Antiviral oligonucleotides having a conserved G4 core sequence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oligonucleotide Synthesis
[0077] DNA synthesizer reagents, controlled-pore glass (CPG)-bound and B-cyanoethyldiisopropylphosphoramidites were purchased from Applied Biosystems (Foster City, Calif.). 2′-O-Methyl B-cyanoethyldiisopropylphosphoramidites were purchased from Chemgenes (Needham, Mass.). Phenoxyacetyl-protected phosphoramadites for RNA synthesis were purchased from BioGenex (Hayward, Calif.).
[0078] Oligonucleotides were synthesized on an automated DNA synthesizer (Applied Biosystems model 380B). 2′-O-Methyl oligonucleotides were synthesized using the standard cycle for unmodified oligonucleotides, except the wait step after pulse delivery of tetrazole and base was increased to 360 seconds. The 3′ base bound to the CPG used to start the synthesis was a 2′-deoxyribonucleotide. After cleavage from the CPG column and deblocking in concentrated ammonium hydroxide at 55° C. (18 hours), the oligonucleotides were purified by precipitation two times out of 0.5 M NaCl solution with...
example 2
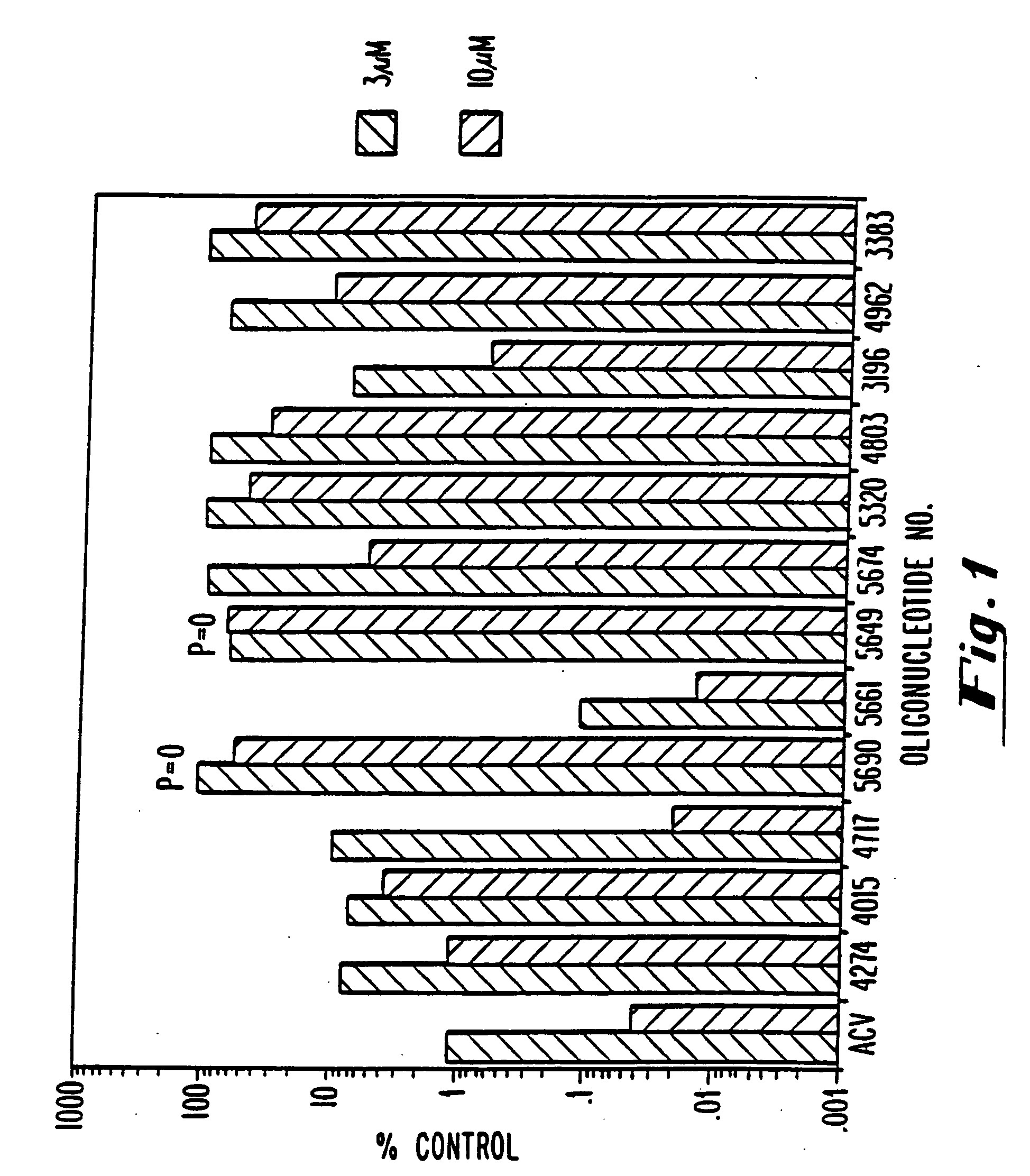
HIV Inhibition Acute HIV Infection Assay
[0079] The human T-lymphoblastoid CEM cell line was maintained in exponential growth phase in RPMI 1640 with 10% fetal calf serum, glutamine, and antibiotics. On the day of the assay, the cells were washed and counted by trypan blue exclusion. These cells (CEM-IIIB) were seeded in each well of a 96-well microtiter plate at 5×103 cells per well. Following the addition of cells to each well, the oligonucleotides were added at the indicated concentrations and serial half log dilutions. Infectious HIV-1IIIB was immediately added to each well at a multiplicity of infection determined to give complete cell killing at 6 days post-infection. Following 6 days of incubation at 37° C., an aliquot of supernatant was removed from each well prior to the addition of the tetrazolium dye XTT to each well. The XTT was metabolized to a formazan product by viable cells and the results calculated spectrophotometrically with a Molecular Devices Vmax Plate Reader. ...
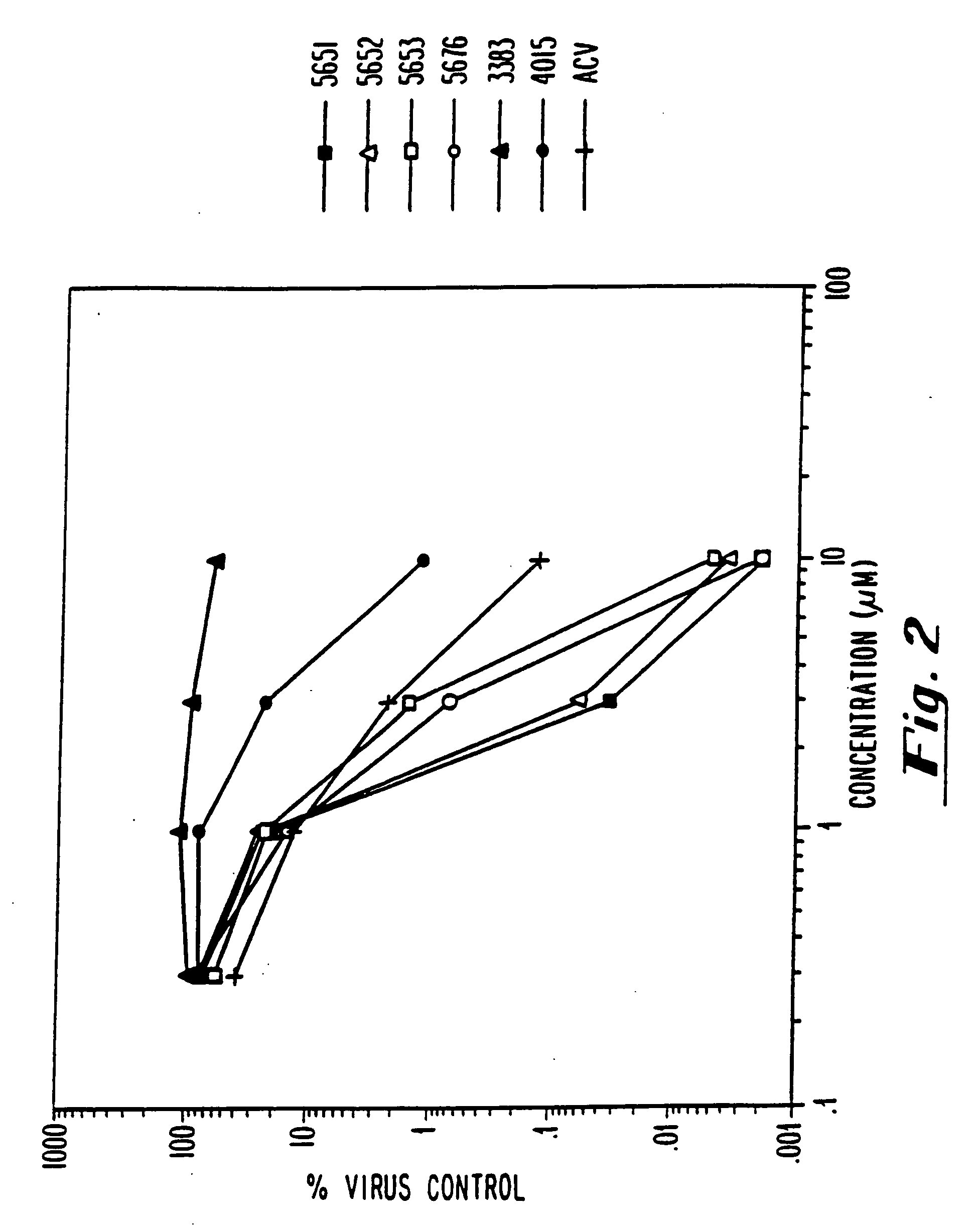
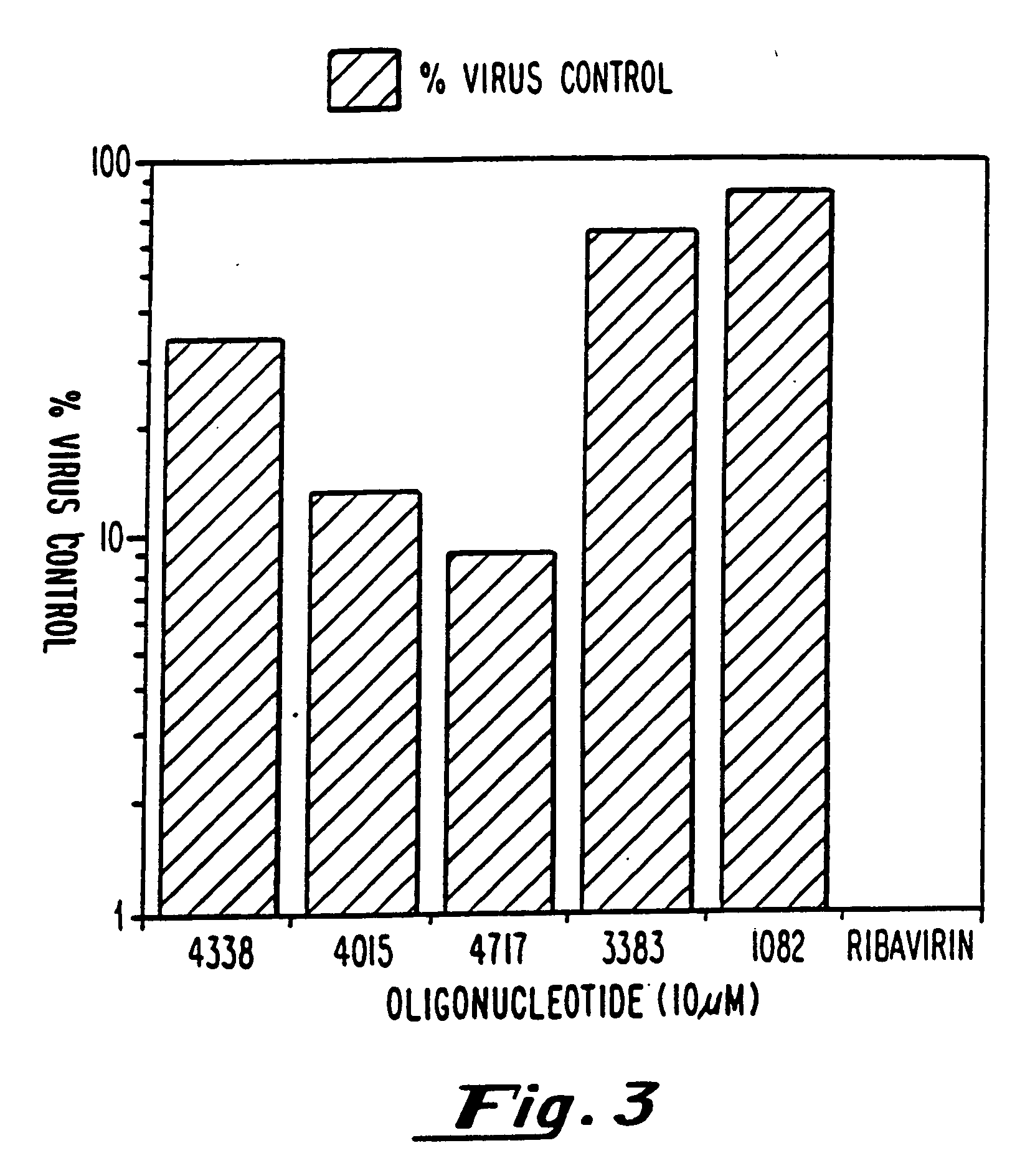
example 3
HSV-1 Inhibition HSV-1 Infection ELISA Assay
[0080] Confluent monolayers of human dermal fibroblasts were infected with HSV-1 (KOS) at a multiplicity of 0.05 pfu / cell. After a 90 minute adsorption at 37° C., virus was removed and culture medium containing oligonucleotide at the indicated concentrations was added. Two days after infection medium was removed and cells fixed by addition of 95% ethanol. HSV antigen expression was quantitated using an enzyme linked immunoassay. Primary reactive antibody in the assay was a monoclonal antibody specific for HSV-1 glycoprotein B. Detection was achieved using biotinylated goat anti-mouse IgG as secondary antibody followed by reaction with streptavidin conjugated B-galactosidase. Color was developed by addition of chlorophenol red B-D-galactopyranoside and absorbance at 570 nanometers was measured. Results are expressed as percent of untreated control.
[0081] Confluent monolayers of human dermal fibroblasts were infected wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com