Extended release pharmaceutical formulations of s-adenosylmethionine
A technology of delayed release and preparation, which is applied in the directions of drug combination, animal repellent, plant growth regulator, etc., can solve the problems such as the application of delayed release SAMe has not been reported, and the application of delayed release SAMe has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Example 1: Delayed release monolithic matrix tablet
[0215] A formulation containing SAMe, magnesium aluminosilicate, light liquid paraffin and magnesium stearate is prepared by mixing the ingredients and compressing with a semi-automatic tablet machine. During the entire manufacturing process, the humidity is kept below 30% and the temperature is kept at 20-25°C. Table 1-1 below lists the proportions of each component.
[0216] Table 1-1: SAMe formulations containing liquid paraffin
[0217] excipient
[0218] The formulations in Table 1-1 enable the manufacture of SAMe tablets with a total excipient content of less than 30%. The granules used in this formulation have good fluidity and no sticking picking during compression.
Embodiment 2
[0219] Example 2: Slugging method
[0220] To improve the compressibility of the SAMe formulation of Example 1, a granulation step (impact) was employed. SAMe was mixed with liquid paraffin and magnesium aluminosilicate. The resulting powdered mixture was loaded into a V-blender and mixed at 50 RPM for 10 minutes. Half the amount of magnesium stearate (see Table 2-1 below) 2.97 g was added to the V-blender and mixed for another 10 minutes.
[0221] The resulting powder was passed through a 20# sieve. The mixture was compressed into 400-500 mg of small pieces having a hardness of approximately 8-9 kp. The small pieces were then ground, passed through a 30# sieve, and blended with the rest of the magnesium stearate (2.97 g). The resulting mixture was then pressed to a hardness of 12-15 kp.
[0222] Table 2-1: Recipe for making SAMe cores with liquid paraffin
[0223] excipient
mg / tablet
% (wt)
SAMe
800
...
Embodiment 3
[0228] Embodiment 3: coating test
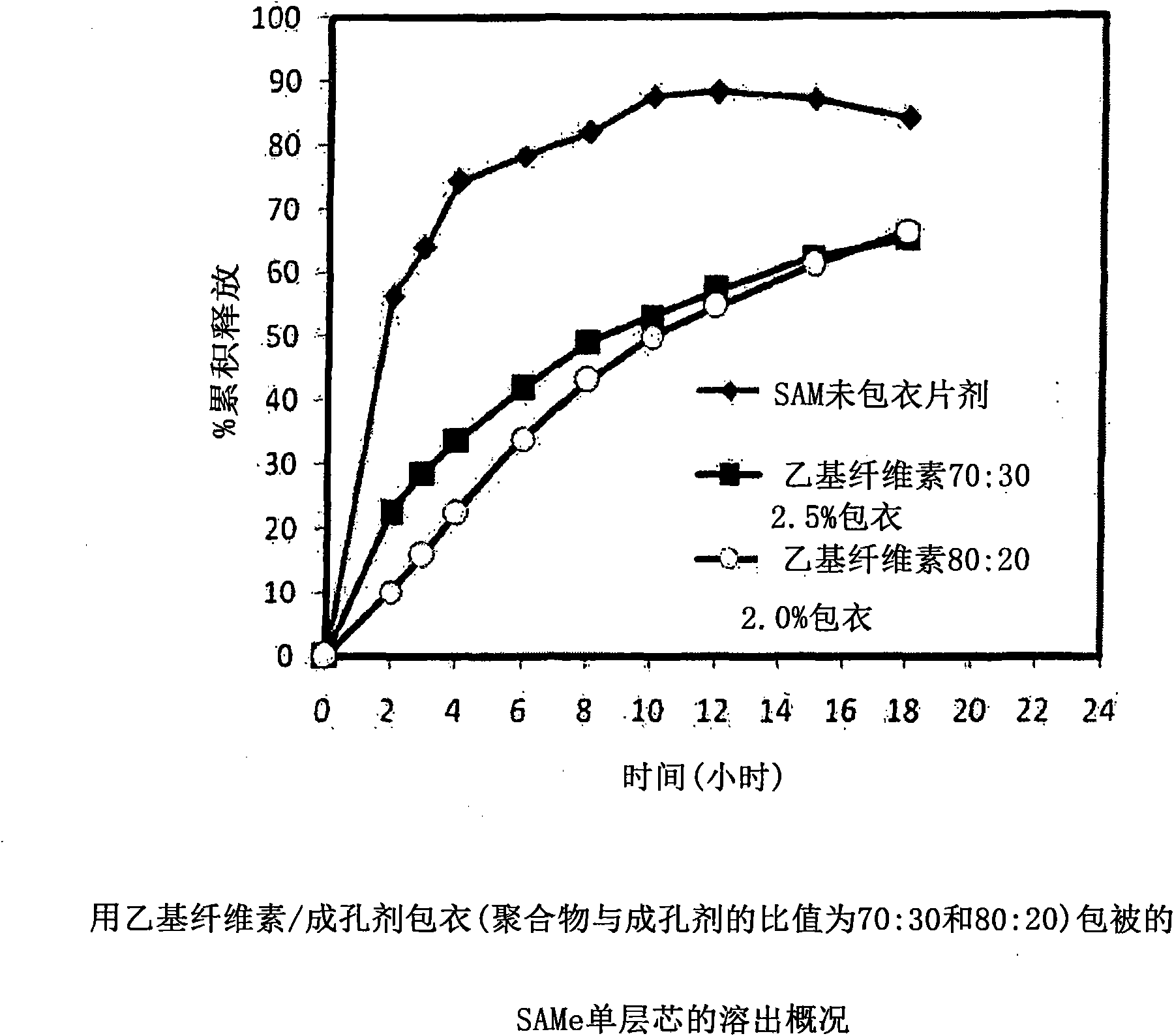
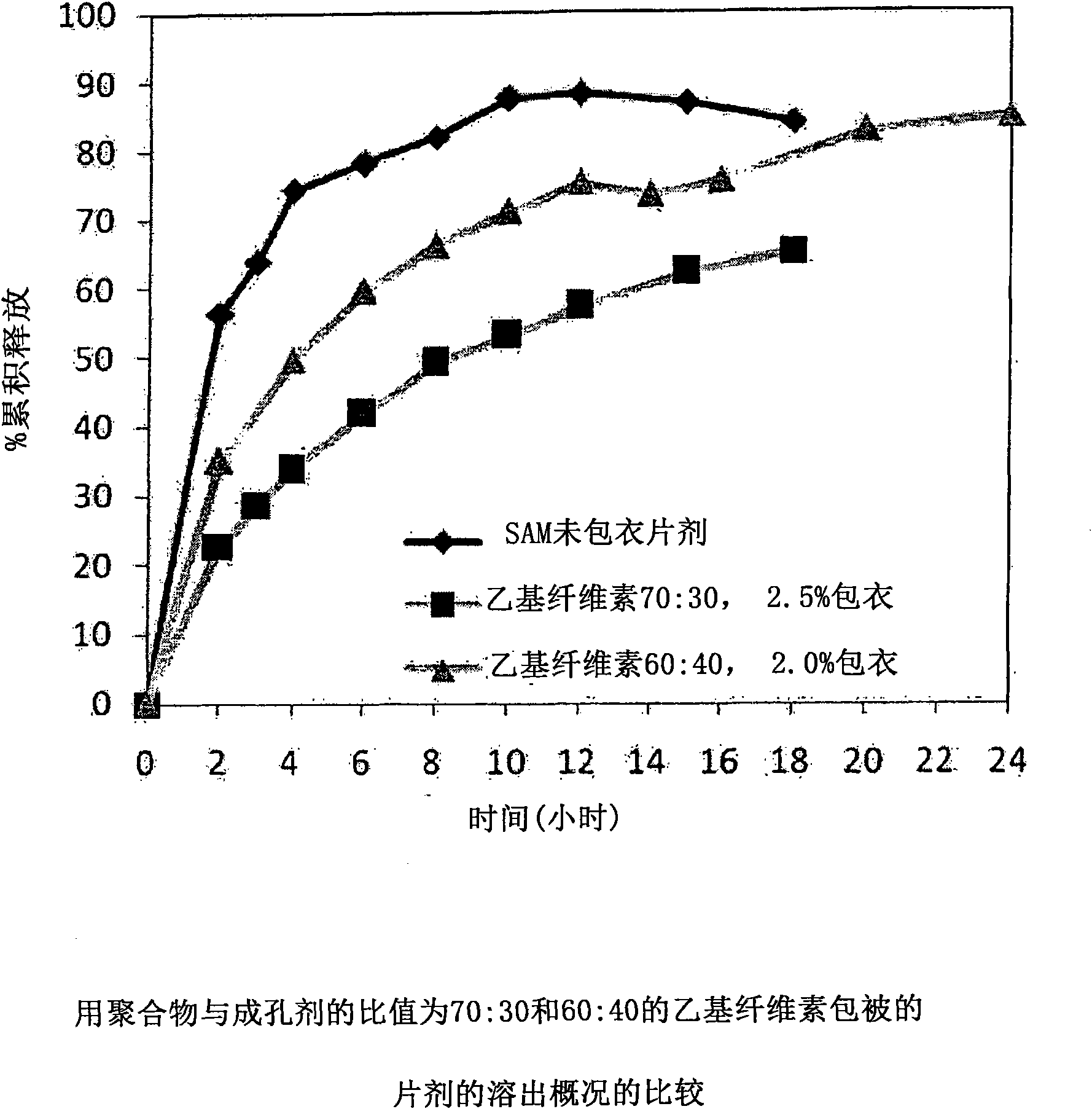
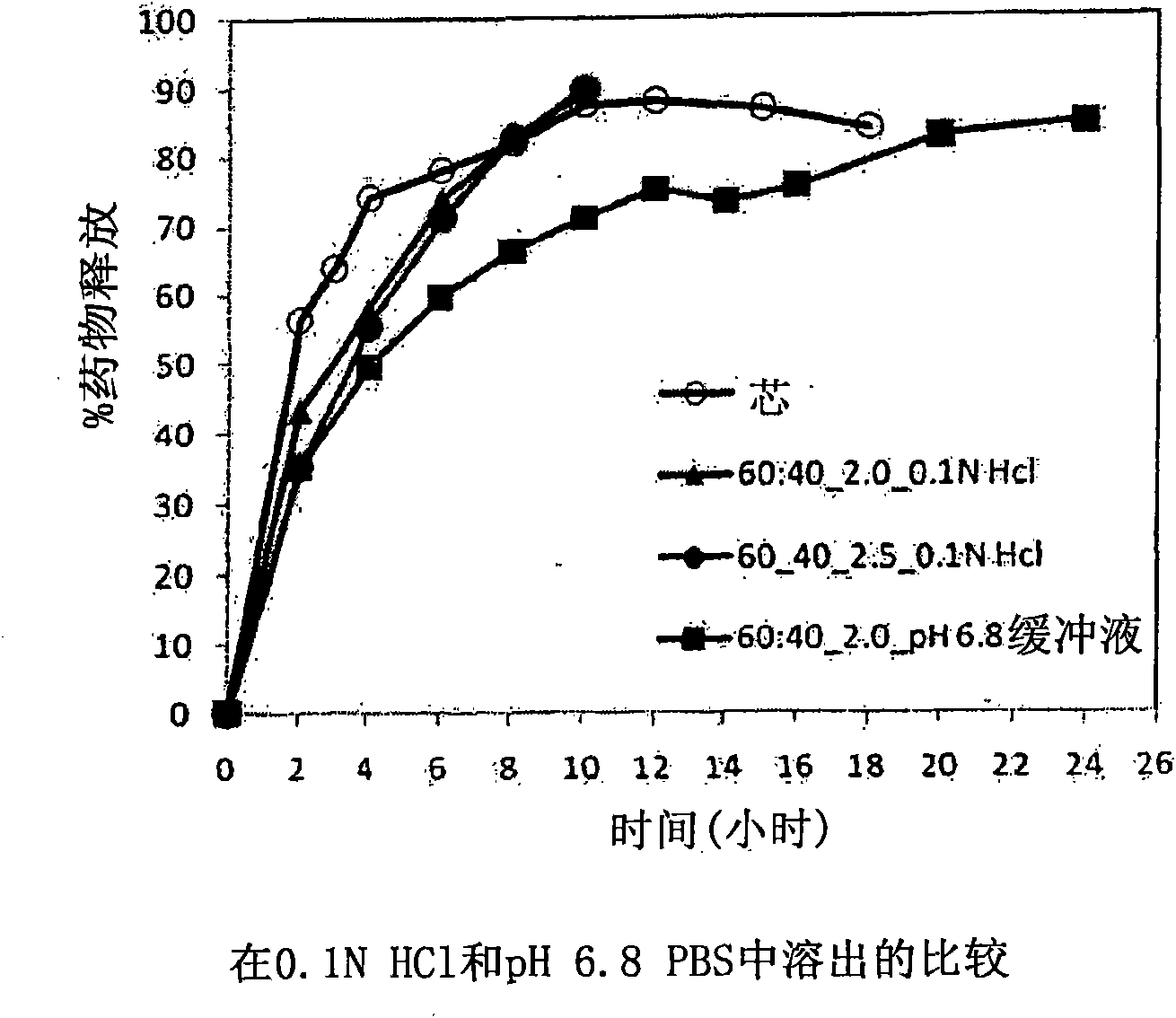
[0229] with different amounts of pore formers ( A combination of pore forming agent, sodium alginate and purified stearic acid) was coated with an ethylcellulose coating of matrix core SAMe tablets as described in Example 2 above. The ethylcellulose portion of the coating is a combination of purified water, Ethyocel 20cP standard premium ethylcellulose and 28% ammonium hydroxide. The coatings were tested at weight ratios of 100:0 (ethylcellulose:pore former), 80:20 and 70:30. Tablets were either uncoated or coated with a 2.5% 70:30 or 80:20 ethylcellulose composition. Dissolution was tested in pH 6.8 PBS buffer. The results are summarized in Table 3-1.
[0230] Table 3-1: Dissolution Results of Uncoated and Coated Tablets at pH 6.8
[0231] time (hours)
uncoated core
With ethylcellulose 70:30*,
2.5%** coated tablet
80:20*, 2.0%** coating
the tablet
2
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com