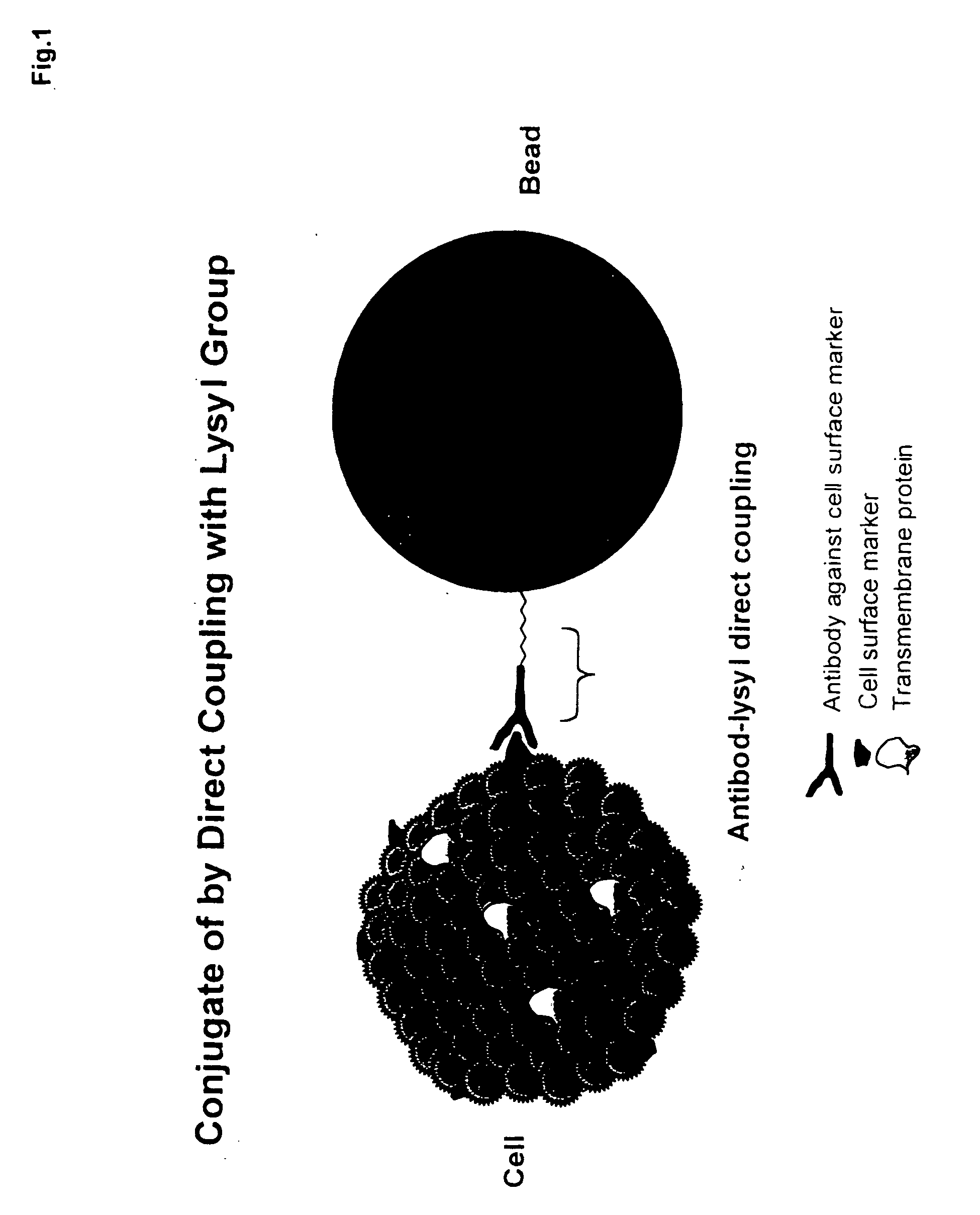
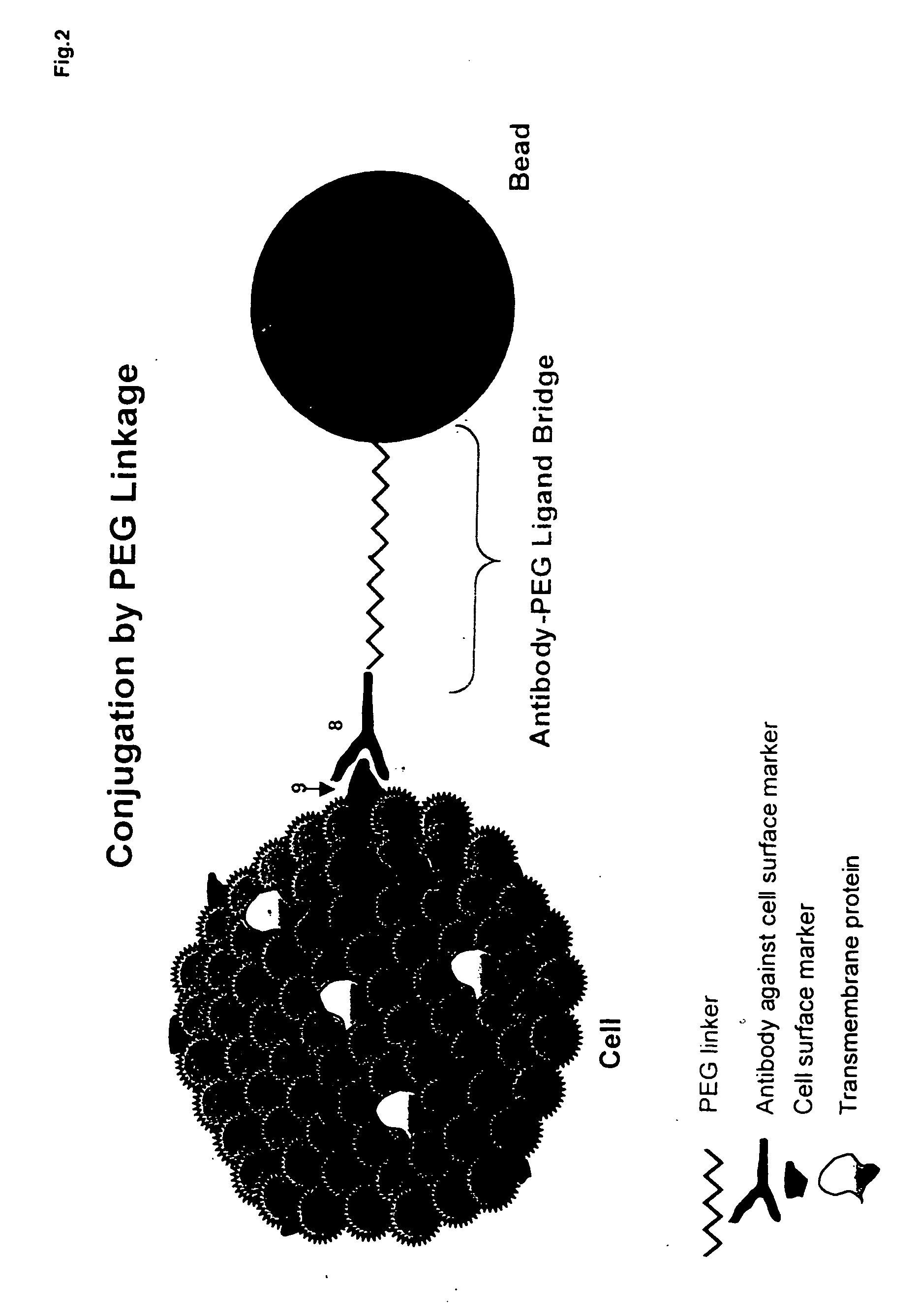
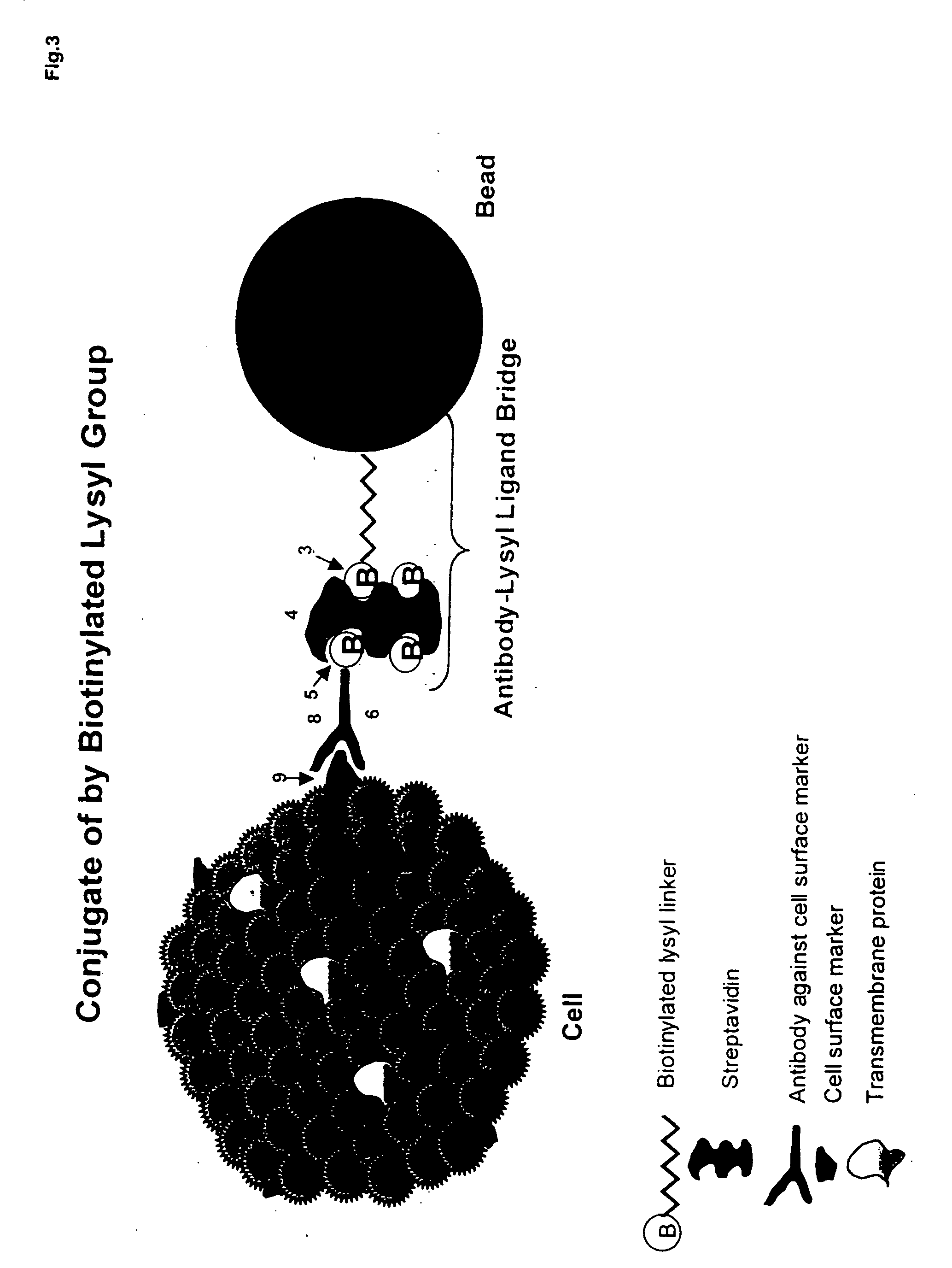
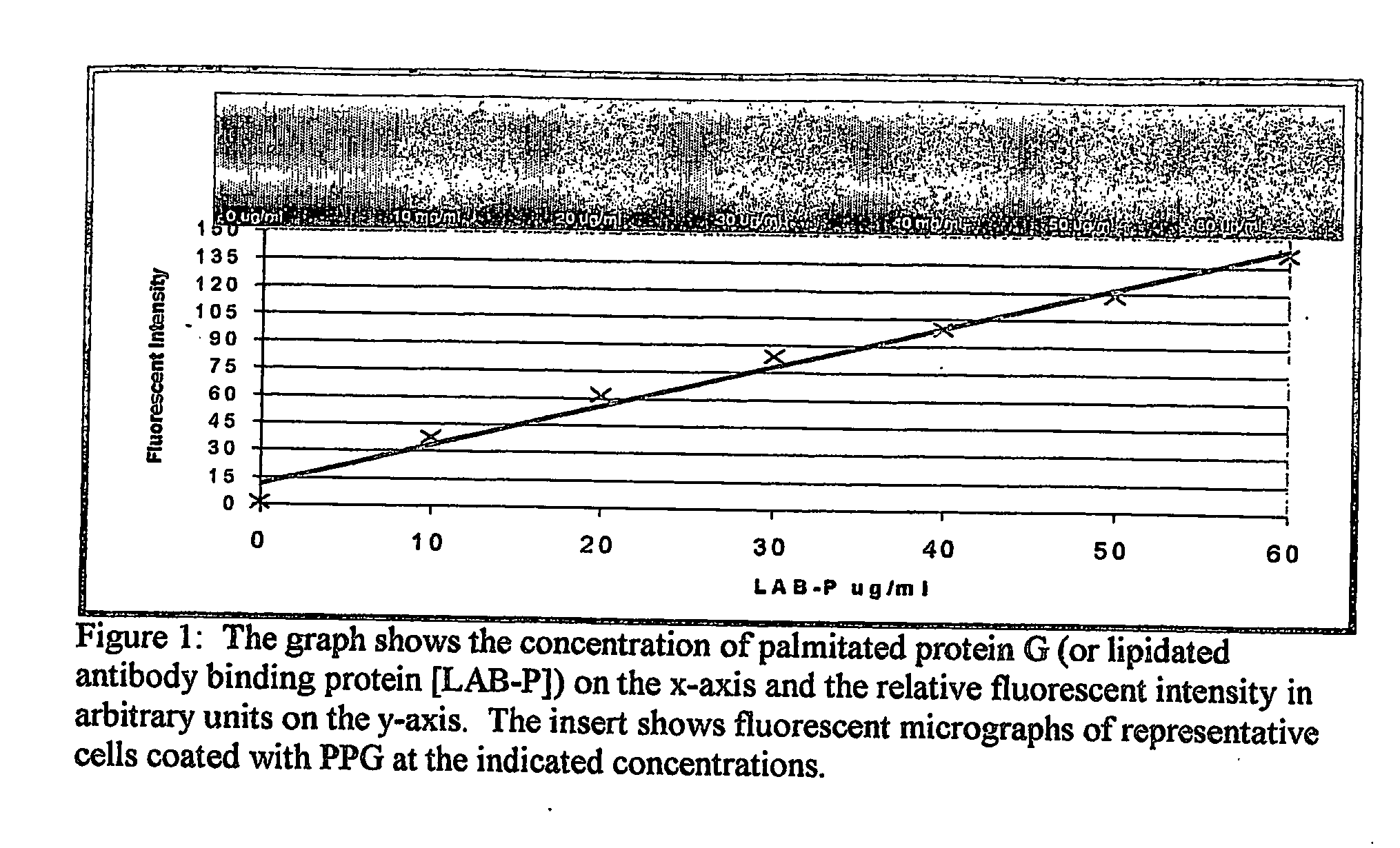
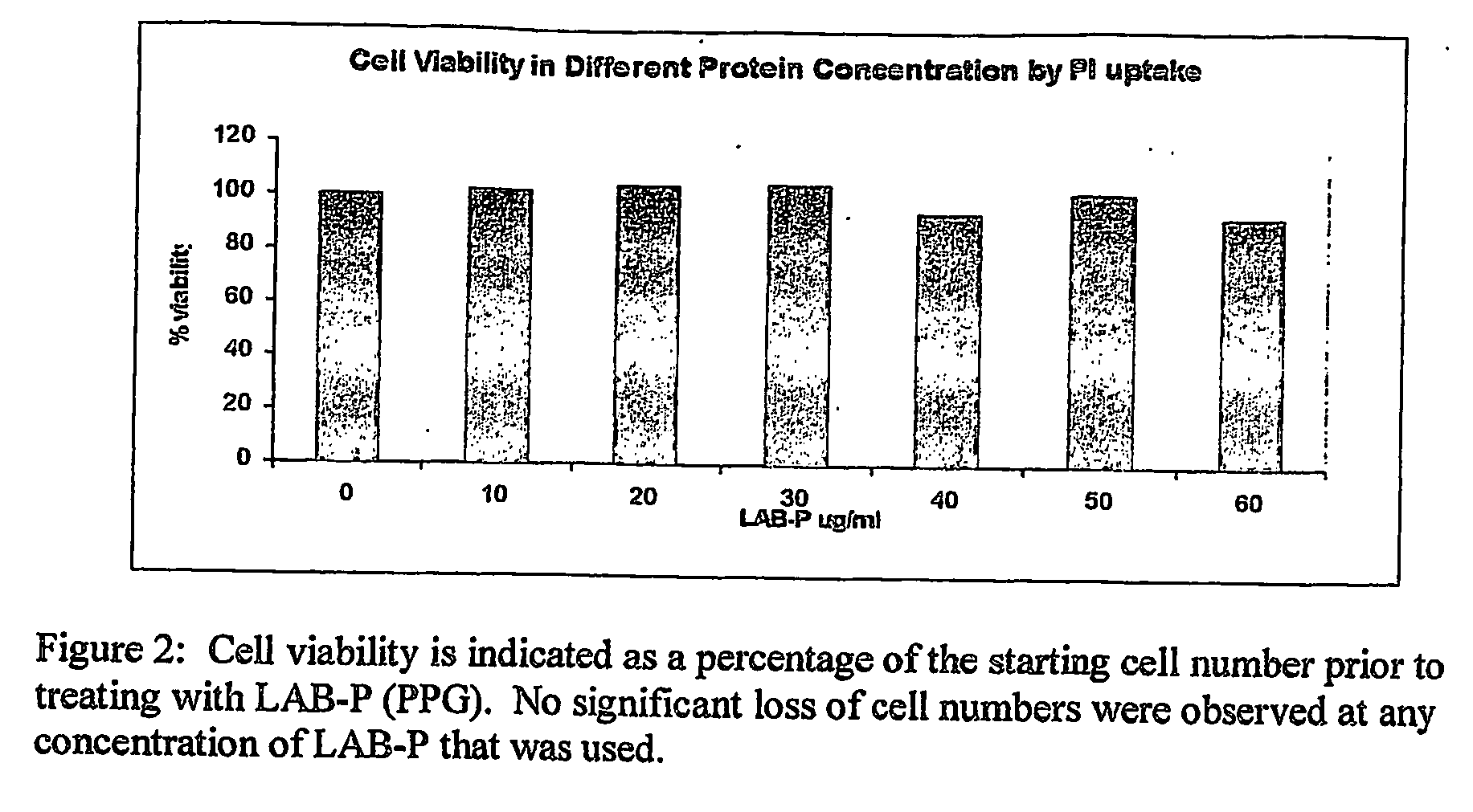
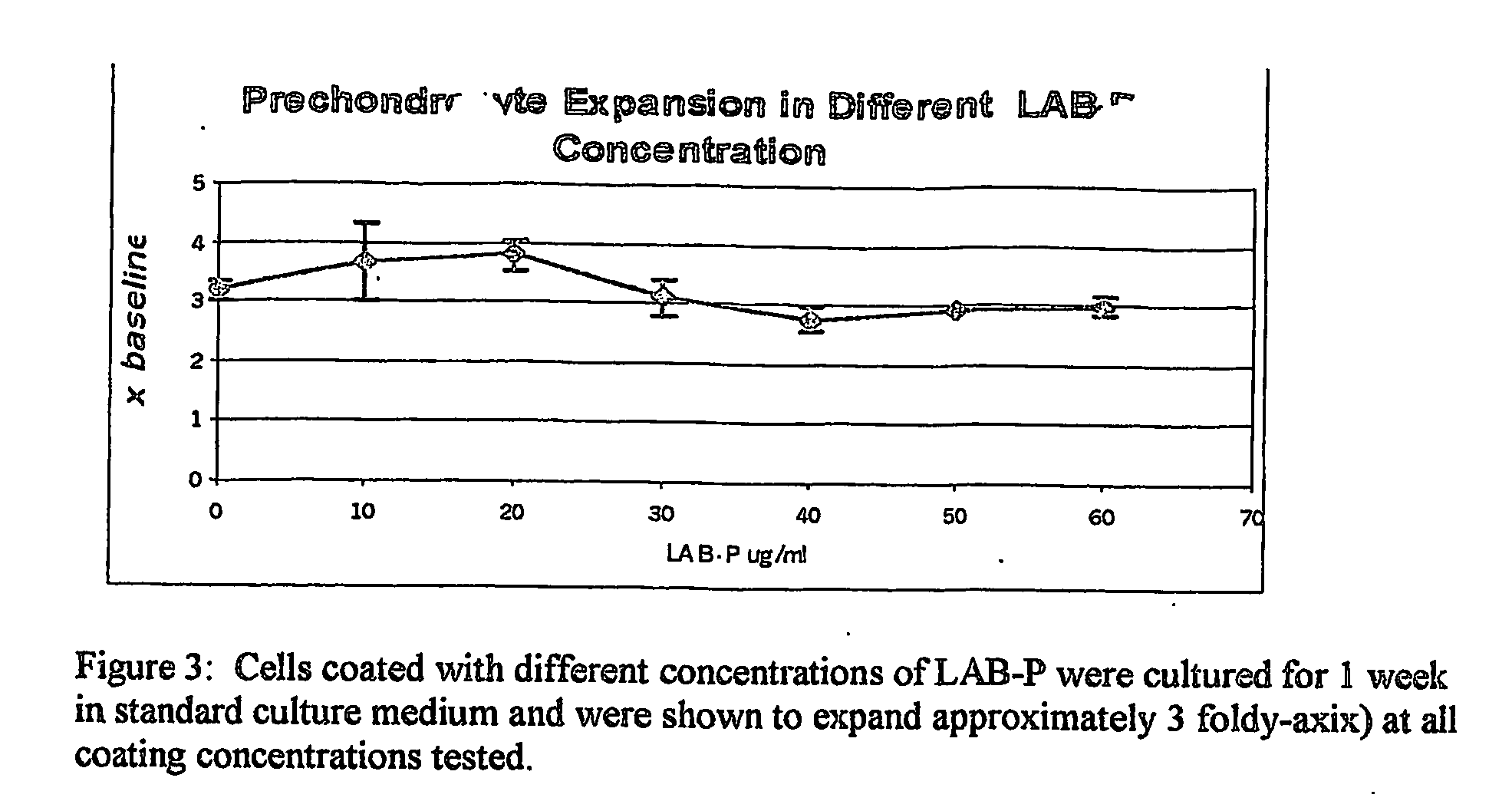
Patents
Literature
1252 results about "Cell therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
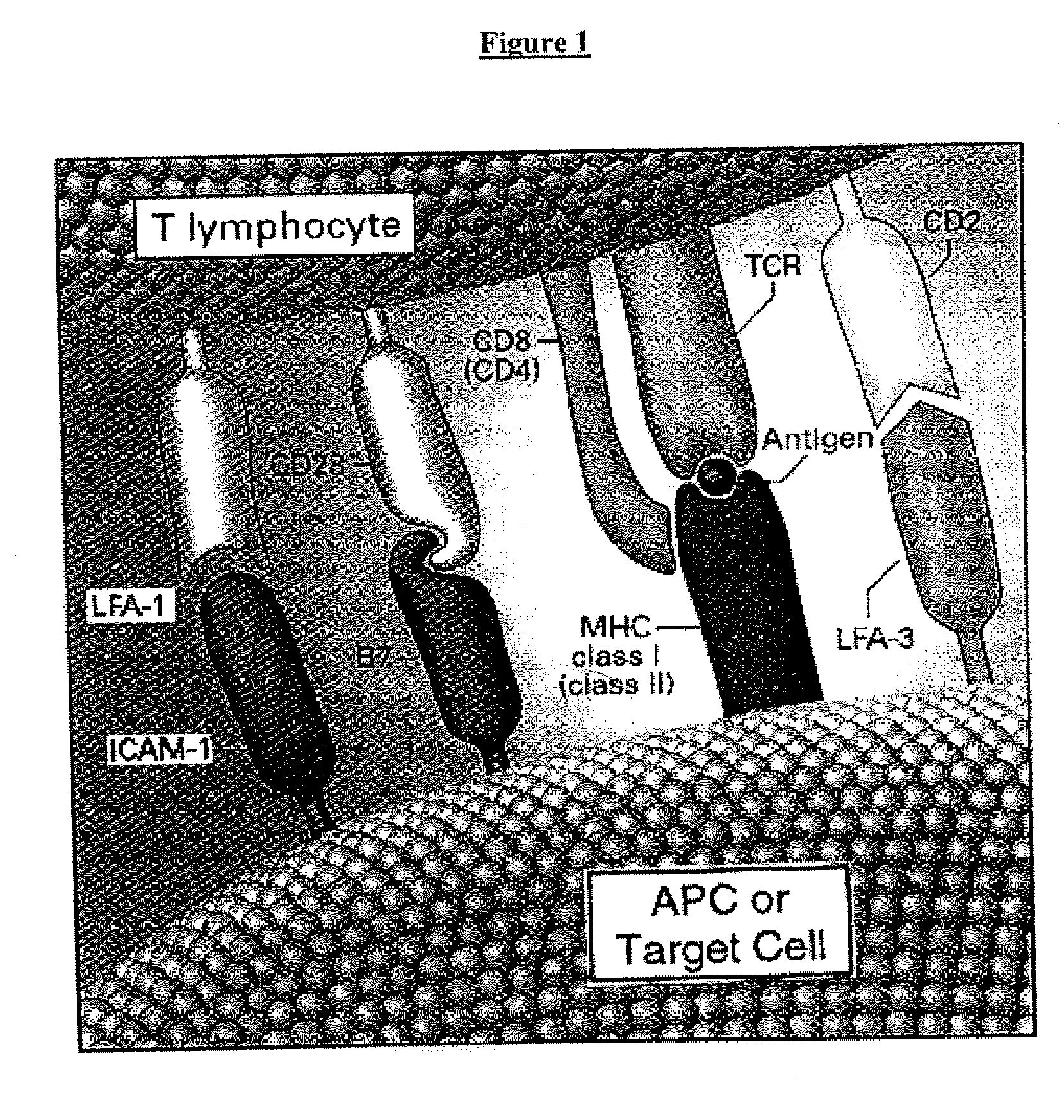
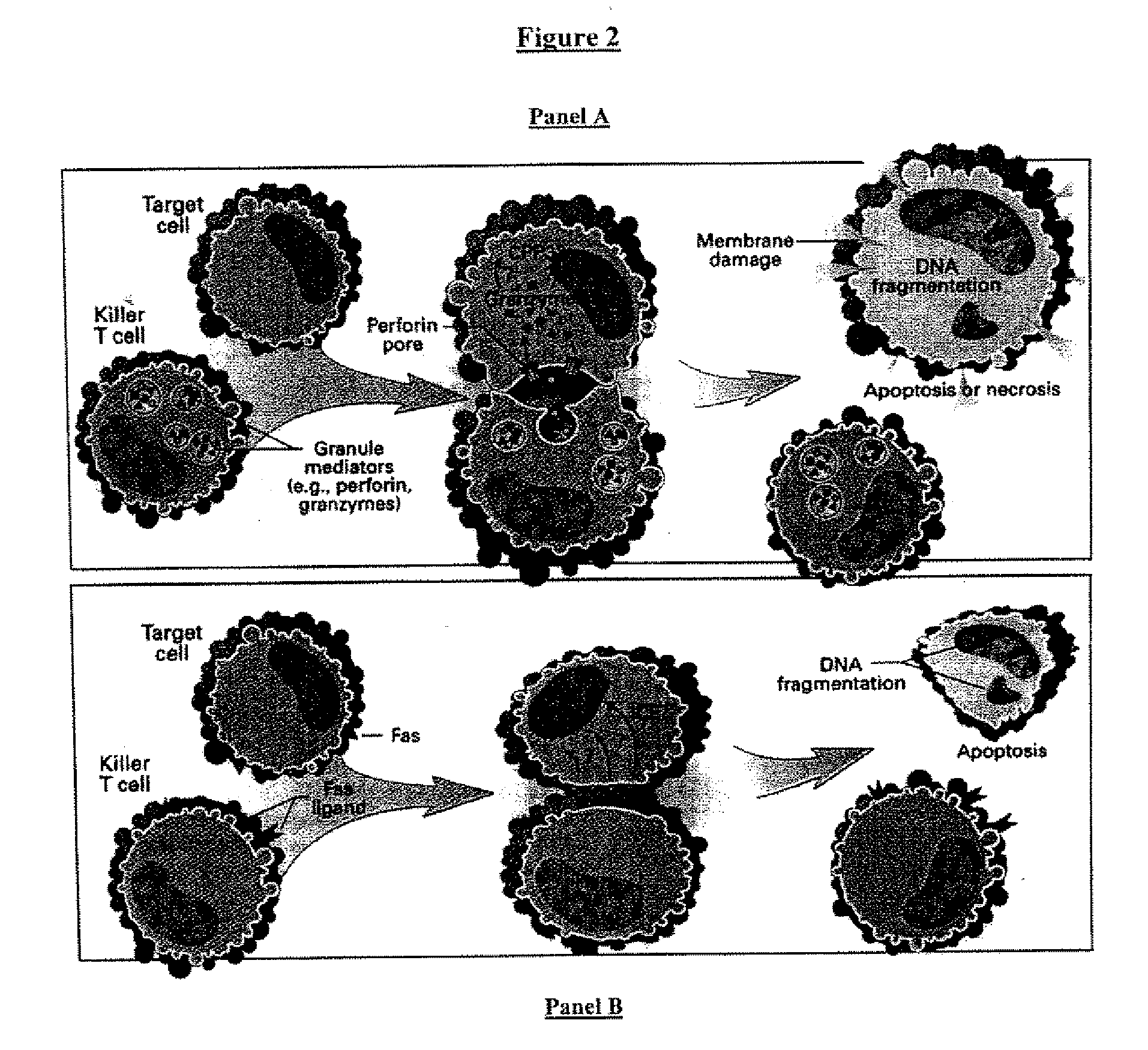
Cell therapy (also called cellular therapy or cytotherapy) is therapy in which cellular material is injected, grafted or implanted into a patient; this generally means intact, living cells. For example, T cells capable of fighting cancer cells via cell-mediated immunity may be injected in the course of immunotherapy.
Defined media for stem cell culture
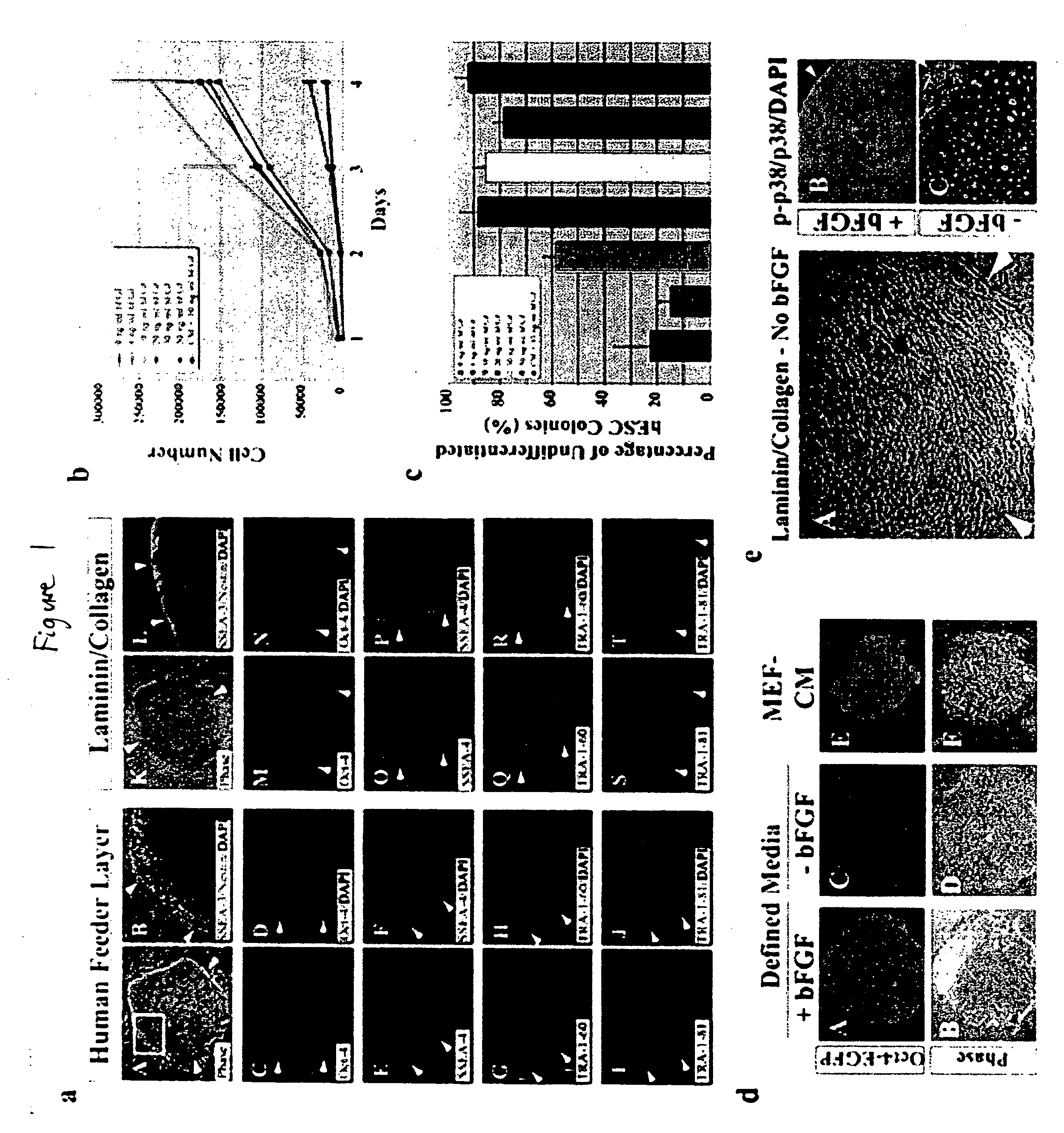
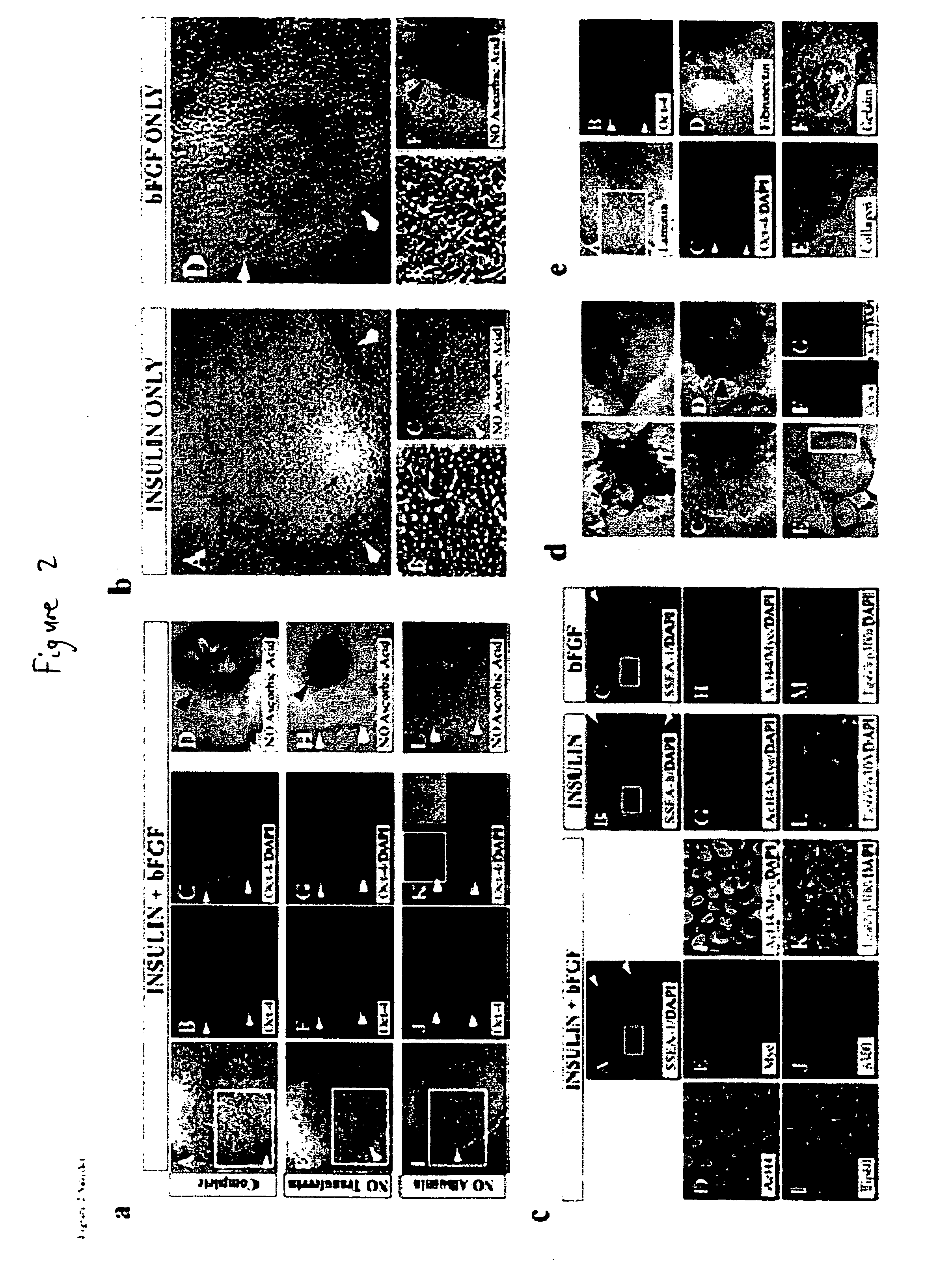
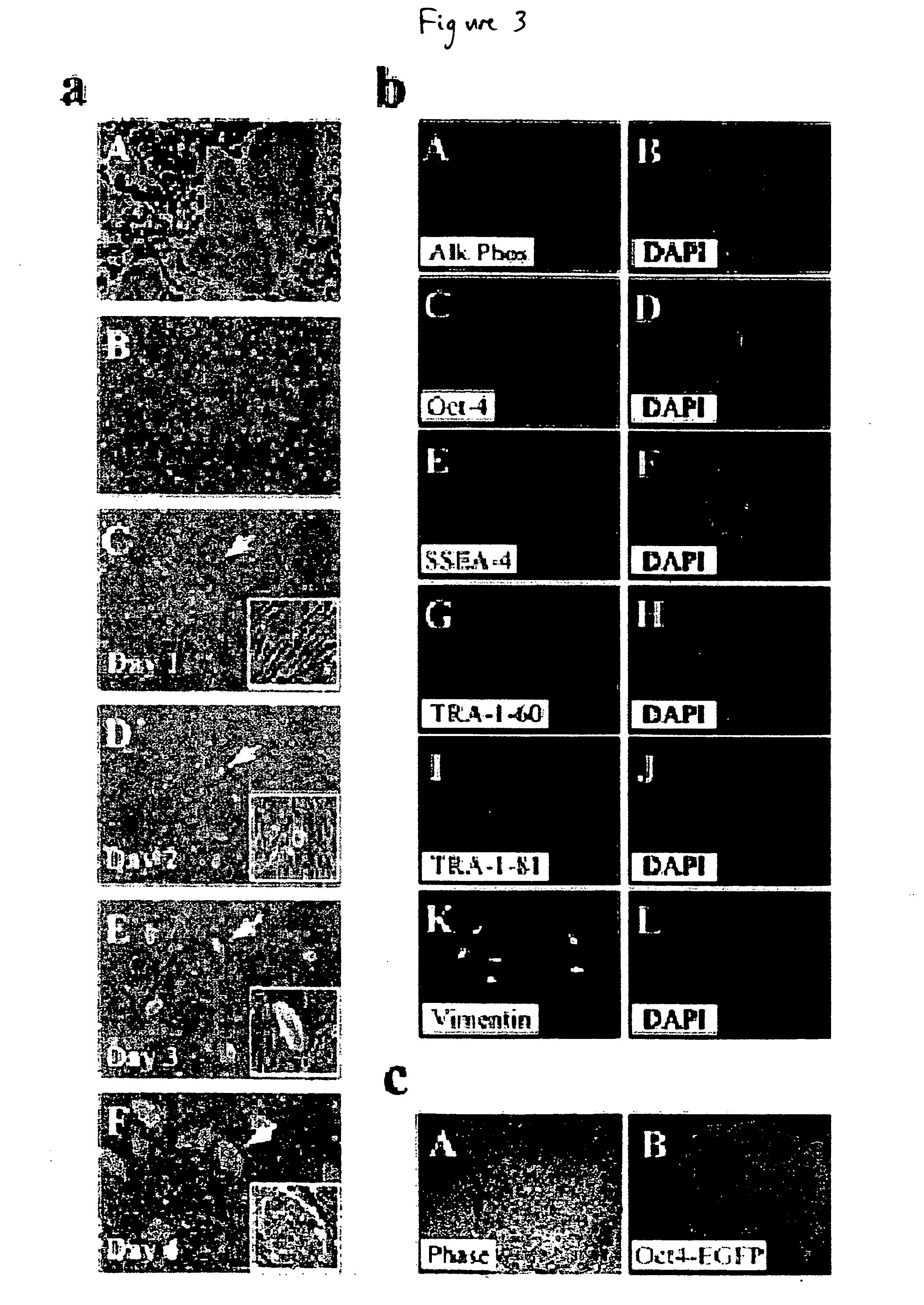
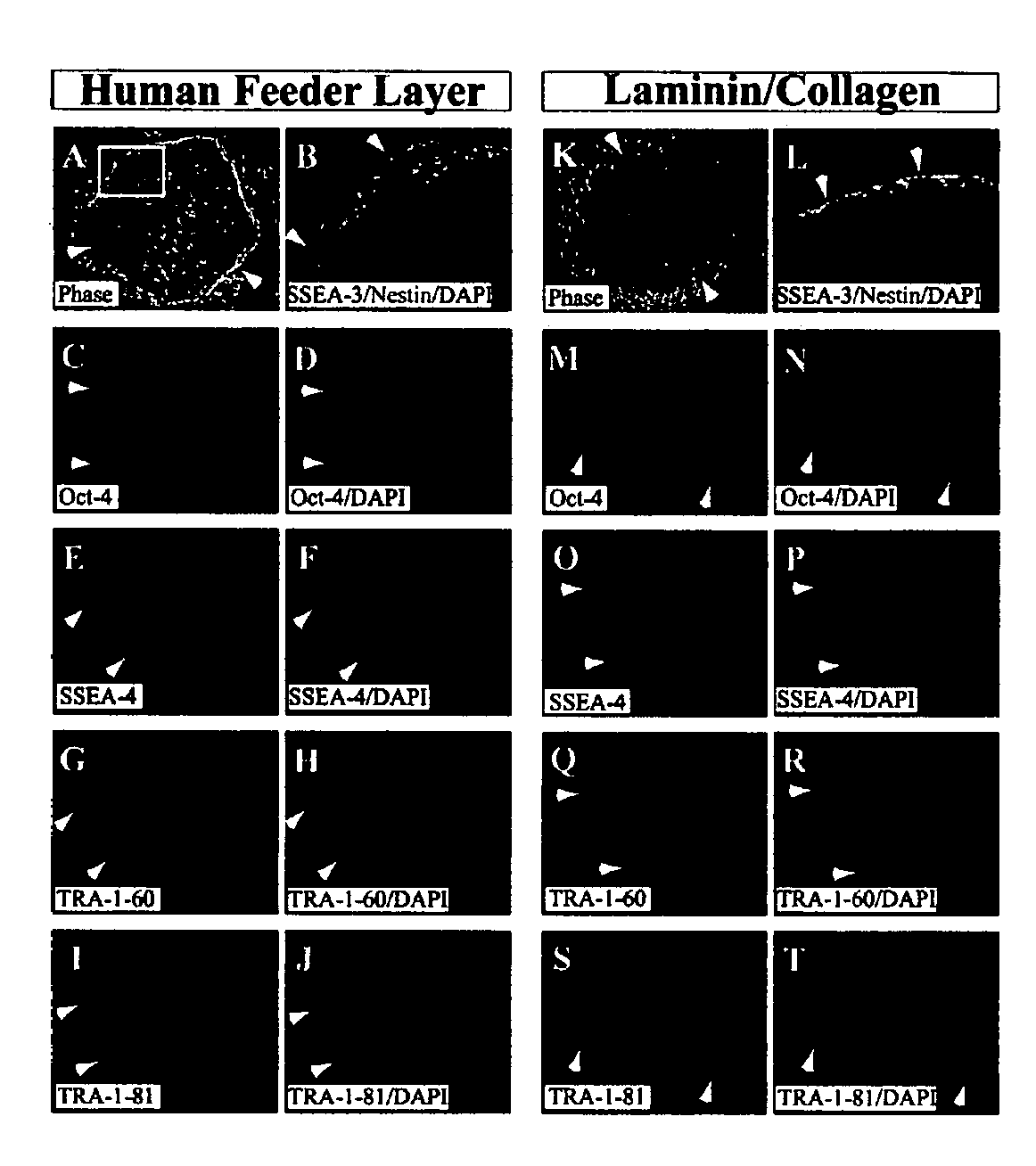
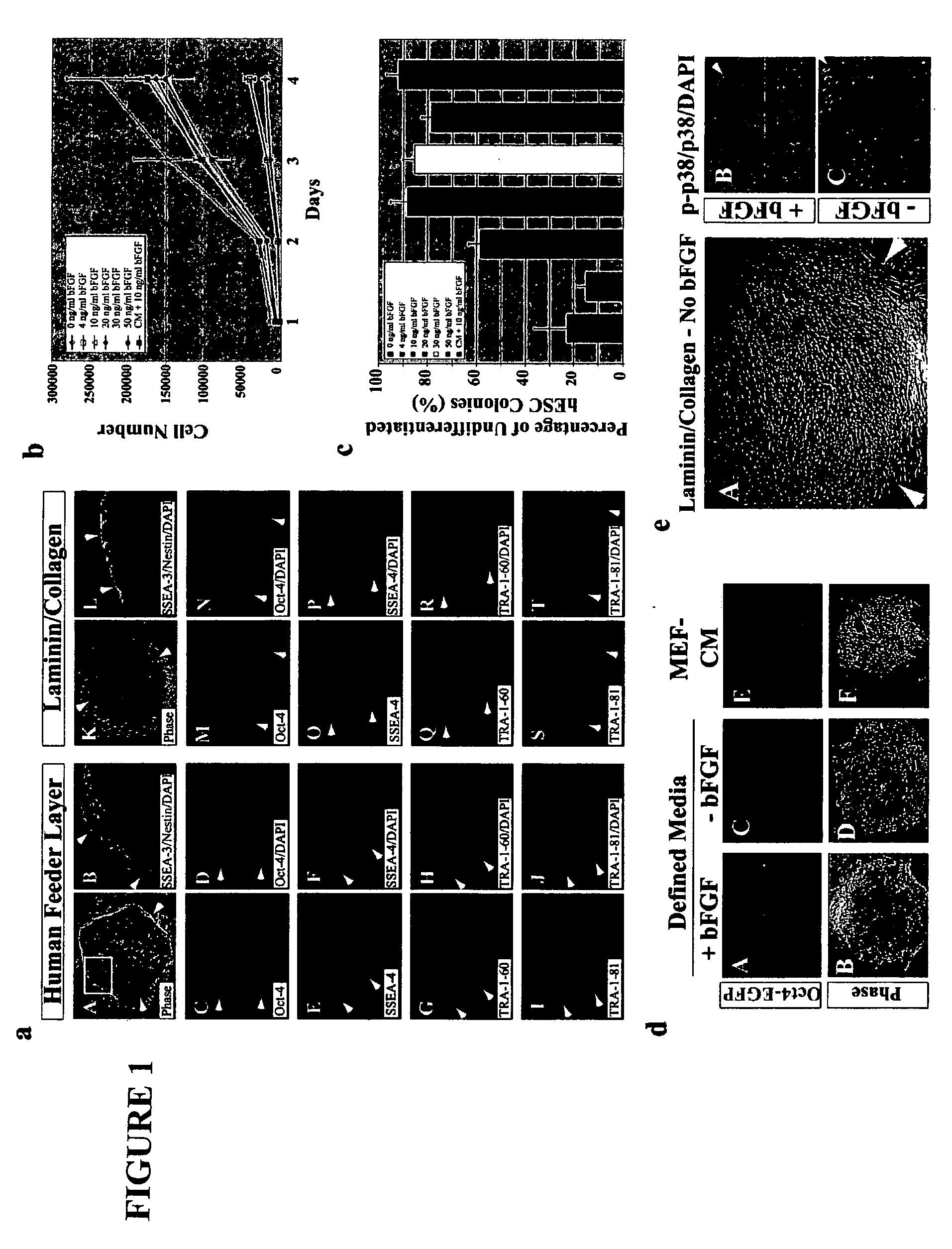
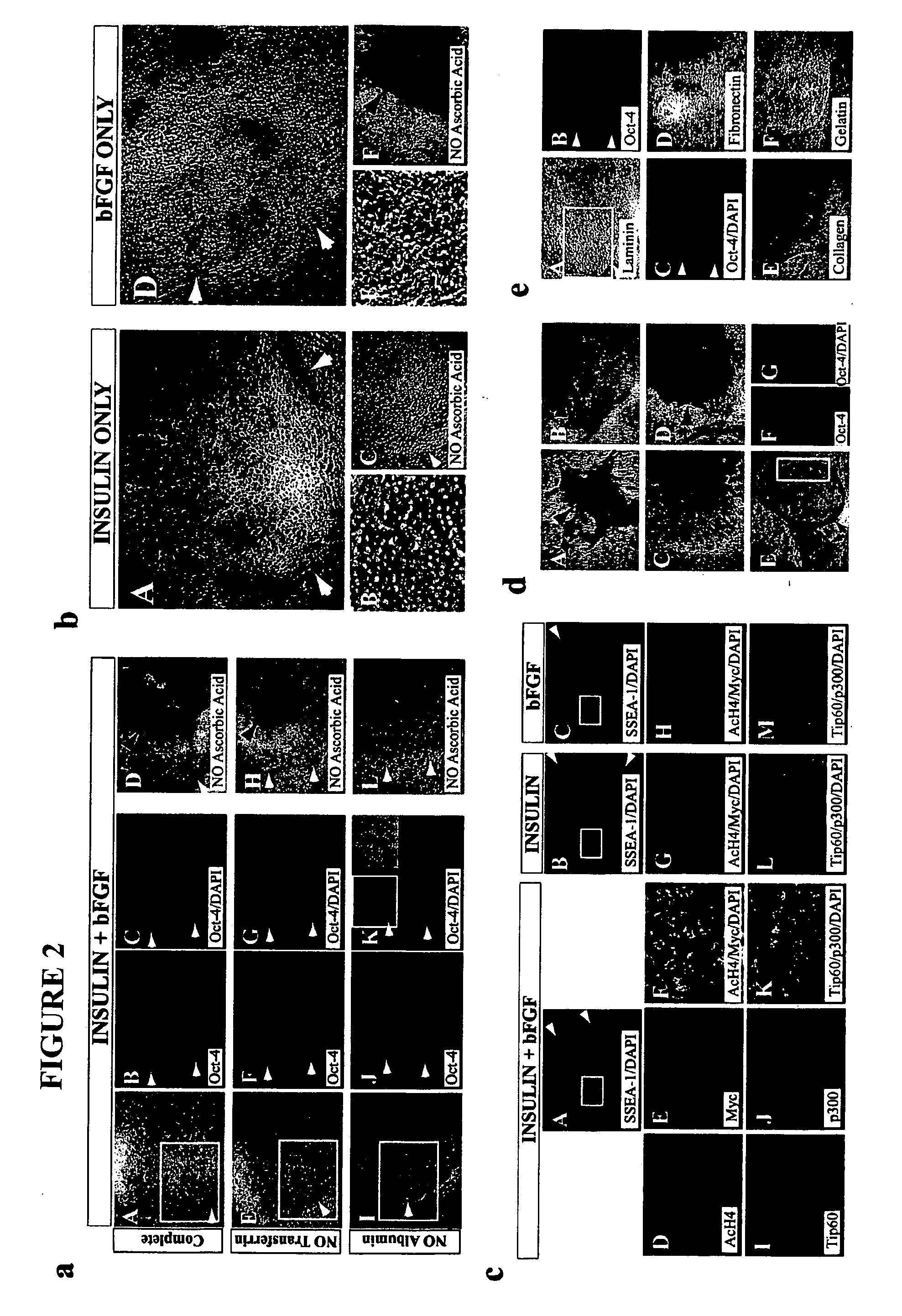
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Adoptive cell therapy with young T cells
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
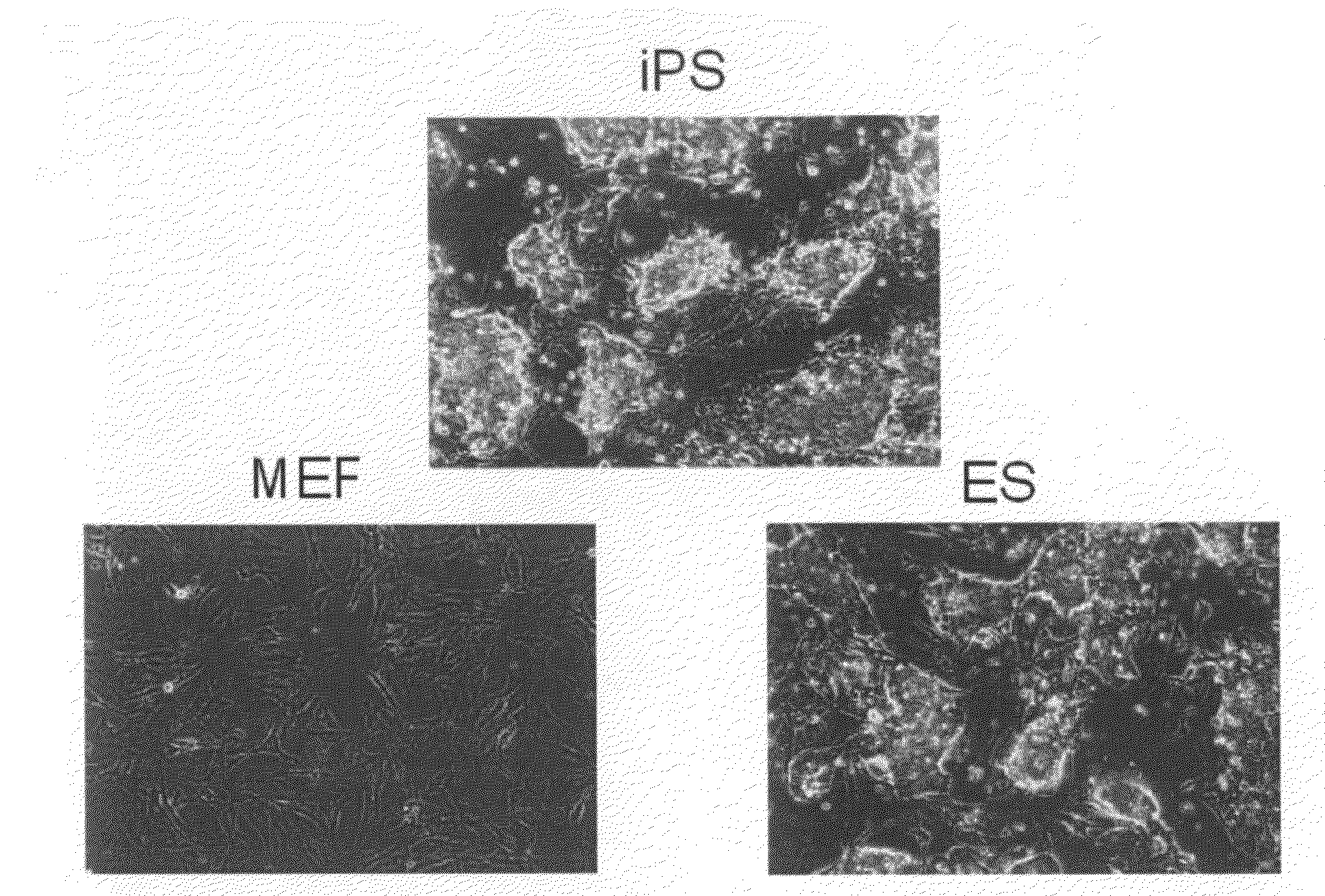
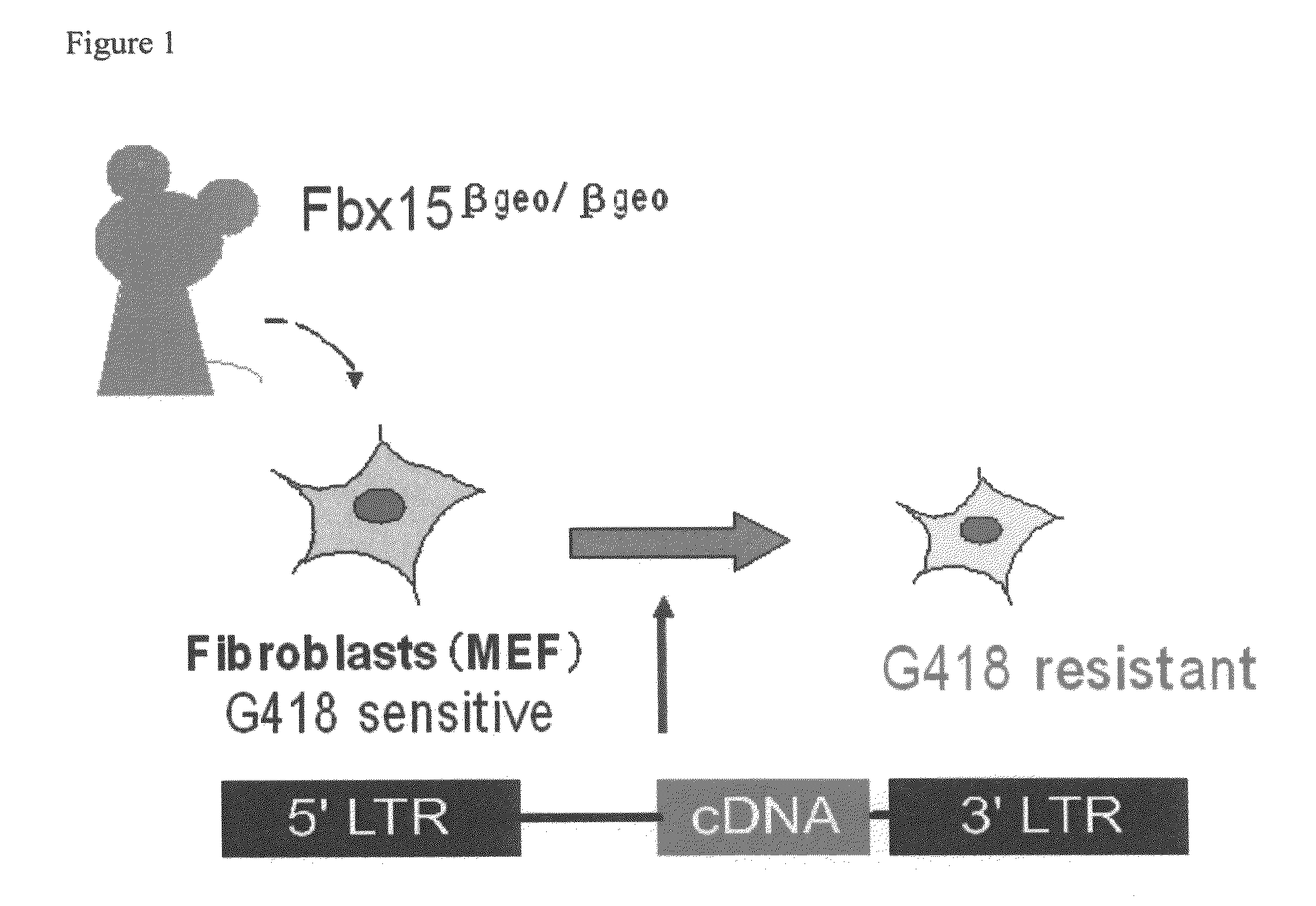
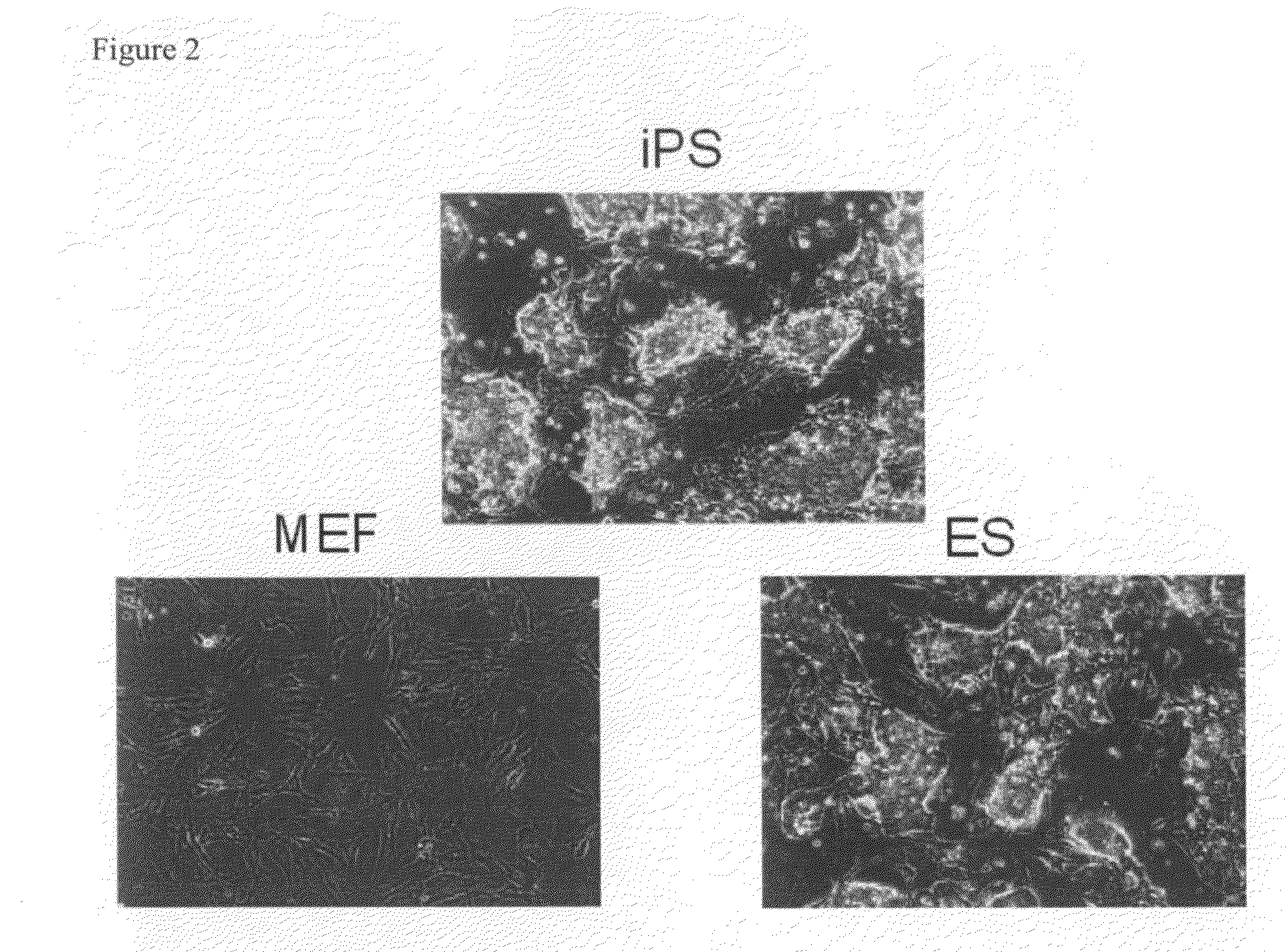
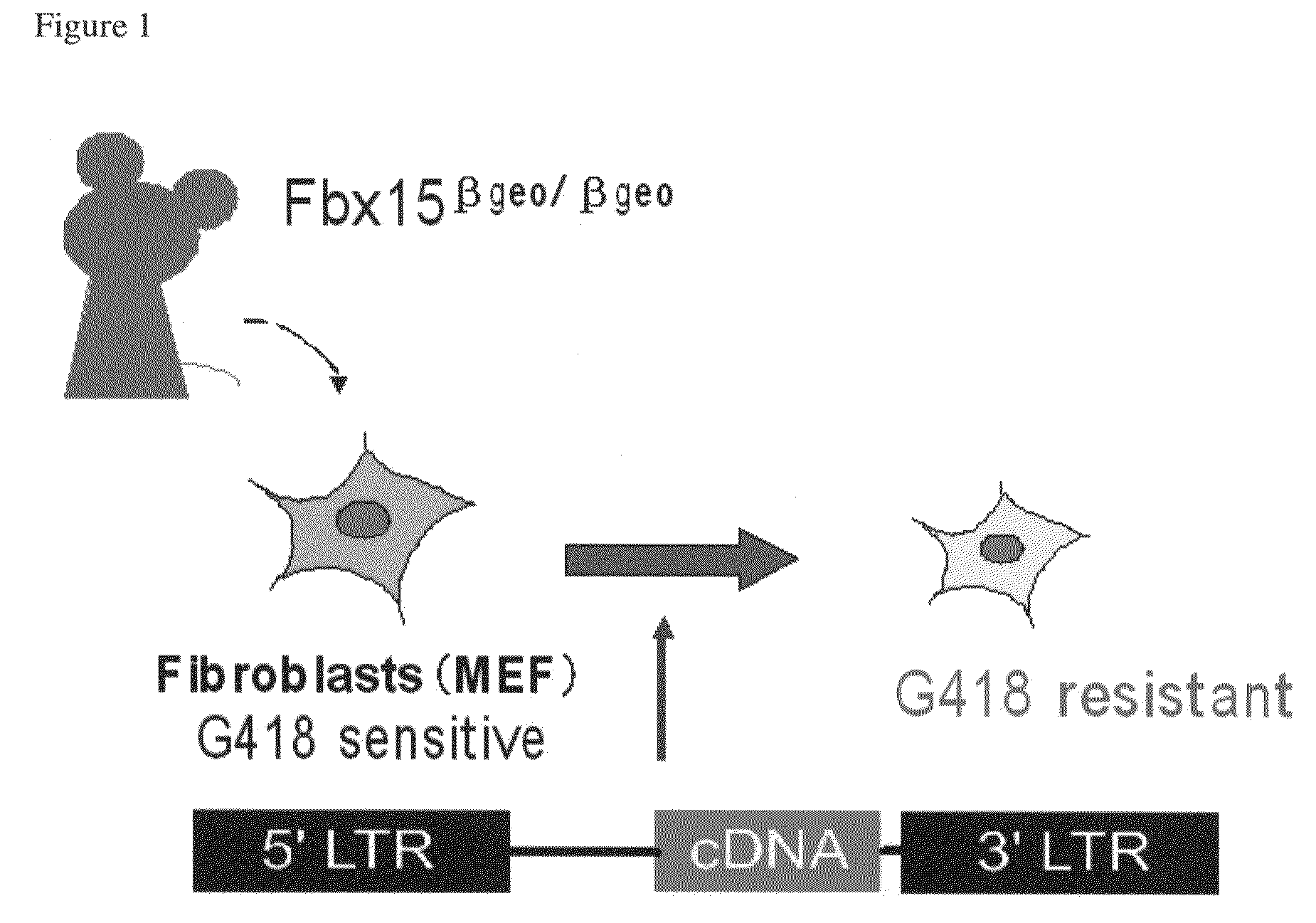
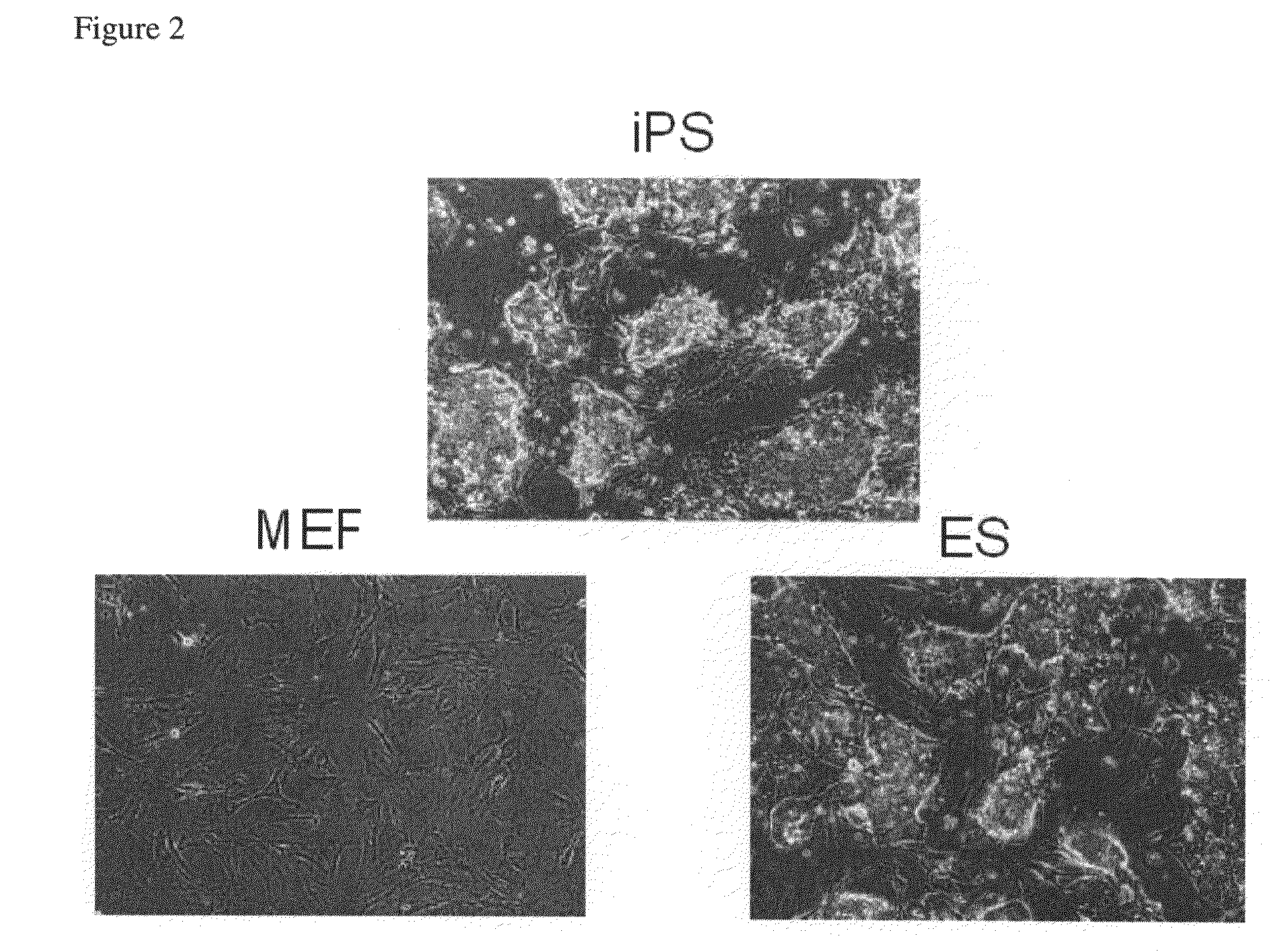
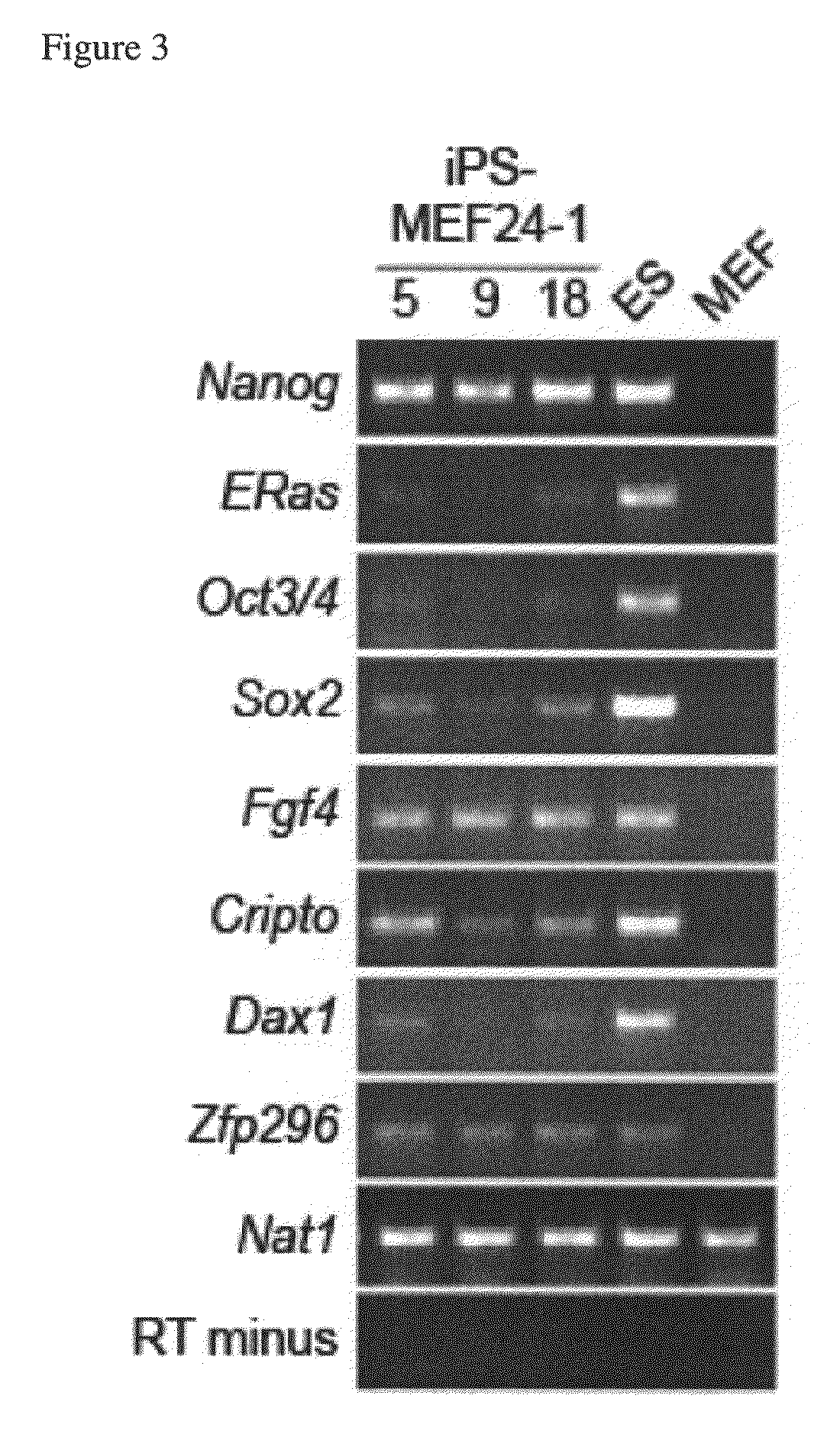
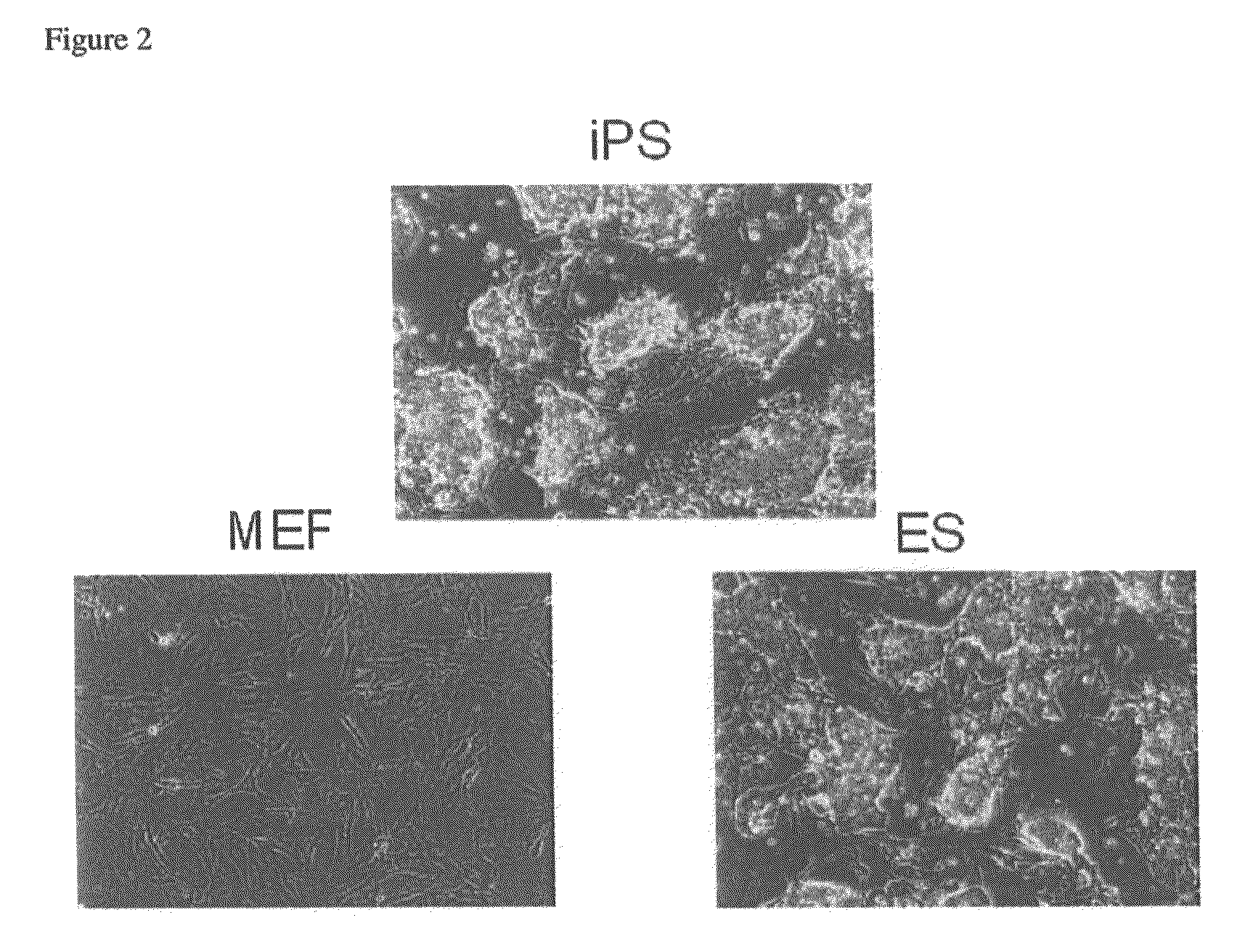
Nuclear reprogramming factor and induced pluripotent stem cells
InactiveUS20090227032A1Easy to prepareEffective isolationGenetically modified cellsPeptidesNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
Somatic cell reprogramming by retroviral vectors encoding Oct3/4. Klf4, c-Myc and Sox2
ActiveUS8129187B2Easy to prepareEffective isolationGenetically modified cellsArtificial cell constructsNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
Adoptive cell therapy with young t cells
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
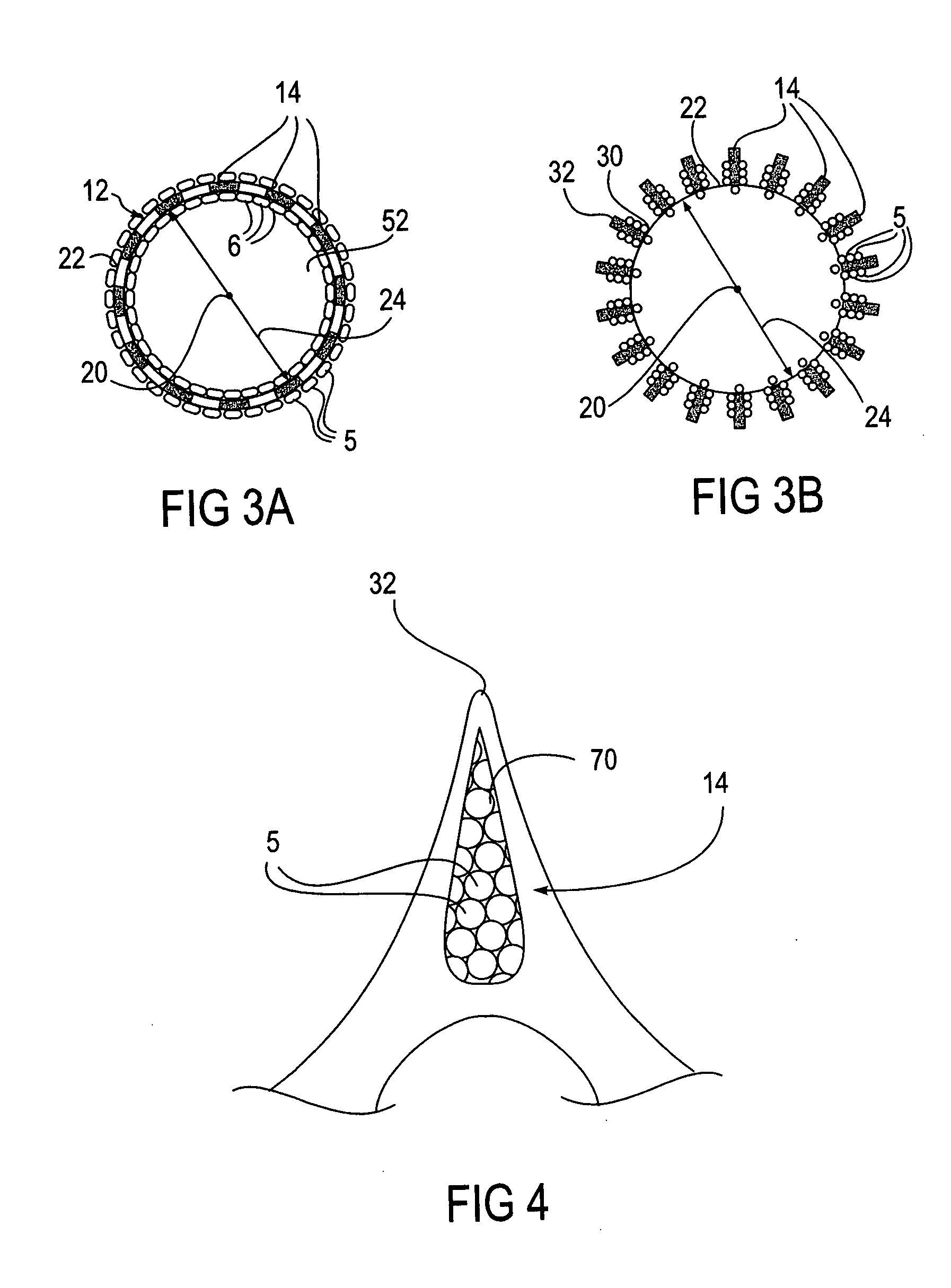
Cell seeded expandable body
InactiveUS20050096731A1Promote regenerationAugment tissue repairStentsSurgeryInsertion stentBody cavity use
Devices, systems and methods for treating medical conditions using cell therapy via body lumens. Localized delivery is achieved with the use of a stent-like expandable body seeded with cells. The expandable body is expanded to contact at least a portion of the inner walls of the body lumen and the cells, cellular products and / or other therapeutic agents are delivered to the surrounding tissue. The therapeutic benefit provided is dependent on the type of cells used and the features of the expandable body.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
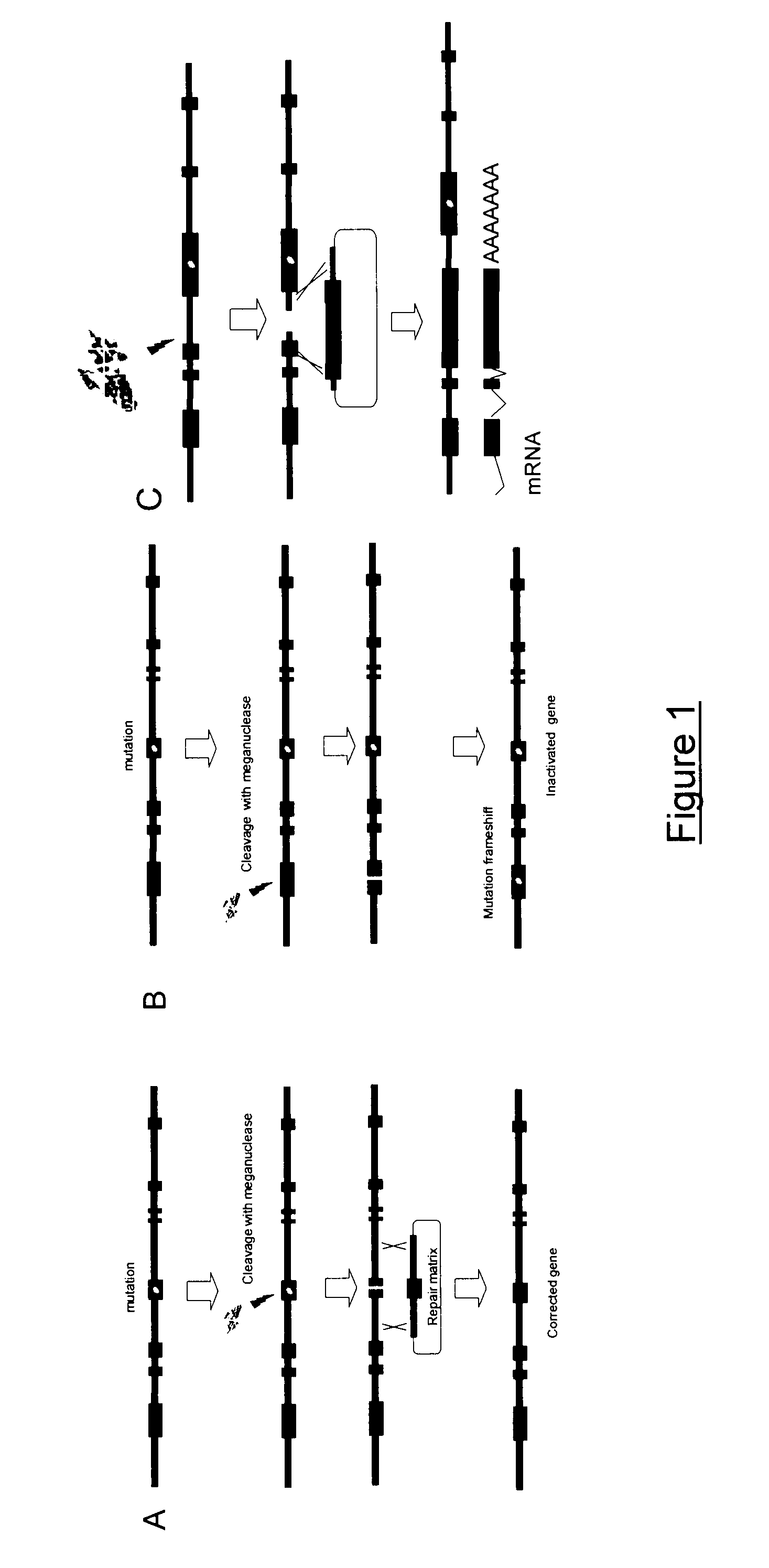
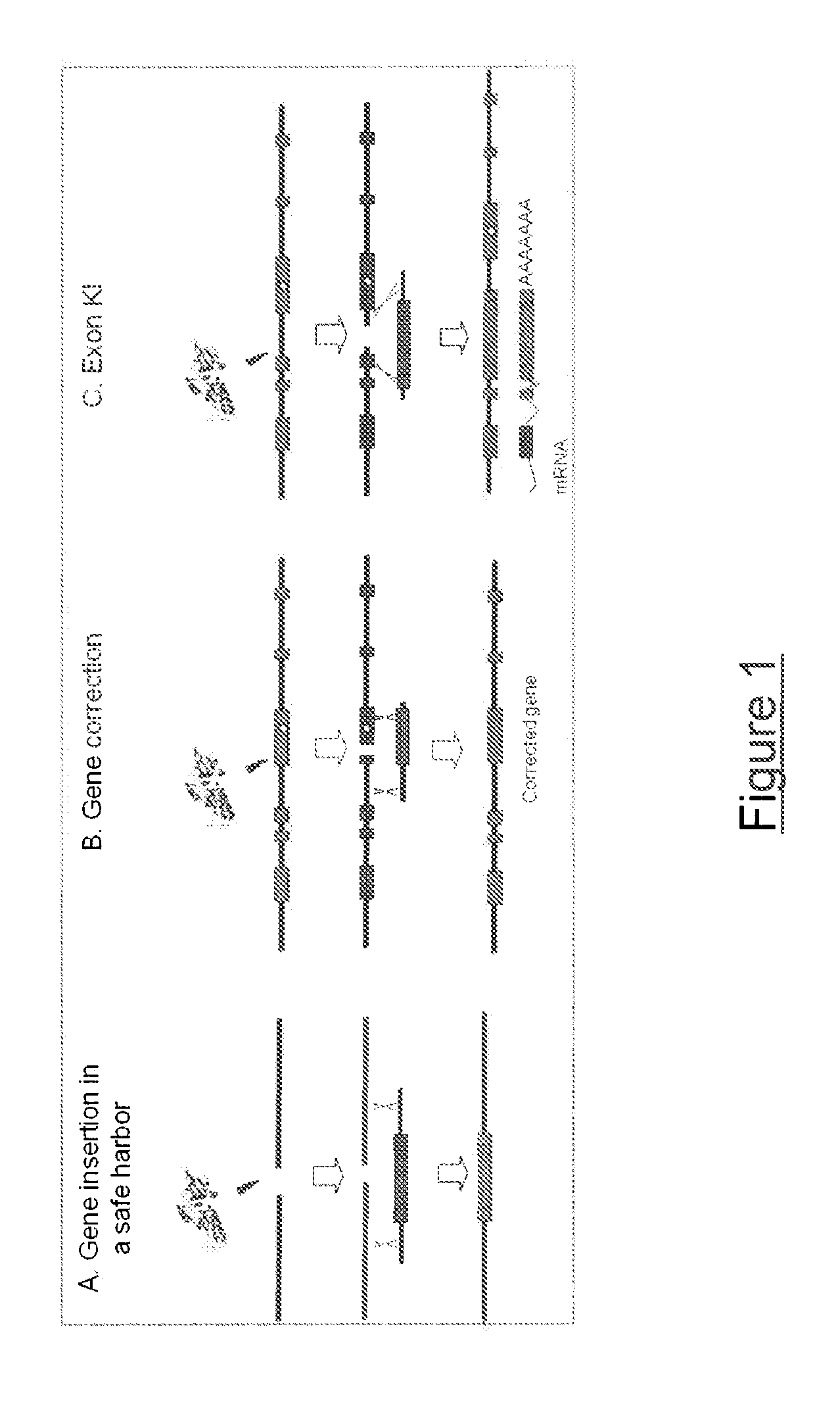
Meganuclease variants cleaving a DNA target sequence from the rhodopsin gene and uses thereof
InactiveUS20130183282A1Recovery functionInhibit expressionFusion with DNA-binding domainSugar derivativesA-DNANuclease
The invention relates to meganuclease variants which cleave a DNA target sequence from the human Rhodopsin gene (RHO), to vectors encoding such variants, to a cell, an animal or a plant modified by such vectors and to the use of these meganuclease variants and products derived therefrom for genome therapy, ex vivo (gene cell therapy) and genome engineering including therapeutic applications and cell line engineering.
Owner:CELLECTIS SA
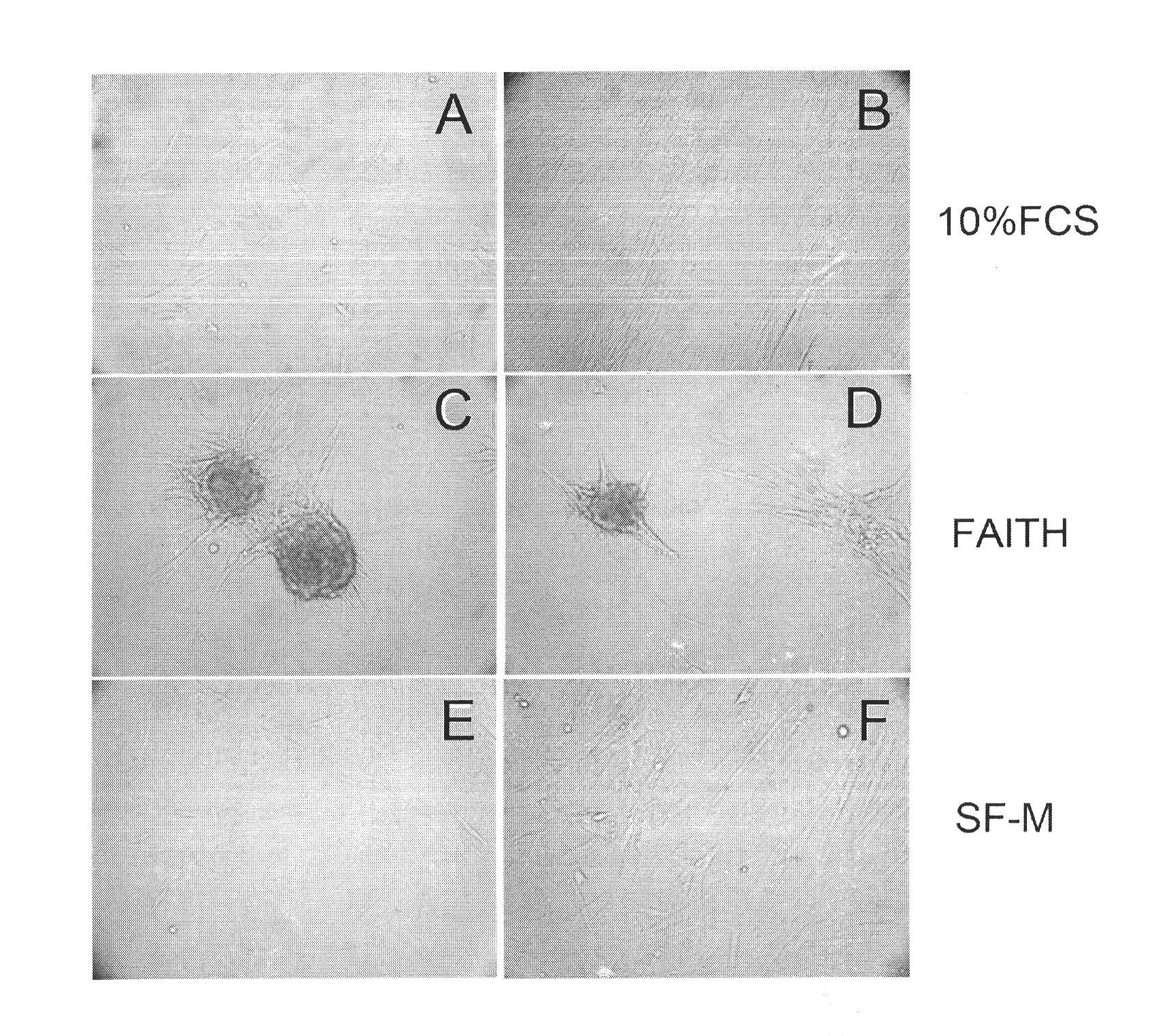
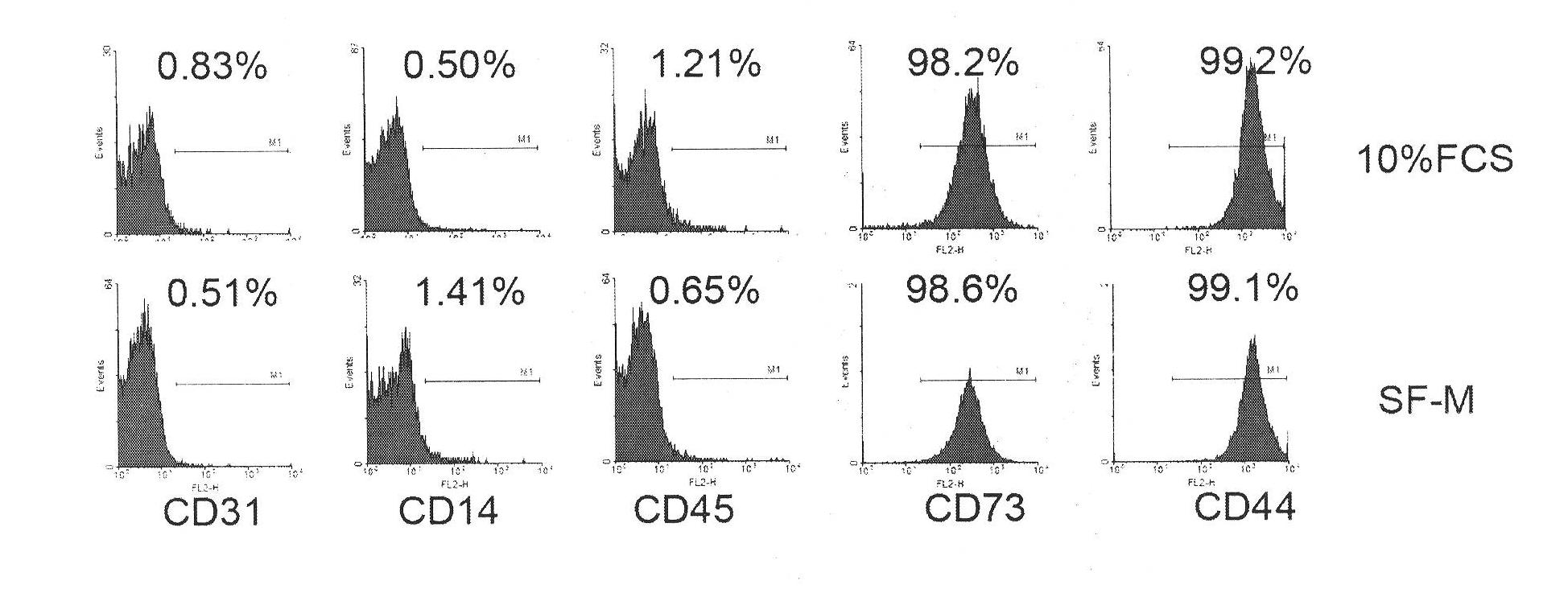
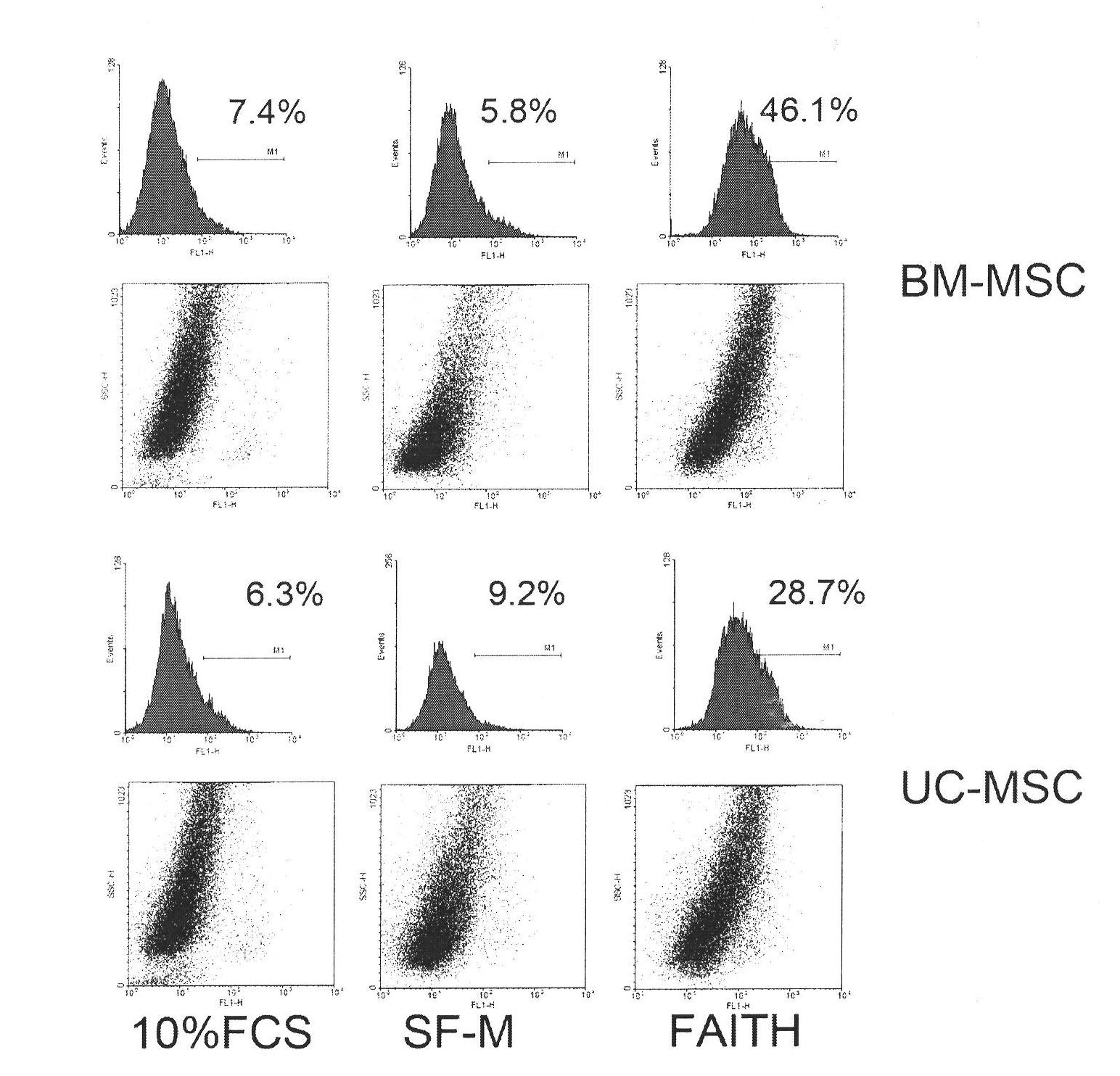
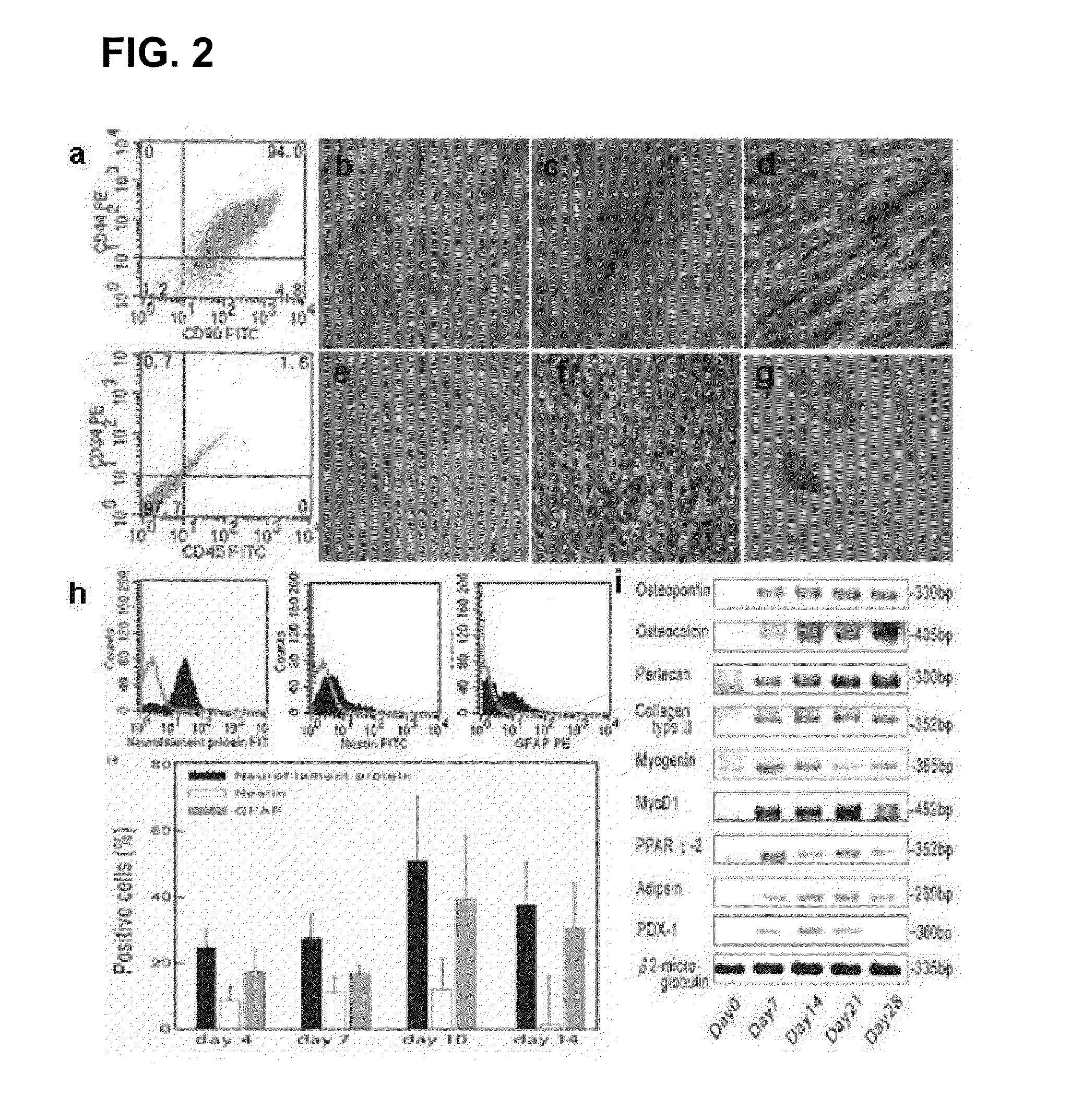
Culture medium for culturing mesenchymal stem cells
InactiveCN101984048AThe chemical composition is clear and clearThe possibility of causing a qualitative differenceSkeletal/connective tissue cellsLipid formationProtein molecules
The invention discloses a culture medium for culturing mesenchymal stem cells. The culture medium comprises improved minimum medium IMDM, cell growth factor combination, insulin, trace elements, lipid substances, vitamin substances, hormone substances, protein molecules, amino acid substances and other compounds. The culture medium provided by the invention does not contain serum or plasma (comprising animal or human serum or plasma), thus, when the system is used for culturing the mesenchymal stem cells, zoonosis caused by the animal serum or plasma in the cell therapy process can be avoided, and the risk of human immunity reaction which is possibly caused in the cell therapy process can be avoided. The culture medium has specific chemical constituent, thus the possibility of different cultured mesenchymal stem cell properties caused by the difference of culture systems can be avoided. The culture medium is not only suitable for culturing the mesenchymal stem cells of human marrow and umbilical cord resources but also suitable for culturing the mesenchymal stem cells of other tissues or other animals, and has the advantage of wide application range.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Meganuclease variants cleaving a DNA target sequence from the dystrophin gene and uses thereof
InactiveUS20130145487A1Expression is sufficient and stableNo impact on the expression of other genesSugar derivativesBacteriaA-DNANuclease
The invention relates to meganuclease variants which cleave a DNA target sequence from the human dystrophin gene (DMD), to vectors encoding such variants, to a cell, an animal or a plant modified by such vectors and to the use of these meganuclease variants and products derived therefrom for genome therapy, ex vivo (gene cell therapy) and genome engineering including therapeutic applications and cell line engineering. The invention also relates to the use of meganuclease variants for inserting therapeutic transgenes other than DMD at the dystrophin gene locus, using this locus as a safe harbor locus. The invention also relates to the use of meganuclease variants for using the dystrophin gene locus as a landing pad to insert and express genes of interest.
Owner:CELLECTIS SA
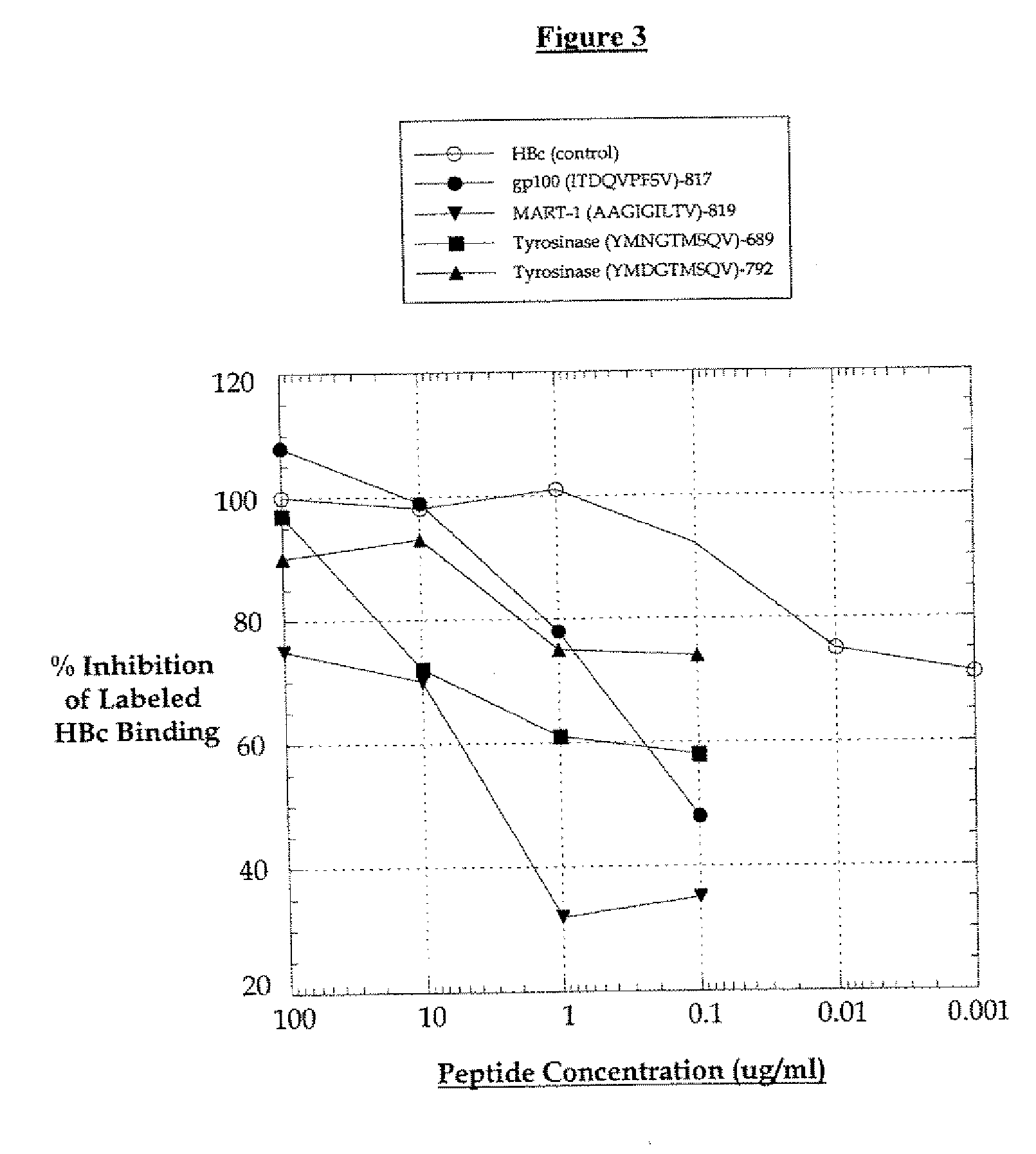
Purification of antigen-specific T cells
InactiveUS20060134125A1Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAbnormal tissue growth
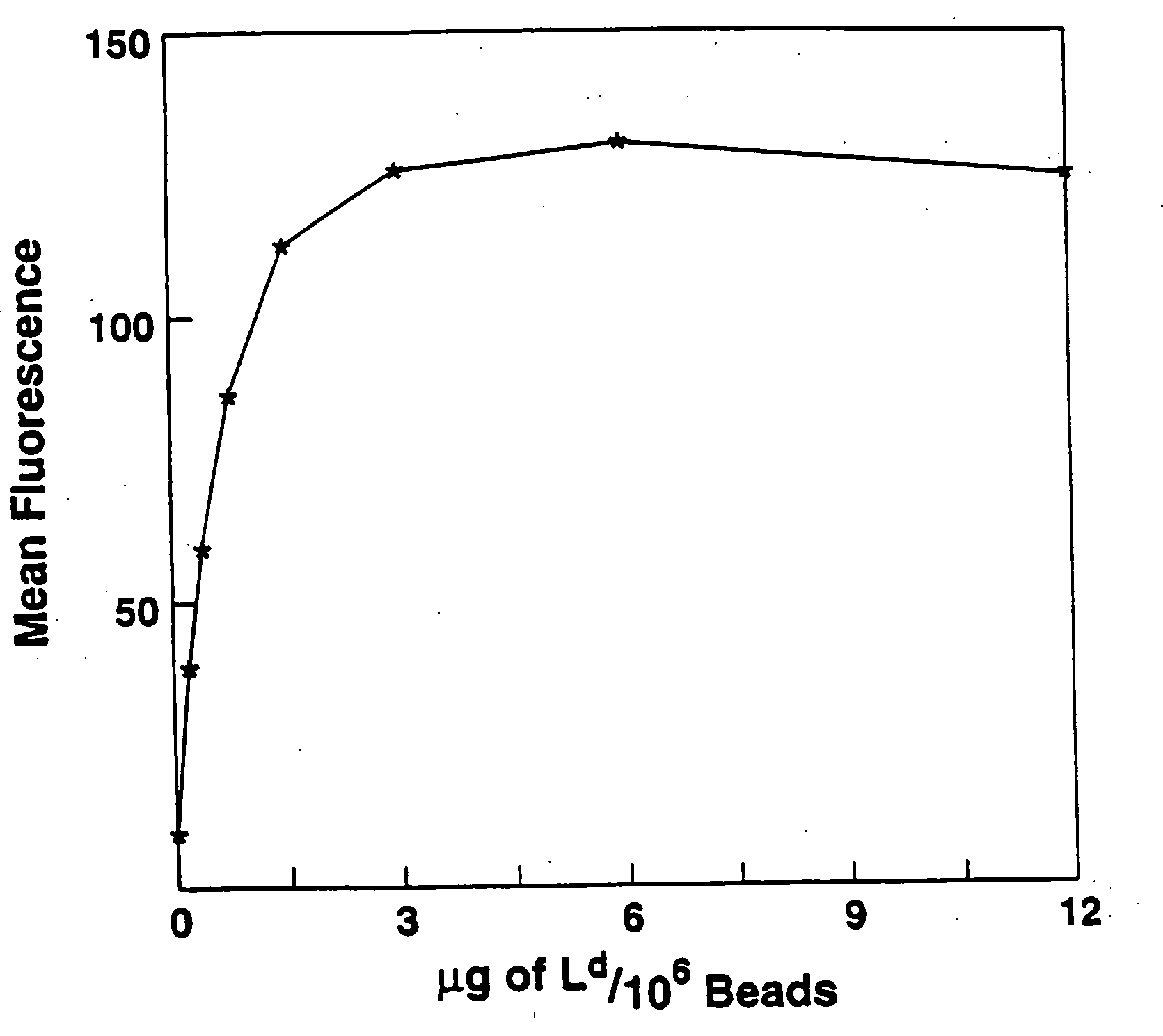
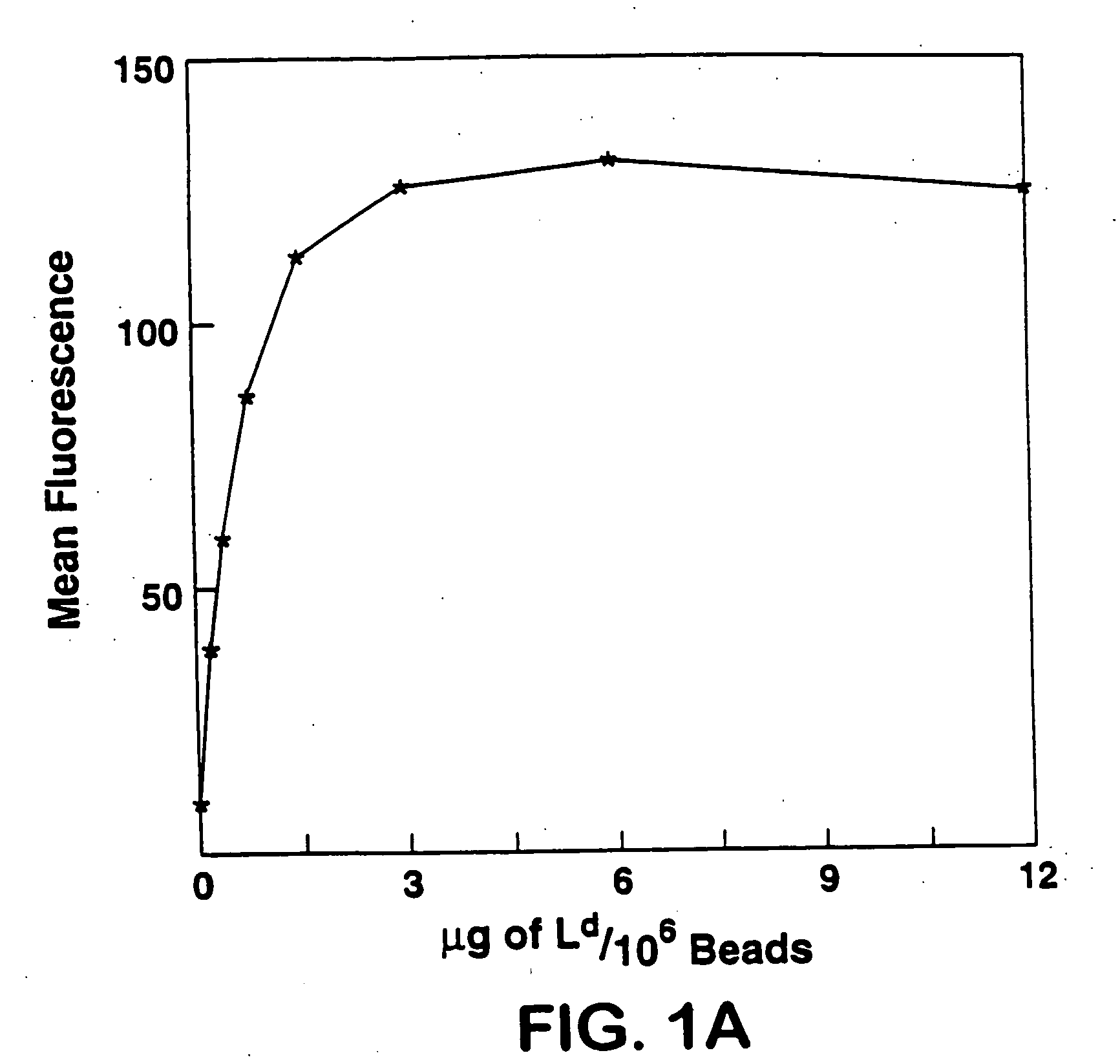
A new method to capture, purify and expand antigen-specific T lymphocytes has been developed using magnetic beads coated with recombinant MHC class I molecules. This method was optimized using homogenous populations of naive T cells purified from mice transgenic for the 2C T cell receptor (TCR). These T cells were captured on beads coated with MHC class I molecules and the relevant antigenic peptides. MHC and peptide specificity was confirmed by the usage of irrelevant MHC peptide combinations. An enrichment of 800 to 1600 fold was measured, using 2C T cells mixed with irrelevant T cells, starting from a 2C T cell frequency of 1 / 3000. The same approach was used to purify antigen-specific CD8+ T cells from total CD8+ T cells from naive mice. The recovered cells could be expanded and specifically kill target cells in vitro; they had a significant effect in vivo as well. We expect this procedure to be suitable to purify and expand in vitro tumor- and virus-specific killer T cells for use in cell therapy.
Owner:ORTHO PHARMA
Enhanced stem cell therapy and stem cell production through the administration of low level light energy
ActiveUS20110060266A1Efficient productionGood effectElectrotherapyElectrical/wave energy microorganism treatmentDiseaseTreatment effect
Methods of enhancing stem cell therapy through the administration of low level light energy are provided in several embodiments. Some embodiments comprise irradiating stem cells before or after implantation at a target tissue having loss of function due to damage or disease, with a resultant increase in the efficacy of the cell therapy. In some embodiments, light energy enhances one or more of the viability, proliferation, migration or engraftment of the stem cells, thereby enhancing the therapeutic effects of the irradiated cells during cell therapy.
Owner:PHOTOTHERA IP HLDG
A cell therapy method for the treatment of tumors
T cell responses are often diminished in humans with a compromised immune system. We have developed a method to isolate, stimulate and expand naïve cytotoxic T lymphocyte precursors (CTLp) to antigen-specific effectors, capable of lysing tumor cells in vivo. This ex vivo protocol produces fully functional effectors. Artificial antigen presenting cells (AAPCs; Drosophila melanogaster) transfected with human HLA class I and defined accessory molecules, are used to stimulate CD8+ T cells from both normal donors and cancer patients. The class I molecules expressed to a high density on the surface of the Drosophila cells are empty, allowing for efficient loading of multiple peptides that results in the generation of polyclonal responses recognizing tumor cells endogenously expressing the specific peptides. The responses generated are robust, antigen-specific and reproducible if the peptide epitope is a defined immunogen. This artificial antigen expression system can be adapted to treat most cancers in a significant majority of the population.
Owner:JANSSEN PHARMA INC
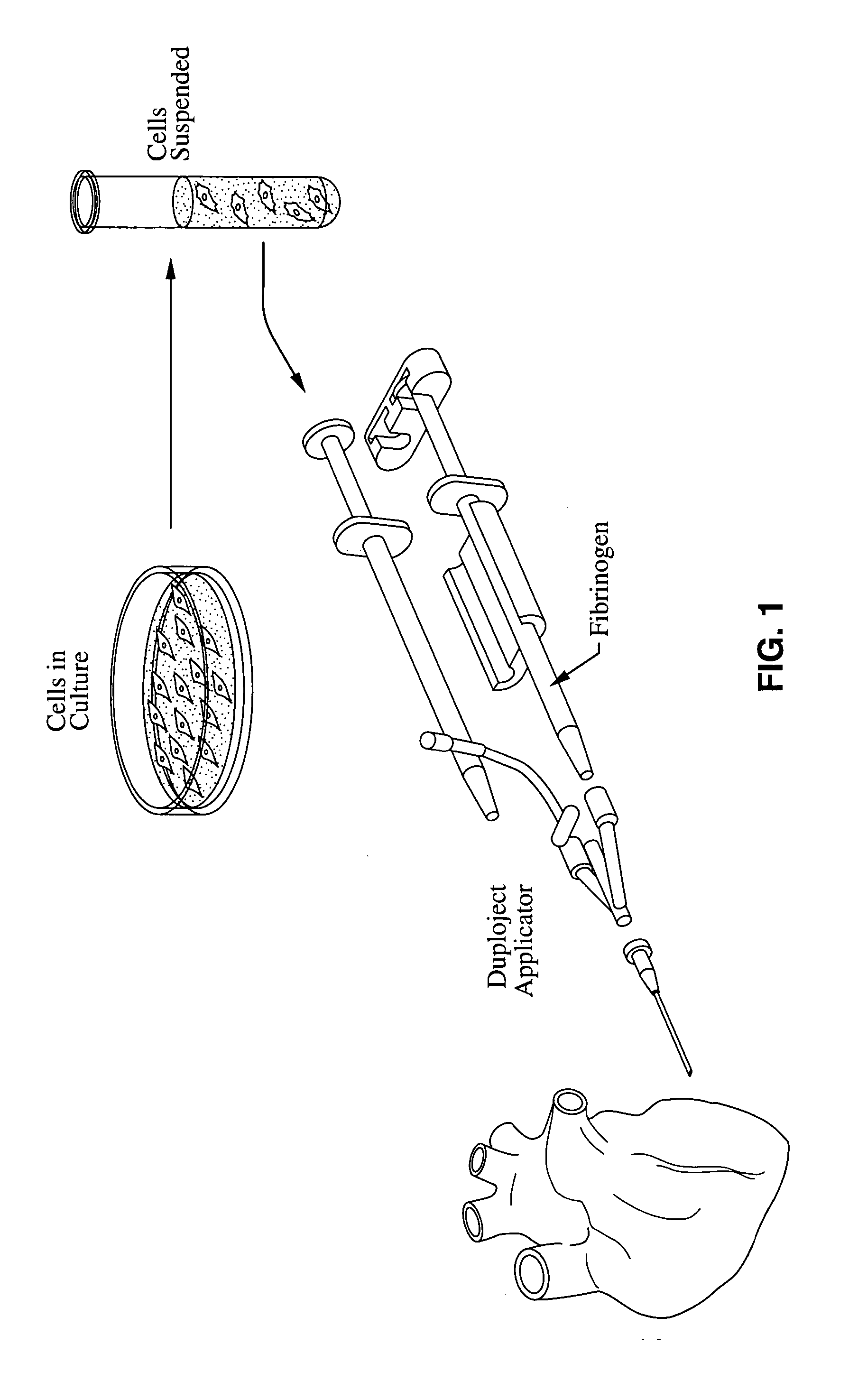
Material compositions and related systems and methods for treating cardiac conditions
InactiveUS20050271631A1Preventing negative remodelingHigh retention rateBiocideSurgical adhesivesInjectable polymersFibrin glue
A medical condition associated with a cardiac structure is treated by injecting an injectable polymer agent into the cardiac structure such that a therapeutic mechanical scaffolding is formed within the cardiac structure itself. In particular, the injectable scaffolding agent is a fibrin glue agent and is injected into regions of damaged myocardium such as ischemic tissue or infarct. LV wall dysfunction may also be treated by injecting the scaffolding agent into the LV wall. Cell therapy may be combined with the injection of fibrin glue or other injectable polymer scaffold agent. The polymeric forms of the agent may be injectable as precursor materials that polymerize as a scaffold in-situ within the cardiac structure. In other modes, polymer agents are injected in order to provide therapeutic angiogenesis, or to induce deposition of cells within the injected area, such as by providing the polymer with fragment E or RDG binding sites, respectively.
Owner:RGT UNIV OF CALIFORNIA
Method to enhance progenitor or genetically-modified cell therapy
InactiveUS20060167501A1Enhance capillary growthReduce arrhythmiaBiocideElectrotherapyProgenitorDisease
A method is provided including selecting a patient suffering from a condition, administering cells to the patient selected from the group consisting of: progenitor cells and genetically-modified cells, applying an electrical current to a site of the patient in a vicinity of nervous tissue, and configuring the current to stimulate the nervous tissue. Other embodiments are also described.
Owner:MEDTRONIC INC
Nuclear reprogramming factor and induced pluripotent stem cells
ActiveUS20100062533A1Easy to prepareEffective isolationGenetically modified cellsPeptidesNuclear reprogrammingCell therapy
The present invention relates to a nuclear reprogramming factor having an action of reprogramming a differentiated somatic cell to derive an induced pluripotent stem (iPS) cell. The present invention also relates to the aforementioned iPS cells, methods of generating and maintaining iPS cells, and methods of using iPS cells, including screening and testing methods as well as methods of stem cell therapy. The present invention also relates to somatic cells derived by inducing differentiation of the aforementioned iPS cells.
Owner:KYOTO UNIV
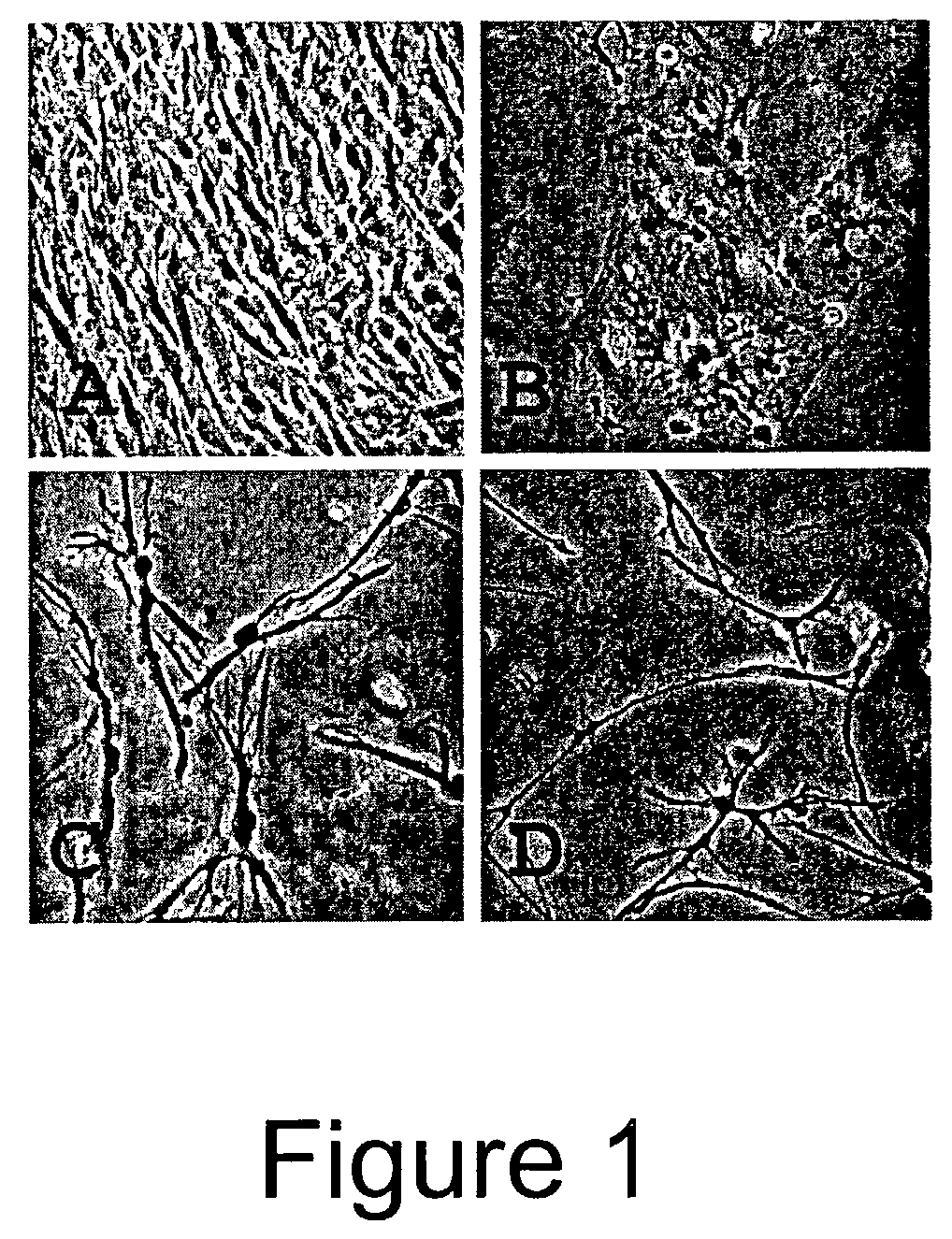
Trans-differentiation and re-differentiation of somatic cells and production of cells for cell therapies
The invention provides a method for effecting the trans-differentiation of a somatic cell, i.e., the conversion of a somatic cell of one cell type into a somatic cell of a different cell type. The method is practiced by culturing a somatic cell in the presence of at least one agent selected from the group consisting of (a) cytoskeletal inhibitors and (b) inhibitors of acetylation, and (c) inhibitors of methylation, and also culturing the cell in the presence of agents or conditions that induce differentiation to a different cell type. The method is useful for producing histocompatible cells for cell therapy.
Owner:ADVANCED CELL TECH INC
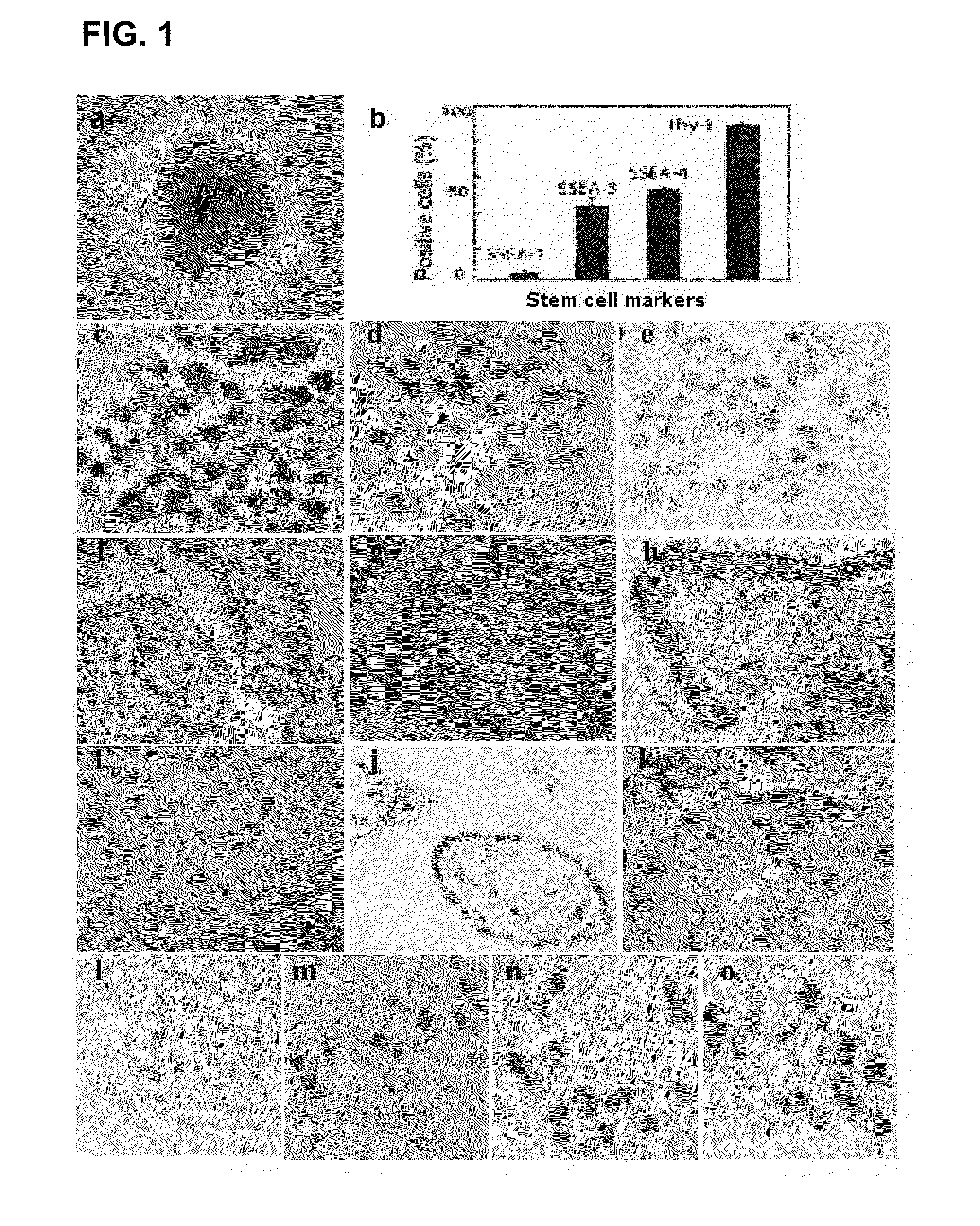
Human trophoblast stem cells and use thereof
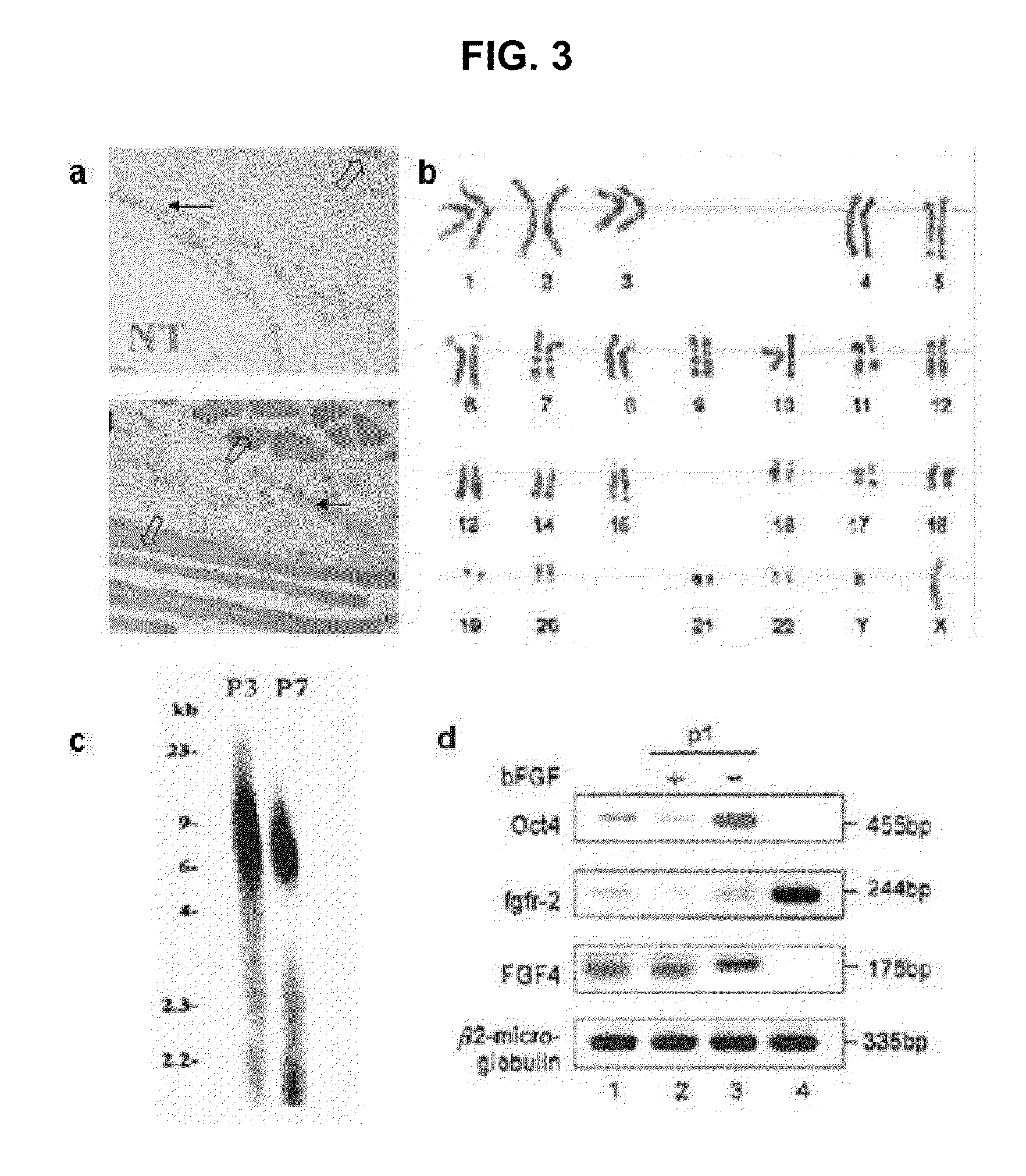
Existence of human trophoblast stem (hTS) cells has been suspected but unproved. The isolation of hTS cells is reported in the early stage of chorionic villi by expressions of FGF4, FGFR-2, Oct4, Thy-1, and stage-specific embryonic antigens distributed in different compartments of the cell. hTS cells are able to derive into specific cell phenotypes of the three primitive embryonic layers, produce chimeric reactions in mice, and retain a normal karyotype and telomere length. In hTS cells, Oct4 and fgfr-2 expressions can be knockdown by bFGF. These facts suggest that differentiation of the hTS cells play an important role in implantation and placentation. hTS cells could be apply to human cell differentiation and for gene and cell-based therapies.
Owner:ACCELERATED BIOSCI
Magneto-cymatic therapeutic face mask
A face mask that delivers magneto-cymatic therapy to a person's face to treat wrinkles and to tone facial muscles. The device consists of a mask worn over the face. Housed within the mask is an array of small transducers delivering cymatic vibrations. At the center of each transducer is a strong permanent magnet which delivers the magnetic therapy. Both the cymatic vibrations and the magnetic field are delivered concurrently. In addition, the magnetic field serves as a conduit for the cymatic vibrations providing a magnetic inductive coupling with the cells in the facial tissue to assist in restoring the cells to their proper resonant frequency.
Owner:TAB LICENSING
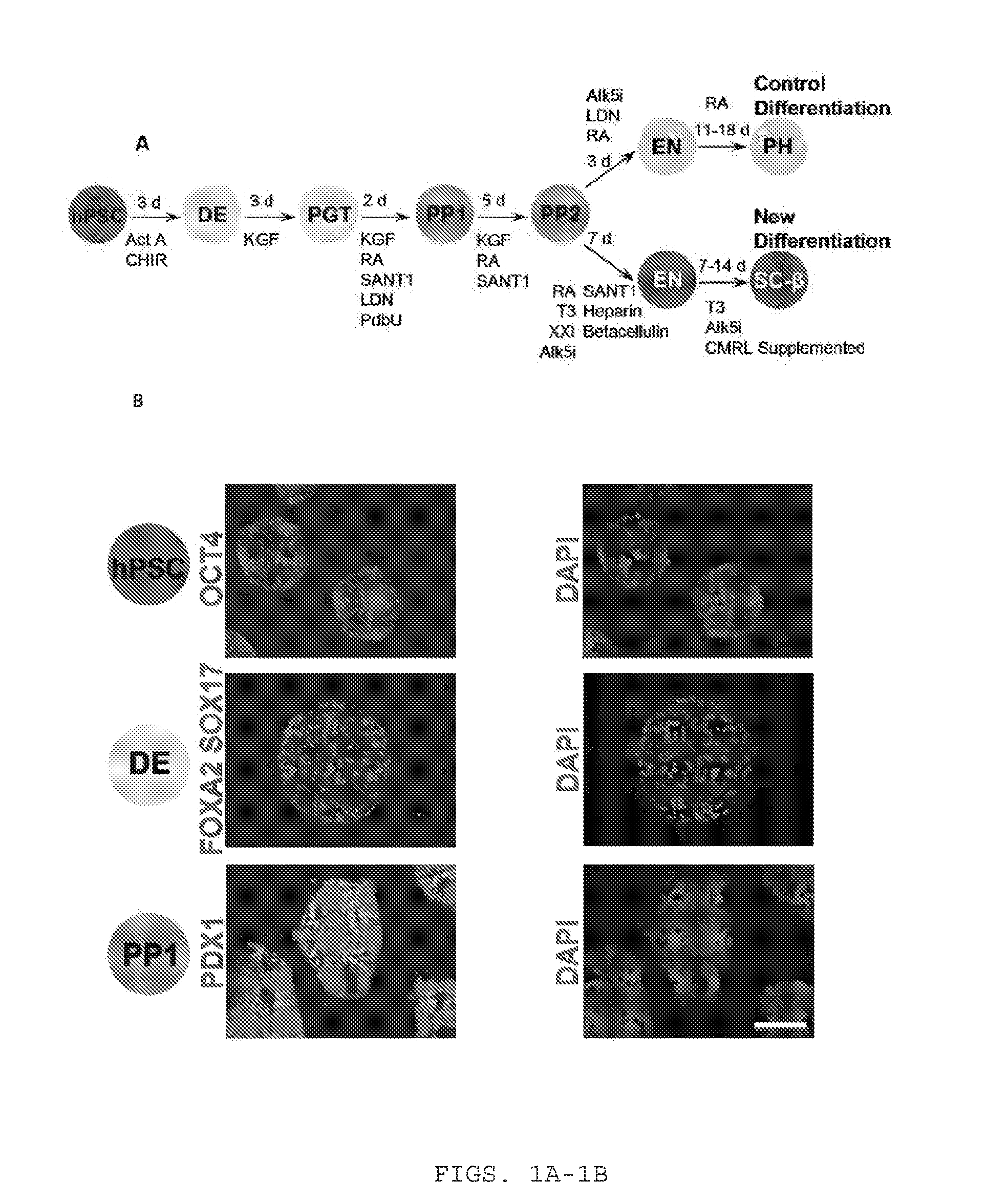
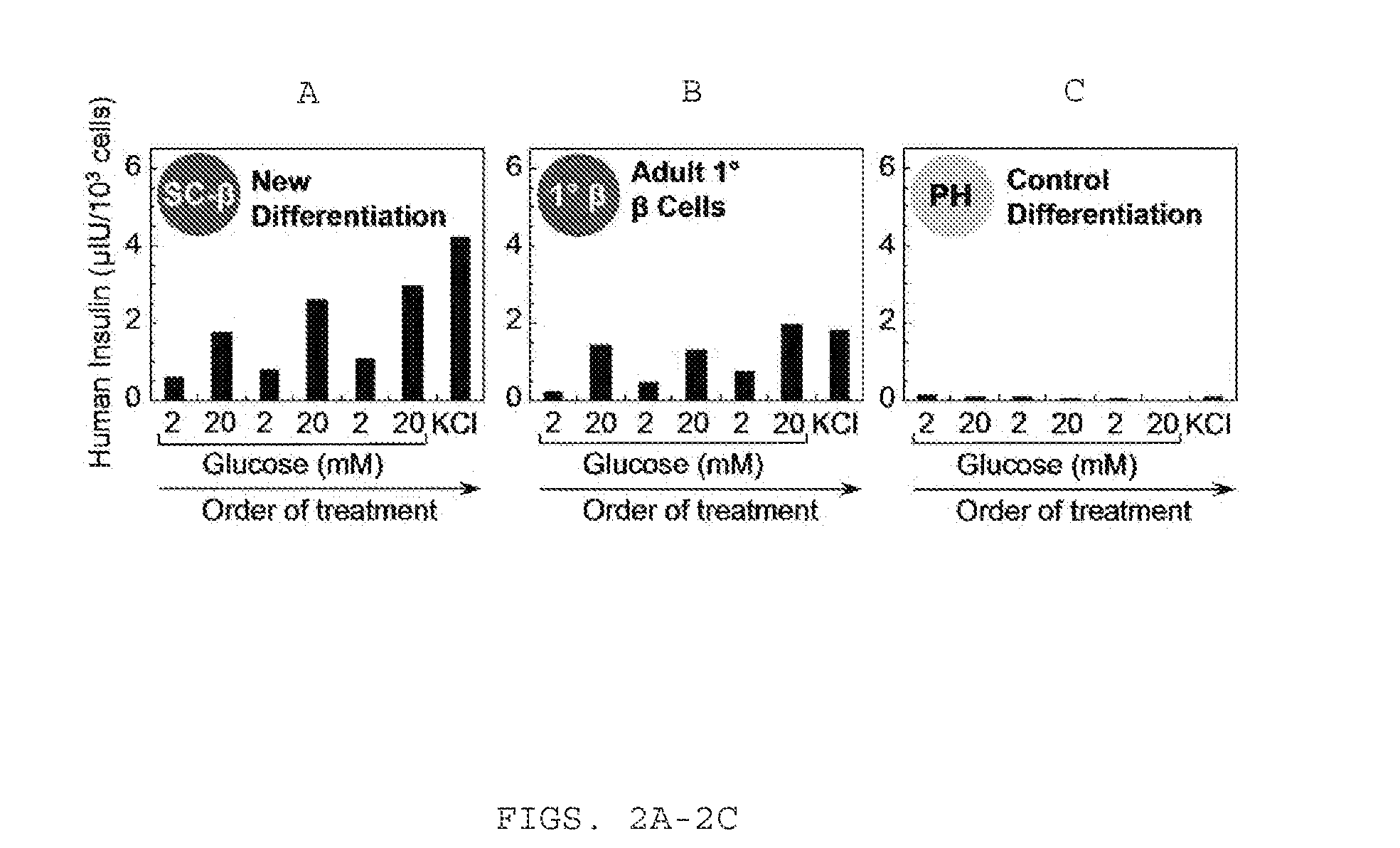
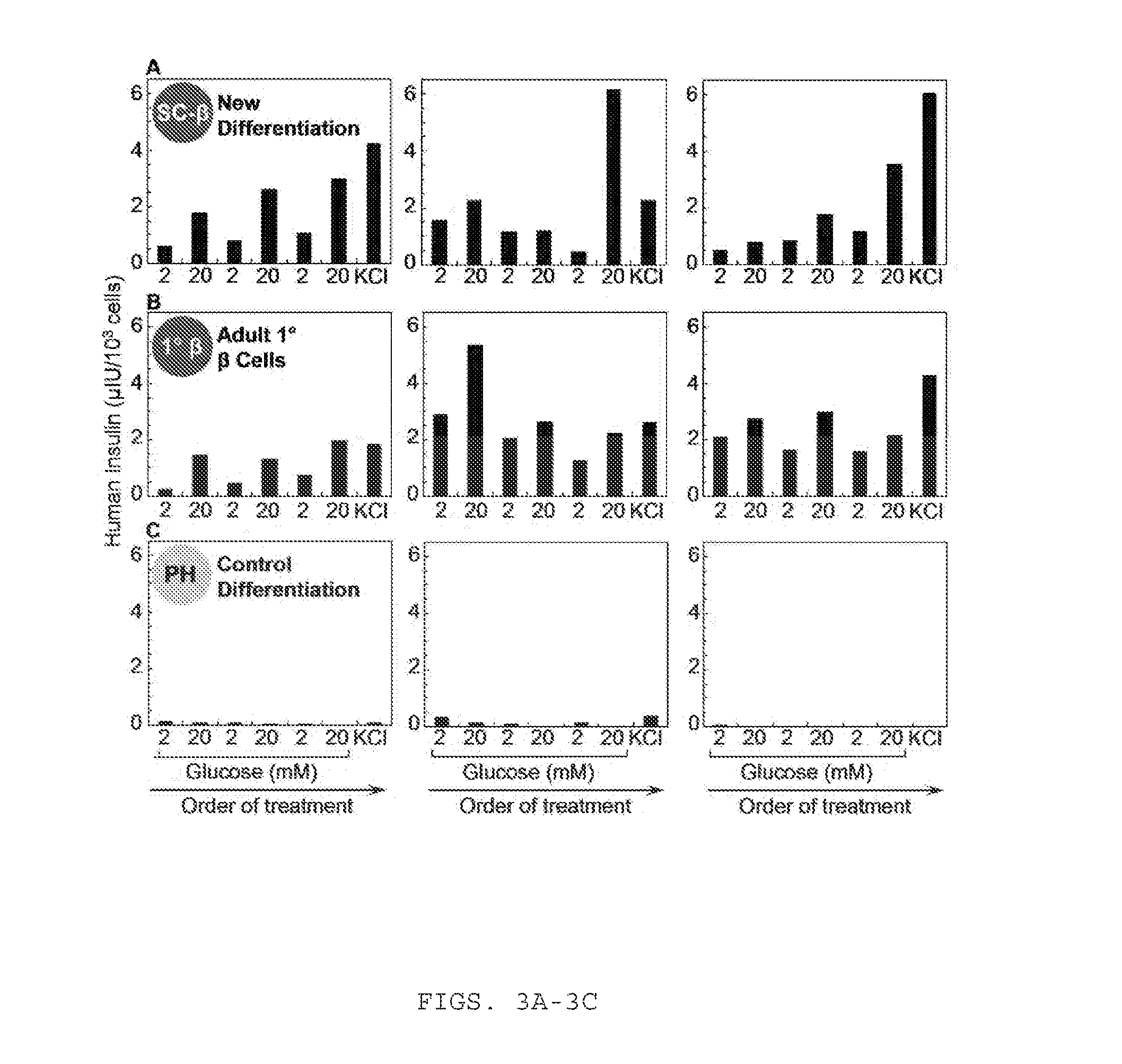
Sc-beta cells and compositions and methods for generating the same
ActiveUS20150218522A1Ameliorate hyperglycemiaMetabolism disorderPancreatic cellsInducer CellsCell therapy
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
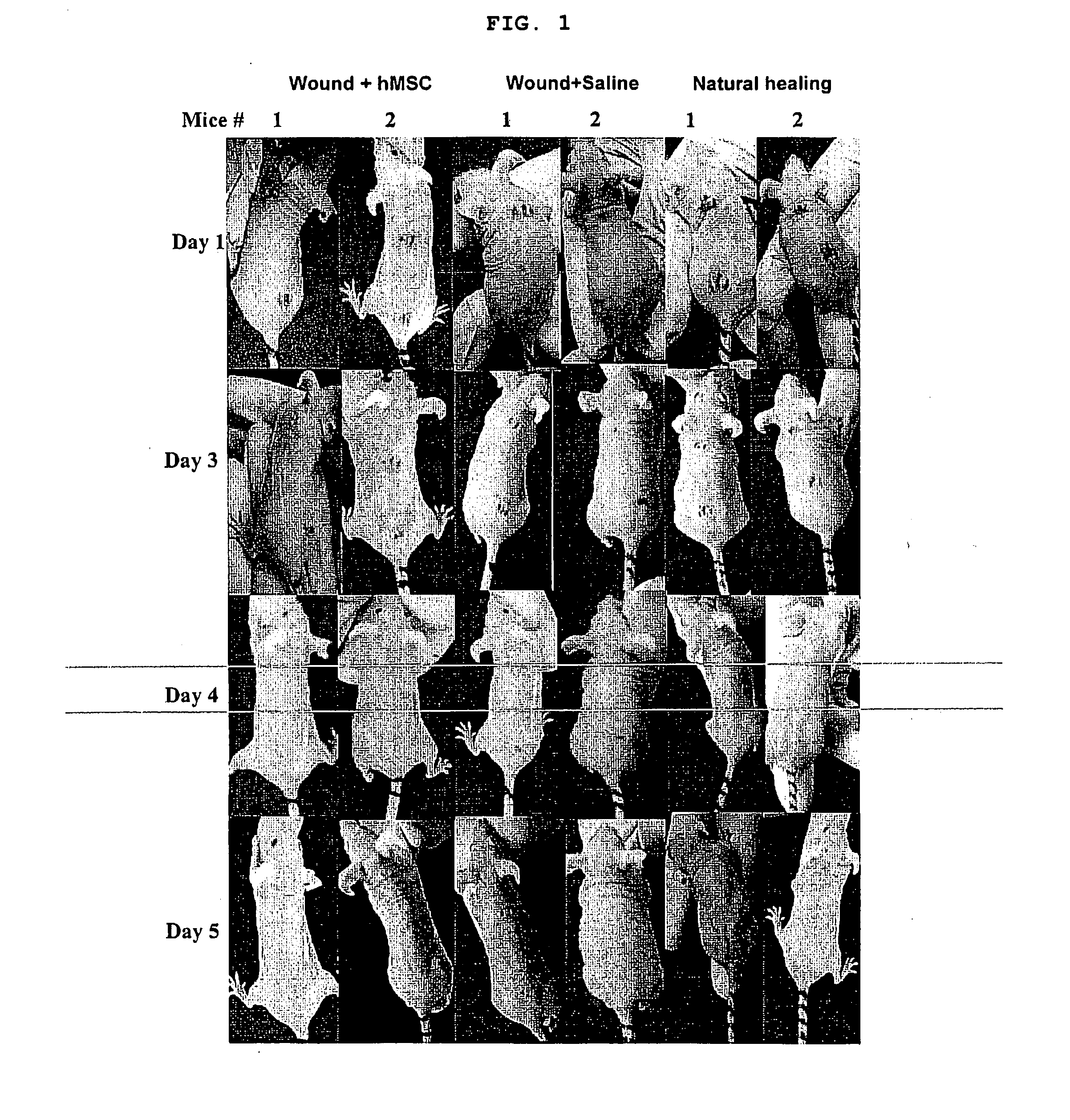
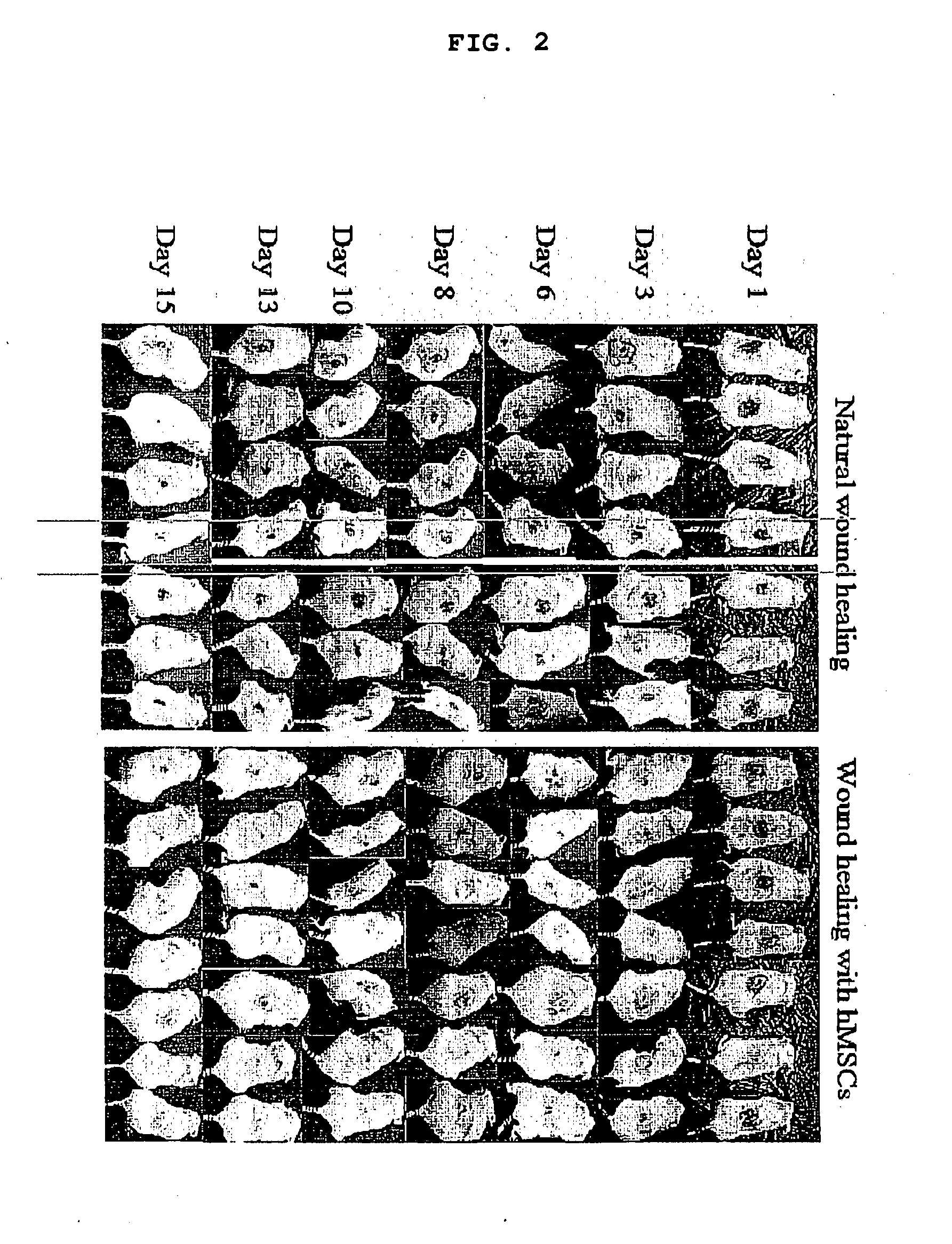
Use of stem cells for wound healing
InactiveUS20110020291A1Promote wound healingMinimizing formation of scar tissueBiocideEpidermal cells/skin cellsInjury mouthChronic wound
Cells, compositions, and methods of cell therapy for administering a therapeutically effective amount of stem cells or cell concentrate to achieve accelerated wound healing of normal and chronic wounds, while minimizing the formation of scar tissue.
Owner:RUTGERS THE STATE UNIV
Trans-Differentiation And Re-Differentiation Of Somatic Cells And Production Of Cells For Cell Therapies
InactiveUS20100120079A1Low costNew functionMicrobiological testing/measurementNervous system cellsCulture cellAcetylation
The invention provides a method for effecting the trans-differentiation of a somatic cell, i.e., the conversion of a somatic cell of one cell type into a somatic cell of a different cell type. The method is practiced by culturing a somatic cell in the presence of at least one agent selected from the group consisting of (a) cytoskeletal inhibitors and (b) inhibitors of acetylation, and (c) inhibitors of methylation, and also culturing the cell in the presence of agents or conditions that induce differentiation to a different cell type. The method is useful for producing histocompatible cells for cell therapy.
Owner:PAGE RAYMOND +2
Biodegradable polymer-ligand conjugates and their uses in isolation of cellular subpolulations and in cryopreservation, culture and transplantation of cells
InactiveUS20050100877A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCell-Extracellular MatrixECM Protein
The invention discloses a biodegradable particle-cell composition having at least one biodegradable particle, at least one receptive group covalently linked thereto, and a cell anchored thereto. The particle can be polylactide, a polylactide-lysine copolymer, polylactide-lysine-polyethylene glycol copolymer, starch, or collagen. The receptive group can be an antibody, a fragment of an antibody, an avidin, a streptavidin, or a biotin moiety. Moreover, the particle can also have extracellular matrix components other than collagen. The particle-cell compositions can be used for selection of cells from a population, for cell culture of anchorage-dependent cells, for cryopreservation of anchorage-dependent cells, and for transplantation as a cell therapy.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Cell therapy for chronic stroke
A method of treating stroke in a patient who has undergone a stroke comprising administering at least 2 million suitable neuronal cells to at least one brain area involved in the stroke. The method comprises the step of using a twist drill or a burr to form a hole in the skull through which the cells could be administered. Exemplary cells are hNT neuronal cells, HCN-1 cells, fetal pig cells, neural crest cells, neural stem cells, or a combination thereof. Also disclosed herein is a pharmaceutical composition of 95% pure hNT neuronal cells, which composition further includes a vial containing PBS and human neuronal cells. This vial is provided in a container with liquid nitrogen, whereby the composition is frozen and maintained at -170° C. before use. Also disclosed are methods of improving speech, cognitive, sensory, and motor function in a person who has experienced brain damage which interferes with function by administering a sterile composition of a sufficient number of neuronal cells or neural stem cells to the damaged area. Also disclosed is a method of replacing central nervous cells lost to neurodegenerative disease, trauma, ischemia or poisoning.
Owner:LAYTON BIOSCI +1
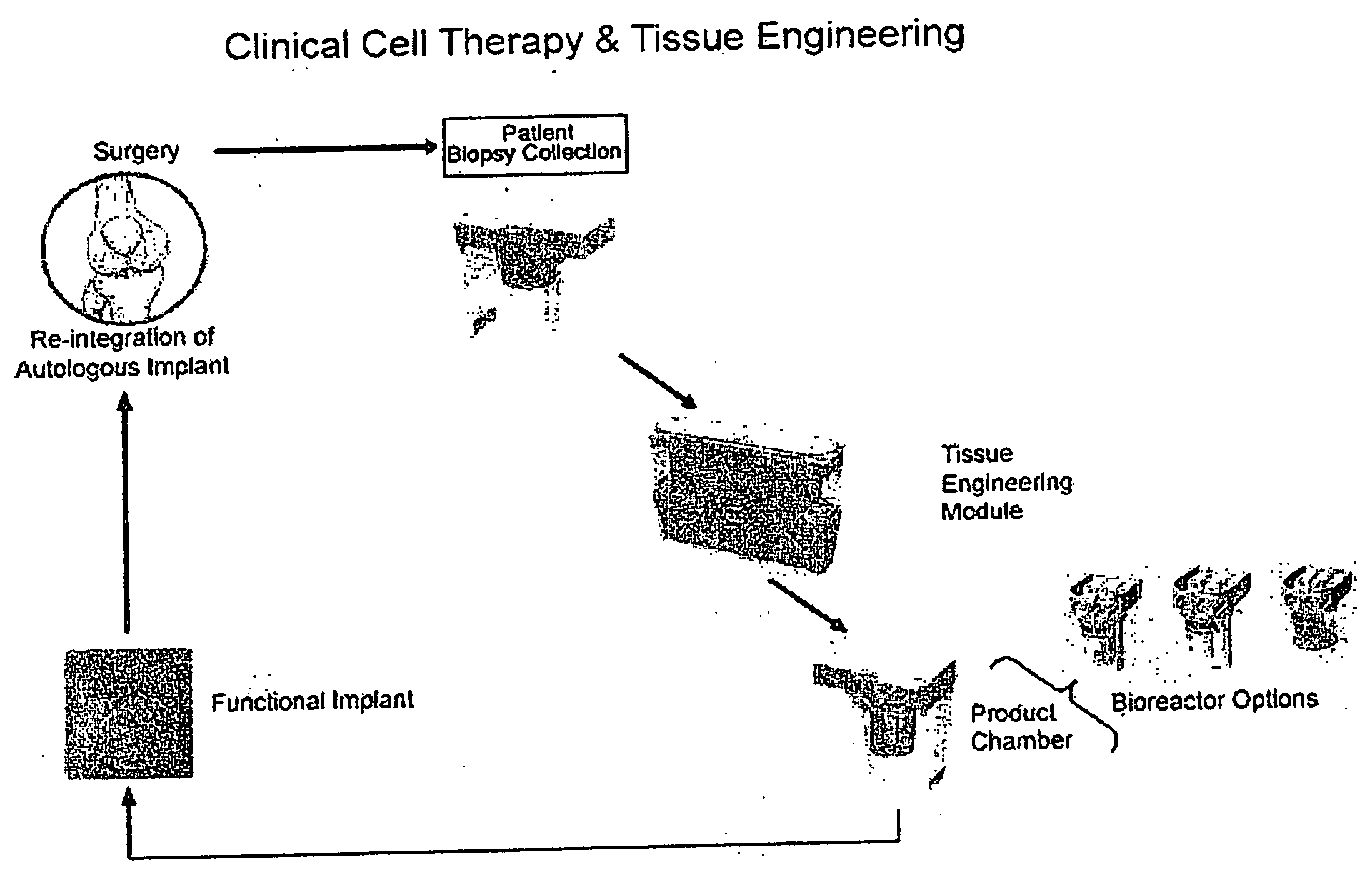
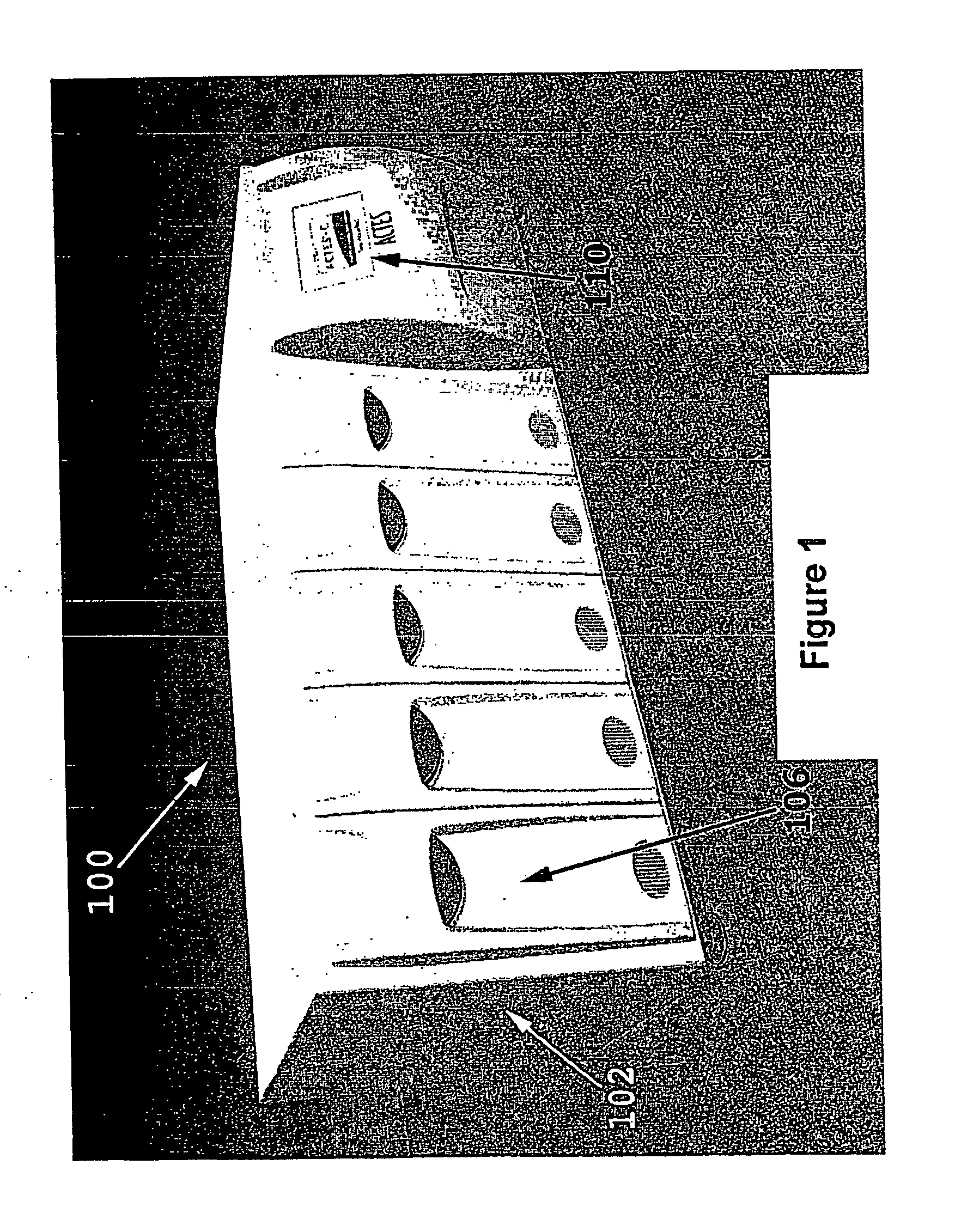
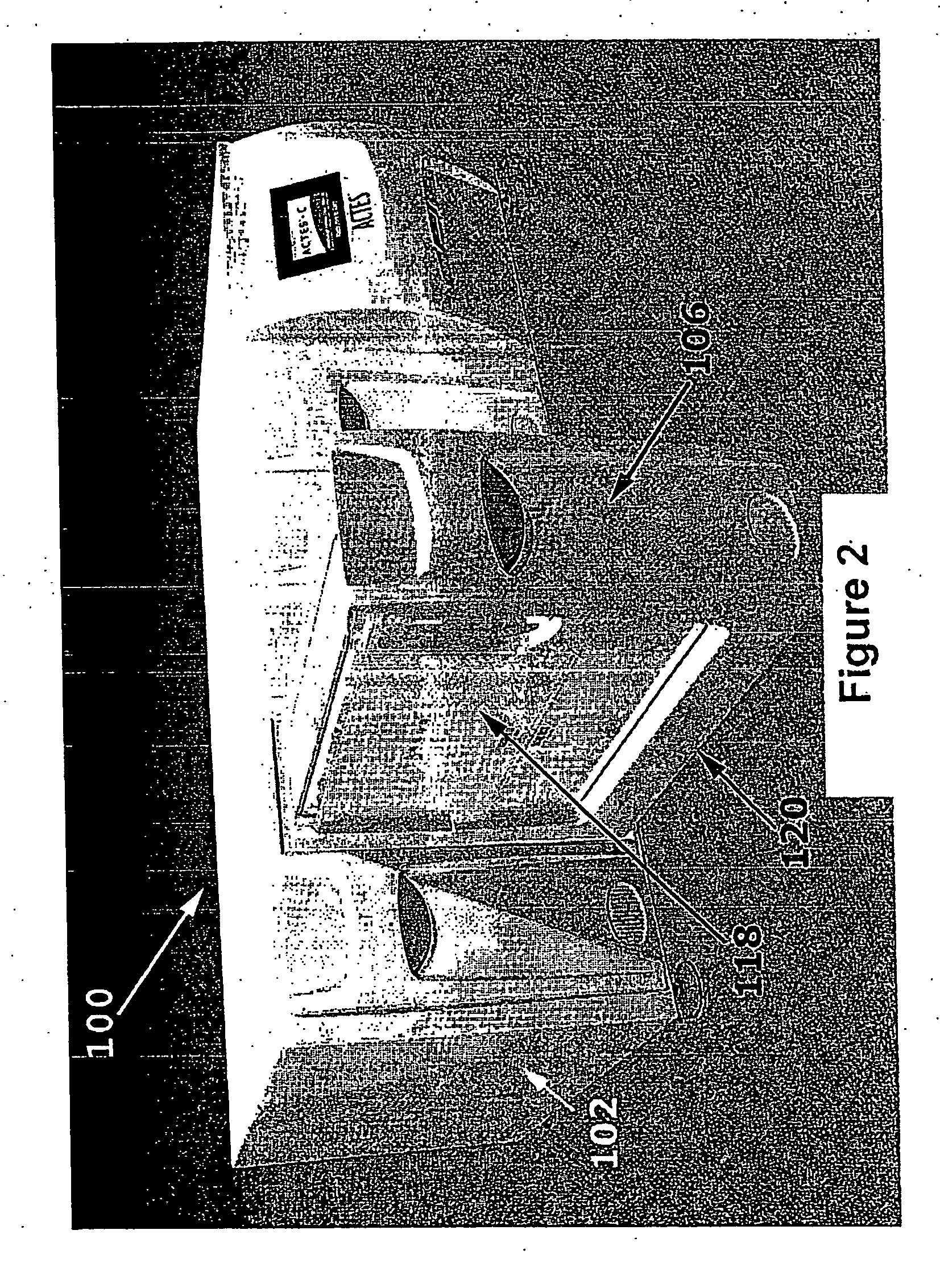
Advanced Tissue Engineering System
ActiveUS20080113426A1Avoid inherent hazardLow costBioreactor/fermenter combinationsBiological substance pretreatmentsSingle implantProduct selection
The invention is an automated advanced tissue engineering system that comprises a housing in which one or more tissue engineering modules are accomodated together with a central microprocessor that controls functioning of the tissue engineering modules. In one embodiment, the tissue engineering module comprises a housing supporting one or more bioreactor chamber assemblies and a fluid reservoir operationally engageable with the housing. The bioreactor chamber assemblies may be selected depending on the end product option desired and may include, for example, a cell therapy bioreactor chamber, a single implant bioreactor chamber and a multiple (mosaic) implant bioreactor chamber.
Owner:MILLENIUM BIOLOGIX TECH +1
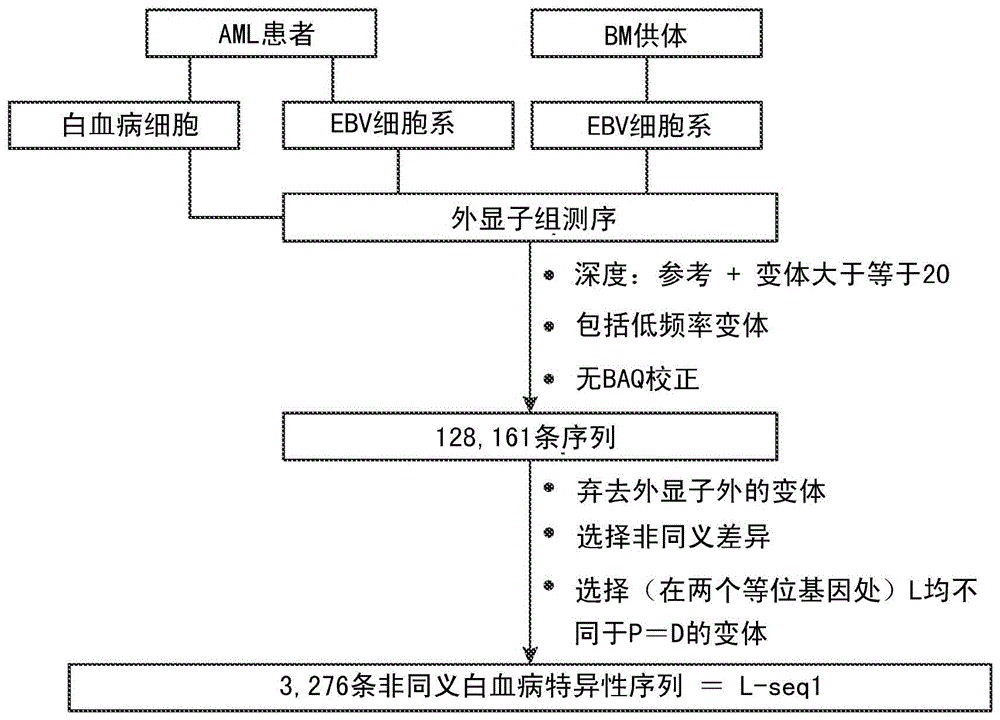
Personalized cancer vaccines and adoptive immune cell therapies
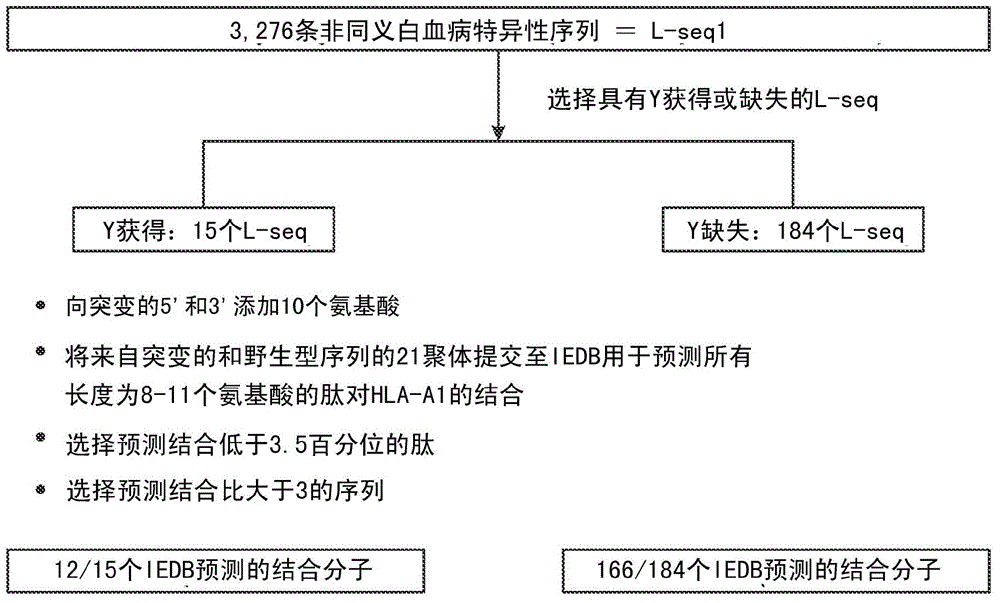
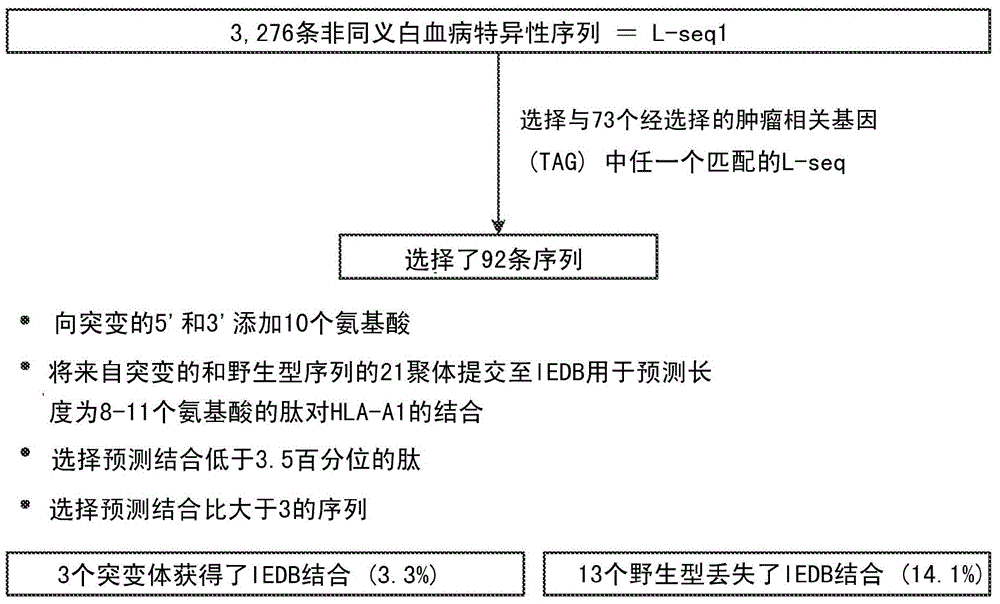
InactiveCN104662171AMicrobiological testing/measurementBiological material analysisCancer cellWild type
Cancer antigens containing mutations in an expressed gene of cancer cells from a cancer patient are identified. Sequences from cancer cells obtained using a parallel sequencing platform are selected by comparing to the patient's normal genes or to normal genes from an HLA-matched individual. Sequences are further selected by identifying an HLA supertype of the cancer patient and selecting for that HLA supertype, sequences that have a particular amino acid at the mutant position and / or corresponding wild-type position in the effected gene. Peptides containing cancer antigens (i.e., mutations—once a mutation is defined, what makes it an immunogen is its ability to induce an immune response) are optionally tested for binding to HLA antigens of the cancer patient. Peptides containing the cancer antigens are evaluated for activating T cells (e.g., helper T lymphocytes and cytotoxic T lymphocytes (CTL)) cell lines from the cancer patient or from an HLA-matched donor. The cancer antigen(s) identified for a cancer patient are used to prepare a cancer vaccine and to treat the cancer patient.
Owner:PERSIMMUNE
Chimeric antigen receptors (CAR) and methods for making and using the same
InactiveUS20170158749A1Facilitate cell targetingReduce off-target cytotoxicity of cellAntibody mimetics/scaffoldsMammal material medical ingredientsAntigenCAR T-cell therapy
Chimeric antigen receptors (CARs) and CAR-expressing T cells are provided that can specifically target cells that express an elevated level of a target antigen. Likewise, methods for specifically targeting cells that express elevated levels of antigen (e.g., cancer cells) with CAR T-cell therapies are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cartilage-derived stem cells and applications thereof
The invention relates to methods of isolating adult stem cells, to the cells thus isolated and to applications thereof. More specifically, the invention relates to isolated adult stem cells, which are derived from dedifferentiated chondrocytes, which can be differentiated and which can give rise to a series of cell lineages, as well as to specific markers present in said cells, such as cell surface antigens. The cells provided by the present invention can be used, for example, in cell therapy and in the search for and development of novel medicaments.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
Cell targeting methods and compositions
In certain aspects, the invention relates to cell delivery compositions comprising a progenitor cell and a targeting moiety, and methods related thereto. Such compositions and methods may be used, for example, in administering a targeted cell therapy to a subject.
Owner:CAPLAN ARNOLDI
Cell freezing medium, freezing resuscitation method and application thereof
ActiveCN109221082AAvoid damageImprove survival rateDead animal preservationSkeletal/connective tissue cellsEpsilon-PolylysineAlcohol
The invention provides a cell freezing medium. The cell freezing medium comprises acylated epsilon polylysine, polyhydric alcohols and equilibration buffer. According to the cell freezing medium disclosed by the invention, the polyhydric alcohols and acylated epsilon polylysine replace toxic dimethyl sulfoxide so as to serve as cryoprotective agents, so that damage to cells in the freezing resuscitation process is reduced, and the cell survival rate is improved. The invention further provides a method for performing freezing resuscitation on cells by utilizing the cell freezing medium and application of the method for freezing stem cells and immune cells. The method is simple and feasible to operate and is suitable for large-scale clinical application of cell therapy, and great convenienceis brought to clinical application of the cell freezing technology.
Owner:SHANGHAI CRYOWISE MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com