Methylbenzofuran quinoline type biological probe, and preparation method and application thereof
A technology of furoquinoline and biological probes, which is applied in the field of biological probes and can solve problems such as no clear conclusions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
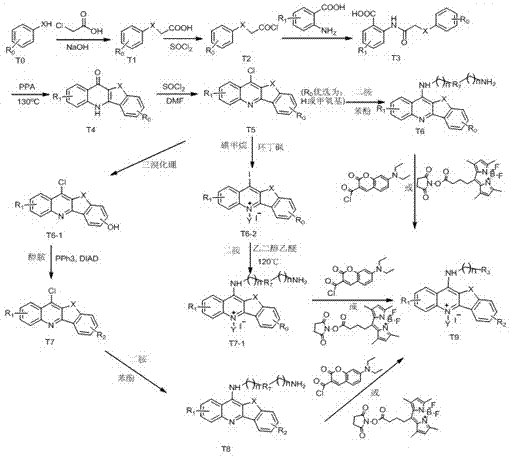
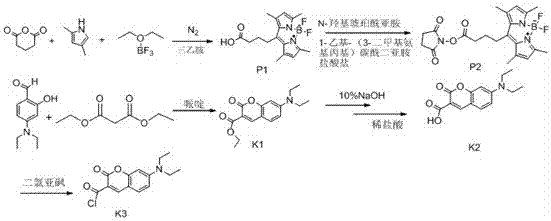
[0071] Example 1: Compound 2T6 Synthesis
[0072] Dissolve 0.3mol of chloroacetic acid in 60ml of water, adjust the pH to 9 with sodium hydroxide, then add 0.2mol of phenol, and reflux at 100°C to obtain compound T1, then add thionyl chloride for chlorination reaction to obtain compound T2, evaporate Thionyl chloride solvent was removed to obtain a brown liquid, which was then condensed with anthranilic acid to obtain T3, and then PPA was preheated to 130°C and added to T3 for a compound reaction to obtain compound T4, and T4 was mixed with thionyl chloride at 80 Chlorination reaction was carried out at reflux at ℃ to obtain compound T5, and then the purified T5 was added to a single-necked bottle, and phenol was added to react overnight at 60°C, then 1,4-butanediamine was added to react at 120°C for 12 hours, and finally the product was passed through silica gel Column purification to obtain compound 2T6 .
[0073] Yield: 82%; Melting point: 158-159°C; 1 H NMR: (400 MHz, ...
Embodiment 2
[0076] Embodiment 2: the synthesis of compound 4T7
[0077] Dissolve 0.3mol of chloroacetic acid in 60ml of water, adjust the pH to 9 with sodium hydroxide, then add 0.2mol of p-methoxyphenol, and reflux at 100°C to obtain compound T1, and then add thionyl chloride for chlorination reaction to obtain Compound T2, evaporate the thionyl chloride solvent to obtain a brown liquid, and then conduct condensation reaction with anthranilic acid to obtain T3, then preheat PPA to 130°C and add T3 for compound reaction to obtain compound T4, and combine T4 with chlorinated Sulfoxide was refluxed at 80°C for chlorination reaction to obtain compound T5, and then the purified T5 was methylated with methyl iodide in a sulfolane solvent system to obtain compound T6, and T6-2 was added to a single-necked bottle , using ethylene glycol ether as a solvent, and reacting with N,N-bis(3-aminopropyl)methylamine at 120°C for 30 minutes, and finally adding a large amount of anhydrous ether, precipitat...
Embodiment 3
[0081] Embodiment 3: the synthesis of compound 5T8
[0082] The method is the same as in Example 2, except that T5 is used as a solvent in dichloromethane, and boron tribromide is used to remove the methyl group in the methoxy group to obtain compound T6-1. React with N,N-dimethylethanolamine under anaerobic conditions, obtain compound T7-1 after purification by silica gel column, add T7-1 into a single-necked bottle, and add phenol to react overnight at 60°C, then add 1, 4-Butanediamine was reacted at 120° C. for 12 h, and finally the product was purified by silica gel column to obtain compound 5T8.
[0083] Yield: 84%; Melting point: 176-177°C; 1 H NMR: (400 MHz, CDCl 3 )δ 8.15 (d, J=8.3 Hz,1H),7.94 (d, J=8.4 Hz, 1H), 7.78 (d, J=2.5 Hz, 1H), 7.61 (t, J=7.2 Hz, 1H), 7.42-7.36 (m, 2H), 7.19 (dd, J = 8.9, 2.6 Hz, 1H), 5.73 (s, 1H), 4.14 (t, J = 5.6 Hz, 2H), 3.99 (t, J = 6.5 Hz , 2H), 2.80-2.74 (m, 4H), 2.34 (s, 6H), 1.87-1.76 (m, 2H), 1.67-1.60 (m, 2H); ESI+APCI-MS m / z: 393...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com