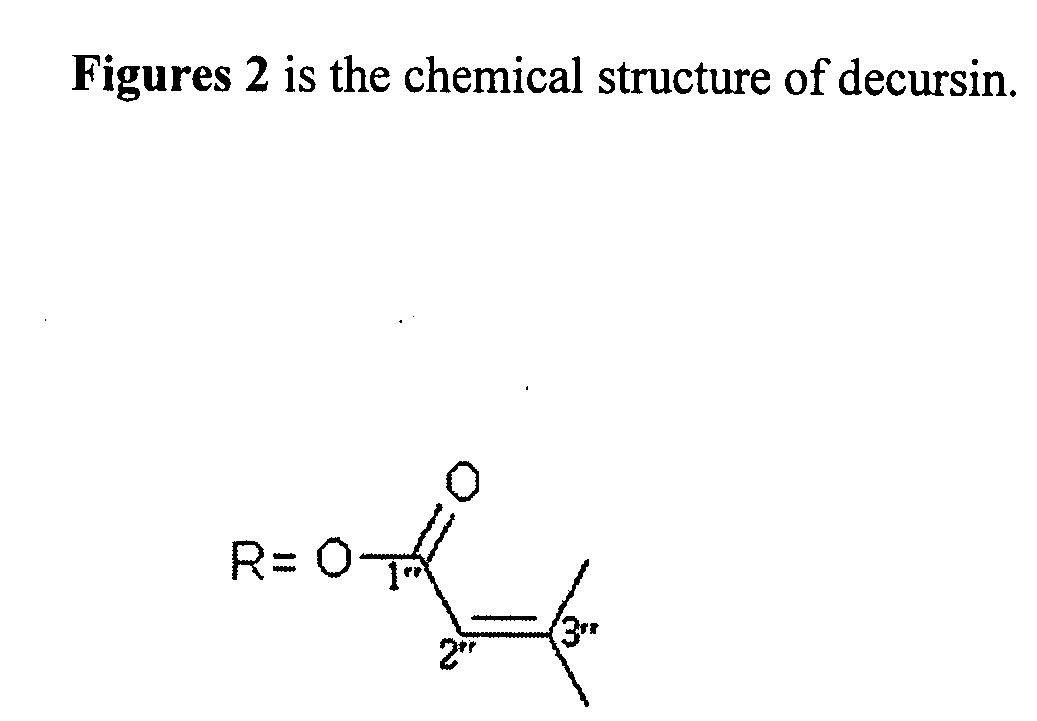Patents
Literature
207 results about "Joint disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for determining meniscal size and shape and for devising treatment
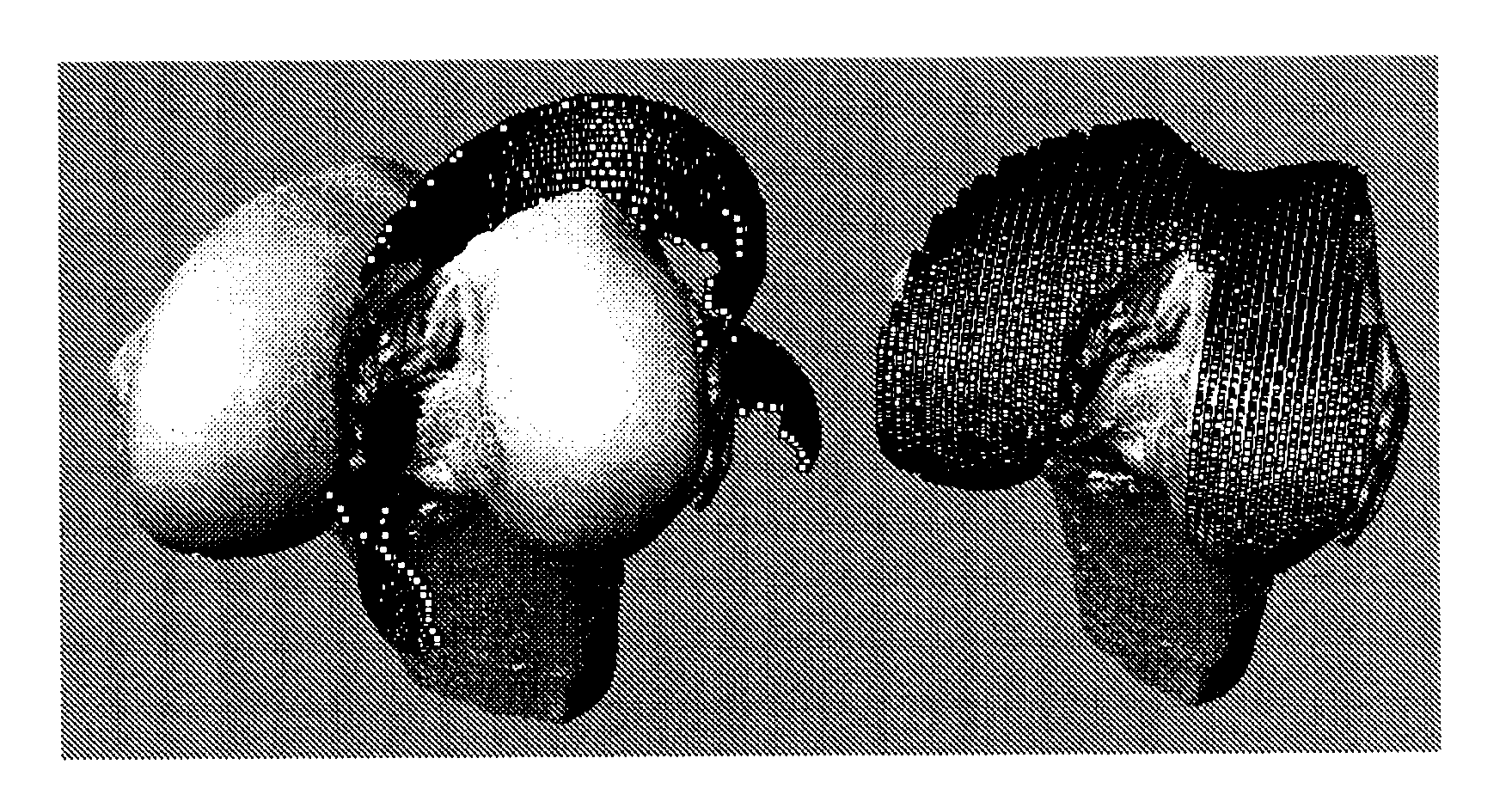
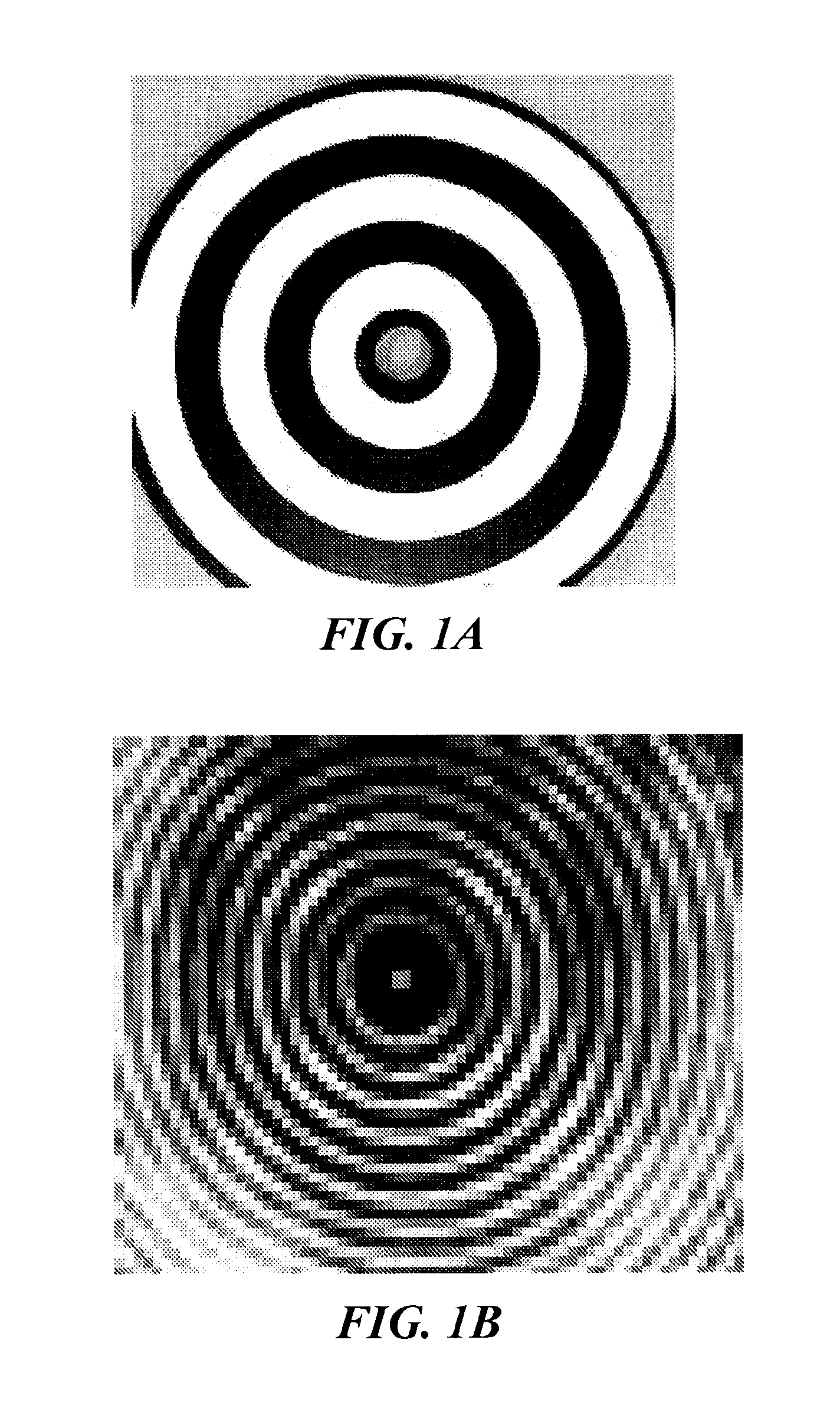
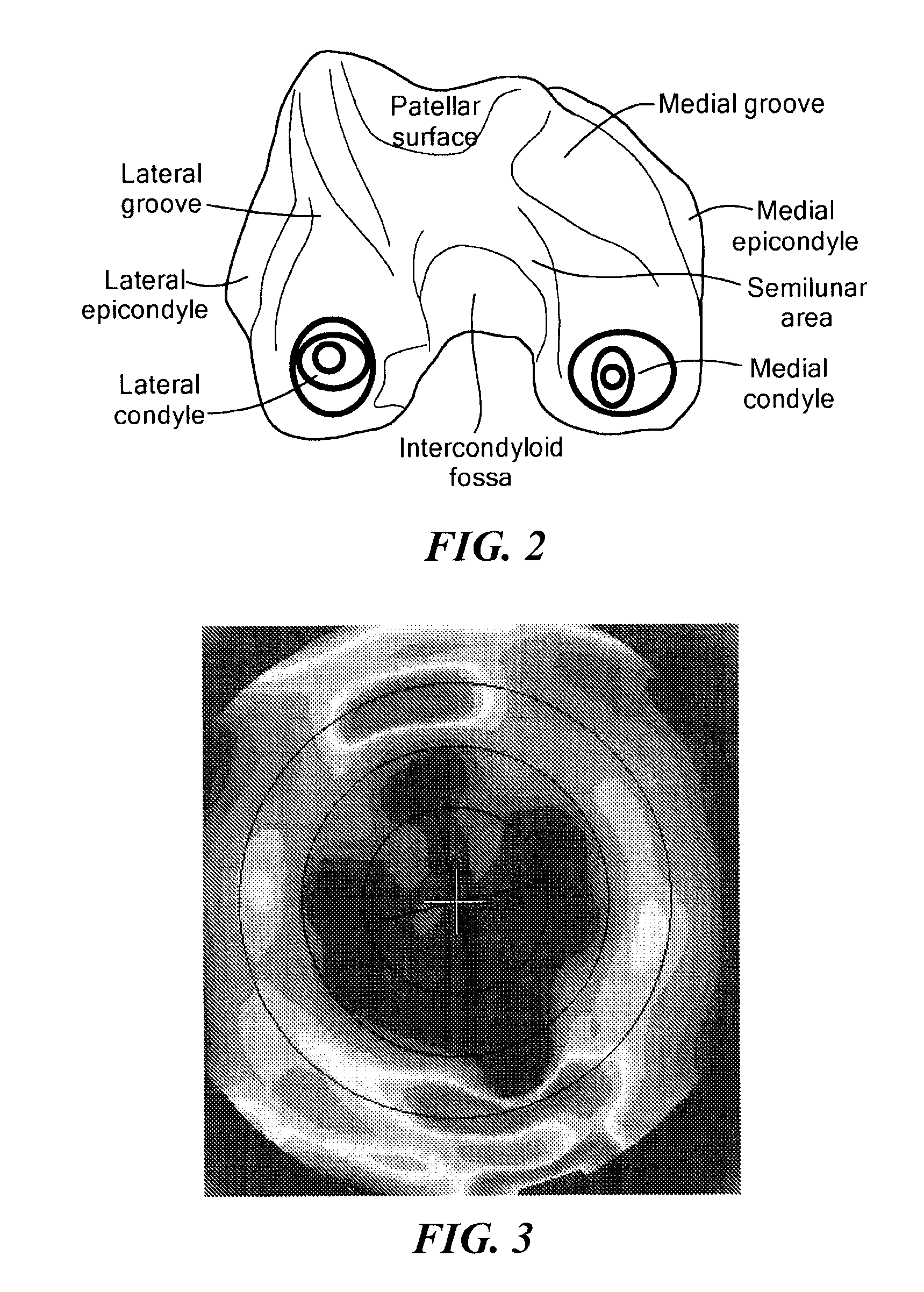
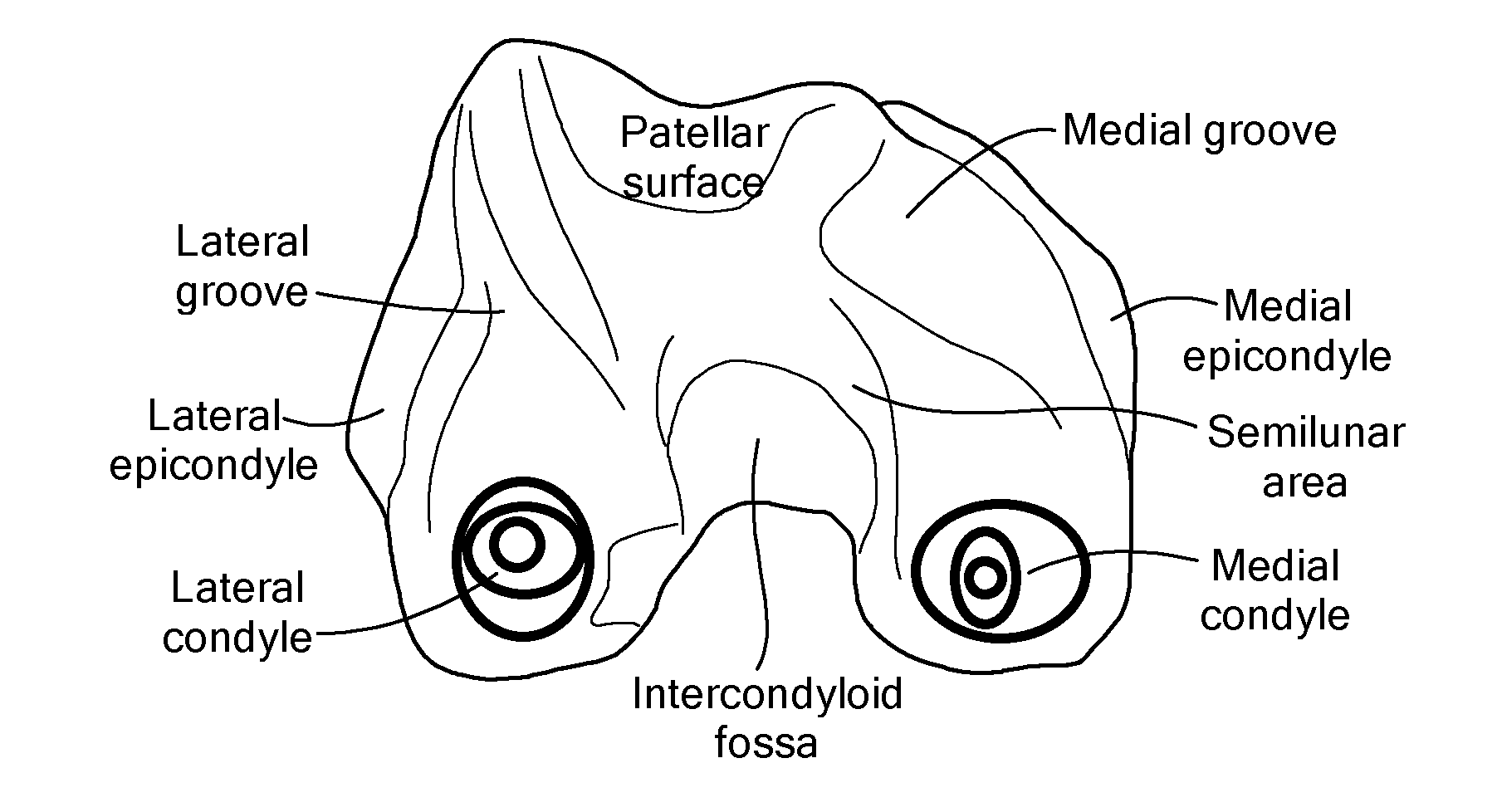
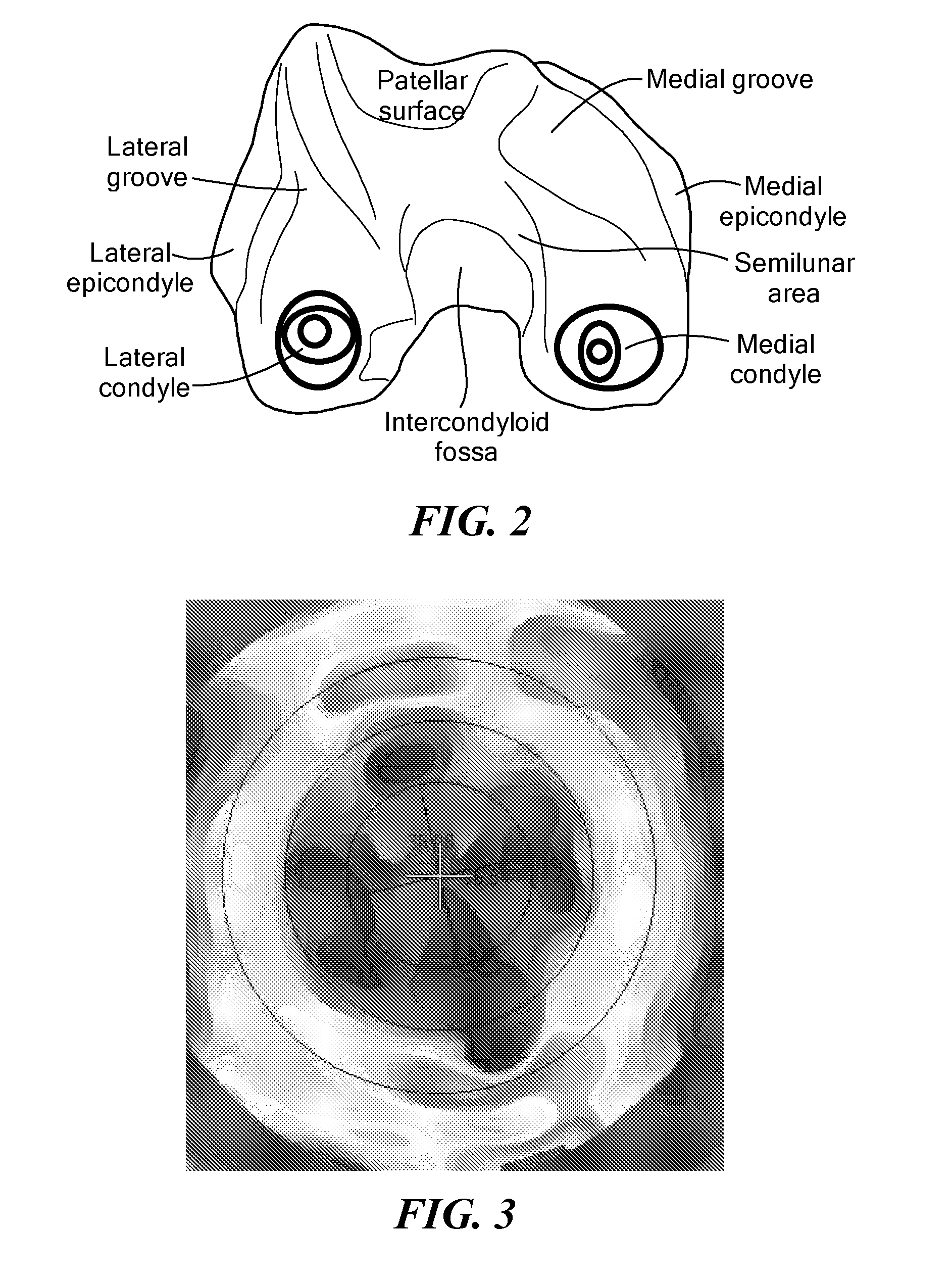
The present invention relates to methods for determining meniscal size and shape for use in designing therapies for the treatment of various joint diseases. The invention uses an image of a joint that is processed for analysis. Analysis can include, for example, generating a thickness map, a cartilage curve, or a point cloud. This information is used to determine the extent of the cartilage defect or damage and to design an appropriate therapy, including, for example, an implant. Adjustments to the designed therapy are made to account for the materials used.
Owner:CONFORMIS
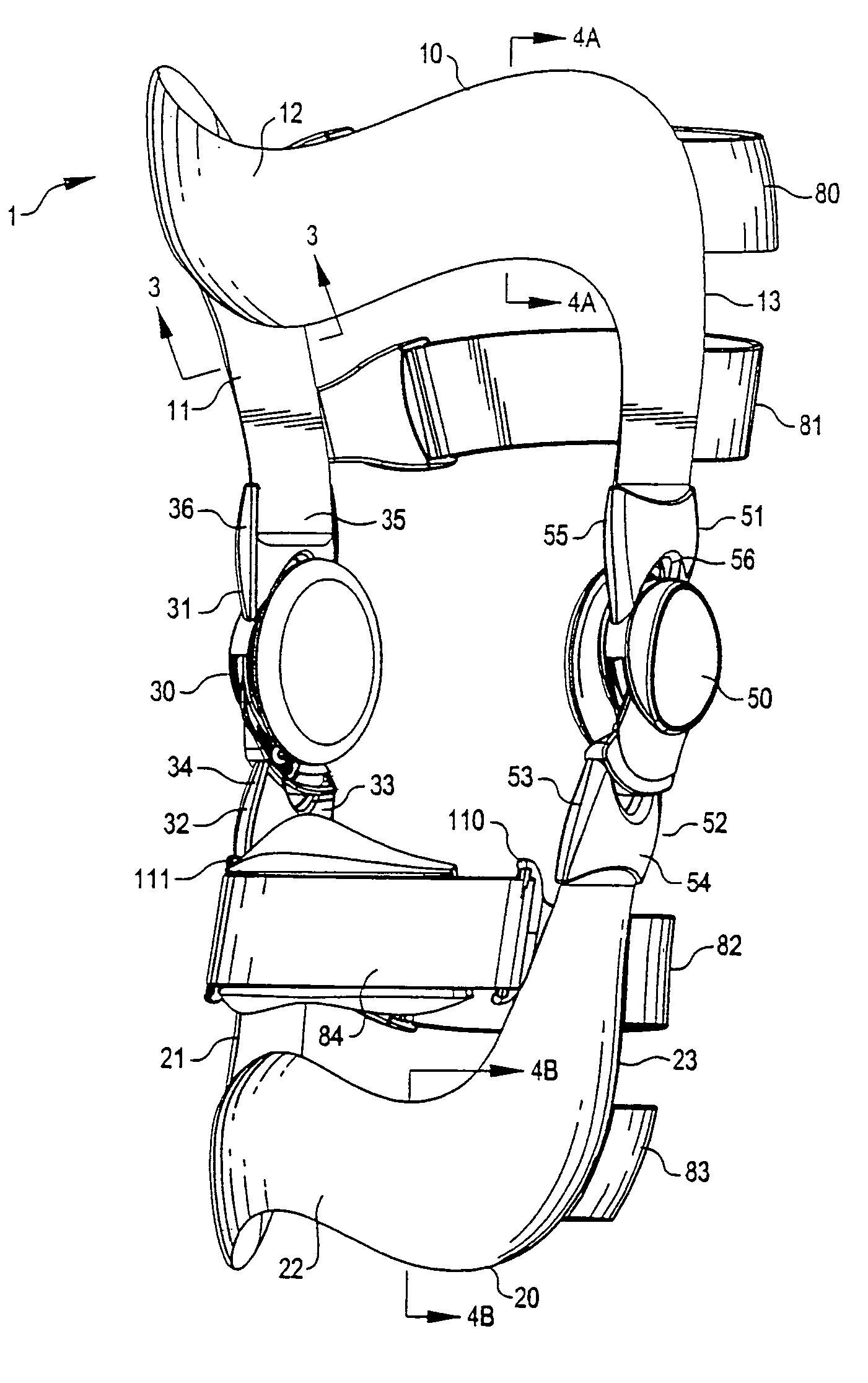
Anatomically designed orthopedic knee brace
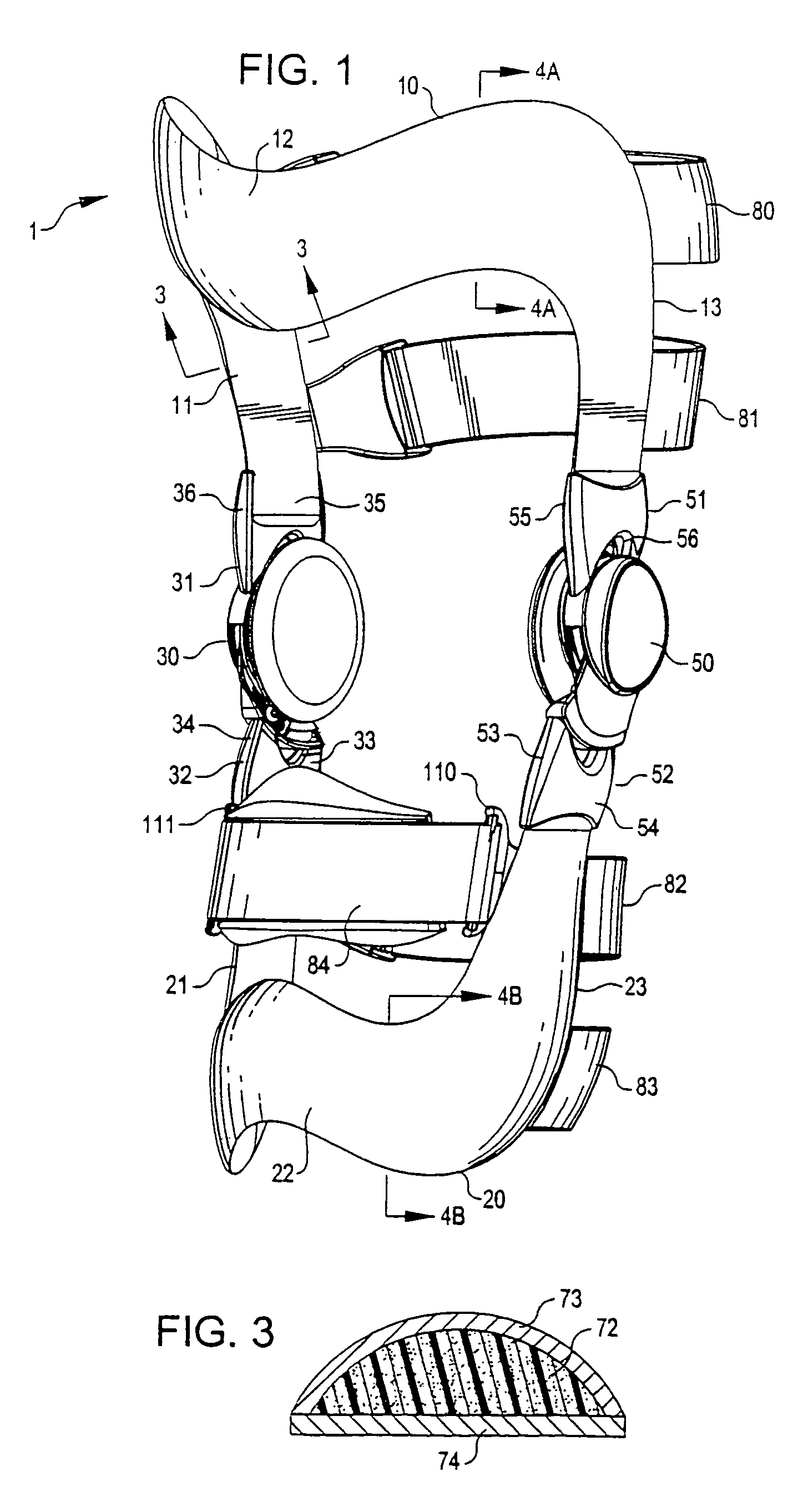
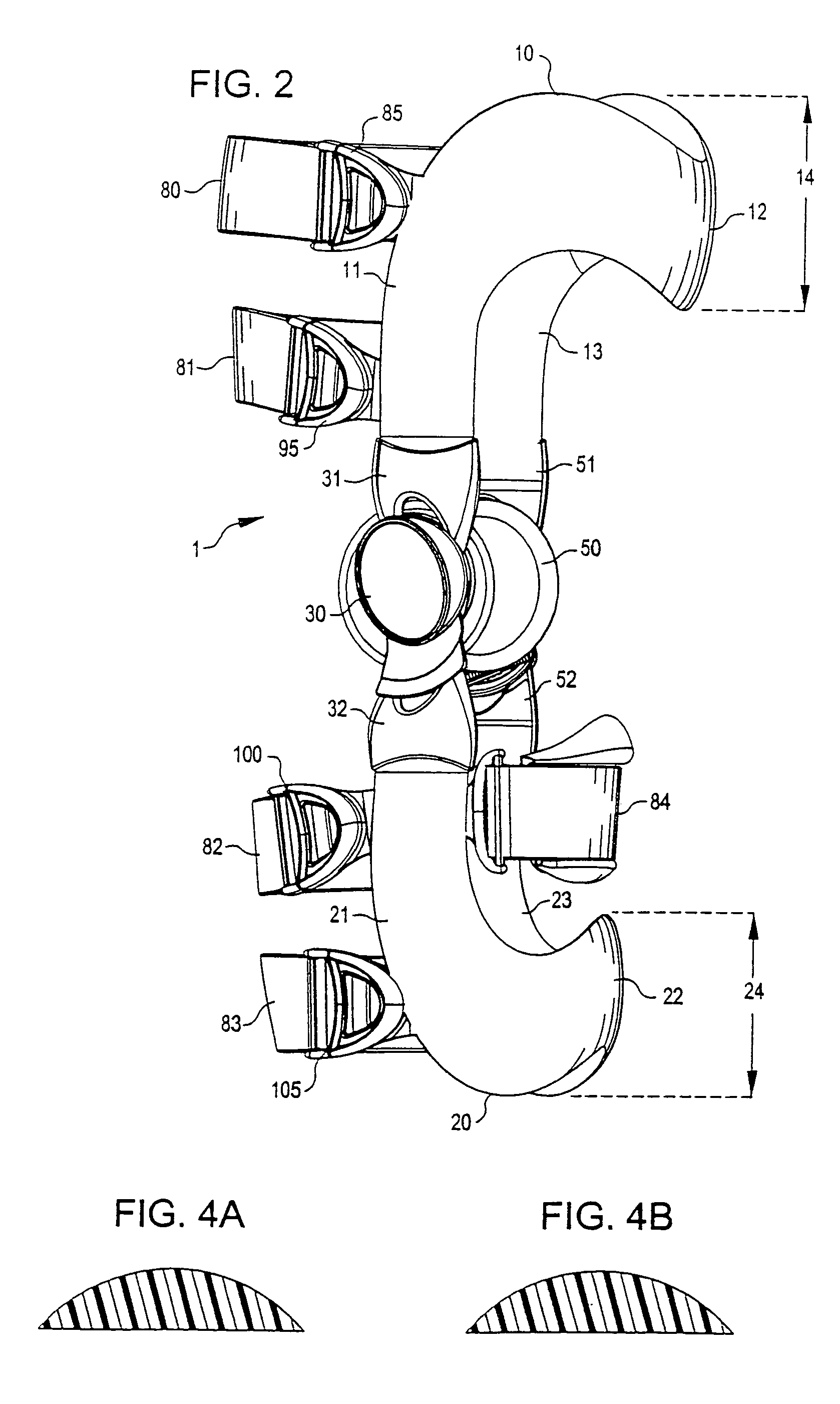
InactiveUS7201728B2Novel and effectiveAccurately prescribingNon-surgical orthopedic devicesKnee proceduresTibia
An orthopedic knee brace provides an apparatus for accurately prescribing the anatomical motion of the human knee. The orthopedic knee brace is used for treatment and rehabilitation following surgery to the knee, protection for a surgically repaired knee, and protection for an uninjured knee, among other applications. The orthopedic knee brace actively prescribes asymmetric three-dimensional anatomic motion in six degrees of freedom of the wearer's knee. The rigid connections between the thigh and calf engaging members and the medial and lateral hinges provide the ability of the orthopedic knee brace to prescribe asymmetric three-dimensional anatomic motion in six degrees of freedom by actively prescribing flexion and extension, abduction and adduction, internal / external rotation, anterior / posterior translation, medial / lateral translation, and proximal / distal translation between a femur and a tibia of a wearer's leg. In alternate embodiments a single-hinge brace design is provided for treatment and prevention of osteoarthritis and other joint diseases and conditions.
Owner:OSSUR HF
Methods for Determining Meniscal Size and Shape and for Devising Treatment
The present invention relates to methods for determining meniscal size and shape for use in designing therapies for the treatment of various joint diseases. The invention uses an image of a joint that is processed for analysis. Analysis can include, for example, generating a thickness map, a cartilage curve, or a point cloud. This information is used to determine the extent of the cartilage defect or damage and to design an appropriate therapy, including, for example, an implant. Adjustments to the designed therapy are made to account for the materials used.
Owner:CONFORMIS
Apparatus for the treatment of disorders of tissue and/or the joints
InactiveUS6048302APromote healingSecure supportElectrotherapySurgeryElectromagnetic fieldBiomedical engineering
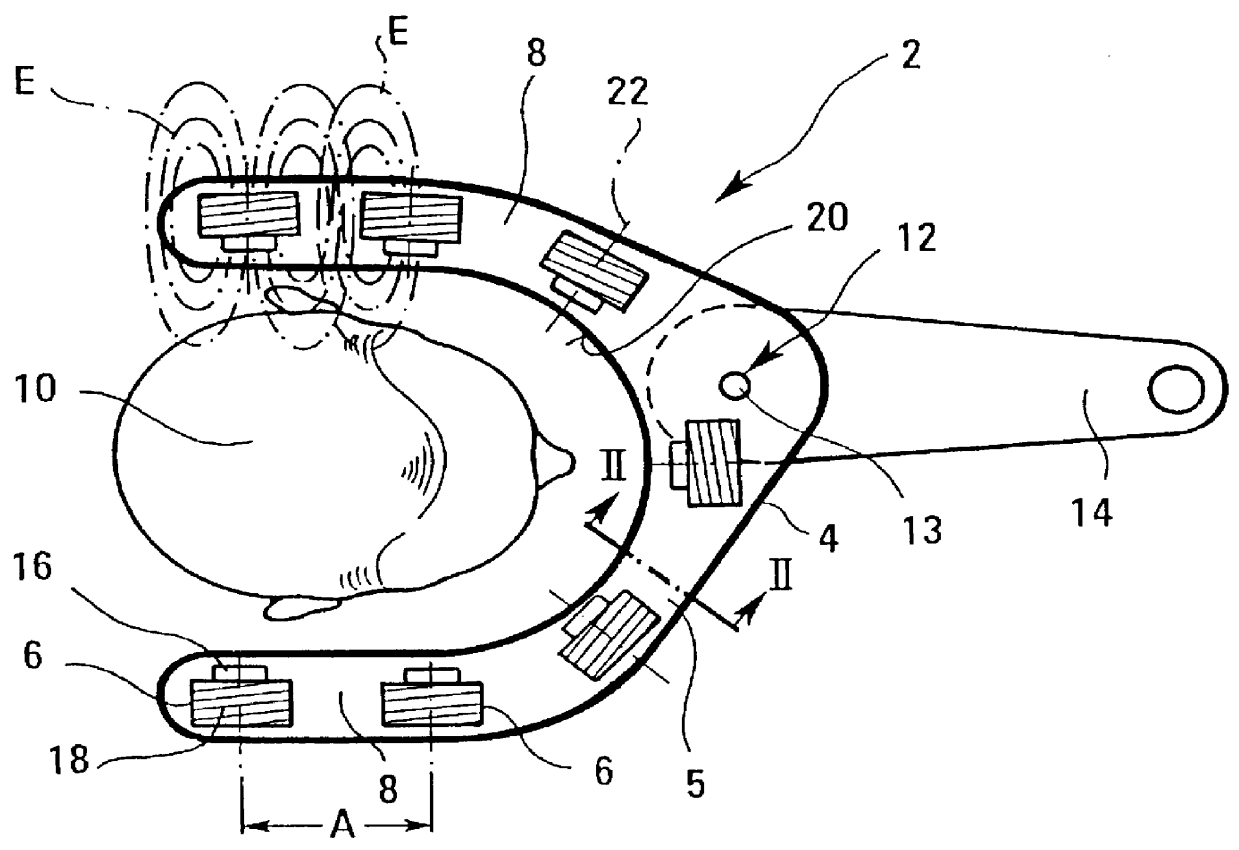
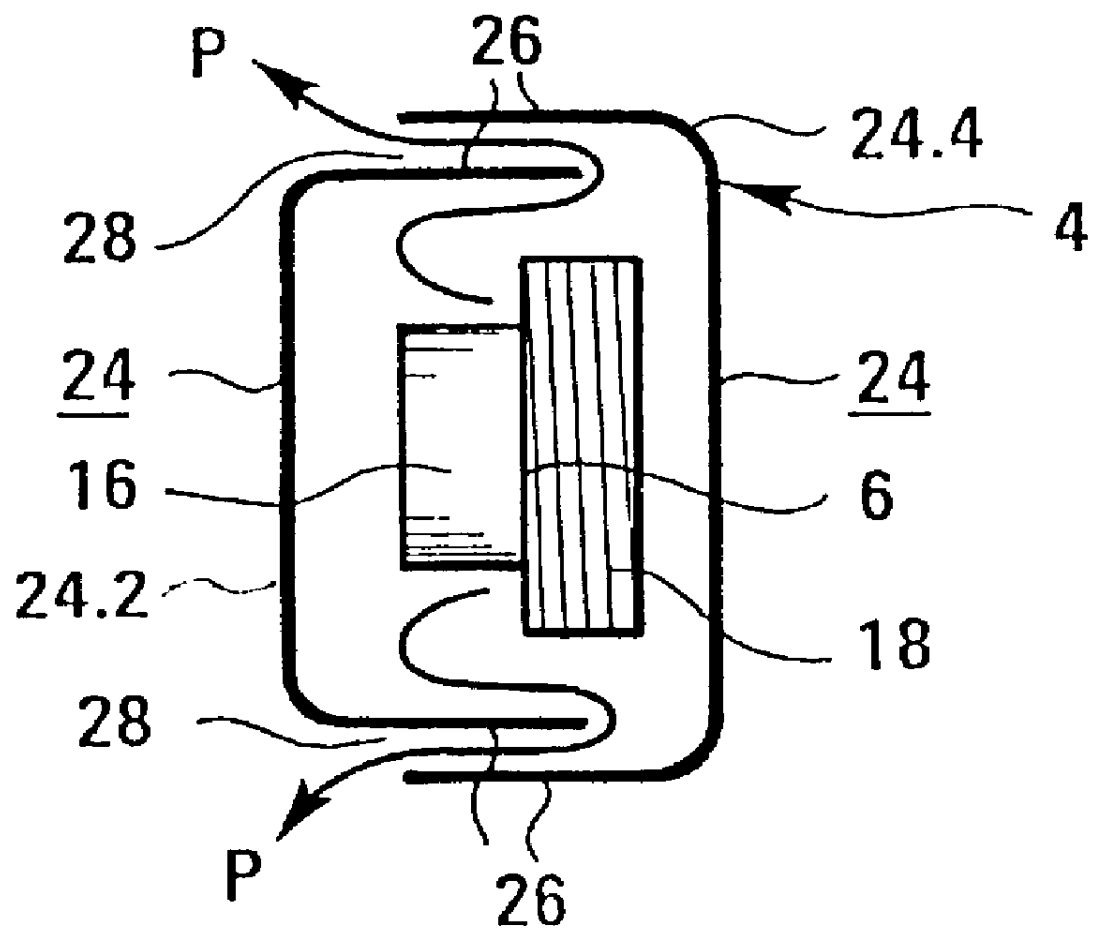
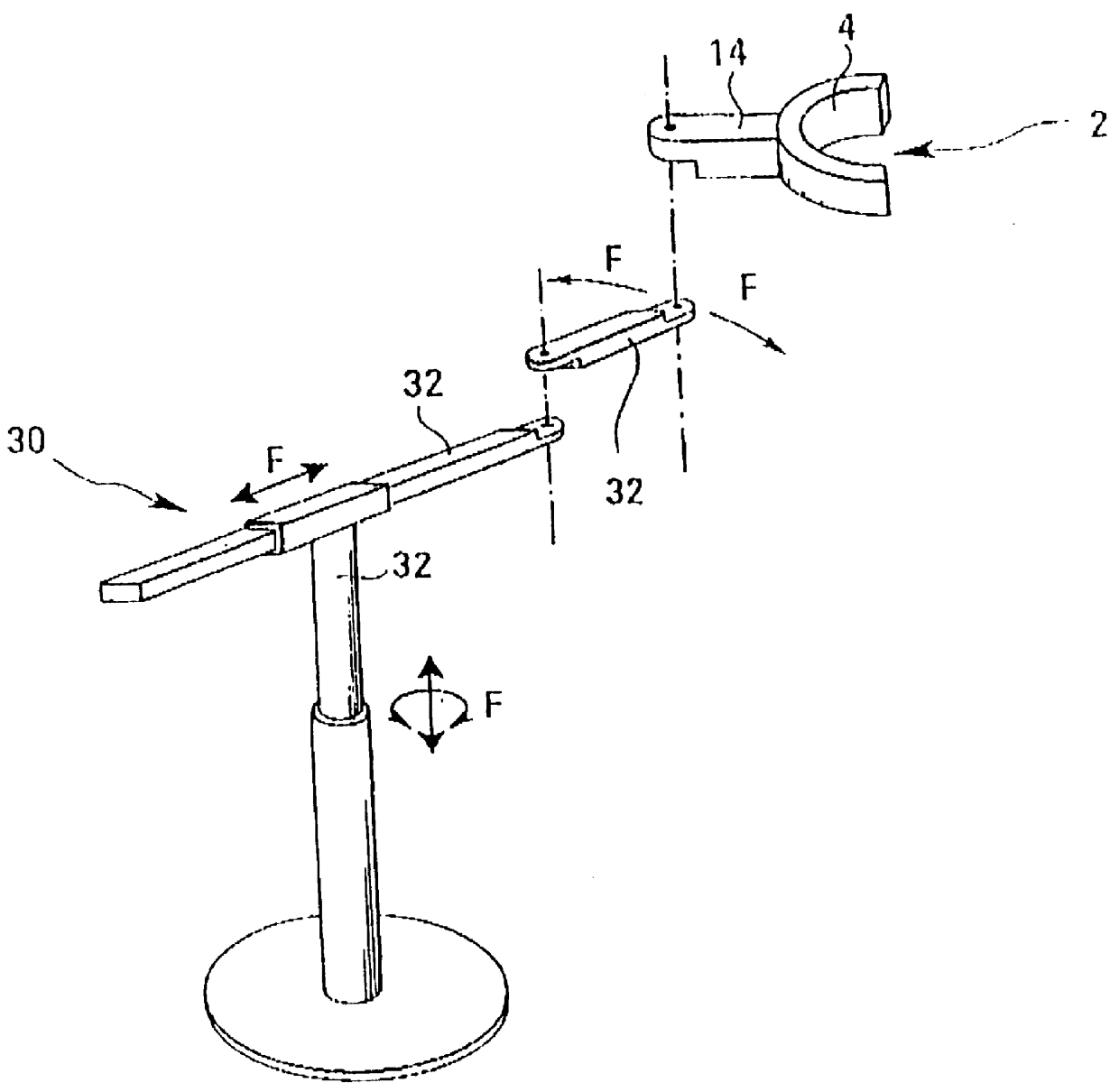
An apparatus for the treatment of disorders of tissue and / or the joints in the area of the jaw or neck of a patient (10), particularly for the treatment of periodontosis, is depicted, wherein the apparatus comprises a housing (4) that surrounds at least the area of the jaw or neck to be treated. A number of coils (6) is also provided for generating at least one electromagnetic field (E) which can be applied to the area to be treated. The coils (6) are arranged in the interior (5) of the housing (4).
Owner:BIO MAGNETIC THERAPY SYST
Osteoclast inhibitors for joint conditions
ActiveUS9610300B2Improve bioavailabilityPhosphorous compound active ingredientsPill deliveryNitrogenAnesthesia
Oral dosage forms of osteoclast inhibitors, such as nitrogen-containing bisphosphonates, can be used to treat or alleviate pain or related conditions.
Owner:ANTECIP BIOVENTURES II
Oral Chinese medicine for curing osteoarthropathy
InactiveCN1400007AGood analgesic effectImprove solubilityAnthropod material medical ingredientsAntipyreticDiseaseCurative effect
Owner:郭好建
Methods and compositions for the treatment and prevention of degenerative joint disorders
InactiveUS20060240037A1Treat and reduce and prevent degenerative joint disorderHigh expressionOrganic active ingredientsBiocideSecreted substanceSecretion
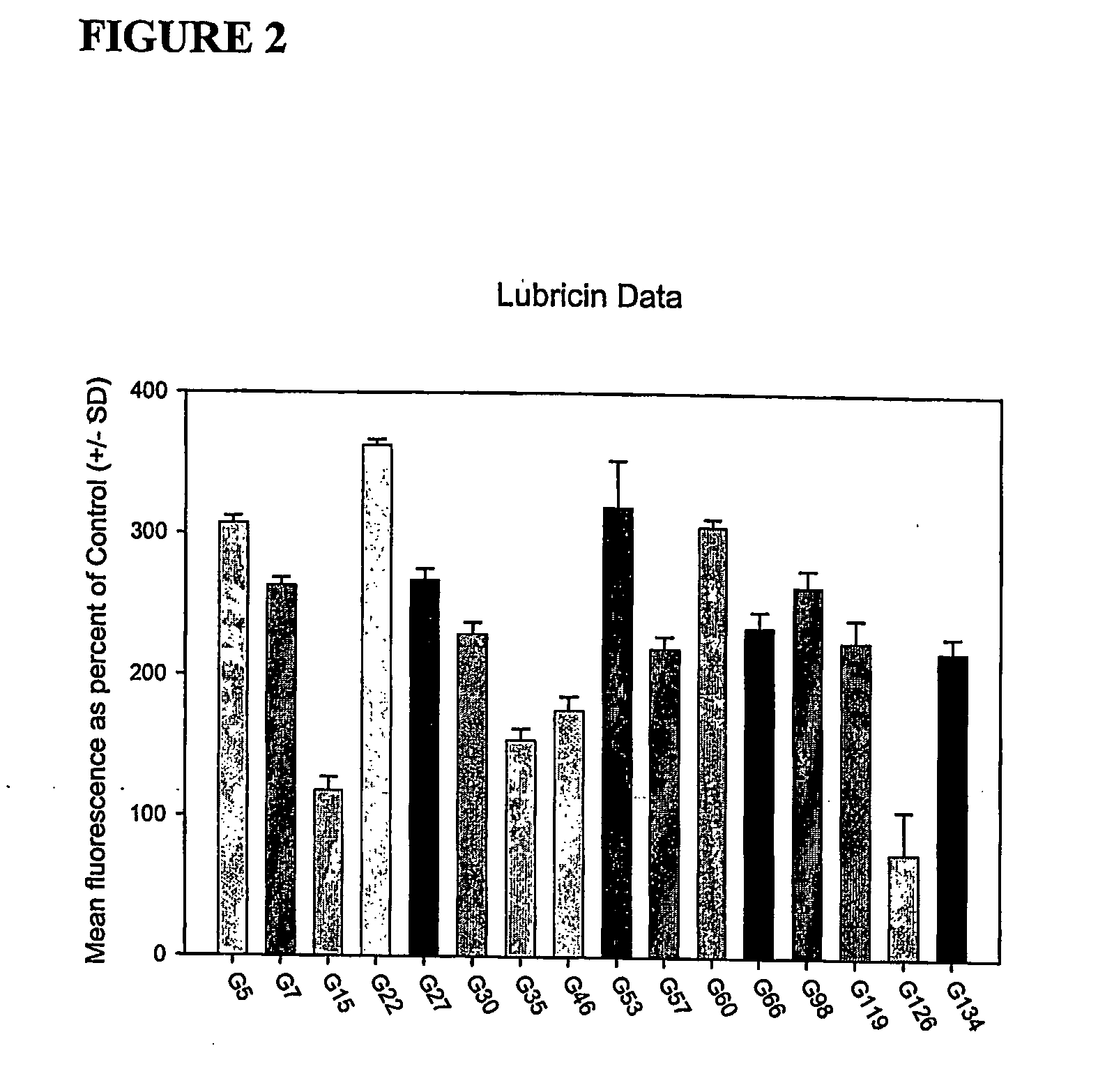
The present invention features methods and compositions for the treatment, reduction, and prevention of degenerative joint disorders by administering to a mammal a joint enhancing composition. According to this invention, the composition increases the level of expression and secretion of endogenous lubricin such that the joints in the mammal being treated are lubricated. This composition can be administered alone or in combination with one or more therapeutic agents.
Owner:MUCOSAL THERAPEUTICS
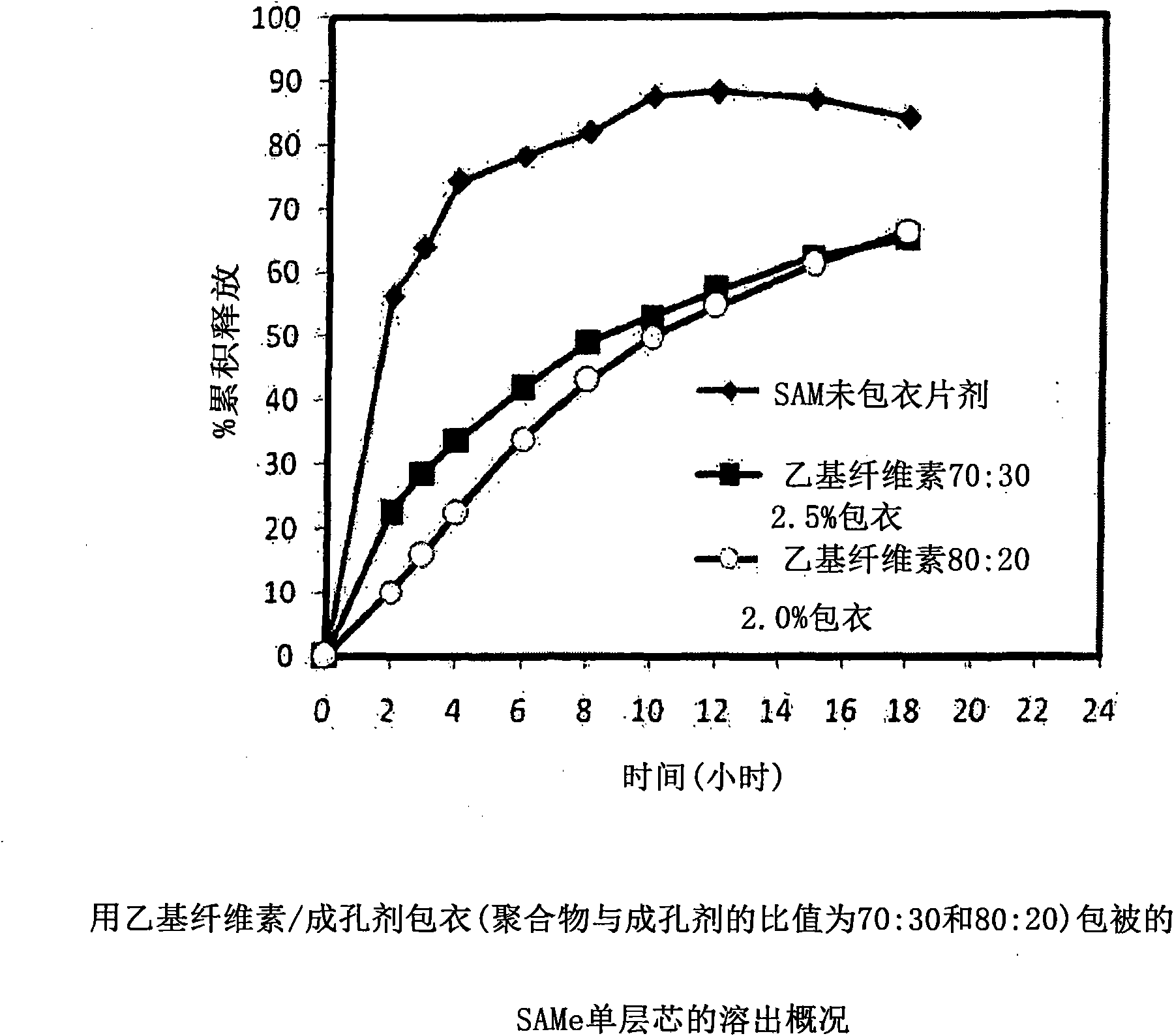
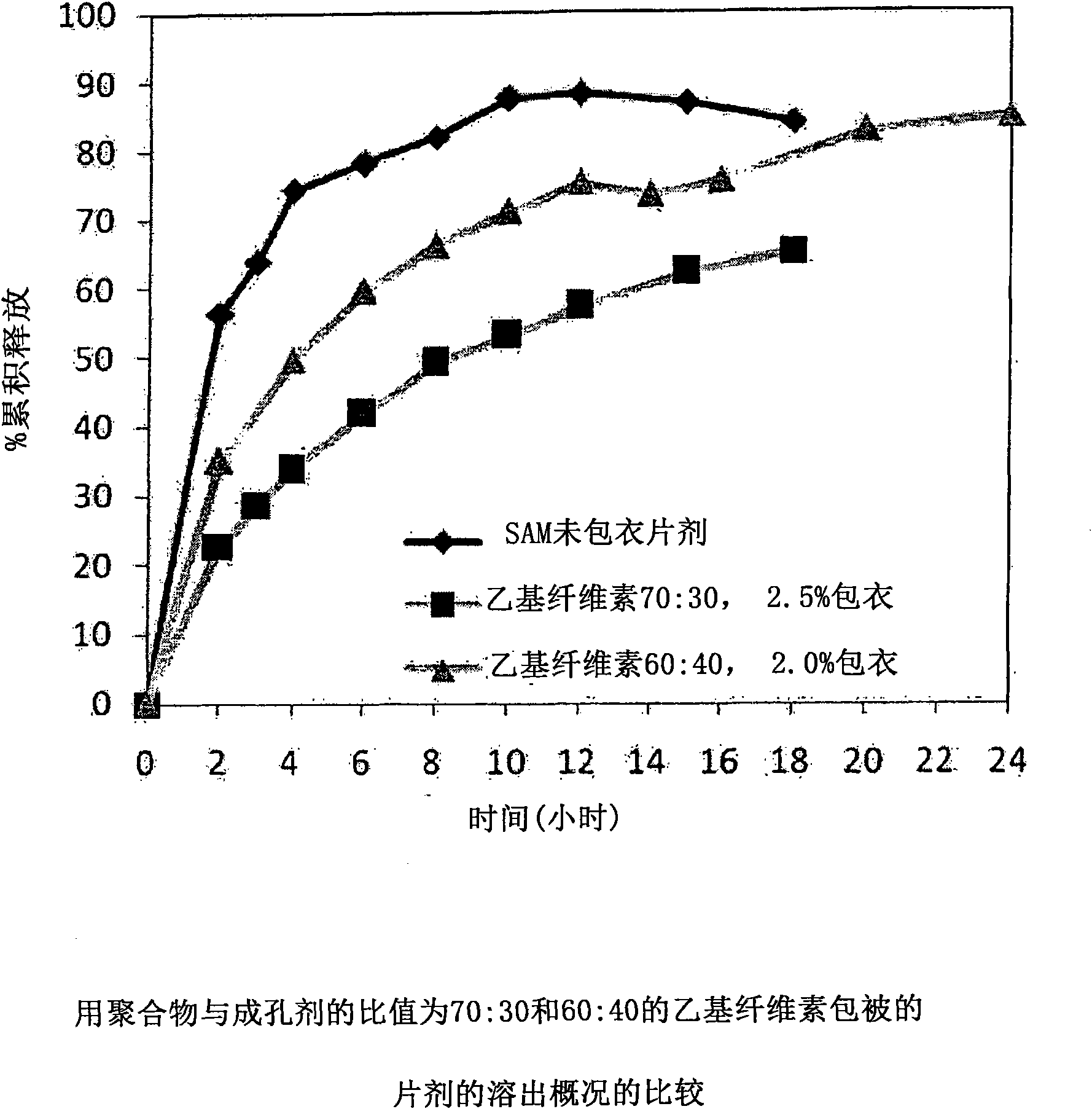
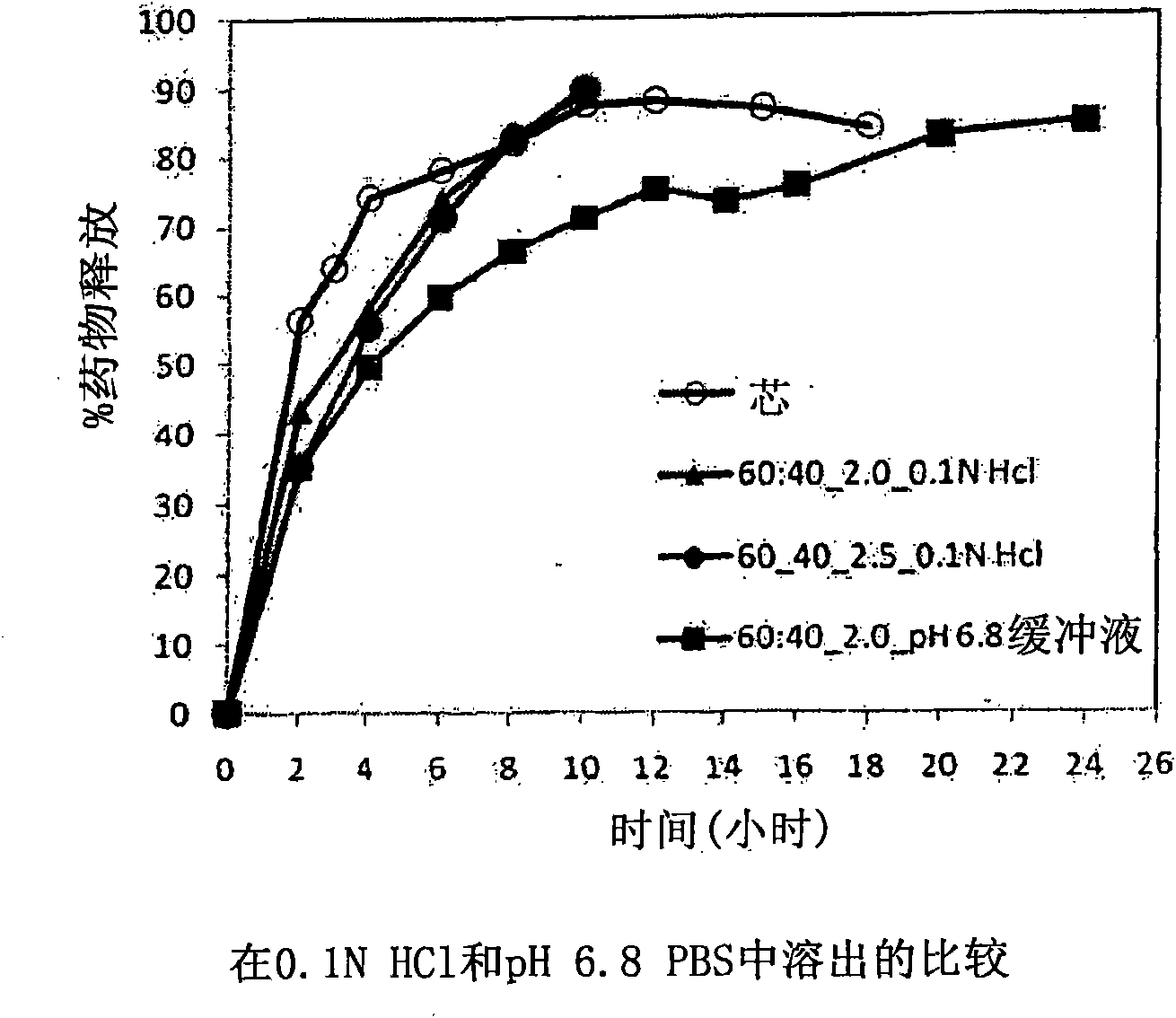
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
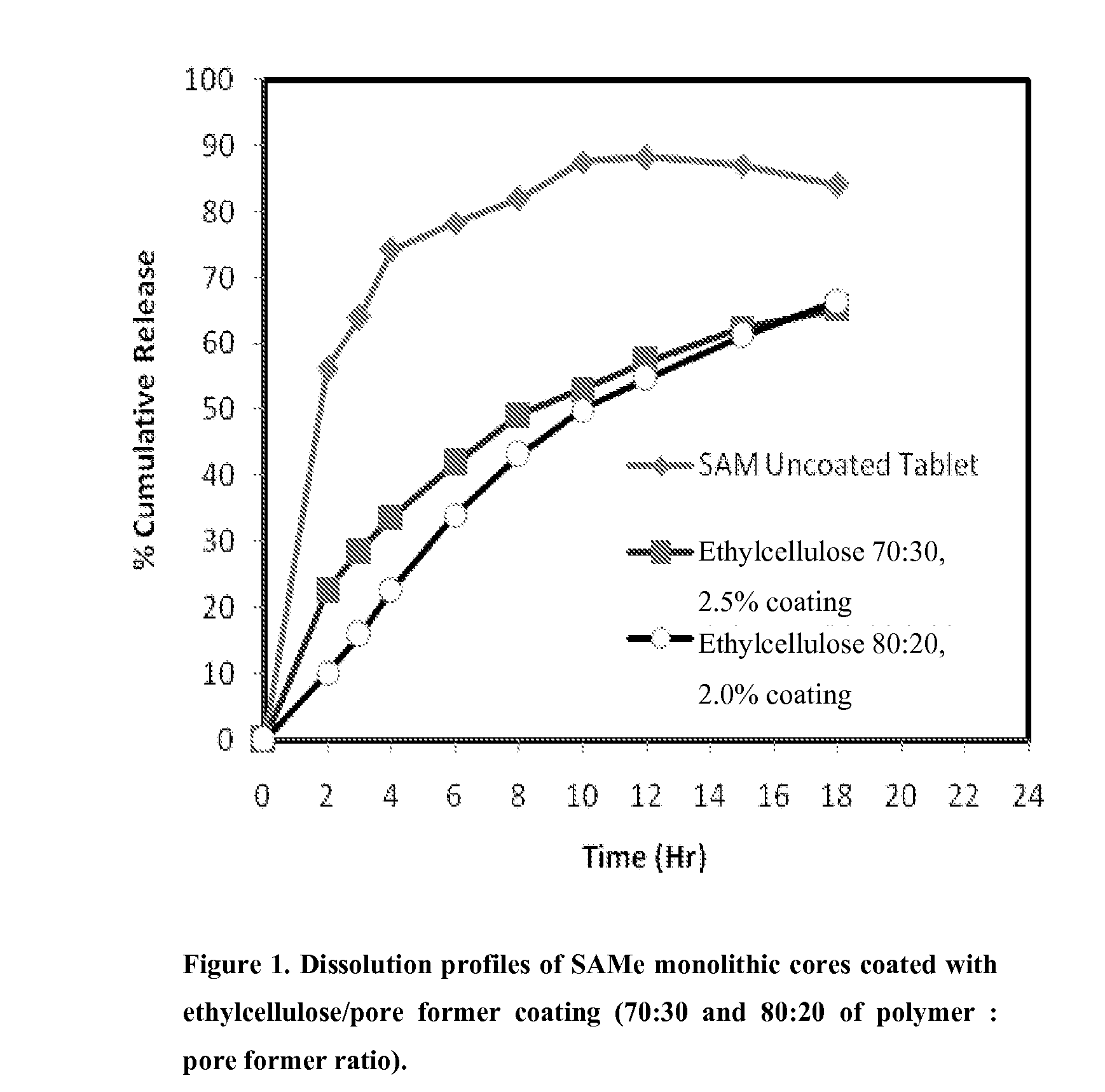
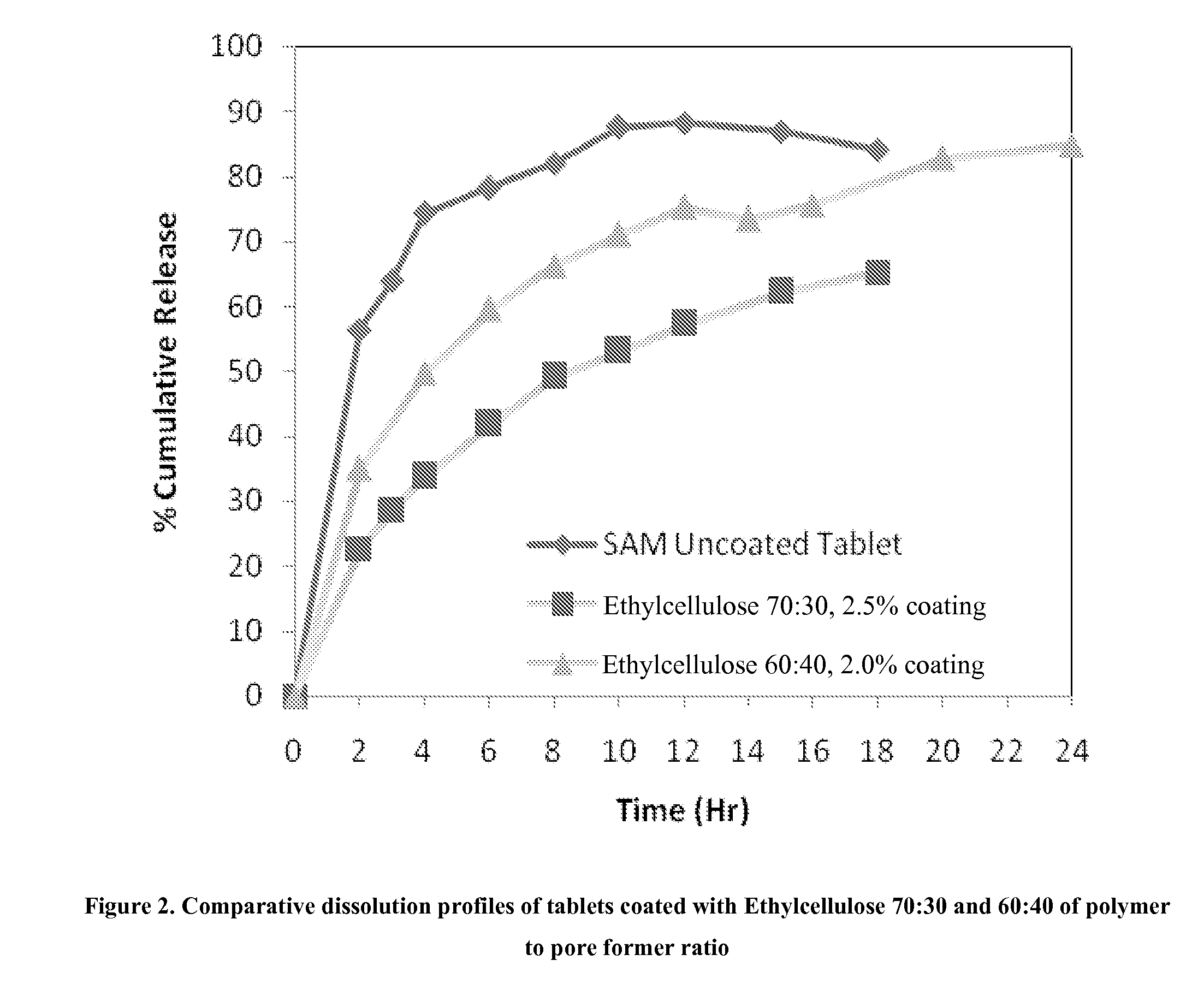
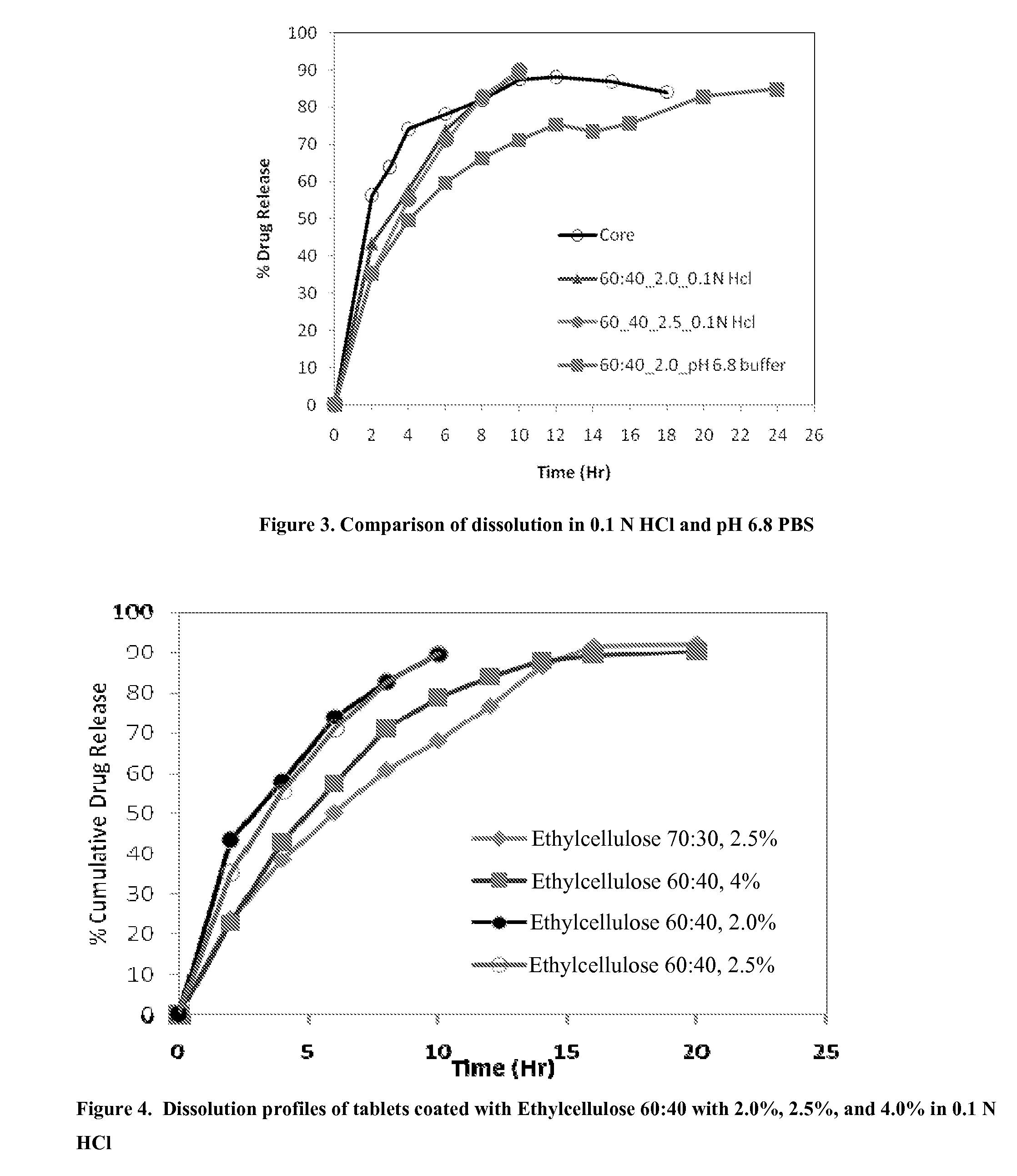
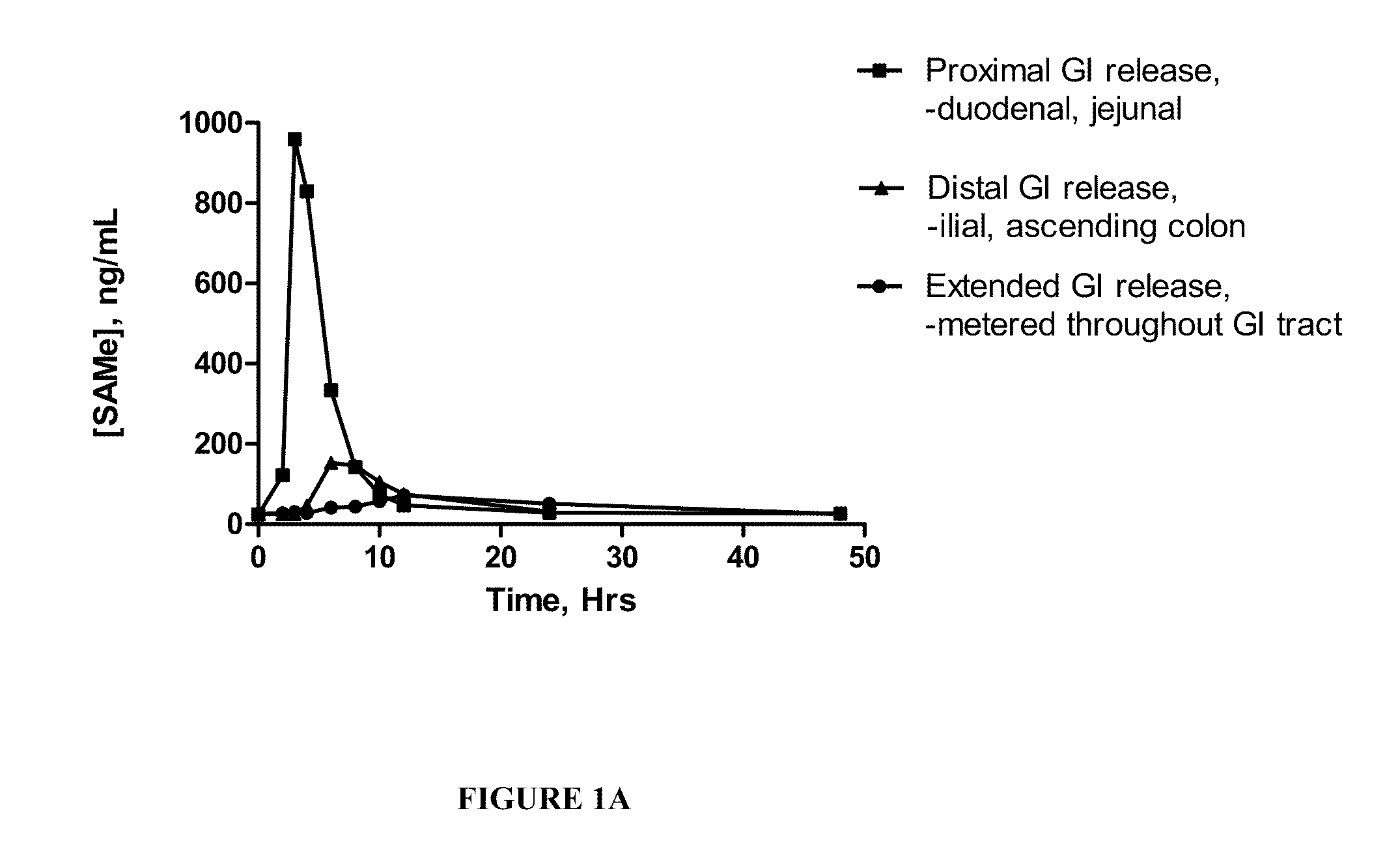
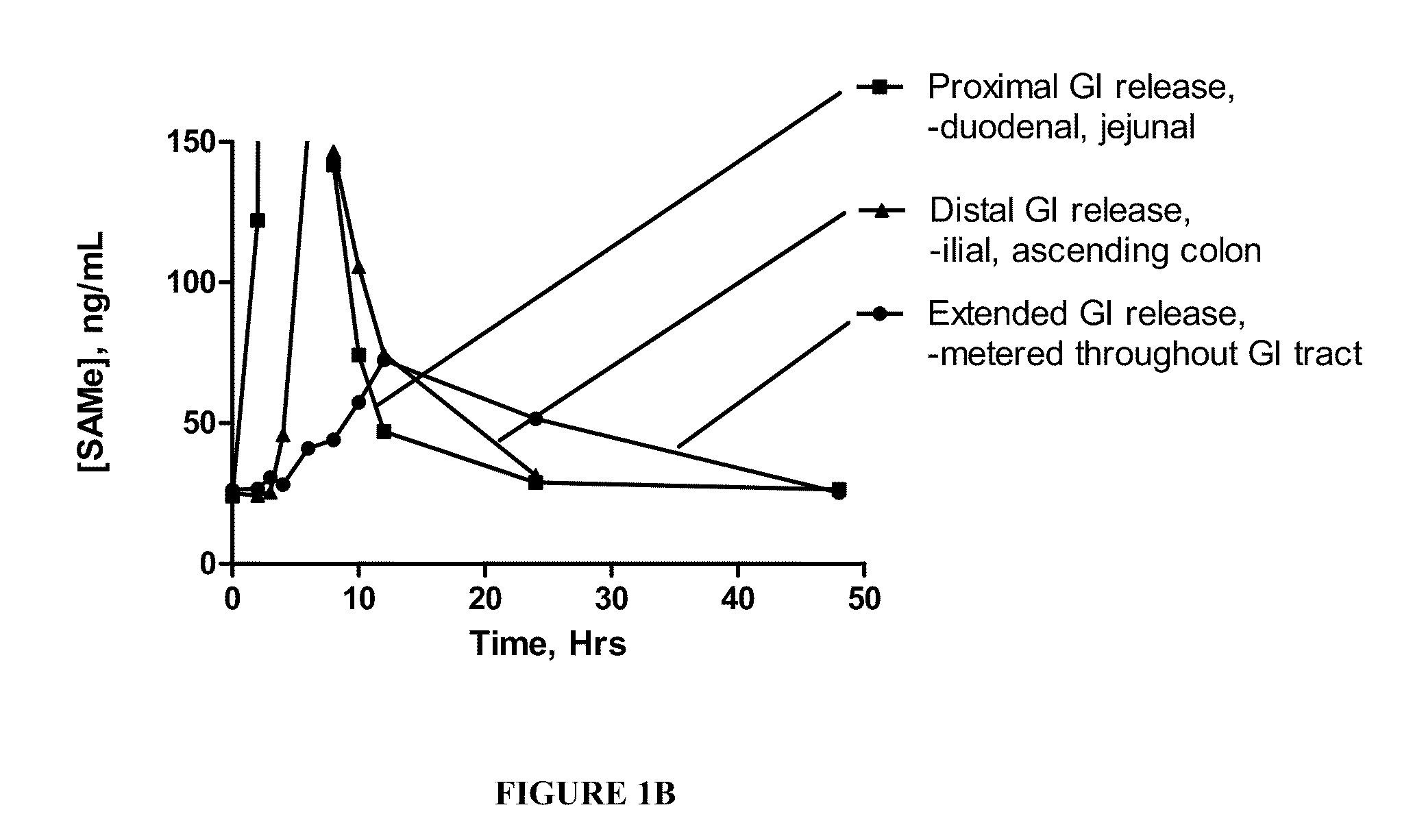
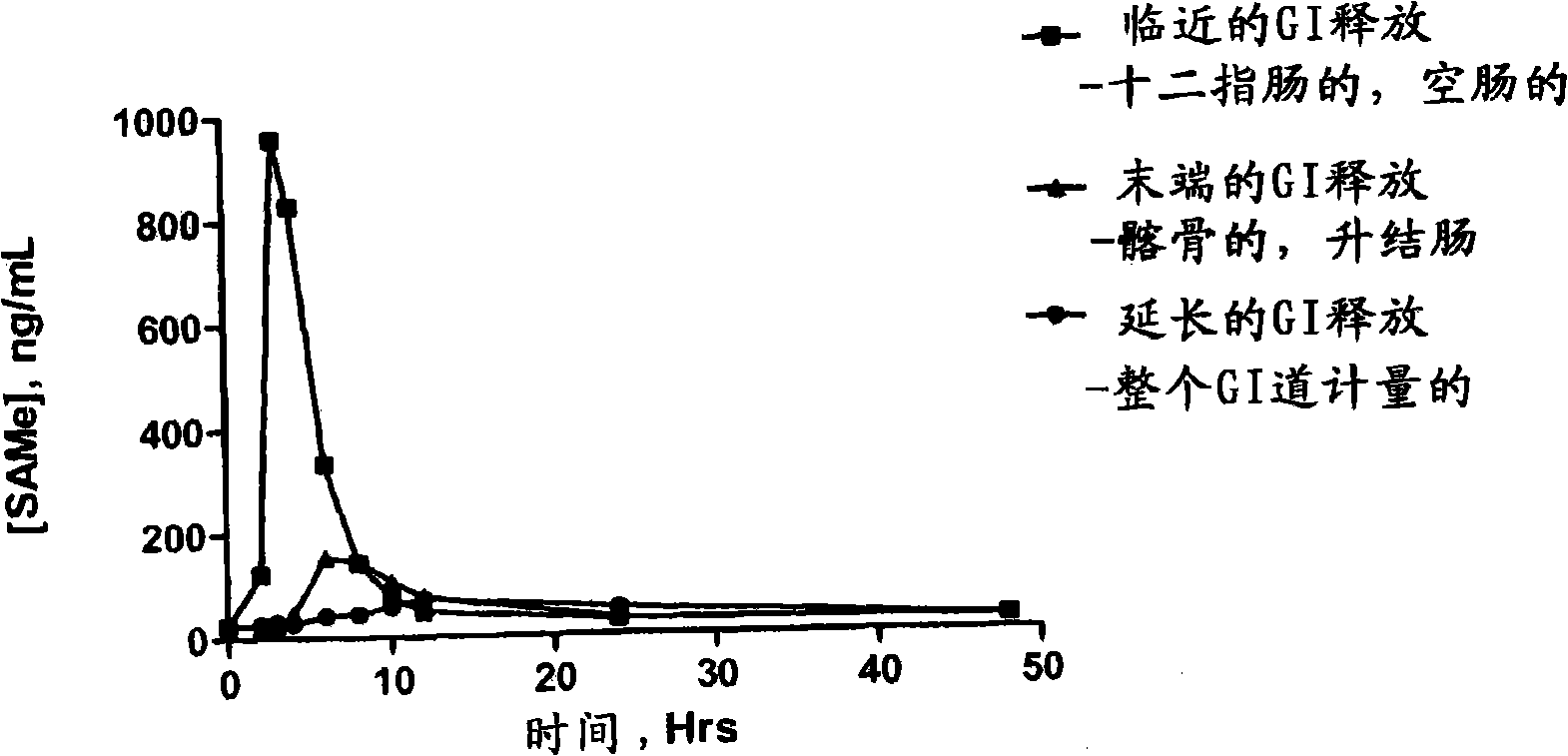
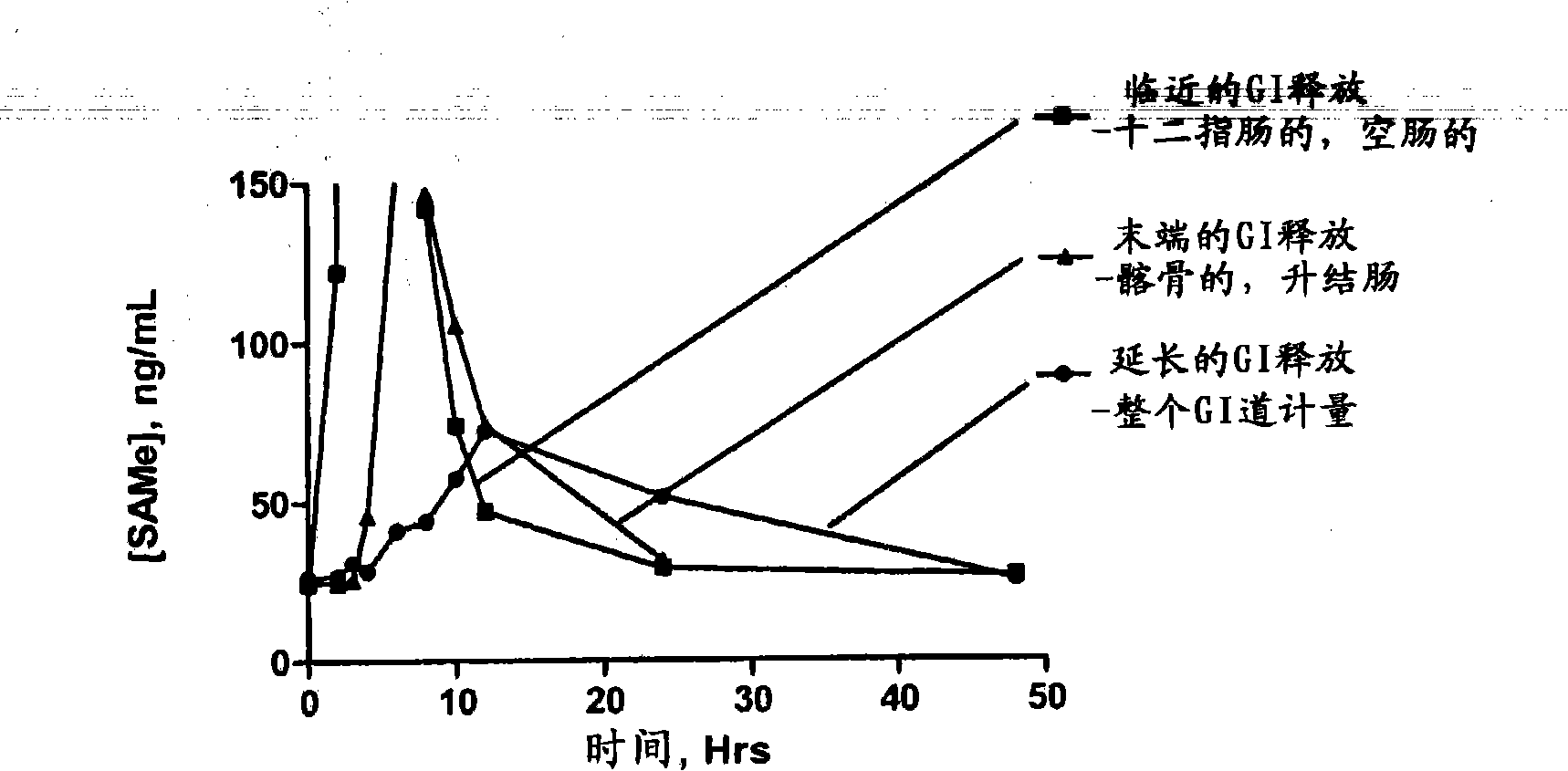
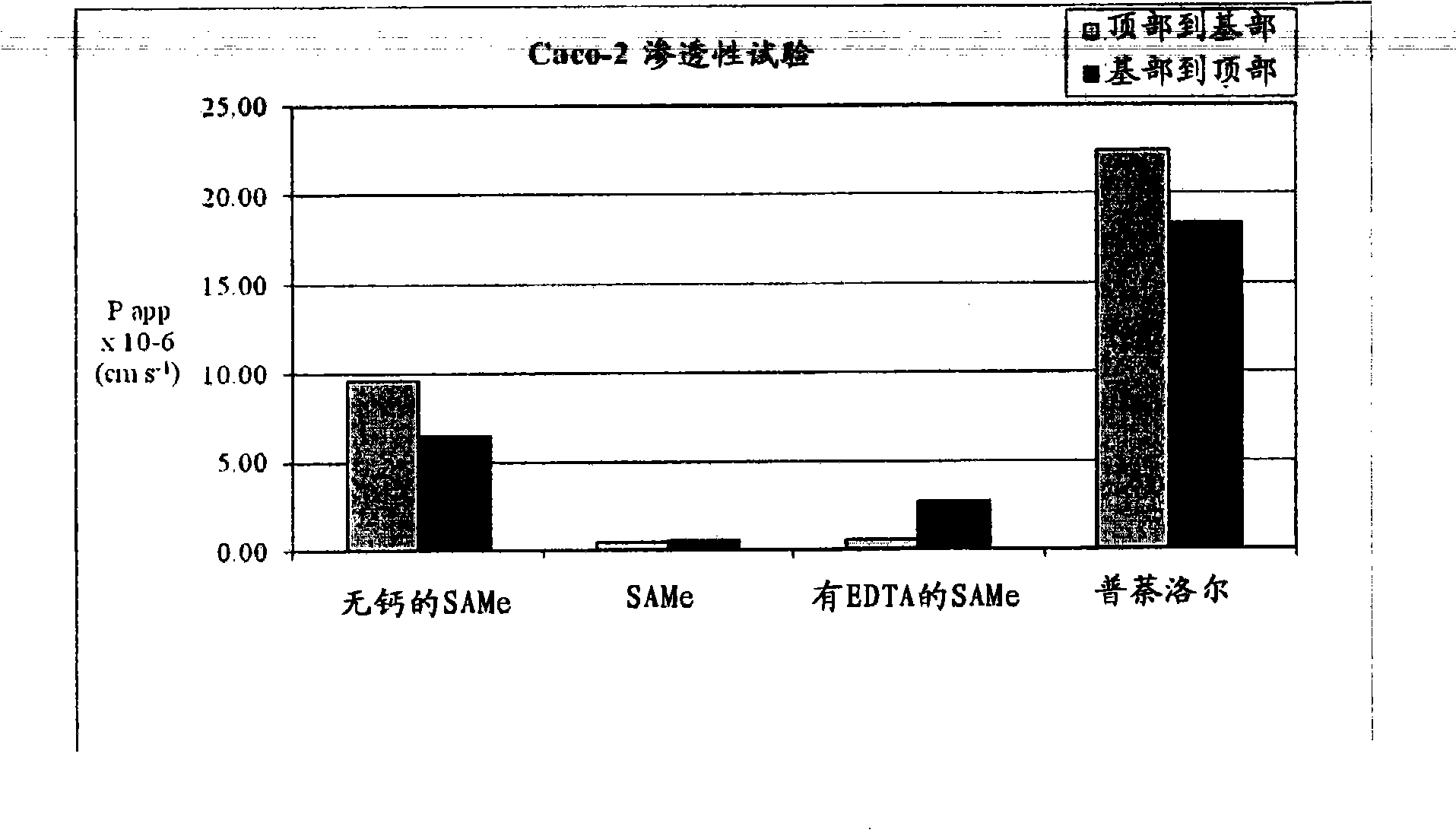
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
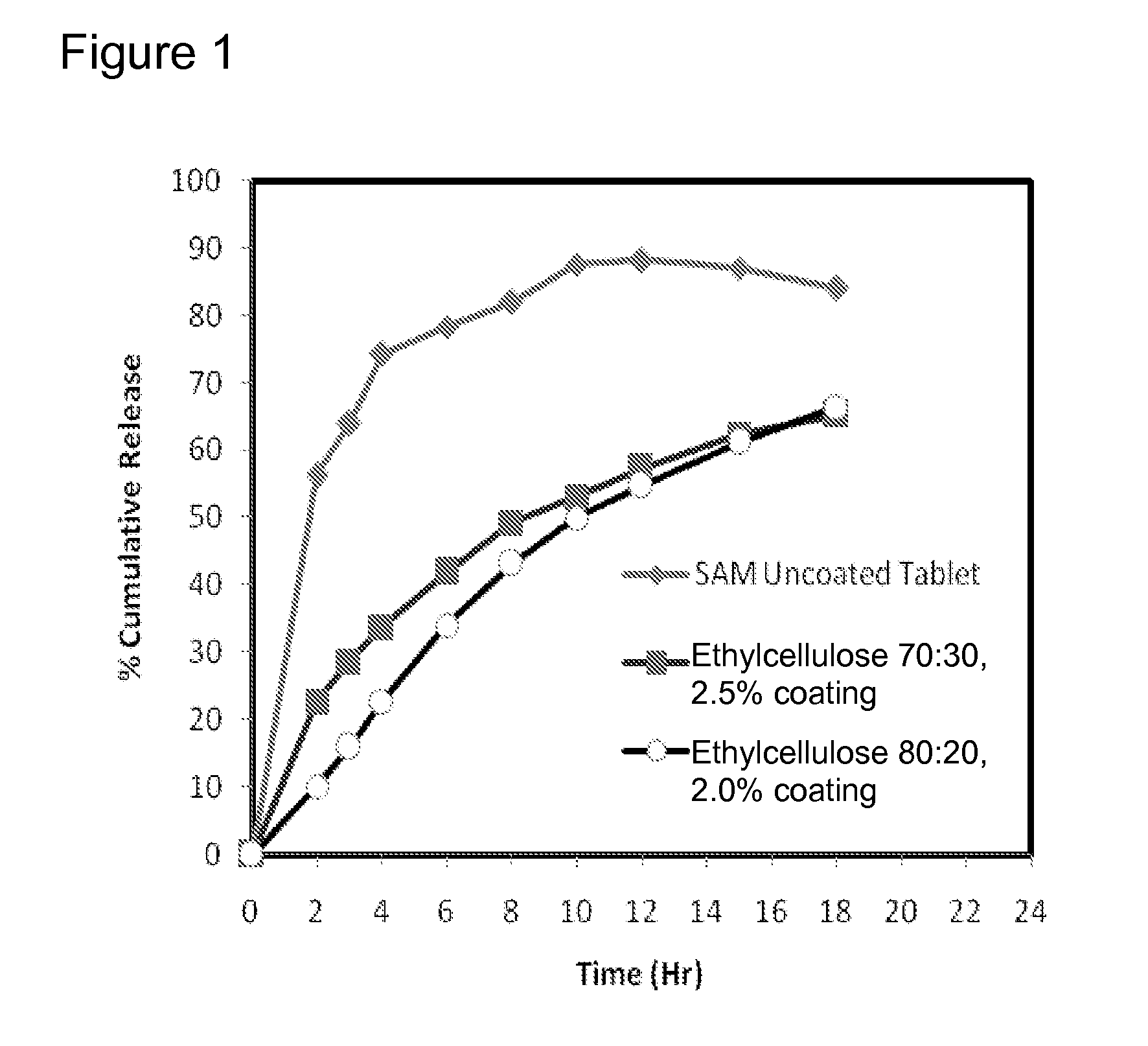
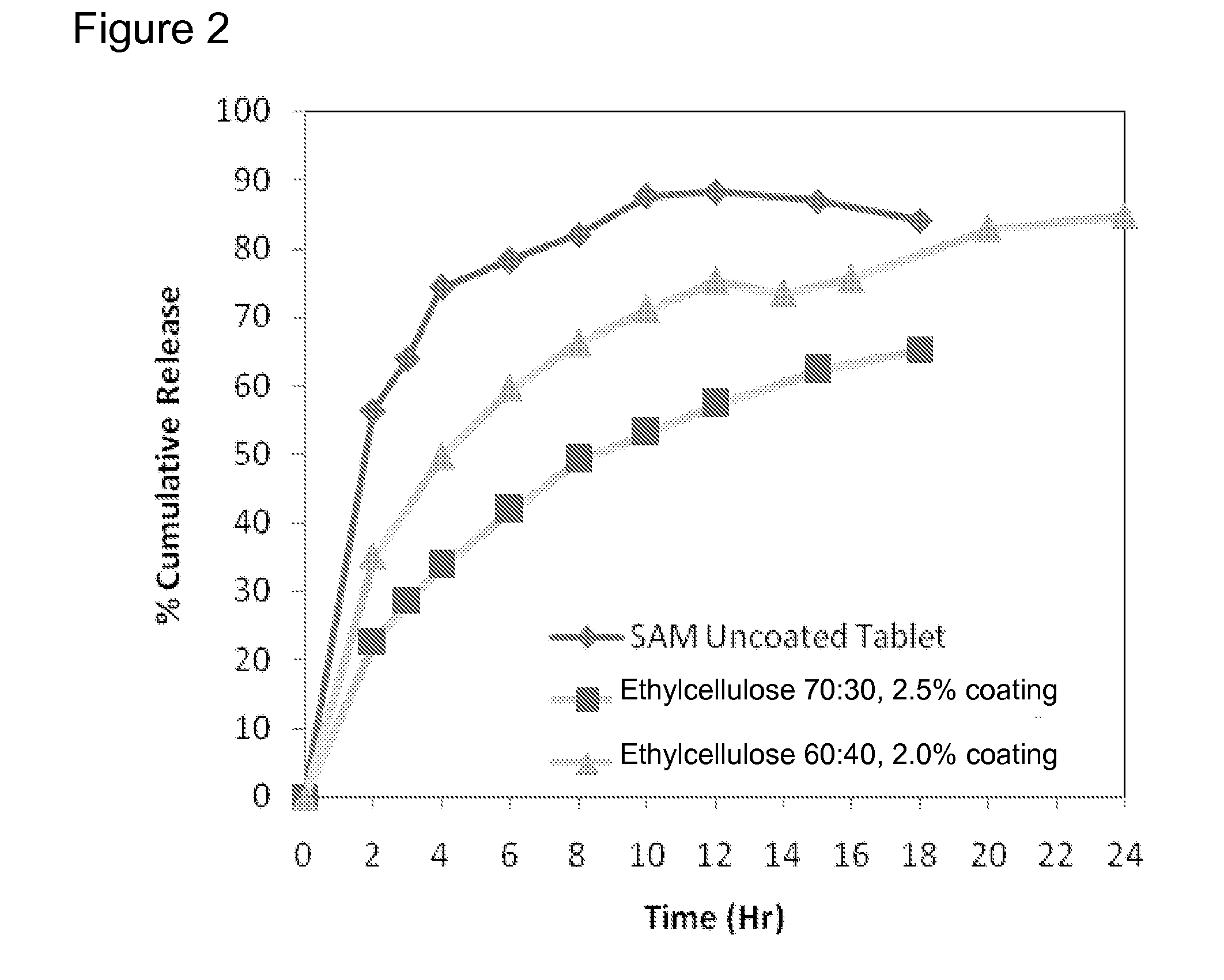
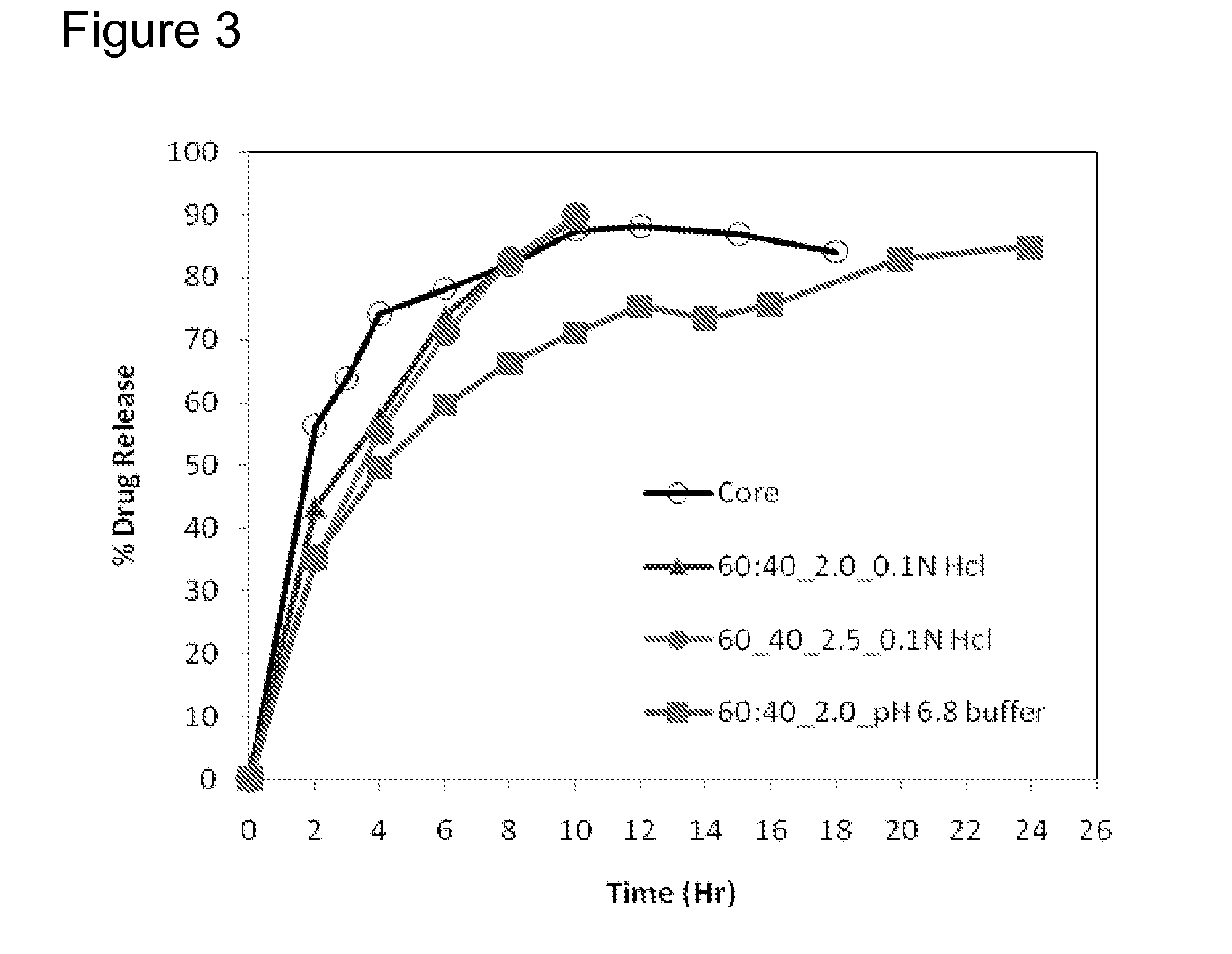
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Composition and methods for treating or preventing degenerative joint and cardiovascular conditions
A therapeutic composition and methods for the treatment and prevention of a degenerative joint disorder and / or a cardiovascular disease comprising polycosanols, glucosamine and chondroitin are disclosed.
Owner:TRX PHARMA
Remedies for joint diseases
InactiveUS6608043B1Effective treatmentSuppressing matrix metalloproteinaseBiocideSenses disorderDiseaseSynovial Cell
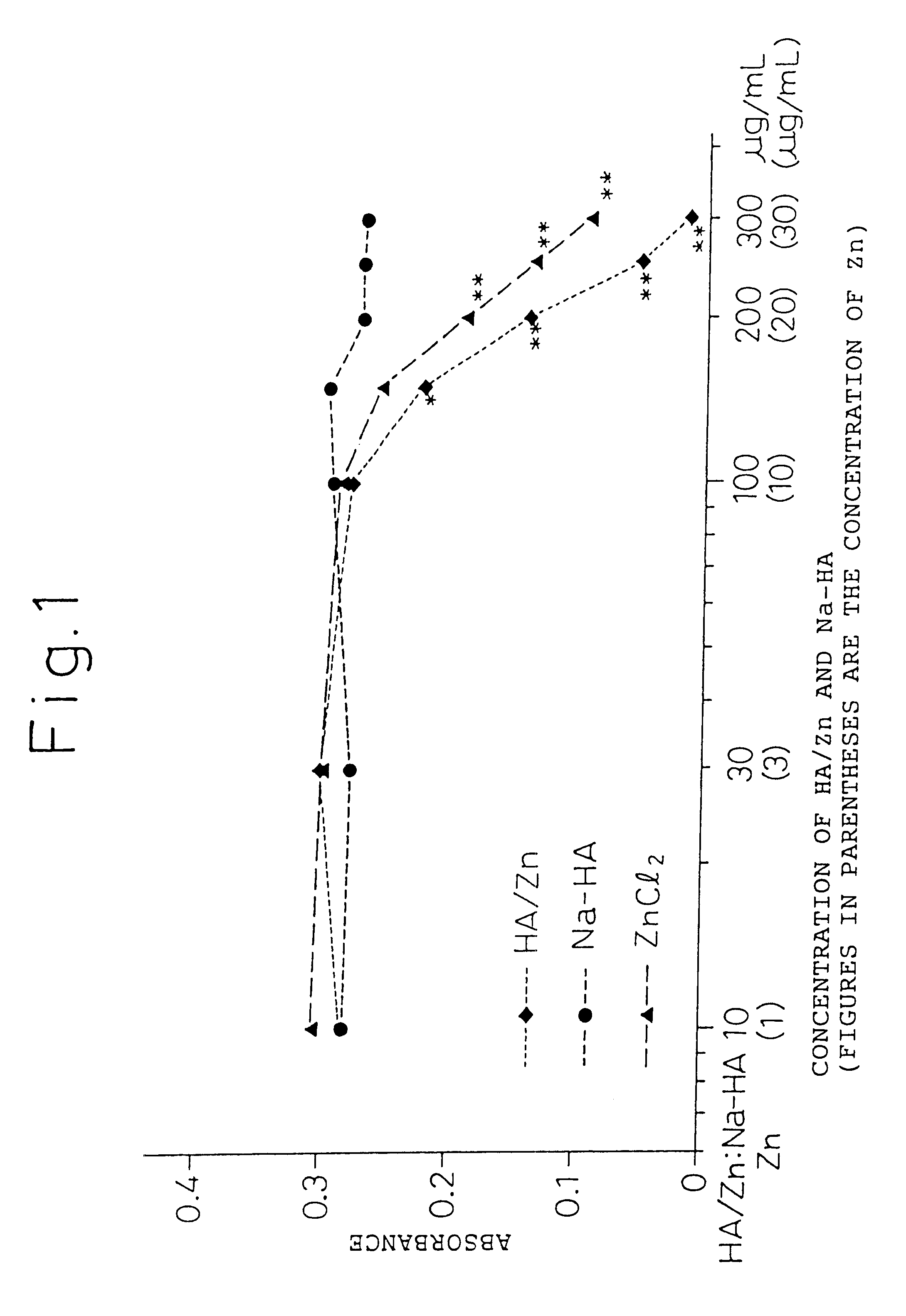
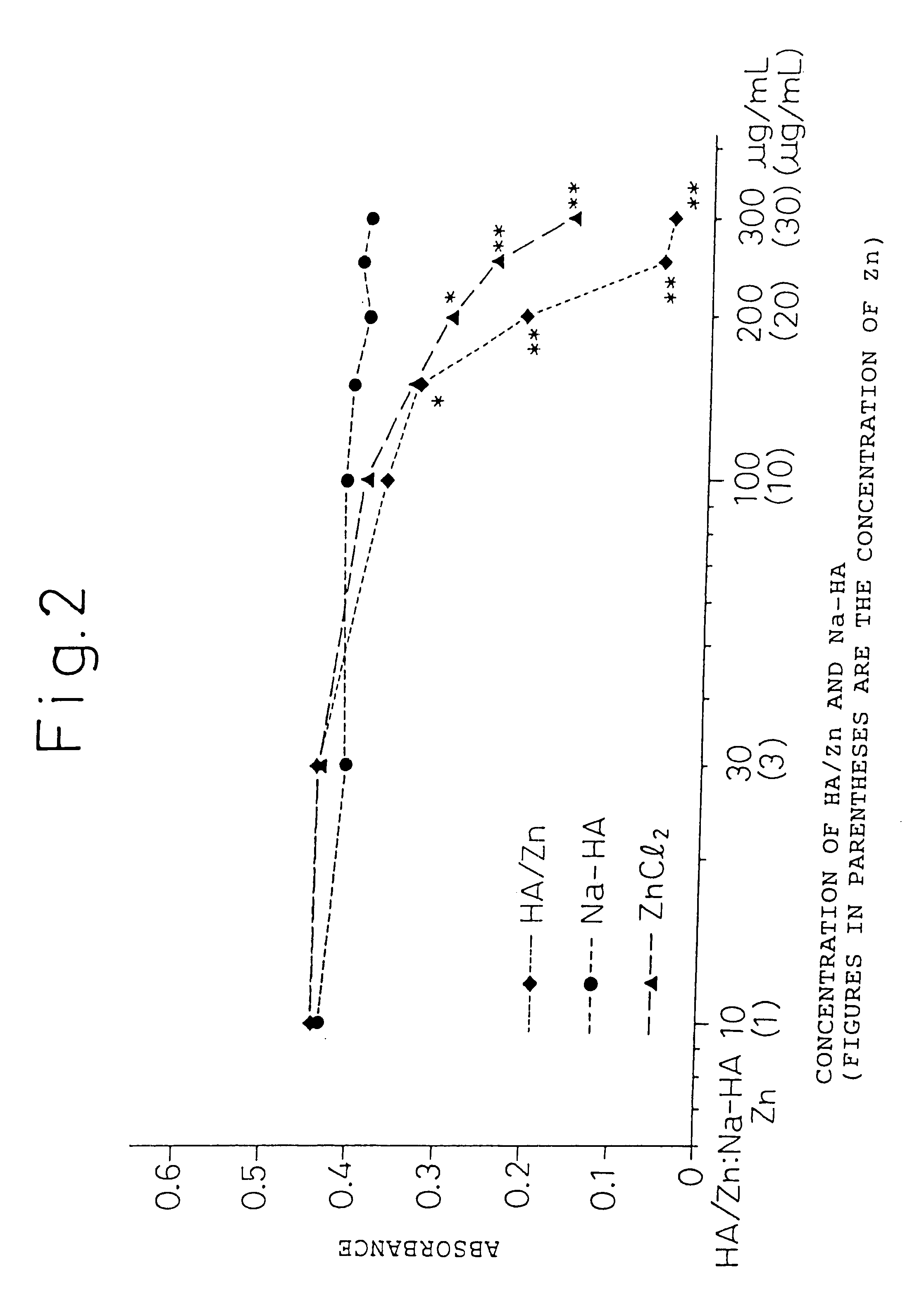
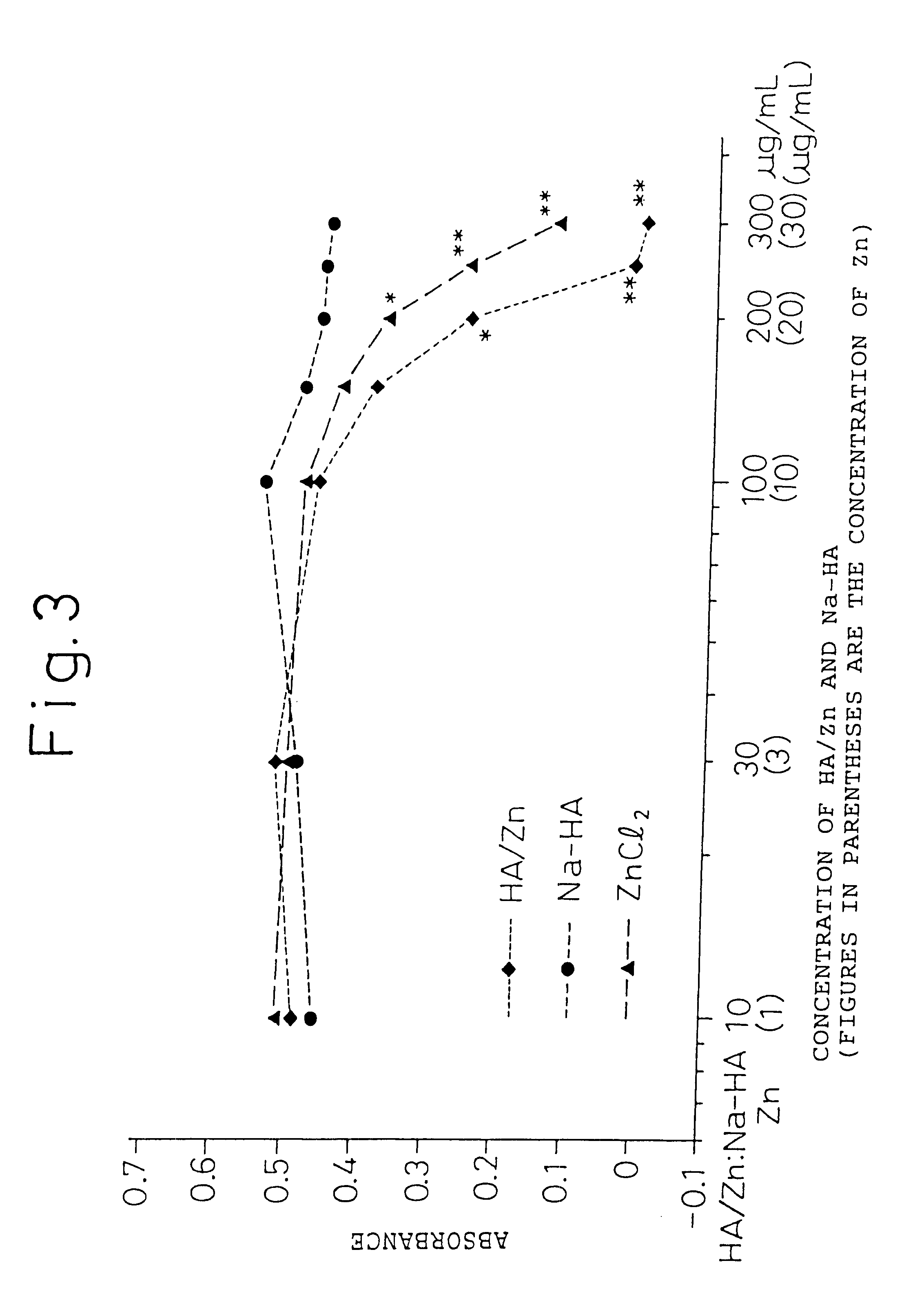
The present invention provides an agent for treatment of arthritic diseases such as rheumatoid arthritis that has for its active ingredient a complex of hyaluronic acid and zinc. This complex synergistically inhibits proliferation of synovial cells and suppresses matrix metalloproteinase MMP-9, which is produced by synovial cells, as compared with its constituents, hyaluronic acid and zinc, alone.
Owner:TAKATA SEIYAKU +1
Bone and/or joint disease-associated genes
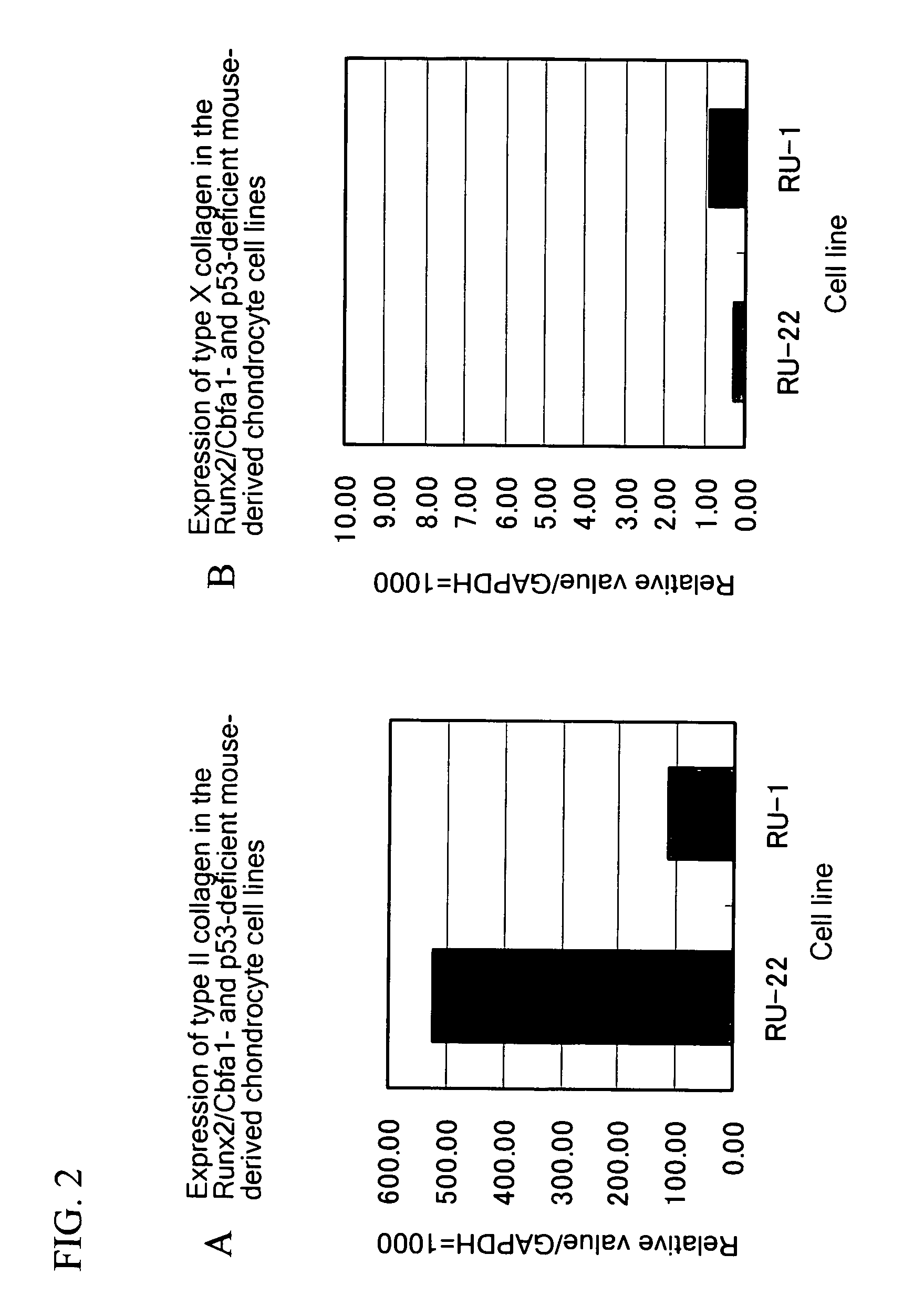
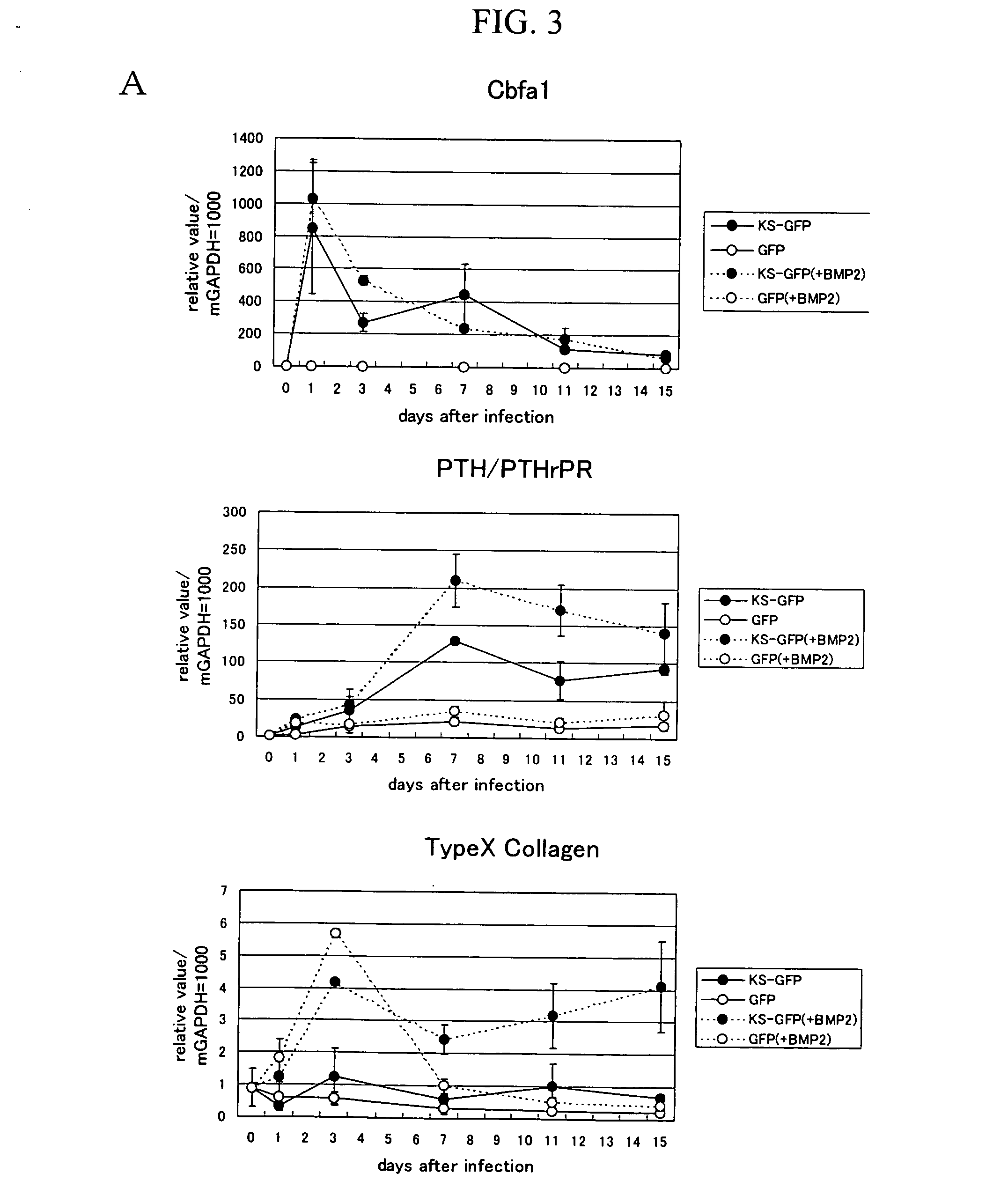
InactiveUS20070028314A1Increase and decrease levelPromote differentiationPeptide/protein ingredientsAntipyreticPolynucleotideJoint disease
This invention provides a method for obtaining a gene involved in regulation of cartilage differentiation, in which a transcription factor, preferably Runx2 / Cbfa1, is forcedly expressed in a cell that is deficient such transcription factor, preferably in a Runx2 / Cbfa1-deficient chondrocyte, and the gene, the expression of which is thereby induced, is selected using DNA chip analysis, subtraction, or other means as well as a Runx2 / Cbfa1-deficient chondrocyte useful for carrying out such method. The invention also provides a polynucleotide obtained by such method, a polypeptide encoded by such polynucleotide, an antibody against such polypeptide, a recombinant vector comprising such polynucleotide, a transformant comprising such recombinant DNA vector, a cell expressing such polypeptide, a transgenic animal of such polynucleotide, an animal model of a bone and / or joint disease (preferably osteoarthritis), a method for screening for a therapeutic agent and / or prophylactic agent for a bone and / or joint disease (preferably osteoarthritis) using the aforementioned objects, a candidate compound for a therapeutic agent and / or prophylactic agent selected by such method, a pharmaceutical composition for a bone and / or joint disease (preferably osteoarthritis), and a method for diagnosing such disease.
Owner:KOMORI TOSHIHISA +1
Medicine for treating bone joint disease and its preparation method
ActiveCN101185718AEffective treatmentEliminate pathological changesPowder deliveryAerosol deliveryDiseaseSide effect
The invention relates to a medicine for the treatment of bone and joint diseases and the preparation method, the invention is prepared by the raw materials with the following mixing ratio of parts by weight: 20 to 40 parts of clematis root, 15 to 30 parts of Szechuan lovage rhizome, 15 to 30 parts of danshen root, 5 to 20 parts of incised notopterygium rhizome, 5 to 20 parts of doubleteeth pubescent angilica root, 20 to 40 parts of Chinese angelica, 15 to 30 parts of safflower, 15 to 30 parts of common clubmoss herb, 10 to 25 parts of common floweringquince fruit, 15 to 35 parts of garden balsam stem, 10 to 35 parts of twotooth achyranthes root, 15 to 30 parts of rhubarb, 15 to 35 parts of slenderstyle acanthopanax bark, 10 to 35 parts of beautiful sweetgum fruit, 5 to 15 parts of Chinese honeylocust abnormal fruit, 10 to 25 parts of yanhusuo, 15 to 35 parts of Chinese starjasmine stem, 15 to 35 parts of sargentgloryvine stem, 10 to 35 parts of ovientvine, 10 to 35 parts of Ningpo yam rhizome, 25 to 40 parts of kudzuvine root, 20 to 35 parts of herba siegesbeckiae, 5 to 20 parts of manchurian wildginger, 5 to 20 parts of red paeony root, 10 to 25 parts of malaytea scurfpea fruit, 5 to 30 parts of fructus litseae, 5 to 35 parts of pine nodular branch, 5 to 25 parts of pricklyash peel and 10 to 35 parts of argy wormwood leaf. The invention has good efficacy on various bone and joint diseases, no toxicity or side effects, so the invention has very good market prospect.
Owner:王淋
Pharmaceutical composition comprising chito-oligomers
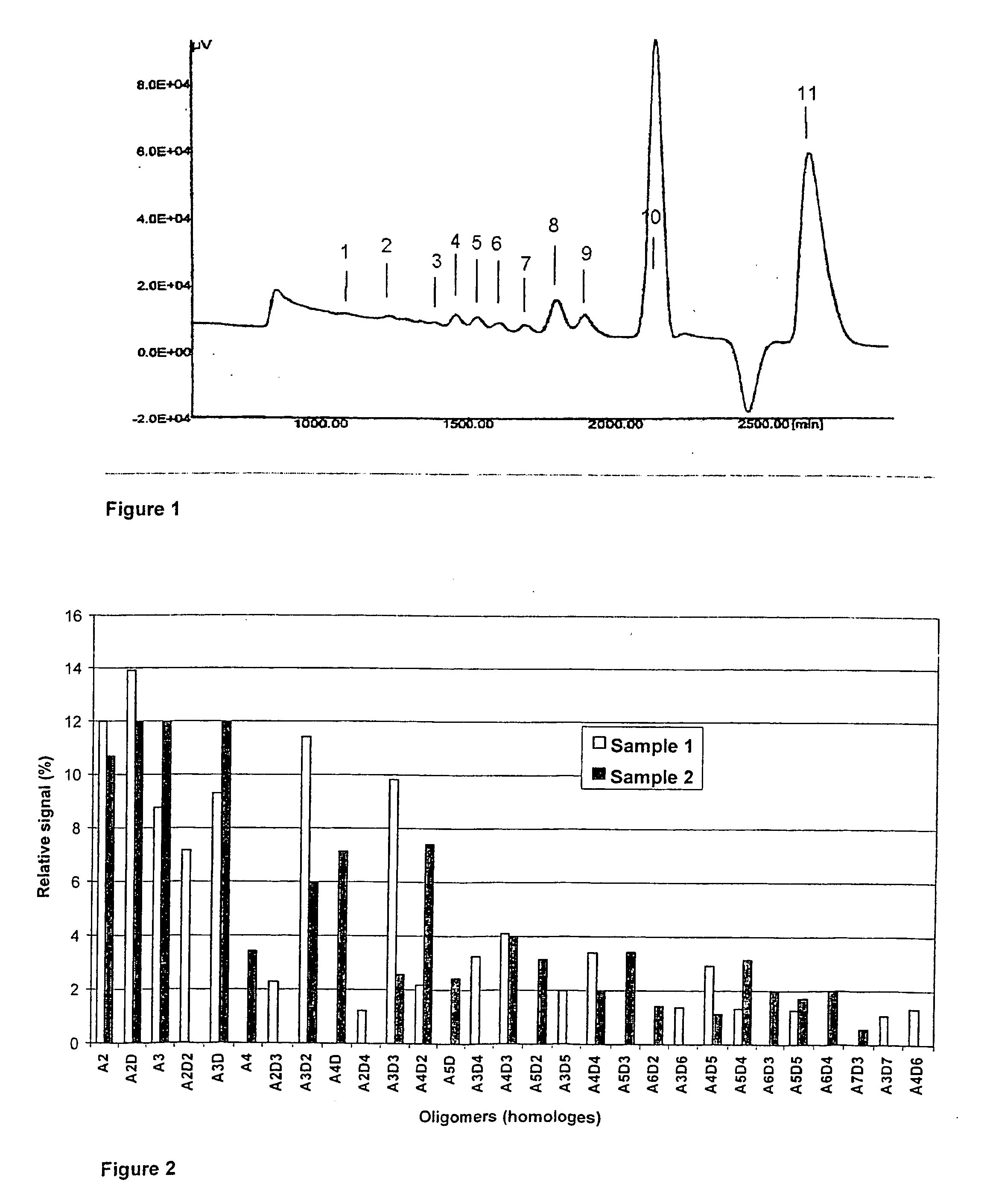
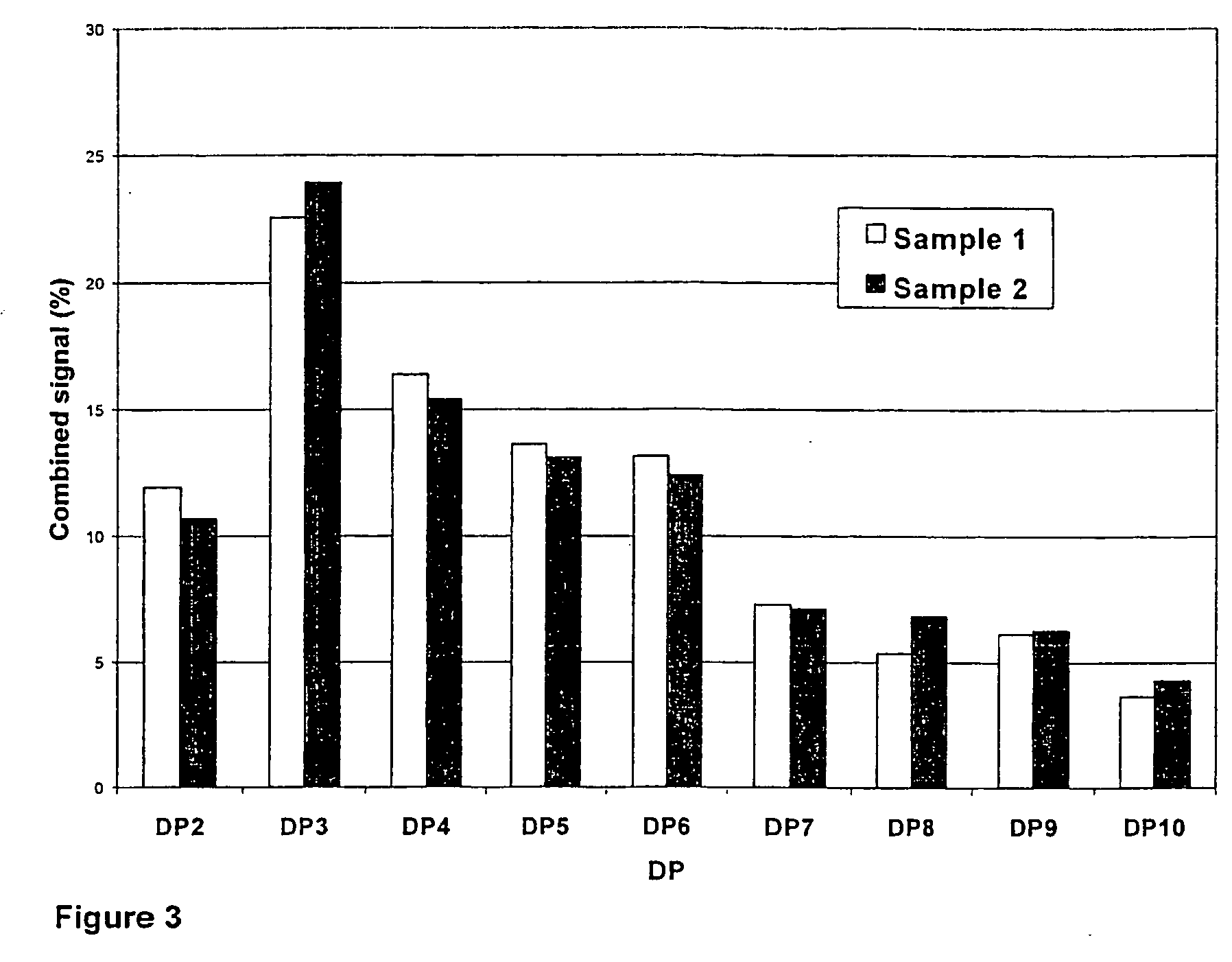
ActiveUS20050004073A1Good results in alleviating the symptoms of joint disordersReduce inflammationEsterified saccharide compoundsBiocideDiseaseOligomer
Compositions are provided comprising chito-oligomers obtainable from chitin, comprising oligomers of N-acetyl glucosamine (NAG) and glucosamine, wherein at least 50% of the oligomers have a chain length of about 2-50, and the degree of deacetylation of the oligomers is in the range of about 0-70%, preferably about 30-50%. The compositions are highly useful as pharmaceutical compositions for treatment of joint disorders such as rheumatoid arthritis and osteoarthritis. Also provided are methods for treatment of joint disorders and treatment against inflammatory activity.
Owner:GENIS EHF
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090197824A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Viscosupplementation with algal polysaccharides in the treatment of arthritis
A joint-lubricating composition for treating degenerative joint disorders is provided, comprising algal polysaccharides in an aqueous solution, wherein the polysaccharides are stable and non-immunogenic. Further provided is a method for treating a joint disorder by injecting into the joint a viscous aqueous solution of a polysaccharide, wherein disorder is, for example, arthritis, and the polysaccharide originates, for example, from a red alga.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
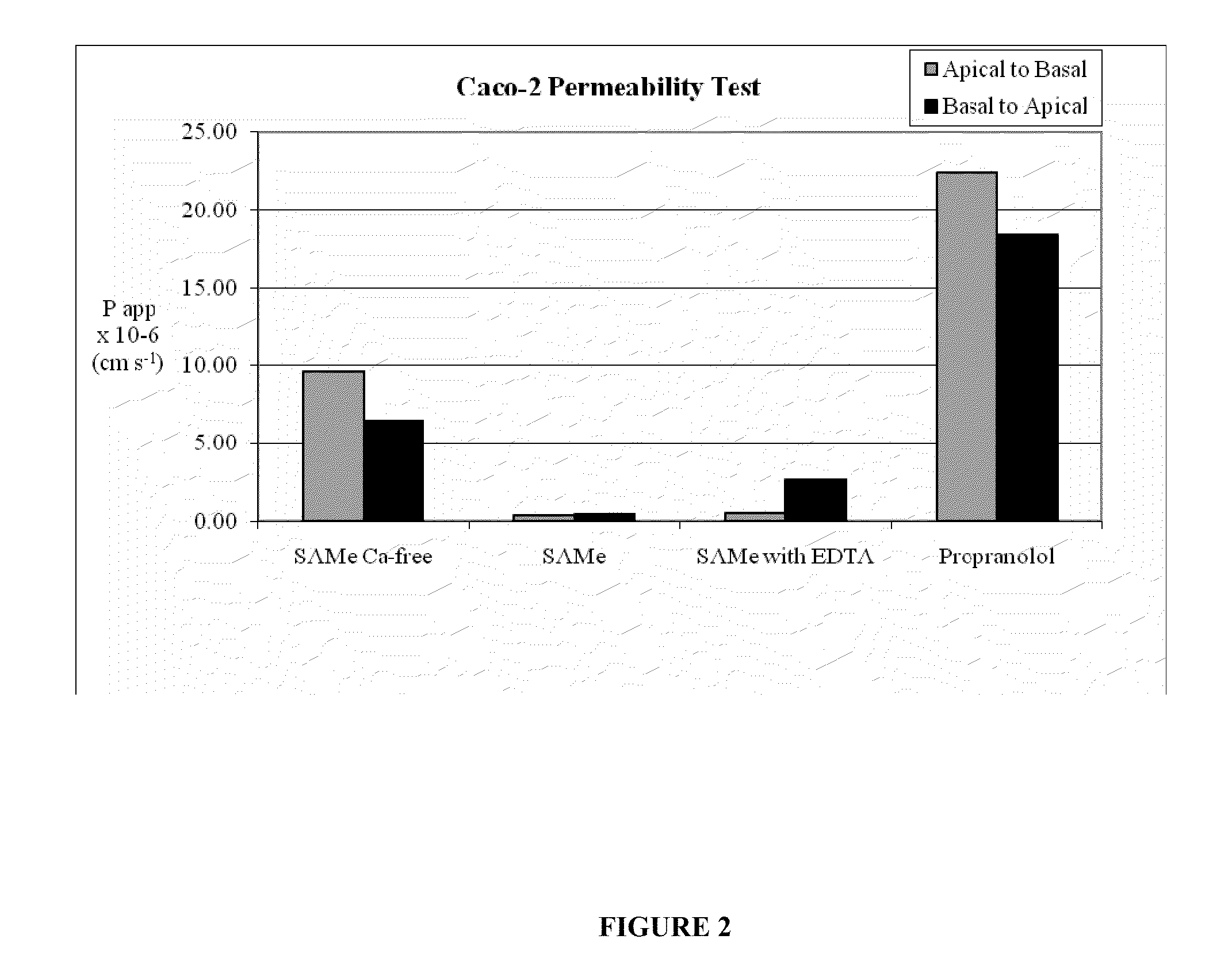
S-adenosylmethionine formulations with enhanced bioavailability
InactiveUS20110027342A1Reduce penetrationPromote absorptionBiocideNervous disorderImmunologic disordersS-Adenosyl-l-methionine
The invention relates to compositions and methods to enhance the absorption of S-adenosylmethionine (SAMe) and to methods of treating various disorders or diseases using non-parenteral SAMe formulations with enhanced-absorption and improved bioavailability. The enhanced bioavailability formulations may be used to treat a variety of diseases or disorders, such as for example, psychiatric disorders including, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, depressive disorders (e.g. major clinical depression) and dysthymia; as well as treating liver disorders, cancer, autoimmune disorders, inflammatory disorders, joint disorders, gastrointestinal disorders and cardiovascular disease.
Owner:MSI METHYLATION SCI
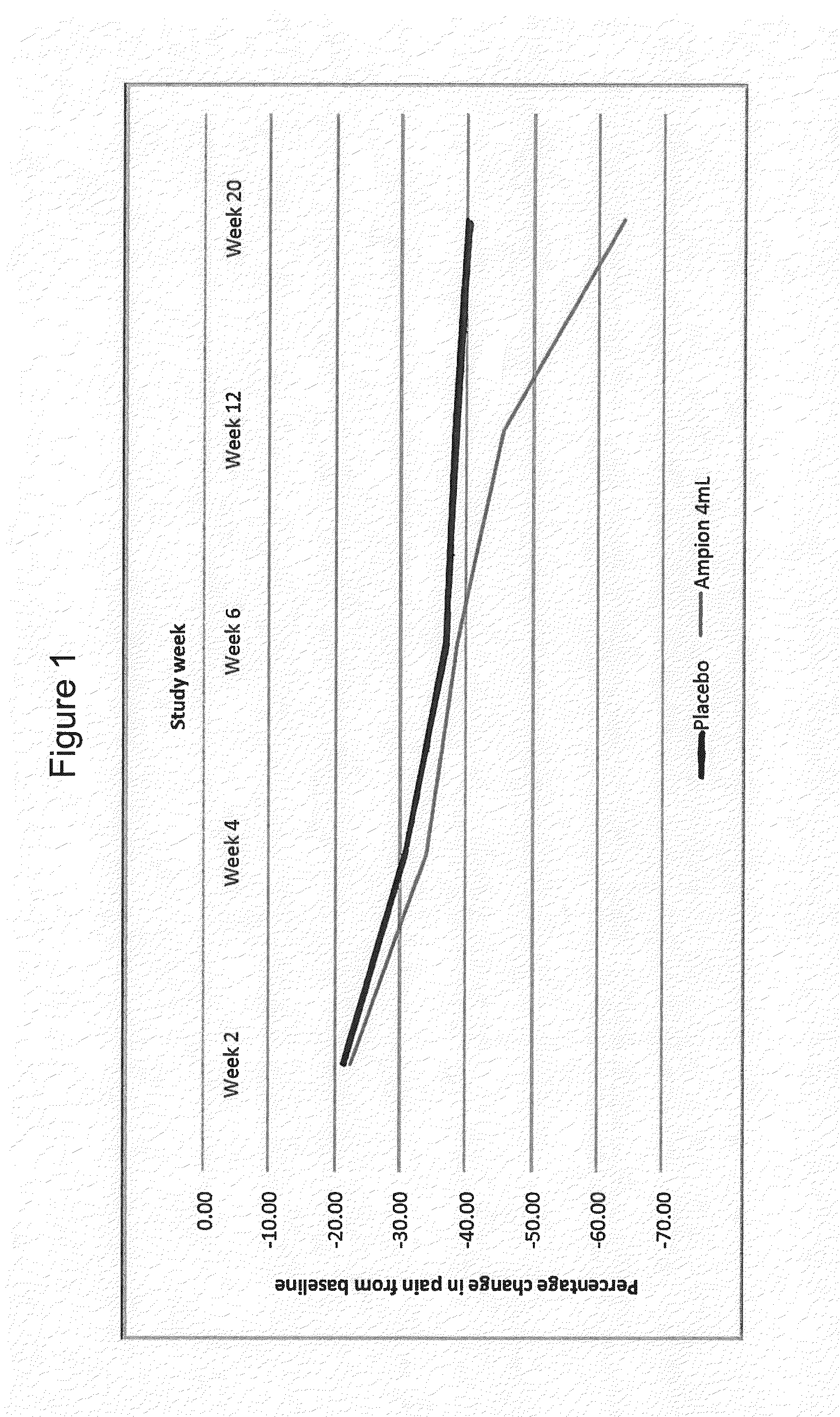
Treatment of joint conditions
ActiveUS20160045493A1Improve throughputLimit deliveryOrganic active ingredientsPeptide/protein ingredientsDiseaseDosing regimen
The invention provides a method of treating a joint condition. The method comprises administering a multi-dose regimen of a pharmaceutical composition comprising a diketopiperazine with amino acid side chains of aspartic acid and alanine (DA-DKP). The invention also provides a method of treating osteoarthritis with multiple doses of a low-molecular weight fraction of human serum albumin.
Owner:AMPIO PHARMA
Polymer gel containing hyaluronic acid and collagen, and its use in joints
Formulations and methods for treating joints, such as temporomandibular joint disorders, osteoarthritis of the knee, hip and other types of inflammatory joint diseases. The method involves identifying specific matrix metalloproteinases (MMPs) that may be responsible for degrading the soft tissues of the joint in question, identifying the specific component of the joint the MMP(s) are targeting, and injecting a polymer gel with the component the MMP(s) seek to destroy, thus preserving the joint and allowing time to heal. These formulations typically require a mixture of glycosoaminoglycans and collagen proteins. One formulation in particular includes both hyaluronic acid and at least type I collagen.
Owner:PRESCOTT ALBERT G
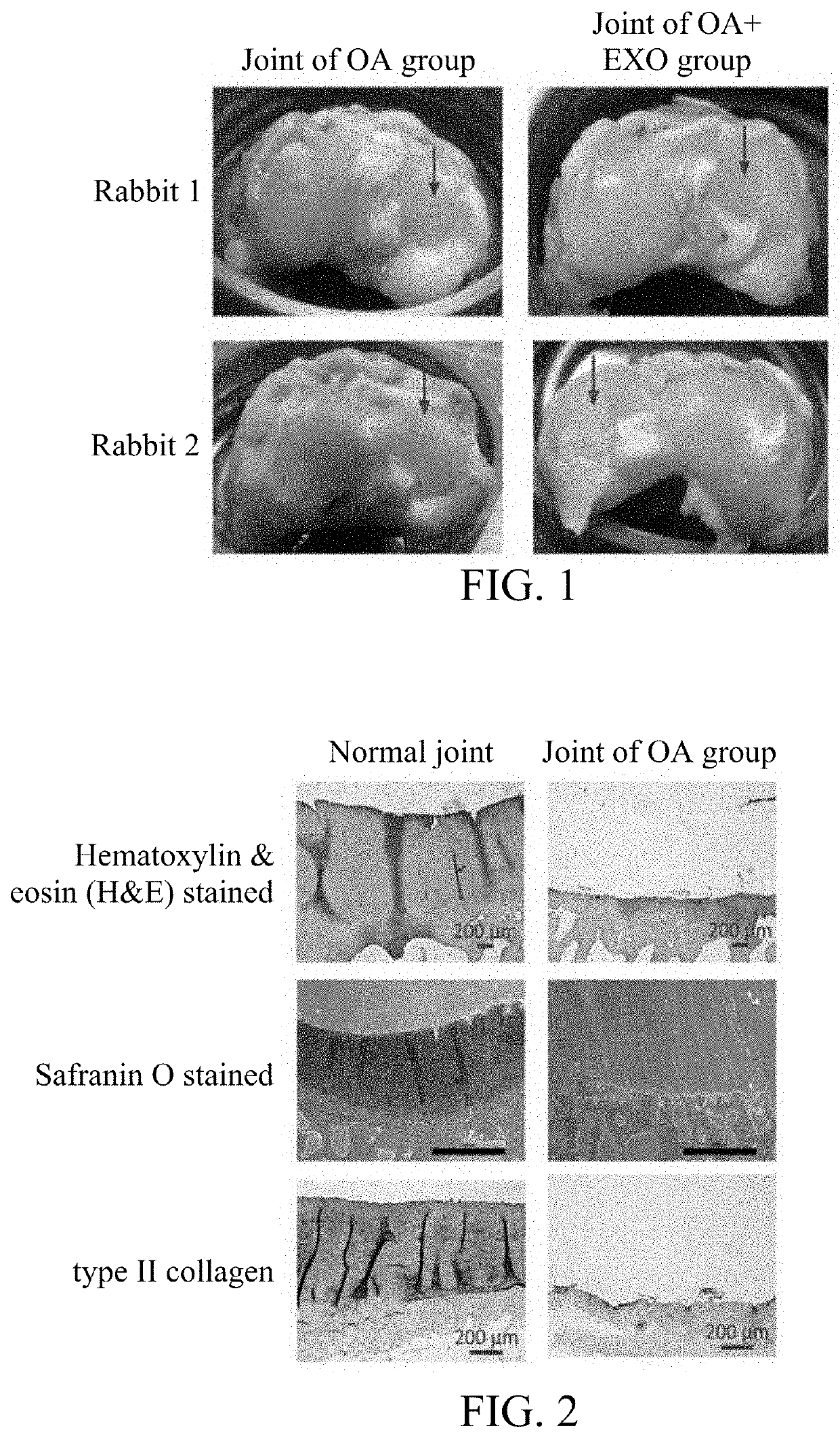
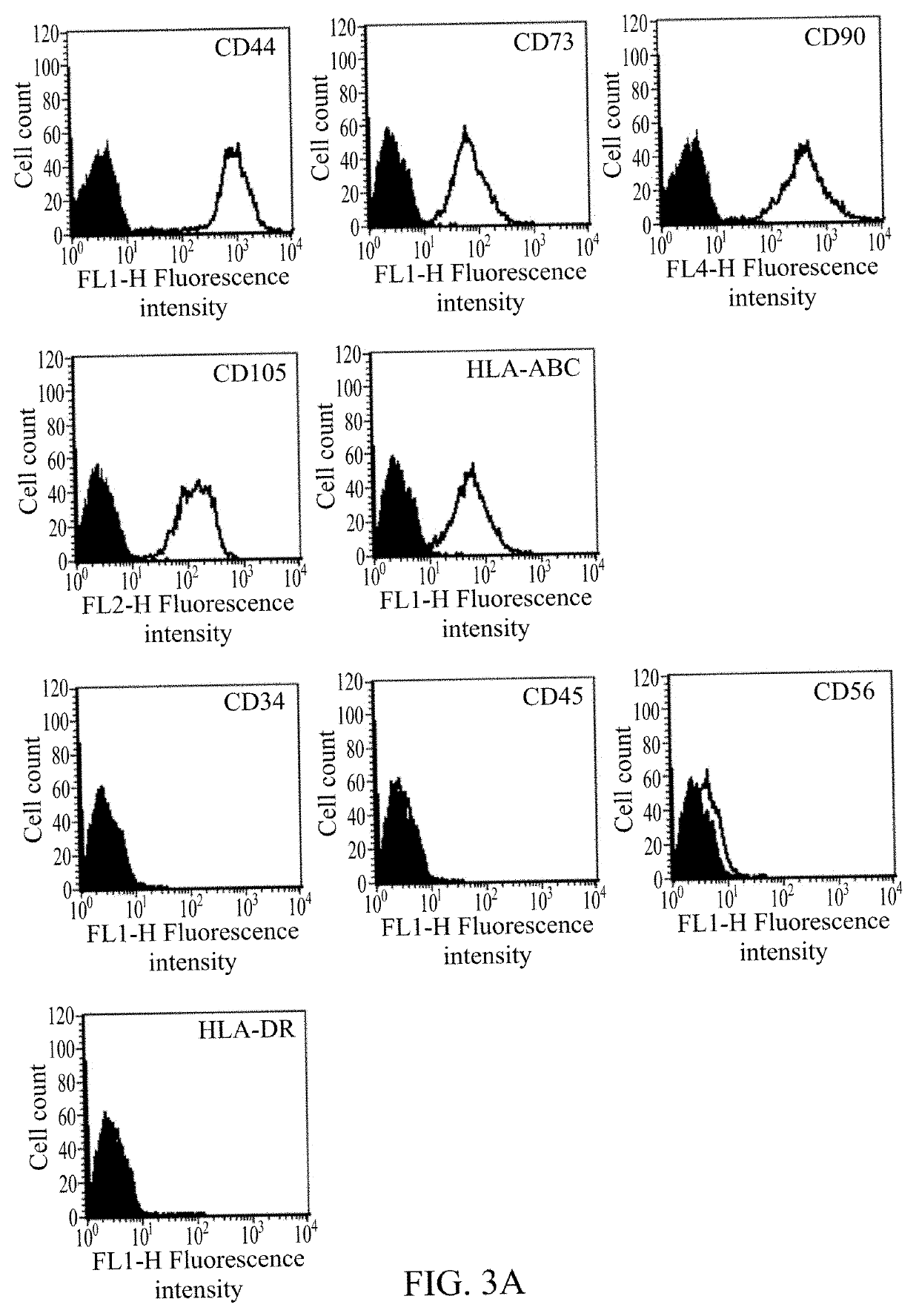
Mesenchymal stem cell derived exosomes and method for preventing or treating a joint disorder by administering a composition comprising the same
Provided is a pharmaceutical composition including an effective amount of mesenchymal stem cell derived exosomes. Also provided is a use of the pharmaceutical composition for preserving cartilage tissue, promoting cartilage regeneration and repairing damaged joints, thereby preventing or treating joint disorders in a subject in need thereof.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
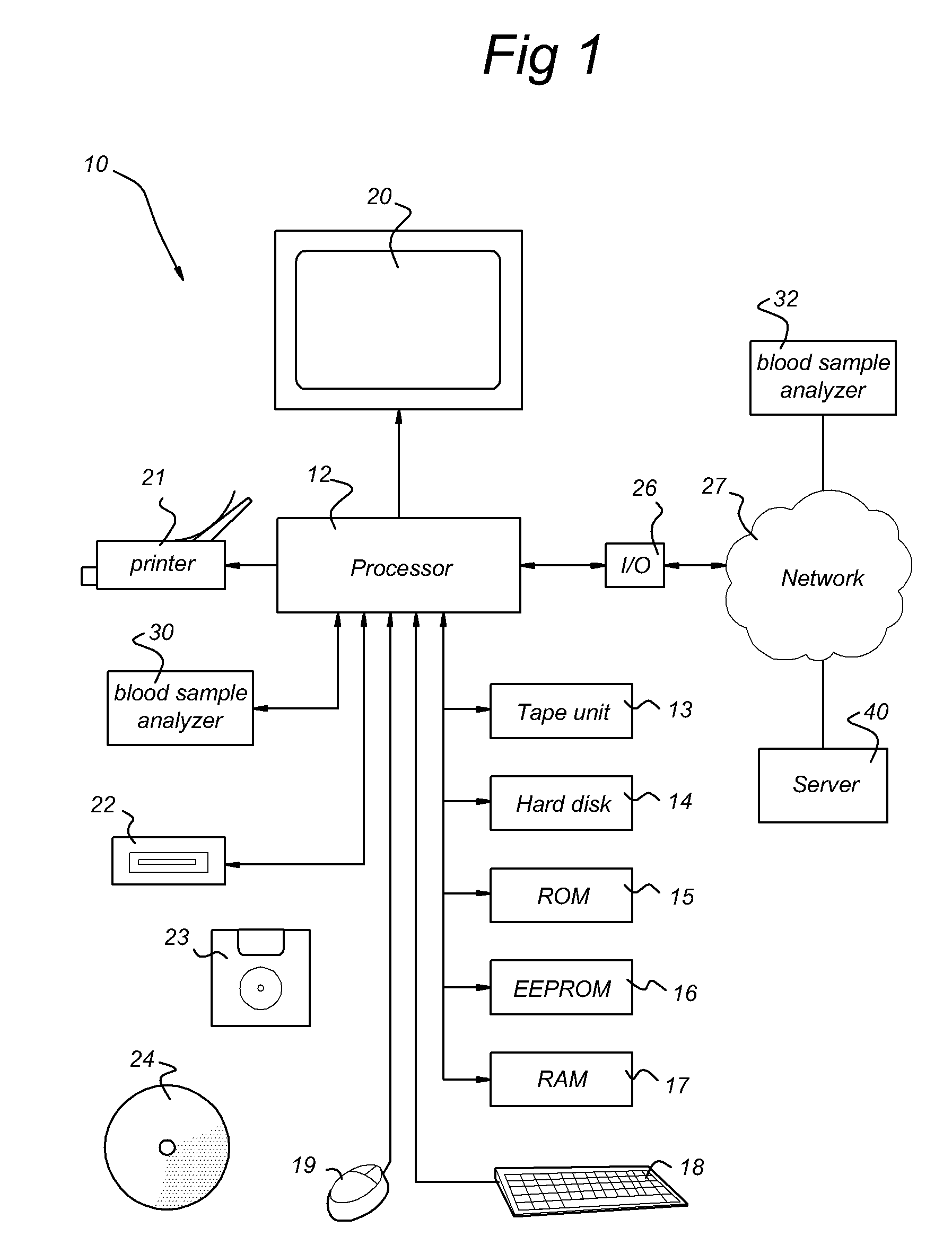
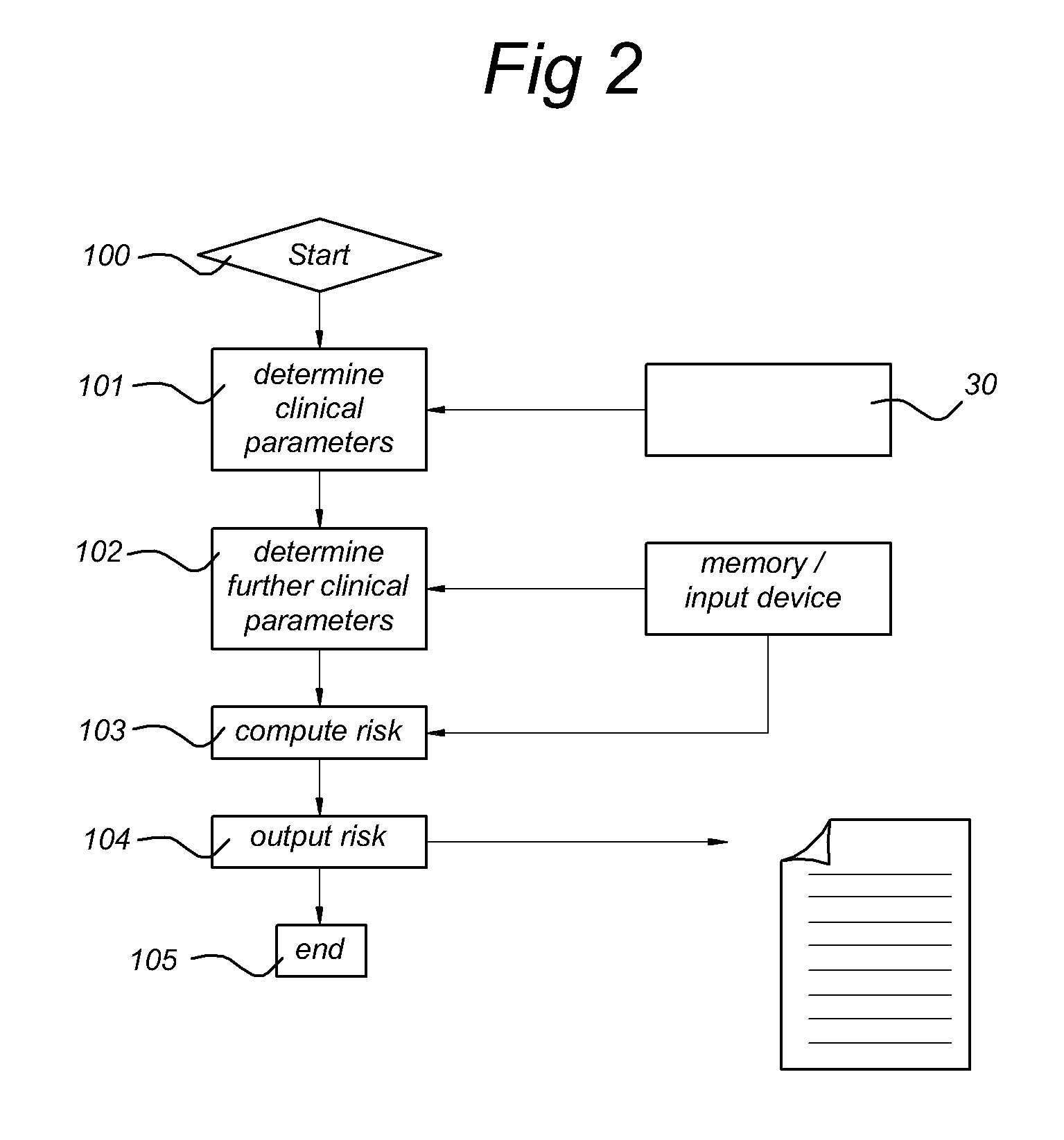
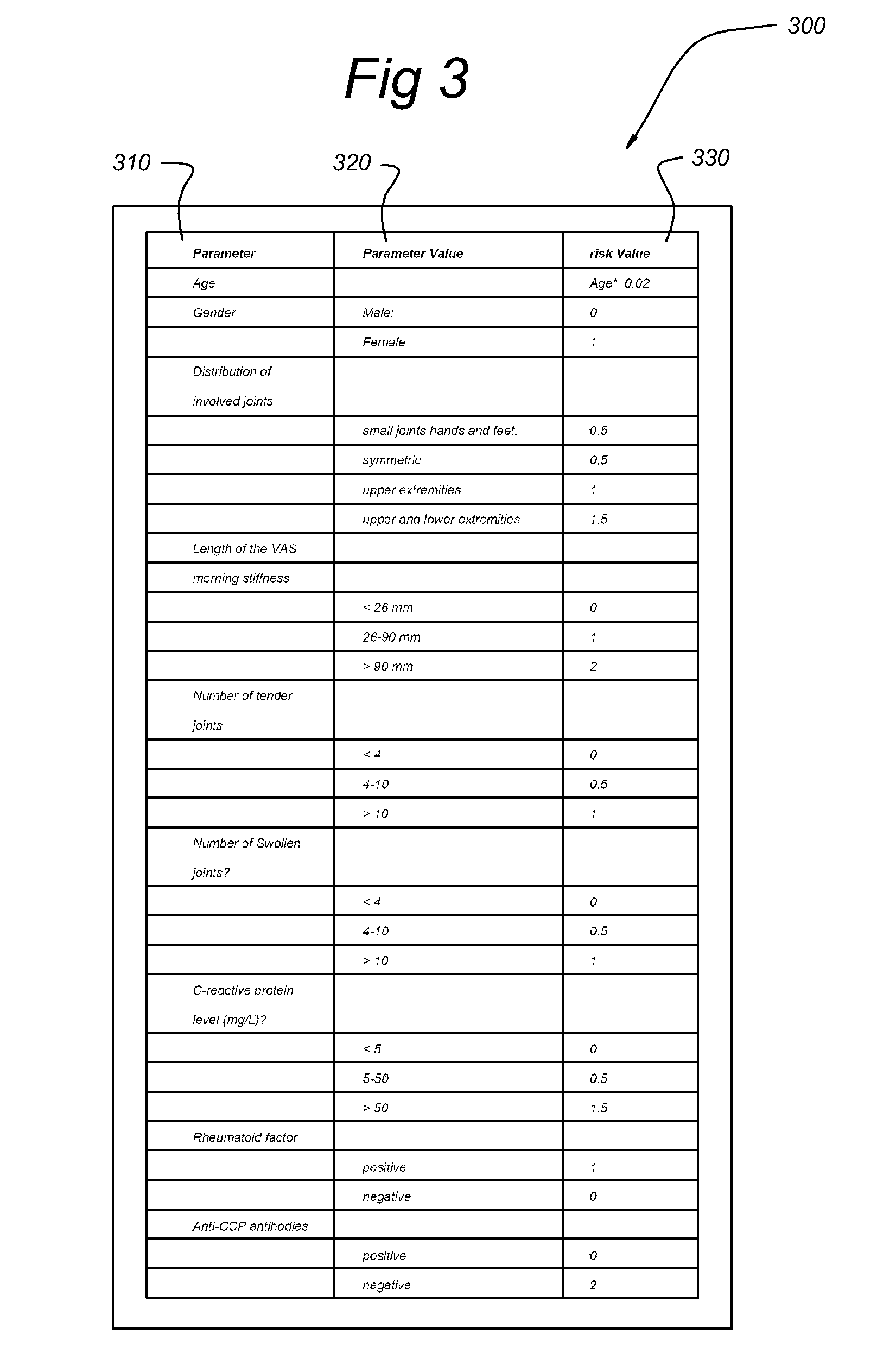
Systems and methods for predicting an individual's risk of developing rheumatoid arthritis
Methods for predicting the likelihood of development of rheumatoid arthritis for individuals that present with recent-onset undifferentiated arthritis. The methods are based on the determination of a set of clinical parameter values and determining a predicted risk for developing rheumatoid arthritis by correlating the parameter values with predefined risk values associated with ranges of parameter values. Parameters values that are decisive for the risk for developing rheumatoid arthritis may include serum levels of C-reactive protein, Rheumatoid factors, anti-CCP antibodies, as well as age, gender, localization of the joint complaints, length of morning stiffness, and number of tender and / or swollen joints. The method may be performed by a computer. The invention further relates to a computer, a sample analyser and a computer program product for performing the method and a data carrier with the computer program product.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Nutritional preparation comprising ribose and folic acid and medical use thereof
Trauma, surgery, inflammation, subfertility, lactation problems, gut disorders, infant nutrition, cancer, arthritis and other joint problems, vascular problems and cardio-or cerebro vascular problems, ischaemia, aging, impaired immune function, burns, sepsis, malnutrition, problems with liver or kidneys, malaria, cystic fibrosis, migraine, neurological problems, respiratory infections, improvement of sports results, muscle soreness, drug intoxication and pain can be treated with a nutritional composition containing effective amounts of ribose and folic acid, optionally combined with other components such as niacin, histidine, glutamine, orotate, vitamin B6 and other components.
Owner:NV NUTRICIA
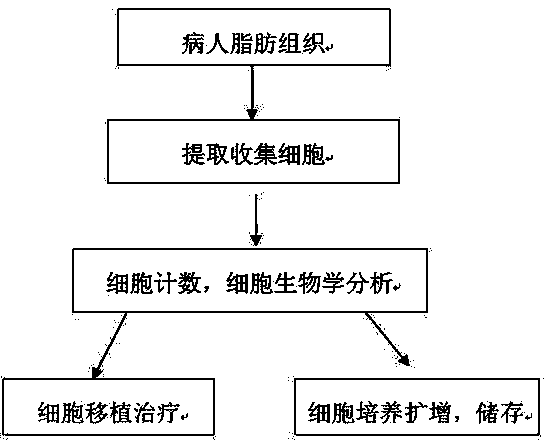
Application of autologous adipose-derived mesenchymal stem cells in preparation of medicines for treating joint diseases
InactiveCN103550256AGood differentiation potentialInhibit inflammationAntipyreticAnalgesicsArticular cavityJoint disease
The invention relates to an application of autologous adipose-derived mesenchymal stem cells in preparation of medicines for treating joint diseases. The application is characterized in that adipose-derived mesenchymal stem cells (Ad-MSCs) separated and extracted from autologous adipose tissues of the patient are injected to the diseased articular cavity in a large dose once. The adopted mesenchymal stem cells have immunophenotyping and representing biological marks of Ad-MSC through flow cytometry authentication, and can be directionally induced and differentiated to osteoblast and chondrocyte. Primary cells are directly injected to the articular cavity of the diseased joint to treat various joint diseases. With the adoption of the autologous adipose-derived mesenchymal stem cells as the primary cells, the cells have better differentiative potential and the ability of inhibiting inflammation and immunoregulation ability. The adipose-derived mesenchymal stem cell transplant provides a novel effective means for treating osteoarticular diseases. The method is simple and feasible, and the quantity of cells collected is great and the trauma is small. The autologous mesenchymal stem cells of the patient are applied without invading related ethics, so that the risk of immunological rejection, infectious diseases and the like is avoided.
Owner:南京优而生物科技发展有限公司
Extended release pharmaceutical formulations of s-adenosylmethionine
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. majorclinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Compositions for use in cartilage breakdown
InactiveUS20160120891A1Improving joint healthPreventing and treating cartilage breakdownBiocideHydroxy compound active ingredientsHydroxytyrosolVeterinary medicine
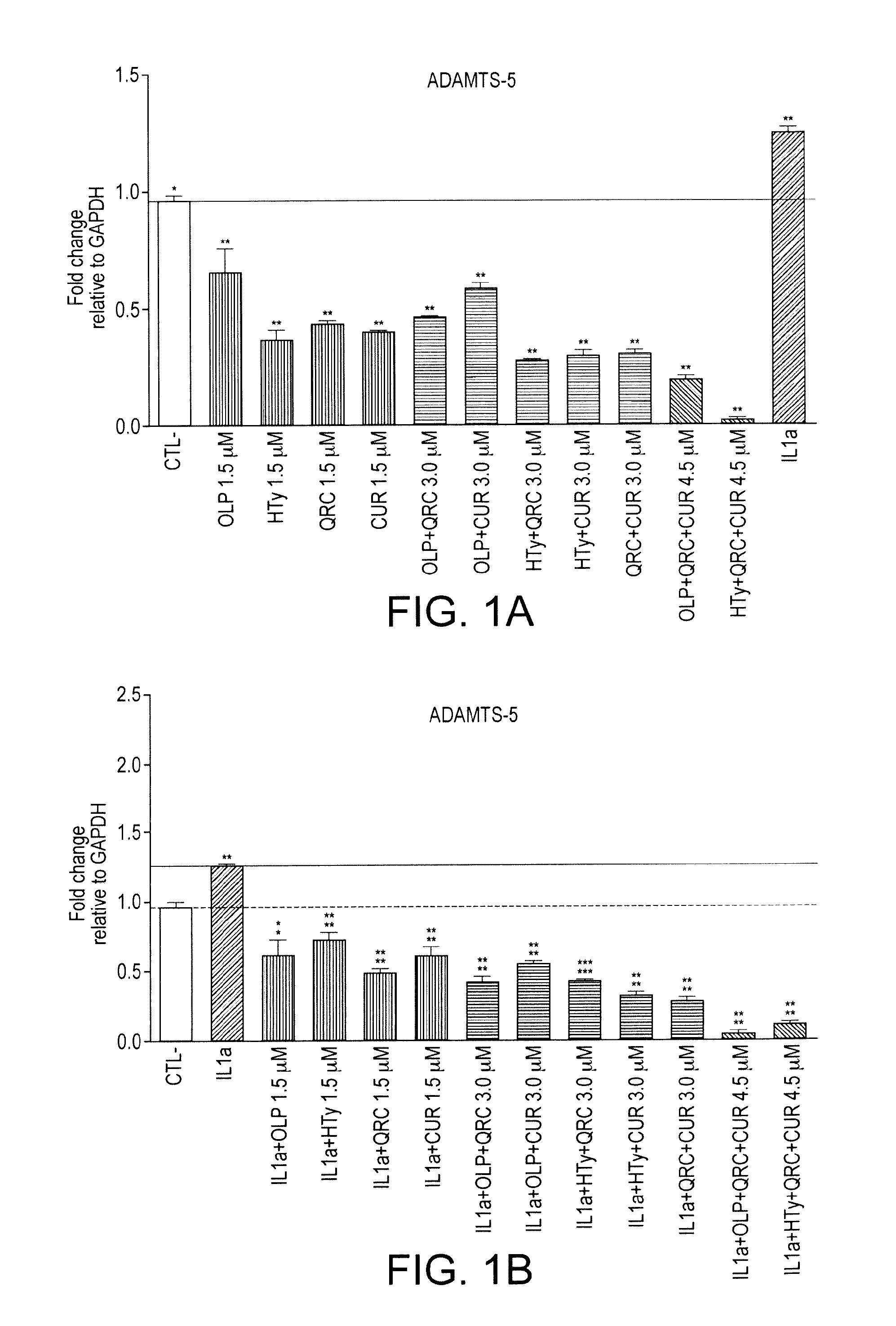
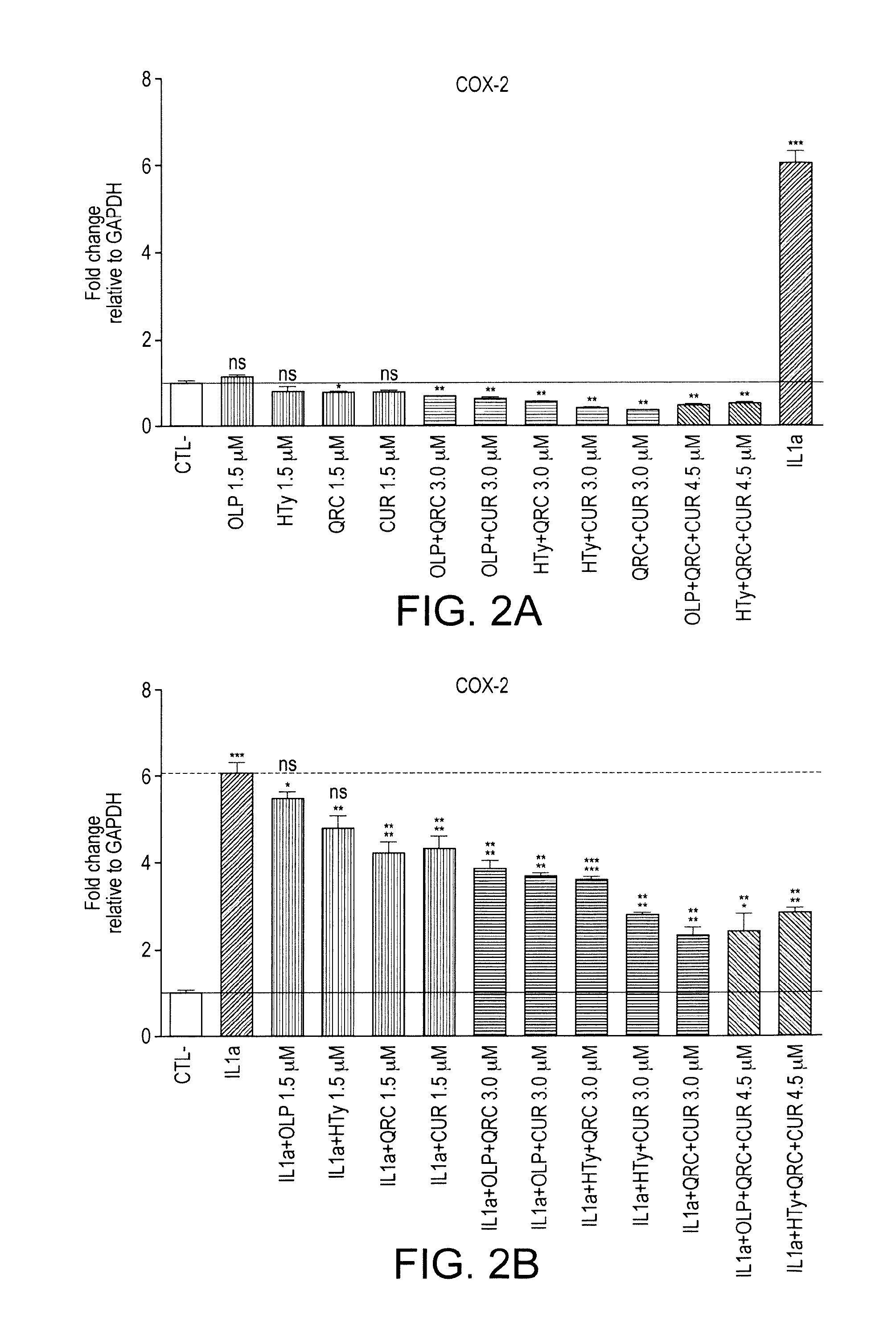
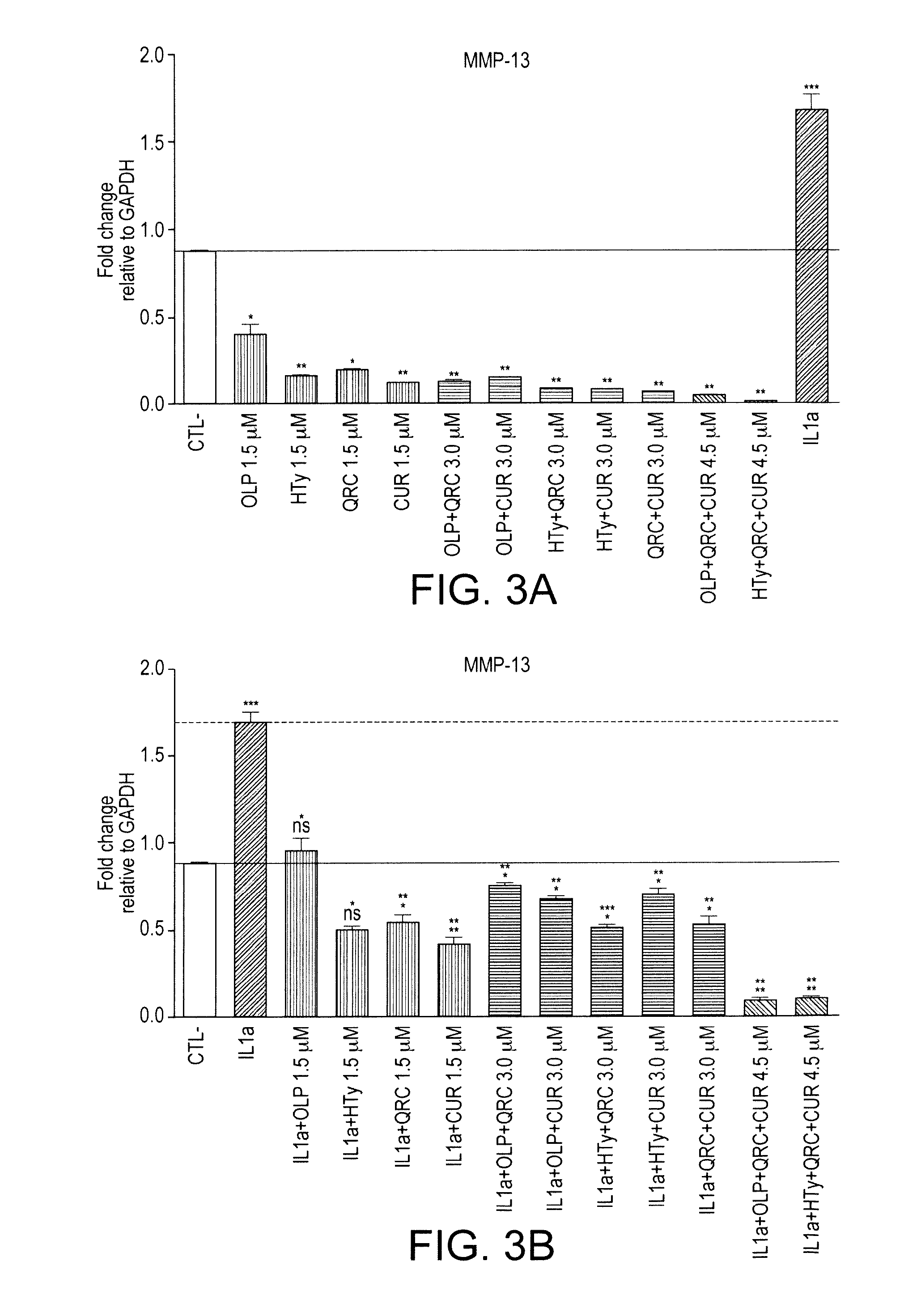
The present invention relates to use of a composition comprising oleuropein and / or hydroxytyrosol for maintenance joint health or prevention or treatment of joint disorders. In particular, the invention relates to oleuropein for use to prevent or treat cartilage breakdown.
Owner:SOC DES PROD NESTLE SA
Healthcare agent for pet joints and preparation method thereof
InactiveCN106620657APromote recoveryRelieve inflammationPeptide/protein ingredientsSkeletal disorderArthritisFeed additive
The invention discloses a healthcare agent for pet joints and belongs to the technical field of feed additives. The healthcare agent uses chondroitin sulfate, glucosamine hydrochloride, dimethyl sulfone and collagen as the core components and vitamins and mineral elements as the auxiliary materials and is applicable to pets with arthritis, caused by excessively fast growth and development or maldevelopment, movement damage, senile joint degeneration and the like, of different degrees. The healthcare agent can promote bone health, increase pet immunity, improve pet bone joint diseases and increase pet living quality.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method for preparing low molecular weighted glucidamin collagen composition, its product and use
InactiveCN1875988AGood knee arthritisCervical spondylosis is goodOrganic active ingredientsCosmetic preparationsDiseaseUltrafiltration
The invention provides a process for preparing low molecular weight glycosaminoglycan collagen compound comprising the following steps, choosing material and cleaning, disintegrating, homogenating, cracking proteolytic enzyme through catalytic decomposition, sterilizing and disinfecting, filtering for entrapment, concentrating, drying and packaging. The obtained compound can be used for treating joint diseases such as cervical vertebra disease and gonarthritis.
Owner:周立军
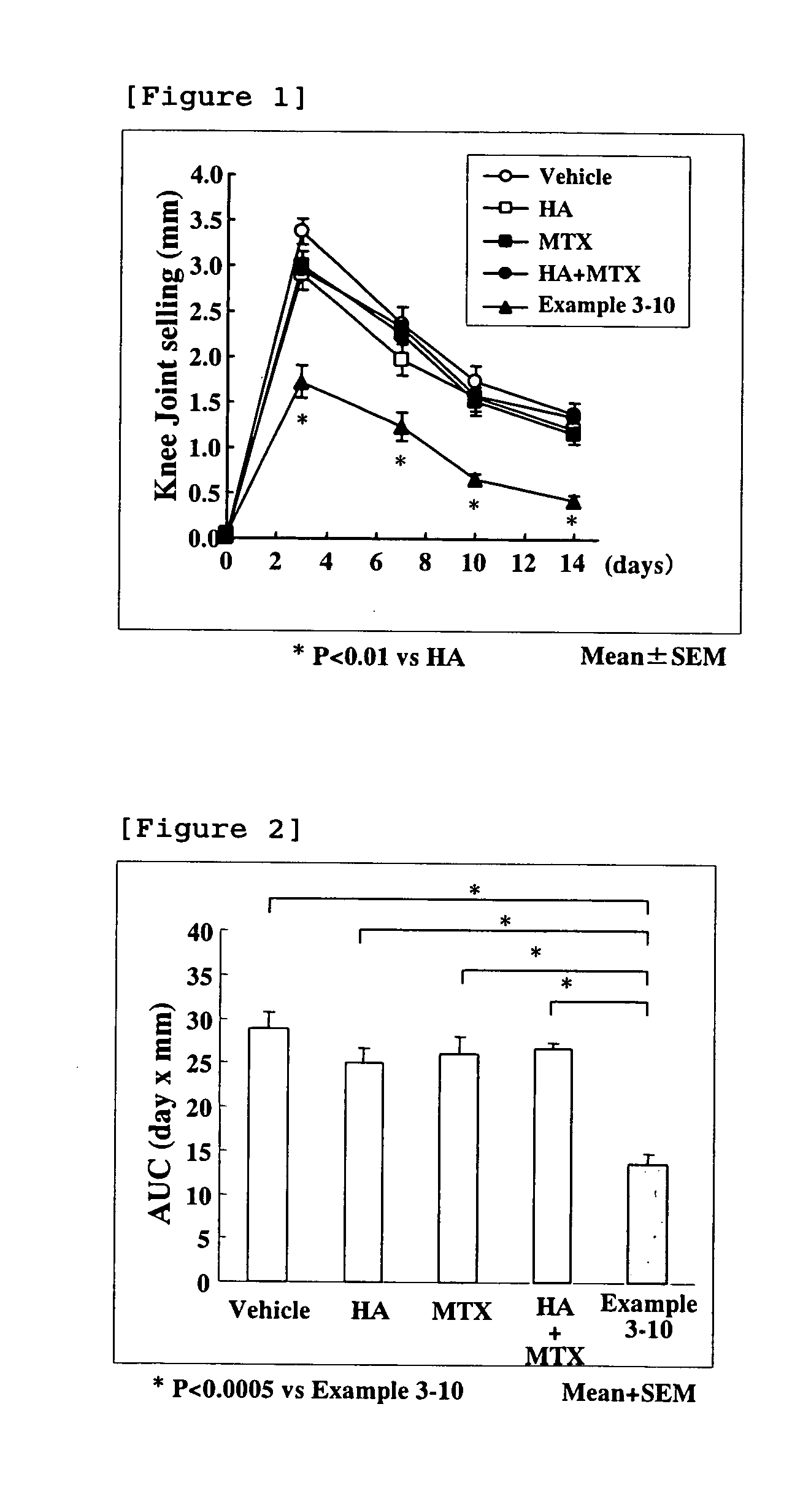
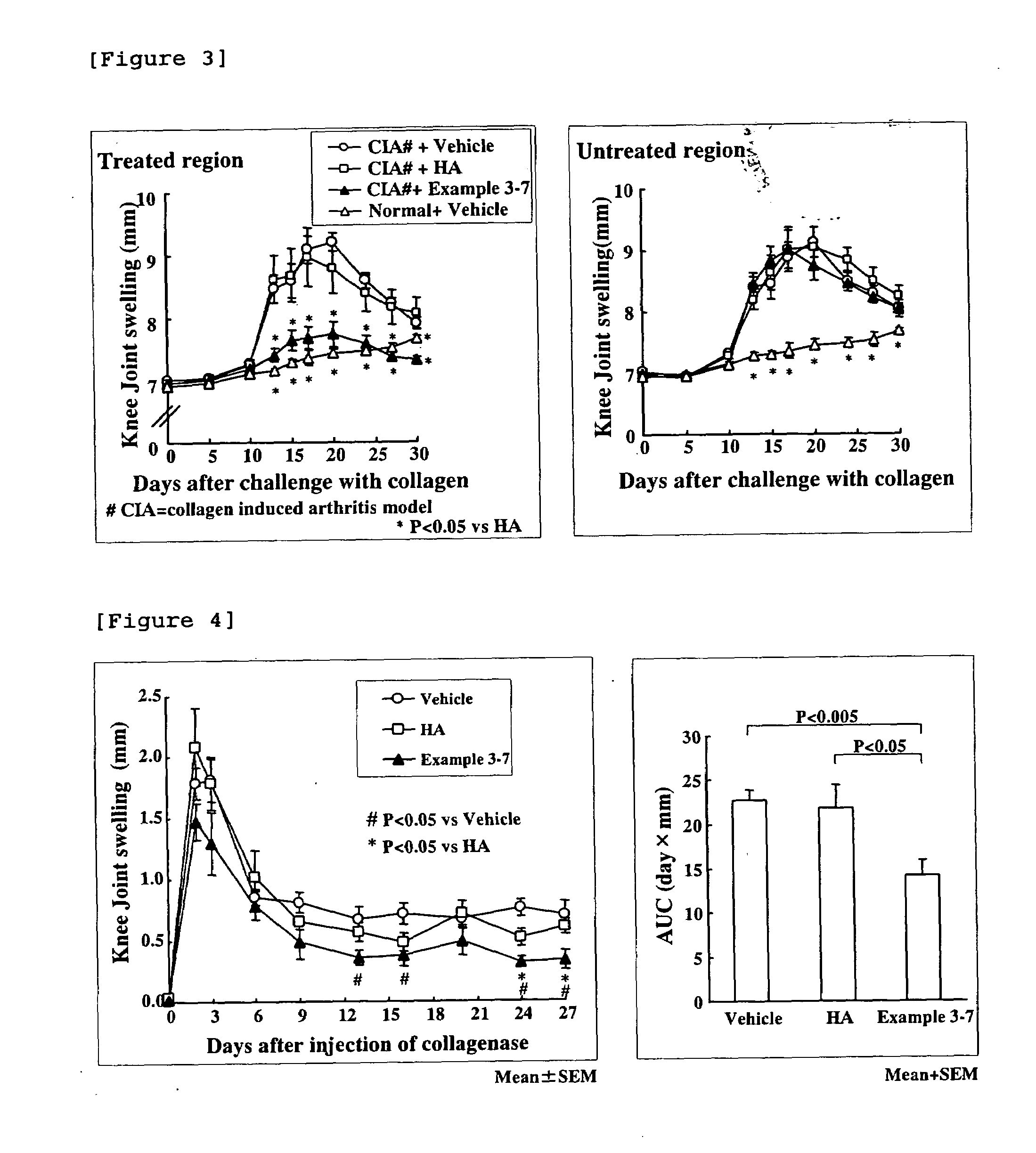
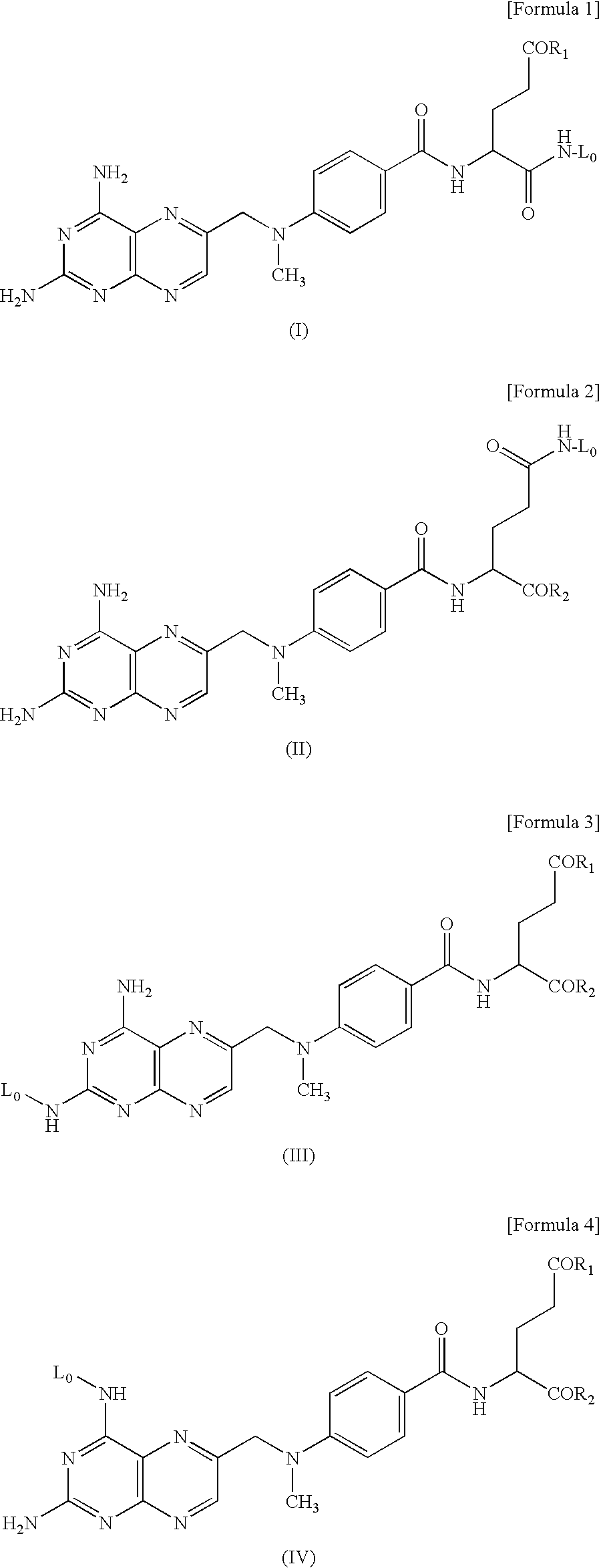
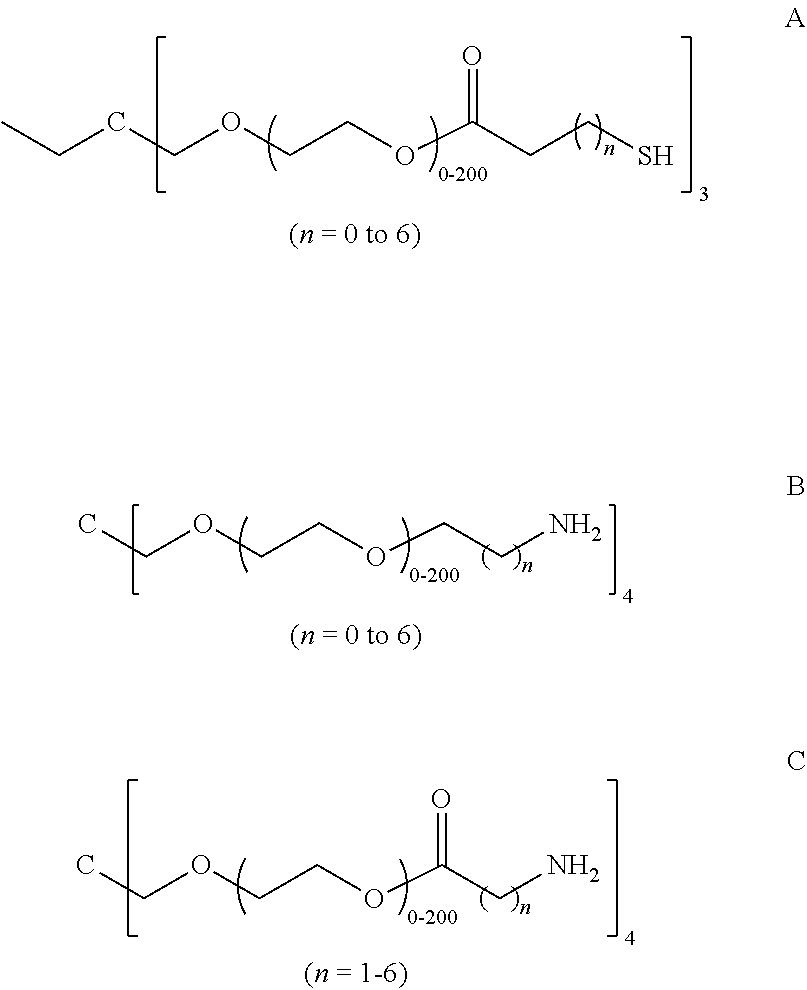
Hyaluronic Acid-Methotrexate Conjugate
An object of the present invention is to provide a hyaluronic acid-methotrexate conjugate useful as a therapeutic drug for joint disorders. There is provided a hyaluronic acid-methotrexate conjugate useful for the treatment of joint disorders, wherein methotrexate is conjugated with a hydroxy group of hyaluronic acid through a linker containing a peptide chain consisting of 1 to 8 amino acids, and the linker is conjugated with the hyaluronic acid through a carbamate group.
Owner:DENKA CO LTD
Solid polyglycol-based biocompatible pre-formulation
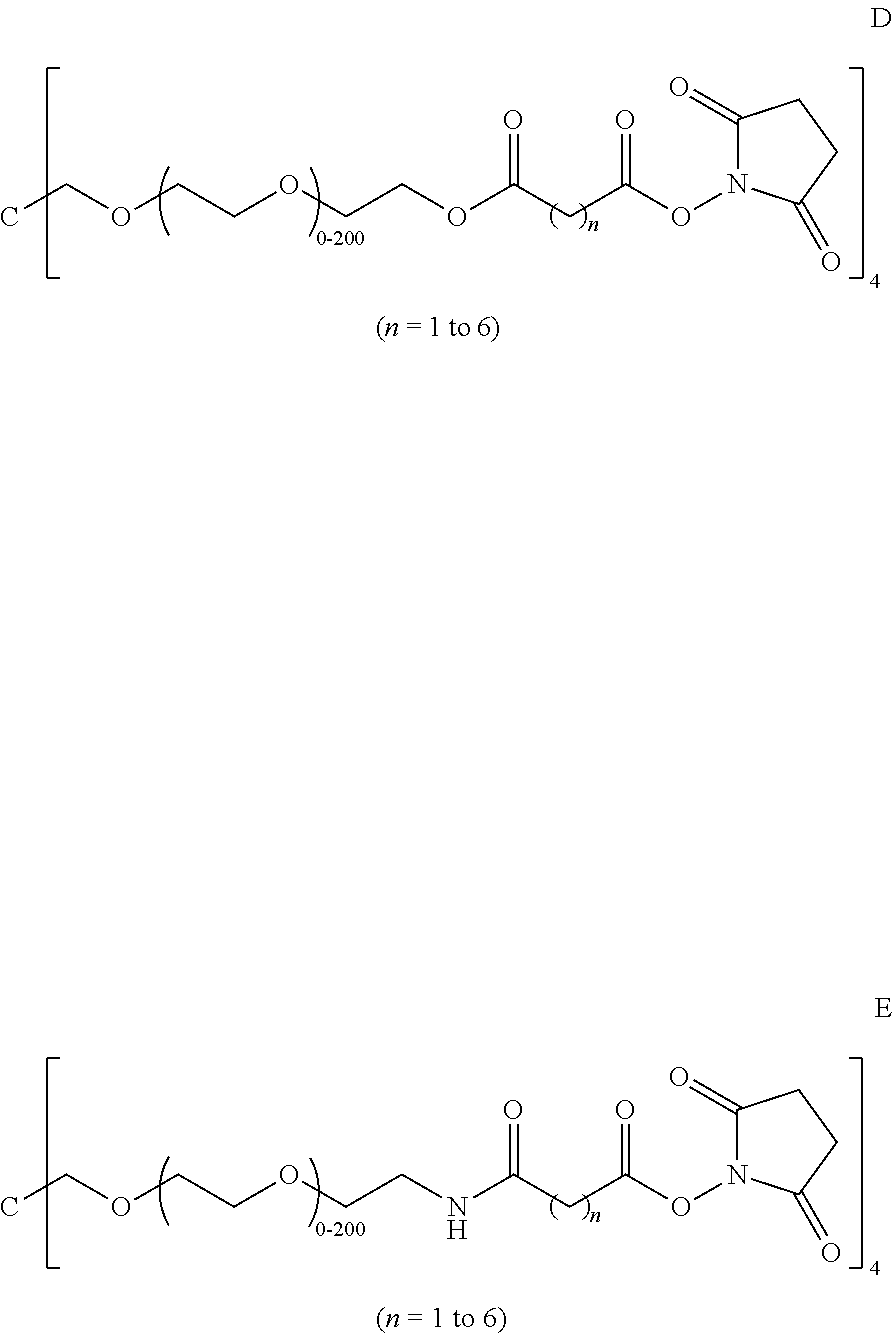
ActiveUS20140271528A1Facilitate cross-linkingHeavy metal active ingredientsBiocideMedicineCompound (substance)
Provided herein are pre-formulations forming a biocompatible hydrogel polymer comprising at least one nucleophilic compound or monomer unit, at least one electrophilic compound or monomer unit, and optionally a therapeutic agent and / or viscosity enhancer. In some embodiments, the biocompatible hydrogel polymer covers a wound in a mammal and adheres to the surrounding skin tissue. In other embodiments, the hydrogel polymer is delivered into a joint space to treat joint disease or navicular disease.
Owner:C P MEDICAL CORP
S-adenosylmethionine formulations with enhanced bioavailability
InactiveCN102695514AGood obedienceImprove side effect performanceOrganic active ingredientsNervous disorderImmunologic disordersS-Adenosyl-l-methionine
The invention relates to compositions and methods to enhance the absorption of S- adenosylmethionine (SAMe) and to methods of treating various disorders or diseases using non-parenteral SAMe formulations with enhanced-absorption and improved bioavailability. The enhanced bioavailability formulations may be used to treat a variety of diseases or disorders, such as for example, psychiatric disorders including, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, depressive disorders (e.g. major clinical depression) and dysthymia; as well as treating liver disorders, cancer, autoimmune disorders, inflammatory disorders, joint disorders, gastrointestinal disorders and cardiovascular disease.
Owner:MSI METHYLATION SCI
Composition and methods for the treatment of joint pain using Angelica gigas Nakai extract and powder as combined with Glucosamine Sulfate, or Chondroitin Sulfate and HCL, or MSM, or aspirin, or Celedrin, and as combinations thereof in powder, pill, capsule, spray, liquid, and gelcap form
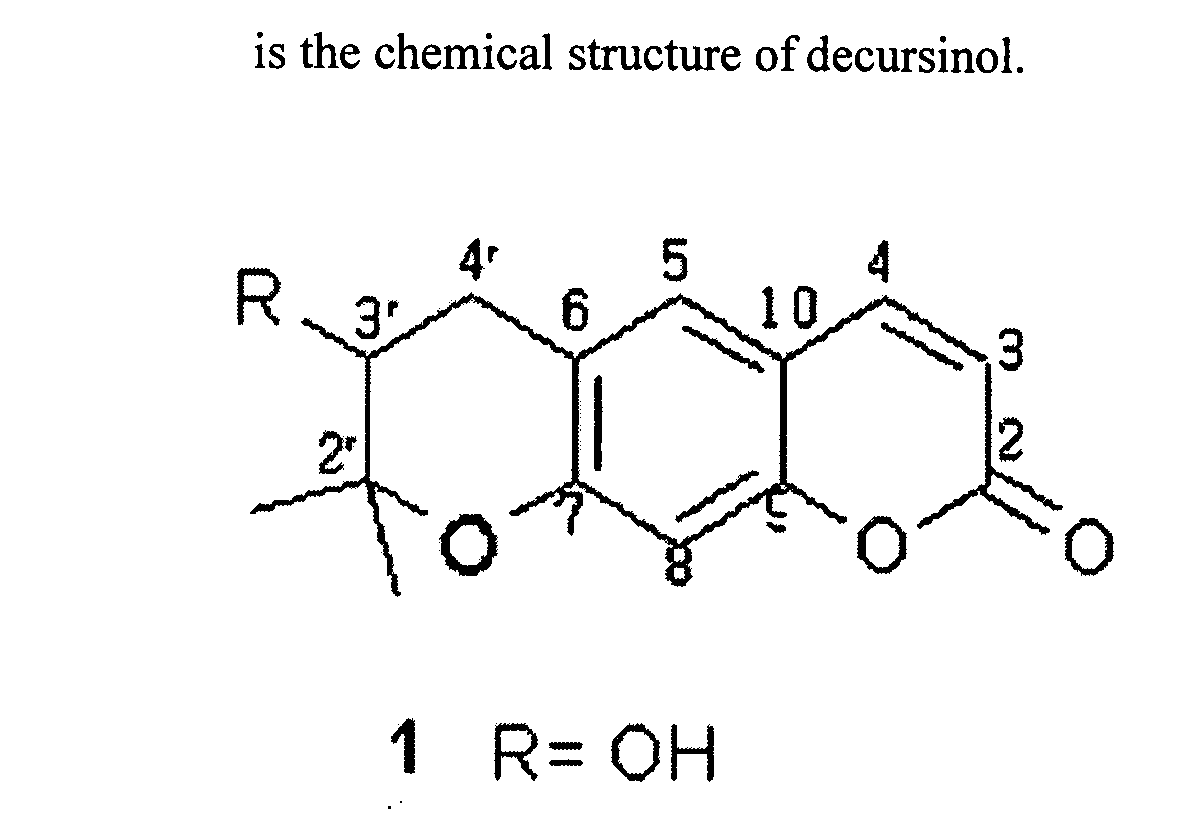
The present invention relates to composition comprising dry powder of decursinol, decursin, and their related derivates isolated from the roots of Angelica gigas Nakai (Korean Angelica) plant and at least one of joint improvement agents such as glucosamine, chondroitin, or methylsulfonylmethane (MSM). The present invention is directed to methods, combinations, and compositions for treating, preventing or reducing the joint pain or the symptoms associated with, or related to, a joint disorder or disease in a subject in need thereof.
Owner:JEFFERS MICHAEL GREGORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com