Patents
Literature
157 results about "Risperidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Risperidone is used to treat certain mental/mood disorders (such as schizophrenia, bipolar disorder, irritability associated with autistic disorder).
Combinations of 5-ht2a inverse agonists and antagonists with antipsychotics
InactiveUS20090053329A1Achieve effectQuick effectCompounds screening/testingBiocideSide effectAntipsychotic drug therapy
Combinations of 5-HT2A inverse agonists or antagonists such as pimavanserin with antipsychotics such as risperidone are shown to induce a rapid onset of antipsychotic action and increase the number of responders when compared to therapy with the antipsychotic alone. These effects can be achieved at a low dose of the antipsychotic, thereby reducing the incidence of side effects. The combinations are also effective at decreases the incidence of weight gain and increased glucose or prolactin levels caused by the antipsychotic.
Owner:ACADIA PHARMA INC
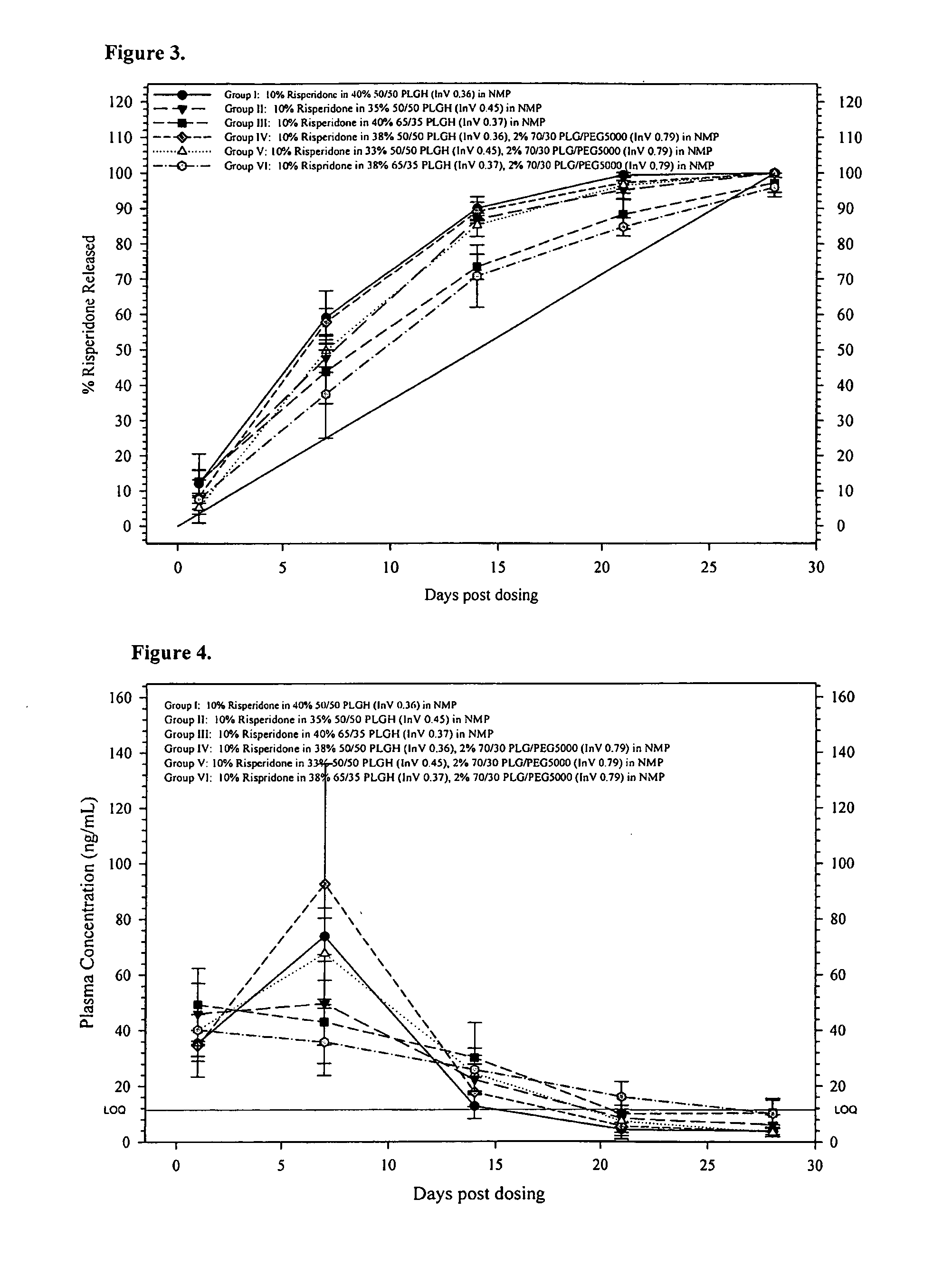
Sustained delivery formulations of risperidone compounds
ActiveUS20100266655A1Improve bioavailabilityLeast riskBiocideOrganic active ingredientsMetaboliteOrganic fluid
The present invention relates to a risperidone sustained release delivery system for treatment of medical conditions relating delusional psychosis, schizophrenia, bipolar disorder, psychotic depression, obsessive-compulsion disorder, Tourette syndrome, and autistic spectrum disorders. The sustained release delivery system includes a flowable composition containing risperidone, a metabolite, or a prodrug thereof and an implant containing risperidone, a metabolite, or a prodrug thereof. The flowable composition may be injected into tissue whereupon it coagulates to become the solid or gel, monolithic implant. The flowable composition includes a biodegradable, thermoplastic polymer, an organic liquid, and risperidone, a metabolite, or a prodrug thereof.
Owner:INDIVIOR UK
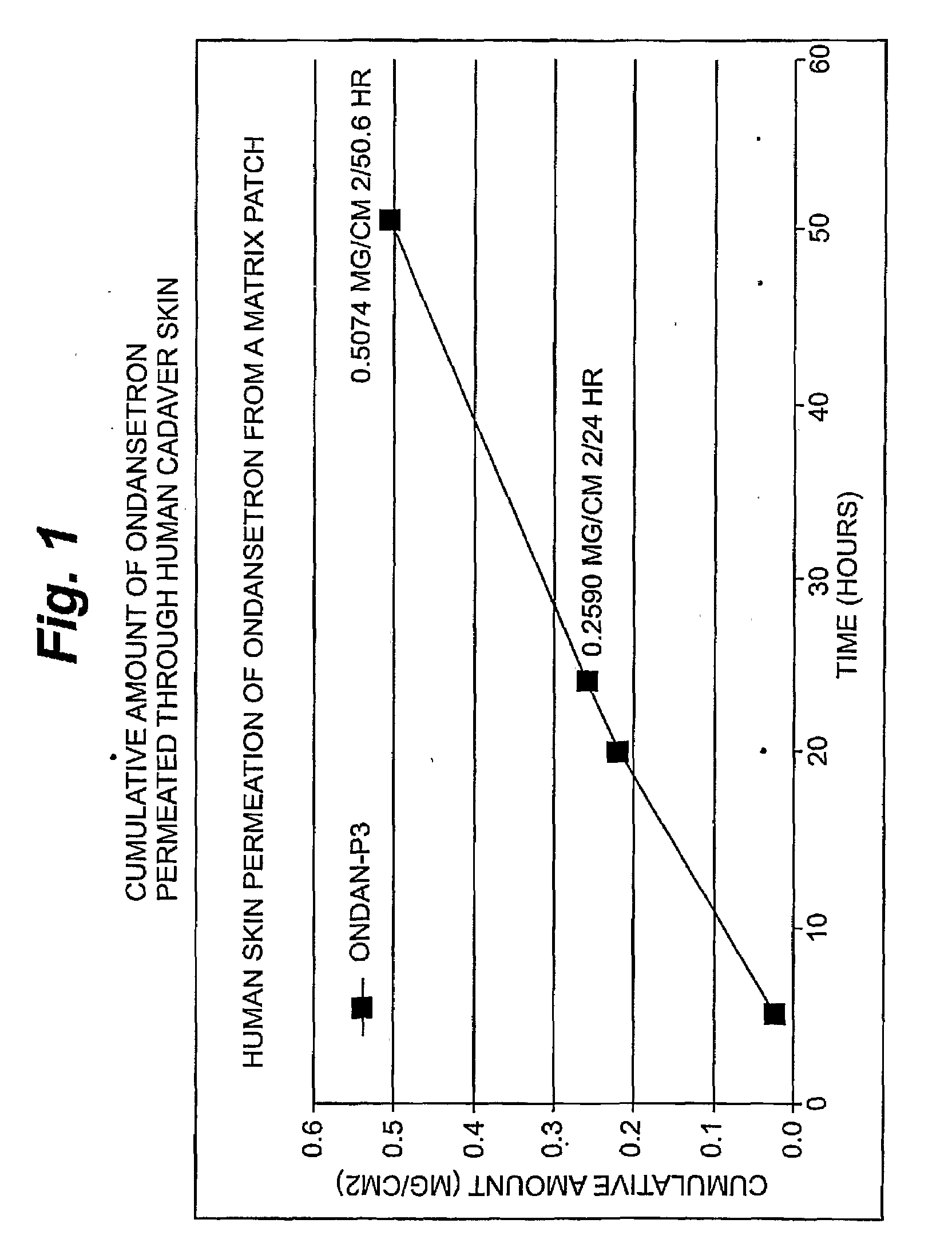
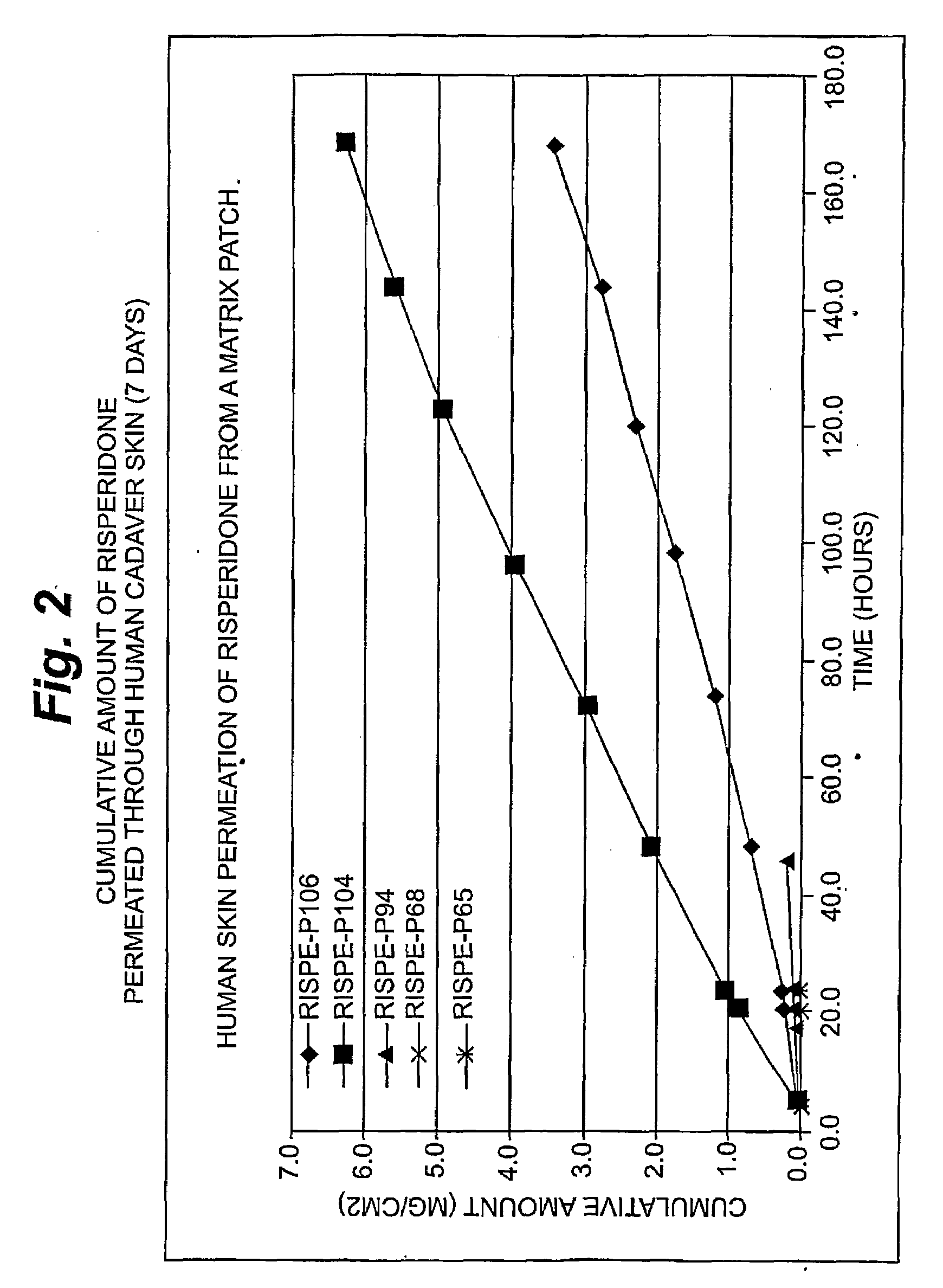
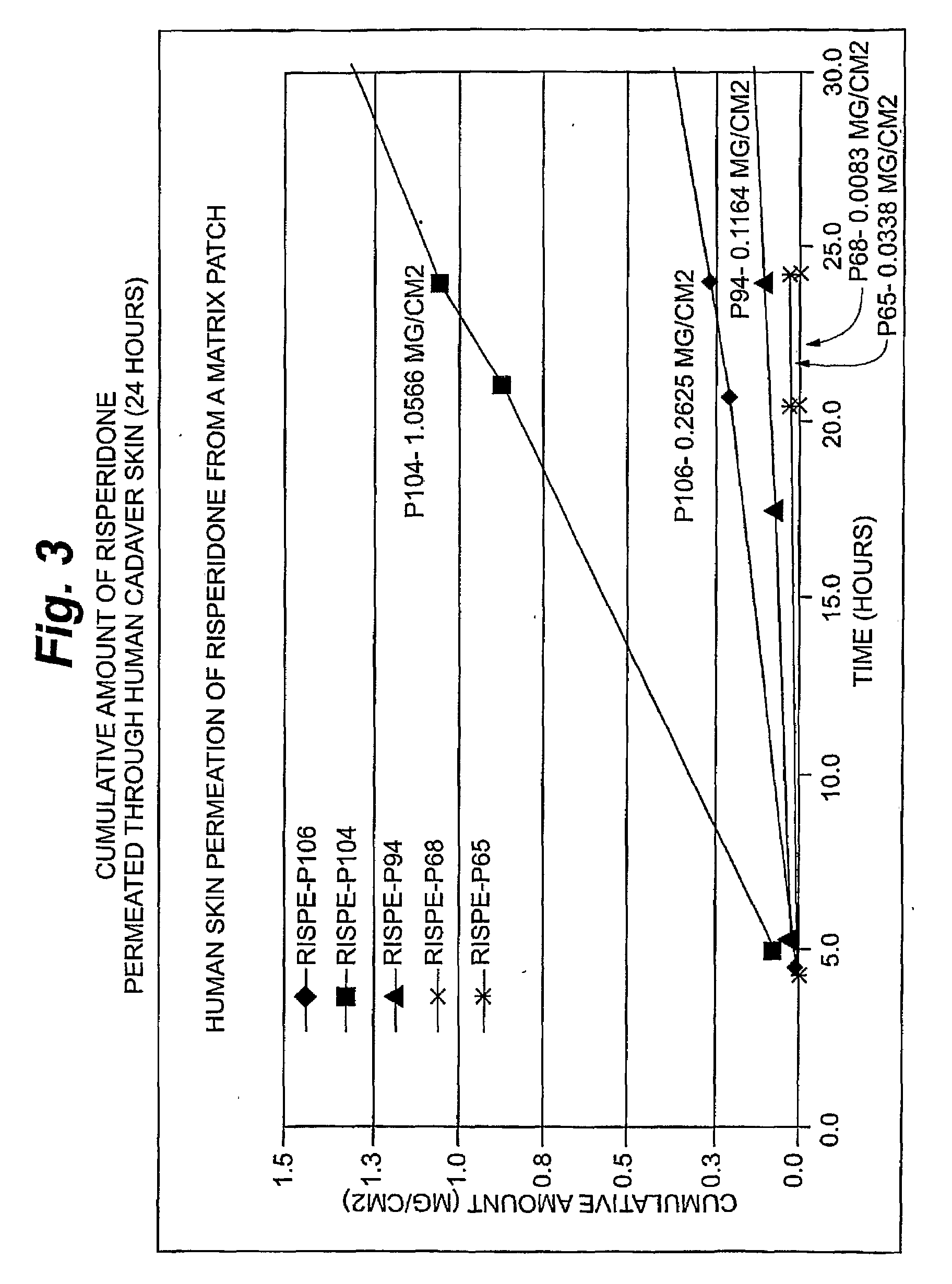
Transdermal Delivery of Hydrophobic Bioactive Agents
InactiveUS20080262445A1Improve solubilityImprove permeabilityBiocideAdhesive dressingsSerotoninFlumazenil
A method and related compositions, including the use of N-acyl derivatives of sarcosine, provide for the delivery of bioactive agents through tissue surfaces such as the skin. The method and composition are particularly well suited for hydrophobic active agents such as serotonin (5HT3) receptor antagonists (e.g., ondansetron), antipsychotic agents (e.g., risperidone), benzodiazepines (e.g., flumazenil), and progestins (e.g., levonorgestrel).
Owner:DERMATRENDS INC
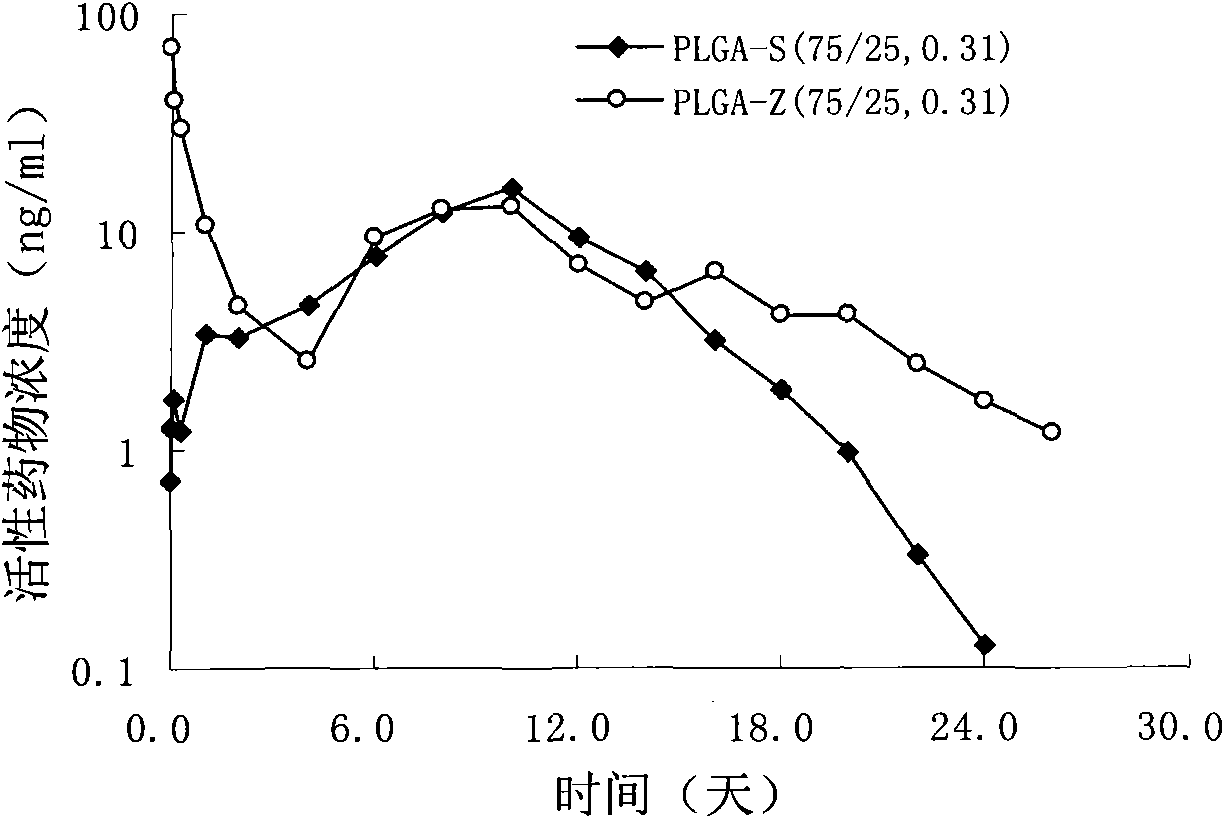
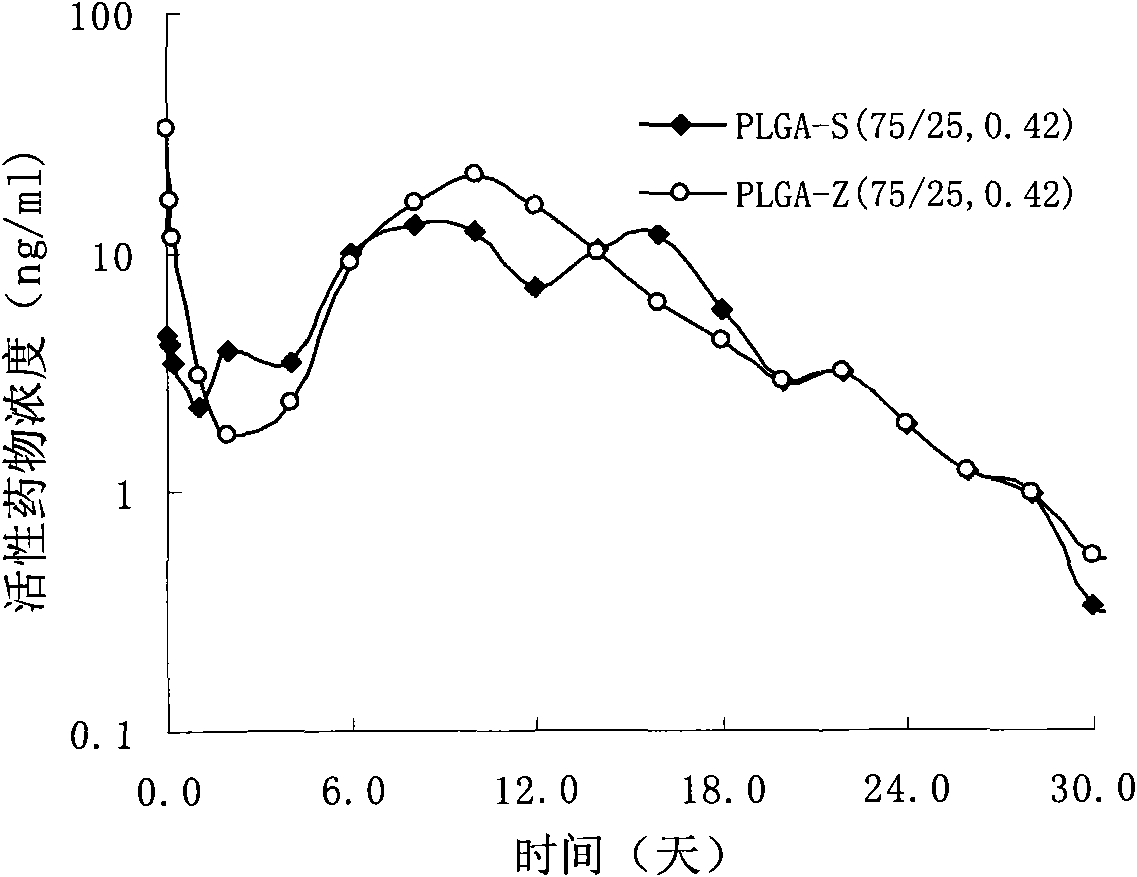
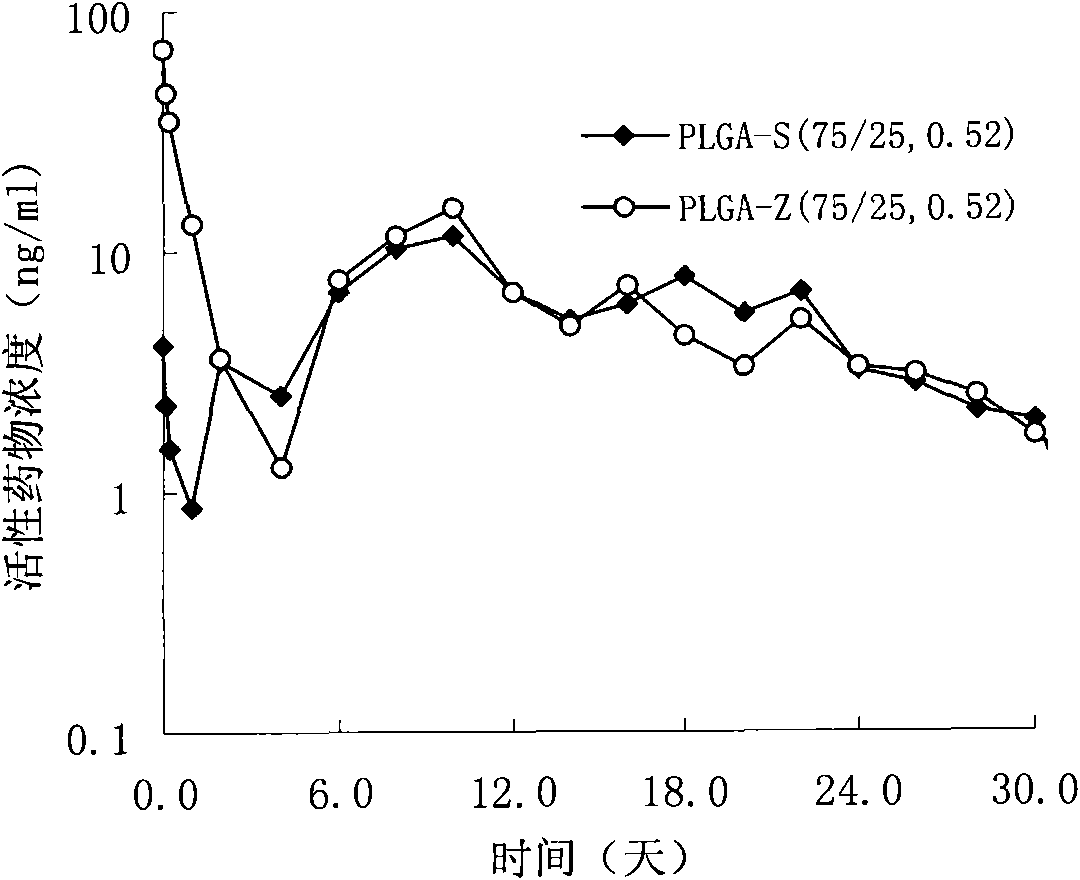
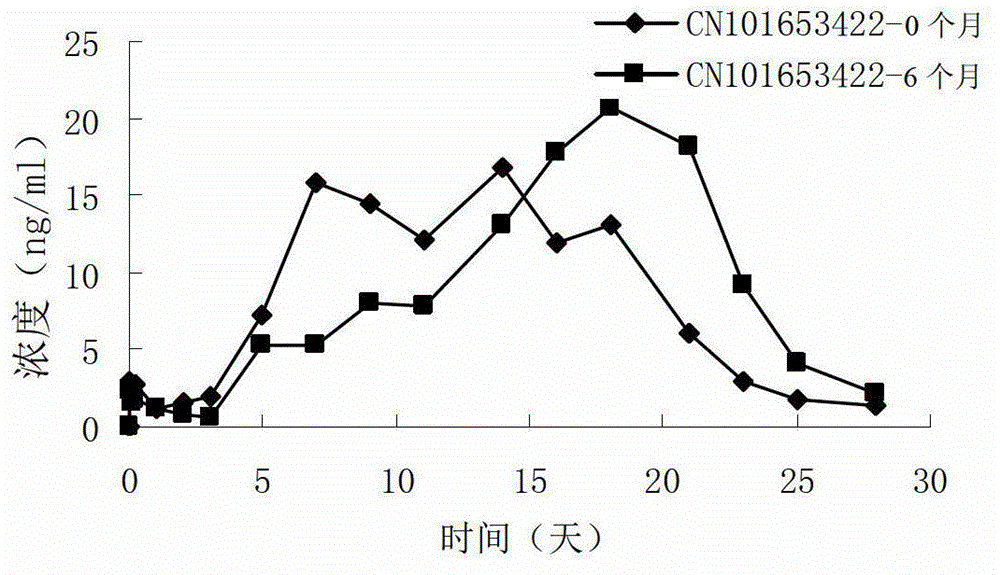
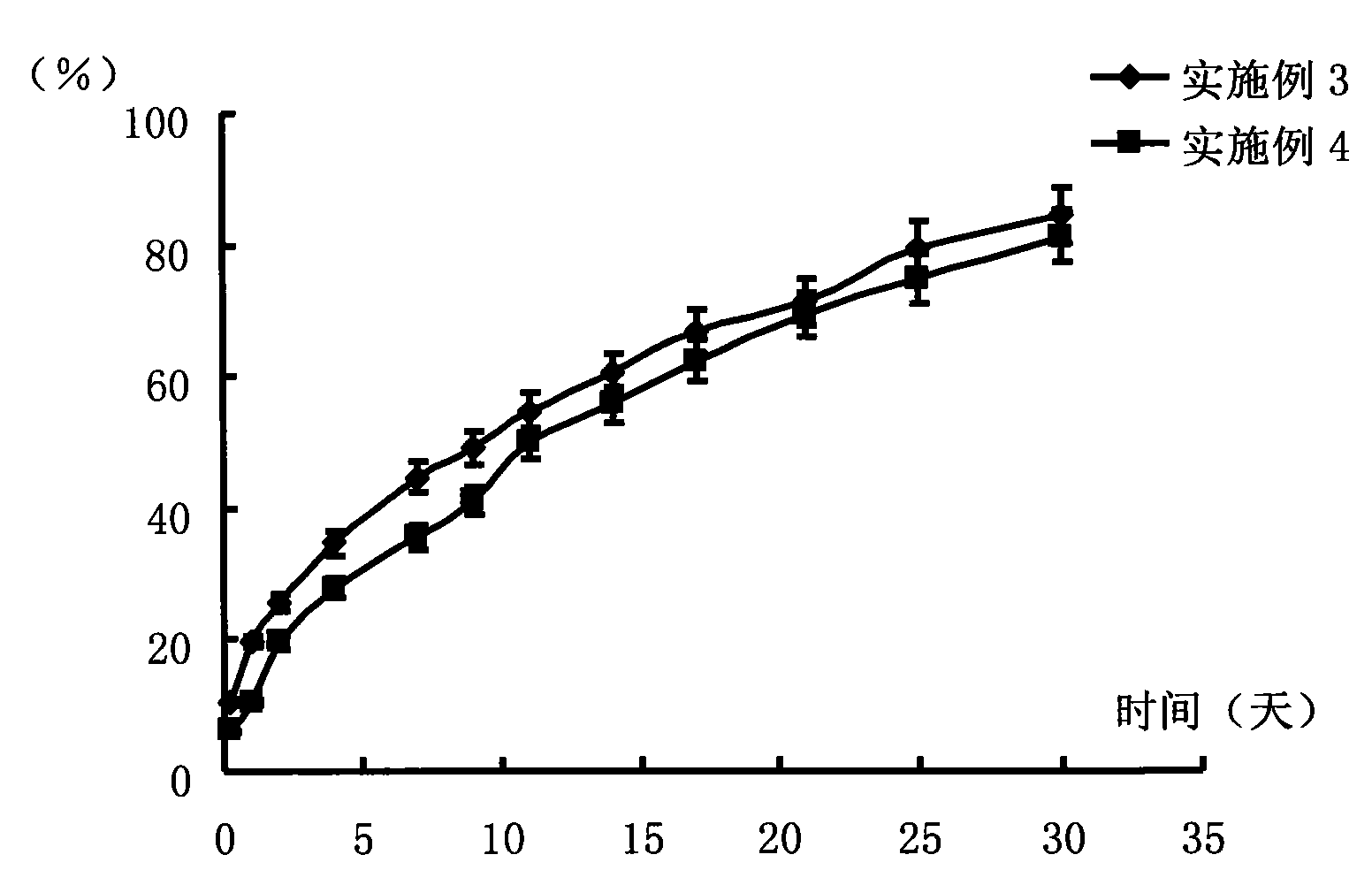
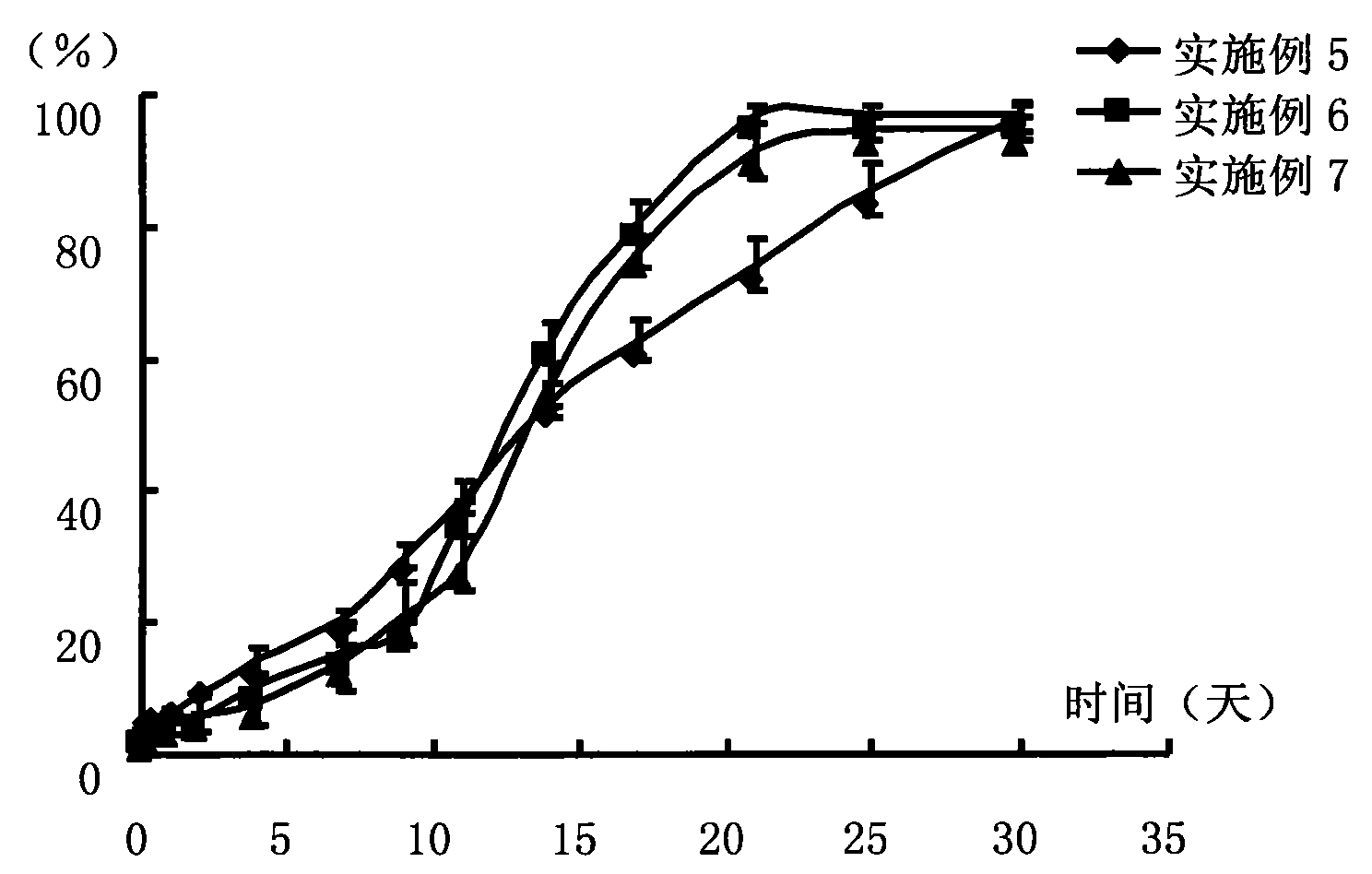
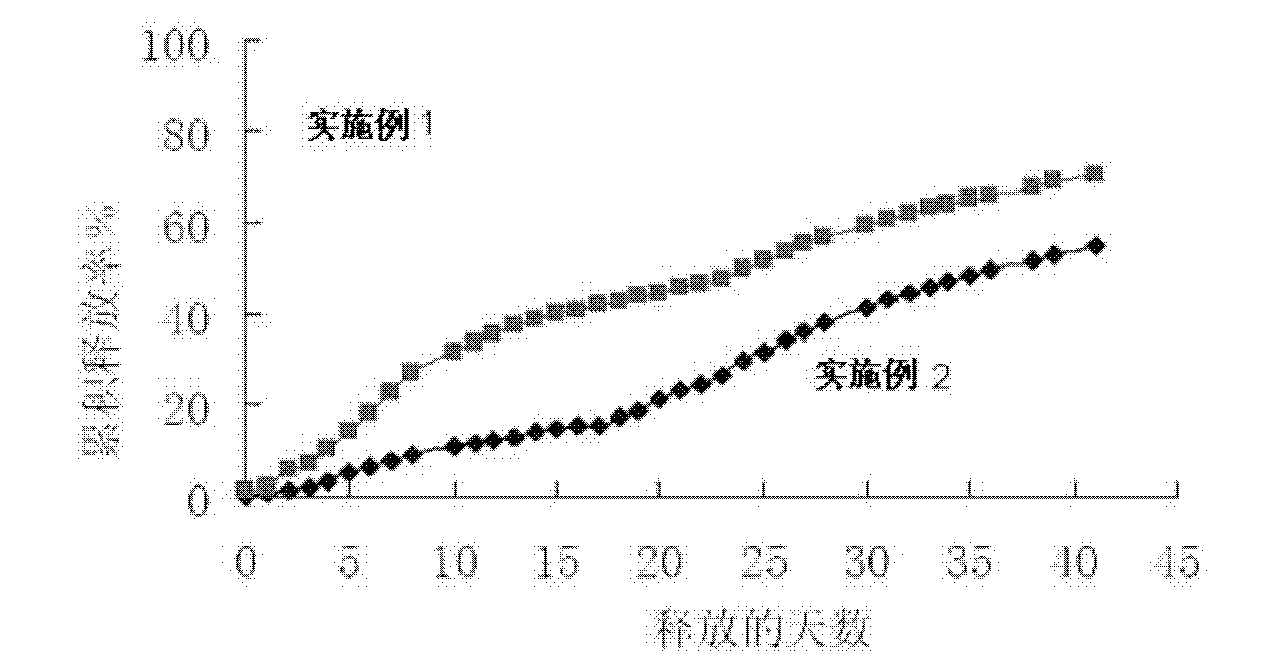
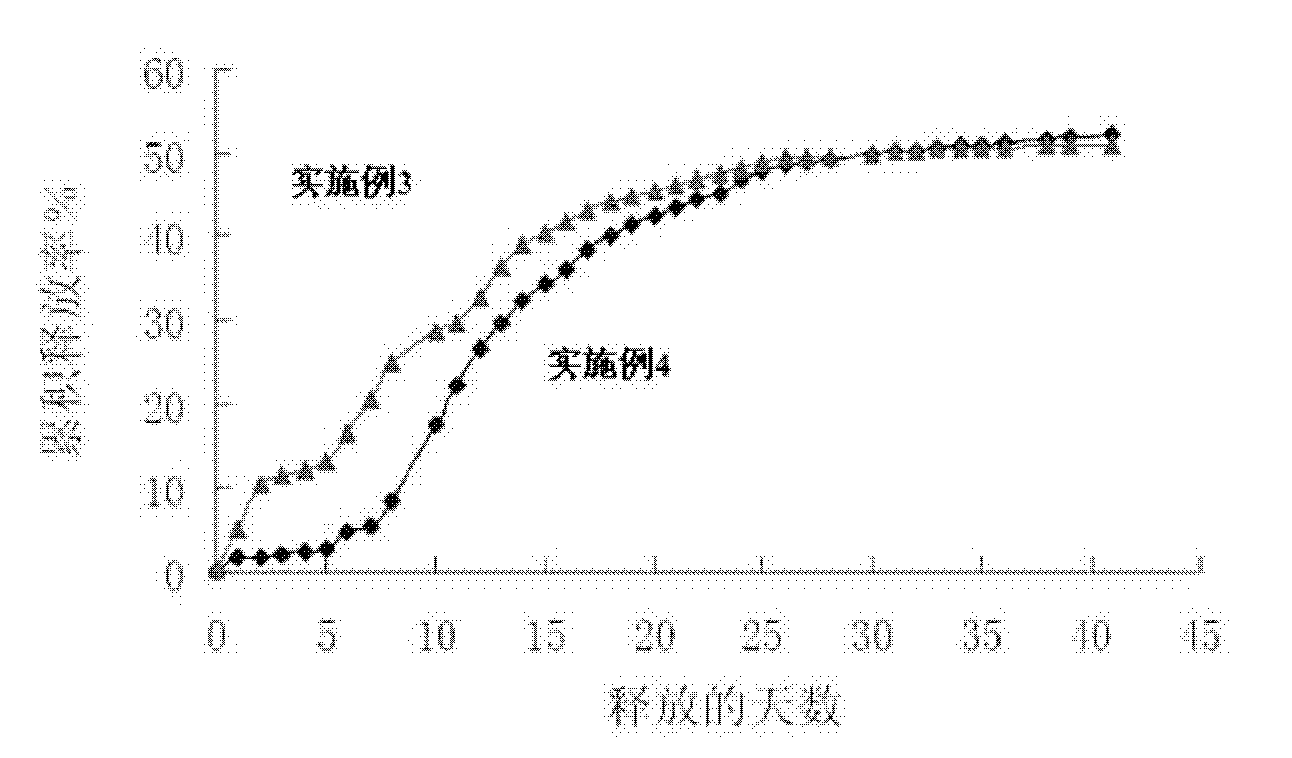
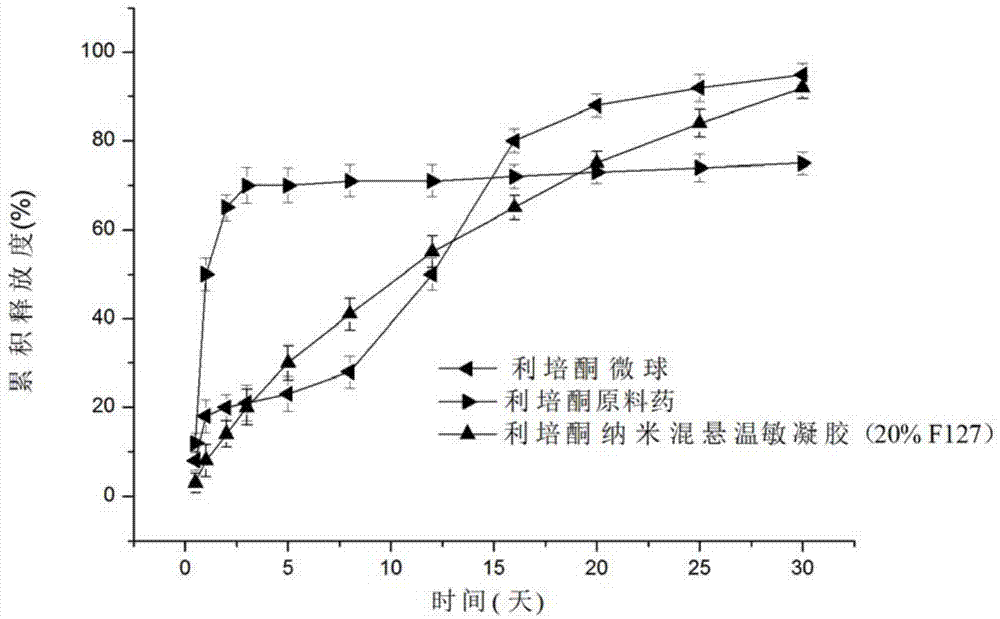
Risperidone slow-release microsphere, preparation method and application thereof
ActiveCN101653422AHigh drug loadingImprove complianceOrganic active ingredientsNervous disorderBlood concentrationMicrosphere
The invention provides a risperidone slow-release microsphere, a preparation method and an application thereof. The microsphere comprises risperidone or 9-hydroxy risperidone or the salt thereof and anon-end-capped lactide-glycollide copolymer. The risperidone slow-release microsphere provided by the invention has higher medicine-carrying quantity, no in-vivo sudden-release phenomenon, stable blood concentration and no medicine release lag period, reduces the administration frequency of a patient greatly, reduces the administration volume of each time, enhances the conformance of the patientand reduces the generation of adverse reactions.
Owner:SHANDONG LUYE PHARMA CO LTD +1
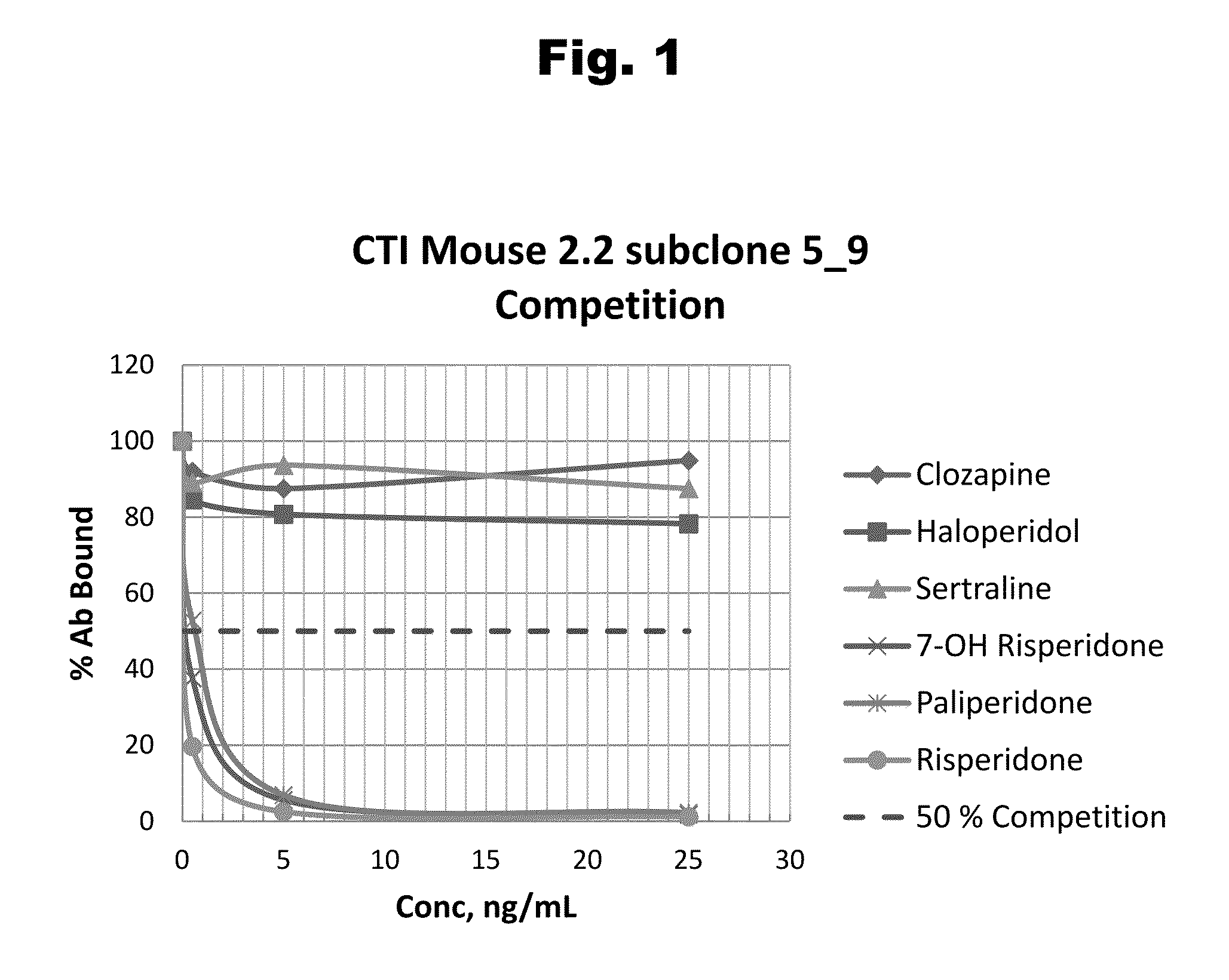
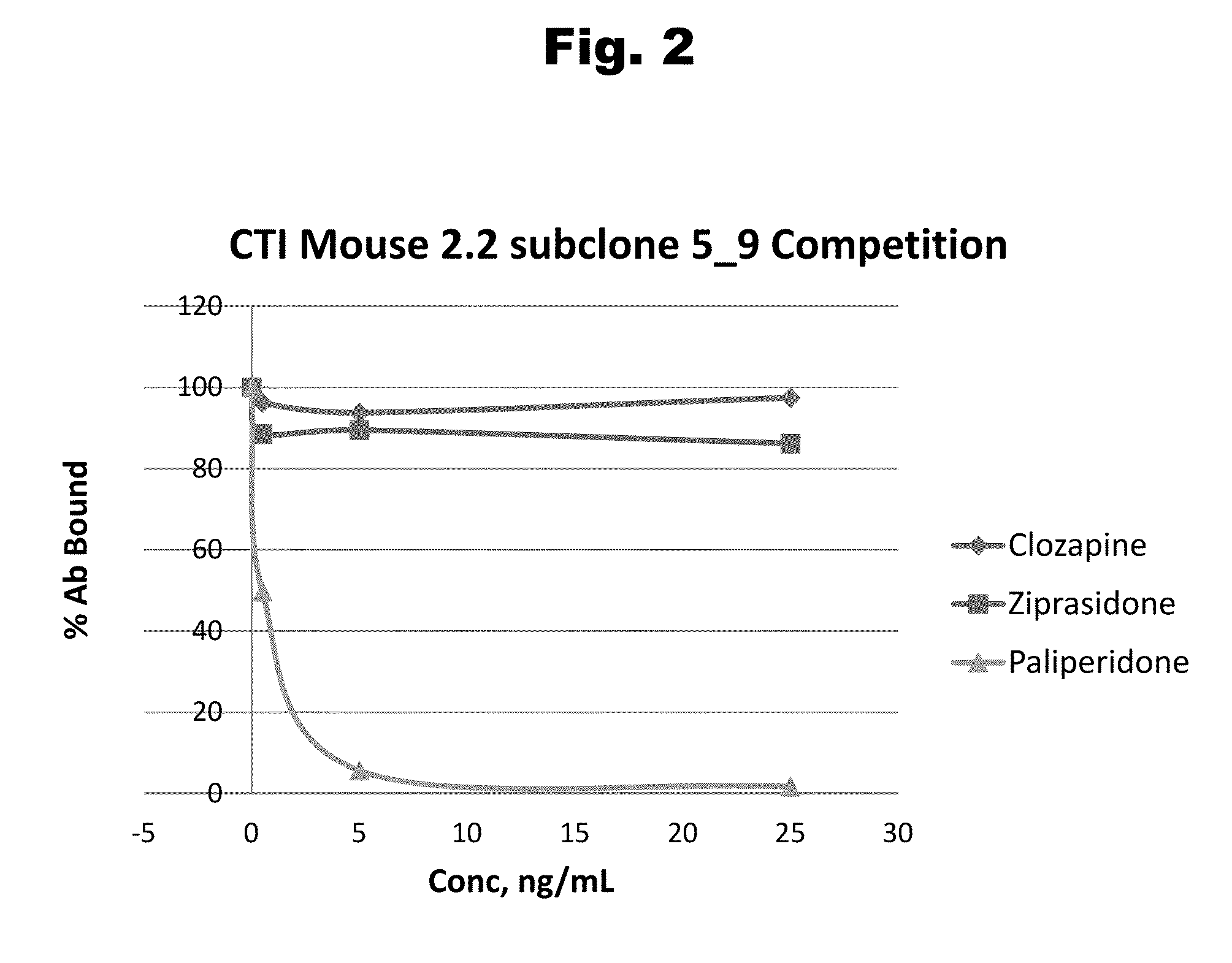
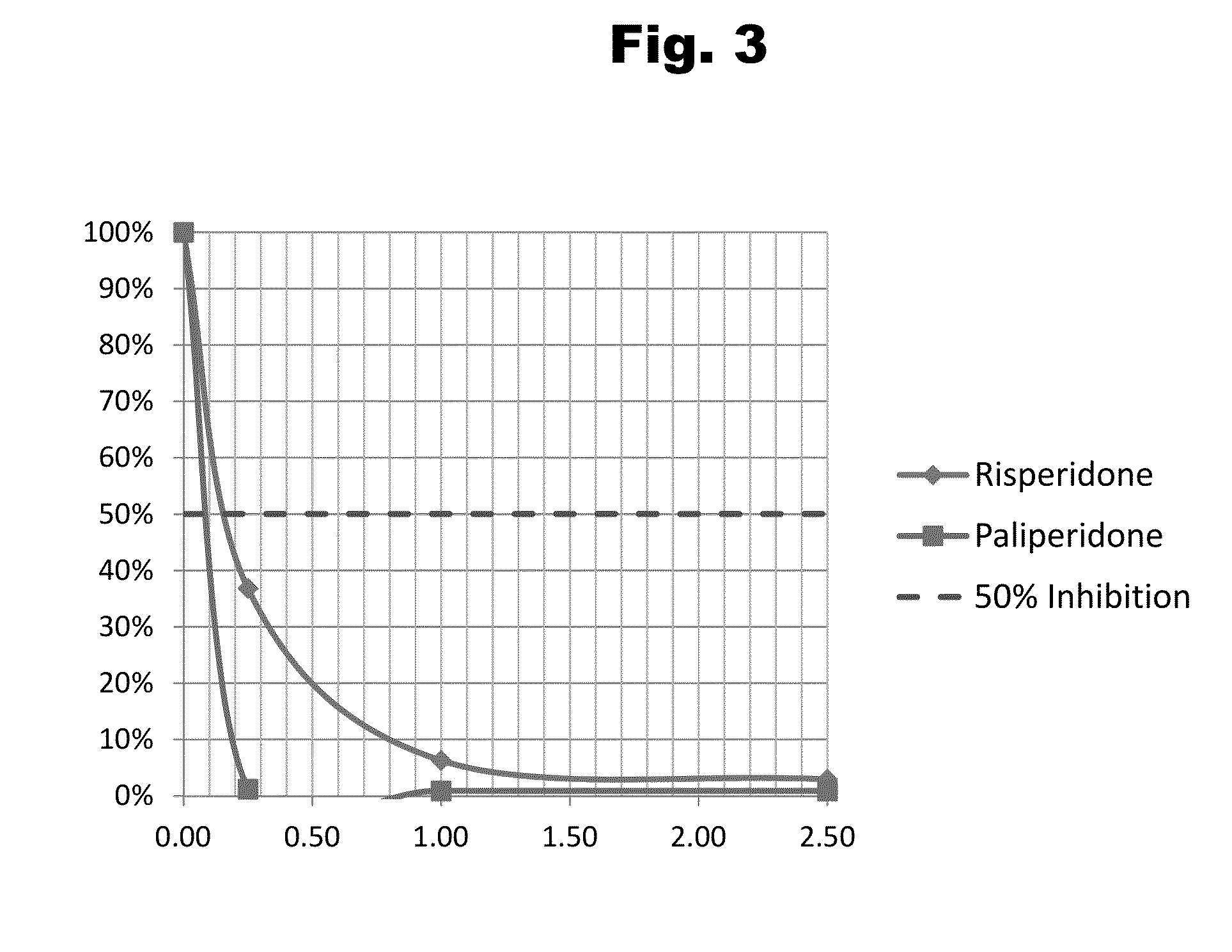
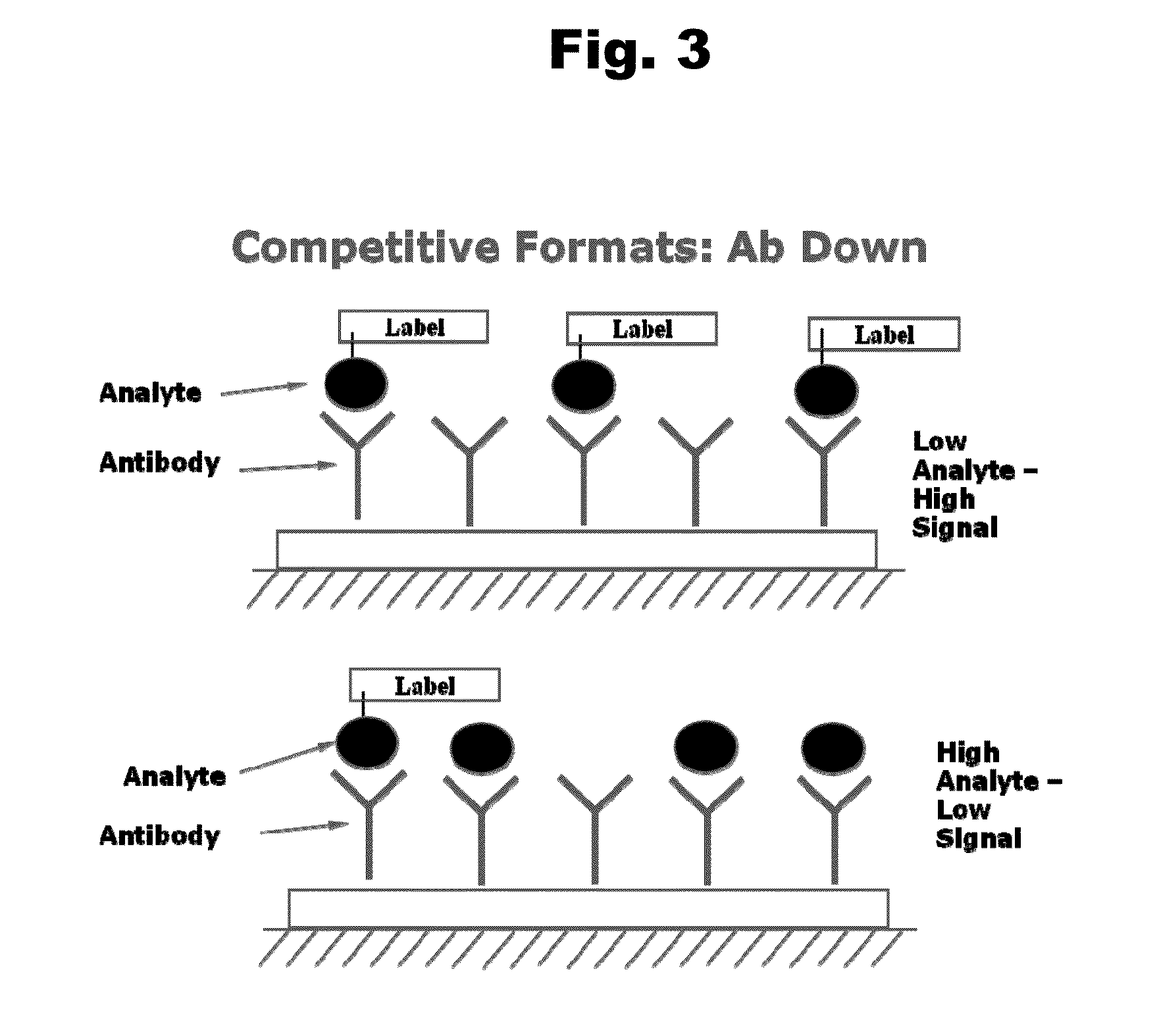
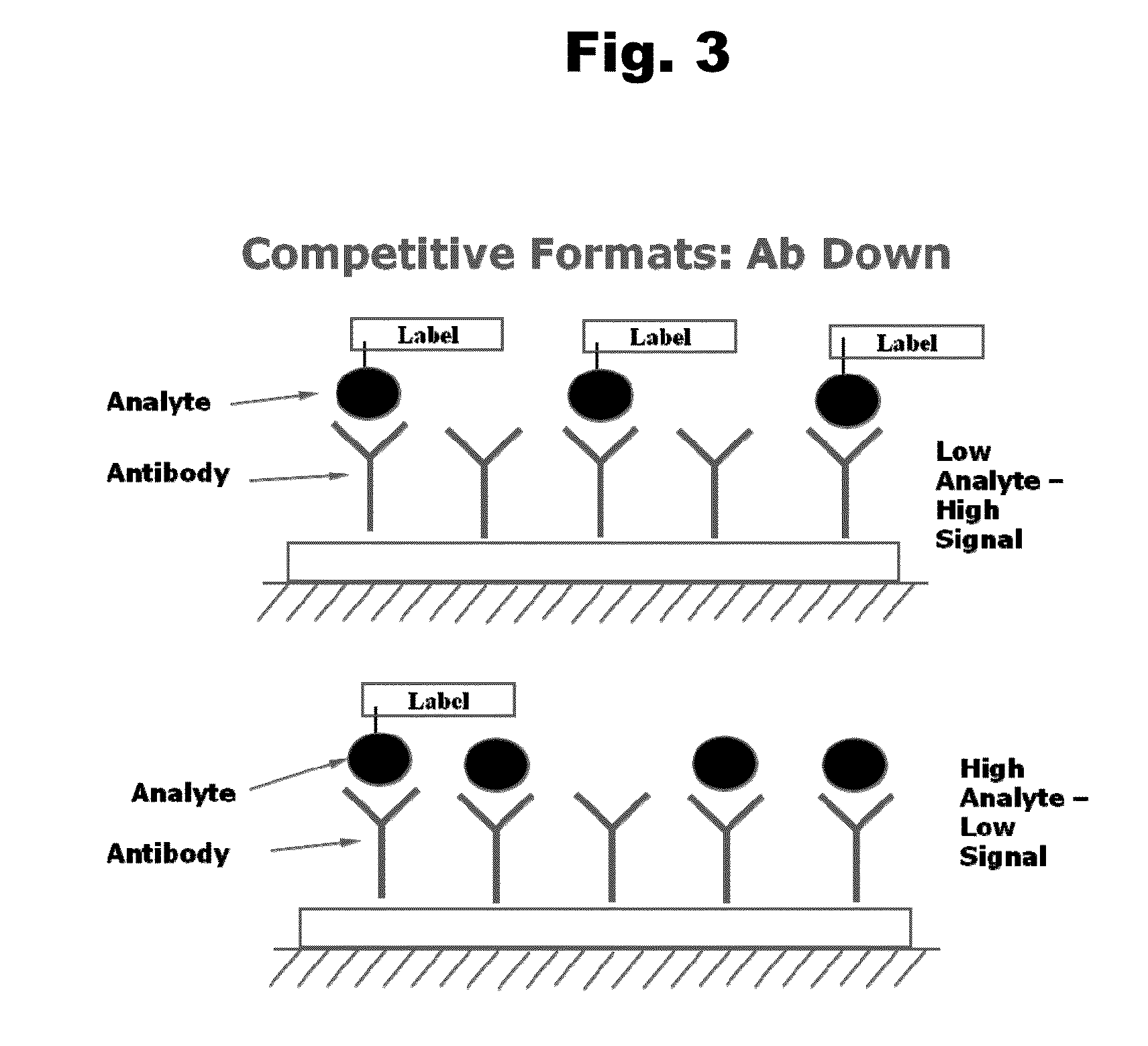
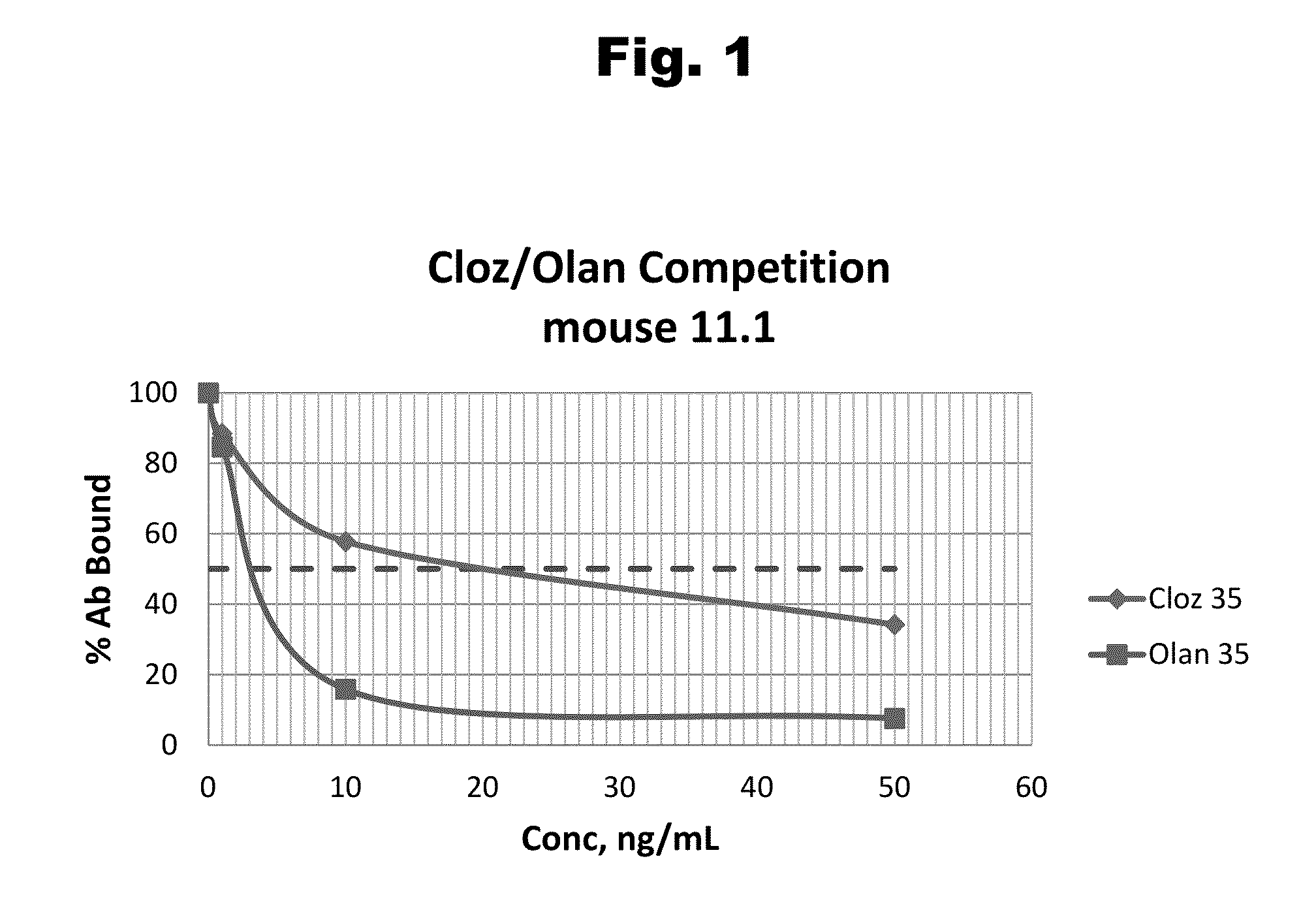
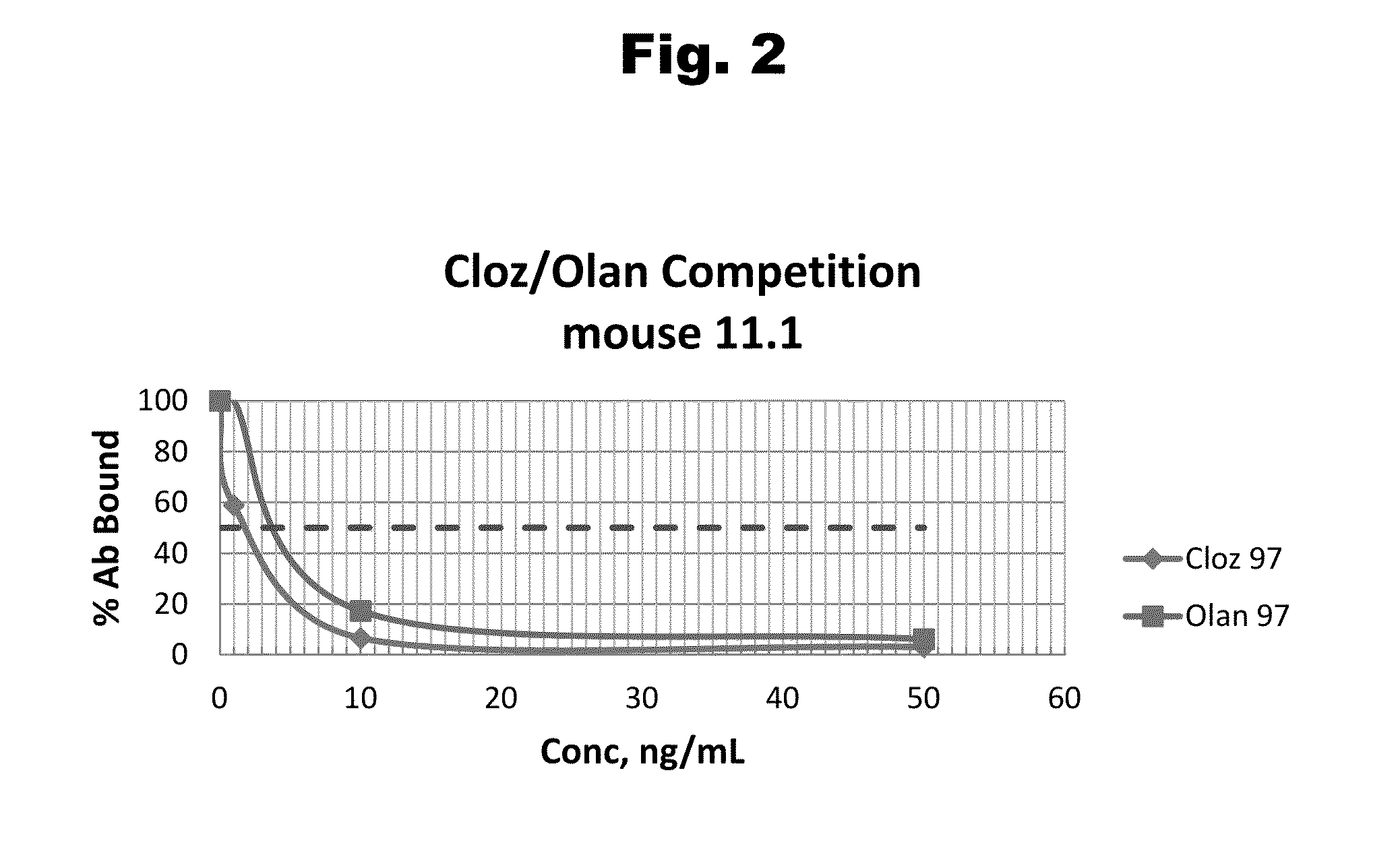
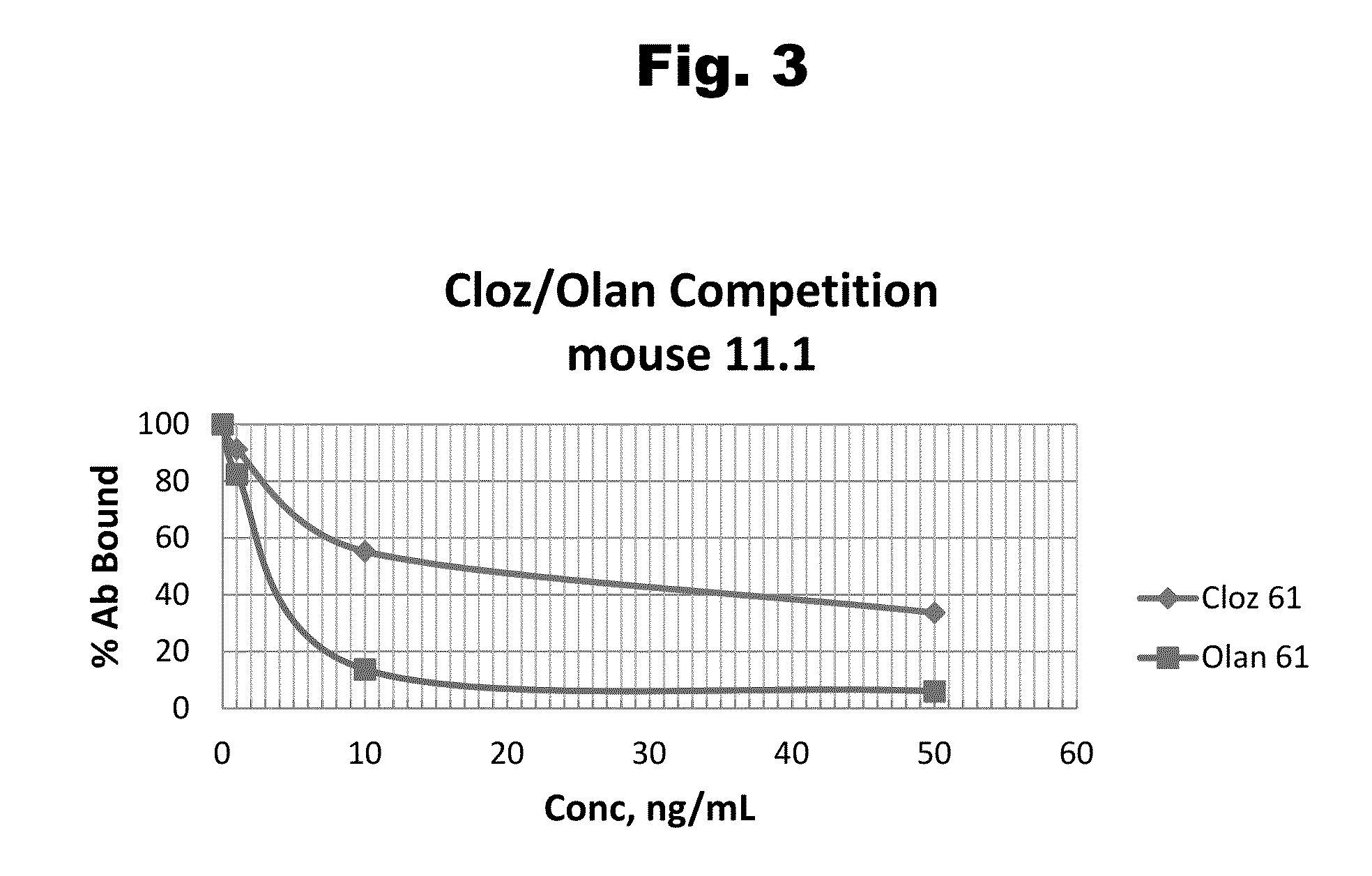
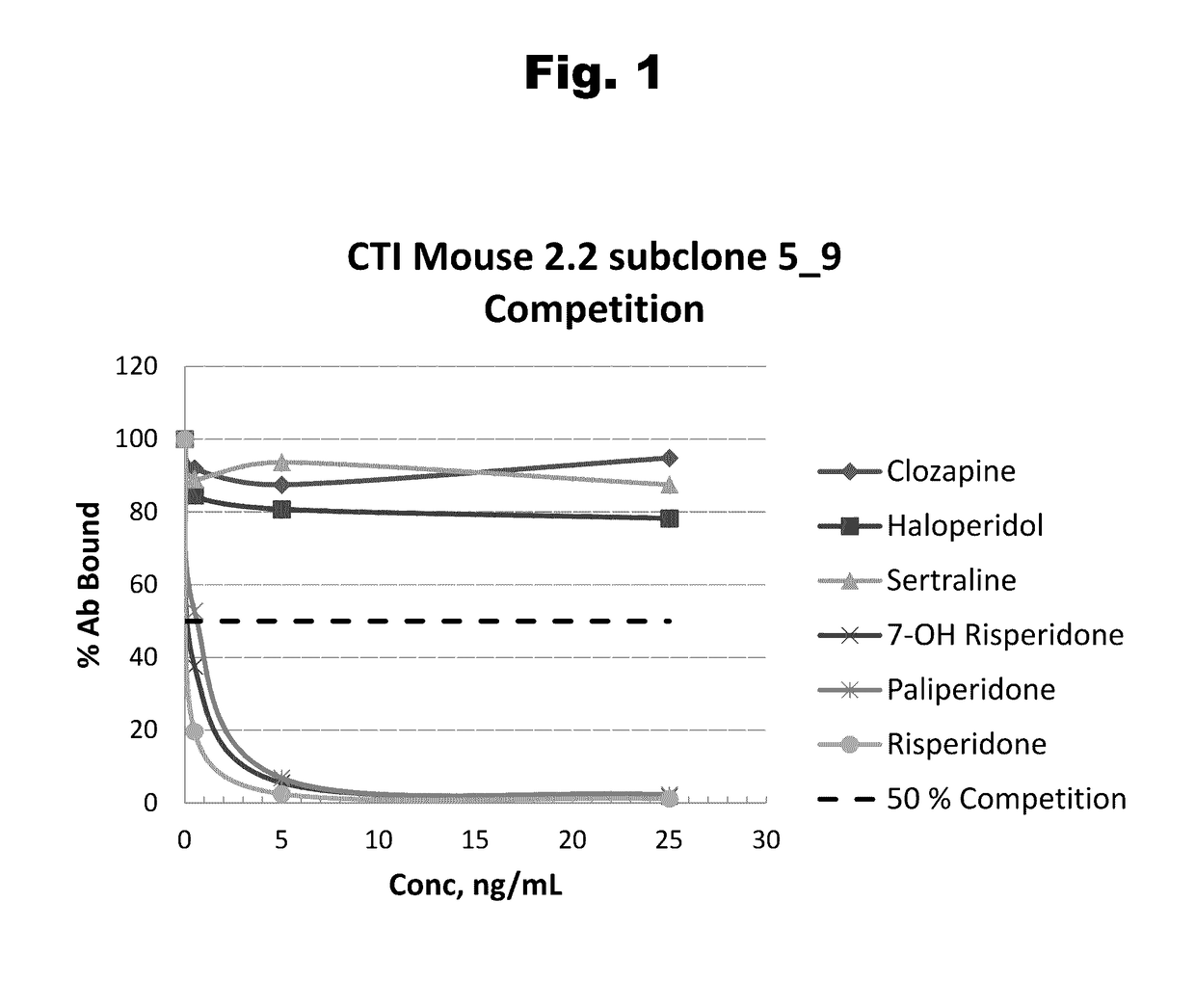
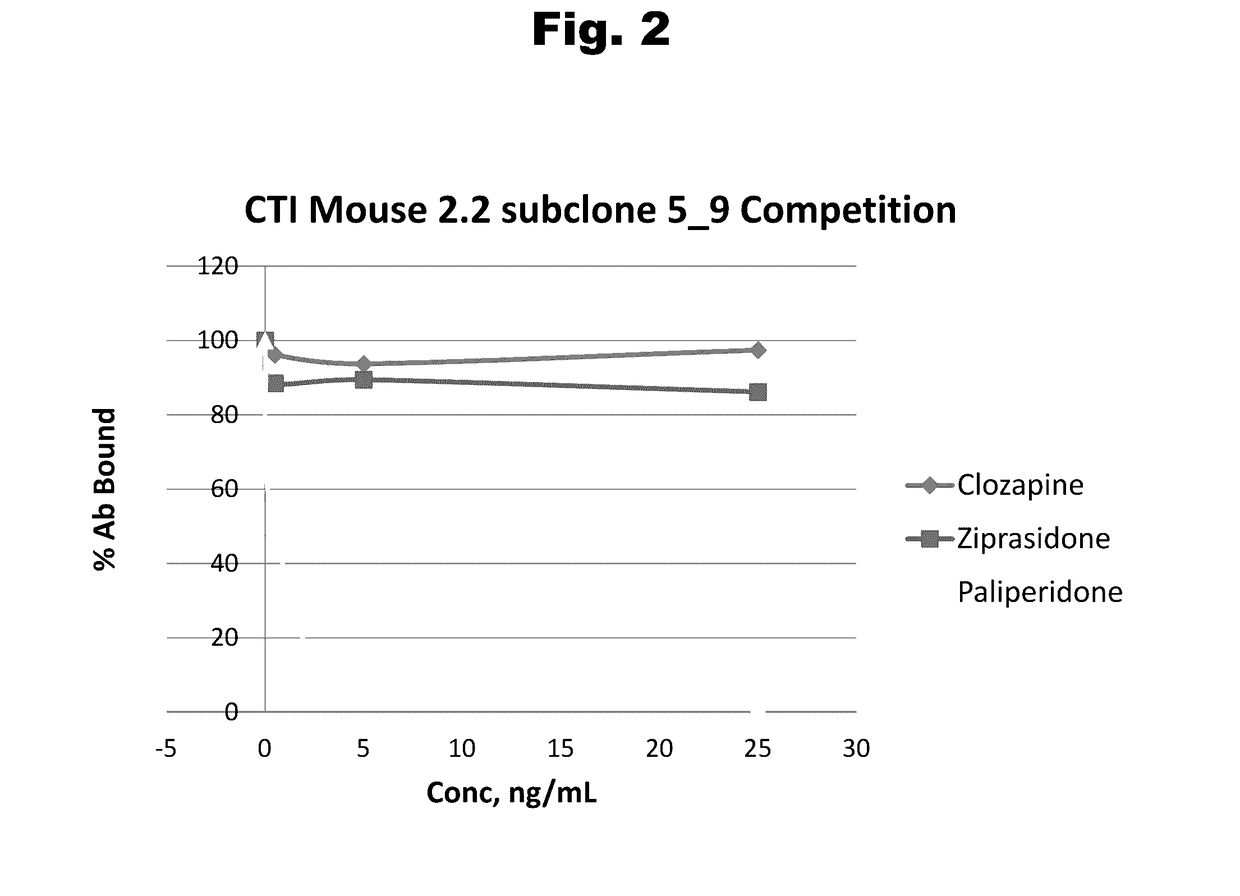
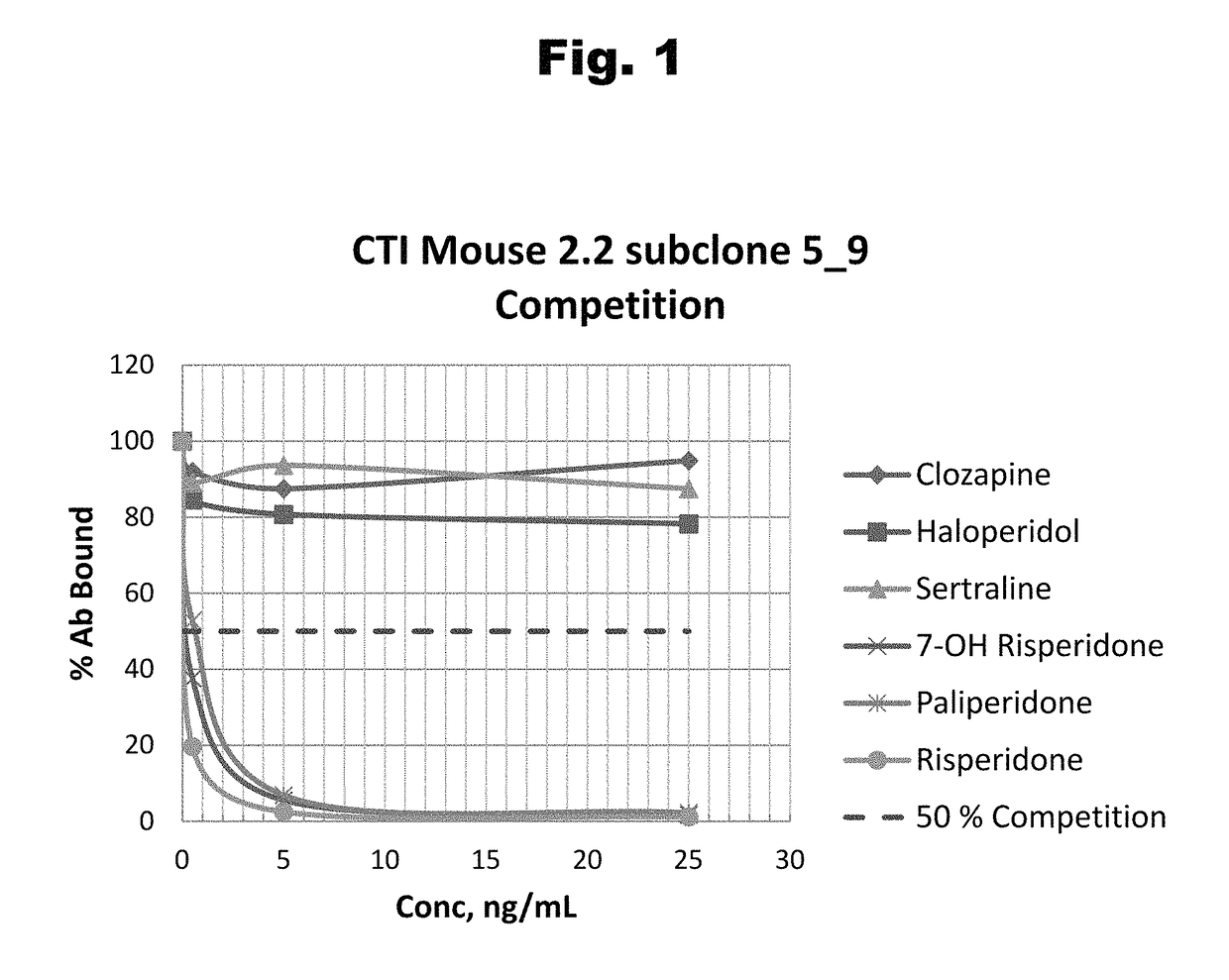
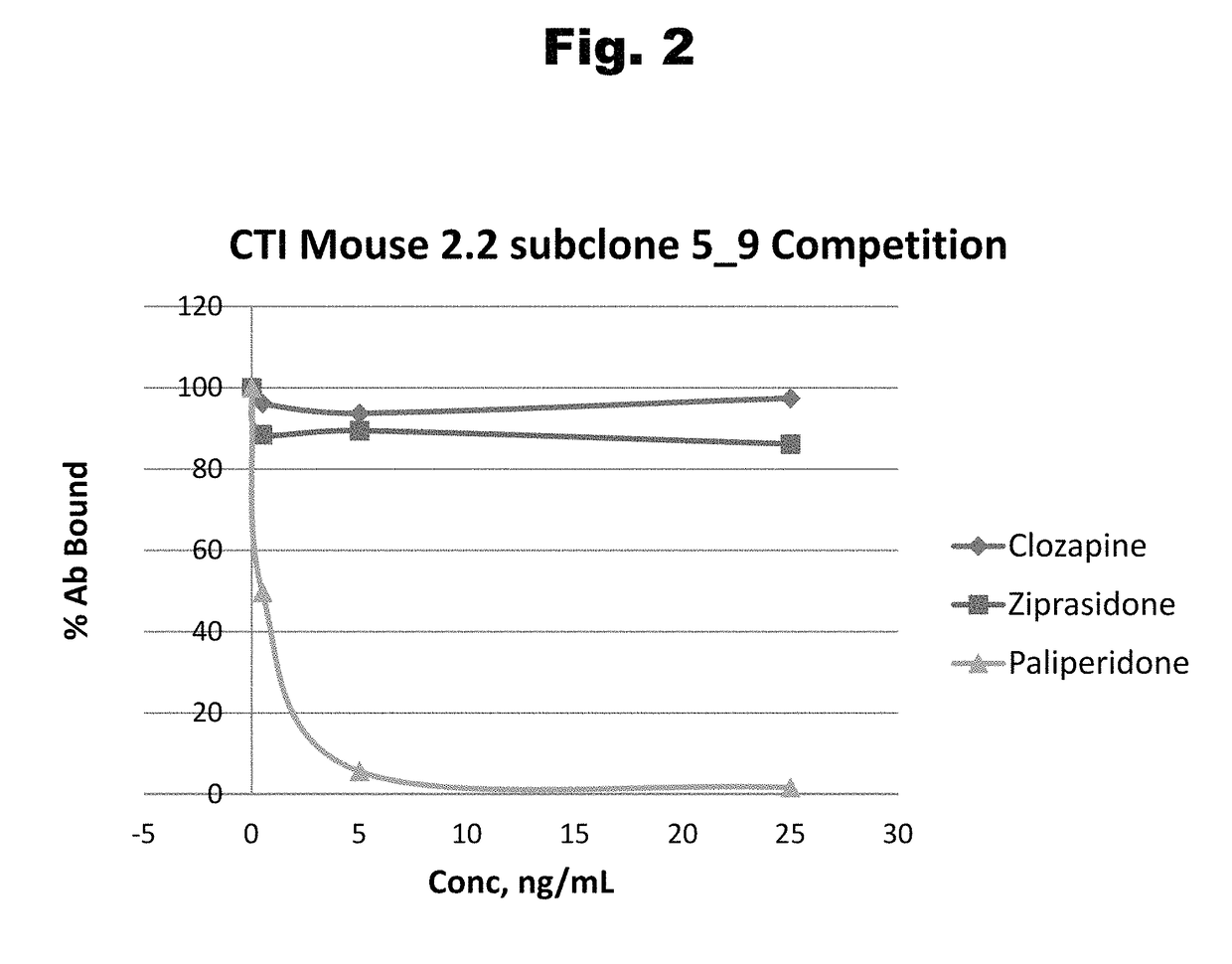
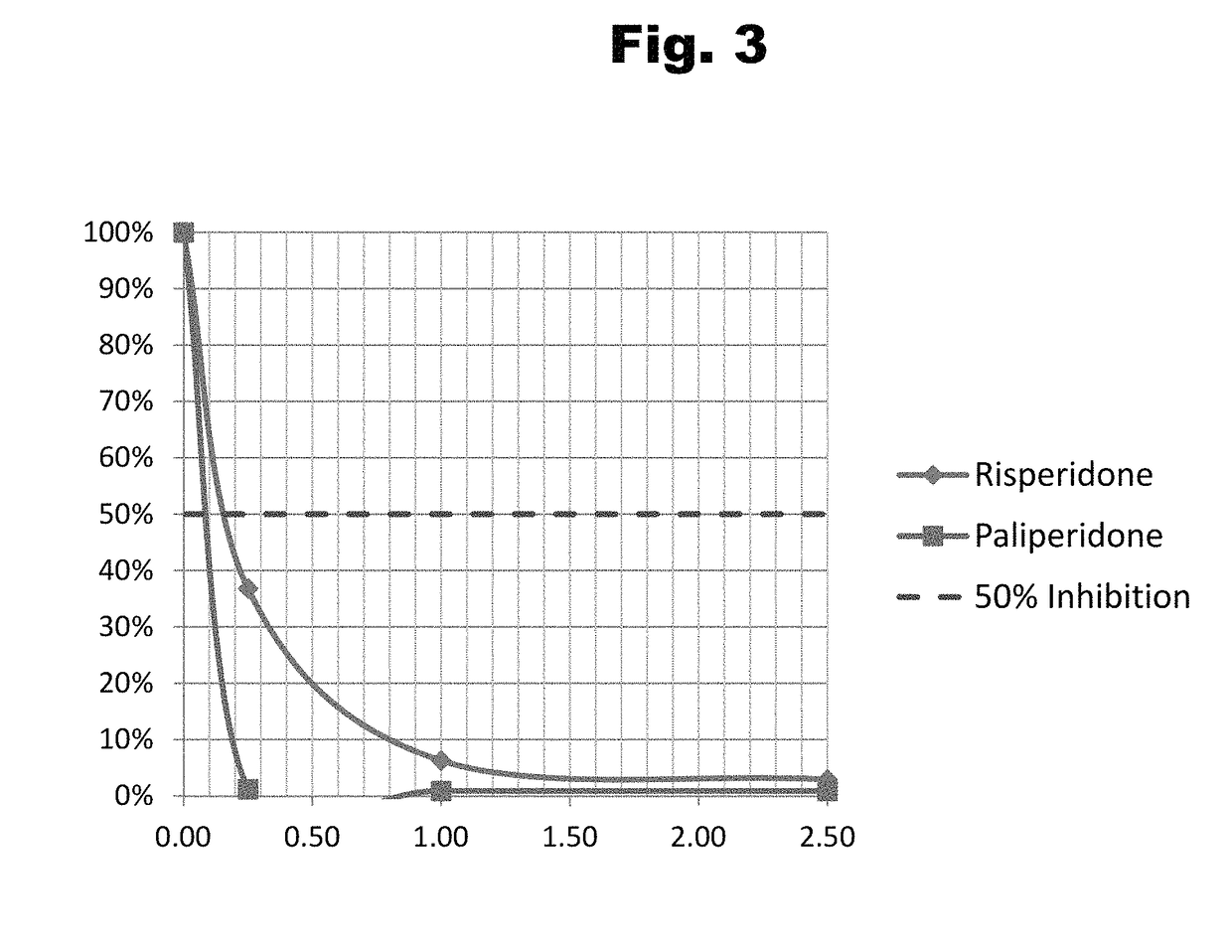
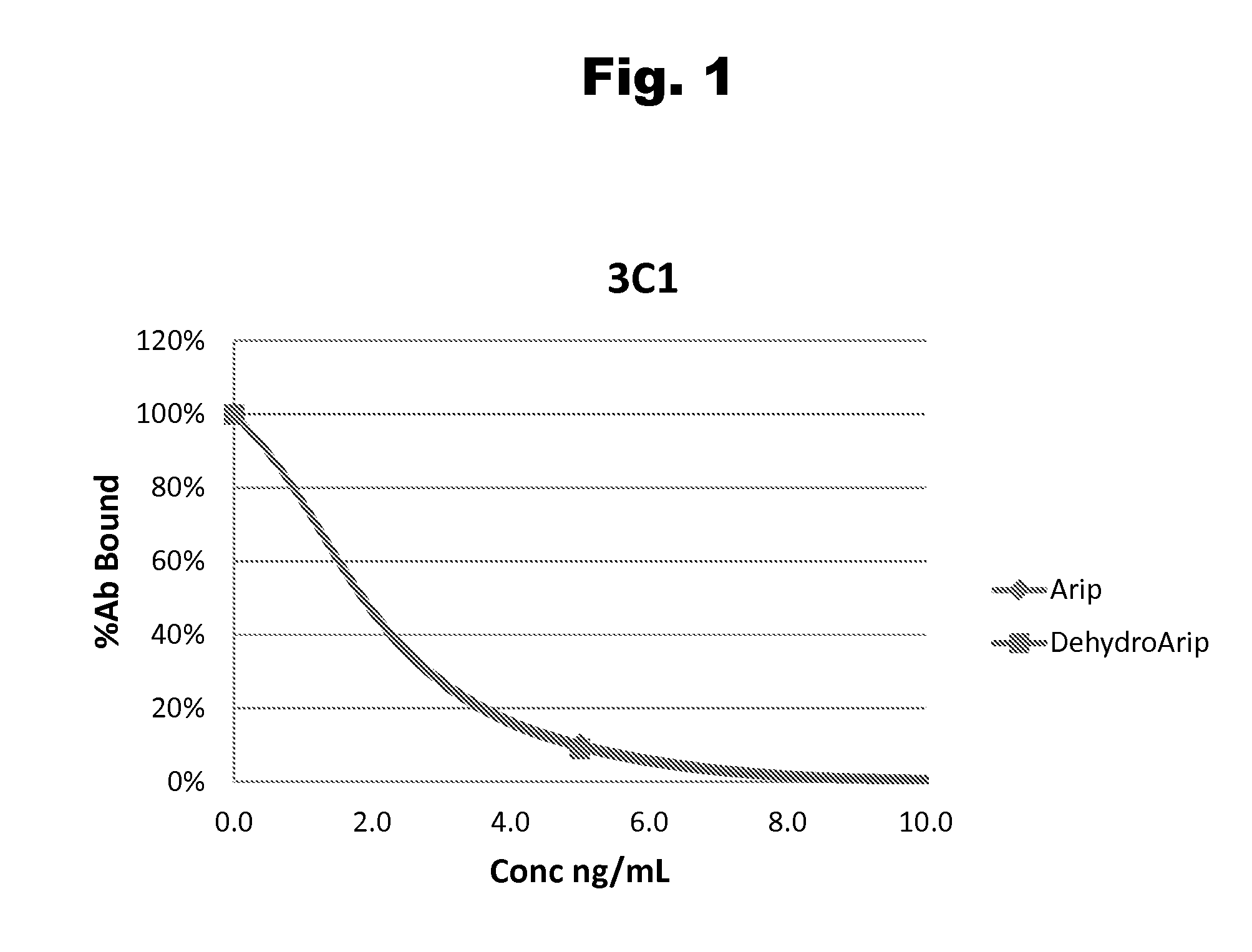
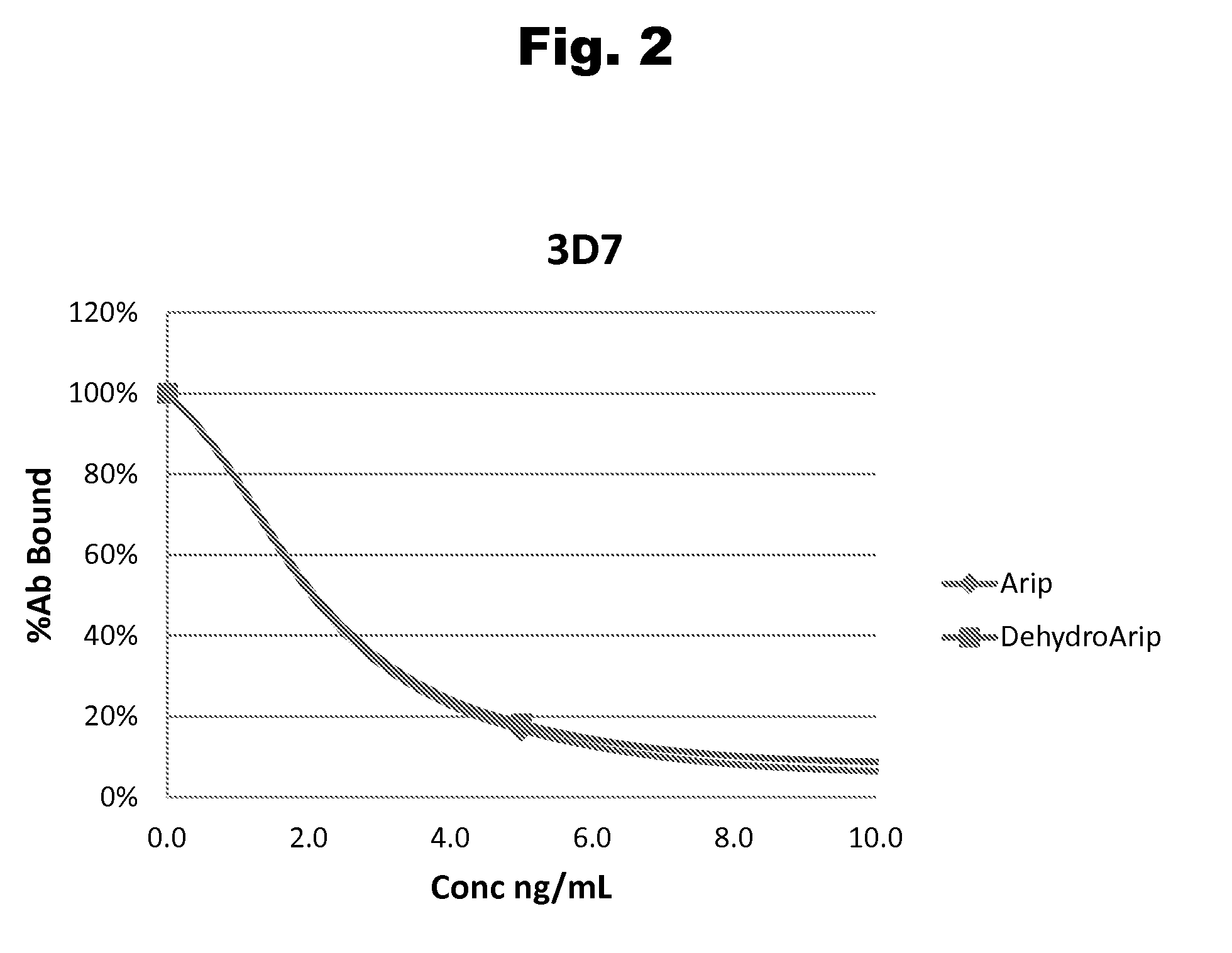
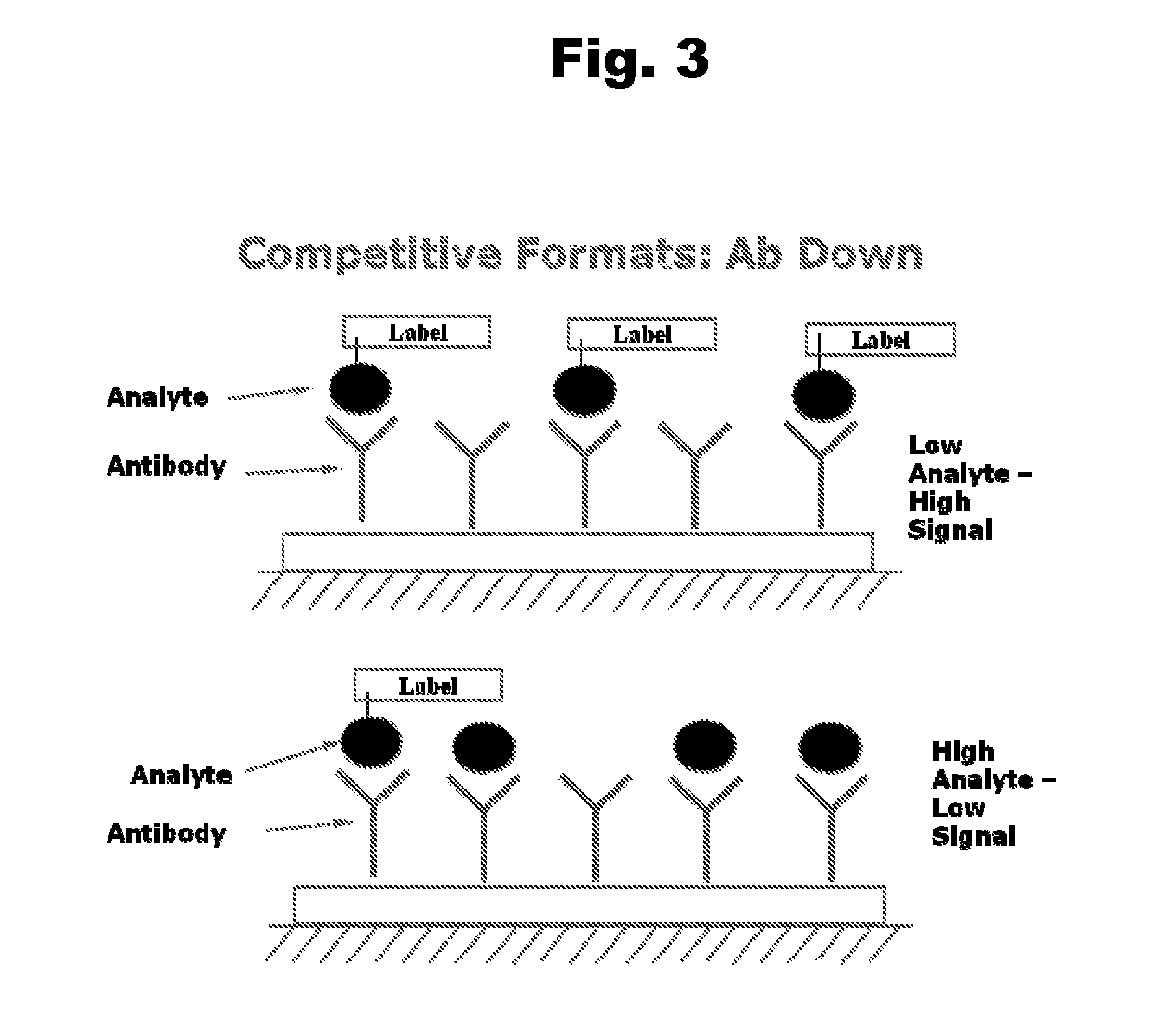
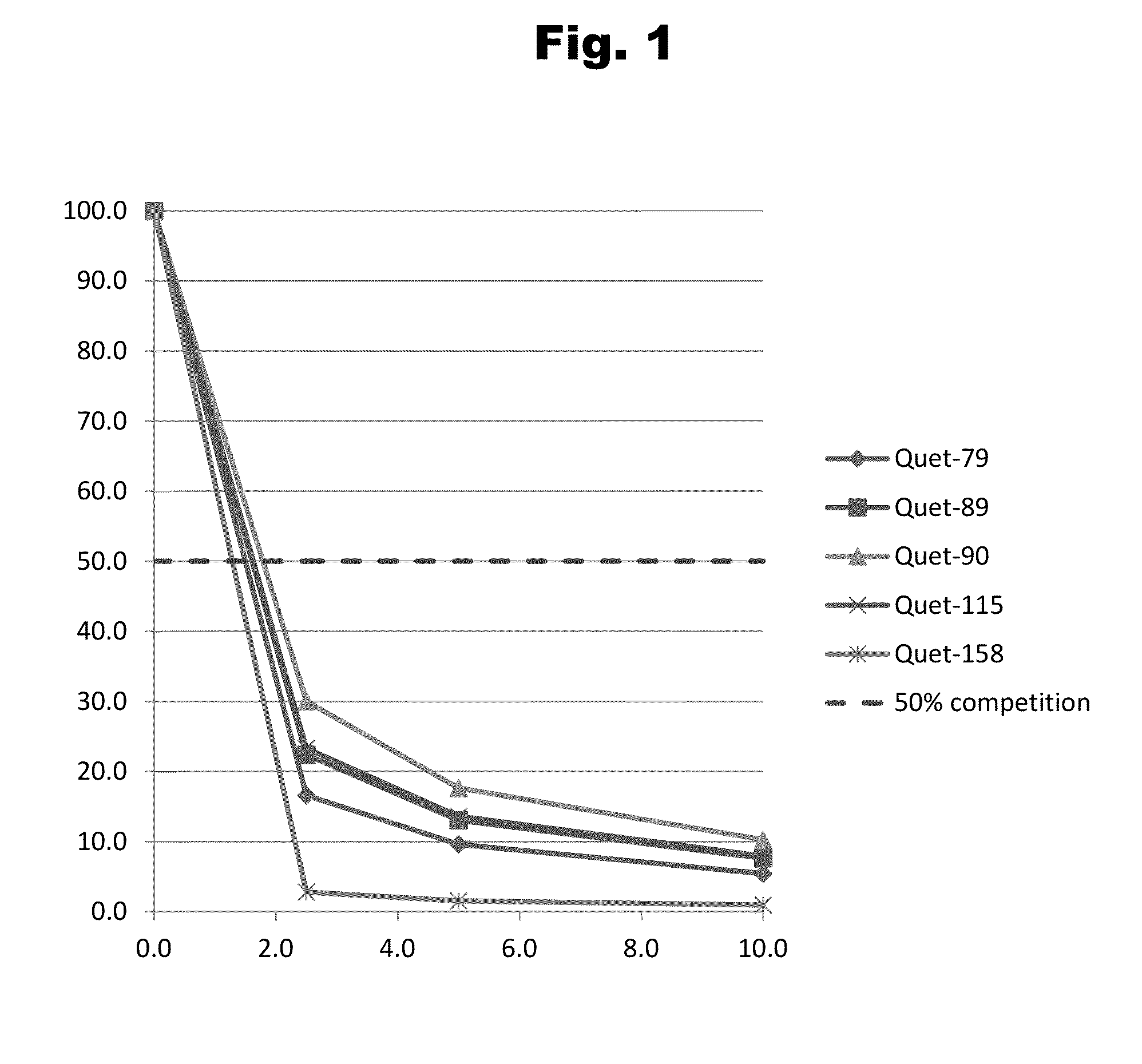
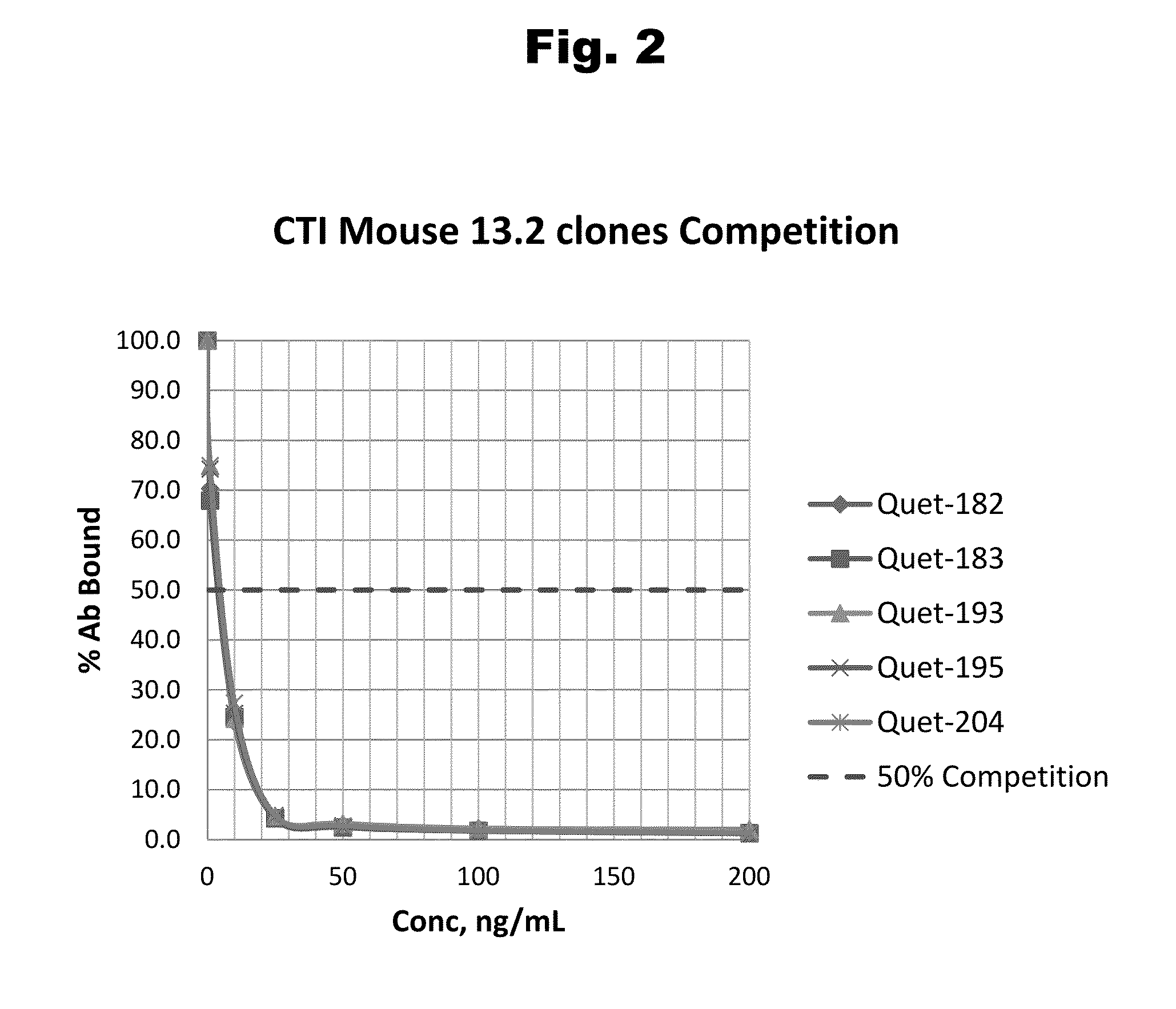
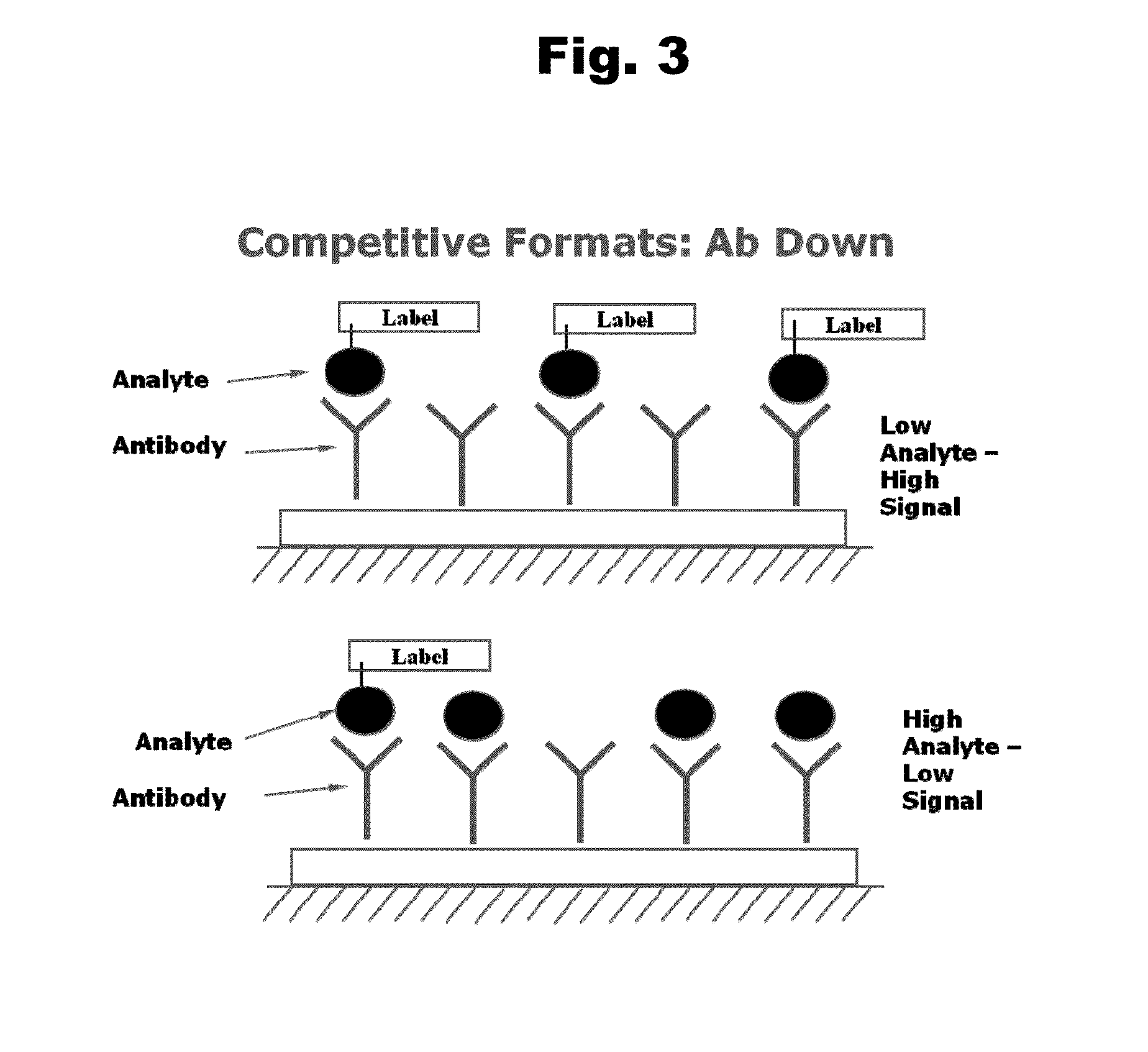
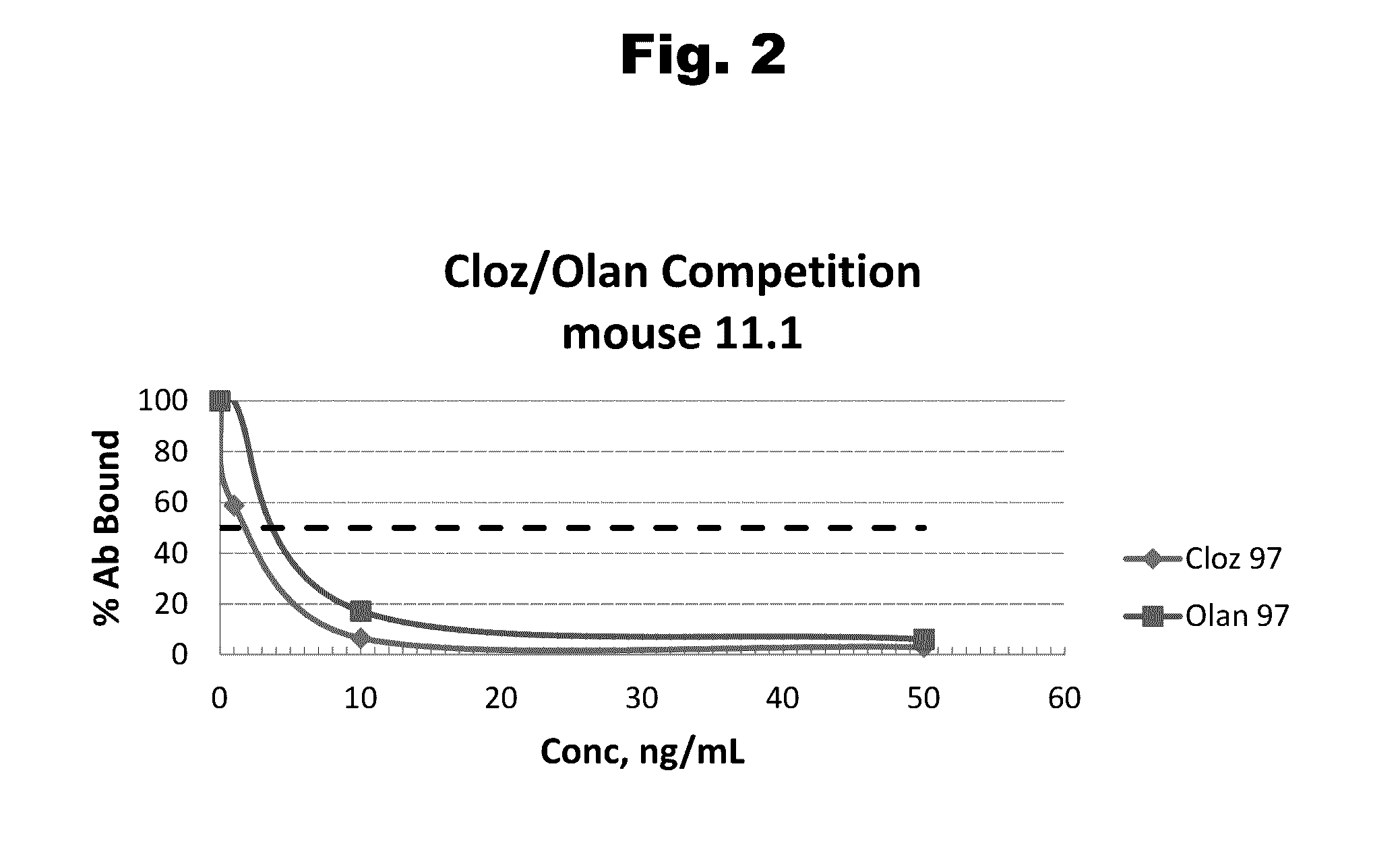
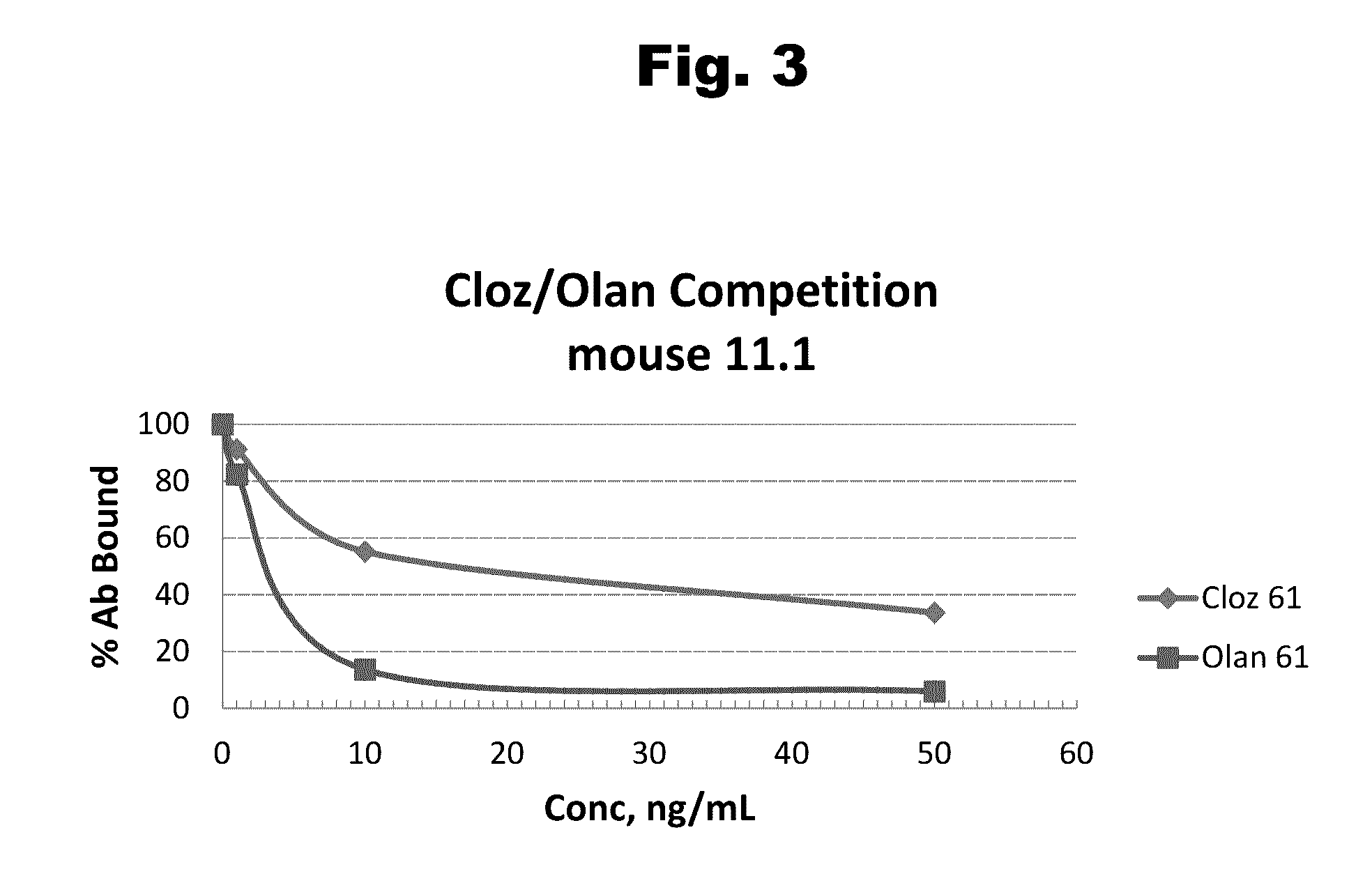
Antibodies to Risperidone Haptens and Use Thereof
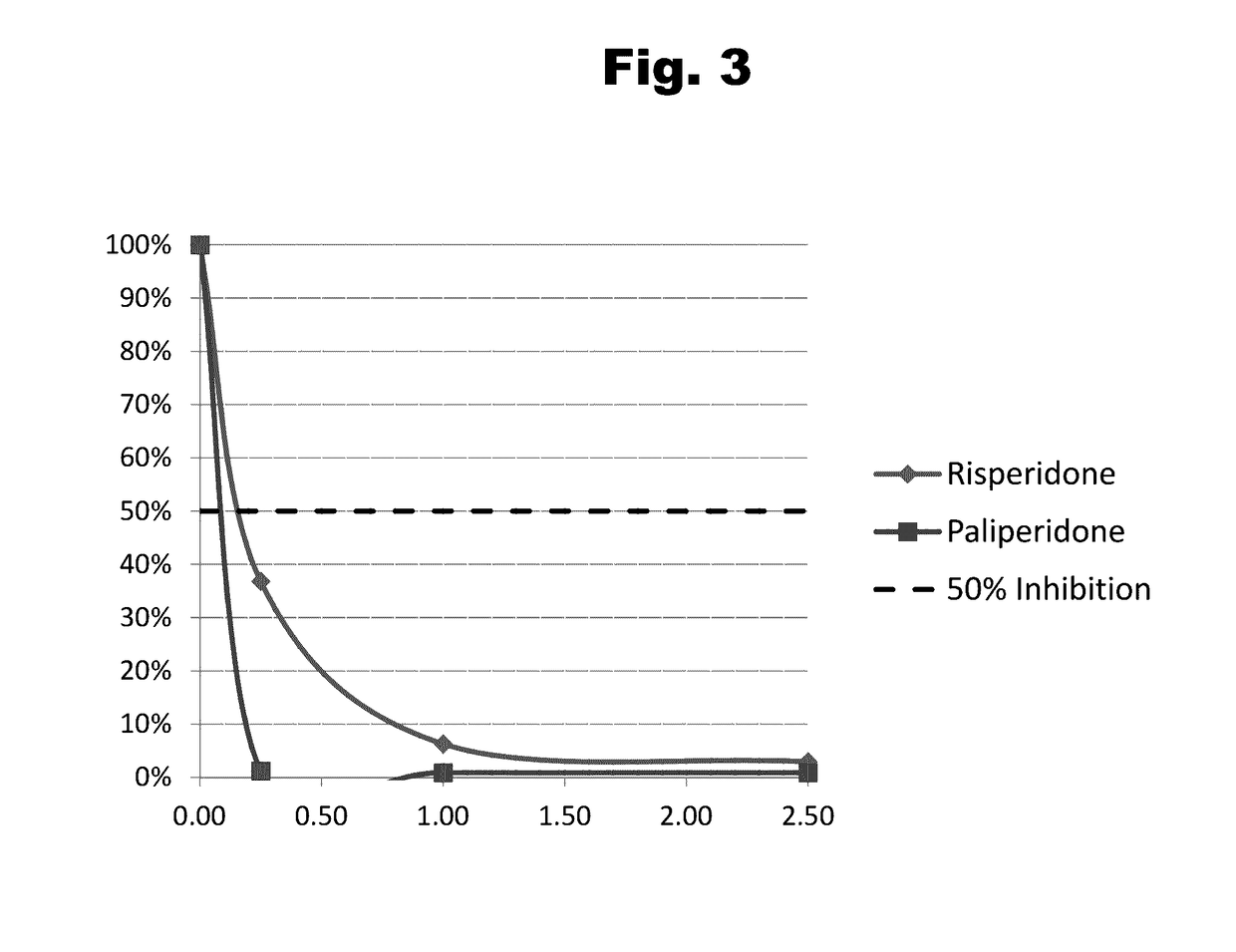
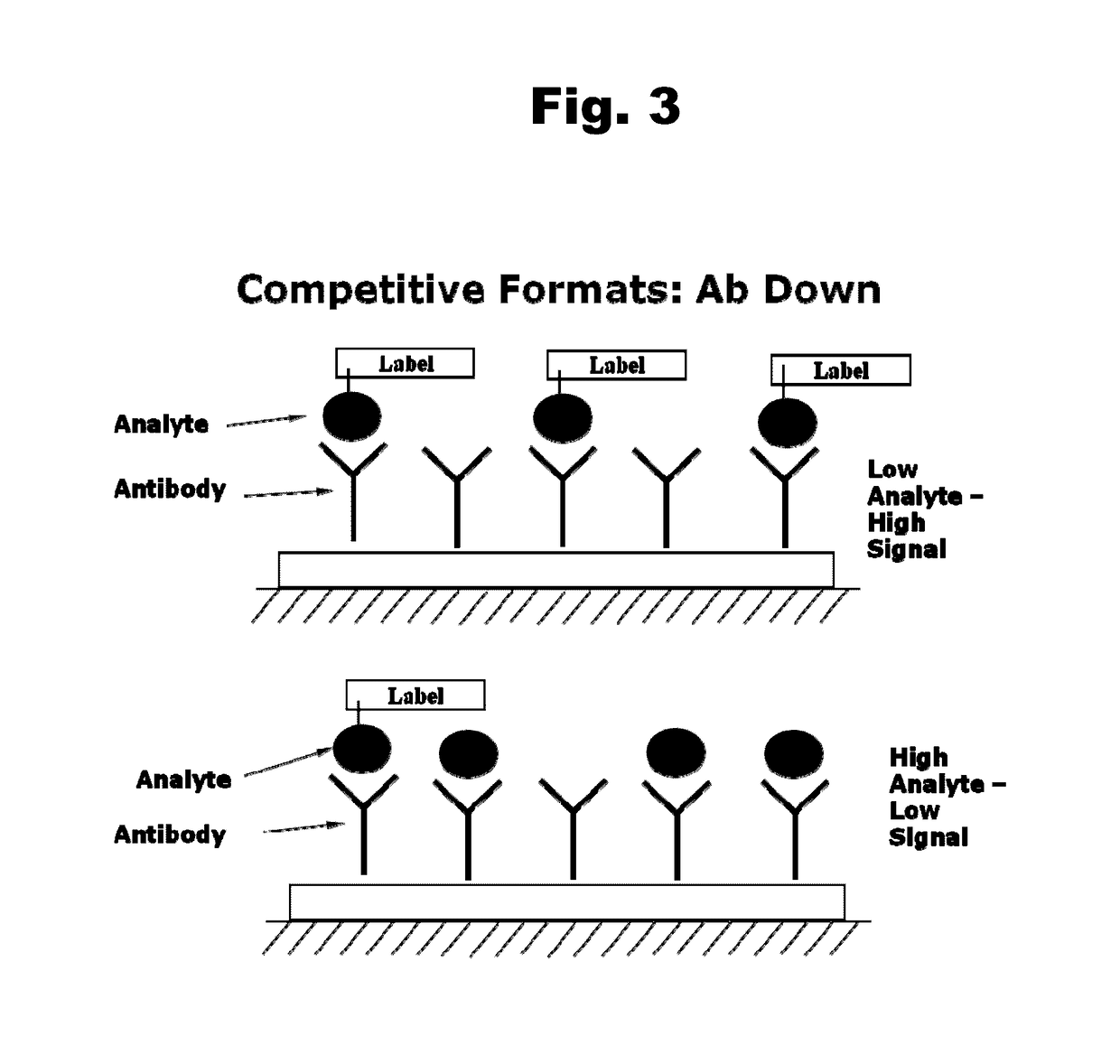
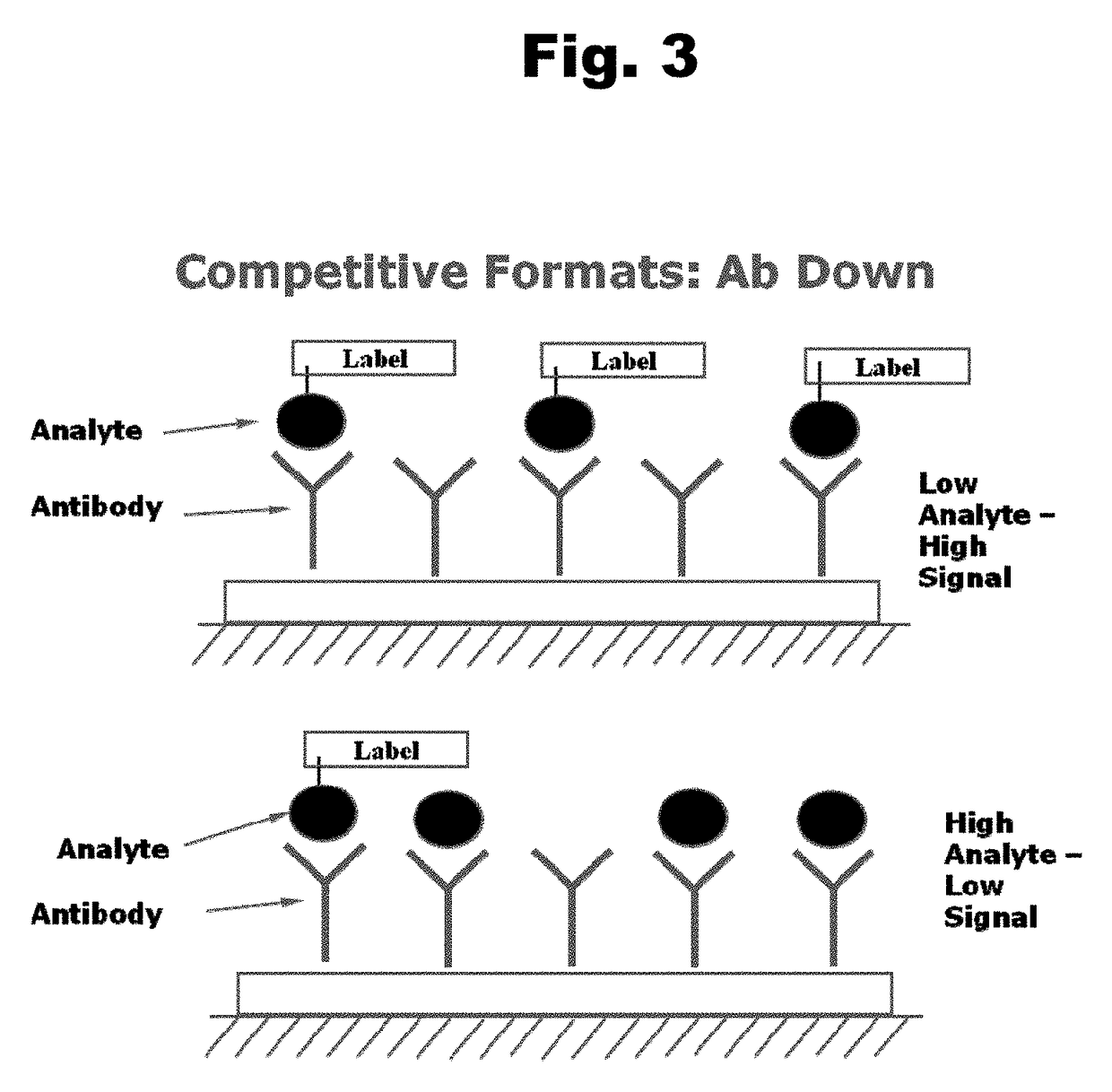
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Sustained-release microsphere containing risperidone and preparation method thereof
InactiveCN101292960AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsNervous disorderMicrosphereAntipsychotic Medications
The invention relates to a sustained-release microsphere of antipsychotic drug risperidone and the preparation method thereof.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
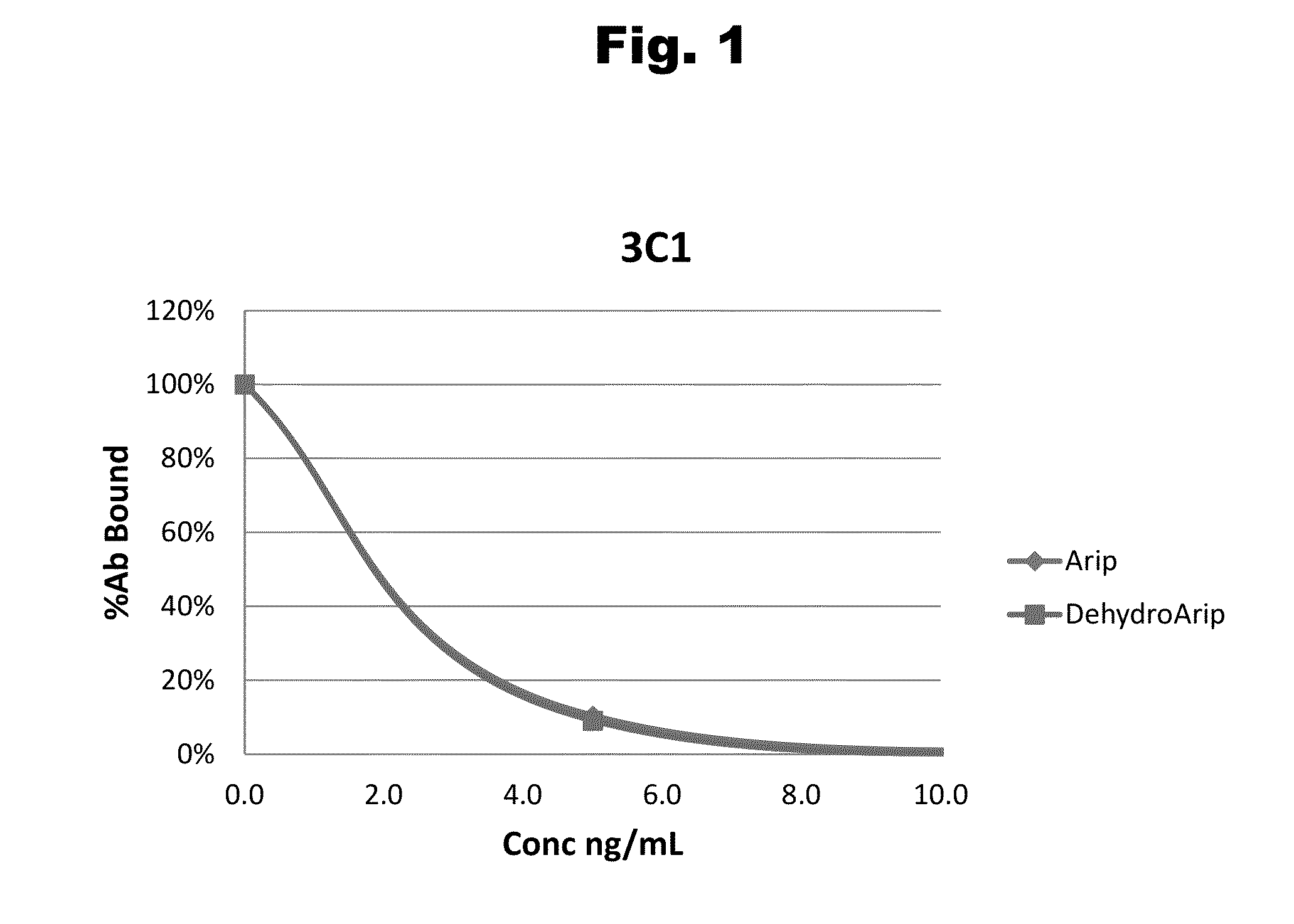
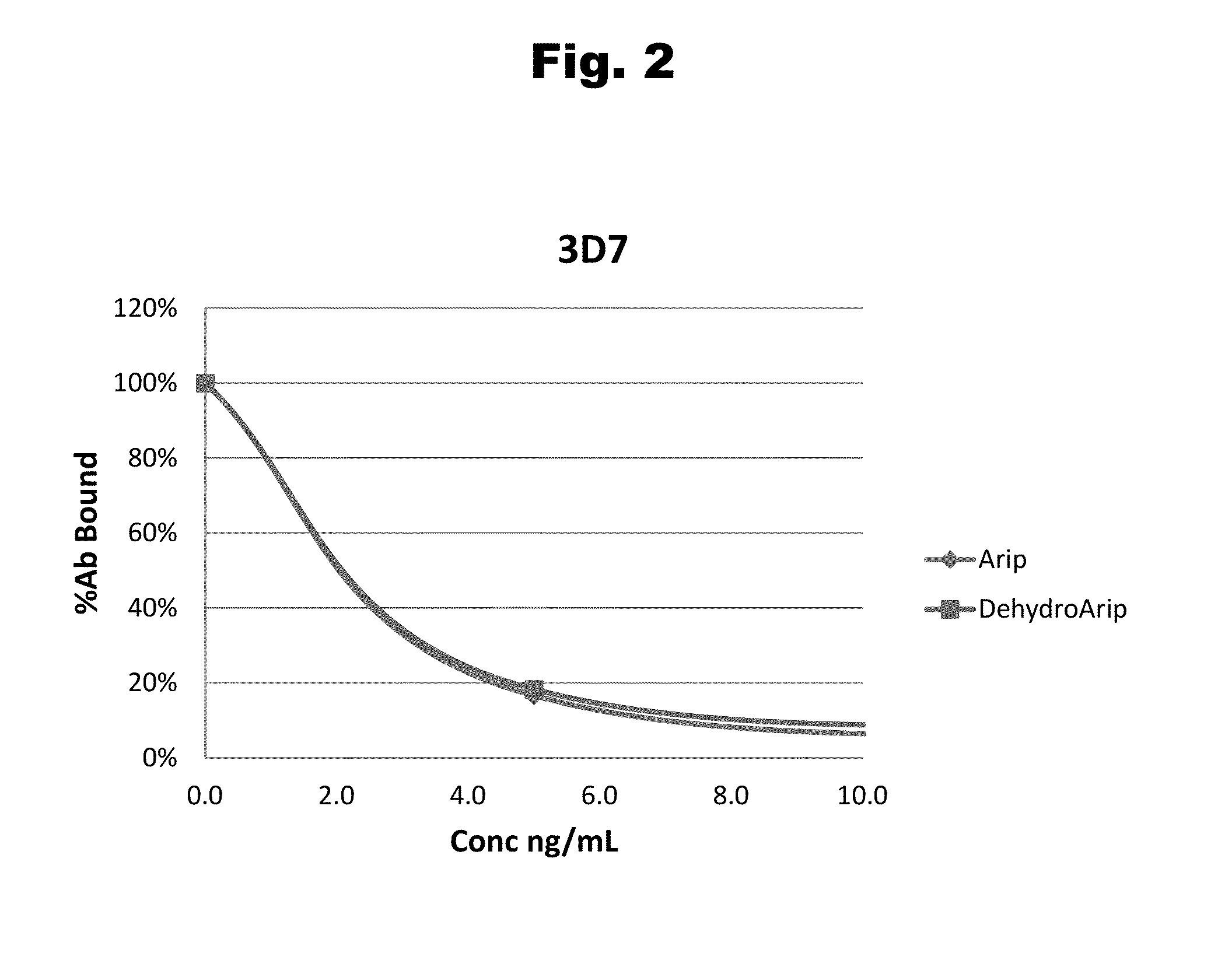
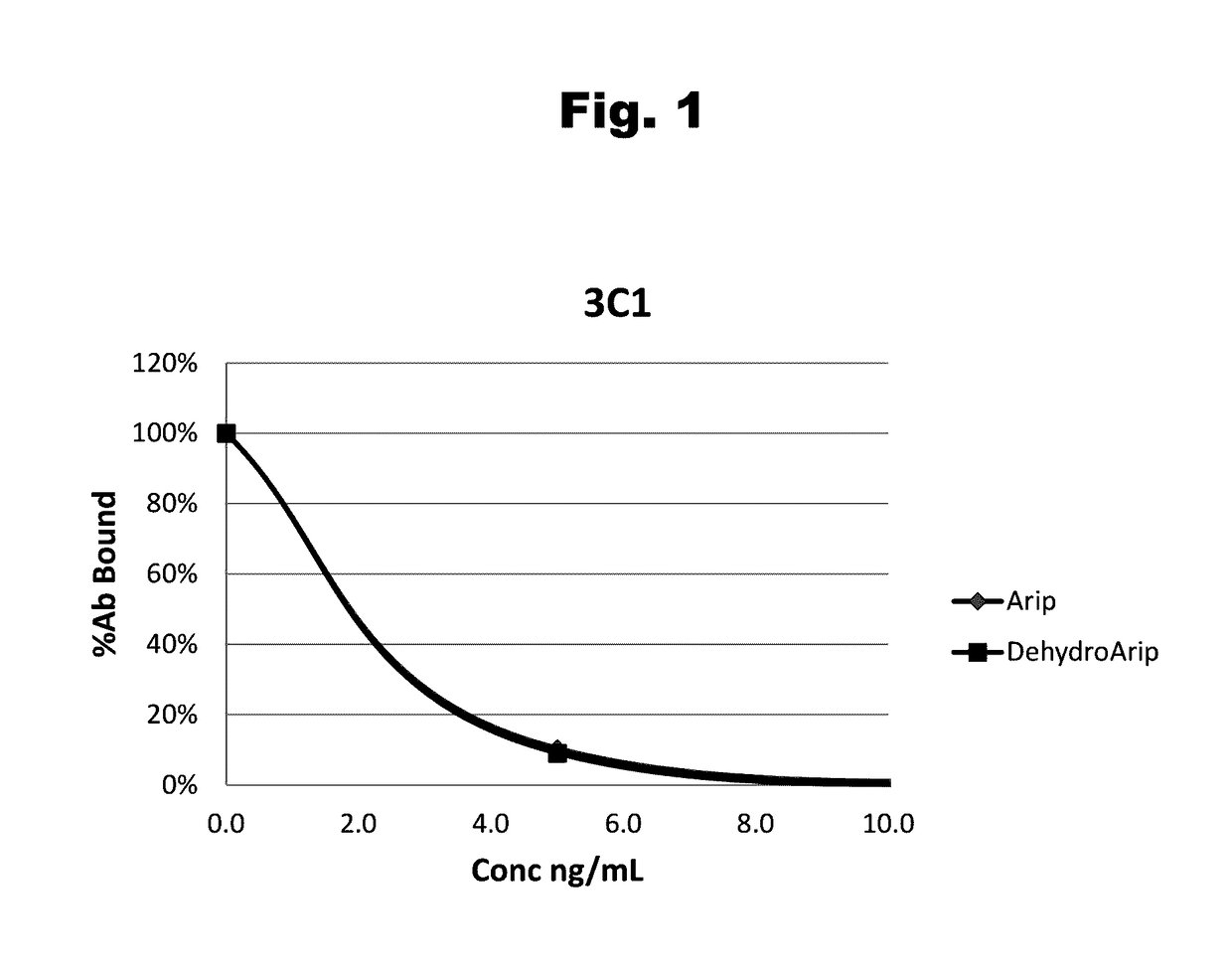
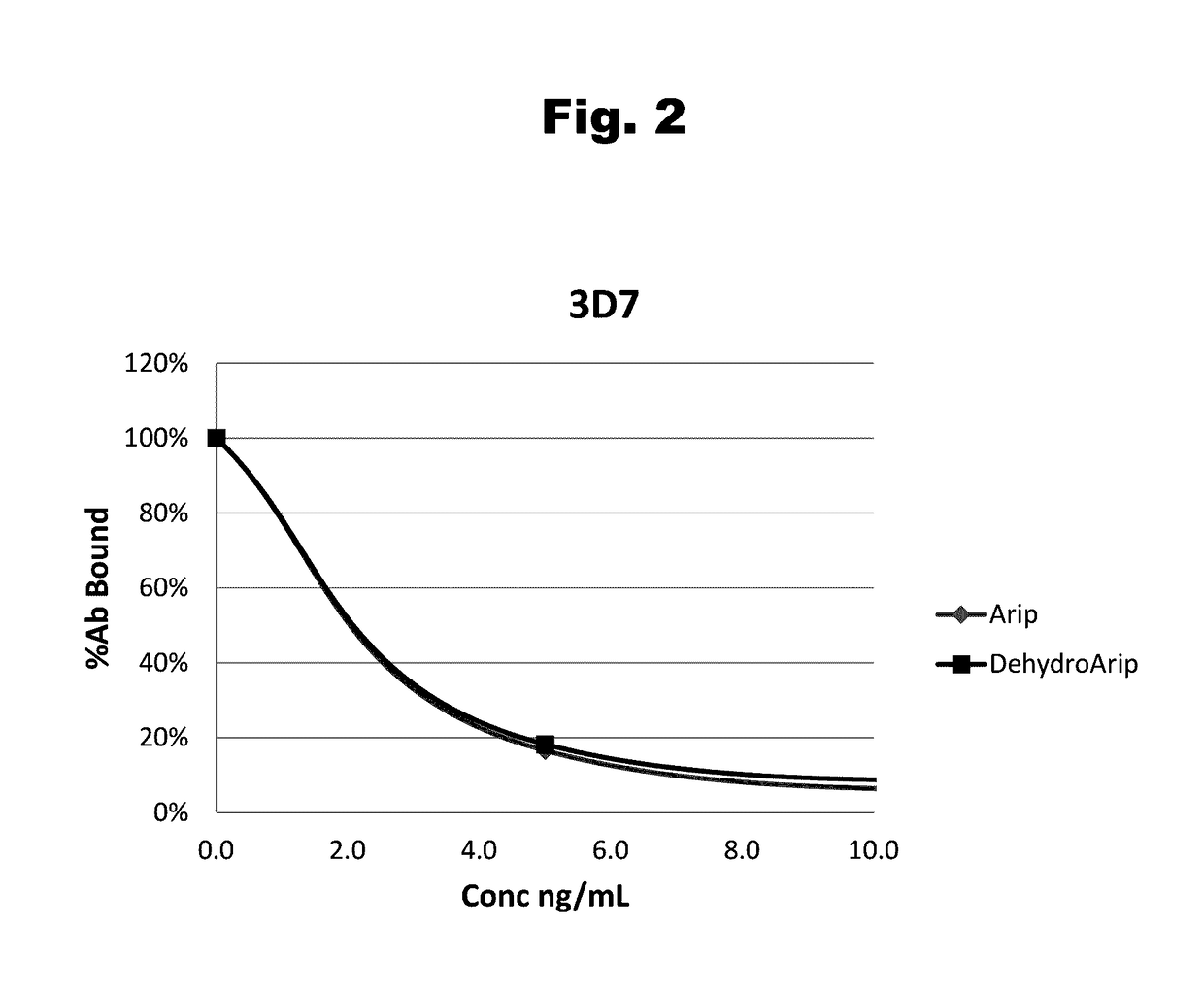
Antibodies to Aripiprazole Haptens and Use Thereof
ActiveUS20140057299A1Bioreactor/fermenter combinationsBiological substance pretreatmentsQuetiapineHapten
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
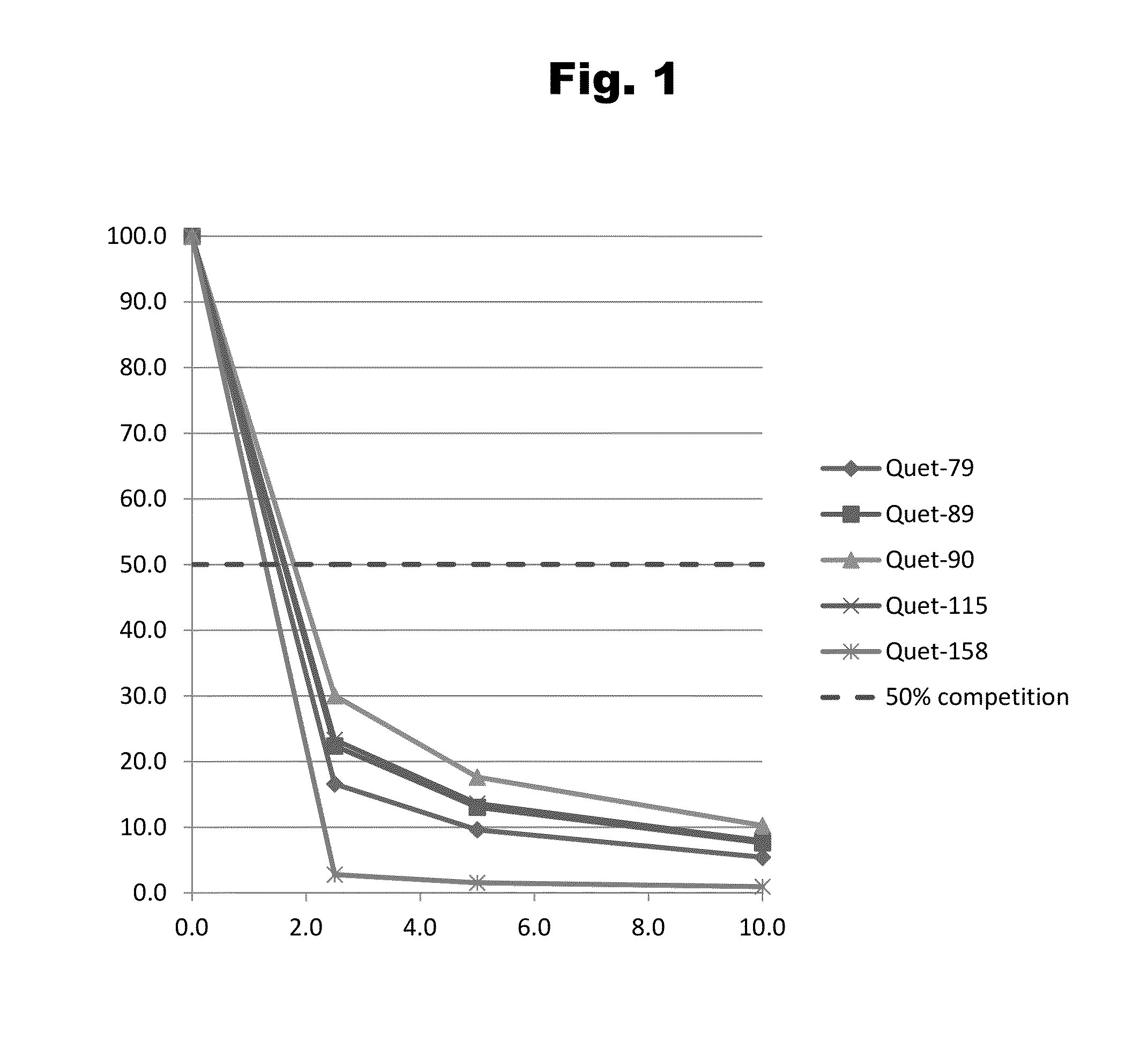
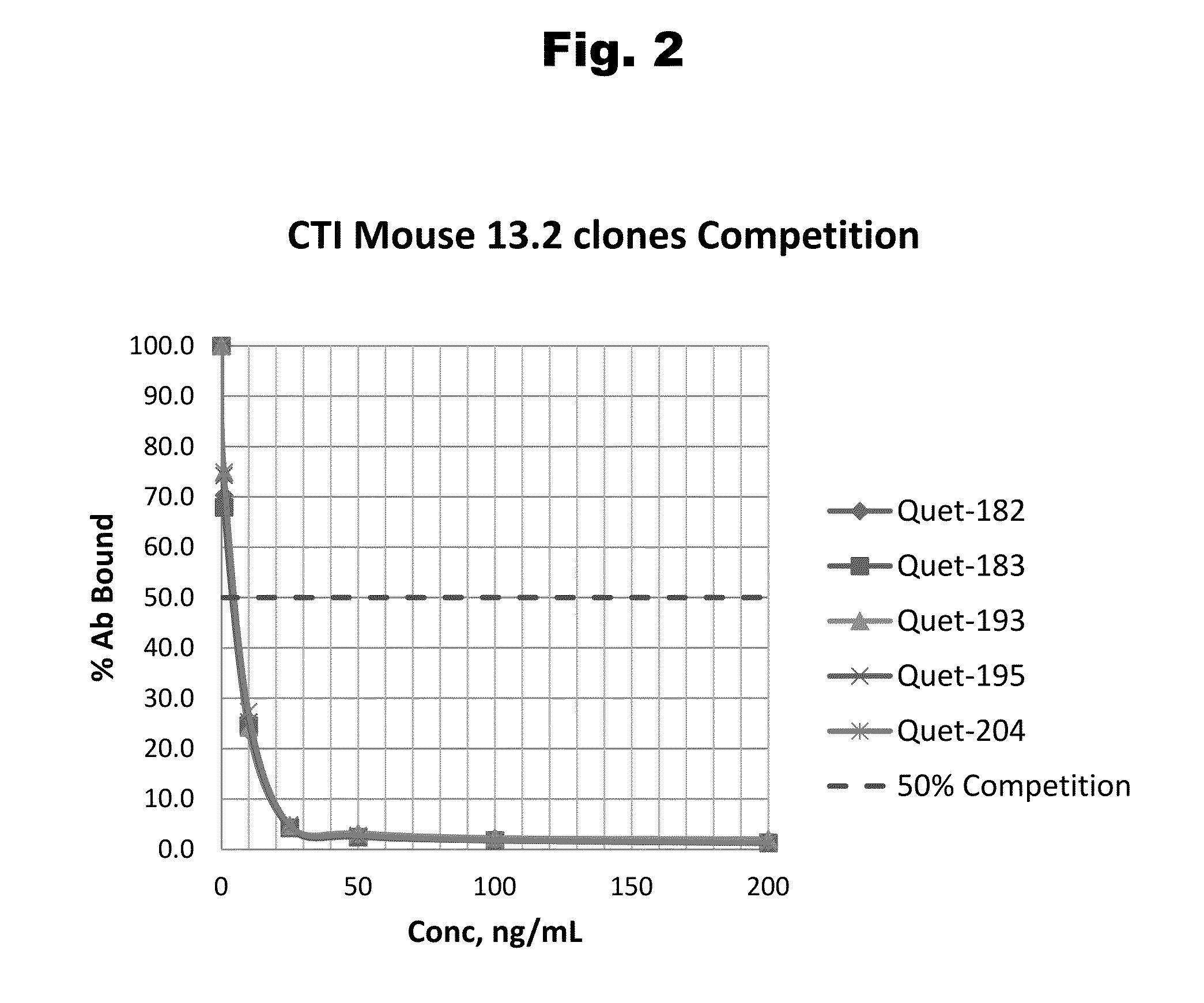
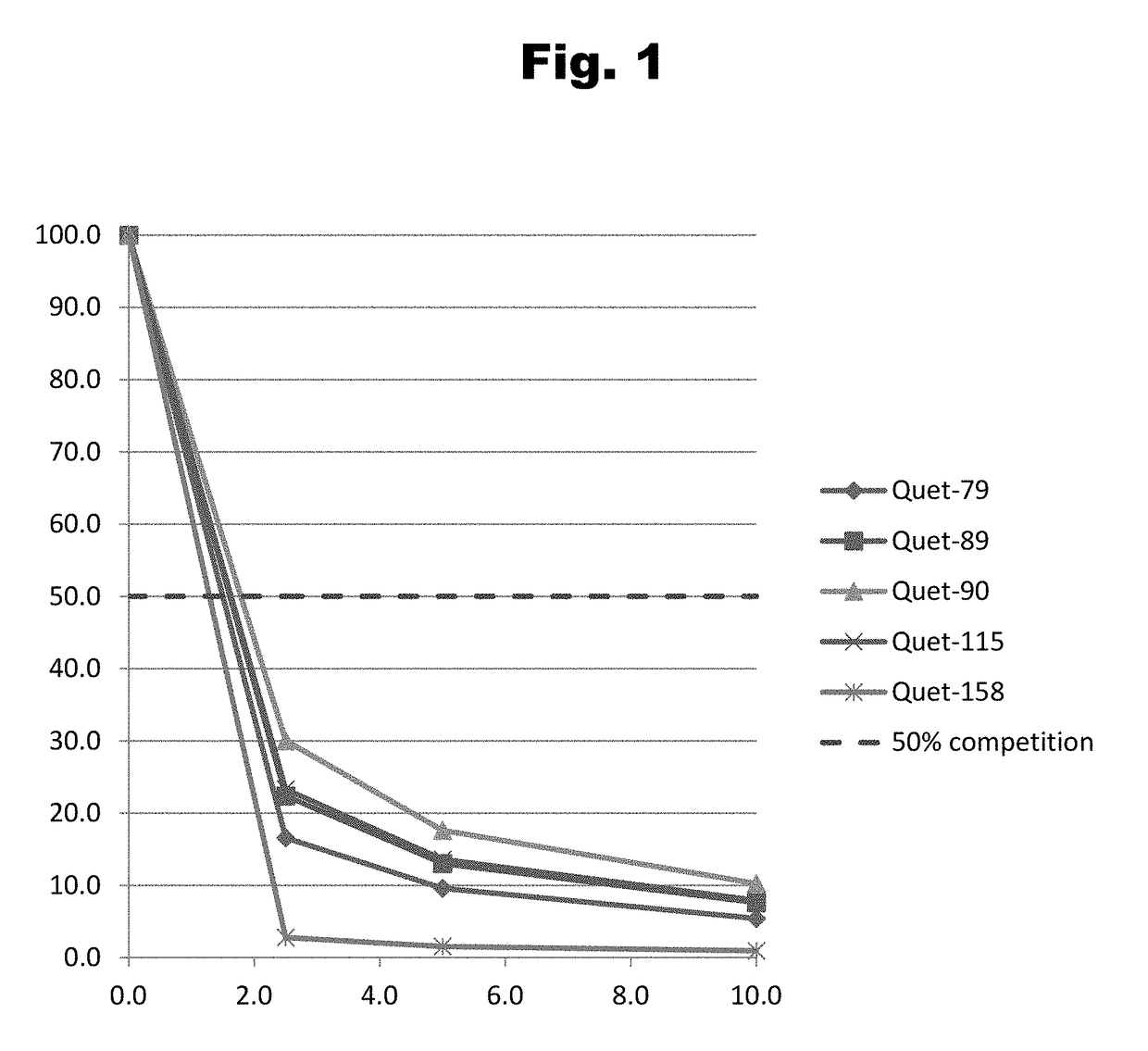
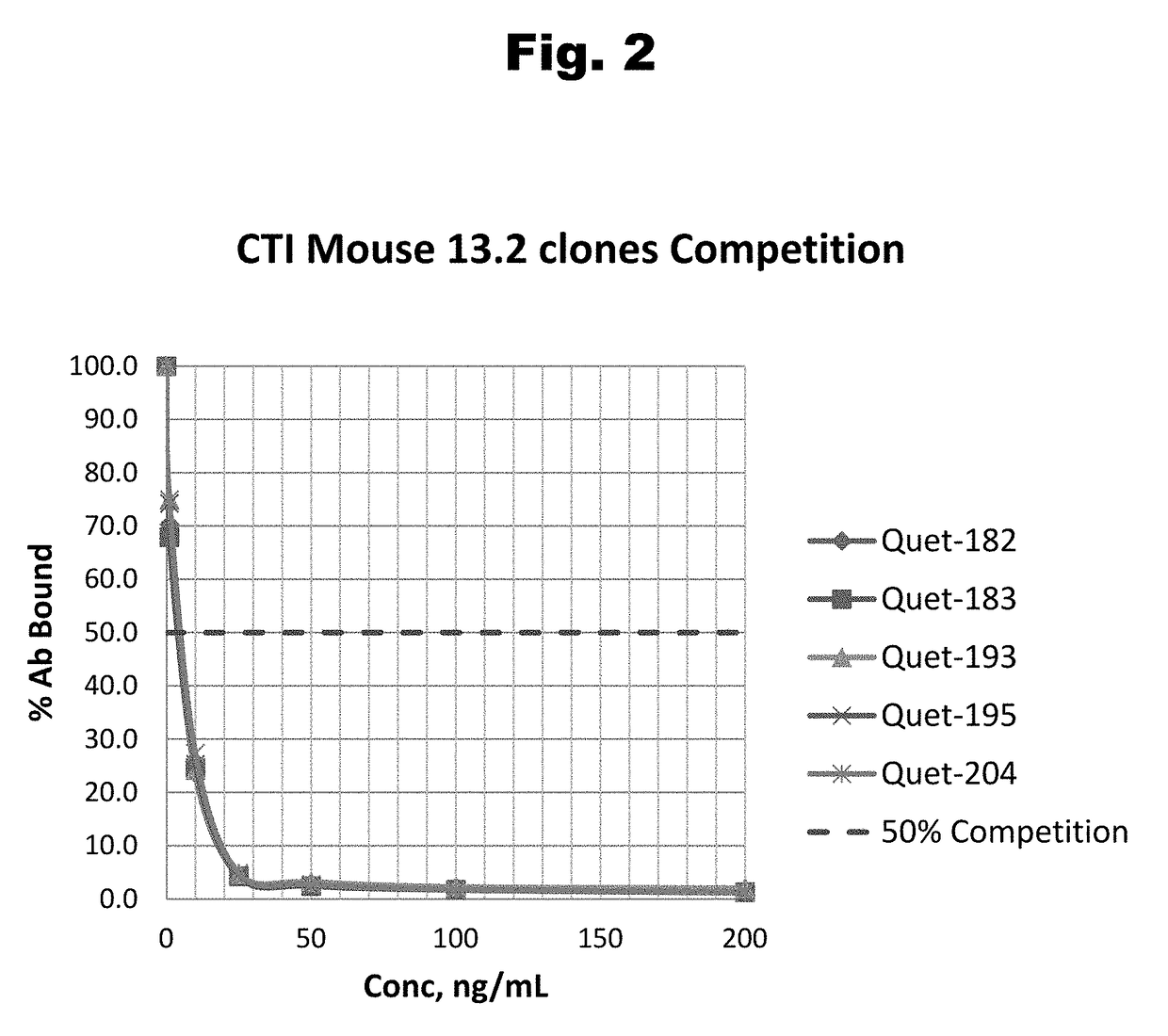
Antibodies to Quetiapine Haptens and Use Thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Risperidone sustained release microsphere composition
ActiveCN103338752ANo release lagEasy to produceOrganic active ingredientsPowder deliveryLactideMicrosphere
A risperidone sustained release microsphere formulation is provided. The microsphere formulation comprise risperidone or 9-hydroxy risperidone or salts thereof, and a polymer blend having a first uncapped lactide-glycolide copolymer and a second uncapped lactide-glycolide copolymer, in which the first uncapped lactide-glycolide copolymer is a copolymer with a high intrinsic viscosity and the second uncapped lactide-glycolide copolymer is a copolymer with a low intrinsic viscosity. The sustained release microsphere formulation according to an embodiment of the present disclosure is suitable for large-scale industrialized production with improved stability, tthe in vivo release behavior of which will not change after long-term storage.
Owner:SHANDONG LUYE PHARMA CO LTD +1
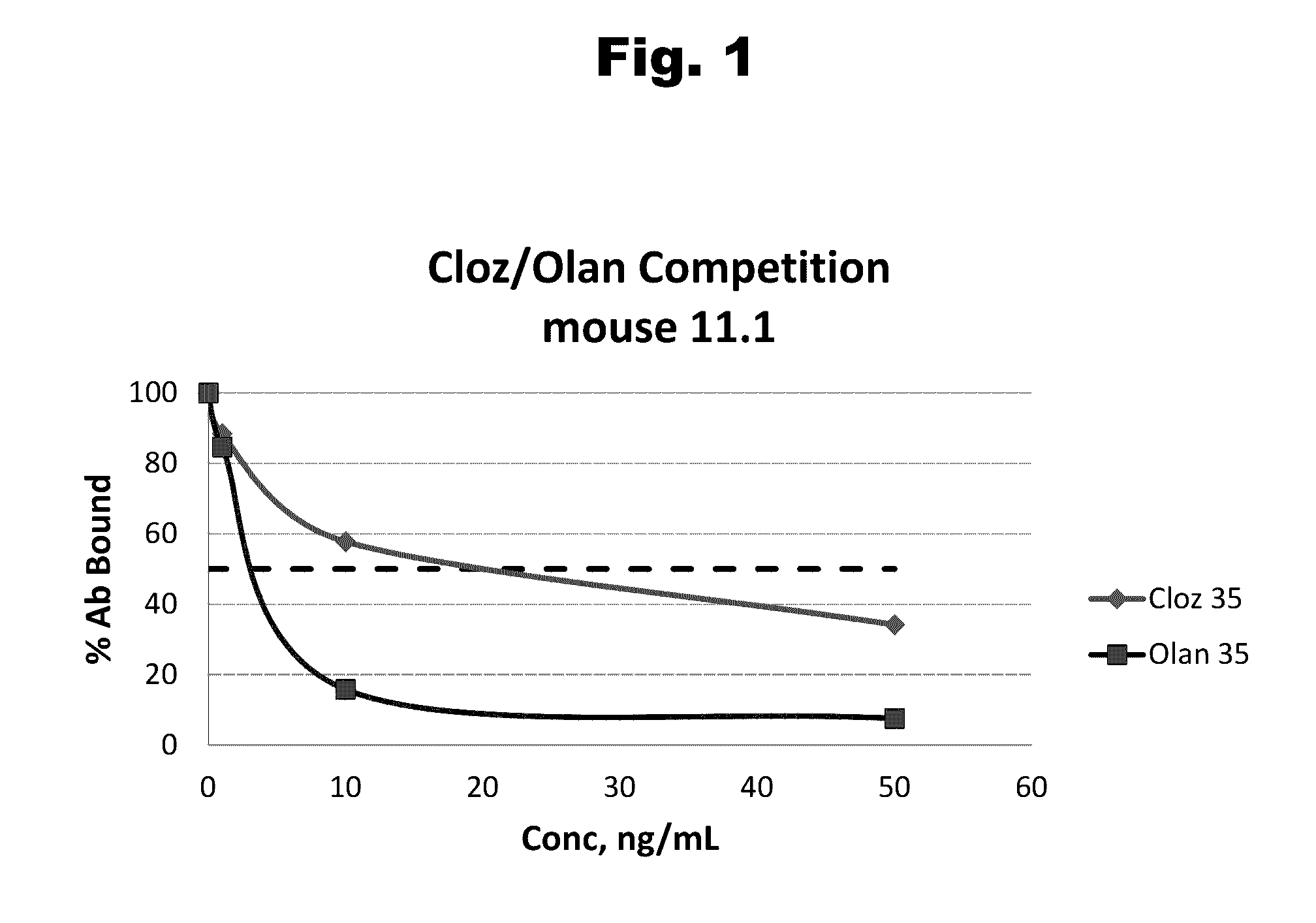
Antibodies to Olanzapine and Use Thereof
Disclosed is an antibody which binds to olanzapine, which can be used to detect olanzapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of olanzapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Compositions of 5-ht3 antagonists and dopamine d2 antagonists for treatment of dopamine-associated chronic conditions
InactiveUS20080004291A1Relieve distressPoor quality of lifeBiocideOrganic active ingredientsDisease5-HT3 antagonist
The present invention provides novel compositions comprising a combination of a 5-HT3 receptor antagonist and a selective dopamine D2 receptor antagonist for the treatment of obsessive, impulsive and compulsive behavioral activities and other dopamine pathway-associated disorders or conditions. Preferably, the pharmaceutical compositions of the present invention comprise amounts of the 5-HT3 receptor antagonist ondansetron and a selective dopamine D2 receptor antagonist, such as risperidone or olanzapine, that are sufficient to control a subjects obsessive, impulsive and compulsive behavioral activities. Kits comprising the combination of antagonists for the treatment of addictive disorders such as alcohol dependence are also provided.
Owner:TRANSCEPT PHARMA
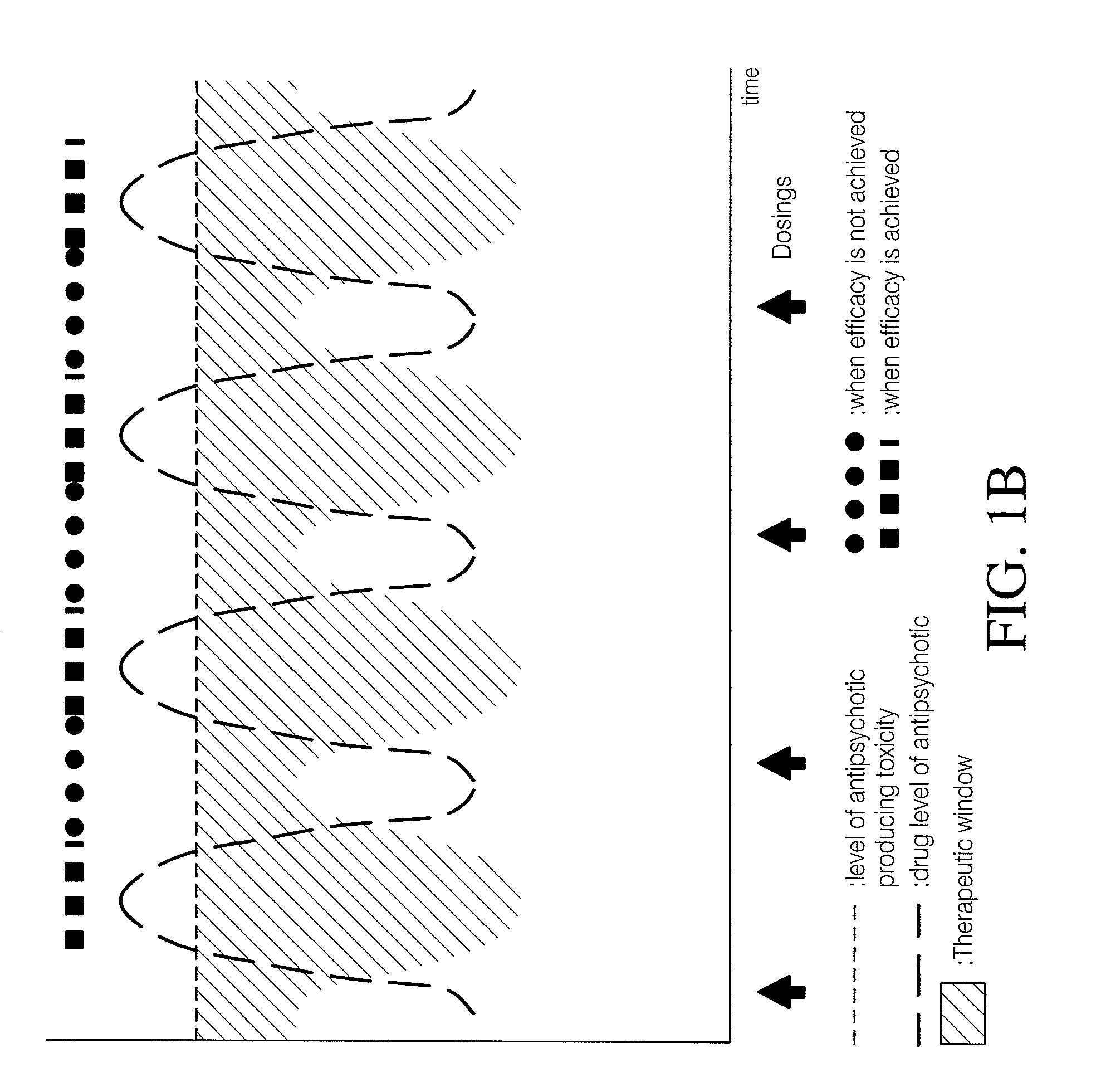
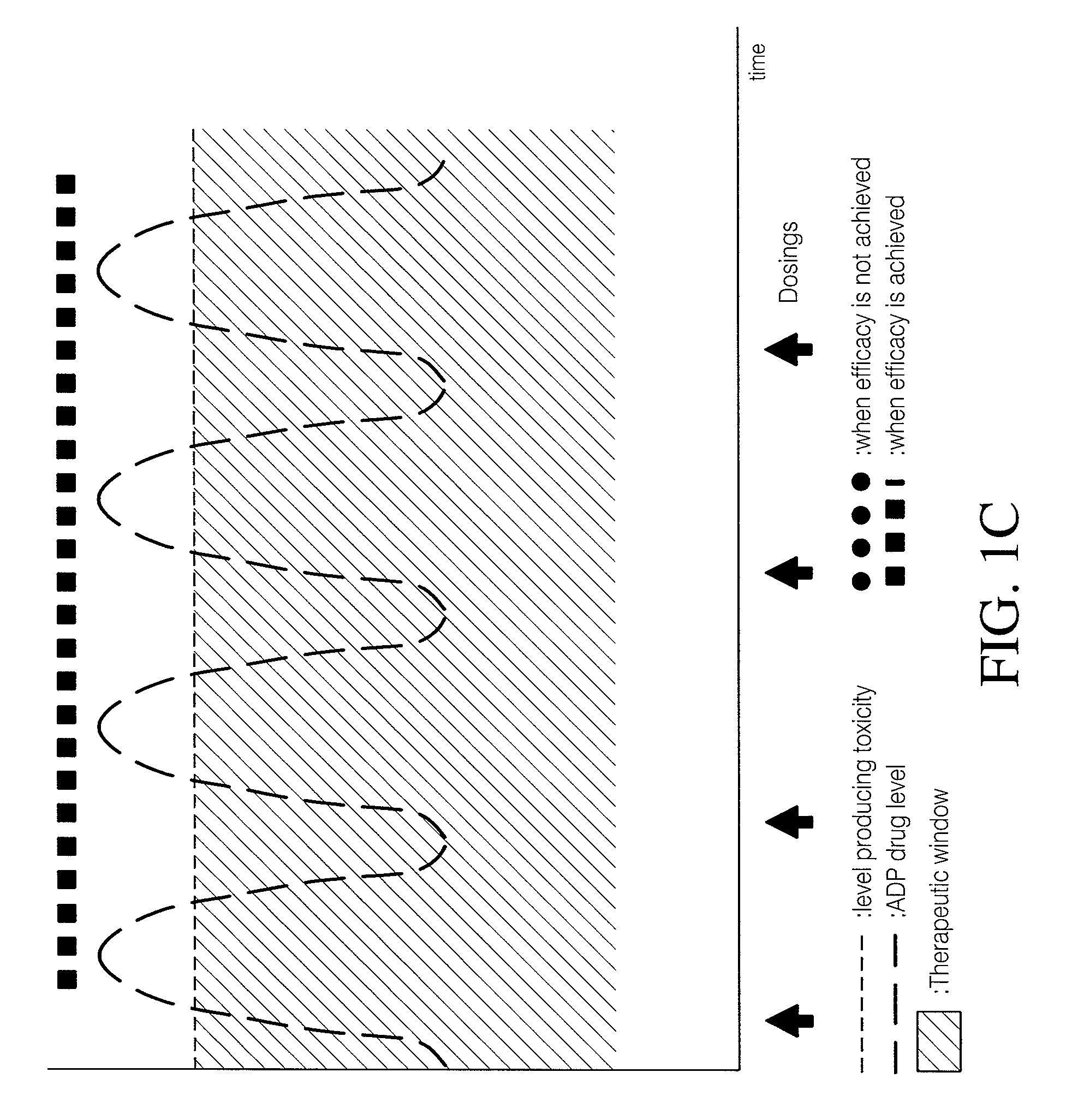
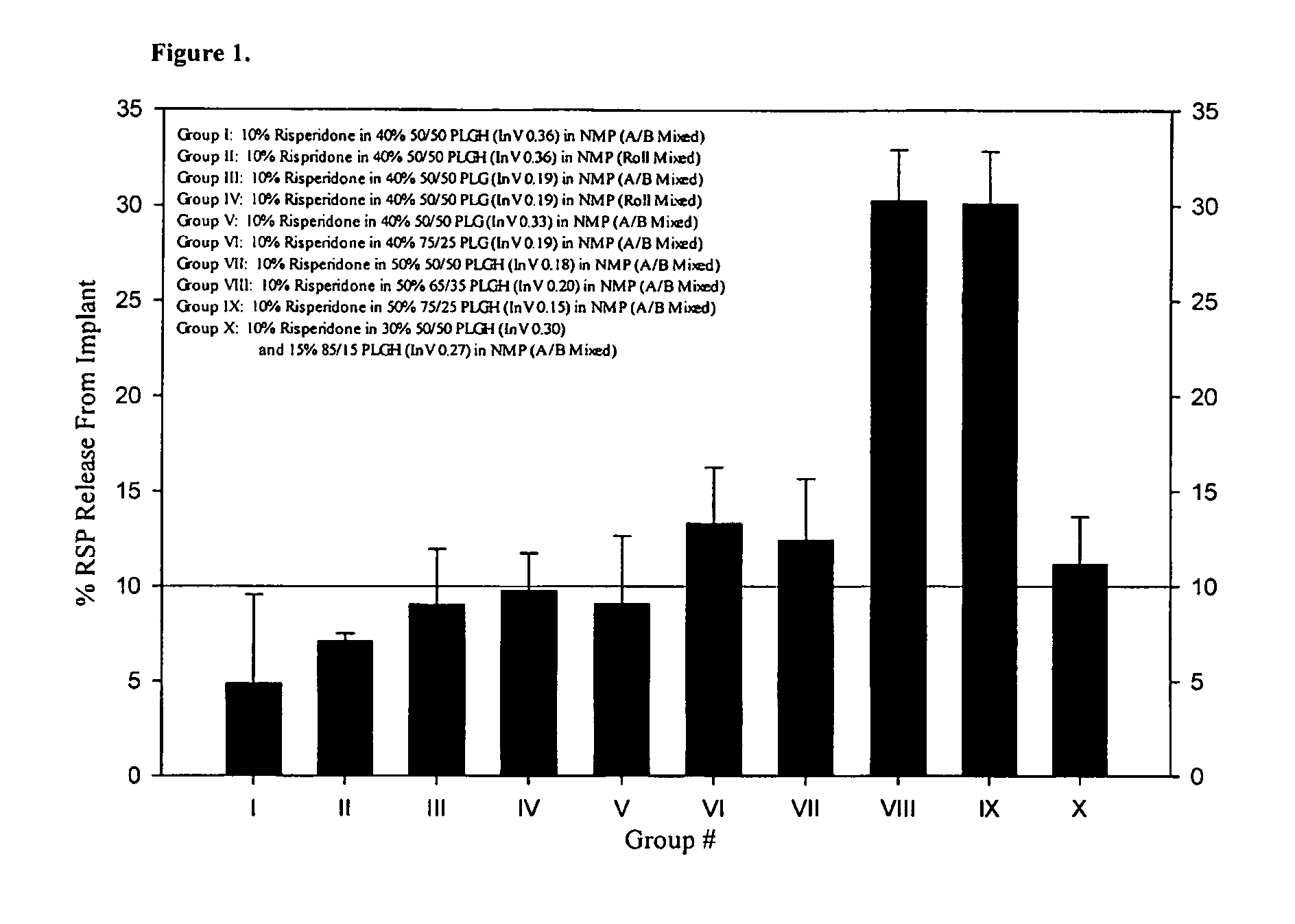
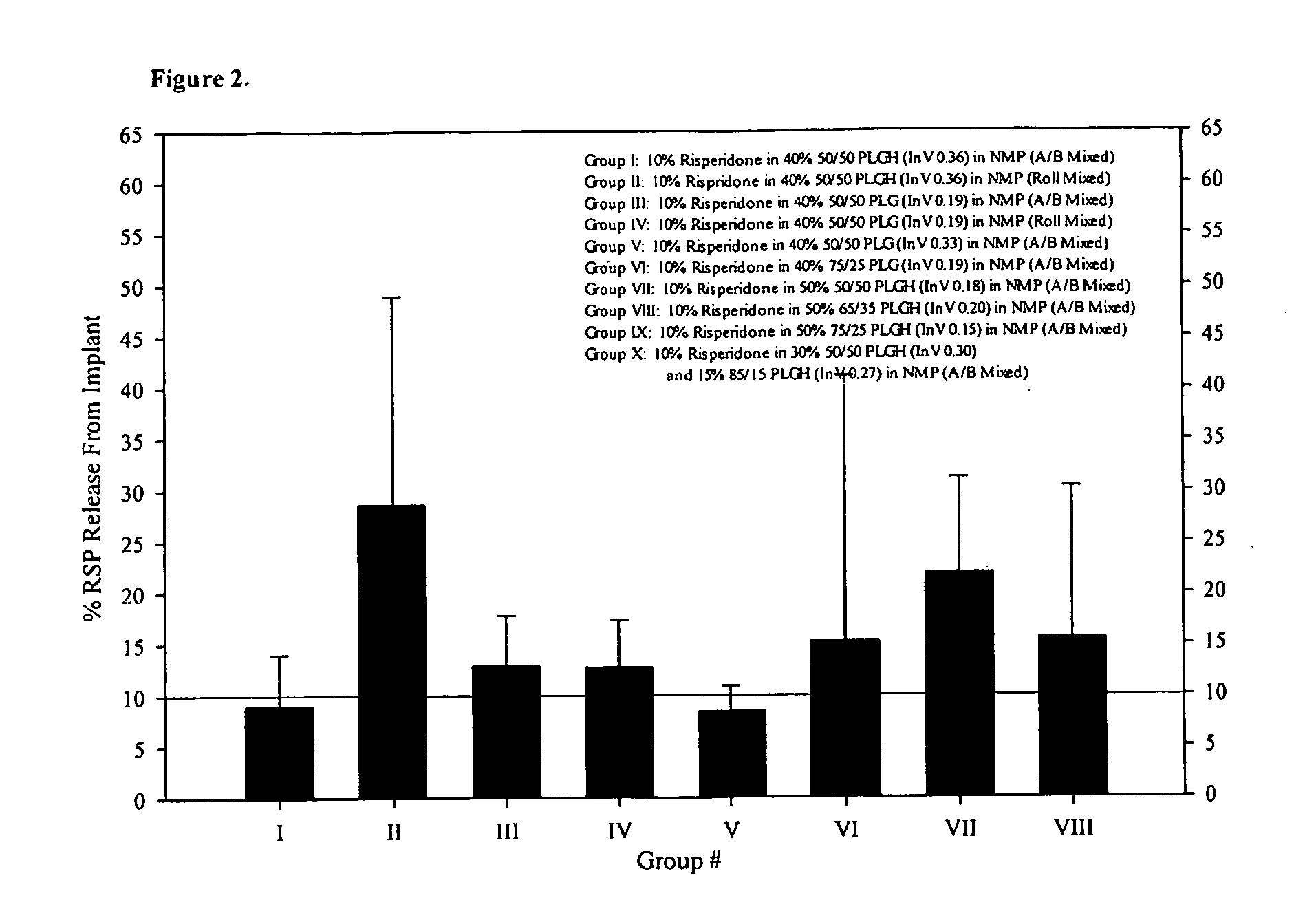
Implantable device, formulation and method for anti-psychotic therapy using risperidone
An subcutaneously implantable device for delivering Risperidone to a patient includes a pump, a compartment configured to store a pharmaceutical formulation, and a volume of the pharmaceutical formulation loaded in the compartment, the pharmaceutical formulation including Risperidone solvated or suspended in a pharmaceutically acceptable solvent in concentration of at least 50 milligrams per milliliter. The implanted pump may then deliver a therapeutically effective dose of Risperidone to the patient.
Owner:MICROSOLUTIONS
Antipsychotic Injectable Depot Composition
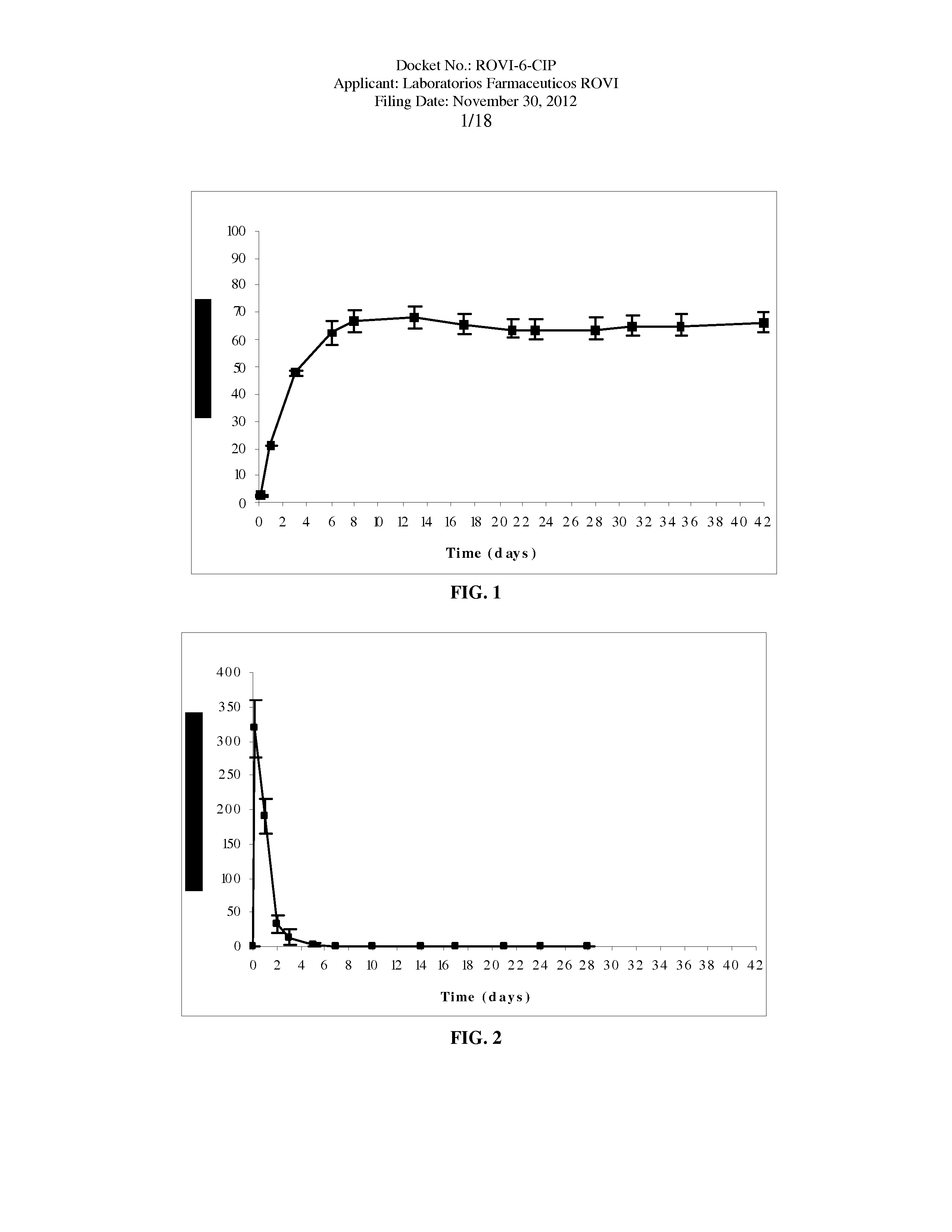
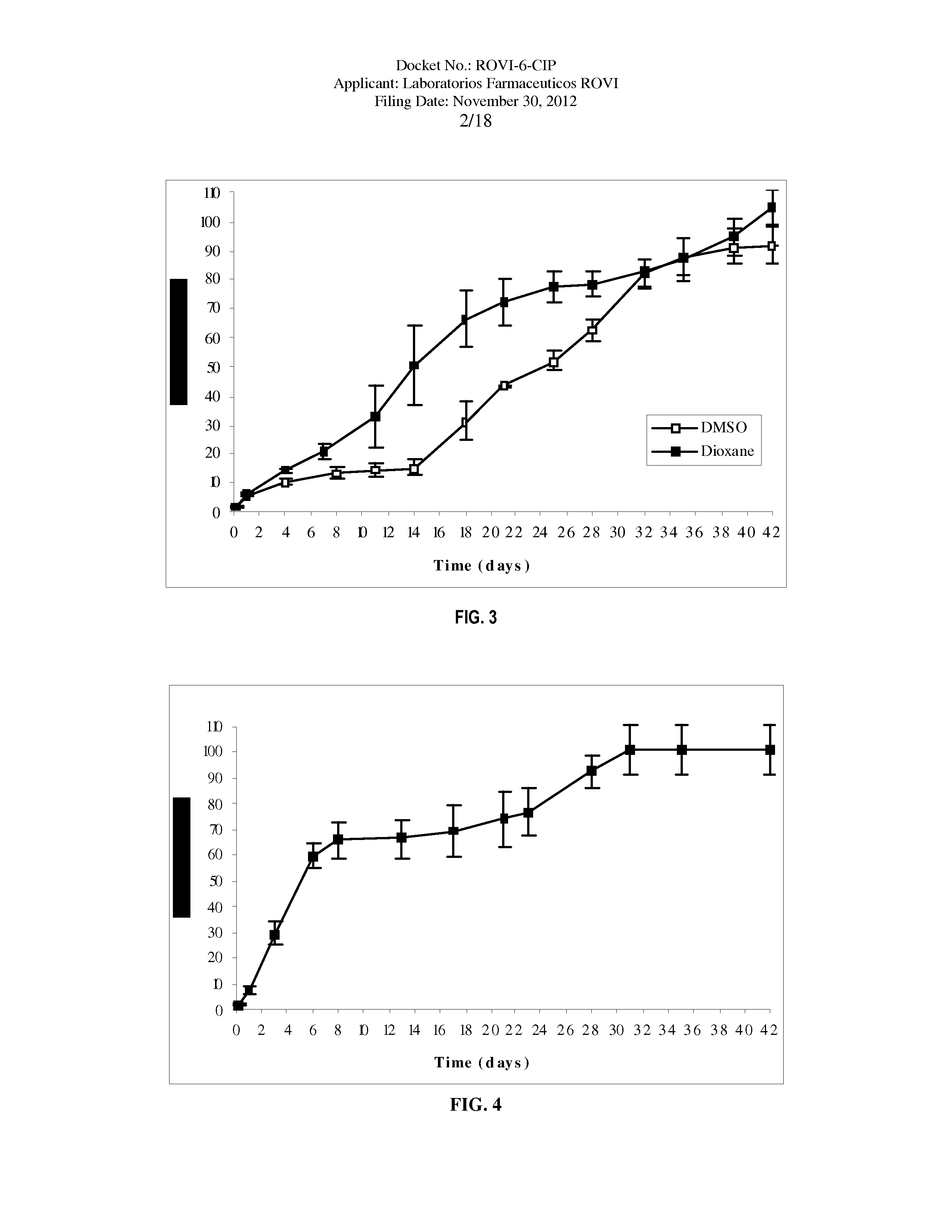
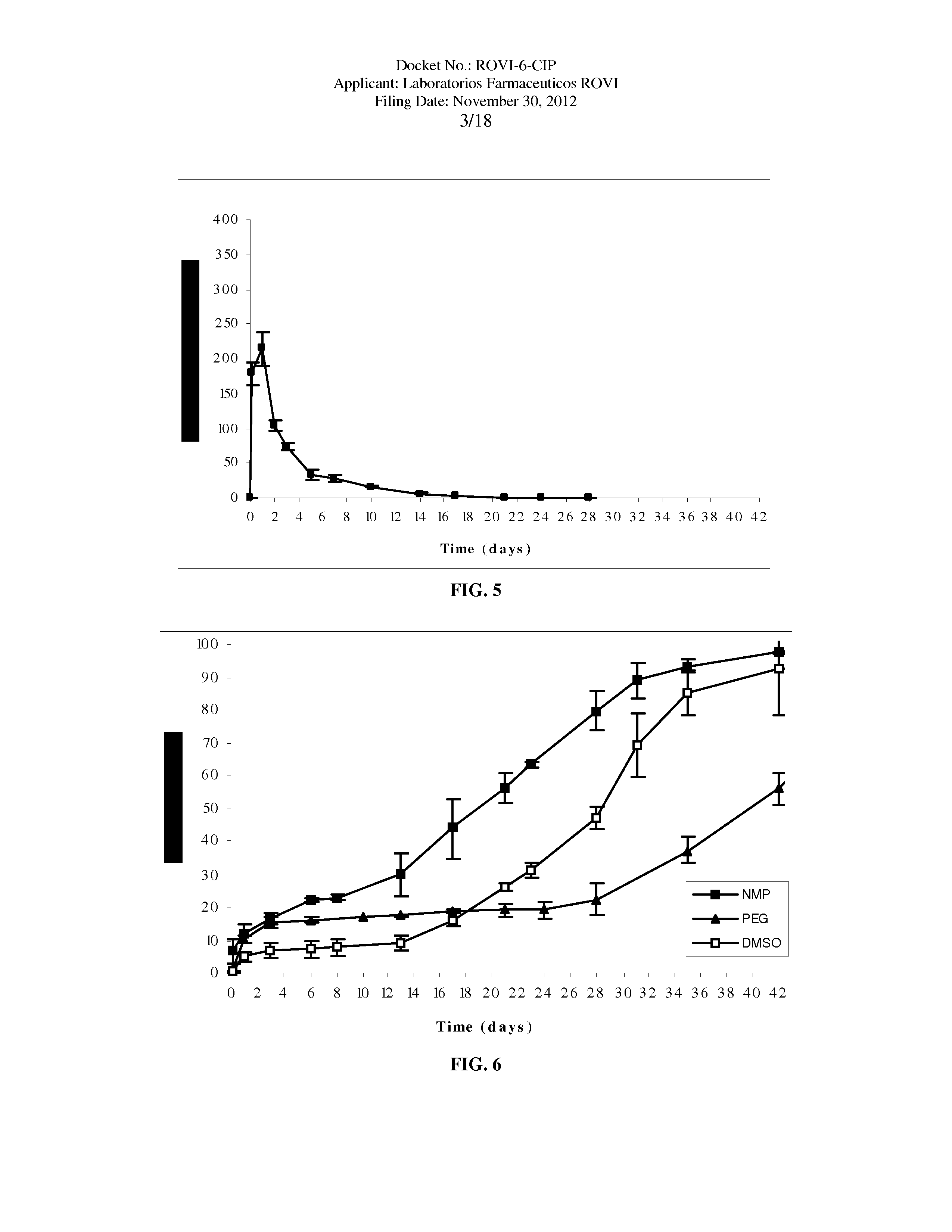
The present invention is directed to a composition that can be used to deliver an antipsychotic drug such as risperidone as an injectable in-situ forming biodegradable implant for extended release providing therapeutic plasma levels from the first day. The composition is in the form of drug suspension on a biodegradable and biocompatible copolymer or copolymers solution using water miscible solvents that is administered in liquid form. Once the composition contacts the body fluids, the polymer matrix hardens retaining the drug, forming a solid or semisolid implant that releases the drug in a continuous manner. Therapeutic plasma levels of the drug can be achieved since the first day up to at least 14 days or more even up to at least four weeks.
Owner:LAB FARM ROVI SA
Risperidone sustained-release gel injection and preparation method thereof
ActiveCN101584652AImprove complianceExcellent drug release performanceOrganic active ingredientsNervous disorderMass ratioIn vivo
The invention discloses a risperidone sustained-release gel injection and preparation method thereof. The risperidone sustained-release gel injection is composed of risperidone or analogue thereof, biological degradable polymer and biocompatible dissolvent, wherein the mass ratio between risperidone or analogue thereof and sum of biological degradable polymer and biocompatible dissolvant is 1:3-67. Continuous and constant-speed release of risperidone is up to several weeks immediately after the injection is injected into appropriate part in vivo, thereby improving compliance therapy in psychotic with risperidone analogue.
Owner:SHANGAI PHARMA GRP CO LTD +1
Antibodies to risperidone and use thereof
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to aripiprazole haptens and use thereof
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to risperidone haptens and use thereof
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
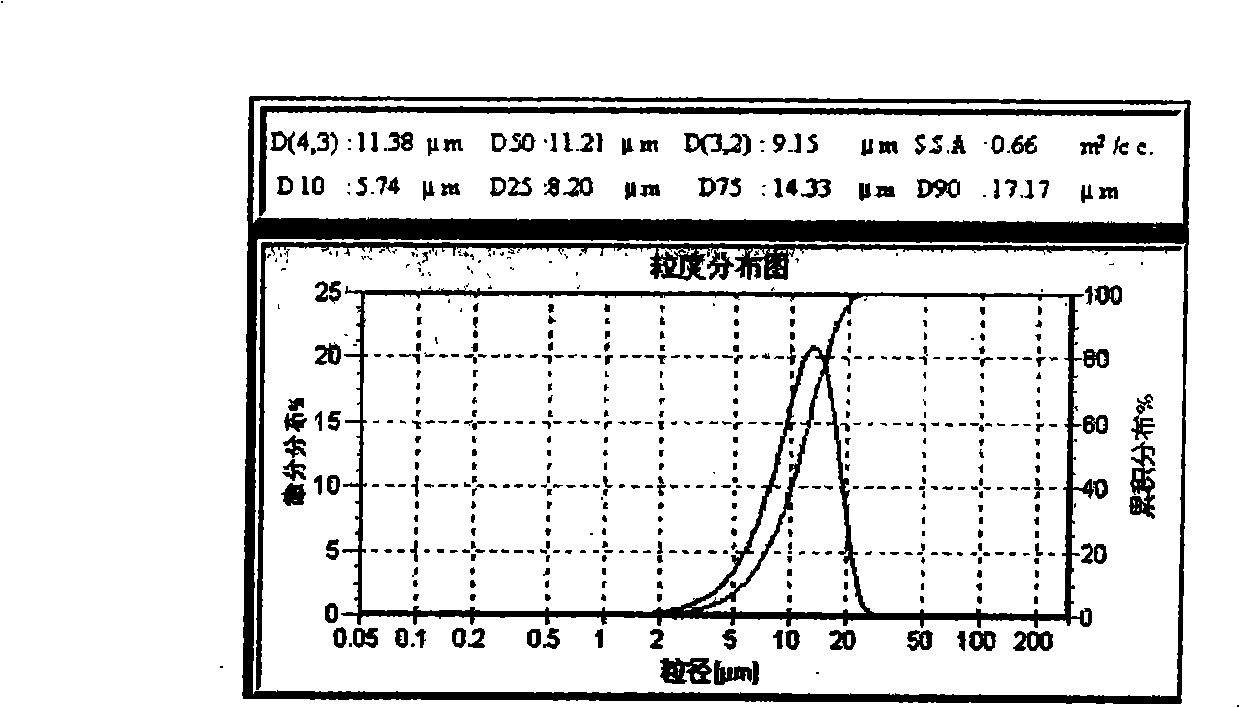
Microsphere preparation method for adjusting and controlling release behavior of risperidone microspheres and for controlling size thereof
InactiveCN102188384ASmooth and rounded surfaceParticles are regular and non-adhesiveOrganic active ingredientsPowder deliveryMicrosphereOil phase
The invention relates to a microsphere preparation method for adjusting and controlling the release behavior of risperidone microspheres and for controlling the size thereof, belonging to the technical field of nano-medicaments. The preparation method comprises the steps of: firstly, adding a sustained-release or controlled-release functional material to one or the mixture of dichloromethane, ethyl acetate and acetonitrile, swirling and dissolving to obtain an organic solution of the sustained-release or controlled-release functional material; then adding particles containing risperidone and an alkaline matter to the organic solution of the sustained-release or controlled-release functional material, i.e. stirring or swirling in the oil phase to evenly disperse to form even suspension; and adding the suspension to SPG (Shirasu Porous Glass) membrane emulsion with different bore diameters, enabling the suspension to enter brine containing a surfactant through the membrane emulsion by adjusting the pressure, stirring to form microspheres, and transferring into the brine for solidifying to finish preparation. The biodegradable risperidone microsphere sustained-release preparation prepared by the invention has quick effect taking speed and long drug release time, the particle size of the microspheres is controllable, the envelop rate is high, and the quality of the risperidone microspheres is controllable.
Owner:SHANGHAI JIAO TONG UNIV
Antibodies to aripiprazole and use thereof
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Oral instant membrane of risperidone and preparation method thereof
InactiveCN101632651AQuick effectImprove medication complianceOrganic active ingredientsNervous disorderDifficult swallowingOrally disintegrating tablet
The invention relates to an oral instant membrane of risperidone and a preparation method thereof, thereby being used for improving the using performance of the risperidone. The technical scheme is as follows: the instant membrane comprises 2-60 parts by weight of risperidone, 25-98 parts by weight of water-soluble pharmaceutical polymer excipients, additives and 0.1-25 parts by weight of water. The oral instant membrane solves the problems that the existing risperidone is clinically difficult to swallow, is not applicable to children and elderly patients for administration and can not be conveniently taken under the situation of having no water. The oral instant membrane can be dissolved on the tongue, the hidden trouble of choking can not be happened no matter how young a baby is, the active ingredients can be absorbed sublingually and absorbed by buccal mucosa, and the onset of action is more rapid in comparison with orally disintegrating tablets. In addition, the oral instant membrane has good compliance, thereby being particularly suitable for elderly people, children and patients with difficult swallowing.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Antibodies to quetiapine and use thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
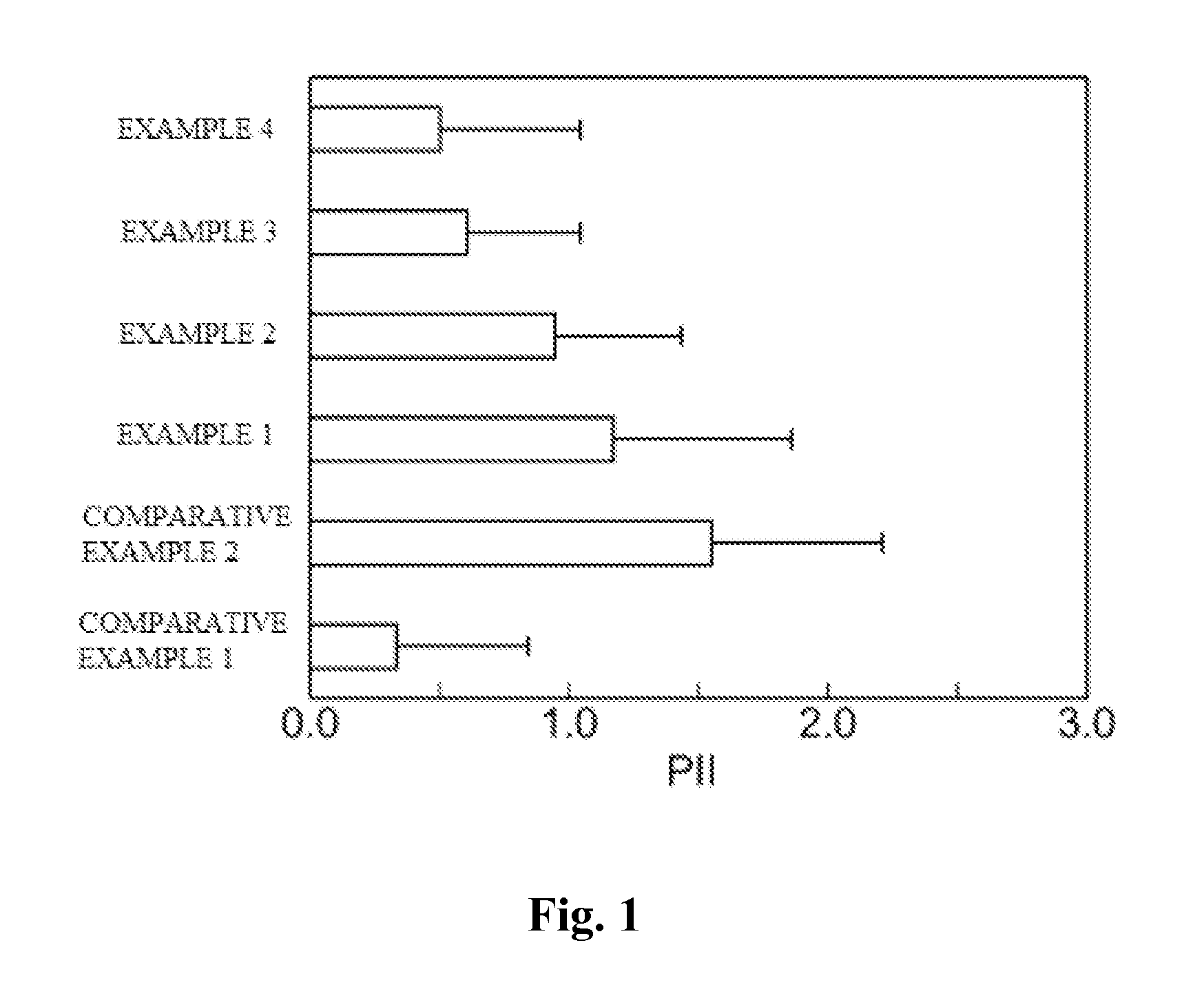
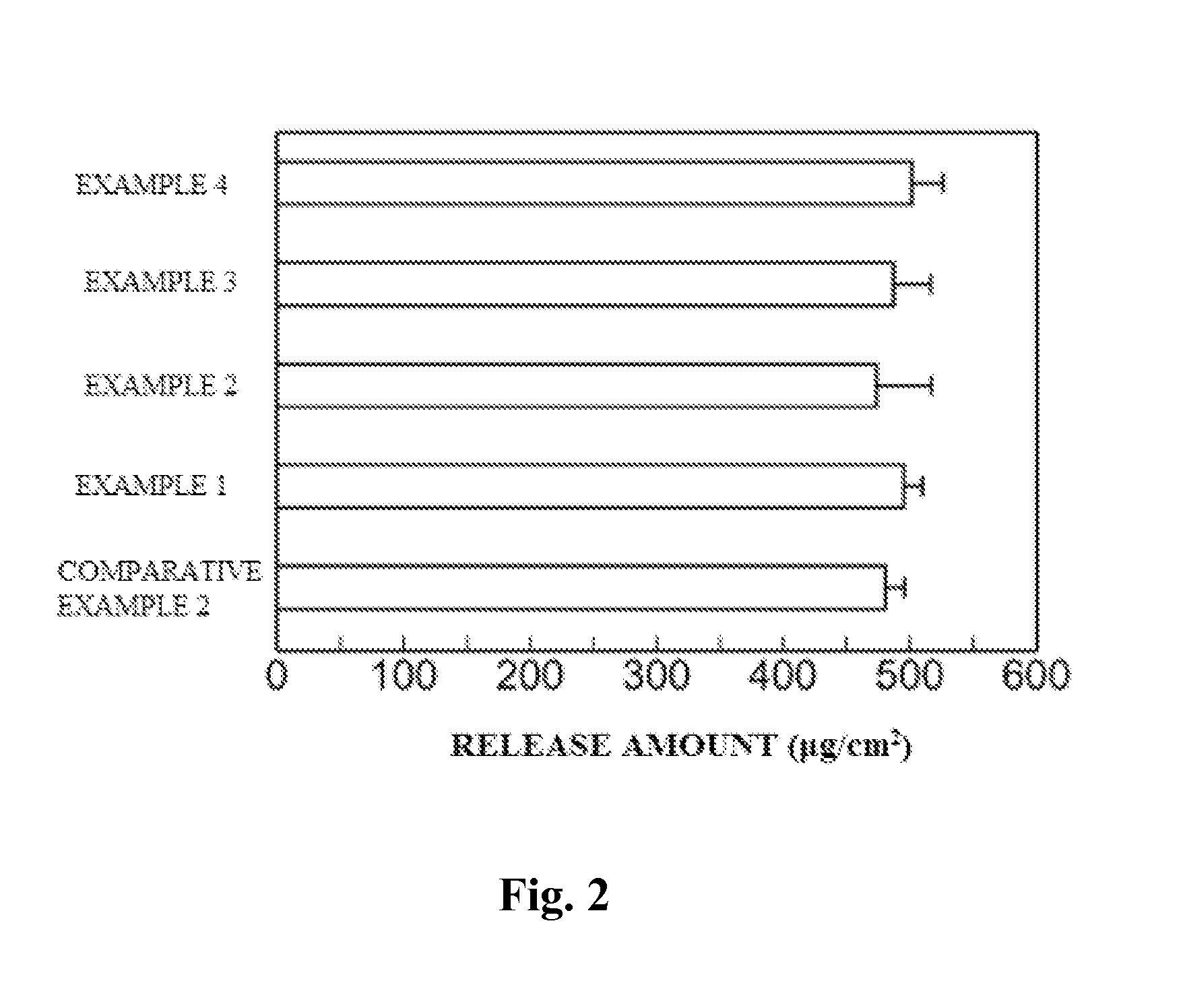
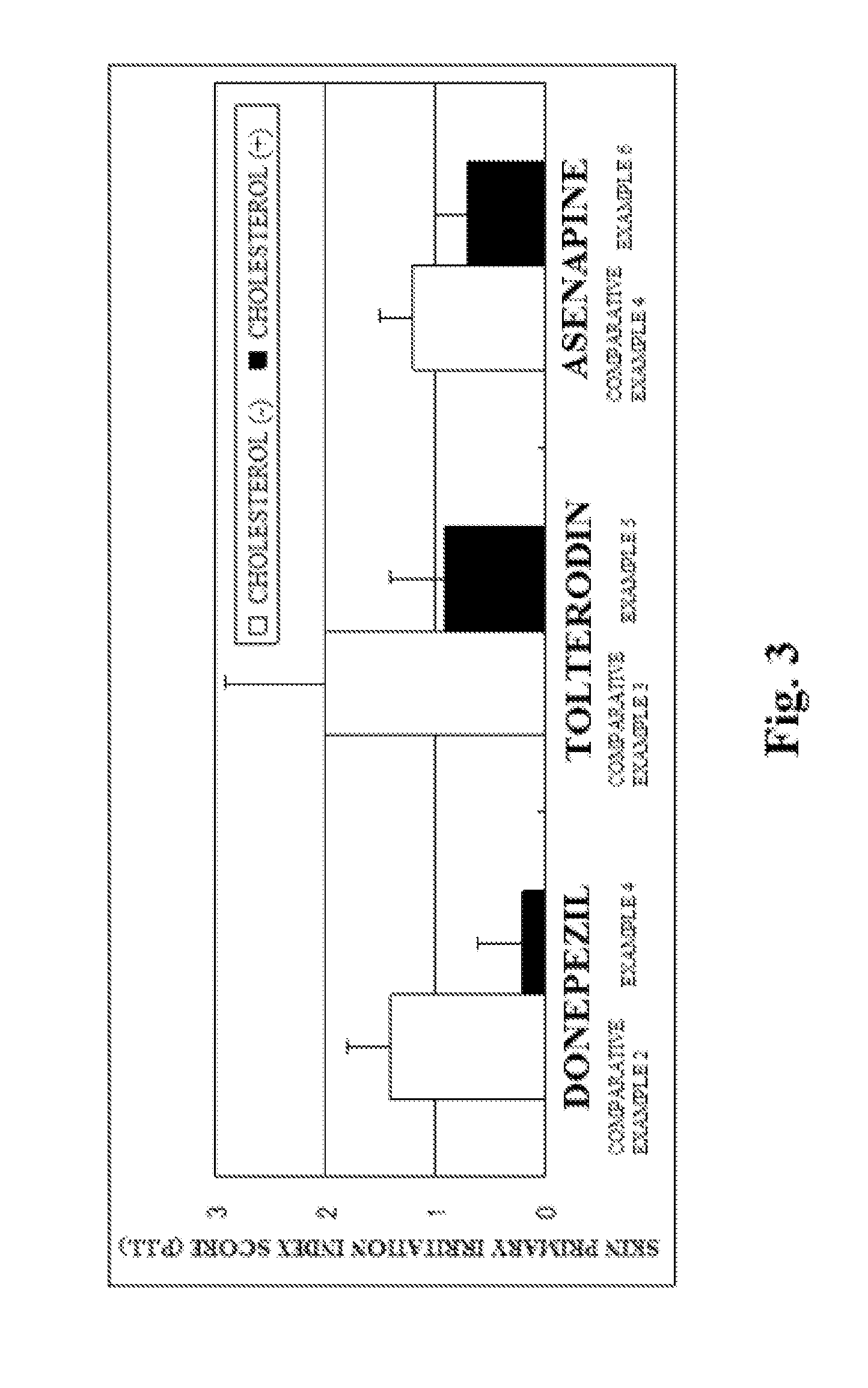
Skin irritation suppressant and transdermal preparation
InactiveUS20130053357A1Sufficient reduction effectReduce skin effectOrganic active ingredientsBiocideCholesterol derivativeCitalopram
Provided is a skin irritation suppressant for transdermal preparations, having a sufficient reduction effect of skin irritation due to a drug. Also provided is a transdermal preparation comprising the skin irritation suppressant. One embodiment of the invention is a skin irritation suppressant for suppressing the skin irritation due to a drug and a pharmaceutical ingredient to be used in a transdermal preparation other than the drug, the skin irritation suppressant comprising a sterol compound selected from the group consisting of cholesterol, cholesterol derivatives and cholesterol analogs, and the drug is one or more basic drugs selected from the group consisting of tolterodine, asenapine, bisoprolol, risperidone, nicotine and citalopram, and their pharmaceutically acceptable salts.
Owner:HISAMITSU PHARM CO INC
Antibodies to quetiapine haptens and use thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to olanzapine and use thereof
Disclosed is an antibody which binds to olanzapine, which can be used to detect olanzapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of olanzapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
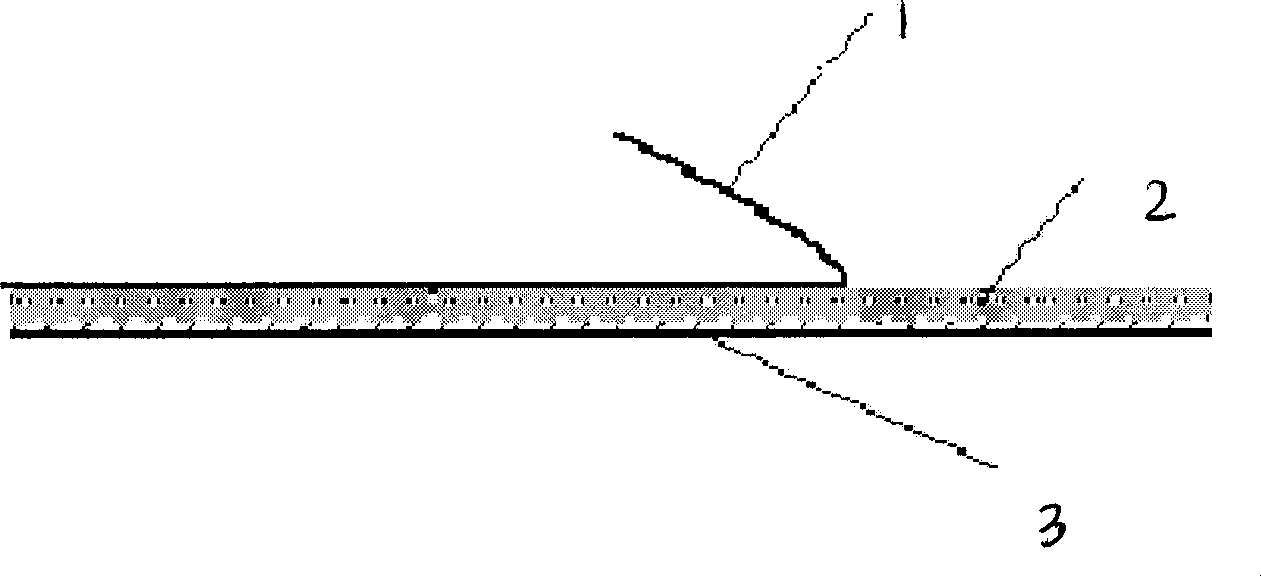
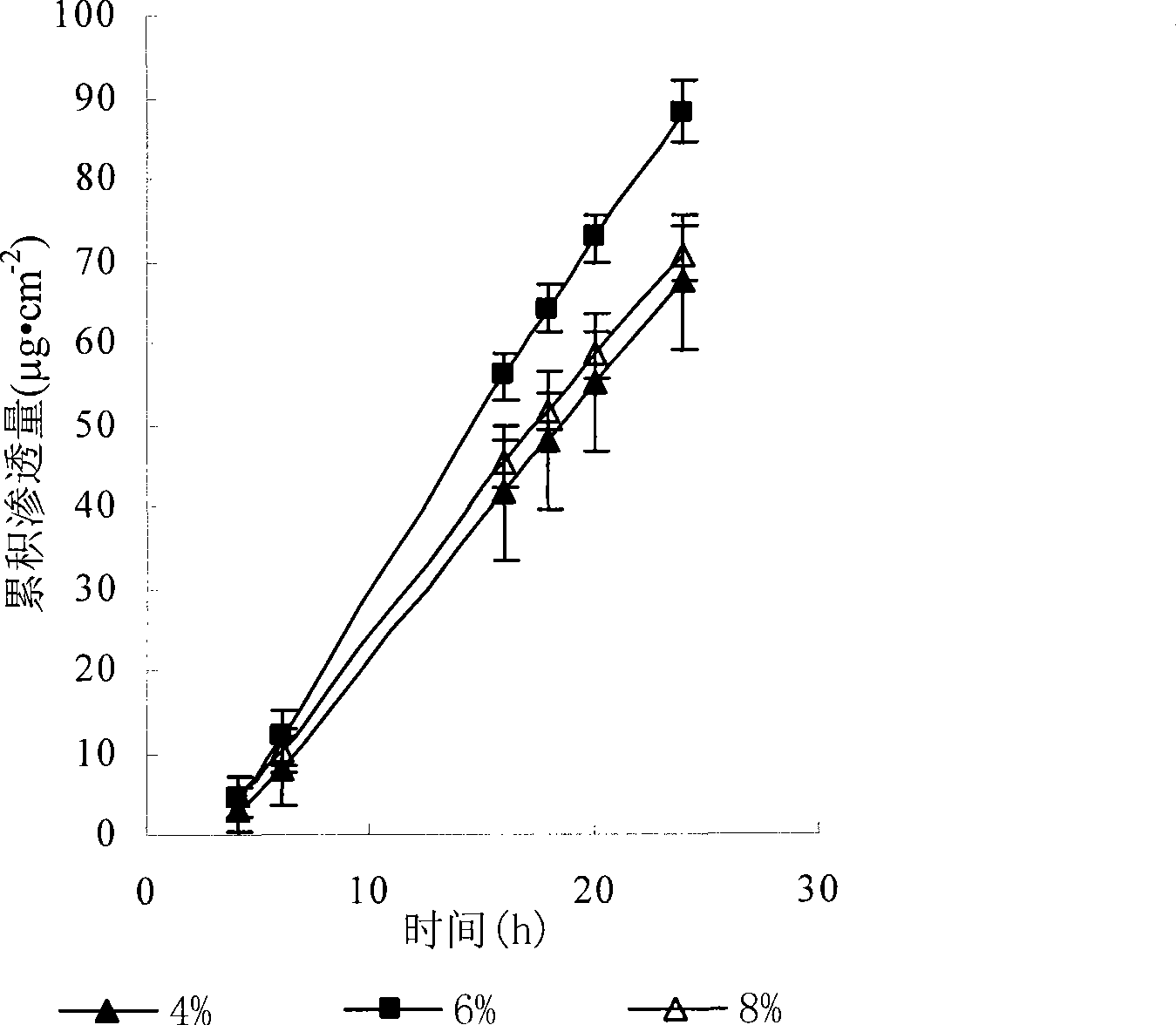
Risperidone percutaneous absorption paster
InactiveCN101366705AProlong the action timeLittle side effectsOrganic active ingredientsNervous disorderTectorial membraneSide effect
The invention discloses a risperidone transdermal absorbing patch, which consists of a back lining layer, a protective film and a substrate layer which is arranged between the back lining layer and the protective film and contains medicines. The compositions of the substrate layer containing the medicines by weight percent are 65 to 95 percent of polyacrylate pressure sensitive adhesive, 4 to 15 percent of risperidone and 1 to 20 percent of transdermal enhancer. The patch delivers the risperidone by means of transdermal impregnation, can prolong the acting time of the medicine, maintains stable blood drug level, reduces the side effect of the medicine, is convenient to use, and can be taken as a medicine for treating various mental sickness such as schizophrenia, mania or dementia and so on.
Owner:ZHEJIANG UNIV
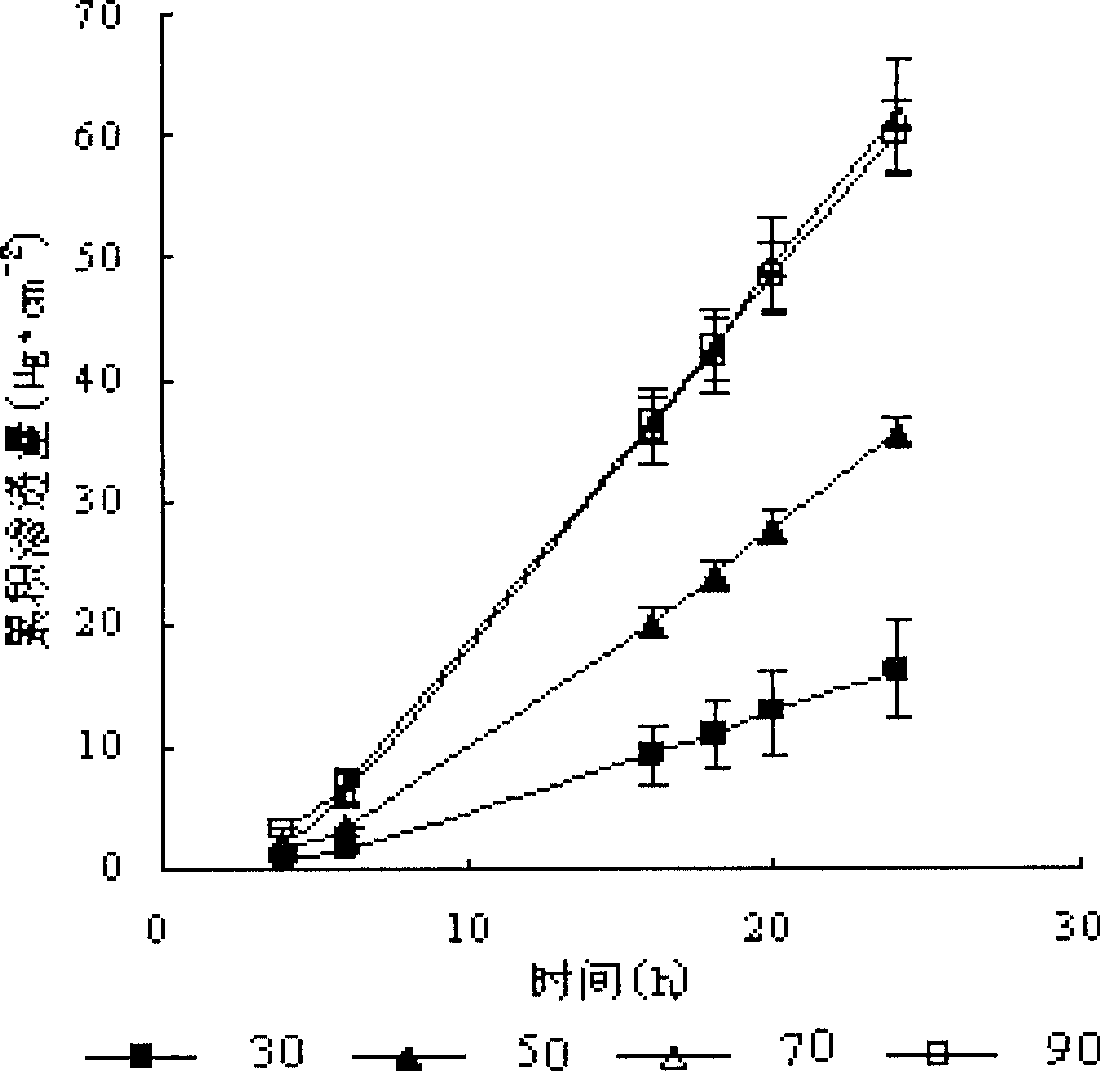
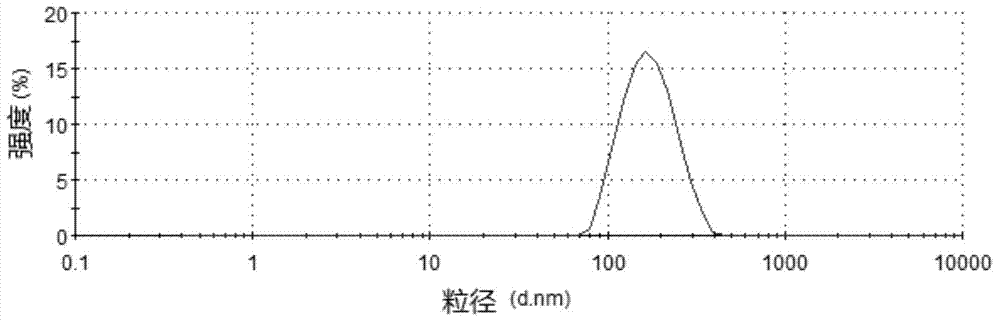
Risperidone nano-suspension temperature sensitive gel and its preparation method
InactiveCN104288091AImprove complianceIncrease dissolution rateOrganic active ingredientsNervous disorderWater basedPatient compliance
The invention discloses a risperidone nano-suspension temperature sensitive gel and its preparation method. Each 100ml of the nanosuspension temperature sensitive gel contains 0.1-10g of risperidone, 0.1-5g of a stabilizer, 1-50g of a temperature sensitive gel material, 0-5g of an additive, and the balance of a water-based solvent. The risperidone nano-suspension temperature sensitive gel is a temperature sensitive controlled-release in situ gel. The above dosage form makes risperidone highly dispersed, so the gel has the advantages of good drug load and good stability; after the gel is administrated in a liquid form, phase transition occurs in applied sites, and the liquid becomes non-chemically-crosslinked semisolid gel, so the gel has good histocompatibility and improves the drug dissolution rate; and the administrated gel has a long retention time in the applied sites as a drug reservoir, so the gel has a slow release effect, prolongs the drug release time, improves the drug bioavailability, reduces the administration frequency and improves the drug effect and the patient compliance.
Owner:HENAN UNIV OF SCI & TECH
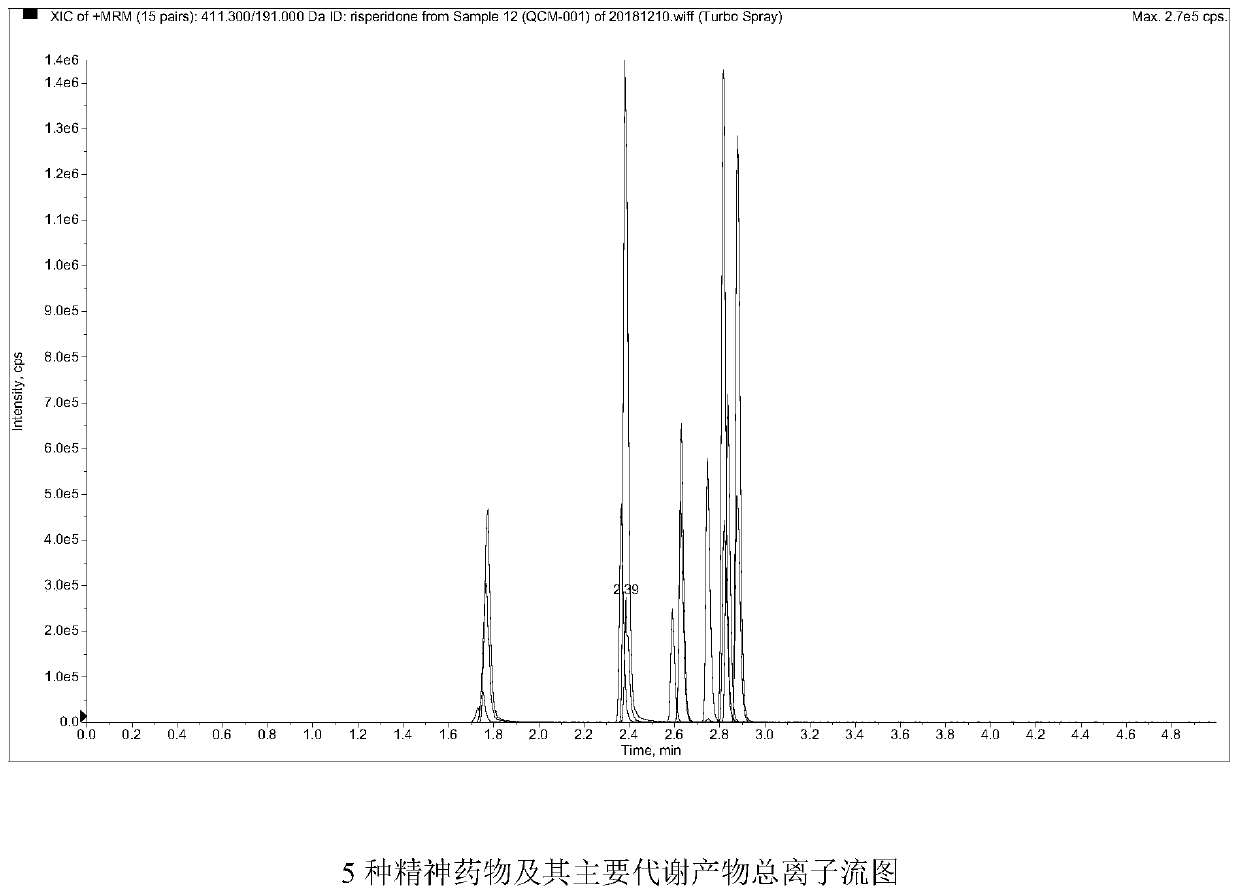
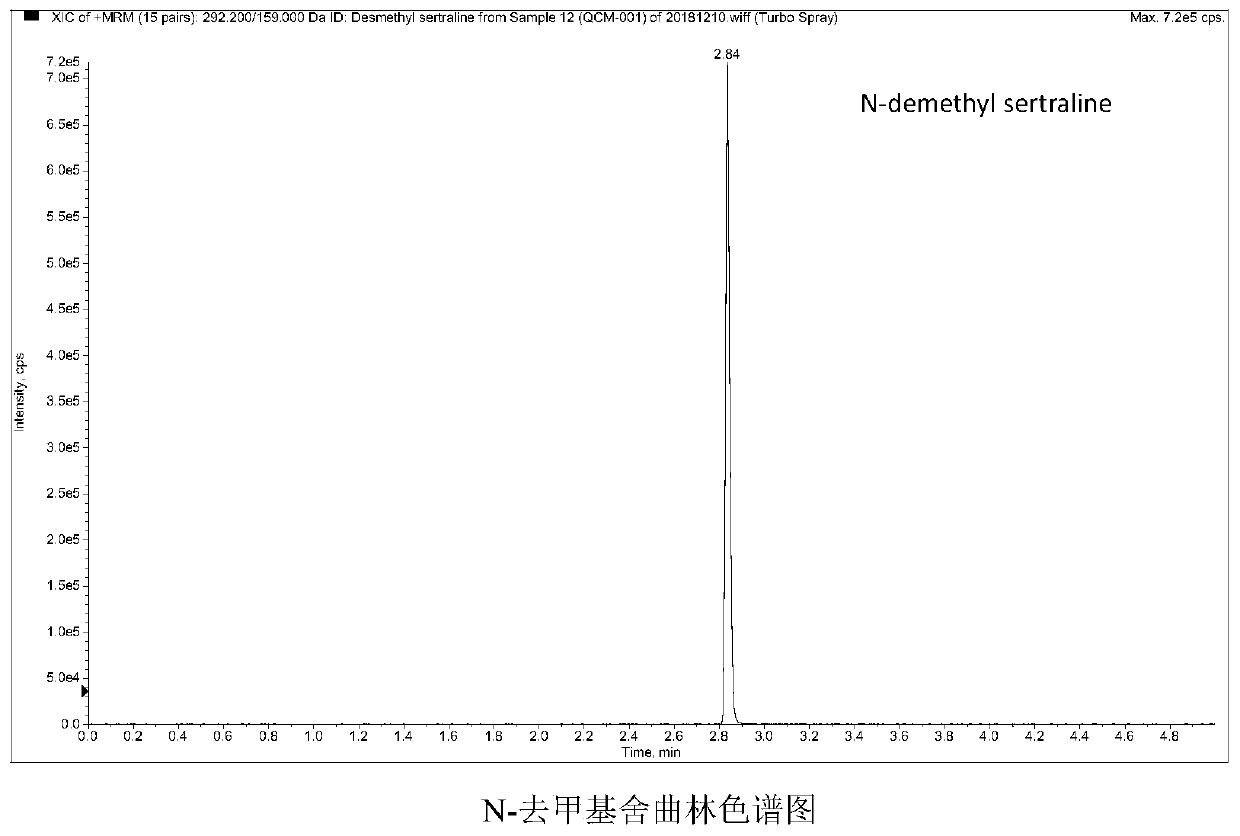
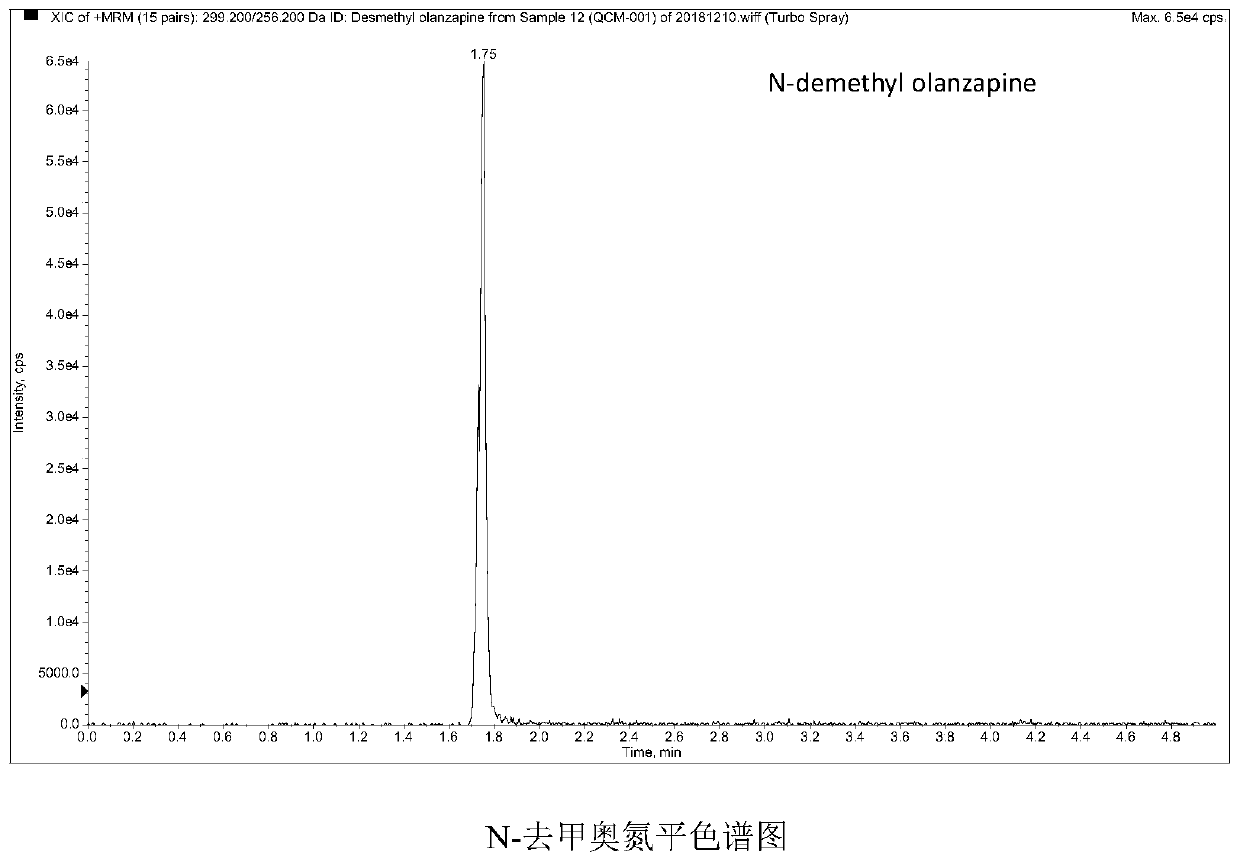
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Method for preparing 6-fluoro-3-(4- piperidyl)-1,2-benzo isoxazole hydrochlorate
ActiveCN101328173APrevent participation in responseSolve the problems of many types, complicated operation and low yieldNervous disorderOrganic chemistryCyclopropanationPetroleum ether
The invention relates to a method for preparing a hydrochloride of 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole. The method is to subject 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to cyclopropanation and salifying in an aprotic solvent containing an alkali metal hydroxdide, wherein the molar ratio of the 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to the alkali metal hydroxide is 1 to between 1 and 3. The method can effectively prevent F atoms on para positions from participating in the reaction and avoid the production of a dipolymer (V), thereby avoiding the production of a dipolymer (VI) during the following preparation of risperidone. The method crystallizes crystals of a target substance directly by salifying, thereby solving the problems of use of a plurality of kinds of organic solvents, complex operation and low yield due to the extraction by toluene, condensation, and crystallization of petroleum ether. The yield rate of the target substance is more than 80 percent, and the purity of target substance is more than 99 percent.
Owner:CSPC OUYI PHARM CO LTD
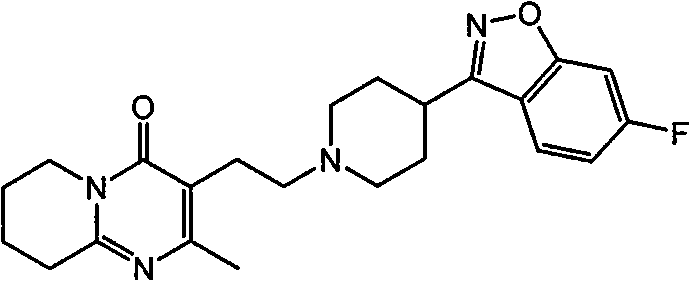
Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
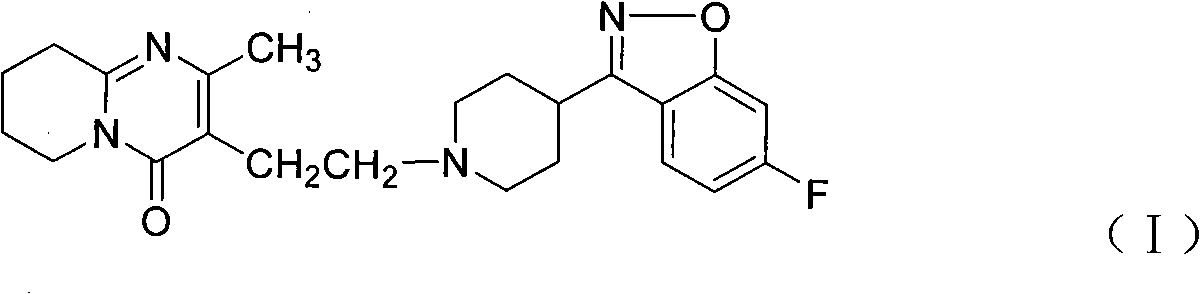
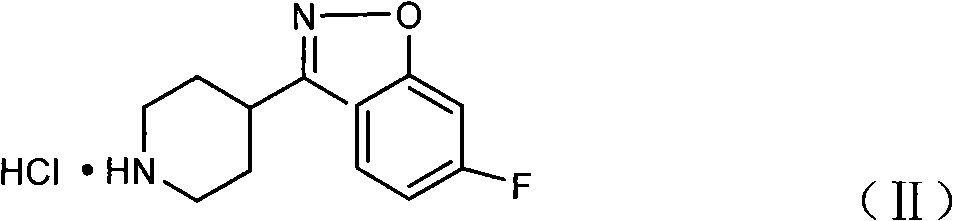
InactiveUS6897308B1Inexpensive and economicalHigh purityNervous disorderOrganic chemistryHydrogenMetal catalyst
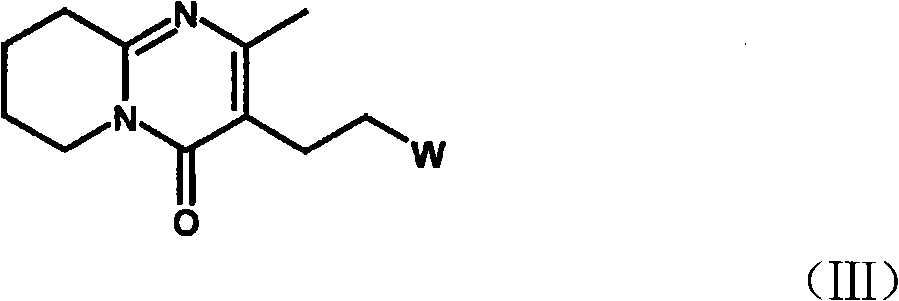
A process for the preparation of 3-substituted ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2,-a]pyrimidin-4-one of the formula IIB: where X may be halo, acyloxy, or sulfonyloxy such as tosyloxy or mesyloxy, an intermediate in the synthesis of the anti-psychotic risperidone. The process comprises hydrogenation of 3-substituted ethyl-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one in aqueous inorganic acid medium at atmospheric to 60 psi at 0-100° C. in the presence of a metal catalyst and the product is isolated. A process for the preparation of risperidone of the formula I: comprising condensation of 3-substituted ethyl-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2,-a]pyrimidin-4-one with 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole in water in the presence of an inorganic base at 25-100° C. and the product is isolated.
Owner:RPG LIFE SCIENCES
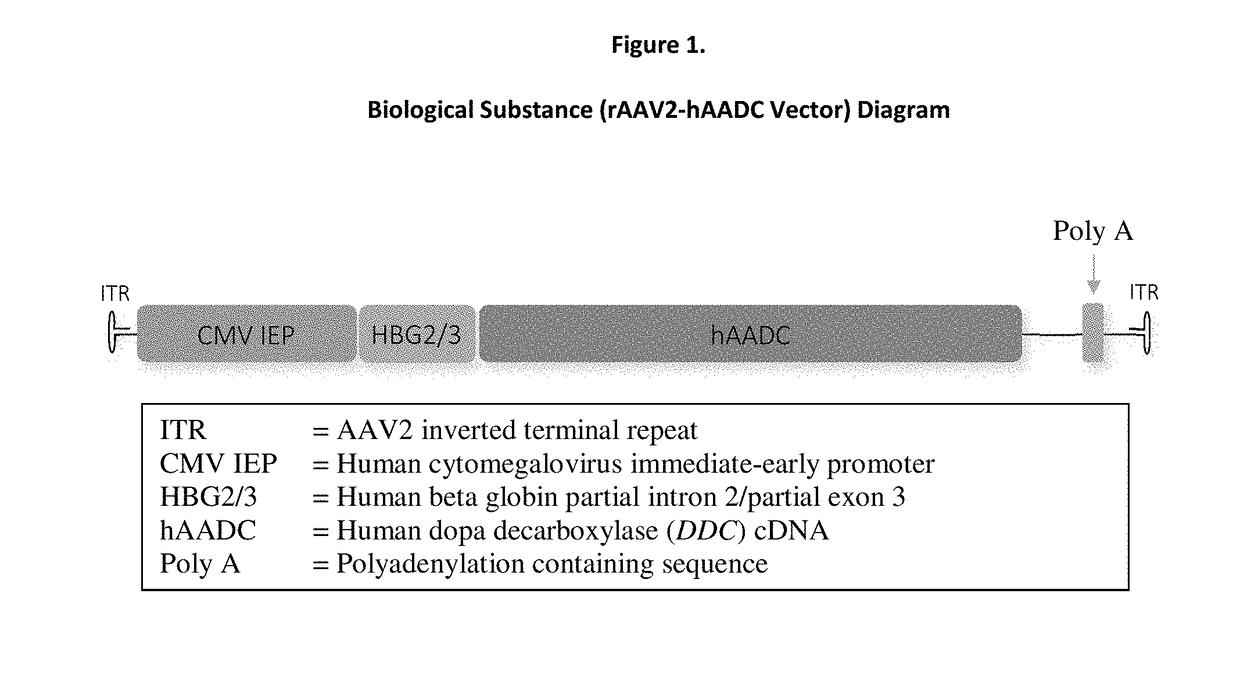
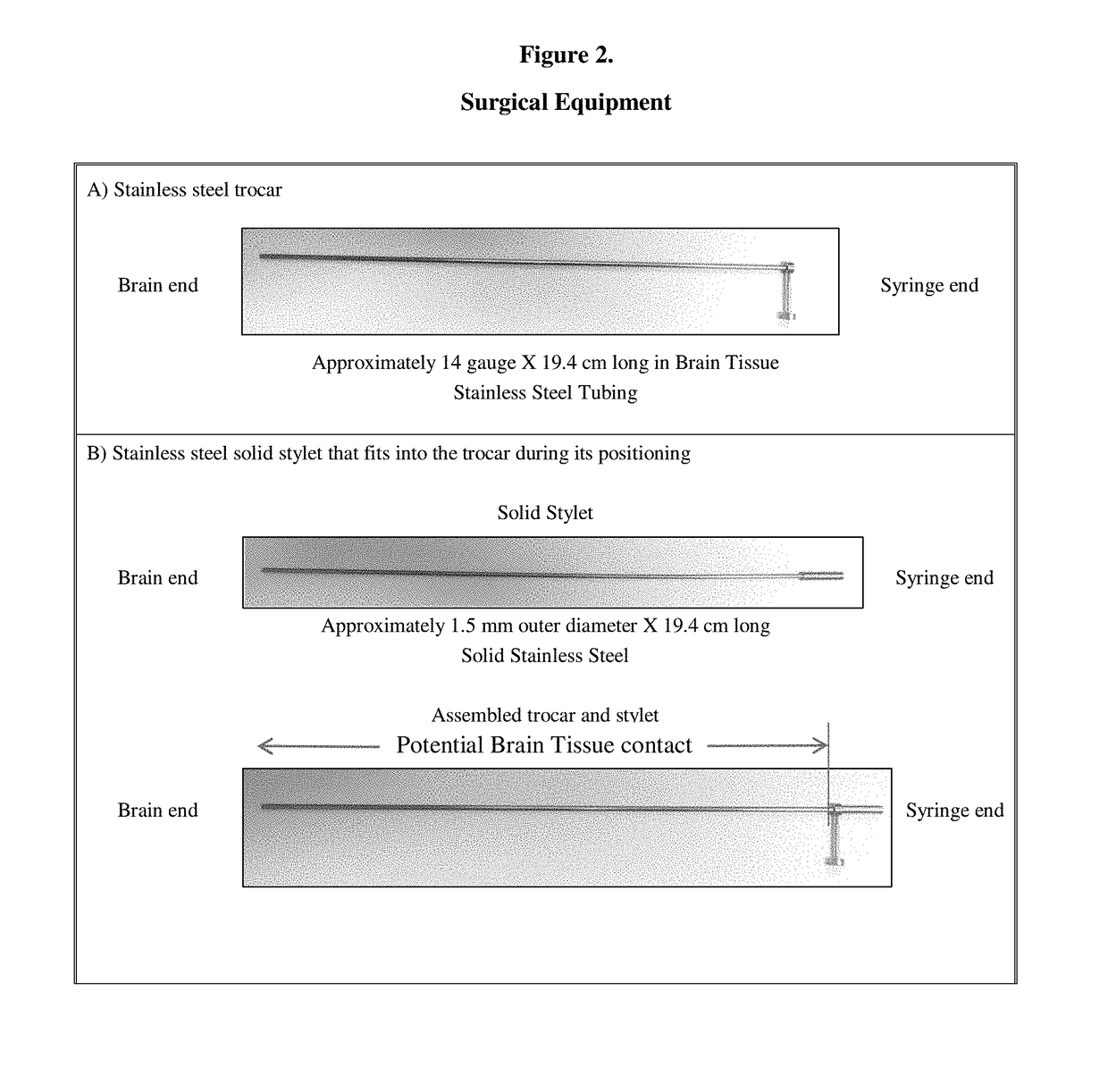
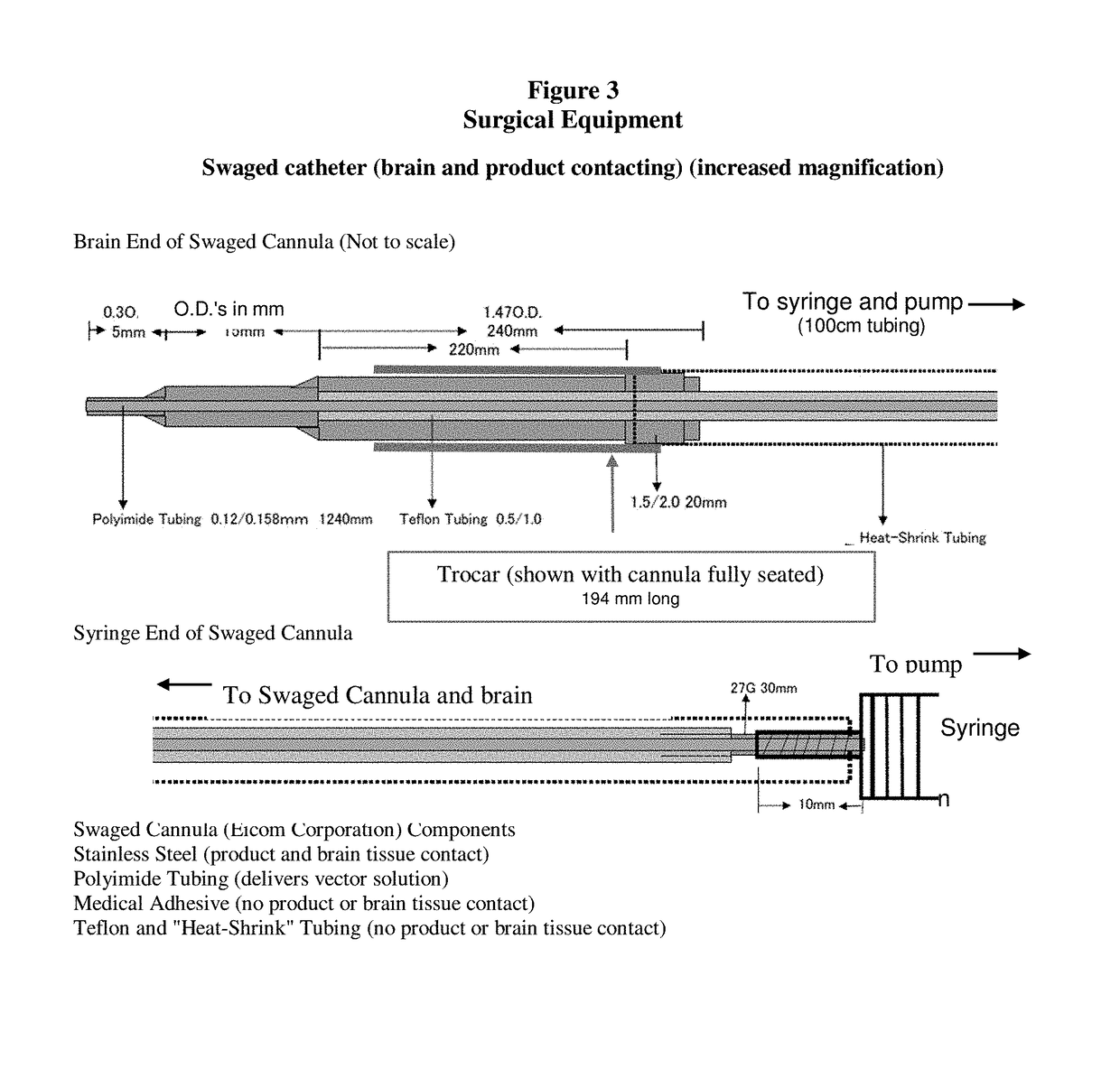
Gene therapy for aadc deficiency
The present invention is directed to compositions and methods for treating aromatic L-amino acid decarboxylase (AADC) deficiency. This invention includes a method of treating AADC deficiency in a pediatric subject, comprising the steps of: (a) providing a pharmaceutical formulation comprising an rAAV2-hAADC vector, (b) stereotactically delivering the pharmaceutical formulation to at least one target site in the brain of the subject in a dose of an amount at least about 1.8×1011 vg; wherein delivering the pharmaceutical formulation to the brain is optionally by frameless stereotaxy, and optionally wherein the dose is an amount of at least about 2.4×1011 vg and in some embodiments wherein the pharmaceutical formulation comprises a rAAV2-hAADC vector concentration of about 5.7×1011 vg / mL. This invention is also directed to methods for treating aromatic L-amino acid decarboxylase (AADC) deficiency, wherein the method optionally further comprises the step of administering a therapeutically effective dose of dopamine-antagonist to the subject such as risperidone. This invention is also directed to methods for treating aromatic L-amino acid decarboxylase (AADC) deficiency, wherein the method optionally comprises providing a pharmaceutical formulation comprising an rAAV2-hAADC vector, and empty capsids.
Owner:NAT TAIWAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka.patsnap.com/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00001.png)
![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka.patsnap.com/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00002.png)
![Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Process for the preparation of anti-psychotic 3-[2-[-4-(6-fluoro-1,2-benziosoxazol-3-yl)-1-piperidinyl] ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one](https://images-eureka.patsnap.com/patent_img/6ef6b91a-1550-486d-94a6-449c3ba4f3bd/US06897308-20050524-C00003.png)