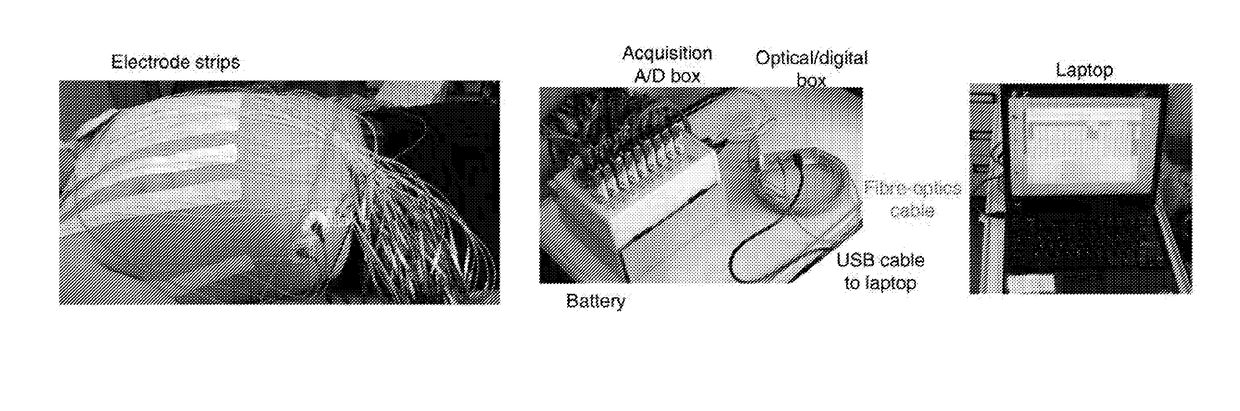
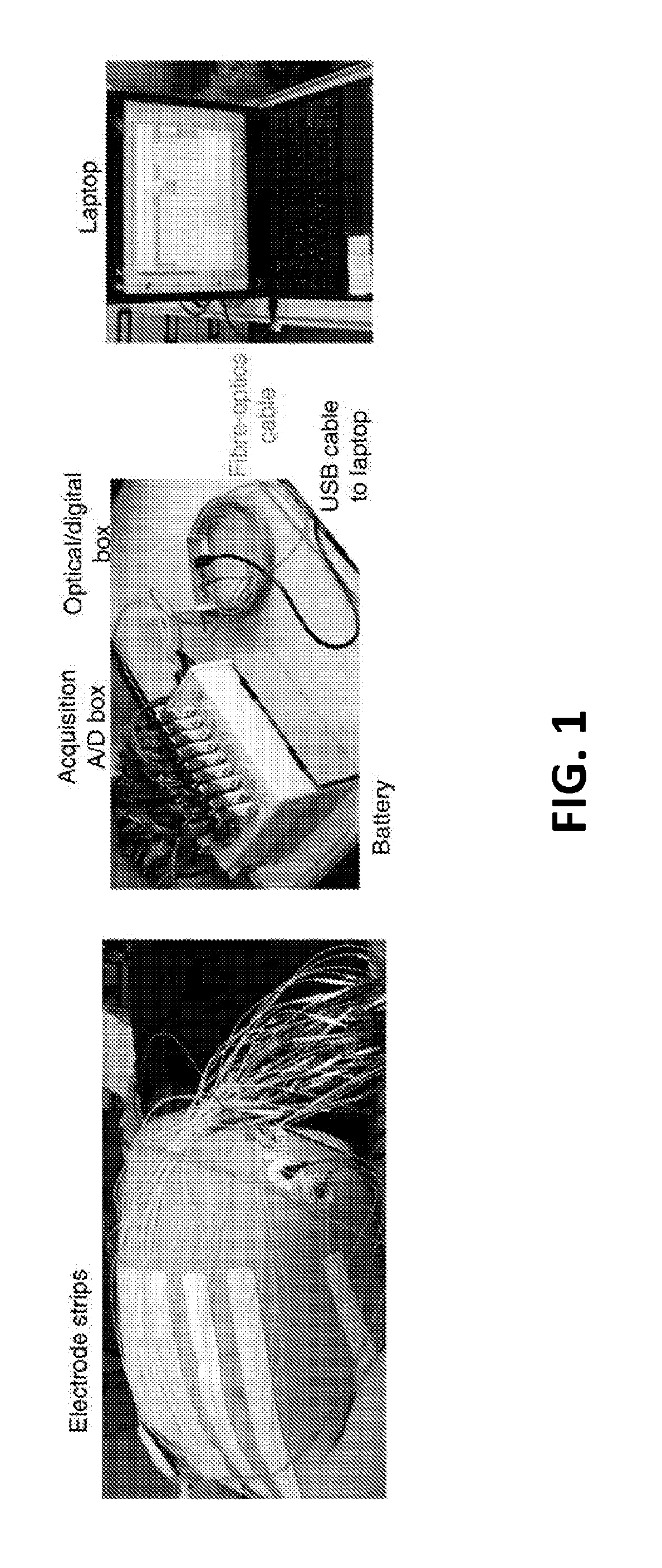
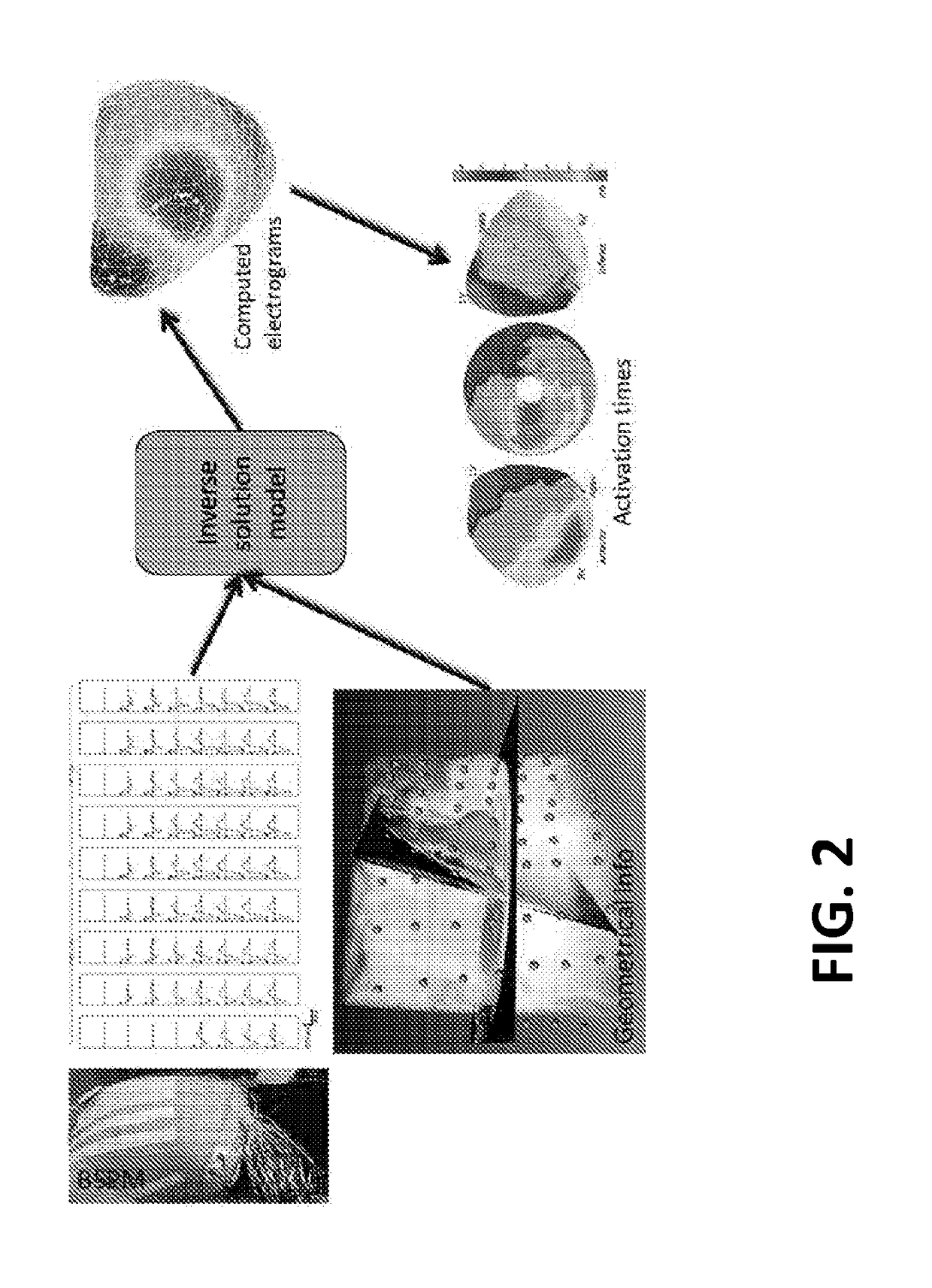
Patents
Literature
71 results about "Cardiac structure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
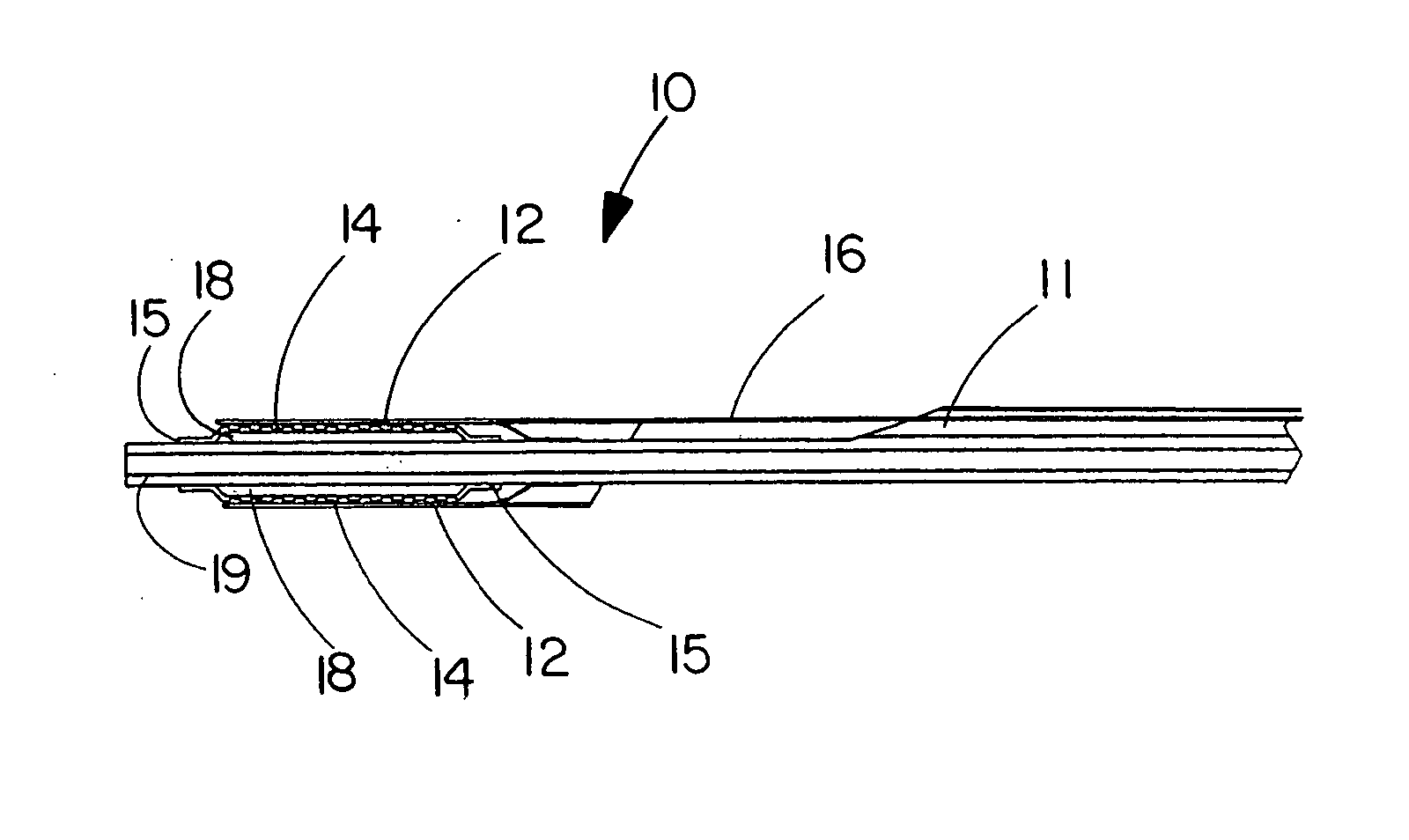
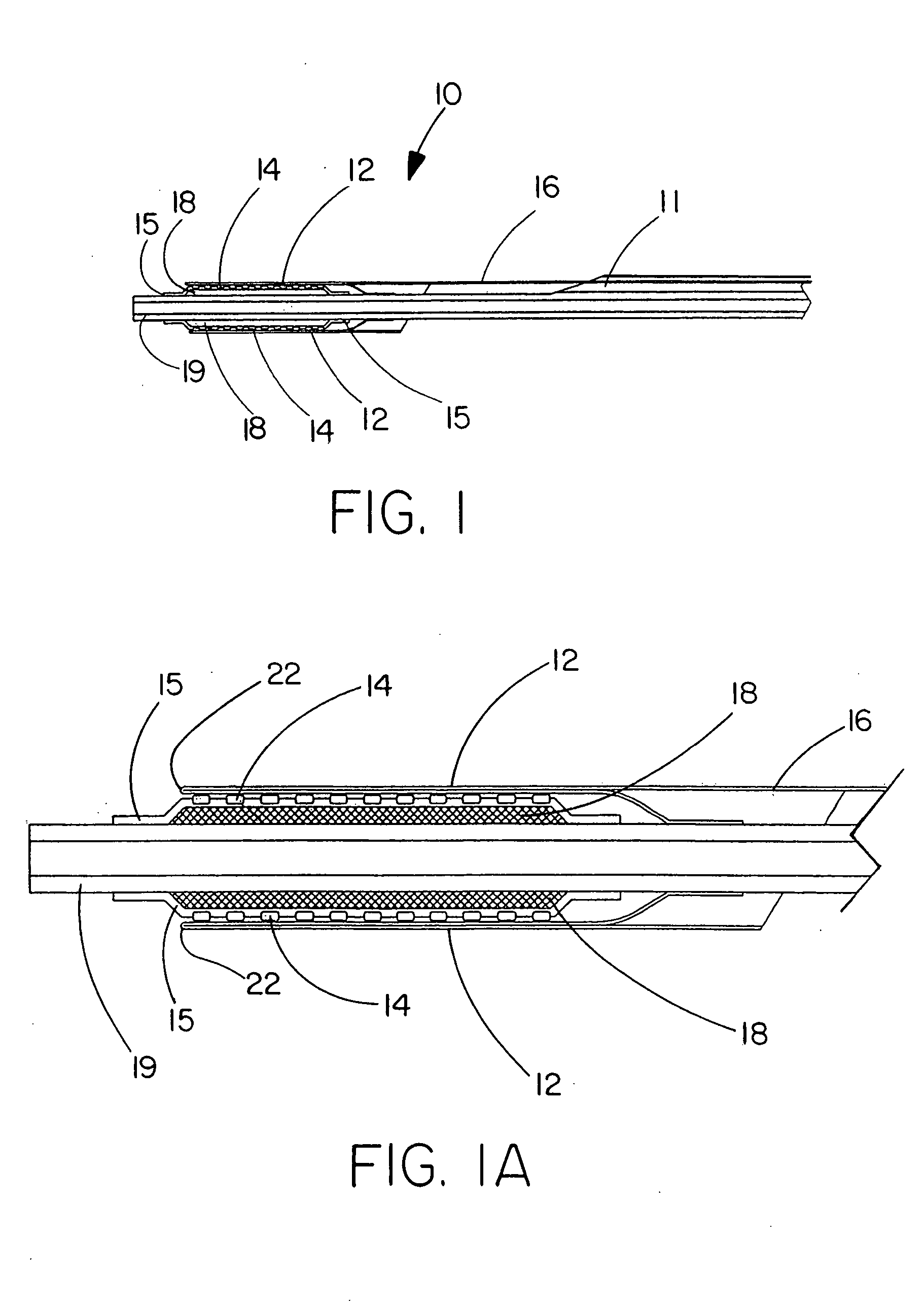
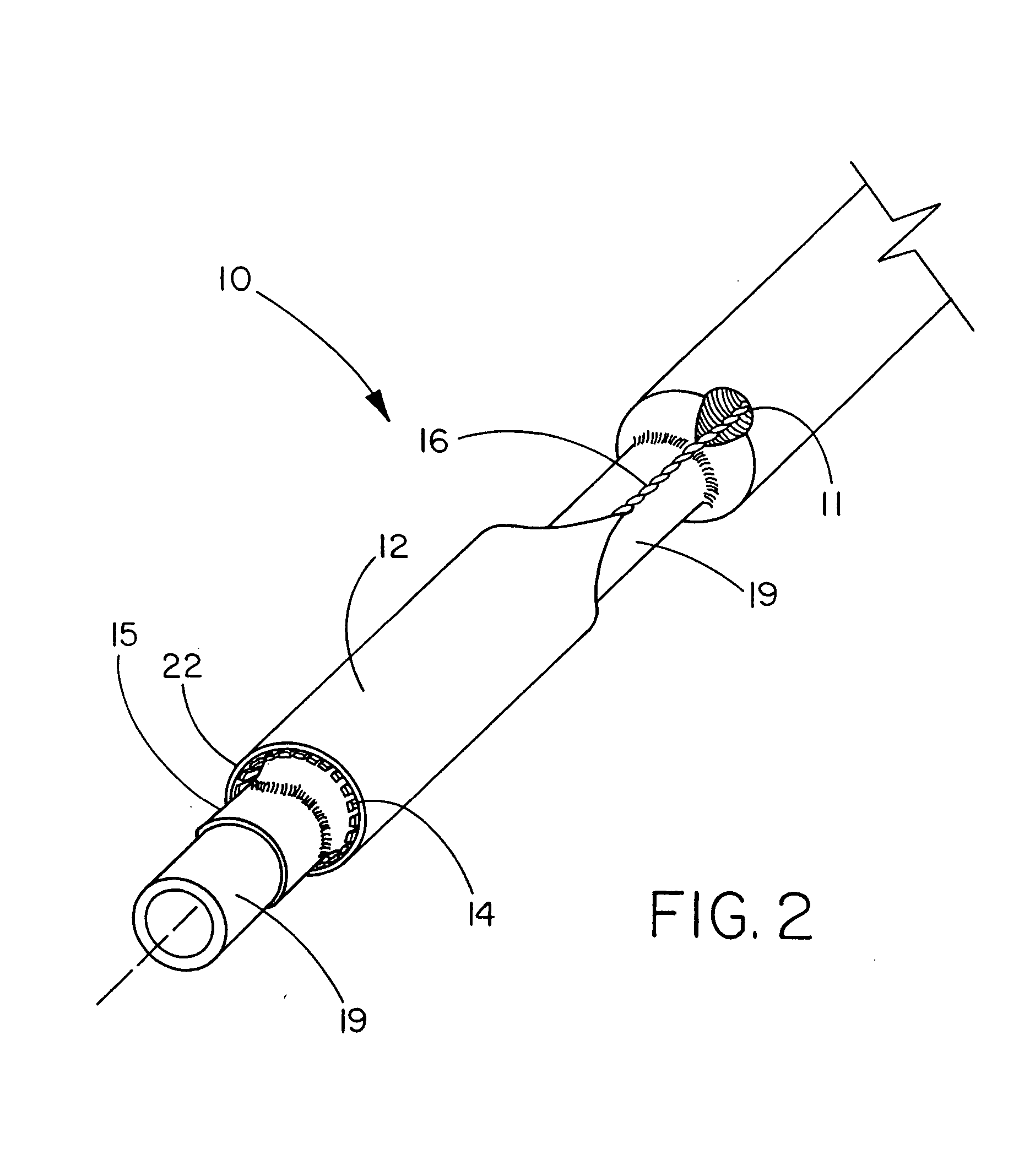
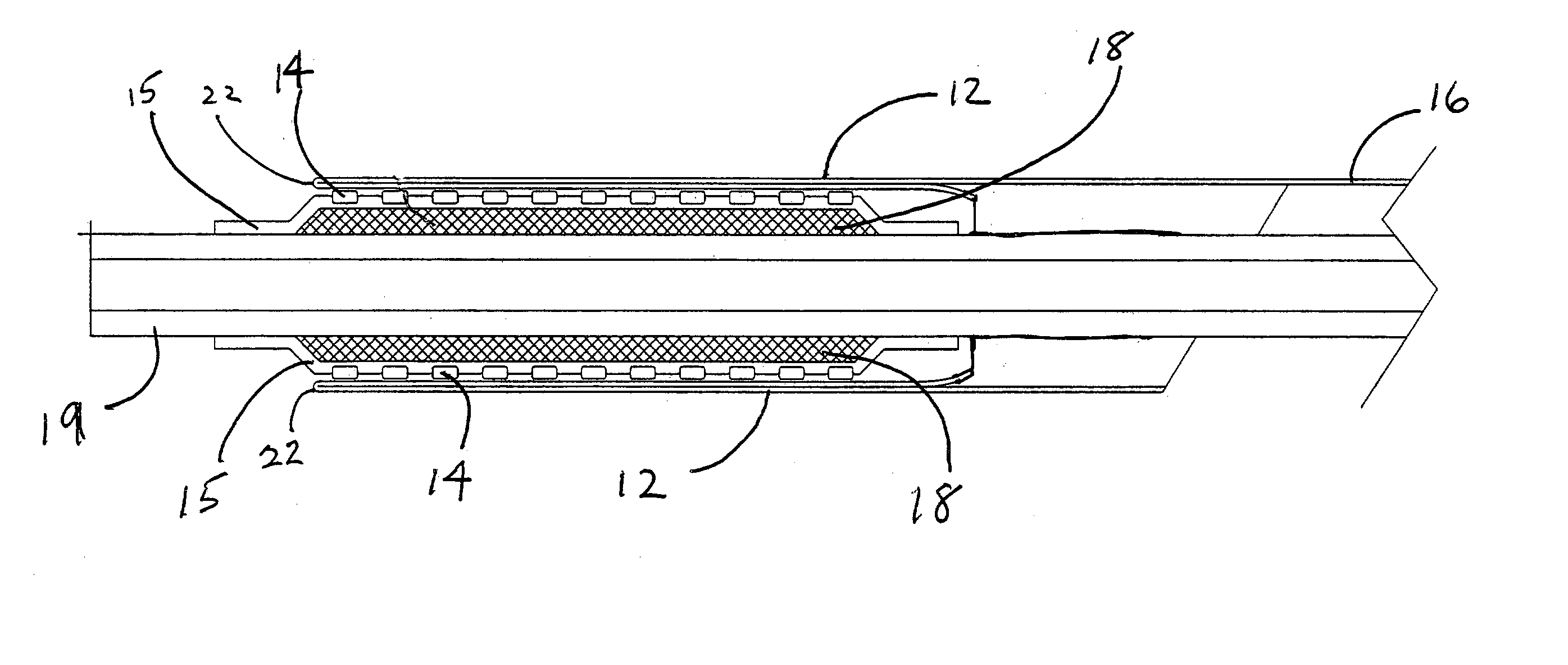
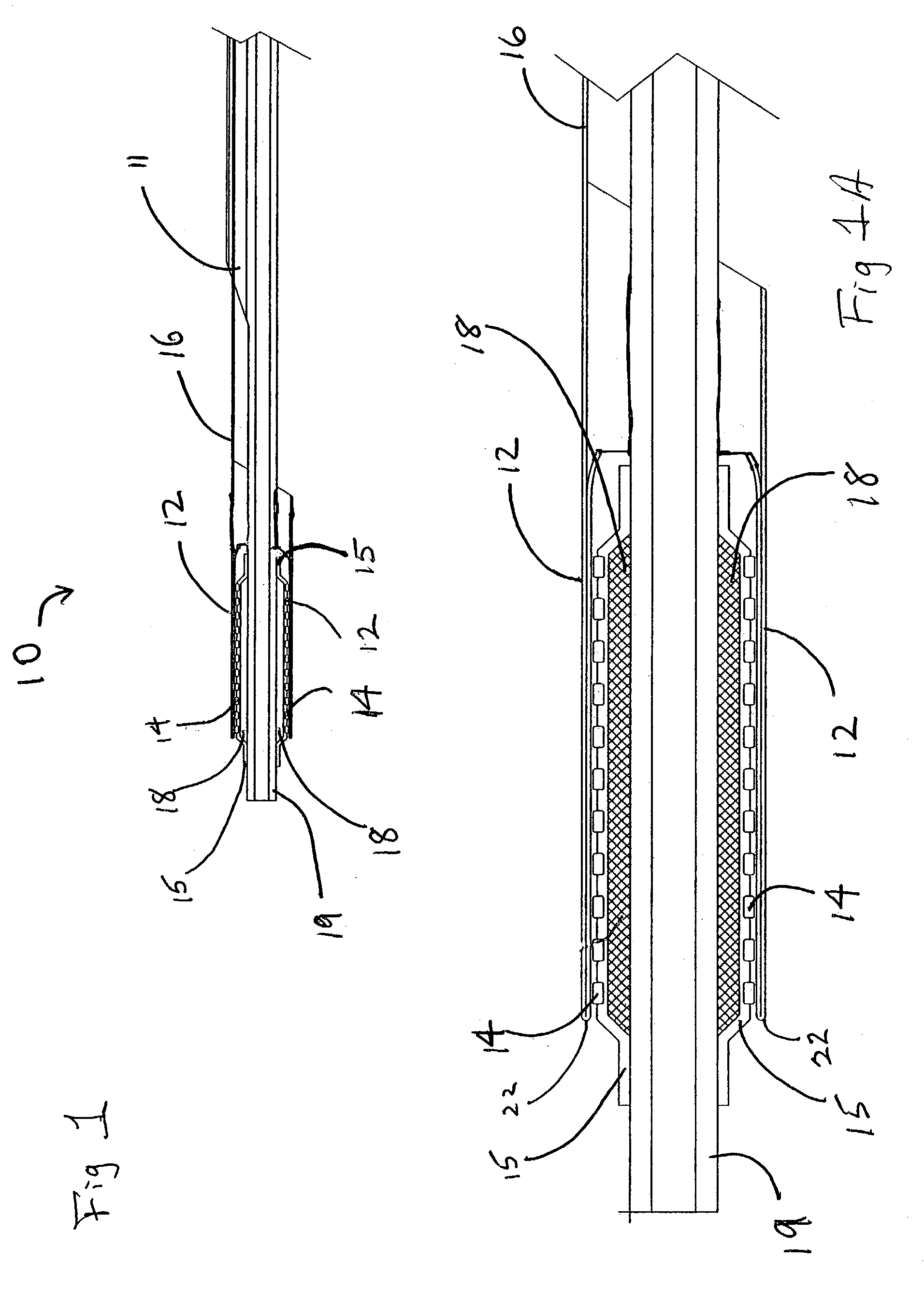
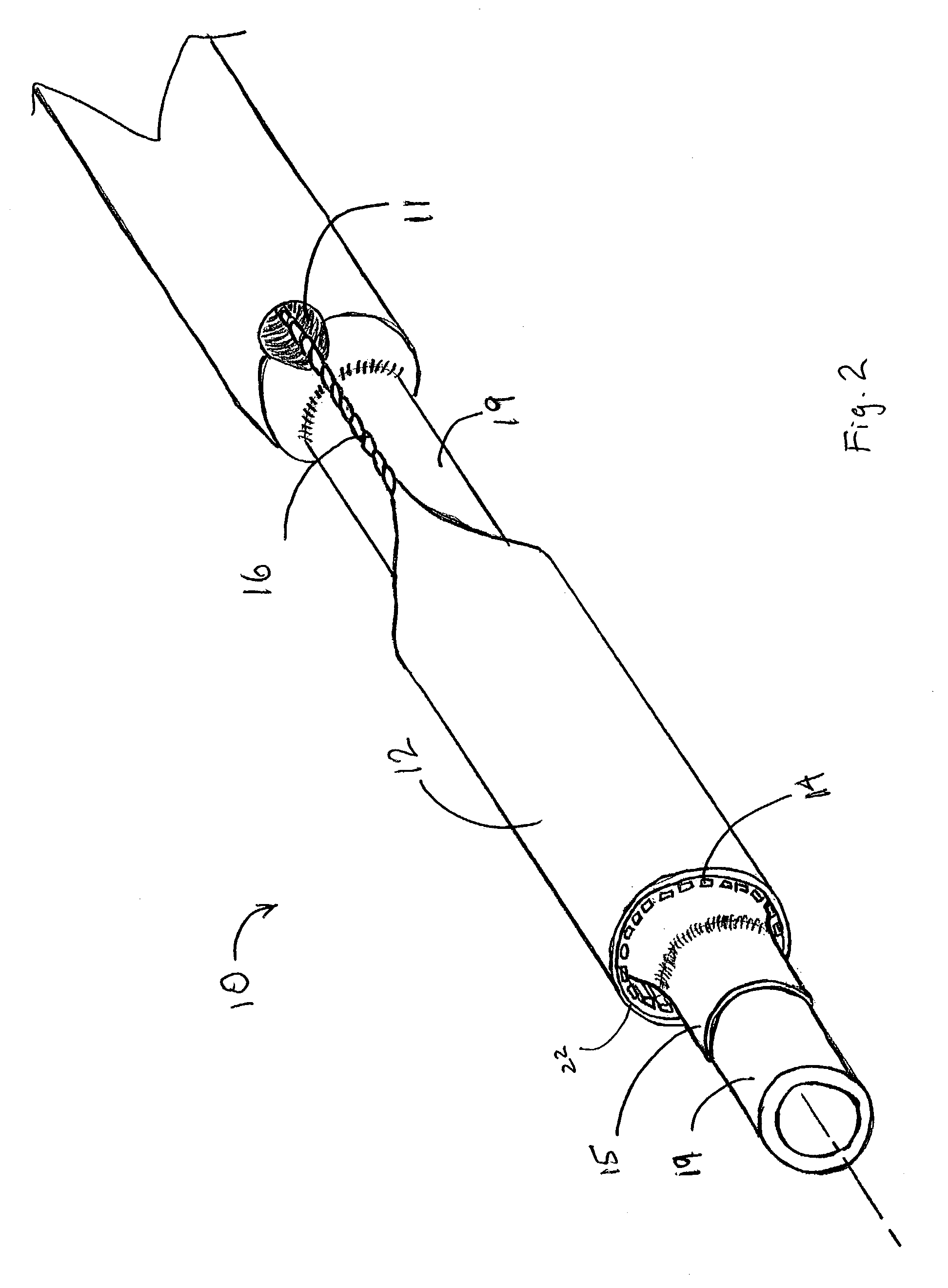
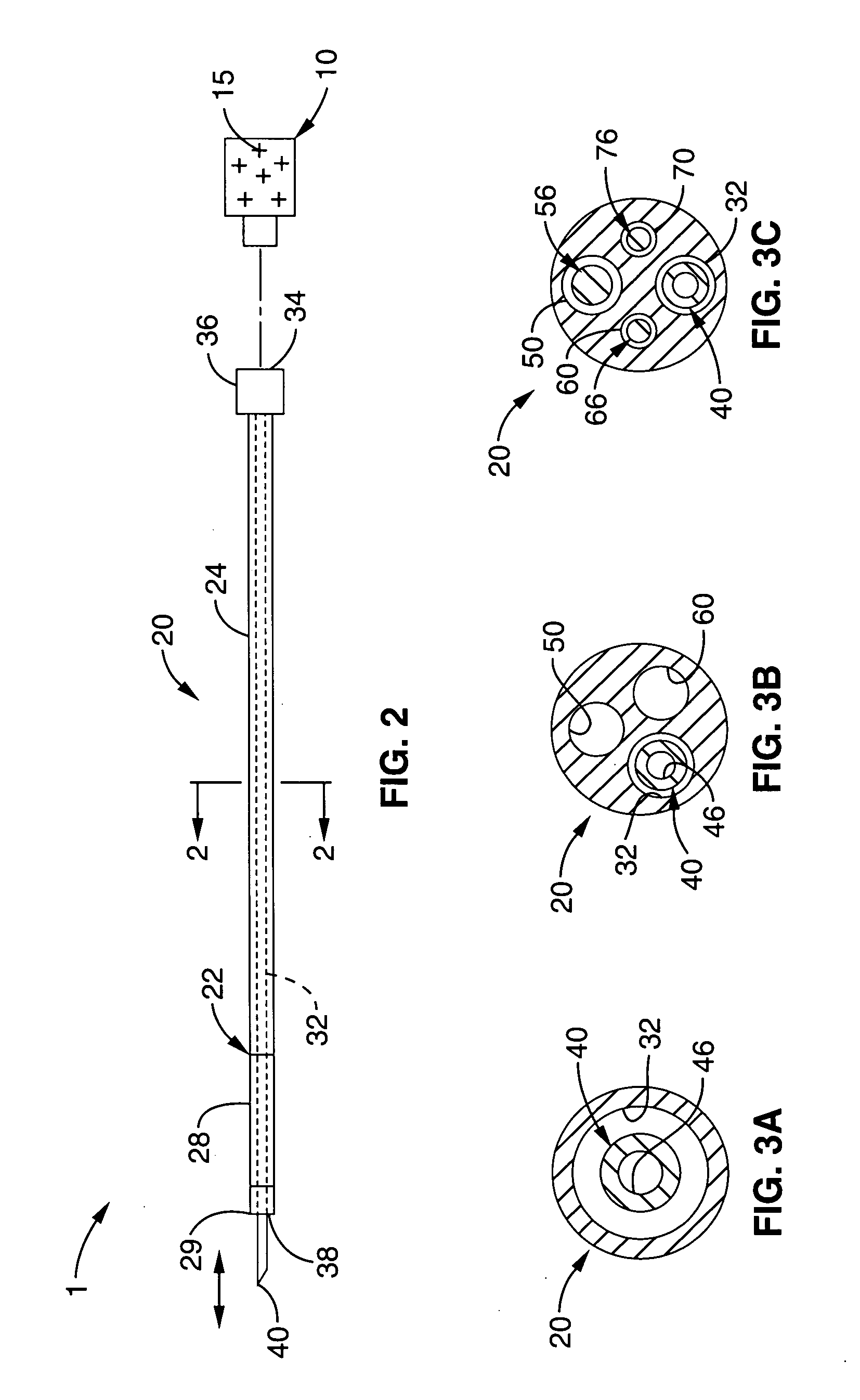
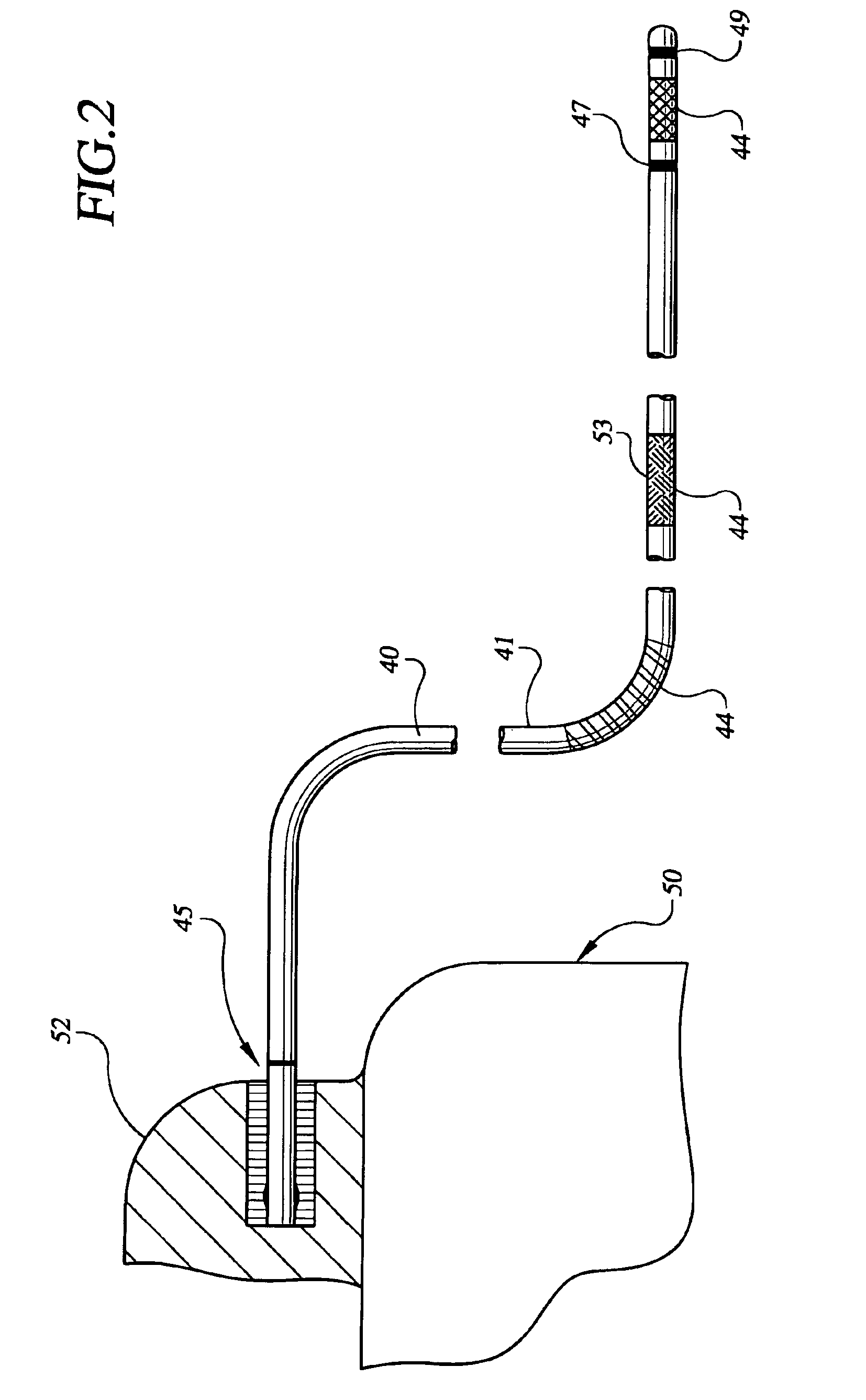
Deployment system for an expandable device
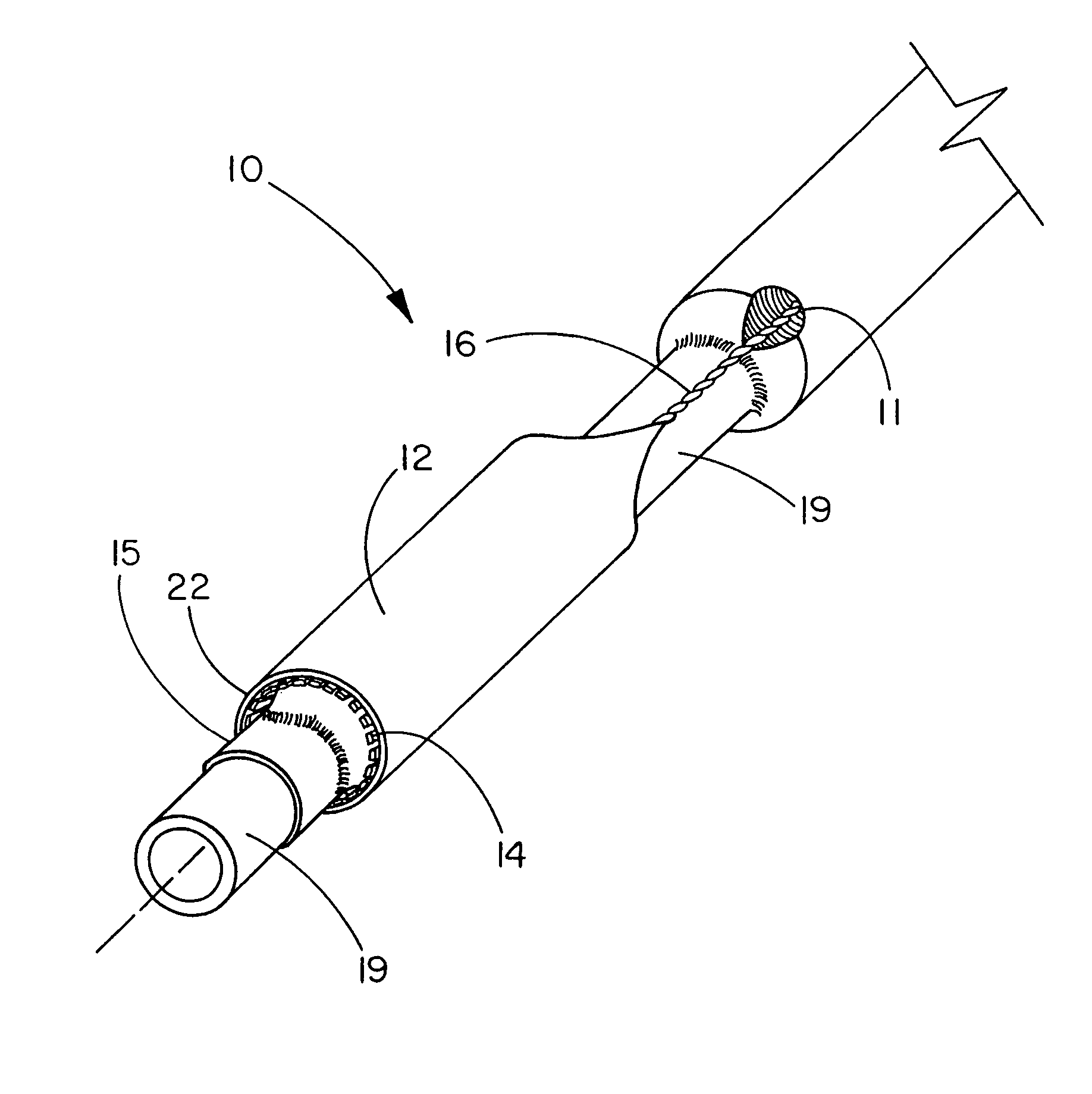
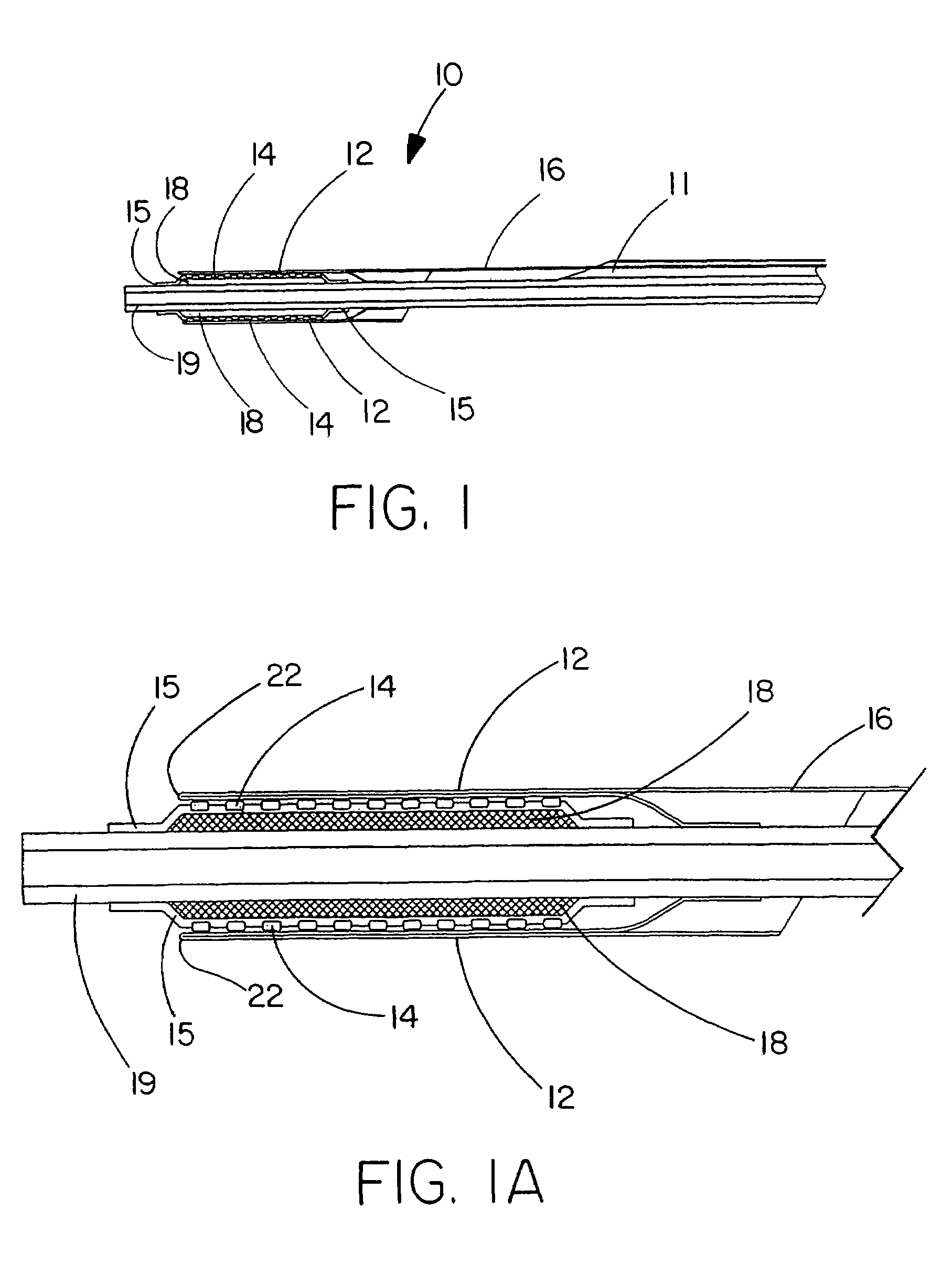
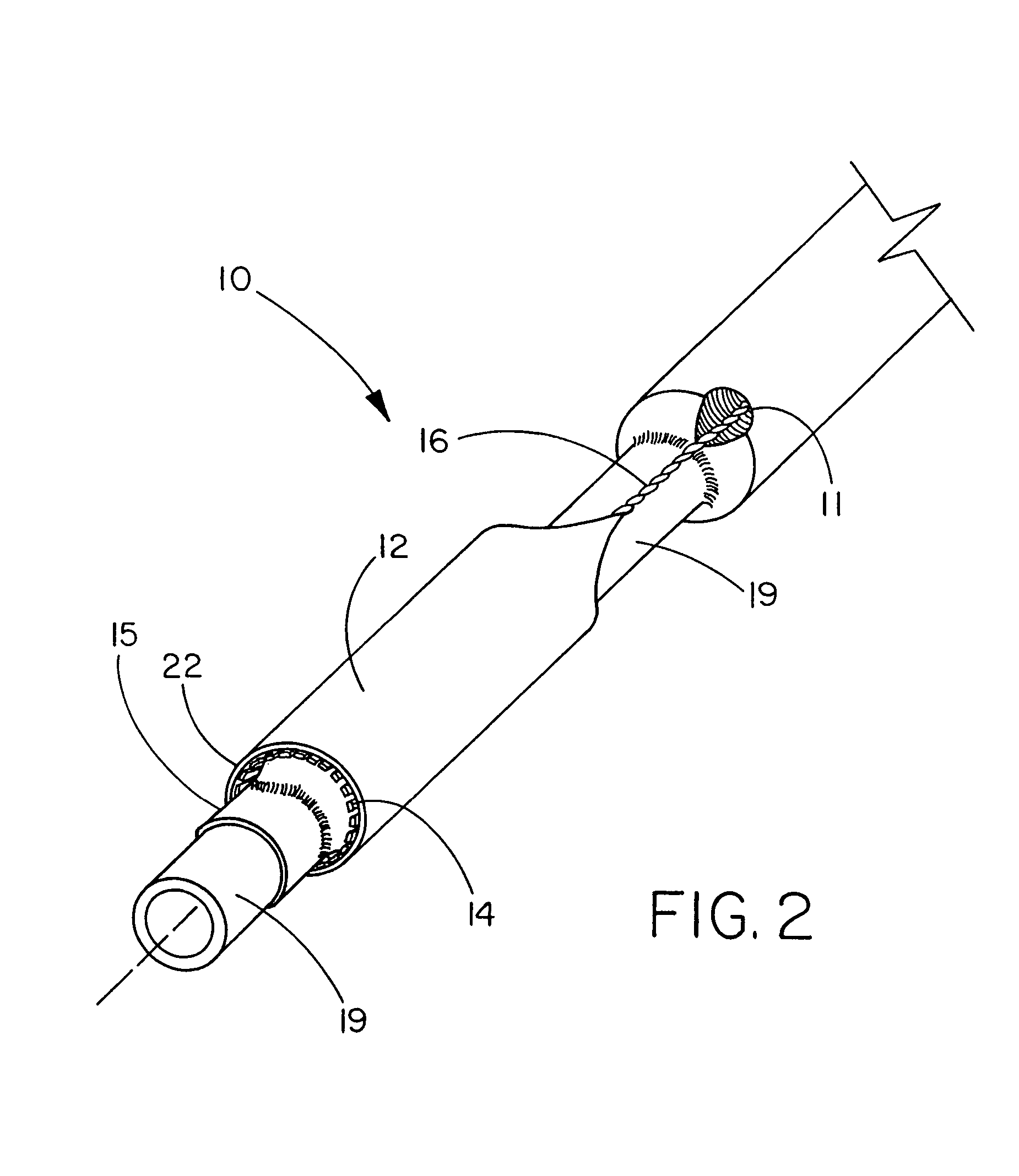
The present invention is directed to a deployment system for an endoluminal device. The deployment system includes a confining sheath placed around a compacted endoluminal device. A deployment line is provided in the system that is an integral extension of the sheath. As the deployment line is actuated, the sheath retracts from around the compacted endoluminal device. As the sheath retracts from around the endoluminal device, material from the sheath may be converted into deployment line. Once the sheath is retracted from around the compacted endoluminal device, the endoluminal device expands in configuration and repairs vascular or cardiac structures of an implant recipient. Any remaining sheath material is removed from the implantation site along with the deployment line. The deployment system also includes an endo-prosthesis mounting member placed between the endoluminal device and an underlying catheter. The endo-prosthesis mounting member serves to cushion and retain the endoluminal device when constrained by the sheath and may assist in expansion of the endoluminal device when unconstrained by the sheath. The present invention is also directed to a deployment system having a deployment assembly that simultaneously expands an endo-prosthesis mounting member while removing a sheath from an expandable medical device.
Owner:WL GORE & ASSOC INC
Surgical perforation device with curve
Owner:BOSTON SCI MEDICAL DEVICE LTD
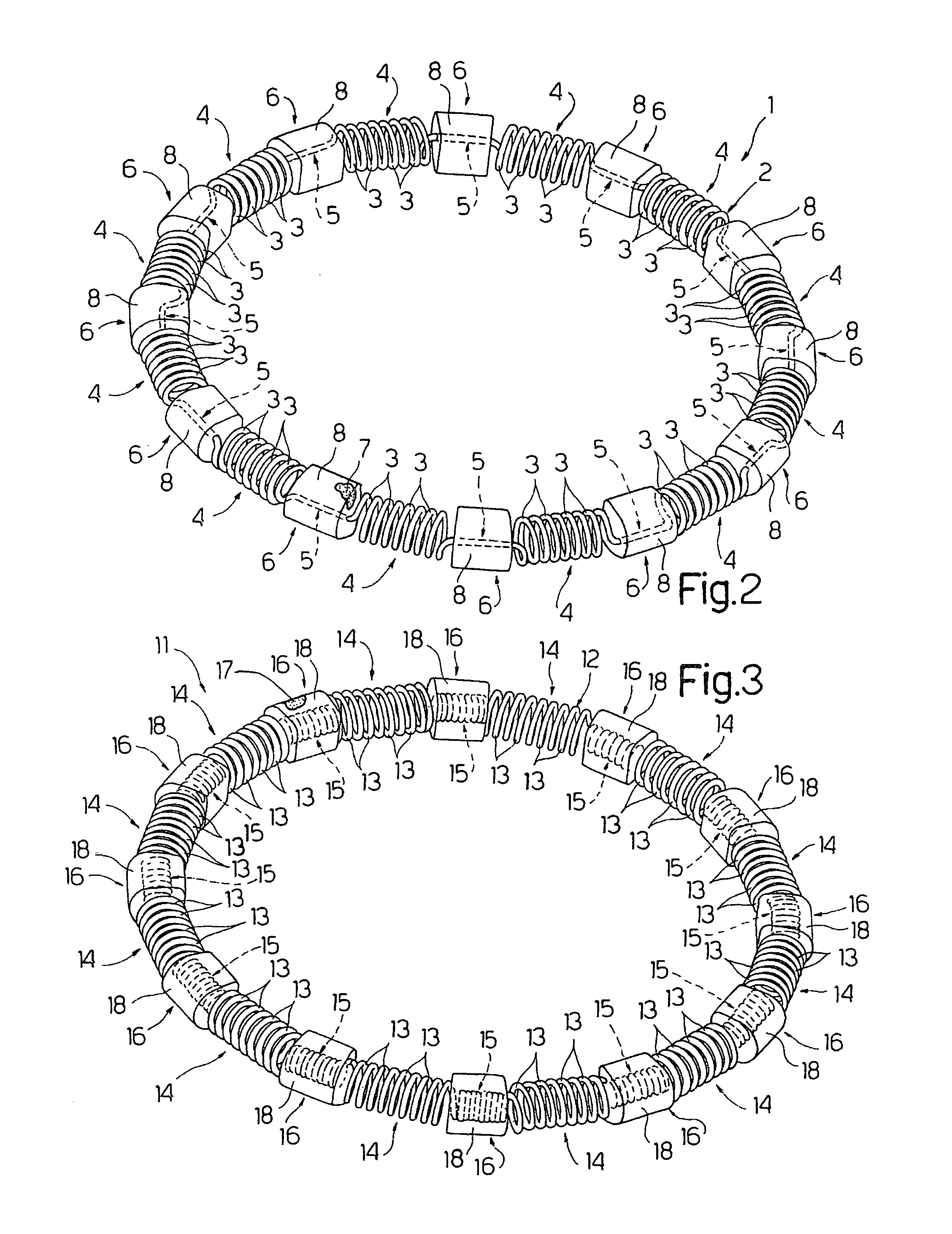
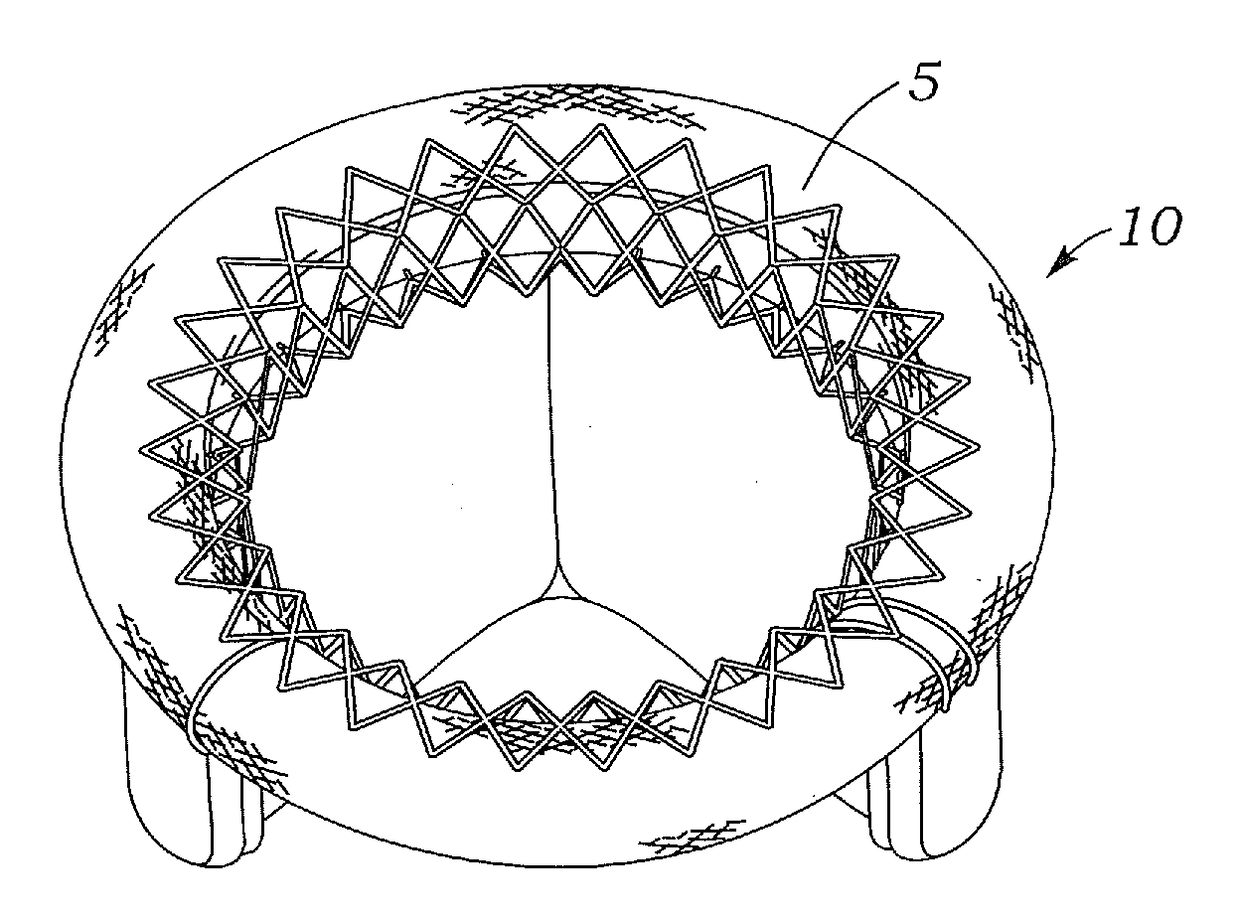
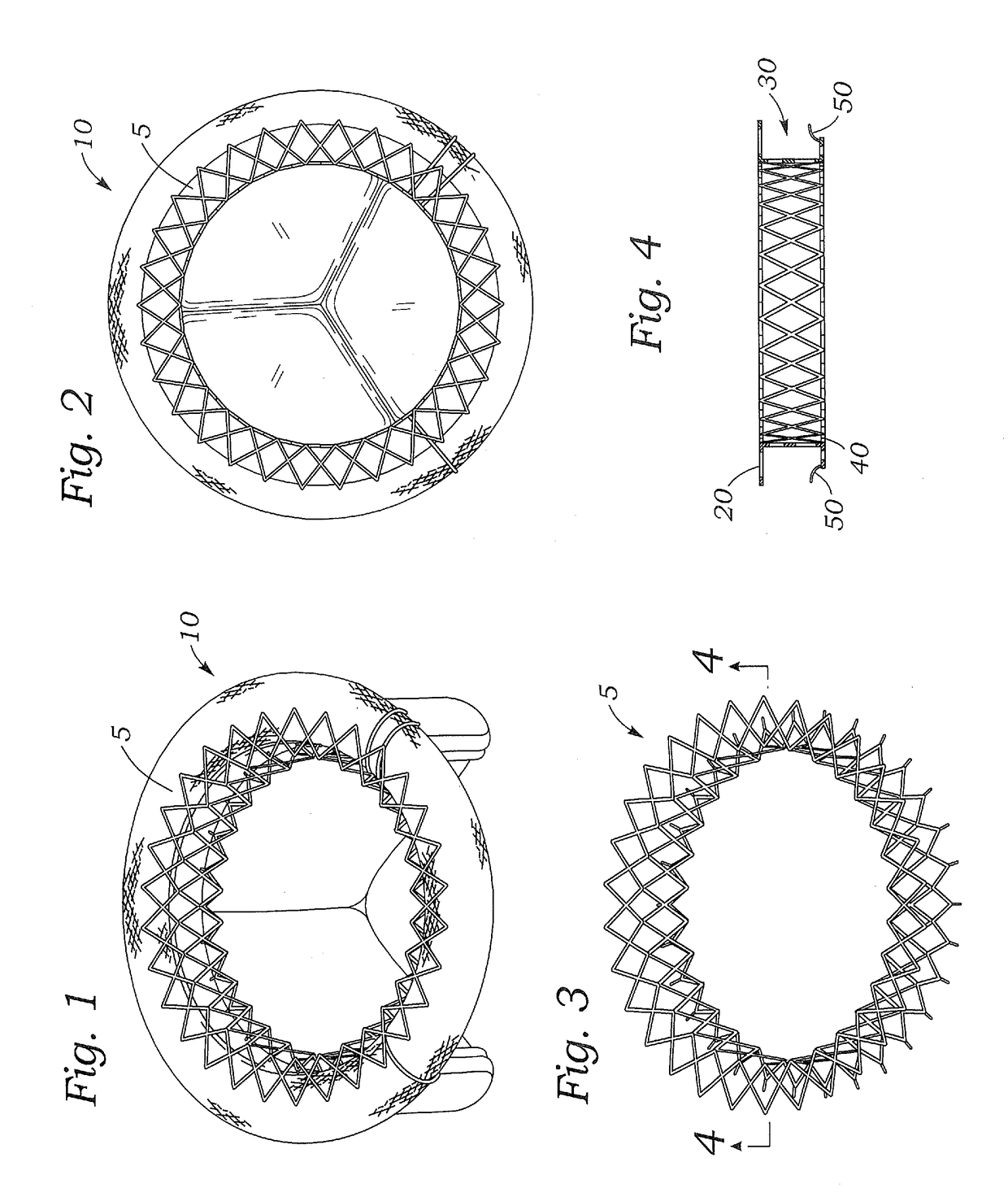
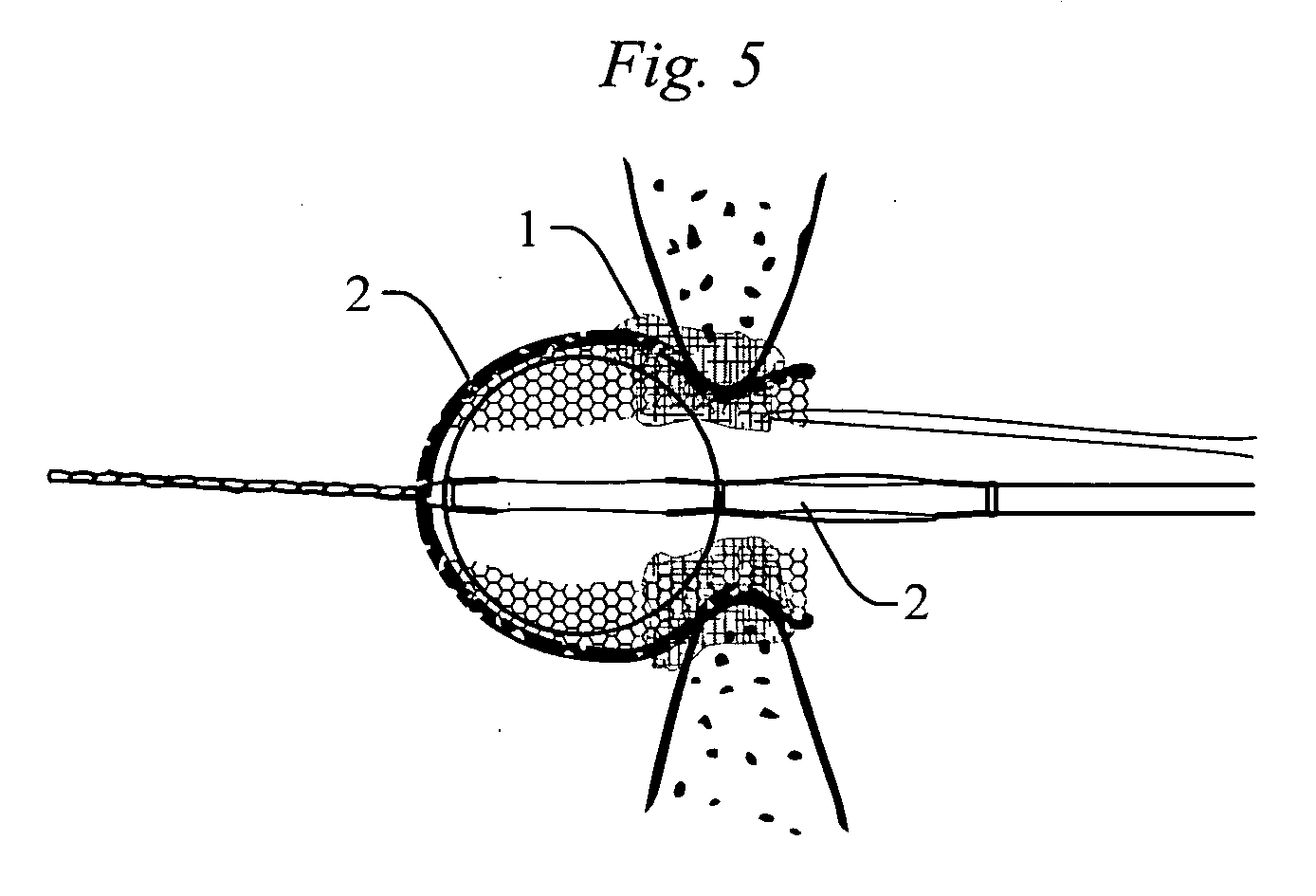
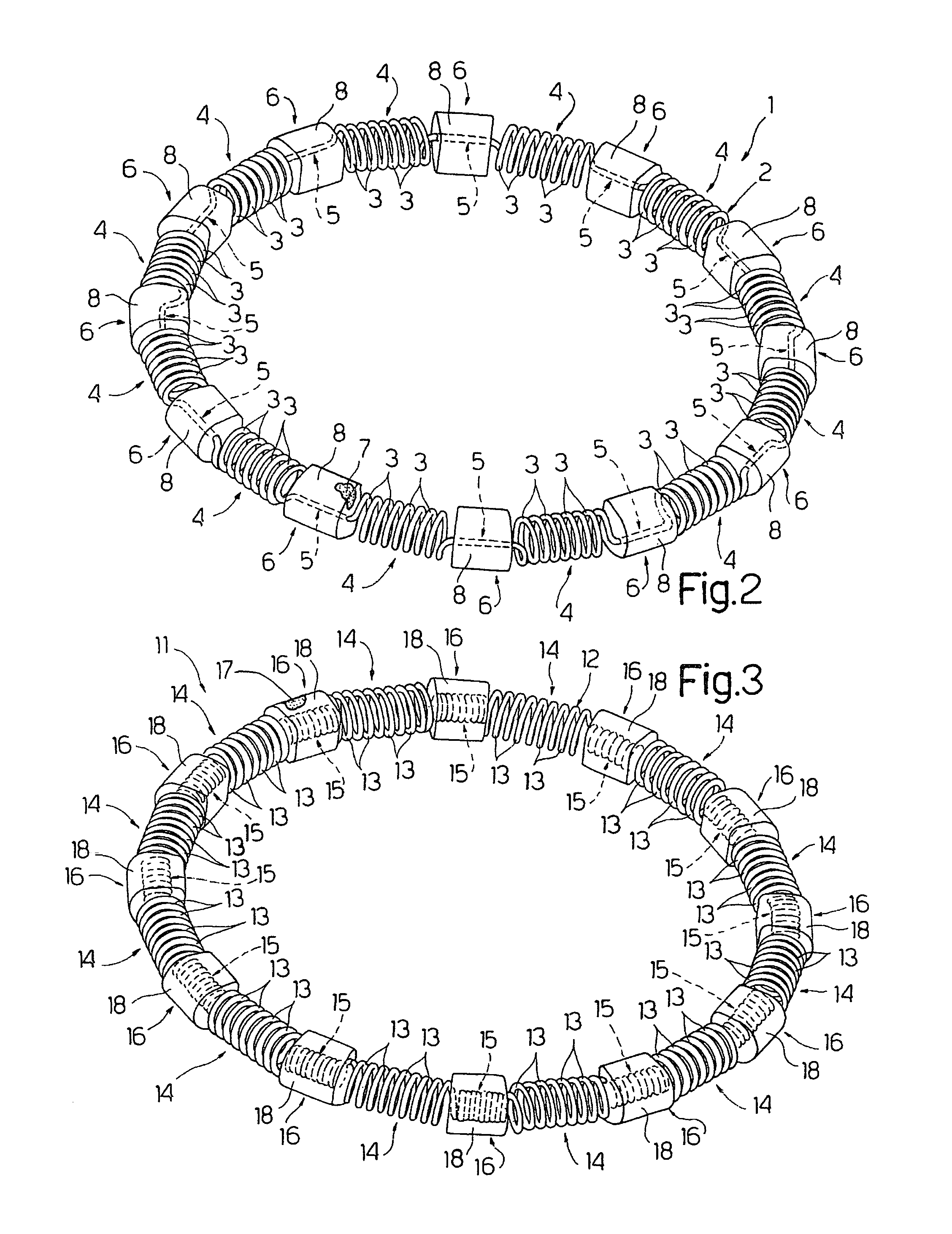
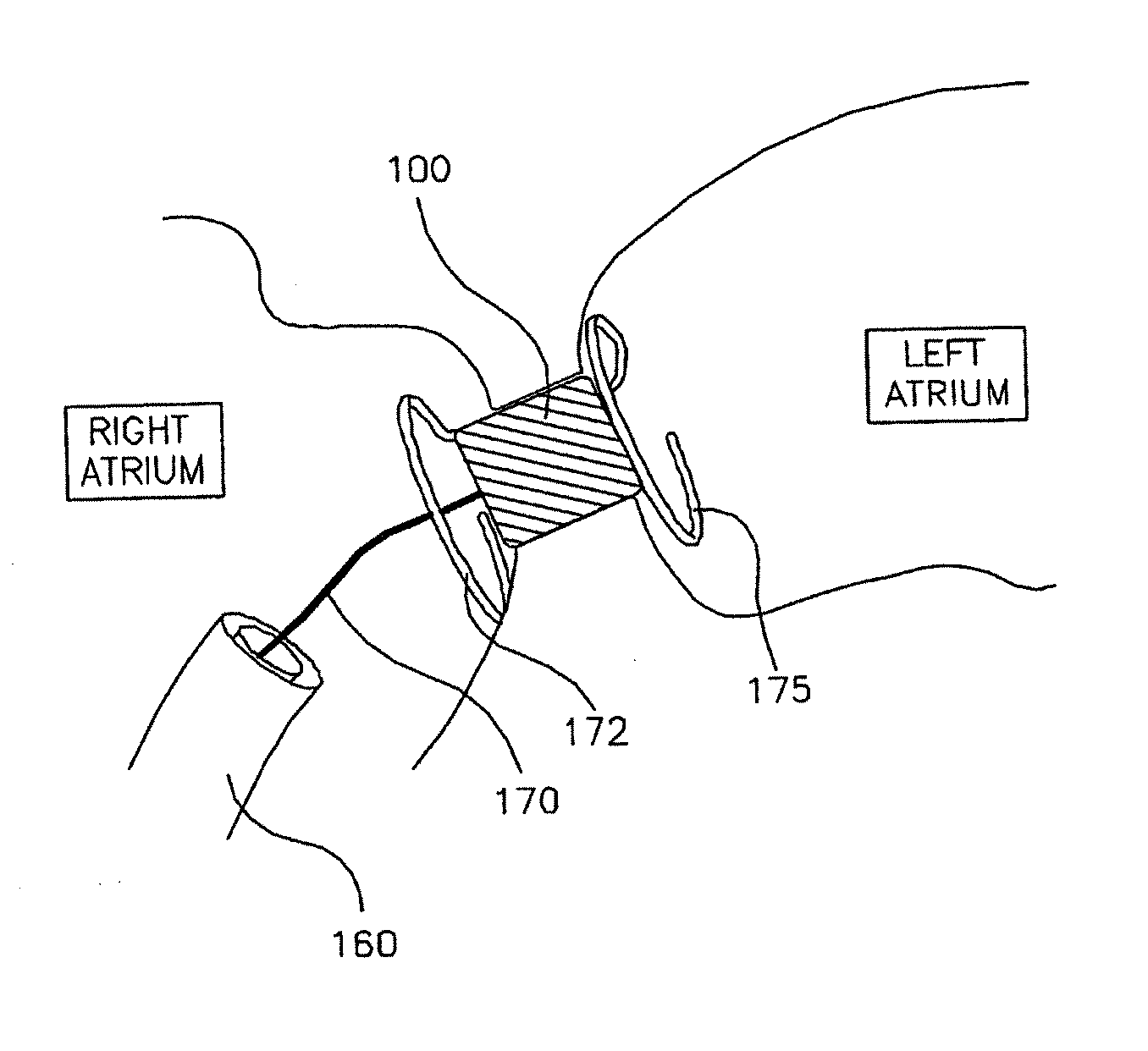
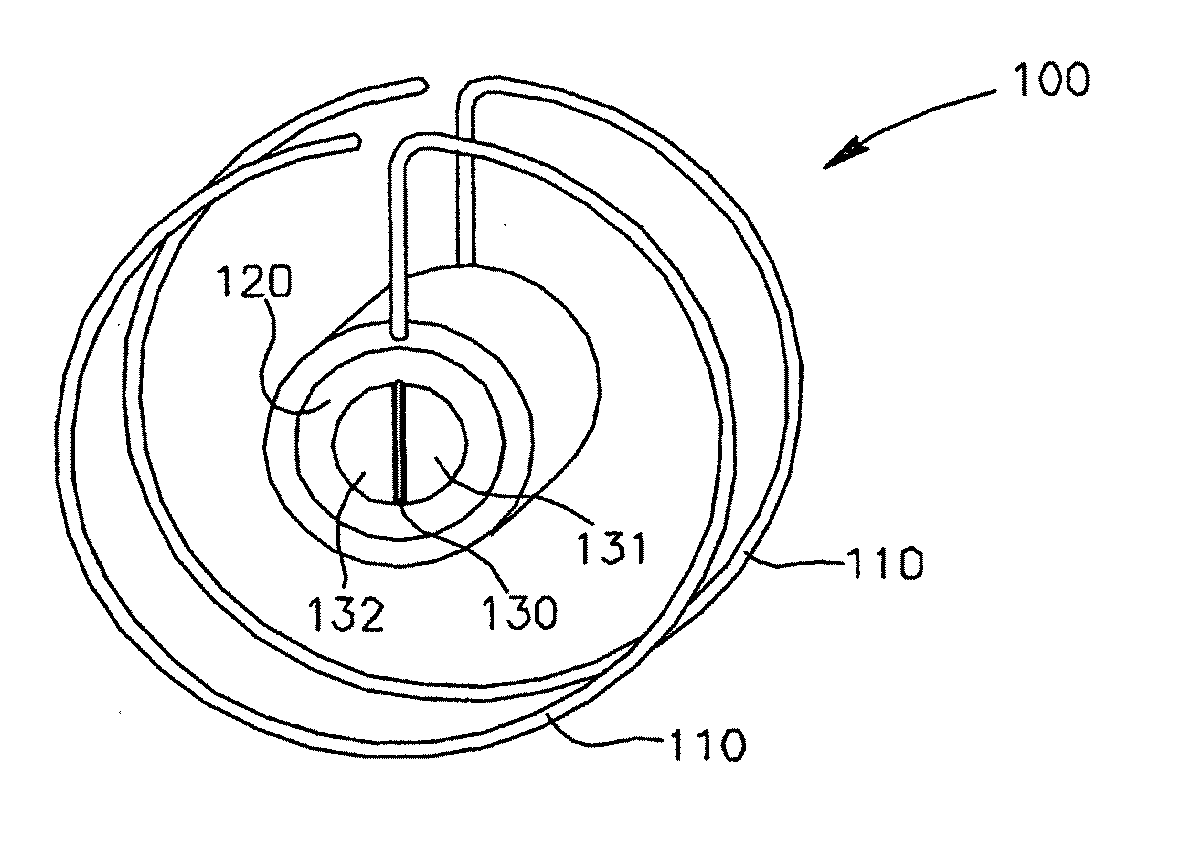
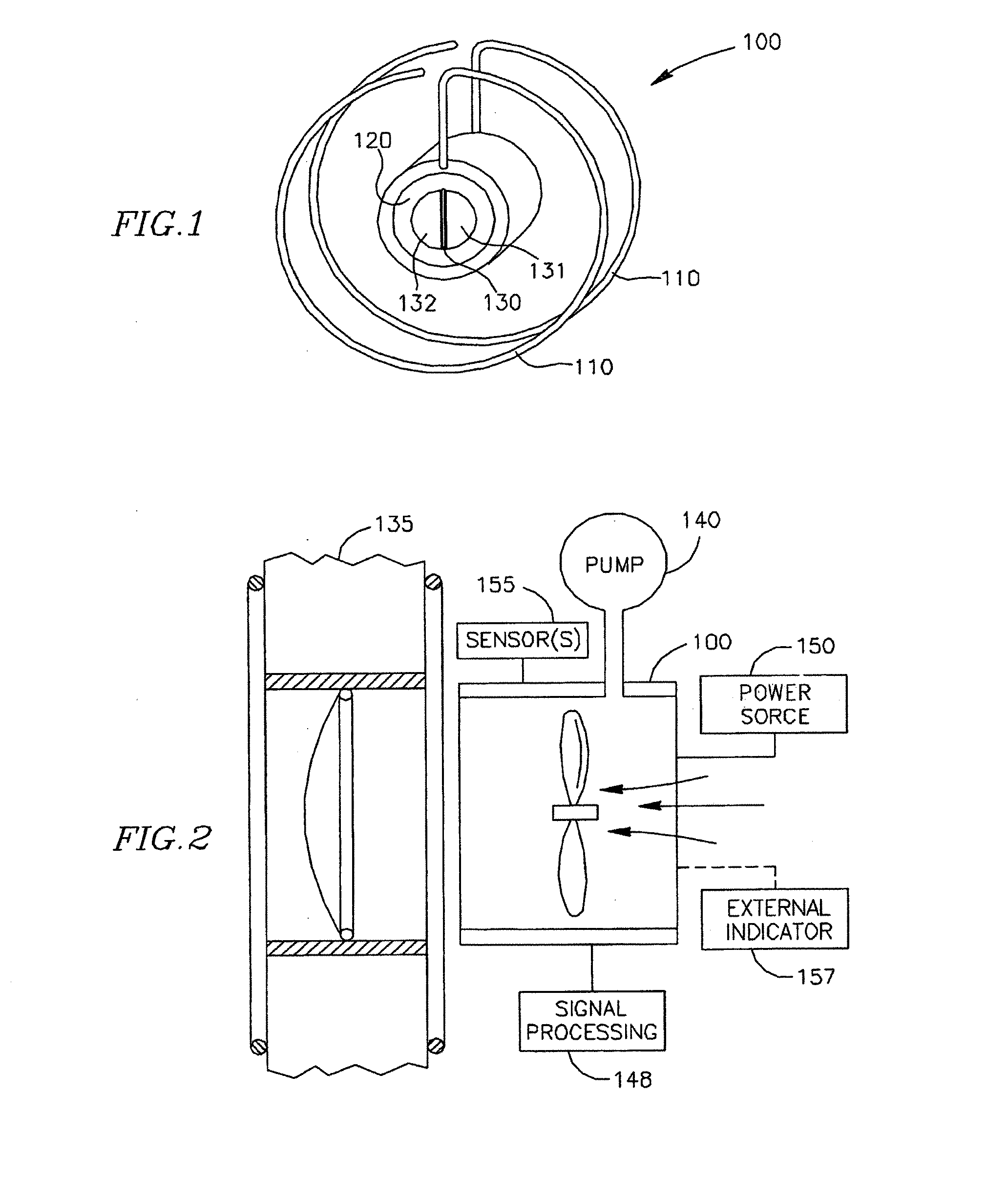
Intracardiac device for restoring the functional elasticity of the cardiac structures, holding tool for the intracardiac device, and method for implantation of the intracardiac device in the heart
ActiveUS20100030014A1Minimize tissutal reactionReduce thrombogenic capacityAnnuloplasty ringsBandagesCardiac cyclePlasma viscosity
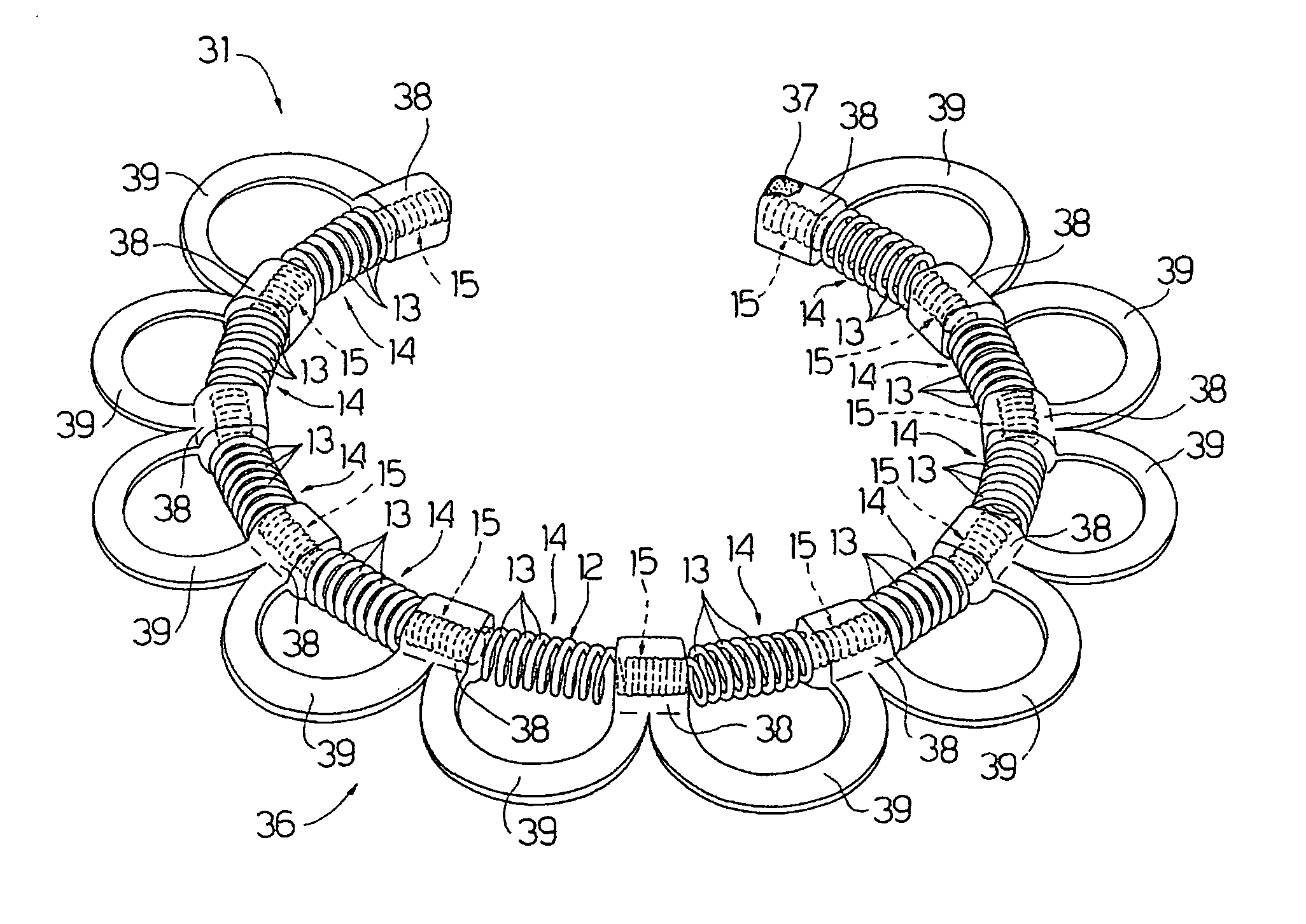
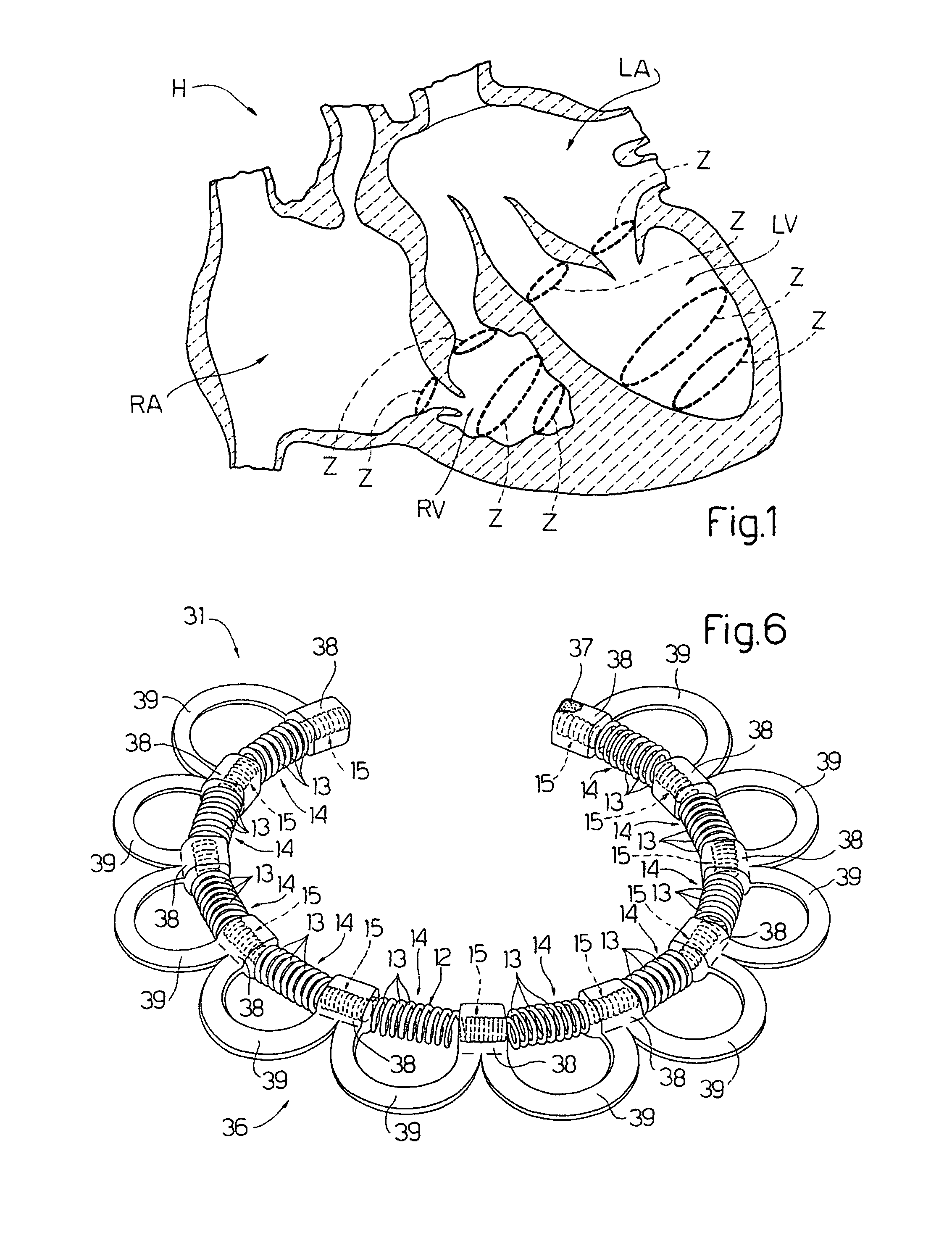
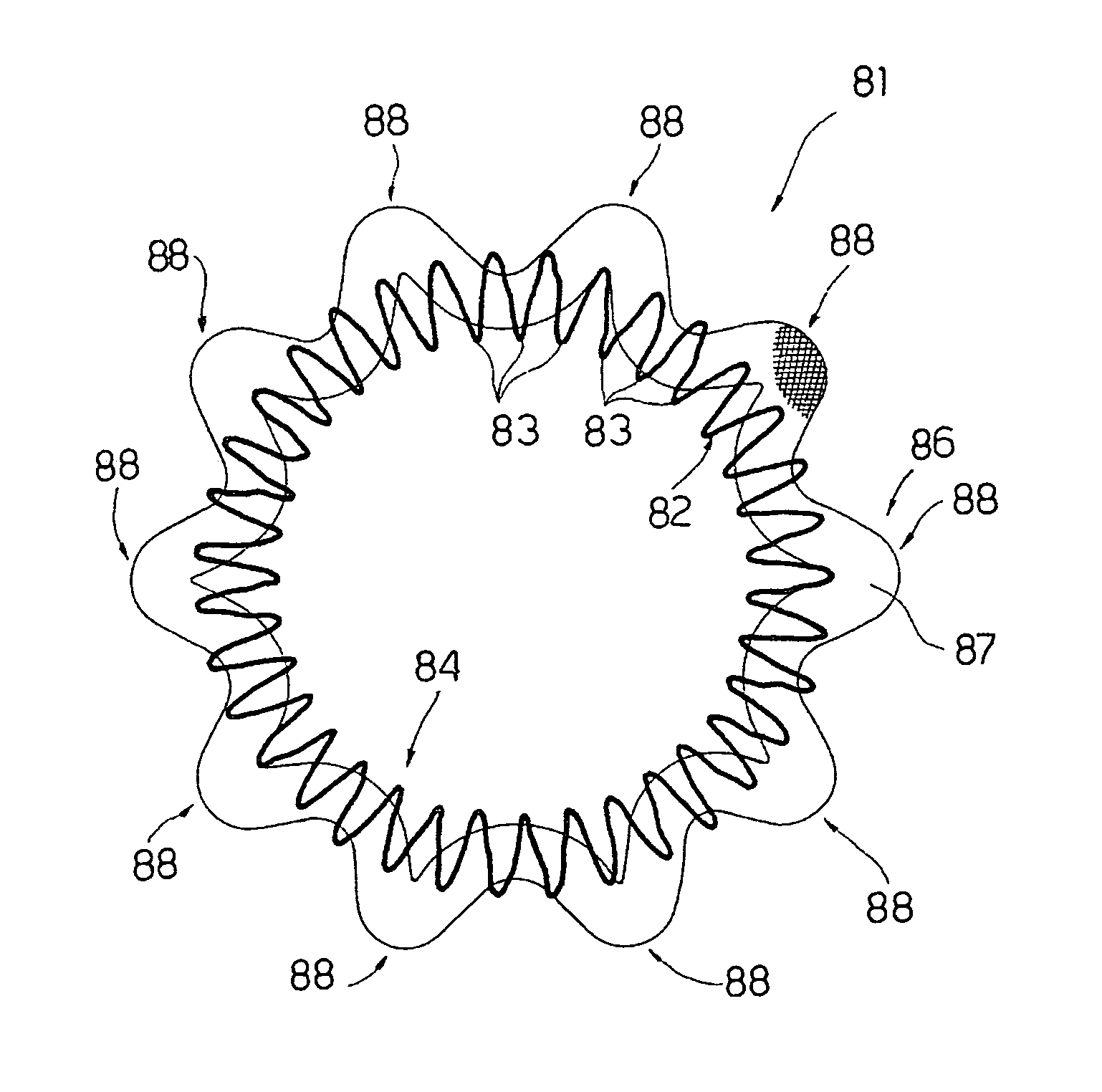
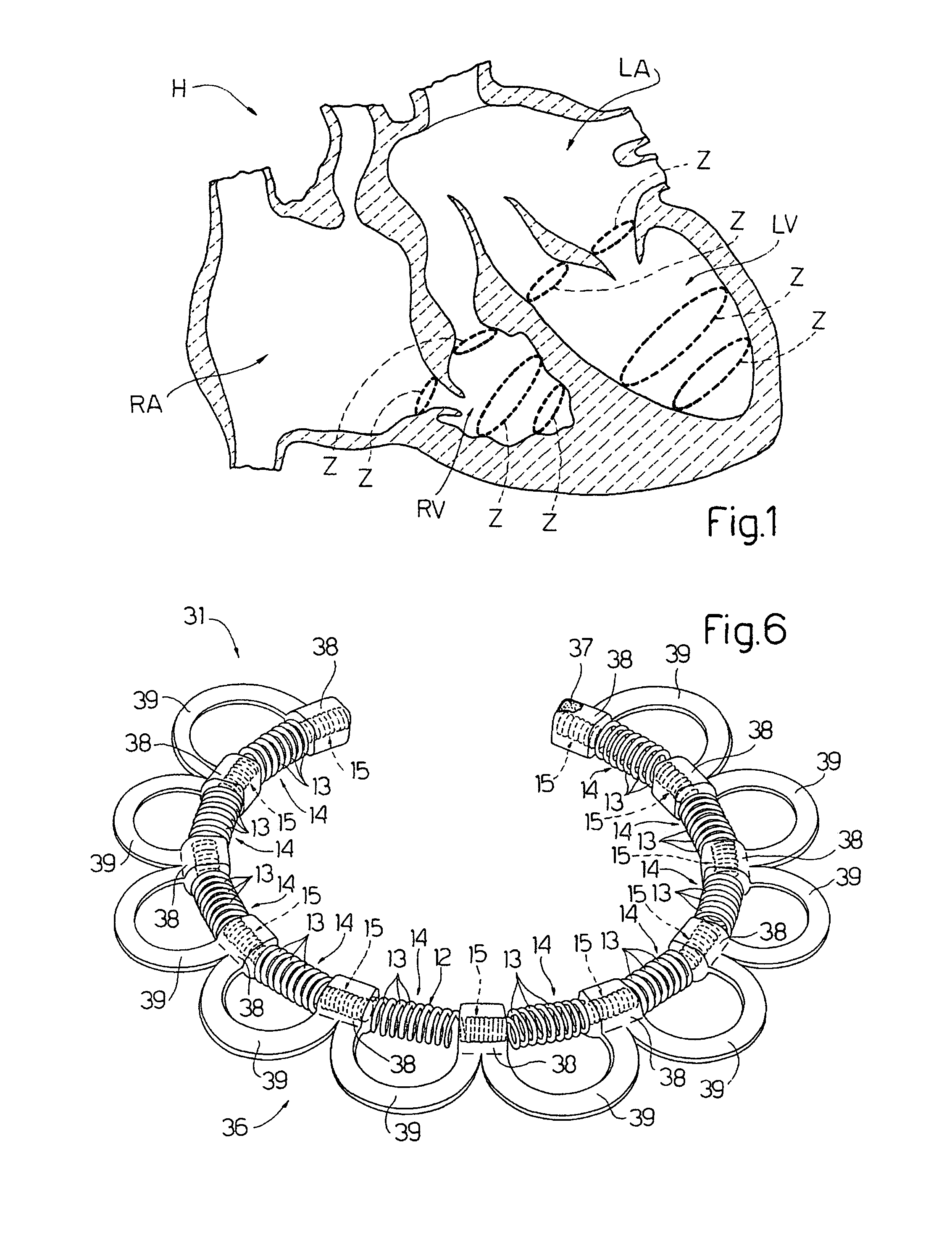
An intracardiac device for restoring the functional elasticity of the cardiac structures, in particular for the treatment of cardiomyopathies and or valvulopathies, by storing energy from the cardiac structures and ceding energy to the cardiac structures during the cardiac cycle, has an elongated shape, is at least partially wound in coils and is attachable to a cardiac structure; the coils are selected in material, number and dimension so as to allow an elastic elongation of the intracardiac device higher than 10% of the rest length of the intracardiac device and are exposed, in use, to the blood flow.
Owner:CUBE
Stem cell therapy for cardiac valvular dysfunction
InactiveUS20080050347A1Raise transfer toReduce needBiocidePeptide/protein ingredientsSexual dysfunctionTricuspid valve function
Disclosed are methods, compounds and compositions useful for treatment of a patient with valvular dysfunction. The invention relates to using stem cells, modified stem cells, derivatives thereof, and agents stimulatory to stem cells in order to substantially ameliorate, and in some cases induce a therapeutic benefit, to a patient suffering from a dysfunction of the mitral, aortic, tricuspid, or pulmonary valve. In some embodiments the invention treats the valve dysfunction itself, whereas in other embodiments treatment of associated cardiac structures is performed. Furthermore, in other embodiments the invention permits physiological compensation for the valve dysfunction, prolonging the time until surgical intervention is needed.
Owner:MEDISTEM LAB
Surgical perforation device with curve
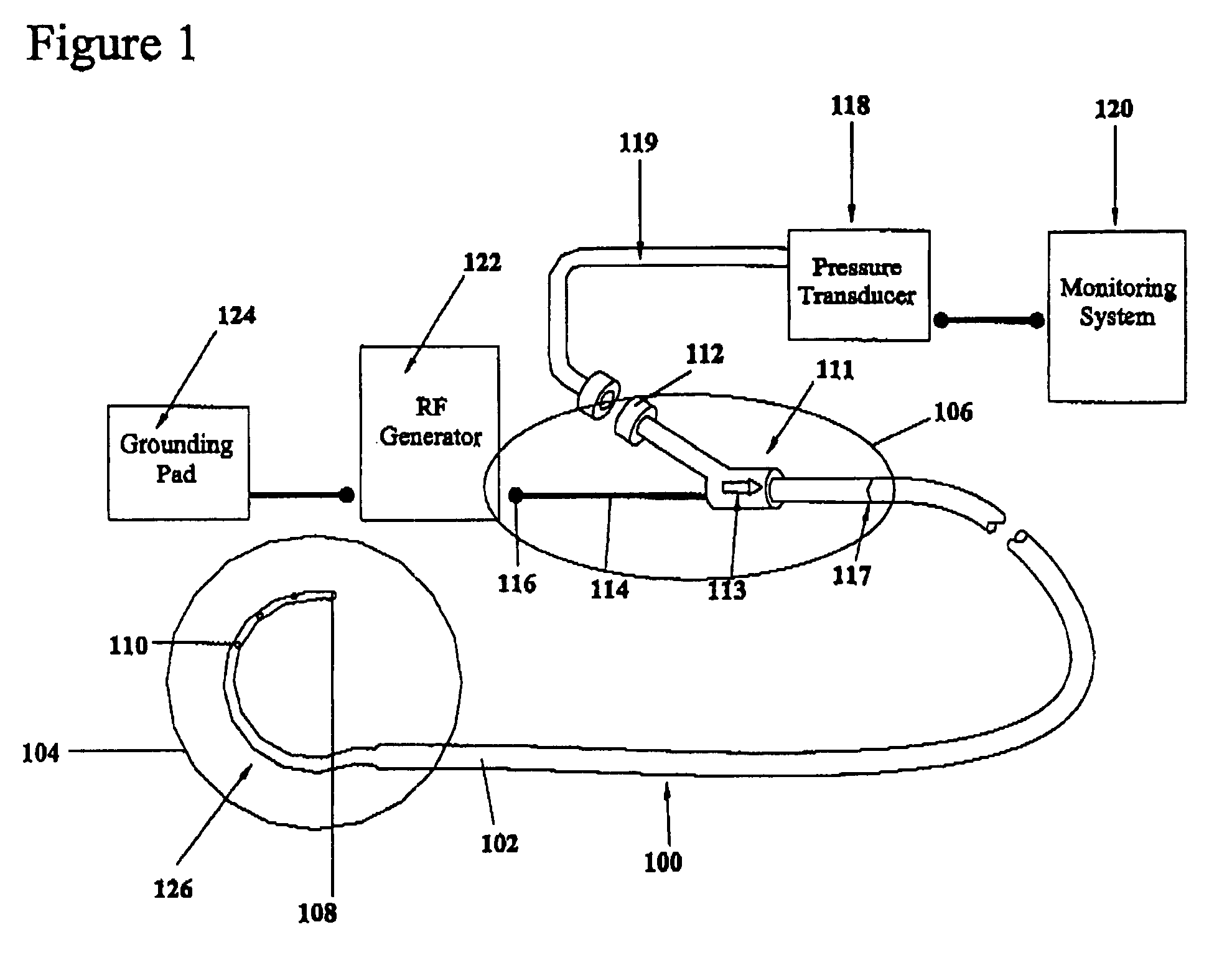
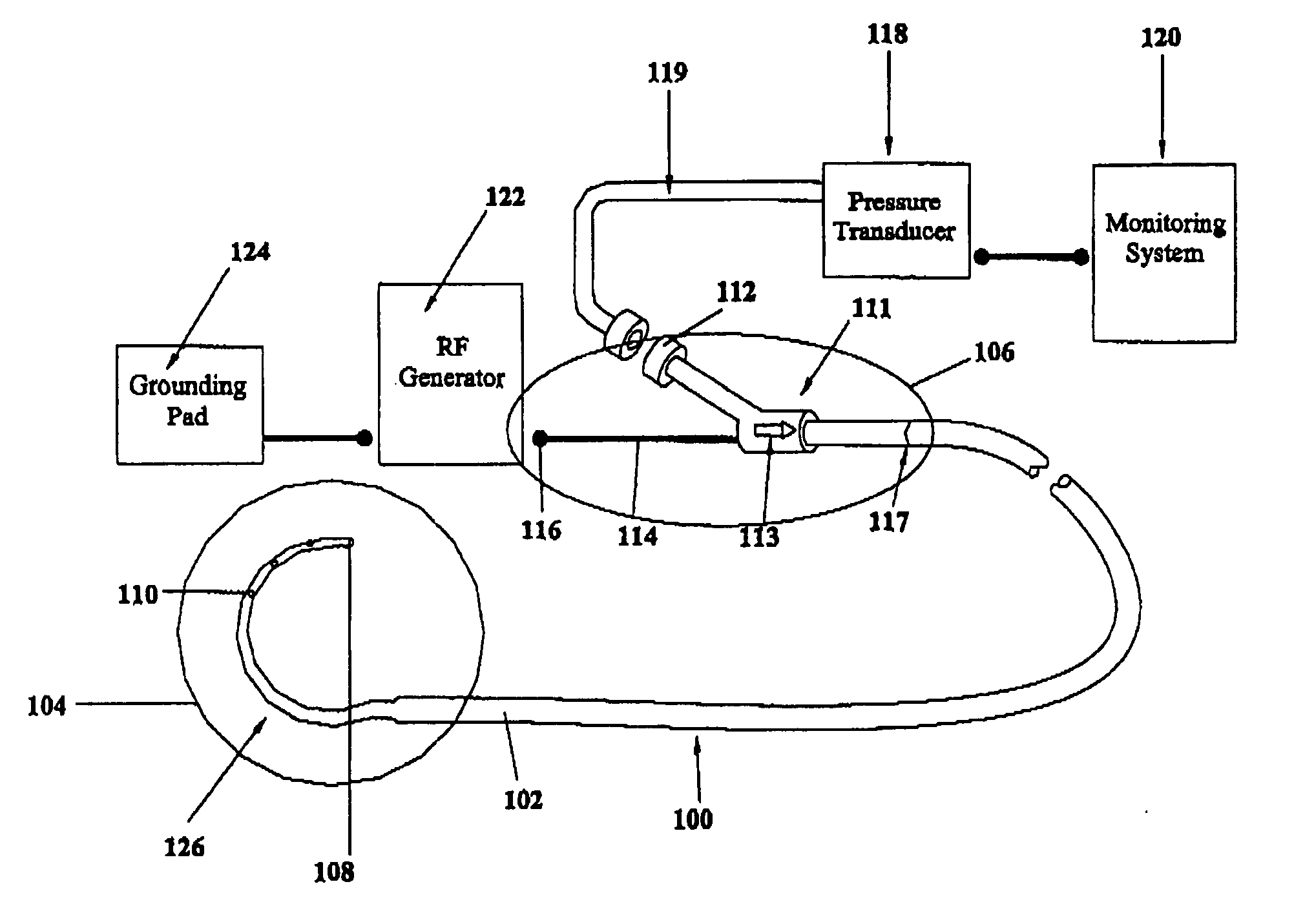
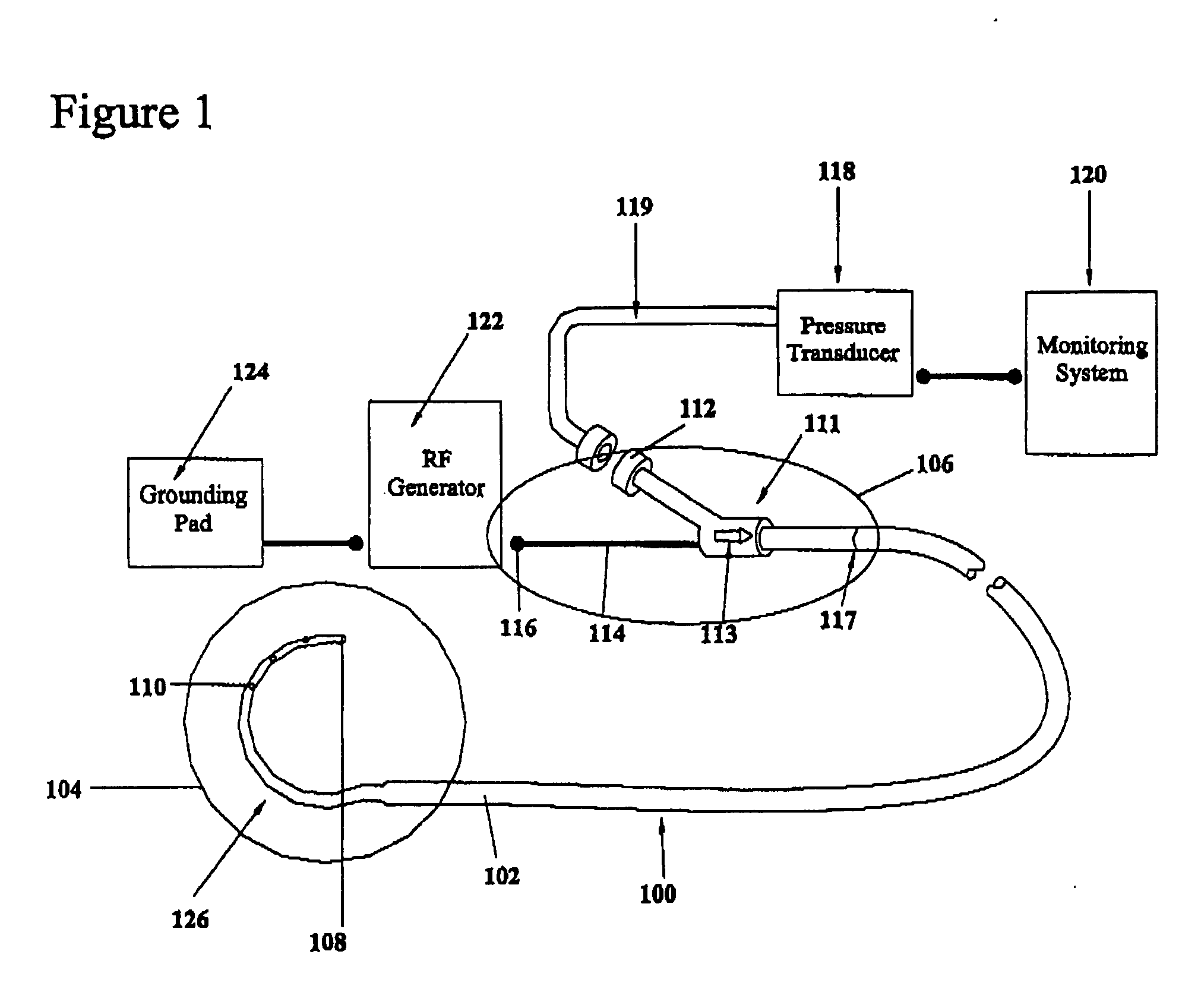
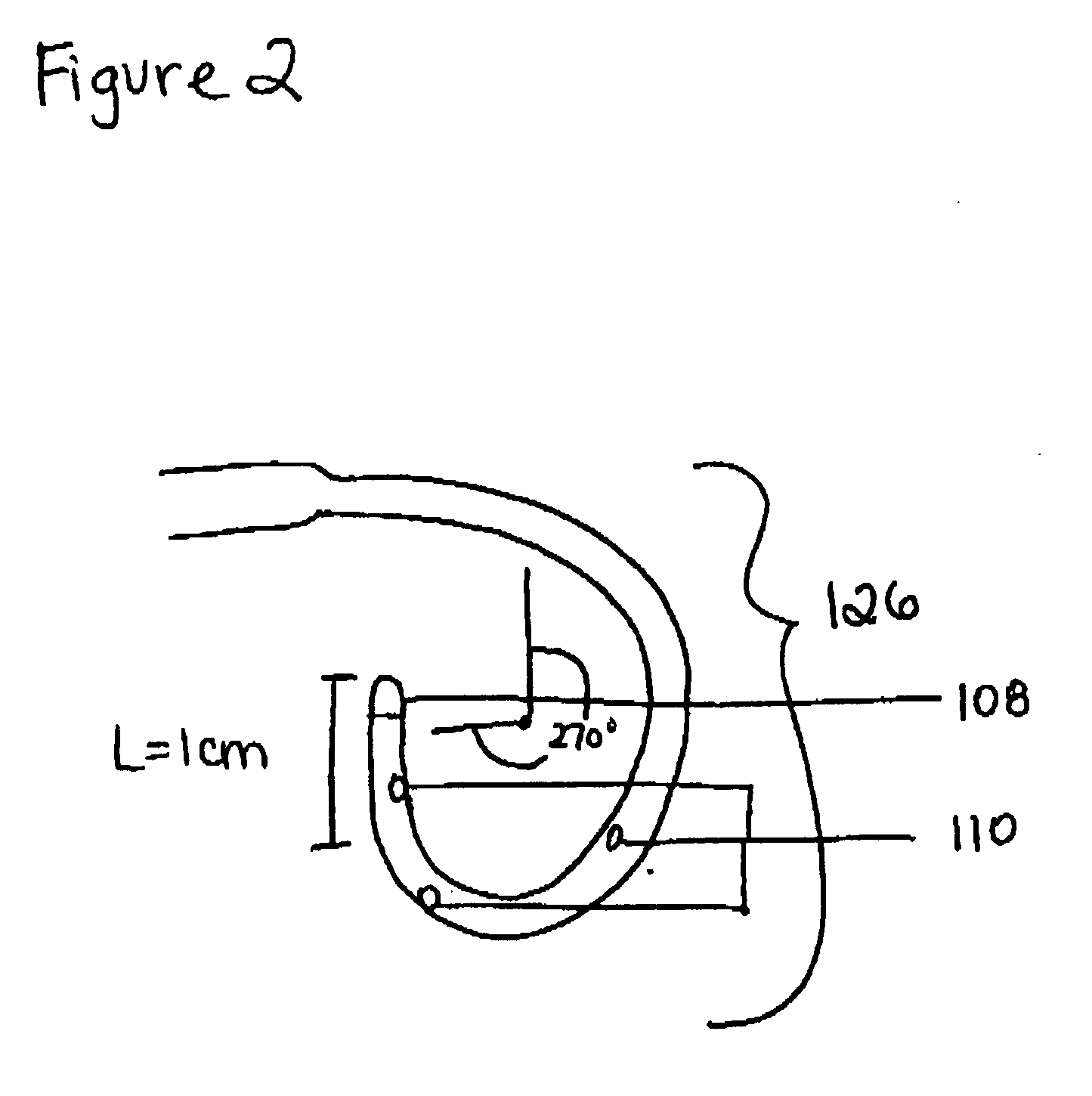
A device for creating a surgical perforation with a functional distal tip for creating a controlled perforation. The functional tip may comprise at least one active electrode for creating the perforation through the application of Radio Frequency (RF) energy. The device is curved in order to decrease the likelihood of injury to structures of a patient such as inadvertent cardiac perforation when used in transseptal perforation procedures. The device is introduced into the right atrium, and the functional tip is then positioned against the atrial septum. Energy is applied to create the perforation. The tip is advanced toward the left atrium to create the perforation and as it advances into the left atrium, the device takes on its curved shape to direct the tip away from cardiac structures. The position of the tip of the device can be determined in response to pressure sensed at the tip and determined by a monitor.
Owner:BOSTON SCI MEDICAL DEVICE LTD
Deployment system for an endoluminal device
The present invention is directed to a deployment system for an endoluminal device. The deployment system includes a confining sheath placed around a compacted endoluminal device. A deployment line is provided in the system that is an integral extension of the sheath. As the deployment line is actuated, the sheath retracts from around the compacted endoluminal device. As the sheath retracts from around the endoluminal device, material from the sheath may be converted into deployment line. Once the sheath is retracted from around the compacted endoluminal device, the endoluminal device expands in configuration and repairs vascular or cardiac structures of an implant recipient. Any remaining sheath material is removed from the implantation site along with the deployment line.
Owner:WL GORE & ASSOC INC
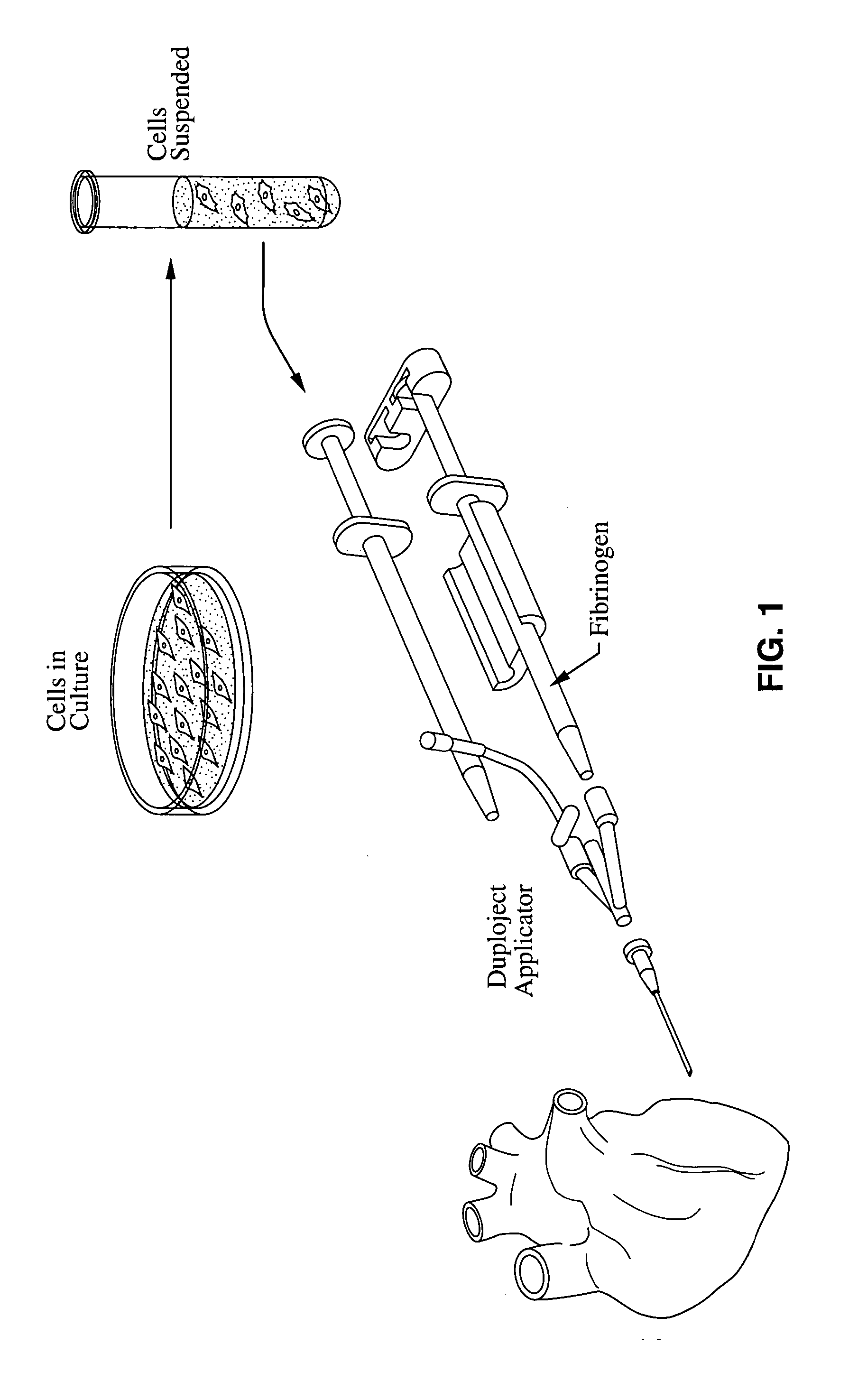
Material compositions and related systems and methods for treating cardiac conditions
InactiveUS20050271631A1Preventing negative remodelingHigh retention rateBiocideSurgical adhesivesInjectable polymersFibrin glue
A medical condition associated with a cardiac structure is treated by injecting an injectable polymer agent into the cardiac structure such that a therapeutic mechanical scaffolding is formed within the cardiac structure itself. In particular, the injectable scaffolding agent is a fibrin glue agent and is injected into regions of damaged myocardium such as ischemic tissue or infarct. LV wall dysfunction may also be treated by injecting the scaffolding agent into the LV wall. Cell therapy may be combined with the injection of fibrin glue or other injectable polymer scaffold agent. The polymeric forms of the agent may be injectable as precursor materials that polymerize as a scaffold in-situ within the cardiac structure. In other modes, polymer agents are injected in order to provide therapeutic angiogenesis, or to induce deposition of cells within the injected area, such as by providing the polymer with fragment E or RDG binding sites, respectively.
Owner:RGT UNIV OF CALIFORNIA
Apparatus and method for stabilizing an implantable lead
InactiveUS6961621B2Improve stabilityGood adhesionTransvascular endocardial electrodesExternal electrodesCannula deviceLead system
A method and system for stabilizing an implantable lead system employs a lead having one or more sensing, pacing, or shocking electrodes. A sleeve arrangement of the lead includes one or more first locations comprising a first material that substantially prevents or inhibits tissue in-growth between the first locations and cardiac tissue contacting the first locations. The sleeve arrangement further includes one or more adhesion sites provided at one or more of the first locations. The adhesion sites promote tissue in-growth or attachment between the adhesion sites and cardiac tissue contacting the adhesion sites. The cardiac tissue may represent tissue of a cardiac structure of the heart or coronary vasculature of the heart.
Owner:CARDIAC PACEMAKERS INC
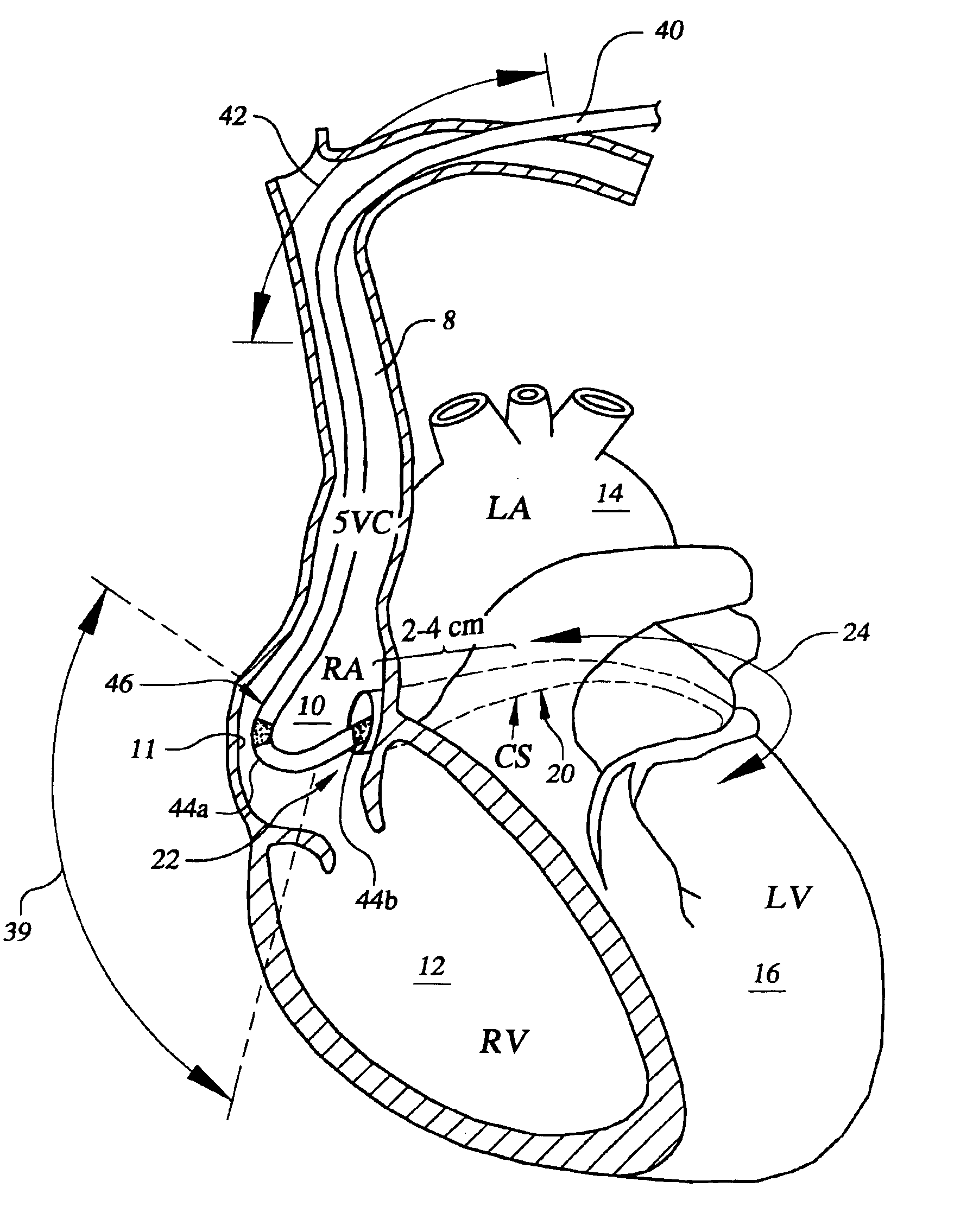
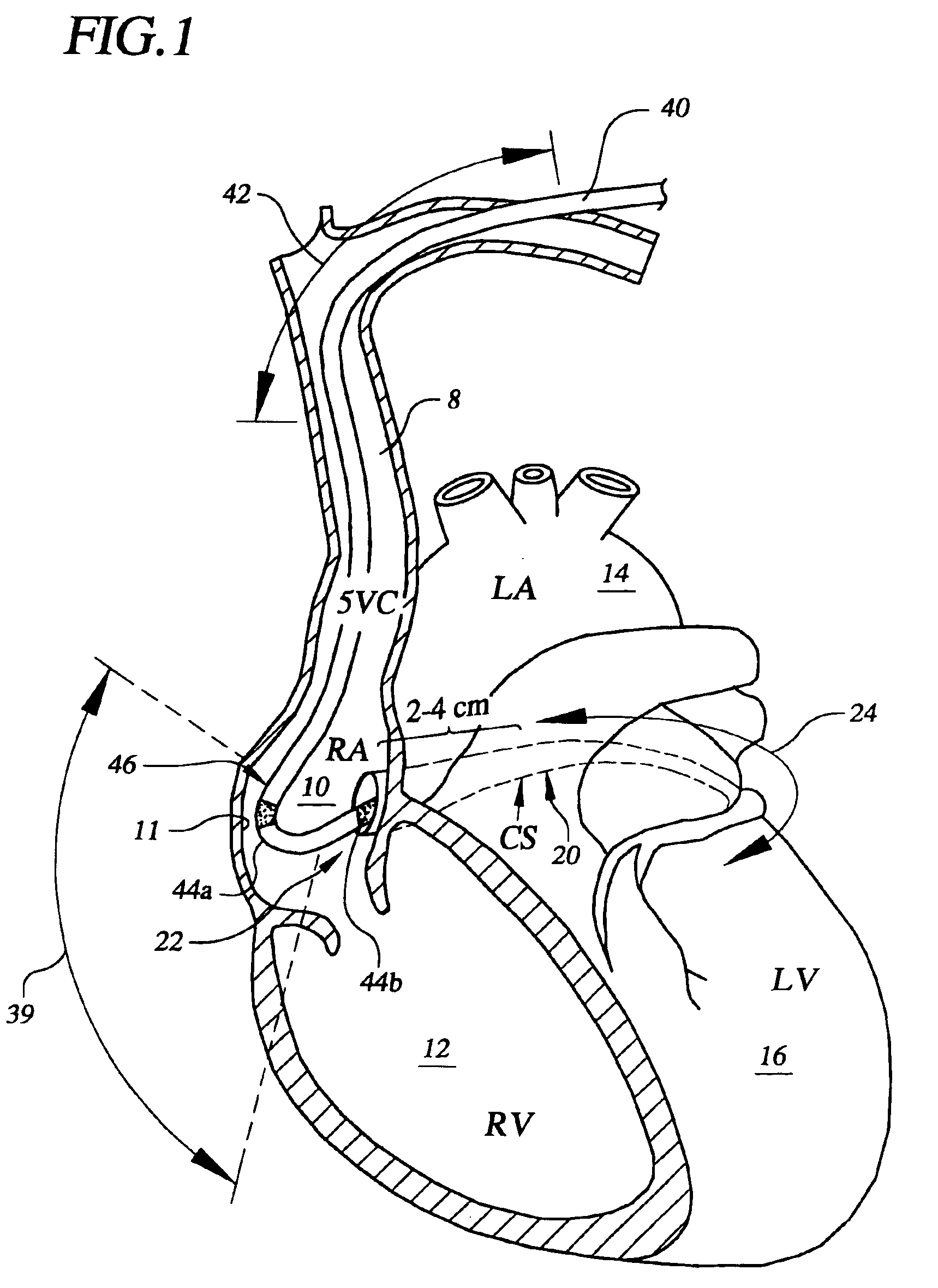
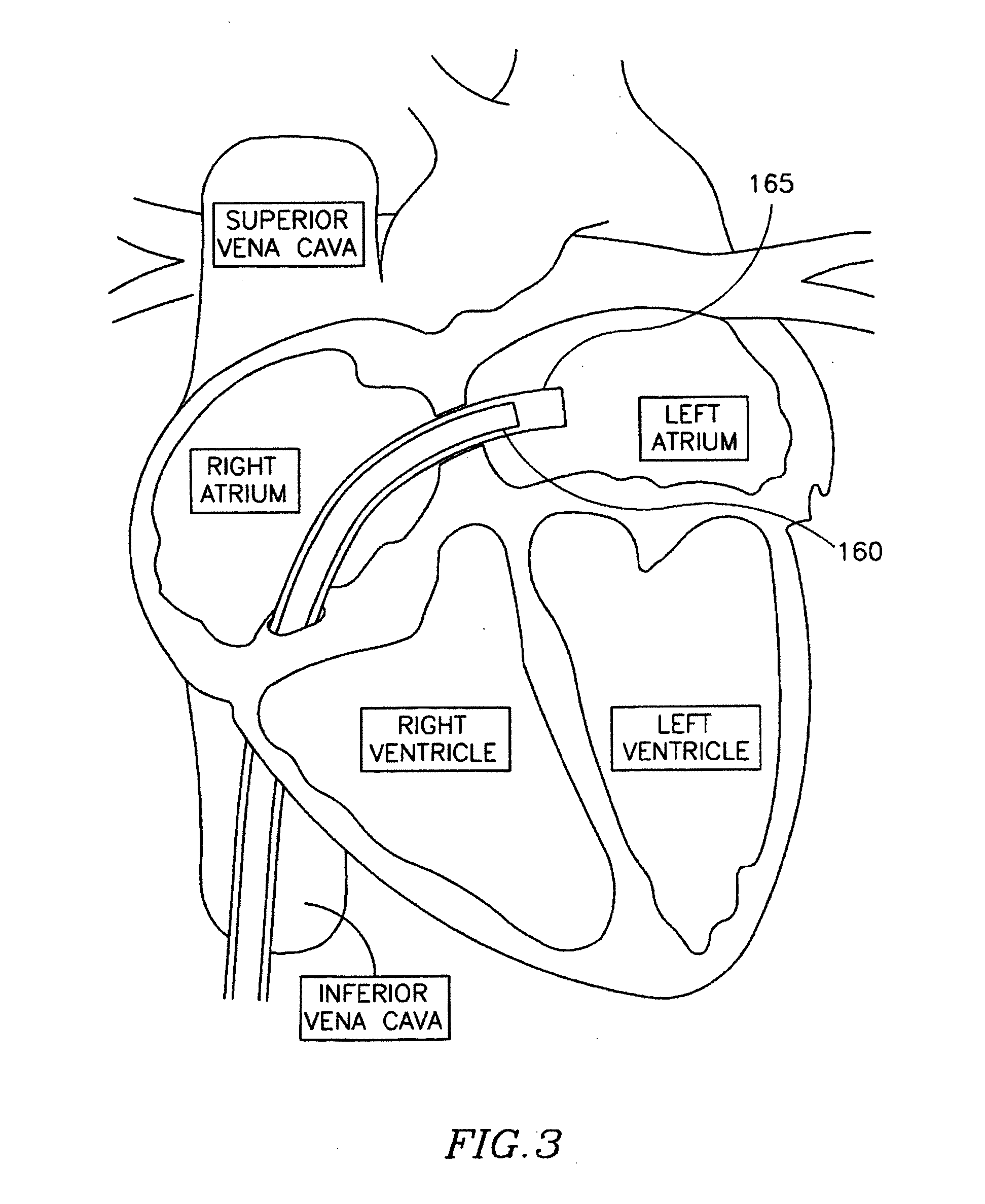
Spacer for securing a transcatheter valve to a bioprosthetic cardiac structure
InactiveUS20170281337A1Reduce the overall diameterBalloon catheterAnnuloplasty ringsDocking stationProsthesis
A spacer for creating a docking station for a transcatheter heart valve is provided. The spacer changes an effective diameter and / or a shape of an implanted bioprosthetic structure such as a bioprosthetic heart valve or annuloplasty ring, providing a supporting structure into which the transcatheter valve expands without over expanding. The spacer may be deployed through an interventional technique either through transseptal access, transfemoral access, or transapical access and is typically deployed at least in part on an inflow portion of the implanted bioprosthetic structure.
Owner:EDWARDS LIFESCIENCES CORP
Extracting ultrasound summary information useful for inexperienced users of ultrasound
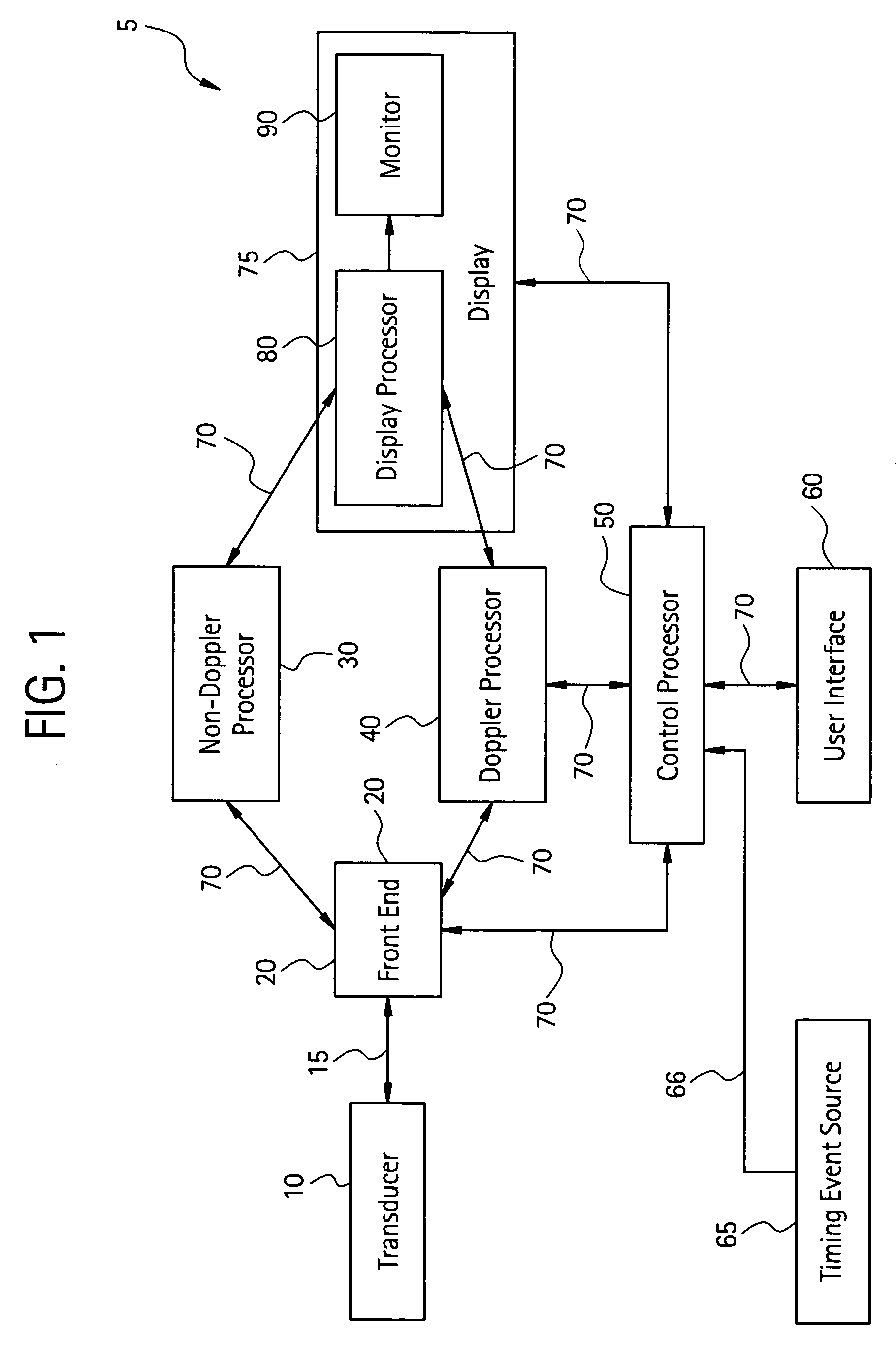
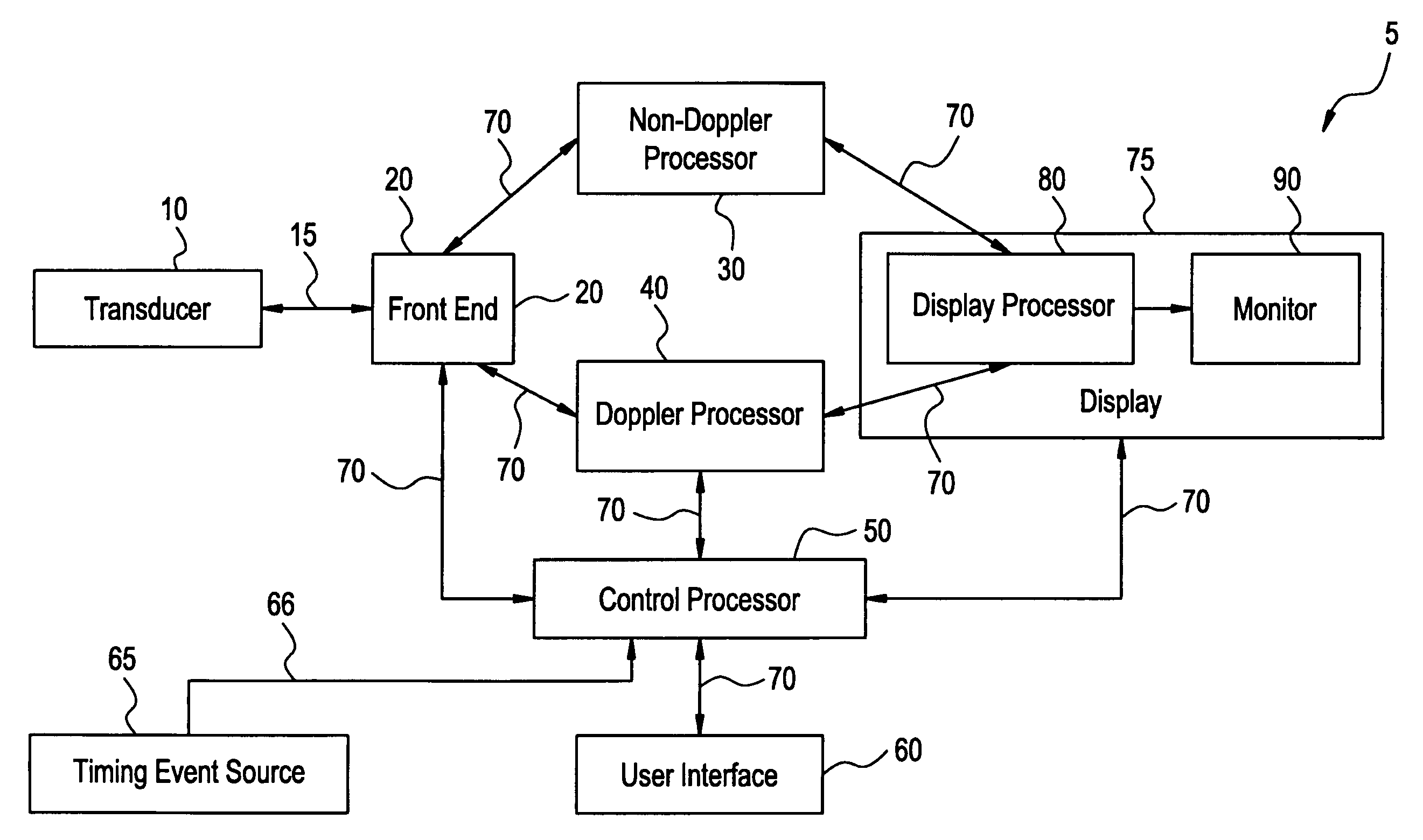
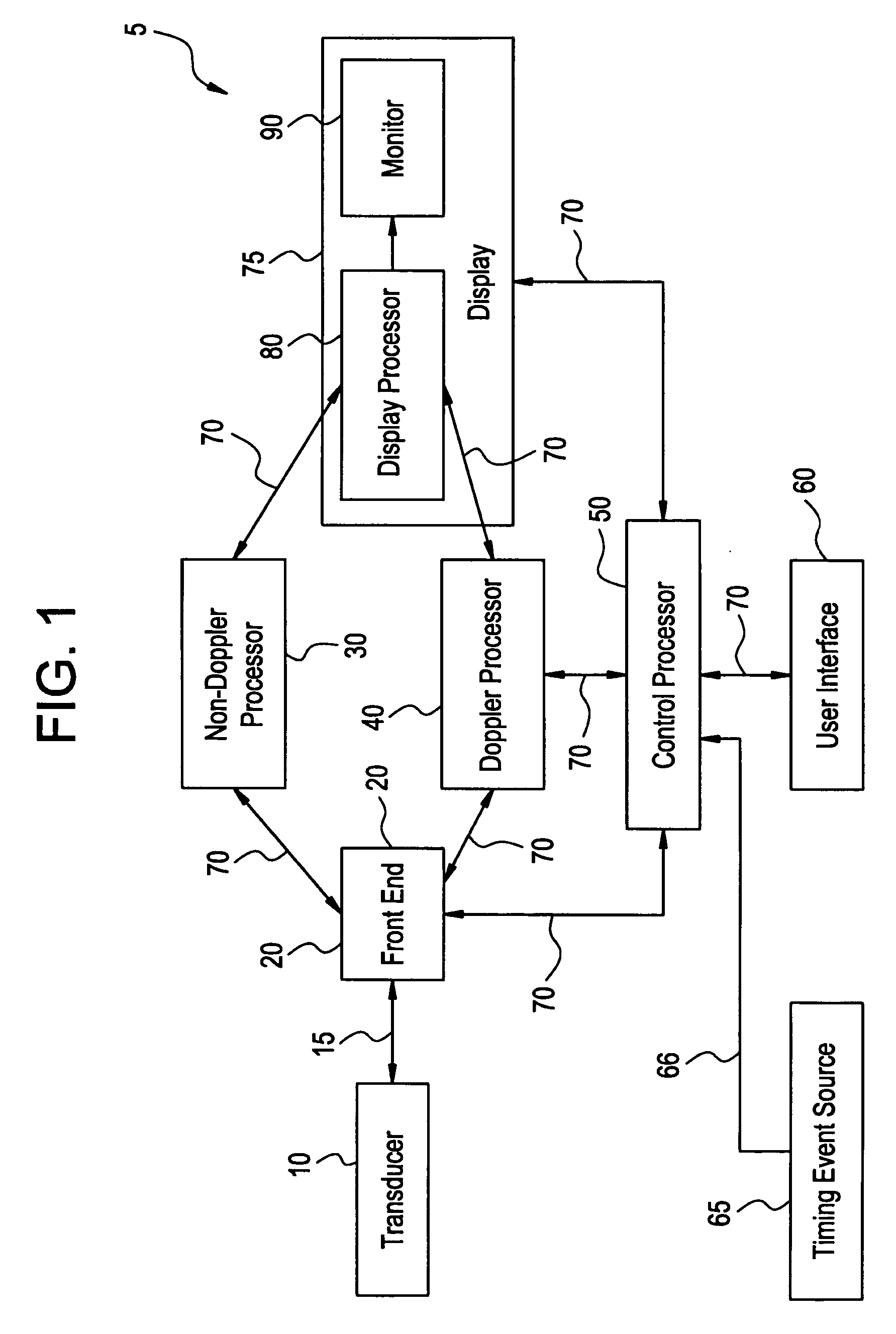
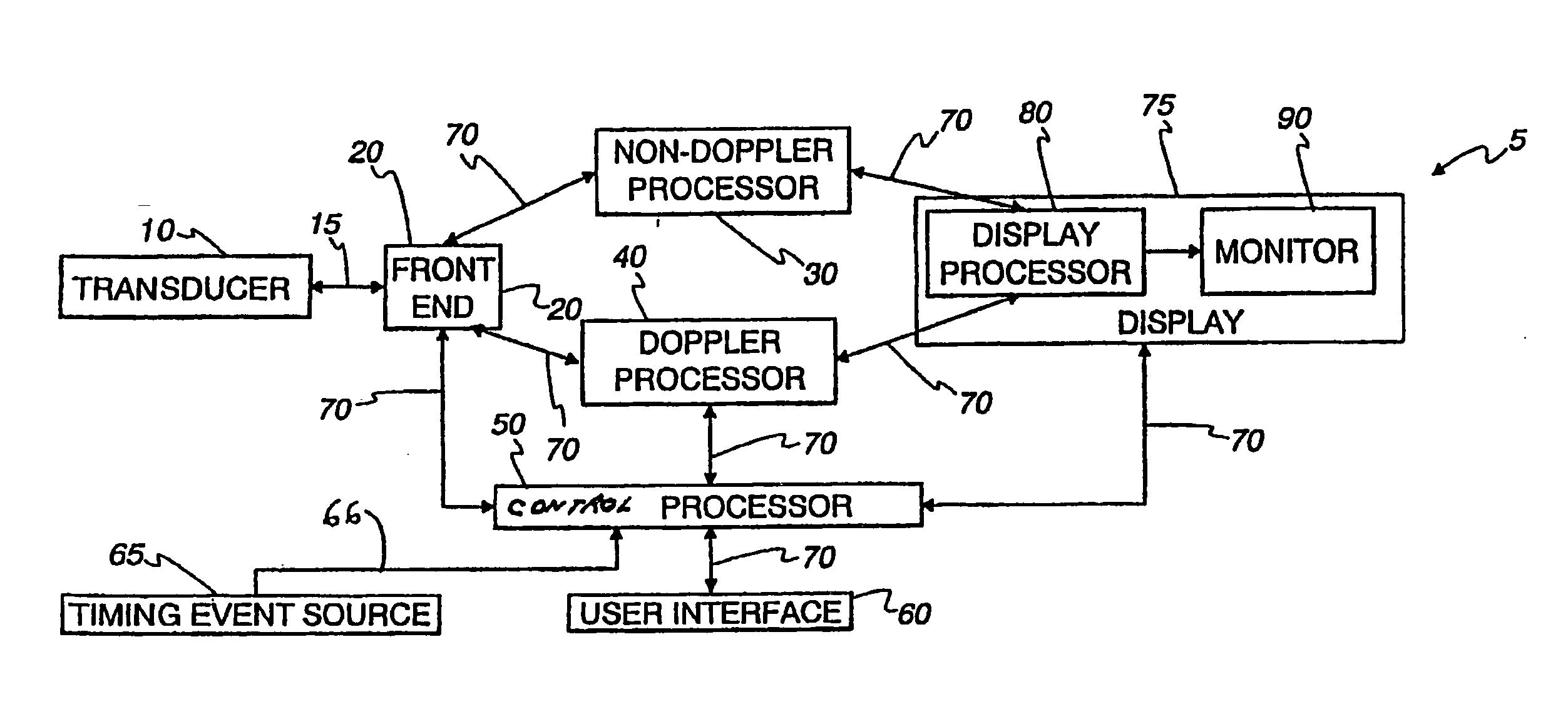
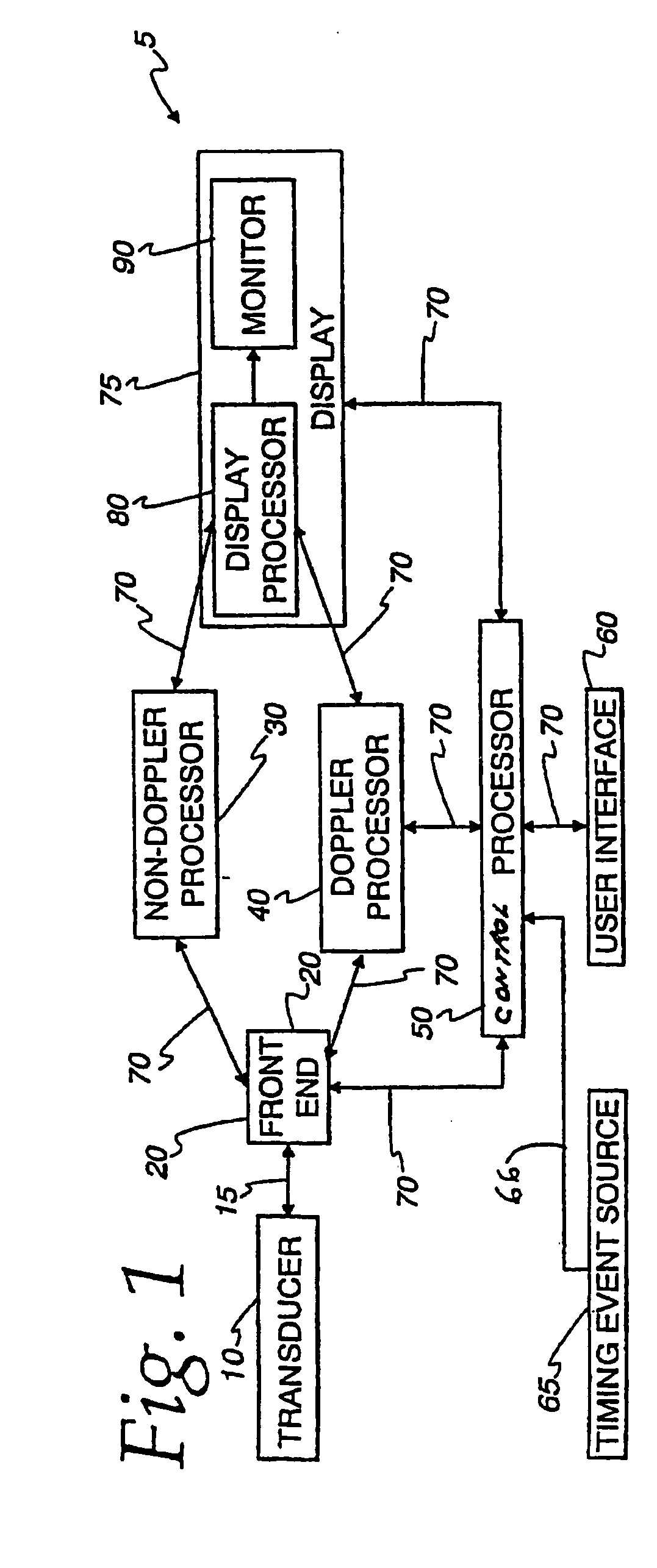
InactiveUS20060058609A1Organ movement/changes detectionInfrasonic diagnosticsAnatomical landmarkRelevant information
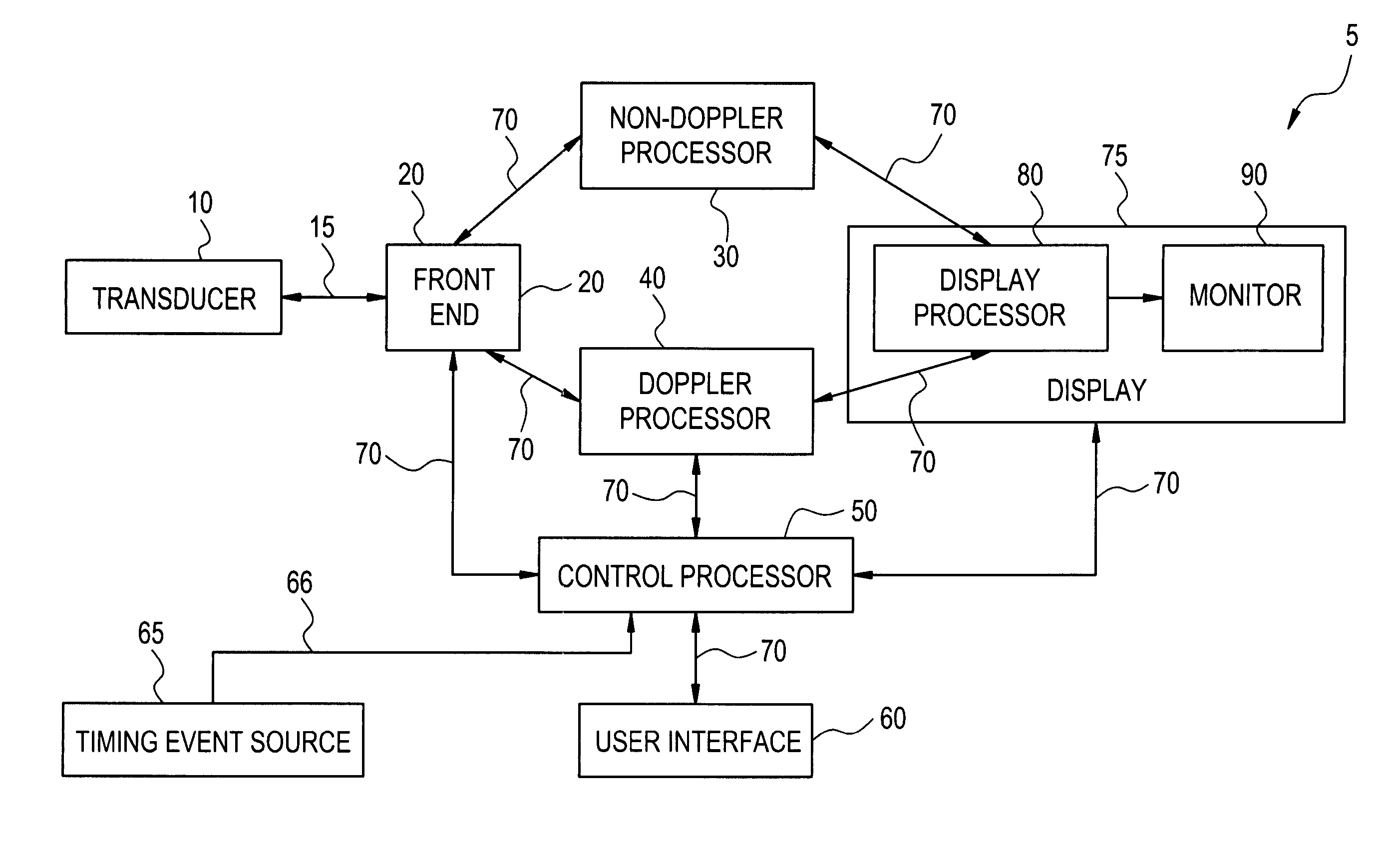
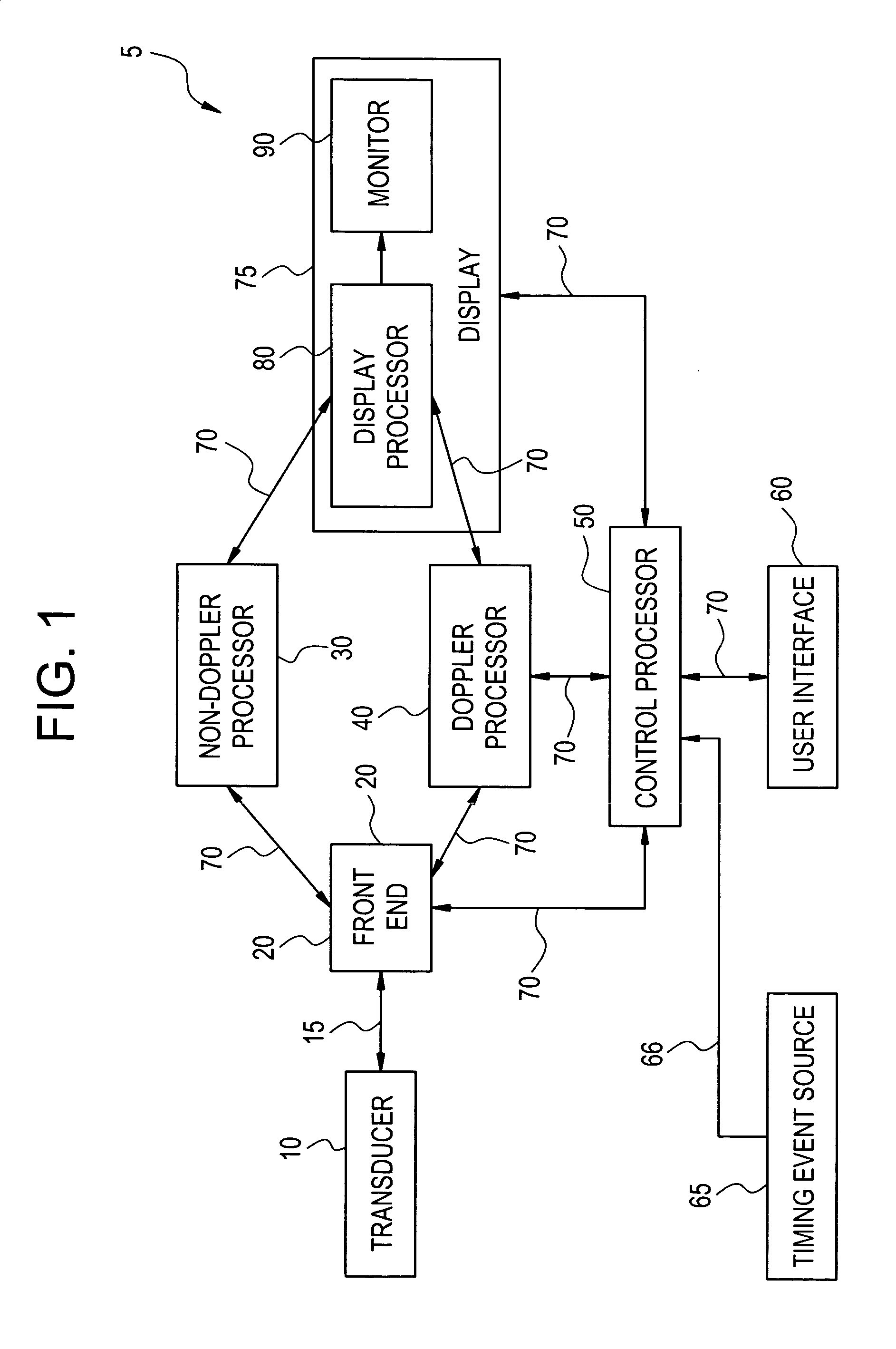
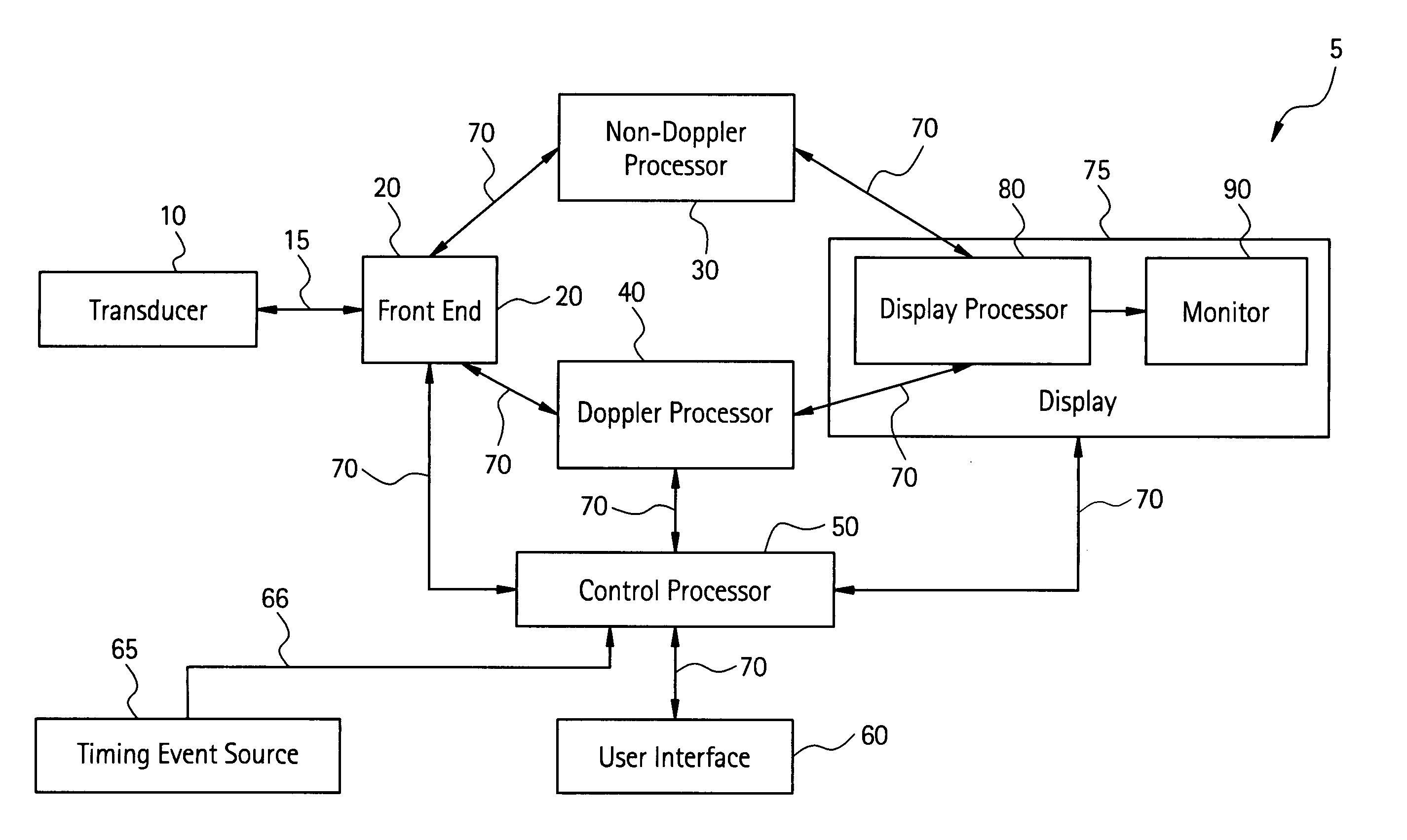
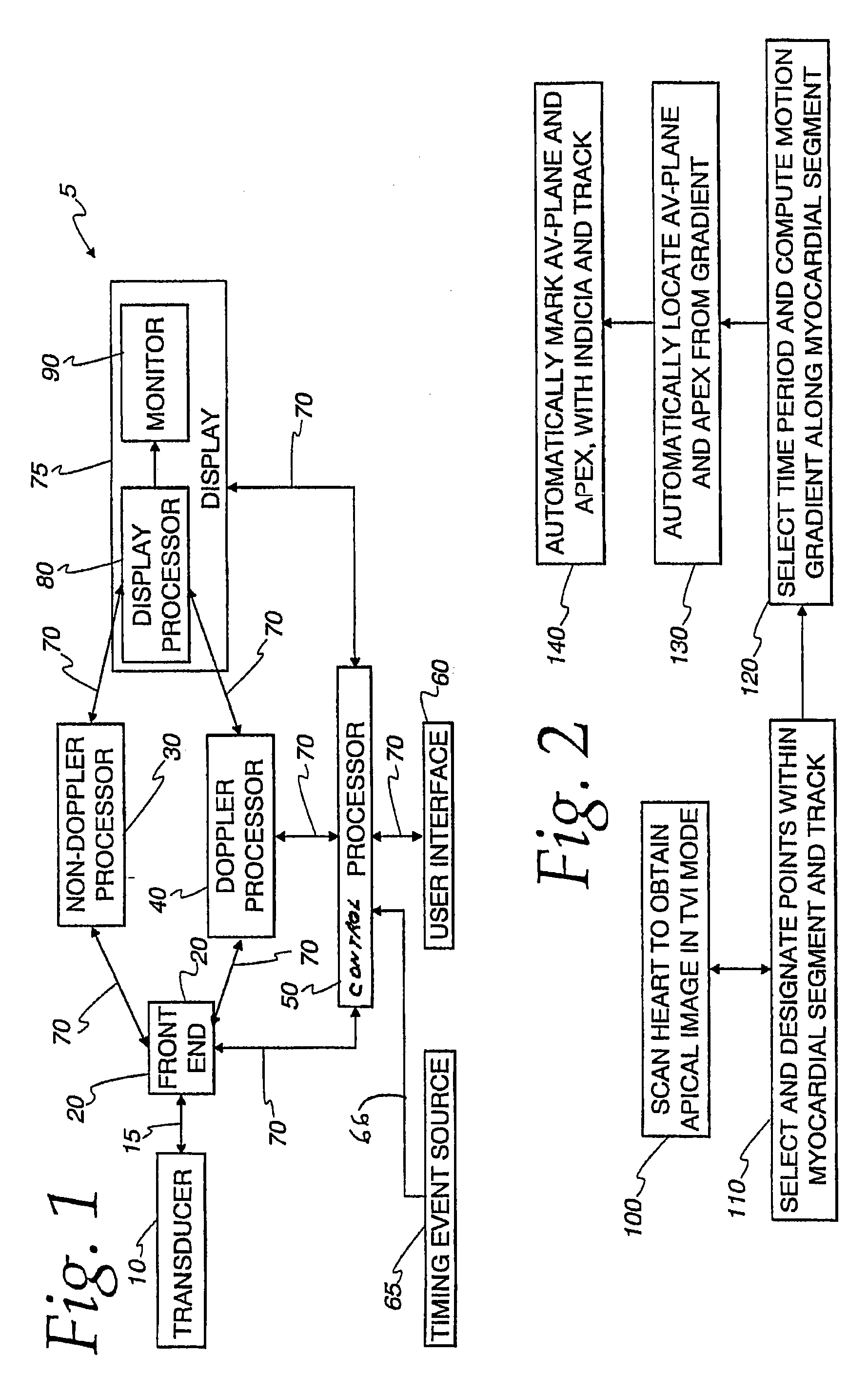
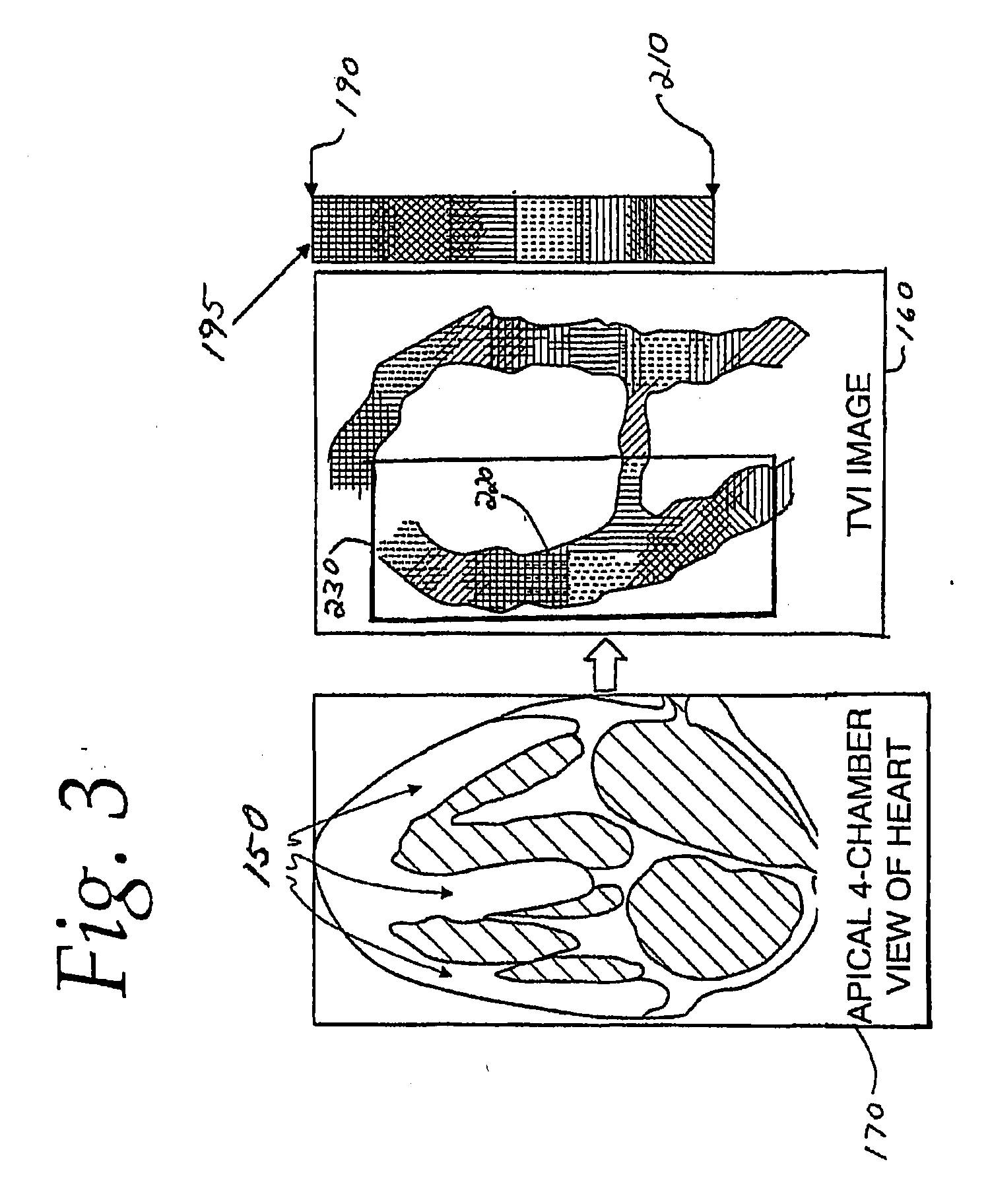
The present invention relates to a method and apparatus for generating an image responsive to moving cardiac structure and blood, and extracting clinically relevant information based on anatomical landmarks located within the heart. One embodiment of the present invention comprises at least one processor responsive to signals received from the heart used to acquire an apical view of the heart, generate an image of the apical view on a display, automatically identify an AV-plane of the heart and generate a clinical executive report using the identified AV-plane.
Owner:GENERAL ELECTRIC CO
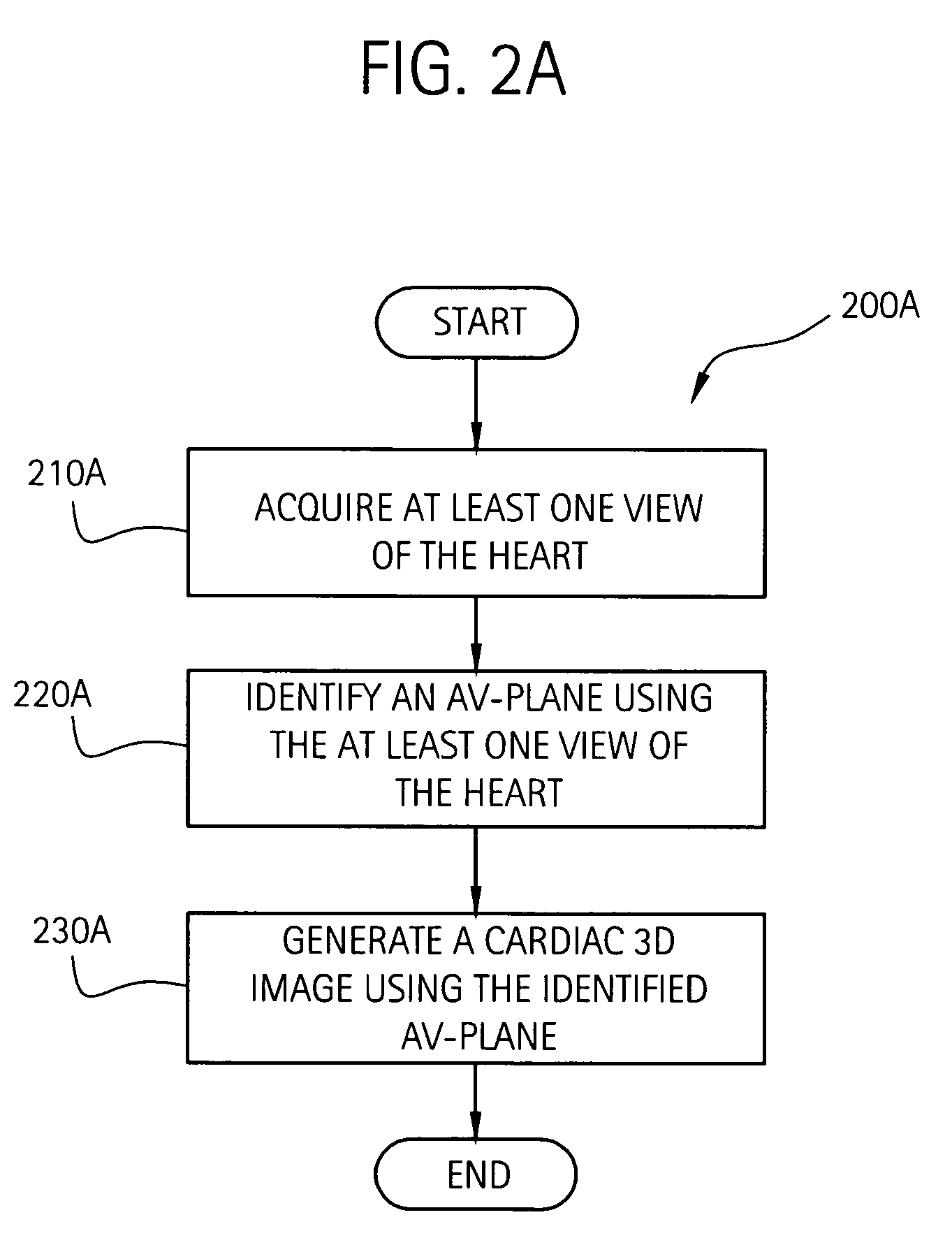
Three dimensional atrium-ventricle plane detection
InactiveUS20060058675A1Organ movement/changes detectionHeart/pulse rate measurement devicesAnatomical landmarkRelevant information
The present invention relates to a method and apparatus for generating at least a 3D image responsive to moving cardiac structure and blood, and extracting clinically relevant information based on anatomical landmarks located within the heart. One embodiment of the present invention comprises at least a front end and at least one processor. The front-end is arranged to transmit ultrasound waves into the moving cardiac structure and blood of a heart and generate received signals in response to ultrasound waves backscattered from the said moving cardiac structure and blood. The at least one processor responsive to the received signals to acquire 3D ultrasound data containing at least one view of the heart, identify an AV-plane using the at least one acquired view, and generate a cardiac 3D image of at least a portion of the heart using at least one identified AV-plane. At least the 3D image may be displayed to a user.
Owner:GENERAL ELECTRIC CO
Optimizing ultrasound acquisition based on ultrasound-located landmarks
InactiveUS20060058674A1Blood flow measurement devicesOrgan movement/changes detectionUltrasound deviceAnatomical landmark
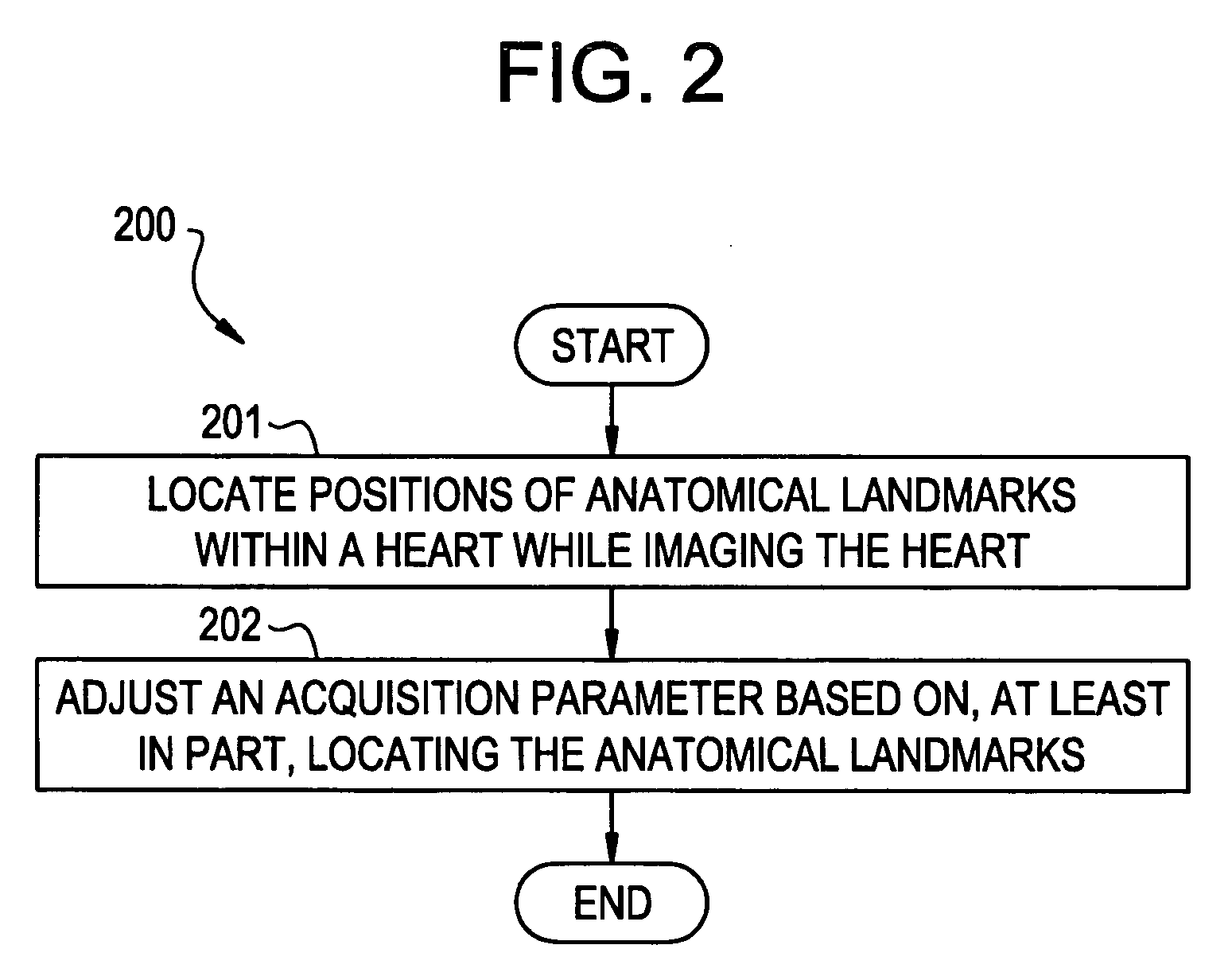
An ultrasound device is disclosed that includes a method and apparatus for generating an image responsive to moving cardiac structure and blood, and for adjusting at least one acquisition parameter based on, at least in part, locating at least one anatomical landmark within a heart. At least one processor, responsive to signals received from the heart, locates anatomical landmarks within the cardiac structure, generate position information of the anatomical landmarks, and adjusts at least one acquisition parameter. The landmarks and acquisition parameters may be displayed to a user of the ultrasound device.
Owner:GENERAL ELECTRIC CO
Ultrasound location of anatomical landmarks
InactiveUS20070167771A1Simple methodOrgan movement/changes detectionInfrasonic diagnosticsAnatomical landmarkSonification
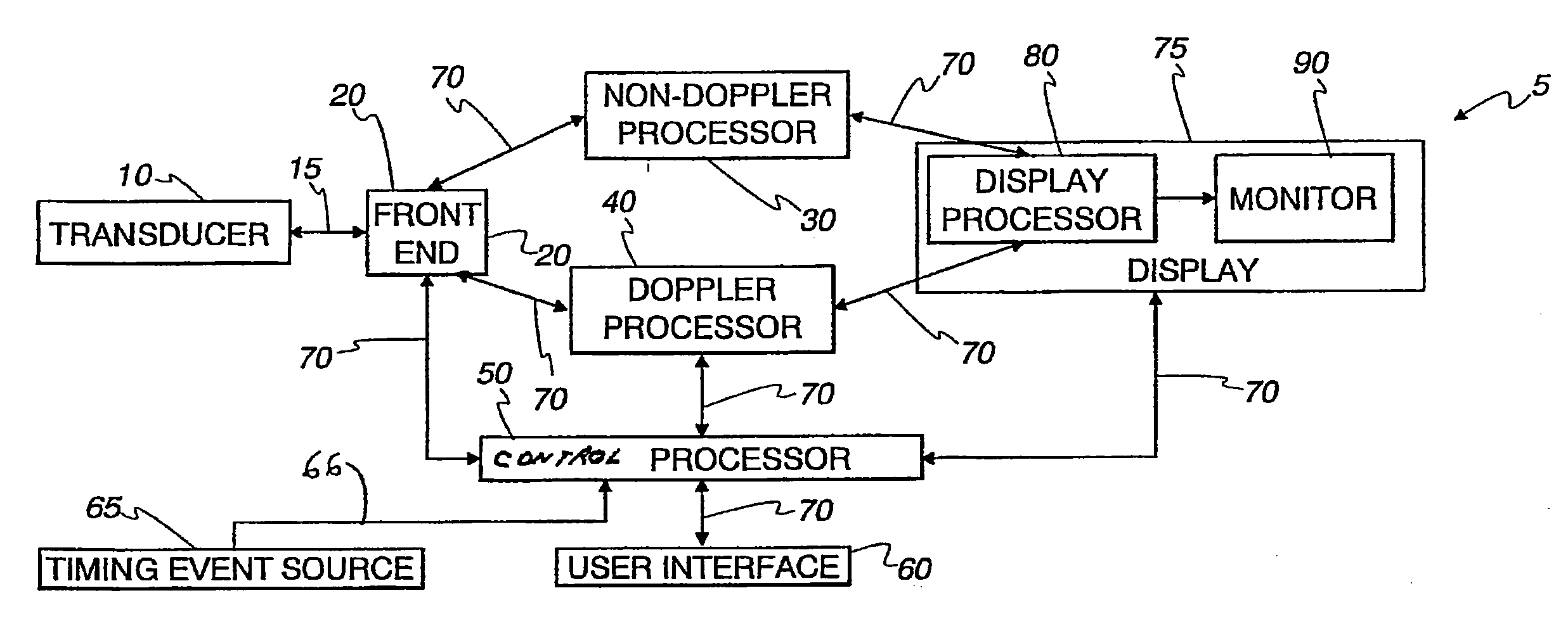
An ultrasound machine is disclosed that includes a method and apparatus for generating an image responsive to moving cardiac structure and for locating anatomical landmarks of the heart by generating received signals in response to ultrasound waves transmitted into and then backscattered from the moving cardiac structure over a time period. A processor is responsive to the received signals to generate a set of analytic parameter values representing movement of the cardiac structure over the time period and analyzes elements of the set of analytic parameter values to automatically extract position information of the anatomical landmarks. A display is arranged to overlay indicia onto the image corresponding to the position information of the anatomical landmarks. The positions of the anatomical landmarks are tracked in real-time.
Owner:GE MEDICAL SYST GLOBAL TECH CO LLC
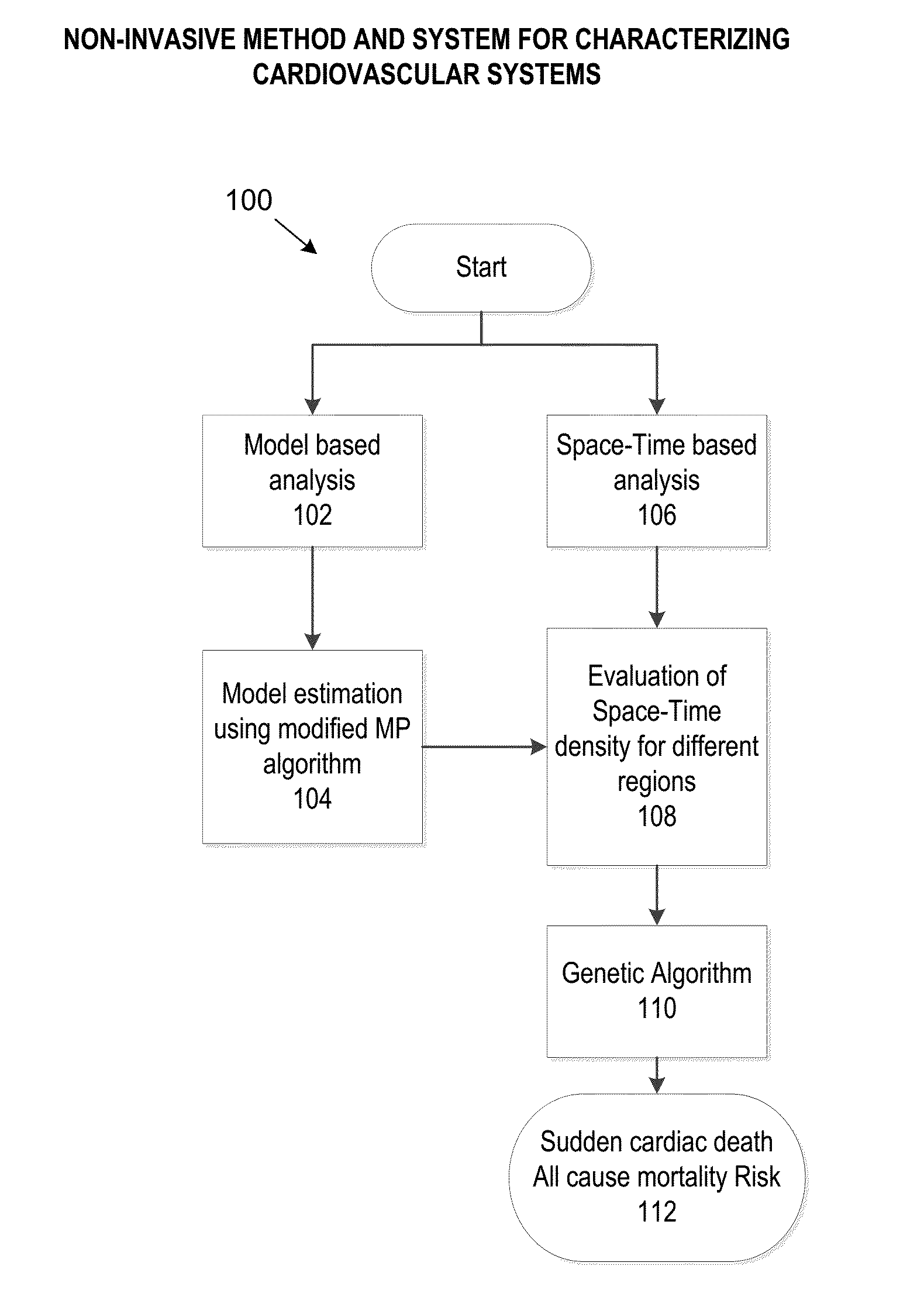
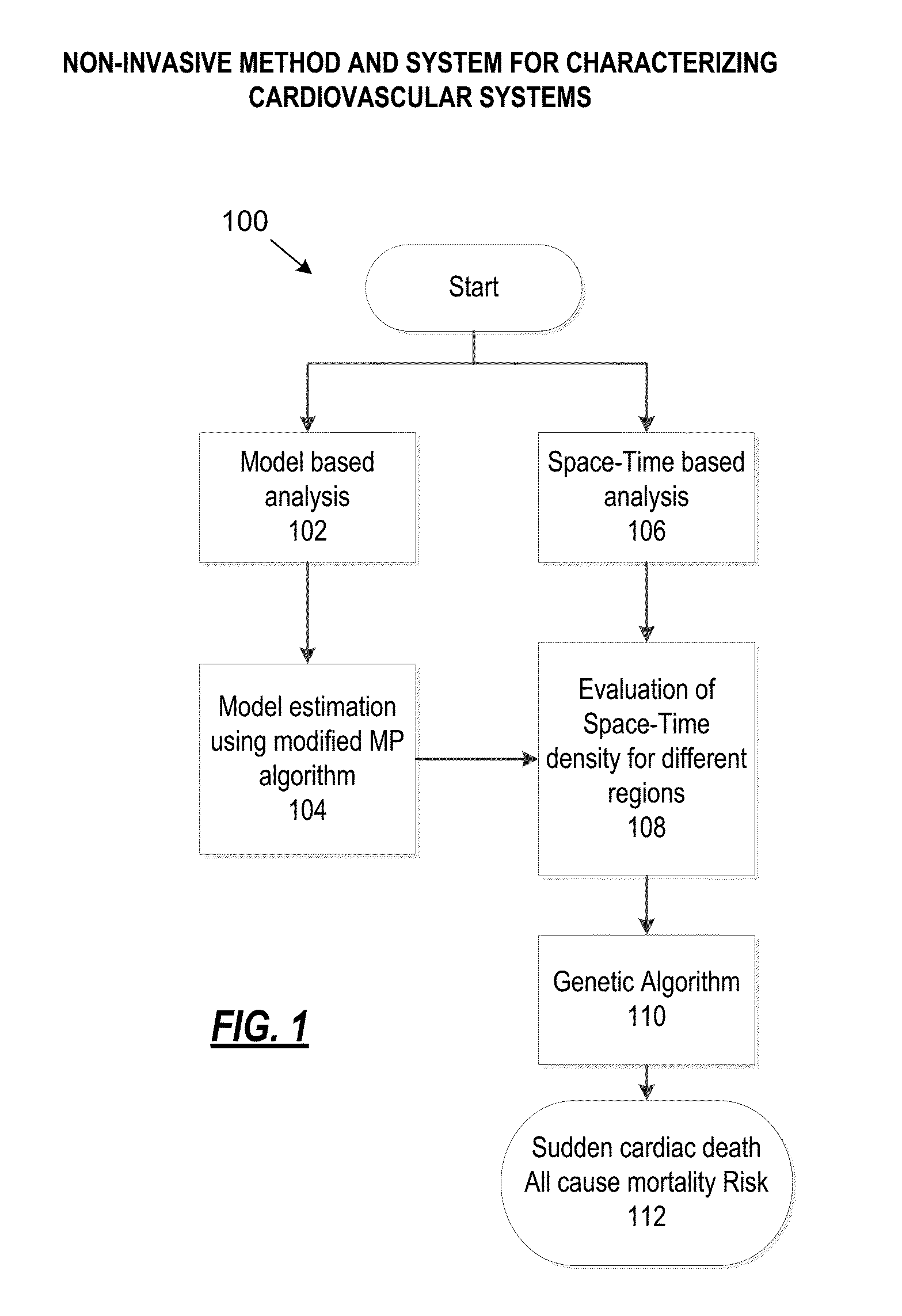
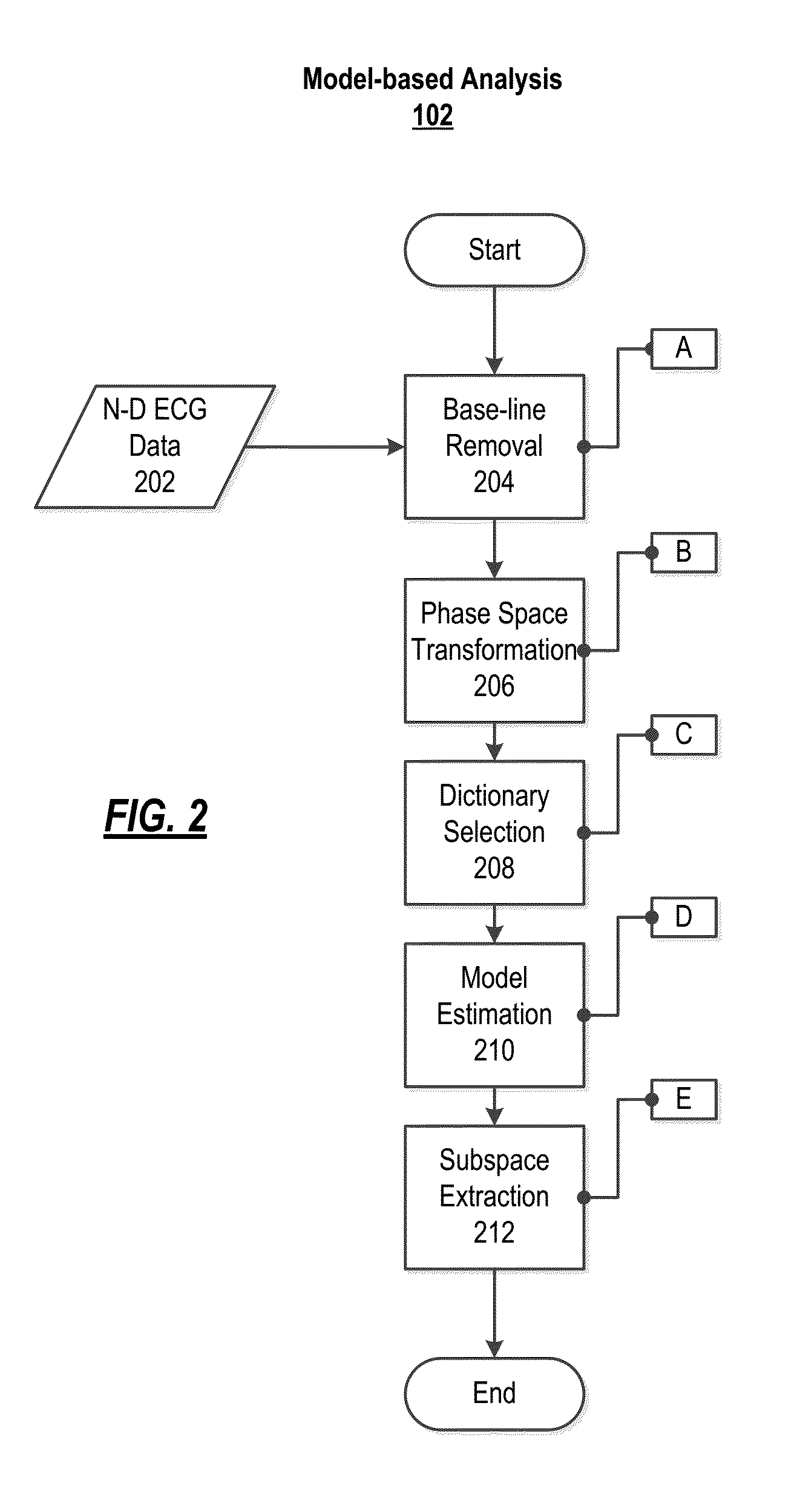
Non-invasive method and system for characterizing cardiovascular systems
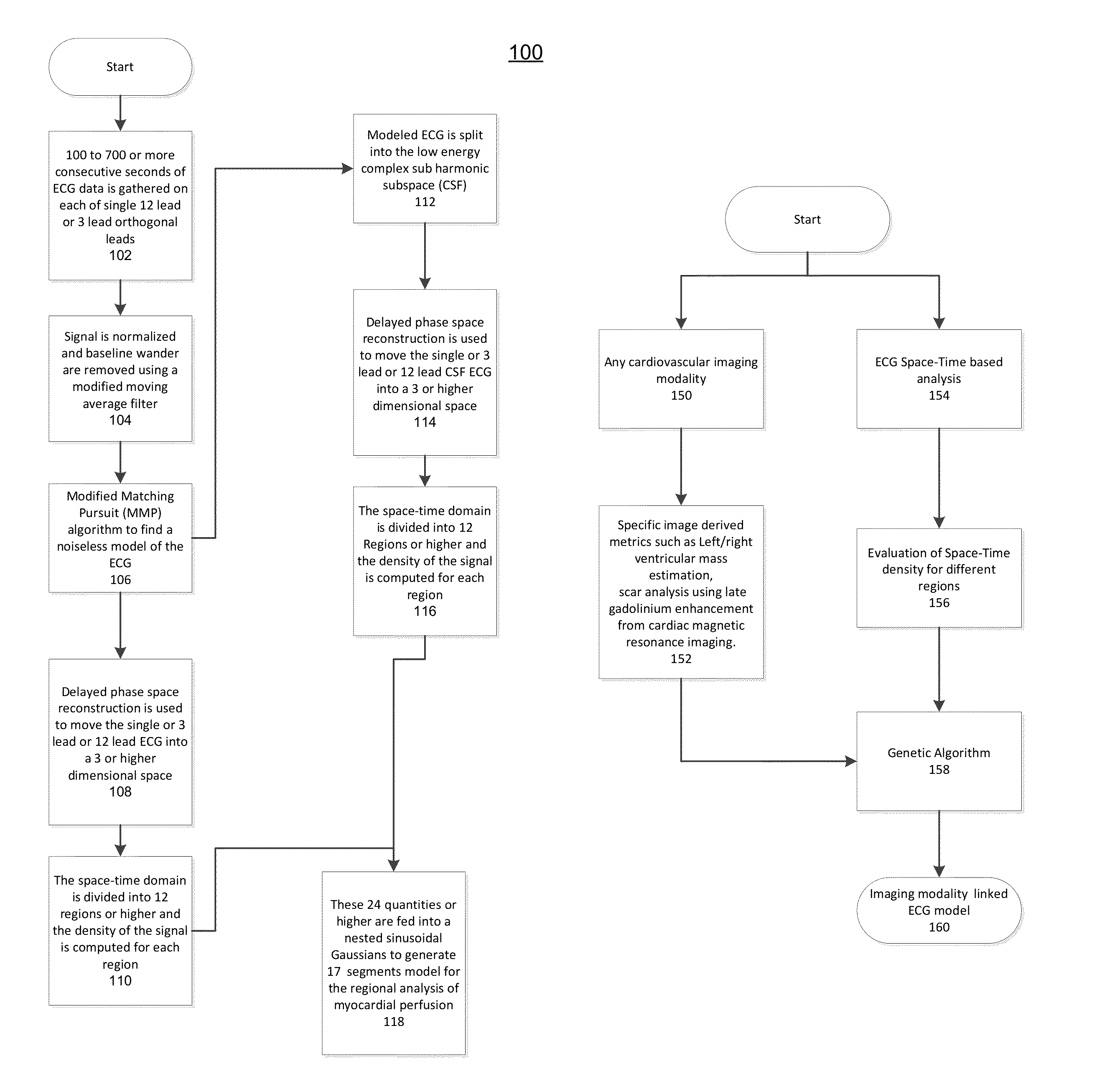
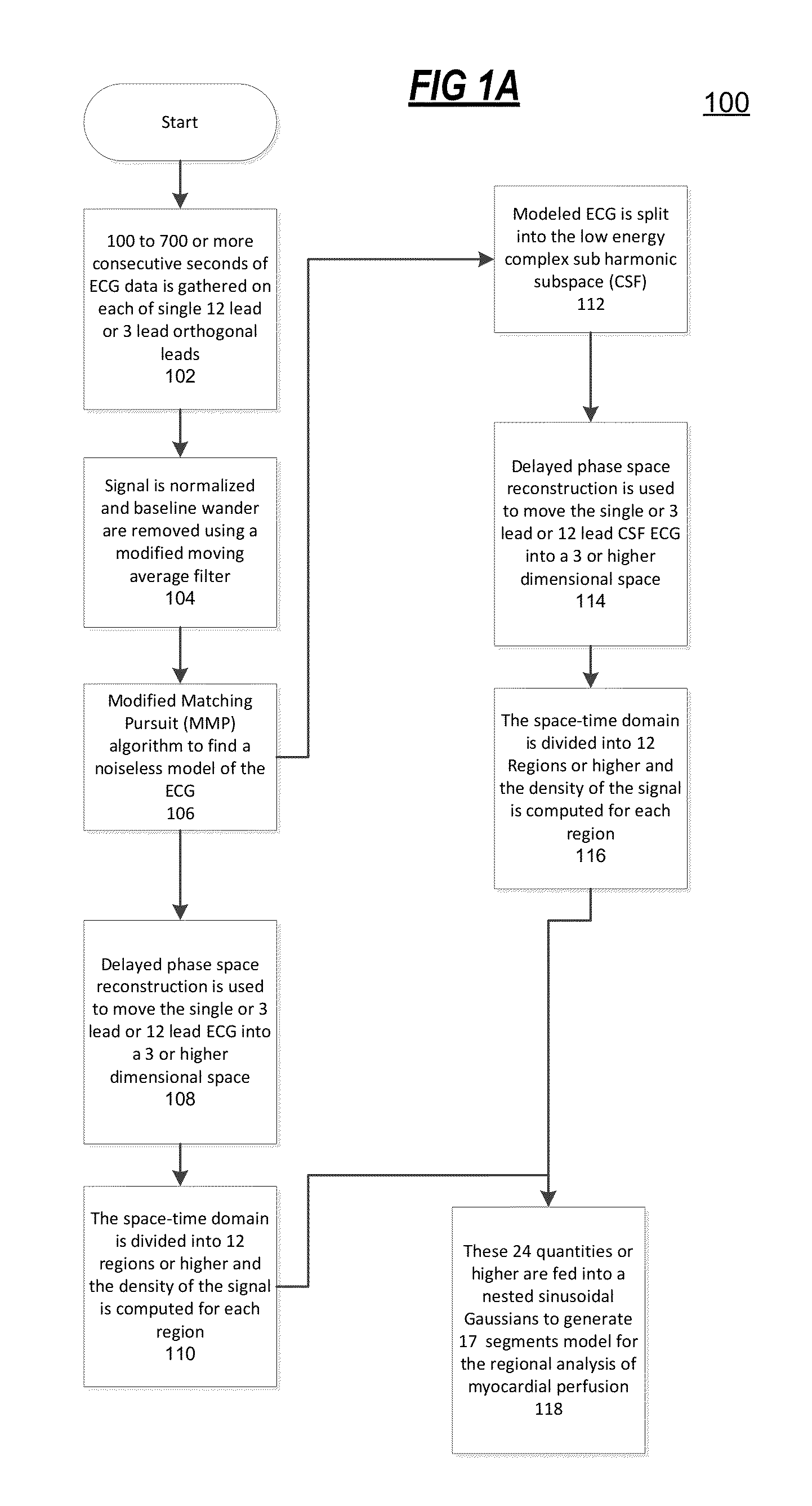
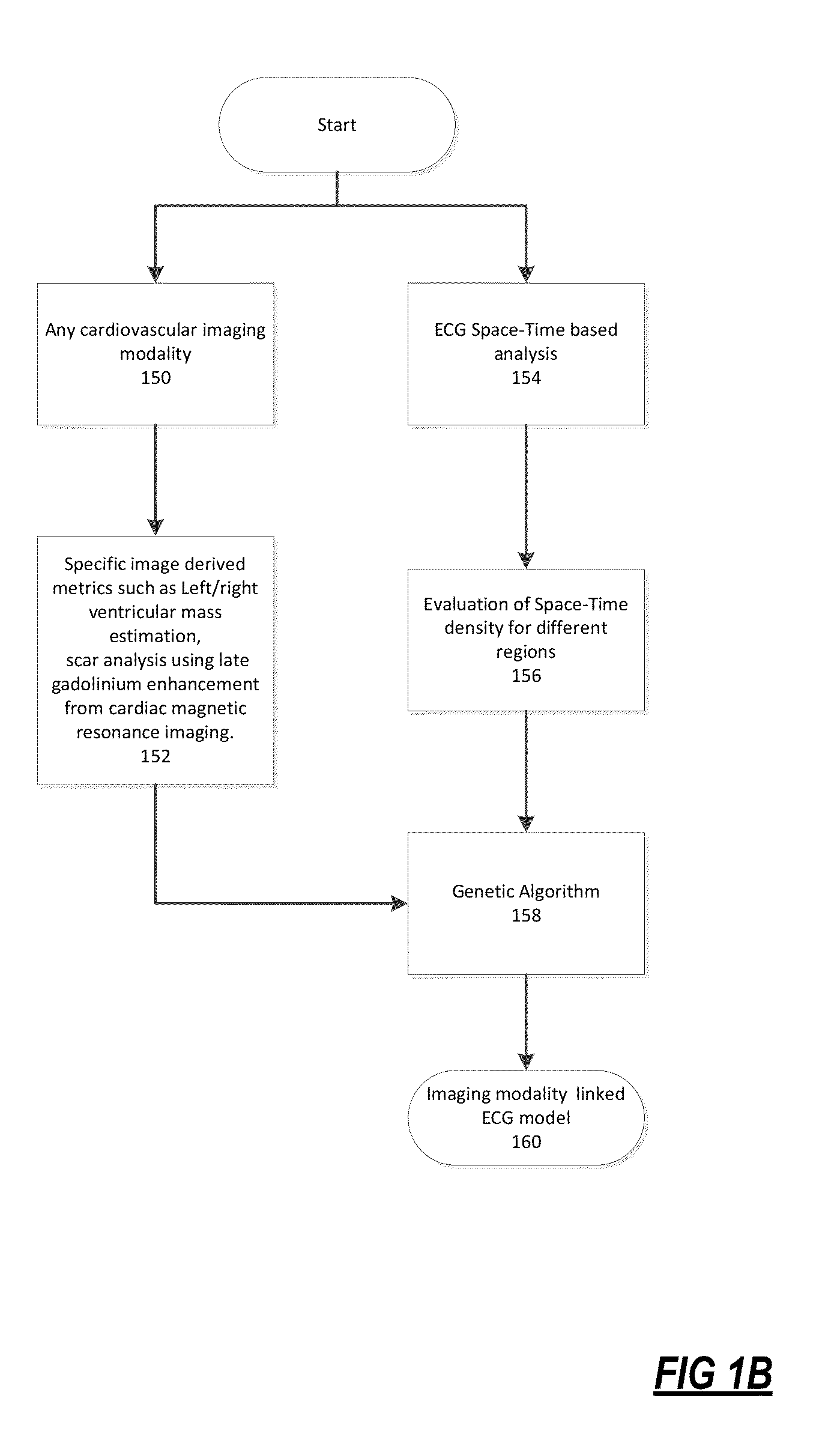
The present disclosure uses physiological data, ECG signals as an example, to evaluate cardiac structure and function in mammals. Two approaches are presented, e.g., a model-based analysis and a space-time analysis. The first method uses a modified Matching Pursuit (MMP) algorithm to find a noiseless model of the ECG data that is sparse and does not assume periodicity of the signal. After the model is derived, various metrics and subspaces are extracted to image and characterize cardiovascular tissues using complex-sub-harmonic-frequencies (CSF) quasi-periodic and other mathematical methods. In the second method, space-time domain is divided into a number of regions, the density of the ECG signal is computed in each region and inputted into a learning algorithm to image and characterize the tissues.
Owner:ANALYTICS FOR LIFE
Increasing the efficiency of quantitation in stress echo
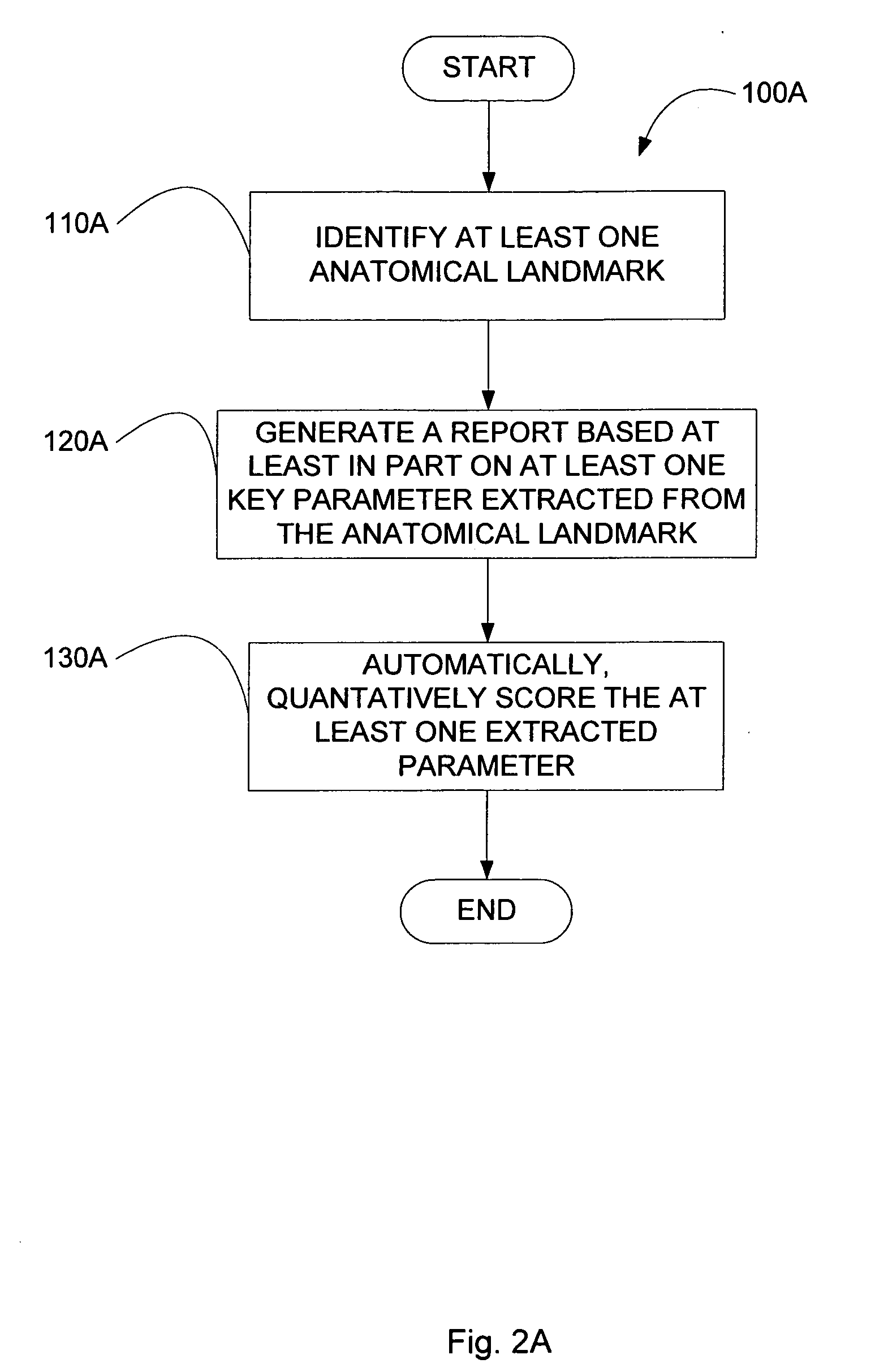
InactiveUS20060058610A1Improve efficiencyImage enhancementImage analysisAnatomical landmarkSonification
The present invention relates to a method and apparatus for extracting ultrasound summary information useful for increasing efficiency of quantitation of a stress echo examination performed using an ultrasound machine. One embodiment of the present invention comprises a front-end arranged to transmit ultrasound waves into moving cardiac structure and blood and generate received signals in response to the ultrasound waves backscattered from moving cardiac structure and blood. At least one processor responsive to the received signals identifies at least one anatomical landmark within the heart, generates a report based at least in part on one key parameter extracted from the anatomical landmark, and scores the at least one extracted parameter. The anatomical landmarks, reports, and scores may be displayed to a user.
Owner:GENERAL ELECTRIC CO
Method and apparatus for occluding a physiological opening
InactiveUS20120010644A1Avoid suture related injuryInvention is often bulkyBalloon catheterDilatorsAdhesiveBiological activation
A method and apparatus for suture-less placement of an occluding patch in which release of the device can be accelerated. In one embodiment, an inactive form of an adhesive is applied on the area of the patch that will come into contact with cardiac tissue; this allows for the introduction and necessary manipulation of the catheter system until activation of the adhesive occurs. In accordance with one aspect of the invention, the adhesive properties of certain polymeric materials are relied upon rather than their ability to cure or harden into a specific shape. In another embodiment, the patch is immediately released utilizing a detaching mechanism on the balloon or the balloon catheter which supports the patch; the patch along with the inflated balloon remain on the cardiac structure occluding the opening.
Owner:SIDERIS ELEFTHERIOS B +1
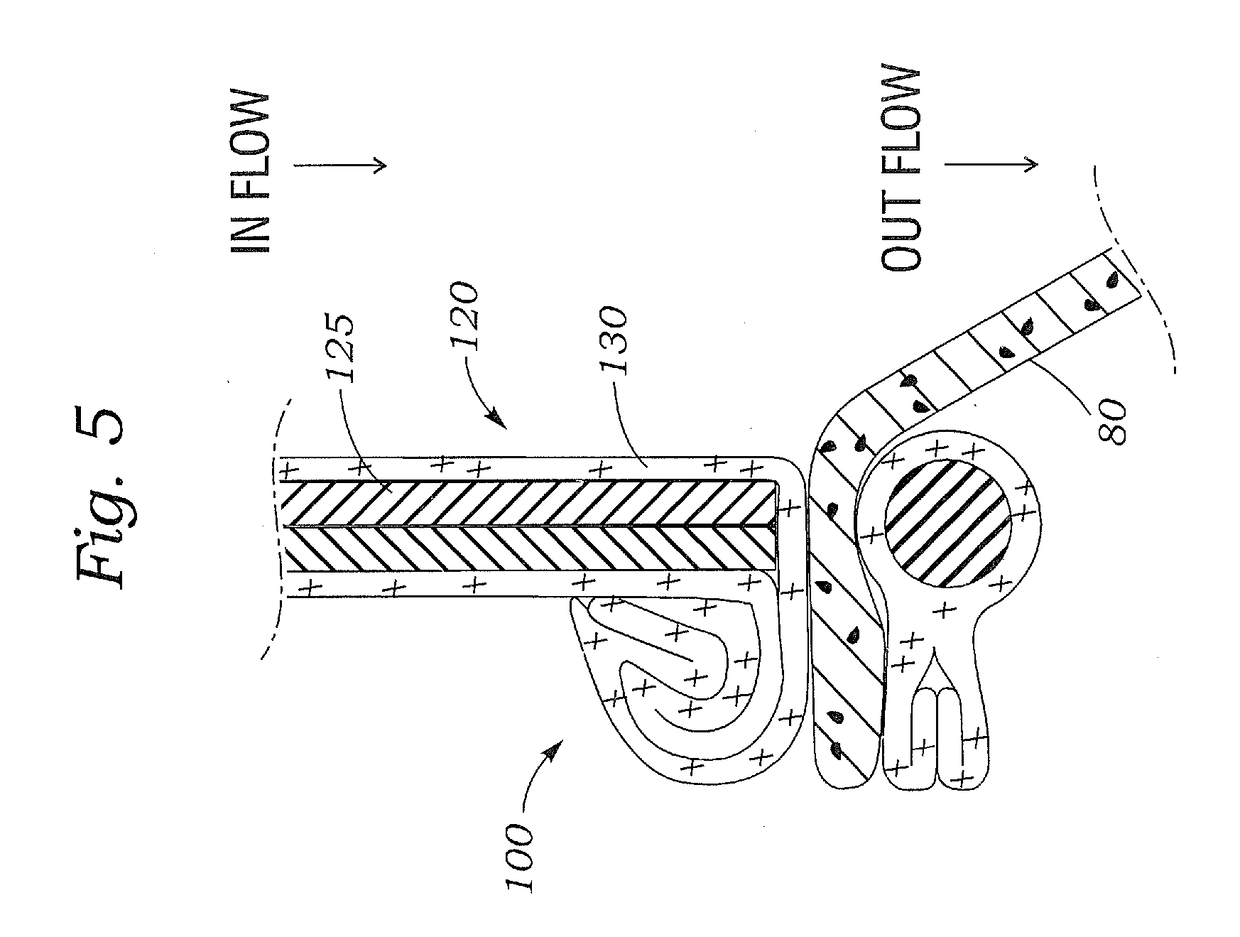
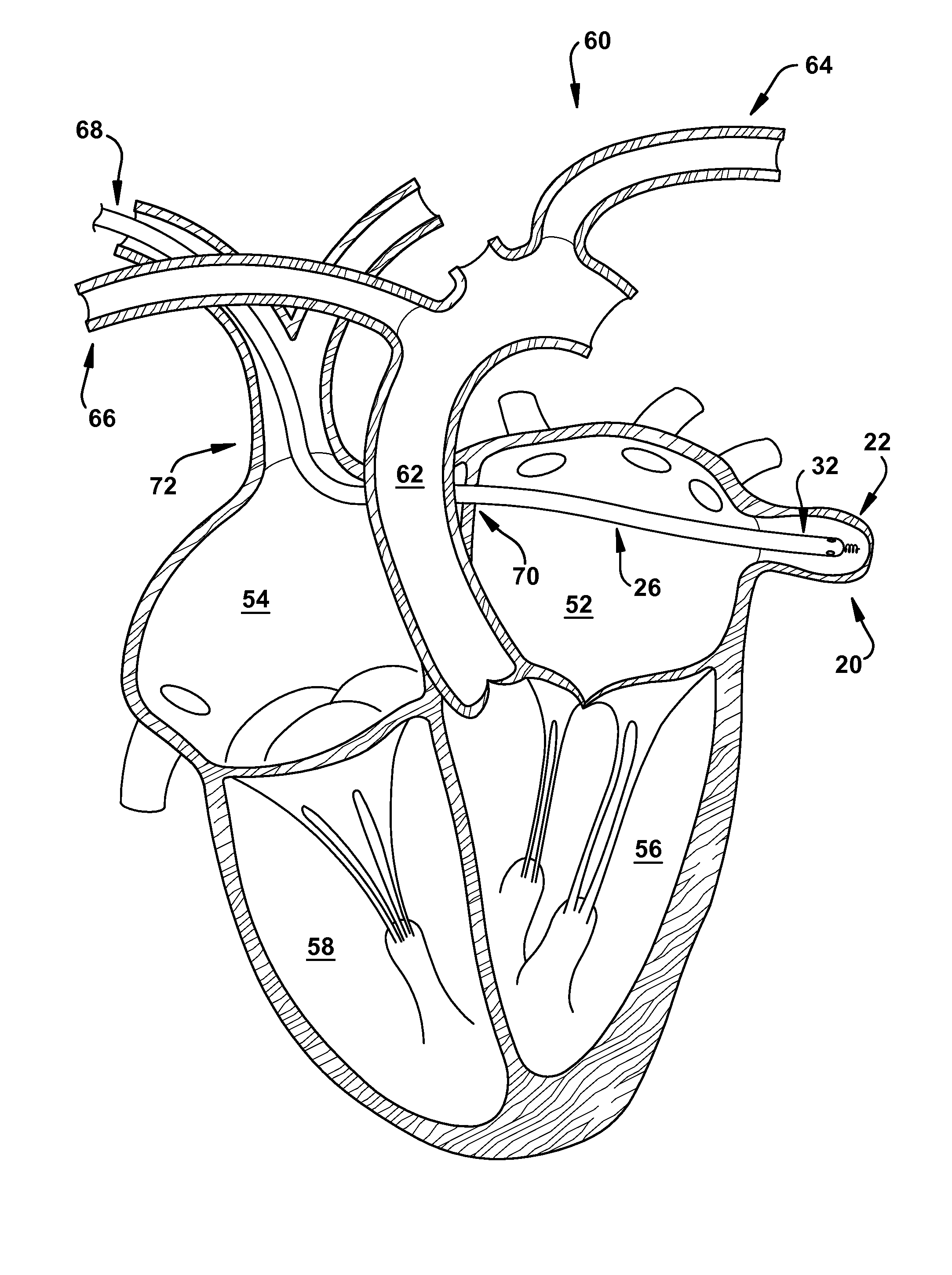
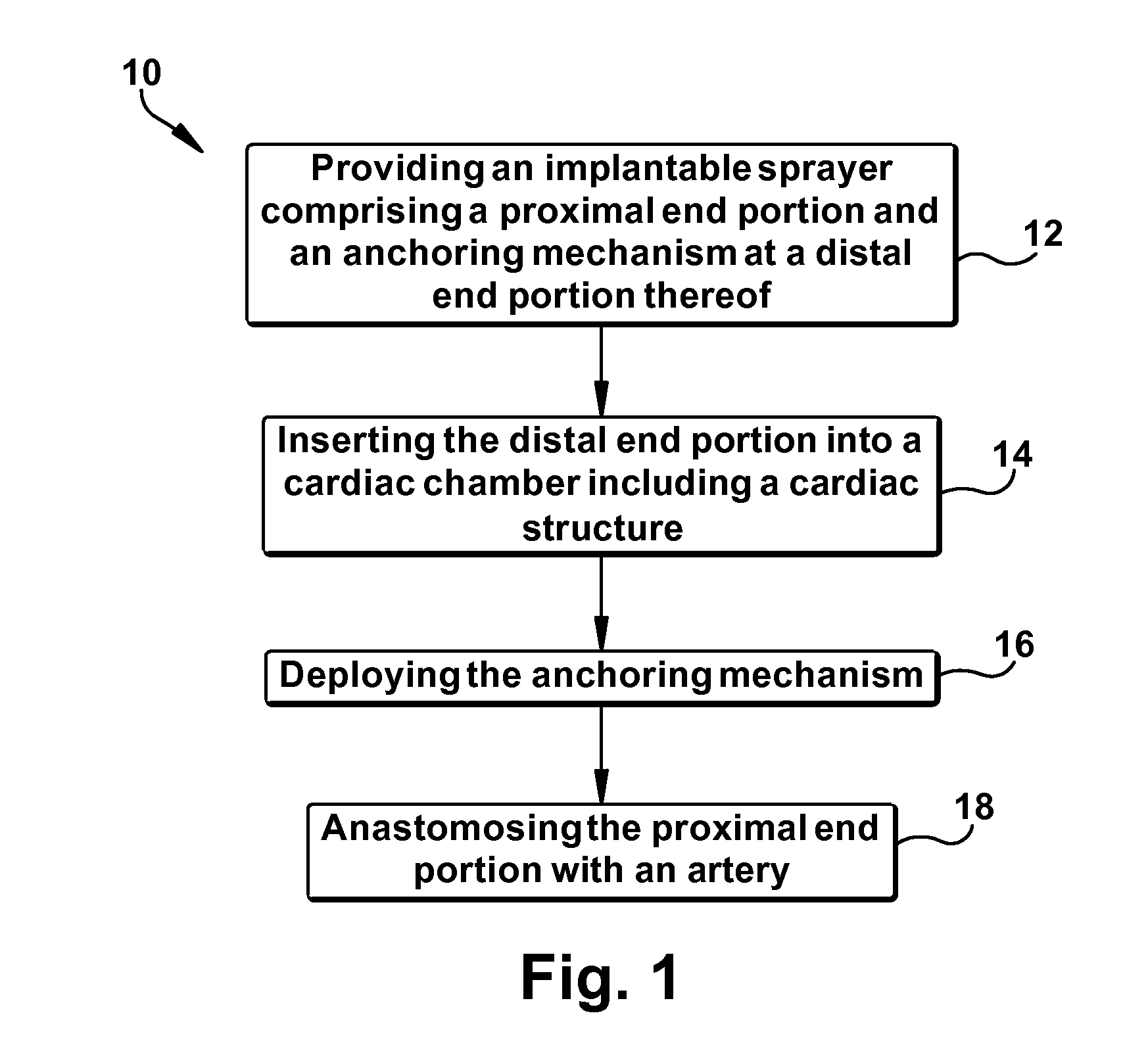
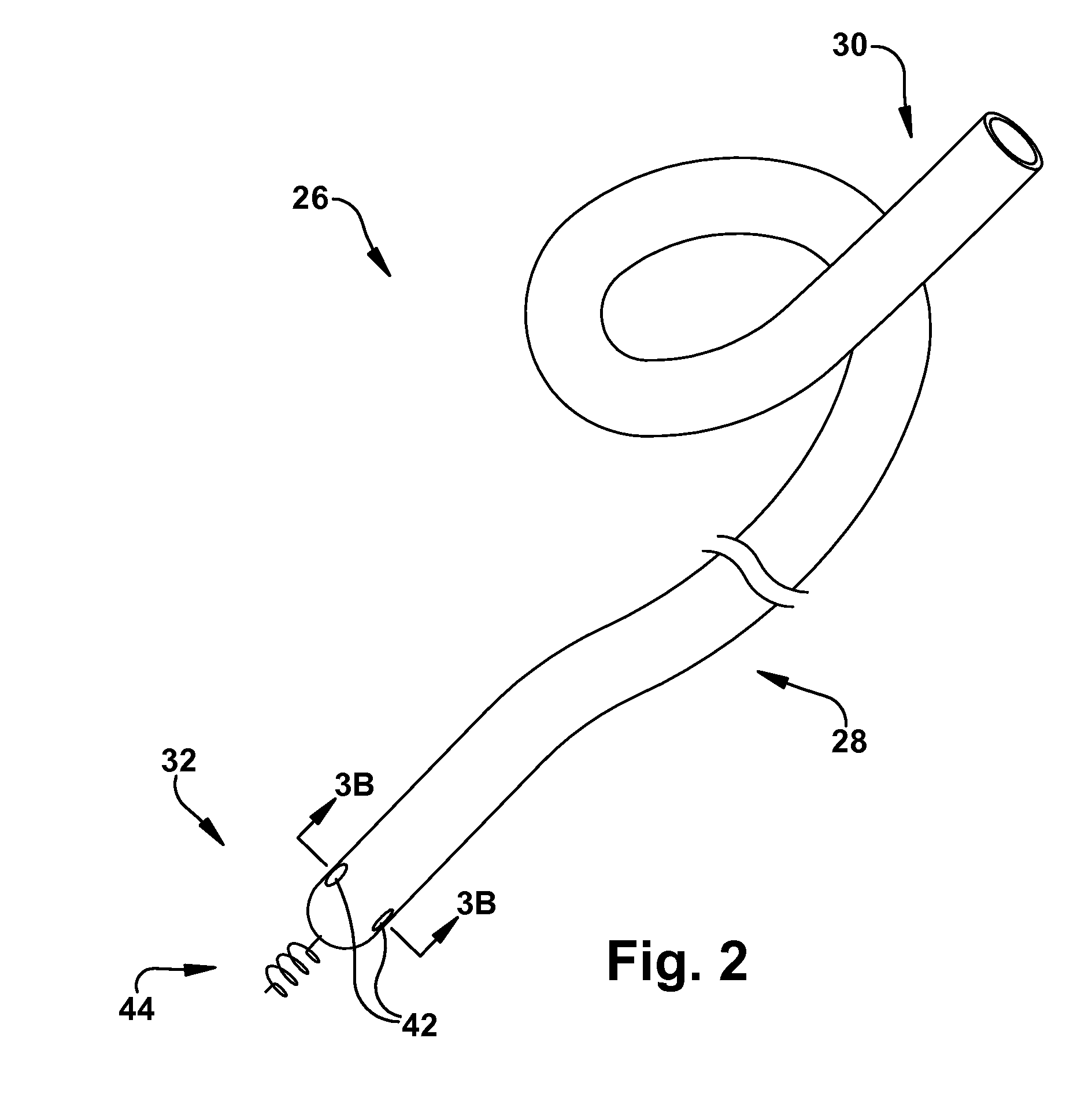
Method for increasing blood flow in or about a cardiac or other vascular or prosthetic structure to prevent thrombosis
ActiveUS8597225B2Promote circulationPrevent and mitigate blood stasisHeart valvesSurgeryProsthesisThrombus
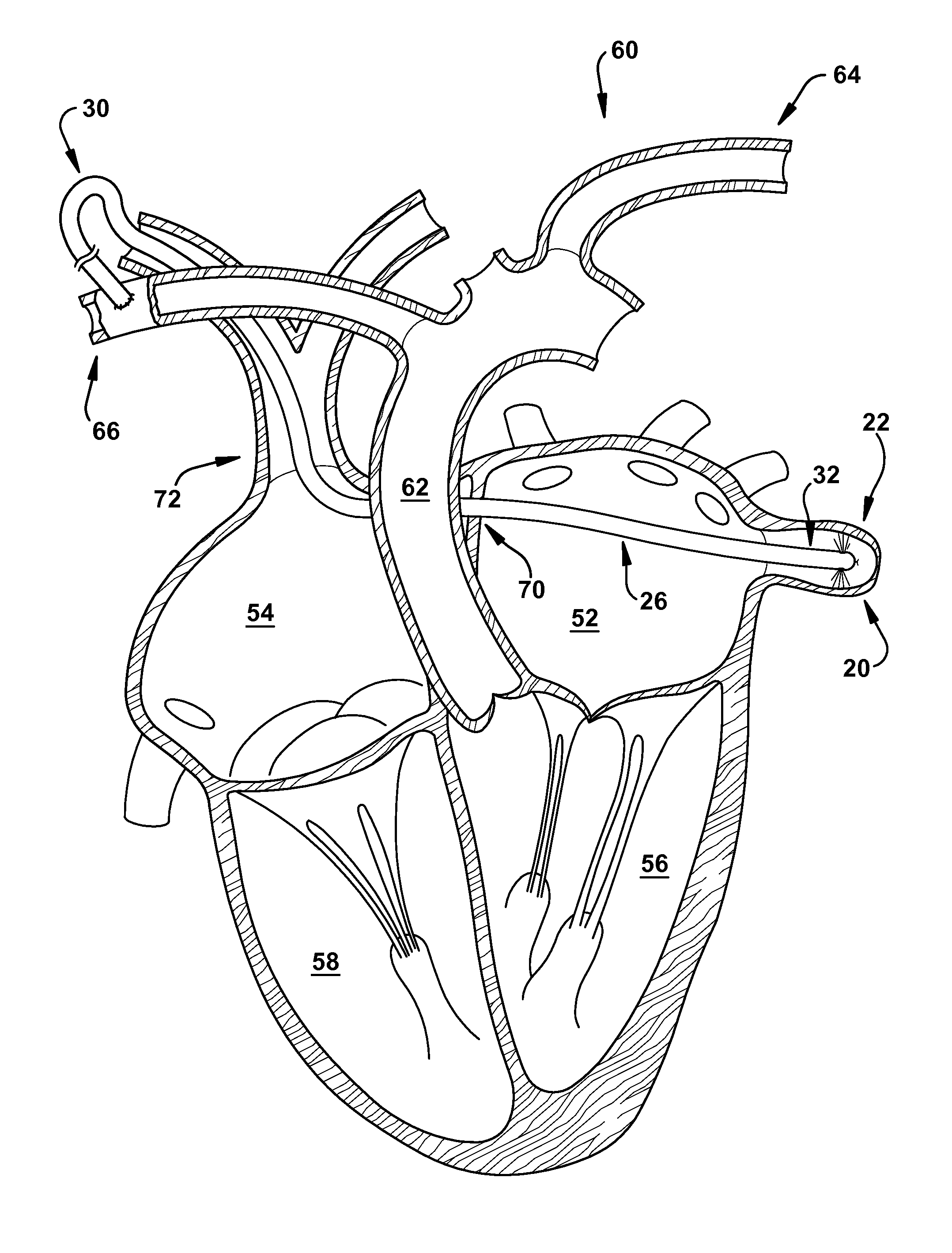
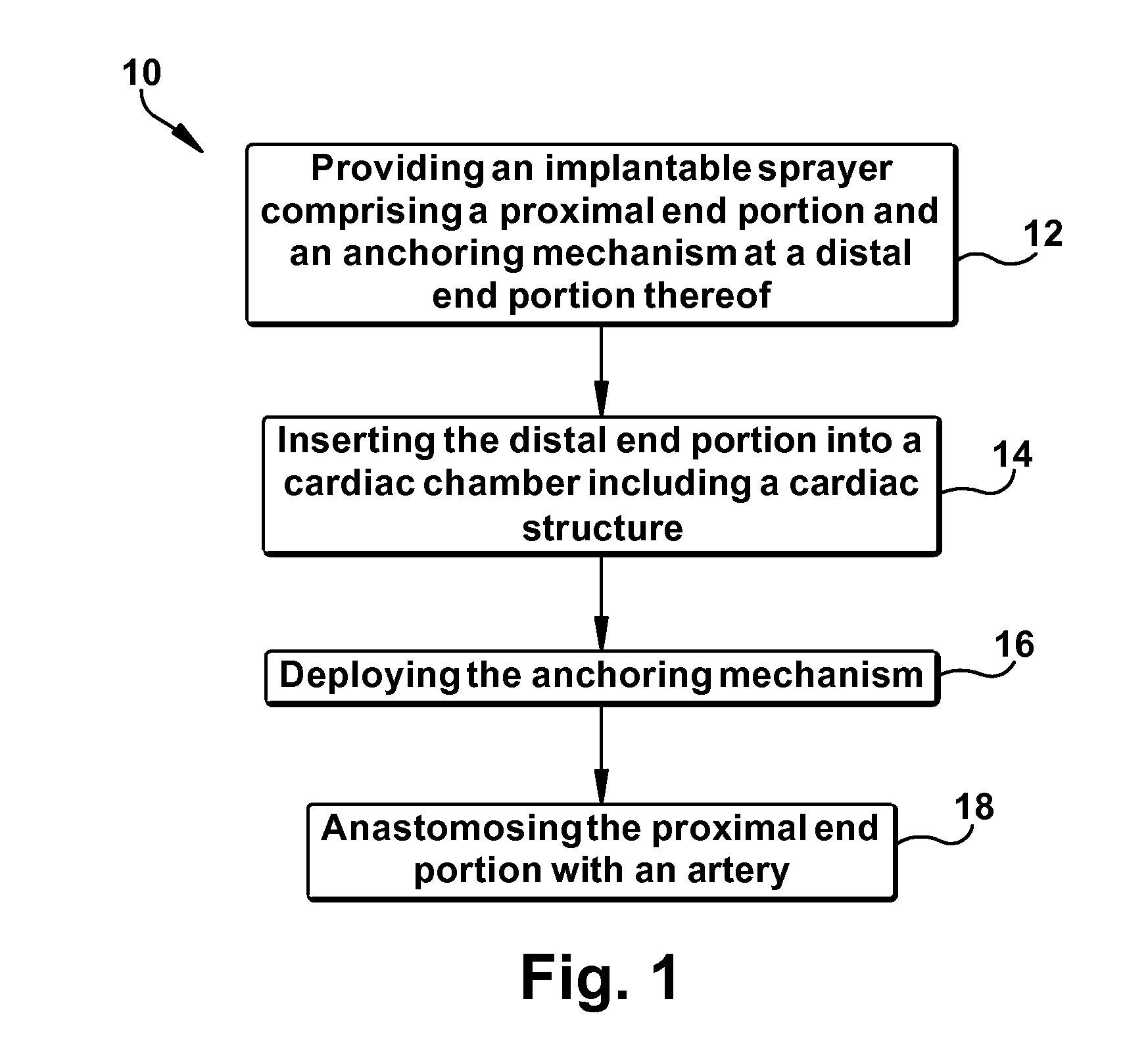
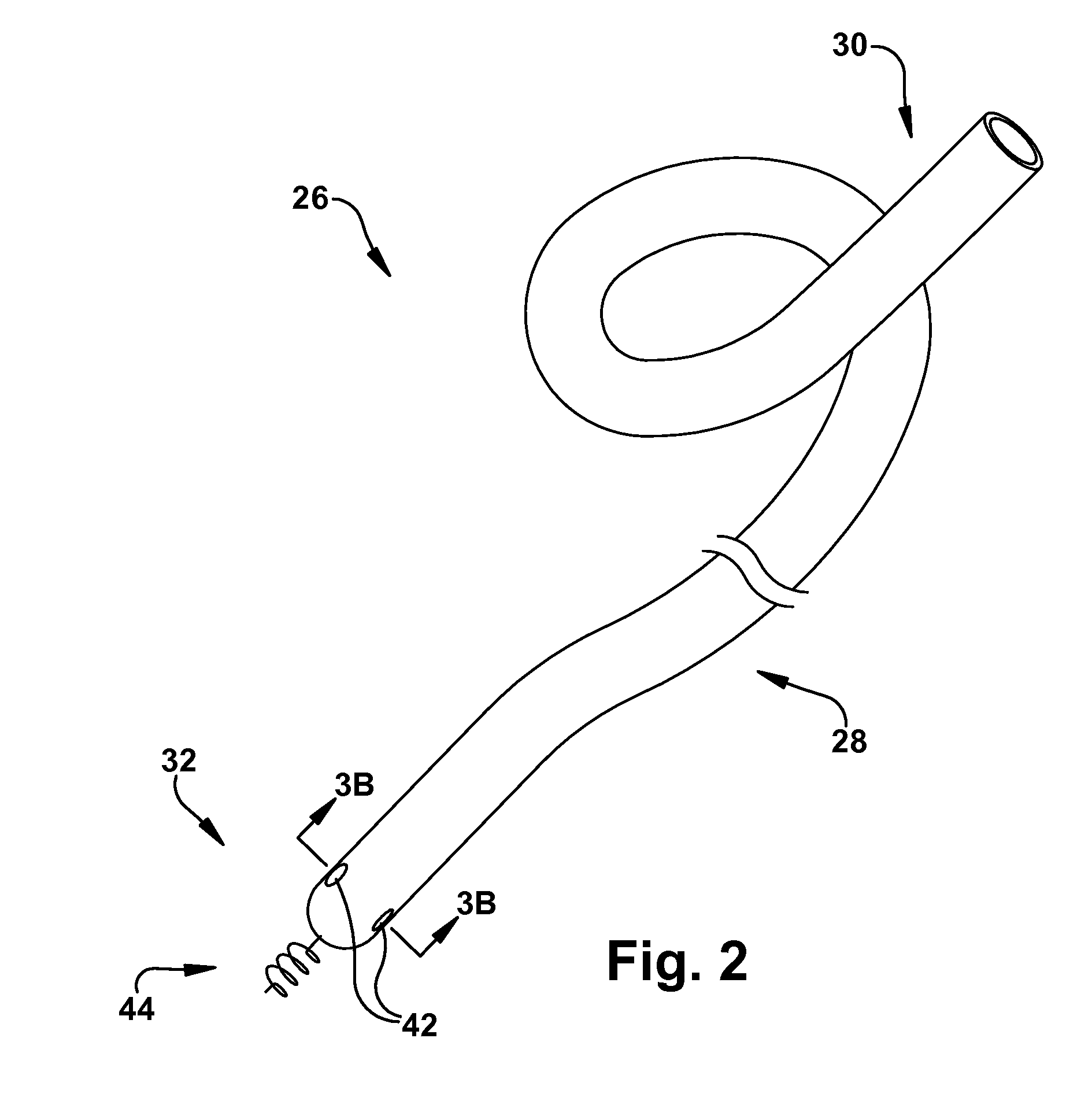
A method is provided for increasing blood flow in or about a cardiac structure to prevent thrombosis. One step of the method includes providing an implantable sprayer having an elongated tubular body with proximal and distal end portions. The distal end portion includes at least one opening and an anchoring mechanism. The distal end portion of the implantable sprayer is inserted into a cardiac chamber that includes the cardiac structure. The anchoring mechanism is then deployed so that the distal end portion of the implantable sprayer is secured in or about the cardiac structure. Next, the proximal end portion is anastomosed with an artery so that blood flows through the elongated tubular body of the implantable sprayer and is sprayed out of the at least one opening to continuously circulate blood in or about the cardiac structure.
Owner:THE CLEVELAND CLINIC FOUND
Method for increasing blood flow in or about a cardiac or other vascular or prosthetic structure to prevent thrombosis
ActiveUS20120022427A1Increase blood flowPrevent thrombosisOther blood circulation devicesHeart valvesThrombusProsthesis
A method is provided for increasing blood flow in or about a cardiac structure to prevent thrombosis. One step of the method includes providing an implantable sprayer having an elongated tubular body with proximal and distal end portions. The distal end portion includes at least one opening and an anchoring mechanism. The distal end portion of the implantable sprayer is inserted into a cardiac chamber that includes the cardiac structure. The anchoring mechanism is then deployed so that the distal end portion of the implantable sprayer is secured in or about the cardiac structure. Next, the proximal end portion is anastomosed with an artery so that blood flows through the elongated tubular body of the implantable sprayer and is sprayed out of the at least one opening to continuously circulate blood in or about the cardiac structure.
Owner:THE CLEVELAND CLINIC FOUND
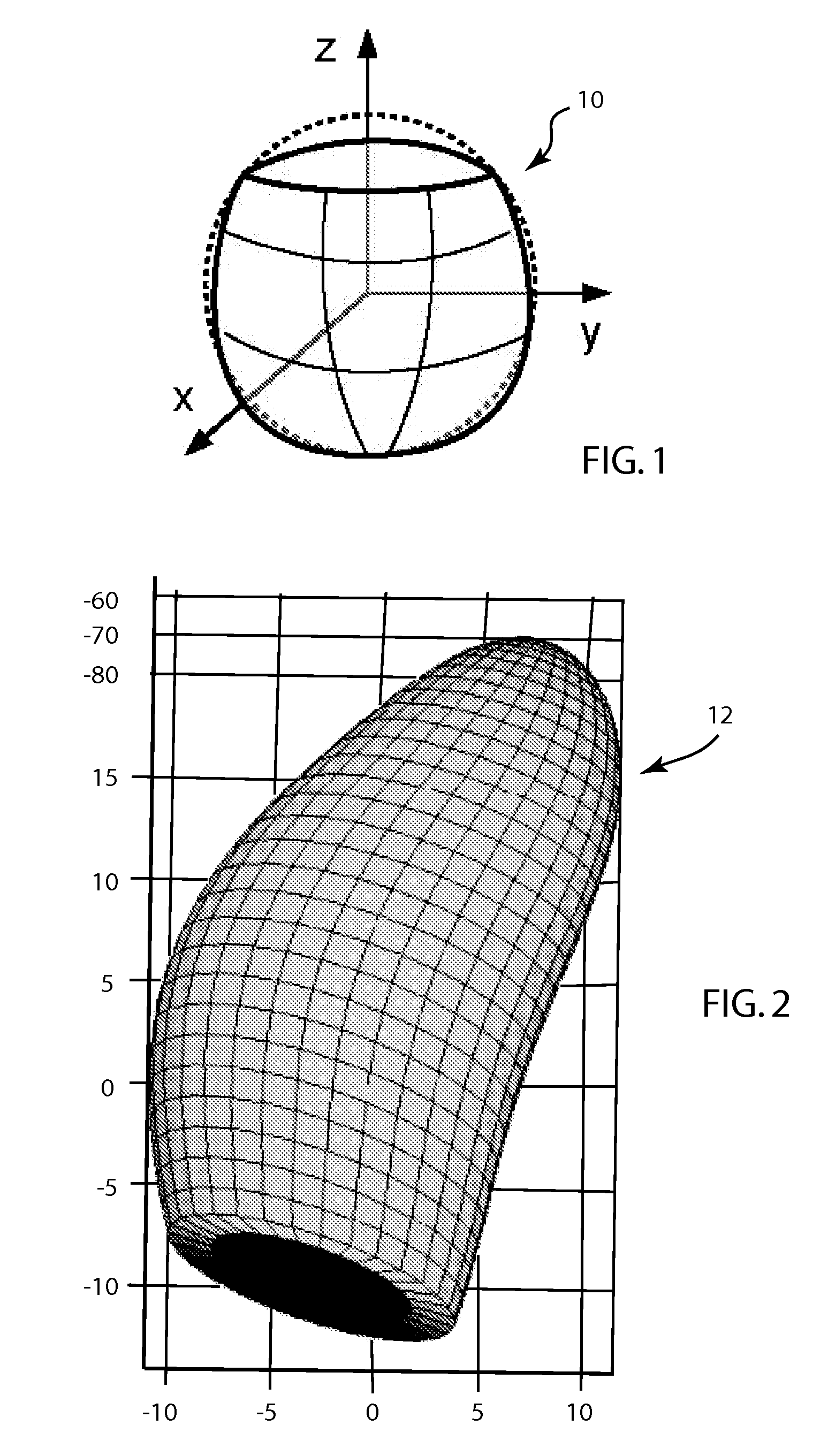
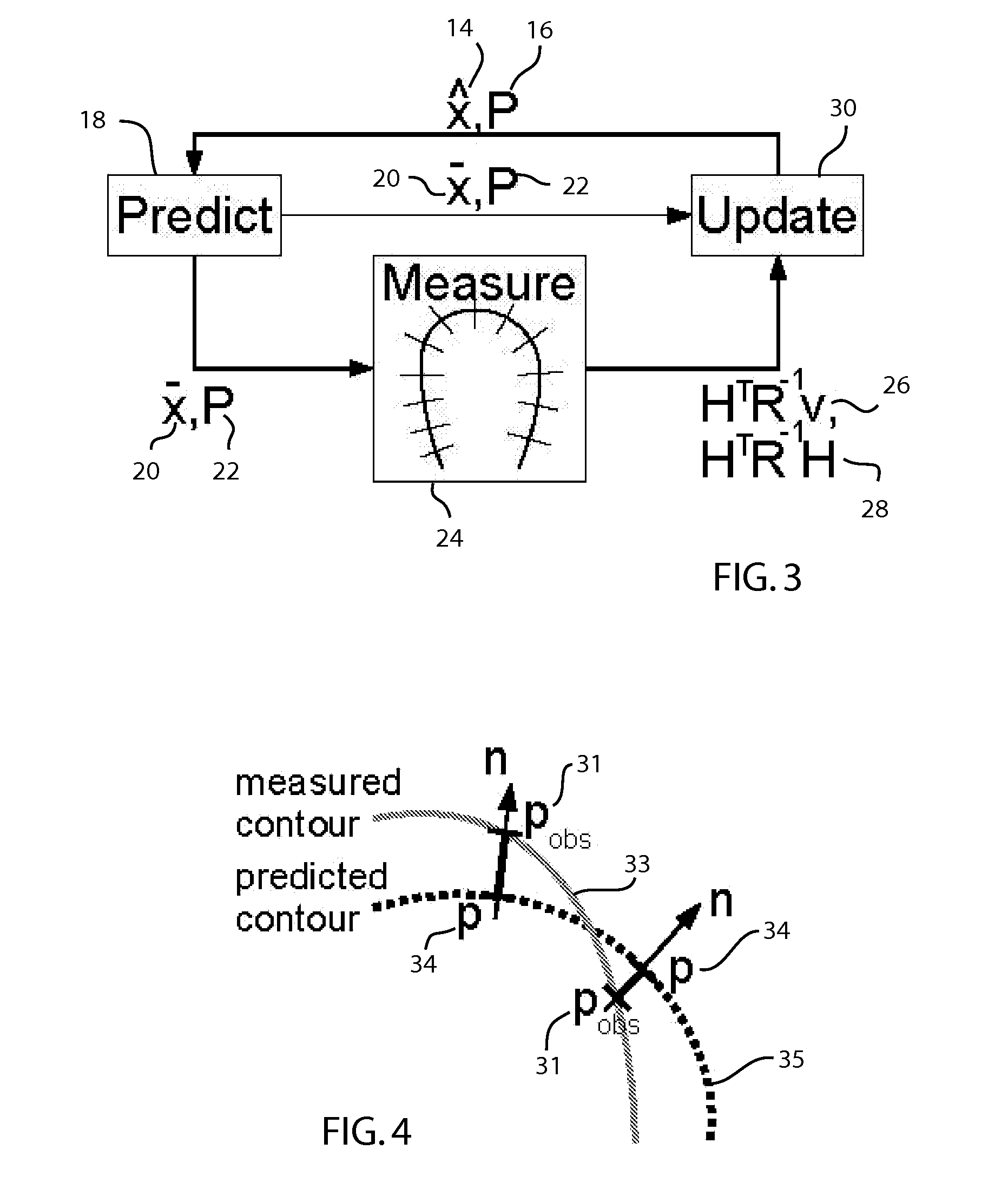
Method for real-time tracking of cardiac structures in 3D echocardiography
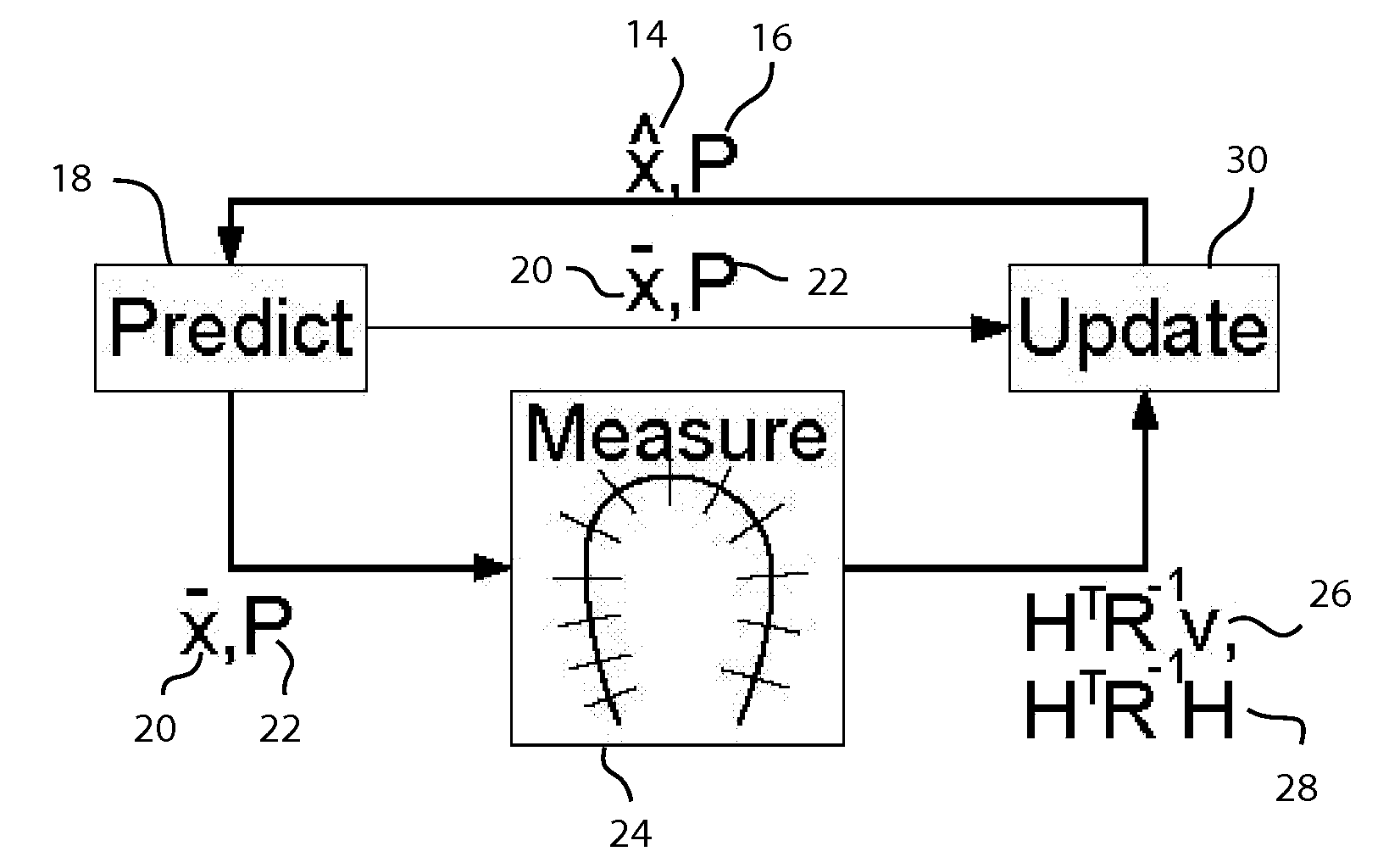
A method for tracking motion and shape changes of a deformable model in a volumetric image sequence. The method is operable to predict the surface of a space from a 3D image, such as cardiac structures from a 3D ultrasound. The shape and position of a deformable model is predicted for each frame of an ultrasound image. Edge detection is then performed for each predicted point on the deformable model perpendicular to the model surface. The distances between the predicted and measured edges for the deformable model are measurements for a Kalman filter. The measurements are coupled with noise values that specify the spatial uncertainty of the edge detection. The measurement data are subsequently summed together in information space and combined with the prediction in the Kalman filter to estimate the position and deformation for the deformable model. The deformable model is then updated to generate an updated surface model.
Owner:GENERAL ELECTRIC CO
Intracardiac device for restoring the functional elasticity of the cardiac structures, holding tool for the intracardiac device, and method for implantation of the intracardiac device in the heart
ActiveUS8337390B2Minimize tissutal reactionReduce thrombogenic capacityAnnuloplasty ringsTubular organ implantsCardiac cyclePlasma viscosity
An intracardiac device for restoring the functional elasticity of the cardiac structures, in particular for the treatment of cardiomyopathies and or valvulopathies, by storing energy from the cardiac structures and ceding energy to the cardiac structures during the cardiac cycle, has an elongated shape, is at least partially wound in coils and is attachable to a cardiac structure; the coils are selected in material, number and dimension so as to allow an elastic elongation of the intracardiac device higher than 10% of the rest length of the intracardiac device and are exposed, in use, to the blood flow.
Owner:CUBE SRL
Method and system for characterizing cardiovascular systems from single channel data
ActiveUS20150216426A1Cumbersome to executeBroaden applicationMedical simulationElectrocardiographyDiseaseCardiac defects
Methods to identify and risk stratify disease states, cardiac structural defects, functional cardiac deficiencies induced by teratogens and other toxic agents, pathological substrates, conduction delays and defects, and ejection fraction using single channel biological data obtained from the subject. A modified Matching Pursuit (MP) algorithm may be used to find a noiseless model of the data that is sparse and does not assume periodicity of the signal. After the model is derived, various metrics and subspaces are extracted to characterize the cardiac system. In another method, space-time domain is divided into a number of regions (which is largely determined by the signal length), the density of the signal is computed in each region and input to a learning algorithm to associate them to the desired cardiac dysfunction indicator target.
Owner:ANALYTICS FOR LIFE
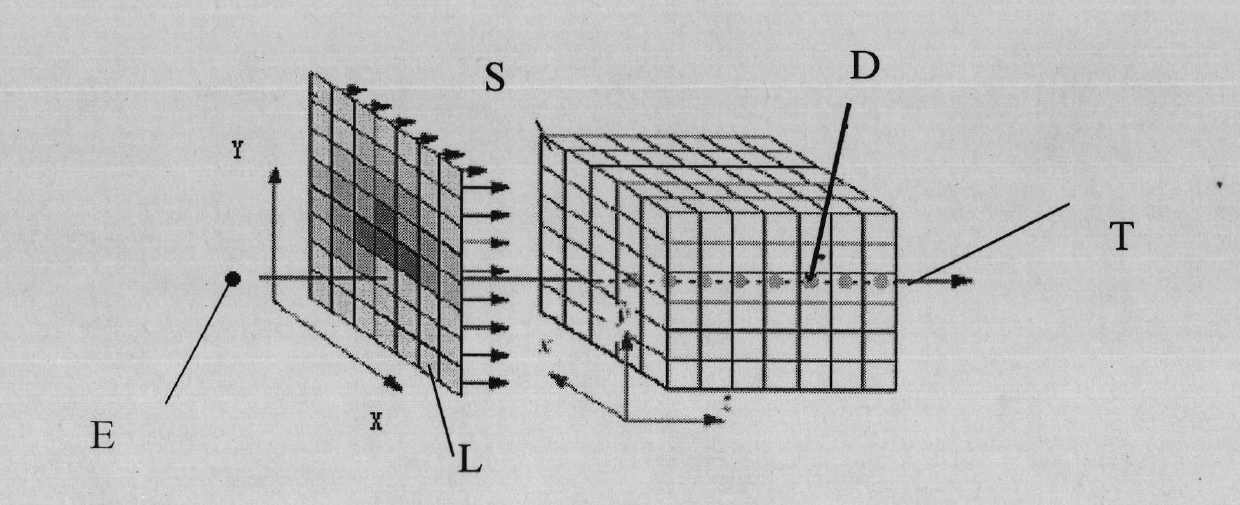
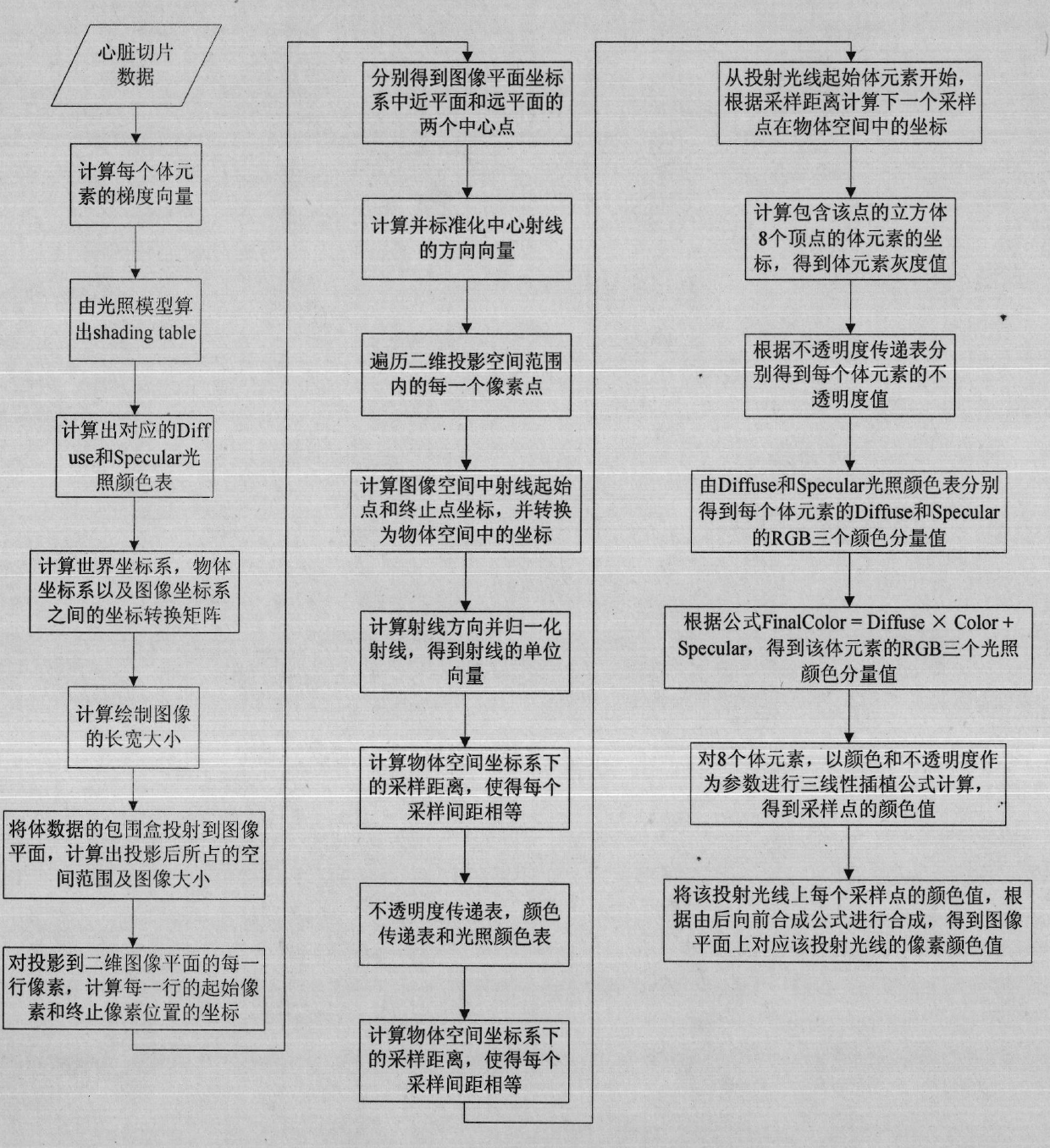
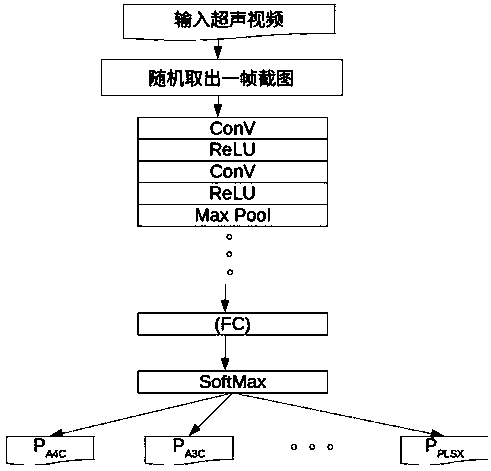
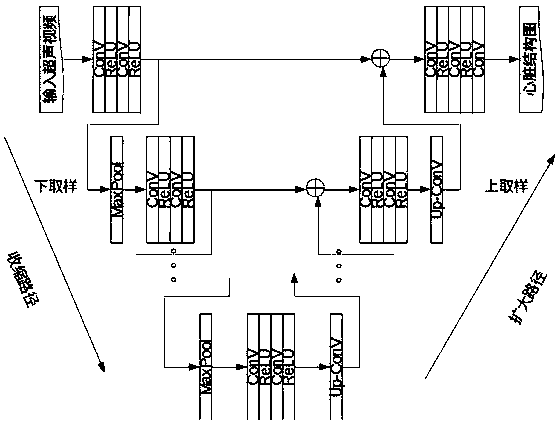
Method for visualizing three-dimensional anatomical tissue structure model of human heart based on ray cast volume rendering algorithm
The invention discloses a method for visualizing a three-dimensional anatomical tissue structure model of a human heart based on a ray cast volume rendering algorithm which, relates to the method for visualizing the three-dimensional anatomical model of the human heart and solves the problem of incapability of visualizing the anatomical tissue structure model of the heart in the prior art. The method comprises the following steps of: (1) obtaining a heart three-dimensional volume data set; (2) selecting a sampling point; (3) calculating a gradient of each volume element in a heart three-dimensional volume data set space; (4) obtaining brightness of each volume element in the heart three-dimensional volume data set space under light; (5) calculating an opacity value and a color value of each volume element; (6) obtaining the opacity value and the color value of each volume element; (7) obtaining pixel point color values corresponding to projection light beams on an image plane; and (8) rendering an image of the heart three-dimensional anatomical tissue structure model according to each pixel point color value on the image plane. The invention lays the foundation for exactly simulating the structure appearance and the behaving function of the heart.
Owner:HARBIN INST OF TECH
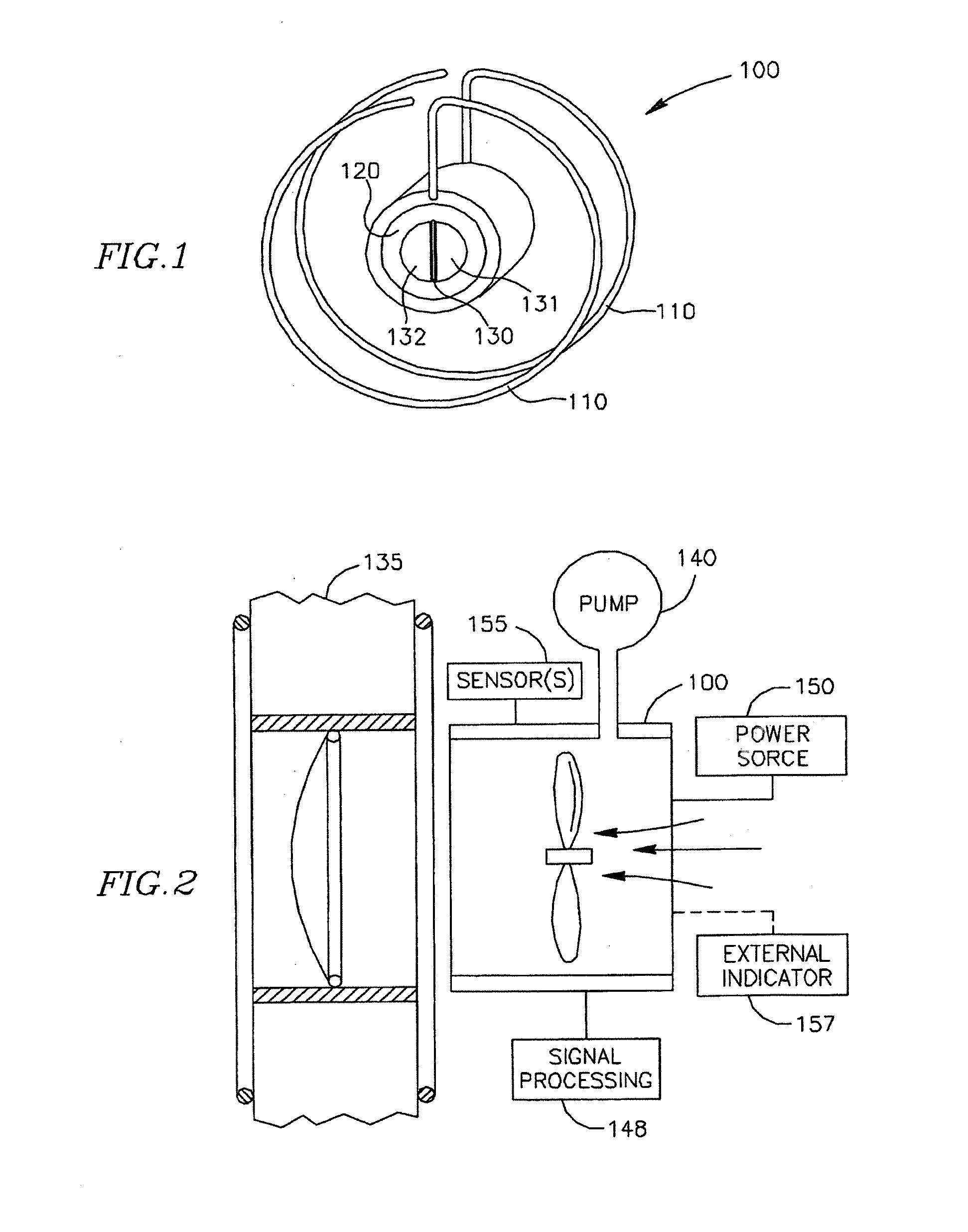
Methods and apparatus for reducing localized circulatory system pressure
InactiveUS20110218477A1Reduces increased diastolic pressurePreventing further deteriorationHeart valvesWound drainsSystoleElectrical battery
The present invention is thus directed to methods and apparatus for decreasing pressure in a first portion of a vessel of the cardiac structure of a patient by implanting a shunt communicating with an area outside said first portion, whereby a volume of blood sufficient to reduce pressure in said first portion is released. Preferably, the first portion comprises the left ventricle and the pressure reduced is the end diastolic pressure, which is accomplished by having the shunt communicate with the left ventricle so a small volume of blood is released from the left ventricle to reduce the end diastolic pressure. Most preferably, the shunt selectively permits flow when a pressure differential between the left ventricle and another chamber of a heart above a threshold pressure, whereby shunting is prevented during left ventricular systole, or, alternatively, selectively permits flow when a pressure differential between the left ventricle and another chamber of a heart is between a lower threshold and a higher threshold, whereby shunting is again prevented during left ventricular systole. In certain embodiments a semi-passive check-valve is controlled and actuated by an external signal, either using a signal generated by an intra-corporeal electrical battery or an externally coupled energy source. In certain embodiments, the shunt has a pump with an input connected to the left ventricle, or other portion with excessive pressure, and an output connected to a volume of lower pressure. The preferred method of implanting the shunt to effect the present invention is by deploying a tubular element having two ends and a tissue affixation element disposed at each of said ends via a catheter, preferably, the fixation element is a shape retaining metallic material that returns to its original shape as part of the retention aspect of its function. In preferred embodiments of the apparatus, the tubular element is comprised of a biologically inert non-metallic material.
Owner:WAVE LTD V
Method and System for Pericardium Based Model Fusion of Pre-operative and Intra-operative Image Data for Cardiac Interventions
ActiveUS20130294667A1Improve accuracyImage enhancementImage analysisHeart chamberComputing tomography
A method and system for model based fusion pre-operative image data, such as computed tomography (CT), and intra-operative C-arm CT is disclosed. A first pericardium model is segmented in the pre-operative image data and a second pericardium model is segmented in a C-arm CT volume. A deformation field is estimated between the first pericardium model and the second pericardium model. A model of a target cardiac structure, such as a heart chamber model or an aorta model, extracted from the pre-operative image data is fused with the C-arm CT volume based on the estimated deformation field between the first pericardium model and the second pericardium model. An intelligent weighted average may be used improve the model based fusion results using models of the target cardiac structure extracted from pre-operative image data of patients other than a current patient.
Owner:SIEMENS HEALTHCARE GMBH
Methods and apparatus for reducing localized circulatory system pressure
InactiveUS20110218478A1Reduces increased diastolic pressurePreventing further deteriorationHeart valvesControl devicesSystoleDifferential pressure
The present invention is thus directed to methods and apparatus for decreasing pressure in a first portion of a vessel of the cardiac structure of a patient by implanting a shunt communicating with an area outside said first portion, whereby a volume of blood sufficient to reduce pressure in said first portion is released. Preferably, the first portion comprises the left ventricle and the pressure reduced is the end diastolic pressure, which is accomplished by having the shunt communicate with the left ventricle so a small volume of blood is released from the left ventricle to reduce the end diastolic pressure. Most preferably, the shunt selectively permits flow when a pressure differential between the left ventricle and another chamber of a heart above a threshold pressure, whereby shunting is prevented during left ventricular systole, or, alternatively, selectively permits flow when a pressure differential between the left ventricle and another chamber of a heart is between a lower threshold and a higher threshold, whereby shunting is again prevented during left ventricular systole. In certain embodiments a semi-passive check-valve is controlled and actuated by an external signal, either using a signal generated by an intra-corporeal electrical battery or an externally coupled energy source. In certain embodiments, the shunt has a pump with an input connected to the left ventricle, or other portion with excessive pressure, and an output connected to a volume of lower pressure. The preferred method of implanting the shunt to effect the present invention is by deploying a tubular element having two ends and a tissue affixation element disposed at each of said ends via a catheter, preferably, the fixation element is a shape retaining metallic material that returns to its original shape as part of the retention aspect of its function. In preferred embodiments of the apparatus, the tubular element is comprised of a biologically inert non-metallic material.
Owner:WAVE LTD V
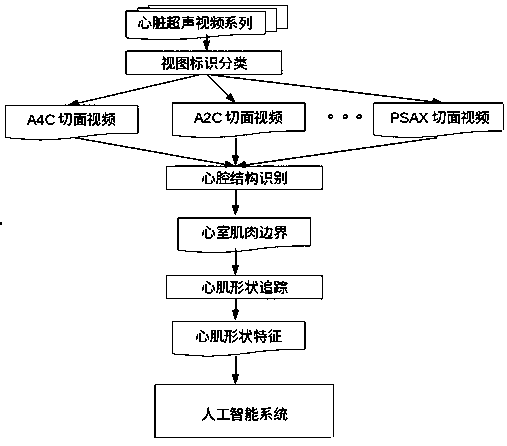
Heart disease risk prediction system
InactiveCN109377470AImprove living qualityLow costImage enhancementImage analysisIschemic heartNerve network
The invention discloses a heart disease risk prediction system, comprising a computer vision pipeline for processing cardiac ultrasound, wherein the computer vision pipeline for processing cardiac ultrasound comprises a view identification classification, a U-Net convolution neural network for cardiac structure identification, cardiac muscle shape tracking, cardiac muscle shape feature vector extraction, electrocardiogram feature data extraction, clinical feature data extraction, depth learning network architecture and data acquisition and training to predict the probability; Through artificial intelligence to assist automatic ultrasound image recognition and diagnosis, Multidimensional myocardial speckle tracking and prediction of ischemic heart failure were calculated, The combination of medical history, clinical data and biomarkers realizes disease prediction on the machine learning platform, and establishes the first artificial intelligence-assisted accurate, sensitive, efficient,automated and scalable cardiovascular screening system, which realizes low-cost, high-accuracy heart disease prediction and early diagnosis system.
Owner:任昊星
System for providing an electrical activity map
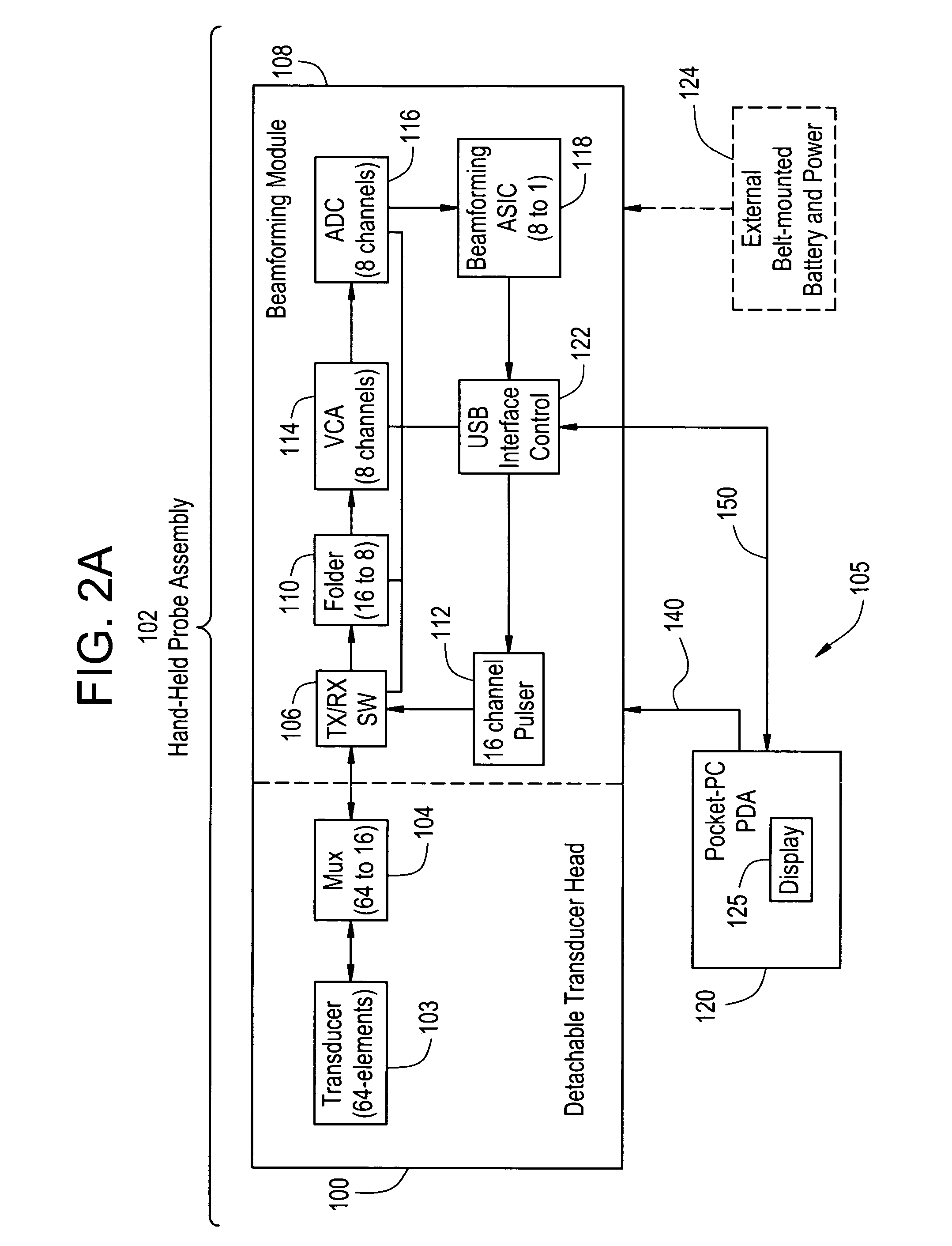
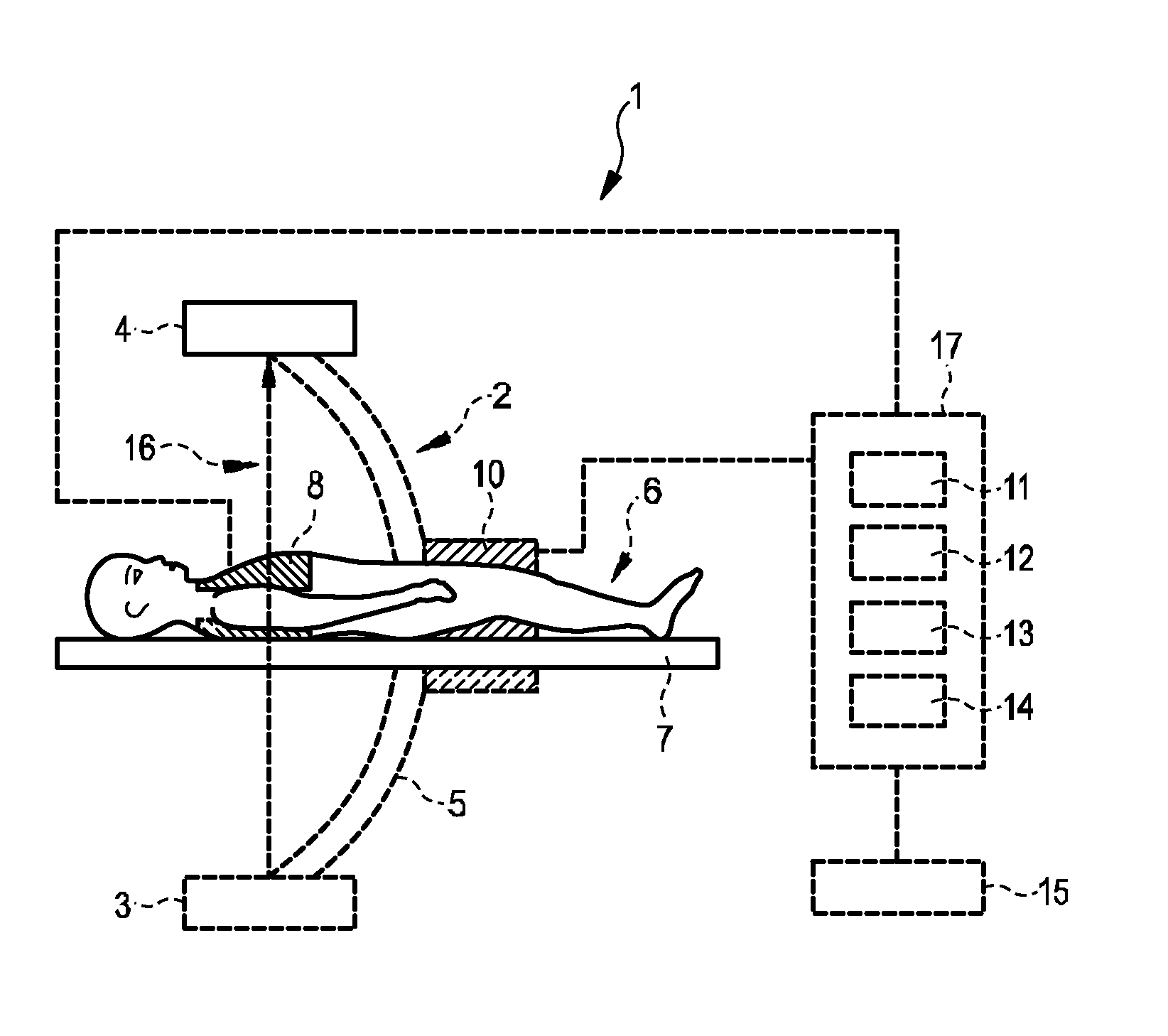
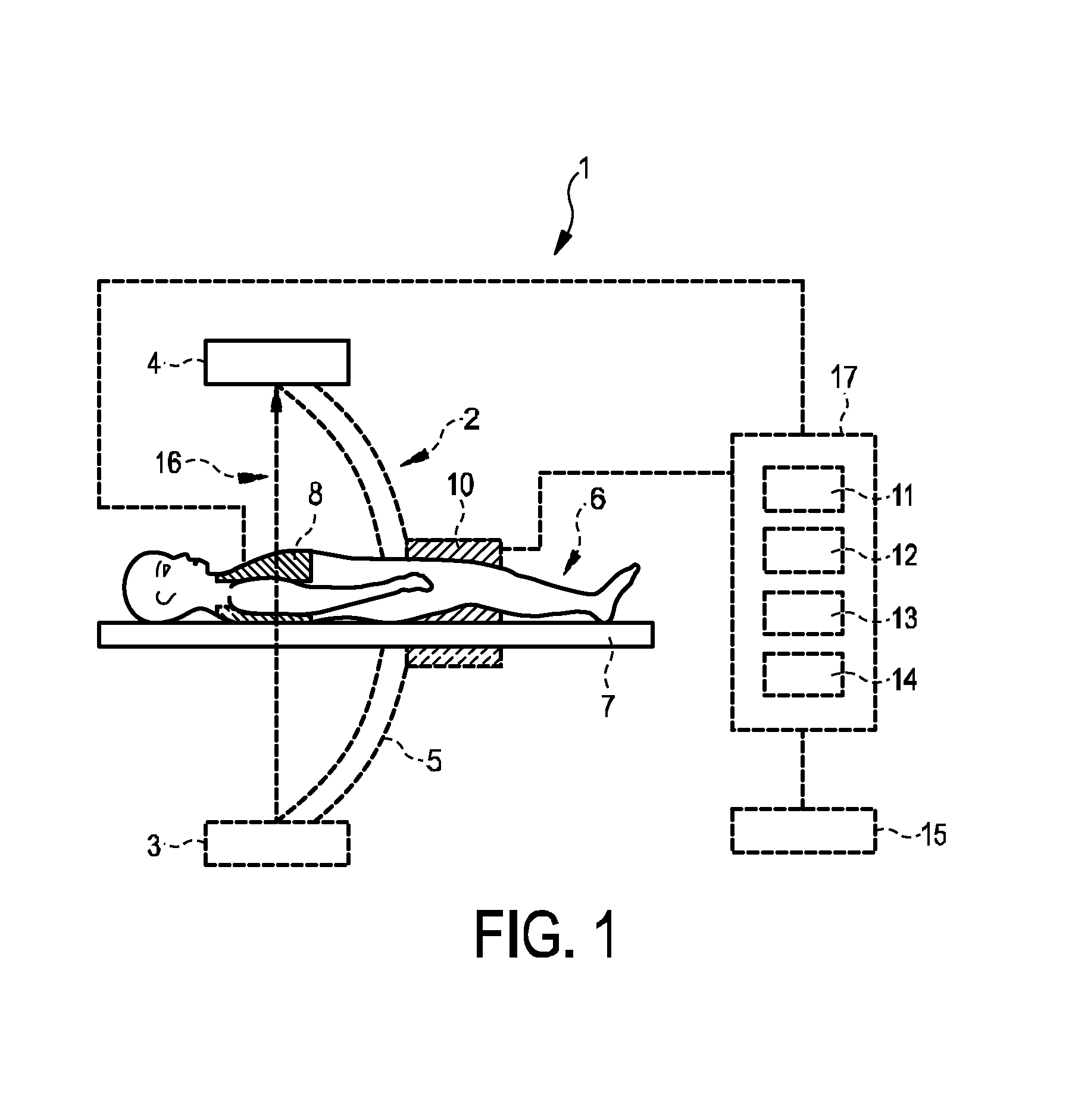
ActiveUS20150038862A1Quality improvementImprove accuracyUltrasonic/sonic/infrasonic diagnosticsMedical imagingElectricityProjection image
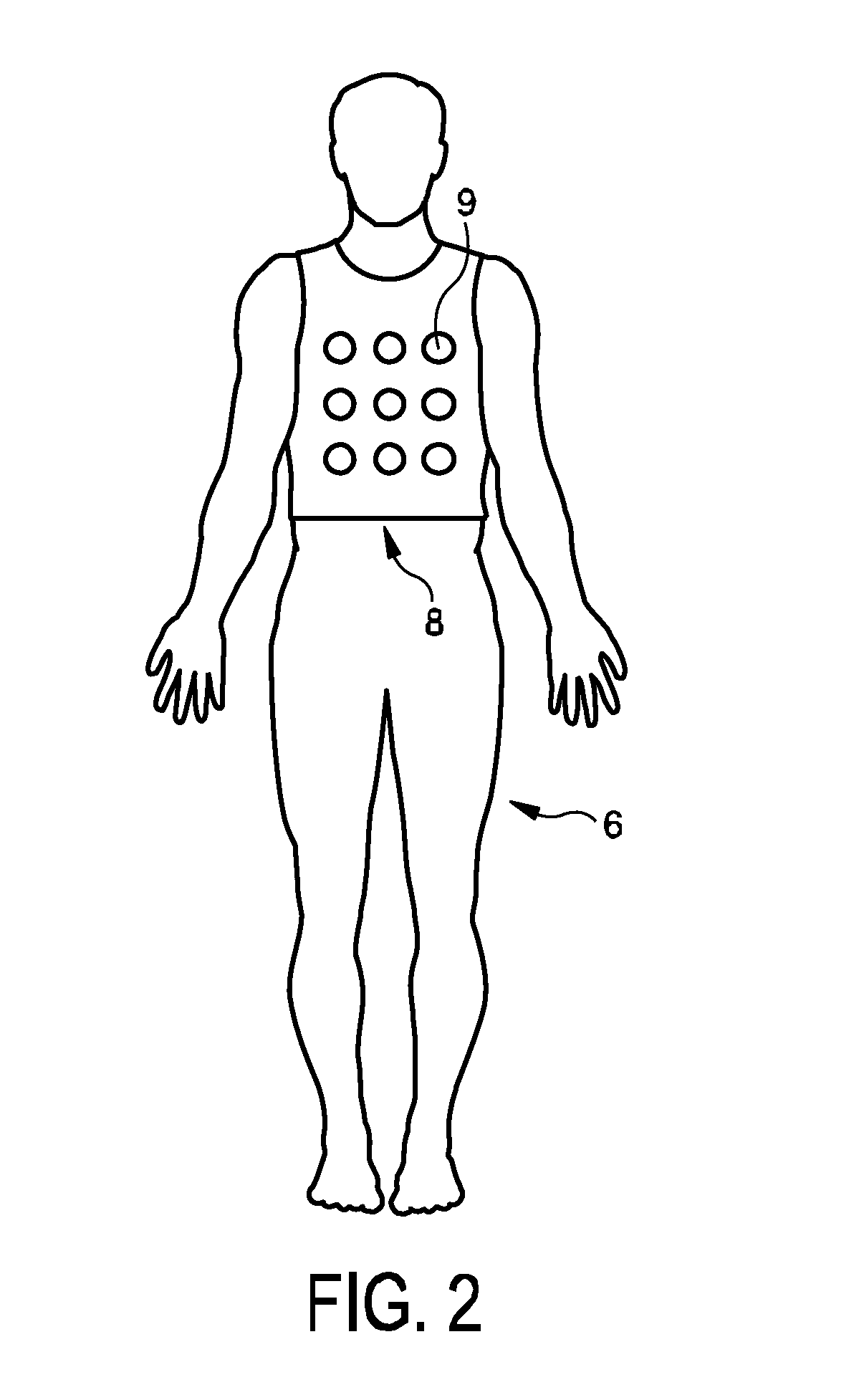
The invention relates to a system for providing an electrical cardiac activity map by means of electrical signals acquired by a plurality of surface electrodes on an outer surface of a living being (6). A cardiac structure position determination unit (12) determines a position of a cardiac structure, in particular, of the epicardial surface, based on provided projection images, wherein an anatomical cardiac model is adapted to the projection images. The projection images may be provided by an x-ray C-arm system (2). An electrical activity map determination unit (13) determines the electrical activity map at the cardiac structure based on the electrical signals, the positions of the plurality of surface electrodes also determined from the projection images, and the determined position of the cardiac structure. This allows determining the electrical activity map at the cardiac structure with high accuracy, without necessarily needing, for instance, an x-ray computed tomography system that would apply a relatively high x-ray radiation dose to the living being. The electrodes may be comprised in a vest (8).
Owner:KONINKLJIJKE PHILIPS NV
Deployment system for an expandable device
The present invention is directed to a deployment system for an endoluminal device. The deployment system includes a confining sheath placed around a compacted endoluminal device. A deployment line is provided in the system that is an integral extension of the sheath. As the deployment line is actuated, the sheath retracts from around the compacted endoluminal device. As the sheath retracts from around the endoluminal device, material from the sheath may be converted into deployment line. Once the sheath is retracted from around the compacted endoluminal device, the endoluminal device expands in configuration and repairs vascular or cardiac structures of an implant recipient. Any remaining sheath material is removed from the implantation site along with the deployment line. The deployment system also includes an endo-prosthesis mounting member placed between the endoluminal device and an underlying catheter. The endo-prosthesis mounting member serves to cushion and retain the endoluminal device when constrained by the sheath and may assist in expansion of the endoluminal device when unconstrained by the sheath.The present invention is also directed to a deployment system having a deployment assembly that simultaneously expands an endo-prosthesis mounting member while removing a sheath from an expandable medical device.
Owner:WL GORE & ASSOC INC
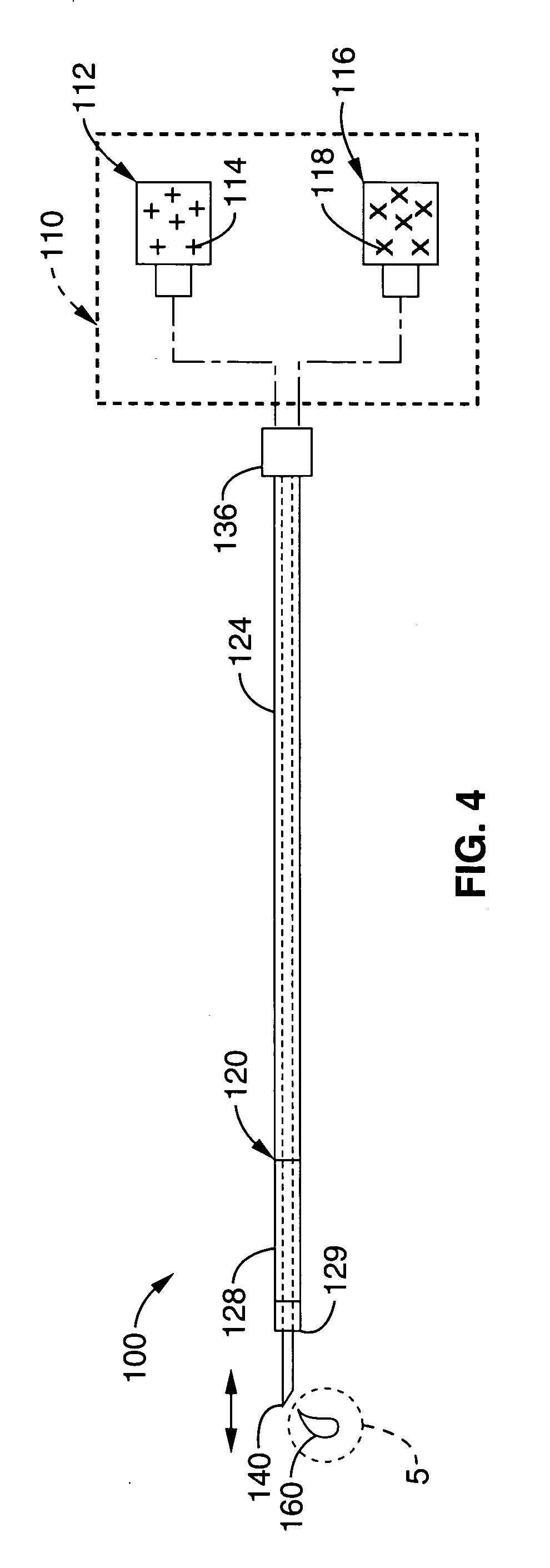
An imaging toolbox for guiding cardiac resynchronization therapy implantation from patient-specific imaging and body surface potential mapping data
The present invention is directed to a method for combining assessment of different factors of dyssynchrony into a comprehensive, non-invasive toolbox for treating patients with a CRT therapy device. The toolbox provides high spatial resolution, enabling assessment of regional function, as well as enabling derivation of global metrics to improve patient response and selection for CRT therapy. The method allows for quantitative assessment and estimation of mechanical contraction patterns, tissue viability, and venous anatomy from CT scans combined with electrical activation patterns from Body Surface Potential Mapping (BSPM). This multi-modal method is therefore capable of integrating electrical, mechanical, and structural information about cardiac structure and function in order to guide lead placement of CRT therapy devices. The method generates regional electro-mechanical properties overlaid with cardiac venous distribution and scar tissue. The fusion algorithm for combining all of the data suggests cardiac segments and routes for implantation of epicardial pacing leads.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Simulation system of cardiac function, simulation method of cardiac function, simulation program of cardiac function, and composite material sheet
ActiveUS20100318326A1Simple designImprove impaired cardiac functionMedical simulationAnalogue computers for chemical processesFiberCardiac feature
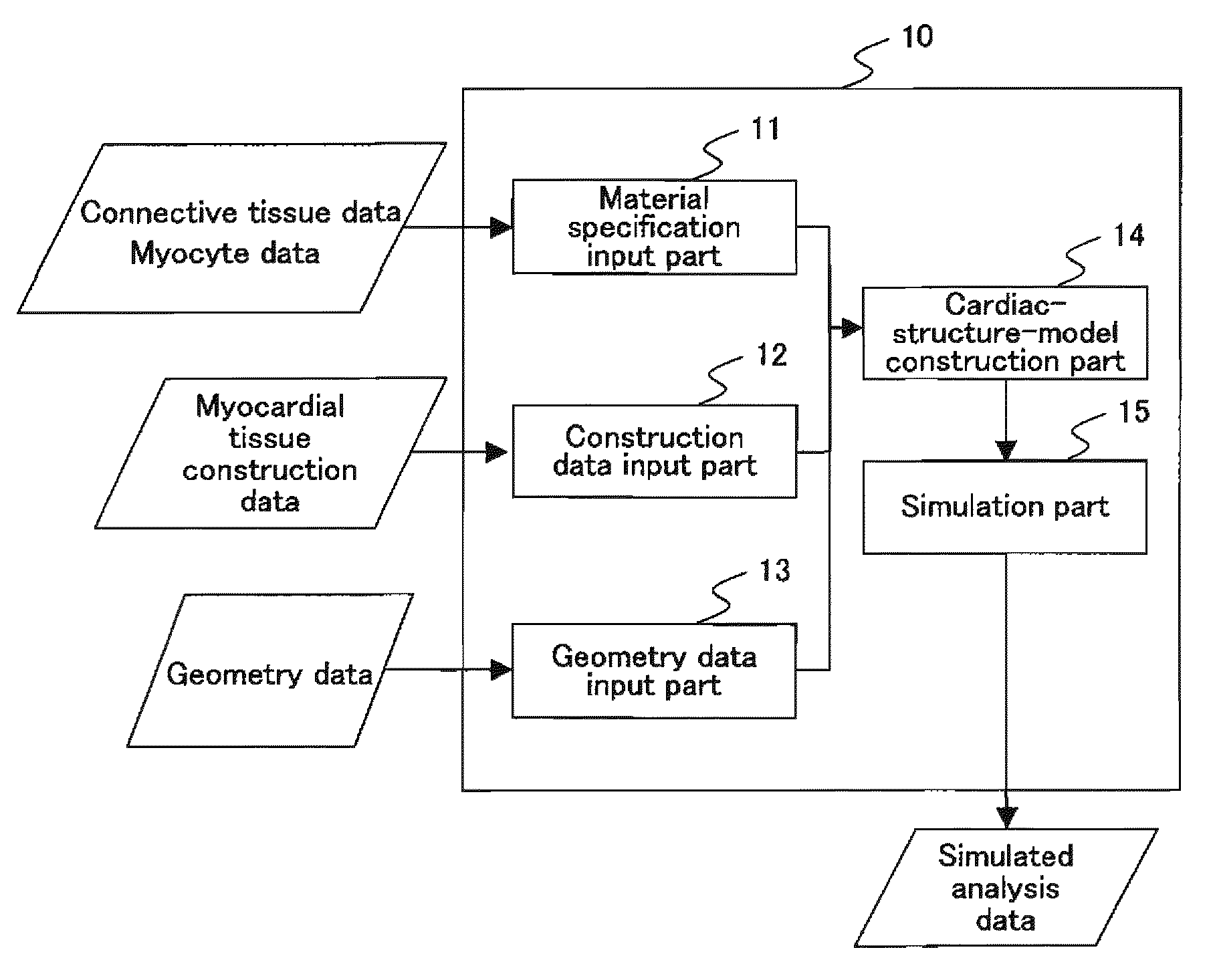
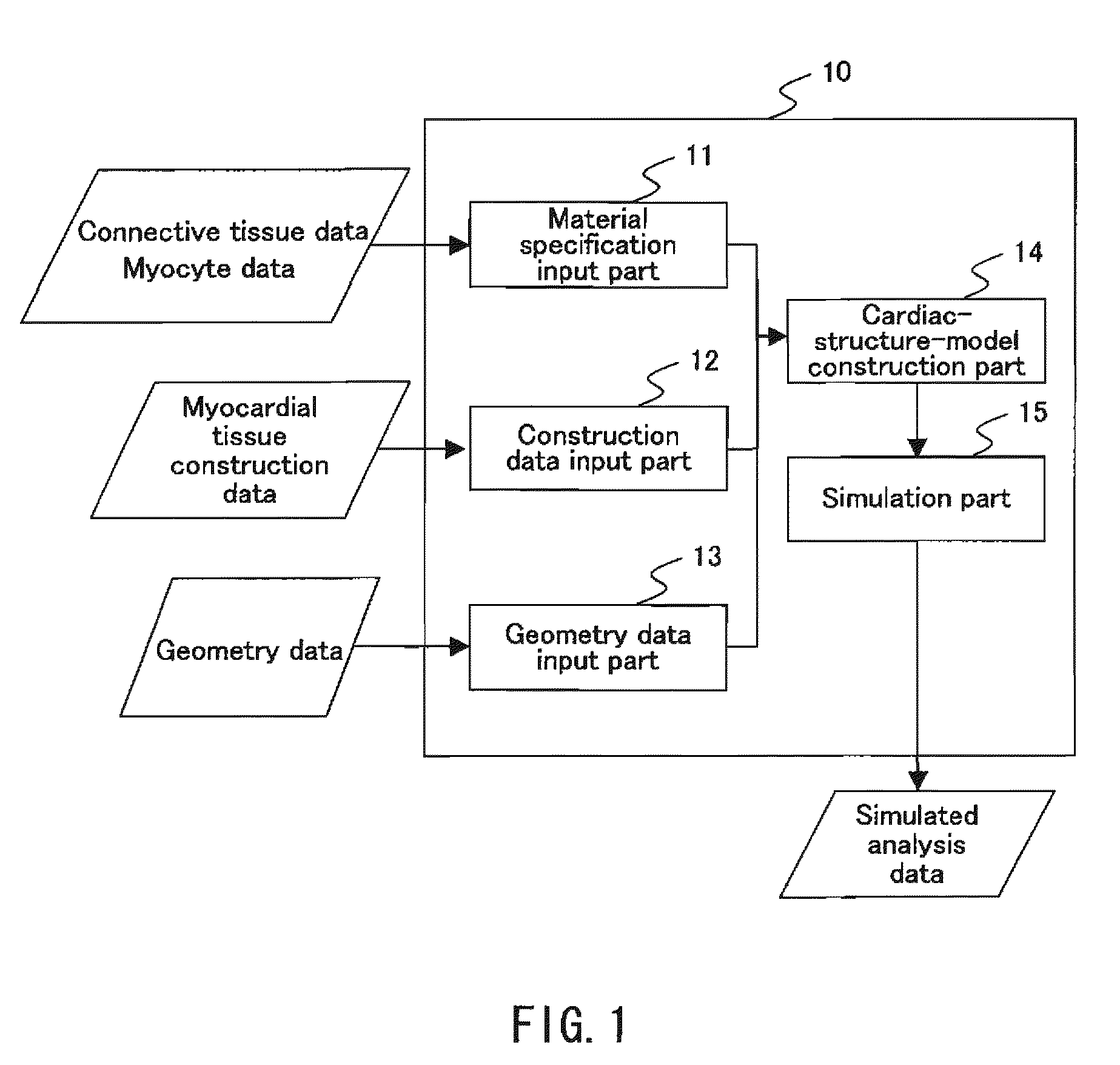
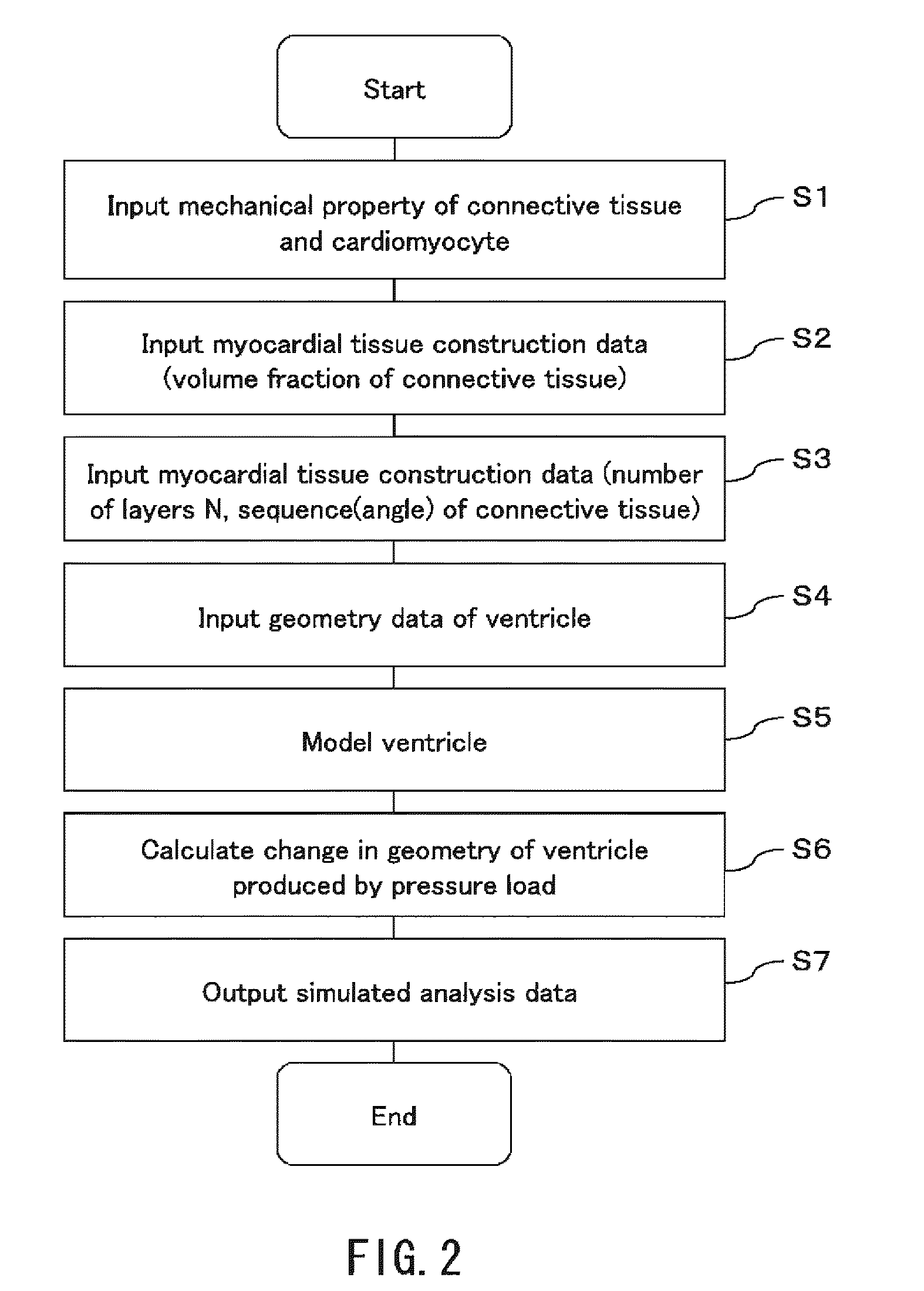
To provide a simulation system of cardiac function utilizing a cardiac structure model which is generated based on an appropriate composite material view representing the myocardial tissue. A simulation system of cardiac function to predict a change in cardiac geometry using a cardiac structure model contains a material specification input part 11 to determine both connective tissue data and myocyte data, a geometry data input part 13 to input geometry data of three-dimensional geometry of a heart, and a cardiac-structure-model construction part 14 wherein a cardiac structure model assumes assembly of finite elements based on continuum data of three-dimensional geometry defined by geometry data and made of composite material containing matrix and reinforcement fiber, and possesses mechanical properties of reinforcement fiber reflecting mechanical properties of connective tissue data and mechanical properties of matrix reflecting mechanical properties of myocyte data. The simulation system also contains a simulation part 15 which predicts a change of geometry of the cardiac structure model produced by pressure load utilizing finite element method with computation.
Owner:YAMAMOTO SHOJI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com