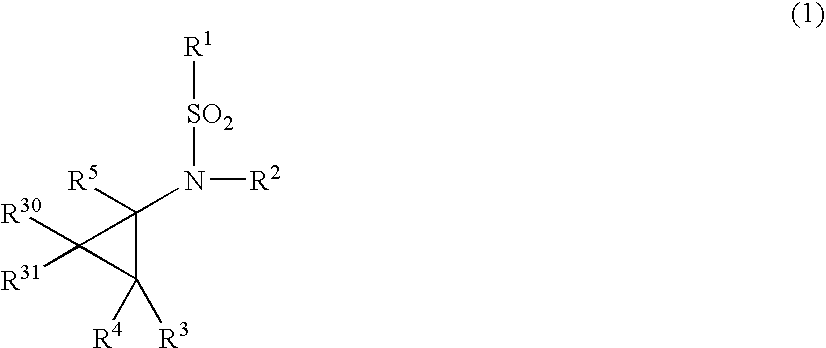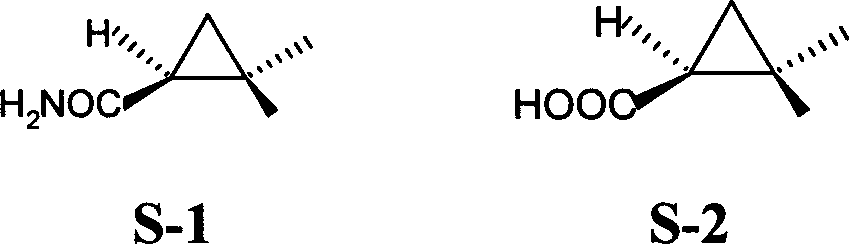Patents
Literature
161 results about "Cyclopropanation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics (ciprofloxacin, sparfloxacin, etc.). However the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
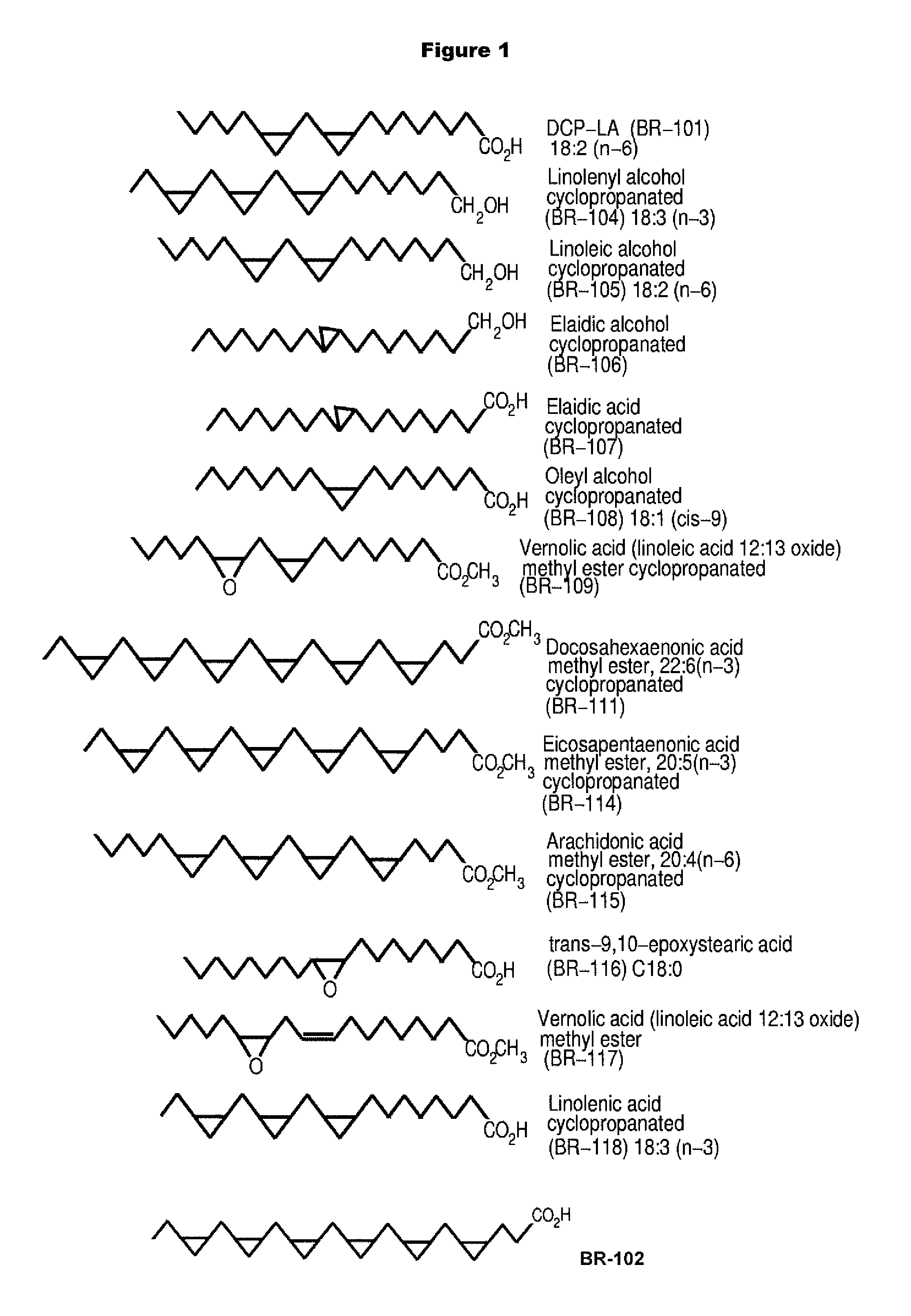
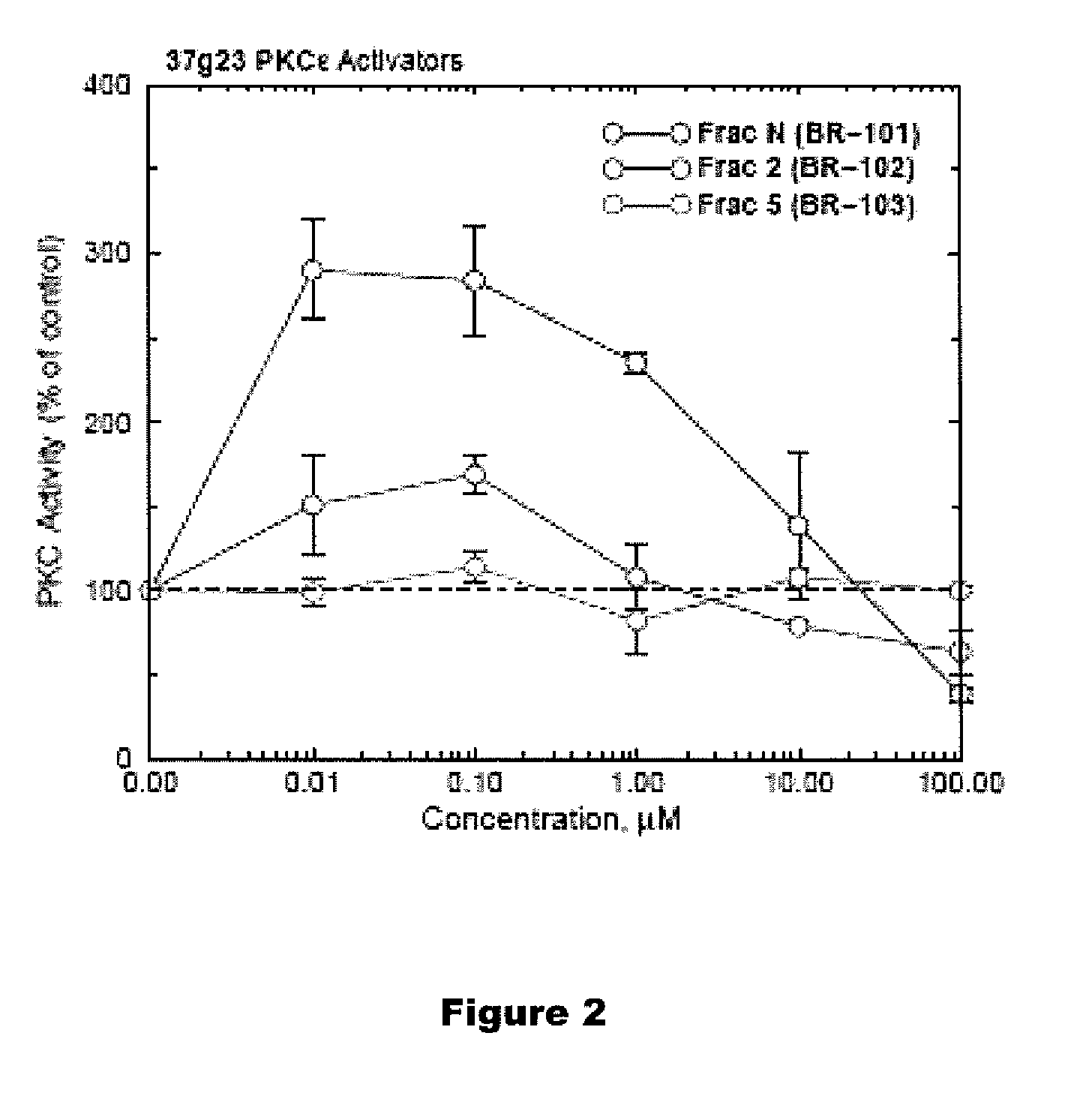
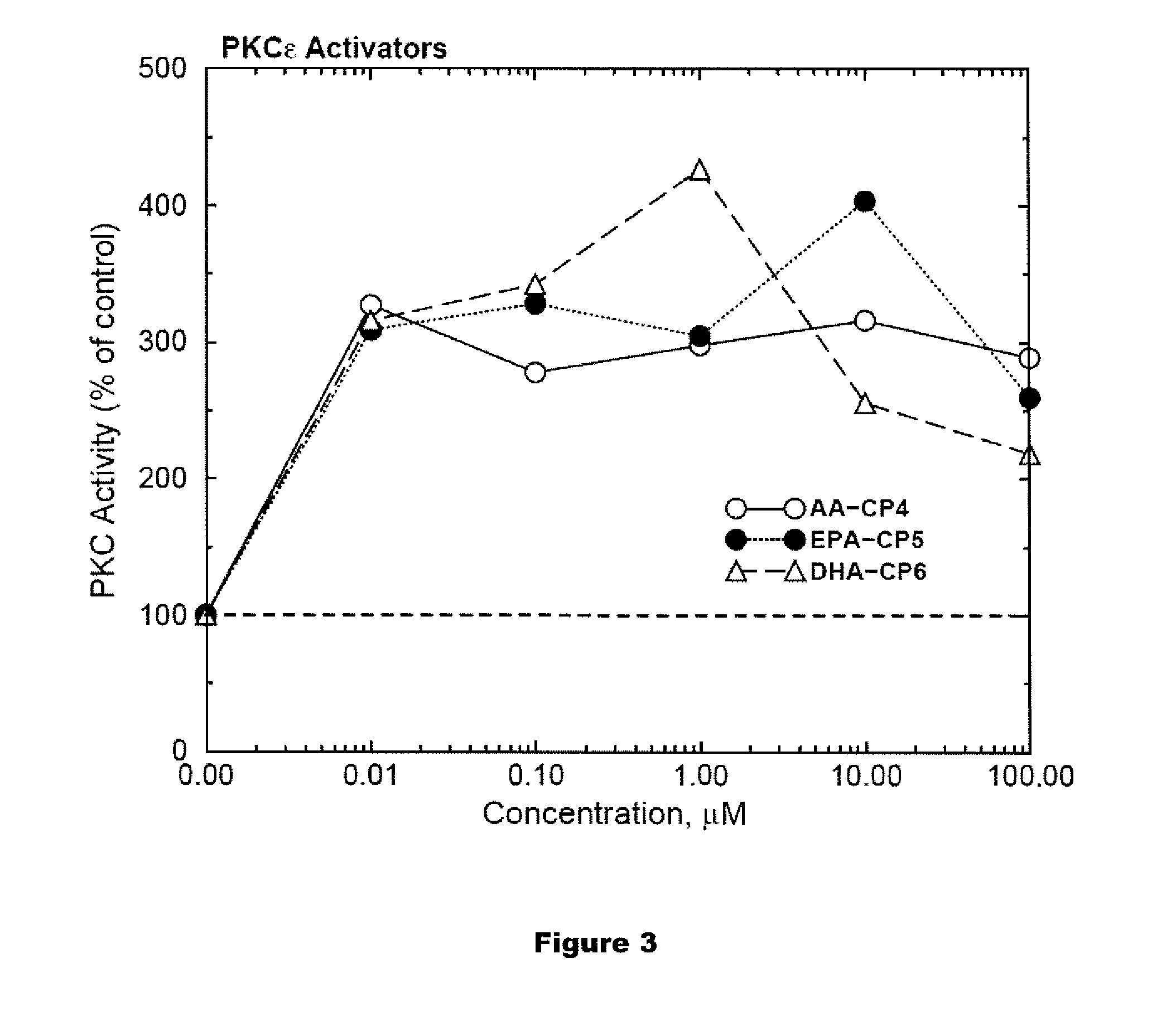
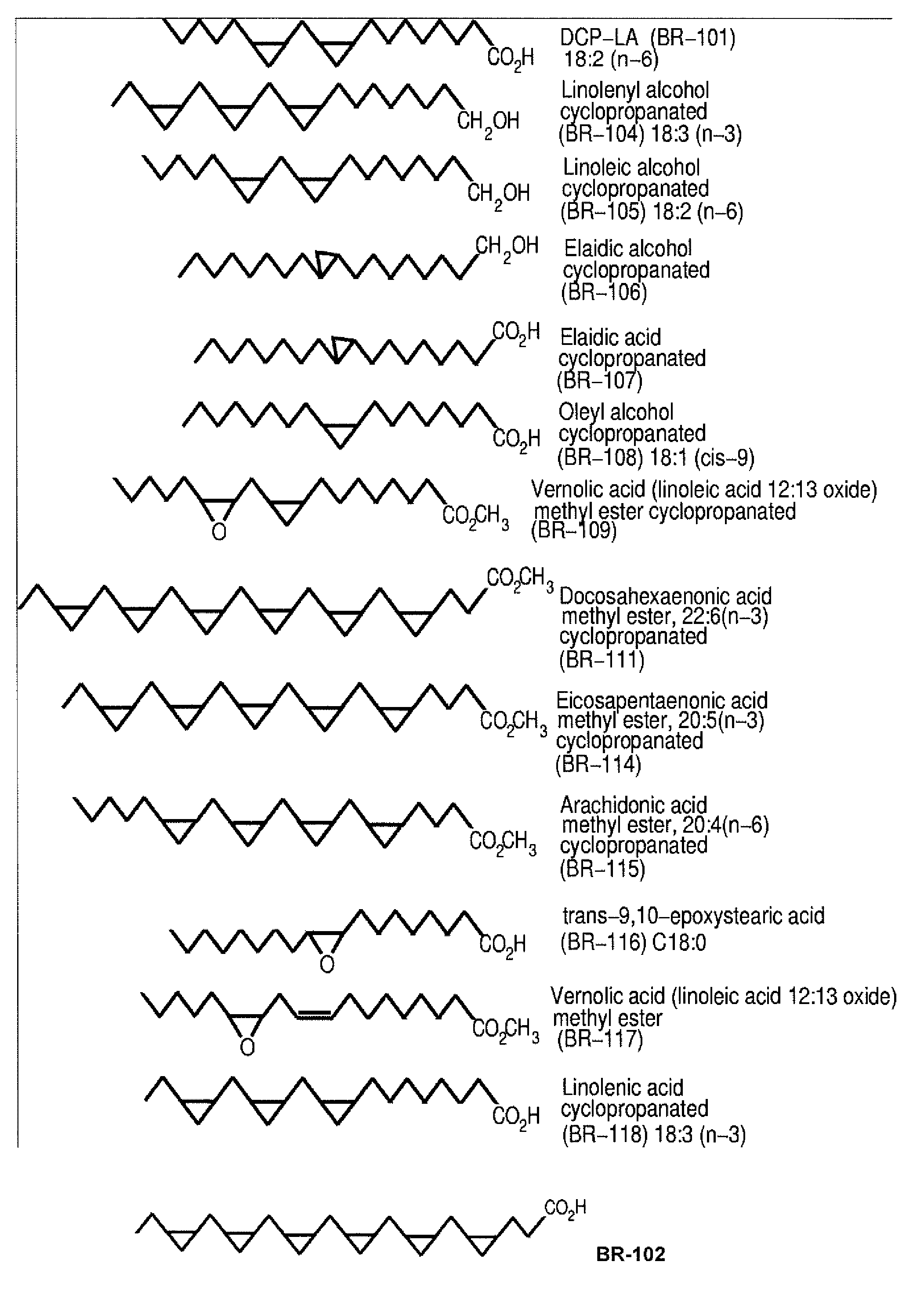
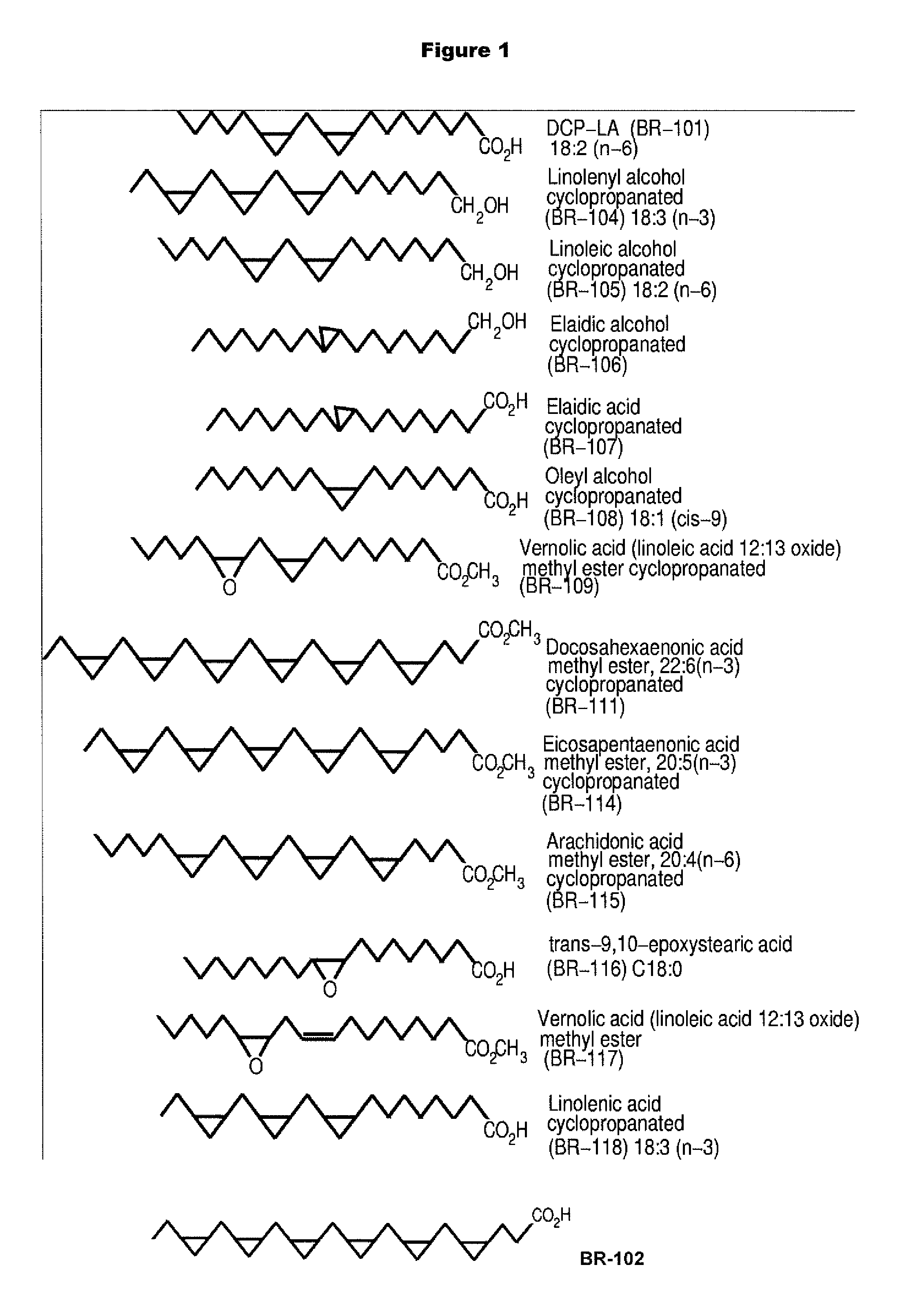
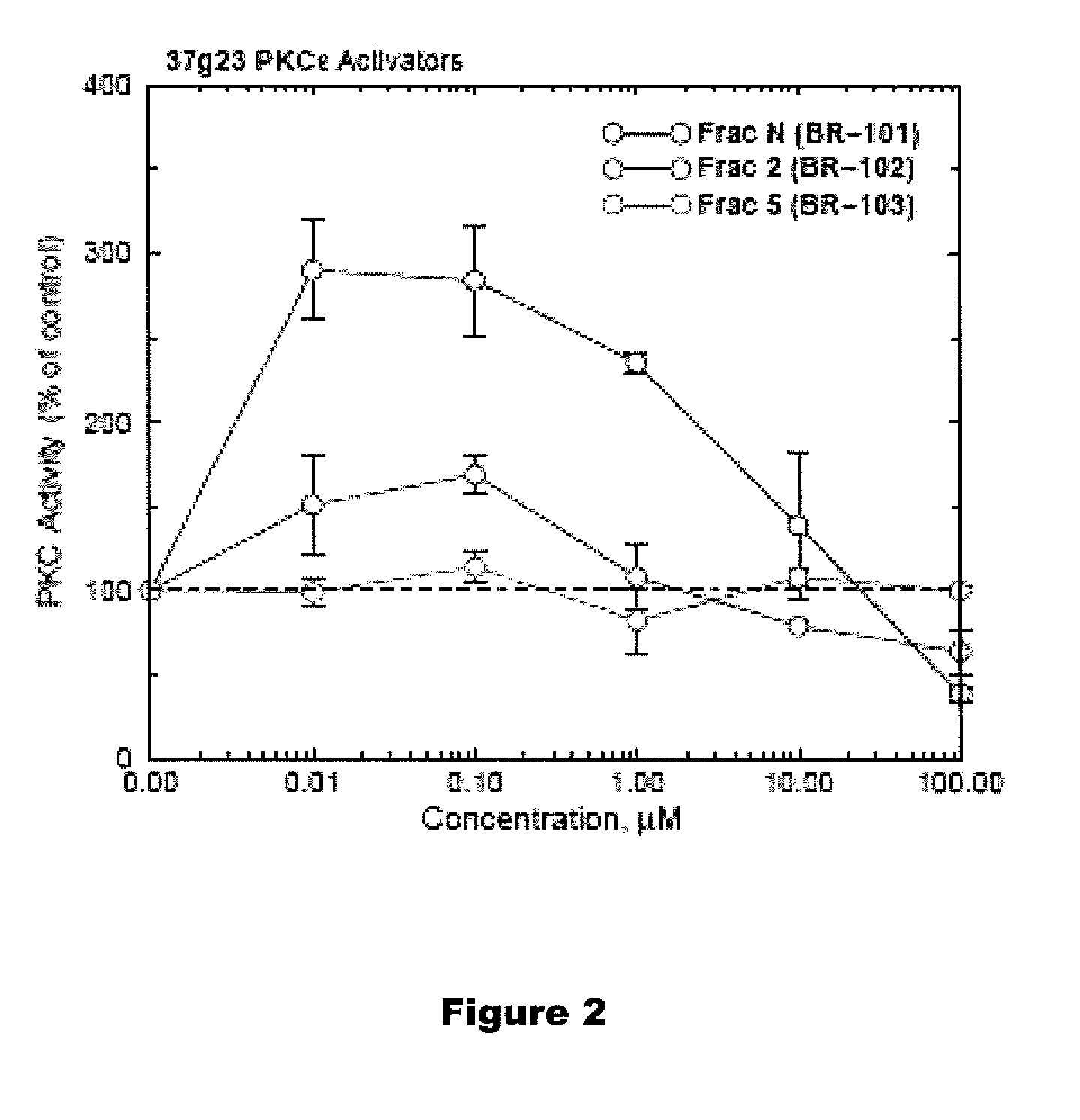
PKC-Activating Compounds for the Treatment of Neurodegenerative Diseases
The present invention relates to methods of activate an isoform of protein kinase C (PKC) for the treatment of neurological diseases including Alzheimer's disease and stroke using cyclopropanated or epoxidized derivatives of mono- and polyunsaturated fatty acids. The present invention also relates to methods of reducing neurodegeneration using cyclopropanated or epoxidized derivatives of mono- and polyunsaturated fatty acids.
Owner:COGNITIVE RES ENTERPRISES INC
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS7754902B2High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
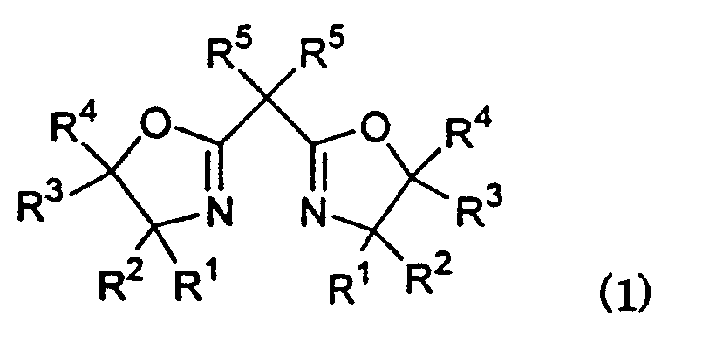
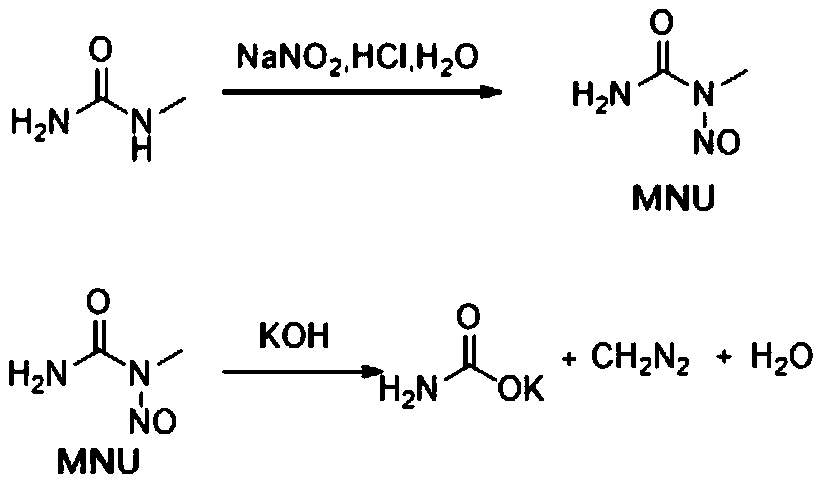
Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one
InactiveUS20100168463A1High yieldEasy to separateOrganic compound preparationCarboxylic acid esters preparationArylAcid hydrolysis
A method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one comprising subjecting a cyclopropane compound of the formula (1):(wherein, R1 represents an alkyl group, R2 represents an alkyl group having carbon atom(s) of 1 to 10, a haloalkyl group having carbon atom(s) of 1 to 10, or an aryl group having carbon atoms of 6 to 10 optionally substituted by an alkyl group having carbon atom(s) of 1 to 10, and when R2 represents the alkyl group, R1 and R2 are optionally the same or different each other.) to any of the following reactions a), b) and c):a) an acid treatment reaction after an alkali hydrolysis reactionb) an acid hydrolysis reactionc) an enzyme hydrolysis reaction , then, removing an aqueous layer.
Owner:SUMITOMO CHEM CO LTD
Ruthenium(II) catalysts for use in stereoselective cyclopropanations
ActiveUS20070270593A1High stereoselectivityHigh yieldRuthenium organic compoundsCobalt organic compoundsProtonationSalen ligand
Chiral ruthenium catalysts comprising salen and alkenyl ligands are provided for stereoselective cyclopropanation, and methods of cyclopropanation are provided. The chiral ruthenium catalyst is prepared in situ by combining an alkenyl ligand, a deprotonated chiral salen ligand, and a ruthenium (II) metal. A preferred catalyst is prepared in situ by combining 2,3-dihydro-4-venylbenzofuran, deprotonated 1,2-cyclohexanediamino-N,N′-bis(3,5-di-t-butyl-salicylidene) and RuCl2(p-cymene)]2.
Owner:VANDA PHARMA INC
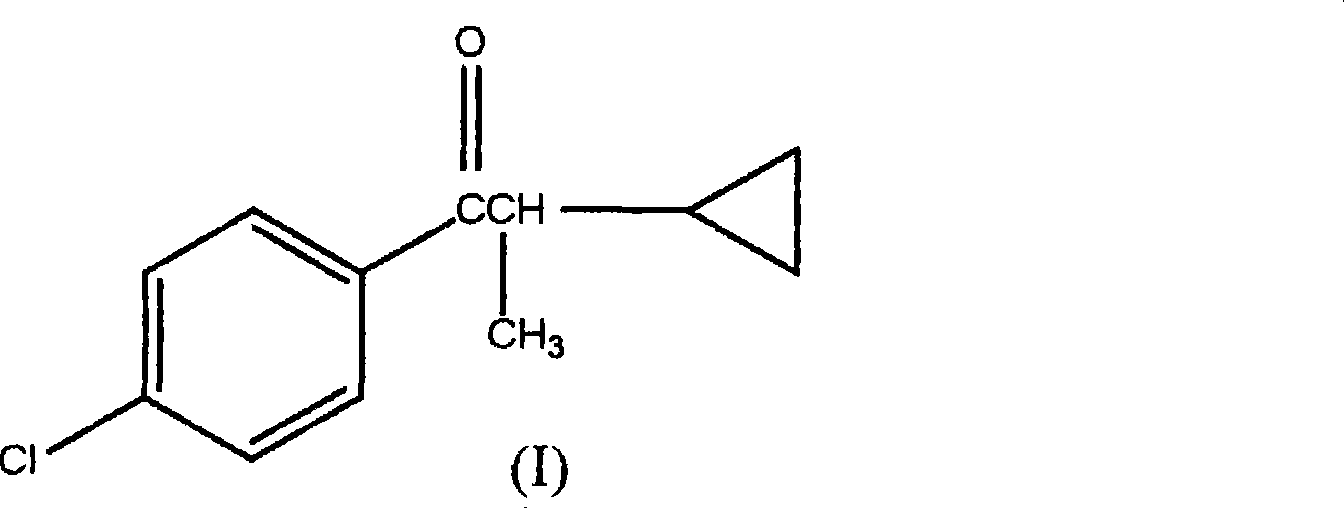
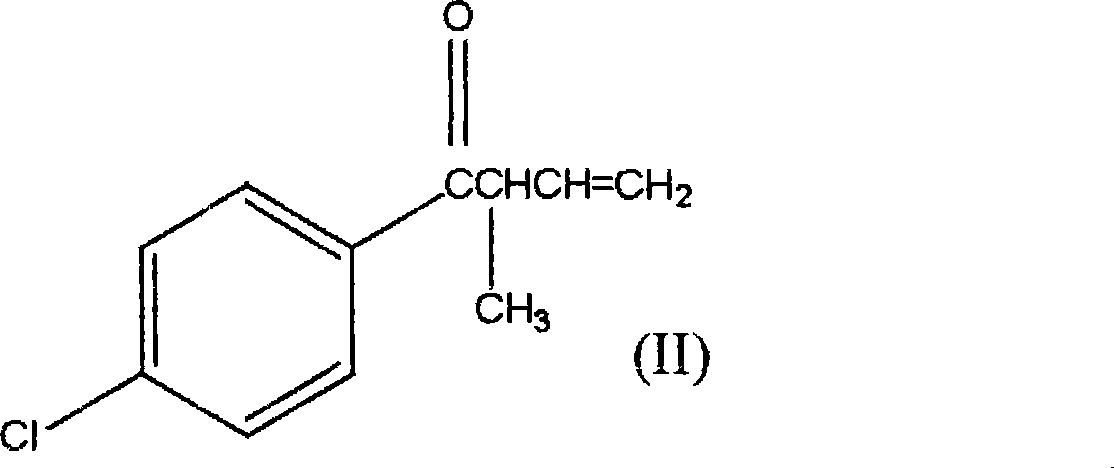
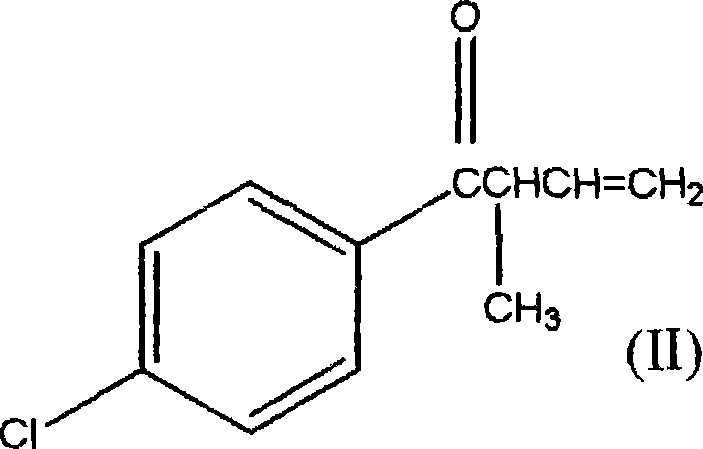
1-(4-chlorophenyl)-2-cyclopropyl-1-acetone and preparation method for intermediate thereof
ActiveCN101125807ALow costImprove responseOrganic compound preparationCarbonyl compound preparation by condensationOrganic solventCrotyl chloride
The invention relates to a preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1- rolactone(I) and cyclopropanation of 1-(4-chlorphenyl)-2-methyl-3-butylene-1-ketone(II); and the 1-(4-chlorphenyl)-2-methyl-3-butylene-1-ketone(II) is produced by the reaction of parachloronitrile, crotyl chloride, zinc powder in tetrahydrofuran and alchlor and alchlor can also not be applied; (II) is reacted with dibromomethane, zinc powder in organic solvent and cuprous chloride to produce 1-(4-chlorphenyl)-2-cyclopropyl-1- rolactone(I). Midbody (II) can not be separated and cleaned in the process of reaction.
Owner:常州沃富斯农化有限公司 +2
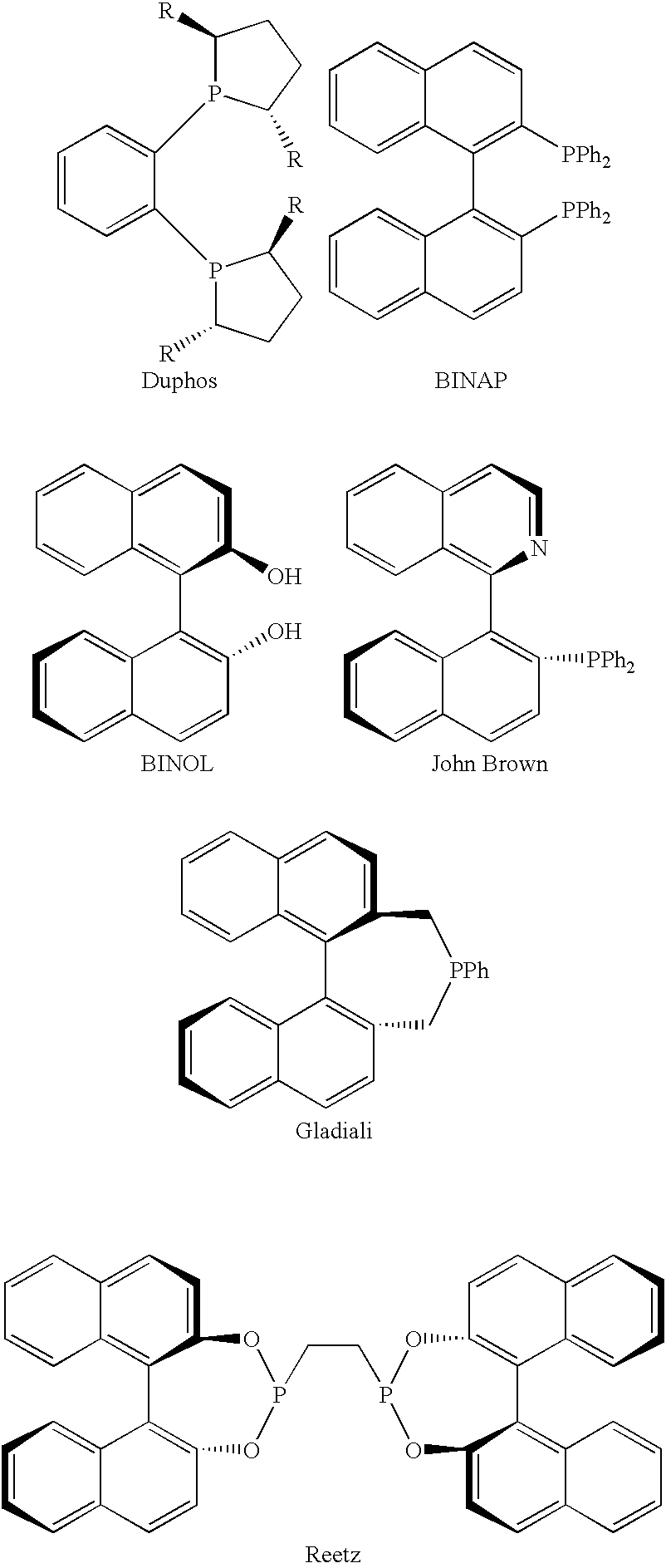
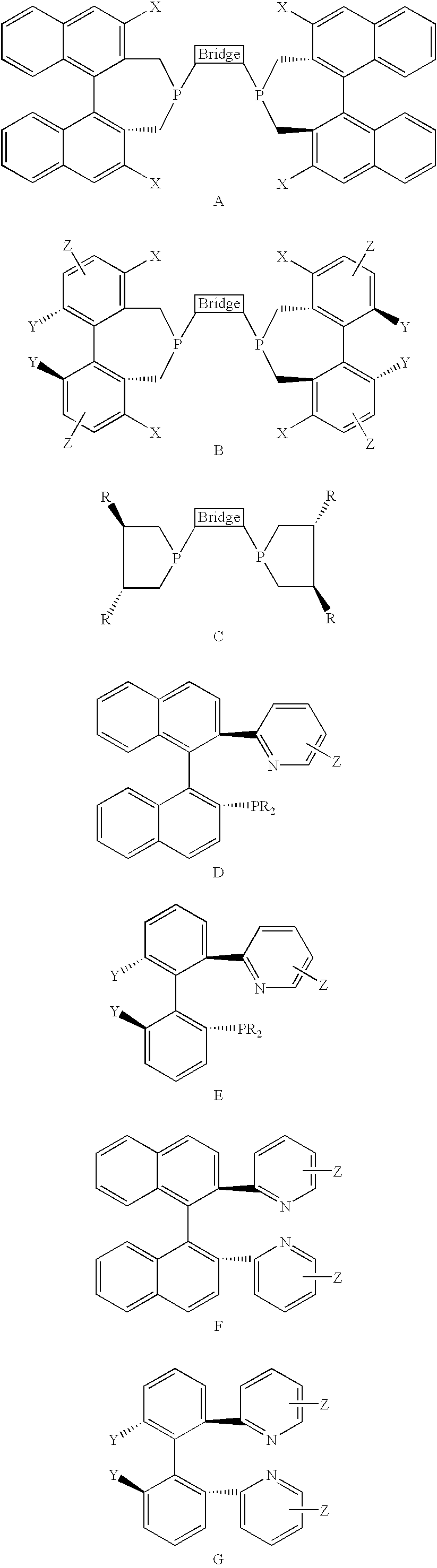
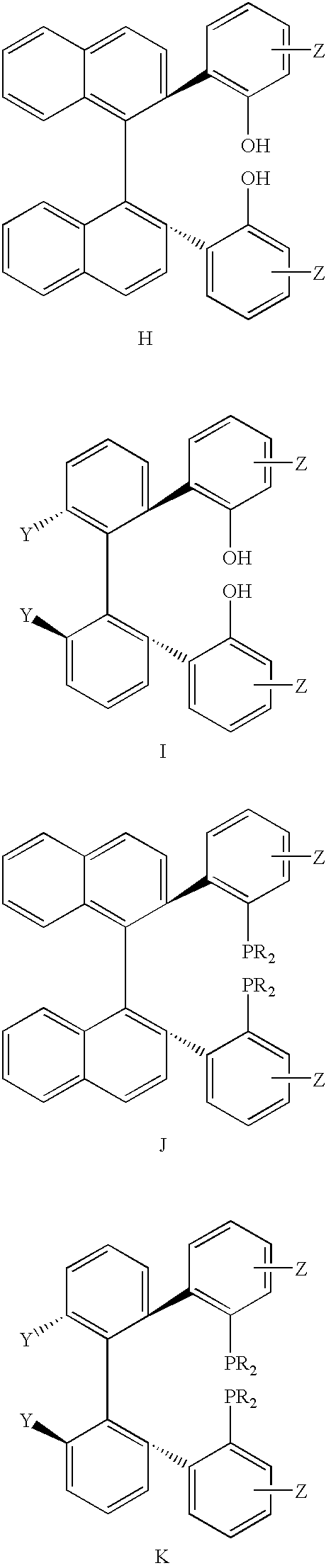
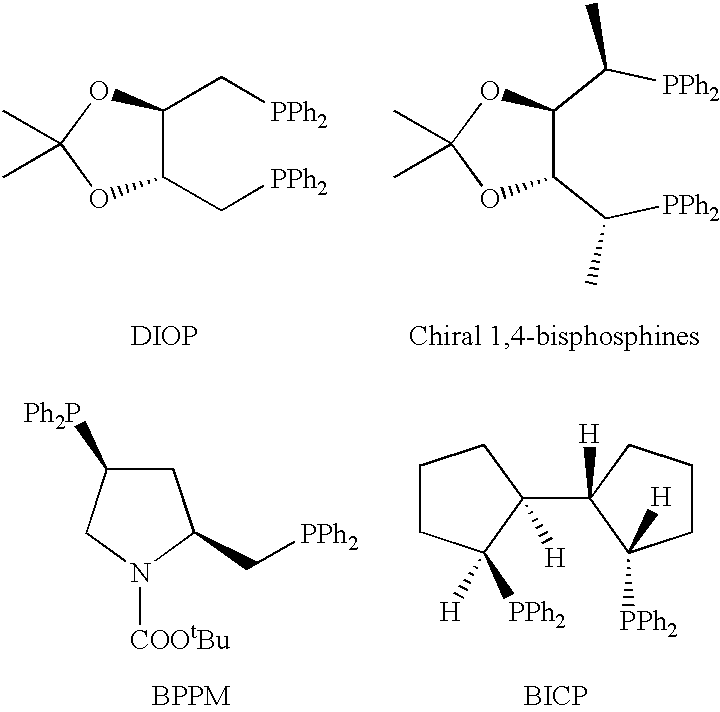
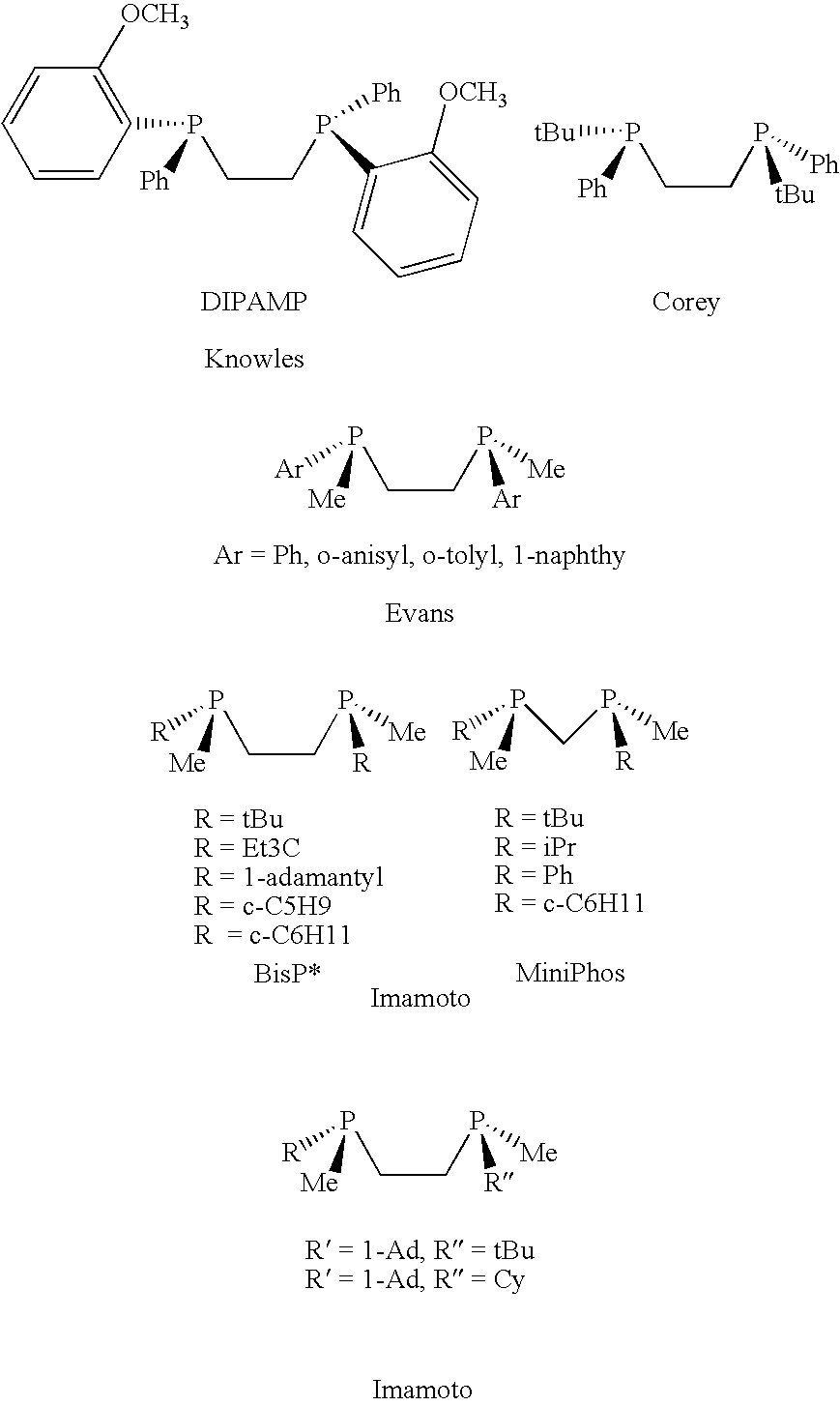
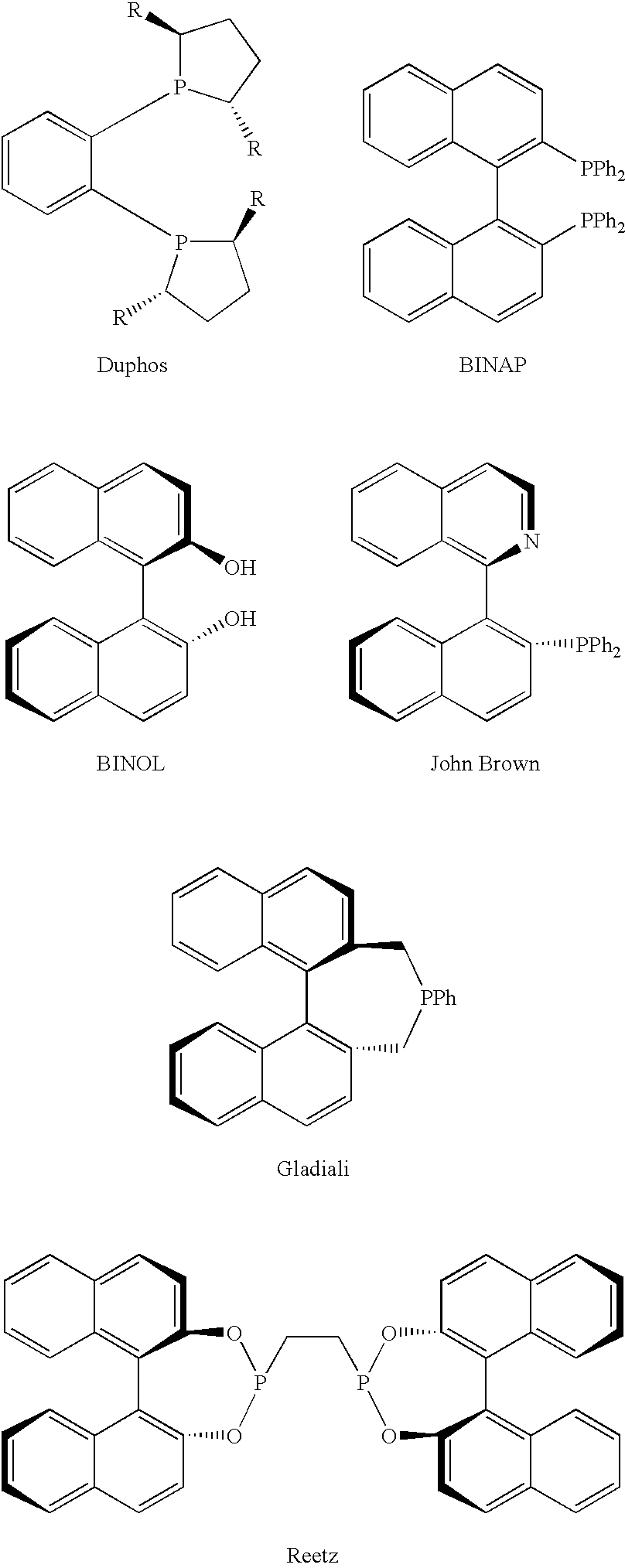
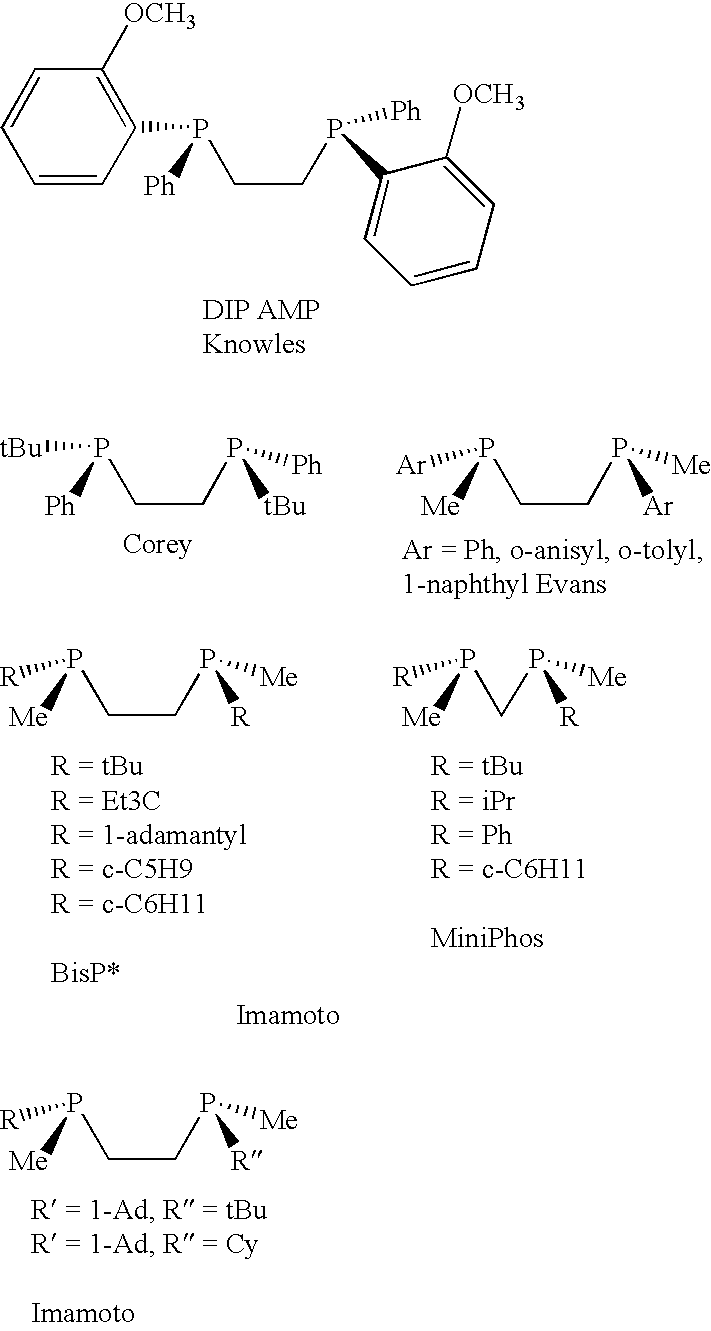
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6525210B1Carboxylic acid amides optical isomer preparationPreparation by carbon monoxide reactionIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
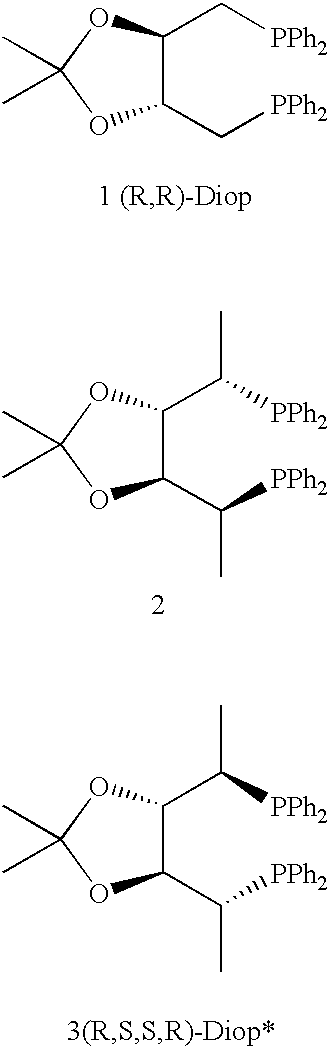
Chiral phosphines, transition metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6576772B1High enantioselectivityEnantioselectivity decreaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include (R,S,S,R)-DIOP*. The ruthenium complex reduces enamide to the corresponding amine with up to 99% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation, hydride transfer, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, isomerization, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
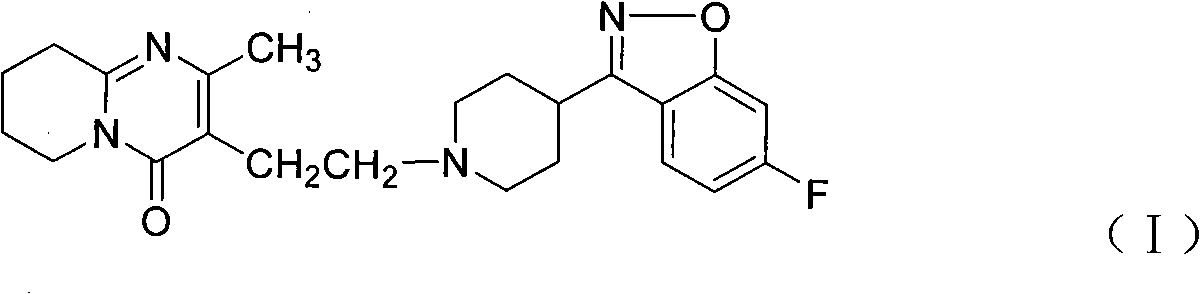
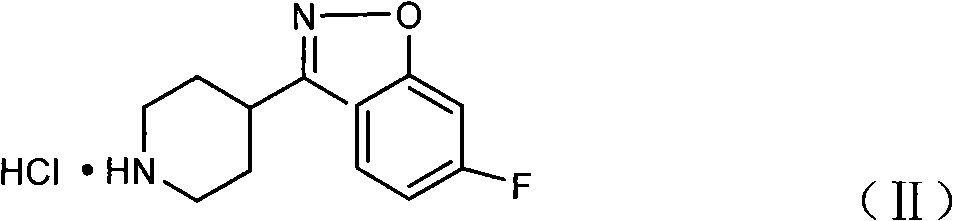
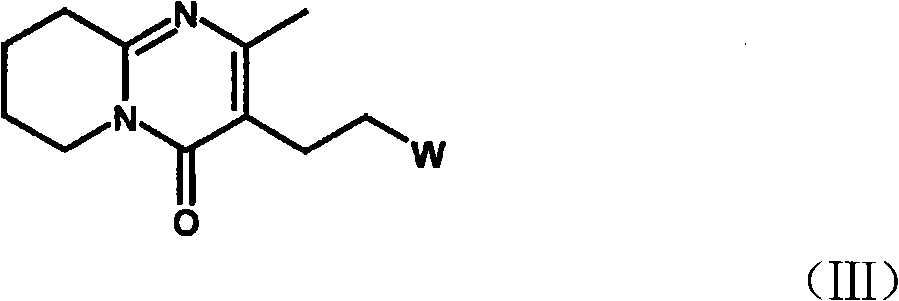
Method for preparing 6-fluoro-3-(4- piperidyl)-1,2-benzo isoxazole hydrochlorate
ActiveCN101328173APrevent participation in responseSolve the problems of many types, complicated operation and low yieldNervous disorderOrganic chemistryCyclopropanationPetroleum ether
The invention relates to a method for preparing a hydrochloride of 6-Fluoro-3-(4-piperidinyl)-1,2-benzisoxazole. The method is to subject 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to cyclopropanation and salifying in an aprotic solvent containing an alkali metal hydroxdide, wherein the molar ratio of the 2,4-difluorophenyl(4-piperidinyl) ketoxime or a hydrochloride thereof to the alkali metal hydroxide is 1 to between 1 and 3. The method can effectively prevent F atoms on para positions from participating in the reaction and avoid the production of a dipolymer (V), thereby avoiding the production of a dipolymer (VI) during the following preparation of risperidone. The method crystallizes crystals of a target substance directly by salifying, thereby solving the problems of use of a plurality of kinds of organic solvents, complex operation and low yield due to the extraction by toluene, condensation, and crystallization of petroleum ether. The yield rate of the target substance is more than 80 percent, and the purity of target substance is more than 99 percent.
Owner:CSPC OUYI PHARM CO LTD
Asymmetric copper compound and cyclopropanation reaction with it
InactiveCN1384105AGroup 1/11 organic compounds without C-metal linkagesOrganic compound preparationStrong acidsCombinatorial chemistry
Owner:SUMITOMO CHEM CO LTD
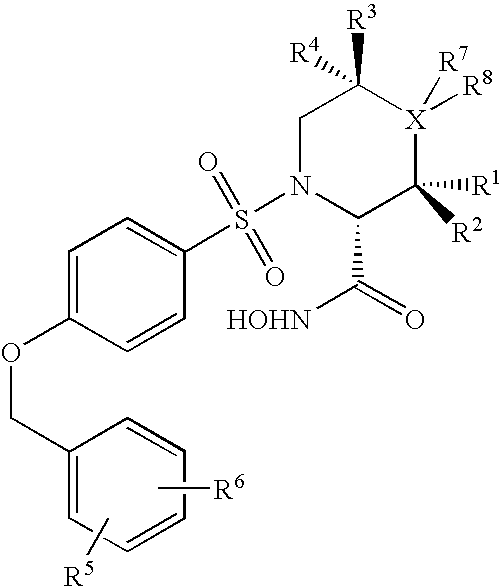
Cyclopropane compounds and pharmaceutical use thereof
InactiveUS20080261994A1Smoothen movement of jointIncrease oral availabilityBiocideOrganic chemistryChemical compoundPharmaceutical drug
The present invention provides a compound having aggrecanase inhibitory activity and MMP-13 inhibitory activity, and useful as a therapeutic agent for osteoarthritis, rheumatoid arthritis and the like, more specifically, a cyclopropane compound of formula (1):wherein R1 is —(CH2)m—X—(CH2)n-A1 etc., wherein m and n are the same or different and each is 0 to 6, X is a single bond, etc. and A1 is a substituted C3-14 hydrocarbon ring group, etc.; R2 and R3 are the same or different and each is a hydrogen atom, —(CH2)p—X1—(CH2)q-A2, etc., wherein p and q are the same or different and each is 0 to 6, X1 is a single bond, etc. and A2 is an optionally substituted C3-14 hydrocarbon ring group, etc.; R4 is —CO2R9, etc., wherein R9 is a hydrogen atom, etc.; and R20 and R21 are the same or different and each is a hydrogen atom, —(CH2)m12—X12—(CH2)m12—R30, etc., wherein m12 and m12 are the same or different and each is 0 to 6, X12 is a single bond, etc. and R30 is a hydrogen atom, etc.; or a prodrug thereof or a pharmaceutically acceptable salt thereof.
Owner:JAPAN TOBACCO INC
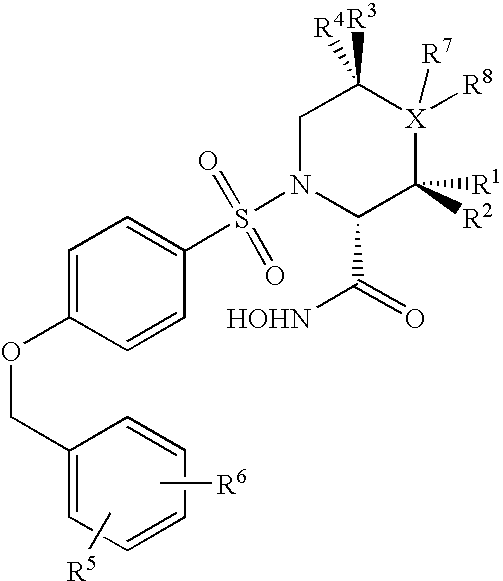
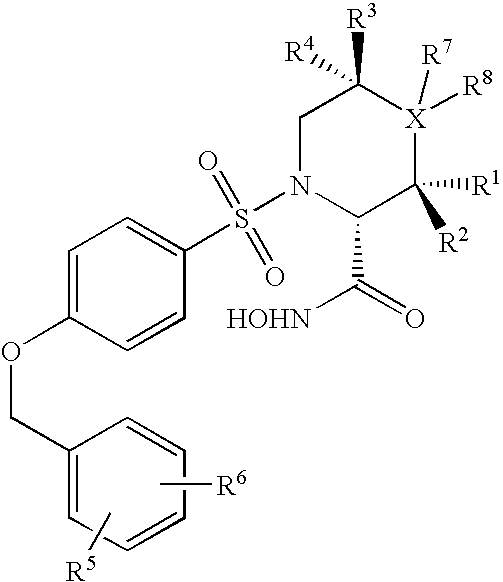
N-substituted-n-sulfonylaminocyclopropane compounds and pharmaceutical use thereof
InactiveUS20050222146A1Superior aggrecanase inhibitory activity activitySmoothen movement of jointBiocideOrganic chemistryHydrogen atomAlkoxy group
The present invention provides a compound having aggrecanase inhibitory activity and MMP-13 inhibitory activity, and useful as a therapeutic agent for osteoarthritis, rheumatoid arthritis and the like, more specifically, a N-substituted-N-sulfonylaminocyclopropane compound of formula (1): wherein R1 is —W-A1—W1-A2, W is —(CH2)m—X—(CH2)n—, wherein W1 is —(CH2)m1—X1—(CH2)n1—, m, m1, n and n1 are the same or different and each is 0 to 6, X and X1 are the same or different and each is a single bond, etc., A1 is an optionally substituted C3-14 hydrocarbon ring group, etc. and A2 is a substituted C3-14 hydrocarbon ring group etc.; R2 is —(CH2)r—CO—R8, etc., wherein r is 0 to 6 and R8 is a C1-6 alkoxy group, etc.; R3 and R4 are the same or different and each is a hydrogen atom, a C1-6 alkyl group, etc.; and R5 is —CO2R21 etc.; R30 and R31 are the same or different and each is a hydrogen atom, etc.; or a prodrug thereof or a pharmaceutically acceptable salt thereof.
Owner:JAPAN TOBACCO INC
Catalyst for cyclopropanizing reaction of olefine and its preparing process
A carried catalyst used for cyclopropanizing 2,5-dimethyl-2,4-hexadiene contains the active component (20-90%) chosen from Fe, Ca, Ni, Ca, Zn, Cr, Mo or Ti, the assistant (0.1-5%) chosen from L, Na or K, and the carrier chosen from alumina silica gel and activated carbon. It is prepared through premodifying carrier, and immersing while adding active component and the organic compound containing hydroxy or carboxyl group and oxygen. Its advantages are low dosage, high output rate, little coking and easy separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
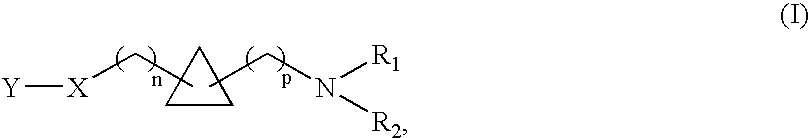
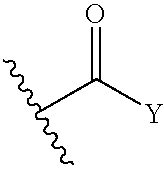
1,1- and 1,2-disubstituted cyclopropane compounds
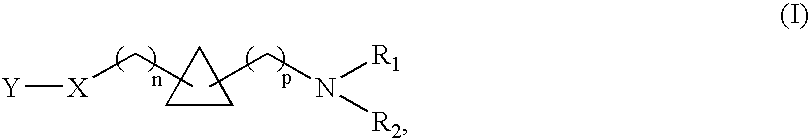
A compound selected from those of formula (I): wherein:p represents an integer of from 0 to 6 inclusive,n represents an integer of from 0 to 6 inclusive,R1, and R2 represent a group selected from hydrogen, alkyl, aryl and arylalkyl, or R1+R2 form together with nitrogen carrying them saturated, monocyclic, or bicyclic system,X represents a group selected from oxygen, sulphur, a group —CH═CH—, methylene, a group of formula —HC═N—O— and a group of formula —O—CH2—CH═CH—, in which groups oxygen is linked to Y of the compounds of formula (I),Y represents a group selected from aryl, heteroaryl, arylalkyl, heteroarylalkyl, —C(O)-A, and —C(S)-A,A represents a group selected from alkyl, aryl, heteroaryl, arylalkyl, heteroarylalkyl, and NR3R4 wherein R3, and R4 represent a group selected from hydrogen, alkyl, aryl, and arylalkyl, or R3+R4 form together with nitrogen carrying them monocyclic, or bicyclic (C3-C10) system,its isomers and addition salts thereof with a pharmaceutically-acceptable acid or base, and medicinal products containing the same which are useful as specific nicotinic ligand of α4β2 receptors.
Owner:ADIR & CO
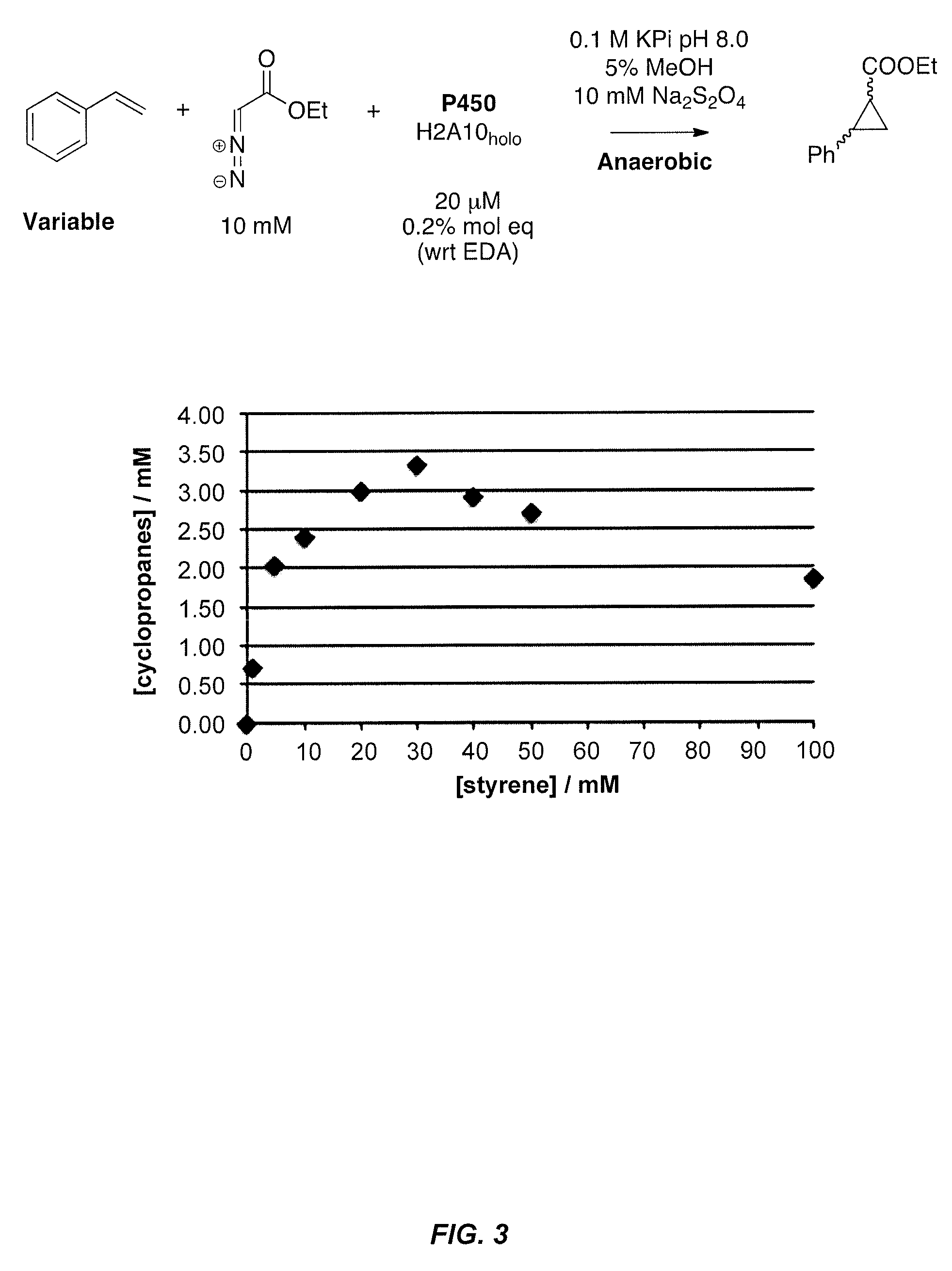
P-450-catalyzed enantioselective cyclopropanation of electron-deficient olefins
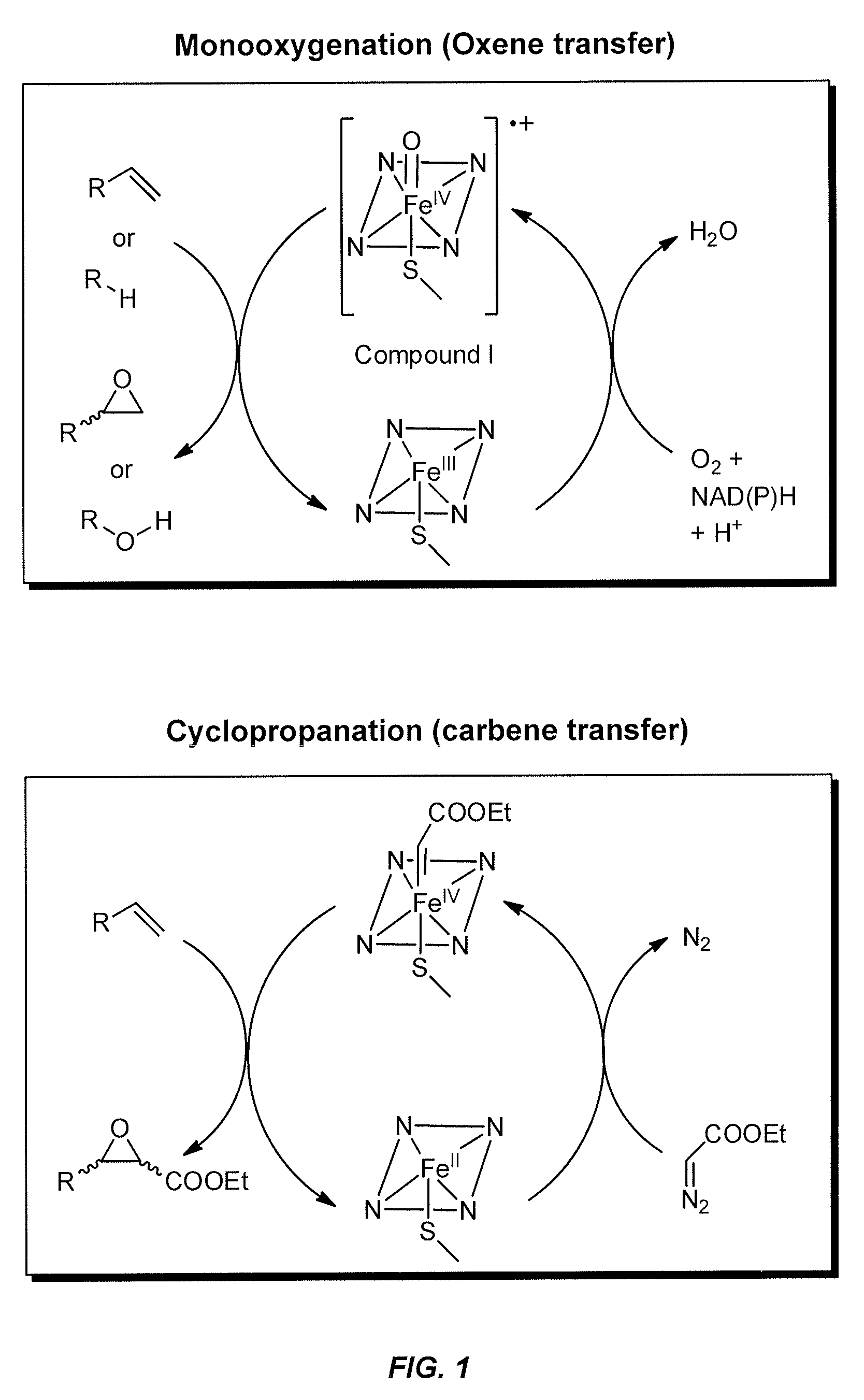
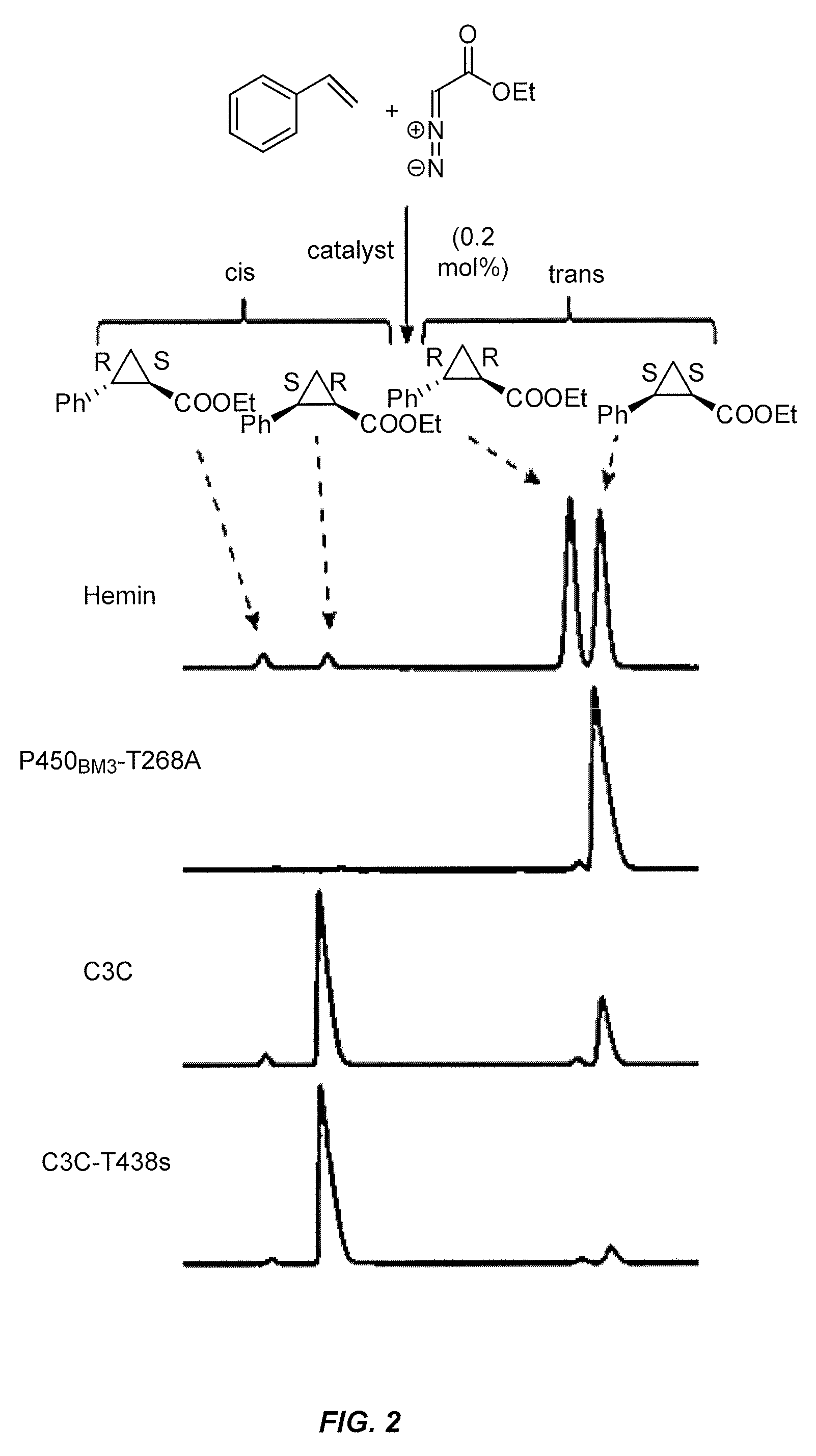
The present invention pertains to the use of engineered variants of enzyme CYP102A, also known as P450-BM3, for cyclopropanation of olefins containing electron-withdrawing groups. One exemplary enzyme variant, referred to as BM3-HStar, contains five mutations away from wild-type P450-BM3, and demonstrates high activity towards cyclopropanation of olefinic substrates using ethyldiazoacetate (EDA) and other carbene transfer reagents. Products of these reactions are potential precursors of levomilnacipran derivatives, a class of compounds that have been shown to be selective inhibitors of monoamine transporters. In addition, cyclopropanation reactions with the P450-BM3 enzyme variants of the invention can be conducted in whole cells expressing the enzyme variants and can proceed under aerobic conditions.
Owner:CALIFORNIA INST OF TECH
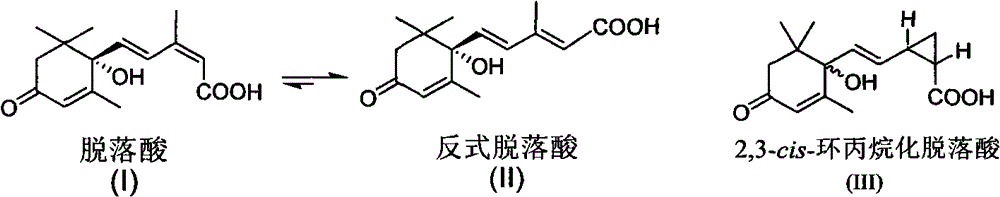
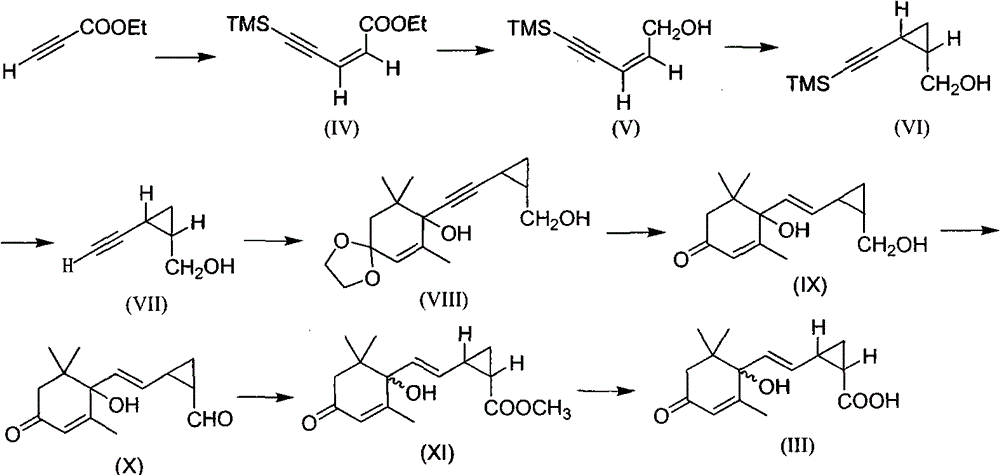
Photostable cis 2, 3-cyclopropanated abscisic acid analogue and preparation method thereof
An abscisic acid (1) is a plant hormone widely existing in a plant and has important physiological function, however, the application range is limited by the high-speed photo-isomerization characteristics. A bioisosteres cis 2, 3-cyclopropanated abscisic acid analogue of the abscisic acid is compounded through 2, 3-cyclopropanation, and the photostability is four times as that of the abscisic acid.
Owner:CHINA AGRI UNIV
PKC-activating compounds for the treatment of neurodegenerative diseases
The present invention relates to methods of activate an isoform of protein kinase C (PKC) for the treatment of neurological diseases including Alzheimer's disease and stroke using cyclopropanated or epoxidized derivatives of mono- and polyunsaturated fatty acids. The present invention also relates to methods of reducing neurodegeneration using cyclopropanated or epoxidized derivatives of mono- and polyunsaturated fatty acids.
Owner:COGNITIVE RES ENTERPRISES INC
Cyclopropane compounds and pharmaceutical use thereof
InactiveUS7351825B2Smoothen movement of jointIncrease oral availabilityBiocideOrganic chemistryChemical compoundPharmaceutical drug
Owner:JAPAN TOBACCO INC
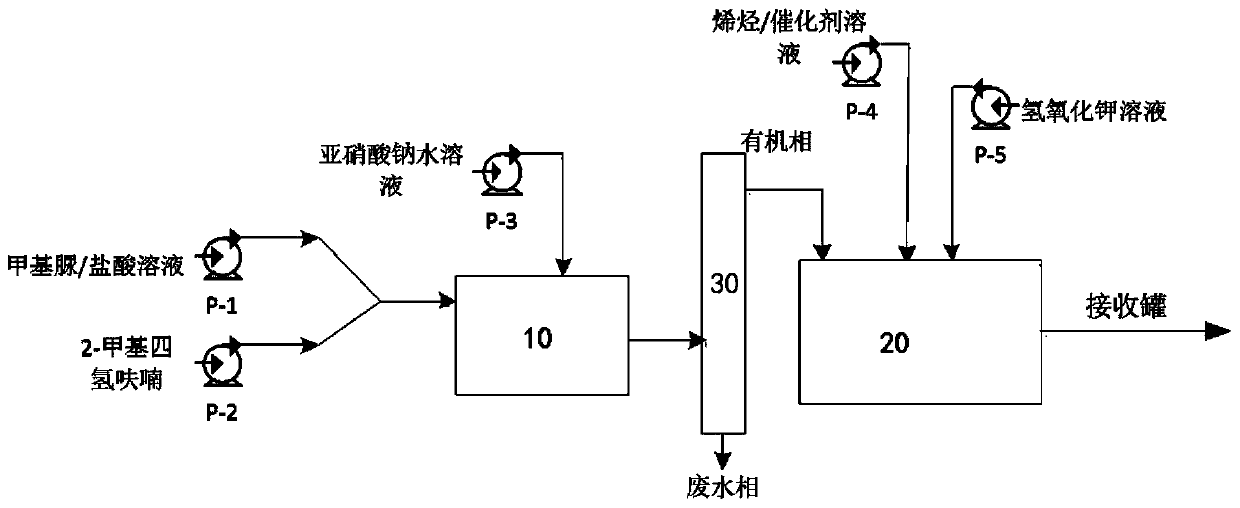
Method and device for continuously synthesizing cyclopropane compounds
PendingCN110577484AReduce transferAvoid the risk of pipeline diversionOrganic chemistryAutomatic controlCyclopropane
The invention discloses a method and a device for continuously synthesizing cyclopropane compounds. The method comprises the following steps of: continuously carrying out a synthesis reaction of a diazomethane precursor in a first reactor, flowing a reaction product of the first reactor into a separator for layering, overflowing an organic phase obtained by layering into a second reactor, continuously consuming the diazomethane precursor in the second reactor to prepare diazomethane, and carrying out an electron-rich mono-olefin cyclopropanation reaction in situ to obtain the cyclopropane compounds. The method can realize automatic control, reduce the transfer of high-risk materials, avoid the risk of pipeline transfer of a diazomethane solution, and effectively improve the production safety, also has simple equipment, can save equipment investment, and can safely and quantitatively realize simultaneous diazomethane generation and olefin cyclopropanation reaction.
Owner:ASYMCHEM LAB TIANJIN
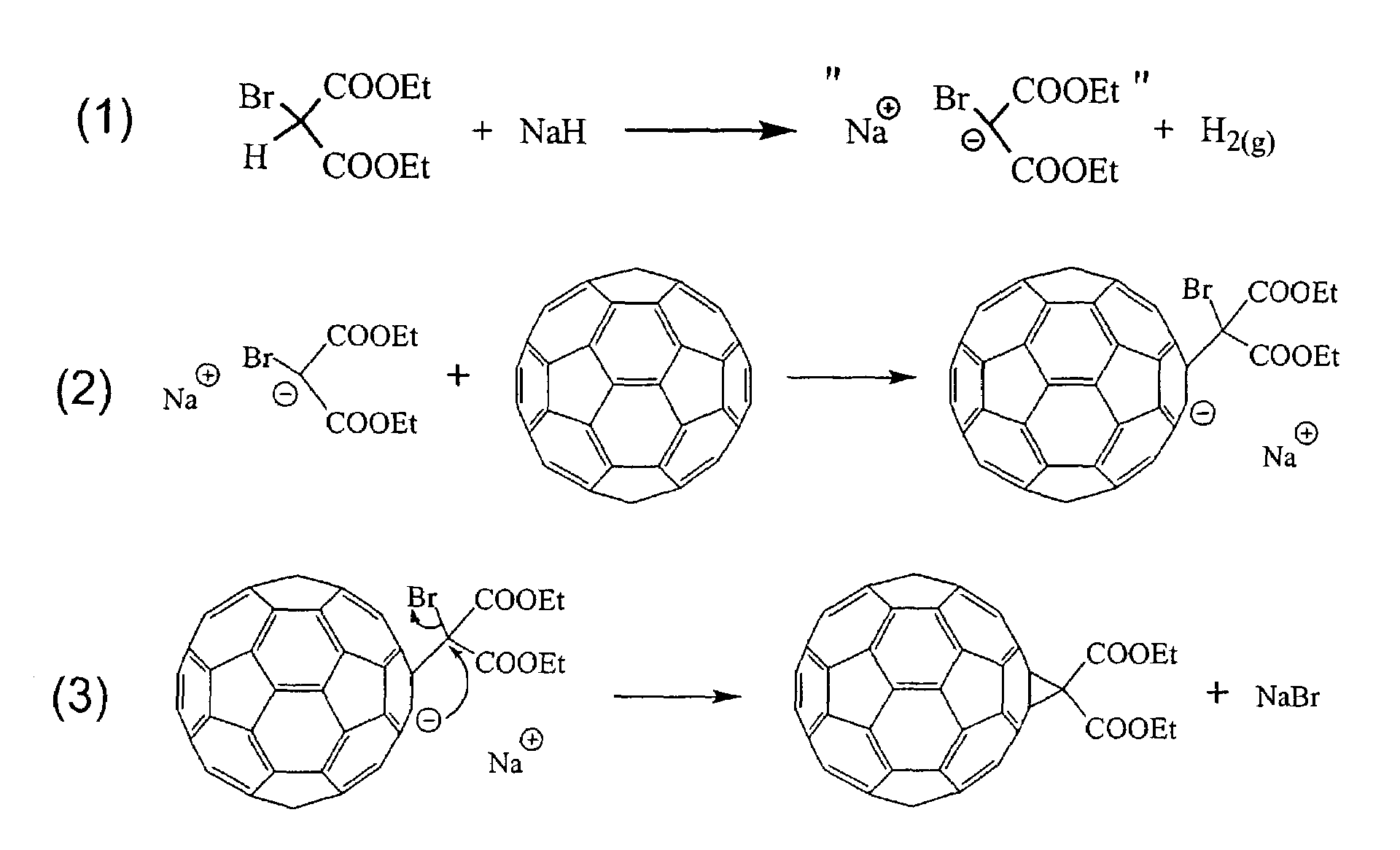
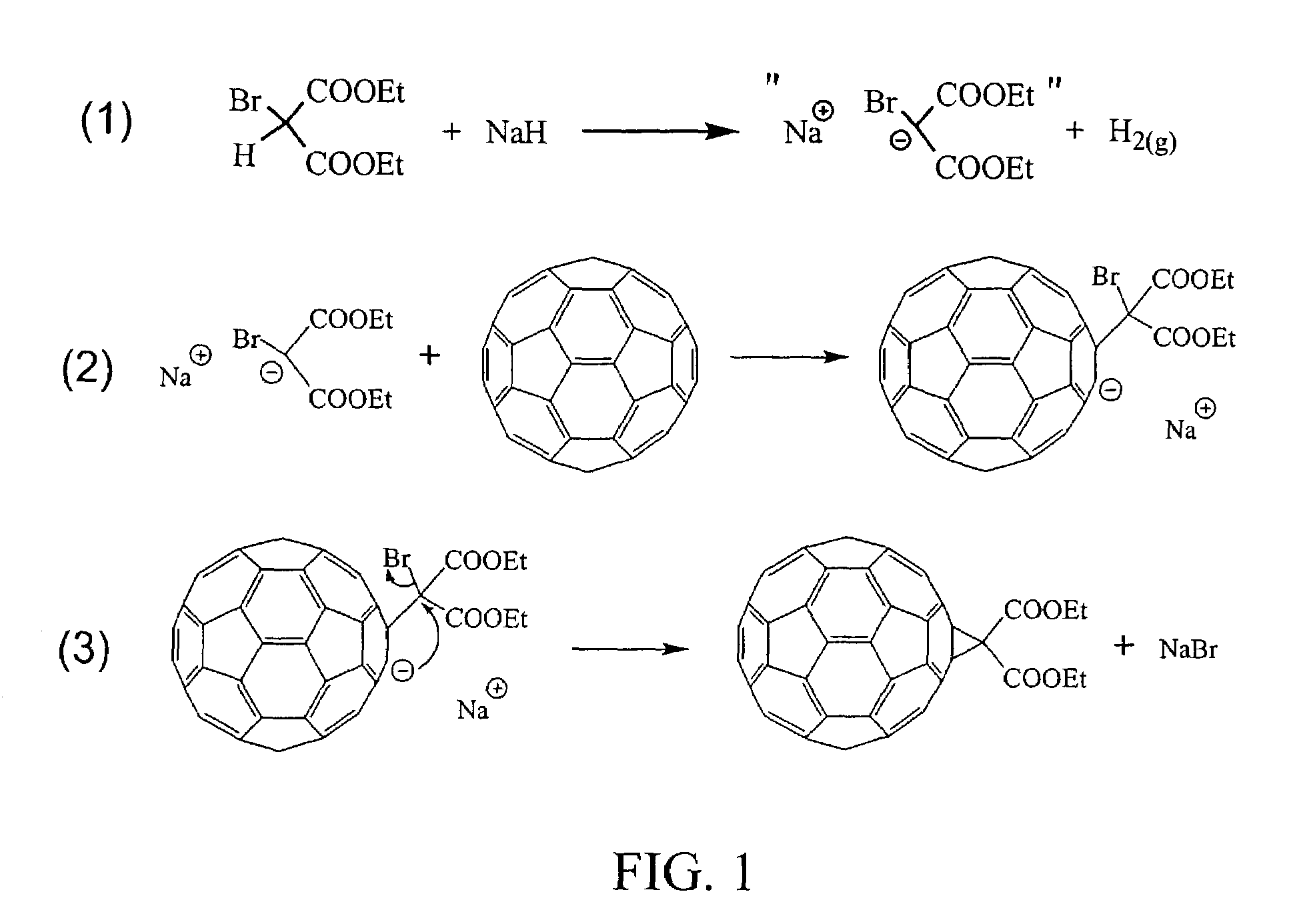
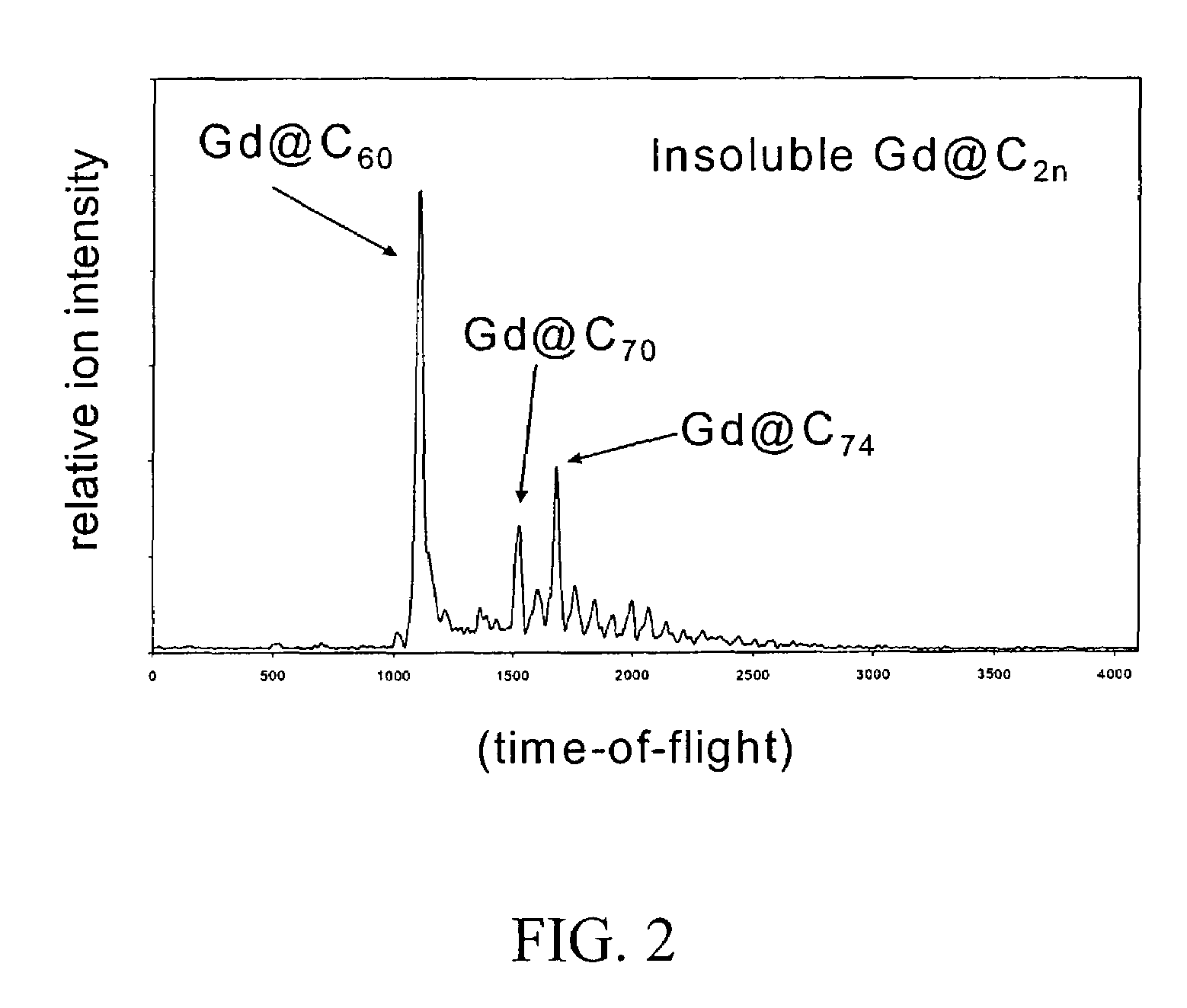
Derivatization and solubilization of insoluble classes of fullerenes
InactiveUS7671230B2Increase the number ofImprove efficiencyMaterial nanotechnologyOrganic compound preparationCarbon nanotubeImproved method
This invention provides improved methods for the derivatization and solubilization of fullerenes, which are particularly useful for those fullerenes that are normally insoluble and which are specifically applied, among others, to endohedral fullerenes, including endohedral metallofullerenes; empty fullerenes, including small-bandgap fullerenes and other insoluble fullerenes and to very high molecular weight fullerenic materials generated in fullerenic soot, including giant fullerenes, fullerenic polymers, carbon nanotubes and metal-carbon nanoencapsulates. More specifically the invention relates to improved methods for cyclopropanation of fullerenes. Specific reaction conditions are provided which allow for cyclopropanation reactions to be successfully performed for the first time on insoluble classes of fullerenes. Also provided is a method for purification of one or more fullerenes from a fullerenic material containing the one or more fullerenes in addition to non-fullerenic carbonaceous material, particularly amorphous carbonaceous material, by derivatizing one or more fullerenes using the methods of the invention and separating soluble derivatizes fullerenes from insoluble materials.
Owner:TDA RES
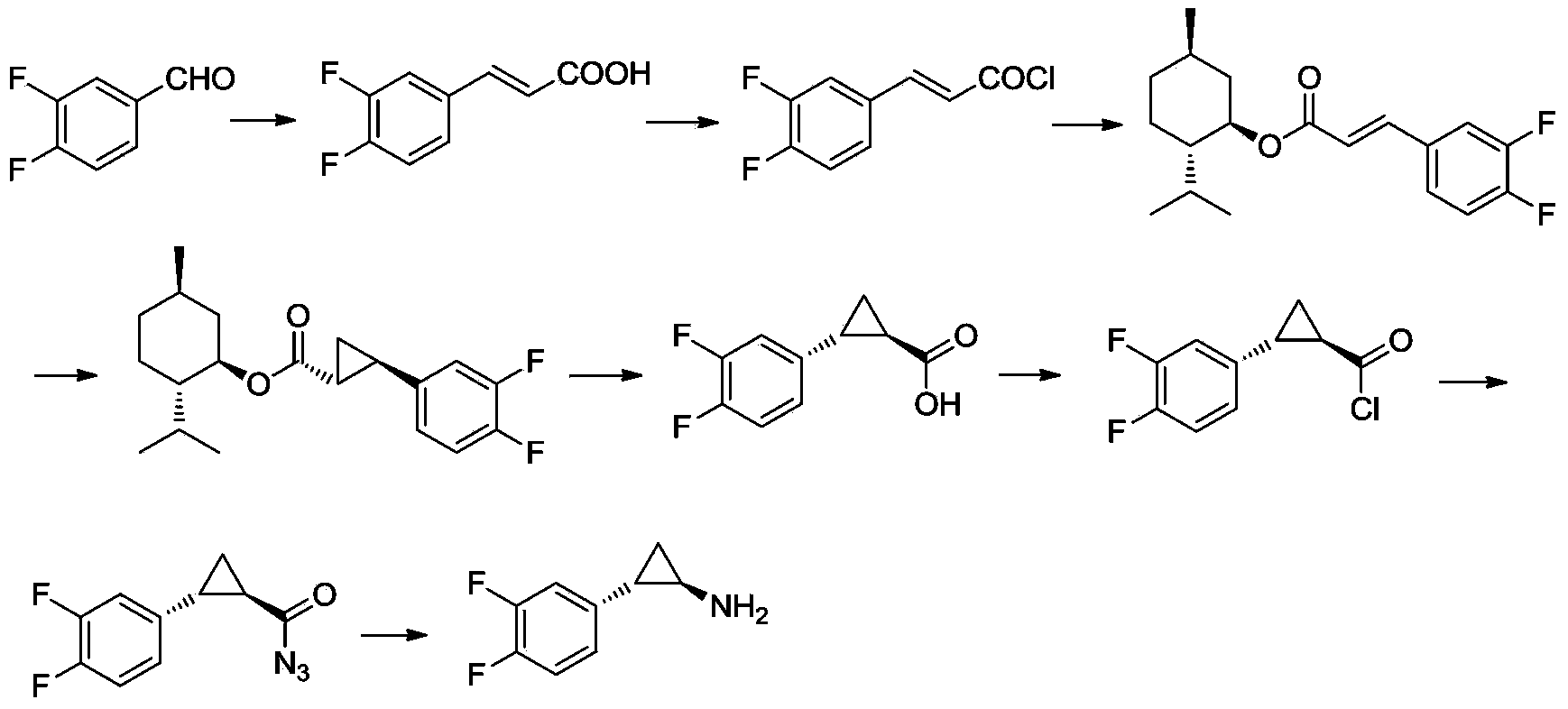
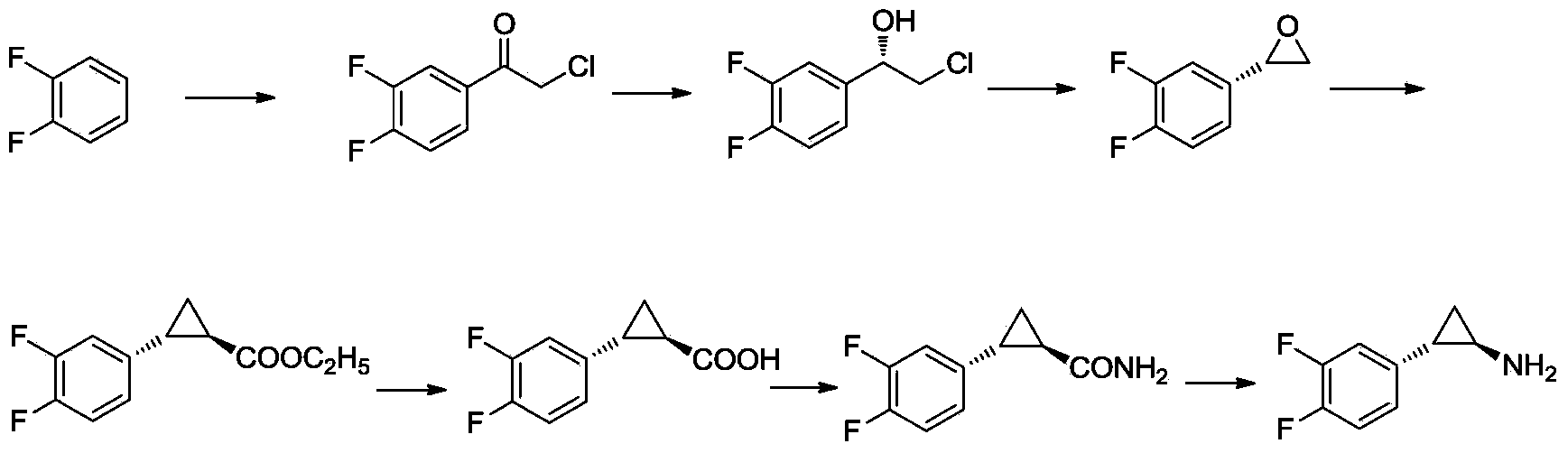
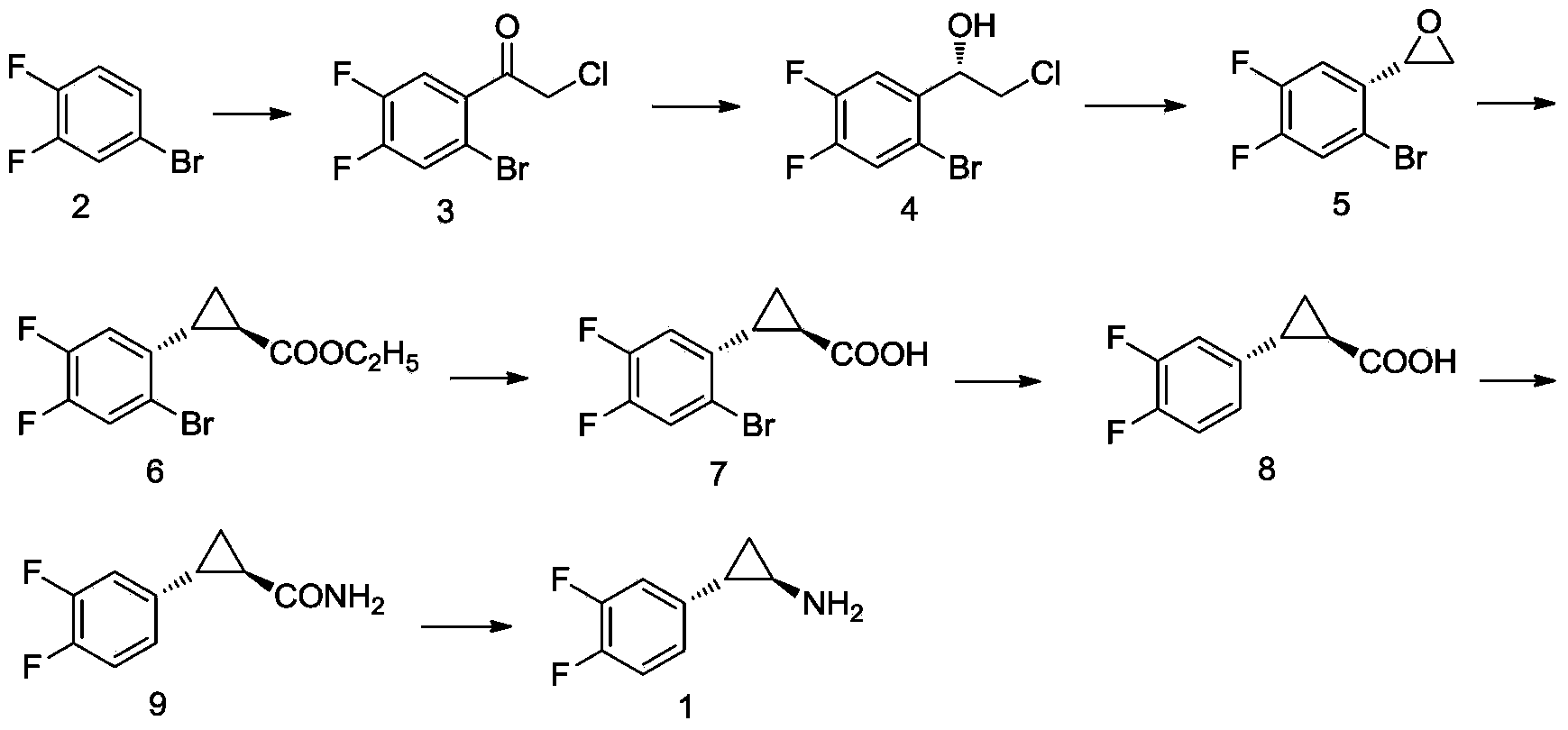
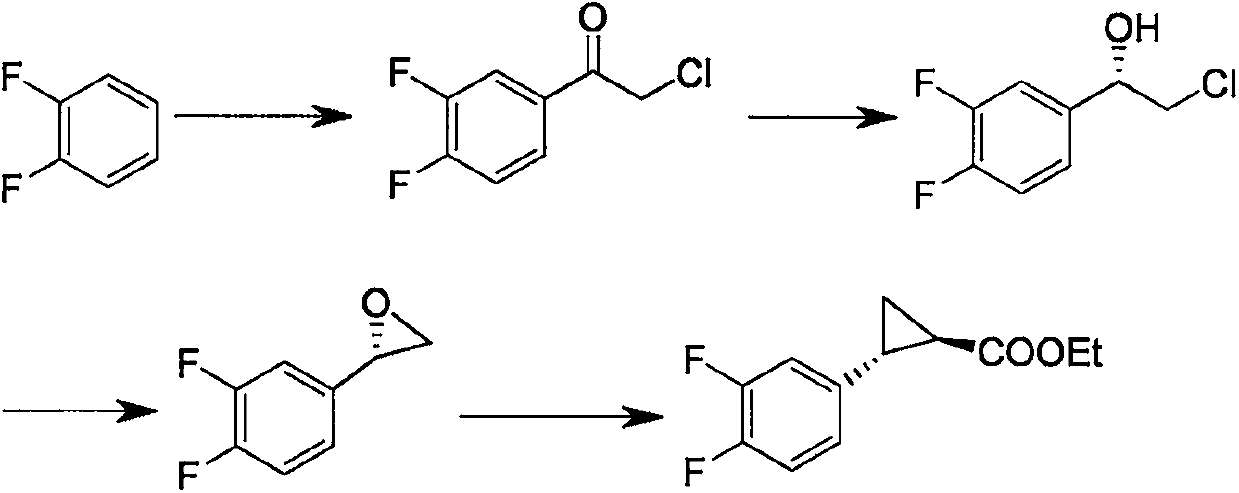
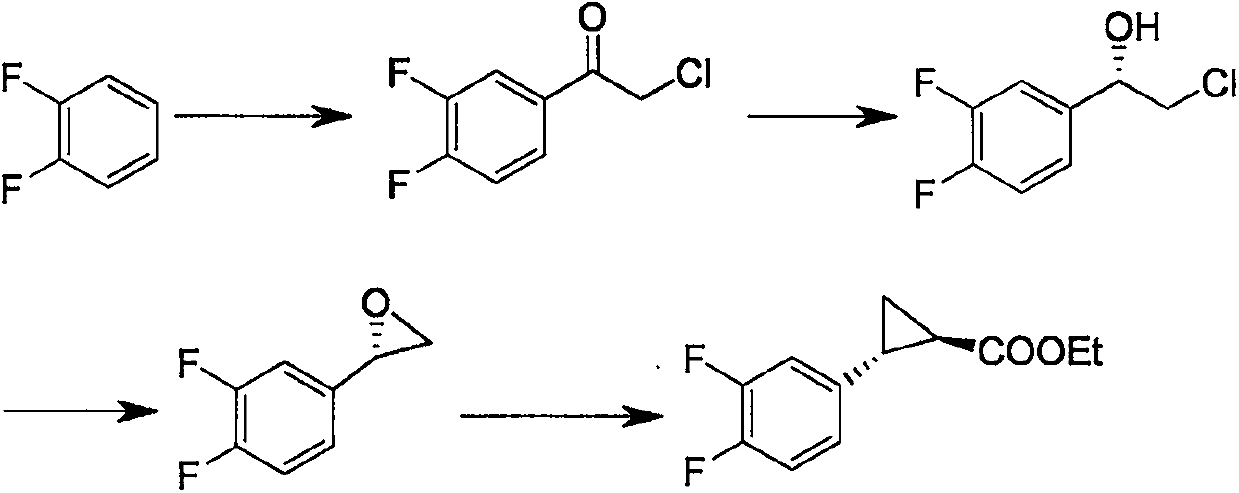
Method for preparing (1R,2S)-2-(3,4-difluorophenyl) -cyclopropylamine
InactiveCN104341310ALow priceSimple stepsPreparation by rearrangement reactionsCombinatorial chemistryCyclopropanation
The invention discloses a method for preparing (1R,2S)-2-(3,4-difluorophenyl)-cyclopropylamine. The method comprises the following steps: carrying out a Fuke acyl reaction on 3,4-difluorobromobenzene (compound 2) which serves as a raw material and chloroacetyl chloride under conditions of lewis acid and certain temperature to obtain a compound 3; carrying out stereoselective reduction to obtain a compound 4; carrying out cyclization, olefination, hydrolyzation and debromination to obtain a compound 8; and preparing amide, and carrying out Hoffmann elimination to obtain a target compound 1. According to the method, the traditional preparation process is improved, the (1R,2S)-2-(3,4-difluorophenyl)-cyclopropylamine can be effectively, simply and conveniently prepared, the total yield is relatively high, the stereoselection is relatively high, and the intermediate in each step is easily purified and stored. The reaction formula in the method is as shown in the specification.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD
P-chiral phospholanes and phosphocyclic compounds and their use in asymmetric catalytic reactions
InactiveUS7169953B2SelectiveGroup 1/11 organic compounds without C-metal linkagesAsymmetric synthesesIsomerizationCycloaddition
Owner:PENN STATE RES FOUND
In vivo and in vitro olefin cyclopropanation catalyzed by heme enzymes
The present invention provides methods for catalyzing the conversion of an olefin to any compound containing one or more cyclopropane functional groups using heme enzymes. In certain aspects, the present invention provides a method for producing a cyclopropanation product comprising providing an olefinic substrate, a diazo reagent, and a heme enzyme; and admixing the components in a reaction for a time sufficient to produce a cyclopropanation product. In other aspects, the present invention provides heme enzymes including variants and fragments thereof that are capable of carrying out in vivo and in vitro olefin cyclopropanation reactions. Expression vectors and host cells expressing the heme enzymes are also provided by the present invention.
Owner:CALIFORNIA INST OF TECH
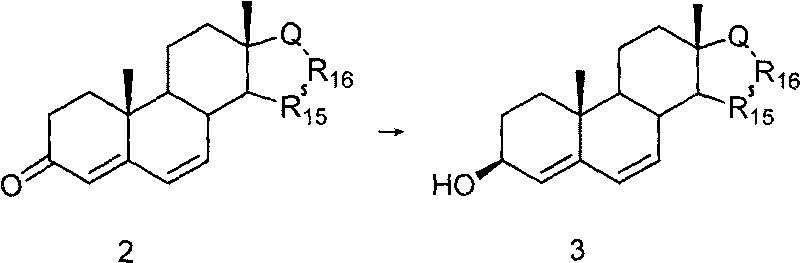
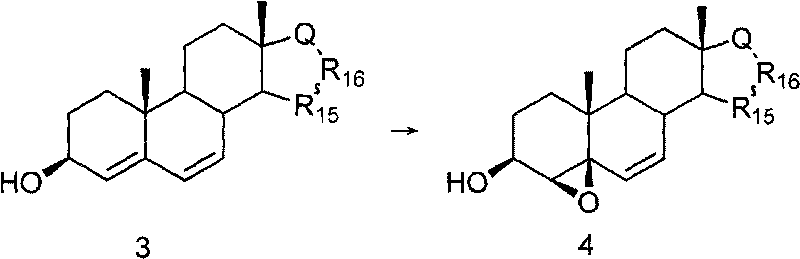
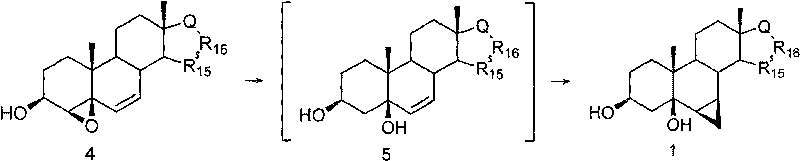
Method for preparing 6beta,7beta-methylene-steride-3beta,5beta-diol
The invention discloses a method for preparing 6beta,7beta-methylene-steride-3beta,5beta-diol, comprising the following steps of: preparing steride-4,6-dien-3beta-ol by using steride-4,6-dien-3beta-one as the raw material through a reductive reaction; preparing 4beta,5beta-epoxy steride-6-alkenyl-3beta-ol by the steride-4,6-dien-3beta-ol through epoxidation; preparing the intermediate steride-6-alkenyl-3beta,5beta-diol by the 4beta,5beta-epoxy steride-6-alkenyl-3beta-ol through a reductive ring opening reaction, and preparing the aimed compound 6beta,7beta-methylene-steride-3beta,5beta-diol by the intermediate through cyclopropanation reaction addition or directly preparing the aimed compound 6beta,7beta-methylene-steride-3beta,5beta-diol by the 4beta,5beta-epoxy steride-6-alkenyl-3beta-ol through cyclopropanation reaction. The invention has the advantages of low cost and easy availability of raw material, easy preparation of 4beta,5beta-epoxy steride-6-alkenyl-3beta-ol, high stereoselectivity for constructing 6beta,7beta-methylene structural unit, less reaction steps, simple operation and high yield, therefore, the method is suitable for industrialized production.
Owner:XIAN UNIV OF SCI & TECH
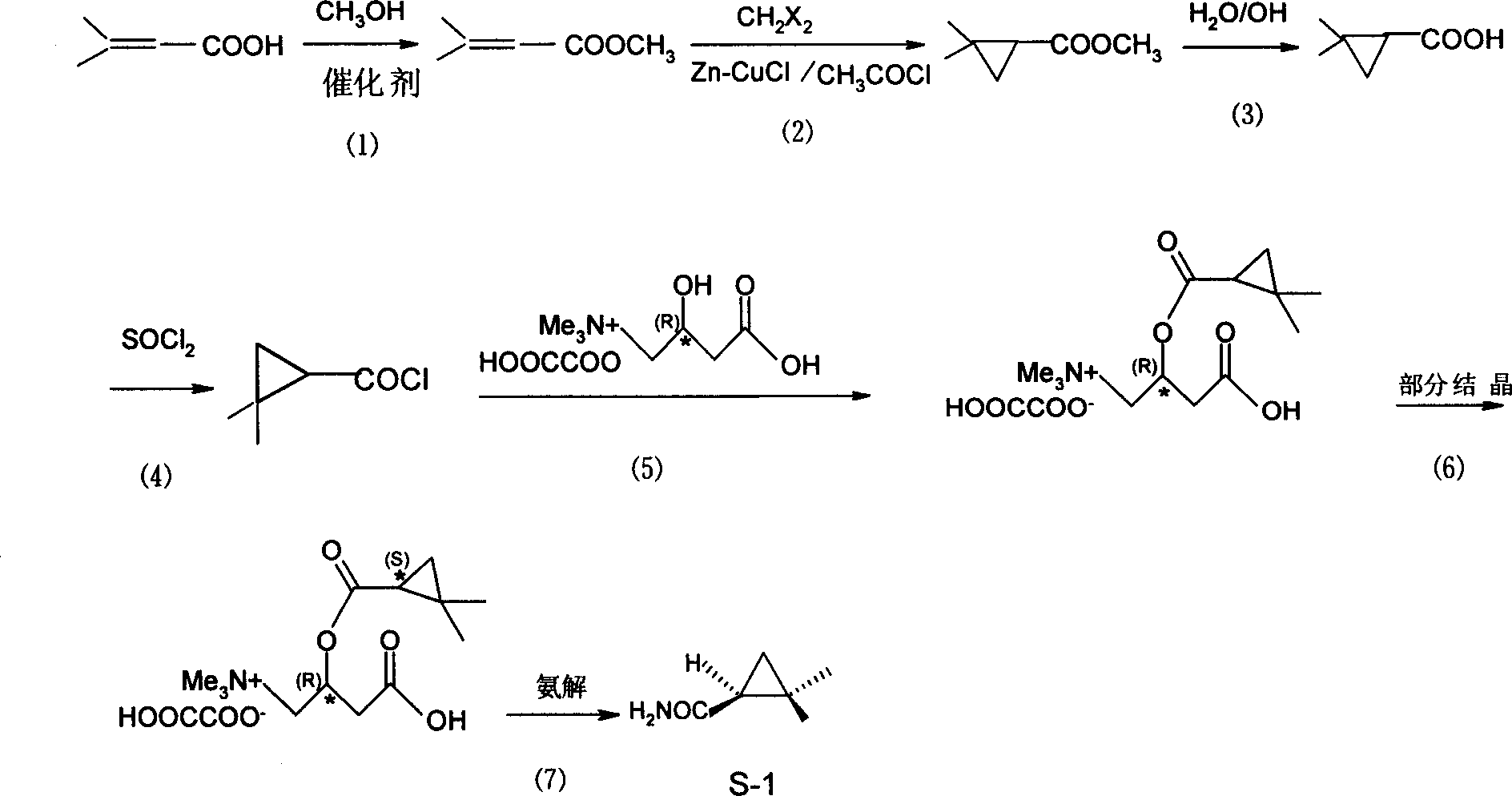
Method ofr synthesizing S-(1)-2.2 dimethylcyclopropane formamide
InactiveCN1562962AShort reaction timeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationFormamideCyclopropanation
This invention relates to process for synthesizing S-(+)-2,2-dimethyl cyclopropane formyl amide, using penteneic acid as main raw material, after procedures of: esterification, cyclopropanization, hydrolyzation, acylation, salifying, partial crystallization and ammonolysis. advantages are: short reaction time, mild reaction condition, simple process, total yield is more than 15%, ee is larger than 95.6%.
Owner:ZHEJIANG UNIV +1
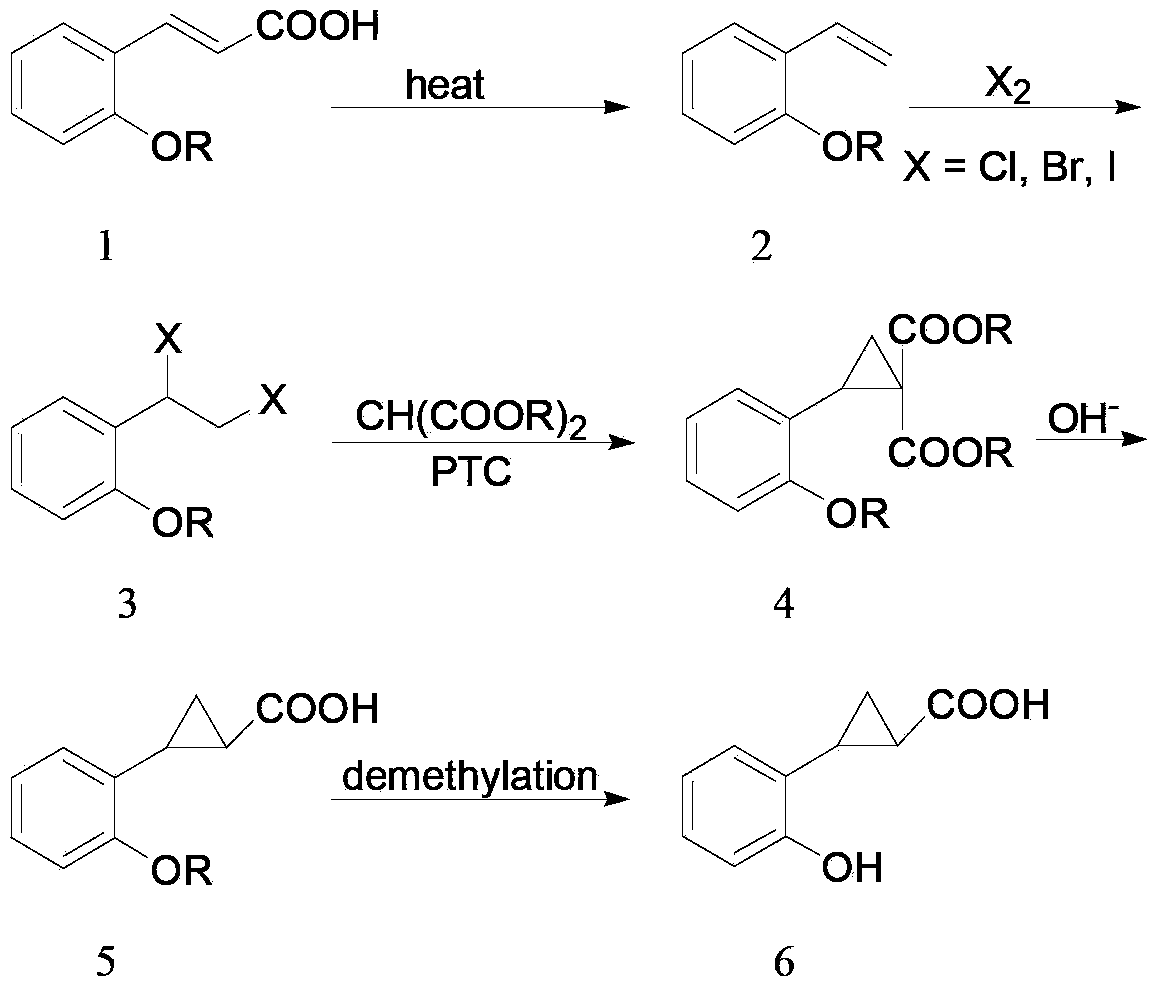
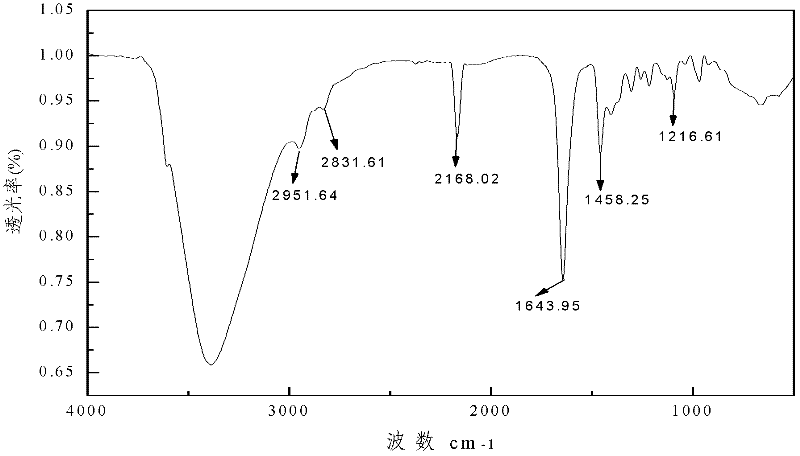
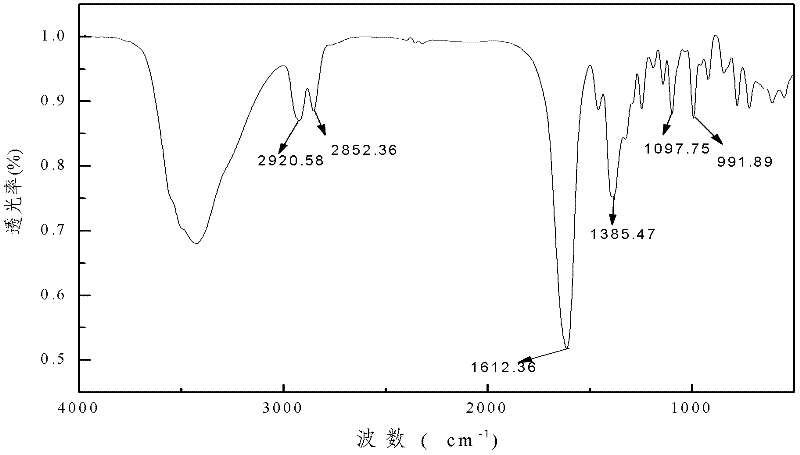
Preparation method of 2-(o-hydroxyphenyl)cyclopropane-1-carboxylic acid
ActiveCN104211598AAvoiding the downsides of the Corey–Chaykovsky reactionMild responseOrganic compound preparationCarboxylic acid esters preparationOrganic reactionCarboxylic acid
A preparation method of 2-(o-hydroxyphenyl)cyclopropane-1-carboxylic acid is provided. With o-alkoxy cinnamic acid as a raw material, 2-(o-alkoxyphenyl)cyclopropane-1,1-ethyl dicarboxylate is obtained through pyrolysis decarboxylation, halogen addition and cyclopropanation, and then the novel tobacco spice 2-(o-hydroxyphenyl)cyclopropane-1-carboxylic acid is prepared through deestering, hydrolysis, demethylation and other steps. The raw material and reagents in the route are all commercial products, so that the raw material is easy to obtain; the reaction steps are all classic organic reactions, especially the cyclopropanation step avoids the use of expensive trimethylsulfoxonium halogen (TMSOX, wherein X is Cl, Br or I) reagents, and thus the cost of production is significantly reduced. Through process optimization, the overall yield of the reaction reaches 53%. The method has the advantages of simple operation, high synthesis yield, low costs of the raw material and the reagents, easy scale production and the like. The 2-(o-hydroxyphenyl)cyclopropane-1-carboxylic acid prepared by the method is mainly used in spice and essence industries.
Owner:SHANGHAI AIPU VEGETABLE TECH +1
Metal complex of novel double piperidine derivative with symmetric structure
InactiveCN102584863AImprove catalytic performanceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzaldehydeBottle
The invention belongs to the field of synthesis of a metal complex, and particularly relates to a metal complex of a novel double piperidine derivative with a symmetric structure. According to the complex, under the protection of nitrogen gas, salt of corresponding metal is added into a reaction bottle provided with a reflux condensing tube; methanol is used as a solvent; the salt and the solvent are stirred until the salt is dissolved to obtain clear solution; double piperidine ligand is added and is heated under stirring until reflux occurs; after the double piperidine ligand is not available in the solution to be reacted, the solution is cooled to room temperature; the solvent is removed under reduced pressure; and residues are recrystallized to obtain the metal complex of the corresponding double piperidine derivative. The metal complex of the double piperidine derivative shows better catalytic performance in catalytic reaction such as epoxidation of olefin, asymmetric epoxidation of olefin, asymmetric addition of benzaldehyde and diethyl zinc and cyclopropanation of styrene.
Owner:HEBEI UNIV OF TECH
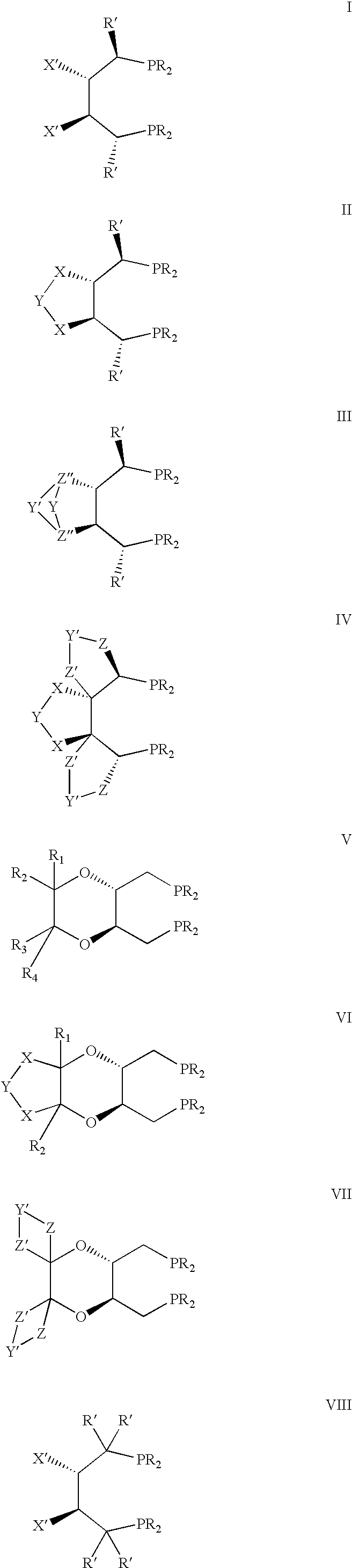
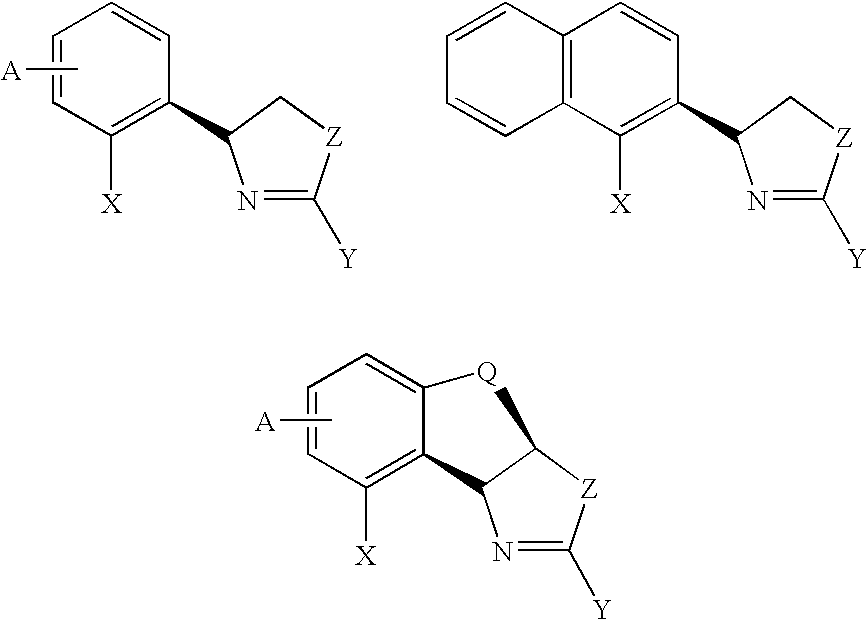
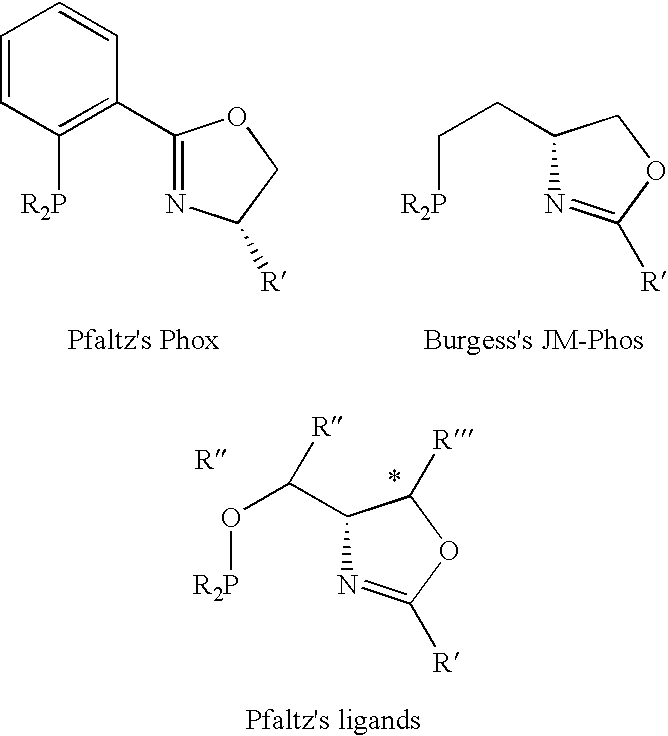
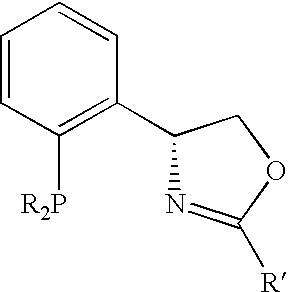
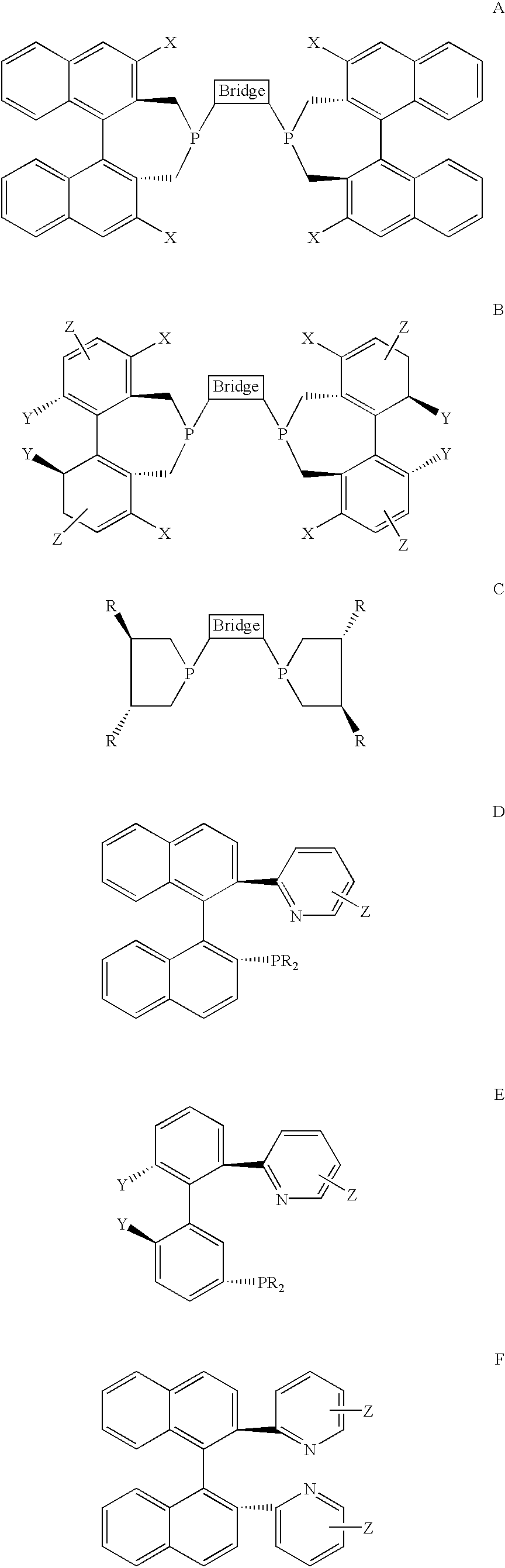
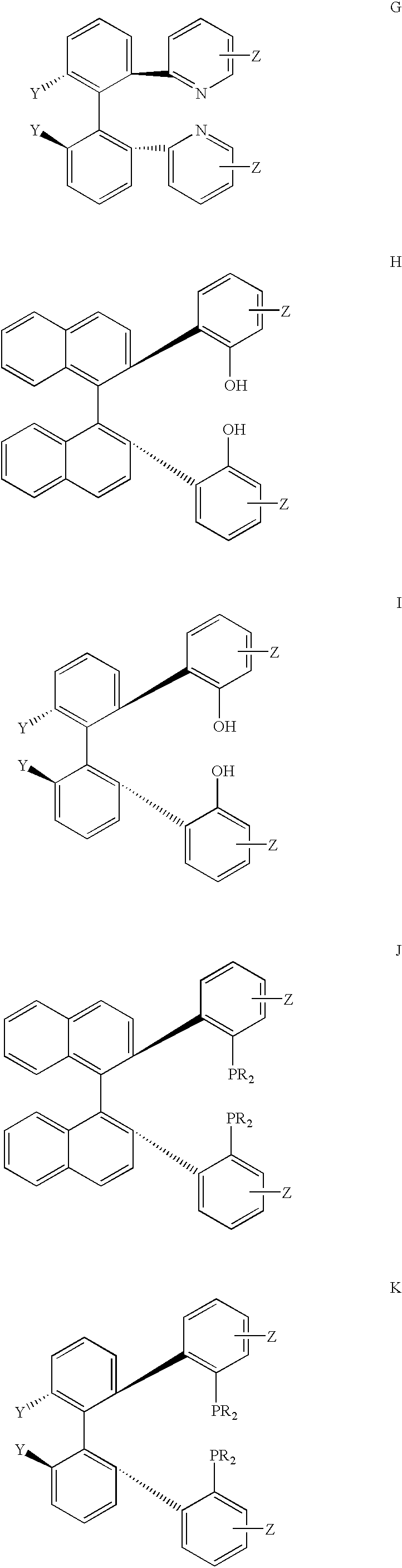
New oxazoline ligands for asymmetric catalysis
InactiveUS20050137403A1Organic compound preparationGroup 8/9/10/18 element organic compoundsEnantiomerHydrosilylation
A chiral ligand represented by the formula and its enantiomer: wherein A, X, Y and Z are as defined in the specification is provided. Also provided is a process of making the chiral ligands and catalysts prepared from these ligands and a transition metal, a salt thereof or a complex thereof. In addition, a method of preparing an asymmetric compound by a transition metal catalyzed asymmetric reaction, such as, hydrogenation, hydride transfer reaction, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, allylic alkylation, epoxidation, cyclopropanation, Diels-Alder reaction, Aldol reaction, ene reaction, Heck reaction and Michael addition is provided.
Owner:PENN STATE RES FOUND
Method for preparing ticagrelor intermediate
InactiveCN107686447AReduce transit lossFew stepsOrganic compound preparationOrganic chemistry methodsTicagrelorGreen cleaning
The invention discloses a method for preparing a ticagrelor intermediate. The method comprises the following steps: taking o-difluorobenzene and chloroacetyl chloride as initial raw materials, carrying out an F-K reaction, a bio-enzyme fermentation technology asymmetric reduction reaction, a ring-closure reaction and a cyclopropanation reaction, so as to obtain the key intermediate (1R, 2R)-2-(3,4-difluorophenyl) cyanocyclopropane carboxylate of the ticagrelor at high yield, high enantioselectivity and high purity. The method can realize industrialized production. The method is low in energy consumption, less in pollution, green, clean, high in yield and high in purity, the cost is reduced, the product quality is stable, and the method is suitable for large-scale stable industrial production.
Owner:XUCHANG HENGSHENG PHARMA
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS20030163003A1Preparation by oxidation reactionsCarboxylic acid amides optical isomer preparationIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
P-chiral phospholanes and phosphocyclic compounds and their use in asymmetric catalytic reactions
InactiveUS7105702B2SelectiveGroup 1/11 organic compounds without C-metal linkagesAsymmetric synthesesIsomerizationCycloaddition
Chiral ligands and metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The metal complexes according to the present invention are useful as catalysts in asymmetric reactions, such as, hydrogenation, hydride transfer, allylic alkylation, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, olefin metathesis, hydrocarboxylation, isomerization, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition; epoxidation, kinetic resolution and [m+n] cycloaddition. Processes for the preparation of the ligands are also described.
Owner:PENN STATE RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one](https://images-eureka.patsnap.com/patent_img/e0dc6f5e-b07f-4208-bddc-b182439c3bda/US20100168463A1-20100701-C00001.png)
![Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one](https://images-eureka.patsnap.com/patent_img/e0dc6f5e-b07f-4208-bddc-b182439c3bda/US20100168463A1-20100701-C00002.png)
![Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one Method of producing 6,6-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one](https://images-eureka.patsnap.com/patent_img/e0dc6f5e-b07f-4208-bddc-b182439c3bda/US20100168463A1-20100701-C00003.png)