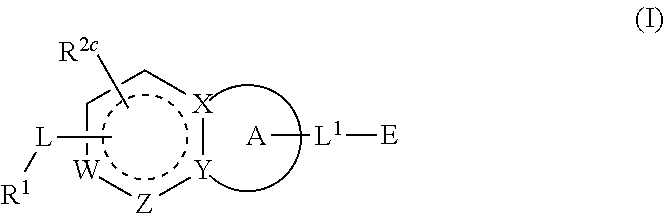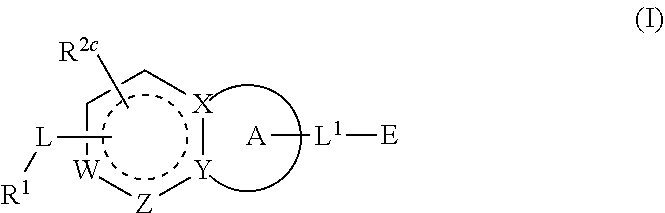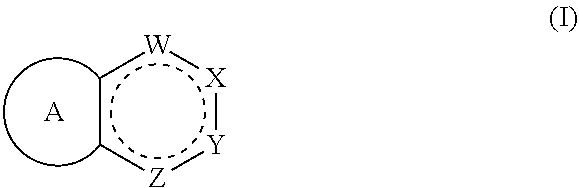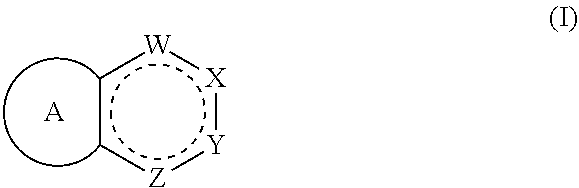Patents
Literature
272 results about "KRAS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
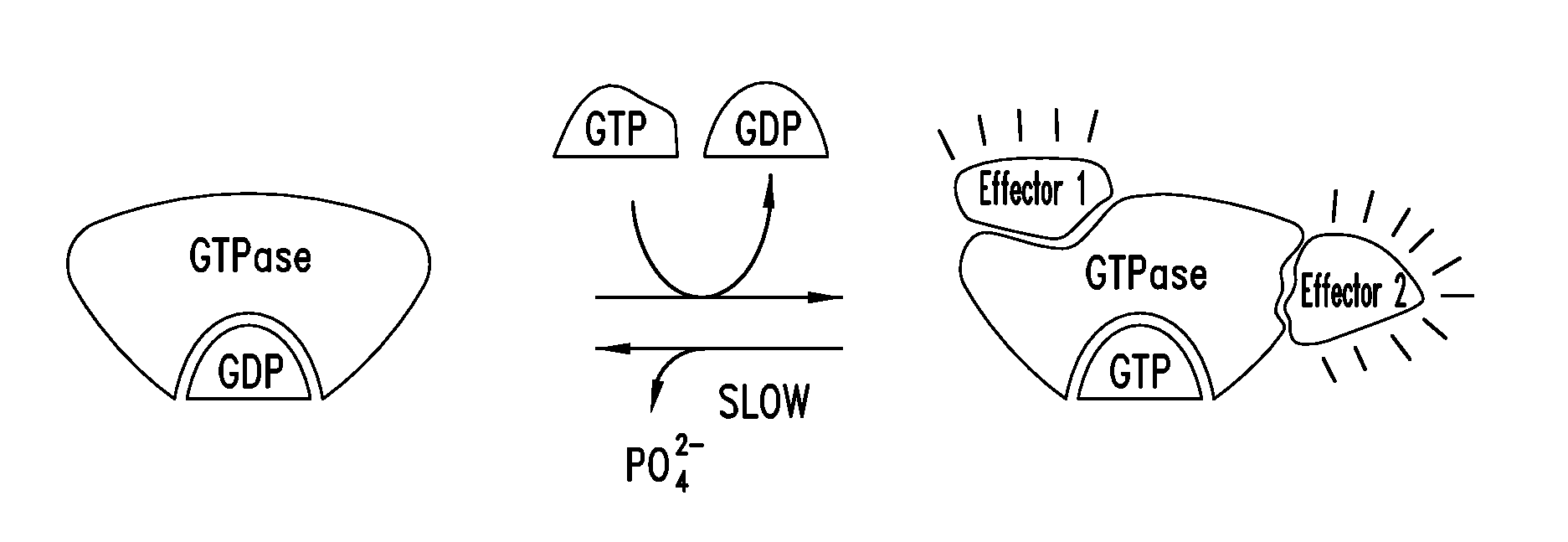
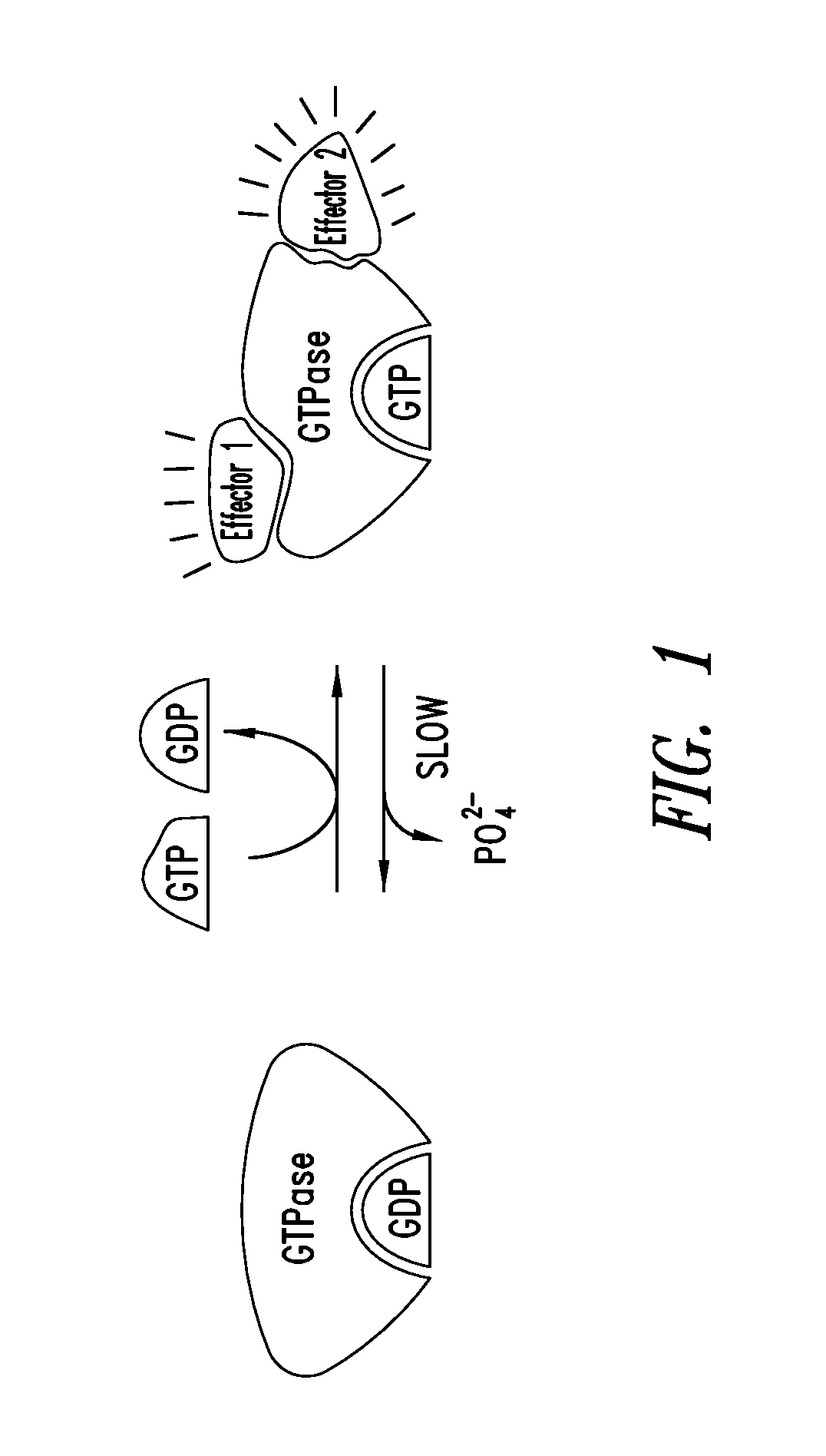
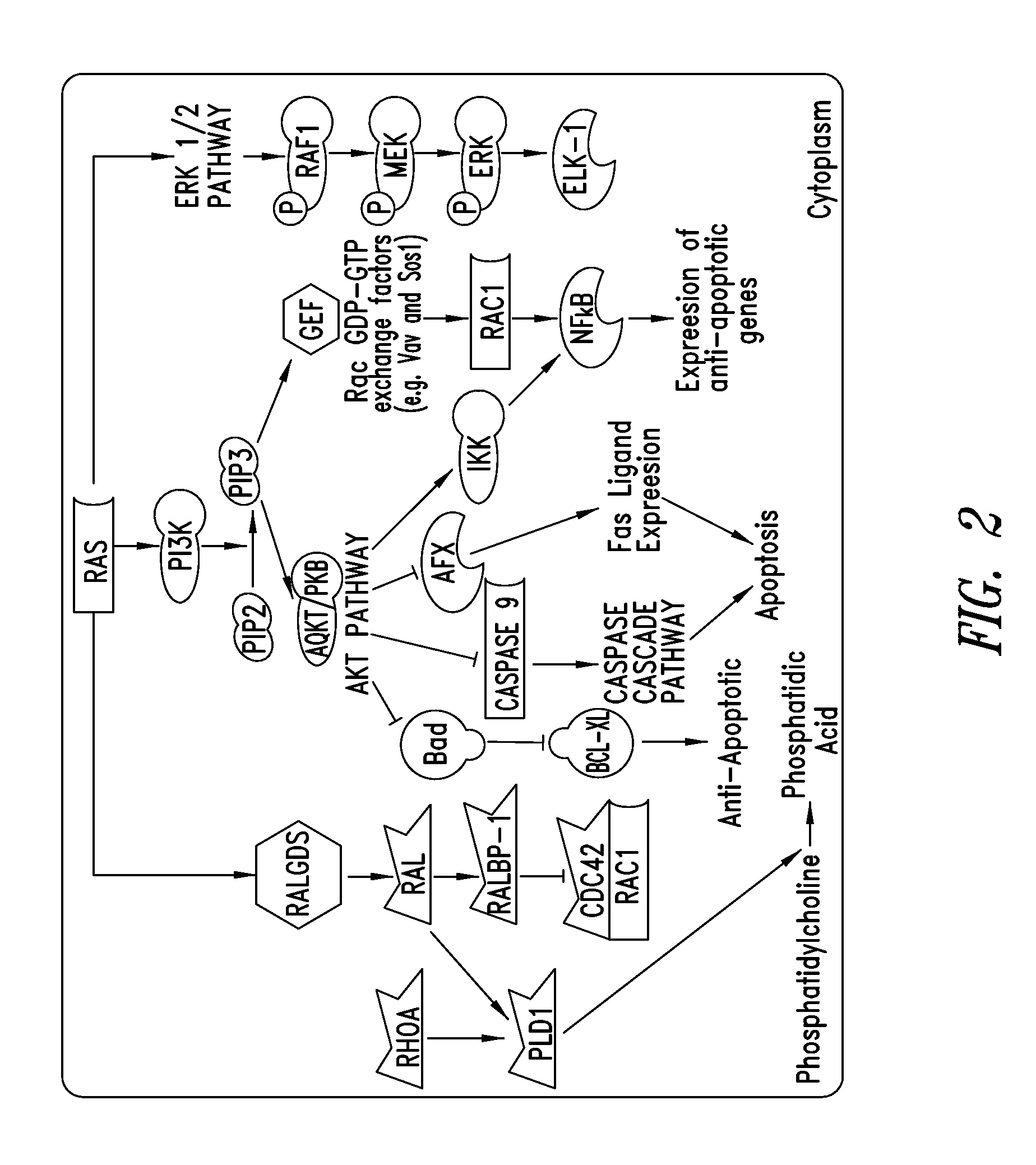
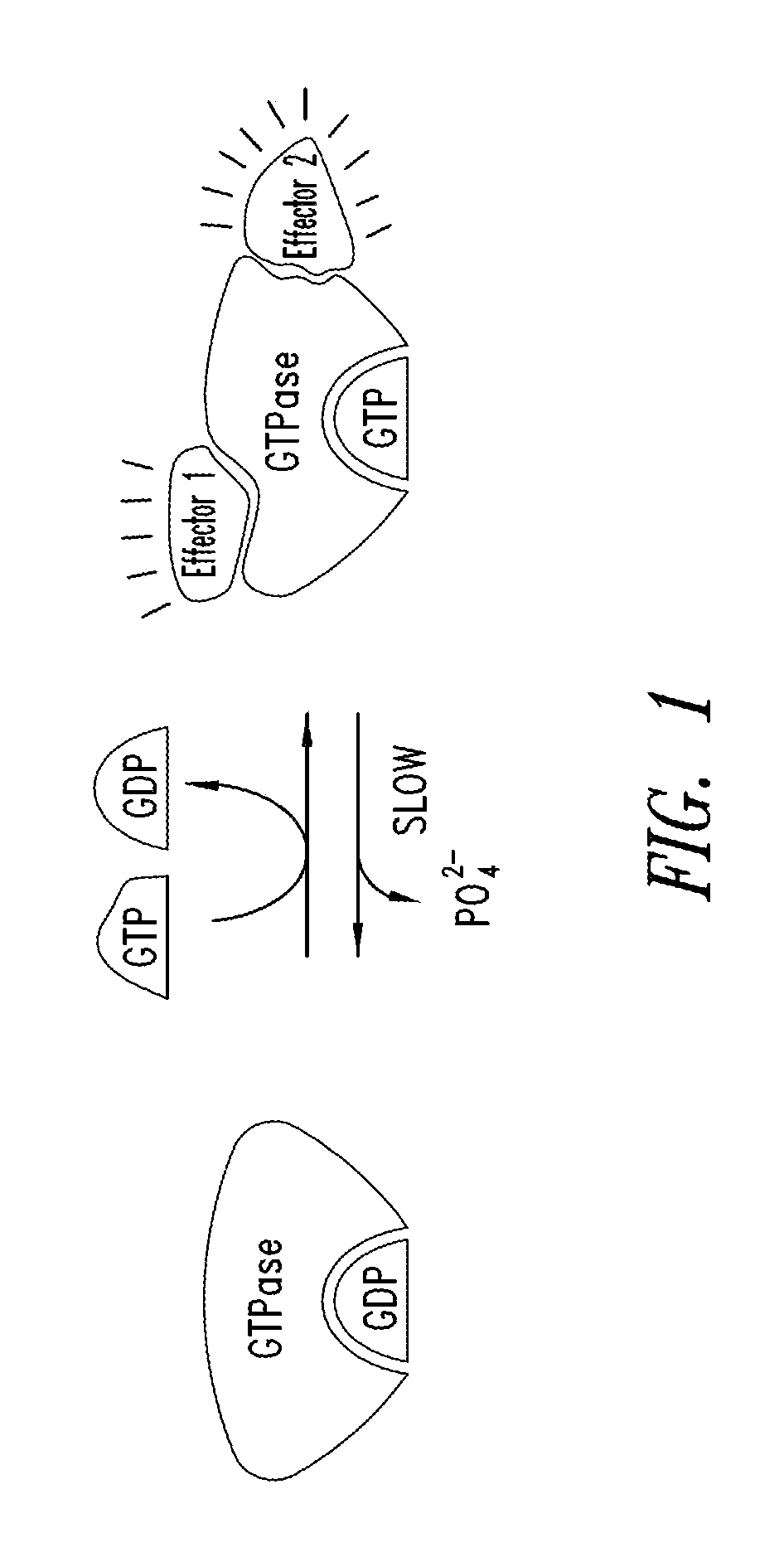
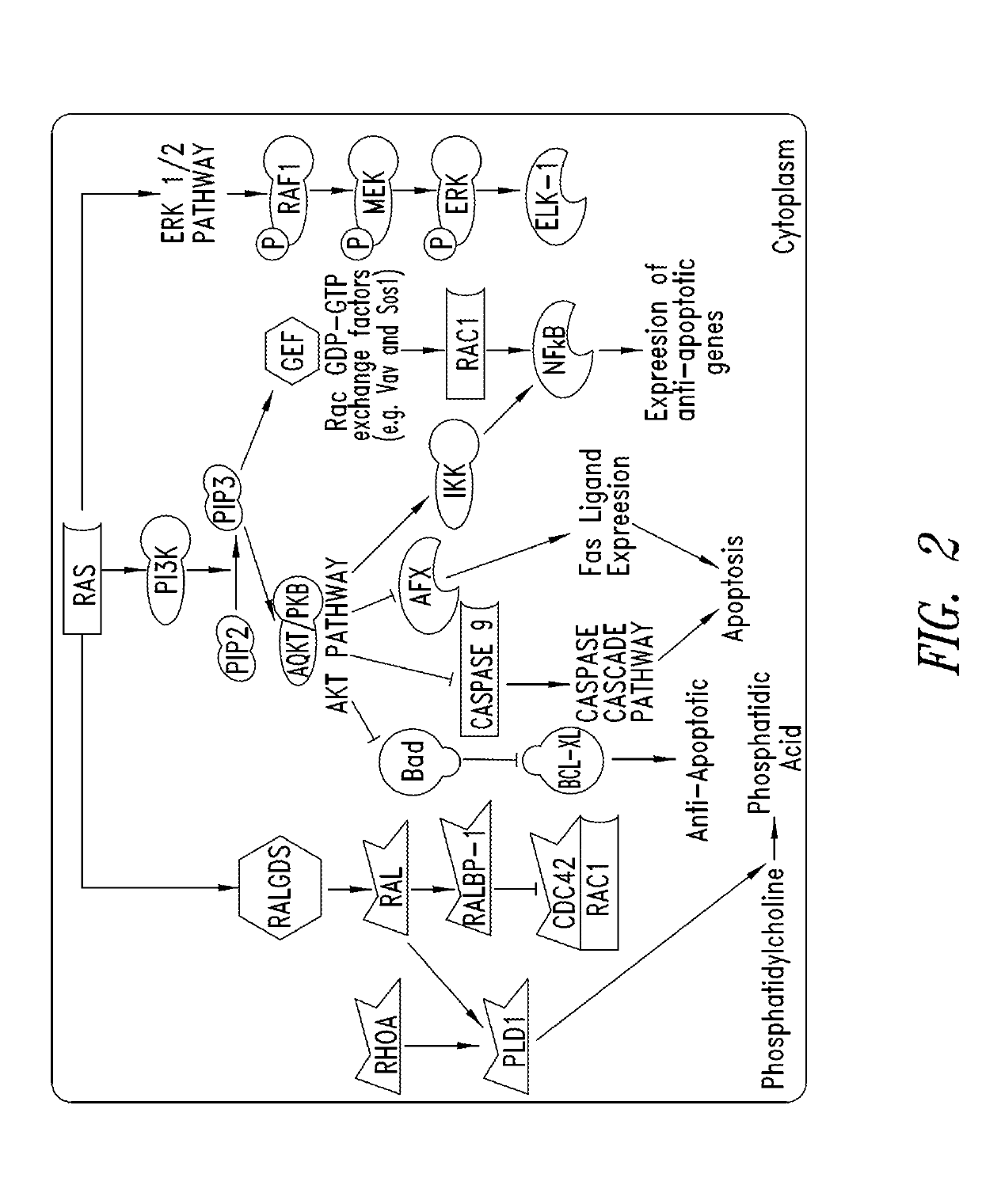
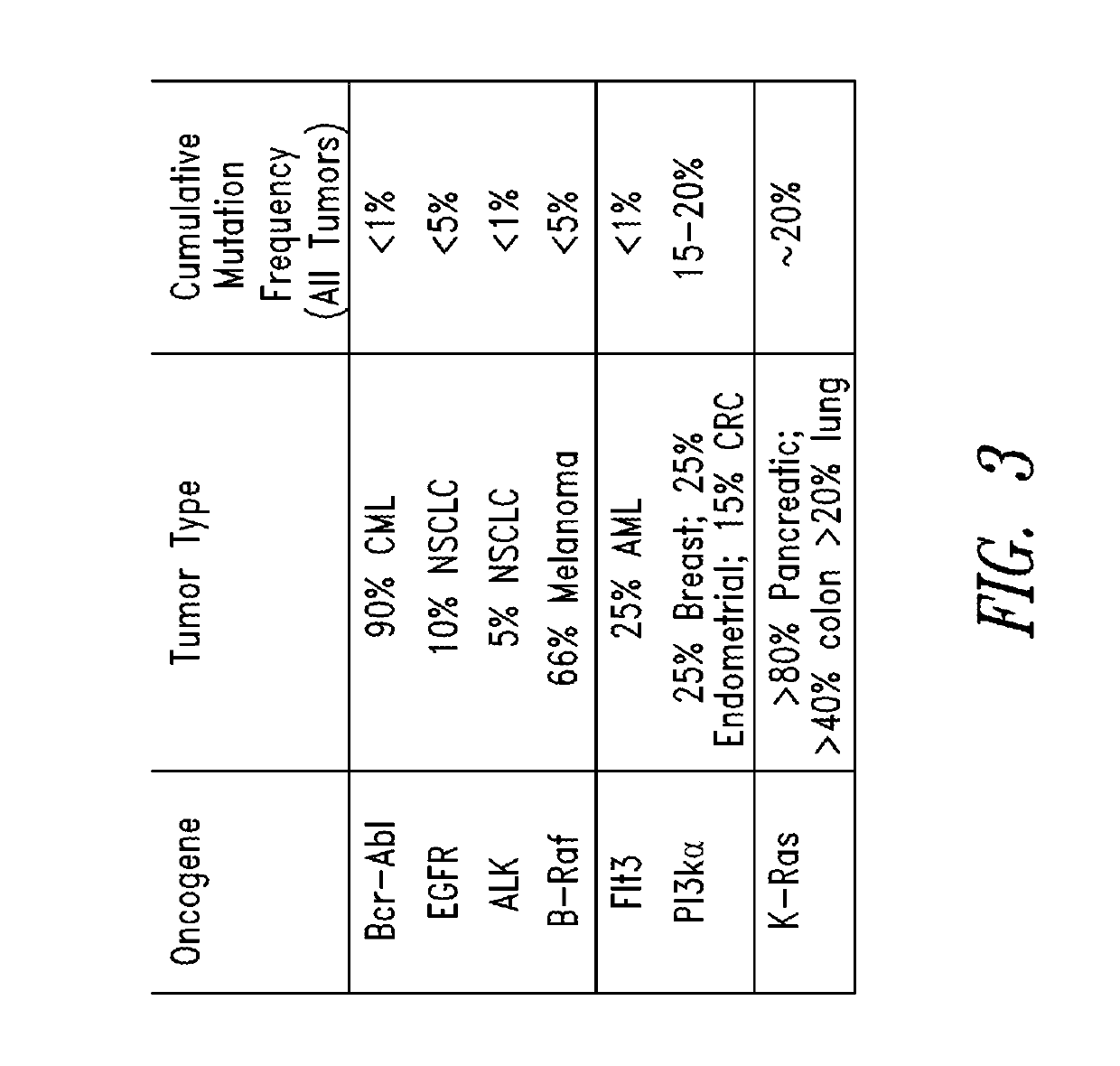
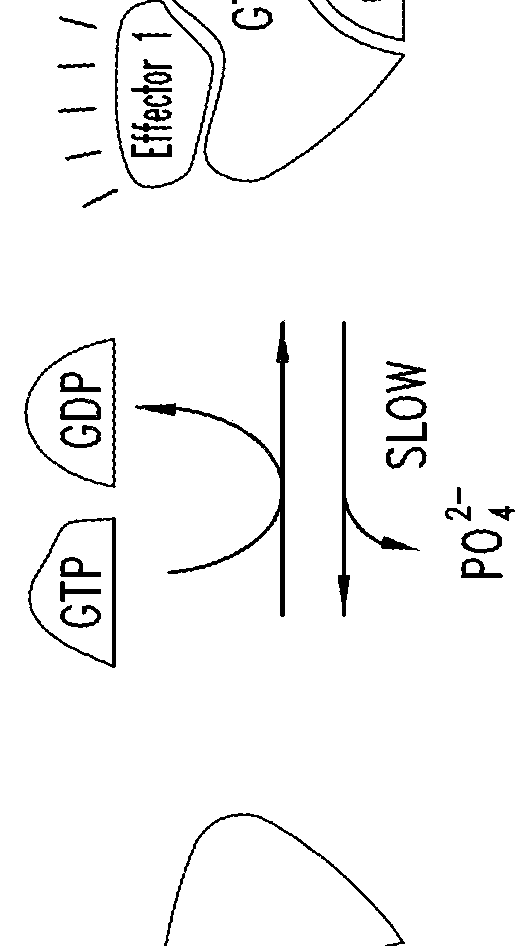
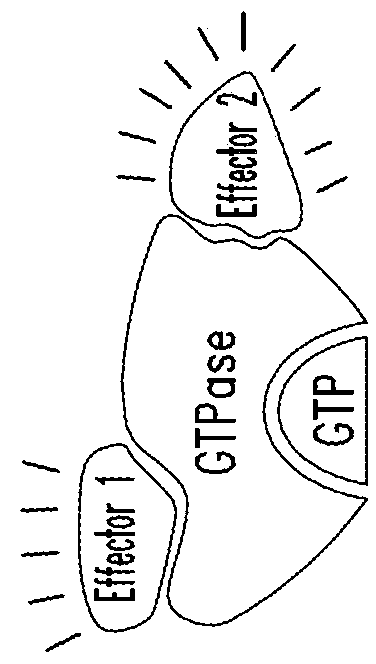
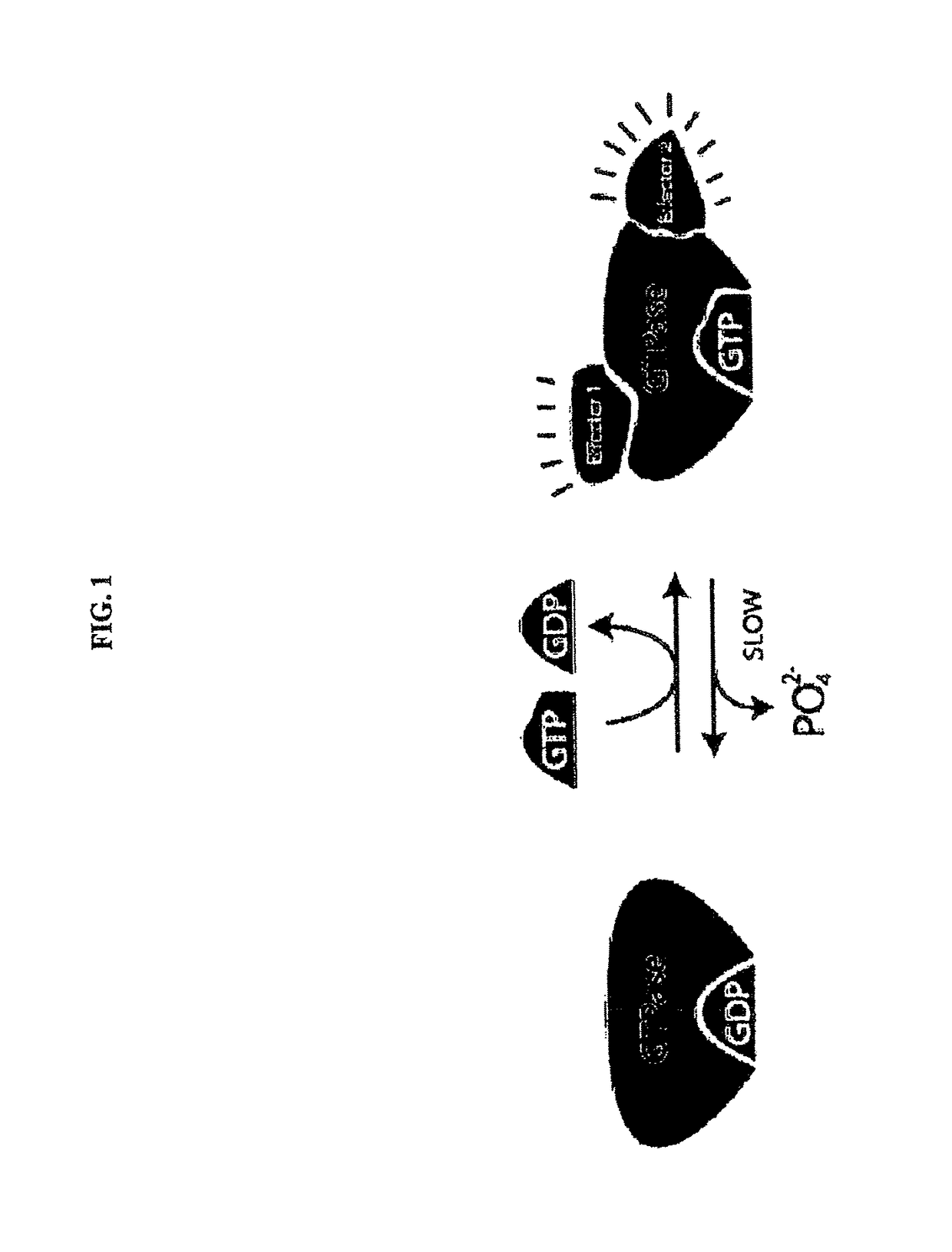
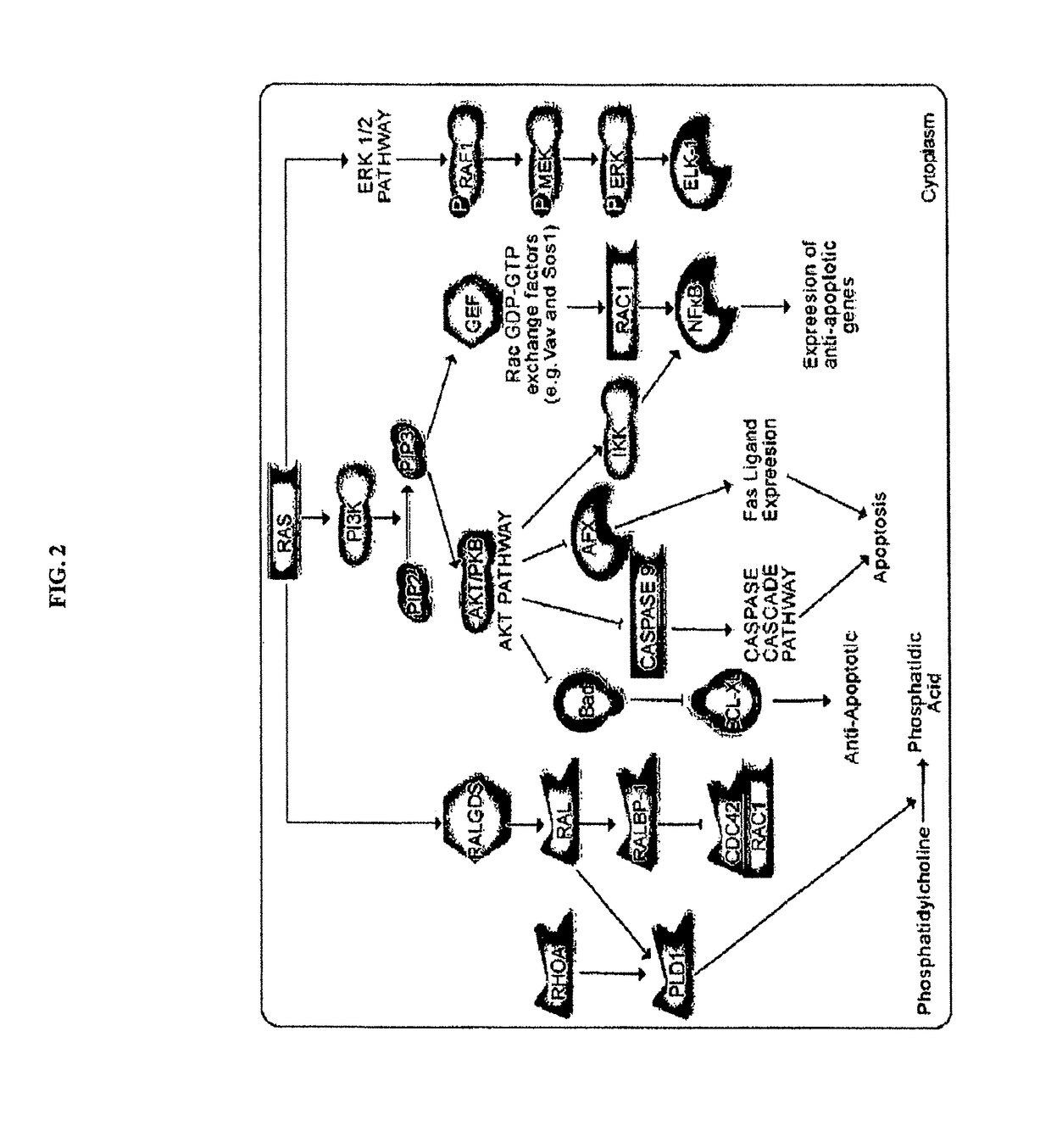
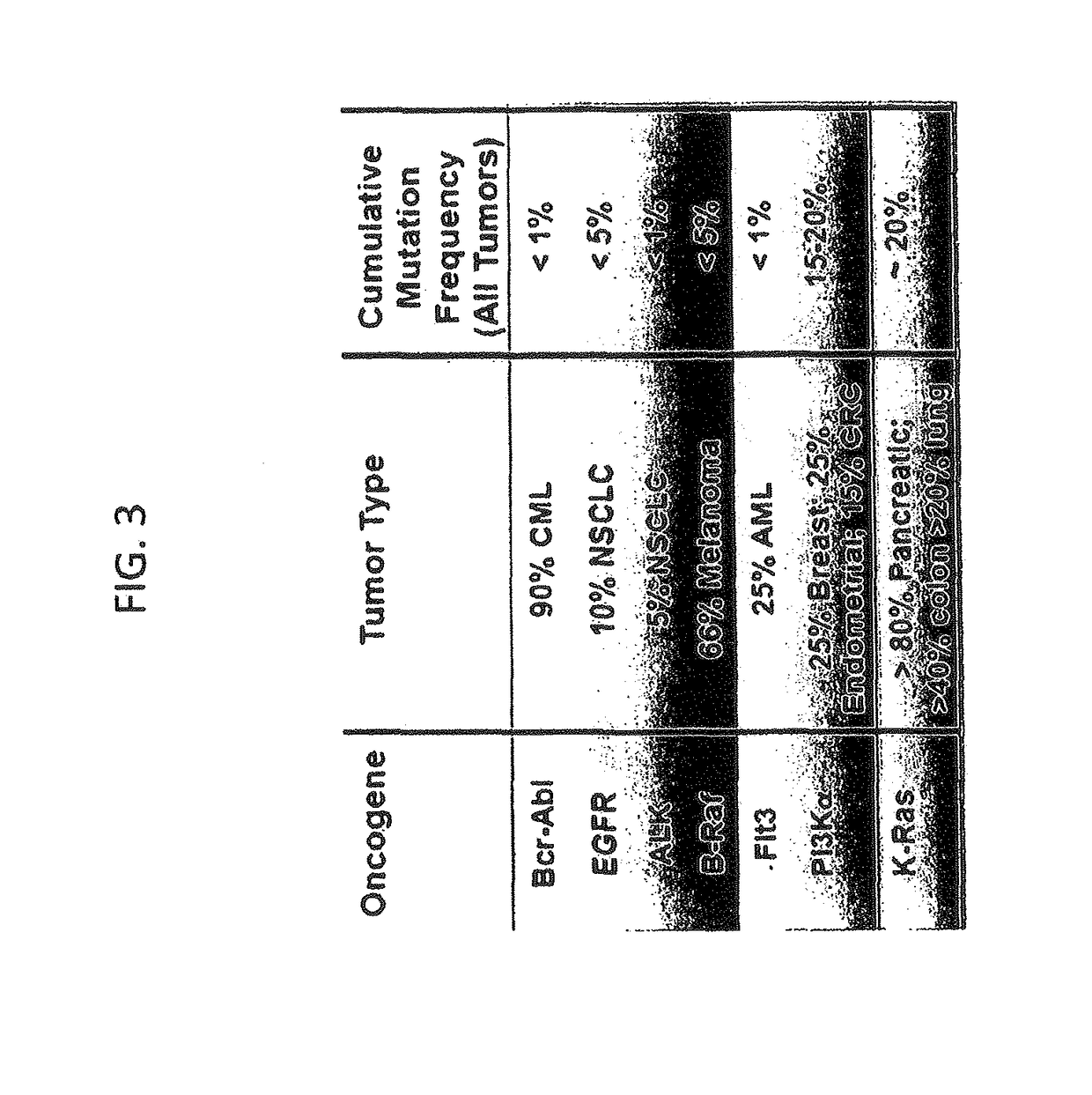
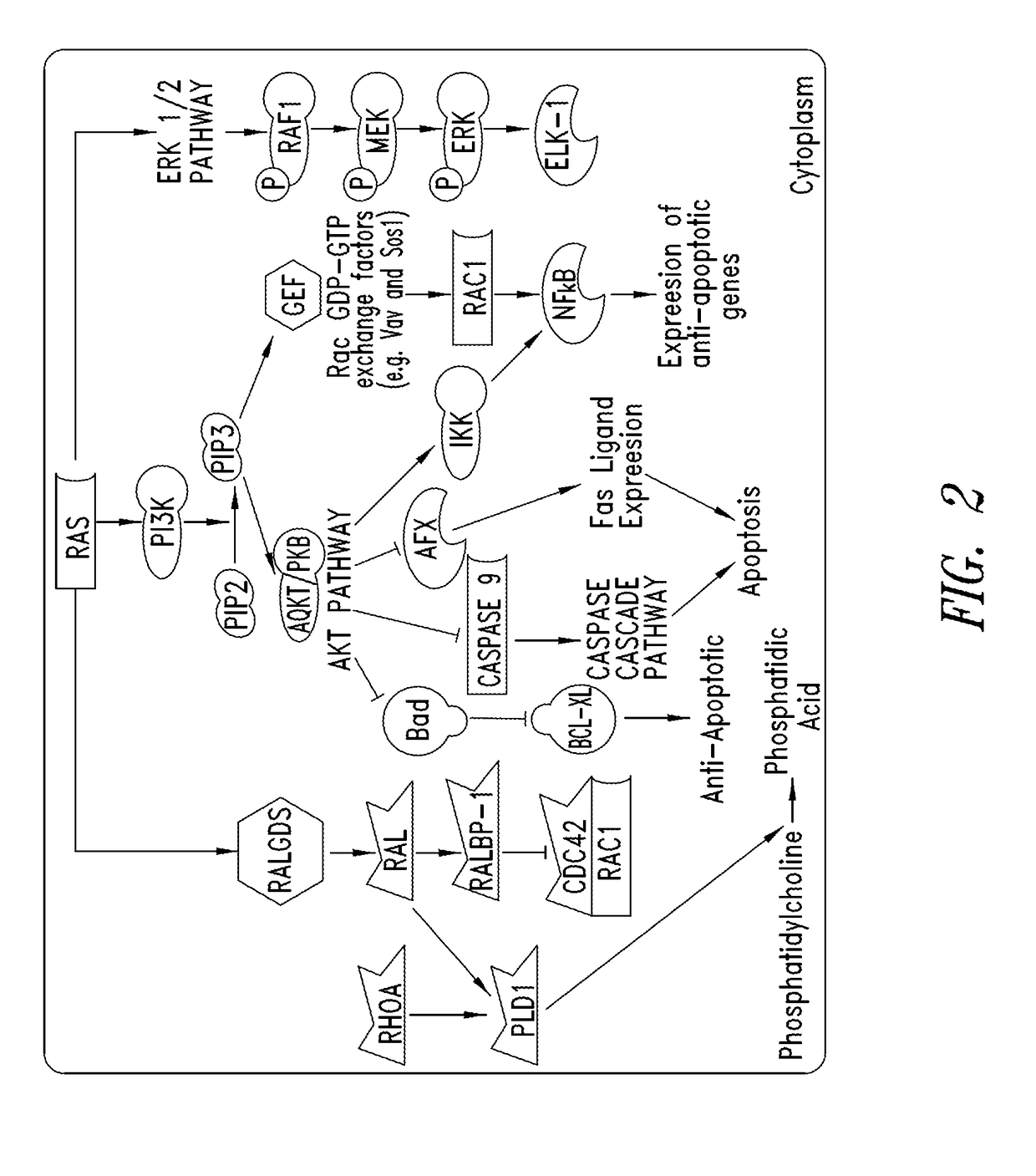
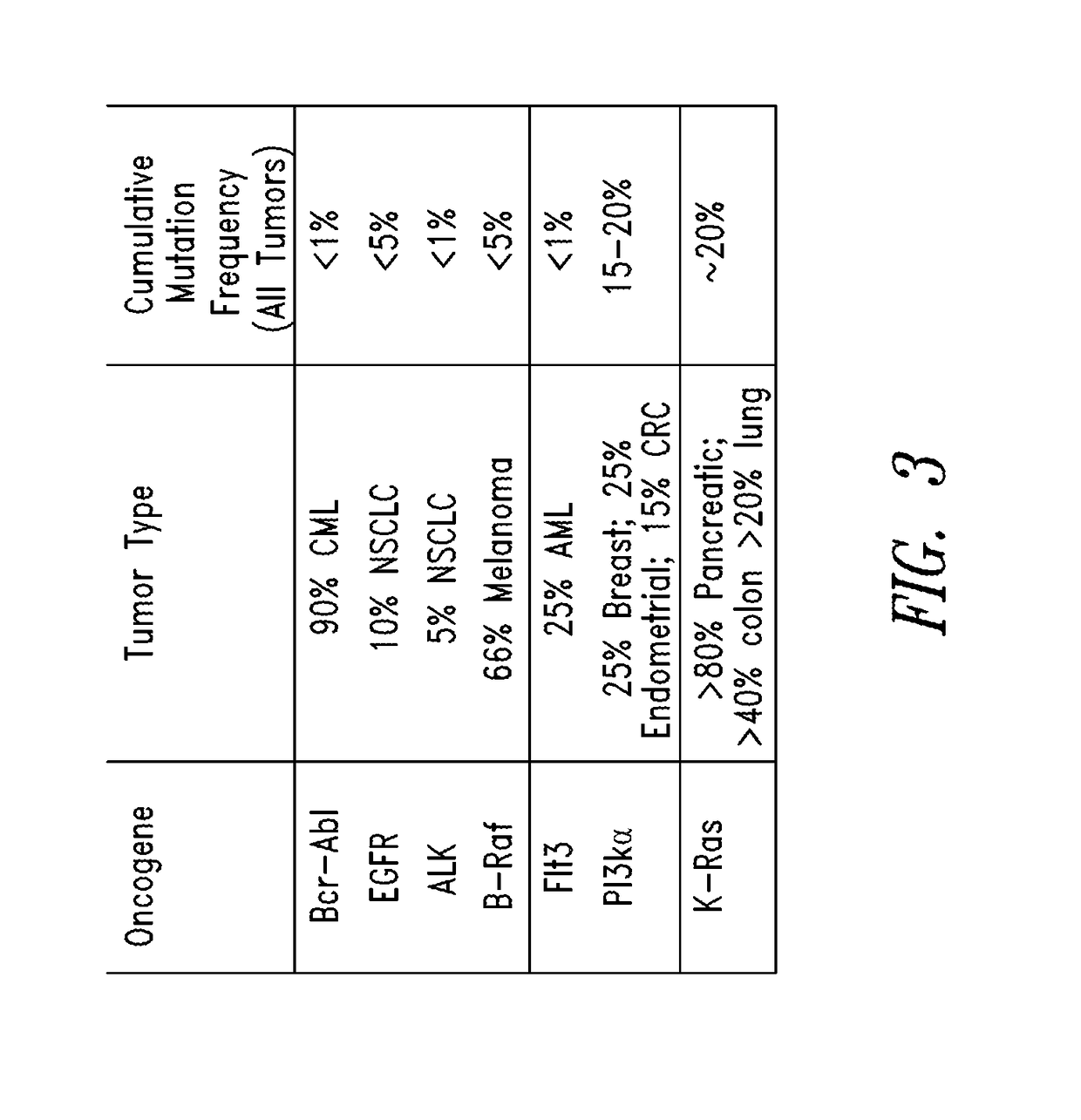
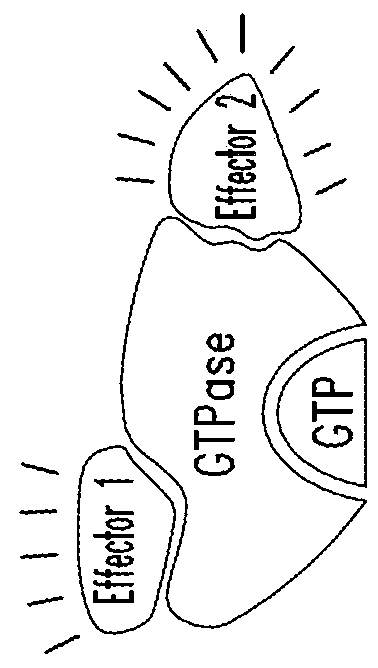
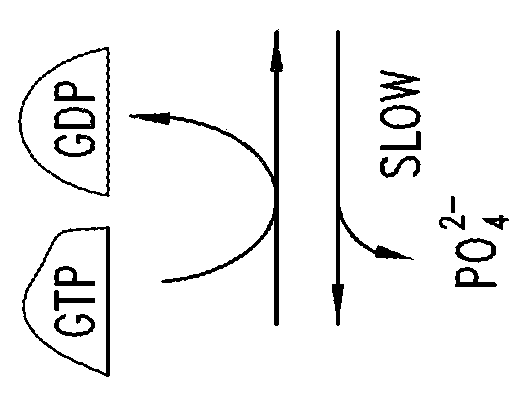
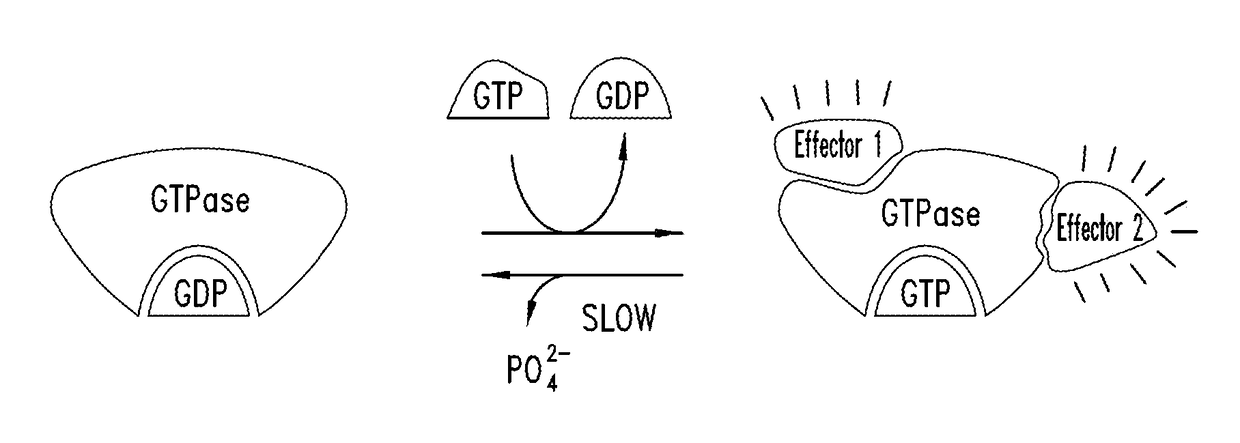
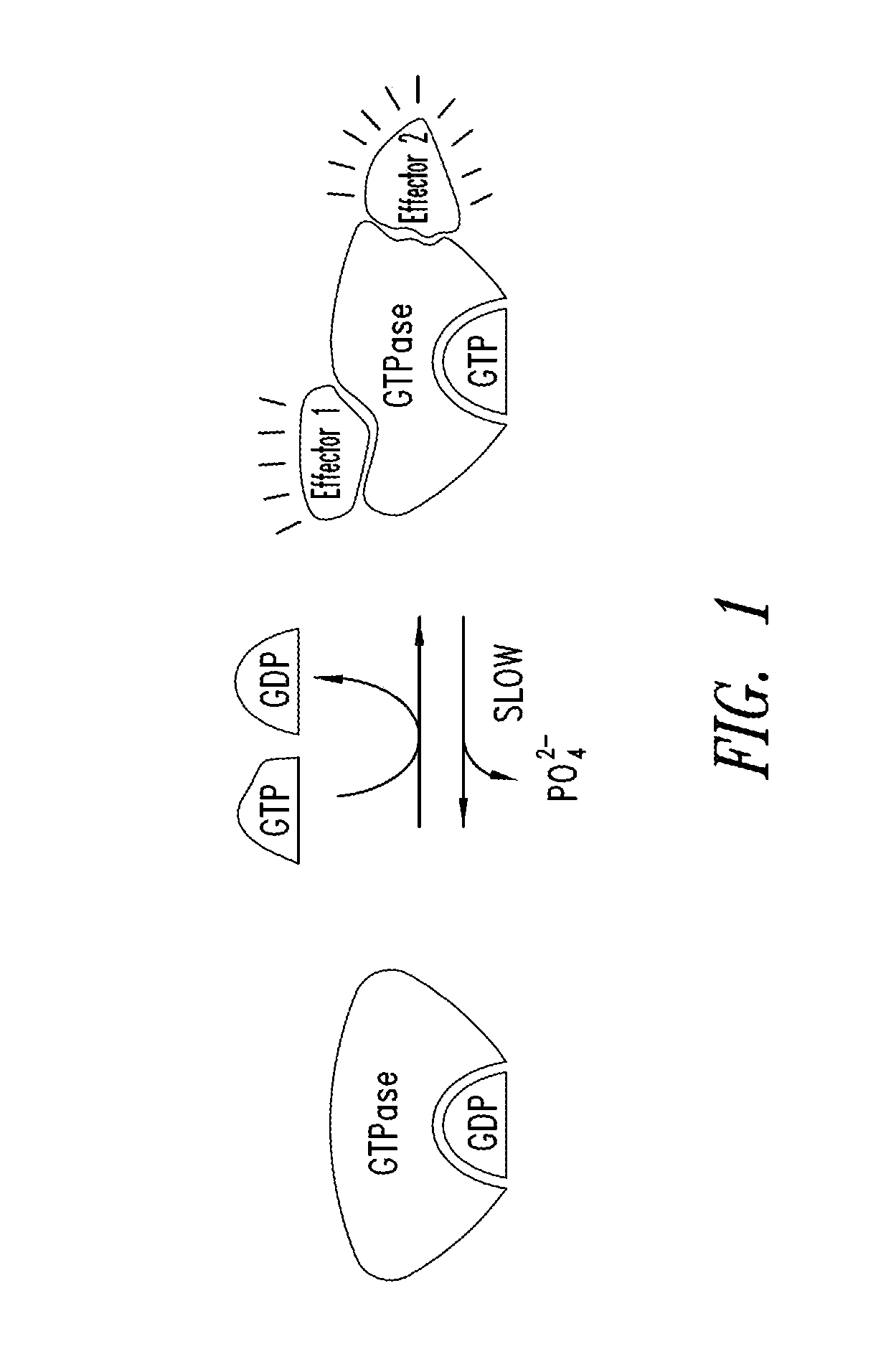
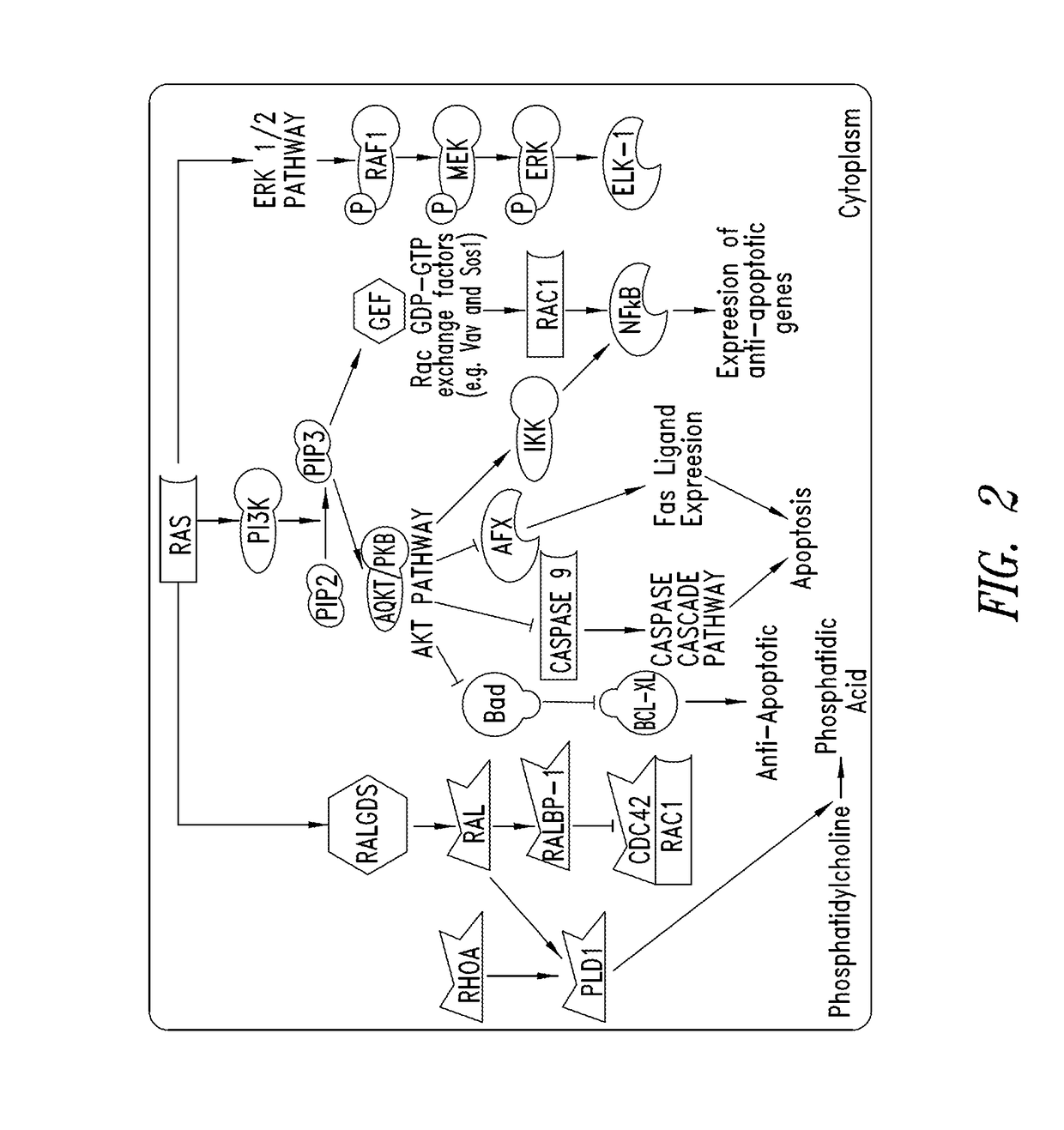
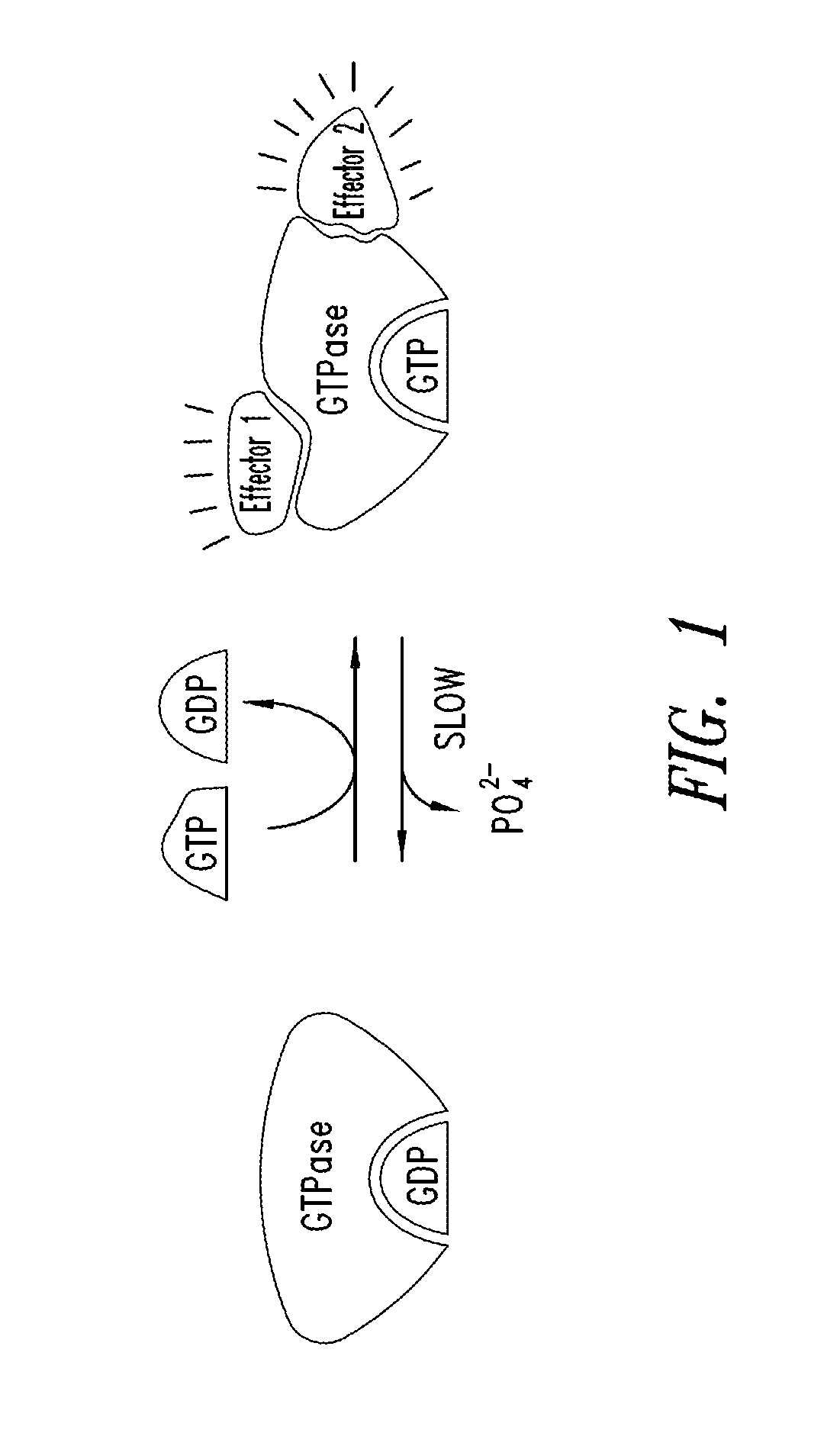
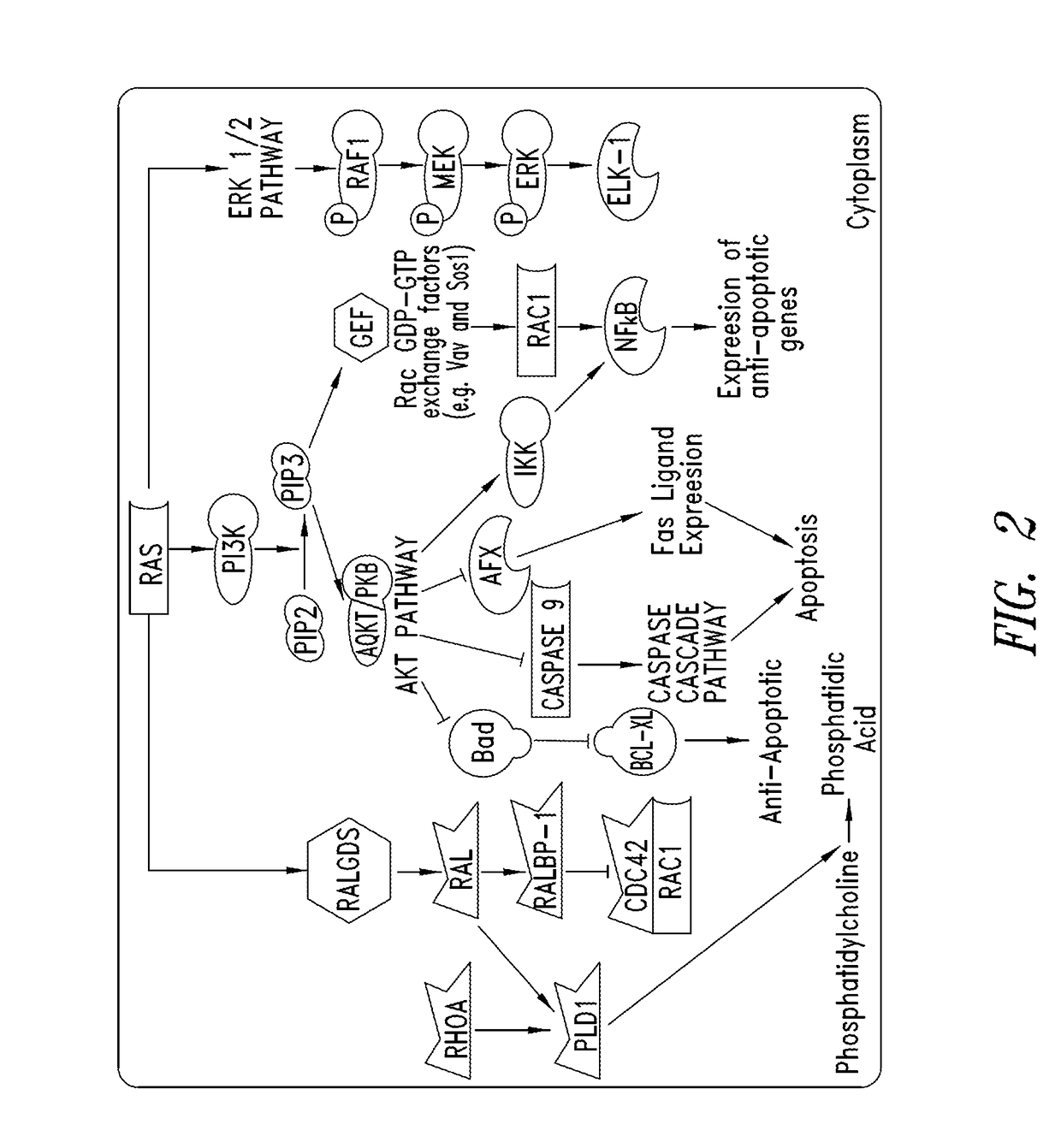
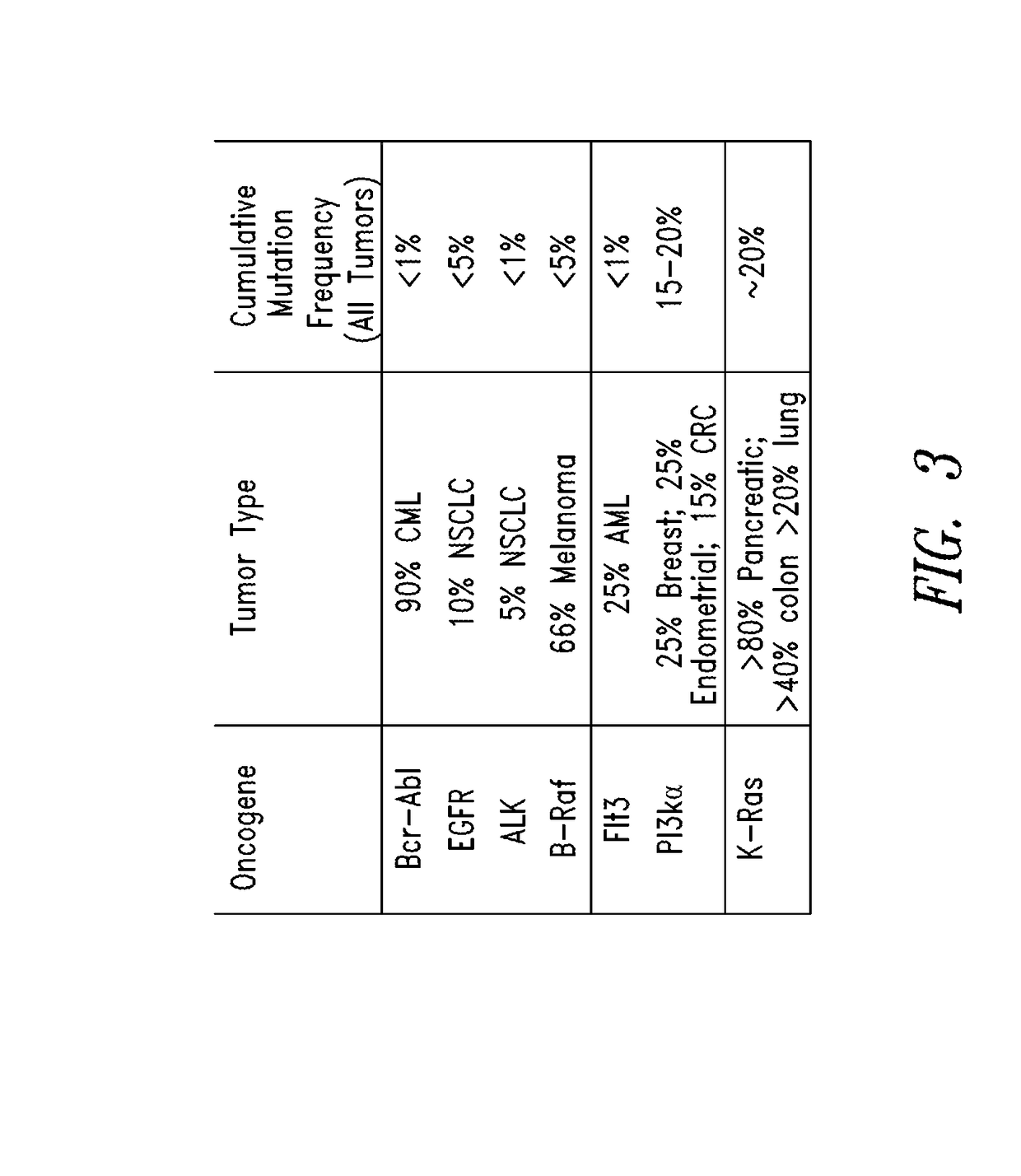
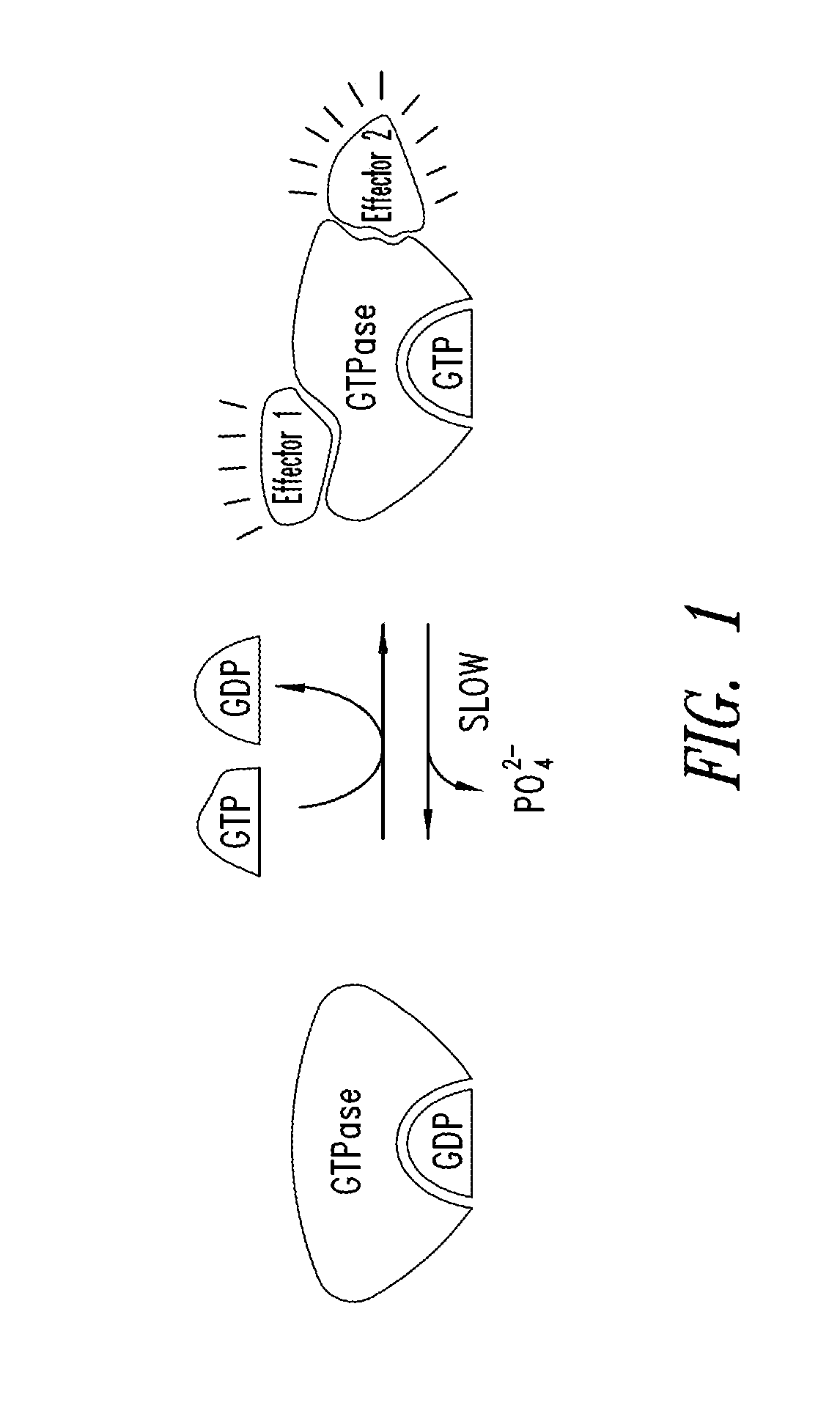
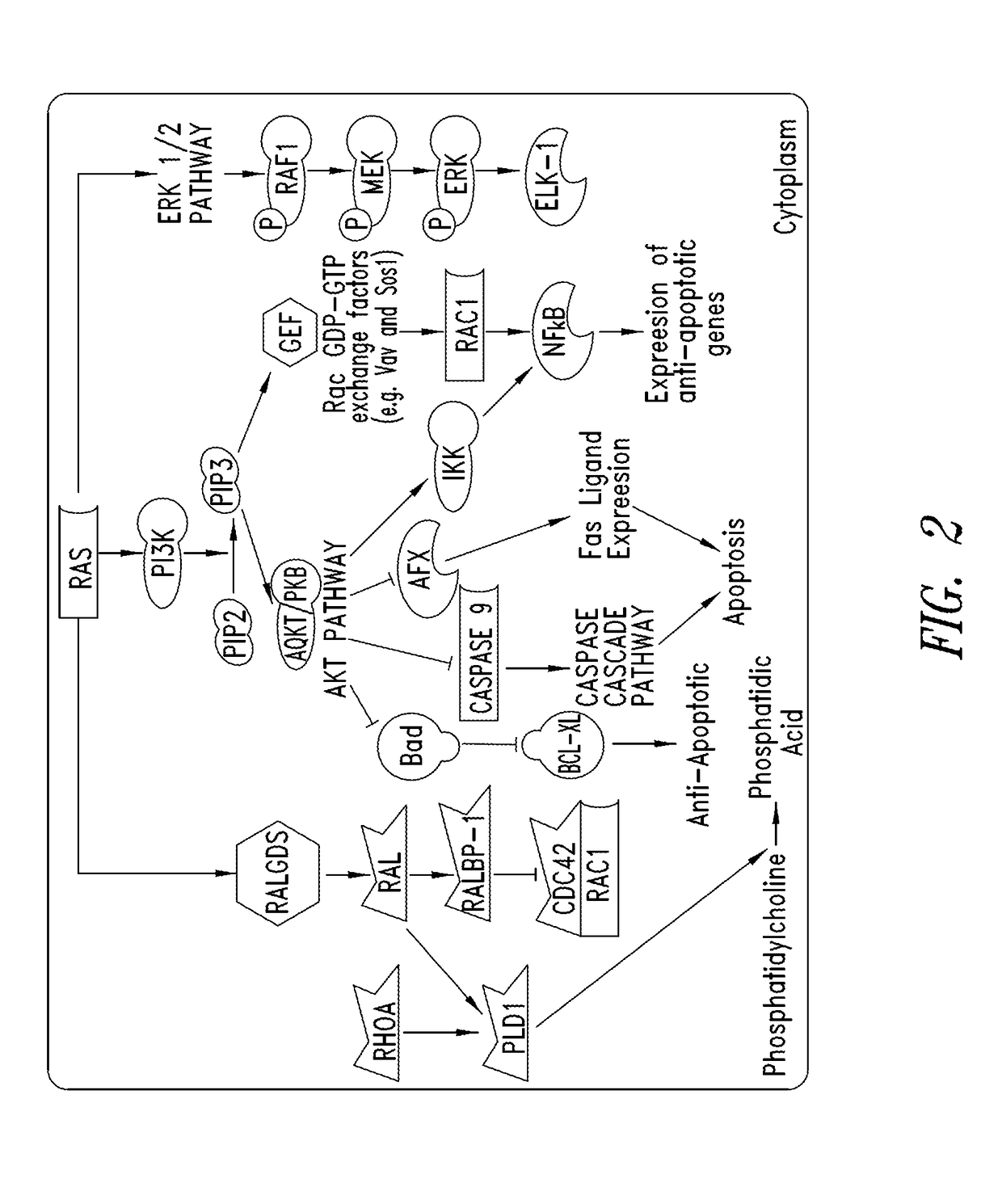
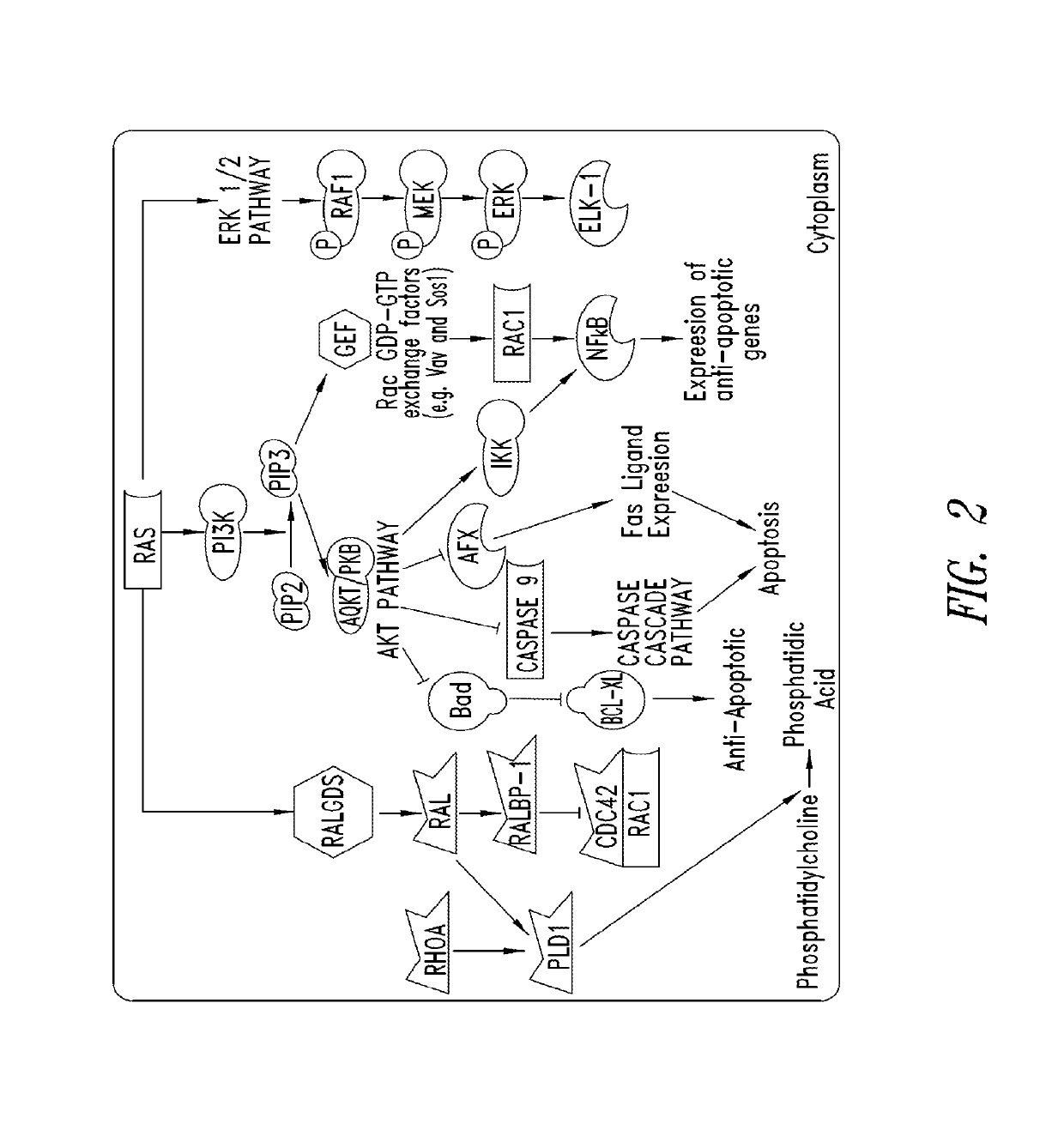
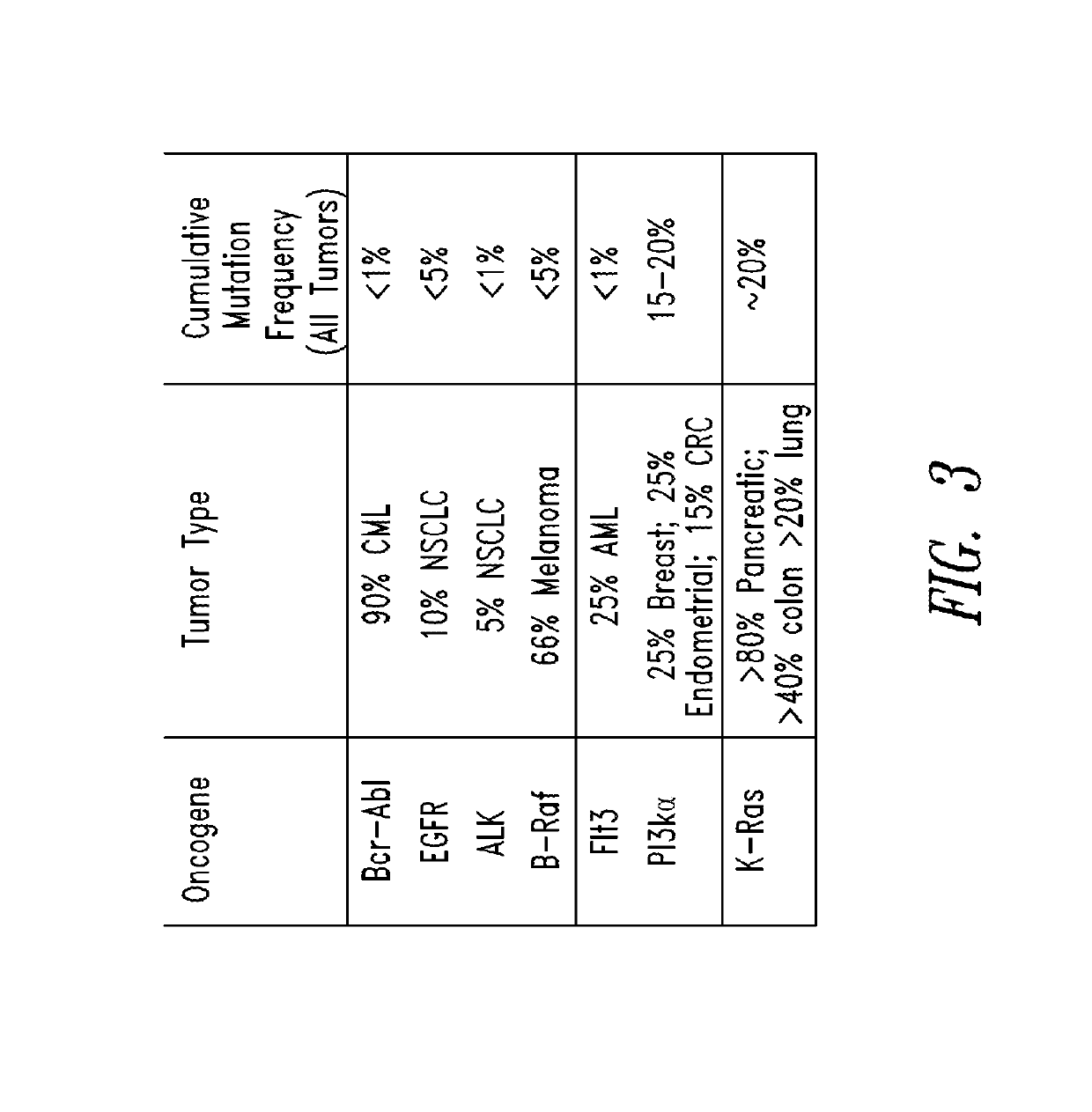
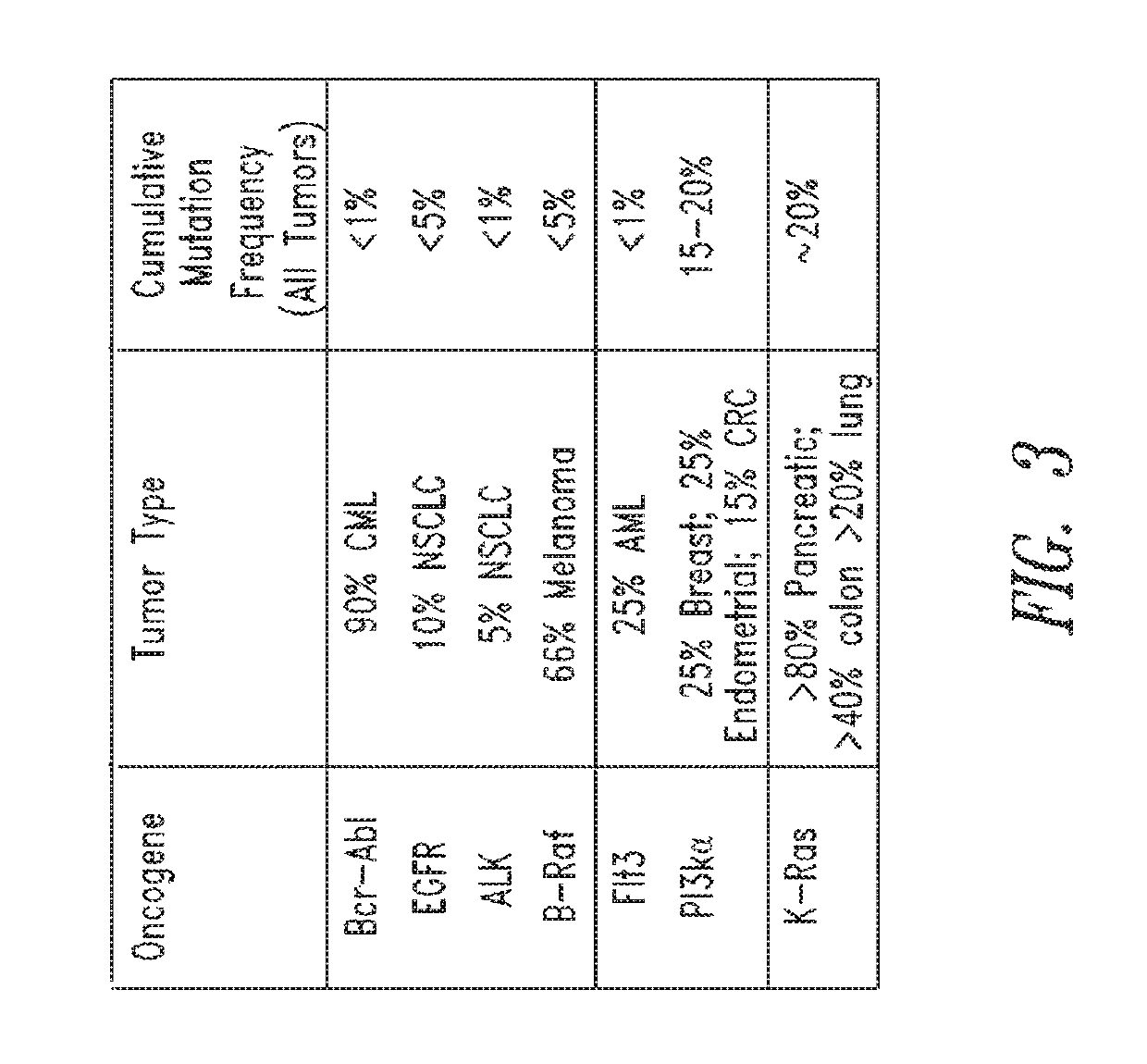
KRAS (K-ras or Ki-ras) is a gene that acts as an on/off switch in cell signalling. When it functions normally, it controls cell proliferation. When it is mutated, negative signalling is disrupted. Thus, cells can continuously proliferate, and often develop into cancer.
Method for predicting sensitivity to EGFR inhibitor
InactiveUS20160002741A1Accurate SensitivityMicrobiological testing/measurementDisease diagnosisKRASWild type
A method for predicting sensitivity to an EGFR inhibitor includes: (a) determining whether there is a KRAS gene-derived nucleic acid or a protein thereof in a blood sample which has been collected from a subject, and whether the KRAS gene-derived nucleic acid or the protein thereof in the blood sample is wild type or mutant; and (b) determining that there is a high possibility that a tumor of the subject is sensitive to an EGFR inhibitor when a wild type KRAS gene-derived nucleic acid or a protein thereof is detected and no mutant KRAS gene-derived nucleic acid or a protein thereof is detected in the blood sample in the process (a).
Owner:TOPPAN PRINTING CO LTD
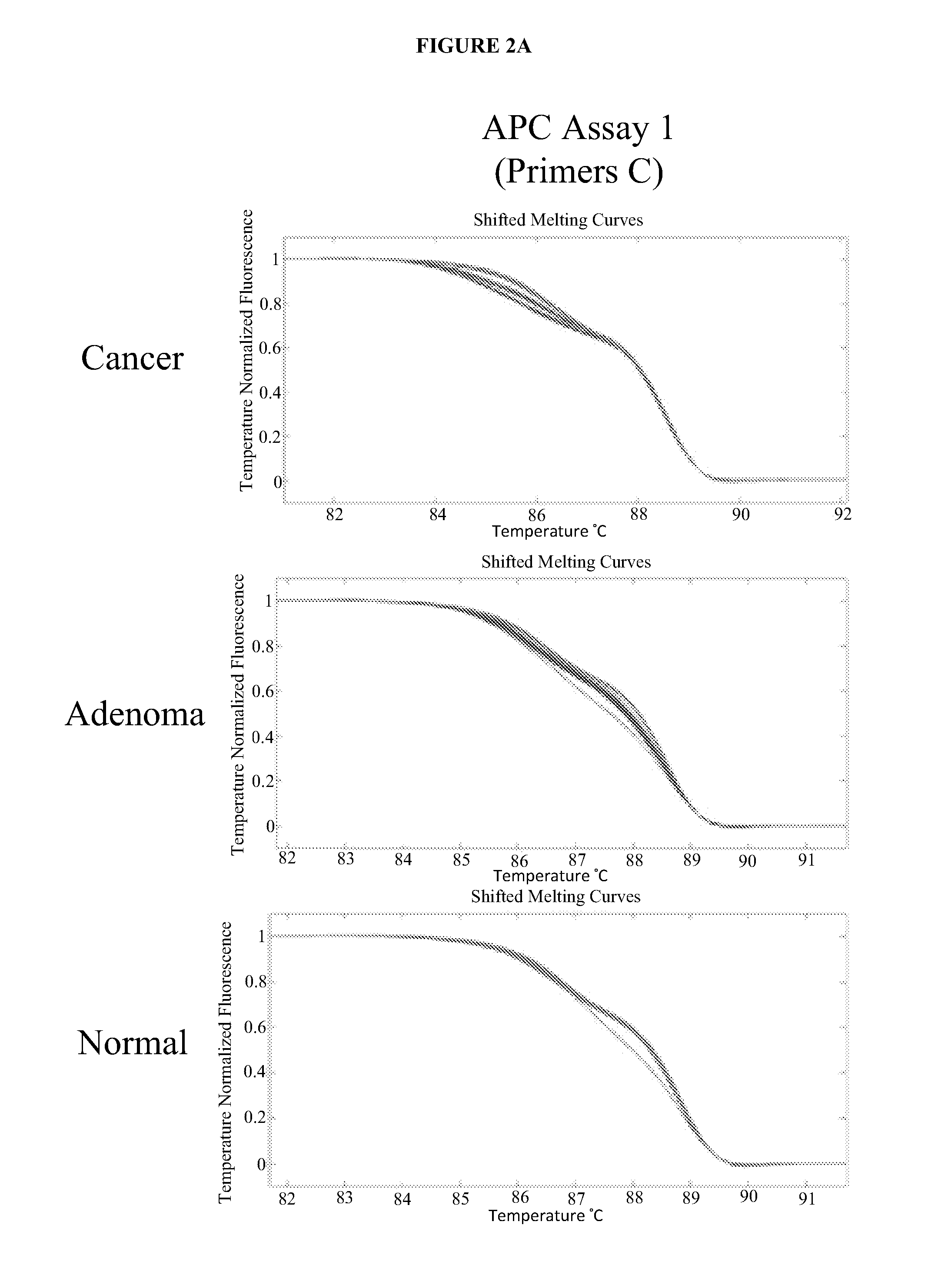
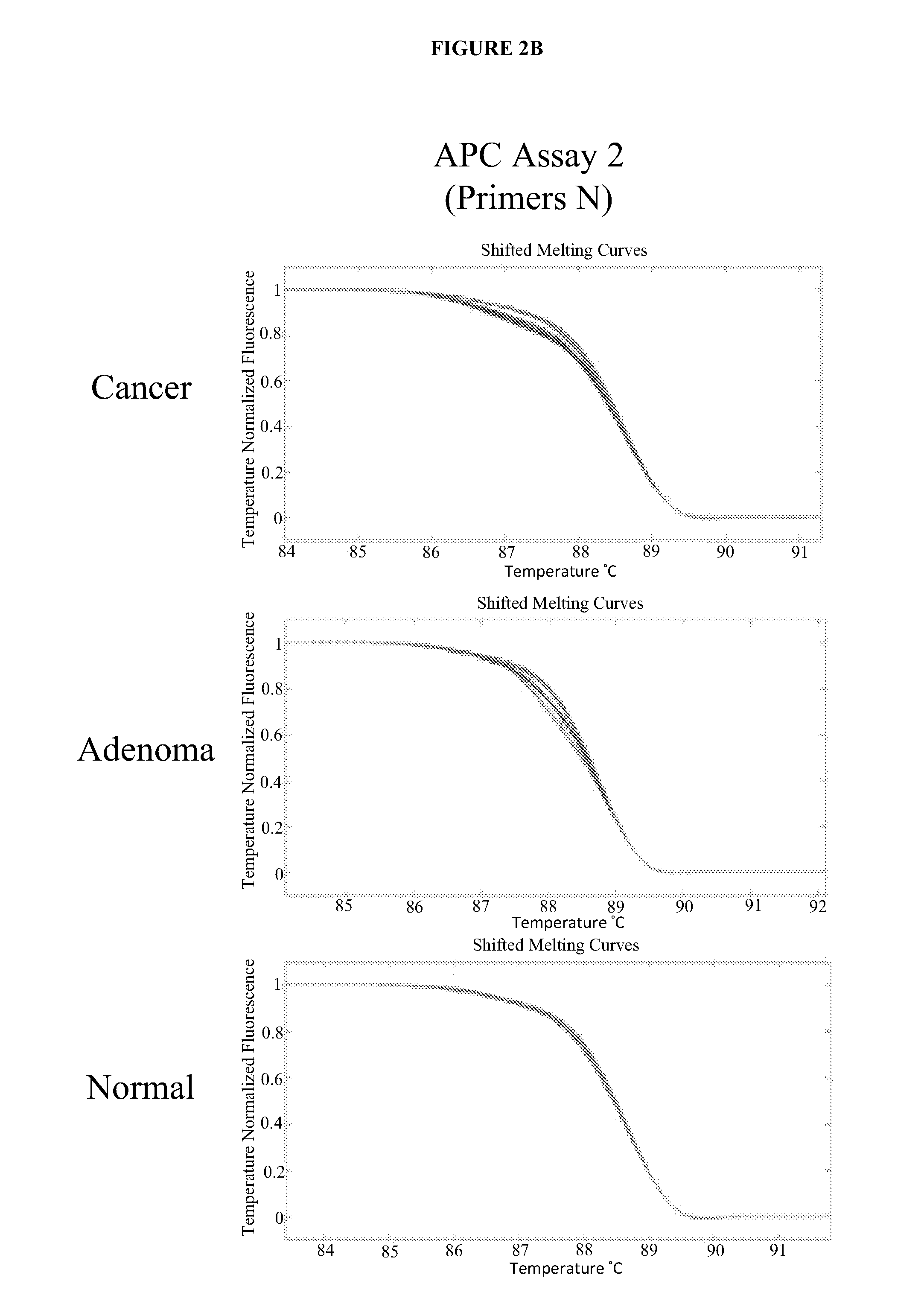
Methods and materials for detecting colorectal cancer and adenoma
InactiveUS20130012410A1High detectionHigh level of sensitivityMicrobiological testing/measurementLibrary screeningFOXE1Mammal
The present invention provides methods and materials related to the detection of colorectal neoplasm-specific markers (e.g., markers associated with colorectal cancer, markers associated with adenoma) in or associated with a subject's stool sample. In particular, the present invention provides methods and materials for identifying mammals (e.g., humans) having a colorectal neoplasm by detecting the presence and level of indicators of colorectal neoplasia such as, for example, long DNA (e.g., quantified by Alu PCR) and the presence and level of tumor-associated gene alterations (e.g., mutations in KRAS, APC, melanoma antigen gene, p53, BRAF, BAT26, PIK3CA) or epigenetic alterations (e.g., DNA methylation) (e.g., CpG methylation) (e.g., CpG methylation in coding or regulatory regions of bmp-3, bmp-4, SFRP2, vimentin, septin9, ALX4, EYA4, TFPI2, NDRG4, FOXE1) in DNA from a stool sample obtained from the mammal.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
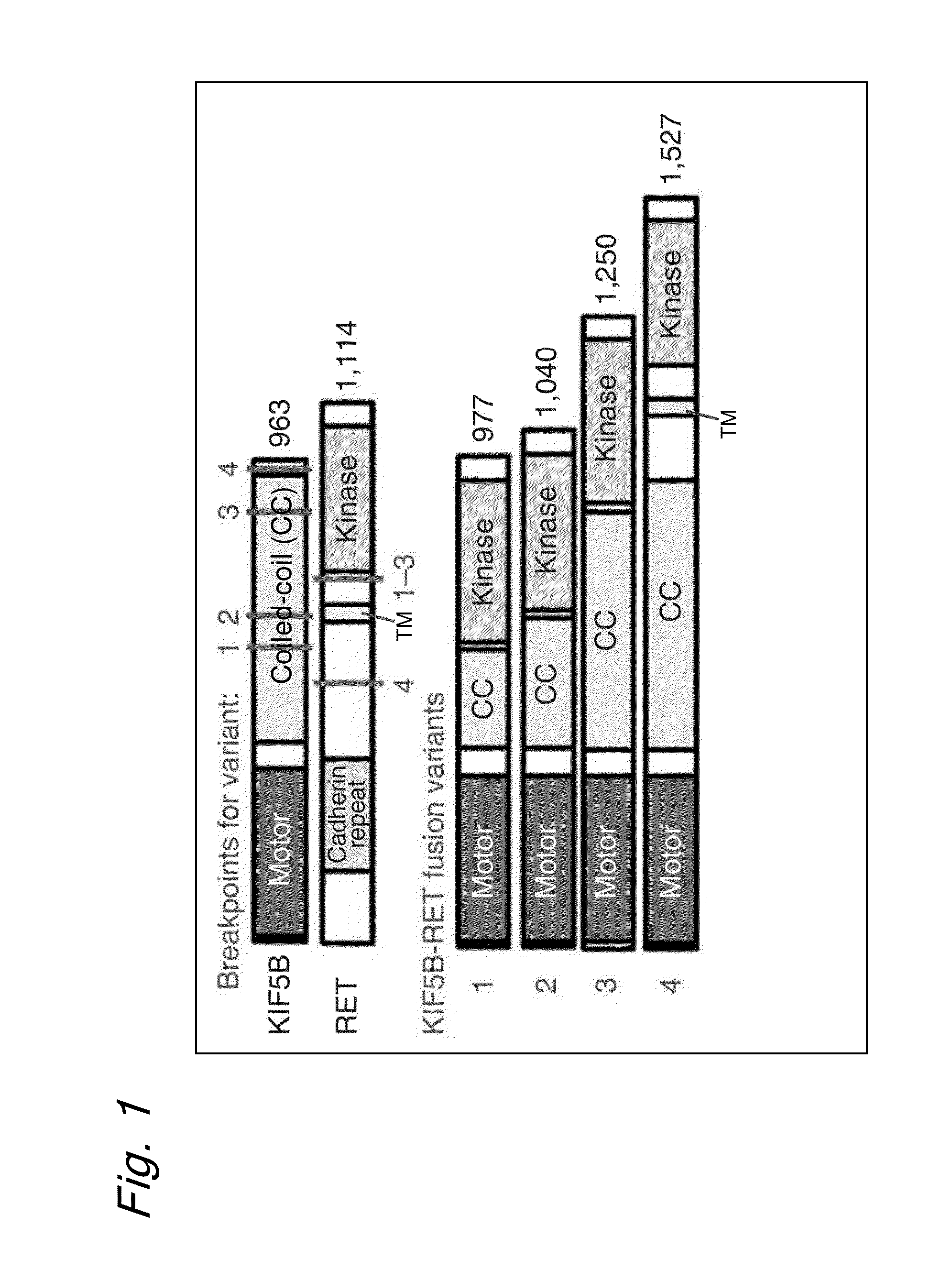
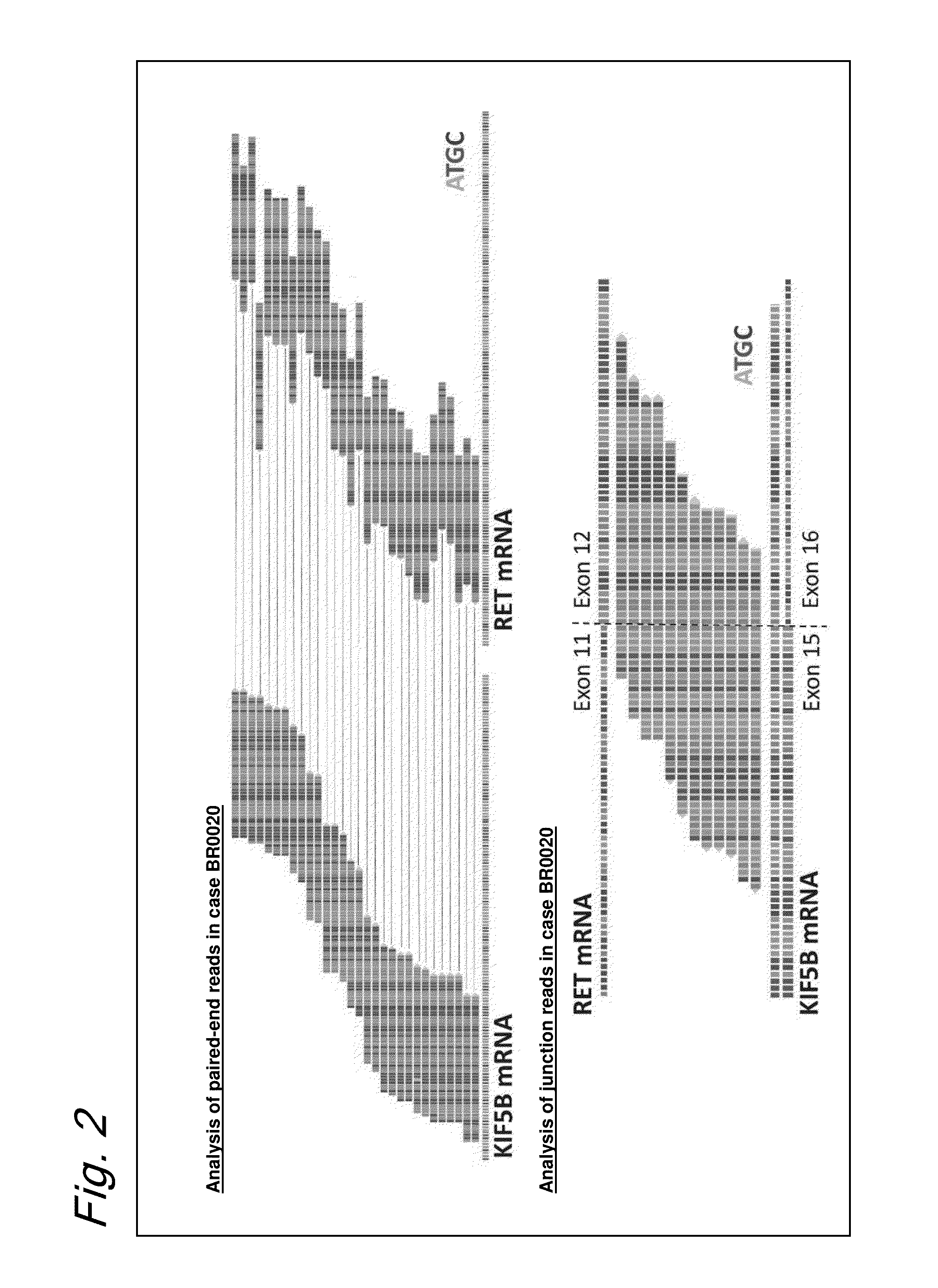
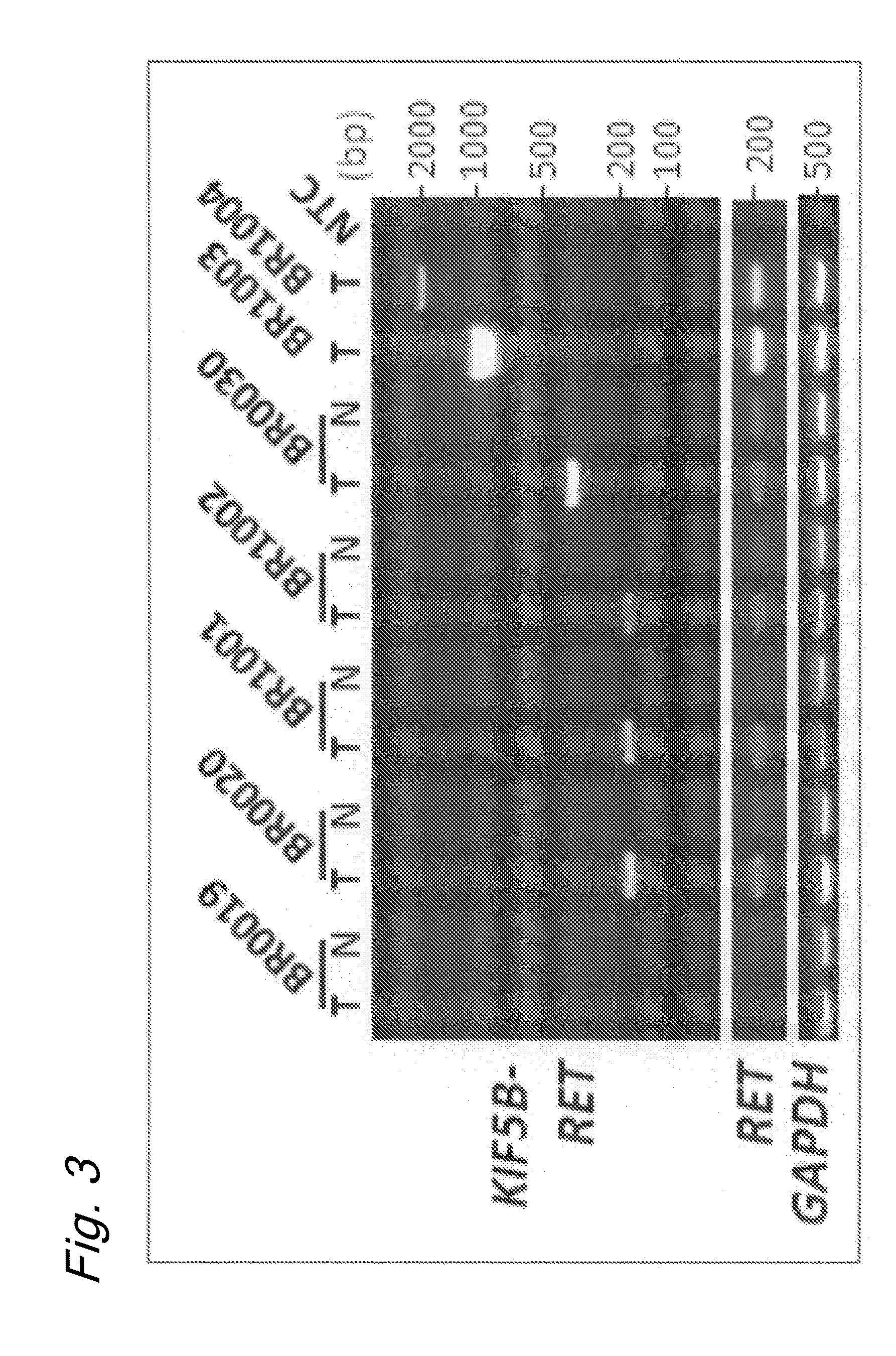
Method for determining effectiveness of cancer treatment by assessing the presence of a KIF5B-RET chimeric gene
ActiveUS9216172B2Prediction of effectivenessEffective treatmentHydrolasesPeptide/protein ingredientsActivating mutationTyrosine-kinase inhibitor
Owner:NAT CANCER CENT
Prognostic biomarkers to predict overall survival and metastatic disease in patients with triple negative breast cancer
The present invention relates to a method for prognosing cancer in a subject with triple negative (TN) breast cancer, whose tumors lack expression of the estrogen receptor (ER), the progesterone receptor (PR) and normal (not amplified) levels of the human epidermal growth factor receptor 2 (HER2). Methods and biomarkers are disclosed that are useful for predicting the overall survival (OS) potential of cancer in a subject with triple negative breast cancer or for predicting metastatic disease in a subject with triple negative breast cancer. For example, the method comprises detecting in a sample from a subject one or more biomarkers selected from the group consisting of ANK3, CD24, EIF1, KLF6, KRAS, KRT1, MAP2K4, SDC4, SLC2A3, STK3, TFAP2C, and WRN. An increase or decrease in one or more biomarkers as compared to a standard is prognostic of OS of TN breast cancer. Likewise, in another example, the method comprises detecting in a sample from a subject one or more biomarkers selected from the group consisting of ANG, DICER1, EIF1, and MSH6. An increase or decrease in one or more biomarkers as compared to a standard is prognostic of metastasis of TN breast cancer.
Owner:VM INST OF RES
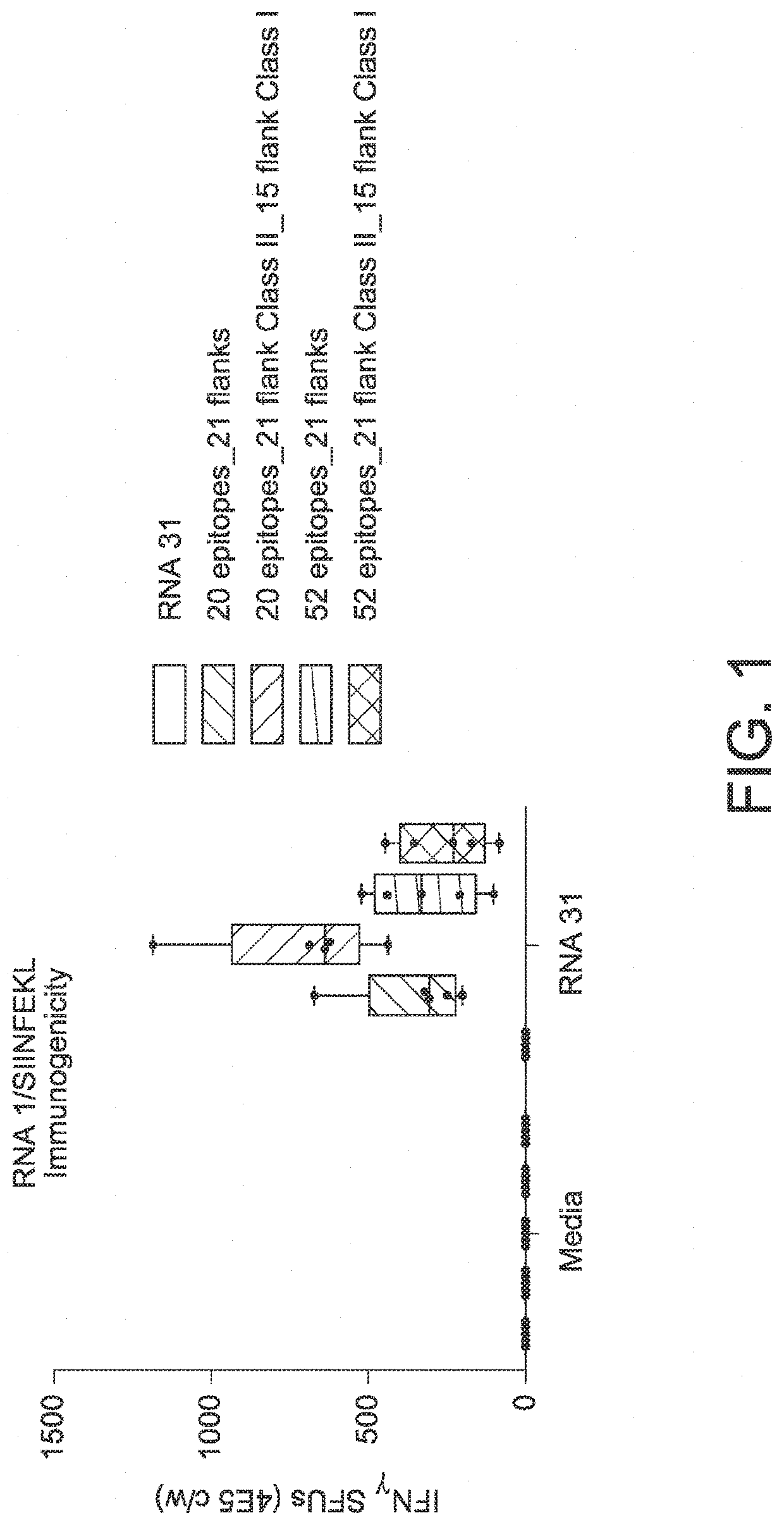
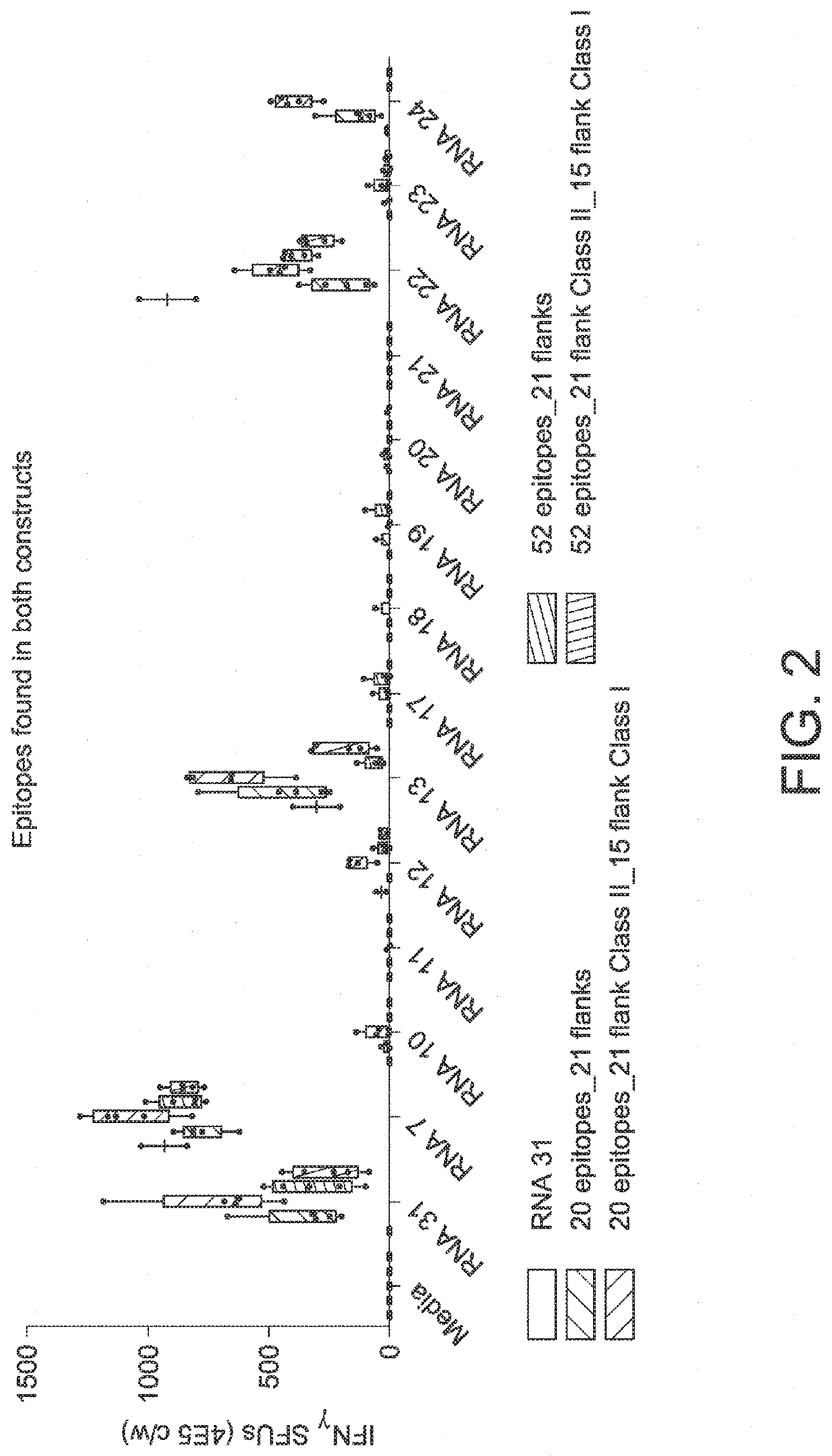
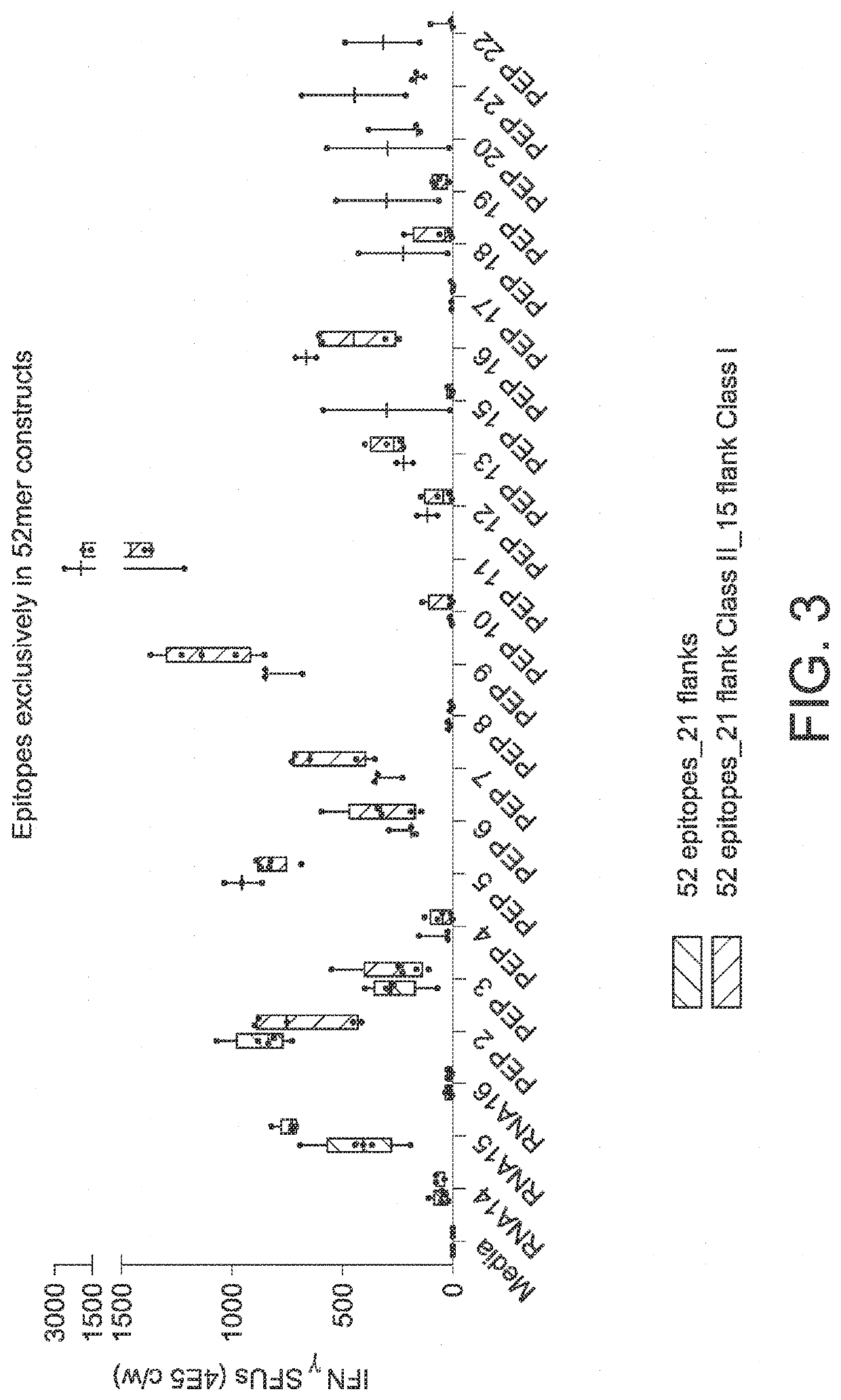
RNA cancer vaccines
PendingUS20190351040A1Balanced immune responseOrganic active ingredientsAntibody ingredientsTetanusAdjuvant
The disclosure relates to cancer ribonucleic acid (RNA) vaccines, as well as methods of using the vaccines and compositions comprising the vaccines. In particular, the disclosure relates to concatemeric mRNA cancer vaccines encoding several cancer epitopes on a single mRNA construct, i.e. poly-epitope mRNA constructs or poly-neo-epitope constructs. The disclosure further relates to p53 and KRAS mutations, as well as incorporation of immune enhancers such as STING, e.g. mRNA constructs further encoding an immune stimulator or adjuvant. The disclosure further relates to inclusion of universal T cell epitopes, such as tetanus or diphtheria toxins to elicit an enhanced immune response.
Owner:MODERNATX INC
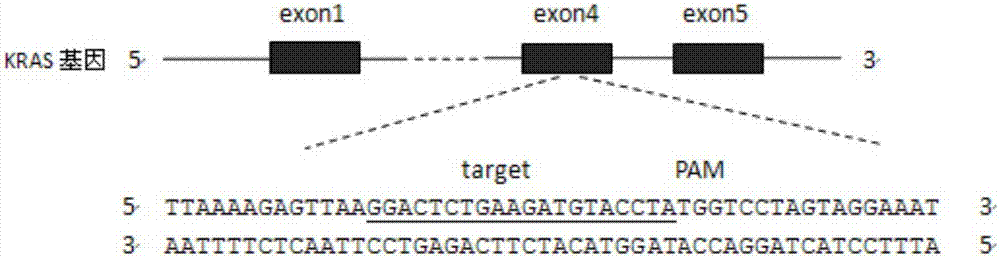
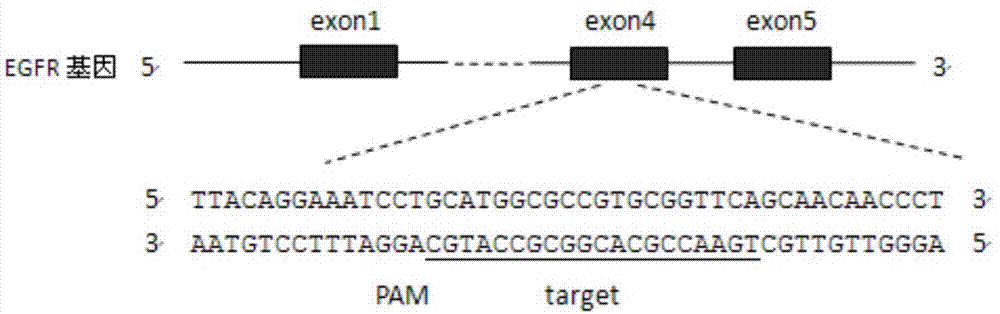
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR (Epidermal Growth Factor Receptor) genes and application thereof
ActiveCN107130000AHigh knockout efficiencyEasy to operateOrganic active ingredientsHydrolasesAbnormal expressionKRAS
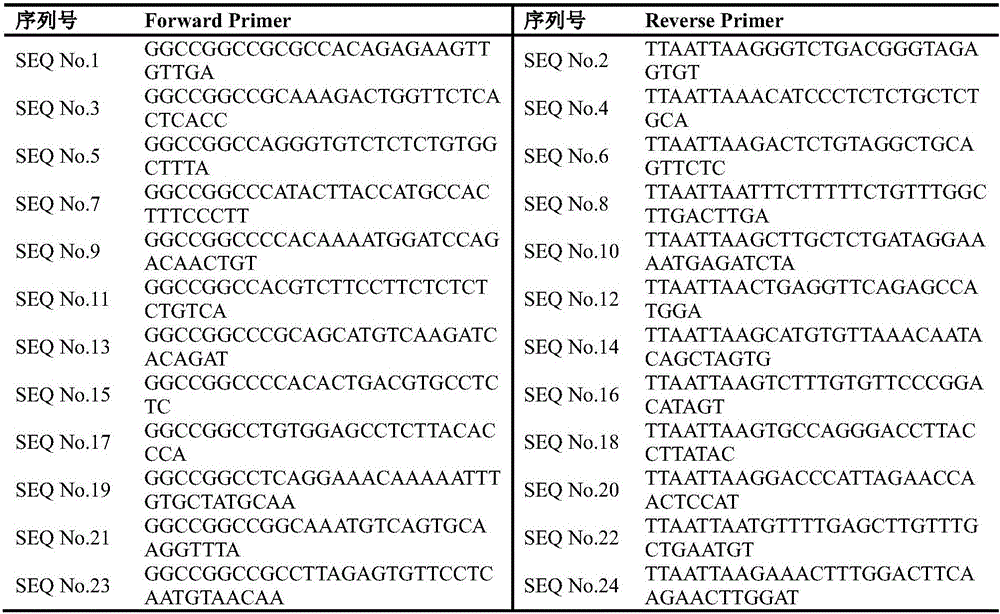
The invention discloses a CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR genes. The system comprises sgRNA for specifically targeting KRAS genes and sgRNA for specifically targeting EGFR genes, wherein a corresponding DNA sequence of the sgRNA for specifically targeting KRAS genes is shown as SEQ ID NO.1 or / and SEQ ID NO.2; and a corresponding DNA sequence of the sgRNA for specifically targeting EGFR genes is shown as SEQ ID NO.11 or / and SEQ ID NO.12. The invention further discloses application of the system in preparation of medicines for treating cancers. The CRISPR-Cas9 system disclosed by the invention is capable of simultaneously and efficiently knocking out two cancer driving factors KRAS and EGFR which are highly-expressed in lung cancer. The system is simple in operation and high in knockout efficiency and is expected to be applied to treatment of the lung cancer. The system disclosed by the invention is applicable to multiple cancers with abnormal expressions of the EGFR and KRAS.
Owner:浙江卫未生物医药科技有限公司
Inhibitors of kras g12c mutant proteins
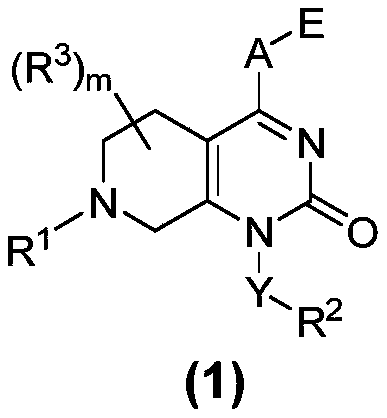
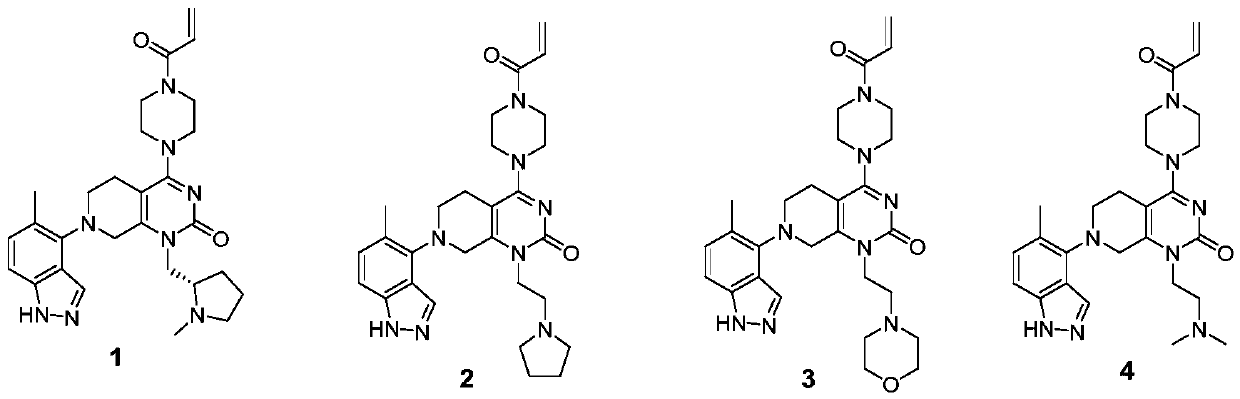
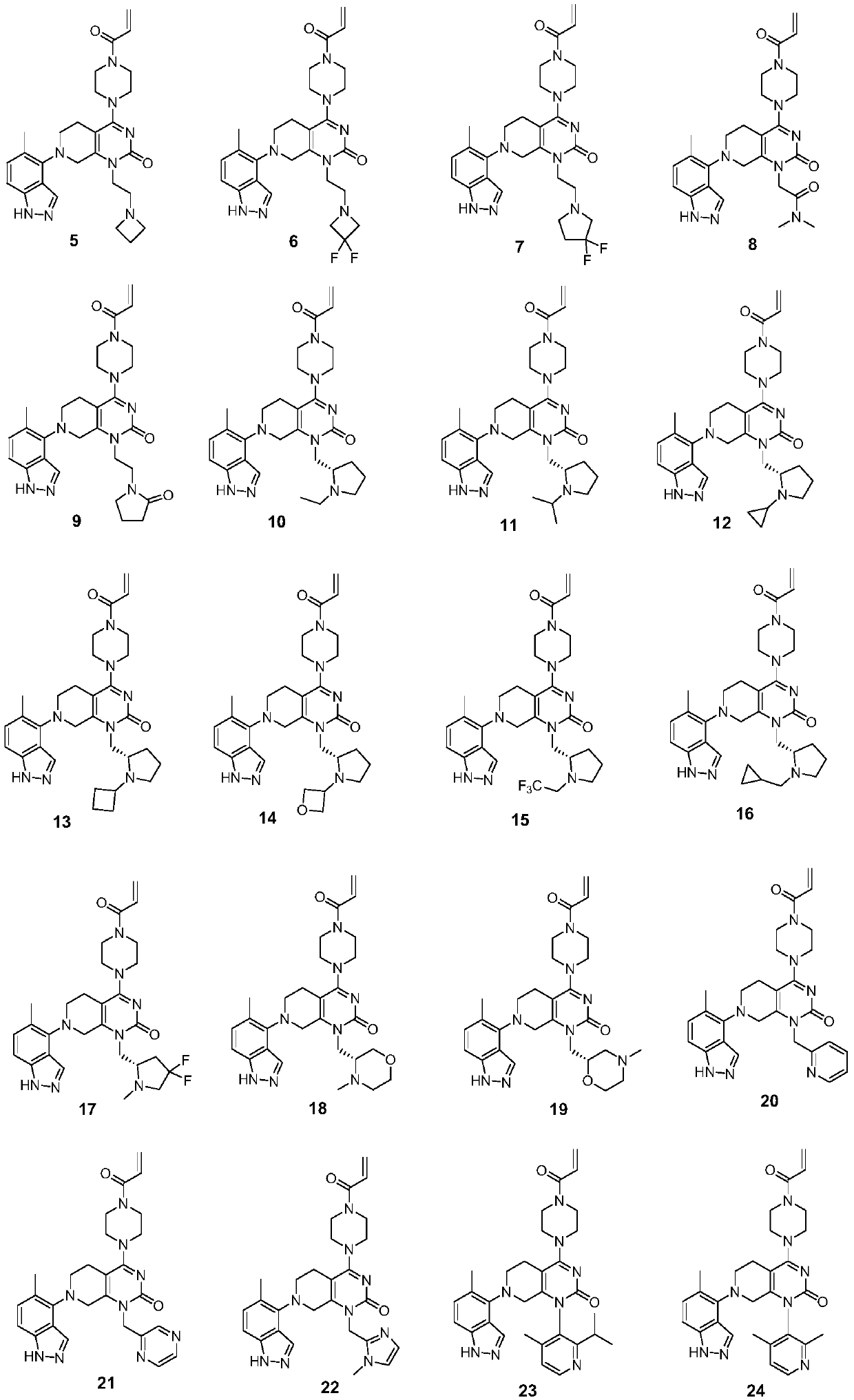
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein A, B R1, R2a, R2b, R3a, R3b, R4a, R4b, G1, G2, m1, m2, L1, L2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): (I) or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof wherein Z, Y R3a, R3b, R4a, R4b, G1, G2, G3, G4, L1, m1, m2, m3, m4, n and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of KRAS G12C mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein R1, R2, R3a, R3b, R4a, R4b, G1, G2, L, m1, m2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein A, X, R3a, R3b, R4a, R4b, G1, G2, G3, G4, L1, L2, m1, m2, m3, m4, and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): (I) or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein A, R3a, R3b, R4a, R4b, G1, G2, G3, G4, m1, m2, m3, m4, L1, L2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
crRNA (Clustered Regularly Interspaced Short Palindromic Repeat-Derived Ribonucleic Acid) for specifically detecting exon mutation of human KRAS genes #2 and #3 based on CRISPR technique
InactiveCN108893529AHigh sensitivityEasy to operateMicrobiological testing/measurementTemperature controlKRAS
The invention discloses crRNA (Clustered Regularly Interspaced Short Palindromic Repeat-Derived Ribonucleic Acid) for specifically detecting exon mutation of human KRAS genes #2 and #3 based on a CRISPR technique. The crRNA is characterized by comprising a CRISPR-Cas13a system as well as crRNA, wherein the crRNA can be combined with the CRISPR-Cas13a system; and the crRNA has a sequence format of5'-Cas13a protein anchoring sequence-crRNA guide sequence-3'. The crRNA has the advantages that according to detection results, whether the human KRAS gene have mutation or not can be judged intuitively through fluorescent reading, high-flux sequencing can be avoided, and the crRNA has the advantages of being low in cost, possible in multi-time detection, high in detection speed, possible in direct result analysis, and the like, and is applicable to large-scale clinical sample detection; the nucleic acid testing technique disclosed by the invention is different from a conventional detection technique which is based on PCR (Polymerase Chain Reaction) techniques, and no complex temperature control instrument or system is needed in a whole reaction process; and three steps of reactions are carried out in a same reaction system, so that operation procedures can be further simplified, and nucleic acid fragments with specific sequences can be detected within two hours.
Owner:江苏博嘉生物医学科技有限公司
Screening method of colorectal cancer treatment prognosis biomarkers
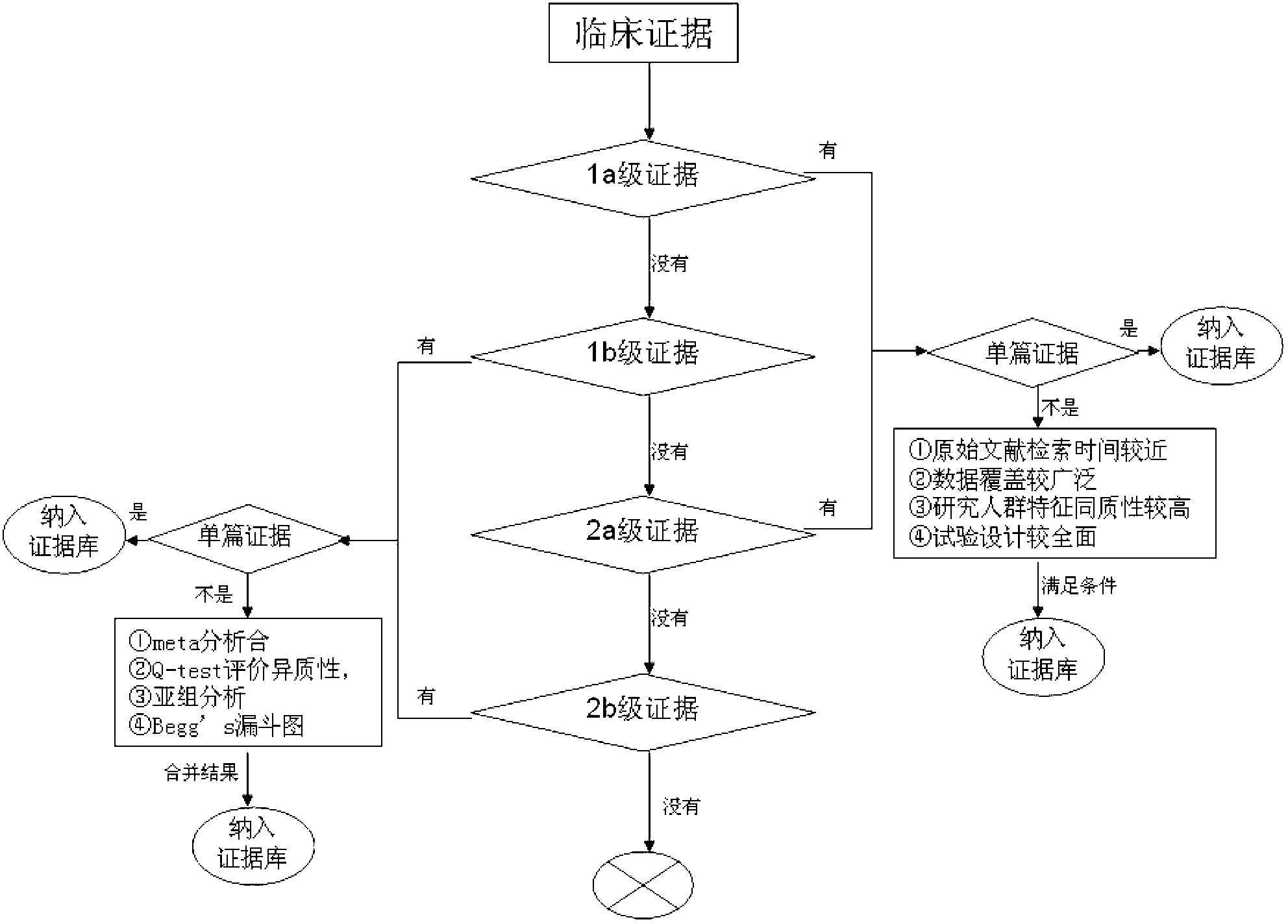
InactiveCN103324846AShorten screening timeReduce screening costsSpecial data processing applicationsDNA/RNA fragmentationKRASPrognosis biomarker
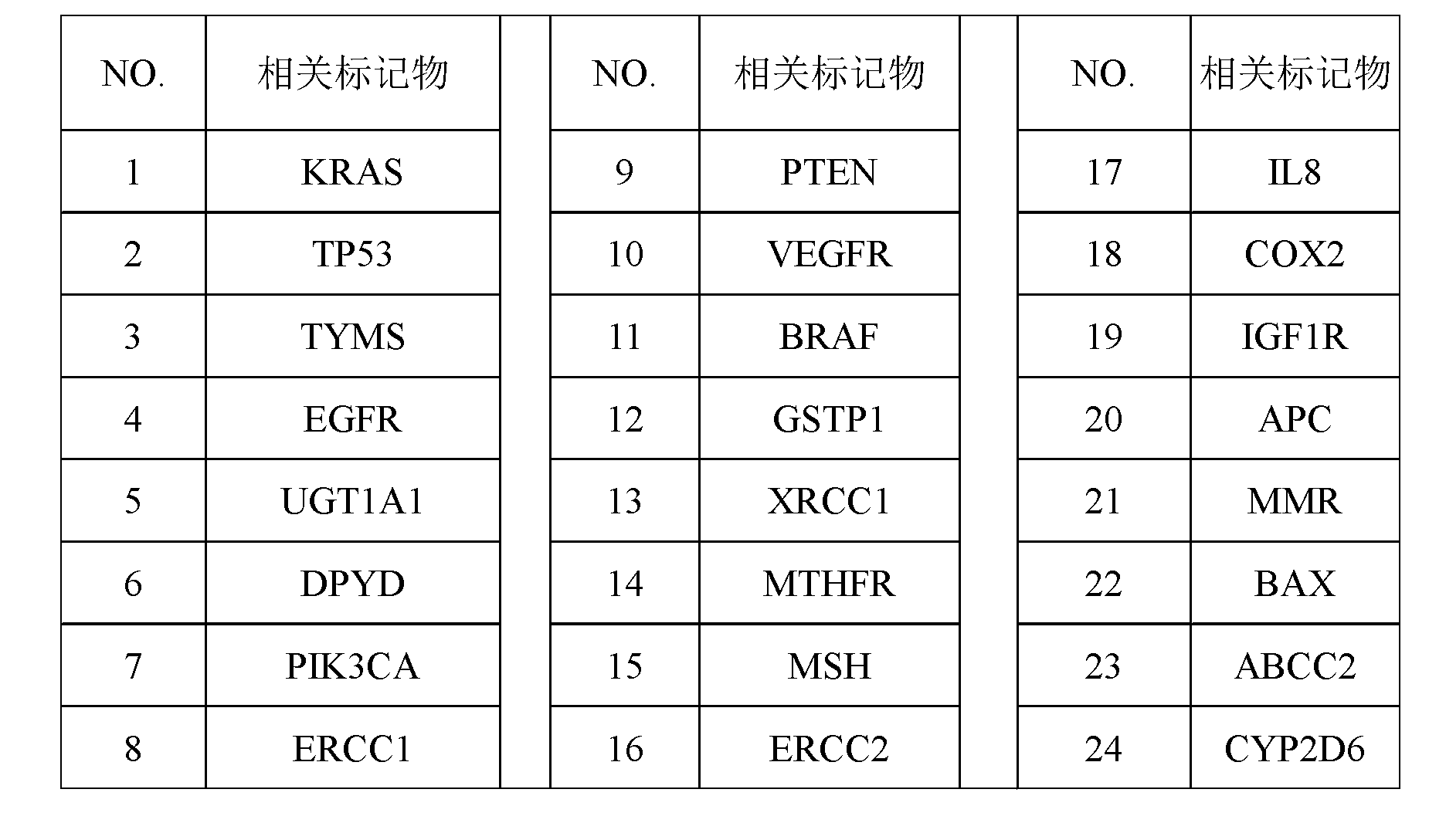
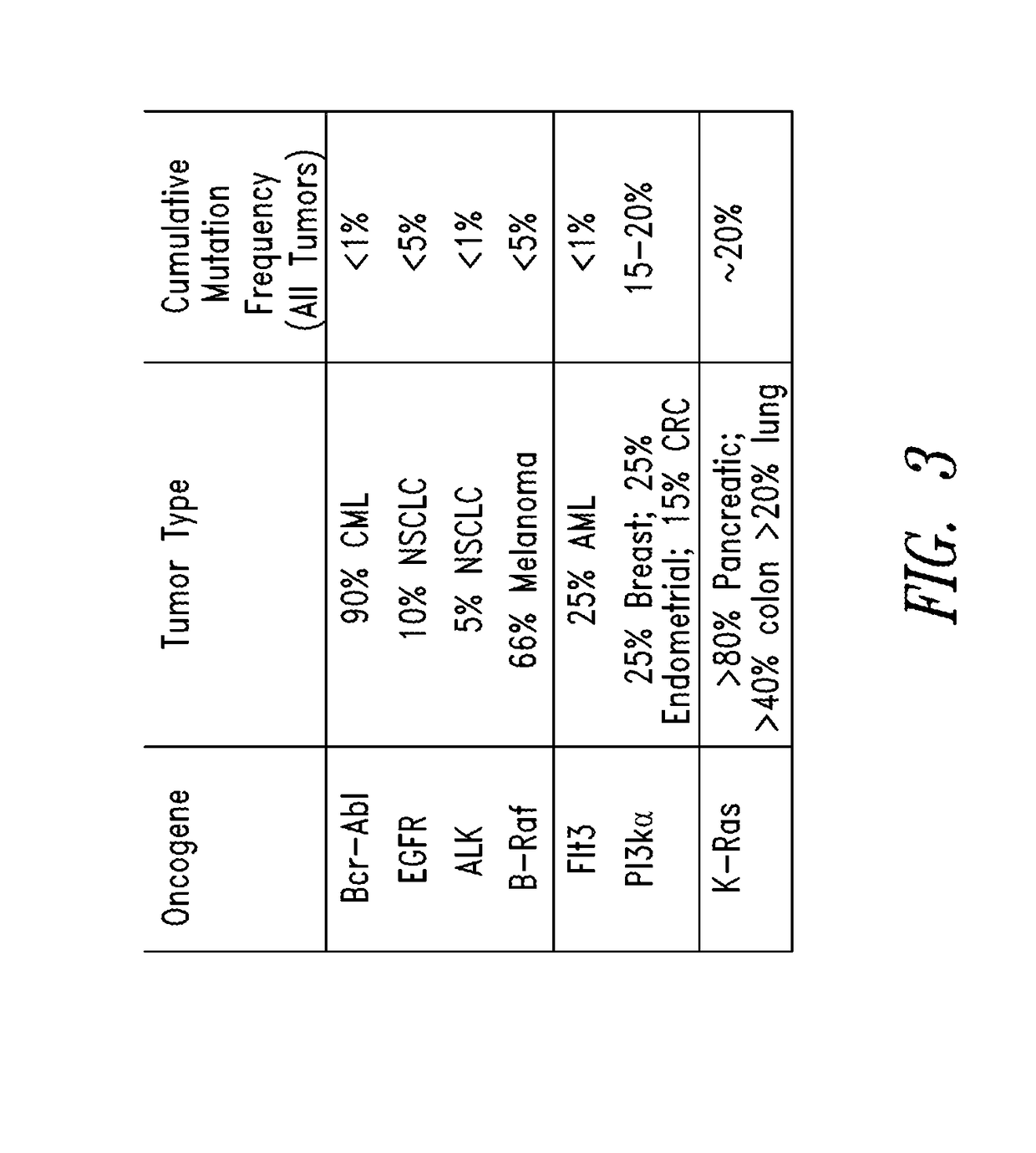
The invention discloses a screening method of colorectal cancer treatment prognosis biomarkers. The screening method includes the following steps of: (1) making a primary search strategy for screening the biomarkers; (2) making a literature adopting and exclusion criterion; (3) analyzing data and primarily screening the biomarkers; (4) making evident grades; (5) making a high quality evident evaluation criterion; (6) performing grading retrieval and quality evaluation on biomarker clinic evident; (7) performing data analyzing and statistics on the biomarker clinic evident; (8) screening the prognosis biomarkers. The invention further provides the colorectal cancer treatment prognosis biomarkers obtained through screening according to the screening method, and the prognosis biomarkers include KRAS, TYMS, TYMS, EGFR, UGT1A1*28, DPYD, PIK3CA, ERCC1, PTEN, VEGFR, BRAF, GST P1, XRCC1, MTHFR, MSI and the like.
Owner:浙江加州国际纳米技术研究院绍兴分院
Method and system for managing a strategic plan via defining and aligning strategic plan elements
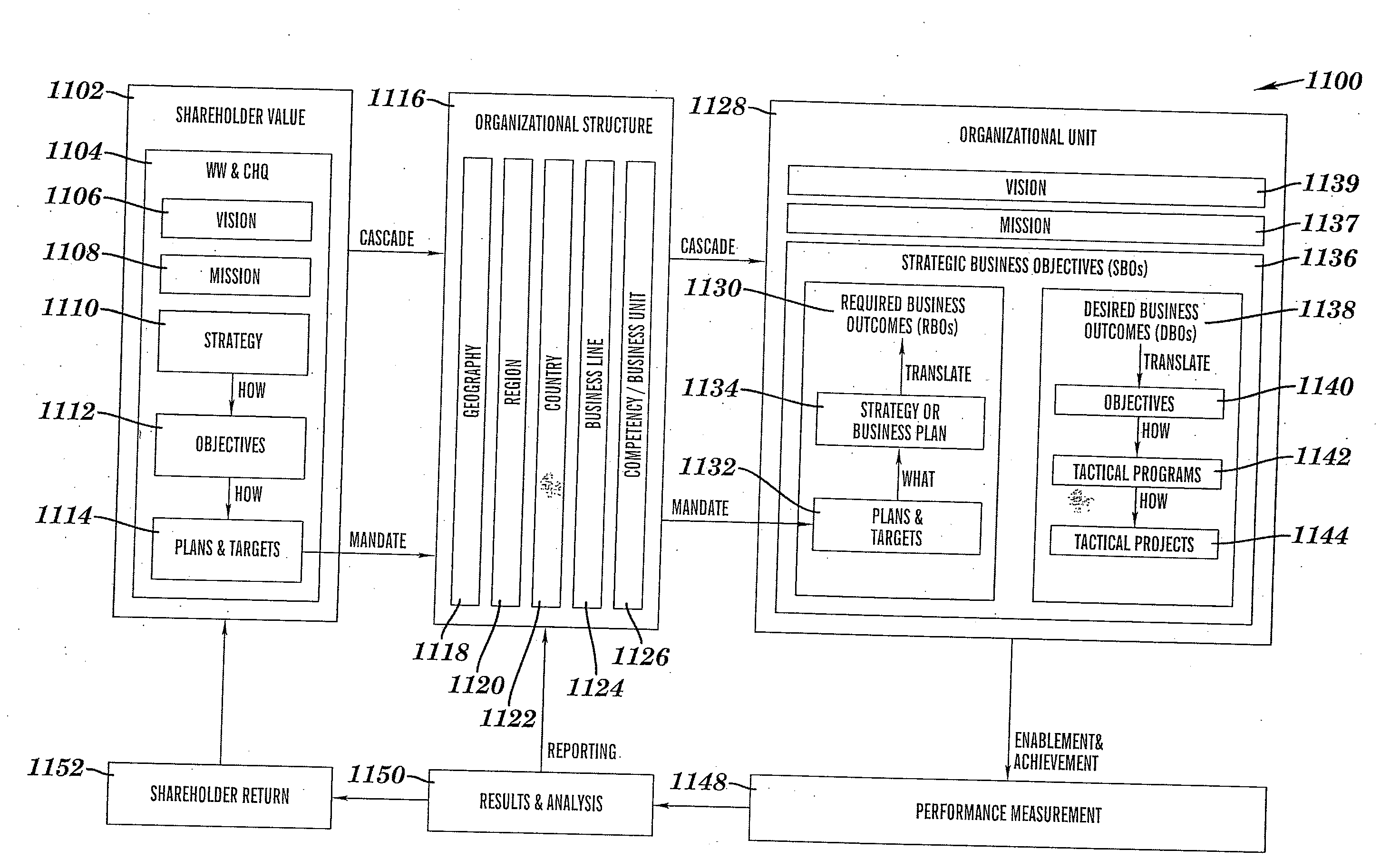
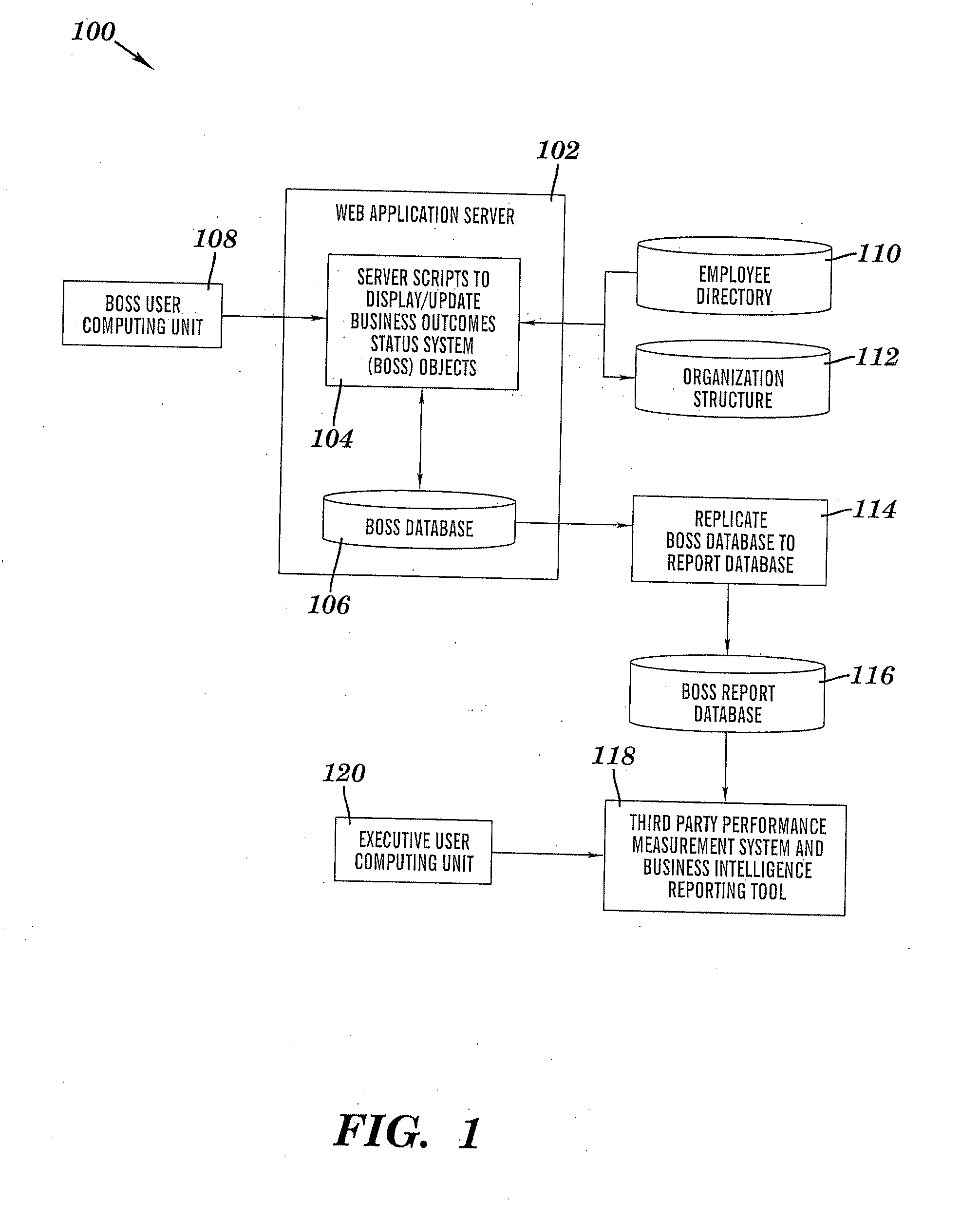
InactiveUS20080281651A1Improve executionFacilitate decision-makingResourcesSpecific program execution arrangementsKRASProgram planning
A method and system for managing a strategic plan via developing and aligning strategy elements that include required business outcomes (RBOs), strategic business objectives (SBOs), and desired business outcomes (DBOs). RBOs are developed and assigned to key result areas (KRAs). RBOs are operational goals required by a higher-level organization, and deliverable via an execution of operational activities of a subordinate organization. KRAs are performance measurement categories included on the subordinate organization's scorecard and the higher-level organization's scorecard. SBOs are developed and assigned to KRAs, resulting in a first alignment of RBOs with SBOs. SBOs are strategic objectives of the subordinate organization. DBOs are developed and assigned to KRAs, resulting in a second alignment of DBOs with SBOs. DBOs are desired results of the subordinate organization. Strategic goals of the higher-level organization are monitored using RBO and DBO statuses and the first and second alignments.
Owner:IBM CORP
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I) or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein R1, R3a, R3b, R4a, R4b, G1, G2, G3, G4, m1, m2, m3, m4, L1, L2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of kras g12c mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein A, R1, R3a, R3b, R4a, R4b, G2, G3, G4, m1, m2, m3, m4, L1, L2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Fused-tricyclic inhibitors of kras and methods of use thereof
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):(I) or a pharmaceutically acceptable salt, stereoisomer or prodrug thereof, wherein A is a heterocyclic or heteroaryl ring, and R1, R2a, R2b, R2c, R3a, R3b, R4a, R4b, A, G1, G2, L1, L2, m1, m2, and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of KRAS G12C mutant proteins
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):or a pharmaceutically acceptable salt, tautomer, stereoisomer or prodrug thereof, wherein B, W, X, Y, R1, R2a, R2b, R3a, R3b, R4a, R4b, G1, G2, m1, m2, L1, L2 and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Inhibitors of RAS and methods of use thereof
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):or a pharmaceutically acceptable salt, stereoisomer or prodrug thereof, wherein A, B, R″, Q, W, X, Y, Z, n1, n2 and are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1 yl)prop-2-en-1-one derivatives and similar compounds as kras g12c modulators for treating cancer
ActiveUS20190382377A1Prevent proliferationOrganic chemistryPharmaceutical delivery mechanismDiseaseBenzoxazole
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, isotopic form or stereoisomer thereof, wherein A is a five-membered heteroaryl comprising 1 or 2 non-adjacent heteroatoms, inclusive of X and Y; W, X, Y, Z, L, L1, E, R1, R2bR2c and the dotted circle are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and compounds for use in methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided. Preferred compounds are e.g. 1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1yl)prop-2-en-1-one derivatives and related compounds such as e.g. the corresponding derivatives with e.g. a benzoimidazole, indole, benzooxazole, imidazopyridine or imidazole core structure, substituted on ring A by e.g. azetidine, pyrrolidine, azepane or bicyclopentane-amine (L1) each substituted by e.g. propenone (E), and the core structure substituted on the six-membered ring with e.g. 3-hydroxynaphthalene or indazole or hydroxy-, alkoxy- and / or fluoro-substituted phenyl (R1).
Owner:ARAXES PHARMA LLC
Fused hetero-hetero bicyclic compounds and methods of use thereof
ActiveUS20190345158A1Prevent proliferationOrganic chemistryPharmaceutical delivery mechanismKRASStereoisomerism
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I), or a pharmaceutically acceptable salt, stereoisomer or prodrug thereof, wherein A, W, X, Y, Z, and are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
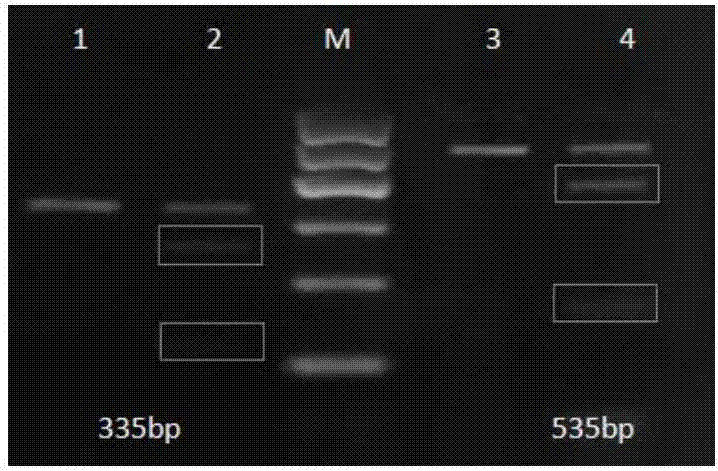
Primer-probe composition, kit and detection method for detecting seven kinds of hot-spot mutation of KRAS gene of humans
ActiveCN104805208AAccurate detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationKRASTyping
The invention discloses a primer-probe composition for detecting seven kinds of hot-spot mutation of KRAS gene of humans. The primer-probe composition comprises 7 pairs of specific primers and one kind of specific probes, wherein the 7 pairs of specific primers are respectively prepared from 7 kinds of ARMS primers and one kind of downstream primer. The invention further discloses a kit containing the primer-probe composition, and a method for detecting mutation by using the kit. The primer-probe composition disclosed by the invention is high in sensitivity and specificity; when the kit is used, the detection processes are all reactions carried out in closed tubes, so that the pollution is significantly reduced, and besides, a UNG enzyme anti-pollution system and an internal control system are added, so that more accurate and stable typing detection can be performed on samples, and the results are guaranteed to be genuine and credible; the detection method is fast, the detection can be completed within 100 minutes, the result judgment is simple and objective, and the analysis is convenient.
Owner:SHANDONG VIGENE BIOSCI
Animal models of pancreatic adenocarcinoma and uses therefor
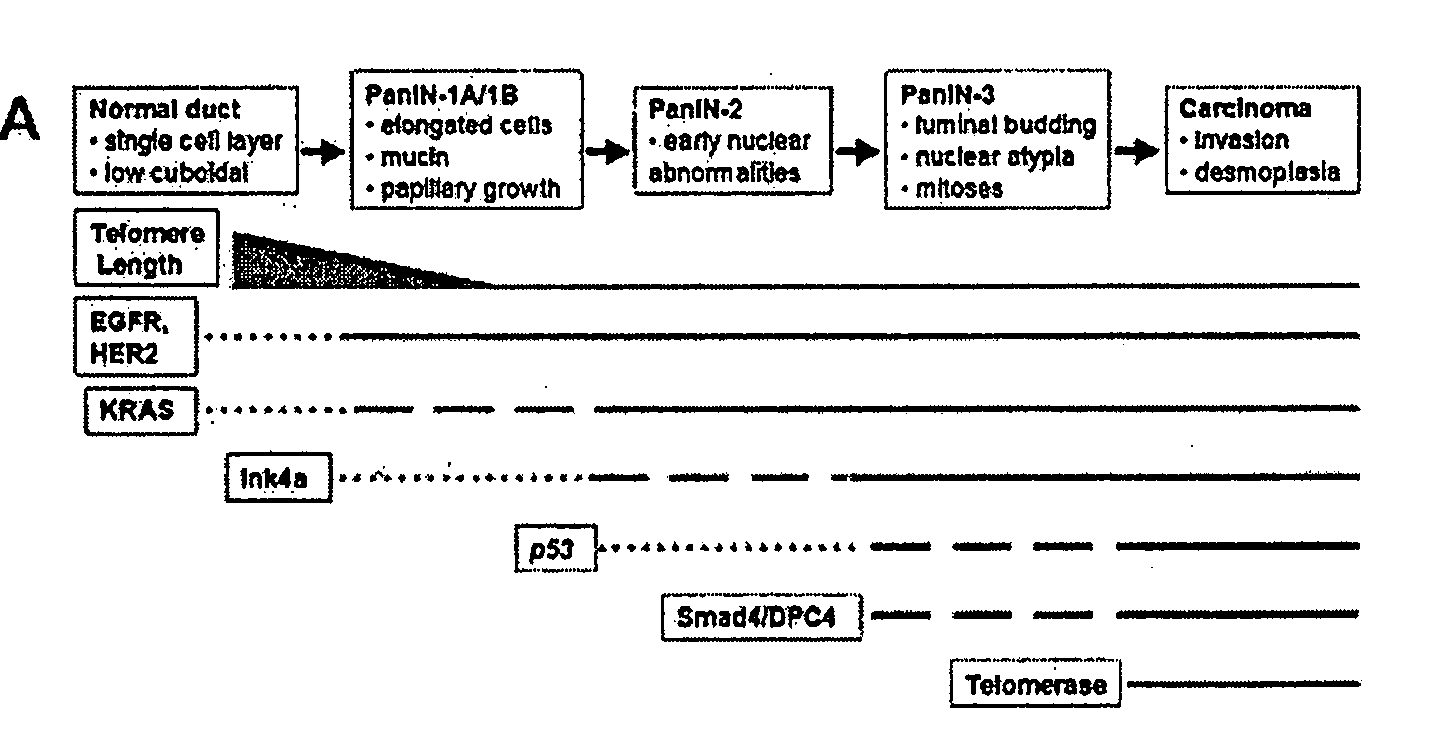
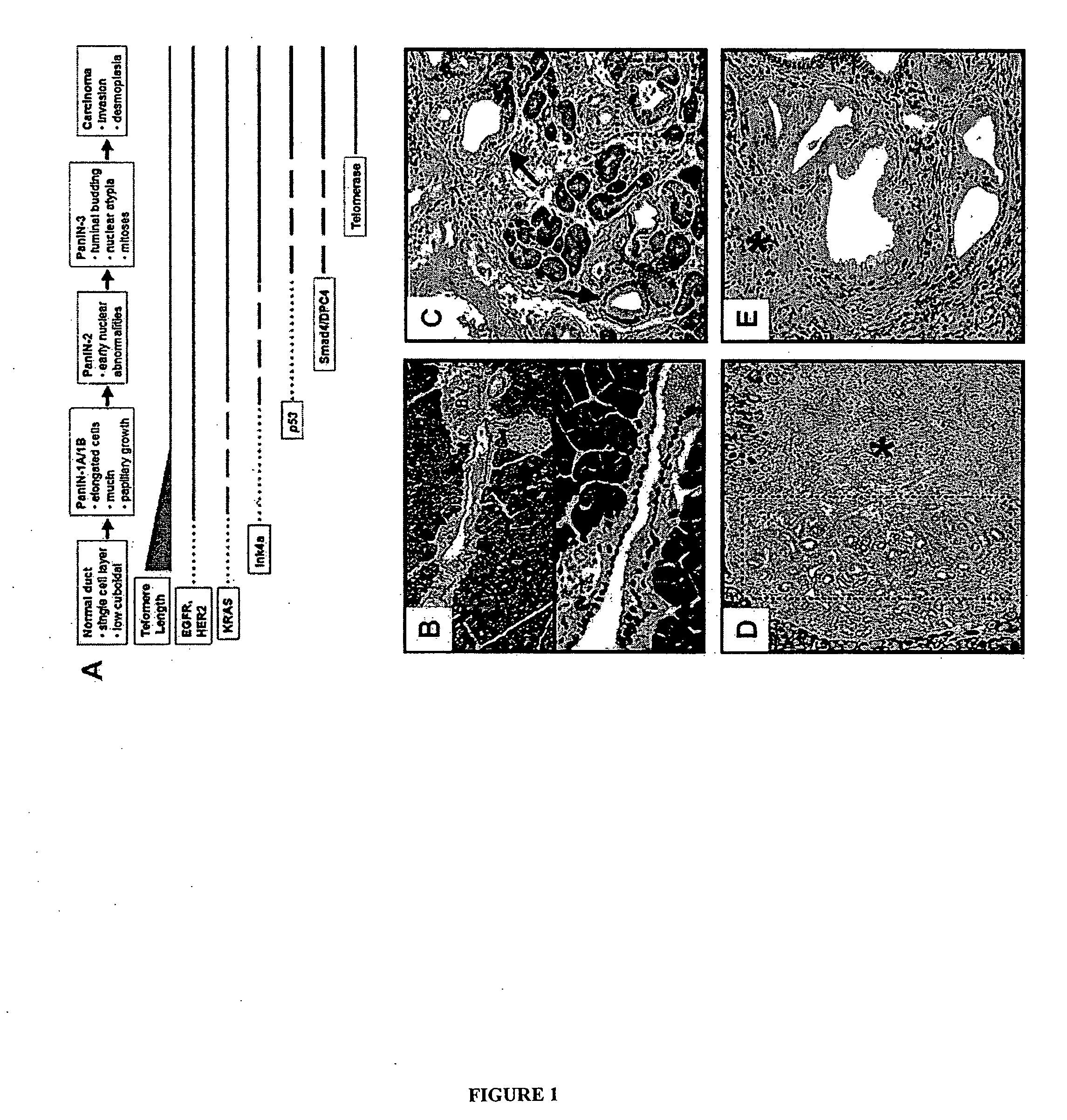
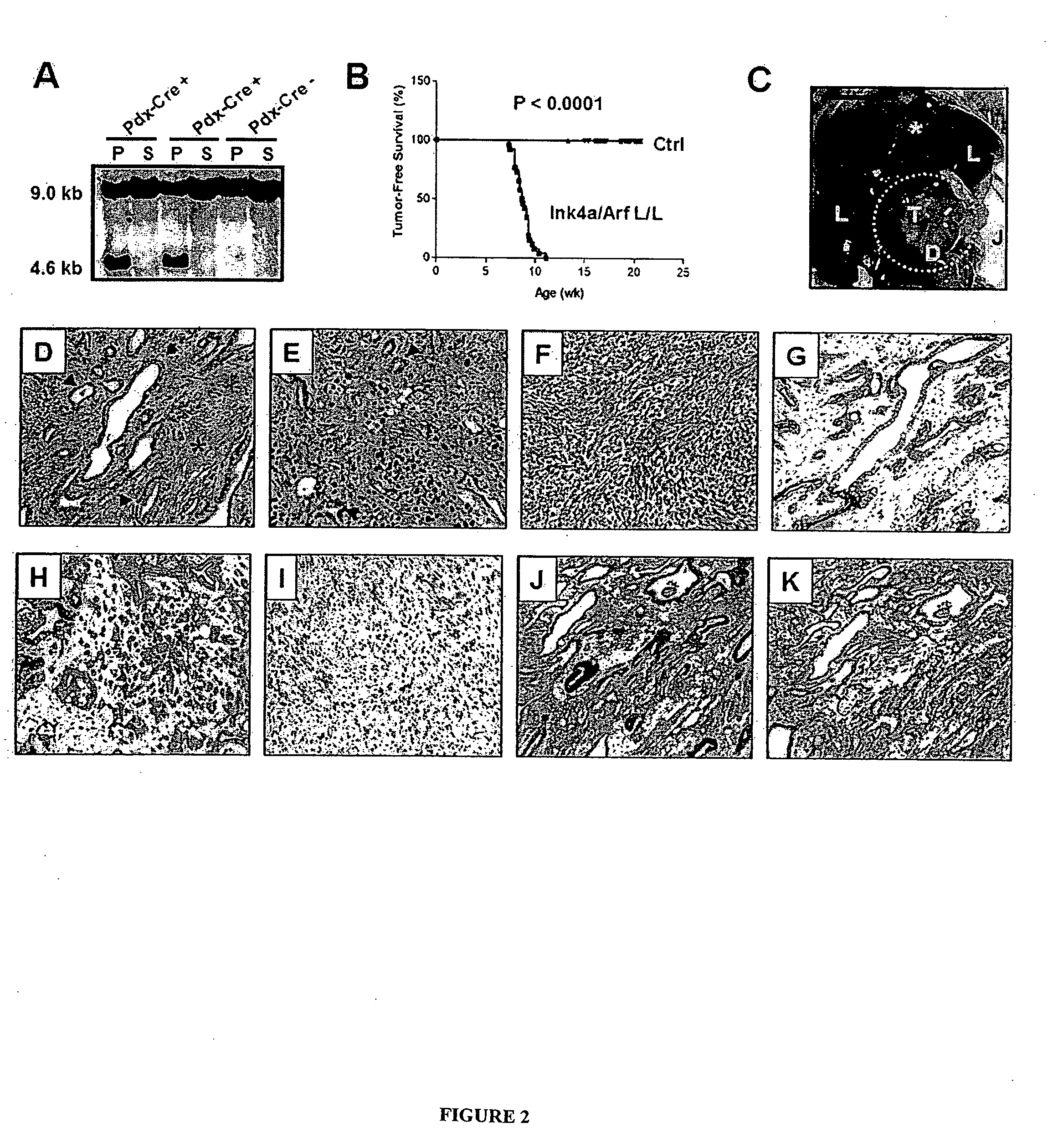
The present invention is based, at least in part, on the generation of an animal model of pancreatic adenocarcinoma which recapitulates the genetic and histological features of human pancreatic adenocarcinoma, including the initiation, maintenance, and progression of the disease. Accordingly, the present invention provides animal models of cancer, e.g., pancreatic adenocarcinoma, wherein an activating mutation of Kras has been introduced, and any one or more known or unknown tumor suppressor genes or loci, e.g., Ink4a / Arf, Ink4a, Arf, p53, Smad4 / Dpc, Lkb1, Brca2, or Mlh1, have been misexpressed, e.g., have been misexpressed leading to decreased expression or non-expression. The animal models of the invention may be used, for example, to identify biomarkers of pancreatic cancer, to identify agents for the treatment or prevention of pancreatic cancer, and to evaluate the effectiveness of potential therapeutic agents.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Non-small cell lung cancer targeted therapy gene detection method
InactiveCN105969857AReduce mutual interferenceStrong specificityMicrobiological testing/measurementCell sensitivityOncogene
The invention discloses a non-small cell lung cancer targeted therapy gene detection method, and belongs to the field of gene detection. The method for detecting 466 mutations of 12 oncogenes is developed by multiplex PCR and high throughput sequencing technologies, wherein the oncogenes are AKT1, ALK, BRAF, EGFR, ERBB4, FGFR1, FGFR2, FGFR3 , KRAS, MET, PIK3CA, and PTEN, and the mutations may be substitutions, insertions and / or deletions of one or more bases. The detection method provided by the invention has the advantages of high detection sensitivity of up to 0.01%, and clear and objective detection results, can be directly used for reflecting specific mutation sites of the relevant gene, directly used for guiding clinical non-small cell lung cancer targeted dosage, and used for early diagnosis or auxiliary diagnosis and screening of cancers as well as post-cancer surveillance.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Use of microvesicles in analyzing kras mutations
Microvesicles are small membrane vesicles that either shed or bud off eukarotic cells. Analysis of the nucleic acid content of microvesicles may be useful in detecting the presence or absence of genetic aberrations. This invention discloses novel methods of diagnosing, prognosing, monitoring, or treating a disease, such as cancer, or other medical condition in a subject involving analyzing one or more nucleic acids contained within an isolated microvesicle for the presence or absence of one or more Kras genetic aberrations.
Owner:THE GENERAL HOSPITAL CORP
Primer for detecting thyroid cancer pathopoiesia related gene variation on basis of high throughput sequencing technology and application of primer
PendingCN109371139AImprove throughputHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationKRASNodular thyroid disease
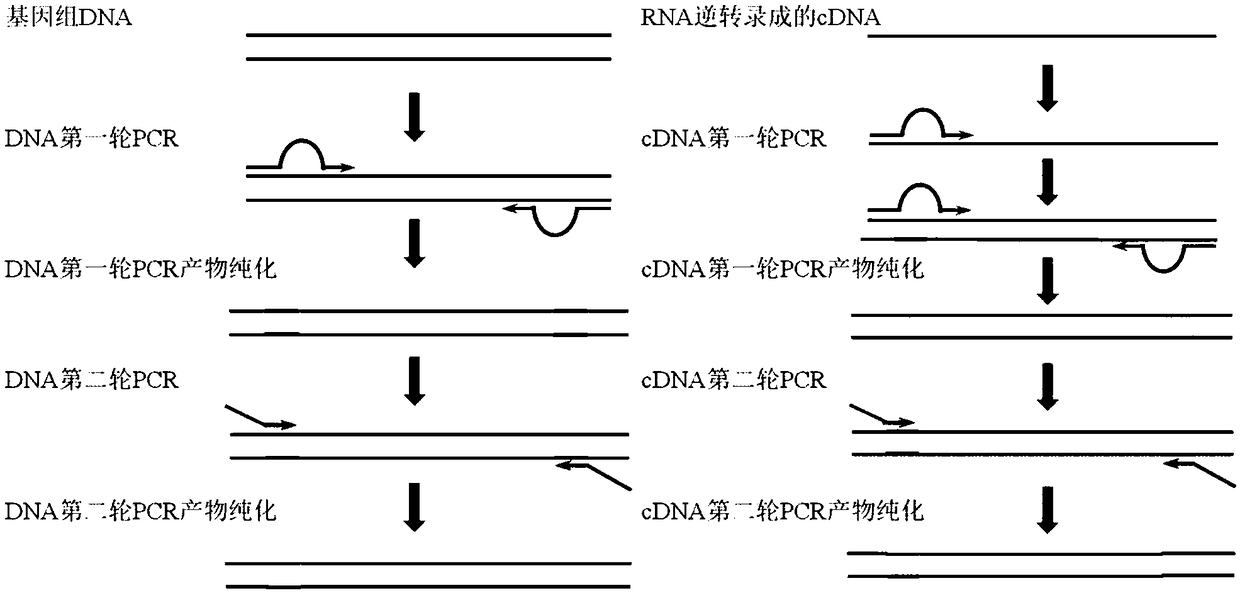
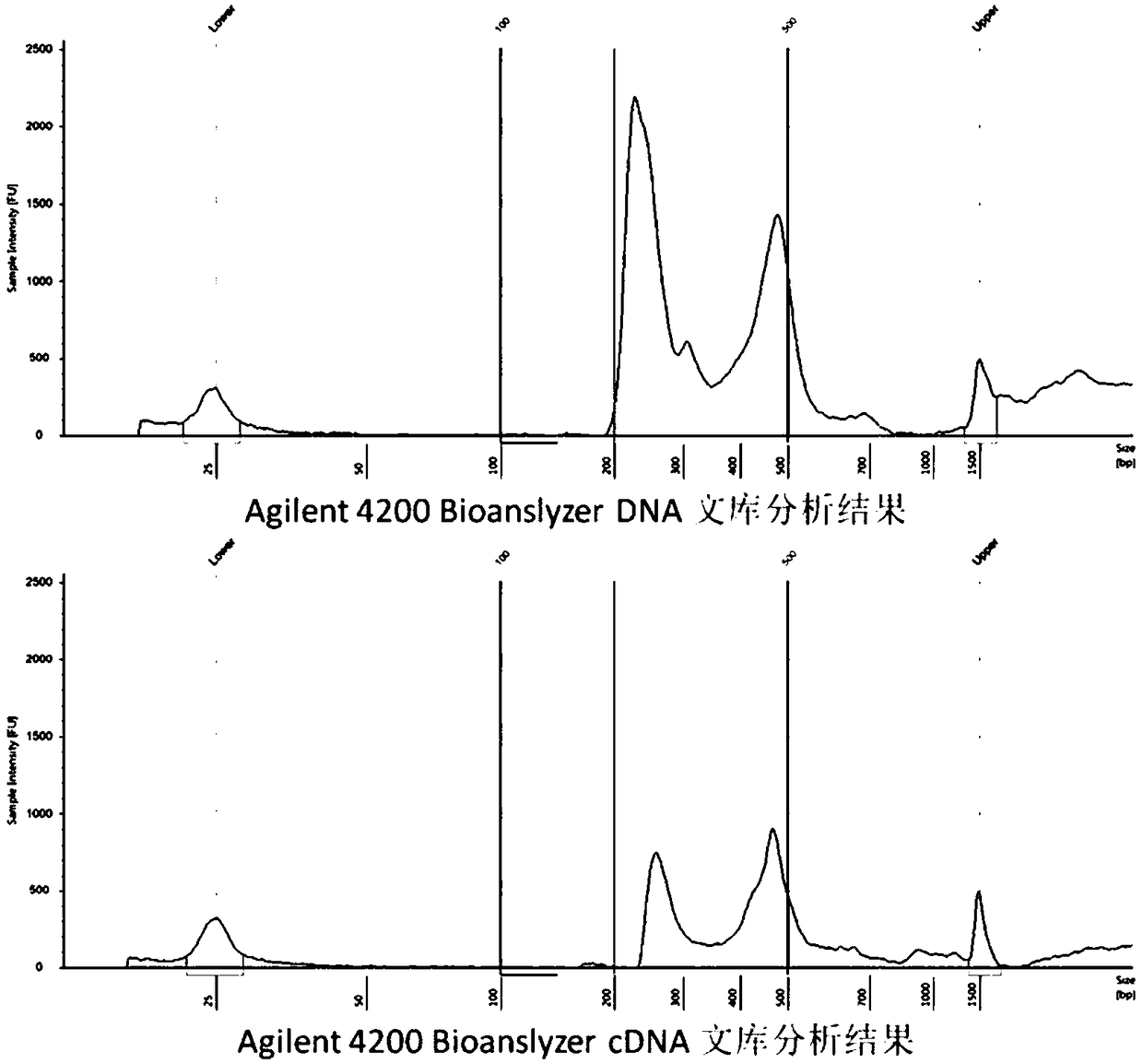
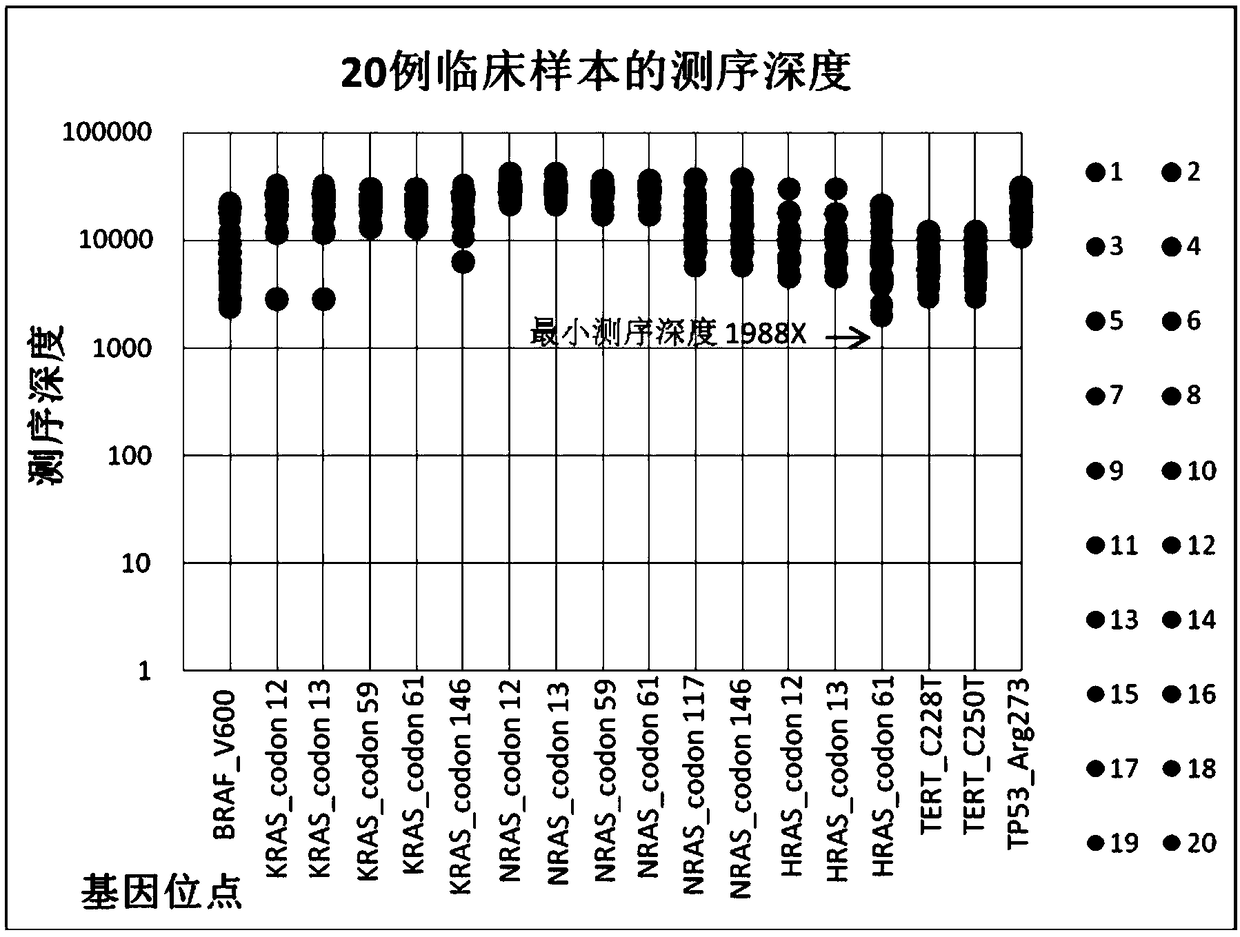
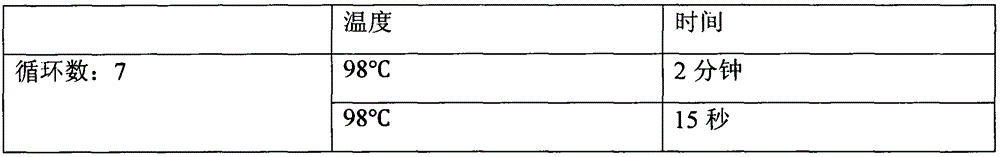
The invention provides a kit for detecting thyroid cancer pathopoiesia related gene variation on the basis of a high throughput sequencing technology. The kit is used for variation detection of thyroid cancer related genes, including 18 mutation sites of 6 genes comprising BRAF, NRAS, KRAS, HRAS, TERT and TP53 and 3 fusion genes comprising CCDC6 / RET, NCOA4 / RET and PAX8 / PPARg. Online sequencing libraries are constructed by two round of multiple PCR, the specific primer is designed for target areas of the nine genes to be used in the first round of multiple PCR, and a general sequencing sequenceprimer is used in the second round of multiple PCR. The invention also discloses a kit, including primer sequences and library systems, for detecting thyroid cancer pathopoiesia related gene variation, and the kit can assist in diagnosing equivocal thyroid sarcoidosis lesions.
Owner:HANGZHOU D A GENETIC ENG
High flux detection method for tumor-targeted drugs related genes mutation, primers and reagent thereof
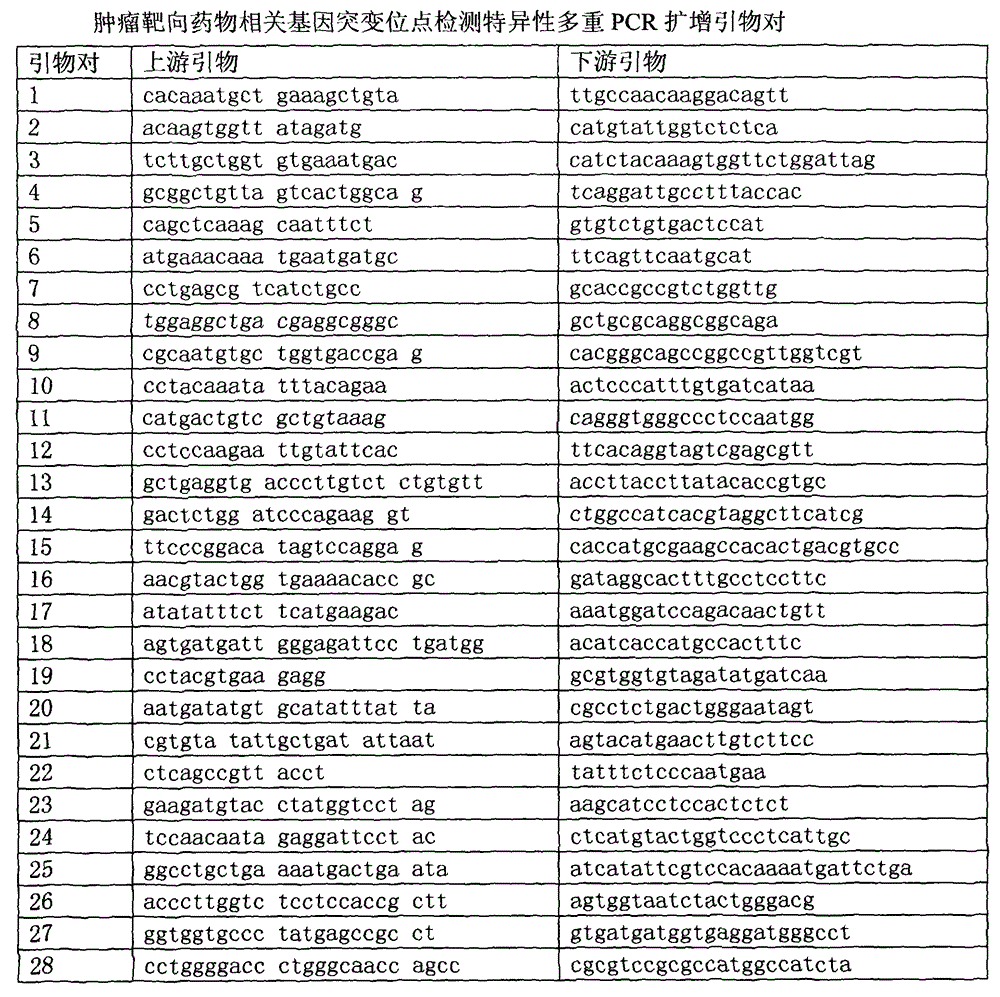
ActiveCN105624274AKnowing about genetic mutationsHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTumor targetWilms' tumor
The invention relates to the field of tumor gene, and discloses a high-flux sequencing detection method for tumor-targeted drugs related genes mutation, a group of primer pairs and a reagent thereof. The method is used for detecting mutation of tumor-targeted drugs related genes in an acceptor, the related genes comprise BRAF, EGFR, KRAS, NRAS, TP53, FGFR3, KIT, PIK3CA, RET, PTEN, and CTNNB1. The method comprises the following steps: extraction of sample DNA, target gene enrichment, primer sequence removal, joint connection, PCR amplification, library homogenization, library detection and high flux sequencing. The disclosed primer pairs enable multiple PCR enrichment and amplification on the tumor-targeted drugs related genes, the method can be used for detecting 115 mutation sites in the above 11 genes, a detection result can be a medication guidance for the tumor-targeted drugs, and provides a basis for exploitation of novel cancer drugs.
Owner:绍兴积准生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com