Anti-tumor immune cell based on ligand-targeted cell conjugate (LTCC) technology as well as preparation method and application of anti-tumor immune cell
A technology of anti-tumor immunity and immune cells, applied in the field of biomedical engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of functionalized nanobodies
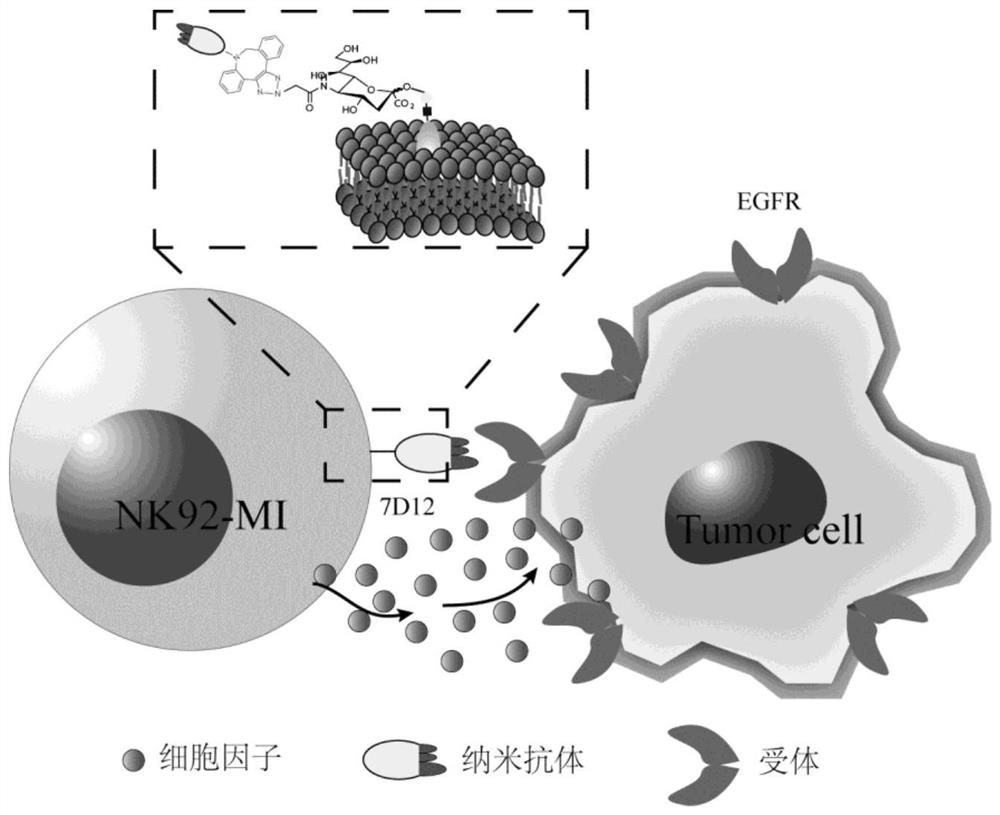
[0039] The preparation of functionalized nanobodies requires three processes, namely nanobody fermentation and purification, chemical synthesis of bio-orthogonal functional groups with triglycine peptides, and Sortase A enzymatic connection.
[0040] Nanobody fermentation purification:
[0041] Take 100 μL of glycerol-preserved Escherichia coli engineered bacteria that can produce Nanobody 7D12 (targeting EGFR) and inoculate them into 25 mL of LB culture containing 100 μg / mL kanamycin for overnight culture activation at 37°C and 200 rpm On the second day, transfer to TB medium containing 100μg / mL kanamycin at 37°C and 200rpm according to 2% inoculum size, and cultivate at 200rpm. The formula of TB is: peptone 12g / L, yeast powder 24g / L, glycerol 5g / L, K 2 HPO 4 ·3H 2 O 16.4g / L, KH 2 PO 4 2.31g / L, pending OD 600 When it is 0.6-0.8, add IPTG with a final concentration of 0.2mM to induce expression, the induction...
Embodiment 2
[0051] Example 2: Effect of azide sugar on the activity of NK cells
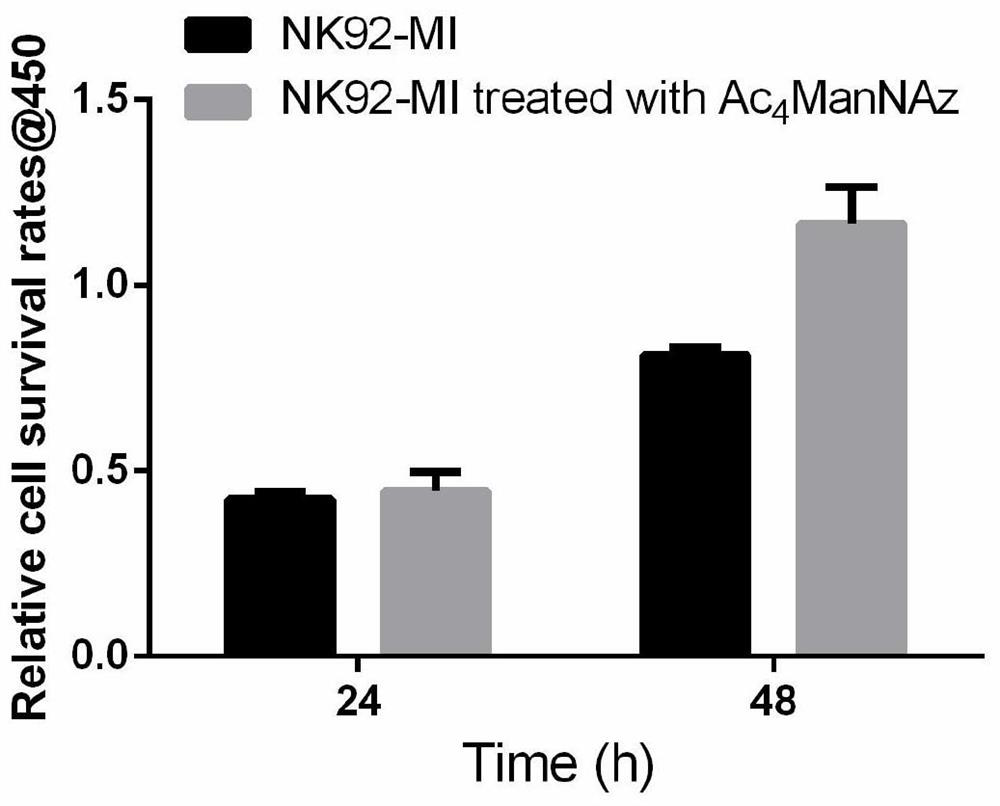
[0052] This example verifies the effect of added unnatural sugar on cell viability. The specific method is: after the unnatural sugar is taken up by the cells, culture for a certain period of time, and then use the CCK8 kit (Biyuntian) to measure the OD 450 , to analyze the difference in cell viability between the sugar-added group and the non-sugar-added group.
[0053] The specific method is as follows: put 1×10 5 NK cells were plated in a 96-well plate, the control group was added with 0.1% DMSO, and the experimental group was added with a final concentration of 50 μM Ac 4 ManNAz (the amount of DMSO is equivalent to 0.1%), and then cultivated in a 37°C cell incubator, and then added CCK8 reagent at 24h and 48h respectively, and detected OD by a microplate reader after incubation for 2h 450 , the result is as image 3 It shows that the addition of non-natural sugar has no toxicity to cells, but has a we...
Embodiment 3
[0054] Example 3: Nanobody modification of immune cells such as NK cells
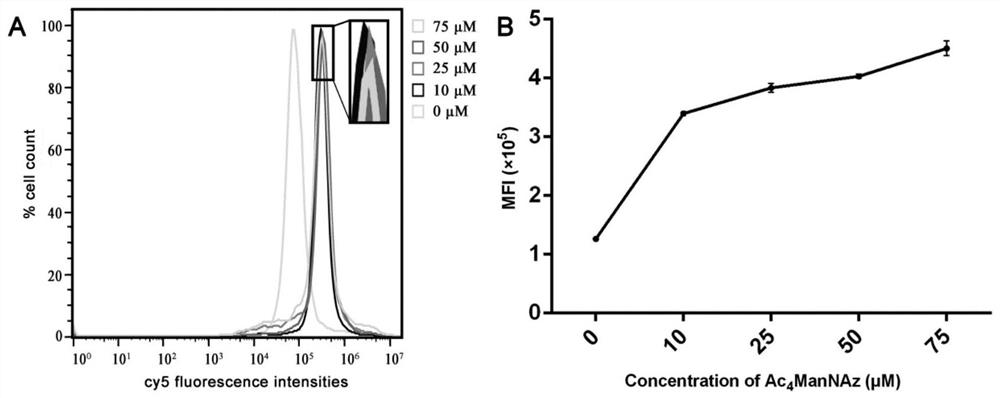
[0055] This example is the acquisition of azide-labeled NK cells and the modification of 7D12. The principle process is as follows figure 1 shown. After the non-natural sugar is taken up by the cells, the bio-orthogonal reactive groups modified on the sugar are loaded onto the cell surface by using glycolysis, sialic acid metabolic pathway and other metabolic glycoengineering processes, and then the cells are collected and injected into the cell surface under similar physiological conditions. Functionalized nanobodies (1×10 4 NK cells were treated with 8-10 μg functionalized nanobody), and reacted at 37°C for 1-1.5h. Verify that cells have been successfully modified with Nanobodies by immunofluorescence.
[0056] The specific method is as follows:
[0057] The above chemically synthesized Ac 4 ManNAz was dissolved in DMSO to make a 50mM mother solution. After filtration and sterilization, it was ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com