Methods for making simvastatin and intermediates
A new technology of vastatin and lovastatin, applied in the fields of synthetic organic chemistry and medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: Chemoenzymatic production of nevastatin
[0175] The following examples describe exemplary protocols of the invention, eg, for the chemoenzymatic production of nevastatin.
[0176] Enzymatic hydrolysis of lovastatin
[0177] The enzyme having the sequence of SEQ ID NO:4 (encoded by SEQ ID NO:3) was evaluated in lovastatin or lovastatin acid at a concentration of 0.1 to 0.5M in 7-10% MeOH / buffer by automated Base was added and the reaction was maintained at pH 9-9.5. For example, on a 500ml scale, in 0.5M lovastatin, a lyophilized preparation with the enzyme SEQ ID NO: 4 (centrifugation supernatant from lysed cells) containing 14mg / ml total protein was observed after 48 hours complete transformation of the substance.
[0178] The reaction mixture was acidified (pH 2), and the precipitate was collected by centrifugation and allowed to dry. The filtrate was extracted with iPrOAc and the organic phase extract was added to the dry cake. The resulting susp...
Embodiment 2
[0188] Embodiment 2: lovastatin esterase analysis
[0189] In one aspect, the invention provides methods comprising enzymatically hydrolyzing lovastatin, lovastatin acid, or lovastatin with a hydrolytic enzyme, e.g., an enzyme of the invention, e.g., SEQ ID NO:4 encoded by SEQ ID NO:3 acid salts to produce triol acids. In one aspect, the invention provides methods comprising enzymatically hydrolyzing lovastatin, lovastatin acid, or lovastatin salt to produce neovastatin.
[0190] The following examples describe exemplary lovastatin esterase assays that can be used to practice the methods of the invention. For example, this exemplary assay can be used to determine whether a method of the invention can be performed with a hydrolytic enzyme, such as an esterase.
[0191] (a) Cell Lysis (Analytical Grade)
[0192] From B-PER (4.5L) (Pierce, #78248), lysozyme (200μL) (Sigma, L-6876; stock solution 10mg / ml) and DNase I (40μL) (Sigma, DN-25; stock solution 5mg / ml) mL), prepare ...
Embodiment 3
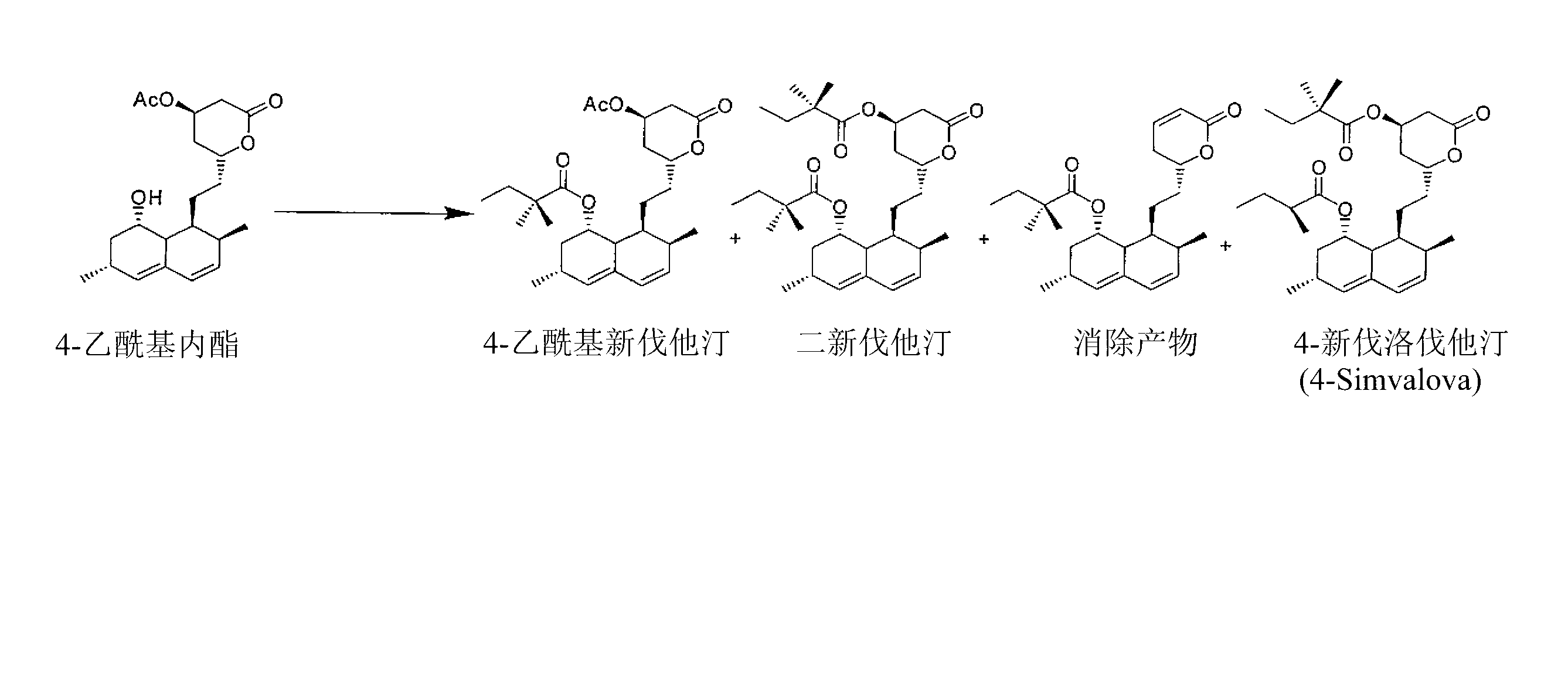
[0230] Embodiment 3: the synthesis of 4-acetyl glycol lactone
[0231] The invention provides a synthetic method for synthesizing 4-acetyl glycol lactone, such as Figure 18 A example.
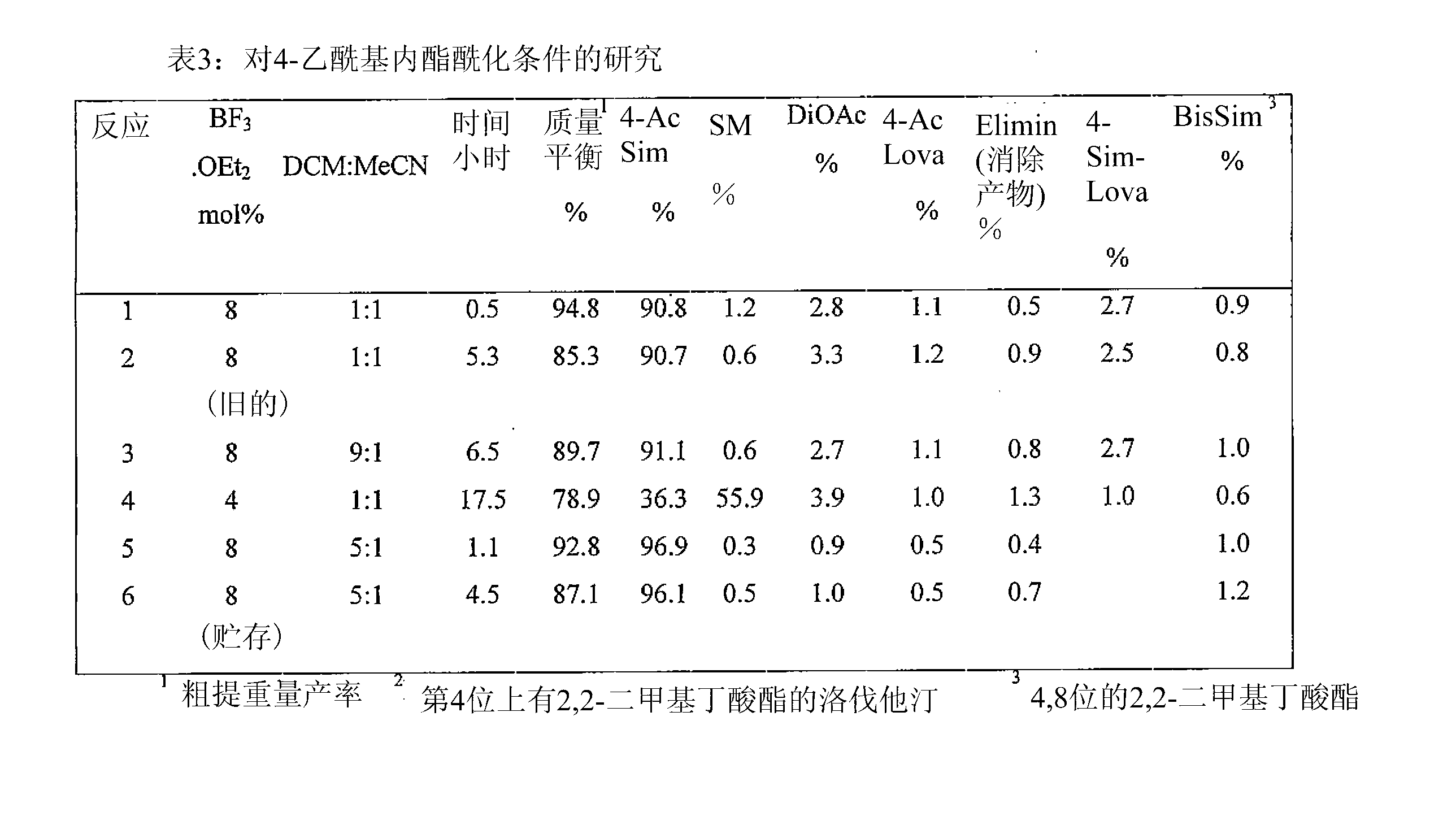
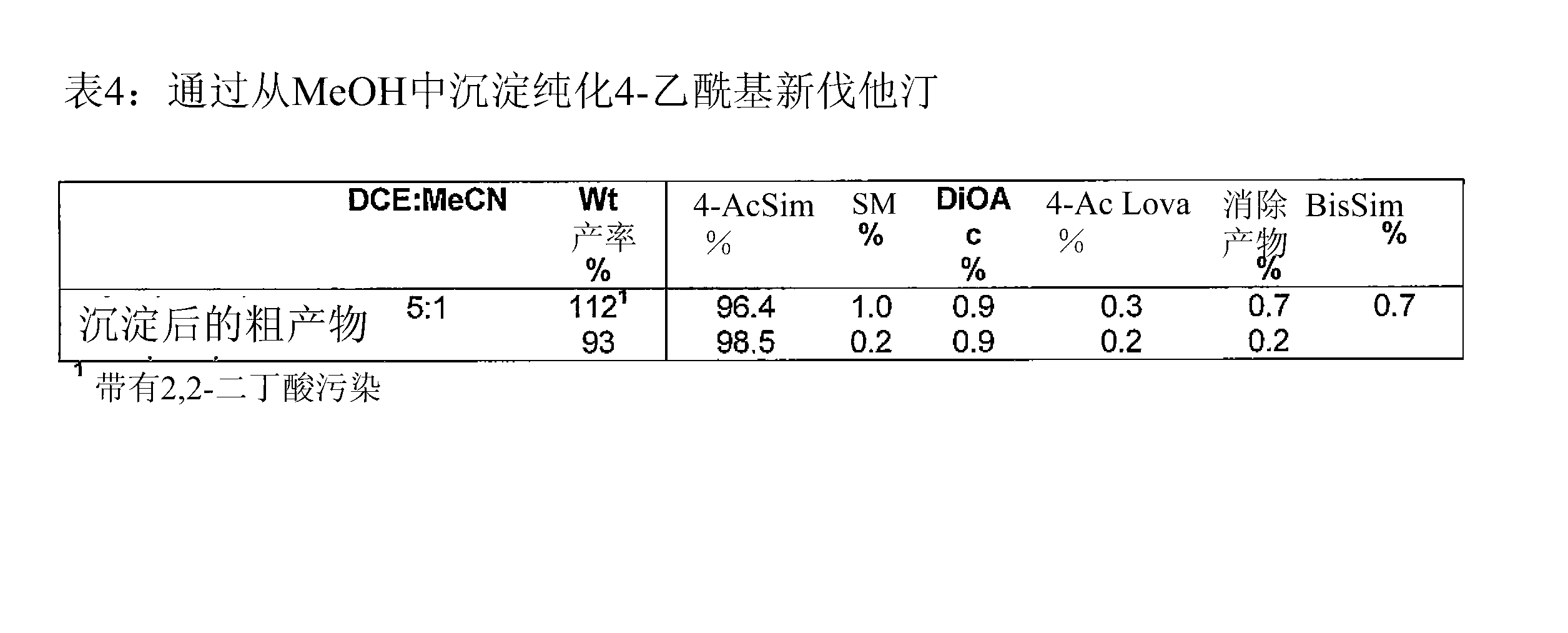
[0232] a. Directly acetylated triol acid (20g level)
[0233] 1. In N 2 Next, the crude triol acid (25.82 g, 59.1 mmol) (Note 1, below) was charged into a dry 500 mL round bottom flask, followed by addition of dry CH 2 C1 2 (200mL). in N 2 The slurry mixture was stirred magnetically at room temperature. DMAP (108 g, 8.8 mmol; 15 mol%) was added, followed by slow addition of acetic anhydride (15.8 mL, 2.8 eq.) via syringe pump over 8.5 hours. At 7.75 hours, an additional portion of DMAP (0.36 g, 2.9 mmol) was added (Remark 2, below).
[0234] 2. Closely monitor the progress of the reaction by HPLC (Remark 3, below).
[0235] 3. After 11 hours, water (5 mL) was added to quench the reaction and the mixture was stored at -20°C before working. Filter the mixture through a pad of celit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com