Patents
Literature
497 results about "Imidazopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An imidazopyridine is a nitrogen containing heterocycle that is also a class of drugs that contain this same chemical substructure. In general, they are GABAA receptor agonists, however recently proton pump inhibitors, aromatase inhibitors, NSAIDs and other classes of drugs in this class have been developed as well. Despite usually being similar to them in effect, they are not chemically related to benzodiazepines. As such, GABAA-agonizing imidazopyridines, pyrazolopyrimidines, and cyclopyrrones are sometimes grouped together and referred to as "nonbenzodiazepines." Imidazopyridines include...
Use of 8-amino-aryl-substituted imidazopyrazines as kinase inhbitors
The present invention relates to 8-amino-aryl-substituted imidazopyrazines which modulate the activity of protein kinases ("PKs"). The compounds of this invention are therefore useful in treating disorders related to abnormal PK activity. Pharmaceutical compositions comprising these compounds, methods of treating diseases utilizing pharmaceutical compositions comprising these compounds and methods of preparing them are also disclosed.
Owner:SUGEN INC
Method for the treatment of dermal lesions caused by envenomation
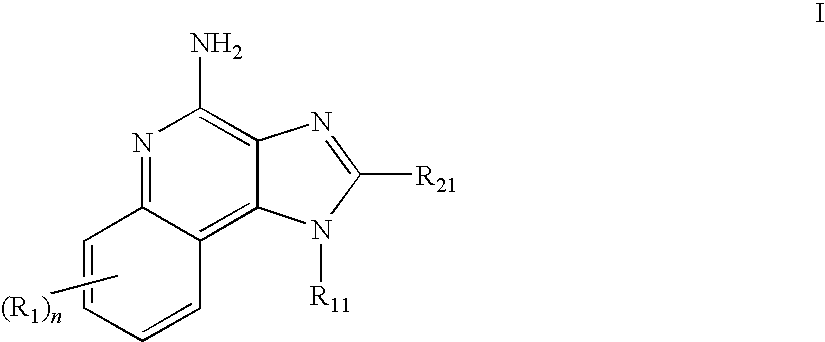
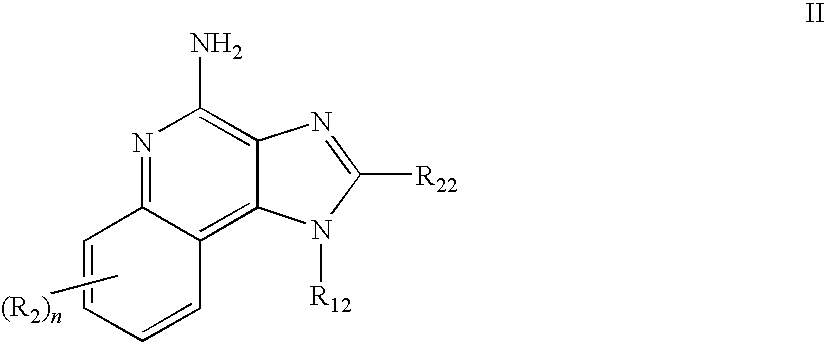
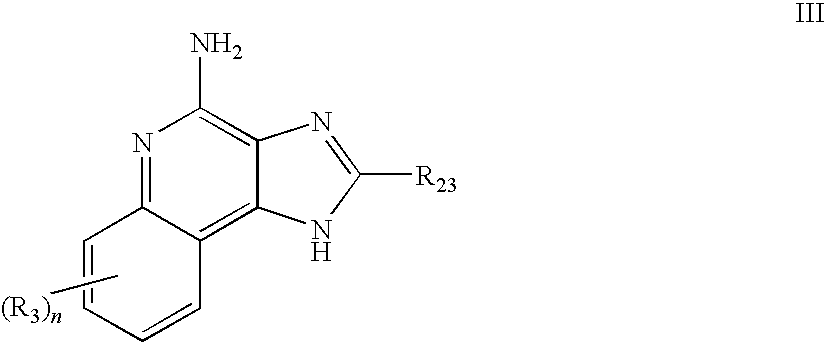
A method of treating dermal lesions caused by envenomation comprising applying a therapeutically effective amount of an immune response modifier compound selected from the group consisting of imidazoquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, oxazolopyridine amines, oxazoloquinoline amines, thiazolopyridine amines, thiazoloquinoline amines and 1,2-bridged imidazoquinoline amines to the site of the lesion is disclosed.
Owner:3M INNOVATIVE PROPERTIES CO
Imidazopyridines and triazolopyridines
The present invention relates to imidazopyridine and triazolopyridine derivatives, pharmaceutical compositions and methods of use thereof.
Owner:WARNER-LAMBERT CO
Aqueous Gel Formulations Containing Immune Response Modifiers
ActiveUS20090163532A1Easy to manufactureExtended stayBiocideOintment deliveryPyridineImidazopyridine
Aqueous gel formulations, including an immune response modifier (IRM), such as those chosen from imidazoquinoline amines, tetrahydroimidazoquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, imidazonaphthyridine amines, imidazotetrahydronaphthyridine amines, oxazoloquinoline amines, thiazoloquinoline amines, oxazolopyridine amines, thiazolopyridine amines, oxazolonaphthyridine amines, thiazolonaphthyridine amines, pyrazolopyridine amines, pyrazoloquinoline amines, tetrahydropyrazoloquinoline amines, pyrazolonaphthyridine amines, tetrahydropyrazolonaphthyridine amines, and 1H-imidazo dimers fused to pyridine amines, quinoline amines, tetrahydroquinoline amines, naphthyridine amines, or tetrahydronaphthyridine amines, are provided. Methods of use and kits are also provided.
Owner:3M INNOVATIVE PROPERTIES CO & 3M CO +1
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
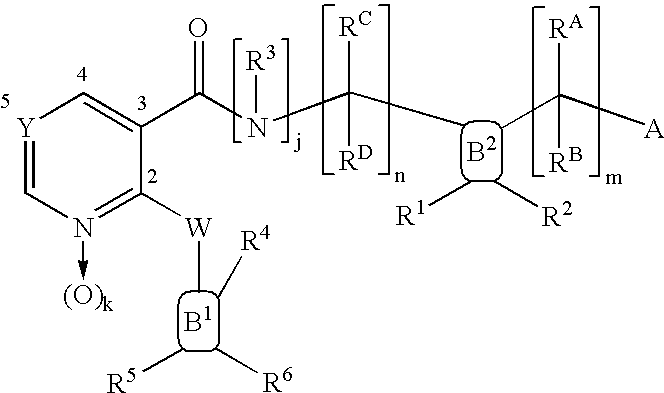
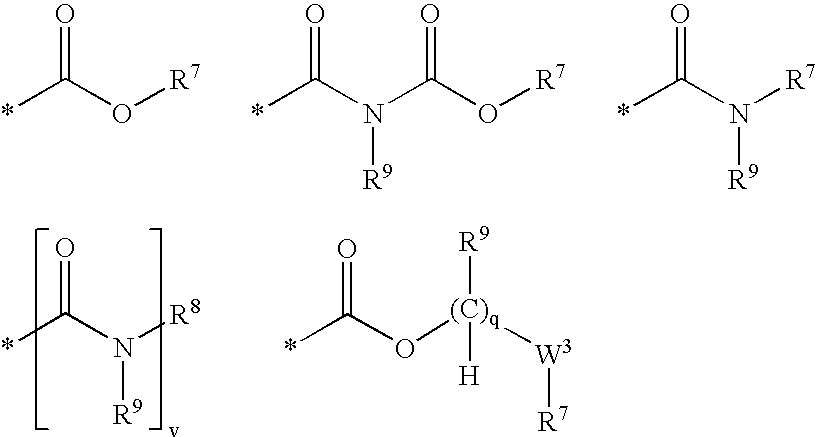
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Methods for the treatment of periodontal disease
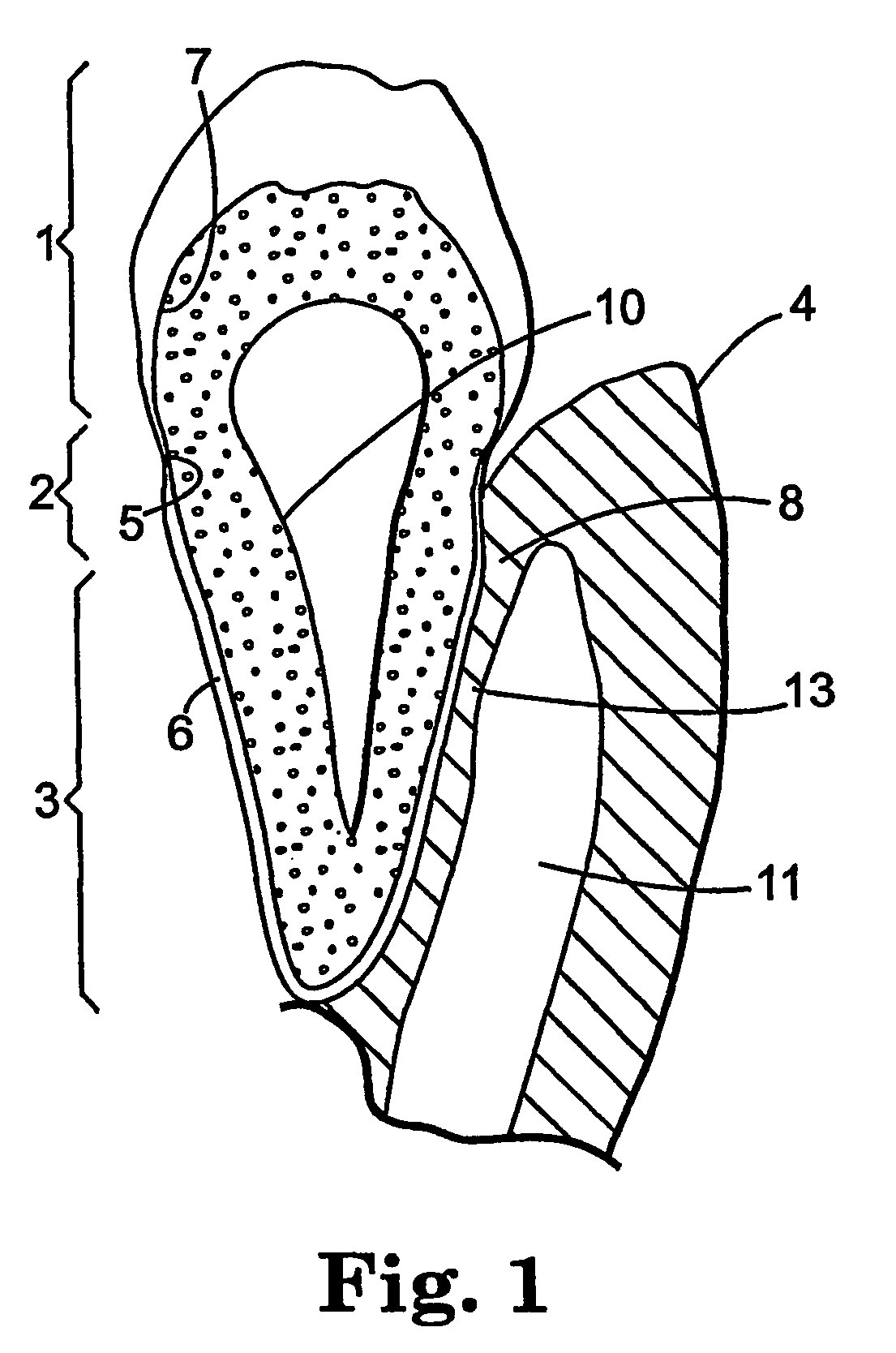
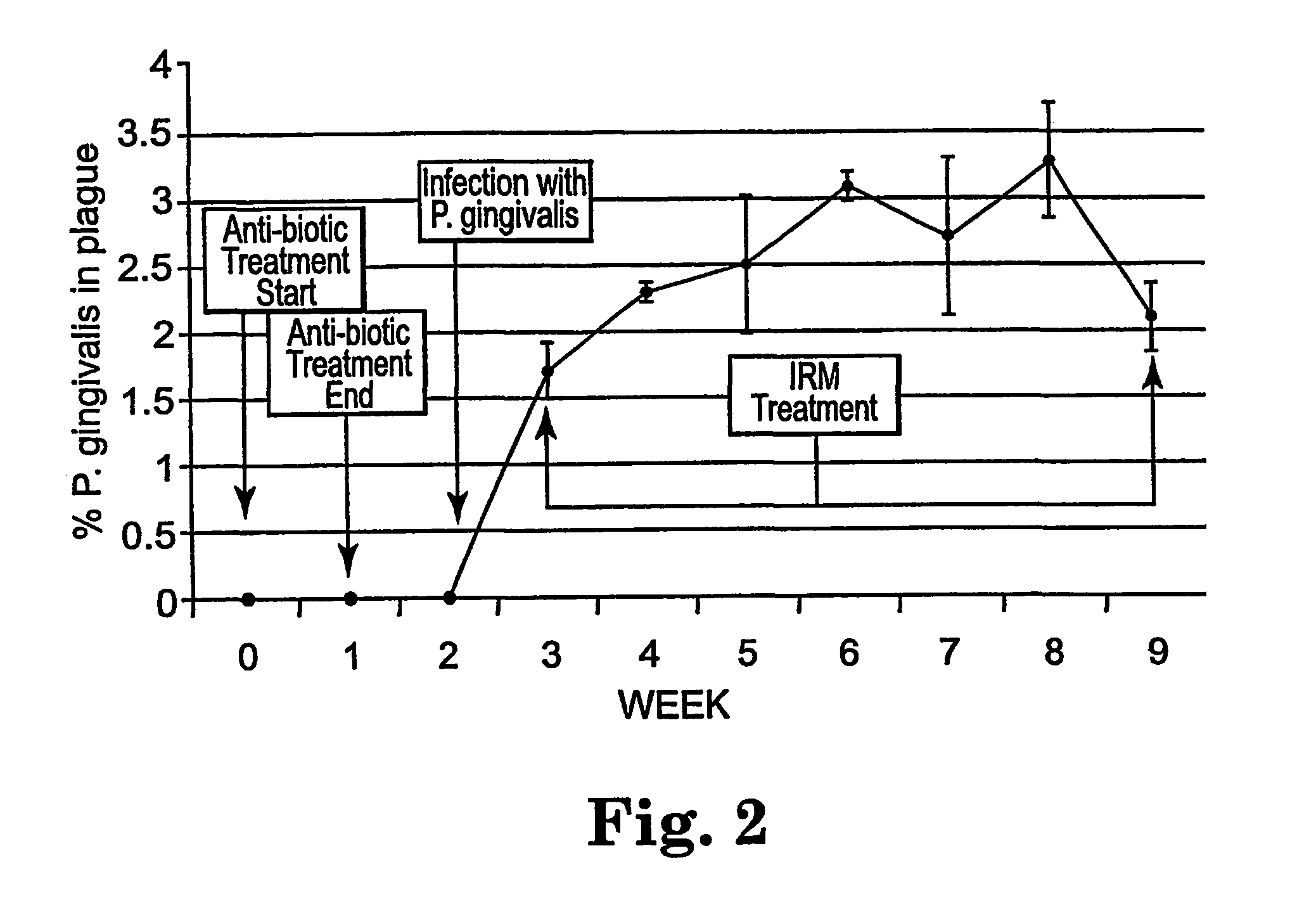
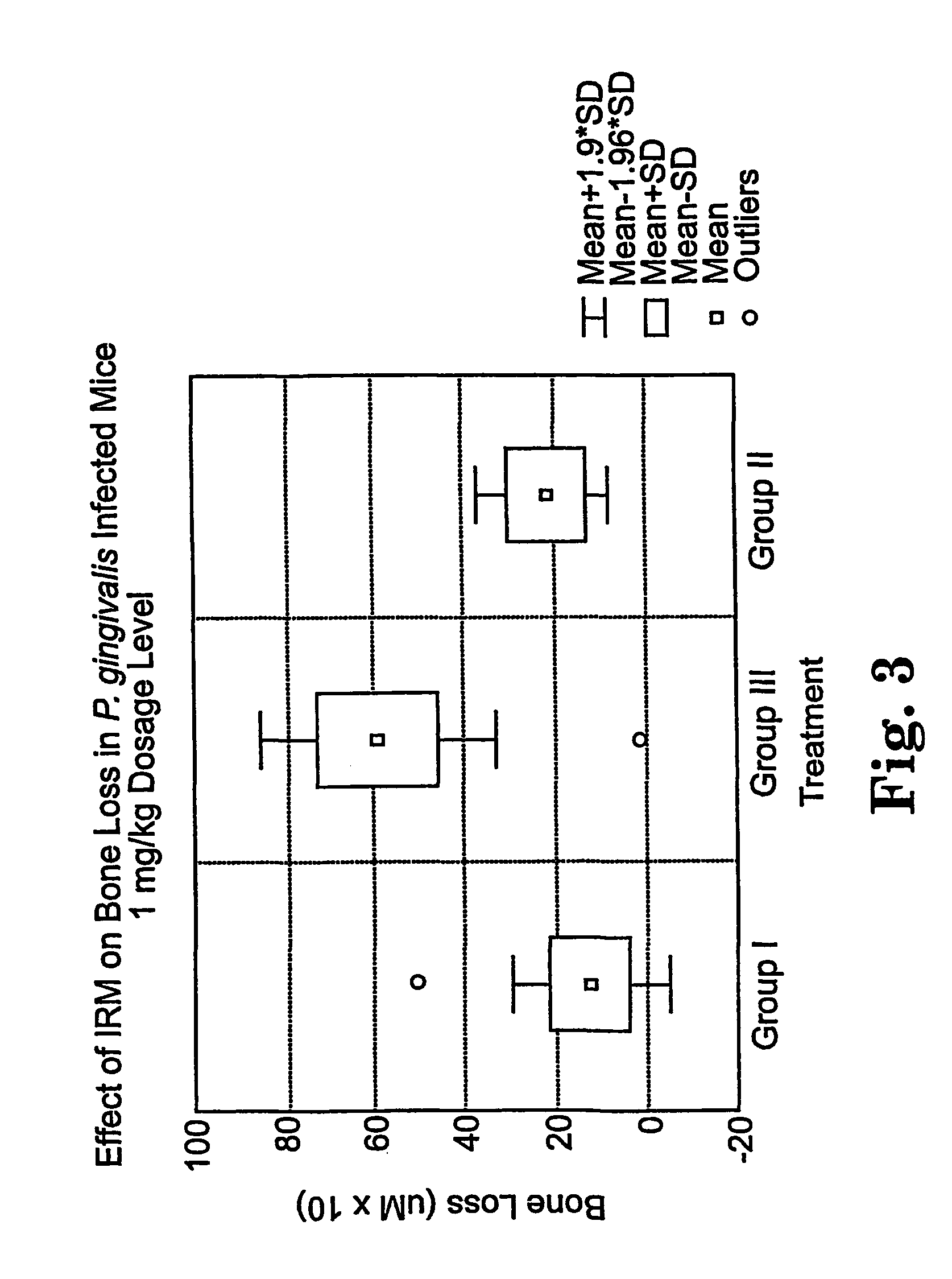
The disclosure provides methods for the treatment and prevention of periodontal disease. In preferred embodiments, the invention provides for local treatment of periodontal tissues with a pharmaceutical composition including an immune response modifier (IRM) selected from the group of immune response modifiers comprising imidazoquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, imidazonaphtyridine amines, oxazoloquinoline amines, thiazoloquinoline amines and 1,2-bridged imidazopyridine amines.
Owner:COLEY PHARMA GRP INC
Dye labeled imidazoquinoline compounds
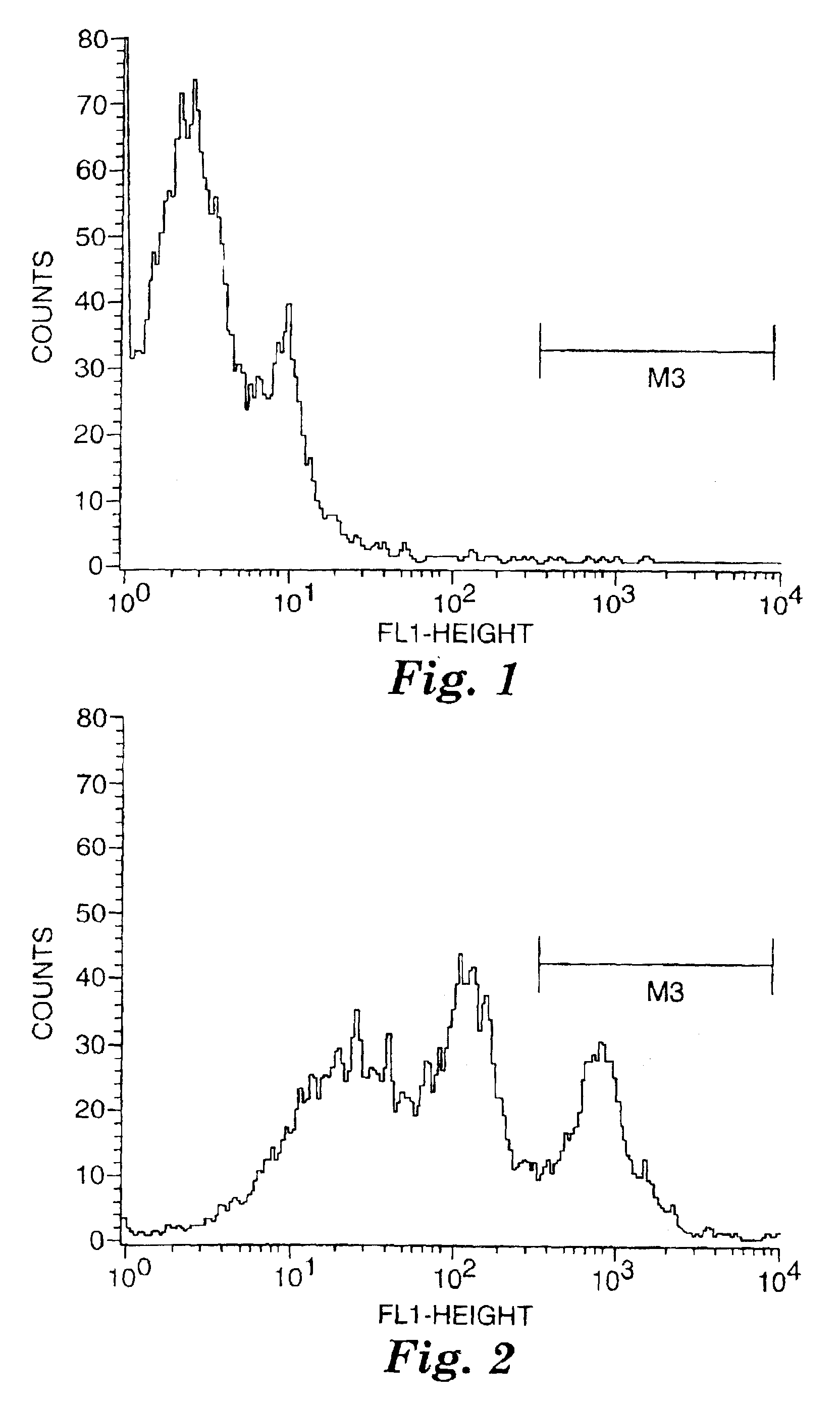
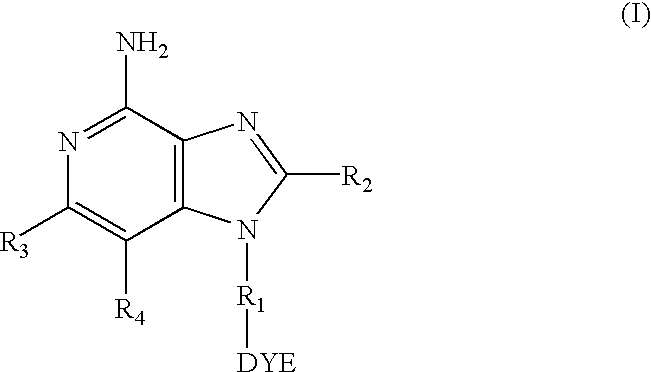
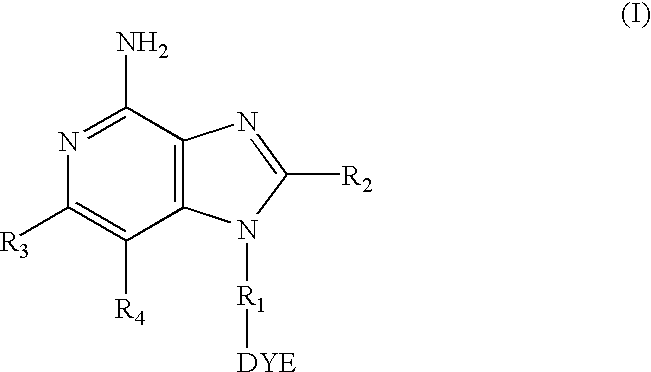
Dye labeled imidazonaphthyridine, imidazopyridine and imidazoquinoline compounds having immune response modulating activity are disclosed. The compounds arc useful, inter alia, for determining the binding and / or receptor sites of the molecules.
Owner:COLEY PHARMA GRP INC
Fused heterocyclic compound
InactiveUS20100029619A1Superior ASK inhibitory activityBiocideNervous disorderEngineeringMethyl group
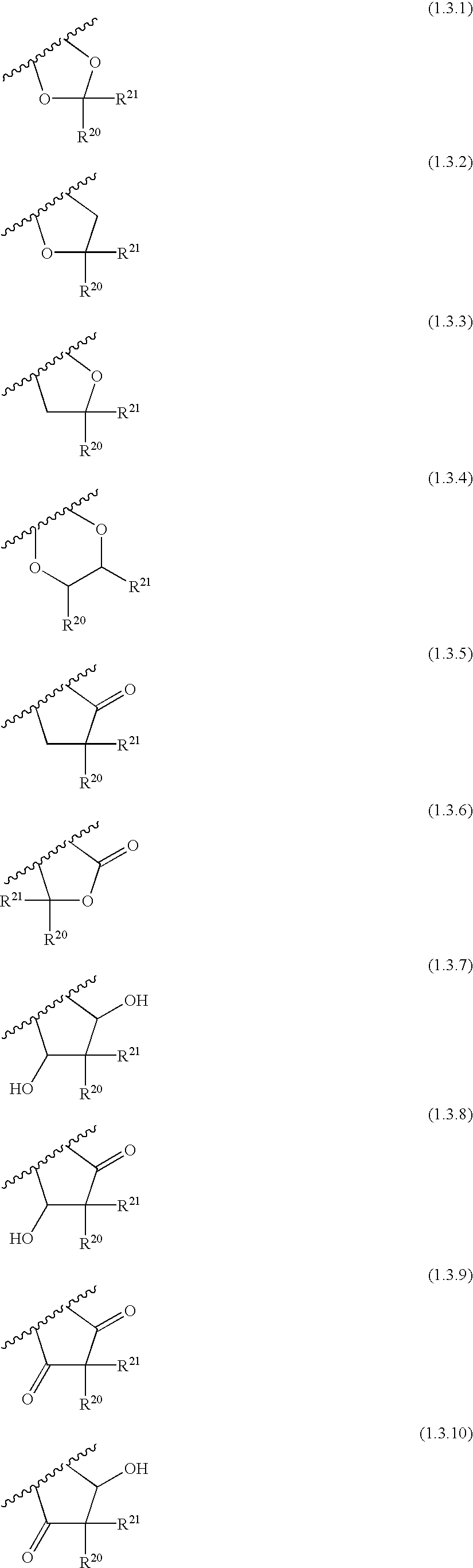
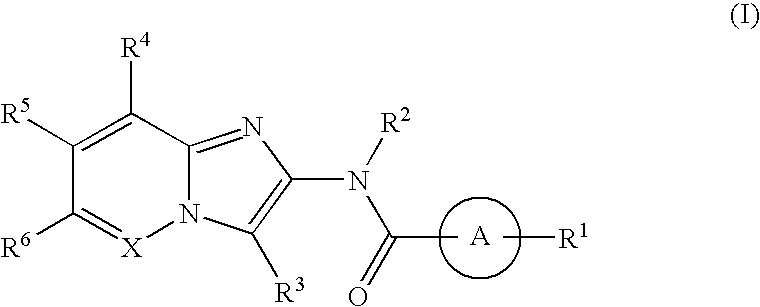
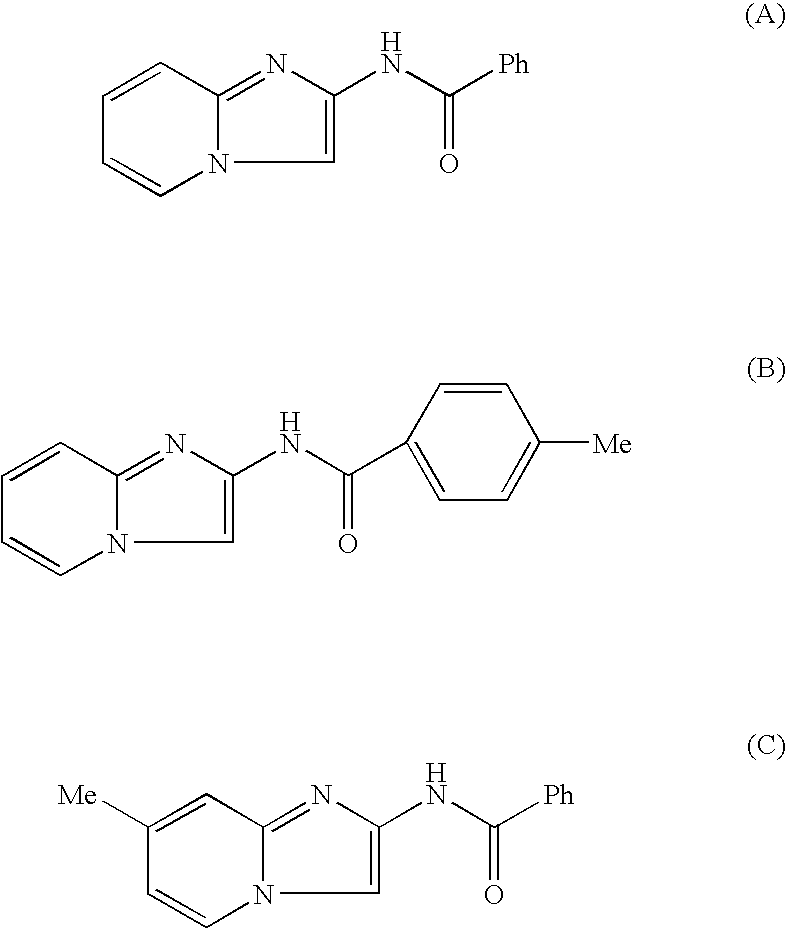
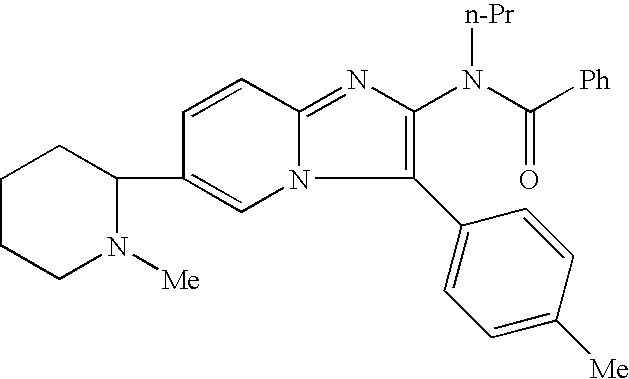
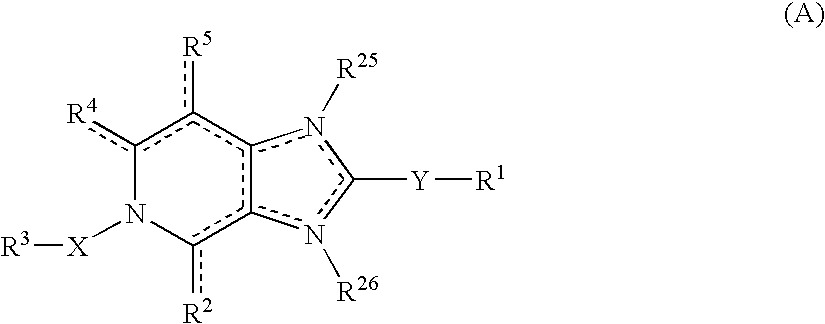
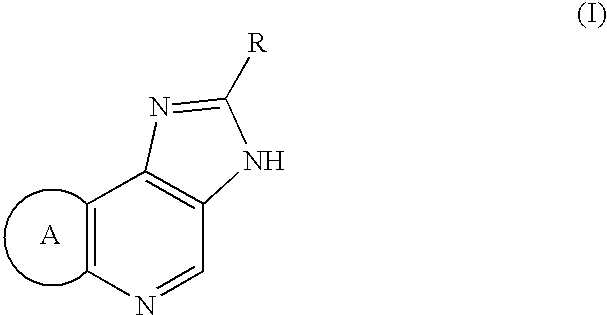
The present invention provides a compound represented by the formula (I):whereinring A is a ring which is optionally further substituted;R1 is a hydrogen atom or a substituent;R2 is a hydrogen atom or a substituent;R3 is a hydrogen atom or a substituent;R4 is a hydrogen atom or a substituent;R5 is a hydrogen atom or a substituent;R6 is a hydrogen atom or a substituent;X is ═N— or ═C(Z)- (Z is a hydrogen atom or a substituent);when X is ═C(Z)-, Z and R6 are optionally bonded to each other to form, together with the carbon atom bonded thereto, an optionally substituted ring,provided that when X is ═CH—, then R6 is not optionally substituted 2-piperidinyl, excluding N-imidazo[1,2-a]pyridin-2-yl-4-methyl-benzamide, N-imidazo[1,2-a]pyridin-2-yl-benzamide and N-(7-methylimidazo[1,2-a]pyridin-2-yl)-benzamide, or a salt thereof, and a pharmaceutical agent containing same.The compound of the present invention has an ASK1 inhibitory action, and is useful as a pharmaceutical agent such as an agent for the prophylaxis or treatment of diabetes, inflammatory diseases and the like, and the like.
Owner:TAKEDA PHARMA CO LTD
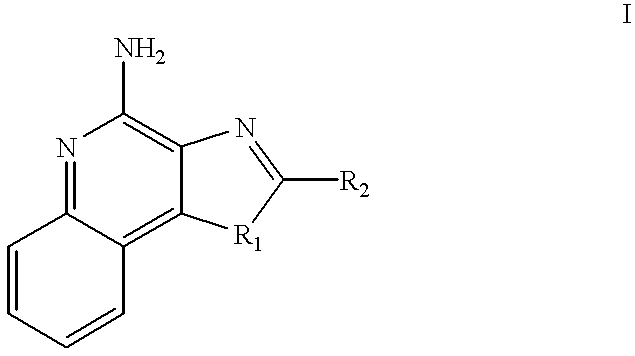
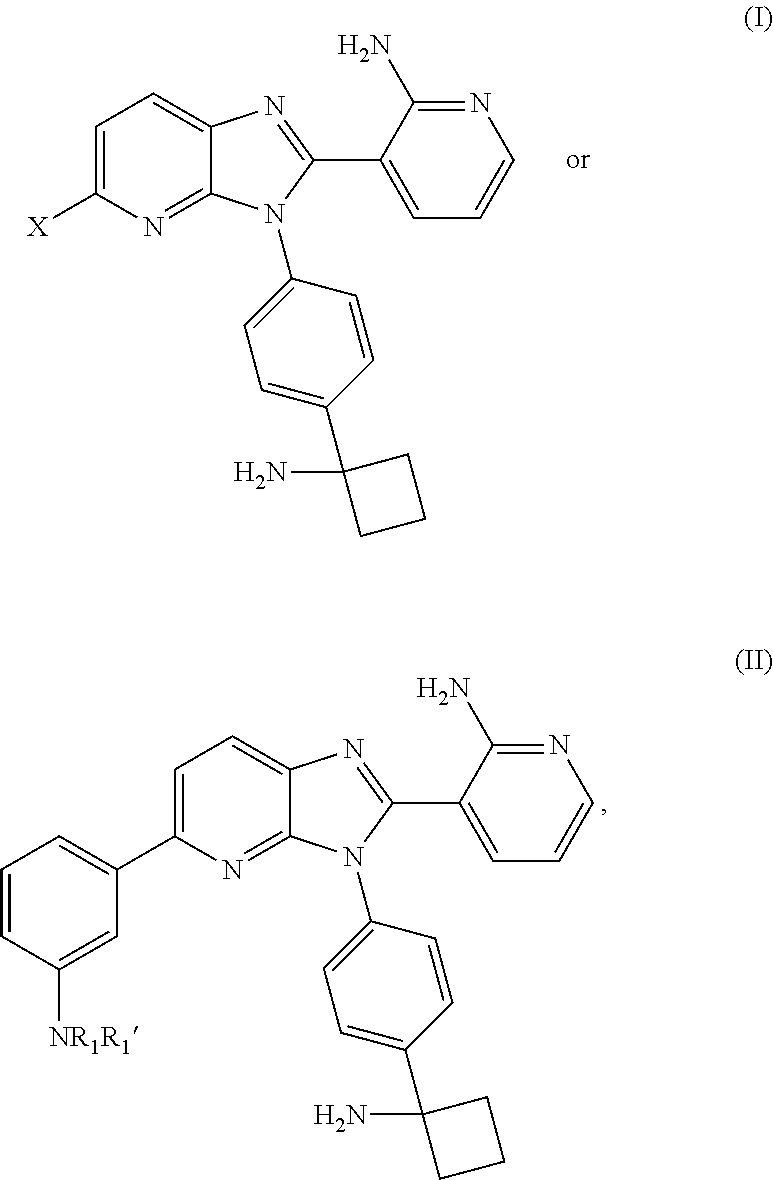
Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD
The invention belongs to the field of medicinal chemical engineering, and relates to application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivatives to prepare medicines for controlling PTSD (posttraumatic stress disorder). Specifically, the invention relates to application of a compound shown as a formula I and pharmaceutically salts thereof to prepare medicines for controlling and / or treating and / or auxiliarily treating PTSD, wherein the compound and the pharmaceutically salts thereof are used as TSPO (translocator protein) ligands. The TSPO ligands such as the compounds shown as the formula I and the pharmaceutically salts are capable of effectively preventing and / or treating and / or auxiliarily treating PTSD, and can be used to prevent medicines for controlling and / or treating and / or auxiliarily treating PTSD.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
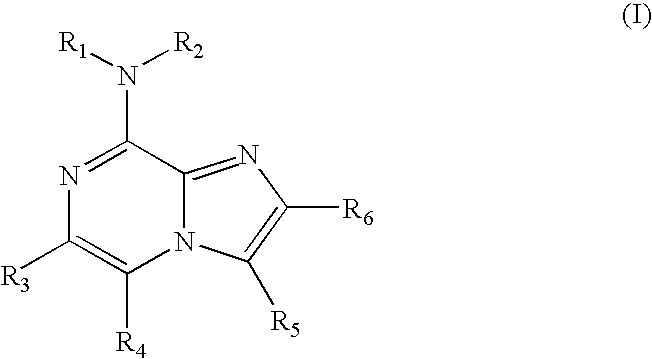
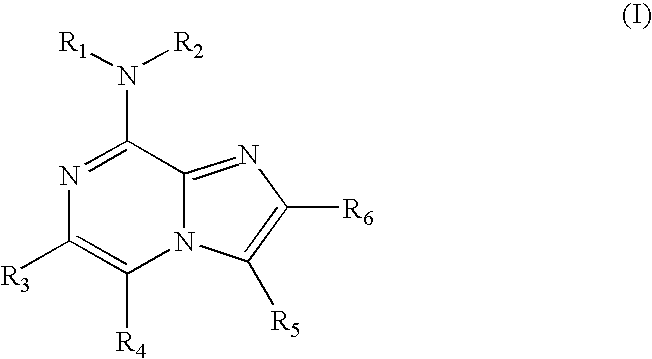
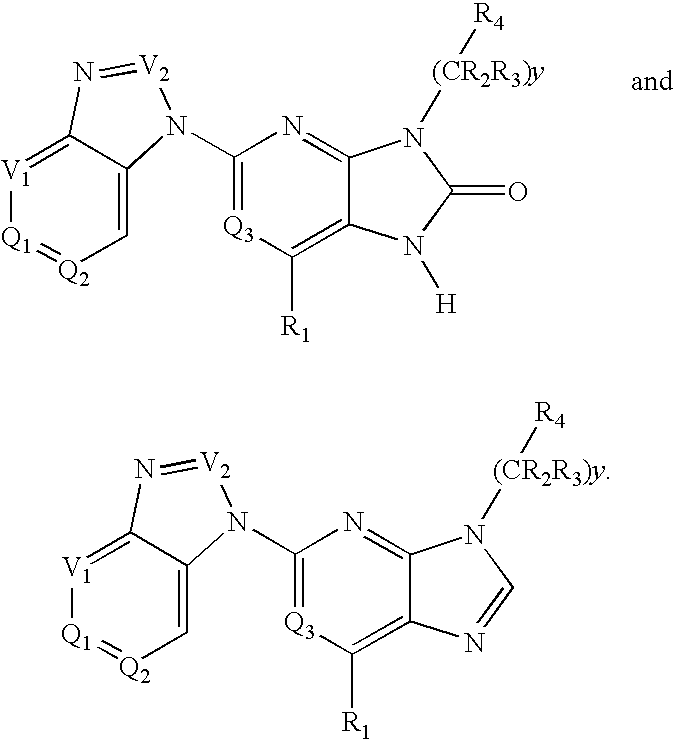
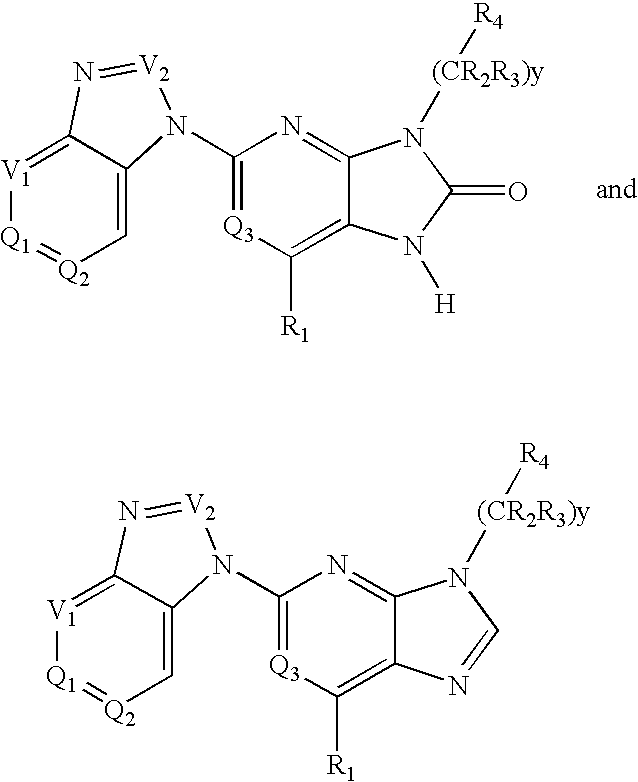
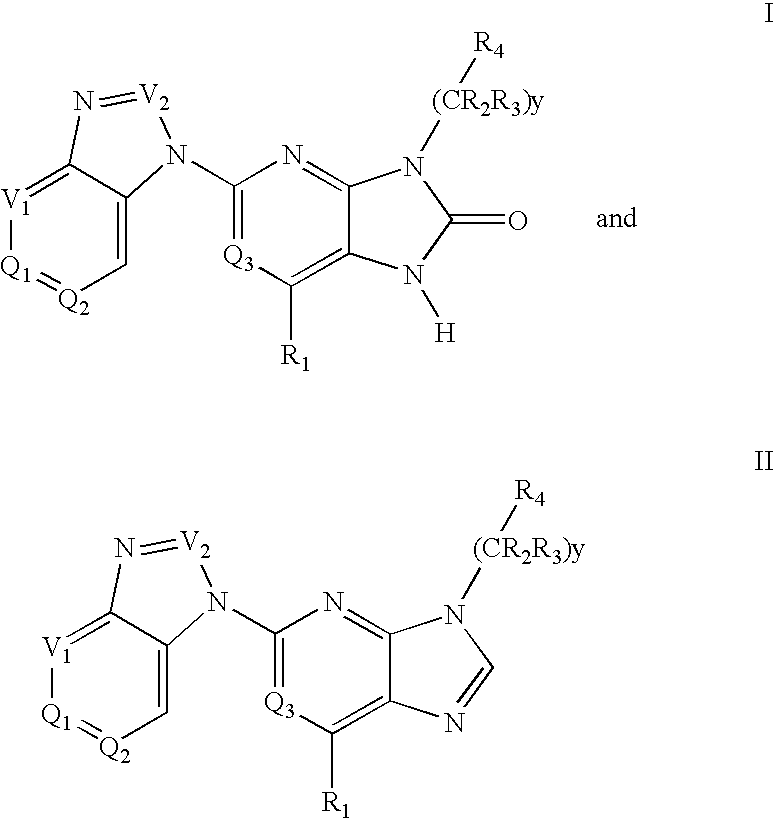
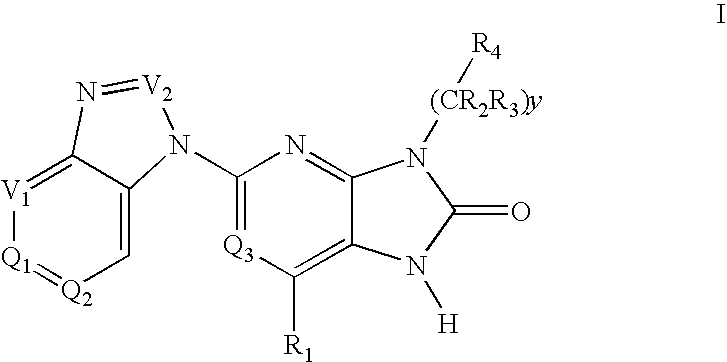
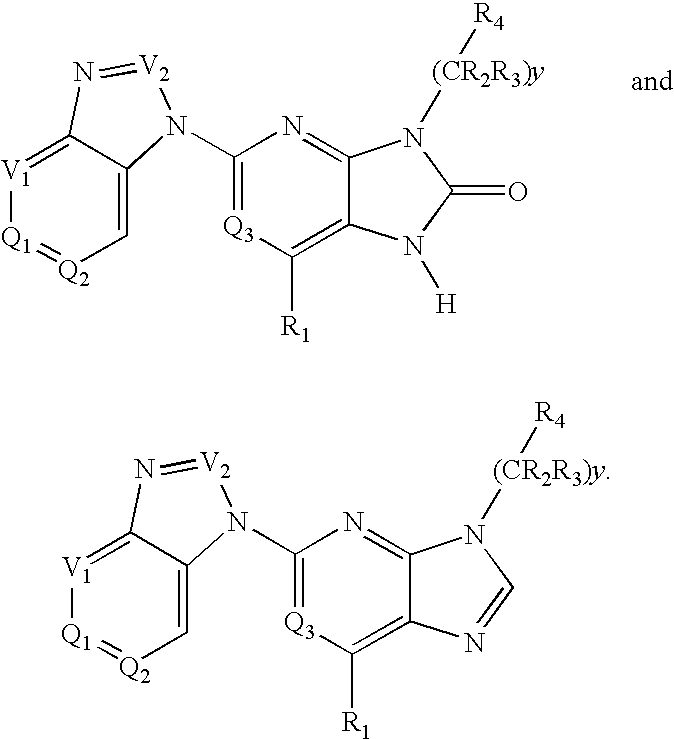
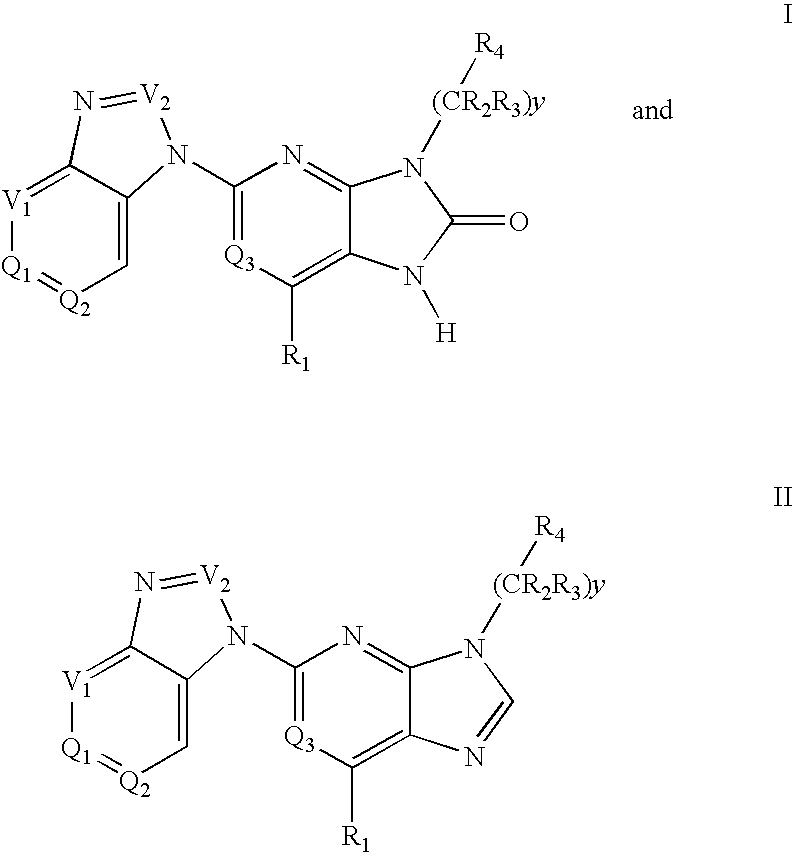
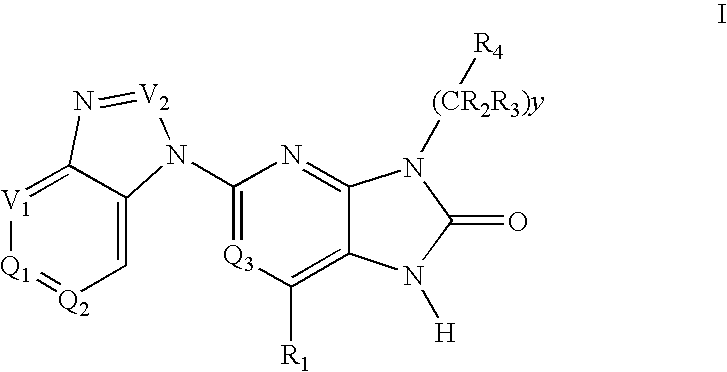
Purine and imidazopyridine derivatives for immunosuppression
The present invention provides novel purine and imidazopyridine derivatives useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formulas:
Owner:WYETH LLC +1
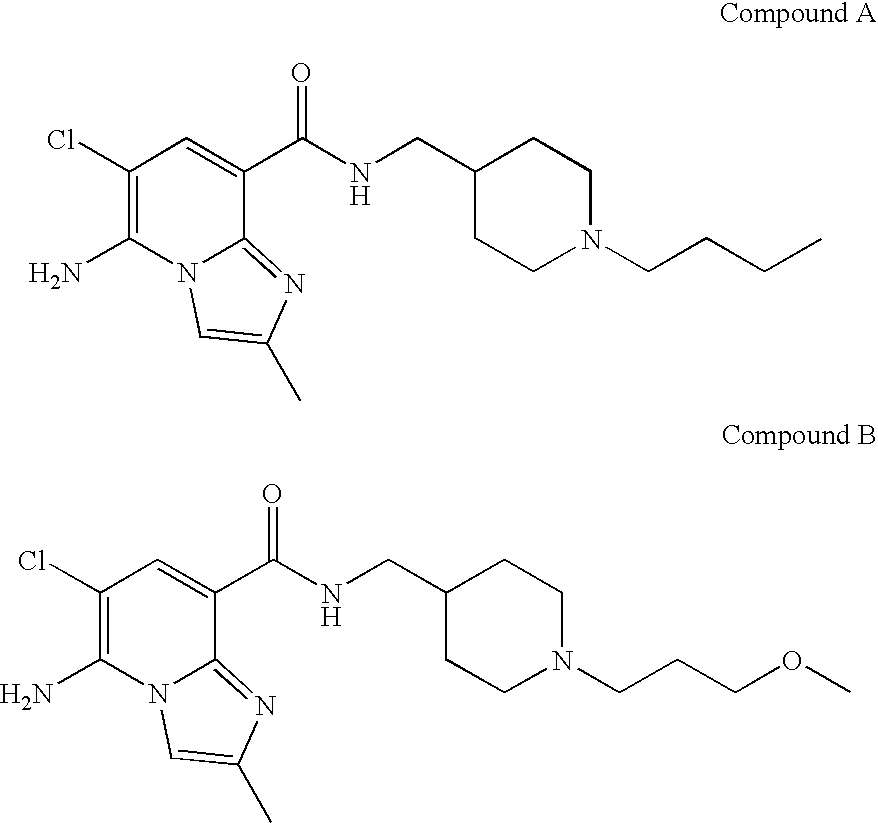
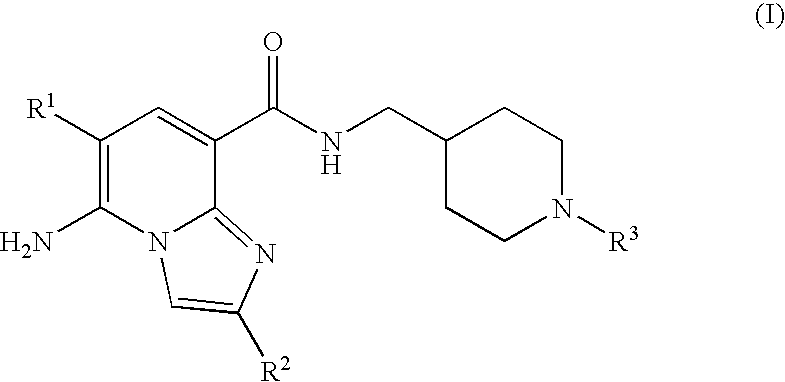
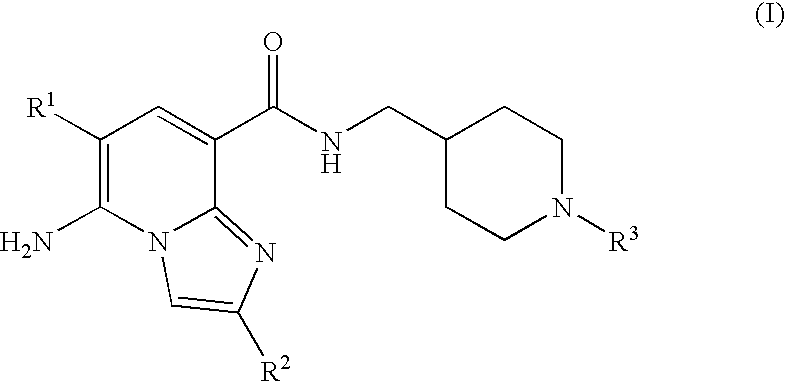
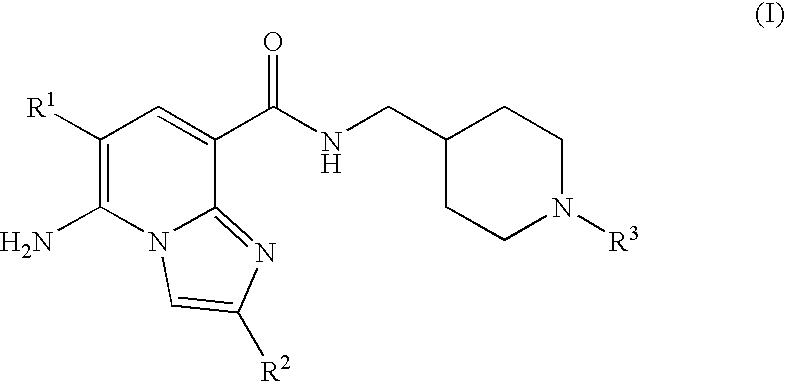
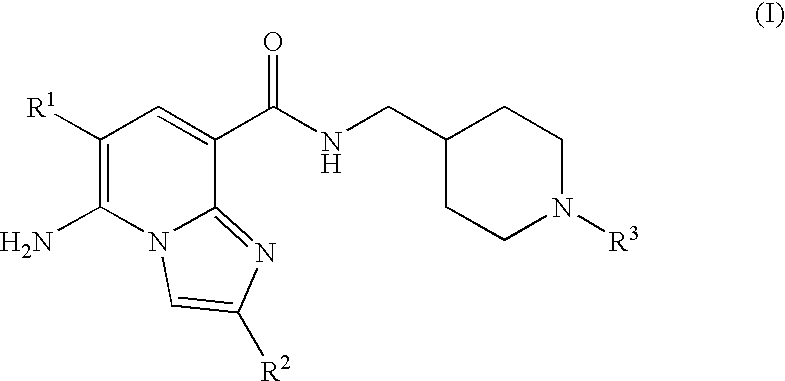
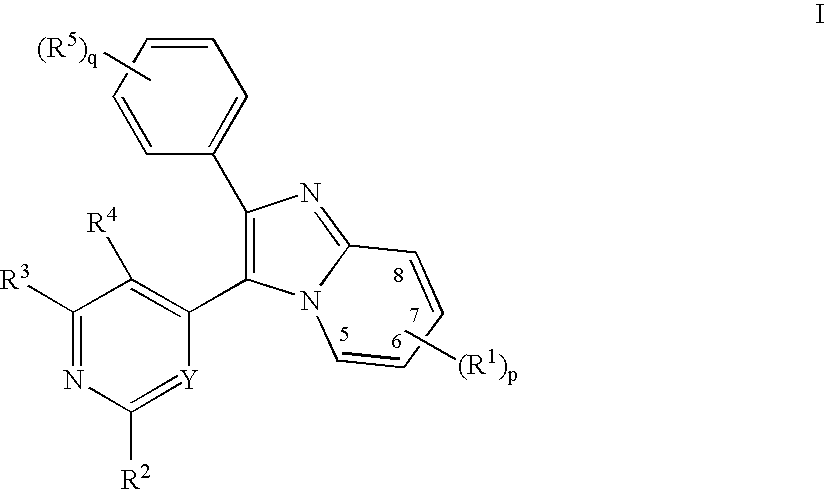
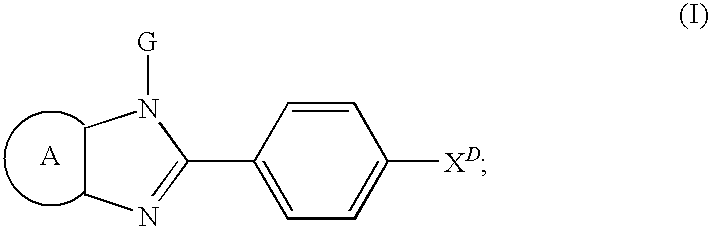
Substituted Imidazopyridinyl-Aminopyridine Compounds
The present invention relates to substituted imidazopyridinyl-aminopyridine compounds and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing substituted imidazopyridinyl-aminopyridine compounds and methods of treating cell proliferative disorders, such as cancer, by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:ARQULE INC
Pharmaceutical formulations comprising an immune response modifier
Pharmaceutical formulations comprising an immune response modifier (IRM) chosen from imidazoquinoline amines, imidazotetrahydroquinoline amines, imidazopyridine amines, 6,7-fused cycloalkylimidazopyridine amines, 1,2-bridged imidazoquinoline amines, thiazolo-quinolineamines, oxazolo-quinolinamines, thiazolo-pyridinamines, oxazolo-pyridinamines, imidazonaphthyridine amines, tetrahydroimidazonaphthyridine amines, and thiazolonaphthyridine amines; a fatty acid; and a hydrophobic, aprotic component miscible with the fatty acid are useful for the treatment of dermal associated conditions. Novel topical formulations are provided. In one embodiment, the topical formulations are advantageous for treatment of actinic keratosis, postsurgical scars, basal cell carcinoma, atopic dermatitis, and warts.
Owner:3M INNOVATIVE PROPERTIES CO
N-substituted piperidinyl-imidazopyridine compounds as 5-HT4 receptor modulators
This invention provides a compound of the formula (I): or a pharmaceutically acceptable salt, amide or ester thereof, wherein R<1 >represents a hydrogen atom or a halogen atom; R<2 >represents a hydrogen atom, etc.; R<3 >represents an alkyl group having from 1 to 10 carbon atoms; said alkyl group of R<3 >is substituted by at least one substituent selected from the group consisting of substituents alpha; said substituents alpha is aryl, hydroxy, oxo, etc.; said aryl having 6 to 10 carbon atoms; said aryl is unsubstituted or substituted by at least one alkyl group having from 1 to 6 carbon atoms; said heterocyclic and the heterocyclic moiety of said heterocycliccarbonyl, both of substituents alpha, are 5- to 10-membered cyclic groups containing from 1 to 4 heteroatoms selected from the group consisting of nitrogen atoms, oxygen atoms and sulfur atoms These compounds have 5-HT4 receptor binding activity, and thus are useful for the treatment of gastroesophageal reflux disease, non-ulcer dyspepsia, functional dyspepsia, irritable bowel syndrome or the like in mammalian, especially humans. This invention also provides a pharmaceutical composition comprising the above compound.
Owner:PFIZER INC
Imidazopyridine compounds as 5-HT4 receptor agonists
This invention provides a compound of the formula (I): or a pharmaceutically acceptable salt thereof, wherein R<1 >represents a hydrogen atom or a halogen atom; R<2 >represents a methyl group or an ethyl group; R<3 >represents a branched alkyl group having from 3 to 6 carbon atoms or an alkyl group having from 3 to 6 carbon atoms substituted by an alkoxy group having from 1 to 6 carbon atoms; with the proviso that when the terminal carbon atom of said alkyl group of R<3 >is substituted by said alkoxy group, said alkyl group is a branched alkyl group. These compounds have 5-HT4 receptor binding activity, and thus are useful for the treatment of gastroesophageal reflux disease, non-ulcer dyspepsia, functional dyspepsia, irritable bowel syndrome or the like in mammalian, especially humans. This invention also provides a pharmaceutical composition comprising the above compound.
Owner:PFIZER INC
Purine and imidazopyridine derivatives for immunosuppression
The present invention provides novel purine and imidazopyridine derivatives useful for the prevention and treatment of autoimmune diseases, inflammatory disease, mast cell mediated disease and transplant rejection. The compounds are of the general formulas:
Owner:PHARMACOPEIA INC CO LIGAND PHARM INC +1
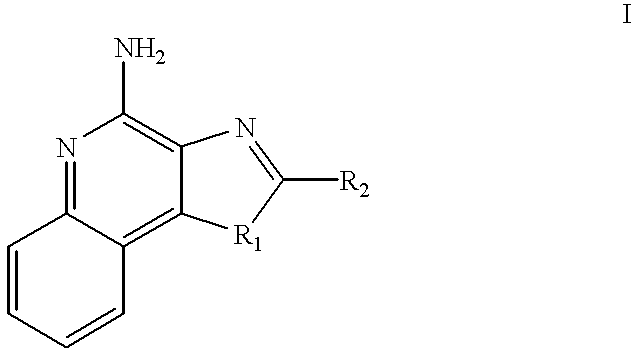
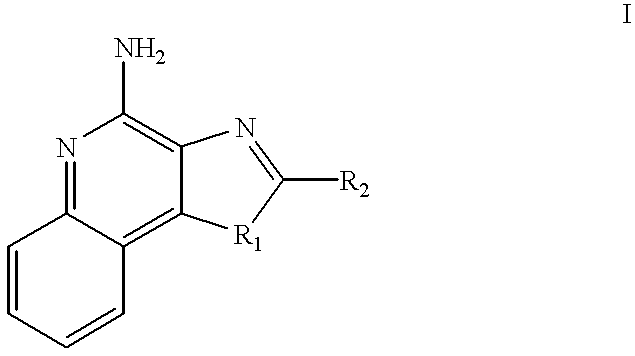
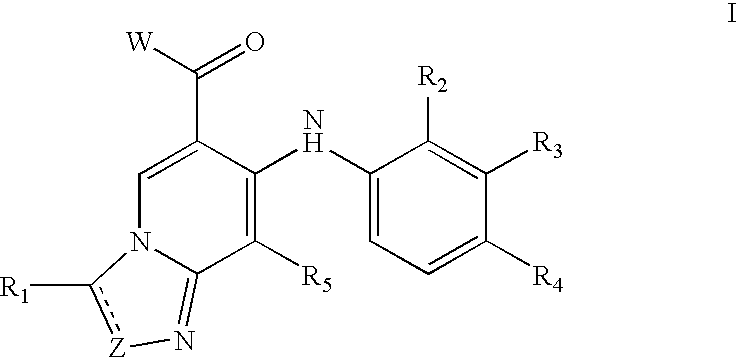
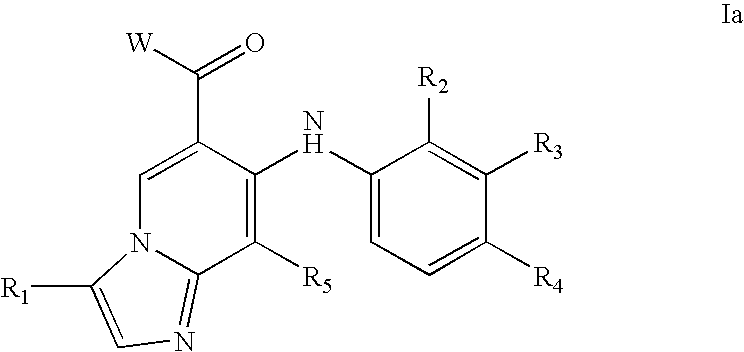
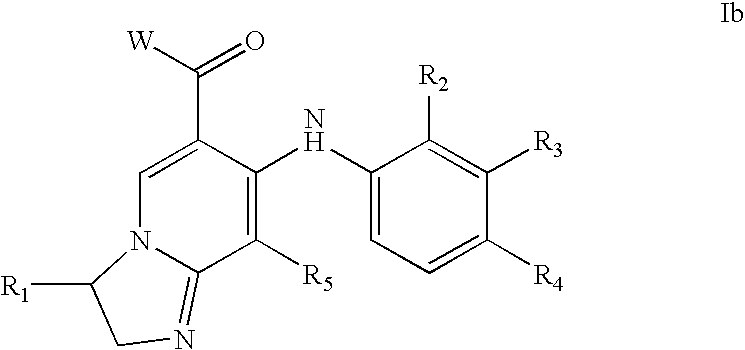
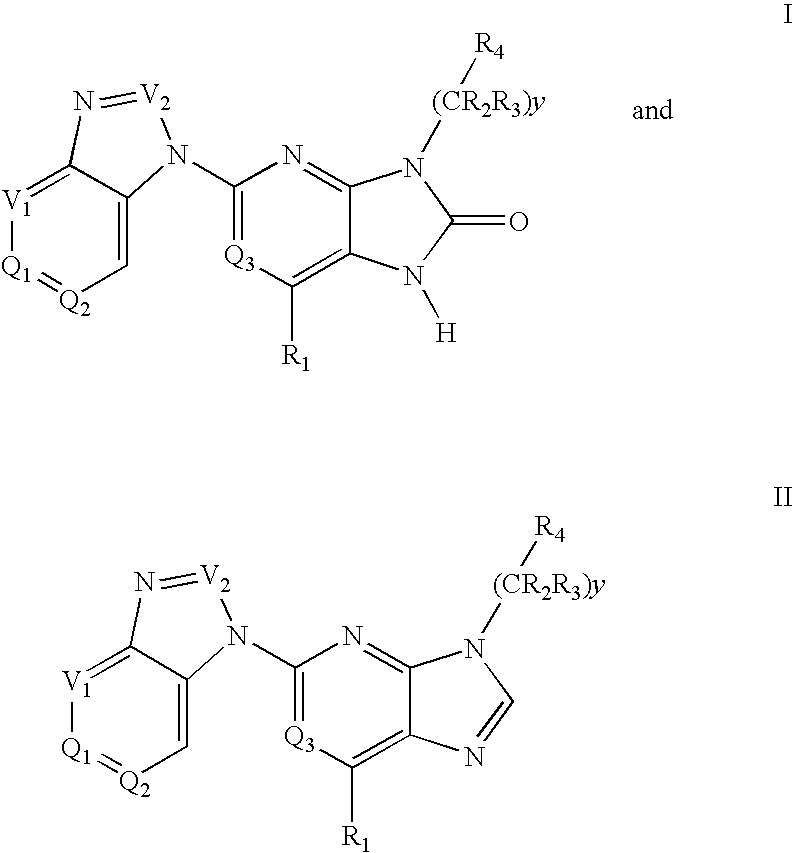
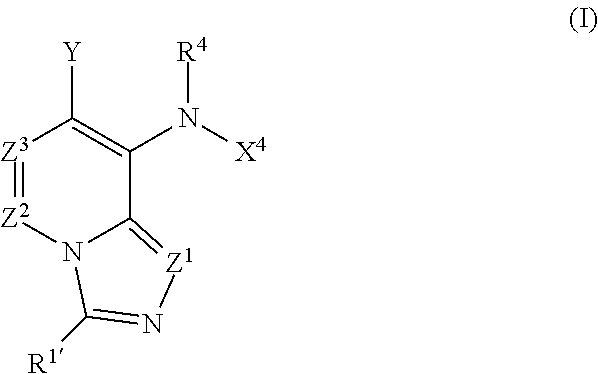
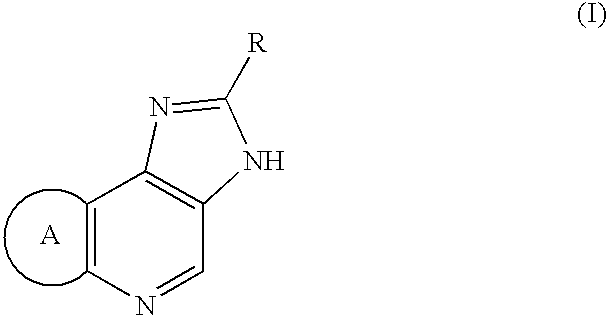
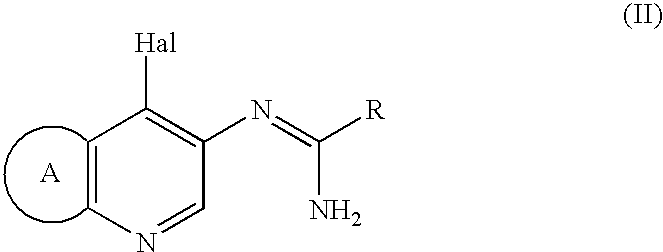
Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
InactiveUS20090325945A1Promote apoptosisInhibiting cell cycle progressionBiocideOrganic chemistryDiseaseMelanoma
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: wherein: J is independently —O— or —NRN1−; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j-M-(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently O—, —S—, —NH—, —NMe-, or —CH2—; each of RP1, RP2, RP5, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be CH═CH—CH═CH—; and additionally RP1 and RP5 taken together may be CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently CH2—, —NRN—, —C(═X)—, or —S(═O)2—; either: exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; either: exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—, or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
8-anilinoimidazopyridines and their use as Anti-cancer and/or Anti-inflammatory agents
The invention relates to imidazopyridines of formula I with anti-cancer and / or anti-inflammatory activity and more specifically to imidazopyridines which inhibit MEK kinase activity. The invention provides compositions and methods useful for inhibiting abnormal cell growth or treating a hyperproliferative disorder, or treating an inflammatory disease in a mammal. The invention also relates to methods of using the compounds for in vitro, in situ, and in vivo diagnosis or treatment of mammalian cells, or associated pathological conditions.
Owner:GENENTECH INC
N-substituted piperidinyl-imidazopyridine compounds as 5-HT4 receptor modulators
InactiveUS6951867B2Inhibitory activityReduced inhibitory activityBiocideNervous disorder5-HT4 receptorOxygen
This invention provides a compound of the formula (I): or a pharmaceutically acceptable salt, amide or ester thereof, wherein R1 represents a hydrogen atom or a halogen atom; R2 represents a hydrogen atom, etc.; R3 represents an alkyl group having from 1 to 10 carbon atoms; said alkyl group of R3 is substituted by at least one substituent selected from the group consisting of substituents α; said substituents α is aryl, hydroxy, oxo, etc.; said aryl having 6 to 10 carbon atoms; said aryl is unsubstituted or substituted by at least one alkyl group having from 1 to 6 carbon atoms; said heterocyclic and the heterocyclic moiety of said heterocycliccarbonyl, both of substituents α, are 5- to 10-membered cyclic groups containing from 1 to 4 heteroatoms selected from the group consisting of nitrogen atoms, oxygen atoms and sulfur atomsThese compounds have 5-HT4 receptor binding activity, and thus are useful for the treatment of gastroesophageal reflux disease, non-ulcer dyspepsia, functional dyspepsia, irritable bowel syndrome or the like in mammalian, especially humans. This invention also provides a pharmaceutical composition comprising the above compound.
Owner:PFIZER INC
Processes for the preparation of imidazo[1,2-a] pyridine derivatives
InactiveUS20060084806A1Easier and economical productionPrevent low temperatureOrganic chemistryCarboxylic acidSolvent
A process for the preparation of imidazo[1,2-a]pyridine derivatives of the formula I: wherein R is hydrogen, halogen or a C1-C4 alkyl group; R1 and R2 are independently hydrogen, a straight or branched C1-C6 alkyl group which is unsubstituted or substituted by one or more halogen atoms, hydroxyl, N(C1-C4 alkyl)2, carbamoyl or C1-C4 alkoxy radicals, a C1-C6 alkyl hydroxy group, a C3-C6 cycloalkyl radical, a benzyl radical, a phenyl radical or R1 and R2 together with the nitrogen atom to which they are bonded are joined together to form a substituted or unsubstituted heterocyclic group optionally containing one or more additional heterocyclic atoms; and R3 and R4 are independently hydrogen, halogen or a C1-C4 alkyl group, or a pharmaceutically acceptable salt thereof, the process comprising (a) reacting an imidazo[1,2-a]pyridine carboxylic acid of the formula II wherein R, R3 and R4 have the aforestated meanings with a halogenating agent in the absence of a solvent to form an acid halide intermediate and (b) reacting the acid halide intermediate with an amine of the formula HNR1R2 wherein R1 and R2 have the aforestated meanings to form the compound of formula I.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Purine and imidazopyridine derivatives for immunosuppression
Owner:WYETH LLC +1
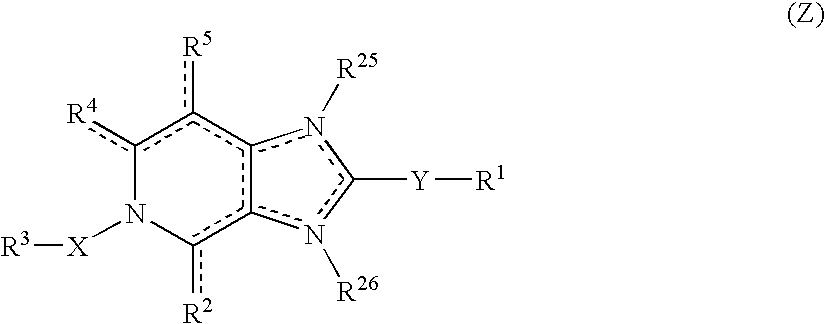
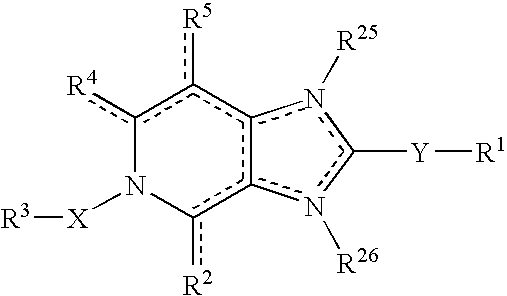
Viral inhibitors
The present invention relates to a pharmaceutical composition for the treatment or prevention of viral infections comprising as an active principle at least one imidazo[4,5-c]pyridine derivative having the general formula (Z): (formula). The invention also relates to processes for the preparation of compounds according to the invention having above mentioned general formula and their use as a medicine or to treat or prevent viral infections.
Owner:JOHNSON & JOHNSON PHARMA RES & DEV LLC +2
Therapeutic Compounds
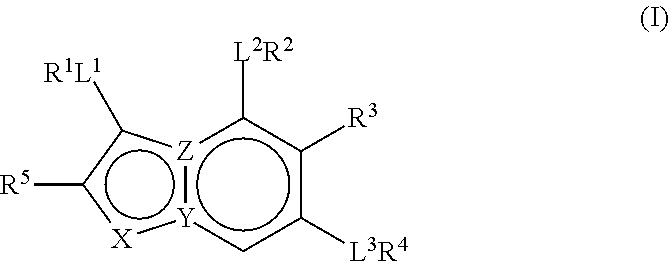
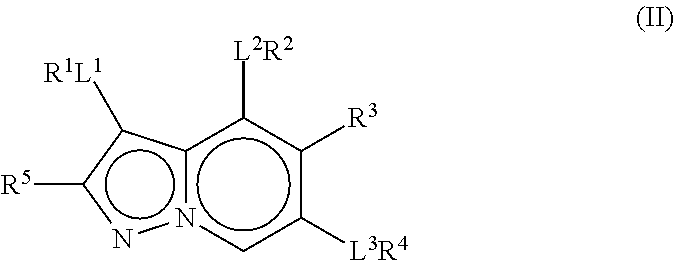
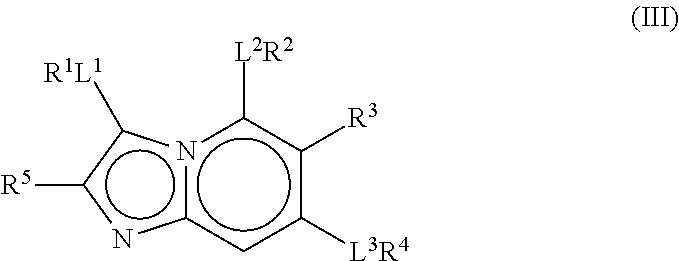
The present invention relates to pyrazolopyridines and imidazopyridines which are inhibitors of the kinase PDK1 and are thus useful for the treatment of myeloproliferative disorders or cancer. The compounds are also useful as inhibitors of other kinases such as FGFR3, NTRK3, RP-S6K and WEE1. Furthermore, the present compounds also selectively inhibit microtubule affinity regulating kinase (MARK) and are therefore useful for the treatment or prevention of Alzheimer's disease.
Owner:MERCK SHARP & DOHME CORP +1
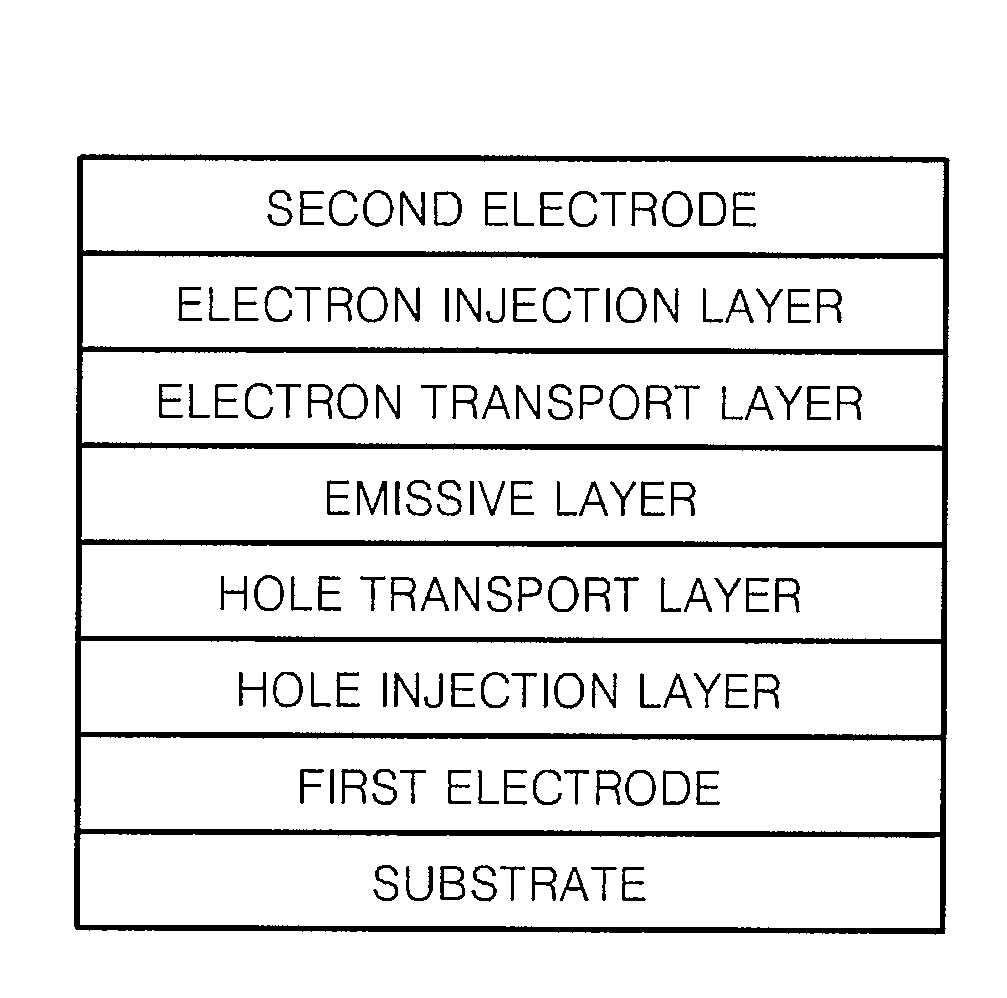
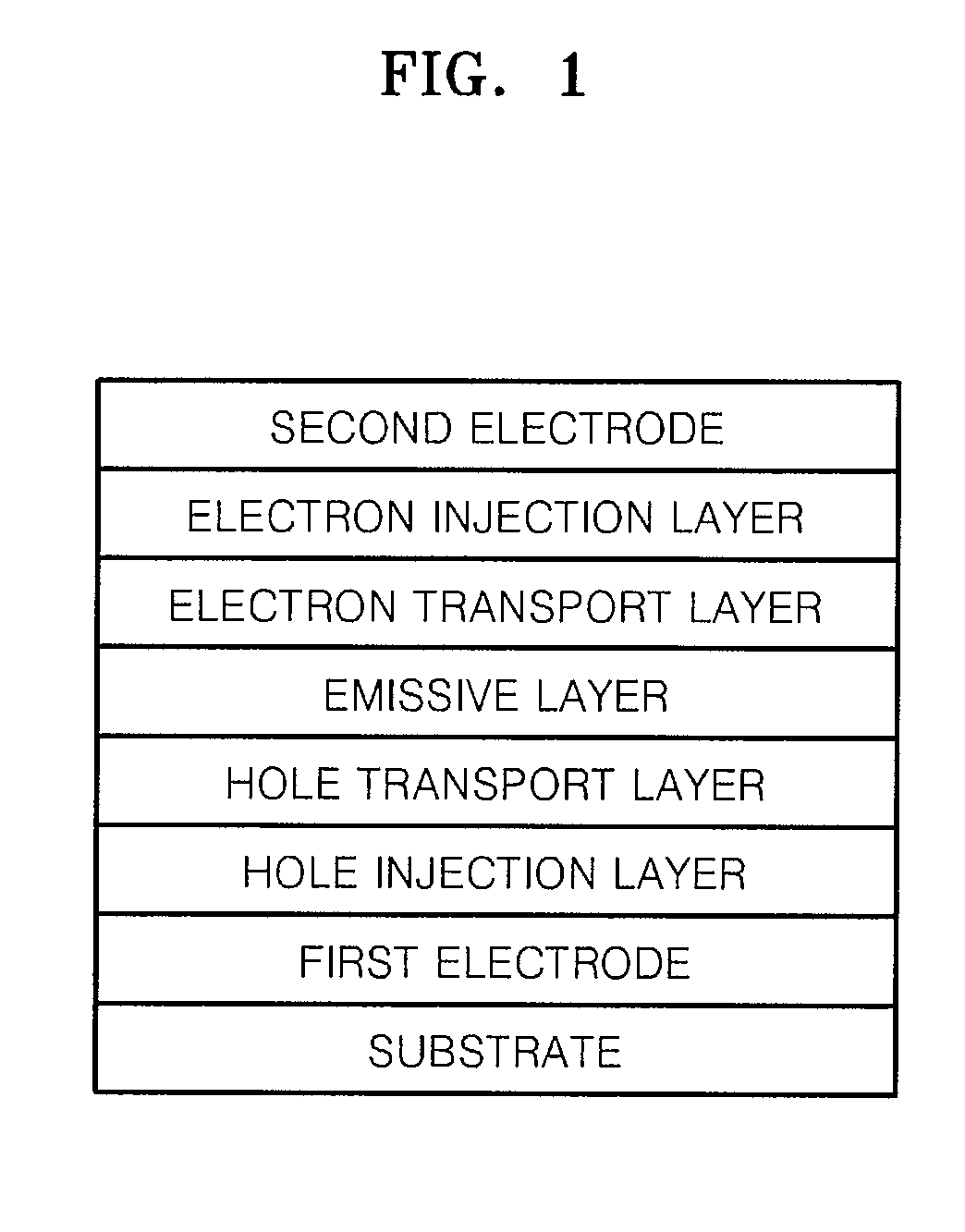
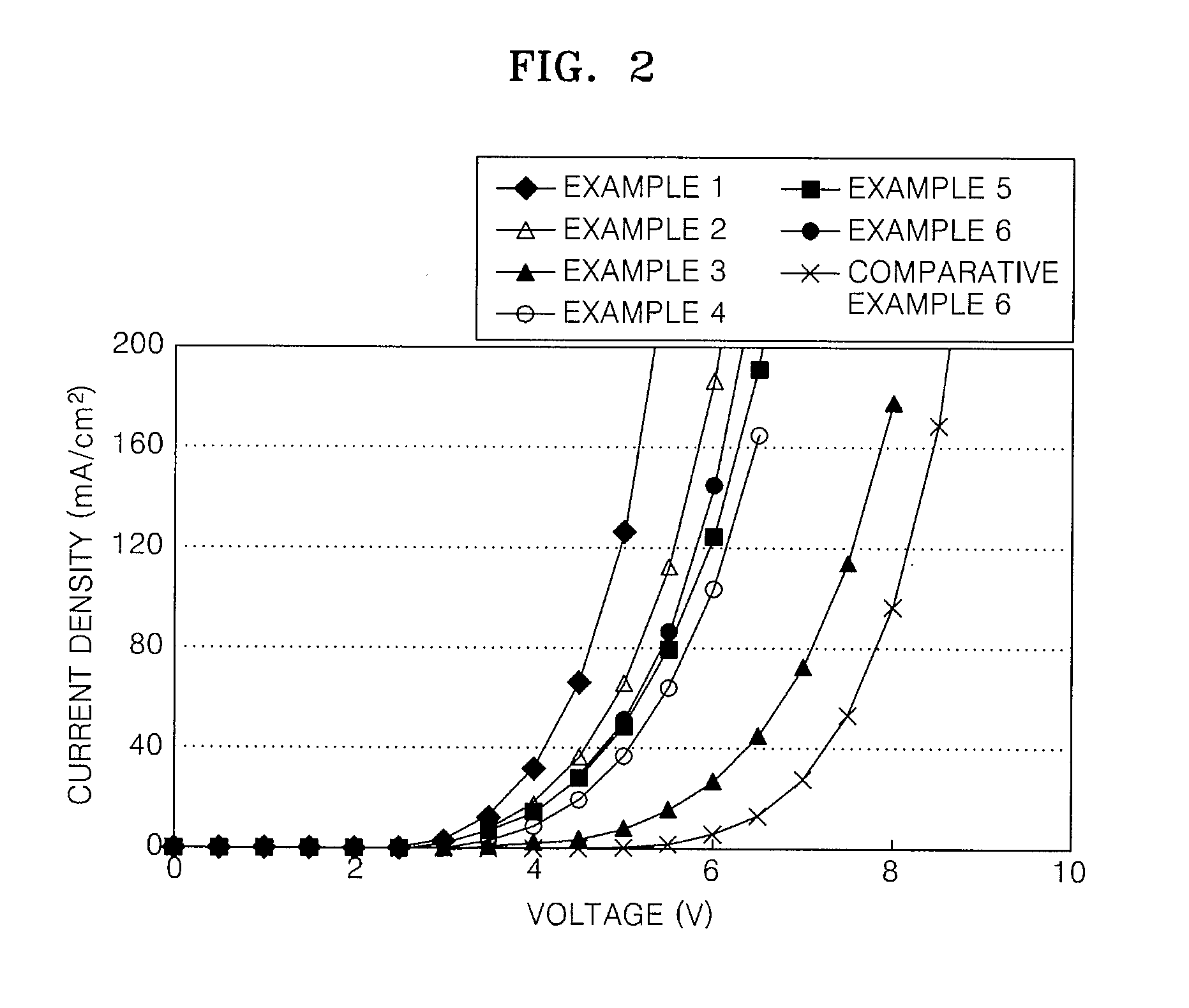
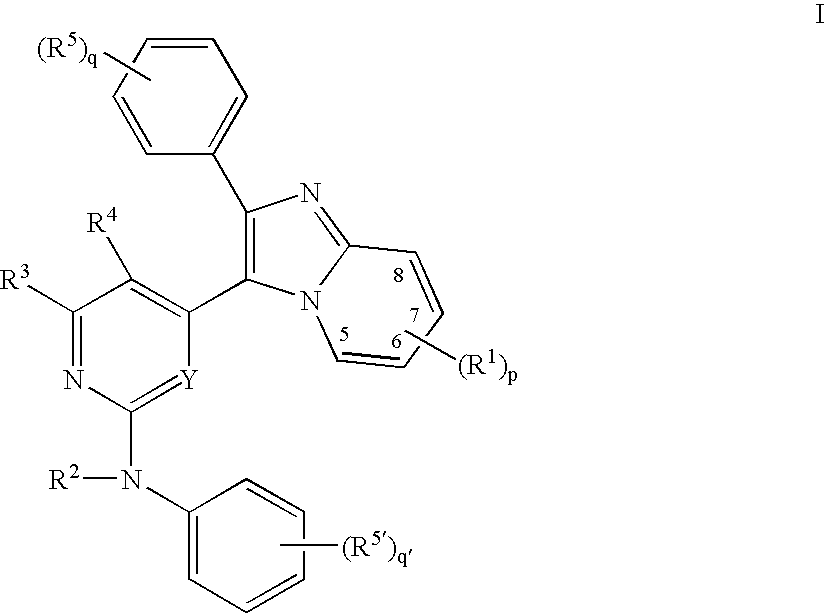
Imidazopyridine-based compound and organic light emitting diode including organic layer comprising the imidazopyridine-based compound
ActiveUS20080125593A1Enhanced electron transport capabilitiesReduce the driving voltageOrganic chemistryDischarge tube luminescnet screensOrganic layerLife time
Imidazopyridine-based compounds and organic light emitting diodes (OLEDs) including organic layers including the imidazopyridine-based compounds are provided. The organic light emitting diodes including organic layers having the imidazopyridine-based compounds have low driving voltages, high efficiencies, high luminance, long life-times and low power consumption.
Owner:SAMSUNG DISPLAY CO LTD
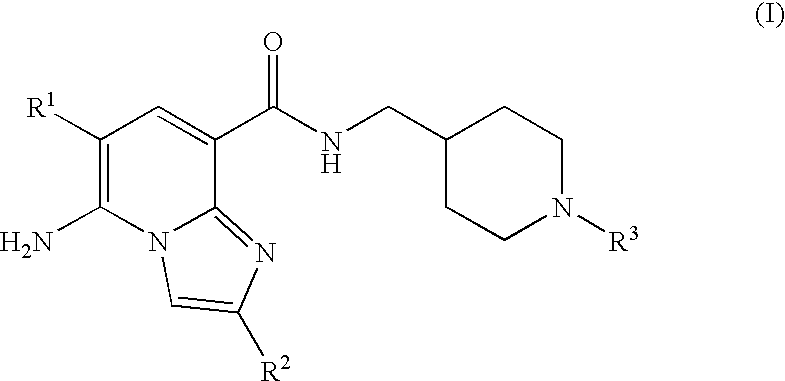
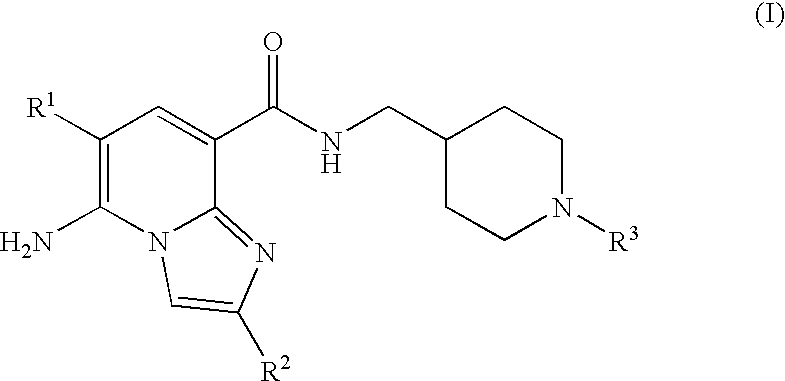
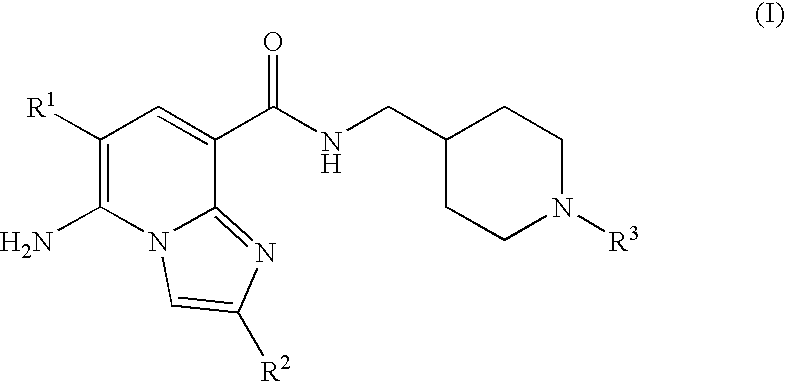
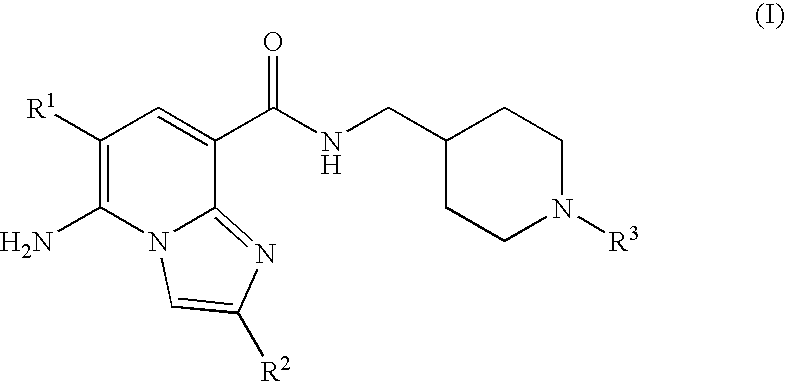
Imidazopyridine Kinase Inhibitors
The present invention provides imidazopyridine compounds, compositions containing the same, as well as processes for the preparation and methods for their use as pharmaceutical agents.
Owner:GLAXO SMITHKLINE LLC
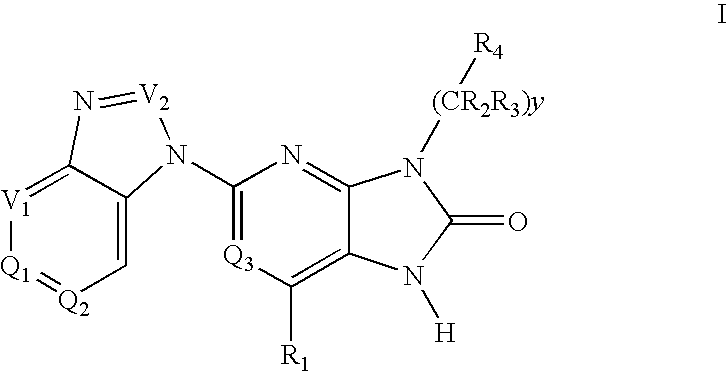
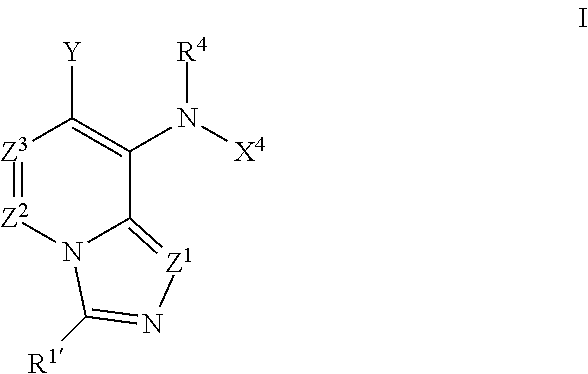
Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
Novel process for producing fused imidazopyridine derivatives and novel crystal form
InactiveUS20030088102A1Efficiently obtainedImprove stabilityNervous disorderOrganic chemistryArylPyridine phosphate
The present invention provides a process of a compound of the formula (I): wherein R is heteroaryl or the like, ring A is a heteroalicyclic group or the like comprising reacting a compound of the formula (II): wherein Hal is halogen and the other symbols are the same as the above, in the presence of a sulfinic acid salt and further in the presence of an acid or a salt with an organic base, and a novel crystal form of 2-(3-isoxazolyl)-3,6,7,9-tetrahydroimidazo[4,5-d]pyrano[4,3-b]pyridine phosphate monohydrate.
Owner:SHIONOGI & CO LTD
Synthesis and Anti-proliferative effect of benzimidazole derivatives
This invention provides for compounds, compositions, and methods that involve anti-proliferative and anti-neoplastic activity in cancer cells. In particular, a series of benzimidazole, purine, imidazopyridine, and imidazopyrizine compounds having selected substitution patterns are disclosed, and the activity of various subject compounds is demonstrated.
Owner:SPELMAN COLLEGE
3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use
The invention discloses 14 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters shown in the general formula I. In the general formula I, AA represents L-Ser residue, L-Glu (OBzl) residue, L-Phe residue, L-Val residue, L-Arg residue, L-Tyr residue, L-Ala residue, L-Trp residue, L-Asn residue, L-Met residue, L-Ile residue, Gly residue, L-Asp (OBzl) residue or L-Leu residue. The invention discloses a preparation method of the 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters, discloses HT-29, K562, A549 and HL60 tumor cell growth inhibition effects of the 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters, further discloses S180-loading mice tumor growth inhibition effects of the 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters, and also discloses use of the 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters as antitumor drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives
The present invention is concerned with aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives of formula I: wherein R1 to R5 are as defined in the specification and pharmaceutically acceptable acid addition salts thereof. This class of compounds has high affinity and selectivity for GABA A α5 receptor binding sites and might be useful as cognitive enhancer or for the treatment of cognitive disorders like Alzheimer's disease.
Owner:F HOFFMANN LA ROCHE & CO AG
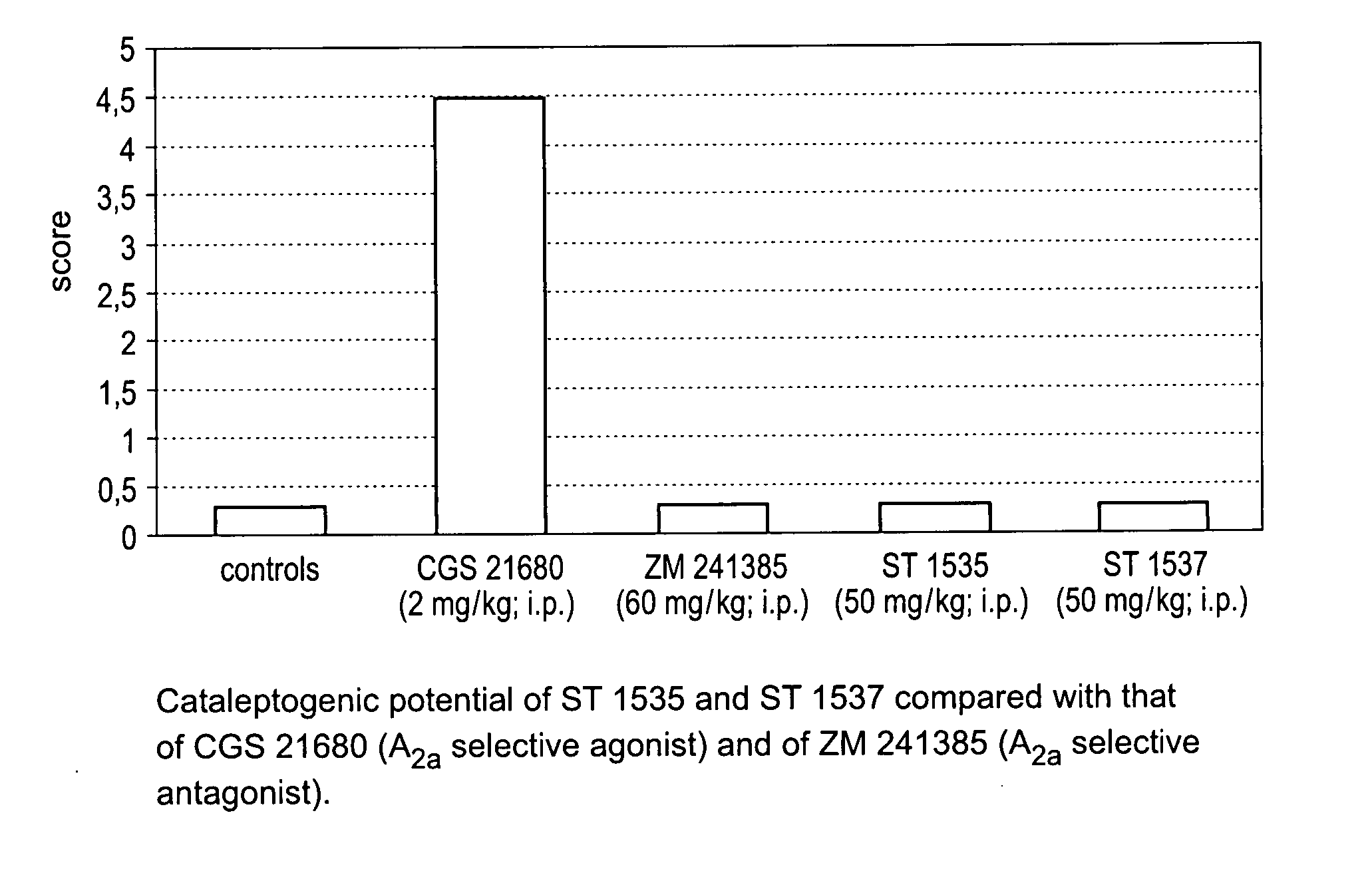
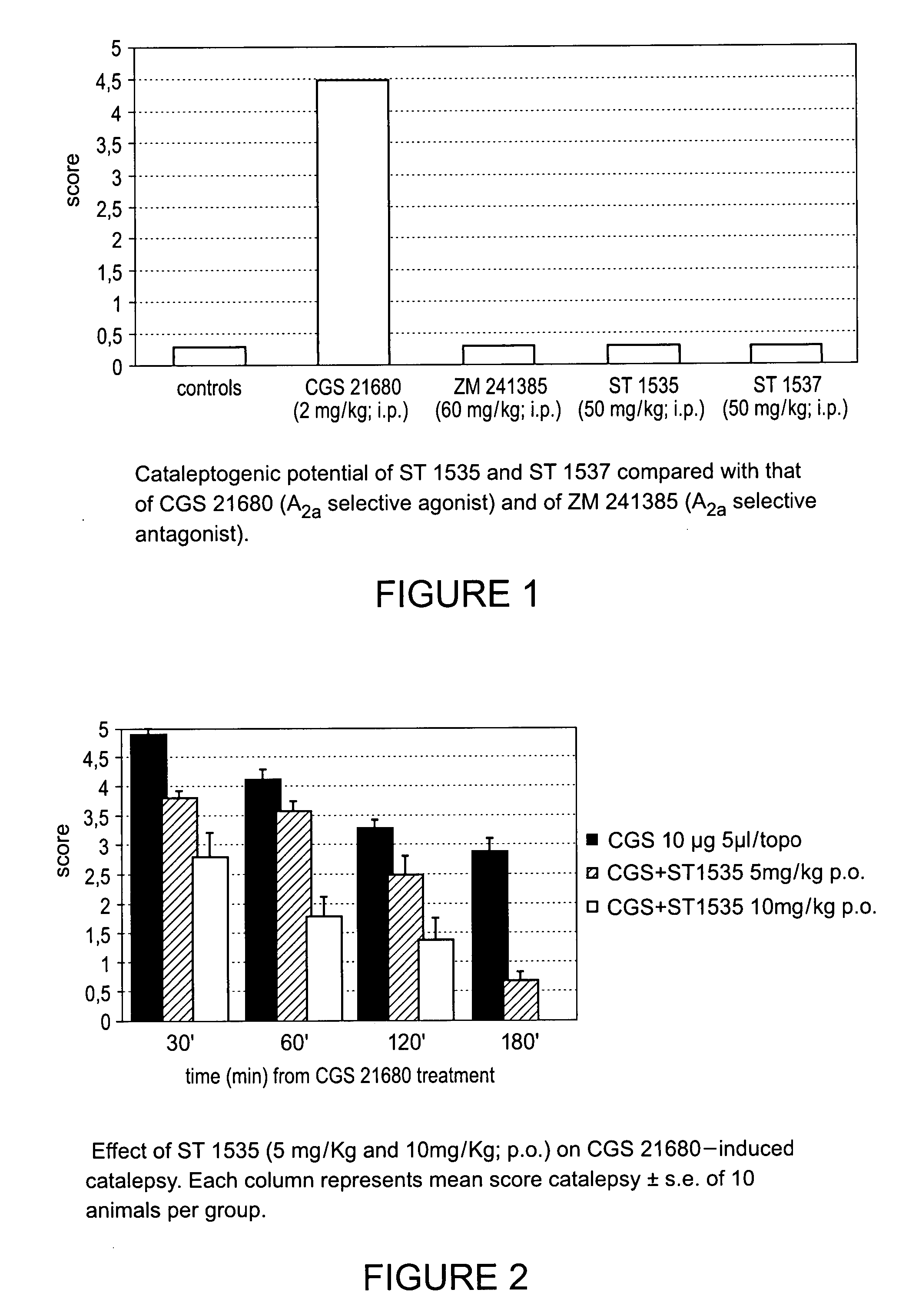
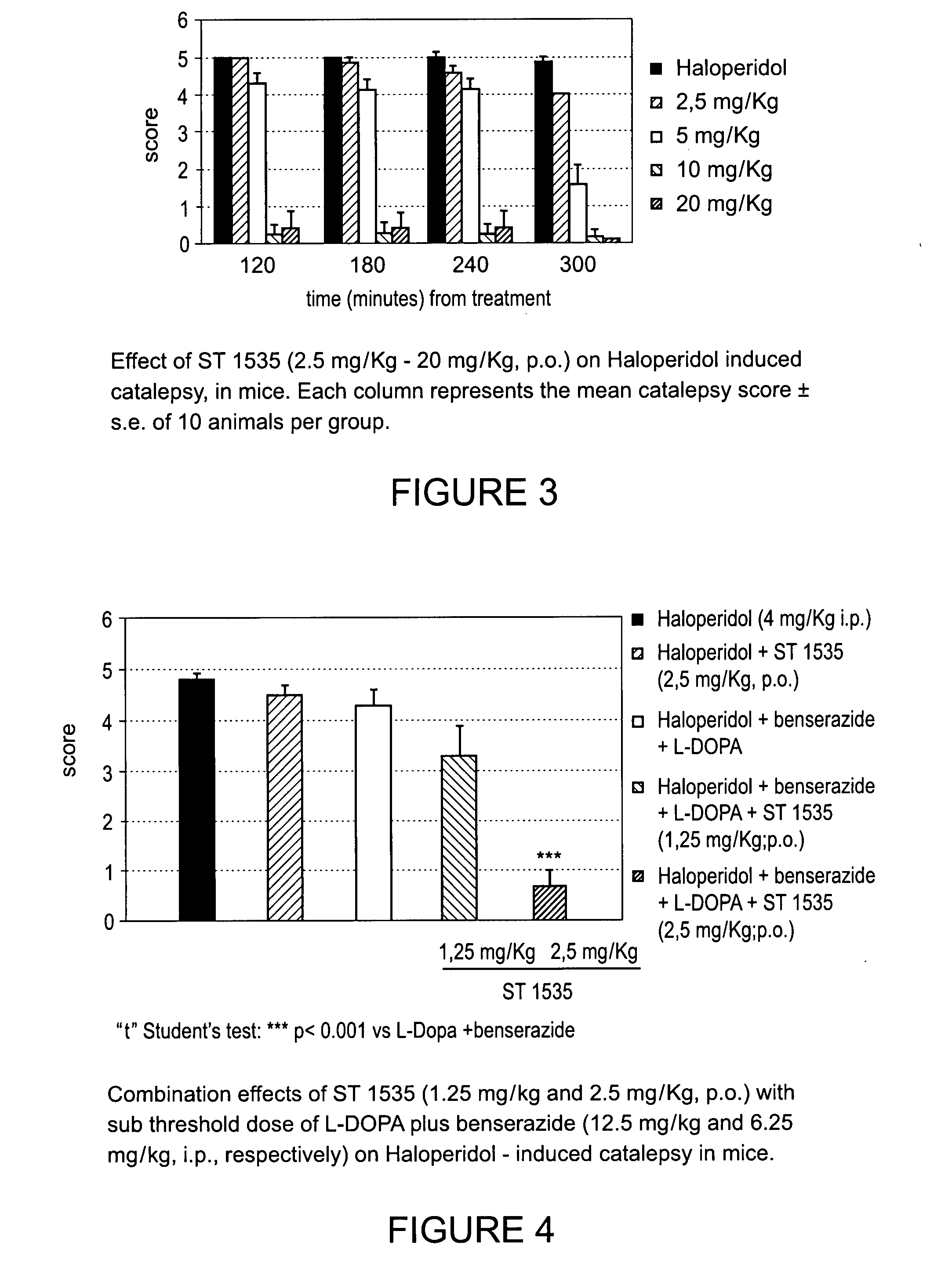
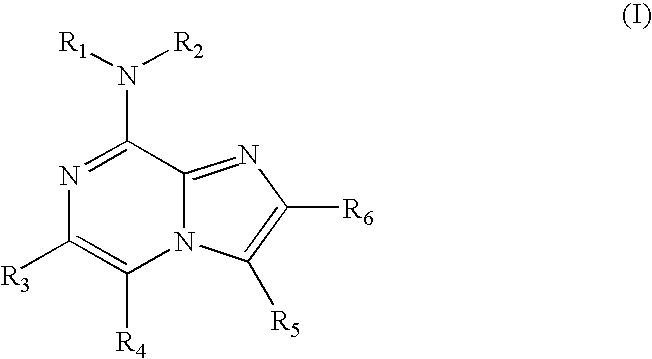
Derivatives of triazoly-imidazopyridine and of the triazolypurines useful as ligands of the adenosine A2a receptor and their use as medicaments
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD](https://images-eureka.patsnap.com/patent_img/dd7fd95c-69c9-47a0-b959-70a3a8b9ca19/HDA00002768079800011.PNG)
![Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD](https://images-eureka.patsnap.com/patent_img/dd7fd95c-69c9-47a0-b959-70a3a8b9ca19/HDA00002768079800012.PNG)
![Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD Application of 2-arylimidazo[1,2-alpha]pyridine-3-acetamide derivative to prepare medicines for controlling PTSD](https://images-eureka.patsnap.com/patent_img/dd7fd95c-69c9-47a0-b959-70a3a8b9ca19/HDA00002768079800021.PNG)
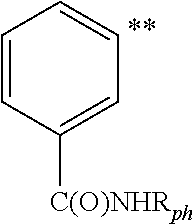
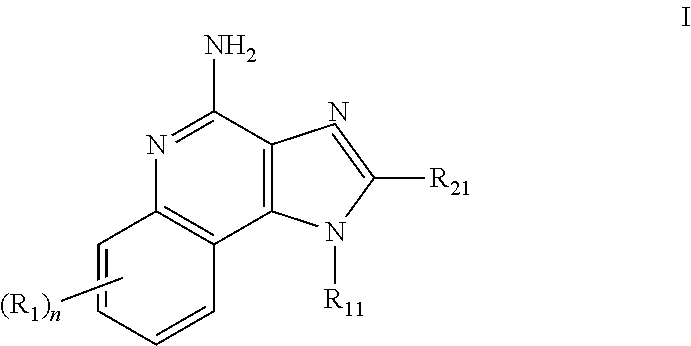
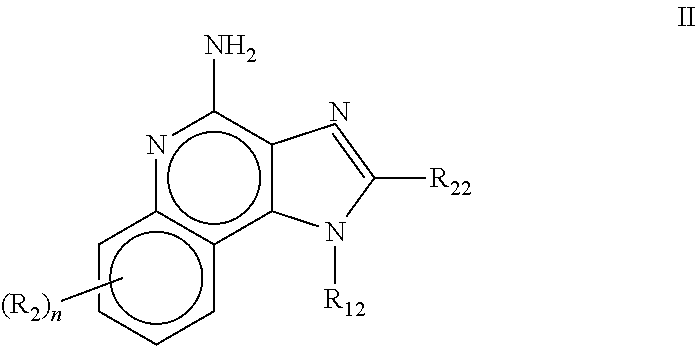
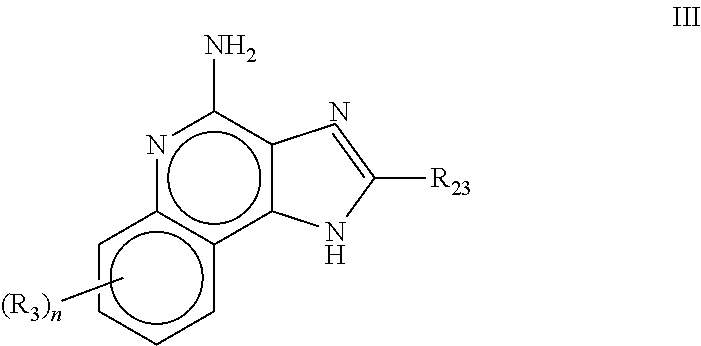
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00001.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00002.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00003.png)
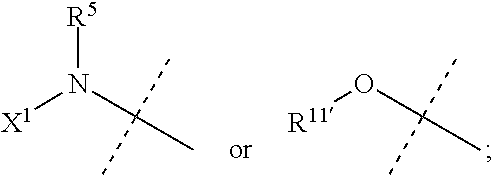
![Processes for the preparation of imidazo[1,2-a] pyridine derivatives Processes for the preparation of imidazo[1,2-a] pyridine derivatives](https://images-eureka.patsnap.com/patent_img/7f805e1a-42a3-469f-a93f-ab2bbc6e01f8/US20060084806A1-20060420-C00001.png)
![Processes for the preparation of imidazo[1,2-a] pyridine derivatives Processes for the preparation of imidazo[1,2-a] pyridine derivatives](https://images-eureka.patsnap.com/patent_img/7f805e1a-42a3-469f-a93f-ab2bbc6e01f8/US20060084806A1-20060420-C00002.png)
![Processes for the preparation of imidazo[1,2-a] pyridine derivatives Processes for the preparation of imidazo[1,2-a] pyridine derivatives](https://images-eureka.patsnap.com/patent_img/7f805e1a-42a3-469f-a93f-ab2bbc6e01f8/US20060084806A1-20060420-C00003.png)
![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00001.png)
![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00002.png)
![Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-B]pyridin-2-one and oxazolo[4, 5-B] pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/56ad8e42-9fd9-4d02-ba70-fb173ab812ce/US07951819-20110531-C00003.png)
![3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use](https://images-eureka.patsnap.com/patent_img/56f44749-4c00-4a88-b868-c17ab9b2c997/HSA00000725950000011.PNG)
![3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use](https://images-eureka.patsnap.com/patent_img/56f44749-4c00-4a88-b868-c17ab9b2c997/BSA00000725949900011.PNG)
![3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use 3H-imidazo[4,5-c]pyridine-6-formyl-amido acid benzyl esters and their synthesis, anti-tumor activity and use](https://images-eureka.patsnap.com/patent_img/56f44749-4c00-4a88-b868-c17ab9b2c997/BSA00000725949900021.PNG)
![Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/646229ad-657d-4e8d-ad57-cde733012e60/US20070179178A1-20070802-C00001.png)
![Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/646229ad-657d-4e8d-ad57-cde733012e60/US20070179178A1-20070802-C00002.png)
![Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives Aryl-isoxazol-4-yl-imidazo[1,2-a]pyridine derivatives](https://images-eureka.patsnap.com/patent_img/646229ad-657d-4e8d-ad57-cde733012e60/US20070179178A1-20070802-C00003.png)