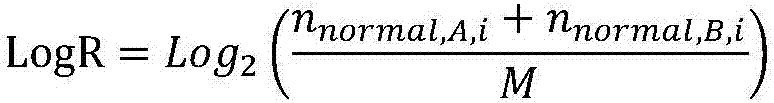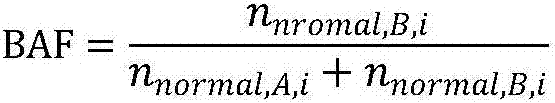Patents
Literature
282 results about "Telomere" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
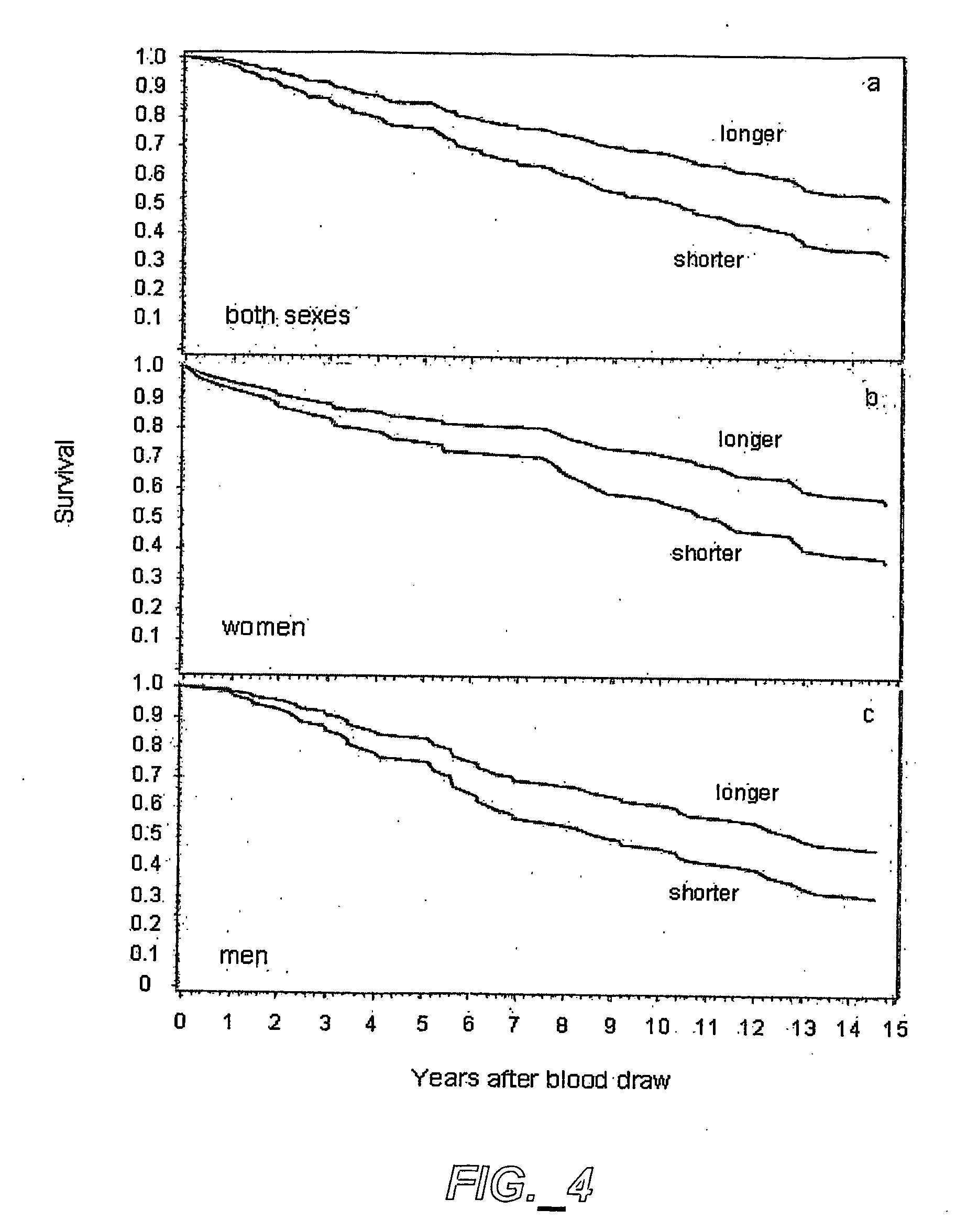
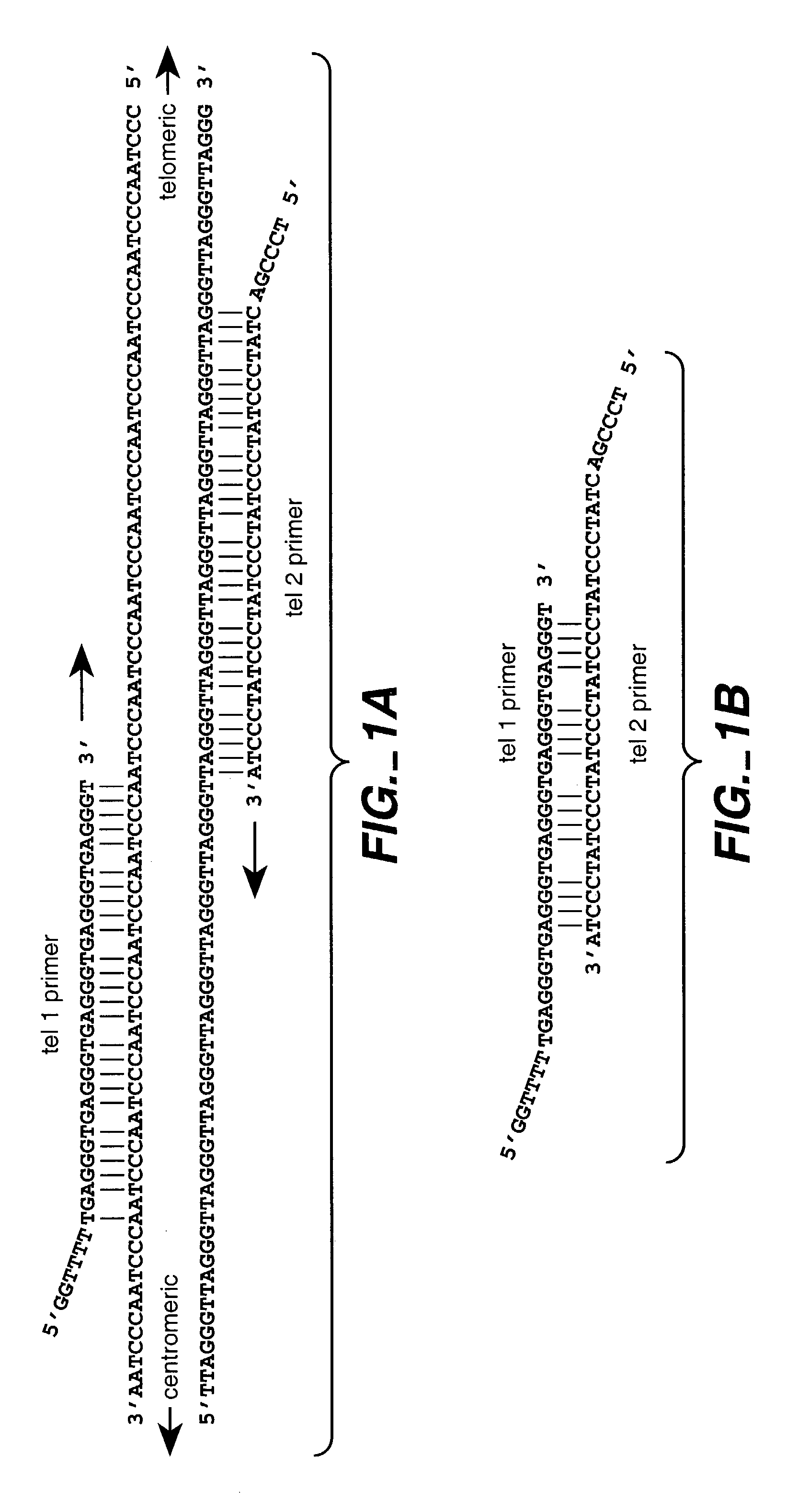
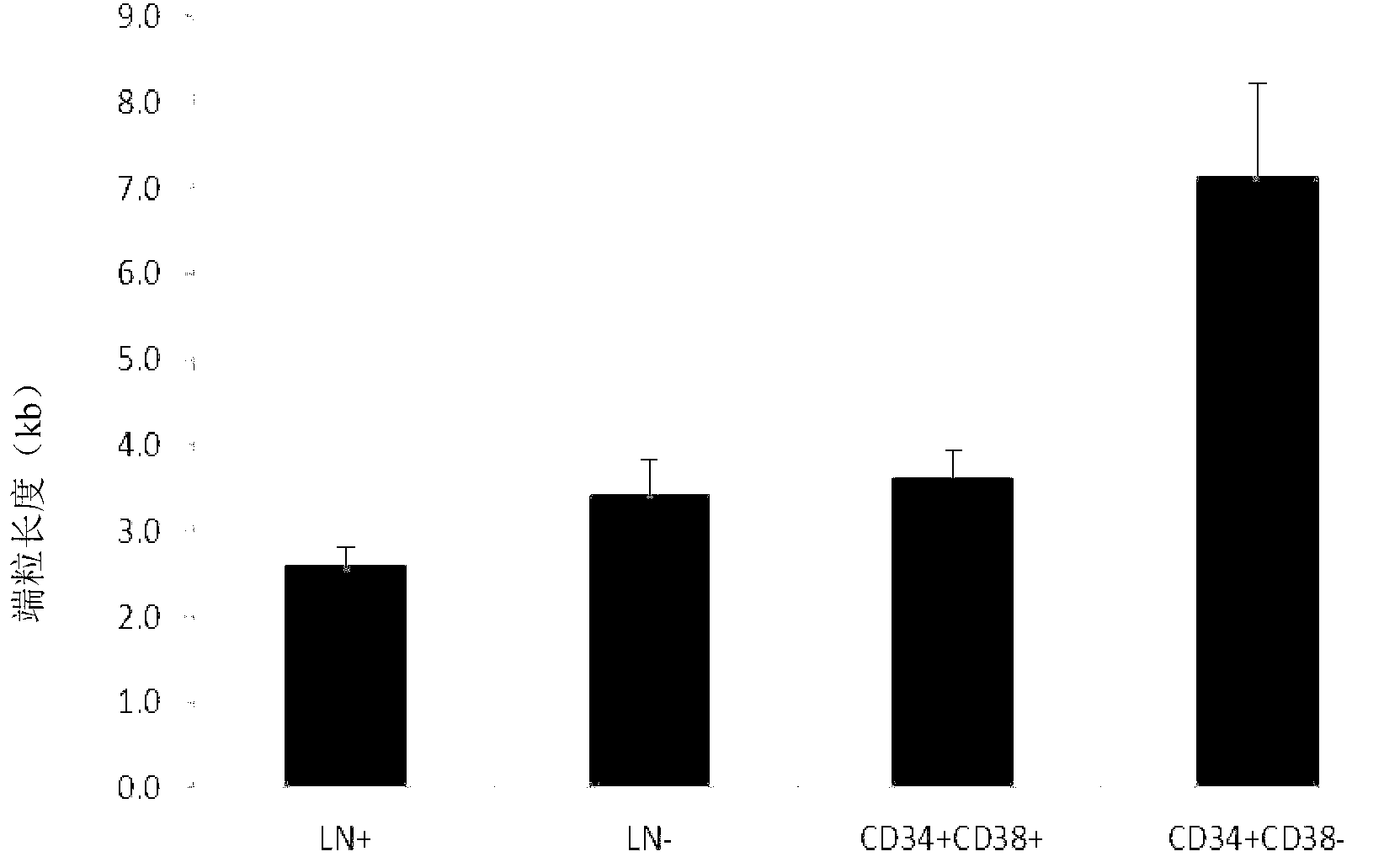
A telomere (/ˈtɛləmɪər/ or /ˈtɪləmɪər/) is a region of repetitive nucleotide sequences at each end of a chromosome, which protects the end of the chromosome from deterioration or from fusion with neighboring chromosomes. Its name is derived from the Greek nouns telos (τέλος) "end" and merοs (μέρος, root: μερ-) "part". For vertebrates, the sequence of nucleotides in telomeres is AGGGTT, with the complementary DNA strand being TCCCAA, with a single-stranded TTAGGG overhang. This sequence of TTAGGG is repeated approximately 2,500 times in humans. In humans, average telomere length declines from about 11 kilobases at birth to fewer than 4 kilobases in old age, with the average rate of decline being greater in men than in women.
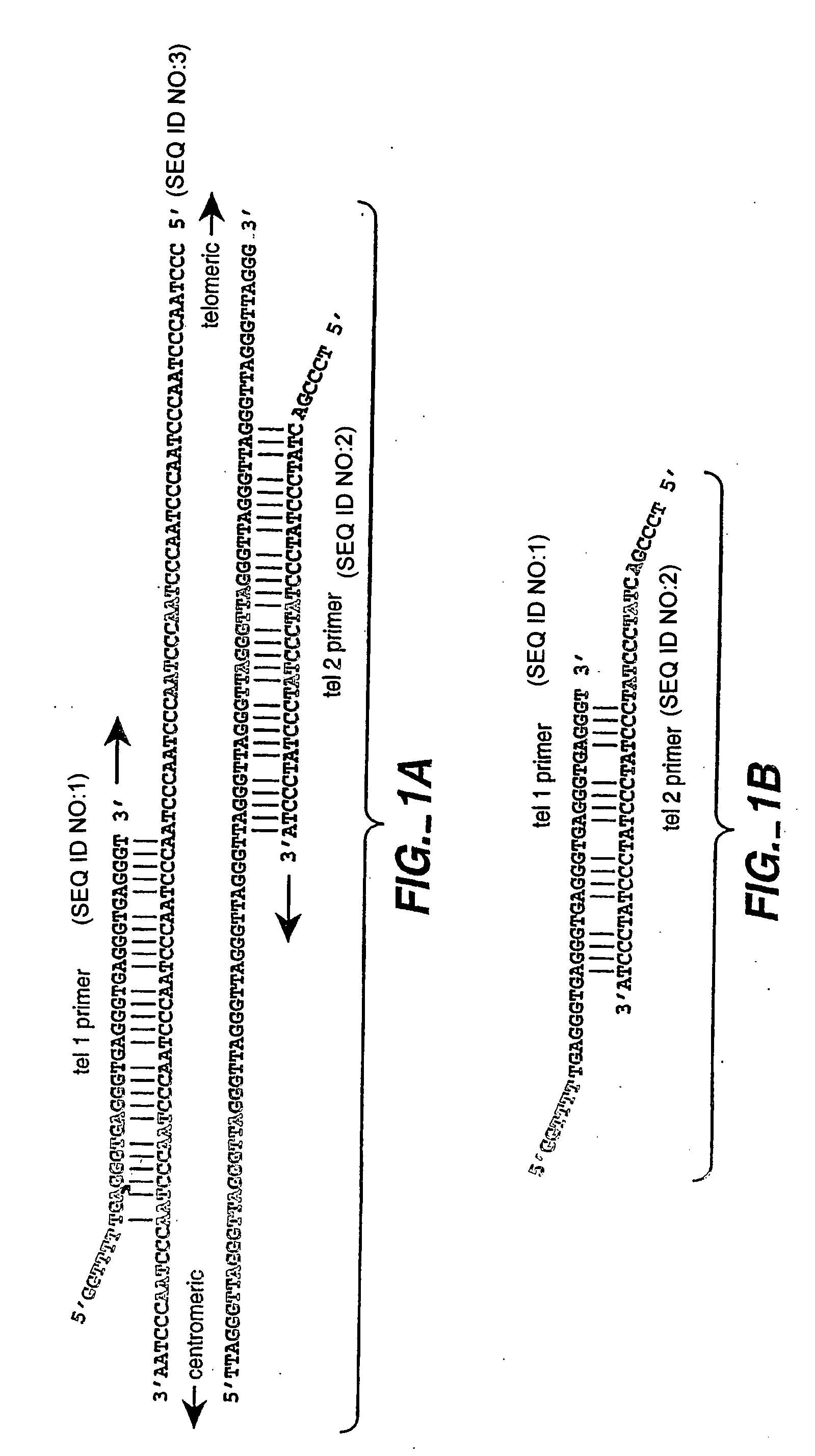
Assays for determining telomere length and repeated sequence copy number
InactiveUS20090298709A1Microbiological testing/measurementLibrary screeningMultiplexingBioinformatics
Methods of detecting copy number of a repeated sequence element, including methods of determining telomere length, are provided. The methods can be multiplexed for detection of repeated sequence element copy number on two or more nucleic acid targets simultaneously. Compositions, kits, and systems related to the methods are also described.
Owner:AFFYMETRIX INC
Diagnosis and treatment of cervical cancer
InactiveUS20080113340A1Organic active ingredientsPeptide/protein ingredientsCervical lesionCervical cell
In certain aspects, the invention relates to methods of diagnosing cervical cancer by using a combination of certain biomarkers such as hTERT, IGFBP-3, transferrin receptor, beta-catenin, Myc-HPV E6 interaction, HPV E7, and telomere length. In other aspects, the invention relates to methods of detecting immortalization of cervical cells by using a combination of certain biomarkers. In yet other aspects, the invention relates to methods of classifying the grade of a cervical lesion for diagnostic and prognostic purposes in a female. In further aspects, the invention relates to methods of treating cervical cancer by administering a therapeutic agent that targets one or more of these biomarkers.
Owner:GEORGETOWN UNIV
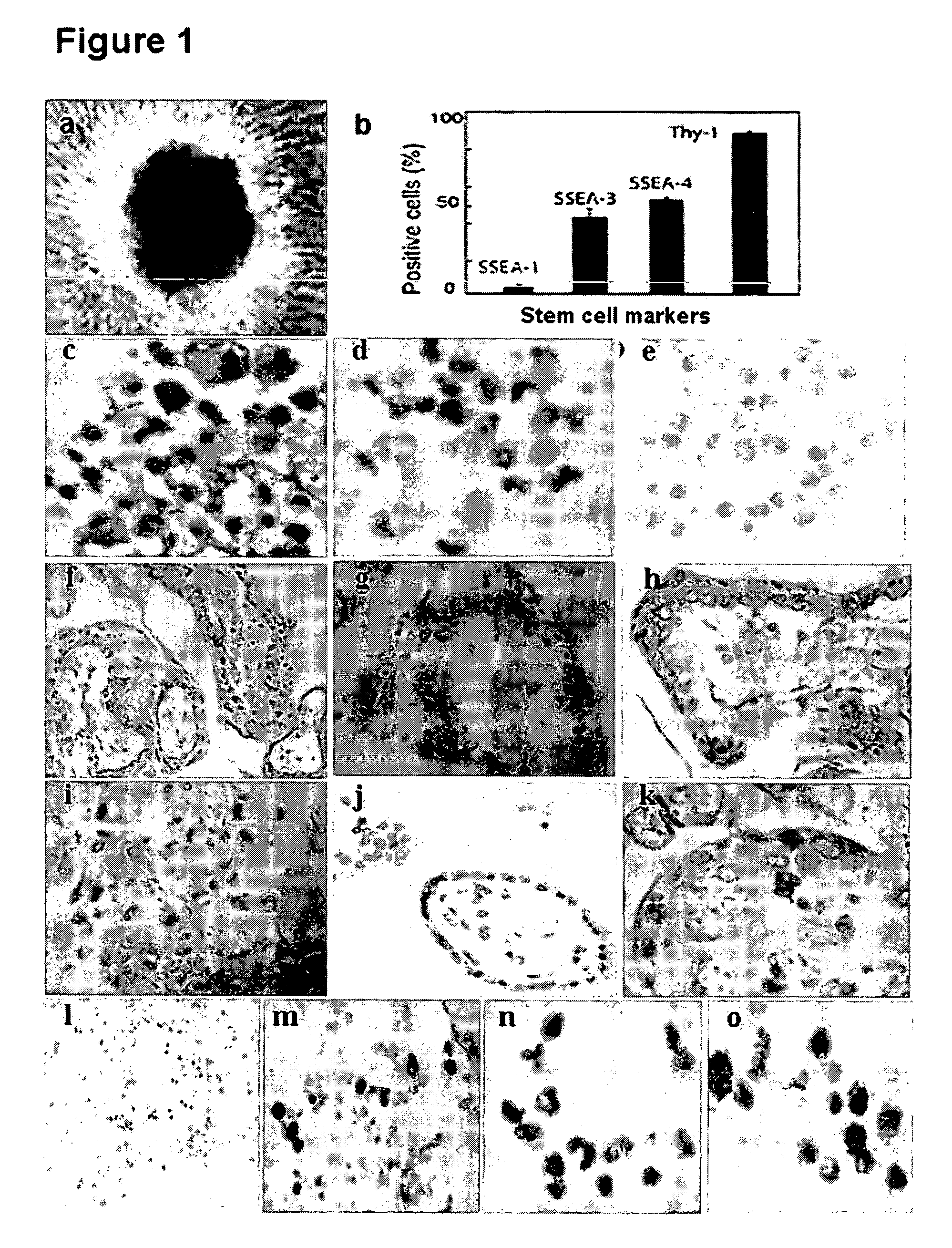
Human trophoblast stem cells and use thereof
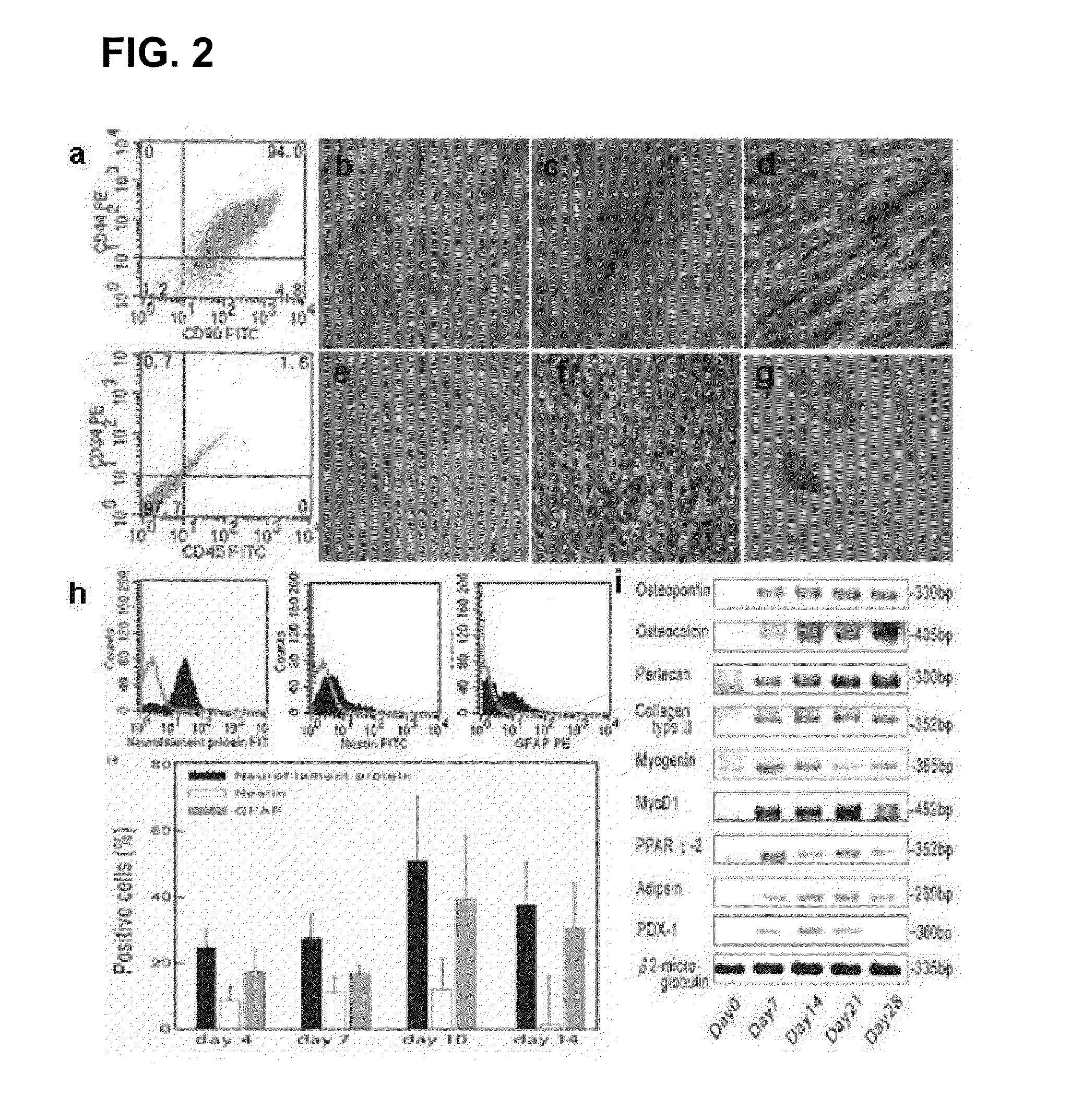
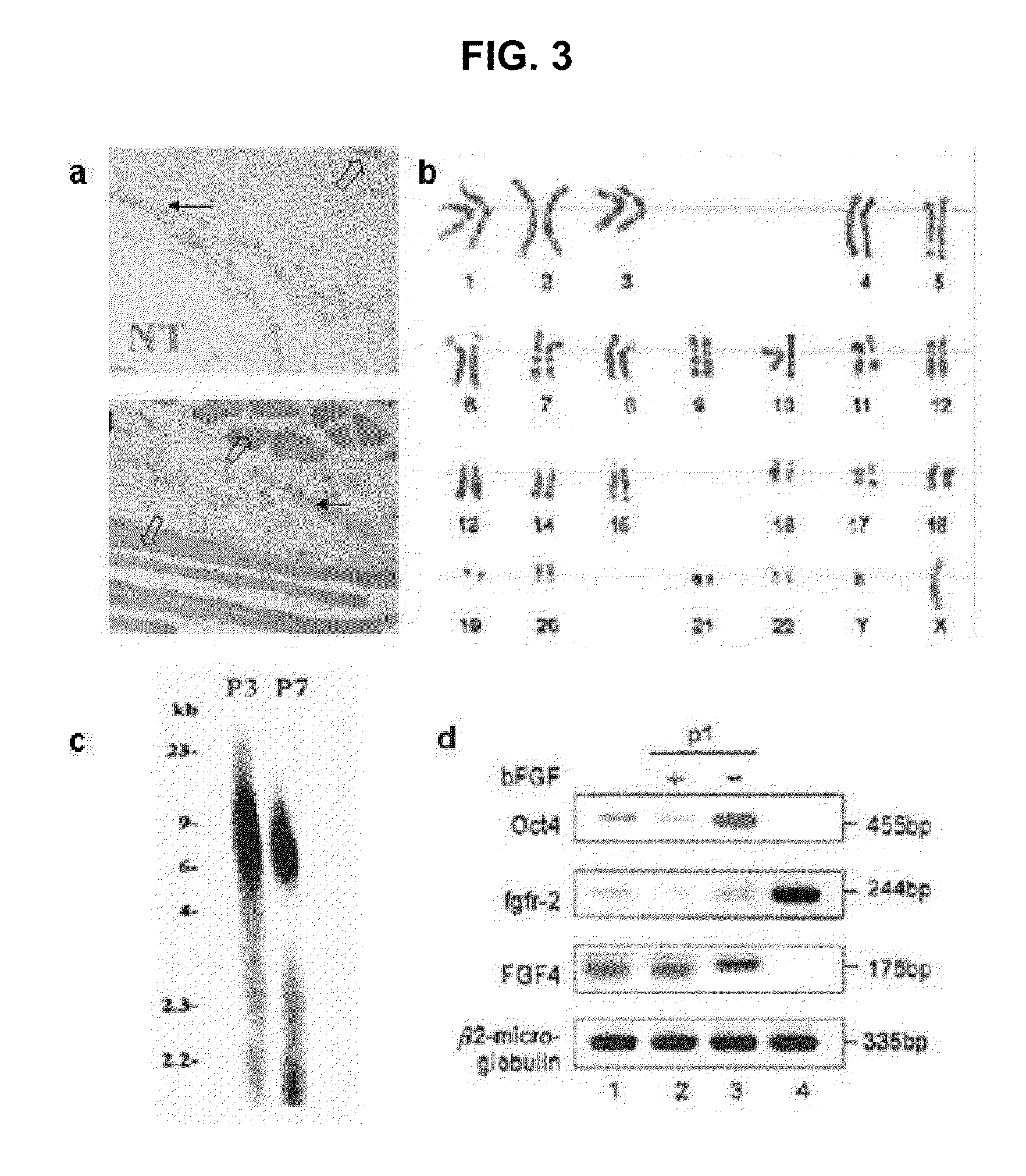
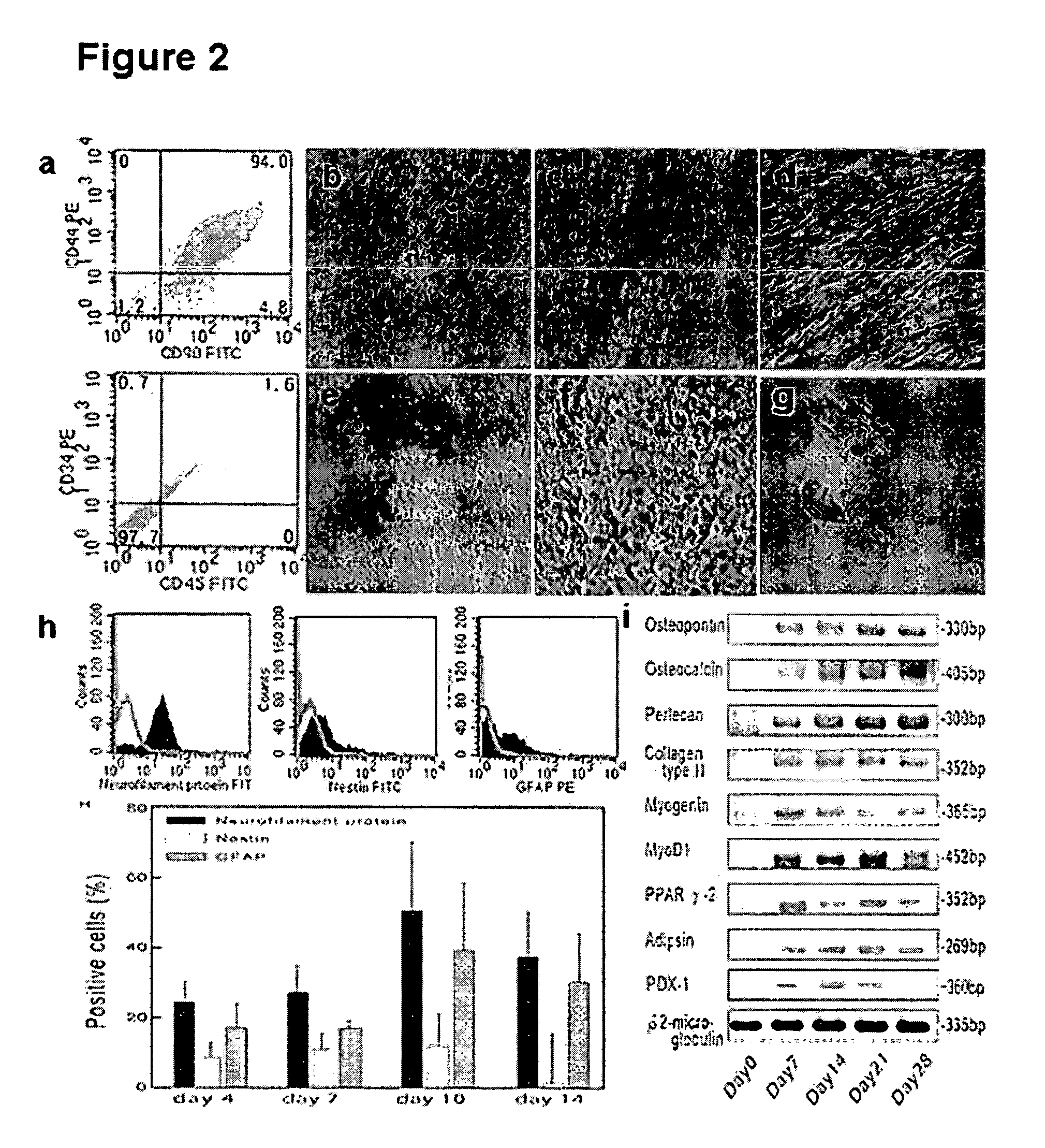
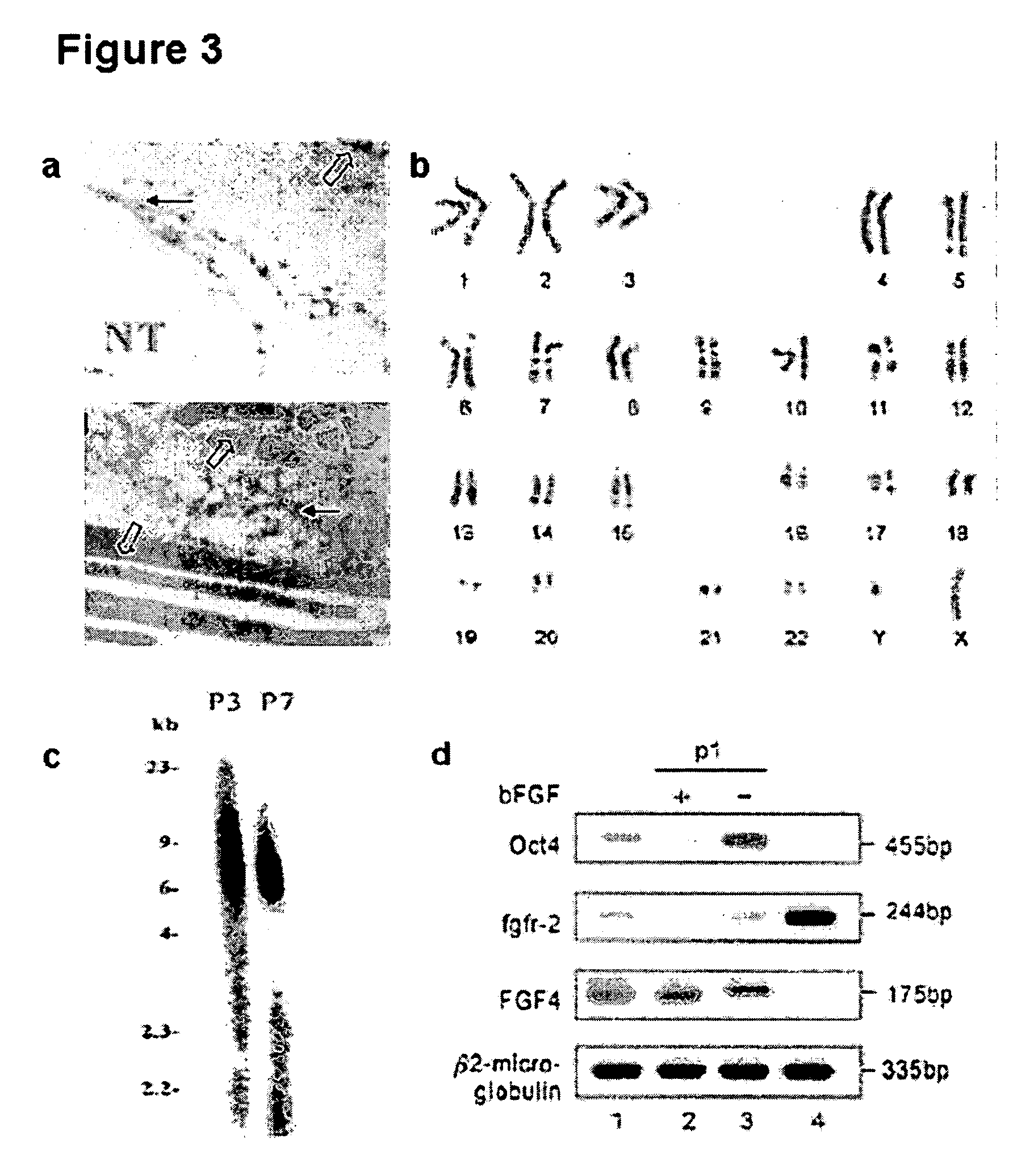
Existence of human trophoblast stem (hTS) cells has been suspected but unproved. The isolation of hTS cells is reported in the early stage of chorionic villi by expressions of FGF4, FGFR-2, Oct4, Thy-1, and stage-specific embryonic antigens distributed in different compartments of the cell. hTS cells are able to derive into specific cell phenotypes of the three primitive embryonic layers, produce chimeric reactions in mice, and retain a normal karyotype and telomere length. In hTS cells, Oct4 and fgfr-2 expressions can be knockdown by bFGF. These facts suggest that differentiation of the hTS cells play an important role in implantation and placentation. hTS cells could be apply to human cell differentiation and for gene and cell-based therapies.
Owner:ACCELERATED BIOSCI
Regulation of epigenetic control of gene expression
Methods are provided for the identification of compounds that selectively modulate epigenetic changes in gene expression. Compounds, compositions, kits or assays devices, and methods are provided for modulating the expression, endogenous levels or the function of small non-coding RNAs cognate to or transcribed by heterochromatic regions subject to epigenetic regulation (i.e., promoters, enhancers, centromeres, telomeres, origins of DNA replication, imprinted loci, or loci marked by dosage-compensation), and for modulating the formation or function of heterochromatin in cells, tissues or animals.
Owner:IONIS PHARMA INC
Method for predicating homologous recombination deficiency mechanism and method for predicating response of patients to cancer therapy
InactiveCN107287285AInnovativeOvercoming the pitfalls of inaccurate forecastsMicrobiological testing/measurementSequence analysisAbnormal tissue growthPolymerase L
The invention discloses a method for predicating a homologous recombination deficiency (HRD) mechanism and a method for predicating response of patients to cancer therapy and relates to the field of biological information predication. The method comprises the step of judging whether a tumor sample has homologous recombination deficiency or not according to one or more comprehensive values in a large-segment INDEL (Insertion / Deletion) fraction, a copy number variation fraction and a tumor mutation load fraction, wherein the comprehensive values can also comprise a loss of heterozygosity variation fraction. By adopting the method disclosed by the invention, predication of a chromosome large-segment structure, a chromosome gene type number, a chromosome gene copy number, a chromosome variation interval and abnormal loss of heterozygosity and chromosome telomeric imbalance is realized, so that an evaluation range is more complete and HRD can be accurately predicated; the comprehensive values also can be used for determining whether the patients have response to a therapeutic regimen containing one or more of a PARP (Poly Adenosine Diphosphate Ribose Polymerase) inhibitor, an DNA (Deoxyribonucleic Acid) injury inhibitor, a topoisomerase II / II+inhibitor, a topoisomerase I inhibitor and radiotherapy; the method is simple and has wide general applicability.
Owner:SHANGHAI ORIGIMED CO LTD
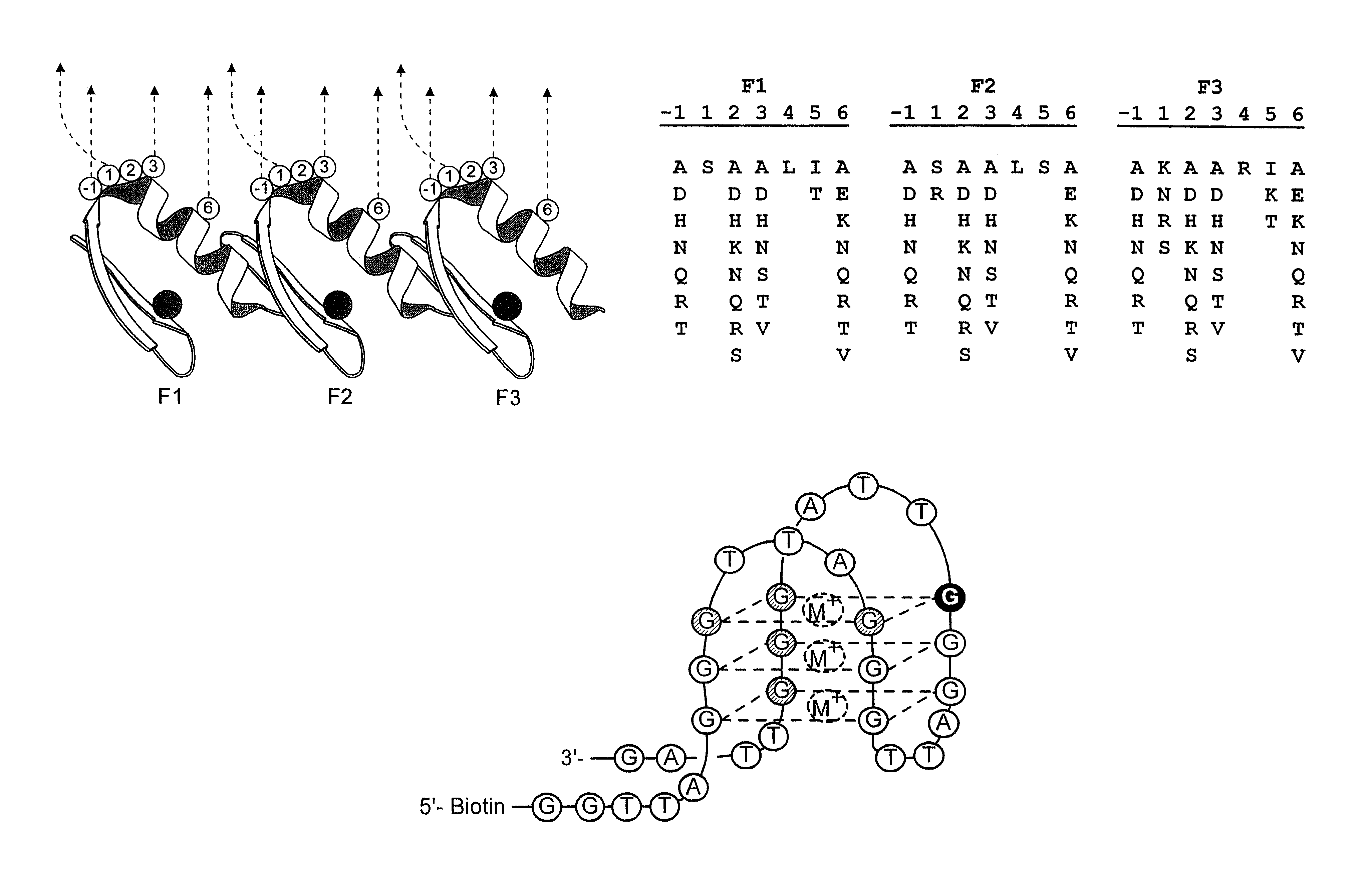
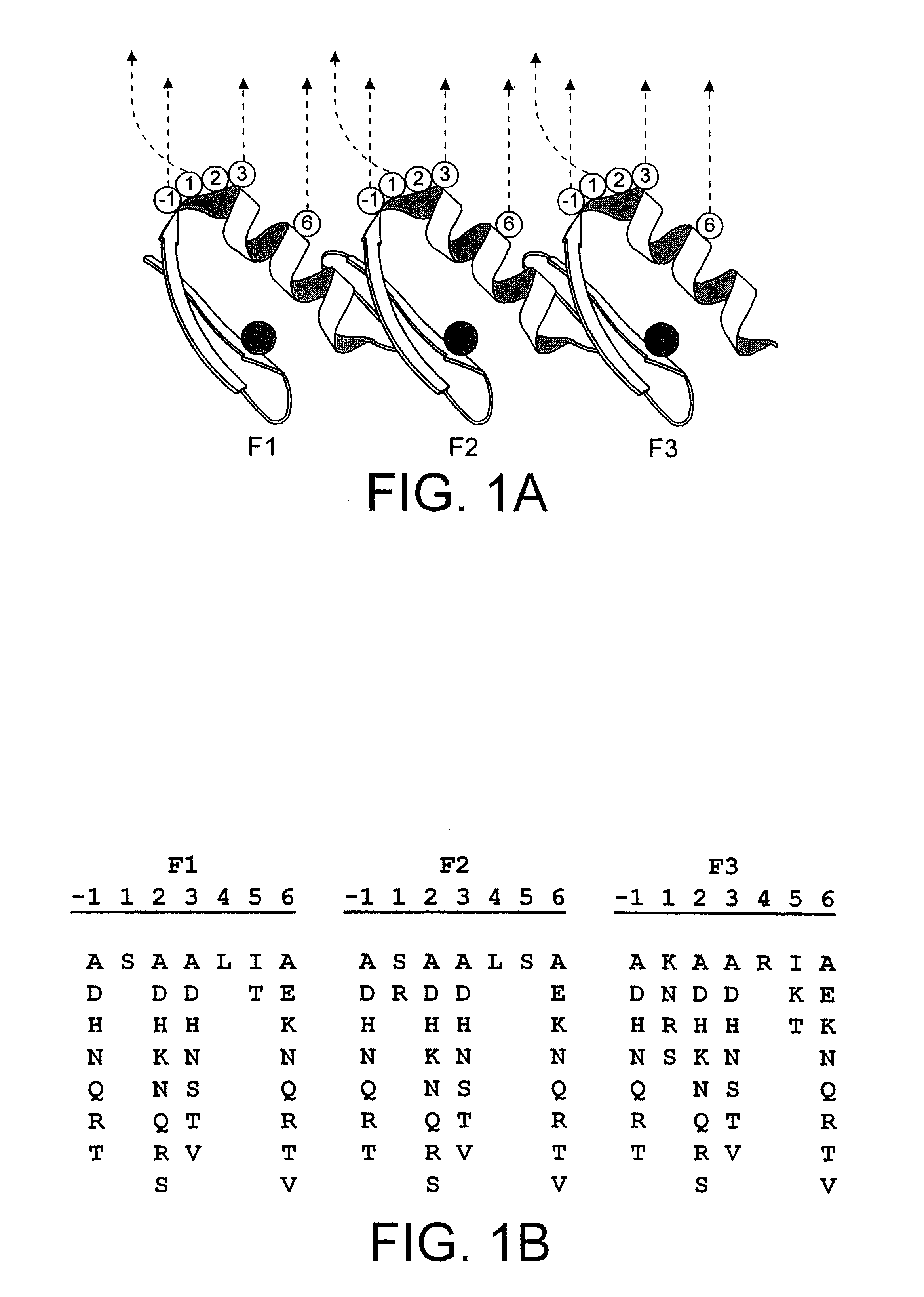
Zinc finger polypeptides capable of binding DNA quadruplexes
InactiveUS6492117B1Facilitates ELISA-based detectionRapid and easily automatedPeptide/protein ingredientsAntibody mimetics/scaffoldsZincZinc finger
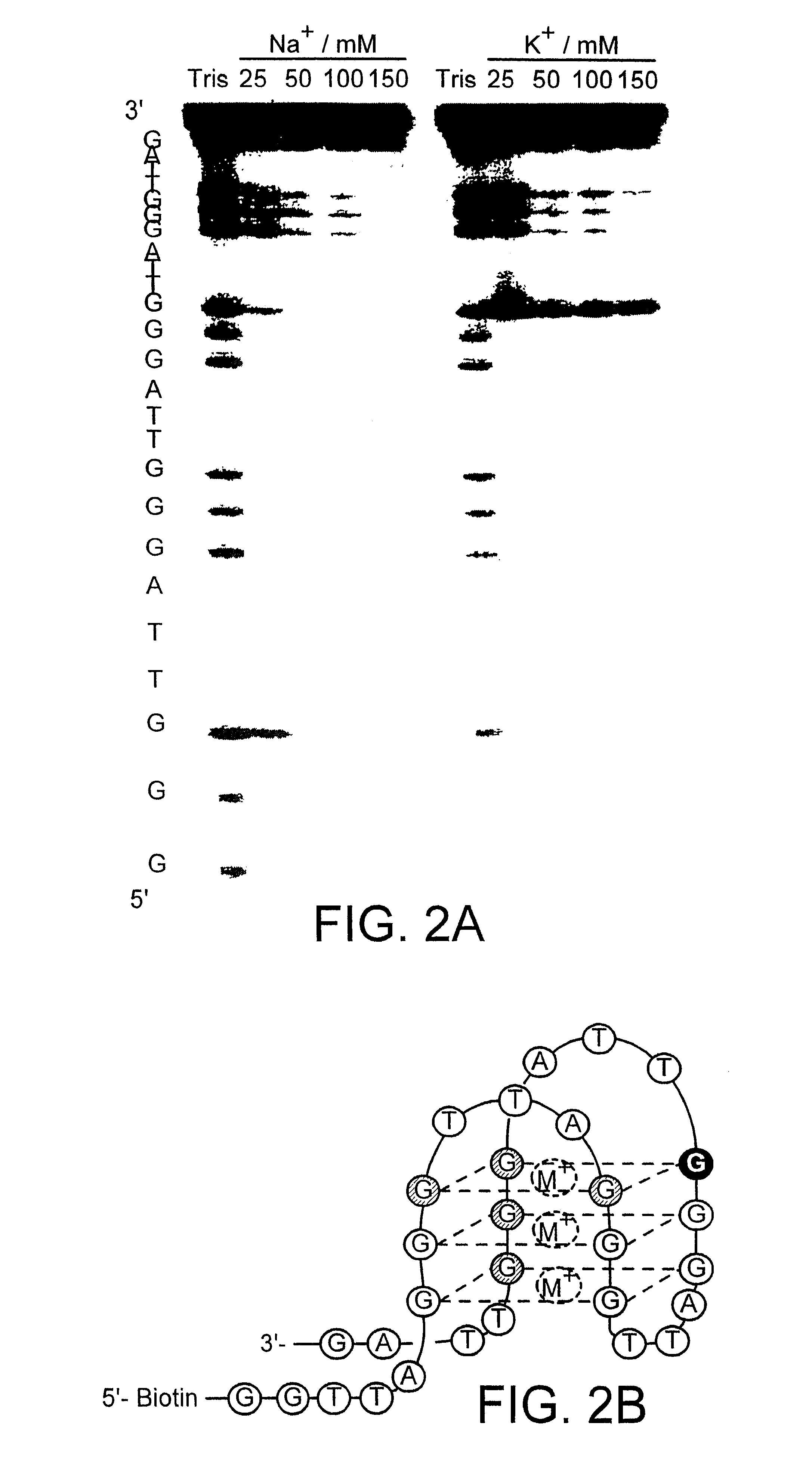
The present invention relates to isolated or purified molecule(s) capable of binding to one or more of telomeric, G-quadruplex, or G-quartet nucleic acid(s).
Owner:GENDAQ +2
Methods of predicting mortality risk by determining telomere length
The present invention provides for methods of determining telomere length of an organism and correlating the measured telomere length with mortality risk associated with telomere length in a population. The presence of shorter telomeres is associated with an increased mortality rate and increased susceptibility to certain types of diseases for an individual member of a human population.
Owner:UNIV OF UTAH RES FOUND
Human trophoblast stem cells and use thereof
Existence of human trophoblast stem (hTS) cells has been suspected but unproved. The isolation of hTS cells is reported in the early stage of chorionic villi by expressions of FGF4, fgfr-2, Oct4, Thy-1, and stage-specific embryonic antigens distributed in different compartments of the cell. hTS cells are able to derive into specific cell phenotypes of the three primitive embryonic layers, produce chimeric reactions in mice, and retain a normal karyotype and telomere length. In hTS cells, Oct4 and fgfr-2 expressions can be knockdown by bFGF. These facts suggest that differentiation of the hTS cells play an important role in implantation and placentation. hTS cells could be apply to human cell differentiation and for gene and cell-based therapies.
Owner:ACCELERATED BIOSCI
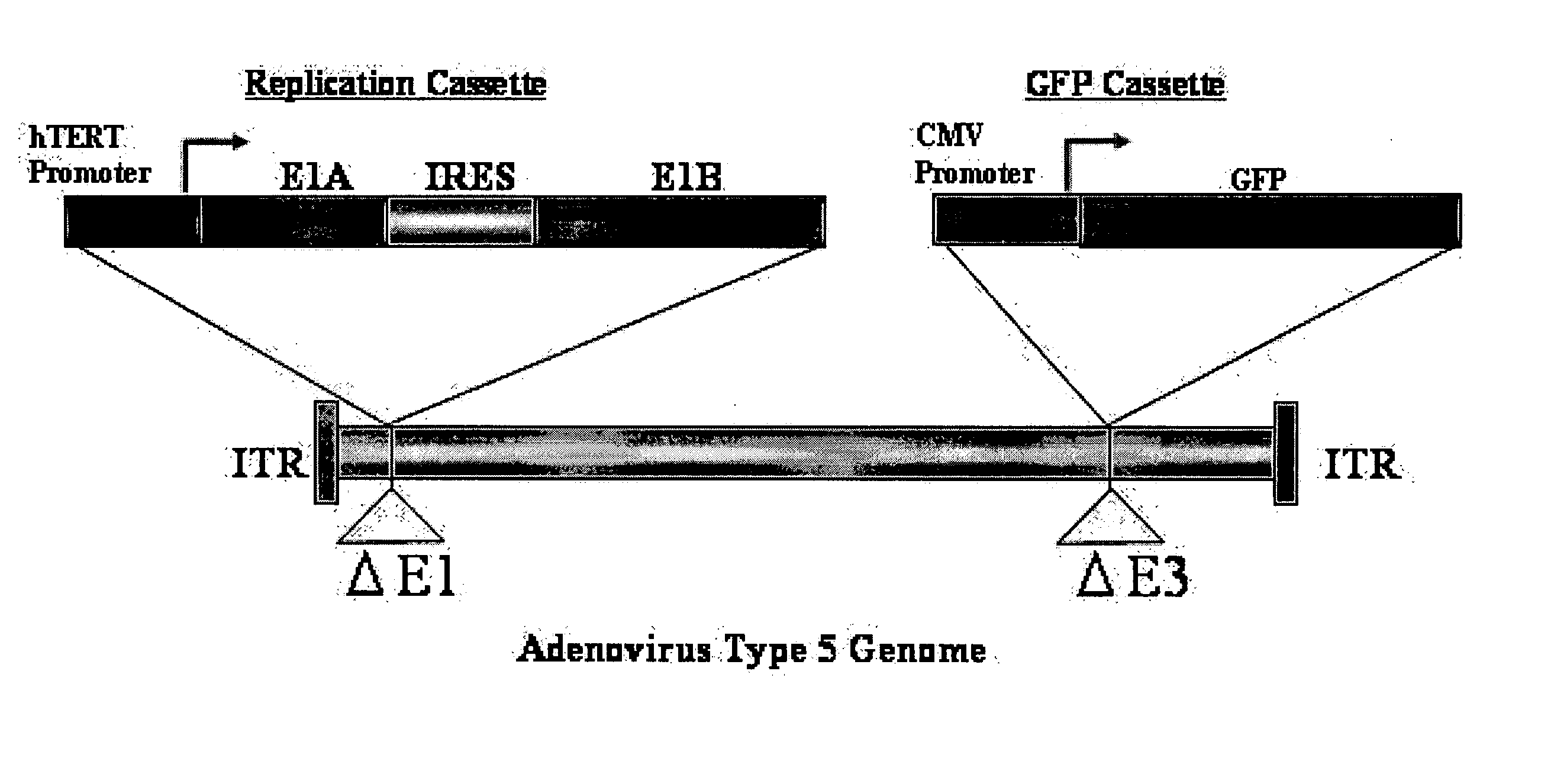
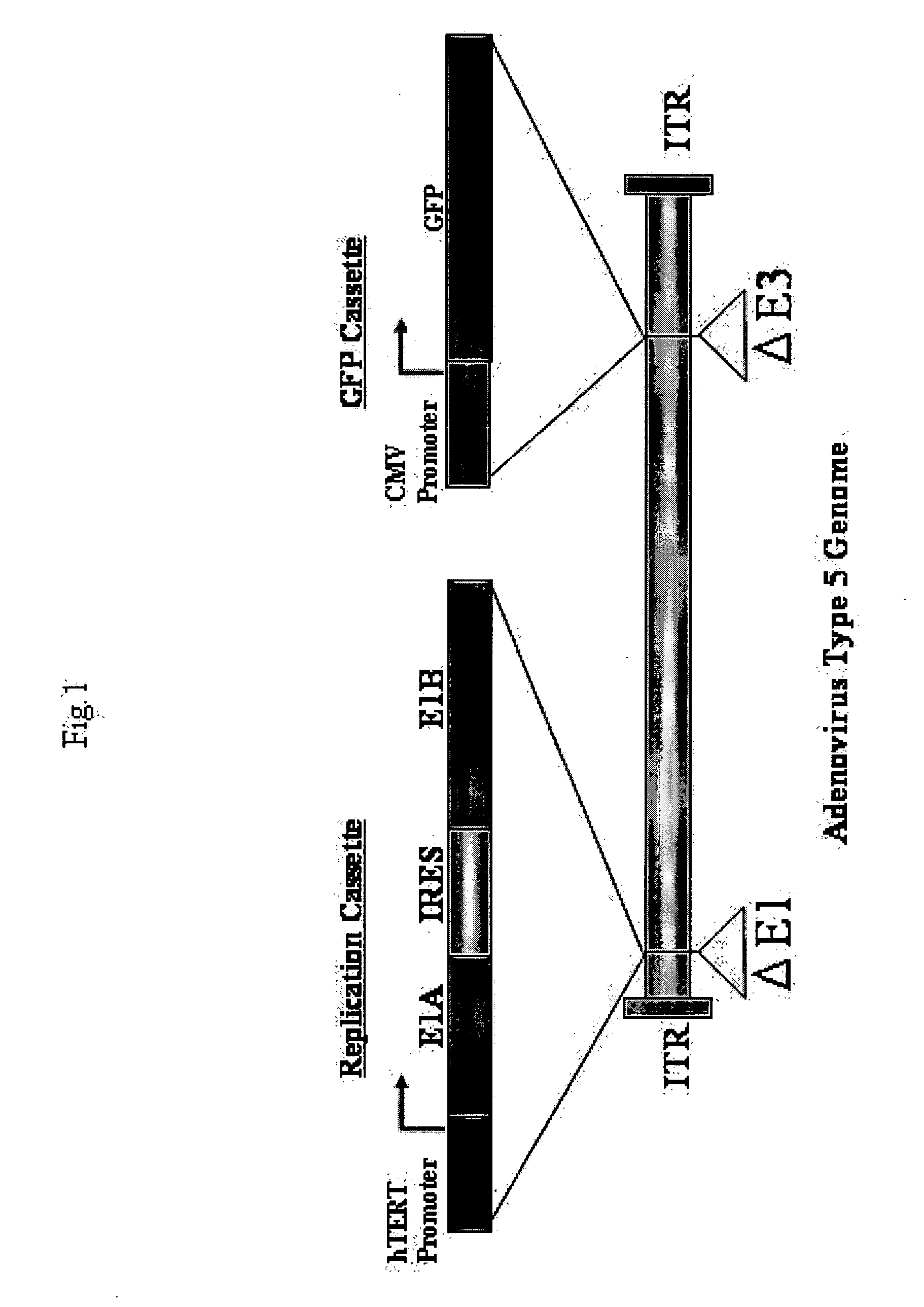
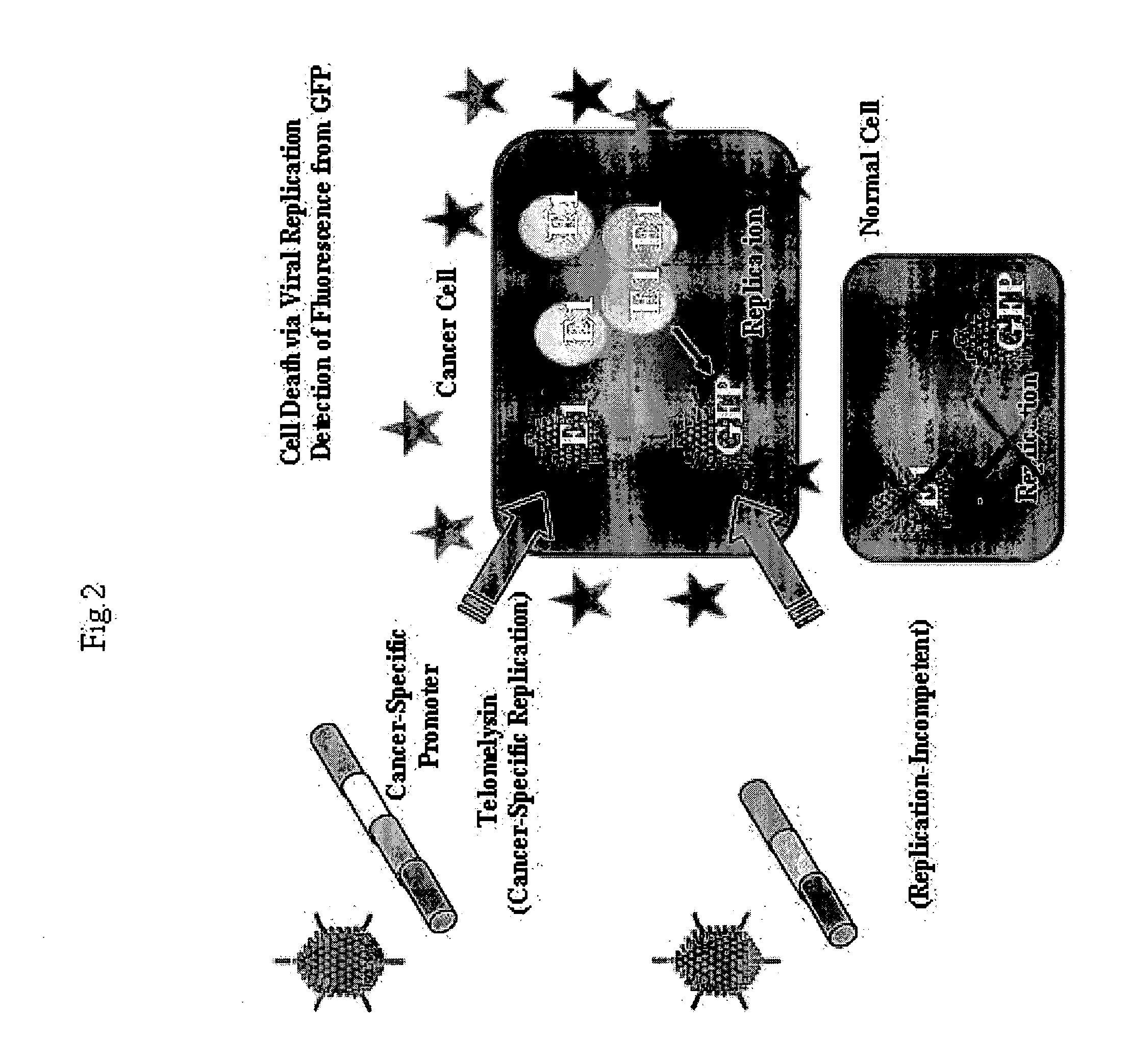
Telomelysin/GFP-expressing recombinant virus
ActiveUS20060067890A1High sensitivityUltrasonic/sonic/infrasonic diagnosticsVirusesTelomeraseCancer cell
The present invention provides a reagent for cancer cell detection or cancer diagnosis. The present invention relates to a reagent for cancer cell detection, comprising a recombinant virus where a replication cassette comprising a promoter from human telomerase, an E1A gene, an IRES sequence and an E1B gene in this order is integrated in E1 region of the viral genome and a labeling cassette comprising a gene encoding a labeling protein and a promoter capable of regulating the expression of the gene encoding the labeling protein is integrated in E3 region of the viral genome.
Owner:ONCOLYS BIOPHARMA
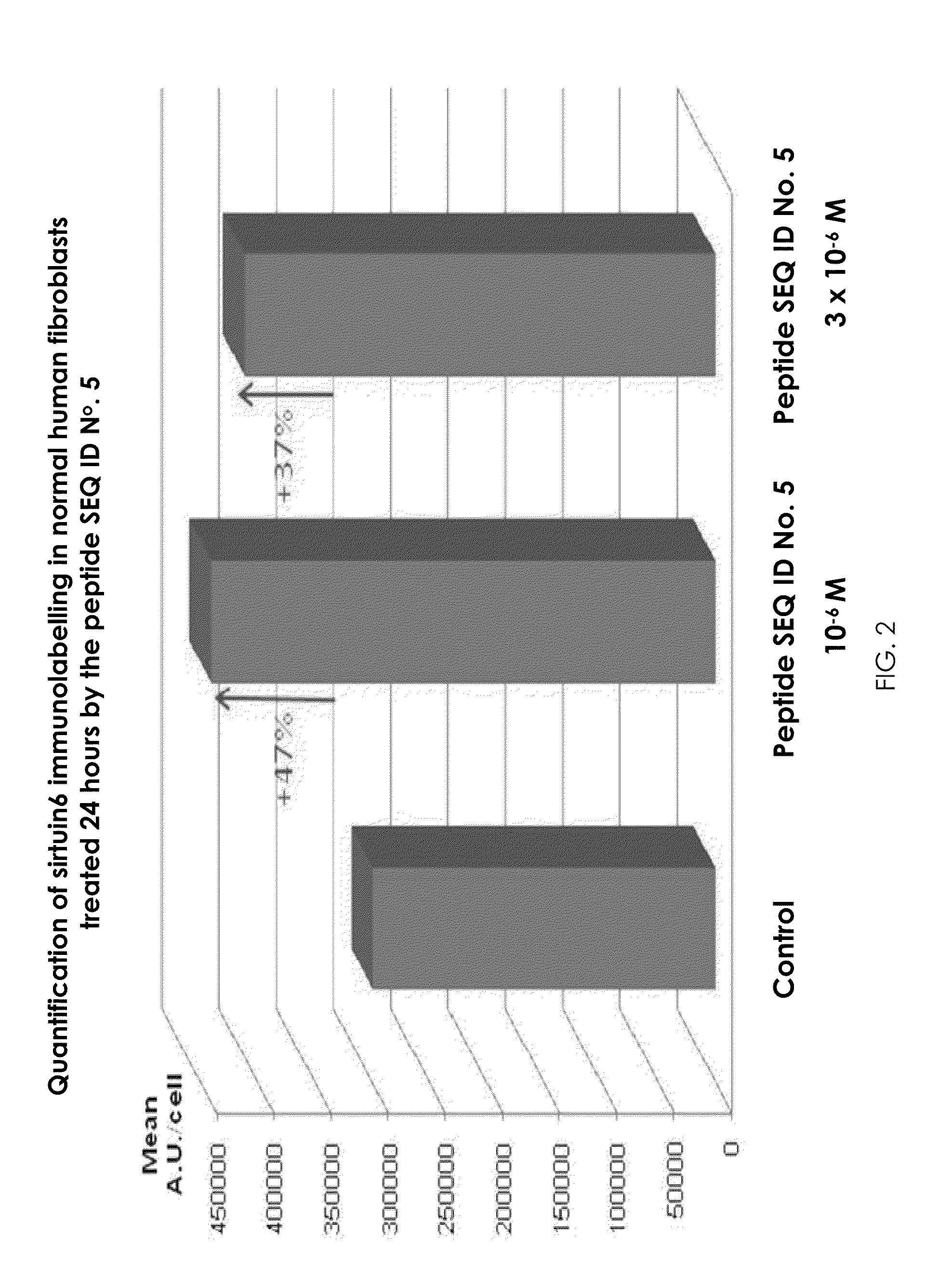
Novel sirtuin 6 activating peptides and cosmetic or pharmaceutical composition containing them
ActiveUS20110318284A1Cosmetic preparationsPeptide/protein ingredientsPhoto agingTraditional medicine
The present invention relates to sirtuin 6 activating peptides, derived from highly conserved regions of human SIRT proteins, and to a cosmetic or pharmaceutical composition comprising at least one sirtuin 6 activating peptide in a physiologically acceptable medium. The invention further relates to the utilization of a cosmetic composition to prevent and / or repair DNA degradation, improve telomere maintenance and reduce cellular senescence. The invention also applies to a cosmetic treatment process intended to prevent and / or treat the cutaneous signs of aging and photo aging.
Owner:ELC MANAGEMENT LLC +1
Reducing non-target nucleic acid dependent amplifications: amplifying repetitive nucleic acid sequences
ActiveUS7695904B2Sugar derivativesMicrobiological testing/measurementNucleic acid sequencingRepetitive Regions
The present invention provides for compositions and methods for amplifying target nucleic acids using nucleic acid primers designed to limit non-target nucleic acid dependent priming events. The present invention permits amplifying and quantitating the number of repetitive units in a repetitive region, such as the number of telomere repetitive units.
Owner:UNIV OF UTAH RES FOUND
Antiviral oligonucleotides having a conserved G4 core sequence
InactiveUS20070015723A1Significant antiviralEffective activityBiocidePeptide/protein ingredientsPhospholipasePhospholipase A
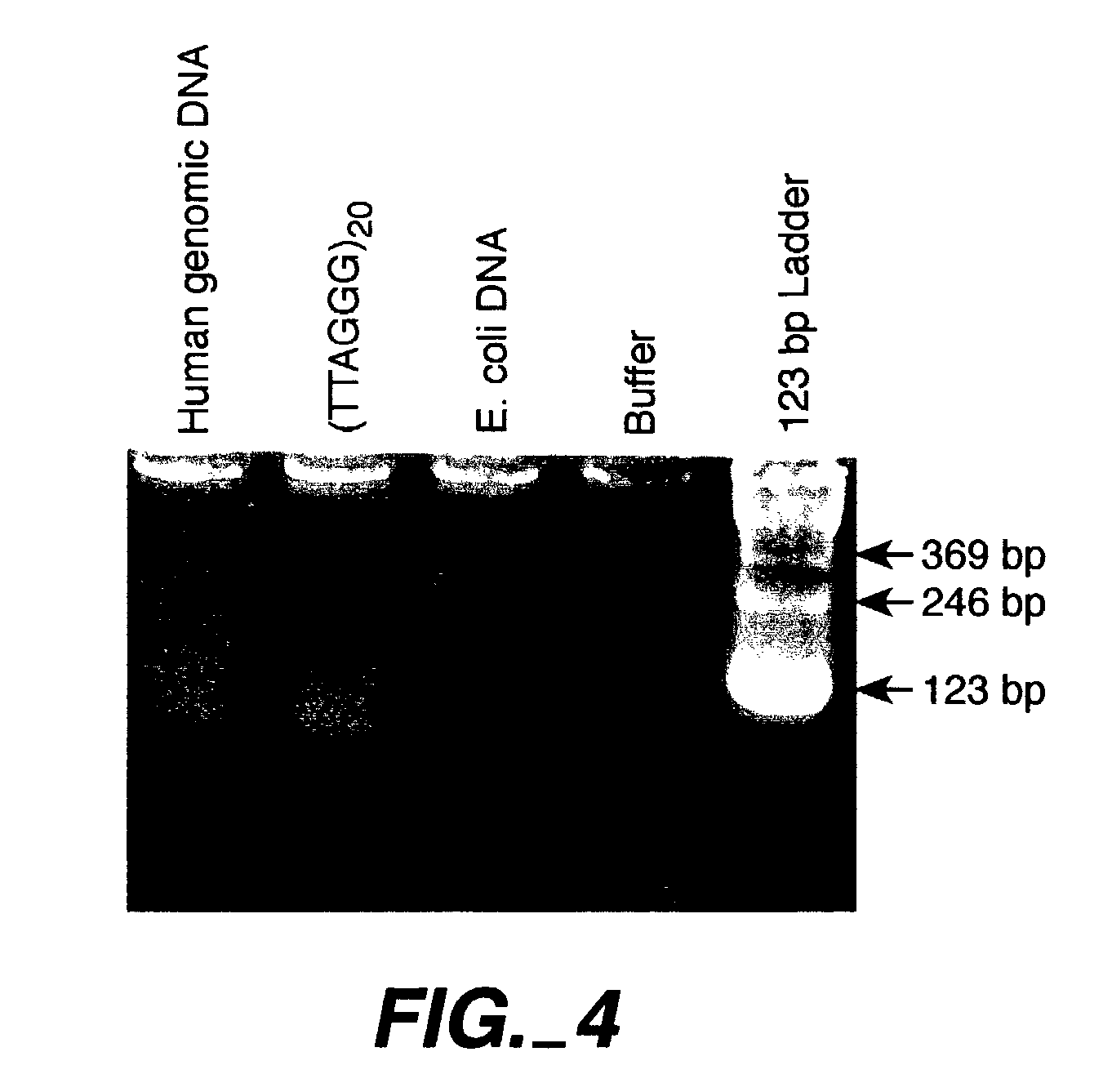
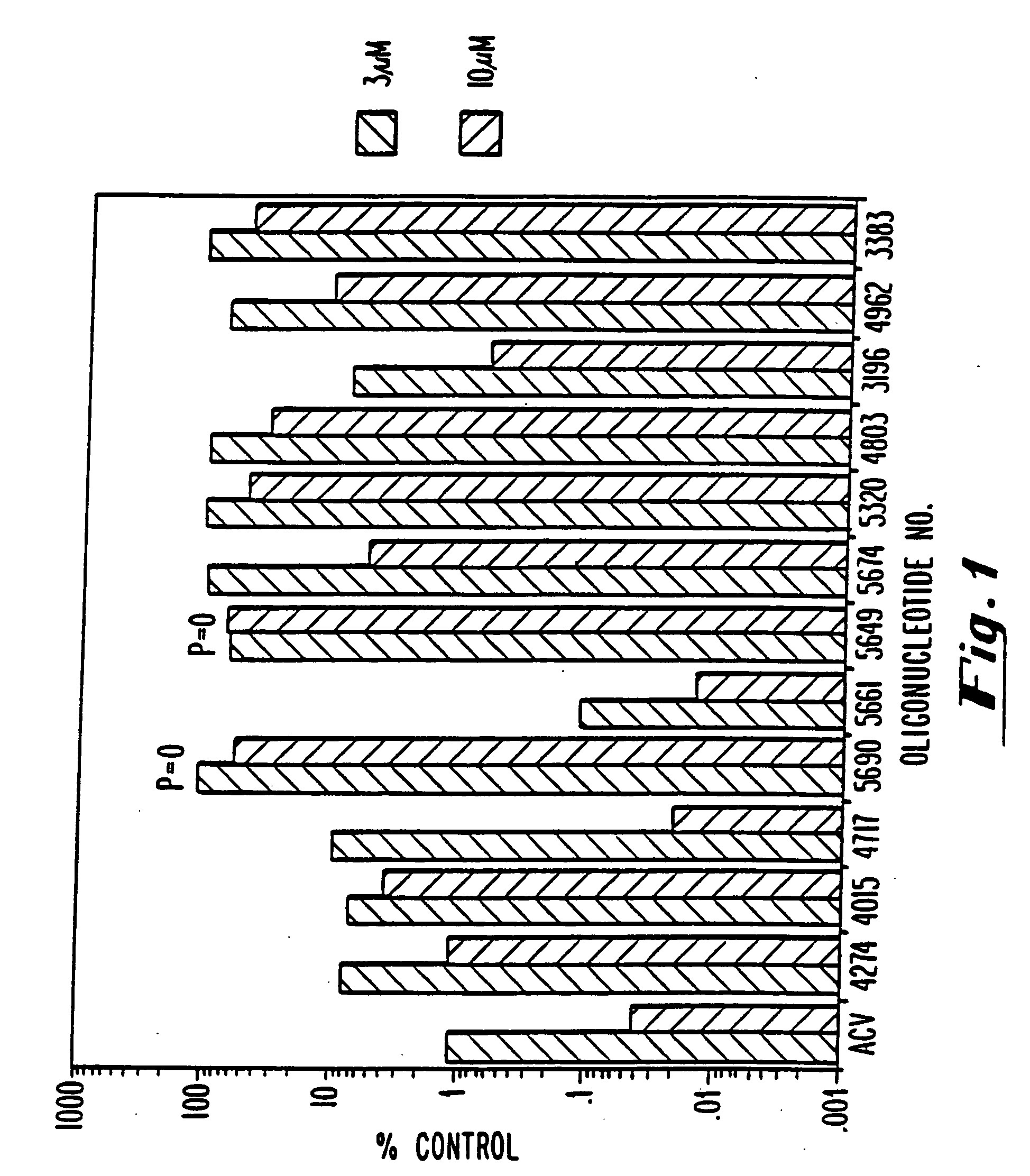
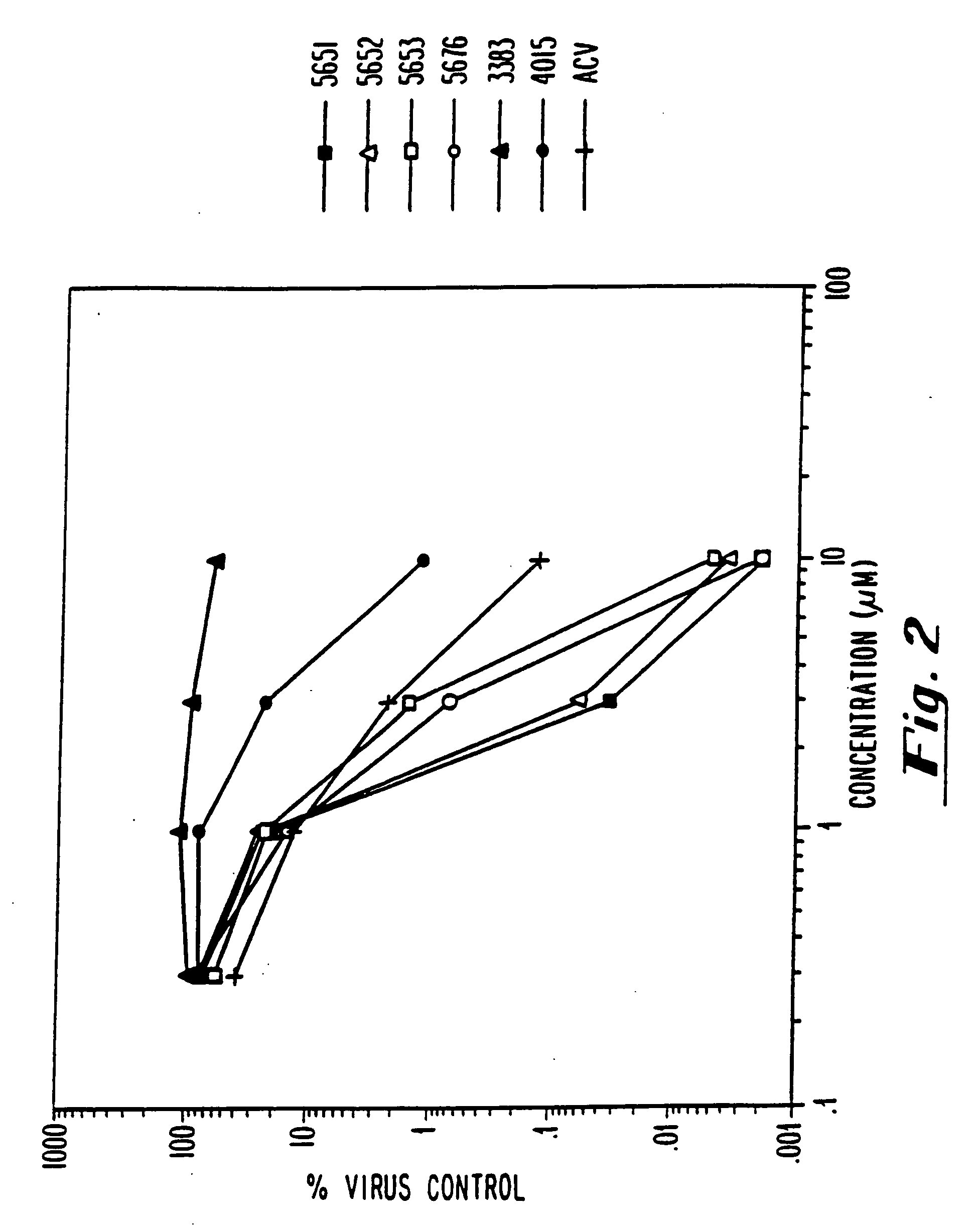
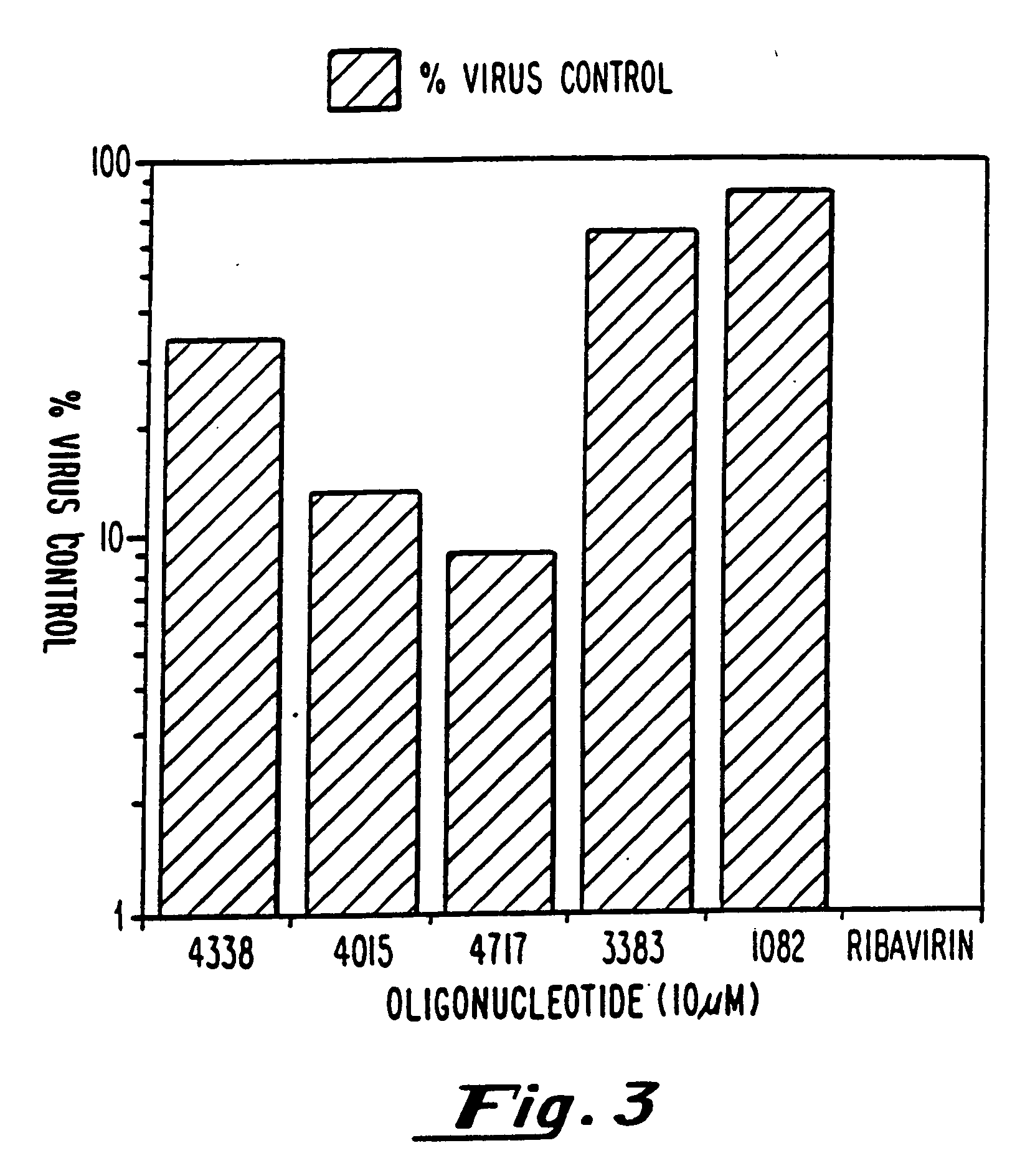
Modified oligonucleotides having a conserved G4 sequence and a sufficient number of flanking nucleotides to significantly inhibit the activity of a virus or phospholipase A2 or to modulate the telomere length of a chromosome are provided. G4 quartet oligonucleotide structures are also provided. Methods of prophylaxis, diagnostics and therapeutics for viral-associated diseases and diseases associated with elevated levels of phospholipase A2 are also provided. Methods of modulating telomere length of a chromosome are also provided; modulation of telomere length is believed to play a role in the aging process of a cell and in control of malignant cell growth.
Owner:IONIS PHARMA INC
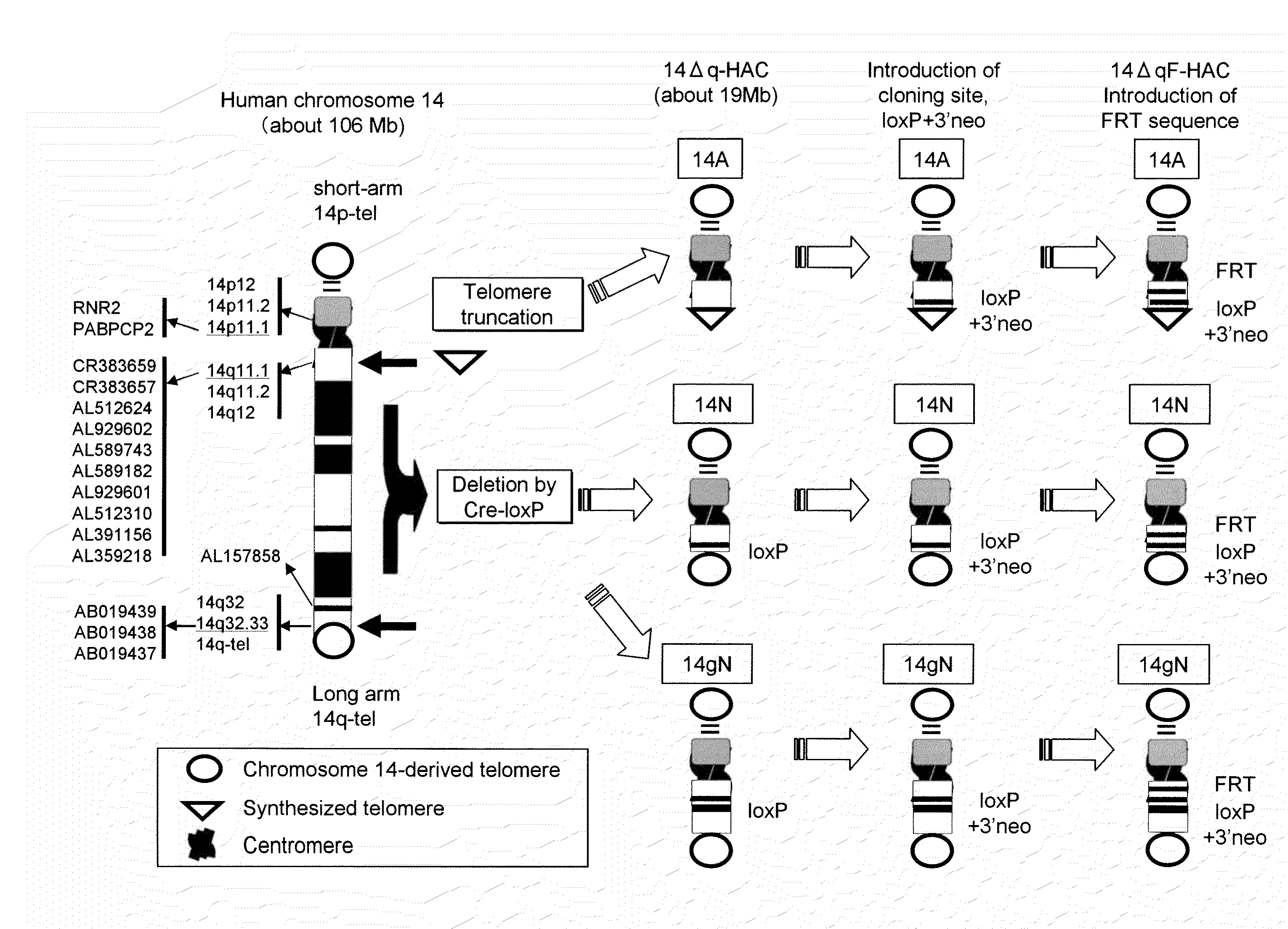
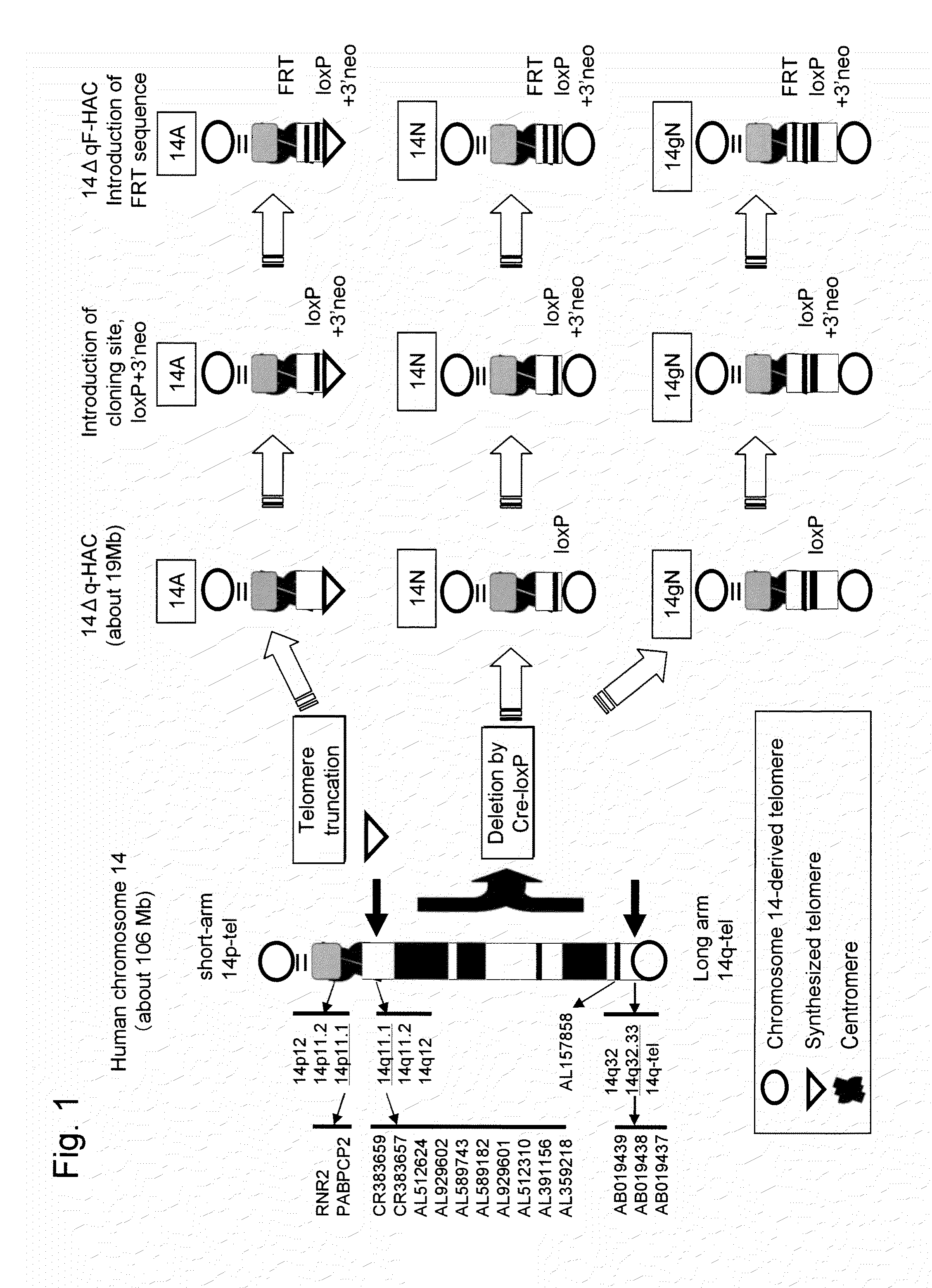
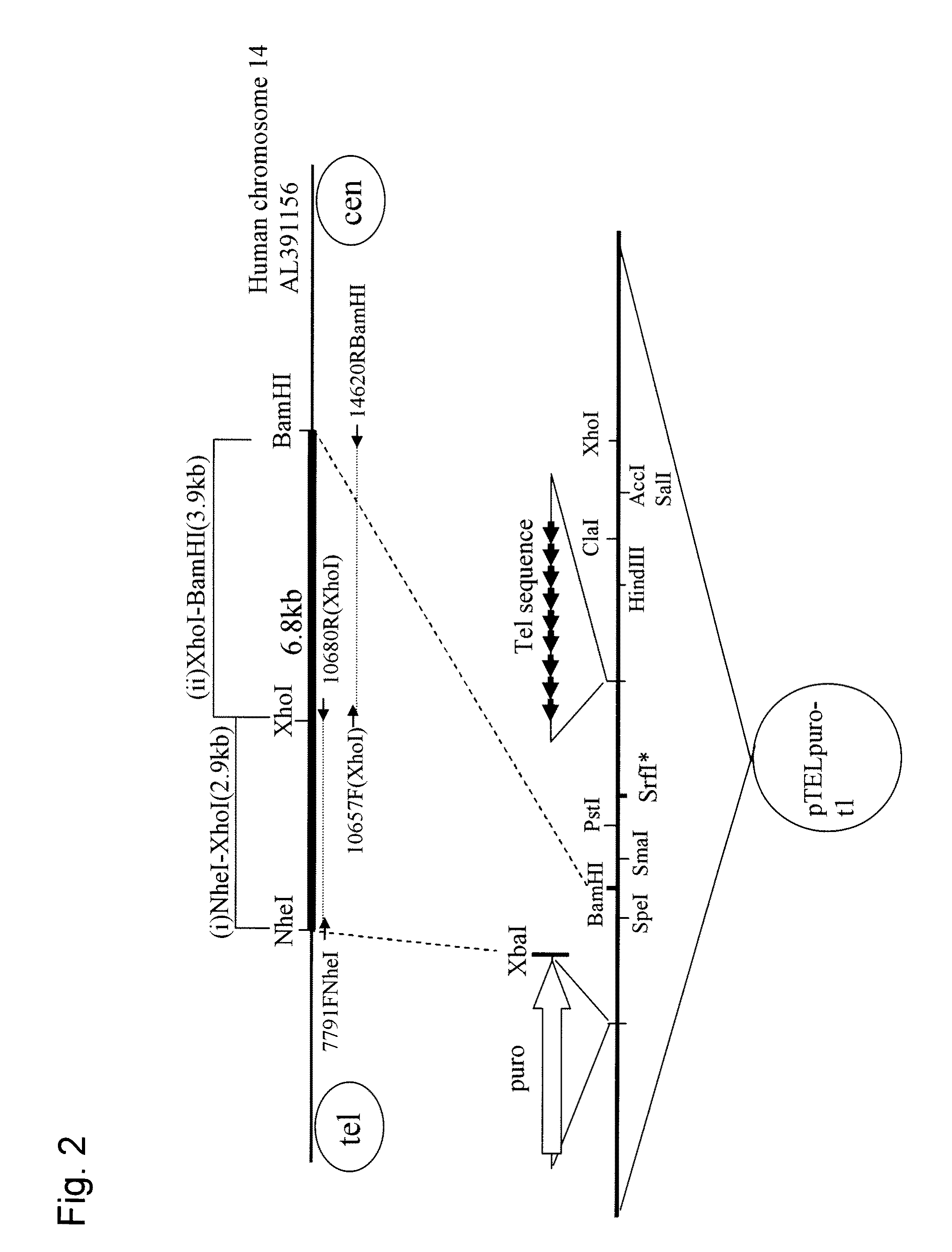
Human artificial chromosome (HAC) vector and human cell medicine comprising same
ActiveUS20100011454A1Improve expression levelPositively affect stable maintenanceBiocideNervous disorderHuman artificial chromosomeTherapeutic protein
This invention relates to a human artificial chromosome (HAC) vector carrying a human chromosome-derived centromere, a subtelomere sequence, and a telomere sequence, to a human cell medicine or human cells comprising the HAC vector, to methods for preparing the HAC vector and human cells, and to methods for producing a therapeutic protein using the HAC vector.
Owner:KYOWA HAKKO KIRIN CO LTD
Diagnosis and treatment of cervical cancer
InactiveUS20070065810A1Reduce expressionReduced gene expressionPeptide/protein ingredientsTransferrinsCervical cellsCervical lesion
In certain aspects, the invention relates to methods of diagnosing cervical cancer by using a combination of certain biomarkers such as hTERT, IGFBP-3, transferrin receptor, beta-catenin, Myc-HPV E6 interaction, HPV E7, and telomere length. In other aspects, the invention relates to methods of detecting immortalization of cervical cells by using a combination of certain biomarkers. In yet other aspects, the invention relates to methods of classifying the grade of a cervical lesion for diagnostic and prognostic purposes in a female. In further aspects, the invention relates to methods of treating cervical cancer by administering a therapeutic agent that targets one or more of these biomarkers.
Owner:GEORGETOWN UNIV
Fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy
ActiveCN103614477AAvoid differencesMicrobiological testing/measurementNeuronal Apoptosis-Inhibitory ProteinFluorescence
The invention relates to a fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplification primers and fluorescent probes. The fluorescent quantitative PCR kit is characterized in that the amplification primers include a pair of shared primers used for specifically amplifying SMN1 (Survival Motor Neuron 1) and SMN2 (Survival Motor Neuron 2) genes, a pair of primers used for specifically amplifying an NAIP (Neuronal Apoptosis Inhibitory Protein) gene and a pair of primers used for specifically amplifying a reference sequence; the fluorescent probes include a fluorescent probe used for specifically detecting the SMN1 gene, a fluorescent probe used for specifically detecting the SMN2 gene, a fluorescent probe used for specifically detecting the NAIP gene and a fluorescent probe used for specifically detecting the reference sequence; the reference sequence is as shown in SEQ NO.14,and is positioned in a GRCh37 / hg19chr5:71107506-71107618 interval positioned on the 78kb position of the telomere side of the NAIP gene. The fluorescent quantitative PCR kit disclosed by the invention remarkably enhances the accuracy and stability of diagnosis by taking the DNA (Desoxvribose Nucleic Acid) sequence positioned at the telomere side of the NAIP gene as the reference sequence.
Owner:SOUTHERN MEDICAL UNIVERSITY
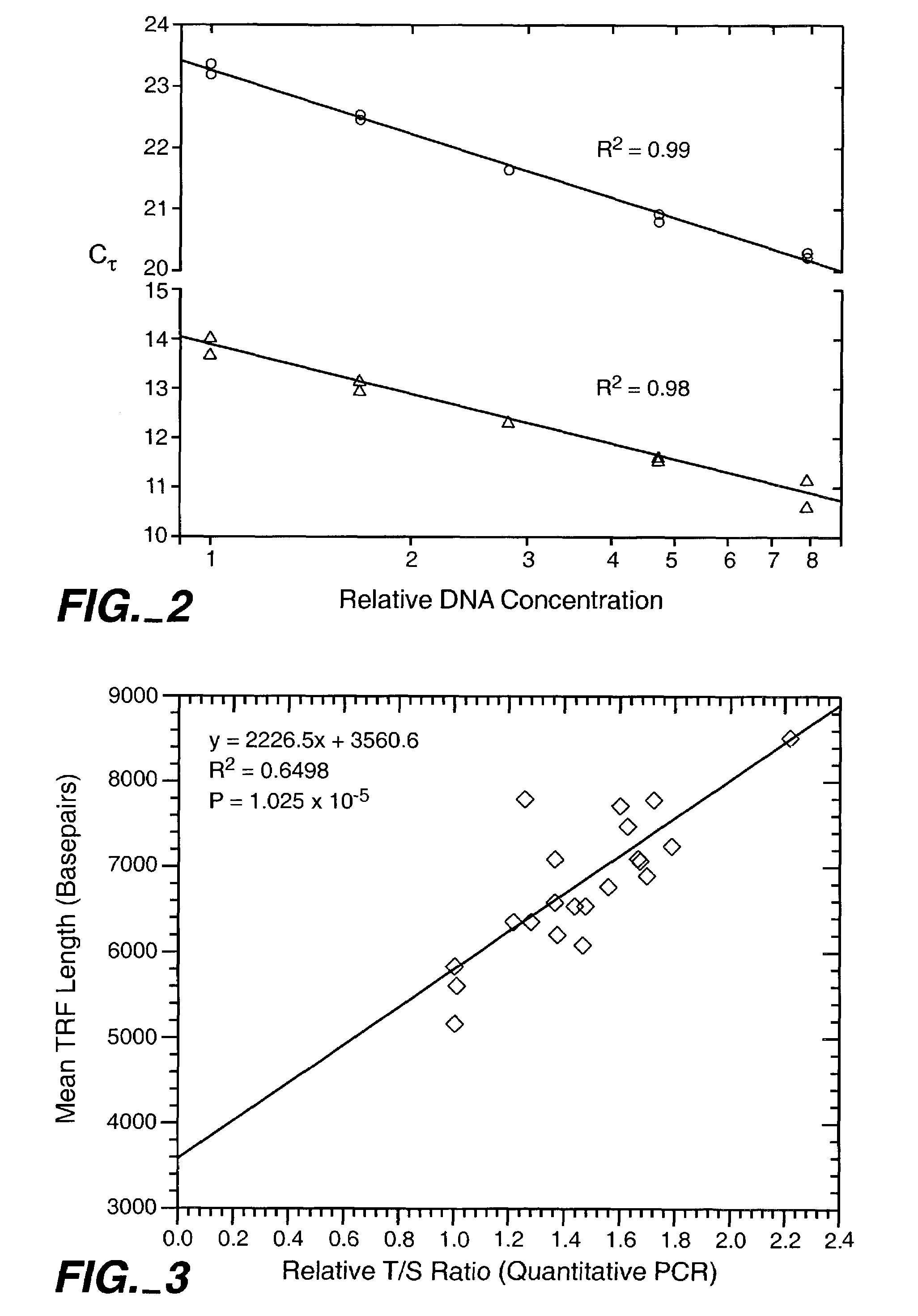
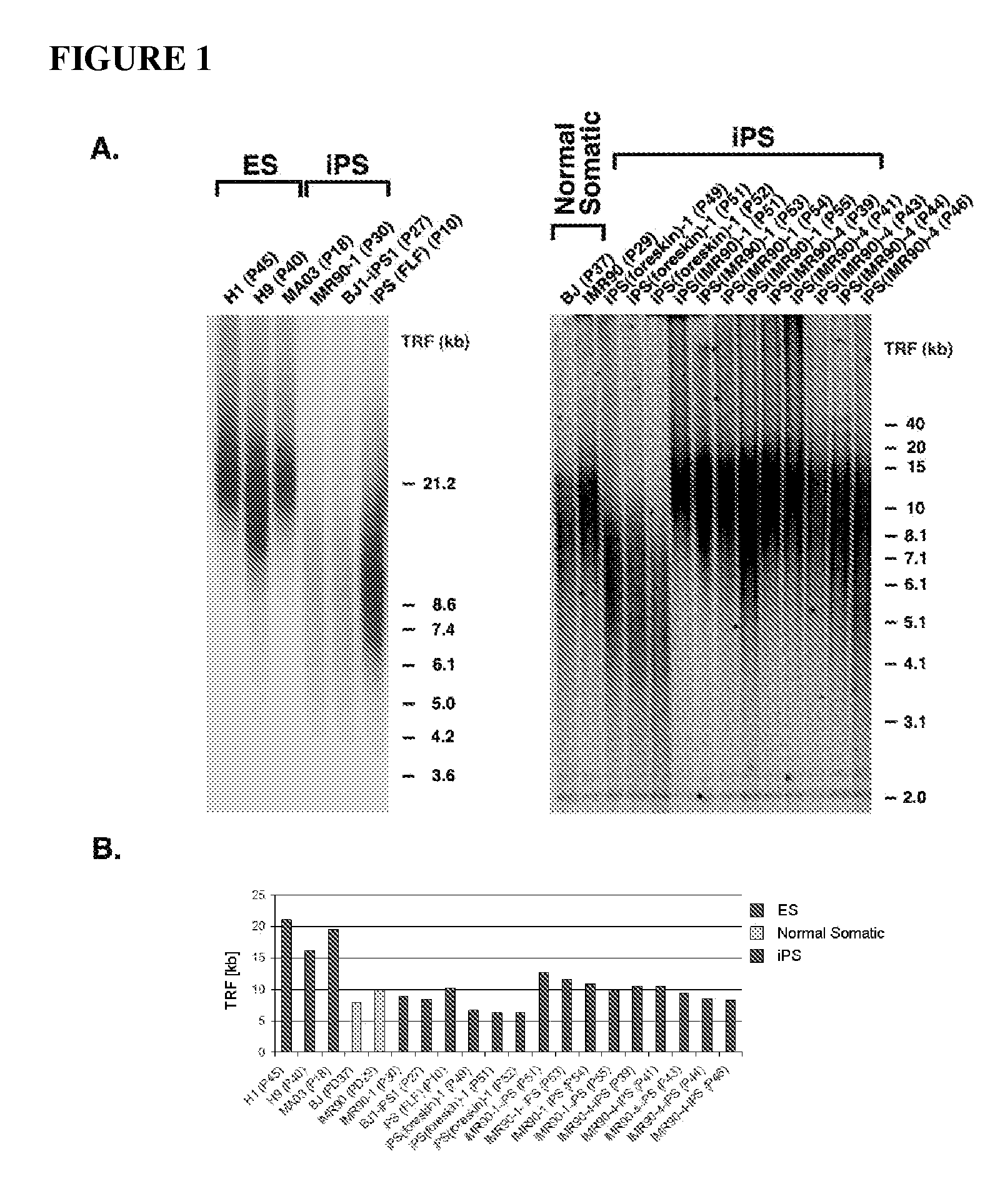
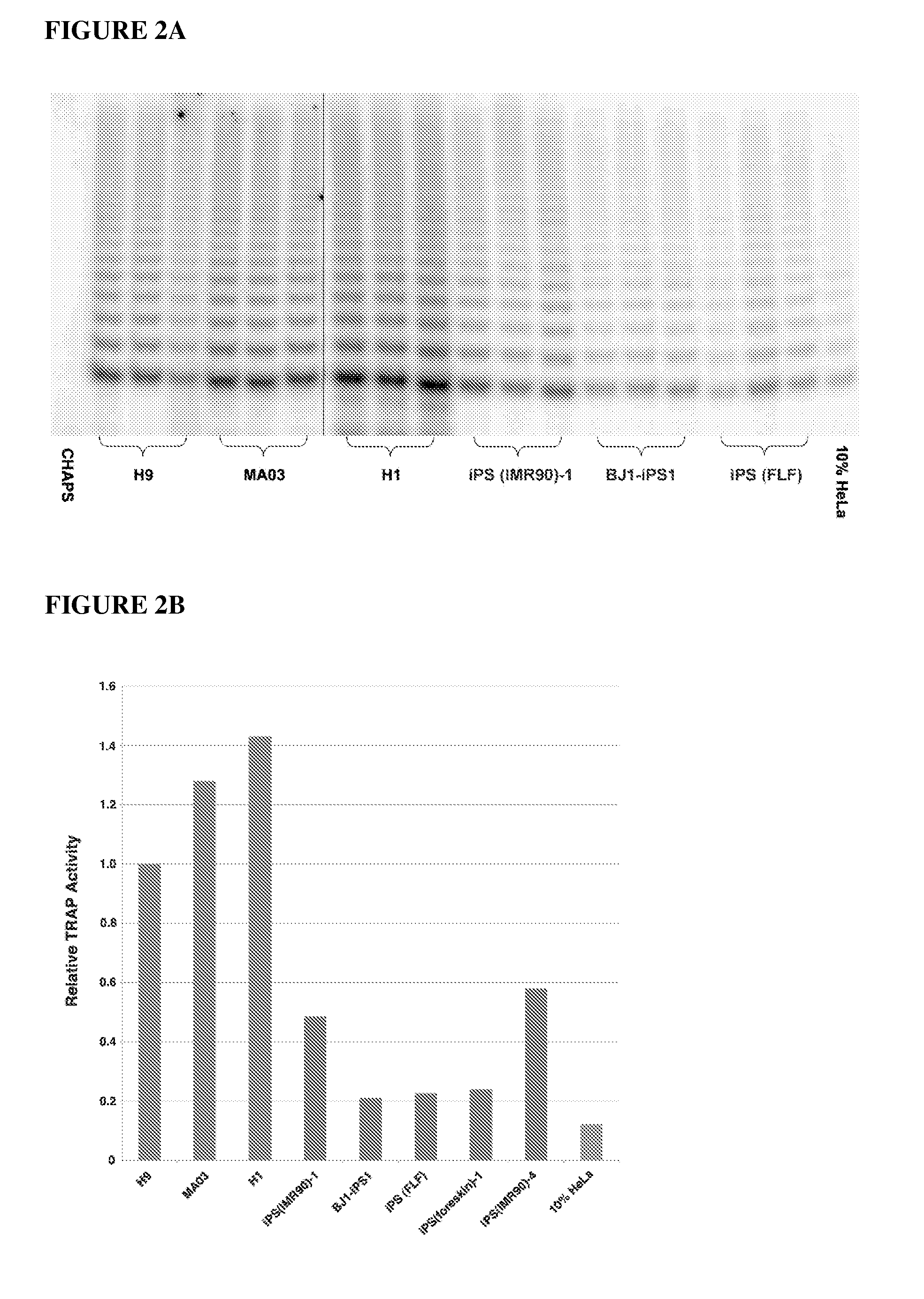
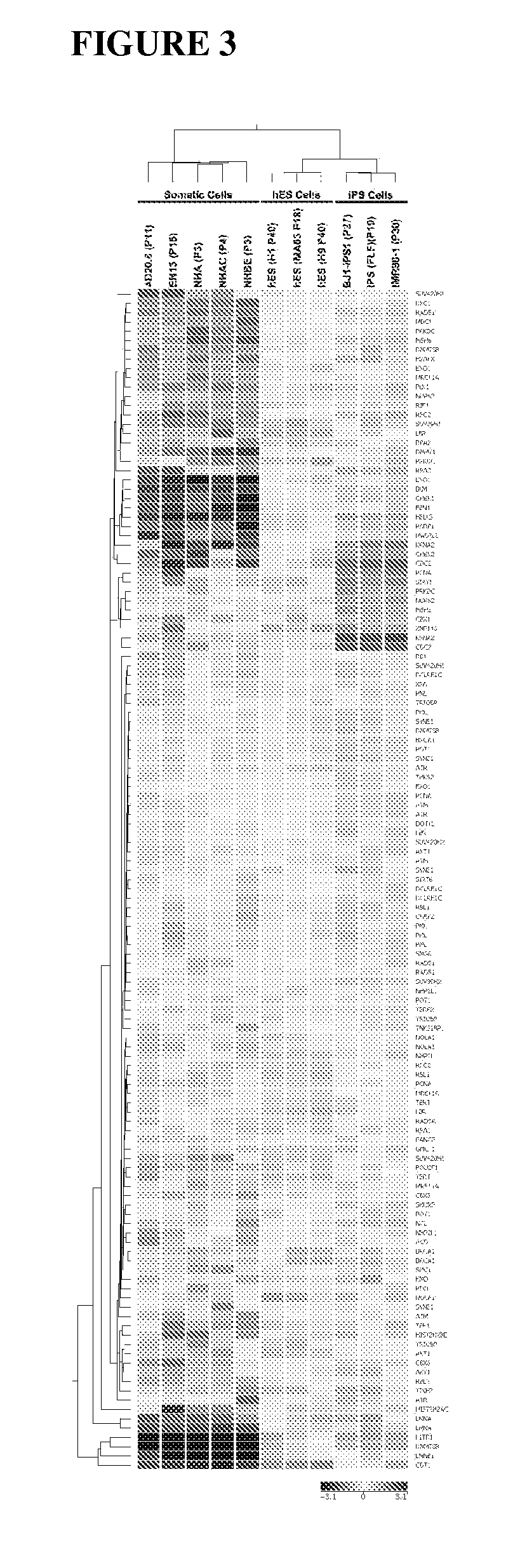
Methods for telomere length and genomic DNA quality control and analysis in pluripotent stem cells
InactiveUS20130011918A1Reduce riskDesired phenotypeMicrobiological testing/measurementLibrary screeningPluripotential stem cellClinical grade
The generation of clinical-grade cell-based therapies from human embryonic stem cells or cells reprogrammed to pluripotency from somatic cells, requires stringent quality controls to insure that the cells have long enough telomeres and resulting cellular lifespan to be clinically useful, and normal gene expression and genomic integrity so as to insure cells with a desired and reproducible phenotype and to reduce the risk of the malignant transformation of cells. Assays useful in identifying human embryonic stem cell lines and pluripotent cells resulting from the transcriptional reprogramming of somatic cells that have embryonic telomere length are described as well as quality control assays for screening genomic integrity in cells expanded and banked for therapeutic use, as well as assays to identify cells capable of abnormal immortalization,
Owner:LINEAGE CELL THERAPEUTICS INC
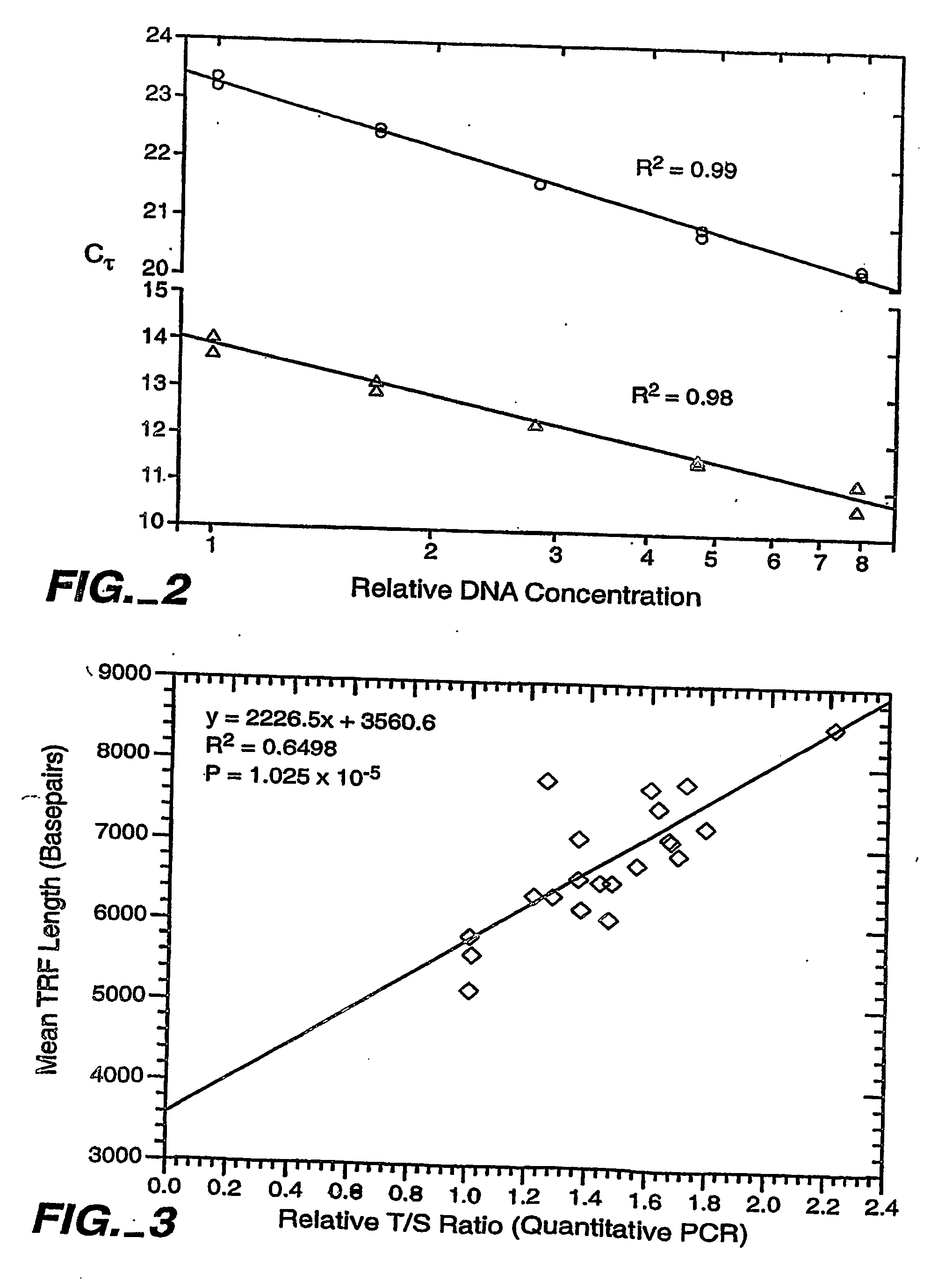
Method for Estimating Telomere Length
InactiveUS20110244462A1Overcome problemsMinimizing handlingMicrobiological testing/measurementMedicineOligonucleotide
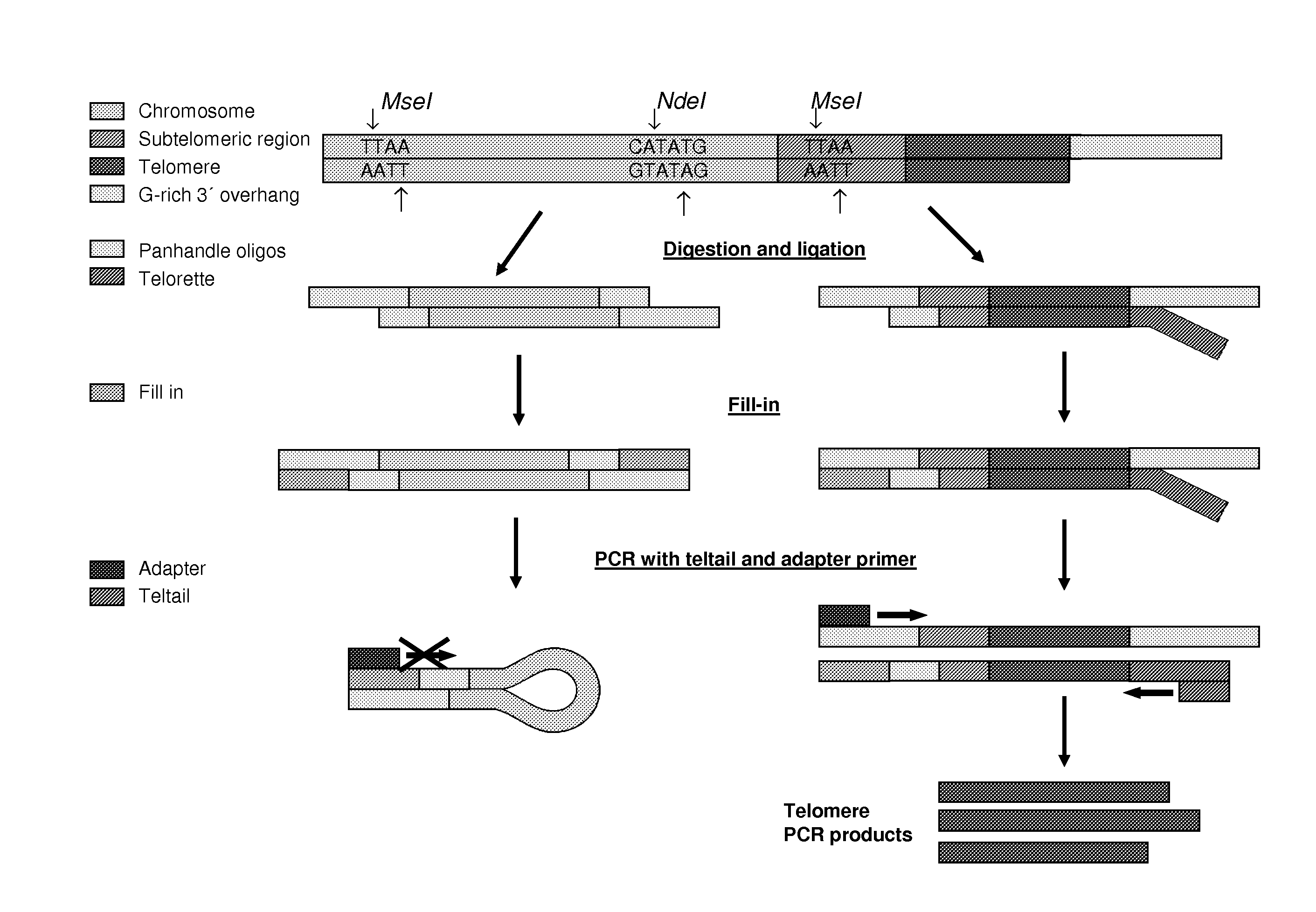
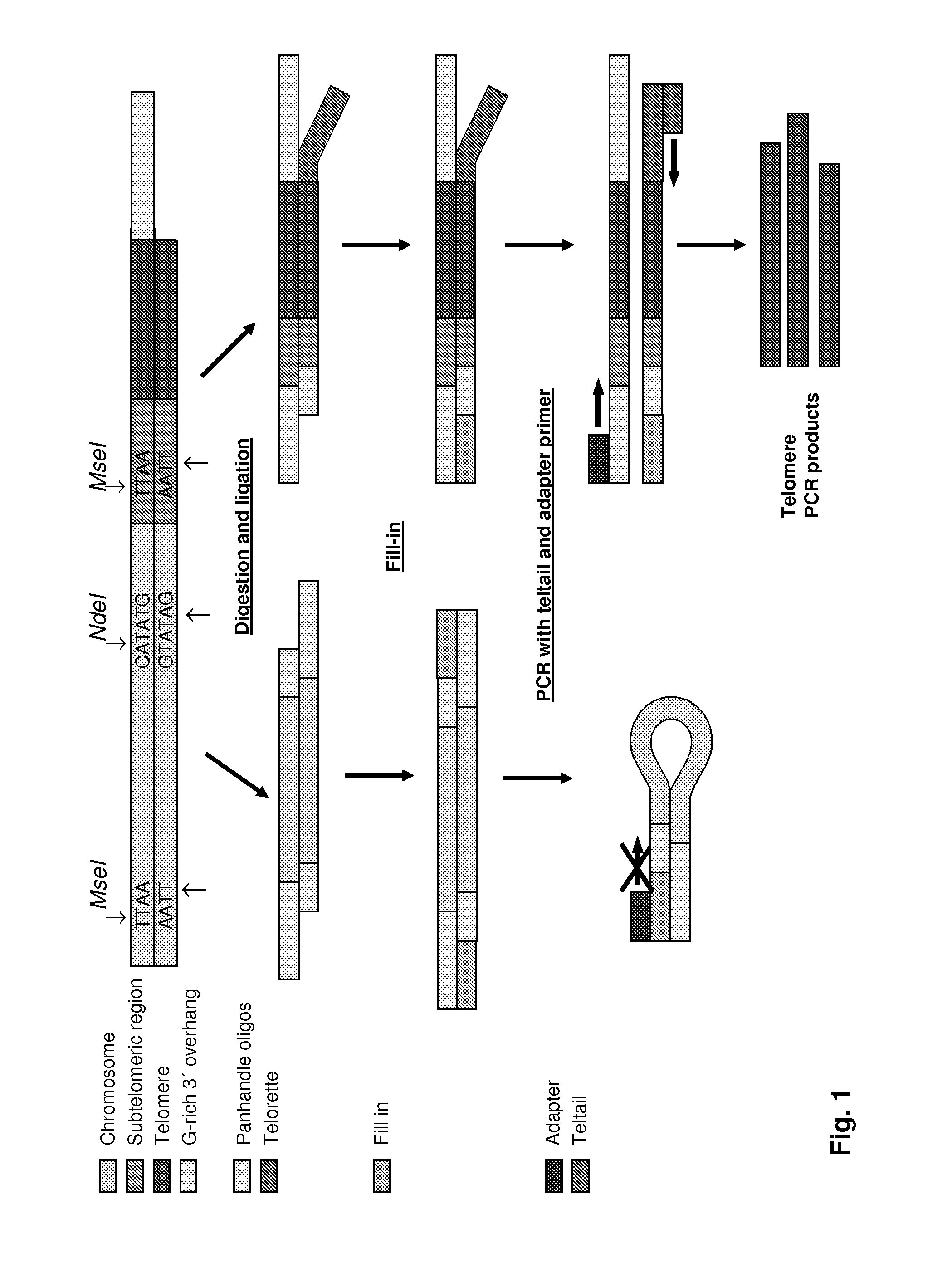
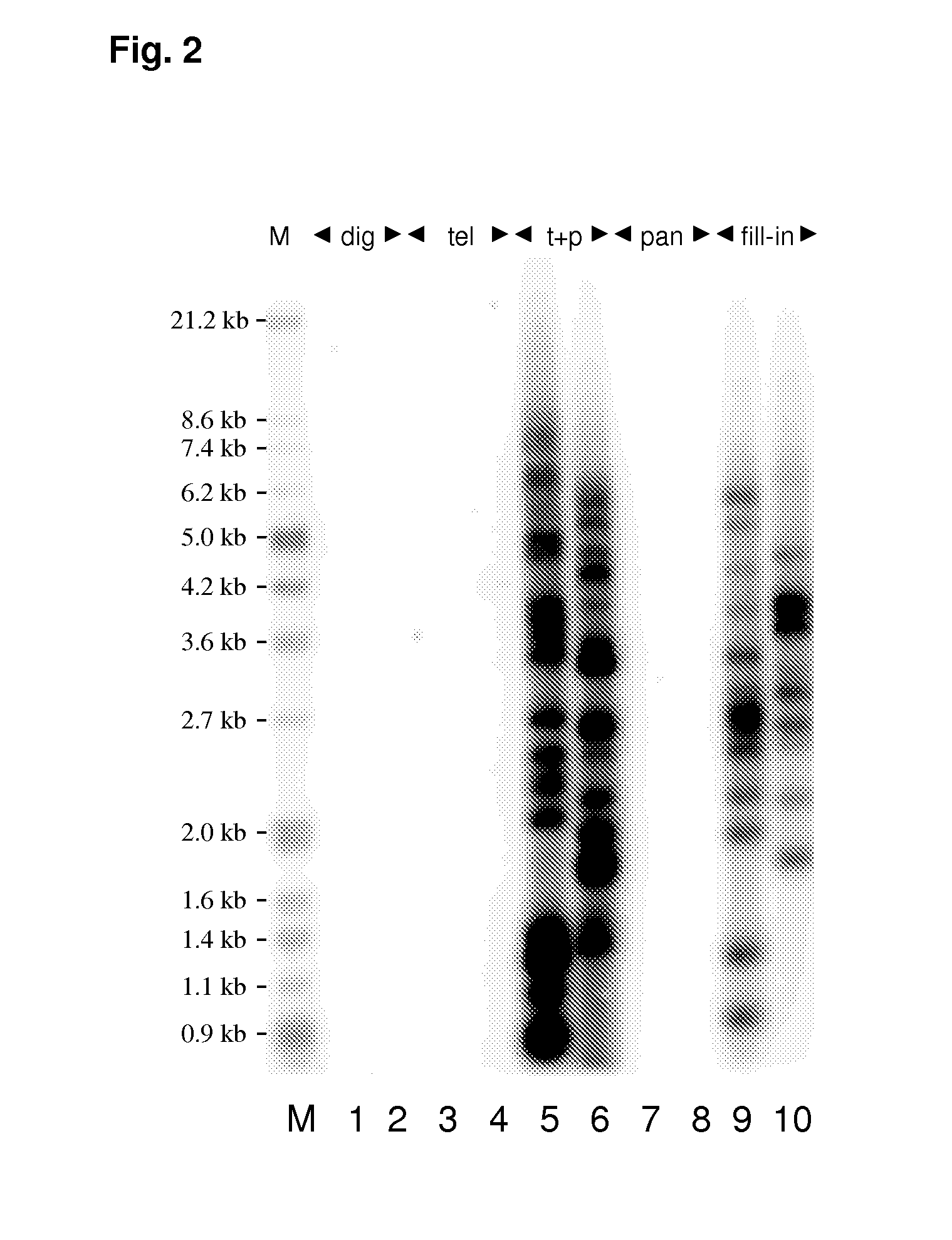
Knowledge about telomere length is highly relevant in cancer and age related research. Currently applied methods for determining telomere length are subject to several drawbacks preventing fast and reliable information concerning telomere length. The present invention relates to a method for determining telomere length which is fast and reliable. The method is PCR based and may advantageously be performed in a “one tube system”, whereby time consuming and inconvenient handling steps are avoided. The method comprises annealing of up- and downstream tags to telomere fragments and subsequent PCR amplification of telomere fragments using primers having a sequence complementary or identical to at least part of the up- and downstream oligonucleotide tags.
Owner:TINA HLDG APS
Peanut oligonucleotide probes and their design method and use method
ActiveCN106987590AImprove general performanceMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMicrosatellite
The invention discloses peanut oligonucleotide probes and their design method and use method. The peanut oligonucleotide probes comprise eight probes having nucleotide sequences shown in the formulas of SEQ ID NO. 1 to NO. 8. The microsatellite and telomere sequences are used for developing the oligonucleotide probes, the novel oligonucleotide probes are combined into a probe set and oligonucleotide types of the peanut cultivar and wild peanut are constructed, an economic, efficient and high universality peanut cytological marker design technique and chromosome recognition technique are built, peanut chromosome markers are enriched, genomes and chromosomes of the peanut cultivar and wild peanut are identified, and chromosomal structural variation of wild peanut species is identified. Through use of a high-throughput small data simplified sequencing (for only measuring the amount of genomic 4Gb data) and bioinformatics analysis, peanut oligonucleotide probe markers are successfully developed. The invention provides a novel effective method for low-cost and high-efficiency development of peanut cytological markers.
Owner:HENAN ACAD OF AGRI SCI
Polycation lipesome telomere enzyme antiseuse oligonucleotide complex and preparation
ActiveCN1936011AHigh inhibition rateImprove antisense efficacyGenetic material ingredientsGene therapyTelomeraseCholesterol
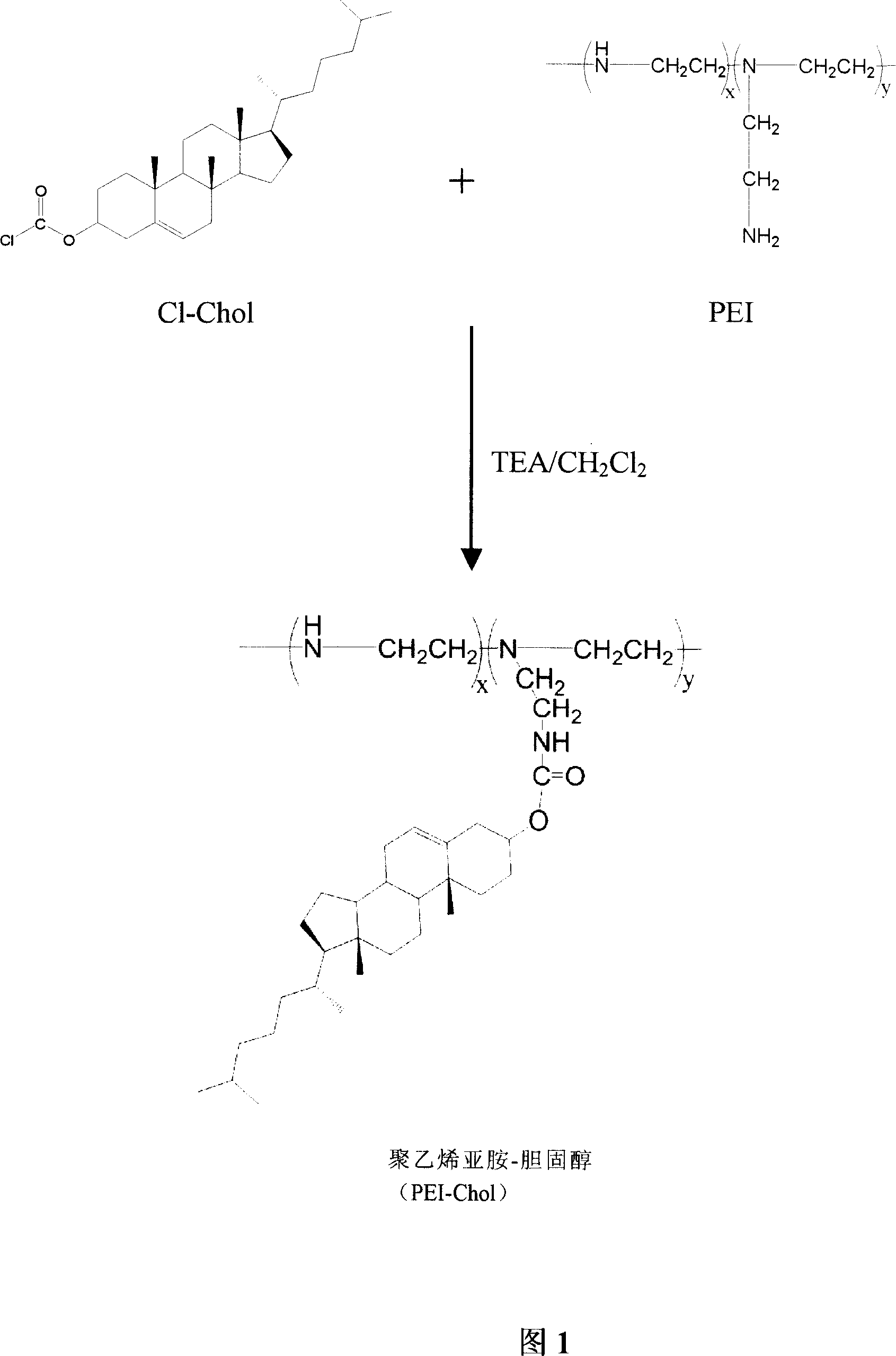
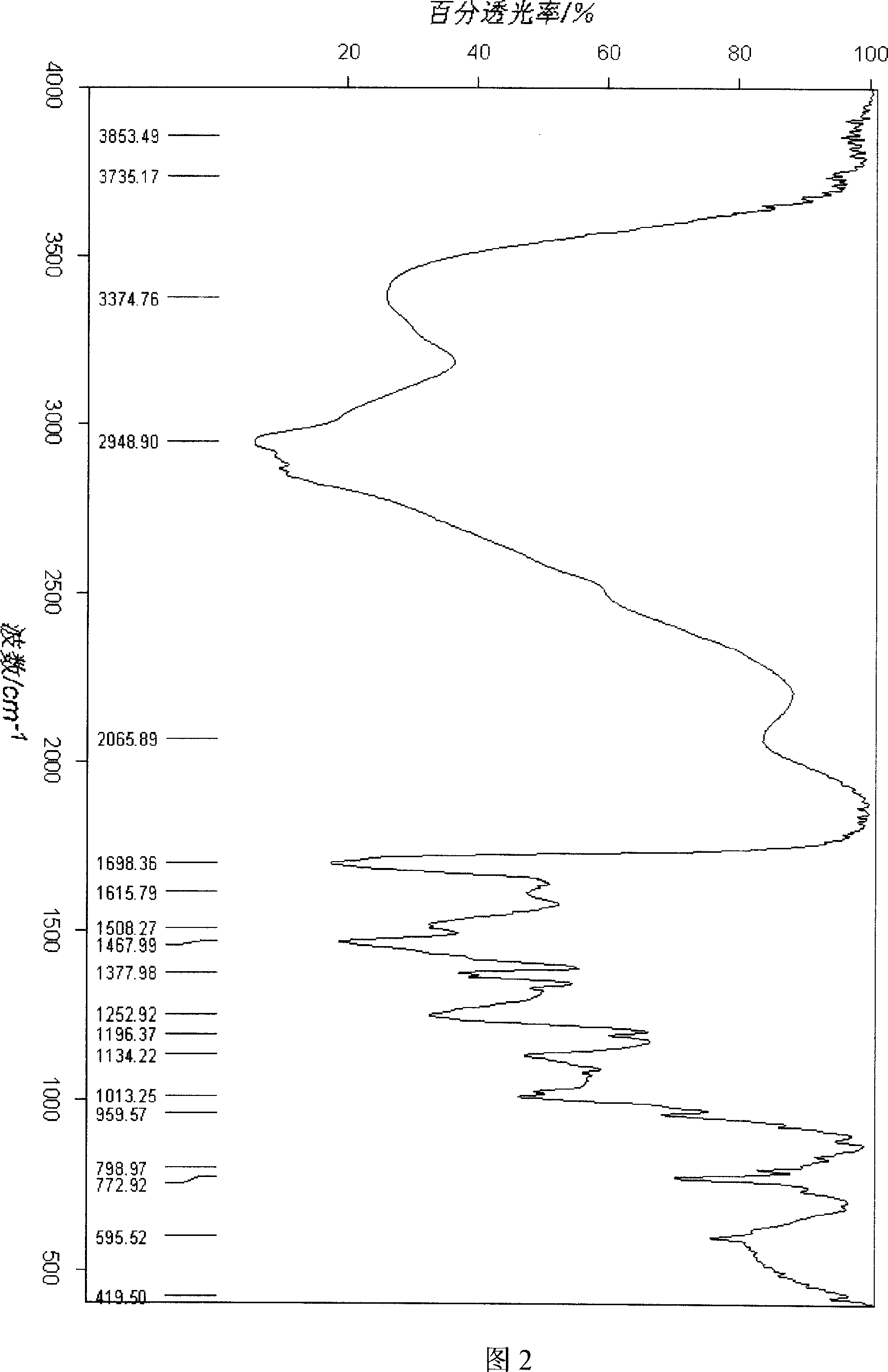
The invention supplies a polycation liposome telomere enzyme antisense oligonucleotide compound that is made up of polycation liposome and antisense oligonucleotide. It uses low molecular weight PEI and acyl chloride cholesterol as raw material to compound PEI-Chol, uses phospholipid, PEI-Chol and cholesterin as film material. The polycation liposome would be made through film dispersion method and reverse evaporation method. The compound could obviously improve ability of cell to absorb anti-hTERT and have higher inhibition ratio than that of cation polymer. The method is simple technology and widely application prospect.
Owner:ZHEJIANG PHARMA COLLEGE +1
Assays for determining telomere length and repeated sequence copy number
InactiveUS20110195864A1Microbiological testing/measurementLibrary screeningMultiplexingBioinformatics
Methods of detecting copy number of a repeated sequence element, including methods of determining telomere length, are provided. The methods can be multiplexed for detection of repeated sequence element copy number on two or more nucleic acid targets simultaneously. Compositions, kits, and systems related to the methods are also described.
Owner:AFFYMETRIX INC
Molecular marker method for avirulence gene of rice blast
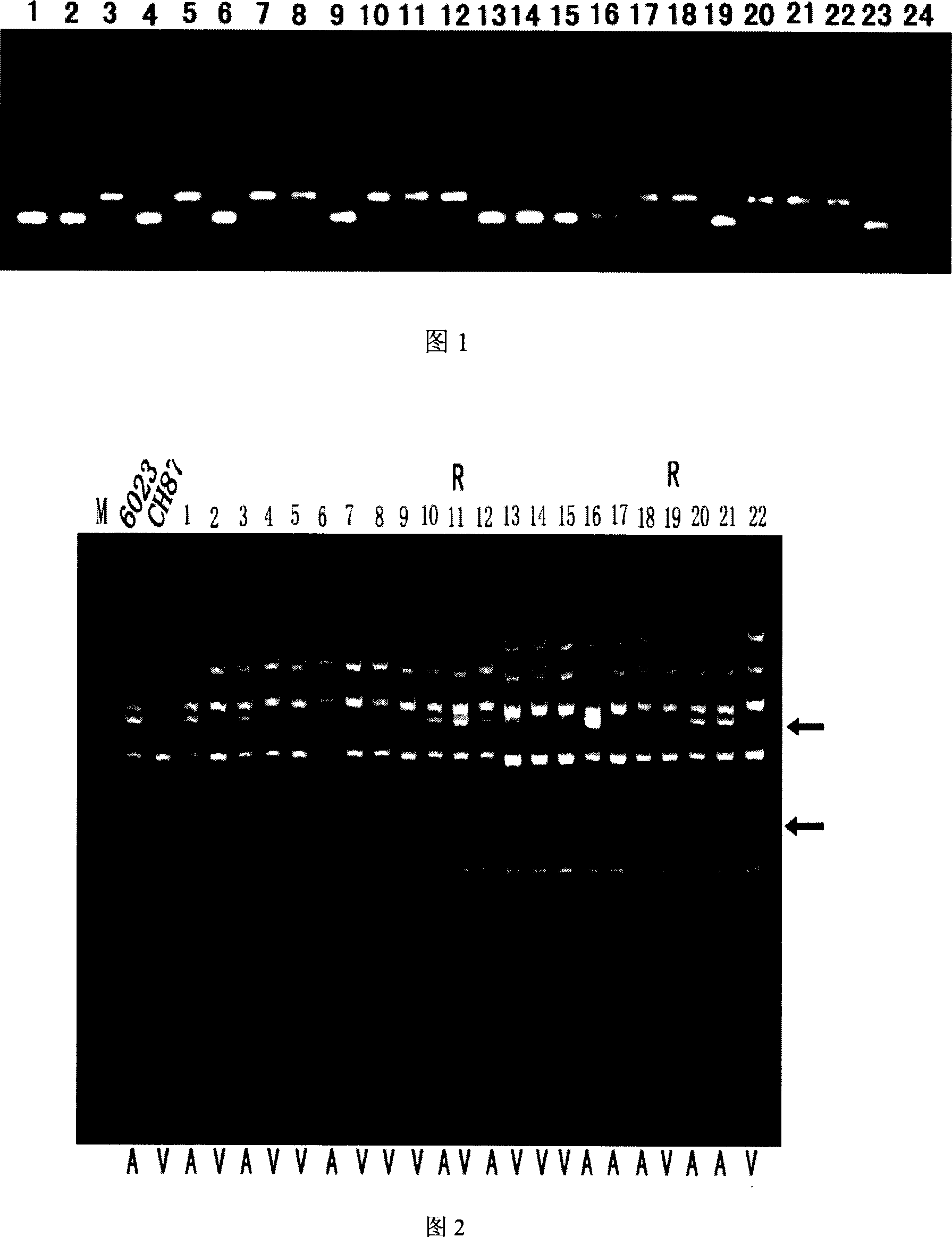
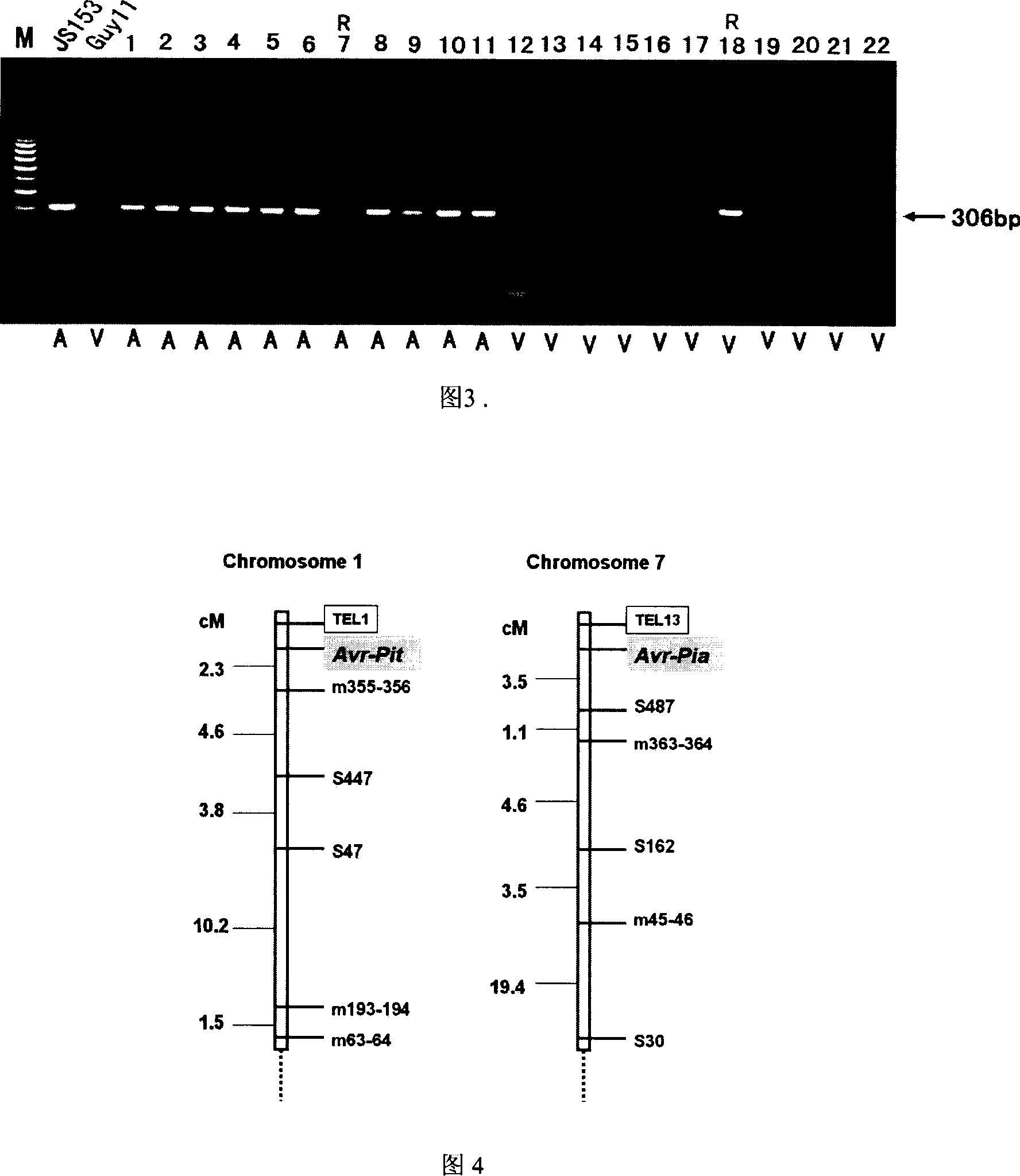
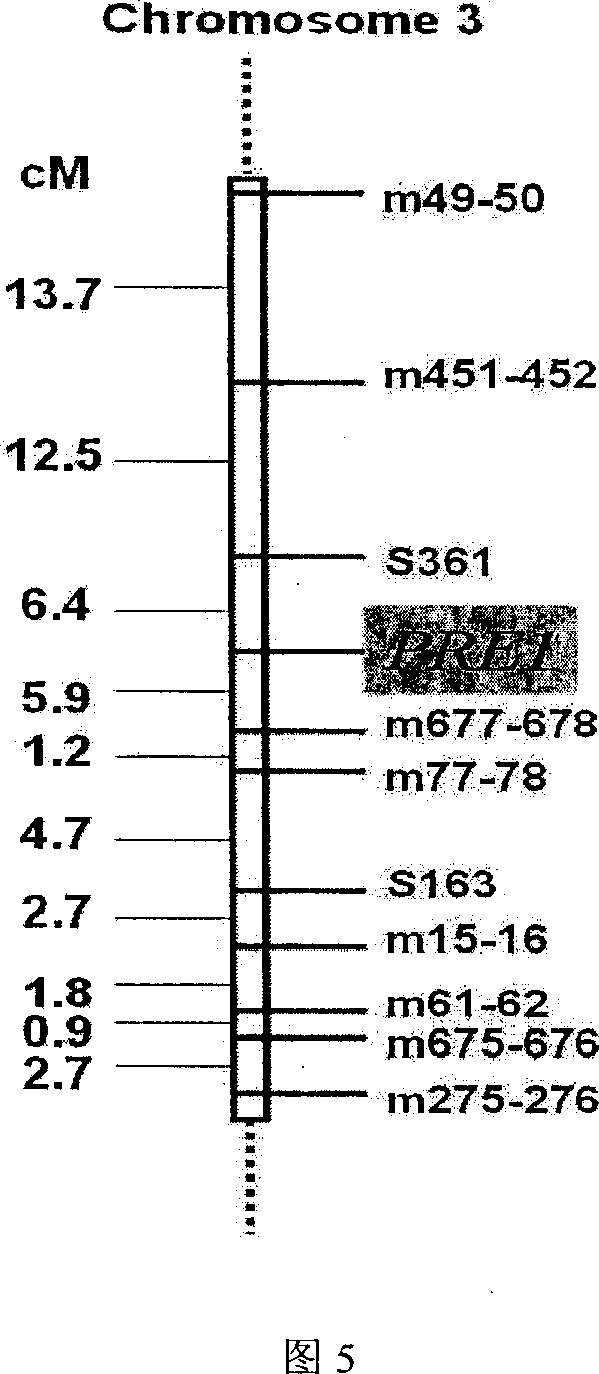
InactiveCN1952177AReveal Toxic ComponentsReveal featuresMicrobiological testing/measurementFermentationPyricularia griseaParental strain
The invention of molecular labeling method of Pyricularia grisea avirulent gene belongs to the field of agricultural biotechnology. The molecular labeling technology includes performing molecular labeling to avirulent gene though assessment from two parental strains and filial generation groups of Pyricularia grisea. M355-356 labeled by SSR near NO. 1 chromosome telomere links with avirulent gene Avr-Pit with a genetic distance of 2.3cm; S487 labeled by RAPD located near NO. 7 chromosome telomere links with avirulent gene Avr-Pia with a genetic distance of 3.5cm; S361 labeled by m677-678 and RAPD located at middle of N0. 3 chromosome telomere links with Pyricularia grisea specified avirulent gene PRE1 with genetic distances of 5.9cm and 6.4. The invention can not only make use of the nontoxic gene marker as gene probe, but also set foundations for further clone of the gene through physical mapping.
Owner:NANJING AGRICULTURAL UNIVERSITY
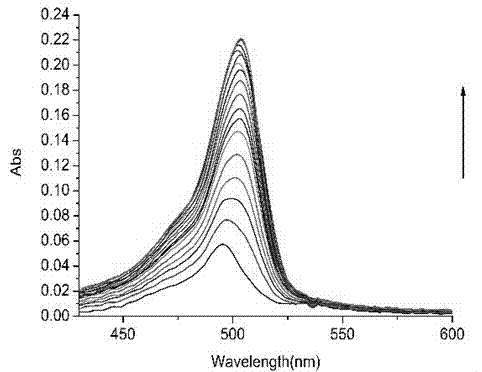
Methylbenzofuran quinoline type biological probe, and preparation method and application thereof
ActiveCN103666452AEasy to makeEasy to storeGroup 3/13 element organic compoundsBiological testingCryptolepineMechanism of action
The invention discloses a benzofuran quinoline type biological probe and a preparation method thereof, and application of the benzofuran quinoline type biological probe in detecting a G-quadruplex structure and in the mechanism of action of cryptolepine and G-quadruplex, wherein the biological probe is high in fluorescence intensity and excellent in fluorescence property, and can be applied to specially detecting the G-quadruplex structure and researching the mechanism of action of a cryptolepine derivative and the G-quadruplex. The invention relates to the research whether the cryptolepine derivative acts on the telomere G-quadruplex or the C-MYCG-quadruplex in the promoter region, or on other G-quadruplex structures in vivo; the detection method has the advantages of simple, convenient and quick operation, good selectivity and obvious visualization; the shortcomings of unavailable in-vivo detection, high price, high requirement equipment, relatively complex technical operations and the like of other detection methods are overcome.
Owner:SUN YAT SEN UNIV
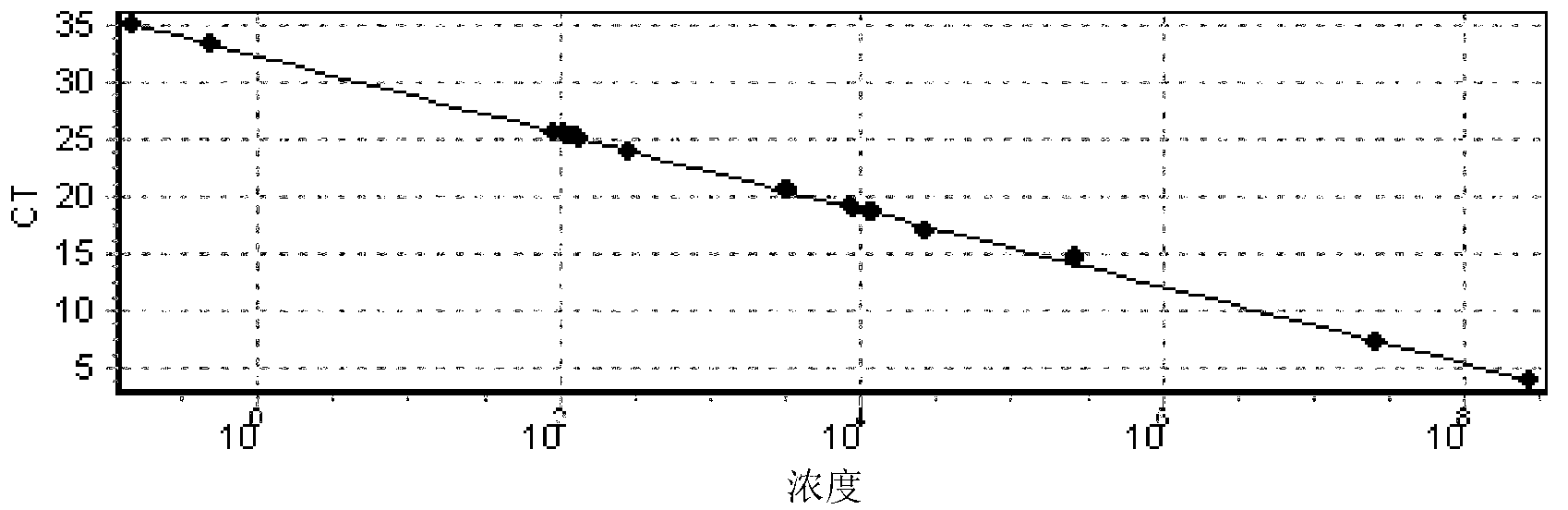
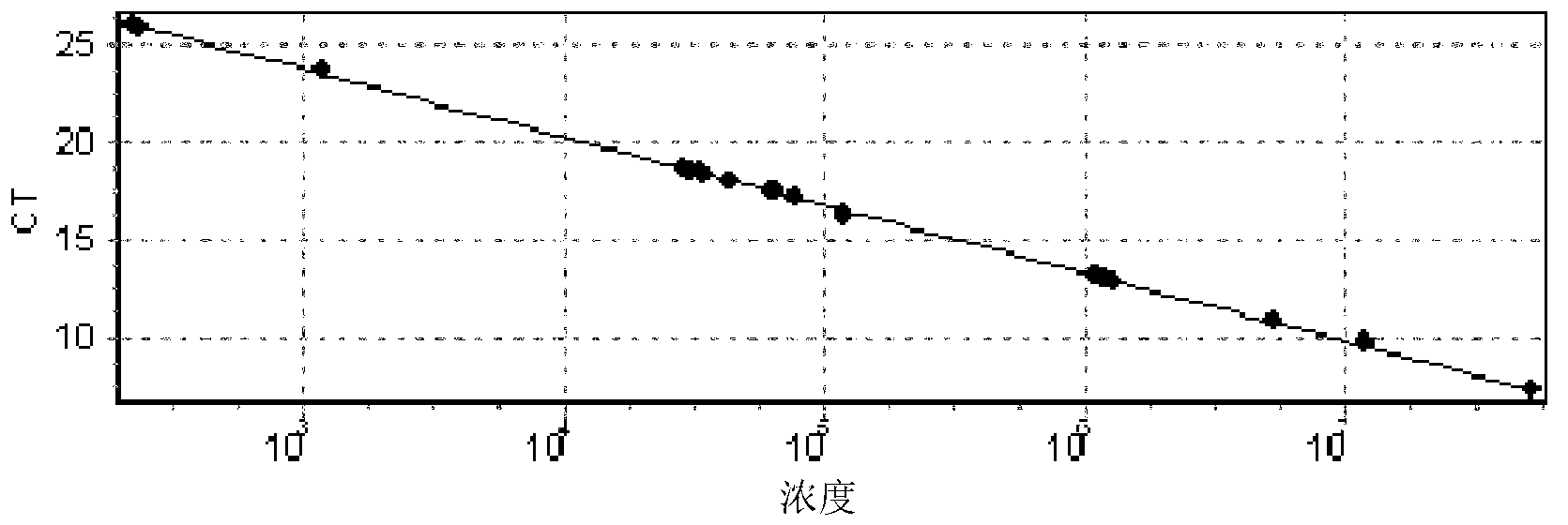
Method for measuring telomere absolute length
InactiveCN103233071AGood repeatabilityMonitor dynamic changesMicrobiological testing/measurementDiseaseOligomer
The invention discloses a method for measuring telomere absolute length. By applying oligomer standard, the telomere absolute length instead of relative length can be measured, which is the largest difference compared with the disclosed real-time quantitative PCR (Polymerase Chain Reaction) method. According to the measuring method, the repeatability is good, few DNA (Deoxyribonucleic Acid) substrates are needed, time is saved, accuracy is realized, and the method is applicable to clinical testing the telomere length and monitoring the dynamic variation, thereby predicting one of disease development and aging indices. The method can be further used for researching preimplantation embryo and stem cell quality.
Owner:南京优而生物科技发展有限公司
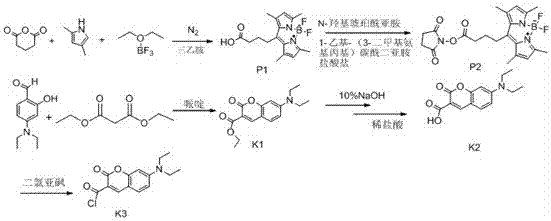
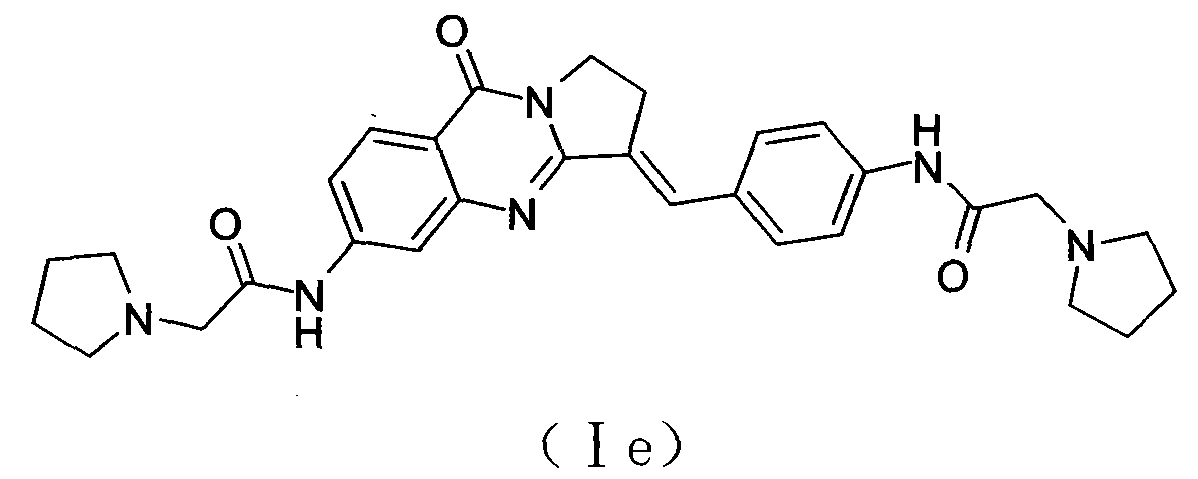
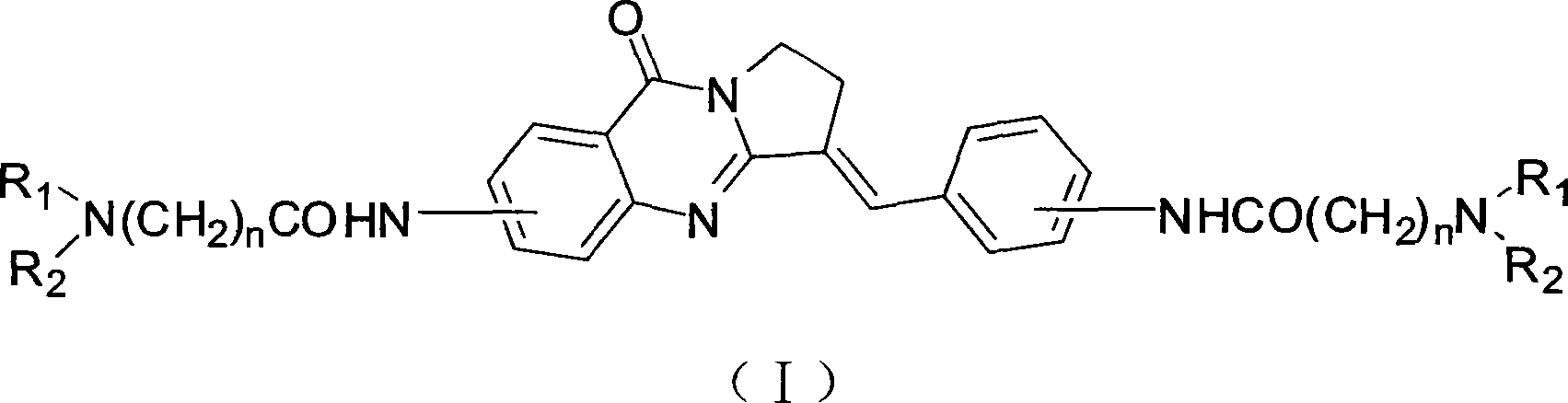
Bisfatty amido substituted quinazolone derivatives as well as preparation method and use thereof as anti-cancer drugs
InactiveCN101250189AGood antitumor activitySmall toxicityOrganic active ingredientsOrganic chemistryQuinoxalineTelomerase
The invention discloses a dual fatty ammonia substituted quinazolinone derivative, represented as formula (I), wherein n is 1, 2, 3, 4 or 5, R1 and R2 are same or different, selected from the alkyl of H and C1-6, and naphthenic group, piperidyl, morpholinyl, piperazinyl or quinoxaline of C3-6. The derivative has strong interaction on the telomere DNA rich with guanine and c-myc DNA of proto-oncogene, strong inhibition on telomere / telomerase of cancer cell and strong inhibition on the c-myc expression of proto-oncogene. The derivative has low toxicity and side effect, which can be developed to a new anti-tumor drug. The derivative has simple preparation method, cheap materials and strong inhibition on various cancer cell lines, which can be prepared into anti-tumor drug, with wide application.
Owner:SUN YAT SEN UNIV
Compositions and methods for analysis of nucleic acids
InactiveUS7270958B2Microbiological testing/measurementFermentationSequence analysisNucleic acid cloning
Disclosed are a number of methods that can be used in a variety of embodiments, including, creation of a nucleic acid terminated at one or more selected bases, sequence analysis of nucleic acids, mapping of sequence motifs within a nucleic acid, positional mapping of nucleic acid clones, and analysis of telomeric regions. The methods utilize double-stranded templates, and in most aspects involve a strand replacement reaction initiated at one or more random or specific locations created in a nucleic acid molecule, and in certain aspects utilizing an oligonucleotide primer.
Owner:RGT UNIV OF MICHIGAN
Saliva-derived measures of telomere abundance and sample collection device
InactiveUS20140370505A1Increased cardiovascular riskHigh riskBioreactor/fermenter combinationsBiological substance pretreatmentsCell typeBiology
This invention provides devices and methods for sample collection. Devices can include (a) a container having an opening and adapted to receive a liquid sample through the opening; (b) a cover configured to reversibly seal the opening; and (c) a capture device configured to be introduced into the container, wherein the capture device is configured to selectively bind cells of a first type and not to substantially bind cells of a second cell type. The sample can be analyzed to make a measure of telomere abundance and the abundance can be correlated to a measure of health.
Owner:TELOME HEALTH
Telomeric protein polypeptide fragment with tumor cell killing activity and application thereof
ActiveCN102942618ABroad-spectrum killing activityGrowth inhibitionPeptide/protein ingredientsGenetic material ingredientsTelomeraseTumor cell apoptosis
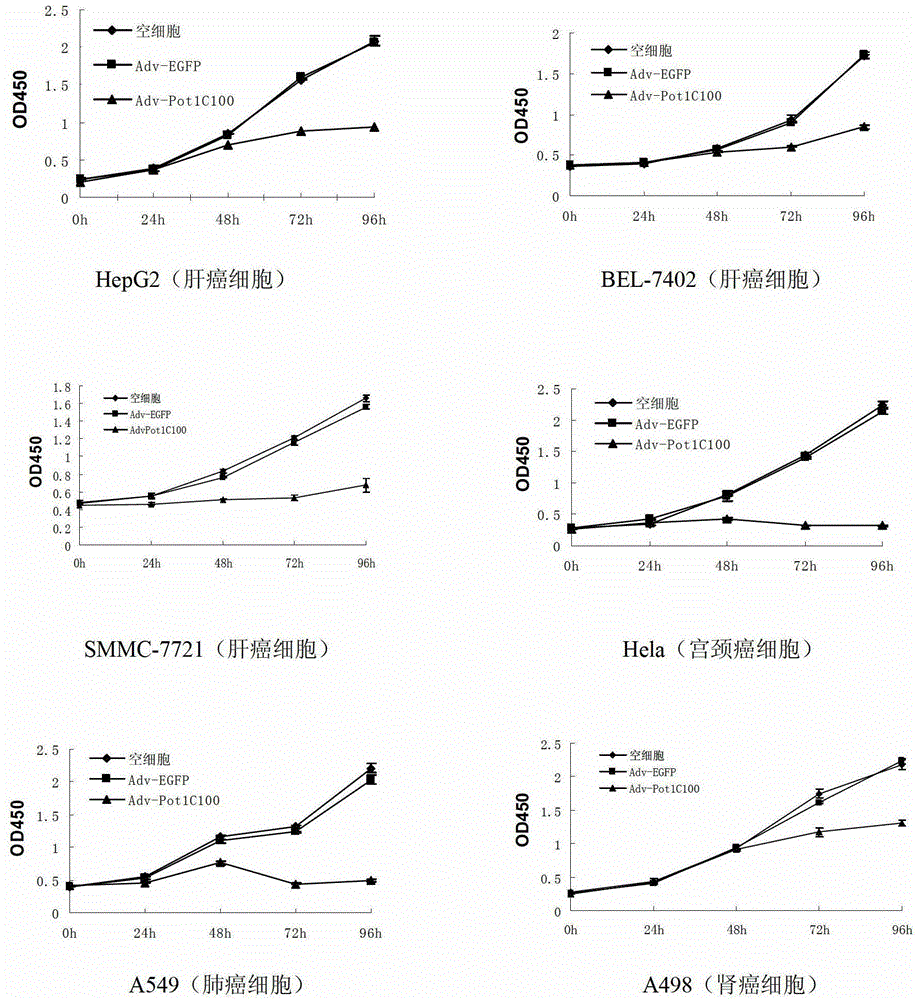
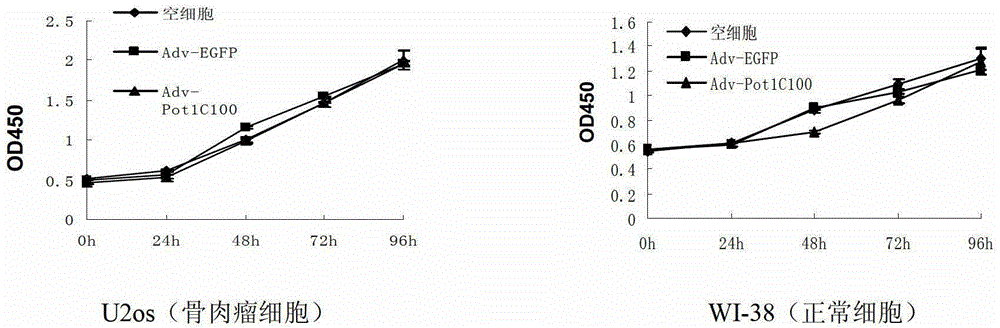
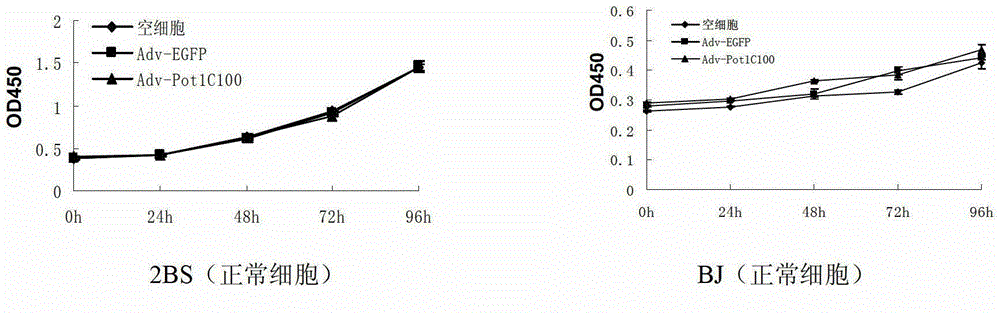
The invention discloses a telomeric protein polypeptide fragment with tumor cell killing activity and an application thereof. The polypeptide provided by the invention is (a) or (b) as follows: (a) polypeptide composed of amino acid sequence as shown in sequence 1 of a sequence table; and (b) polypeptide which is derived from sequence 1 relative to the tumor cell killing activity and replacing and / or missing and / or adding one or more amino acid residues for the amino acid sequence of the sequence 1. The experiment shows that the recombinant adenovirus which expresses the polypeptide provided by the invention can inhibit the growth of the tumor cell telomerase positive, induces the apoptosis of the tumor cell and inhibits the growth of tumors in vitro and vivo. The polypeptide provided by the invention has broad spectrum and specific telomerase positive tumor cell killing activity; and meanwhile, the polypeptide does not affect the growth and survival of normal human cell; therefore, the telomeric protein polypeptide fragment and the application thereof have significant meaning on the research of novel broad spectrum specific anticancer drugs.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Gene scar for characterizing HRD homologous recombination repair defects and identification method
ActiveCN111883211AImprove robustnessImprove inspection accuracyMicrobiological testing/measurementSequence analysisAllele ImbalanceOriginal data
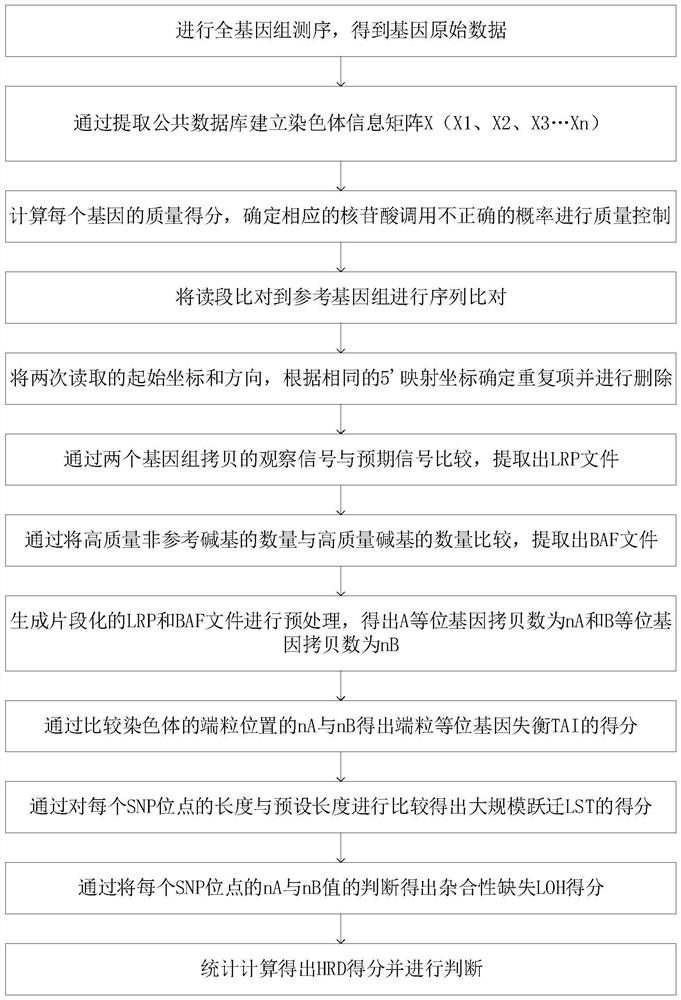
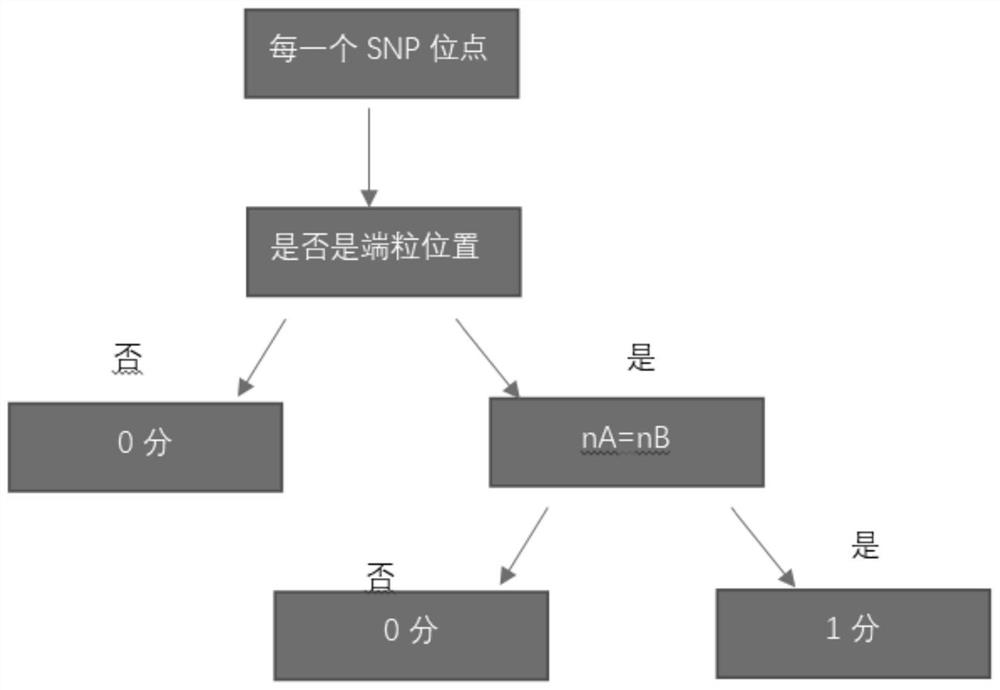
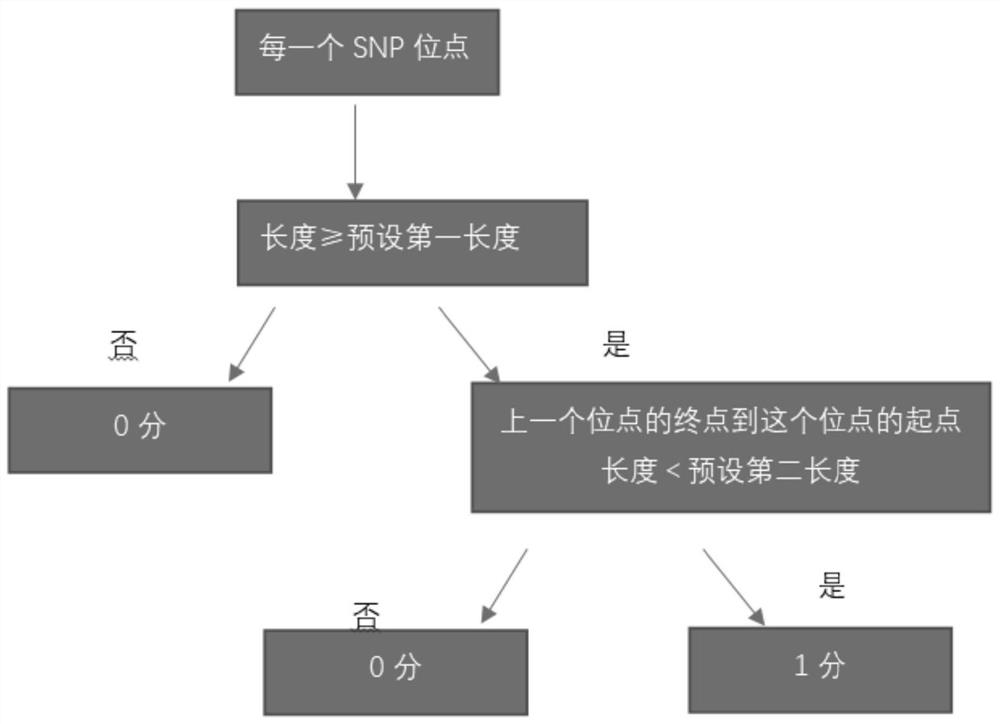
The invention relates to a gene scar for characterizing HRD homologous recombination repair defects and an identification method. The identification method comprises the following steps of step 1, performing whole genome sequencing to obtain gene original data; step 2, establishing a chromosome information matrix X (X1, X2, X3... Xn); step 3, performing data quality control; step 4, performing sequence comparison; step 5, deleting repeated reads; step 6, extracting an LRP file through signals copied by two genomes. step 7, comparing the number of high-quality non-reference bases with the number of the high-quality bases to extract a BAF file; step 8, generating fragmented LRP and BAF files for preprocessing to obtain an A allele copy number nA and a B allele copy number nB; step 9, obtaining a telomere allele imbalance TAI score through the nA and the nB; step 10, obtaining a large-scale transition LST score according to the length of each site; step 11, obtaining a heterozygosity deletion LOH score through judgment of the nA and the nB of each SNP site; and step 12, performing statistical calculation to obtain an HRD score and preforming judgment.
Owner:张哲 +1
Identification method research of wheat-secale cereale L 1BL/1RS translocation line
Secale cereale L is one of the important kindred plants of wheat and has the characteristics of floweriness, multi-powder, eurytopicity, and the like. The 1RS segment of secale cereale L carries resistance genes Lr26, Sr31, Yr9 and Pro8 for resisting leaf rust, stem rust, stripe rust and powdery mildew as well as plant hopper resistant gene Gb2 and a great amount of anti-retrieval genes. The content of soluble fiber of 1BL / 1RS translocation wheat flour is higher than that of ordinary wheat and is beneficial to human body. Related genes of a plurality of excellent traits for increasing the QTL loci, improving the yield traits and increasing the protein content exists on the 1RS. Me1z, Schlegel and Thiele find that partial isogeny relationship exists between 1RS, 1RL, 2RL, 3RS and 5RS and corresponding wheat chromosome arms via ph16 line analysis of addition line, telomere line and the wheat, thereby being capable of producing abnormal addition line, replacement line and translocation line of wheat-secale cereale L. As 1RS can favorably compensate negative effect caused by 1BS defect on the heredity, the wheat 1B / 1R translocation line can be directly applied to wheat breeding. The researched is carried out based thereon.
Owner:李祥
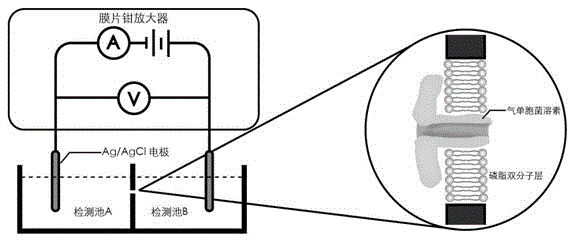
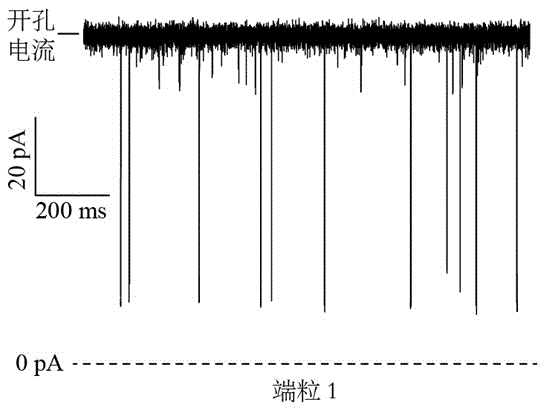
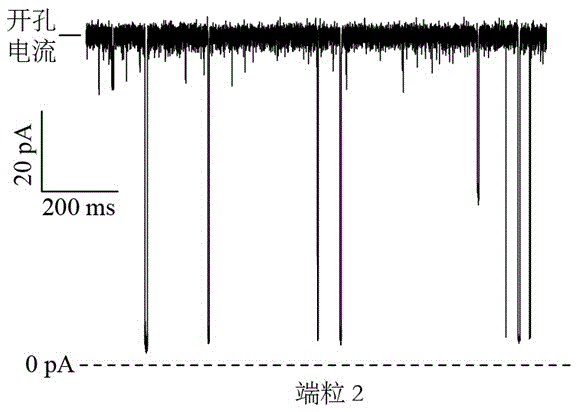
Telomere length detecting method based on biological nano channel of aerolysin
InactiveCN105548261AAccurate measurementDirect real-timeMaterial analysis by electric/magnetic meansLysinEnzyme digestion
The invention discloses a telomere length detecting method based on a biological nano channel of aerolysin. The method comprises the following steps: (1) opening the G-quadruplet structure of telomere DNA of human; (2) preparing a biological nano channel of aerolysin: (a) activating aerolysin; (b) preparing a phospholipid n-decane solution; (c) preparing phospholipid bilayer; (d) forming a biological nano channel of aerolysin; (3) using the biological nano channel of aerolysin to detect the telomere length: (a) collecting monomolecular signal data; (2) analyzing the monomolecular signal data. Compared with the prior art, the provided method has the advantages that the operation is simple and efficient; enzyme digestion, molecular hybridization, or fluorescence labeling is not needed; the consumption of DNA substrate is little; the detection sensitivity and resolution are high; the price is cheap; the absolute length of telomere can be precisely and directly measured in real time; and the method has a positive meaning for bioscience research.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com