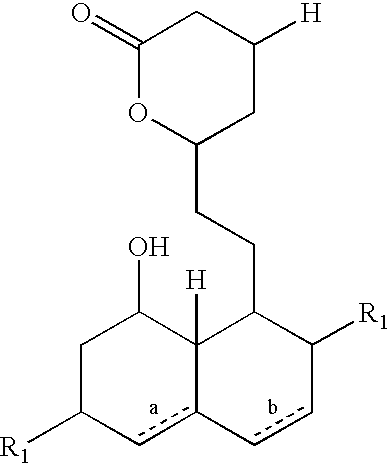
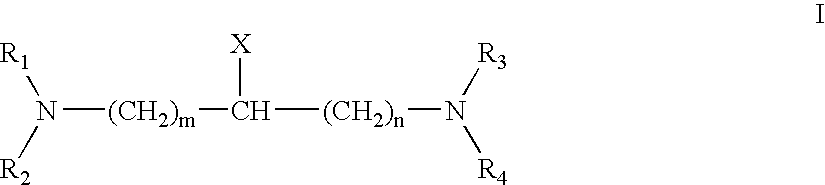
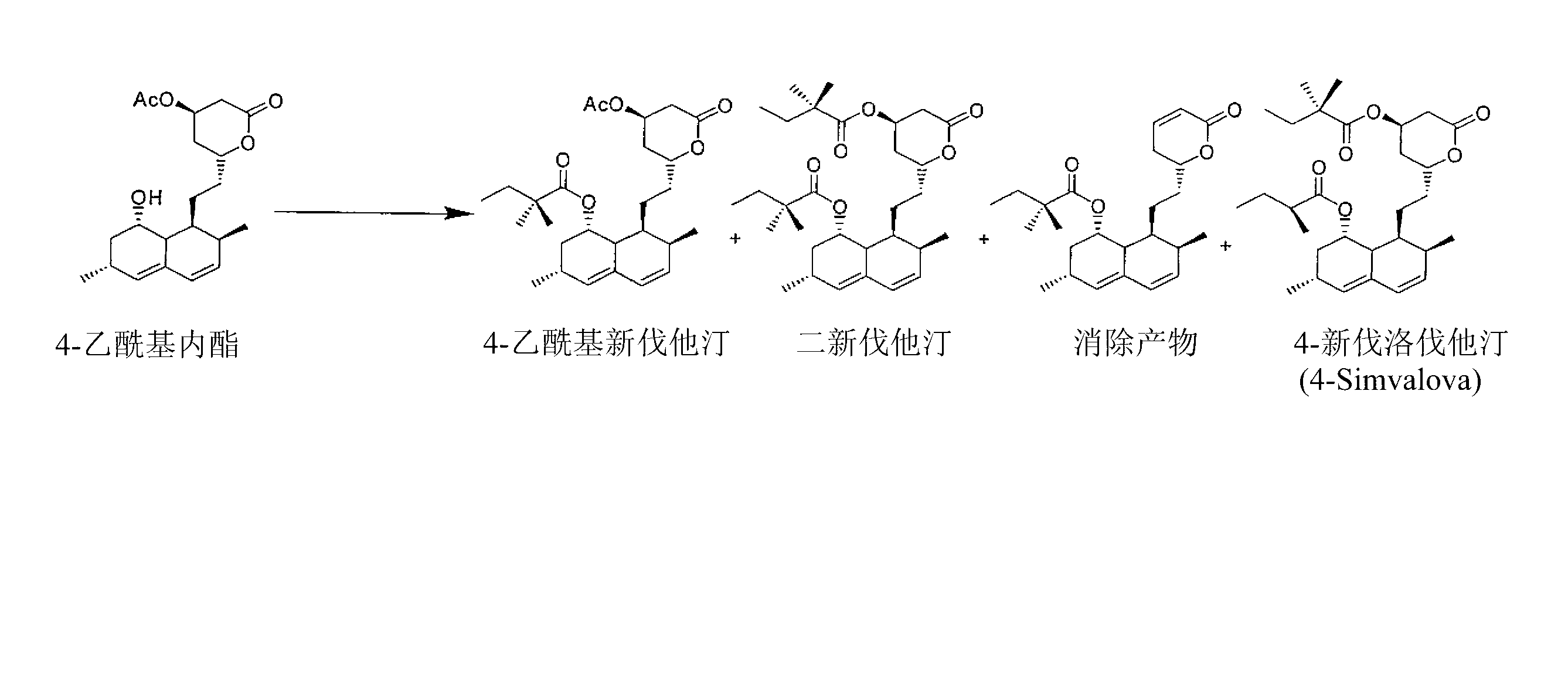
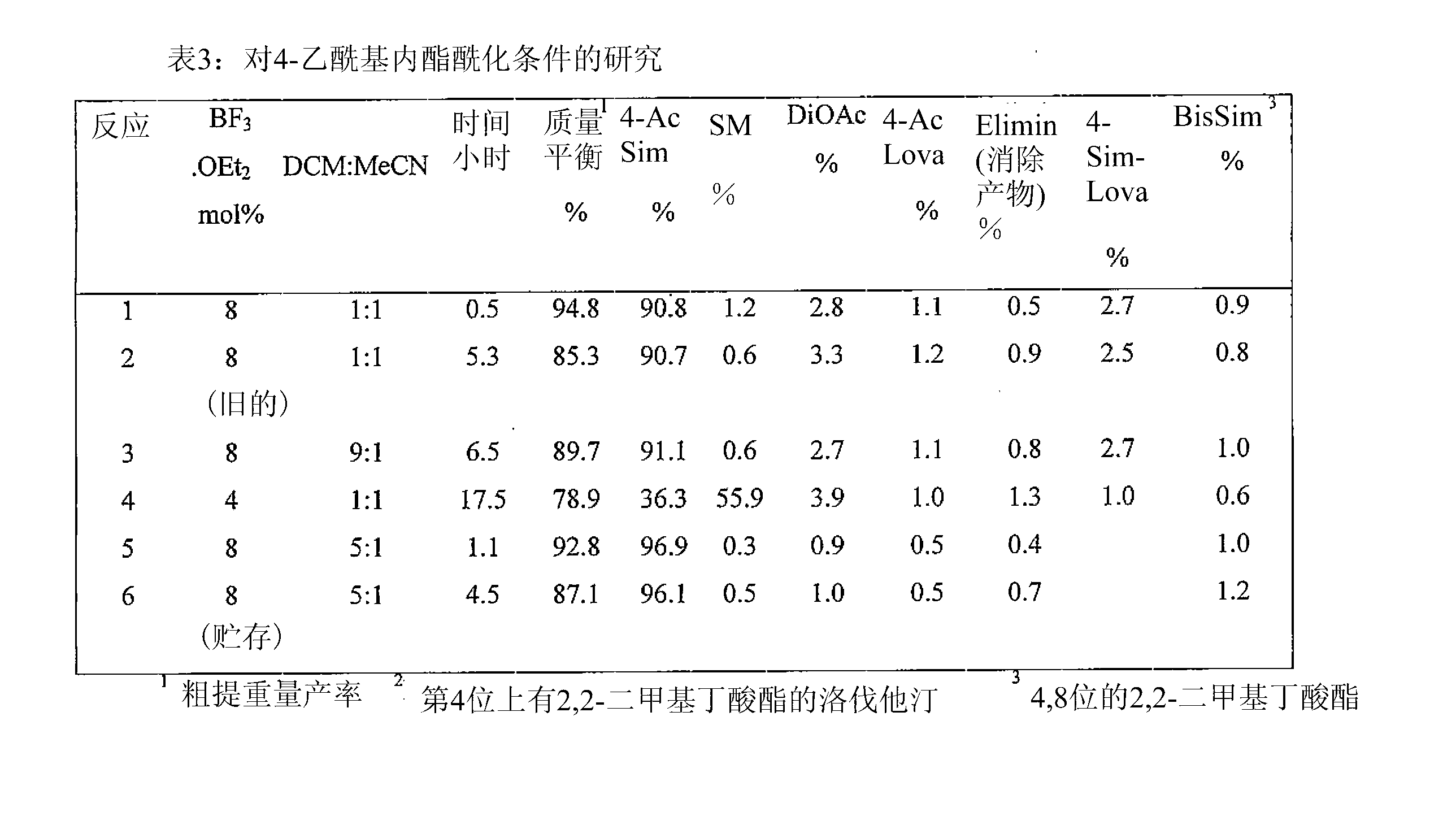
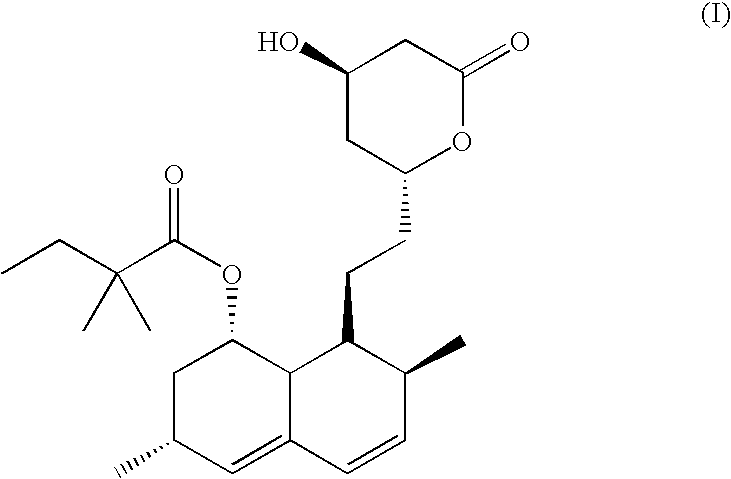
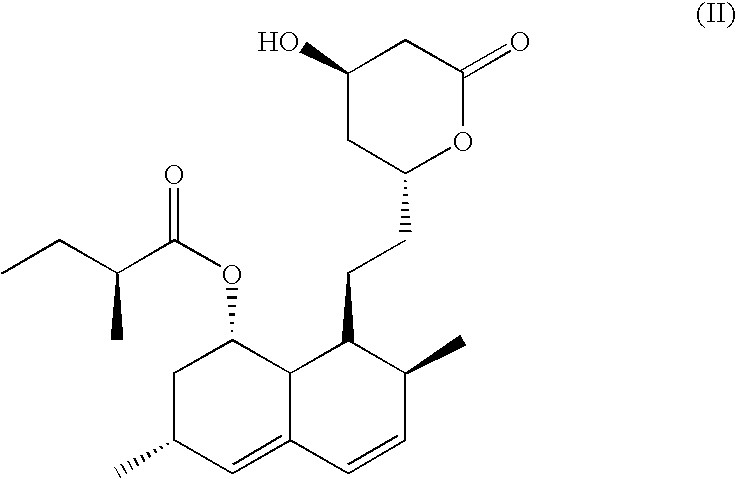
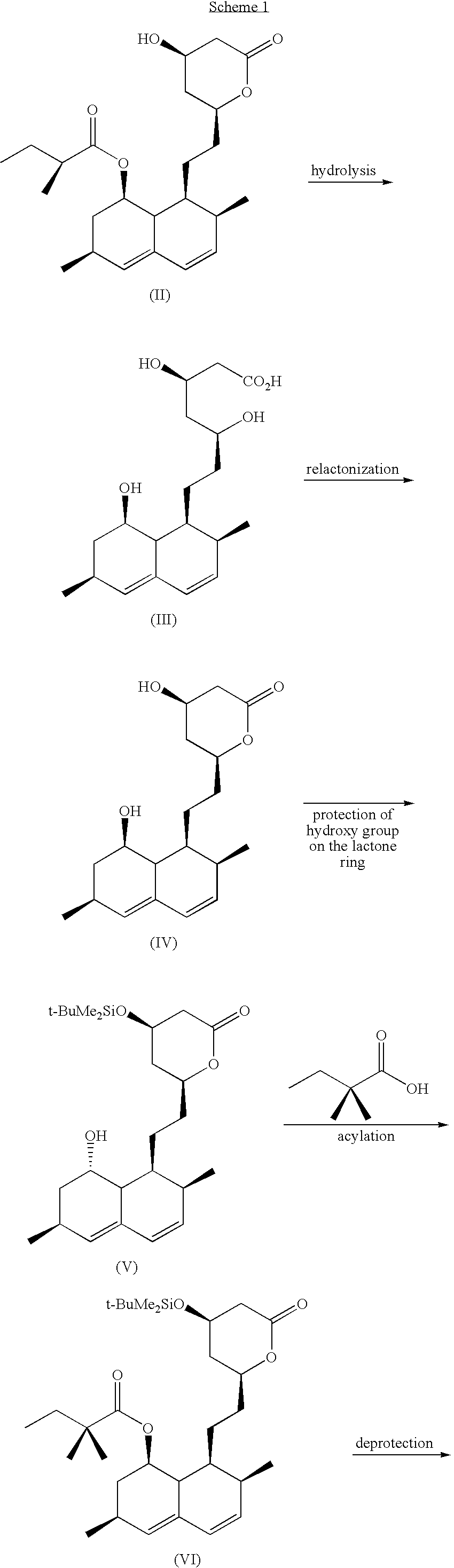
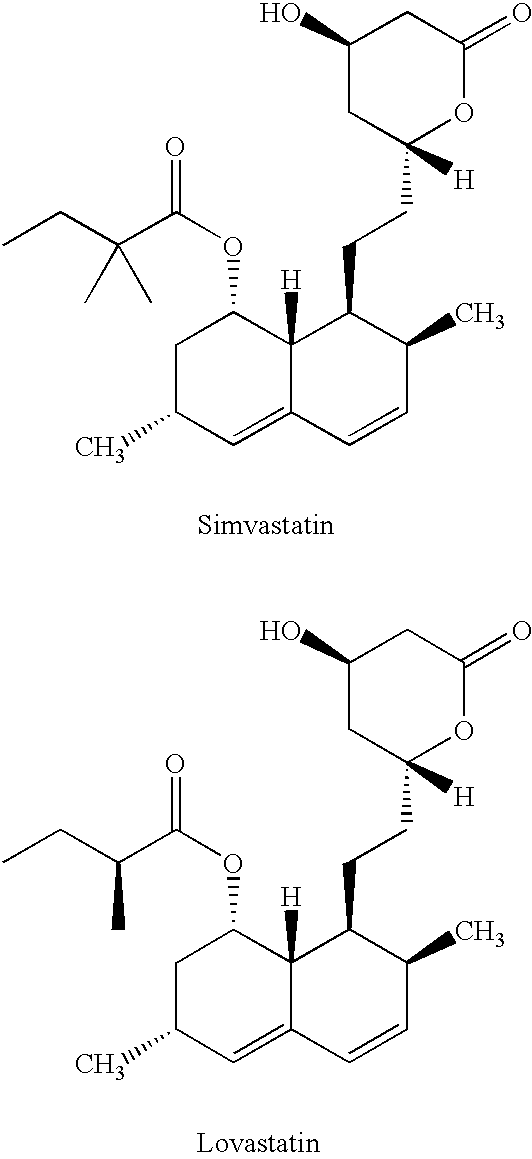
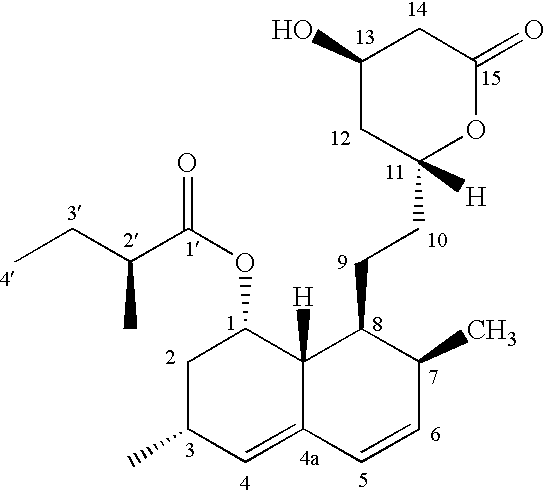
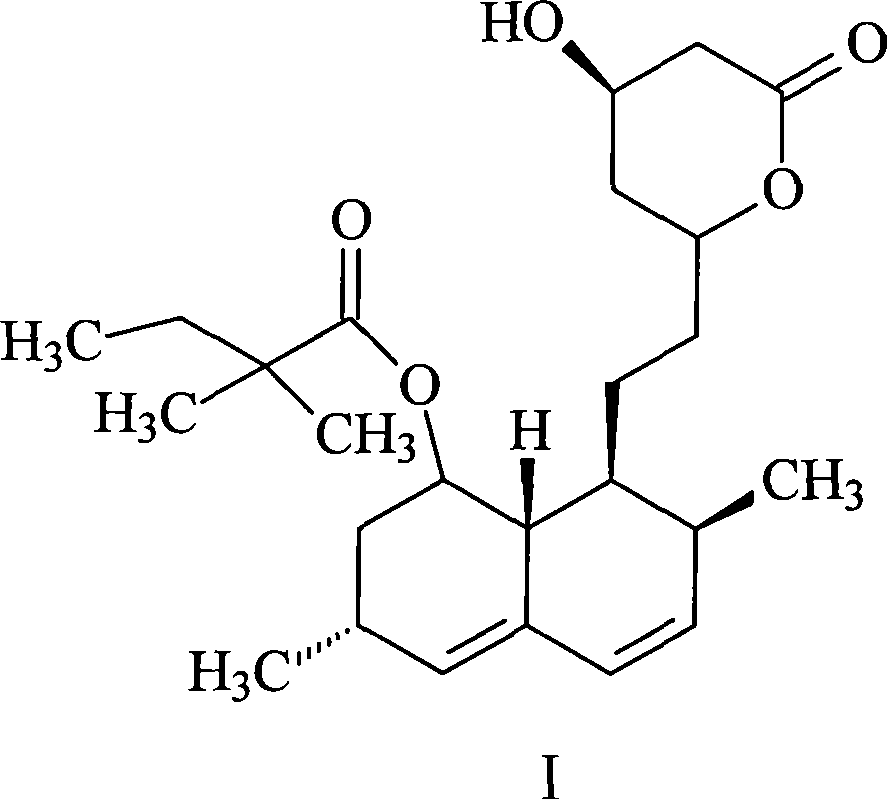
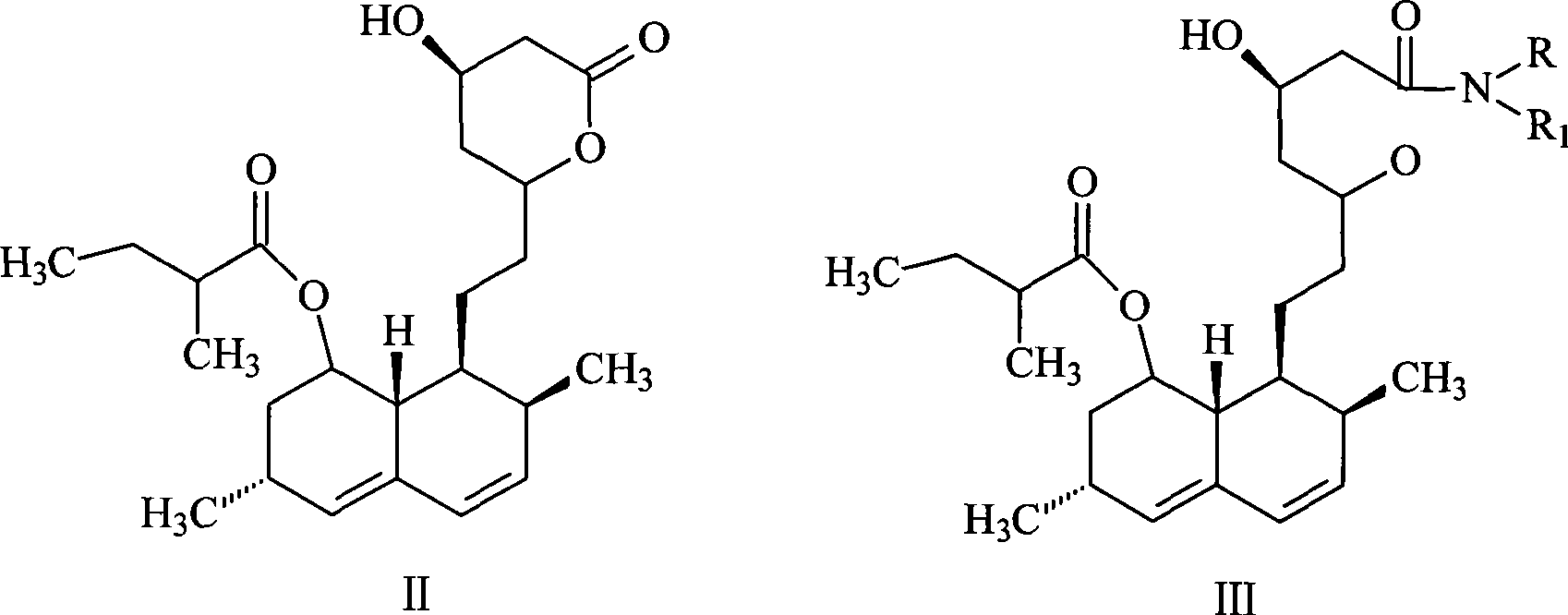
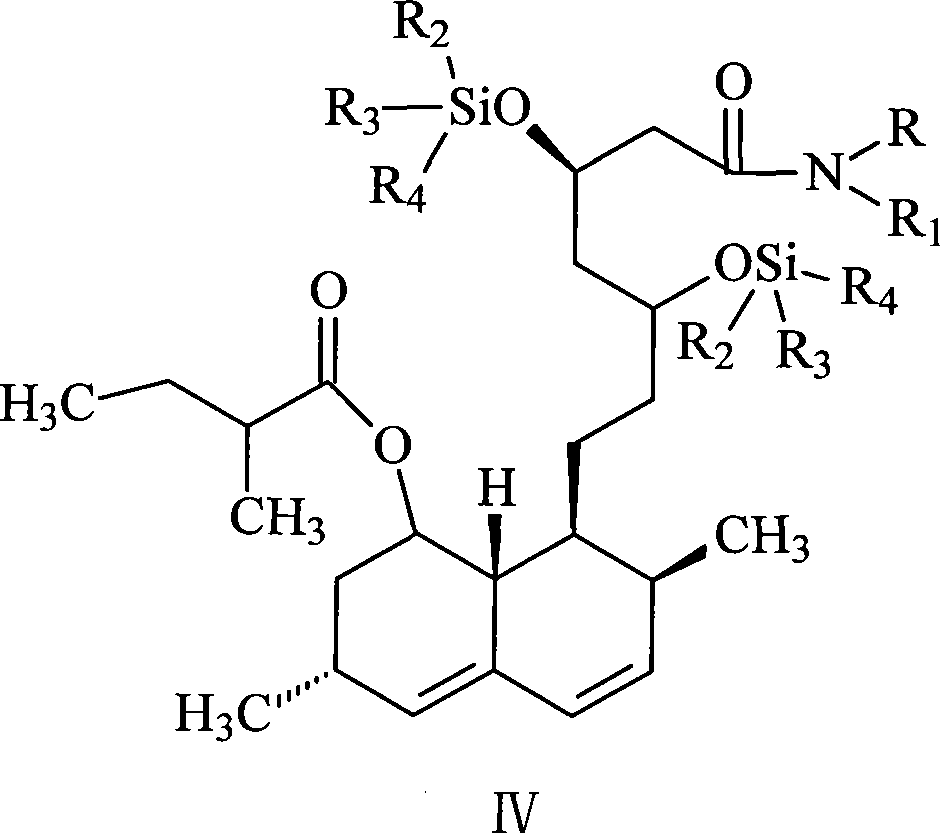
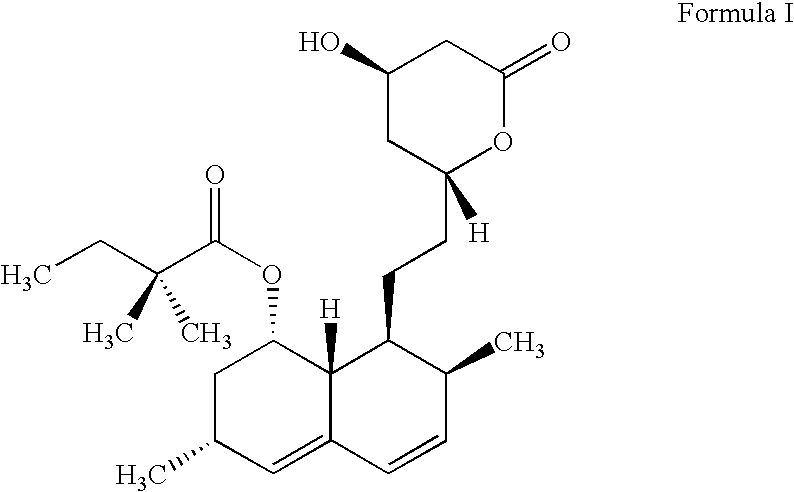
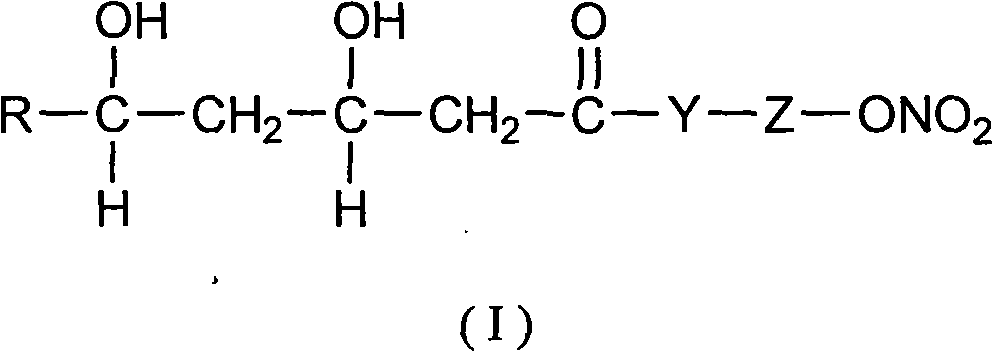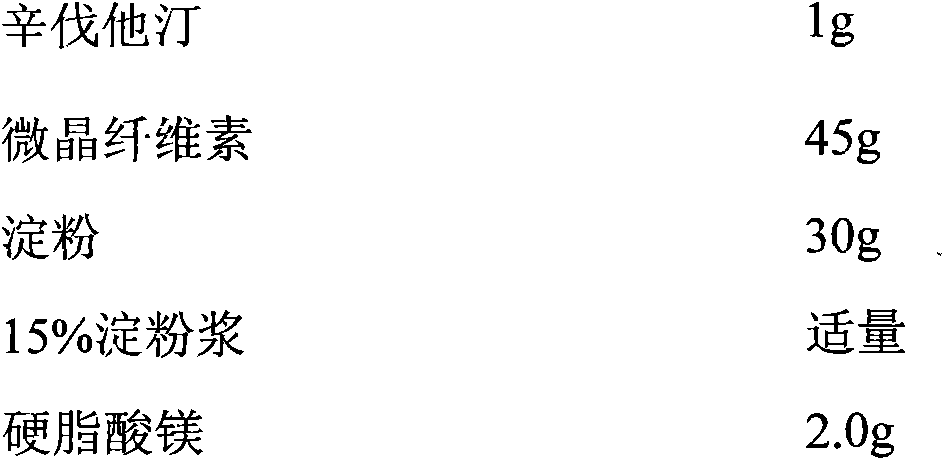Patents
Literature
364 results about "Simvastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
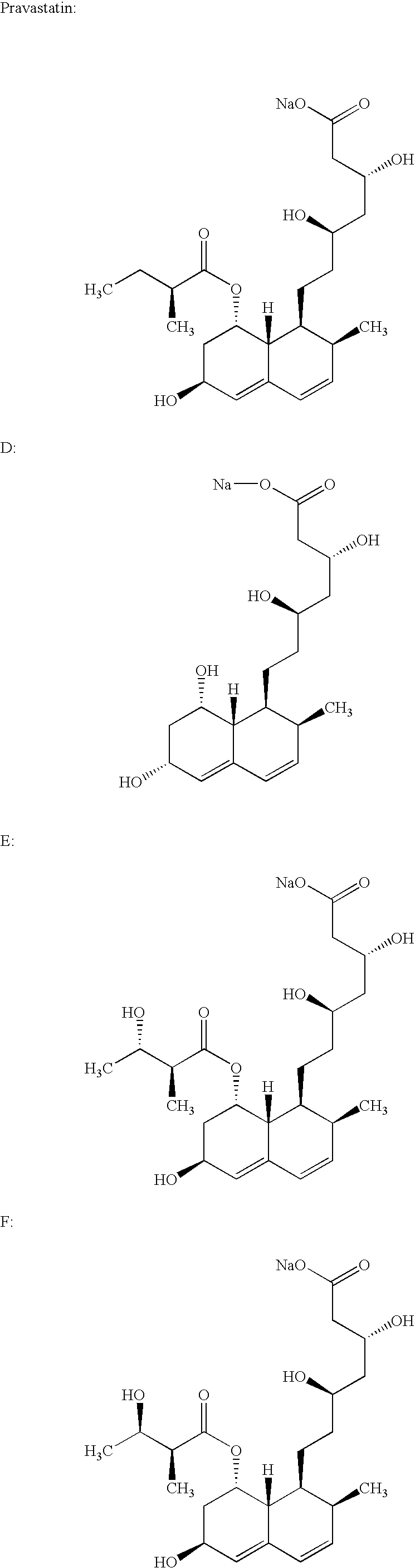
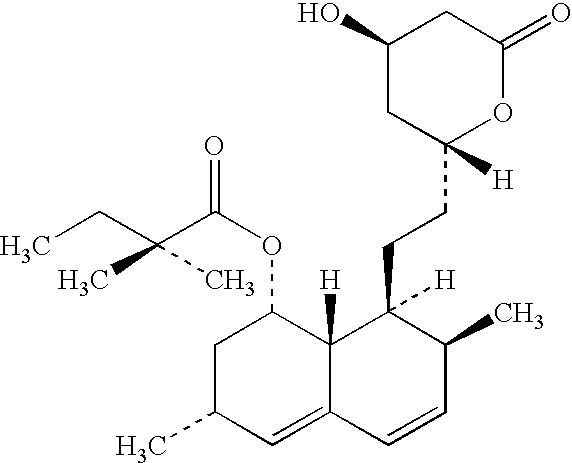
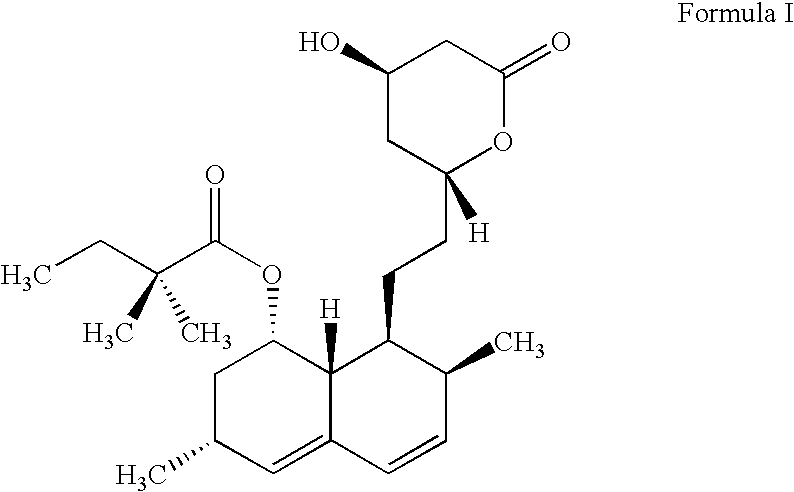
Simvastatin is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood.
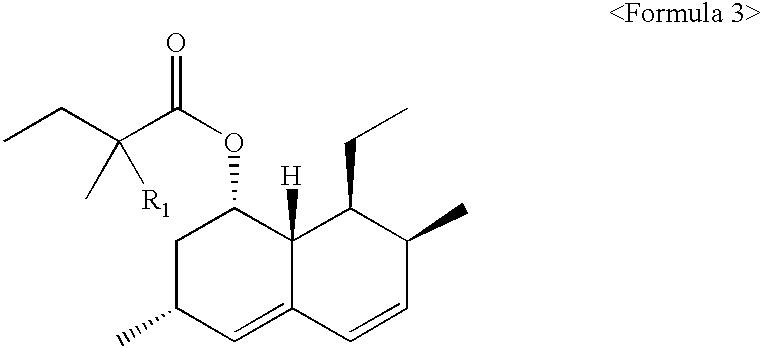
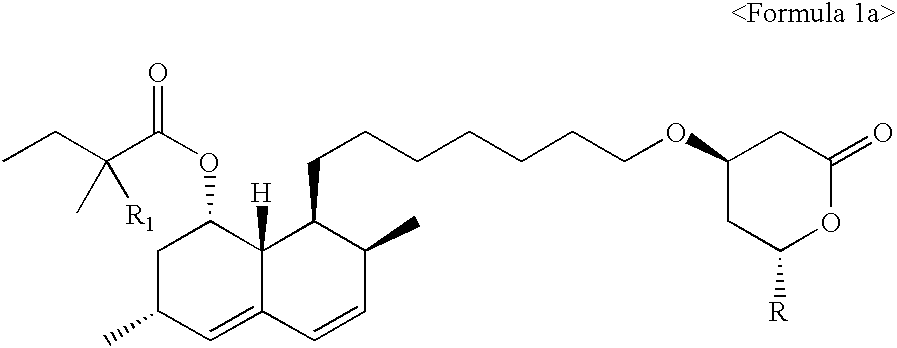
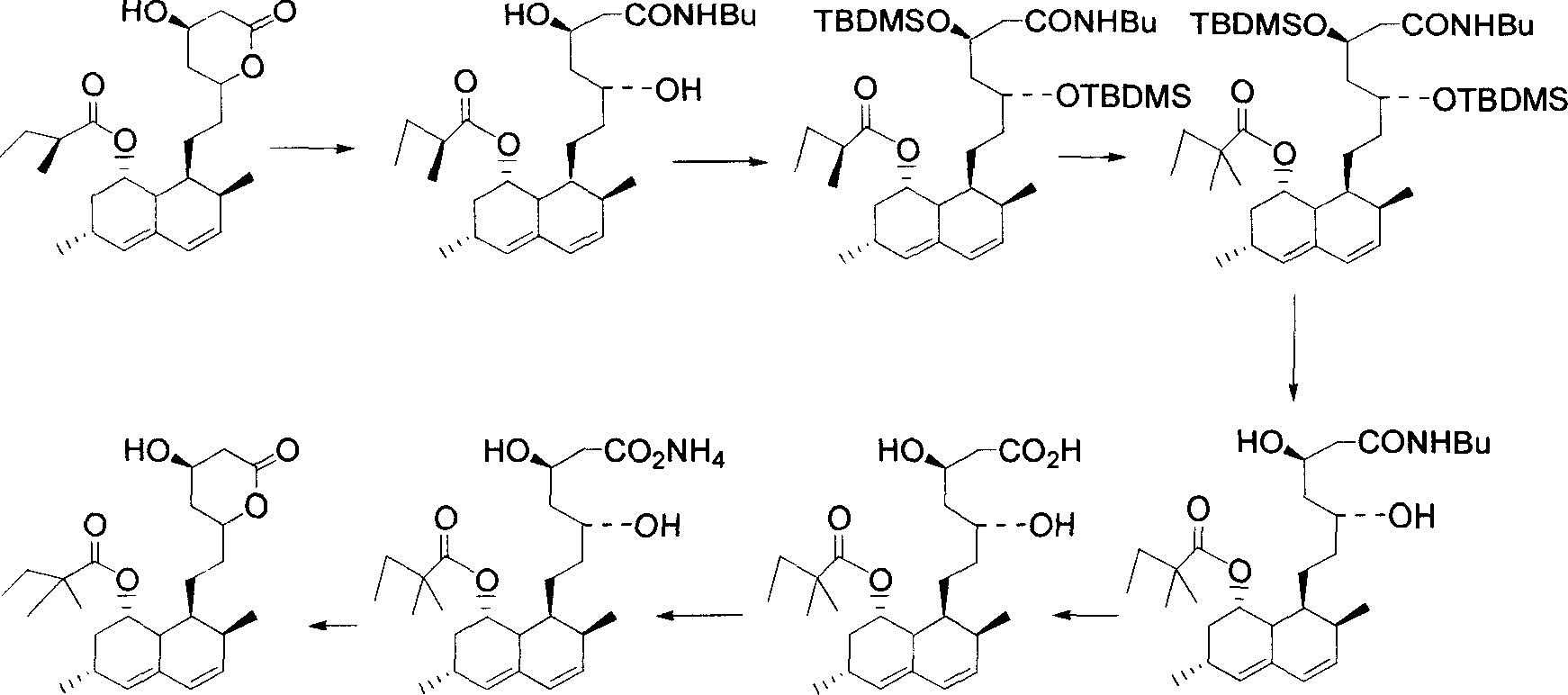
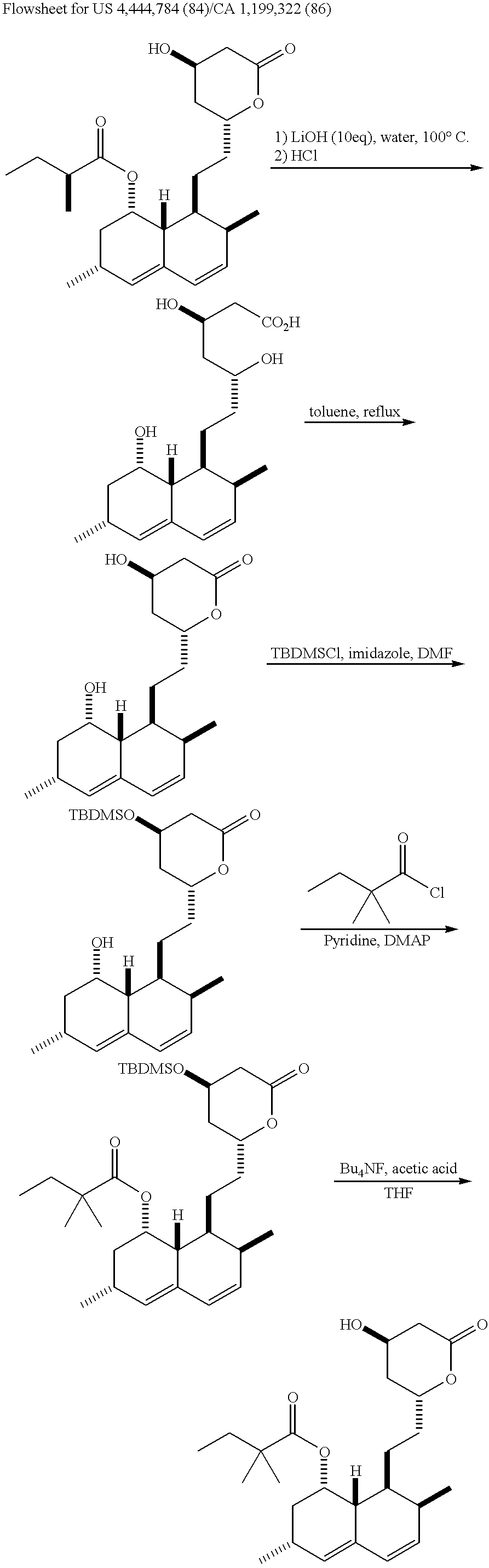
Process for producing simvastatin
InactiveUS6331641B1Improve efficiencyMild conditionsOrganic chemistryBulk chemical productionHMG-CoA reductaseAlcohol
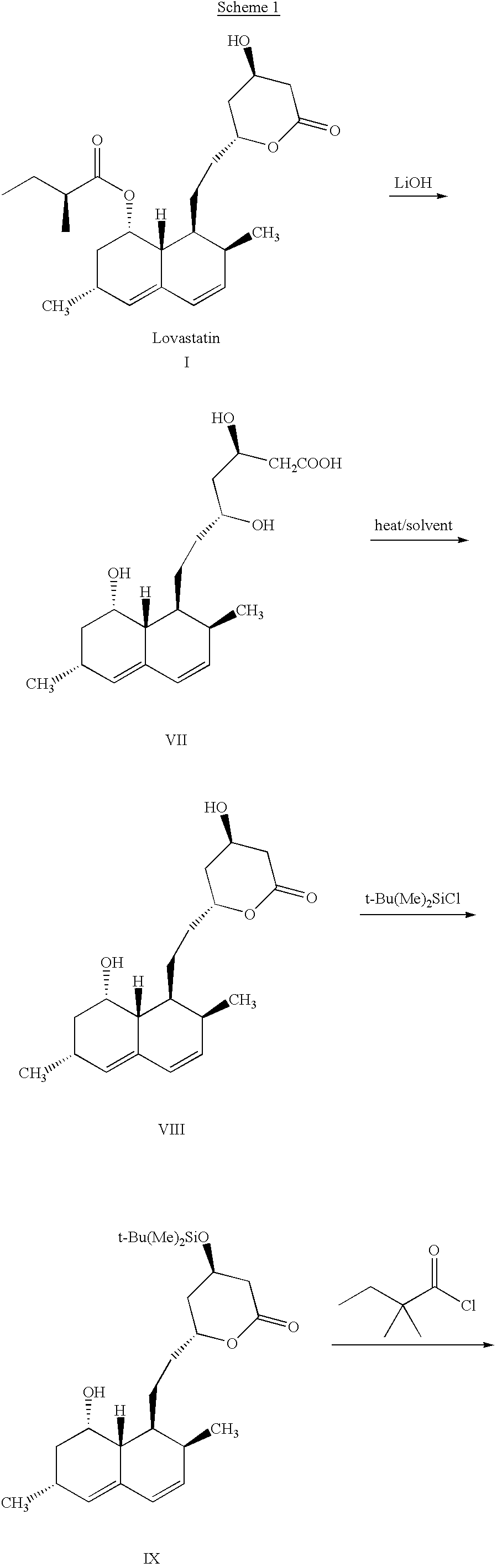
This invention provides an easy and efficient process for producing a simvastatin of great use as an HMG-CoA reductase inhibitor, which comprises deacylation of lovastatin with an inorganic base and a secondary or tertiary alcohol and subjecting the resulting diol lactone to selective protection with a ketal or acetal protective group, acylation and deprotection-lactonization to give simvastatin.
Owner:KANEKA CORP
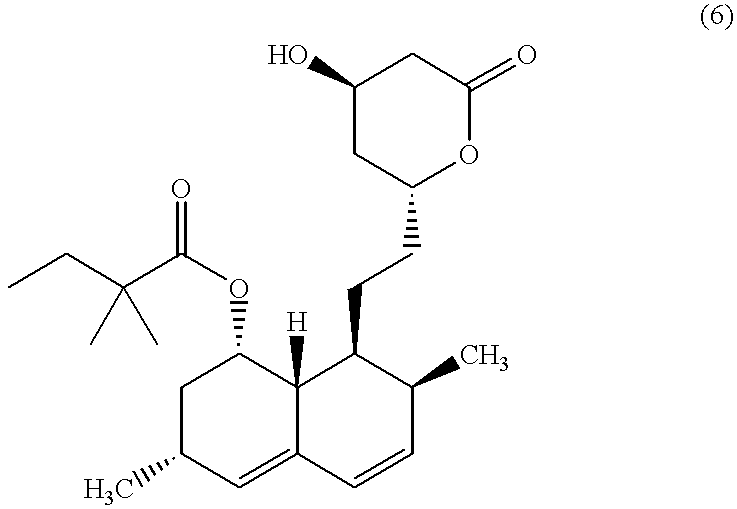
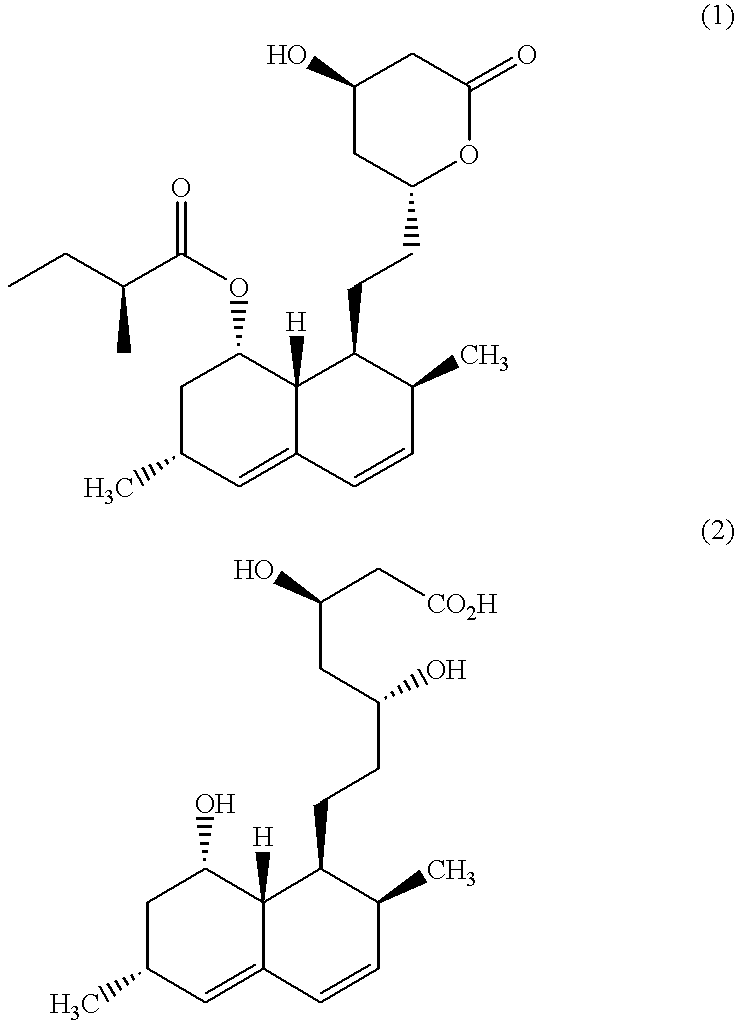
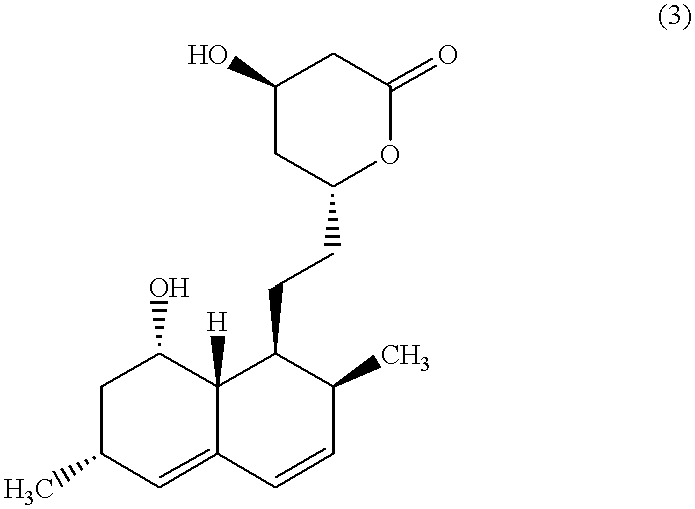
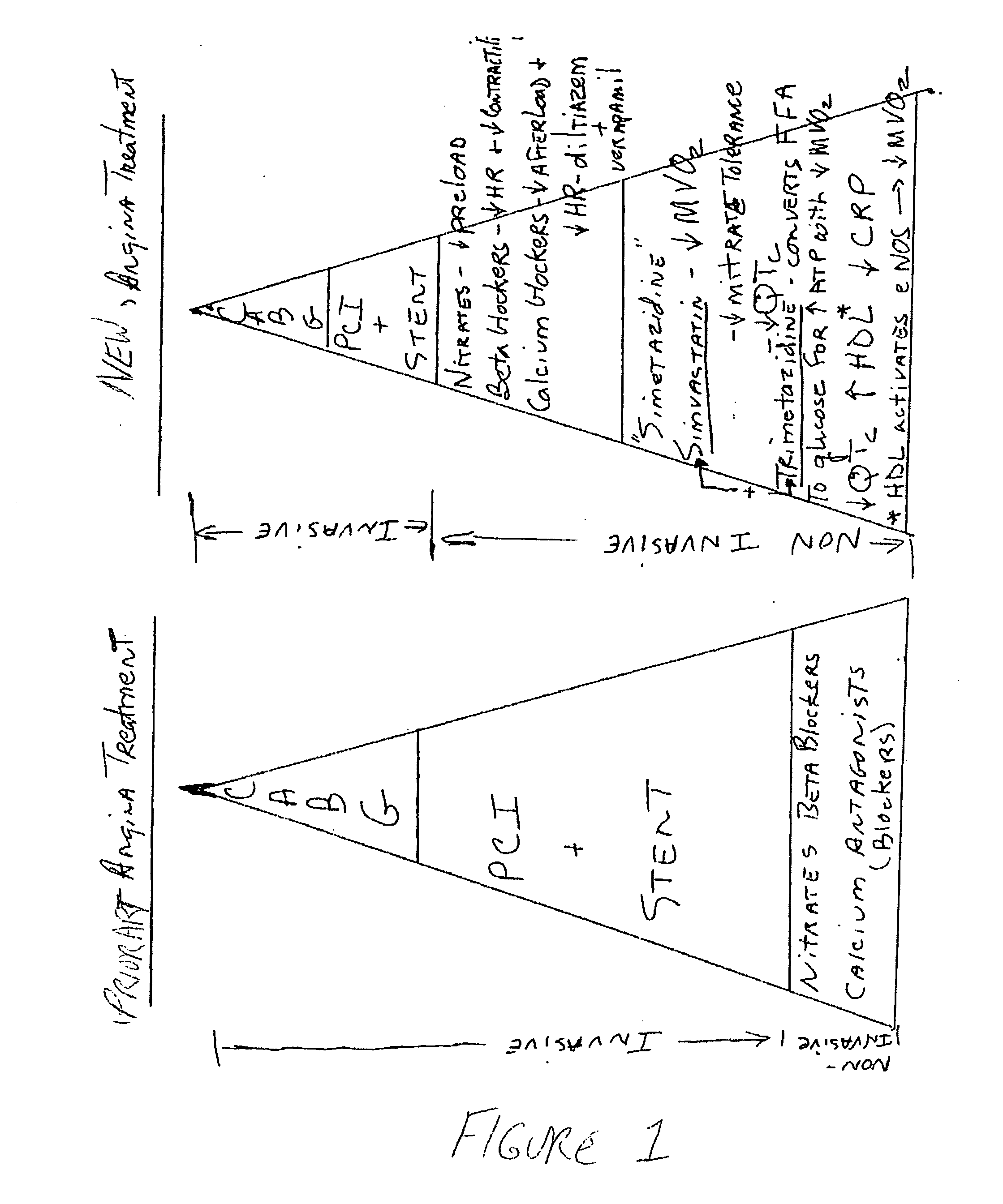
Combination therapy for endothelial dysfunction, angina and diabetes
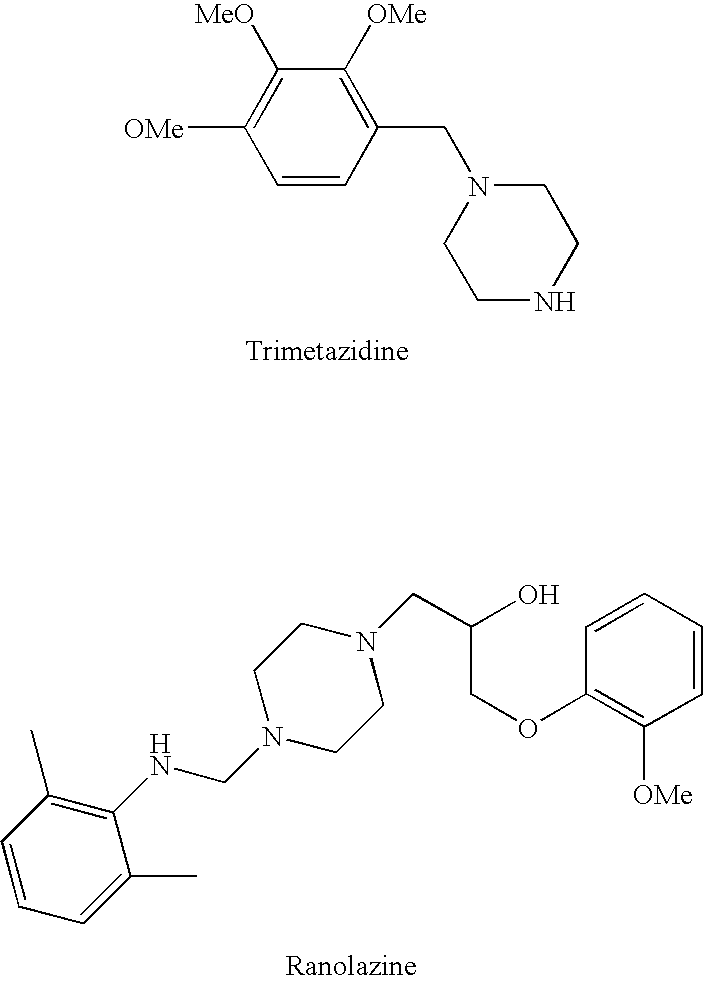
InactiveUS20060205727A1Control blood sugar levelsIncrease productionBiocideMetabolism disorderHMG-CoA reductaseTrimetazidine
The combination of a HMG CoA reductase inhibitor like a statin, such as simvastatin, with a pFox inhibitor such as trimetazidine (“Simetazidine”) is particularly advantageous for treatment of end-stage complications, such as acute coronary syndrome (ACS) and chronic angina, especially in type II diabetics. The combination therapy is also useful in the treatment and / or prevention of chronic heart failure (CHF) and peripheral arterial disease (PAD). The combination of a nitric oxide (NO) mechanism with increased NO production with pFox inhibition simultaneously treats both the effect and the cause of angina. One or more oral hypoglycemic compounds (biguanides, insulin sensitizers, such as thiazolidinediones, α-glucosidase inhibitors, insulin secretagogues, and dipeptidyl peptidase IV inhibitors), protein kinase C (PKC) inhibitors, and acetyl-CoA carboxylase inhibitors can also be used in combination with the HMG CoA reductase inhibitors and / or pFox inhibitors, especially in type II diabetics, to control glucose levels and treat endothelial dysfunction. The drugs can be given in combination (e.g. a single tablet) or in separate dosage forms, administered simultaneously or sequentially. In the preferred form the statin is given in a dose of between 5 and 80 mg / day in two separate doses, and the pFox inhibitor is administered in a sustained or extended dosage formulation at a dose of 20 mg three times a day or 35 mg two times a day. The dose of the oral hypoglycemic, PKC inhibitor, or acetyl-CoA carboxylase inhibitor varies with the type of drug used.
Owner:HONG KONG NITRIC OXIDE
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
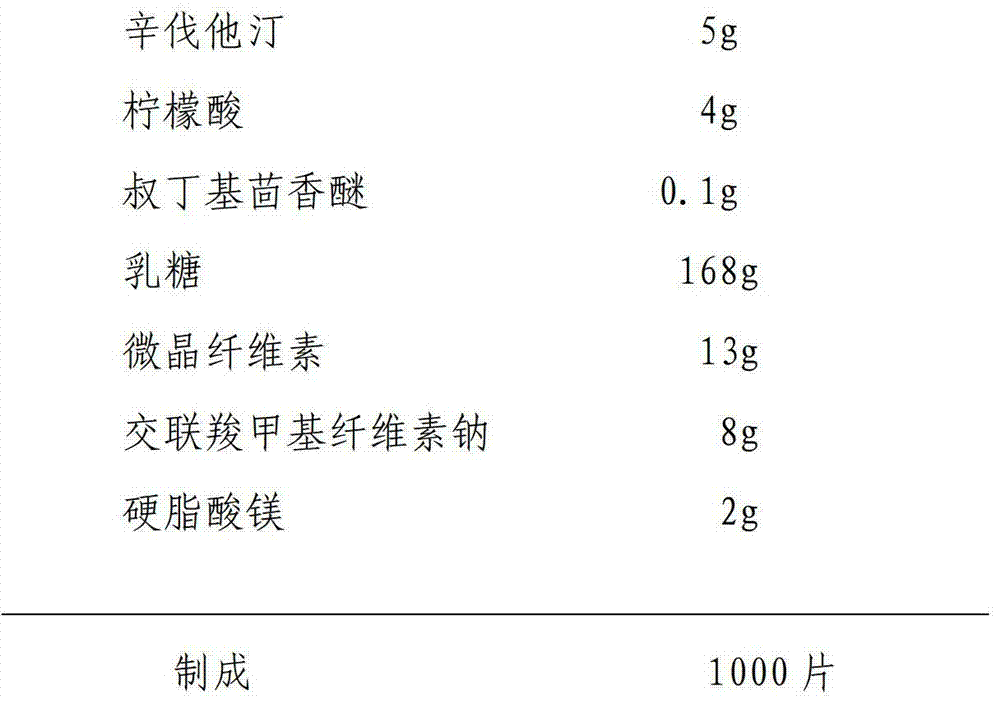
Stable simvastatin oral tablet and preparation method thereof
InactiveCN102091050AEffective isolationGood film formingOrganic active ingredientsMetabolism disorderMedicineAntioxidant
The invention provides a stable simvastatin oral tablet which is prepared from the following components in percentage by weight: 10-20% of simvastatin, 0.01-0.04% of antioxidant, 0.5-2.0% of acidifier, 70-80% of filler, 5-10% of disintegrant, 1-1.2% of lubricant and 0.5-2% of binder. The invention also provides a method for preparing the stable simvastatin oral tablet. By strictly controlling the moisture of a finished product, the doses of the antioxidant and acidifier in the components are obviously reduced, the product stability is better, and the safety is higher.
Owner:CHONGQING KERUI PHARMA GRP
Methods for treating disorders associated with hyperlipidemia in a mammal
The invention is directed to methods for treating hyperlipidemia in a mammal. The methods involve combination therapies using a microsomal triglyceride transfer protein (MTP) inhibitor (for example, BMS-201038 and implitapide) and a HMG-CoA reductase inhibitor (for example simvastatin or atorvastatin). Co-administration of the MTP inhibitor with the HMG-CoA reductase inhibitor produces a therapeutic benefit, for example, a reduction in the concentration of cholesterol and / or triglycerides in the blood stream, but with fewer or reduced side effects than when higher dosages of the MTP inhibitor are used during monotherapy to provide the same or similar therapeutic benefit.
Owner:AEGERION PHARM INC
A kit for simultaneously detecting statin metabolizing gene multisite mutations
The invention relates to a kit for simultaneously detecting statin metabolizing gene multisite mutations. The kit includes primer pairs and probe pairs for detecting APOE, SLCO1B1, CETP, ABCB1 and MTHFR gene sites. MGB (minor groove binder) probes and a real-time fluorescent PCR technique are applied in the kit. The kit has advantages of capability of being time saving and convenient, high sensitivity, capability of allowing positive and negative coincidence rates of a sample to be 99.5% or above, and the like. The kit is mainly used for personalized medication assisted diagnosis of statins such as simvastatin, atorvastatin and pravastatin.
Owner:NINGBO MEIJING MEDICAL TECH
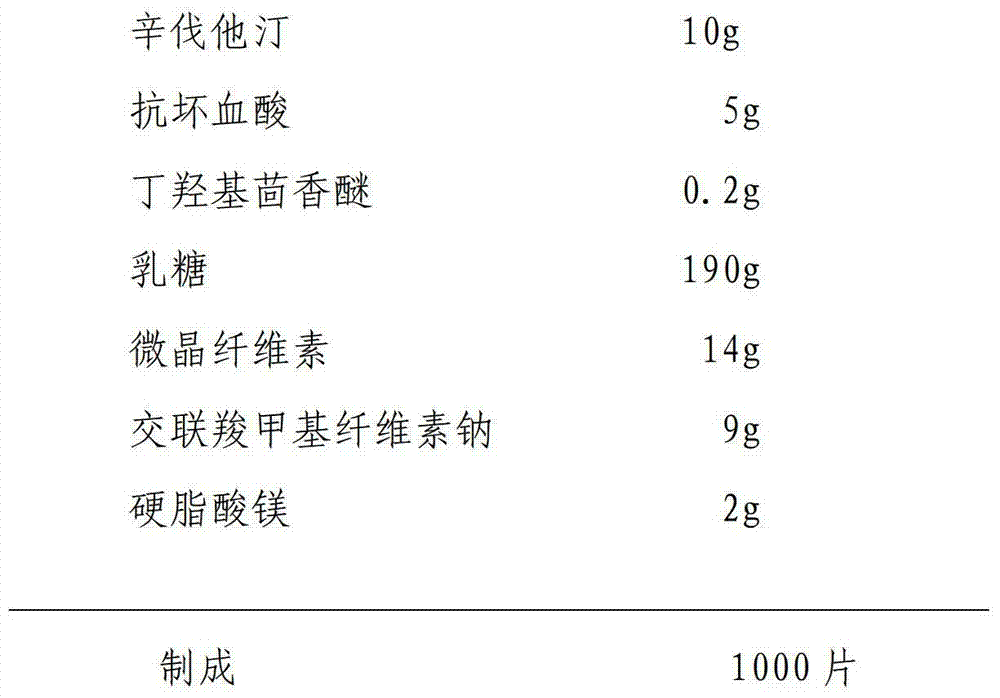
Simvastatin tablet and preparation method thereof
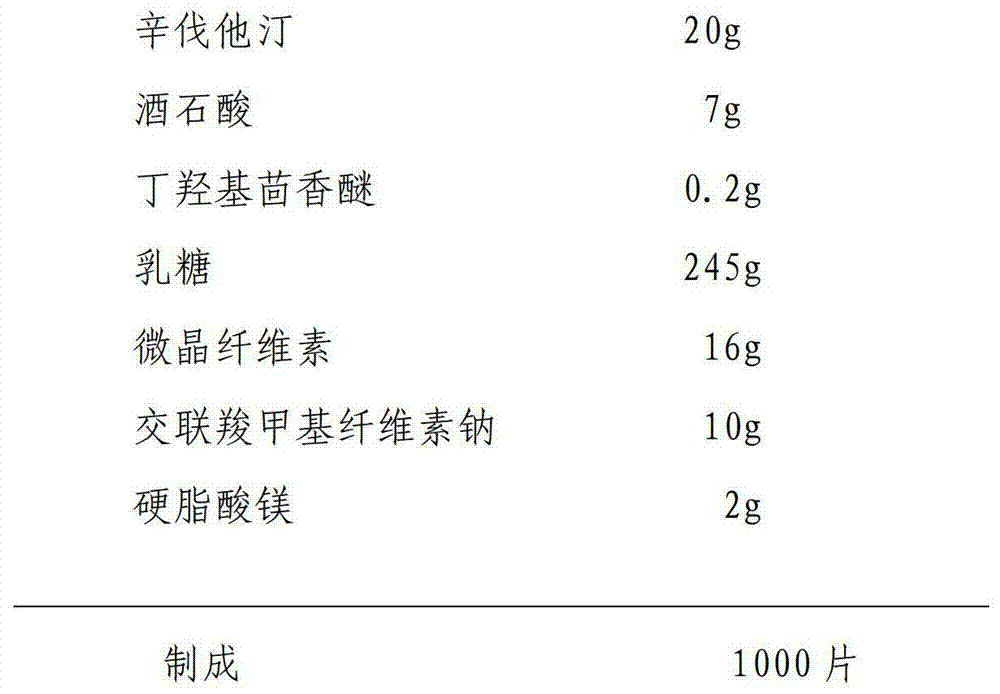
The invention discloses a simvastatin tablet and a preparation method thereof. The simvastatin tablet comprises an active ingredient simvastatin and pharmaceutical excipients, wherein the pharmaceutical excipients are spherical lactose, cross-linked sodium carboxymethyl cellulose, butylhydroxyanisole, hydroxypropyl methyl cellulose, silicon dioxide, magnesium stearate and film coating premix, which are added according to a specific mass ratio and process. The simvastatin and the pharmaceutical excipients are incompatible, and are prone to hydrolysis and oxidation, lactone bonds break to open loop to generate an active metabolite simvastatin hydroxy acid under the high-humidity condition, the intramolecular diene bond is subjected to a slow oxidative copolymerization reaction to generate a dimer or polymer under the high-temperature condition, and the preparation of a stable preparation is greatly difficult. In recent years, with the continuous disclosure of information, the difference between the quality standards of simvastatin tablets produced by different manufacturers is great, wherein the dissolution behavior difference is more significant, so that the situation that the simvastatin tablets have the same name but have different quality is very obvious. The prescription process determined by the study can continuously produce the simvastatin tablet at large scale, and the prepared simvastatin tablet has a good dissolution performance in various PH-value dissolution media, and keeps good stability in the long-term storage process.
Owner:DIAO GRP CHENGDU PHARMA
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
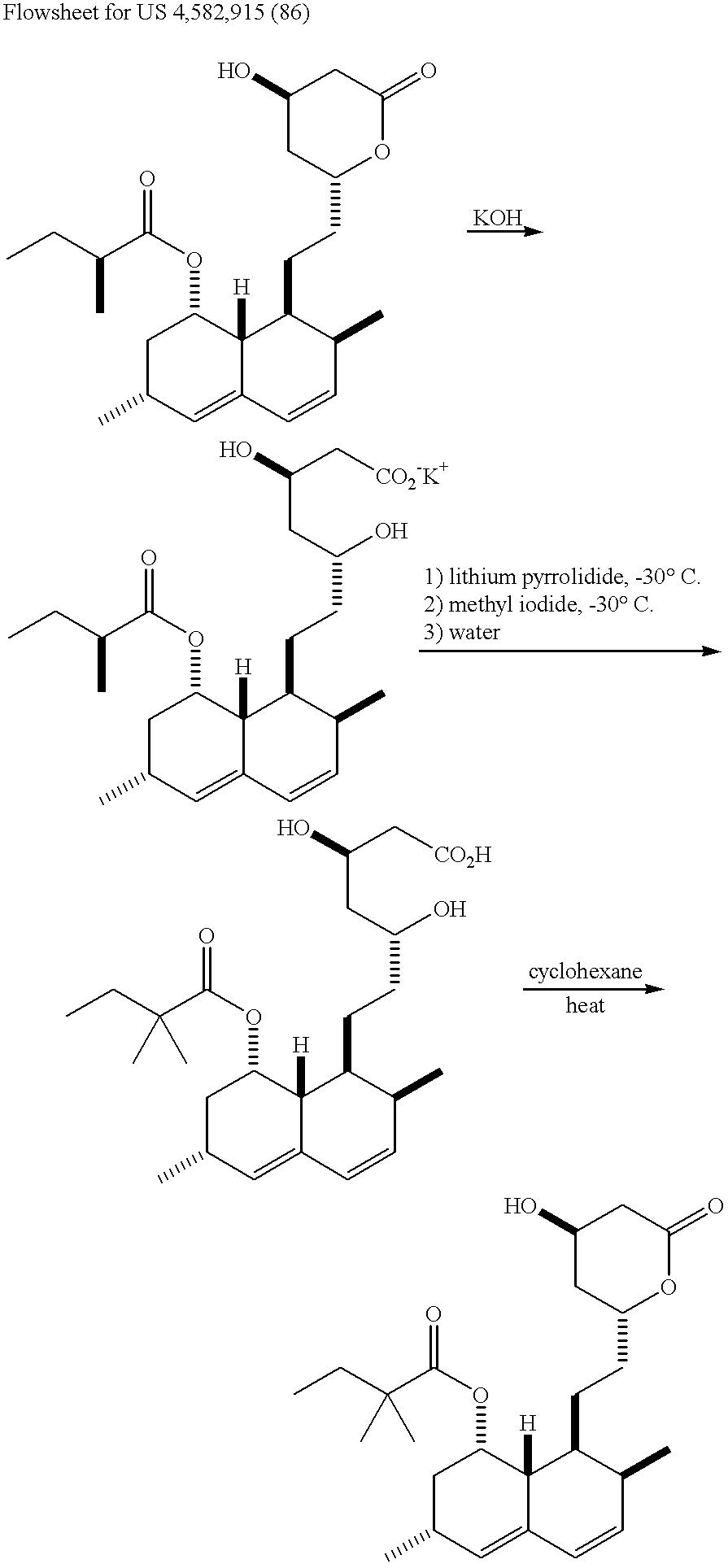
Process of lactonization in the preparation of statins
The present invention relates to a process for preparing lovastatin and simvastatin which comprises (1) performing step of a lactonization of mevinic acid and analog thereof compounds in the presence of a dehydrating agent and without an acid catalyst under nitrogen sweep; and then (2) making step of crystals at a high temperature. In the process of the present invention, lovastatin and simvastatin highly purified can be produced in a high yield and especially, heterodimers formed as a by-product can be reduced in an amount remarkably. Therefore, the process of the present invention is convenient and economical.
Owner:CJ HEALTHCARE CORP
Nanoparticulate statin formulations and novel statin combinations
InactiveUS20080213378A1Improve bioavailabilityHigh dissolution ratePowder deliveryBiocideLovastatinNanoparticle
The present invention is directed to nanoparticulate compositions comprising statin such as lovastatin or simvastatin. The statin particles of the composition have an effective average particle size of less than about 2000 nm. In another aspect of this invention, novel combinations of statins and other cholesterol lowering agents are described and methods of using same are taught.
Owner:ELAN PHRMA INT LTD
Preparation method of simvastatin tablet
InactiveCN104224736ASmall particle sizeLarge specific surface areaOrganic active ingredientsMetabolism disorderPrillDissolution
The invention relates to a preparation method of a simvastatin tablet. The simvastatin tablet is composed of simvastatin, an acidic protecting agent, an antioxidant agent, a filling agent, a disintegrating agent and a lubricant. The preparation method disclosed by the invention comprises the following steps: carrying out low-temperature superfine grinding on a simvastatin raw material and lactose in a weight ratio of 1 to (1-10), so that the particle size is reduced and the specific surface area is increased, and then the raw material is changed from a lipophilic material into a hydrophilic material; uniformly mixing the obtained mixed material with the acidic protecting agent and the antioxidant agent, the rest of lactose, microcrystalline celluloses and crosslinked carboxymethyl cellulose sodium, granulating by using a wet method, and carrying out fluidized drying while controlling the water content of a finished product grains at 1-5%, so that the simvastatin tablet is prepared. The prescription process is simple and easy to control, and prepared preparation is good in content uniformity, high in dissolution rate, good in absorption, and high in bioavailability in comparison with other preparations. The preparation method of the simvastatin tablet disclosed by the invention is good in good in reproducibility, large in productivity, and stable and controllable in quality, and can effectively guarantee the effectiveness of drugs and the safety of drug application of patients.
Owner:哈药集团人民同泰医药股份有限公司
Use of chloride 13-hexyl berberine and chloride 13-hexyl palmatine in preparation of medicine for treating moist tetter, dermatitis and psoriasis
InactiveCN1923199AEffective activityAvoid bad consequencesOrganic active ingredientsAerosol deliverySide effectEczematous rash
The invention relates to a method for using alcaine 13-hexyl berberine, and relative lcaine 13-palmatine to prepare external the drug that resists dermopathy and psoriasis. And it has antibacterial activity and better treatment on eczema, etc. And its emulsion can treat psoriasis with similar effect of Simvastatin, without side effect.
Owner:CHUGOKU IGAKU KAGAKUIN HIFUBIYOU KENKYUSHO
Process for the preparation of simvastatin
InactiveCN1754870APreparation by ester-hydroxy reactionOrganic compound preparationLovastatinAcid catalysis
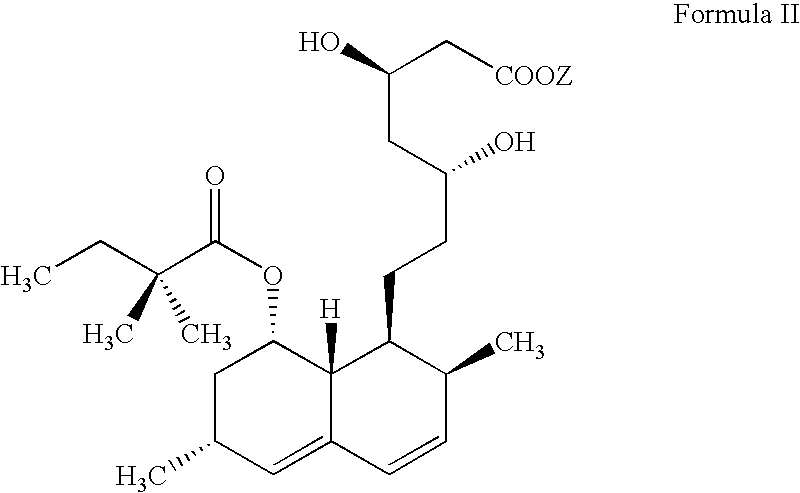
The invention discloses a preparation method for simvastatin. Wherein, using inorganic base to hydrolyze lovastatin and obtain trioxyacidic intermediate with formulate (3); then, etherifying directly the intermediate to prepare simvastatin derivative with formulate (4), taking ring-opening reaction with catalyst to obtain open-loop ester with formulate (6), catalyzing, acidifying, and obtaining the product; or, improving existing technique with this invention, such as, converting the intermediate into ketal intermediate with six membered ring, catalyzing, etherifying to obtain the simvastatin derivative with formulate (8), using acid-catalysis de-preserving reaction to obtain product. This invention simplifies greatly existing technique.
Owner:PFICKER PHARMA
Process for obtaining HMG-CoA reductase inhibitors of high purity
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, Streptomyces, Actinomadura, Micromonospora, some are obtained by treating the fermentation products using the method of chemical synthesis or they are the products of total chemical synthesis. The purity of the active ingredient is an important factor for manufacturing the safe and effective pharmaceutical, especially if the pharmaceutical product must be taken on a longer term basis in the treatment or prevention of high plasma cholesterol. The accumulation of the impurities from the pharmaceuticals of lower purity may cause many side effects during the medical treatment. The present invention relates to a new industrial process for the isolation of HMG-CoA reductase inhibitors using so-called displacement chromatography. Use of the invention enables one to obtain HMG-CoA reductase inhibitors of high purity, with high yields, lower production costs and suitable ecological balance.
Owner:LEK PHARMA D D
Process for producing simvastatin
InactiveCN1290261AEfficient productionOrganic chemistryBulk chemical productionHMG-CoA reductaseAlcohol
A convenient, efficient and industrially favorable process for producing simvastatin which is useful as an HMG-coA reductase inhibitor. This process comprises deacylating lovastatin by treating with an inorganic base and a secondary or tertiary alcohol to thereby form diol lactone, and then selectively protecting, acylating, deblocking and lactonizing the diol lactone by using a ketal or acetal protective group to thereby give simvastatin.
Owner:KANEKA CORP
Process for producing simvastatin
InactiveUS6307066B1Silicon organic compoundsGroup 3/13 element organic compoundsLovastatinBoric acid
A process for manufacturing Simvastatin is provided comprising reacting lovastatin with an organic boronic acid to produce a derivative of lovastatin (lovastatin phenylboronate) methylating the 2-methylbutyryloxy group on the lovastatin derivative to form a 2,2-dimethylbutyryloxy group on the lovastatin derivative and thereafter removing the boronate group to produce simvastatin.
Owner:APOTEX PHARMACHEN INC
Composite nano fiber support material, as well as preparation method and application in bone repairing aspect
InactiveCN102499997AGood biocompatibilityImprove adhesionFilament/thread formingMonocomponent polyesters artificial filamentAdditive ingredientBiocompatibility Testing
The invention belongs to the technical field of bone repairing materials, and relates to a PLGA / HA / S (Poly(Lactic-co-Glycolic Acid) / Hydroxyapatite / Simvastatin) medicament-carrying composite nano fiber support material as well as a preparation method and application in the bone repairing aspect. The material is prepared by preparing a carrier polymer mixed solution containing simvastatin from hydroxyapatite, simvastatin and PLGA, and processing by a high-voltage electrostatic spinning device, wherein the mass ratio of hydroxyapatite to PLGA and simvastatin to PLGA is (1:100)-(1:10) respectively. The physical and chemical performances and degradation rate of the material are controlled by controlling the blending ratio, reaction temperature, time and other factors; the PLGA / HA / S blended medicament-carrying nano fiber has good biocompatibility; the shape of the material is similar to extracellular matrix, thereby being beneficial to the adhesion and growth of cells; and the material has better biodegradability, and can release the main ingredient of bone tissues, namely hydroxyapatite and a medicament for promoting osteogenesis, namely simvastatin, thus the biomaterial is particularly suitable to be used as a bone repairing material.
Owner:JILIN UNIV
Compositions comprising fenofibrate and simvastatin
InactiveUS20060105050A1Substance may accumulateImprove bioavailabilityPowder deliveryBiocideParticulatesHMG-CoA reductase
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor simvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCsimvastatin) of between about 800 and about 29,300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and simvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
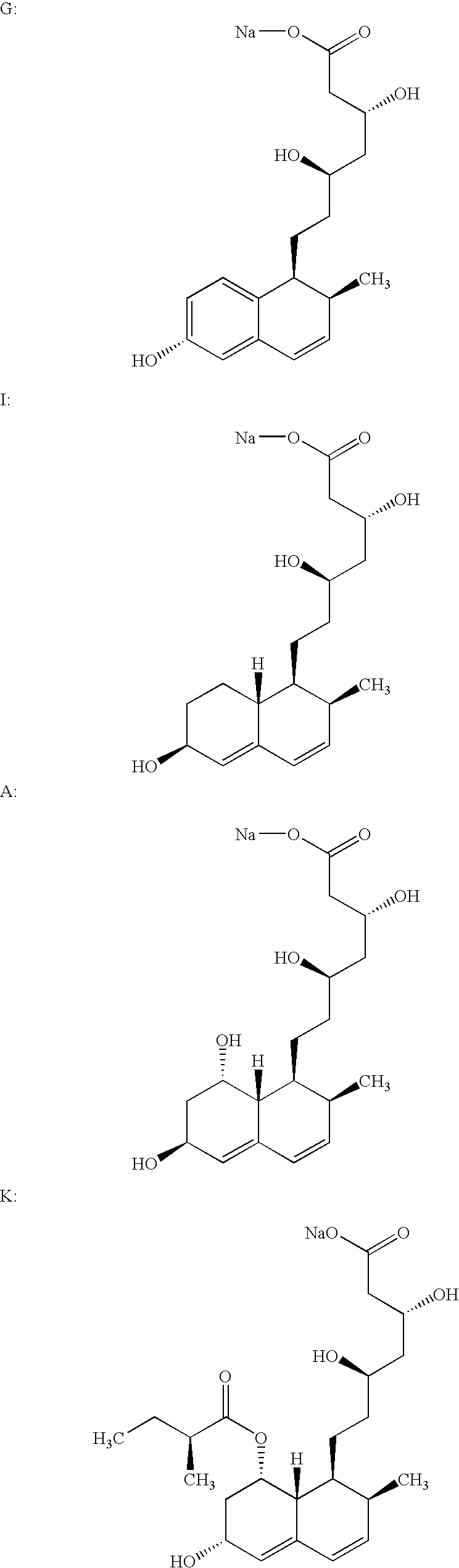
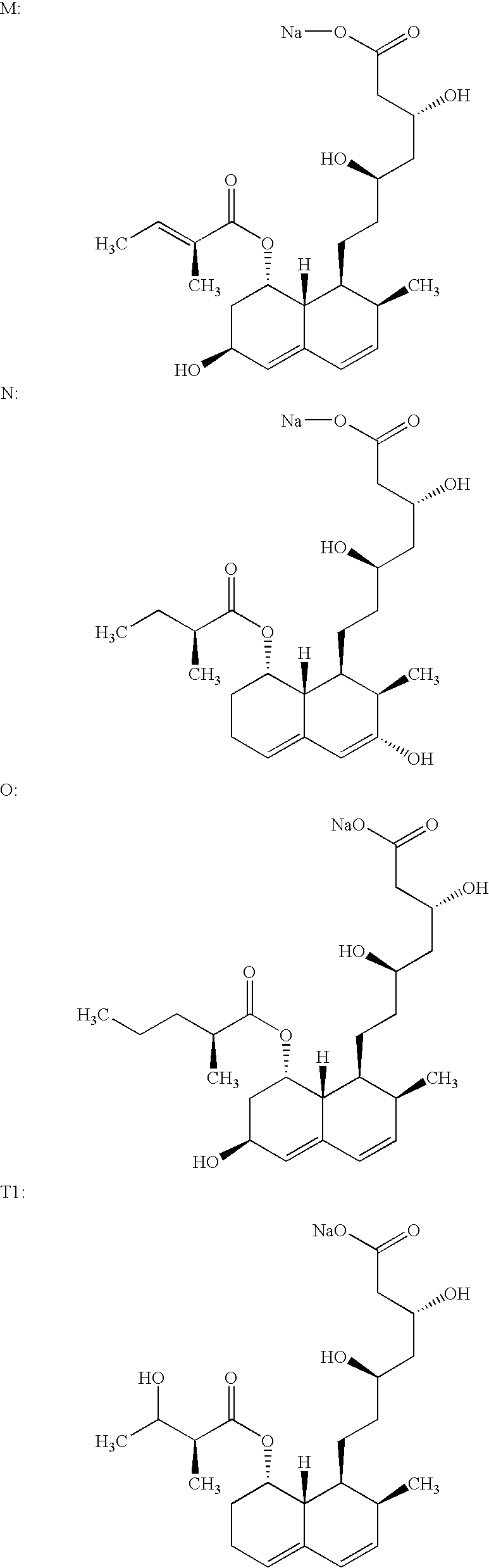
Salts of HMG-CoA reductase inhibitors
InactiveUS6838566B2Isolation and/or purificationLow toxicityOrganic compound preparationCrystallization separationChemical synthesisHMG-CoA reductase
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, some are obtained by treating the fermentation products using the methods of chemical synthesis or they are the products of total chemical synthesis. The present invention relates to the new amine salts of HMG-CoA reductase inhibitors, the preparation thereof, the preparation of pure HMG-CoA reductase inhibitors via amine salts thereof, use of the amine salts of HMG-CoA reductase inhibitors in the process for semisynthetic preparation of HMG-CoA reductase inhibitors, use of the amine salts of HMG-CoA reductase inhibitors in the process for biotechnological modification of HMG-CoA reductase inhibitors as well as the conversion of the amine salts of HMG-CoA reductase inhibitors into the pharmaceutically acceptable salts of the HMG-CoA reductase inhibitors and the conversion of the amine salts of HMG-CoA reductase inhibitors into the HMG-CoA reductase inhibitors in the lactone form.
Owner:LEK PHARMA D D
Methods for making simvastatin and intermediates
The invention provides synthetic chemical and chemoenzymatic methods of producing simvastatin and various intermediates. In one aspect, enzymes such as hydrolases, e.g., esterases, are used in the methods of the invention.
Owner:VERENIUM CORP (US)
Regenerated active artificial implant for osteoporosis therapy and preparation method thereof
The invention discloses a regenerated active artificial implant for osteoporosis therapy and a preparation method thereof. The regenerated active man-made implant comprises a tooth implant with titanium or titanium alloy as a base and an artificial bone joint medical hard tissue implant material. The base material surface is subjected to serial nano-micron treatment and simvastatin-containing improved bionic liquid treatment to form a nano bionic coating structure with regeneration activity and bone mass formation promoting effect; the nano coating crystal grains on the surface of the implant are bone-like hydroxyapatite substance rich in calcium and phosphorous; the nano-micron bionic coating implant has bone regeneration promoting activity and high affinity, and compared with coating-free materials, the nano-micron bionic coating implant has remarkably increased binding force with bone so as to effectively prevent the implant from loose and fallout phenomena; the simvastatin-containing calcium / phosphorus bionic coating implant is a novel medical implant material with bone mass formation promoting effect and regeneration activity.
Owner:ZHEJIANG UNIV
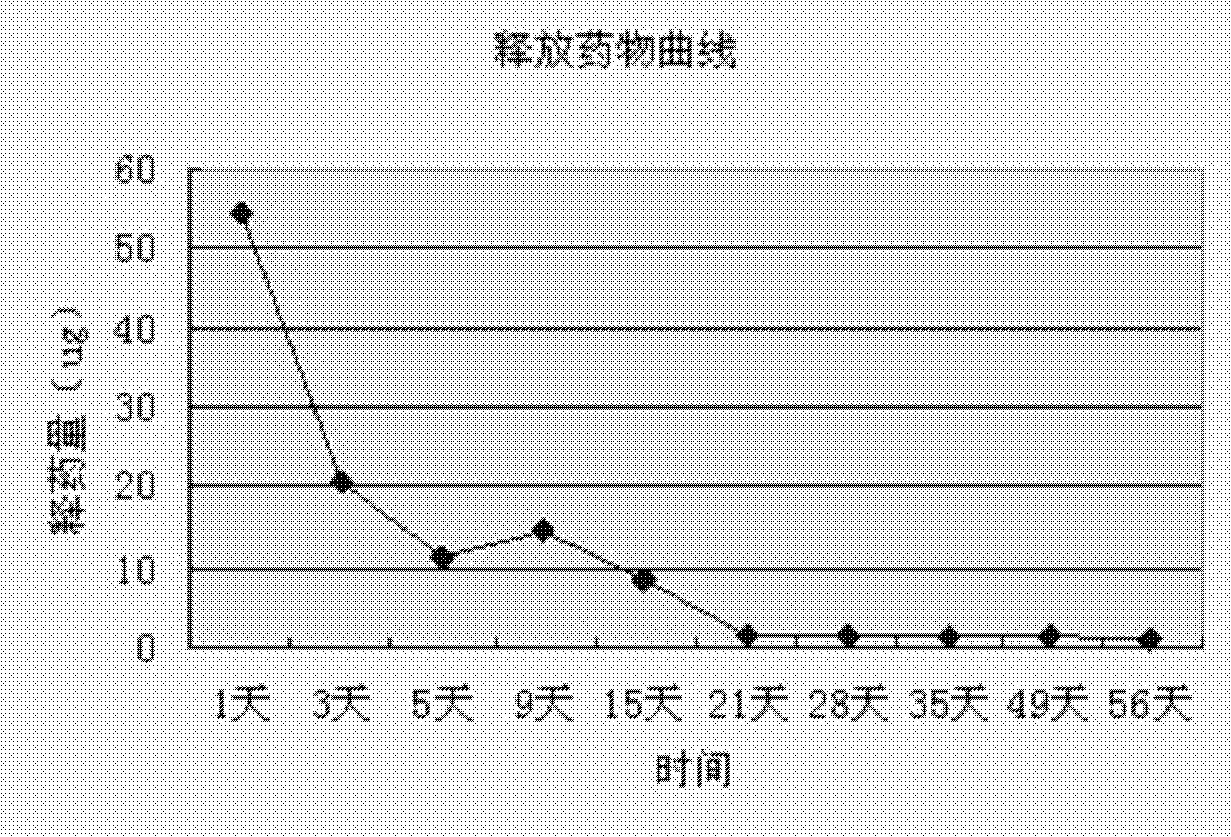
Method of preparing simvastatin sustained-release microsphere carried series
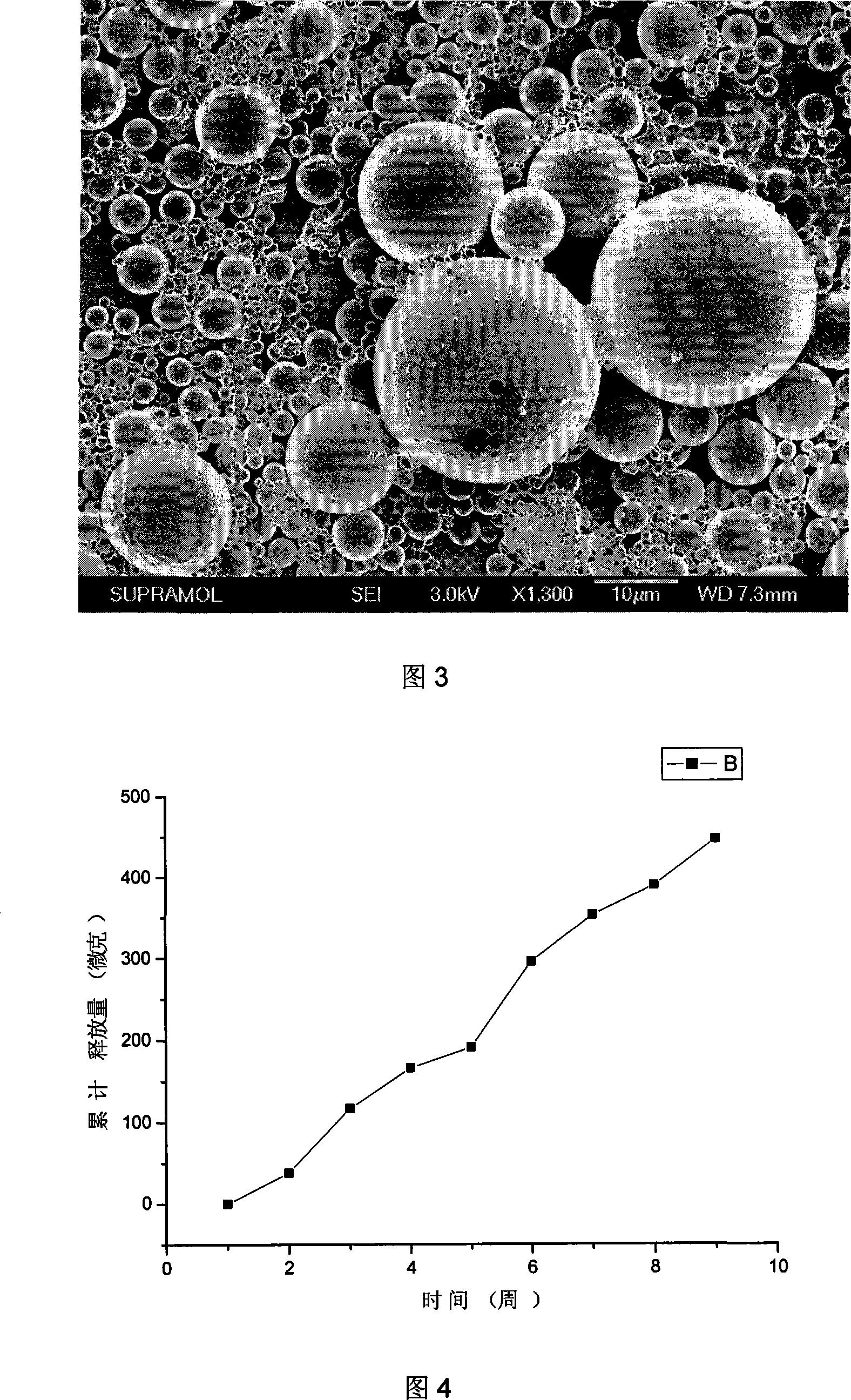
InactiveCN101219119ARelease stabilityLarge adjustment rangeOrganic active ingredientsSurgeryMicrospherePolyvinyl alcohol
The invention relates to a method that a functional drug is enveloped into a polymeric material with biodegradability to form a nano-micron microsphere system. The method comprises that the polymeric material and simvastatin are dissolved in an organic liquor to form uniform dispersion which is then added into an liquor containing emulsifier Tween 80 and biologically nontoxic electrolytic polyvinyl alcohol or sodium dodecyl benzene sulfonate (SDBS), and then the obtained liquor is stirred, evaporated at a reduced pressure, centrifugalized, washed and vacuum dried, finally the simvastatin-contained delayed-release microsphere system is obtained. The surface of the microsphere is smooth and round, the granule thereof is regular without conglutination, and the granule diameter, the drug-loading rate (1-10 percent) and the encapsulation rate (above 40 percent) are all controllable, and the delayed-release time exceeds 2 months. The prepared simvastatin-contained delayed-release microsphere system can be processed into various preparations used in bony tissue absorption or bony defect parts, the microsphere system is degraded at a proper speed, thus the simvastatin can be further released; the degradation of the polymer can provide bony tissue with subsequent recuperating space to complete the repair of the bony defect parts.
Owner:JILIN UNIV
Pharmaceutical composition containing simvastatin
ActiveCN1994296AControl degradationImprove stabilityOrganic active ingredientsMetabolism disorderActive componentPharmaceutical formulation
The invention relates to a drug which contains Simvastatin, wherein it comprises active component as Simvastatin and drug findings. Said drug findings comprise acid pH adjuster whose consumption can reduce pH value not higher than 3. 5 as 2. 5-3. 2. The invention, via pH adjuster, oxidization resistance and other findings can effectively restrain the oxidzation of Simvastatin, with high stability.
Owner:ZHEJIANG JINGXIN PHARMA +1
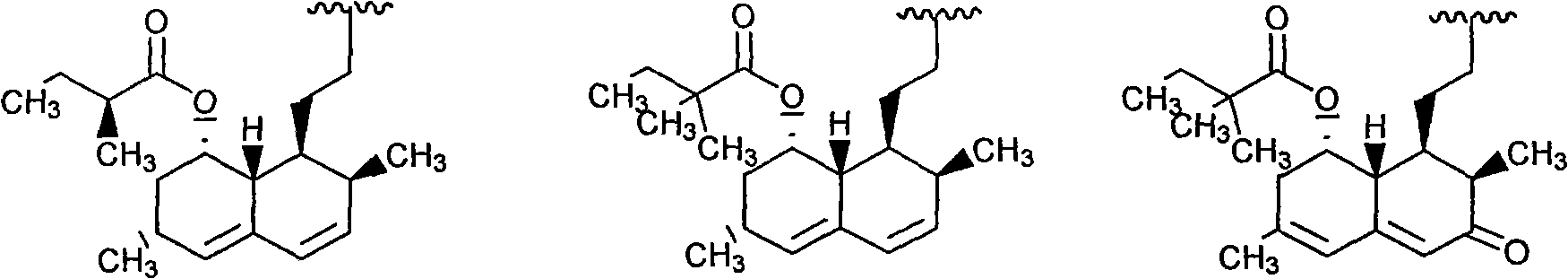
Lovastatin, simvastatin and simvastatin-6-oxide nitro-oxo-derivative and preparation method thereof
The invention describes lovastatin, simvastatin and simvastatin-6-oxide (L-669262) nitro-oxo-derivative of which the general formula is shown in formula (I) and the drugs can increase the non-lipid activity of statins such as anti-inflammatory and the like. The invention also relates to a preparation method of the drugs.
Owner:四川抗菌素工业研究所有限公司
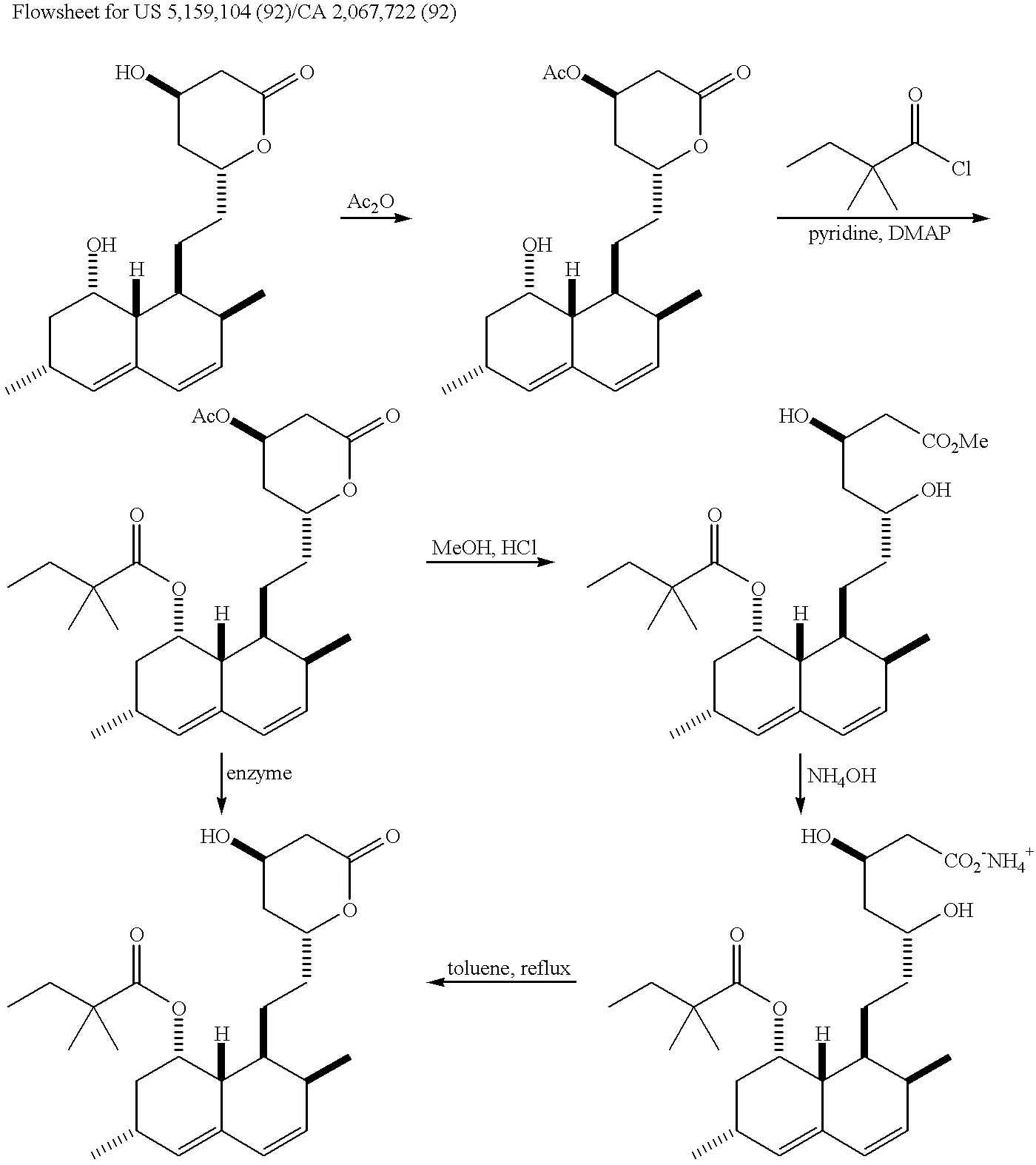
Process for the preparation of simvastatin
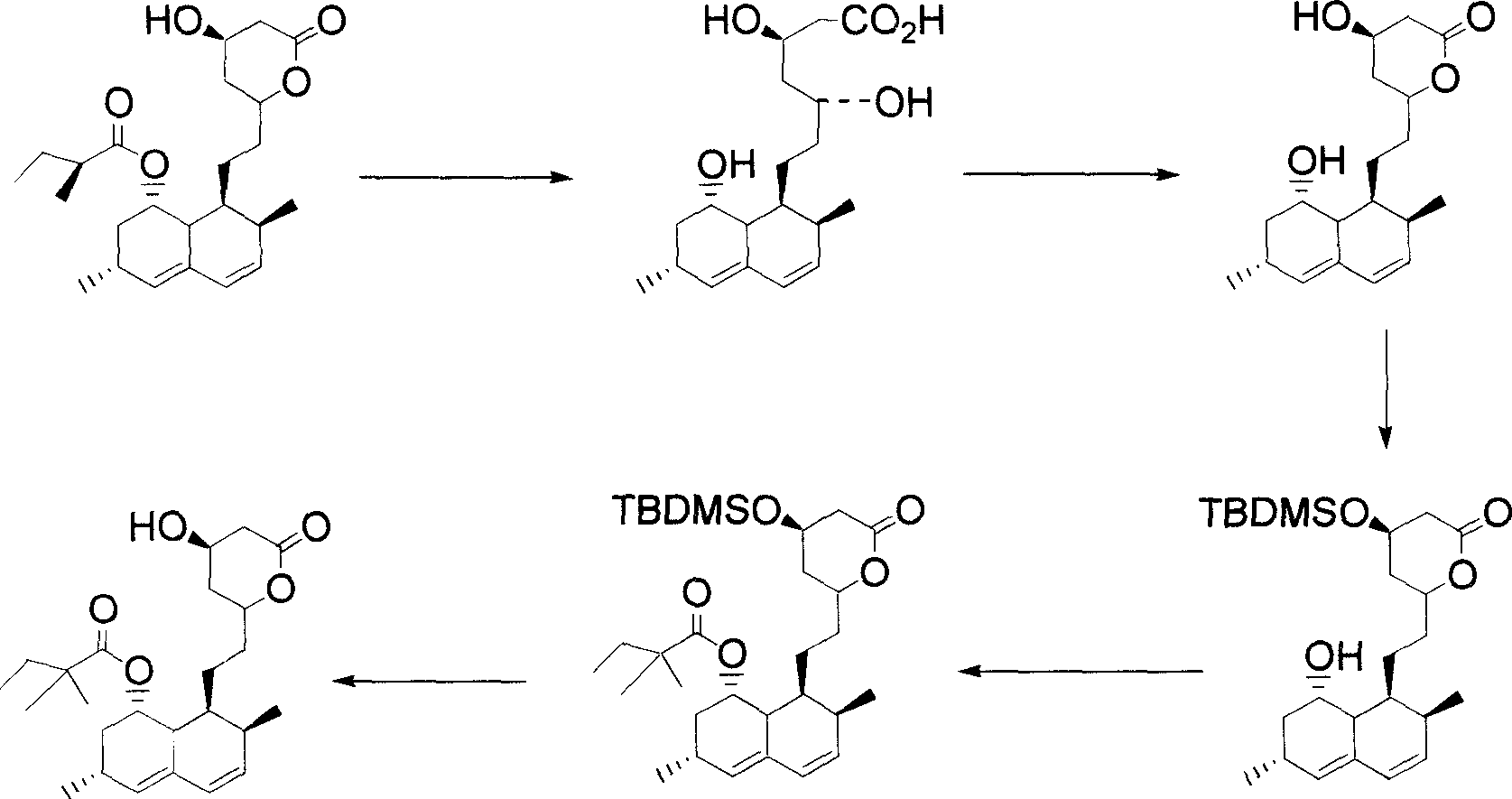
InactiveUS20050080275A1High yieldSpeed up the processOrganic active ingredientsGroup 4/14 element organic compoundsSilyleneLovastatin
Highly pure simvastatin can be prepared economically in a high yield using the method comprising the steps of treating lovastatin with potassium hydroxide dissolved in a mixture of water and methanol to obtain a triol acid; relactonizing the triol acid, and protecting the hydroxy group on the lactone ring; and acylating the resulting compound with 2,2-dimethylbutyryl chloride or 2,2-dimethylbutyryl bromide in the presence of an acylation catalyst in an organic solvent, followed by removing the silyl protecting group on the lactone ring to obtain simvastatin.
Owner:HANMI PHARMA
Process to manufacture simvastatin and intermediates
InactiveUS6506929B1Reduce usageSimplifies isolationOrganic compound preparationPreparation by transesterificationLovastatinLactone formation
A process is disclosed for the preparation of simvastatin which enables highly regio selective C-methylation of the 2'-position group of lovastatin without requiring protection / deprotection of 13-OH of lovastatin and lactone ring opening / closure.
Owner:APOTEX TECH INC
Preparation method of simvastatin
ActiveCN101381356AReduce manufacturing costSimple production processOrganic chemistryMetabolism disorderLovastatinHydrolysis
The invention discloses a method for preparing simvastatin. The method is as follows: a. lovastatin and alkylamine are prepared into lovastatin amide; b. hydroxyl groups in lovastatin amide molecules are protected, and lovastatin amide di-(trimethyl) silyl ether is generated; c. the lovastatin amide dimethyl (trimethyl) silyl ether is subjected to methylation, so as to obtain simvastatin amide di-silyl ether; d. the simvastatin amide di-silyl ether is protected, and simvastatin amide is generated; e. the simvastatin amide is subjected to hydrolysis and is added with ammonia gas, and simvastatin ammonium salt is obtained; and f. the simvastatin ammonium salt is subjected to ring closure to generate the simvastatin. The method takes a composite solvent of tetrahydrofuran and cyclonexane as a solvent for the protective reaction; the lovastatin amide di-(trimethyl) silyl ether can directly perform methylation reaction without alkaline washing and water scrubbing; after water scrubbing of methylate, protective groups are automatically fallen off, thereby the reaction for removing the protective groups is saved and the products can directly perform ammonium salt reaction. The method simplifies the synthesis technique of the simvastatin.
Owner:HEBEI GUOLONG PHARMA CO LTD
Application of HMGCoA reductase inhibitor in preparation of medicine used for treating xerophthalmia
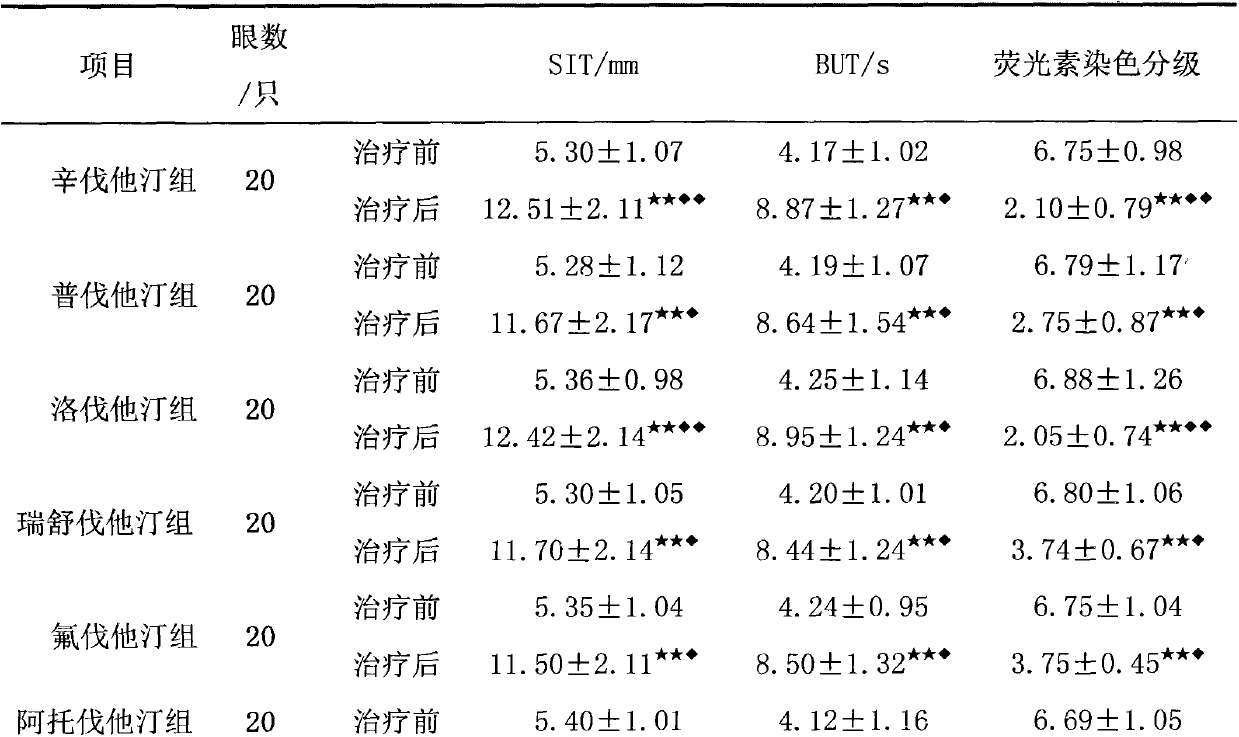
InactiveCN103007284AImprove permeabilityPromote secretionSenses disorderEster active ingredientsSide effectOphthalmic drug
The invention relates to an application of an HMGCoA reductase inhibitor in preparation of a medicine used for treating xerophthalmia and belongs to the field of medicines. The inventor finds that a frequently-used lipid regulation medicine, the HMGCoA reductase inhibitor, can obviously prolong SIT time and BUT time and obviously reduce an FLS score when being used for treating the xerophthalmia, and the HMGCoA reductase inhibitor is better than ciclosporin or artificial tears in the aspect of improving the indexes, wherein simvastatin and lovastatin have the best treatment effect. The HMGCoA reductase inhibitor also can be prepared into oral preparation used for treatment or adjuvant therapy of the xerophthalmia. The HMGCoA reductase inhibitor has a definite curative effect and less toxic and side effects, compliance of a patient is high, and clinical ophthalmic drugs are further enriched.
Owner:闫莹
Process for lactonization to produce simvastatin
Owner:AUROBINDO PHARMA LTD
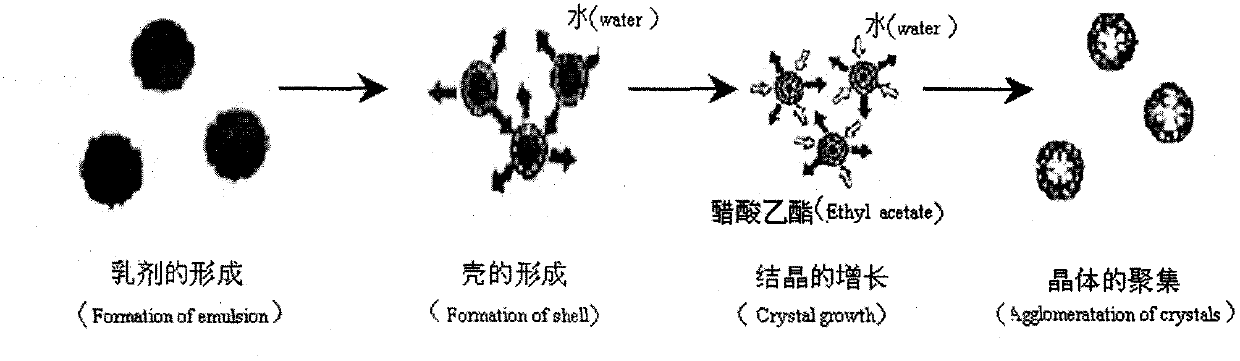
Solid self-microemulsion based on spherical crystallization technique and preparation method thereof
InactiveCN103315960AReduce liver and kidney toxicityAvoid influencePowder deliveryEmulsion deliveryNeogambogic acidCaprylic acid
The invention relates to the medical technology field, and particularly relates to a solid self-microemulsion based on a spherical crystallization technique and a preparation method thereof. The solid self-microemulsion is characterized in that: with the use of the spherical crystallization technique, the solid self-microemulsifying micoparticles are prepared from poorly water soluble drugs in a liquid phase by one step. The solid self-microemulsion with the poorly water soluble drugs comprises the components, by weight: 0.1 to 1.5 g of the poorly water soluble drugs, 4.0 g of a polyoxyethylene hydrogenated castor oil, 2.0 g of capric caprylic triglyceride, 2.0 g of tpropylene glycol, 1.0 ml of ethanol, 4.0 ml of dichloromethane, 0.5 to 1.1 g of ethylcellulose (or Eudragit RS100, RL100), 0.05 g of PEG4000, and 0.5 g of colloidal silicon dioxide. The poorly water soluble drugs include cyclosporine A, fenofibrate, glimepiride, cilnidipine, isradipine, simvastatin, baicalein, neogambogic acid, puerarin, cyclovirobuxine D, silymarin and the like.
Owner:胡容峰
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com