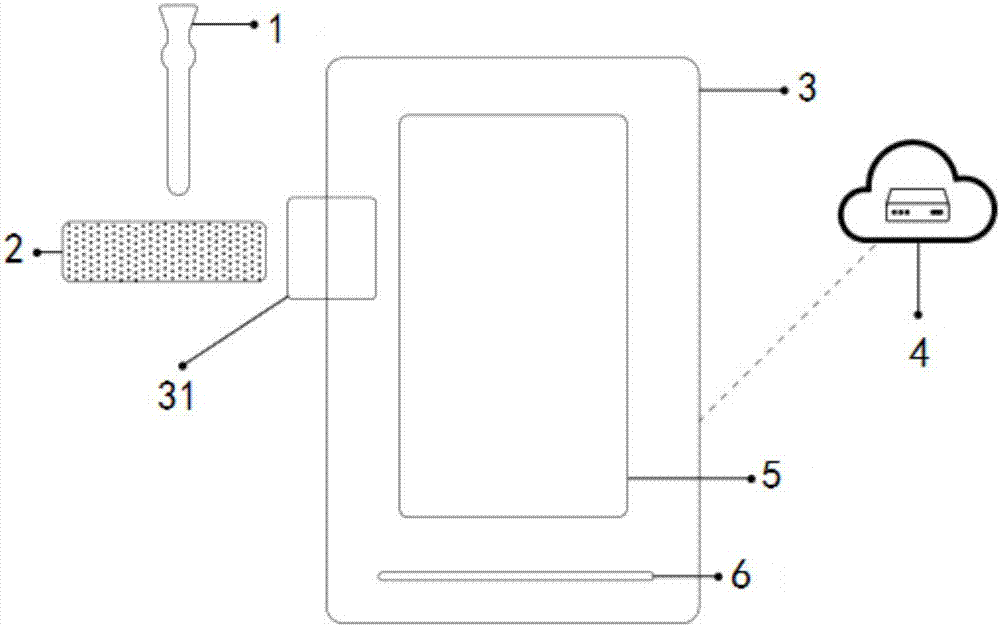
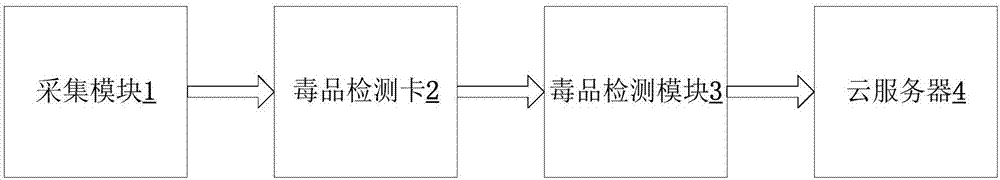
Drug detection device and detection method
InactiveCN107219221ALower detection thresholdImprove color recognitionMaterial analysis by observing effect on chemical indicatorDrug detectionIntelligent control
The invention provides a drug detection device and detection method. The device comprises a shell, a collection module, a drug detection card and a drug detection module, wherein the collection module and the drug detection card are arranged on the outer part of the shell; the drug detection module is arranged in the shell; the collection module, the drug detection card and the drug detection module are connected in sequence; the drug detection module comprises a developing interpretation submodule and an intelligent control submodule, wherein the developing interpretation submodule is used for reading an optical signal detected on the drug detection card; the intelligent control submodule is connected with the developing interpretation submodule for processing the optical signal detected by the developing interpretation submodule to obtain a detection result. According to the device, the developing interpretation submodule is used for replacing human eye identification, a color distinguishing degree for a drug detection card developing result is improved, drug taking detection sensitivity is enhanced, a drug detection threshold value is lowered, and the case handling efficiency of law enforcement officers is improved.
Owner:合肥君匠科技有限公司
Rapid detection method of methionine sulfoxide in amino acid injection
The invention belongs to the technical filed of a drug detection method and particularly relates to a rapid detection method of methionine sulfoxide in amino acid injection. An automatic amino acid analyzer is adopted for detection; only two mobile phases are adopted for separation, R1-R3 are derivative reaction reagents, Hitachi special ion exchange resin is taken as a filling agent, and the calculation is performed by peak area according to an external standard method. Through contrastive study, as a primary standard procedure adopts six mobile phases, the consumption of the reagents is high, and the mobile phases are various. As the two mobile phases are adopted, the consumption of the reagents is low, and a spectrogram is briefer. The test of the primary standard procedure needs 53.8 minutes, and the detection method provided by the invention only needs 29.5 minutes, so that the detection time is greatly reduced, and the efficiency is higher.
Owner:湖北华仁同济药业有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com