Protected tetrasaccharides, their process of preparation and their use as transglucosylase acceptor substrates in chemo-enzymatic synthesis of shigella flexneri specific oligosaccharides
A glucose-based, protected technology for use in the field of O-antigen fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0442] Embodiment 1: the synthesis of formula (I) compound
[0443] Synthesis of common precursors of A, B and C residues (1)
[0444] Allyl 4-O-(2-naphthylmethyl)-α-L-rhamnopyranoside (1)
[0445]
[0446] Acetyl chloride (50 mL, 0.70 mol, 2.5 equiv) was added dropwise to allyl alcohol (610 mL) at 0 °C, the solution was stirred for 25 min, and then L-rhamnose monohydrate (50 g, 277 mmol) was added. The mixture was heated at 70 °C for 2.5 h, then at 40 °C for 15 h. TLC (DCM / MeOH 8:2) trace showed complete conversion of the starting hemiacetal (Rf 0.2) to the less polar product (Rf 0.7). Cool the bath temperature to 0 °C, and by adding NaHCO 3 (102.5 g) to neutralize the solution. The suspension was passed through Pad filtered, evaporated solvent and co-evaporated 3 times with toluene.
[0447] The brown oily residue was dissolved in anhydrous acetone (300 mL), then 2,2-dimethoxypropane (100 mL, 0.81 mol, 3.0 eq) and PTSA (3.04 g, 16 mmol, 0.05 eq) were added sequen...
Embodiment 2
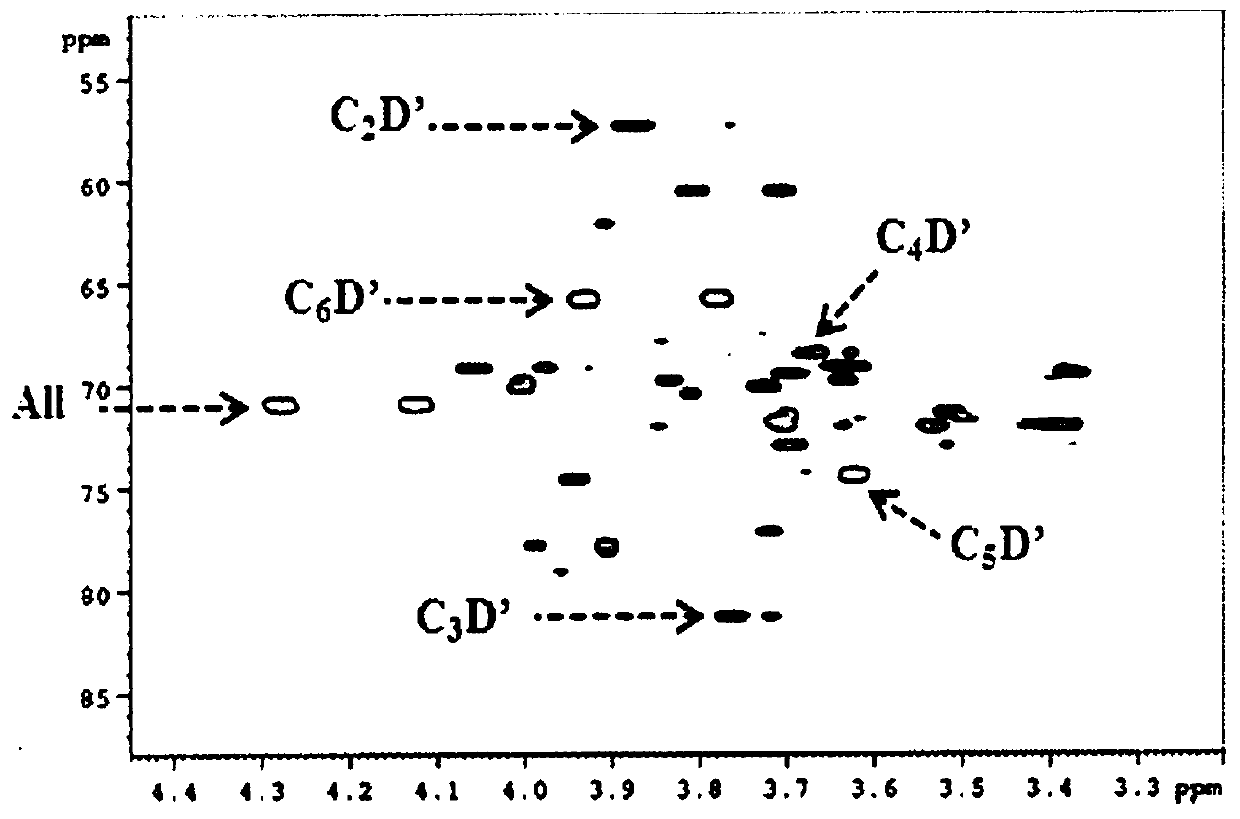
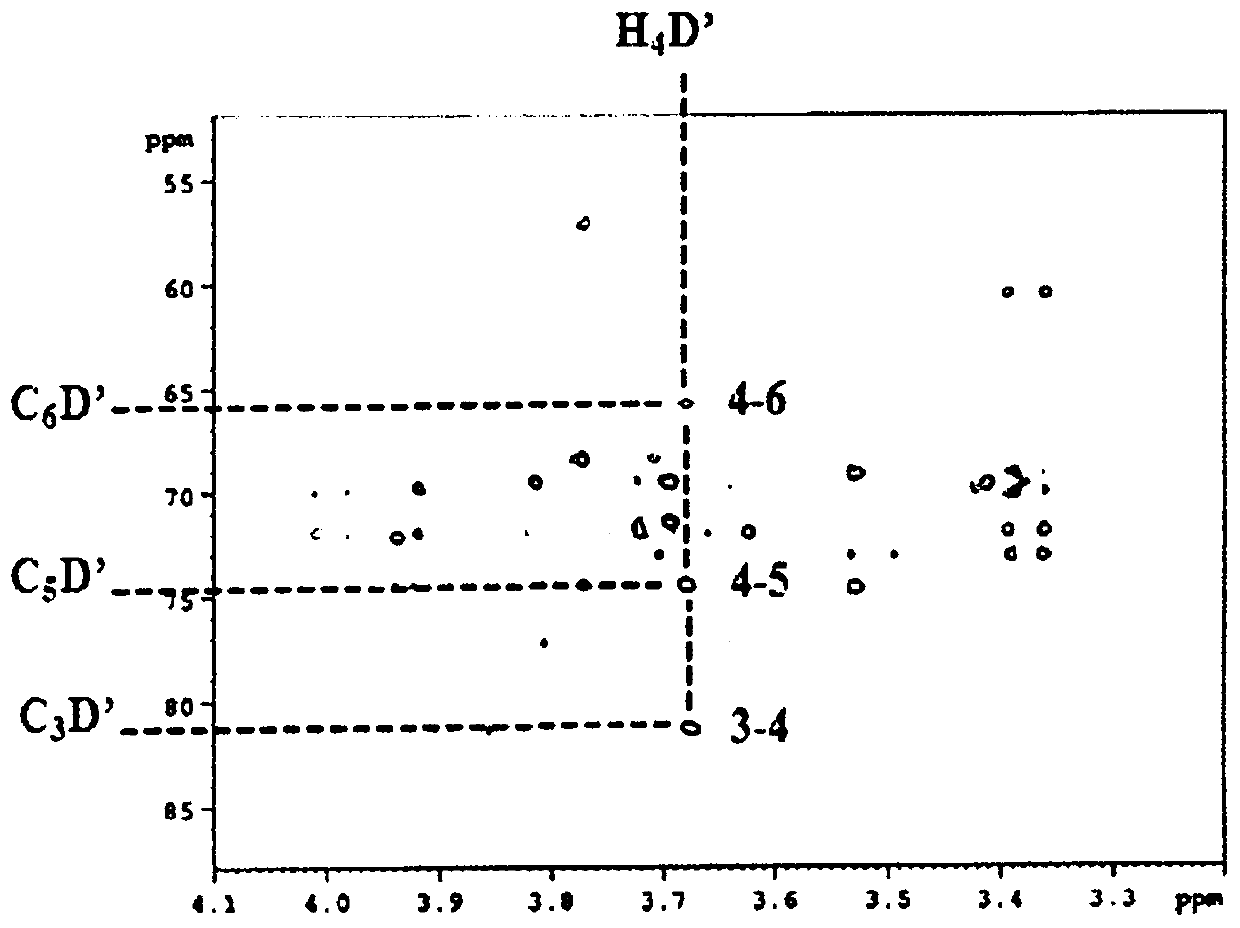
[0570] Example 2: Enzymatic α-D-glucosylation of Compounds of Formula (I) with Branched Sucrase enzyme
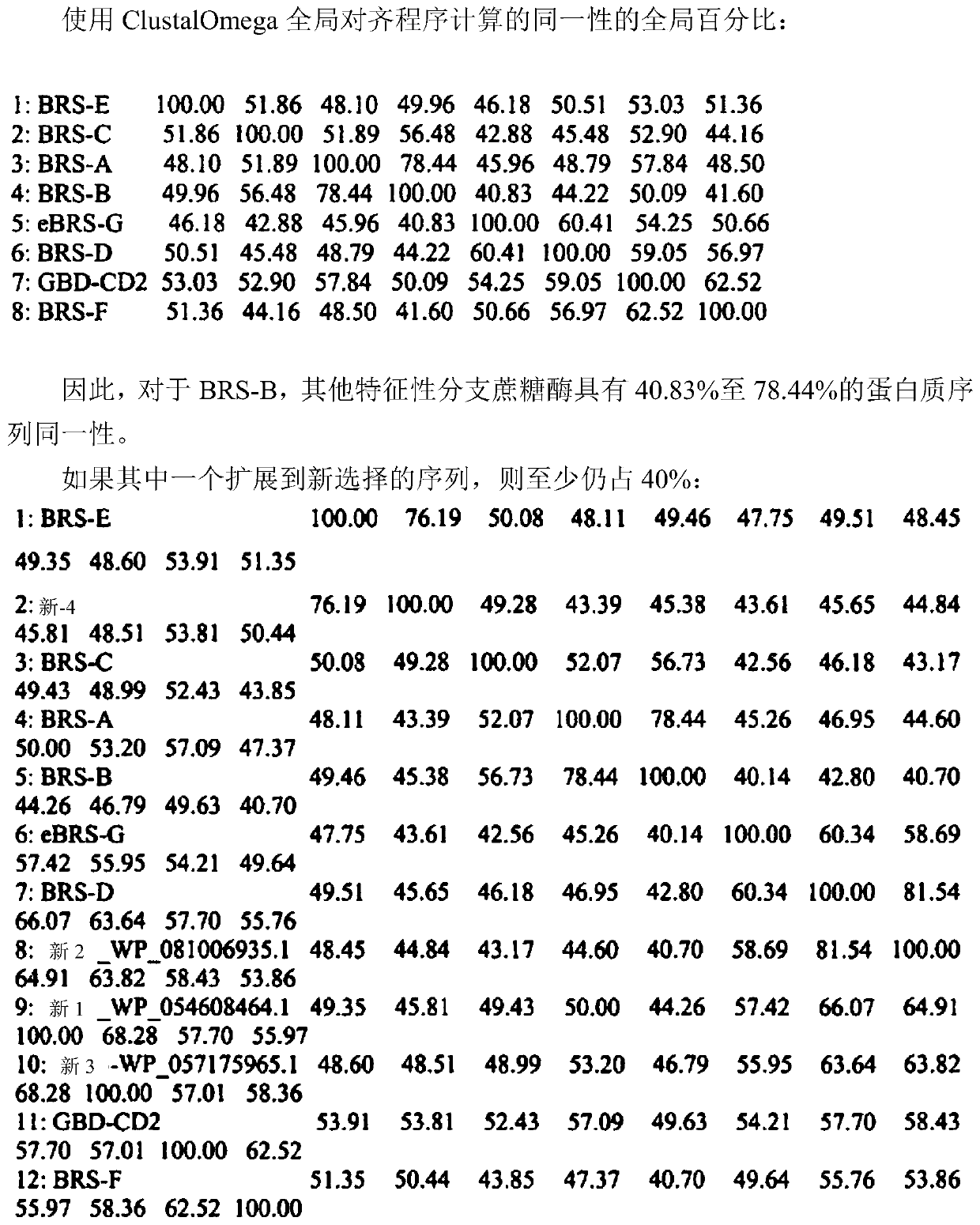
[0571] Table 1 shows some enzymes used in the context of the present invention.
[0572]
[0573]
[0574]
[0575] * Mutations are given relative to the sequence of GBD-CD2 wild type.
[0576] The sequence of the enzyme is as follows:
[0577] SEQ ID NO:1(BRS-E)
[0578] SEQ ID NO:2(BRS-A)
[0579] SEQ ID NO:3(BRS-B-D1)
[0580] SEQ ID NO:4(BRS-B-D2)
[0581] SEQ ID NO:5(BRS-C)
[0582] SEQ ID NO:6(BRS-D)
[0583] SEQ ID NO: 7 (GBD-CD2).
[0584]
[0585]
[0586]
[0587]
[0588]
[0589] Table 2 shows some mutants of BRS-B-D2 used in the context of the present invention.
[0590]
[0591]
[0592]
[0593]
[0594]
[0595]
[0596] Isolation of the brsE gene
[0597] The brsE gene was identified in the Leuconostoc mesenteris KFRI-MG genome (NCBI reference sequence: CP000574) by nucleotide BLAST of the GH70α-transgluc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com