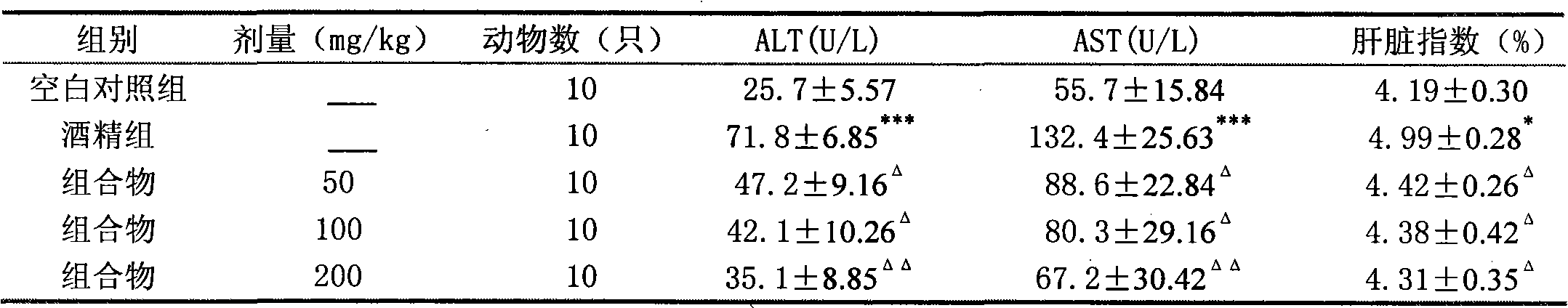
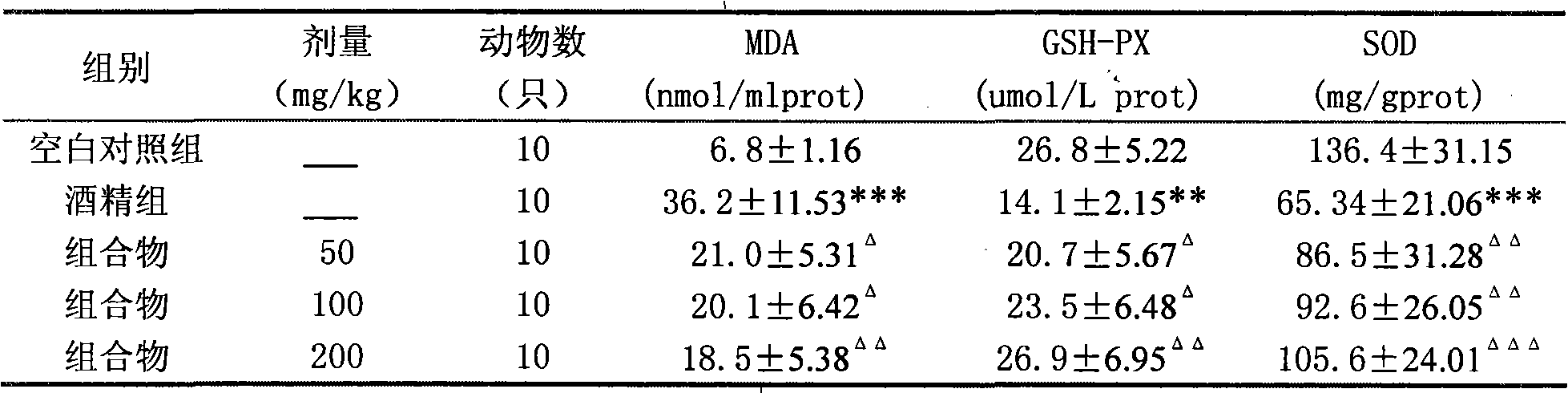
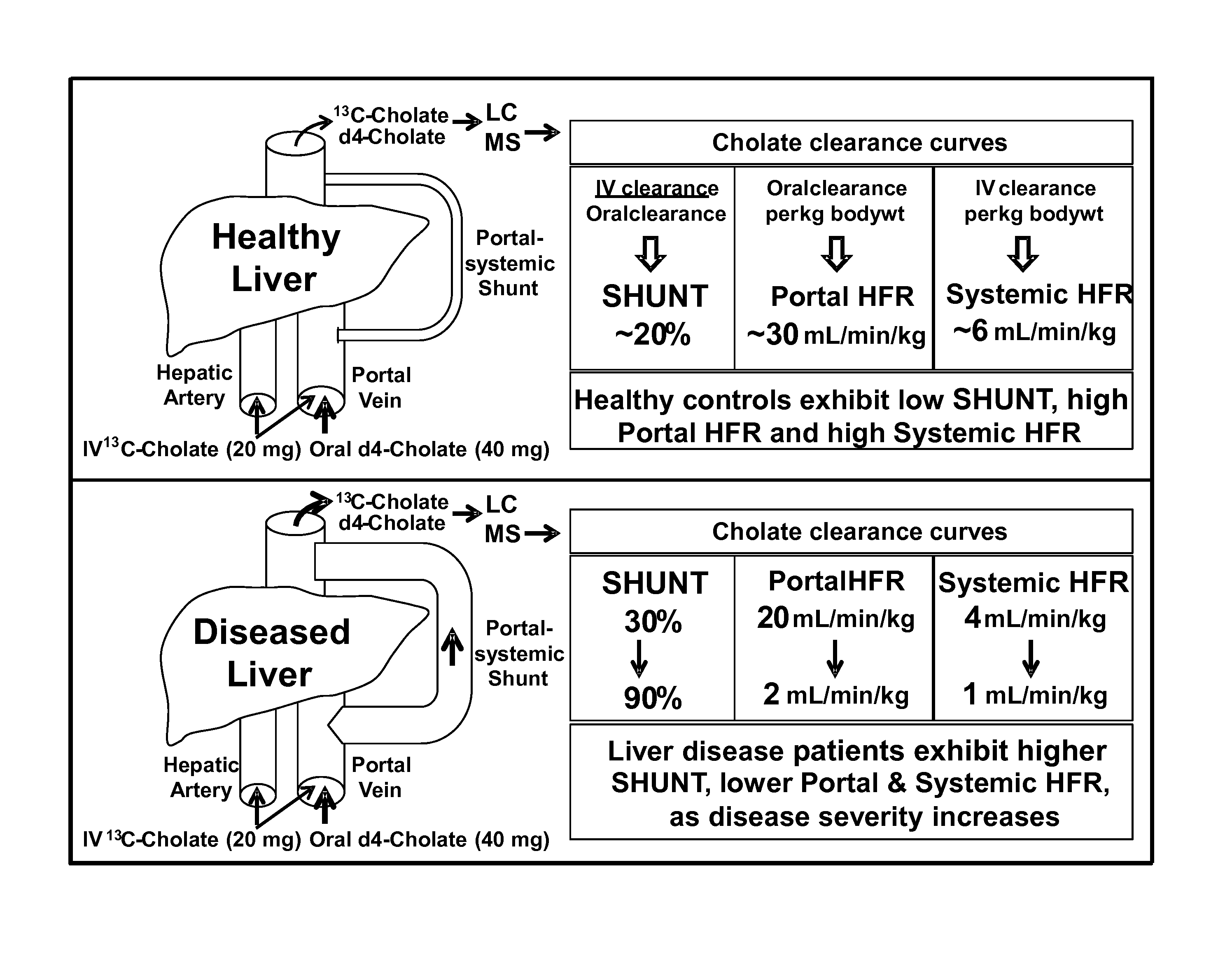
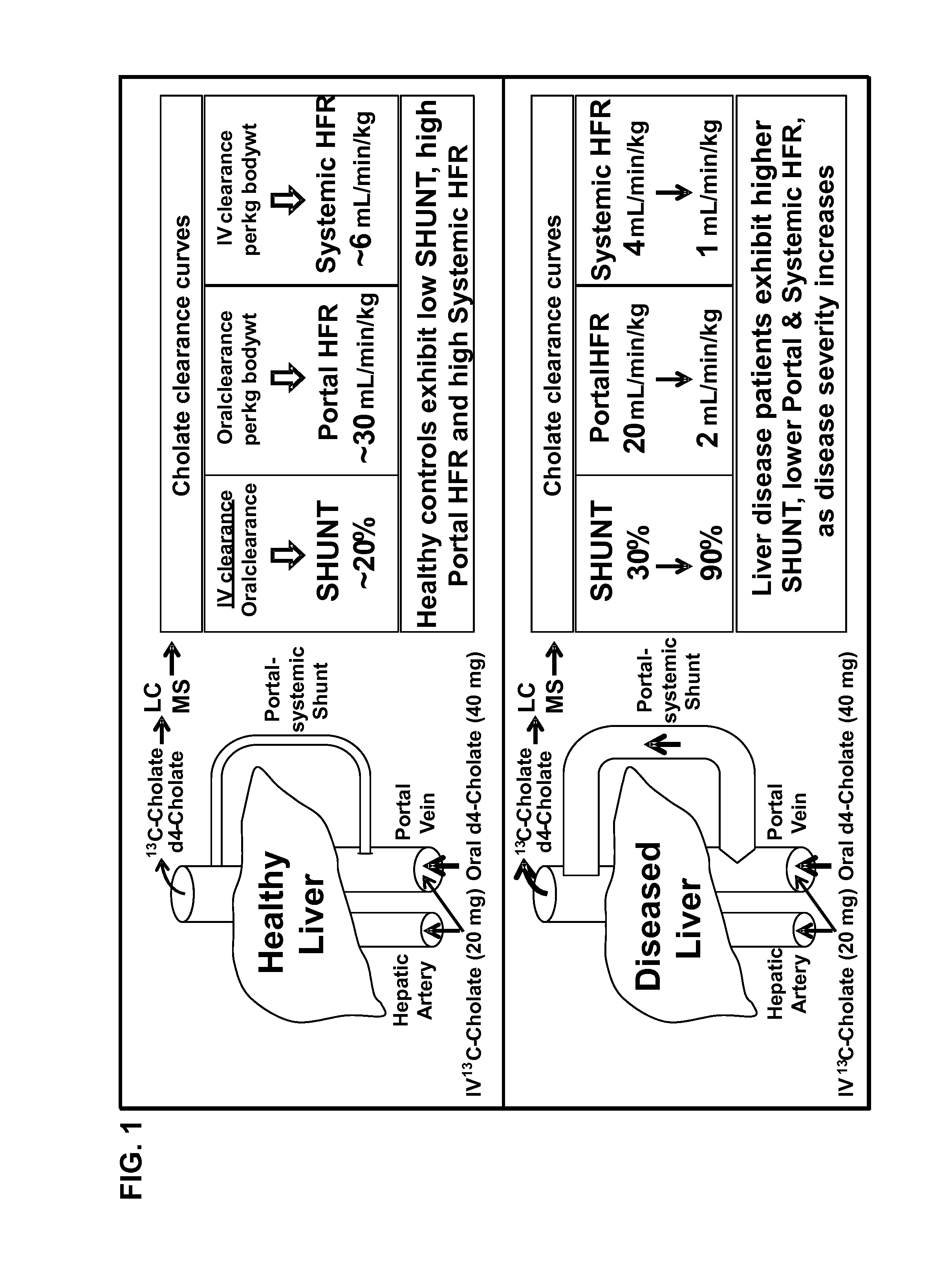
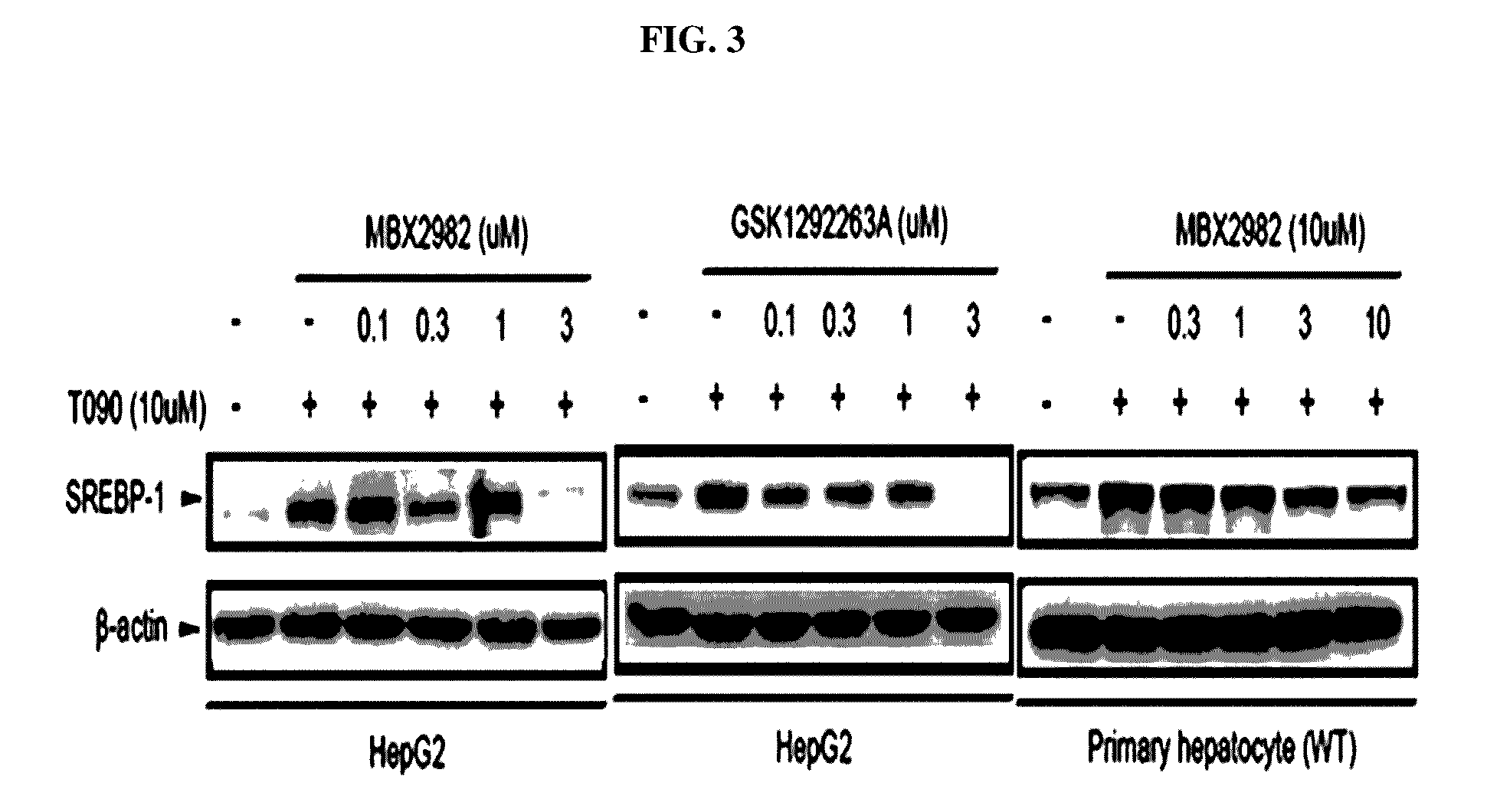
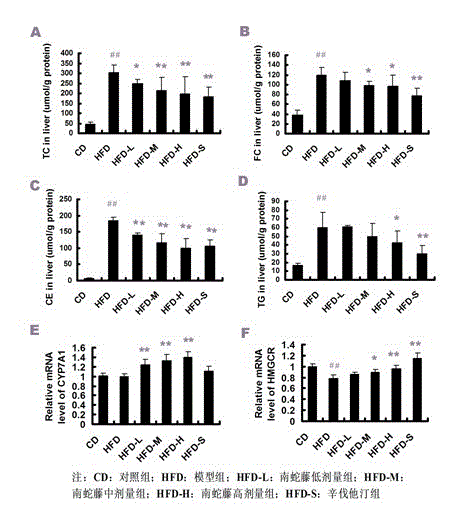
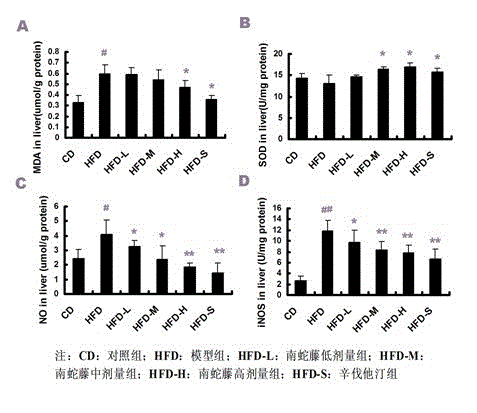
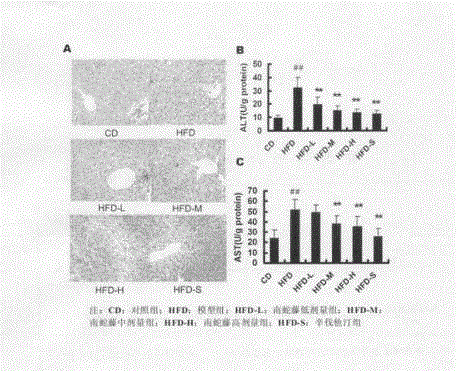
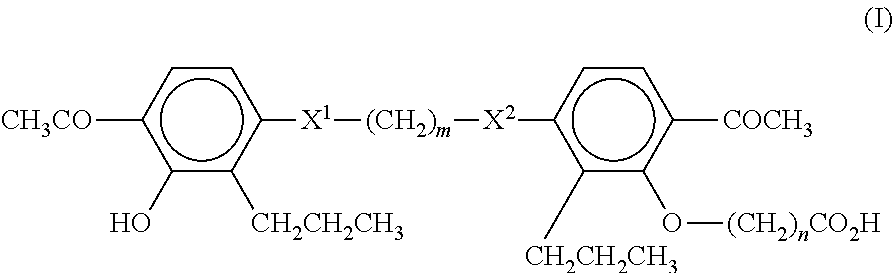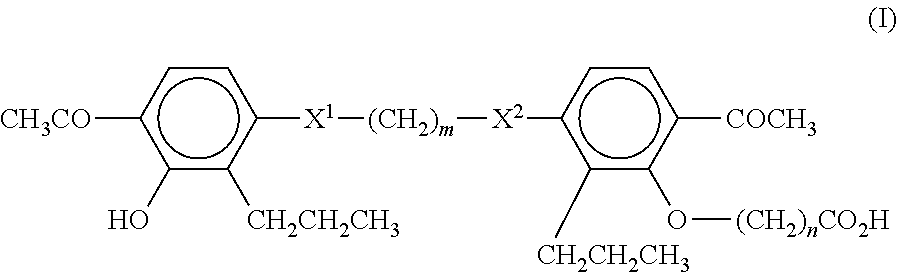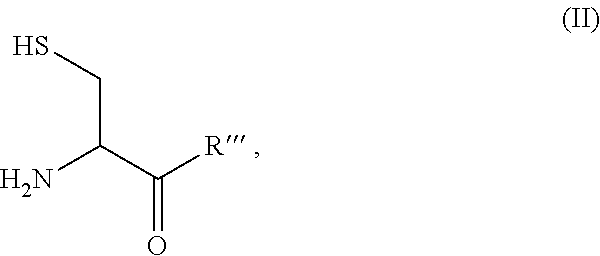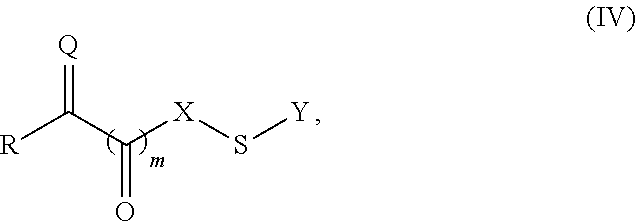Patents
Literature
304 results about "Alcoholic liver disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alcoholic liver disease is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.
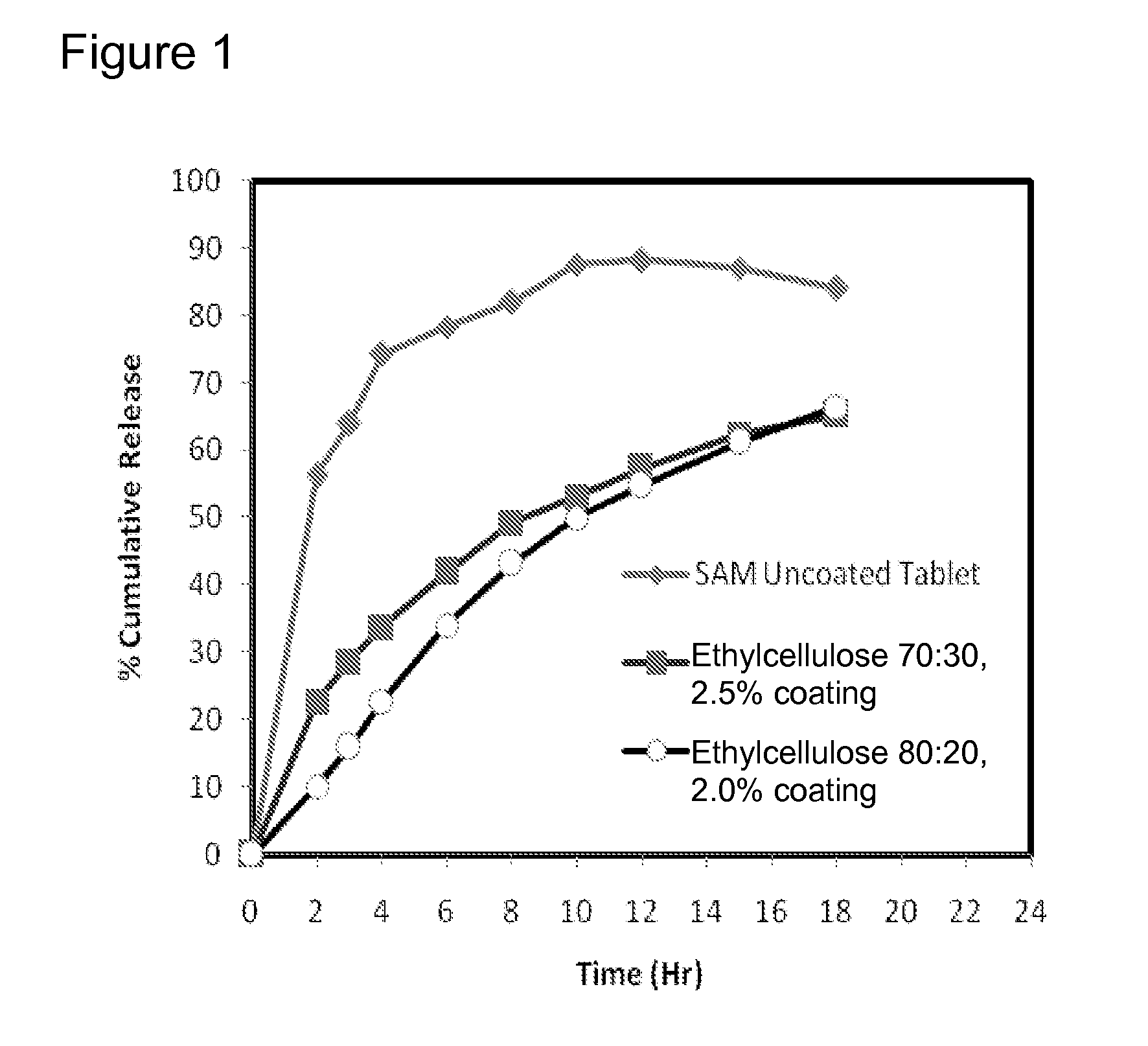
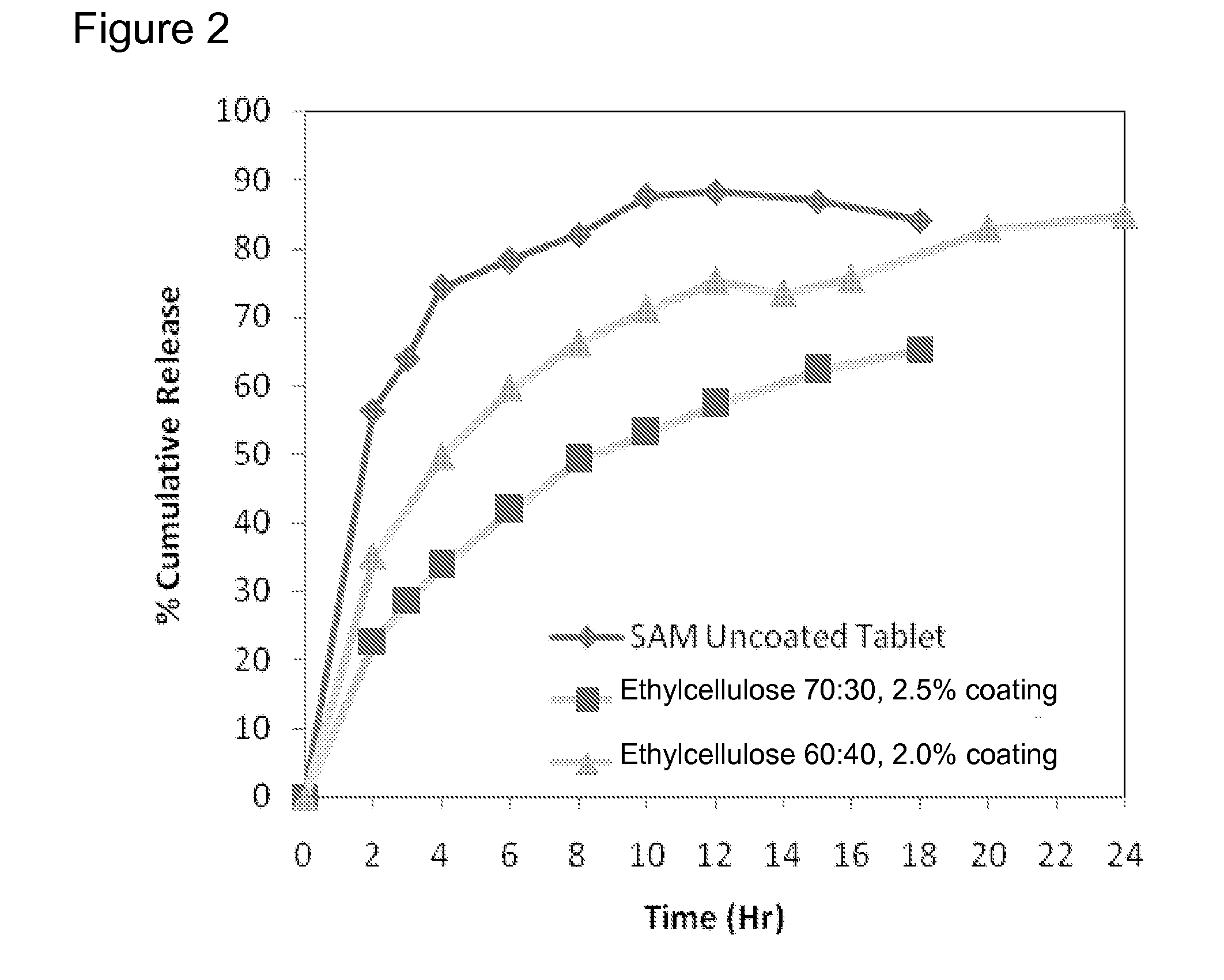
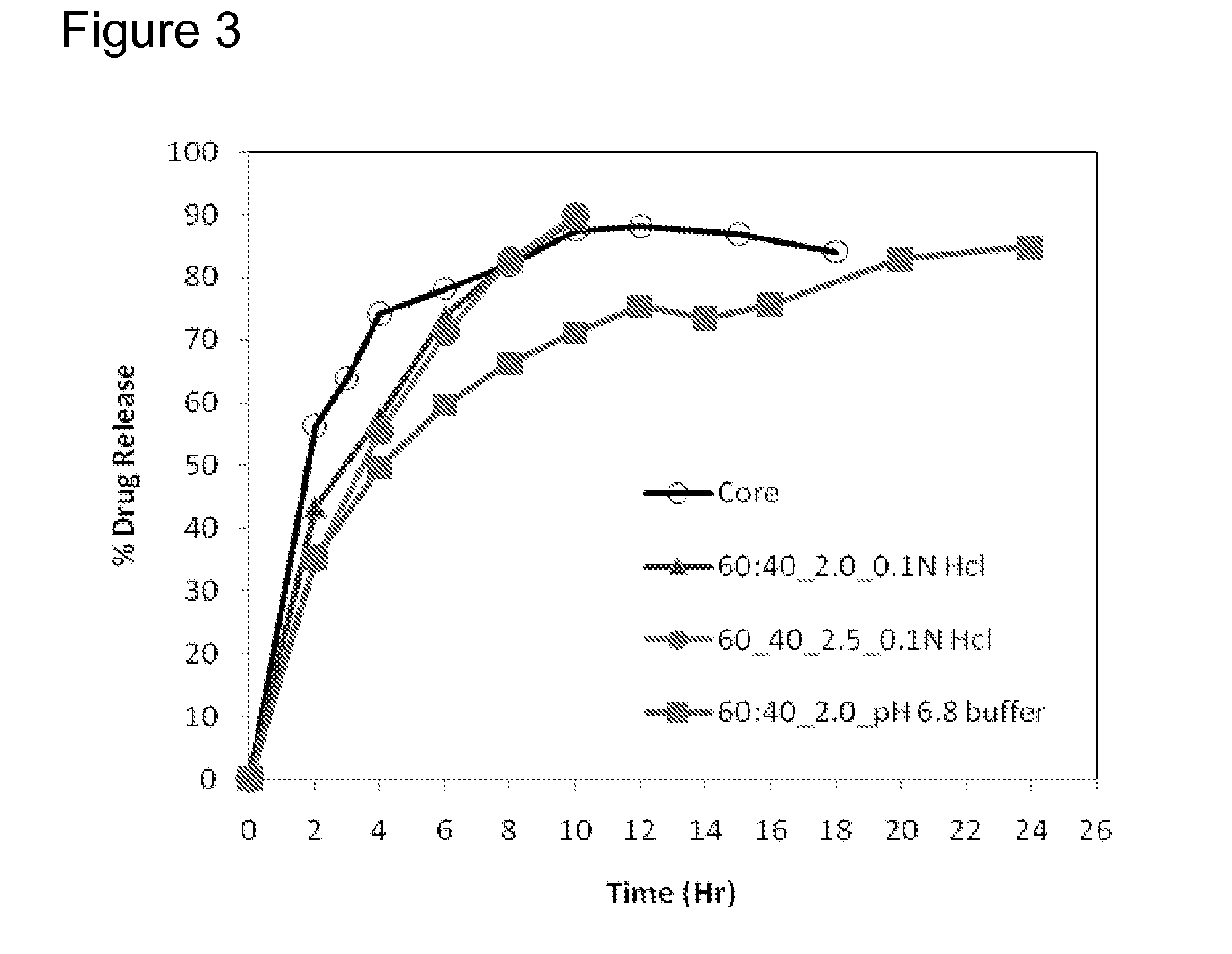
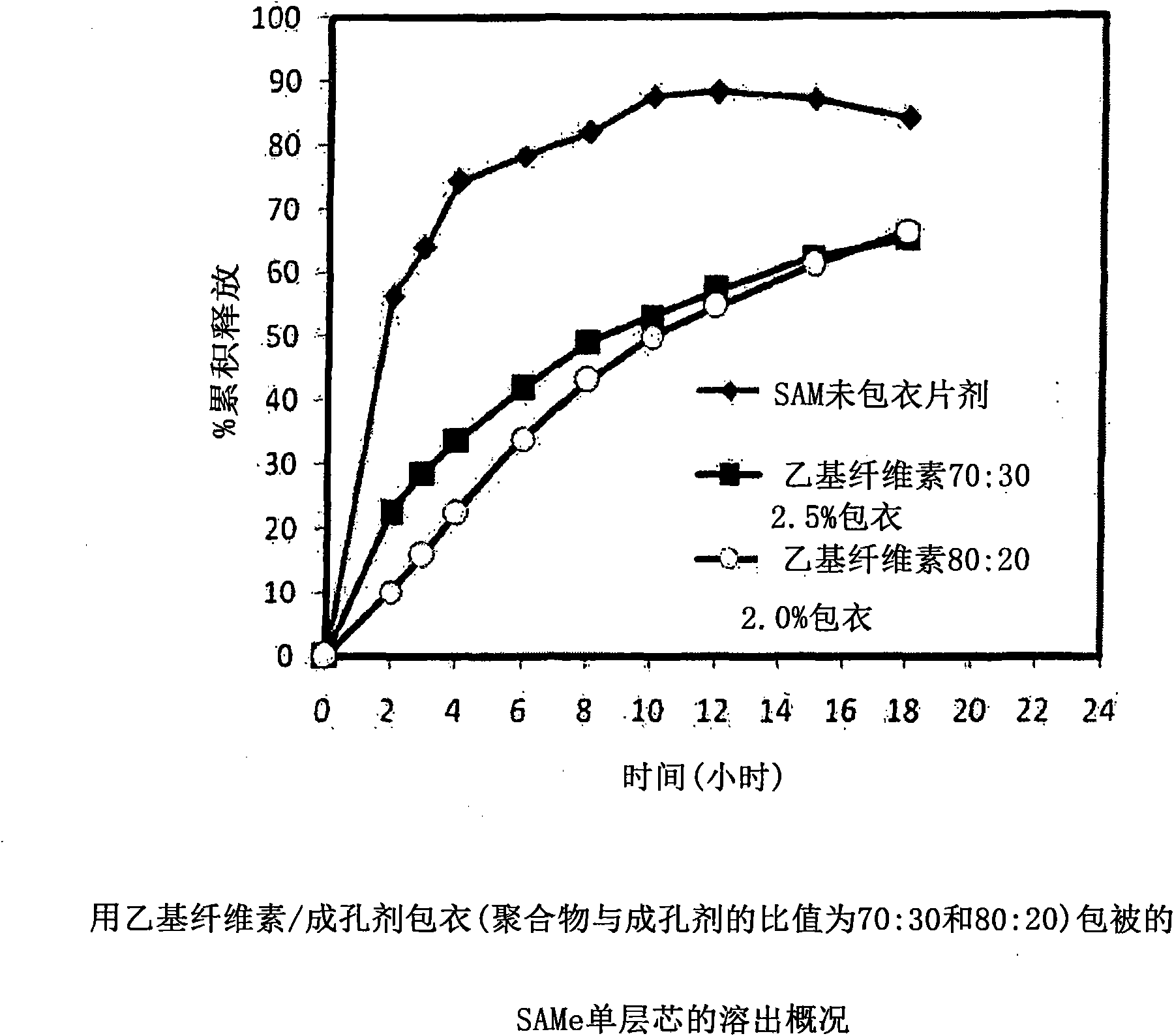
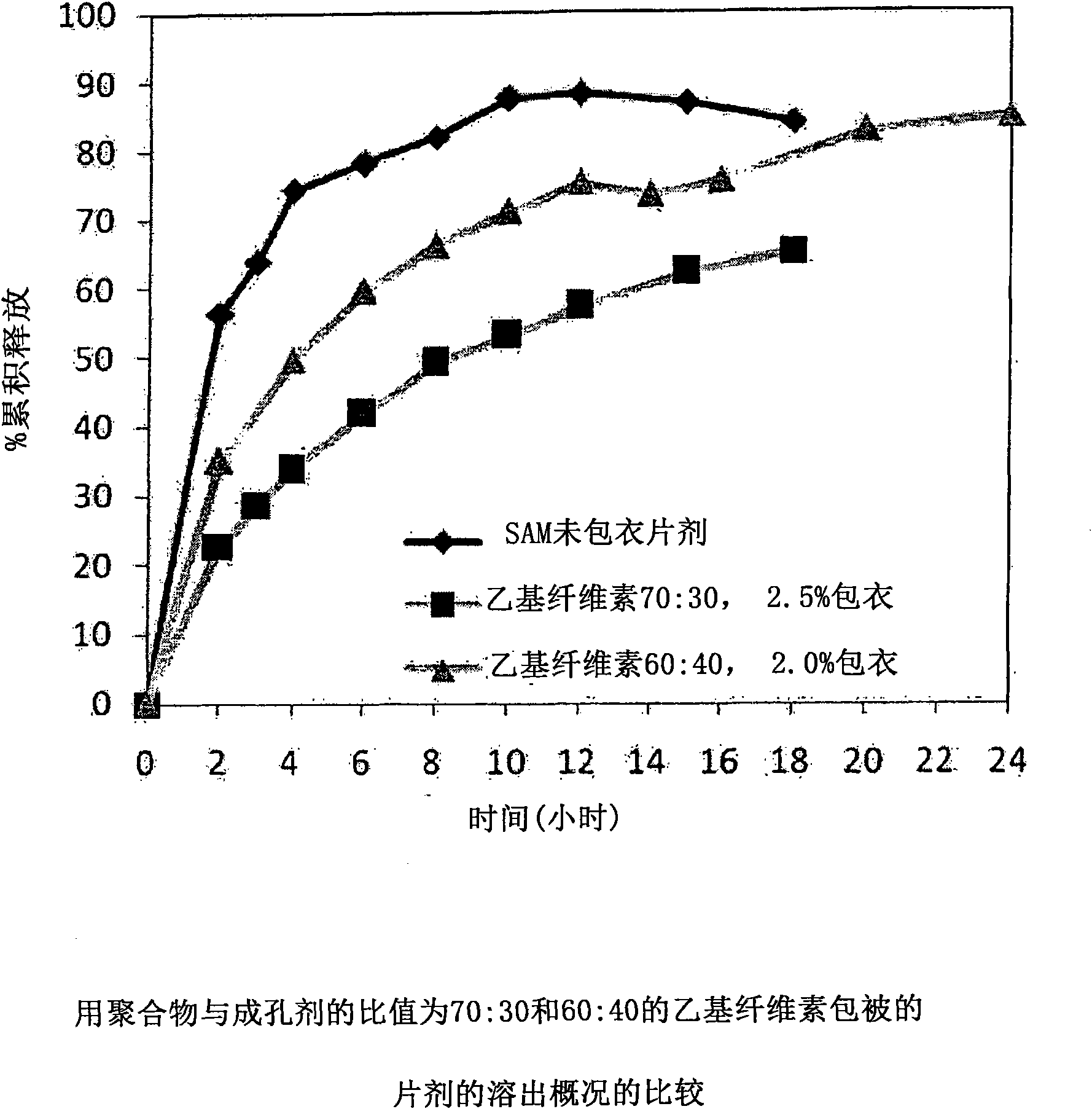
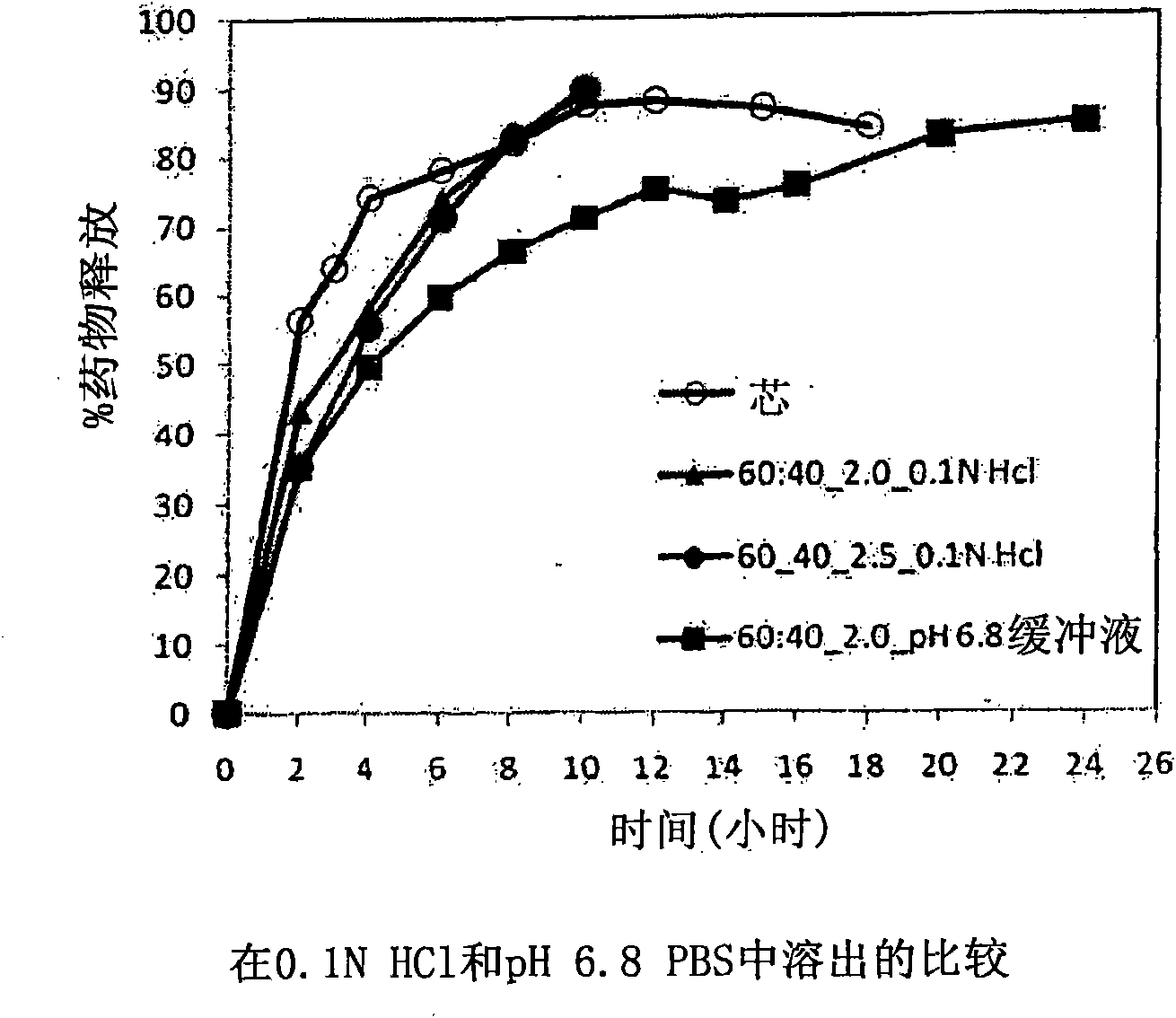
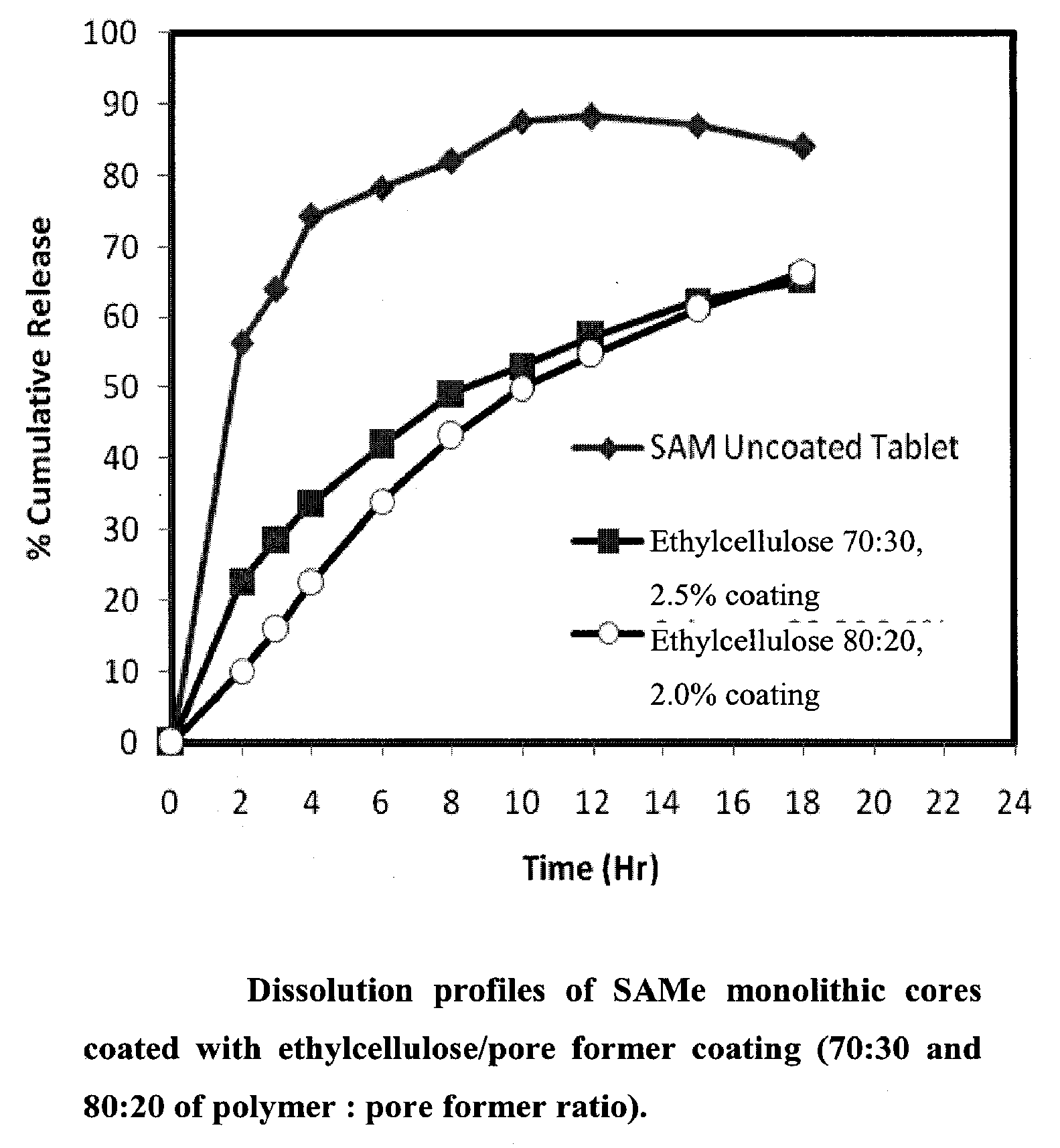
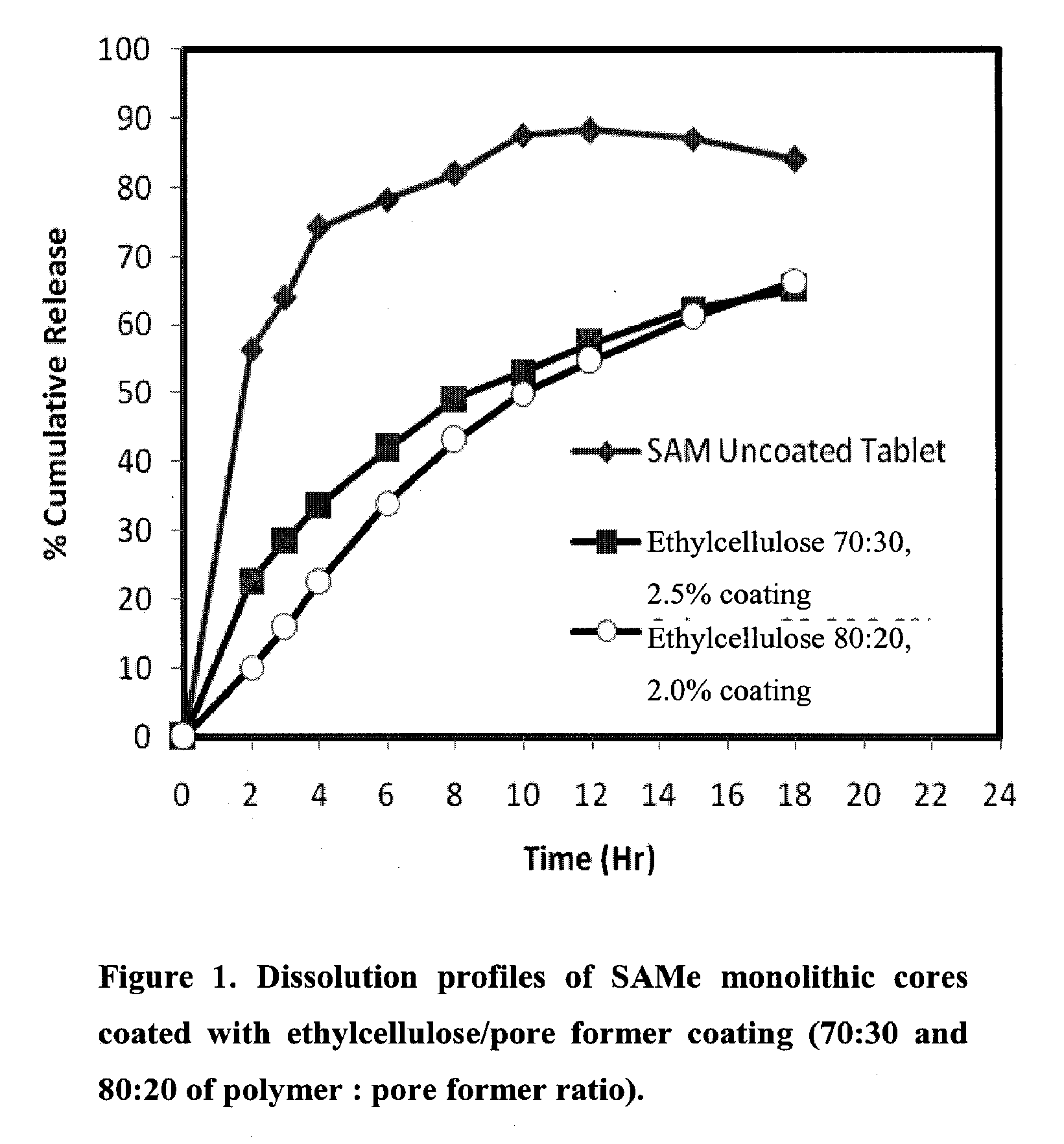
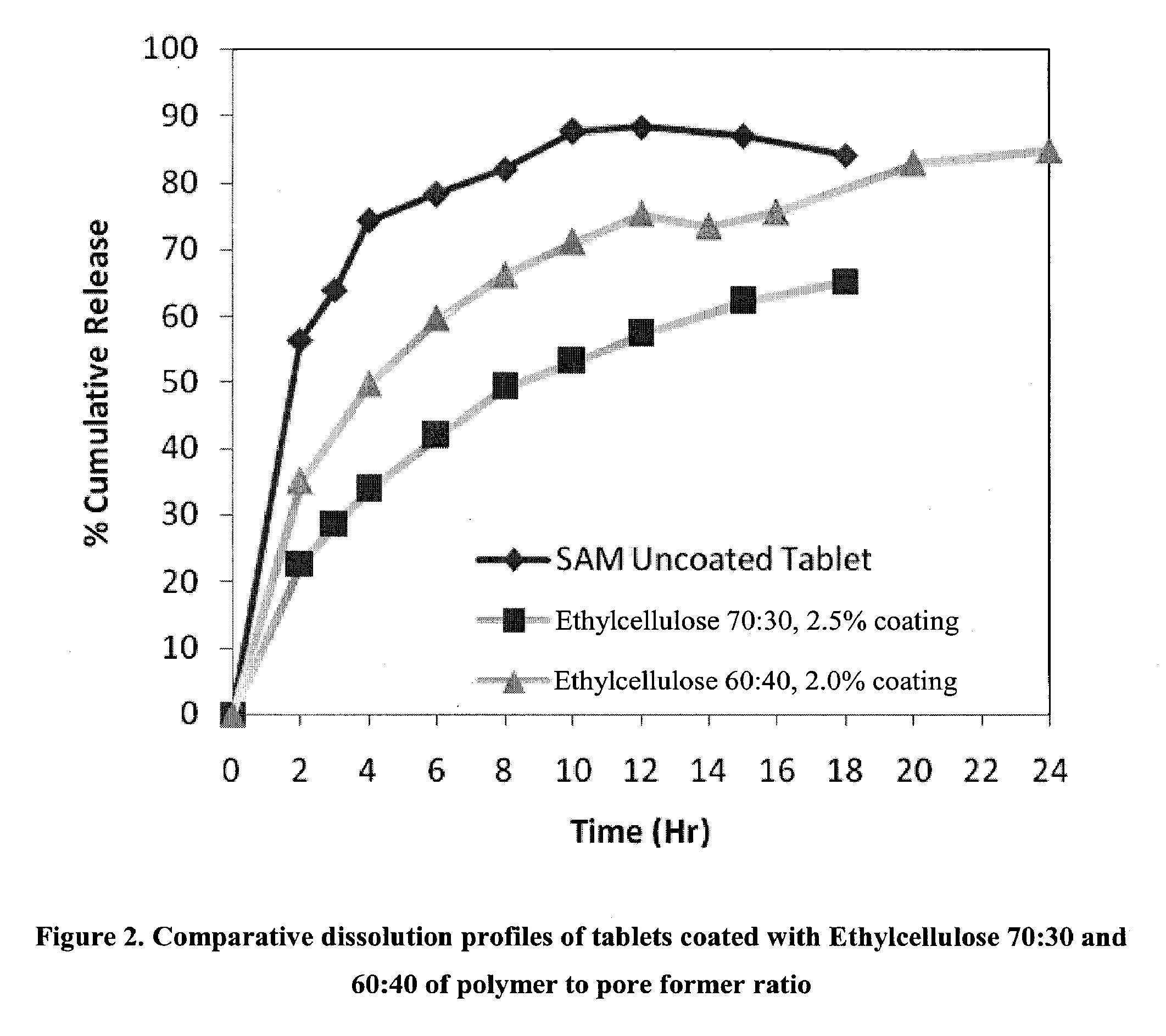
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090088404A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
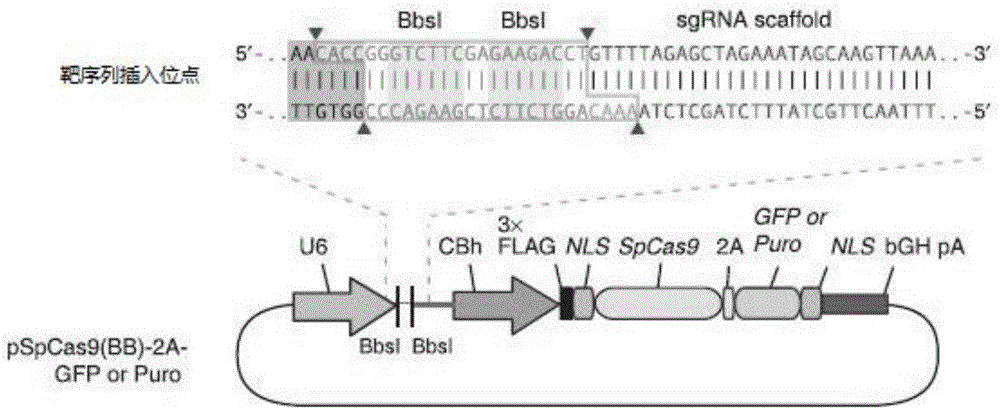
sgRNA sequence for knocking out human CYP2E1, construction method of deficiency cell strain of CYP2E1 and application thereof
ActiveCN106191057AAchieve silencingImproved silence is not completeGenetically modified cellsEnzymesHuman bodyDisease
The present invention provides an sgRNA sequence for knocking out the human CYP2E1 gene. The target DNA sequence of the sgRNA is at least one of the sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2. The present invention also provides a method for knocking out CYP2E1 gene of human embryonic kidney cells, which is adapted to transform CYP2E1 gene in human embryonic kidney cells by using CRISPR / Cas system. The invention also provides a CYP2E1 knockout cell strain. CYP2E1 involves in the important metabolism function of a human body. The CYP2E1 gene knock-out cell strain provided by the invention provides an effective platform for studying the metabolism function of exogenous chemical or exogenous poison in the body, and a powerful tool for studying chronic diseases (such as alcoholic liver disease and diabetes) and tumor-related diseases.
Owner:SUN YAT SEN UNIV
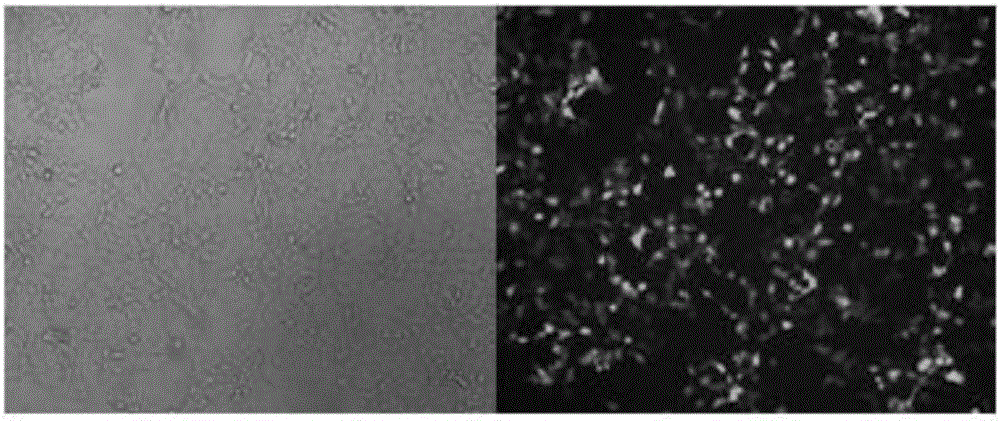
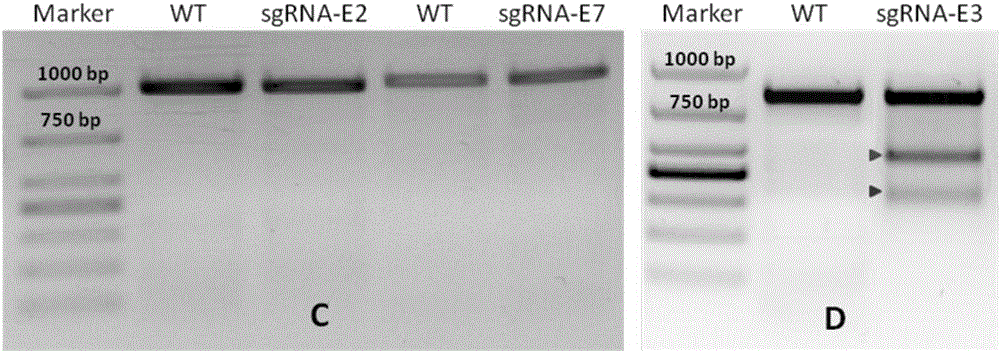
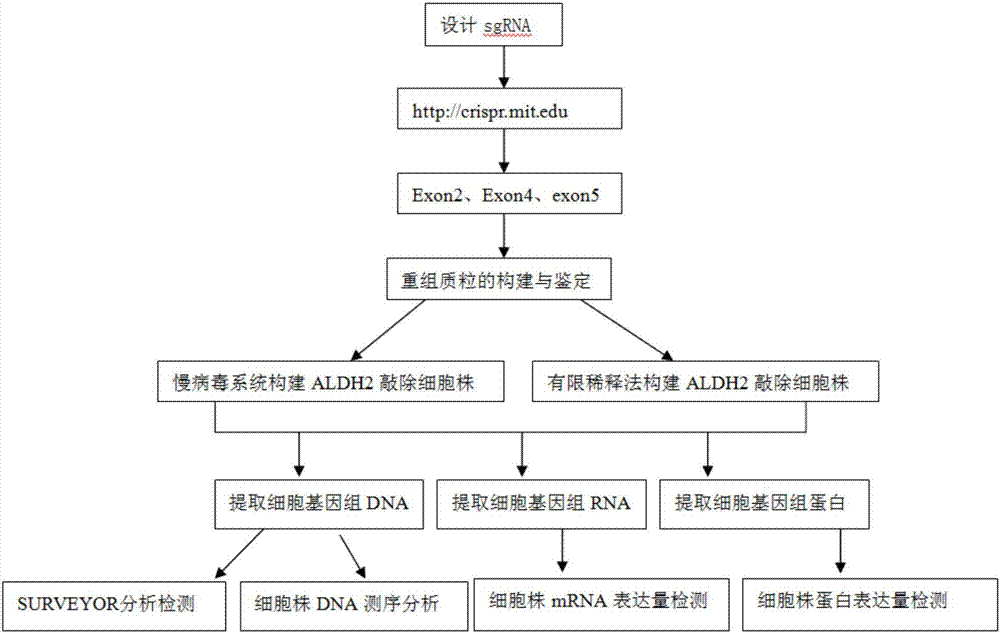
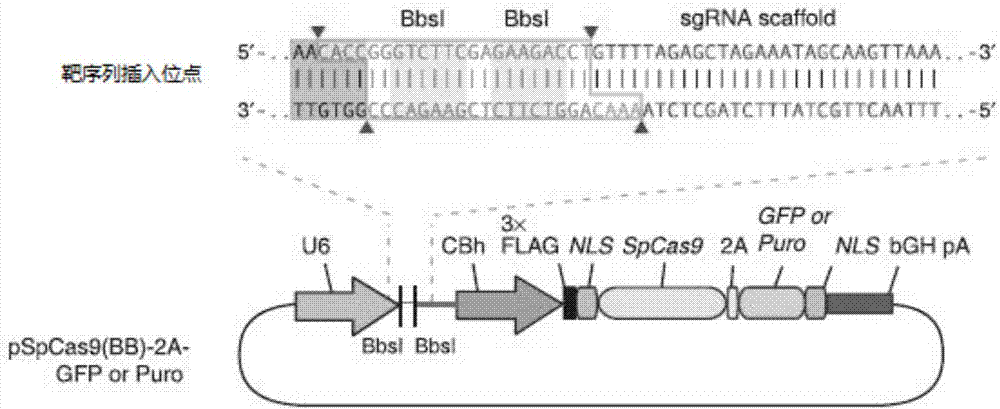
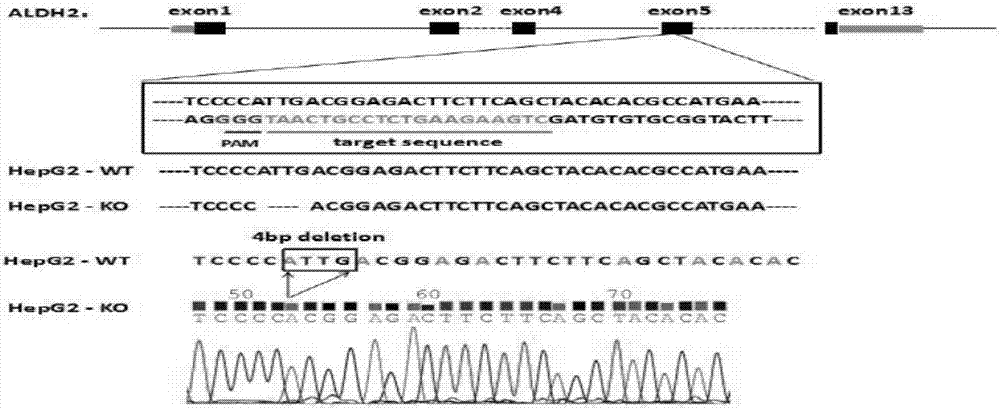
Construction method and application of sg RNA and ALDH2 gene deletion cell strains used for knocking out human ALDH2 gene
ActiveCN107502608AAchieve silencingImproved silence is not completeHydrolasesStable introduction of DNAIn vivoWilms' tumor
The invention provides an sg RNA sequence used for knocking out a human ALDH2 gene;,a target DNA sequence of the sg RNA is at least one of sequences as shown in SEQ ID NO:1, SEQ ID NO:2 and SEQ ID NO:3. The invention further provides a method for knocking out a human hepatoma carcinoma cell ALDH2 gene; the method utilizes a CRISPR / Cas system to modify the ALDH2 gene in a human hepatoma carcinoma cell. The invention further provides two ALDH2 gene deletion cell strains; ALDH2 participates in an important metabolic function of a body. The ALDH2 gene deletion cell strains provided by the invention provide an effective platform for metabolism study, in vivo, of exogenous chemicals or exogenous poisons, so that powerful means are provided for researching chronic diseases (such as alcoholic liver diseases and diabetes) as well as tumor-associated diseases.
Owner:SUN YAT SEN UNIV
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
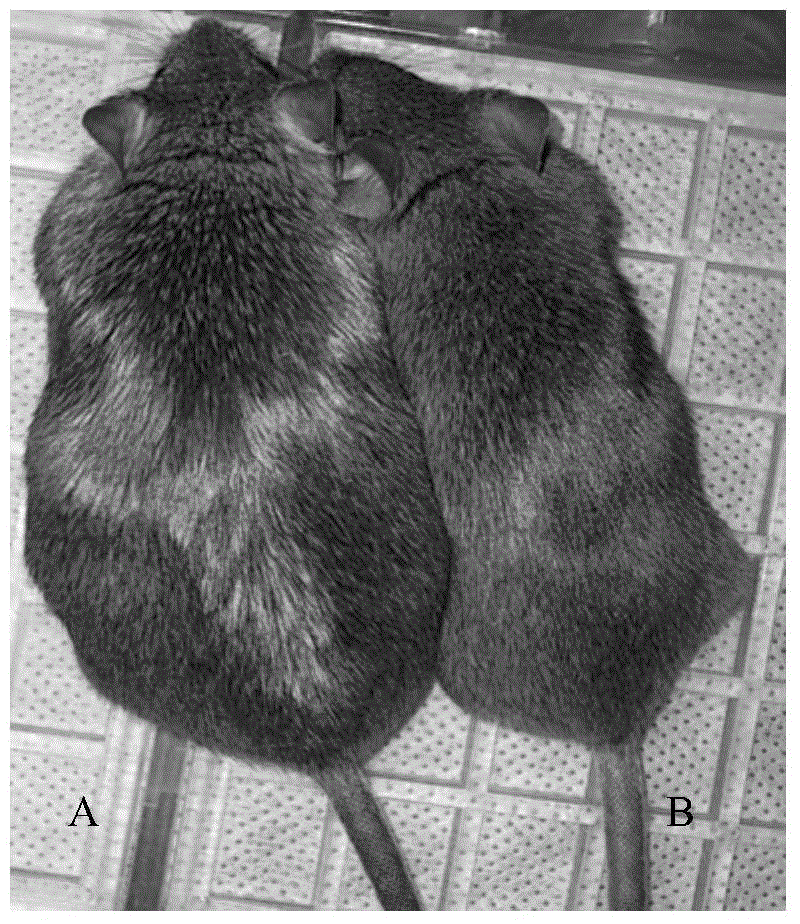
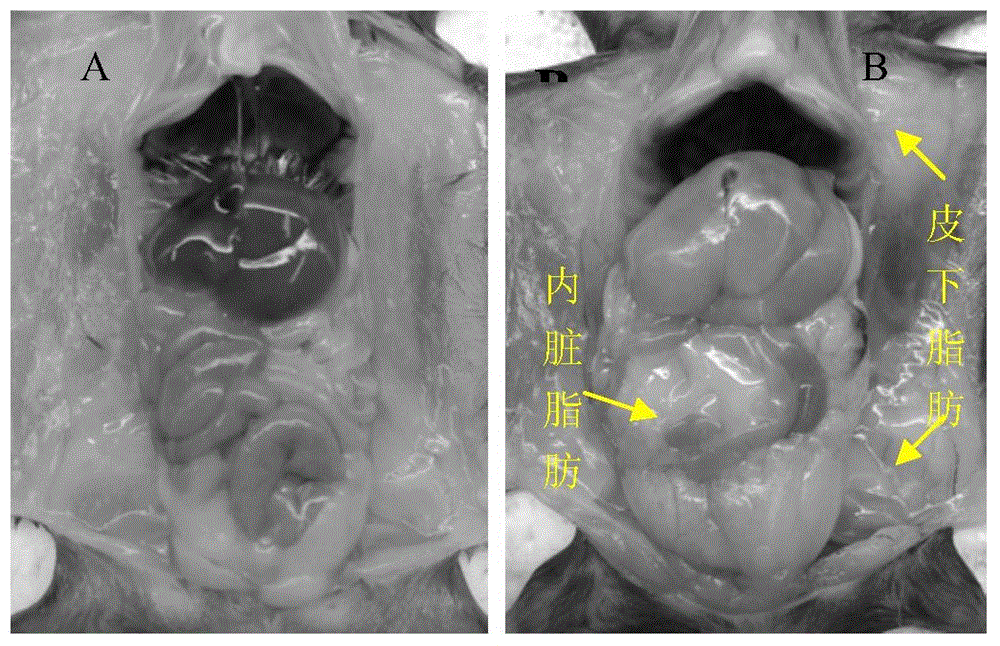
Construction method of cynomolgus macaque model for alcoholic liver disease
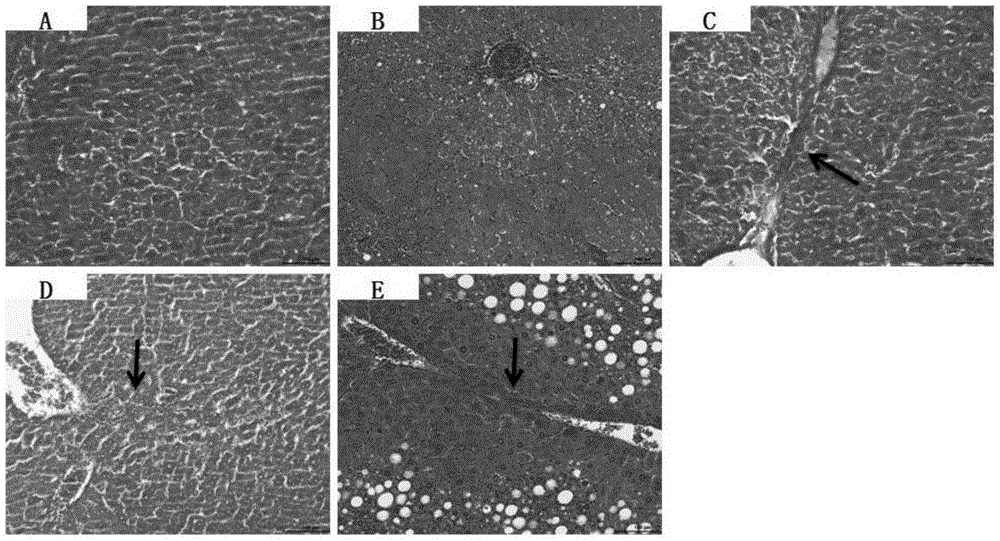
InactiveCN106389394ABuild method time is shortIncrease success rateCompounds screening/testingHalogenated hydrocarbon active ingredientsHigh fatBiology
The invention belongs to a construction method of an animal model, and in particular relates to a construction method of a cynomolgus macaque model for alcoholic liver disease. The construction method of the cynomolgus macaque model for alcoholic liver disease comprises the following steps: screening experimental animals, feeding the screened experimental animals with high-glucose and high-fat feed, and conducting liver biopsy and acquisition of the determined model. The construction method of the cynomolgus macaque model for alcoholic liver disease provided by the invention is short in duration and high in success rate; the construction method is suitable for researching the occurrence mechanism of the alcoholic liver disease and is convenient for screening drugs for treating the alcoholic liver disease and for evaluating the efficacy of the drugs on the alcoholic liver disease; and the construction method has a good application prospect.
Owner:FANGCHENGGANG CHANGCHUN BIOLOGICAL TECH DEV
Pharmaceutical compositions for the treatment/prophylaxis of non-alcoholic fatty liver disease
InactiveUS20120202849A1Lowering/preventing accumulation of fatBiocideOrganic chemistryDiseaseHydroxychloroquine
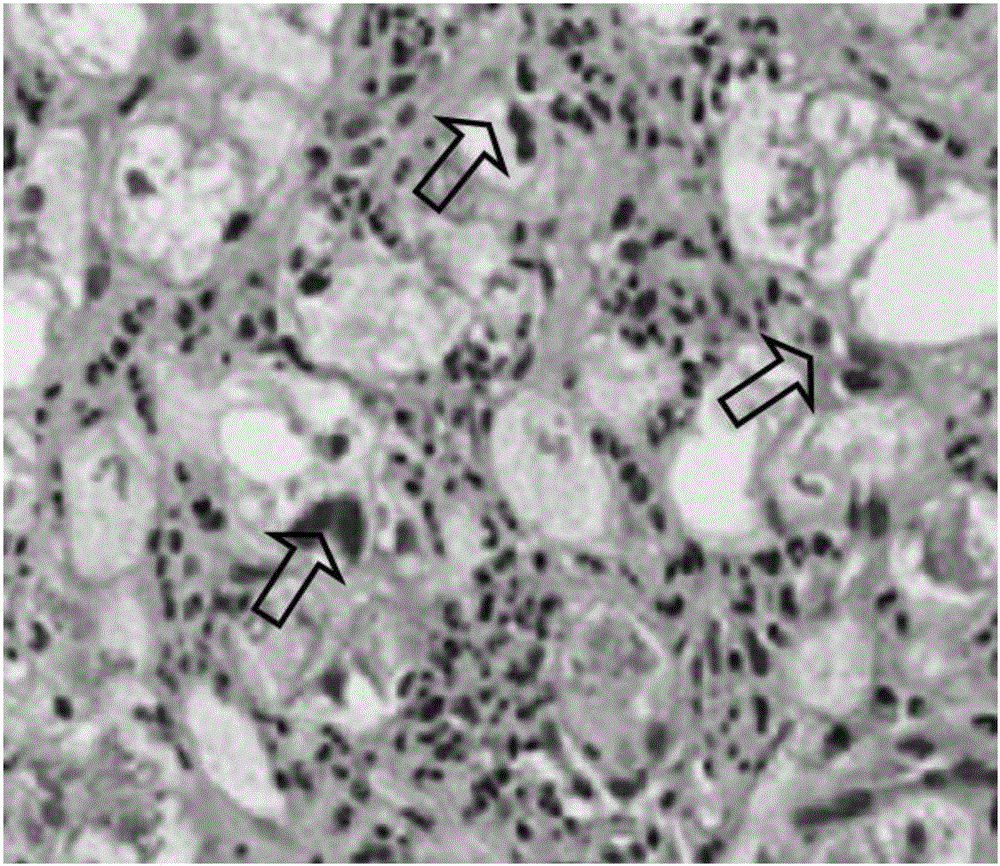
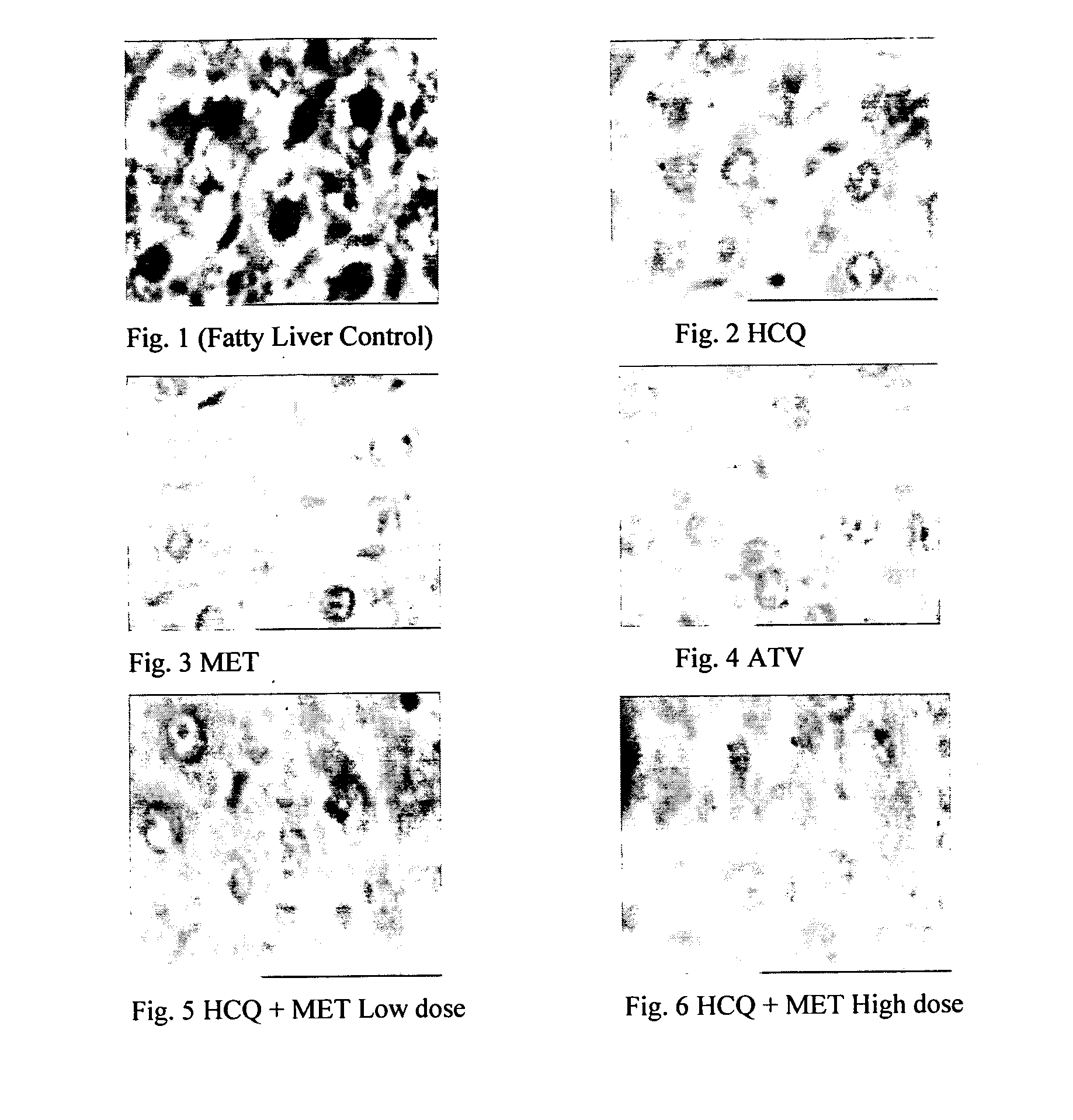
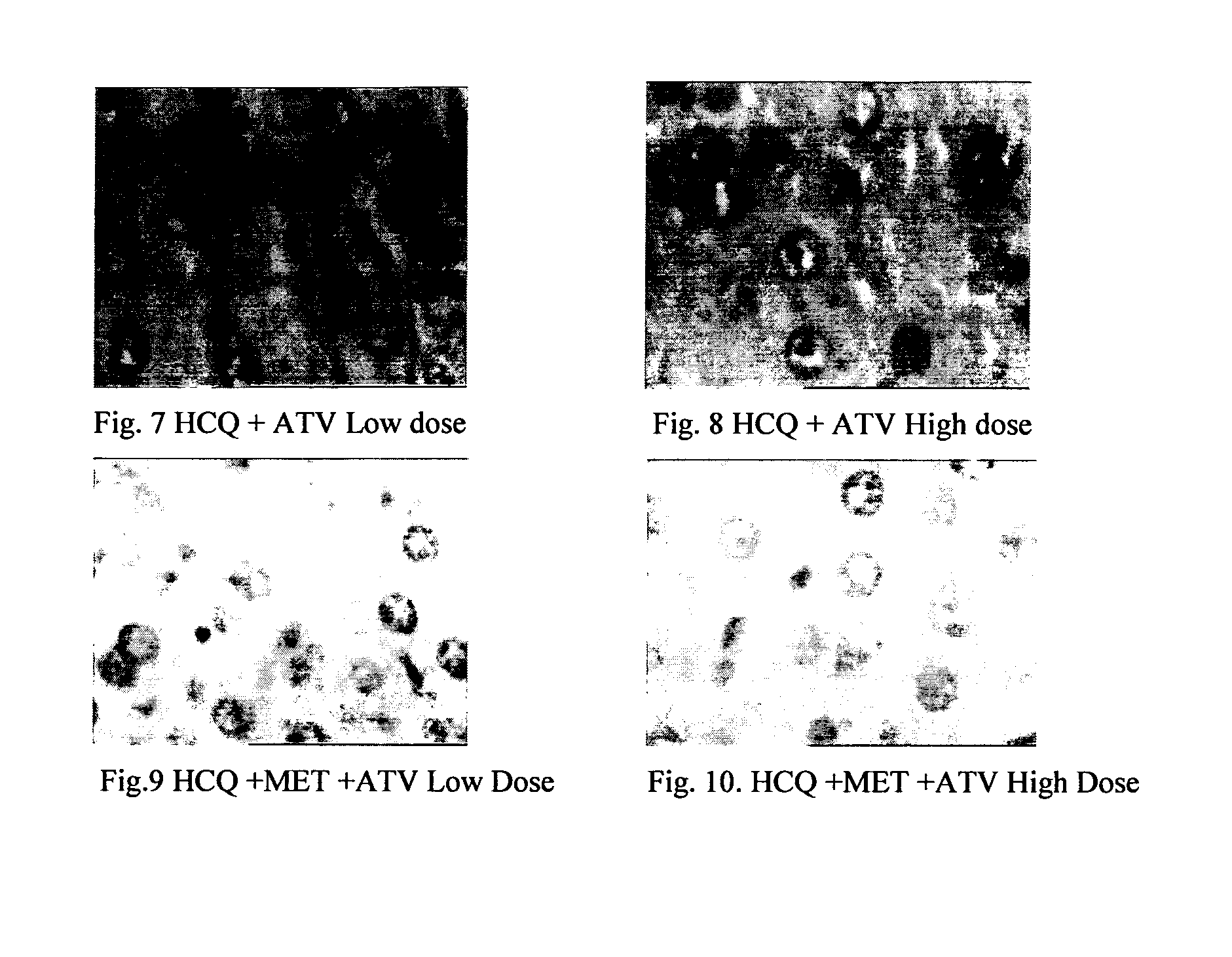
Disclosed herein is a novel synergistic pharmaceutical composition comprising hydroxychloroquine with insulin sensitizing agents and lipid lowering agents such as statins along with pharmaceutical excipients / carriers useful in treating Non-Alcoholic Fatty Liver Disease.
Owner:IPCA LAB LTD
Chinese medicine health product preparation for preventing and treating alcoholic liver disease and preparation method thereof
InactiveCN101502627AMedicinal Flavor SimplifiedSignificant effectDigestive systemPlant ingredientsDrug contentSemen
The invention discloses a traditional Chinese medicine health product preparation for preventing and treating alcohol liver disease and a preparation method. According to the parts by weight, the traditional Chinese medicine health product preparation is prepared by adopting the following raw materials: 250 to 328 parts of pueraria flower, 250 to 328 parts of semen hoveniae, 250 to 328 parts ofradix puerariae, 125 to 164 parts of fructus amomi, 250 to 328 parts of dark plum fruit, 250 to 328 parts of date and 125 to 164 parts of liquoric root. The traditional Chinese medicine health product preparation can effectively prevent the alcohol from causing GSH exhaustion and MDA rising of the liver, reduce fatty degeneration of liver cells and has the function of preventing alcoholic liver damage; the health product preparation has condense medicinal odour, stable and controllable preparation technique and product quality, obvious curative effect and fast effect; the provided formulation has convenient in use, reduces the oral dosage; and in the formulation, all the drug contents are obtained from natural resources, has lower cost and is conveniently to promote and broad masses of the people are willing to adopt the traditional Chinese medicine health product preparation.
Owner:贵阳青青生物科技有限公司
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20090197824A1Act quicklyReduce riskBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Method of treating non-alcoholic fatty liver disease and steatohepatitis
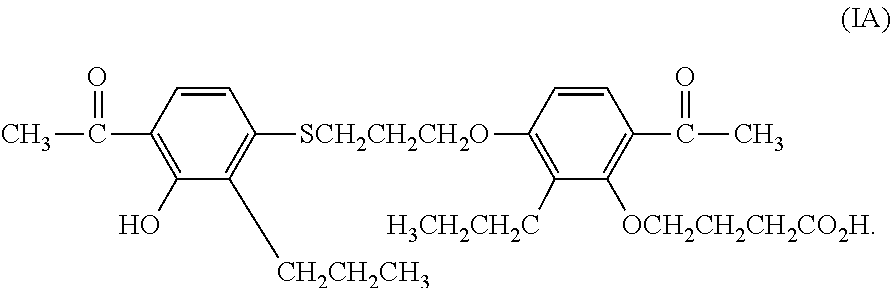
A compound of Formula (I):or a metabolite thereof, or an ester of the compound of Formula (I) or the metabolite thereof, or a pharmaceutically acceptable salt of each thereof, wherein m, n, X1 and X2 are as defined herein, is useful for treating non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH).
Owner:MEDICINOVA INC
Application of medicinal composition to preparation of medicament for preventing and treating alcoholic liver damage and fatty liver and lowering blood fat
ActiveCN102058632AEasy to solveImprove development and utilization valueOrganic active ingredientsDigestive systemSecondary hyperlipidemiaAlcoholic liver damage
The invention discloses an ilicis routundae cortex medicinal composition for preventing and treating alcoholic liver damage, alcoholic fatty liver, non-alcoholic fatty liver and hyperlipidemia. The composition is extracted and refined from natural ilicis routundae cortex and is not chemically modified, and is characterized by comprising substances such as pedunculoside, syringin, rotundicacid and the like. The invention also discloses a preparation method of the ilicis routundae cortex composition and application to preparation of a medicament for preventing and treating the alcoholic liver damage, the alcoholic fatty liver and the non-alcoholic fatty liver and lowering blood fat.
Owner:吉林修正药业新药开发有限公司
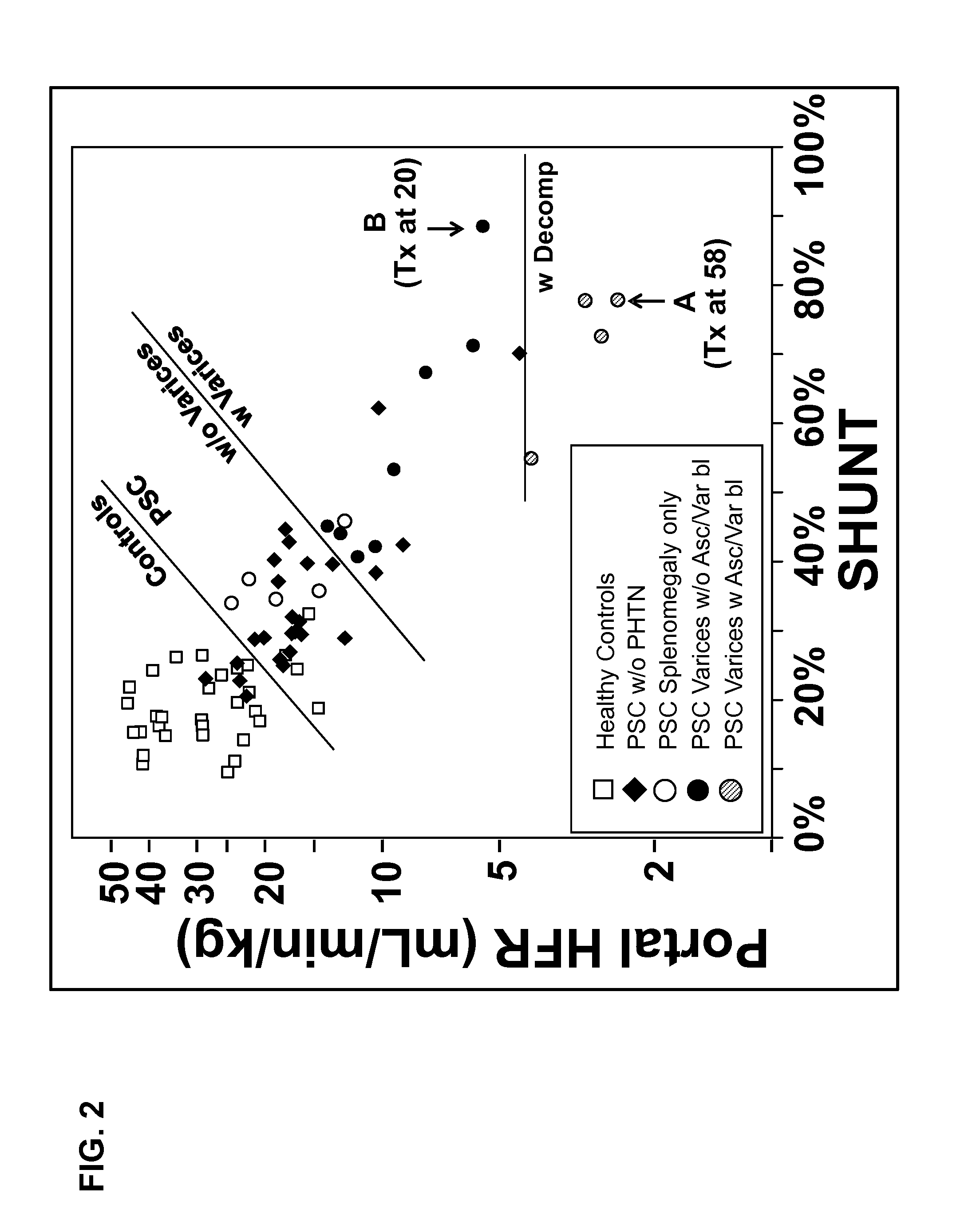
Disease severity index for assessment of chronic liver disease and method for diagnosis of three distinct subtypes of primary sclerosing cholangitis
ActiveUS20140147875A1Low indexOrganic active ingredientsHealth-index calculationDisease severityNon invasive
A Disease Severity Index (DSI) is provided for assessment of chronic liver disease in a patient using non-invasive liver function test results. A DSI was derived from non-invasive liver function test results based on hepatic blood flow. The DSI is used in methods for prediction of clinical outcomes, prediction of response to antiviral treatment, and assessment of progression of chronic liver diseases. Non-invasive methods to diagnose three distinct categories of patients with Primary Sclerosing Cholangitis (PSC) are provided. The methods can be used to diagnose PSC patients as Slow Progressors, Moderate Progressors and Rapid Progressors.
Owner:UNIV OF COLORADO THE REGENTS OF
Treatment of hepatic fibrosis with imatinib mesylate
Disclosed herein is a method for treating hepatic fibrosis comprising administering to a patient in need of such treatment an amount effective to treat hepatic fibrosis of imatinib mesylate. This is based on the ability of imatinib mesylate to down regulate stellate cell activation in culture and in vivo. Hepatic fibrosis is not limited to patients with chronic Hepatitis B, Hepatitis C, non-alcoholic steatophepatitis (NASH), alcoholic liver disease, metabolic liver diseases (Wilson's disease, hemochromatosis), biliary obstruction (congenital or acquired) or liver diseases associated with fibrosis of unknown cause.
Owner:MT SINAI SCHOOL OF MEDICINE
Compositions and methods for reducing hepatotoxicity associated with drug administration and treating non-alcoholic fatty liver disease, non-alcoholic steatohepatitis and associated cirrhosis
InactiveUS20090239831A1Improve efficiencyEnhancing armamentariumBiocideSalicyclic acid active ingredientsActive agentHigh doses
The present invention relates to the discovery that acetylsalicylic acid (ASA or aspirin), salicylic acid (SA) and related salicylate esters and their pharmaceutically acceptable salts, when coadministered in effective amounts with a drug or other bioactive agent which typically (in the absence of the salicylate compound) produces significant hepatotoxicity as a secondary indication, will substantially reduce or even eliminate such hepatotoxicity. Favorable therapeutic intervention results from the use of the present invention having the effect of reducing hepatotoxicity associated with the administration of certain drugs and other bioactive agents and in certain instances of allowing the administration of higher doses of a compound which, without the coadministration, would produce hepatotoxicity which limits or even negates the therapeutic value of the compound. The invention also relates to methods of reducing the likelihood of a patient at risk for non-alcoholic fatty liver diseases (NAFLD), including non-alcoholic steatohepatitis (NASH), or treating NAFLD or NASH including primary NASH, NASH secondary to liver transplantation (NASH post-liver transplantation) or cirrhosis represent alternative aspects of the present invention.
Owner:YALE UNIV
Detecting and Treating Liver Damage
InactiveUS20150247149A1Reducing blocking internalizationShorten the progressAntibody ingredientsDisease diagnosisMicroparticleLiver fibrosis
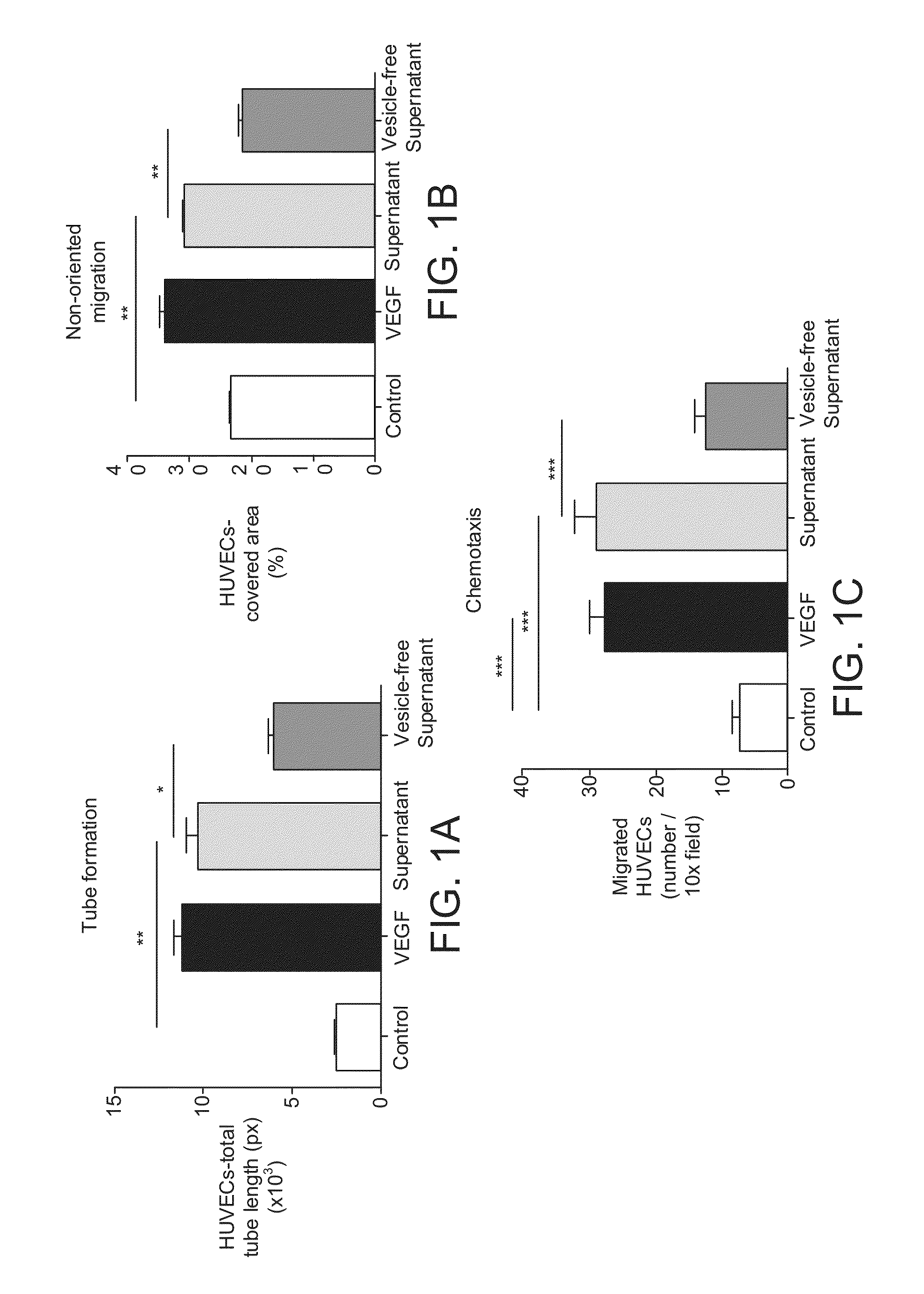
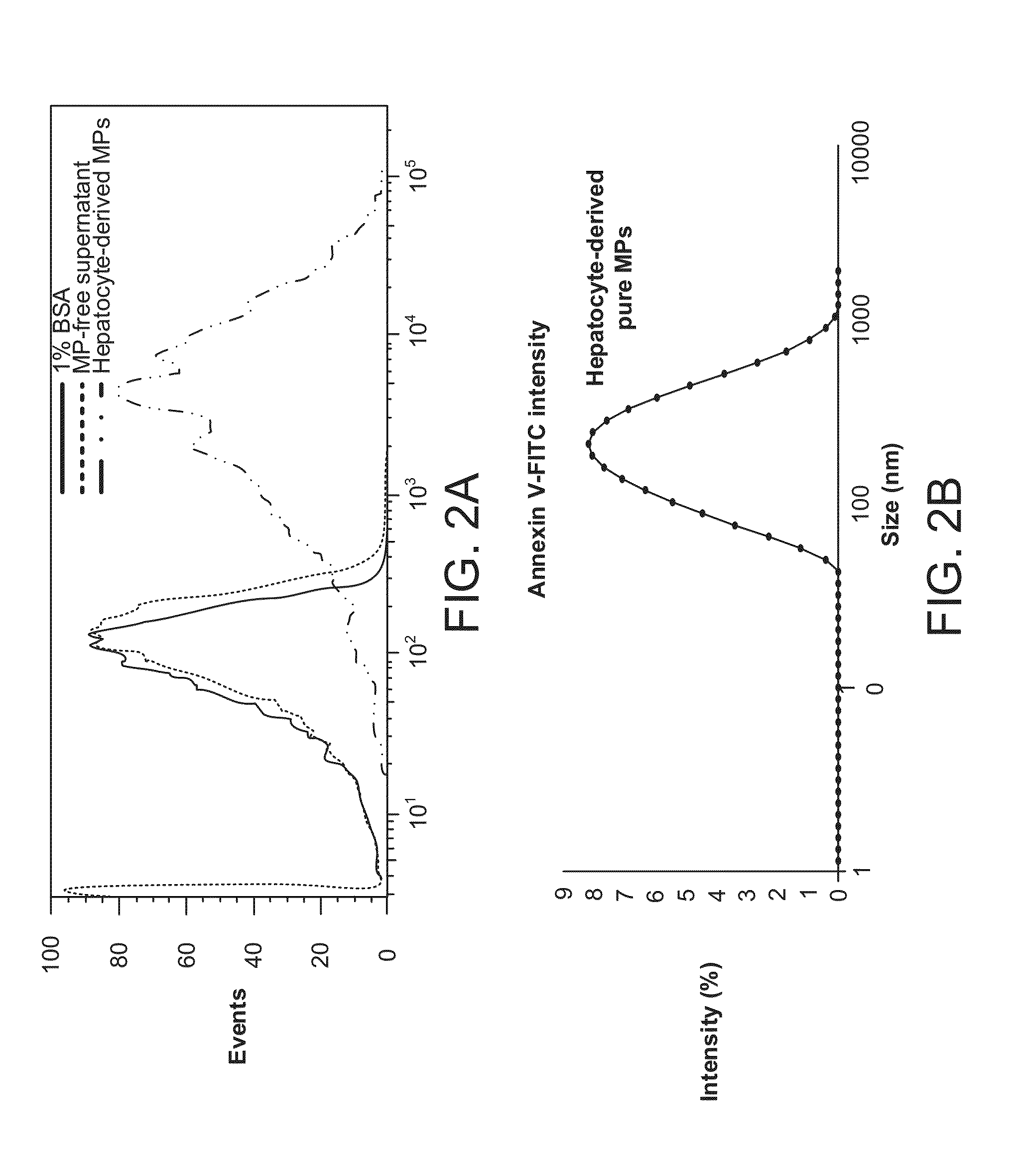
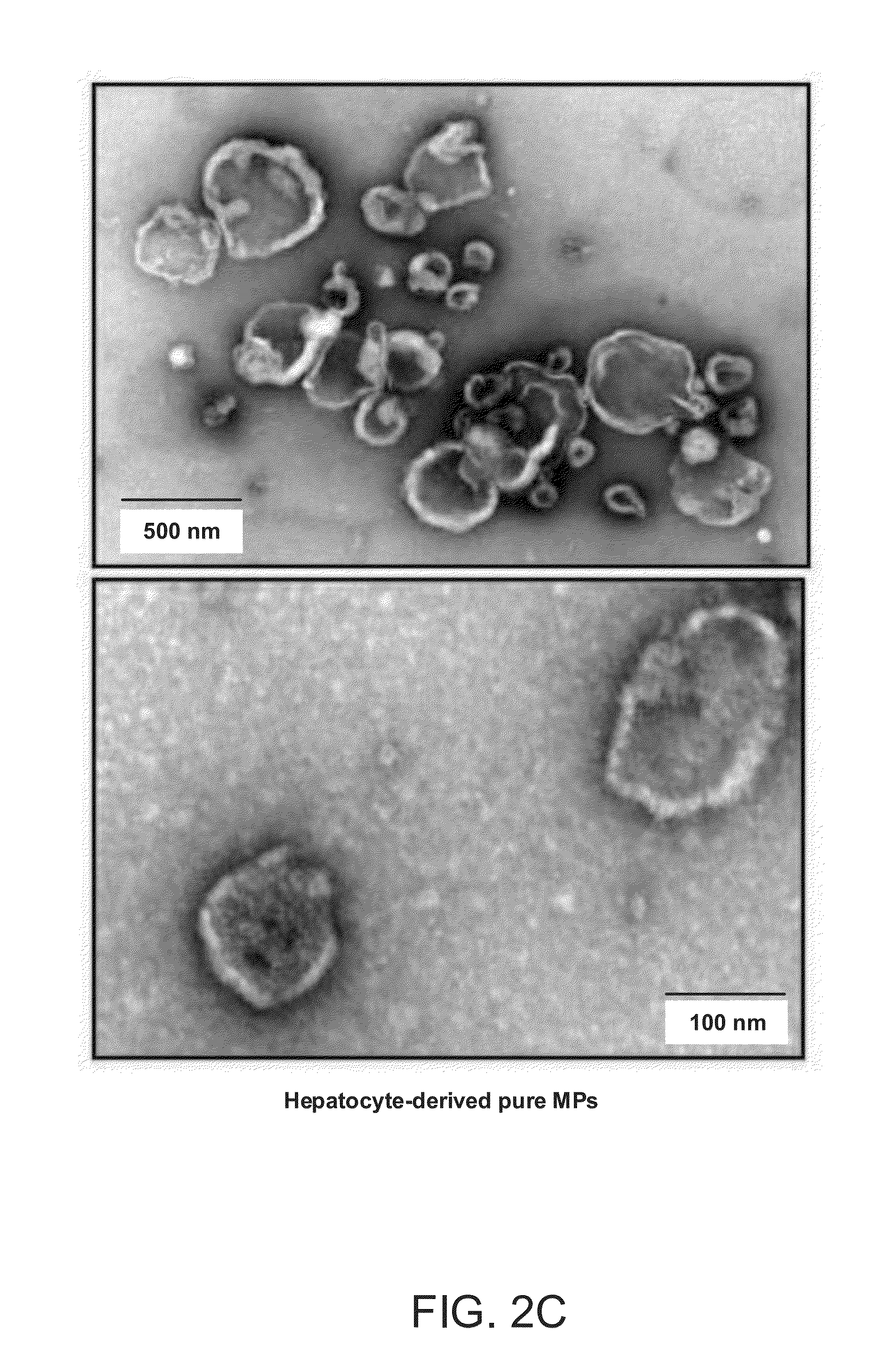
The invention provides a method of detecting, monitoring, assessing and treating non-alcoholic fatty acid liver disease (NAFLD) and associated liver damage in a subject comprising measuring the amount of hepatocyte-derived circulating extracellular vesicles (EVs) and / or microparticle (MPs) in the bodily sample, or the expression level or activity of at least one biomarker expressed or detected in the EVs and / or MPs. The increased amount of EVs or MPs in the bodily sample and / or the increased expression or detection level of the biomarker of interest correlate with the degree or severity of NAFLD, NASH, liver fibrosis, or other associated liver damage, which can be associated with angiogenesis. Prevention and treatment of NAFLD, NASH, liver fibrosis or associated liver damage by reducing EVs or MPs, or targeting the biomarkers expressed in the EVs or MPs are also provided.
Owner:RGT UNIV OF CALIFORNIA
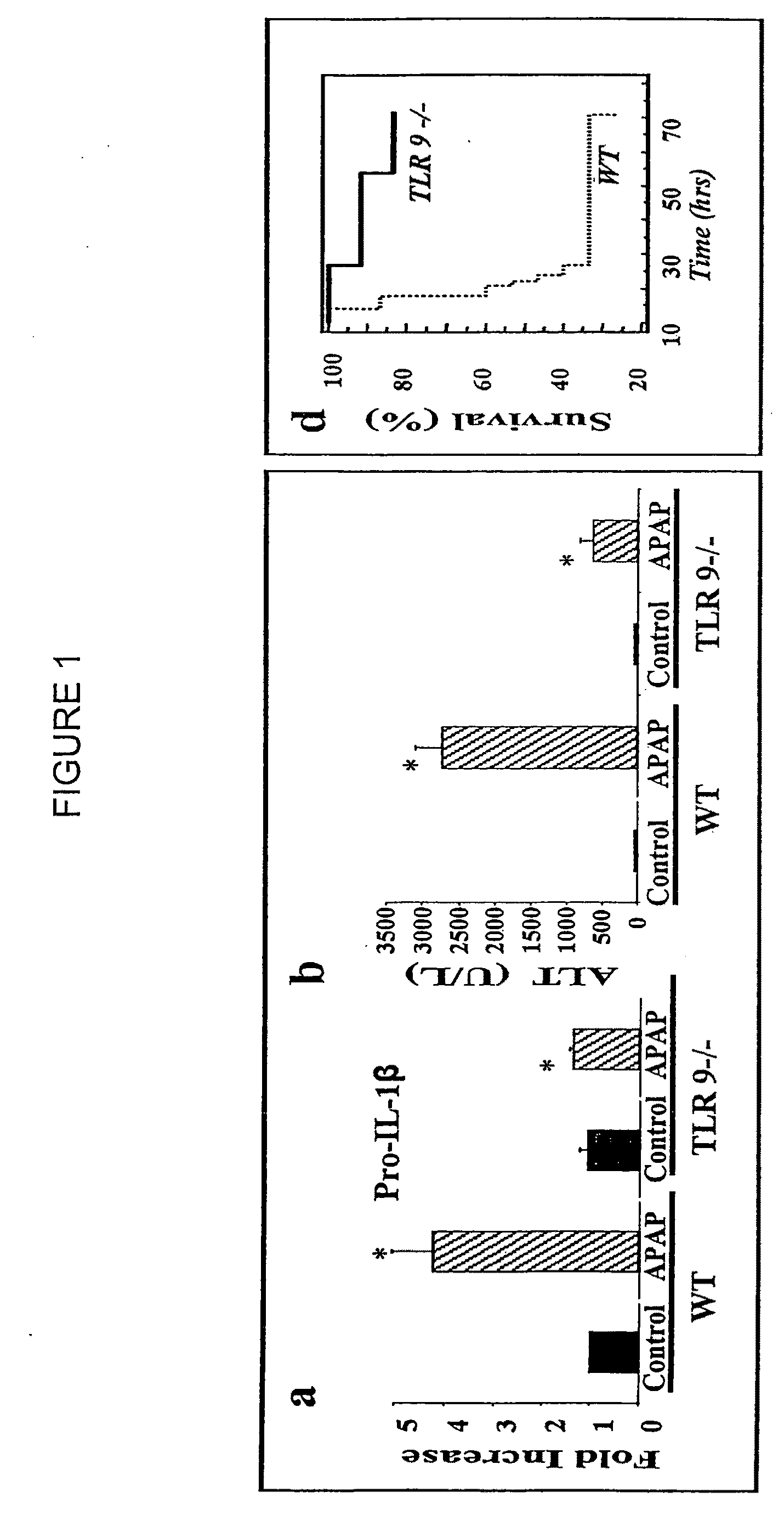
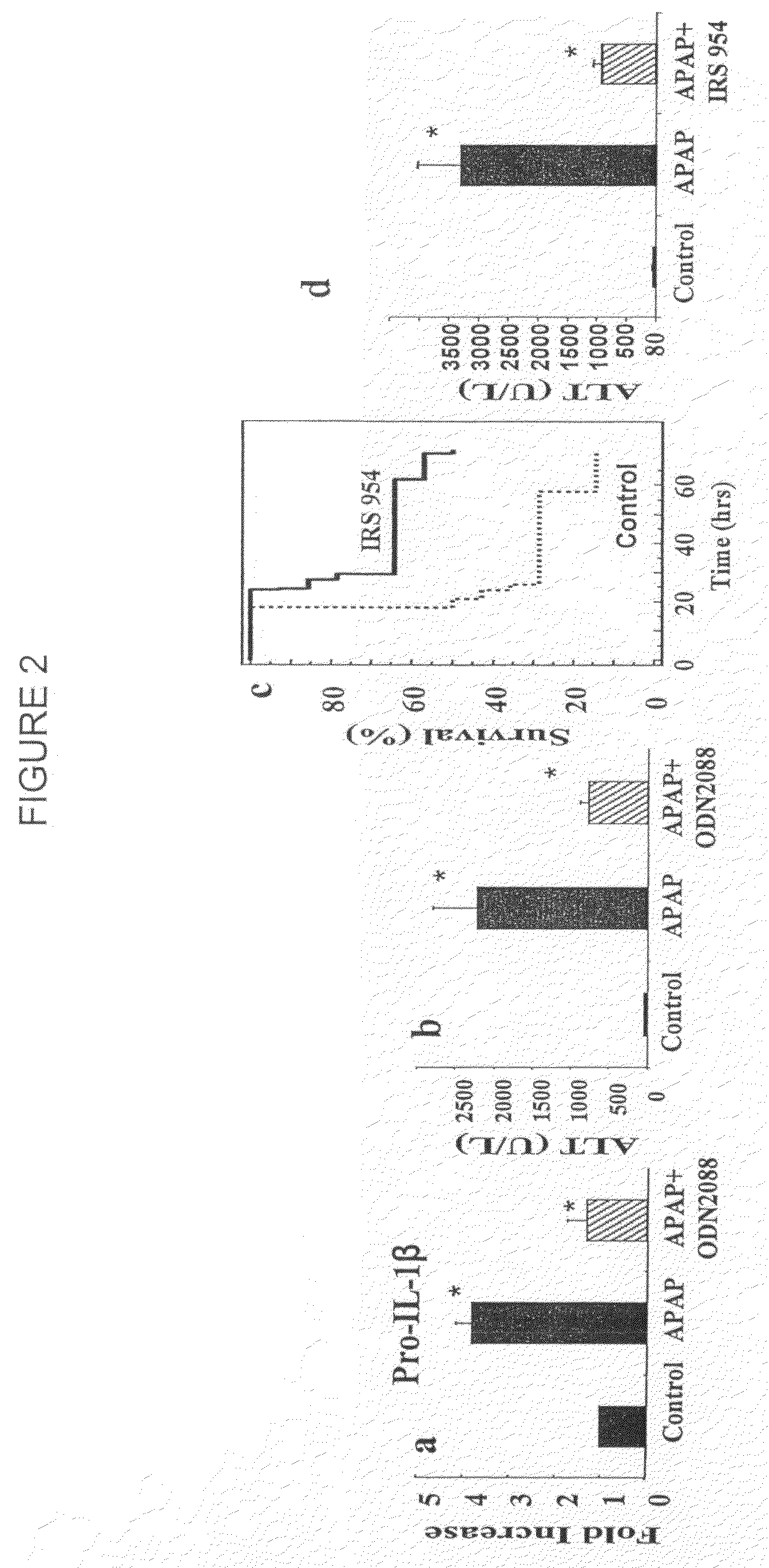
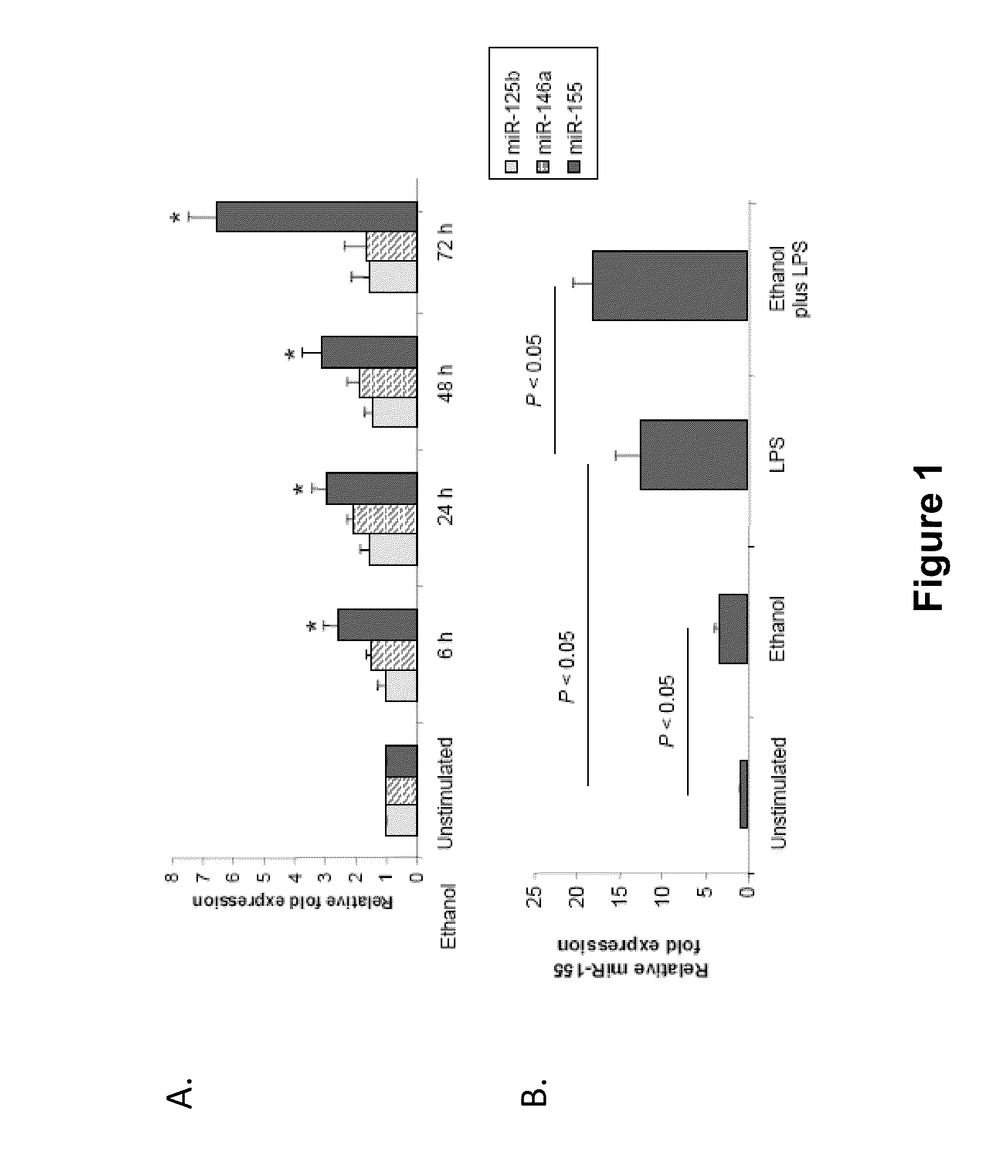
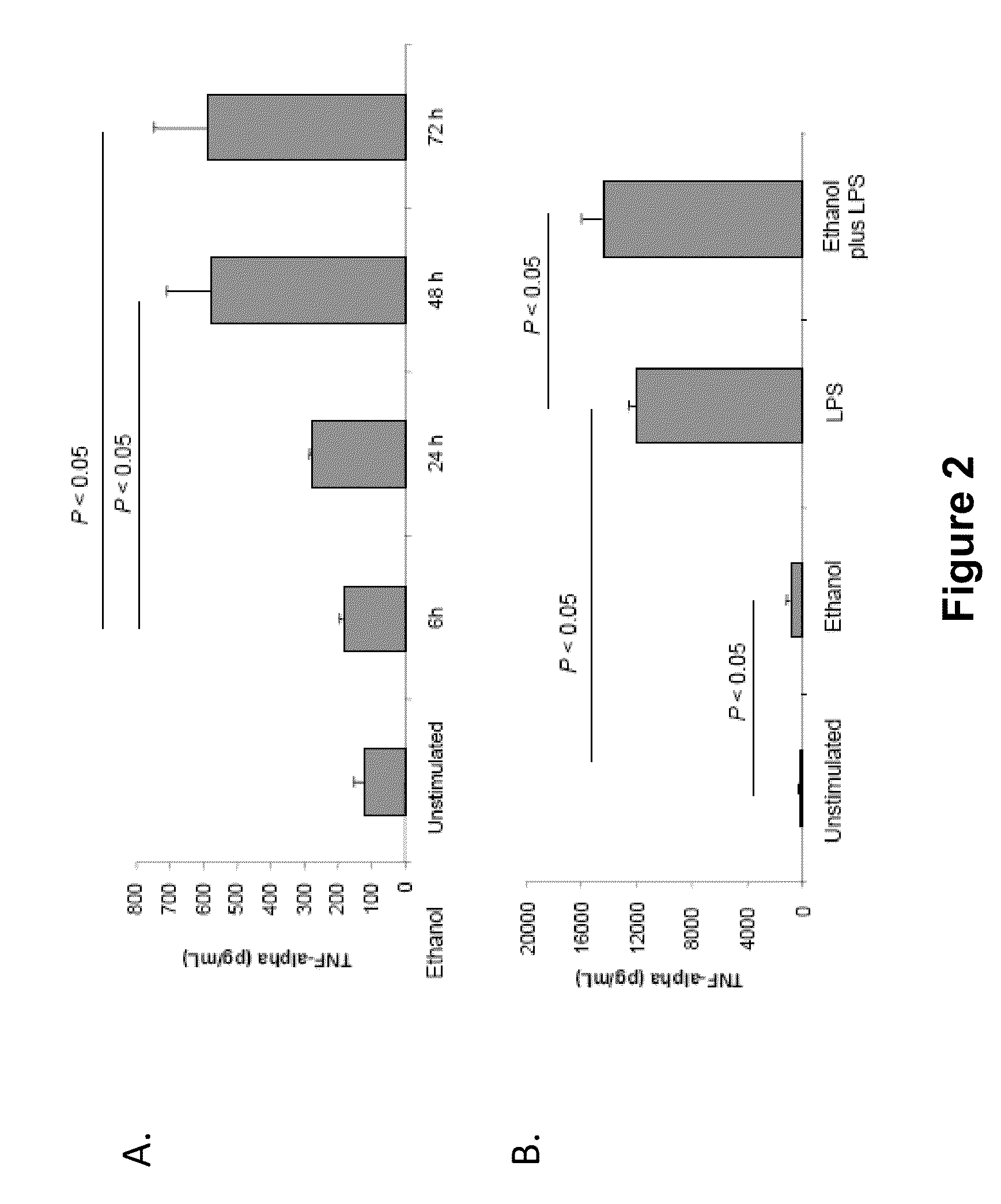
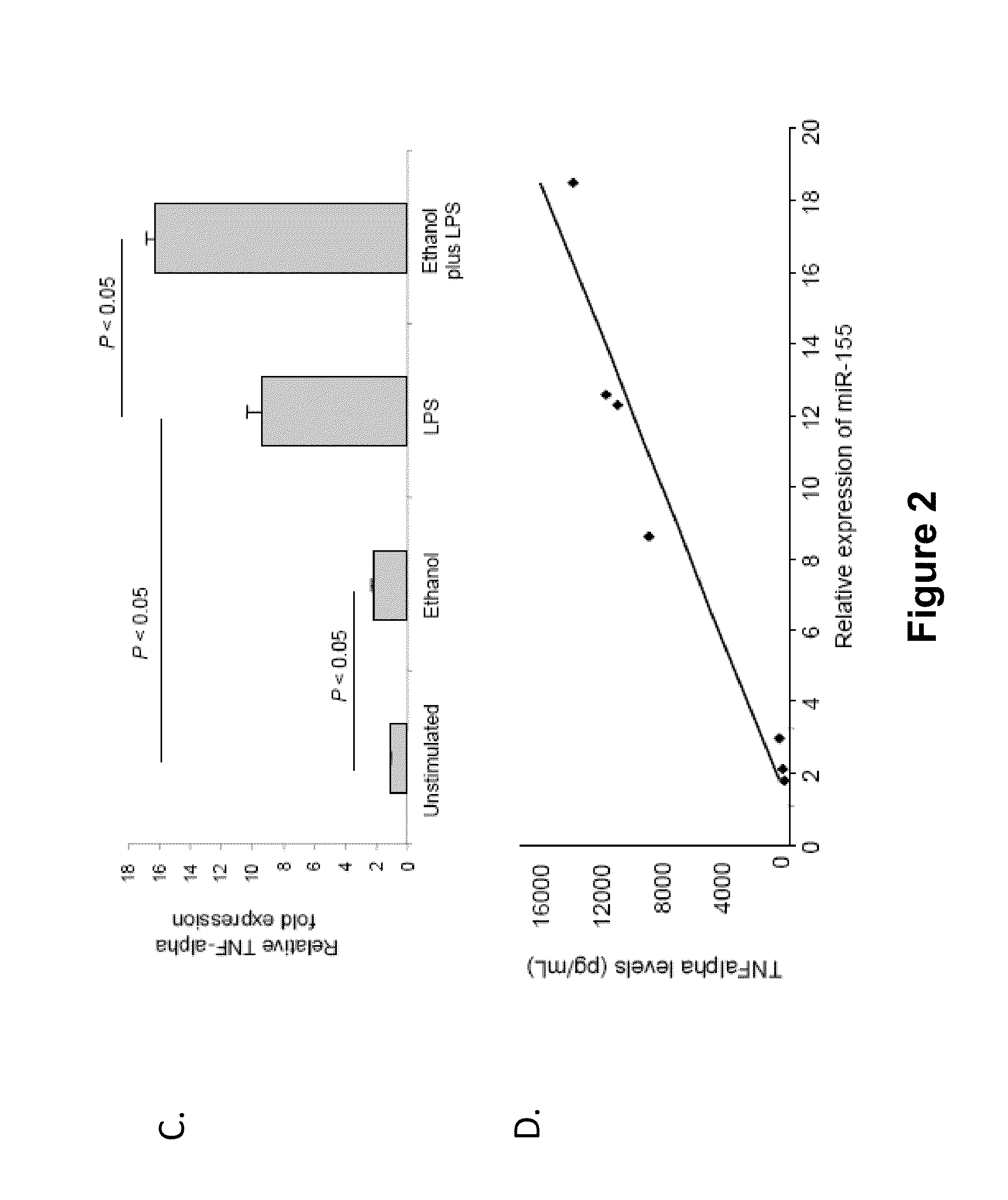
Antagonists of mir-155 for the treatment of inflammatory liver disease
InactiveUS20110293653A1Relieve symptomsAttenuate pathologyOrganic active ingredientsBiocideInflammatory bowel diseaseLiver disease
Provided herein are methods of treating or preventing an inflammatory liver disease in a subject, such as alcoholic liver disease (ALD), by administering to said subject an miR-155 antagonist.
Owner:UNIV OF MASSACHUSETTS
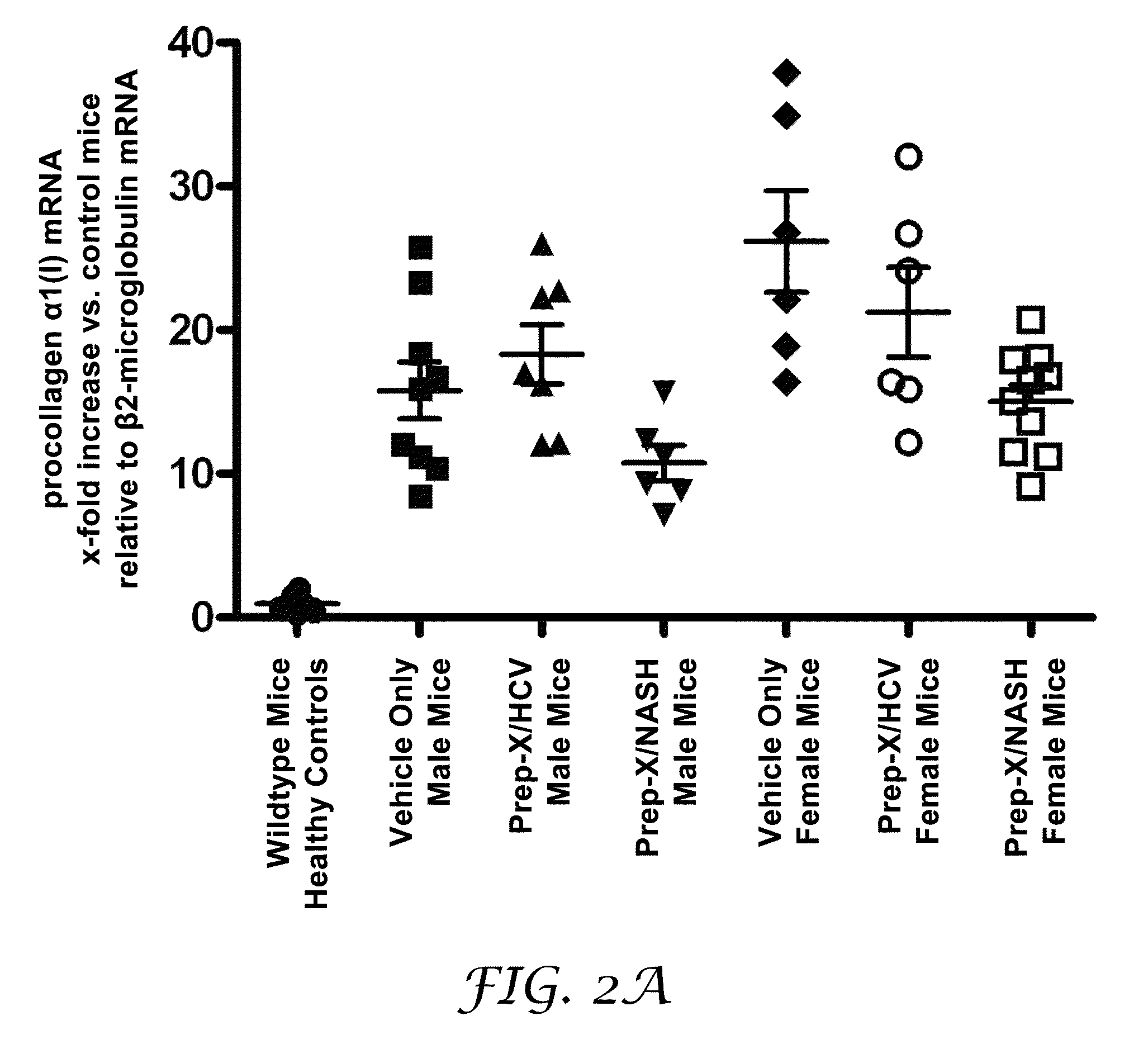
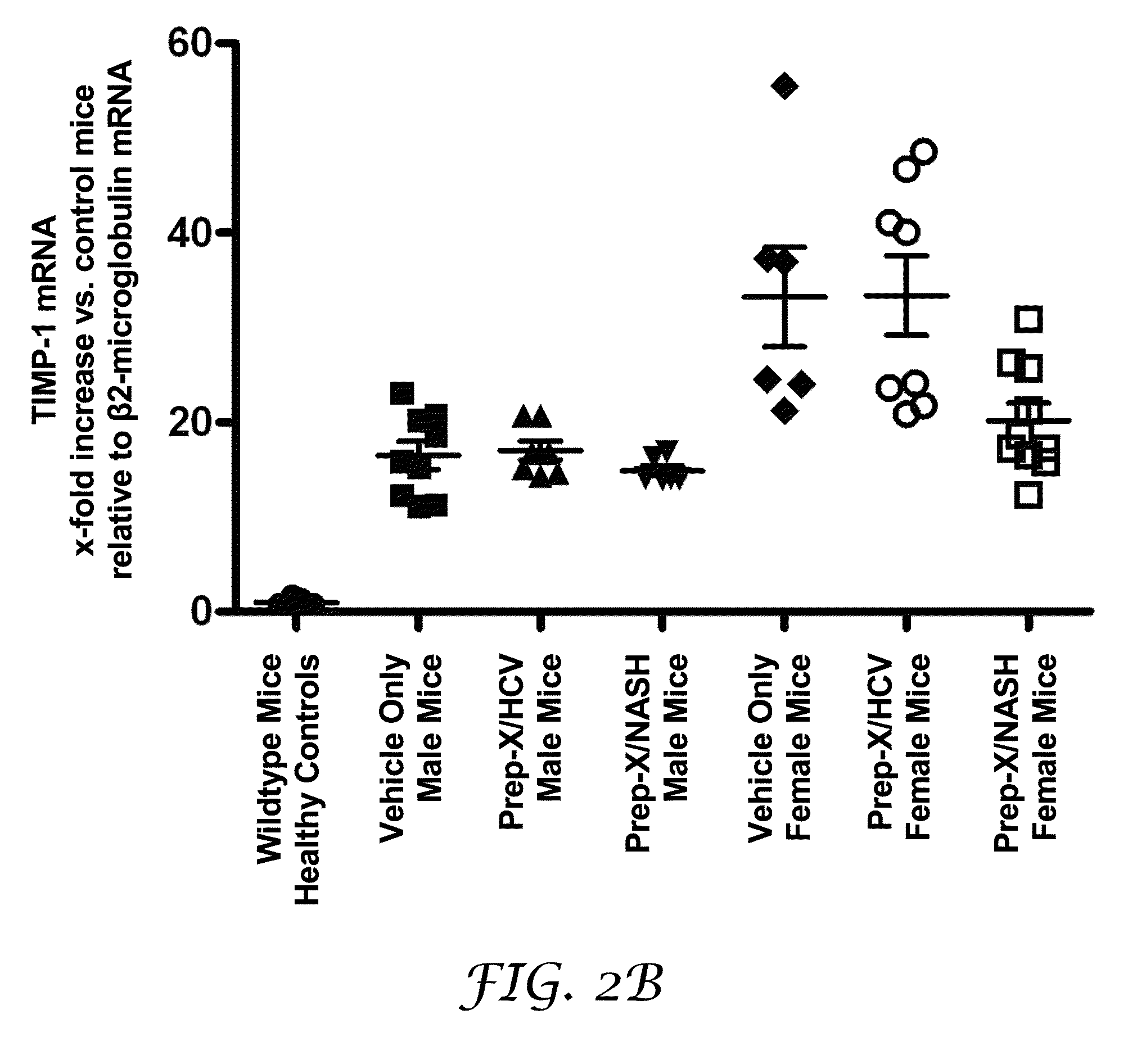
Method for establishing non-alcoholic fatty liver disease combined with viral hepatitis mouse model
InactiveCN104365543AAffect eatingGood repeatabilityViral/bacteriophage medical ingredientsAnimal feeding stuffLiver histologyHigh fat
The invention discloses a method for establishing a non-alcoholic fatty liver disease combined with viral hepatitis mouse model. The method comprises the steps that high-fat feed is used for feeding a C3H / HeN small mouse for 12 weeks, the body weight, the liver weight, transaminase, the glucolipid metabolism index and liver histological change of the small mouse are observed, and a non-alcoholic fatty liver disease small mouse is cultivated. Then 10 PFU of MHV-3 infected mice are selected, and the survival rate, the liver histology and intrahepatic virus replication of the infected mice are observed, so that the non-alcoholic fatty liver disease combined with viral hepatitis mouse model similar to a human disease process is established. The established model provides a forceful tool for virus dynamics, immunological changes and prevention and control measures systematically studying non-alcoholic fatty liver disease combined with viral hepatitis.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Kit for detecting alcoholic liver disease susceptibility
InactiveCN102146438AImprove accuracyImprove throughputMicrobiological testing/measurementEthanol dehydrogenaseLife habit
The invention discloses a kit for detecting alcoholic liver disease susceptibility. The kit detects three genes closely related with an alcoholic liver disease, namely an ADH2 gene of alcohol dehydrogenase, an ALDH2 gene of acetaldehyde dehydrogenase and a CYP2E1 gene of cytochrome P4502E1. The simultaneously detected SNP sites comprise an rs1229984 site of the ADH2 gene, an rs671 site of the ALDH2 and an rs2031920 site of the CYP2E1. Whether a detected crowd carries ''alcoholic liver disease susceptible genes'' can be clearly judged by using the kit, detecting a group of genes and sites related with the alcoholic liver disease susceptibility, using specific primers and probes and combining mononucleotide extension technology and micro array chip technology, and the alcoholic liver disease susceptible crowd is screened from the crowd, so that poor life habits are changed and the purpose of prevention is achieved.
Owner:UNION STEMCELL & GENE ENG +1
Traditional Chinese medicine decoction for dispelling effects of alcohol
InactiveCN103656428ATo promote metabolismReduce ethanol concentrationNervous disorderDigestive systemStomach PainSide effect
The invention discloses a traditional Chinese medicine decoction for dispelling effects of alcohol. The traditional Chinese medicine decoction is prepared from the following raw materials in parts by weight: 10-15 parts of rhizoma galangae, 15-20 parts of flower of kudzuvine, 5-10 parts of liquorice, 12-18 parts of mung bean flower, 10-15 parts of persimmon leaf, 5-10 parts of coralhead plant stem with leaf, 16-22 parts of hovenia dulcis thumb, 10-15 parts of root of rehmannia, 12-18 parts of poria with hostwood, 8-14 parts of elecampane, 10-15 parts of stevia rebaudian, 5-10 parts of nutmeg, 10-15 parts of fingered citron, 15-20 parts of Agastache rugosus, 11-17 parts of medicated leaven, 10-15 parts of citrus peel, 5-10 parts of Penthorum chinense Pursh, 8-14 parts of lalang grass rhizome, 10-15 parts of fructus aurantii and 5-10 parts of mangnolia officinalis. The medicines in the traditional Chinese medicine decoction have the effects of clearing away heat and toxic materials, regulating vital energy and reducing swelling, calming the adverse-rising energy and arresting vomiting, dispelling effects of alcohol, protecting the liver and stomach, benefiting qi for tranquillization and helping to produce saliva and slake thirst, can accelerate alcohol metabolism, reduce the ethanol concentration in blood and prevent the liver and stomach from being injured by alcohol, are used for treating uncomfortable symptoms such as drunk sleep, vomiting acid regurgitation, abdominal distension, stomach pain and mouth parched and tongue scorched caused by insobriety, and the traditional Chinese medicine decoction is exact in curative effect and fast in effectiveness, does not have a toxic or side effect, and also can be used for preventing and treating alcoholic stomach illness and alcoholic liver disease after being drunk for a long time.
Owner:青岛米品品服装有限公司
Methods and formulations for treating chronic liver disease
An anti-inflammatory and anti-fibrotic antioxidant formulation for treatment of hepatic oxidative stress and cirrhosis is disclosed. The antioxidant formulation can further include at least one of a hepatitis C virus-specific or a non-alcoholic steatohepatitis-specific formulation comprising one or more compounds to retard the progression of liver fibrosis and possibly reverse an established fibrosis. Methods of treatment or therapies for treating chronic liver disease and chronic hepatitis are also provided.
Owner:THE MARCUS FOUND
Extended release pharmaceutical formulations of s-adenosylmethionine
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. majorclinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
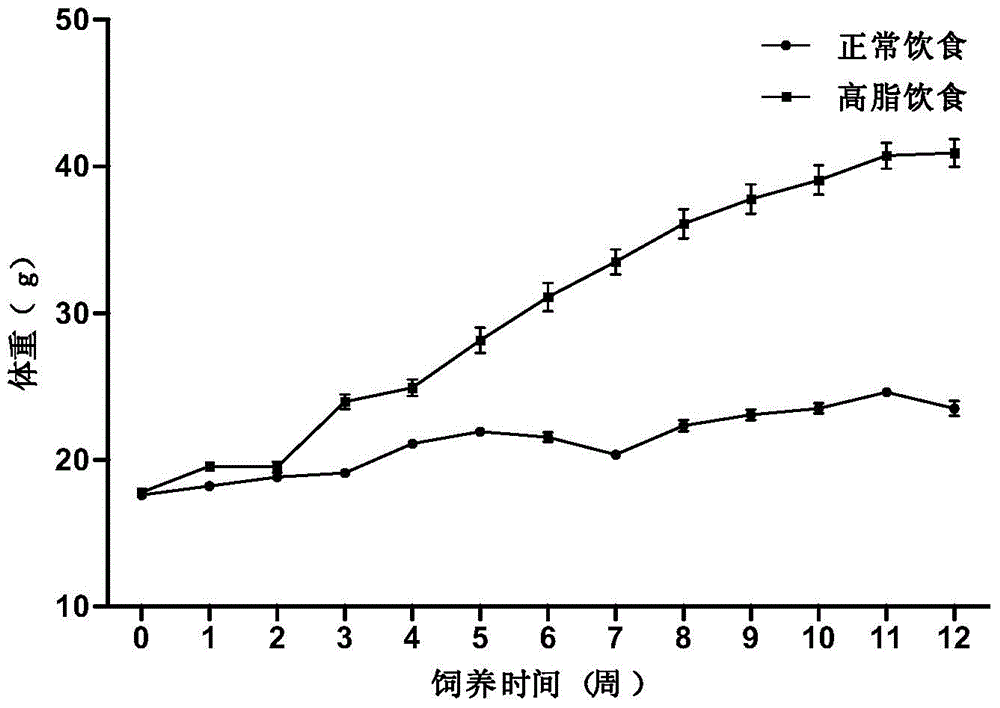
Feed for preparation of alcoholic liver animal model
The invention belongs to the technical field of medical animal model feed and discloses feed for preparation of an alcoholic liver animal model. The feed for mice is prepared from casein, L-cystine, DL-methionine, maltodextrin, meal cellulose, xanthan gum, choline bitartrate, a mineral premix, a vitamin premix, cholesterol, sodium cholate, fish oil, alcohol and water. The feed for preparation of an alcoholic liver animal model can gradually cause mouse alcoholic fatty liver, hepatitis and liver fibrosis. The model has features similar to human diseases and pathology has a certain development process. A modeling method is simple and easy and has a high success rate, a low death rate, good repeatability and a wide application prospect.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
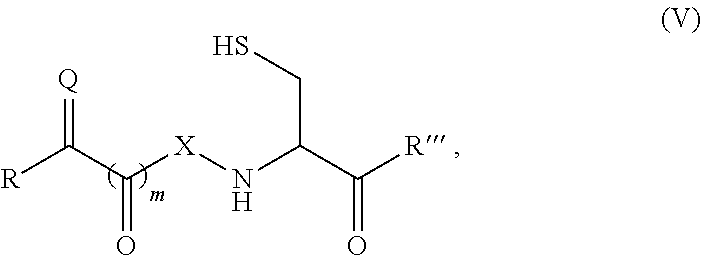
Site-specific modification of proteins through chemical modification enabling protein conjugates, protein dimer formation, and stapled peptides
ActiveUS20110263832A1Diminishment of extentAvoid spreadingHormone peptidesHybrid immunoglobulinsLupus erythematosusAdduct
The present invention generally provides methods for the site-specific modification of peptides, polypeptides, and proteins, e.g., granulocyte macrophage colony-stimulating factor, human superoxide dismutase, annexin, leptin, antibodies and the like, cytokines and chemokines, at their N-termini and at sites at which unnatural aminoacids have been introduced along the protein framework. The modifications described herein can be used for the synthesis and application of the adducts in radio-labeling, molecular imaging and protein therapeutic applications, and the treatment of disorders such as rheumatoid arthritis, lupus erythematosus, psoriasis, multiple sclerosis, type-1 diabetes, Crohn's disease, and systemic sclerosis, Alzheimer disease, cancer, liver disease (e.g., alcoholic liver disease), and cachexia.
Owner:ADVANCED PROTEOME THERAPEUTICS
Application of mixture containing four lactic acid bacteria strains in prevention and/or relieving of alcoholic liver diseases
The invention discloses an application of a mixture containing lactobacillus acidophilus LASW, enterococcus faecium TM39, lactobacillus fermentum LF33 and lactobacillus plantarum BCRC10069 in prevention and / or relieving of alcoholic liver diseases (ALD). The lactobacillus acidophilus LASW, the enterococcus faecium TM39 and the lactobacillus fermentum LF33 are collected in the China Center for Type Culture Collection (CCTCC) with the collection numbers of CCTCC NO:M204083, CCTCC NO:M2012547 and CCTCC NO:M2012548 respectively.
Owner:HUNGKUANG UNIV
Novel application of penthorum chinense pursh extract
The invention belongs to the field of medicines, in particular to a novel application of a penthorum chinense pursh extract. The novel application is an application of the penthorum chinense pursh extract in preparing a medicine for treating alcoholic liver diseases. The extract is an extract obtained after penthorum chinense pursh is extracted by water and deposited by alcohol, and has a definite therapeutic action to the alcoholic liver diseases.
Owner:四川合泰新光医药有限公司
Method and system for reducing the likelihood of developing NASH in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10245288B2Improvements in intestinal dysbiosisReduce intestinal permeabilityUnknown materialsNon-surgical orthopedic devicesDiseaseOxidized low density lipoprotein
A method for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease that involves providing an individual with an effective amount of a composition of bacteria modified using a Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR-associated system (CRISPR-Cas) or Clustered Regularly Interspaced Short Palindromic Repeats from Prevotella and Francisella 1 (CRISPR / Cpf1) system so that the bacteria is able to produce a therapeutically effective amount of anti-bodies to oxidized low density lipoprotein, with the modified bacteria preferably being from the Lactobacillus, Bifidobacterium, and Streptococcus species; and most preferably including L. reuteri bacteria modified using CRISPR-Cas and / or Cpf1 systems so that it is able to survive the conditions in the duodenum and jejunum of the small intestine of a human.
Owner:SEED HEALTH INC
Traditional Chinese medicine composition for treating non-alcoholic fatty liver disease
ActiveCN101919986AHas curative effect in the treatment of non-alcoholic fatty liver diseaseDigestive systemPlant ingredientsSedum sarmentosumSpleen
The invention discloses a traditional Chinese medicine composition for treating non-alcobolic fatty liver disease, which comprises the following bulk pharmaceutical chemicals in parts by weight: 10-20 parts of the root of red-rooted salvia, 10-20 parts of the root of kudzu vine, 20-40 parts of sedum sarmentosum, 10-20 parts of fructus ligustri lucidi, 10-20 parts of rhizoma atractylodis macrocephalae and 5-15 parts of rhizoma wenyujin concisum. The invention has a curing rule of tonifying spleen and clearing damp as well as eliminating blood stasis and dissolving phlegm, hits the pathogenesis of non-alcoholic fatty liver disease in traditional Chinese medical science, thus the composition has the curative effect on treating the non-alcoholic fatty liver disease.
Owner:INCREASEPHARM TIANJIN INST CO LTD
Compound traditional Chinese medicinal effective component preparation for resisting alcoholic fatty liver disease and application thereof
ActiveCN103800352APromote reversalLowers triglyceride (TG) levelsOrganic active ingredientsDigestive systemChlorogenic acidRat model
The invention belongs to the field of traditional Chinese medicines, relates to a compound traditional Chinese medicinal effective component preparation for resisting alcoholic fatty liver disease and application thereof, and in particular relates to a compound traditional Chinese medicinal component preparation comprising geniposide and chlorogenic acid and application thereof. The compound preparation is prepared from geniposide and chlorogenic acid, and can be processed into clinical common preparations by a conventional method, including granules, tablets, capsules and other oral solid preparations. Animal experiments indicate that the compound preparation can remarkably reduce the content of triglyceride of liver tissues of a rat model, and reduce the degeneration degree of liver fat and liver damage degree. The effects of the compound preparation are superior to those of single component. The compound preparation can be used for improving the effect of resisting alcoholic fatty liver, effectively preventing development of non-alcoholic fatty liver, and can be used for treating and preventing non-alcoholic fatty liver, chronic liver damage and other symptoms.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
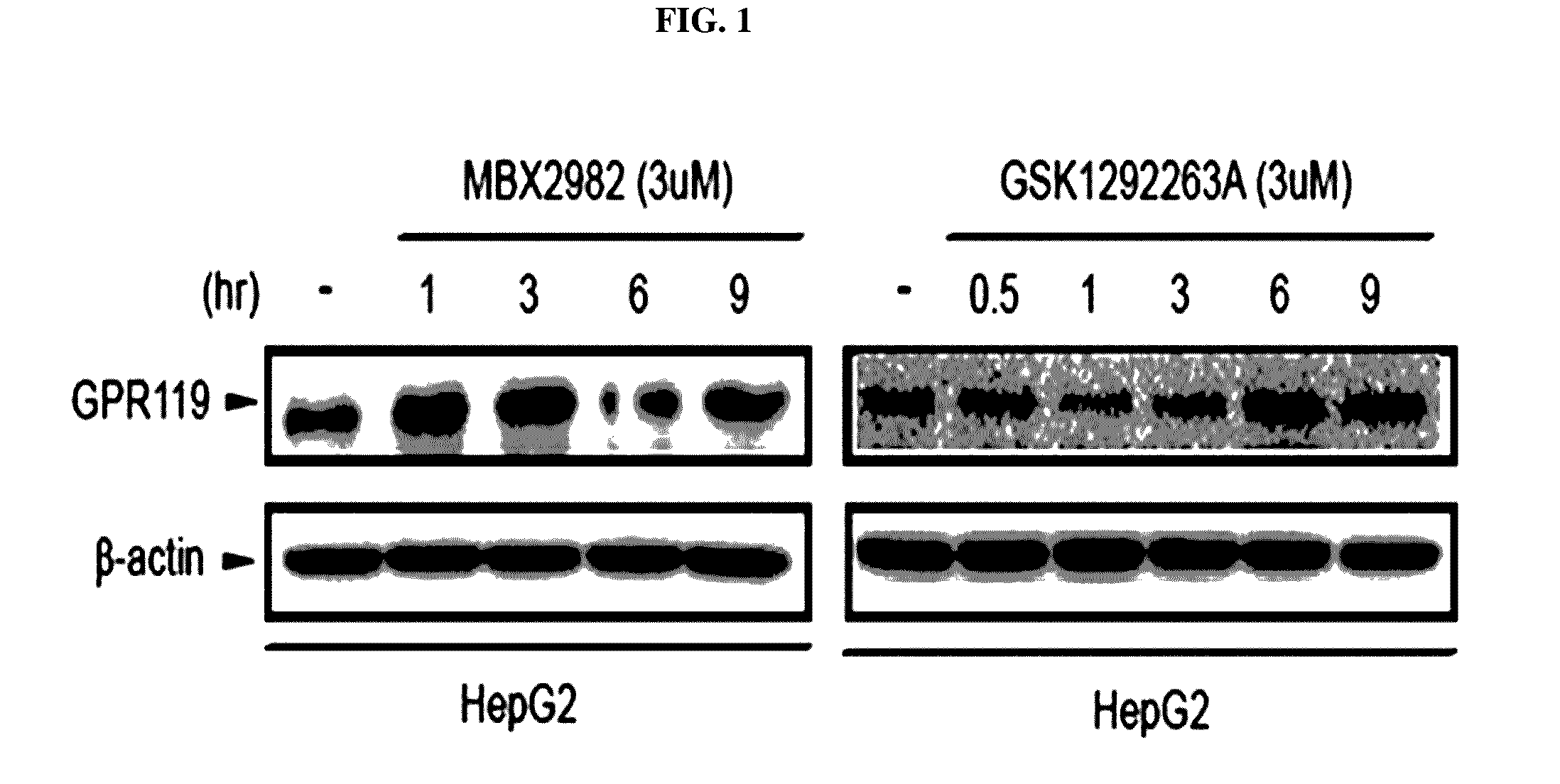
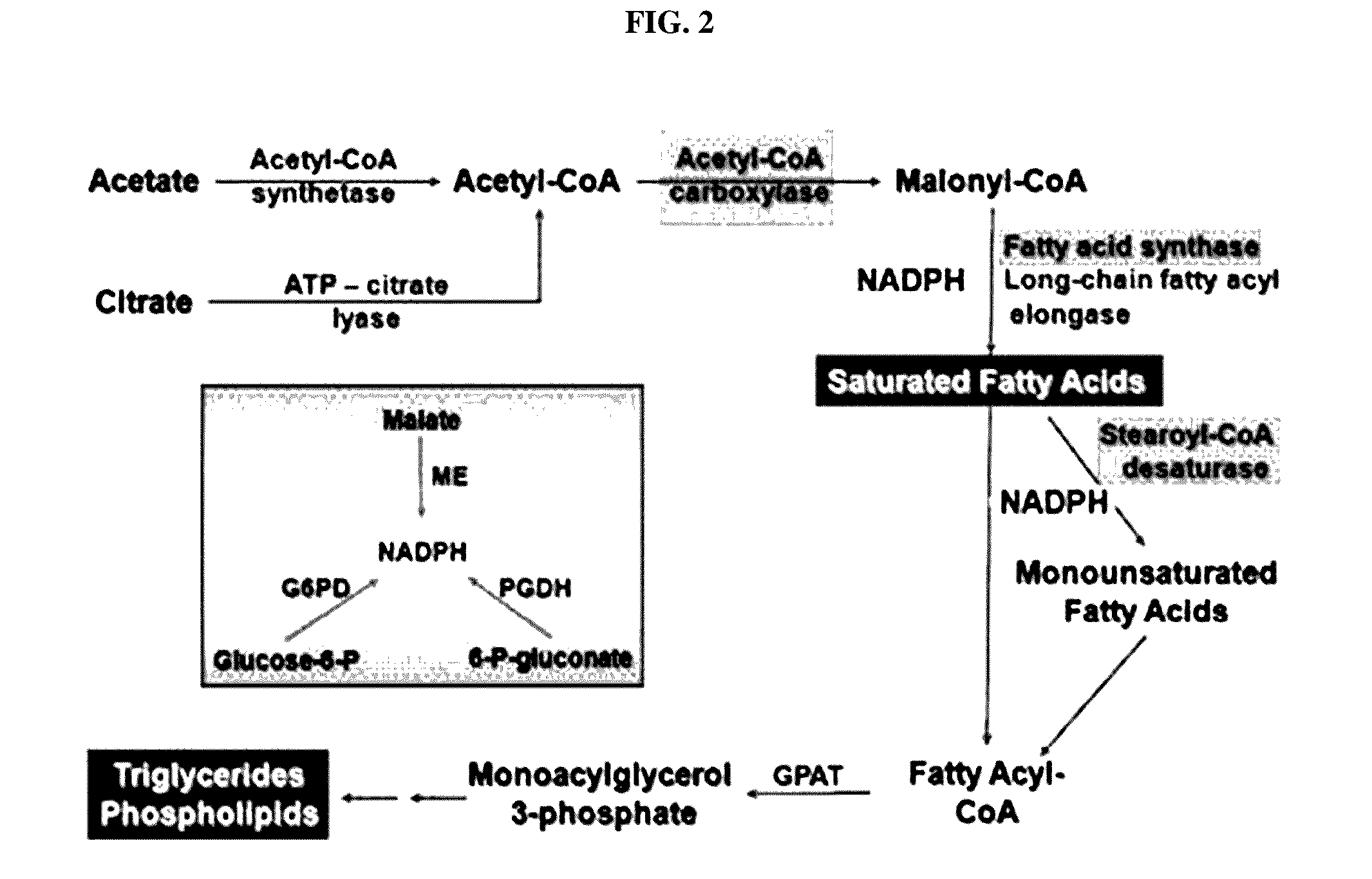
Pharmaceutical composition containing gpr119 ligand as active ingredient for preventing or treating non-alcoholic fatty liver disease
InactiveUS20170049773A1High expressionInhibitory activityOrganic active ingredientsDigestive systemGPR119Diabrezide
The present invention relates to a pharmaceutical composition containing a G protein coupled receptor 119 (GPR119) ligand as an active ingredient for preventing or treating non-alcoholic fatty liver disease. More particularly, it was confirmed that the GPR119 ligand, which has been developed as only an anti-diabetic drug, exhibits superior effects on the treatment of non-alcoholic fatty liver and the signal pathways in hepatocytes therefor differ from the signal pathways in the small intestine and the pancreas exhibiting anti-diabetic effects, whereby the GPR119 ligand can be useful to treat non-alcoholic fatty liver.
Owner:PHARMEDIX CO LTD
Application of celastrus orbiculatus alcohol extract in preparing medicine for treating non-alcoholic fatty liver disease (NAFLD)
InactiveCN103142679AHas antioxidant activityReduce sensitivityDigestive systemPlant ingredientsCelastrus orbiculatusPositive control
The invention discloses an application of a celastrus orbiculatus alcohol extract in preparing a medicine for treating non-alcoholic fatty liver disease (NAFLD). By adopting a high-fat diet induced guinea pig NAFLD model and taking simvastatin as a positive control medicine, the celastrus orbiculatus alcohol extract is orally taken for 8 weeks, then influence of the celastrus orbiculatus alcohol extract on NAFLD is observed, and a corresponding mechanism is explored; and the result indicates that the celastrus orbiculatus alcohol extract can be used for obviously improving the high-fat induced pathological alteration of liver tissue, easing the damage of liver cells, reducing the lipid accumulation in liver and suppressing oxidative stress reaction in liver. According to the invention, a new application of the celastrus orbiculatus alcohol extract is developed, and a new means of treating NAFLD is provided.
Owner:TAISHAN MEDICAL UNIV
Extended Release Pharmaceutical Formulations of S-Adenosylmethionine
InactiveUS20080206333A1Improved pharmacokinetic propertiesAct quicklyBiocideNervous disorderS-Adenosyl-l-methioninePharmaceutical formulation
Extended release formulations of S-methyladenosylmethionine (SAMe) are provided, as are methods of treating various disorders using extended release SAMe formulations. The extended release formulations may be used to treat a variety of disorders, including liver disorders, psychiatric disorders and joint disorders. Thus, extended release SAMe formulations may be used to treat alcoholic liver disease, fatty liver disease, hepatitis, generalized anxiety disorder, obsessive compulsive disorder, post traumatic stress disorder, panic disorder, and depressive disorders such as depression (e.g. major clinical depression) and dysthymia.
Owner:METILEJSHN SAJENSIS INT SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com