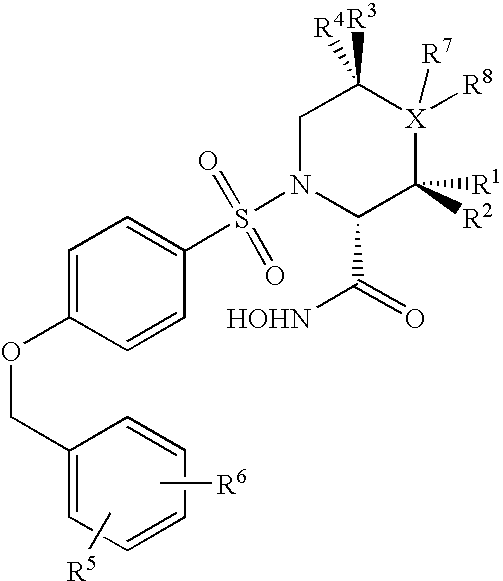
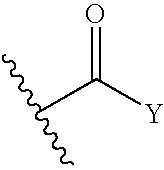
Cyclopropane compounds and pharmaceutical use thereof
a technology of cyclopropane compounds and cyclopropane, which is applied in the direction of amide active ingredients, drug compositions, medical preparations, etc., can solve the problems of oa treatment of drugs that suppress enzymes involved in cartilage destruction, deterioration of patient qol (life quality), and only about 15 life of artificial joints, etc., to achieve the effect of superior aggrecanase inhibitory activity and mmp inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1-1
(2R*,3R*)-3-methylsulfanyl-2-phenyl-butan-2-ol (step 1-1)
[1393]
[1394]To a solution of trans-2,3-dimethyl-2-phenyloxirane (14 g, 76 mmol) in methanol (100 mL) was added 15% aqueous sodium methyl mercaptan solution (61 mL, 190 mmol) under nitrogen atmosphere at room temperature, and the mixture was warmed to 60° C. and stirred for 2 hours. The mixture was concentrated under reduced pressure, and the residue was extracted twice with diethyl ether (50 mL), sequentially washed with water (50 ml) and saturated aqueous sodium chloride solution (50 mL), and dried over sodium sulfate. The resultant mixture was filtrated and the solvent was evaporated to give the title compound (16 g, yield 99%) as a colorless oil.
preparation example 1-2
(1R*,2R*)-(1-chloro-1-methyl-2-methylsulfanyl-propyl)benzene or
(1R*,2R*)-(2-chloro-1-methyl-1-methylsulfanyl-propyl)benzene (step 1-2)
[1395]
[1396]To a solution of (2R*,3R)-3-methylsulfanyl-2-phenyl-butan-2-ol (1.0 g, 5.1 mmol) obtained in Preparation Example 1-1 in toluene (3.0 mL) was added dropwise a solution of thionyl chloride (0.67 mL, 9.2 mmol) in toluene (2.0 mL) under argon atmosphere at 0° C., and the mixture was stirred for 1 hour. The solution was evaporated to give a mixture of two kinds of regioisomers of the title compound (1.0 g, yield 100%) as a yellow oil.
preparation example 1-3
1-(1-chloro-1-methyl-2-methylsulfanyl-ethyl)-4-fluoro-benzene or
1-(2-chloro-1-methyl-1-methylsulfanyl-ethyl)-4-fluoro-benzene (step 1-3)
[1397]
[1398]This procedure was performed according to the method described in Synthesis (1980, 9690-9691).
[1399]While cooling to −40° C., sulfuryl chloride (2.9 mL, 37 mmol) was added dropwise to a solution of dimethyl disulfide (3.3 mL, 37 mmol) in 1,2-dimethoxyethane (20 mL) under nitrogen atmosphere, and the mixture was stirred for 10 min. The obtained solution was added dropwise to a solution of 4-fluoro-α-methylstyrene (10 g, 73 mmol) in 1,2-dimethoxyethane (20 mL) while maintaining a temperature below −30° C. After stirring for 1 hour at room temperature, the solution was evaporated to give a crude product of the title compound. The obtained product was used in the next step without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com